Abstract
Migraine-type photophobia, most commonly described as exacerbation of headache by light, affects nearly 90% of the patients. It is classified as one of the most disabling symptoms that accompany an attack. Using subjective psychophysical assessments, we showed recently that migraine patients are more sensitive to all colors of light during the ictal than during the interictal phase and that control subjects do not experience pain when exposed to different colors of light. Based on these findings, we suggested that color preference is unique to migraineurs (as it was not found in control subjects) rather than migraine phase (as it was found in the ictal and interictal phases). To identify the origin of this photophobia in migraineurs, we compared the electrical waveforms that were generated in the retina and visual cortex of 46 interictal migraineurs to those generated in 42 healthy controls using color-based electroretinography and visual evoked potential paradigms. Unexpectedly, it was the amplitude of the retinal rod-driven b-wave, which was consistently larger (by 14-19% in the light-adapted and 18-34% in the dark-adapted flash ERG) in the migraineurs than in the controls, rather than the retinal cone-driven a-wave or the visual evoked potentials that differ most strikingly between the two groups. Mechanistically, these findings suggest that the inherent hypersensitivity to light among migraine patients may originate in the retinal rods rather than retinal cones or the visual cortex. Clinically, the findings may explain why migraineurs complain that the light is too bright even when it is dim to the extent that non-migraineurs feel as if they live in a cave.
Keywords: Headache, electroretinography, visual evoked potential, night vision, photophobia
Introduction
In ophthalmology, photophobia is commonly defined as an abnormal sensitivity, or intolerance, to light, especially discerned by the eyes (Dorland Medical). This definition, also termed photo-oculodynia, attempts to capture the pathological ocular perception of an otherwise innocuous visual stimuli as noxious or uncomfortable among patients suffering from a variety of ocular diseases [15]. To capture the experience of migraine patients, the definition of photophobia was initially expanded to include discomfort in the eye or head[15], and later modified to reflect more accurately what patients experience as exacerbation of headache by light [26; 27] or a more general aversion to light during an attack[28]; referred here as ‘migraine-type photophobia’. Second only to the headache itself, photophobia is commonly identified by patients as the most disabling symptom of migraine [22; 35; 42] as it repeatedly forces them to stop their work or home responsibilities, in order to be in dark environments. Accordingly, reducing photophobia is now considered by the FDA as a co-primary endpoint [2].
Our understanding of migraine-type photophobia continues to evolve. In a recent series of studies we reported that (a) none of the blind migraine patients who lacked light perception experienced photophobia, whereas those who could detect light tended to complain about worsening of their headache once light conditions were dominated by short wavelengths; (b) during migraine, patients with normal eyesight found white, blue, yellow and red lights to be more painful than green light, and that larger electrical amplitudes were generated by both the retina (a-waves) and visual cortex (N2/P2 complexes) in response to white, blue, yellow and red lights as compared to green [26]; (c) regardless of headache intensity, all colors of light triggered unpleasant autonomic and affective symptoms which we categorized as aversive; and (d) this non-specific aversion to colors occurred more frequently in the ictal than the interictal phase, and more frequently in the interictal phase than in healthy controls [28]. Incorporating these psychophysical and electrophysiological findings in migraine patients with our preclinical anatomical and physiological identification of novel retinothalamocortical, retinohypothalamic-sympathetic and retinohypothalamic-parasympathetic pathways, we initially proposed that (a) in the absence of the optic nerve, migraine-type photophobia could not occur; (b) the exacerbation of headache by light was mediated by retinal projections to thalamic neurons that terminated in several sensory cortices; and (c) the retinal projections to the thalamus originated in melanopsinergic and non-melanopsinergic retinal ganglion cells in migraine patients with normal eyesight, but mainly in melanopsinergic retinal ganglion cells in blind migraine patients [27]. We then concluded that initial activation of retinal cones and cone-driven post-receptor cells and subsequent activation of color-sensitive neurons in the visual cortex were required to mediate the aforementioned distinct color effects [26]. This conclusion is heavily supported by the notion that color perception is generated by the visual cortex in response to signals that originate in retinal cones [11]. Along this line, we also suggested that because the generation of color perception and its processing depend on color-sensitive, cone-opponent neurons in the visual cortex [11], the most reasonable way to explain why control subjects like colored light whereas migraineurs find colored light aversive is by abnormal functioning of the visual cortex [25].
Continuing to probe the mechanisms of migraine-type photophobia, in the current study we have attempted to determine whether it was the cortex, the retina, or both, that were more sensitive to light in migraine patients than in healthy controls, and if it were the retina, which type of photoreceptors were affected. To the best of our knowledge, this is the first study to compare the amplitudes of a- and b-waves of light-, flickering- and dark-adapted electroretinograms (ERGs), as well as the N2/P2 amplitudes of visual-evoked potentials (VEPs) in response to different colors of light in both migraine patients and healthy controls.
Materials and Methods
All study visits took place at Beth Israel Deaconess Medical Center (BIDMC), Boston, MA (September 2010 - June 2017). The BIDMC Committee on Clinical Investigations approved the study and all participants provided written informed consent. Patients were recruited from the BIDMC Comprehensive Headache Center, Neurology Clinic, the primary care clinic at Healthcare Associates and from advertisements at BIDMC and Harvard Medical School. Women and men who were 15-85 years old were potentially eligible for the study if they met the International Classification of Headache Disorders Committee [39] criteria for migraine with or without aura, were able to communicate in English and were willing to attend a visit during an untreated migraine attack. Exclusion criteria included fewer than 5 headache free days per month, chronic head or neck pain not attributed to migraine, chronic use of opioids (≥ 15 days/month for 3 previous consecutive months or longer), or having an ocular disease. For this study an ocular disease was defined as a primary and persisting visual disorder, including disease of the anterior chamber, glaucoma, macular degeneration, retinal degenerative diseases, cones dystrophy, rods dystrophy, achromatopsia (congenital total color blindness), retinitis pigmentosa, Leber’s Congenital Amaurosis, Albinism, night blindness, or cortical blindness due to posterior circulation stroke. Participants were permitted to stop participating in the study at any time.
Electroretinography
Electroretinographies (ERG) responses were recorded in 46 migraine patients and 42 healthy controls. As recommended by the International Society for Clinical Electrophysiology of Vision [24] we used the full-field (ganzfeld) stimulation method as it favors recording stability, reproducibility and reliability by delivering the photic stimuli to all photoreceptors at a relatively homogenous manner. To minimize discomfort, the pupils were not dilated and the ERG recordings were not done through a contact lens electrode. Rather, all ERG recording were done using one disposable, low-impedance silver/nylon corneal recording electrode (DTL Plus) that is comfortable and easy to use, one reference gold cup electrodes placed on the forehead, and a ground silver ear clip electrode placed on the ipsilateral ear. To ensure good ocular contact and proper electrode impedance, we first ran few test stimuli to verify that the recorded waveforms were comparable to the standard ERG. Amplification of signals, fixation point and other technical aspects of our ERG recording, all provided technically by the same ColorDome used in the psychophysical studies, adhered to the guideline of “Standard for clinical electroretinography” as defined in 2015 [24].
Light-adapted, single-flash cones/rods ERG.
Before recording ERG, participants were given 10 minutes to adapt to the background light. The stimulus intensity of each flash of light was 3.0 cd·s·m−2 (calculated as time integrated luminance and measured in photopic candela-seconds per meter square), the duration was 4 msec, and the background luminance was 30 cd·m−2. Using these parameters, each color of light was flashed 9 times (interstimulus interval (ISI) = 1 sec). The 9 flashes of light were divided to 3 series; each averaged the ERG signals recorded over 3 flashed of light. The order of color stimuli was white, blue, green, yellow and red. For comparisons, we measured a-wave amplitude (from baseline to trough), b-wave amplitude (from trough of a-wave to peak of b-wave) and the implicit time (from stimulus onset to peak b-wave) as described in Noseda et al., 2016 [26].
Light-adapted 30 Hz flicker cone ERG.
The 30 Hz flickering ERG consisted of 150 single flashes of light, delivered over a period of 5 seconds. At 30 Hz, rods do not contribute to the ERG signal as they can’t follow this speed [16]. As above, the intensity of each flash of light was 3.0 cd·s·m−2, the duration was 4 msec, and the background illumination was 30 cd·m−2. Using these parameters, each patient was exposed to 3 series of 150 single flashes (29 msec ISI) per color in the same order as above, and the average of the 3 series was used in the data analysis. Because the first few ERGs could represent responses to a single flash, they were discarded. For comparisons, we measured a-wave amplitude (trough), b-wave amplitude (peak) and the implicit time as described in Noseda et al., 2016 [26].
Dark-adapted rod ERG.
Before recording the rod system ERG, patients were kept in total darkness for 20 minutes. To minimize detection by cones, dim flashes of light (0.01 cd·s·m−2, 4 msec) were delivered in the absence of background illumination. As in the light-adapted flash ERG, each color of light was flashed 9 times (ISI = 1.1 sec). The 9 flashes of light were divided to 3 series; each averaged the ERG signals recorded over 3 flashed of light. As above, dark-adapted rod ERGs were repeated with white, blue, green, yellow and red lights.
Visual evoked potential recording
Visual evoked potentials were recorded in all 46 migraine patients and 42 healthy controls. Of these, only 29 migraine patients and 31 healthy controls yielded waveforms with clearly identifiable N1, P1, N2 and P2 deflections. As recommended by the International Society for Clinical Electrophysiology of Vision guideline for VEP standard [29], we elicited full field light flashes using the ColorDome system. Recordings were made using gold-disc surface electrodes. The active electrode was placed on the scalp over the midline of the visual cortex (at point Oz); the reference electrode was place over the midline of the forehead (at point Fz); the ground electrode was placed on the scalp over the vertex (at point Cz) [1]. To establish color-specific VEP, patients were placed in a dimly illuminated room (non-dilated pupils), and presented with five sets of photic stimuli in the following order: white, blue, green, yellow and red. Each set of photic stimuli consisted of 64 flashes of light delivered at 1.1 Hz (frequency), 3.0 cd·s·m−2 (intensity), 4 ms (duration). Analysis time was set at 300 msec. Because peaks of N2 and P2 waves are the most robust components of flash VEP, comparisons between VEP responses in migraine patients and healthy controls were based on their amplitudes and time to peak.
Calibration and quantification of photic stimulation
Repeated calibrations of our ColorDome with the international light technologies photometer (ILT1700) and an Ocean Optics Maya LSL spectrometer were used to verify that the different colors of light were delivered at equal luminance. For example, when the nominal luminance was set at 3 cd·m−2 in the Espion software (Diagnosys LLC), the measured luminance for blue, green, yellow and red was 2.96, 3.0, 3.36, 2.76 cd·m−2, respectively. To ensure that each of these colors appeared to be exactly the same luminance to our participants (i.e., taking into account differences in photopic sensitivity of the human retina), our ColorDome delivered different power with each photic stimulus. When the measured luminance for blue, green, yellow and red was 2.96, 3.0, 3.36, 2.76 cd·m−2, the respective power was 32.2, 1.9, 2.1, and 4.9 W·cm−2/nM.
Statistical Analysis
All analyses were performed using SPSS version 25 (Statistical Package for the Social Sciences, IBM) and SAS version 9.4 (SAS Institute Inc., 2008). Statistical significance was set at p < 0.05. The primary outcome measures were defined as physiological markers attesting to the magnitude of individual responsiveness to the applied visual stimuli, namely the amplitudes of ERG-derived retinal a- and b-waves and VEP-derived cortical N2P2 complexes. Nonparametric Mann-Whitney tests compared the amplitudes of these markers between migraine patients and healthy controls as induced by white, blue, green, yellow and red lights.
Results
Forty-six patients diagnosed with migraine [39], photophobia, and no known ocular diseases, were studied. Their demographics and headache characteristics are brought in Table 1.
Table 1. Demographics and headache characteristics of the study participants.
Continuous data are presented as median [25th percentile – 75th percentile].
| Group | Migraine Patients (n=46) | Healthy Controls (n=42) |
|---|---|---|
| Age | 39 [29 - 49] | 41.5 [27.5 – 52.5] |
| Sex (female, male) | 43 (93%), 3 (7%) | 36 (86%), 6 (14%) |
| History of migraine [years] | 19 [9 - 26.5] | - |
| Average no. of attacks / month | 4 [2.5 – 6.25] | - |
| Average attack duration [hours] | 72 [24 – 72] | - |
| Migraine type (chronic, episodic) | 4 (9%), 41 (89%) | - |
| Unilateral | 34 (74%) | - |
| Pulsatile | 37 (80%) | - |
| Moderate / Severe | 44 (96%) | - |
| Physical activity | 33 (72%) | - |
| Nausea | 41 (89%) | - |
| Phonophobia | 39 (85%) | - |
Electroretinography (ERG) studies in migraineurs
To identify possible retinal contribution to migraine-type photophobia, we sought to determine whether the electrical signal that are generated by the retina in response to light differ in migraine patients as compared to healthy controls. To answer this question we recorded ERG signals from the retina of 46 migraineurs and 42 healthy controls.
Light-adapted single-flash ERG (Fig. 1):
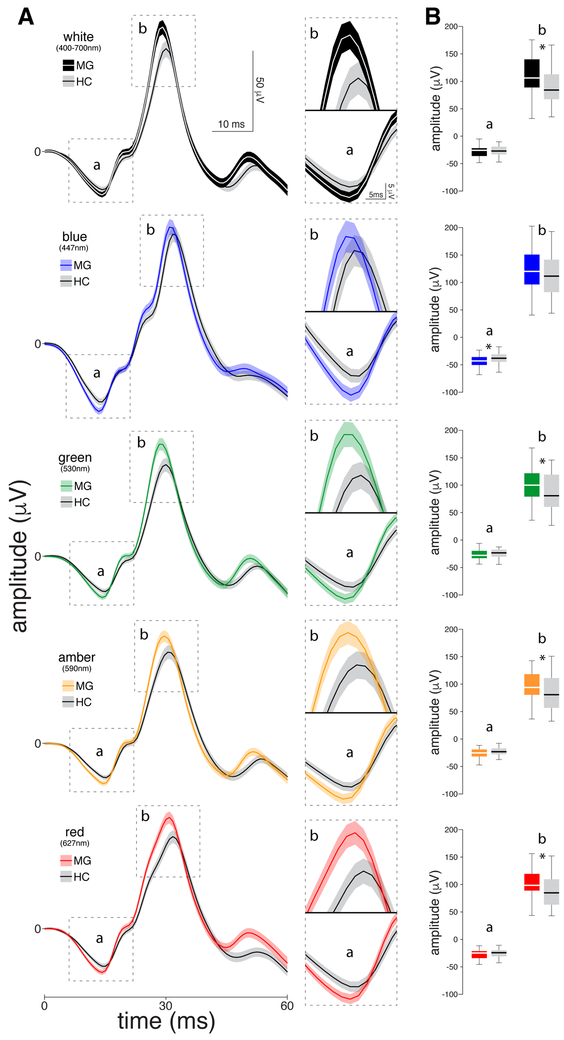
Figure 1.
Chromatic electroretinographies (ERGs) recorded interictally in 46 migraine patients and 42 healthy controls. (A) Left panel: Standard light-adapted single flash ERG waveforms averaged (SEM) across patients and healthy controls in response to 414 and 378 flashes, respectively (nine flashes per participant) of each colour of light. Right panel: Zoomed-in scale of the a- and b-waves per color of light. (B) Boxplots illustrating medians (thick horizontal white lines), interquartile ranges (25th–75th percentile; lower and upper box boundaries) and observations below and above the 25th and 75th percentiles (vertical lines) for a- and b-waves, for migraine patients and healthy controls. Significant differences between the two groups are depicted by asterisks.
In these cone/rod ERGs, the b-wave amplitudes generated by all lights were larger in migraine patients as compared to healthy controls; these differences were statistically significant in response to white (106.21 [88.17 – 140.29] in migraine patients vs. 84.21 [67.15 – 113.94] in healthy controls; p=0.028), green (99.93 [78.75 – 121.88] vs. 80.67 [59.79 – 119.24]; P=0.03), yellow (94.28 [79.54 – 119.39] vs. 80.89 [56.65 – 111.57]; P=0.032) and red (98.40 [87.92 – 119.47] vs. 84.73 [63.04 – 109.92]; P=0.019), but not blue (119.96 [95.94 – 150.89] vs. 111.39 [81.12 - 141.81]; P=0.120). In contrast, the a-wave amplitudes were comparable in migraine patients and healthy controls; this was true for all but the blue light (−43.08 [−50.38 – −35.56] vs. −38.17 [−44.79 – −30.94]; P=0.020).
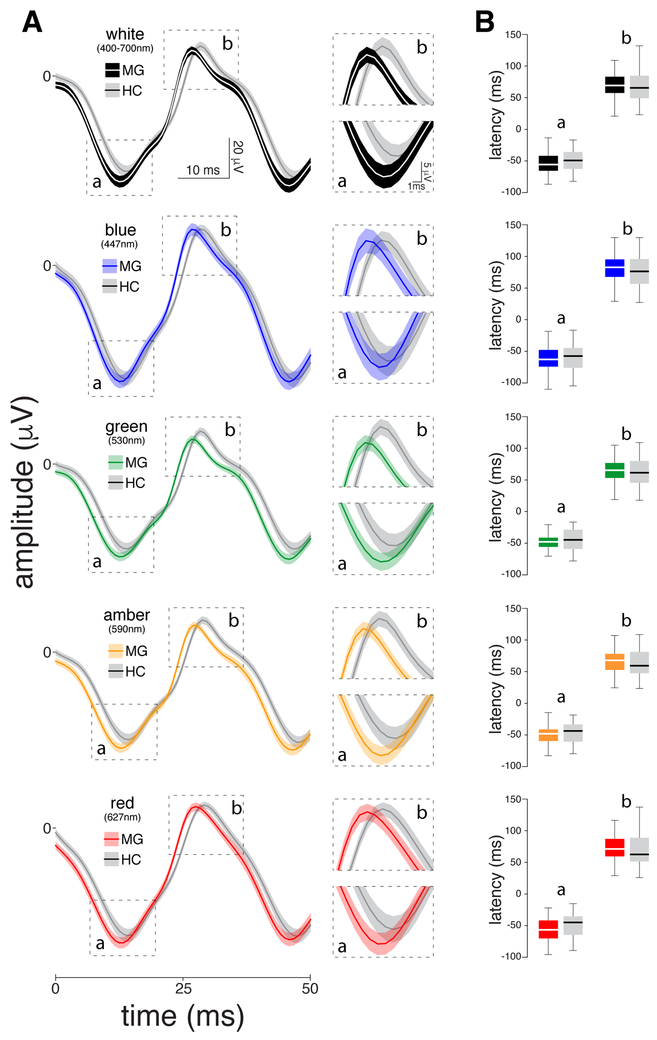
Light-adapted flicker ERG (Fig. 2):
Figure 2.
Chromatic electroretinographies (ERGs) recorded interictally in 46 migraine patients and 42 healthy controls. (A) Left panel: Standard light-adapted 30 Hz flickering ERG waveforms averaged (SEM) across patients and healthy controls. Right panel: Zoomed-in scale of the a- and b-waves per color of light. (B) Boxplots illustrating medians (thick horizontal white lines), interquartile ranges (25th–75th percentile; lower and upper box boundaries) and observations below and above the 25th and 75th percentiles (vertical lines) for a- and b-waves, for migraine patients and healthy controls. Significant differences between the two groups are depicted by asterisks.
In these cones-mediated responses, no significant differences in b-wave or a-wave amplitudes were found between the migraine patients and healthy controls in any of the examined colors (Ps>0.114).
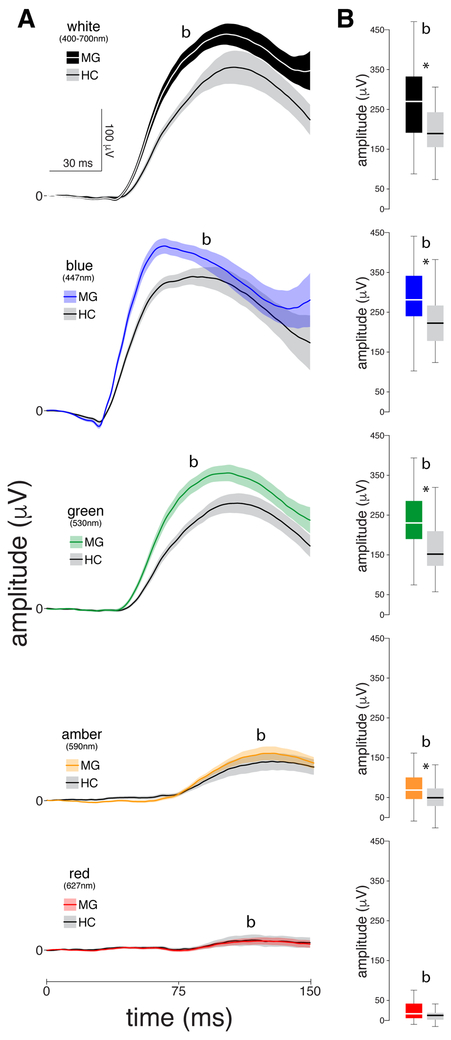
Dark-adapted rod ERG (Fig. 3):
Figure 3.
Chromatic electroretinographies (ERGs) recorded interictally in 46 migraine patients and 42 healthy controls. (A) Standard dark-adapted rod ERG waveforms averaged (SEM) across patients and healthy controls. (B) Boxplots illustrating medians (thick horizontal white lines), interquartile ranges (25th–75th percentile; lower and upper box boundaries) and observations below and above the 25th and 75th percentiles (vertical lines) for b-waves for migraine patients and healthy controls. Significant differences between the two groups are depicted by asterisks.
In these rod ERGs, the b-wave amplitudes generated by all lights were larger in migraine patients as compared to healthy controls; these differences were statistically significant in response to white (272.22 [192.87 – 336.81] in migraine patients vs. 190.81 [151.04 – 246.52] in healthy controls; P=0.028), green (229.98 [186.79 – 285.81] vs. 151.82 [118.10 – 210.38]; P<0.0001), yellow (68.70 [45.70 – 101.43] vs. 49.61 [28.64 – 73.35]; P=0.009) and blue (281.27 [236.11 – 342.83] vs. 222.46 [177.43 – 267.00]; P=0.002), but not red (16.77 [5.64 – 42.41] vs. 12.64 [0.78 – 19.00]; P=0.289).
Visual Evoked Potential (VEP) studies in migraineurs
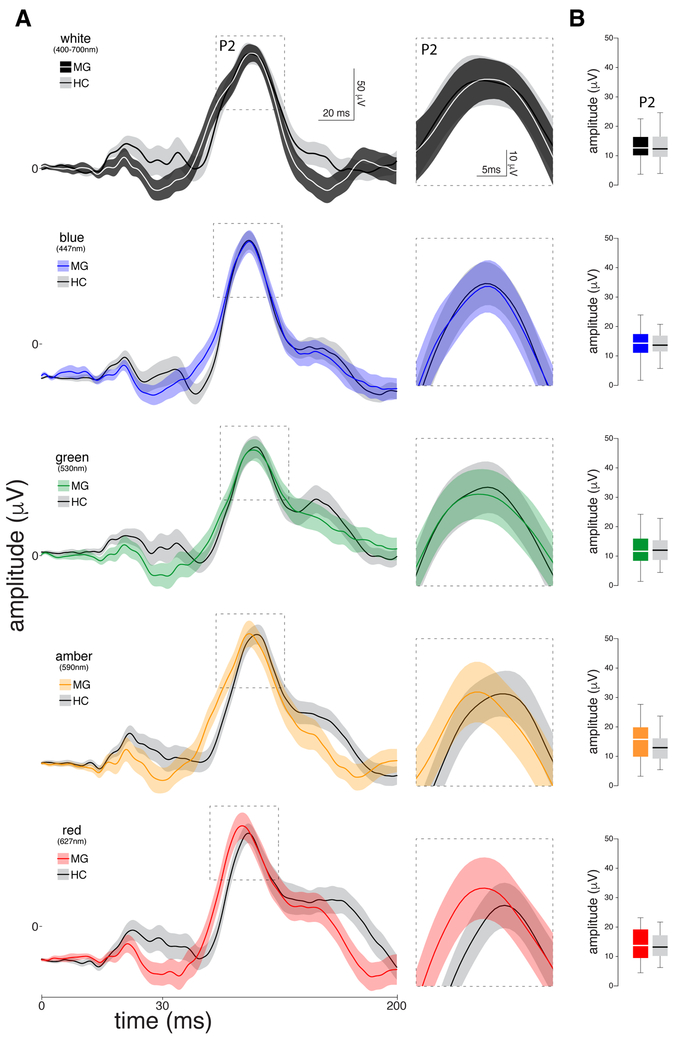
As the leading theory about migraine photophobia suggests that it is a reflection of genetically-determined hyperexcitable cortex, we also sought to determine whether the electrical signal that are generated by the cortex in response to light differ in migraine patients as compared to healthy controls. To answer this question we also recorded standard flash VEP from the same group of participants. Surprisingly, analyses of the N2-P2 amplitude revealed no statistically significant differences between migraine patients and healthy controls (Fig. 4). This was true for all colors of light: white (12.57 [9.94 – 16.40] in migraine patients vs. 12.24 [9.42 – 16.46] in healthy controls; P=0.830), green (11.37 [8.10 – 15.84] vs. 11.81 [7.58 – 15.61]; P=0.947), yellow (15.64 [9.61 – 20.00] vs. 12.80 [8.10 – 16.40]; P=0.222), blue (14.28 [10.89 – 17.37] vs. 13.59 [11.01 – 16.92] ; P=0.520), and red (13.65 [9.23 – 19.54] vs. 13.14 [9.58 – 17.52]; P=0.652).
Figure 4.
Chromatic VEPs recorded interictally in 29 migraine patients and 31 healthy controls. (A) Left panel: Standard flash visual evoked potential waveforms averaged (SEM) in response to each color of light, focusing on the P2 complex. Right panel: Zoomed-in scale of the P2 component. (B) Boxplots illustrating medians (thick horizontal white lines), interquartile ranges (25th–75th percentile; lower and upper box boundaries) and observations below and above the 25th and 75th percentiles (vertical lines) for the P2 complex for migraine patients and healthy controls. Significant differences between the two groups are depicted by asterisks.
Discussion
This is the first study in which the same experimental setup was used to evaluate the sensitivity of the visual system in migraine patients and control subjects. Using standard ERG and VEP stimulation paradigms, we show that the physiological marker that differentiates most strikingly the visual system of the two populations is the amplitude of the retinal rod-driven b-wave. Notably, the two populations did not differ in the amplitudes of the retinal cone-driven a-wave or the cortical N2-P2 VEP. These findings suggest that the inherent hypersensitivity to light among migraine patients, as compared to healthy subjects, may originate in the retinal rods rather than retinal cones or the visual cortex. In contrast, it is cone-driven retinal pathways and the visual cortex, rather than retinal rods that seem to mediate the distinct pattern of exacerbation or alleviation of headache by the different colors of light among migraineurs.
In our recent studies on migraine-type photophobia we showed that the exacerbation of headache by light depends on a novel retinto-thalamo-cortical pathways that originates in melanopsinergic retinal ganglion cells (RGC) in migraine patients who are blind and in both non-melanopsinergic and melanopsinergic RGC in migraine patients with normal eyesight [27]. Subsequently, we showed that among migraine patients with normal eyesight the perception that blue and red exacerbate the headache more than yellow and white and that yellow and white exacerbate the headache more than green, which at low intensities can actually alleviate the headache, originates in retinal cones and finalized in the visual cortex [26]. Comparing migraine patients during ictal vs. interictal phases vs. healthy controls, we just reported that the selective color effects we documented in migraineurs during the ictal phase are also observed in the interictal phase but not in healthy controls (MS# Photophobia 3.5). Accordingly, we proposed that this subject-rather than attack-dependent phenomenon is mediated by visual cortex neurons that ‘generate’ color perception, namely V1 double-opponent cells, and color-encoding cells in V2 and the inferior and posterior inferior temporal cortex (V4). As discussed below, this conclusion is somewhat challenged by the current study.
Unexpectedly, it was the b-wave amplitude - rather than the a-wave amplitude - that most strikingly differentiated migraine patients from the healthy controls. As shown in figures 1-3, the greater b-wave amplitude we recorded in the migraineurs, compared to controls, was apparent in both the light- and dark-adapted single flash ERGs, whereas similar a-wave amplitudes were apparent in the light-adapted single flash and flickering ERGs. Current understanding attributes the origin of the ERG b-wave, the corneal positive deflection, to the activation of Muller, bipolar and potentially amacrine cells in the inner retina [3; 21; 31; 32; 36]. This activation is actuated by rods under light intensities that are too low to trigger cone activation (scotopic conditions), and by both rods and cones under light intensities sufficient for triggering both (photopic conditions)[32]. In contrast, the origin of the a-wave, the corneal negative deflection, is attributed mainly to activation of cones and post-cones retinal pathways [8-10; 30]. Based on the above, we propose that the larger b-wave responses originated in rods rather than cones. This interpretation is further supported by the following: (i) the b-wave amplitude recorded under the dark-adapted condition could not have been generated by cones as the light intensity was below their activation threshold [37; 38]; and (ii) when the stimulus paradigm was such that only cones were activated (30 Hz flickering ERG) [16], a-wave amplitudes were comparable in both groups.
By far, the most surprising finding of this study is that the N2-P2 amplitude of the VEP, although different for the different colors of light, was similar in the migraineurs and healthy controls (Fig. 4). It is surprising because it challenges the notion that migraine-type photophobia originates in what is thought to be genetically-predetermined hyperexcitable visual cortex [17]. While evidence for cortical hyperexcitability in migraineurs is overwhelming and we do not by any mean dispute the data, little evidence exists to support the view that it is the hyperexcitable visual cortex that actually mediates the abnormal sensitivity to light. The origin of the theory that the visual cortex is hyperexcitable could be found in old VEP studies [34]. The introduction of functional imaging techniques to the filed of neuroscience gave rise to a large number of high-quality publications that showed even more convincingly that in the visual cortex of the migraine brain respond more vigorously and/or to lower intensity of stimulation than the visual cortex of the non-migraine brain [20; 23; 33; 41] [6; 7; 13; 18; 19]. Based on these studies it was accepted that the migraine brain, in general, and the visual cortex in particular is hyperexcitable, hyperreactive or hyperresponsive [5; 12; 14; 41; 43], either because of inherent defect in ionic channels that modulate excitability or because of inherent defect in cortical habituation [4; 12; 14; 34; 40]. While these studies are convincing, they lack a correlation with psychophysical assessments of the actual sensitivity of the participants to each of the photic stimuli that were used to measure the cortical responsiveness. Without which, one cannot conclude with certainty that the hyperexcitable visual cortex is the culprit of photophobia. The Boulloche et al., paper [6] exemplifies this point most elegantly as it shows that while activation of the visual cortex by light are always stronger in migraineurs than in controls, the visual discomfort is not. Of relevance to the main finding of the current study is the Martin et al., paper [23] in which the authors showed that when simultaneous psychophysical assessment and fMRI imaging were used to evaluated sensitivity to light and BOLD signals in the visual cortex of migraineurs and controls, it was only the low luminance conditions that differentiate the 2 groups most strikingly (see Fig. 1).
Anecdotally, one of the most common complains of spouses of migraineurs is that they feel like they “live in a cave”. This complaint is attributed to the fact that even when all windows are covered, and the most intense light bulb in the room is 40 Watt - to the extent that it is depressing - it is still too bright and unbearable to their migraine spouse. This repeated complain helps us place the clinical significance of this study in the right context. Because rod signals differentiated migraineurs from controls most significantly, and because rods are the class of retinal photoreceptors we use to detect and process the dimmest of light, we propose here that migraine type photophobia originates in retinal rods and transformed through retinal ganglion cells to brain areas such as the hypothalamus, brainstem and medial thalamus, each of which can contribute to what patients describe as aversion to light.
ACKNOWLEDGEMENTS AND FUNDING
This research was supported by NIH grants R37 NS079678, RO1 NS069847 (RB). This work was conducted with support from Harvard Catalyst ∣ The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The funding sources had no involvement in the study design; collection, analysis, and interpretation of data; the writing of the report; and the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
REFERENCES
- [1].American Encephalographic Society: Guidline for standard electrode position nomenclature. J Clinical Neurophysiol 1994;11(Guideline 13):111–113. [PubMed] [Google Scholar]
- [2].Guidance for industry migraine: developing drugs for acute treatment. In: USdohah services editor. Division of Neurology Products, Center for Drug Evaluation and Research: Food and Drug Administration, 2014. [Google Scholar]
- [3].Abd-El-Barr MM, Pennesi ME, Saszik SM, Barrow AJ, Lem J, Bramblett DE, Paul DL, Frishman LJ, Wu SM. Genetic dissection of rod and cone pathways in the dark-adapted mouse retina. J Neurophysiol 2009;102(3):1945–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Afra J, Cecchini AP, De Pasqua V, Albert A, Schoenen J. Visual evoked potentials during long periods of pattern-reversal stimulation in migraine. Brain 1998;121 (Pt 2):233–241. [DOI] [PubMed] [Google Scholar]
- [5].Aurora SK, Wilkinson F. The brain is hyperexcitable in migraine. Cephalalgia 2007;27(12):1442–1453. [DOI] [PubMed] [Google Scholar]
- [6].Boulloche N, Denuelle M, Payoux P, Fabre N, Trotter Y, Geraud G. Photophobia in migraine: an interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatry 2010;81(9):978–984. [DOI] [PubMed] [Google Scholar]
- [7].Bramanti P, Grugno R, Vitetta A, Di Bella P, Muscara N, Nappi G. Migraine with and without aura: electrophysiological and functional neuroimaging evidence. Funct Neurol 2005;20(1):29–32. [PubMed] [Google Scholar]
- [8].Brown KT, Murakami M. Biphasic Form of the Early Receptor Potential of the Monkey Retina. Nature 1964;204:739–740. [DOI] [PubMed] [Google Scholar]
- [9].Brown KT, Wiesel TN. Analysis of the intraretinal electroretinogram in the intact cat eye. The Journal of physiology 1961;158:229–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brown KT, Wiesel TN. Localization of origins of electroretinogram components by intraretinal recording in the intact cat eye. The Journal of physiology 1961;158:257–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Conway BR. Color vision, cones, and color-coding in the cortex. Neuroscientist 2009;15(3):274–290. [DOI] [PubMed] [Google Scholar]
- [12].Coppola G, Pierelli F, Schoenen J. Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia 2007;27(12):1427–1439. [DOI] [PubMed] [Google Scholar]
- [13].Coppola G, Pierelli F, Schoenen J. Habituation and migraine. Neurobiology of learning and memory 2009;92(2):249–259. [DOI] [PubMed] [Google Scholar]
- [14].Demarquay G, Mauguiere F. Central Nervous System Underpinnings of Sensory Hypersensitivity in Migraine: Insights from Neuroimaging and Electrophysiological Studies. Headache 2016;56(9):1418–1438. [DOI] [PubMed] [Google Scholar]
- [15].Digre KB, Brennan KC. Shedding light on photophobia. J Neuroophthalmol 2012;32(1):68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dodt E Cone electroretinography by flicker. Nature 1951;168(4278):738. [DOI] [PubMed] [Google Scholar]
- [17].Ferrari MD, Klever RR, Terwindt GM, Ayata C, van den Maagdenberg AM. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol 2015;14(1):65–80. [DOI] [PubMed] [Google Scholar]
- [18].Fumal A, Laureys S, Di Clemente L, Boly M, Bohotin V, Vandenheede M, Coppola G, Salmon E, Kupers R, Schoenen J. Orbitofrontal cortex involvement in chronic analgesic-overuse headache evolving from episodic migraine. Brain 2006;129(Pt 2):543–550. [DOI] [PubMed] [Google Scholar]
- [19].Huang J, Cooper TG, Satana B, Kaufman DI, Cao Y. Visual distortion provoked by a stimulus in migraine associated with hyperneuronal activity. Headache 2003;43(6):664–671. [DOI] [PubMed] [Google Scholar]
- [20].Huang J, Delano M, Cao Y. Visual cortical inhibitory function in migraine is not generally impaired: evidence from a combined psychophysical test with an fMRI study. Cephalalgia 2006;26(5):554–560. [DOI] [PubMed] [Google Scholar]
- [21].Jurklies B, Kaelin-Lang A, Niemeyer G. Cholinergic effects on cat retina In vitro: changes in rod- and cone-driven b-wave and optic nerve response. Vision Res 1996;36(6):797–816. [DOI] [PubMed] [Google Scholar]
- [22].Lipton RB, Serrano D, Buse DC, Pavlovic JM, Blumenfeld AM, Dodick DW, Aurora SK, Becker WJ, Diener HC, Wang SJ, Vincent MB, Hindiyeh NA, Starling AJ, Gillard PJ, Varon SF, Reed ML. Improving the detection of chronic migraine: Development and validation of Identify Chronic Migraine (ID-CM). Cephalalgia 2016;36(3):203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Martin H, Sanchez del Rio M, de Silanes CL, Alvarez-Linera J, Hernandez JA, Pareja JA. Photoreactivity of the occipital cortex measured by functional magnetic resonance imaging-blood oxygenation level dependent in migraine patients and healthy volunteers: pathophysiological implications. Headache 2011;51(10):1520–1528. [DOI] [PubMed] [Google Scholar]
- [24].McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 2015;130(1):1–12. [DOI] [PubMed] [Google Scholar]
- [25].Nir R-R, Lee AJ, Huntington S, Noseda R, Bernstein CA, Fulton AB, Bertisch SM, Hovaguimian A, Buettner C, Borsook D, Burstein R. Color-selective photophobia in ictal vs. interictal migraineurs and in healthy controls. Pain 2018;(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Noseda R, Bernstein CA, Nir RR, Lee AJ, Fulton AB, Bertisch SM, Hovaguimian A, Cestari DM, Saavedra-Walker R, Borsook D, Doran BL, Buettner C, Burstein R. Migraine photophobia originating in cone-driven retinal pathways. Brain 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, Burstein R. A neural mechanism for exacerbation of headache by light. Nat Neurosci 2010;13(2):239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Noseda R, Lee AJ, Nir RR, Bernstein CA, Kainz VM, Bertisch SM, Buettner C, Borsook D, Burstein R. Neural mechanism for hypothalamic-mediated autonomic responses to light during migraine. Proc Natl Acad Sci U S A 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Odom JV, Bach M, Brigell M, Holder GE, McCulloch DL, Tormene AP, Vaegan. ISCEV standard for clinical visual evoked potentials (2009 update). Doc Ophthalmol 2010;120(1):111–119. [DOI] [PubMed] [Google Scholar]
- [30].Penn RD, Hagins WA. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature 1969;223(5202):201–204. [DOI] [PubMed] [Google Scholar]
- [31].Pinilla I, Lund RD, Sauve Y. Contribution of rod and cone pathways to the dark-adapted electroretinogram (ERG) b-wave following retinal degeneration in RCS rats. Vision Res 2004;44(21):2467–2474. [DOI] [PubMed] [Google Scholar]
- [32].Robson JG, Frishman LJ. Dissecting the dark-adapted electroretinogram. Doc Ophthalmol 1998;95(3-4):187–215. [DOI] [PubMed] [Google Scholar]
- [33].Sandor PS, Dydak U, Schoenen J, Kollias SS, Hess K, Boesiger P, Agosti RM. MR-spectroscopic imaging during visual stimulation in subgroups of migraine with aura. Cephalalgia 2005;25(7):507–518. [DOI] [PubMed] [Google Scholar]
- [34].Schoenen J, Ambrosini A, Sandor PS, Maertens de Noordhout A. Evoked potentials and transcranial magnetic stimulation in migraine: published data and viewpoint on their pathophysiologic significance. Clin Neurophysiol 2003;114(6):955–972. [DOI] [PubMed] [Google Scholar]
- [35].Smetana GW. The diagnostic value of historical features in primary headache syndromes: a comprehensive review. Archives of internal medicine 2000;160(18):2729–2737. [DOI] [PubMed] [Google Scholar]
- [36].Stockton RA, Slaughter MM. B-wave of the electroretinogram. A reflection of ON bipolar cell activity. J Gen Physiol 1989;93(1):101–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sugita Y, Suzuki H, Tasaki K. Human rods are acting in the light and cones are inhibited in the dark. Tohoku J Exp Med 1989;157(4):365–372. [DOI] [PubMed] [Google Scholar]
- [38].Sugita Y, Tasaki K. The activation of cones in scotopic and rods in photopic vision. Tohoku J Exp Med 1988;156(4):311–317. [DOI] [PubMed] [Google Scholar]
- [39].The International Classification of Headache Disorders re. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33(9):629–808. [DOI] [PubMed] [Google Scholar]
- [40].Valeriani M, Fierro B, Brighina F. Brain excitability in migraine: hyperexcitability or inhibited inhibition? Pain 2007;132(1-2):219–220; author reply 220-212. [DOI] [PubMed] [Google Scholar]
- [41].Vincent M, Pedra E, Mourao-Miranda J, Bramati IE, Henrique AR, Moll J. Enhanced interictal responsiveness of the migraineous visual cortex to incongruent bar stimulation: a functional MRI visual activation study. Cephalalgia 2003;23(9):860–868. [DOI] [PubMed] [Google Scholar]
- [42].Walters AB, Smitherman TA. Development and Validation of a Four-Item Migraine Screening Algorithm Among a Nonclinical Sample: The Migraine-4. Headache 2016;56(1):86–94. [DOI] [PubMed] [Google Scholar]
- [43].Welch KM. Contemporary concepts of migraine pathogenesis. Neurology 2003;61(8 Suppl 4):S2–8. [DOI] [PubMed] [Google Scholar]