Abstract
Pairing vagus nerve stimulation (VNS) with movements or sounds can direct robust plasticity in motor or auditory cortex, respectively. The degree of map plasticity is influenced by the intensity and pulse width of VNS, number of VNS-event pairings, and the interval between each pairing. It is likely that these parameters interact, influencing optimal implementation of VNS pairing protocols. We varied VNS intensity, number of stimulations, and inter-stimulation interval (ISI) to test for interactions among these parameters. Rats were implanted with a vagus nerve stimulating cuff and randomly assigned to one of three treatment groups to receive 20 days of VNS paired with a 9 kHz tone: 1) Fast VNS: 50 daily pairings of 400 μA VNS with a 30 s ISI; 2) Dispersed VNS: 50 daily pairings of 400 μA VNS with a 180 s ISI; and 3) Standard VNS: 300 daily pairings of 800 μA VNS with a 30 s ISI. Following 20 days of VNS-tone pairing, multi-unit recordings were conducted in primary auditory cortex (A1) and receptive field properties were analyzed. Increasing ISI (Dispersed VNS) did not lead to an enhancement of cortical plasticity. Reducing the current intensity and number of stimulations (Fast VNS) resulted in robust cortical plasticity, using 6 times fewer VNS pairings than the Standard protocol. These findings reveal an interaction between current intensity, stimulation number, and ISI and identify a novel VNS paradigm that is substantially more efficient than the previous standard paradigm.
Keywords: Vagal nerve stimulation, stimulation parameters, current intensity, inter-stimulation interval, cortical plasticity, auditory cortex
Introduction
Repeated pairing of vagus nerve stimulation (VNS) with sensory or motor events induces large-scale expansion of cortical representations (Engineer et al., 2011, 2015; Porter et al., 2012; Shetake et al., 2012; Hulsey et al., 2016). In addition to these effects on map plasticity in healthy subjects, paired VNS enhances rehabilitation in models of neurological damage. A preclinical study in an animal model of tinnitus demonstrated that pairing tones with VNS can eliminate both the behavioral and neurophysiological correlates of tinnitus (Engineer et al., 2011). In animal models of stroke, traumatic brain injury and spinal cord injury, pairing VNS with a deficit-related task improves recovery of motor function beyond that of rehabilitation alone (Khodaparast et al., 2013, 2014, 2016, Hays et al., 2014a, 2016; Pruitt et al., 2016; Ganzer et al., 2018). Based on this robust and specific enhancement of plasticity and rehabilitation, VNS has emerged as a strategy to improve recovery in chronic stroke and tinnitus patients (De Ridder et al., 2014; Dawson et al., 2016; Tyler et al., 2017). While the initial clinical results are encouraging, optimization of VNS pairing paradigms could lead to greater compliance, faster therapeutic recovery, and better outcomes for patients.
A number of studies investigating the relationship between VNS efficacy and intensity have found that increasing VNS intensity results in an inverted-U response. Pairing VNS with tone presentation drives plasticity in auditory cortex at moderate current intensities, but not at low or high intensities (Borland et al., 2016; Loerwald et al., 2017). Studies investigating VNS-mediated enhancement of memory (Clark et al., 1995, 1998) and hippocampal plasticity (Zuo et al., 2007) reveal similar responsiveness that is selective for moderate current intensities. Taken together, these findings demonstrate VNS efficacy is critically sensitive to current intensity.
In addition to VNS current intensity, stimulation number and inter-stimulation interval (ISI) have also been explored to optimize VNS efficacy. Lengthening the interval between VNS-tone pairings increases the magnitude of plasticity evoked by VNS in auditory cortex (Borland et al., 2017). Significantly more plasticity is produced when the interval between VNS-tone pairings is 120 s compared to 8 s. Presenting only 50 VNS-tone pairings per day fails to drive plasticity when delivered at 800 μA, whereas 300 pairings per day drives robust plasticity (Borland et al., 2017). These results demonstrate that independently modifying these parameters alters the magnitude of plasticity induced, but it is unknown how these parameters interact with each other and other parameters to alter efficacy.
To investigate the interactions among VNS parameters, we compared the amount of plasticity driven by different VNS-tone pairing paradigms by varying current intensity, number of stimulations, and ISI. In contrast with previous reports, we found that lengthening ISI does not enhance plasticity at lower current intensities. Finally, we report that reducing the current intensity and number of daily stimulations while holding ISI constant drives plasticity equivalent to our standard stimulation parameters in substantially less time each day. These findings reveal an interaction between VNS intensity, number of stimulations, and ISI that yields a stimulation paradigm substantially more efficient at generating cortical plasticity than the previous standard paradigm.
Experimental procedures
This study, including design, statistical methodology, and planned comparisons, was preregistered on Open Science Framework (DOI 10.17605/OSF.IO/3Y7U8) in compliance with the Transparency and Openness Promotion guidelines (Alberts et al., 2015). All handling, housing, stimulation, and surgical procedures were approved by The University of Texas at Dallas Institutional Animal Care and Use Committee. One hundred twenty-one 3 – 6 month old Sprague Dawley female rats were housed in a 12:12 hour reversed light-dark cycle. Rats were randomly assigned to one of three VNS-treated groups. Following cuff implantation, rats received 20 days of VNS-tone pairing after which multi-unit recordings were collected in A1 using standard microelectrode recording techniques to assess A1 responsiveness to a range of tones. Recordings were also conducted in 12 of these rats who were not implanted and did not receive daily auditory stimuli to serve as naïve controls. Seventy-six rats were excluded from analysis according to predefined criteria, as detailed in the Auditory Cortex Recordings section below.
Vagus nerve surgery
A custom made platinum iridium bipolar cuff electrode was implanted around the left cervical vagus nerve as described previously (Engineer et al., 2011, 2015; Shetake et al., 2012; Hays et al., 2014a, 2014b, Khodaparast et al., 2014, 2016, Borland et al., 2016, 2017; Pruitt et al., 2016; Hulsey et al., 2016; Loerwald et al., 2017). In brief, rats were anesthetized with ketamine hydrochloride (80 mg/kg) and xylazine (10 mg/kg) administered intraperitoneally and supplemented as needed to maintain a state of areflexia. Body temperature was maintained at 37° C throughout the surgery. The vagus nerve was exposed and isolated via blunt dissection. A cuff electrode was implanted surrounding the vagus nerve with 2 leads tunneled subcutaneously to connect with a 2-channel connector fixed with acrylic to the skull. Nerve activation was confirmed immediately after implanting the cuff electrode by observation of a ≥ 5% drop in blood oxygen saturation in response to a 10 s stimulation train of 30 Hz VNS consisting of 800 μA, 100 μs biphasic pulses as in previous studies (Borland et al., 2016, 2017; Loerwald et al., 2017). If stimulation failed to evoke changes in oxygen saturation, the cuff was adjusted or replaced. Buprenex (0.03 mg/kg) and atipamezole hydrochloride (0.5 mg/kg) were administered to manage pain and facilitate recovery, respectively. Rats received amoxicillin (10 mg) and rimadyl (2 mg) tablets for 2 days following surgery and were allowed to recover for at least 5 days before beginning VNS.
Vagus Nerve Stimulation and Tone Pairing
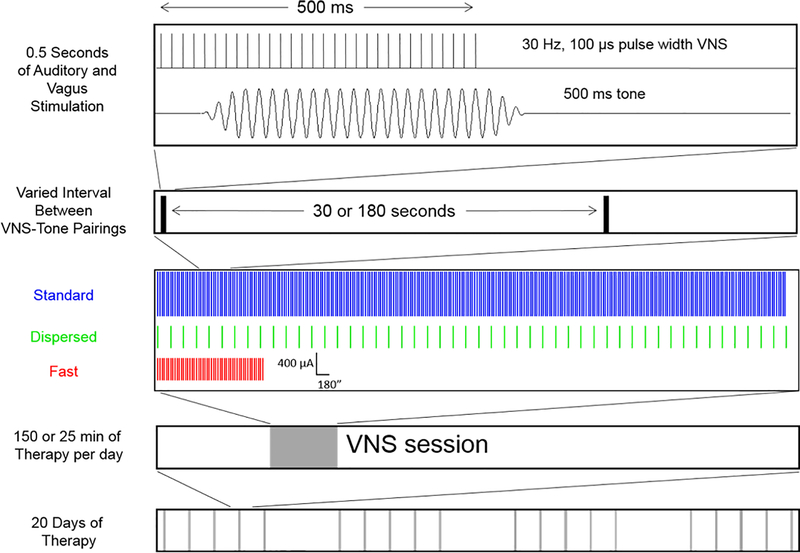
Rats were exposed to presentations of a 500 ms, 9 kHz 50 dB tone paired with VNS daily for 20 days. Five millisecond ramps were added to the beginning and end of each tone to eliminate acoustic transients. Depending on the experimental conditions, VNS consisted of 500 ms, 30 Hz trains of 100 μs biphasic pulses delivered at either 400 μA or 800 μA. The number of pairings was either 50 or 300 and the interval between pairings was either 30 or 180 s, depending on the experimental group. To prevent rats from anticipating when stimulation would occur, VNS sessions were designed to present 300 pairings every 15 s for the Standard and Fast groups and every 90 s for the Dispersed group with each event having a probability of 0.5 resulting in 300 stimulations with an average ISI of 30 or 180 s, respectively..
Auditory Cortex Recordings
Multi-unit recordings were conducted from primary auditory cortex (A1) according to standard techniques (Engineer et al., 2011; Borland et al., 2016, 2017; Loerwald et al., 2017). 24–72 hrs after the last day of pairing, rats were anesthetized with sodium pentobarbital (50 mg/kg). Rats were monitored throughout the recording and supplemented with additional anesthesia as necessary to maintain a state of areflexia. A tracheal tube was inserted to facilitate respiration and a cisternal drain was used to mitigate brain swelling. A section of skull was removed to expose right auditory cortex. The dura was removed and a thin layer of silicone oil was applied to prevent desiccation. Four parylene-coated tungsten microelectrodes (1.5–2.5 MΩ, FHC) were inserted 600 – 700 μm below the surface of cortex to target layer IV. Neural signals were amplified using an RA16PA preamplifier (Tucker-Davis Technologies) and digitized at 24.414 ks/s with 16-bit resolution using an RZ5 BioAmp processor (Tucker-Davis Technologies) and subsequently filtered with a 300 to 3,000 Hz bandpass filter and further amplified 20,000 times using Brainware (Jan Schnupp). A 600 mV threshold was applied to amplified voltage signals for spike detection. For electrical and acoustic isolation, recordings were conducted in a foam-lined, doubled-walled sound attenuated chamber. 1296 pure tones spanning 81 frequencies across 5 octaves (1 – 32 kHz) and 16 intensities (0 – 75 dB) were presented in a randomly interleaved fashion every 500 ms via a speaker placed 10 cm away from the left ear. Multi-unit activity was recorded in response to each tone with Brainware and each recording site was logged on a digitized image of the cortex. A site was considered to be in A1 based on tonotopy and the following criteria: onset latency of 5 – 25 ms, peak latency of 10 – 40 ms, end of peak latency of 20 – 60 ms, and response strength of at least 50% increase in firing rate over spontaneous firing rate. Upon completion of the recordings, vagus nerve activation by the cuff electrode was again confirmed by observation of either a decrease in blood oxygen saturation or cessation of breathing if stable oxygen saturation baseline levels were unable to be obtained. Nineteen animals failed to exhibit a drop in oxygen saturation after mapping and were consequently excluded from analysis. Twenty-two animals were excluded due to complications during auditory cortex recordings that prevented the complete mapping of A1 such as cortical damage or inability to detect spiking activity. Nineteen animals died during the recording procedure. Sixteen animals died or were euthanized before completion of 20 days of VNS-tone pairing.
Data analysis
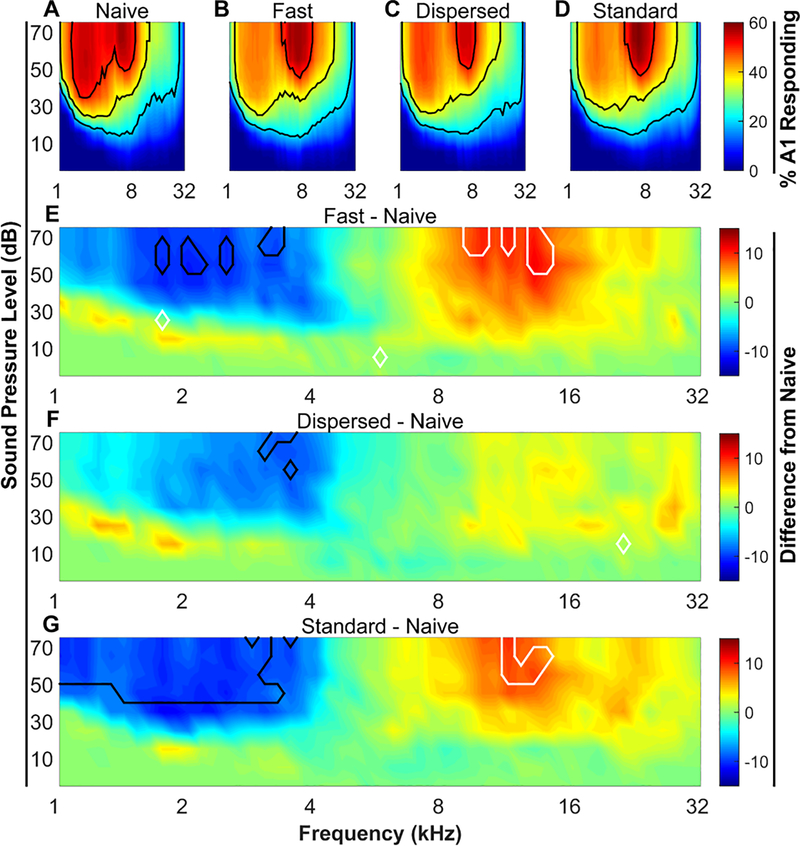
Auditory evoked neural responses were analyzed using a custom, fully-automated Matlab program to quantify receptive field and response characteristics at each site. The percent of area of A1 that responded to each of the 1296 combinations of tone frequency and intensity was quantified for each rat, as in previous studies (Engineer et al., 2011, 2015, Borland et al., 2016, 2017; Loerwald et al., 2017). Cortical reorganization was evaluated by subtracting the averaged group response of experimentally naïve rats from the averaged group response of rats that received VNS paired with a 9 kHz tone. The percent of area responding and extent of cortical reorganizations were measured for each animal. Group averages were compared across conditions using one-way ANOVAs followed by Tukey’s test for multiple comparisons when appropriate to determine significant differences in receptive field organization. For figure 2, a Benjamini-Hochberg correction was used to control the false discovery rate (Benjamini et al., 2001).
Figure 2. VNS-tone pairing reorganizes receptive fields in primary auditory cortex.
(A – D) Percent of area of A1 responding to each of the 1296 tones. Black contour lines denote 20%, 35%, and 50% of area responding. (E – G) Area responding difference plots. Naïve percent of area responding values were subtracted from each of the 3 VNS treated groups. Black and white contour lines denote areas of significant (P < 0.05) decreases and increases relative to naïve, respectively. N’s are 12, 11, 11, & 11 for naïve, Fast VNS, Dispersed VNS, and Standard VNS, respectively.
Results
Experimental design
To assess the efficacy of different VNS-tone pairing regimens, we evaluated changes in A1 receptive fields after 20 days of VNS-tone pairing. Receptive fields were measured by recording multi-unit activity throughout auditory cortex in response to presentation of pure tones ranging in frequency and intensity that span the rat hearing range. Sites were identified as being in A1 based on response characteristics and tonotopy. The percent of area of A1 responding was calculated for each sound. All maps were tonotopically organized, as indicated by a significant correlation between the anterior-posterior location and the binary logarithm of the characteristic frequency (average R2 across all animals = 0.68 ± 0.02). As in previous studies, rats receiving VNS-tone pairing were mapped 1 – 3 days after 20 days of pairing 500 ms VNS pulse trains with 500 ms 9 kHz, 50 dB tones (Engineer et al., 2011; Borland et al., 2016, 2017; Loerwald et al., 2017). The size of A1 was unaffected by VNS (Kruskal-Wallis, H(3) = .791; P > 0.05; mean ± SEM area in mm2 = 1.67 ± 0.13, 1.74 ± .10, 1.75 ± 0.23, 1.78 ± .13 for naïve, Fast, Dispersed, and Standard groups, respectively). There was no difference in the number of A1 sites recorded from or the density of recording sites in A1 across groups (Number of sites: One-way ANOVA, F(3, 41) = 0.57, P = 0.64; Sampling density: One-way ANOVA F(3, 41) = 0.13, P = 0.94).
Previous studies varying VNS parameters have found that 300 daily pairings of 800 μA with a 30 s ISI drive significant cortical plasticity (Borland et al., 2016, 2017; Loerwald et al., 2017). Therefore, one group of rats received these parameters (referred to as ‘Standard VNS’) as a reference point to assess any gains made in VNS efficacy with other regimens (Fig. 1). We tested the effectiveness of increasing ISI with 50 stimulations of 400 μA current intensity (referred to as ‘Dispersed VNS’) to match the session duration of Standard VNS. To explore a potential interaction between intensity and stimulation number, we also tested 400 μA current intensity, 50 stimulation VNS paradigm with a 30 s ISI resulting in a reduced daily session length (referred to as ‘Fast VNS’). Data from all measures of plasticity reported come from standard normal distributions (Kolmogorov-Smirnov test, P > 0.05) with equal variance (Bartlett’s test, P > 0.05).
Figure 1. Schematic of VNS-tone paring paradigm and experimental groups.
With each 500 ms tone presentation, a 30 Hz, 500 ms train of electrical stimulation consisting of 100 μs biphasic pulses at varied intensities was delivered to the left vagus nerve via a cuff electrode. VNS paradigms were ‘Fast’ (50 pairings of 400 μA VNS delivered every 30 s); ‘Dispersed’ (50 pairings of 400 μA VNS delivered every 180 s); or ‘Standard’ (300 pairings of 800 μA VNS delivered every 30 s). Rats received VNS paired with a 9 kHz, 50 dB tone for 20 days.
Reducing the number of stimulations and intensity of VNS drives robust plasticity
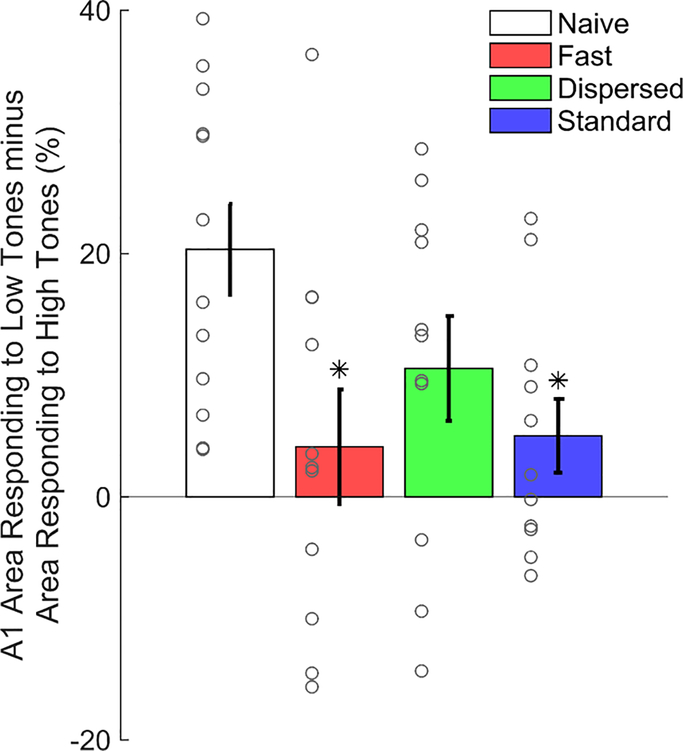
Nine kHz tones presented at 50 dB sound pressure level (SPL) elicits responses in 32.6 ± 3.1% of A1 in Naïve animals while 2 kHz tones at 50 dB SPL elicits responses in 54.8 ± 2.9% of A1 (Fig. 2A). To summarize the effects of different VNS regimens on cortical reorganization, we computed the difference between the percent of area of A1 responding to low frequency tones (2 – 4 kHz) and the percent of A1 responding to high frequency tones (8 – 16 kHz) (Fig. 3) as in previous studies (Borland et al., 2016, 2017; Loerwald et al., 2017). A1 responsiveness in naïve animals has a bias towards low frequency tones (Naïve low – high (%) = 20.4 ± 3.7). After 20 days of VNS-tone pairing this low frequency bias was significantly diminished (One-way ANOVA, F(3, 41) = 3.62, P = 0.02, η2 = 0.21, Fig. 3). Consistent with previous studies (Engineer et al., 2011; Borland et al., 2016, 2017; Loerwald et al., 2017), Standard VNS reduced responsiveness to low – high tones to 5.02 ± 3.04 % (Tukey HSD, P = 0.04). This reduction in low frequency bias is characterized by an increased responsiveness to 8 – 16 kHz tones (area outlined in white in Fig. 2G) accompanied by a decrease in responsiveness to 2 – 4 kHz tones (area outlined in black in Fig. 2G) relative to naïve. No correlation was observed between map size and A1 responsiveness to low – high tones (R2 = .08, P > 0.05). These results corroborate previous studies demonstrating that Standard VNS paired with tones drives robust plasticity in auditory cortex.
Figure 3. VNS diminishes low frequency tone bias.
Subtracting responses to high frequency tones (8 – 16 kHz, 50 dB) from responses to low frequency tones (2 – 4 kHz, 50 dB) reveals a low frequency bias in naïve rats. Both Fast and Standard VNS significantly reduce this bias so that responsiveness to low and high frequency tones is similar. Data from individual animals is represented as gray circles. * = P < 0.05 by Tukey’s test for multiple comparisons.
Reducing the number of stimulations has been shown to significantly weaken VNS-mediated enhancement of plasticity (Borland et al., 2017). However, a number of studies have demonstrated that high VNS current intensities also lead to a decrease in efficacy (Clark et al., 1995, 1998, 1999; Zuo et al., 2007; Borland et al., 2016). Therefore, to investigate a potential interaction between stimulation number and current intensity, we reduced the stimulation intensity from 800 μA to 400 μA and the number of stimulations from 300 to 50 (Fast VNS) (Fig. 1). Fast VNS reduced the bias towards low frequency tones that was observed in naïve rats to 4.12 ± 4.7% (Tukey HSD, P = 0.03). Similar to Standard VNS, this was characterized by a suppression responsiveness to 2 – 4 kHz tones and an enhancement of responsiveness to 8 – 16 kHz tones (Fig. 2E). This result indicates that short VNS-tone pairing sessions can drive robust plasticity if delivered at lower current intensities and may suggest that the Standard VNS intensity (i.e. 800 μA) is initially too strong before adaptation reduces activation to appropriate levels.
Increasing the interval between VNS-tone pairings abolishes VNS-mediated plasticity
Previous evidence indicates that increasing the interval between 800 μA current intensity stimulations leads to an enhancement of plasticity (Borland et al., 2017). Therefore, we hypothesized that the effect of 400 μA, 50-stim VNS may be enhanced by lengthening the interval from 30 s to 180 s (Dispersed VNS). Dispersed VNS had little effect on responsiveness to any frequency range relative to naive (Figure 2F), with a Tukey post hoc test revealing no significant difference from any other group (Fig 2G). This result demonstrates that lengthening the stimulation interval at lower current intensity levels diminished the effectiveness of VNS.
We investigated whether changes in threshold or bandwidth could underlie VNS-dependent plasticity. No differences in threshold or bandwidth 10, 20, and 40 dB above threshold at sites with characteristic frequencies between 8 and 16 kHz were observed across groups (One way ANOVA, Threshold: F(3, 41) = 0.32, P = 0.81; Bandwidth 10, 20, 30, and 40 dB above threshold: F(3, 41) = 0.51, 0.53, 0.16, and 0.45 respectively, all P > 0.65). This suggests that alterations in tuning selectivity do not underlie VNS-dependent plasticity, but rather VNS-dependent represent suprathreshold increases in responsiveness across the tonotopic gradient.
These findings indicate that 1) reducing both the number of stimulations and current intensity of VNS produces plasticity more quickly (25 vs. 150 minutes), and 2) increasing the interval between stimulations at these reduced settings is insufficient to enhance receptive field plasticity.
Discussion
Previous studies have demonstrated that VNS efficacy is sensitive to both the timing and amount of VNS delivered (Hays et al., 2014b; Borland et al., 2016, 2017; Ganzer et al., 2018). Here, we report that reducing the number of VNS stimulations to 50 and reducing the stimulation intensity to 400 μA drives plasticity equivalent to Standard VNS (Table 1, 300 stimulations at 800 μA). In contrast to previous findings, lengthening the stimulation interval under these conditions failed to enhance plasticity. These findings indicate an interaction between stimulation intensity, number, and ISI and identify a novel VNS paradigm capable of driving targeted plasticity in much less time than previously required.
Table 1.
The effects of stimulation parameters on the magnitude of VNS-dependent plasticity
| Study | Intensity (mA) | Pulse width (μs) | Interstimulus Interval (s) | Number of Pairings per Day | % of Standard Parameter Effect |
|---|---|---|---|---|---|
| Present Study | 0.4 | 100 | 30 | 300 | 106 ± 31 |
| 0.4 | 100 | 180 | 50 | 64 ± 28 | |
| Borland et al., 2016 | 0.4 | 100 | 30 | 300 | 101 ± 46 |
| 1.2 | 100 | 30 | 300 | 33 ± 35 | |
| 1.6 | 100 | 30 | 300 | 12 ± 33 | |
| Loerwald et al., 2017 | 0.2 | 100 | 300 | 300 | 21 ± 17 |
| 0.2 | 500 | 300 | 300 | 72 ± 13 | |
| Borland et al., 2018 | 0.8 | 100 | 8 | 300 | 72 ± 21 |
| 0.8 | 100 | 1200 | 300 | 102 ± 32 | |
| 0.8 | 100 | 50 | 50 | 46 ± 16 |
Previous studies using VNS and deep brain stimulation to direct plasticity used 300 stimulations per day for many weeks (Kilgard and Merzenich, 1998; Puckett et al., 2007; Engineer et al., 2011, 2015; Shetake et al., 2012; Borland et al., 2016, 2017; Loerwald et al., 2017) to parallel the number of rewarded daily trials performed by monkeys in classic studies investigating cortical plasticity induced by operant conditioning (Recanzone et al., 1993). The current study replicates findings from previous studies that tone-paired VNS under these conditions can drive plasticity in auditory cortex. Furthermore, it demonstrates that it is possible to produce equivalent large-scale map plasticity with six times fewer trials if the VNS current paired with tones is at the low end of the effective range for producing plasticity (Table 1, Borland et al., 2016; Loerwald et al., 2017). This finding is consistent with several studies indicating that neuromodulator-dependent plasticity can be induced with a relatively small number of pairings (Bakin and Weinberger, 1996; Froemke et al., 2007). Spike-timing-dependent plasticity paradigms can induce both LTP and LTD with only 40 pairings if the pairings coincide with neuromodulator release (He et al., 2015). Receptive field plasticity can be induced in A1 neurons when tones are paired with LC stimulation with as few as 30 pairings (Martins and Froemke, 2015). Moreover, the present study corroborates evidence that 50 daily parings of VNS, if timed appropriately with rehabilitation, can enhance plasticity and improve recovery after spinal cord injury (Ganzer et al., 2018).
In the present study, 50 daily VNS-tone pairings delivered at 400 μA results in robust plasticity when the interval between pairings is short. However, this finding stands in contrast with a previous study demonstrating that 50 daily VNS-tone pairings at a higher current intensity (800 μA) drives little plasticity, while 300 pairings at the same intensity drives robust plasticity (Table 1, Borland et al., 2017). This apparent discrepancy may be explained by initial over-activation of VNS targets with 800 μA stimulation. With repeated stimulation, however, activation of downstream targets may adapt to levels appropriate for plasticity. In support of this notion, VNS-tone pairing only drives plasticity at moderate current intensities, beyond which the effect of VNS diminishes (Borland et al., 2016). Similarly, cortical function has been shown to demonstrate an inverted-U response to increasing levels of neuromodulator concentration (Arnsten, 2011). Therefore, the ability of Fast VNS in the current study to drive plasticity may be attributed to the mitigation of initial over-activation and may reveal a form of desensitization elicited by 800 μA after prolonged (> 50) stimulation. Alternatively, 400 μA may be a more optimal stimulation intensity than 800 μA, requiring fewer pairings to drive similar amounts of plasticity than 800 μA. However, a previous study showed that 400 μA and 800 μA VNS elicit comparable degrees of plasticity when all other conditions are held constant (Borland et al., 2016). These findings suggest that the most effective VNS regimens will be those which maintain adaptation of the entire system within an optimal level of activation.
It has previously been demonstrated that lengthening the interval between VNS stimulations increases the extent of plasticity expressed (Table 1, Borland et al., 2017), contrasting with findings from this study. However, a number of additional parameter differences exist between studies including the total number of daily pairings (300 vs 50), stimulation intensity (800 vs 400 μA), and interval length (120 vs 180 s) that may contribute to the disparate findings. Nonetheless, the observation from the current study that Dispersed VNS was less effective at generating plasticity demonstrates that shorter intervals are required at weaker stimulation intensities. Considering the observed effectiveness of Fast VNS, this may suggest that reducing the interval engages a form of rapid sensitization. Taken together with previous findings, results from this study suggest two forms of adaptation influenced by the interaction of the timing and amount of VNS: a rapid sensitization occurring on a timescale of < 180 s and a concurrent slower desensitization resulting from prolonged (> 50) stimulation.
Predicting how parameters will interact under a range of parameter values requires understanding the forms of adaptation associated with each parameter. VNS enhances plasticity by engaging neuromodulatory networks which are known to exhibit sensitization and desensitization (Schmidt et al., 2000; Ferguson, 2001; Xu, 2004). Monoaminergic and cholinergic neuromodulator systems, mediated predominately through G-protein coupled receptors (GPCRs), contribute to several forms of both cellular and network level adaptation including adaptation and autoregulation within neuromodulatory nuclei, presynaptic adaptation at neuromodulatory terminals in cortex, postsynaptic adaptation at cortical excitatory and/or inhibitory neurons, and network adaptation in local cortical circuits. For instance, persistent receptor activation on the timescale of one to two hours leads to a downregulation of cell surface receptors (Valiquette et al., 1990; Ferguson and Caron, 1998). Therefore, it would be expected that longer daily sessions would be more affected by this form of desensitization than shorter sessions, consistent with the finding that Fast VNS, but not Dispersed VNS, can elicit significant plasticity. However, in cases where stronger current intensities are used, such as in Standard VNS, reduced receptor density associated with longer sessions may be offset by enhanced signaling through the remaining receptors. Alternatively, shorter ISI’s may contribute to increased accumulation of second messenger levels. Persistent receptor activation has been shown to increase second messengers levels (Violin et al., 2008), although the temporal dynamics of second messenger signals vary with the duration and concentration of available ligand (January et al., 1997). Therefore, it is likely that multiple VNS parameters interact to influence signal transduction processes in unique ways.
One neuromodulatory system of particular interest in the context of VNS is the noradrenergic locus coeruleus (LC). VNS enhances spiking activity in the LC phasically in response to brief (500 ms), acute VNS (Hulsey et al., 2017), and persistently in response to either longer (30s) acute applications (Groves et al., 2005) or chronic (14 – 90 days) stimulation (Manta et al., 2009). Both LC activity and noradrenergic transmission have been shown to be involved in learning and plasticity (Edeline et al., 2011; He et al., 2015; Martins and Froemke, 2015). Synaptically evoked discharge rates of LC neurons are non-adapting in response to increasing stimulation frequency of VNS (Hulsey et al., 2017), suggesting the locus of adaptation is likely in cortex. Pairing direct stimulation of LC with tones induces receptive field plasticity in auditory cortex and perceptual changes by attenuating tonic GABAergic transmission (Martins and Froemke, 2015). A similar network mechanism may be utilized by VNS as network reorganization is correlated with cortical disinhibition (Chen et al., 2015). Additional neuromodulator systems which display regulation by VNS and may be involved in its plasticity-enhancing effects include acetylcholine, serotonin, and dopamine (Manta et al., 2009, 2013; Hulsey et al., 2016). Future studies investigating the network and cellular mechanisms of these putative forms of adaptation will allow them to be further exploited for optimization of VNS.
This study demonstrates that a shortened VNS paradigm can produce robust neural plasticity when delivered at the appropriate stimulation intensity and interval. This finding reveals a VNS paradigm capable of driving substantial, experience-dependent plasticity 6 times faster than previously determined. Reducing daily VNS session length is expected to enhance clinical utility. The complex interactions between multiple parameters suggest that future study may lead to additional paradigms that generate plasticity more quickly or produce a greater degree of plasticity.
Acknowledgements
We would like to thank Jayant Kuvari, Collin Chandler, Corinne Kelly, Alan Carroll, and Emily Jensen for help with electrophysiological recordings, Abigail Berry for help with running VNS pairing sessions, Ruby Solorzano for help with cuff electrode implants, and John Buell, Lena Sadler, Son Pham, Revanth Vedula, Jeremy Samuel, and Jordan Chen for help with electronics construction. This work was supported by NIH R01NS085167, R01NS094384, and the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) Electrical Prescriptions (ElectRx) program under the auspices of Dr. Doug Weber and Eric Van Gieson through the Space and Naval Warfare Systems Center, Pacific Cooperative Agreement No. HR0011-15-2-0017 and N66001-15-2-4057 and the DARPA BTO Targeted Neuroplasticity Training (TNT) program under the auspices of Dr. Doug Weber and Dr. Tristan McClure-Begley through the Space and Naval Warfare Systems Center, Pacific Grant/Contract No. N66001-17-2-4011. MPK is a consultant for, and has a financial interest in, MicroTransponder, Inc., which is developing therapies using VNS. KWL, MSB, EPB, RLR, and SAH report no biomedical financial interests or potential conflicts of interest.
References
- Alberts B, Cicerone RJ, Fienberg SE, Kamb A, McNutt M, Nerem RM, Schekman R, Shiffrin R, Stodden V, Suresh S, Zuber MT, Pope BK, Jamieson KH (2015) Self-correction in science at work. Science (80- ) 348:1420–1422 Available at: http://science.sciencemag.org/content/sci/348/6242/1422.full.pdf. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT (2011) Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry 69:e89–99 Available at: 10.1016/j.biopsych.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM (1996) Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci U S A 93:11219–11224 Available at: http://www.pnas.org/content/93/20/11219.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I (2001) Controlling the false discovery rate in behavior genetics research. Behav Brain Res 125:279–284 Available at: http://www.stat.purdue.edu/~doerge/BIOINFORM.D/FALL06/BenjaminiandYFDR.pdf%5Cnhttp://engr.case.edu/ray_soumya/mlrg/controlling_fdr_benjamini95.pdf. [DOI] [PubMed] [Google Scholar]
- Borland MS, Engineer CT, Vrana WA, Moreno NA, Engineer ND, Vanneste S, Sharma P, Pantalia MC, Lane MC, Rennaker RL, Kilgard MP (2017) The Interval Between VNS-Tone Pairings Determines the Extent of Cortical Map Plasticity. Neuroscience 369:76–86 Available at: http://www.ncbi.nlm.nih.gov/pubmed/29129793%0Ahttp://linkinghub.elsevier.com/retrieve/pii/S0306452217307911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland MS, Vrana WA, Moreno NA, Fogarty EA, Buell EP, Sharma P, Engineer CT, Kilgard MP (2016) Cortical Map Plasticity as a Function of Vagus Nerve Stimulation Intensity. Brain Stimul 9:117–123 Available at: http://www.sciencedirect.com/science/article/pii/S1935861X15011250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SX, Kim AN, Peters AJ, Komiyama T (2015) Subtype-specific plasticity of inhibitory circuits in motor cortex during motor learning. Nat Neurosci 18:1109–1115 Available at: http://www.nature.com/doifinder/10.1038/nn.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KB, Krahl SE, Smith DC, Jensen RA (1995) Post-training Unilateral Vagal Stimulation Enhances Retention Performance in the Rat. Neurobiol Learn Mem 63:213–216 Available at: http://linkinghub.elsevier.com/retrieve/pii/S1074742785710246. [DOI] [PubMed] [Google Scholar]
- Clark KB, Naritoku DK, Smith DC, Browning R a, Jensen R a (1999) Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci 2:94–98 Available at: http://www.nature.com/doifinder/10.1038/4600. [DOI] [PubMed] [Google Scholar]
- Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, Jensen RA (1998) Posttraining Electrical Stimulation of Vagal Afferents with Concomitant Vagal Efferent Inactivation Enhances Memory Storage Processes in the Rat. Neurobiol Learn Mem 70:364–373 Available at: http://linkinghub.elsevier.com/retrieve/pii/S1074742798938631. [DOI] [PubMed] [Google Scholar]
- Dawson J, Pierce D, Dixit A, Kimberley TJ, Robertson M, Tarver B, Hilmi O, McLean J, Forbes K, Kilgard MP, Rennaker RL, Cramer SC, Walters M, Engineer N (2016) Safety, Feasibility, and Efficacy of Vagus Nerve Stimulation Paired With Upper-Limb Rehabilitation After Ischemic Stroke. Stroke 47:143–150 Available at: http://stroke.ahajournals.org/lookup/doi/10.1161/STROKEAHA.115.010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D, Vanneste S, Engineer ND, Kilgard MP (2014) Safety and Efficacy of Vagus Nerve Stimulation Paired With Tones for the Treatment of Tinnitus: A Case Series. Neuromodulation Technol Neural Interface 17:170–179 Available at: http://doi.wiley.com/10.1111/ner.12127. [DOI] [PubMed] [Google Scholar]
- Edeline J-M, Manunta Y, Hennevin E (2011) Induction of selective plasticity in the frequency tuning of auditory cortex and auditory thalamus neurons by locus coeruleus stimulation. Hear Res 274:75–84 Available at: 10.1016/j.heares.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Engineer CT, Engineer ND, Riley JR, Seale JD, Kilgard MP (2015) Pairing speech sounds with vagus nerve stimulation drives stimulus-specific cortical plasticity. Brain Stimul 8:637–644 Available at: 10.1016/j.brs.2015.01.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP (2011) Reversing pathological neural activity using targeted plasticity. Nature 470:101–104 Available at: http://www.nature.com/doifinder/10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53:1–24 Available at: http://www.ncbi.nlm.nih.gov/pubmed/11171937. [PubMed] [Google Scholar]
- Ferguson SSG, Caron MG (1998) G protein-coupled receptor adaptation mechanisms. Semin Cell Dev Biol 9:119–127. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE (2007) A synaptic memory trace for cortical receptive field plasticity. Nature 450:425–429 Available at: https://www.nature.com/articles/nature06289.pdf. [DOI] [PubMed] [Google Scholar]
- Ganzer PD, Darrow MJ, Meyers EC, Solorzano BR, Ruiz AD, Robertson NM, Adcock KS, James JT, Jeong HS, Becker A, Goldberg MP, Pruitt DT, Hays SA, Kilgard MP, Rennaker RL (2018) Closed-loop Neuroprosthesis Restores Network Connectivity and Motor 2 Control after Spinal Cord Injury. Elife:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves DA, Bowman EM, Brown VJ (2005) Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci Lett 379:174–179 Available at: http://linkinghub.elsevier.com/retrieve/pii/S0304394005000029. [DOI] [PubMed] [Google Scholar]
- Hays SA, Khodaparast N, Hulsey DR, Ruiz A, Sloan AM, Rennaker RL, Kilgard MP (2014a) Vagus Nerve Stimulation During Rehabilitative Training Improves Functional Recovery After Intracerebral Hemorrhage. Stroke 45:3097–3100 Available at: http://stroke.ahajournals.org/lookup/doi/10.1161/STROKEAHA.114.006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, Khodaparast N, Ruiz A, Sloan AM, Hulsey DR, Rennaker RL, Kilgard MP (2014b) The timing and amount of vagus nerve stimulation during rehabilitative training affect poststroke recovery of forelimb strength. Neuroreport 25:682–688 Available at: http://www.ncbi.nlm.nih.gov/pubmed/24818637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, Ruiz A, Bethea T, Khodaparast N, Carmel JB, Rennaker RL, Kilgard MP (2016) Vagus nerve stimulation during rehabilitative training enhances recovery of forelimb function after ischemic stroke in aged rats. Neurobiol Aging 43:111–118 Available at: 10.1016/j.neurobiolaging.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Huertas M, Hong SZ, Tie X, Hell JW, Shouval H, Kirkwood A (2015) Distinct Eligibility Traces for LTP and LTD in Cortical Synapses. Neuron 88:528–538 Available at: 10.1016/j.neuron.2015.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsey DR, Hays SA, Khodaparast N, Ruiz A, Das P, Rennaker RL, Kilgard MP (2016) Reorganization of Motor Cortex by Vagus Nerve Stimulation Requires Cholinergic Innervation. Brain Stimul 9:174–181 Available at: http://www.sciencedirect.com/science/article/pii/S1935861X16000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA (2017) Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol 289:21–30 Available at: 10.1016/j.expneurol.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- January B, Seibold A, Whaley B, Hipkin RW, Lin D, Schonbrunn A, Barber R, Clark RB (1997) beta2-adrenergic receptor desensitization, internalization, and phosphorylation in response to full and partial agonists. J Biol Chem 272:23871–23879 Available at: http://www.ncbi.nlm.nih.gov/pubmed/9295336 [Accessed January 4, 2018]. [DOI] [PubMed] [Google Scholar]
- Khodaparast N, Hays SA, Sloan AM, Fayyaz T, Hulsey DR, Rennaker RL, Kilgard MP (2014) Vagus Nerve Stimulation Delivered During Motor Rehabilitation Improves Recovery in a Rat Model of Stroke. Neurorehabil Neural Repair 28:698–706 Available at: http://journals.sagepub.com/doi/10.1177/1545968314521006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, Rennaker RL, Kilgard MP (2013) Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol Dis 60:80–88 Available at: 10.1016/j.nbd.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Khodaparast N, Kilgard MP, Casavant R, Ruiz A, Qureshi I, Ganzer PD, Rennaker RL, Hays SA (2016) Vagus Nerve Stimulation During Rehabilitative Training Improves Forelimb Recovery After Chronic Ischemic Stroke in Rats. Neurorehabil Neural Repair 30:676–684 Available at: http://journals.sagepub.com/doi/10.1177/1545968315616494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM (1998) Cortical map reorganization enabled by nucleus basalis activity. Science 279:1714–1718 Available at: http://www.ncbi.nlm.nih.gov/pubmed/9497289. [DOI] [PubMed] [Google Scholar]
- Loerwald KW, Borland MS, Rennaker RL, Hays SA, Kilgard MP (2017) The interaction of pulse width and current intensity on the extent of cortical plasticity evoked by vagus nerve stimulation. Brain Stimul:1–7 Available at: http://linkinghub.elsevier.com/retrieve/pii/S1935861X17309634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manta S, Dong J, Debonnel G, Blier P (2009) Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J Psychiatry Neurosci 34:272–280 Available at: http://www.ncbi.nlm.nih.gov/pubmed/19568478. [PMC free article] [PubMed] [Google Scholar]
- Manta S, El Mansari M, Debonnel G, Blier P (2013) Electrophysiological and neurochemical effects of long-term vagus nerve stimulation on the rat monoaminergic systems. Int J Neuropsychopharmacol 16:459–470 Available at: http://ijnp.oxfordjournals.org/content/16/2/459.abstract. [DOI] [PubMed] [Google Scholar]
- Martins ARO, Froemke RC (2015) Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat Neurosci 18:1483–1492 Available at: 10.1038/nn.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter BA, Khodaparast N, Fayyaz T, Cheung RJ, Ahmed SS, Vrana WA, Rennaker RL, Kilgard MP (2012) Repeatedly Pairing Vagus Nerve Stimulation with a Movement Reorganizes Primary Motor Cortex. Cereb Cortex 22:2365–2374 Available at: https://academic.oup.com/cercor/article-lookup/doi/10.1093/cercor/bhr316. [DOI] [PubMed] [Google Scholar]
- Pruitt DT, Schmid AN, Kim LJ, Abe CM, Trieu JL, Choua C, Hays SA, Kilgard MP, Rennaker RL (2016) Vagus Nerve Stimulation Delivered with Motor Training Enhances Recovery of Function after Traumatic Brain Injury. J Neurotrauma 33:871–879 Available at: http://online.liebertpub.com/doi/10.1089/neu.2015.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckett AC, Pandya PK, Moucha R, Dai W, Kilgard MP (2007) Plasticity in the Rat Posterior Auditory Field Following Nucleus Basalis Stimulation. J Neurophysiol 98:253–265 Available at: http://jn.physiology.org/cgi/doi/10.1152/jn.01309.2006. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM (1993) Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci 13:87–103 Available at: http://www.ncbi.nlm.nih.gov/pubmed/8423485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Frings M, Mono M-L, Guo Y, Weernink PAO, Evellin S, Han L, Jakobs KH (2000) G Protein-coupled Receptor-induced Sensitization of Phospholipase C Stimulation by Receptor Tyrosine Kinases. J Biol Chem 275:32603–32610 Available at: http://www.jbc.org/content/275/42/32603.full.pdf. [DOI] [PubMed] [Google Scholar]
- Shetake JA, Engineer ND, Vrana WA, Wolf JT, Kilgard MP (2012) Pairing tone trains with vagus nerve stimulation induces temporal plasticity in auditory cortex. Exp Neurol 233:342–349 Available at: 10.1016/j.expneurol.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Tyler R, Cacace A, Stocking C, Tarver B, Engineer N, Martin J, Deshpande A, Stecker N, Pereira M, Kilgard M, Burress C, Pierce D, Rennaker R, Vanneste S (2017) Vagus Nerve Stimulation Paired with Tones for the Treatment of Tinnitus: A Prospective Randomized Double-blind Controlled Pilot Study in Humans. Sci Rep 7:11960 Available at: http://www.nature.com/articles/s41598-017-12178-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiquette M, Bonin H, Hnatowich M, Caron MG, Lefkowitz RJ, Bouvier M (1990) Involvement of tyrosine residues located in the carboxyl tail of the human beta 2-adrenergic receptor in agonist-induced down-regulation of the receptor. Proc Natl Acad Sci U S A 87:5089–5093 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=54267&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violin JD, DiPilato LM, Yildirim N, Elston TC, Zhang J, Lefkowitz RJ (2008) β 2 -Adrenergic Receptor Signaling and Desensitization Elucidated by Quantitative Modeling of Real Time cAMP Dynamics. J Biol Chem 283:2949–2961 Available at: http://www.jbc.org/lookup/doi/10.1074/jbc.M707009200. [DOI] [PubMed] [Google Scholar]
- Xu G-P (2004) Chronic Morphine Sensitizes the Brain Norepinephrine System to Corticotropin-Releasing Factor and Stress. J Neurosci 24:8193–8197 Available at: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.1657-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Smith DC, Jensen RA (2007) Vagus nerve stimulation potentiates hippocampal LTP in freely-moving rats. Physiol Behav 90:583–589 Available at: http://linkinghub.elsevier.com/retrieve/pii/S0031938406004963. [DOI] [PMC free article] [PubMed] [Google Scholar]