Abstract
Rationale:
The heart contains abundant interstitial and perivascular fibroblasts. Traditional views suggest that, under conditions of mechanical stress, cytokines, growth factors and neurohumoral mediators stimulate fibroblast activation, inducing extracellular matrix protein synthesis, and promoting fibrosis and diastolic dysfunction. Members of the Transforming Growth Factor (TGF)-β family are upregulated and activated in the remodeling myocardium and modulate phenotype and function of all myocardial cell types through activation of intracellular effector molecules, the Smads, and through Smad-independent pathways.
Objectives:
To examine the role of fibroblast-specific TGF-β/Smad3 signaling in the remodeling pressure-overloaded myocardium.
Methods and Results:
We examined the effects of cell-specific Smad3 loss in activated periostin-expressing myofibroblasts using a mouse model of cardiac pressure overload, induced through transverse aortic constriction (TAC). Surprisingly, myofibroblast-specific Smad3 knockout (FS3KO) mice exhibited accelerated systolic dysfunction following pressure overload, evidenced by an early 40% reduction in ejection fraction after 7 days of TAC. Accelerated systolic dysfunction in pressure-overloaded FS3KO mice was associated with accentuated matrix degradation and generation of collagen-derived matrikines, accompanied by cardiomyocyte myofibrillar loss and apoptosis, and by enhanced macrophage-driven inflammation. In vitro, TGF-β1, TGF-β2 and TGF-β3 stimulated a Smad3-dependent matrix-preserving phenotype in cardiac fibroblasts, suppressing matrix metalloproteinase (MMP)3 and MMP8 synthesis and inducing tissue inhibitor of metalloproteinases (TIMP)1. In vivo, administration of an MMP8 inhibitor attenuated early systolic dysfunction in pressure-overloaded FS3KO mice, suggesting that the protective effects of activated cardiac myofibroblasts in the pressure-overloaded myocardium are, at least in part, due to suppression of MMPs and activation of a matrix-preserving program. MMP8 stimulation induces a pro-inflammatory phenotype in isolated macrophages.
Conclusions:
In the pressure-overloaded myocardium, TGF-β/Smad3-activated cardiac fibroblasts play an important protective role, preserving the extracellular matrix network, suppressing macrophage-driven inflammation and attenuating cardiomyocyte injury. The protective actions of the myofibroblasts are mediated, at least in part, through Smad-dependent suppression of matrix-degrading proteases.
Subject Terms: Fibrosis, Growth factors/Cytokines, Inflammation, Pathophysiology, Remodeling
Keywords: Fibroblast, TGF-β, Smad, macrophage, matrix metalloproteinases, cardiac remodeling, extracellular matrix, inflammation
INTRODUCTION
The adult mammalian heart contains a large pool of fibroblast-like interstitial cells that can be activated following injury to repair the myocardium, but also participate in maladaptive cardiac fibrosis1,2,3. Most cardiac pathologic conditions are associated with expansion of the interstitial fibroblast population. Following myocardial infarction, cardiomyocyte necrosis triggers an inflammatory cascade that clears the wound from dead cells, and leads to activation of reparative myofibroblasts that secrete collagens, preserving the structural integrity of the ventricle4,5,. Genetic cardiomyopathies, valvular diseases, metabolic conditions such as diabetes and obesity, and myocarditis are also associated with expansion and activation of fibroblasts, and with increased deposition of extracellular matrix (ECM) proteins in the cardiac interstitium. In conditions associated with cardiac pressure overload, resident fibroblast lineages proliferate and undergo activation6,7. Stimulation of mechanosensitive pathways activates a matrix-synthetic program in cardiac fibroblasts, leading to a progressive increase in interstitial collagen content, associated with diastolic dysfunction. However, the molecular signals responsible for fibroblast activation in the pressure-overloaded heart remain poorly understood.
Extensive evidence suggests that members of the Transforming Growth Factor (TGF)-β superfamily critically modulate fibroblast phenotype and are involved in the pathogenesis of tissue fibrosis8,9,10. TGF-βs signal by sequentially transphosphorylating type II and type I receptors, activating downstream signaling cascades that may involve a family of intracellular effectors, the Smads, or Smad-independent pathways11. Evidence derived from global loss-of-function models and in vitro experiments suggest that Smad3 plays an important role in tissue fibrosis in a wide range of pathophysiologic conditions12,13,14,15,16,17. In the injured and remodeling myocardium, global Smad3 loss attenuated cardiac fibrosis, reducing interstitial collagen deposition12,13,16,17. However, considering the broad effects of Smad3 signaling in all cell types involved in cardiac remodeling, the relative significance of fibroblast-specific Smad3 signaling remains unclear. In the current study, we tested the hypothesis that fibroblast-specific Smad3 signaling may promote fibrotic remodeling and diastolic dysfunction in the pressure-overloaded myocardium, using cell-specific loss-of-function approaches in vivo, and in vitro experiments. Surprisingly, myofibroblast-specific Smad3 loss accelerated systolic dysfunction following pressure overload, accentuating matrix degradation, promoting myofibrillar loss in cardiomyocytes, and stimulating inflammatory macrophage activation. The protective effects of myofibroblast-specific Smad3 signaling are mediated through activation of a matrix-preserving program that inhibits matrix metalloproteinase (MMP)-driven ECM degradation, and attenuates the pro-inflammatory actions of MMPs. Our findings challenge traditional views on the role of myofibroblasts as detrimental effectors of cardiac fibrosis and provide the first direct experimental evidence suggesting protective actions of activated myofibroblasts in the pressure-overloaded myocardium. TGF-β/Smad3-dependent activation of fibroblast subpopulations may afford protection in the pressure-overloaded myocardium, mediated at least in part through preservation of the cardiac ECM.
METHODS
All supporting data are available within the article and the online supplement.
Detailed methodology is provided in the online supplement
We generated mice with Smad3 loss in activated myofibroblasts (FS3KO), using the periostin Cre driver. Postn-Cre mice were bred with Smad3 fl/fl mice18,19 to generate FS3KO animals and corresponding Smad3 fl/fl controls. A mouse model of cardiac pressure overload induced through transverse aortic constriction (TAC) was used. In vitro experiments were performed using isolated primary cardiac fibroblasts and macrophages.
RESULTS
TGF-βs, but not Bone Morphogenetic Proteins (BMP), or angiotensin II activate Smad3 signaling in isolated cardiac fibroblasts.
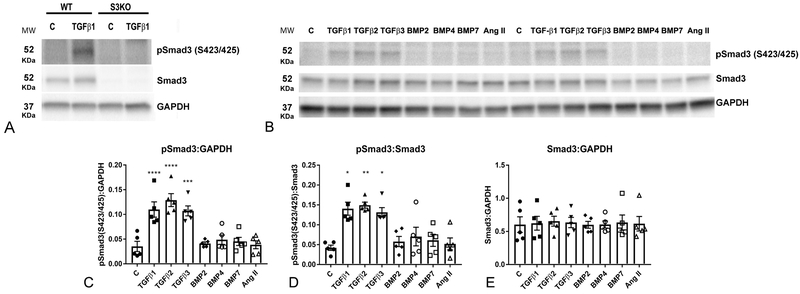
Cardiac pressure overload induces myocardial expression of TGF-β isoforms and downstream activation of TGF-β/Smad cascades20. In order to explore the signals that activate Smad3 specifically in cardiac fibroblasts, we assessed Smad3 phosphorylation in fibroblasts stimulated with members of the TGF-β superfamily, or with angiotensin II. We documented the specificity of the anti-pSmad3 antibody using Smad3 KO cells (Figure 1A). After 30 minutes of stimulation, all three TGF-β isoforms significantly increased the ratio of pSmad3:Smad3 expression without significantly affecting total Smad3 protein (Figure 1B-E). In contrast, BMP-2, BMP-4 and BMP-7 had no effects on Smad3 phosphorylation. Angiotensin II, a critical mediator in cardiac remodeling, has been suggested to signal by stimulating TGF-β/Smad321,22. However, in cardiac fibroblasts, angiotensin II did not affect Smad3 phosphorylation after 30 min of stimulation (Figure 1B-E), suggesting that its reported effects on Smad3 signaling may be indirect.
Figure 1: All three TGF-β isoforms, but not angiotensin II, BMP2, BMP4 and BMP7, activate Smad3 in cardiac fibroblasts.
A. The specificity of the antibodies to p-Smad3 and Smad3 was validated using fibroblasts from WT and global Smad3 KO hearts (S3KO). B. Representative western blotting experiment demonstrates that TGF-β1 (10ng/ml), -β2 (10ng/ml) and -β3 (10ng/ml) increase Smad3 phosphorylation at the S423/S425 sites after 30 min of stimulation. In contrast, BMP2 (50ng/ml), BMP4 (50ng/ml), BMP7 (50ng/ml) and angiotensin II (50ng/ml) do not activate Smad3. C-E: Quantitative analysis shows that TGF-βs significantly increase p-Smad3 levels (C) and the pSmad3:Smad3 ratio (D), without affecting Smad3 expression (E). (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 vs. control, n=4).
Generation of mice lacking Smad3 in activated myofibroblasts.
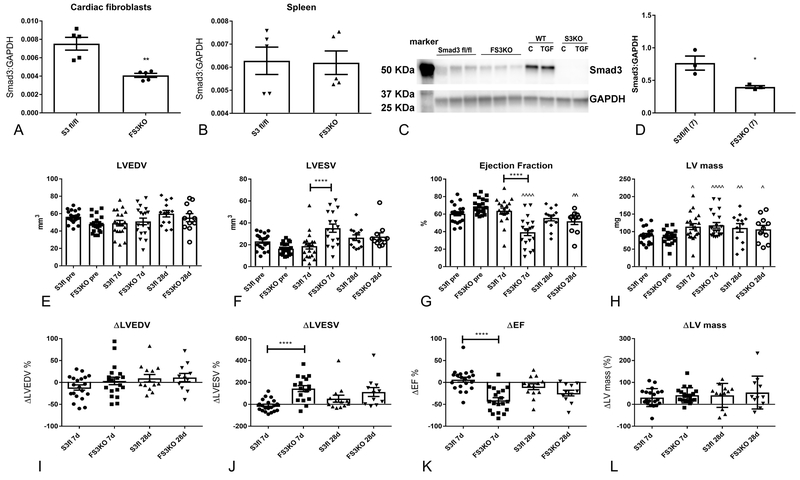
In order to generate FS3KO mice, we used the periostin Cre driver, as previously described19. Following 7 days of pressure overload, cardiac fibroblasts harvested from FS3KO mice had a marked reduction in Smad3 mRNA levels, when compared with fibroblasts from Smad3 fl/fl mice (Figure 2A). In contrast, no significant differences in Smad3 mRNA expression were noted in the spleen of FS3KO and Smad3 fl/fl animals (Figure 2B). Effective Smad3 deletion was confirmed at the protein level. Western blotting demonstrated that fibroblasts harvested from FS3KO mice had markedly lower Smad3 expression (Figure 2C-D). The specificity of the anti-Smad3 antibody was validated using cells from Smad3 KO mice (Figure 2C).
Figure 2: Fibroblast-specific Smad3 loss accelerates systolic dysfunction following cardiac pressure overload.
Mice with loss of Smad3 in activated myofibroblasts (FS3KO) were generated using the periostin-Cre driver. A-D: Documentation of cell-specific Smad3 knockdown in FS3KO mice. A: Cardiac fibroblasts isolated from pressure-overloaded hearts after 7 days of TAC exhibited markedly lower Smad3 mRNA expression, when compared with Smad3 fl/fl animals (**p<0.01, n=5/group). In contrast, pressure-overloaded FS3KO mice and corresponding Smad3 fl/fl animals had comparable levels of Smad3 in the spleen (B). C: Western blotting showed markedly reduced Smad3 protein in fibroblasts harvested from pressure-overloaded FS3KO hearts, when compared with fibroblasts from Smad3 fl/fl hearts after 7 days of TAC (*p<0.05, n=3/group). Specificity of the Smad3 antibody was validated using cells from mice with global Smad3 loss (S3KO). E-L: Echocardiography was used to assess the effects of fibroblast-specific Smad3 loss on cardiac remodeling following pressure overload. E, I: FS3KO mice and Smad3 fl/fl had no statistically significant differences in LVEDV. E-L: After 7-28 days of pressure overload, control Smad3 fl/fl animals exhibited left ventricular hypertrophy in the absence of significant systolic dysfunction. In contrast, FS3KO mice exhibited a marked >40% reduction in ejection fraction after 7 days of TAC (****p<0.0001 vs. Smad3 fl/fl), suggesting accelerated systolic dysfunction (G, K). After 28 days of TAC, FS3KO and not Smad3 fl/fl mice exhibited depressed systolic function (^^p<0.01 vs. corresponding baseline); however there was no significant difference in ejection fraction between FS3KO and Smad3 fl/fl, reflecting late dysfunction in Smad3 fl/fl mice. E, L: The increase in LV mass was not significantly different between Smad3 fl/fl and FS3KO mice after 7-28 days of TAC (^p<0.05, ^^p<0.01, ^^^^p<0.0001 vs. corresponding baseline) (n=12-21 mice/group).
Myofibroblast-specific Smad3 loss accelerates systolic dysfunction following pressure overload.
FS3KO and Smad3 fl/fl mice underwent TAC protocols. 3 FS3KO mice and 1 Smad3 fl/fl mouse died during the peri-operative period. The right:left carotid flow ratio was not different between FS3KO and Smad3 fl/fl mice (FS3KO: 8.3±0.5, Smad3 fl/fl: 8.89±0.59, p=NS, n=30–33/group), reflecting comparable pressure gradients. FS3KO and Smad3 fl/fl mice exhibited comparable mortality following pressure overload (Supplemental Figure IA). After 7 days of pressure overload, FS3KO mice had significantly higher HW:BW and LW:BW ratio than corresponding Smad3 fl/fl controls (Supplemental Figure IB-C), suggesting worse heart failure. However, no significant differences in HW:BW and LW:BW were noted after 28 days of pressure overload (Supplemental Figure ID-E). The effects of fibroblast-specific Smad3 loss on remodeling of the pressure-overloaded myocardium were assessed using echocardiography (Figure 2E-L). Baseline geometry and function were not significantly different between FS3KO and Smad3 fl/fl hearts. Consistent with previously published work in wildtype C57/BL6J mice20, Smad3 fl/fl animals exhibited early development of cardiac hypertrophy in the absence of significant systolic dysfunction (Figure 2E-L). Surprisingly, fibroblast-specific Smad3 loss was associated with a marked (>40%) early reduction in ejection fraction after 7 days of pressure overload (change in ejection fraction in FS3KO: −41.5%±5.8, vs. Smad3 fl/fl +8.7±6%, p<0.0001; Figure 2G, K), suggesting accelerated systolic dysfunction. After 28 days of TAC, FS3KO but not Smad3 fl/fl mice had persistent systolic dysfunction (^^p<0.01 vs. baseline); however, there was no significant difference in ejection fraction between FS3KO and Smad3 fl/fl groups, likely reflecting progressive dysfunction in control animals. The increase in LV mass was comparable between Smad3 fl/fl and FS3KO animals (Figure 2H, L).
To assess diastolic function, we used mitral inflow Doppler and tissue Doppler imaging. No significant effects of myofibroblast-specific Smad3 loss on indicators of diastolic function were noted at baseline and after 7–28 days of pressure overload (Supplemental figure II)
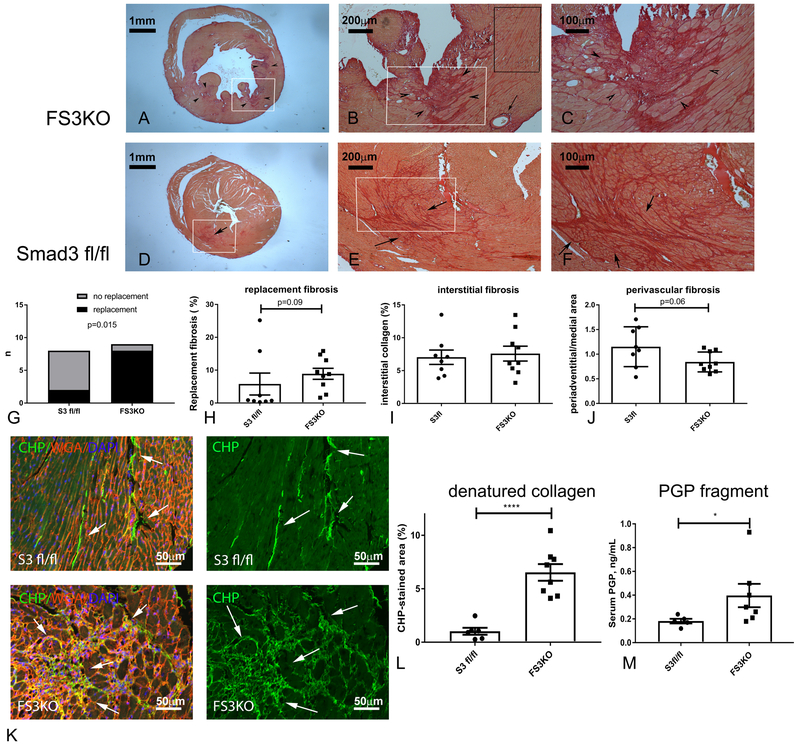
Accelerated systolic dysfunction in FS3KO mice undergoing TAC protocols is associated with ECM fragmentation.
In order to investigate the basis for accelerated dysfunction in FS3KO mice, we performed systematic histological analysis. In wildtype mice TAC typically causes interstitial and perivascular fibrosis, in the absence of significant replacement fibrosis (Figure 3)20. Surprisingly, after 7 days of pressure overload, 8 out of 9 FS3KO mice exhibited evidence of replacement fibrosis (defined as >2.5% replacement of myocardium with collagen-based scar) (p=0.015 vs. Smad3 fl/fl animals, Figure 3A-G). Quantitative analysis showed that the increase in the area of replacement fibrosis in FS3KO mice did not reach statistical significance (Figure 3H, p=0.09). Because Smad3 signaling is linked with activation of a matrix-synthetic program in fibroblasts, we also quantitatively assessed interstitial and perivascular fibrosis in myocardial areas with no evidence of replacement fibrosis. The effects of fibroblast-specific Smad3 loss on perivascular and interstitial fibrosis did not reach statistical significance after 7 days of pressure overload (Figure 3I-J). Next, we asked, whether the detrimental effects of fibroblast-specific Smad3 loss are due to loss of matrix-preserving functions of the activated fibroblasts, leading to increased fragmentation of the cardiac ECM. We used two distinct strategies to study collagen breakdown. First, labeling with collagen hybridizing peptide (CHP), which specifically binds to denatured collagen23 was compared between Smad3 fl/fl and FS3KO hearts. FS3KO animals had a marked increase in the interstitial areas labeled by CHP (Figure 3K-L). Second, mass spectrometry demonstrated that circulating levels of the Pro-Gly-Pro peptide, a collagen-derived matrikine with pro-inflammatory properties24 were increased in FS3KO mice (Figure 3M). Thus, fibroblast-specific loss of Smad3 was associated with robust evidence of ECM degradation.
Figure 3: Fibroblast-specific Smad3 loss accentuates matrix degradation in the pressure-overloaded heart, promoting release of collagen-derived matrikines.
A-J: Sirius red staining was used to identify the collagen network in pressure-overloaded hearts. A-F: In the TAC model, cardiac pressure overload is typically associated with interstitial and perivascular fibrosis (arrows), in the absence of replacement fibrosis. FS3KO mice exhibited foci of replacement fibrosis (arrowheads); in contrast Smad3 fl/fl animals had predominantly interstitial and perivascular collagen deposition (arrows). G. Quantitative analysis of replacement fibrosis areas showed that after 7 days of TAC, 8 of 9 FS3KO mice had >2.5% of the left ventricular myocardium replaced by scar (p=0.015 vs. Smad3 fl/fl mice, n=8-9/group). H: The difference in the area of replacement fibrosis between pressure-overloaded FS3KO and Smad3 fl/fl mice did not reach statistical significance (p=0.09). I: Quantitative analysis of interstitial fibrosis was performed in areas with no evidence of replacement fibrosis (black rectangle, B), and showed no significant differences between Smad3 fl/fl and FS3KO animals. J: FS3KO and Smad3 fl/fl mice did not have a statistically significant difference in the mean ratio of peri-adventitial collagen-stained area:medial area, an indicator of perivascular fibrosis (p=0.06). K-M: Fibroblast-specific Smad3 loss accentuates matrix degradation. In order to study the effects of fibroblast-specific Smad3 loss on collagen fragmentation and denaturation we used two distinct methods: a) fluorescent staining with collagen hybridizing peptide (CHP), which binds only to unfolded denatured collagen fibers (K, arrows), and b) mass spectrometry to assess levels of the collagen-derived matrikine Pro-Gly-Pro (PGP). FS3KO mice had extensive collagen denaturation, evidenced by a marked increase in the CHP-stained area in the pressure-overloaded cardiac interstitium after 7 days of TAC (K, arrows; M, ****p<0.0001, n=7-8/group). M: Mass spectrometry of serum samples after 7 days of TAC showed that FS3KO mice had a 2-fold increase in generation of PGP (*p<0.05, n=5/group).
After 28 days of TAC, FS3KO mice had a trend towards increased replacement fibrosis that did not reach statistical significance (Supplemental Figure IIIA-C). Interstitial fibrosis was not different between Smad3 fl/fl and FS3KO mice (Supplemental Figure IIID-E). Fibroblast-specific Smad3 loss significantly attenuated perivascular fibrosis (Supplemental Figure IIIF-G). Western blotting analysis showed no significant effects of fibroblast-specific Smad3 loss on collagen III protein levels after 28 days of TAC (Supplemental figure IIIH-I).
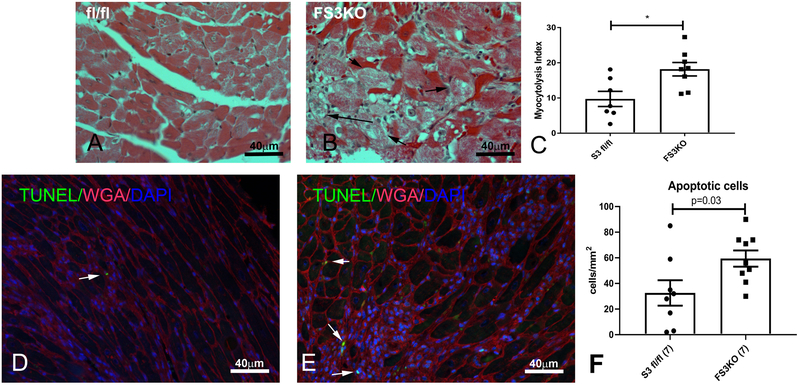
Myofibroblast-specific Smad3 loss accentuates degenerative changes in pressure-overloaded cardiomyocytes.
In order to explore the basis for early systolic dysfunction in pressure-overloaded FS3KO mice, we studied cardiomyocyte morphology. After 7 days of TAC, FS3KO mice exhibited significant myocytolysis, characterized by degenerative changes and myofibrillar loss in cardiomyocytes (Figure 4A-B). Quantitative analysis demonstrated that the myocytolysis index was higher in FS3KO mice (p<0.05) (Figure 4C). FS3KO mice also had a higher number of TUNEL+ apoptotic cells in the pressure-overloaded myocardium (Figure 4D-F). After 28 days of TAC, the severity of myocytolysis and the number of apoptotic cells were comparable between FS3KO and Smad3 fl/fl mice (Supplemental figure IV). This likely reflected the progressive increase in cardiomyocyte degeneration in Smad3 fl/fl animals.
Figure 4: Fibroblast-specific Smad3 loss is associated with increased cardiomyocyte injury after 7 days of TAC.
A-B: Hematoxylin-eosin staining demonstrated that many cardiomyocytes in pressure-overloaded FS3KO mice exhibited significant myofibrillar loss (myocytolysis, arrows). C: Quantitative analysis showed that FS3KO animals had a significantly higher myocytolysis index (*p<0.05, n=8–9). D-E: TUNEL staining was used to identify apoptotic cells in pressure overloaded hearts (arrows). Quantitative analysis showed that after 7 days of TAC, FS3KO mice had a significantly higher density of apoptotic cells (F), when compared with corresponding Smad3 fl/fl mice.
Taken together our findings (Figures 2-4) suggest that myofibroblast-specific Smad3 protects the pressure-overloaded myocardium and prevents early systolic dysfunction, by preserving the cardiac ECM, thus attenuating cardiomyocyte degeneration.
Myofibroblast-specific Smad3 loss accentuates macrophage-driven inflammation.
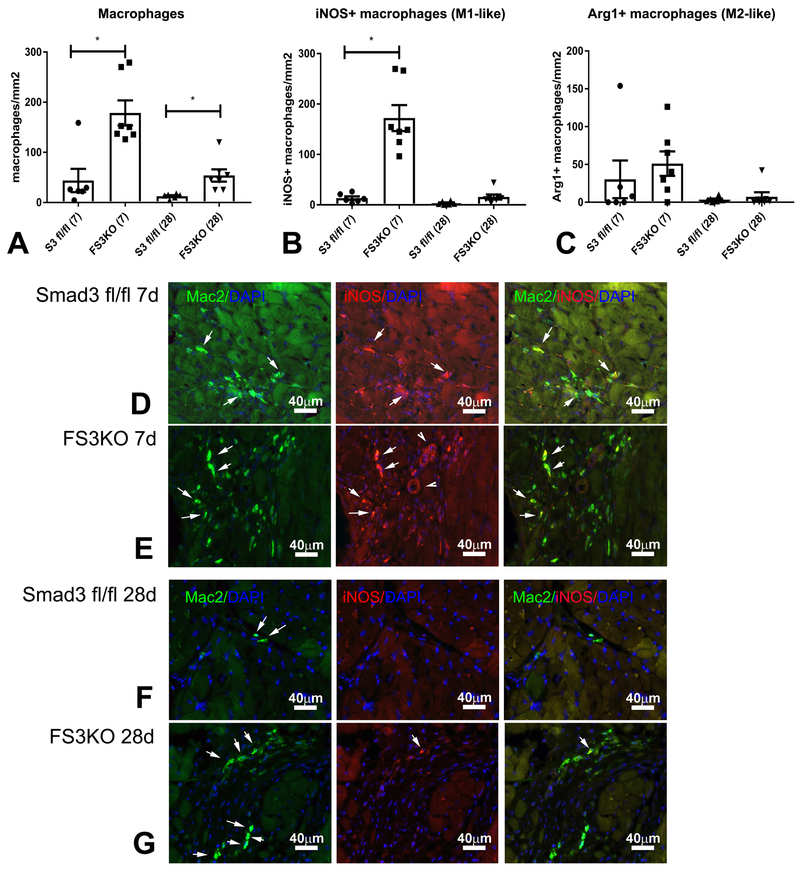
Because macrophage-mediated inflammation has been implicated in dysfunction of the remodeling myocardium25, and generation of matrix fragments may trigger macrophage recruitment, we examined the effects of myofibroblast-specific Smad3 loss on myocardial macrophage infiltration (Figure 5). After 7–28 days of TAC, FS3KO mice exhibited markedly higher macrophage infiltration in comparison to Smad3 fl/fl animals (Figure 5A, p<0.05). The majority of infiltrating macrophages expressed iNOS suggesting a pro-inflammatory phenotype (Figure 5B, D-G). In contrast, the density of Arg1+ macrophages was comparable between groups (Figure 5C). These findings suggest that fibroblast Smad3 signaling attenuates inflammation in the pressure-overloaded heart.
Figure 5: Fibroblast-specific Smad3 loss accentuates macrophage-driven inflammation in the pressure-overloaded myocardium.
A. Mac2 immunofluorescence was used to identify macrophages in the remodeling myocardium. FS3KO mice exhibited a marked increase in macrophage density after 7-28 days of TAC (*p<0.05 vs. Smad3 fl/fl, n=6-7/group). B. Dual immunofluorescence for Mac2 and iNOS was used to identify pro-inflammatory M1-like macrophages. The density of iNOS+ macrophages was significantly higher in FS3KO mice after 7 days of TAC (*p<0.05 vs. Smad3 fl/fl). The number of pro-inflammatory macrophages in FS3KO mice was significantly reduced after 28 days of TAC when compared to the 7-day timepoint (^p<0.05 vs. FS3KO 7d, n=7/group). C. Dual immunofluorescence for Mac2 and Arginase-1 (Arg1) was used to identify anti-inflammatory M2-like macrophages. The density of Arg1+ macrophages was comparable between groups. D-G: Representative images show Mac2/iNOS dual fluorescence (arrows) in Smad3 fl/fl and FS3KO mice after 7 and 28 days of TAC.
Myofibroblast-specific Smad3 loss accentuates MMP8 expression, promoting a matrix-degrading environment.
Next, we explored the molecular basis for the protective effects of myofibroblast-specific Smad3 on the pressure-overloaded myocardium. Because synthesis of structural and matricellular ECM proteins is an important function of activated fibroblasts in the remodeling heart26, we compared the gene expression profiles of fibroblasts harvested from Smad3 fl/fl and FS3KO hearts after 7 days of pressure overload. FS3KO and Smad3 fl/fl fibroblasts had comparable mRNA synthesis of type I collagen, fibronectin, TGF−β1 and of the matricellular proteins tenascin-C, thrombospondin-1 and −2, SPARC, osteopontin and CCN2 (Supplemental figure V).
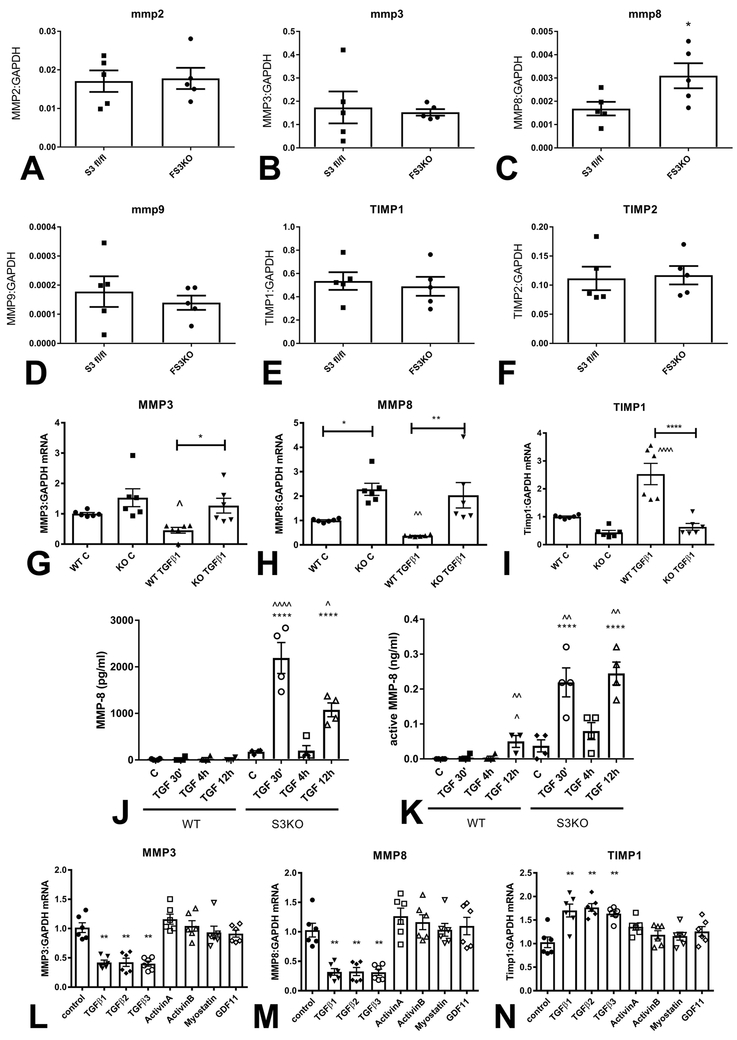
In order to examine the role of fibroblast Smad3 in regulating expression of proteases and anti-proteases involved in ECM metabolism, we compared MMP and TIMP expression between fibroblasts harvested from pressure-overloaded FS3KO and Smad3 fl/fl mice. FS3KO fibroblasts exhibited markedly increased MMP8 synthesis, when compared with Smad3 fl/fl cells. In contrast, MMP2, MMP3, MMP9, TIMP1 and TIMP2 expression was comparable between groups (Figure 6A-F). In vivo, immunohistochemical staining showed that FS3KO mice had significantly higher density of MMP8+ interstitial cells after 7 days of TAC (Supplemental figure VI).
Figure 6: Smad3 signaling mediates the matrix-preserving effects of TGF-β in cardiac fibroblasts.
Fibroblasts harvested from FS3KO and Smad3 fl/fl hearts after 7 days of TAC had comparable MMP2 (A) and MMP3 (B) mRNA expression levels, but exhibited markedly elevated MMP8 expression (C, *p<0.05, n=5/group). MMP9 (D), TIMP1 (E) and TIMP2 (F) expression levels were comparable between Smad3 fl/fl and FS3KO fibroblasts. G-I: Effects of TGF-β on MMP and TIMP expression in cardiac fibroblasts cultured in collagen pads are dependent on Smad3. In WT cells, TGF-β1 stimulation suppressed synthesis of MMP3 (G) and MMP8 (H) and induced TIMP-1 (I) expression (**p<0.01 vs. control, n=6/group). In contrast, TGF-β1 had no significant effects on MMP3, MMP8 and TIMP1 expression in Smad3 KO fibroblasts (^p<0.05, ^^p<0.01 vs. corresponding control). J-K: WT cardiac fibroblasts had low levels of MMP8 protein and activity in the supernatant, in the presence or absence of TGF-β. In contrast, Smad3 KO cells exhibited marked release of MMP8 protein and activity 30 min and 12h after TGF-β1 stimulation (****p<0.0001 vs. corresponding WT cells; ^p<0.05, ^^p<0.01, ^^^^p<0.0001 vs. unstimulated cells, n=4/group). The findings suggested that Smad3 signaling restrains release of MMP8 protein and activity by cardiac fibroblasts. L-N: Effects of Smad3 activating members of the TGF-β superfamily on expression of MMP3, MMP8 and TIMP1. Only TGF-β1, β2 and β3, and not activin-A, activin-B, myostatin and GDF-11 suppressed MMP3 (L) and MMP8 (M) expression, and induced TIMP-1 synthesis (N) in fibroblasts cultured in collagen pads (**p<0.01 vs. control, n=5/group).
Smad3 mediates matrix-preserving actions of TGF-β.
In vitro experiments using mouse cardiac fibroblasts cultured in collagen pads demonstrated that Smad3 KO cells exhibited markedly higher baseline MMP8 mRNA expression than WT fibroblasts and showed trends towards higher MMP3 and lower TIMP-1 expression (Figure 6G-I), TGF-β1 stimulation suppressed MMP3 and MMP8 in WT cells, but not in Smad3 KO fibroblasts (Figure 6G-H). Moreover, TGF-β1 induced TIMP-1 in WT cells but had no effect on TIMP-1 expression in Smad3 KO fibroblasts (Figure 6I). Next, we examined whether Smad3 loss also affects release of active MMP8 protein by cardiac fibroblasts. WT fibroblasts secreted very low amounts of MMP8 protein, in the presence or absence of TGF-β1. In contrast, Smad3 KO cells released large amounts of MMP8 after TGF-β1 stimulation (Figure 6J). An MMP8 activity assay confirmed that Smad3 signaling restrains release of active MMP8 by stimulated fibroblasts (Figure 6K). Taken together, our experiments suggest that Smad3 mediates potent matrix-preserving actions of TGF-β.
TGF-βs, but not activins, myostatin and GDF-11 directly stimulate a matrix-preserving program.
Next, we examined whether the matrix-preserving effects of TGF-β1 are shared by other members of the TGF-β superfamily, known to activate Smad3 signaling. All 3 TGF-β isoforms, but not activins, myostatin, and GDF-11 suppressed MMP3 and MMP8 synthesis by cardiac fibroblasts (Figure 6L-M). Moreover, only TGF-βs induced TIMP-1 synthesis (Figure 6N).
Effects of non-Smad pathways on MMP:TIMP expression.
In addition to their Smad-mediated effects, TGF-βs act through Smad-independent pathways, such as Akt, p38 MAPK, and Erk MAPK. In order to explore the role of Smad3 in TGF-β-mediated activation of Akt, p38 and Erk, we compared responses between WT and Smad3 KO fibroblasts. TGF-β1 stimulation rapidly activated Akt, p38 and Erk in both WT and Smad3 KO fibroblasts (Supplemental figure VIIA-F). In order to investigate the role of Smad-independent pathways in regulating the protease/anti-protease balance, we examined the effects of the Erk inhibitor U0126 and the p38 MAPK inhibitor SB203580. In WT cells, Erk inhibition, but not p38 MAPK inhibition abrogated the suppressive effects of TGF-β1 on MMP3 and MMP8 synthesis. In contrast, in Smad3 KO cells, Erk inhibition did not affect MMP3 and MMP8 synthesis suggesting that the effects of Erk in mediating the suppressive effects of TGF-β1 are not independent of Smad3. In WT cells, both Erk and p38 MAPK inhibition attenuated TGF-β1-induced TIMP-1 synthesis. In contrast, in Smad3 KO cells neither the Erk inhibitor, not the p38 MAPK inhibitor had any effects on TIMP-1 levels, suggesting that the effects of Erk and p38 MAPK on TIMP-1 expression were Smad3-dependent (Supplemental figure VII G-I).
MMP8 inhibition attenuates systolic dysfunction in pressure-overloaded FS3KO mice.
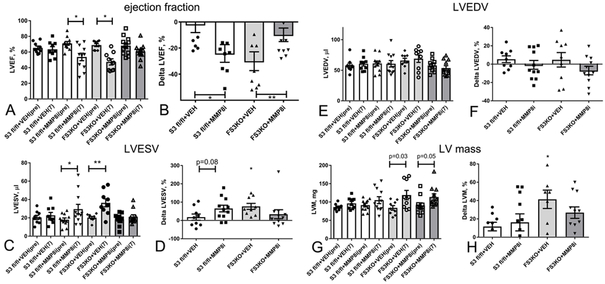
In order to examine whether the in vivo protective effects of fibroblast-specific Smad3 signaling are mediated through activation of a matrix-preserving program, we treated FS3KO mice and Smad3 fl/fl animals undergoing TAC protocols with an MMP8 inhibitor27. Administration of the inhibitor attenuated systolic dysfunction in FS3KO animals after 7 days of TAC (Figure 7A-B) and reduced the increase in LVESV (Figure 7C-D), without exerting any significant effects on LVEDV (Figure 7E-F) and on LV mass (Figure 7G-H). Surprisingly, treatment of Smad3 fl/fl mice with the MMP8 inhibitor worsened LV function after 7 days of TAC (Figure 7A-B). These findings suggest that an optimal functional response of the myocardium to a pressure load requires balanced matrix-degrading activity: both excessive MMP8 expression (in FS3KO animals) and very low levels of MMP8 (in inhibitor-treated Smad3 fl/fl mice) suppress systolic function.
Figure 7: MMP8 inhibition attenuates systolic dysfunction in pressure-overloaded FS3KO mice.
A-B. FS3KO mice exhibited significant early reduction in ejection fraction after 7 days of TAC (*p<0.05 vs. baseline). Administration of an MMP8 inhibitor, but not vehicle attenuated systolic dysfunction in FS3KO mice. Surprisingly, MMP8 inhibition caused reduction of ejection fraction in Smad3 fl/fl mice undergoing TAC protocols, suggesting that both excessive MMP8 (in FS3KO mice) and very low MMP8 activity (in control mice treated with inhibitor) have detrimental effects. C-D: MMP8 inhibition also attenuated the increase in LVESV noted in pressure-overloaded FS3KO mice. E-F: There were no significant effects of MMP8 inhibition on LVEDV. G-H: MMP8 inhibition did not significantly affect the effects of fibroblast-specific Smad3 loss on left ventricular mass (LVM). (*p<0.05, **p<0.01, n=9-13/group).
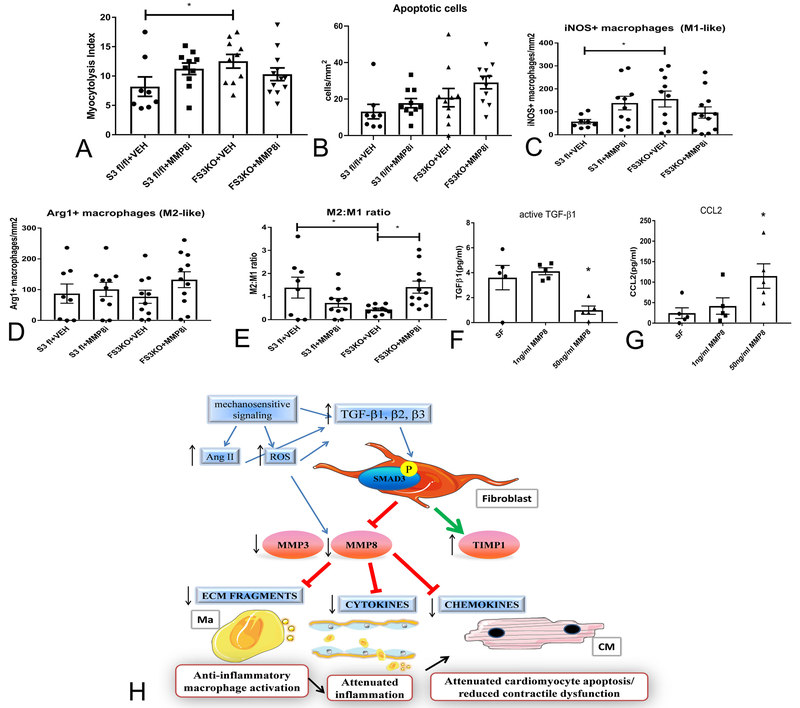
In order to explore the cellular mechanism for the effects of MMP8 on the remodeling myocardium, we performed histological analysis of the myocardium in animals treated with the inhibitor or vehicle. Myocytolysis and density of apoptotic cells were not significantly different between groups (Figure 8A-B). However, quantitative analysis of the iNOS+ macrophages (M1-like) and Arg1+ macrophages (M2-like) showed that FS3KO mice exhibit a reduction in the M2:M1 cell ratio, and that treatment with the MMP8 inhibitor attenuated the reduction in the M2:M1 ratio (Figure 8C-E). In vitro, stimulation of bone marrow macrophages with recombinant MMP8 (50 ng/ml) significantly reduced active TGF-β1 levels in the supernatant, while increasing release of the pro-inflammatory chemokine CCL2 (Figure 8F-G). Thus, MMP8 exerts direct actions on macrophages, promoting a pro-inflammatory phenotype.
Figure 8: MMP8 promotes a pro-inflammatory macrophage phenotype in vitro and in vivo.
A-C: Treatment with the MMP8 inhibitor did not improve the myocytolysis score in FS3KO mice (A) and did not affect the density of apoptotic cells (B) in the pressure-overloaded myocardium after 7 days of TAC. Although the effects of MMP8 inhibition on the density of iNOS+ M1-like macrophages (C) and Arg1+ M2-like macrophages (D) did not reach statistical significance, administration of the inhibitor increased the ratio of M2 like to M1 like cells (E, *p<0.05). In vitro, recombinant MMP8 (50ng/ml) markedly reduced levels of active TGF-β1 (F) and increased levels of the pro-inflammatory chemokine CCL2 (G) in the supernatant of isolated bone marrow macrophages (*p<0.05, n=5/group). H: Schematic cartoon illustrating the protective effects of TGF-β/Smad3-activated fibroblasts in the pressure-overloaded myocardium. Mechanical stress stimulates angiotensin II (Ang II) and generates reactive oxygen species (ROS), activating proteases. TGF-β-mediated stimulation of fibroblasts promotes a matrix-preserving program, suppressing MMP3 and MMP8 expression and inducing TIMP1 synthesis. Reduced matrix degradation decreases release of collagen-derived matrikines and attenuates macrophage-driven inflammation protecting cardiomyocytes from death and dysfunction. Moreover, restrained MMP activity may inhibit inflammation by decreasing formation of bioactive cytokine and chemokine molecules. The cartoon was designed using Servier Medical Art (https:smart.servier.com).
DISCUSSION
In patients with heart disease, myocardial fibrosis is consistently associated with adverse prognosis. In heart failure with preserved ejection fraction (HFpEF) patients, cardiac fibrosis was associated with increased baseline levels of biomarkers reflecting heart failure severity, and with worse outcome28. In patients with left ventricular systolic dysfunction, myocardial fibrosis was a powerful predictor of ventricular arrhythmic risk, in both ischemic and non-ischemic cardiomyopathy groups29. Considering that cardiac myofibroblasts are the main source of ECM proteins in the remodeling heart, the close association between myocardial fibrosis and adverse outcome led to a unidimensional view of cardiac myofibroblasts, as detrimental cellular effectors that mediate dysfunction in myocardial infarction and in chronic heart failure. However, this oversimplified paradigm on the role of myofibroblasts in cardiac remodeling overlooks their reparative functions. In many cardiac pathologic conditions, fibroblast expansion is not the primary cause of disease, but represents activation of a reparative process in response to cardiomyocyte injury. The reparative role of fibroblasts has been well-documented in myocardial infarction30,5,19. In adult mammals, repair of the infarcted myocardium requires generation of a well-organized scar, comprised of well-aligned arrays of activated myofibroblasts that secrete a collagen-based matrix. Our current study demonstrates for the first time that the protective functions of cardiac fibroblasts are not limited to myocardial infarction, but also play an important role in restraining ECM degradation, in inhibiting inflammation, and in preventing cardiomyocyte injury and systolic dysfunction in injurious responses not associated with significant cardiomyocyte necrosis. Using cell-specific approaches in vivo, we documented that in the pressure-overloaded heart, Smad-dependent myofibroblast activation protects stressed cardiomyocytes from death, and attenuates macrophage-driven inflammation, by stimulating a matrix-preserving program.
The protective functions of myofibroblasts in the pressure-overloaded myocardium are mediated through activation of a matrix-preserving program.
Surprisingly, loss of the critical fibrogenic effector Smad3 in activated myofibroblasts, accelerated systolic dysfunction in the pressure-overloaded myocardium. Impaired systolic function in FS3KO mice was associated with accentuated collagen degradation and release of pro-inflammatory collagen-derived matrikines (Figure 3). Increased collagen fragmentation and denaturation in pressure-overloaded FS3KO mice was accompanied by loss of myofibrillar content in cardiomyocytes (Figure 4) and by enhanced macrophage-driven inflammation (Figure 5). Assessment of gene expression in fibroblasts isolated directly from pressure-overloaded hearts demonstrated that Smad3 absence was associated with accentuated expression of the collagenase MMP8 (Figure 6). In vivo, administration of an MMP8 inhibitor protected FS3KO hearts from the development of early systolic dysfunction, documenting that the matrix-degrading effects of fibroblast-specific Smad3 loss mediate systolic dysfunction. Thus, our findings suggest a novel conceptual paradigm on the role of myofibroblasts in the pressure overloaded myocardium (Figure 8H). Activation of mechanosensitive signaling in response to a pressure load is a potent stimulus for induction and activation of MMPs31. TGF-β/Smad3 activation suppresses MMP synthesis and stimulates TIMP expression, playing a critical role in preservation of the cardiac ECM under conditions of mechanical stress and attenuating release of matrix fragments that may promote inflammation and accentuate cardiomyocyte injury. In the absence of Smad3, unrestrained MMP activity in response to pressure overload, and subsequent release of collagen-derived matrikines may promote dysfunction.
What are the cellular mechanisms of MMP8-driven cardiac dysfunction in remodeling FS3KO mice?
Several cellular mechanisms may explain the link between excessive MMP8 expression and systolic dysfunction in the pressure-overloaded heart. First, MMP-driven degradation of the pericellular ECM may deprive pressure-overloaded cardiomyocytes of critical survival signals promoting their apoptosis under conditions of mechanical stress. Second, MMP8 is known to generate pro-inflammatory matrix fragments that promote chemotactic recruitment and activation of myeloid cells32. Our in vivo experiments demonstrated that FS3KO mice had increased levels of the pro-inflammatory collagen-derived matrikine PGP (Figure 3), a chemotactic fragment generated through MMP8 actions32,33. Third, MMP8 may trigger proteolytic activation of cytokines and chemokines, thus stimulating inflammation. MMP8 has been reported to exhibit tumor necrosis factor (TNF)- β converting enzyme activity, cleaving the TNF-β prodomain to generate the active cytokine27. Moreover, MMP-8 is implicated in CXC chemokine processing, leading to generation of potent leukocyte chemoattractants34,35. Our in vitro experiments demonstrated that stimulation with recombinant MMP8 promotes a pro-inflammatory macrophage phenotype, increasing CCL2 release and attenuating levels of bioactive TGF-β. MMP8-driven accentuation of inflammation may explain, at least in part, the transient effects of fibroblast-specific Smad3 loss on cardiac function, which are not associated with long-term consequences on cardiac remodeling. Thus, the detrimental effects of excessive MMP8 on the remodeling myocardium may involve both activation of macrophage-driven inflammation and increased susceptibility of cardiomyocytes to stress-induced death. Moreover, considering the abundant expression of MMP8 by neutrophils and monocytes, attenuation of dysfunction through MMP8 inhibition in pressure-overloaded FS3KO mice may reflect actions on leukocytes, and not inhibition of fibroblast-specific MMP8 activity.
In contrast to the protective effects of MMP8 neutralization in pressure-overloaded FS3KO animals, MMP8 inhibition worsened cardiac function in Smad3 fl/fl mice. These effects may reflect protective actions of low amounts of MMP8 under conditions of stress. Complete absence of MMP8 in mice perturbed resolution of inflammation following wound healing34, and exacerbated inflammation, in a model of endotoxin-induced lung injury36. Tight regulation of MMP8 activity may be important to protect tissues from damage under conditions of stress: both excessive MMP8 expression and very low levels of MMP8 may have deleterious effects.
The effects of TGF-β/Smad3 signaling on fibroblast phenotype in vitro and in vivo.
Our recently published work examined the effects of fibroblast TGF-β/Smad3 signaling in myocardial infarction19. We demonstrated that fibroblast-specific Smad3 loss impairs repair of the infarcted heart and is associated with a high incidence of late cardiac rupture and with accentuated adverse post-infarction remodeling. The catastrophic effects of fibroblast-specific Smad3 loss were caused by perturbations in scar organization, due to reduced fibroblast integrin expression, and defective activation of downstream reparative signals. Considering the massive loss of cardiomyocytes in myocardial infarction and the negligible regenerative capacity of the adult mammalian heart, the requirement for functional activated myofibroblasts in the infarcted heart is perhaps not unexpected. The findings of the current study are much more surprising, suggesting that fibroblast-specific TGF-β/Smad3 signaling is protective in a model of non-reparative cardiac fibrosis induced by pressure overload. Thus, even in the absence of significant cardiomyocyte death, activation of fibroblasts may be required in order to prevent matrix fragmentation, preserving ECM structure and protecting stressed cardiomyocytes from injury.
In contrast to our observations, Khalil et al. recently reported that fibroblast-specific Smad3 exerts detrimental effects on the pressure overloaded heart, mediating fibrotic remodeling8. The seemingly conflicting messages of the two studies can be explained by differences in research design and in interpretation of the findings. First, our study examined a larger number of animals, of both genders; we have systematically assessed the functional and histological consequences of fibroblast-specific Smad3 loss at 2 different timepoints, after 7 and 28 days of pressure overload. In contrast, Khalil and co-workers examined only male mice; functional analysis was performed at a late timepoint (12 weeks after TAC) and early effects of Smad3 loss were not studied. Second, although Khalil et al. did not report data on diastolic function parameters in FS3KO animals, their findings suggested no significant effects of combined fibroblast-specific Smad2 and Smad3 loss on diastolic function. Thus, conclusions regarding the adverse consequences of fibroblast-specific Smad-dependent signaling were not based on functional data. Third, the studies used different approaches to delete Smad3 in cardiac fibroblasts. We have used a constitutive periostin-Cre driver; considering the absence of periostin expression in normal resident cardiac fibroblasts and the selective induction of periostin in activated injury site myofibroblasts, this approach specifically targets activated fibroblasts in the remodeling myocardium, as demonstrated by our group and other investigators. Khalil et al. used an inducible periostin Mer-Cre-Mer driver and achieved almost complete Smad3 loss in fibroblasts (in contrast to a 50% reduction in our study). However, administration of tamoxifen for Cre induction and gene deletion may modulate the baseline response to pressure overload.
The functional pluralism of fibroblasts in the remodeling myocardium.
Our study challenges the prevailing dogma that fibroblasts are unidimensional cells that mediate fibrosis and dysfunction. Cardiac fibroblasts are versatile cells that may acquire distinct phenotypes in response to tissue injury, exerting a wide range of inflammatory, hypertrophic and cytoprotective actions. The functional diversity of cardiac fibroblasts may reflect, at least in part, the phenotypic heterogeneity of interstitial cell populations, which are comprised of functionally distinct subsets37. The diverse functions of cardiac fibroblasts have been appreciated in the dynamic environment of the infarcted heart26. In myocardial ischemia and reperfusion, fibroblasts have been suggested to protect cardiomyocytes from ischemic death38. During the early pro-inflammatory phase of infarct healing, fibroblasts may act as inflammatory and matrix-degrading cells, producing cytokines, chemokines and proteases39, and stimulating leukocyte recruitment. A recently published study suggested that fibroblast-derived GM-CSF synthesis may activate myeloid cells triggering leukocyte recruitment in the infarct40. Infarct fibroblasts have also been reported to participate in engulfment of apoptotic cells and in negative regulation of the post-infarction inflammatory response41. During the proliferative phase of infarct healing, fibroblasts undergo myofibroblast conversion, and synthesize ECM proteins; formation of aligned arrays of activated myofibroblasts is critical for repair of the infarcted heart and protects from adverse dilative remodeling19.
In the pressure-overloaded heart, activated fibroblasts have been implicated in cardiomyocyte hypertrophy. Fibroblast-specific signaling through the zinc-containing transcription factor Kruppel-like factor (Klf)-5 was essential for adaptive hypertrophy in the pressure-overloaded heart42. Activation of a hypertrophic program in pressure-overloaded cardiomyocytes may be driven, at least in part, through secretion of miRNA-enriched exosomes by cardiac fibroblasts43.
Conclusions.
Our findings document the protective functions of activated fibroblasts in the remodeling myocardium. In the pressure-overloaded heart, activation of neurohumoral and pro-inflammatory signals in response to mechanical stress induces expression and activation of MMPs44,45. Our findings suggest that activation of TGF-β/Smad3 signaling in cardiac myofibroblasts restrains matrix-degrading activity, preventing release of pro-inflammatory collagen-derived matrikines, suppressing macrophage-driven inflammation, and attenuating cardiomyocyte injury (Figure 8H). Under conditions of stress, the heart is highly dependent on myofibroblast-mediated preservation of the ECM to maintain function. These observations have important therapeutic implications for heart failure patients. Targeting activated fibroblasts in patients with heart failure may also abrogate protective actions of interstitial cells, thus exacerbating the adverse consequences of the underlying injurious process. In contrast to the primary injurious role of fibroblasts in fibrotic conditions affecting other systems, such as scleroderma46 or idiopathic pulmonary fibrosis47, myocardial fibrosis often reflects a reparative/protective response to injury. In many heart failure patients, direct inhibition of fibroblast activity may have detrimental effects. Moreover, targeting TGF-β cascades in patients with myocardial disease may carry significant risks, by interfering with important homeostatic or reparative functions. Thus, successful implementation of anti-fibrotic strategies in patients with myocardial fibrotic remodeling may require identification of patient subsets with excessive or dysregulated fibroblast activation.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Cardiac pressure overload is associated with fibroblast activation and expansion of the perivascular and interstitial extracellular matrix (ECM) network.
Transforming Growth Factor (TGF)-β signaling cascades activate fibroblasts in vitro and in vivo.
Activated fibroblasts are considered key effector cells in the pathogenesis of myocardial cardiac fibrosis, and essential contributors to the pathophysiology of cardiac dysfunction.
Activated fibroblasts may be therapeutic targets in patients with heart failure.
What New Information Does This Article Contribute?
In contrast to prevailing dogma, activated cardiac myofibroblasts protect the heart from pressure-overload-induced remodeling, through Smad3-dependent preservation of the cardiac ECM.
Activation of TGF-β/Smad3 signaling in cardiac myofibroblasts suppresses collagenase synthesis, reducing generation of pro-inflammatory matrikines, and attenuating macrophage-driven inflammation.
Non-selective targeting of activated fibroblasts in failing hearts may accentuate cardiac dysfunction under conditions of mechanical stress by abrogating key reparative signals.
TGF-β signaling is activated following tissue injury and modulates phenotype and function of all cell types involved in repair and remodeling, by stimulating Smad-dependent and Smad-independent cascades. The cell-specific actions of TGF-βs in cardiac remodeling remain poorly understood. In the heart subjected to pressure overload, activation of TGF−β/Smad cascades in fibroblasts may be involved in the pathogenesis of cardiac remodeling, fibrosis and dysfunction. Using myofibroblast-specific Smad3 knockout mice and a wide range of in vitro models, we demonstrate protective actions of activated fibroblasts in a mouse model of cardiac pressure overload. Activation of Smad3 signaling in cardiac myofibroblasts protects the heart from pressure-overload induced systolic dysfunction, preserves cardiac extracellular matrix, prevents generation of pro-inflammatory matrix fragments, inhibits cardiomyocyte injury, and attenuates macrophage-driven inflammation. The protective effects of activated myofibroblasts are mediated through Smad3-dependent stimulation of a matrix-preserving program. Our findings suggest that, in contrast to traditional views suggesting deleterious effects of activated fibroblasts in failing hearts, subpopulations of the activated myofibroblasts may exert protective actions on the remodeling myocardium, by inhibiting mechanical stress-mediated extracellular matrix degradation. Thus, non-selective targeting of cardiac fibroblasts in failing hearts may abrogate key protective signals.
ACKNOWLEDGMENTS
The authors are grateful to Ms. Marta Paz García for her help with histopathological staining.
SOURCES OF FUNDING
This work was supported by NIH grants R01 HL76246 and R01 HL85440, and by Department of Defense grants PR151134 and PR151029. Dr. Simon J Conway is supported by R01 HL135657. The Proteomics core used a Triple quadrupole mass spectrometer system that was purchased through NIH SIG grant 1S10RR027701. Dr. Shuaibo Huang is supported by China Scholarship Council grant 201603170222. Dr. Bijun Chen is supported by an American Heart Association post-doctoral grant.
Nonstandard Abbreviations and Acronyms:
- Akt
protein kinase B
- Arg1
arginase 1
- BMP
bone morphogenetic proteins
- CCL2
C-C motif chemokine ligand 2
- CCN2
connective tissue growth factor
- CHP
collagen hybridizing peptide
- DT
deceleration time
- ECM
extracellular matrix
- Erk
extracellular signal-regulated kinases
- FS3KO
fibroblast-specific Smad3 knockout mice
- GDF-11
growth differentiation factor-11
- HW
heart weight
- I.P.
intraperitoneal injection
- iNOS
inducible nitric oxide synthase
- KO
knockout
- LVEDV
left ventricular end diastolic volume
- LVESV
left ventricular end systolic volume
- LW
lung weight
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinase
- OPN
osteopontin
- PGP
proline-glycine-proline
- Postn
periostin gene
- Smad
small mothers against decapentaplegic
- SPARC
secreted protein acidic and rich in cysteine
- TAC
transverse aortic constriction
- TDI
tissue Doppler imaging
- TGF
transforming growth factor
- TIMP
tissue inhibitor of metalloproteinase
- TNF
tumor necrosis factor
- TSP
thrombospondin
- VEH
vehicle
- WGA
wheat germ agglutinin
- WT
wild type
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA and Tallquist MD. Revisiting Cardiac Cellular Composition. Circ Res. 2016;118:400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tallquist MD and Molkentin JD. Redefining the identity of cardiac fibroblasts. Nat Rev Cardiol. 2017;14:484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Souders CA, Bowers SL and Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cleutjens JP, Verluyten MJ, Smiths JF and Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–38. [PMC free article] [PubMed] [Google Scholar]
- 5.Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, SC JL, Aronow BJ, Tallquist MD and Molkentin JD. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nature communications. 2016;7:12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore-Morris T, Guimaraes-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu Y, Dalton ND, Cedenilla M, Gomez-Amaro R, Zhou B, Brenner DA, Peterson KL, Chen J and Evans SM. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest. 2014;124:2921–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, Kamran P, Muller AM, Volz KS, Tang Z, Red-Horse K and Ardehali R. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res. 2014;115:625–35. [DOI] [PubMed] [Google Scholar]
- 8.Khalil H, Kanisicak O, Prasad V, Correll RN, Fu X, Schips T, Vagnozzi RJ, Liu R, Huynh T, Lee SJ, Karch J and Molkentin JD. Fibroblast-specific TGF-beta-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest. 2017;127:3770–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei Y, Kim TJ, Peng DH, Duan D, Gibbons DL, Yamauchi M, Jackson JR, Le Saux CJ, Calhoun C, Peters J, Derynck R, Backes BJ and Chapman HA. Fibroblast-specific inhibition of TGF-beta1 signaling attenuates lung and tumor fibrosis. J Clin Invest. 2017;127:3675–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biernacka A, Dobaczewski M and Frangogiannis NG. TGF-beta signaling in fibrosis. Growth Factors. 2011;29:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y and Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. [DOI] [PubMed] [Google Scholar]
- 12.Divakaran V, Adrogue J, Ishiyama M, Entman ML, Haudek S, Sivasubramanian N and Mann DL. Adaptive and maladptive effects of SMAD3 signaling in the adult heart after hemodynamic pressure overloading. Circ Heart Fail. 2009;2:633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biernacka A, Cavalera M, Wang J, Russo I, Shinde A, Kong P, Gonzalez-Quesada C, Rai V, Dobaczewski M, Lee DW, Wang XF and Frangogiannis NG. Smad3 Signaling Promotes Fibrosis While Preserving Cardiac and Aortic Geometry in Obese Diabetic Mice. Circ Heart Fail. 2015;8:788–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB and Gauldie J. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol. 2004;173:2099–108. [DOI] [PubMed] [Google Scholar]
- 15.Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85:47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF and Frangogiannis NG. Essential Role of Smad3 in Infarct Healing and in the Pathogenesis of Cardiac Remodeling. Circulation. 2007;116:2127–38. [DOI] [PubMed] [Google Scholar]
- 17.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF and Frangogiannis NG. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Pangas SA, Jorgez CJ, Graff JM, Weinstein M and Matzuk MM. Redundant roles of SMAD2 and SMAD3 in ovarian granulosa cells in vivo. Mol Cell Biol. 2008;28:7001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong P, Shinde AV, Su Y, Russo I, Chen B, Saxena A, Conway SJ, Graff JM and Frangogiannis NG. Opposing Actions of Fibroblast and Cardiomyocyte Smad3 Signaling in the Infarcted Myocardium. Circulation. 2018;137:707–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia Y, Lee K, Li N, Corbett D, Mendoza L and Frangogiannis NG. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol. 2009;131:471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang XR, Chung AC, Yang F, Yue W, Deng C, Lau CP, Tse HF and Lan HY. Smad3 mediates cardiac inflammation and fibrosis in angiotensin II-induced hypertensive cardiac remodeling. Hypertension. 2010;55:1165–71. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Huang XR, Canlas E, Oka K, Truong LD, Deng C, Bhowmick NA, Ju W, Bottinger EP and Lan HY. Essential role of Smad3 in angiotensin II-induced vascular fibrosis. Circ Res. 2006;98:1032–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zitnay JL, Li Y, Qin Z, San BH, Depalle B, Reese SP, Buehler MJ, Yu SM and Weiss JA. Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides. Nature communications. 2017;8:14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaggar A, Jackson PL, Noerager BD, O’Reilly PJ, McQuaid DB, Rowe SM, Clancy JP and Blalock JE. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol. 2008;180:5662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kingery JR, Hamid T, Lewis RK, Ismahil MA, Bansal SS, Rokosh G, Townes TM, Ildstad ST, Jones SP and Prabhu SD. Leukocyte iNOS is required for inflammation and pathological remodeling in ischemic heart failure. Basic Res Cardiol. 2017;112:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinde AV and Frangogiannis NG. Fibroblasts in myocardial infarction: A role in inflammation and repair. J Mol Cell Cardiol. 2014;70C:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee EJ, Han JE, Woo MS, Shin JA, Park EM, Kang JL, Moon PG, Baek MC, Son WS, Ko YT, Choi JW and Kim HS. Matrix metalloproteinase-8 plays a pivotal role in neuroinflammation by modulating TNF-alpha activation. J Immunol. 2014;193:2384–93. [DOI] [PubMed] [Google Scholar]
- 28.Schelbert EB, Fridman Y, Wong TC, Abu Daya H, Piehler KM, Kadakkal A, Miller CA, Ugander M, Maanja M, Kellman P, Shah DJ, Abebe KZ, Simon MA, Quarta G, Senni M, Butler J, Diez J, Redfield MM and Gheorghiade M. Temporal Relation Between Myocardial Fibrosis and Heart Failure With Preserved Ejection Fraction: Association With Baseline Disease Severity and Subsequent Outcome. JAMA Cardiol. 2017;2:995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Disertori M, Rigoni M, Pace N, Casolo G, Mase M, Gonzini L, Lucci D, Nollo G and Ravelli F. Myocardial Fibrosis Assessment by LGE Is a Powerful Predictor of Ventricular Tachyarrhythmias in Ischemic and Nonischemic LV Dysfunction: A Meta-Analysis. JACC Cardiovasc Imaging. 2016;9:1046–1055. [DOI] [PubMed] [Google Scholar]
- 30.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW 2nd, Conway SJ, Aronow BJ, Robbins J and Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blain EJ. Mechanical regulation of matrix metalloproteinases. Front Biosci. 2007;12:507–27. [DOI] [PubMed] [Google Scholar]
- 32.Lin M, Jackson P, Tester AM, Diaconu E, Overall CM, Blalock JE and Pearlman E. Matrix metalloproteinase-8 facilitates neutrophil migration through the corneal stromal matrix by collagen degradation and production of the chemotactic peptide Pro-Gly-Pro. Am J Pathol. 2008;173:144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koelink PJ, Overbeek SA, Braber S, Morgan ME, Henricks PA, Abdul Roda M, Verspaget HW, Wolfkamp SC, te Velde AA, Jones CW, Jackson PL, Blalock JE, Sparidans RW, Kruijtzer JA, Garssen J, Folkerts G and Kraneveld AD. Collagen degradation and neutrophilic infiltration: a vicious circle in inflammatory bowel disease. Gut. 2014;63:578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutierrez-Fernandez A, Inada M, Balbin M, Fueyo A, Pitiot AS, Astudillo A, Hirose K, Hirata M, Shapiro SD, Noel A, Werb Z, Krane SM, Lopez-Otin C and Puente XS. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8). FASEB J. 2007;21:2580–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tester AM, Cox JH, Connor AR, Starr AE, Dean RA, Puente XS, Lopez-Otin C and Overall CM. LPS responsiveness and neutrophil chemotaxis in vivo require PMN MMP-8 activity. PLoS One. 2007;2:e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez-Lopez A, Aguirre A, Lopez-Alonso I, Amado L, Astudillo A, Fernandez-Garcia MS, Suarez MF, Batalla-Solis E, Colado E and Albaiceta GM. MMP-8 deficiency increases TLR/RAGE ligands S100A8 and S100A9 and exacerbates lung inflammation during endotoxemia. PLoS One. 2012;7:e39940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frangogiannis NG. The Functional Pluralism of Fibroblasts in the Infarcted Myocardium. Circ Res. 2016;119:1049–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abrial M, Da Silva CC, Pillot B, Augeul L, Ivanes F, Teixeira G, Cartier R, Angoulvant D, Ovize M and Ferrera R. Cardiac fibroblasts protect cardiomyocytes against lethal ischemia-reperfusion injury. J Mol Cell Cardiol. 2014;68:56–65. [DOI] [PubMed] [Google Scholar]
- 39.Saxena A, Chen W, Su Y, Rai V, Uche OU, Li N and Frangogiannis NG. IL-1 Induces Proinflammatory Leukocyte Infiltration and Regulates Fibroblast Phenotype in the Infarcted Myocardium. J Immunol. 2013;191:4838–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anzai A, Choi JL, He S, Fenn AM, Nairz M, Rattik S, McAlpine CS, Mindur JE, Chan CT, Iwamoto Y, Tricot B, Wojtkiewicz GR, Weissleder R, Libby P, Nahrendorf M, Stone JR, Becher B and Swirski FK. The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes. J Exp Med. 2017;214:3293–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakaya M, Watari K, Tajima M, Nakaya T, Matsuda S, Ohara H, Nishihara H, Yamaguchi H, Hashimoto A, Nishida M, Nagasaka A, Horii Y, Ono H, Iribe G, Inoue R, Tsuda M, Inoue K, Tanaka A, Kuroda M, Nagata S and Kurose H. Cardiac myofibroblast engulfment of dead cells facilitates recovery after myocardial infarction. J Clin Invest. 2017;127:383–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, Shindo T, Sano M, Otsu K, Snider P, Conway SJ and Nagai R. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 2010;120:254–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J and Thum T. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124:2136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piccoli MT, Gupta SK, Viereck J, Foinquinos A, Samolovac S, Kramer FL, Garg A, Remke J, Zimmer K, Batkai S and Thum T. Inhibition of the Cardiac Fibroblast-Enriched lncRNA Meg3 Prevents Cardiac Fibrosis and Diastolic Dysfunction. Circ Res. 2017;121:575–583. [DOI] [PubMed] [Google Scholar]
- 45.Kandalam V, Basu R, Moore L, Fan D, Wang X, Jaworski DM, Oudit GY and Kassiri Z. Lack of tissue inhibitor of metalloproteinases 2 leads to exacerbated left ventricular dysfunction and adverse extracellular matrix remodeling in response to biomechanical stress. Circulation. 2011;124:2094–105. [DOI] [PubMed] [Google Scholar]
- 46.Leask A Matrix remodeling in systemic sclerosis. Seminars in immunopathology. 2015;37:559–63. [DOI] [PubMed] [Google Scholar]
- 47.Ahluwalia N, Shea BS and Tager AM. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am J Respir Crit Care Med. 2014;190:867–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.