This article analyzes outcomes in a large cohort of patients with hepatocellular cancer with microvascular invasion who either were or were not treated with adjuvant transarterial chemoembolization after radical resection, aiming to help oncologists choose an appropriate adjuvant treatment for these patients based on risk‐benefit assessments.
Keywords: Transarterial chemoembolization, Hepatocellular carcinoma, Microvascular invasion, Propensity score matching, Prognosis
Abstract
Background.
Patients with hepatocellular carcinoma (HCC) and microvascular invasion (mVI) have shown dismal postoperative prognosis; however, whether adjuvant transarterial chemoembolization (TACE) can improve their outcomes remains unclear.
Materials and Methods.
We retrospectively identified 549 eligible patients to form the crude cohort and adopted propensity score matching method to assemble another cohort of 444 patients with similar baseline characteristics. We assessed the effects of adjuvant TACE by stratified analyses and multivariate Cox analyses in two cohorts.
Results.
There was significant interaction between tumor size and adjuvant TACE with respect to overall survival (OS; p = .006 for interaction). In the matched cohort, patients who received adjuvant TACE showed higher rates of 5‐year OS (72.4% vs. 50.9%, p = .005) and 5‐year recurrence‐free survival (50.5% vs. 36.4%, p = .003) in the tumor ≤5 cm subgroup, but not in the tumor >5 cm subgroup (32.3% vs. 24.9%, p = .350 and 18.8% vs. 19.7%, p = .180). The independent protective role of adjuvant TACE on OS was observed in patients with tumor ≤5 cm (adjusted odds ratio [OR] = 0.59, 95% confidence interval [CI] 0.36–0.97) but not in patients with tumor >5 cm (adjusted OR = 1.17, 95% CI 0.84–1.62). The effects of adjuvant TACE did not change materially while the analysis was performed in the crude cohort.
Conclusion.
For patients with HCC and mVI, adjuvant TACE was associated with improved outcomes, but not for those with tumor >5 cm, according to the current protocol.
Implications for Practice.
The outcomes of patients with hepatocellular carcinoma and microvascular invasion who received adjuvant transarterial chemoembolization were inconsistent in this study. According to the current protocol, adjuvant transarterial chemoembolization was associated with improved prognosis in patients with microvascular invasion, except for those with tumor >5 cm. Multivariate Cox models confirmed adjuvant transarterial chemoembolization was an independent protective factor in the tumor ≤5 cm subgroup but not in the tumor >5 cm subgroup.
Introduction
Vascular invasion (VI), which can occur as either macrovascular or microvascular invasion (mVI), is an aggressive form of hepatocellular carcinoma (HCC) and is currently one of the most important predictive factors for postoperative prognosis [1], [2]. Macro VI can be detected preoperatively using imaging techniques and is already used in the American Joint Committee on Cancer and Barcelona Clinic Liver Cancer staging systems to determine treatment strategies for HCC [3], [4]. Recently, an increasing amount of studies have suggested that the presence of mVI also predicts a worse prognosis after radical resection [2], [5], [6]. Because mVI can only be determined by histological examination after liver resection, prevention of recurrence by adjuvant treatment may be an important option to improve the prognosis of patients with this characteristic.
Previously, some studies have indicated that postoperative adjuvant TACE is a promising method that can significantly improve the prognosis of patients with HCC [7], [8], [9], but others have reported mixed results [10], [11]. As the understanding of HCC classification gradually improved, some researchers suggested that postoperative adjuvant TACE provides benefits only for certain high‐risk subgroups of patients, such as those with macro VI [12], [13], [14], [15]. This further greatly aroused our interest to study the role of adjuvant TACE for patients with mVI.
In the present study, we retrospectively analyzed the outcomes of a large cohort of HCC patients with mVI who were or were not treated with adjuvant TACE after radical resection. We aimed to provide an accurate estimation of postoperative adjuvant TACE, which may help oncologists choose an appropriate adjuvant treatment for these patients based on risk–benefit assessments.
Materials and Methods
Study Population
This retrospective study evaluated patient data from our electronic medical records; patient identities were anonymized prior to analysis. Adult patients who underwent liver resection for primary HCC from January 2007 to December 2012 at Zhongshan Hospital were identified. Patients who also met the following criteria were enrolled: (a) received radical resection (the radical resection criterion was the same as previously described [16]); (b) no extrahepatic metastasis; (c) macro VI was not present, and mVI was diagnosed by postoperative pathological examinations; (d) liver function returned to Child‐Pugh class A/B, with a serum bilirubin level ≤ 1.5 times the upper normal limit, alanine aminotransferase and aspartate aminotransferase levels ≤2 times the upper normal limit; (e) no serious major organ dysfunction; (f) tumor did not relapse within 1 month after surgery; and (g) patients only received operation or operation plus adjuvant TACE before tumor recurrence. Finally, a total of 549 patients were enrolled as the crude cohort in the present study. The flow chart of the entire process is shown in supplemental online Figure 1.
Ethics Approval and Consent to Participate
The study protocol was approved by the Zhongshan Hospital Research Ethics Committee. Written informed consent was obtained from every HCC patient before each treatment. The entire study complied with the ethical guidelines of the 1975 Declaration of Helsinki (as revised in Brazil in 2013).
Histopathological Evaluation
mVI was defined as microscopic tumor invasion that was identified in the portal and hepatic veins of the surrounding liver tissue and was contiguous with the tumor edge. The histological grade was assessed according to the Edmondson‐Steiner grading system based on the highest grade in a specimen [17]. The results for the above variables were determined using histopathological reports that had been stored in a prospectively maintained computerized clinical database.
Adjuvant TACE
All patients received liver function test, serum alpha‐fetoprotein (AFP) test, and enhanced magnetic resonance imaging or computed tomography scans of the abdomen at 1 month after the operation. For patients who had not been diagnosed with tumor recurrence, adjuvant TACE was recommended by the attending physician after adequately informing them of the potential benefits and risks of this treatment. Finally, a total of 303 patients underwent adjuvant TACE, compared with the remaining 246 patients who did not undergo this regimen.
Adjuvant TACE was conducted using the Seldinger method for the remnant liver. Chemotherapeutic agents, including doxorubicin hydrochloride (10 mg) or pharmorubicin (20–30 mg/m3), were slowly infused through the right and left hepatic arteries, followed by an emulsion of lipiodol (5–10 mL).
In the current study, we reported side effects of adjuvant TACE, according to the Clavien‐Dindo standardized classification system, which was recommended in its current form for use in retrospective studies [18].
Follow‐Up and Endpoint
All patients were periodically followed up to prospectively monitor the recurrence of HCC. Generally, testing for serum AFP and ultrasonography were performed every 3 months thereafter. Additionally, dynamic contrast‐enhanced magnetic resonance imaging scans of the abdomen were performed 1 month after the operation and every 6‐12 months thereafter. Whenever recurrence was confirmed, further treatment was immediately administered as described in detail earlier [19], [20].
The primary outcome of interest was overall survival (OS), which was defined as time from the operation to death or last follow‐up. Secondary outcome was recurrence‐free survival (RFS), defined as time from the operation to tumor recurrence diagnosis or last follow‐up. Follow‐up data for all patients were summarized at the end of December 2014, resulting in a median observation time of 29 months.
Variables Studied
Potential variables related to the prognosis of HCC patients were selected based on previous studies [19], [21]. Tumor size, number, and capsular were confirmed based on a histopathological examination. Tumor size (≤5 cm; >5 cm) was further categorized based on cutoff values similar to those used in the Milan criteria or tumor‐node‐metastasis classification of malignant tumours [4], [22]. The extent of resection (minor, <3 segments; major, ≥3 segments) was based on Couinaud's nomenclature [23]. The data of anatomic resection and blood transfusion were obtained from surgical records and anesthesia records, respectively. Age was categorized by the median value. Other continuous variables, including hepatitis B virus (HBV) DNA, total bilirubin, gamma‐glutamyl transferase (GGT), and AFP levels, were categorized using cutoff values provided by clinical references. For patients with HBV DNA >5 × 102 IU/mL, antiviral therapy was performed immediately, and the indicator was re‐evaluated 1 month later to maintain the viral replication at a low level.
Statistical Analysis
We used propensity score matching (PSM) to identify a cohort of patients with similar baseline characteristics. The propensity score was estimated by a nonparsimonious multivariable logistic‐regression model with adjuvant TACE as the dependent variable and all the baseline characteristics listed in Table 1 as covariates. Patients were matched by a 1:1 ratio using the nearest neighbor method with a caliber of 0.05.
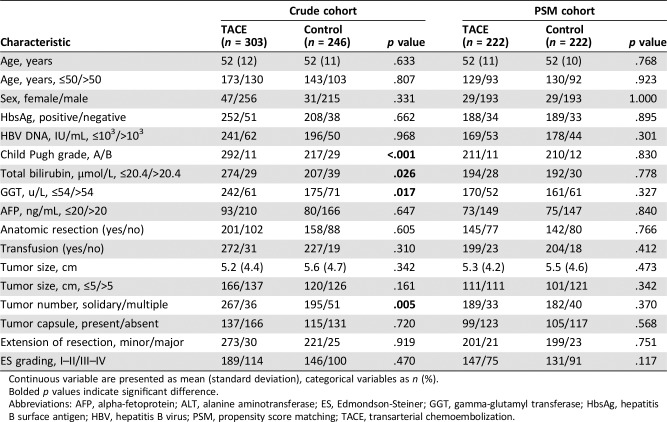
Table 1. Baseline characteristics of the study patients according to treatment groups.
Continuous variable are presented as mean (standard deviation), categorical variables as n (%).
Bolded p values indicate significant difference.
Abbreviations: AFP, alpha‐fetoprotein; ALT, alanine aminotransferase; ES, Edmondson‐Steiner; GGT, gamma‐glutamyl transferase; HbsAg, hepatitis B surface antigen; HBV, hepatitis B virus; PSM, propensity score matching; TACE, transarterial chemoembolization.
Comparisons between two groups were performed with the use of chi‐square test or Student's t test. Then, univariate analyses were performed with the cause‐specific Cox proportional hazards models. We calculated odds ratio (OR) for risk of OS by stratified analysis, and the p value for an interaction was calculated based on a log likelihood ratio test that compared two nested models. Cumulative OS and RFS curves were assessed by Kaplan‐Meier method, and differences between groups were compared by log‐rank test. Finally, we characterized independent association between adjuvant TACE and OS by multivariate Cox proportional hazards models, progressively adjusting for confounding factors.
The results were expressed as OR and 95% confidence intervals (CI). A two‐sided significance level of 0.05 was used to evaluate statistical significance. Statistical analyses were performed using the R software (http://www.R-project.org, version 3.4.3, R Development Team, Vienna, Austria).
Results
Table 1 shows the clinical characteristics of the study population grouped by adjuvant TACE. In the crude cohort, 303 (55.2%) patients received postoperative adjuvant TACE, and the remaining 246 (44.8%) patients did not receive adjuvant TACE. Patients in TACE group had higher rates of Child‐Pugh grade A, GGT ≤54 u/L, total bilirubin ≤20.4 μmol/L, and solidary tumor than those in control group (all p < .05). With the use of PSM, 222 patients who received postoperative adjuvant TACE were matched with 222 patients who received only operation. After matching, all the covariates were well balanced between the two groups (all p > .05).
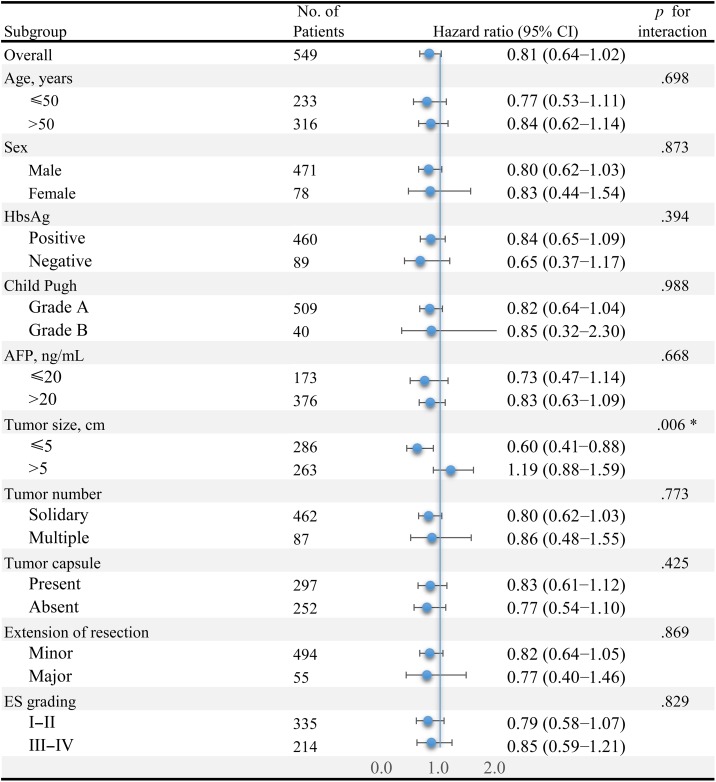
In the overall study population, a total of 291 participants died at the end of follow‐up. Univariate analysis (supplemental online Table 1) showed that the association between adjuvant TACE and OS was close to the level of significance (OR = 0.81, 95% CI 0.64–1.02, p = .069). Stratified analyses according to patient characteristics are shown in Figure 1. Effects were similar across most subgroups; however, the benefit of adjuvant TACE among patients with tumor ≤5 cm appeared to be more significantly pronounced than that among patients with tumor >5 cm (OR = 0.60 vs. 1.19, p = .006 for interaction). Therefore, the association between adjuvant TACE and OS was explored independently in different tumor size subgroups.
Figure 1.
Adjuvant transarterial chemoembolization (TACE) on overall survival in prespecified or exploratory subgroups. Odds ratio (OR) of adjuvant TACE on overall survival was significantly different in different tumor size subgroups (OR = 0.60 in tumor size ≤5 cm subgroup and OR = 1.19 in tumor size >5 cm subgroup, p = .006 for interaction). In addition, adjuvant TACE showed consistent protective effect on overall survival in all of the remaining subgroups (all p > .05 for interaction).
Abbreviations: AFP, alpha‐fetoprotein; CI, confidence interval; ES, Edmonson‐Steiner; HbsAg, hepatitis B surface antigen.
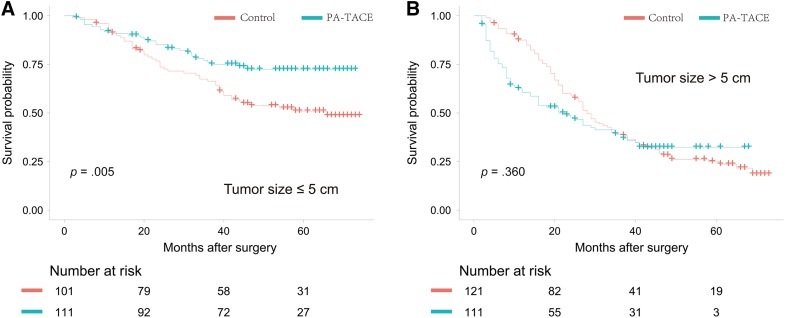
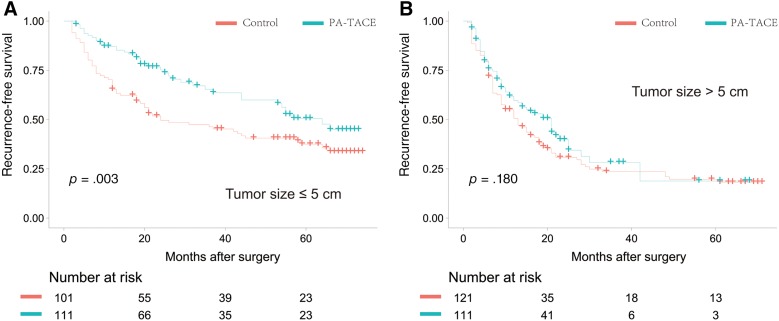
During unadjusted analysis in the PSM cohort, patients who received adjuvant TACE had significantly higher OS than patients who did not receive TACE in the tumor ≤5 cm subgroup (5‐year OS, 72.4% vs. 50.9%, p = .005; Fig. 2A); however, no significant difference was found in the tumor >5 cm subgroup (5‐year OS, 32.3% vs. 24.9%, p = .360; Fig. 2B). RFS was also significantly higher among patients who received adjuvant TACE in the tumor ≤5 cm subgroup (5‐year RFS, 50.5% vs. 36.4%, respectively, p = .003; Fig. 3A) but did not differ significantly between the two groups in the tumor >5 cm subgroup (5‐year RFS, 18.8% vs. 19.7%, p = .180; Fig. 3B). In the crude cohort, both OS and RFS of patients receiving adjuvant TACE were significantly higher in the tumor ≤5 cm subgroup. However, no significant treatment effect of adjuvant TACE on OS or RFS was found in the tumor ≤5 cm subgroup (supplemental online Figs. 2, 3).
Figure 2.
Overall survival curves of patients in the propensity score matching cohort. (A): For individuals with tumor size ≤5 cm, the 1‐, 3‐, and 5‐year overall survival rates were 90.1%, 66.4%, and 50.9%, respectively, in the group without adjuvant treatment and 91.8%, 76.1%, and 72.4%, respectively, in the group with adjuvant treatment. (B): For individuals with tumor size >5 cm, the 1‐, 3‐, and 5‐year overall survival rates were 87.5%, 39.2%, and 24.9%, respectively, in the group without adjuvant treatment and 60.5%, 39.1%, and 32.3%, respectively, in the group with adjuvant treatment.
Abbreviations: OS, overall survival; PA‐TACE, postoperative adjuvant transarterial chemoembolization.
Figure 3.
Recurrence‐free survival curves of patients in the propensity score matching cohort. (A): For individuals with tumor size ≤5 cm, the 1‐, 3‐, and 5‐year recurrence‐free survival rates were 64.4%, 45.3%, and 36.4%, respectively, in the group without adjuvant treatment and 87.2%, 65.6%, and 50.5%, respectively, in the group with adjuvant treatment. (B): For individuals with tumor size >5 cm, the 1‐, 3‐, and 5‐year recurrence‐free survival rates were 50.6%, 23.6%, and 19.7%, respectively, in the group without adjuvant treatment and 59.6%, 28.2%, and 18.8%, respectively, in the group with adjuvant treatment.
Abbreviation: PA‐TACE, postoperative adjuvant transarterial chemoembolization.
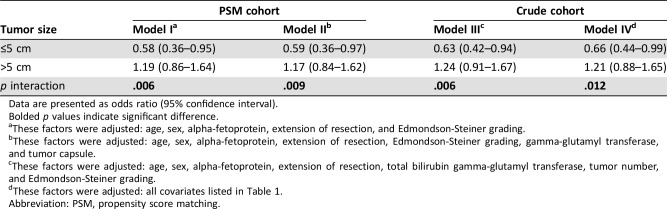
After adjusting potential confounding factors found during univariate analysis, in the PSM cohort, the OR of adjuvant TACE for OS was 0.58 (95% CI 0.36–0.95) in individuals with tumor ≤5 cm and 1.19 (95% CI 0.86–1.64) in those with tumor >5 cm (model I, p = .006 for interaction; Table 2). Even when progressively adjusted for more confounding factors, the OR of adjuvant TACE in individuals with tumor ≤5 cm remained significantly different than that in those with tumor >5 cm (model II, 0.59 vs. 1.17, p = .009 for interaction). The results did not change materially during analysis in the crude cohort after adopting different adjustment strategies (model III, 0.63 vs. 1.24, p = .006 for interaction; model IV, 0.66 vs. 1.21, p = .012 for interaction).
Table 2. The results of multivariate Cox regression analyses of overall survival stratified by tumor size.
Data are presented as odds ratio (95% confidence interval).
Bolded p values indicate significant difference.
These factors were adjusted: age, sex, alpha‐fetoprotein, extension of resection, and Edmondson‐Steiner grading.
These factors were adjusted: age, sex, alpha‐fetoprotein, extension of resection, Edmondson‐Steiner grading, gamma‐glutamyl transferase, and tumor capsule.
These factors were adjusted: age, sex, alpha‐fetoprotein, extension of resection, total bilirubin gamma‐glutamyl transferase, tumor number, and Edmondson‐Steiner grading.
These factors were adjusted: all covariates listed in Table 1.
Abbreviation: PSM, propensity score matching.
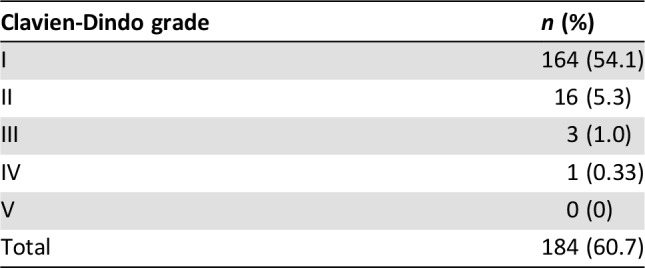
Short‐term side effects of 303 patients who received adjuvant TACE were described according to the Clavien‐Dindo classification (Table 3). Although the overall incidence of complications is up to 60.7%, the vast majority were Clavien‐Dindo grade 1 (54.1%), including fever, nausea or vomiting, pain, etc. A total of 16 (5.3%) patients experienced grade 2 complications, including mild liver function abnormalities, pancreatitis and leukopenia, and were cured by pharmacologic treatment. Meanwhile, a total of three (1.0%) patients experienced grade 3 complications, of whom two had biliary system complications and were cured by endoscopic retrograde cholangio‐pancreatography. Another case had liver abscess and was cured by puncture drainage. Only one patient (0.33%) experienced acute liver failure and was cured by comprehensive treatment in intensive care unit. No death due to complications was observed in the current study.
Table 3. Short‐term side effects of adjuvant transarterial chemoembolization measured by Clavien‐Dindo classification.
Discussion
Resection is considered to be a “curative” treatment for HCC. However, the long‐term survival of these patients remains far from promising; even for early tumors, the recurrence rate is up to 60% [24]. As more studies confirmed the dismal relationship between mVI and postoperative outcomes, adjuvant treatments were expected to bring a better prognosis for them. In the current study, the significant improvement in long‐term outcomes with adjuvant TACE, along with a manageable safety profile, suggested that it could be an option for the treatment of patients with HCC accompanied by mVI but not for those with tumor >5 cm.
With the advancement of pathological diagnosis technology, a number of cases with HCC accompanied by mVI were detected. Previous studies have shown that the prevalence of mVI in specimens obtained from liver transplantation or resection can reach 59.0% [25]. Even in those that meet the Milan Criteria, 50.0% of them exhibited mVI [26]. Results from a multicenter study showed that the frequency of mVI was 33.8% in patients with HCC within 2 cm [27]. Currently, mVI is considered to be the direct evidence of intrahepatic metastasis and a high risk of postoperative recurrence [26]. Some scholars have proposed that mVI is superior to Milan Criteria in predicting postoperative outcomes [2]. Therefore, assessment of the value of adjuvant treatment for patients with this characteristic is essential to improve their prognosis.
Previously, TACE has long been used as an effective locoregional therapy for unresectable [28] or recurrent HCC [29]. It is encouraging that our results added that postoperative adjuvant TACE was associated with higher RFS and OS in patients with tumor size ≤5 cm accompanied by mVI. These data are in agreement with previous studies, which also showed that adjuvant TACE significantly improved postoperative prognosis in patients with multiple tumors [30], poor differentiation [31], or portal vein tumor thrombus [12], [13], [14], [32]. Generally, many HCC recurrences are likely to be related to minimal residual tumors that may not have been detected before or during surgery and micro metastases that may have been shed from tumor masses removed during operation. Adjuvant TACE could therefore decrease the recurrence risk with chemotherapy drugs and block the blood supply of “residual lesions” [33]. In fact, a recent study also suggested a favorable effect of three cycles of adjuvant TACE on the prognosis in HCC patients with mVI [34]. However, the authors did not elaborate on whether the treatment effect was consistent in patients with different characteristics. In addition, as the authors are concerned, selection bias is a major drawback for this kind of retrospective study. In the current study, we used PSM to balance significant differences in some baseline characteristics and stratified analysis to improve the conclusion. Finally, we found one cycle can achieve similar treatment effect with less liver damage and fewer medical expenses in those patients with tumor ≤5 cm, not only in the whole cohort but also in the matched cohort.
With the advantage of interaction analyses, we also suggested that postoperative adjuvant TACE failed to provide long‐term advantages for patients with tumor size >5 cm accompanied by mVI. This result was robust when we ruled out various potential confounding factors by corresponding Cox multivariate regression models in the crude and matched cohorts. Inconsistent with our findings, a few studies showed adjuvant TACE benefit in patients with large tumors [33], [35]. Several factors may explain the differences between the results in the present study and those in previous trials involving patients without mVI. First, for large tumors with mVI, they are likely to have more unrecognized microscopic metastases. Because angiogenic factors peak after TACE [36], some of the micrometastases that have not been eliminated may also be potentially stimulated. Consistently, another study also suggested that TACE is not beneficial for tumors larger than 5 cm [37]. Second, the regimen of adjuvant TACE varied, and questions concerning the exact choice, optimum timing, and preferred cycles remained unanswered. We performed adjuvant TACE only once at 4–6 weeks after surgery to minimize the adverse effects of this procedure in depressing the host immunity and damaging liver function [38]. Some studies also achieved satisfactory results in patients with [34] or without [14], [39], [40], [41] mVI by repeating this treatment twice or more. Recently, a meta‐analysis, including 3,325 patients, demonstrated that patients' prognosis was not improved by repeated courses [35]. For the adjuvant TACE, the absence of direct evidence of residual tumors contributes to inconsistent outcomes by bringing diverse effects on tumor cells, microenvironment, and immune surveillance [42], [43]. Based on these conflicting and uncertain results, the benefit of increasing the frequency of adjuvant TACE should be interpreted with caution. Instead of increasing frequency, exploring new indicators or adopting other adjuvant treatments may be beneficial for patients with tumor size >5 cm accompanied by mVI, which needs the validation of more prospective clinical trials.
In clinical practice, adjuvant therapy in HCC represents an area of high unmet medical need, and attempts to address this need have not proved successful. Studies of potential adjuvant treatment modalities, such as sorafenib [44], interferon alpha [45], vitamin K2 [46], I‐131‐Lipiodol [47], and systemic chemotherapy [48], have been inconclusive in terms of efficacy and safety or have not been supported with a high level of evidence. Thus, international guidelines do not recommend any of them as a universal treatment after potentially curative resection but recommend that larger trials with lower risk of systematic error be undertaken. In this large observational study, tumor size can confound and complicate the clinical prognosis, contributing to adjuvant TACE in this setting being controversial. As more researchers suggested that each postoperative therapy has its own indication, further refinement is necessary to identify the subset of patients that is most likely to benefit from each adjuvant treatment. Moving forward, there needs to be an emphasis on validating whether these adjuvant regimens will bring benefits for patients with HCC larger than 5 cm accompanied by mVI.
The nature of this retrospective study remains an issue in this type of analysis and might attenuate effect estimates for adjuvant TACE. Therefore, we used PSM to eliminate the heterogeneity between the two groups. In addition, a consensus is urgently needed regarding the definition of mVI [49], [50]. Although all pathological specimens were reviewed by two pathologists to reduce interobserver variability, intraobserver variability may still exist because this analysis was based on data from a single institution. Finally, the different combination of antitumor regimens, including anthracycline, platinum compounds, mitomycin C, and 5‐fluorouracil during TACE, demonstrated similar survival benefits [51], [52]. However, whether the results are applicable in adjuvant TACE needs further exploration.
Conclusion
In the presence of mVI, which is an accepted prognostic factor of HCC, postoperative adjuvant TACE reduces the risk of tumor recurrence and death for HCC less than 5 cm. However, failure of long‐term advantages for patients with tumor size larger than 5 cm may call for more optimized application of this regimen.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This work was supported in part by Program of Shanghai Subject Chief Scientist (16XD1400800) and the National Natural Science Foundation of China (8157301, 81802893, 81502487).
Contributed equally
Author Contributions
Conception/design: Jian Zhou, Jia Fan, Qinghai Ye
Provision of study material or patients: Bo Zhang, Binghai Zhou, Wentao Zhang, Jian Zhou, Jia Fan
Collection and/or assembly of data: Shuang Liu, Hui Li, Lei Guo
Data analysis and interpretation: Shuang Liu, Hui Li, Qinghai Ye
Manuscript writing: Shuang Liu, Hui Li, Qinghai Ye
Final approval of manuscript: Shuang Liu, Hui Li, Lei Guo, Qinghai Ye
Disclosures
The authors indicated no financial relationships.
References
- 1.Jonas S, Bechstein WO, Steinmuller T et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology 2001;33:1080–1086. [DOI] [PubMed] [Google Scholar]
- 2.Lim KC, Chow PK, Allen JC et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg 2011;254:108–113. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology 2011;53:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egner JR. AJCC cancer staging manual. JAMA 2010;304:1726–1727. [Google Scholar]
- 5.Shah SA, Greig PD, Gallinger S et al. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg 2006;202:275–283. [DOI] [PubMed] [Google Scholar]
- 6.Sumie S, Kuromatsu R, Okuda K et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol 2008;15:1375–1382. [DOI] [PubMed] [Google Scholar]
- 7.Zhong C, Guo RP, Li JQ et al. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for stage III A hepatocellular carcinoma. J Cancer Res Clin Oncol 2009;135:1437–1445. [DOI] [PubMed] [Google Scholar]
- 8.Xi T, Lai EC, Min AR et al. Adjuvant transarterial chemoembolization after curative resection of hepatocellular carcinoma: A non‐randomized comparative study. Hepatogastroenterology 2012;59:1198–1203. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Wen TF, Yan LN et al. Liver resection versus liver resection plus TACE for patients with hepatocellular carcinoma beyond Milan criteria. J Surg Res 2017;209:8–16. [DOI] [PubMed] [Google Scholar]
- 10.Izumi R, Shimizu K, Iyobe T et al. Postoperative adjuvant hepatic arterial infusion of lipiodol containing anticancer drugs in patients with hepatocellular carcinoma. Hepatology 1994;20:295–301. [PubMed] [Google Scholar]
- 11.Jiang JH, Guo Z, Lu HF et al. Adjuvant transarterial chemoembolization after curative resection of hepatocellular carcinoma: Propensity score analysis. World J Gastroenterol 2015;21:4627–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai T, Chen J, Xie ZB et al. The efficacy and safety of postoperative adjuvant transarterial embolization and radiotherapy in hepatocellular carcinoma patients with portal vein tumor thrombus. Onco Targets Ther 2016;9:3841–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan J, Zhou J, Wu ZQ et al. Efficacy of different treatment strategies for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol 2005;11:1215–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng BG, He Q, Li JP et al. Adjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Surg 2009;198:313–318. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Guo L, Li H et al. Postoperative adjuvant trans‐arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg Oncol 2018;25:2098–2104. [DOI] [PubMed] [Google Scholar]
- 16.Shah SA, Cleary SP, Wei AC et al. Recurrence after liver resection for hepatocellular carcinoma: Risk factors, treatment, and outcomes. Surgery 2007;141:330–339. [DOI] [PubMed] [Google Scholar]
- 17.Edmondson HA, Steiner PE. Primary carcinoma of the liver. A study of 100 cases among 48,900 necropsies. Cancer 1954;7:462–503. [DOI] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun HC, Zhang W, Qin LX et al. Positive serum hepatitis B e antigen is associated with higher risk of early recurrence and poorer survival in patients after curative resection of hepatitis B‐related hepatocellular carcinoma. J Hepatol 2007;47:684–690. [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Li X, Li H et al. Is the Hong Kong Liver Cancer staging system the best guide for hepatitis B virus‐related hepatocellular carcinoma patients with multiple tumors? Oncotarget 2016;7:51598–51607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S, Li X, Li H et al. Longer duration of the Pringle maneuver is associated with hepatocellular carcinoma recurrence following curative resection. J Surg Oncol 2016;114:112–118. [DOI] [PubMed] [Google Scholar]
- 22.Mazzaferro V, Regalia E, Doci R et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–700. [DOI] [PubMed] [Google Scholar]
- 23.Couinaud C. The anatomy of the liver [in French]. Ann Ital Chir 1992;63:693–697. [PubMed] [Google Scholar]
- 24.Roayaie S, Obeidat K, Sposito C et al. Resection of hepatocellular cancer ≤2 cm: Results from two Western centers. Hepatology 2013;57:1426–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unek T, Karademir S, Arslan NC et al. Comparison of Milan and UCSF criteria for liver transplantation to treat hepatocellular carcinoma. World J Gastroenterol 2011;17:4206–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sumie S, Nakashima O, Okuda K et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol 2014;21:1002–1009. [DOI] [PubMed] [Google Scholar]
- 27.Shindoh J, Andreou A, Aloia TA et al. Microvascular invasion does not predict long‐term survival in hepatocellular carcinoma up to 2 cm: Reappraisal of the staging system for solitary tumors. Ann Surg Oncol 2013;20:1223‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vetter D, Wenger JJ, Bergier JM et al. Transcatheter oily chemoembolization in the management of advanced hepatocellular carcinoma in cirrhosis: Results of a Western comparative study in 60 patients. Hepatology 1991;13:427–433. [PubMed] [Google Scholar]
- 29.Takayasu K, Wakao F, Moriyama N et al. Postresection recurrence of hepatocellular carcinoma treated by arterial embolization: Analysis of prognostic factors. Hepatology 1992;16:906–911. [DOI] [PubMed] [Google Scholar]
- 30.Dong ZR, Zhang PF, Wang CH et al. Postoperative adjuvant transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond the Milan criteria: A retrospective analysis. Am J Cancer Res 2015;5:450–457. [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Z, Du G, Pang Y et al. Adjuvant transarterial chemoembolization after radical resection contributed to the outcomes of hepatocellular carcinoma patients with high‐risk factors. Medicine 2017;96:e7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Q, Wang J, Sun Y et al. Efficacy of postoperative transarterial chemoembolization and portal vein chemotherapy for patients with hepatocellular carcinoma complicated by portal vein tumor thrombosis—A randomized study. World J Surg 2006;30:2004–2011. [DOI] [PubMed] [Google Scholar]
- 33.Sun JJ, Wang K, Zhang CZ et al. Postoperative adjuvant transcatheter arterial chemoembolization after r0 hepatectomy improves outcomes of patients who have hepatocellular carcinoma with microvascular invasion. Ann Surg Oncol 2016;23:1344–1351. [DOI] [PubMed] [Google Scholar]
- 34.Ye JZ, Chen JZ, Li ZH et al. Efficacy of postoperative adjuvant transcatheter arterial chemoembolization in hepatocellular carcinoma patients with microvascular invasion. World J Gastroenterol 2017;23:7415–7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao M, Zhu Z, Wang H et al. Adjuvant transarterial chemoembolization for patients after curative resection of hepatocellular carcinoma: A meta‐analysis. Scand J Gastroenterol 2017;52:624–634. [DOI] [PubMed] [Google Scholar]
- 36.Poon RT, Lau C, Yu WC et al. High serum levels of vascular endothelial growth factor predict poor response to transarterial chemoembolization in hepatocellular carcinoma: A prospective study. Oncol Rep 2004;11:1077–1084. [PubMed] [Google Scholar]
- 37.Tong Y, Li Z, Liang Y et al. Postoperative adjuvant TACE for patients of hepatocellular carcinoma in AJCC stage I: Friend or foe? A propensity score analysis. Oncotarget 2017;8:26671–26678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z, Ren Z, Chen Y et al. Adjuvant transarterial chemoembolization for HBV‐related hepatocellular carcinoma after resection: A randomized controlled study. Clin Cancer Res 2018;24:2074–2081. [DOI] [PubMed] [Google Scholar]
- 39.Li F, Guo Z, Zhang Y et al. Postoperative adjuvant arterial chemoembolization improves the survival of hepatitis B virus‐related hepatocellular carcinoma: A retrospective control study. Ir J Med Sci 2015;184:753–759. [DOI] [PubMed] [Google Scholar]
- 40.Li Q, Wang J, Sun Y et al. Postoperative transhepatic arterial chemoembolization and portal vein chemotherapy for patients with hepatocellular carcinoma: A randomized study with 131 cases. Dig Surg 2006;23:235–240. [DOI] [PubMed] [Google Scholar]
- 41.Liu C, Sun L, Xu J et al. Clinical efficacy of postoperative adjuvant transcatheter arterial chemoembolization on hepatocellular carcinoma. World J Surg Oncol 2016;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai EC, Lo CM, Fan ST et al. Postoperative adjuvant chemotherapy after curative resection of hepatocellular carcinoma: A randomized controlled trial. Arch Surg 1998;133:183–188. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Feng GS, Zheng CS et al. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol 2004;10:2878–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruix J, Takayama T, Mazzaferro V et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): A phase 3, randomised, double‐blind, placebo‐controlled trial. Lancet Oncol 2015;16:1344–1354. [DOI] [PubMed] [Google Scholar]
- 45.Sun HC, Tang ZY, Wang L et al. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV‐related hepatocellular carcinoma: A randomized clinical trial. J Cancer Res Clin Oncol 2006;132:458–465. [DOI] [PubMed] [Google Scholar]
- 46.Haruhiko Y, Yasushi S, Masatoshi K et al. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology 2011;54:532–540. [DOI] [PubMed] [Google Scholar]
- 47.Chung AY, Ooi LL, Machin D et al. Adjuvant hepatic intra‐arterial iodine‐131‐lipiodol following curative resection of hepatocellular carcinoma: A prospective randomized trial. World J Surg 2013;37:1356–1361. [DOI] [PubMed] [Google Scholar]
- 48.Ono T, Yamanoi A, Nazmy El Assal O et al. Adjuvant chemotherapy after resection of hepatocellular carcinoma causes deterioration of long‐term prognosis in cirrhotic patients: Metaanalysis of three randomized controlled trials. Cancer 2001;91:2378–2385. [PubMed] [Google Scholar]
- 49.Sparrelid E, Del Chiaro M. Microvascular invasion in hepatitis B virus‐related hepatocellular carcinoma: Another step toward preoperative evaluation? JAMA Surg 2016;151:364. [DOI] [PubMed] [Google Scholar]
- 50.Fan L, Mac MT, Frishberg DP et al. Interobserver and intraobserver variability in evaluating vascular invasion in hepatocellular carcinoma. J Gastroenterol Hepatol 2010;25:1556–1561. [DOI] [PubMed] [Google Scholar]
- 51.Reidy DL, Schwartz JD. Therapy for unresectable hepatocellular carcinoma: Review of the randomized clinical trials‐I: Hepatic arterial embolization and embolization‐based therapies in unresectable hepatocellular carcinoma. Anticancer Drugs 2004;15:427–437. [DOI] [PubMed] [Google Scholar]
- 52.Sahara S, Kawai N, Sato M et al. Prospective evaluation of transcatheter arterial chemoembolization (TACE) with multiple anti‐cancer drugs (epirubicin, cisplatin, mitomycin c, 5‐fluorouracil) compared with TACE with epirubicin for treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol 2012;35:1363–1371. [DOI] [PubMed] [Google Scholar]