Abstract
Dietary oxalate is plant-derived and may be a component of vegetables, nuts, fruits, and grains. In normal individuals, approximately half of urinary oxalate is derived from the diet and half from endogenous synthesis. The amount of oxalate excreted in urine plays an important role in calcium oxalate stone formation. Large epidemiological cohort studies have demonstrated that urinary oxalate excretion is a continuous variable when indexed to stone risk. Thus, individuals with oxalate excretions >25 mg/day may benefit from a reduction of urinary oxalate output. The 24-h urine assessment may miss periods of transient surges in urinary oxalate excretion, which may promote stone growth and is a limitation of this analysis. In this review we describe the impact of dietary oxalate and its contribution to stone growth. To limit calcium oxalate stone growth, we advocate that patients maintain appropriate hydration, avoid oxalate-rich foods, and consume an adequate amount of calcium.
Keywords: calcium oxalate, crystalluria, kidney stones, oxalate
INTRODUCTION
The amount of oxalate excreted in urine is a critical factor in calcium oxalate (CaOx) stone growth (24, 48). Urinary oxalate is derived from two major sources: the diet and endogenous synthesis. The contribution of dietary oxalate to stone formation has been difficult to assess accurately for reasons that include 1) the requirement to control the intake of oxalate, calcium, and other nutrients to accurately identify factors influencing oxalate excretion, 2) the difficulty of estimating oxalate intake, 3) unexplained variability in oxalate absorption and secretion, 4) variability in oxalate content of foods due to environmental/growth conditions, 5) oxalate’s interactions with dietary calcium, 6) the influence of oxalate-degrading microbes, and 7) a lack of clinical studies showing that decreasing oxalate intake lowers the frequency of stone recurrence. In this review we discuss dietary oxalate absorption, degradation, and excretion and its potential impact on kidney stone growth. Additionally, future directions for research on the contribution of dietary oxalate to stone disease are addressed.
DIETARY OXALATE AND STONE RISK
Two common tools used over the last few decades to understand the role of dietary oxalate in stone risk are food frequency questionnaires and 24-h urine collections. Both of these tools offer valuable insight into a patient’s oxalate intake and risk for stone disease, respectively. However, they do not fully assess the substantial risk that may be associated with sporadic intake of large amounts of dietary oxalate. Food frequency questionnaires have been used to examine prospective stone formation in three separate long-term cohorts. These studies showed a minor risk associated with dietary oxalate intake and stone disease [relative risk 1.21 (men) and 1.22 (older women)] (51). Despite infrequent consumption, cooked and raw spinach was listed as the major source of dietary oxalate in these cohorts. Consumption of a normal portion of spinach (50–100 g) will result in a load of ~500–1,000 mg of dietary oxalate and significantly increase urinary oxalate excretion (21, 23). The contribution of dietary oxalate to stone risk may have been marginalized in these studies due to 1) an inability of participants to accurately recall how often they consumed foods containing large amounts of oxalate over the course of 1 mo, 2) variability in the amount of oxalate-rich food consumed by a participant, and 3) variability in the absorption of the ingested oxalate. In other studies these same investigators showed a greater influence of dietary calcium than dietary oxalate on kidney stone risk (negative correlation) (3, 9, 52). This response was hypothesized to be due to reduced crystalline oxalate in the gut, with low calcium intake resulting in more oxalate absorption and heightened urinary excretion.
Twenty-four-hour urinary oxalate excretion has been used to assess the risk of developing an incident kidney stone. Curhan and Taylor reported a three- to fourfold increase in the relative risk of forming a stone in their health professional cohort compared with groups with either the lowest or highest oxalate excretion (8). Thus the relative risk of stone disease was much higher when 24-h urine oxalate analyses were examined than when food frequency questionnaires were used. In males with oxalate excretion in the normal range, even small increases in urinary oxalate excretion (5 mg/day) doubled kidney stone risk, illustrating that oxalate excretion is a continuous variable. Importantly, Curhan and Taylor also recognized that “24 hour urine collections give a picture of oxalate excretion in a day but it doesn’t provide information regarding low and high periods.” The concept of a diet-induced transient surge in oxalate excretion was previously reported by Erickson et al. (11) in individuals receiving a dietary calcium load. We subsequently showed similar surges in oxalate excretion following oxalate loads (20, 34). Thus it is certainly conceivable that the impact of an oxalate surge following an oxalate-rich meal may not be detected by a 24-h urine collection. Such a surge could be associated with a transient increase in urine supersaturation with CaOx. Prochaska et al. recently showed that moderate changes in relative supersaturation of urine with CaOx are associated with large changes in stone risk (46). Thus it appears that an oxalate excretion >25 mg/day is an increased risk factor for stones and that >40 mg/day should be considered an indicator of primary or secondary hyperoxalurias. Additional studies are warranted to investigate this further.
DIETARY OXALATE AND ITS INTESTINAL ABSORPTION
Oxalate is absorbed by both para- and transcellular mechanisms, and their relative contributions may vary in different intestinal segments (14). SLC26 transporters are thought to play an important role in transcellular transport, with SLC26A3 regulating intestinal absorption (14). The results of a study of 30 non-stone-forming individuals who underwent an absorption test in triplicate indicated that ~5–10% of a soluble oxalate load (50-mg load of sodium [13C2]oxalate) is absorbed. However, absorption can range from 1 to 20%, with a large inter- and intraindividual variability (56). The amount of oxalate absorbed in such a test has been reported to be higher in stone-forming than non-stone-forming individuals (19). However, uncertainties remain, as it is not known how well absorption of the sodium oxalate in this load replicates absorption of food-derived oxalate. In addition, the impact of calcium absorption, gastrointestinal motility, colonization with components of the fecal microbiome including Oxalobacter formigenes, and other potential modifiers is not well defined.
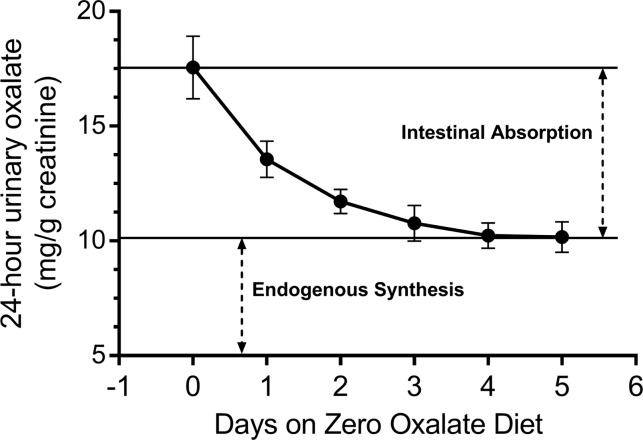
The intestinal absorption of dietary oxalate is largely affected by the solubility (bioavailability) of ingested oxalate. Humans ingest on average 15–25 mmol of calcium per day compared with 1–3 mmol of oxalate, suggesting that, in the intestine, the bulk of oxalate is insoluble, crystalline CaOx. Crystalline oxalate is eliminated in the fecal stream; thus the pool of oxalate that may be absorbed is in a soluble form. The effect of the solubility of oxalate in food was illustrated by Tang et al., who compared urinary excretion of oxalate after ingestion of two substances with similar oxalate content, cinnamon and turmeric, but different degrees of oxalate solubility (50). The estimated oxalate absorption from turmeric, enriched in soluble oxalate, was 8.2% compared with 2.6% from cinnamon, mainly composed of crystalline oxalate. In Fig. 1, the change in oxalate excretion of 12 individuals who switched from a self-selected diet (day 0) to an oxalate-free formula diet is shown (21). Notably, it takes several days for oxalate to be cleared from the gut. This was confirmed by oxalate analyses in the stool of two individuals in whom oxalate was detected on day 4 but not on day 6 (22).
Fig. 1.
Reduction of urinary oxalate excretion of 12 healthy subjects consuming a diet lacking oxalate. Relative amounts of urinary oxalate derived from intestinal absorption and endogenous synthesis are shown. Values are means (SD). [Data were calculated from Holmes et al. (21).]
OXALATE DEGRADATION BY GUT BACTERIA
Studies on the degradation of oxalate in the gut by microorganisms have focused on O. formigenes, which utilizes oxalate as its predominant source of energy and carbon. Colonization with this organism has been reported to decrease the risk of recurrent CaOx stone formation by 70% (29). We found that colonization with this organism resulted in a reduction of urinary oxalate excretion only when subjects ingested a diet low in calcium (400 mg) and moderately high in oxalate (250 mg) (28). A diet low in calcium, as previously mentioned, is known to be a risk factor for stone formation (9), and thus colonization may be protective in this setting. Rat studies have demonstrated that O. formigenes stimulates oxalate secretion from plasma to the gut (2, 17), which may attenuate urinary oxalate excretion and limit stone risk. While initial studies focused on this organism, it is important to recognize that the fecal microbiome contains a number of other organisms that can support O. formigenes survival, metabolize oxalate, and also influence gut transport. Thus, more research focusing on the collective influence of the microbiome in oxalate degradation and transport and how it supports oxalate degradation by O. formigenes is needed (40, 42).
DIETARY OXALATE AND URINARY OXALATE EXCRETION
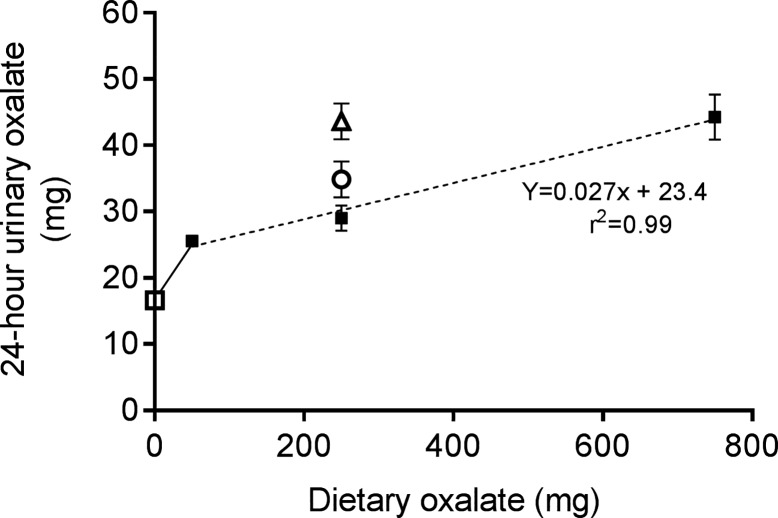
Despite its limitations, a 24-h urine collection is the best available tool to assess the contribution of dietary oxalate to urinary oxalate excretion. Ideally, the intake of dietary oxalate and calcium should be optimally controlled, but this is essentially impractical in patients. It would also be preferable to control other dietary constituents, including hydroxyproline and vitamin C, that may influence the endogenous production of oxalate. Several studies demonstrate that increasing dietary calcium decreases urinary oxalate excretion in individuals on controlled diets (37, 39, 55). We have shown that reducing dietary calcium from 1,000 to 400 mg/day on a 250 mg/day oxalate diet increases mean oxalate excretion by 20.3% in colonized individuals and 50.3% in noncolonized individuals (Fig. 2) (28). This finding underscores the influence of dietary calcium, as well as colonization status, on urinary oxalate excretion. Figure 2 further illustrates the relationship between dietary oxalate and urinary oxalate excretion and reveals that, over a large range of dietary oxalate intakes, 50–750 mg/day, for every 100 mg of oxalate consumed on a 1,000 mg/day calcium diet, urinary oxalate increases by 2.7 mg. With diets containing <50 mg of oxalate per day, the proportion of dietary oxalate absorbed increases more sharply (21). This could result from the majority of the dietary oxalate being soluble at low concentrations or segmental differences in absorption in the small intestine, where paracellular uptake may predominate (31).
Fig. 2.
Urinary oxalate excretion of healthy subjects on oxalate- and calcium-controlled diets: constellations of 39 individuals (19 male, 20 female, 22–43 yr old) from 4 previously published dietary-controlled studies in our laboratory (28, 32, 33, 35). Dotted line represents line of best fit for 1,000-mg calcium diets (■) and 50- to 750-mg dietary oxalate intake. Urinary oxalate excretion increases 2.7 mg for every 100 mg of dietary oxalate ingested. At 250 mg of oxalate intake, data points demonstrate urinary oxalate excretion at 400 mg of calcium in Oxalobacter formigenes-colonized (○) and -noncolonized (∆) individuals. □, Urinary oxalate excretion of individuals on an oxalate-free formula diet (21). Values are means (SD).
DIETARY OXALATE AND RENAL OXALATE HANDLING
The absorbed dietary oxalate is delivered to the kidney, where it is filtered and either secreted or reabsorbed. The relative contributions of these processes with respect to urinary oxalate excretion are not well defined. Bergsland and associates measured the fractional oxalate excretion of normal subjects and CaOx stone-forming individuals on a carefully controlled diet with relatively low oxalate content, 92 mg/day (4). The fractional excretion of oxalate was >1 in some of the stone-forming individuals but none of the controls. This indicates that some oxalate secretion occurs in this setting, even with low oxalate consumption. We previously reported the presence of prominent oxalate secretory fluxes in normal subjects and CaOx kidney stone-forming individuals treated with relatively high soluble oxalate loads (20, 34). Oxalate secretion has also been reported in patients with type I primary hyperoxaluria, and CaOx crystals have been identified in renal proximal tubules of this cohort (58). It is clear that further investigations are needed to assess the impact of dietary oxalate (low and high amounts) on renal oxalate handling, especially responses to higher levels of dietary oxalate consumption.
DIETARY OXALATE AND FOOD INTAKE RECOMMENDATIONS
Efforts to prevent CaOx stone formation should be directed at limiting the supersaturation of urine with CaOx, which is known to impact stone risk (46). Patients need to be aware that excessive oxalate intake and low calcium ingestion in a single meal may transiently increase oxalate absorption and influence the supersaturation of urine with CaOx. A practical strategy is limited consumption of high-oxalate-containing foods, as depicted in Table 1, and maintenance of a normal dietary calcium intake. Patients with enteric hyperoxaluria may be susceptible to development of oxalate nephropathy with high levels of oxalate consumption. Oxalate nephropathy may also occur in individuals with normal gastrointestinal function when they consume foods with a high content of oxalate, especially in its soluble form (1, 7, 10, 16, 49). This oxalate deposition appears to be exacerbated with the onset of chronic kidney disease. Foods implicated include peanuts, rhubarb, star fruit, and “juiced” vegetables. Another approach for patients is consumption of the Dietary Approaches to Stop Hypertension (DASH) diet, which has been shown to be effective in reducing CaOx supersaturation (44).
Table 1.
Simplified dietary instructions to limit urinary oxalate excretion
| Avoid | Limit | Ingest |
|---|---|---|
| Spinach | Potato (<100 g) | Calcium with each meal (300–400 mg) |
| Chard | Chocolate | |
| Rhubarb | Nuts | |
| Star fruit | Beets | |
| Bran |
Administration of enzyme preparations that theoretically lower intestinal oxalate content has also been explored for lowering urinary oxalate excretion. Hoppe and colleagues showed that O. formigenes was well tolerated but did not significantly reduce urinary oxalate excretion in patients with primary hyperoxaluria (25). Furthermore, O. formigenes did not reduce urinary oxalate levels in a randomized phase II/III trial (26). One potential approach to reduce urinary oxalate is the use of ALLN-177, a novel oral enzyme therapy that has been shown to reduce urinary oxalate excretion by degrading intestinal dietary oxalate in humans (36).
FUTURE DIRECTIONS
Since dietary oxalate consumption may play a role in kidney stone growth, better methods of measuring oxalate intake are needed. One approach that warrants evaluation is the utilization of image-based methods (smart phone) to monitor food intake and to estimate oxalate and calcium intake (6). This approach may prove to be more accurate than food frequency questionnaires and written dietary records. If this approach is validated, it is possible that apps could also be developed to enhance dietary compliance.
Another important factor in this process is the role of CaOx crystals in stone formation. Evan and associates elegantly demonstrated the role of Randall’s plaque in idiopathic CaOx stone formation (12, 13). They did not detect CaOx crystals in the nephron of their cohort. However, the subjects were in a unique state: fasted, hydrated, and under general anesthesia. There is a need to address whether surges in oxalate delivery to the kidney with an oxalate-rich meal could result in CaOx crystal formation. Using a nanoparticle analyzer, investigators found nanocrystals of CaOx in human urine (15, 18). Such crystals (<1 µm and not detectable by conventional methods) could be delivered to the urinary space, attach to Randall’s plaque, and result in CaOx stone growth.
Additionally, crystals could stimulate immune responses (5, 27, 30, 38, 43, 47, 53). Although this has not been shown in histology from patients, experimental models have illustrated that CaOx crystals stimulate production of monocyte chemoattractant protein-1 in renal epithelial cells (41, 54). This may likely occur in an effort to remove crystals via the monocyte/macrophage system. We previously determined that a small cohort of CaOx stone-forming individuals have suppressed monocyte cellular energetics (57). We further showed that oxalate causes similar responses in monocytes from healthy subjects (45). Thus, an understanding of the role of dietary oxalate in the generation of immune responses could be of value and warrants further investigation using animal models or dietary feeding studies in humans.
CONCLUSIONS
Dietary oxalate may have an important influence on the risk of formation of CaOx kidney stones. The extent of the impact is difficult to quantify, as current assessments do not focus on transient increases in oxalate excretion that occur with dietary oxalate consumption. A more real-time dietary assessment tool is needed to better quantify such responses. Reduced intake of high-oxalate-containing foods and normal intake of dietary calcium may be a practical method for attenuating CaOx supersaturation and, thus, may limit stone risk in kidney stone-forming individuals.
GRANTS
This study was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-106284 (T. Mitchell), DK-87967 (J. Knight), DK-115833 (K. D. Wood), DK-62284 (D. G. Assimos), and DK-50466 and DK-54468 (R. P. Holmes).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.M., J.K., and R.P.H. prepared figures; T.M., D.G.A., and R.P.H. drafted manuscript; T.M., P.K., T.R., K.D.W., J.K., D.G.A., and R.P.H. edited and revised manuscript; T.M., P.K., T.R., K.D.W., J.K., D.G.A., and R.P.H. approved final version of manuscript.
REFERENCES
- 1.Albersmeyer M, Hilge R, Schröttle A, Weiss M, Sitter T, Vielhauer V. Acute kidney injury after ingestion of rhubarb: secondary oxalate nephropathy in a patient with type 1 diabetes. BMC Nephrol 13: 141, 2012. doi: 10.1186/1471-2369-13-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arvans D, Jung YC, Antonopoulos D, Koval J, Granja I, Bashir M, Karrar E, Roy-Chowdhury J, Musch M, Asplin J, Chang E, Hassan H. Oxalobacter formigenes-derived bioactive factors stimulate oxalate transport by intestinal epithelial cells. J Am Soc Nephrol 28: 876–887, 2017. doi: 10.1681/ASN.2016020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr RG, Wentowski CC, Curhan GC, Somers SC, Stampfer MJ, Schwartz J, Speizer FE, Camargo CA Jr. Prospective study of acetaminophen use and newly diagnosed asthma among women. Am J Respir Crit Care Med 169: 836–841, 2004. doi: 10.1164/rccm.200304-596OC. [DOI] [PubMed] [Google Scholar]
- 4.Bergsland KJ, Zisman AL, Asplin JR, Worcester EM, Coe FL. Evidence for net renal tubule oxalate secretion in patients with calcium kidney stones. Am J Physiol Renal Physiol 300: F311–F318, 2011. doi: 10.1152/ajprenal.00411.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boonla C, Hunapathed C, Bovornpadungkitti S, Poonpirome K, Tungsanga K, Sampatanukul P, Tosukhowong P. Messenger RNA expression of monocyte chemoattractant protein-1 and interleukin-6 in stone-containing kidneys. BJU Int 101: 1170–1177, 2008. doi: 10.1111/j.1464-410X.2008.07461.x. [DOI] [PubMed] [Google Scholar]
- 6.Boushey CJ, Spoden M, Zhu FM, Delp EJ, Kerr DA. New mobile methods for dietary assessment: review of image-assisted and image-based dietary assessment methods. Proc Nutr Soc 76: 283–294, 2017. doi: 10.1017/S0029665116002913. [DOI] [PubMed] [Google Scholar]
- 7.Chen CL, Fang HC, Chou KJ, Wang JS, Chung HM. Acute oxalate nephropathy after ingestion of star fruit. Am J Kidney Dis 37: 418–422, 2001. doi: 10.1053/ajkd.2001.21333. [DOI] [PubMed] [Google Scholar]
- 8.Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int 73: 489–496, 2008. doi: 10.1038/sj.ki.5002708. [DOI] [PubMed] [Google Scholar]
- 9.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 328: 833–838, 1993. doi: 10.1056/NEJM199303253281203. [DOI] [PubMed] [Google Scholar]
- 10.Ellis D, Lieb J. Hyperoxaluria and genitourinary disorders in children ingesting almond milk products. J Pediatr 167: 1155–1158, 2015. doi: 10.1016/j.jpeds.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Erickson SB, Cooper K, Broadus AE, Smith LH, Werness PG, Binder HJ, Dobbins JW. Oxalate absorption and postprandial urine supersaturation in an experimental human model of absorptive hypercalciuria. Clin Sci (Lond) 67: 131–138, 1984. doi: 10.1042/cs0670131. [DOI] [PubMed] [Google Scholar]
- 12.Evan A, Lingeman J, Coe FL, Worcester E. Randall’s plaque: pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int 69: 1313–1318, 2006. doi: 10.1038/sj.ki.5000238. [DOI] [PubMed] [Google Scholar]
- 13.Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, Sommer AJ, Paterson RF, Kuo RL, Grynpas M. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 111: 607–616, 2003. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freel RW, Whittamore JM, Hatch M. Transcellular oxalate and Cl− absorption in mouse intestine is mediated by the DRA anion exchanger Slc26a3, and DRA deletion decreases urinary oxalate. Am J Physiol Gastrointest Liver Physiol 305: G520–G527, 2013. doi: 10.1152/ajpgi.00167.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao J, Xue J-F, Xu M, Gui B-S, Wang F-X, Ouyang J-M. Comparison of physiochemical properties of nano- and microsized crystals in the urine of calcium oxalate stone patients and control subjects. J Nanomater 2014: 790473, 2014. doi: 10.1155/2014/790473. [DOI] [Google Scholar]
- 16.Getting JE, Gregoire JR, Phul A, Kasten MJ. Oxalate nephropathy due to “juicing”: case report and review. Am J Med 126: 768–772, 2013. doi: 10.1016/j.amjmed.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Hatch M, Cornelius J, Allison M, Sidhu H, Peck A, Freel RW. Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int 69: 691–698, 2006. doi: 10.1038/sj.ki.5000162. [DOI] [PubMed] [Google Scholar]
- 18.He JY, Deng SP, Ouyang JM. Morphology, particle size distribution, aggregation, and crystal phase of nanocrystallites in the urine of healthy persons and lithogenic patients. IEEE Trans Nanobioscience 9: 156–163, 2010. doi: 10.1109/TNB.2010.2045510. [DOI] [PubMed] [Google Scholar]
- 19.Hesse A, Schneeberger W, Engfeld S, Von Unruh GE, Sauerbruch T. Intestinal hyperabsorption of oxalate in calcium oxalate stone formers: application of a new test with [13C2]oxalate. J Am Soc Nephrol 10, Suppl 14: S329–S333, 1999. [PubMed] [Google Scholar]
- 20.Holmes RP, Ambrosius WT, Assimos DG. Dietary oxalate loads and renal oxalate handling. J Urol 174: 943–947, 2005. doi: 10.1097/01.ju.0000169476.85935.e2. [DOI] [PubMed] [Google Scholar]
- 21.Holmes RP, Goodman HO, Assimos DG. Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int 59: 270–276, 2001. doi: 10.1046/j.1523-1755.2001.00488.x. [DOI] [PubMed] [Google Scholar]
- 22.Holmes RP, Goodman HO, Assimos DG. Dietary oxalate and its intestinal absorption. Scanning Microsc 9: 1109–1118, 1995. [PubMed] [Google Scholar]
- 23.Holmes RP, Kennedy M. Estimation of the oxalate content of foods and daily oxalate intake. Kidney Int 57: 1662–1667, 2000. doi: 10.1046/j.1523-1755.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- 24.Holmes RP, Knight J, Assimos DG. Lowering urinary oxalate excretion to decrease calcium oxalate stone disease. Urolithiasis 44: 27–32, 2016. doi: 10.1007/s00240-015-0839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoppe B, Groothoff JW, Hulton SA, Cochat P, Niaudet P, Kemper MJ, Deschênes G, Unwin R, Milliner D. Efficacy and safety of Oxalobacter formigenes to reduce urinary oxalate in primary hyperoxaluria. Nephrol Dial Transplant 26: 3609–3615, 2011. doi: 10.1093/ndt/gfr107. [DOI] [PubMed] [Google Scholar]
- 26.Hoppe B, Niaudet P, Salomon R, Harambat J, Hulton SA, Van’t Hoff W, Moochhala SH, Deschênes G, Lindner E, Sjögren A, Cochat P. A randomised phase I/II trial to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Pediatr Nephrol 32: 781–790, 2017. doi: 10.1007/s00467-016-3553-8. [DOI] [PubMed] [Google Scholar]
- 27.Huang MY, Chaturvedi LS, Koul S, Koul HK. Oxalate stimulates IL-6 production in HK-2 cells, a line of human renal proximal tubular epithelial cells. Kidney Int 68: 497–503, 2005. doi: 10.1111/j.1523-1755.2005.00427.x. [DOI] [PubMed] [Google Scholar]
- 28.Jiang J, Knight J, Easter LH, Neiberg R, Holmes RP, Assimos DG. Impact of dietary calcium and oxalate, and Oxalobacter formigenes colonization on urinary oxalate excretion. J Urol 186: 135–139, 2011. doi: 10.1016/j.juro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufman DW, Kelly JP, Curhan GC, Anderson TE, Dretler SP, Preminger GM, Cave DR. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J Am Soc Nephrol 19: 1197–1203, 2008. doi: 10.1681/ASN.2007101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan SR. Role of renal epithelial cells in the initiation of calcium oxalate stones. Nephron, Exp Nephrol 98: e55–e60, 2004. doi: 10.1159/000080257. [DOI] [PubMed] [Google Scholar]
- 31.Knauf F, Ko N, Jiang Z, Robertson WG, Van Itallie CM, Anderson JM, Aronson PS. Net intestinal transport of oxalate reflects passive absorption and SLC26A6-mediated secretion. J Am Soc Nephrol 22: 2247–2255, 2011. doi: 10.1681/ASN.2011040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knight J, Assimos DG, Easter L, Holmes RP. Metabolism of fructose to oxalate and glycolate. Horm Metab Res 42: 868–873, 2010. doi: 10.1055/s-0030-1265145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knight J, Easter LH, Neiberg R, Assimos DG, Holmes RP. Increased protein intake on controlled oxalate diets does not increase urinary oxalate excretion. Urol Res 37: 63–68, 2009. doi: 10.1007/s00240-009-0170-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knight J, Holmes RP, Assimos DG. Intestinal and renal handling of oxalate loads in normal individuals and stone formers. Urol Res 35: 111–117, 2007. doi: 10.1007/s00240-007-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knight J, Jiang J, Assimos DG, Holmes RP. Hydroxyproline ingestion and urinary oxalate and glycolate excretion. Kidney Int 70: 1929–1934, 2006. doi: 10.1038/sj.ki.5001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langman CB, Grujic D, Pease RM, Easter L, Nezzer J, Margolin A, Brettman L. A double-blind, placebo controlled, randomized phase 1 cross-over study with ALLN-177, an orally administered oxalate degrading enzyme. Am J Nephrol 44: 150–158, 2016. doi: 10.1159/000448766. [DOI] [PubMed] [Google Scholar]
- 37.Liebman M, Costa G. Effects of calcium and magnesium on urinary oxalate excretion after oxalate loads. J Urol 163: 1565–1569, 2000. doi: 10.1016/S0022-5347(05)67680-X. [DOI] [PubMed] [Google Scholar]
- 38.Lieske JC, Toback FG. Interaction of urinary crystals with renal epithelial cells in the pathogenesis of nephrolithiasis. Semin Nephrol 16: 458–473, 1996. [PubMed] [Google Scholar]
- 39.Lieske JC, Tremaine WJ, De Simone C, O’Connor HM, Li X, Bergstralh EJ, Goldfarb DS. Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int 78: 1178–1185, 2010. doi: 10.1038/ki.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu M, Koh H, Kurtz ZD, Battaglia T, PeBenito A, Li H, Nazzal L, Blaser MJ. Oxalobacter formigenes-associated host features and microbial community structures examined using the American Gut Project. Microbiome 5: 108, 2017. doi: 10.1186/s40168-017-0316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Z, Wang T, Yang J, Wang S, Yang W, Liu J, Ye Z. Calcium oxalate monohydrate crystals stimulate monocyte chemoattractant protein-1 and transforming growth factor β1 expression in human renal epithelial cells. Mol Med Rep 5: 1241–1244, 2012. doi: 10.3892/mmr.2012.813. [DOI] [PubMed] [Google Scholar]
- 42.Miller AW, Oakeson KF, Dale C, Dearing MD. Effect of dietary oxalate on the gut microbiota of the mammalian herbivore Neotoma albigula. Appl Environ Microbiol 82: 2669–2675, 2016. doi: 10.1128/AEM.00216-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulay SR, Kulkarni OP, Rupanagudi KV, Migliorini A, Darisipudi MN, Vilaysane A, Muruve D, Shi Y, Munro F, Liapis H, Anders HJ. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J Clin Invest 123: 236–246, 2013. doi: 10.1172/JCI63679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noori N, Honarkar E, Goldfarb DS, Kalantar-Zadeh K, Taheri M, Shakhssalim N, Parvin M, Basiri A. Urinary lithogenic risk profile in recurrent stone formers with hyperoxaluria: a randomized controlled trial comparing DASH (Dietary Approaches to Stop Hypertension)-style and low-oxalate diets. Am J Kidney Dis 63: 456–463, 2014. doi: 10.1053/j.ajkd.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 45.Patel M, Yarlagadda V, Adedoyin O, Saini V, Assimos DG, Holmes RP, Mitchell T. Oxalate induces mitochondrial dysfunction and disrupts redox homeostasis in a human monocyte derived cell line. Redox Biol 15: 207–215, 2018. doi: 10.1016/j.redox.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prochaska M, Taylor E, Ferraro PM, Curhan G. Relative supersaturation of 24-hour urine and likelihood of kidney stones. J Urol 199: 1262–1266, 2018. doi: 10.1016/j.juro.2017.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhee E, Santiago L, Park E, Lad P, Bellman GC. Urinary IL-6 is elevated in patients with urolithiasis. J Urol 160: 2284–2288, 1998. doi: 10.1016/S0022-5347(01)62311-5. [DOI] [PubMed] [Google Scholar]
- 48.Robertson WG. Potential role of fluctuations in the composition of renal tubular fluid through the nephron in the initiation of Randall’s plugs and calcium oxalate crystalluria in a computer model of renal function. Urolithiasis 43, Suppl 1: 93–107, 2015. doi: 10.1007/s00240-014-0737-1. [DOI] [PubMed] [Google Scholar]
- 49.Sasaki M, Murakami M, Matsuo K, Matsuo Y, Tanaka S, Ono T, Mori N. Oxalate nephropathy with a granulomatous lesion due to excessive intake of peanuts. Clin Exp Nephrol 12: 305–308, 2008. doi: 10.1007/s10157-008-0046-5. [DOI] [PubMed] [Google Scholar]
- 50.Tang M, Larson-Meyer DE, Liebman M. Effect of cinnamon and turmeric on urinary oxalate excretion, plasma lipids, and plasma glucose in healthy subjects. Am J Clin Nutr 87: 1262–1267, 2008. doi: 10.1093/ajcn/87.5.1262. [DOI] [PubMed] [Google Scholar]
- 51.Taylor EN, Curhan GC. Oxalate intake and the risk for nephrolithiasis. J Am Soc Nephrol 18: 2198–2204, 2007. doi: 10.1681/ASN.2007020219. [DOI] [PubMed] [Google Scholar]
- 52.Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol 15: 3225–3232, 2004. doi: 10.1097/01.ASN.0000146012.44570.20. [DOI] [PubMed] [Google Scholar]
- 53.Umekawa T, Chegini N, Khan SR. Oxalate ions and calcium oxalate crystals stimulate MCP-1 expression by renal epithelial cells. Kidney Int 61: 105–112, 2002. doi: 10.1046/j.1523-1755.2002.00106.x. [DOI] [PubMed] [Google Scholar]
- 54.Umekawa T, Tsuji H, Uemura H, Khan SR. Superoxide from NADPH oxidase as second messenger for the expression of osteopontin and monocyte chemoattractant protein-1 in renal epithelial cells exposed to calcium oxalate crystals. BJU Int 104: 115–120, 2009. doi: 10.1111/j.1464-410X.2009.08374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Unruh GE, Voss S, Sauerbruch T, Hesse A. Dependence of oxalate absorption on the daily calcium intake. J Am Soc Nephrol 15: 1567–1573, 2004. doi: 10.1097/01.ASN.0000127864.26968.7F. [DOI] [PubMed] [Google Scholar]
- 56.von Unruh GE, Voss S, Sauerbruch T, Hesse A. Reference range for gastrointestinal oxalate absorption measured with a standardized [13C2]oxalate absorption test. J Urol 169: 687–690, 2003. doi: 10.1016/S0022-5347(05)63993-6. [DOI] [PubMed] [Google Scholar]
- 57.Williams J, Holmes RP, Assimos DG, Mitchell T. Monocyte mitochondrial function in calcium oxalate stone formers. Urology 93: 224.e1–224.e6, 2016. doi: 10.1016/j.urology.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Worcester EM, Evan AP, Coe FL, Lingeman JE, Krambeck A, Sommers A, Phillips CL, Milliner D. A test of the hypothesis that oxalate secretion produces proximal tubule crystallization in primary hyperoxaluria type I. Am J Physiol Renal Physiol 305: F1574–F1584, 2013. doi: 10.1152/ajprenal.00382.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]