Abstract
In this paper, we show that the nonlinear growth rate of particles in a supersaturated solution or supercooled melt, as well as the rate of removal of crystals from the metastable liquid of a crystallizer, significantly change the size-distribution function of crystals. Taking these rates into account, we present a complete analytical solution of the integro-differential model describing the transient nucleation of solid particles and their evolution in a metastable liquid. The distribution function and metastability degree (supersaturation or supercooling) are found by means of the separation of variables and saddle-point methods. The nonlinear growth rates of crystals in supersaturated solutions and supercooled melts (single-component and binary) are summarized and compared with experimental data.
This article is part of the theme issue ‘Heterogeneous materials: metastable and non-ergodic internal structures’.
Keywords: nucleation, evolution of particulate assemblage, metastable liquid, removal of crystals, phase transformation
1. Introduction
In actual practice, the nucleation and evolution of a polydisperse ensemble of crystals are complicated by their removal from a metastable liquid as well as by the presence of mass sources or heat sinks [1–4]. In addition, the growth rate of crystals in supersaturated solutions and supercooled melts is a nonlinear function of the supersaturation (supercooling) of the metastable liquid [5–11]. Namely, experimental works [5–8] demonstrate that the growth rate V of crystals is close to parabolic with increasing the supersaturation Δ. In other words, the frequently used linear approximation V (τ) = β*Δ(τ) does not well describe the real experiments (here β* and τ represent the kinetic coefficient and time). The analytical theory recently developed for supercooled single-component and binary melts [9,10] as well as for supersaturated solutions [11] shows that the crystal growth rate represents the following nonlinear function of metastability degree Δ and time τ
| 1.1 |
and
where λl is the thermal conductivity, ρs is the density of the solid phase, L is the latent heat of phase transition, m is the liquidus slope, k is the equilibrium partition coefficient, C0 is the solute concentration far from the growing crystal, D is the diffusion coefficient, and Cp is the concentration at saturation. To find the rate Rg (g/h cm2) of the increase in the mass of growing crystals per unit time per unit area, let us write out the mass of crystals as M = ρsNυ, where N is the total number of crystals and υ is the volume of a single particle. Next considering the case of spherical crystals υ = 4πr3/3 one can find the mass growth rate as dM/dτ = 4πρsNr2V (τ), where V (τ) is given by expressions (1.1). Now introducing Rg = (4πr2N)−1 dM/dτ = ρsV (τ), we come to
| 1.2 |
where the third line of expression (1.1) is taken into account. As the growth rate (1.2) depends on Δ and τ one can use a desupersaturation curve Δ(τ) or its inverse function τ(Δ) to plot the growth rate (1.2) as a function of a single variable V (τ) or V (Δ). Using such a curve experimentally found by Garside et al. [6] (see their fig. 5), we compare in figure 1 the nonlinear growth rates (1.1) and (1.2) with experimental data on the nucleation and evolution of potassium sulfate in a laboratory scale fluidized bed crystallizer.
Figure 1.
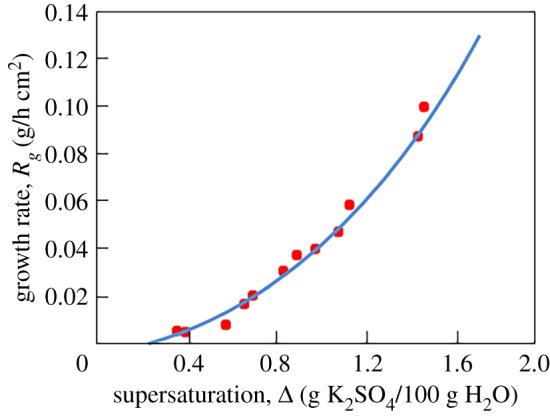
Growth rate Rg of potassium sulfate crystals in a fluidized bed crystallizer (symbols designate the experimental data [6] and the solid curve is plotted in accordance with expression (1.2)). Here χ3 = 0.049 h−1[Δ]−1, κ = 0.082 g/(h cm2[Δ]) and [Δ] denotes the dimension of supersaturation shown on the horizontal axis.
Taking into account the aforesaid, we note that the evolution of a polydisperse ensemble of particles in a metastable liquid should be described by means of an integro-differential model of kinetic and balance equations, which includes the nonlinear growth rates of spherical crystals (1.1). In addition, this model is complicated by the presence of mass sources (heat sinks) and the removal of product crystals in the case of the industrial crystallizer. With that in mind, we develop a new theoretical approach for analytical description of the evolution of a particulate assemblage in a supersaturated solution or a supercooled melt of the industrial crystallizer.
2. The model of nonlinear dynamics of crystals in a crystallizer
Consider the process of evolution of a polydisperse ensemble of particles in the metastable liquid (supersaturated solution or supercooled melt) of a crystallizer. Owing to the mass flux QC(τ) introduced from outside (or the heat flux Qθ(τ) removed from the system) the supersaturation (supercooling) necessary for crystal growth is maintained in the liquid. Taking into account the withdrawal rate of product crystals from the metastable liquid of a crystallizer, let us write down the kinetic equation for the particle-radius distribution function f(r, τ) describing the evolution of a polydisperse assemblage of particles
| 2.1 |
where V (τ) = dr/dτ is determined by expression (1.1), r is the radial coordinate, h(r) is the crystal withdrawal rate (h represents the inverse of the mean residence time of particles of radius r in the metastable liquid), r* is the radius of critical nuclei that are capable to appear in a supersaturated or supercooled system, and D is the coefficient of mutual Brownian diffusion. For the sake of simplicity, we consider the case of constant rate h and D(τ) = d1V (τ), where d1 stands for a pertinent factor [4,12–14].
The mass (heat) balance equation for supersaturated (supercooled) liquids reads as
| 2.2 |
where C is the solute concentration of the supersaturated solution, θ is the temperature of the supercooled melt, ρm and Cm are the density and specific heat of the mixture, respectively, and LV = ρsL. Note that the first line of expression (2.2) corresponds to supersaturated solutions, whereas the second line describes a phase transformation in supercooled melts.
The initial distribution function f0(r) as well as the initial metastability degree Δ0 = ΔC0 (Δθ0) should be known, i.e.
| 2.3 |
The flux of crystals overcoming the critical nucleation barrier and the distribution function of withdrawn crystals determine the boundary conditions
| 2.4 |
where I is the rate of nucleation, rp is the radius of withdrawn crystals (product crystals), and Δ designates the current metastability degree Δ = ΔC = C − Cp or Δ = Δθ = θp − θ in cases of supersaturated or supercooled liquids. Here C and θ stand for the current values of solute concentration and temperature, and θp represents the phase transition temperature of a supercooled melt.
Our further analysis is devoted to the study of the influence of the nonlinear contribution to the growth rate of individual crystals (which is proportional to τΔ(τ) in (1.1)) on the evolution of a polydisperse ensemble of particles in a metastable liquid of a crystallizer.
3. Analytical solution
For the convenience of further analysis, the mathematical model (2.1)–(2.4) can be rewritten in dimensionless form as (parameter χ plays the role of χ1, χ2 or χ3 introduced in expression (1.1))
| 3.1 |
| 3.2 |
| 3.3 |
where (p and ϕ(w) are given in §2 in [15] for supersaturated solutions and supercooled melts for the Meirs and Weber–Volmer–Frenkel–Zeldovich kinetics), x and t represent the dimensionless coordinate and time, respectively, F(x, t) and w(t) are the dimensionless distribution function and supersaturation (supercooling), respectively, x0 is the reduced radius of withdrawn crystals, Q is the dimensionless mass (heat) flux, and all the rest parameters are introduced in [15] (in the case of supercooled liquids the initial supersaturation ΔC0 should be replaced by the initial supercooling Δθ0).
To use the method of separation of variables to solve equation (3.1), we make the boundary conditions for the distribution function homogeneous by means of the following substitution:
| 3.4 |
After that, the problem (3.1), (3.3) takes the form
| 3.5 |
| 3.6 |
| 3.7 |
Now representing the reduced distribution function F1(x, t) as a product of two functions solely dependent on x and t, F1(x, t) = X(x)T(t), and taking into account that X(0) − u0X′(0) = 0 and X(x0) = 0, we obtain the eigenvalues nk and eigenfunctions Xk(x) in the form
| 3.8 |
Expanding functions ν(x, t) and F1(x, 0) in series in the eigenfunctions Xk(x)
and substituting (3.8) into (3.5), we get
| 3.9 |
where νk(t) and F0k are determined by expressions (3.9) and (3.10) in [15], and
| 3.10 |
Now taking into account that the derivative S′(t) > 0 (S(t) reaches a maximum at the upper limit of integration), we estimate the integral contribution in (3.9) on the basis of the saddle-point method for a Laplace-type integral as [16] (u0≪1)
| 3.11 |
Combining (3.9) and (3.11), we have
| 3.12 |
where we introduced a new unknown function
| 3.13 |
Now substituting (3.12) into the mass (heat) balance equation (3.2) and keeping in mind expression (3.4), we arrive at the following Cauchy problem for the determination of the integral function U(t)
| 3.14 |
Here the following notations are introduced
Let us especially note that the solution of the Cauchy problem (3.14) determines the integral function U(t). Then the metastability degree w(t) can be easily found as a derivative of this function, i.e. w(t) = U′(t). It means that the dimensionless particle size distribution function F(x, t) is also known by means of expressions (3.4) and (3.12) and takes the form
| 3.15 |
4. Summary and conclusion
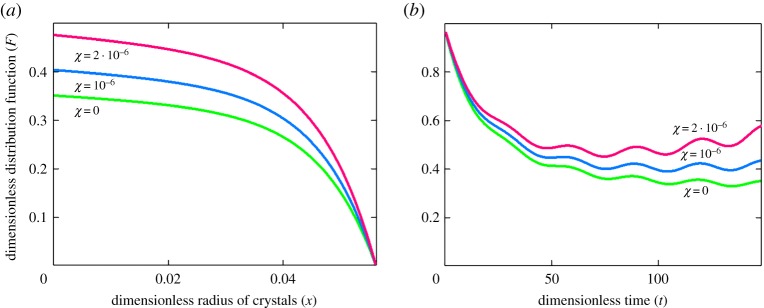
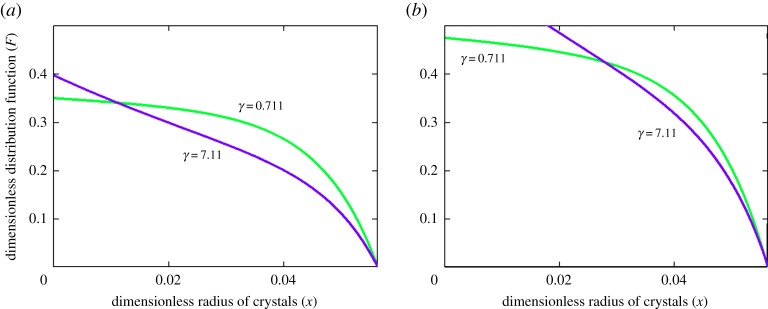
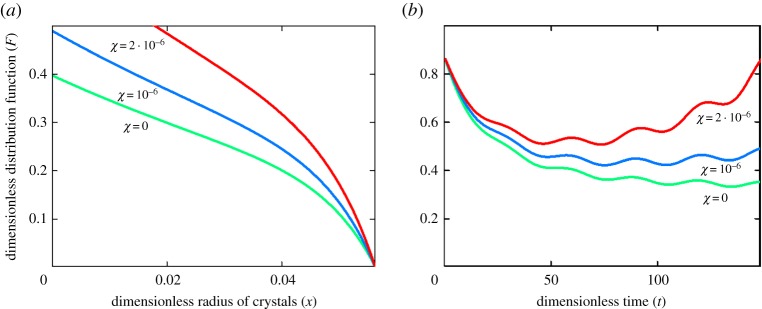
Figures 2–4 illustrate the particle-size distribution function plotted in accordance with the analytical solution (3.15) (physical parameters of the system under consideration are given in [15], Q(t) = A(1 − sin(ωt)/2), A = 0.005, and ω = π/15). The first important conclusion is the fact that the size distribution of crystals strongly depends on the parameter χ (compare curves at different χ in figures 2 and 3a,b). It is easily seen that the nonlinear growth rate of particles in a metastable liquid of crystallizer (expressions (1.1)) substantially changes the particle-size distribution. Our calculations show that the relative error may exceed tens of per cent in the course of time for real metastable liquids. As this takes place, the greatest difference of nonlinear growth law occurs for sufficiently small particles at a fixed time (figures 2a and 3a) and at essentially large times for particles of a given size (figures 2b and 3b). From the physical point of view, the nonlinear growth law (1.1) with non-zero χ means that the growth rates of evolving crystals become lower as compared with the case χ = 0. In other words, we have more crystals in the supercooled (supersaturated) liquid of a crystallizer, which grow longer until they are removed from the system (until they reach a given size x0).
Figure 2.
Dimensionless distribution function versus dimensionless coordinate x ((a) t = 100) and time t ((b) x = 0.01) for γ = 0.711.
Figure 4.
Dimensionless distribution function versus dimensionless coordinate x at time t = 100 for χ = 0 (a) and χ = 2 · 10−6 (b).
Figure 3.
Dimensionless distribution function versus dimensionless coordinate x ((a) t = 100) and time t ((b) x = 0.01) for γ = 7.11.
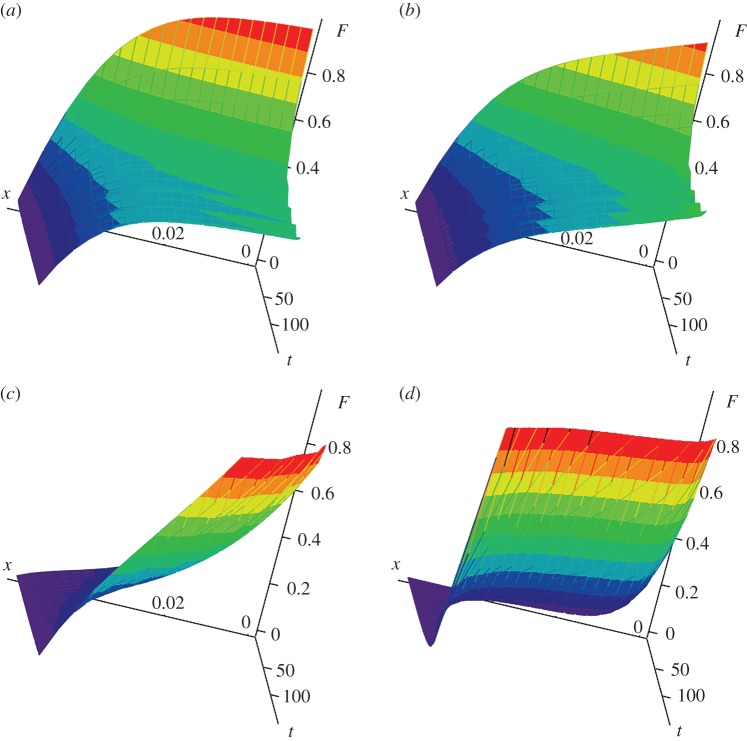
The second important conclusion is the influence of the crystal withdrawal rate h (which is proportional to the parameter γ) on the distribution of growing crystals. Indeed, with an increase in the rate of removal of crystals (γ increases), the number of large particles decreases and the number of small particles increases. Note that this effect takes place in both cases of linear and nonlinear growth rates (figure 4a,b). In other words, with the evolution of a metastable system with the same supercooling (supersaturation), when the number of larger crystals decreases, the number of smaller crystals increases and vice versa. Therefore, the distribution functions shown in figure 4 at various γ have a point of intersection. In addition, the three-dimensional dependencies F of x and t plotted in figure 5 show that the surface of the distribution function is inverted with an increase in the rate of removal of crystals (with increasing γ and h). In this case, the mean residence time of crystals in the metastable liquid of a crystallizer decreases and the surface of the distribution function bends in the direction of smaller times.
Figure 5.
Dimensionless distribution function versus dimensionless coordinate x and time t for χ = 10−6 and γ = 0.711 (a), γ = 7.11 (b), γ = 71.1 (c) and γ = 711 (d).
In summary, the particle-size distribution function strongly depends on two principal effects influencing the evolution of particulate assemblages in metastable liquids: (i) the nonlinear growth laws of crystals (expressions (1.1)) and (ii) the crystal withdrawal rate from a crystallizer. The theory under consideration, which takes into account these important physical effects should be used for a more precise description of similar phase transition phenomena when the evolution of crystals or other aggregates plays a decisive role. Such processes and phenomena as polymerization and crystallization in continuous apparatuses [17], evolution of protein and insulin crystals [18–20], evolution of aggregates in colloids and magnetic fluids [21–23], crystal growth in mushy layers [24–26], dissolution of dispersed solids, evaporation of a polydisperse mist, combustion of a solid dispersed fuel [27–29] may be mentioned among others.
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed equally to the present research article.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Russian Science Foundation (grant no. 18-19-00008).
References
- 1.Buyevich YuA, Natalukha IA. 1994. Unsteady processes of combined polymerization and crystallization in continuous apparatuses. Chem. Eng. Sci. 49, 3241–3247. ( 10.1016/0009-2509(94)E0052-R) [DOI] [Google Scholar]
- 2.Volmer U, Raisch J. 2001. H∞-Control of a continuous crystallizer. Control Eng. Practice 9, 837–845. ( 10.1016/S1474-6670(17)38528-2) [DOI] [Google Scholar]
- 3.Rachah A, Noll D, Espitalier F, Baillon F. 2015. A mathematical model for continuous crystallization. Math. Meth. Appl. Sci. 39, 1101–1120. ( 10.1002/mma.3553) [DOI] [Google Scholar]
- 4.Alexandrov DV. 2014. Nucleation and crystal growth kinetics during solidification: the role of crystallite withdrawal rate and external heat and mass sources. Chem. Eng. Sci. 117, 156–160. ( 10.1016/j.ces.2014.06.012) [DOI] [Google Scholar]
- 5.Mullin JW, Gaska C. 1969. The growth and dissolution of potassium sulphate crystals in a fluidized bed crystallizer. Can. J. Chem. Eng. 47, 483–489. ( 10.1002/cjce.5450470514) [DOI] [Google Scholar]
- 6.Garside J, Gaska C, Mullin JW. 1972. Crystal growth rate studies with potassium sulphate in a fluidized bed crystallizer. J. Cryst. Growth 13/14, 510–516. ( 10.1016/0022-0248(72)90290-4) [DOI] [Google Scholar]
- 7.Mullin JW, Gaska C. 1973. Potassium sulfate crystal growth rates in aqueous solution. J. Chem. Eng. Data 18, 217–220. ( 10.1021/je60057a030) [DOI] [Google Scholar]
- 8.Mullin JW, Osman MM. 1973. Nickel ammonium sulfate crystal growth rates in aqueous solution. J. Chem. Eng. Data 18, 353–355. ( 10.1021/je60059a035) [DOI] [Google Scholar]
- 9.Alexandrov DV. 2018. Nucleation and evolution of spherical crystals with allowance for their unsteady-state growth rates. J. Phys. A: Math. Theor. 51, 075102 ( 10.1088/1751-8121/aaa5b7) [DOI] [Google Scholar]
- 10.Alexandrov DV, Alexandrova IV. 2019. On the theory of the unsteady-state growth of spherical crystals in metastable liquids. Phil. Trans. R. Soc. A 377, 20180209 ( 10.1098/rsta.2018.0209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexandrov DV, Nizovtseva IG, Alexandrova IV. 2019. On the theory of nucleation and nonstationary evolution of a polydisperse ensemble of crystals. Int. J. Heat Mass Trans. 128, 46–53. ( 10.1016/j.ijheatmasstransfer.2018.08.119) [DOI] [Google Scholar]
- 12.Alexandrov DV. 2014. Nucleation and crystal growth in binary systems. J. Phys. A: Math. Theor. 47, 125102 ( 10.1088/1751-8113/47/12/125102) [DOI] [Google Scholar]
- 13.Alexandrov DV, Malygin AP. 2014. Nucleation kinetics and crystal growth with fluctuating rates at the intermediate stage of phase transitions. Modelling Simul. Mater. Sci. Eng. 22, 015003 ( 10.1088/0965-0393/22/1/015003) [DOI] [Google Scholar]
- 14.Alexandrov DV, Nizovtseva IG. 2014. Nucleation and particle growth with fluctuating rates at the intermediate stage of phase transitions in metastable systems. Proc. R. Soc. A 470, 20130647 ( 10.1098/rspa.2013.0647) [DOI] [Google Scholar]
- 15.Makoveeva EV, Alexandrov DV. 2018. A complete analytical solution of the Fokker-Planck and balance equations for nucleation and growth of crystals. Phil. Trans. R. Soc. A 376, 20170327 ( 10.1098/rsta.2017.0327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fedoruk MV. 1977. Saddle-point method. Moscow, Russia: Nauka. [Google Scholar]
- 17.Ivanov AA, Alexandrova IV, Alexandrov DV. 2019. Phase transformations in metastable liquids combined with polymerization. Phil. Trans. R. Soc. A 377, 20180215 ( 10.1098/rsta.2018.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barlow DA. 2009. Theory of the intermediate stage of crystal growth with applications to protein crystallization. J. Cryst. Growth 311, 2480–2483. ( 10.1016/j.jcrysgro.2009.02.035) [DOI] [Google Scholar]
- 19.Barlow DA. 2017. Theory of the intermediate stage of crystal growth with applications to insulin crystallization. J. Cryst. Growth 470, 8–14. ( 10.1016/j.jcrysgro.2017.03.053) [DOI] [Google Scholar]
- 20.Alexandrov DV, Nizovtseva IG. 2019. On the theory of crystal growth in metastable systems with biomedical applications: protein and insulin crystallization. Phil. Trans. R. Soc. A 377, 20180214 ( 10.1098/rsta.2018.0214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buyevich YA, Ivanov AO. 1993. Kinetics of phase separation in colloids: I. Formation of initial aggregates. Physica A 192, 375–390. ( 10.1016/0378-4371(93)90044-5) [DOI] [Google Scholar]
- 22.Buyevich YA, Ivanov AO. 1993. Kinetics of phase separation in colloids II. Non-linear evolution of a metastable colloid. Physica A 193, 221–240. ( 10.1016/0378-4371(93)90027-2) [DOI] [Google Scholar]
- 23.Ivanov AO, Zubarev AYu. 1998. Non-linear evolution of a system of elongated drop-like aggregates in a metastable magnetic fluid. Physica A 251, 348–367. ( 10.1016/S0378-4371(97)00561-X) [DOI] [Google Scholar]
- 24.Alexandrov DV, Ivanov AA. 2009. Solidification of a ternary melt from a cooled boundary, or nonlinear dynamics of mushy layers. Int. J. Heat Mass Trans. 52, 4807–4811. ( 10.1016/j.ijheatmasstransfer.2009.05.029) [DOI] [Google Scholar]
- 25.Alexandrov DV, Malygin AP. 2011. Convective instability of directional crystallization in a forced flow: the role of brine channels in a mushy layer on nonlinear dynamics of binary systems. Int. J. Heat Mass Trans. 54, 1144–1149. ( 10.1016/j.ijheatmasstransfer.2010.11.008) [DOI] [Google Scholar]
- 26.Alexandrov DV, Ivanov AA, Alexandrova IV. 2018. Analytical solutions of mushy layer equations describing directional solidification in the presence of nucleation. Phil. Trans. R. Soc. A 376, 20170217 ( 10.1098/rsta.2017.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buyevich YuA, Korolyova NA, Natalukha IA. 1993. Modelling of unsteady combustion regimes for polydisperse fuels I. Instability and auto-oscillations. Int. J. Heat Mass Trans. 36, 2223–2231. ( 10.1016/S0017-9310(05)80153-X) [DOI] [Google Scholar]
- 28.Buyevich YuA, Korolyova NA, Natalukha IA. 1993. Modelling of unsteady combustion regimes for polydisperse fuels II. Parametrically controlled combustion. Int. J. Heat Mass Trans. 36, 2233–2238. ( 10.1016/s0017-9310(05)80154-1) [DOI] [Google Scholar]
- 29.Buyevich YuA, Goldobin YuM, Yasnikov GP. 1994. Evolution of a particulate system governed by exchange with its environment. Int. J. Heat Mass Trans. 37, 3003–3014. ( 10.1016/0017-9310(94)90354-9) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.