Abstract
Background
Ventilator‐associated pneumonia (VAP) is defined as pneumonia developing in people who have received mechanical ventilation for at least 48 hours. VAP is a potentially serious complication in these patients who are already critically ill. Oral hygiene care (OHC), using either a mouthrinse, gel, toothbrush, or combination, together with aspiration of secretions, may reduce the risk of VAP in these patients.
Objectives
To assess the effects of oral hygiene care on incidence of ventilator‐associated pneumonia in critically ill patients receiving mechanical ventilation in hospital intensive care units (ICUs).
Search methods
We searched the following electronic databases: Cochrane Oral Health’s Trials Register (to 17 December 2015), the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2015, Issue 11), MEDLINE Ovid (1946 to 17 December 2015), Embase Ovid (1980 to 17 December 2015), LILACS BIREME Virtual Health Library (1982 to 17 December 2015), CINAHL EBSCO (1937 to 17 December 2016), Chinese Biomedical Literature Database (1978 to 14 January 2013), China National Knowledge Infrastructure (1994 to 14 January 2013), Wan Fang Database (January 1984 to 14 January 2013) and VIP Database (January 2012 to 4 May 2016). We searched ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform for ongoing trials to 17 December 2015. We placed no restrictions on the language or date of publication when searching the electronic databases.
Selection criteria
We included randomised controlled trials (RCTs) evaluating the effects of OHC (mouthrinse, swab, toothbrush or combination) in critically ill patients receiving mechanical ventilation for at least 48 hours.
Data collection and analysis
At least two review authors independently assessed search results, extracted data and assessed risk of bias in included studies. We contacted study authors for additional information. We pooled data from trials with similar interventions and outcomes. We reported risk ratio (RR) for dichotomous outcomes and mean difference (MD) for continuous outcomes, using random‐effects models unless there were fewer than four studies.
Main results
We included 38 RCTs (6016 participants). There were four main comparisons: chlorhexidine (CHX) mouthrinse or gel versus placebo/usual care; toothbrushing versus no toothbrushing; powered versus manual toothbrushing; and comparisons of oral care solutions. We assessed the overall risk of bias as low in five trials (13%), high in 26 trials (68%), and unclear in seven trials (18%). We did not consider the risk of bias to be serious when assessing the quality of evidence (GRADE) for VAP incidence, but we downgraded other outcomes for risk of bias.
High quality evidence from 18 RCTs (2451 participants, 86% adults) shows that CHX mouthrinse or gel, as part of OHC, reduces the risk of VAP compared to placebo or usual care from 24% to about 18% (RR 0.75, 95% confidence intervals (CI) 0.62 to 0.91, P = 0.004, I2 = 35%). This is equivalent to a number needed to treat for an additional beneficial outcome (NNTB) of 17 (95% CI 9 to 50), which indicates that for every 17 ventilated patients in intensive care receiving OHC including chlorhexidine, one outcome of VAP would be prevented. There is no evidence of a difference between CHX and placebo/usual care for the outcomes of mortality (RR 1.09, 95% CI 0.96 to 1.23, P = 0.20, I2 = 0%, 14 RCTs, 2014 participants, moderate quality evidence), duration of mechanical ventilation (MD ‐0.09 days, 95% CI ‐1.73 to 1.55 days, P = 0.91, I2 = 36%, five RCTs, 800 participants, low quality evidence), or duration of intensive care unit (ICU) stay (MD 0.21 days, 95% CI ‐1.48 to 1.89 days, P = 0.81, I2 = 9%, six RCTs, 833 participants, moderate quality evidence). There is insufficient evidence to determine the effect of CHX on duration of systemic antibiotics, oral health indices, caregivers' preferences or cost. Only two studies reported any adverse effects, and these were mild with similar frequency in CHX and control groups.
We are uncertain as to the effects of toothbrushing (± antiseptics) on the outcomes of VAP (RR 0.69, 95% CI 0.44 to 1.09, P = 0.11, I2 = 64%, five RCTs, 889 participants, very low quality evidence) and mortality (RR 0.87, 95% CI 0.70 to 1.09, P = 0.24, I2 = 0%, five RCTs, 889 participants, low quality evidence) compared to OHC without toothbrushing (± antiseptics). There is insufficient evidence to determine whether toothbrushing affects duration of mechanical ventilation, duration of ICU stay, use of systemic antibiotics, oral health indices, adverse effects, caregivers' preferences or cost.
Only one trial (78 participants) compared use of a powered toothbrush with a manual toothbrush, providing insufficient evidence to determine the effect on any of the outcomes of this review.
Fifteen trials compared various other oral care solutions. There is very weak evidence that povidone iodine mouthrinse is more effective than saline/placebo (RR 0.69, 95% CI 0.50 to 0.95, P = 0.02, I2 = 74%, three studies, 356 participants, high risk of bias), and that saline rinse is more effective than saline swab (RR 0.47, 95% CI 0.37 to 0.62, P < 0.001, I2 = 84%, four studies, 488 participants, high risk of bias) in reducing VAP. Due to variation in comparisons and outcomes among trials, there is insufficient evidence concerning the effects of other oral care solutions.
Authors' conclusions
OHC including chlorhexidine mouthwash or gel reduces the risk of developing ventilator‐associated pneumonia in critically ill patients from 24% to about 18%. However, there is no evidence of a difference in the outcomes of mortality, duration of mechanical ventilation or duration of ICU stay. There is no evidence that OHC including both antiseptics and toothbrushing is different from OHC with antiseptics alone, and some weak evidence to suggest that povidone iodine mouthrinse is more effective than saline/placebo, and saline rinse is more effective than saline swab in reducing VAP. There is insufficient evidence to determine whether powered toothbrushing or other oral care solutions are effective in reducing VAP. There is also insufficient evidence to determine whether any of the interventions evaluated in the studies are associated with adverse effects.
Plain language summary
Oral hygiene care for critically ill patients to prevent ventilator‐associated pneumonia
Review question
What are the effects of oral hygiene care on the incidence of ventilator‐associated pneumonia in critically ill patients receiving mechanical ventilation in hospital intensive care units (ICUs)? We aimed to summarise all the available appropriate research in order to identify evidence‐based care for these vulnerable patients.
Background
Critically ill people, who may be unconscious or sedated while they are treated in ICUs, often need to have machines to help them breathe (ventilators). The use of these machines for more than 48 hours may result in ventilator‐associated pneumonia (VAP). VAP is a potentially serious complication in these patients who are already critically ill.
Oral hygiene care, using a mouthrinse, gel, toothbrush, or combination, together with suctioning secretions, may reduce the risk of VAP in these patients.
Study characteristics
This review of studies was carried out through Cochrane Oral Health, and the evidence is current up to 17 December 2015.
We included 38 research studies but only a few (13%) of the studies were well conducted and described.
All of the studies took place in ICUs in hospitals. In total there were 6016 participants randomly allocated to treatment. Participants were critically ill and required assistance from nursing staff for their oral hygiene care. Most of the studies involved adults only, but the participants were children in three of the studies, and newborns in one study.
We grouped studies into four main comparisons.
1. Chlorhexidine antiseptic mouthrinse or gel compared to placebo (treatment without the active ingredient chlorhexidine) or usual care, (with or without toothbrushing) 2. Toothbrushing compared with no toothbrushing (with or without antiseptics) 3. Powered compared with manual toothbrushing 4. Oral care solutions with other solutions
Key results
We found high quality evidence that chlorhexidine, either as a mouthrinse or a gel, reduces the risk of VAP from 24% to about 18%. For every 17 people on ventilators for more than 48 hours in intensive care, the use of oral hygiene care including chlorhexidine will prevent one person developing VAP. However, we found no evidence that oral hygiene care with chlorhexidine makes a difference to the numbers of patients who die in ICU, or to the number of days on mechanical ventilation or days in ICU.
We have only limited evidence on the effects of toothbrushing (with or without antiseptics) and oral care without toothbrushing (with or without antiseptics) on the risk of developing VAP. Three studies showed some weak evidence of a reduction in VAP with povidone iodine antiseptic mouthrinse compared to placebo/saline. Four studies showed some weak evidence of a reduction in VAP with saline rinse compared to saline swab.
There was insufficient evidence to determine whether any of the interventions evaluated in the studies are associated with any unwanted side effects.
Quality of the evidence
The evidence presented was limited by how well the included studies were done and reported. Only 13% of the studies were well conducted and well described. For a number of outcomes, there was not enough information to draw a solid conclusion.
Summary of findings
Summary of findings for the main comparison. Chlorhexidine (mouthrinse or gel) versus placebo/usual care for critically ill patients to prevent ventilator‐associated pneumonia.
| Chlorhexidine (mouthrinse or gel) versus placebo/usual care for critically ill patients to prevent ventilator‐associated pneumonia (VAP) | ||||||
|
Patient or population: critically ill adults and children receiving mechanical ventilation
Settings: intensive care units (ICU)
Intervention: chlorhexidine (mouthrinse or gel) Comparison: placebo or usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control (placebo or usual care) | Chlorhexidine (mouthrinse or gel) | |||||
| Ventilator‐associated pneumonia Follow‐up: mean 1 month | 243 per 10001 | 180 per 1000 (148 to 221) | RR 0.75 (0.62 to 0.91) | 2451 (18 studies) | ⊕⊕⊕⊕ high | This equates to an NNTB of 17 (95% CI 9 to 50) |
| Mortality Follow‐up: mean 1 month | 222 per 10001 | 242 per 1000 (213 to 273) | RR 1.09 (0.96 to 1.23) | 2014 (14 studies) | ⊕⊕⊕⊝ moderate2 | |
| Duration of ventilation Days of ventilation required Follow‐up: mean 1 month | The mean duration of ventilation in the control groups ranged from 7 to 18 days | The mean duration of ventilation in the intervention groups was 0.09 days fewer (1.73 fewer to 1.55 more) | 800 (5 studies) | ⊕⊕⊝⊝ low3 | ||
| Duration of ICU stay Follow‐up: mean 1 month | The mean duration of ICU stay in the control groups ranged from 10 to 24 days | The mean duration of ICU stay in the intervention groups was 0.21 days more (1.48 fewer to 1.89 more) | 833 (6 studies) | ⊕⊕⊕⊝ moderate 4 |
||
| Adverse effects | Most of the studies did not provide information on adverse events. Information on adverse events were identified from 2 studies. One study stated there were none, the other study reported on mild reversible irritation of the oral mucosa | ⊕⊝⊝⊝ very low5 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
1Assumed risk is based on the median event rate in the control groups of the included studies.
2Downgraded one level due to serious risk of bias: eight studies at high risk of bias, four at unclear risk of bias and three at low risk of bias. The sensitivity analysis based on three low‐risk‐of‐bias studies gave similar effect estimate (RR = 1.13), but further research may change this estimate.
3Downgraded two levels due to serious imprecision and serious risk of bias: two studies at high risk of bias, three at low risk of bias. The sensitivity analysis based on three studies at low risk of bias gave an effect estimate of 0.84 days, which is not clinically important in the context of median duration of 12 days.
4Downgraded one level due to serious imprecision.
5Downgraded three levels due to very serious imprecision and serious inconsistency: only two studies reported on this outcome, and they did not report data adequately to enable us to evaluate the risk of adverse events.
Summary of findings 2. Toothbrushing (± antiseptics) versus no toothbrushing (± antiseptics) for critically ill patients to prevent ventilator‐associated pneumonia.
| Toothbrushing (± antiseptics) versus no toothbrushing (± antiseptics) for critically ill patients to prevent ventilator‐associated pneumonia (VAP) | ||||||
|
Patient or population: critically ill patients receiving mechanical ventilation
Settings: intensive care units (ICUs)
Intervention: toothbrushing (± chlorhexidine) Comparison: no toothbrushing (± chlorhexidine) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No toothbrushing | Toothbrushing | |||||
|
Incidence of VAP Follow‐up: mean 1 month |
367 per 10001 | 253 per 1000 (161 to 400) | RR 0.69 (0.44 to 1.09) | 889 (5 studies)2 | ⊕⊝⊝⊝ very low3 | |
| Mortality Follow‐up: mean 1 month | 236 per 10001 | 205 per 1000 (165 to 257) | RR 0.87 (0.70 to 1.09) | 889 (5 studies)2 | ⊕⊕⊝⊝ low4 | |
| Duration of ventilation Follow‐up: mean 1 month | The mean duration of ventilation in the control groups ranged from 9.8 to 10 days | The mean duration of ventilation in the intervention groups was 0.11 days fewer (0.90 fewer to 0.68 more) | 644 (3 studies) | ⊕⊕⊝⊝ low5 | ||
| Duration of ICU stay Follow‐up: mean 1 month | The mean duration of ICU stay in the control groups ranged from 13 to 15 days | The mean duration of ICU stay in the intervention groups was 1.82 days fewer (3.95 fewer to 0.32 more) | 583 (2 studies) | ⊕⊝⊝⊝ very low6 | ||
| Adverse effects | Most of the studies did not provide information on adverse events. Information on adverse events was identified from one study which stated there was none. | ⊕⊝⊝⊝ very low7 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
1Assumed risk is based on the outcomes in the control groups of the included studies 2Three studies compared toothbrushing + chlorhexidine with chlorhexidine alone, one study compared toothbrushing with no toothbrushing (no chlorhexidine in either group), another study compared toothbrushing + povidone iodine with povidone iodine alone. 3Downgraded three levels due to serious imprecision, substantial heterogeneity (I2 = 64%) and very serious risk of bias: five studies at high risk of bias. 4Downgraded two levels due to very serious risk of bias: five studies at high risk of bias. 5Downgraded two levels due to very serious risk of bias: three studies at high risk of bias. 6Downgraded three levels due to very serious imprecision and serious risk of bias: two studies at high risk of bias. 7Downgraded three levels due to very serious imprecision and serious inconsistency: only one study reported on this outcome, with data which did not enable us to evaluate the risk of adverse events.
Background
Description of the condition
Patients in intensive care units (ICUs) in hospital frequently require mechanical ventilation because their ability to breathe unassisted is impaired due to trauma, or as a result of a medical condition or recent surgery. These critically ill patients are also dependent on hospital staff to meet their needs for nutrition and hygiene, including oral hygiene.
Overall, the research suggests that oral health deteriorates following admission to a critical care unit (Terezakis 2011). Intubation and critical illness reduce oral immunity, may be associated with mechanical injury of the mouth or respiratory tract, increase the likelihood of dry mouth, and the presence of the endotracheal tube may also make access for oral care more difficult (Alhazzani 2013; Labeau 2011). Dental plaque accumulates rapidly in the mouths of critically ill patients and as the amount of plaque increases, colonisation by microbial pathogens is likely (Fourrier 1998; Scannapieco 1992). Plaque colonisation may be exacerbated in the absence of adequate oral hygiene care and by the drying of the oral cavity due to prolonged mouth opening, which reduces the buffering and cleansing effects of saliva. In addition, the patient's normal defence mechanisms for resisting infection may be impaired (Alhazzani 2013; Terpenning 2005). Dental plaque is a complex biofilm which, once formed, is relatively resistant to chemical control, requiring mechanical disruption (such as toothbrushing) for maximum impact (Marsh 2010).
One of the complications that may develop in ventilated patients is ventilator‐associated pneumonia (VAP). VAP is generally defined as a pneumonia developing in a patient who has received mechanical ventilation for at least 48 hours (ATS Guideline 2005). It is thought that the endotracheal tube, which delivers the necessary oxygen to the patient, may also act as a conduit for pathogenic bacteria, which multiply in the oral cavity and move down the tube into the lungs. Micro‐aspiration of pharyngeal secretions may also occur around an imperfect seal of the cuff of the endotracheal tube in a ventilated patient. Several studies have shown that micro‐aspiration contributes to the development of nosocomial pneumonia (Azoulay 2006; Mojon 2002; Scannapieco 1992).
VAP is a relatively common nosocomial infection in critically ill patients, with a reported prevalence ranging between 6% and 52% (Apostolopoulou 2003; Edwards 2009), with some indications that incidence is decreasing as understanding of the risk factors and preventative measures improves. A recent study estimated the attributable mortality of VAP to be 10% (Melsen 2011). Cohort studies have found that duration of ICU stay is increased in patients who develop VAP, but it is unclear whether this is cause or effect (Apostolopoulou 2003; Cook 1998).
Antibiotics, administered either intra‐orally as topical pastes or systemically, have been used to prevent VAP, and these interventions are evaluated in other Cochrane systematic reviews (D'Amico 2009; Selim 2010). Topical antibiotic pastes have been shown to be effective but are not widely used because of the risk of developing antibiotic‐resistant organisms (Panchabhai 2009). However, overuse of antibiotics is associated with the development of multidrug‐resistant pathogens and therefore there is merit in using other approaches for preventing infections such as VAP.
Description of the intervention
This systematic review evaluates various types of oral hygiene care as a means of reducing the incidence of VAP in critically ill patients receiving mechanical ventilation for at least 48 hours. Oral hygiene care is promoted in clinical guidelines as a means of reducing the incidence of VAP, but the evidence base is limited (Tablan 2004).
Oral hygiene care includes the use of mouthrinses (water, saline, antiseptics) applied either as sprays, liquids, or with a swab, with or without toothbrushing (either manual or powered) and toothpaste, to remove plaque and debris from the oral cavity. Oral hygiene care also involves suction to remove excess fluid, toothpaste, and debris, and may be followed by the application of an antiseptic gel. Antiseptics are broadly defined to include saline, chlorhexidine, povidone iodine, cetylpyridium, and possibly others, (but exclude antibiotics).
How the intervention might work
Patients on mechanical ventilation often have a very dry mouth due to prolonged mouth opening, which may be exacerbated by the side effects of medications used in their treatment. In healthy individuals, saliva functions to maintain oral health through its lubricating, antibacterial, and buffering properties (Labeau 2011), but patients on ventilators lack sufficient saliva for this to occur, and the usual stimuli for saliva production are absent.
Routine oral hygiene care is designed to remove plaque and debris, as well as replacing some of the functions of saliva, moistening and rinsing the mouth. Toothbrushing, with either a manual or powered toothbrush, removes plaque from teeth and gums and disrupts the biofilm within which plaque bacteria multiply (Whittaker 1996; Zanatta 2011). It is hypothesised that using an antiseptic, such as chlorhexidine gluconate or povidone iodine, as either a rinse or a gel, may further reduce the bacterial load or delay a subsequent increase in bacterial load.
However, it is important, that during oral hygiene care, the plaque and debris are removed from the oral cavity with care in order to avoid aspiration of contaminated fluids into the respiratory tract. Raising the head of the bed, and careful use of appropriately‐maintained closed suction systems, together with an appropriately‐fitted cuff around the endotracheal tube are other important aspects of care of critically ill patients that are not part of this systematic review.
Why it is important to do this review
Cochrane Oral Health undertook an extensive prioritisation exercise in 2014 to identify a core portfolio of titles that were the most clinically important reviews to maintain on the Cochrane Library (Worthington 2015). The periodontal expert panel identified this review as a priority topic (Cochrane OHG priority review portfolio).
Other Cochrane Reviews have evaluated the use of topical antibiotic pastes applied to the oral cavity (selective oral decontamination D'Amico 2009), probiotics (Hao 2015), and systemic antibiotics (Selim 2010) to prevent VAP. Other published reviews have evaluated aspects of oral hygiene care, such as toothbrushing (Alhazzani 2013) or use of chlorhexidine (Pineda 2006), and broader reviews have noted the lack of available evidence (Berry 2007; Shi 2004). Clinical guidelines recommend the use of oral hygiene care, but there is a lack of available evidence as a basis for specifying the essential components of such care (Muscedere 2008; Tablan 2004). Hypersensitivity is a rare but potentially severe side effect of chlorhexidine. In view of recent reports in the UK of two cases of serious adverse events associated with irrigation of dry socket with chlorhexidine mouthrinse (Pemberton 2012), establishing the safety of oral hygiene care including chlorhexidine is also important.
The goal of this Cochrane Review was to evaluate all oral hygiene care interventions (excluding the use of antibiotics) used in ICU for patients on ventilators for at least 48 hours, to determine the effects of oral hygiene care on the development of VAP. We planned to summarise all the available research in order to facilitate the provision of evidence‐based care for these vulnerable patients.
Objectives
To assess the effects of oral hygiene care on incidence of ventilator‐associated pneumonia in critically ill patients receiving mechanical ventilation in hospital intensive care units (ICUs).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of oral hygiene care interventions. We did not consider quasi‐randomised studies for inclusion.
Types of participants
Critically ill patients in hospital settings receiving mechanical ventilation for a minimum of 48 hours, without ventilator‐associated pneumonia or respiratory infection at baseline. We included trials where only some of the participants were receiving mechanical ventilation if the outcome of ventilator‐associated pneumonia was reported, and data were available for those who had been treated with mechanical ventilation for a minimum of 48 hours and then developed nosocomial pneumonia.
We included trials where participants were undergoing a surgical procedure that involved mechanical ventilation (e.g. cardiac surgery) only if the oral hygiene care was given during the period of mechanical ventilation that had a minimum duration of 48 hours. We excluded trials where patients received a single preoperative dose of antibacterial rinse or gargle, and received mechanical ventilation only for the duration of the surgery, with no further mechanical ventilation and oral hygiene care during the postoperative period.
Types of interventions
Intervention group: received clearly‐defined oral care procedures such as nurse‐assisted toothbrushing, oral and pharyngeal cavity rinse, decontamination of oropharyngeal cavities with antiseptics;
Control group: received no treatment, placebo, 'usual care', or a different specific oral hygiene care procedure.
We excluded trials where the intervention being evaluated was a type of suction system or variation of method, timing, or place where mechanical ventilation was introduced (e.g. emergency room or ICU).
We excluded trials of selective decontamination using topical antibiotics administered to the oral cavity or oropharynx, because these interventions are covered in another Cochrane Review (D'Amico 2009). We also excluded trials of probiotics administered to prevent respiratory infections, as these are covered in a separate review (Hao 2015).
Types of outcome measures
We included studies that aimed to assess at least one of our primary outcomes.
Primary outcomes
Incidence of VAP (defined as pneumonia developing in a patient who has received mechanical ventilation for at least 48 hours)
Mortality (either ICU mortality if these data were available, or 30‐day mortality)
Secondary outcomes
Duration of mechanical ventilation or ICU stay, or both
Systemic antibiotic use
Oral health indices such as gingival index, plaque index, bleeding index, periodontal index, etc.
Adverse effects of the interventions
Caregivers' preferences for oral hygiene care
Economic data
Search methods for identification of studies
To identify studies for this review, we developed detailed search strategies for each database searched. These were based on the search strategy developed for MEDLINE Ovid but revised appropriately for each database. The search strategy used a combination of controlled vocabulary and free‐text terms. The Embase subject search was linked to Cochrane Oral Health's filter for identifying clinical trials in EMBASE Ovid.
Electronic searches
We searched the following electronic databases.
Cochrane Oral Health's Trials Register (searched 17 December 2015) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 11) in the Cochrane Library (searched 17 December 2015) (Appendix 2);
Ovid MEDLINE (1946 to 17 December 2015) (Appendix 3);
Ovid Embase (1980 to 17 December 2015) (Appendix 4);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 17 December 2015) (Appendix 5);
LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database; from 1982 to 17 December 2015) (Appendix 6);
Chinese Biomedical Literature Database (1978 to 14 January 2013) (Appendix 7);
China National Knowledge Infrastructure (1994 to 14 January 2013) (Appendix 8);
Wan Fang Database (1984 to 14 January 2013) (Appendix 9);
VIP Database (January 2012 to 4 May 2016) (Appendix 10).
We included all relevant publications irrespective of language. For this update, we did not conduct searches of the Chinese Biomedical Literature Database, the China National Knowledge Infrastructure or the Wan Fang Database. We found these databases to be adequately covered by searches of the VIP Database.
Searching other resources
We searched the following trials registries for ongoing studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 17 December 2015) (see Appendix 11);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 17 December 2015) (see Appendix 12).
We manually checked all the references lists of the included studies to identify any additional studies.
We contacted the first or corresponding authors of the included studies, other experts in the field, and manufacturers of oral hygiene products to request unpublished relevant information.
Data collection and analysis
Selection of studies
At least two of six review authors independently examined each title and abstract of articles obtained from the searches. We resolved disagreements by discussion. We linked multiple reports from a study, and designated the report with the most complete follow‐up data as the primary source of data.
We obtained copies of potentially relevant reports and examined them in detail to determine whether the study fulfilled the eligibility criteria. We resolved any queries by discussion. We attempted to contact study authors to obtain additional information as necessary. We excluded studies when the only information available was from the abstract and this was insufficient to enable full assessment of risk of bias.
Data extraction and management
At least two of six review authors independently extracted data from each included study onto predesigned structured data extraction forms. We resolved any disagreements by discussion. We extracted the following items:
General characteristics of the study: authors, year of publication, country where the study was performed, funding, language of publication, study duration, citation, contact details for the authors and identifier.
Specific trial characteristics: we collected basic study design characteristics: sequence generation, allocation sequence concealment, blinding, incomplete outcome data and selective outcome reporting, etc., and presented them in the table of 'Characteristics of included studies. We included verbatim quotes on the first three issues from original reports.
Participants: total number, setting, age, sex, country, ethnicity, socio‐demographic details (e.g. education level), diagnostic criteria for VAP and the presence of comorbid conditions.
Interventions: we collected details of all experimental and control interventions, such as dosages for drugs used and routes of delivery, format for oral hygiene care, timing and duration of the oral care procedures. We also collected information on any co‐interventions administered.
Outcomes: we collected the incidence of VAP or other respiratory diseases and mortality (directly and indirectly attributable), duration of mechanical ventilation, duration of ICU stay, systemic antibiotic use, oral health indices, and adverse outcomes resulting from the interventions, etc. We specified all outcome variables in terms of definition, timing, units and scales.
Other results: we also collected summary statistics, sample size, key conclusions, comments and any explanations provided for unexpected findings by the study authors. We contacted the lead authors of included studies if there were issues to be clarified.
Assessment of risk of bias in included studies
At least two of six review authors assessed the risk of bias of each included study, using the Cochrane domain‐based, two‐part tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We contacted study authors for clarification or missing information where necessary. We resolved any disagreements concerning risk of bias by discussion. We completed a 'Risk of bias' table for each included study. For each domain of risk of bias, we described what was reported to have happened in the study in order to provide a rationale for the second part, which involved assigning a judgement of 'low risk' of bias, 'high risk' of bias, or 'unclear risk' of bias.
For each included study, we assessed the following seven domains of risk of bias.
Random sequence generation (selection bias): use of simple randomisation (e.g. random‐number table, computer‐generated randomisation, central randomisation by a specialised unit), restricted randomisation (e.g. random permuted blocks), stratified randomisation and minimisation were assessed as low risk of bias. Other forms of simple randomisation such as repeated coin‐tossing, throwing dice or dealing cards were also considered as low risk of bias (Schulz 2002). Where a study report used the phrase 'randomised' or 'random allocation' but with no further information, we assessed it as unclear for this domain.
Allocation concealment (selection bias): use of centralised/remote allocation, pharmacy‐controlled randomisation and sequentially‐numbered, sealed, opaque envelopes were assessed as low risk of bias. If a study report did not mention allocation concealment, we assessed it as unclear for this domain.
Blinding of participants and personnel (performance bias): participants in included studies were in intensive care and on mechanical ventilation and were therefore unlikely to be aware of the treatment group to which they were assigned. We therefore assessed caregiver and outcome assessor blinding. Where no placebo was used, caregivers would be aware of the assigned intervention and this would introduce a risk of performance bias. If a study was described as double‐blind and a placebo was used, we assumed that caregivers and outcome assessors were blinded to the allocated treatment. If blinding was not mentioned and no placebo was used, we assumed that no blinding of caregivers occurred and we assessed this domain as being at high risk of bias.
Blinding of outcome assessment (detection bias): if outcome assessor blinding was not mentioned in the trial report, we assessed this domain as being at unclear risk of bias.
Incomplete outcome data (attrition bias): where the overall rate of attrition was high, we assessed the risk of attrition bias as high. If numbers of participants and/or reasons for exclusion were different in each arm of the study, we assessed the risk of attrition bias as high. If numbers of participants randomised or evaluated in each arm of the study were not reported, we assessed this domain as unclear.
Selective reporting (reporting bias): if the study did not report outcomes stated in the Methods section, or reported outcomes without estimates of variance, we assessed this as being at high risk of reporting bias.
Other bias: any other potential source of bias that might feasibly alter the magnitude of the effect estimate, e.g. baseline imbalance between study arms in important prognostic factors (e.g. clinical pulmonary infection scores (CPIS), antibiotic exposure), early stopping of the trial, or co‐interventions or differences in other treatment between study arms. We described any other potential sources of bias and assessed their risk of bias.
We summarised the risks of bias as follows.
| Risk of bias | Interpretation | In outcome | In included studies |
| Low risk of bias | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
We present the 'Risk of bias' graphically by: (a) proportion of studies with each judgement (low, high, or 'unclear risk of bias) for each domain, and (b) cross‐tabulation of judgements by study and by domain.
Measures of treatment effect
For dichotomous outcomes, we computed the effect measure as the risk ratio (RR) together with the 95% confidence interval (CI). For continuous outcomes, we used the mean difference (MD) with 95% CI to estimate the summary effect. If different scales were used, we calculated standardised mean differences.
Unit of analysis issues
The unit of analysis was the participant. The indices of plaque and gingivitis were measured as mean values for the participants. Episodes of care were also related back to individual participants.
Dealing with missing data
We contacted the lead author of studies requesting that they supply any missing data. We planned to obtain missing standard deviations using the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
To detect heterogeneity among studies in a meta‐analysis, we applied a Chi2 test with a 0.10 level of significance as the cut‐off value. We quantified the impact of statistical heterogeneity using the I2 statistic. To interpret the results, we used the thresholds of I2 recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
If considerable heterogeneity existed, we investigated it, using subgroup analyses to investigate possible differences between the studies.
Assessment of reporting biases
Only a proportion of research projects conducted are ultimately published in an indexed journal and become easily identifiable for inclusion in systematic reviews. Reporting biases arise when the reporting of research findings is influenced by the nature and direction of the findings of the research. We investigated and attempted to minimise potential reporting biases in this review, including publication bias, time lag bias, multiple (duplicate) publication bias, and language bias.
Where there were more than 10 studies in an outcome, we constructed a funnel plot. We planned to investigate the asymmetry in the funnel plot (indicating possible publication bias) by undertaking statistical analysis using the methods introduced by Egger 1997 (continuous outcome) and Rücker 2008 (dichotomous outcome) (such analysis would have been done in Stata).
Data synthesis
We undertook meta‐analyses for similar comparisons and the same outcomes across studies. We used random‐effects models providing there were four or more trials in any one meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We proposed one subgroup analysis a priori. We decided to undertake a subgroup analysis according to whether participants' teeth were cleaned or not, as we hypothesised that antiseptics would be less effective if toothbrushing was not used to disrupt dental plaque biofilm.
Sensitivity analysis
To determine whether the intervention effects of oral hygiene care were robust, we planned sensitivity analyses to assess the effect on the estimates of effect of studies with questionable diagnostic criteria for VAP, studies with high risk of bias, or by changing our assumptions about missing data.
If the results had not changed substantially in sensitivity analyses, we would have regarded our conclusions as stable with a higher degree of certainty. If sensitivity analyses had identified particular factors that greatly influenced the conclusions of the review, we would have explored the plausible causes of the uncertainties and interpreted the results with more caution.
Summary of findings
We adopted the GRADE system for evaluating quality of the evidence of systematic reviews (Guyatt 2008; Higgins 2011), using the software GRADEprofiler. We included the following outcomes in the 'Summary of findings' tables: incidence of VAP, mortality, duration of ventilation, duration of ICU stay, and adverse effects. We assessed the quality of the body of evidence with reference to the overall risk of bias of the included studies, the directness of the evidence, the consistency of the results, the precision of the estimates, and the risk of publication bias. We classified the quality of the body of evidence into four categories: high, moderate, low and very low.
Results
Description of studies
Results of the search
For this review update, after removal of duplicates, we identified 317 records from electronic databases and other resources. At least two review authors screened all records against the review inclusion criteria. We discarded 253 records and requested full‐text copies of 64 references. At least two review authors assessed these papers to determine their eligibility, and from these, we deemed 38 studies eligible for inclusion.
Three previously included studies (Grap 2004; McCartt 2010; Needleman 2011) have been excluded from this update (see Characteristics of excluded studies for details).Two studies are awaiting classification because we have not yet obtained adequate information about them. The study flow diagram is shown in Figure 1.
1.
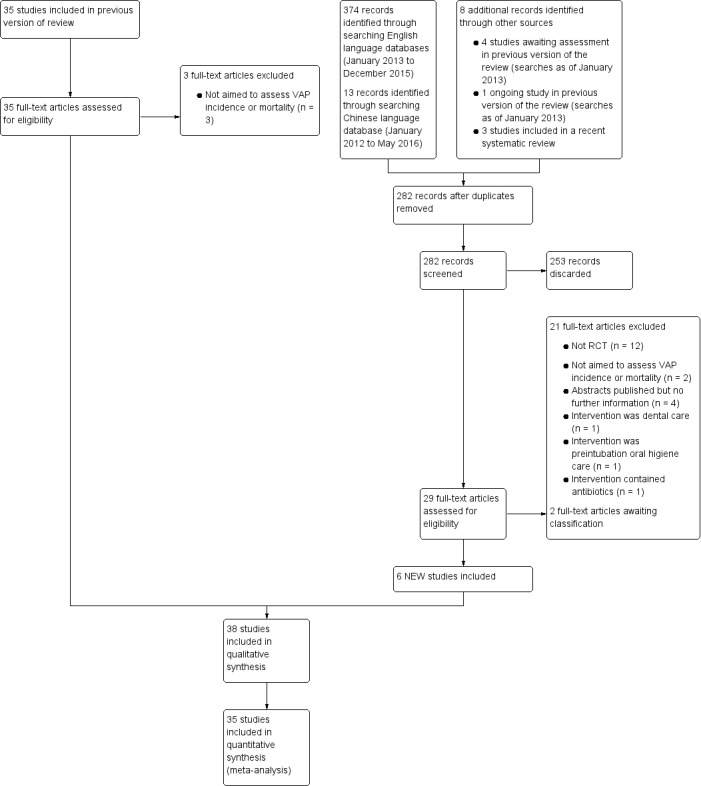
Study flow diagram
Included studies
We included 38 RCTs in this review.
Setting
Eight of the included studies were conducted in the USA (Bopp 2006; DeRiso 1996; Fields 2008; Grap 2011; Munro 2009; Prendergast 2012; Scannapieco 2009; Stefanescu 2013), nine in China (Chen 2008; Feng 2012; Hu 2009; Long 2012; Mo 2016; Tang 2013; Xu 2007; Xu 2008; Zhao 2012), five in Brazil (Bellissimo‐Rodrigues 2009; Caruso 2009; Jacomo 2011; Kusahara 2012a; Meinberg 2012), four in France (Fourrier 2000; Fourrier 2005; Seguin 2006; Seguin 2014) and three in Spain (Lorente 2012; Pobo 2009; Roca Biosca 2011), two in India (Panchabhai 2009; Sebastian 2012), two in Australia (Berry 2011; Berry 2013), and one each in Croatia (Cabov 2010), Taiwan (Yao 2011), Thailand (Tantipong 2008), Turkey (Ozcaka 2012), the Netherlands (Koeman 2006).
All studies took place in ICUs in hospitals. Most of the studies were two‐arm parallel group RCTs, but five studies had three arms (Berry 2011; Berry 2013; Scannapieco 2009; Seguin 2006; Xu 2007), and one study had four arms (Munro 2009).
Participants
There were 6016 participants randomly allocated to treatment in 37 RCTs, and the other trial (Fields 2008) did not state how many participants were included. The criteria for inclusion in these studies generally specified no prior intubation, no clinically‐apparent pneumonia at baseline (other than Sebastian 2012, where most of the children admitted to ICU had pneumonia already and criteria of the Centers for Disease Control (CDC) were strictly applied to diagnose subsequent VAP), and an expected requirement for mechanical ventilation for a minimum of 48 hours. Participants were critically ill and required assistance from nursing staff for their oral hygiene care. In three of the included studies, participants were children (Jacomo 2011; Kusahara 2012a; Sebastian 2012); in one study, participants were neonates (Stefanescu 2013); and in the remaining studies, only adults participated.
In six studies, participants were either medical or surgical patients (Berry 2013; Koeman 2006; Meinberg 2012; Mo 2016; Munro 2009; Panchabhai 2009); in another five studies, participants were described as trauma patients (Grap 2011; Prendergast 2012; Scannapieco 2009; Seguin 2006; Seguin 2014); six studies recruited surgical patients only (Chen 2008; DeRiso 1996; Jacomo 2011; Kusahara 2012a; Yao 2011; Zhao 2012); nine studies recruited medical patients only (Cabov 2010; Fields 2008; Fourrier 2000; Fourrier 2005; Ozcaka 2012; Sebastian 2012; Stefanescu 2013; Tang 2013; Tantipong 2008); and in the remaining 12 studies, it was not clearly stated whether participants were medical, surgical, or trauma cases.
Nine of the included studies (Fields 2008; Fourrier 2000; Grap 2011; Lorente 2012; Munro 2009; Ozcaka 2012; Pobo 2009; Prendergast 2012; Roca Biosca 2011) specifically excluded edentulous participants, and the remaining studies did not report whether or not participants were dentate.
Classification of the interventions
We classified the interventions into three broad groups.
Chlorhexidine
Chlorhexidine solution (applied as mouthrinse, spray or on a swab)
Chlorhexidine gel
-
Toothbrushing
Powered
Manual
-
Other solutions
Povidone iodine
Saline
Bicarbonate
Triclosan
Furacilin
Listerine
Biotene OralBalance
These interventions were used either singly or in combinations. We evaluated the following comparisons.
Chlorhexidine versus placebo/usual care, with or without toothbrushing (19 studies: Bellissimo‐Rodrigues 2009; Berry 2011; Bopp 2006; Cabov 2010; Chen 2008; DeRiso 1996; Fourrier 2000; Fourrier 2005; Grap 2011; Jacomo 2011; Koeman 2006; Kusahara 2012a; Meinberg 2012; Munro 2009; Ozcaka 2012; Panchabhai 2009; Scannapieco 2009; Sebastian 2012; Tantipong 2008)
Toothbrushing versus no toothbrushing (in addition to usual care) (eight studies: Bopp 2006; Fields 2008; Lorente 2012; Long 2012; Munro 2009; Pobo 2009; Roca Biosca 2011; Yao 2011)
Powered toothbrushing versus manual toothbrushing (one study: Prendergast 2012)
-
Other solutions (15 studies)
Saline (Caruso 2009; Hu 2009; Mo 2016; Seguin 2006; Tang 2013; Xu 2007; Xu 2008)
Bicarbonate (Berry 2011; Berry 2013)
Povidone iodine (Feng 2012; Seguin 2006; Seguin 2014)
Triclosan (Zhao 2012)
Furacilin (Feng 2012)
Listerine (Berry 2013)
Biotene OralBalance (Stefanescu 2013)
There was some variation between the studies in the number of episodes of OHC per day, with most of the studies (79%) delivering two to four episodes of care daily. Thirteen studies (Berry 2011; Bopp 2006; DeRiso 1996; Fields 2008; Hu 2009;Jacomo 2011; Kusahara 2012a; Panchabhai 2009; Prendergast 2012; Scannapieco 2009; Xu 2007; Xu 2008; Yao 2011) delivered two episodes of OHC a day, nine studies (Bellissimo‐Rodrigues 2009; Cabov 2010; Fourrier 2000; Fourrier 2005; Long 2012; Lorente 2012; Munro 2009; Pobo 2009; Sebastian 2012) had three episodes a day, and eight studies (Chen 2008; Feng 2012; Koeman 2006; Meinberg 2012; Mo 2016; Ozcaka 2012; Tantipong 2008; Zhao 2012) had four episodes a day. One study delivered OHC every two hours (Berry 2013), another only once (Grap 2011), and in the remaining three studies it is unclear (Caruso 2009; Roca Biosca 2011; Tang 2013).
In some of the included studies, the intervention described as 'placebo' may have had some antibacterial activity, but this was considered by the trialists to be negligible compared to the active intervention. Placebo interventions included saline (Chen 2008; Feng 2012; Hu 2009; Ozcaka 2012; Seguin 2006; Tantipong 2008), potassium permanganate (Panchabhai 2009), half‐strength hydrogen peroxide (Bopp 2006), water/alcohol mixture (DeRiso 1996; Jacomo 2011), placebo gel (Fourrier 2005; Koeman 2006; Kusahara 2012a; Meinberg 2012; Sebastian 2012), base solution (Scannapieco 2009) or water (Berry 2011; Berry 2013). In one trial, the nature of the placebo was not specified (Bellissimo‐Rodrigues 2009).
In eight studies, the control group received usual/standard care (Caruso 2009; Fields 2008; Fourrier 2000; Hu 2009; Grap 2011; Munro 2009; Seguin 2006; Yao 2011) (for specific details see Characteristics of included studies), and in three studies, there was a head‐to‐head comparison between two potentially active interventions (Pobo 2009; Prendergast 2012; Roca Biosca 2011).
Measures of primary outcomes
Incidence of VAP
The primary outcome of our review is ventilator‐associated pneumonia (VAP), defined as pneumonia developing in a person who has been on mechanical ventilation for at least 48 hours. VAP was fully reported by 34 of the included studies (Bellissimo‐Rodrigues 2009; Berry 2011; Berry 2013; Bopp 2006; Cabov 2010; Caruso 2009; Chen 2008; DeRiso 1996; Feng 2012; Fourrier 2005; Grap 2011; Hu 2009; Jacomo 2011; Koeman 2006; Kusahara 2012a; Long 2012; Lorente 2012; Meinberg 2012; Mo 2016; Ozcaka 2012; Panchabhai 2009; Pobo 2009; Prendergast 2012; Scannapieco 2009; Sebastian 2012; Seguin 2006; Seguin 2014; Stefanescu 2013; Tang 2013; Tantipong 2008; Xu 2007; Xu 2008; Yao 2011; Zhao 2012). One study reported only that there was no difference in VAP between the two arms of the study (Roca Biosca 2011). One study reported that the VAP rate dropped to zero in the intervention group but the control group event rate was not reported (Fields 2008). Two studies reported the outcome of nosocomial pneumonia, but it was not clear in the trial reports whether all those who developed this outcome had been on mechanical ventilation for at least 48 hours (Fourrier 2000; Hu 2009). We sought clarification from the trial authors but have so far received no further data.
Diagnostic criteria for the outcome of ventilator‐associated pneumonia were specified in 33 studies. Seventeen studies used Pugin's criteria (Cook 1998; Pugin 1991), which form the basis of the CPIS score, based on the presence of an infiltrate on chest radiograph, plus two or more of the following: temperature greater than 38.5º C or less than 35º C, white blood cell count greater than 11,000/mm3 or less than 4000/mm3, mucopurulent or purulent bronchial secretions, or more than 20% increase in fraction of inspired oxygen required to maintain saturation above 92% (Berry 2011; Berry 2013; Cabov 2010; Caruso 2009; Fourrier 2000; Fourrier 2005; Grap 2011; Koeman 2006; Kusahara 2012a; Meinberg 2012; Munro 2009; Pobo 2009; Scannapieco 2009; Seguin 2006; Seguin 2014; Tantipong 2008; Yao 2011). In Ozcaka 2012, no specific criteria were reported, but communication with the author confirmed that participants with new pulmonary infiltrates or opacities on the chest X‐ray were prediagnosed VAP and lower tracheal mini‐bronchoalveolar lavage (mini‐BAL) samples were taken and then participants were diagnosed according to CPIS criteria. Those who had a score of six or more and the presence of 104 or more colony‐forming units/mL of a target potential respiratory bacterial pathogen (PRP) in mini‐BAL were diagnosed with VAP.
A further six studies used the CDC criteria as described in Horan 2008 (Bellissimo‐Rodrigues 2009; DeRiso 1996; Fields 2008; Jacomo 2011; Panchabhai 2009; Sebastian 2012). Stefanescu 2013 used CDC criteria for diagnosis of neonatal VAP.
Six studies used the criteria of the Chinese Society of Respiratory Diseases: presence of new infiltrates on chest radiographs developed after 48 hours of mechanical ventilation with any two of the following items: (a) temperature greater than 38º C, (b) change in characteristics of bronchial secretions from mucoid to mucopurulent or purulent, (c) white cell count greater than 10,000/mm3, (d) positive culture of tracheal aspirate or positive culture of bronchoalveolar lavage fluid or both, or (e) arterial oxygen tension/inspiratory fraction of oxygen PaO2/FiO2 decreased over 30% within the period of ventilation (Chen 2008; Feng 2012; Mo 2016; Tang 2013; Xu 2007; Xu 2008).
Hu 2009 reported the outcome of VAP based on clinical examination plus three criteria: chest radiograph, white cell count and culture of the aspirate from lower respiratory tract (but no precise parameters were specified). In Lorente 2012, the diagnosis of VAP was made by an expert panel blinded to the allocated intervention, but the diagnostic criteria were not specified. Prendergast 2012 had a single diagnostic criterion of a new or worsening pulmonary infiltrate on chest radiograph. Two studies used positive culture from the lower respiratory tract as criteria for diagnosis of VAP (Long 2012; Zhao 2012).
The remaining two studies with the outcome of VAP did not report their diagnostic criteria (Bopp 2006; Roca Biosca 2011).
Mortality
Twenty‐four studies reported the outcome of mortality, either as ICU mortality or 30‐day mortality (Bellissimo‐Rodrigues 2009; Cabov 2010; Caruso 2009; Fourrier 2000; Fourrier 2005; Jacomo 2011; Kusahara 2012a; Long 2012; Lorente 2012; Meinberg 2012; Mo 2016; Munro 2009; Ozcaka 2012; Panchabhai 2009; Pobo 2009; Prendergast 2012; Scannapieco 2009; Sebastian 2012; Seguin 2006; Seguin 2014; Stefanescu 2013; Tang 2013; Tantipong 2008; Yao 2011). Where ICU mortality was reported, we used these data; where ICU mortality was not reported, we used 30‐day mortality.
Measures of secondary outcomes
Duration of ventilation
Sixteen studies reported this outcome (Bellissimo‐Rodrigues 2009; Caruso 2009; Fourrier 2000; Fourrier 2005; Hu 2009; Koeman 2006; Long 2012; Lorente 2012; Ozcaka 2012; Pobo 2009; Prendergast 2012; Scannapieco 2009; Seguin 2006; Tang 2013; Xu 2008; Zhao 2012). Berry 2013, Jacomo 2011, Meinberg 2012 and Sebastian 2012 reported the median duration of ventilation or the range for each group or both, but we could not combine these data in a meta‐analysis. Unless explicitly reported otherwise, we have assumed that all studies used similar methods to calculate these data including participants who died. Stefanescu 2013 only reported a P value for the difference between groups in duration of ventilation.
Duration of ICU stay
There were 15 studies reporting this outcome (Bellissimo‐Rodrigues 2009; Bopp 2006; Caruso 2009; Fourrier 2000; Fourrier 2005; Koeman 2006; Kusahara 2012a; Lorente 2012; Ozcaka 2012; Panchabhai 2009; Pobo 2009; Prendergast 2012; Seguin 2006; Seguin 2014; Zhao 2012). Berry 2013, Jacomo 2011, Meinberg 2012, and Sebastian 2012 reported the median ICU stay and the range for each group, but we could not combine these data in a meta‐analysis. Unless explicitly reported otherwise, we have assumed that all studies used similar methods to calculate these data including participants who died.
Systemic antibiotic therapy
There were five studies that reported some measure of systemic antibiotic use. DeRiso 1996 reported the number of participants in each group who required treatment of an infection with systemic antibiotics during their ICU stay; Seguin 2014 reported the number of participants who were treated with antibiotics; and Fourrier 2005 and Scannapieco 2009 both reported the mean number of days of systemic antibiotic use in the intervention and control groups. Berry 2013 only reported a P value for the difference among groups in antibiotic administration.
Oral health indices
Plaque indices were mentioned as outcomes in four studies (Ozcaka 2012; Roca Biosca 2011; Scannapieco 2009; Yao 2011). Complete data for plaque indices were reported in one study (Ozcaka 2012), and were supplied by the corresponding author of another study (Yao 2011). Scannapieco 2009 reported this outcome in graphs only, and Roca Biosca 2011 did not report any estimate of variance, so we could not use these data in this review.
Adverse effects
Only two of the included studies reported adverse effects of the interventions (Seguin 2014; Tantipong 2008); five studies reported that there were no adverse effects (Berry 2011; Berry 2013; Jacomo 2011; Ozcaka 2012; Sebastian 2012), and Stefanescu 2013 reported no significant difference between groups with respect to adverse events in buccal mucosa. The remaining studies did not mention adverse effects in their reports.
Excluded studies
In this update, we excluded 24 studies for the reasons summarised below. Three studies that we included in the previous version of the review are excluded from this version (Grap 2004; McCartt 2010; Needleman 2011).
Twelve studies were excluded because they were not RCTs (Buckley 2013; Darnell 2015; Gu 2013; Labeau 2013; Liao 2015; Maury 2015; Pelucchi 2013; Sands 2015; Seo 2011; Swartz 2015; Tattevin 2015; Yun 2011).
Five studies were excluded because they did not attempt to assess the incidence of VAP or mortality (Baradari 2012; Grap 2004; Kusahara 2012b; McCartt 2010; Needleman 2011).
Four studies were reported as abstracts only and our attempts to find a full publication or obtain sufficient data to enable inclusion in this review were unsuccessful (Anon 2012; Jafari 2007; MacNaughton 2004; Pivkina 2014).
Bellissimo‐Rodrigues 2014 was excluded because the intervention was dental care (e.g. treatment of caries, tooth extraction), not oral hygiene care.
Munro 2015 was excluded because the intervention was oral hygiene care prior to, not during, mechanical ventilation.
Fan 2015 was excluded because the CHX solution used for interventions contained antibiotics.
For further information, see the Characteristics of excluded studies table, which also provides information on studies excluded in the last version of this review.
Risk of bias in included studies
Allocation
Sequence generation
Twenty‐eight of the included studies clearly described a random method of sequence generation and we assessed them at low risk of bias for this domain. The remaining 10 studies stated that allocation was random but provided no further details and we therefore assessed them at unclear risk of bias for this domain (Caruso 2009; Feng 2012; Fields 2008; Long 2012; Panchabhai 2009; Roca Biosca 2011; Tang 2013; Xu 2007; Xu 2008; Zhao 2012).
Allocation concealment
Allocation concealment was clearly described in 19 of the included studies and we assessed them at low risk of bias for this domain. In 18 studies, allocation concealment was not described in sufficient detail to determine risk of bias and we rated these studies at unclear risk of bias (Cabov 2010; Caruso 2009; Chen 2008; Feng 2012; Fourrier 2000; Grap 2011; Long 2012; Lorente 2012; Mo 2016; Munro 2009; Panchabhai 2009; Sebastian 2012; Tang 2013; Tantipong 2008; Xu 2007; Xu 2008; Yao 2011; Zhao 2012). We assessed Bopp 2006 at high risk of bias because the allocation was not concealed from the researchers.
The risk of selection bias based on combined assessment of these two domains was high in one study (Bopp 2006), unclear in 20 studies (Cabov 2010; Caruso 2009; Chen 2008; Feng 2012; Fields 2008; Fourrier 2000; Grap 2011; Long 2012; Lorente 2012; Mo 2016; Munro 2009; Panchabhai 2009; Roca Biosca 2011; Sebastian 2012; Tang 2013; Tantipong 2008; Xu 2007; Xu 2008; Yao 2011; Zhao 2012), and low in the remaining 17 studies.
Blinding
Twelve studies were described as double blind and we assessed them at low risk of performance bias (Bellissimo‐Rodrigues 2009; Cabov 2010; DeRiso 1996; Fourrier 2005; Jacomo 2011; Koeman 2006; Kusahara 2012a; Meinberg 2012; Ozcaka 2012; Scannapieco 2009; Sebastian 2012; Seguin 2014). There was insufficient information to determine whether blinding occurred in two studies (Caruso 2009; Zhao 2012). In the remaining 24 studies, blinding of the participants and their caregivers to the allocated treatment was not possible because the active and control treatments were so different, and no placebos were used. We assessed these studies at high risk of performance bias.
Blinding of outcome assessment was possible in all of the included studies and was described in 22 studies (Bellissimo‐Rodrigues 2009; Berry 2011; Berry 2013; Cabov 2010; Caruso 2009; DeRiso 1996; Fourrier 2000; Fourrier 2005; Hu 2009; Jacomo 2011; Kusahara 2012a; Lorente 2012; Meinberg 2012; Ozcaka 2012; Panchabhai 2009; Pobo 2009; Prendergast 2012; Scannapieco 2009; Sebastian 2012 ; Seguin 2014; Tantipong 2008; Yao 2011), which we assessed as being at low risk of detection bias. One of the included studies reported no blinding of outcome assessment and we assessed it at high risk of detection bias (Bopp 2006). In the remaining 15 studies, there was insufficient information provided and we rated the risk of detection bias as unclear.
Incomplete outcome data
In the studies included in this review loss of participants during the course of the study is to be expected, as these critically ill people leave the intensive care unit either because they recover and no longer require mechanical ventilation, or because they die from their illness. In 25 of the included studies, either all the randomised participants were included in the outcome, or the number of losses/withdrawals and the reasons given were similar in both arms of the study, and we assessed these studies at low risk of attrition bias (Bellissimo‐Rodrigues 2009; Bopp 2006; Cabov 2010; Caruso 2009; Chen 2008; Feng 2012; Fourrier 2005; Jacomo 2011; Koeman 2006; Kusahara 2012a; Long 2012; Lorente 2012; Meinberg 2012; Mo 2016; Ozcaka 2012; Pobo 2009; Sebastian 2012 ; Seguin 2006; Seguin 2014, Stefanescu 2013; Tang 2013; Xu 2007; Xu 2008; Yao 2011; Zhao 2012).
We rated nine of the included studies at high risk of attrition bias, because the numbers and reasons for withdrawal/exclusion were different in each arm of the study, or because the number of participants withdrawn or excluded from the outcomes evaluation was high and insufficient information was provided (Berry 2011; Berry 2013; Fields 2008; Grap 2011; Hu 2009; Munro 2009; Prendergast 2012; Roca Biosca 2011; Scannapieco 2009). In the remaining four studies there was insufficient information available to determine the risk of attrition bias.
Selective reporting
Twenty‐six of the included studies reported the outcomes specified in their Methods section in full, or this information was supplied by trial authors, and we assessed these studies at low risk of reporting bias (Bellissimo‐Rodrigues 2009; Berry 2011; Cabov 2010; Caruso 2009; DeRiso 1996; Feng 2012; Fourrier 2000; Fourrier 2005; Koeman 2006; Kusahara 2012a; Long 2012; Lorente 2012; Mo 2016; Ozcaka 2012; Panchabhai 2009; Pobo 2009; Prendergast 2012; Sebastian 2012; Seguin 2006; Seguin 2014; Stefanescu 2013; Tang 2013; Xu 2007; Xu 2008; Yao 2011; Zhao 2012).
Three studies did not report all the outcomes specified in their Methods sections (Grap 2011; Meinberg 2012; Roca Biosca 2011), two studies reported outcomes as percentages only, with unclear denominators for each arm (Berry 2013; Hu 2009), and one study did not report the number of participants evaluated (Fields 2008). We rated these six trials at high risk of reporting bias.
We assessed the remaining six trials at unclear risk of reporting bias, because there was insufficient information reported to make a clear judgement (Bopp 2006; Chen 2008; Koeman 2006; Munro 2009; Scannapieco 2009; Tantipong 2008).
Other potential sources of bias
We rated five studies at high risk of other bias. Three studies were stopped early (Berry 2011; Meinberg 2012; Pobo 2009). Berry 2011 was stopped due to withdrawal of one of the investigational products by a regulatory authority; Pobo 2009 was stopped after 37% of the planned 400 participants had been recruited because there appeared to be no difference between the study arms in the outcome of VAP. Meinberg 2012 was stopped due to "futility"; however we are unsure whether this was the main problem. Grap 2011 did not report baseline data for each randomised treatment group but the trial report noted that there was a "statistically significant difference in gender and CPIS score between groups at baseline", and we considered that this difference was likely to have biased the results. In Scannapieco 2009 the imputations used for the missing data were unclear and the pre‐study exposure to systemic antibiotics was greater in the control group, so we assessed this study at high risk of other bias.
In 12 studies, we rated the risk of other bias as unclear (Berry 2013; Chen 2008; Fields 2008; Kusahara 2012a; Long 2012; Panchabhai 2009; Roca Biosca 2011; Stefanescu 2013; Tang 2013; Tantipong 2008; Yao 2011; Zhao 2012). The reasons for this are as follows:
In Berry 2013 ineligible participants were included in the ITT analysis, but reasons for ineligibility in each group were not given;
The participants in the treatment group in Chen 2008 received a co‐intervention that was not given to the control group;
In both Fields 2008 and Roca Biosca 2011 the study reports contained insufficient information for us to be confident that study methodology was robust;
In Stefanescu 2013 more infants in the control group received a complete course of antenatal steroids compared to infants in the Biotene OralBalance group (P = 0.045). A complete course of antenatal steroids improves antenatal lung maturity and function and may reduce the risk of VAP. This imbalance is likely to lead to an underestimate of the benefit of the active treatment;
In Kusahara 2012a there was a statistically significant difference in the age of the children in each arm of the study and we are unclear whether this is associated with potential bias;
Panchabhai 2009 reported baseline characteristics only for those participants completing the study;
In Tang 2013, a detailed description about the intervention methods and frequency of oral care in each group was not reported.
Tantipong 2008 included participants treated in different units of the hospital where care and co‐interventions are likely to have been different;
In Yao 2011 there is no information as to how the edentulous participants in each arm were treated;
Long 2012 and Zhao 2012 reported the criteria for VAP diagnosis as being positive culture of lower respiratory tract secretions, with no other criteria, and it is unclear if this would have introduced a bias in these unblinded studies.
We assessed the remaining 21 studies at low risk of other bias.
Overall risk of bias
Overall, we rated just five of the included studies (13%) at low risk of bias for all domains (Bellissimo‐Rodrigues 2009; Fourrier 2005; Koeman 2006; Ozcaka 2012; Seguin 2014), and seven studies (18%) were at unclear risk of bias for at least one domain. Over two‐thirds of the included studies (26 studies, 68%) were at high risk of bias in at least one domain (see Figure 2; Figure 3).
2.
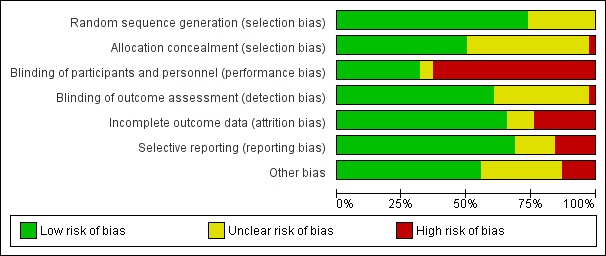
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
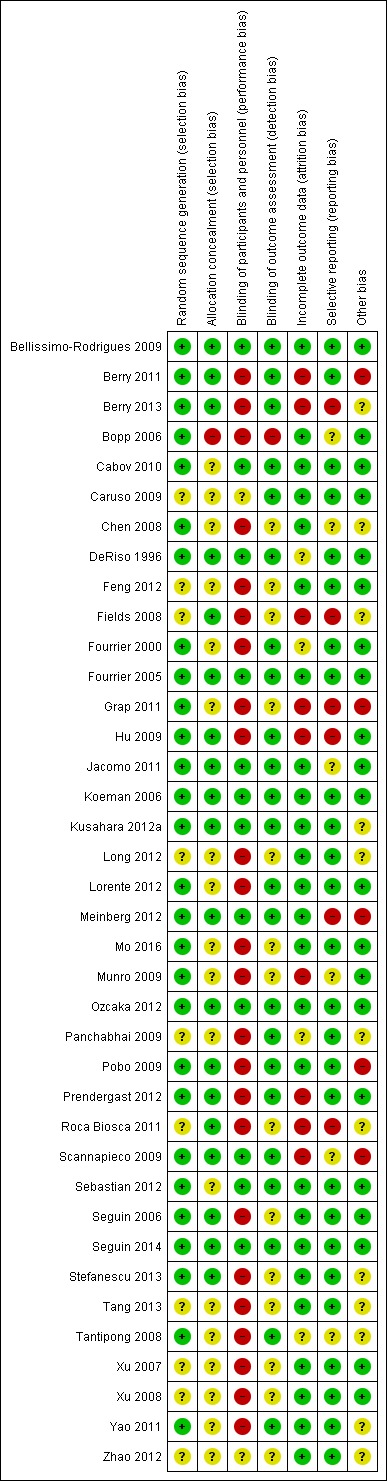
Risk of bias summary graph: review authors' judgements about each risk of bias item for each included study
Effects of interventions
Comparison 1: Chlorhexidine versus placebo/usual care (with or without toothbrushing)
Chlorhexidine antiseptic was evaluated in 19 studies included in this review, but only 18 studies could be included in meta‐analysis for VAP. One study was a very small pilot study with no usable outcome data (Bopp 2006, n = 5).
Concentration of the chlorhexidine used was 2% in three studies (Koeman 2006; Tantipong 2008; Meinberg 2012), 1% in one study (Sebastian 2012), 0.20% in five studies (Berry 2011; Cabov 2010; Fourrier 2000; Fourrier 2005; Panchabhai 2009), unclear in one study (Chen 2008), and 0.12% in the remaining studies.
We assessed 10 of the 19 studies at high risk of bias (Berry 2011; Bopp 2006; Chen 2008; Fourrier 2000; Grap 2011; Meinberg 2012; Munro 2009; Panchabhai 2009; Scannapieco 2009; Tantipong 2008), four studies at low risk of bias (Bellissimo‐Rodrigues 2009; Fourrier 2005; Koeman 2006; Ozcaka 2012), and the remaining five studies at unclear risk of bias.
We subgrouped these studies according to whether chlorhexidine was administered as a liquid mouthrinse or a gel, and whether chlorhexidine was used in conjunction with toothbrushing or not.
Incidence of VAP
Overall, the meta‐analysis of 18 studies (nine studies at high risk of bias, five at unclear risk of bias and four at low risk of bias) showed a reduction in VAP with use of chlorhexidine (risk ratio (RR) 0.75, 95% confidence interval (CI) 0.62 to 0.91, P = 0.004, I2 = 35%; 2451 participants) (Analysis 1.1). This equates to a number needed to treat for an additional beneficial outcome (NNTB) of 17 (95% CI 9 to 50).
1.1. Analysis.
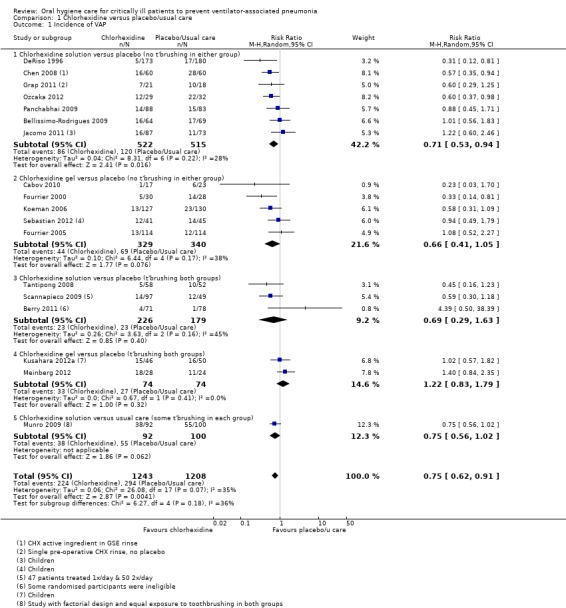
Comparison 1 Chlorhexidine versus placebo/usual care, Outcome 1 Incidence of VAP.
Seven studies (1037 participants) compared chlorhexidine solution (0.12% or 0.2%) with either placebo (six studies) or 'usual care' (Grap 2011) without toothbrushing. Six of these studies reported the use of a swab, either to clean the mouth prior to chlorhexidine application or to ensure that the chlorhexidine solution was applied to all oral surfaces. In the remaining study (Chen 2008) the mode of application is unclear. The meta‐analysis showed a reduction in VAP in the chlorhexidine group (RR 0.71, 95% CI 0.53 to 0.94, P = 0.02, I2 = 28%) (Analysis 1.1, Subgroup 1.1.1).
A further five studies (669 participants) compared chlorhexidine gel (0.2% or 2%) with placebo (no toothbrushing in either group) and the meta‐analysis showed a similar reduction in VAP associated with chlorhexidine gel (RR 0.66, 95% CI 0.41 to 1.05, P = 0.08, I2 = 38%) (Analysis 1.1, Subgroup 1.1.2).
Three studies (405 participants) compared chlorhexidine solution (2%, 0.12% or 0.2%) with placebo (with toothbrushing in both groups). The meta‐analysis showed no evidence of a difference in VAP between the groups group (RR 0.69, 95% CI 0.29 to 1.63, P = 0.40, I2 = 45%) (Analysis 1.1, Subgroup 1.1.3).
Two further studies (Meinberg 2012; Kusahara 2012a, including 52 adults and 96 children), at high and unclear risk of bias, compared chlorhexidine gel (2% and 0.12%) with placebo (with toothbrushing in both groups) and found no difference in the incidence of VAP (RR 1.22, 95% CI 0.83 to 1.79, P = 0.32, I2 = 0%) (Analysis 1.1, Subgroup 1.1.4).
Munro 2009 reported results from some of the participants randomised into a study with a factorial design. This study showed a reduction in VAP that did not attain statistical significance (P = 0.06) associated with the use of chlorhexidine, where exposure to toothbrushing was equal in both groups (Analysis 1.1, Subgroup 1.1.5).
The pilot study by Bopp 2006 also showed a reduction in VAP associated with chlorhexidine (Additional Table 7).
1. Other outcome data from included studies.
| Comparison | Number of participants | Outcome | Data | Effect estimate (95% CI) |
| Listerine versus sodium bicarbonate versus sterile water (Berry 2013) | Listerine group: 127; Sodium bicarbonate group: 133; Sterile water group: 138 | Duration of mechanical ventilation | No significant difference between groups in median ventilation hours (81 hours, SD 1058) | |
| Duration of ICU stay | No significant difference between groups in median length of ICU stay (5 days, SD 29) | |||
| Systemic antibiotic use | No significant difference between groups (P = 0.21) | |||
| Adverse events | No adverse events were reported associated with interventions | |||
| CHX + toothbrushing versus control (Bopp 2006) | CHX + toothbrushing group: 2; Control group:3 | Incidence of VAP | 0 cases in CHX + toothbrushing group and 1 case in control group | |
| Duration of ventilation | Mean 5.5 days (SD 0.3896) in toothbrushing group and mean 5 days (SD 0.8051) in control group | |||
| Duration of ICU stay | Mean 18 days (SD 1.6695) in toothbrushing group and mean 10.3 days (SD 2.6971) in control group | |||
| CHX versus placebo (Koeman 2006) | CHX: 127; Placebo:130 | Mortality |
HR | HR 1.12 (95% CI 0.72 to 1.17) |
| CHX versus placebo (Meinberg 2012) | CHX group: 28; Placebo group: 24 | Duration of mechanical ventilation | Median days in CHX group 8.5 (interquartile range, 7.3 to 14.7) and median days in placebo group 6 (4 to 12.7) (P = 0.17) | |
| Duration of ICU stay | Median days in CHX group 12 (interquartile range, 9 to 29) and median days in placebo group 11 (5 to 16) (P = 0.36) | |||
| Powered toothbrush + CHX versus CHX alone (Roca Biosca 2011) | Powered toothbrush group: 29; CHX alone group: 32 | Plaque index | Mean in toothbrush group 1.68 and mean in control group 1.91; no estimates of variance but reported that P = 0.7 (no difference) | |
| Incidence of VAP | OR 0.78 (95% CI 0.36 to 1.68, P = 0.56) | |||
| CHX (once daily or twice daily) versus placebo (Scannapieco 2009) | CHX 1x/day group: 47; CHX 2x/day group: 50; Placebo group: 49 | Plaque index | No difference between the 3 groups (data presented graphically) | |
| Biotene OralBalance versus control (Stefanescu 2013) | Biotene OralBalance group: 20; Control group: 21 | Duration of mechanical ventilation | No difference between groups (P = 0.77) | |
| Adverse events | No significant difference between groups with respect to adverse events in buccal mucosa |
CHX = chlorhexidine; CI = confidence interval; CPIS = Clinical Pulmonary Infection Score; HR = hazard ratio; ICU = intensive care unit; OR = odds ratio; P = probability; SD = standard deviation; VAP = ventilator‐associated pneumonia
Mortality
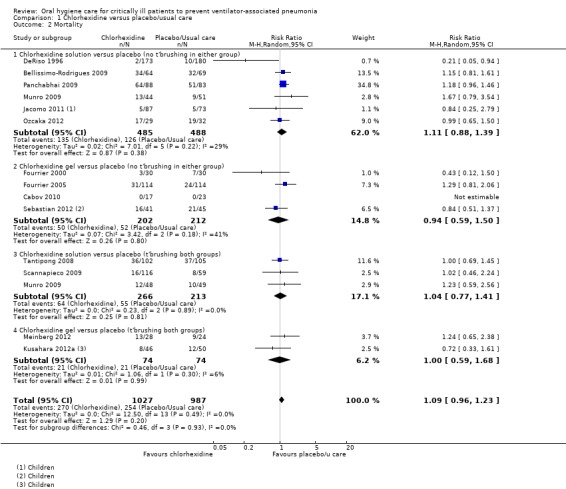
The outcome of mortality was reported in 14 studies (2014 participants), and overall the meta‐analysis showed no evidence of a difference between chlorhexidine and placebo/usual care with minimal heterogeneity (RR 1.09, 95% CI 0.96 to 1.23, P = 0.20 , I2 = 0%) (Analysis 1.2). Nor was there evidence of a difference in mortality between (P = 0.93) or within the subgroups (chlorhexidine mouthrinse/gel with or without toothbrushing) (Analysis 1.2; Additional Table 7).
1.2. Analysis.
Comparison 1 Chlorhexidine versus placebo/usual care, Outcome 2 Mortality.
Duration of ventilation
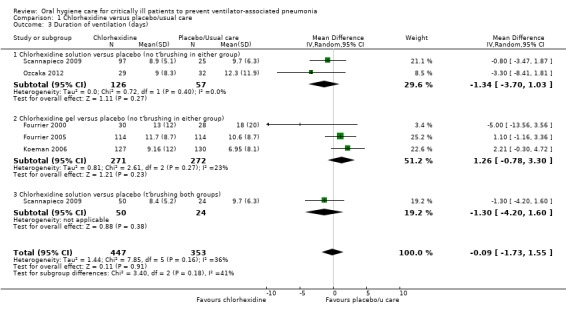
From the five studies (800 participants) that reported data in a way that could be combined in meta‐analysis, there is no evidence of a difference in the duration of ventilation (days) between groups receiving chlorhexidine compared to those receiving placebo/usual care (mean difference (MD) ‐0.09 days, 95% CI ‐1.73 to 1.55 days, P = 0.91, I2 = 36%) (Analysis 1.3). There was no evidence of a difference in duration of ventilation in any of the subgroups.
1.3. Analysis.
Comparison 1 Chlorhexidine versus placebo/usual care, Outcome 3 Duration of ventilation (days).
A further study (Meinberg 2012), comparing chlorhexidine gel and placebo, also found no difference in duration of ventilation (Additional Table 7).
Duration of ICU stay
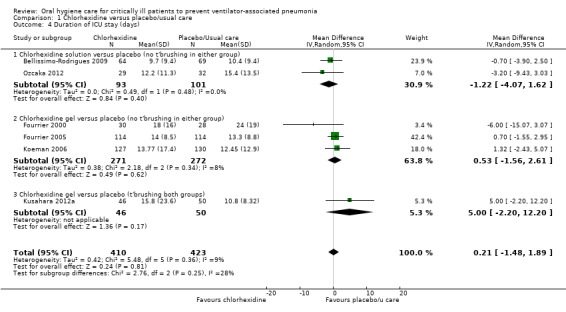
There was no evidence of a difference between those receiving chlorhexidine compared to placebo/usual care in the outcome of duration of ICU stay (days) (MD 0.21 days, 95% CI ‐1.48 to 1.89 days, P = 0.81, I2 = 9%; six RCTs, 833 participants). There was no evidence of a difference in two subgroups (Analysis 1.4, Subgroup 1.4.1; Subgroup 1.4.2) and insufficient evidence to determine whether or not there was a difference in Analysis 1.4, Subgroup 1.4.3.
1.4. Analysis.
Comparison 1 Chlorhexidine versus placebo/usual care, Outcome 4 Duration of ICU stay (days).
Another study (Meinberg 2012) compared chlorhexidine gel with placebo and also found no difference in duration of ICU stay (Additional Table 7).
Use of systemic antibiotics
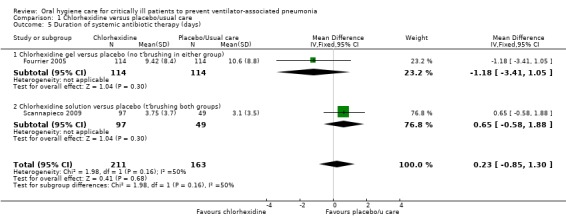
Two trials (374 participants) reported this outcome, but there was insufficient evidence to determine whether or not there is a difference in duration of systemic antibiotic therapy between the chlorhexidine and control groups (MD 0.23 days, 95% CI ‐0.85 to 1.30, P = 0.68, I2 = 50%; fixed‐effect model). There was moderate heterogeneity, probably due to the differences between the two studies in the mode of chlorhexidine used (Analysis 1.5).
1.5. Analysis.
Comparison 1 Chlorhexidine versus placebo/usual care, Outcome 5 Duration of systemic antibiotic therapy (days).
Oral health indices: plaque index
Two of the studies in this group reported the outcome of plaque index (Ozcaka 2012; Scannapieco 2009), but only Ozcaka 2012 reported numerical data. Neither study found a difference in plaque indices between the chlorhexidine and control groups (Analysis 1.6; Additional Table 7).
1.6. Analysis.
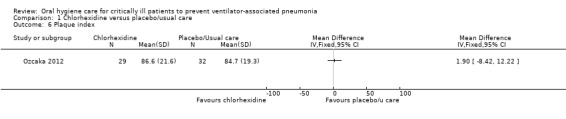
Comparison 1 Chlorhexidine versus placebo/usual care, Outcome 6 Plaque index.
Adverse effects
Two studies in this group reported adverse effects. Tantipong 2008 found mild reversible irritation of the oral mucosa in 10% of the chlorhexidine participants compared to 1% of the control group participants (Analysis 1.7). Berry 2011 stated that there were no adverse events in either group.
1.7. Analysis.
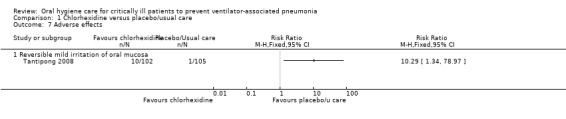
Comparison 1 Chlorhexidine versus placebo/usual care, Outcome 7 Adverse effects.
Adverse effects were not mentioned in the other studies in this group.
Other outcomes
The outcomes of caregivers' preferences and cost were not reported.
Heterogeneity
The moderate statistical heterogeneity found for the outcome of VAP incidence is likely to be due to clinical differences between these studies, attributable to variability in the frequency, application method, volume, and concentration of chlorhexidine solution (Analysis 1.1).
In Subgroup 1.1.1, six of the seven studies used a placebo control and the volume of chlorhexidine (either 0.12% or 0.2%) used varied between 10 and 50 ml administered either two, three, or four times daily. One study used a single application by swab of a very small volume of chlorhexidine preoperatively (Grap 2011). One of the seven studies was in children aged from birth to 14 years (Jacomo 2011); the other studies recruited adults.
In Subgroup 1.1.2, there was also moderate heterogeneity, that may be due to variations in the way the intervention was delivered. Three of the five studies in this subgroup administered 0.2% chlorhexidine gel three times daily following rinsing of the mouth and aspiration of rinse (Cabov 2010; Fourrier 2000; Fourrier 2005). The other two studies used a gel with higher chlorhexidine concentration (2% and 1% respectively) and applied the gel using a swab (Koeman 2006; Sebastian 2012).
Sensitivity analysis
For the primary outcomes, we conducted a sensitivity analysis excluding studies at high risk of bias. The estimate remained similar for both VAP incidence (RR 0.79, 95% CI 0.60 to 1.04, P = 0.09, I2 = 28%; 1414 participants) compared with 0.75, and mortality (RR 0.99, 95% CI 0.78 to 1.24, P = 0.92, I2 = 18%; 1157 participants) compared with 1.09 (Analyses not shown).
A meta‐analysis of the three studies of children (342 participants, aged from 3 months to 15 years) provided no evidence that chlorhexidine compared to placebo showed a difference in the outcomes of VAP (RR 1.04, 95% CI 0.72 to 1.51, P = 0.82, I2 = 0%) or mortality (RR 0.81, 95% CI 0.54 to 1.20, P = 0.29, I2 = 0%) (Jacomo 2011; Kusahara 2012a; Sebastian 2012) (Analyses not shown).
In addition, we also carried out sensitivity analyses by grouping the included studies by chlorhexidine concentration. Results of these subgroup analyses suggest no evidence of a difference between subgroups or any dose‐response relationship in either incidence of VAP (P = 0.83) or mortality (P = 0.59) (Analyses not shown).
Publication bias
Each of the subgroups in this comparison contained a small number of studies and it was therefore not appropriate to produce a funnel plot to investigate possible publication bias.
Comparison 2: Toothbrushing versus no toothbrushing (with or without antiseptics)
The eight studies included in this comparison (Bopp 2006; Fields 2008; Long 2012; Lorente 2012; Munro 2009; Pobo 2009; Roca Biosca 2011; Yao 2011) had toothbrushing as part of the intervention versus no toothbrushing in the control group. The studies were all at high risk of bias. Three studies used powered toothbrushes (Pobo 2009; Roca Biosca 2011), and five used manual toothbrushes. Bopp 2006 was a very small pilot study (n = 5) and the data from this study are recorded in Additional Table 7; Fields 2008 reported no numerical data at all. Roca Biosca 2011 did not report data for each arm of the study and we were not able to obtain these data from the authors. Available data from this study are recorded in Additional Table 7.
Incidence of VAP
There was no evidence of a difference in the incidence of VAP due to toothbrushing in the combined meta‐analysis of five studies (RR 0.69, 95% CI 0.44 to 1.09, P = 0.11 , I2 = 64%, 889 participants, high risk of bias) (Analysis 2.1) or the combined meta‐analysis of four studies for chlorhexidine (RR 0.77, 95% CI 0.50 to 1.21, P = 0.26, I2 = 62%, 828 participants, high risk of bias) (analysis not shown) (Lorente 2012; Munro 2009; Pobo 2009; Yao 2011), with the substantial statistical heterogeneity likely to be explained by the differences between the studies in exposure to antiseptics.
2.1. Analysis.
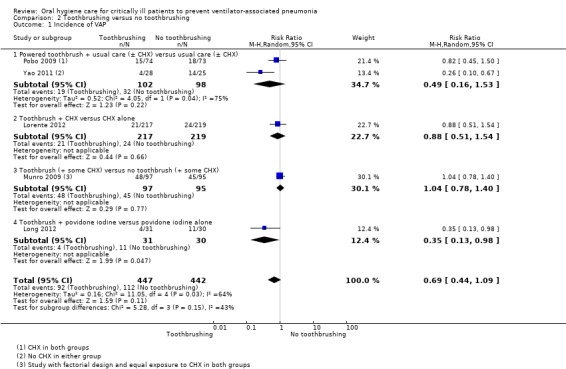
Comparison 2 Toothbrushing versus no toothbrushing, Outcome 1 Incidence of VAP.
One small study ( (Yao 2011); 53 participants) at high risk of bias, compared usual care plus the addition of twice daily toothbrushing with a powered toothbrush, to usual care alone, and found a reduction in VAP. The usual‐care intervention comprised the participant's bed being elevated 30° to 45°, hypopharyngeal suctioning, lips moistened with 'toothette' swab and water, then further hypopharyngeal suctioning. A second study with 147 participants, also assessed at high risk of bias (Pobo 2009), compared powered toothbrushing plus usual care including chlorhexidine, with usual care alone, and found no difference in the outcome of VAP. The combined estimate from these studies showed no difference in the incidence of VAP (RR 0.49, 95% CI 0.16 to 1.53, P = 0.22, I2 = 75%) (Analysis 2.1, Subgroup 2.1.1), with the heterogeneity probably due to the additional exposure to chlorhexidine in both groups of only one of the studies.
In Lorente 2012 (436 participants), where the intervention group received toothbrushing with a manual toothbrush as well as chlorhexidine, compared to chlorhexidine alone in the control group, there was no evidence of a difference in the incidence of VAP between the intervention and control groups (Analysis 2.1, Subgroup 2.1.2).
Munro 2009, a study with a factorial design in 192 participants, compared toothbrushing with no toothbrushing (equal exposure to chlorhexidine in both arms), and reported no difference in the development of VAP (Analysis 2.1, Subgroup 2.1.3).
A further study (Long 2012; 61 participants) compared toothbrushing plus povidone iodine with povidone iodine alone, and found some evidence for a benefit for toothbrushing (Analysis 2.1, Subgroup 2.1.4). The results of this study have not been replicated, so should be interpreted with caution.
Bopp 2006 was a very small pilot study (n = 5) of toothbrushing versus none, and the data are reported in Additional Table 7. There were no numerical outcome data in the study by Fields 2008; the report makes the statement that "the VAP rate dropped to zero within a week of beginning the every 8 hours toothbrushing regimen in the intervention group." This rate of zero incidence of VAP was reportedly sustained for six months. Roca Biosca 2011 recruited 117 participants and reported a summary estimate for the outcome of VAP, with no difference between powered toothbrushing and no toothbrushing (Additional Table 7).
Mortality
Five studies (889 participants) evaluated the effect of toothbrushing, as an addition to oral care, on the outcome of mortality (Long 2012; Lorente 2012; Munro 2009; Pobo 2009; Yao 2011). The comparisons were different in each trial and there was no evidence of a difference in mortality with or without toothbrushing (RR 0.87, 95% CI 0.70 to 1.09, P = 0.24 , I2 = 0%) (Analysis 2.2).
2.2. Analysis.
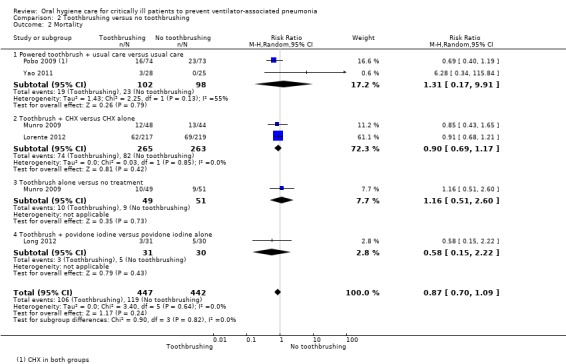
Comparison 2 Toothbrushing versus no toothbrushing, Outcome 2 Mortality.
Duration of ventilation
Meta‐analysis of two trials of chlorhexidine (583 participants) reported the outcome of mean duration of mechanical ventilation, and showed no difference associated with toothbrushing (MD ‐0.85 days, 95% CI ‐2.43 to 0.73 days, P = 0.29, I2 = 0%; fixed‐effect model) (Analysis 2.3). A further trial of povidone iodine also failed to show a benefit for toothbrushing for this outcome (Long 2012).
2.3. Analysis.
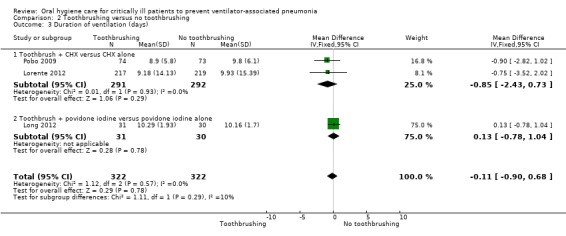
Comparison 2 Toothbrushing versus no toothbrushing, Outcome 3 Duration of ventilation (days).
Duration of ICU stay
Meta‐analysis of two trials (583 participants) that reported the outcome of mean duration of ICU stay found no evidence of a difference between the groups (MD ‐1.82 days, 95%CI ‐3.95 to 0.32 days, P = 0.10, I2 = 0%, fixed‐effect model, Analysis 2.4). The data from Bopp 2006 are reported in Additional Table 7.
2.4. Analysis.
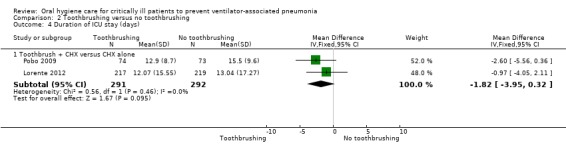
Comparison 2 Toothbrushing versus no toothbrushing, Outcome 4 Duration of ICU stay (days).
Use of systemic antibiotics
This outcome was not reported by any of the studies in this group.
Oral health indices: plaque score
One study (Yao 2011) also reported the outcome of plaque score in each group after seven to eight days. The study failed to show evidence of reduced plaque in the toothbrushing group (Analysis 2.5).
2.5. Analysis.
Comparison 2 Toothbrushing versus no toothbrushing, Outcome 5 Plaque score.
Roca Biosca 2011 reported plaque scores, without any estimates of variance. The trial report also stated that there was no difference between the groups (Additional Table 7).
Adverse effects
Pobo 2009 reported that there were no adverse effects reported in either arm of the study and none of the other studies in this comparison mentioned adverse effects.
Other outcomes
The outcomes of caregivers' preferences and cost were not reported.
Comparison 3: Powered toothbrushing versus manual toothbrushing
One small study of 78 participants (Prendergast 2012), assessed at high risk of bias, compared the use of a powered toothbrush as a component of 'comprehensive oral care' with a control group receiving manual toothbrushing and standard oral care.
In this study there was no difference between the intervention and control groups for the outcomes of incidence of VAP, mortality or mean duration of ventilation or ICU stay (Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4). There were no adverse effects mentioned in this study. The outcomes of oral health indices, systemic antibiotic therapy, caregivers' preferences for oral hygiene care or cost were not reported in the study.
3.1. Analysis.
Comparison 3 Powered toothbrush versus manual toothbrush, Outcome 1 Incidence of VAP.
3.2. Analysis.
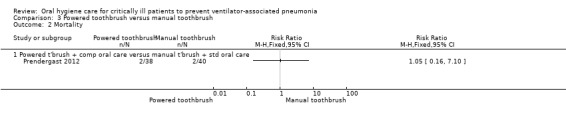
Comparison 3 Powered toothbrush versus manual toothbrush, Outcome 2 Mortality.
3.3. Analysis.
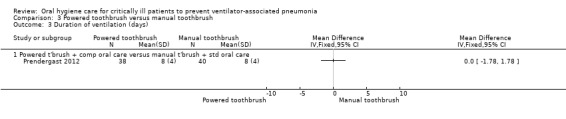
Comparison 3 Powered toothbrush versus manual toothbrush, Outcome 3 Duration of ventilation (days).
3.4. Analysis.
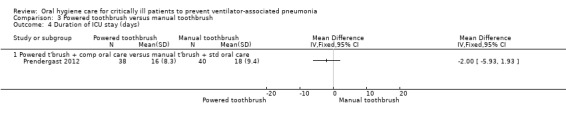
Comparison 3 Powered toothbrush versus manual toothbrush, Outcome 4 Duration of ICU stay (days).
Comparison 4: Other oral care solutions
Thirteen studies were included in this comparison, with a total of 2702 participants randomised to treatments (Berry 2011; Berry 2013; Caruso 2009; Feng 2012; Hu 2009; Mo 2016; Seguin 2006; Seguin 2014; Stefanescu 2013; Tang 2013; Xu 2007; Xu 2008; Zhao 2012). Twelve of these studies were at high risk of bias and Seguin 2014 was at low risk of bias. The studies evaluated the effects of other solutions with a potential antiseptic effect on the outcomes of VAP, mortality, duration of ventilation, and duration of ICU stay.
Incidence of VAP
Three studies (356 participants) compared povidone iodine rinse with a saline rinse or placebo (Feng 2012; Seguin 2006; Seguin 2014). They showed evidence of a reduction in VAP (RR 0.69, 95% CI 0.50 to 0.95, P = 0.02, I2 = 74%, fixed‐effect model) (Analysis 4.1, Subgroup 4.1.1).
4.1. Analysis.
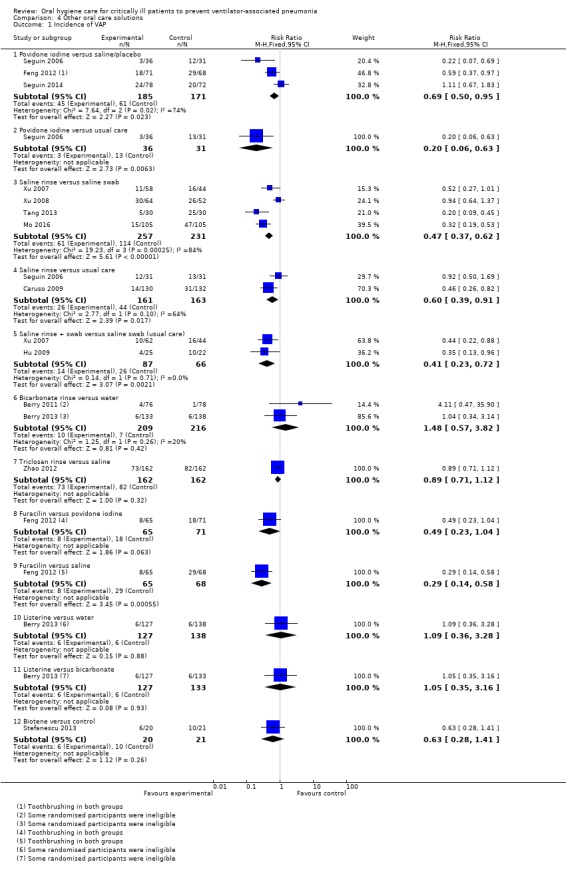
Comparison 4 Other oral care solutions, Outcome 1 Incidence of VAP.
Seguin 2006 (67 participants) also compared povidone iodine rinse with usual care (suction alone with no rinse) and found a reduction in VAP (Analysis 4.1, Subgroup 4.1.2). The result of this study has not been replicated, so should be interpreted with caution.
Four studies (488 participants) (Mo 2016; Tang 2013; Xu 2007; Xu 2008), all at high risk of bias, which compared a saline rinse with a saline‐soaked swab found some weak evidence that saline rinse reduced the incidence of VAP (RR 0.47, 95% CI 0.37 to 0.62, P < 0.001, I2 = 84%, fixed‐effect model) (Analysis 4.1, Subgroup 4.1.3).
Two studies (Caruso 2009; Seguin 2006; 324 participants), both at high risk of bias, compared a saline rinse with usual care (no rinse) and found a reduction in VAP (RR 0.60, 95% CI 0.39 to 0.91, P = 0.02, I2 = 64%, fixed‐effect model) (Analysis 4.1, Subgroup 4.1.4). While this result should be interpreted cautiously due to the high risk of bias, there appears to be some evidence that the use of a saline rinse prior to aspiration of secretions was associated with reduction of VAP.
Hu 2009 and Xu 2007, both at high risk of bias, compared both saline rinse plus swab, with a saline‐soaked swab alone (usual care) and found some very weak evidence (from 153 participants) that the combined rinse plus swab reduced the incidence of VAP (RR 0.41, 95% CI 0.23 to 0.72, P = 0.002, I2 = 0%, fixed‐effect model) (Analysis 4.1, Subgroup 4.1.5).
Two studies (Berry 2011; Berry 2013; 425 participants), both at high risk of bias, compared bicarbonate rinse plus toothbrushing with a water rinse plus toothbrushing and found no evidence of a difference in the incidence of VAP (RR 1.48, 95% CI 0.57 to 3.82, P = 0.42, I2 = 20%, fixed‐effect model) (Analysis 4.1, Subgroup 4.1.6).
A single study compared triclosan rinse with saline rinse and found no difference in the outcome of VAP over the duration of the study (Zhao 2012) (Analysis 4.1, Subgroup 4.1.7). The results of this study have not been replicated, so should be interpreted with caution.
A single three‐arm study compared povidone iodine, furacilin and usual care (Feng 2012). It found both antiseptics combined with toothbrushing were more effective than usual care (Analysis 4.1, Subgroup 4.1.1 and Analysis 4.1, Subgroup 4.1.9) with little difference between the two antiseptic solutions (Analysis 4.1, Subgroup 4.1.8).
A single study (Berry 2013; 265 participants), comparing Listerine with water, and Listerine with bicarbonate, found no evidence of a difference in VAP incidence (Analysis 4.1, Subgroups 4.1.10 and 4.1.11).
Another single study (Stefanescu 2013; 41 participants) compared Biotene OralBalance with control and found no difference in incidence of VAP (Analysis 4.1, Subgroup 4.1.12).
Mortality
Seven studies reported mortality in the following comparisons (Analysis 4.2).
4.2. Analysis.
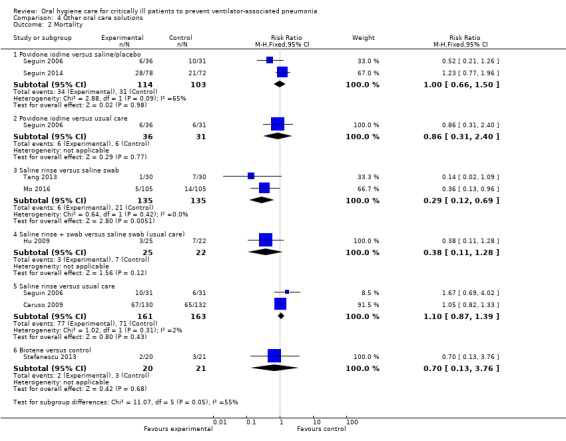
Comparison 4 Other oral care solutions, Outcome 2 Mortality.
Povidone iodine versus saline/placebo: two studies (217 participants) (RR 1.00, 95% CI 0.66 to 1.50, P = 0.98, I2 = 65%; fixed‐effect model), no evidence to suggest a difference in mortality.
Povidone iodine versus usual care: single study (67 participants), no evidence to suggest a difference.
Saline rinse versus saline swab: two studies (270 participants) (RR 0.29, 95% CI 0.12 to 0.69; P = 0.005, I2 = 0%; fixed‐effect model), suggesting a significant reduction in mortality for saline rinse.
Saline rinse versus usual care: two studies (324 participants) (RR 1.10, 95% CI 0.87 to 1.39, P = 0.43, I2 = 2%; fixed‐effect model), no evidence to suggest a difference in mortality.
Saline rinse plus swab versus saline swab (usual care): single study (47 participants), no evidence to suggest a difference.
Biotene OralBalance versus control: single study (41 participants), no evidence to suggest a difference.
Duration of ventilation
Six studies reported duration of ventilation (days) in the following comparisons (Analysis 4.3).
4.3. Analysis.
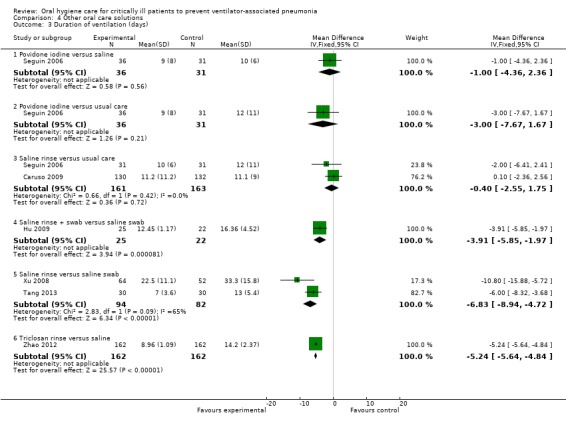
Comparison 4 Other oral care solutions, Outcome 3 Duration of ventilation (days).
Povidone iodine versus saline/placebo: single study (67 participants), no evidence to suggest a difference.
Povidone iodine versus usual care: single study (67 participants), no evidence to suggest a difference.
Saline rinse versus usual care: two studies (324 participants) (MD ‐0.40 days, 95% CI ‐2.55 to 1.75 days, P = 0.72, I2 = 0%), no evidence to suggest a difference in duration of ventilation.
Saline rinse plus swab versus saline swab (usual care): single study (47 participants) suggesting a statistically significant effect in favour of shorter duration for the saline rinse plus swab.
Saline rinse versus saline swab: two studies (176 participants) (MD ‐6.83 days, 95% CI ‐8.94 to ‐4.72 days; P < 0.00001, I2 = 65%) suggest saline rinse leads to shorter duration of ventilation.
Triclosan rinse versus saline: single study (324 participants) suggesting that triclosan leads to shorter duration of ventilation than saline.
Berry 2013, comparing Listerine with water, and Listerine with bicarbonate, found no difference among groups in median ventilation hours. Another study (Stefanescu 2013), comparing Biotene OralBalance and control, also found no difference between groups in duration of ventilation. (Additional Table 7)
Duration of ICU stay
Four studies reported duration of ICU stay (days) in the following comparisons (Analysis 4.4).
4.4. Analysis.
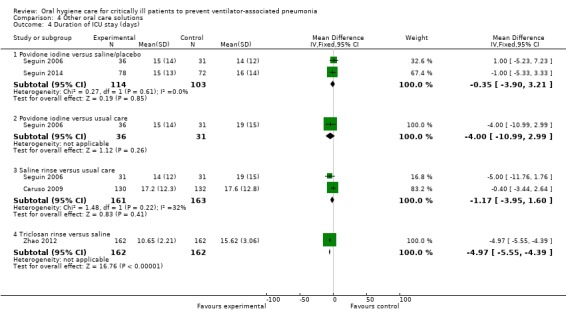
Comparison 4 Other oral care solutions, Outcome 4 Duration of ICU stay (days).
Povidone iodine versus saline/placebo: two studies (217 participants) (MD ‐0.35 days, 95% CI ‐3.90 to 3.21 days, P = 0.85, I2 = 0%; fixed‐effect model), no evidence to suggest a difference.
Povidone iodine versus usual care: single study (67 participants), no evidence to suggest a difference.
Saline rinse versus usual care: two studies (324 participants) (MD ‐1.17 days, 95% CI ‐3.95 to 1.60 days, P = 0.41, I2 = 32%; fixed‐effect model), no evidence to suggest a difference in duration of ICU stay.
Triclosan rinse versus saline: single study (324 participants), suggesting that triclosan leads to shorter stay in ICU than saline.
Another study (Berry 2013), comparing Listerine with water, and Listerine with bicarbonate, found no difference among groups in median ICU length of stay (Additional Table 7).
Use of systemic antibiotics
Seguin 2014, comparing povidone iodine and placebo, showed no evidence of a difference in the number of participants treated with systemic antibiotics (Analysis 4.5). Berry 2013, comparing Listerine with water, and Listerine with bicarbonate, found no difference among groups in antibiotic administration. See Additional Table 7.
4.5. Analysis.
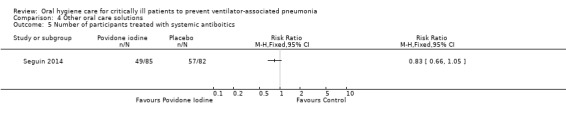
Comparison 4 Other oral care solutions, Outcome 5 Number of participants treated with systemic antiboitics.
Adverse effects
Seguin 2014 found no evidence of a difference in the occurrence of acute respiratory distress syndrome, agitation and/or hypertension, epistaxis, oxygen desaturation and aspiration (Analysis 4.6). Berry 2013 found no adverse events associated with interventions. Stefanescu 2013, comparing Biotene OralBalance and control, found no significant difference between groups with respect to adverse events in buccal mucosa. See Additional Table 7.
4.6. Analysis.
Comparison 4 Other oral care solutions, Outcome 6 Adverse effects.
Discussion
Summary of main results
We included 38 randomised controlled trials in this updated review and these studies evaluate four main groups of interventions in the oral hygiene care of critically ill patients receiving mechanical ventilation for at least 48 hours in intensive care units.
Chlorhexidine (CHX) antiseptic versus placebo/usual care (with or without toothbrushing)
There is high quality evidence from 18 RCTs that the use of chlorhexidine (either as a mouthrinse or a gel) reduces the incidence of ventilator‐associated pneumonia (VAP) from 24% to about 18% (Table 1). There is no evidence that use of chlorhexidine is associated with a difference in mortality (moderate‐quality evidence), duration of mechanical ventilation (low‐quality evidence) or duration of ICU stay (moderate quality evidence). There is insufficient evidence to determine the effect of chlorhexidine on the other secondary outcomes of this review.
Toothbrushing versus no toothbrushing (with or without antiseptics)
Based on five RCTs (very low quality evidence), we found no evidence of a difference in the incidence of VAP due to toothbrushing. There is also no evidence for a difference between toothbrushing or no toothbrushing for the outcomes of mortality (low quality evidence), duration of ventilation (low quality evidence) or duration of ICU stay (very low quality evidence) (Table 2).
Oral care with powered toothbrush versus oral care with manual toothbrush
From the single study in this comparison, there is insufficient evidence to determine the effects of powered versus manual toothbrushing on the outcomes of VAP, mortality, duration of mechanical ventilation or duration of ICU stay.
Oral care with other solutions
The studies in this comparison, most of which are at high overall risk of bias, made different comparisons. For the reduction of VAP, there is some weak evidence that povidone iodine rinse is more effective than saline/placebo, use of saline rinse is more effective than saline swab, use of both a saline swab and a saline rinse may be more effective than a saline swab alone, and use of saline rinse may be more effective than usual care. There is no evidence of a difference between bicarbonate rinse and a water rinse.
For the outcome of mortality, we found no evidence of a difference between povidone iodine rinse and saline/placebo or between saline rinse and usual care. We found some very weak evidence of a difference between saline rinse and saline swab.
For the duration of ventilation, we found no evidence of a difference between saline rinse and usual care, and some weak evidence that saline rinse leads to shorter duration of ventilation compared to saline swab. For the duration of ICU stay, we found no evidence of a difference between povidone iodine and saline/placebo or between saline rinse and usual care.
Overall completeness and applicability of evidence
In this review, we have included studies that compared active oral hygiene care interventions with either placebo or usual care. We recognise that the use of a placebo is a better control comparison in research studies because it enables the masking of caregivers to which group participants are in active or control group, thus eliminating some possible performance bias. However, we chose to include pragmatic studies where 'usual care' was the control comparator, despite recognising that in many instances 'usual care' was not specified and may have varied between participants and between individual caregivers. Where there was no blinding, we assessed studies as being at high risk of performance and detection bias.
There are some other variables which may have influenced the outcomes in the included studies. These include the number of episodes of OHC a day, the 'dose' of the antiseptic, and whether participants were dentate or edentulous. Most of the studies (79%) stated that they delivered between two and four episodes of OHC per day. Nine studies specified that edentulous people were excluded, one study focused on newborns, but most of the included studies did not report whether or not participants were dentate. We investigated whether there was a dose‐response effect and could find no evidence for this.
We also recognise that participation in a research study is likely to have a positive effect on the performance of 'usual care', improving both the quality of care and compliance with routine practice ‐ a Hawthorne effect (McCarney 2007). The combination of a 'usual care' control group, the absence of caregiver blinding in most cases, and the Hawthorne effect of being part of a study may have reduced the observed difference in effect between the active and control interventions in these studies. Two of the studies noted that care was recorded in patient notes, but none of the studies included in this review reported compliance with oral hygiene care protocols.
Another area of variability between the studies (and possibly also between studies and usual practice) is the diagnosis of VAP, which is at least partly subjective and may be based on variable diagnostic criteria. Most of the included studies (33/38) stated the criteria used to diagnose VAP, of which the two most common were some version of the clinical pulmonary infection score (CPIS) based on Pugin's criteria (Cook 1998; Pugin 1991) (17 studies) and Centers for Disease Control (CDC) criteria as described in Horan 2008 (six studies). Six studies conducted in China used Chinese Society of Respiratory Diseases (CSRD) criteria for diagnosis of VAP (Chen 2008; Feng 2012; Mo 2016; Tang 2013; Xu 2007; Xu 2008).
Currently there is no clearly accepted gold standard for the diagnosis of VAP, and when different criteria are applied to the same cohort of patients, the estimated VAP prevalence could vary widely (Klompas 2007). In light of the limited sensitivity and specificity of the traditional VAP diagnosis, the US Centers for Disease Control (CDC) has recently developed a new surveillance criterion, ventilator‐associated event (VAE), to incorporate all complications (including VAP) leading to the worsening of gas exchange in mechanically‐ventilated patients. However, the advent of a more objective and definitive diagnosis of VAP may depend on further development of biomarker technologies, which may not occur in the near future. (Waters 2015)
Although this review found evidence that the use of chlorhexidine as part of oral care reduces the incidence of VAP, there was no evidence of a reduction in mortality. This is in contrast to a review by Price 2014, which claimed that CHX is possibly associated with increased mortality. There has been some debate in the literature about the attributable mortality of VAP, but a recent survival analysis of nearly 4500 patients found that ICU mortality attributable to VAP was about 1% on day 30 (Bekaert 2011), which might explain our findings.
This review has not found evidence that oral care including both toothbrushing and chlorhexidine is different from oral care with chlorhexidine alone in reducing VAP. Only one of the trials of toothbrushing which reported the outcome of VAP also reported plaque levels as an indicator of the effectiveness of the toothbrushing carried out in this trial (Yao 2011). This small trial (53 participants), which we assessed at high risk of bias, did not use chlorhexidine in either group, and found a reduction in both plaque and VAP in the powered toothbrushing group compared to the no‐toothbrushing group. Three other trials of toothbrushing in our meta‐analysis (Lorente 2012 (manual), Munro 2009 (manual), Pobo 2009 (powered toothbrush), with a combined total of 775 participants included exposure to chlorhexidine in both intervention and control groups. Assessed at unclear, high and high risks of bias respectively, meta‐analysis of these three trials showed no evidence of a difference in the outcome of VAP. A further study (Roca Biosca 2011), included in this review and also at high risk of bias, could not be included in the meta‐analysis, but also found no difference between oral care with chlorhexidine and toothbrushing and oral care with chlorhexidine alone. All five of these studies described the toothbrushing intervention in detail, and noted that nurses delivering the intervention received specific training. While the presence of ventilator tubes in the mouths of trial participants makes effective toothbrushing difficult, it seems likely that, despite this, the toothbrushing intervention was carried out thoroughly within these trials.
Earlier cohort studies noted that patients in ICU who developed VAP were likely to have increased length of stay in the ICU (Apostolopoulou 2003; Cook 1998). However, our Cochrane Review has not evaluated duration of ICU stay in patients who develop VAP. The studies in our review reported mean length of ICU stay and the standard deviation for each arm of the study. We have combined these in meta‐analyses based on an assumption that the duration of ICU stay in each arm of each trial follows an approximately normal distribution. In fact, the distribution of duration of stay in ICU is likely to be skewed, and the means are likely to be a poor indicator of the effect of oral hygiene care on duration of ICU stay.
Our review has not looked at the outcome of cost of interventions. However, it is likely that the additional cost of using an antiseptic mouthrinse or gel is low in comparison with the cost of the antibiotics used to treat VAP. One study reported the cost of the chlorhexidine gluconate solution by participant was USD 3.15 (Jacomo 2011), while the cost associated with a single incident of VAP was estimated at USD 10,000 to 40,000 (Hillier 2013; Waters 2015). Reducing the incidence of VAP using relatively inexpensive additions to usual care is likely to be cost‐effective, as well as avoiding additional morbidity for the patient.
The increasing incidence of bacteria which are resistant to current antibiotics is of concern worldwide, and one of the reasons for bacterial resistance is the overuse of systemic antibiotics (Gyssens 2011). Oral hygiene care using antiseptics such as chlorhexidine, to reduce the risk of VAP, could potentially also result in a reduced requirement for these patients to be treated with systemic antibiotics. Because only four of the 38 studies included in this review provided data about the duration of antibiotic use in study participants, we do not have sufficient information to determine whether there was any effect on systemic antibiotic use.
It is interesting that only one of the 19 studies that evaluated chlorhexidine reported adverse reactions to chlorhexidine (mild reversible irritation of the oral mucosa) (Tantipong 2008). Hypersensitivity is a rare but potentially severe side effect of chlorhexidine (Pemberton 2012). In over 2000 participants included in these studies there was no report of hypersensitivity to chlorhexidine. However, it is notable that in six of the included studies (DeRiso 1996; Jacomo 2011; Kusahara 2012a; Ozcaka 2012; Scannapieco 2009; Sebastian 2012), a prior history of hypersensitivity to chlorhexidine was an exclusion criterion during participant recruitment. In view of recent reports in the UK of two cases of serious adverse events associated with irrigation of dry socket with chlorhexidine mouthrinse, it is recommended that all members of the dental team prescribing chlorhexidine products are aware of the potential for both minor and serious adverse side effects (Pemberton 2012).
Quality of the evidence
All the included studies were prospective, randomised controlled trials, but only five of them (13%) were assessed at low risk of bias (Bellissimo‐Rodrigues 2009; Fourrier 2005; Koeman 2006; Ozcaka 2012; Sebastian 2012) for all domains. However, we did not consider that the impact of bias reduced our confidence in the outcome of VAP incidence. Although more than two‐thirds of included studies had a high risk of bias for at least one domain, sensitivity analysis by risk of bias did not alter the size or direction of the effect for VAP (see Table 1). This provides support for our decision to consider the quality of the evidence for this outcome to be high. In contrast, we downgraded the quality of evidence for duration of ventilation and stay in ICU, because we could not rule out bias having a greater impact on these resource use outcomes. Most studies did not provide information on adverse events, and the scant information we could obtain from two studies prompted us to downgrade the quality of evidence to very low.
Potential biases in the review process
In order to reduce the risk of publication bias, we conducted a broad search for both published and unpublished studies, with no restrictions on language. We searched the reference lists of included studies and contacted many of the study authors in order to obtain information that was not included in the published reports. We also searched the reference lists of other published reviews of oral hygiene care for critically ill patients.
For this review we also chose very broad inclusion criteria, which has resulted in a clinically heterogeneous group of studies including adults, children and neonates, and a range of indications for ICU care, including medical conditions, surgery and trauma where patients were ventilated for over 48 hours. In some of the included studies, the precise details of what was involved in the oral hygiene care intervention were poorly described, making it difficult to determine the similarity between studies in oral hygiene care practices. There was also potential variation in the methods used for intubation and for the calculation of duration outcomes (e.g. duration of mechanical ventilation, duration of ICU stay) (Contentin 2014), both of which were not always clearly specified.
One other potential bias in this review is the variation in and the subjective nature of criteria/methods used for VAP diagnosis (Klompas 2007). Also, we have made a number of changes to the methods of this review since the publication of the protocol (see Differences between protocol and review). Some of these changes were clarifications, and some were to take account of other Cochrane Reviews published or in preparation, to avoid unnecessary duplication of effort. We acknowledge that post hoc changes to the review methods may introduce a risk of bias into this review.
Agreements and disagreements with other studies or reviews
A previous meta‐analysis by Pineda 2006 found that the use of chlorhexidine for oral decontamination did not reduce the incidence of nosocomial pneumonia. However, their meta‐analysis included only four studies and the outcome was nosocomial pneumonia rather than VAP. Another systematic review by Labeau 2011 included 14 studies of either chlorhexidine or povidone iodine antiseptics and found that the use of antiseptics as part of oral hygiene care reduced the incidence of VAP by approximately one‐third. Our review confirmed these findings.
One recent systematic review (Price 2014) has looked at the effects of selective digestive/oropharyngeal decontamination and topical oropharyngeal chlorhexidine on the prevention of death in general intensive care, and claimed that CHX is possibly associated with increased mortality (odds ratio (OR) 1.25, 95% CI 1.05 to 1.50). Reasons for the discrepancy between this finding and ours mainly include differences in the review scope (e.g. whether focused on adults, general intensive care only) and review methodology (e.g. inclusion of studies for which only abstracts are available). With less strict eligibility criteria for settings and participants and more stringent inclusion criteria for the reporting and methodology of primary studies, we believe that our finding is more generalisable and reflects the current evidence base. More trials are needed of the association between CHX usage and ICU mortality, to provide more insight into this issue.
Two more recent systematic reviews have looked at the effects of chlorhexidine with different concentrations. One claimed that the use of higher concentration chlorhexidine was associated with higher mortality (Klompas 2014), and the other stated that chlorhexidine with the concentration of 0.12% had the best effect in reducing VAP incidence (Zhang 2013). However, these findings were all based on trivial differences in point estimates, with wide confidence intervals for each estimate and statistically non‐significant differences between concentrations. The results of our sensitivity analyses do not support the dose‐response relationships that they proposed, and confirm that differences between concentrations were statistically non‐significant.
Two published meta‐analyses of toothbrushing to reduce VAP included four trials and found no evidence for a difference in incidence of VAP, again possibly due to low statistical power (Alhazzani 2013; Gu 2012). Our review reaches similar conclusions.
Authors' conclusions
Implications for practice.
Effective oral hygiene care is important for ventilated patients in intensive care to reduce ventilator‐associated pneumonia. The definition of oral hygiene care varied among the studies included in this review, but common elements include cleaning of the teeth and gums with a swab or gauze, removing secretions using suction, and rinsing the mouth. There is evidence from our review that oral hygiene care incorporating chlorhexidine mouthrinse or gel is effective in reducing the development of ventilator‐associated pneumonia in adults in intensive care. We found no evidence of an association between oral hygiene care and mortality, duration of mechanical ventilation, and duration of ICU stay. For the other comparisons assessed in this review, fewer studies contributed evidence and consequently the quality of the body of evidence was lower.
Implications for research.
Although the included studies provided some evidence for the benefits of oral hygiene care for critically ill patients to prevent ventilator‐associated pneumonia, incomplete reporting of studies is a major limitation. More consistent use of the CONSORT statement for reporting of randomised controlled trials (CONSORT 2012) would increase the value of research.
Detailed reporting of methods, such as generation of allocation sequence, allocation concealment, and numbers and reasons for withdrawals and exclusions.
Use of a placebo where possible to enable blinding.
Full reporting of methods used to diagnose ventilator‐associated pneumonia.
Reporting of adverse effects of interventions.
Further trials of oral hygiene care (including use of manual or powered toothbrushes, or swabs) should report both measures of effectiveness of plaque removal and prevention of ventilator‐associated pneumonia. They should also state explicitly whether those patients who have died during the study are included in the calculation of duration outcomes (e.g. duration of ICU stay, duration of mechanical ventilation).
Future studies may also consider adopting the new definitions and diagnostic criteria (ventilator‐associated event, VAE) recently developed by the US CDC (Waters 2015), which is likely to overcome the limitations of traditional VAP diagnosis and facilitate high quality synthesis of research findings.
Feedback
Mortality data for chlorhexidine, 23 November 2016
Summary
Hua et al examined the effect of chlorhexidine (CHX) on mortality (analysis 1.2) and found no benefit of CHX compared to placebo (risk ratio (RR) 1.09, 95% confidence interval (CI) 0.96 to 1.23).a Those results differ to those derived in a recent meta‐analysis by our group (odds ratio (OR) 1.25, 95% CI 1.05 to 1.50).b The review authors specifically discussed this and suggested that this discrepancy could be accounted for by differences in review methodology.
We fully agree. We sought to explore the effect of CHX (and selective digestive or oropharygneal decontamination) on mortality in general adult intensive care units so we excluded studies on cardiac surgery patients and children. We did include a study by MacNaughton et al that was published only as an abstractc. Hua et al suggested that our inclusion of this abstract might contribute to the observed difference in the two pooled estimates. However, removal of this study from our meta‐analysis leads to a very similar result (OR 1.29, 95% CI 1.07 to 1.56).
When considering the studies included in both our reviews, there were three studies that both Hua et al and we identified but handled differently. We wonder if this accounts for much of the observed discrepancy.
Berry et ald: the primary outcome of this study was bacterial growth at day 4. Berry et al accordingly excluded patients who had died within 96 hours from their analysis; this is shown in Figure 1 of their paper. Although Hua et al state that they used intensive care unit (ICU) mortality when available, they appear to have used these 96‐hour mortality data in their pooled estimates. Berry et al did not publish ICU mortality data but we managed to obtain the data from them.
Koeman et ale: the authors omitted mortality data from this moderately large (relevant arms consisting of 257 patients) and robust trial from their pooled estimates. We included these data. Of note, other meta‐analyses on this subject that have examined mortality have included this study in their pooled estimates; Hua et al are unique in choosing to exclude it (references on request).
Munro et alf Hua et al chose to use the “day 3 analysis sample” rather than obtaining intention‐to‐treat data. This means they have included only 192 patients out of an enrolled population of 547 (the largest randomised controlled trial on this subject published to date). In their paper, Munro et al explained how this group came about: “Of the 547 enrolled patients, 249 were still endotracheally intubated on study day 3; of these, 209 patients had complete day 3 CPIS [Clinical Pulmonary Infection Score] data. Because of missing values on some of the components of the CPIS, only 192 patients had CPIS values on both days 1 and 3, and their data could be analyzed completely.” Accordingly, 298 patients have been excluded either owing to extubation or death and 57 patients have been excluded owing to lack of data for a scoring system that is irrelevant to the outcome of death.
In summary, the authors have, in our view, three relevant omissions in their dataset. These might be for reasons such as being unable to contact authors, as indeed was the case for one of our included studiesg. Nevertheless, there has been substantial attrition of potentially available data in their pooled estimate and accordingly we question if they are correct in their claim that their result “reflects the current evidence base”.
I do not have any affiliation with or involvement in any organisation with a financial interest in the subject matter of my comment.
References:
aHua F, Xie H, Worthington HV, Furness S, Zhang Q, Li C. Oral hygiene care for critically ill patients to prevent ventilator‐associated pneumonia. Cochrane Database of Systematic Reviews 2016, Issue 10. Art. No.: CD008367. DOI: 10.1002/14651858.CD008367.pub3. bPrice R, MacLennan G, Glen J. Selective digestive or oropharyngeal decontamination and topical oropharyngeal chlorhexidine for prevention of death in general intensive care: systematic review and network meta‐analysis. BMJ 2014; 348: g2197. cMacNaughton PD, Bailey J, Donlin N, Branfield P, Williams A, Rowswell H. A randomised controlled trial assessing the efficacy of oral chlorhexidine in ventilated patients. Intensive Care Medicine 2004; 30: S12. dBerry AM, Davidson PM, Masters J, Rolls K, Ollerton R. Effects of three approaches to standardized oral hygiene to reduce bacterial colonization and ventilator associated pneumonia in mechanically ventilated patients: A randomised control trial. International Journal of Nursing Studies 2011; 48: 681‐8. eKoeman M, Van der Ven AJ, Hak E, Joore HC, Kaasjager K, De Smet AM, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator‐associated pneumonia. American Journal of Respiratory Critical Care Medicine 2006; 173: 1348‐55. fMunro CL, Grap MJ, Jones DJ, McClish DK, Sessler CN. Chlorhexidine, toothbrushing and preventing ventilator associated pneumonia in critically ill adults. American Journal of Critical Care 2009; 18: 428‐38. gPanchabhai TS, Dangayach NS, Krishnan A, Kothari VM, Karnad DR. Oropharyngeal cleansing with 0.2% chlorhexidine for prevention of nosocomial pneumonia in critically ill patients. Chest 2009; 135: 1150‐6.
Reply
We thank Price and colleagues for their feedback on our reviewa. We know that the association between oral care using CHX and ICU mortality has been a hot topic in recent years, with lots of new discussions in the literature.b,c,d,
In the Discussion section of our review, we mentioned the Price 2014 reviewc and said, “Reasons for the discrepancy between this finding and ours mainly include differences in the review scope (e.g. whether focused on adults, general intensive care only) and review methodology (e.g. inclusion of studies for which only abstracts are available).”
Firstly, in their review Price et alc excluded trials carried out on cardiac surgery patients and children, while our review did not have such restrictions. Also, one of our main inclusion criteria for trials and participants was mechanical ventilation for a minimum of 48 hours, but Price et alc did not state such a requirement. Therefore the questions that these two reviews tried to answer were essentially different. For instance, the DeRiso 1996 studye, which enrolled only cardiac surgery patients, was included in our review but not in Price 2014c. If Price 2014 were to include this study, their pooled OR would become 1.19 (95% CI 0.96 to 1.46). Actually, other similar systematic reviewsb,f have also included DeRiso 1996. In addition, according to a post‐hoc subgroup analysis, we found that the mortality results of the adult trials and the child trials included in our Analysis 1.2 were not significantly different (P = 0.14), indicating that analysing these two types of trials separately may not be necessary.
Secondly, there is potential risk in directly using unpublished trial data from previous systematic reviews. Such data may or may not be trustworthy. One perfect example here is that for the same MacNaughton 2004 studyg, an abstract that did not clearly report results for mortality, data used in Price 2014 (29/101 for treatment, 29/93 for control)c and Klompas 2014 (36/91 for treatment, 33/88 for control)b differed substantially. In terms of Koeman 2006, we noticed that both Price et alc and Klompas et alb used data provided in a previous systematic reviewf, but without verifying the data by contacting original authors. As documented in our 'Characteristics of included studies' table, we tried to contact Koeman et alh for data confirmation but failed (invalid email address). Plus, if we used the same Koeman data as in Price 2014c, our pooled RR would remain similar (1.11, 95% CI 0.99 to 1.25).
We noticed that for multiple studies, including Berry 2011i and Munro 2009j, the data that we used for Analysis 1.2 were different from those used in the Price review [3]. As all of these data were extracted for the previously published version of our reviewk, we re‐examined this data and agree that the inclusion of the 96‐hour mortality data in Berry 2011 is inappropriate and we have therefore decided to exclude these data from our Analysis 1.2. After this revision, our pooled RR remains 1.09 (95% CI 0.96 to 1.23). As to other studies included in this analysis, no mistakes in the use of data were found so we do not plan to make other modifications.
As described in our Abstract and Methods section, the pre‐determined primary objective of our review was to assess the effects of oral hygiene care on the incidence of ventilator‐associated pneumonia (VAP) in critically ill patients receiving mechanical ventilation in ICUs. The participants that we were interested in were those who received mechanical ventilation for at least 48 hours and therefore were at risk of developing VAP. Thus, our use of the Day 3 sample data in Munro 2009j was reasonable. The same reason (pre‐determined PICO) can also explain the differences between our review and Price 2014c for the data of Panchabhai 2009l, Cabov 2010m and Bellissimo‐Rodrigues 2009n. Authors of the previous version of our reviewk obtained relevant data that met our criteria from the original authors of those studies.
Thank you for your interest in our work.
References
aHua F, Xie H, Worthington HV, Furness S, Zhang Q, Li C. Oral hygiene care for critically ill patients to prevent ventilator‐associated pneumonia. Cochrane Database of Systematic Reviews 2016, Issue 10. Art. No.: CD008367. DOI: 10.1002/14651858.CD008367.pub3. bKlompas M, Speck K, Howell MD, Greene LR, Berenholtz SM. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: systematic review and meta‐analysis. JAMA Internal Medicine 2014; 174: 751‐61. cPrice R, MacLennan G, Glen J, Su DC. Selective digestive or oropharyngeal decontamination and topical oropharyngeal chlorhexidine for prevention of death in general intensive care: systematic review and network meta‐analysis. BMJ 2014; 348: g2197. dKlompas M, Li LL, Kleinman K, Szumita PM, Massaro AF. Associations between ventilator bundle components and outcomes. JAMA Internal Medicine 2016; 176: 1277‐83. eDeRiso AJ, 2nd, Ladowski JS, Dillon TA, Justice JW, Peterson AC. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest 1996; 109: 1556‐61. fChan EY, Ruest A, Meade MO, Cook DJ. Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and meta‐analysis. BMJ 2007; 334: 889. gMacNaughton P, Bailey J, Donlin N, Branfield P, Williams A, Rowswell H. A randomised controlled trial assessing the efficacy of oral chlorhexidine in ventilated patients. Intensive Care Medicine 204; 30: S12. hKoeman M, Van der ven AJ, Hak E, Joore HC, Kaasjager K, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator‐associated pneumonia. American Journal of Respiratory Critical Care Medicine 2006; 173: 1348‐55. iBerry AM, Davidson PM, Masters J, Rolls K, Ollerton R. Effects of three approaches to standardized oral hygiene to reduce bacterial colonization and ventilator associated pneumonia in mechanically ventilated patients: a randomised control trial. International Journal of Nursing Studies 2011; 48: 681‐8. jMunro CL, Grap MJ, Jones DJ, McClish DK, Sessler CN. Chlorhexidine, toothbrushing, and preventing ventilator‐associated pneumonia in critically ill adults. American Journal of Critical Care 2009; 18: 428‐37; quiz 438. kShi Z, Xie H, Wang P, Zhang Q, Wu Y, Chen E, et al. Oral hygiene care for critically ill patients to prevent ventilator‐associated pneumonia. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No.: CD008367 DOI: 10.1002/14651858.CD008367.pub2. lPanchabhai TS, Dangayach NS, Krishnan A, Kothari VM, Karnad DR. Oropharyngeal cleansing With 0.2% chlorhexidine for prevention of nosocomial pneumonia in critically iII patients an open‐label randomized trial with 0.01% potassium permanganate as control. Chest 2009; 135: 1150‐6. mCabov T, Macan D, Husedzinovic I, Skrlin‐Subic J, Bosnjak D, Sestan‐Crnek S, et al. The impact of oral health and 0.2% chlorhexidine oral gel on the prevalence of nosocomial infections in surgical intensive‐care patients: a randomized placebo‐controlled study. Wien Klin Wochenschr 2010; 122: 397‐404. nBellissimo‐Rodrigues F, Bellissimo‐Rodrigues WT, Viana JM, Teixeira CA, Nicolini E, Auxiliadora‐Martins M, et al. Effectiveness of oral rinse with chlorhexidine in preventing nosocomial respiratory tract infections among intensive care unit patients. Infection Control & Hospital Epidemiology 2009; 30: 952‐8.
Contributors
Comment: Richard Price, Graeme MacLennan, John Glen and Brian H Cuthbertson on behalf of the SuDDICU collaboration (Selective Decontamination of the Digestive tract in critically ill patients treated in Intensive Care Unit).
Reply: Review authors Fang Hua, Susan Furness and Helen Worthington.
What's new
| Date | Event | Description |
|---|---|---|
| 12 June 2017 | Amended | Data entry error corrected in Analysis 1.1.3. This resulted in a very small change to the results. |
History
Protocol first published: Issue 2, 2010 Review first published: Issue 8, 2013
| Date | Event | Description |
|---|---|---|
| 6 March 2017 | Feedback has been incorporated | See Feedback section for comments regarding different interpretations in other reviews of the effects of chlorhexidine on mortality in critically ill patients. |
| 6 March 2017 | Amended | Edits to Analysis 1.2 and 4.2. In response to the feedback, review authors decided that mortality data derived from trial flow diagrams in Berry 2011 and Berry 2013 should not be used. This does not change the mortality results: chlorhexidine versus placebo, RR 1.09 (95% CI 0.96 to 1.23). Removal of Berry 2011 and 2013 mortality data also means no conclusions can be drawn about the effect on mortality of bicarbonate rinse versus water, Listerine versus water or Listerine versus bicarbonate (previous analyses 4.2.6, 4.2.7, 4.2.8). |
| 20 July 2016 | New citation required but conclusions have not changed | 6 new studies included. 3 previously included studies now excluded. Some changes to Methods (see 'Differences between protocol and review' section). |
| 17 December 2015 | New search has been performed | Search updated. |
| 27 November 2013 | Amended | Minor typographical error. |
Acknowledgements
We would like to acknowledge the contributions of Zongdao Shi, Ping Wang, Yan Wu, E Chen, Linda Ng and Ian Needleman as authors of the original review published in 2013 (Shi 2013).
We would also like to thank Anne Littlewood, Information Specialist of Cochrane Oral Health (COH), for refining search strategies and providing searching results from the databases of COH's Trials Register, CENTRAL, MEDLINE, Embase, LILACS, CINAHL, ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform. For help with the translations of foreign papers, our thanks go to Sepideh Banava (Persian), Kim Kun Hyung (Korean), Luisa Fernandez‐Mauleffinch (Portuguese) and Roca Biosca (Spanish).
For the 2016 update, we would like to thank Anne‐Marie Glenny, Laura MacDonald and Janet Lear at Cochrane Oral Health. We would also like to thank our copy editor Kate Cahill, external referees Martin Ashley, John Moore and Graeme Maclennan, and Toby Lasserson from the Cochrane Editorial Unit.
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
#1 ((critical* AND ill*):ti,ab) AND (INREGISTER) #2 ((depend* and patient*):ti,ab) AND (INREGISTER) #3 (("critical care" or " intensive care" or ICU or CCU):ti,ab) AND (INREGISTER) #4 ((intubat* or ventilat*):ti,ab) AND (INREGISTER) #5 ((#1 or #2 or #3 or #4)) AND (INREGISTER) #6 ((pneumonia or "nosocomial infect*" or VAP):ti,ab) AND (INREGISTER) #7 (#5 and #6) AND (INREGISTER)
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor Critical illness this term only #2 (critical* in All Text near/6 ill* in All Text) #3 (depend* in All Text near/6 patient* in All Text) #4 MeSH descriptor Critical care this term only #5 (intensive‐care in All Text or "intensive care" in All Text or critical‐care in All Text or "critical care" in All Text) #6 ICU in Title, Abstract or Keywords #7 ((intubat* in All Text near/5 patient* in All Text) or (ventilat* in All Text near/5 patient* in All Text)) #8 (#1 or #2 or #3 or #4 or #5 or #6 or #7) #9 (VAP in Title, Abstract or Keywords or VAP in Title, Abstract or Keywords) #10 "nosocomial infection*" in Title, Abstract or Keywords #11 MeSH descriptor Pneumonia, Ventilator‐Associated this term only #12 pneumonia in All Text #13 (#9 or #10 or #11 or #12) #14 MeSH descriptor Oral health this term only #15 MeSH descriptor Oral hygiene explode all trees #16 MeSH descriptor Dentifrices explode all trees #17 MeSH descriptor Mouthwashes explode all trees #18 MeSH descriptor Periodontal diseases explode all trees #19 periodont* in All Text #20 ("oral care" in All Text or "oral health" in All Text or oral‐health in All Text or "mouth care" in All Text or "oral hygien*" in All Text or oral‐hygien* in All Text or "dental hygien*" in All Text or decontaminat* in All Text) #21 (mouthwash* in All Text or mouth‐wash* in All Text or mouth‐rins* in All Text or mouthrins* in All Text or "oral rins*" in All Text or oral‐rins* in All Text or "artificial saliva" in All Text or "saliva substitut*" in All Text or ( (denture* in All Text near/6 clean* in All Text) or toothpaste* in All Text) or dentifrice* in All Text) #22 (#14 or #15 or #16 or #17 or #18 or #19 or #20 or #21) #23 (#8 and #13) #24 (#22 and #23)
Appendix 3. MEDLINE Ovid search strategy
1. CRITICAL ILLNESS/ 2. (critical$ adj5 ill$).mp. 3. (depend$ adj5 patient$).mp. 4. INTENSIVE CARE/ 5. ("intensive care" or intensive‐care or "critical care" or critical‐care).mp. 6. ICU.mp. or CCU.ti,ab. 7. ((intubat$ or ventilat$) adj5 patient$).mp. 8. or/1‐7 9. PNEUMONIA, VENTILATOR‐ASSOCIATED/ 10. pneumonia.ti,ab. 11. VAP.ti,ab. 12. "nosocomial infection".mp. 13. or/9‐12 14. exp ORAL HYGIENE/ 15. exp DENTIFRICES/ 16. MOUTHWASHES/ 17. ANTI‐INFECTIVE AGENTS, LOCAL/ 18. Cetylpyridinium/ 19. Chlorhexidine/ 20. Povidone‐Iodine/ 21. ("oral care" or "mouth care" or "oral hygien$" or oral‐hygien$ or "dental hygien$").ti,ab. 22. (mouthwash$ or mouth‐wash$ or mouth‐rins$ or mouthrins$ or "oral rins$" or oral‐rins$ or toothpaste$ or dentifrice$ or toothbrush$ or chlorhexidine$ or betadine$ or triclosan$ or cepacol or Corsodyl or Peridex or Hibident or Prexidine or Parodex or Chlorexil or Peridont or Eludril or Perioxidin or Chlorohex or Savacol or Periogard or Chlorhexamed or Nolvasan or Sebidin or Tubulicid or hibitane).mp. 23. (antiseptic$ or antiinfect$ or "local microbicide$" or "topical microbicide$").mp. 24. or/14‐23 25. 8 and 13 and 24
Appendix 4. Embase Ovid search strategy
1. CRITICAL ILLNESS/ 2. (critical$ adj5 ill$).mp. 3. (depend$ adj5 patient$).mp. 4. INTENSIVE CARE/ 5. ("intensive care" or intensive‐care or "critical care" or critical‐care).mp. 6. (ICU or CCU).ti,ab. 7. ((intubat$ or ventilat$) adj5 patient$).mp. 8. or/1‐7 9. PNEUMONIA, VENTILATOR‐ASSOCIATED/ 10. pneumonia.ti,ab. 11. VAP.ti,ab. 12. "nosocomial infection".mp. 13. or/9‐12 14. exp ORAL HYGIENE/ 15. exp DENTIFRICES/ 16. MOUTHWASHES/ 17. ANTI‐INFECTIVE AGENTS, LOCAL/ 18. Cetylpyridinium/ 19. Chlorhexidine/ 20. Povidone‐Iodine/ 21. ("oral care" or "mouth care" or "oral hygien$" or oral‐hygien$ or "dental hygien$").ti,ab. 22. (mouthwash$ or mouth‐wash$ or mouth‐rins$ or mouthrins$ or "oral rins$" or oral‐rins$ or toothpaste$ or dentifrice$ or toothbrush$ or chlorhexidine$ or betadine$ or triclosan$ or cepacol or Corsodyl or Peridex or Hibident or Prexidine or Parodex or Chlorexil or Peridont or Eludril or Perioxidin or Chlorohex or Savacol or Periogard or Chlorhexamed or Nolvasan or Sebidin or Tubulicid or hibitane).mp. 23. (antiseptic$ or antiinfect$ or "local microbicide$" or "topical microbicide$").mp. 24. or/14‐23 25. 8 and 13 and 24
The above subject search was linked to Cochrane Oral Health's filter for EMBASE via OVID:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ 16. HUMAN/ 17. 16 and 15 18. 15 not 17 19. 14 not 18
Appendix 5. CINAHL EBSCO search strategy
S25 S14 and S24 S24 S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 S23 (antiseptic* or antiinfect* or "local microbicide*" or "topical microbicide*") S22 (mouthwash* or mouth‐wash* or mouth‐rins* or mouthrins* or "oral rins*" or oral‐rins* or toothpaste* or dentifrice* or toothbrush* or chlorhexidine* or betadine* or triclosan* or cepacol or Corsodyl or Peridex or Hibident or Prexidine or Parodex or Chlorexil or Peridont or Eludril or Perioxidin or Chlorohex or Savacol or Periogard or Chlorhexamed or Nolvasan or Sebidin or Tubulicid or hibitane) S21 ("oral care" or "mouth care" or "oral hygien*" or oral‐hygien* or "dental hygien*") S20 (MH Povidone‐Iodine) S19 (MH Chlorhexidine) S18 (MH "Antiinfective Agents, Local") S17 MH MOUTHWASHES S16 (MH "DENTIFRICES+") S15 (MH "Oral Hygiene+") S14 S8 AND S13 S13 S9 or S10 or S11 or S12 S12 TI pneumonia or AB pneumonia S11 MH PNEUMONIA, VENTILATOR‐ASSOCIATED S10 TI "nosocomial infection" and AB "nosocomial infection" S9 TI VAP or AB VAP S8 S1 or S2 or S3 or S4 or S5 or S6 or S7 S7 ((intubat* N5 patient*) or (ventilat* N5 patient*)) S6 TI ICU or AB ICU or TI CCU or AB CCU S5 (intensive‐care or "intensive care" or critical‐care or "critical care") S4 MH CRITICAL CARE S3 (depend* N6 patient*) S2 (critical* N6 ill*) S1 MH CRITICAL ILLNESS
Appendix 6. LILACS BIREME Virtual Health Library search strategy
(Mh Critical illness or "Enfermedad Crítica" or "Estado Terminal" or "critical illness$" or Mh Intensive care or "Cuidados Intensivos" or "Terapia Intensiva" or "critical care" or "intensive care" or "ICU" or "CCU" or intubate$ or ventilate$) [Words] and (Mh Pneumonia, Ventilator‐Associated or "Neumonia Asociada al Ventilador" or "Pneumonia Associada à Ventilação Mecânica" or (ventilator AND pneumonia)) [Words] and (Mh Oral hygiene or "oral hygiene" or "Higiene Bucal" or "oral care" or "mouth care" or mouthwash$ or mouthrins$ or toothpaste$ or dentifrice$ or chlorhexidine or betadine or triclosan or Clorhexidina or Clorexidina or "Antisépticos Bucales" or "Antissépticos Bucais" or "Cepillado Dental" or "Escovação Dentária" or antiseptic$ or antiinfective$)
Appendix 7. Chinese Biomedical Literature Database search strategy
#1 缺省[智能]:危重 ‐限定:1978‐2012
#2 缺省:ICU ‐限定:1978‐2012
#3 缺省:VAP ‐限定:1978‐2012
#4 缺省:插管 ‐限定:1978‐2012
#5 #4 or #3 or #2 or #1
#6 缺省:口腔护理
#7 缺省[智能]:口腔清洁
#8 缺省:口腔卫生
#9 缺省[智能]:刷牙
#10 #9 or #8 or #7 or #6
#11 #10 and #5
#12 缺省[智能]:随机
#13 缺省:随机对照
#14 #13 or #12
#15 #14 and #11
Appendix 8. China National Knowledge Infrastructure search strategy
#1 数据库:中国期刊全文数据库 检索条件:((题名=VAP) 或者 (摘要=ICU) 或者 (题名=危重))并且(摘要=呼吸机相关性肺炎) 或者 (摘要=插管) (模糊匹配);2003‐2012;全部期刊;时间排序; 单库检索
#2 数据库:中国期刊全文数据库 检索条件: (题名=口腔护理) 或者 (摘要=口腔去污染) 或者 (题名=口腔清洁) 或者 (摘要=刷牙) 或者 (主题=口腔卫生) (模糊匹配);时间排序; 单库检索(结果中检索)
#3 数据库:中国期刊全文数据库 检索条件: (题名=随机对照) 或者 (摘要=随机) 或者 (题名=随机对照实验) 或者 (摘要=随机分配) 或者 (主题=随机隐藏) (模糊匹配);时间排序; 单库检索(结果中检索)
Appendix 9. Wan Fang Database search strategy
1. ((全部字段 =(模糊匹配) "危重") ) ;按相关度排序
2. ((全部字段 =(模糊匹配) "ICU") ) ;按相关度排序
3. ((全部字段 =(模糊匹配) "VAP") ) ;按相关度排序
4. ((全部字段 =(模糊匹配) "口腔") ) ;按相关度排序
5. ((全部字段 =(模糊匹配) "刷牙") ) ;按相关度排序
6. ((全部字段 =(模糊匹配) "去污染") ) ;按相关度排序
7. ((全部字段 =(模糊匹配) "洗必泰") ) ;按相关度排序
8. ((全部字段 =(模糊匹配) "口腔冲洗") ) ;按相关度排序
9. ((全部字段 =(模糊匹配) "危重") ) 或 ((全部字段 =(模糊匹配) "ICU") ) 或 ((全部字段 =(模糊匹配) "VAP") )
10. ((全部字段 =(模糊匹配) "口腔") ) 或 ((全部字段 =(模糊匹配) "刷牙") ) 或 ((全部字段 =(模糊匹配) "去污染") ) 或 ((全部字段 =(模糊匹配) "洗必泰") ) 或 ((全部字段 =(模糊匹配) "口腔冲洗") )
11. ( ((全部字段 =(模糊匹配) "口腔") ) 或 ((全部字段 =(模糊匹配) "刷牙") ) 或 ((全部字段 =(模糊匹配) "去污染") ) 或 ((全部字段 =(模糊匹配) "洗必泰") ) 或 ((全部字段 =(模糊匹配) "口腔冲洗") ) ) 与 ( ((全部字段 =(模糊匹配) "口腔") ) 或 ((全部字段 =(模糊匹配) "刷牙") ) 或 ((全部字段 =(模糊匹配) "去污染") ) 或 ((全部字段 =(模糊匹配) "洗必泰") ) 或 ((全部字段 =(模糊匹配) "口腔冲洗") ) 与 ((全部字段 =(模糊匹配) "危重") ) 或 ((全部字段 =(模糊匹配) "ICU") ) 或 ((全部字段 =(模糊匹配) "VAP") ) )
Appendix 10. VIP search strategy
(R=口腔 AND R=肺炎 AND R=随机) limited to (核心期刊 AND Time=2012‐2016)
Appendix 11. ClinicalTrials.gov search strategy
ventilator and pneumonia and "oral hygiene"
Appendix 12. WHO International Clinical Trials Registry Platform search strategy
ventilator and pneumonia and "oral hygiene"
Data and analyses
Comparison 1. Chlorhexidine versus placebo/usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of VAP | 18 | 2451 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.62, 0.91] |
| 1.1 Chlorhexidine solution versus placebo (no t'brushing in either group) | 7 | 1037 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.53, 0.94] |
| 1.2 Chlorhexidine gel versus placebo (no t'brushing in either group) | 5 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.41, 1.05] |
| 1.3 Chlorhexidine solution versus placebo (t'brushing both groups) | 3 | 405 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.29, 1.63] |
| 1.4 Chlorhexidine gel versus placebo (t'brushing both groups) | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.83, 1.79] |
| 1.5 Chlorhexidine solution versus usual care (some t'brushing in each group) | 1 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.56, 1.02] |
| 2 Mortality | 14 | 2014 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.96, 1.23] |
| 2.1 Chlorhexidine solution versus placebo (no t'brushing in either group) | 6 | 973 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.88, 1.39] |
| 2.2 Chlorhexidine gel versus placebo (no t'brushing in either group) | 4 | 414 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.59, 1.50] |
| 2.3 Chlorhexidine solution versus placebo (t'brushing both groups) | 3 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.77, 1.41] |
| 2.4 Chlorhexidine gel versus placebo (t'brushing both groups) | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.59, 1.68] |
| 3 Duration of ventilation (days) | 5 | 800 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐1.73, 1.55] |
| 3.1 Chlorhexidine solution versus placebo (no t'brushing in either group) | 2 | 183 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐3.70, 1.03] |
| 3.2 Chlorhexidine gel versus placebo (no t'brushing in either group) | 3 | 543 | Mean Difference (IV, Random, 95% CI) | 1.26 [‐0.78, 3.30] |
| 3.3 Chlorhexidine solution versus placebo (t'brushing both groups) | 1 | 74 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐4.20, 1.60] |
| 4 Duration of ICU stay (days) | 6 | 833 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐1.48, 1.89] |
| 4.1 Chlorhexidine solution versus placebo (no t'brushing in either group) | 2 | 194 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐4.07, 1.62] |
| 4.2 Chlorhexidine gel versus placebo (no t'brushing in either group) | 3 | 543 | Mean Difference (IV, Random, 95% CI) | 0.53 [‐1.56, 2.61] |
| 4.3 Chlorhexidine gel versus placebo (t'brushing both groups) | 1 | 96 | Mean Difference (IV, Random, 95% CI) | 5.0 [‐2.20, 12.20] |
| 5 Duration of systemic antibiotic therapy (days) | 2 | 374 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.85, 1.30] |
| 5.1 Chlorhexidine gel versus placebo (no t'brushing in either group) | 1 | 228 | Mean Difference (IV, Fixed, 95% CI) | ‐1.18 [‐3.41, 1.05] |
| 5.2 Chlorhexidine solution versus placebo (t'brushing both groups) | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | 0.65 [‐0.58, 1.88] |
| 6 Plaque index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Reversible mild irritation of oral mucosa | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Toothbrushing versus no toothbrushing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of VAP | 5 | 889 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.44, 1.09] |
| 1.1 Powered toothbrush + usual care (± CHX) versus usual care (± CHX) | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.16, 1.53] |
| 1.2 Toothbrush + CHX versus CHX alone | 1 | 436 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.51, 1.54] |
| 1.3 Toothbrush (+ some CHX) versus no toothbrush (+ some CHX) | 1 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.78, 1.40] |
| 1.4 Toothbrush + povidone iodine versus povidone iodine alone | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.13, 0.98] |
| 2 Mortality | 5 | 889 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.09] |
| 2.1 Powered toothbrush + usual care versus usual care | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.17, 9.91] |
| 2.2 Toothbrush + CHX versus CHX alone | 2 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.69, 1.17] |
| 2.3 Toothbrush alone versus no treatment | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.51, 2.60] |
| 2.4 Toothbrush + povidone iodine versus povidone iodine alone | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.15, 2.22] |
| 3 Duration of ventilation (days) | 3 | 644 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.90, 0.68] |
| 3.1 Toothbrush + CHX versus CHX alone | 2 | 583 | Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐2.43, 0.73] |
| 3.2 Toothbrush + povidone iodine versus povidone iodine alone | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.78, 1.04] |
| 4 Duration of ICU stay (days) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Toothbrush + CHX versus CHX alone | 2 | 583 | Mean Difference (IV, Fixed, 95% CI) | ‐1.82 [‐3.95, 0.32] |
| 5 Plaque score | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Powered toothbrush versus usual care | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Powered toothbrush versus manual toothbrush.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of VAP | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Powered t'brush + comp oral care versus manual t'brush + std oral care | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Powered t'brush + comp oral care versus manual t'brush + std oral care | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Duration of ventilation (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Powered t'brush + comp oral care versus manual t'brush + std oral care | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Duration of ICU stay (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Powered t'brush + comp oral care versus manual t'brush + std oral care | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Other oral care solutions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of VAP | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Povidone iodine versus saline/placebo | 3 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.50, 0.95] |
| 1.2 Povidone iodine versus usual care | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.63] |
| 1.3 Saline rinse versus saline swab | 4 | 488 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.37, 0.62] |
| 1.4 Saline rinse versus usual care | 2 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.39, 0.91] |
| 1.5 Saline rinse + swab versus saline swab (usual care) | 2 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.23, 0.72] |
| 1.6 Bicarbonate rinse versus water | 2 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.57, 3.82] |
| 1.7 Triclosan rinse versus saline | 1 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.12] |
| 1.8 Furacilin versus povidone iodine | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.23, 1.04] |
| 1.9 Furacilin versus saline | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.14, 0.58] |
| 1.10 Listerine versus water | 1 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.36, 3.28] |
| 1.11 Listerine versus bicarbonate | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.35, 3.16] |
| 1.12 Biotene versus control | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.28, 1.41] |
| 2 Mortality | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Povidone iodine versus saline/placebo | 2 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.66, 1.50] |
| 2.2 Povidone iodine versus usual care | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.31, 2.40] |
| 2.3 Saline rinse versus saline swab | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.12, 0.69] |
| 2.4 Saline rinse + swab versus saline swab (usual care) | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.11, 1.28] |
| 2.5 Saline rinse versus usual care | 2 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.87, 1.39] |
| 2.6 Biotene versus control | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.13, 3.76] |
| 3 Duration of ventilation (days) | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Povidone iodine versus saline | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐4.36, 2.36] |
| 3.2 Povidone iodine versus usual care | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐7.67, 1.67] |
| 3.3 Saline rinse versus usual care | 2 | 324 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐2.55, 1.75] |
| 3.4 Saline rinse + swab versus saline swab | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐3.91 [‐5.85, ‐1.97] |
| 3.5 Saline rinse versus saline swab | 2 | 176 | Mean Difference (IV, Fixed, 95% CI) | ‐6.83 [‐8.94, ‐4.72] |
| 3.6 Triclosan rinse versus saline | 1 | 324 | Mean Difference (IV, Fixed, 95% CI) | ‐5.24 [‐5.64, ‐4.84] |
| 4 Duration of ICU stay (days) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Povidone iodine versus saline/placebo | 2 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐3.90, 3.21] |
| 4.2 Povidone iodine versus usual care | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐10.99, 2.99] |
| 4.3 Saline rinse versus usual care | 2 | 324 | Mean Difference (IV, Fixed, 95% CI) | ‐1.17 [‐3.95, 1.60] |
| 4.4 Triclosan rinse versus saline | 1 | 324 | Mean Difference (IV, Fixed, 95% CI) | ‐4.97 [‐5.55, ‐4.39] |
| 5 Number of participants treated with systemic antiboitics | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Acute respiratory distress syndrome | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.62, 195.61] |
| 6.2 Agitation and/or hypertension | 1 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.12, 1.86] |
| 6.3 Epistaxis | 1 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.63] |
| 6.4 Oxygen desaturation | 1 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.06, 15.17] |
| 6.5 Aspiration | 1 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 70.07] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bellissimo‐Rodrigues 2009.
| Methods | Study design: RCT, 2 parallel groups Location: Sao Paulo, Brazil Number of centres: 1 Study period: March 2006 to February 2008 Funding source: Not stated |
|
| Participants | Setting: ICU in tertiary care hospital Inclusion criteria: All patients admitted to ICU with expected stay > 48 hours. Not all participants received mechanical ventilation Exclusion criteria: Previous chlorhexidine sensitivity, pregnancy, formal indication for chlorhexidine use, prescription of another oral topical medication Number randomised: 200 (only 133 on ventilators) Number evaluated: 194 Baseline characteristics: ‐ Intervention group: Age: median 62.5 (17 ‐ 89) M/F: 47/51; APACHE II Score: median 17 (5 ‐ 35) ‐ Control group: Age: median 54.0 (15 ‐ 85) M/F: 51/45; APACHE II Score: median 19 (5 ‐ 41) |
|
| Interventions |
Comparison: 0.12% chlorhexidine solution versus placebo Experimental group (n = 64 on vent): 0.12% chlorhexidine solution applied orally 3 times daily. Oral hygiene was conducted by nurses specially trained in the protocol. 3 times daily after mechanical cleaning of the mouth by a nurse, 15 ml of study solution was applied and attempts made to distribute solution over all oral surfaces Control group (n = 69 on vent): The same protocol was conducted with the placebo solution, which was identical in colour, consistency, smell and taste |
|
| Outcomes | 1. Respiratory tract infections (VAP for those on ventilators) 2. Respiratory tract infection‐free survival time 3. Time from ICU admission to first RTI 4. Duration of mechanical ventilation 5. Length of ICU stay 6. Total mortality 7. Mortality due to RTI 8. Antibiotic use 9. Microbiological culture of endotracheal secretions 10. Adverse effects |
|
| Notes | Sample size calculation: "to have sufficient power to detect a 69% difference in incidence of VAP with α = 5% and β = 20% it was estimated that 96 patients per group were required" Only 133/194 of patients evaluated received mechanical ventilation Email sent 3 September 2012. Reply received The Cochrane calculator was used to calculate the SD value for duration of mechanical ventilation, but the SD obtained seemed inappropriate and was therefore not used in data synthesis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised" Method of sequence generation not described but undertaken by pharmacy |
| Allocation concealment (selection bias) | Low risk | "only the pharmacist knew which code numbers corresponded to which type of solution" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/200 participants were excluded from the analysis. 1 control participant needed to receive chlorhexidine treatment, and further 3 in control group and 2 in experimental group were excluded due to protocol violation. Unlikely to have introduced a bias |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported |
| Other bias | Low risk | No other sources of bias identified |
Berry 2011.
| Methods | Study design: Feasibility study – single‐blind parallel‐group RCT with 3 groups Location: Australia Number of centres: 1 Study period: Not stated Funding source: Hospital |
|
| Participants | Setting: A 20‐bed adult intensive care unit in a university hospital Inclusion criteria: All intubated patients admitted to the unit were considered for inclusion in the study provided they met the following criteria: able to be randomised within 12 hours of intubation, aged over 15 years and next‐of‐kin able to give informed consent Exclusion criteria: Patients were ineligible for study participation if they: required specific oral hygiene procedures in relation to maxillofacial trauma or dental trauma/surgery; had been in the ICU previously during the current period of hospitalisation; received irradiation or chemotherapy on admission to the ICU or in the preceding 6 weeks; or suffered an autoimmune disease. Informed consent was obtained for all participants and agreement to participate could be withdrawn at any time Number randomised: 225 (71, 76, 78 in Groups 1, 2, 3) Number evaluated: 109 (33, 33, 43 in Groups 1, 2, 3) Group 1 (chlorhexidine 0.2% aqueous) group: Age: 58.2 ± 19.4; M/F: 35/36; APACHE II Score: 22.8 ± 7.8 Group 2 (sodium bicarbonate mouthwash rinsed 2‐hourly): Age: 60.4 ± 17.5; M/F: 42/24; APACHE II Score: 22.0 ± 7.5 Group 3 (sterile water rinsed 2‐hourly): Age: 59.1 ± 18.1; M/F: 44/34; APACHE II Score: 21.6 ± 7.8 |
|
| Interventions |
Comparison: Chlorhexidine 0.2% versus water versus sodium bicarbonate Group 1: Twice daily irrigation with chlorhexidine 0.2% aqueous oral rinse with 2‐hourly irrigation with sterile water Group 2: Sodium bicarbonate mouthwash rinsed 2‐hourly Group 3: sterile water rinsed 2‐hourly (used as the control in this review) "All treatment options included a comprehensive cleaning of the mouth using a soft, pediatric toothbrush 3 times a day" |
|
| Outcomes | 3 outcome variables were reported: 1. Microbial colonisation of dental plaque (or gums in edentulous participants) 2. Incidence of VAP 3. Adverse events |
|
| Notes | Sample size calculation: Feasibility study to inform sample size calculation for main study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomisation into one of three groups according to a balanced randomisation table prepared by biostatistician" |
| Allocation concealment (selection bias) | Low risk | Study packs were identical in outward appearance and allocation remained blinded until study pack opened by attending nurse |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: Blinding not possible, but non‐blinding of caregivers may have introduced a risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Microbiologist and radiologists who assessed outcomes were blinded to allocated treatment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 102/225 participants evaluated. High rate of attrition and reasons varied in each group. Death rate higher in Group B, breach of inclusion criteria more likely in Groups B & C |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | High risk | Study stopped early due to withdrawal of investigational product by regulator |
Berry 2013.
| Methods | Study design: 3‐arm parallel‐group RCT Location: Australia Number of Centres: 1 Study period: Not stated Funding source: Pfizer provided product only |
|
| Participants | Setting: ICU in a 1000‐bed tertiary referral teaching hospital Inclusion criteria: admitted to ICU, able to be randomised within 12 hours of intubation; aged over 15 years; next‐of‐kin able to give informed consent Exclusion criteria: required specific oral hygiene procedures following facio‐maxillary or dental trauma/surgery; had received irradiation or chemotherapy on admission to the ICU or in the preceding 6 weeks; diagnosed with autoimmune disease; had previous ICU admission during current period of hospitalisation Number randomised: 398 (Group A: 138; Group B: 133; Group C: 127) Number evaluated: 398 (Group A: 138; Group B: 133; Group C: 127); however, 11% of these participants were ineligible Baseline characteristics: ‐ Group A: Age: 58.82 (16.7); M/F: 84/54; APACHE II Score: 20.86 (7.7) ‐ Group B: Age: 54.93 (19.5); M/F: 79/54; APACHE II Score: 21.38 (8.0) ‐ Group C: Age: 59.96 (18.0); M/F: 73/54; APACHE II Score: 21.21 (8.0) |
|
| Interventions |
Comparison: Sterile water versus sodium bicarbonate versus Listerine Group A: Control – sterile water mouth rinses, 20 ml every 2 hours. Group B: Sodium bicarbonate mouth wash (6.5 g/L sterile water), 20 ml every 2 hours. Group C: Listerine mouth wash, 20 ml instilled twice a day and sterile water every 2 hours for remaining time All 3 groups received mechanical cleaning of the oral cavity with a small, soft‐bristled toothbrush and general‐purpose toothbrush 3 times a day. Curved‐tip dental syringes were used to instill mouth rinses. During the study period, VAP preventive measures including head of the bed elevation, stress ulcer prophylaxis and endotracheal cuff occlusive pressure between 22 and 30 cm H2O were maintained. |
|
| Outcomes | 1. Incidence of VAP 2.Dental plaque colonisation 3. Systemic antibiotic administration (unclear if systemic) 4. Adverse effects |
|
| Notes | Sample size calculation: reported for inhibition of microbial growth on dental plaque, not VAP Emailed study investigator 10 April 2016 for publication details or full unpublished study data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation numbers were computer generated” |
| Allocation concealment (selection bias) | Low risk | “Nurses were blinded to the study option until the study packs were opened” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Nurses were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Microbiologists … and … radiologists also blinded to the treatment code” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 245 randomised participants (62%) were no longer in the study on the 4th day; Intention‐to‐treat analysis was used but unsure how and whether appropriate |
| Selective reporting (reporting bias) | High risk | VAP data were only presented as percentages; 24 participants died within 4 days but unclear how many died after that; exact data for systemic antibiotic administration was not reported. |
| Other bias | Unclear risk | Ineligible patients were included in the ITT but reasons for ineligibility in each group were not given |
Bopp 2006.
| Methods | Study design: Pilot study, 2‐arm RCT Location: USA Number of centres: 1 Study period: February 2002 to August 2002 Funding source: Grant from American Dental Hygienists' Association's Institute for Oral Health |
|
| Participants | Setting: Critical care unit Inclusion criteria: Orally and nasally intubated patients entering critical care unit Exclusion criteria: Taking metronidazole, history of allergy to chlorhexidine, sensitive to alcohol, risk for endocarditis, history of other serious illness (specified), those with pneumonia Number randomised: 5 Number evaluated: 5 Baseline characteristics: ‐ Intervention group: Age: 40, range 28 ‐ 52; M/F: 0/2 ‐ Control group: Age: 73.7, range 62 ‐ 81; M/F: 2/1 |
|
| Interventions |
Comparison: 0.12% chlorhexidine + suction toothbrush versus suction swab + hydrogen peroxide Experimental group (n = 2): Twice daily oral hygiene care with 0.12% chlorhexidine gluconate during intubation period plus oral cleaning with PlaqVac suction toothbrush Control group (n = 3): Standard oral care 6 times daily using a suctioning soft foam swab and half‐strength hydrogen peroxide, plus oral lubricant |
|
| Outcomes | Microbial colonisation VAP, mortality | |
| Notes | Sample size calculation: This was a pilot study. Data were not used in meta‐analysis on advice of statistician Email sent to contact author 14 November 2012, reply received 19 November 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomly assigned to either control or experimental treatment by the flip of a coin" |
| Allocation concealment (selection bias) | High risk | Coin toss was undertaken by researcher. No allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible. Reply from contact author "they were not blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Reply from contact author "they were not blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in outcome evaluation |
| Selective reporting (reporting bias) | Unclear risk | VAP planned and reported in this pilot study. Microbial culture data not reported per person, and mortality is also reported |
| Other bias | Low risk | No other sources of bias detected |
Cabov 2010.
| Methods | Study design: 2 parallel‐arm RCT Location: Croatia Number of centres: 1 Study period: March 2008 to December 2008 Funding source: Supported by Croatian Ministry of Science Education and Sports Grant number 065‐1080057‐0429 |
|
| Participants | Setting: Surgical ICU in university hospital Inclusion criteria: Aged > 18 years, medical condition suggesting hospitalisation in ICU > 3 days, eventual requirement for mechanical ventilation by oropharyngeal or nasotracheal ventilation Exclusion criteria: Number randomised: 60. 40 of the 60 participants (17 and 23 in each group) were on mechanical ventilation Number evaluated: 60 Baseline characteristics: ‐Intervention group: Age: 57 ± 16; M/F: 19/11 ‐Control group: Age: 52 ± 19; M/F: 20/10 |
|
| Interventions |
Comparison: Chlorhexidine gel versus placebo Experimental group (n = 17): 3 times daily, following standard oral care comprising rinsing mouth with bicarbonate isotonic serum, followed by gentle oropharyngeal sterile aspiration, participants received application of 0.2% chlorhexidine gel applied by nurses to dental gingival and oral surfaces using a sterile gloved finger Control group (n = 23): Standard oral care, 3 times daily as above followed by administration of placebo gel In both groups gel was left in place and oral cavity was not rinsed |
|
| Outcomes | Simplified acute physiological score (SAPS), dental status, dental plaque, plaque culture, nosocomial infections, mortality | |
| Notes | Sample size calculation: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomized into two groups using a computer‐generated balanced randomization table" |
| Allocation concealment (selection bias) | Unclear risk | Unclear who conducted the allocation and whether it was concealed from the investigators |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in outcome evaluations |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported |
| Other bias | Low risk | No other sources of bias identified |
Caruso 2009.
| Methods | Study design: 2‐arm RCT Location: Brazil Number of centres: 1 Study period: August 2001 to December 2004 Funding source: Not stated |
|
| Participants | Setting: Closed medical surgical ICU unit in oncologic hospital Inclusion criteria: Patients aged > 18 years expected to need mechanical ventilation for > 72 hours through orotracheal or tracheotomy tube Exclusion criteria: Previous mechanical ventilation within past month, mechanical ventilation for > 6 hours prior to study enrolment, contraindication to bronchoscopy and expected to die or stop treatment within 48 hours Number randomised: 262 Number evaluated: 262 Baseline characteristics: ‐ Intervention group: Age: 65 ± 14 years; M/F: 66/64 ‐ Control group: Age: 63 ± 6 years; M/F: 70/62 |
|
| Interventions |
Comparison: Saline rinse versus usual care Experimental group (n = 130): Instillation of 8 ml of isotonic saline prior to tracheal suctioning, which was conducted by respiratory therapists Control group (n = 132): Tracheal suction alone with no saline instillation Aspirations were carried out when 1 of the following occurred: visible airway secretion into endotracheal tube, discomfort or participant asynchrony, noisy breathing, increased peak expiratory pressures, or decreased tidal volume during ventilation attributed to airway secretion |
|
| Outcomes | 1. Incidence of VAP 2. Duration of ventilation in ICU 3. Length of stay in ICU 4. ICU mortality 5. Tracheal colonisation 6. Suctions per day, chest radiographs |
|
| Notes | Sample size calculation: Estimated that 130 participants per group required to give 80% power with α = 5% to detect a decrease in VAP from 30% to 15% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" No details of method of sequence generation provided in report |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Attending physicians and nurses blinded to study group. Intervention carried out by respiratory therapists available on ICU 24/7 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment conducted by physicians and nurses blinded to allocated treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in outcome evaluation |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported in full |
| Other bias | Low risk | No other sources of bias identified |
Chen 2008.
| Methods | Study design: A single‐centre RCT with 2 parallel groups Location: China Number of centres: 1 surgical ICU in provincial hospital Study period: Not stated Funding source: External |
|
| Participants | Inclusion criteria: Admission into the ICU, orally intubated, receiving mechanical ventilation ≥ 7 days, without oral and lung disease Exclusion criteria: Using hormone therapy; with diabetes Number randomised: 120 Number evaluated: 120 ‐ Intervention group: n = 60; mean age: 42.0 ± 9.0; M/F: 39/21 ‐ Control group: n = 60; mean age: 40.0 ± 8.0; M/F: 45/15 Baseline characteristics were comparable |
|
| Interventions |
Comparison: Oral care + chlorhexidine rinse versus saline rinse Intervention group: Oral cavity irrigated with 50 ml GSE rinse (chlorhexidine + extracts of grapefruit + FE enzyme) then aspirated off, 4 times a day, and routine oral nursing care was given once a day after the first irrigation Control group: Oral irrigation with 50 ml saline, 4 times a day, without the combination of routine oral care |
|
| Outcomes | 3 outcome variables were reported: 1. Incidence of VAP after 7 days of mechanical ventilation 2. Incidence of oral inflammation (ulceration and herpes) 3. Change in bacteria colonisation: the throat swab cultures at baseline and after treatment |
|
| Notes | GSE rinse: We are advised by reviewers from China that GSE rinse should be treated as chlorhexidine + 2 potentially active other antiseptics Diagnosis of VAP was according to Chinese Society of Respiratory Diseases criteria Information translated from Chinese paper by Shi Zongdao and colleagues |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised into different groups according to a randomised number table |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not described and not possible. Difference between intervention and control means caregivers would be aware of who was in each group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information on throat swab culture result (baseline and after treatment) |
| Other bias | Unclear risk | The treatment group received co‐intervention of routine oral nursing care once daily, but this was not done in the control group |
DeRiso 1996.
| Methods | Study design: Parallel‐group RCT Location: Indiana, USA Number of centres: 1 Study period: Not stated Funding source: The study was supported by a grant from the August Tomusk Foundation |
|
| Participants | Setting: Surgical ICU for postoperative cardiac surgery Inclusion criteria: Patients undergoing cardiac surgery which required cardiopulmonary bypass Exclusion criteria: Intra‐operative death, preoperative infection or intubation, pregnancy, heart and lung transplant recipients, known hypersensitivity to chlorhexidine Number randomised: Unclear Number evaluated: 353 (173 in chlorhexidine group and 180 in control) Baseline characteristics: ‐ Intervention group: Age: 64.1 ± 0.86; M/F: 119/54 ‐ Control group: Age: 63.5 ± 0.84; M/F: 123/57 |
|
| Interventions |
Comparison: Chlorhexidine oral rinse versus placebo Experimental group: 0.5 fl ounce (approx 15 ml) of 0.12% chlorhexidine (+ 11.6% ethanol (Proctor & Gamble)) mouthrinse used as oropharyngeal rinse and "rigorously applied" to buccal, pharyngeal, gingival tongue and tooth surfaces for 30 seconds twice daily Control group: Placebo mouthrinse identical in appearance containing base solution and 3.2% ethanol (1/3 of concentration of active solution) All participants also received the standard oral care of the ICU (systemic antibiotics, pressor agents and nutritional support as deemed necessary) |
|
| Outcomes | 5 outcome variables were reported: 1. Nosocomial infection rates (upper & lower RTI, UTI, fungaemias, line sepsis, wound & blood infection, other infection) 2. Non‐prophylactic antibiotic use 3. Length of stay in hospital 4. Duration of intubation 5. Mortality |
|
| Notes | Sample size calculation: Not reported Unclear duration of mechanical ventilation. Unable to contact author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "..the pharmacy randomised the patients to either experimental or control group by means of computer driven random number generator" |
| Allocation concealment (selection bias) | Low risk | Allocation was performed in pharmacy and solutions with identical appearance were dispensed for use in ICU |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind. Quote: "…matching placebo…Both were packaged in 120‐mL brown bottles and labelled 'Oral Rinse Solution: Peridex/Placebo Trial Solution' with a 1‐week expiration date" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of people originally randomised to treatment or control groups not stated |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported (no data for length of stays, duration of ventilation) |
| Other bias | Low risk | No other sources of bias identified |
Feng 2012.
| Methods | Study design: A single‐centre RCT with 3 parallel groups (2 groups included in this review) Location: China Number of centres: 1 ICU in a city hospital Study period: February 2009 to January 2011 Funding source: Not stated |
|
| Participants | Inclusion criteria: Entry ICU, with orotracheal intubation and ventilation Exclusion criteria: Pulmonary infection, stomatitis or oral tumours before intubation, accompanied by ulcer of the digestive tract, malignant tumours of the body, taking steroids > 3 days, diabetes Number randomised: 204 Number evaluated: 204 Intervention group: 0.05% povidone iodine: n = 71; mean age: 43.7 ± 8.1 years Intervention group: 1/5000 furacilin: n = 65; mean age: 38.5 ± 11.6 years Control group: Saline n = 68; mean age: 40.3 ± 8.5 years Baseline characteristics: Not specified |
|
| Interventions |
Comparison: Povidone iodine + toothbrushing versus saline + toothbrushing Group A (n = 71): Toothbrushing along the slits between the teeth with 0.05% povidone iodine by nurses, then the oropharyngeal cavity was rinsed with 50 ml of the solution and it was suctioned out completely. This procedure was repeated 4 times a day Group B: Toothbrushing along the slits between the teeth with 1/5000 furacilin (antibiotic) by nurses. Excluded from this review Control group (n = 68): Toothbrushing along the slits between the teeth with 0.9% saline by nurses, then the oropharyngeal cavity was rinsed with 50 ml of the saline and it was suctioned out completely. This procedure was repeated 4 times a day |
|
| Outcomes | 4 outcome variables were reported: 1. Incidence of VAP 2. Rates of oral ulcer or herpes, or both 3. Oral cleanliness ‐ no odour, no foreign bodies and visually clean surfaces of tube and equipment 4. Throat swab culture |
|
| Notes | Diagnosis of VAP was according to Chinese Society of Respiratory Diseases criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were divided into three groups according to randomisation principle" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not described and not possible for the caregivers who would be aware of who was in each group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in the outcome evaluation |
| Selective reporting (reporting bias) | Low risk | The results were fully reported |
| Other bias | Low risk | No other sources of bias identified |
Fields 2008.
| Methods | Study design: Parallel‐group RCT Location: Akron Ohio, USA Number of centres: 1 Study period: October 2005 to March 2006 Funding source: Internal hospital funding |
|
| Participants | Setting: 24‐bed stroke, neurological and medical ICU Inclusion criteria: Any mechanically‐ventilated patient on the stroke/medical ICU intubated in the hospital for < 24 hours , no previous diagnosis of pneumonia Exclusion criteria: Patients with prior tracheotomies, younger than 18 years, AIDS secondary to immunocompromised systems, edentulous patients Number randomised: Not stated Number evaluated: Not stated Baseline characteristics: Not reported |
|
| Interventions |
Comparison: Toothbrushing 8‐hourly versus usual care Experimental group: Nurse brushed patient's teeth, tongue and hard palate for > 1 minute, then used toothette swab to swab patient's teeth, tongue and hard palate for > 1 minute, then apply moisturiser to lips. Mouth and pharynx were suctioned as needed using catheter which was replaced every 24 hours. Oral assessment every 12 hours. Oral care kit #2 provided for each participant, with worksheet #2 Control group: Usual care (unspecified) which could include up to 2 toothbrushings daily and toothette mouthcare as needed. Nurses used oral care kit #1 and worksheet #1 |
|
| Outcomes | 1. Incidence of VAP | |
| Notes | Sample size calculation: "Desired sample size was 200 ventilator dependent patients or 2000 ventilator days" Email sent to authors 3 September 2012 requesting numbers of patients treated. No reply received. Trial included in text as narrative only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "..a plastic bin labelled 1‐350, containing sealed envelopes which each had either worksheet #1 or #2, plus information about the trial to give to families". No mention of whether envelopes were sequentially numbered. Method of sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Allocation contained in sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible, both nurses and participants would have known allocated treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome of VAP assessed by infection control nurse. Unclear whether this person was blinded to allocated treatment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The study neither reports the number of participants randomised nor the number analysed |
| Selective reporting (reporting bias) | High risk | No numerical data were reported in this paper. VAP incidence was not reported by treatment group or with any measure of variance |
| Other bias | Unclear risk | Insufficient information in the trial report to produce confidence in the methodology of this trial |
Fourrier 2000.
| Methods | Study design: Single‐blind RCT Location: Lille, France Number of centres: 1 Study period: June 1997 to July 1998 Funding source: Not stated |
|
| Participants | Setting: Adult ICU Inclusion criteria: Patients admitted to ICU aged > 18 years, medical condition likely to require ICU stay of 5 days, requiring mechanical ventilation by oropharyngeal or nasopharyngeal intubation or tracheostomy Exclusion criteria: Edentulous patients Number randomised: 60 Number evaluated: 58 Baseline characteristics: ‐ Intervention group: Age: 51.2 ± 15.2; M/F: 19/11; SAPS II Score: 37 ± 15 ‐ Control group: Age: 50.4 ± 15.5; M/F: 19/11; SAPS II Score: 33 ± 13 |
|
| Interventions |
Comparison: Rinse + chlorhexidine gel versus rinse alone Experimental group: After mouth rinsing and oropharyngeal aspiration, 0.2% chlorhexidine gel was applied to dental and gingival surfaces of the patient using glove‐protected finger. Intervention 3 times daily Control group: Mouthrinsing with bicarbonate isotonic serum followed by gentle oropharyngeal aspiration 4 times daily during ICU stay Participants were allowed to eat and drink freely |
|
| Outcomes | 1. Incidence of nosocomial infections 2. Dental status (DMFT/CAO) 3. Amount of dental plaque (Loe & Silness Index) 4. Plaque bacterial culture |
|
| Notes | Sample size calculation: Not reported Investigators verified antibacterial activity of chlorhexidine gel in vitro prior to study Unclear numbers on mechanical ventilation developing VAP. Email sent 14 November 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...patients were randomized into two groups according to a computer‐generated balanced randomization table" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to determine whether or not the allocation of the sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible as no placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Bacteriologist blinded to randomisation code, and evaluation of nosocomial infections done by hygienist nurse and physician not aware of the treatment given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many participants are included in the evaluation of the outcomes |
| Selective reporting (reporting bias) | Low risk | Planned outcome of nosocomial infection, dental plaque, and colonisation reported |
| Other bias | Low risk | Groups appear similar at baseline. No other sources of bias identified |
Fourrier 2005.
| Methods | Study design: A multicentre double‐blind placebo‐controlled study with 2 parallel groups Location: France Number of centres: 6 ICUs (3 in university hospitals & 3 in general hospitals) Study period: January 2001 to September 2002 Funding source: Partial funding from Programme Hospitalier de Recherche Clinique PHRC (French Ministry of Health) |
|
| Participants | Inclusion criteria: Age > 18 years and a medical condition suggesting an ICU stay at least 5 days and the requirement for mechanical ventilation by orotracheal or nasotracheal intubation. Only patients hospitalised for 48 hours before admission in the ICU could be included Exclusion criteria: Patients with a tracheostomy tube at recruitment; completely edentulous; suffering from facial trauma; post‐surgical and requiring specific oropharyngeal care; known allergy to chlorhexidine Age group: Mean 61.0 SD 14.7, 61.1 years SD 14.9 in each group Number randomised: 228 Number evaluated: 228 (ITT) Intervention group: Age: 61.1 ± 14.9; M/F: 73/41; SAPS II Score: 45.0 ± 17.5 Control group: Age: 61.0 ± 14.7; M/F: 83/31; SAPS II Score: 45.2 ± 17.5 |
|
| Interventions |
Comparison: Chlorhexidine gel versus placebo Intervention (n = 114): After mouthrinsing and aspiration, plaque antiseptic decontamination of gingival and dental plaque with a 0.2% chlorhexidine gel provided by nurses at least 3 times a day during the entire ICU stay Control (n = 114): A placebo gel, same usage as that of plaque antiseptic decontamination "Toothbrushing was not allowed in the protocol" |
|
| Outcomes | The following variables were reported and compared: 1. Incidence of VAP 2. Incidence of VAP (%) per 1000 days of mechanical ventilation 3. Incidence of VAP (%) per 1000 days of intubation 5. Mortality from day 0 to day 28 6. ICU days (mean ± SD) 7. Days of intubation (mean ± SD) 8. Antibiotic days (mean ± SD) |
|
| Notes | Sample size calculation: Calculation provided based on expected incidence of nosocomial infections of 30% in placebo group and 15% in treatment group. Planned interim analysis to determine effects of interventions, and study stopped based on pre‐planned stopping rule after this interim analysis Email sent to author 14 November 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomly assigned … block randomization stratified by site" |
| Allocation concealment (selection bias) | Low risk | "all randomization lists were held in sealed envelopes in the pharmacy departments of the 6 centres" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The placebo gel was indistinguishable by colour, taste or odour from the tested agent. The investigators were unaware of participants' assignments |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant in intervention group was excluded and the reason was clearly explained. ITT analysis |
| Selective reporting (reporting bias) | Low risk | All planned outcomes clearly defined and reported |
| Other bias | Low risk | No other sources of bias identified. Although this study was stopped early, interim analysis was planned in protocol and carried out appropriately |
Grap 2011.
| Methods | Study design: RCT Location: Virginia USA Number of centres: 2 units in same hospital, Level 1 trauma centre Study period: Not stated Funding source: Triservice Nursing research programme grant TSNRP MDA‐905‐03‐TS02 |
|
| Participants | Setting: Surgical trauma ICU & neuroscience ICU Inclusion criteria: Patients intubated within 12 hours of admission to trauma centre (intubation may have occurred in emergency department, in the field or in pre‐hospital setting) Exclusion criteria: Previous endotracheal tube placed in 48 hours prior to admission, clinical diagnosis of pneumonia on admission, burn injuries, edentulous persons Number randomised: 152, 7 lost, enrolled sample 145 (71/74) (only 75 were still intubated after 48 hours) Number evaluated: At 48 or 72 hours = 60 (36/24) (for VAP) 39 (21/18) Baseline characteristics: Not reported for each randomised group in total Those with 48/72 hour data: ‐ Experimental group: n = 36, M/F 27/9, APACHE II 70.69 ± 30.14 ‐ Control group: n = 24, M/F 11/13, APACHE II 60.46 ± 23.45 |
|
| Interventions |
Comparison: Chlorhexidine applied by swab versus usual care Experimental group: 1 x 5 ml dose of chlorhexidine 0.12% applied to all areas of oral cavity by swab within 12 hours prior to intubation. All participants received usual oral comfort care (details not reported) Control group: Usual oral comfort care |
|
| Outcomes | 1. Incidence of VAP 2. CPIS score 3. APACHE III 4. TRISS 5. Oral Health (DMFT) |
|
| Notes | Sample size calculation: Not reported (but pilot study published in 2004) Email sent and reply received to clarify the data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The subjects were randomised to a treatment group or control group using a block randomisation scheme" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible because no placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Huge attrition, and reasons for losses not described for each group. Conclusions based on 39/152 (26%) of those originally randomised to treatment or control |
| Selective reporting (reporting bias) | High risk | Primary outcome planned was development of VAP but inclusion criteria used in this study meant that fewer than half those randomised were at risk of developing VAP |
| Other bias | High risk | Study report notes statistically significant difference in gender and CPIS score between groups at baseline. No baseline characteristics data reported for each randomised group, and likely that important prognostic factors e.g. place of intubation, surgery, may have been different in each group |
Hu 2009.
| Methods | Study design: RCT Location: Beijing, China Number of centres: 1 Study period: Not stated Funding source: No external funding |
|
| Participants | Setting: ICU in second affiliated hospital of PLA General Hospital Inclusion criteria: Patients in ICU receiving mechanical ventilation Exclusion criteria: Unclear Number randomised: 47 Number evaluated: Unclear Baseline characteristics: Not reported for each randomised group in total Those with 48/72 hour data: ‐ Experimental group: n = 25, M/F 16/9, age range 19 ‐ 68 ‐ Control group: n = 22, M/F 13/9, age range 22 ‐ 60 |
|
| Interventions |
Comparison: Saline swab + rinse versus saline swab Experimental group: Lips, teeth, tongue and palate were swabbed with a saline saturated cotton ball and the oral cavity was rinsed with saline twice daily Control group: Lips, teeth, tongue and palate were swabbed with saline saturated cotton ball twice daily |
|
| Outcomes | VAP, mortality, days on ventilator, days in hospital, halitosis, ulceration | |
| Notes | Information translated from Chinese paper by Shi Zongdao and colleagues. Unable to confirm outcome data with trial authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Email from author "the sequence was generated by using a random number table" |
| Allocation concealment (selection bias) | Low risk | Email from author "allocation was concealed using opaque envelopes numbered with inclusion sequence" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and caregivers were not blinded to interventions received |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Email from author "the outcome assessors were a group of nurses not involved with the interventions". Probably blinded to allocated treatment group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of participants included in the outcome assessments at each time point is unclear. VAP reported as percentages only |
| Selective reporting (reporting bias) | High risk | All planned outcomes reported but as percentages only |
| Other bias | Low risk | No other sources of bias identified |
Jacomo 2011.
| Methods | Study design: Double‐blind placebo‐controlled RCT (NCT00829842) Location: Sao Paulo, Brazil Number of centres: 1 Study period: February 2006 to February 2008 Funding source: Not stated |
|
| Participants | Setting: Tertiary care hospital paediatric ICU Inclusion criteria: Children with congenital heart disease undergoing cardiac surgery with or without cardiopulmonary bypass, admitted to paediatric ICU for postoperative care Exclusion criteria: Pre‐operative pneumonia, hypersensitivity to chlorhexidine, congenital or acquired immunodeficiency, refusal to participate Number randomised: 164 Number evaluated: 160 (4 intra‐operative deaths) Baseline characteristics: ‐ Intervention group: Age: median12.2 (0 ‐ 176 months); M/F: 42/45 ‐ Control group: Age: median 10.8 (0 ‐ 204 months); M/F: 35/38 |
|
| Interventions |
Comparison: Chlorhexidine (gargle or swab) versus placebo Experimental group: Oral hygiene with 0.12% chlorhexidine gluconate solution, administered pre‐operatively and twice daily postoperatively. 0.3 ml/kg of body weight were used in children aged > 6 years, who gargled for 30 seconds avoiding ingestion. In younger children and intubated postoperative patients solution was applied to oral mucosa, gingival, tongue and tooth surfaces for 30 seconds with a spatula wrapped in gauze Control group: Received the same treatment with placebo solution that looked and tasted the same All participants received orotracheal intubation and prophylactic systemic antibiotics intravenously for 48 hours |
|
| Outcomes | 1. Incidence of nosocomial pneumonia 2. Incidence of VAP 3. Duration of intubation 4. Need for reintubation 5. Time to development of pneumonia 6. Length of paediatric ICU/hospital stay 7. 28‐day mortality |
|
| Notes | Sample size calculation: Estimated that 160 participants would detect a reduction in 50% in incidence of nosocomial pneumonia (31% to 15.5%) with α = 0.05 & β = 0.20 NCT 00829842 at ClinicalTrials.gov |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "..randomized to the experimental or control groups by means of a list generated by a computerized system that uses a random number generator to produce customized sets of random numbers" |
| Allocation concealment (selection bias) | Low risk | "The randomisation list was held in the hospital pharmacy and all investigators were unaware of patients assignments" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind. Texture, colour, and flavour of placebo similar to active solution, placed in similar containers and labelled A or B |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind. "..the diagnosis of nosocomial pneumonia was made independently by the PICU physicians and an infection control practitioner blinded to the patient's group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants in each group died and were therefore excluded from pneumonia outcomes |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes clearly reported but unclear how many trial participants were ventilated for at least 48 hours |
| Other bias | Low risk | No other sources of bias identified |
Koeman 2006.
| Methods | Study design: A multicentre randomised double‐blind placebo‐controlled trial with 3 parallel groups Location: 2 university hospitals and 3 general hospitals in the Netherlands Number of centres: 5 hospitals (2 surgical and 5 mixed ICUs) Study period: February 2001 to March 2003 Funding source: ZONMw Netherlands Organization for Health Research and Development (project number 2200.0046) |
|
| Participants | Inclusion criteria: Consecutive adult patients (> 18 years of age) needing mechanical ventilation for at least 48 hours were included within 24 hours after intubation and start of mechanical ventilation Exclusion criteria: A pre‐admission immunocompromised status, pregnancy, and if the physical condition did not allow oral application of study medication Age group: Not stated Number randomised: 385 Number evaluated: 379 Group A: Chlorhexidine group: n = 127; mean age: 60.9 ± 15.3; M/F: 71/57; APACHE II: 22.2 ± 7.02 Group B: Chlorhexidine/COL group: n = 128; mean age: 62.4 ± 19.1; M/F: 66/61; APACHE II: 23.7 ± 7.38 Group C: Control group: n = 130; mean age: 62.1 ± 15.9; M/F: 93/37; APACHE II: 21.8 ± 7.43 |
|
| Interventions |
Comparison: Chlorhexidine (in petroleum jelly) versus petroleum jelly alone Group A: Chlorhexidine group (n = 127): Oral decontamination with chlorhexidine (2%) in Vaseline petroleum jelly Group B: Chlorhexidine/COL group (n = 128): Oral decontamination with chlorhexidine plus colistin antibiotic chlorhexidine/colistin (CHX/COL 2%/2%) in Vaseline petroleum jelly Group C: Control (n = 130): Oral decontamination with Vaseline petroleum jelly Trial medication was administered 4 times daily, after removing remnants of the previous dose with a gauze moistened with saline. Approximately 2 cm of paste, approximately 0.5 g, was put on a gloved fingertip and administered to each side of the buccal cavity |
|
| Outcomes | The following outcome variables were reported for each group: 1. Incidence of VAP 2. Incidence of early onset VAP 3. Days ventilated (mean ± SD) 4. ICU stay (mean ± SD) 5. Days in hospital after ICU discharge (mean ± SD) 6. Changes of endotracheal colonisation through cultures in 3 time windows after ventilation, 1 ‐ 3 days, 5 ‐ 8 days and 9 ‐ 12 days respectively |
|
| Notes | Sample size calculation: Reported in paper together with planned sequential analysis Only Group A and Group C included in this review Email sent to author 26 August 2016 requesting mortality data but failed due to invalid email address |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomly assigned to one of three study groups by computerised randomisation schedule. Randomization was stratified by hospital" |
| Allocation concealment (selection bias) | Low risk | The interventions were produced by an independent unit and we considered allocation was concealed from the research team. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, placebo controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind, placebo controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study was discontinued in 6 participants, 5 participants withdrew consent, 1 due to adverse event. Intention‐to‐treat analysis included all participants for primary outcome |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported |
| Other bias | Low risk | Unlikely |
Kusahara 2012a.
| Methods | Study design: Double‐blind placebo‐controlled RCT Location: Sao Paulo, Brazil Number of centres: 1, tertiary care hospital affiliated with Federal University of Sao Paulo Brazil Study period: 36 months dates not stated Funding source: Funded by a grant from Fundacao de Amparo a Pesquisa do Estado de Sao Paulo (04‐13361‐2) |
|
| Participants | Setting: PICU Inclusion criteria: Children admitted to PICU likely to require ventilation within 24 hours of admission Exclusion criteria: Newborn, confirmed diagnosis of pneumonia at admission, known hypersensitivity to chlorhexidine, tracheostomy, duration of ventilation < 48 hours, intubated for > 24 hours prior to PICU admission Number randomised: 96 (46/50) Number evaluated: 96, at day 2 (44/45), at day 4 23/23 Baseline characteristics: ‐ Intervention group: Age: 12 ± 49.75 months; M/F: 28/18 ‐ Control group: Age: 34.5 ± 58.8 months; M/F: 32/18 |
|
| Interventions |
Toothbrushing + 0.12% chlorhexidine gel versus toothbrushing + placebo Experimental group: Oral care with toothbrushing and oral gel containing chlorhexidine twice daily (08:00 & 20:00 hours). Mouth was divided into 4 quadrants and each brushed in a defined pattern. With child in lateral position, gel was applied directly to toothbrush, and all tooth surfaces (vestibular, lingual, occlusal and incisal) were cleaned and ventral surface of tongue was brushed posterior to anterior. Each quadrant was rinsed with water and excess fluid and debris were removed with continuous suction. Finally oral foam applicator was immersed in the gel and applied all over the gingival surfaces of the participant Control group: Oral care with toothbrushing and placebo oral gel twice daily. With child in lateral position, gel was applied directly to toothbrush, and all tooth surfaces (vestibular, lingual, occlusal and incisal) were cleaned and ventral surface of tongue was brushed posterior to anterior. Each quadrant was rinsed with water and excess fluid and debris were removed with continual suction. Finally oral foam applicator was immersed in the gel and applied all over the gingival surfaces of the participant |
|
| Outcomes | 1. Incidence of VAP 2. Duration of ventilation in PICU 3. Length of stay in PICU 4. Hospital mortality 5. Tracheal colonisation with Gram +ve & ‐ve organisms |
|
| Notes | Sample size calculation: Reported that this was not done "due to the absence of previous research on this population" Email correspondence with Prof Pedreira confirmed that Pedreira 2009 and Kusahara 2012a both refer to the same study. NCT 01083407 & NCT0410682 at ClinicalTrials.gov |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "..randomised into two groups using a balanced randomisation table generated by True Epistat Program" |
| Allocation concealment (selection bias) | Low risk | Both chlorhexidine and identical placebo gels were supplied by pharmacy in identical containers and only the pharmacist was aware of the gel type for each participant |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind. Identical placebo used so that neither participants nor clinical staff were aware of allocated treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind. Only the pharmacist was aware of the gel type for each participant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in the outcome evaluation |
| Selective reporting (reporting bias) | Low risk | One primary and 4 secondary outcomes reported in full |
| Other bias | Unclear risk | Statistically significant difference in mean age of children in each group. This may have introduced a bias |
Long 2012.
| Methods | Study design: A single‐centre RCT with 2 parallel groups Location: China Number of centres: 1 ICU in the university hospital Study period: February 2010 to March 2012 Funding source: Program for masters degree |
|
| Participants | Inclusion criteria: Patients admitted to ICU, with oral intubation, receiving mechanical ventilation ≥ 48 hours, age ≥ 18 years, patients or their relatives agreed to participate in the study Exclusion criteria: Intubated in emergency e.g. after cardiac arrest, operations upon the oral cavity, trauma of the respiratory tract, with severe bleeding or coagulation disorders Number randomised: 70 Number evaluated: 61 (the other 9 were death or ventilation < 48 hours) ‐ Intervention group: Mean age: 60.06 ± 10.71 years, M/F 20/11, APACHE 17.94 ± 1.24 ‐ Control group: Mean age: 63.67 ± 10.02 years, M/F 18/12, APACHE 18.23 ± 0.57 |
|
| Interventions |
Comparison: Povidone iodine + toothbrushing versus povidone iodine alone Experimental group (n = 31): Modified oral nursing method: swab with 0.1% povidone iodine immediately before intubation, then toothbrushing and rinsing with 0.1 povidone iodine, 3 times a day Control group (n = 30): Usual oral nursing method: swab with cotton balls soaked with 0.1% povidone iodine |
|
| Outcomes | 3 outcome variables were available: 1. Incidence of VAP 2. Mortality 3. Ventilation days |
|
| Notes | Microbial examinations for the aspirate secretions obtained from inferior respiratory tract every day after intubation were referred for diagnosis of VAP | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...patients were randomly assigned into 2 groups, observing group and control group with 35 cases in each group" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not described and not possible for the caregivers who would be aware of who was in each group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 randomised participants were excluded from analysis, numbers and reasons similar for each group |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Unclear risk | Only the results of microbial examination of the aspirate secretions from the inferior respiratory tract as tool of VAP diagnosis may not be enough |
Lorente 2012.
| Methods | Study design: Parallel‐group RCT Location: Tenerife, Spain Number of centres: 1 Study period: August 2010 to August 2011 Funding source: Hospital funding |
|
| Participants | Setting: Medical/surgical ICU Inclusion criteria: Consecutive patients undergoing invasive mechanical ventilation for at least 24 hours Exclusion criteria: Edentulous, aged < 18 years, pregnant, HIV positive, white blood cells < 1000 cells/mm3, solid or haematological tumour, immunosuppressive therapy, mechanical ventilation duration < 24 hours Number randomised: 436 (217/219) Number evaluated: 436 Baseline characteristics: ‐ Intervention group: Age: 61.0 ± 15.6 years; M/F: 146/71 ‐ Control group: Age: 60.4 ± 16.6 years; M/F: 145/74 |
|
| Interventions |
Toothbrushing + 0.12% chlorhexidine gel versus chlorhexidine alone Experimental group (n = 217): Oral cleansing performed with 0.12% chlorhexidine impregnated gauze, and oral cavity injection, followed by manual brushing of the teeth with a brush impregnated with 0.12% chlorhexidine (tooth by tooth on the anterior and posterior surfaces, the gum line and the tongue for a period of 90 seconds) Control group (n = 219): Oral cleansing performed with 0.12% chlorhexidine impregnated gauze, and oral cavity injection only In both groups nurse performed oral care every 8 hours. First endotracheal cuff pressure was tested, oropharyngeal secretions were aspirated, then chlorhexidine impregnated gauze was used to cleanse the teeth, tongue and mucosal surfaces, followed by injection of 10 ml 0.12% of chlorhexidine digluconate into oral cavity, and finally after 30 seconds the OParea was suctioned |
|
| Outcomes | 1. Incidence of VAP 2. Duration of ventilation 3. ICU mortality 4. Tracheal colonisation with Gram +ve & ‐ve organisms 5. Antibiotic exposure |
|
| Notes | Sample size calculation: Estimated that 218 participants required in each group to give 80% power and α error of 5%, to show a reduction in VAP from 15% to 7.5% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "..a list of random numbers generated with Excel software (Microsoft, Seattle, WA)" |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The diagnosis of VAP was made by an expert panel, blinded to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants are included in the outcome evaluations |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported in full |
| Other bias | Low risk | No other sources of bias identified |
Meinberg 2012.
| Methods | Study design: Parallel‐group RCT Location: Brazil Number of centres: 1 Study period: July 2007 to December 2009 Funding source: Not stated |
|
| Participants | Setting: surgical ICU Inclusion criteria: > 18 years, receiving mechanical ventilation within 24 hours of admission, expected to require ventilation for > 72 hours. Exclusion criteria: Aspiration pneumonia, tracheostomy, pregnancy and immunosuppression Number randomised: 52 (28/24) Number evaluated: 52 (28/24) Baseline characteristics: ‐ Intervention group: Age: 40.1 ± 14.6 years; APACHE II 17.9 ± 4.5 ‐ Control group: Age: 41.0 ± 19.0 years; APACHE II 16.7 ± 6.8 |
|
| Interventions |
Comparison: Toothbrushing + 2% chlorhexidine gel versus toothbrushing + placebo gel Experimental group (n = 28): Toothbrushing plus chlorhexidine gel 2% 4 times daily Control group (n = 24): Toothbrushing plus placebo gel 4 times daily |
|
| Outcomes | 1. VAP 2. Mortality 2. ICU mortality 3. Duration of intubation 4. Duration of ICU stay 5. Duration of hospital stay |
|
| Notes | Errors in numbers reported for duration of intubation in Table 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | This was undertaken by the pharmacist |
| Allocation concealment (selection bias) | Low risk | "only the pharmacist responsible for preparing the solutions and for the randomisation process knew the contents of the distributed gel tubes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "only the pharmacist responsible for preparing the solutions and for the randomisation process knew the contents of the distributed gel tubes" "placebo group (gel with same colour and consistency)" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "only the pharmacist responsible for preparing the solutions and for the randomisation process knew the contents of the distributed gel tubes" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | High risk | Adverse events not reported. Data not fully reported. Data errors |
| Other bias | High risk | Study terminated due to 'futility'. Reason for termination unclear |
Mo 2016.
| Methods | Study design: 2‐group parallel RCT Location: China Number of centres: 1 Study period: December 2012 to May 2015 Funding source: Not reported |
|
| Participants | Setting: Department of Cardio‐Thoracic Surgery Inclusion criteria: mechanical ventilation > 48 hours Exclusion criteria: Patients with pulmonary infections or oral diseases Number randomised: 210 (Gp A: 105; Gp B: 105) Number evaluated: 210 (Gp A: 105; Gp B: 105) Baseline characteristics: ‐ Gp A: Age: 59.14 (12.06); M/F: 60/45 ‐ Gp B: Age: 56.71 (10.53); M/F: 68/37 |
|
| Interventions |
Comparison: Saline rinse versus saline swab (usual care) Gp A: Rinse with saline for 10 minutes each time, 4 times per day Gp B: Swab with saline 4 times per day |
|
| Outcomes | 1. Incidence of VAP 2. Mortality |
|
| Notes | Sample size calculation: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomised patients to the experimental and control group using a random number table” |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not described and not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in analysis |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No other sources of bias identified |
Munro 2009.
| Methods | Study design: A single‐centre RCT with 4 parallel groups Location: 3 ICUs in large urban University Medical Centre, Virginia, USA Number of centres: 3 (ICUs) Study period: Not stated Funding source: Grant NIH R01 NR07652 |
|
| Participants | Inclusion criteria: Critically ill adults (> 18) in 3 intensive care units were enrolled within 24 hours of intubation. All patients older than 18 years (n = 10,913) in medical, surgical/trauma, and neuroscience ICUs were screened for inclusion Exclusion criteria: Clinical diagnosis of pneumonia at the time of intubation, edentulous patients, patients who had a previous endotracheal intubation during the current hospital admission Group 1: 26/18 M/F, age mean 46.1 (18.2) Group 2: 28/21 M/F, age mean 47.1 (15.7) Group 3: 28/20 M/F, age mean 47.3 (18.8) Group 4: 37/14 M/F, age mean 46.8 (16.4) Number randomised: 547 (but 355 subsequently excluded due to pneumonia at baseline) Number evaluated: 192 |
|
| Interventions |
Comparison: Chlorhexidine swab versus toothbrushing versus both versus usual care Group 1: (n = 44) a 0.12% solution of chlorhexidine gluconate (chlorhexidine) 5 mL by oral swab twice daily (at 10 AM and 10 PM) Group 2: (n = 49) toothbrushing (manual toothbrush) 3 times a day (at 9 AM, 2 PM, and 8 PM), detailed toothbrushing protocol followed quadrant by quadrant Group 3: (n = 48) combination care (toothbrushing 3 times a day and chlorhexidine every 12 hours) Group 4: (n = 51) control (usual care) |
|
| Outcomes | VAP measured by CPIS score, also dichotomised at days 1, 3, 5, 7 Mortality (died during hospitalisation) |
|
| Notes | Median length of stay and stay in ICU were presented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A randomized controlled 2 × 2 factorial experimental design was used...Patients were randomly assigned to 1 of 4 treatments". "Patients were randomized to treatment within each ICU according to a permuted block design developed by the biostatistician (D.K.M.) before the start of the study" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 355/547 (65%) of those originally randomised were excluded from the analysis at day 3 because they were found to have pneumonia at baseline |
| Selective reporting (reporting bias) | Unclear risk | VAP reported as percentages only and denominator unclear |
| Other bias | Low risk | No other sources of bias identified |
Ozcaka 2012.
| Methods | Study design: double‐blind placebo‐controlled RCT Location: Izmir, Turkey Number of centres: 1 Study period: November 2007 to November 2009 Funding source: "The study was funded solely by the institutions of the authors" |
|
| Participants | Setting: respiratory ICU Inclusion criteria: patients aged 18 or over, admitted to respiratory ICU expecting to require ventilation for > 48 hours Exclusion criteria: witnessed episode of aspiration, confirmed diagnosis of post‐obstructive pneumonia, known hypersensitivity to chlorhexidine, diagnosed thrombocytopenia, pregnancy, oral mucositis, readmission to same ICU, expected survival < 1 week, edentulism Number randomised: 66 Number evaluated: 61 Baseline characteristics: ‐ Intervention group: age: 60.5 ± 14.7 years ‐ Control group: age: 56.0 ± 18.2 years |
|
| Interventions |
Comparison: Chlorhexidine solution versus saline Experimental group (n = 32): oral mucosa was swabbed with 0.2% chlorhexidine on sponge pellets, 4 times daily. Excess rinse was suctioned from patient's mouth after 1 minute Control group (n = 34): oral mucosa was swabbed with saline on sponge pellets, 4 times daily. Excess rinse was suctioned from patient's mouth after 1 minute Deep suctioning was performed in both groups every 6 hours and following position changes to remove pooled secretions from around the cuff of the endotracheal tube |
|
| Outcomes | 1. Incidence of VAP 2. Mortality 3. Duration of ventilation in ICU 4. Length of stay in ICU 5. Presence of potential respiratory pathogens in minibronchoalveolar lavage |
|
| Notes | Sample size calculation: Estimated that 28 participants would be required in each group to give 81% power with α of 5%, to show a reduction in VAP from 70% to 30% Email sent 22 January 2013 and reply received 29 January 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation prepared a set of subject identification (SID) numbers which had assigned treatment" Description unclear, but involvement of statistician suggests this was well done |
| Allocation concealment (selection bias) | Low risk | "Study nurse obtained the SID number when the patient was enrolled" Allocation was probably concealed and not able to be anticipated by investigators |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Assignment of treatment was blinded to patients and to all investigators, including periodontist, .... respiratory ICU physicians and outcome statisticians" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Assignment of treatment was blinded to patients and to all investigators, including periodontist, .... respiratory ICU physicians and outcome statisticians" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 66 participants randomised, 1 secondary exclusion from each group, and 2 and 1 early deaths in chlorhexidine and control groups, respectively. Unlikely to have introduced a bias |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No other sources of bias identified |
Panchabhai 2009.
| Methods | Study design: open‐label RCT Location: Mumbai, India Number of centres: 1 Study period: 8 months ‐ dates not stated Funding source: Not stated |
|
| Participants | Setting: ICU (mixed medical and surgical), tertiary care hospital Inclusion criteria: all patients admitted to ICU during study period who signed consent Exclusion criteria: pregnant women, those with pneumonia at baseline, those for whom oral care was contraindicated, those with allergy to chlorhexidine Number randomised: 512 Number evaluated: 471 (only 88/83 = 171 on mechanical ventilation) Baseline characteristics (given for 471 who completed the trial only): ‐ Intervention group: age: 35.2 ± 15.9; M/F: 136/88; APACHE II Score: 12 ± (9 ‐ 17) ‐ Control group: age: 36.9 ± 16.2; M/F: 171/76; APACHE II Score: 14 ± (9 ‐ 19) |
|
| Interventions |
Comparison: Chlorhexidine versus potassium permanganate Experimental group (n = 250): Oral and pharyngeal suction of pooled secretions followed by swabbing of the oral cavity, teeth, palate, buccal spaces, posterior pharyngeal wall, and hypopharynx with normal saline.Then oropharyngeal cleansing, following the same procedure, twice daily with 0.2% chlorhexidine solution Control group (n = 262): Oral and pharyngeal suction of pooled secretions followed by swabbing of the oral cavity, teeth, palate, buccal spaces, posterior pharyngeal wall, and hypopharynx with normal saline.Then oropharyngeal cleansing twice daily, following the same procedure, with 0.01% potassium permanganate solution Non‐intubated participants, rinsed with water, then rinsed and gargled with 10 ml of study solution. No eating/drinking for 1 hour postintervention |
|
| Outcomes | 1. Incidence of nosocomial pneumonia 2. Day of development of pneumonia 3. Mortality (hospital) 4. Duration of ICU stay |
|
| Notes | Sample size calculation: "This study had a statistical power of 75% to detect a 50% reduction in the incidence of nosocomial pneumonia in the study group with 95% level of confidence. Assuming the incidence of pneumonia in the control group was 16%, 506 subjects were required" Email sent to author 14 November 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "..randomly assigned to treatment .... by concealed simple random sampling" No details of sequence generation provided |
| Allocation concealment (selection bias) | Unclear risk | "..concealed simple randomisation" Unclear whether allocation was concealed from researchers |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label RCT |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Open‐label RCT but "two independent, blinded reviewers made the diagnosis of nosocomial pneumonia" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 26/250 (10%) and 15/262 (5.7%) were excluded from the analysis in the chlorhexidine and control groups respectively. Reasons given were ICU stay < 48 hours, 14/250 versus 6/262, and protocol violation 12/250 and 9/262 respectively |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported in full |
| Other bias | Unclear risk | Baseline parameters only reported for those who completed the study |
Pobo 2009.
| Methods | Study design: Prospective, single‐blind, randomised trial with parallel groups Location: Spain Number of centres: 1 ICU at a hospital Study period: Not stated Funding source: This work was supported by Fondo de Investigaciones Sanitarias (FISS 06/060), Centro de Investigación Biomédica en Red Enfermedades Respiratorias (06/06/36), and the Agency for the Administration of University and Research Grants (2005/SGR/920) |
|
| Participants | Inclusion criteria: Intubated adults without evidence of pulmonary infection, expected to remain ventilated for > 48 hours. Randomised within 12 hours of intubation Exclusion criteria: Edentulous, suspicion of pneumonia at time of intubation or evidence of massive aspiration during intubation, tracheostomy (or expected within 48 hours), recent enrolment in other trials, pregnancy, and chlorhexidine allergy Age group: Adults Intervention group: n = 74; age: 55.3 ± 17.9; M/F: 49/25; mean APACHE II Score: 18.8 ± 7.1 Control group: n = 73; age: 52.6 ± 17.2; M/F: 46/27; mean APACHE II Score: 18.7 ± 7.3 Number randomised: 147 (74 in toothbrush group and 73 in standard care group) Number evaluated: 147 |
|
| Interventions |
Comparison: Powered toothbrush + standard oral care versus standard oral care alone Group 1 (n = 74): Standard oral care plus toothbrush group: besides the standard oral care, toothbrushing was performed tooth by tooth, on anterior and posterior surfaces, and along the gumline, the tongue was also brushed. A powered toothbrush was used (Braun Oral B AdvancePower 450 TX, Braun GmbH). This procedure was repeated once every 8 hours Group 2 (n = 73): Standard oral care: maintaining head elevation at 30°. After aspiration of oropharyngeal secretions and adjustment of endotracheal cuff pressure, a gauze containing 20 ml of 0.12% chlorhexidine digluconate was applied to all the oral surfaces including tongue and mucosal surface, and 10 ml of 0.12% chlorhexidine digluconate was injected into oral cavity, being aspirated after 30 seconds, repeated every 8 hours |
|
| Outcomes | The following outcome variables were reported for each group: 1. Incidence of VAP 2. Incidence of suspected VAP per 1000 days of mechanical ventilation 3. Mean days of mechanical ventilation (mean ± SD) 4. ICU length of stay (mean ± SD) 5. Mortality |
|
| Notes | In the review, the standard oral care group was viewed as intervention with chlorhexidine and the other group was viewed as control with toothbrushing Sample size calculation: Estimated that 200 participants would be required in each group to show a 50% reduction in VAP with 80% power and α error of 5%. After 147 of planned 400 participants were randomised, the study was stopped by the steering committee due to no difference in VAP between the groups NCT 00842478 at ClinicalTrials.gov |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by means of a computer‐generated list, stratified for antibiotic use at admission |
| Allocation concealment (selection bias) | Low risk | The list was concealed in opaque sealed envelopes opened by the nurse within 12 hours of intubation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible. Participants unlikely to be aware of treatment, but caregivers were aware |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators and attending physicians were blinded to assigned groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. All randomised participants included in the analysis |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported, including adverse events |
| Other bias | High risk | Study stopped early after recruitment of 147 of planned 400 participants because no differences between groups were found and revised estimates indicated that 1500 participants would need to be recruited to show a difference. Numbers not feasible in this centre |
Prendergast 2012.
| Methods | Study design: Prospective, randomised trial with 2 parallel groups. NCT 00518752 Location: USA Number of centres: 1 neuroscience ICU at a tertiary medical centre Study period: August 2007 to August 2009 Funding source: Not stated |
|
| Participants | Inclusion criteria: All patients aged at least 18 years admitted to neuroscience ICU, intubated within 24 hours of admission Exclusion criteria: Pregnancy, edentulous, aged < 18 years, facial fractures or trauma affecting oral cavity, unstable cervical fractures, anticipated extubation within 24 hours, grim prognosis Intervention group: n = 38; age: 54 ± 17.8; M/F: 19/19 Control group: n = 40; age: 51 ± 18.4; M/F: 23/17 Number randomised: 78 (38 in comprehensive group and 40 in standard care group) Number evaluated: Variable (fewer than 11 participants/group) |
|
| Interventions |
Comparison: Powered toothbrush + comprehensive oral care versus manual toothbrush + standard oral care Group 1 (n = 38): Tongue scraping using a low‐profile tongue scraper with posterior to anterior sweeping motion across the dorsal surface of the tongue. Then toothbrushing with Oral B vitality powered toothbrush + Biotene (non‐foaming) toothpaste for 2 minutes, then a liberal application or Oral Balance gel. Care performed twice daily Group 2 (n = 40): Standard oral care: using manual paediatric toothbrush, toothpaste with 1000 ppm fluoride with SLS and water‐based inert lubricant (KY jelly). Care performed twice daily |
|
| Outcomes | The following outcome variables were reported for each group: 1. Oral and sputum cultures every 48 hours 2. Incidence of suspected VAP (day 2 ‐ 6) 3. ICU length of stay (mean ± SD) 4. Mortality |
|
| Notes | Sample size calculation: Not reported NCT 00518752 at ClinicalTrials.gov |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "..randomized ... using a computer generated list maintained in a separate locked cabinet" |
| Allocation concealment (selection bias) | Low risk | "..list was maintained in a separate locked cabinet from enrolment forms to prevent manipulation of eligibility judgements" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Diagnosis of VAP by examination of chest radiographs, by physicians blinded to allocated treatment (information in Prendergast dissertation) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear how many were assessed at each time point but paper states that "less than 11 patients in each group at each time point" |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported |
| Other bias | Low risk | No other sources of bias identified |
Roca Biosca 2011.
| Methods | Study design: Single‐blind RCT Location: Tarragona, Spain Number of centres: 1 Study period: June 2006 to May 2009 Funding source: Grant from Health Investigation Fund (FISS 06/060) |
|
| Participants | Setting: ICU (14‐bed) Inclusion criteria: Adults aged > 18 years, requiring mechanical ventilation for at least 48 hours, no pneumonia at baseline, at least 2 premolars and 1 incisor, consenting to take part Exclusion criteria: Edentulous, suspected pneumonia < 18 years, requiring < 48 hours mechanical ventilation, tracheotomy, moribund (death expected within 72 hours) allergic to chlorhexidine Number randomised: 147 Number evaluated: Not stated Baseline characteristics: Report states that there were no differences in gender, age, diagnosis, APACHE scores between the groups at baseline. No supporting data reported |
|
| Interventions |
Comparison: Powered toothbrush + standard oral care versus standard oral care alone Experimental group: RASPALL ‐ Standard oral hygiene protocol + powered toothbrush. Participant was elevated to 35°, oropharyngeal secretions were aspirated, intubation cuff pressure checked, then teeth, tongue and oral cavity cleaned with swab soaked in 10 ml 0.12% chlorhexidine digluconate. Solution left for 30 seconds then excess was aspirated. All tooth surfaces then brushed using a powered toothbrush Control group: Standard oral hygiene protocol alone as described for treatment group |
|
| Outcomes | 4 outcome variables planned: 1. Plaque index (Loe & Silness) days 1, 5 and 10 2. Plaque cultures 3. VAP (reported as NAV) 4. Halitosis |
|
| Notes | Sample size calculation: Not reported Translated from Portuguese by Luisa Fernandez‐Mauleffinch Email to authors sent 14 November 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Group assignment was done randomly by sealed envelope" Method of sequence generation not described |
| Allocation concealment (selection bias) | Low risk | "Group assignment was done randomly by sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study described as single blind but unclear who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Numbers of participants included in outcome of plaque index were 74 and 73 at day 0, 60 and 57 at day 5, and 29 and 32 at day 10 for toothbrush and control groups respectively. Reasons for missing outcome data are extubation, need for tracheotomy, VAP, death or intubation for total of 28 days. No information as to numbers missing by group for each reason |
| Selective reporting (reporting bias) | High risk | Planned outcomes of plaque index and microbiological culture reported but data for VAP and halitosis in each group not reported |
| Other bias | Unclear risk | Insufficient information in trial report to be clear about potential for other bias |
Scannapieco 2009.
| Methods | Study design: A randomised, double‐blind, placebo‐controlled clinical trial Location: USA Number of centres: 1 18‐bed trauma ICU Study period: March 2004 until November 2007 Funding source: USPH grant R01DE‐14685 from the National Institute of Dental and Craniofacial Research |
|
| Participants | Inclusion criteria: Those admitted to the ICU who were expected to be intubated and mechanically ventilated within 48 hours of admission Exclusion criteria: A witnessed aspiration suspected with chemical pneumonitis; a confirmed diagnosis of post‐obstructive pneumonia e.g. advanced lung cancer; a known hypersensitivity to chlorhexidine; absence of consent; a diagnosed thrombocytopenia (platelet count < 40 and/or a INR > 2, or other coagulopathy); a do‐not‐intubate order; children < 18 years; pregnant women; legal incarceration; transfer from another ICU; oral mucositis; immunosuppression either HIV‐ or drug‐induced e.g. organ transplant patients or those on long‐term steroid therapy; and readmission to the ICU Number randomised: 175 Number evaluated: 146 Intervention group (chlorhexidine 1): n = 47; mean age: 44.8 ± 19.9; M/F: 43/15; mean APACHE II Score: 18.5 ± 4.1 Intervention group (chlorhexidine 2): n = 50; mean age: 47.6 ± 19.1; M/F: 44/14; mean APACHE II Score: 19.7 ± 6.1 Control group: n = 49; mean age: 50.0 ± 22.5; M/F: 36/23; mean APACHE II Score: 19.1 ± 6.1 |
|
| Interventions |
Comparison: Chlorhexidine twice per day + toothbrush versus chlorhexidine once per day + toothbrush versus placebo + toothbrush Intervention group: Chlorhexidine (0.12% CHX gluconate) was applied using a rinse‐saturated oral foam applicator (Sage Products, Cary, IL, USA) once a day (placebo at other time) Intervention group: Chlorhexidine (0.12% CHX gluconate) was applied using a rinse‐saturated oral foam applicator (Sage Products, Cary, IL, USA) twice a day (in the morning at about 8 AM and in the evening at about 8 PM) Control group: Placebo was applied using a rinse‐saturated oral foam applicator twice per day All groups had routine oral care using a suction toothbrush (Sage Products, Cary, IL, USA) twice a day and as needed to brush teeth and the surface of the tongue or approximately 1 ‐ 2 minutes, and applying suction at completion and as needed during the brushing |
|
| Outcomes | 1. Incidence of VAP (diagnosed as the presence of more than 104 CFU of pathogen/ml of bqBAL fluid) 2. Death 3. Days ventilated 4. Days in hospital 5. Antibiotic use |
|
| Notes | Sample size calculation: Estimated that 53 participants per arm would give 90% power to detect a 505 decrease in colonisation. For outcomes 2 ‐ 5, the P values were for 3‐group comparisons NCT00123123 at ClinicalTrials.gov |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A web‐based enrolment system which allocated randomised participant identification numbers |
| Allocation concealment (selection bias) | Low risk | The oral topical treatment for each box was formulated and prepared by the hospital pharmacy. Sealed envelopes containing a random number were generated in blocks of 6 to provide concealment of participant assignment from the investigators |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Assignment of treatment was blinded to patients and all investigators including outcome assessors, statisticians and care providers" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Assignment of treatment was blinded to patients and all investigators including outcome assessors, statisticians and care providers" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 175 participants were randomised, microbiological baseline data were available for 146 participants, 115 had full data at 48 hours. > 20% dropouts in all groups. ITT analysis used for 175 participants but unclear what imputation was used to account for losses |
| Selective reporting (reporting bias) | Unclear risk | Planned microbiological outcomes were reported only in graphs with no data presented |
| Other bias | High risk | Problems with data analysis due to unclear denominator and imputations. Pre‐study antibiotic exposure higher in control group |
Sebastian 2012.
| Methods | Study design: Double‐blind stratified placebo‐controlled RCT Location: New Delhi, India Number of centres: 1 Study period: November 2007 to April 2009 Funding source: Indian Council of Medical Research Grant. Chlorhexidine gel and placebo supplied by ICPA Health Products Limited |
|
| Participants | Setting: Paediatric ICU (6 beds) Inclusion criteria: Patients aged 3 months to 15 years who required orotracheal or nasotracheal intubation and mechanical ventilation. Patients with pneumonia at baseline were also included as these made up 66% of patient population Exclusion criteria: Patients mechanically ventilated for > 48 hours prior to paediatric ICU admission, those with tracheostomies, with inaccessible oral cavities, or with known hypersensitivity to chlorhexidine Number randomised: 86 (41/45) Number evaluated: 86 Baseline characteristics: ‐ Intervention group: Age: 13/41, 3 ‐ 12 months; 28/41, 1 year ‐ 15 years; M/F: 23/18 ‐ Control group: Age: 15/45, 3 ‐ 12 months; 30/45, 1 year ‐ 15 years; M/F: 27/18 |
|
| Interventions |
Comparison: Chlorhexidine gel versus placebo Experimental group (n = 41): Oral cavity was suctioned to remove secretions then mucosal surfaces were cleaned with saline soaked gauze. Then 0.75 cm 1% chlorhexidine gel was applied to each side of the mouth using a standardised disposable applicator Control group (n = 45): Oral cavity was suctioned to remove secretions then mucosal surfaces were cleaned with saline soaked gauze. Then 0.75 cm placebo gel was applied to each side of the mouth using a standardised disposable applicator Care was repeated every 8 hours |
|
| Outcomes | 1. Incidence of VAP 2. Length of stay in ICU 3. Duration of hospital stay 4. Hospital mortality 5. Type and antibiotic sensitivity of organisms cultured |
|
| Notes | Sample size calculation: Estimated that 91 participants per group were required to give 80% power with α = 5% to detect a reduction in VAP from 40% to 20% NCT00597688 at ClinicalTrials.gov This study included participants with pneumonia at baseline and used age‐appropriate CDC criteria to diagnose VAP |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible participants were stratified into 1 of 4 groups based on age group and presence or pneumonia at baseline. Within each stratum participants were randomised to receive either chlorhexidine or placebo gel. "..the random sequence was generated for each stratum using STATA 9.0 in blocks of 6" |
| Allocation concealment (selection bias) | Unclear risk | No details about how the allocation was communicated to the researchers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in the ITT analysis |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported. Medians and IQRs (as reported) are the correct statistic for a skewed distribution but cannot be combined in meta‐analysis |
| Other bias | Low risk | Paper states that "the funding agency did not have any role in the study design, data collection and analysis, decision to publish or preparation of the manuscript" |
Seguin 2006.
| Methods | Study design: 3‐arm parallel RCT Location: Rennes, France Number of centres: 1 Study period: August 2001 to January 2003 Funding source: Not stated |
|
| Participants | Setting: Surgical ICU Inclusion criteria: Adults (> 18 years) with closed head trauma admitted to ICU and expected to need mechanical ventilation for at least 2 days Exclusion criteria: Admitted > 12 hours after initial trauma, those with facial, thoracic, abdominal or spinal injuries, known history of reaction to iodine or of respiratory disease, chest infiltrates at admission or need for curative antibiotics Number randomised: 110 (38/36/36) Number evaluated: 98 (36/31/31) Baseline characteristics: ‐ Iodine group: Age: 38 ± 17 years; M/F: 28/10 ‐ Saline group: Age: 38 ± 16 years; M/F: 24/12 ‐ Control group: Age: 41 ± 18 years; M/F: 23/13 |
|
| Interventions |
Comparison: Povidone Iodine versus saline versus usual care (no rinse) Iodine group (n = 38): Nasopharynx and oropharynx rinsed 4‐hourly with 20 ml of 10% povidone iodine aqueous solution (Betadine oral rinse solution) reconstituted in a 60 ml solution with sterile water, followed by aspiration of oropharyngeal secretions Saline group (n = 36): Nasopharynx and oropharynx rinsed 4‐hourly with 60 ml saline, followed by aspiration of oropharyngeal secretions Control group (n = 36): Standard regimen without any instillation but with aspiration of oropharyngeal secretions For all participants the suction catheters were inserted as distally as possible. Procedures were reported on patients chart |
|
| Outcomes | 1. Incidence of VAP ‐ early and late onset 2. Duration of ventilation in surgical ICU 3. Length of stay in surgical ICU 4. Surgical ICU mortality |
|
| Notes | Sample size calculation: Estimated that 30 participants in each group would provide 80% power with α error = 5% to detect a reduction in VAP from 50% to 20% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to received one of three regimens according to computer‐generated random number codes kept in sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned to received one of three regimens according to computer‐generated random number codes kept in sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear information about blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 randomised participants (11%) excluded from analysis. 6 participants (1/3/2 in each group) were withdrawn because unexpected recovery meant that they were not on mechanical ventilation for 48 hours and a further 6 participants (1/2/3) died. Unlikely to have introduced a bias |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported in full |
| Other bias | Low risk | No other sources of bias identified |
Seguin 2014.
| Methods | Study design: 2‐arm parallel group RCT Location: France Number of centres: 6 Study period: May 2008 to May 2011 Funding source: French Ministry of Health |
|
| Participants | Setting: ICU Inclusion criteria: > 18 years, closed traumatic brain injury (Glasgow Coma Score ≤ 8), expected mechanical ventilation ≥ 48 hours. Protocol amended to include patients with cerebral haemorrhage. Exclusion criteria: patients in whom oral care procedure could not be performed within 12 hours after intubation, or had tetraplegia, facial trauma, pulmonary contusion involving > 1 lobe, aspiration pneumonia, current curative antimicrobial therapy, known allergy to povidone‐iodine, pregnancy. Number randomised: 179 (Povidone‐Iodine: 91; Control: 88) Number evaluated: 150 (Povidone‐Iodine: 78; Control: 72) Baseline characteristics: ‐ Povidone Iodine*: Age: 48 (19); M/F: 60/25; SAPS II Score: 47 (11) ‐ Control*: Age: 48 (18); M/F: 64/18; SAPS II Score: 46 (12) * data presented on participants analysed |
|
| Interventions |
Comparison: Povidone‐Iodineversus Placebo Povidone Iodine: Betadine 10% oral antiseptic solution portioned in identical vials containing 125 mL of product. Participants received nasopharynx and oropharynx rinsing with 20 mL of povidone iodine (10%) using a 60 mL syringe (final concentration 3.3%). The solution was progressively injected in the buccal and pharyngeal cavities and regularly suctioned during 2 minutes, every 4 hours. The protocol was continued until extubation or until day 30. Placebo: used as above. |
|
| Outcomes | 1. Incidence of VAP 2. VAP as time to first occurrence 3. Incidence of early (< 7 days) and late (≥ 7 days) VAP 4. Incidence density of VAP per 1000 ventilator days 5. ICU and 90‐day mortality 6. Duration of ICU and hospital stay 7. Number of ventilation‐free days 8. Oropharayngeal and tracheal colonisation by potentially pathogenic microorganisms 9. Incidence of ventilator‐associated tracheobronchitis 10. Incidence of acute respiratory distress syndrome 11. Events of other nosocomial infections 12. Systemic antibiotic use 13. Adverse effects: agitation/hypertension, epistaxis, oxygen desaturation, aspiration, others |
|
| Notes | Sample size calculation: reported for VAP | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was centralized and performed by the pharmacy of the coordinating centre, stratified by centre and by type of patients (trauma or cerebral haemorrhage), and equilibrated by blocks of 4”. Probably done well using computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | “Randomization was centralized and performed by the pharmacy of the coordinating centre, stratified by centre and by type of patients (trauma or cerebral haemorrhage), and equilibrated by blocks of 4”. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The placebo was identical to povidone‐iodine in terms of colour, small and texture. Both povidone‐iodine and placebo were portioned in identical vials” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “An independent diagnosis validation committee. . blindly classified each patient as positive or negative for VAP.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16% attrition rate for VAP incidence but the numbers and reasons for lost to follow‐up were similar in each group. |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported. |
| Other bias | Low risk | No other sources of bias identified |
Stefanescu 2013.
| Methods | Study design: Two‐arm parallel group RCT Location: USA Number of centres: 1 Study period: June 2010 to January 2012 Funding source: Forsyth Medical Center Sara Lee for Women’s Health and WFSM Department of Peadiatric Research Funds |
|
| Participants | Setting: neonatal ICU Inclusion criteria: extremely low birth weight, gestational age ≤ 28 weeks, receipt of mechanical ventilation of at least 3 days in the first week of life and in the interval between days 7 and 10 of life; a parent provided written informed consent Exclusion criteria: chromosomal or major congenital anomaly, the attending physician did not intend to provide full medical support. Number randomised: 41 (Biotene: 20; Control: 21) Number evaluated: 41 (Biotene: 20; Control: 21) Baseline characteristics: ‐ Biotene: (Median gestational age: 24 weeks (24 ‐ 25); M/F: 7/13) ‐ Control: (Median gestational age: 25 weeks (24 ‐ 25); M/F: 11/10) |
|
| Interventions |
Comparison: Biotene versus Control Biotene: Timed oral care performed using sterile foam‐tip swabs with OralBalance Gel from 2 ml single use twist‐tip vials, and involved hygiene of buccal mucosa, tongue and areas around endotracheal tube, every 4 hours from enrolment to final extubation. Control: Timed oral care performed using sterile foam tip swabs with sterile water from 2 ml single use twist‐tip vials, and involved hygiene of buccal mucosa, tongue and areas around endotracheal tube, every 4 hours from enrolment to final extubation. All infants received VAP bundling, consisting of good hand hygiene and use of gloves when handling respiratory secretions, head of bed elevation, avoidance of routine use of saline with tracheal suctioning process, and weekly change of ventilator circuits |
|
| Outcomes | 1. Incidence of VAP 2. Number of VAP per 1000 ventilator days 3. Mortality 4. Length of hospital stay 5. Duration of mechanical ventilation 6. Micro‐organism colonisation in tracheal aspirate 7. Adverse effects |
|
| Notes | Sample size calculation: not reported, a pilot study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “We used blocked randomisation with varying block size” Probably done using computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | “Group assignments were provided in sealed envelopes which were kept secure by the investigational pharmacist, who was responsible for identifying the group to which each randomised patient was allocated” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “the primary limitation to our study was that we did not blind the staff to the intervention” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "All radiographs where VAP was suspected were reviewed with the paediatric radiologists who were blinded to individual study assignment” Potential for bias in deciding whether VAP is suspected |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Unclear risk | More infants in the control group received a complete course of antenatal steroids compared to infants in the Biotene group (P = 0.045). A complete course of antenatal steroids improves neonatal lung maturity and function and may reduce the risk of VAP. This imbalance is likely to lead to an underestimate of the benefit of the active treatment |
Tang 2013.
| Methods | Study design: RCT Location: Adult ICU(China) Number of centres: 1 Study period: 14 months (dates not given) Funding source: Unclear |
|
| Participants | Setting: adult ICU Inclusion criteria: All patients admitted to the ICU with receipt of mechanical ventilation of at least 48 hours were assessed for inclusion in the study.. Exclusion criteria: unclear Number randomised: 60 (Gp A: 30; Gp B: 30) Number evaluated: 60 (Gp A: 30; Gp B: 30) Baseline characteristics: Age: 56 (13.22) ; M/F: 38/22 "Age and sex comparable between groups" |
|
| Interventions |
Comparison: Saline rinse vs saline swab Gp A: rinse oral cavity with saline Gp B: saline swab with saline cotton ball |
|
| Outcomes | 1. VAP 2. Mortality 3. Duration of ventilation |
|
| Notes | Sample size calculation: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in analysis |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Unclear risk | The authors did not give a detailed description about the intervention methods and frequency of oral care in each group |
Tantipong 2008.
| Methods | Study design: A single‐centre RCT with 2 parallel groups Location: Thailand Number of centres: 1 tertiary care university hospital Study period: January 2006 through March 2007 Funding source: Thailand Research Fund and Faculty of Medicine Siriraj Hospital |
|
| Participants | Inclusion criteria: Eligible patients were adults aged ≧ 18 years who were hospitalised in intensive care units (36 beds) or general medical wards (240 beds) at Siriraj Hospital and who received mechanical ventilation Exclusion criteria: Patients who had pneumonia at enrolment or who had a chlorhexidine allergy Number randomised: 207 Number evaluated: 207 (110 participants received mechanical ventilation for > 48 hours) ‐ Experimental group: n = 102; age: 56.5 ± 20.1; M/F: 50/52; mean APACHE II Score: 16.7 ± 7.9 ‐ Control group: n = 105; age: 60.3 ± 19.1; M/F: 51/54; mean APACHE II Score: 18.2 ± 8.1 Participants' demographic characteristics between groups did not differ significantly |
|
| Interventions |
Comparison: Toothbrush + chlorhexidine versus toothbrush + placebo Experimental group (n = 102): received oral care 4 times a day with brushing the teeth, suctioning any oral secretions, and rubbing the oropharyngeal mucosa with 15 ml of a 2% chlorhexidine solution, until their endotracheal tubes were removed Control group (n = 105): Underwent the same oral care procedure with normal saline solution |
|
| Outcomes | The following outcome variables were reported for each group: 1. Incidence of VAP 2. Number of cases of VAP per 1000 ventilator days 3. Incidence of VAP for participants who received mechanical ventilation for > 2 days 4. Overall mortality 5. Mean days of mechanical ventilation (mean ± SD) 6. Rate of irritation of oral mucosa |
|
| Notes | Sample size calculation: Estimated that 108 participants required in each group to give 80% power to detect a 50% decrease in VAP with 5% Type 1 error | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "..randomized..... by stratified randomization according to sex and hospital location of eligible patient" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned and probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded as chlorhexidine solution had different odour and taste from saline |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The assessors who determined whether a participant developed pneumonia were unaware of the participant's study group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All randomised participants included in outcome evaluation but only 53% of participants on ventilators for > 2 days and therefore at risk of VAP |
| Selective reporting (reporting bias) | Unclear risk | Planned outcome VAP but not all participants at risk and information unclear. Mortality reported |
| Other bias | Unclear risk | Only 60% of study participants received ventilation in ICU and only 53% of participants received mechanical ventilation for > 48 hours. Likely that nursing care protocols were different in general medical wards compared to ICUs |
Xu 2007.
| Methods | Study design: Parallel‐group RCT Location: Nanjing, China Number of centres: 1 Study period: December 2004 to June 2006 Funding source: No external funding |
|
| Participants | Setting: ICU in drum tower hospital of Nanjing University Inclusion criteria: Critically ill adult patients in ICU receiving mechanical ventilation Exclusion criteria: Patients with severe oral diseases, mechanical ventilation for > 24 hours prior to study entry, those who refused oral care protocol Number randomised: 164 Number evaluated: 164 Baseline characteristics: Not reported for each randomised group |
|
| Interventions |
Comparison: Saline swab versus saline rinse versus both Experimental group A (n = 58): Rinsing the oropharyngeal cavity with saline for 5 ‐ 10 seconds, followed by suction aspiration, repeated 5 ‐ 10 times twice daily for 7 days Experimental group B (n = 62): Both wipe and rinse as above, twice daily for 7 days Control group (n = 44): Usual care ‐ wiping the oropharyngeal cavity with saline‐soaked cotton ball twice daily for 7 days |
|
| Outcomes | VAP, stomatitis, fungal infection | |
| Notes | Diagnosis of VAP was according to Chinese Society of Respiratory Diseases criteria Information translated from Chinese paper by Shi Zongdao and colleagues |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated" but no details of sequence generation described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in outcome evaluation |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Low risk | No other sources of bias identified |
Xu 2008.
| Methods | Study design: Parallel‐group RCT Location: Shandong, China Number of centres: 1 Study period: No stated Funding source: No external funding |
|
| Participants | Setting: ICU of the second hospital of Shandong University Inclusion criteria: Adults entering ICU receiving mechanical ventilation expected to last > 48 hours Exclusion criteria: Patients with pulmonary infections Number randomised: 116 Number evaluated: 116 Baseline characteristics: Not reported for each randomised group |
|
| Interventions |
Comparison: Saline rinse versus saline swab Experimental group (n = 64): Rinse of the oropharyngeal cavity with saline for 5 ‐ 10 seconds, followed by suction aspiration and repeated 5 ‐ 10 times, twice daily Control group (n = 52): Standard oral care comprising scrubbing with a cotton ball soaked in saline, twice daily |
|
| Outcomes | VAP, duration of ventilation (days) | |
| Notes | Diagnosis of VAP was according to Chinese Society of Respiratory Diseases criteria Information translated from Chinese paper by Shi Zongdao and colleagues |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated". Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in the outcome evaluation |
| Selective reporting (reporting bias) | Low risk | Both outcomes listed in Methods are reported in the Results section |
| Other bias | Low risk | No other sources of bias identified |
Yao 2011.
| Methods | Study design: Single‐blind pilot RCT (NCT 00604916) Location: Taiwan Number of centres: 1 Study period: March to November 2007 Funding source: Grants from Taiwan National Science Council and career development grant from National Health Research Institutes |
|
| Participants | Setting: Surgical ICU Inclusion criteria: Intubated and ventilated postoperative patients expected to be in ICU > 48 hours and expected to require mechanical ventilation for 48 ‐ 72 hours with nasal or endotracheal intubation Exclusion criteria: Patients with pneumonia at baseline Number randomised: 53 Number evaluated: 53 (VAP), day 3 ‐ 4 50, day 7 ‐ 8 42 Baseline characteristics: ‐ Intervention group: Age: 60.7 ± 16.0; M/F: 17/11; APACHE II Score: 19.6 ± 5.2 ‐ Control group: Age: 60.5 ± 16.5; M/F: 17/8; APACHE II Score: 19.4 ± 4.4 |
|
| Interventions |
Comparison: Oral care + toothbrushing twice a day versus usual oral care Experimental group: Standardised oral care protocol twice daily for 15 ‐ 20 minutes for 7 days from trained intervention nurse. Bed elevated 30° to 45°, hypopharyngeal suctioning, mouth moistened with 5 ‐ 10 ml purified water, buccal surfaces of teeth cleaned with powered toothbrush and lingual tooth surfaces and tongue, gums and mucosa massaged with soft paediatric toothbrush. Oral cavity then cleaned with toothette swab connected to a suction tube and rinsed with 50 ml water + hypopharyngeal suctioning Control group: Received oral care protocol, twice daily for 10 ‐ 15 minutes provided by same trained intervention nurse. Participants elevated, hypopharyngeal suctioning, lips moistened with toothette swab and water, then further hypopharyngeal suctioning |
|
| Outcomes | 1. Oral Assessment Guide (OAG) score 2. Plaque score (Turesky‐Gilmore‐Glickman modification of Quigley‐Hein plaque index with disclosing dye. Recorded 1 tooth from each quadrant (prioritising premolars and incisors) scores summed) 3. Duration of ventilation 4. Length of ICU stay 5. Incidence of VAP (defined as CPIS > 6) 4. Mortality (ICU) |
|
| Notes | Sample size calculation: Pilot study NCT 00604916 at ClinicalTrials.gov Email sent to author 14 November 2012. Reply received 12 December 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomized using a computer generated randomization table" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in trial report Unclear whether allocation was concealed from researchers prior to assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Experimental group received toothbrushing (both powered and manual) and control group did not, so blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessed blinded to allocated treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | VAP outcome assessed in all randomised participants. For oral health and plaque outcomes 8/28 (experimental) and 7/25 (control) participants lost (transferred to ward) and 2/28 participants in experimental group died |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported, but denominators unclear for VAP and mortality. However this information was supplied by email from the authors |
| Other bias | Unclear risk | 3/28 (11%) and 1/25 (4%) participants in experimental and control groups were edentulous. Unclear how the intervention and outcomes were applied in these participants |
Zhao 2012.
| Methods | Study design: A single‐centre RCT with 2 parallel groups Location: China Number of centres: 1 surgical ICU in city hospital Study period: May 2010 to April 2011 Funding source: Not stated |
|
| Participants | Inclusion criteria: Admission into the ICU, orally intubated, receiving mechanical ventilation Exclusion criteria: Not specified Number randomised: 324 (162 per group) Number evaluated: 324 Age group: Mean 66.25 ± 15.28 Baseline characteristics were comparable |
|
| Interventions |
Comparison: Yikou (triclosan) rinse versus saline Experimental group: Oral cavity swab with 15 ml of Yikou gargle (triclosan is main ingredient), 4 times a day Control group: Oral cavity swab with normal saline, 4 times a day Secretions were aspirated using suction once daily and sent to lab for culture |
|
| Outcomes | 3 outcome variables were available: 1. Incidence of VAP in < 4 days of ventilation and within 4 ‐ 10 days of ventilation 2. Mechanical ventilation days 3. ICU stay days 4. Culture of the samples taking from oropharyngeal cavity and inferior respiratory tract (Table 3, detection rates of microbial pathogens before and after oral nursing care were listed) |
|
| Notes | Diagnosis of VAP was mainly determined by microbial examination of the aspirate secretions from the inferior respiratory tract, which was performed every day | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided into 2 groups" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described and unclear whether Yikou and saline had the same appearance and odour |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The main results were all reported |
| Selective reporting (reporting bias) | Low risk | The results were fully reported |
| Other bias | Unclear risk | Only the results of microbial examination of the aspirate secretions from the inferior respiratory tract as tool of VAP diagnosis was mentioned and its diagnostic efficacy may not be enough |
APACHE II = Acute Physiology and Chronic Health Evaluation II; CAO = caries/absent/occluded; CDC = Centers for Disease Control; CHX = chlorhexidine; CPIS = Clinical Pulmonary Infection Score; DMFT = decayed/missing/filled teeth; ED = emergency department; ICU = intensive care unit; INR = international normalised ratio; IQRs = interquartile ranges; ITT = intention‐to‐treat; M/F = male/female; PICU = paediatric intensive care unit; ppm = parts per million; RCT = randomised controlled trial; RTI = respiratory tract infection; SAPS = Simplified Acute Physiologic Score; SD = standard deviation; SLS = sodium lauryl sulfate; TRISS = Trauma Injury Severity Score; UTI = urinary tract infection; VAP = ventilator‐associated pneumonia
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abusibeih 2010 | Quasi‐randomised trial |
| Anon 2012 | Abstract only; insufficient information to assess |
| Baradari 2012 | Not aimed to assess VAP incidence or mortality |
| Bellissimo‐Rodrigues 2014 | Intervention was dental care, not dental hygiene care |
| Bordenave 2011 | Identified from ClinicalTrials.gov website as ongoing study but email from contact author on 8 November 2012 confirmed that this study did not proceed due to lack of funding |
| Buckley 2013 | Not RCT |
| Chao 2009 | Not RCT |
| Darnell 2015 | Not RCT |
| Epstein 1994 | The participants involved in the study were not critically ill |
| Fan 2012 | The ingredients of the mouthwash used in the trial were not reported, so we could not judge the mouthwash containing antibiotics or not |
| Fan 2015 | The CHX solution used in interventions contained antibiotics |
| Ferozali 2007 | The target population was long‐term care residents, not critically ill people in hospitals |
| Genuit 2001 | Not RCT |
| Grap 2004 | Not aimed to assess VAP incidence or mortality |
| Gu 2013 | Not RCT |
| Guo 2007 | RCT, but patients had lung trauma (injury before receiving the oral nursing intervention) |
| Houston 2002 | Likely that fewer than 10% of study participants had mechanical ventilation for a minimum of 48 hours |
| Jafari 2007 | Abstract only; insufficient information to assess |
| Kusahara 2012b | Not aimed to assess VAP incidence or mortality |
| Labeau 2013 | Not RCT |
| Lai 1997 | RCT of critically ill people, unclear how many were on mechanical ventilation, outcome candidiasis |
| Li 2011 | Participants allocated to groups by alternation (not RCT) |
| Li 2012 | The mouthwash Kouitai used in the trial contains both chlorhexidine and metronidazole, and the latter is an antibiotic |
| Liang 2007 | The participants involved in the study did not use mechanical ventilation |
| Liao 2015 | Not RCT |
| Liwu 1990 | Clinical controlled trial, not an RCT |
| MacNaughton 2004 | Abstract only; insufficient information to assess |
| Maury 2015 | Not RCT |
| McCartt 2010 | Not aimed to assess VAP incidence or mortality |
| McCoy 2012 | Not RCT |
| Munro 2015 | Intervention was preintubation oral hygiene care |
| Needleman 2011 | Not aimed to assess VAP incidence or mortality |
| Ogata 2004 | The target population was patients about to receive orotracheal intubation, they were not on mechanical ventilation. Study about gargling with povidone iodine before oral intubation to reduce the transport of bacteria into the trachea, not oral care intervention in critically ill patients to reduce VAP |
| Pawlak 2005 | Not RCT |
| Pelucchi 2013 | Not RCT, Italian systematic review |
| Pivkina 2014 | Abstract only; insufficient information to assess |
| Sands 2015 | Not RCT |
| Santos 2008 | Email reply from Dr Santos stated that “The nurse put the first admission on biotene and the second admission on cetylpyridium, the third admission on biotene and so on.” Alternation as an allocation method is not truly random and therefore this study was excluded |
| Segers 2006 | The participants involved in the study did not use mechanical ventilation |
| Seo 2011 | Not RCT |
| Swartz 2015 | Not RCT |
| Tattevin 2015 | Not RCT |
| Ueda 2004 | The target population was people in nursing homes, not critically ill people in hospitals |
| Wang 2006 | Quasi‐randomised controlled trial |
| Wang 2012 | The interventions being tested in the experimental group included elevation of the head of the bed, closed endotracheal suctioning in addition to oral nursing care, which is outside the scope of the review |
| Yin 2004 | RCT aiming to improve oral cleanliness. Unlikely that participants received mechanical ventilation |
| Yun 2011 | Not RCT |
| Zouka 2010 | Abstract only, insufficient information to include in review. Emailed contact author 6 November 2012 without response |
RCT = randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Dahiya 2012.
| Methods | RCT |
| Participants | 70 "adult patients (>18 years) admitted to ICU on mechanical ventilation for <24 hours". The meaning of this sentence is unclear. It is possible that the patients did not receive mechanical ventilation for more than 48 hours and thus did not meet the definition of VAP |
| Interventions | 0.2% chlorhexidine gluconate solution versus hydrogen peroxide solution |
| Outcomes | VAP, oropharyngeal colonisation |
| Notes | Emailed study investigator 4th March 2016 for publication details or full unpublished study data |
NCT 01657396.
| Methods | RCT ‐ 3‐arm parallel‐group study |
| Participants | Adults in intensive care units in Alberta, Canada |
| Interventions | SAGE Q care (commercial package) versus SAGE Q care plus chlorhexidine versus standard oral hygiene care |
| Outcomes | VAP, frequency of oral care procedures, oral assessment score, duration of ICU and hospital stay, ICU and hospital mortality, antimicrobial utilisation, acquisition of antimicrobial‐resistant organisms |
| Notes | Information on trial register website suggests trial completed but no study results or publication posted. Principle investigator contacted but no further details about the study obtained |
ICU = intensive care unit; OA = oral assessment; RCT = randomised controlled trial; VAP = ventilator‐associated pneumonia
Differences between protocol and review
We clarified the criteria for studies eligible to be included in this review.
Participants in trials should not have a respiratory infection at baseline.
The interventions to be included in this review must include an oral hygiene care component. We excluded trials where the intervention being evaluated was a type of suction system or variation of method, timing, or place where mechanical ventilation was introduced (e.g. emergency room or ICU).
Minimum duration of mechanical ventilation of 48 hours, in order for the diagnosis of nosocomial pneumonia, either during the period of ventilation or within 48 hours of extubation, to be considered ventilator‐associated pneumonia.
Outcome of mortality defined as either all‐cause ICU mortality or, where this was not available, all‐cause 30‐day mortality. We considered that the effect of the underlying condition(s) on mortality would be similar in each randomised treatment group during this period.
In order to avoid duplication, we excluded trials where the intervention was selective decontamination of the digestive tract with antibiotics, as these interventions are included in another Cochrane Review (D'Amico 2009).
Likewise, we excluded trials where the intervention was probiotics, as these interventions are included in another Cochrane Review (Hao 2015).
We updated the text in the Methods section of this review about the 'Risk of bias' assessment in line with the latest version of the Cochrane Handbook for Systematic Reviews of Interventions, and we added more details about the process followed.
For this 2016 update:
As the purpose of this systematic review is to determine the effects of oral hygiene care on the development of VAP in a group of very ill patients in intensive care, we excluded studies that reported only intermediate outcomes, such as microbial colonisation or CPIS scores, because the relationship between these outcomes and VAP or mortality is unclear.
We dropped the outcome 'microbial colonisation'. We excluded studies that only reported this outcome, and not VAP incidence or mortality, so an analysis of this outcome for the included studies would lead to selective reporting. Additionally, most traditional criteria for VAP diagnosis already incorporate results of microbial colonisation laboratory tests (Waters 2015).
We undertook a subgroup analysis for a dose‐response relationship for chlorhexidine, as recent research suggests a possible relationship between chlorhexidine dose and mortality/effectiveness in VAP reduction (Klompas 2014; Zhang 2013).
We used the risk ratio (RR) rather than the odds ratio (OR) for the binary data, in line with current Cochrane Oral Health policy, as this made interpretation of the results easier.
We only searched the VIP database for Chinese studies, because the previous search strategies are no longer valid.
We added the outcomes reported in the 'Summary of findings tables' to the Methods section.
Contributions of authors
Conceiving and designing the initial review: HX Conducting and writing the initial review: HX, QZ, HW, SF Co‐ordinating the update: FH, HW, SF Developing search strategy and undertaking searches for the update: FH, HX, CL Screening search results for the update: FH, HX, QZ, HW, SF, CL Extracting data and assessing risk of bias for the update: FH, HX, QZ, HW, SF, CL Analysing and interpreting data for the update: FH, HX, HW, SF, CL Writing the review update: FH, HX, HW, SF, CL Approving the final review update prior to submission: FH, HX, QZ, HW, SF, CL
Sources of support
Internal sources
-
West China College of Stomatology of Sichuan University and the Chinese Cochrane Center, China.
This review was supported by the West China College of Stomatology, Sichuan University academically and in manpower resource; statistical analysis was supported by the Chinese Cochrane Center
The University of Manchester, UK.
-
Manchester Academic Health Sciences Centre (MAHSC), UK.
Cochrane Oral Health is supported by MAHSC and the NIHR Manchester Biomedical Research Centre
External sources
CMB funding SR0510, Project of Development of Systematic Review supported by Chinese Medical Board of New York, USA.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (ohg.cochrane.org/partnerships‐alliances). Contributors over the past year have been the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Fang Hua: none known Huixu Xie: none known Helen V Worthington: none known. Professor Worthington is a Co‐ordinating Editor with Cochrane Oral Health Susan Furness: none known. Mrs Furness is an editor with Cochrane Oral Health Qi Zhang: none known Chunjie Li: none known
Edited (no change to conclusions)
References
References to studies included in this review
Bellissimo‐Rodrigues 2009 {published data only}
- Bellissimo‐Rodrigues F, Bellissimo‐Rodrigues WT, Viana JM, Teixeira GC, Nicolini E, Auxiliadora‐Martins M, et al. Effectiveness of oral rinse with chlorhexidine in preventing nosocomial respiratory tract infections among intensive care unit patients. Infection Control & Hospital Epidemiology 2009;30(10):952‐8. [DOI] [PubMed] [Google Scholar]
Berry 2011 {published data only}
- Berry AM, Davidson PM, Masters J, Rolls K, Ollerton R. Effects of three approaches to standardized oral hygiene to reduce bacterial colonization and ventilator associated pneumonia in mechanically ventilated patients: A randomised control trial. International Journal of Nursing Studies 2011;48(6):681‐8. [DOI] [PubMed] [Google Scholar]
Berry 2013 {published data only}
- Berry A M. A comparison of Listerine and sodium bicarbonate oral cleansing solutions on dental plaque colonisation and incidence of ventilator associated pneumonia in mechanically ventilated patients: a randomised control trial. Intensive & critical care nursing 2013;29(5):275‐81. [DOI] [PubMed] [Google Scholar]
Bopp 2006 {published data only}
- Bopp M, Darby M, Loftin KC, Broscious S. Effects of daily oral care with 0.12% chlorhexidine gluconate and a standard oral care protocol on the development of nosocomial pneumonia in intubated patients: a pilot study. Journal of Dental Hygiene 2006;80(3):9. [PubMed] [Google Scholar]
Cabov 2010 {published and unpublished data}
- Cabov T, Macan D, Husedzinovic I, Skrlin‐Subic J, Bosnjak D, Sestan‐Crnek S, et al. The impact of oral health and 0.2% chlorhexidine oral gel on the prevalence of nosocomial infections in surgical intensive‐care patients: a randomized placebo‐controlled study. Wiener Klinische Wochenschrift 2010;122(13‐14):397‐404. [DOI] [PubMed] [Google Scholar]
Caruso 2009 {published data only}
- Caruso P, Denari S, Ruiz SAL, Demarzo SE, Deheinzelin D. Saline instillation before tracheal suctioning decreases the incidence of ventilator‐associated pneumonia. Critical Care Medicine 2009;37(1):32‐8. [DOI] [PubMed] [Google Scholar]
Chen 2008 {published data only}
- Chen QL, Ye XF, Jiang YZ, Yan MQ. Application of new oral care method to orotracheal intubation. Fujian Medical Journal 2008;30(5):155‐7. [Google Scholar]
DeRiso 1996 {published data only}
- DeRiso AJ 2nd, Ladowski JS, Dillon TA, Justice JW, Peterson AC. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest 1996;109(6):1556‐61. [DOI] [PubMed] [Google Scholar]
Feng 2012 {published data only}
- Feng S, Sun X, Chen Y. Application of different mouthwashes in oral nursing for patients with orotracheal intubation. China Medicine and Pharmacy 2012;8(2):100‐1. [Google Scholar]
Fields 2008 {published data only}
- Fields LB. Oral care intervention to reduce incidence of ventilator‐associated pneumonia in the neurologic intensive care unit. Journal of Neuroscience Nursing 2008;40(5):291‐8. [DOI] [PubMed] [Google Scholar]
Fourrier 2000 {published data only}
- Fourrier F, Cau‐Pottier E, Boutigny H, Roussel‐Delvallez M, Jourdain M, Chopin C. Effects of dental plaque antiseptic decontamination on bacterial colonization and nosocomial infections in critically ill patients. Intensive Care Medicine 2000;26(9):1239‐47. [DOI] [PubMed] [Google Scholar]
Fourrier 2005 {published data only}
- Fourrier F, Dubois D, Pronnier P, Herbecq P, Leroy O, Desmettre T, et al. Effect of gingival and dental plaque antiseptic decontamination on nosocomial infections acquired in the intensive care unit: a double‐blind placebo‐controlled multicenter study. Critical Care Medicine 2005;33(8):1728‐35. [DOI] [PubMed] [Google Scholar]
Grap 2011 {published data only}
- Grap MJ, Munro CL, Hamilton VA, Elswick RK Jr, Sessler CN, Ward KR. Early, single chlorhexidine application reduces ventilator‐associated pneumonia in trauma patients. Heart & Lung 2011;40(5):e115‐22. [DOI] [PubMed] [Google Scholar]
Hu 2009 {published data only}
- Hu X, Chen X. Application of improved oral nursing method to orotracheal intubation. Chinese Journal of Misdiagnostics 2009;9(17):4058‐9. [Google Scholar]
Jacomo 2011 {published data only}
- Jacomo AD, Carmona F, Matsuno AK, Manso PH, Carlotti AP. Effect of oral hygiene with 0.12% chlorhexidine gluconate on the incidence of nosocomial pneumonia in children undergoing cardiac surgery. Infection Control & Hospital Epidemiology 2011;32(6):591‐6. [DOI] [PubMed] [Google Scholar]
Koeman 2006 {published data only}
- Koeman M, Ven AJ, Hak E, Joore HC, Kaasjager K, Smet AG, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator‐associated pneumonia. American Journal of Respiratory & Critical Care Medicine 2006;173(12):1348‐55. [DOI] [PubMed] [Google Scholar]
- Koeman M, Ven AJ, Hak E, Joore JC, Kaasjager HA, Smet AM, et al. Less ventilator‐associated pneumonia after oral decontamination with chlorhexidine; a randomised trial. Nederlands Tijdschrift voor Geneeskunde 2008;152(13):752‐9. [PubMed] [Google Scholar]
Kusahara 2012a {published data only}
- Kusahara DM, Peterlini MA, Pedreira ML. Oral care with 0.12% chlorhexidine for the prevention of ventilator‐associated pneumonia in critically ill children: Randomised, controlled and double blind trial. International Journal of Nursing Studies 2012;49(11):1354‐63. [DOI] [PubMed] [Google Scholar]
- Kusahara DM, Peterlini MAS, Pedreira MLG. Randomized, controlled and double blinded trial of oral decontamination with 0.12% chlorhexidine for the prevention of ventilator‐associated pneumonia in children. Pediatric Critical Care Medicine 2011;12(3, Suppl 1):A16. [Google Scholar]
- Pedreira MLG, Kusahara DM, Carvalho WB, Nunez SC, Peterlini MAS. Oral care interventions and oropharyngeal colonization in children receiving mechanical ventilation. American Journal of Critical Care 2009;18(4):319‐29. [DOI] [PubMed] [Google Scholar]
Long 2012 {published data only}
- Long Y, Mou G, Zuo Y, lv F, Feng Q, Du J. Effect of modified oral nursing method on the patients with orotracheal intubation. Journal of Nurses Training 2012;27(24):2290‐3. [Google Scholar]
Lorente 2012 {published data only}
- Lorente L, Lecuona M, Jimenez A, Palmero S, Pastor E, Lafuente N, et al. Ventilator‐associated pneumonia with or without toothbrushing: a randomized controlled trial. European Journal of Clinical Microbiology and Infectious Diseases 2012;31(10):2621‐9. [DOI] [PubMed] [Google Scholar]
Meinberg 2012 {published data only}
- Meinberg MC, Cheade M de F, Miranda AL, Fachini MM, Lobo SM. The use of 2% chlorhexidine gel and toothbrushing for oral hygiene of patients receiving mechanical ventilation: effects on ventilator‐associated pneumonia [Uso de clorexidina 2% gel e escovacao mecanica na higiene bucal de pacientes sob ventilacao mecanica: efeitos na pneumonia associada a ventilador]. Revista Brasileira de Terapia Intensiva 2012;24(4):369‐74. [PUBMED: 23917935] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mo 2016 {published data only}
- Mo ZD, Li XL, Ke JY, Wu JP, Chen XW. Analysis of risk factors in ventilator‐associated pneumonia and preventive effect of oral care. Chinese Journal of Nosocomiology 2016;26(3):698‐699, 705. [Google Scholar]
Munro 2009 {published data only}
- Munro C, Grap M, Sessler C, McClish D. Effect of oral care interventions on dental plaque in mechanically ventilated ICU adults. American Journal of Critical Care 2007;16(3):309. [Google Scholar]
- Munro CL, Grap MJ, Jones DJ, McClish DK, Sessler CN. Chlorhexidine, toothbrushing, and preventing ventilator‐associated pneumonia in critically ill adults. American Journal of Critical Care 2009;18(5):428‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozcaka 2012 {published data only}
- Ozcaka O, Basoglu OK, Buduneli N, Tasbakan MS, Bacakoglu F, Kinane DF. Chlorhexidine decreases the risk of ventilator‐associated pneumonia in intensive care unit patients: a randomized clinical trial. Journal of Periodontal Research 2012;47(5):584‐92. [DOI] [PubMed] [Google Scholar]
Panchabhai 2009 {published data only}
- Panchabhai TS, Dangayach NS, Krishnan A, Kothari VM, Karnad DR. Oropharyngeal cleansing with 0.2% chlorhexidine for prevention of nosocomial pneumonia in critically ill patients: an open‐label randomized trial with 0.01% potassium permanganate as control. Chest 2009;135(5):1150‐6. [DOI] [PubMed] [Google Scholar]
Pobo 2009 {published data only}
- Pobo A, Lisboa T, Rodriguez A, Sole R, Magret M, Trefler S, et al. A randomized trial of dental brushing for preventing ventilator‐associated pneumonia. Chest 2009;136(2):433‐9. [DOI] [PubMed] [Google Scholar]
Prendergast 2012 {published data only}
- Prendergast V. Safety and efficacy of oral care for intubated neuroscience intensive care patients. Doctoral Dissertation Series 2012‐3. Lund, Sweden: Lund University, 2012:1‐86. [Google Scholar]
- Prendergast V, Hagell P, Hallberg IR. Electric versus manual tooth brushing among neuroscience ICU patients: is it safe?. Neurocritical Care 2011;14(2):281‐6. [DOI] [PubMed] [Google Scholar]
- Prendergast V, Hallberg IR, Jakobsson U, Renvert S, Moran A, Gonzalez O. Comparison of oropharyngeal and respiratory nosocomial bacteria between two methods of oral care: A randomized controlled trial. Journal of Neuroscience and Neurosurgical Nursing 2012;1(1):10‐18. [Google Scholar]
- Prendergast V, Jakobsson U, Renvert S, Hallberg IR. Effects of a standard versus comprehensive oral care protocol among intubated neuroscience ICU patients: results of a randomized controlled trial. Journal of Neuroscience Nursing 2012;44(3):134‐46. [DOI] [PubMed] [Google Scholar]
Roca Biosca 2011 {published data only}
- Roca Biosca A, Anguera Saperas L, García Grau N, Rubio Rico L, Velasco Guillén MC. Prevention of mechanical ventilator‐associated pneumonia: a comparison of two different oral hygiene methods. Enfermería Intensiva 2011;22(3):104‐11. [DOI] [PubMed] [Google Scholar]
Scannapieco 2009 {published data only}
- Scannapieco FA, Yu J, Raghavendran K, Vacanti A, Owens SI, Wood K, et al. A randomized trial of chlorhexidine gluconate on oral bacterial pathogens in mechanically ventilated patients. Critical Care 2009;13(4):R117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sebastian 2012 {published data only}
- Sebastian MR, Lodha R, Kapil A, Kabra SK. Oral mucosal decontamination with chlorhexidine for the prevention of ventilator‐associated pneumonia in children ‐ a randomized, controlled trial. Pediatric Critical Care Medicine 2012;13(5):e305‐10. [DOI] [PubMed] [Google Scholar]
Seguin 2006 {published data only}
- Seguin P, Tanguy M, Laviolle B, Tirel O, Malledant Y. Effect of oropharyngeal decontamination by povidone‐iodine on ventilator‐associated pneumonia in patients with head trauma. Critical Care Medicine 2006;34(5):1514‐9. [DOI] [PubMed] [Google Scholar]
Seguin 2014 {published data only}
- Seguin P, Laviolle B, Dahyot‐Fizelier C, Dumont R, Veber B, Gergaud S, et al. Effect of oropharyngeal povidone‐iodine preventive oral care on ventilator‐associated pneumonia in severely brain‐injured or cerebral hemorrhage patients: a multicenter, randomized controlled trial. Critical Care Medicine 2014;42:1‐8. [DOI] [PubMed] [Google Scholar]
Stefanescu 2013 {published data only}
- Stefanescu BM, Hétu C, Slaughter JC, O'Shea TM, Shetty AK. A pilot study of Biotene OralBalance gel for oral care in mechanically ventilated preterm neonates. Contemporary Clinical Trials 2013;35(2):33‐9. [DOI] [PubMed] [Google Scholar]
Tang 2013 {published data only}
- Tang J, Chen SL, Deng JL. Efficacy of mouth cavity irrigation in prevention of ventilator‐associated pneumonia. Chinese Journal of Nosocomiology 2013;23(17):4119‐21. [Google Scholar]
Tantipong 2008 {published data only}
- Silvestri L, Saene HK, Milanese M, Zei E, Blazic M. Prevention of ventilator‐associated pneumonia by use of oral chlorhexidine. Infection Control & Hospital Epidemiology 2009;30(1):101‐3. [DOI] [PubMed] [Google Scholar]
- Tantipong H, Morkchareonpong C, Jaiyindee S, Thamlikitkul V. Randomized controlled trial and meta‐analysis of oral decontamination with 2% chlorhexidine solution for the prevention of ventilator‐associated pneumonia. Infection Control & Hospital Epidemiology 2008;29(2):131‐6. [DOI] [PubMed] [Google Scholar]
Xu 2007 {published data only}
- Xu J, Feng B, He L, Shen H, Chen XY. Influence of different oral nursing methods on ventilator‐associated pneumonia and oral infection in the patients undergoing mechanical ventilation. Journal of Nursing Science 2007;7(22):56‐7. [Google Scholar]
Xu 2008 {published data only}
- Xu HL. Application of improved oral nursing method to the prevention of ventilator‐associated pneumonia. Journal of Qilu Nursing 2008;14(19):15‐6. [Google Scholar]
Yao 2011 {published data only}
- Yao L, Chang C, Wang C, Chen C. Effect of an oral care protocol in preventing ventilator associated pneumonia in ICU patients. Critical Care 2008;12(Suppl 2):Abs No P48. [Google Scholar]
- Yao LY, Chang CK, Maa SH, Wang C, Chen CC. Brushing teeth with purified water to reduce ventilator‐associated pneumonia. Journal of Nursing Research 2011;19(4):289‐97. [DOI] [PubMed] [Google Scholar]
Zhao 2012 {published data only}
- Zhao Y. Research on application of Yikou gargle in prevention of ventilation associated pneumonia. Chinese Journal of Nosocomiology 2012;23(22):5232‐3. [Google Scholar]
References to studies excluded from this review
Abusibeih 2010 {published data only}
- Abusibeih A, Lev A. Randomized control trial comparing oral care methods and VAP. Intensive Care Medicine 2010;36:S329. [Google Scholar]
Anon 2012 {published data only}
- Anon. EB57 Development of an oral care‐based programme for prevention of ventilator‐associated pneumonia. Critical Care Nurse 2012;32(2):e34. [Google Scholar]
Baradari 2012 {published data only}
- Baradari AG, Khezri HD, Arabi S. Comparison of antibacterial effects of oral rinses chlorhexidine and herbal mouth wash in patients admitted to intensive care unit. Bratislavske Lekarske Listy 2012;113(9):556‐60. [DOI] [PubMed] [Google Scholar]
Bellissimo‐Rodrigues 2014 {published data only}
- Bellissimo‐Rodrigues WT, Menegueti MG, Gaspar GG, Nicolini EA, Auxiliadora‐Martins M, Basile‐Filho A, et al. Effectiveness of a dental care intervention in the prevention of lower respiratory tract nosocomial infections among intensive care patients: a randomized clinical trial. Infection Control and Hospital Epidemiology 2014; Vol. 35, issue 11:1342‐8. [DOI] [PubMed]
Bordenave 2011 {published data only}
- Bordenave C. Evaluation of the effectiveness of a protocol of intensification of mouth care (teeth brushing and chlorhexidine 0.12%) on the colonisation of tracheal aspirations in intubated and ventilated patients in intensive care. Recherche en Soins Infirmiers 2011;2011(106):92‐8. [PubMed] [Google Scholar]
Buckley 2013 {published data only}
- Buckley MS, Dzierba AL, Smithburger PL, McAllen KJ, Jordan CJ, Kane‐Gill SL. Chlorhexidine for the prevention of ventilator associated pneumonia in critically ill adults. Journal of Infection Prevention 2013;14:162‐9. [Google Scholar]
Chao 2009 {published data only}
- Chao YC, Chen Y, Wang KK, Lee R, Tsai H. Removal of oral secretion prior to position change can reduce the incidence of ventilator‐associated pneumonia for adult ICU patients: a clinical controlled trial study. Journal of Clinical Nursing 2009;18(1):22‐8. [DOI] [PubMed] [Google Scholar]
Darnell 2015 {published data only}
- Darnell J. EB44 TMI: Reducing ventilator‐associated pneumonia in the surgical intensive care unit. Critical Care Nurse 2015;35:e20. [Google Scholar]
Epstein 1994 {published data only}
- Epstein J, Ransier A, Lunn R, Spinelli J. Enhancing the effect of oral hygiene with the use of a foam brush with chlorhexidine. Oral Surgery, Oral Medicine, Oral Pathology 1994;77(3):242‐7. [DOI] [PubMed] [Google Scholar]
Fan 2012 {published data only}
- Fan T. The effect of a modified oral nursing care method on prevention of ventilator associated pneumonia. Nursing Practice and Research 2012;9(12):99‐100. [Google Scholar]
Fan 2015 {published data only}
- Fan YL, Chang QF, Zhang L. Research of oropharyngeal cleaning methods for ventilator associated pneumonia. Chinese Journal of Nosocomiology 2015;25(12):2777‐9. [Google Scholar]
Ferozali 2007 {published data only}
- Ferozali F, Johnson G, Cavagnaro A. Health benefits and reductions in bacteria from enhanced oral care. Special Care in Dentistry 2007;27(5):168‐76. [DOI] [PubMed] [Google Scholar]
Genuit 2001 {published data only}
- Genuit T, Bochicchio G, Napolitano LM, McCarter RJ, Roghman MC. Prophylactic chlorhexidine oral rinse decreases ventilator‐associated pneumonia in surgical ICU patients. Surgical Infections 2001;2(1):5‐18. [DOI] [PubMed] [Google Scholar]
Grap 2004 {published data only}
- Grap MJ, Munro CL, Elswick RK Jr, Sessler CN, Ward KR. Duration of action of a single, early oral application of chlorhexidine on oral microbial flora in mechanically ventilated patients: a pilot study. Heart & Lung 2004;33(2):83‐91. [DOI] [PubMed] [Google Scholar]
Gu 2013 {published data only}
- Gu W J, Liu J C. Toothbrushing for critically ill mechanically ventilated patients: a duplicated trial included?. Critical Care Medicine 2013;41(7):e137‐8. [DOI] [PubMed] [Google Scholar]
Guo 2007 {published data only}
- Guo MJ. Study on the new oral nursing for the patient of acute lung brain‐storm through tracheal. Chinese Journal of Mordern Nursing 2007;13(26):2537‐8. [Google Scholar]
Houston 2002 {published data only}
- Houston S, Hougland P, Anderson JJ, LaRocco M, Kennedy V, Gentry LO. Effectiveness of 0.12% chlorhexidine gluconate oral rinse in reducing prevalence of nosocomial pneumonia in patients undergoing heart surgery. American Journal of Critical Care 2002;11(6):567‐70. [PubMed] [Google Scholar]
Jafari 2007 {published data only}
- Jafari S, Ranjbar H, Kamrani F, Alavi‐Majd H, Yaghmaei F. Effects of chlorhexidine and normal saline on dental plaque formation in ICU patients: a comparative study. Journal of Nurse Midwifery 2007;17(56):36‐43. [Google Scholar]
Kusahara 2012b {published data only}
- Kusahara D M, Friedlander L T, Peterlini M A S, Pedreira M L G. Oral care and oropharyngeal and tracheal colonization by Gram‐negative pathogens in children. Nursing in critical care 2012;17:115‐22. [DOI] [PubMed] [Google Scholar]
Labeau 2013 {published data only}
- Labeau SO, Blot SI. Oral care for mechanically ventilated patients involving toothbrushing. Critical Care Medicine 2013;41(7):e136‐7. [DOI] [PubMed] [Google Scholar]
Lai 1997 {published data only}
- Lai M, Huang H. Toothpaste mouth care for critically ill patients. Journal of Nursing Science 1997;12(2):106‐7. [Google Scholar]
Li 2011 {published data only}
- Li W, Ma X, Peng Y, Cao J, Loo WTY, Hao L, et al. Application of a Nano‐antimicrobial film to prevent ventilator‐associated pneumonia: A pilot study. African Journal of Biotechnology 2011;10(10):1926‐31. [Google Scholar]
Li 2012 {published data only}
- Li S, Zhang H, Zhang B. Application of different oral nursing methods to prevent ventilation associated pneumonia. Practical Clinical Medicine 2012;13(5):92‐3. [Google Scholar]
Liang 2007 {published data only}
- Liang YL, Lu LQ, Liang JT. Oral cavity nursing study in endotracheal intubation with the Baihu decoction. Journal of Nurses Training 2007;1(22):79‐80. [Google Scholar]
Liao 2015 {published data only}
- Liao YM, Tsai JR, Chou FH. The effectiveness of an oral health care program for preventing ventilator‐associated pneumonia. Nursing in Critical Care 2015;20(2):89‐97. [DOI] [PubMed] [Google Scholar]
Liwu 1990 {published data only}
- Liwu A. Oral hygiene in intubated patients. The Australian Journal of Advanced Nursing 1990;7(2):4‐7. [PubMed] [Google Scholar]
MacNaughton 2004 {published data only}
- MacNaughton PD, Bailey J, Donlin N, Branfield P, Williams A, Rowswell H. A randomised controlled trial assessing the efficacy of oral chlorhexidine in ventilated patients (029). Intensive Care Medicine 2004;30(Suppl):S12. [Google Scholar]
Maury 2015 {published data only}
- Maury E, London J, Offenstadt G. Community‐acquired pneumonia. New England Journal of Medicine 2015;372(3):292. [DOI] [PubMed] [Google Scholar]
McCartt 2010 {published data only}
- McCartt PAM. Effect of Chlorhexidine Oral Spray versus Mechanical Toothbrushing and Chlorhexindine Rinse in Decreasing Ventilator Associated Pneumonia in Critically Ill Adults [PhD thesis]. Gainesville, Florida, USA: University of Florida, 2010:91. [Google Scholar]
McCoy 2012 {published data only}
- McCoy T, Fields W, Kent N. Evaluation of emergency department evidence‐based practices to prevent the incidence of ventilator‐acquired pneumonia. Journal of Nursing Care Quality 2012;27(1):83‐8. [DOI] [PubMed] [Google Scholar]
Munro 2015 {published data only}
- Munro CL, Grap MJ, Sessler CN, Elswick RK Jr, Mangar D, Karlnoski‐Everall R, et al. Preintubation application of oral chlorhexidine does not provide additional benefit in prevention of early‐onset ventilator‐associated pneumonia. Chest 2015;147(2):328‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Needleman 2011 {published data only}
- Needleman IG, Hirsch NP, Leemans M, Moles DR, Wilson M, Ready DR, et al. Randomized controlled trial of toothbrushing to reduce ventilator‐associated pneumonia pathogens and dental plaque in a critical care unit. Journal of Clinical Periodontology 2011;38(3):246‐52. [DOI] [PubMed] [Google Scholar]
Ogata 2004 {published data only}
- Ogata J, Minami K, Miyamoto H, Horishita T, Ogawa M, Sata T, et al. Gargling with povidone‐iodine reduces the transport of bacteria during oral intubation. Canadian Journal of Anaesthesia 2004;51(9):932‐6. [DOI] [PubMed] [Google Scholar]
Pawlak 2005 {published data only}
- Pawlak D, Semar R, Cantos K. Improving frequency of oral care in the medical intensive care unit. American Journal of Infection Control 2005;33(5):E147. [Google Scholar]
Pelucchi 2013 {published data only}
- Pelucchi G, Ciucur M, Giacovelli M, Lucchini A, Luongo M. L'igiene del cavo orale [Italian]. SCENARIO: Official Italian Journal of ANIARTI 2013;30:23‐34. [Google Scholar]
Pivkina 2014 {published data only}
- Pivkina AI, Gusarov VG, Zhivotneva I V, Bodunova GE. Oral care in ventilated patients‐can we improve it?. Intensive Care Medicine 2014;40:S28. [Google Scholar]
Sands 2015 {published data only}
- Sands KM, Twigg JA, Wise MP. Oral hygiene with chlorhexidine in critically ill patients. JAMA Internal Medicine 2015;175(2):316. [DOI] [PubMed] [Google Scholar]
Santos 2008 {published data only}
- Santos PS, Mello WR, Wakim RCS, Paschoal MG. Use of oral rinse with enzymatic system in patients totally dependent in the intensive care unit [Uso de solução bucal com sistema enzimático em pacientes totalmente dependentes de cuidados em unidade de terapia intensiva]. Revista Brasileira de Terapia Intensiva 2008;20(2):154‐9. [PubMed] [Google Scholar]
Segers 2006 {published data only}
- Segers P, Speekenbrink RG, Ubbink DT, Ogtrop ML, Mol BA. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial. JAMA 2006;296(20):2460‐6. [DOI] [PubMed] [Google Scholar]
Seo 2011 {published data only}
- Seo H‐K, Choi E‐H, Kim J‐H. The effect of oral hygiene for ventilator‐associated pneumonia (VAP) incidence. Journal of Korean Critical Care Nursing 2011;4(2):1. [Google Scholar]
Swartz 2015 {published data only}
- Swartz AW. Community‐acquired pneumonia. New England Journal of Medicine 2015;372(3):293. [DOI] [PubMed] [Google Scholar]
Tattevin 2015 {published data only}
- Tattevin P, Levy HG, Gould IM. Community‐acquired pneumonia. New England Journal of Medicine 2015;372(3):293. [DOI] [PubMed] [Google Scholar]
Ueda 2004 {published data only}
- Ueda K, Yamada Y, Toyosato A, Nomura S, Saitho E. Effects of functional training of dysphagia to prevent pneumonia for patients on tube feeding. Gerodontology 2004;21(2):108‐11. [DOI] [PubMed] [Google Scholar]
Wang 2006 {published data only}
- Wang MM. The improvement of oral care in ICU intubated patients. Journal of Mudanjiang Medical College 2006;27(5):80‐1. [Google Scholar]
Wang 2012 {published data only}
- Wang YX, He LY, Gao MR, Wang ZW, Li XY. Application of oral nursing care bundle to prevent VAP for critically surgically ill patients. Chinese Archives of General Surgery 2012;6(5):448‐50. [Google Scholar]
Yin 2004 {published data only}
- Yin XR, Liao Y. The improvement of the oral care for patients with orotracheal intubation. West China Medical Journal 2004;19(3):482. [Google Scholar]
Yun 2011 {published data only}
- Yun H‐Y, Lee E‐S, Kim J‐Y, Kim H‐S, Kim K‐A, Kim E‐S, et al. Effect of tooth‐brushing on oral health and ventilator‐associated pneumonia of critically ill patients. Journal of Korean Critical Care Nursing 2011;4(2):1. [Google Scholar]
Zouka 2010 {published data only}
- Zouka M, Soultati I, Hari H, Pourzitaki C, Paroutsidou G, Thomaidou E, et al. Oral dental hygiene and ventilator‐associated pneumonia prevention in an ICU setting: Comparison between two methods (preliminary data of a randomised prospective study). Intensive Care Medicine 2010;36:S103. [Google Scholar]
References to studies awaiting assessment
Dahiya 2012 {published data only}
- Dahiya U. Decontamination with chlorhexidine gluconate reduces the incidence of ventilator associated pneumonia. Nursing Journal of India 2012;103:89‐91. [PubMed] [Google Scholar]
NCT 01657396 {unpublished data only}
Additional references
Alhazzani 2013
- Alhazzani W, Smith O, Muscedere J, Medd J, Cook D. Toothbrushing for critically ill mechanically ventilated patients: a systematic review and meta‐analysis of randomized trials evaluating ventilator‐associated pneumonia. Critical Care Medicine 2013;41(2):646‐55. [DOI] [PubMed] [Google Scholar]
Apostolopoulou 2003
- Apostolopoulou E, Bakakos P, Katostaras T, Gregorakos L. Incidence and risk factors for ventilator‐associated pneumonia in 4 multidisciplinary intensive care units in Athens. Respiratory Care 2003;48(7):681‐8. [PubMed] [Google Scholar]
ATS Guideline 2005
- American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia. American Journal of Respiratory and Critical Care Medicine 2005;171(4):388‐416. [DOI] [PubMed] [Google Scholar]
Azoulay 2006
- Azoulay E, Timsit JF, Tafflet M, Lassence A, Darmon M, Zahar JR, et al. Candida colonization of the respiratory tract and subsequent pseudomonas ventilator‐associated pneumonia. Chest 2006;129(1):110‐7. [DOI] [PubMed] [Google Scholar]
Bekaert 2011
- Bekaert M, Timsit JF, Vansteelandt S, Depuydt P, Vésin A, Garrouste‐Orgeas M, et al. Attributable mortality of ventilator‐associated pneumonia: a reappraisal using causal analysis. American Journal of Respiratory and Critical Care Medicine 2011;184(10):1133‐9. [DOI] [PubMed] [Google Scholar]
Berry 2007
- Berry AM, Davidson PM, Masters J, Rolls K. Systematic literature review of oral hygiene practices for intensive care patients receiving mechanical ventilation. American Journal of Critical Care 2007;16(6):552‐62. [PubMed] [Google Scholar]
CONSORT 2012
- Altman DG, Moher D, Schultz KF. Improving the reporting of randomised trials: The CONSORT statement and beyond. Statistics in Medicine 2012;31(25):2985‐97. [DOI] [PubMed] [Google Scholar]
Contentin 2014
- Contentin L, Ehrmann S, Giraudeau B. Heterogeneity in the definition of mechanical ventilation duration and ventilator‐free days. American Journal of Respiratory and Critical Care Medicine 2014;189(8):998‐1002. [DOI] [PubMed] [Google Scholar]
Cook 1998
- Cook DJ, Walter SD, Cook RJ, Griffith LE, Guyatt GH, Leasa D, et al. Incidence of and risk factors for ventilator‐associated pneumonia in critically ill patients. Annals of Internal Medicine 1998;29(6):433‐46. [DOI] [PubMed] [Google Scholar]
D'Amico 2009
- D'Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E, Liberati A. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD000022.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2009
- Edwards JR, Peterson KD, Mu Y, Banerjee S, Allen‐Bridson K, Morrell G, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. American Journal of Infection Control 2009;37(10):783‐805. [DOI] [PubMed] [Google Scholar]
Fourrier 1998
- Fourrier F, Duvivier B, Boutigny H, Roussel‐Delvallez M, Chopin C. Colonization of dental plaque: a source of nosocomial infections in intensive care unit patients. Critical Care Medicine 1998;26(2):301‐8. [DOI] [PubMed] [Google Scholar]
Gu 2012
- Gu WJ, Gong YZ, Pan L, Ni YX, Liu JC. Impact of oral care with versus without toothbrushing on the prevention of ventilator‐associated pneumonia: a systematic review and meta‐analysis of randomized controlled trials. Critical Care 2012;16(5):R190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gyssens 2011
- Gyssens IC. Antibiotic policy. International Journal of Antimicrobial Agents 2011;38 Suppl:11‐20. [PUBMED: 22018989] [DOI] [PubMed] [Google Scholar]
Hao 2015
- Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD006895.pub3] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hillier 2013
- Hillier B, Wilson C, Chamberlain D, King L. Preventing ventilator‐associated pneumonia through oral care, product selection, and application method: a literature review. AACN Advanced Critical Care 2013;24(1):38‐58. [DOI] [PubMed] [Google Scholar]
Horan 2008
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care‐associated infection and criteria for specific types of infections in the acute care setting. American Journal of Infection Control 2008;36(5):309‐32. [DOI] [PubMed] [Google Scholar]
Klompas 2007
- Klompas M. Does this patient have ventilator‐associated pneumonia?. JAMA 2007;297(14):1583‐93. [DOI] [PubMed] [Google Scholar]
Klompas 2014
- Klompas M, Speck K, Howell MD, Greene LR, Berenholtz SM. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: Systematic review and meta‐analysis. JAMA Internal Medicine 2014;174(5):751‐61. [DOI: 10.1001/jamainternmed.2014.359.] [DOI] [PubMed] [Google Scholar]
Labeau 2011
- Labeau SO, Vyver K, Brusselaers N, Vogelaers D, Blot SI. Prevention of ventilator‐associated pneumonia with oral antiseptics: a systematic review and meta‐analysis. Lancet Infectious Diseases 2011;11(11):845‐54. [DOI] [PubMed] [Google Scholar]
Marsh 2010
- Marsh PD. Microbiology of dental plaque biofilms and their role in oral health and caries. Dental Clinics of North America 2010;54(3):441‐54. [DOI] [PubMed] [Google Scholar]
McCarney 2007
- McCarney R, Warner J, Iliffe S, Haselen R, Griffin M, Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Medical Research Methodology 2007;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Melsen 2011
- Melsen WG, Rovers MM, Koeman M, Bonten MJ. Estimating the attributable mortality of ventilator‐associated pneumonia from randomized prevention studies. Critical Care Medicine 2011;39(12):2736‐42. [DOI] [PubMed] [Google Scholar]
Mojon 2002
- Mojon P. Oral health and respiratory infection. Journal of the Canadian Dental Association 2002;68(6):340‐6. [PubMed] [Google Scholar]
Muscedere 2008
- Muscedere J, Dodek P, Keenan S, Fowler R, Cook D, Heyland D. Comprehensive evidence‐based clinical practice guidelines for ventilator‐associated pneumonia: prevention. Journal of Critical Care 2008;23(1):126‐37. [DOI] [PubMed] [Google Scholar]
Pemberton 2012
- Pemberton MN, Gibson J. Chlorhexidine and hypersensitivity reactions in dentistry. British Dental Journal 2012;213(11):547‐50. [DOI] [PubMed] [Google Scholar]
Pineda 2006
- Pineda LA, Saliba RG, Solh AA. Effect of oral decontamination with chlorhexidine on the incidence of nosocomial pneumonia: a meta‐analysis. Critical Care 2006;10(1):R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Price 2014
- Price R, MacLennan G, Glen J, SuDDICU Collaboration. Selective digestive or oropharyngeal decontaminationand topical oropharyngeal chlorhexidine for preventionof death in general intensive care: systematic review and network meta‐analysis. BMJ 2014;348:g2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pugin 1991
- Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM. Diagnosis of ventilator‐associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic 'blind' bronchoalveolar lavage fluid. American Review of Respiratory Disease 1991;143(5 Pt 1):1121‐9. [DOI] [PubMed] [Google Scholar]
Scannapieco 1992
- Scannapieco FA, Stewart EM, Mylotte JM. Colonization of dental plaque by respiratory pathogens in medical intensive care patients. Critical Care Medicine 1992;20(6):740‐5. [DOI] [PubMed] [Google Scholar]
Schulz 2002
- Schulz KF, Grimes DA. Generation of allocation sequences in randomised trials: chance, not choice. Lancet 2002;359(9305):515‐9. [DOI] [PubMed] [Google Scholar]
Selim 2010
- Selim AG, Balalis G, Bhaskar B, Driel ML. Antibiotics for ventilator‐associated pneumonia. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD004267] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2004
- Shi ZD, Yu JR, Luo R, He Y, Liu XC, Chen E. Effect of oral nursing care: a systematic review. Chinese Journal of Evidence Based Medicine 2004;4(12):837‐46, 858. [Google Scholar]
Tablan 2004
- Tablan OC, Anderson L, Besser R, Bridges C, Hajjeh R. Guidelines for preventing healthcare‐associated pneumonia: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. Morbidity & Mortality Weekly Report. Recommendations & Reports 2004;55 (RR‐3):1‐36. [PubMed] [Google Scholar]
Terezakis 2011
- Terezakis E, Needleman I, Kumar N, Moles D, Agudo E. The impact of hospitalization on oral health: a systematic review. Journal of Clinical Periodontology 2011;38(7):628‐36. [DOI] [PubMed] [Google Scholar]
Terpenning 2005
- Terpenning M. Geriatric oral health and pneumonia risk. Clinical Infectious Diseases 2005;40(12):1807‐10. [DOI] [PubMed] [Google Scholar]
Waters 2015
- Waters B, Muscedere J. A 2015 update on ventilator‐associated pneumonia: new insights on its prevention, diagnosis, and treatment. Current Infectious Disease Reports 2015;17(8):1‐9. [DOI] [PubMed] [Google Scholar]
Whittaker 1996
- Whittaker CJ, Klier CM, Kolenbrander PE. Mechanisms of adhesion by oral bacteria. Annual Review of Microbiology 1996;50:513‐52. [DOI] [PubMed] [Google Scholar]
Worthington 2015
- Worthington H, Clarkson J, Weldon J. Priority oral health research identification for clinical decision‐making. Evidence‐based Dentistry 2015;16(3):69‐71. [DOI] [PubMed] [Google Scholar]
Zanatta 2011
- Zanatta FB, Bergoli AD, Werle SB, Antoniazzi RP. Biofilm removal and gingival abrasion with medium and soft toothbrushes. Oral Health and Preventive Dentistry 2011;9(2):177‐83. [PubMed] [Google Scholar]
Zhang 2013
- Zhang TT, Tang SS, Fu LJ. The effectiveness of different concentrations of chlorhexidine for prevention of ventilator‐associated pneumonia: a meta‐analysis. Journal of Clinical Nursing 2014;23(11‐12):1461‐75. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Shi 2010
- Shi Z, Xie H, Wang P, Wu Y, Chen E, Ng L, Worthington HV, Singer M, Needleman I. Oral hygiene care for critically ill patients to prevent ventilator associated pneumonia. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD008367] [DOI] [PubMed] [Google Scholar]
Shi 2013
- Shi Z, Xie H, Wang P, Zhang Q, Wu Y, Chen E, Ng L, Worthington HV, Needleman I, Furness S. Oral hygiene care for critically ill patients to prevent ventilator‐associated pneumonia. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD008367.pub2] [DOI] [PubMed] [Google Scholar]


