Abstract
Background:
Humans are ubiquitously exposed to flame retardants, including organophosphate esters (OPEs), through direct contact with consumer products or exposure through household dust. Children are at increased risk because of their proximity to dust, hand-to-mouth activity, and the importance of childhood as a critical period in neurodevelopment.
Objectives:
To quantify differences in exposure levels between mothers and children (three to six years of age), we analyzed urinary metabolites of OPEs. We additionally assessed the ability of silicone wristbands (measuring ambient exposure) to predict urinary metabolite concentrations.
Methods:
We selected 32 mother and child dyads from an existing cohort. Participants provided baseline urine samples and wore wristbands for one week. After the first week, they returned their wristbands and provided a second urine sample. During the second week, participants wore a second wristband that they returned at the end of week two with a third and final urine sample.
Results:
We found significantly higher levels of bis(1,3-dichloro-2-propyl) phosphate (BDCIPP) (p < 0.001) and lower levels of bis(1-chloro-2-isopropyl) I-hydroxy-2-propyl phosphate (BCIPHIPP) (p < 0.001) in children’s urine samples compared to mothers’ samples at baseline. We found that triphenylphosphate (TPHP), tris(1,3-dichloroisopropyl) phosphate (TDCIPP), and tris(1-chloro-2-propyl) phosphate (TCIPP) measured in wristbands predicted their respective metabolite levels in urine.
Conclusion:
Children had higher levels than mothers for two of six flame retardant metabolites measured in urine. Generally, wristband measurements positively predicted internal dose. As little is known about the health effects of OPEs on child development, future research is needed to determine the impact of differential exposure.
Keywords: Flame retardants, Exposure assessment, Wristband sampling, Organophosphate esters (OPEs), Organophosphate flame retardants (OPFRs)
1. Introduction
In the U.S., flame retardants have been added to a wide variety of consumer products, including furniture, textiles, electronics, and construction materials, to meet flammability regulations (Affairs, 2000; Herbstman and Mall, 2014). Brominated flame retardants such as polybrominated diphenyl ethers (PBDEs) comprised a large majority of commercially-used flame retardants in polyurethane foam used in furniture prior to 2005 (Stapleton et al., 2012). Because of their persistence in the environment and their potentially harmful effects (e.g. neurobehavioral outcomes) (Darnerud et al., 2001; Palm et al., 2002; Eskenazi et al., 2009; Roze et al., 2009; Herbstman et al., 2010; Gascon et al., 2011; Shy et al., 2011; Gascon et al., 2012), industry voluntarily ended production of penta- and octa-BDE commercial mixtures in 2005 and deca-BDE production in 2013 (van der Veen and de Boer, 2012; EPA, 2014; Howard, 2014).
This phase-out prompted an increase in the use of other flame retardants to meet flammability standards, including organophosphate esters (OPEs) (also referred to as PFRs (phosphorous flame retardants) and OPFRs (organophosphate flame retardants)) to replace PBDEs in consumer products (Dodson et al., 2012; Stapleton et al., 2012). OPEs can also be used as plasticizers, and have since become pervasive, with measurable levels in indoor dust and air (Marklund et al., 2005; Meeker and Stapleton, 2010; Sugeng et al., 2017), outdoor air (Salamova et al., 2014), water and sediments (Venier et al., 2014; Peverly et al., 2015), wildlife (Greaves and Letcher, 2014), and humans (Kucharska et al., 2015; Van den Eede et al., 2015; Hammel et al., 2016; Liu et al., 2016; Braun, 2017).
Flame retardant exposure appears to be higher in children than in adults (Butt et al., 2014; Cequier et al., 2015; Butt et al., 2016; Cowell et al., 2017), possibly because of increased hand-to-mouth activity, a higher body surface area to internal mass ratio (affecting internal dose), and closer proximity to accumulated dust. Because the few first years of life are a critical period of susceptibility with respect to neurodevelopment, children’s flame retardant exposure occurring in early childhood may have more detrimental effects than exposure later in life. OPEs have shown neurotoxicity in laboratory models (Matthews et al., 1993; Dishaw et al., 2011; Patisaul et al., 2013; Pillai et al., 2014), and early studies have reported associations between OPE exposure and behavioral problems and impaired cognitive performance in children (Hutter et al., 2013; Lipscomb et al., 2017).
In the present study, we compared exposure levels in mothers and children within the same household using urine samples to measure individual internal dose. We hypothesized that we would find higher levels of OPEs in urine samples of children compared with their mothers. Because we measured both personalized ambient exposure to OPEs in wristbands and OPE metabolite concentrations in urine at multiple time points for each individual, we had the opportunity to additionally investigate the ability of wristband concentrations of parent compounds to predict urinary metabolite concentrations in a subset of OPEs.
2. Materials and methods
2.1. Participants
We selected 32 mother and child dyads from a previously established cohort to participate in a study to assess flame retardant levels at three time points (Cowell et al., 2017) within a behavioral intervention trial (Gibson et al., 2018). Families were selected from the Sibling-Hermanos cohort (N = 125), which began in 2008, consisting of Dominican and African-American mothers and children from Northern Manhattan and the South Bronx. Briefly, mothers in this study were previously enrolled in the Columbia Center for Children’s Environmental Health Mothers and Newborns birth cohort between 1998 and 2006 (Miller et al., 2001 ). When subsequently pregnant with a singleton, these women were invited to enroll an additional child in the Sibling-Hermanos cohort and were followed prospectively (Cowell et al., 2017). Among participants with children between three and five years of age in 2015, we invited N = 32 (from a total of N = 125) women and their children to participate in the current study. Measurements for the present study were collected when these children were between three and six years old. We describe the results of the intervention in a separate report (Gibson et al., 2018), but analyses described in this report are unlikely to be impacted by the effect of the intervention results.
2.2. Sample collection
All adults gave informed consent for themselves and their children before sample collection. At three visits (baseline, after week 1, after week 2), we collected a spot urine sample from the mother and her child. Visits occurred between 12:00 p.m. and 10:30 p.m., at the convenience of the mothers. The relatively short half-lives (4–8 h) of OPE metabolites in urine allow us to look at sequential measures that represent only recent exposure (Lynn et al., 1981; Burka et al., 1991; Hammel et al., 2016).
Two sizes of commercially available silicone wristbands were purchased (a larger size for mothers than for children) (24hourwristbands.com, Houston, TX), soaked with ethyl acetate, hexane and methanol at 30 ° c to remove analytical interferences prior to use, and stored at −20 ° c after treatment (O’Connell et al., 2014). Participants were asked to wear their wristbands continuously for a designated 7-day period through sleeping, bathing, and any other daily activities, beginning on the morning of day 1 and removing the wristband at the same time on morning on day 7. The wristbands were stored in amber jars at −20 ° c until extraction.
2.3. Laboratory analysis
2.3.1. Wristbands
Wristband and urine samples were extracted and analyzed using methods published previously for OPEs in wristbands and urine (Cooper et al., 2011; Butt et al., 2014; O’Connell et al., 2014; Kile et al., 2016). As previously described, compounds were removed from the silicone wristband with two rounds of 100 mL of ethyl acetate, which was combined and reduced to nominally 300 μL with nitrogen evaporators (Turbo-VapL, Biotage, Charlotte, NC and N-EVAPIII, OrganomationAssociates Inc., Berlin, MA) (O’Connell et al., 2014). To remove background of carbohydrates, lipids, and proteins, we added 3 mL of acetonitrile to each sample and loaded extracts onto 500 mg C18 solid phase extraction (SPE) cartridges pre-rinsed with 6 mL of acetonitrile at 3 mL/min (Supelco, Bellefonte, PA) (Lehotay et al., 2001; Forsberg et al., 2011 ). Samples were loaded at 1.8 mL/min and finally eluted with 9 mL of acetonitrile at 3 mL/min using a Rapid Trace, automated SPE work station (Biotage, Uppsala, Sweden). We reduced the 9mL extracts as above with Turbo-Vapsto 0.5 mL, and solvent-exchanged them to hexane to be more amenable to GC analysis (O’Connell et al., 2014; Kile et al., 2016).
We fortified aliquots with perylene-d12 at 500 ng as the internal standard prior to analysis and analyzed samples by gas chromatography mass spectrometry (GC-MS) with an Agilent 7890A gas chromatograph (Santa Clara, CA) interfaced with an Agilent 5975C mass spectrometer equipped with a triple axis detector (Kile et al., 2016). In all instrument blanks (1B, n = 38), extraction blank (n = 1)and SPE blank (n = 1), all target analytes were below the limits of detection (LODs). LODs were determined using an established EPA method (Environmental Protection Agency 1195; Kile et al., 2016). We ran the lowest standard that resulted in a signal to noise ratio of 15:1 for each compound 7 times, and we multiplied the resulting standard deviation with the Student’s t value corresponding to the appropriate degree of freedom for a 99% confidence interval (Kile et al., 2016). Wristband LODs ranged from 0.72 ng/g (TPHP) to 6.00 ng/g (TCIPP), with higher LODs in children’s wristbands because of their smaller mass. Wristband concentrations are reported as analyte mass per wristband mass (nanograms per gram (ng/g)). This is equivalent to nanograms per wristband standardized by wristband size (all mothers wore large and all children wore small wristbands, thus the variation remained constant across the study). We measured four OPEs in wristbands (triphenylphosphate (TPHP), tris(1,3-dichloroisopropyl) phosphate (TDCIPP, also called Tris), tris-(2-chloroethyl) phosphate (TCEP), and tris(1-chloro-2propyl) phosphate (TCIPP)).
Some wristbands were damaged, never worn, or not returned by the participants. Families missing at least one wristband from mother or child did not have their wristbands sent to the laboratory for analysis. Though mixed effect models do allow for some missingness, families with no wristband measurements could not contribute to the prediction models introduced in Section 2.4.
2.3.2. Urine samples
We used a digital hand-held refractometer (Atago) to measure specific gravity for each urine sample. Using methods described previously, 5.0 mL of urine was spiked individually with d-BDCIPP, d-DPHP, and d-TDCIPP as internal standards and combined with a sodium acetate buffer and an enzyme solution, then incubated overnight at 37 ° c. The flame retardant metabolites were extracted via mixed-mode anion-exchange solid-phase extraction and measured using atmospheric pressure chemical ionization liquid chromatography-tandem mass spectrometry (Agilent Technologies, Model 6410) (Cooper et al., 2011). d-BDClPP, d-DPHP, and d-TDCIPP recoveries averaged 107%, 69%, and 69%, respectively. The method detection limits (MDLs) were calculated using 3 times the standard deviation of the blanks normalized to the volume of urine extracted. MDLs ranged from 0.08 ng/mL (ip-DPHP and tbutyl-DPHP) to 0.64 ng/mL (BCIPHIPP) for urinary metabolites. Urinary concentrations are reported as analyte mass per volume (nanograms per milliliter (ng/mL)), normalized by specific gravity, which will partially control for differences between consumption and excretion rates of water per body weight in adults and children, to account for urinary dilution (Meeker et al., 2011). The normalized values are included in regression models. This analysis includes six OPE metabolites measured in urine (bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), bis( I-chloro-2-propyl) phosphate (BCIPP), bis(l -chloro-2-isopropyl) I-hydroxy-2-propyl phosphate (BCI-PHIPP), diphenylphosphate (DPHP), and two alkylated DPHPs (ip-DPHP and tbutyl-DPHP)). Isopropyl triphenylphosphate (ip-TPHP) and t-butyl triphenylphosphate (tbutyl-TPHP), the parent compounds for ip-DPHP and tbutyl-DPHP, respectively, were not measured (Butt et al., 2014). Relationships between parent compounds and metabolites are described in Table 1.
Table 1.
Relationships between measured analytesin wristbands and metabolites in urine.
| Parent Compound | Urinary Metabolite |
|---|---|
| TDCIPP | BDC1PP |
| TPHP | DPHP |
| TCIPP | BC1PP |
| BCIPHIPP | |
| TCEP | Not measured |
| Not measured | ip-DPHP |
| Not measured | tbutyl-DPHP |
2.4. Data analysis
Measurements below the MDL for urine and the LOD for wristbands were assigned the sample-specific MDL or LOD/√2. Because no direct comparisons were made between maternal and child wristbands of different sizes, we did not normalize their differing LODs. Analyses were conducted for analytes with detection frequency >50%. We used individual concentrations of wristband OPEs and urinary OPE metabolites.
Flame retardant concentrations were not normally distributed. We log-transformed all concentrations in regression models and used non-parametric tests for correlation and differences between mothers and children (sensitivity analysis). We used mixed-effect models for repeated measures (three urine samples and two wristbands within each individual), with household included as a multilevel random effect to account for correlated observations within families. To evaluate the difference in OPE exposure between mothers and children, we analyzed pre-intervention urinary metabolites only (to prohibit any potentially biasing effects of intervention) with a mother/child categorical variable included as a fixed effect (N = 30 pairs). To evaluate the influence of household (shared environment), we estimated intra-class correlations (ICCs) from these models as the variance attributable to the random effect of being in the same family divided by the total variance. Models presented are unadjusted as no true confounders can exist (i.e., nothing can cause mother/child status). Additional adjustment for number of handwashes per day (a potential mediator) did not meaningfully alter the direction, magnitude, or significance of unadjusted effect estimates.
Models to predict urinary metabolites included wristband concentrations as fixed effects and controlled for mother/child status as a confounder. Wristband concentrations during week 1 of the study predicted concentrations in the urine sample collected after week 1; wristband concentrations during week 2 of the study predicted concentrations in the urine sample collected after week 2. In this way, variation in parent compounds and urinary metabolites due to the effectiveness of the intervention was reflected in both the wristband and urine concentrations, effectively controlling for intervention. Additional adjustment for intervention group and week did not meaningfully alter the effect estimate. We used mixed-effect regression models with mothers and children nested within households, with both independent (wristband concentrations) and dependent (urinary concentrations) variables log-transformed. Instead of interpreting the beta coefficients of these log-log models as effect sizes, as is standard in environmental epidemiology, we interpret them as the percent change in the outcome associated with a one percent increase in the exposure. This is a common interpretation in econometrics, referred to as “elasticity”(Wooldridge, 2015). All mixed-effect models use empirical standard errors. Because these models include urinary metabolites and wristband analytes at multiple time points (urine samples after week 1 and week 2 and both wristbands), we present concentration distributions averaged over the course of the study.
As a sensitivity analysis, we further compared mothers and children using Wilcoxon signed-rank tests on paired data to examine differences in concentrations within families, restricting these tests to baseline urinary metabolites. We performed statistical analyses in SAS statistical software (version 9.4; SAS Institute Inc.,Cary, NC) and in R (version 12.11.0; R Development Core Team, 2010); statistical tests were conducted at the 0.05 significance level.
3. Results
Thirty-two mothers and thirty children provided baseline urine samples. Nineteen families returned all four wristbands (two maternal, two child) from both weeks of the study, for a total of 76 wristbands analyzed. Thirteen families were missing at least one wristband. We observed no significant demographic or behavioral differences between those families who returned all wristbands and those who did not. The mean age of mothers in the study was 32 years, and the mean age of children was 5 years. Mothers and children differed significantly on average number of handwashes per day (p-value < 0.05). Characteristics of study participants are provided in Table 2.
Table 2.
Characteristics of study participants at baseline.
| Variable | Urine Samples |
|||
|---|---|---|---|---|
| Overalla |
Mothersa |
Childrena |
pb |
|
| n = 62 | n = 32 | n = 30 | ||
| Child Sex | – | |||
| Male | 18(60.0%) | |||
| Female | – | – | 12(40.0%) | |
| Ethnicity | 0.99 | |||
| African American | 24 (38.7%) | 12 (40.0%) | 12(37.5%) | |
| Dominican American | 38 (61.3%) | 18 (60.0%) | 20 (62.5%) | |
| Child Age (years) | – | – | 4.8 (0.8) | – |
| Maternal Age (years) | – | 32.6 (4.1) | – | – |
| Avg Times Hands Washed/Day | 0.03 | |||
| 1–2 | 6 (9.7%) | 2 (6.3%) | 4(13.3%) | |
| 3–5 | 29 (46.8%) | 11 (34.4%) | 18(60.0%) | |
| 6–8 | 14 (22.6%) | 8 (25.0%) | 6 (20.0%) | |
| 9+ | 13 (21.0%) | 11 (34.4%) | 2 (6.7%) | |
| Avg Hours/Day Spent in Home | 0.43 | |||
| 1–4 | 21 (33.9%) | 12 (37.5%) | 9 (30.0%) | |
| 5–6 | 18 (29.0%) | 7(21.9%) | 11 (36.7%) | |
| 7+ | 23 (37.1) | 13 (40.6%) | 10(33.3%) | |
| Maternal Education | – | |||
| Some high school | – | 12 (37.5%) | – | |
| High school degree | – | 15 (46.9%) | – | |
| Some college | – | 5(15.6%) | – | |
Values are mean (standard deviation) or number (%).
P-values are from paired t-test or Fisher’s exact test for differences between mothers and children.
Detection of OPE metabolites in urine was high, ranging from 100% detection of DPHP, BDCIPP, and ip-DPHP to 84% detection of BCIPP in maternal samples and from 100% (DPHP, BDCIPP, and ip-DPHP) to 90.7% (BCIPP) in children’s samples over the course of the study (Table 3). TPHP was detected in 100% of maternal and child wristbands. TCIPP was detected in 44.7% of maternal wristbands and 73.7% of children’s. Quantiles of OPE metabolites over the course of the study are given in Table 3 (See Appendix, Supplemental Table 1 for baseline concentrations in urine).
Table 3.
Average concentrations of urinary metabolites and wristband analytes over the course of the study.
| Urinary metabolite (ng/mL)a | Mothers (n = 96) |
Children (n = 90) |
||||||||||
| Percentiles |
Percentiles |
|||||||||||
| MDLb | # (%) < MDLb. c | 25th | 50th | 75th | Max | MDLb | # (%) < MDLb,c | 25th | 50th | 75th | Max | |
| BC1PP | 0.15 | 15 (16) | 0.3 | 0.7 | 1.5 | 13.9 | 0.15 | 8 (9) | 0.3 | 0.9 | 2.3 | 17.1 |
| DPHP | 0.45 | – | 1.6 | 2.5 | 4.2 | 39.8 | 0.45 | – | 2.0 | 3.2 | 6.7 | 74.7 |
| BDC1PP | 0.19 | – | 0.6 | 1.1 | 2.2 | 7.8 | 0.19 | – | 1.2 | 2.6 | 5.4 | 40.4 |
| BC1PH1PP | 0.64 | 2 (2) | 0.5 | 0.9 | 1.5 | 6.9 | 0.64 | 4 (5) | 0.4 | 0.6 | 1.0 | 4.2 |
| ip-DPHP | 0.08 | – | 4.2 | 6.7 | 10.9 | 46.0 | 0.08 | – | 5.0 | 7.7 | 12.0 | 46.7 |
| tbutyl-DPHP | 0.08 | 2 (2) | 0.2 | 0.2 | 0.4 | 1.8 | 0.08 | 1 (1) | 0.2 | 0.3 | 0.5 | 2.7 |
| Mothers (n = 38) |
Children (n = 38) |
|||||||||||
| Percentiles |
Percentiles |
|||||||||||
| Wristband analyte (ng/g) | LODc | # (%) < LODd, e | 25th | 50th | 75th | Max | LODc | # (%) < LODd, e | 25th | 50th | 75th | Max |
| TCEP | 3.27 | 4 (11) | 68.3 | 108.5 | 173.0 | 719 | 3.83 | 9 (24) | 31.7 | 64.5 | 115.0 | 656 |
| TCIPP | 5.12 | 21 (55) | 3.6 | 4.2 | 252.0 | 929 | 6.00 | 10 (26) | 4.2 | 297.5 | 778.0 | 3490 |
| TDCIPP | 5.00 | 1 (3) | 85.6 | 163.5 | 352.0 | 1060 | 5.85 | 1 (3) | 135.0 | 390.5 | 658.0 | 2460 |
| TPHP | 0.72 | – | 166.0 | 399.0 | 529.0 | 2230 | 0.72 | – | 195.0 | 440.0 | 976.0 | 3050 |
Urinary metabolites are normalized to specific gravity.
MDL = method detection limit of the analyte; # (%) < MDL = number and percentage of participants with concentrations below the MDL.
Percent calculated is # < MDL divided by 96 for mothers and by 90 for children.
LOD = method detection limit of the analyte; # (%) < LOD = number and percentage of participants with concentrations below the LOD.
Percent calculated is # < LOD divided by 38 for mothers and for children.
3.1. Exposure by maternal/child status
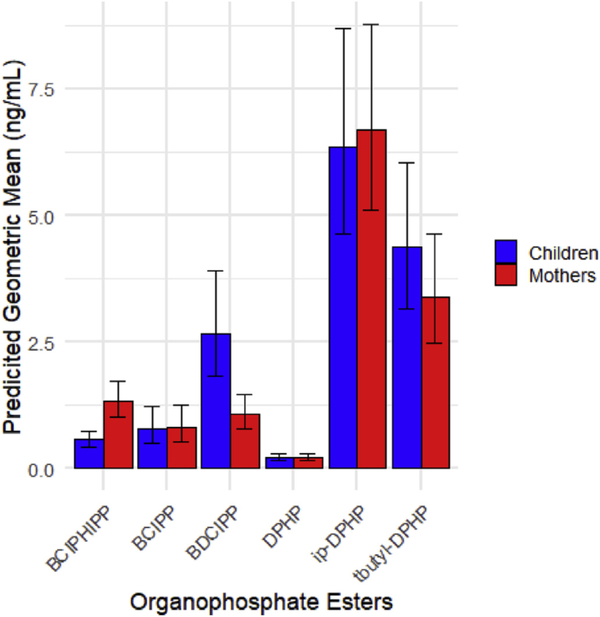
Predicted geometric means of OPE metabolites in urine from mixed effect models are depicted in Fig. 1. BDCIPP, the metabolite of TDCIPP, was 151.15% higher in children than in mothers (p-value<O.001; 95% Cl (55.80, 304.87)). BCIPHIPP, a urinary metabolite of TCIPP, was 53.22% lower in children than in mothers (p-value < 0.001; 95% Cl (−68.88,−29.69)). BCIPP, another metabolite of TCIPP, DPHP, a metabolite of TPHP, and ip-DPHP, whose parent was not measured, were all lower in children but non-significant. Tbutyl-DPHP, whose parent was not measured, was higher in children but non-significant (Table 4). The paired Wilcoxon singed-rank tests used as a sensitivity analysis for differences between maternal and child flame retardant concentrations yielded similar results (results not shown).
Fig.1.
Geometric means of urinary metabolites in mothers and children at baseline (n = 30 pairs). Geometric means and 95% confidence intervals for flame retardant metabolites measured in urine in children and mothers. Bars represent geometric mean in mothers and children. Error bars represent 95% confidence interval. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 4.
Baseline differences between OPE in urine for mothers and children.
| Metabolite | Parent Status1 | % Difference | 95% CI |
|---|---|---|---|
| BC1PP | Child | −4.29 | (−37.16, 44.77) |
| Mother | REF | REF | |
| DPHP | Child | −0.42 | (−33.28, 48.62) |
| Mother | REF | REF | |
| BDC1PP | Child | 151.15 | (55.80, 304.87) |
| Mother | REF | REF | |
| BCIPH1PP | Child | −53.22 | (−68.88, −29.69) |
| Mother | REF | REF | |
| ip-DPHP | Child | 11.51 | (−38.34, 26.99) |
| Mother | REF | REF | |
| tbutyl-DPHP | Child | 32.94 | (−18.15, 115.89) |
| Mother | REF | REF |
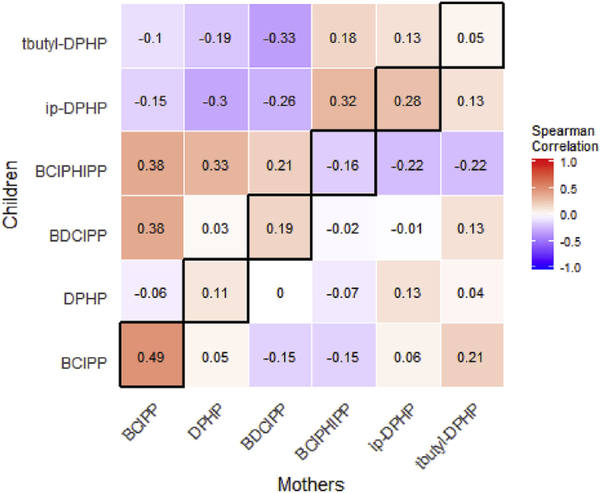
OPE concentrations in mothers and children within households were not strongly correlated across metabolites (Fig. 2). BCIPP had a correlation coefficient of 0.48 (p < 0.01) in mothers and children at baseline, but coefficients for the remaining OPEs were between −0.16 and 0.28 and all non-significant (DPHP, r = 0.11, p =.55; BDCIPP, r =0.19, p =0.32; BCIPHIPP, r= −0.16, p=O.40; ip-DPHP, r = 0.28, p = 0.14, tbuty1-DPHP, r = 0.05, p = 0.79). Intraclass correlations (ICCs) from mixed effect models for DPHP, BCIPHIPP, and tbutyl-DPHP were all zero, meaning that household membership did not explain any of the variance in these metabolites. BCIPP had the highest ICC (0.54), followed by ip-DPHP (0.16) and BDCIPP (0.07).
Fig.2.
Correlation across maternal and child urine samples at baseline (n 30 pairs). Diagonal (highlighted with black border) represents direct comparisons of the same metabolites in mothers and children. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. Urinary metabolite prediction
A one percent increase in TDCIPP measured in wristbands was associated with a 0.33% increase in its metabolite BDCIPP (p = 0.001; 95% CI = (0.14, 0.52)). A one percent increase in TCIPP was marginally associated with a 0.05% increase in BCIPHIPP (p = 0.08; 95% CI = (−0.01, 0.12)) and non-significantly associated with a 0.11% increase in BCIPP (p =.10; Cl = (−0.02, 0.23). A one percent increase in TPHP was marginally associated with a 0.14% decrease in DPHP (p =.06; Cl = (−0.29, 0.01 (Table 5). Additional adjustment for intervention arm and week did not substantially change the magnitude, direction, or significance of effect estimates.
Table 5.
Elasticity between wristband concentrations and urinary metabolites across weeks 1 and 2.
| Dependent Variable | Independent Variable | Elasticitya | 95% CI | P-value |
|---|---|---|---|---|
| DPHP | TPHP | −0.14 | (−0.29, 0.01) | 0.06 |
| BCIPP | TCIPP | 0.11 | (−0.02, 0.23) | 0.10 |
| BCIPHIPP | TCIPP | 0.05 | (−0.01, 0.12) | 0.08 |
| BDCIPP | TDCIPP | 0.33 | (0.14, 0.52) | 0.001 |
Elasticity is defined as the percent change in the dependent variable when the independent variable increases by one percent (Wooldridge, 2015).
4. Discussion
In this study, we aimed to compare OPE exposure between mothers and children living in the same household using urinary metabolites. The results of this study support the hypothesis that children have higher exposure than mothers to some, though not all, flame retardants, as measured by internal dose, but they also display high variability within and between OPEs. In urine samples, children had higher levels of two of the six OPE metabolites measured, with significantly higher levels of BDCIPP (a metabolite of TDCIPP).
Baseline concentrations of most metabolites of OPEs in urine were higher in our participants than in an exposure assessment conducted on paired mothers and toddlers from North Carolina (Butt et al., 2014). Mothers and children in our study had higher levels of BCIPP, DPHP, ip-DPHP, and tbutyl-DPHP, but lower levels of BDCIPP than mothers and children observed by Butt et al. Compared to a separate study of adults in North Carolina, our study found higher levels of DPHP, BCIPP, and BDCIPP, but lower levels of BCIPHIPP in maternal urine (Hammel et al., 2016). Mothers and children in our study had higher median levels of DPHP, but lower median levels of BDCIPP compared with mother-toddler pairs in California (Butt et al., 2016). Median levels of BCIPP and BCIPHIPP were higher in children in our study than in a study of younger children between the ages of 3 and 29 months in Queensland, Australia (0.68 and 0.93 ng/mL, respectively), but median BDCIPP was lower in children in our study (He et al., 2018a). Along with demographic and geographic differences between these cohorts, age trends in OPE exposure have been observed, with exposure to most OPEs decreasing with age (He et al., 2018b).
Using ICC to examine the extent of intra-familial correlation in flame retardant level, we found that family membership explained no variation in urinary metabolite levels for half of the OPEs measured (DPHP, BCIPHIPP, and tbutyl-DPHP). Ambient exposure of two individuals in the same home is expected to be more similar than the same individuals’ metabolite levels, as the latter are affected by internal metabolism. Urinary excretion rates and pathways differ substantially between adults and children, explaining the lack of household similarities in urinary metabolite levels. While wristbands measure only ambient exposure, urine samples incorporate exposure and excretion capacity. Because of these biological differences and likely similarities in ambient exposure to mothers and children within households collected by wristbands, wristband sampling may reflect more similar exposure patterns in comparison to urine, particularly because mothers and children in this study spent the majority of their time at home. The two metabolites with significant differences between mothers and children (BDCIPP and BCIPHIPP) had ICCs of 0.07 and zero, respectively, indicating that in instances where children have significantly higher (or lower) flame retardant levels than mothers, family membership cannot adequately characterize exposure.
We unexpectedly observed higher concentrations of BCIPHIPP (a metabolite of TCIPP) in maternal urine samples in comparison to children. BCIPP (TCIPP’s other metabolite) was also non-significantly higher in mothers, though the magnitude was much smaller. TCIPP in wristbands was more strongly correlated with BCIPP (mothers: r = 0.43, p = 0.08; children: r = 0.58, p = 0.02) than with BCIPHIPP (mothers: r = 0.22, p = 0.38; children: r = 0.10, p = 0.73) throughout the study. Thus, we believe that BCIPP in our population more closely captures individual exposure to TCIPP. Hammel et al. detected BCIPHIPP in 100% and BCIPP in only 18% of urine samples from study participants (whereas we detected BCIPHIPP in 96% and BCIPP in 90%) and found significant correlation between TCIPP measured in wristbands and BCIPHIPP (r = 0.62, p-value < 0.0001), but not between TCIPP and BCIPP. Because BCIPP was rarely detected, it was not included in their analyses (Hammel et al., 2016). This could indicate differences in exposure magnitude, measurement (e.g., different MDLs of analytes), or differential metabolism of TCIPP across populations, though the latter is unlikely to account for such a large difference.
Using wristband measures of parent compound to predict urinary metabolites within individuals, we found that TCIPP predicted a marginally significant increase in both measured metabolites, BCIPP and BCIPHIPP, and TDCIPP predicted a significant increase in BDCIPP. All effect estimates were less than one, meaning that the change in the urinary metabolite was less than proportional (a one percent increase in the independent variable associated with a one percent increase in the dependent variable would give a beta coefficient of one) in response to the change in the wristband concentration. This makes biological sense, as metabolic processes within an individual will also influence urinary metabolite levels.
Increasing TPHP was surprisingly associated with a marginally significant decrease (beta = −0.14, p = 0.07) in DPHP. TPHP in wristbands and DPHP in urine were not correlated in mothers (r = −0.22, p = 0.37) or children (r = 0.01; p=0.96) in our study. Hammel et al. observed this as well, and explained that other chemicals in addition to TPHP (including monosubstituted iso-propylatedtriaryl phosphate (mono-ITP), 2-ethylhexyl diphenyl phosphate (EH-DPHP), and isodecyldiphenyl phosphate (id-DPHP)) may metabolize to form DPHP, explaining the lack of association (Hammel et al., 2016). Diet, nail polish, and plastic bottles are additional sources of TPHP exposure that would not be captured by wristbands (Mendelsohn et al., 2016). Further, correlations between wristband analytes and urine metabolites may be more highly variable in children than in mothers due to issues of compliance, as children are more likely to take off their wristbands, play with them, or drop them. No questions about wristband compliance were included in the questionnaire.
While this analysis evaluated differences between maternal and child flame retardant exposure, it is important to remember that these observations were made within the context of a behavioral intervention study designed to reduce exposure (Gibson et al., 2018). Because our assessment of differences between OPE levels in mothers and children was conducted on baseline measurements, the intervention’s effectiveness cannot affect our interpretation. The prediction models of urine levels included measurements across the intervention. As the intervention was designed to affect external exposure and not internal processes, we believe that all variability due to the effectiveness of the intervention in the predictive models (which include both week 1 and week 2 of the trial) was captured by the wristband concentrations, and models were unaffected by additional adjustment for intervention arm and week.
Limitations of this study include the study’s small sample size, lack of a baseline wristband, and participants’ compliance. With the small sample size, it is possible that observed differences between mothers and children do not reflect the larger population, but as this pattern has been observed in varied populations (Butt et al., 2014; Cowell et al., 2017); our study confirms previous findings. Further, the small sample size allowed for more robust data collection. Because no wristbands were worn before the study began, we cannot use them to investigate differences in wristband analytes between mothers and children and we cannot use baseline urine samples in the prediction model. Our questionnaire did not address wristband compliance, i.e., it did not ask if wristbands were worn continuously over the week. Wristbands that were lost or damaged were not included in the analysis, further reducing the sample size.
5. Conclusion
We found higher levels of BDCIPP and tbutyl-DPHP in children’s urine sampled than in mothers’. This supports previous findings of higher exposure to flame retardants in children than adults. We found that wristband concentrations of TCIPP and TDCIPP predicted their respective metabolites. Little is known about the health effects of OPEs on child development. As more research is conducted, efforts to reduce OPE exposure should acknowledge differential exposure levels between children and adults and aim to safeguard children during a critical period in neurodevelopment.
Supplementary Material
HIGHLIGHTS.
We measured OPE exposure in maternal and child wristbands and urine (metabolites).
Children were more exposed than mothers to BDCIPP and tbutyl-DPHP, an alkylated diphenylphosphate.
TPHP, TDCIPP, and TCIPP measured in wristbands predicted their own metabolite levels.
Acknowledgements
We thank the families that participated in this study. This work was supported by the John Merk Fund and the National Institutes of Health [grant numbers ROI ES021806, P30 ES009089, and T32 ES023772].
Footnotes
Declarations of interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chemosphere.2018.12.008.
References
- Affairs, C. D. o. C., 2000. Technical Bulletin 117: Requirements, Test Procedure and Apparatus for Testing the Flame Retardance of Resilient Filling Materials Used in Upholstered Furniture. http://www.bearhfti.ca.govlindustry/117.pdf. (Accessed 7 July 2017).
- Braun JM, 2017. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol 13 (3), 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burka LT., Sanders JM, Herr DW, Matthews HB, 1991. Metabolism of tris(2-chloroethyl) phosphate in rats and mice. Drug Metab. Dispos 19 (2), 443–447. [PubMed] [Google Scholar]
- Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM, 2014. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoatein urine from paired mothers and toddlers. Environ. Sci. Technol 48 (17), 10432–10438. [DOI] [PubMed] [Google Scholar]
- Butt CAM., Hoffman K Chen A, Lorenzo A, Congleton J Stapleton HM, 2016. Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoicacid (TBBA) in mother-toddler pairs from California and New Jersey. Environ. Int 94 (Suppl. C), 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cequier E, Sakhi AK, Marce RM, Becher G, Thomsen C, 2015. Human exposure pathways to organophosphate triesters - a biomonitoring study of mother-child pairs. Environ. Int 75, 159–165. [DOI] [PubMed] [Google Scholar]
- Cooper EM, Covaci A, van Nuijs AL, Webster TF, Stapleton HM, 2011. Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem 401 (7), 2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell WJ, Stapleton HM, Holmes D, Calero L, Tobon C, Perzanowski M, Herbstman JB, 2017. Prevalence of historical and replacement brominated flame retardant chemicals in New York City homes. Emerg. Contamin [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnerud PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M, 2001. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ. Health Perspect 109 (Suppl. 1 ), 49–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishaw LV, Powers CM, Ryde LT, Roberts SC, Seidler FJ, Slotkin TA, Stapleton HM, 2011. Is the PentaBDE replacement, tris (1,3-dichloropropyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC 12 cells. Toxicol. Appl. Pharmacol 256 (3), 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Perovich LJ., Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JC, Rudel RA, 2012. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ. Sci. Technol 46 (24), 13056–13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency, E. (1195). Definition and procedure for determination of the Method Detection Limit. 40 CFR Part 136, Appendix B, Revision 1.11: 343–345
- EPA, 2014. An Alternatives Assessment for the Flame Retardant Decabromodiphenyl Ether (DecaBDE). E. P. Agency. [Google Scholar]
- Eskenazi B, Marks A, Chevrier J, Harley K, Bradman A, Sjodin A, 2009. Associations between maternal PBDE serum concentrations and child neurodevelopment in the chamacos cohort. Epidemiology 20 (6), S94–S95. [Google Scholar]
- Forsberg ND, Wilson CR, Anderson KA, 2011. Determination of parent and substituted polycyclic aromatic hydrocarbons in high-fat salmon using a modified QuEChERS extraction, dispersive SPE and GC-MS. J. Agric. Food Chem 59 (15), 8108–8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon M, Fort M, Martinez D, Carsin AE, Forns J, Grimalt JO, Marina LS., Lertxundi N, Sunyer J, Vrijheid M, 2012. Polybrominated diphenyl ethers (PBDEs) in breast milk and neuropsychological development in infants. Environ. Health Perspect 120 (12), 1760–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon M, Vrijheid M, Martinez D, Forns J, Grimalt JO, Torrent M, Sunyer J, 2011. Effects of pre and postnatal exposure to low levels of polybromodiphenylethers on neurodevelopment and thyroid hormone levels at 4 years of age. Environ. Int 37 (3), 605–611. [DOI] [PubMed] [Google Scholar]
- Gibson EA, Stapleton HM, Calero L, Holmes D, Burke K, Martinez R, Cortes B, Nematollahi A, Evans D, Herbstman JB, 2018. Flame retardant exposure assessment: findings from a behavioral intervention study. J. Expo. Sci. Environ. Epidemiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves AK, Letcher RJ, 2014. Comparative body compartment composition and in ovo transfer of organophosphate flame retardants in North American Great Lakes herring gulls. Environ. Sci. Technol 48 (14), 7942–7950. [DOI] [PubMed] [Google Scholar]
- Hammel SC, Hoffman K, Webster TF, Anderson KA, Stapleton HM, 2016. Measuring personal exposure to organophosphate flame retardants using silicone wristbands and hand wipes. Environ. Sci. Technol 50 (8), 4483–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, English K, Baduel C, Thai P, Jagals P, ware R.s., Li Y, Wang X, Sly PD, Mueller JF, 2018a. Concentrations of organophosphate flame retardants and plasticizers in urine from young children in Queensland, Australia and associations with environmental and behavioural factors. Environ. Res 164, 262–270. [DOI] [PubMed] [Google Scholar]
- He C, Toms LL., Thai P, Van den Eede N, Wang X, Li Y, Baduel C, Harden FA, Heffernan AL, Hobson P, Covaci A, Mueller JF, 2018b. Urinary metabolites of organophosphate esters: concentrations and age trends in Australian children. Environ. Int 111, 124–130. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Mall JK, 2014. Developmental exposure to polybrominated diphenyl ethers and neurodevelopment. Curr. Environ. Health Rep 1 (2), 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY, Perera F, 2010. Prenatal exposure to PBDEs and neurodevelopment. Environ. Health Perspect 118 (5), 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard GJ, 2014. Chemical alternatives assessment: the case of flame retardants. Chemosphere 116, 112–117. [DOI] [PubMed] [Google Scholar]
- Hutter HP, Haluza D, Piegler K, Hohenblum R, Frohlich M, Scharf S, Uhl M, Damberger B, Tappler P, Kundi M, Wallner P, Moshammer H, 2013. Semivolatile compounds in schools and their influence on cognitive performance of children. Int. J. Occup. Med. Environ. Health 26 (4), 628–635. [DOI] [PubMed] [Google Scholar]
- Kile ML, Scott RP, O’Connell SG, Lipscomb S, MacDonald M, McClelland M, Anderson KA, 2016. Using silicone wristbands to evaluate preschool children’s exposure to flame retardants. Environ. Res 147, 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharska A, Cequier E, Thomsen C, Becher G, Covaci A, Voorspoels S, 2015. Assessment of human hair as an indicator of exposure to organophosphate flame retardants. Case study on a Norwegian mother-child cohort. Environ. Int 83, 50–57. [DOI] [PubMed] [Google Scholar]
- Lehotay SJ, Lightfield AR, Harman-Fetcho JA, Donoghue DJ, 2001. Analysis of pesticide residues in eggs by direct sample introduction/gas chromatography/ tandem mass spectrometry. J. Agric. Food Chem 49 (10), 4589–4596. [DOI] [PubMed] [Google Scholar]
- Lipscomb ST, McClelland MM, MacDonald M, Cardenas A, Anderson KA, Kile ML, 2017. Cross-sectional study of social behaviors in preschool children and exposure to flame retardants. Environ. Health 16 (1), 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LY., He K, Hites RA, Salamova A, 2016. Hair and nails as noninvasive biomarkers of human exposure to brominated and organophosphate flame retardants. Environ. Sci. Technol 50 (6), 3065–3073. [DOI] [PubMed] [Google Scholar]
- Lynn RK, Wong K, Garvie-Gould C, Kennish JM, 1981. Disposition of the flame retardant, tris(1,3-dichloro-2-propyl) phosphate, in the rat. Drug Metab. Dispos 9 (5), 434–441. [PubMed] [Google Scholar]
- Marklund A, Andersson B, Haglund P, 2005. Organophosphorus flame retardants and plasticizers in air from various indoor environments. J. Environ. Monit 7 (8), 814–819. [DOI] [PubMed] [Google Scholar]
- Matthews HB, Eustis SL, Haseman J, 1993. Toxicity and carcinogenicity of chronic exposure to tris(2-chloroethyl)phosphate. Fund. Appl. Toxicol 20 (4), 477–485. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Stapleton HM, 2010. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ. Health Perspect 118 (3), 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Yang T, Ye X, Calafat AM, Hauser R, 2011. Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environ. Health Perspect 119 (2), 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn E, Hagopian A, Hoffman K, Butt CM, Lorenzo A, Congleton J, Webster TF, Stapleton HM, 2016. Nail polish as a source of exposure to triphenyl phosphate. Environ. Int 86, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL, Chew GL, Bell CA, Biedermann SA, Aggarwal M, Kinney PL, Tsai WY, Whyatt RM, Perera FP, Ford JC, 2001. Prenatal exposure, maternal sensitization, and sensitization in utero to indoor allergens in an inner-city cohort. Am. J. Respir. Crit. Care Med 164 (6), 995–1001. [DOI] [PubMed] [Google Scholar]
- O’Connell SG, Kincl LD., Anderson KA, 2014. Silicone wristbands as personal passive samplers. Environ. Sci. Technol 48 (6), 3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm A, Cousins IT, Mackay D, Tysklind M, Metcalfe C, Alaee M, 2002. Assessing the environmental fate of chemicals of emerging concern: a case study of the polybrominated diphenyl ethers. Environ. Pollut 117 (2), 195–213. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, Stapleton HM, 2013. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster(R) 550 in rats: an exploratory assessment. J. Biochem. Mol. Toxicol 27 (2), 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peverly AA, O’Sullivan C, Liu LY., Venier M, Martinez A, Hornbuckle KC, Hites RA, 2015. Chicago’s Sanitary and Ship Canal sediment: polycyclic aromatic hydrocarbons, polychlorinated biphenyls, brominated flame retardants, and organophosphate esters. Chemosphere 134, 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai HK, Fang M, Beglov D, Kozakov D, Vajda S, Stapleton HM, Webster TF, Schlezinger JJ, 2014. Ligand binding and activation of PPARgamma by Firemaster(R) 550: effects on adipogenesis and osteogenesis in vitro. Environ. Health Perspect 122 (11), 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roze E, Meijer L, Bakker A, Van Braeckel KN, Sauer PJ, Bos AF, 2009. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ. Health Perspect 117 (12), 1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamova A, Hermanson MH, Hites RA, 2014. Organophosphate and halogenated flame retardants in atmospheric particles from a European Arctic site. Environ. Sci. Technol 48 (11), 6133–6140. [DOI] [PubMed] [Google Scholar]
- Shy CC, Huang HL, Chang-Chien GP, Chao HR, Tsou T.c., 2011. Neurodevelopment of infants with prenatal exposure to polybrominated diphenyl ethers. Bull. Environ. Contam. Toxicol 87 (6), 643–648. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A, 2012. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ. Sci. Technol 46 (24), 13432–13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugeng EJ, Leonards PEG, van de Bor M, 2017. Brominated and organophosphorus flame retardants in body wipes and house dust, and an estimation of house dust hand-loadings in Dutch toddlers. Environ. Res 158, 789–797. [DOI] [PubMed] [Google Scholar]
- Van den Eede N, Heffernan AL, Aylward LL, Hobson P, Neels H, Mueller JF, Covaci A, 2015. Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ. Int 74, 1–8. [DOI] [PubMed] [Google Scholar]
- van der Veen 1, de Boer J, 2012. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88 (10), 1119–1153. [DOI] [PubMed] [Google Scholar]
- Venier M, Dove A, Romanak K, Backus S, Hites R, 2014. Flame retardants and legacy chemicals in Great Lakes’ water. Environ. Sci. Technol 48 (16), 9563–9572. [DOI] [PubMed] [Google Scholar]
- Wooldridge JM, 2015. Introductory Econometrics: aModern Approach. Nelson Ed ucation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.