Highlights
-
•
Growing list of PRRs that sense pathogenic fungal conserved moieties.
-
•
Enhanced immune responses or fungal evasion mechanisms occur through PRR crosstalk.
-
•
Increasing evidence of potent inhibitory mechanisms during PRR signalling.
-
•
PRR targeting strategies to boost immune responses to pathogenic fungi.
Keywords: Pathogen recognition receptor, C-type lectin-like receptor, Toll-like receptor, Nod-like receptor, Crosstalk
Abstract
Over the last decade, invasive fungal infections have emerged as a growing threat to human health worldwide and novel treatment strategies are urgently needed. In this context, investigations into host-pathogen interactions represent an important and promising field of research. Antigen presenting cells such as macrophages and dendritic cells are strategically located at the frontline of defence against potential invaders. Importantly, these cells express germline encoded pattern recognition receptors (PRRs), which sense conserved entities from pathogens and orchestrate innate immune responses. Herein, we review the latest findings regarding the biology and functions of the different classes of PRRs involved in pathogenic fungal recognition. We also discuss recent literature on PRR collaboration/crosstalk and the mechanisms involved in inhibiting/regulating PRR signalling. Finally, we discuss how the accumulated knowledge on PRR biology, especially Dectin-1, has been used for the design of new immunotherapies against fungal infections.
1. Introduction
Invasive fungal infections have become a major healthcare issue in recent years, with unacceptably high morbidity and mortality levels particularly in immunocompromised patients [1]. Further research in this area is required as there is still no commercial vaccine, the number of antifungal drugs is limited and fungal pathogens are developing drug resistance [2]. A limited number of fungi including Candida albicans, Aspergillus fumigatus, Cryptococcus neoformans and Pneumocystis jirovecii cause life-threatening conditions in humans [1]. However, invasive fungal infections from other species are currently on the rise including C. glabrata, C. parapsilosis, C. tropicalis and the multi-drug resistant C. auris [3,4]. Therefore, new effective antifungal drugs, immunotherapies and vaccines are urgently needed. An in-depth understanding of host-pathogen interactions is required to help design novel anti-fungal immunotherapies.
Myeloid immune cells such as monocytes, macrophages and dendritic cells (DCs) are the first line of defence during infection. They sense invaders using an array of innate immune receptors termed pattern recognition receptors (PRRs) [5]. PRRs detect conserved structural motifs from microbes and endogenous stress signals called microbe-, pathogen-or damage-associated molecular patterns (MAMPs, PAMPs or DAMPs respectively). The main families of PRRs include Toll-like receptors (TLRs), C-type lectin-like receptors (CLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) and retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs). Following recognition of their respective ligands, these receptors induce innate immune responses for immediate protection or to orchestrate the activation of adaptive immunity [5,6].
Over the last two decades, the biology of PRRs in antifungal immunity has been extensively studied [7,8]. These PRRs sense various fungal cell wall components such as β-glucans, mannans, mannoproteins and chitin as well as fungal-derived RNA and unmethylated DNA (Table 1) [9]. Following ligand binding, PRRs shape immune responses by initiating various signalling cascades which result in fungal internalisation via phagocytosis, cytokine production and/or production of reactive nitrogen and oxygen species (RNS and ROS, respectively) [6,8]. Numerous reports have recently started to unravel the complexity of PRR crosstalk during fungal infections [10,11]. Investigation of potential PRR collaboration/antagonism is essential to develop new therapies to direct the innate immune system towards efficient protection while preventing adverse effects. In this review, we discuss recent findings on PRR-induced anti-fungal immunity with an emphasis on receptor crosstalk/interactions, negative regulation, and the potential for development of novel immunotherapies.
Table 1.
PRR Recognition of Fungal Pathogens/Ligands.
| PRR | Localisation | Cell Types | Motif/Adaptor | Fungal Ligand/Pathogen |
|---|---|---|---|---|
| CLR | ||||
| Dectin-1 | Cell surface | Monocytes, MØ, DCs, neutrophils, mast cells, subset of T lymphocytes | hemITAM | ß-glucans |
| Zymosan | ||||
| Candida spp. | ||||
| A. fumigatus | ||||
| Pneumocystis spp. | ||||
| Coccidioides spp. | ||||
| Fonsecaea spp. | ||||
| T. rubrum | ||||
| Dectin-2 | Cell surface | Monocytes, MØ, DCs, neutrophils | ITAM-FcRγ | Mannose |
| Zymosan | ||||
| Candida spp. | ||||
| Malassezia spp. | ||||
| A. fumigatus | ||||
| P. brasiliensis | ||||
| Pneumocystis spp. | ||||
| C. posadasii | ||||
| F. pedrosoi | ||||
| H. capsulatum | ||||
| T. rubrum | ||||
| C. neoformans | ||||
| Mincle | Cell surface | Monocytes, MØ, DCs, neutrophils, mast cells, some subsets of B cells | ITAM-FcRγ | α-mannose |
| C. albicans | ||||
| A. fumigatus | ||||
| Pneumocystis spp. | ||||
| Fonsecaea spp. | ||||
| Malassezia spp. | ||||
| Mcl | Cell surface | Monocytes, MØ, DCs, neutrophils, mast cells | ITAM-FcRγ | C. albicans |
| B. dermatitidis | ||||
| DC-SIGN | Cell surface | MØ, DCs, activated B cells | Tyrosine-based motif, LSP1 | Mannose |
| C. albicans | ||||
| A. fumigatus | ||||
| T. rubrum | ||||
| MR | Cell surface | MØ, Kupffer cells, endothelial cells | Tyrosine-based motif, FcRγ? | Mannose |
| C. albicans | ||||
| C. neoformans | ||||
| P. carinii | ||||
| C. immitis | ||||
| P. brasiliensis | ||||
| H. capsulatum | ||||
| T. rubrum | ||||
| TLR | ||||
| TLR2 | Cell surface | Monocytes, MØ, DCs, mast cells, neutrophils | MyD88, Mal | Phospholipomannans |
| β-glucans | ||||
| Zymosan | ||||
| C. albicans | ||||
| Alternaria | ||||
| A. fumigatus | ||||
| TLR4 | Cell surface, Endosome | Monocytes, MØ, DCs, mast cells, neutrophils, B lymphocytes, intestinal epithelium | MyD88, Mal, TRIF, TRAM | C. albicans |
| A. fumigatus | ||||
| TLR6 | Cell surface | Monocytes, MØ, mast cells, B lymphocytes | MyD88, Mal | Zymosan |
| TLR7 | Endosome | Monocytes, MØ, DCs, B lymphocytes | MyD88 | Candida spp. |
| TLR9 | Endosome | Monocytes, MØ, DCs, B lymphocytes | MyD88 | Unmethylated DNA with CpG motif |
| NLR | ||||
| NLRP3 | Cytoplasm | Monocytes, DCs, MØ, neutrophils, T and B lymphocytes, epithelial cells | ASC, Caspase-1 | C. albicans |
| A. fumigatus | ||||
| C. neoformans | ||||
| Malassezia spp | ||||
| P. brasiliensis | ||||
| S. schenckii | ||||
| H. capsulatum | ||||
| NLRP4 | Cytoplasm | DCs, MØ | TBK1 | C. albicans |
| NLRP10 | Cytoplasm | DCs, MØ, epithelial cells, T lymphocytes | ASC, Caspase-1 | C. albicans |
| NOD1 | Cytoplasm | Monocytes, DCs, MØ, T and B lymphocytes, intestinal epithelium | RIP2 | A. fumigatus |
| NOD2 | Cytoplasm | Monocytes, DCs, MØ, T and B lymphocytes | CARD9, RIP2 | Chitin |
| C. parapsilosis | ||||
| A. fumigatus | ||||
| RLR | ||||
| MDA5 | Cytoplasm | Monocytes, DCs, MØ, fibroblasts, epithelial cells, endothelial cells, B lymphocytes | CARDs, MAVS | C. albicans |
2. CLRs
Members of the CLR family contain a conserved C-type lectin-like domain (CTLD), which in many cases binds carbohydrates or Ca2+ [12]. Some of the best described CLRs during anti-fungal immunity are Dectin-1, the Dectin-2 cluster (Dectin-2, Mincle and Mcl), the mannose receptor (MR) and DC-specific ICAM-3 grabbing non-integrin (DC-SIGN), which are mainly expressed on myeloid cells (Table 1). In addition, collectins such as mannose binding lectin (MBL) and surfactant proteins A and B (SP-A and SP-D) have been reported to play a role in anti-fungal immunity [13]. The roles of these receptors and their signalling pathways have been expertly reviewed elsewhere [6,[13], [14], [15]]. Briefly, Dectin-2, Mincle and Mcl associate with the immunoreceptor tyrosine-based activation motif (ITAM)-containing FcRγ signalling chain while Dectin-1 contains a hemITAM in its cytoplasmic tail. Dectin-2 and Mincle have both been shown to form complexes with Mcl. Following ligand binding the hemITAM/ITAM are phosphorylated, Syk is recruited and canonical NFκB signalling is activated via a Card9/Bcl10/Malt1 complex (Fig. 1). Dectin-1 also activates NFAT and IRF1/5, and a non-canonical NFκB pathway via NFκB inducing kinase (NIK). In addition, DC-SIGN and Dectin-1 both activate RAF1, leading to phosphorylation of TLR or SYK-induced p65 at Ser276. This finely tunes NFκB-induced cytokine responses [6,14,15]. The MR receptor lacks known signalling motifs in its cytoplasmic tail, however it was recently shown to bind the FcRγ signalling chain. MR activation by Mycobacterium tuberculosis (M.tb) results in recruitment of Grb2 leading to activation of the Rac/Pak/Cdc-42 signalling cascade and recruitment of SHP-1 thereby limiting PI3K activity [16]. Whether this occurs in response to fungal pathogens remains to be determined. While many CLRs are important for anti-fungal immunity we will focus here on Dectin-1 and the Dectin-2 gene cluster.
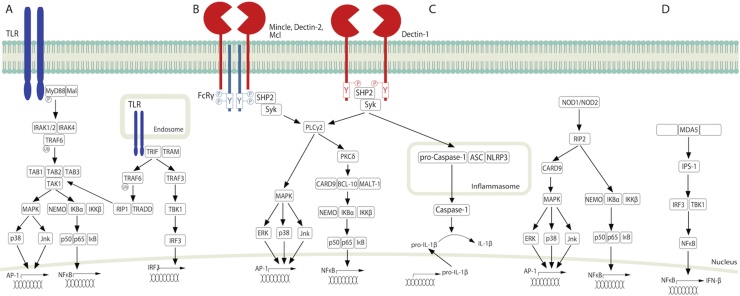
Fig. 1.
PRR signalling pathways. (A) Following TLR-mediated ligand recognition, MyD88 is recruited to the TLR and a signalling cascade involving IRAK-4, IRAK-1/2, TRAF6, TGF-β-activated kinase 1 (TAK-1), TAK1-binding protein 1 (TAB1), TAB2, TAB3 complex and IκB kinase (IKK)-β is initiated. IKK-β phosphorylates the NFκB inhibitory protein, IκBa, causing its degradation thereby facilitating NFκB nuclear translocation and transcription of proinflammatory cytokines. TLR3, 4, 7, 9, 10 and 13 signal in endosomes via the TRIF-dependent pathway. Ligand-induced TLR activation initiates a TRIF, TRAF3, TBK1 and IRF-3 cascade. Alternatively, TRIF can activate a TRAF6, RIP1, TRADD, TAK-1, NFκB pathway. (B) CLRs signal either by associating with the ITAM-containing FcRγ signalling chain (Dectin-2, Mincle, Mcl) or through a hemITAM in its cytoplasmic tail (Dectin-1). Following ligand recognition, the hemITAM/ITAM are phosphorylated and Syk is recruited. A signalling cascade involving PLCγ, PKCδ and a Card9/Bcl10/Malt1 complex is then initiated. This leads to IKKβ-mediated degradation of IκB to induce nuclear translocation of NFκB (p50, p65). MAPK pathways (ERK, p38 and Jnk) are also activated downstream of PLCγ to induce subsequent AP-1 activation. (C) NLRP3 inflammasome is activated via a CLR-Syk pathway. NLRP3 recruits ASC and pro-Caspase-1 to form an inflammasome complex. This leads to caspase-1 activation and Caspase-1-mediated cleavage of pro-IL-1β into functional IL-1β. Additionally, non-inflammasome forming NLRs such as NOD1 and NOD2 signal via RIP2 to activate the NEMO-IKBα-IKKβ complex to induce p50, p65, IκB and subsequent NFκB activation. In addition, a CARD9-MAPK pathway (ERK, p38 and Jnk) is activated resulting in subsequent AP-1 activation. (D) MDA5 activation by viral RNA, signals through IPS-1 to activate IRF3 and TBK-1, which in turn activates NFκB to induce IFN-β. Whether this occurs in response to fungal pathogens remains to be determined.
Dectin-1 binds to ß-glucans from various fungal pathogens (Table 1). Dectin-1 signalling is important for multiple anti-fungal responses including phagocytosis, ROS production, cytokine production, inflammasome activation and Th1 and Th17 responses [6,14,15]. Dectin-1 deficiency has important functional consequences during anti-fungal immunity, fungal allergy and colitis. For example, Dectin-1 KO mice were more susceptible to infections with C. albicans [17], C. glabrata [18], A. fumigatus [19] and Coccidioides immitis [20]. In addition, when C. tropicalis is present in the gut, Dectin-1 protects against colitis development by limiting fungal invasion [21]. However, in the absence of opportunistic fungi in the gut, inhibition of Dectin-1 protects against colitis due to reduced antimicrobial peptide production resulting in Lactobacilli murinus overgrowth and T regulatory cell expansion [22]. Further, during fungal allergy to A. fumigatus, Dectin-1 contributes to lung immunopathology by inducing IL-22 [23]. Thus the role of Dectin-1 during fungal-related diseases is complex and context specific. In agreement with various murine studies, patients with Dectin-1 polymorphisms have been shown to have increased susceptibility to mucocutaneous candidiasis [24], aspergillosis [25,26], increased Candida colonization [27], increased severity of ulcerative colitis [21] and an altered gut microbiome [28].
Dectin-2 and Mincle bind fungal cell wall mannans (Table 1), and interaction of Mcl with Dectin-2 increased recognition of α-mannans. However distinct hydrophilic and lipophilic ligands from the fungal cell walls of Malassezia spp. were identified for Dectin-2 and Mincle, respectively [29]. Dectin-2 is important for anti-fungal immune responses including cytokine production, phagocytosis, ROS production and induction of Th1 and Th17 responses [30] and Mincle is involved in cytokine/chemokine production in response to Malassezia spp. and C. albicans [31,32]. Dectin-2 deficient mice were more susceptible to infections with C. albicans and C. glabrata [30,33,34], while Mincle deficient mice displayed increased fungal burdens following infection with C. albicans and P. carinii [31,35]. Mcl deficient mice developed severe colitis due to defective innate immune responses to C. tropicalis in the gut and impaired tissue repair following fungal invasion [36]. In addition, Dectin-2 has been implicated in allergic reactions to house dust mite and A. fumigatus. House dust mites and A. fumigatus induce production of pro-inflammatory lipid mediators of asthma, such as cysteinyl leukotrienes which in turn induces a Th2 immune response [37,38]. Further, Dectin-2 polymorphisms have been associated with susceptibility to pulmonary cryptococcosis [39] and aspergillosis [26] and polymorphisms in these CLRs have been associated with an altered gut microbiome [28].
3. TLRs
TLRs are the best characterised PRR family for pathogen recognition. They are evolutionary conserved transmembrane proteins that sense extracellular and intracellular pathogens in endosomes and lysosomes. The TLRs contain N-terminal leucine-rich repeats (LRRs) and a C-terminal Toll/IL-1R homology (TIR) domain. Most TLRs form homodimers, except TLR2, which forms a heterodimer with TLR1 or TLR6. TLRs signal through the adapter proteins Myeloid differentiation primary response 88 (MyD88), MyD88 adapter-like (Mal), TIR-domain-containing adapter-inducing IFN-β (TRIF) and TRIF-related adaptor molecule (TRAM). The roles of the TLRs and their signalling pathways have been expertly reviewed elsewhere [5,40]. Briefly, TLR signalling usually occurs via two distinct pathways, the MyD88-dependent and the TRIF-dependent pathways (Fig. 1). MyD88 is utilised by all TLRs except for TLR3. Following ligand recognition, MyD88 is recruited to the TLR and a signalling cascade is initiated that culminates in NFκB nuclear translocation and transcription of proinflammatory cytokines (Fig. 1). Endosomal TLRs signal via the TRIF-dependent pathway to induce IRF-3 nuclear translocation and transcription of type I IFN genes (Fig. 1) [5,40].
TLRs play an important role in recognising various fungal pathogens/ligands (Table 1). TLR2 has been shown to be recruited to zymosan-containing phagosomes [41]. In addition, zymosan and various fungal pathogens induce TLR2 or TLR2/TLR6-mediated cytokine responses [[41], [42], [43], [44]]. Further, TLR2 has been shown to induce C. albicans-mediated monocyte apoptosis via activation of caspase-8 and caspase-3 [45]. While immunocompetent TLR2, TLR4 and MyD88 deficient mice did not display increased susceptibility to invasive aspergillosis compared to wild type mice [46], another study showed that loss of both TLR2 and TLR4 resulted in severely impaired neutrophil recruitment [47]. SNPs in the TLR2 binding partners, TLR1 and TLR6, have been associated with susceptibility to invasive aspergillosis [26,48]. Interestingly, a synergistic combination of TLR2/6 and TLR9 agonists (Pam2-ODN) as an inhaled treatment protected immunocompromised mice against a broad range of pathogens including A. fumigatus [49].
TLR4 recognises O-linked mannosyl chains in the cell wall of C. albicans [50]. TLR4 KO mice were more susceptible to systemic infection with C. albicans due to reduced chemokine responses and impaired neutrophil recruitment while cytokine responses were normal in TLR4 KO macrophages [51]. In response to A. fumigatus conidia but not hyphae, cytokine (TNF, IL-1α, IL-1β) production was reduced in TLR4 KO cells. TLR4-mediated signalling was lost during germination of A. fumigatus, which could be a potential means for A. fumigatus to evade host innate defences [52]. A link between a TLR4 haplotype and invasive aspergillosis has been suggested by Bochud et al. [53].
TLR7 recognises fungal RNA while TLR9 binds fungal unmethylated CpG DNA (Table 1) [54,55]. Various fungal pathogens/ligands have been shown to induce cytokines (IFN-ß, IFN- α, TNF, IL-12p40) in a TLR7, TLR9 and MyD88-dependent manner [[54], [55], [56]], however TLR9 KO mice were no more susceptible than WT mice to C. albicans infection [56] suggesting receptor redundancy. Interestingly, polymorphisms in TLR7, 8 and 9 were recently associated with aspergillosis [26].
4. NLRs
NLRs represent another arm of the innate immune system with the ability to detect PAMPs derived from internalised microbial components. The critical function of NLRs/inflammasomes during fungal infections have been expertly reviewed elsewhere [57]. NLRs are structurally composed of a C-terminal ligand sensing series of leucine rich repeats (LRR), a central oligomerisation NACHT domain and an N-terminal protein-protein domain (CARD or pyrin domain). NLRs are categorised into two major family subsets, NOD1/2 and the inflammasome-forming NLRs [58]. Inflammasome multiprotein complexes are generally composed of an NLR such as NLRP3 or NALP3, ASC and Caspase proteins. Inflammasome activation culminates in Caspase-mediated cleavage of pro-IL-1β and pro-IL-18 into functional IL-1β and IL-18 (Fig. 1) [58]. IL-1β and IL-18 have both been reported to be critical for the control of fungal infections [59,60].
Several fungal pathogens have been shown to induce Caspase-1-mediated IL-1β production via NLRP3 (Table 1) [57]. Importantly, it was reported that transition of C. albicans from bud to hyphal form was a key event for NLRP3 activation and IL-1β production [61]. In addition, C. albicans was shown to induce macrophage cell death via NLRP3-mediated pyroptosis [62]. A. fumigatus-induced secretion of IL-1β and IL-18 involved NLRP3, AIM2, ROS production and potassium efflux [63]. Nlrp3, Asc, Caspase-1 KO mice and/or Nlrp3 Aim2 double KO mice were more susceptible to infection with A. fumigatus, C. albicans, C. neoformans, P. brasiliensis and S. schenckii [[63], [64], [65], [66], [67]]. Critically, a polymorphism in NLRP3 was associated with impaired C. albicans-induced IL-1β production and increased occurrence of recurrent vulvovaginal candidiasis (RVVC) [68]. Besides NLRP3, the NLRC4 inflammasome was shown to control oral mucosal C. albicans infection and to protect against systemic dissemination of C. albicans via inflammatory cell recruitment and induction of pro-inflammatory cytokines and antimicrobial peptides [69].
The function of non-inflammasome forming NLRs such as NOD1 and NOD2, during fungal infections has not been investigated as thoroughly as the other NLRs. A. fumigatus has been shown to induce expression of NOD1, NOD2 and the downstream signalling molecule RIP2. NOD1 and NOD2 were shown to mediate A. fumigatus-induced cytokine release [70,71]. However, NOD1 deficient mice were protected against invasive aspergillosis due to enhanced Dectin-1 expression, ROS and cytokine production [72]. NOD2 polymorphisms were not increased in patients with Candida infections and Candida-induced cytokine responses were unaffected in patients with NOD2 polymorphisms [73]. NOD2 has since been shown to be involved in recognising chitin and C. parapsilosis and inducing downstream cytokine responses [74,75].
5. RLRs
The RLR family is composed of three major receptors namely RIG-I, melanoma differentiation factor 5 (MDA5) and laboratory of genetics and physiology 2 (LGP2) which recognise a vast array of RNA viruses and orchestrate innate immune responses mainly through the production of type I IFNs (Fig. 1) [76]. Although extensively studied in the context of antiviral immunity, the involvement of RLRs in anti-fungal immunity has only recently emerged. C. albicans hyphae was shown to induce IFIHI (MDA5) in macrophages (Table 1) and Mda5 deficient cells produced lower IFN-β levels in response to C. albicans compared to WT cells. Furthermore, the study also revealed a strong association with missense variations in the IFIH1 gene and susceptibility to systemic candidiasis [77]. Further studies are required to fully unravel the complex roles of the CLRs, TLRs, NLRs and RLRs during anti-fungal immunity.
6. PRR collaboration/interactions
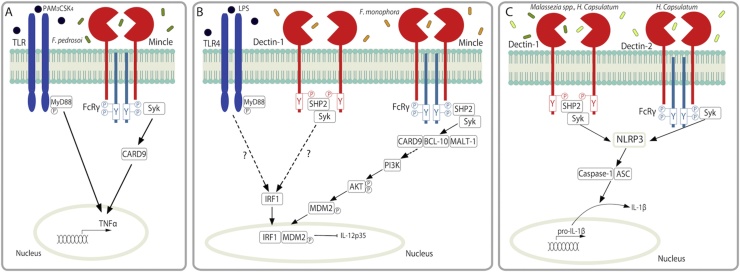
Fungal pathogens engage multiple PRRs and interactions between these receptors/pathways can result in an enhanced immune response to clear the pathogen [78], or in some cases can help the pathogen evade the immune response [10]. Some clear examples of PRR interactions in response to Fonsecaea spp., the leading cause of the chronic skin condition chromoblastomycosis, have been described. For example, F. pedrosoi conidia were shown to induce a weak Mincle-mediated TNF response, however artificial TLR co-engagement induced a more robust TNF response that resulted in improved pathogen clearance (Fig. 2) [79]. In a proof of principle study, the authors demonstrated that topical application of imiquimod, a TLR7 agonist, to the skin lesions of four patients with chromoblastomycosis resulted in a marked improvement of their lesions [80]. In agreement with this, another report showed that F. pedrosoi conidia induced a very weak inflammatory response, however the muriform induced a more pronounced pro-inflammatory response. Phagocytosis of the muriform but not the conidia was partially dependent on Dectin-1 and FcγR [81]. While co-engagement of a TLR with F. pedrosoi conidia may have therapeutic benefits [78,80], it is possible that co-engagement of a TLR could induce excess inflammation in cases where the muriform is prevalent. Furthermore, Wevers et al. [10] showed that Mincle engagement by F. monophora inhibited Th1 cell differentiation. The authors demonstrated that F. monophora can induce IL-12p70 production via Dectin-1, however signalling via Mincle counteracts this response and blocks IL12A transcription and subsequent Th1 responses (Fig. 2). Similarly, Shiqueira et al. demonstrated that F. pedrosoi conidia and muriform inhibited LPS and IFN-γ-induced IL-12 production [81]. In addition, Wuthrich et al. [82] showed that F. pedrosoi-induced Th17 responses were mediated by Dectin-2 and to a lesser extent Dectin-1 while Mincle inhibited these Th17 responses. Unlike the enhanced TNF response observed following co-engagement of a TLR, T cell responses were not augmented [82]. These data indicate that the immune response to Fonsecaea spp. involves complex interactions between different PRRs.
Fig. 2.
PRR collaboration. (A) F. pedrosoi engages Mincle to induce a weak TNF response however artificial engagement of a TLR such as TLR2 by Pam3CSK4 induces a more robust TNF response. (B) F. monophora is recognised by Mincle and leads to CARD9-BCL-10-MALT-1 complex formation through SHP2-Syk activation. This leads to PI3K activation and AKT phosphorylation, rather than NFκB activation. AKT phosphorylates MDM2, which promotes translocation to the nucleus. MDM2 associates with Dectin-1- or LPS-induced IRF1 and the ubiquitin ligase activity of MDM2 is activated. MDM2 targets IRF1 for degradation thereby blocking IL-12p35 activation. (C) Malassezia spp. and H. capsulatum activate Dectin-1 and Dectin-2 in a Syk-dependent manner to induce NLRP3-mediated IL-1β production through Caspase-1 and ASC signalling to induce Th1/Th17 responses.
Several other examples of PRR crosstalk regulating cytokine production in response to fungal pathogens have been identified. For example, Dectin-1 and Complement receptor 3 (CR3) have been shown to collaborate in response to H. capsulatum. Dectin-1 and CR3 co-localised to lipid rafts and induced enhanced activation of a Syk-JNK-AP-1 pathway that resulted in robust TNF and IL-6 production. In addition, Dectin-1 and CR3 cooperated to produce a protective adaptive anti-fungal immune response to H. capsulatum in vivo [83]. Dectin-1 has also been shown to form a complex with Galectin-3 resulting in TNF production in response to C. albicans [84]. Furthermore, Dectin-1 has been shown to interact with members of the tetraspanin family (CD63 and CD37). CD37 and Dectin-1 co-localised on the surface of antigen presenting cells. Dectin-1-induced IL-6 and IL-6-mediated antibody responses were increased during infection with C. albicans in the absence of CD37 resulting in increased pathogen clearance [85,86].
In addition to crosstalk during cytokine responses, PRRs co-operate during ligand recognition and phagocytosis. For example, Dectin-1 was recently shown to control TLR9 trafficking to ß-glucan-, A. fumigatus- and C. albicans-containing phagosomes [87]. Interestingly, Wu and colleagues linked NOD2 and TLR2 by demonstrating that A. fumigatus-induced NOD2 expression was partially mediated by TLR2 [88]. Furthermore, Wagener et al. [75] showed that digested chitin particles are phagocytosed by the mannose receptor, and NOD2 and TLR9 co-localize with internalised chitin leading to the production of IL-10 [75]. In addition, our group recently demonstrated that NOD2 and TLR7 co-localised with internalised C. parapsilosis leading to the production of IL-27, although the ligand responsible for this has not yet been identified [74].
Furthermore, CLRs have been linked with inflammasome activation. Dectin-1 induced NLRP3-mediated IL-1β production in response to Malassezia furfur (Fig. 2) [89]. In addition, Dectin-1/Syk-mediated inflammasome-dependent IL-1β production in response to C. albicans was responsible for inducing Th-17 responses [90]. In agreement with this, another report demonstrated that Caspase-1 and ASC-deficient mice displayed reduced Th1/Th17 responses [91]. Furthermore, H. capsulatum induced NLRP3 inflammasome-activation and IL-1ß production via Dectin-2. Dectin-1 was not involved in this process in the presence of Dectin-2, however in the absence of Dectin-2, Dectin-1 induced IL-1ß production although to a lesser degree than Dectin-2 [92]. These data clearly demonstrate that fungal pathogens induce co-ordinated PRR-mediated anti-fungal responses.
7. Negative regulation of PRR-induced signalling
As with all inflammatory responses, fungal responses require negative feedback mechanisms to control the level of inflammation. Recent reports have identified new feedback mechanisms that are activated during anti-fungal immunity, some of which could be potential therapeutic targets. Various negative regulators of CLRs have been discussed in detail in [11], therefore we will focus here on negative feedback mechanisms that have not been reviewed in detail elsewhere.
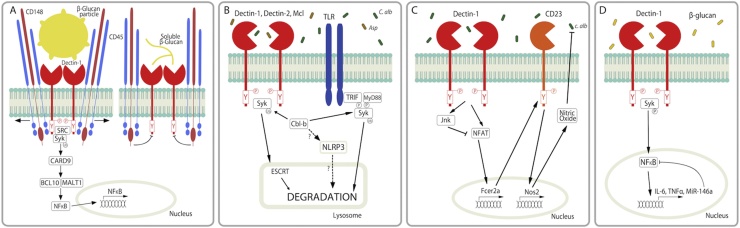
Dectin-1 binds both soluble and particulate β-glucans, however Dectin-1 signalling is only induced in response to particulate β-glucans. This is due to the formation of a “phagocytic synapse” and exclusion of the tyrosine phosphatases (CD45 and CD148) from this synapse upon binding of particulate β-glucans. Soluble β-glucans are unable to exclude the tyrosine phosphatases therefore Dectin-1 signalling is blocked by the inhibitory activity of these phosphatases (Fig. 3) [93]. As a negative feedback mechanism, phagocytosis of particulate β-glucans following Dectin-1 binding results in reduced Dectin-1 signalling and cytokine responses. Indeed, cytokine production in response to the particulate Dectin-1 ligands, zymosan and curdlan, was significantly increased upon treatment with a phagocytosis inhibitor [94,95]. In agreement with this, poorly immunogenic β-glucan microparticles induced enhanced Dectin-1-mediated cytokine production in the presence of phagocytosis inhibitors [96]. These data indicate that Dectin-1 only responds when it encounters an intact microbe. In addition, delayed phagocytosis due to larger microbe size will likely increase Dectin-1-mediated responses while smaller microbes that are phagocytosed quicker will likely promote weaker Dectin-1-mediated responses.
Fig. 3.
Negative Regulation of PRR-induced signalling. (A) Dectin-1 binds β-glucan particles (such as yeasts) to form the “phagocytic synapse”. The physical interaction between particulate β-glucan and Dectin-1 results in exclusion of CD45 and CD148 tyrosine phosphatases from the synapse. This facilitates Dectin-1 signalling via Src/Syk activation. Soluble β-glucans are unable to exclude the tyrosine phosphatases from the synapse therefore Dectin-1 signalling is blocked by the inhibitory activity of CD45 and CD148. (B) CLR activation results in their ubiquitination and degradation in a Syk-dependent manner. Cbl-b mediates ubiquitination of the activated CLRs through Syk. The ubiquitinated CLRs are then sorted into lysosomes for degradation by an endosomal sorting complex required for transport (ESCRT) system. Additionally, Cbl-b has been shown to target MyD88 and TRIF for degradation following phsophorylation by Syk. Lastly, Cbl-b potentially targets NLRP3 for degradation. (C) C. albicans is recognised by Dectin-1, leading to activation of NFAT and Jnk1. NFAT induces CD23 expression and production of nitric oxide. In the absence of Jnk1, NFAT activation, CD23 and nitric oxide levels are increased compared to WT cells. (D) β-glucans induce expression of miR-146a via a Dectin-1-Syk-NFκB pathway. MiR-146a negatively regulates Dectin-1 signalling by supressing NFκB activation.
Three reports independently showed that the E3 ubiquitin ligase Casitas B–lineage lymphoma protein b (Cbl-b) promoted ubiquitination of activated Dectin-1, Dectin-2, Mcl and Syk. The ubiquitinated CLRs were then targeted for lysosomal-mediated degradation, thereby limiting anti-fungal responses (Fig. 3). C. albicans- and A. fumigatus-induced cytokine/chemokine and ROS responses were increased in Cbl-b KO cells and mice. Cbl-b KO mice cleared C. albicans and A. fumigatus infections better than WT mice [[97], [98], [99]]. In addition, Cbl-b targeted MyD88 and Trif for degradation downstream of a TLR-CD11b pathway. In agreement with this, TLR responses were enhanced in the absence of CD11b or Cbl-b [100,101]. It has also been suggested that Cbl-b inhibits NLRP3 inflammasome activation by targeting NLRP3 for ubiquitination although the data supporting this claim is yet unpublished [102]. Additionally, we previously showed that another family member, c-Cbl, is phosphorylated in response to zymosan [103], however whether c-Cbl also targets fungal-related PRRs and associated molecules for degradation is currently unknown. Cbl-b therefore represents a potential therapeutic target during systemic Candida and Aspergillus infections, however as Cbl-b has many targets, the potential for undesirable side effects such as hyperinflammation must be taken into account.
Interestingly, Zhao and colleagues demonstrated that Jnk1 suppresses anti-fungal immunity through a novel mechanism. A Dectin-1/NFAT pathway induced expression of a CLR, CD23 and production of nitric oxide in Jnk1 deficient macrophages (Fig. 3). Other CLRs and TLRs were largely unchanged in the absence of Jnk1 [104]. Fcer2a, which encodes CD23, is found in a gene cluster with Cd209a (DC-SIGN). It is a low affinity receptor for IgE and cystic fibrosis patients with allergic bronchopulmonary aspergillosis have been shown to display increased sensitivity to IL-4 which results in increased CD23 expression on B cells and heightened CD4+ Th2 responses to A. fumigatus allergens [105]. Zhao et al. showed that in addition to binding IgE, CD23 can also bind both the yeast and hyphal forms of C. albicans. Increased CD23 expression and nitric oxide production resulted in increased Candida killing and improved survival in Jnk1 deficient mice infected with C. albicans. Mouse and human cells treated with Jnk inhibitors displayed increased Candida clearance indicating that Jnk1 represents another potential therapeutic target [104].
MicroRNAs (miRNAs) are small noncoding RNAs that regulate gene expression of mRNAs containing complementary sequences. They have been shown to play important roles in regulating inflammatory responses [106]. Some recent reports have identified roles for miRNAs in regulating anti-fungal immune responses and PRR-induced responses. ß-glucan from the C. albicans cell wall was recently shown to induce expression of miR-146a, miR-30-5p and miR-210-3p via a Dectin-1-Syk-NFκB pathway. The authors went on to show that miR-146a is a negative feedback regulator of Dectin-1 signalling as miR-146a suppressed Dectin-1-induced NFκB activation and IL-6 and TNF production (Fig. 3) [107]. In contrast, C. glabrata has been shown to down-regulate miR-146a expression [108]. Another study showed that heat killed C. albicans induced various miRNAs including miR-155, miR-455, miR-125a and miR-146a [109]. In addition, various TLRs involved in antifungal immunity, including TLR2, TLR4, TLR7 and TLR9, have been shown to induce a range of miRNAs such as miR-125a, miR-155, miR-146a, miR-132, miR-147, miR-9, miR-223 and others [[109], [110], [111], [112]]. Various TLR associated molecules have been identified as direct targets of miRNAs such as MYD88, IRAK1/2 and TRAF6 [112]. In addition, miR-125a has been shown to target NOD1 [113] while miR-223 and miR-9 suppress NLRP3 expression/activation [114,115]. Clearly, miRNAs are involved in regulating the PRR-induced pathways during anti-fungal immunity, however, further study is required in this area.
8. Harnessing PRRs for the development of novel immunotherapies against fungal infections: the case of Dectin-2
One of the ultimate goals behind investigations into host-pathogen interactions is to identify new immunotherapy strategies. This may involve stimulation or interference with PRR signalling or specifically targeting fungal antigens to DCs to improve immune-mediated protection. This is particularly relevant for vaccine research where extensive efforts have been undertaken to design better vaccine delivery tools and novel adjuvants with increased immune reactivity and lower toxicity. Numerous investigations have assessed the potential of fungal cell wall components to induce protective immunity to various diseases including infectious and autoimmune diseases as well as cancer. In particular, Dectin-1 agonists such as β-glucans have been extensively studied for their potent immunostimulatory properties. In addition, antibodies against Dectin-1 have been used to target fungal antigens to DCs and T cells have been modified to express a Dectin-1-chimeric antigen receptor. These could represent interesting strategies for future immunotherapies against fungal infections. A full description of all the studies in this area is beyond the scope of this review, therefore we will focus on select fungal-related studies.
Various in vitro studies have demonstrated the potential of β-glucan preparations to enhance immune responses to fungal pathogens. For example, curdlan has been shown to boost A. fumigatus-induced pro-inflammatory cytokine production and β-glucan was shown to amplify microbial toxicity of human neutrophils to both C. albicans and C. glabrata [116,117]. Similarly, several in vivo studies demonstrated the therapeutic potential of β-glucan administration. In combination with normal antifungal treatments, intravenous administration of β-glucan to patients infected with P. brasiliensis resulted in reduced serum antibody levels and increased P. brasiliensis-associated CD4+ T cell responses [118]. In addition, the β-glucan Laminarin conjugated to the diphtheria toxoid CRM197 was shown to induce protection in mice systemically infected with C. albicans via the induction of high levels of β-glucan antibodies [119]. in vivo imaging studies also revealed that treatment with the same Laminarin-CRM vaccine-conjugate, in combination with MF59 adjuvant, could protect mice against vaginal C. albicans infection [120]. Additionally, another study showed that Laminarin-conjugates could ameliorate antibody-mediated immunity to C. albicans in mice [121]. Oral administration of β-glucan has also been shown to increase protection against intestinal inflammation and C. albicans colonisation in mice [122]. Another interesting study showed that administration of whole glucan particles (WGP) could significantly increase survival of mice challenged with A. fumigatus. This was associated with reduced fungal burden in both brain and kidney and elevated cytokine expression in the WGP treated mice [123]. Follow-up studies from the same group showed that WGP-vaccinated mice challenged intravenously with Coccidioides posadasii had reduced mortality rates and lower fungal burden in the lung, liver and spleen [124]. Taken together, these findings highlight Dectin-1 agonists as promising candidates for the development of immunotherapies to treat fungal infections in humans. However, it remains to be elucidated whether treatments using β-glucan in combination with selected activators or inhibitors of other PRR pathways could represent an interesting strategy to further enhance immune protection.
Some groups have evaluated the potential of Dectin-1 antibodies as a delivery system to specifically target antigens such as ovalbumin and haemagglutinin to DCs and subsequently increase protective immune responses to infectious agents. Anti-Dectin-1-antigen conjugates have been shown to trigger potent CD4+ and CD8+ T cell responses [[125], [126], [127]]. This therefore represents an interesting strategy to improve vaccine efficacy to microbial pathogens. However, it remains to be determined whether this approach will have any impact against fungal pathogens.
Finally, CAR T cell technology was recently exploited to generate cytotoxic T cells specific for fungal pathogens. A novel Dectin-1-chimeric antigen receptor (D-CAR) was bioengineered using the extracellular domain of Dectin-1 to redirect T-cell specificity towards fungal β-glucan moieties [128]. It was shown that D-CAR+ T cells could inhibit A. fumigatus hyphae formation in vitro through recognition of β-glucan. In addition, immunosuppressed mice treated with D-CAR+ T cells exhibited reduced pulmonary A. fumigatus burden when compared with mice administered with control CD19-specific CAR+ T cells. Thus, these findings represent a promising step for the development of novel efficient immunotherapies using host-pathogen interactions to control fungal infections in immunocompromised individuals.
9. Conclusions
Collaborative efforts from the scientific community have provided an important body of literature on PRR biology that will be extremely valuable for the development of immunotherapies to treat fungal infections. In recent years, it has become evident that PRR crosstalk and inhibitory feedback mechanisms help finely tune anti-fungal immune responses. While much progress has been made to identify PRR crosstalk and inhibitory mechanisms, further work is required to fully unravel the complexities of these PRR interactions. A better understanding of PRR biology/interactions will be invaluable to help develop immunotherapies for fungal infections and other inflammatory diseases.
Funding
SJO is funded by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant Number 099953/Z/12/Z).
References
- 1.Brown G.D., Denning D.W., Gow N.A., Levitz S.M., Netea M.G., White T.C. Hidden killers: human fungal infections. Sci. Transl. Med. 2012;4(165) doi: 10.1126/scitranslmed.3004404. 165rv13. [DOI] [PubMed] [Google Scholar]
- 2.Denning D.W., Bromley M.J. Infectious disease. How to bolster the antifungal pipeline. Science. 2015;347(6229):1414–1416. doi: 10.1126/science.aaa6097. [DOI] [PubMed] [Google Scholar]
- 3.Alangaden G.J. Nosocomial fungal infections: epidemiology, infection control, and prevention. Infect. Dis. Clin. North Am. 2011;25(1):201–225. doi: 10.1016/j.idc.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Morales-Lopez S.E., Parra-Giraldo C.M., Ceballos-Garzon A., Martinez H.P., Rodriguez G.J., Alvarez-Moreno C.A., Rodriguez J.Y. Invasive infections with multidrug-resistant yeast Candida auris, Colombia. Emerg. Infect. Dis. 2017;23(1):162–164. doi: 10.3201/eid2301.161497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Bryant C.E., Orr S., Ferguson B., Symmons M.F., Boyle J.P., Monie T.P. International union of basic and clinical pharmacology. XCVI. Pattern recognition receptors in health and disease. Pharmacol. Rev. 2015;67(2):462–504. doi: 10.1124/pr.114.009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garth J.M., Steele C. Innate lung defense during invasive Aspergillosis: new mechanisms. J. Innate. Immun. 2017;9(3):271–280. doi: 10.1159/000455125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Netea M.G., Joosten L.A., van der Meer J.W., Kullberg B.J., van de Veerdonk F.L. Immune defence against Candida fungal infections. Nat. Rev. Immunol. 2015;15(10):630–642. doi: 10.1038/nri3897. [DOI] [PubMed] [Google Scholar]
- 9.Gow N.A.R., Latge J.P., Munro C.A. The fungal cell wall: structure, biosynthesis, and function. Microbiol. Spectr. 2017;5(3) doi: 10.1128/microbiolspec.FUNK-0035-2016. [DOI] [PubMed] [Google Scholar]
- 10.Wevers B.A., Kaptein T.M., Zijlstra-Willems E.M., Theelen B., Boekhout T., Geijtenbeek T.B., Gringhuis S.I. Fungal engagement of the C-type lectin mincle suppresses dectin-1-induced antifungal immunity. Cell Host Microbe. 2014;15(4):494–505. doi: 10.1016/j.chom.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Ostrop J., Lang R. Contact, collaboration, and conflict: signal integration of Syk-coupled C-type lectin receptors. J. Immunol. 2017;198(4):1403–1414. doi: 10.4049/jimmunol.1601665. [DOI] [PubMed] [Google Scholar]
- 12.Zelensky A.N., Gready J.E. The C-type lectin-like domain superfamily. FEBS J. 2005;272(24):6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 13.Vautier S., MacCallum D.M., Brown G.D. C-type lectin receptors and cytokines in fungal immunity. Cytokine. 2012;58(1):89–99. doi: 10.1016/j.cyto.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Geijtenbeek T.B., Gringhuis S.I. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 2009;9(7):465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geijtenbeek T.B., Gringhuis S.I. C-type lectin receptors in the control of T helper cell differentiation. Nat. Rev. Immunol. 2016;16(7):433–448. doi: 10.1038/nri.2016.55. [DOI] [PubMed] [Google Scholar]
- 16.Rajaram M.V.S., Arnett E., Azad A.K., Guirado E., Ni B., Gerberick A.D., He L.Z., Keler T., Thomas L.J., Lafuse W.P., Schlesinger L.S. M. tuberculosis-initiated human mannose receptor signaling regulates macrophage recognition and vesicle trafficking by FcRgamma-chain, Grb2, and SHP-1. Cell Rep. 2017;21(1):126–140. doi: 10.1016/j.celrep.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor P.R., Tsoni S.V., Willment J.A., Dennehy K.M., Rosas M., Findon H., Haynes K., Steele C., Botto M., Gordon S., Brown G.D. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 2007;8(1):31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S.M., Shen H., Zhang T., Huang X., Liu X.Q., Guo S.Y., Zhao J.J., Wang C.F., Yan L., Xu G.T., Jiang Y.Y., An M.M. Dectin-1 plays an important role in host defense against systemic Candida glabrata infection. Virulence. 2017:1–14. doi: 10.1080/21505594.2017.1346756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werner J.L., Metz A.E., Horn D., Schoeb T.R., Hewitt M.M., Schwiebert L.M., Faro-Trindade I., Brown G.D., Steele C. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 2009;182(8):4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viriyakosol S., Jimenez Mdel P., Gurney M.A., Ashbaugh M.E., Fierer J. Dectin-1 is required for resistance to coccidioidomycosis in mice. MBio. 2013;4(1):e00597–12. doi: 10.1128/mBio.00597-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iliev I.D., Funari V.A., Taylor K.D., Nguyen Q., Reyes C.N., Strom S.P., Brown J., Becker C.A., Fleshner P.R., Dubinsky M., Rotter J.I., Wang H.L., McGovern D.P., Brown G.D., Underhill D.M. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336(6086):1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang C., Kamiya T., Liu Y., Kadoki M., Kakuta S., Oshima K., Hattori M., Takeshita K., Kanai T., Saijo S., Ohno N., Iwakura Y. Inhibition of Dectin-1 signaling Ameliorates Colitis by inducing Lactobacillus-mediated regulatory T cell expansion in the intestine. Cell Host Microbe. 2015;18(2):183–197. doi: 10.1016/j.chom.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Lilly L.M., Gessner M.A., Dunaway C.W., Metz A.E., Schwiebert L., Weaver C.T., Brown G.D., Steele C. The beta-glucan receptor dectin-1 promotes lung immunopathology during fungal allergy via IL-22. J. Immunol. 2012;189(7):3653–3660. doi: 10.4049/jimmunol.1201797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferwerda B., Ferwerda G., Plantinga T.S., Willment J.A., van Spriel A.B., Venselaar H., Elbers C.C., Johnson M.D., Cambi A., Huysamen C., Jacobs L., Jansen T., Verheijen K., Masthoff L., Morre S.A., Vriend G., Williams D.L., Perfect J.R., Joosten L.A., Wijmenga C., van der Meer J.W., Adema G.J., Kullberg B.J., Brown G.D., Netea M.G. Human dectin-1 deficiency and mucocutaneous fungal infections. N. Engl. J. Med. 2009;361(18):1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sainz J., Lupianez C.B., Segura-Catena J., Vazquez L., Rios R., Oyonarte S., Hemminki K., Forsti A., Jurado M. Dectin-1 and DC-SIGN polymorphisms associated with invasive pulmonary Aspergillosis infection. PLoS One. 2012;7(2):e32273. doi: 10.1371/journal.pone.0032273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skonieczna K., Styczynski J., Krenska A., Stawinski P., Ploski R., Derwich K., Badowska W., Wysocki M., Grzybowski T. Massively parallel targeted resequencing reveals novel genetic variants associated with aspergillosis in paediatric patients with haematological malignancies. Pol. J. Pathol. 2017;68(3):210–217. doi: 10.5114/pjp.2017.71528. [DOI] [PubMed] [Google Scholar]
- 27.Plantinga T.S., van der Velden W.J., Ferwerda B., van Spriel A.B., Adema G., Feuth T., Donnelly J.P., Brown G.D., Kullberg B.J., Blijlevens N.M., Netea M.G. Early stop polymorphism in human DECTIN-1 is associated with increased candida colonization in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 2009;49(5):724–732. doi: 10.1086/604714. [DOI] [PubMed] [Google Scholar]
- 28.Bonder M.J., Kurilshikov A., Tigchelaar E.F., Mujagic Z., Imhann F., Vila A.V., Deelen P., Vatanen T., Schirmer M., Smeekens S.P., Zhernakova D.V., Jankipersadsing S.A., Jaeger M., Oosting M., Cenit M.C., Masclee A.A., Swertz M.A., Li Y., Kumar V., Joosten L., Harmsen H., Weersma R.K., Franke L., Hofker M.H., Xavier R.J., Jonkers D., Netea M.G., Wijmenga C., Fu J., Zhernakova A. The effect of host genetics on the gut microbiome. Nat. Genet. 2016;48(11):1407–1412. doi: 10.1038/ng.3663. [DOI] [PubMed] [Google Scholar]
- 29.Ishikawa T., Itoh F., Yoshida S., Saijo S., Matsuzawa T., Gonoi T., Saito T., Okawa Y., Shibata N., Miyamoto T., Yamasaki S. Identification of distinct ligands for the C-type lectin receptors Mincle and Dectin-2 in the pathogenic fungus Malassezia. Cell Host Microbe. 2013;13(4):477–488. doi: 10.1016/j.chom.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Ifrim D.C., Bain J.M., Reid D.M., Oosting M., Verschueren I., Gow N.A., van Krieken J.H., Brown G.D., Kullberg B.J., Joosten L.A., van der Meer J.W., Koentgen F., Erwig L.P., Quintin J., Netea M.G. Role of Dectin-2 for host defense against systemic infection with Candida glabrata. Infect. Immun. 2014;82(3):1064–1073. doi: 10.1128/IAI.01189-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wells C.A., Salvage-Jones J.A., Li X., Hitchens K., Butcher S., Murray R.Z., Beckhouse A.G., Lo Y.L., Manzanero S., Cobbold C., Schroder K., Ma B., Orr S., Stewart L., Lebus D., Sobieszczuk P., Hume D.A., Stow J., Blanchard H., Ashman R.B. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J. Immunol. 2008;180(11):7404–7413. doi: 10.4049/jimmunol.180.11.7404. [DOI] [PubMed] [Google Scholar]
- 32.Yamasaki S., Matsumoto M., Takeuchi O., Matsuzawa T., Ishikawa E., Sakuma M., Tateno H., Uno J., Hirabayashi J., Mikami Y., Takeda K., Akira S., Saito T. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc. Natl. Acad. Sci. U. S. A. 2009;106(6):1897–1902. doi: 10.1073/pnas.0805177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saijo S., Ikeda S., Yamabe K., Kakuta S., Ishigame H., Akitsu A., Fujikado N., Kusaka T., Kubo S., Chung S.H., Komatsu R., Miura N., Adachi Y., Ohno N., Shibuya K., Yamamoto N., Kawakami K., Yamasaki S., Saito T., Akira S., Iwakura Y. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32(5):681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Ifrim D.C., Quintin J., Courjol F., Verschueren I., van Krieken J.H., Koentgen F., Fradin C., Gow N.A., Joosten L.A., van der Meer J.W., van de Veerdonk F., Netea M.G. The role of Dectin-2 for host defense against disseminated Candidiasis. J. Interferon Cytokine Res. 2016;36(4):267–276. doi: 10.1089/jir.2015.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kottom T.J., Hebrink D.M., Jenson P.E., Nandakumar V., Wuthrich M., Wang H., Klein B., Yamasaki S., Lepenies B., Limper A.H. The interaction of pneumocystis with the C-type lectin receptor mincle exerts a significant role in host defense against infection. J. Immunol. 2017;198(9):3515–3525. doi: 10.4049/jimmunol.1600744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang T., Pan D., Zhou Z., You Y., Jiang C., Zhao X., Lin X. Dectin-3 deficiency promotes colitis development due to impaired antifungal innate immune responses in the gut. PLoS Pathog. 2016;12(6):e1005662. doi: 10.1371/journal.ppat.1005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrett N.A., Maekawa A., Rahman O.M., Austen K.F., Kanaoka Y. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J. Immunol. 2009;182(2):1119–1128. doi: 10.4049/jimmunol.182.2.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrett N.A., Rahman O.M., Fernandez J.M., Parsons M.W., Xing W., Austen K.F., Kanaoka Y. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J. Exp. Med. 2011;208(3):593–604. doi: 10.1084/jem.20100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu X.P., Wang R.Y., Wang X., Cao Y.H., Chen Y.Q., Zhao H.Z., Wu J.Q., Weng X.H., Gao X.H., Sun R.H., Zhu L.P. Dectin-2 polymorphism associated with pulmonary cryptococcosis in HIV-uninfected Chinese patients. Med. Mycol. 2015;53(8):810–816. doi: 10.1093/mmy/myv043. [DOI] [PubMed] [Google Scholar]
- 40.Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Front. Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Underhill D.M., Ozinsky A., Hajjar A.M., Stevens A., Wilson C.B., Bassetti M., Aderem A. The toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401(6755):811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 42.Jouault T., Ibata-Ombetta S., Takeuchi O., Trinel P.A., Sacchetti P., Lefebvre P., Akira S., Poulain D. Candida albicans phospholipomannan is sensed through toll-like receptors. J. Infect. Dis. 2003;188(1):165–172. doi: 10.1086/375784. [DOI] [PubMed] [Google Scholar]
- 43.Mambula S.S., Sau K., Henneke P., Golenbock D.T., Levitz S.M. Toll-like receptor (TLR) signaling in response to Aspergillus fumigatus. J. Biol. Chem. 2002;277(42):39320–39326. doi: 10.1074/jbc.M201683200. [DOI] [PubMed] [Google Scholar]
- 44.Shin S.H., Kim Y.H., Jin H.S., Kang S.H. Alternaria induces production of thymic stromal lymphopoietin in nasal fibroblasts through toll-like receptor 2. Allergy Asthma Immunol. Res. 2016;8(1):63–68. doi: 10.4168/aair.2016.8.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dreschers S., Saupp P., Hornef M., Prehn A., Platen C., Morschhauser J., Orlikowsky T.W. Reduced PICD in monocytes mounts altered neonate immune response to Candida albicans. PLoS One. 2016;11(11):e0166648. doi: 10.1371/journal.pone.0166648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dubourdeau M., Athman R., Balloy V., Huerre M., Chignard M., Philpott D.J., Latge J.P., Ibrahim-Granet O. Aspergillus fumigatus induces innate immune responses in alveolar macrophages through the MAPK pathway independently of TLR2 and TLR4. J. Immunol. 2006;177(6):3994–4001. doi: 10.4049/jimmunol.177.6.3994. [DOI] [PubMed] [Google Scholar]
- 47.Meier A., Kirschning C.J., Nikolaus T., Wagner H., Heesemann J., Ebel F. Toll-like receptor (TLR) 2 and TLR4 are essential for Aspergillus-induced activation of murine macrophages. Cell Microbiol. 2003;5(8):561–570. doi: 10.1046/j.1462-5822.2003.00301.x. [DOI] [PubMed] [Google Scholar]
- 48.Kesh S., Mensah N.Y., Peterlongo P., Jaffe D., Hsu K., Van Den Brink M., O’Reilly R., Pamer E., Satagopan J., Papanicolaou G.A. TLR1 and TLR6 polymorphisms are associated with susceptibility to invasive aspergillosis after allogeneic stem cell transplantation. Ann. N. Y. Acad. Sci. 2005;1062:95–103. doi: 10.1196/annals.1358.012. [DOI] [PubMed] [Google Scholar]
- 49.Leiva-Juarez M.M., Ware H.H., Kulkarni V.V., Zweidler-McKay P.A., Tuvim M.J., Evans S.E. Inducible epithelial resistance protects mice against leukemia-associated pneumonia. Blood. 2016;128(7):982–992. doi: 10.1182/blood-2016-03-708511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Netea M.G., Gow N.A., Munro C.A., Bates S., Collins C., Ferwerda G., Hobson R.P., Bertram G., Hughes H.B., Jansen T., Jacobs L., Buurman E.T., Gijzen K., Williams D.L., Torensma R., McKinnon A., MacCallum D.M., Odds F.C., Van der Meer J.W., Brown A.J., Kullberg B.J. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and toll-like receptors. J. Clin. Invest. 2006;116(6):1642–1650. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Netea M.G., Van Der Graaf C.A., Vonk A.G., Verschueren I., Van Der Meer J.W., Kullberg B.J. The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J. Infect. Dis. 2002;185(10):1483–1489. doi: 10.1086/340511. [DOI] [PubMed] [Google Scholar]
- 52.Netea M.G., Warris A., Van der Meer J.W., Fenton M.J., Verver-Janssen T.J., Jacobs L.E., Andresen T., Verweij P.E., Kullberg B.J. Aspergillus fumigatus evades immune recognition during germination through loss of toll-like receptor-4-mediated signal transduction. J. Infect. Dis. 2003;188(2):320–326. doi: 10.1086/376456. [DOI] [PubMed] [Google Scholar]
- 53.Bochud P.Y., Chien J.W., Marr K.A., Leisenring W.M., Upton A., Janer M., Rodrigues S.D., Li S., Hansen J.A., Zhao L.P., Aderem A., Boeckh M. Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N. Engl. J. Med. 2008;359(17):1766–1777. doi: 10.1056/NEJMoa0802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biondo C., Signorino G., Costa A., Midiri A., Gerace E., Galbo R., Bellantoni A., Malara A., Beninati C., Teti G., Mancuso G. Recognition of yeast nucleic acids triggers a host-protective type I interferon response. Eur. J. Immunol. 2011;41(7):1969–1979. doi: 10.1002/eji.201141490. [DOI] [PubMed] [Google Scholar]
- 55.Ramirez-Ortiz Z.G., Specht C.A., Wang J.P., Lee C.K., Bartholomeu D.C., Gazzinelli R.T., Levitz S.M. Toll-like receptor 9-dependent immune activation by unmethylated CpG motifs in Aspergillus fumigatus DNA. Infect. Immun. 2008;76(5):2123–2129. doi: 10.1128/IAI.00047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miyazato A., Nakamura K., Yamamoto N., Mora-Montes H.M., Tanaka M., Abe Y., Tanno D., Inden K., Gang X., Ishii K., Takeda K., Akira S., Saijo S., Iwakura Y., Adachi Y., Ohno N., Mitsutake K., Gow N.A., Kaku M., Kawakami K. Toll-like receptor 9-dependent activation of myeloid dendritic cells by deoxynucleic acids from Candida albicans. Infect. Immun. 2009;77(7):3056–3064. doi: 10.1128/IAI.00840-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van de Veerdonk F.L., Joosten L.A., Netea M.G. The interplay between inflammasome activation and antifungal host defense. Immunol. Rev. 2015;265(1):172–180. doi: 10.1111/imr.12280. [DOI] [PubMed] [Google Scholar]
- 58.Zhong Y., Kinio A., Saleh M. Functions of NOD-like receptors in human diseases. Front. Immunol. 2013;4:333. doi: 10.3389/fimmu.2013.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vonk A.G., Netea M.G., van Krieken J.H., Iwakura Y., van der Meer J.W., Kullberg B.J. Endogenous interleukin (IL)-1 alpha and IL-1 beta are crucial for host defense against disseminated candidiasis. J. Infect. Dis. 2006;193(10):1419–1426. doi: 10.1086/503363. [DOI] [PubMed] [Google Scholar]
- 60.Stuyt R.J., Netea M.G., Verschueren I., Fantuzzi G., Dinarello C.A., Van Der Meer J.W., Kullberg B.J. Role of interleukin-18 in host defense against disseminated Candida albicans infection. Infect. Immun. 2002;70(6):3284–3286. doi: 10.1128/IAI.70.6.3284-3286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joly S., Ma N., Sadler J.J., Soll D.R., Cassel S.L., Sutterwala F.S. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J. Immunol. 2009;183(6):3578–3581. doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wellington M., Koselny K., Sutterwala F.S., Krysan D.J. Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryot. Cell. 2014;13(2):329–340. doi: 10.1128/EC.00336-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karki R., Man S.M., Malireddi R.K., Gurung P., Vogel P., Lamkanfi M., Kanneganti T.D. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe. 2015;17(3):357–368. doi: 10.1016/j.chom.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gross O., Poeck H., Bscheider M., Dostert C., Hannesschlager N., Endres S., Hartmann G., Tardivel A., Schweighoffer E., Tybulewicz V., Mocsai A., Tschopp J., Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459(7245):433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 65.Goncalves A.C., Ferreira L.S., Manente F.A., de Faria C., Polesi M.C., de Andrade C.R., Zamboni D.S., Carlos I.Z. The NLRP3 inflammasome contributes to host protection during Sporothrix schenckii infection. Immunology. 2017;151(2):154–166. doi: 10.1111/imm.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lei G., Chen M., Li H., Niu J.L., Wu S., Mao L., Lu A., Wang H., Chen W., Xu B., Leng Q., Xu C., Yang G., An L., Zhu L.P., Meng G. Biofilm from a clinical strain of Cryptococcus neoformans activates the NLRP3 inflammasome. Cell Res. 2013;23(7):965–968. doi: 10.1038/cr.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feriotti C., de Araujo E.F., Loures F.V., da Costa T.A., Galdino N.A.L., Zamboni D.S., Calich V.L.G. NOD-like receptor P3 inflammasome controls protective Th1/Th17 immunity against pulmonary Paracoccidioidomycosis. Front. Immunol. 2017;8:786. doi: 10.3389/fimmu.2017.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lev-Sagie A., Prus D., Linhares I.M., Lavy Y., Ledger W.J., Witkin S.S. Polymorphism in a gene coding for the inflammasome component NALP3 and recurrent vulvovaginal candidiasis in women with vulvar vestibulitis syndrome. Am. J. Obstet. Gynecol. 2009;200(3) doi: 10.1016/j.ajog.2008.10.039. 303 e1-6. [DOI] [PubMed] [Google Scholar]
- 69.Tomalka J., Ganesan S., Azodi E., Patel K., Majmudar P., Hall B.A., Fitzgerald K.A., Hise A.G. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog. 2011;7(12):e1002379. doi: 10.1371/journal.ppat.1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y., Wu J., Xin Z., Wu X. Aspergillus fumigatus triggers innate immune response via NOD1 signaling in human corneal epithelial cells. Exp. Eye Res. 2014;127:170–178. doi: 10.1016/j.exer.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 71.Li Z.Z., Tao L.L., Zhang J., Zhang H.J., Qu J.M. Role of NOD2 in regulating the immune response to Aspergillus fumigatus. Inflamm. Res. 2012;61(6):643–648. doi: 10.1007/s00011-012-0456-4. [DOI] [PubMed] [Google Scholar]
- 72.Gresnigt M.S., Jaeger M., Subbarao Malireddi R.K., Rasid O., Jouvion G., Fitting C., Melchers W.J.G., Kanneganti T.D., Carvalho A., Ibrahim-Granet O., van de Veerdonk F.L. The absence of NOD1 enhances killing of Aspergillus fumigatus through modulation of Dectin-1 expression. Front. Immunol. 2017;8:1777. doi: 10.3389/fimmu.2017.01777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van der Graaf C.A., Netea M.G., Franke B., Girardin S.E., van der Meer J.W., Kullberg B.J. Nucleotide oligomerization domain 2 (Nod2) is not involved in the pattern recognition of Candida albicans. Clin. Vaccine Immunol. : CVI. 2006;13(3):423–425. doi: 10.1128/CVI.13.3.423-425.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patin E.C., Jones A.V., Thompson A., Clement M., Liao C.T., Griffiths J.S., Wallace L.E., Bryant C.E., Lang R., Rosenstiel P., Humphreys I.R., Taylor P.R., Jones G.W., Orr S.J. IL-27 induced by select Candida spp. via TLR7/NOD2 signaling and IFN-beta production inhibits fungal clearance. J. Immunol. 2016;197(1):208–221. doi: 10.4049/jimmunol.1501204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wagener J., Malireddi R.K., Lenardon M.D., Koberle M., Vautier S., MacCallum D.M., Biedermann T., Schaller M., Netea M.G., Kanneganti T.D., Brown G.D., Brown A.J., Gow N.A. Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS Pathog. 2014;10(4):e1004050. doi: 10.1371/journal.ppat.1004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y., Olagnier D., Lin R. Host and viral modulation of RIG-I-mediated antiviral immunity. Front. Immunol. 2016;7:662. doi: 10.3389/fimmu.2016.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jaeger M., van der Lee R., Cheng S.C., Johnson M.D., Kumar V., Ng A., Plantinga T.S., Smeekens S.P., Oosting M., Wang X., Barchet W., Fitzgerald K., Joosten L.A.B., Perfect J.R., Wijmenga C., van de Veerdonk F.L., Huynen M.A., Xavier R.J., Kullberg B.J., Netea M.G. The RIG-I-like helicase receptor MDA5 (IFIH1) is involved in the host defense against Candida infections. Eur. J. Clin. Microbiol. Infect. Dis. 2015;34(5):963–974. doi: 10.1007/s10096-014-2309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sousa Mda G., Reid D.M., Schweighoffer E., Tybulewicz V., Ruland J., Langhorne J., Yamasaki S., Taylor P.R., Almeida S.R., Brown G.D. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe. 2011;9(5):436–443. doi: 10.1016/j.chom.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.da Gloria Sousa M., Reid D.M., Schweighoffer E., Tybulewicz V., Ruland J., Langhorne J., Yamasaki S., Taylor P.R., Almeida S.R., Brown G.D. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe. 2011;9(5):436–443. doi: 10.1016/j.chom.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Sousa Mda G., Belda W., Jr., Spina R., Lota P.R., Valente N.S., Brown G.D., Criado P.R., Benard G. Topical application of imiquimod as a treatment for chromoblastomycosis. Clin. Infect. Dis. 2014;58(12):1734–1737. doi: 10.1093/cid/ciu168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Siqueira I.M., de Castro R.J.A., Leonhardt L.C.M., Jeronimo M.S., Soares A.C., Raiol T., Nishibe C., Almeida N., Tavares A.H., Hoffmann C., Bocca A.L. Modulation of the immune response by Fonsecaea pedrosoi morphotypes in the course of experimental chromoblastomycosis and their role on inflammatory response chronicity. PLoS Negl. Trop. Dis. 2017;11(3):e0005461. doi: 10.1371/journal.pntd.0005461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wuthrich M., Wang H., Li M., Lerksuthirat T., Hardison S.E., Brown G.D., Klein B. Fonsecaea pedrosoi-induced Th17-cell differentiation in mice is fostered by Dectin-2 and suppressed by mincle recognition. Eur. J. Immunol. 2015;45(9):2542–2552. doi: 10.1002/eji.201545591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang J.H., Lin C.Y., Wu S.Y., Chen W.Y., Chu C.L., Brown G.D., Chuu C.P., Wu-Hsieh B.A. CR3 and Dectin-1 collaborate in macrophage cytokine response through association on lipid rafts and activation of Syk-JNK-AP-1 pathway. PLoS Pathog. 2015;11(7):e1004985. doi: 10.1371/journal.ppat.1004985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Esteban A., Popp M.W., Vyas V.K., Strijbis K., Ploegh H.L., Fink G.R. Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. Proc. Natl. Acad. Sci. U. S. A. 2011;108(34):14270–14275. doi: 10.1073/pnas.1111415108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meyer-Wentrup F., Figdor C.G., Ansems M., Brossart P., Wright M.D., Adema G.J., van Spriel A.B. Dectin-1 interaction with tetraspanin CD37 inhibits IL-6 production. J Immunol. 2007;178(1):154–162. doi: 10.4049/jimmunol.178.1.154. [DOI] [PubMed] [Google Scholar]
- 86.van Spriel A.B., Sofi M., Gartlan K.H., van der Schaaf A., Verschueren I., Torensma R., Raymakers R.A., Loveland B.E., Netea M.G., Adema G.J., Wright M.D., Figdor C.G. The tetraspanin protein CD37 regulates IgA responses and anti-fungal immunity. PLoS Pathog. 2009;5(3):e1000338. doi: 10.1371/journal.ppat.1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khan N.S., Kasperkovitz P.V., Timmons A.K., Mansour M.K., Tam J.M., Seward M.W., Reedy J.L., Puranam S., Feliu M., Vyas J.M. Dectin-1 controls TLR9 trafficking to phagosomes containing beta-1,3 glucan. J. Immunol. 2016;196(5):2249–2261. doi: 10.4049/jimmunol.1401545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu J., Zhang Y., Xin Z., Wu X. The crosstalk between TLR2 and NOD2 in Aspergillus fumigatus keratitis. Mol. Immunol. 2015;64(2):235–243. doi: 10.1016/j.molimm.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 89.Kistowska M., Fenini G., Jankovic D., Feldmeyer L., Kerl K., Bosshard P., Contassot E., French L.E. Malassezia yeasts activate the NLRP3 inflammasome in antigen-presenting cells via Syk-kinase signalling. Exp. Dermatol. 2014;23(12):884–889. doi: 10.1111/exd.12552. [DOI] [PubMed] [Google Scholar]
- 90.Cheng S.C., van de Veerdonk F.L., Lenardon M., Stoffels M., Plantinga T., Smeekens S., Rizzetto L., Mukaremera L., Preechasuth K., Cavalieri D., Kanneganti T.D., van der Meer J.W., Kullberg B.J., Joosten L.A., Gow N.A., Netea M.G. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J. Leukoc. Biol. 2011;90(2):357–366. doi: 10.1189/jlb.1210702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van de Veerdonk F.L., Joosten L.A., Shaw P.J., Smeekens S.P., Malireddi R.K., van der Meer J.W., Kullberg B.J., Netea M.G., Kanneganti T.D. The inflammasome drives protective Th1 and Th17 cellular responses in disseminated candidiasis. Eur. J. Immunol. 2011;41(8):2260–2268. doi: 10.1002/eji.201041226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chang T.H., Huang J.H., Lin H.C., Chen W.Y., Lee Y.H., Hsu L.C., Netea M.G., Ting J.P., Wu-Hsieh B.A. Dectin-2 is a primary receptor for NLRP3 inflammasome activation in dendritic cell response to Histoplasma capsulatum. PLoS Pathog. 2017;13(7):e1006485. doi: 10.1371/journal.ppat.1006485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goodridge H.S., Reyes C.N., Becker C.A., Katsumoto T.R., Ma J., Wolf A.J., Bose N., Chan A.S., Magee A.S., Danielson M.E., Weiss A., Vasilakos J.P., Underhill D.M. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature. 2011;472(7344):471–475. doi: 10.1038/nature10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brown G.D., Herre J., Williams D.L., Willment J.A., Marshall A.S., Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J. Exp. Med. 2003;197(9):1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosas M., Liddiard K., Kimberg M., Faro-Trindade I., McDonald J.U., Williams D.L., Brown G.D., Taylor P.R. The induction of inflammation by dectin-1 in vivo is dependent on myeloid cell programming and the progression of phagocytosis. J. Immunol. 2008;181(5):3549–3557. doi: 10.4049/jimmunol.181.5.3549. [DOI] [PubMed] [Google Scholar]
- 96.Hernanz-Falcon P., Joffre O., Williams D.L., Reis e Sousa C. Internalization of Dectin-1 terminates induction of inflammatory responses. Eur. J. Immunol. 2009;39(2):507–513. doi: 10.1002/eji.200838687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu L.L., Luo T.M., Xu X., Guo Y.H., Zhao X.Q., Wang T.T., Tang B., Jiang Y.Y., Xu J.F., Lin X., Jia X.M. E3 ubiquitin ligase Cbl-b negatively regulates C-type lectin receptor-mediated antifungal innate immunity. J. Exp. Med. 2016;213(8):1555–1570. doi: 10.1084/jem.20151932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wirnsberger G., Zwolanek F., Asaoka T., Kozieradzki I., Tortola L., Wimmer R.A., Kavirayani A., Fresser F., Baier G., Langdon W.Y., Ikeda F., Kuchler K., Penninger J.M. Inhibition of CBLB protects from lethal Candida albicans sepsis. Nat. Med. 2016;22(8):915–923. doi: 10.1038/nm.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao Y., Tang J., Guo H., Zhao Y., Tang R., Ouyang S., Zeng Q., Rappleye C.A., Rajaram M.V., Schlesinger L.S., Tao L., Brown G.D., Langdon W.Y., Li B.T., Zhang J. Targeting CBLB as a potential therapeutic approach for disseminated candidiasis. Nat. Med. 2016;22(8):906–914. doi: 10.1038/nm.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bachmaier K., Toya S., Gao X., Triantafillou T., Garrean S., Park G.Y., Frey R.S., Vogel S., Minshall R., Christman J.W., Tiruppathi C., Malik A.B. E3 ubiquitin ligase Cblb regulates the acute inflammatory response underlying lung injury. Nat. Med. 2007;13(8):920–926. doi: 10.1038/nm1607. [DOI] [PubMed] [Google Scholar]
- 101.Han C., Jin J., Xu S., Liu H., Li N., Cao X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat. Immunol. 2010;11(8):734–742. doi: 10.1038/ni.1908. [DOI] [PubMed] [Google Scholar]
- 102.Liu Q., Guo H., Xiao Y., Zhang J. E3 ubiquitin ligase Cbl-b inhibits NLRP3 inflammasome activation by targeting NLRP3 for ubiquitination. J. Immunol. 2014;192(1 Supplement 122.2) [Google Scholar]
- 103.Orr S.J., Burg A.R., Chan T., Quigley L., Jones G.W., Ford J.W., Hodge D., Razzook C., Sarhan J., Jones Y.L., Whittaker G.C., Boelte K.C., Lyakh L., Cardone M., O’Connor G.M., Tan C., Li H., Anderson S.K., Jones S.A., Zhang W., Taylor P.R., Trinchieri G., McVicar D.W. LAB/NTAL facilitates fungal/PAMP-induced IL-12 and IFN-gamma production by repressing beta-catenin activation in dendritic cells. PLoS Pathog. 2013;9(5):e1003357. doi: 10.1371/journal.ppat.1003357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao X., Guo Y., Jiang C., Chang Q., Zhang S., Luo T., Zhang B., Jia X., Hung M.C., Dong C., Lin X. JNK1 negatively controls antifungal innate immunity by suppressing CD23 expression. Nat. Med. 2017;23(3):337–346. doi: 10.1038/nm.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Knutsen A.P., Hutchinson P.S., Albers G.M., Consolino J., Smick J., Kurup V.P. Increased sensitivity to IL-4 in cystic fibrosis patients with allergic bronchopulmonary aspergillosis. Allergy. 2004;59(1):81–87. doi: 10.1046/j.1398-9995.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 106.Pasquinelli A.E. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat. Rev. Genet. 2012;13(4):271–282. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 107.Du L., Chen X., Duan Z., Liu C., Zeng R., Chen Q., Li M. MiR-146a negatively regulates dectin-1-induced inflammatory responses. Oncotarget. 2017;8(23):37355–37366. doi: 10.18632/oncotarget.16958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arghavan B., Sharifi M., Shafiee M., Mohammadi R. Evaluation of miR-146a expression level in macrophages exposed to Candida glabrata. Curr. Med. Mycol. 2016;2(2):16–19. doi: 10.18869/acadpub.cmm.2.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Monk C.E., Hutvagner G., Arthur J.S. Regulation of miRNA transcription in macrophages in response to Candida albicans. PLoS One. 2010;5(10):e13669. doi: 10.1371/journal.pone.0013669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bazzoni F., Rossato M., Fabbri M., Gaudiosi D., Mirolo M., Mori L., Tamassia N., Mantovani A., Cassatella M.A., Locati M. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc. Natl. Acad. Sci. U. S. A. 2009;106(13):5282–5287. doi: 10.1073/pnas.0810909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu G., Friggeri A., Yang Y., Park Y.J., Tsuruta Y., Abraham E. miR-147, a microRNA that is induced upon toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc. Natl. Acad. Sci. U. S. A. 2009;106(37):15819–15824. doi: 10.1073/pnas.0901216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.O’Neill L.A., Sheedy F.J., McCoy C.E. MicroRNAs: the fine-tuners of toll-like receptor signalling. Nat. Rev. Immunol. 2011;11(3):163–175. doi: 10.1038/nri2957. [DOI] [PubMed] [Google Scholar]
- 113.Kang H., Park Y., Lee A., Seo H., Kim M.J., Choi J., Jo H.N., Jeong H.N., Cho J.G., Chang W., Lee M.S., Jeon R., Kim J. Negative regulation of NOD1 mediated angiogenesis by PPARgamma-regulated miR-125a. Biochem. Biophys. Res. Commun. 2017;482(1):28–34. doi: 10.1016/j.bbrc.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 114.Haneklaus M., Gerlic M., Kurowska-Stolarska M., Rainey A.A., Pich D., McInnes I.B., Hammerschmidt W., O’Neill L.A., Masters S.L. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1beta production. J. Immunol. 2012;189(8):3795–3799. doi: 10.4049/jimmunol.1200312. [DOI] [PubMed] [Google Scholar]
- 115.Wang Y., Han Z., Fan Y., Zhang J., Chen K., Gao L., Zeng H., Cao J., Wang C. MicroRNA-9 inhibits NLRP3 inflammasome activation in human atherosclerosis inflammation cell models through the JAK1/STAT signaling pathway. Cell Physiol. Biochem. 2017;41(4):1555–1571. doi: 10.1159/000470822. [DOI] [PubMed] [Google Scholar]
- 116.Zhu C.C., Zhao G.Q., Lin J., Hu L.T., Xu Q., Peng X.D., Wang X., Qiu S. Dectin-1 agonist curdlan modulates innate immunity to Aspergillus fumigatus in human corneal epithelial cells. Int. J. Ophthalmol. 2015;8(4):690–696. doi: 10.3980/j.issn.2222-3959.2015.04.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bonfim-Mendonca Pde S., Ratti B.A., Godoy Jda S., Negri M., Lima N.C., Fiorini A., Hatanaka E., Consolaro M.E., de Oliveira Silva S., Svidzinski T.I. Beta-glucan induces reactive oxygen species production in human neutrophils to improve the killing of Candida albicans and Candida glabrata isolates from vulvovaginal candidiasis. PLoS One. 2014;9(9):e107805. doi: 10.1371/journal.pone.0107805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Meira D.A., Pereira P.C., Marcondes-Machado J., Mendes R.P., Barraviera B., Pellegrino Junior J., Rezkallah-Iwasso M.T., Peracoli M.T., Castilho L.M., Thomazini I., Da Silva C.L., Foss N.T., Curi P.R. The use of glucan as immunostimulant in the treatment of paracoccidioidomycosis. Am. J. Trop. Med. Hyg. 1996;55(5):496–503. doi: 10.4269/ajtmh.1996.55.496. [DOI] [PubMed] [Google Scholar]
- 119.Torosantucci A., Bromuro C., Chiani P., De Bernardis F., Berti F., Galli C., Norelli F., Bellucci C., Polonelli L., Costantino P., Rappuoli R., Cassone A. A novel glyco-conjugate vaccine against fungal pathogens. J. Exp. Med. 2005;202(5):597–606. doi: 10.1084/jem.20050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pietrella D., Rachini A., Torosantucci A., Chiani P., Brown A.J., Bistoni F., Costantino P., Mosci P., d’Enfert C., Rappuoli R., Cassone A., Vecchiarelli A. A beta-glucan-conjugate vaccine and anti-beta-glucan antibodies are effective against murine vaginal candidiasis as assessed by a novel in vivo imaging technique. Vaccine. 2010;28(7):1717–1725. doi: 10.1016/j.vaccine.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 121.Lipinski T., Fitieh A., St Pierre J., Ostergaard H.L., Bundle D.R., Touret N. Enhanced immunogenicity of a tricomponent mannan tetanus toxoid conjugate vaccine targeted to dendritic cells via Dectin-1 by incorporating beta-glucan. J. Immunol. 2013;190(8):4116–4128. doi: 10.4049/jimmunol.1202937. [DOI] [PubMed] [Google Scholar]
- 122.Jawhara S., Habib K., Maggiotto F., Pignede G., Vandekerckove P., Maes E., Dubuquoy L., Fontaine T., Guerardel Y., Poulain D. Modulation of intestinal inflammation by yeasts and cell wall extracts: strain dependence and unexpected anti-inflammatory role of glucan fractions. PLoS One. 2012;7(7):e40648. doi: 10.1371/journal.pone.0040648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clemons K.V., Danielson M.E., Michel K.S., Liu M., Ottoson N.C., Leonardo S.M., Martinez M., Chen V., Antonysamy M.A., Stevens D.A. Whole glucan particles as a vaccine against murine aspergillosis. J. Med. Microbiol. 2014;63(Pt 12):1750–1759. doi: 10.1099/jmm.0.079681-0. [DOI] [PubMed] [Google Scholar]
- 124.Clemons K.V., Antonysamy M.A., Danielson M.E., Michel K.S., Martinez M., Chen V., Stevens D.A. Whole glucan particles as a vaccine against systemic coccidioidomycosis. J. Med. Microbiol. 2015;64(10):1237–1243. doi: 10.1099/jmm.0.000138. [DOI] [PubMed] [Google Scholar]
- 125.Carter R.W., Thompson C., Reid D.M., Wong S.Y., Tough D.F. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J. Immunol. 2006;177(4):2276–2284. doi: 10.4049/jimmunol.177.4.2276. [DOI] [PubMed] [Google Scholar]
- 126.Duluc D., Joo H., Ni L., Yin W., Upchurch K., Li D., Xue Y., Klucar P., Zurawski S., Zurawski G., Oh S. Induction and activation of human Th17 by targeting antigens to dendritic cells via dectin-1. J. Immunol. 2014;192(12):5776–5788. doi: 10.4049/jimmunol.1301661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ni L., Gayet I., Zurawski S., Duluc D., Flamar A.L., Li X.H., O’Bar A., Clayton S., Palucka A.K., Zurawski G., Banchereau J., Oh S. Concomitant activation and antigen uptake via human dectin-1 results in potent antigen-specific CD8+ T cell responses. J. Immunol. 2010;185(6):3504–3513. doi: 10.4049/jimmunol.1000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kumaresan P.R., Manuri P.R., Albert N.D., Maiti S., Singh H., Mi T., Roszik J., Rabinovich B., Olivares S., Krishnamurthy J., Zhang L., Najjar A.M., Huls M.H., Lee D.A., Champlin R.E., Kontoyiannis D.P., Cooper L.J. Bioengineering T cells to target carbohydrate to treat opportunistic fungal infection. Proc. Natl. Acad. Sci. U. S. A. 2014;111(29):10660–10665. doi: 10.1073/pnas.1312789111. [DOI] [PMC free article] [PubMed] [Google Scholar]