Abstract
Hot flashes (HFs), defined as transient sensations of heat, sweating, flushing, anxiety, and chills lasting for 1–5 min, constitute one of the most common symptoms of menopause among women though only a few seek treatment for these. The basis of HFs lies in abnormal hypothalamic thermoregulatory control resulting in abnormal vasodilatory response to minor elevations of core body temperature. Recent data suggest an important role for calcitonin gene-related peptide, hypothalamic kisspeptin, neurokinin B and dynorphin signal system, serotonin, norepinephrine in causation of HFs in addition to estrogen deficiency which plays a cardinal role. The mainstay of treatment includes hormonal replacement therapy, selective serotonin, and norepinephrine reuptake inhibitors in addition to lifestyle modification. In this review, we address common issues related to menopause HFs and suggest a stepwise approach to their management.
KEYWORDS: Hormone replacement therapy, hot flashes, menopause
INTRODUCTION
More than 80% of women experience hot flashes (HFs) during menopause. Defined by transient sensations of heat, sweating, flushing, anxiety, and chills lasting for 1–5 min, HFs can cause considerable distress especially when severe and frequent.[1,2] However, these are often ignored due in part to their relatively benign nature. In this review, we discuss epidemiology, pathophysiology, diagnosis as well as management of menopausal HFs.
EPIDEMIOLOGY
HFs are extremely common affecting approximately 85% of menopausal women. Approximately 55% of women experience HFs as they enter transition phase toward menopause indicated by the beginning of menstrual irregularity. These increase in incidence and severity as women enter menopause becoming most troublesome during late menopause transition followed by gradual decline.[3,4,5,6] As menopausal age in Indian women is approximately 4–5 years lower than western women,[7] HFs may occur earlier in Indian women. The mean duration of HFs is determined to be 5.2 years in one meta-analysis.[5] In another meta-analysis (n = 35455), mean duration of HFs was estimated to be 4 years,[6] while two studies[8,9] found a median duration of 7.4 years for the presence of HFs. Approximately 25% of women continue to experience HFs after 5 years of attaining menopause, one-third of women continue to experience HFs even after 10 years of menopause[2] and 8% of women continue to experience HFs even after 20 years of menopause.[10,11] Studies have shown geographical boundaries to affect the prevalence of HFs with the highest frequency in Turkish women (97%), followed by Australian (83%), European (76.5%), and North American (58.8%) women in that order. HFs are reported by 47% of women living in South America and 45% of women in Asia.[12] Tepper et al.[13] (n = 1455) reported four distinct patterns of menopausal vasomotor symptoms (VMS). Nearly 44% of women start having VMS approximately 11 years before their final menstrual period. Among them, VMS decline in approximately 42.3% and persist at high frequency in 57.5% of women. Approximately 29% of women experience HFs toward the end of the menstrual period with later decline while 27% of women experience HFs at a persistently low frequency. Studies have found that despite having distressing symptoms only one out of every four women with menopausal VMS actually seeks medical advice.[14,15]
Risk factors
Among various factors reported to be associated with increase in incidence of HFs, a consistent association has been found only with obesity, African descent, lower socioeconomic status, presence of premenstrual syndrome, sedentary lifestyle, and smoking.[16,17] Recently, the role of genetic factors in causation of HFs is being highlighted. In one study,[18] evaluating 11,078,977 single-nucleotide polymorphisms (SNPs) in 17695 women, SNPs in intronic regions of tachykinin receptor 3 gene, which codes for neurokinin B neuropeptide receptor (NK3R), were significantly (Odd's ratio: 1.5) associated with menopausal VMS. However, lot remains to be done as far as the role of genetic factors in elucidation of menopausal VMS is considered.
PATHOPHYSIOLOGY
Despite decades of research, the exact pathophysiological basis of HFs remains elusive. Whatsoever, the mechanisms HFs are characterized by excessive vasodilatation of peripheral vasculature to lose heat in setting of abnormal hypothalamic thermoneutral zone. While normal women initiate mechanisms of heat loss, once core body temperature increases by 0.4°C, women with HFs initiate vasodilatory response with a much smaller increase in core body temperature. It is peripheral vasodilatory response that results in profuse sweating and sensation of intense heat.[19,20,21]
During HFs, there is increase in blood flow along with hyperthermia in major portions of the body. The maximum increase in temperature occurs in digits and toes where temperature may increase from normal of 20°C–33°C, though the symptoms of hot flushes are most intense in upper torso (head, neck, and upper chest). The peripheral vasodilation results in loss of heat with lowering of core body temperature and abolition of flush. The chills which accompany HFs are a compensatory response to bring lowered core body temperature to normal.[19,20,21]
The underlying cause for HFs is abnormality in hypothalamic thermoregulatory mechanisms. As HFs are associated with menopause and improve following estrogen therapy, estrogen deficiency seems to play definite role in their causation. However, precise role of estrogen deficiency remains to be elucidated. There is no correlation between serum estrogen levels and frequency and severity of HFs. Furthermore, HFs cease with time after menopause when estrogen levels are further declining. Thus, the rate of decline of estrogen levels rather than actual decrease may be more important in causation of HFs.[21,22,23] The prior priming of brain by estrogens also seems to play an important role in the generation of HFs as women with ovarian dysgenesis develop HFs only after withdrawal of estrogen replacement therapy.[21] A potential role for other pituitary hormones, gonadotropins, and anti-mullerian hormones has been suggested but never proven.[21]
A role of serotonin has been suggested by several authors.[21] Estrogens stimulate the production of serotonin and endorphins, and there is 50% decrease in levels of serotonin after menopause corresponding to declining estrogen levels. Decrease in serotonin results in increase in levels of norepinephrine which disturbs hypothalamic thermostat. Several indirect observations also suggest a role for serotonin and norepinephrine in the generation of HFs.[21] These include (a) favorable response of HFs to selective serotonin reuptake inhibitors (SSRIs); (b) increased plasma levels of main brain metabolite of norepinephrine during HFs;[24] (c) reduction in HFs by Clonidine (α2 adrenergic antagonist), a drug which decreases brain norepinephrine levels;[25] and (d) Yohimbine (α2 adrenergic agonist), a drug which increases brain levels of norepinephrine may trigger HFs.
Recent studies have suggested a role for hypothalamic kisspeptin, neurokinin B and dynorphin (KND) signal system in genesis of HFs.[25] Neurons of this system control secretion of gonadotropins and project to median preoptic area which is the main hypothalamic thermoregulatory center.[26] In experiment animals, central administration of kisspeptin increases rat tail skin temperature similar to that induced by estrogen deficiency.[27] The findings of induction of HFs in postmenopausal women by the administration of neurokinin B[28] coupled with evidence of increased expression of neurokinin B in postmortem brain samples of postmenopausal women also suggest a role for KND system in induction of HFs.[29]
Another neurotransmitter postulated to have a role in HFs is calcitonin gene-related peptide (CGRP). Localized to sensory fibers, it is the most potent vasodilator in man. Animal studies have suggested a role for CGRP in HFs. Intravenous CGRP causes greater increase in skin temperature of ovariectomized rats compared to controls.[30,31]
The vasodilatory effect of CGRP is independent of histamine, bradykinin, prostaglandin, or epinephrine. In fact, HFs do not respond to antagonists of any of these substances.[32] Cutaneous distribution of CGRP follows the distribution of sensory nerves which can explain why flashes prominently affect upper torso.[33] CGRP is found in cholinergic sympathetic nerves of sweat glands and local administration of CGRP enhances methacholine-induced sweating in a dose-dependent manner.[34] All these facts suggest a role for CGRP in genesis of HFs and future studies will help to delineate its role in a better manner.
CLINICAL SYMPTOMS
A typical HF begins as a sudden unexpected sensation of intense heat involving face and upper chest which rapidly involves the entire body. The sensation lasts from 2 to 4 min and is associated with profuse sweating, chills, palpitation, and anxiety. Their frequency varies from occasional attacks in a week to once or twice each hour. HFs often present as night sweats and are associated sleep disturbances. When severe these are associated with interference in activities of daily living and have a negative impact on quality of life.[1,35] The approach to diagnosis and evaluation of HFs is summarized in Figure 1.
Figure 1.
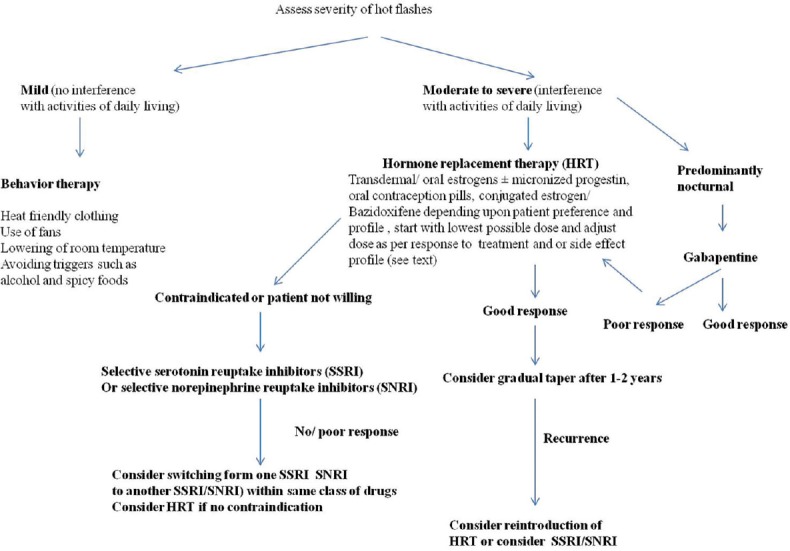
Step-wise approach to management of menopausal hot flashes
MANAGEMENT
The management of HFs is guided by their frequency and severity. The severity of HFs can be graded as (a) mild (no interference with usual daily activities), (b) moderate (interfere with usual daily activities to some extent), and (c) severe (when usual daily activities cannot be performed).[35] The further management protocols can be directed according to the severity of HFs, presence of associated menopausal symptoms such as depression personal choice of patient and presence or absence of any contraindications to hormonal replacement therapy.
Management of mild hot flashes
For most women with HFs of mild severity, the modification of daily life may suffice. The various ways which often prove helpful include use of fans, lowering of room temperature, avoidance of triggers such as alcohol, spices, and use of clothes with are heat and sweat-friendly.[36] Other options which may be tried with some efficacy include Vitamin E in low doses, weight loss, and cognitive behavior therapy.[37,38]
Management of moderate to severe hot flashes
Hormonal replacement therapy
Hormonal replacement therapy (HRT) either with estrogen alone (following hysterectomy) or both estrogen and progestin (in the presence of uterus to avoid endometrial hyperplasia) is recommended as first-line therapy for Rx of moderate-to-severe menopausal HFs[39] provided women is <60 years of age and does not have contraindication to HRT such as past stroke or breast cancer or venous thromboembolism, coronary artery disease, active liver disease, unexplained vaginal bleeding, known thrombophilic state, and active gall bladder disease. HRT benefits both HFs and other symptoms of menopause such as mood changes, sleep problems, vaginal atrophy, dyspareunia, and joint aches.[40,41,42,43]
The route (oral/transdermal/subcutaneous implant or intravaginal) of estrogen therapy should be individualized in liaison with the patient. The efficacy of oral 17-β estradiol (1 mg daily), transdermal 17-β estradiol (0.05 mg daily), and conjugated estrogen (0.625 mg daily) is almost the same with abolition of HFs in approximately 80% of women.[44] As transdermal formulations have lower risk of thromboembolism and stroke, vaginal bleeding and breast tenderness, these may be used as initial treatment. One can start with 0.025 mg estradiol transdermal preparation or oral estrogen (0.5 mg estradiol daily) depending on the patient's preference. If required, dose is increased at monthly intervals to 0.05 mg transdermal estradiol or 1 mg oral estradiol. For severe HFs, 0.05 mg transdermal estradiol can be used from the beginning. As transdermal preparation avoid hepatic first mass metabolism, it can be used in women taking enzyme-inducing drugs.[45] Transdermal preparation is also preferred in women on thyroid hormone replacement therapy as oral estrogens increase thyroid bindings globulins thereby decreasing the amount of free thyroxine.[46]
Women with intact uterus need progestins along with estrogens to prevent endometrial hyperplasia and carcinoma. To avoid irregular bleeding, preferred treatment includes natural micronized progestin 200 mg daily for 12 days a month in pre and perimenopausal women. For women intolerant to cyclic progestin therapy due to mood swings/bloating, daily therapy may be better. For women intolerant to oral progestin therapy, low dose levonorgestrel-releasing intrauterine device can be advised. For women without uterus, estrogen alone is used as only advantage of the use of progesterone is avoidance of endometrial hyperplasia.
In the United States, conjugated estrogen (0.45 mg) in combination with bazedoxifene (20 mg) (a selective estrogen receptor modulator) is available for women who cannot tolerate estrogen or progestin therapy. Theoretically, this combination has agonist effect on bone, antagonist effect on endometrium and no effect on the breast. However, future studies are needed to check the efficacy of bazedoxifene.[47]
HRT is used for a maximum duration of 5 years or up to 60 years of age. It can be stopped in 40%–50% of women within 1 year and 65%–75% within 2 years of Rx.[48,49] For women, who develop recurrence of HFs, nonhormonal treatment options are used first followed by extended HRT (beyond 60 or even 65 years of age) shall nonhormonal treatment options fail.[50]
Once a decision to stop HRT is taken, it is preferable to taper the drugs over 3–6 months. For women who develop recurrence of severe HFs, more gradual taper, over 6 months to 1 year, may be advisable.[51,52] Low-dose oral contraceptive pill (OCP) containing 20 μg of ethinyl estradiol may be used instead of HRT in perimenopausal women (40–50 years of age) who desire contraception and have heavy bleeding. Once the woman reaches the age of 50–51 years, consideration should be given to either complete stoppage of OCP or replacement with HRT. Routine mammography and breast examination are recommended for women on HRT.[53]
Nonhormonal drug treatment
For women, who have contraindications or cannot tolerate HRT, nonhormonal drugs are used for the management of HFs.[40,54] The various nonhormonal treatment options include.
Selective serotonin reuptake inhibitors and selective norepinephrine reuptake inhibitors
SSRIs and SNRIs are the most effective drugs for HFs after HRT.[55,56] Among the various SSRIs available, paroxetine (7.5–25 mg daily) and escitalopram (20 mg daily) are used as first choice, while fluoxetine and sertraline are not effective.[57,58] The duration of therapy needed to demonstrate benefit on HFs is lower than the duration required to demonstrate their antidepressant effect. SNRIs (venlafaxine [37.5 mg daily increased to 75 mg daily after 1 week] and desvenlafaxine) are equally effective in the management of HFs.[59]
Gabapentin may be a better choice for women with predominant nocturnal HFs for its added benefit on the maintenance of sleep cycle. It is as effective as venlafaxine, but patients often prefer venlafaxine due to better tolerance profile of later.[60] The dosage of gabapentin needs to be individualized. While a single 100–300 mg bedtime dose may suffice for predominantly nocturnal HFs, a dose of 300 mg three times a day may be required for severe and frequent HFs. To decrease sedation, it is preferable to start with 100 mg daily with gradual increase by 100 mg every 3 days to maximally effective or tolerated dose.[55] While switching therapy from SSRI/SNRI to gabapentin, it is preferable to continue SSRI/SNRI for 1st 2 weeks while gabapentin is being introduced and taking its full effect. The other drugs (clonidine and pregabalin) are used only infrequently for the management of HFs.
For women, who do not respond to one SSRI/SNRI, use another SSRI/SNRI itself before switching to another class of drugs. In case, these are ineffective or not tolerated, consider administration of gabapentin.
Other treatment options
These include single 500 mg intramuscular dose of depot medroxyprogesterone acetate once every few months/10 mg norethindrone acetate daily.[61,62]
Special considerations
Women with breast cancer
Factors which contribute to HFs in women with breast cancer include bilateral oophorectomy, chemotherapy-induced ovarian failure, tamoxifen (HFs in 80% which are severe in 30%) and aromatase inhibitors. SSRI/SNRI are the drugs of choice as HRT is contraindicated in breast cancer. However, paroxetine and fluoxetine should not be used with Tamoxifen as these are potent inhibitors of CYP2D6 which catalyses conversion of Tamoxifen to its active metabolites.[63,64,65]
Future therapies
Neurokinin 3 receptor antagonist – Although initial results of treatment with MLE4901 (a neurokinin 3 receptor [NK3R] antagonist) are promising, more studies are needed before NK3R antagonists can be recommended as nonhormonal agents for the treatment of HFs.[66]
Stellate ganglion block is known to alleviate HFs especially of moderate to severe intensity.[67,68] However, more studies need to be done before this procedure can be used routinely.
Conventional therapies
These include cognitive behavioral therapy, plant-based therapies, weight loss, evening primrose oil, flaxseed, ginseng, wild yam, black cohosh, progesterone creams, medicinal Chinese herbs, reflexology, and magnetic devices. However, these are either ineffective or their efficacy remains to be proven in large clinical trials.[69,70,71] Various treatment options have been summarized in Table 1.
Table 1.
Treatment options for menopausal hot flashes
| Nature of therapy | Benefits on hot flashes | Current status | Comments |
|---|---|---|---|
| Transdermal estrogen + micronized progestin or oral estrogen + micronized progestin | Definite | 1st choice therapy for Rx of moderate to severe hot flashes in women with intact uterus Transdermal preparation has lower risk of thromboembolism, vaginal bleeding, stroke, and breast tenderness and lacks hepatic first pass metabolism |
Past history of stroke/breast cancer/venous thromboembolic event, coronary artery disease, active liver disease, unexplained vaginal bleeding, active gall bladder disease |
| Transdermal/oral estrogen alone | Definite | 1st choice therapy for Rx of moderate to severe hot flashes in women without uterus Transdermal preparation has lower risk of thromboembolism, vaginal bleeding, stroke, and breast tenderness and lacks hepatic first pass metabolism |
Same as above |
| SSRIs (paroxetine and escitalopram) | Definite | Rx of moderate to severe hot flashes in women who cannot tolerate hormonal therapy or in whom hormonal therapy is contraindicated | Paroxetine should be avoided in women on tamoxifen |
| SNRIs | Definite | Rx of moderate to severe hot flashes in women who cannot tolerate hormonal therapy or in whom hormonal therapy is contraindicated SNRIs are equally effective to SSRIs |
May be used as alternative treatment option for women who cannot tolerate SSRIs/who develop intolerable side effects to SSRIs |
| Gabapentin | Definite | Useful in women with predominantly nocturnal moderate to severe hot flashes | Sedation is main side effect |
| 500 mg intramuscular dose of depot medroxyprogesterone acetate once every few months | Definite | More effective than venlafaxine | May be used in women who have contraindications to estrogen therapy |
| Tibolone | Definite | A synthetic steroid widely used in Europe, it controls hot flashes and has beneficial effect on bone metabolism | Associated with higher risk of stroke |
| Conjugated estrogen+bazedoxifene | Studies are needed | Theoretically, this combination has agonist effect on bone, antagonist effect on endometrium and no effect on breast | |
| Oral contraceptive pills | Definite | May be used in perimenopausal women in (40-50 years of age) who desire contraception and have heavy bleeding | Past history of stroke/breast cancer/venous thromboembolic event, coronary artery disease, active liver disease, unexplained vaginal bleeding, active gall bladder disease |
| Cognitive behavior therapy | Benefits distress and sleep problems, but effects on hot flashes per se is not certain | Often used without any proven benefit | |
| Stellate ganglion block | Beneficial in some trials | Future studies are needed before this procedure can be used routinely | |
| Weight loss | Some benefits in overweight patients | Must be tried in obese postmenopausal women with hot flashes | |
| Exercise | Uncertain | May trigger hot flashes through elevation of core body temperature | |
| Plant based therapies (soybean, chickpeas, lentils for isoflavones; flaxseed, lentils, grains, fruits, and vegetables for lignans) | Uncertain | Concern of risk of breast cancer | |
| Flaxseed/evening primrose oil | Nil | Not recommended | |
| Acupuncture | Benefits hot flashes, sleep problems, sweating, emotional and physical symptoms | One randomized controlled trail (n=70) showed definite benefit for acupuncture in menopausal hot flashes | Future trails will delineate it role in a better manner |
SSRIs: Selective serotonin reuptake inhibitors, SNRIs: Selective norepinephrine reuptake inhibitors
Acupuncture
Acupuncture has been used for the management of menopausal HFs for quite some time but without much evidence.[72,73] However, a recently conducted RCT[74] in Danish population (n = 70), showed the efficacy of acupuncture in statistically significant amelioration of HFs, general sweating, day-and-night sweats, menopausal-specific sleeping problems, emotional and physical symptoms as well as skin and hair symptoms. The beneficial effects of acupuncture were seen 3 weeks into treatment and most of the participants tolerated the procedure well. However, this study[74] had relatively small sample size (n = 70; cases = 36; controls = 34), follow-up was of short duration (5 weeks) and placebo group was not well-defined. Future studies employing larger sample size will further help in delineation of the role of acupuncture in menopausal HFs.
CONCLUSION
HFs continues to be an important symptom in peri- and post-menopausal women. A careful approach to diagnosis and management using predefined algorithms often alleviate HFs and improve quality of life of women with HFs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.de Zambotti M, Colrain IM, Javitz HS, Baker FC. Magnitude of the impact of hot flashes on sleep in perimenopausal women. Fertil Steril. 2014;102:1708–150. doi: 10.1016/j.fertnstert.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: Evidence from the Penn Ovarian Aging Study cohort. Menopause. 2014;21:924–32. doi: 10.1097/GME.0000000000000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ACOG practice bulletin no. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202–16. doi: 10.1097/01.AOG.0000441353.20693.78. [DOI] [PubMed] [Google Scholar]
- 4.Reed SD, Lampe JW, Qu C, Copeland WK, Gundersen G, Fuller S, et al. Premenopausal vasomotor symptoms in an ethnically diverse population. Menopause. 2014;21:153–8. doi: 10.1097/GME.0b013e3182952228. [DOI] [PubMed] [Google Scholar]
- 5.Col NF, Guthrie JR, Politi M, Dennerstein L. Duration of vasomotor symptoms in middle-aged women: A longitudinal study. Menopause. 2009;16:453–7. doi: 10.1097/gme.0b013e31818d414e. [DOI] [PubMed] [Google Scholar]
- 6.Politi MC, Schleinitz MD, Col NF. Revisiting the duration of vasomotor symptoms of menopause: A meta-analysis. J Gen Intern Med. 2008;23:1507–13. doi: 10.1007/s11606-008-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahuja M. Age of menopause and determinants of menopause age: A PAN India survey by IMS. J Midlife Health. 2016;7:126–31. doi: 10.4103/0976-7800.191012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter MS, Gentry-Maharaj A, Ryan A, Burnell M, Lanceley A, Fraser L, et al. Prevalence, frequency and problem rating of hot flushes persist in older postmenopausal women: Impact of age, body mass index, hysterectomy, hormone therapy use, lifestyle and mood in a cross-sectional cohort study of 10,418 British women aged 54-65. BJOG. 2012;119:40–50. doi: 10.1111/j.1471-0528.2011.03166.x. [DOI] [PubMed] [Google Scholar]
- 9.Avis NE, Crawford SL, Greendale G, Bromberger JT, Everson-Rose SA, Gold EB, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531–9. doi: 10.1001/jamainternmed.2014.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeleke BM, Davis SR, Fradkin P, Bell RJ. Vasomotor symptoms and urogenital atrophy in older women: A systematic review. Climacteric. 2015;18:112–20. doi: 10.3109/13697137.2014.978754. [DOI] [PubMed] [Google Scholar]
- 11.Huang AJ, Grady D, Jacoby VL, Blackwell TL, Bauer DC, Sawaya GF. Persistent hot flushes in older postmenopausal women. Arch Intern Med. 2008;168:840–6. doi: 10.1001/archinte.168.8.840. [DOI] [PubMed] [Google Scholar]
- 12.Makara-Studzińśka MT, Kryś-Noszczyk KM, Jakiel G. Epidemiology of the symptoms of menopause – An intercontinental review. Prz Menopauzalny. 2014;13:203–11. doi: 10.5114/pm.2014.43827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tepper PG, Brooks MM, Randolph JF, Jr, Crawford SL, El Khoudary SR, Gold EB, et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause. 2016;23:1067–74. doi: 10.1097/GME.0000000000000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randolph JF, Jr, Sowers M, Bondarenko I, Gold EB, Greendale GA, Bromberger JT, et al. The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab. 2005;90:6106–12. doi: 10.1210/jc.2005-1374. [DOI] [PubMed] [Google Scholar]
- 15.Kronenberg F. Hot flashes: Epidemiology and physiology. Ann N Y Acad Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. [DOI] [PubMed] [Google Scholar]
- 16.Gold EB, Sternfeld B, Kelsey JL, Brown C, Mouton C, Reame N, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152:463–73. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 17.Whiteman MK, Staropoli CA, Langenberg PW, McCarter RJ, Kjerulff KH, Flaws JA. Smoking, body mass, and hot flashes in midlife women. Obstet Gynecol. 2003;101:264–72. doi: 10.1016/s0029-7844(02)02593-0. [DOI] [PubMed] [Google Scholar]
- 18.Crandall CJ, Manson JE, Hohensee C, Horvath S, Wactawski-Wende J, LeBlanc ES, et al. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the women's health initiative study. Menopause. 2017;24:252–61. doi: 10.1097/GME.0000000000000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman RR. Menopausal hot flashes: Mechanisms, endocrinology, treatment. J Steroid Biochem Mol Biol. 2014;142:115–20. doi: 10.1016/j.jsbmb.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sturdee DW, Reece BL. Thermography of menopausal hot flushes. Maturitas. 1979;1:201–5. doi: 10.1016/0378-5122(79)90009-4. [DOI] [PubMed] [Google Scholar]
- 21.Sturdee DW, Hunter MS, Maki PM, Gupta P, Sassarini J, Stevenson JC, et al. The menopausal hot flush: A review. Climacteric. 2017;20:296–305. doi: 10.1080/13697137.2017.1306507. [DOI] [PubMed] [Google Scholar]
- 22.McLaren HC. The induced menopause. J Obstet Gynaecol Br Empire. 1941;48:23–40. [Google Scholar]
- 23.Chakravarti S, Collins WP, Newton JR, Oram DH, Studd JW. Endocrine changes and symptomatology after oophorectomy in premenopausal women. Br J Obstet Gynaecol. 1977;84:769–75. doi: 10.1111/j.1471-0528.1977.tb12491.x. [DOI] [PubMed] [Google Scholar]
- 24.Freedman RR. Biochemical, metabolic, and vascular mechanisms in menopausal hot flashes. Fertil Steril. 1998;70:332–7. doi: 10.1016/s0015-0282(98)00137-x. [DOI] [PubMed] [Google Scholar]
- 25.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 26.Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166:680–97. doi: 10.1016/j.neuroscience.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinsey-Jones JS, Li XF, Knox AM, Lin YS, Milligan SR, Lightman SL, et al. Corticotrophin-releasing factor alters the timing of puberty in the female rat. J Neuroendocrinol. 2010;22:102–9. doi: 10.1111/j.1365-2826.2009.01940.x. [DOI] [PubMed] [Google Scholar]
- 28.Franco-Cereceda A, Gennari C, Nami R, Agnusdei D, Pernow J, Lundberg JM, et al. Cardiovascular effects of calcitonin gene-related peptides I and II in man. Circ Res. 1987;60:393–7. doi: 10.1161/01.res.60.3.393. [DOI] [PubMed] [Google Scholar]
- 29.Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update. 2014;20:485–500. doi: 10.1093/humupd/dmu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noguchi K, Senba E, Morita Y, Sato M, Tohyama M. Alpha-CGRP and beta-CGRP mRNAs are differentially regulated in the rat spinal cord and dorsal root ganglion. Brain Res Mol Brain Res. 1990;7:299–304. doi: 10.1016/0169-328x(90)90080-w. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi T, Ushijima O, Chen JT, Shiraki M, Ohta T, Kiyoki M, et al. Basal tail skin temperature elevation and augmented response to calcitonin gene-related peptide in ovariectomized rats. J Endocrinol. 1995;146:431–7. doi: 10.1677/joe.0.1460431. [DOI] [PubMed] [Google Scholar]
- 32.Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–6. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- 33.Goodman EC, Iversen LL. Calcitonin gene-related peptide: Novel neuropeptide. Life Sci. 1986;38:2169–78. doi: 10.1016/0024-3205(86)90568-0. [DOI] [PubMed] [Google Scholar]
- 34.Landis SC, Fredieu JR. Coexistence of calcitonin gene-related peptide and vasoactive intestinal peptide in cholinergic sympathetic innervation of rat sweat glands. Brain Res. 1986;377:177–81. doi: 10.1016/0006-8993(86)91205-9. [DOI] [PubMed] [Google Scholar]
- 35.Barnabei VM, Cochrane BB, Aragaki AK, Nygaard I, Williams RS, McGovern PG, et al. Menopausal symptoms and treatment-related effects of estrogen and progestin in the women's health initiative. Obstet Gynecol. 2005;105:1063–73. doi: 10.1097/01.AOG.0000158120.47542.18. [DOI] [PubMed] [Google Scholar]
- 36.Freedman RR. Physiology of hot flashes. Am J Hum Biol. 2001;13:453–64. doi: 10.1002/ajhb.1077. [DOI] [PubMed] [Google Scholar]
- 37.Barton DL, Loprinzi CL, Quella SK, Sloan JA, Veeder MH, Egner JR, et al. Prospective evaluation of Vitamin E for hot flashes in breast cancer survivors. J Clin Oncol. 1998;16:495–500. doi: 10.1200/JCO.1998.16.2.495. [DOI] [PubMed] [Google Scholar]
- 38.Ziaei S, Kazemnejad A, Zareai M. The effect of Vitamin E on hot flashes in menopausal women. Gynecol Obstet Invest. 2007;64:204–7. doi: 10.1159/000106491. [DOI] [PubMed] [Google Scholar]
- 39.The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of the North American menopause society. Menopause. 2017;24:728–53. doi: 10.1097/GME.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 40.Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, et al. Treatment of symptoms of the menopause: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975–4011. doi: 10.1210/jc.2015-2236. [DOI] [PubMed] [Google Scholar]
- 41.North American Menopause Society. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause. 2012;19:257–71. doi: 10.1097/gme.0b013e31824b970a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, et al. Postmenopausal hormone therapy: An endocrine society scientific statement. J Clin Endocrinol Metab. 2010;95:s1–66. doi: 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shifren JL, Schiff I. Role of hormone therapy in the management of menopause. Obstet Gynecol. 2010;115:839–55. doi: 10.1097/AOG.0b013e3181d41191. [DOI] [PubMed] [Google Scholar]
- 44.Maclennan AH, Broadbent JL, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev. 2004;4:CD002978. doi: 10.1002/14651858.CD002978.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mattson RH, Cramer JA, Darney PD, Naftolin F. Use of oral contraceptives by women with epilepsy. JAMA. 1986;256:238–40. [PubMed] [Google Scholar]
- 46.Ginsburg ES, Mello NK, Mendelson JH, Barbieri RL, Teoh SK, Rothman M, et al. Effects of alcohol ingestion on estrogens in postmenopausal women. JAMA. 1996;276:1747–51. doi: 10.1001/jama.1996.03540210055034. [DOI] [PubMed] [Google Scholar]
- 47.Lobo RA, Pinkerton JV, Gass ML, Dorin MH, Ronkin S, Pickar JH, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril. 2009;92:1025–38. doi: 10.1016/j.fertnstert.2009.03.113. [DOI] [PubMed] [Google Scholar]
- 48.Berman RS, Epstein RS, Lydick E. Risk factors associated with women's compliance with estrogen replacement therapy. J Womens Health. 1997;6:219–26. doi: 10.1089/jwh.1997.6.219. [DOI] [PubMed] [Google Scholar]
- 49.Grady D, Sawaya GF. Discontinuation of postmenopausal hormone therapy. Am J Med. 2005;118(Suppl 12B):163–5. doi: 10.1016/j.amjmed.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 50.North American Menopause Society. The North American Menopause Society statement on continuing use of systemic hormone therapy after age 65. Menopause. 2015;22:693. doi: 10.1097/GME.0000000000000492. [DOI] [PubMed] [Google Scholar]
- 51.Haimov-Kochman R, Barak-Glantz E, Arbel R, Leefsma M, Brzezinski A, Milwidsky A, et al. Gradual discontinuation of hormone therapy does not prevent the reappearance of climacteric symptoms: A randomized prospective study. Menopause. 2006;13:370–6. doi: 10.1097/01.gme.0000186663.36211.c0. [DOI] [PubMed] [Google Scholar]
- 52.Aslan E, Bagis T, Kilicdag EB, Tarim E, Erkanli S, Kuscu E. How best is to discontinue postmenopausal hormone therapy: Immediate or tapered? Maturitas. 2007;56:78–83. doi: 10.1016/j.maturitas.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 53.Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262–70. doi: 10.1056/NEJMcp0708481. [DOI] [PubMed] [Google Scholar]
- 54.Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of the North American Menopause Society. Menopause. 2015;22:1155–72. doi: 10.1097/GME.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 55.Loprinzi CL, Sloan J, Stearns V, Slack R, Iyengar M, Diekmann B, et al. Newer antidepressants and gabapentin for hot flashes: An individual patient pooled analysis. J Clin Oncol. 2009;27:2831–7. doi: 10.1200/JCO.2008.19.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson HD, Vesco KK, Haney E, Fu R, Nedrow A, Miller J, et al. Nonhormonal therapies for menopausal hot flashes: Systematic review and meta-analysis. JAMA. 2006;295:2057–71. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 57.Simon JA, Portman DJ, Kaunitz AM, Mekonnen H, Kazempour K, Bhaskar S, et al. Low-dose paroxetine 7.5 mg for menopausal vasomotor symptoms: Two randomized controlled trials. Menopause. 2013;20:1027–35. doi: 10.1097/GME.0b013e3182a66aa7. [DOI] [PubMed] [Google Scholar]
- 58.Suvanto-Luukkonen E, Koivunen R, Sundström H, Bloigu R, Karjalainen E, Häivä-Mällinen L, et al. Citalopram and fluoxetine in the treatment of postmenopausal symptoms: A prospective, randomized, 9-month, placebo-controlled, double-blind study. Menopause. 2005;12:18–26. doi: 10.1097/00042192-200512010-00006. [DOI] [PubMed] [Google Scholar]
- 59.Loprinzi CL, Barton DL, Sloan JA, Novotny PJ, Dakhil SR, Verdirame JD, et al. Mayo clinic and north central cancer treatment group hot flash studies: A 20-year experience. Menopause. 2008;15:655–60. doi: 10.1097/gme.0b013e3181679150. [DOI] [PubMed] [Google Scholar]
- 60.Bordeleau L, Pritchard KI, Loprinzi CL, Ennis M, Jugovic O, Warr D, et al. Multicenter, randomized, cross-over clinical trial of venlafaxine versus gabapentin for the management of hot flashes in breast cancer survivors. J Clin Oncol. 2010;28:5147–52. doi: 10.1200/JCO.2010.29.9230. [DOI] [PubMed] [Google Scholar]
- 61.Casper RF, Alapin-Rubillovitz S. Progestins increase endogenous opioid peptide activity in postmenopausal women. J Clin Endocrinol Metab. 1985;60:34–6. doi: 10.1210/jcem-60-1-34. [DOI] [PubMed] [Google Scholar]
- 62.Schiff I. The effects of progestins on vasomotor flushes. J Reprod Med. 1982;27:498–502. [PubMed] [Google Scholar]
- 63.Jin Y, Hayes DF, Li L, Robarge JD, Skaar TC, Philips S, et al. Estrogen receptor genotypes influence hot flash prevalence and composite score before and after tamoxifen therapy. J Clin Oncol. 2008;26:5849–54. doi: 10.1200/JCO.2008.16.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jones SE, Cantrell J, Vukelja S, Pippen J, O'Shaughnessy J, Blum JL, et al. Comparison of menopausal symptoms during the first year of adjuvant therapy with either exemestane or tamoxifen in early breast cancer: Report of a tamoxifen exemestane adjuvant multicenter trial substudy. J Clin Oncol. 2007;25:4765–71. doi: 10.1200/JCO.2007.10.8274. [DOI] [PubMed] [Google Scholar]
- 65.Santen RJ, Stuenkel CA, Davis SR, Pinkerton JV, Gompel A, Lumsden MA, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102:3647–61. doi: 10.1210/jc.2017-01138. [DOI] [PubMed] [Google Scholar]
- 66.Prague JK, Roberts RE, Comninos AN, Clarke S, Jayasena CN, Nash Z, et al. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: A phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:1809–20. doi: 10.1016/S0140-6736(17)30823-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walega DR, Rubin LH, Banuvar S, Shulman LP, Maki PM. Effects of stellate ganglion block on vasomotor symptoms: Findings from a randomized controlled clinical trial in postmenopausal women. Menopause. 2014;21:807–14. doi: 10.1097/GME.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Othman AH, Zaky AH. Management of hot flushes in breast cancer survivors: Comparison between stellate ganglion block and pregabalin. Pain Med. 2014;15:410–7. doi: 10.1111/pme.12331. [DOI] [PubMed] [Google Scholar]
- 69.Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: A review of randomized, controlled trials. Ann Intern Med. 2002;137:805–13. doi: 10.7326/0003-4819-137-10-200211190-00009. [DOI] [PubMed] [Google Scholar]
- 70.Nedrow A, Miller J, Walker M, Nygren P, Huffman LH, Nelson HD. Complementary and alternative therapies for the management of menopause-related symptoms: A systematic evidence review. Arch Intern Med. 2006;166:1453–65. doi: 10.1001/archinte.166.14.1453. [DOI] [PubMed] [Google Scholar]
- 71.Lethaby A, Marjoribanks J, Kronenberg F, Roberts H, Eden J, Brown J. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev. 2013;12:CD001395. doi: 10.1002/14651858.CD001395.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dodin S, Blanchet C, Marc I, Ernst E, Wu T, Vaillancourt C, et al. Acupuncture for menopausal hot flushes. Cochrane Database Syst Rev. 2013;7:CD007410. doi: 10.1002/14651858.CD007410.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith CA, Carmady B. Acupuncture to treat common reproductive health complaints: An overview of the evidence. Auton Neurosci. 2010;157:52–6. doi: 10.1016/j.autneu.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 74.Lund KS, Siersma V, Brodersen J, Waldorff FB. Efficacy of a standardised acupuncture approach for women with bothersome menopausal symptoms: A pragmatic randomised study in primary care (the ACOM study) BMJ Open. 2019;9:e023637. doi: 10.1136/bmjopen-2018-023637. [DOI] [PMC free article] [PubMed] [Google Scholar]


