Abstract
Background
Lung volume reduction surgery (LVRS) performed to treat patients with severe diffuse emphysema was reintroduced in the nineties. Lung volume reduction surgery aims to resect damaged emphysematous lung tissue, thereby increasing elastic properties of the lung. This treatment is hypothesised to improve long‐term daily functioning and quality of life, although it may be costly and may be associated with risks of morbidity and mortality. Ten years have passed since the last version of this review was prepared, prompting us to perform an update.
Objectives
The objective of this review was to gather all available evidence from randomised controlled trials comparing the effectiveness of lung volume reduction surgery (LVRS) versus non‐surgical standard therapy in improving health outcomes for patients with severe diffuse emphysema. Secondary objectives included determining which subgroup of patients benefit from LVRS and for which patients LVRS is contraindicated, to establish the postoperative complications of LVRS and its morbidity and mortality, to determine which surgical approaches for LVRS are most effective and to calculate the cost‐effectiveness of LVRS.
Search methods
We identified RCTs by using the Cochrane Airways Group Chronic Obstructive Pulmonary Disease (COPD) register, in addition to the online clinical trials registers. Searches are current to April 2016.
Selection criteria
We included RCTs that studied the safety and efficacy of LVRS in participants with diffuse emphysema. We excluded studies that investigated giant or bullous emphysema.
Data collection and analysis
Two independent review authors assessed trials for inclusion and extracted data. When possible, we combined data from more than one study in a meta‐analysis using RevMan 5 software.
Main results
We identified two new studies (89 participants) in this updated review. A total of 11 studies (1760 participants) met the entry criteria of the review, one of which accounted for 68% of recruited participants. The quality of evidence ranged from low to moderate owing to an unclear risk of bias across many studies, lack of blinding and low participant numbers for some outcomes. Eight of the studies compared LVRS versus standard medical care, one compared two closure techniques (stapling vs laser ablation), one looked at the effect of buttressing the staple line on the effectiveness of LVRS and one compared traditional 'resectional' LVRS with a non‐resectional surgical approach. Participants completed a mandatory course of pulmonary rehabilitation/physical training before the procedure commenced. Short‐term mortality was higher for LVRS (odds ratio (OR) 6.16, 95% confidence interval (CI) 3.22 to 11.79; 1489 participants; five studies; moderate‐quality evidence) than for control, but long‐term mortality favoured LVRS (OR 0.76, 95% CI 0.61 to 0.95; 1280 participants; two studies; moderate‐quality evidence). Participants identified post hoc as being at high risk of death from surgery were those with particularly impaired lung function, poor diffusing capacity and/or homogenous emphysema. Participants with upper lobe‐predominant emphysema and low baseline exercise capacity showed the most favourable outcomes related to mortality, as investigators reported no significant differences in early mortality between participants treated with LVRS and those in the control group (OR 0.87, 95% CI 0.23 to 3.29; 290 participants; one study), as well as significantly lower mortality at the end of follow‐up for LVRS compared with control (OR 0.45, 95% CI 0.26 to 0.78; 290 participants; one study). Trials in this review furthermore provided evidence of low to moderate quality showing that improvements in lung function parameters other than forced expiratory volume in one second (FEV1), quality of life and exercise capacity were more likely with LVRS than with usual follow‐up. Adverse events were more common with LVRS than with control, specifically the occurrence of (persistent) air leaks, pulmonary morbidity (e.g. pneumonia) and cardiovascular morbidity. Although LVRS leads to an increase in quality‐adjusted life‐years (QALYs), the procedure is relatively costly overall.
Authors' conclusions
Lung volume reduction surgery, an effective treatment for selected patients with severe emphysema, may lead to better health status and lung function outcomes, specifically for patients who have upper lobe‐predominant emphysema with low exercise capacity, but the procedure is associated with risks of early mortality and adverse events.
Plain language summary
Lung volume reduction surgery for adults with diffuse emphysema
Review question
Does lung volume reduction surgery improve lung function and quality of life, without leading to an increased chance of death, higher rates of illness after the procedure and higher costs for patients with severe emphysema, and which surgical methods lead to the best results in these patients?
Background
Emphysema causes severe damage to the lungs, which leads to breathing problems. Lung volume reduction surgery (LVRS) may help improve symptoms by removing the most diseased and non‐functioning parts of the lung. However, this procedure has been the centre of much controversy with its possible benefit being outweighed by potential harms and costs.
Study characteristics
This review examined the research published up to the 14th of April, 2016, and identified 11 studies involving 1760 participants. Eight of the studies compared LVRS versus standard medical care, one compared two closure techniques (stapling vs laser ablation), one looked at the effect of buttressing the staple line on the effectiveness of LVRS and one compared a traditional approach to LVRS with a 'non‐resectional' surgical approach. All participants completed a mandatory course of pulmonary rehabilitation/physical training before the procedure commenced.
Key results
This review found that people undergoing LVRS were at increased risk of death at three months after the procedure. By the end of follow‐up, death rates were lower for participants treated with LVRS than for those given standard medical care. Participants who were characterised by poor lung function with a particular distribution of diseased tissue in their lungs were at higher risk of death at three months and throughout one large study. One study identified a group of participants who responded better to LVRS than other participants, making them especially suitable for this treatment. The benefit of surgery for surviving participants was significant in terms of quality of life, exercise capacity and lung function, but costs of the procedure are relatively high, and patients had a greater chance of adverse events after the procedure.
Quality of the evidence
The quality of the data reported is low to moderate in nature owing to some methodological issues of the trials (lack of blinding, unclear risk of bias). The results presented in this review are largely dominated by one influential study, which accounted for 68% of the participants.
Summary of findings
for the main comparison.
| Lung volume reduction surgery for diffuse emphysema | ||||||
| Patient or population: patients with diffuse emphysema Setting: hospitals Intervention: lung volume reduction surgery Comparison: standard medical care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with surgery | |||||
| Early mortality (90 days) | 13 per 1000 | 77 per 1000 (42 to 138) | OR 6.16 (3.22 to 11.79) | 1489 (5 RCTs) | ⊕⊕⊕⊝ MODERATEa | |
| Long‐term mortality (> 36 months) | 547 per 1000 | 478 per 1000 (424 to 534) | OR 0.76 (0.61 to 0.95) | 1280 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa | Substantial differences in follow‐up between the 2 trials measuring this construct |
| Change in total scores SGRQ (end of follow‐up) | End of treatment control group mean SGRQ scores ranged from 57 units to 62.1 units | Mean SGRQ score in the LVRS group was ‐13.78 units lower (‐15.75 to ‐11.78) | ‐ | 1326 (2 RCTs) | ⊕⊕⊕⊝ MODERATEb | Lower score indicates better quality of life. A difference of 4 units or more is thought to be clinically important. |
| Walking distance (end of follow‐up) | Control group walking distance ranged from 303 to 350 metres (in the 4 studies reporting 6MWD) | Standardised mean walking distance in the LVRS group was 0.70 standard deviations higher (0.42 to 0.98) | ‐ | 215 (5 RCTs) | ⊕⊕⊝⊝ LOWc,d | Four studies reported 6MWD test and 1 shuttle walking test. 0.7 standard deviations equates to approximately 70 metres for 6MWD. |
| FEV1 (end of follow‐up) | Control group FEV1 ranged from 0.64 L to 0.7 L FEV1 | Mean FEV1 in the LVRS group was 0.2 L higher (0.13 to 0.28) | ‐ | 188 (4 RCTs) | ⊕⊕⊝⊝ LOWc,e | |
| RV (end of follow‐up) | Control group predicted RV ranged from 213% to 258% predicted | Mean predicted RV in the LVRS group was 44.28% less (‐57.80 to ‐30.75) | ‐ | 177 (4 RCTs) | ⊕⊕⊝⊝ LOWa,c | |
| TLC (end of follow‐up) | Control group predicted RV ranged from 127% to 149% predicted | Mean predicted TLC in the LVRS group was ‐14.83% less (‐20.50 to ‐9.15) | ‐ | 178 (4 RCTs) | ⊕⊕⊝⊝ LOWa,c | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 6MWD: six‐minute walking distance; CI: confidence interval; FEV1: forced expired volume in one second; L: litre; OR: odds ratio; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; RV: residual volume; SGRQ: St George's Respiratory Questionnaire; SMD: standardised mean difference; TLC: total lung capacity. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded owing to overall high rates of high and unclear risk of bias in all trials.
bDowngraded owing to risk of performance and detection bias: Studies were not blinded and SGRQ is dependent on patients' subjective responses.
cDowngraded owing to imprecision: low participant number.
dDowngraded owing to risk of performance and detection bias: Studies were not blinded and 6MWD is effort dependent.
eDowngraded owing to risk of performance and detection bias: Studies were not blinded and FEV1 is effort dependent.
Background
Chronic obstructive pulmonary disease (COPD), one of the leading causes of mortality in the world (Lozano 2012), is a heterogeneous group of diseases that show similar symptoms and include contrasting and overlapping underlying disease processes (Stockley 2009). Most patients with COPD are given a diagnosis of chronic bronchitis, chronic inflammation of the central airways, emphysema or impaired and damaged lung parenchyma epithelium; most commonly, they show symptoms relating to both chronic bronchitis and emphysema (Kim 2008; Tuder 2003). Chronic obstructive pulmonary disease is a chronic progressive disease that is largely preventable and is characterised by hyperinflation and decreased elasticity of the airways resulting from structural degradation and inflammation of lung tissue; in patients with COPD, efficient gas exchange between the alveoli and the blood is impaired (Bourdin 2009; Sharafkhaneh 2008).
Patients with severe emphysema have limited treatment options as a result of extensive damage to the airways (Berger 2010; Russi 1997). One available treatment is lung volume reduction surgery (LVRS), in which unhealthy damaged parts of the lung are resected, leading to improved mechanical efficiency of healthy parts of the lung, and subsequently more efficient gas exchange. However, LVRS is a complicated procedure with significant associated risks. This review set out to determine the effectiveness of LVRS, to define the mortality and morbidity related to LVRS and to identify optimal surgical techniques.
This review is an update of previous Cochrane reviews (Hensley 1999; Tiong 2006), which identified several studies, including the very large NETT 2003 trial. A considerable amount of time has passed since the last version of the review, prompting us to revisit literature published since that time. The current review focuses only on surgical lung volume reduction; lung volume reduction through endoscopic/bronchoscopic procedures will be addressed in a separate Cochrane review (van Agteren 2016).
Description of the condition
Emphysema, one of the main conditions of COPD, is characterised by destruction of the extracellular matrix in the walls of the smaller airways and the lung parenchyma (Sharafkhaneh 2008). Emphysema can be defined by disease distribution, as well as location. An emphysematous lung can show a homogenous or heterogeneous (regional) pattern of pathological lesions, which can impact lung parameters characteristic of emphysema differently (e.g. dynamic lung volume) (Boutou 2015; Mair 2009). Weder 1997 developed a more specific classification of emphysema that divides patients into three classes: markedly heterogeneous, intermediately heterogeneous and homogenous. Furthermore, emphysema can be divided into subtypes based on the unit of lung anatomy in which the emphysema is predominantly present (Hogg 2002).
Centrilobular emphysema: most closely associated with smoking and results from dilation and destruction of respiratory bronchioles. Lesions associated with centrilobular emphysema are located predominantly in the upper lung.
Panlobular emphysema: found mainly in the lower lobes and often associated with a genetic (alpha1‐anti‐trypsin) deficiency.
Paraseptal emphysema: occurs in the periphery of the lobules, specifically in the subpleural region.
Emphysema, which develops as the result of an interplay of various processes, is fuelled predominantly by exposure to cigarette smoke or other noxious particles (e.g. air pollutants) (Stockley 2009). Constant exposure to noxious particles leads to oxidative stress, a proteinase‐antiproteinase imbalance, increased apoptosis and chronic inflammation, all leading to gradual destruction of the lung tissue (Bagdonas 2015; Demedts 2006; Kirkham 2013; Suki 2003; Taraseviciene‐Stewart 2008).
The consistent destruction of healthy lung tissue results in the classic physiological characteristics of severe emphysema: hyperinflation of lungs, loss of elastic recoil, loss of surface area for gas exchange and flow limitation (Ferguson 2006; Ingenito 2005; Papandrinopoulou 2012). Emphysema causes a decrease in elastic recoil pressure and an increase in lung compliance. This in turn causes static and dynamic hyperinflation of the lungs, which limits airflow and results in clinical outcomes of lower functional capacity, higher levels of dyspnoea and limited exercise performance. Respiratory symptoms can worsen drastically, leading to physiological deterioration. These respiratory exacerbations can be triggered by a variety of factors and become more frequent in patients with severe emphysema (Wedzicha 2003). Patients with emphysema often have to deal with a significant number of concurrent diseases, including (lung) cancer, cardiovascular disease, anxiety, depression, hypertension and chronic infection (Sin 2006; Smith 2014), which further significantly affect patient quality of life (QoL) and disease manifestations.
Severe emphysema is diffuse by nature, meaning that emphysematous lesions can be found throughout the lung and are not localised. The focus of this review will be confined to diffuse emphysema. Giant bullous emphysema, which is a separate entity pathologically and radiologically (Mura 2005), is treated by a different surgical procedure known as bullectomy; therefore we will exclude this condition from the current review.
Description of the intervention
Lung volume reduction surgery was resurrected by Joel Cooper and his colleagues at the Washington University School of Medicine in the 1990s as treatment for patients with advanced COPD in which emphysema is the predominant feature (Cooper 1995). Three main surgical access techniques may be used for LVRS: median sternotomy, the technique used by Cooper; the less invasive video‐assisted thoracoscopic surgery (VATS); and thoracotomy (Russi 1997). A detailed description of these surgical procedures can be found in Fessler 2003.
Median sternotomy allows access to the pleural space by creating a vertical inline incision across the sternum. The incision is made just below the sternal notch and extends to the tip of the xyphoid process; then a sternal saw is used to split the sternum. The surgeon will usually proceed to operate on the worst affected lung, as determined by preoperative imaging, through resection of unhealthy lung tissue and use of unilateral or bilateral stapling to close the open lung tissue. Cooper suggested that staple lines should be secured with bovine reinforcement strips to prevent air leakage ‐ one of the most frequent complications in pulmonary resection. Furthermore, a pleural tent can be used (Venuta 1998) to ensure that no air leakage occurs.
Video‐assisted thoracoscopic surgery, a less invasive form of surgery than median sternotomy (MS), was initially used for simple diagnostic and therapeutic procedures (Brodsky 2000). Video‐assisted thoracoscopic surgery allows the surgeon to gain access to all parts of the lung via placement of trocars and completion of a procedure that requires only small incisions to be made. Trocars are generally placed between the seventh and eighth intercostal spaces and between the fourth and fifth intercostal spaces to allow access to the camera and the surgical instruments, respectively (Harris 1995). Video‐assisted thoracoscopic surgery allows, in addition to stapling, the use of newer techniques to shrink lung volume. Specifically, thermal energy can be applied to facilitate reduction via the use of a neodymium: yttrium‐aluminium‐garnet (Nd: YAG) laser (Wakabayashi 1995).
Unilateral or bilateral thoracotomy is performed to a lesser extent than the surgical approaches already described (Klepetko 1999). Thoracotomy incisions are often made in the fourth intercostal space for upper lobe and in the fifth or sixth intercostal space for lower lobe emphysema, and provide especially good access to the lower lobes.
How the intervention might work
Yusen 1996, in line with Cooper 1995, proposed that removal of diseased and functionless lung may improve the function of the remaining lung by:
increasing elastic recoil pressure, thereby increasing expiratory airflow;
decreasing the degree of hyperinflation, resulting in improved diaphragm and chest wall mechanics; and
decreasing the inhomogeneity of regional ventilation and perfusion, leading to improved alveolar gas exchange and increased effectiveness of ventilation in maintaining blood gas levels.
Zoumot 2015 adds that lung volume reduction can result in decreased asynchronous movement of different chest wall compartments, leading to improved ventilatory mechanics. The overall result of the procedure is improvement of the 'fit' of the lung in relation to the chest wall (Fessler 1998). This notion has been supported by studies examining respiratory mechanics after LVRS (Degano 2004; Hamnegård 2006; Teschler 1996). As the main aim of LVRS is improvement of respiratory mechanics, substantial improvement can still be expected in carefully selected patients with homogenous emphysema despite the fact that LVRS traditionally is not recommended for these patients (Weder 2009).
Why it is important to do this review
The burden of chronic illness is rising (Halbert 2006; Mannino 2007), with COPD currently the third leading cause of death (Lozano 2012). Healthcare costs related to COPD in general rise with disease severity, specifically owing to (exacerbation‐related) hospitalisations (Dal Negro 2008; Perera 2012). Lung volume reduction surgery might significantly benefit patients in the short and long term through improvements in exercise capability, dyspnoea, QoL and survival time (Teschler 1999). This will lead to an overall increase in the capability of disease management for patients who undergo LVRS. Finding effective treatments to help patients with severe emphysema to manage their illness, thereby preventing them from coming to hospital, can have a tremendously positive impact on the healthcare system and the lives of individual patients.
Objectives
The primary objective of this review was to gather all available evidence from randomised controlled trials comparing the effectiveness of lung volume reduction surgery (LVRS) versus non‐surgical standard therapy in improving health outcomes for patients with severe diffuse emphysema.
Secondary objectives were as follows.
To determine which subgroup of patients benefit from LVRS
To determine for which patients LVRS is contraindicated
To establish the postoperative complications of LVRS
To define morbidity and mortality related to LVRS
To determine which surgical approaches for LVRS are most effective
To calculate the cost‐effectiveness of LVRS
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that studied the safety, efficacy and/or cost efficiency of LVRS in patients with diffuse emphysema.
Types of participants
Participants with severe diffuse emphysema. We excluded studies that recruited participants with giant or bullous emphysema.
Types of interventions
We considered any of the variety of approaches and techniques used in LVRS for emphysema, including:
median sternotomy with bilateral stapling of non‐functional lung tissue with bovine reinforcement strips or pleural tenting technique;
video‐assisted thoracoscopic surgery (VATS) with neodymium: yttrium‐aluminium‐garnet (Nd: YAG) laser ablation to contract non‐functional tissue;
median sternotomy with unilateral stapling to resect approximately 20% of non‐functional tissue; and
Video‐assisted thoracoscopic surgery with unilateral laser ablation of non‐functional tissue.
Control groups consisted of usual follow‐up or different surgical techniques. We did not include in this review studies that focused on bronchoscopic lung volume reduction (BLVR) procedures.
Types of outcome measures
Primary outcomes
Short‐term (90 days) and long‐term (> 36 months) mortality
Quality of life (e.g. St George Respiratory Questionnaire (SGRQ))
Secondary outcomes
Lung function parameters (e.g. forced expiratory volume in one second (FEV1))
Exercise performance (e.g. six‐minute walk distance (6MWD))
Hospital utilisation (e.g. perioperative length of stay, re‐admission rate (hospitalisations, emergency department visits))
Adverse events (e.g. persistent air leaks, pneumothorax, dyspnoea)
Cost‐benefit analysis of LVRS
Search methods for identification of studies
Electronic searches
The previously published version of this review included searches up to September 2008. The search period for this update was September 2008 to April 2016. We identified trials by using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (see Appendix 1 for details). We searched all records in the Specialised Register coded as 'COPD' using the strategy presented in Appendix 2, with no restrictions on language or type of publication.
Searching other resources
We reviewed reference lists of all primary studies and review articles to look for additional references. We contacted authors of identified trials and asked them to identify other published and unpublished studies. We searched online clinical trials registers, including the ISRCTN registry, the UK Clinical Trials Gateway, ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform, to look for ongoing and recently completed studies.
Data collection and analysis
Selection of studies
We identified potentially relevant articles and retrieved titles, abstracts and key words through the search strategy. Two review authors (JA and KC) worked together to determine whether potentially relevant articles met the inclusion criteria for RCTs of LVRS for emphysema. We obtained full‐text copies of those articles. Upon reviewing article texts, we determined which studies should be included or excluded. We resolved disagreements by consensus following discussion with a third review author (BS).
Data extraction and management
Two review authors (JA and KC) independently extracted the data from included studies using a standardised data extraction form before entering data into Review Manager 5.3. Review authors also corresponded with study authors to request missing or raw data as required. Extracted data included study characteristics and risk of bias of the interventions, as well as details and outcomes.
Assessment of risk of bias in included studies
Two independent review authors (JA and KC) independently evaluated risk of bias (ROB) according to recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This evaluation consisted of random sequence allocation, allocation concealment, blinding of participants and outcome assessors, incomplete outcome data, selective outcome reporting and other potential threats to validity. We assessed ROB for each domain as low (low risk of bias), high (high risk of bias) or unclear (uncertain risk of bias), as per the guidelines given in Table 8.5a of the Cochrane Handbook for Systematic Reviews of Interventions. During assessment, we resolved conflicts by consensus or by referral to a third party.
Measures of treatment effect
We analysed outcomes as continuous or dichotomous data using standard statistical techniques with a fixed‐effect model up to the end of follow‐up.
For continuous outcomes, we used weighted mean difference and 95% confidence intervals.
For dichotomous outcomes, we used the Mantel‐Haenszel method to calculate an odds ratio (OR) with 95% confidence intervals (CIs). We did not use Peto ORs in the updated review, as large effects sizes are underestimated by this method.
We attempted to calculate from pooled ORs the numbers needed to treat for an additional harmful effect (NNTHs) for postoperative mortality, taking control group event rate data as baseline risk. We have reported these alongside the results of outcomes for which we have undertaken this calculation.
Unit of analysis issues
In the case of multi‐arm trials, we included each pair‐wise comparison separately but divided out shared intervention groups approximately evenly among the comparators. However, if intervention groups were deemed similar enough to be pooled, we combined the groups using appropriate formulas, as stated in the Cochrane Handbook for Systematic Reviews of Interventions: Table 7.7a for continuous data, and Chapter 16.5.4 for dichotomous data (Higgins 2011).
Dealing with missing data
We evaluated missing information regarding participants on an as‐available case analysis basis, as described in Chapter 16.1.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When statistics essential for analysis were missing (e.g. group means and standard deviations for both groups were not reported) and could not be calculated from other data, we attempted to contact the study authors to obtain data. We assumed that loss of participants that occurred before baseline measurements were performed had no effect on eventual outcome data of the study. We assessed and discussed any losses that occurred after the baseline measurement was taken.
Assessment of heterogeneity
We measured statistical heterogeneity by using the I² statistic and by visually inspecting the data.
Assessment of reporting biases
When a minimum of 10 studies were included, we explored potential reporting biases by using a funnel plot. When we included fewer than 10 studies, we extrapolated potential reporting biases within the other bias section in the risk of bias tables.
Data synthesis
'Summary of findings' table
We created a 'Summary of findings' table that includes the following outcomes.
Early mortality (90 days).
Long‐term mortality (> 36 months).
Change in total SGRQ scores (at end of follow‐up).
Walking distance (at end of follow‐up).
FEV1 (at end of follow‐up).
Residual volume (RV) (at end of follow‐up).
Total lung capacity (TLC) (at end of follow‐up).
We combined data by using Review Manager software, version 5.3. We reported studies by intention‐to‐treat (ITT) analysis. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) (GRADEpro GDT) to assess the quality of a body of evidence as it relates to studies that contributed data to meta‐analyses for prespecified outcomes. We adhered to the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and used GRADEpro software. We justified all decisions to downgrade or upgrade the quality of studies by using footnotes, and, when necessary, we made comments to aid the reader's understanding of the review.
Subgroup analysis and investigation of heterogeneity
We planned the first subgroup analysis per comparator, specifically:
surgical technique (MS and VATS) versus standard medical care;
LVRS with stapling versus Nd: YAG laser ablation; and
LVRS with or without buttressing of the staple line.
Furthermore, we planned several additional post hoc analyses.
High‐ versus low‐risk participants.
Distribution of emphysema (upper vs non‐upper lobe) and exercise capacity (high vs low) of participants.
We conducted these subgroup analyses for primary outcomes only.
Sensitivity analysis
We planned re‐analyses of data with a random‐effects model when the I² statistic exceeds 50% (Higgins 2011). We reported both fixed‐effect and random‐effects analyses when these yielded discordant results.
Results
Description of studies
We have provided a full description of each study in the Characteristics of included studies table.
Results of the search
We identified a total of 462 citations through electronic literature searches (search dates: all years to April 2016). Since the last search, we retrieved 111 citations (Figure 1). We have provided a breakdown of the total search history in Table 2. We identified two new trials (Clarenbach 2015; Pompeo 2012) since the last update of this review in 2006, and we added data on long‐term follow‐up reported by two other trials (CLVR 2005; NETT 2003).
1.
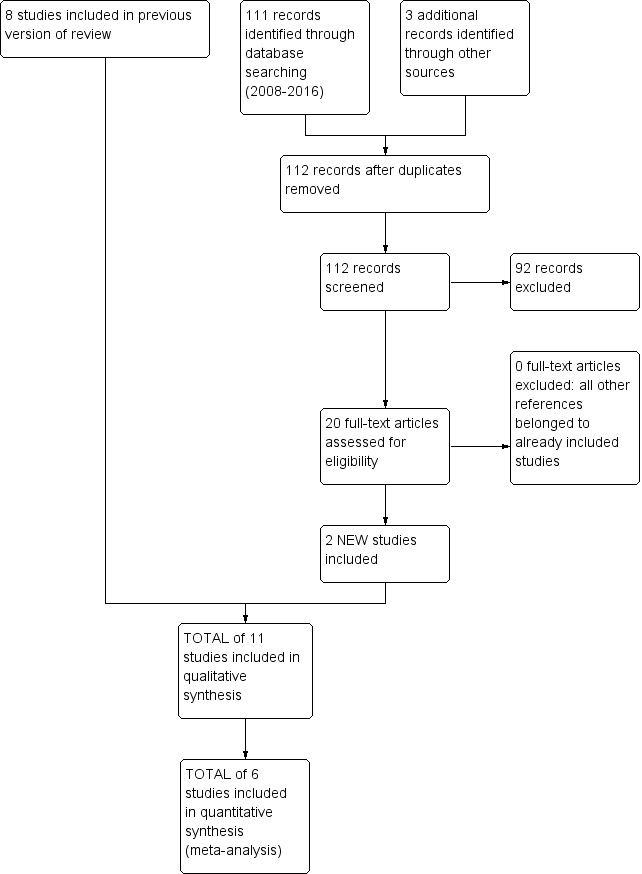
Study flow diagram: review update.
1. Search history.
| Search dates | Detail |
| 1. All years to December 1999 | References identified: 65 Full‐text articles retrieved: 13 Unique studies identified: 13 Studies failing to meet review entry criteria: 12 Studies meeting entry criteria: 1 |
| 2. December 1999 to September 2005 | References identified: 253 Full‐text articles retrieved: 45 Unique studies identified: 11 Studies failing to meet review entry criteria: 4 Studies meeting entry criteria: 7 Total number of included studies (sum previous number and new included studies): 8 |
| 3. September 2005 to October 2006 | References identified: 24 Full‐text articles retrieved: 7 Unique studies identified: 0 (the references were subsequent publications of either NETT 2003 or Hillerdal 2005) |
| 4. October 2006 to September 2007 | References identified: 7 Full‐text articles retrieved: 3 Unique studies identified: 0 (the references were subsequent publications of NETT 2003) |
| 5. September 2007 to May 2016 | References identified: 112 Full‐text articles retrieved: 19 Unique studies identified: 0 (the references were subsequent publications of NETT 2003 and CLVR 2005) |
Included studies
Study designs
All included studies were RCTs, four of which were conducted in the United States (Criner 1999; McKenna 1996; NETT 2003; OBEST 2005) and two in Canada (CLVR 2005; Goldstein 2003). One study took place in the United Kingdom (Geddes 2000), one in Switzerland (Clarenbach 2015), one in Italy (Pompeo 2012) and one in Sweden (Hillerdal 2005). The final study took place across three countries (Stammberger 2000: Switzerland, Austria and Germany).
Participants
A total of 1760 participants were randomised to a total of 11 studies. Two studies failed to describe screening procedures (McKenna 1996; Stammberger 2000). In the remaining trials, screening procedures excluded approximately 71% of screening populations (Table 3).
2. Study populations.
| Study ID | Screened | Entered (% Screened) |
| CLVR 2005 | 406 | 62 (15) |
| Clarenbach 2015 | 40 | 30 (75) |
| Criner 1999 | 200 | 37 (19) |
| Geddes 2000 | 174 | 48 (28) |
| Goldstein 2003 | 328 | 55 (17) |
| Hillerdal 2005 | 304 | 106 (35) |
| McKenna 1996 | Unclear | 72 (unclear) |
| NETT 2003 | 3777 | 1218 (32) |
| OBEST 2005 | 332 | 35 (11) |
| Pompeo 2012 | Unclear | 63 (unclear) |
| Stammberger 2000 | Unclear | 74 (unclear) |
| Total | 5561 | 1591 (29)* *Excludes data from McKenna 1996, Pompeo 2012 and Stammberger 2000. |
All participants were given a diagnosis of severe emphysema, which was confirmed by computed tomography (CT) (CLVR 2005; Criner 1999; Geddes 2000; Hillerdal 2005; McKenna 1996; NETT 2003; OBEST 2005; Pompeo 2012; Stammberger 2000), by lung ventilation/perfusion (V/Q) scans (Goldstein 2003) or by a combination of the two (Clarenbach 2015). All participants had to have significant airflow obstruction, and criteria of studies ranged between FEV1 < 30% and < 40% of predicted value. Participants had to show severe airflow obstruction and hyperinflation of the lung as indicated by a TLC > 100% predicted (criteria in studies ranged between > 100% predicted and > 120% predicted) and an RV > 150% predicted (criteria in studies ranged between > 150% predicted and > 200% predicted). Furthermore, hypercapnia and hypertension were exclusion criteria in all trials. Clarenbach 2015, NETT 2003 and OBEST 2005 also required that participants must be able to cover a minimum of 140 meters or 492 feet walking distance. See Characteristics of included studies for the inclusion criteria per study.
The mean age of trial participants ranged between 58.9 and 69 years and was reported in all but one paper (Geddes 2000 reported a median instead of a mean age of 60 for the medical group and 62 for the surgery group). Most of the randomised participants (68%) included in this review were recruited to NETT 2003 (1218 participants). The other studies had a considerably smaller number of participants (Clarenbach 2015: 30, CLVR 2005: 62, Criner 1999: 37, Geddes 2000: 48, Goldstein 2003: 55, Hillerdal 2005: 93, McKenna 1996: 72, OBEST 2005: 35, Pompeo 2012: 63 Stammberger 2000: 65).
Interventions
Presurgical and postsurgical pulmonary rehabilitation
In many of the included studies, a prerequisite for study entry was completion of a course of pulmonary rehabilitation, which was routinely undertaken by participants in usual medical care treatment groups or was completed as an additional part of postintervention treatment (Clarenbach 2015; CLVR 2005; Criner 1999; Geddes 2000; Goldstein 2003; Hillerdal 2005; NETT 2003; OBEST 2005). This additional aspect of care usually incorporated educational, nutritional and physical exercise components (Criner 1999; Geddes 2000; NETT 2003). In only one of the studies was rehabilitation not undertaken as part of the study protocol (Stammberger 2000). McKenna 1996 offered rehabilitation to participants in both treatment groups , postoperatively. One study, Pompeo 2012, indicated that included patients needed to show severe disability despite maximum medical therapy, which includes pulmonary rehabilitation.
Surgical techniques
Eight studies compared a surgical technique with a control group (Clarenbach 2015; CLVR 2005; Criner 1999; Geddes 2000; Goldstein 2003; Hillerdal 2005; NETT 2003; OBEST 2005). In these studies, LVRS was performed as VATS or median sternotomy (MS). CLVR 2005 and Criner 1999 reported that one technique was used exclusively (MS). In NETT 2003, 70% of procedures were MS, and the remainder were performed as VATS. Geddes 2000 reported that either MS or thoracoscopy was used. In Goldstein 2003, most surgical procedures were performed as VATS, with MS undertaken at the discretion of the attending surgeon, whereas in Hillerdal 2005 and OBEST 2005, MS was the predominant surgical intervention and VATS was used in a few cases. Clarenbach 2015 and Pompeo 2012 exclusively used VATS.
McKenna 1996, Pompeo 2012 and Stammberger 2000 did not compare LVRS with a medical control group. McKenna 1996 compared two different resection techniques using VATS, stapled lung reduction and laser bullectomy (via Nd: YAG), and Stammberger 2000 compared LVRS with or without buttressing using bovine pericardium.
Pompeo 2012 compared "traditional" resectional LVRS with a non‐resectional surgical approach performed on awake participants. They performed the non‐resectional technique via VATS; this involved pushing down the most seriously damaged portions of the lung, grasping the redundant lung edges and stapling the plicated lung area to form a linear, uninterrupted suture. The aim was to reduce lung volume by 20% to 30%.
Control group interventions
Seven studies compared a surgical intervention versus usual medical care. This entailed optimised medical therapy for all but Hillerdal 2005, in which the control group was given a prolonged physical conditioning intervention. Criner 1999 included an additional three‐month pulmonary rehabilitation course in the control group. Geddes 2000 also vaccinated all participants against influenza and pneumococcus.
Outcomes
Mortality
All but two studies (Clarenbach 2015; Stammberger 2000) reported mortality at a variety of follow‐up times. OBEST 2005 insufficiently reported mortality for the medical group, making it impossible to determine mortality at the end of follow‐up.
Baseline quality of life
Investigators used a variety of measures to determine quality of life, specifically:
SGRQ: Hillerdal 2005 and NETT 2003 reported baseline mean QoL scores for the SGRQ.
Chronic Respiratory Questionnaire (CRQ): Goldstein 2003, CLVR 2005 and OBEST 2005 used the CRQ, but only the former reported baseline values (separated per domain).
Short Form‐36 (SF‐36): Hillerdal 2005 and NETT 2003 reported baseline values for the SF‐36 separated per domain. OBEST 2005 reported the baseline utility score for the SF‐36. CLVR 2005 and McKenna 1996 did report data from the SF‐36 but did not report baseline values. Pompeo 2012 reported values on the physical functioning domain of the SF‐36. Criner 1999 mentioned the SF‐36 in a figure (page 2020, top of the page) as part of the data collection but did not mention the SF‐36 anywhere else in the text.
Quality of Well Being Scale (QWB): NETT 2003 mentioned baseline values for the QWB.
San Diego Shortness of Breath Questionnaire (SOBQ): NETT 2003 reported baseline values for the SOBQ.
Sickness Impact Profile: Criner 1999 reported baseline values for the SIP.
These questionnaires can be divided into two categories: general QoL and disease‐specific QoL questionnaires. SGRQ, CRQ and SOBQ are questionnaires that measure QoL that have a specific focus on respiratory disease; SF‐36, QWB and SIP measure general QoL. Clarenbach 2015 did not report on QoL.
Baseline lung function
All studies but one reported a variety of lung function measures at baseline. The severity of emphysema across studies indicated that trial populations suffered significant functional impairment.
Average baseline FEV1 (0.65 to 0.82 L) was similar across all groups, and mean % predicted values (25% to 33%) were similar across studies reporting FEV1. Geddes 2000 reported median values of 0.74 L and 0.75 L for surgery and control, respectively.
Mean total lung capacity (TLC) % predicted (124.5% to 151%) was reported by Clarenbach 2015, CLVR 2005, Criner 1999, Goldstein 2003, Hillerdal 2005, NETT 2003, Pompeo 2012 and Stammberger 2000. Geddes 2000 reported median values of 136% and 129% for surgery and medical care, respectively. OBEST 2005 and McKenna 1996 provided only mean TLC in litres.
Mean residual volume (RV) % predicted at baseline was between 217% and 287% (reported by Criner 1999, Goldstein 2003, Hillerdal 2005, NETT 2003, Pompeo 2012 and Stammberger 2000. Geddes 2000 reported median values of 226% and 220% for surgery and medical care, respectively. CLVR 2005, OBEST 2005 and McKenna 1996 reported only RV in litres. Clarenbach 2015 did not report baseline RV.
All but two studies (Clarenbach 2015; Goldstein 2003) reported baseline partial arterial pressure of oxygen (PaO2) and carbon dioxide (PaCO2). Geddes 2000 reported median values and Goldstein 2003 did not provide baseline values for PaO2 and PaCO2. Clarenbach 2015 reported only oxygen saturation as measured by blood analysis (SaO2) %.
Clarenbach 2015, CLVR 2005, Criner 1999, Goldstein 2003, OBEST 2005, NETT 2003 and McKenna 1996 mentioned baseline mean values for diffusing capacity of the lungs for carbon monoxide (DLCO) or transfer factor for carbon monoxide (TLCO). Geddes 2000 reported median values. Hillerdal 2005 mentioned DLCO in text but did not report the values. Stammberger 2000 and Pompeo 2012 did not measure DLCO (and did not mention it in text).
Exercise capacity
Researchers measured exercise capacity via walking distance or cycle ergometry. Clarenbach 2015, CLVR 2005, Criner 1999, Goldstein 2003, NETT 2003, OBEST 2005 and Pompeo 2012 reported baseline values for six‐minute walking distance (6MWD), and Hillerdal 2005 and Geddes 2000 used the shuttle walking test to determine walking distance. Briefly, the 6MWD measures the distance a patient is able to walk in a period of six minutes on a flat hard surface. The shuttle walk requires patients to walk a set distance of 10 metres between cones within a time period marked by auditory beeps. The auditory beeps decrease in time, requiring the patient to walk faster the more (s)he progresses. Average mean walking distance reported in these studies ranged between 260 metres and 340 metres. Geddes 2000 reported a median baseline shuttle walk of 210 metres for LVRS versus 220 metres for control. Average shuttle walk distance in the Hillerdal 2005 study was 237 metres for LVRS versus 198 metres for control. Furthermore, cycle ergometry was used in the following studies: Goldstein 2003, Hillerdal 2005, NETT 2003 and Criner 1999.
Hospital utilisation
Clarenbach 2015, CLVR 2005, OBEST 2005, Goldstein 2003, NETT 2003 and Pompeo 2012 reported hospitalisation rates after the start of the trial. Criner 1999 mentioned hospitalisation rates for the LVRS group before and after surgery but did not compare this group with the control group. McKenna 1996 and Stammberger 2000 reported on operating times between the two procedures and length of stay for each group.
Adverse events
All studies but CLVR 2005 and OBEST 2005 reported adverse events resulting from different surgical procedures.
Cost‐effectiveness of LVRS
Only NETT 2003 and CLVR 2005 reported on cost‐effectiveness of LVRS versus medical care. NETT 2003 reported quality‐adjusted life‐years (QALYs) up to six years, as well as direct medical costs, and CLVR 2005 reported QALYs calculated via the Health Utility Index (HUI3) up to two years after the trial.
Duration of follow‐up
Study authors described variation in the follow‐up of participants. Two studies (Clarenbach 2015 and Criner 1999) reported outcome assessments at three months postoperatively. OBEST 2005 reported values for up to six months. Geddes 2000, Goldstein 2003 and Hillerdal 2005 reported data for up to 12 months post intervention. CLVR 2005 reported most data for a 24‐month follow‐up, with the exception of long‐term follow‐up for survival of eight to 10 years. NETT 2003 reported outcomes at an average follow‐up of three years, with some new data on QoL and mortality at follow‐up of six years. Pompeo 2012 reported most data for follow‐up until 24 months but provided rates of survival for up to 48 months.
Excluded studies
The main reason for exclusion of screened studies involved problems related to their design. Daniel 1996, Keenan 1996, Kotloff 1996, Little 1995, Nickoladze 1992 and Wakabayashi 1995 were case series studies. Martinez 1997, O'Brien 1999, Sciurba 1996, Szekely 1997, Tan 2000 and Teschler 1996 were prospective case series. Pompeo 2000 was an RCT but included participants with bullous emphysema, causing this study to be excluded from this review.
Risk of bias in included studies
We have provided in Figure 2 an overview of our judgements of the risk of bias of each study.
2.
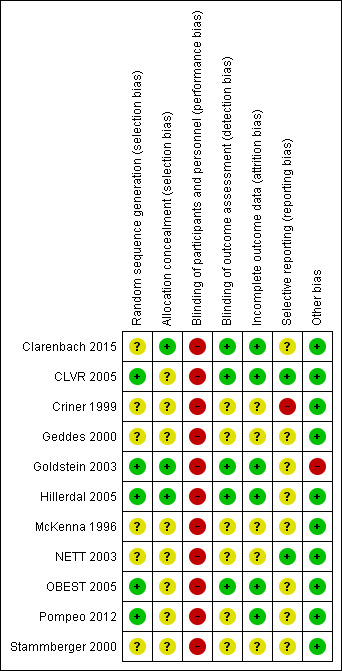
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Random sequence generation was adequate in CLVR 2005, Goldstein 2003, Hillerdal 2005, OBEST 2005 and Pompeo 2012. The other studies had unclear risk of selection bias owing to random sequence generation. Clarenbach 2015, Criner 1999, Geddes 2000, McKenna 1996, NETT 2003 and Stammberger 2000 mentioned randomisations in the text but did not specify the specific methods used nor by whom these were conducted.
Clarenbach 2015, Goldstein 2003 and Hillerdal 2005 reported information on allocation concealment and were rated to be at low risk of selection bias. All other studies had an unclear status, as they did not report on allocation concealment (Criner 1999; Geddes 2000; McKenna 1996; NETT 2003; Pompeo 2012; Stammberger 2000) or indicated only that the allocation lists were kept at a separate data centre without further specification (CLVR 2005; OBEST 2005).
Blinding
Lung volume reduction surgery does not lend itself to blinding within ethical guidelines. None of the studies comparing LVRS versus standard medical care specifically reported on blinding of participants and personnel, which, in light of the expected lack of blinding, led to assessment of high risk of performance bias.
Clarenbach 2015, CLVR 2005, Goldstein 2003, Hillerdal 2005 and OBEST 2005 indicated that outcome assessment was performed by staff who were unaware of allocation of groups, thereby having low risk of detection bias. The other studies (Criner 1999; Geddes 2000; McKenna 1996; NETT 2003; Pompeo 2012; Stammberger 2000) did not mention blinding of outcome assessment; therefore, we assigned these studies unclear risk of detection bias.
Incomplete outcome data
Five out of 11 studies (Criner 1999; Geddes 2000; McKenna 1996; NETT 2003; Stammberger 2000) did not provide a sufficient description of handling missing outcome data from questionnaires (if any). Hillerdal 2005, Pompeo 2012; and Clarenbach 2015 reported attrition but did not report the presence or absence of missing data from questionnaires. CLVR 2005, Goldstein 2003 and OBEST 2005 reported attrition and percentages of participants with missing outcome data.
Selective reporting
Most studies (Criner 1999; Geddes 2000; Goldstein 2003; Hillerdal 2005; McKenna 1996; OBEST 2005; Stammberger 2000) did not publish a prespecified protocol, making it difficult to judge selective reporting; we assessed these studies as having unclear risk of reporting bias. Criner 1999 indicated that investigators used the SF‐36, but they did not report on it in the text, leading to assessment of high risk of reporting bias.
CLVR 2005 performed a pilot study in which study authors stated most of the variables of interest. Lack of a formal protocol made it difficult to assess whether study authors stuck to the specific variables tested in the pilot study. NETT 2003 published an extensive document on the rationale behind the trial, indicating the main variables of interest. Clarenbach 2015 and Pompeo 2012 were registered on clinicaltrials.gov, but performed a per‐protocol analysis rather than an ITT, possibly introducing a source of bias.
Other potential sources of bias
Clarenbach 2015, CLVR 2005, Geddes 2000, Hillerdal 2005, McKenna 1996, NETT 2003, OBEST 2005, Pompeo 2012 and Stammberger 2000 are not at risk for other potential biases. Criner 1999 reported potential risk of cross‐over effects, as participants in the medical group were allowed to cross over to the treatment group after completion of the follow‐up period by the control group. As the study authors separately reported results including and excluding cross‐over participants, review authors assessed this study as having low risk of other bias. Goldstein 2003 indicated that lack of a sham surgery group may have led to some placebo effects, but this was a problem in all groups owing to lack of blinding. Furthermore, this study may have conducted selective recruitment, as participants were referred by respiratory physicians, and a physician and a surgeon reassessed those wishing to proceed and made the final decision regarding eligibility, causing this study to be rated at high risk of bias .
Effects of interventions
See: Table 1
We will present the results for effects of the intervention separately per comparator. First, we will discuss all studies comparing LVRS versus standard medical care (Comparison 1). This will be followed by the trial comparing stapled lung reduction versus laser ablation (Comparison 2) and the trial determining the effect of buttressing the staple line (Comparison 3). Finally, we will discuss the trial comparing traditional resectional LVRS with awake non‐resectional LVRS (Comparison 4).
LVRS versus usual medical care (Comparison 1)
Mortality (Analyses 1.1 to 1.5)
Data from five clinical trials were available for outcomes reporting mortality at different endpoints (Figure 3). Early mortality (90 days) was significantly higher for participants treated with LVRS than for those given standard care (odds ratio (OR) 6.16, 95% confidence interval (CI) 3.22 to 11.79; 1489 participants; five studies; moderate‐quality evidence). Long‐term mortality (> 36 months), however, favoured LVRS over control (OR 0.76, 95% CI 0.61 to 0.95; 1280 participants; two studies; moderate‐quality evidence). We did not include Criner 1999 and OBEST 2005 in the meta‐analysis. Criner 1999 did not specifically mention mortality in the control group but did report on mortality for all participants treated with LVRS (including cross‐over participants), which was 9.4% (three of 32). OBEST 2005 did not provide sufficient detail to allow determination of mortality.
3.
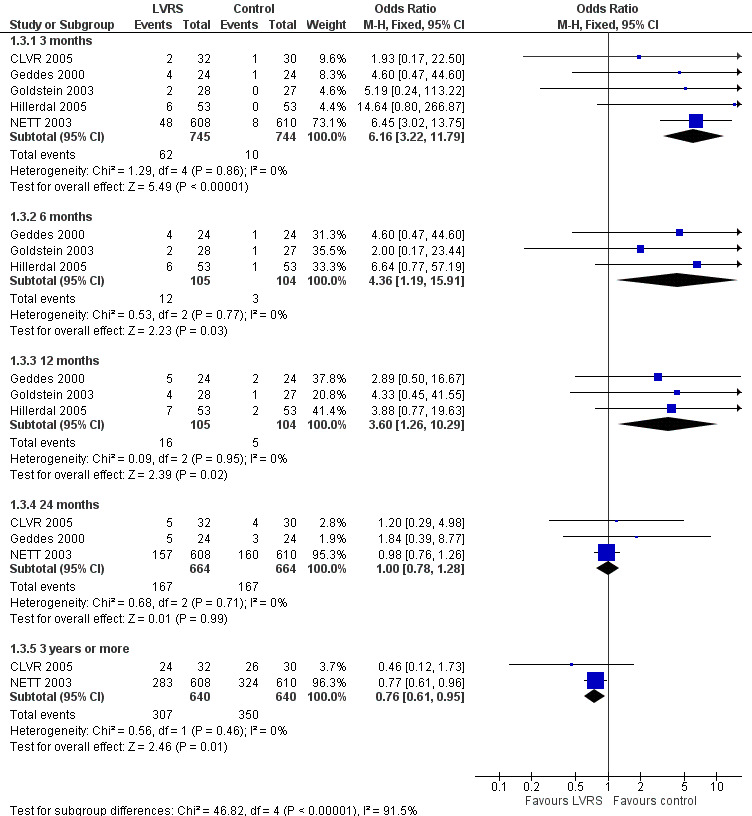
Forest plot of comparison: 1 Surgery versus control, outcome: 1.3 Overall mortality (stratified by follow‐up period).
Mortality in subgroups: high risk versus non‐high risk
NETT 2003 re‐analysed data on the basis of risk of early mortality identified ad hoc by an independent data and safety monitoring board. The monitoring board determined that high‐risk' candidates were those with low FEV1 predicted (< 20%) and either low carbon monoxide diffusing capacity (< 20% predicted) or homogeneous emphysema at baseline. Stratifying mortality for high risk versus non‐high risk shows a significant difference in mortality at completion of follow‐up (24 months) for the high risk subgroup (OR 2.00, 95% CI 1.02 to 3.92) and no differences in mortality for the non‐high risk subgroup (OR 0.86, 95% CI 0.64 to 1.14; Figure 4). NETT 2003 furthermore reported on 90‐day mortality for the risk subgroups, showing that the high risk subgroup of participants (N = 140), as well as the non‐high risk group, had considerably higher 90‐day mortality than participants in the control group (OR 57.24, 95% CI 3.38 to 968.54; and OR 3.65, 95% CI 1.65 to 8.09, respectively). Two other trials (Geddes 2000 and Hillerdal 2005) also revised entry criteria following identification of characteristics that suggested higher risk of postoperative mortality (Geddes 2000: DLCO < 30% predicted and low exercise capacity; Hillerdal 2005: DLCO ≤ 20% predicted). However, these trials did not provide sufficient information to justify pooling of their results with the results of NETT 2003. The NNTH is six for high risk and 38 for non‐high risk subgroups.
4.
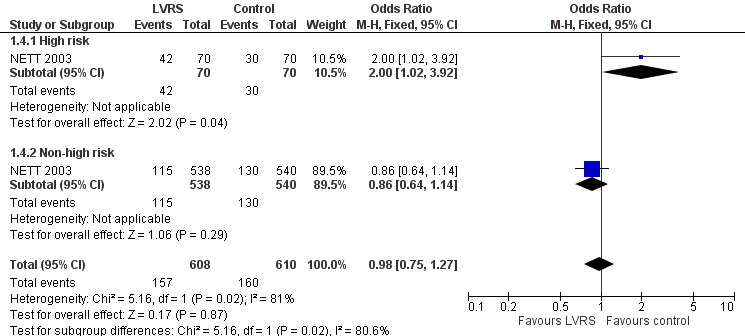
Forest plot of comparison: 1 Surgery versus control, outcome: 1.4 Overall mortality (stratified by risk, to end of follow‐up).
A recent publication on mortality of the high risk subgroup showed that after 14 years, almost all participants from the LVRS (96%) and medical treatment (97%) 'high risk' group in the NETT trial have died. Although mortality was higher for the LVRS group at the start of the trial, the mortality curves crossed at around 4.4 years, and afterwards showed a non‐significant trend favouring the LVRS group. Overall survival for both groups did not significantly differ (P = 0.95), with median survival in the LVRS and medical treatment groups reported as 2.14 (95% CI 1.20 to 4.07) and 3.12 (95% CI 2.79 to 4.37) years, respectively.
Mortality in non‐high risk subgroups: emphysema location and exercise capacity
End of follow‐up
NETT 2003 post hoc defined subgroups for non‐high risk participants on the basis of specific participant characteristics that could influence the efficacy of LVRS: presence of upper or non‐upper lobe‐predominant emphysema and/or low or high postrehabilitation exercise capacity. Participants with upper lobe‐predominant emphysema with a low exercise capacity at baseline in the LVRS group had lower mortality than those in the medical group (OR 0.45, 95% CI 0.26 to 0.78; 290 participants). Participants with non‐upper lobe‐predominant emphysema and low exercise capacity in the LVRS group had higher mortality at the end of follow‐up (OR 2.28, 95% CI 1.12 to 4.64; 257 participants) than those in the control group. For the remaining categories, upper lobe with high exercise capacity and non‐upper lobe with high exercise capacity did not show significant differences in mortality between LVRS and control groups (OR 0.88, 95% CI 0.53 to 1.46; 419 participants; and OR 0.75, 95% CI 0.38 to 1.47; 149 participants). See Figure 5 for an overview of mortality based on subgroup.
5.
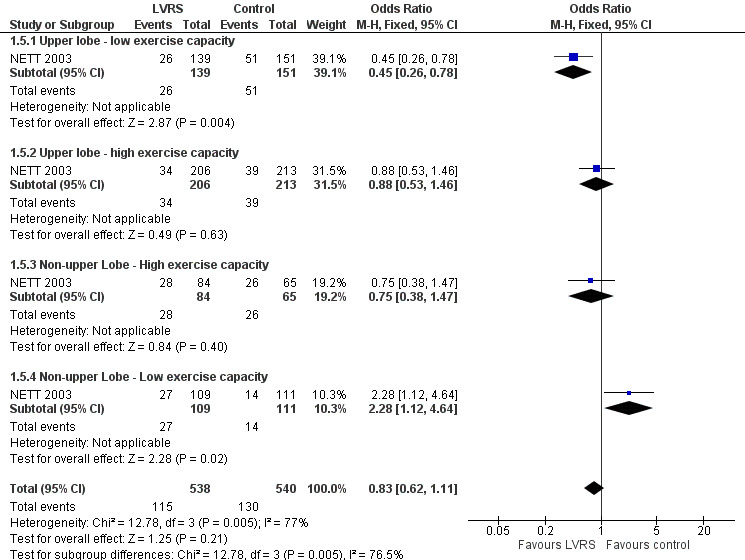
Forest plot of comparison: 1 Surgery versus control, outcome: 1.5 Overall mortality (stratified by subgroup, to end of follow‐up).
Follow‐up after 4.3 years showed that the survival benefit of LVRS remained for participants with upper lobe‐predominant emphysema with low exercise capacity (risk ratio (RR) 0.57; P = 0.01). Mortality was similar at the end of follow‐up for the other three subgroups.
Three‐month follow‐up
NETT 2003 provided differences in mortality at three months for each of the subgroups. Participants with upper lobe‐predominant emphysema, regardless of baseline exercise status, did not show significantly higher mortality at 90 days when treated with LVRS compared with those in the control group: low exercise capacity, OR 0.87, 95% CI 0.23 to 3.29; 290 participants; high exercise capacity, OR 3.17, 95% CI 0.63 to 15.86; 419 participants. Participants with non‐upper lobe‐predominant emphysema and low exercise capacity did not show significantly higher early mortality when treated with LVRS versus control (OR 12.68, 95% CI 0.71 to 226.19; 149 participants). The only subgroup of participants showing significantly higher early mortality when treated with LVRS compared with similar participants in the control group consisted of participants with non‐upper lobe emphysema and high exercise capacity (OR 12.35, 95% CI 1.57 to 97.37; 220 participants).
Mortality in subgroups: residual volume
Finally, post hoc re‐analysis of NETT 2003 data based on mortality indicators recently discovered via bronchoscopic lung volume reduction (BLVR) showed that participants who underwent LVRS with residual volume > 225% predicted had higher mortality at 24 months than participants with residual volume < 225% predicted (P value not reported). We found no such difference in mortality among participants who received standard medical care.
Quality of life (Analyses 1.6 to 1.10)
Disease‐specific quality of life
Studies including SGRQ, SF‐36, CRQ and QWB used a variety of health status measurements focused on disease‐specific quality of life. Censored mean changes in SGRQ from NETT 2003 were drawn from surviving participants. We pooled this information with ITT data from the much smaller Hillerdal 2005 study, noting a difference in mean change from baseline in total SGRQ scores at end of follow‐up (‐13.78 SGRQ units, 95% CI ‐15.75 to ‐11.80; 1324 participants; moderate‐quality evidence) significantly favoured LVRS over standard medical care (Figure 6). Furthermore, Hillerdal 2005 reported that baseline SGRQ scores were inversely related to changes in the domains of SGRG (P < 0.05), indicating that participants with the lowest QoL scores at baseline showed greatest improvement at the end of follow‐up. Data from NETT 2003 and Hillerdal 2005 were also pooled for six‐ and 12‐month follow‐up for total scores on SGRQ, indicating favourable QoL scores for LVRS at six months (mean difference (MD) ‐13.48, 95% CI ‐15.13 to ‐11.84) and at 12 months (MD ‐13.77, 95% CI ‐15.75 to ‐11.80).
6.
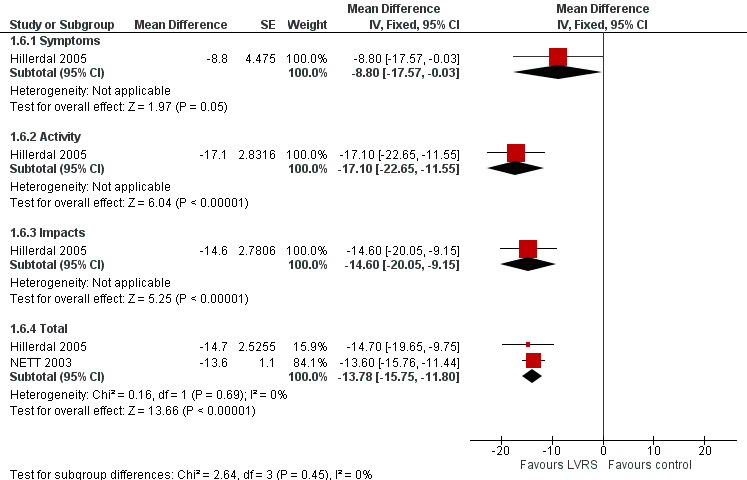
Forest plot of comparison: 1 Surgery versus control, outcome: 1.6 Change in SGRQ (end of follow‐up).
As a countermeasure to the potential overestimation of effect mediated as a continuous variable, NETT 2003 reported long‐term follow‐up data on the number of participants with an increase, no change or a decrease in their quality of life, describing those who had died or whose data were missing as unchanged/deteriorated. Clinically significant improvement, defined as a decrease in SGRQ greater than eight units, was significantly greater for the LVRS group than for the control group up to 4 years (P < 0.01). Goldstein 2003 and CLVR 2005 reported significant differences in favour of surgery on all four domains of a different questionnaire ‐ the CRQ ‐ at 12 and 24 months, respectively. OBEST 2005 reported six‐month follow‐up for the CRQ, which showed significantly better outcomes in three (dyspnoea, fatigue and mastery) of the four domains.
Disease‐specific quality of life in subgroups
As reported previously, NETT 2003 reported dichotomised data on quality of life (clinically significant improvement defined as a decrease in SGRQ scores greater than eight units) for each of the subgroups. Analysis of subgroups stratified by risk revealed no significant difference in the number of responders in the LVRS group compared with the control group among high risk participants (OR 12.01, 95% CI 0.66 to 218.88). However, significantly more non‐high risk participants showed clinically significant improvement on SGRQ compared with those in the medical group (OR 5.06, 95% CI 3.31 to 7.72). The number of responders reporting quality of life from subgroups based on emphysema location and exercise capacity favoured the LVRS group over the medical care control group in all but one category (non‐upper lobe with high exercise capacity ‐ no difference; OR 1.35, 95% CI 0.48 to 3.81). Long‐term data indicate that among participants with upper lobe‐predominant emphysema with low exercise capacity, a significant decrease in SGRQ remained significant for five years.
General quality of life
Geddes 2000 reported significant median changes in total SF‐36 scores at six and 12 months post randomisation favouring surgery, whereas Hillerdal 2005 and CLVR 2005 reported mean domain differences for all domains of this health status measurement. CLVR 2005 specifically used quality‐adjusted values to derive a total change score over the course of the trial (24 months). We pooled these quality‐adjusted values over 24 months with completion of follow‐up data (12 months) from Hillerdal 2005 to discover that SF‐36 scores significantly favoured LVRS for all but one (emotional role) domain of the SF‐36, indicating an overall significant advantage for the surgical group over the medical care group. OBEST 2005 reported six‐month differences between LVRS and medical care on the SF‐36 but did not provide enough information for inclusion in the meta‐analysis. However, investigators reported the composition scores of the two overall domains (mental vs physical) at six months, revealing no differences for either score.
NETT 2003 conducted long‐term (six‐year) follow‐up on the Quality of Wellbeing Scale for 114 LVRS participants and 122 medical care participants (out of 608 and 610 participants, respectively) who entered the trial early enough to be followed up before the trial ended. This follow‐up imputed missing data to counteract the significant problems associated with loss of follow‐up for measures such as quality of life. On the imputed model, the surgery group showed significantly better quality of life over the medical care group for all follow‐up years (P < 0.001). This analysis further indicated that LVRS produced about 3.6 quality‐adjusted extra months of life compared with medical care. Criner 1999 used the SIP to determine general quality of life and found that at three months, the LVRS group showed better quality of life (P < 0.008) versus baseline, but this was not the case for the control group.
Change in exercise capacity (Analysis 1.11)
Walking distance
Clarenbach 2015, Criner 1999, Goldstein 2003 and OBEST 2005 provided information on 6MWD, and Hillerdal 2005 used the shuttle walking test to determine walking distance for participants at a variety of follow‐up times. Pooling of data from these studies revealed that participants in the LVRS group showed significantly better improvement in walking distance compared with those in the medical control group at the end of follow‐up (standardised mean difference (SMD) 0.70, 95% CI 0.42 to 0.98; 215 participants; five studies; I2 = 51%; low‐quality evidence). CLVR 2005 reported quality‐adjusted scores for 6MWD over the total 24‐month period (cannot be pooled with the other results) that favoured the LVRS group (P = 0.02). In CLVR 2005, 22 of 32 participants in the LVRS group (69%) compared with eight of 30 participants in the medical care group had higher 6MWD scores over the two‐year period (P < 0.0009). Geddes 2000 reported median values for the shuttle walk test at three‐, six‐ and 12‐month follow‐up but failed to find a difference between LVRS and medical care at the end of follow‐up (P = 0.26). NETT 2003 reported that non‐high risk participants who underwent LVRS showed significant improvement in 6MWD compared with control participants (P < 0.001).
Cycle ergometry
Intention‐to‐treat data from NETT 2003 indicate that LVRS‐treated participants were more likely to have improved baseline exercise capacity as measured by cycle ergometry than those treated with usual medical care at one (23% vs 5%), two (15% vs 3%) and three years (9% vs 1%) (P < 0.001) at each follow‐up time. Criner 1999 found no significant differences in exercise capacity between the medical care group and the LVRS group at three months. Goldstein 2003 reported a significant increase in peak incremental exercise power of 13 Watts (P < 0.05) for LVRS (44 ± 2) versus control (31 ± 2) at six months. Hillerdal 2005 found a significant mean difference of 9 W (95% CI 0 to 18) at 12 months favouring LVRS. CLVR 2005, Geddes 2000 and OBEST 2005 did not report values for cycle ergometry.
Lung function outcomes (Outcomes 1.12 to 1.16)
FEV1
We pooled results from Criner 1999, Goldstein 2003, Hillerdal 2005 and OBEST 2005 showing improvement in FEV1 (in litres) until end of follow‐up, which significantly favoured LVRS (MD 0.20, 95% CI 0.13 to 0.28; 188 participants; four studies; low‐quality evidence). Furthermore, NETT 2003 reported non‐ITT data for this outcome. The validity of statistically significant differences of 8.5%, 6.3% and 5.4% predicted at six, 12 and 24 months should be weighed against the censored nature of the data, whereby the proportion of randomised participants contributing to this outcome diminished from 66% to 54% to 31% over the course of two‐year follow‐up. NETT 2003 also reported the number of participants achieving percentage changes, describing those who had died or whose data were missing as unchanged/deteriorated. Participants were more likely to demonstrate an increase in FEV1 post LVRS than those in the control group at six, 12 and 24 months.
CLVR 2005 reported quality‐adjusted scores for FEV1 over the 24‐month period favouring the LVRS group (P = 0.007). Geddes 2000 reported only median values and interquartile ranges (IQRs), among which only three‐month values significantly favoured LVRS, with no difference in FEV1 between groups at six‐ and 12‐month follow‐up. Clarenbach 2015 found that LVRS led to a change in baseline FEV1 % predicted of 8.1% compared with a decrease of 1.6% for control, with a total positive effect of +9.7% favouring LVRS over control at three months (P < 0.001).
Absolute residual volume
Data from four studies (Clarenbach 2015; Criner 1999; Goldstein 2003; Hillerdal 2005) could be pooled at end of follow‐up to compare RV % predicted. Participants in the LVRS group had significantly lower % predicted RV at the end of follow‐up compared with the medical group (MD ‐44.28, 95% CI ‐57.80 to ‐30.75; 177 participants; four studies; low‐quality evidence). Geddes 2000 provided median values for three, six and 12 months, all of which favoured LVRS. OBEST 2005 reported a mean difference of ‐1.32 L favouring the LVRS group (P = 0.0012).
Absolute total lung capacity
We pooled data from four studies (Clarenbach 2015; Criner 1999; Goldstein 2003; Hillerdal 2005) showing that LVRS led to a significant reduction in total lung capacity % predicted (MD ‐14.83%, 95% CI ‐20.50 to ‐9.15; 178 participants; four studies; low‐quality evidence). Geddes 2000 did not report a difference at the end of follow‐up (P = 0.17). OBEST 2005 reported a mean difference of ‐1.11 L favouring the LVRS group (P = 0.0019).
Arterial blood gases
Criner 1999 and OBEST 2005 measured PaCO2 in mmHg, and Hillerdal 2005 in kPA; therefore, we could not pool the data indicating that LVRS led to a significantly lower PaCO2 compared with medical treatment (SMD ‐0.43, 95% CI ‐0.78 to ‐0.08). Criner 1999 measured PaO2 in mmHg,and Hillerdal 2005 in kPa; we pooled data to find an SMD of 0.10 (95% CI ‐0.30 to 0.50). NETT 2003 found a significant reduction in PaCO2 and increase in PaO2 for LVRS compared with medical treatment at all moments in follow‐up (six, 12 and 24 months). CLVR 2005 did not find significant differences in PaCO2 and PaO2 over the two years. Geddes 2000 found no differences in PaCO2 (P = 0.70) and PaO2 values (P = 0.08) between LVRS participants and those given medical care.
Carbon monoxide diffusing capacity
Clarenbach 2015 reported a non‐significant % change to end of follow‐up of median ‐1 (IQR ‐4 to 0) for control versus 5.0 (IQR 1‐7) for LVRS (P = 0.06). CLVR 2005 did not report the final values of DLCO in their final paper but did report values in the intermittent report for CLVR 2005 and OBEST 2005. CLVR 2005 found an increase of 1.615 mL/min/mmHg (P = 0.067), and OBEST 2005 found a decrease of ‐0.21 mL/min/mmHg, between control and LVRS (P = 0.85). Criner 1999 reported final values for DLCO, with control showing 59% predicted (standard deviation (SD) 17) and LVRS showing 55% predicted (SD 22). Changes between eight weeks post rehabilitation and three months of follow‐up were significant only for the LVRS condition (P = 0.05). Goldstein 2003 reported final values for DLCO, with control showing 33% predicted (SE 2) and surgery showing 37% predicted (SE 2); these findings were not significant between groups. Geddes 2000, Hillerdal 2005 and NETT 2003 did not report on DLCO values at the end of follow‐up.
Adverse events
Clarenbach 2015 reported that two participants in the LVRS group developed a pneumothorax and one developed a persistent fistula. Criner 1999 reported that two participants required intubation and mechanical ventilation for an exacerbation and one developed pneumonia during the follow‐up period. Geddes 2000 reported that three participants had persistent air leaks and two developed an infection. Goldstein 2003 reported a variety of adverse events during hospitalisation for surgery, with two participants requiring prolonged ventilation, one significant bleeding and one a sternal dehiscence. Furthermore, these researchers reported on 10 participants with prolonged air leakage, six benign dysrhythmias, six respiratory tract infection, six transient confusion, two small bowel ileus, two vocal cord dysfunction and one a transient ischaemic attack. During 12‐month follow‐up, investigators noted only ischaemic heart disease (one LVRS, one control) and respiratory tract infection (30 LVRS, 35 control).
NETT 2003 conducted a non‐randomised comparison of the effects of two surgical techniques and of different buttressing materials used in the study on postoperative air leaks, but found no significant difference in duration or prevalence of air leaks. NETT 2003 found that COPD exacerbations (P = 0.0005) and time to first exacerbation (P < 0.0002) were reduced in the LVRS group versus the medical group, specifically among those with a big improvement in FEV1 (P = 0.04). Furthermore, NETT 2003 reported major pulmonary morbidity in 29.8% and cardiovascular morbidity in 20.0% of participants, and pointed out that 58.7% of participants in the LVRS group developed at least one complication.
Hospital utilisation (Outcome 1.17)
NETT 2003 reported long‐term hospitalisation rates and revealed no difference in mean hospitalisations between LVRS and control groups for zero to 12 months (MD ‐0.15, 95% CI ‐0.33‐0.03) or for 25 to 36 months (MD ‐0.15, 95% CI ‐0.33 to 0.03). The mean difference favours LVRS between 13 and 24 months (MD ‐0.20, 95% CI ‐0.34 to ‐0.06). Goldstein 2003 reported on four re‐admissions during the follow‐up period of 12 months in the surgical group and zero in the control group. NETT 2003 furthermore performed a non‐randomised comparison between VATS and median sternotomy and found that more participants who had VATS (80.9%) than median sternotomy (70.5%) were living independently after 30 days (P = 0.02). The other studies did not report data on hospital utilisation. Mean emergency room visits after the trial were significantly different for 13 to 24 months at ‐0.2 days (95% CI ‐0.34 to ‐0.06) favouring LVRS, but not for at zero to 12 months and after 24 months. Clarenbach 2015 reported an average hospitalisation time of 14 days (range, 7 to 28 days) for the LVRS group.
Cost‐effectiveness (Outcome 1.18)
NETT 2003 provided data at different time points that were available for those surviving and contributing data at 12, 24 and 36 months. Direct medical costs (in 1000 United States Dollar (USD)) significantly favoured medical therapy between zero and 12 months (MD 45.41, 95% CI 40.05 to 50.77; N = 1066) and between 13 and 24 months (MD 79.09, 95% CI 76.12 to 82.06; N = 1066). Total costs of health care favoured medical therapy between zero and 12 months (MD 48.15, 95% CI 42.10 to 54.20; N = 1066), but between 13 and 24 months, LVRS on average was cheaper than medical therapy (MD ‐8.10, 95% CI ‐11.85 to ‐4.35; N = 831). Direct (MD ‐2.10, 95% CI ‐5.19 to 0.99; N = 455) and total costs of care (MD ‐3.65, 95% CI ‐7.74 to 0.44; N = 455) were not significantly different between conditions for 25 and 36 months. At three years, the average cost of LVRS was around 36,000 USD more expensive than medical therapy (P < 0.001). Furthermore, a non‐randomised comparison between VATS and median sternotomy showed that VATS had lower costs of hospitalisation (P = 0.03) and total medical and non‐medical costs (P = 0.005) compared with median sternotomy.
CLVR 2005 found that the mean cost for LVRS was 49.776 Canadian Dollar (CAD) versus 28.119 CAD for the medical group over the total two‐year period. As they found a 0.21 QALY increase for the LVRS group over the medical group, this led to a cost‐effectiveness ratio of 133.900 CAD per QALY. NETT 2003 found similar results ($190.000 USD at three years and $140.000 USD at five years) but projected costs for 10 years between $54.000 USD and $58.000 USD per QALY gained for the overall population. Over six years of follow‐up, LVRS produced an average of 0.30 QALYs in the NETT 2003 study. Projections up to 10 years for the group of high responders ‐ participants with upper lobe‐predominant emphysema and low exercise capacity ‐ were as low as $48,000 USD per QALY.
Stapled lung reduction versus laser bullectomy (Comparison 2)
One study reported data for this comparison (McKenna 1996).
Mortality
In the laser group, one participant died of respiratory failure three months after surgery. One participant died of other causes six weeks after surgery, and another died during sleep three months after surgery. In the staple‐treated group, one died after surgery as a result of a contralateral tension pneumothorax.
Disability and health status
The Medical Outcomes Study (MOS) SF‐36 quality of life questionnaire was reported to improve significantly. Breathlessness showed improvement in dyspnoea by more than one grade in 26 of 39 in the staple‐treated group (66%) compared with eight of 33 (24%) in the laser‐treated group (P < 0.003). The supplemental oxygen requirement was reduced from 25 to 12 participants in the laser group and from 27 to five in the staple‐treated group.
Lung function
The mean improvement in FEV1 at six months was 0.09 L (13.4%, SD 5.5) for the laser group and 0.22 L (32.9%, SD 4.8) for the staple‐treated group (P < 0.01). Forced vital capacity (FVC) increased similarly: laser 0.13 L (6%, SD 3); staple 0.35 L (21%, SD 6) (P = 0.07). Improvement in FEV1 and FVC from baseline was statistically significant (P < 0.006) only in the staple‐treated group. Residual volume, gas exchange and blood gases were not reported.
Exercise performance
Exercise performance was not reported.
Adverse events
Air leaks that persisted for longer than seven days were not statistically different between the two groups: 11 in the laser‐treated group and 19 in the staple‐treated group. One participant in each group underwent reoperation for closure of a persistent air leak. The re‐admission rate was not reported. One participant in the staple‐treated group had a suspected tension pneumothorax in the contralateral lung. Additionally, the postoperative death in this group was due to a contralateral tension pneumothorax. Six participants in the laser group developed delayed pneumothorax compared with none in the staple‐treated group (P < 0.005). One participant in the staple‐treated group and none in the laser‐treated group experienced deep vein thrombosis.
Hospital stay
The mean hospital stay was 11 (SD = 12) days and 13 (SD = 11) days for the laser‐ and staple‐treated groups, respectively. Postoperative infection was not reported.
Cost‐effectiveness
No data on cost‐effectiveness were reported.
Buttressed versus non‐buttressed stapling devices (Comparison 3)
One study reported data for this comparison (Stammberger 2000).
Mortality
Two participants in the control group died on the third day after surgery, but neither of these deaths was related to the surgical technique.
Quality of life
No data on quality of life were reported.
Lung function outcomes
We noted no significant differences between treatment groups for TLC, RV or FEV1 % of predicted.
Exercise capacity
Investigators reported no data on exercise capacity.
Hospital utilisation
We noted no significant differences in hospital stay between buttressed and non‐buttressed treatment groups (12.7 vs 15.7 days, respectively; P = 0.14).
Adverse events
A higher percentage of participants in the non‐buttressed group (77%) versus the buttressed group (39%) had persistent air leaks (P < 0.001). Air leak duration (P = 0.002) and drainage time (P = 0.045) favoured the buttressed group. Researchers reported no significant differences between treatment groups in the number of participants with pneumothorax (five vs seven in treatment and control groups, respectively). Three and four participants, respectively, in the buttressed and control groups had to undergo reoperation following these leaks.
Cost‐effectiveness
Investigators provided no data on cost‐effectiveness.
Non‐awake resectional LVRS versus awake non‐resectional LVRS (Comparison 4)
Mortality
Pompeo 2012 indicated that one operative death occurred in the traditional LVRS condition versus none in the non‐resectional LVRS condition; this finding was non‐significant (P = 1.0). No differences in survival at 36 months were determined via Kaplan‐Meier curves (P = 0.5).
Quality of life
Pompeo 2012 reported that the physical functioning subscale of the SF‐36 showed no difference between traditional LVRS and the non‐resectional condition at 24‐month follow‐up. Scores on the SF‐36 were significantly improved for both groups from baseline to end of follow‐up (P < 0.0009).
Exercise capacity
Investigators reported no overall difference in exercise capacity between LVRS and the non‐resectional condition (P = 0.17), but exercise capacity was increased for both groups compared with baseline (P < 0.0009).
Lung function parameters
At six months, median change from baseline in FEV1 was 0.29 L in the traditional LVRS group (P < 0.00001) compared with 0.28 L (P < 0.00001) in the non‐resectional group (between‐group difference; P = 0.81). At 24 months, 63% of participants in the LVRS group and 54% in the non‐resectional group had a change from baseline FEV1 greater than 0.1 L; this finding did not differ significantly between groups (P = 0.48). Study authors described no significant differences between groups at 24‐month follow‐up (P = 0.55). Both RV and TLC were significantly improved at 24 months compared with baseline for the traditional LVRS group and the non‐resectional group (P = 0.0009), but investigators reported no significant differences from baseline in PaO2 or PaCO2 for either condition at the end of follow‐up.
Adverse events
Participants in the traditional LVRS group had a significantly greater number of adverse events (16 participants) compared with those in the awake group (seven participants; P = 0.019). Adverse events included air leaks (six in the non‐resectional group vs 15 in the traditional LVRS group), atrial fibrillations (one in the non‐resectional group vs three in the traditional group) and pneumonia (one case in the traditional LVRS group). Researchers noted no differences between the two groups in the need for contralateral treatment in the case of deterioration.
Hospital utilisation
Median stay was shorter in the non‐resectional group (median 6.0 days) than in the traditional LVRS group (median 7.5 days; P = 0.04). Furthermore, more participants in the non‐resectional group than in the traditional LVRS group were discharged earlier (66% vs 32%; P = 0.01). Time spent in the recovery room was less in the non‐resectional group than in the traditional LVRS group, 93 min (SD = 43) versus 228 min (SD = 68), P < 0.0001. None of the participants in either group required intensive care unit admission.
Cost‐effectiveness
Researchers did not report data on cost‐effectiveness.
Discussion
Summary of main results
This updated review identified two new studies and found extra citations for two previously included studies, leading to changes to the conclusions of this review. Short‐term mortality was overall higher for lung volume reduction surgery (LVRS) than for control, but long‐term mortality favoured LVRS. Participants identified post hoc as having high risk of early death from surgery were those with particularly impaired lung function and poor diffusing capacity and/or homogenous emphysema, but these participants did not show higher mortality at the end of follow‐up (i.e. initial higher mortality was offset by later lower mortality). Participants with upper lobe‐predominant emphysema and low exercise capacity benefited the most from LVRS, as they showed no increased short‐term mortality and more favourable long‐term mortality. Improvements in lung function, quality of life and exercise capacity were more likely with LVRS than with usual follow‐up. Although LVRS leads to an overall increase in quality‐adjusted life‐years (QALYs), the procedure is relatively costly overall.
The findings in terms of mortality in the identified subgroups merit consideration, as statistical significance for any one of them may be a function of the numerous categorisations (six in total) of study participants, rather than reflecting a true difference predicted by participant disposition. These subgroups furthermore were tested in only one trial, although the Geddes 2000 and Hillerdal 2005 trials revised entry criteria similarly to the high risk subgroup identified in NETT 2003 after determining higher risk of early mortality. It is unlikely that additional studies of similar statistical power to NETT 2003 will be conducted, making it difficult to confirm that these are valid distinctions to make in deciding which patients stand to gain the most benefit, and which are at greatest risk of postoperative death.
Additional outcomes of clinical importance, including quality of life, exercise capacity and lung function, overall favoured LVRS. Statistically significant differences in quality of life scores favouring LVRS at the end of follow‐up indicated that improvements persisted over a long time. The decrease of 13.6 units for NETT 2003 and 14.7 for Hillerdal 2005 on the St George's Respiratory Questionnaire (SGRQ) is clearly in excess of the minimum clinically important difference (a reduction of 4 points) for this questionnaire (Jones 2005; Welling 2015). The censored nature of available continuous outcome data means however that the observed difference in favour of surgically treated patients may overestimate the true effect. Dichotomised data from NETT 2003 incorporated data from all randomised participants, indicating that participants treated by LVRS were more likely to experience clinically important improvements in SGRQ scores, exercise capacity and lung function than those treated with usual medical care. Although differences for these endpoints favoured surgery throughout the study, the number of participants with improvements in these variables in the LVRS groups was reduced over time as a result of death and/or withdrawal. Given the progressive nature of chronic obstructive pulmonary disease (COPD), the decline in health status and lung function and the poor survival observed in long‐term pharmacotherapy studies (Bale 2008; Burge 2000), this could imply that surgery mitigates deterioration in the health of these patients in the long run.
Current information on exacerbations and hospitalisations is available via resource utilisation as a measurement of the cost of treatment in NETT 2003. These data suggest no difference in the mean number of emergency visits between groups at 12, 24 and 36 months. Optimised medical therapy administered to both LVRS and control groups during study monitoring may have reduced the potential for LVRS to modify this endpoint. Furthermore, the number of exacerbations and time until first exacerbation are reduced after LVRS. Cost analysis undertaken in NETT 2003 indicates that LVRS was associated with high costs at 12 months of follow‐up (by an average of around 45,000 USD for both direct (insurer related) and total (pertaining to all costs including those of carers and individuals undergoing treatment) medical costs). By three years, the total costs of LVRS were significantly higher than those incurred by participants given medical therapy. Calculation of QALYs in CLVR 2005 and NETT 2003 revealed that LVRS is a relatively costly procedure, as the procedure costs more than the standard $100.000 dollars per QALY gained threshold at end of follow‐up. Projections up to 10 years however paint a more favourable cost benefit, with projections as low as $54,000 USD for the overall population and $48,000 USD for the group of high responders. Given that costs should be weighed against benefit and harm, the judgement of whether this intervention is indeed cost‐effective should be made against two considerations: first, that the effects of surgery may be overestimated because of the absence of a placebo arm in these studies; and conversely, that favourable effects sustained throughout the study could be expected to have outlasted a placebo effect.
The relative merits of two of the most commonly employed surgical techniques (video‐assisted thoracoscopic surgery (VATS) and median sternotomy (MS)) have been assessed as a randomised comparison within one of the studies. In most trials, the decision to perform one technique over the other was left to the discretion of the attending surgeon. In a small substudy (N = 148), randomisation between MS and VATS occurred at a small number of the study centres in NETT 2003. These findings indicate that air leak and 30 day mortality rates were similar between the two randomised groups (P = 0.08 and 0.39, respectively). Although investigators noted no significant differences between groups in terms of within‐hospital costs, they reported a significant difference in total medical costs of treatment in favour of VATS. However, this difference represents only a snapshot of the total costs of treatment in this study, and the longer‐term costs of surgery appeared to fluctuate in relation to those of usual medical care.
The two studies comparing laser versus stapling (McKenna 1996) and buttressing versus use of a non‐buttressed staple line (Stammberger 2000) provide some evidence for the use of specific resection techniques. Using a stapler rather than a neodymium: yttrium‐aluminium‐garnet (Nd: YAG) laser led to better quality of life (QoL), less oxygen use after the procedure and better improvement in forced expiratory volume in one second (FEV1). Butrressing the staple line led to fewer air leaks and shorter air leak duration and drainage time compared with control.
Finally, the Pompeo 2012 trial added further evidence for the favourable results of traditional LVRS, with favourable results for quality of life, lung function outcomes and exercise capacity, and showed that traditional LVRS was comparable with a unique innovative awake non‐resectional method for performing LVRS, without leading to higher risk of death.
Overall completeness and applicability of evidence
Studies recruited highly selected patient populations (on average 29% of the screening population), and this statistic bears testimony to the fact that people with a particular type of emphysema are considered most likely to benefit and therefore are the most operable (Yusen 2003; Zoumot 2014). This is supported by trials assessing patient eligibility. Akuthota 2012 conducted an analysis on a COPD patient database and found that up to 15% of the patient population could be eligible for LVRS on the basis of NETT 2003 criteria, highlighting that LVRS is an option only for a subgroup of emphysematous patients.
Pulmonary rehabilitation adds an additional layer of selection and introduces an important aspect of presurgical and post surgical management. Benefits of respiratory rehabilitation in COPD include reduced daily functional impairment as measured by health‐related quality of life and exercise capacity (McCarthy 2015). Although these effects are consistently superior to 'usual follow‐up' in the context of randomised studies, the take‐up of LVRS may be hindered in part by the large numbers of patients who do not complete pulmonary rehabilitation following initial referral (Cockram 2006; Garrod 2006). In the context of LVRS, pulmonary rehabilitation may play an important role in establishing optimal care before a decision is made on whether to proceed with surgery, especially if the outcome of rehabilitation is delay in or even rejection of surgery. Subsequent to the procedure, it may be advantageous that in the phase of postsurgical recovery, a routine of sustained physical activity and self‐management is established. The study design for most of the trials in this review incorporated pulmonary rehabilitation programmes in both intervention and control groups; therefore, the significant impact of surgery on exercise capacity at long‐term follow‐up may reflect a favourable effect of LVRS in improving exercise tolerance. Although the enhanced lifestyle and exercise intervention may have limited the generalisability of review findings, this review, may in fact be an accurate reflection of the rigorous screening procedures recommended in guidance for LVRS (NICE 2005; NICE 2010).
Limited data from studies comparing the different available surgical techniques may also hinder the provision of clear guidance as to the risks and benefits of particular procedures. NETT 2003 provided a non‐randomised comparison between VATS and MS, indicating some advantages of VATS over MS; Stammberger 2000 showed that buttressing the staple line has advantages over non‐stapling; and McKenna 1996 indicated that stapling was superior to laser ablation, but overall the evidence comparing different methods is not strong.
Quality of the evidence
This review is based on a total of 11 trials that have contributed data on a range of clinically relevant endpoints, including postoperative mortality, determination of whether the risk carried by this intervention is sufficient to justify withholding its use and information on well‐defined patients who can derive benefit from surgery. Evidence from NETT 2003 dominates this review and has largely influenced the wider debate about whether the cost of LVRS is justified in terms of the health benefit the intervention confers weighed against the potential harm it poses to those who opt to undergo the procedure. Although the other trials are substantially smaller, their results further strengthen the evidence provided by NETT 2003 and add valuable information on a large number of important endpoints. Specifically, the long‐term survival data provided by CLVR 2005 strengthen conclusions regarding the mortality risk associated with LVRS.
The quality of evidence provided in this review ranges from low to moderate (see Table 1 for an overview). We graded data on early and long‐term mortality as moderate because of the overall high rates of unclear and high risk of bias, and thus risk of methodological flaws, found in all trials. Specific focus on mortality data for subgroups identified in NETT 2003 reveals that the evidence is of low quality because of the small number of participants included in each subgroup.
We rated the quality of evidence provided for quality of life, measured via the SGRQ, as moderate, and the quality of evidence on exercise capacity, specifically measured via the six‐minute walking distance (6MWD), as low. We downgraded evidence on quality of life, as the SGRQ is a subjective measure and can thus be subject to performance and detection bias in unblinded trials. We downgraded the quality of evidence for 6MWD as a result of the small participant number (and thus resulting imprecision), and the fact that the 6MWD is effort‐dependent. Similarly, we downgraded the quality of evidence for FEV1, as this is an effort‐dependent measure, and only a small participant number was available for this outcome. We rated the quality of evidence for remaining lung function parameters ‐ RV and TLC ‐ as low because meta‐analysis of the small participant number was possible for this outcome, and because overall rates of unclear and high risk of bias were high in all trials.
Potential biases in the review process
This review has several limitations that are important to address. First is the issue of subgroup analysis. Provision of LVRS will likely be limited to those participants who do not fall into the high risk categorisation of the NETT 2003. We were unable to test the validity of this definition across trials because of the absence of stratified data in the remaining trials (although Geddes 2000 and Hillerdal 2005 seemed to report similar findings). Additional work in those who have shown a survival benefit (i.e. those with predominantly upper lobe emphysema and low exercise capacity from NETT 2003) would help to confirm this finding. Studies using eligibility criteria similar to NETT 2003, which have been conducted more recently (Clark 2014; Ginsburg 2011), however, provide evidence to support the beneficial effects of LVRS for the subgroups identified by NETT.
Second is the issue of censored data. Meta‐analysis of variables likely to feature in a clinical setting such as exercise capacity and lung function should ideally reflect the metrics on which they would be measured on a day‐to‐day basis (i.e. % predicted, L/min, metres). However, the primary data available for NETT 2003 are reported as dichotomised endpoints (i.e. the number of participants achieving an increase in FEV1 and exercise capacity). A balance is needed between adequate adjustment for missing data and analysis and reporting of data in a clinically meaningful way.
Agreements and disagreements with other studies or reviews
Results of the randomised controlled trials (RCTs) included in this review are largely in line with the results of early prospective studies indicating favourable results of LVRS in selected patients (Ciccone 2003; Daniel 1996; Keenan 1996; Kotloff 1996; Little 1995; Martinez 1997; O'Brien 1999; Pompeo 2000; Sciurba 1996; Tan 2000; Teschler 1996; Wakabayashi 1995). None of these studies, however, matched the power and rigorousness obtained in the NETT 2003 trial and the other RCTs featured in this review (with the notable exception of the RCT by Pompeo 2000, which was excluded from this review, as it included patients with bullous emphysema).
More recent trials have found similar improvements (e.g. functional outcomes), as reported in this review, complemented by improved survival. Ginsburg 2011, using the entry criteria defined by NETT 2003, found no operative or 90 day mortality and a 0.95 probability of survival at three years. Clark 2014 reported on a range of functional outcomes showing clear improvement after both bilateral and unilateral LVRS, and showed no additional mortality in patients treated unilaterally (as opposed to bilaterally, with a 90 day mortality rate of 21.7%).
Other reviews on the topic of LVRS (Huang 2011; Pompeo 2014; Zahid 2011) have come to the same conclusion as was reached for the specific outcomes measured in this review. Huang 2011 furthermore included the Moser 2008 (fibrin sealant vs no sealant) and Rathinam 2009 (BioGlue vs buttressing of staple line) studies looking at different sealants, and concluded that fibrin sealant reduces air leakage and chest tube drainage duration and that results for BioGlue are comparable with those reported for buttressing of the staple line.
Authors' conclusions
Implications for practice.
Evidence available to‐date indicates that LVRS can lead to improved health outcomes and improvement in disease status (e.g. reduction in frequency of exacerbations) for selected patients with severe emphysema. Specifically, patients must have completed a course of pulmonary rehabilitation and must have had their candidacy for surgery established through high‐resolution computed tomography if disease severity and distribution of emphysema are to be determined (DeCamp 2008). Patient selection ideally involves a multi‐disciplinary team consisting of respiratory physicians, radiologists and surgeons (Rathinam 2014). NETT 2003 (supported in part by Geddes 2000 and Hillerdal 2005) findings reveal that the subgroups of participants identified as being at high risk of death from surgery are those with particularly impaired lung function and poor diffusing capacity and/or homogeneous emphysema. Evidence furthermore points out that LVRS seems particularly effective for patients with heterogeneous upper lobe‐predominant emphysema (and low baseline exercise capacity).
This review has highlighted that lung volume reduction surgery confers risk of early‐stage postoperative death, but the degree to which patients are at risk could be predicted by specific characteristics. Guidance on the estimate of differential risk is drawn from a post hoc analysis performed in one large study conducted in the USA (NETT 2003). In this trial, long‐term follow‐up of the high risk participants suggested that the initial increase in the odds of death did not remain significant and crossed after 4.3 years ‐ a finding further supported by a Canadian trial (CLVR 2005).
The results presented in this review are not new (most trials were completed over 10 years ago), but remaining therapeutic nihilism for this specific set of vulnerable patients (Zoumot 2014) is thought to be due to misconceptions about the safety of the procedure (McNulty 2014). More recent observational research (Clark 2014; Ginsburg 2011) suggests that improved patient selection and maturity of procedures performed in highly specialised centres may have led to a substantially lower risk of death over time. This suggestion holds promise that the positive health outcomes of LVRS may be even further improved compared with the results presented in this review.
Implications for research.
Additional work in this area should consider the effects of LVRS on hospitalisation, and the requirement for oral steroid and antibiotic therapy in the management of exacerbations of COPD, and should seek to determine whether LVRS slows this rate of decline in health, as measured by lung function, quality of life and frequency of hospital admission. Furthermore, it would be interesting to see more centres that routinely perform LVRS publish mortality and morbidity data for their patients, in accordance with the example of Clark 2014 and Ginsburg 2011, preferably via the use of controlled studies, thereby further strengthening the evidence for the potential effectiveness of LVRS in selected patients.
Although the palliative effects of LVRS have been shown, uptake of this procedure is limited because of the costs involved and perceived mortality (Lenfant 2006; McNulty 2014). This fact, among others, has pushed towards the development of techniques that can help to deliver the benefits of LVRS without the risk of death and costs associated with it. Recent advantages in the field of non‐surgical bronchoscopic techniques have sparked hope for patients with emphysematous destruction of lung tissue who are unresponsive to medical therapy, do not meet the strict criteria for LVRS or do not wish to undergo surgery (Ingenito 2008). Non‐surgical techniques and interventions used to perform bronchoscopic lung volume reduction (BLVR) are distinct but aim to achieve the same result: increasing the mechanical efficiency of the lung, thereby improving the health status of the patient (Fessler 2008; Maxfield 2004). By aiming to achieve similar results as those witnessed in LVRS but without the potential morbidity, mortality and costs involved, these treatments may prove to be a valuable addition to or substitute for LVRS in the treatment of patients with severe emphysema. Several studies have been done or are currently under way on an array of techniques, including one‐way valves (Davey 2015; Ninane 2012; Sciurba 2010; Toma 2003; Venuta 2005), endobronchial coils (Deslée 2016; Klooster 2014; Klooster 2014a; Shah 2013; Zoumot 2015), airway stents (Choong 2006; Higuchi 2006; Shah 2011), sealants (Come 2015; Criner 2009; Refaely 2010) and vapour ablation (Herth 2016); these techniques may open up a new frontier in the treatment of patients with severe emphysema. Future research should compare the effectiveness of these new BLVR techniques versus LVRS in the treatment of severe emphysema, as, for instance, is planned for the CELEB study.
What's new
| Date | Event | Description |
|---|---|---|
| 14 April 2016 | New citation required and conclusions have changed | Newly available data on long‐term survival from CLVR and NETT and the inclusion of 2 new studies (Clarenbach 2015; Pompeo 2012) have led to changes in the conclusions provided in this updated review that further confirm the already reasonably positive findings of the original review. |
| 14 April 2016 | New search has been performed | The literature search was updated: 21 references were added to the NETT study. The previous review included the OBEST and CLVR studies as a single study (Miller 2005). However, review authors used only CLVR preliminary data. As the trial has been completed, we have separated the 2 individual studies in this version of the review and have incorporated additional data from CLVR (2 citations). |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 4 July 2008 | Amended | We have converted this review to new review format. |
| 1 September 2006 | New search has been performed | A new search run in September 2006 revealed 7 references to studies already included in the review. |
| 8 August 2006 | New citation required and conclusions have changed | This review was updated with the addition of 7 new studies (Criner 1999; Geddes 2000; Goldstein 2003; Hillerdal 2005; Miller 2005; NETT 2003; Stammberger 2000). Most randomised participants were drawn from 1 large multi‐centre trial. Altered review conclusions stated that LVRS conferred benefit in the long term in terms of exercise capacity and quality of life in surviving patients, but that in the short term, risk of death within 3 months of surgery was increased. By the end of follow ‐up, risk of death was not significantly different. The subgroup of participants at greatest risk of postsurgical death had particularly low lung capacity, and risk of death in these patients was significantly higher at all time points. |
Acknowledgements
We are grateful to the Information Specialist of the Cochrane Airways Group editorial base (Liz Stovold) for ongoing support and expertise in information science. We are grateful to the Cochrane Airways Group of Australia, and to the 'Nederlands Astma Fonds' for providing grants to assist with completion of the previous version of this review.
Chris Cates was the Editor for this review and commented critically on the review.
The Background and Methods sections of this review are based on a standard template used by the Cochrane Airways Group.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (The Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Appendix 2. Search strategy to identify relevant trials from the CAGR
#1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All
#2 MeSH DESCRIPTOR Bronchitis, Chronic
#3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)
#4 COPD:MISC1
#5 (COPD OR COAD OR COBD):TI,AB,KW
#6 emphysem*
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 surgery
#9 "lung volume reduction"
#10 "lung reduction"
#11 "volume reduction"
#12 LVRS
#13 LVR
#14 "reduction pneumoplasty"
#15 pneumonectomy
#16 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15
#17 #7 and #16
[In search line #4, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, COPD]
Data and analyses
Comparison 1. Surgery versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early mortality (90 days) | 5 | 1489 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.16 [3.22, 11.79] |
| 2 Long‐term mortality (> 36 months) | 2 | 1280 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.61, 0.95] |
| 3 Overall mortality (stratified by follow‐up period) | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 3 months | 5 | 1489 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.16 [3.22, 11.79] |
| 3.2 6 months | 3 | 209 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.36 [1.19, 15.91] |
| 3.3 12 months | 3 | 209 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.60 [1.26, 10.29] |
| 3.4 24 months | 3 | 1328 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.78, 1.28] |
| 3.5 3 years or more | 2 | 1280 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.61, 0.95] |
| 4 Overall mortality (stratified by risk, to end of follow‐up) | 1 | 1218 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.75, 1.27] |
| 4.1 High risk | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [1.02, 3.92] |
| 4.2 Non‐high risk | 1 | 1078 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.64, 1.14] |
| 5 Overall mortality (stratified by subgroup, to end of follow‐up) | 1 | 1078 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.62, 1.11] |
| 5.1 Upper lobe ‐ low exercise capacity | 1 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.26, 0.78] |
| 5.2 Upper lobe ‐ high exercise capacity | 1 | 419 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.53, 1.46] |
| 5.3 Non‐upper Lobe ‐ High exercise capacity | 1 | 149 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.38, 1.47] |
| 5.4 Non‐upper Lobe ‐ Low exercise capacity | 1 | 220 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.12, 4.64] |
| 6 Change in SGRQ (end of follow‐up) | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 6.1 Symptoms | 1 | Mean Difference (Fixed, 95% CI) | ‐8.8 [‐17.57, ‐0.03] | |
| 6.2 Activity | 1 | Mean Difference (Fixed, 95% CI) | ‐17.1 [‐22.65, ‐11.55] | |
| 6.3 Impacts | 1 | Mean Difference (Fixed, 95% CI) | ‐14.6 [‐20.05, ‐9.15] | |
| 6.4 Total | 2 | Mean Difference (Fixed, 95% CI) | ‐13.78 [‐15.75, ‐11.80] | |
| 7 Change in SGRQ (total score, stratified by follow‐up period) | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 7.1 6 months | 2 | Mean Difference (Fixed, 95% CI) | ‐13.48 [‐15.13, ‐11.84] | |
| 7.2 12 months | 2 | Mean Difference (Fixed, 95% CI) | ‐13.77 [‐15.75, ‐11.80] | |
| 8 SGRQ responders (stratified by risk, to end of follow‐up) | 1 | 749 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.21 [3.43, 7.92] |
| 8.1 High risk | 1 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.01 [0.66, 218.88] |
| 8.2 Non‐high risk | 1 | 643 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.06 [3.31, 7.72] |
| 9 SGRQ responders (stratified by subgroup, to end of follow‐up) | 1 | 643 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.14 [3.37, 7.83] |
| 9.1 Upper Lobe ‐ Low exercise capacity | 1 | 176 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.38 [3.73, 18.85] |
| 9.2 Upper Lobe ‐ High exercise capacity | 1 | 253 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.67 [2.95, 10.88] |
| 9.3 Non‐upper Lobe ‐ High exercise capacity | 1 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.35 [1.98, 27.29] |
| 9.4 Non‐upper Lobe ‐ Low exercise capacity | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.48, 3.81] |
| 10 Difference on SF‐36 (end of follow‐up) | 3 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 10.1 Physical functioning | 2 | Mean Difference (Fixed, 95% CI) | 18.82 [12.61, 25.03] | |
| 10.2 Role Physical | 2 | Mean Difference (Fixed, 95% CI) | 21.52 [8.96, 34.09] | |
| 10.3 Bodily pain | 2 | Mean Difference (Fixed, 95% CI) | 9.92 [3.06, 16.78] | |
| 10.4 General Health | 3 | Mean Difference (Fixed, 95% CI) | 12.01 [6.56, 17.46] | |
| 10.5 Vitality | 2 | Mean Difference (Fixed, 95% CI) | 10.46 [2.69, 18.24] | |
| 10.6 Social functioning | 2 | Mean Difference (Fixed, 95% CI) | 15.77 [6.21, 25.33] | |
| 10.7 Role emotional | 2 | Mean Difference (Fixed, 95% CI) | 10.07 [‐2.15, 22.28] | |
| 10.8 Mental health | 2 | Mean Difference (Fixed, 95% CI) | 12.55 [5.76, 19.35] | |
| 11 Walking Distance (Mtrs, end of follow‐up) | 5 | 215 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.42, 0.98] |
| 12 FEV1 (L, end of follow‐up) | 4 | 188 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.13, 0.28] |
| 13 RV (% predicted, end of follow‐up) | 4 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐44.28 [‐57.80, ‐30.75] |
| 14 Mean number of emergency‐room visits | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 0‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 13‐24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 25‐36 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 PA02 (mm Hg, end of follow‐up) | 2 | 97 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.30, 0.50] |
| 16 PAC02 (mm Hg, end of follow‐up) | 3 | 132 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.78, ‐0.08] |
| 17 TLC (% predicted, end of follow‐up) | 4 | 178 | Mean Difference (IV, Fixed, 95% CI) | ‐14.83 [‐20.50, ‐9.15] |
| 18 Mean direct medical costs and total healthcare‐related costs according to time after randomisation (USD 000s) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18.1 Direct Medical Costs 0‐12 Months after randomisation (USD 000s) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Total Costs 0‐12 months after randomisation (USD 000s) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.3 Direct medical costs 13‐24 months after randomisation (USD 000s) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.4 Total costs 13‐24 months after randomisation (USD 000s) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.5 Direct medical costs 25‐36 months after randomisation (USD 000s) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.6 Total costs 25‐36 months after randomisation (USD 000s) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
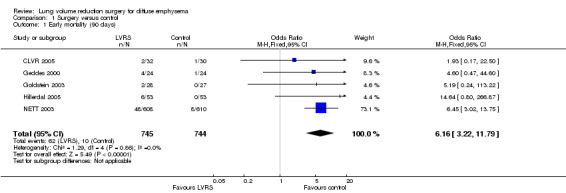
Comparison 1 Surgery versus control, Outcome 1 Early mortality (90 days).
1.2. Analysis.
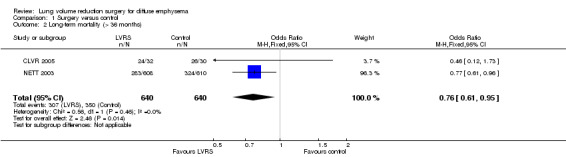
Comparison 1 Surgery versus control, Outcome 2 Long‐term mortality (> 36 months).
1.3. Analysis.
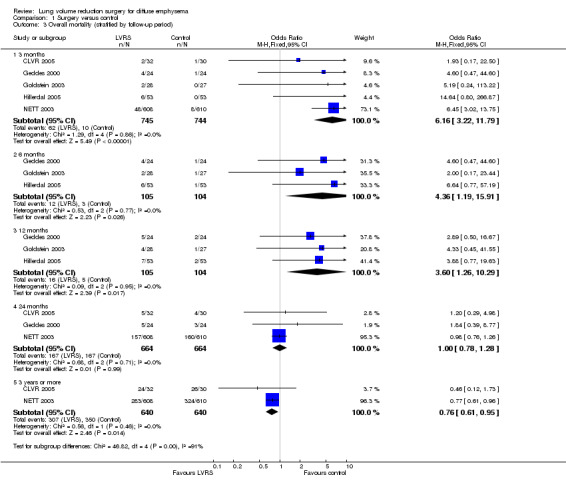
Comparison 1 Surgery versus control, Outcome 3 Overall mortality (stratified by follow‐up period).
1.4. Analysis.
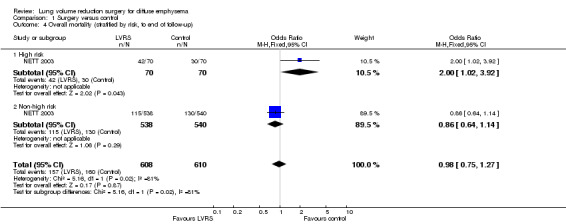
Comparison 1 Surgery versus control, Outcome 4 Overall mortality (stratified by risk, to end of follow‐up).
1.5. Analysis.
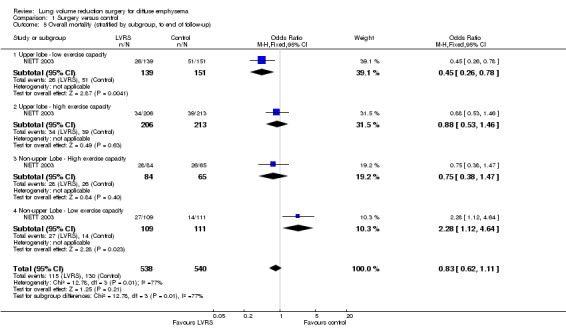
Comparison 1 Surgery versus control, Outcome 5 Overall mortality (stratified by subgroup, to end of follow‐up).
1.6. Analysis.
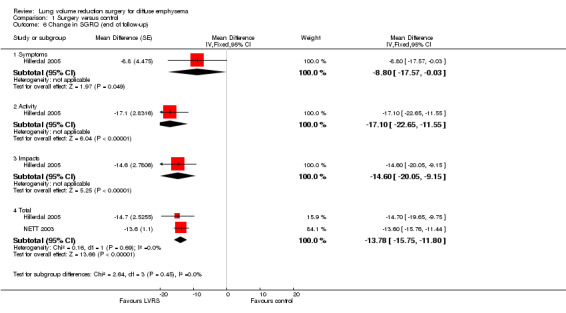
Comparison 1 Surgery versus control, Outcome 6 Change in SGRQ (end of follow‐up).
1.7. Analysis.
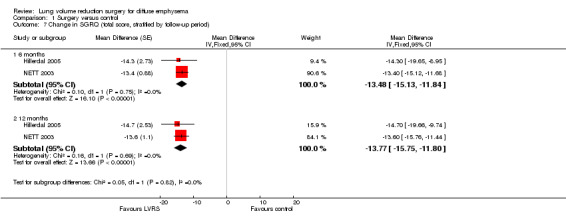
Comparison 1 Surgery versus control, Outcome 7 Change in SGRQ (total score, stratified by follow‐up period).
1.8. Analysis.
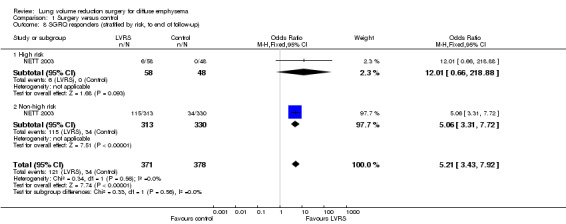
Comparison 1 Surgery versus control, Outcome 8 SGRQ responders (stratified by risk, to end of follow‐up).
1.9. Analysis.
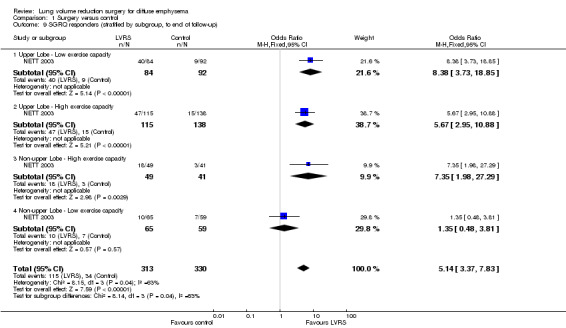
Comparison 1 Surgery versus control, Outcome 9 SGRQ responders (stratified by subgroup, to end of follow‐up).
1.10. Analysis.
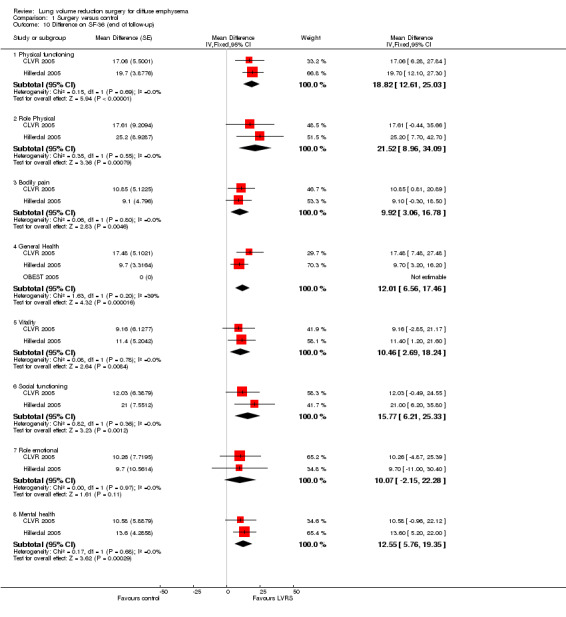
Comparison 1 Surgery versus control, Outcome 10 Difference on SF‐36 (end of follow‐up).
1.11. Analysis.
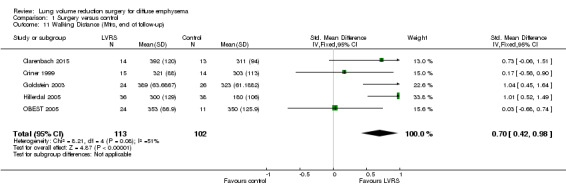
Comparison 1 Surgery versus control, Outcome 11 Walking Distance (Mtrs, end of follow‐up).
1.12. Analysis.
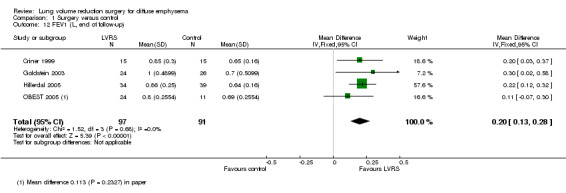
Comparison 1 Surgery versus control, Outcome 12 FEV1 (L, end of follow‐up).
1.13. Analysis.
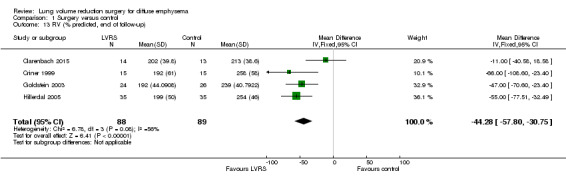
Comparison 1 Surgery versus control, Outcome 13 RV (% predicted, end of follow‐up).
1.14. Analysis.
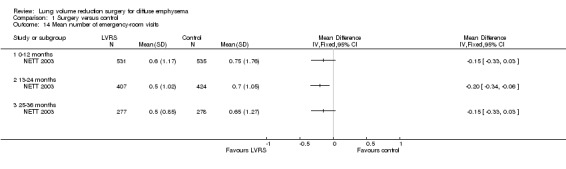
Comparison 1 Surgery versus control, Outcome 14 Mean number of emergency‐room visits.
1.15. Analysis.
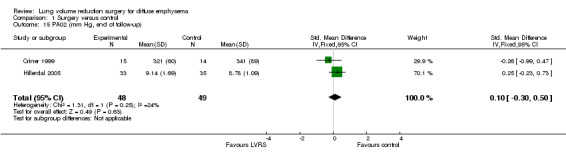
Comparison 1 Surgery versus control, Outcome 15 PA02 (mm Hg, end of follow‐up).
1.16. Analysis.
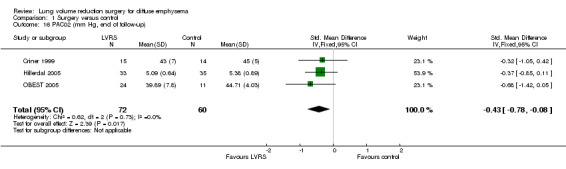
Comparison 1 Surgery versus control, Outcome 16 PAC02 (mm Hg, end of follow‐up).
1.17. Analysis.
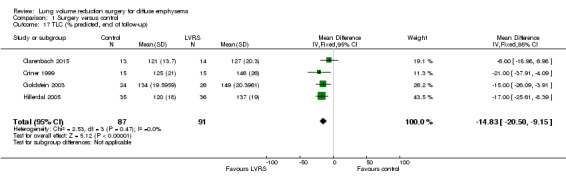
Comparison 1 Surgery versus control, Outcome 17 TLC (% predicted, end of follow‐up).
1.18. Analysis.
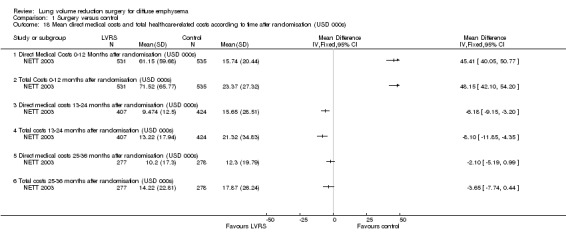
Comparison 1 Surgery versus control, Outcome 18 Mean direct medical costs and total healthcare‐related costs according to time after randomisation (USD 000s).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Clarenbach 2015.
| Methods | Randomised controlled trial Method of randomisation: not described Allocation concealment: performed via opaque envelopes Outcome assessor blinding: not described Withdrawals/Dropouts: fully accounted for | |
| Participants | Screened: 40
Randomised: 30 Completed: 27 (LVRS 14; control 13) Mean age in years: 63 Diagnosis: CT scan Emphysema: heterogeneous and homogenous Diseases included: not stated Entry criteria (similar to NETT): FEV1 ≤ 45% predicted (≥ 15% predicted among participants ≥ 70 years of age); TLC ≥ 100% predicted; RV ≥ 150% predicted; partial pressure of resting arterial carbon dioxide ≤ 60 mmHg; resting partial pressure of arterial oxygen ≥ 45 mm Hg; ability to walk ≥ 140 metres (m) in 6 minutes; ability to complete 3 minutes on bicycle ergometer; abstinence from smoking for 6 months before randomisation Exclusion criteria: concurrent medical conditions precluding surgery or that might interrupt follow‐up Baseline QoL: not stated 6‐minute walk, metres: 326 for LVRS vs 287 for control FEV1 in % predicted (SD): 27.8 (7.2) for LVRS vs 26.2 (5.9) for control RV: not stated TLC in % predicted (SD): 124.5 (9.1) for LVRS vs 137.2 (19.8) for control PaO2: not stated PaCO2: not stated DLCO median % predicted (IQR): 35 (27 to 39) for LVRS vs 33 (31 to 38) for control |
|
| Interventions | CT scan and perfusion scintigraphy were used to determine the target area, after which surgery was conducted via VATS. For participants with homogenous emphysema, LVRS was performed in the upper lobes. | |
| Outcomes | ‐ Assessment of endothelial function by FMD ‐ Determination of systemic inflammation ‐ Blood pressure and heart rate ‐ Daily physical activity and physical activity level ‐ Exercise capacity ‐ Lung function values including FEV1, FVC, RV, DLCO % predicted |
|
| Notes | Support was received from "Lunge Zurich." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was mentioned, but methods were not described. |
| Allocation concealment (selection bias) | Low risk | "Allocation concealment was performed by the use of sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | LVRS does not permit blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All measurements were analyzed by one examiner, who was blinded to the randomization protocol (M.K.)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was published, but trial was registered at Clinicaltrials.gov: NCT 01020344. Data analysis was performed on a per‐protocol basis. |
| Other bias | Low risk | No evidence of contamination was found other than that reported above, but post hoc analysis was performed to adjust for imbalance in baseline characteristics. |
CLVR 2005.
| Methods | Randomised clinical trial Method of randomisation: not described Allocation concealment: conducted off‐site at a data co‐ordinating centre Outcome assessor blinding: described Withdrawals/Dropouts: fully accounted for | |
| Participants | Screened: 467
Randomised: 62 (LVRS 32; control 30)
Completed: 59 (LVRS 29; control 30)
Mean age in years: 63.6
Diagnosis: CT and VP scan
Emphysema: heterogeneous and homogenous
Diseases included: not stated
Entry criteria: advanced emphysema, CRQ < 4, < 80 years, 15% to 40 FEV1 % predicted, FEV1 response to bronchodilator < 30% predicted and 300 mL, TLC and RV > 120% predicted, RV > 200% predicted, RV/TLC ratio % predicted 60%, PaCO2 < 55 mmHg, 17 to 32 BMI, compliance with rehabilitation Exclusion criteria: mechanical ventilation, antitrypsin deficiency, bullous emphysema, bronchiectasis, obliterated pleural space, pulmonary node, prednisolone > 10 mg, hypertension > 30 mmHg, life expectancy < 1 year, registered for lung transplant Baseline SF‐36 utility score (SD): 0.648 (0.110) for LVRS vs 0.622 (0.128) for control * CRQ: 3.42 (0.98) for LVRS vs 3.37 (0.79) for control * 6‐minute walk, metres: 340 for LVRS vs 319 for control FEV1 in litres (% predicted): 0.73 (25) for LVRS vs 0.65 (23) for control RV in litres: 5.4 for LVRS vs 5.37 for control TLC in litres (% predicted): 8.2 (136) for LVRS vs 7.78 (138) for control PaO2 in mmHg: 687.38 for LVRS vs 65.93 for control * PaCO2 in mmHg: 45.93 for LVRS vs 45.46 for control * DLCO in mL/min/mmHg (% predicted): 8.18 (31) for LVRS vs 8.92 (35) for control * Conditional data obtained from Miller 2005 paper. Final values for complete cohort in CLVR study not reported |
|
| Interventions | LVRS via median sternotomy vs usual medical care Optimal care standardised (including PR, bronchodilators, vaccination, steroids and antibiotics) Pulmonary rehabilitation: 6 week course before randomisation (and continued for the duration of the study in both groups) Participants followed up for 2 years post randomisation |
|
| Outcomes | Difference QALYs by HUI, morbidity; lung function; quality of life (SF‐36, CRDQ); 6MWD | |
| Notes | "The Canadian Institute of Health Research (CIHR), MT‐14386, funded the Canadian Lung Volume Reduction Surgery Study trial." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Surgeon stratification and blocking were performed at each centre (2 to 4 group blocks). |
| Allocation concealment (selection bias) | Unclear risk | Study authors reported that allocation was concealed, as it was performed at the data co‐ordinating centre, but did not describe methods. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | LVRS does not lend itself to blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study authors reported, “Every effort was made to blind those persons who were administering outcome measures tests to the allocation group of the study participants. However, as in all surgical clinical trials, blinding was difficult to ensure in all instances.” Furthermore, they added, “A trained individual who was blinded to the patient treatment allocation administered all measurement.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors reported attrition and percentages of participants with missing outcome data for SF‐36 and 6MWD. |
| Selective reporting (reporting bias) | Low risk | Prespecified protocol was not available for comparison, but pilot study was published. |
| Other bias | Low risk | Low ‐ No other bias was detected. |
Criner 1999.
| Methods | Prospective, randomised controlled trial. Allows cross‐over of participants from medical to surgical arm after they had completed evaluation after 3 additional months of medical therapy and rehabilitation. Method of randomisation: not described Allocation concealment: unclear Outcome assessor blinding: not described Withdrawals/Dropouts: fully accounted for | |
| Participants | Screened: 200
Randomised: 37 (LVRS 19; control 18) Completed: LVRS 19; control 18 Mean age in years: 59 Diagnosis: CT scan Emphysema: diffuse, heterogeneous, bullae < 5 cm Entry criteria: non‐smokers (≥ 6 months); symptomatic despite optimised medical therapy; NYHA Classes III and IV; evidence of airflow obstruction and hyperinflation by pulmonary function studies (i.e. FEV1 < 30% of predicted, postbronchodilator administration, FRC or TLC > 120% of predicted), hyperinflation documented by chest X‐ray and diffuse bullous emphysema documented by high‐resolution computed tomography (CT) scan, decreased or absent perfusion documented in planned resected lung tissue by quantitative perfusion lung scan Exclusion criteria: severe and refractory hypoxaemia (PaO2/FiO2 ratio < 150); severe hypercapnic respiratory failure requiring mechanical ventilation; presence of significant cardiovascular disease; presence of severe pulmonary hypertension (mean pulmonary artery pressure > 35 mmHg); severe debilitated state with total body weight < 70% of ideal body weight; presence of significant extrapulmonary end‐organ dysfunction expected to limit survival; psychosocial dysfunction; continued smoking Baseline QoL: not reported (SF‐36 was administered to participants but was not reported on) 6‐minute walk in metres (SD): 260 (92) for LVRS vs 273 (90) for control FEV1 in litres (% predicted): 0.69 (28) for LVRS vs 0.72 (29) for control RV in litres (% predicted): 4.9 (253) for LVRS vs 4.4 (230) for control TLC in litres (% predicted): 7.0 (140) for LVRS vs 6.8 (135) for control PaO2: not stated PaCO2 in mmHg: 46.50 for LVRS vs 46.40 for control DLCO in L/min/mmHg (SD): 1.97 (0.6) for LVRS vs 1.9 (0.66) for control |
|
| Interventions | LVRS via MS and bilateral stapling resection vs usual medical care (including pulmonary rehabilitation) Pulmonary rehabilitation: 8‐week programme with additional 3 months for participants randomised to control. PR had educational, physical, psychosocial supportive components. All participants had individualised programmes based on exercise test results. Outcomes were assessed after 8 weeks of outpatient pulmonary rehabilitation, and 3 months after additional pulmonary rehabilitation or LVRS. |
|
| Outcomes | Lung function (performed according to ATS guidelines); arterial blood gases; Sickness Impact Profile (SIP); mortality | |
| Notes | No funding source was stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no other information available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | LVRS does not lend itself to blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Methods of handling missing outcome data from questionnaires (if any) not described |
| Selective reporting (reporting bias) | High risk | No protocol available. SF‐36 mentioned but not reported on. |
| Other bias | Low risk | Potential risk of cross‐over effects, but results reported separately. Study authors note: "The crossover design of the study may potentially bias the results toward LVRS because patients on the rehabilitation arm may not be as motivated as patients on the LVRS arm given the often dramatic account of LVRS in the lay press." |
Geddes 2000.
| Methods | Randomised parallel‐group trial Method of randomisation: not described Allocation concealment: off‐site Outcome assessor blinding: not described Withdrawals/Dropouts: fully accounted for | |
| Participants | Screened: 174
Randomised: 48 (LVRS 24; control 24) Completed: 47 Median age in years: 61 Diagnosis: CT scan Emphysema: no restriction on pattern and distribution Entry criteria: CT‐confirmed severe emphysema; < 75 years; FEV1 > 500 mL; CS dose < 10 mg/d Exclusion criteria: O2 use > 18 hours/d; asthma; previous thoracic surgery or other serious medical conditions Baseline SF‐36 median in units (IQR): 50 (40 to 59) for LVRS vs 51 (48 to 56) for control Median shuttle walk in metres: 210 for LVRS vs 220 for control Median FEV1 in litres: 0.74 for LVRS vs 0.75 for control Median RV % predicted: 226 for LVRS vs 220 for control Median TLC % predicted: 136 for LVRS vs 129 for control Median PaO2 in mmHg: 74 for LVRS vs 70 for control Median PaCO2 in mmHg: 37 for LVRS vs 38 for control Median DLCO % predicted: 36 for LVRS vs 37 for control |
|
| Interventions | LVRS via median sternotomy or thoracoscopy vs continued medical care Continued medical care included rehabilitation and optimised drug therapy. Pulmonary rehabilitation: 6‐week programme consisting of physical, occupational health and nutritional education components Participants were telephoned to encourage them to adhere with the exercise programme. Outcome assessment took place at 3‐, 6‐ and 12‐monthly intervals. |
|
| Outcomes | Mortality; FEV1; FVC; TLC; RV; shuttle‐walking distance and quality of life; inspiratory and expiratory mouth pressures; arterial blood gas values | |
| Notes | Supported by research funding from the Royal Brompton Hospital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed by an ‘independent institute’, but specific method was not given. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | LVRS does not lend itself to blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Methods of handling missing outcome data from questionnaires (if any) not described |
| Selective reporting (reporting bias) | Unclear risk | Prespecified protocol not available for comparison |
| Other bias | Low risk | Low ‐ No other bias was detected. |
Goldstein 2003.
| Methods | Randomised controlled trial Method of randomisation: random numbers table, block randomisation in groups of 4 Allocation concealment: adequate Outcome assessor blinding: described Withdrawals/Dropouts: fully accounted for | |
| Participants | Screened: 328
Randomised: 55 (LVRS 28, control 27) Completed: 50 (LVRS 24, control 26) Mean age in years: 65 Diagnosis: CT or V/Q scan Emphysema: heterogeneous distribution Entry criteria: < 75 years; FEV1 < 40% predicted; TLC > 120% predicted; evidence of heterogenous emphysema on CT or V/Q scan Exclusion criteria: asthma; prior lung surgery; pleural disease; contraindications for surgery; inability to attend PR or follow‐up; pulmonary hypertension Baseline QoL: not reported (SF‐36 administered to participants, but not reported on) 6‐minute walk in metres (SE): 387 (15) for LVRS vs 372 (17) for control FEV1 in litres (% predicted): 0.8 (33) for LVRS vs 0.7 (32) for control RV in % predicted: 228 for LVRS vs 253 for control TLC in % predicted: 142 for LVRS vs 155 for control PaO2: not stated PaCO2: not stated DLCO: not stated |
|
| Interventions | LVRS via video‐assisted thoracic surgery (VATS) (or less often by median sternotomy at the discretion of the surgeon) vs ongoing medical treatment including pulmonary rehabilitation. A short course of pulmonary rehabilitation was offered to participants in the surgery group. Pulmonary rehabilitation: 6‐week programme with supervised physical exercise, educational and psychosocial components Outcomes assessed at 3, 6, 9 and 12 months after randomisation |
|
| Outcomes | Quality of life (measured by the CRDQ); 6‐minute walking distance, submaximal cycle endurance time; FEV1; FEV1/FVC; RV; FRC; TLC; mortality | |
| Notes | "This study was supported, in part, by the Physician's Services Incorporated Foundation (Ontario, Canada) and by West Park Healthcare Centre Foundation." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table, block randomisation in groups of 4 |
| Allocation concealment (selection bias) | Low risk | Study authors reported that allocation was concealed, with the physician and surgeon remaining unaware of the arm to which the participant would be allocated; however, they did not describe methods. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | LVRS does not lend itself to blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistants who were blind to the participant's group allocation conducted all outcome assessments at 3, 6, 9 and 12 months after randomisation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data due to treatment complications displayed graphically; attrition also reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available for comparison. |
| Other bias | High risk | Possible selective recruitment, as participants were referred by respiratory physicians, and a physician and a surgeon reassessed those wishing to proceed and made the final decision regarding eligibility; no other information was provided. Furthermore, no sham‐surgery group, which might lead to some placebo effects (as mentioned by study authors) |
Hillerdal 2005.
| Methods | Randomised controlled trial Method of randomisation: randomised number lists (blocks of 4) Allocation concealment: off‐site, concealed from participants (adequate) Outcome assessor blinding: not described Withdrawals/Dropouts: fully accounted for | |
| Participants | Screened: 304 (eligible: 114)
Randomised: 106 (LVRS 53; control 53)
Completed: 83 (LVRS 42; control 41)
Mean age in years: 62
Diagnosis: CT scan
Emphysema: diffuse
Major exclusions: asthma or bronchitis; smoking; DLCO ≤ 20% predicted age; sequelae of pleurisy/pleural adhesions; long‐term OCS treatment Baseline SGRQ total score in units (SD): 59.1 (13.3) for LVRS vs 58.7 (15.5) for control Shuttle walk in metres (SD): 237 (122) for LVRS vs 198 (104) for control FEV1 in litres (% predicted): 0.72 (26) for LVRS vs 0.69 (27) for control RV in % predicted: 255 for LVRS vs 267 for control TLC in % predicted: 135 for LVRS vs 142 for control PaO2 in kPa: 8.83 for LVRS vs 8.79 for control PaCO2 in kPa: 5.31 for LVRS vs 5.39 for control DLCO: not stated |
|
| Interventions | Bilateral LVRS by median sternotomy (N = 42) or video‐assisted thoracoscopy (N = 3) vs continued physical training. Physical training offered to both treatment groups Study duration: participants followed up for 1 year post randomisation |
|
| Outcomes | Mortality, lung function, withdrawal, quality of life (SGRQ and SF‐36), exercise capacity (6‐minute walk test and shuttle walk test) | |
| Notes | "Supported by a generous grant from the Swedish Heart‐Lung Foundation" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table with block randomisation in groups of 4 |
| Allocation concealment (selection bias) | Low risk | Lists kept at the study computer centre, but methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors reported, “…randomisation procedure was concealed from the participants,” but it is unclear if the ‘procedure’ refers just to the method of randomisation or actual blinding of participants throughout the study period. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Questionnaires and other data sent to the ‘computer centre’, where secretaries unaware of participants' surgical status ‘processed and fed the data into the computer’ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not available for comparison |
| Other bias | Low risk | No other bias detected |
McKenna 1996.
| Methods | Randomised controlled trial of 2 interventions Method of randomisation: not described Allocation concealment: not described Outcome assessor blinding: not blinded Withdrawals/Dropouts: fully accounted for | |
| Participants | Screened: not stated
Eligible: 72
Randomised: 72 (laser 33; staple 39)
Completed: 62 (laser 26; staple 36)
Mean age in years (SD): 67 (7)
Diagnosis: CT scan
Emphysema: diffuse, heterogeneous, bullae < 5 cm
Diseases included: not stated
Major exclusions: smoking, no prior thoracic surgery, age > 75 years, CO2 retention > 55 mmHg, severe cardiac disease, cancer within the past 5 years, ventilator dependency, presence of a lung mass, bullae > 5 cm Baseline SF‐36: not stated 6MWD (SD): not tested FEV1 in litres (SD): 0.7 (0.2) for laser vs 0.7 (0.2) for staple RV in litres (SD): 5.1 (1.1) for laser vs 5.4 (0.2) for staple TLC in litres (SD): 7.6 (1.4) for laser vs 7.9 (1.3) for staple PaO2 in mmHg: 65 for laser vs 66 for staple PaCO2 in mmHg: 43 for laser vs 44 for staple DLCO in mL/min/mmHg: 5.4 for laser vs 8.6 for staple |
|
| Interventions | Laser Extent: bilateral/unilateral ‐ unsure Approach: thoracoscipic Resection method: Nd: YAG (neodymium yttrium‐aluminium‐garnet laser) Non‐surgical: postop pulmonary rehab continued for 2 to 3 weeks after discharge Medications: not clear Stapling Extent: unilateral Approach: thoracoscipic Resection method: stapling with bovine pericardium reinforcement Non‐surgical: postoperative pulmonary rehabilitation continued for 2 to 3 weeks after discharge Medications: not clear Other: All participants in both groups were educated and were encouraged to join a Better Breathers Club. | |
| Outcomes | Morbidity, air leaks, delayed pneumothorax, FEV1, MOS‐36, operation time (hours), length of stay, supplemental oxygen therapy, repiratory failure | |
| Notes | "Supported in part by Department of Education grant DEf603‐ 91 ER61227 and National Institutes of Health grant R01192" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned but methods not described |
| Allocation concealment (selection bias) | Unclear risk | Unclear ‐ Study authors mention that “…patients were blindly randomized…”, but methods were not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | LVRS does not lend itself to blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Methods of handling outcome data missing from questionnaires (if any) not described |
| Selective reporting (reporting bias) | Unclear risk | Prespecified published protocol not available for comparison |
| Other bias | Low risk | No other biases identified |
NETT 2003.
| Methods | Randomised controlled trial Method of randomisation: computer‐generated random number sequence Allocation concealment: adequate Outcome assessor blinding: not described | |
| Participants | Screened: 3777
Randomised: 1218 (LVRS 608; control 610)
Mean age in years: 66.6
Emphysema: heterogeneous and homogenous
Diagnosis: CT scan
Entry criteria: FEV1 ≤ 45 % predicted (≥ 15 % predicted among participants ≥ 70 years of age); TLC ≥ 100% predicted; RV ≥ 150% predicted; partial pressure of resting arterial carbon dioxide ≤ 60 mmHg; resting partial pressure of arterial oxygen ≥ 45 mmHg; ability to walk ≥ 140 metres in 6 minutes; ability to complete 3 minutes on a bicycle ergometer; abstinence from smoking for 6 months before randomisation Exclusion criteria: concurrent medical conditions precluding surgery or that might interrupt follow‐up SGRQ total score in units (SD): 52.5 (12.6) for LVRS vs 53.6 (12.7) for control 6MWD in metres (SD): 370.78 (95.28) for LVRS vs 377.55 (96.32) for control FEV1 in % predicted (SD): 26.8 (7.4) for LVRS vs 26.7 (7.0) for control RV in % predicted (SD): 220.5 (49.9) for LVRS vs 223.4 (48.9) for control TLC in % predicted (SD): 128 (15.3) for LVRS vs 128.5 (15.0) for control PaO2 in mmHg: 64.5 for LVRS vs 64.2 for control PaCO2 in mmHg: 43.3 for LVRS vs 43.0 for control DLCO in % predicted: 28.3 for laser vs 28.4 for control |
|
| Interventions | LVRS via VATS or MS vs usual medical care according to ATS recommendations Usual care tailored to each participant in the control group included smoking cessation (for those resuming smoking during course of the study); drug therapies (including CS and inhaled bronchodilators); LTOT; immunisations; and continued pulmonary rehabilitation. Pulmonary rehabilitation: 3 phases: prerandomisation (6 to 10 weeks); post randomisation: 8 to 9 weeks) and long‐term maintenance (duration of the trial). PR consisted of physical, educational and psychosocial components (including nutritional counselling). Offered to both treatment groups |
|
| Outcomes | Mortality; exercise capacity; quality of life; FEV1; FVC; RV; cost; complications; length of hospital stay | |
| Notes | Funding received by National Heart, Lung and Blood Institute (NHLBI) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned but methods not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study is indicated to be unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Methods of handling outcome data missing from questionnaires (if any) not described |
| Selective reporting (reporting bias) | Low risk | Prespecified published protocol is not available for comparison, but rationale behind the trial is published. |
| Other bias | Low risk | No other biases identified |
OBEST 2005.
| Methods | Randomised clinical trial Method of randomisation: not described Allocation concealment: conducted off‐site at a data co‐ordinating centre Outcome assessor blinding: described Withdrawals/Dropouts: fully accounted for | |
| Participants | Screened: 332
Randomised: 35 (LVRS 24; control 11)
Completed: 29 (LVRS 20; control 9)
Mean age in years: 63.9
Diagnosis: CT scan
Emphysema: heterogeneous
Diseases included: not stated Entry criteria: advanced emphysema, MRC > 1, < 75 years, < 40 FEV1 % predicted, FEV1 response to bronchodilator < 30% pred and 300 mL, TLC and RV > 125% pred, PCO2 < 55 mmHg, heterogeneous emphysema by CT, 75% to 125% ideal body weight, compliance with rehabilitation, 6MWD > 492 feet Exclusion criteria: mechanical ventilation, antitrypsin deficiency, bullous emphysema, bronchiectasis, obliterated pleural space, pulmonary node > 0.7 cm, prednisolone > 10 mg, hypertension > 30 mmHg, life expectancy < 2 years, registered for lung transplant Baseline SF‐36 utility score in units (SD): 0.687 (0.121) for LVRS vs 0.673 (0.078) for control 6MWD in metres (SD): 309.98 (99.06) for LVRS vs 330.40 (96.32) for control FEV1 in litres (SD): 0.65 (0.02) for LVRS vs 0.78 (0.02) for control RV in litres (SD): 5.68 (0.28) for LVRS vs 4.9 (0.26) for control TLC in litres (SD): 6.71 (2.34) for LVRS vs 8.42 (2.57) for control PaO2 in mmHg: 69.42 for LVRS vs 69.18 for control PaCO2 in mmHg: 43.88 for LVRS vs 42.46 for control DLCO in mL/min/mmHg: 6.71 for laser vs 8.42 for staple |
|
| Interventions | LVRS via median sternotomy vs usual medical care at 5 of 6 centres. The other centre performed VATS. Optimal care standardised (including PR, bronchodilators, vaccination, steroids and antibiotics) Pulmonary rehabilitation: 6‐week course before randomisation (continued for the duration of the study in both groups) Participants followed up for 6 months |
|
| Outcomes | Morbidity; lung function; quality of life (CRDQ); exercise capacity; withdrawal | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Low ‐ randomisation in 6 participant blocks for each referring centre |
| Allocation concealment (selection bias) | Unclear risk | Unclear ‐ Study authors reported that allocation was concealed, as it was performed at the Data Co‐ordinating Centre, but they did not describe methods. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | LVRS does not lend itself to blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low ‐ Study authors reported, “Every effort was made to blind those persons who were administering outcome measures tests to the allocation group of the study participants. However, as in all surgical clinical trials, blinding was difficult to ensure in all instances.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low ‐ Study authors reported attrition and percentages of participants with missing outcome data for SF‐36 and 6MWD |
| Selective reporting (reporting bias) | Unclear risk | Unclear ‐ Published protocol is not available for comparison |
| Other bias | Low risk | Low ‐ No other bias was detected. |
Pompeo 2012.
| Methods | Randomised clinical trial Method of randomisation: not described Allocation concealment: conducted off‐site at a data co‐ordinating centre Outcome assessor blinding: described Withdrawals/Dropouts: fully accounted for | |
| Participants | Screened: 87
Randomised: 63 (LVRS 31; control 32)
Completed: 62 (laser 30; staple 32)
Age in years (SD): LVRS 65 (7) and control 64 (9)
Diagnosis: CT scan
Emphysema: diffuse, upper lobe predominant
Inclusion: severe smoking‐related emphysema with upper lobe predominance; severe disability despite maximised medical therapy, including respiratory rehabilitation with Modified Medical Research Council dyspnoea grade 3; no clinically significant sputum production, bronchiectasis or asthma; postbronchodilator FEV1 40% predicted; plethysmographic RV 180% predicted with TLC > 120% predicted; peak systolic pulmonary artery pressure < 50 mmHg on colour Doppler echocardiography; arterial carbon dioxide < 50 mmHg; diffusion capacity of carbon monoxide > 20% predicted; quit smoking for at least 4 months;
age 80 years; ASA score 3; body mass index > 18 and < 29; no unstable angina or ventricular arrhythmia; no comorbid condition that would significantly increase operative risk or negatively affect participation in a vigorous respiratory rehabilitation programme; no neoplastic disease with life expectancy < 12 months; no previous pleurodesis or thoracotomy in the hemithorax targeted for LVRS
Major exclusions: smoking, no prior thoracic surgery, age > 75 years, CO2 retention > 55 mmHg, severe cardiac disease, cancer within the past 5 years, ventilator dependency, presence of a lung mass, bullae > 5 cm Baseline SF‐36 units (SD): 29 (13) for LVRS vs 28 (13) for control 6MWD in metres (SD): 329 (98) for LVRS vs 300 (329) for control FEV1 in litres (% predicted): 0.78 (27) for LVRS vs 0.82 (29) for control RV in % predicted: 229 for LVRS vs 217 for control TLC in % predicted: 140 for LVRS vs 129 for control PaO2 in mmHg: 67 for LVRS vs 68 for control PaCO2 in mmHg: 41 for LVRS vs 41 for control DLCO: not stated |
|
| Interventions | Control: LVRS via VATS Experimental: non‐resectional technique performed via VATS, which involves pushing down the most damaged parts of the lung, grasping the redundant lung edges and stapling the plicated lung area to form a linear, uninterrupted suture | |
| Outcomes | Primary: hospital stay, FEV1 Secondary: 90 day mortality, ratio of arterial oxygen tension to fraction of inspired oxygen (PaO2/FiO2), arterial carbon dioxide tension (PaCO2), RV, FVC, 6MWD, MMRC, SF‐36 | |
| Notes | Study authors state that they "have nothing to disclose with regard to commercial support." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was carried out centrally by means of a computer generated sequence of casual numbers in which treatment arms were assigned to paired and unpaired numbers, respectively." |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | LVRS does not lend itself to blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Study was registered at ClinicalTrials.gov site (trial No. NCT00566839). Per‐protocol instead of intention‐to‐treat analysis; 1 participant converted from VATS to open approach; 2 participants who could not tolerate awake procedure excluded |
| Other bias | Low risk | No other bias other than that reported above. Study groups relatively well matched in main baseline data |
Stammberger 2000.
| Methods | Randomised controlled trial Method of randomisation: not described Allocation concealment: not clear Blinding of assessors: not described | |
| Participants | Screened: not reported
Randomised: 74 (65 analysed: buttressed group 32; non‐buttressed group 33)
Completed: 65 (LVRS 32; control 33)
Mean age in years: 60
Diagnosis: CT scan
Entry criteria: radiological evidence of emphysema; breathlessness leading to impaired quality of life; consent; FEV1 < 35% predicted; optimal medical therapy; candidature for lung transplantation
Major exclusions: current smoker; > 75 years; 'vanishing lung' on CT; TLCO < 20% predicted; hypercapnia; pulmonary hypertension; bronchiectasis; chest infection; OCS therapy > 15 mg/d Baseline QoL: not tested 6MWD: not tested FEV1 in litres (% predicted): 0.77 (27) for buttressing vs 0.76 (27) for control RV in % predicted: 287 for LVRS vs 284 for control TLC in L (% predicted): 8.15 (139) for buttressing vs 8.45 (138) for control PaO2 in mmHg: 65.3 for LVRS vs 64.2 for control PaCO2 in mmHg: 40.1 for LVRS vs 41.3 for control DLCO: not stated |
|
| Interventions | Buttressed vs non‐buttressed stapling device in LVRS procedures Pulmonary rehabilitation not undertaken as part of study protocol |
|
| Outcomes | Length of hospital stay; FEV1; dyspnoea; PaO2 and PaCO2; complications; mortality | |
| Notes | "Supported by grant 3200‐043358;95.1 from the Swiss National Science Fund and by a grant from the Zurich Lung League" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Unclear ‐ no mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of attempted blinding for participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing outcome data not mentioned |
| Selective reporting (reporting bias) | Unclear risk | No online protocol available |
| Other bias | Low risk | No other biases identified |
6MWD: 6‐minute walk distance; ATS: American Thoracic Society; BMI: body mass index; CRDQ/CRQ: Chronic Respiratory Disease Questionnaire; CS: corticosteroid; CT: computed tomography; DLCO: diffusing capacity of the lungs for carbon monoxide; FEV1: forced expiratory volume in one second; FiO2: fraction of inspired oxygen; FRC: functional residual capacity; FVC: forced expiratory vital capacity; HUI: Health Utilities Index; IQR: interquartile range; LTOT: long‐term oxygen therapy; LVRS: lung volume reduction surgery; mmHg: millimetres of mercury; MS: median sternotomy; MOS‐36: Medical Outcomes Study‐36 questionnaire; MRC: Medical Research Council breathlessness scale; NYHA: New York Heart Association; OCS: oral corticosteroids; PaCO2: partial pressure of carbon dioxide; PaO2: partial pressure of oxygen; PR: pulmonary rehabilitation; QALY: quality‐adjusted life‐year; QoL: quality of life; RV: residual volume; SD: standard deviation; SGRQ: St George's Respiratory Questionnaire; SF‐36: Short‐Form 36 questionnaire; TLC: total lung capacity; TLCO: transfer factor for carbon monoxide; VP: ventilation/perfusion; V/Q scan: ventilation/perfusion scan; VATS: video‐assisted thoracic surgery.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Daniel 1996 | Case series |
| Keenan 1996 | Case series |
| Kotloff 1996 | Case series |
| Little 1995 | Case series |
| Martinez 1997 | Prospective case series |
| Moser 2008 | Participants acted as own controls. |
| Nickoladze 1992 | Case series in patients with bullous emphysema |
| O'Brien 1999 | Prospective case series |
| Pompeo 2000 | Includes diffuse bullous emphysema (with heterogeneous distribution) |
| Rathinam 2009 | Participants acted as own controls. |
| Sciurba 1996 | Prospective case series |
| Szekely 1997 | Prospective case series with retrospective review of preoperative characteristics |
| Tan 2000 | Prospective case series (not randomised) |
| Teschler 1996 | Prospective case series |
| Wakabayashi 1995 | Case series using laser pneumoplasty |
Characteristics of studies awaiting assessment [ordered by study ID]
Goodnight 2001.
| Methods | |
| Participants | People with severe emphysema |
| Interventions | LVRS vs medical care |
| Outcomes | Quality of life, lung function, mortality, exercise capacity |
| Notes | Interim analysis of data presented at 2001 ATS conference. No follow‐up publication identified |
Characteristics of ongoing studies [ordered by study ID]
CELEB.
| Trial name or title | Lung volume reduction in COPD ‐ surgery vs endobronchial valves |
| Methods | Randomised parallel trial. Follow‐up: unknown Estimated enrolment: 152 participants |
| Participants | Patients suffering from heterogeneous emphysema |
| Interventions | Lung volume reduction surgery vs endobronchial valves |
| Outcomes |
Primary: change in iBODE score (a composite of BMI, FEV1, MRC dyspnoea score and shuttle walk test distance) 1 year post procedure Secondary
|
| Starting date | April 2016 |
| Contact information | Dr Nick Hopkinson |
| Notes | ISRCTN19684749 |
Differences between protocol and review
The protocol did not take into account the subgroups identified by NETT 2003. Owing to the significance of this trial (most likely no other trial on LVRS will have the power of this trial), we decided to take the identified subgroups into account in our analyses, although they were identified ad hoc and post hoc.
High‐risk versus low‐risk patients: Participants with an FEV1 of 20% or less predicted with a low carbon monoxide diffusing capacity (≤ 20%) or a homogenous pattern of emphysema were found to have higher risk of mortality.
Further analyses based on distribution of emphysema (upper vs non‐upper lobe) and exercise capacity (high vs low) of participants.
Furthermore, the protocol specified end of follow‐up as the endpoint for mortality. Owing to significant differences between trials at the end of follow‐up, we have changed this outcome to early (90 days) and late (> 36 months) mortality.
We have updated the Methods section to bring the review into line with current Cochrane best practice methods (e.g. inclusion of a 'Summary of findings' table and GRADE assessments).
Contributions of authors
JA: 2016 review update: assessment of studies, data extraction, data entry and write‐up. KC: 2016 review update: assessment of studies, data extraction and write‐up. LT: 2006 review update: in charge of 2006 protocol, editing of write‐up. BS: 2016 review update: supervision and editing of write‐up.
Michael Hensley was primary author on the first version of this review and was responsible for initiating the review.
Sources of support
Internal sources
The review authors declare that no such funding was received for this systematic review, Other.
External sources
Nederlands Astma Fonds, Netherlands.
Australasian Cochrane Airways Network, Australia.
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Clarenbach 2015 {published data only}
- Clarenbach CF, Sievi NA, Brock M, Schneiter D, Weder W, Kohler M. Lung volume reduction surgery and improvement of endothelial function and blood pressure in patients with chronic obstructive pulmonary disease. A randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2015;192(3):307‐14. [DOI: 10.1164/rccm.201503-0453OC] [DOI] [PubMed] [Google Scholar]
CLVR 2005 {published data only}
- Agzarian J, Miller JD, Kosa SD, Malthaner R, Tan L, Canadian Volume Reduction Surgery Study Group. Long‐term survival analysis of the Canadian Lung Volume Reduction Surgery trial. Annals of Thoracic Surgery 2013;96(4):1217‐22. [DOI: 10.1016/j.athoracsur.2013.04.077] [DOI] [PubMed] [Google Scholar]
- Davis P, Miller J, Malthaner R, CLVRS Group. Quality of life (QoL) preserved after lung reduction surgery (LVRS): the Canadian trial (CLVR) [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 2805. [Google Scholar]
- Malthaner RA, Miller JD. Lung volume reduction surgery: results of a Canadian pilot study. Canadian Lung Volume Reduction Surgery Study Group. Canadian Journal of Surgery 2000;43(5):377‐83. [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Berger RL, Malthaner RA, Celli BR, Goldsmith CH, Ingenito EP, et al. Lung volume reduction surgery versus medical treatment: for patients with advanced emphysema. Chest 2005;127(4):1166‐77. [DOI] [PubMed] [Google Scholar]
- Miller JD, Coughlin MD, Edey L, Miller P, Sivji Y. Equipoise and the ethics of the Canadian Lung Volume Reduction Surgery Trial study: should there be a randomized, controlled trial to evaluate lung volume reduction surgery?. Canadian Respiratory Journal 2000;7(4):329‐32. [DOI] [PubMed] [Google Scholar]
- Miller JD, Malthaner RA, Goldsmith CH, Goeree R, Higgins D, Cox PG, et al. A randomized clinical trial of lung volume reduction surgery versus best medical care for patients with advanced emphysema: a two‐year study from Canada. Annals of Thoracic Surgery 2006;81(1):314‐20. [DOI] [PubMed] [Google Scholar]
- Miller JD, Moore‐Cox A, Malthaner R, Tan L, Road J, et al. Quality of life after lung volume reduction: a randomised controlled trial. Chest 2003;124(4):167s. [Google Scholar]
Criner 1999 {published data only}
- Criner GJ, Cordova FC, Furukawa S, Kuzma AM, Travaline JM, Leyenson V, et al. Prospective randomized trial comparing bilateral lung volume reduction surgery to pulmonary rehabilitation in severe chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1999;160(6):2018‐27. [DOI] [PubMed] [Google Scholar]
Geddes 2000 {published data only}
- Cleverley JR, Desai SR, Wells AU, Koyama H, Eastick S, Schmidt MA, et al. Evaluation of patients undergoing lung volume reduction surgery: ancillary information available from computed tomography. Clinical Radiology 2000;55:45‐50. [DOI] [PubMed] [Google Scholar]
- Davies MG, Koyama H, Hensel DM, Pastorino U, Pepper J, Goldstraw P, et al. Lung volume reduction surgery in pulmonary emphysema: results of a randomized, controlled trial. American Journal of Respiratory and Critical Care Medicine 2000;161:585. [Google Scholar]
- Geddes D, Davies M, Koyama H, Hansell D, Pastorino U, Pepper J, et al. Effect of lung‐volume‐reduction surgery in patients with severe emphysema. The New England Journal of Medicine 2000;343:239‐45. [DOI] [PubMed] [Google Scholar]
- Goldstraw P, Davies MG, Koyama H, Hansell DM, Bott J, et al. Lung volume reduction surgery in pulmonary emphysema; results of a randomised, controlled trial. Thorax 1999;54:S15. [Google Scholar]
Goldstein 2003 {published data only}
- Dolmage TE, Goldstein RS, Todd TR, Guyatt G, Rooy S, Krip B, et al. The influence of lung volume reduction surgery (LVRS) on exercise in patients with COPD. American Thoracic Society 99th International Conference. 2003.
- Dolmage TE, Waddell TK, Maltais F, Guyatt GH, Todd TRJ, Keshavjee S, et al. The influence of lung volume reduction surgery on exercise in patients with COPD. The European Respiratory Journal 2004;23:269‐74. [DOI] [PubMed] [Google Scholar]
- Goldstein RS, Todd TR, Guyatt G, Keshavjee S, Dolmage TE, Rooy S, et al. Influence of lung volume reduction surgery (LVRS) on health related quality of life in patients with chronic obstructive pulmonary disease. Thorax 2003;58:405‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RS, Todd TRJ, Keshavjee S, Dolmage TE, Rooy S, Guyatt GH, et al. The influence of lung volume reduction surgery (LVRS) on health related quality of life in patients with chronic obstructive pulmonary disease. American Thoracic Society 99th International Conference. 2003. [DOI] [PMC free article] [PubMed]
Hillerdal 2005 {published data only}
- Hillerdal G, Lofdahl CG, Strom K, Skoogh BE, Jorfeldt L, Nilsson F, et al. Comparison of lung volume reduction surgery and physical training on health status and physiologic outcomes: a randomized controlled clinical trial. Chest 2005;128(5):3489‐99. [DOI] [PubMed] [Google Scholar]
- Hillerdal G, Lofsahl CG, Strom K, Skoogh BE. Volume reducing surgery in pulmonary emphysema compared to exercise training: a randomised study. The European Respiratory Journal 2001;18:355s. [Google Scholar]
- Lofdahl CG, Hillerdal G, Strom K. Randomized controlled trial of volume reduction surgery ‐ preliminary results up to 12 months. American Journal Respiratory & Critical Care Medicine 2000;161:A585. [Google Scholar]
McKenna 1996 {published data only}
- McKenna RJ Jr, Brenner M, Gelb AF, Mullin M, Singh N, Peters H, et al. A randomized, prospective trial of stapled lung reduction versus laser bullectomy for diffuse emphysema. The Journal of Thoracic & Cardiovascular Surgery 1996;111:317‐21. [DOI] [PubMed] [Google Scholar]
NETT 2003 {published data only}
- Anonymous. Rationale and design of The National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. The National Emphysema Treatment Trial Research Group. Chest 1999;116(6):1750‐61. [DOI] [PubMed] [Google Scholar]
- Anonymous. Rationale and design of the National Emphysema Treatment Trial (NETT): a prospective randomized trial of lung volume reduction surgery. Journal of Thoracic & Cardiovascular Surgery 1999;118(3):518‐28. [DOI] [PubMed] [Google Scholar]
- Armstrong H, Bartels M, Thomashow B. Does lung volume reduction surgery improve chronotropic incompetence in chronic obstructive pulmonary disease (COPD) patients?. Respiratory Medicine 2011;38(Suppl 55):464. [Google Scholar]
- Armstrong HF, Gonzalez‐Costello J, Jorde UP, Ginsburg ME, Layton AM, Thomashow BM, et al. The effect of lung volume reduction surgery on chronotropic incompetence. Respiratory Medicine 2012;106(10):1389‐95. [DOI] [PubMed] [Google Scholar]
- Becker C. With bated breath. Though Medicare will begin reimbursing for surgery to aid some emphysema patients, questions remain over benefit, cost. Modern Healthcare 2003;33(41):40‐1. [PubMed] [Google Scholar]
- Benzo R, Farrell MH, Chang C‐CH, Martinez FJ, Kaplan R, Reilly J, et al. Integrating health status and survival data: the palliative effect of lung volume reduction surgery. American Journal of Respiratory and Critical Care Medicine 2009;180(3):239‐46. [DOI: 10.1164/rccm.200809-1383OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzo R, Heinzer R, Kaplan R, Martinez F, Wise R, Make B, et al. Effect of lung volume reduction surgery (LVRS) on the decline of health related quality of life (HRQL) [Abstract]. American Thoracic Society International Conference, May 18‐23, 2007, San Francisco, California. 2007:A812.
- Calverley PM. Closing the NETT on lung volume reduction surgery. Thorax 2003;58(8):651‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carino T, Sheingold S, Tunis S, Carino T, Sheingold S, Tunis S. Using clinical trials as a condition of coverage: lessons from the National Emphysema Treatment Trial. Clinical Trials 2004;1(1):108‐14. [DOI] [PubMed] [Google Scholar]
- Chandra D, Wise RA, Kulkarni HS, Benzo RP, Criner G, Make B, et al. Optimizing the 6‐min walk test as a measure of exercise capacity in COPD. Chest 2012;142(6):1545‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E. Surgical treatment of end stage emphysema. Minerva Anestesiologica 2004;70(5):307‐12. [PubMed] [Google Scholar]
- Criner GJ, Belt P, Sternberg AL, Mosenifar Z, Make BJ, Utz JP, et al. Effects of lung volume reduction surgery on gas exchange and breathing pattern during maximum exercise. Chest 2009;135(5):1268‐79. [10.1378/chest.08‐1625] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criner GJ, Cordova F, Sternberg AL, Martinez FJ. The National Emphysema Treatment Trial (NETT) part II: lessons learned about lung volume reduction surgery. American Journal of Respiratory and Critical Care Medicine 2011;184(8):881‐93. [DOI: 10.1164/rccm.201103-0455CI] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criner GJ, Sternberg AL. National Emphysema Treatment Trial: the major outcomes of lung volume reduction surgery in severe emphysema. Proceedings of the American Thoracic Society 2008;5(4):393‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz J, Caldeira J, Cravino J Cruz J, Caldeira J, Cravino J. Surgery for volume reduction in chronic pulmonary obstructive disease. Revista Portuguesa de Cirurgia Cardio‐torácica e Vascular 2003;10(2):55‐60. [PubMed] [Google Scholar]
- DeCamp MM, Blackstone EH, Naunheim KS, Krasna MJ, Wood DE, Meli YM, et al. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Annals of Thoracic Surgery 2006;82(1):197‐207. [DOI] [PubMed] [Google Scholar]
- Fan VS, Giardino ND, Blough DK, Kaplan RM, Ramsey SD, Fishman AP, et al. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD: Journal of Chronic Obstructive Pulmonary Disease 2008;5(2):105‐16. [DOI] [PubMed] [Google Scholar]
- Fan VS, Ramsey SD, Giardino ND, Make BJ, Emery CF, Diaz PT, et al. NETT 1999. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Archives of Internal Medicine 2007;167(21):2345‐53. [DOI] [PubMed] [Google Scholar]
- Fan VS, Ramsey SD, Make BJ, Martinez FJ. Physiologic variables and functional status independently predict COPD hospitalizations and emergency department visits in patients with severe COPD. COPD: Journal of Chronic Obstructive Pulmonary Disease 2007;4(1):29‐39. [DOI: 10.1080/15412550601169430] [DOI] [PubMed] [Google Scholar]
- Ferrer NB, Mamary AJ, Gaughan JP, DeCamp M, Krasna M, Mosenifar Z, et al. Changes In Respiratory Muscle Strength After Lung Volume Reduction Surgery For Severe Emphysema. D22. CHRONIC OBSTRUCTIVE PULMONARY DISEASE: NOVEL OUTCOME MEASURES AND TREATMENTS. American Thoracic Society, 2010, 2010:A5422. [Google Scholar]
- Fisher MR, Criner GJ, Fishman AP, Hassoun PM, Minai OA, Scharf SM, et al. Estimating pulmonary artery pressures by echocardiography in patients with emphysema. European Respiratory Journal 2007;30(5):914‐21. [DOI] [PubMed] [Google Scholar]
- Fishman Al, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. A randomized trial comparing lung‐volume‐reduction surgery with medical therapy for severe emphysema. The New England Journal of Medicine 2003;348(21):2059‐73. [DOI] [PubMed] [Google Scholar]
- Kaplan A. Lung volume reduction surgery vs medical therapy for severe emphysema. Primary Care Respiratory Journal 2007;16(4):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R, Reilly J, Mohsenifar Z. Measurement of health‐related quality of life in the national emphysema treatment trial (NETT) [Abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A220. [Google Scholar]
- Kaplan RM, Ries AL, Reilly J, Mohsenifar Z. Measurement of health‐related quality of life in the national emphysema treatment trial. Chest 2004;126(3):781‐9. [DOI] [PubMed] [Google Scholar]
- Kaplan RM, Sun Q, Naunheim KS, Ries AL. Long‐term follow‐up of high‐risk patients in the National Emphysema Treatment Trial. Annals of Thoracic Surgery 2014;98(5):1782‐9. [DOI] [PubMed] [Google Scholar]
- Kaplan RM, Sun Q, Ries AL. Quality of well‐being outcomes in the national emphysema treatment trial. Chest 2015;147(2):377‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim V, Criner G, Chatila W, Martin U, Krachman S. Effects of lung volume reduction surgery (LVRS) on the correlation between awake gas exchange and nocturnal oxygenation in patients with severe emphysema [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:C36, Poster: J55.
- Kim V, Kretschman DM, Sternberg AL, DeCamp MM Jr, Criner GJ. Weight gain after lung reduction surgery is related to improved lung function and ventilatory efficiency. American Journal of Respiratory and Critical Care Medicine 2012;186(11):1109‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozora E, Emery CF, Ellison MC, Wamboldt FS, Diaz PT, Make B. Improved neurobehavioral functioning in emphysema patients following lung volume reduction surgery compared with medical therapy. Chest 2005;128(4):2653‐63. [DOI] [PubMed] [Google Scholar]
- Krachman SL, Chatila W, Martin UJ, Nugent T, Crocetti J, Gaughan J, et al. Effects of lung volume reduction surgery on sleep quality and nocturnal gas exchange in patients with severe emphysema. Chest 2005;128(5):3221‐8. [DOI] [PubMed] [Google Scholar]
- Krahnke JS. Residual volume > 225% predicted is associated with higher mortality after LVRS in the Nett cohort. American Journal of Respiratory and Critical Care Medicine 2014;189:A3050. [Google Scholar]
- Lee SM, Wise R, Sternberg AL, Tonascia J, Piantadosi S, National Emphysema Treatment Trial Research Group. Methodological issues in terminating enrollment of a subgroup of patients in a multicenter randomized trial. Clinical Trials 2004;1(3):326‐38. [DOI] [PubMed] [Google Scholar]
- Make B, Tolliver R, Christensen P, Karla S, MacIntyre N, Ries A. Pulmonary rehabilitation improves exercise capacity and dyspnea in the National Emphysema Treatment Trial (NETT). American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A254. [Google Scholar]
- Martinez FJ, Andrel A, Benditt J, Naunheim K, Criner G, Make B, et al. Six month change in modified BODE (mBODE) predicts mortality after lung volume reduction surgery (LVRS). Proceedings of the American Thoracic Society. 2006:A118 [poster J59].
- Martinez FJ, Andrel A, Benditt J, Naunheim K, Make B, Criner G, et al. Change in modified BODE (mBODE) in severe emphysema patients treated medically or with lung volume reduction surgery (LVRS. Proceedings of the American Thoracic Society. 2006:A119 [Poster J60].
- Martinez FJ, Foster G, Curtis JL, Criner G, Weinmann G, Fishman A, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. American Journal of Respiratory & Critical Care Medicine 2006;173(12):1326‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna Jr RJ, Benditt JO, DeCamp M, Deschamps C, Kaiser L, Lee SM, et al. Safety and efficacy of median sternotomy versus video‐assisted thoracic surgery for lung volume reduction surgery. Journal of Thoracic & Cardiovascular Surgery 2004;127(5):1350‐60. [DOI] [PubMed] [Google Scholar]
- Mohsenifar Z, Lee SM, Diaz P, Criner G, Sciurba F, Ginsburg M, et al. Single‐breath diffusing capacity of the lung for carbon monoxide: a predictor of PaO2, maximum work rate, and walking distance in patients with emphysema. Chest 2003;123(5):1394‐400. [DOI] [PubMed] [Google Scholar]
- National Emphysema Treatment Trial Research Group. A randomized trial comparing lung‐volume‐reduction surgery with medical therapy for severe emphysema. The New England Journal of Medicine 2003;348(21):2059‐73. [DOI] [PubMed] [Google Scholar]
- National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung‐volume‐reduction surgery. The New England Journal of Medicine 2001;345(15):1075‐83. [DOI] [PubMed] [Google Scholar]
- Naunheim KS. Update on lung volume reduction. Journal of Surgical Research 2004;117(1):134‐43. [DOI] [PubMed] [Google Scholar]
- Naunheim KS, DeCamp MM, Wood DE, Deschamps C, Mosenifar Z, Benditt JO, et al. Long‐term results from the National Emphysema Treatment Trial. The Society of Thoracic Surgeons 42nd Annual Meeting; Chicago, Illinois. 2005:Abstract No. 02.
- Naunheim KS, Wood DE, Krasna MJ, DeCamp MM, Ginsburg ME, McKenna RJ, et al. Predictors of operative mortality and cardiopulmonary morbidity in the National Emphysema Treatment Trial. The Journal of Thoracic and Cardiovascular Surgery 2006;131(1):43‐53. [DOI] [PubMed] [Google Scholar]
- Naunheim KS, Wood DE, Mohsenifar Z, Sternberg AL, Criner GJ, DeCamp MM, et al. Long‐term follow‐up of patients receiving lung‐volume‐reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Annals of Thoracic Surgery 2006;82(2):385‐7. [DOI] [PubMed] [Google Scholar]
- Piantadosi S. A prospective randomized trial of lung volume reduction surgery. Journal of Cardiopulmonary Rehabilitation 2000;20(1):24‐36. [DOI] [PubMed] [Google Scholar]
- Ramsey SD, Berry K, Etzioni R, Kaplan RM, Sullivan SD, Wood DE. Cost effectiveness of lung‐volume‐reduction surgery for patients with severe emphysema. The New England Journal of Medicine 2003;348(21):2092‐102. [DOI] [PubMed] [Google Scholar]
- Ramsey SD, Blough DK, Sullivan SD, NETT 1999. A forensic evaluation of the National Emphysema Treatment Trial using the expected value of information approach. Medical Care 2008;46(5):542‐8. [DOI] [PubMed] [Google Scholar]
- Ramsey SD, Kaplan RM, Schwartz JS. Economic analysis of lung volume reduction surgery as part of the National Emphysema Treatment Trial. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A786. [Google Scholar]
- Ramsey SD, Shroyer AL, Sullivan SD, Wood DE. Updated evaluation of the cost‐effectiveness of lung volume reduction surgery. Chest 2007;131(3):823‐32. [DOI: 10.1378/chest.06-1790] [DOI] [PubMed] [Google Scholar]
- Ramsey SD, Sullivan SD. Evidence, economics and emphysema: Medicare's long journey with lung volume reduction surgery. Health Affairs 2005;24(1):55‐67. [DOI] [PubMed] [Google Scholar]
- Ramsey SD, Sullivan SD, Kaplan RM, Wood DE, Chiang YP, Wagner JL. Economic analysis of lung volume reduction surgery as part of the National Emphysema Treatment Trial. NETT Research Group. The Annals of Thoracic Surgery 2001;71(3):995‐1002. [DOI] [PubMed] [Google Scholar]
- Reilly J, Moy M, Kaplan R, Diaz P, Benditt J, Criner G, Lee S. Predictors of improved health‐related quality of life (QOL) following pulmonary rehabilitation in the National Emphysema Treatment Trial (NETT). American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A503. [Google Scholar]
- Ries A, Christensen P, Kalra S, MacIntyre N, Make B. Pulmonary rehabilitation in the National Emphysema Treatment Trial (NETT): effect of prior rehab experience and use of satellite centers. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A13. [Google Scholar]
- Ries AL, Make BJ, Lee SM, Krasna MJ, Bartels M, Crouch R, et al. The effects of pulmonary rehabilitation in the national emphysema treatment trial. Chest 2005;128(6):3799‐809. [DOI] [PubMed] [Google Scholar]
- Robbins HY, Bulman WA, Jellen PA, Brogan FL, Ginsburg ME, Sonett J, et al. Improved Outcomes After Lung Volume Reduction After NETT. D22. CHRONIC OBSTRUCTIVE PULMONARY DISEASE: NOVEL OUTCOME MEASURES AND TREATMENTS. American Thoracic Society, 2010, 2010:A5417. [Google Scholar]
- Russi EW, Bloch KE, Weder W. Lung volume reduction surgery: what can we learn from the National Emphysema Treatment Trial? [Editorial]. The European Respiratory Journal 2003;22(4):571‐3. [DOI] [PubMed] [Google Scholar]
- Sciurba F, Criner GJ, Lee SM, Mohsenifar Z, Shade D, Slivka W, et al. Six‐minute walk distance in chronic obstructive pulmonary disease: reproducibility and effect of walking course layout and length. American Journal of Respiratory & Critical Care Medicine 2003;167(11):1522‐7. [DOI] [PubMed] [Google Scholar]
- Snyder ML, Goss CH, Neradilek B, Polissar NL, Mosenifar Z, Wise RA, et al. Changes in arterial oxygenation and self‐reported oxygen use after lung volume reduction surgery. American Journal of Respiratory and Critical Care Medicine 2008;178(4):339‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland ER, Make BJ. Maximum exercise as an outcome in COPD: minimal clinically important difference. COPD: Journal of Chronic Obstructive Pulmonary Disease 2005;2(1):137‐41. [DOI] [PubMed] [Google Scholar]
- Teschler H, Stamatis G. NETT confirms clear survival advantages by lung volume reduction in predominantly apical emphysema with reduced ability to cope with stress. Pneumologie 2003;57(7):361‐2. [DOI] [PubMed] [Google Scholar]
- Washko GR, Fan VS, Ramsey SD, Mohsenifar Z, Martinez F, Make BJ, et al. The effect of lung volume reduction surgery on chronic obstructive pulmonary disease exacerbations. American Journal of Respiratory and Critical Care Medicine 2008;177(2):164‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washko GR, Fan VS, Ramsey SD, Reilly JJ. The effect of lung volume reduction surgery on acute exacerbations of chronic obstructive pulmonary disease. American Thoracic Society International Conference, May 18‐23. 2007:A812.
- Weitzenblum E. Surgery and lung volume reduction in emphysema ‐ Part A: final results of the American National Emphysema Treatment Trial multicentric study. Revue Des Maladies Respiratoires 2003;20(5 II):6S35‐40. [Google Scholar]
OBEST 2005 {published data only}
- Miller JD, Berger RL, Malthaner RA, Celli BR, Goldsmith CH, Ingenito EP, et al. Lung volume reduction surgery vs medical treatment for patients with advanced emphysema. Chest 2005;127(4):1166‐77. [DOI] [PubMed] [Google Scholar]
Pompeo 2012 {published data only}
- Pompeo E, Rogliani P, Tacconi F, Dauri M, Saltini C, Novelli G, et al. Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery. The Journal of Thoracic and Cardiovascular Surgery 2012;143(1):47‐54. [DOI] [PubMed] [Google Scholar]
Stammberger 2000 {published data only}
- Stammberger U, Klepetko W, Stamatis G, Hamacher J, Schmid RA, Wisser W, et al. Buttressing the staple line in lung volume reduction surgery: a randomized three‐center study. The Annals of Thoracic Surgery 2000;70:1820‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Daniel 1996 {published data only}
- Daniel TM, Chan BBK, Bhasker V, Parekh JS, Walters PE, Reeder J, et al. Lung volume reduction surgery: case selection, operative technique, and clinical results. Annals of Surgery 1996;223:526‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keenan 1996 {published data only}
- Keenan RJ, Landreneau RJ, Sciurba FC, Ferson PF, Holbert JM, Brown ML, et al. Unilateral thoracoscopic surgical approach for diffuse emphysema. Journal of Thoracic and Cardiovascular Surgery 1996;111:308‐16. [DOI] [PubMed] [Google Scholar]
Kotloff 1996 {published data only}
- Kotloff RM, Tino G, Bavaria JE, Palevsky HI, Hansen‐Flaschen J, Wahl PM, et al. Bilateral lung volume reduction surgery for advanced emphysema: a comparison of median sternotomy and thoracoscopic approaches. Chest 1996;110:1399‐406. [DOI] [PubMed] [Google Scholar]
Little 1995 {published data only}
- Little AG, Swain JA, Nino JJ, Prabhu RD, Schlachter MD, Barcia TC. Reduction pneumoplasty for emphysema. Annals of Surgery 1995;222:365‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez 1997 {published data only}
- Martinez FJ, Montes de Oca M, Whyte RI, Stetz J, Gay SE, Celli BR. Lung‐volume reduction improves dyspnea, dynamic hyperinflation, and respiratory muscle function. American Journal of Respiratory and Critical Care Medicine 1997;155:1984‐90. [DOI] [PubMed] [Google Scholar]
Moser 2008 {published data only}
- Moser C, Opitz I, Zhai W, Rousson V, Russi EW, Weder W, et al. Autologous fibrin sealant reduces the incidence of prolonged air leak and duration of chest tube drainage after lung volume reduction surgery: a prospective randomized blinded study. The Journal of Thoracic and Cardiovascular Surgery 2008;136(4):843‐9. [DOI] [PubMed] [Google Scholar]
Nickoladze 1992 {published data only}
- Nickoladze MD. Functional results of surgery for bullous emphysema. Chest 1992;101:119‐22. [DOI] [PubMed] [Google Scholar]
O'Brien 1999 {published data only}
- O'Brien GM, Furukawa S, Kuzma AM, Corodva F, Criner GJ. Improvements in lung function, exercise, and quality of life in hypercapneic COPD patients after lung volume reduction surgery. Chest 1999;115:75‐84. [DOI] [PubMed] [Google Scholar]
Pompeo 2000 {published data only}
- Mineo TC, Ambrogi A, Pompeo E, Elia S, Mineo D, Bollero P, Nofroni I. Impact of lung volume reduction surgery versus rehabilitation on quality of life. The European Respiratory Journal 2004;23:275‐80. [DOI] [PubMed] [Google Scholar]
- Mineo TC, Ambrogi V, Pompeo E, Bollero P, Mineo D, Nofroni I. Body weight and nutritional changes after reduction pneumoplasty for severe emphysema: a randomised study. Journal of Thoracic and Cardiovascular Surgery 2002;124(4):660‐7. [DOI] [PubMed] [Google Scholar]
- Pompeo E, Marino M, Nofroni I, Matteucci G, Mineo TC. Reduction pneumoplasty versus respiratory rehabilitation in severe emphysema: a randomized study. Pulmonary Emphysema Research Group. The Annals of Thoracic Surgery 2000;70:948‐53. [DOI] [PubMed] [Google Scholar]
Rathinam 2009 {published data only}
- Rathinam S, Naidu BV, Nanjaiah P, Loubani M, Kalkat MS, Rajesh PB. BioGlue and Peri‐strips in lung volume reduction surgery: pilot randomised controlled trial. Journal of Cardiothoracic Surgery 2009;4:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sciurba 1996 {published data only}
- Sciurba FC, Rogers RM, Keenan RJ, Slivka WA, Gorcsan J, Ferson PF, et al. Improvement in pulmonary function and elastic recoil after lung‐reduction surgery for diffuse emphysema. The New England Journal of Medicine 1996;334:1095‐9. [DOI] [PubMed] [Google Scholar]
Szekely 1997 {published data only}
- Szekely LA, Oelberg DA, Wright C, Johnson DC, Wain J, Trotman‐Dickenson B, et al. Preoperative predictors of operative morbidity and mortality in COPD patients undergoing bilateral lung volume reduction surgery. Chest 1997;111:550‐8. [DOI] [PubMed] [Google Scholar]
Tan 2000 {published data only}
- Tan AL, Unruh HW, Mink SN. Lung volume reduction surgery for the treatment of severe emphysema: a study in a single Canadian institution. Canadian Journal of Surgery 2000;43(5):369‐76. [PMC free article] [PubMed] [Google Scholar]
Teschler 1996 {published data only}
- Teschler H, Stamatis G, El‐Raouf Farhat AA, Meyer FJ, Costabel U, Konietzko N. Effect of surgical lung volume reduction on respiratory muscle function in pulmonary emphysema. The European Respiratory Journal 1996;9:1779‐84. [DOI] [PubMed] [Google Scholar]
Wakabayashi 1995 {published data only}
- Wakabayashi A. Thoracoscopic laser pneumoplasty in the treatment of diffuse bullous emphysema. The Annals of Thoracic Surgery 1995;60:936‐42. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Goodnight 2001 {published data only}
- Goodnight White S, Jones JW, Baaklini WA, Soltero E, Smithwick P, Sharafkhaneh A, et al. Lung volume reduction surgery (LVRS) in patients with severe emphysema: 1 year follow‐up. American Journal of Respiratory and Critical Care Medicine 2001;163:A486. [Google Scholar]
References to ongoing studies
CELEB {published data only}
- Lung volume reduction in COPD ‐ surgery vs endobronchial valves. Ongoing study April 2016.
Additional references
Akuthota 2012
- Akuthota P, Litmanovich D, Zutler M, Boiselle PM, Bankier AA, Roberts DH, et al. An evidence‐based estimate on the size of the potential patient pool for lung volume reduction surgery. The Annals of Thoracic Surgery 2012;94(1):205‐11. [DOI: 10.1016/j.athoracsur.2012.03.047] [DOI] [PubMed] [Google Scholar]
Bagdonas 2015
- Bagdonas E, Raudoniute J, Bruzauskaite I, Aldonyte R. Novel aspects of pathogenesis and regeneration mechanisms in COPD. International Journal of COPD 2015;10:995‐1013. [DOI: 10.2147/COPD.S82518] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bale 2008
- Bale G, Martinez‐Camblor P, Burge PS, Soriano JB. Long‐term mortality follow‐up of the ISOLDE participants: causes of death during 13 years after trial completion. Respiratory Medicine 2008;102(10):1468‐72. [DOI: 10.1016/j.rmed.2008.04.001] [DOI] [PubMed] [Google Scholar]
Berger 2010
- Berger RL, Decamp MM, Criner GJ, Celli BR. Lung volume reduction therapies for advanced emphysema. Chest 2010;138(2):407‐15. [DOI: 10.1378/chest.09-1822] [DOI] [PubMed] [Google Scholar]
Bourdin 2009
- Bourdin A, Burgel P‐R, Chanez P, Garcia G, Perez T, Roche N. Recent advances in COPD: pathophysiology, respiratory physiology and clinical aspects, including comorbidities. European Respiratory Review 2009;18(114):198‐212. [DOI: ] [DOI] [PubMed] [Google Scholar]
Boutou 2015
- Boutou AK, Zoumot Z, Nair A, Davey C, Hansell DM, Jamurtas A, et al. The impact of homogeneous versus heterogeneous emphysema on dynamic hyperinflation in patients with severe COPD assessed for lung volume reduction. Journal of Chronic Obstructive Pulmonary Disease 2015;0:1‐8. [DOI: 10.3109/15412555.2015.1020149] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brodsky 2000
- Brodsky JB, Cohen E. Video‐assisted thoracoscopic surgery. Current Opinion in Anaesthesiology 2000;13(1):41‐45. [DOI] [PubMed] [Google Scholar]
Burge 2000
- Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. British Medical Journal 2000;320:1297‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choong 2006
- Choong CK, Phan L, Massetti P, Haddad FJ, Martinez C, Roschak E, et al. Prolongation of patency of airway bypass stents with use of drug‐eluting stents. Journal of Thoracic and Cardiovascular Surgery 2006;131(1):60‐4. [DOI: 10.1016/j.jtcvs.2005.07.057] [DOI] [PubMed] [Google Scholar]
Ciccone 2003
- Ciccone AM, Meyers BF, Guthrie TJ, Davis GE, Yusen RD, Lefrak SS, et al. Long‐term outcome of bilateral lung volume reduction in 250 consecutive patients with emphysema. The Journal of Thoracic and Cardiovascular Surgery 2003;125(3):513‐25. [DOI: 10.1067/mtc.2003.147] [DOI] [PubMed] [Google Scholar]
Clark 2014
- Clark SJ, Zoumot Z, Bamsey O, Polkey MI, Dusmet M, Lim E, et al. Surgical approaches for lung volume reduction in emphysema. Clinical Medicine 2014;14(2):122‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cockram 2006
- Cockram J, Cecins N, Jenkins S. Maintaining exercise capacity and quality of life following pulmonary rehabilitation. Respirology 2006;11(1):98‐104. [DOI: 10.1111/j.1440-1843.2006.00791.x] [DOI] [PubMed] [Google Scholar]
Come 2015
- Come CE, Kramer MR, Dransfield MT, Abu‐Hijleh M, Berkowitz D, Bezzi M, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. European Respiratory Journal 2015;April:ERJ‐02056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 1995
- Cooper JD, Trulock EP, Triantafillou AN, Patterson GA, Pohl MS, Deloney PA, et al. Bilateral pneumectomy (volume reduction) chronic obstructive pulmonary disease. General Thoracic Surgery 1995;109(1):106‐19. [DOI: 10.1016/S0022-5223(95)70426-4] [DOI] [PubMed] [Google Scholar]
Criner 2009
- Criner GJ, Pinto‐Plata V, Strange C, Dransfield M, Gotfried M, Leeds W, et al. Biologic lung volume reduction in advanced upper lobe emphysema: phase 2 results. American Journal of Respiratory and Critical Care Medicine 2009;179(9):791‐8. [DOI: 10.1164/rccm.200810-1639OC] [DOI] [PubMed] [Google Scholar]
Dal Negro 2008
- Dal Negro R. Optimizing economic outcomes in the management of COPD. International Journal of Chronic Obstructive Pulmonary Disease 2008;3(1):1‐10. [PUBMED: PMC2528207] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davey 2015
- Davey C, Zoumot Z, Jordan S, Carr DH, Polkey MI, Shah PL, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR‐HIFi trial): study design and rationale. Thorax 2015;70(3):288‐90. [DOI: 10.1136/thoraxjnl-2014-205127] [DOI] [PMC free article] [PubMed] [Google Scholar]
DeCamp 2008
- DeCamp MM Jr, Lipson D, Krasna M, Minai OA, McKenna RJ Jr, Thomashow BM. The evaluation and preparation of the patient for lung volume reduction surgery. Proceedings of the American Thoracic Society 2008;5(4):427‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Degano 2004
- Degano B, Brouchet L, Rami J, Arnal JF, Escamilla R, Hermant C, et al. Improvement after lung volume reduction surgery: a role for inspiratory muscle adaptation. Respiratory Physiology and Neurobiology 2004;139(3):293‐301. [DOI: 10.1016/j.resp.2003.11.002] [DOI] [PubMed] [Google Scholar]
Demedts 2006
- Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respiratory Research 2006;7(53):1‐10. [DOI: 10.1186/1465-9921-7-53] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deslée 2016
- Deslée G, Mal H, Dutau H, Bourdin A, Vergnon JM, Pison C, et al. Lung volume reduction coil treatment vs usual care in patients with severe emphysema: the REVOLENS randomized clinical trial. JAMA 2016;315(2):175‐84. [DOI] [PubMed] [Google Scholar]
Ferguson 2006
- Ferguson GT. Why does the lung hyperinflate?. Proceedings of the American Thoracic Society 2006;3:176‐9. [DOI: 10.1513/pats.200508-094DO] [DOI] [PubMed] [Google Scholar]
Fessler 1998
- Fessler HE, Permutt S. Lung volume reduction surgery and airflow limitation. American Journal of Respiratory and Critical Care Medicine 1998;157(3):715‐22. [DOI] [PubMed] [Google Scholar]
Fessler 2003
- Fessler HE, Reilly JJ, Sugarbaker DJ. Lung Volume Reduction Surgery for Emphysema. Boca Raton: CRC Press, 2003. [Google Scholar]
Fessler 2008
- Fessler HE, Scharf SM, Ingenito EP, McKenna RJ, Sharafkhaneh A. Physiologic basis for improved pulmonary function after lung volume reduction. Proceedings of the American Thoracic Society 2008;5(4):416‐20. [DOI: 10.1513/pats.200708-117ET] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garrod 2006
- Garrod R, Marshall J, Barley E, Jones PW. Predictors of success and failure in pulmonary rehabilitation. The European Respiratory Journal 2006;27(4):788‐94. [DOI: 10.1183/09031936.06.00130605] [DOI] [PubMed] [Google Scholar]
Ginsburg 2011
- Ginsburg ME, Thomashow BM, Yip CK, DiMango AM, Maxfield RA, Bartels MN, et al. Lung volume reduction surgery using the NETT selection criteria. The Annals of Thoracic Surgery 2011;91(5):1556‐61. [DOI: 10.1016/j.athoracsur.2011.01.054] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 2 February 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Halbert 2006
- Halbert RJ, Natoli JL, Badamgarev E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta‐analysis. European Respiratory Journal 2006;28:523‐32. [DOI: 10.1183/09031936.06.00124605] [DOI] [PubMed] [Google Scholar]
Hamnegård 2006
- Hamnegård CH, Polkey MI, Thylen A, Nilsson F, Schersten H, Bake B. Effect of lung volume reduction surgery for emphysema on diaphragm function. Respiratory Physiology and Neurobiology 2006;150(2):182‐90. [DOI: 10.1016/j.resp.2005.03.010] [DOI] [PubMed] [Google Scholar]
Harris 1995
- Harris RJ, Kavuru MS, Rice TW, Kirby TJ. The diagnostic and therapeutic utility of thoracoscopy: a review. Chest 1995;108(3):828‐41. [DOI] [PubMed] [Google Scholar]
Herth 2016
- Herth FJF, Valipour A, Shah PL, Eberhardt R, Grah C, Egan J, et al. Segmental volume reduction using thermal vapour ablation in patients with severe emphysema: 6‐month results of the multicentre, parallel‐group, open‐label, randomised controlled STEP‐UP trial. The Lancet Respiratory Medicine 2016;4(3):185‐93. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Chichester (UK): The Cochrane Collaboration; John Wiley & Sons, 2011. [Google Scholar]
Higuchi 2006
- Higuchi T, Reed A, Oto T, Holsworth L, Ellis S, Bailey MJ, et al. Relation of interlobar collaterals to radiological heterogeneity in severe emphysema. Thorax 2006;61(5):409‐13. [DOI: 10.1136/thx.2005.051219] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hogg 2002
- Hogg JC, Senior RM. Chronic obstructive pulmonary disease c2: pathology and biochemistry of emphysema. Thorax 2002;57:830‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2011
- Huang W, Wang WR, Deng B, Tan YQ, Jiang GY, Zhou HJ, et al. Several clinical interests regarding lung volume reduction surgery for severe emphysema: meta‐analysis and systematic review of randomized controlled trials. Journal of Cardiothoracic Surgery 2011;6(1):148. [DOI: 10.1186/1749-8090-6-148] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ingenito 2005
- Ingenito EP, Tsai LW, Majumdar A, Suki B. On the role of surface tension in the pathophysiology of emphysema. American Journal of Respiratory and Critical Care Medicine 2005;171:300‐4. [DOI: 10.1164/rccm.200406-770PP] [DOI] [PubMed] [Google Scholar]
Ingenito 2008
- Ingenito EP, Wood DE, Utz JP. Bronchoscopic lung volume reduction in severe emphysema. Proceedings of the American Thoracic Society 2008;5(4):454‐60. [DOI: 10.1513/pats.200707-085ET] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2005
- Jones PW. St. George's respiratory questionnaire: MCID. COPD: Journal of Chronic Obstructive Pulmonary Disease 2005;2(1):75‐9. [DOI] [PubMed] [Google Scholar]
Kim 2008
- Kim V, Rogers TJ, Criner GJ. New concepts in the pathobiology of chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2008;5:478‐85. [DOI: 10.1513/pats.200802-014ET] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkham 2013
- Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest 2013;144(1):266‐73. [DOI: 10.1378/chest.12-2664] [DOI] [PubMed] [Google Scholar]
Klepetko 1999
- Klepetko W. Surgical aspects and techniques of lung volume reduction surgery for severe emphysema. European Respiratory Journal 1999;13:919‐25. [DOI] [PubMed] [Google Scholar]
Klooster 2014
- Klooster K, Hacken NHT, Franz I, Kerstjens HAM, Rikxoort EM, Slebos D‐J. Lung volume reduction coil treatment in chronic obstructive pulmonary disease patients with homogeneous emphysema: a prospective feasibility trial. Respiration 2014;88(2):116‐25. [DOI: 10.1159/000362522] [DOI] [PubMed] [Google Scholar]
Klooster 2014a
- Klooster K, Hacken NHT, Slebos D‐J. The lung volume reduction coil for the treatment of emphysema: a new therapy in development. Expert Review of Medical Devices 2014;11(5):481‐9. [DOI: 10.1586/17434440.2014.929490] [DOI] [PubMed] [Google Scholar]
Lenfant 2006
- Lenfant C. Will lung volume reduction surgery be widely applied?. The Annals of Thoracic Surgery 2006;82(2):385‐7. [DOI: 10.1016/j.athoracsur.2006.06.049] [DOI] [PubMed] [Google Scholar]
Lozano 2012
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095‐128. [DOI: 10.1016/S0140-6736(12)61728-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mair 2009
- Mair G, Miller JJ, McAllister D, Maclay J, Connell M, Murchison JT, et al. Computed tomographic emphysema distribution: relationship to clinical features in a cohort of smokers. European Respiratory Journal 2009;33(3):536‐42. [DOI: 10.1183/09031936.00111808] [DOI] [PubMed] [Google Scholar]
Mannino 2007
- Mannino DM, Braman S. The epidemiology and economics of chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2007;4(7):502‐6. [DOI: 10.1513/pats.200701-001FM] [DOI] [PubMed] [Google Scholar]
Maxfield 2004
- Maxfield RA. New and emerging minimally invasive techniques for lung volume reduction. Chest 2004;125(2):777‐83. [DOI] [PubMed] [Google Scholar]
McCarthy 2015
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD003793.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McNulty 2014
- McNulty W, Jordan S, Hopkinson NS. Attitudes and access to lung volume reduction surgery for COPD: a survey by the British Thoracic Society. BMJ Open Respiratory Research 2014;1(1):e000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mura 2005
- Mura M, Zompatori M, Mussoni A, Fasano L, Grazia Pacilli AM, Ferro O, et al. Bullous emphysema versus diffuse emphysema: a functional and radiologic comparison. Respiratory Medicine 2005;99:171‐8. [DOI] [PubMed] [Google Scholar]
NICE 2005
- National Institute of Clinical Effectiveness. Lung reduction volume surgery for advanced emphysema ‐ guidance (IPG 114), 2005. https://www.nice.org.uk/guidance/ipg114/resources/lung‐reduction‐volume‐surgery‐for‐advanced‐emphysema‐304015645 (accessed 30 September 2016).
NICE 2010
- National Institute for Clinical Excellence (NICE). Chronic obstructive pulmonary disease. National clinical guideline for management of chronic obstructive pulmonary disease in adults in primary and secondary care, 2010. http://www.nice.org.uk/CG101 (accessed 4 July 2016).
Ninane 2012
- Ninane V, Geltner C, Bezzi M, Foccoli P, Gottlieb J, Welte T, et al. Multicentre European study for the treatment of advanced emphysema with bronchial valves. European Respiratory Journal 2012;39(6):1319‐25. [DOI: 10.1183/09031936.00019711] [DOI] [PubMed] [Google Scholar]
Papandrinopoulou 2012
- Papandrinopoulou A, Tzouda V, Tsoukalas G. Lung compliance and chronic obstructive pulmonary disease. Pulmonary Medicine 2012;2012:1‐6. [DOI: 10.1155/2012/542769] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perera 2012
- Perera PN, Armstrong EP, Sherrill DL, Skrepnek GH. Acute exacerbations of COPD in the United States: inpatient burden and predictors of costs and mortality. COPD: Journal of Chronic Obstructive Pulmonary Disease 2012;9(2):131‐41. [DOI: 10.3109/15412555.2011.650239] [DOI] [PubMed] [Google Scholar]
Pompeo 2014
- Pompeo E. Lung volume reduction surgery for emphysema treatment: state‐of‐the‐art and perspectives. ISRN Pulmonology 2014;2014:418092. [DOI: 10.1155/2014/418092] [DOI] [Google Scholar]
Rathinam 2014
- Rathinam S, Oey I, Steiner M, Spyt T, Morgan MD, Waller DA. The role of the emphysema multidisciplinary team in a successful lung volume reduction surgery programme. European Journal Cardio‐Thoracic Surgery 2014;46(6):1021‐6. [DOI: 10.1093/ejcts/ezu129] [DOI] [PubMed] [Google Scholar]
Refaely 2010
- Refaely Y, Dransfield M, Kramer MR, Gotfried M, Leeds W, McLennan G, et al. Biologic lung volume reduction therapy for advanced homogeneous emphysema. European Respiratory Journal 2010;36(1):20‐7. [DOI: 10.1183/09031936.00106009] [DOI] [PubMed] [Google Scholar]
Russi 1997
- Russi EW, Stammberger U, Weder W. Lung volume reduction surgery for emphysema. European Respiratory Journal 1997;10:208‐18. [DOI] [PubMed] [Google Scholar]
Sciurba 2010
- Sciurba FC, Ernst A, Herth FJF, Strange C, Criner GJ, Marquette CH, et al. A randomized study of endobronchial valves for advanced emphysema. The New England Journal of Medicine 2010;363(13):1233‐44. [DOI: 10.1056/NEJMoa0900928] [DOI] [PubMed] [Google Scholar]
Shah 2011
- Shah PL, Slebos DJ, Cardoso PFG, Cetti E, Voelker K, Levine B, et al. Bronchoscopic lung‐volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham‐controlled, multicentre trial. Lancet 2011;378(9795):997‐1005. [DOI: 10.1016/S0140-6736(11)61050-7] [DOI] [PubMed] [Google Scholar]
Shah 2013
- Shah PL, Zoumot Z, Singh S, Bicknell SR, Ross ET, Quiring J, et al. Endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET): a randomised controlled trial. The Lancet: Respiratory Medicine 2013;1(3):233‐40. [DOI: 10.1016/S2213-2600(13)70047-X] [DOI] [PubMed] [Google Scholar]
Sharafkhaneh 2008
- Sharafkhaneh A, Hanania NA, Kim V. Pathogenesis of emphysema. Proceedings of the American Thoracic Society 2008;5:475‐7. [DOI: 10.1513/pats.200708-126ET] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sin 2006
- Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: role of comorbidities. European Respiratory Journal 2006;28(6):1245‐57. [PUBMED: 17138679] [DOI] [PubMed] [Google Scholar]
Smith 2014
- Smith MC, Wrobel JP. Epidimiology and clinical impact of major comorbidities in patients with COPD. International Journal of COPD 2014;9:871‐88. [DOI: 10.2147/COPD.S49621] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stockley 2009
- Stockley RA, Mannino D, Barnes PJ. Burden and pathogenesis of chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2009;6:524‐6. [DOI: 10.1513/pats.200904-016DS] [DOI] [PubMed] [Google Scholar]
Suki 2003
- Suki B, Lutchen KR, Ingenito EP. On the progressive nature of emphysema: roles of proteases, inflammation, and mechanical forces. American Journal of Respiratory and Critical Care Medicine 2002;168:516‐21. [DOI] [PubMed] [Google Scholar]
Taraseviciene‐Stewart 2008
- Taraseviciene‐Stewart L, Voelkel NF. Molecular pathogenesis of emphysema. The Journal of Clinical Investigation 2008;118(2):394‐402. [DOI: 10.1172/JCI31811] [DOI] [PMC free article] [PubMed] [Google Scholar]
Teschler 1999
- Teschler H, Thompson AB, Stamatis G. Short‐ and long‐term functional results after lung volume reduction surgery for severe emphysema. European Respiratory Journal 1999;13:1170‐6. [DOI] [PubMed] [Google Scholar]
Toma 2003
- Toma TP, Hopkinson NS, Hillier J, Hansell DM, Morgan C, Goldstraw PG, et al. Bronchoscopic volume reduction with valve implants in patients with severe emphysema. Lancet 2003;361(9361):931‐3. [DOI: 10.1016/S0140-6736(03)12762-6] [DOI] [PubMed] [Google Scholar]
Tuder 2003
- Tuder RM, Macgrath S, Neptune E. The pathobiological mechanisms of emphysema models: what do they have in common?. Pulmonary Pharmacology and Therapeutics 2003;16:67‐78. [DOI: 10.1016/S1094-5539(02)00099-8] [DOI] [PubMed] [Google Scholar]
van Agteren 2016
- Agteren JEM, Hnin K, Carson KV, Grosser D, Smith BJ. Bronchoscopic lung volume reduction procedures for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD012158] [DOI] [PMC free article] [PubMed] [Google Scholar]
Venuta 1998
- Venuta F, Giucomo T, Rendina EA, Ricci C, Coloni GF. Thoracoscopic pleural tent. The Annals of Thoracic Surgery 1998;66(5):1833‐4. [DOI] [PubMed] [Google Scholar]
Venuta 2005
- Venuta F, Giacomo T, Rendina EA, Ciccone AM, Diso D, Perrone A, et al. Bronchoscopic lung‐volume reduction with one‐way valves in patients with heterogenous emphysema. The Annals of Thoracic Surgery 2005;79(2):411‐6. [DOI: 10.1016/j.athoracsur.2004.07.048] [DOI] [PubMed] [Google Scholar]
Weder 1997
- Weder W, Thurnheer R, Stammberger U, Bärge M, Russi EW, Bloch KE. Radiologic emphysema morphology is associated with outcome after surgical lung volume reduction. The Annals of Thoracic Surgery 1997;64(2):313‐20. [DOI: ] [DOI] [PubMed] [Google Scholar]
Weder 2009
- Weder W, Tutic M, Bloch KE. Lung volume reduction surgery in non heterogeneous emphysema. Thoracic Surgery Clinics 2009;19(2):193‐9. [DOI: 10.1016/j.thorsurg.2009.03.002] [DOI] [PubMed] [Google Scholar]
Wedzicha 2003
- Wedchiza JA, Donaldson GC. Exacerbations of chronic obstructive pulmonary disease. Respiratory Care 2003;48(12):1204‐15. [PubMed] [Google Scholar]
Welling 2015
- Welling JBA, Hartman JE, Hacken NHT, Klooster K, Slebos D‐J. The minimal important difference for the St George's Respiratory Questionnaire in patients with severe COPD. St. George's Respiratory Questionnaire: MCID. 2015;46(6):1598‐604. [DOI] [PubMed] [Google Scholar]
Yusen 1996
- Yusen RD, Lefrak SS. Evaluation of patients with emphysema for lung volume reduction surgery. Washington University Emphysema Surgery Group. Seminars in Thoracic and Cardiovascular Surgery 1996;8(1):83‐93. [PubMed] [Google Scholar]
Yusen 2003
- Yusen RD, Lefrak SS, Gierada DS, Davis GE, Meyers BF, Patterson GA, et al. A prospective evaluation of lung volume reduction surgery in 200 consecutive patients. Chest 2003;123(4):1026‐37. [DOI] [PubMed] [Google Scholar]
Zahid 2011
- Zahid I, Sharif S, Routledge T, Scarci M. Is lung volume reduction surgery effective in the treatment of advanced emphysema?. Interactive Cardiovascular and Thoracic Surgery 2011;12(3):480‐6. [DOI: 10.1510/icvts.2010.252213] [DOI] [PubMed] [Google Scholar]
Zoumot 2014
- Zoumot Z, Jordan S, Hopkinson NS. Emphysema: time to say farewell to therapeutic nihilism. Thorax 2014;69(11):973‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zoumot 2015
- Zoumot Z, Kemp SV, Singh S, Bicknell SR, McNulty WH, Hopkinson NS, et al. Endobronchial coils for severe emphysema are effective up to 12 months following treatment: medium term and cross‐over results from a randomised controlled trial. PLoS One 2015;10(4):1‐13. [DOI: 10.1371/journal.pone.0122656] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Hensley 1999
- Hensley M, Coughlan JL, Davies HR, Gibson P. Lung volume reduction surgery for diffuse emphysema. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD001001; PUBMED: 10796577] [DOI] [PubMed] [Google Scholar]
Tiong 2006
- Tiong LU, Gibson PG, Hensley MJ, Hepworth R, Laserson TJ, Smith B, et al. Lung volume reduction surgery for diffuse emphysema. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD001001.pub2] [DOI] [PubMed] [Google Scholar]


