This secondary analysis of the Exenatide-PD trial assesses whether patients with Parkinson disease have augmented activity in brain insulin and protein kinase B signaling pathways.
Key Points
Question
How might neuronal-derived exosomes be used to explore the molecular mechanisms by which an experimental intervention exerts clinical effects on motor function?
Findings
In this seconday analysis of a randomized clinical trial, serum samples from 60 participants in the Exenatide-PD trial were used to isolate neuronal-derived exosomes to evaluate levels of the brain insulin-signaling proteins and downstream effectors protein kinase B (Akt) and mechanistic target of rapamycin. After 48 and 60 weeks of subcutaneous drug administration, patients with Parkinson disease treated with exenatide had greater activation of brain insulin signaling proteins and downstream effectors compared with baseline and patients in the placebo group.
Meaning
These results are suggestive of target engagement of brain insulin, protein kinase B, and mechanistic target of rapamycin signaling pathways by exenatide and provide a mechanistic context for the clinical findings of the trial; these techniques could have widespread application across a large number of trials in central nervous system diseases.
Abstract
Importance
Exenatide, a glucagon-like peptide 1 agonist used in type 2 diabetes, was recently found to have beneficial effects on motor function in a randomized, placebo-controlled trial in Parkinson disease (PD). Accumulating evidence suggests that impaired brain insulin and protein kinase B (Akt) signaling play a role in PD pathogenesis; however, exploring the extent to which drugs engage with putative mechnisms in vivo remains a challenge.
Objective
To assess whether participants in the Exenatide-PD trial have augmented activity in brain insulin and Akt signaling pathways.
Design, Setting, and Participants
Serum samples were collected from 60 participants in the single-center Exenatide-PD trial (June 18, 2014, to June 16, 2016), which compared patients with moderate PD randomized to 2 mg of exenatide once weekly or placebo for 48 weeks followed by a 12-week washout period. Serum extracellular vesicles, including exosomes, were extracted, precipitated, and enriched for neuronal source by anti–L1 cell adhesion molecule antibody absorption, and proteins of interest were evaluated using electrochemiluminescence assays. Statistical analysis was performed from May 1, 2017, to August 31, 2017.
Main Outcomes and Measures
The main outcome was augmented brain insulin signaling that manifested as a change in tyrosine phosphorylated insulin receptor substrate 1 within neuronal extracellular vesicles at the end of 48 weeks of exenatide treatment. Additional outcome measures were changes in other insulin receptor substrate proteins and effects on protein expression in the Akt and mitogen-activated protein kinase pathways.
Results
Sixty patients (mean [SD] age, 59.9 [8.4] years; 43 [72%] male) participated in the study: 31 in the exenatide group and 29 in the placebo group (data from 1 patient in the exenatide group were excluded). Patients treated with exenatide had augmented tyrosine phosphorylation of insulin receptor substrate 1 at 48 weeks (0.27 absorbance units [AU]; 95% CI, 0.09-0.44 AU; P = .003) and 60 weeks (0.23 AU; 95% CI, 0.05-0.41 AU; P = .01) compared with patients receiving placebo. Exenatide-treated patients had elevated expression of downstream substrates, including total Akt (0.35 U/mL; 95% CI, 0.16-0.53 U/mL; P < .001) and phosphorylated mechanistic target of rapamycin (mTOR) (0.22 AU; 95% CI, 0.04-0.40 AU; P = .02). Improvements in Movement Disorders Society Unified Parkinson’s Disease Rating Scale part 3 off-medication scores were associated with levels of total mTOR (F4,50 = 5.343, P = .001) and phosphorylated mTOR (F4,50 = 4.384, P = .04).
Conclusions and Relevance
The results of this study are consistent with target engagement of brain insulin, Akt, and mTOR signaling pathways by exenatide and provide a mechanistic context for the clinical findings of the Exenatide-PD trial. This study suggests the potential of using exosome-based biomarkers as objective measures of target engagement in clinical trials using drugs that target neuronal pathways.
Introduction
Previous work1 has explored the use of extracellular vesicles (EVs) harvested from peripheral blood and enriched for neuronal origin to measure neuropathological changes in vivo over time. Extracellular vesicles (including exosomes) are nanosized membranous particles secreted by virtually all cells, including neurons,2 that circulate in blood and contain variable cellular cargo representative of their origin, which can be significantly altered depending on the physiologic state of the parent cell.3 Extracellular vesicles can cross the blood-brain barrier; thus, EVs of neuronal origin can be selectively isolated by targeting neuronal antigens, such as the neuronal cell adhesion molecule and the L1 cell adhesion molecule (L1CAM), embedded in the vesicle membrane. Several studies4,5,6 have used neuronal-derived EVs isolated by L1CAM immunocapture to quantify levels of pathogenic proteins contained within them and have found that they can successfully distinguish between disease states and healthy controls in Alzheimer disease and Parkinson disease (PD). The potential utility of this technique in revealing target engagement and mechanism of action of central nervous sysrem drugs in clinical trials is increasingly being recognized.
A variety of novel targets for neuroprotection have been identified and are actively being pursued in clinical trials for neurodegenerative diseases. Among these targets, the identification of metabolic dysfunction in PD is of major interest7,8; evidence from epidemiologic studies and animal-toxin models of PD suggest that impaired insulin signaling may play a role in the pathogenesis.9,10,11,12,13,14,15,16,17,18,19,20 In the brain, insulin modulates neuronal cell survival via 2 downstream pathways: the phosphoinositide 3-kinase–protein kinase B (Akt) and mitogen-activated protein kinase (MAPK) pathways (Figure 1). Diminished insulin signaling reduces the activity of Akt, modulating the activity of numerous kinases, including mechanistic target of rapamycin (mTOR), glycogen synthase kinase 3β (GSK-3β), and forkhead box protein O1, which regulate processes involved in PD pathogenesis,21 such as α-synuclein degradation,22,23 mitochondrial biogenesis, and modulation of inflammatory and oxidative stress pathways.24
Figure 1. Proposed Scheme for the Neuroprotective Effects of Glucagon-Like Peptide 1 (GLP-1) in Neurons.
The cross-talk with insulin receptor signaling pathways and shared downstream effectors is shown. The formation and source of extracellular vesicles can be used as a source of biomarkers, showing the initial inward budding of the plasma membrane. This membrane fuses to form an early endosome, which then accumulates cytoplasmic molecules. This results in the formation of multivesicular bodies before fusing with the plasma membrane, releasing their contents into the extracellular environment. Akt indicates protein kinase B; Bcl-2, B-cell lymphoma 2; BAD, Bcl-2 antagonist of death; Bcl-XL, B-cell lymphoma 2 extralarge; Bim, Bcl-2-like protein 11; cAMP, cyclic adenosine monophosphate; CREB, cAMP response element-binding protein; Erk1/2, extracelluar signal-related kinase; FoxO1/O3, forkhead box O1/O3; GRB2, growth factor receptor-bound protein 2; GSK-3β, glycogen synthase 3β; IDE, insulin-degrading enzyme; IL-1α, interleukin 1α; IRS-1, insulin receptor signaling substrate 1; MAPK, mitogen-associated protein kinase; mTOR, mechanistic target of rapamycin; mTORC1, mTOR complex 1; mTORC2, mTOR complex 2; NF-kB, nuclear factor–κB; PI3-K, phosphoinositide 3-kinase; PKA, protein kinase A; S6k, serine kinase β-1; TNF-α, tumor necrosis factor α; Tyr, tyrosine residue. Figure provided by Dr Athauda.
Insulin signaling relies on the stability of insulin receptor substrate 1 (IRS-1), which acts as the first node in the cascade, and its activity is regulated through a number of serine and tyrosine phosphorylation sites.25 Although tyrosine IRS-1 phosphorylations are needed for insulin-evoked responses, serine phosphorylations primarily deactivate IRS-1 and attenuate insulin signaling.26,27,28,29 Prior studies in postmortem tissue from patients with PD,30,31 Alzheimer disease,27,28,29 and multiple system atrophy15 have identified elevated IRS-1 phosphorylation at serine positions 616 (IRS-1 p-S616) and 312 (IRS-1 p-S312) as being associated with attenuated insulin signaling, supporting their use as biomarkers of neuronal insulin resistance. Of importance, the reversal and restoration of insulin signaling by exogenous insulin or insulin-sensitizing agents led to improved cell survival and functional improvements.32,33,34
Although alternative markers of insulin resistance are available through neuroimaging and cerebrospinal fluid studies, measuring brain insulin-signaling markers in peripheral blood represents a rational, easily accessible, and practical method for assessing time-dependent changes. Previous studies used plasma neuronal-derived EVs to demonstrate decreased tyrosine phosphorylated IRS-1 (IRS-1 p-Tyr) and increased levels of IRS-1 phosphorylated at serine 312 (IRS-1 p-S312)35 in patients with Alzheimer disease, closely mimicking the pattern observed in autopsy,36 and have found that therapeutic interventions that target insulin signaling can significantly alter these IRS-1 phosphorylations.37 Taken together, these findings suggest that IRS-1 and downstream signaling mediators in neuronal-derived EVs could be used as biomarkers of brain insulin resistance in neurodegenerative diseases.
Glucagon-like peptide 1 (GLP-1) agonists are used for type 2 diabetes treatment and activate similar pathways to insulin to improve glucose homeostasis.38 The GLP-1 signaling pathways also indirectly promote and restore neuronal insulin signaling,39,40 reducing serine IRS-1 phosphorylation and monomeric α-synuclein load, preserving dopaminergic neurons, and attenuating cell death in rodent models of multiple systems atrophy and Alzheimer disease.32,41 A proposed mechanism of action of GLP-1 agonists in neurons is also shown in Figure 1.
Exenatide, the first synthetic GLP-1 agonist, was recently studied for potential disease-modifying effects in a randomized, placebo-controlled clinical trial in patients with moderate PD, finding positive effects on motor severity (measured after overnight dopaminergic medication withdrawal) that were sustained 12 weeks beyond the period of exenatide exposure.42 Given its positive clinical effects in the trial and preclinical data suggesting modulation of insulin signaling as its main mechanism of action, our a priori hypothesis was that exenatide-treated compared with placebo-treated participants would show changes in IRS-1 p-Tyr signaling proteins in neuronal-enriched EVs, suggesting activation of brain insulin signaling pathways. Our analysis used patient serum samples to identify changes in insulin signaling biomarkers in neuronal-enriched EVs during multiple time points.
Methods
Patients and Study Design
The Exenatide-PD trial (a randomized, double-blind, placebo-controlled, single center, 60-week trial of exenatide once weekly for the treatment of moderate-severity PD43) (NCT01971242) was performed from June 18, 2014, to June 16, 2016, to assess the effects of exenatide on disease progression for 60 weeks.42 The trial enrolled 60 men and women between 25 and 75 years of age with idiopathic PD44 who were receiving dopaminergic treatment. Patients were randomized to self-inject either 2 mg of exenatide (n = 31) or placebo (n = 29) once weekly for 48 weeks, followed by drug withdrawal and a final visit 12 weeks later. At each visit, patients were assessed using the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) and provided blood samples after an overnight withdrawal from PD medication. The study was coordinated by the University College London Comprehensive Clinical Trials Unit, London, United Kingdom. Patients consented to future analysis of all samples collected during the trial as part of the original trial consent process. This trial was approved by the Brent National Health Service Research Ethics Committee, London, United Kingdom. All patients provided written informed consent, and all data were deidentified.
Outcomes
On the basis of previous literature,35,36,39,41 our a priori hypothesis was that exenatide treatment would activate insulin signaling pathways detectable as a change in IRS-1 p-Tyr at the end of 48 weeks of treatment with exenatide. Additional exploratory outcomes were (1) differences between exenatide and placebo in other related IRS-1 signaling proteins and (2) downstream effectors of the Akt and MAPK pathways—the 2 pathways primarily involved in GLP-1 and insulin signaling. We further hypothesized that these changes would be associated with the positive motor effects seen in the clinical trial. Because of the limited amount of serum samples available, we were able to assess only a limited candidate group of biomarkers and selected Akt, extracellular signal-related kinase (Erk), total p38 (t-p38) MAPK, phospho p38 MAPK, c-Jun N-terminal kinase (JNK), GSK-3β, and mTOR.
Serum Sample Collection
Whole blood samples were collected in accordance with preprocessing guidelines for EV-based biomarker analysis.45,46 Samples from baseline, week 24, week 48, and week 60 were analyzed.
Isolation of EVs and Enrichment for Neuronal Origin
Investigators at the National Institute on Aging who performed EV isolation and protein quantifications were masked to exenatide and placebo treatment allocation. A detailed description of the methods and evidence for neuronal enrichment has been previously published.47 A 2-step method of particle precipitation to increase EV concentration was followed by immune capture for neuronal surface antigen L1CAM to selectively isolate extracellular vesicles enriched for neuronal origin.1,48
Quantification of EV Insulin Signaling Proteins
Extracellular vesicles in suspension were lysed with the addition of 260 μL of Mammalian Protein Extraction Reagent (M-PER; Thermo Scientific). Proteins in the lysate were quantified by electrochemiluminescence using the Mesoscale Discovery platform and kits, including IRS-1 p-Tyr (catalog No. N45CA-1), IRS-1 p-S616 and IRS-1 p-S312 (catalog No. K150HLD-2), and total (t-) and phosphorylated (p-) forms of Akt (catalog No. K15177D-2), mTOR (catalog No. K15170D-2), GSK-3β (catalog No. K15109D-2), p38 MAPK, Erk1/2, and JNK (catalog No. K15157D-2). The IRS-1 p-Tyr, IRS-1 p-S312, and IRS-1 p-S616 assays had the same capture but different detection antibodies (for p-S616 monoclonal antibody cell signaling 2386s was used).
All assays were conducted in duplicate, and the mean coefficients of variance were less than 10%. In all total protein assays, recombinant protein supplied by the manufacturer was used to calculate a standard curve and convert the electrochemiluminescence signal into concentrations. For phosphoproteins, the electrochemiluminescence signal was used for the analysis. All electrochemiluminescence values for total proteins were above the lowest limit of quantification and within the linear range of the curve. All samples from repeated visits of a given patient were included on the same plate to avoid within-subject variability caused by plate to plate variability. Plate to plate variability was assessed using an internal standard (EVs from a control patient; between-plate coefficients of variance were <10%).
Statistical Analysis
Analyses were performed using SPSS statistical software, version 21.0 (IBM Corp). Biomarker values were natural log transformed to avoid skewness. To assess the effect of exenatide on a given biomarker, a linear mixed-effects model was used, with treatment groups (exenatide vs placebo), time, baseline biomarker, and EV concentration as fixed effects and participant identification treated as a random effect. The inclusion of EV concentration (determined by NanoSight) as a covariate enabled normalization for differential EV yield in different samples, as done previously.37
To assess whether changes in biomarker levels were associated with the effect of treatment on disease progression, multiple linear regression of change in MDS-UPDRS Part 3 off-medication scores was fitted with change in biomarker levels, treatment group, EV concentration, and the interaction of biomarker change and treatment group as independent variables. Changes in MDS-UPDRS part 3 scores were defined as differences from baseline to 48 or 60 weeks. P < .05 was considered to be statistically significant. Statistical analysis was performed from May 1, 2017, to August 31, 2017.
Results
Patient Characteristics
Sixty patients (mean [SD] age, 59.9 [8.4] years; 43 [72%] male) participated in the study: 31 in the exenatide group and 29 in the placebo group. Data from 1 patient were excluded from the analysis because of extreme outlying values despite log transformation (eFigure 1 in the Supplement). Patient demographics and baseline characteristics were generally similar between the 2 groups (eTable 1 in the Supplement), although exenatide-treated participants were slightly older, had higher baseline MDS-UPDRS Part 3 scores, and had slightly lower levodopa equivalent dose than placebo-assigned participants. Comparison of biomarkers (log transformed) at baseline were similar between the 2 groups (Figure 2).
Figure 2. Baseline Biomarker Profile in the Exenatide and Placebo Groups.
Bars represent mean adjusted for differences in extracellular vesicle concentration; Error bars represent SE. Akt indicates protein kinase B; AU, absorbance units at 450 nm; ErK, extracellular signal-related kinase; GSK-3β, glycogen synthase 3β; IRS-1, insulin receptor substrate 1; JNK, c-Jun N-terminal kinase; MAPK, mitogen-associated protein kinase; mTOR, mechanistic target of rapamycin; p-, phosphorylated; t-, total.
Association of Exenatide With Biomarker Changes
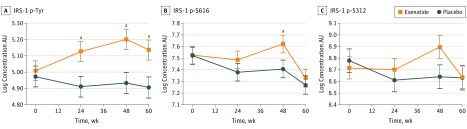
Exenatide-treated patients had an early and sustained increase in IRS-1 p-Tyr compared with placebo-assigned participants, resulting in a significant adjusted between-group difference at 24 weeks (0.22 absorbance unit [AU]; 95% CI, 0.04-0.39; P = .02), 48 weeks (0.27 AU; 95% CI, 0.09-0.44; P = .003), and 60 weeks (0.23 AU; 95% CI, 0.05-0.41; P = .01) (Figure 3A).
Figure 3. Association of Exenatide With Phosphorylation of Insulin Receptor Signaling Substrate 1 (IRS-1) Proteins .
All values are means (SEMs [error bars]) adjusted for differences in extracellular vesicle concentration and baseline biomarker values. AU, absorbance units; IRS-1 p-S312, IRS-1 phosphorylated at serine residue 312; IRS-1 p-S616, IRS-1 phosphorylated at serine residue 616; and IRS-1 p-Tyr, IRS-1 phosphorylated at tyrosine residues.
aP < .05.
By 48 weeks, there was also an (unexpected) increase in IRS-1 p-S616 of 0.096 AU (95% CI, −0.16 to 0.36) in the exenatide group compared with a decrease in the placebo group of −0.12 AU (95% CI, −0.37 to 0.15), resulting in a significant adjusted between-group difference of 0.22 AU (95% CI, 0.03-0.43; P = .047) (Figure 3B). A similar increase in IRS-1 p-S312 in the exenatide group at 48 weeks was observed, although the adjusted difference between the 2 groups (0.26 AU; 95% CI, −0.03 to 0.54; P = .07) did not reach significance (Figure 3C). These differences disappeared at 60 weeks.
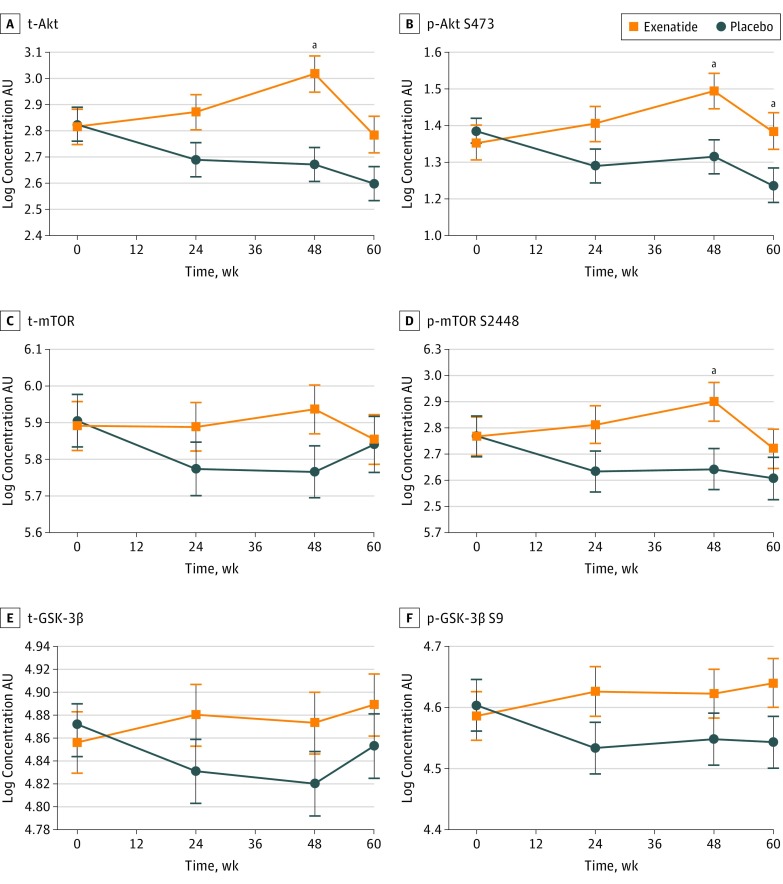
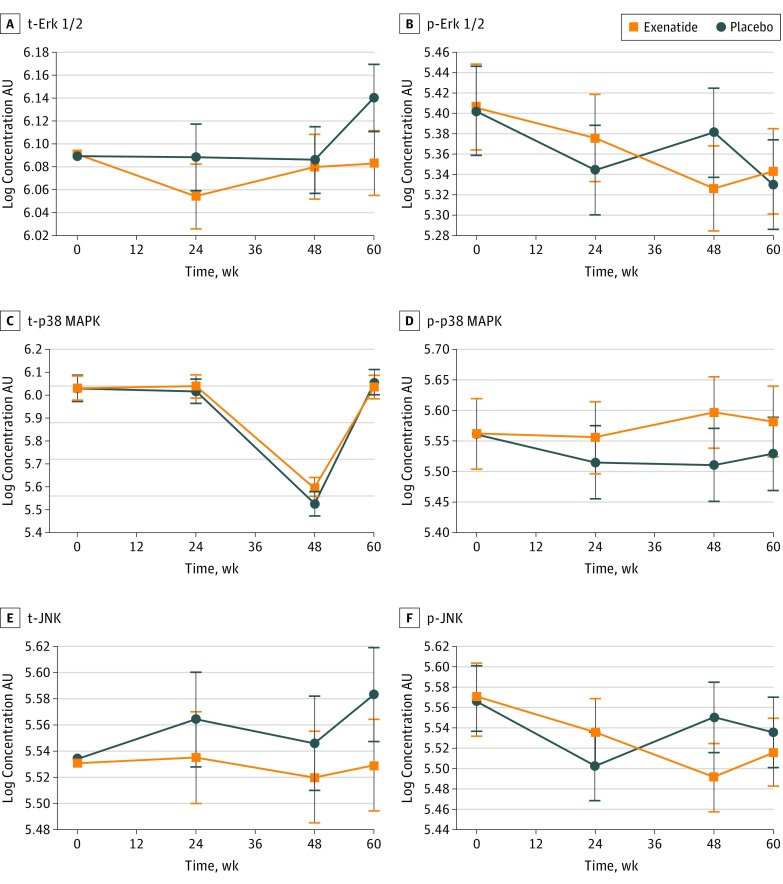
We observed significant increases in t-Akt, p-Akt S473, and p-mTOR S2448 at 48 weeks in the exenatide group compared with the placebo group, resulting in adjusted between-group differences of 0.35 U/mL (95% CI, 0.16-0.53 U/mL; P < .001) for t-Akt, 0.18 U/mL (95% CI, 0.04-0.31 U/mL; P = .008) for p-Akt S473, and 0.22 AU (95% CI, 0.04-0.40 AU; P = .02) for p-mTOR (Figure 4A, B, and D); p-Akt S473 was still significantly elevated in the exenatide group at 60 weeks (ie, 12 weeks after drug cessation; 0.15 AU; 95% CI, 0.01-0.28 AU; P = .03). No significant increases in t-mTOR (0.17 AU; 95% CI, −0.02 to 0.36; P = .09), t-GSK-3β (0.05 AU; 95% CI, −0.03 to 0.13 AU; P = .18), and p-GSK-3β S9 (0.08 AU; 95% CI, −0.04 to 0.19 AU; P = .20) were found at 48 weeks (Figure 4C, E, and F). There were no significant changes in t- and p-p38 MAPK, Erk1/2, and JNK between the 2 groups at any time points (Figure 5).
Figure 4. Association of Exenatide With Downstream Targets of Insulin Receptor Signaling Substrate 1 (IRS-1) .
All values are adjusted means (SEMs [error bars]). p-Akt S473 indicates phosphorylated AKT S473; p-GSK-3β S9, phosphorylated glycogen synthase 3β S9; p-mTOR S2448, phosphorylated mechanistic target of rapamycin S2448; t-Akt, total Akt; t-GSK-3β, total glycogen synthase 3β; and t-mTOR, total mechanistic target of rapamycin.
aP < .05.
Figure 5. Association of Exenatide With the MAPK/ERK Pathway and Downstream Effectors .
All values are adjusted means (SEMs [error bars]). p-Erk indicates phosphorylated extracellular signal-related kinase; p-JNK, phosphorylated c-Jun N-terminal kinase; p-p38 MAPK, phosphorylated p38 mitogen-activated protein kinase; t-Erk, total extracellular signal-related kinase; t-JNK, total c-Jun N-terminal kinase; and t-p38 MAPK, total p38 mitogen-activated protein kinase.
Association of Biomarkers With Clinical Scores
Consistent with the hypothesis that motor advantages seen with exenatide may relate (at least in part) to activation of the insulin, Akt, and mTOR cascades, we found that at 48 weeks changes in the levels of certain EV biomarkers significantly determined change in MDS-UPDRS Part 3 scores. These biomarkers were IRS-1 p-S616 (F4,46 = 7.181, P < .001), t-mTOR (F4,50 = 5.343, P = .001), and p-mTOR S2448 (F4,50 = 4.384, P = .04). Changes in biomarker levels and change in MDS-UPDRS Part 3 scores at 48 and 60 weeks are presented in the eTable 2 in the Supplement.
There were also corresponding significant interaction terms for group × change in biomarker for IRS-1 p-S616 (β = −11.15; 95% CI, −19.43 to −2.86; P = .009), t-mTOR (β = −9.22; 95% CI, −16.23 to −2.20; P = .01), and p-mTOR S2448 (β = −7.83; 95% CI, −15.62 to −0.04; P = .049). This finding indicates that at 48 weeks exenatide-related improvements in MDS-UPDRS Part 3 scores in the exenatide-treated group were significantly associated with changes in these biomarkers (eFigures 2 and 3 in the Supplement). There was no significant association between MDS-UPDRS part 3 scores and the levels of the other biomarkers tested.
In the clinical trial, clinical advantages in motor scores persisted at 60 weeks (ie, 12 weeks after drug cessation). At 60 weeks, the regression models assessing the associations between changes in EV biomarkers and change in MDS-UPDRS Part 3 scores were statistically significant for t-mTOR (F4,47 = 4.924, P = .002), with a corresponding significant interaction term for t-mTOR (β = −10.05; 95% CI, −17.95 to −2.16; P = .01). Although this finding was nonsignificant, changes in p-Akt S473 were potentially associated with changes in motor scores (F4,50 = 2.191, P = .08; interaction term β = −10.46; P = .047) (eFigures 4 and 5 in the Supplement). There was no significant association between MDS-UPDRS Part 3 scores and t-Akt, t- and p-GSK-3β, p38 MAPK, Erk 1/2, or JNK and no significant group × change in biomarker interactions.
Discussion
The current study demonstrates the potential use of EVs harvested from peripheral blood samples and enriched for neuronal origin as a source of biomarkers to gauge molecular responses to therapeutic interventions in clinical trials for neurologic disorders.1,37 Our results suggest that exenatide treatment may be associated with augmented brain insulin signaling pathways, as evidenced by tyrosine phosphorylation of IRS-1 and activated downstream Akt and mTOR signaling. Furthermore, in view of the significant interaction effects, we also found that the beneficial motor advantages seen at 48 and 60 weeks in the exenatide group may be (at least partially) explicable by concomitant activation of mTOR signaling. Although there are some inconsistencies in the association between the clinical improvements and some of the upstream biomarker changes, these findings provide further support to our a priori hypothesis relating to one of the potential mechanisms through which treatment with exenatide may confer clinical benefits in PD. They also provide further support for the association between insulin resistance and PD pathogenesis.
Although GLP-1 receptor stimulation can directly activate Akt,38 our findings that the observed exenatide-associated changes in IRS-1 were accompanied by changes in Akt and mTOR suggest that modulation of insulin signaling at multiple levels may better account for the observed effects. Although exenatide was associated with increased IRS-1 p-Tyr in neurons as we hypothesized, we also found that exenatide was associated with increased IRS-1 p-S616 and IRS-1 p-S312, particularly between 24 and 48 weeks, possibly because of negative feedback via sustained mTORC1 activation (see eResults in the Supplement for detailed discussion).
We found the changes in IRS-1 p-Tyr were also associated with increased t-Akt and p-Akt S473, and an association was observed between persistent motor benefits at 60 weeks and elevation of p-Akt S473. Our results are consistent with previous suggestions that pharmacologic upregulation of the Akt pathway may underlie the neuroprotective effects of many putative disease-modifying strategies49,50 and mediates exenatide-induced effects on cellular proliferation and differentiation,51 neurotrophism,52 and inhibition of inflammation53 and apoptosis.54,55 As a master regulator of cellular function, Akt signaling maintains a critical balance between proapoptotic and antiapoptotic pathways and has been identified as a major contributor to neurodegeneration in PD,24,56 influencing α-synuclein aggregation.57 A previous study58 found that activated forms of Akt are greatly reduced in substantia nigra dopaminergic neurons from patients with PD. Thus, restoration of normal functioning of the Akt pathway is one plausible mechanism to explain the clinical effects of exenatide.
Our findings are also in keeping with previous studies59,60,61,62,63,64,65 that support a neuroprotective role for mTOR in PD; mTOR (composed of 2 complexes: mTORC1, primarily phosphorylated on S2448, and mTORC2, phosphorylated predominantly on S248166) is a downstream target of Akt, and our results demonstrated that exenatide-treated patients had increased t-mTOR and p-mTOR S2448, whereas changes in t-mTOR were associated with beneficial clinical effects. Activation of Akt and mTOR signaling in dopaminergic neurons promotes regrowth of axons after nigrostriatal degeneration59 and prevents neuronal loss in toxin models of PD,60 whereas several toxin-based models of PD report that suppression of mTOR signaling induces oxidative stress.61,62,63 Despite others reporting that inhibition of mTOR (with rapamycin or its derivatives) is neuroprotective in models of PD64,65 (perhaps reflecting the differing roles of individual complexes), it may be that it is the loss of the regulation of mTOR activity that can have negative effects on neuronal physiologic mechanisms, and thus it may be that upstream restoration of mTOR signaling60 may be therapeutically beneficial in PD.
We did not find any significant association between exenatide and the MAPK pathway. Although some studies67,68 have found that stimulation of MAPK signaling is involved in mediating the neuroprotective effects of exenatide, others have found that exenatide treatment does not affect phosphorylation of MAPK signaling kinases69,70 and that MAPK signaling is not necessary for the effects of exenatide on cell survival.54,71,72 Our data suggest that the MAPK pathway is less likely to be involved in any beneficial effects of exenatide in PD.
Although the data from this study support the notion that exenatide-associated effects on the insulin and Akt signaling pathway in neurons were associated with clinical benefit, whether these changes are ultimately associated with modification of disease pathologic mechanisms is still uncertain. Insulin resistance is associated with decreased expression of surface dopamine transporters in the striatum73,74 and reduced dopamine turnover75; therefore, reversing this could lead to better dopaminergic transmission (and, therefore, a functional benefit). Conversely, reversal and restoration of dysfunctional neuronal insulin signaling in cultured cells and animals using GLP-1 agonists have been associated with reduction in cell death, aggregation of toxic oligomers, and inflammation, suggesting a disease-modifying effect that may also be reflected by functional improvements.32,33,41,76 Another possibility is that the clinical improvement and biomarker changes were produced in parallel through independent mechanisms of action of exenatide: GLP-1 stimulation is known to increase intracellular cyclic adenosine monophosphate,77 which can inhibit serine phosphorylation of IRS-1 (thereby producing biomarker changes), and upregulates the expression and activity of tyrosine hydroxylase,78 the rate-limiting enzyme in the synthesis of dopamine (thereby producing a clinical symptomatic effect). We deem this possibility as less likely given the associations between clinical and biomarker changes as well as the persistence of biomarker changes after the washout.
Beyond these effects on insulin signaling, a further potential mechanism of action of exenatide that was not captured by the methods used in this study may relate to an anti-inflammatory effect of GLP-1 receptor stimulation on microglial cells and consequent reduction of conversion of astrocytes to the neurotoxic A1 subtype.79 The methods for isolating EVs enriched for astrocytic origin have been recently reported,80,81 which raises the possibility of exploring mechanisms that involve astrocytes in future studies. Studies isolating EVs of different central nervous system cells of origin may be able to determine the relative magnitude of effects of exenatide on insulin signaling in neurons vs actions that involve microglia and astrocytes. Furthermore, although we excluded patients with concurrent diabetes from this study (based on hemoglobin A1c levels), patients with PD and peripheral insulin resistance may still have been included and the clinical improvement may be partially attributable to exenatide restoring peripheral insulin sensitivity. Central and peripheral insulin resistance are interrelated, but dissociable, and insulin-signaling molecules in different subpopulations of EVs may be used to disentangle their relative contributions in drug effects in future clinical trials.82
Limitations
Our approach to EV isolation has some limitations. It is widely recognized that no technique is perfect for EV isolation and removal of soluble content; however, combining 2 techniques (ie, particle precipitation and immune capture, as done here) is preferable to each one alone.45 Moreover, selectively isolating neuronal-derived EVs relies on immunoprecipitation using antibodies against L1CAM, a cell surface marker highly (but not exclusively) expressed on neurons that has been accepted as a neuronal marker. Given that the insulin signaling, Akt, and mTOR pathways are not specific to neurons and the residual contamination of some nonneuronal EVs, it is not possible to assert that the effects of exenatide on EV biomarkers is solely attributable to neurons. Amelioration of insulin resistance in nonneuronal tissues may thus be a contributory factor to the reported results. Although this issue is of no concern when assaying proteins that are only neuronally expressed, further work to optimize the isolation of EVs of pure neuronal origin will further assist future assessments of drug actions in which both central and peripheral mechanisms may theoretically contribute to clinical effects.
Conclusions
We present, to our knowledge, the first biomarker evidence that peripherally administered exenatide may engage and normalize brain insulin signaling in association with activation of Akt and mTOR cascades in PD. Furthermore, exenatide-related changes in EV biomarkers were significantly associated with clinical improvements and could potentially be further used to assess target engagement and treatment response for this class and other classes of drugs. The use of neuronal origin–enriched EVs obtained from peripheral sources provides a simple, practical method for elucidating target engagement that should be further investigated in prospective clinical trials of putative disease-modifying interventions.
eFigure 1. Extreme Outlier for IRS-1p Tyr
eFigure 2. Interaction Plots for IRS-1 Proteins at 48 Weeks According to Treatment Allocation
eFigure 3. Interaction Plots for Downstream Target Proteins at 48 Weeks According to Treatment Allocation
eFigure 4. Interaction Plots for IRS-1 Proteins at 60 Weeks According to Treatment Allocation
eFigure 5. Interaction Plots for Downstream Target Proteins at 60 Weeks According to Treatment Allocation
eTable 1. Comparison of Baseline Patient Characteristics and Demographics
eTable 2. Association of Change in Biomarker Level and Progression of PD at 48 and 60 Weeks
eResults. Supplementary Results
References
- 1.Mustapic M, Eitan E, Werner JK Jr, et al. . Plasma Extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes. Front Neurosci. 2017;11:278. doi: 10.3389/fnins.2017.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fauré J, Lachenal G, Court M, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31(4):642-648. doi: 10.1016/j.mcn.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 3.Bellingham SA, Guo BB, Coleman BM, Hill AF. Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front Physiol. 2012;3:124. doi: 10.3389/fphys.2012.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiandaca MS, Kapogiannis D, Mapstone M, et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement. 2015;11(6):600-607.e1. doi: 10.1016/j.jalz.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goetzl EJ, Kapogiannis D, Schwartz JB, et al. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J. 2016;30(12):4141-4148. doi: 10.1096/fj.201600816R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi M, Liu C, Cook TJ, et al. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014;128(5):639-650. doi: 10.1007/s00401-014-1314-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Pablo-Fernández E, Breen DP, Bouloux PM, Barker RA, Foltynie T, Warner TT. Neuroendocrine abnormalities in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2017;88(2):176-185. doi: 10.1136/jnnp-2016-314601 [DOI] [PubMed] [Google Scholar]
- 8.Song J, Kim J. Degeneration of dopaminergic neurons due to metabolic alterations and Parkinson’s disease. Front Aging Neurosci. 2016;8:65. doi: 10.3389/fnagi.2016.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aviles-Olmos I, Limousin P, Lees A, Foltynie T. Parkinson’s disease, insulin resistance and novel agents of neuroprotection. Brain. 2013;136(Pt 2):374-384. doi: 10.1093/brain/aws009 [DOI] [PubMed] [Google Scholar]
- 10.Bassil F, Fernagut P-O, Bezard E, Meissner WGT. Insulin, IGF-1 and GLP-1 signaling in neurodegenerative disorders: targets for disease modification? Prog Neurobiol. 2014;118:1-18. doi: 10.1016/j.pneurobio.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 11.Driver JA, Smith A, Buring JE, Gaziano JM, Kurth T, Logroscino G. Prospective cohort study of type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care. 2008;31(10):2003-2005. doi: 10.2337/dc08-0688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schernhammer E, Hansen J, Rugbjerg K, Wermuth L, Ritz B. Diabetes and the risk of developing Parkinson’s disease in Denmark. Diabetes Care. 2011;34(5):1102-1108. doi: 10.2337/dc10-1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Q, Park Y, Huang X, et al. Diabetes and risk of Parkinson’s disease. Diabetes Care. 2011;34(4):910-915. doi: 10.2337/dc10-1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris JK, Zhang H, Gupte AA, Bomhoff GL, Stanford JA, Geiger PC. Measures of striatal insulin resistance in a 6-hydroxydopamine model of Parkinson’s disease. Brain Res. 2008;1240:185-195. doi: 10.1016/j.brainres.2008.08.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold SE, Lucki I, Brookshire BR, et al. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis. 2014;67:79-87. doi: 10.1016/j.nbd.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi J-Y, Jang E-H, Park C-S, Kang J-H. Enhanced susceptibility to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity in high-fat diet-induced obesity. Free Radic Biol Med. 2005;38(6):806-816. doi: 10.1016/j.freeradbiomed.2004.12.008 [DOI] [PubMed] [Google Scholar]
- 17.Kleinridders A, Cai W, Cappellucci L, et al. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc Natl Acad Sci U S A. 2015;112(11):3463-3468. doi: 10.1073/pnas.1500877112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris JK, Bomhoff GL, Stanford JA, Geiger PC. Neurodegeneration in an animal model of Parkinson’s disease is exacerbated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2010;299(4):R1082-R1090. doi: 10.1152/ajpregu.00449.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris JK, Bomhoff GL, Gorres BK, et al. Insulin resistance impairs nigrostriatal dopamine function. Exp Neurol. 2011;231(1):171-180. doi: 10.1016/j.expneurol.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Zhai Y-Q, Xu L-L, et al. Metabolic inflammation exacerbates dopaminergic neuronal degeneration in response to acute MPTP challenge in type 2 diabetes mice. Exp Neurol. 2014;251:22-29. doi: 10.1016/j.expneurol.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 21.Athauda D, Foltynie T. Insulin resistance and Parkinson’s disease: a new target for disease modification? Prog Neurobiol. 2016;145-146:98-120. doi: 10.1016/j.pneurobio.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 22.Gao S, Duan C, Gao G, Wang X, Yang H. Alpha-synuclein overexpression negatively regulates insulin receptor substrate 1 by activating mTORC1/S6K1 signaling. Int J Biochem Cell Biol. 2015;64:25-33. doi: 10.1016/j.biocel.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 23.Sharma SK, Chorell E, Steneberg P, Vernersson-Lindahl E, Edlund H, Wittung-Stafshede P. Insulin-degrading enzyme prevents α-synuclein fibril formation in a nonproteolytical manner. Sci Rep. 2015;5:12531. doi: 10.1038/srep12531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jha SK, Jha NK, Kar R, Ambasta RK, Kumar P. p38 MAPK and PI3K/AKT signalling cascades in Parkinson’s disease. Int J Mol Cell Med. 2015;4(2):67-86. [PMC free article] [PubMed] [Google Scholar]
- 25.Gual P, Le Marchand-Brustel Y, Tanti J-F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005;87(1):99-109. doi: 10.1016/j.biochi.2004.10.019 [DOI] [PubMed] [Google Scholar]
- 26.Herschkovitz A, Liu Y-F, Ilan E, Ronen D, Boura-Halfon S, Zick Y. Common inhibitory serine sites phosphorylated by IRS-1 kinases, triggered by insulin and inducers of insulin resistance. J Biol Chem. 2007;282(25):18018-18027. doi: 10.1074/jbc.M610949200 [DOI] [PubMed] [Google Scholar]
- 27.Copps KD, Hancer NJ, Opare-Ado L, Qiu W, Walsh C, White MF. Irs1 serine 307 promotes insulin sensitivity in mice. Cell Metab. 2010;11(1):84-92. doi: 10.1016/j.cmet.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giraud J, Leshan R, Lee Y-H, White MF. Nutrient-dependent and insulin-stimulated phosphorylation of insulin receptor substrate-1 on serine 302 correlates with increased insulin signaling. J Biol Chem. 2004;279(5):3447-3454. doi: 10.1074/jbc.M308631200 [DOI] [PubMed] [Google Scholar]
- 29.Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE. 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem. 2001;276(50):46912-46916. doi: 10.1074/jbc.C100483200 [DOI] [PubMed] [Google Scholar]
- 30.Bassil F, Canron M-H, Vital A, Bezard E, Fernagut P-O, Meissner WG Brain insulin resistance in Parkinson’s disease [abstract]. Mov Disord 2017;32(suppl 2). http://www.mdsabstracts.org/abstract/brain-insulin-resistance-in-parkinsons-disease/. Accessed November 26, 2018.
- 31.Sekar S, Taghibiglou C. Elevated nuclear phosphatase and tensin homolog (PTEN) and altered insulin signaling in substantia nigral region of patients with Parkinson’s disease. Neurosci Lett. 2018;666:139-143. doi: 10.1016/j.neulet.2017.12.049 [DOI] [PubMed] [Google Scholar]
- 32.Bomfim TR, Forny-Germano L, Sathler LB, et al. . An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease–associated Aβ oligomers. J Clin Invest. 2012;122(4):1339-1353. doi: 10.1172/JCI57256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long-Smith CM, Manning S, McClean PL, et al. The diabetes drug liraglutide ameliorates aberrant insulin receptor localisation and signalling in parallel with decreasing both amyloid-β plaque and glial pathology in a mouse model of Alzheimer’s disease. Neuromolecular Med. 2013;15(1):102-114. doi: 10.1007/s12017-012-8199-5 [DOI] [PubMed] [Google Scholar]
- 34.Xu W, Yang Y, Yuan G, Zhu W, Ma D, Hu S. Exendin-4, a glucagon-like peptide-1 receptor agonist, reduces Alzheimer disease-associated tau hyperphosphorylation in the hippocampus of rats with type 2 diabetes. J Investig Med. 2015;63(2):267-272. doi: 10.1097/JIM.0000000000000129 [DOI] [PubMed] [Google Scholar]
- 35.Kapogiannis D, Boxer A, Schwartz JB, et al. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 2015;29(2):589-596. doi: 10.1096/fj.14-262048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talbot K, Wang H-Y, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316-1338. doi: 10.1172/JCI59903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eitan E, Tosti V, Suire CN, et al. . In a randomized trial in prostate cancer patients, dietary protein restriction modifies markers of leptin and insulin signaling in plasma extracellular vesicles. Aging Cell. 2017;16(6):1430-1433. doi: 10.1111/acel.12657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma MK, Jalewa J, Hölscher C. Neuroprotective and anti-apoptotic effects of liraglutide on SH-SY5Y cells exposed to methylglyoxal stress. J Neurochem. 2014;128(3):459-471. doi: 10.1111/jnc.12469 [DOI] [PubMed] [Google Scholar]
- 39.Sandoval D, Sisley SR. Brain GLP-1 and insulin sensitivity. Mol Cell Endocrinol. 2015;418(Pt 1):27-32. doi: 10.1016/j.mce.2015.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Y-W, Hsieh TH, Chen K-Y, et al. Glucose-dependent insulinotropic polypeptide ameliorates mild traumatic brain injury-induced cognitive and sensorimotor deficits and neuroinflammation in rats. J Neurotrauma. 2016;33(22):2044-2054. doi: 10.1089/neu.2015.4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bassil F, Canron M-H, Vital A, et al. Insulin resistance and exendin-4 treatment for multiple system atrophy. Brain. 2017;140(5):1420-1436. doi: 10.1093/brain/awx044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Athauda D, Maclagan K, Skene SS, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10103):1664-1675. doi: 10.1016/S0140-6736(17)31585-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.clinicaltrials.gov A Randomised, Double Blind, Placebo Controlled, Single Centre, 60 Week Trial of Exenatide Once Weekly for the Treatment of Moderate Severity Parkinson's Disease. NCT01981242. https://clinicaltrials.gov/ct2/show/NCT01981242. Accessed November 27, 2018.
- 44.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181-184. doi: 10.1136/jnnp.55.3.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coumans FAW, Brisson AR, Buzas EI, et al. Methodological guidelines to study extracellular vesicles. Circ Res. 2017;120(10):1632-1648. doi: 10.1161/CIRCRESAHA.117.309417 [DOI] [PubMed] [Google Scholar]
- 46.Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2(1):20360. doi: 10.3402/jev.v2i0.20360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mustapic M, Eitan E, Werner JK Jr, et al. . Plasma extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes. Front Neurosci. 2017;11:278. doi: 10.3389/fnins.2017.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sáenz-Cuesta M, Arbelaiz A, Oregi A, et al. Methods for extracellular vesicles isolation in a hospital setting. Front Immunol. 2015;6:50. doi: 10.3389/fimmu.2015.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh S, Mishra A, Mishra SK, Shukla S. ALCAR promote adult hippocampal neurogenesis by regulating cell-survival and cell death-related signals in rat model of Parkinson’s disease like-phenotypes. Neurochem Int. 2017;108:388-396. doi: 10.1016/j.neuint.2017.05.017 [DOI] [PubMed] [Google Scholar]
- 50.Yue P, Gao L, Wang X, Ding X, Teng J. Intranasal administration of GDNF protects against neural apoptosis in a rat model of Parkinson’s disease through PI3K/Akt/GSK3β pathway. Neurochem Res. 2017;42(5):1366-1374. doi: 10.1007/s11064-017-2184-1 [DOI] [PubMed] [Google Scholar]
- 51.Wang Q, Li L, Xu E, Wong V, Rhodes C, Brubaker PL. Glucagon-like peptide-1 regulates proliferation and apoptosis via activation of protein kinase B in pancreatic INS-1 beta cells. Diabetologia. 2004;47(3):478-487. doi: 10.1007/s00125-004-1327-5 [DOI] [PubMed] [Google Scholar]
- 52.Zhou H, Li D, Shi C, et al. Effects of exendin-4 on bone marrow mesenchymal stem cell proliferation, migration and apoptosis in vitro. Sci Rep. 2015;5:12898. doi: 10.1038/srep12898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ceriello A, Novials A, Ortega E, et al. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care. 2013;36(8):2346-2350. doi: 10.2337/dc12-2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang M-D, Huang Y, Zhang G-P, et al. Exendin-4 improved rat cortical neuron survival under oxygen/glucose deprivation through PKA pathway. Neuroscience. 2012;226:388-396. doi: 10.1016/j.neuroscience.2012.09.025 [DOI] [PubMed] [Google Scholar]
- 55.Gao H, Zeng Z, Zhang H, et al. . The glucagon-like peptide-1 analogue liraglutide inhibits oxidative stress and inflammatory response in the liver of rats with diet-induced non-alcoholic fatty liver disease. Biol Pharm Bull. 2015;38(5):694-702. doi: 10.1248/bpb.b14-00505 [DOI] [PubMed] [Google Scholar]
- 56.Greene LA, Levy O, Malagelada C. Akt as a victim, villain and potential hero in Parkinson’s disease pathophysiology and treatment. Cell Mol Neurobiol. 2011;31(7):969-978. doi: 10.1007/s10571-011-9671-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim SR, Ries V, Cheng H-C, et al. Age and α-synuclein expression interact to reveal a dependence of dopaminergic axons on endogenous Akt/PKB signaling. Neurobiol Dis. 2011;44(2):215-222. doi: 10.1016/j.nbd.2011.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malagelada C, Jin ZH, Greene LA. RTP801 is induced in Parkinson’s disease and mediates neuron death by inhibiting Akt phosphorylation/activation. J Neurosci. 2008;28(53):14363-14371. doi: 10.1523/JNEUROSCI.3928-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SR, Chen X, Oo TF, et al. Dopaminergic pathway reconstruction by Akt/Rheb-induced axon regeneration. Ann Neurol. 2011;70(1):110-120. doi: 10.1002/ana.22383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Q, Liu C, Liu W, et al. Rotenone induction of hydrogen peroxide inhibits mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways, leading to neuronal apoptosis. Toxicol Sci. 2015;143(1):81-96. doi: 10.1093/toxsci/kfu211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi J-S, Park C, Jeong J-W. AMP-activated protein kinase is activated in Parkinson’s disease models mediated by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Biochem Biophys Res Commun. 2010;391(1):147-151. doi: 10.1016/j.bbrc.2009.11.022 [DOI] [PubMed] [Google Scholar]
- 62.Rodríguez-Blanco J, Martín V, García-Santos G, et al. Cooperative action of JNK and AKT/mTOR in 1-methyl-4-phenylpyridinium-induced autophagy of neuronal PC12 cells. J Neurosci Res. 2012;90(9):1850-1860. doi: 10.1002/jnr.23066 [DOI] [PubMed] [Google Scholar]
- 63.Selvaraj S, Sun Y, Watt JA, et al. Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. J Clin Invest. 2012;122(4):1354-1367. doi: 10.1172/JCI61332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang J, Jiang J, Zuo Y, Gu Z. Rapamycin protects the mitochondria against oxidative stress and apoptosis in a rat model of Parkinson’s disease. Int J Mol Med. 2013;31(4):825-832. doi: 10.3892/ijmm.2013.1280 [DOI] [PubMed] [Google Scholar]
- 65.Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J Neurosci. 2010;30(3):1166-1175. doi: 10.1523/JNEUROSCI.3944-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Copp J, Manning G, Hunter T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 2009;69(5):1821-1827. doi: 10.1158/0008-5472.CAN-08-3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng Y, Su L, Zhong X, et al. Exendin-4 promotes proliferation and differentiation of MC3T3-E1 osteoblasts by MAPKs activation. J Mol Endocrinol. 2016;56(3):189-199. doi: 10.1530/JME-15-0264 [DOI] [PubMed] [Google Scholar]
- 68.Zheng A, Cao L, Qin S, Chen Y, Li Y, Zhang D. Exenatide regulates substrate preferences through the p38γ MAPK pathway after ischaemia/reperfusion injury in a rat heart. Heart Lung Circ. 2017;26(4):404-412. doi: 10.1016/j.hlc.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 69.Candeias E, Sebastião I, Cardoso S, et al. Brain GLP-1/IGF-1 signaling and autophagy mediate exendin-4 protection against apoptosis in type 2 diabetic rats. Mol Neurobiol. 2018;55(5):4030-4050. doi: 10.1007/s12035-017-0622-3 [DOI] [PubMed] [Google Scholar]
- 70.Mukai E, Fujimoto S, Sato H, et al. Exendin-4 suppresses SRC activation and reactive oxygen species production in diabetic Goto-Kakizaki rat islets in an Epac-dependent manner. Diabetes. 2011;60(1):218-226. doi: 10.2337/db10-0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Y, Tweedie D, Mattson MP, Holloway HW, Greig NH. Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J Neurochem. 2010;113(6):1621-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang C, Chen X, Ding X, He Y, Gu C, Zhou L. Exendin-4 promotes beta cell proliferation via PI3k/Akt signalling pathway. Cell Physiol Biochem. 2015;35(6):2223-2232. doi: 10.1159/000374027 [DOI] [PubMed] [Google Scholar]
- 73.Jones KT, Woods C, Zhen J, Antonio T, Carr KD, Reith MEA. Effects of diet and insulin on dopamine transporter activity and expression in rat caudate-putamen, nucleus accumbens, and midbrain. J Neurochem. 2017;140(5):728-740. doi: 10.1111/jnc.13930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stouffer MA, Woods CA, Patel JC, et al. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun. 2015;6:8543. doi: 10.1038/ncomms9543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baladi MG, Horton RE, Owens WA, Daws LC, France CP. Eating high fat chow decreases dopamine clearance in adolescent and adult male rats but selectively enhances the locomotor stimulating effects of cocaine in adolescents. Int J Neuropsychopharmacol. 2015;18(7):pyv024. doi: 10.1093/ijnp/pyv024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang Y, Ma D, Xu W, et al. Exendin-4 reduces tau hyperphosphorylation in type 2 diabetic rats via increasing brain insulin level. Mol Cell Neurosci. 2016;70:68-75. doi: 10.1016/j.mcn.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 77.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci U S A. 1987;84(10):3434-3438. doi: 10.1073/pnas.84.10.3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim KS, Park DH, Wessel TC, Song B, Wagner JA, Joh TH. A dual role for the cAMP-dependent protein kinase in tyrosine hydroxylase gene expression. Proc Natl Acad Sci U S A. 1993;90(8):3471-3475. doi: 10.1073/pnas.90.8.3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yun SP, Kam T-I, Panicker N, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med. 2018;24(7):931-938. doi: 10.1038/s41591-018-0051-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goetzl EJ, Mustapic M, Kapogiannis D, et al. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J. 2016;30(11):3853-3859. doi: 10.1096/fj.201600756R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goetzl EJ, Schwartz JB, Abner EL, Jicha GA, Kapogiannis D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann Neurol. 2018;83(3):544-552. doi: 10.1002/ana.25172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mullins RJ, Diehl TC, Chia CW, Kapogiannis D. Insulin resistance as a link between amyloid-beta and tau pathologies in Alzheimer’s disease. Front Aging Neurosci. 2017;9:118. doi: 10.3389/fnagi.2017.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Extreme Outlier for IRS-1p Tyr
eFigure 2. Interaction Plots for IRS-1 Proteins at 48 Weeks According to Treatment Allocation
eFigure 3. Interaction Plots for Downstream Target Proteins at 48 Weeks According to Treatment Allocation
eFigure 4. Interaction Plots for IRS-1 Proteins at 60 Weeks According to Treatment Allocation
eFigure 5. Interaction Plots for Downstream Target Proteins at 60 Weeks According to Treatment Allocation
eTable 1. Comparison of Baseline Patient Characteristics and Demographics
eTable 2. Association of Change in Biomarker Level and Progression of PD at 48 and 60 Weeks
eResults. Supplementary Results