Abstract
Couples influence each other’s mental and physical health. This review focuses on how couples’ relationships, the partners’ individual and joint vulnerabilities, and their health behaviors influence health through changes in the gut microbiota, metabolism, and immune function. Couples’ shared stressors and emotions and their intertwined lifestyles and routines serve to promote common disease risks in part through parallel changes in their gut microbiotas. Marital discord, stress, and depression have strong bidirectional links, fueling one another. Chronic marital stress and depression can elevate the risk for obesity, metabolic syndrome, and cardiovascular disease by altering resting energy expenditure, insulin production, and triglyceride responses following unhealthy meals. During stressful times, health behaviors typically suffer—and sleep disturbances, poor diets, and sedentary behavior all influence these metabolic pathways while also promoting gut dysbiosis. Dysbiosis increases intestinal permeability (gut leakiness), providing a mechanistic pathway from marital distress and depression to heightened inflammation and accelerated aging. Age-related changes in the gut microbiota’s composition and gut leakiness foster immunosenescence, as well as the progression of inflamm-aging; these age-related risks may be altered by stress and depression, diet, sleep, exercise habits, and developmental shifts in emotion regulation strategies. Consideration of the strong mutual influences that partners have on each other’s mood and health behaviors, as well as the biological pathways that underlie these influences, provides a new way to view marriage’s health implications.
Keywords: Marriage, gut microbiota, inflamm-aging, gut dysbiosis, immunosenescence, health behaviors
Couples influence each other’s mental and physical health. Recent studies have demonstrated notable spousal concordance in gene expression patterns, cellular immune profiles, and inflammation (1–3). Furthermore, couples’ intestinal microbial communities – or gut microbiotas - and health behaviors are also more similar to each other than those of unrelated partners, providing common pathways for shared disease risks (4, 5).
Marital discord, stress, and depression have strong bidirectional links, and they alter health behaviors and biological pathways in ways that compromise health (6). Even in satisfying marriages, couples’ interconnected health behaviors can be beneficial or harmful, as couples’ diets, exercise habits, and sleep unite to influence the gut microbiota. In addition, developmental shifts in goals and emotion regulation strategies may shape couples’ relationships, and may interplay with age-related changes in the gut microbiota to extend couples’ healthy years or hasten decline (Figure 1).
Figure 1.
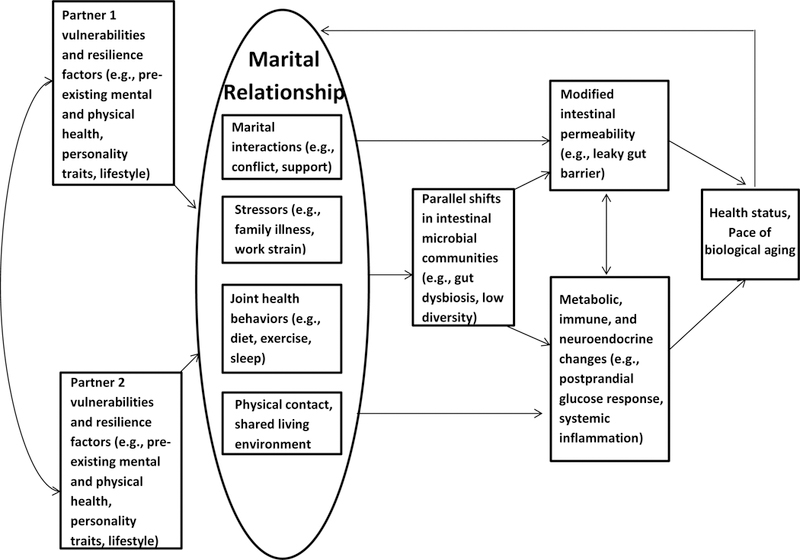
Conceptual model summarizing the paths by which the marital relationship may influence health and aging via the gut. Often interrelated, both partners’ characteristics—e.g., their own prior mental and physical health, personality traits, and lifestyles—affect the relationship. In turn, partners’ marital interactions, the stressors they face together and individually, their health behaviors, as well as their physical contact and living environment have physiological consequences. Namely, they may augment intestinal permeability, as well as metabolic, immune, and neuroendocrine function directly, or indirectly, in part through changes in intestinal microbial communities. Thereafter, these mutually reinforcing physiological cascades shape partners’ health status and set the pace of aging. Ultimately, changes in health and biological aging feed back to affect life with one’s partner.
Spousal Similarities: Gene Expression, Immune Profiles, and the Gut Microbiota
Recent studies have provided mechanistic insights into spouses’ shared disease risks. Gene expression, shaped by both genetic and environmental regulators, plays a fundamental role in determining biological function. In this context it is significant that across a sizable number of genes, husbands’ and wives’ gene expression patterns exhibited noteworthy transcriptional similarity, with much smaller variances in couples than random pairs (1). For example, a gene that has been associated with coronary artery disease (CAD) and hypercholesterolemia was among those that showed the “couple effect” of transcription; spousal concordance has been demonstrated for CAD risk and cholesterol, among many others (5).
Another research team used a systems-level approach to determine cellular immune profiles for 670 healthy people (2). Cohabitation had the strongest influence: couples living together had 50% less variation in 54 different immune parameters than did unrelated pairs. This effect was independent of age, and extended to stimulated cytokine production. In fact, the authors concluded that living together had a stronger relationship to immune system parameters than acute and untreated gastroenteritis.
The bacteria and viruses that inhabit the gut help to educate the immune system, and thus it is no surprise that an analysis of cohabiting couples’ stool samples revealed that they also share similar gut microbiotas (4). Physical interaction promotes microbial sharing, and couples’ intimate behaviors—touching, kissing, and sex—ensure microbial transfers (7). Importantly, factors including diet, drugs, and body measurements account for over 20% of between-person gut microbiota variability – a large sphere of influence for couples’ shared living space and lifestyle patterns; in contrast, genetics have only a minor influence, less than 2% (4).
As the number of bacterial cells closely mirrors the number of human cells in the human body, microbiota variability has real-world implications (8). Even after controlling for age, gender, diet, and host genetics, microbiota data accounted for substantial variability in body mass index (25%), fasting glucose (22%), high-density lipoprotein (36%), waist circumference (29%), waist-hip ratio (24%), and lactose consumption (36%) (4). For example, even when people eat identical foods, their blood glucose responses are highly variable, and largely pathogenic bacteria phyla positively correlate with glucose responses – demonstrating the microbiota’s influence on metabolism (9). Thus, between-person microbiota variability may help to explain metabolic differences.
This within-couple microbiota similarity (4) parallels some of the spousal linkages reported for major coronary risk factors (diastolic blood pressure, triglycerides, total and low-density lipoprotein cholesterol, body mass index, and waist-to-hip ratio), as well as health conditions (diabetes, metabolic syndrome, hypertension, arthritis, cancer, asthma, peptic ulcer disease, and physician-diagnosed hay fever) (5). The spousal ties are sizable: people whose partners have asthma or peptic ulcer disease have a 70% or greater increased risk themselves for these conditions, even after controlling for partners’ age, smoking, and obesity (10), while a spouse’s hypertension increases the partner’s risk two-fold (11), and a partner’s arthritis almost triples the odds (12). New analyses from the UK Understanding Society panel showed spousal concordance for adiposity measures, blood pressure, heart rate, and two inflammatory markers, C-reactive protein (CRP) and fibrinogen (3).
Dyadic Pathways: Couples’ Shared Stressors, Emotions, and Health Behaviors
Beyond the effects of a common living environment and physical touch, couples’ shared stressors may help to drive biological convergence and compound health risks (Figure 1). Whether they encounter a stressful event together, e.g., when a child is sick, or bring home their work strain, partners tend to feel similarly stressed (13). According to ecological momentary studies, bad moods are particularly contagious in close proximity—i.e., when couples are physically together, or have experienced the same stressor and work jointly on the problem (14, 15).
Happy and unhappy marriages can both transmit stress via mood contagion, perhaps through distinct routes. In the daily diaries of people with chronic pain and their spouses, happier wives reported greater emotional distress on days when their spouse was suffering more compared to days with lower suffering (16). On the other hand, marital discord can fuel negative affect reciprocity, as unhappier spouses’ negative moods and cortisol patterns tracked more closely together than those of happier couples (17).
Stress can drain marital resources. Among dual-earner couples, spouses were less responsive to each other after more demanding work days compared to less stressful days (14). The relationship faces greatest risks when both partners are stressed, and among couples who lack strong conflict resolution skills (13). Marital discord carries its own health consequences, promoting additional stress and depression (5). In turn, having a depressed partner doubles the risk of becoming depressed (10).
The stress that accompanies a troubled marriage promotes adverse health behaviors. However, couples’ convergence or interdependence can also prove problematic in untroubled marriages if their shared behaviors are unhealthy. Couples’ diet, sleep, exercise, smoking, and alcohol consumption show significant associations (5), and each of these behaviors influences the microbiota (18).
In particular, apart from the sleep-disrupting effects of marital conflict, sharing a bed or a sleep routine may be sufficient to spread health risks between partners. Indeed, couples share remarkable similarity in their sleep patterns: when partners’ minute-by-minute night actigraphy was compared, more than half the time, when a person was awake during the night, the partner was too (19). Although partners with more similar bedtimes and higher minute-to-minute sleep concordance report greater relationship satisfaction and fewer mood symptoms (19, 20), actigraphy assessments reveal that sleep is more restless with a bed partner than alone (21). Accordingly, people whose partners report chronic sleep problems have higher inflammation (22) as well as poorer self-rated health and higher levels of depressive symptoms than those with well-rested partners (21, 23).
The Gut Microbiota: Inflammation and Health
A healthy gut microbiota is diverse – a term that captures both the number of distinct bacterial species (i.e., richness) and the relative abundance, or evenness, of the species. The goal is homeostatic equilibrium, with pathogenic populations counterbalanced by commensal, i.e., beneficial, populations. A greater number of species as well as an even distribution prevents the overgrowth or dominance of a particular species and supports healthy metabolic and immune function. People with low bacterial richness have greater adiposity, insulin resistance, and dyslipidemia, as well as a more pronounced inflammatory phenotype compared to those with high bacterial richness; additionally, among those with lower richness, obese individuals also gain more weight over time (24). Low diversity in the bacterial community structure makes the host more vulnerable when challenged or perturbed (Figure 1).
Some evidence suggests that depressed patients’ microbiotas are less diverse than those of controls (25). In fact, the microbiota composition may promote depression; transferring fecal matter from people with a major depression diagnosis to microbiota-deficient rats induced depressive-like behavior in the rats (25). This link is relevant for marriage: both syndromal depression and depressive symptoms are strongly associated with marital distress (26). Troubled marriages provide fertile soil for depression, and depression promotes poorer marital quality, both pathways that can impact the microbiota (26).
The bidirectional communication between the gut and brain -- the gut-brain axis -- involves multiple depression- and stress-responsive pathways including the sympathetic nervous system, the hypothalamic-pituitary-adrenal axis, the vagus nerve, and the immune system. Through these diverse pathways depression and stress can provoke gut dysbiosis (adverse changes in gut microbiota profiles). Microbes fortify the gut barrier, ensuring that food particles, toxins, and pathogens do not enter the bloodstream, but gut dysbiosis can disrupt barrier integrity, producing intestinal permeability (27). Translocation of bacterial endotoxin (lipopolysaccharide, LPS) from the gut microbiota to blood circulation – the result of a “leaky gut”-- stimulates systemic inflammatory responses (28, 29).
Long-term diets have major effects on the microbiota (7). Typical Western-style diets (high intake of red meat, refined sugars, and saturated fat) generate lower overall microbial diversity than plant-based diets like the Mediterranean diet (18). The Western diet can increase microbial populations that are more efficient in harvesting energy from food, facilitating weight gain. Furthermore, high saturated fat diets induce gut dysbiosis, and promote gut permeability (18). Couples are highly concordant on diet, particularly total fat (30), providing one prime pathway to shared risk. Stress has been associated with poorer diets, e.g., greater reliance on high-fat, high-sugar comfort foods, and couples in troubled marriages have poorer diets (31).
To investigate increased gut permeability as one potential mechanistic pathway from marital distress to heightened inflammation, we examined LPS-binding protein (LBP), a surrogate marker of microbial translocation, in healthy married couples. The couples discussed a marital disagreement during two separate sessions; behavioral coding of these interactions provided data on hostile marital behaviors, a hallmark of marital distress. Participants with more hostile marital interactions had less healthy diets as well as higher LBP than those who were less hostile. Higher LBP was associated with greater CRP production; for example, compared to low LBP participants (25th percentile), those in the 75th percentile had 79% higher CRP (31).
The gut microbiota can also impact energy balance, glucose metabolism, and obesity-related inflammation, in part through gut leakiness (32). Recent work from our lab has shown that stress and a mood disorder history alter metabolic responses to high-fat meals (33–35). In one study, couples ate a high-fat meal and then discussed a marital disagreement during each of two visits (34). When combined with a mood disorder history, men and women who had more hostile marital interactions had lower post-meal energy expenditure, and this disparity was clinically meaningful: 128 kcal, a difference that could add ~7.7 pounds/year. Furthermore, higher levels of hostile behaviors among those who had a mood disorder history were also associated with higher post-meal insulin compared to other participants. Higher insulin levels stimulate food intake and visceral fat accumulation (36), and thus would act in tandem with lower energy expenditure to promote obesity.
In addition to unhealthy diets, marital discord, stress, and depression, disturbed sleep, and sedentary lifestyles influence the gut microbiota and promote intestinal permeability, and thus enhance inflammation. During stressful times, health behaviors often suffer; therefore, sleep disturbances, poor diets, and sedentary behavior may interact with marital discord and depression to further exacerbate gut dysbiosis and bacterial translocation (18).
Sleep and its Links to Metabolism and the Gut Microbiota
Sleep problems elevate risks for diabetes, cardiovascular disease, cancer, and premature death (e.g., 37), perhaps in part through their effects on metabolism and weight (38). Both sleep restriction and circadian disruption—sleep’s misalignment with the body’s internal clock—alter appetite hormones in a way that makes people hungrier and eat more (38). The gut microbiota may mediate this effect, because gut microbes also affect production of leptin, an appetite-stimulating hormone (7). Following a night of poor sleep, people also tend to eat more food high in saturated fat and simple carbohydrates, exacerbating the effects of circadian misalignment and sleep loss (39).
Even overweight people on a calorie-restricted diet who slept poorly lost less fat than those on the same diet who slept well, suggesting that sleep disruption slows fat metabolism regardless of calorie intake (38). Moreover, healthy people who were restricted to 4 h of sleep for 6 nights showed a 40% decrease in glucose clearance, comparable to diabetic levels (38); conversely, extending the sleep of chronically short sleepers enhanced glucose metabolism (38).
Likewise, circadian misalignment decreases insulin sensitivity, apart from and compounded by the effects of sleep loss (38). Glucose metabolism does not appear to habituate to sleep disruptions (38), suggesting that the risks accumulate over time. As described earlier, microbiota data account for 22% of the variability in fasting glucose (4).
A theoretical review conceptualized sleep loss and acute circadian disruption as physiological stressors, noting that periods of shorter or no sleep often lead to increases in afternoon and early evening cortisol levels, which promote visceral fat storage and elevate circulating glucose (39). Insofar as sleep dysregulation triggers a stress response, this review also proposed that alterations in the gut microbiota may help to explain sleep-related risks for obesity and metabolic diseases. As discussed, gut microbes help to maintain metabolism, nutrient absorption, and the intestinal barrier, and stress and high-fat diets induce gut dysbiosis (18). The sleep-dysbiosis link has support in rodent models: within 5 days of sleep deprivation, intestinal bacteria appeared in rats’ mesenteric lymph nodes, outside the intestine, implying rapid bacterial translocation (39). Mice with genetically induced circadian disruption also had lower gut microbiota diversity compared to wildtypes (40). Unhealthy diet may further exacerbate these effects: in mice fed a high-fat diet, gut microbes themselves showed altered circadian rhythms, which in turn were associated with changes in the mice’s own circadian clock gene expression and weight gain (39, 41).
Taken together, when marital hostility disrupts sleep, it likely triggers maladaptive gut microbiota responses and metabolic cascades as well. In turn, sleep loss can interfere with partners’ ability to read each other’s emotions and fuel hostility (22, 42), forming a risky cycle. Further, a few nights of shorter sleep prime larger inflammatory responses to marital conflict (22), suggesting that the physiological stress of sleep restriction may interact with marital stress to heighten health risks.
Exercise and the Gut Microbiota
Physical activity benefits the microbiota in multiple ways (43). Exercise increases gut motility, and faster colonic transit times increase microbiota diversity. Regular physical activity changes the microbial community’s composition and also modulates bacterial metabolism via production of beneficial short-chain fatty acids, which helps prevent gut barrier dysfunction and limits the resulting systemic inflammation (43, 44).
Spouses tend to engage in activities with similar frequency (5). Further, partners who had been living together for two or more years were twice as likely to both be physically inactive and sedentary (45).
Marriage, Aging, and Gut Microbiota
Marriage, Emotions, and Aging
The microbiota-mediated health consequences of marriage may compound over time and with age. As middle-aged and older adults prune their more distant social ties (46), the marital relationship assumes an increasingly central role in coloring the emotions of daily life. With biological aging, physiological responsiveness to stressors becomes dysregulated (47), further exacerbating the health risks of marital stress. Indeed, in two separate marital disagreement studies, the effect sizes of healthy older couples’ immune reactivity to conflict were medium to large (48), whereas effects in healthy younger couples were small to medium (49). Associations between post-conflict appraisals and wound healing emerged only among middle-aged and older partners, not younger couples (50).
Longitudinal data reveal consonant patterns: older adults in strained marriages had steeper declines in self-rated health over eight years compared to younger and happily married counterparts (51). In a 20-year prospective study, couples’ greater anger and stonewalling (i.e., discussion-blocking) predicted steeper increases in self-reported physical symptoms over time, but were unrelated to initial symptoms, suggesting that the scars of a chronically stressed marriage may appear years later (52).
According to social-emotional aging theories, as people perceive less time to live, they focus on maximizing emotional well-being and meaningful experiences with loved ones (46). To serve this goal, over the course of adulthood people increasingly shift away from confrontation, to avoiding and reframing potential stressors (47). Consistent with theory, older couples tend to feel closer and more satisfied in their marriages than younger couples (53). Older couples’ positive affect is less disrupted by marital disagreement compared to their younger counterparts’, and older adults rate their spouses’ behavior as more positive than independent raters judge them to be (50, 54). On the other hand, corresponding patterns in conflict behaviors and autonomic reactivity to marital disagreement have produced equivocal results (55–58).
Beyond this, the closeness that might emerge from years of life together can also confer health risks. Although older adults report fewer stressors on average (53), the increasing intertwinement of partners’ goals and routines with older age may boost shared stress, and draw them closer to partners’ suffering. Even the most comforting relationships may promote poor health when partners bond around unhealthy behaviors such as smoking, drinking excessively, or dining on high-fat, sugary foods. A longer history of these behaviors could pile on the risks, particularly in aging bodies. Thus, it is unclear whether social-emotional advantages and the joy and support of a close marriage in older age may offset the magnified health risks of marital strain or closeness. The unique contributions of older age and a longer marriage also remain unknown.
Stress, Depression, and the Microbiota: A Marital Path to Accelerated Aging
When marital stress or depression disrupts the homeostatic relationship between the microbiota and the host, exaggerated inflammation can occur (59). Mouse studies have documented multiple adverse effects of stress on the gut microbiota, including alterations in bacterial composition, decreased diversity, increased gut leakiness, and heightened inflammation (29). Similarly, compared to controls, the gut microbiotas of depressed patients differed in composition, with less diversity and greater leakiness (25, 60). Paralleling these stress- and depression-related patterns, aging is accompanied by less diversity in the gut microbiota’s bacterial composition, as well as increased gut bacteria translocation, and these changes promote the persistent age-associated low-grade inflammation, termed “inflamm-aging,” that contributes to age-associated frailty, morbidity, and mortality (28, 61).
Indeed, the decline in microbiota diversity may be a stronger factor in age-related frailty than chronological age (62, 63). For example, among 728 female twins (M=63 years old), frailty – independent of chronological age -- predicted distinctive genera in the microbiota community, and was also associated with lower diversity (64). In fact, the microbiota’s composition may impact the rate of aging (Figure 1), in tandem with age-linked changes in lifestyle, nutrition, frailty, and inflammation (62, 63). Higher levels of LBP, reflecting greater gut leakiness, have been associated with poorer physical function and higher levels of inflammation, even among healthy older adults (61).
The independent and joint functionality of the host and the microbiota influence health status via an inflammatory phenotype, and thus help to set the pace of biological aging by speeding or impeding inflamm-aging (62). Importantly, unlike chronological age, functional status -- of both the host and microbes – may be modified via health behaviors (diet, sleep, and exercise), as well as stress and depression. After a disturbance, the gut microbiota community typically returns to the pre-disturbance “core” composition. However, severe or repetitive insults may produce long-term—perhaps permanent—changes in the core composition, leading to problematic alterations in the commensal gut microbiota that regulate local and systemic inflammation and immunity and maintain gut barrier function. As described earlier, greater marital hostility predicted heightened gut leakiness (31). Thus, persistent marital strain may create long-term maladaptive changes in the microbiota.
Conclusion
Troubled marriages set the stage for poorer mental and physical health. However, we have also described how happy marriages can increase risk through couples’ shared health habits. Indeed, the new evidence showing couples’ concordance on gene expression, cellular immune profiles, and microbiotas highlights key pathways that influence spousal similarities in disease risk. As noted, these are interconnected pathways; the immune system serves as a major communication path between the brain and the gut (65), and the gut microbiota appears to play a central mediational role while influencing both. Factors that negatively impact the microbiota’s composition and diversity can also induce dysregulated immune responses, accelerate inflamm-aging, and thus promote the development of inflammatory diseases (66). Additionally, the immune system has a role in limiting bacterial translocation and the associated inflammation. This interplay between the immune system and the gut microbiota is important for homeostasis; dysbiosis promotes systemic inflammation (66). In this intricate web, inflammation can fuel depression, and, in turn, marital stress and depression boost inflammation (67).
The bidirectional gut-immune-brain paths have been well-documented in rodent models, but the corresponding human biobehavioral data are scant; human studies have been primarily cross-sectional and observational. Future work using prospective longitudinal studies and experimental designs is needed to understand how these interconnected cascades unfold in couples, and future research should carefully consider the role of selection effects. For example, a history of depression increases stress responsiveness and heightens risk for marital discord as well as gut dysbiosis (31, 68, 69). Furthermore, while this review has focused primarily on presence, absence, and abundance of gut microorganisms, their genome – termed microbiome, and functionality – studied with metabolomics – are also critical targets for future research and intervention.
Although we have emphasized risk-related pathways, couples’ concordance also may be leveraged for both partners’ benefit. For example, addressing one person’s sleep problems may promote both partners’ sleep health: treating a person’s sleep apnea improved the partner’s sleep, and the co-sleeping partner bolstered treatment adherence (21, 70, 71). In addition, positive changes in one spouse’s behavior can prompt change in their partner. Data from the English Longitudinal Study of Ageing, a population-based study of middle-aged and older adults, showed that a person was more likely to stop smoking, increase physical activity, and lose 5% or more of their weight if their partner made the same positive change (72).
Moreover, one spouse can benefit from an intervention delivered to their partner, a “ripple effect” (73). For example, in a trial that evaluated how intentional weight loss affected cardiovascular outcomes in overweight people with type 2 diabetes, spouses of intervention group participants lost more weight than the partners of usual care condition participants, and the spouses’ weight loss was significantly correlated (73). Similarly, husbands of women in the low-fat intervention arm of the Women’s Health Trial reduced their body fat and weight more than the husbands of control arm women (74).
Couples clearly influence each other’s mental and physical health. New studies have revealed the deep roots of biological concordance that span gene expression patterns, cellular immune profiles, inflammation, and the gut microbiota. Couples’ emotions, their relationship satisfaction, and their interconnected health behaviors all interplay with age-related biological changes. Through these pathways, a couple’s partnership shapes their shared journey toward healthy or unhealthy aging.
Acknowledgments:
Work on this project was supported in part by NIH grants K05 CA172296, R01 AG057032, K99 AG056667, and T32 DE014320.
Abbreviations:
- CAD
coronary artery disease
- CRP
C-reactive protein
- LPS
lipopolysaccharide
- LBP
LPS-binding protein
Footnotes
Conflicts of Interest: All authors declare no conflicts of interest.
References
- 1.Tang K, Zhang W. Transcriptional similarity in couples reveals the impact of shared environment and lifestyle on gene regulation through modified cytosines. PeerJ 2016;4:e2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carr EJ, Dooley J, Garcia-Perez JE, Lagou V, Lee JC, Wouters C, Meyts I, Goris A, Boeckxstaens G, Linterman MA, Liston A. The cellular composition of the human immune system is shaped by age and cohabitation. Nat Immunol 2016;17:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davillas A, Pudney S. Concordance of health states in couples: Analysis of self-reported, nurse administered and blood-based biomarker data in the uk understanding society panel. J Health Econ 2017;56:87–102. [DOI] [PubMed] [Google Scholar]
- 4.Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, Shilo S, Lador D, Vila AV, Zmora N, Pevsner-Fischer M, Israeli D, Kosower N, Malka G, Wolf BC, Avnit-Sagi T, Lotan-Pompan M, Weinberger A, Halpern Z, Carmi S, Fu J, Wijmenga C, Zhernakova A, Elinav E, Segal E. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018;555:210–5. [DOI] [PubMed] [Google Scholar]
- 5.Kiecolt-Glaser JK, Wilson SJ. Lovesick: How couples’ relationships influence health. Annu Rev Clin Psychol 2017;13:421–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiecolt-Glaser JK, Newton TL. Marriage and health: His and hers. Psychol Bull 2001;127:472–503. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med 2018;24:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLOS Biology 2016;14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, Suez J, Mahdi JA, Matot E, Malka G, Kosower N, Rein M, Zilberman-Schapira G, Dohnalova L, Pevsner-Fischer M, Bikovsky R, Halpern Z, Elinav E, Segal E. Personalized nutrition by prediction of glycemic responses. Cell 2015;163:1079–94. [DOI] [PubMed] [Google Scholar]
- 10.Hippisley-Cox J, Coupland C, Pringle M, Crown N, Hammersley V. Married couples’ risk of same disease: Cross sectional study. BMJ 2002;325:636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hippisley-Cox J, Pringle M. Are spouses of patients with hypertension at increased risk of having hypertension? A population-based case-control study. Br J Gen Pract 1998;48:1580–3. [PMC free article] [PubMed] [Google Scholar]
- 12.Stimpson JP, Peek MK. Concordance of chronic conditions in older Mexican American couples. Preventing chronic disease 2005;2:A07. [PMC free article] [PubMed] [Google Scholar]
- 13.Neff LA, Karney BR. Stress crossover in newlywed marriage: A longitudinal and dyadic perspective. J Marriage Fam 2007;69:594–607. [Google Scholar]
- 14.Repetti R, Wang SW, Saxbe D. Bringing it all back home: How outside stressors shape families’ everyday lives. Curr Dir Psychol Sci 2009;18:106–11. [Google Scholar]
- 15.Berg CA, Wiebe DJ, Butner J. Affect covariation in marital couples dealing with stressors surrounding prostate cancer. Gerontology 2011;57:167–72. [DOI] [PubMed] [Google Scholar]
- 16.Monin JK, Levy BR, Kane HS. To love is to suffer: Older adults’ daily emotional contagion to perceived spousal suffering. J Gerontol B Psychol Sci Soc Sci 2017;72:383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxbe D, Repetti RL. For better or worse? Coregulation of couples’ cortisol levels and mood states. J Pers Soc Psychol 2010;98:92–103. [DOI] [PubMed] [Google Scholar]
- 18.Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2015;7:17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunn HE, Buysse DJ, Hasler BP, Begley A, Troxel WM. Sleep concordance in couples is associated with relationship characteristics. Sleep 2015;38:933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen JH. Couples’ sleep and psychological distress: A dyadic perspective. J Gerontol B Psychol Sci Soc Sci 2017;73:30–9. [DOI] [PubMed] [Google Scholar]
- 21.Troxel WM, Robles TF, Hall M, Buysse DJ. Marital quality and the marital bed: Examining the covariation between relationship quality and sleep. Sleep Med Rev 2007;11:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson SJ, Jaremka LM, Fagundes CP, Andridge R, Peng J, Malarkey WB, Habash D, Belury MA, Kiecolt-Glaser JK. Shortened sleep fuels inflammatory responses to marital conflict: Emotion regulation matters. Psychoneuroendocrinology 2017;79:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strawbridge WJ, Shema SJ, Roberts RE. Impact of spouses’ sleep problems on partners. Sleep 2004;27:527–31. [DOI] [PubMed] [Google Scholar]
- 24.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jorgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clement K, Dore J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T, Bork P, Wang J, Ehrlich SD, Pedersen O. Richness of human gut microbiome correlates with metabolic markers. Nature 2013;500:541–6. [DOI] [PubMed] [Google Scholar]
- 25.Kelly JR, Borre Y, C OB, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF, Dinan TG. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 2016;82:109–18. [DOI] [PubMed] [Google Scholar]
- 26.Beach SRH. The couple and family discord model of depression: Updates and future directions. In: Agnew CR, South SC, editors. Interpersonal relationships and health: Social and clinical psychological mechanisms New York, NY, US: Oxford University Press; 2014. p. 133–55. [Google Scholar]
- 27.Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J, Foley KP, Schertzer JD, Larché MJ, Davidson DJ, Verdú EF, Surette MG, Bowdish DME. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host & Microbe 21:455–66.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim KA, Jeong JJ, Yoo SY, Kim DH. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiology 2016;16. [DOI] [PMC free article] [PubMed]
- 29.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav Immun 2011;25:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaetano Gd, Zito F, van Dongen M, Siani A, Krogh V, Donati MB, Dirckx C, Lorgeril Md, Cappuccio FP, Jozef A, Quacquaruccio G, Castelnuovo AD, Iacoviello L Cardiovascular risk factors and global risk of fatal cardiovascular disease are positively correlated between partners of 802 married couples from different European countries. Thromb Haemost 2017;98:648–55. [PubMed] [Google Scholar]
- 31.Kiecolt-Glaser JK, Wilson SJ, Bailey M, Andridge R, Peng J, Jaremka L, Fagundes CP, Malarkey WB, Laskowski B, Belury MA. Marital distress, depression, and a leaky gut: Translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinolgy 2018;98:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newsholme P, Homem de Bittencourt PI Jr. Gut associated bacteria are critical to metabolism, inflammation and health. Curr Opin Clin Nutr Metab Care 2016;19:245–9. [DOI] [PubMed] [Google Scholar]
- 33.Kiecolt-Glaser JK, Fagundes CP, Andridge R, Peng J, Malarkey WB, Habash D, Belury MA. Depression, daily stressors and inflammatory responses to high-fat meals: When stress overrides healthier food choices. Mol Psychiatry 2017;22:476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiecolt-Glaser JK, Jaremka L, Andridge R, Peng J, Habash D, Fagundes CP, Glaser R, Malarkey WB, Belury MA. Marital discord, past depression, and metabolic responses to high-fat meals: Interpersonal pathways to obesity. Psychoneuroendocrinology 2015;52:239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiecolt-Glaser JK, Habash DL, Fagundes CP, Andridge R, Peng J, Malarkey WB, Belury MA. Daily stressors, past depression, and metabolic responses to high-fat meals: A novel path to obesity. Biol Psychiatry 2015;77:653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab 2010;21:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur Heart J 2011;32:1484–92. [DOI] [PubMed] [Google Scholar]
- 38.McHill AW, Wright KP Jr. Role of sleep and circadian disruption on energy expenditure and in metabolic predisposition to human obesity and metabolic disease. Obes Rev 2017;18 Suppl 1:15–24. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds AC, Paterson JL, Ferguson SA, Stanley D, Wright KP Jr., Dawson D The shift work and health research agenda: Considering changes in gut microbiota as a pathway linking shift work, sleep loss and circadian misalignment, and metabolic disease. Sleep Med Rev 2017;34:3–9. [DOI] [PubMed] [Google Scholar]
- 40.Everson CA, Toth LA. Systemic bacterial invasion induced by sleep deprivation. Am J Physiol Regul Integr Comp Physiol 2000;278:R905–16. [DOI] [PubMed] [Google Scholar]
- 41.Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, Pierre JF, Heneghan AF, Nadimpalli A, Hubert N, Zale E, Wang Y, Huang Y, Theriault B, Dinner AR, Musch MW, Kudsk KA, Prendergast BJ, Gilbert JA, Chang EB. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host & Microbe 2015;17:681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasler BP, Troxel WM. Couples’ nighttime sleep efficiency and concordance: Evidence for bidirectional associations with daytime relationship functioning. Psychosom Med 2010;72:794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cook MD, Allen JM, Pence BD, Wallig MA, Gaskins HR, White BA, Woods JA. Exercise and gut immune function: Evidence of alterations in colon immune cell homeostasis and microbiome characteristics with exercise training. Immunol Cell Biol 2016;94:158–63. [DOI] [PubMed] [Google Scholar]
- 44.Ticinesi A, Lauretani F, Milani C, Nouvenne A, Tana C, Del Rio D, Maggio M, Ventura M, Meschi T. Aging gut microbiota at the cross-road between nutrition, physical frailty, and sarcopenia: Is there a gut-muscle axis? Nutrients 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The NS, Gordon-Larsen P. Entry into romantic partnership is associated with obesity. Obesity (Silver Spring) 2009;17:1441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carstensen LL. Evidence for a life-span theory of socioemotional selectivity. Curr Dir Psychol Sci 1995;4:151–6. [Google Scholar]
- 47.Charles ST. Strength and vulnerability integration: A model of emotional well-being across adulthood. Psychol Bull 2010;136:1068–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiecolt-Glaser JK, Glaser R, Cacioppo JT, MacCallum RC, Snydersmith M, Kim C, Malarkey WB. Marital conflict in older adults: Endocrinological and immunological correlates. Psychosom Med 1997;59:339–49. [DOI] [PubMed] [Google Scholar]
- 49.Kiecolt-Glaser JK, Malarkey WB, Chee M, Newton T, Cacioppo JT, Mao H, Glaser R. Negative behavior during marital conflict is associated with immunological down-regulation. Psychosom Med 1993;55:395–409. [DOI] [PubMed] [Google Scholar]
- 50.Wilson SJ, Andridge R, Peng J, Bailey BE, Malarkey WB, Kiecolt-Glaser JK. Thoughts after marital conflict and punch biopsy wounds: Age-graded pathways to healing. Psychoneuroendocrinology 2017;85:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Umberson D, Williams K, Powers DA, Liu H, Needham B. You make me sick: Marital quality and health over the life course. J Health Soc Behav 2006;47:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haase CM, Holley SR, Bloch L, Verstaen A, Levenson RW. Interpersonal emotional behaviors and physical health: A 20-year longitudinal study of long-term married couples. Emotion 2016;16:965–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rook KS, Charles ST. Close social ties and health in later life: Strengths and vulnerabilities. Am Psychol 2017;72:567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Story TN, Berg CA, Smith TW, Beveridge R, Henry NJ, Pearce G. Age, marital satisfaction, and optimism as predictors of positive sentiment override in middle-aged and older married couples. Psychol Aging 2007;22:719–27. [DOI] [PubMed] [Google Scholar]
- 55.Smith TW, Uchino BN, Berg CA, Florsheim P, Pearce G, Hawkins M, Henry NJ, Beveridge RM, Skinner MA, Ko KJ, Olsen-Cerny C. Conflict and collaboration in middle-aged and older couples: II. Cardiovascular reactivity during marital interaction. Psychol Aging 2009;24:274–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levenson RW, Carstensen LL, Friesen WV, Ekman P. Emotion, physiology, and expression in old age. Psychol Aging 1991;6:28–35. [DOI] [PubMed] [Google Scholar]
- 57.Smith TW, Berg CA, Florsheim P, Uchino BN, Pearce G, Hawkins M, Henry NJ, Beveridge RM, Skinner MA, Olsen-Cerny C. Conflict and collaboration in middle-aged and older couples: I. Age differences in agency and communion during marital interaction. Psychol Aging 2009;24:259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carstensen LL, Gottman JM, Levenson RW. Emotional behavior in long-term marriage. Psychol Aging 1995;10:140–9. [DOI] [PubMed] [Google Scholar]
- 59.Reber SO, Siebler PH, Donner NC, Morton JT, Smith DG, Kopelman JM, Lowe KR, Wheeler KJ, Fox JH, Hassell JE Jr., Greenwood BN, Jansch C, Lechner A, Schmidt D, Uschold-Schmidt N, Fuchsl AM, Langgartner D, Walker FR, Hale MW, Lopez Perez G, Van Treuren W, Gonzalez A, Halweg-Edwards AL, Fleshner M, Raison CL, Rook GA, Peddada SD, Knight R, Lowry CA. Immunization with a heat-killed preparation of the environmental bacterium mycobacterium vaccae promotes stress resilience in mice. Proc Natl Acad Sci U S A 2016;113:E3130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maes M, Kubera M, Leunis JC, Berk M. Increased IgA and IgM responses against gut commensals in chronic depression: Further evidence for increased bacterial translocation or leaky gut. J Affect Disord 2012;141:55–62. [DOI] [PubMed] [Google Scholar]
- 61.Stehle JR Jr., Leng X, Kitzman DW, Nicklas BJ, Kritchevsky SB, High KP. Lipopolysaccharide-binding protein, a surrogate marker of microbial translocation, is associated with physical function in healthy older adults. J Gerontol A Biol Sci Med Sci 2012;67:1212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaiserman AM, Koliada AK, Marotta F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res Rev 2017;35:36–45. [DOI] [PubMed] [Google Scholar]
- 63.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O’Sullivan O, Fitzgerald GF, Deane J, O’Connor M, Harnedy N, O’Connor K, O’Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O’Toole PW. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012;488:178–84. [DOI] [PubMed] [Google Scholar]
- 64.Jackson MA, Jeffery IB, Beaumont M, Bell JT, Clark AG, Ley RE, O’Toole PW, Spector TD, Steves CJ. Signatures of early frailty in the gut microbiota. Genome Med 2016;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dinan TG, Cryan JF. Microbes, immunity, and behavior: Psychoneuroimmunology meets the microbiome. Neuropsychopharmacology 2017;42:178–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen S, Wong CH. Bugging inflammation: Role of the gut microbiota. Clin Transl Immunology 2016;5:e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: Depression fans the flames and feasts on the heat. Am J Psychiatry 2015;172:1075–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hammen C Generation of stress in the course of unipolar depression. J Abnorm Psychol 1991;100:555–61. [DOI] [PubMed] [Google Scholar]
- 69.Husky M, Mazure C, Maciejewski P, Swendsen J. Past depression and gender interact to influence emotional reactivity to daily life stress. Cognit Ther Res 2009;33:264–71. [Google Scholar]
- 70.Baron KG, Smith TW, Czajkowski LA, Gunn HE, Jones CR. Relationship quality and CPAP adherence in patients with obstructive sleep apnea. Behav Sleep Med 2009;7:22–36. [DOI] [PubMed] [Google Scholar]
- 71.Beninati W, Harris CD, Herold DL, Shepard JW Jr. The effect of snoring and obstructive sleep apnea on the sleep quality of bed partners. Mayo Clin Proc 1999;74:955–8. [DOI] [PubMed] [Google Scholar]
- 72.Jackson SE, Steptoe A, Wardle J. The influence of partner’s behavior on health behavior change: The English longitudinal study of ageing. JAMA Intern Med 2015;175:385–92. [DOI] [PubMed] [Google Scholar]
- 73.Gorin AA, Wing RR, Fava JL, Jakicic JM, Jeffery R, West DS, Brelje K, Dilillo VG, Look AHERG. Weight loss treatment influences untreated spouses and the home environment: Evidence of a ripple effect. Int J Obes (Lond) 2008;32:1678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.White E, Hurlich M, Thompson RS, Woods MN, Henderson MM, Urban N, Kristal A. Dietary-changes among husbands of participants in a low-fat dietary intervention. Am J Prev Med 1991;7:319–25. [PubMed] [Google Scholar]


