Abstract
While formaldehyde (HCHO) was likely generated in Earth's prebiotic atmosphere by ultraviolet light, electrical discharge, and/or volcano-created lightning, HCHO could not have accumulated in substantial amounts in prebiotic environments, including those needed for prebiotic processes that generate nucleosidic carbohydrates. HCHO at high concentrations in alkaline solutions self-reacts in the Cannizzaro reaction to give methanol and formate, neither having prebiotic value. Here, we explore the possibility that volcanic sulfur dioxide (SO2) might have generated a reservoir for Hadean HCHO by a reversible reaction with HCHO to give hydroxymethanesulfonate (HMS). We show that salts of HMS are stable as solids at 90°C and do not react with themselves in solution, even at high (>8 M) concentrations. This makes them effective stores of HCHO, since the reverse reaction slowly delivers HCHO back into an environment where it can participate in prebiotically useful reactions. Specifically, we show that in alkaline borate solutions, HCHO derived from HMS allows formation of borate-stabilized carbohydrates as effectively as free HCHO, without losing material to Cannizzaro products. Further, we show that SO2 can perform similar roles for glycolaldehyde and glyceraldehyde, two intrinsically unstable carbohydrates that are needed by various models as precursors for RNA building blocks. Zircons from the Hadean show that the Hadean mantle likely provided volcanic SO2 at rates at least as great as the rates of atmospheric HCHO generation, making the formation of Hadean HMS essentially unavoidable. Thus, hydroxymethylsulfonate adducts of formaldehyde, glycolaldehyde, and glyceraldehyde, including the less soluble barium, strontium, and calcium salts, are likely candidates for prebiotically useful organic minerals on early Earth.
Key Words: Borate, Organic minerals, Formaldehyde, Sulfur dioxide, Cannizzaro reaction, Hydroxymethanesulfonate, Origins of life, Prebiotic chemistry
1. Introduction
A recent trend in efforts toward understanding the origin of life seeks models that incorporate geochemical and geophysical features of the early Earth environment into reaction schemes that involve organic molecules (Benner et al., 2010). For example, minerals on early Earth might have acted as catalysts for organic transformations in prebiotic reactions, as potential reactants, and as species that controlled organic reactivity. An example of the first are clays that catalyze organic reactions (Ferris, 2005). For the second, the phosphoborate mineral lüneburgite performs controlled phosphorylation of nucleosides (Kim et al., 2016). For the last, borate minerals were likely useful to control prebiotic reaction sequences to make carbohydrates (Ricardo et al., 2004; Scorei and Cimpoiasu, 2006; Amaral et al., 2008; Šponer, 2008; Cossetti et al., 2010; Pepi et al., 2010; Kim et al., 2011; Kopetzki and Antonietti, 2011; Saladino et al., 2011; Delidovich et al., 2013; Furukawa et al., 2013; da Silva and Holm, 2014).
Further, prebiotic chemistry could have benefitted from organic minerals, solids having defined compositions that contain reduced carbon atoms (as opposed to carbonates). These are scarce on modern Earth, as only the least soluble and most refractory organic minerals (e.g., mellite, Benner et al., 2000) escape rapid consumption on a modern biology-rich Earth. However, on a prebiotic Earth free of life, organic minerals unknown on modern Earth may have been reservoirs for organic species that would otherwise have been too reactive to accumulate in sufficient amounts to be useful for prebiotic synthesis.
Any discussion of prebiotic minerals creates a problem since a mineral cannot be simply a substance of defined composition (i.e., a chemical compound) but must also be naturally occurring. To prevent the proliferation of mineral names, the International Mineralogical Association carefully reviews claims of a mineralogical discovery, resolves issues of priority, and certifies the selection of a name. None of this is possible, of course, when discussing prebiotic minerals that can exist on only an abiological world but are rapidly eaten on a biology-rich planet like modern Earth. To reflect this fact, “mineral” is placed within quotation marks when it refers to prebiotic minerals.
For example, Keefe, Miller, Sutherland, and others have suggested that ferrocyanide species might have been prebiotic “mineral” species able to stabilize cyanide, a prebiotically valuable species that self-reacts. This organic “mineral” might have provided nitrogen and carbon resources for prebiotic chemistry (Keefe and Miller, 1995; Patel et al., 2015). Likewise, the Benner group suggested that a branch pentose-borate complex might have been an organic “mineral” reservoir of organic species having the same molecular formula as ribose (Ricardo et al., 2004; Li et al., 2005; Benner et al., 2010, 2012; Kim et al., 2011; Neveu et al., 2013; Furukawa et al., 2015). Indeed, borate-ribose complexes have been productively used in the formation of nucleosides (Becker et al., 2016).
Atmospheric components must, however, be considered as we add geochemistry to models of prebiotic Earth. The Hadean almost certainly had more volcanic activity than today, as radioactive decay and impact energy then was greater than today. Studies of Hadean zircons show that after formation of its iron core, Earth likely had a mantle as oxidizing as today, above the fayalite-magnetite-quartz (FMQ) redox buffer (Trail et al., 2011). This implies that Hadean volcanoes generated large amounts of sulfur dioxide (SO2), which in water gives sulfite or bisulfite (HSO3-, pKa ≈ 6.97) anions. SO2 can also deliver elemental sulfur (S8) and sulfate (SO4) by disproportionating in hydrothermal fluids (Kusakabe et al., 2000).
Today, sulfite is oxidized to sulfate in our dioxygen-rich atmosphere. However, a more reducing Hadean atmosphere lacking O2 would have allowed bisulfite and its minerals (e.g., hannebachite (CaSO3)2 ½ H2O) (Rai et al., 1991) to accumulate (Marion et al., 2013).
Bisulfite reacts with carbonyl-containing compounds to form stable adducts, including with formaldehyde (HCHO), which was likely generated in Earth's prebiotic atmosphere containing water vapor and CO2 by the action of ultraviolet light, silent and nonsilent electrical discharge, and volcanic lightning (Cleaves, 2008). HCHO and SO2 react to give hydroxymethanesulfonate (HMS = HOCH2SO3−) as an addition product. On modern Earth, HMS is formed in aerosols whenever HCHO and SO2 come together (Graedel and Weschler, 1981). The addition is reversible, with an equilibrium constant at pH values above the pKa of bisulfite ([SO3−]tot·[HCHO]tot/[HOCH2SO3−] ≈ 2 × 10−4 M) (Sorensen and Andersen, 1970; Boyce and Hoffmann, 1984; Dong and Dasgupta, 1986; Kok et al., 1986). The unimolecular rate constant generating HCHO from HMS at high pH is ∼43 s−1 (Sorensen and Andersen, 1970).
Formaldehyde (HCHO) is a key prebiotic precursor for carbohydrates, perhaps best known in the “formose reaction” (Butlerov, 1861; Kim et al., 2011; Appayee and Breslow, 2014). Unfortunately, the formose process requires high concentrations of HCHO under alkaline conditions (pH 10–11) to get started. Under these conditions, HCHO reacts with itself to produce methanol and formate in a process known as the Cannizzaro reaction (Cannizzaro, 1853). Neither of these products has prebiotic value. Further, once HCHO is exhausted, carbohydrates can suffer intercomponent reactions to give complex mixtures that also lack clear prebiotic utility. Particularly easily destroyed are glycolaldehyde and glyceraldehyde, compounds that some models for prebiotic nucleoside synthesis require in large amounts (Powner et al., 2009).
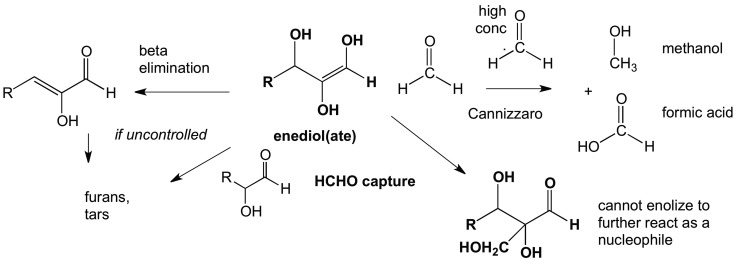
Formaldehyde (HCHO) is especially useful in prebiotic chemistry because of its ability to trap enediolates of carbonyl-containing (C = O) compounds, preventing beta elimination, hydride shift (Appayee and Breslow, 2014), reaction of enediolates with higher carbohydrates, and other processes that give “tars.” At ∼10 mM concentrations, HCHO is sufficiently reactive as electrophile to compete with these processes (Fig. 1), even though it exists largely in a hydrated form. For example, in a quantitative study, Kim et al. (2011) showed that low concentrations of HCHO would react as the preferred electrophile with any enediol formed by any aldose or ketose in formose-like processes, even though in aqueous solution it is predominately in its hydrated form (H2C(OH)2). Only when HCHO was consumed did the “yellowing” characteristic of formose processes begin (Ricardo et al., 2006).
FIG. 1.
Formaldehyde (HCHO) can capture enediol(ate)s before they react to give complex mixtures of unproductive species. However, at high concentrations, HCHO disproportionates in a bimolecular Cannizzaro reaction to yield unproductive methanol and formate. Both problems can be mitigated if an organic mineral can be found that slowly bleeds HCHO into a prebiotic reaction mixture.
Other carbonyl-containing processes are likewise in need of stabilization. For example, glycolaldehyde was undoubtedly produced in the Hadean atmosphere as well, albeit at lower concentrations (Löb, 1913). Glycolaldehyde can act catalytically to fix formaldehyde. This avoids the first, extremely slow, step in the formose process, the (presumed) direct dimerization of HCHO to form glycolaldehyde, something that requires high concentrations of HCHO. Glycolaldehyde reacts readily with itself to form tetroses (Kim et al., 2011). However, if HCHO is present, the enediolate of glycolaldehyde is productively captured to form glyceraldehyde. Likewise, if HCHO is present, the enediolate of glyceraldehyde also can escape tar formation. If borate is present, its binding to adjacent hydroxyl groups (1,2-diols) further controls downstream reactions, diminishing “tar” formation.
These considerations provide relevance to the work reported here. Simple calculations suggest that the amount of Hadean volcanic SO2 matched or exceeded the amount of Hadean atmospheric HCHO (Pinto et al., 1980) and, certainly, the amounts of Hadean glycolaldehyde and glyceraldehyde. Thus, the conclusion is inescapable that HCHO generated in the Hadean atmosphere was largely captured on the surface by SO2 to give HMS.
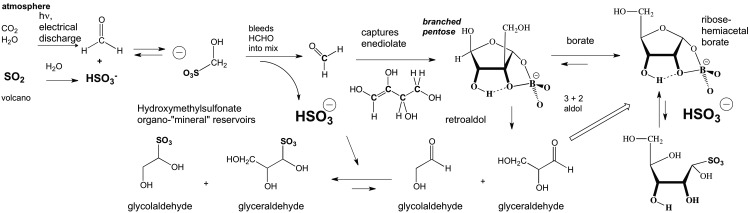
This raises questions. First, we might ask whether this is “bad” for prebiotic chemistry, because it removes HCHO needed in prebiotic schemes. Or is it “good,” because HMS can accumulate and, because of the reversibility of the reaction that forms it, provide a continuous source of HCHO, which would otherwise be unavailable for prebiotic chemistry (Fig. 2)? Is it possible that the reverse dissociation to give HCHO and bisulfite would give HCHO in an aquifer at high enough concentrations to control undesirable enediolate reactivities, but not so high as to waste material in the Cannizzaro reaction, whose rate scales with the square of HCHO concentration? Last, can bisulfite also provide stable reservoirs of higher carbohydrates, such as glycolaldehyde and glyceraldehyde?
FIG. 2.
Possible prebiotic reactions involving volcanic SO2, HCHO, and other lower carbohydrates. Note how the reaction with ribose involves competition with ring closed forms, here the furanose. Therefore, the equilibrium concentration favors the bisulfite addition product with ribose considerably less than with HCHO, glycolaldehyde, or glyceraldehyde. The same is the case with ketoses. The stereochemistry shown is arbitrary.
We report here a study of this reaction manifold using the sodium salt of 13C-labeled HMS. Dihydroxyacetone (DHA) was used as a model “lower” carbohydrate in sodium carbonate-bicarbonate buffers at pH 10.5, reasonable from serpentinizing basalts. We show that HMS, by slowly dissociating to give free HCHO, can capture the enediolate of DHA before it reacts with itself to generate 4-hydroxymethyl-1,3,4,5-tetrahydroxy pentane-2-one. We then show how bisulfite might react reversibly with other unstable carbohydrates to provide reservoirs useful in prebiotic synthesis.
2. Materials and Experimental Methods
2.1. Materials
Materials used were deuterium oxide (D2O; Acros), chloroform-D (CDCl3; Cambridge Isotope Laboratories), sodium deuteroxide (NaOD; Acros Organic), hydroxylmethyl sulfonate (CH3NaO4S; Aldrich), dihydroxyacetone (C3H6O3; Aldrich), glycolaldehyde (C2H4O2; 0.0476 M, Omicron, C-13 labeled), sodium bisulfite (NaHSO3; Fisher), paraformaldehyde (HO-(CH2O)n H, C-13 labeled; Sigma), erythrulose (C4H8O4; Sigma-Aldrich), glyceraldehyde (C3H6O3; Sigma), boric acid (Sigma-Aldrich), and sodium carbonate (Fisher).
2.2. Experimental methods
2.2.1. Synthesis of 13C-labeled sodium hydroxymethanesulfonate
13C-Labeled paraformaldehyde (30 mg, 1 mmol, Sigma) was treated with sodium bisulfite (1 mmol, 104 mg, Fisher) in deionized water (1 mL) at 65°C. The powder did not immediately dissolve, so the pH of the mixture was slowly raised by dropwise adding 1 M NaOH (Fisher) until the solution became clear. The mixture was then lyophilized to obtain the sodium salt of HMS, which gave a 13C NMR signal at 74.51 ppm (CH3OH standard at 49.5 ppm, 75 MHz, room temperature). Neither methanol nor formic acid, the Cannizzaro products, were seen.
2.2.2. Reaction of 13C-labeled sodium hydroxymethanesulfonate with dihydroxyacetone
A sample of the DHA (0.5 mmol), which gives the same reactive enediolate as glyceraldehyde, was dissolved in borate-carbonate buffer (1.1 M Na2CO3, 0.278 M H3BO3, 1 mL D2O, pH ≈10.5, all at 25°C) with 13C-labeled HMS (1.0 mmol). A parallel reaction was run without HMS but with an equivalent amount of 13C-labeled formaldehyde (1.0 mmol). The reaction mixture samples were incubated at 65°C and room temperature, respectively, for 24 h. The samples were then directly analyzed by 13C NMR spectroscopy at room temperature.
2.2.3. Parallel large-scale reaction of unlabeled HMS with DHA
A mixture of DHA (0.90 g, 10 mmol) and unlabeled HMS (4.10 g, 30 mol, 3 fold excess) was dissolved in degassed borate-carbonate buffer (200 mL H2O, 1.1 M Na2CO3, 0.278 M H3BO3, pH ≈ 10.5, all at 25°C). This mixture was then incubated with stirring at 65°C for 2 days and at room temperature for 10 days under an N2 atmosphere.
To prepare for proton NMR analysis, the mixture was neutralized by ion exchange resin (Dowex-50, H+ form, Acros), which released the carbonate as carbon dioxide. The resin was then removed by filtration and the filtrate lyophilized. The borate ions were then removed by addition of CH3OH (100 mL), followed by evaporation of the trimethylborate formed by rotary evaporation to give a reddish-brown solid. The samples were dried further overnight under high vacuum and analyzed by NMR.
2.2.4. Derivatization of the carbohydrate products
For further analysis, portions of the residues were dissolved in pyridine (100 mL) containing dimethylaminopyridine (250 mg), treated with acetic anhydride (15 mL), and incubated at room temperature (1 day). Solvents were removed by rotary evaporation, the residue was treated with aqueous 1 M HCl at room temperature, and water was added. Acetates were extracted with CH2Cl2, and the aqueous phase containing pyridinium hydrochloride was discarded. The CH2Cl2 was removed by rotary evaporation to give a reddish residue. The products were separated by silica gel column chromatography (ethyl acetate:hexane gradient = 1:2 to 2:1; Fluka silica gel, 230–400 mesh), and the effluent was collected in fractions. Each fraction was evaporated, and the residue was weighed to estimate yields and characterized by 1H NMR. To identify each fraction, standard materials were used as comparison.
2.2.5. Mass spectral analysis
The products of the reactions between unlabeled HMS and unlabeled HCHO were analyzed before acetate derivatization (but after removing carbonate and borate) by mass spectrometry (Agilent 6220 ESI-TOF with Agilent 1100 LC; Mass Spectrometry Services, University of Florida) using electrospray ionization (ESI) and time-of-flight (TOF) analysis. The samples were prepared as a solution of acidified methanol (0.1% formic acid); injecting of 5 μL of sample into the electrospray source was done at a rate of 200 mL/min. ESI settings were as follows: voltage 4000 V, source temperature 350°C, and cone voltage of 60 V. The TOF analyzer was scanned over m/z 30–3200 with a 2 s integration time (data not shown).
3. Results
3.1. Comparing the 13C spectra of the product manifolds obtained by reaction of dihydroxyacetone with 13C-formaldehyde (HCHO) directly, or with (H13CHO) delivered in situ from 13C-hydroxymethanesulfonate
Nuclear magnetic resonance was first used to test the hypothesis that HMS can replace formaldehyde (HCHO) in reactions that may simulate the formation of carbohydrates in borate-containing effluents emerging from serpentinizing igneous basalts. This reaction has previously been studied in quantitative detail (Kim et al., 2011; Neveu et al., 2013), where it was shown that DHA or glyceraldehyde enolize in sodium carbonate-borate buffer (pH ≈ 10.5) to give a common enediolate. This enediolate reacts with HCHO to give erythrulose, which, under guidance from borate, enolizes to give another enediolate. This reacts with a second molecule of HCHO to give either a branched tetrose (2-hydroxymethyl-2,3 dihydroxypropanal) or erythrulose. The branched tetrose has no enolizable protons but suffers a crossed-Cannizzaro reduction with HCHO to give 2-hydroxymethyl-1,2,3 trihydroxypropane, a dead-end product.
Erythrulose, as the principal product, can enolize, however. The resulting enediolate was shown by Kim et al. (2011) to react at its less hindered center to give a mixture of erythro and threo stereoisomers of branched pentoses (C5). The 13C NMR spectra of these species arising from H13CHO is characteristic of the product ratio, especially in the collection of NMR signals at 60–65 ppm (MeOH standard at 49.5).
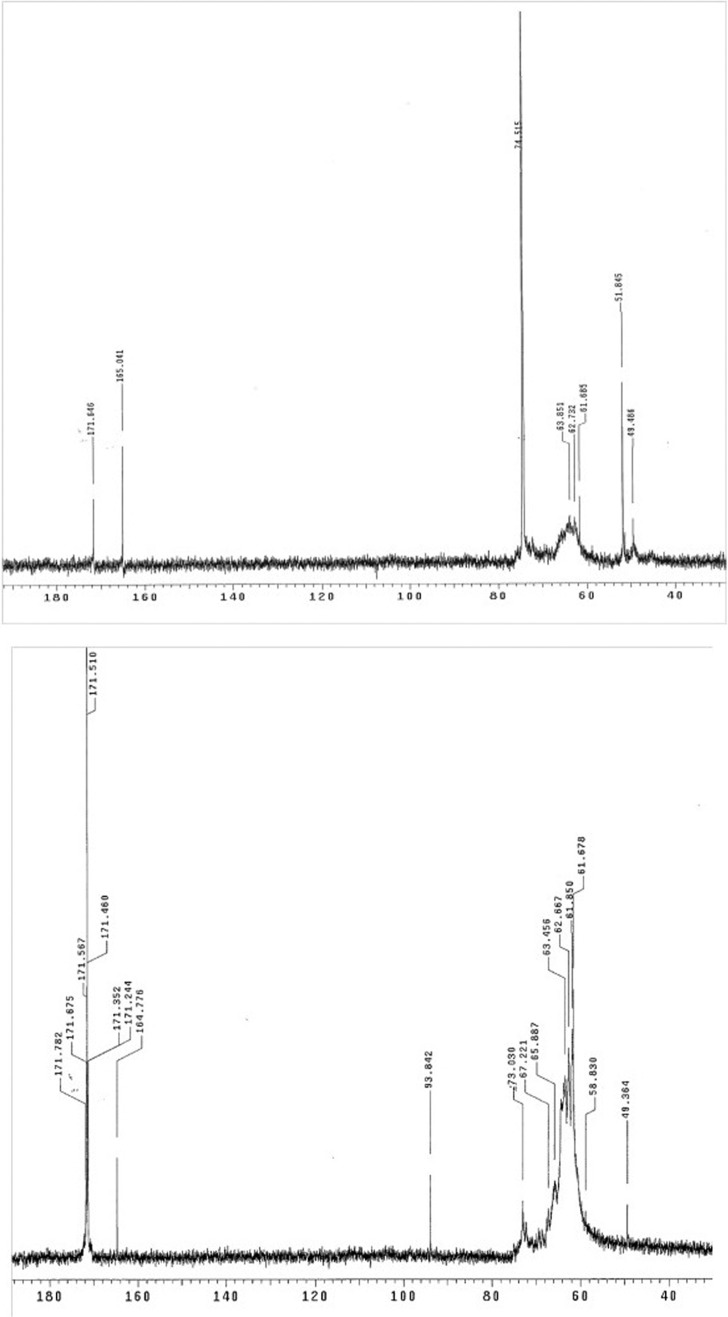
The Kim et al. (2011) experiment was repeated, and repeated in parallel where H13CHO was replaced by 13C-HMS. The NMR spectra are strikingly similar (Fig. 3). This indicates that H13CHO added to solution directly can be replaced by H13CHO “bled” into the medium by the unimolecular decomposition of HMS. The similarity of the manifolds of 13C signals showed that the product ratios were quantitatively identical, regardless of the source of the H13CHO. Further, unlike when H13CHO was used, very few Cannizzaro products (methanol at 49.5 ppm and formic acid at ∼171 ppm) were seen. This suggested that the concentration of H13CHO bled from HMS was never high enough to allow this reaction (bimolecular in HCHO) to occur to a substantial degree.
FIG. 3.
Comparison by 13C NMR spectroscopy of the reaction of DHA with H13CHO “bled” into the reaction mixture from 13C-labeled HMS (top, signal at ∼74.5) or H13CHO added directly (bottom). 13C-HMS resonates at 74.2 ppm. Reactions were run in NaCO3 (1.1 M all at 65°C) H3BO3 (0.278 M) buffer at pH 10.4. Note that the manifold of peaks arising from 13CH2OH units in the branched pentoses at 60–65 ppm are essentially the same. Also note the presence of substantially more HCOOH (171 ppm, ionized to give formate) from the Cannizzaro reaction when free H13CHO is added directly. MeOH (49.5) was added as an internal standard. The pH-dependent signal from carbonate buffer is at ∼165 ppm.
3.2. Stability of sodium hydroxymethanesulfonate
To estimate the thermal stability of salts of HMS, a sample of sodium HMS (6.55 g) was weighed and then heated at 90°C. The weights of the material were measured over a 24 h period. After 24 h, ∼3.5% loss of mass was observed, entirely due to dehydration. Thus, this form of the organic “mineral” was stable to thermal degradation under these conditions.
3.3. Solubility of hydroxymethanesulfonate minerals
Aliquots (1.0 mL) of a saturated solution of sodium HMS (∼0.95 g/mL) were treated with aliquots (0.25 mL) of 1 M solutions of the chlorides of barium, strontium, calcium, and magnesium as their chloride salts (all at 25°C). Precipitates were observed for the first three divalent cations, but not with magnesium. The barium HMS was the least soluble of the three. A sample of this was prepared, 1 g was suspended in pure water (at 25°C), a 200 μL sample was withdrawn, and the residue was weighed to give a solubility of 386 g/L. In parallel, no precipitation was seen with any transition metal tested, including manganese, iron, cobalt, nickel, copper, or zinc, all as divalent cations.
3.4. Reaction of sulfite with glycolaldehyde, glyceraldehyde, dihydroxyacetone, and ribose
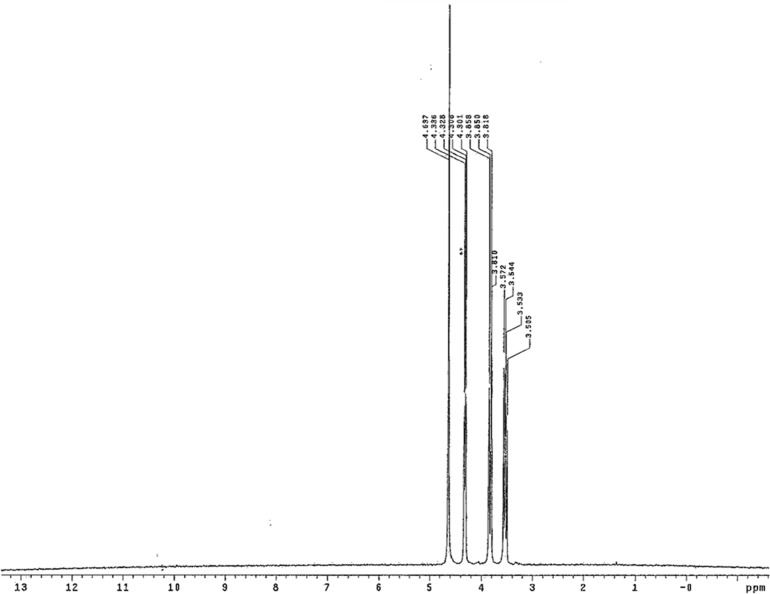
We then determined whether other prebiotically useful carbonyl compounds that are unstable, especially with respect to self-reaction, might be stabilized as bisulfite addition products. The 1H NMR spectrum of a 1:1 mixture of glycolaldehyde and sodium bisulfite was seen to contain exclusively the bisulfite adduct (Fig. 4, all at 25°C). These data indicate an equilibrium constant strongly favoring adduct formation. The same conclusion was compelled by the 13C NMR spectrum of a 1:1 mixture of 13C1-labeled glyceraldehyde and sodium bisulfite in D2O (Fig. 5).
FIG. 4.
1H NMR spectra of 1:1 mixture of glycolaldehyde and sodium bisulfite, obtained in D2O at 25°C. Glycolaldehyde has proton resonances at 4.9 ppm (triplet, C1 of the hydrate) and 3.3 ppm (doublet, C2 of the hydrate, data not shown). Both are missing in the spectrum, which has new resonances at 4.3 and 3.85 ppm (C1, adduct, split by C2 proton, two diastereomers) and 3.5 ppm (C2). Notice also the coupling due to the new stereogenenic center. Absence of detectable amounts of glycolaldehyde suggests that adduct had consumed more than 99% of the substrate.
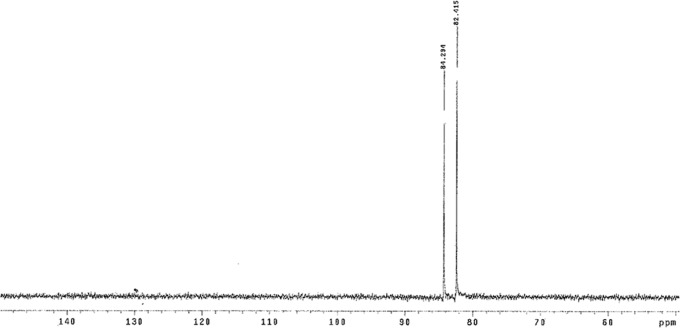
FIG. 5.
13C NMR spectra of a 1:1 mixture of 1-13C-labeled D,L-glyceraldehyde and sodium bisulfite, obtained in D2O at a pH of 4.76 at 25°C. The two resonances (84.3 and 82.4) are assigned to be the two diastereomeric adducts in a ratio of ∼47:53. Glyceraldehyde itself had a single resonance at 89.95 ppm (data not shown), which is assigned to the hydrated species. Again, undetectable of a resonance at 89.95 ppm suggests that adduct had consumed more than 99% of the substrate.
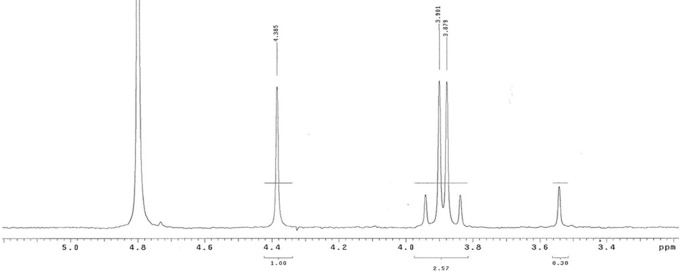
Dihydroxyacetone (DHA) also forms an adduct with bisulfite. Here, however, adduct formation was not complete with equal amounts of DHA and bisulfite (10 mM each). Instead, the ratio of the bisulfite adduct to the free DHA was approximately 2:1 (Fig. 6). This allowed ready calculation of an equilibrium constant of 1.6 × 10−3 M. This incomplete formation of the adduct is consistent with a model where steric hindrance opposes addition of nucleophiles to the central carbonyl group, and is reflected as well in the smaller amount of acetone hydrate compared to acetaldehyde hydrate in equilibrium.
FIG. 6.
1H NMR spectra of DHA plus sodium bisulfite (in a 1:1 mixture, 10 mM each), obtained in D2O at 25°C. DHA alone has a proton resonance of 4.38 (with water set at 4.8 ppm; the peak at 3.7 is the hydrate of DHA). Here, the presence of free and adducted DHA allows an equilibrium constant to be calculated, at ≈1.6 × 10-3M. The integral shows that the (2.57 = integral for total adduct protons, versus 1.3 = integral for total DHA proton, or 2:1 adduct:free DNA) yields an equilibrium constant [DHA][bisulfite]/[adduct] = 1.6 × 10−3 M.
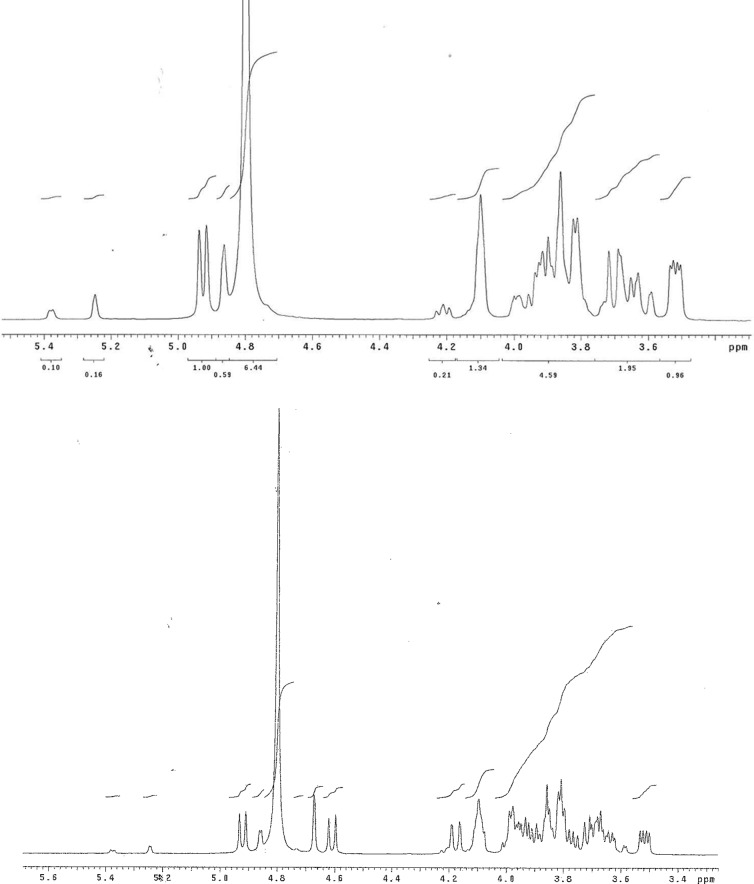
Finally, the ability of ribose to form a bisulfite adduct was estimated by 1H NMR in D2O containing bisulfite (10 mM) (Fig. 7). As with DHA, a substantial amount of the carbonyl compound remains. The precise amount was difficult to estimate, as ribose itself has four forms in equilibrium (alpha and beta forms of the furanose and pyranose), each giving different NMR signals. However, 1 × 10−3 M is not a bad estimate for the equilibrium constant for the addition of bisulfite to ribose. Here, although ribose nominally has an aldehyde, bisulfite addition must compete with cyclization to form the closed furanose and pyranose rings.
FIG. 7.
1H NMR spectra of ribose (top) and ribose plus sodium bisulfite (a 1:1 mixture, 10 mM each), obtained in D2O (set at 4.8 ppm) at 25°C. Attention is drawn to the peaks at 4 4.0–4.92, 5.25, and 5.38, which are the alpha and beta isomers of the furanose and pyranose forms. The new resonances at 4.6 (doublet) and 4.67 (broad) are the bisulfite addition products (C1). Note that these represent perhaps only 20–30%% of the total. The estimated equilibrium constant [ribose][bisulfite]/[adduct] ≈ 2 × 10−2 M.
4. Discussion
Subareal volcanism from today's Earth generates ca. 2 × 1011 mol/year of sulfur dioxide (SO2) (Andres and Kasgnoc, 1998). If we assume that the Hadean mantle had similar oxygen fugacity at the relevant time (Trail et al., 2011), this likely represents a lower limit on the rate of SO2 production during the period relevant to prebiotic synthesis. This is similar to the classical estimate of 1011mol/year for the annual Hadean production of HCHO (Pinto et al., 1980).
Thus, it seems reasonable to assume that most HCHO produced in the Hadean atmosphere ended up in the hydrosphere as HMS. The HMS may have been formed in aerosol droplets in the atmosphere itself, but certainly would have occurred in the hydrosphere, capturing HCHO and SO2 that rained into it. Only if the amount of Hadean volcanism is severely overestimated or the rate of Hadean HCHO formation severely underestimated should we expect free HCHO in large amounts to serve as a prebiotic reagent.
The results reported here show that these estimates do not preclude HCHO as a prebiotic precursor. Via its dissociation reaction, HMS is shown by these experiments to be suitable as a continuous source of HCHO at concentrations high enough to participate in various prebiotic processes, here, the borate-moderated formose process that generates 5-carbon carbohydrates from glycolaldehyde (Kim et al., 2011). Here, bleeding of HCHO into an aqueous environment is high enough to trap enediolates formed from carbohydrates produced at pH values likely to be obtained in serpentinizing rocks, preventing them from suffering a range of destructive reactions. The key result here is the experimental demonstration that the ratio of products in the borate-moderate formose reaction, judged by 13C labeling, is the same whether HCHO is added directly or is bled into the reaction via dissociation of HMS. These products are mainly erythro- and threo-branched pentoses (Kim et al., 2011).
As Orgel pointed out some time ago (Orgel, 2004), substantial concentrations of HCHO did not likely accumulate on a Hadean Earth in any case. At the alkaline conditions expected from serpentinization, high concentrations of HCHO are destroyed by the Cannizzaro reaction to give methanol and formate, both essentially useless as prebiotic precursors. The results reported here show that if HCHO is bled (slowly released) from a HMS reservoir, its steady-state concentration is insufficient to support the Cannizzaro reaction.
The effectiveness of enediolate trapping depends on the rate of formation of the enediolate compared to the rate of formation of HCHO by dissociation of HMS, which in turn depend on the relevant reaction rate constants and the concentrations of the species involved. The rate constant for cleavage of HMS is 43 s−1 (Sorensen and Andersen, 1970), at least 2 orders of magnitude higher than the rate constant for the enolization of glyceraldehyde at pH 10.5 (Kim et al., 2011), a reasonable pH for serpentinizing basalts. Thus, efficient capture of enediolates by HCHO generated from HMS is likely in a prebiotic scenario that incorporates both chemistry and geology.
Of course, the high water solubility of the sodium salt of HMS (∼1 g/mL at 25°C) means that it could accumulate in an arid desert environment only. However, the calcium salt, the strontium salt, and especially the barium salt of HMS are rather insoluble in water. Direct experiments showed that the barium bis(hydroxymethanesulfonate) (BaC2H6S2O8, assuming no hydration) has a solubility in water at 25°C of 386 g/L, essentially the same as that of halite (the mineral form of NaCl). These “minerals” are as yet unknown. Certainly on contemporary Earth, with its panversal biosphere, any such HMS minerals would be rapidly consumed by microorganisms.
Interestingly, bisulfite derived from volcanic SO2 appears able to perform the same role for Hadean atmospheric glycolaldehyde (Löb, 1913) and glyceraldehyde. The addition of bisulfite to these alpha-hydroxy aldehydes, while also reversible, appears to go largely to completion with an equilibrium constant of less than ∼10−5 M. Glycolaldehyde and glyceraldehyde in substantial concentrations are invoked in several models for the Hadean origin of ribonucleoside derivatives, including those of Powner et al. (2009). While accumulations of large amounts of free glycolaldehyde and free glyceraldehyde required by these models seems unlikely due to their self-reaction, these models may now be revisited where the precursors are the bisulfite addition products of glycolaldehyde and glyceraldehyde.
The bisulfite addition products of DHA (where the C═O unit is in the form of a ketone) and ribose are thermodynamically less stable. For the first, this is understood under a steric model. Just as the hydrate of DHA is less stable than the hydrate of glyceraldehyde, so is its bisulfite addition product. The relatively little bisulfite addition product formed by ribose is understood by its need to compete with the formation of cyclic forms of this pentose. In the presence of borate, this competition is even less favorable.
This hypothesis has implications for the mineralogy of early Earth. As noted in the introduction, sulfur (IV) on contemporary Earth is readily oxidized in the dioxygen-containing atmosphere. Therefore, while sulfate minerals are widespread today on Earth, sulfite minerals are not. Exceptions include calcium sulfite (hannebachite, soluble at 25°C to 0.043 g/L, less than the solubility of calcium sulfate dihydrate, which is the evaporate mineral gypsum), scotlandite (lead sulfite), gravegliaite (manganese sulfite), and orschallite (calcium sulfate-sulfite) (Weidenthaler et al., 1993). The interaction of aldehyde on the surface of sulfite minerals led to the adduct formation (Pitsch et al., 2000). Thus, this work should stimulate this overlooked area of mineralogy and help guide the exploration of other rocky planets.
Acknowledgments
We are indebted to colleagues at the Foundation for Applied Molecular Evolution for assistance, and Jonathan Allison at the MS Services of the Department of Chemistry, University of Florida, for MS data. The material is based in part upon work supported by NASA under award NNX14AK37G. Any opinions, findings, conclusions, or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Aeronautics and Space Administration. This project/publication was also made possible through the support of a grant from the John Templeton Foundation 54466. The opinions expressed in this publication are those of the author(s) and do not necessarily reflect the views of the John Templeton Foundation.
Abbreviations Used
- DHA
dihydroxyacetone
- ESI
electrospray ionization
- HMS
hydroxymethanesulfonate
- TOF
time-of-flight
Associate Editor: David Deamer
References
- Amaral A.F., Marques M.M., da Silva J.A.L., and da Silva J.J.R.F. (2008) Interactions of D-ribose with polyatomic anions, and alkaline and alkaline-earth cations: possible clues to environmental synthesis conditions in the pre-RNA world. New Journal of Chemistry 32:2043–2049 [Google Scholar]
- Andres R.J. and Kasgnoc A.D. (1998) Time-Averaged Inventory of Volcanic Sulfur Emissions [online dataset]. Global Emissions Inventory Activity, Version 1. https://gcmd.nasa.gov/KeywordSearch/Metadata.do?Portal=idn_ceos&KeywordPath=%5BKeyword%3D%27IGBP%27%5D&OrigMetadataNode=GCMD&EntryId=GEIA_SO2_VOLCANO&MetadataView=Full&MetadataType=0&lbnode=mdlb4 [Google Scholar]
- Appayee C. and Breslow R. (2014) Deuterium studies reveal a new mechanism for the formose reaction involving hydride shifts. J Am Chem Soc 136:3720–3723 [DOI] [PubMed] [Google Scholar]
- Becker S., Thoma I., Deutsch A., Gehrke T., Mayer P., Zipse H., and Carell T. (2016) A high-yielding, strictly regioselective prebiotic purine nucleoside formation pathway. Science 352:833–836 [DOI] [PubMed] [Google Scholar]
- Benner S.A., Devine K.G., Matveeva L.N., and Powell D.H. (2000) The missing organic molecules on Mars. Proc Natl Acad Sci USA 97:2425–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner S.A., Kim H.-J., Kim M.-J., and Ricardo A. (2010) Planetary organic chemistry and the origins of biomolecules. In The Origin of Life, 4th ed., edited by D. Deamer, and J.W. Szostak, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 67–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner S.A., Kim H.J., and Carrigan M.A. (2012) Asphalt, water, and the prebiotic synthesis of ribose, ribonucleosides, and RNA. Acc Chem Res 45:2025–2034 [DOI] [PubMed] [Google Scholar]
- Boyce S.D. and Hoffmann M.R. (1984) Kinetic and mechanism of the formation of hydroxymethanesulfonic acid at low pH. J Phys Chem 88:4740–4746 [Google Scholar]
- Butlerov A. (1861) Bildung einer zuckerartigen Substanz durch Synthese. Annalen Chemie 120:295–298 [Google Scholar]
- Cannizzaro S. (1853) Ueber den der Benzoësäure entsprechenden Alkohol. Justus Liebigs Ann Chem 88:129–130 [Google Scholar]
- Cleaves H.J. (2008) The prebiotic geochemistry of formaldehyde. Precambrian Res 164:111–118 [Google Scholar]
- Cossetti C., Crestini C., Saladino R., and di Mauro E. (2010) Borate minerals and RNA stability. Polymers 2:211–228 [Google Scholar]
- da Silva J.A.L. and Holm N.G. (2014) Borophosphates and silicophosphates as plausible contributors to the emergence of life. J Colloid Interface Sci 431:250–254 [DOI] [PubMed] [Google Scholar]
- Delidovich I.V., Taran O.P., and Parmon V.N. (2013) Plausible prebiotic synthesis of aldopentoses from simple substrates, glycolaldehyde and formaldehyde. Paleontological Journal 47:1093–1096 [Google Scholar]
- Dong S. and Dasgupta P.K. (1986) On the formaldehyde-bisulfite hydroxymethane sulfonate equilibrium. Atmos Environ 20:1635–1637 [Google Scholar]
- Ferris J.P. (2005) Mineral catalysis and prebiotic synthesis: montmorillonite-catalyzed formation of RNA. Elements 1:145–149 [Google Scholar]
- Furukawa Y., Horiuchi M., and Kakegawa T. (2013) Selective stabilization of ribose by borate. Orig Life Evol Biosph 43:353–361 [DOI] [PubMed] [Google Scholar]
- Furukawa Y., Kim H.J., Hutter D., and Benner S.A. (2015) Abiotic regioselective phosphorylation of adenosine with borate in formamide. Astrobiology 15:259–267 [DOI] [PubMed] [Google Scholar]
- Graedel T.E. and Weschler C.J. (1981) Chemistry within aqueous atmospheric aerosols and raindrops. Rev Geophys 19:505–539 [Google Scholar]
- Keefe A.D. and Miller S.L. (1995) Was ferrocyanide a prebiotic reagent. Orig Life Evol Biosph 26:111–129 [DOI] [PubMed] [Google Scholar]
- Kim H.J., Ricardo A., Illangkoon H.I., Kim M.J., Carrigan M.A., Frye F., and Benner S.A. (2011) Synthesis of carbohydrates in mineral-guided prebiotic cycles. J Am Chem Soc 133:9457–9468 [DOI] [PubMed] [Google Scholar]
- Kim H.J., Furukawa Y., Kakegawa T., Bita A., Scorei R., and Benner S.A. (2016) Evaporite borate-containing mineral ensembles make phosphate available and regiospecifically phosphorylate ribonucleosides: borate as a multifaceted problem solver in prebiotic chemistry. Angew Chem Int Ed Engl 55:15816–15820 [DOI] [PubMed] [Google Scholar]
- Kok G.L., Gitlin S.N., and Lazrus A.L. (1986) Kinetics of the formation and decomposition of hydroxymethanesulfonate. J Geophys Res: Atmospheres 91:2801–2804 [Google Scholar]
- Kopetzki D. and Antonietti M. (2011) Hydrothermal formose reaction. New Journal of Chemistry 35:1787–1794 [Google Scholar]
- Kusakabe M., Komoda Y., Takano B., and Abiko T. (2000) Sulfur isotopic effects in the disproportionation reaction of sulfur dioxide in hydrothermal fluids: implications for the δ34S variations of dissolved bisulfate and elemental sulfur from active crater lakes. Journal of Volcanology and Geothermal Research 97:287–307 [Google Scholar]
- Li Q., Ricardo A., Benner S.A., Winefordner J.D., and Powell D.H. (2005) Desorption/ionization on porous silicon mass spectrometry studies on pentose-borate complexes. Anal Chem 77:4503–4508 [DOI] [PubMed] [Google Scholar]
- Löb W. (1913) Uber das Verhalten des Formamids unter der Wirkung der stillen Entladung Ein Beitrag zur Frage der Stickstoff-Assimilation. Berichte der Deutschen Chemischen Gesellschaft 46:684–697 [Google Scholar]
- Marion G.M., Kargel J.S., Crowley J.K., and Catling D.C. (2013) Sulfite sulfide sulfate carbonate equilibria with applications to Mars. Icarus 223:342–351 [Google Scholar]
- Neveu M., Kim H.J., and Benner S.A. (2013) The “strong” RNA world hypothesis. Fifty years old. Astrobiology 13:391–403 [DOI] [PubMed] [Google Scholar]
- Orgel L.E. (2004) Prebiotic chemistry and the origin of the RNA world. Crit Rev Biochem Mol Biol 39:99–123 [DOI] [PubMed] [Google Scholar]
- Patel B.H., Percivalle C., Ritson D.J., Duffy C.D., and Sutherland J.D. (2015) Common origins of RNA, protein and lipid precursors in a cyanosulfidic proto metabolism. Nat Chem 7:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepi F., Garzoli S., Tata A., and Giacomello P. (2010) Low-energy collisionally activated dissociation of pentose–borate complexes. Int J Mass Spectrom 289:76–83 [Google Scholar]
- Pinto J., Gladstone G., and Yung Y. (1980) Photochemical production of formaldehyde in Earth's primitive atmosphere. Science 210:183–185 [DOI] [PubMed] [Google Scholar]
- Pitsch S., Krishnamurthy R., and Arrhenius G. (2000) Concentration of simple aldehydes by sulfite-containing double-layer hydroxide minerals: implications for biopoesis. Helv Chem Acta 83:2398–2411 [DOI] [PubMed] [Google Scholar]
- Powner M.W., Gerland B., and Sutherland J.D. (2009) Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 459:239–242 [DOI] [PubMed] [Google Scholar]
- Rai D., Felmy A.R., Fulton R.W., and Moore D.A. (1991) An aqueous thermodynamic model for Ca 2+−SO32- ion interactions and the solubility product of crystalline CaSO3· ½H2O. J Solution Chem 20:623–632 [Google Scholar]
- Ricardo A., Carrigan M.A., Olcott A.N., and Benner S.A. (2004) Borate minerals stabilize ribose. Science 303:196. [DOI] [PubMed] [Google Scholar]
- Ricardo A., Frye F., Carrigan M.A., Tipton J.D., Powell D.H., and Benner S.A. (2006) 2- Hydroxymethylboronate as a reagent to detect carbohydrates. Application to the analysis of the formose reaction. J Org Chem 71:9503–9505 [DOI] [PubMed] [Google Scholar]
- Saladino R., Barontini M., Cossetti C., di Mauro E., and Crestini C. (2011) The effects of borate minerals on the synthesis of nucleic acid bases, amino acids and biogenic carboxylic acids from formamide. Orig Life Evol Biosph 41:317–330 [DOI] [PubMed] [Google Scholar]
- Scorei R. and Cimpoiasu V.M. (2006) Boron enhances the thermostability of carbohydrates. Orig Life Evol Biosph 36:1–11 [DOI] [PubMed] [Google Scholar]
- Sorensen P.E. and Andersen V.S. (1970) The formaldehyde-hydrogen sulphite system in alkaline aqueous solution. Kinetics, mechanisms, and equilibria. Acta Chem Scand 21:1301–1306 [Google Scholar]
- Šponer J.E., Sumpter B.G., Leszczynski J., Šponer J., and Fuentes-Cabrera M. (2008) Theoretical study on the factors controlling the stability of the borate complexes of ribose, arabinose, lyxose, and xylose. Chemistry 14:9990–9998 [DOI] [PubMed] [Google Scholar]
- Trail D., Watson E.B., and Tallby N.D. (2011) The oxidation state of Hadean magmas and implications for early Earth's atmosphere. Nature 480:79–82 [DOI] [PubMed] [Google Scholar]
- Weidenthaler C., Tillmanns E., and Hentschel G. (1993) Orschallite, Ca3(SO3)2·SO4·12H2O, a new calcium-sulfite-sulfate-hydrate mineral. Mineral Petrol 48:167–177 [Google Scholar]