This phase 3 randomized clinical trial of 340 patients with locally advanced non–small cell lung cancer compares outcomes of treatment with prophylactic cranial irradiation vs observation.
Key Points
Question
Does prophylactic cranial irradiation (PCI) improve survival in patients with locally advanced non–small cell lung cancer (LA-NSCLC) compared with observation alone?
Findings
In this prospective randomized phase 3 clinical trial including 340 evaluable patients with LA-NSCLC, PCI decreased the 5- and 10-year rate of brain metastases and improved 5- and 10-year disease-free survival, but did not improve overall survival compared with observation alone.
Meaning
Although this study did not meet its primary end point of overall survival, the long-term results reveal many important PCI findings, which will benefit future trials.
Abstract
Importance
Brain metastasis (BM) rates are high in locally advanced non–small cell lung cancer (LA-NSCLC), approaching rates seen in small cell lung cancer, where prophylactic cranial irradiation (PCI) is standard of care. Although PCI decreases the incidence of BM in LA-NSCLC, a survival advantage has not yet been shown.
Objective
To determine if PCI improves survival in LA-NSCLC.
Design, Setting, and Participants
Radiation Therapy Oncology Group (RTOG) 0214 was a randomized phase 3 clinical trial in stage III NSCLC stratified by stage (IIIA vs IIIB), histologic characteristics (nonsquamous vs squamous) and therapy (no surgery vs surgery). The study took place at 291 institutions in the United States, Canada, and internationally. Of 356 patients with stage III NSCLC entered onto this study, 16 were ineligible; therefore, 340 patients were randomized.
Intervention for Clinical Trials
Observation vs PCI.
Main Outcomes and Measures
The primary outcome was overall survival (OS). The secondary end points were disease-free survival (DFS) and incidence of BM.
Results
Of the 340 total participants, mean (SD) age was 61 years; 213 of the participants were men and 127 were women. The median follow-up time was 2.1 years for all patients, and 9.2 years for living patients. The OS for PCI was not significantly better than observation (hazard ratio [HR], 0.82; 95% CI, 0.63-1.06; P = .12; 5- and 10-year rates, 24.7% and 17.6% vs 26.0% and 13.3%, respectively), while the DFS (HR, 0.76; 95% CI, 0.59-0.97; P = .03; 5- and 10-year rates, 19.0% and 12.6% vs 16.1% and 7.5% for PCI vs observation) and BM (HR, 0.43; 95% CI, 0.24-0.77; P = .003; 5- and 10-year rates, 16.7% vs 28.3% for PCI vs observation) were significantly different. Patients in the PCI arm were 57% less likely to develop BM than those in the observation arm. Younger patients (<60 years) and patients with nonsquamous disease developed more BM. On multivariable analysis, PCI was associated with decreased BM and improved DFS, but not improved OS. Multivariable analysis within the nonsurgical arm suggests that PCI effectively prolongs OS, DFS, and BM.
Conclusions and Relevance
In patients with stage III LA-NSCLC without progression of disease after therapy, PCI decreased the 5- and 10-year rate of BM and improved 5- and 10-year DFS, but did not improve OS. Although this study did not meet its primary end point, the long-term results reveal many important findings that will benefit future trials. Identifying the appropriate patient population and a safe intervention is critical.
Trial Registration
ClinicalTrials.gov identifier: NCT00048997
Introduction
Combined-modality therapy, including chemotherapy, irradiation, and/or surgery, has resulted in improved survival of locally advanced non–small cell lung cancer (LA-NSCLC). This lengthened survival is associated with increased incidence of brain metastases (BM),1,2,3,4,5,6,7,8,9,10,11,12 which can have a devastating impact on survival and quality of life (QOL). Rates of BM reported in NSCLC studies are as high as 55%,13,14,15,16,17,18,19,20 approaching rates seen in small cell lung cancer (SCLC), where prophylactic cranial irradiation (PCI) is standard of care. Although PCI decreases the incidence of BM in LA-NSCLC,21,22,23 a survival advantage has not yet been shown.
The NRG Oncology/Radiation Therapy Oncology Group (RTOG) led a study of PCI in LA-NSCLC after definitive primary therapy. The primary end point of the study was overall survival (OS), with secondary end points including disease-free survival (DFS) and development of BM. This is an updated long-term analysis of the previously reported preliminary results.24
Methods
Patient Population
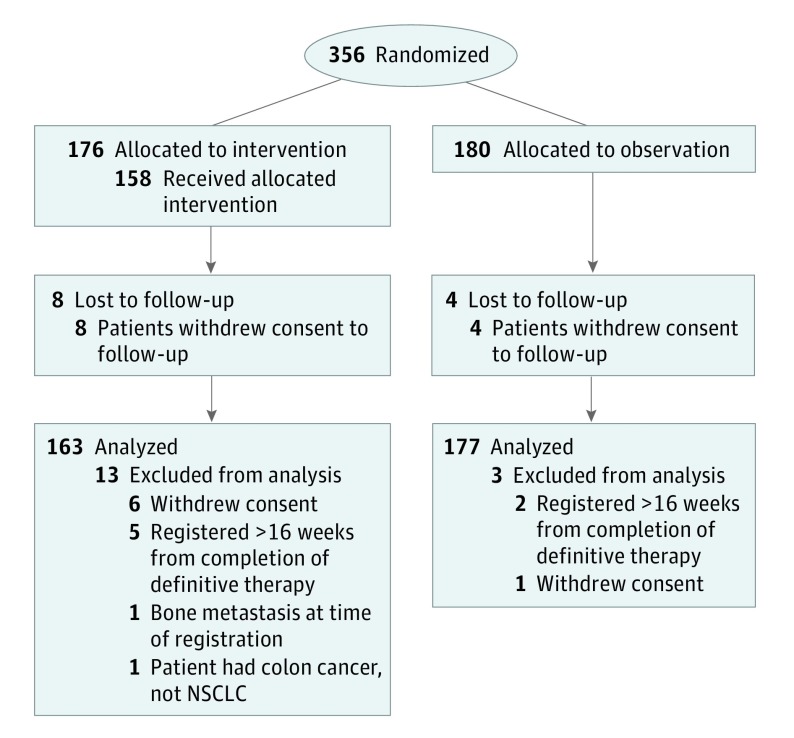
Patients with stage IIIA/B NSCLC without disease progression after completing definitive locoregional therapy with irradiation and/or surgery with or without chemotherapy were randomized to PCI or observation (Figure 1). Patients were restaged and enrolled within 16 weeks of completing definitive therapy. Patients were restaged with a computed tomography (CT) scan of the chest/abdomen and magnetic resonance imaging (MRI) of the brain within six weeks of study entry. Brain CT with contrast was allowed if MRI was contraindicated. Patients could have no evidence of progressive intrathoracic disease, BM, or extracranial metastases. All institutions obtained institutional review board approval prior to patient recruitment and all patients signed approved informed consents prior to trial enrollment. The trial protocol is available in Supplement 1.
Figure 1. Participant Enrollment Flow Diagram.
NSCLC indicates non–small cell lung cancer.
Treatment and Follow-up
Patients were stratified by stage (IIIA or B), histologic characteristics (nonsquamous or squamous), and therapy (surgery or no surgery) and then randomized to PCI or observation. Patients randomized to PCI were treated with 2 Gy/fraction, five days per week, to 30 Gy. Acute PCI toxic events were evaluated using the Common Terminology Criteria (CTC) version 2.0 grading criteria. Late PCI toxic events were evaluated using the RTOG/European Organisation for Research and Treatment of Cancer (EORTC) Late Toxicity Criteria. Patients were followed up 6 months from start of PCI, every 6 months for 2 years and then yearly. Brain imaging with MRI or CT was performed at 6 and 12 months and then annually thereafter.
Study Design and End Points
The primary end point of this study was OS, and secondary end points included DFS and BM. Overall survival failure events were defined as death due to any cause. Disease-free survival failure events were defined as the earliest event of death due to any cause, local progression, regional metastasis, distant metastasis, or second primary. Brain metastases failure events were defined as any evidence of BM. Time to event was measured from date of randomization to date of failure or date of most recent follow-up if no failure occurred.
Statistical Methods
The rates of OS and DFS were estimated using Kaplan-Meier method,25 and BMs were estimated using cumulative incidence function.26 The comparisons between PCI and observation were based on log-rank tests27,28 stratified by American Joint Committee on Cancer (AJCC) stage (IIIA vs IIIB); prior surgery (no vs yes); histologic characteristics (nonsquamous vs squamous) and Zubrod performance status (0 vs >0). Cox proportional hazards models29 were used to evaluate the impact of treatment on OS and DFS after adjusting for all these factors and age (<60 vs ≥60 years). Cause-specific hazard approach was used to analyze BM in presence of death without BM as a competing event.30 Subgroup analysis was performed based on prespecified stratification factors at randomization when the heterogeneity of treatment effects was supported by tests for interaction (eg, prior surgery) or of interest (eg, histology).31 All statistical comparisons were considered statistically significant at a 2-sided P < .05, and no multiple comparison adjustments were made. All statistical analyses were run with SAS, 9.4 (SAS Institute Inc) software.
Results
This study opened on September 19, 2002, and closed owing to poor accrual on August 30, 2007. This report includes all data reported as of December 15, 2016. All data analysis took place from December 1, 2017 to January 15, 2018. A total of 527 deaths and a projected accrual of 1058 participants were targeted to detect a 20% risk reduction (hazard ratio [HR], 0.8, observation arm as reference) in death with 80% power and a 1-sided significance level of P = .025. The total accrual at study closure was 356 patients. Among 356 patients entered, 9 patients (7 PCI, 2 observation) were ineligible and 7 patients (6 PCI, 1 observation) withdrew consent. Therefore 340 patients were eligible for this study. The median follow-up time was 2.1 years (range, 0.1-12.6 years) for all patients, and 9.2 years for 63 patients still alive. The pretreatment characteristics were evenly distributed between the 2 arms except Zubrod performance status. The majority of patients received a platinum doublet chemotherapy regimen.
Primary End Point
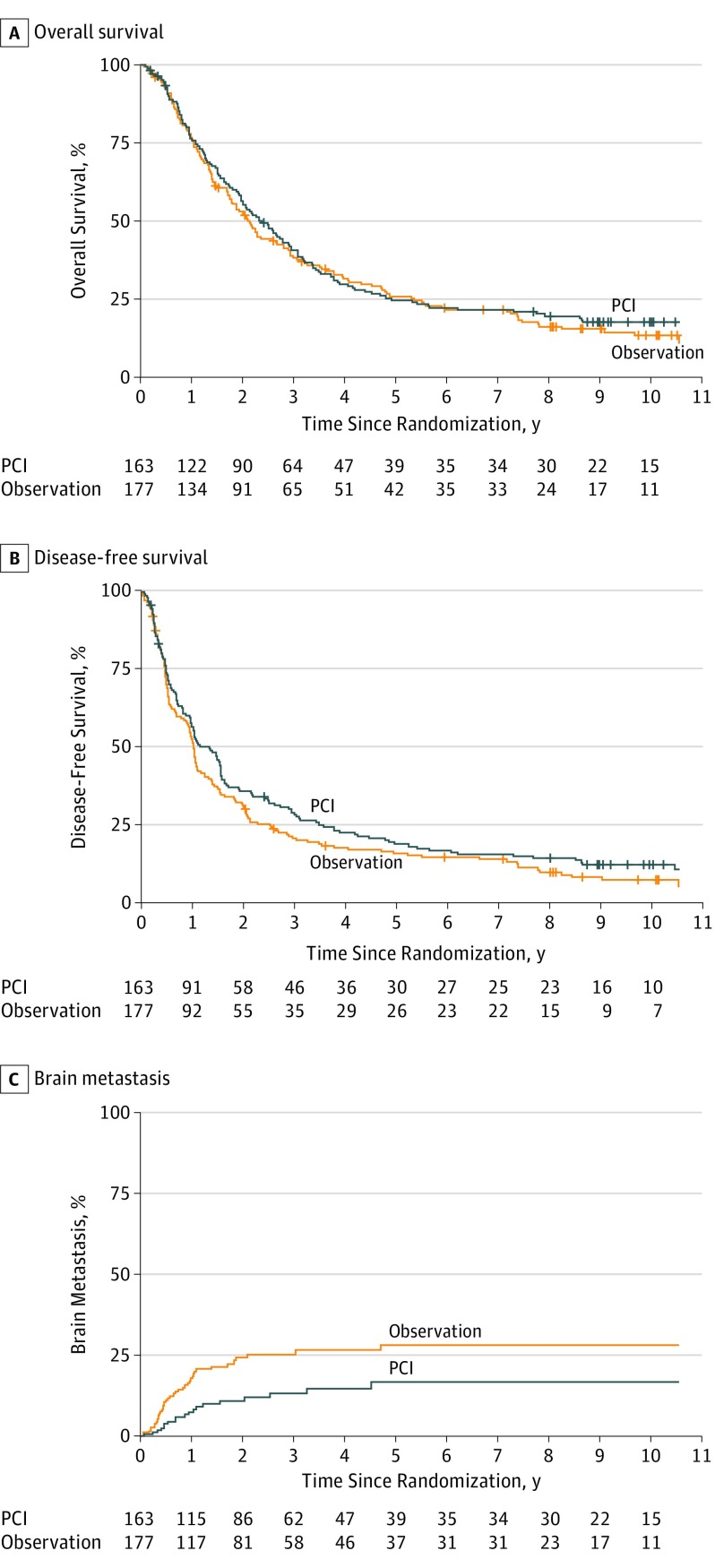
At the time of this analysis, 277 deaths had occurred in 340 evaluable patients, which provided approximately 45% power to detect the targeted difference in OS. Patients died primarily due to their lung cancer. The survival estimates and HR indicated that there appeared to be no improvement in survival with the use of PCI. Five-year and 10-year OS rates were 24.7% and 17.6% for PCI and 26.0% and 13.3% for observation, respectively (Table 1 and Figure 2). Estimated median survival (MS) duration was 2.4 years (95% CI, 2.0-2.9) and 2.1 years (95% CI, 1.7-2.7) for PCI and observation, respectively. The HR for PCI vs observation was 0.82 (95% CI, 0.63-1.06; P = .12).
Table 1. Outcome Estimates for Entire Study.
| Outcome by Time | PCI (n = 163) | Observation (n = 177) | PCI vs Observation, HR (95% CI)a | P Valueb | ||
|---|---|---|---|---|---|---|
| No. at Risk | Event Estimate % (95% CI) | No. at Risk | Event Estimate % (95% CI) | |||
| Overall survival | 0.82 (0.63-1.06) | .12 | ||||
| 2 y | 90 | 56.3 (48.3-63.6) | 91 | 53.0 (45.3-60.1) | ||
| 5 y | 39 | 24.7 (18.3-31.6) | 42 | 26.0 (19.6-32.8) | ||
| 10 y | 15 | 17.6 (12.1-23.9) | 11 | 13.3 (8.4-19.4) | ||
| MST (95% CI) | 2.4 y (2.0-2.9) | 2.1 y (1.7-2.7) | ||||
| No. of events | 131 | 146 | ||||
| Disease-free survival | 0.76 (0.59-0.97) | .03 | ||||
| 2 y | 58 | 36.1 (28.7-43.5) | 55 | 31.5 (24.7-38.4) | ||
| 5 y | 30 | 19.0 (13.3-25.4) | 26 | 16.1 (11.0-22.0) | ||
| 10 y | 10 | 12.6 (8.0-18.3) | 7 | 7.5 (4.0-12.5) | ||
| MST (95% CI) | 1.3 y (1.0-1.6) | 1.0 (0.9-1.1) | ||||
| No. of events | 141 | 159 | ||||
| Brain metastasis | 0.43 (0.24-0.77) | .003 | ||||
| 2 y | 86 | 10.9 (6.7-17.6) | 81 | 24.3 (18.1-32.0) | ||
| 5 y | 39 | 16.7 (10.6-25.9) | 37 | 28.3 (21.2-37.2) | ||
| 10 y | 15 | 16.7 (10.6-25.9) | 11 | 28.3 (21.2-37.2) | ||
| MST (95% CI) | Not reached | Not reached | ||||
| No. of events | 20 | 40 | ||||
Abbreviations: AJCC, American Joint Committee on Cancer; HR, hazard ratio; MST, median survival time; PCI, prophylactic cranial irradiation.
From stratified Cox proportional hazard model, stratified by AJCC stage (IIIA vs IIIB); prior surgery (no vs yes); histologic characteristics (nonsquamous vs squamous) and Zubrod performance status (0 vs >0).
From stratified log-rank test, stratified by AJCC stage (IIIA vs IIIB); prior surgery (no vs yes); histologic characteristics (nonsquamous vs squamous) and Zubrod performance status (0 vs >0).
Figure 2. PCI vs Observation in Patients with Locally Advanced Non–Small Cell Lung Cancer.
PCI indicates prophylactic cranial irradiation.
DFS and BM
There were 300 DFS events at the time of analysis. Five-year and 10-year DFS rates were 19.0% and 12.6% for PCI and 16.1% and 7.5% for observation, respectively (P = .03) (Table 1 and Figure 2). The HR for PCI vs observation was 0.76 (95% CI, 0.59-0.97). There were 60 BM events and the 5-year and 10-year BM rates were 16.7% in the PCI arm and 28.3% in the observation arm (P = .004) (Table 1 and Figure 2). The corresponding HR was 0.43 (95% CI, 0.24-0.77). These results continued to be statistically significant.
Prognostic Variables
Multivariable Cox proportional hazards models were performed (Table 2). Surgery, Zubrod performance status, and age were associated with OS. Surgery was associated with DFS. A multivariable model of BM was also performed, but owing to the limited number of events, the results should be interpreted with caution. Use of PCI remained strongly associated with decreased risk of developing BM; histological characteristics and age also appeared to be important factors. Younger patients (<60 years) and patients with nonsquamous cancer were more likely to develop BM.
Table 2. Cox Proportional Hazard Multivariable Analysis Results for Entire Study.
| Covariate | Comparison | Overall Survival | Disease-Free Survival | Brain Metastasis | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Treatment arm | PCI | 0.84 (0.66-1.07) | .16 | 0.78 (0.62-0.99) | .04 | 0.44 (0.25-0.77 | .01 |
| Observation (RL) | 1 [Reference] | ||||||
| Age | ≥60 years | 1.39 (1.08-1.79) | .01 | 1.14 (0.90 1.45 | .29 | 0.57 (0.34-0.97) | .04 |
| <60 years | 1 [Reference] | ||||||
| Zubrod | >0 | 1.34 (1.05-1.72) | .02 | 1.18 (0.93-1.49) | .17 | 1.00 (0.59-1.70) | .99 |
| 0 | 1 [Reference] | ||||||
| Prior surgery | Yes | 0.82 (0.62-1.07) | .14 | 0.79 (0.61-1.02) | .07 | 0.65 (0.36-1.19) | .16 |
| No | 1 [Reference] | ||||||
| AJCC stage | IIIB | 1.17 (0.91-1.50) | .22 | 1.23 (0.96-1.56) | .10 | 0.88 (0.51-1.54) | .65 |
| IIIA | 1 [Reference] | ||||||
| Histologic characteristics | Squamous | 1.08 (0.84-1.39) | .56 | 1.01 (0.80-1.29) | .91 | 0.20 (0.08-0.46) | .01 |
| Nonsquamous | 1 [Reference] | ||||||
Abbreviations: AJCC, American Joint Committee on Cancer; HR, hazard ratio; PCI, prophylactic cranial irradiation.
Patterns of First Failure
A total of 114 and 132 patients in the PCI and observation arms, respectively, experienced some sort of treatment failure. Failing locally was defined as failure at the primary site; regionally was defined as the regional lymph nodes, and distantly was any site beyond local or regional. Of these, 11 (10%) patients in the PCI arm and 30 (23%) patients in the observation arm experienced treatment failure in the brain as the first site of failure, of which, ten (9%) and 28 (21%) were isolated events, respectively. Among patients whose treatments failed, 75 (66%) in the PCI arm and 68 (52%) in the observation arm first experienced local or regional failure; 62 (54%) in the PCI arm and 80 (61%) in the observation arm first experienced distant failure; 23 (20%) in the PCI arm and 17 (13%) in the observation arm first experienced local/regional and distant failure; and 9 (8%) PCI and 16 (12%) observation patients developed a second primary tumor prior to experiencing any other failure (eFigure 1 in Supplement 2).
Subgroup Analysis
There were 225 patients (66%) who did not have surgery. Table 3 details the OS between the PCI and observation arms in this subgroup. As evidenced by the data in Table 3, there were significant differences in OS between the 2 arms, with an MS of 2.3 years for PCI and 1.9 years for observation, respectively. The rates of DFS were significantly different between the 2 arms. For BM, the rates for PCI were significantly lower than those for observation. Multivariable analysis of OS (eTable 1 in Supplement 2) revealed PCI patients had lower risks of death (HR, 0.73; 95% CI, 0.54-0.98; P = .04). Zubrod performance status (>0) was found to be significantly associated with increased risk of death. A lower risk of DFS events was associated with PCI (HR, 0.70; 95% CI, 0.52-0.93; P = .01). For BM, PCI patients were less likely to develop BM than those in observation (HR, 0.34; 95% CI, 0.17-0.68; P = .002). In addition, younger (<60 years) patients and patients with nonsquamous cancers had higher rates of BM.
Table 3. Outcome Estimates, by Prior Surgery Status.
| Outcome by Time | No. at Risk | Event Estimate % (95% CI) | No. at Risk | Event Estimate % (95% CI) | PCI vs Observation, HR (95% CI)a | P Valueb | |
|---|---|---|---|---|---|---|---|
| No Prior Surgery | |||||||
| PCI (n = 106) | Observation (n = 119) | ||||||
| Overall survival | 0.70 (0.52-0.96) | .03 | |||||
| 2 y | 60 | 58.3 (48.2-67.1) | 54 | 47.7 (38.3-56.4) | |||
| 5 y | 23 | 22.8 (15.2-31.3) | 21 | 19.5 (12.7-27.3) | |||
| 10 y | 9 | 16.6 (10.1-24.5) | 5 | 8.9 (4.2-15.7) | |||
| MST (95% CI) | 2.3 y (2.0-3.0) | 1.9 (1.4-2.2) | |||||
| No. of events | 85 | 102 | |||||
| Disease-free survival | 0.69 (0.51-0.93) | .01 | |||||
| 2 y | 38 | 36.6 (27.5-45.8) | 28 | 24.0 (16.7-32.0) | |||
| 5 y | 18 | 17.8 (11.1-25.8) | 12 | 10.7 (5.9-17.1) | |||
| 10 y | 6 | 11.7 (6.4-18.9) | 3 | 4.9 (1.8-10.2) | |||
| MST (95% CI) | 1.1 y (0.8-1.6) | 1.0 (0.6-1.0) | |||||
| No. of events | 92 | 110 | |||||
| Brain metastasis | 0.36 (0.18-0.72) | .01 | |||||
| 2 y | 57 | 9.5 (5.0-17.5) | 47 | 27.0 (19.2-37.2) | |||
| 5 y | 23 | 13.0 (7.3-22.8) | 19 | 31.0 (22.1-42.3) | |||
| 10 y | 9 | 13.0 (7.3-22.8) | 5 | 31.0 (22.1-42.3) | |||
| MST (95% CI) | Not reached | Not reached | |||||
| No. of events | 11 | 29 | |||||
| Prior Surgery | |||||||
| PCI (n = 57) | Observation (n = 58) | ||||||
| Overall survival | 1.15 (0.72-1.84) | .56 | |||||
| 2 y | 30 | 52.6 (39.0-64.6) | 37 | 63.8 (50.1-74.7) | |||
| 5 y | 16 | 28.1 (17.2-40.0) | 21 | 38.9 (26.3-51.2) | |||
| 10 y | 6 | 19.3 (10.3-30.4) | 6 | 22.1 (11.9-34.4) | |||
| MST (95% CI) | 2.5 y (1.4-3.4) | 3.1 y (2.2-5.3) | |||||
| No. of events | 46 | 44 | |||||
| Disease-free survival | 0.95 (0.60-1.51) | .84 | |||||
| 2 y | 20 | 35.1 (23.1-47.4) | 27 | 46.6 (33.4-58.7) | |||
| 5 y | 12 | 21.1 (11.6-32.4) | 14 | 27.0 (16.2-38.9) | |||
| 10 y | 4 | 14.0 (6.6-24.3) | 4 | 12.9 (5.1-24.4) | |||
| MST (95% CI) | 1.5 y (1.0-1.6) | 1.6 y (1.0-2.6) | |||||
| No. of events | 49 | 49 | |||||
| Brain metastasis | 0.66 (0.24-1.84) | .43 | |||||
| 2 y | 29 | 13.6 (6.3-28.0) | 34 | 19.1 (10.7-32.7) | |||
| 5 y | 16 | 22.6 (11.3-42.0) | 18 | 23.1 (13.1-39.0) | |||
| 10 y | 6 | 22.6 (11.3-42.0) | 6 | 23.1 (13.1-39.0) | |||
| MST (95% CI) | Not reached | Not reached | |||||
| No. of events | 8 | 11 | |||||
Abbreviations: AJCC, American Joint Committee on Cancer; HR, hazard ratio; MST, median survival time; PCI, prophylactic cranial irradiation.
From stratified Cox proportional hazard model, stratified by AJCC stage (IIIA vs IIIB); histologic characteristics (nonsquamous vs squamous) and Zubrod performance status (0 vs >0).
From stratified log-rank test, stratified by AJCC stage (IIIA vs IIIB); histologic characteristics (nonsquamous vs squamous) and Zubrod performance status (0 vs >0).
For subgroup analysis of the 115 patients treated with surgery, there was no difference between the 2 arms for OS, MS, DFS, or BM. Multivariable analysis (eTable2 in Supplement 2) revealed that older age (≥60 years) and stage IIIB cancer were associated with increased risks of death. In addition, patients with stage IIIB cancer had higher risk of DFS events, and patients with nonsquamous cancer had higher risk of BM.
There was no difference between PCI and observation among the 225 patients with nonsquamous histologic characteristics with respect to OS (eTable3 in Supplement 2). Similar to the main analysis, there was a significant benefit for DFS (HR, 0.72; 95% CI, 0.53-0.98; P = .04) and BM (HR, 0.43; 95% CI, 0.25-0.78; P = .01) with the use of PCI. There were 115 patients with squamous histologic characteristics. There was no difference in OS and DFS between the 2 arms. There was an insufficient number of events within the BM end point for subgroup analysis in this population.
PCI-Related Toxic Events
Grade 3 acute PCI toxic events occurred in 6 (4%) of the cases in the PCI arm, which included fatigue, hematologic, ataxia, depression, and headache. There was 1 (1%) grade 4 acute PCI toxic event reported (depression). Five (3%) patients in the PCI arm reported grade 3 late PCI toxic events. The only additional grade 3 late PCI toxic event, not reported initially, was soft-tissue necrosis. Neurocognitive function findings were previously published,32,33 however, with longer follow-up, there was insufficient data for further analysis.
Discussion
The NRG Oncology/RTOG 0214 randomized clinical trial was developed to address the apparent increasing incidence of BM in patients who are living longer with improved control of locoregional disease with advanced radiation and surgery techniques and who are experiencing fewer extracranial distant metastases with multidrug chemotherapy. Studies have shown that those with adenocarcinoma or nonsquamous disease16,18,34,35 were at higher risk of BM. Higher BM rates are also associated with greater extent of disease.5,17,36,37 Additionally, studies with trimodality therapy including surgery reported the highest rates of BM.2,3,5,6,7,9,10,12 The NRG Oncology/RTOG 0214 trial was therefore stratified by histologic characteristics (squamous or nonsquamous), stage (IIIA or IIIB), and therapy for primary disease (surgery or no surgery) to minimize potential bias in estimating overall treatment effect due to any heterogeneous treatment effects within these subgroups. Although this study was closed prematurely owing to poor accrual, it still represents the largest randomized clinical trial to evaluate PCI in LA-NSCLC to our knowledge. With one-third of the planned sample size, the current long-term report was only able to provide approximately 45% power to detect the hypothesized effect size in OS. Not surprisingly, this long-term analysis failed to show a statistically significant improvement in OS, although the observed magnitude (HR, 0.82) was numerically similar to the hypothesized (HR, 0.8). In addition, with more events than the initial report, this study shows that PCI improves DFS and decreases the risk of BM by 57%. A number of randomized and nonrandomized studies have consistently shown that PCI is associated with decreased risk of BM in patients with LA-NSCLC.2,7,9,10,12,21,22,23,38,39
On multivariable analysis, observation, nonsquamous cancer, and age (<60 years) were associated with higher risk of BM, while performance status, surgery, and stage were not. There was no difference in BM rates between patients with stage IIIA and IIIB disease. However, data regarding volume of disease and extent of nodal involvement were not available. Ceresoli et al5 reported borderline significance of bulky mediastinal disease (nodes >2 cm) and the incidence of BM. Robnett et al17 reported 2-year actuarial incidence of BM of 36% with stage IIIB disease and 29% with stage II/IIIA disease. Wang et al34 conducted a more extensive analysis of impact of nodal disease on BM in 223 patients treated surgically with stage IIIA/B disease. Brain metastases were greater in patients with more lymph nodes and nodal regions involved.
In multivariable analysis of the patients studied by Ceresoli et al,5 age younger than 60 years was associated with an increased risk of BM (31% vs 9%, P = .03). Carolan et al14 showed that 25.6% of patients younger than 60 years experienced first failure in the brain compared with 11.4% of patients 60 years or older. In a review of 4 SWOG studies, patients younger than 50 years of age were at increased risk for developing BM with a hazard ratio of 1.8 (P = .046).13 Review of the Metropolitan Detroit Cancer Surveillance System showed the highest incidence of BM in patients with lung cancer occurred in patients between the ages of 40 and 49 years.40 Other series have not shown an increased risk of BM with young age.1,17
The reliability of subset analyses in this trial are limited by the low numbers, although the results are informative and may guide future trials. The rates of OS and DFS were evaluated in subsets of patients with nonsquamous cancer, with surgery, and without surgery for primary disease. Patients with nonsquamous disease had more BMs than those with squamous disease. The use of PCI significantly decreased the risk of BM (HR, 0.43; 95% CI, 0.24-0.78; P = .01) and a DFS event (HR, 0.72; 95% CI, 0.53-0.98; P = .04). Perhaps there was no difference between PCI and observation among the 225 patients with nonsquamous cancer with respect to OS; as in a subgroup of patients, targeted therapies can be effective as salvage treatment, including for BM, especially new-generation targeted agents.41,42,43,44,45,46
Although it was expected that patients with surgery for treatment of primary disease would have a higher rate of BM and would therefore be more likely to benefit from PCI, this was not shown in this trial. Contrary to what was anticipated, subset analyses showed that patients with surgery had a relatively low rate of BM, and the risk did not appear to be influenced by PCI. A possible explanation is that patients in the NRG Oncology/RTOG 0214 trial who were undergoing surgery had more favorable disease than in reported trimodality trials. The proportion of patients with subclinical or gross N2 disease is not known, although it is likely that patients treated surgically had lower-volume disease, including bulk of nodal disease and number of nodal stations, relative to the nonsurgical patients in this trial.
Subset analysis of patients treated nonsurgically showed higher incidence of BM in the observation arm at 5 and 10 years (31%) than in the observation arm of the surgery group (23%). This may be owing to higher risk of BM in the nonsurgical group owing to disease burden. Median OS (2.3 years vs 1.9 years, P = .03) and DFS (1.1 years vs 1.0 years; P = .01) were better with PCI in nonsurgical patients. On multivariable analysis, PCI and favorable performance status were associated with improved OS, and PCI was associated with improved DFS in the nonsurgical subset. The therapeutic effect of PCI, if any, appears to be mainly driven by patients treated without surgery. Multivariable analysis within this patient population suggests that PCI effectively prolongs OS (HR, 0.73; P = .04) and DFS (HR, 0.70; P = .01), and decreases BM (HR, 0.34; P = .002). The original study design assumed the MS time for operable and inoperable patients as 38 and 17 months, respectively, and hypothesized detection of a 20% relative risk reduction among both operable and inoperable patients (HR, 0.8, observation arm as reference level). Had all patients in this study been treated without surgery, the corresponding subgroup analysis suggests that PCI would have effectively reduced the relative risk of death by 30% (HR, 0.70). We had hypothesized that the incidence rates of BM would improve from 23.4% to 15.0% by using PCI. This long-term analysis supports this hypothesis in both the overall population (28.3% to 16.7%, HR, 0.43) and in patients treated without surgery (31.0% to 13.0%, HR, 0.36).
Of the patients who experienced failure, 10% in the PCI arm and 23% in the observation arm experienced failure in the brain as the first site of failure, of which 9% and 21% were isolated events, respectively. With the advent of stereotactic radiosurgery for BM, it is likely that many of these patients were treated with stereotactic radiosurgery, thereby potentially confounding the OS results, more so in the observation arm. Furthermore, the decrease in BM, especially as the first site of failure, likely contributed to the improvement in DFS seen in the PCI arm. Since BMs have such a profound impact on QOL, it is likely that this benefit in DFS would translate into improved QOL. Unfortunately, we do not have enough long-term data on QOL to confirm this potentially important finding.
The main difference between the preliminary analysis and this long-term update is the improvement in DFS. With longer follow-up, DFS of PCI patients became statistically significantly better than observation (HR, 0.76, 95% CI, 0.59-0.97; P = .03; 5- and 10-year rates 19.0% and 12.6% vs 16.1% and 7.5% for PCI vs observation). Although OS was also improved with longer follow-up, it was not significant.(HR, 0.82; 95% CI, 0.63-1.06; P = .12; 5- and 10-year rates, 24.7% and 17.6% vs 26.0% and 13.3%, for PCI vs observation) The lack of patients accrued likely contributed to the lack of OS benefit. Unfortunately, it is unlikely with even longer follow-up that OS will show a difference in this study because of the limited numbers of patients still alive.
It is the risk-benefit ratio that helps to determine the advisability of a particular treatment. The risks of PCI are mainly related to the effects on neurocognitive function, which we and others have previously reported.2,7,32,33,47,48,49,50 Effective strategies to decrease these risks include the use of the neuroprotectant memantine51 and PCI with hippocampal avoidance.52,53,54,55 Both of these strategies have shown promising results when treating known BM with whole brain radiotherapy. Prospective randomized phase 2/3 studies will be addressing these same strategies in SCLC, where PCI is part of standard of care56,57,58,59 and may be appropriate for select patients with LA-NSCLC and high risk of BM.
The challenge in the future is to exploit the therapeutic ratio of benefits vs risks. Patients most likely to benefit from PCI are those at highest risk of developing BM. These patients would include those treated without surgery or with poor risk features such as nonsquamous cancer, young age, and high-volume disease. To minimize the risk, promising strategies include hippocampal avoidance radiotherapy techniques and/or neuroprotectants (memantine). Furthermore, as the standard treatment of unresectable stage III disease is in the process of change with the addition of consolidation immunotherapy,60,61 this has to be taken into account for future trials.
Limitations
The main limitation of this study was the lack of patient accrual, which likely contributed to the lack of OS benefit for the entire cohort. If the planned sample size had been accrued, then there may have been enough statistical power to detect the hypothesized effect size in OS, as the observed magnitude (HR, 0.82) was numerically similar to the hypothesized (HR, 0.8). This study, the largest randomized phase 3 clinical trial to evaluate PCI in LA-NSCLC to our knowledge, is currently part of an international collaborative effort, which is performing an individual patient data meta-analysis of similar randomized studies. It is hoped that this meta-analysis will have the power to detect an OS advantage for PCI, as was the case for the establishment of PCI in SCLC.56
Conclusions
In conclusion, this final analysis did not show an overall survival benefit, although PCI improved DFS and decreased the risk of BM in patients with LA-NSCLC. It is very unlikely that a single definitive study with and without PCI for NSCLC will ever be completed, although establishing an accepted means of prevention of BM remains important. Currently, the most effective therapy to prevent BMs is PCI. Identifying the appropriate patient population and a safe intervention is critical.
Trial protocol.
eTable 1. Cox proportional hazard multivariable analysis results, for no prior surgery subset
eTable 2. Cox proportional hazard multivariate analysis results, for prior surgery subset
eTable 3. Outcome estimates, by histology
eFigure 1. Cumulative Incidences of First Failures by Event Types
Data Sharing Statement
References
- 1.Chen AM, Jahan TM, Jablons DM, Garcia J, Larson DA. Risk of cerebral metastases and neurological death after pathological complete response to neoadjuvant therapy for locally advanced nonsmall-cell lung cancer: clinical implications for the subsequent management of the brain. Cancer. 2007;109(8):1668-1675. doi: 10.1002/cncr.22565 [DOI] [PubMed] [Google Scholar]
- 2.Pöttgen C, Eberhardt W, Grannass A, et al. . Prophylactic cranial irradiation in operable stage IIIA non small-cell lung cancer treated with neoadjuvant chemoradiotherapy: results from a German multicenter randomized trial. J Clin Oncol. 2007;25(31):4987-4992. doi: 10.1200/JCO.2007.12.5468 [DOI] [PubMed] [Google Scholar]
- 3.Mamon HJ, Yeap BY, Jänne PA, et al. . High risk of brain metastases in surgically staged IIIA non-small-cell lung cancer patients treated with surgery, chemotherapy, and radiation. J Clin Oncol. 2005;23(7):1530-1537. doi: 10.1200/JCO.2005.04.123 [DOI] [PubMed] [Google Scholar]
- 4.Keith B, Vincent M, Stitt L, et al. . Subsets more likely to benefit from surgery or prophylactic cranial irradiation after chemoradiation for localized non-small-cell lung cancer. Am J Clin Oncol. 2002;25(6):583-587. doi: 10.1097/00000421-200212000-00011 [DOI] [PubMed] [Google Scholar]
- 5.Ceresoli GL, Reni M, Chiesa G, et al. . Brain metastases in locally advanced nonsmall cell lung carcinoma after multimodality treatment: risk factors analysis. Cancer. 2002;95(3):605-612. doi: 10.1002/cncr.10687 [DOI] [PubMed] [Google Scholar]
- 6.Law A, Karp DD, Dipetrillo T, Daly BT. Emergence of increased cerebral metastasis after high-dose preoperative radiotherapy with chemotherapy in patients with locally advanced nonsmall cell lung carcinoma. Cancer. 2001;92(1):160-164. doi: [DOI] [PubMed] [Google Scholar]
- 7.Stuschke M, Eberhardt W, Pöttgen C, et al. . Prophylactic cranial irradiation in locally advanced non-small-cell lung cancer after multimodality treatment: long-term follow-up and investigations of late neuropsychologic effects. J Clin Oncol. 1999;17(9):2700-2709. doi: 10.1200/JCO.1999.17.9.2700 [DOI] [PubMed] [Google Scholar]
- 8.Choi NC, Carey RW, Daly W, et al. . Potential impact on survival of improved tumor downstaging and resection rate by preoperative twice-daily radiation and concurrent chemotherapy in stage IIIA non-small-cell lung cancer. J Clin Oncol. 1997;15(2):712-722. doi: 10.1200/JCO.1997.15.2.712 [DOI] [PubMed] [Google Scholar]
- 9.Albain KS, Rusch VW, Crowley JJ, et al. . Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi: 10.1200/JCO.1995.13.8.1880 [DOI] [PubMed] [Google Scholar]
- 10.Strauss GM, Herndon JE, Sherman DD, et al. . Neoadjuvant chemotherapy and radiotherapy followed by surgery in stage IIIA non-small-cell carcinoma of the lung: report of a Cancer and Leukemia Group B phase II study. J Clin Oncol. 1992;10(8):1237-1244. doi: 10.1200/JCO.1992.10.8.1237 [DOI] [PubMed] [Google Scholar]
- 11.Weiden PL, Piantadosi S. Preoperative chemotherapy (cisplatin and fluorouracil) and radiation therapy in stage III non-small-cell lung cancer: a phase II study of the Lung Cancer Study Group. J Natl Cancer Inst. 1991;83(4):266-273. doi: 10.1093/jnci/83.4.266 [DOI] [PubMed] [Google Scholar]
- 12.Skarin A, Jochelson M, Sheldon T, et al. . Neoadjuvant chemotherapy in marginally resectable stage III M0 non-small cell lung cancer: long-term follow-up in 41 patients. J Surg Oncol. 1989;40(4):266-274. doi: 10.1002/jso.2930400413 [DOI] [PubMed] [Google Scholar]
- 13.Gaspar LE, Chansky K, Albain KS, et al. . Time from treatment to subsequent diagnosis of brain metastases in stage III non-small-cell lung cancer: a retrospective review by the Southwest Oncology Group. J Clin Oncol. 2005;23(13):2955-2961. doi: 10.1200/JCO.2005.08.026 [DOI] [PubMed] [Google Scholar]
- 14.Carolan H, Sun AY, Bezjak A, et al. . Does the incidence and outcome of brain metastases in locally advanced non-small cell lung cancer justify prophylactic cranial irradiation or early detection? Lung Cancer. 2005;49(1):109-115. doi: 10.1016/j.lungcan.2004.12.004 [DOI] [PubMed] [Google Scholar]
- 15.Albain KS, Crowley JJ, Turrisi AT III, et al. . Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: a Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol. 2002;20(16):3454-3460. doi: 10.1200/JCO.2002.03.055 [DOI] [PubMed] [Google Scholar]
- 16.Andre F, Grunenwald D, Pujol JL, et al. . Patterns of relapse of N2 non–small cell lung carcinoma patients treated with preoperative chemotherapy: should prophylactic cranial irradiation be reconsidered? Cancer. 2001;91:2394-2400. doi: [DOI] [PubMed] [Google Scholar]
- 17.Robnett TJ, Machtay M, Stevenson JP, Algazy KM, Hahn SM. Factors affecting the risk of brain metastases after definitive chemoradiation for locally advanced non-small-cell lung carcinoma. J Clin Oncol. 2001;19(5):1344-1349. doi: 10.1200/JCO.2001.19.5.1344 [DOI] [PubMed] [Google Scholar]
- 18.Cox JD, Scott CB, Byhardt RW, et al. . Addition of chemotherapy to radiation therapy alters failure patterns by cell type within non-small cell carcinoma of lung (NSCCL): analysis of radiation therapy oncology group (RTOG) trials. Int J Radiat Oncol Biol Phys. 1999;43(3):505-509. doi: 10.1016/S0360-3016(98)00429-5 [DOI] [PubMed] [Google Scholar]
- 19.Komaki R, Scott CB, Sause WT, et al. ; Radiation Therapy Oncology Group. Eastern Cooperative Oncology Group . Induction cisplatin/vinblastine and irradiation vs. irradiation in unresectable squamous cell lung cancer: failure patterns by cell type in RTOG 88-08/ECOG 4588. Int J Radiat Oncol Biol Phys. 1997;39(3):537-544. doi: 10.1016/S0360-3016(97)00365-9 [DOI] [PubMed] [Google Scholar]
- 20.Arriagada R, Le Chevalier T, Quoix E, et al. . ASTRO (American Society for Therapeutic Radiology and Oncology) plenary: Effect of chemotherapy on locally advanced non-small cell lung carcinoma: a randomized study of 353 patients. GETCB (Groupe d’Etude et Traitement des Cancers Bronchiques), FNCLCC (Féderation Nationale des Centres de Lutte contre le Cancer) and the CEBI trialists. Int J Radiat Oncol Biol Phys. 1991;20(6):1183-1190. doi: 10.1016/0360-3016(91)90226-T [DOI] [PubMed] [Google Scholar]
- 21.Cox JD, Stanley K, Petrovich Z, Paig C, Yesner R. Cranial irradiation in cancer of the lung of all cell types. JAMA. 1981;245(5):469-472. doi: 10.1001/jama.1981.03310300023013 [DOI] [PubMed] [Google Scholar]
- 22.Umsawasdi T, Valdivieso M, Chen TT, et al. . Role of elective brain irradiation during combined chemoradiotherapy for limited disease non-small cell lung cancer. J Neurooncol. 1984;2(3):253-259. doi: 10.1007/BF00253278 [DOI] [PubMed] [Google Scholar]
- 23.Russell AH, Pajak TE, Selim HM, et al. . Prophylactic cranial irradiation for lung cancer patients at high risk for development of cerebral metastasis: results of a prospective randomized trial conducted by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1991;21(3):637-643. doi: 10.1016/0360-3016(91)90681-S [DOI] [PubMed] [Google Scholar]
- 24.Gore EM, Bae K, Wong SJ, et al. . Phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small-cell lung cancer: primary analysis of radiation therapy oncology group study RTOG 0214. J Clin Oncol. 2011;29(3):272-278. doi: 10.1200/JCO.2010.29.1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;35:457-481. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 26.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Hoboken, New Jersey: John Wiley & Sons; 2011. [Google Scholar]
- 27.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163-170. [PubMed] [Google Scholar]
- 28.Kim K, Tsiatis AA. Study duration for clinical trials with survival response and early stopping rule. Biometrics. 1990;46(1):81-92. doi: 10.2307/2531632 [DOI] [PubMed] [Google Scholar]
- 29.Cox DR. Regression models and life-tables (with discussion). J R Stat Soc B. 1972;34:187-200. [Google Scholar]
- 30.Prentice RL, Kalbfleisch JD, Peterson AV Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34(4):541-554. doi: 10.2307/2530374 [DOI] [PubMed] [Google Scholar]
- 31.Gail M, Simon R. Testing for qualitative interactions between treatment effects and patient subsets. Biometrics. 1985;41(2):361-372. doi: 10.2307/2530862 [DOI] [PubMed] [Google Scholar]
- 32.Sun A, Bae K, Gore EM, et al. . Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: neurocognitive and quality-of-life analysis. J Clin Oncol. 2011;29(3):279-286. doi: 10.1200/JCO.2010.29.6053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gondi V, Paulus R, Bruner DW, et al. . Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: pooled secondary analysis of Radiation Therapy Oncology Group randomized trials 0212 and 0214. Int J Radiat Oncol Biol Phys. 2013;86(4):656-664. doi: 10.1016/j.ijrobp.2013.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komaki R, Cox JD, Stark R. Frequency of brain metastasis in adenocarcinoma and large cell carcinoma of the lung: correlation with survival. Int J Radiat Oncol Biol Phys. 1983;9(10):1467-1470. doi: 10.1016/0360-3016(83)90319-X [DOI] [PubMed] [Google Scholar]
- 35.Perez CA, Pajak TF, Simpson JR, et al. . Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Cancer. 1987;59:1874-1881. doi: [DOI] [PubMed] [Google Scholar]
- 36.Wang SY, Ye X, Ou W, Lin YB, Zhang BB, Yang H. Risk of cerebral metastases for postoperative locally advanced non-small-cell lung cancer. Lung Cancer. 2009;64(2):238-243. doi: 10.1016/j.lungcan.2008.08.012 [DOI] [PubMed] [Google Scholar]
- 37.Komaki R, Scott CB, Byhardt R, et al. . Failure patterns by prognostic group determined by recursive partitioning analysis (RPA) of 1547 patients on four radiation therapy oncology group (RTOG) studies in inoperable nonsmall-cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 1998;42(2):263-267. doi: 10.1016/S0360-3016(98)00213-2 [DOI] [PubMed] [Google Scholar]
- 38.Li N, Zeng ZF, Wang SY, et al. . Randomized phase III trial of prophylactic cranial irradiation versus observation in patients with fully resected stage IIIA-N2 nonsmall-cell lung cancer and high risk of cerebral metastases after adjuvant chemotherapy. Ann Oncol. 2015;26(3):504-509. doi: 10.1093/annonc/mdu567 [DOI] [PubMed] [Google Scholar]
- 39.De Ruysscher D, Dingemans AC, Praag J, et al. . Prophylactic cranial irradiation versus observation in radically treated stage III non-small-cell lung cancer: a randomized phase III NVALT-11/DLCRG-02 Study. J Clin Oncol. 2018;36(23):2366-2377. doi: 10.1200/JCO.2017.77.5817 [DOI] [PubMed] [Google Scholar]
- 40.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865-2872. doi: 10.1200/JCO.2004.12.149 [DOI] [PubMed] [Google Scholar]
- 41.Wu YL, Ahn MJ, Garassino MC, et al. . CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol. 2018;36(26):2702-2709. doi: 10.1200/JCO.2018.77.9363 [DOI] [PubMed] [Google Scholar]
- 42.Zhang I, Zaorsky NG, Palmer JD, Mehra R, Lu B. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol. 2015;16(13):e510-e521. doi: 10.1016/S1470-2045(15)00013-3 [DOI] [PubMed] [Google Scholar]
- 43.Johung KL, Yeh N, Desai NB, et al. . Extended survival and prognostic factors for patients with ALK-rearranged non-small-cell lung cancer and brain metastasis. J Clin Oncol. 2016;34(2):123-129. doi: 10.1200/JCO.2015.62.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerber NK, Yamada Y, Rimner A, et al. . Erlotinib versus radiation therapy for brain metastases in patients with EGFR-mutant lung adenocarcinoma. Int J Radiat Oncol Biol Phys. 2014;89(2):322-329. doi: 10.1016/j.ijrobp.2014.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reungwetwattana T, Nakagawa K, Cho BC, et al. . CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018;JCO2018783118. [DOI] [PubMed] [Google Scholar]
- 46.Costa DB, Shaw AT, Ou SH, et al. . Clinical experience with crizotinib in patients with advanced ALK-rearranged non–small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33(17):1881-1888. doi: 10.1200/JCO.2014.59.0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arriagada R, Le Chevalier T, Borie F, et al. . Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst. 1995;87(3):183-190. doi: 10.1093/jnci/87.3.183 [DOI] [PubMed] [Google Scholar]
- 48.Gregor A, Cull A, Stephens RJ, et al. ; United Kingdom Coordinating Committee for Cancer Research (UKCCCR) and the European Organization for Research and Treatment of Cancer (EORTC) . Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: results of a multicentre randomised trial. Eur J Cancer. 1997;33(11):1752-1758. doi: 10.1016/S0959-8049(97)00135-4 [DOI] [PubMed] [Google Scholar]
- 49.Wolfson AH, Bae K, Komaki R, et al. . Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81(1):77-84. doi: 10.1016/j.ijrobp.2010.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Péchoux C, Laplanche A, Faivre-Finn C, et al. ; Prophylactic Cranial Irradiation (PCI) Collaborative Group . Clinical neurological outcome and quality of life among patients with limited small-cell cancer treated with two different doses of prophylactic cranial irradiation in the intergroup phase III trial (PCI99-01, EORTC 22003-08004, RTOG 0212 and IFCT 99-01). Ann Oncol. 2011;22(5):1154-1163. doi: 10.1093/annonc/mdq576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown PD, Pugh S, Laack NN, et al. ; Radiation Therapy Oncology Group (RTOG) . Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429-1437. doi: 10.1093/neuonc/not114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gutiérrez AN, Westerly DC, Tomé WA, et al. . Whole brain radiotherapy with hippocampal avoidance and simultaneously integrated brain metastases boost: a planning study. Int J Radiat Oncol Biol Phys. 2007;69(2):589-597. doi: 10.1016/j.ijrobp.2007.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gondi V, Pugh SL, Tome WA, et al. . Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810-3816. doi: 10.1200/JCO.2014.57.2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kazda T, Jancalek R, Pospisil P, et al. . Why and how to spare the hippocampus during brain radiotherapy: the developing role of hippocampal avoidance in cranial radiotherapy. Radiat Oncol. 2014;9:139. doi: 10.1186/1748-717X-9-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Péchoux CL, Sun A, Slotman BJ, De Ruysscher D, Belderbos J, Gore EM. Prophylactic cranial irradiation for patients with lung cancer. Lancet Oncol. 2016;17(7):e277-e293. doi: 10.1016/S1470-2045(16)30065-1 [DOI] [PubMed] [Google Scholar]
- 56.Aupérin A, Arriagada R, Pignon JP, et al. ; Prophylactic Cranial Irradiation Overview Collaborative Group . Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med. 1999;341(7):476-484. doi: 10.1056/NEJM199908123410703 [DOI] [PubMed] [Google Scholar]
- 57.Meert AP, Paesmans M, Berghmans T, et al. . Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer. 2001;1:5. doi: 10.1186/1471-2407-1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slotman B, Faivre-Finn C, Kramer G, et al. ; EORTC Radiation Oncology Group and Lung Cancer Group . Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357(7):664-672. doi: 10.1056/NEJMoa071780 [DOI] [PubMed] [Google Scholar]
- 59.Schild SE, Foster NR, Meyers JP, et al. ; North Central Cancer Treatment Group . Prophylactic cranial irradiation in small-cell lung cancer: findings from a North Central Cancer Treatment Group Pooled Analysis. Ann Oncol. 2012;23(11):2919-2924. doi: 10.1093/annonc/mds123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators . Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 61.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators . Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342-2350. doi: 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
eTable 1. Cox proportional hazard multivariable analysis results, for no prior surgery subset
eTable 2. Cox proportional hazard multivariate analysis results, for prior surgery subset
eTable 3. Outcome estimates, by histology
eFigure 1. Cumulative Incidences of First Failures by Event Types
Data Sharing Statement