Summary
Patterning the dorsal surface of the Drosophila blastoderm embryo requires Decapentaplegic (Dpp) and Screw (Scw), two BMP family members. Signaling by these ligands is regulated at the extracellular level by the BMP binding proteins Sog and Tsg. We demonstrate that Tsg and Sog play essential roles in transporting Dpp to the dorsal-most cells. Furthermore, we provide biochemical and genetic evidence that a heterodimer of Dpp and Scw, but not the Dpp homodimer, is the primary transported ligand and that the heterodimer signals synergistically through the two type I BMP receptors Tkv and Sax. We propose that the use of broadly distributed Dpp homodimers and spatially restricted Dpp/Scw heterodimers produces the biphasic signal that is responsible for specifying the two dorsal tissue types. Finally, we demonstrate mathematically that heterodimer levels can be less sensitive to changes in gene dosage than homodimers, thereby providing further selective advantage for using heterodimers as morphogens.
Introduction
A fundamental issue that organisms face during development is how to reliably reproduce spatially restricted patterns of gene expression from initial broad instructive cues. This problem is especially acute during early Drosophila development, in which cell fate in the dorsal half of the embryo is determined by the morphogen Decapentaplegic (Dpp) during a very short developmental time window. Dpp is a bone morphogenetic protein (BMP) type ligand of the TGF-β superfamily (Padgett et al., 1987). It requires the activity of Screw (Scw), a second BMP-type factor (Arora et al., 1994), to pattern dorsal embryonic cells into two primary tissues, the extraembryonic amnioserosa that is derived from the dorsal midline cells and requires high levels of BMP signaling, and the dorsal ectoderm that comes from dorsal lateral cells and requires lower levels of signaling (Ferguson and Anderson, 1992a; Wharton et al., 1993).
A key unresolved issue with respect to this patterning process is how the BMP activity gradient is generated. BMP signals are transduced by a heteromeric complex of type I and II receptors that phosphorylate members of the Smad family of transcription factors (reviewed in Massague and Chen [2000]). Once phosphorylated, the R-Smads form a complex with a common Smad (Co-Smad) and translocate to the nucleus, where they regulate target gene activity. In Drosophila, Punt is the type II BMP receptor for early embryonic patterning (Letsou et al., 1995; Ruberte et al., 1995), while Thick veins (Tkv) and Saxophone (Sax) are type I receptors that act synergistically to transduce the Dpp and Scw signals, respectively (Haerry et al., 1998; Neul and Ferguson, 1998; Nguyen et al., 1998). Recently, the output of these ligands and receptors has been visualized in the early embryo using antibodies that specifically recognize either the phosphorylated form of Mad (p-Mad), the primary Drosophila BMP R-Smad, or a specific nuclear conformation of the Co-Smad Medea (Dorfman and Shilo, 2001; Raftery and Sutherland, 2003; Ross et al., 2001; Rushlow et al., 2001). These studies revealed that, during an interval of approximately 40 min, a sharp transition in p-Mad accumulation develops between those 10–12 cells that receive very high levels of BMP signal at the dorsal midline and more lateral cells that receive low levels of signal. As a result, the response to the BMP signal is highly localized near the dorsal midline.
The mechanism that generates the sharp transition between cells that receive high versus low BMP signal involves the activity of three additional extracellular proteins, Short gastrulation (Sog), Twisted gastrulation (Tsg), and Tolloid (Tld) (Ferguson and Anderson, 1992b; Francois et al., 1994; Marques et al., 1997; Mason et al., 1994; Ross et al., 2001; Shimell et al., 1991). Both Sog and Tsg are BMP binding proteins that can form a tripartite complex with Dpp, blocking it from binding to the receptor, while Tld cleaves Sog when it is bound to Dpp and releases the ligand for signaling (Marques et al., 1997; Ross et al., 2001; Shimmi and O’Connor, 2003). Scw is produced uniformly (Arora et al., 1994), while Dpp is produced only in cells within the dorsal half of the embryo (Ray et al., 1991). A current hypothesis is that Sog, Tsg, and Tld act to transport BMPs to the dorsal midline (Decotto and Ferguson, 2001; Holley et al., 1996; Marques et al., 1997; Ross et al., 2001). Sog is produced ventral-laterally and establishes a gradient over the dorsal region (Francois et al., 1994; Srinivasan et al., 2002). This gradient establishes the directionality of net Sog flux bound with BMPs, which diffuse from the lateral region toward the dorsal midline (Eldar et al., 2002; Holley et al., 1996).
While this mechanism can, in principle, explain the localized signaling output, several of its basic tenets remain untested. First, does one or more of the BMP ligands actually concentrate at the dorsal midline? Second, is the output of the two type I receptors coordinated to produce a synergistic signal, and, if so, how is this coordination achieved? Third, what properties of the network compensate for reductions in gene dos-age, thereby resulting in viable heterozygous mutants for sog, tsg, tld, and scw? In this report, we investigate these issues using both experimental and computational approaches. We find that the key for understanding all three issues is the formation of heteromeric complexes. Using a genomic epitope-tagged Dpp construct, we find that Dpp protein localizes in a narrow dorsal stripe along those midline cells that accumulate high levels of p-Mad. This protein localization depends not only on Sog and Tsg function but also on Scw, contradicting a prerequisite for reliable patterning that emerged from a previous mathematical model (Eldar et al., 2002). We show, both in tissue culture and in early embryo extracts, that Dpp and Scw form heterodimers and that the signaling activity of the Dpp/Scw heterodimer is much higher on a molar basis than either Dpp or Scw homodimers. Furthermore, we use RNAi to demonstrate that the synergistic signal produced by the heterodimer requires the activity of both Tkv and Sax. Using coimmunoprecipitation, we find that the Dpp/ Scw heterodimer shows significantly higher affinity for Sog and Tsg than does either homodimer and that the Dpp/Scw heterodimer also stimulates processing of Sog by Tld to a much greater extent than does either homodimer. Furthermore, mathematical analysis demonstrates that heteromer formation at the level of ligands (Dpp/Scw) and modulators (Sog/Tsg) can significantly buffer BMP signaling against fluctuations in gene dosage, thereby contributing to the stability and reproducibility of the early tissue patterning process.
Results
Dpp Protein Accumulates at the Dorsal Midline
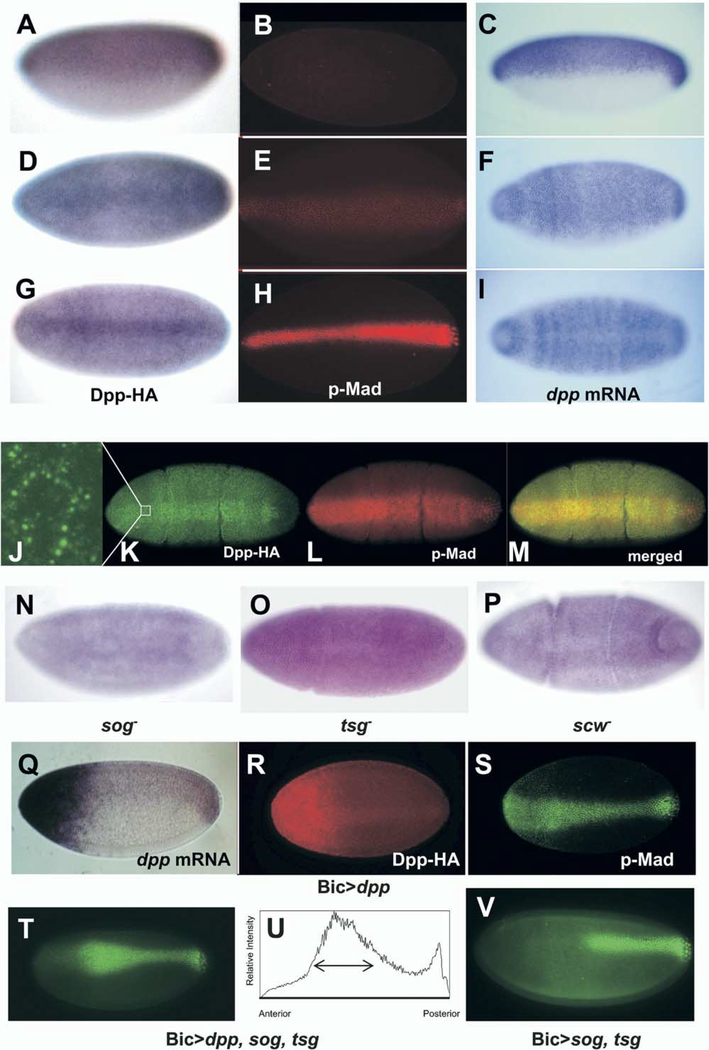
Patterning within the dorsal domain of the Drosophila embryo requires the two BMP-type ligands Dpp and Scw. Transcription of dpp begins in the precellular blastoderm embryo at stage 5 in a broad dorsal-on ventral-off pattern (Ray et al., 1991) (Figures 1C, 1F, and 1I). This transcriptional profile continues during cellularization, and then, just prior to gastrulation, expression in the dorsal-most cells fades (Figure 1I). During this same time interval, transcription of sew is uniform throughout the entire embryo and then declines as gastrulation commences (Arora et al. [1994] and data not shown). Unlike these broad patterns of transcription, the distribution of cells receiving high levels of BMP signal, as determined by accumulation of p-Mad, is much more limited (Dorfman and Shilo, 2001; Ross et al., 2001) (Figures 1B, 1E, and 1H). In the early blastoderm, p-Mad accumulation is below the level of detection. At the mid- to late-cellularization stage, p-Mad first becomes visible in a 16–18-cell-wide stripe centered about the dorsal midline. Just prior to gastrulation, this stripe refines to an intensely stained stripe, approximately 8–12 cells in width, centered on the dorsal midline.
Figure 1.
Dpp-HA Protein Forms a Dorsal Stripe during Cellularization of the Blastoderm Embryo
(A-I) Dpp-HA staining in a Dpp-HA transgenic embryo (A, D, and G), p-Mad staining (B, E, and H), and in situ hybridization of dpp mRNA (C, F, and I) in a wt embryo. Early (A-C), middle (D-F), and late (G-I) blastoderm stages, lateral view (A-C) and dorsal view (D-I). Note that Dpp protein is localized in the dorsal half of embryo at the early blastoderm stage, as is dpp mRNA. As cellularization proceeds, Dpp protein is concentrated at the dorsal midline. Also note that Dpp protein accumulates dorsally with the same kinetics that p-Mad staining does.
(J-M) Early gastrulation embryos double stained for Dpp HA (J and K, green) and pMad (L, red). Dpp-HA is seen in numerous punctate structures ([J], high-magnification view of boxed area in [K]).
(N-P) Dorsal view of Dpp-HA staining in sog (N), tsg (O), and scw (P) mutant embryos. No localization of dpp-HA into a dorsal stripe is observed. Instead, staining remains broad throughout the dorsal region.
(Q) Lateral view of dpp mRNA in Gal4-Bic>UAS-dpp-HA embryo. Strong expression of dpp was induced in the anterior end and endogenous dpp mRNA can be seen in the posterior part of embryo.
(R) Dorsal view of HA staining in Gal4-Bic>UAS-dpp-HA embryo. Dpp-HA forms a dorsal stripe even though dpp-HA is expressed only in the anterior end.
(S) Dorsal view of p-Mad staining in the same embryo of (R).
(T) Dorsal view of Gal4-Bic > UAS-dpp, sog, tsg.
(U) The p-Mad image of dorsal-most five cells in (D) was analyzed using ImageJ.
(V) Dorsal view of Gal4-Bic>UAS-sog, tsg. The anterior half of p-Mad staining is lost.
One explanation for the discrepancy between the broad dpp and scw mRNA expression patterns and the narrow distribution of cells that accumulate high levels of p-Mad has been that one or both of the extracellular ligands preferentially accumulates in the dorsal-most region through the combined action of the Sog, Tsg, and Tld proteins. To examine this, we generated a genomic hemmaglutinin (HA)-tagged form of Dpp. Transgenic flies containing genomic dpp-HA are able to rescue the dorsal patterning defects of dpp embryonic lethal alleles, indicating that the epitope tag does not interfere with the function of the gene product. As shown in Figures 1A, 1D, and 1G, the Dpp protein distribution changes with time inversely to the RNA (Figures 1C, 1F, and 1I). At the precellular stage, the mRNA and protein distributions show equivalent, broad dorsal-on and ventral-off patterns. During late cellularization, an enrichment of the Dpp-HA protein begins within the dorsal-most domain similar to the p-Mad profile (Figure 1D versus Figure 1E). Just prior to gastrulation, Dpp-HA becomes highly enriched in a dorsal midline stripe that is approximately the same width as the p-Mad stripe and noticeably absent in the mRNA profile (Figure 1G versus Figure 1H, Figure 1K versus Figures 1L and 1I). A higher magnification view (Figure 1J) reveals that the most intense stain at this stage is found in numerous punctate structures. We infer that these punctate structures are sites of internalizing Dpp associated with receptors.
Dorsal Midline Accumulation of Dpp-HA Requires Sog and Tsg
We next examined the distribution of the Dpp protein in various mutant backgrounds. It has been suggested that the Sog and Tsg proteins facilitate the flux of Dpp toward the dorsal domain via formation of a high-affinity complex with Dpp (Decotto and Ferguson, 2001; Eldar et al., 2002; Ross et al., 2001). This complex prevents Dpp from binding to receptors in the dorsal lateral region and facilitates its diffusion toward the dorsal side via the flux of Sog from its ventral lateral site of synthesis. If this model is correct, then accumulation of Dpp in the dorsal midline should be eliminated in sog and tsg mutant backgrounds. As shown in Figures 1N and 1O, homozygous sog or tsg mutant embryos fail to accumulate Dpp-HA in a dorsal stripe. In contrast, heterozygous mutant embryos still show dorsal stripe Dpp-HA accumulation (data not shown). Taken together, these findings are consistent with a facilitated diffusion model in which Sog and Tsg transport Dpp from lateral regions and move it to the dorsal midline. However, these findings on their own are also consistent with Sog and Tsg simply promoting degradation of Dpp in the dorsal lateral region. To determine if Sog and Tsg actually facilitate ligand redistribution, we used the Gal4-UAS system to overexpress Dpp-HA in the anterior end of the embryo. This results in a large amount of Dpp protein accumulating within the anterior region (Figure 1Q). The p-Mad accumulation, however, is still concentrated at the midline, although there is an expansion in the width of the p-Mad stripe at the anterior end (Figure 1S). Interestingly, some Dpp-HA protein still accumulates in a dorsal midline stripe in the posterior (Figure 1R). In this case, since the only source of Dpp-HA is from the anterior of the embryo, the dorsal midline accumulation cannot have arisen solely by degradation but must reflect movement of some Dpp-HA from its anterior site of synthesis and secretion. When Sog and Tsg are cooverexpressed with Dpp, there is inhibition of p-Mad accumulation at the anterior end followed by dramatically increased p-Mad accumulation at approximately 40% embryo length (Figures 1T and 1U). In contrast, when both Sog and Tsg are coexpressed in the absence of ectopic Dpp, inhibition of p-Mad accumulation extends to approximately 50% of embryo length (Figure 1V), and there is no increase in p-Mad accumulation. These p-Mad profiles cannot have arisen by Sog and Tsg enhancing turnover of ligand. Rather, it is more consistent with Sog and Tsg facilitating the redistribution of Dpp away from the anterior domain.
Scw Is Required for Dpp Localization and Forms a Heterodimer with Dpp
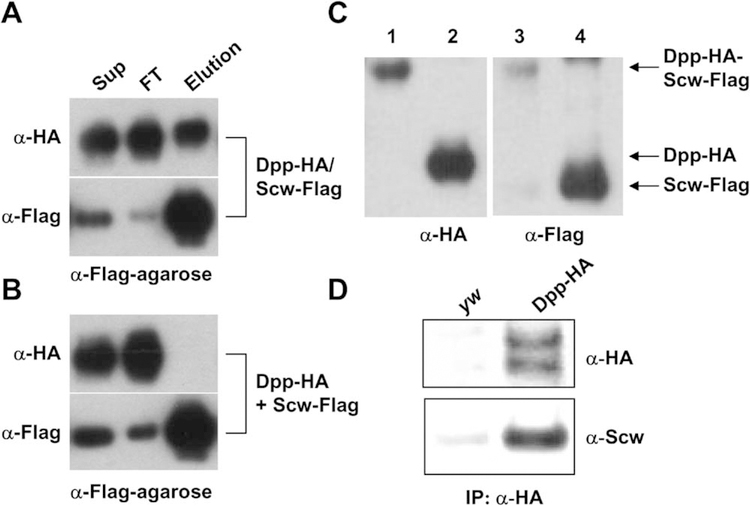
Surprisingly, we found that scw mutant embryos also failed to localize Dpp-HA to the dorsal midline (Figure 1P). The simplest explanation for this observation is that both Dpp and Scw proteins are contained in a complex with Sog and Tsg. Previous stoichiometric analysis of the vertebrate BMP/Chordin/Tsg tripartite complex indicates that one dimeric BMP molecule binds together with a Tsg dimer and a single Chordin molecule (Oelgeschlager et al., 2000). If an equivalent complex is formed by the Drosophila homologs, then the simplest explanation for the dependence of Dpp- HA localization on Scw is that the preferred ligand for binding to Sog and Tsg is a heterodimer of Dpp and Scw. To determine whether Scw and Dpp form heterodimers, we coexpressed differentially tagged forms of each protein in Drosophila Schneider S2 cells. When the conditioned media of cells coexpressing HA-tagged Dpp and Flag-tagged Scw was immunoprecipitated with anti-Flag (Figure 2A) or anti-HA antibody-coupled beads (data not shown), the two proteins were coimmunoprecipitated. However, when conditioned media from separately transfected cells are mixed (i.e., Dpp-HA homodimer and Scw-Flag homodimer), no coprecipitation was observed (Figure 2B). This demonstrates that these proteins are not simply aggregating or being brought together via bridging with some other molecule in the conditioned media. Rather, the requirement for coexpression is consistent with the view that heterodimers are formed in the endoplasmic reticulum (Gray and Mason, 1990) via a disulfide bridge linkage. To further demonstrate the physical association between these proteins, we ran the immunoprecipitated complex on nonreducing gels and found that a fraction of the Scw-Flag comigrated with the Dpp-HA at approximately 39 kd (Figure 2C), consistent with the presence of a disulfide crosslinked dimer, as has been seen for other BMP family heterodimers.
Figure 2.
Dpp and Scw Form Heterodimers
(A) HA-tagged Dpp and Flag-tagged Scw were coexpressed in S2 cells, and the conditioned medium was purified through anti-Flag M2 column. Conditioned medium (Sup), flowthrough fraction (FT), and eluted fraction by Flag peptide (Elution) were blotted and probed with anti-HA and anti-Flag antibodies. Dpp-HA was eluted from the Flag affinity column together with Scw-Flag.
(B) A mixture of Dpp-HA and Scw-Flag homodimers were loaded and eluted from the Flag affinity column. All the Dpp-HA was in the flowthrough.
(C) Purified Dpp-HA/Scw-Flag complex was analyzed by nonreduced (lanes 1 and 3) and reduced conditions (lanes 2 and 4). These gels were blotted and probed with anti-HA (lanes 1 and 2) and anti-Flag antibodies (lanes 3 and 4). The monomers of Dpp-HA and Scw-Flag show the distinct molecular weights of 25 kDa and 22 kDa, respectively (lanes 2 and 4); however, Dpp-HA/Scw-Flag shows exactly the same molecular weight of 39 kDa under nonreducing conditions (lanes 1 and 3), indicating that Dpp-HA and Scw-Flag form heterodimers.
(D) Coimmunoprecipitation of Dpp-HA and Scw in the embryo. One to three hour embryos of control (yw flies) or Dpp-HA transgenic flies were collected, and the protein extracts were precipitated with anti-HA antibody-coupled beads, then analyzed by Western blotting using anti-HA and anti-Scw antibodies. Scw protein came down together with Dpp-HA, indicating that Dpp and Scw form heterodimers in the embryo. The double Dpp band is caused by cleavage of the pro-protein at either one of two different maturation sites (O.S. and M.B.O., unpublished data).
Although these results show that Dpp and Scw can form heterodimers in tissue culture cells, we sought evidence for their presence in the blastoderm embryo when dorsal patterning takes place. To this end, we immunoprecipitated Dpp-HA from 1–3 hr embryonic extracts, and, as shown in Figure 2D, Scw was found to coprecipitate with Dpp-HA. These findings suggest that a fraction of the Dpp in early embryos is contained in a heterodimer with Scw.
The Dpp/Scw Heterodimer Signals Synergistically through the Type I Receptors Tkv and Sax
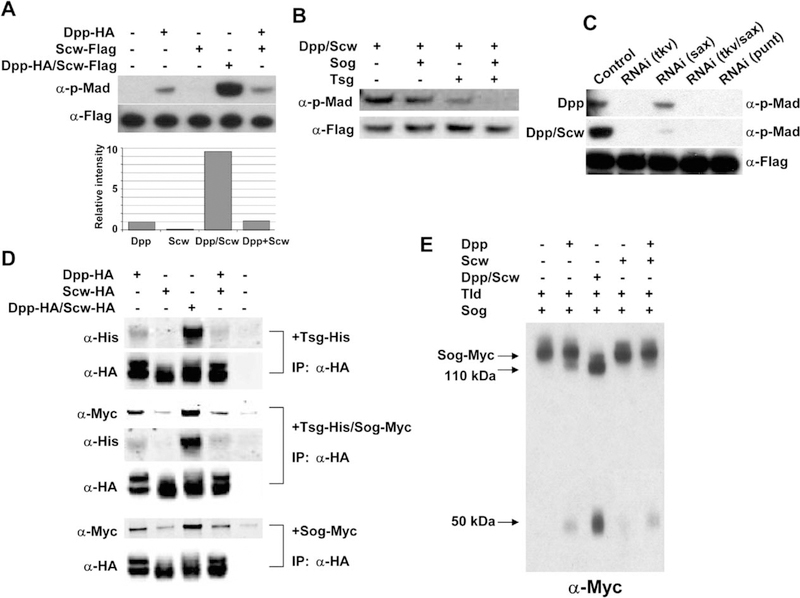
Previously, activated forms of the Drosophila BMP type I receptors Tkv and Sax have been shown to produce a synergistic signal when coexpressed in either embryos or wing imaginal discs (Haerry et al., 1998; Neul and Ferguson, 1998; Nguyen et al., 1998). The mechanism by which these receptors are activated in vivo to produce a synergistic signal is unclear. The present view is that the active signaling complex of TGF-β superfamily receptors is a tetramer composed of two type II receptors and two type I receptors bound to a dimer of ligand (Massague and Chen, 2000). Our finding that Dpp and Scw form heterodimers suggests a mechanism for producing a synergistic signal if the heterodimer preferentially binds to and activates a heteromeric complex of the Tkv and Sax receptors. To determine whether the Dpp/Scw heterodimer can produce a synergistic signal through Tkv/Sax, we used a tissue culture assay for measuring BMP signaling in S2 cells (Ross et al., 2001; Shimmi and O’Connor, 2003). S2 cells endogenously express Punt, the primary BMP type II receptor, as well as Tkv and Sax, and Mad is phosphorylated in a concentration-dependent manner when these cells are incubated with BMP ligands. Using this system, we addressed the relative signaling strengths of Dpp and Scw homodimers versus heterodimers. We found that the heterodimer was approximately 10-fold more potent than an equimolar level of Dpp homodimer (Figure 3A). In this assay, Dpp homodimers produce reasonable signals; Scw homodimers are inefficient, producing only 10%−20% of the Dpp signal; and a synergistic signal is not produced by simply mixing homodimers (Figure 3A, lane 5). This suggests that higher-order complexes of homodimers with receptors cannot result in synergistic receptor activation under our culture conditions. Since Sog and Tsg are also required to establish high levels of BMP signaling in vivo, we further tested whether either or both of these components (in various concentrations) might be able to stimulate a synergistic signal. In no case were we able to produce such a condition. Rather, a high dose of Sog or Tsg gave slight inhibition, and Sog/Tsg together showed a synergistic inhibition of Dpp/Scw heterodimer signaling similar to that reported previously for Dpp homodimers (Ross et al., 2001; Shimmi and O’Connor, 2003) (Figure 3B and data not shown).
Figure 3.
Dpp/Scw Heterodimer Shows Enhanced Signaling Compared to Homodimers and Enhanced Interactions with Sog and Tsg
(A) Comparisons of BMP activities of each ligand in a cell-based signaling assay. BMP signalings of equivalent amounts of Dpp homodimer, Scw homodimer, Dpp/Scw heterodimer, or a mixture of Dpp and Scw homodimers were measured. Dpp/Scw heterodimer has approximately 10-fold higher activity than Dpp homodimers. Scw homodimers produce only low-level signals, and a mixture of Dpp and Scw homodimers showed an activity similar to Dpp homodimers alone.
(B) Sog and Tsg synergistically inhibit Dpp/Scw heterodimer signaling. Dpp/Scw were preincubated with Sog and/or Tsg, and their BMP activities were measured.
(C) Determination of receptor requirements for homo- and heterodimer signaling by RNAi analysis. Dpp homodimer signals primarily through Tkv as a type I receptor; in contrast, synergistic signaling by Dpp/Scw heterodimer requires both Tkv and Sax as type I receptors.
(D) Coimmunoprecipitation of Tsg and/or Sog with ligands. Dpp/Scw heterodimer binds with higher affinity to Sog and Tsg than does either homodimer.
(E) Comparisons of each ligand as a catalyst for Sog cleavage by Tld.
To determine if synergistic signaling requires both Tkv and Sax or can be produced by a single receptor in response to heterodimer binding, we treated the S2 cells with dsRNA targeted against different receptors. We have shown previously that Dpp signals primarily through Tkv in S2 cells (Shimmi and O’Connor, 2003). In contrast, we find here that the heterodimer requires both Tkv and Sax to produce a synergistic signal, since phosphorylation of Mad is severely compromised when the levels of either receptor are reduced by RNAi treatment (Figure 3C). Both the homodimer and the heterodimer absolutely require the type II receptor Punt for signaling (Figure 3C, lane 5). We conclude from these results that the Dpp/Scw heterodimer is able to produce a synergistic signal that requires both Tkv and Sax.
The Dpp/Scw Heterodimer Is a Preferred Binding Partner for Sog/Tsg and Preferentially Stimulates Sog Cleavage by Tld
The facilitated transport model that leads to Dpp accumulation at the embryonic dorsal midline requires complex formation with Sog and Tsg as well as digestion of the complex by Tld to release the ligand for signaling (Eldar et al., 2002; Marques et al., 1997; Ross et al., 2001; Shimmi and O’Connor, 2003). The dependence of Dpp-HA dorsal localization on Scw suggests that the heterodimer might be the preferred substrate for Tsg/Sog binding. To test this hypothesis, we carried out coimmunoprecipitation experiments using Sog, Tsg, or a combination of the two with either homo- or heterodimers of the Dpp and Scw ligands. As shown in Figure 3D, the heterodimer has significantly higher affinity for Sog, Tsg, and the Tsg/Sog complex than does either homodimer. Of the homodimers, Dpp has higher affinity for Sog and Tsg than does Scw, which is unable to efficiently bind to either Tsg or Sog or to a combination of the two. In addition, mixing of the two homodimers with Tsg and Sog showed a profile similar to that of Dpp alone, suggesting that a multimeric high-affinity complex of homodimers with Sog and Tsg does not form. We also tested the ability of homo and heterodimers to stimulate cleavage of Sog. Once again, heterodimers are the best catalyst for stimulating Sog cleavage by Tld, while Dpp shows moderate ability and the Scw homodimer shows very little capacity to stimulate processing (Figure 3E).
The Broadly Distributed Dpp Homodimers Produce a Low Level Signal
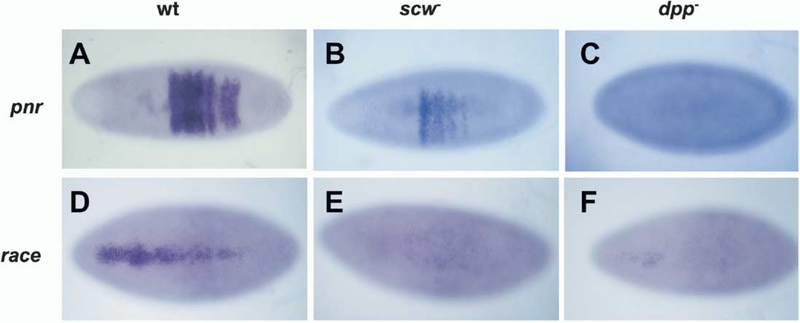
In a scw mutant, Dpp homodimers are broadly distributed (Figure 1P), and we expect that they should pro-duce a less localized, low-level signal compared to heterodimers. Since we could not detect evidence for this signal by pMad staining, we examined the expression profiles of race and pnr in dpp and scw mutant backgrounds. Activation of race requires the highest level of BMP signal, while pnr requires only a low-level signal (Ashe et al., 2000; Rushlow et al., 2001) We find that, in a dpp mutant, expression of both target genes is eliminated (Figures 4C and 4F). However, in a scw mutant embryo, expression of race is eliminated, while pnr expression is maintained, albeit in a slightly narrower weaker pattern. (Figures 4B and 4E). These findings are consistent with the cell culture observation that Dpp homodimers produce low levels of signal relative to the heterodimer and suggest that Dpp homodimers provide a functional signal in the dorsal lateral regions that are fated to become dorsal ectoderm.
Figure 4.
Analysis of Target Gene Activation in dpp and scw Mutant Backgrounds
(A-C) A dorsal view of pnr mRNA at blastoderm stage. Wild-type (A), scw mutant (B), and dpp mutant embryos (C). pnr is expressed in scw but not dpp mutant embryos.
(D-F) Dorsal view of race mRNA in wild-type embryo (D), scw mutant (E), and dpp mutant embryos (F). race is not expressed in either scw or dpp mutant embryo.
Heterodimer Formation Compensates for Perturbations
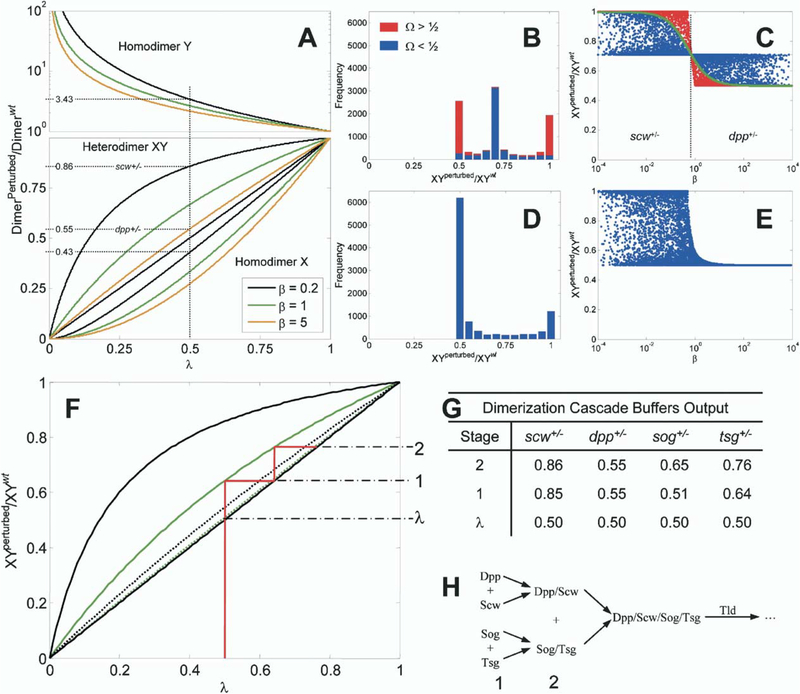
A previous model for BMP patterning demonstrated Dpp and Scw accumulation at the dorsal midline as the result of facilitated transport of the BMPs by the inhibitors Sog and Tsg (Eldar et al., 2002). Experimentally, it was found that the patterning is robust with respect to heterozygous mutations of scw+/−, tsg+/−, sog+/−, and tld+/−, yielding dorsal region patterning that is indistinguishable when compared to wild-type. However, in the mathematical model, this robustness could only be achieved through the decoupling of Dpp and Scw by the action of Sog and Tsg, thereby leading to independent and parallel mechanisms for the localization of the Scw and Dpp homodimers (Eldar et al., 2002). Our data show that Dpp and Scw localization is highly coupled and that Dpp and Scw form a heterodimer in vivo that patterns the dorsal midline. These observations suggest that robustness of patterning with respect to gene dose is achieved by an alternative mechanism. To examine how the Dpp/Scw heterodimer affects the robustness of BMP patterning, we formulated a kinetic scheme that includes all possible dimerization reactions between Dpp and Scw and analyzed the sensitivity of the heterodimer output to changes in the monomer input.
The resulting kinetic equations for the evolution of the five species (two monomers and three dimers) are shown (Figure 5), where X represents Scw and Y represents Dpp. The steady-state level of each species is found by setting the time derivatives equal to zero and solving the resulting algebraic equations. To measure the degree of compensation gained by forming heterodimers of Dpp and Scw, the production rate (ϕ) of one monomer is perturbed (e.g., mutant) from its wild-type (wt) value by either increasing or decreasing the rate at which it is supplied to the system. The effect of the perturbation is measured by , which represents the ratio of the output level of the heterodimer in the perturbed or mutant case to the wt case. If this ratio equals 1, then the output of Dpp/Scw in the perturbed case is equal to the base case (perfect compensation), whereas, if this ratio is <1 (>1), there is a decrease (increase) in heterodimer output for a given change of input.
Figure 5.
Analysis of Dimerization Reactions
The analysis of Equations 1–5 shows that the steady-state solutions depend upon three combinations of the nine system parameters (Figure 5). First, we define , which is the ratio of the wild-type production rates for monomer Dpp to monomer Scw. Second, we define the perturbation parameter as the ratio of the production rate for monomer Scw in the perturbed state to the production rate of Scw in the wt state. Lastly, we define , which is the ratio of the overall heterodimer formation rate to twice the geometric mean of the overall homodimer formation rates. This leads to the dimensionless Equations 6–10, which can be solved to obtain the dependence of the heterodimer and homodimer ratios on system parameters given in Equations 13–15. The simplest case occurs for Ω = 1/2, which arises, for instance, when all the dimerization steps occur at the same rates. Then the change in the outputs to perturbations of the input is given by:
| (16) |
| (17) |
| (18) |
| (19) |
| (20) |
| (21) |
Plots of the heterodimer and homodimer ratios as a function of λ for different values of β are shown in Figure 6A.
Figure 6.
Heterodimer Formation Compensates for Reductions of Input
(A) Ratio of dimer output for reductions of a monomer input. Heterodimer, homodimer X, and homodimer Y are shown for Ω = 1/2, and β = 0.2 (black), 1 (green), or 5 (orange). An example calculation is shown for a 50% (λ = 1/2) reduction of Scw and for Dpp.
(B and C) Histogram and β dependence of computational results of 10,000 simulations with randomly chosen parameter values for Ω ≠ 1/2. Red data correspond to solutions in which heterodimer formation is dominant (Ω > 1/2), and blue data are for homodimer-dominant processes (Ω < 1/2). A green line for Ω = 1/2 is shown in (C) for reference.
(D and E) Same as (B-(C) except for Sog/Tsg case with linear degradation and no homodimer formation.
(F-H) Binding cascade enhances robustness to reductions of input monomer. (F) Heterodimer ratios for Dpp/Scw (black) and Sog/Tsg complex (green) are shown for Ω = 1/2, β = 0.2 (solid), and β = 5 (dotted). An example dimerization and binding cascade (solid red line) leading to robust BMP signaling is shown for the case of tsg+/− embryos. This demonstrates graphically the enhanced buffering obtained by the sequence of binding reactions shown in (H). The perturbation λ and output from each stage are shown on the right of (F). Example output results for each stage are shown in (G) for scw+/−, dpp+/−, sog+/−, and tsg+/− mutants for given values of Ω = 1/2, β = 0.2, 5.
Dpp/Scw Formation Compensates for Reductions of Scw but Not for Reductions of Dpp
Experimentally, we have observed that p-Mad signaling is unchanged in scw+/− embryos but is extremely low in dpp+/− embryos (data not shown). To explain the advantage of heterodimer formation as simply as possible, suppose that Ω = 1/2, β = 1/5 (Scw monomer production is five times as large as for Dpp), and λ = 1/2. Then the heterodimer ratio (Equation 17) can be determined from Figure 6A by drawing a vertical line at λ = 1/2. From the intersections, one sees that , , and so the heterodimer Dpp/Scw is reduced by only 14% for a 50% reduction in the level of Scw.
The same is not true for reductions of Dpp. By redefining the symbols X and Y to be Dpp and Scw, respectively, and redefining and , we can determine the level of compensation in heterodimer formation for the reductions in Dpp expected in dpp+/− embryos. Now, β = 5, and the ratios can be determined as before, which leads to , , and . Thus, the Dpp/Scw levels are reduced by 45% for a 50% reduction in the levels of Dpp.
In general, Ω ≠ 1/2, and solutions for the heterodimer ratio are given by Equation 13. To determine the extent of buffering for Ω ≠ 1/2, we varied each of the reaction rates, transport coefficients, and production rates over five orders of magnitude in the known range for biologically feasible rate constants. For each set of parameters, the heterodimer ratio is calculated for λ = 1/2 (a 50% reduction in the input of one of the monomers). A histogram of the computational results (Figure 6B) and the ratio of the heterodimer versus β are shown (Figure 6C). Data points and histogram columns corresponding to Ω > 1/2 (heterodimer formation dominant) are shown in red, while blue corresponds to Ω < ½ (homodimer formation dominant). The case Ω = 1/2 is shown as a solid green line for reference. Interestingly, for small , which applies for reductions in Scw, the heterodimer ratio is bounded between a minimum of or ~0.71 and a maximum of one. Thus, regardless of parameter values chosen, heterodimer formation buffers reductions of gene dosage for scw+/− embryos if Scw is in slight excess. For large β (β > 0.71), which corresponds to reductions of Dpp, the output of Dpp/Scw is more sensitive to reductions in the input, and the ratio of mutant to wt lies between a minimum of 0.5 (no compensation) and a maximum of 0.71. This clearly shows that, for any parameter values, the system is less sensitive to reductions of Scw than of Dpp when measured by the level of the Dpp/Scw heterodimer.
Discussion
The suggestion that facilitated transport of a BMP signaling molecule (Holley et al., 1996) might be the primary mechanism that generates pattern within the dorsal domain of the Drosophila blastoderm embryo was a conceptual breakthrough, since it could account for the paradoxical abilities of Sog and Tsg to have both positive and negative effects on patterning. However, there was no direct evidence that either Dpp or Scw actually concentrated to the midline. In addition, it did not explain the roles of Dpp and Scw in producing the restricted high-level signaling output at the midline, as measured by p-Mad accumulation, nor did it explain how a lower level of signal was maintained in the more lateral regions to help fate the future dorsal ectoderm. Lastly, it was not apparent how the system achieves resiliency to changes in gene dosages of certain components. The experimental and computational observations described here address these issues.
Heterodimers Help Establish Peak Signal Level by Synergistic Activation of Tkv and Sax
One of our primary findings is that Dpp and Scw form heterodimers both in tissue culture and in vivo and that these heterodimers are able to synergistically stimulate phosphorylation of Mad in cell culture. Since the Dpp/Scw heterodimers have highest affinity for Sog and Tsg, we infer that the heterodimer is the primary ligand transported dorsally by Sog and Tsg, resulting in high levels of p-Mad accumulation at the dorsal midline just prior to gastrulation. Consistent with this view, we find that Dpp localization to the midline depends on Scw.
In addition to heterodimers being the preferred translocated species, the heterodimer model also explains the mechanism by which Scw contributes to dorsal patterning. This issue has been enigmatic since scw and its receptor, Sax, are expressed ubiquitously in the early embryo, yet signal output is limited to dorsal cells. In addition, misexpression of Scw or activated Sax produces very limited effects in most tissues, while misexpression of Dpp or activated Tkv results in very dramatic consequences. A partial resolution to this issue was suggested by the finding that coexpression of activated Sax and activated Tkv in embryos or imaginal discs produces a synergistic signal impling that both the Sax and Tkv signals are necessary for a robust output (Haerry et al., 1998; Neul and Ferguson, 1998; Nguyen et al., 1998). However, it has remained unclear whether endogenous, nonactivated receptors can produce a synergistic signal in response to ligands. As we describe here, the formation of a heterodimer between Dpp and Scw resolves these issues. In tissue culture assays, Scw homodimers produce very limited signal, while Dpp homodimers produce a moderate signal requiring only the Tkv receptor. The differential signaling ability of each homodimer explains their nonequivalence in producing patterning abnormalities when mis-expressed during development. In contrast, the Dpp/Scw heterodimer is able to produce a synergistic phosphorylation of Mad that requires both the Tkv and Sax receptors; simply mixing homodimers is not sufficient. These observations demonstrate that synergistic signaling occurs at the level of receptor-mediated Mad phosphorylation and not through integration of separate signals at downstream targets. The molecular mechanism by which the Tkv and Sax receptors produce a synergistic output remains unclear.
Although the original role for Scw in dorsal patterning invoked formation of a heterodimer as the primary signaling species (Arora et al., 1994), this model fell into disfavor because ventral injection of scw mRNA or ventral expression of scw from the twist promoter can partially rescue amnioserosa formation (Neul and Ferguson, 1998; Nguyen et al., 1998). Since disulfide- linked heterodimer formation of TGF-β type ligands is known to occur in the Golgi during the secretion process (Gray and Mason, 1990), ventral expression of Scw without Dpp should preclude formation of heterodimers, and, therefore, any rescuing activity should be brought about by homodimers. Although some rescue was observed in these experiments, it is important to note that even multiple copies of ventrally expressed Scw did not lead to viability. In contrast, a single copy of Scw expressed in the dorsal domain using the tld promoter gave complete viability and fertility (Arora et al., 1994). In addition, these experiments assume that there is no internalization within the dorsal domain of Scw homodimers followed by isomerization with Dpp and resecretion. This possibility is mechanistically very similar to models in which Dpp is proposed to undergo transcytosis (Entchev et al., 2000). Therefore, while ventral overexpression of Scw homodimers may have some ability to compensate for loss of Scw dorsally, normal patterning is most efficiently achieved when Scw is expressed in a domain in which heterodimers can form.
Heterodimers and Homodimers Generate a Biphasic Signaling Profile
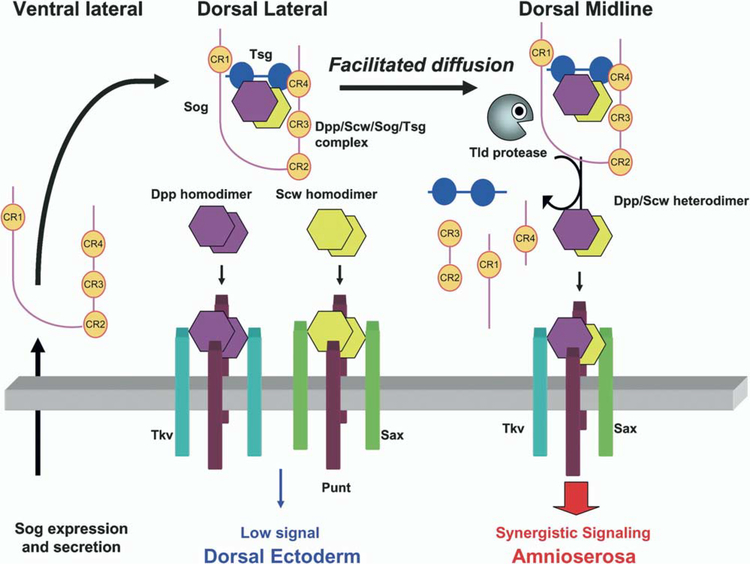
BMP-directed patterning of dorsal blastoderm cells ultimately results in the specification of of two tissues, amnioserosa and dorsal ectoderm. In general, these tissues derive from cells receiving high and low BMP signal, respectively. Whether there are additional cell fate subdivisions specified within the steep signaling transition zone (Raftery and Sutherland, 2003) is not clear, although cells can discriminate subtle signaling differences as evidenced by the slightly wider expression pattern of the BMP target genes rho and usp compared to zen and race (Ashe et al., 2000; Rushlow et al., 2001). Although both Dpp and Scw are required to establish the high point of signaling necessary to specify amnioserosa, only Dpp is needed to specify dorsal ectoderm. This is consistent with our observations that the Dpp/Scw heterodimer will be preferentially concentrated at the midline because of its high affinity for Sog and Tsg. In contrast, Dpp and Scw homodimers will be more broadly distributed because of their lower affinities for Sog and Tsg. Although we cannot directly distinguish the different species in vivo, analysis of downstream target genes in a scw mutant embryo revealed that there is sufficient BMP activity to activate pnr transcription, but its pattern is very wide, consistent with the observed broad distribution of Dpp homodimers. In the wild-type case, Dpp and Scw homodimers, together with a small number of heterodimers that escape from Sog and Tsg, may contribute to signaling in the lateral ectoderm, since the pnr signal is stronger in wild-type than in scw mutants. These homodimers also likely signal in a repressive manner to prevent ectopic transcription of neurogenic genes within the dorsal domain (Biehs et al., 1996). Thus, patterning of dorsal tissue appears to take advantage of the differing properties of homo- and heterodimers to establish a biphasic signaling state. Specifically, selective transport of the heterodimer and synergistic receptor signaling produce a restricted high point and amnioserosa cell fate, while Dpp and Scw homodimers generate a broad low level of signal that help fate the future dorsal ectoderm and restrict neurogenic activity to more lateral regions (Figure 7). It is likely that the full specification of dorsal ectoderm does not occur until a second round of dpp transcription takes place after germ band extension (Dorfman and Shilo, 2001). It is also likely that additional components help reinforce the formation of the biphasic state, since recent genetic data indicate that tight localization of Dpp to the midline requires an initial phase of low-level Dpp signal reception (E.L. Ferguson, personal communication). The suggestion is that this initial low-level Dpp signal induces expression of an additional component that participates in the localization process. The identity of this component remains elusive.
Figure 7.
Schematic Model for Patterning Dorsal Tissues in the Drosophila Embryo
Homodimers and heterodimers of Dpp and Scw are produced throughout the dorsal domain. Sog is expressed and secreted in the ventral lateral region and diffuses toward the dorsal side. Dpp and Scw homodimers do not bind to Sog and Tsg with high affinity. As a result, they are free to bind with receptors, but they produce only low-level signals that can activate targets such as pnr. In contrast, Dpp/Scw heterodimers bind with high affinity to Sog and Tsg. Net flux of the complex toward the dorsal midline leads to an increase in the heterodimer concentration at the midline. Tld processes Sog at the midline to release the ligand, which then binds to a receptor complex containing both Sax and Tkv. This complex produces a synergistic high signal that activates high-level response genes such as race and leads to specification of the amnioserosa.
Lastly, we note that employment of heterodimers in early embryonic patterning may be a common theme. In zebrafish, both BMP2b and BMP7 are required for dorsal-ventral patterning, and loss-of-function mutations in each gene exhibit identical severely dorsalized phenotypes. Since this phenotype is not enhanced in double mutants and overexpression of these two gene products reveals synergy in the ventralization of wildtype embryos, it has been suggested that BMP2a/BMP7 heterodimers are the primary molecules that specify ventral cell fates in this organism (Schmid et al., 2000). These observations further highlight the overall similarity in the molecular components used to pattern the early zebrafish and Drosophila embryos (Holley and Ferguson, 1997; Schier, 2001).
Implications of Heterodimer Formation for Robustness
Use of the Dpp/Scw heterodimer provides the patterning system with an effective buffer at a very early step in dorsal cell fate specification. Buffering for reductions of Scw or Dpp is predominantly determined by the relative monomer production rates, and if Scw is in slight excess with respect to Dpp, reductions in the levels of Scw will have little effect on the output Dpp/Scw heterodimer, regardless of the specific choices of parameters (Figure 6C).
Patterning is also resilient to reductions of Sog and Tsg (data not shown, Eldar et al. [2002]). We have shown that Sog and Tsg have synergistic BMP binding activity and that the concentration of Sog/Tsg in the PV space is governed by the interaction of reaction and diffusion. However, the kinetic equations for Sog/Tsg complex formation are similar to Equations 1–3 (see Supplemental Data available with this article online). The Sog/Tsg ratio can be computed as described for Dpp/Scw to determine the compensation in this subsystem (Figures 6D and 6E), and the results are different from those for Dpp/Scw. Now there are two distinct solution regions, one for small β (Figure 6E, left) where many choices of parameters provide significant compensation for reductions of gene dosage, and one for large β where there is virtually no compensation. Because the behavior for large β and small β is very different, this analysis can explain the compensation for reductions in either Sog or Tsg but not both. This suggests that other mechanisms must be involved to explain the experimentally observed resilience in both sog and tsg heterozygous embryos. These could include the following: (1) the spatial separation of Sog and Tsg expression, (2) downstream kinetic mechanisms that compensate after Sog/Tsg formation, or (3) both. Both may contribute, but we focus on the possible effects of compensation in downstream kinetic interactions.
After Sog/Tsg formation, the next step downstream is the binding of the inhibitor Sog/Tsg to Dpp/Scw. Experimentally, we observe that Tolloid cleavage of Sog is greatly enhanced when bound to Dpp/Scw (Figure 3E) and is enhanced in the presence of Tsg (Shimmi and O’Connor, 2003). In addition, a previous mathematical model of BMP patterning suggested that cleavage of Sog only when bound in the complex Sog/BMP is a requirement for the system to exhibit resilience to changes in gene dose of sog, tsg, or scw (Eldar et al., 2002). These data support the idea that Dpp/Scw transported from the broad dorsal region must be released from the Sog/Tsg/Dpp/Scw complex. Interestingly, the local dynamics of Sog/Tsg + Dpp/Scw complex formation are completely analogous to the local dynamics for Sog + Tsg complex formation. This suggests that, if the level of Dpp/Scw or Sog/Tsg is decreased from the original wt levels, the output complex Sog/Tsg/Dpp/Scw would be less affected. Taken together, the Sog/Tsg and Sog/Tsg/Dpp/Scw steps lead to a cascade in which the compensation in the first step is enhanced in the second step. In effect, the output from one complex formation stage becomes the input substrate for the next stage. Of course, the level of buffering achieved depends on the system parameters, as illustrated in Figures 6F–6H for a two-level cascade. The output from stage one is found for the initial reduction (λ = 1/2), and a horizontal line is drawn to y = x line since the output from 1 becomes the input to stage 2. Example results for scw+/− dpp+/−, sog+/−, and tsg+/− are summarized in Figure 6H. Here, a hierarchy is easily seen and suggests that patterning would be most compensated for reduction of Scw, followed by Tsg, then Sog, and lastly Dpp. Of course, other downstream steps may also contribute to compensation.
In reality, patterning involves diffusive transport as well, but our analysis shows how a cascade of stages can produce compensation in the kinetic steps. When our full BMP patterning model that incorporates transport is compared to a previous model mediated by homodimers and monomers, there are approximately 100 times more “robust” hits when scw+/−, sog+/−, tsg+/−, and tld+/− cases are considered (our unpublished data). In principle, the binding cascade analysis extends to other systems and can be used to explore other changes of input, including overexpression of a protein.
Experimental Procedures
Fly Stocks
Genomic Dpp-HA lines were obtained by the injection of Casper4-genomic dpp-HA using standard protocols. The tsgXB86, sogP, dppH48 flies were used as null mutants. Df(2L)OD16 was used as scw null mutant. Bicoid-Gal4 flies were from Ethan Bier. UAS-dppHA, UAS-sog, tsg flies were described previously (Ross et al., 2001).
Constructs
Dpp-HA, Tsg-His, Sog-Myc, Tld-HA, and Mad-Flag for tissue culture transfections were described previously (Ross et al., 2001; Shimmi and O’Connor, 2003). For generation of Scw-Flag, the Flag tag sequence (KLDYKDDDDK) was inserted between P297 and Q298 of scw cDNA by PCR, and the resulting fragment was subcloned into an actin promoter vector for protein production in S2 cells. For genomic dpp-HA, an 8 kb EcoRI fragment containing the entire dpp coding sequence and intron of the RE transcript species (Flybase) was cloned into bluescript II (Stratagene). The triple HA epitope (RIFYPYDVPDYAGYPYDVPDYAGSYPYDVPDYAAQC) was inserted in-frame as a NotI fragment into an engineered NotI site positioned between D486 and T487 of the Dpp coding sequence.
Immunostaining and In Situ Hybridization
Rabbit anti-phospho Mad antibody was a gift from P. ten Dijke and used as previously described (Shimmi and O’Connor, 2003). AntiRat HA monoclonal antibody 3F10 (Roche) was used at 1/500 dilution. Secondary antibodies against rat IgG were either alkaline phosphatase coupled (Promega) or Alex 486 coupled (Molecular Probes) and were used at 1/1000 or 1/500, respectively. In situ hybridization of dpp, pnr, and race to whole-mount embryos were performed with digoxigenin-labeled RNA probes and visualized with alkaline phosphatase precipitates as previously described (Ross et al., 2001).
Production and Purification of Recombinant Proteins
Drosophila S2 cells were used for producing recombinant proteins as previously described (Ross et al., 2001). To obtain Dpp/Scw heterodimers, 10 μg of HA-tagged dpp and 5 μg Flag-tagged scw DNAs were cotransfected into S2 cells. The supernatants were collected 5 days after transfection and applied to anti-Flag M2 antibody-coupled agarose (Sigma), and Dpp/Scw heterodimers were eluted by the application of Flag peptide. Reverse phase chromatography (C8) was used to separate DppHA/ScwFlag heterodimers from Scw-Flag homodimers, and most fractions of affinity-purified sample were found to contain Dpp/Scw heterodimers but not Scw homodimers. Since these highly purified Dpp/Scw heterodimers yielded exactly same results in signaling assays as the less-pure Flag affinity-purified Dpp/Scw heterodimers, Flag affinity-purified samples were used in most experiments.
Cell-Based Signaling Assay and RNA Interference
A cell-based assay for BMP signaling was described previously (Ross et al., 2001; Shimmi and O’Connor, 2003). For RNA interference, the PCR primers used to make templates for the production of dsRNA for the Tkv, Sax, and Punt receptors were as previously described (Shimmi and O’Connor, 2003). S2 cells transfected by flag-mad were incubated with dsRNA of tkv, sax, tkv/sax, or punt. Three days after transfection, cells were split and incubated with each ligand for 3 hr, and cell extracts were analyzed by Western blotting. Protein samples were run on 4–12 gradient NuPAGE gels (Invitrogen) and developed for immunoblotting as described previously (Shimmi and O’Connor, 2003).
Immunoprecipitation
To detect Dpp/Scw heterodimers in vivo, 1–3 hr embryos from Dpp-HA transgenic flies or control yw flies were collected, dechorinized with 50% bleach, and stored at −80°C for further use. One gram of embryos was homogenized in a Pyrex ground glass homogenizer (Fisher 7725–13) in 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% BSA containing 1× protease inhibitor cocktail (Roche). After spinning down the homogenates, the collected supernatants were incubated overnight at 4°C with biotinylated anti-HA 3F10 antibody (Roche) and Avidin-coupled agarose (Pierce). The beads were washed extensively and then extracted with 1× SDS-PAGE sample buffer at 90°C for 10 min. Dpp-HA and Scw proteins were detected with anti-HA (1/1000) and anti-Scw (1/100) (a gift from Kavita Arora) antibodies respectively. For detection of Sog and Tsg interactions with ligands, equal amounts of Dpp-HA, Scw-HA, coexpressed Dpp-HA/Scw-HA, or a mixture of Dpp-HA and Scw-HA were incubated with Sog-Myc and/or Tsg-His for 3 hr at 25°C. The samples were then incubated overnight at 4°C with anti-HA antibody-coupled beads. The beads were washed and extracted by 1× SDS-PAGE sample buffer at 90°C for 10 min, then probed with anti-HA 12CA5 (1/1000), anti-Myc A14 (Santa Cruz Biotechnology, 1/1000), and anti-His (Qiagen, 1/1000) antibodies.
In Vitro Cleavage Assay
Mixtures of purified Sog-myc and Tld-HA were incubated with the equivalent amounts of Dpp-HA, Scw-Flag, Dpp-HA/Scw-Flag heterodimer, or a mixture of Dpp-HA and Scw-Flag for 12 hr at 25°C in the presence of 1× reaction buffer, and Sog fragments were analyzed by Western blotting using anti-Myc antibody as described previously (Shimmi and O’Connor, 2003).
Supplementary Material
Acknowledgments
We thank MaryJane Shimell and David Zhitomirsky for help in producing the Dpp-HA and Scw-HA genomic transgenes. We thank Kavita Arora for the gift of Scw antibody. The manuscript was improved by the thoughtful editing of MaryJane Shimell and Guillermo Marques and several anonymous reviewers. We thank Yu Chiun Wang, Chip Ferguson, and Arthur Lander for communication of results prior to publication. This work was funded in part by PHS grant GM29123 (to H.O.). D.U. is supported by the NIH Biotechnology Training Grant. M.B.O. is an Investigator with the Howard Hughes Medical Institute.
Footnotes
Supplemental Data
Supplemental Data include supplemental text and can be found with this article online at http://www.cell.com/cgi/content/full/120/6/873/DC1/.
References
- Arora K, Levine MS, and O’Connor MB (1994). The screw gene encodes a ubiquitously expressed member of the TGF-beta family required for specification of dorsal cell fates in the Drosophila embryo. Genes Dev. 8, 2588–2601. [DOI] [PubMed] [Google Scholar]
- Ashe HL, Mannervik M, and Levine M (2000). Dpp signaling thresholds in the dorsal ectoderm of the Drosophila embryo. Development 127, 3305–3312. [DOI] [PubMed] [Google Scholar]
- Biehs B, Francois V, and Bier E (1996). The Drosophila short gastrulation gene prevents Dpp from autoactivating and suppressing neurogenesis in the neuroectoderm. Genes Dev. 10, 2922–2934. [DOI] [PubMed] [Google Scholar]
- Decotto E, and Ferguson EL (2001). A positive role for Short gastrulation in modulating BMP signaling during dorsoventral patterning in the Drosophila embryo. Development 128, 3831–3841. [DOI] [PubMed] [Google Scholar]
- Dorfman R, and Shilo BZ (2001). Biphasic activation of the BMP pathway patterns the Drosophila embryonic dorsal region. Development 128, 965–972. [DOI] [PubMed] [Google Scholar]
- Eldar A, Dorfman R, Weiss D, Ashe H, Shilo BZ, and Barkai N (2002). Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature 419, 304–308. [DOI] [PubMed] [Google Scholar]
- Entchev EV, Schwabedissen A, and Gonzalez-Gaitan M (2000). Gradient formation of the TGF-beta homolog Dpp. Cell 103, 981–991. [DOI] [PubMed] [Google Scholar]
- Ferguson EL, and Anderson KV (1992a). Decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophila embryo. Cell 71, 451–461. [DOI] [PubMed] [Google Scholar]
- Ferguson EL, and Anderson KV (1992b). Localized enhancement and repression of the activity of the TGF-beta family member, decapentaplegic, is necessary for dorsal-ventral pattern formation in the Drosophila embryo. Development 114, 583–597. [DOI] [PubMed] [Google Scholar]
- Francois V, Solloway M, O’Neill JW, Emery J, and Bier E (1994). Dorsal-ventral patterning of the Drosophila embryo depends on a putative negative growth factor encoded by the short gastrulation gene. Genes Dev. 8, 2602–2616. [DOI] [PubMed] [Google Scholar]
- Gray AM, and Mason AJ (1990). Requirement for activin A and transforming growth factor-beta 1 pro-regions in homodimer assembly. Science 247, 1328–1330. [DOI] [PubMed] [Google Scholar]
- Haerry TE, Khalsa O, O’Connor MB, and Wharton KA (1998). Synergistic signaling by two BMP ligands through the SAX and TKV receptors controls wing growth and patterning in Drosophila. Development 125, 3977–3987. [DOI] [PubMed] [Google Scholar]
- Holley SA, and Ferguson EL (1997). Fish are like flies are like frogs: conservation of dorsal-ventral patterning mechanisms. Bioessays 19, 281–284. [DOI] [PubMed] [Google Scholar]
- Holley SA, Neul JL, Attisano L, Wrana JL, Sasai Y, O’Connor MB, De Robertis EM, and Ferguson EL (1996). The Xenopus dorsalizing factor noggin ventralizes Drosophila embryos by preventing DPP from activating its receptor. Cell 86, 607–617. [DOI] [PubMed] [Google Scholar]
- Letsou A, Arora K, Wrana JL, Simin K, Twombly V, Jamal J, Staehling-Hampton K, Hoffmann FM, Gelbart WM, Massague J, et al. (1995). Drosophila Dpp signaling is mediated by the punt gene product: a dual ligand-binding type II receptor of the TGF beta receptor family. Cell 80, 899–908. [DOI] [PubMed] [Google Scholar]
- Marques G, Musacchio M, Shimell MJ, Wunnenberg-Stapleton K, Cho KW, and O’Connor MB (1997). Production of a DPP activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell 91, 417–426. [DOI] [PubMed] [Google Scholar]
- Mason ED, Konrad KD, Webb CD, and Marsh JL (1994). Dorsal midline fate in Drosophila embryos requires twisted gastrulation, a gene encoding a secreted protein related to human connective tissue growth factor. Genes Dev. 8, 1489–1501. [DOI] [PubMed] [Google Scholar]
- Massague J, and Chen YG (2000). Controlling TGF-beta signaling. Genes Dev. 14, 627–644. [PubMed] [Google Scholar]
- Neul JL, and Ferguson EL (1998). Spatially restricted activation of the SAX receptor by SCW modulates DPP/TKV signaling in Drosophila dorsal-ventral patterning. Cell 95, 483–494. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Park S, Marques G, and Arora K (1998). Interpretation of a BMP activity gradient in Drosophila embryos depends on synergistic signaling by two type I receptors, SAX and TKV. Cell 95, 495–506. [DOI] [PubMed] [Google Scholar]
- Oelgeschlager M, Larrain J, Geissert D, and De Robertis EM (2000). The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature 405, 757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett RW, St Johnston RD, and Gelbart WM (1987). A transcript from a Drosophila pattern gene predicts a protein homologous to the transforming growth factor-beta family. Nature 325, 81–84. [DOI] [PubMed] [Google Scholar]
- Raftery LA, and Sutherland DJ (2003). Gradients and thresholds: BMP response gradients unveiled in Drosophila embryos. Trends Genet. 19, 701–708. [DOI] [PubMed] [Google Scholar]
- Ray RP, Arora K, Nusslein-Volhard C, and Gelbart WM (1991). The control of cell fate along the dorsal-ventral axis of the Drosophila embryo. Development 113, 35–54. [DOI] [PubMed] [Google Scholar]
- Ross JJ, Shimmi O, Vilmos P, Petryk A, Kim H, Gaudenz K, Hermanson S, Ekker SC, O’Connor MB, and Marsh JL (2001). Twisted gastrulation is a conserved extracellular BMP antagonist. Nature 410, 479–483. [DOI] [PubMed] [Google Scholar]
- Ruberte E, Marty T, Nellen D, Affolter M, and Basler K (1995). An absolute requirement for both the type II and type I receptors, punt and thick veins, for dpp signaling in vivo. Cell 80, 889–897. [DOI] [PubMed] [Google Scholar]
- Rushlow C, Colosimo PF, Lin MC, Xu M, and Kirov N (2001). Transcriptional regulation of the Drosophila gene zen by competing Smad and Brinker inputs. Genes Dev. 15, 340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier AF (2001). Axis formation and patterning in zebrafish. Curr. Opin. Genet. Dev 11, 393–404. [DOI] [PubMed] [Google Scholar]
- Schmid B, Furthauer M, Connors SA, Trout J, Thisse B, Thisse C, and Mullins MC (2000). Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development 127, 957–967. [DOI] [PubMed] [Google Scholar]
- Shimell MJ, Ferguson EL, Childs SR, and O’Connor MB (1991). The Drosophila dorsal-ventral patterning gene tolloid is related to human bone morphogenetic protein 1. Cell 67, 469–481. [DOI] [PubMed] [Google Scholar]
- Shimmi O, and O’Connor MB (2003). Physical properties of Tld, Sog, Tsg, and Dpp protein interactions are predicted to help create a sharp boundary in Bmp signals during dorsoventral patterning of the Drosophila embryo. Development 130, 4673–4682. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Rashka KE, and Bier E (2002). Creation of a Sog morphogen gradient in the Drosophila embryo. Dev. Cell 2, 91–101. [DOI] [PubMed] [Google Scholar]
- Wharton KA, Ray RP, and Gelbart WM (1993). An activity gradient of decapentaplegic is necessary for the specification of dorsal pattern elements in the Drosophila embryo. Development 117, 807–822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.