Abstract
STUDY QUESTION
What is the association between perimenarchal exposure to total suspended particulate (TSP) in air, menstrual irregularity phenotypes and time to menstrual cycle regularity?
SUMMARY ANSWER
Exposures to TSP during high school are associated with slightly increased odds of menstrual irregularity and longer time to regularity in high school and early adulthood.
WHAT IS KNOWN ALREADY
The menstrual cycle is responsive to hormonal regulation. Particulate matter air pollution has demonstrated hormonal activity. However, it is not known if air pollution is associated with menstrual cycle regularity.
STUDY DESIGN, SIZE, DURATION
Cross sectional study of 34 832 of the original 116 430 women (29.91%) enrolled in 1989 from the Nurses’ Health Study II (NHSII). The follow-up rate for this analytic sample was 97.76% at the 1991 survey.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Annual averages of TSP were available for each year of high school attendance. We created three case definitions including high school menstrual irregularity and androgen excess. The time to menstrual cycle regularity was reported by participants as <1 year, 1–2 years, 3–4 years, 5 years or longer, or never on the baseline questionnaire. Odds ratios and 95% confidence intervals (CI) were calculated for 45 μg/m3 increases in TSP exposure, adjusted for risk factors for menstrual irregularity.
MAIN RESULTS AND THE ROLE OF CHANCE
In multivariable adjusted models, we observed that for every 45 μg/m3 increase in average high school TSP there was an increased odds (95%CI) of 1.08 (1.03–1.14), 1.08 (1.02–1.15) and 1.10 (0.98–1.25) for moderate, persistent, and persistent with androgen excess irregularity phenotypes, respectively. TSP was also associated with a longer time to cycle regularity, with stronger results among women with older ages at menarche and those living in the Northeast or the West.
LIMITATIONS, REASONS FOR CAUTION
The outcomes of menstrual regularity and time to cycle regularity were retrospectively assessed outcomes and may be susceptible to recall bias. There is also the potential for selection bias, as women had to live until 2011 to provide addresses.
WIDER IMPLICATIONS OF THE FINDINGS
Temporal exposure to air pollution in the adolescent and early adulthood window may be especially important, given its association with phenotypes of menstrual irregularity. The data from this study agrees with existing literature regarding air pollution and reproductive tract diseases.
STUDY FUNDING/COMPETING INTEREST(S)
Shruthi Mahalingaiah: Reproductive Scientist Development Program HD000849, and a research grant from the Boston University Department of Obstetrics and Gynecology, Stacey Missmer: R01HD57210 from the National Institute of Child Health and Human Development and the Massachusetts Institute of Technology Center for Environmental Health Sciences Translational Pilot Project Program, R01CA50385 from the National Cancer Institute, Jaime Hart and Francine Laden: 5R01ES017017 from the National Institute for Environmental Health Sciences, Jaime Hart: P30 ES00002 from the National Institute for Environmental Health Sciences at the National Institute of Health, The Nurses’ Health Study II is supported by infrastructure grant UM1CA176726 from the National Cancer Institute, NIH, U.S. Department of Health and Human Services The authors have no conflicts of interest to declare.
Keywords: air pollution, menstrual cycle, menstrual irregularity, polycystic ovary syndrome, menarche
Introduction
The menstrual cycle is a marker of physiologic and reproductive health; its regularity is affected by hormones, stress, and environmental factors (Wang et al., 1976; Miyauchi et al., 1992; Chung et al., 2005; Ouyang et al., 2005). Regular menstrual cycles take 2–5 years to be established in the majority of adolescent girls due to maturation of the hypothalamic-pituitary-ovarian (HPO) axis. After this initialization, disorders of the HPO axis and endocrine system can affect regularity (Legro et al., 2000). Furthermore, menstrual irregularity in conjunction with biochemical or clinical androgen excess, is a hallmark of polycystic ovary syndrome (PCOS), one of the most common causes of infertility (Ansari et al., 2008). Androgen excess in PCOS is noted by symptoms of hirsutism, acne, and male-pattern balding, or can be noted by biochemical androgen excess on serum laboratory evaluation (Ehrmann, 2005). Women with menstrual irregularity and androgen excess are at an increased lifetime risk of multiple chronic health problems including high cholesterol (Huang et al., 2015), cardiovascular disease (Solomon et al., 2002) and diabetes (Solomon et al., 2001; Wang et al., 2011).
As a possible source of endocrine disruption, air pollution exposure has previously been examined in the context of gynecologic diseases and infertility (Wang et al., 2005; Oh et al., 2008; Radwan et al., 2016; Carre et al., 2017). Particulate matter (PM) from air pollution is comprised of a variety of different chemicals and is characterized by particle diameter. Particle composition is varied and depends on emission sources (Laden et al., 2000). Total suspended particulate (TSP) is comprised of particles measuring up to 50 microns (Wedding et al., 1977). Previous studies show reductions in fertility with exposures to nitrogen oxides (NOx) and three size fractions of PM (Nieuwenhuijsen et al., 2014). It has also been found to be associated with lower live birth rates (Perin et al., 2010), lower fecundability ratio (Slama et al., 2013), lower IVF pregnancy rate (Legro et al., 2010), reductions in term birth weight (Morello-Frosch et al., 2010) and higher risk of miscarriage (Mohorovic et al., 2010; Faiz et al., 2012).
Human studies showing that metabolites from tobacco, such as cadmium and cotinine, are found in follicular fluid suggest that inhaled exposures and their metabolites may possibly cross the lung-blood barrier, circulate and deposit at the level of the developing egg (Younglai et al., 2002; Al-Saleh et al., 2009; Benedict et al., 2011). Laboratory studies have also demonstrated that diesel exhaust particles have hormonal activity in estrogenic and androgenic activity assays (Wang et al., 2005; Misaki et al., 2008; Oh et al., 2008; Sidlova et al., 2009). In particular, high school and adolescence may be a sensitive time window for endocrine disruption (Golub et al., 2004; McBride et al., 2005; Rubino and Parolaro, 2008).
Although perinatal and pre-pubertal exposure to androgens are known exposures that predispose to menstrual irregularity in animal models and humans (Hewlett et al., 2016), we know of no study that has examined air pollution exposures in the perimenarchal time window in relation to menstrual irregularity and time to regularity. Because exposure to air pollution near the period of menstrual stabilization may affect future menstrual regularity, the objective of this study is to examine the association between perimenarchal total suspended particulates (TSP), menstrual irregularity phenotypes (including androgen excess), and time to cycle regularity in the Nurses’ Health Study II (NHSII).
Materials and Methods
Study population and case ascertainment
All study participants were members of the NHSII, a prospective cohort started in 1989 when 116 430 female nurses aged 25–42 years completed a baseline questionnaire. At enrollment, the women resided in 14 states (California, Connecticut, Indiana, Iowa, Kentucky, Massachusetts, Michigan, Missouri, New York, North Carolina, Ohio, Pennsylvania, South Carolina, and Texas), but have subsequently moved to all 50 states. Participants are followed via biennial follow-up questionnaires with response rates over 90%, and each collects updated information on incidence of disease and on a variety of dynamic lifestyle characteristics. As part of the web version of the 2011 questionnaire, women were asked to provide the address they lived at the longest during high school. Women were excluded from the current study if they did not provide a high school address (N = 77 506), if they did not provide information on menstrual irregularity on the baseline questionnaire (N = 3962), or if they were missing information on potential confounders (N = 378). After these exclusions, there were a total of 34 832 women available for analysis (29.91%).
Early menstrual cycle characteristics were ascertained retrospectively on the 1989 baseline questionnaire. Women were asked the following question, ‘What was the pattern of your menstrual cycles (excluding time around pregnancies or when using oral contraceptives) during high school?' and 'During ages 18–22?' Respondents had the following options for both time periods: very regular (±3 days), regular, usually irregular, always irregular, no periods. Women were also asked, ‘How many years after the onset of your menstrual periods did your cycles become regular?’ The options for this question were <1 year, 1–2 years, 3–4 years, 5 years or longer, and never.
Androgen excess was ascertained by self-report of hirsutism or acne. On the baseline questionnaire, women were asked to report if they had ever had a physician diagnosis of ‘severe teenage acne’ and to provide the year of diagnosis. A similar question on the 1991 questionnaire ascertained if women had ever had a physician diagnosis of hirsutism and asked them to report the year of diagnosis as before 1989, between 1989 and 1990, or in 1991 or later. We included all reports of hirsutism self-reported to have occurred before 1989.
We created three case definitions: two case definitions to classify increasing levels of irregularity and a third case definition combining irregularity with androgen excess. These three case definitions used are as follows: (i) Moderate irregularity defined by ‘always irregular in high school or ages 18–22’, (ii) Persistent irregularity defined by ‘always irregular in high school and ages 18–22’ and (iii) Moderate irregularity with Androgen Excess, defined by ‘always irregular in high school or ages 18–22 and severe teenage acne or hirsutism before 1989’. There were too few cases to examine the most extreme potential phenotype of persistent irregularity with androgen excess. The time to menstrual cycle regularity was based on the participants’ responses to the baseline questionnaire.
Exposure assessment
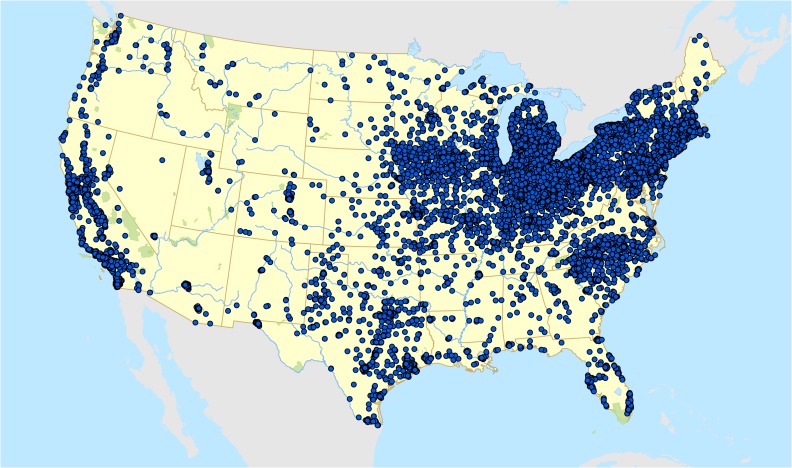
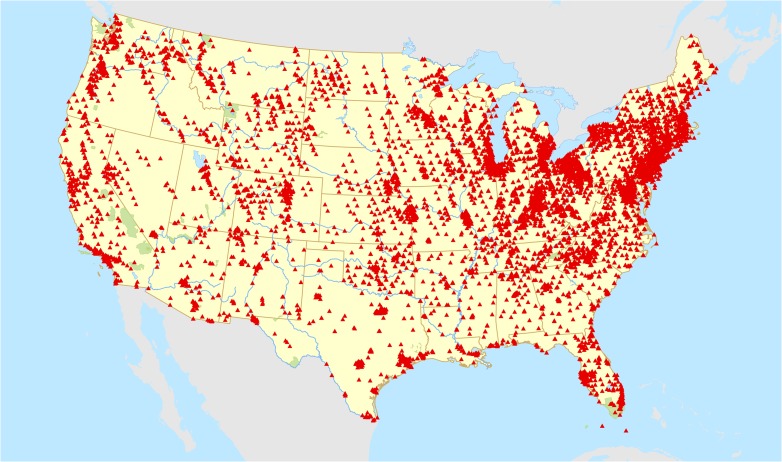
Residential address information for the longest address lived during high school was geocoded to obtain latitude and longitude (Fig. 1). Information on total suspended particulate (TSP) monitoring locations and concentrations was obtained directly from the US Environmental Protection Agency for the years the NHSII participants were in high school (1960–1983, Fig. 2). A 4-year average of TSP levels were calculated from the monitoring location closest to each residential address for the calendar years in which each participant was 14, 15, 16, 17 and 18.
Figure 1.
Geocoded high school residential addresses of 34 832 women in the Nurses’ Health Study II (NHSII).
Figure 2.
Locations of US Environmental Protection Agency total suspended particulate matter (TSP) monitors 1960–1980.
Potential confounders
We examined possible confounding by a priori selected risk factors for menstrual irregularity: age at questionnaire completion in 1989 (in months), race (white/other), body mass index at age 18 (BMI-continuous), reported somatotype at ages 5 and 10 (categories from 1 to 9), cigarettes smoked per day before age 15 (0, 1–4, 5 or more), hours per week of strenuous, moderate, or walking physical activity during high school, and in a subset of women with information on high school diet, we included data on high school consumption of soda and diet soda (Harris et al., 2016) and calculated overall diet quality as measured by the 2010 Alternative Healthy Eating Index (AHEI), (McCullough and Willett, 2006; Chiuve et al., 2012). To determine if each variable was also associated with exposure, we conducted linear regressions including each potential confounder. Only variables associated with both the exposure and outcome were included in multivariable models.
Statistical analysis
Logistic regression was used to calculate odds ratios and 95% confidence intervals for the association between menstrual irregularity (dichotomous) and each participant’s 4-year average of TSP exposure during high school (continuous). Odds ratios are presented scaled to the interquartile range (45 μg/m3) in exposure. All dose-responses were checked for deviations from linearity using cubic regression splines, and continuous effect estimates are only presented for those that did not deviate from linearity. Each of the three menstrual irregularity phenotypes (moderate, persistent and moderate with androgen excess) were modeled separately. To determine the impacts of distance from a monitor on our estimates, in sensitivity analyses, we restricted our models to the subset of women living within 10 km (N = 19 082) of a TSP monitor for whom we would expect less exposure misclassification.
Multinomial regression was used to determine odds ratios and 95% confidence intervals for the association of continuous high school TSP exposures (scaled to the interquartile range) and time to menstrual regularity (ordinal). An alpha level of 0.05 was used to determine statistical significance and cubic regression splines were used to assess deviations from linearity.
Stratified analyses were performed to explore potential effect measure modification of the odds ratios by age at menarche, age in 1989 and geographic region. The composition of TSP likely varies by region, which may be associated with different effects on menstrual irregularity. Age at questionnaire completion in 1989 may be a proxy for the ability to recall specific menstrual characteristics during adolescence, and age at menarche is both a proxy for time between menarche and our high-school measures of exposure and early or late onset may be a marker for endocrine disruption. The statistical significance of each potential effect modifier was assessed with multiplicative interaction terms. All statistical analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC).
Ethical approval
The study was approved by the Institutional Review Boards (IRB) of Boston University School of Medicine/Boston Medical Center and Brigham and Women’s Hospital.
Results
A total of 34 842 women were available for analysis. The demographic characteristics at the 1989 baseline questionnaire for the overall analytic sample, as well as the lower and upper quartiles of exposure, are presented in Table I. The average age of the participants in the analytic cohort at enrollment was 34.8 years (standard deviation (SD) = 4.6) years, while the average BMI (SD) at age 18 was 21.3 (3.2) kg/m2. The population was mostly Caucasian (97.6%) with an age at menarche of 12 years or more (75.8%), with an average of 3.7 (2.8) hours/week of strenuous physical activity during high school. The majority of women in this cohort reached menstrual regularity within 2 years of menarche (70.4%). The cohort had a mean AHEI score of 46.2 (9.8) during high school, and most were former or never smokers who did not smoke in high school. As shown in Supplementary Table SI, women excluded from the analyses were largely similar to those who were included in the analyses. Excluded women were far more likely to have lived outside of the United States at age 15 (26.8% versus 8.3%).
Table I.
Selected characteristics of the 34 832 women in the Nurses’ Health Study II (NHSII) included in the analysis overall and for the lower and upper quartiles of average high school exposure to total suspended particulate matter (TSP).
| Characteristic | Overall | TSP < 65.5 | TSP > 110.0 |
|---|---|---|---|
| N | 34 832 | 8722 | 8719 |
| Age in 1989 (years), Mean ± SD | 34.8 ± 4.6 | 31.2 ± 3.7 | 38.2 ± 3.6 |
| Race, N (%) | |||
| White | 33 982 (97.6) | 8540 (97.9) | 8471 (97.2) |
| Other races | 850 (2.4) | 182 (2.1) | 248 (2.8) |
| Average total suspended particulate (TSP, μg/m3), ages 14–18, Mean ± SD | 90.1 ± 32.6 | 54.3 ± 8.2 | 135.3 ± 22.4 |
| Average distance to the nearest TSP monitor (km), Mean ± SD | 19.3 ± 31.3 | 16.0 ± 31.7 | 22.4 ± 30.2 |
| High school Alternative Healthy Eating Index, Mean ± SD | 46.2 ± 9.8 | 46.8 ± 10.1 | 45.8 ± 9.8 |
| High school physical activity (hours/week), Mean ± SD | |||
| Strenuous | 3.7 ± 2.8 | 4.0 ± 2.8 | 3.5 ± 2.7 |
| Moderate | 3.3 ± 2.3 | 3.5 ± 2.3 | 3.2 ± 2.3 |
| Walking | 2.1 ± 1.9 | 1.8 ± 1.8 | 2.4 ± 2.0 |
| Body mass index (BMI, kg/m2) at age 18, Mean ± SD | 21.3 ± 3.2 | 21.3 ± 3.2 | 21.2 ± 3.2 |
| Age at menarche, N (%) | |||
| <12 | 8340 (23.9) | 2003 (23.0) | 2143 (24.6) |
| 12 | 10 715 (30.8) | 2629 (30.1) | 2668 (30.6) |
| 13 | 9648 (27.7) | 2441 (28.0) | 2494 (28.6) |
| ≥14 | 6025 (17.3) | 1616 (18.5) | 1393 (16.0) |
| Missing or not known | 104 (0.3) | 33 (0.4) | 21 (0.2) |
| Number of Years until Menstrual Regularity, N (%) | |||
| <1 | 15 933 (45.7) | 3846 (44.1) | 4054 (46.5) |
| 1–2 | 8597 (24.7) | 2202 (25.3) | 2154 (24.7) |
| 3–4 | 2397 (6.9) | 643 (7.4) | 550 (6.3) |
| 5+ | 4069 (11.7) | 907 (10.4) | 1111 (12.7) |
| Never | 3660 (10.5) | 1084 (12.4) | 805 (9.2) |
| Missing | 176 (0.5) | 40 (0.5) | 45 (0.5) |
| Cigarettes per day during high school, N (%) | |||
| 0 | 33 136 (95.1) | 8232 (94.4) | 8336 (95.6) |
| 1–4 | 1096 (3.2) | 317 (3.6) | 245 (2.8) |
| ≥5 | 600 (1.7) | 173 (2.0) | 138 (1.6) |
| Region of residence in high school | |||
| Northeast | 13 243 (38.0) | 3883 (44.5) | 3407 (39.1) |
| Midwest | 12 845 (36.9) | 2572 (29.5) | 3144 (36.1) |
| West | 4139 (11.9) | 766 (8.8) | 1335 (15.3) |
| South | 4343 (12.5) | 1489 (17.1) | 692 (7.9) |
| Missing | 262 (0.8) | 12 (0.1) | 141 (1.6) |
Participants in the upper quartile of TSP exposure tended to be slightly older, have larger increases in BMI between age 18 and 1989, and live in the West compared to women in the lowest quartile of exposure. The distributions of TSP averaged over high school (ages 14–18) and by year of age are presented in Supplementary Table SII. The ranges of exposure were similar for all ages. The mean (SD) exposure during high school was 90.1 μg/m3 (32.6 μg/m3), and range was 10.1–319.7 μg/m3. Altogether, there were 4882 cases of moderate irregularity, 3074 of persistent irregularity and 686 cases of moderate irregularity with androgen excess.
BMI at age 18, smoking status at ages 15–18, physical activity during high school, and high school diet and soda consumption were all statistically significantly associated with high school TSP, and were therefore included in all multivariable models. The associations between average high school TSP exposure and the presence of a menstrual irregularity phenotype are presented in Table II. We did not observe deviations from linearity, and therefore present linear dose-responses scaled to an interquartile range (45 μg/m3) increase in exposure. In basic models adjusted only for age and race, for every 45 μg/m3 increase in TSP exposure during high school, there was a greater odds (95%CI) of having moderate irregularity, persistent irregularity and moderate irregularity with androgen excess of 8% (3,14%), 8% (2,15%) and 11% (−2,25%), respectively. The odds ratios remained robust to adjustment in multivariable models that did or did not include high school diet (Table II). Results were unchanged when restricting to women who lived in closer proximity to an air pollution monitor (Supplementary Table SIII).
Table II.
Associations of a 45 μg/m3 increase in participant high school total suspended particulate exposure with several menstrual irregularity phenotypes among 34 832 women in the Nurses’ Health Study II (NHSII).
| Moderate irregularity | Persistent irregularity | Moderate irregularity with androgen excess | |
|---|---|---|---|
| OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| Cases | 4882 | 3074 | 686 |
| Covariates included | |||
| Basica | 1.08 (1.03, 1.14) | 1.08 (1.02, 1.15) | 1.11 (0.98, 1.25) |
| Multivariableb | 1.08 (1.03, 1.14) | 1.08 (1.02, 1.15) | 1.10 (0.98, 1.25) |
| Multivariable + high school dietc | 1.06 (0.99, 1.13) | 1.09 (1.00, 1.18) | 1.06 (0.90, 1.25) |
Note: Moderate irregularity is defined as always irregular in high school or ages 18–22, persistent irregularity is defined as always irregular in high school and ages 18–22, and moderate irregularity with androgen excess is defined as always irregular in high school or ages 18–22 and severe teenage acne or hirsutism before 1989.
aAdjusted for age and race.
bAdjusted for age, race, BMI at age 18, smoking status at ages 15–18 and physical activity during high school (walking, moderate and vigorous MET-hours per week).
cAdditionally adjusted for consumption of soda and diet soda during high school and overall diet quality (AHEI); models with high school diet have an N = 19 094 and 2752, 1736 and 407 cases.
There was no statistically significant effect modification by age at menarche, age in 1989, or region of residence (Supplementary Table SIV). However, there was a suggestion of greater odds of menstrual irregularity among the older age categories for the moderately irregular and persistently irregular phenotypes. Conversely, this trend reversed for the moderately irregular menses with androgen excess phenotype.
Each 45 μg/m3 increase of average TSP exposure was associated in the basic model with a 1.04 increased odds (95%CI: 1.01, 1.07) of being in a longer time to regularity category (Table III). In the multivariable adjusted multinomial model the OR was also 1.04 (95%CI: 1.00, 1.07). There was no evidence of effect modification of these associations by age in 1989. Age at menarche and region of residence during high school did statistically significantly modify the association. Longer times to regularity were observed for older ages at menarche and in the Western and Northeast regions.
Table III.
Associations between participant high school total suspended particulate exposure (scaled to the interquartile range of a 45 μg/m3) on time to menstrual regularity among 34 656 women in the Nurses’ Health Study II (NHSII).
| Multinomial regression OR (95%CI) |
|
|---|---|
| Covariates included | |
| Basica | 1.04 (1.01, 1.07) |
| Multivariableb | 1.04 (1.00, 1.07) |
| Multivariable + high school dietc | 1.04 (0.99, 1.08) |
aAdjusted for age and race.
bAdditionally adjusted for BMI at age 18, smoking status at ages 15–18, and physical activity during high school (walking, moderate and vigorous MET-hours per week).
cAdditionally adjusted for consumption of soda and diet soda during high school and overall diet quality (AHEI); Models with high school diet have an N = 19 011.
Discussion
We assessed the association between air pollution exposure and menstrual irregularity in the high school and early adulthood time window. We observed that for every interquartile range (45 μg/m3) increase in average TSP during high school, there was a greater odds of menstrual irregularity for all three phenotypes examined: moderate, persistent and moderate with androgen excess. The associations between high school TSP exposure and menstrual irregularity were maintained across all severity categories and remained robust after multivariable adjustment. Increasing exposures were also associated with longer times to menstrual irregularity. In analyses stratified by age at menarche, there was a suggestion of increasing effect sizes for the moderately irregular and persistently irregular phenotypes and statistically significant modification in time to menstrual regularity at older menarchal ages. Time to menstrual regularity also differed by region of residence, with effects observed only in the Northeastern and Western regions.
While the effect sizes found in this study are small and strictly suggestive, they are consistent with the literature regarding air pollution and reproductive tract diseases in this cohort (Mahalingaiah et al., 2014a, b, 2016). Menstrual irregularity alone has been previously used as a proxy for PCOS in analyses within the NHSII, and this definition has provided insights into the long-term health outcomes associated with menstrual irregularity, including cardiovascular disease and diabetes (Solomon et al., 2001, 2002). A previous study has validated the self-reported menstrual regularity responses in the NHSII and found that concordance was high between reporting of regular versus irregular and length of menstrual cycles (Solomon et al., 2002). The prevalence of moderate menstrual irregularity in the high school time window or ages 18–22 in our study was 14% (4882/34 842) within the analytic sample. Other studies report population prevalence as high as 50% for unselected adolescent girls (Hickey et al., 2011), 31% in university students (Cakir et al., 2007) and 24% in unselected 27–34-year-old women (March et al., 2010).
In this current study, the only pollutant widely available throughout the US during our study time window is total suspended particulate matter (TSP), which is a measure of all size fractions of particulate and agnostic to the composition of particulates. In the Harvard Six Cities Studies, it was found that silicon from soil, lead from motor vehicle exhaust, and selenium from coal combustion were common in the fine particulate matter in six cities across the US (Laden et al., 2000). Other elements found in some cities but not others included vanadium from fuel oil, chlorine from salt, and manganese and other metals. Further compositional analyses is warranted before additional inferences are drawn, as measurements from the Harvard study were from 1979 to 1980, and the sources of TSP are both time and geographically varying.
There is no literature that we are aware of on the association of air pollution and menstrual regularity or age at menarche. Previous studies have reported that air pollution exposure may be associated with adverse fetal growth (Glinianaia et al., 2004; Dadvand et al., 2013; Pedersen et al., 2013; Winckelmans et al., 2015). The literature on tobacco smoking reports that passive smoke exposure in childhood is associated with a longer time to menstrual cycle regularity (Dossus et al., 2012), although for those exposed in utero via maternal active or passive exposure, the association reported is with an earlier age at menarche (Behie and O’Donnell, 2015; Zhang et al., 2015).
In the current study, we found that for the moderately irregular and persistently irregular phenotypes and for time to menstrual regularity, odds of menstrual irregularity was greater as the age of menarche increased. Of note, this trend reversed in the category of women reporting moderate irregularity with androgen excess, as increased exposure to TSP was associated with an earlier age at menarche. A recent review by Carre et al. (2017) documents several studies suggesting that air pollution may be associated with infertility. They found that the male gamete has been extensively studied, but relatively few studies have been carried out on the female gamete (i.e. the oocyte and its functional status in ovulation and subsequent menstruation). In light of our findings regarding menstrual cycle irregularity, further investigation into the effects of air pollution on the female gamete is warranted.
In our further analyses, we observed slightly higher odds of longer time to cycle regularity with increases in TSP during high school. This is consistent with a study by Huang et al. (2017) which found that greater PM10 exposure in Hong Kong was associated with later pubertal and breast development in girls. It is also in line with a study by Radwan et al. (2016) reporting that PM10 may have antiestrogenic effects in their Polish cohort Laboratory studies on the endocrine effects of various sources of air pollution also demonstrate activity at the estrogen-receptor (Oh et al., 2008), progesterone-receptor (Wang et al., 2005), aryl hydrocarbon-receptor (Bidgoli et al., 2012).
This study must be interpreted in the context of several limitations. The outcomes of menstrual regularity in high school and ages 18–22 and time to cycle regularity were retrospectively assessed, in some cases decades after the event, on the baseline survey in 1989. As such, recall bias leading to misclassification of these two outcomes is a concern; however, this recall bias is likely non-differential with respect to TSP exposure, which would likely bias our results towards the null. There is also the potential for selection bias, as women had to live until 2011 to provide addresses. If women with higher levels of exposure and irregular cycles were less likely to have survived to complete the 2011 questionnaire, this could lead to bias. There did not appear to be differences in times to menstrual regularity or the other outcomes among women included and excluded from our analyses (Supplementary Table SI). Our use of the nearest TSP monitor likely lead to exposure misclassification. However, results were similar in analyses restricted to women living within 10 km of a monitor. Due to the timing of high school for these participants, we were unable to examine the association between smaller PM size fractions or PM composition on menstrual regularity and time to regularity. Another potential source of exposure measurement error may be that the women moved during high school or did not spend the majority of their time at their home address. Although we were able to adjust for many known risk factors for menstrual irregularity that did confound the association, residual confounding cannot be ruled out, as it is possible that other factors we were unable to control for, such as socioeconomic status during high school, may be associated with both the exposure and outcome. Lastly, the results of this study may not generalize to women of different ages, race/ethnicities or other locations, as the composition and sources of exposure may be very different than those assessed for this population.
This study also has several notable strengths, including a large sample size and the use of time- and location-specific exposure assessment for a critical time window. We were also able to include several variables in our models that are known to be associated with menstrual regularity and were associated with exposure to ambient TSP in this population, such as physical activity and body mass index.
In conclusion, we observed greater odds of menstrual irregularity during high school and ages 18–22 with TSP exposures during high school. Further studies are needed to provide more detailed exposure assessment of size fractions and compositional analyses combined with prospective collection of pubertal and menstrual characteristics.
Supplementary data
Supplementary data are available at Human Reproduction online.
Supplementary Material
Authors’ roles
S.M., S.A.M., J.J.C. and J.E.H. have made substantial contributions at all levels, from conception and design to data analysis and interpretation, revisions and final edits. S.M. drafted the article and all authors critically revised it for intellectual content. F.L. was involved in design of the study. J.C. approved the analysis plan and was included per the Nurses’ Health Study committee determination. The final version of this article was approved by all authors for publication.
Funding
The work for this paper was supported by the following: Shruthi Mahalingaiah: Reproductive Scientist Development Program HD000849, and a research grant from the Boston University Department of Obstetrics and Gynecology, Stacey Missmer: R01HD57210 from the National Institute of Child Health and Human Development and the Massachusetts Institute of Technology Center for Environmental Health Sciences Translational Pilot Project Program, R01CA50385 from the National Cancer Institute, Jaime Hart and Francine Laden: 5R01ES017017 from the National Institute for Environmental Health Sciences, Jaime Hart: P30 ES00002 from the National Institute for Environmental Health Sciences at the National Institute of Health, The Nurses’ Health Study II is supported by infrastructure grant UM1CA176726 from the National Cancer Institute, NIH, U.S. Department of Health and Human Services
Conflict of interest
None declared.
References
- Al-Saleh I, El-Doush I, Arif J, Coskun S, Jaroudi K, Al-Shahrani A, El-Din Mohamed G. Levels of DNA adducts in the blood and follicular fluid of women undergoing in vitro fertilization treatment and its correlation with the pregnancy outcome. Bull Environ Contam Toxicol 2009;84:23–28. [DOI] [PubMed] [Google Scholar]
- Ansari M, Kotselidis C, Watson I, Kirkham C, Lujan M, Jarvis K Lee-TM: a non-trivial benchmark suite for transactional memory. Algorithms and Architectures for Parallel Processing, Proceedings, 2008;5022: 196–207.
- Behie AM, O’Donnell MH. Prenatal smoking and age at menarche: influence of the prenatal environment on the timing of puberty. Hum Reprod 2015;30:957–962. [DOI] [PubMed] [Google Scholar]
- Benedict MD, Missmer SA, Vitonis AF, Cramer DW, Meeker JD. Cotinine concentrations in follicular fluid as a measure of secondhand tobacco smoke exposure in women undergoing in vitro fertilization: inter-matrix comparisons with urine and temporal variability. Chemosphere 2011;84:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidgoli SA, Khorasani H, Keihan H, Sadeghipour A, Mehdizadeh A. Role of endocrine disrupting chemicals in the occurrence of benign uterine leiomyomata: special emphasis on AhR tissue levels. Asian Pac J Cancer Prev 2012;13:5445–5450. [DOI] [PubMed] [Google Scholar]
- Cakir M, Mungan I, Karakas T, Girisken I, Okten A. Menstrual pattern and common menstrual disorders among university students in Turkey. Pediatr Int 2007;49:938–942. [DOI] [PubMed] [Google Scholar]
- Carre J, Gatimel N, Moreau J, Parinaud J, Leandri R. Does air pollution play a role in infertility? A systematic review. Environ Health 2017;16:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung FF, Yao CC, Wan GH. The associations between menstrual function and life style/working conditions among nurses in Taiwan. J Occup Health 2005;47:149–156. [DOI] [PubMed] [Google Scholar]
- Dadvand P, Parker J, Bell ML, Bonzini M, Brauer M, Darrow LA, Gehring U, Glinianaia SV, Gouveia N, Ha EH et al. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect 2013;121:267–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossus L, Kvaskoff M, Bijon A, Fervers B, Boutron-Ruault MC, Mesrine S, Clavel-Chapelon F. Determinants of age at menarche and time to menstrual cycle regularity in the French E3N cohort. Ann Epidemiol 2012;22:723–730. [DOI] [PubMed] [Google Scholar]
- Ehrmann DA. Polycystic ovary syndrome. N Engl J Med 2005;352:1223–1236. [DOI] [PubMed] [Google Scholar]
- Faiz AS, Rhoads GG, Demissie K, Kruse L, Lin Y, Rich DQ. Ambient air pollution and the risk of stillbirth. Am J Epidemiol 2012;176:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinianaia SV, Rankin J, Bell R, Pless-Mulloli T, Howel D. Particulate air pollution and fetal health: a systematic review of the epidemiologic evidence. Epidemiology 2004;15:36–45. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Jerome CP. Endocrine disruption in adolescence: immunologic, hematologic, and bone effects in monkeys. Toxicol Sci 2004;82:598–607. [DOI] [PubMed] [Google Scholar]
- Harris HR, Willett WC, Vaidya RL, Michels KB. Adolescent dietary patterns and premenopausal breast cancer incidence. Carcinogenesis 2016;37:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett M, Chow E, Aschengrau A, Mahalingaiah S. Prenatal Exposure to Endocrine Disruptors: A Developmental Etiology for Polycystic Ovary Syndrome. Reproductive Sciences 2016. doi: 10.1177/1933719116654992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey M, Doherty DA, Atkinson H, Sloboda DM, Franks S, Norman RJ, Hart R. Clinical, ultrasound and biochemical features of polycystic ovary syndrome in adolescents: implications for diagnosis. Hum Reprod 2011;26:1469–1477. [DOI] [PubMed] [Google Scholar]
- Huang R, Zheng J, Li S, Tao T, Ma J, Liu W. Characteristics and contributions of hyperandrogenism to insulin resistance and other metabolic profiles in polycystic ovary syndrome. Acta Obstet Gynecol Scand 2015;94:494–500. [DOI] [PubMed] [Google Scholar]
- Huang JV, Leung GM, Schooling CM. The association of air pollution with pubertal development: evidence from Hong Kong’s ‘Children of 1997’ birth cohort. Am J Epidemiol 2017;185:914–923. [DOI] [PubMed] [Google Scholar]
- Laden F, Neas LM, Dockery DW, Schwartz J. Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ Health Perspect 2000;108:941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro RS, Lin HM, Demers LM, Lloyd T. Rapid maturation of the reproductive axis during perimenarche independent of body composition. J Clin Endocrinol Metab 2000;85:1021–1025. [DOI] [PubMed] [Google Scholar]
- Legro RS, Sauer MV, Mottla GL, Richter KS, Li X, Dodson WC, Liao D. Effect of air quality on assisted human reproduction. Hum Reprod 2010;25:1317–1324. [DOI] [PubMed] [Google Scholar]
- Mahalingaiah S, Hart JE, Laden F, Aschengrau A, Missmer SA. Air pollution exposures during adulthood and risk of endometriosis in the Nurses’ Health Study II. Environ Health Perspect 2014. a;122:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingaiah S, Hart JE, Laden F, Terry KL, Boynton-Jarrett R, Aschengrau A, Missmer SA. Air pollution and risk of uterine leiomyomata. Epidemiology 2014. b;25:682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingaiah S, Hart JE, Laden F, Farland LV, Hewlett MM, Chavarro J, Aschengrau A, Missmer SA. Adult air pollution exposure and risk of infertility in the Nurses’ Health Study II. Hum Reprod 2016;31:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 2010;25:544–551. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Bell RL, Rodd ZA, Strother WN, Murphy JM. Adolescent alcohol drinking and its long-range consequences. Studies with animal models. Recent Dev Alcohol 2005;17:123–142. [DOI] [PubMed] [Google Scholar]
- McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: the alternate healthy eating index. Public Health Nutr 2006;9:152–157. [DOI] [PubMed] [Google Scholar]
- Misaki K, Suzuki M, Nakamura M, Handa H, Iida M, Kato T, Matsui S, Matsuda T. Aryl hydrocarbon receptor and estrogen receptor ligand activity of organic extracts from road dust and diesel exhaust particulates. Arch Environ Contam Toxicol 2008;55:199–209. [DOI] [PubMed] [Google Scholar]
- Miyauchi F, Nanjo K, Otsuka K. [Effects of night shift on plasma concentrations of melatonin, LH, FSH and prolactin, and menstrual irregularity]. Sangyo Igaku 1992;34:545–550. [DOI] [PubMed] [Google Scholar]
- Mohorovic L, Petrovic O, Haller H, Micovic V. Pregnancy loss and maternal methemoglobin levels: an indirect explanation of the association of environmental toxics and their adverse effects on the mother and the fetus. Int J Environ Res Public Health 2010;7:4203–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello-Frosch R, Jesdale BM, Sadd JL, Pastor M. Ambient air pollution exposure and full-term birth weight in California. Environ Health 2010;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ, Basagana X, Dadvand P, Martinez D, Cirach M, Beelen R, Jacquemin B. Air pollution and human fertility rates. Environ Int 2014;70:9–14. [DOI] [PubMed] [Google Scholar]
- Oh SM, Ryu BT, Chung KH. Identification of estrogenic and antiestrogenic activities of respirable diesel exhaust particles by bioassay-directed fractionation. Arch Pharm Res 2008;31:75–82. [DOI] [PubMed] [Google Scholar]
- Ouyang F, Perry MJ, Venners SA, Chen C, Wang B, Yang F, Fang Z, Zang T, Wang L, Xu X et al. Serum DDT, age at menarche, and abnormal menstrual cycle length. Occup Environ Med 2005;62:878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen M, Giorgis-Allemand L, Bernard C, Aguilera I, Andersen AM, Ballester F, Beelen RM, Chatzi L, Cirach M, Danileviciute A et al. Ambient air pollution and low birthweight: a European cohort study (ESCAPE). Lancet Respir Med 2013;1:695–704. [DOI] [PubMed] [Google Scholar]
- Perin PM, Maluf M, Czeresnia CE, Nicolosi Foltran Januario DA, Nascimento Saldiva PH. Effects of exposure to high levels of particulate air pollution during the follicular phase of the conception cycle on pregnancy outcome in couples undergoing in vitro fertilization and embryo transfer. Fertil Steril 2010;93:301–303. [DOI] [PubMed] [Google Scholar]
- Radwan M, Jurewicz J, Polanska K, Sobala W, Radwan P, Bochenek M, Hanke W. Exposure to ambient air pollution—does it affect semen quality and the level of reproductive hormones? Ann Hum Biol 2016;43:50–56. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. Long lasting consequences of cannabis exposure in adolescence. Mol Cell Endocrinol 2008;286:S108–S113. [DOI] [PubMed] [Google Scholar]
- Sidlova T, Novak J, Janosek J, Andel P, Giesy JP, Hilscherova K. Dioxin-like and endocrine disruptive activity of traffic-contaminated soil samples. Arch Environ Contam Toxicol 2009;57:639–650. [DOI] [PubMed] [Google Scholar]
- Slama R, Bottagisi S, Solansky I, Lepeule J, Giorgis-Allemand L, Sram R. Short-term impact of atmospheric pollution on fecundability. Epidemiology 2013;24:871–879. [DOI] [PubMed] [Google Scholar]
- Solomon CG, Hu FB, Dunaif A, Rich-Edwards J, Willett WC, Hunter DJ, Colditz GA, Speizer FE, Manson JE. Long or highly irregular menstrual cycles as a marker for risk of type 2 diabetes mellitus. JAMA 2001;286:2421–2426. [DOI] [PubMed] [Google Scholar]
- Solomon CG, Hu FB, Dunaif A, Rich-Edwards JE, Stampfer MJ, Willett WC, Speizer FE, Manson JE. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab 2002;87:2013–2017. [DOI] [PubMed] [Google Scholar]
- Wang CF, Lasley BL, Lein A, Yen SS. The functional changes of the pituitary gonadotrophs during the menstrual cycle. J Clin Endocrinol Metab 1976;42:718–728. [DOI] [PubMed] [Google Scholar]
- Wang ET, Calderon-Margalit R, Cedars MI, Daviglus ML, Merkin SS, Schreiner PJ, Sternfeld B, Wellons M, Schwartz SM, Lewis CE et al. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet Gynecol 2011;117:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xie P, Kettrup A, Schramm KW. Inhibition of progesterone receptor activity in recombinant yeast by soot from fossil fuel combustion emissions and air particulate materials. Sci Total Environ 2005;349:120–128. [DOI] [PubMed] [Google Scholar]
- Wedding JB, Mcfarland AR, Cermak JE. Large particle collection characteristics of ambient aerosol samplers. Environ Sci Technol 1977;11:387–390. [Google Scholar]
- Winckelmans E, Cox B, Martens E, Fierens F, Nemery B, Nawrot TS. Fetal growth and maternal exposure to particulate air pollution—more marked effects at lower exposure and modification by gestational duration. Environ Res 2015;140:611–618. [DOI] [PubMed] [Google Scholar]
- Younglai EV, Foster WG, Hughes EG, Trim K, Jarrell JF. Levels of environmental contaminants in human follicular fluid, serum, and seminal plasma of couples undergoing in vitro fertilization. Arch Environ Contam Toxicol 2002;43:121–126. [DOI] [PubMed] [Google Scholar]
- Zhang B, Shi H, Wang Q, Zhang Z, Li M. Maternal passive smoking during pregnancy and age of menarche in daughters: a study of elementary and middle school students in Shanghai. Asia Pac J Public Health 2015;27:14S–20S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.