Abstract
Objectives. To evaluate the association of state dense breast notification (DBN) laws with use of supplemental tests and cancer diagnosis after screening mammography.
Methods. We examined screening mammograms (n = 1 441 544) performed in 2014 and 2015 among privately insured women aged 40 to 59 years living in 9 US states that enacted DBN laws in 2014 to 2015 and 25 US states with no DBN law in effect. DBN status at screening mammography was categorized as no DBN, generic DBN, and DBN that mandates notification of possible benefits of supplemental screening (DBN+SS). We used logistic regression to examine the change in rate of supplemental ultrasound, magnetic resonance imaging, breast biopsy, and breast cancer detection.
Results. DBN+SS laws were associated with 10.5 more ultrasounds per 1000 mammograms (95% CI = 3.0, 17.6 per 1000; P = .006) and 0.37 more breast cancers detected per 1000 mammograms (95% CI = 0.05, 0.69 per 1000; P = .02) compared with no DBN law. No significant differences were found for generic DBN laws in either ultrasound or cancer detection.
Conclusions. DBN legislation is associated with increased use of ultrasound and cancer detection after implementation only when notification of the possible benefits of supplemental screening is required.
Breast density is recognized as a risk factor for breast cancer,1 and dense breast tissue can decrease mammographic sensitivity, leading to diagnostic delay.2,3 This is of particular concern to women in their 40s and 50s, for whom the prevalence of dense breasts ranges from 38% to 57%.4 Concerns about the low sensitivity of screening mammography among women with dense breast tissue have led to an increased interest in informing women of the limits of mammography and incorporating additional modalities such as ultrasound and magnetic resonance imaging (MRI) into the screening algorithm. Although the use of breast ultrasound or MRI after screening mammography improves cancer detection among average-risk women with dense breasts,5–9 it results in higher false-positive screenings and has not been demonstrated to improve survival.7,10 Adding to these concerns, prior cost-effectiveness evaluations have indicated that supplemental ultrasound screening is not cost-effective under a wide range of assumptions and screening strategies.11,12
In response to concerns about the reduced efficacy of mammography among women with increased breast density, 36 states have enacted dense breast notification (DBN) laws.13 These laws typically require that mammogram reports provided to women include information regarding whether they have increased breast density.14 Although almost all state notifications suggest communication with a provider (e.g., This information about the results of your mammogram is given to you to raise your awareness and to inform your conversations with your doctor. Together, you can decide which screening options are right for you), some states further require that information about the potential benefits of supplemental screening be included in the notification (e.g., You may benefit from supplementary screening tests, which may include a breast ultrasound screening or a breast MRI examination, or both, depending on your individual risk factors).13 It is notable that the legislative response to concerns about screening among women with dense breasts has focused on providing information to women rather than on requiring insurance coverage of supplemental tests. Although the impact of legislation requiring insurance coverage for cancer screening has been widely studied,15,16 the impact of laws that communicate medical information has not been well evaluated.
Although reports have indicated that use of supplemental ultrasound increased in certain states after DBN laws were introduced,17,18 whether these laws are associated with population-level changes in breast cancer biopsy or breast cancer detection is unknown. To address these knowledge gaps, we conducted a retrospective cohort study of women aged 40 to 59 years receiving a screening mammography and assessed associations between 2 categories of state DBN laws and receipt of any supplemental ultrasound, MRI, or breast biopsy, and cancer detection. Given the strong association between breast density and age, we also assess potential differences in such effects by age at mammography.
METHODS
We conducted a difference-in-differences analysis using administrative claims data (2013–2016) to assess the changes in relevant outcomes in 9 states that implemented DBN laws during this period compared with changes in a comparison group of 25 states that did not implement DBN laws.
Data Source and Study Population
We used administrative claims data obtained through a secure portal from the Blue Cross Blue Shield (BCBS) Alliance for Health Research, with members in all 50 US states, to identify women aged 40 to 59 years who underwent screening mammography from January 1, 2014, through December 31, 2015. Screening ultrasound and MRI were covered benefits during the period studied but were still subject to copayments, coinsurance and deductibles. Women were required to have continuous enrollment with primary medical coverage in a BCBS plan in the 9 months before screening mammography, the full calendar year of screening, and in the 9 months after screening. Screening mammography was identified from claims data by adapting a validated algorithm.19 Specifically, we omitted women who received a diagnostic mammogram or breast-related diagnosis or procedure in the 9 months before the index mammogram. Detailed inclusion criteria are available in Figure A (available as a supplement to the online version of this article at http://www.ajph.org).
Because we relied on the introduction of new DBN laws to identify effects, we excluded women residing in states that had laws in place during the first 2 quarters of our study period or that implemented a law within the last 2 quarters of our study period. We also omitted women residing in states that had legislation requiring insurance coverage for supplemental screening during the study period, because of potential confounding and the timing of legislation outside of our study period (Figure B, available as a supplement to the online version of this article at http://www.ajph.org). We excluded mammograms performed in a transition period (DBN law in effect 1–5 months) out of concern that providers might experience delays in implementing DBN and that near-term ultrasound appointments might not have been available during this period.20 These exclusion criteria resulted in 9 states that implemented DBN laws during our study period and 25 control states with no law in effect.
Type of DBN Legislation
On the basis of the member’s state of residence and date of mammogram, we characterized DBN status as no DBN law in effect (25 states, Figure B), generic DBN law with no mention of the possible benefits of supplemental screening (5 states: AZ, OH, MN, ND, TN), and DBN law with mandated notification of the possible benefits of supplemental screening (DBN+SS; 4 states: MA, MI, MO, RI). The exact language of the notification in these 9 states is in Table A (available as a supplement to the online version of this article at http://www.ajph.org).
Supplemental Tests and Cancer Detection
We assessed receipt of supplemental breast ultrasound, MRI, and biopsy within 4 months after index screening mammography and breast cancer detection within 9 months after screening mammography. Receipt of supplemental testing was identified using International Classification of Diseases, Ninth and Tenth Revisions procedure and diagnosis codes, Current Procedural Terminology codes, and Healthcare Common Procedure Coding System codes (Table B, available as a supplement to the online version of this article at http://www.ajph.org). We considered receipt of either screening or diagnostic supplemental ultrasound or MRI because information in claims did not allow us to distinguish screening tests from diagnostic tests during the study period. Breast ultrasound, MRI, and biopsy received on the same day as index mammogram were included as supplemental tests. We adapted a validated algorithm to identify incident invasive breast cancer, requiring a breast cancer diagnosis within 4 months after screening mammography and breast-directed surgery within 9 months after screening.21
Statistical Analysis
We compared unadjusted outcomes for mammograms provided during a preimplementation period when no study state had a DBN law (January 1, 2014–June 30, 2014) with a postimplementation period during which all 9 study states had DBN laws in place (July 1, 2015–December 31, 2015). We limited the evaluation of unadjusted outcomes to a comparison of the first and last 6 months of the study period to avoid confounding the effect of law implementation with secular trends in outcomes.
Logistic regression models included variables indicating whether a DBN or a DBN+SS law was in effect, age (40–49 vs 50–59 years), a linear time variable reflecting number of months since the beginning of the study period, and state of residence. By including state in our models, we implicitly considered whether DBN was associated with changes in outcomes within states before and after implementation, controlling for changes in states with no DBN law. This controls for time-invariant characteristics between states. We also assessed the interaction of DBN laws with age as a proxy indicator for breast density because younger women are more likely to have dense breasts. A key assumption of our study design is that in the absence of policy change, that is, no law, DBN and DBN+SS states would have experienced similar trends in outcomes. We tested this assumption by estimating a model predicting each outcome using binary month indicators interacted with the 2 DBN groups before implementation of any DBN laws in our study cohort.
In all models, we used clustered robust standard errors (clustering on state of residence) and transformed predicted probabilities to adjusted rates per 1000 mammograms with 95% confidence intervals (CIs).22,23 All analyses were performed using Microsoft SQL Server Management Studio v17.0 (Microsoft Corp, Redmond, WA) and Stata version 14.1 (Stata Corp, College Station, TX). Statistical tests were 2-sided, with significance set at α = .05.
RESULTS
We identified 1 441 544 screening mammograms among 1 160 079 distinct women aged 40 to 59 years from January 1, 2014, through December 31, 2015, living in 34 US states.
Effects on Screening
The unadjusted rate of supplemental testing or cancer detection per 1000 mammograms did not change significantly in states that did not adopt a DBN law (Table 1). Among states that adopted a generic DBN law, there was a very small but statistically significant 0.81 per 1000 increase in supplemental MRI (95% CI = 0.10, 1.5 per 1000). States that adopted a DBN+SS law experienced a statistically significant 11.2 per 1000 mammograms increase in supplemental ultrasound (95% CI = 8.6, 13.7 per 1000; Table 1), with no statistically significant changes in other outcomes studied. For each of the 4 outcomes, parallel trends tests indicated that in the first 6 months of the study, there were no significant differences in trends across the 3 categories of DBN law.
TABLE 1—
Unadjusted Supplemental Testing and Cancer Detection Rates per 1000 Mammograms Before and After Implementation of DBN Laws: 34 US States, January 1, 2014–December 31, 2015
| Type of DBN law | Before DBN Implementation (Jan 1–Jun 30, 2014) (95% CI) | After DBN Implementation (Jul 1–Dec 31, 2015) (95% CI) | Absolute Change (95% CI) |
| No DBN | |||
| Ultrasound | 74.4 (74.1, 75.4) | 74.4 (73.3, 75.5) | −0.01 (−1.5, 1.5) |
| MRI | 4.37 (41.0, 46.6) | 4.30 (4.04, 4.58) | −0.07 (−0.46, 0.31) |
| Biopsy | 17.0 (16.5, 17.5) | 16.1 (15.6, 16.6) | −0.89 (−1.6, 0.14) |
| Cancer detection | 2.31 (2.12, 2.52) | 2.05 (1.86, 2.24) | −0.27 (−0.54, 0.06) |
| Generic DBN | |||
| Ultrasound | 72.3 (70.4, 74.3) | 68.9 (66.9, 70.9) | −3.4 (−6.2, 0.67) |
| MRI | 3.81 (3.37, 4.30) | 4.62 (4.10, 5.18) | 0.81* (0.10, 1.5) |
| Biopsy | 15.8 (14.9, 16.8) | 15.8 (14.8, 16.8) | −0.07 (−1.4, 1.3) |
| Cancer detection | 2.06 (1.74, 2.43) | 2.24 (1.88, 2.64) | 0.17 (−0.17, 0.33) |
| DBN+SS | |||
| Ultrasound | 71.3 (69.7, 72.9) | 82.5 (79.5, 85.8) | 11.2* (8.6, 13.7) |
| MRI | 4.05 (3.67, 4.47) | 4.35 (3.89, 4.86) | 0.30 (−0.32, 0.92) |
| Biopsy | 18.7 (17.8, 19.5) | 17.1 (16.1, 18.0) | −1.6 (−2.9, 0.35) |
| Cancer detection | 2.24 (1.96, 2.56) | 2.60 (2.04, 3.35) | 0.35 (−0.11, 0.83) |
Note. CI = confidence interval; DBN = dense breast notification; DBN+SS = dense breast notification with mandated notification of the possible benefits of supplemental screening; MRI = magnetic resonance imaging. The study sample contained 1 441 544 mammograms representing 1 160 079 women, with 51.9% and 48.1% receiving a mammogram in 2014 and 2015 respectively. Among women with 2 mammograms in our sample, the average time between mammograms was 13 months (range = 10–23 months), with only 1.2% of women receiving the second mammogram less than 12 months after the first. Rates are per 1000 screening mammograms.
P < .05.
In adjusted analysis, the baseline supplemental ultrasound rate, defined as the rate for all mammograms provided when no DBN law was in effect, was 74.3 per 1000 mammograms (95% CI = 73.8, 74.7 per 1000). The increase in supplemental ultrasound was significantly higher among screening mammograms performed in states with DBN+SS laws than among those performed in states with no law (10.5 per 1000; 95% CI = 2.95, 17.6 per 1000; P = .006) as well as among states with generic DBN laws (9.2 per 1000; 95% CI = 1.31, 16.7 per 1000; P = .02). We found no significant differences in the supplemental MRI rate across categories of DBN status (Table 2). The baseline biopsy rate was 17.0 per 1000 mammograms (95% CI = 16.8, 17.3 per 1000). Most biopsies performed (72.9%) were among women who received supplemental ultrasound or MRI; however, we identified no significant differences in biopsy across categories of DBN law (Table 2).
TABLE 2—
Association of State DBN Laws with Supplemental Testing and Cancer Detection, Adjusted Rates per 1000 Screening Mammograms: 34 US States, January 1, 2014–December 31, 2015
| Ultrasound | MRI | Biopsy | Cancer Detection | |||||
| Rate (95% CI) | P | Rate (95% CI) | P | Rate (95% CI) | P | Rate (95% CI) | P | |
| Overall predicted means | ||||||||
| Type of DBN law | ||||||||
| No law (Ref) | 71.1 (70.5, 71.7) | 4.09 (4.04, 4.16) | 16.6 (16.5, 16.7) | 2.11 (2.04, 2.19) | ||||
| Generic DBN | 72.3 (69.2, 75.5) | .48 | 4.58 (4.11, 5.05) | .07 | 17.1 (16.1, 18.1) | .50 | 2.19 (1.95, 2.43) | .59 |
| DBN+SS | 81.5 (74.6, 88.5) | .006 | 4.55 (3.91, 5.20) | .19 | 16.3 (15.4, 17.2) | .65 | 2.48 (2.20, 2.76) | .022 |
| Age, y | ||||||||
| 40–49 (Ref) | 93.0 (92.1, 93.8) | 4.44 (4.21, 4.67) | 19.3 (19.0, 19.5) | 1.61 (1.50, 1.72) | ||||
| 50–59 | 61.9 (61.3, 62.5) | < .001 | 4.26 (4.11, 5.20) | .35 | 15.4 (15.2, 17.2) | < .001 | 2.61 (2.49, 2.72) | < .001 |
| Predicted differences in means | ||||||||
| Generic DBN vs no law | 1.25 (−2.26, 4.77) | .48 | 0.48 (−0.04, 0.99) | .07 | 0.45 (−0.63, 1.54) | .41 | 0.08 (−0.21, 0.37) | .59 |
| DBN+SS vs no law | 10.5 (2.95, 17.6) | .006 | 0.46 (−0.23, 1.15) | .19 | −0.29 (−1.27, 0.70) | .57 | 0.37 (0.05, 0.69) | .022 |
| DBN+SS vs generic DBN | 9.2 (1.31, 16.7) | .025 | −0.02 (−0.81, 0.77) | .96 | −0.74 (−1.98, 049) | .24 | 0.29 (−0.02, 0.60) | .07 |
Note. CI = confidence interval; DBN = dense breast notification; DBN+SS = dense breast notification with mandated notification of the possible benefits of supplemental screening; MRI = magnetic resonance imaging. The study sample contained 1 441 544 mammograms representing 1 160 079 women. This represents 570 635 mammograms among women aged 40–49 years and 870 909 mammograms among women aged 50–59 years. Adjusted rates are per 1000 screening mammograms.
Effects on Cancer Detection
The baseline cancer detection rate was 2.22 per 1000 mammograms (95% CI = 2.13, 2.30 per 1000). The cancer detection rate in states with no law in effect was 2.11 per 1000 (95% CI = 2.04, 2.19 per 1000), compared with states with generic DBN laws (2.19 per 1000; 95% CI = 1.95, 2.43 per 1000) and states with DBN+SS laws (2.48 per 1000; 95% CI = 2.20, 2.76 per 1000; Table 2). The increase in the cancer detection rate reached significance for mammograms performed in states with DBN+SS laws compared with states with no law in effect (difference = 0.37 per 1000; 95% CI = 0.05, 0.69 per 1000; P = .02), but the increase did not reach significance compared with states with generic DBN laws (0.29 per 1000; 95% CI = −0.02, 0.60 per 1000; P = .07).
Differential Effects by Age
Overall, younger women were significantly more likely to receive ultrasound and biopsy after screening mammography (Table 2). Cancer detection was significantly lower among younger women.
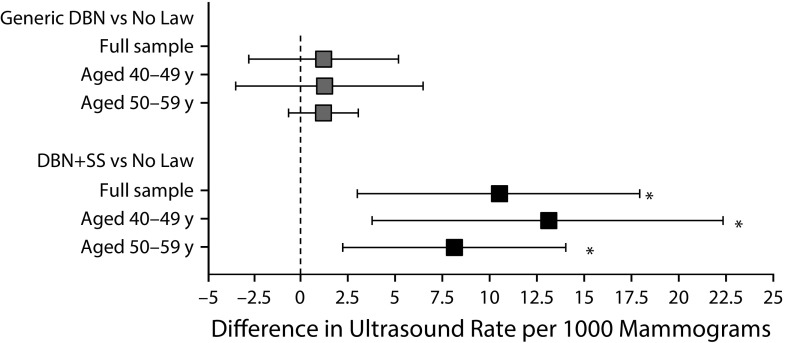
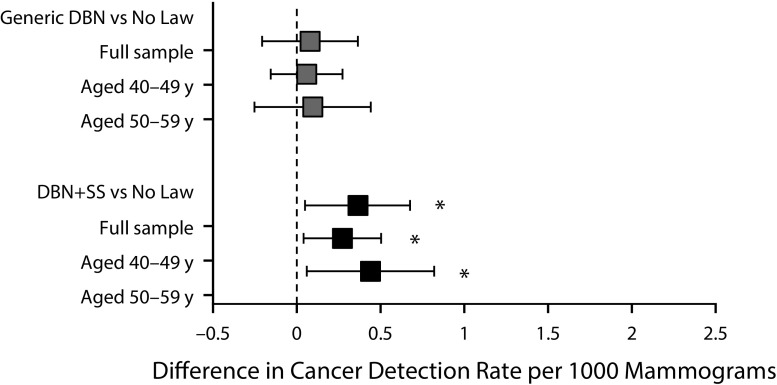
The increase in the rate of supplemental ultrasound (Figure 1) and cancer detection (Figure 2) in states with DBN+SS laws compared with states with no law was significant for both age groups. When we tested the interaction between type of DBN law and age, we identified small yet significant age effects for the magnitude of change in supplemental MRI and biopsy. Among younger women aged 40 to 49 years, we found a significant increase in the rate of supplemental MRI (0.94 per 1000; 95% CI = 0.15, 1.75 per 1000; P = .02) for mammograms performed in states with DBN+SS laws compared with states with no law in effect; however, among women aged 50 to 59 years, an increase in the rate of supplemental MRI was identified only for mammograms performed in states with generic DBN laws compared with states with no law in effect (Figure C, available as a supplement to the online version of this article at http://www.ajph.org). For biopsy, the only significant increase occurred among women aged 50 to 59 years living in states with generic DBN laws compared with states with no law (Figure C).
FIGURE 1—
Difference in Supplemental Ultrasound Rate by Type of DBN Law, Overall and by Age: 34 US States, January 1, 2014–December 31, 2015
Note. DBN = dense breast notification; DBN+SS = dense breast notification with mandated notification of the possible benefits of supplemental screening.
*P < .05.
FIGURE 2—
Difference in Cancer Detection Rate by Type of DBN Law, Overall and by Age: 34 US States, January 1, 2014–December 31, 2015
Note. DBN = dense breast notification; DBN+SS = dense breast notification with mandated notification of the possible benefits of supplemental screening.
*P < .05.
DISCUSSION
In this study assessing the impact of state DBN laws, we found significant increases in the use of supplemental breast ultrasound and the cancer detection rate after screening mammography within 6 to 18 months of states’ implementation of DBN+SS laws. Although statistically significant, the 10.5 per 1000 mammograms increase in the use of supplemental ultrasound in states with DBN+SS laws suggests a relatively small incremental uptake rate among women with dense breasts. Prior work has reported that approximately 38% to 57% of women aged 40 to 59 years have heterogeneously dense or extremely dense breasts,4 suggesting that for every 1000 women receiving a screening mammogram, at least 380 were required to be notified about the increased risks of dense breasts and the potential benefits of supplemental screening. Notably, this low uptake is for an insured population with plans that cover breast ultrasounds and is among women already receiving a screening mammogram, suggesting that these women perceive some benefits to breast cancer screening.
Despite the relatively low uptake in supplemental ultrasound, we observed a significant increase in cancer detection (0.37 per 1000 mammograms) in states with DBN+SS laws. This finding suggests that the type of information delivered in the notification has an impact on utilization and potentially on clinical outcomes. Women in states with DBN+SS laws may be more likely to approach their provider regarding supplemental testing or may perceive more benefits of supplemental testing. In addition, providers in these states may be more likely than other providers to recommend additional testing. Overall, our findings suggest that if one of the goals of DBN laws is to suggest that women discuss supplemental imaging with their provider and thereby increase utilization among women at the highest risk, the specific information delivered is important.
Overall, we found increases in ultrasound rates similar to those identified by Horný et al.17,18 It is reassuring that although the studies are not directly comparable because of the differences in population age, states studied, follow-up time, and methodological approach, they also found relatively small increases in ultrasound use. Our study further examined changes in biopsy and breast cancer detection rates and differences in receipt of supplemental testing and other outcomes by age.
In addition to the unclear language of many notification laws,13 there are several reasons why mandated DBN may lead to relatively low uptake of services. Clinical uncertainty surrounds the utility of supplemental ultrasound and MRI screening; therefore, clinicians may be less likely to recommend them to women.24 Moreover, DBN laws may be more difficult to enforce than other policies. For example, since 1986 federal law has required the distribution of Centers for Disease Control and Prevention vaccine information sheets noting the risks and benefits to vaccine recipients (or their parents). Yet, 1 study indicated that in 1998 only about 69% of pediatricians self-reported that they distributed these sheets.25 Others have commented on whether and how communication mandates interfere with patient–provider relations. For example, there has been significant controversy related to state laws that limit specific communication between providers and patients related to gun ownership26 and other laws that require certain information be provided during counseling to women before abortion.27
Although we found limited clinical impact except in situations in which the DBN law specifically mentions the benefit of supplemental screening, these laws may empower women to make more informed decisions about whether to pursue supplemental testing. We were not able to directly determine whether DBN led to more women inquiring about the increased risk of breast cancer among women with dense breasts, but in a survey of screening-age women, residents of a state with DBN were more likely to have discussed their breast density with a health care provider than were residents of other states (67% vs 43%).28 However, about 50% of primary care physicians in California were not aware of the California law 10 months after implementation, and only 5% felt “completely comfortable” answering questions about breast density.29
In some cases, perhaps because of past mammogram findings, providers may order an ultrasound and mammogram for the same day; if this practice is affected by DBN laws, it will be included in our estimates. Considering only the last 6 months of data for our sample, the proportion of ultrasounds occurring on the same day as screening mammograms was 15.4%, 14.3%, and 13.8% in no DBN, generic DBN, and DBN+SS states, respectively. This result suggests that although this occurrence is not rare, the majority of supplemental screening ultrasounds are not conducted on the same day as mammography.
In part, DBN laws were passed in response to observational cohort studies that indicated that supplemental screening led to improved cancer detection among high-risk women, albeit alongside high recall and false-positive rates.5 A recent US Preventive Services Task Force review summarized extant studies, noting that in the best-rated studies, supplemental ultrasound resulted in additional cancer detection of 4.4 per 1000, with a recall rate of 14%.30 In the context of the low incremental uptake of supplemental ultrasound found in our study, the 0.37 increase in cancer detection per 1000 mammograms we found is relatively large, although the 95% confidence interval of 0.05, 0.69 includes relatively small increases. In a hypothetical population of 1 million women aged 40 to 59 years, we would expect an additional 370 cancers detected in the 6 to 18 months after implementation of DBN+SS legislation, although the lower bound of the confidence interval suggests this could be as low as 50 cancers per 1 million women. The point estimate indicating a high rate of cancer identification found relative to ultrasound uptake may be due to providers recommending ultrasounds only for the highest risk women. For example, when counseling patients, providers may consider available information on breast cancer risk, including patient age, family history of breast cancer, and the woman’s reproductive history. Finally, it is important to note that even though some DBN laws were associated with the diagnosis of additional breast cancer, our study does not examine whether this leads to a decrease in cancer morbidity and mortality.30
In addition to possible benefits, consideration of potential adverse consequences of DBN is warranted. Health system costs may be substantial, although our results indicate that these may be less than originally hypothesized because the increase in ultrasound and biopsy was less than anticipated. However, women who receive DBN may experience increased anxiety, regardless of whether they ultimately receive supplemental imaging. In 1 convenience sample of women with dense breasts, 43% reported that knowledge of their breast density increased their anxiety about getting breast cancer.31
This study is not without limitations. The BCBS administrative claims data do not contain clinical information on individual breast density or cancer staging or enough information to distinguish screening from diagnostic tests. Because of the relatively short follow-up period (maximum 18 months after implementation), we could not evaluate breast cancer morbidity or mortality or determine whether the effect of DBN laws increased over time. We limited our analyses to individuals who remained continuously enrolled in an insurance plan for 2 years or more. To the extent that the effect of DBN laws differs among individuals who switch plans, our study will not capture these effects. We used claims-based algorithms that were originally validated in older women aged 65 years or older to identify receipt of screening mammography as well as to identify invasive screening-detected cancer in a population of women aged 40 to 59 years. Moreover, our study period resulted in analysis of only 9 states with mandatory DBN laws.
This study provides useful information to state policymakers considering DBN legislation with the goal of increasing awareness and early breast cancer detection. We find that laws that specifically mention the potential benefit of supplemental imaging are associated with small increases in use of breast ultrasound after screening mammography as well as increases in breast cancer detection. Some states may consider amending existing laws to include this language.
ACKNOWLEDGMENTS
Financial support for the research and the work was awarded to C. P. Gross by the American Cancer Society (grant RSGI-15-151-01) and to I. B. Richman by the Yale Center for Clinical Investigation (grant KL2-TR001862).
This work was previously presented at the 2018 American Society of Clinical Oncology Annual Meeting.
Note. The funder was not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication. The authors participate in the BCBS Alliance for Health Research. The Blue Cross Blue Shield Association established the Alliance to engage leading US health care researchers in collaborative efforts to use BCBS companies’ commercial insurance claims data to explore critical health care issues to improve the health of Americans. The BCBS Alliance for Health Research provides researchers with use of a secure data portal to access a limited data set drawn from BCBS Axis, a large collection of commercial insurance claims, medical professional, and cost of care information. BCBS Association is an association of independent BCBS companies.
CONFLICTS OF INTEREST
C. P. Gross has received research funding from 21st Century Oncology, Johnson & Johnson, and Pfizer. In addition, C. P. Gross has received travel reimbursement from Flatiron Health. I. B. Richman has received funding under contract with the Centers for Medicare and Medicaid Services. No other disclosures are reported.
HUMAN PARTICIPANT PROTECTION
The study protocol was approved by the Yale University Human Investigation Committee.
Footnotes
See also Fedewa, p. 660.
REFERENCES
- 1.Nelson HD, Zakher B, Cantor A et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and meta-analysis. Ann Intern Med. 2012;156(9):635–648. doi: 10.1059/0003-4819-156-9-201205010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandelson MT, Oestreicher N, Porter PL et al. Breast density as a predictor of mammographic detection: comparison of interval-and screen-detected cancers. J Natl Cancer Inst. 2000;92(13):1081–1087. doi: 10.1093/jnci/92.13.1081. [DOI] [PubMed] [Google Scholar]
- 3.Kerlikowske K, Zhu W, Tosteson AN et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162(10):673–681. doi: 10.7326/M14-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sprague BL, Gangnon RE, Burt V et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10):dju255. doi: 10.1093/jnci/dju255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg WA, Blume JD, Cormack JB et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299(18):2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corsetti V, Houssami N, Ghirardi M et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7):1021–1026. doi: 10.1016/j.ejca.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Siu AL. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279–296. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 8.Weinstein SP, Localio AR, Conant EF, Rosen M, Thomas KM, Schnall MD. Multimodality screening of high-risk women: a prospective cohort study. J Clin Oncol. 2009;27(36):6124–6128. doi: 10.1200/JCO.2009.24.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283(2):361–370. doi: 10.1148/radiol.2016161444. [DOI] [PubMed] [Google Scholar]
- 10.Berg WA, Zhang Z, Lehrer D et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404. doi: 10.1001/jama.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sprague BL, Stout NK, Schechter C et al. Benefits, harms, and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162(3):157–166. doi: 10.7326/M14-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas JS, Kaplan CP. The divide between breast density notification laws and evidence-based guidelines for breast cancer screening: legislating practice. JAMA Intern Med. 2015;175(9):1439–1440. doi: 10.1001/jamainternmed.2015.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kressin NR, Gunn CM, Battaglia TA. Content, readability, and understandability of dense breast notifications by state. JAMA. 2016;315(16):1786–1788. doi: 10.1001/jama.2016.1712. [DOI] [PubMed] [Google Scholar]
- 14.Are You Dense Advocacy, Inc. Are you dense? Advocacy because your life matters. 2018. Are You Dense? Advocacy website. Available at https://www.areyoudenseadvocacy.org. Accessed March 13, 2019.
- 15.Sommers BD, Gunja MZ, Finegold K, Musco T. Changes in self-reported insurance coverage, access to care, and health under the Affordable Care Act. JAMA. 2015;314(4):366–374. doi: 10.1001/jama.2015.8421. [DOI] [PubMed] [Google Scholar]
- 16.Bitler MP, Carpenter CS. Health insurance mandates, mammography, and breast cancer diagnoses. Am Econ J Econ Policy. 2016;8(3):39–68. doi: 10.1257/pol.20120298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horný M, Cohen AB, Duszak R, Jr, Christiansen CL, Shwartz M, Burgess JF., Jr Dense breast notification laws: impact on downstream imaging after screening mammography. Med Care Res Rev. 2018;1077558717751941 doi: 10.1177/1077558717751941. [DOI] [PubMed] [Google Scholar]
- 18.Horný M, Shwartz M, Duszak R, Jr, Christiansen CL, Cohen AB, Burgess JF., Jr Characteristics of State policies impact health care delivery: an analysis of mammographic dense breast notification and insurance legislation. Med Care. 2018;56(9):798–804. doi: 10.1097/MLR.0000000000000967. [DOI] [PubMed] [Google Scholar]
- 19.Fenton JJ, Zhu W, Balch S, Smith-Bindman R, Fishman P, Hubbard RA. Distinguishing screening from diagnostic mammograms using Medicare claims data. Med Care. 2014;52(7):e44–e51. doi: 10.1097/MLR.0b013e318269e0f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology. 2012;265(1):59–69. doi: 10.1148/radiol.12120621. [DOI] [PubMed] [Google Scholar]
- 21.Fenton JJ, Onega T, Zhu W et al. Validation of a Medicare claims-based algorithm for identifying breast cancers detected at screening mammography. Med Care. 2016;54(3):e15–e22. doi: 10.1097/MLR.0b013e3182a303d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cameron AC, Miller DL. A practitioner’s guide to cluster-robust inference. J Hum Resour. 2015;50(2):317–372. [Google Scholar]
- 23.Bertrand M, Duflo E, Mullainathan S. How much should we trust differences-in-differences estimates? Q J Econ. 2004;119(1):249–275. [Google Scholar]
- 24.Slanetz PJ, Freer PE, Birdwell RL. Breast-density legislation—practical considerations. N Engl J Med. 2015;372(7):593–595. doi: 10.1056/NEJMp1413728. [DOI] [PubMed] [Google Scholar]
- 25.Davis TC, Fredrickson DD, Arnold CL et al. Childhood vaccine risk/benefit communication in private practice office settings: a national survey. Pediatrics. 2001;107(2):E17. doi: 10.1542/peds.107.2.e17. [DOI] [PubMed] [Google Scholar]
- 26.Parmet WE, Smith JA, Miller MJ. Wollschlaeger v. Governor of Florida—the First Amendment, physician speech, and firearm safety. N Engl J Med. 2016;374(24):2304–2307. doi: 10.1056/NEJMp1605740. [DOI] [PubMed] [Google Scholar]
- 27.An Overview of Abortion Laws. 2018. Available at https://www.guttmacher.org/state-policy/explore/overview-abortion-laws. Accessed April 9, 2018.
- 28.Rhodes DJ, Breitkopf CR, Ziegenfuss JY, Jenkins SM, Vachon CM. Awareness of breast density and its impact on breast cancer detection and risk. J Clin Oncol. 2015;33(10):1143–1150. doi: 10.1200/JCO.2014.57.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khong KA, Hargreaves J, Aminololama-Shakeri S, Lindfors KK. Impact of the California breast density law on primary care physicians. J Am Coll Radiol. 2015;12(3):256–260. doi: 10.1016/j.jacr.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melnikow J, Fenton JJ, Whitlock EP et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2016;164(4):268–278. doi: 10.7326/M15-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moothathu NS, Philpotts LE, Busch SH, Gross CP, Staib LH, Hooley RJ. Knowledge of density and screening ultrasound. Breast J. 2017;23(3):323–332. doi: 10.1111/tbj.12734. [DOI] [PubMed] [Google Scholar]