Abstract
A putative source of inappropriate immune activation that drives human immunodeficiency virus (HIV)-1 immunopathogenesis is the gastrointestinal tract. Even with effective antiretroviral treatment, residual activation persists. We hypothesized that an oral probiotic could improve the residual immune activation in chronic treated HIV-1 infection, and tested a Bacillus coagulans GBI-30, 6086 capsule probiotic in HIV-1-infected persons with suppressed viremia on stable antiretroviral therapy in a 3-month double-blind placebo-controlled trial (10 probiotic, 7 placebo). The Gastrointestinal Symptom Rating Scale (GSRS) was administered monthly. Blood was tested at the start and end of placebo/probiotic administration for viremia, CD4+ T cell percentage/concentration, soluble (s)CD14, soluble intestinal fatty acid binding protein, sCD163, D-dimer, C-reactive protein (CRP), interleukin-8, and tumor necrosis factor-α. All participants maintained viremia <40 RNA copies/ml. The probiotic was safe and well tolerated, and appeared to improve chronic gastrointestinal symptoms. Its administration was associated with a significant increase in the percentage of blood CD4+ T cells compared to placebo (+2.8% versus −1.8%, p=0.018) although CD4+ T cell concentrations were generally unchanged in both groups. None of the biomarkers showed significant changes on probiotic treatment or between-group differences in change (although significance was borderline for a greater sCD163 drop in the probiotic versus placebo group, p=0.05). Some biomarkers showed significant correlations to each other, particularly D-dimer with CRP and sCD14 with tumor necrosis factor (TNF)-α. These data demonstrate the safety and possible benefit of this probiotic for residual inflammation in treated HIV-1 infection, although further study will be required to determine the immune pathways involved.
Introduction
Untreated human immunodeficiency virus type 1 (HIV-1) infection generally leads to progressive loss of CD4+ T cells, and growing evidence indicates a potential central role of gut-associated lymphoid tissue. This compartment, which contains the majority of total body lymphocytes,1 is massively depleted by direct HIV-1 infection of CD4+ T cells in early acute infection,2 does not recover after acute infection, and poorly reconstitutes with combination antiretroviral treatment (cART) that restores blood CD4+ T cell levels.3 It has been suggested that this persisting defect leads to systemic translocation of gut bacteria causing the immune activation that drives chronic disease progression.4 Alternatively, the depletion of key regulatory CD4+ iNKT cells in the gut5 or ongoing viral replication in this compartment6 drives inappropriate immune activation.
As a major reservoir of immune cells that are constantly exposed to antigens from food, microbial flora, and pathogens, the gut is a major determinant of systemic immune processes. Gut cells interact with antigens through pattern recognition receptors such as toll-like receptors (TLRs), setting the net state of inflammation.7 Many normal bacterial flora provide antiinflammatory signals, while pathogens can drive inflammatory danger signals. It is evident that the complex gut flora profoundly influences health and disease; for example, different profiles are associated with obesity and nonobesity in monozygotic twins,8 and vary with diseases ranging from autoimmunity9 to cancer.10
Administering probiotics to alter gut microbial flora has been considered for health and disease. In the simian immunodeficiency virus (SIV)-macaque model of AIDS, probiotic administration to antiretroviral-treated macaques increased reconstitution of colonic CD4+ T cells, reducing inflammation-associated fibrosis and increasing antigen-presenting cell frequency and function.11 For human HIV-1 infection probiotic data are very limited, and probiotics have not been tested in infected persons on effective cART who have significant residual immune activation. Here we study the safety and immune effects of the probiotic Bacillus coagulans GBI-30, 6086 (GanedinBC30), a gram-positive spore-forming bacterium with potential immunomodulatory effects.12–15
Materials and Methods
Study design
This was a randomized, double-blind, placebo-controlled study in persons with chronic HIV-1 infection who were receiving effective cART. Twenty-four subjects were randomized to placebo or probiotic and monitored for 90 days. The study was performed under a BioMed Central Institutional Review Board (San Diego, CA) approved protocol and registered with ClinicalTrials.gov (NCT01184456).
Study subjects
Adults with documented HIV seropositivity were recruited from greater metropolitan Los Angeles. Inclusion criteria included blood CD4+ T cell counts ≥250 cells/mm3 and plasma viremia measurements <50 HIV RNA copies/ml for at least 6 months, high functioning Karnofsky score ≥60%, baseline (within 30 days of study entry) blood values of absolute neutrophils >1,000 cells/mm3, hemoglobin >9 g/dl, platelets >75,000/mm3, creatinine <1.5×the upper limit of normal, AST and ALT <3×the upper limit of normal, and bilirubin <2 mg/dl. Exclusion criteria included pregnancy, active infections, treatment with other probiotics, any antibiotic therapy within 30 days, immunosuppressive drug treatments, malabsorption syndrome, and liver disease. The first subject and last subjects were enrolled on August 9, 2010 and February 18, 2011, with the last follow-up on May 13, 2011.
Probiotic administration
For 24 participants 1:1 randomization was predetermined to receive placebo or probiotic (GanedinBC30). Subjects received a daily capsule containing either placebo or 2 billion colony-forming units of BC30 (Bacillus coagulans GBI-30, 6086), with instructions to swallow the capsule with water at the same time each day, regardless of meals. Adherence to the study protocol was determined by caplet counts. Both care providers and participants were blinded.
Clinical follow-up
Subjects received physical examinations at days 0, 30, 60, and 90. At each visit they were administered the Gastrointestinal Symptoms Rating Scale (GSRS) survey.16 Blood was obtained at days 0 and 90 for assessment of CD4+ T cell counts, viremia, and biomarker testing.
Proinflammatory blood biomarkers
Testing for serum C-reactive protein (CRP), interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor (TNF)-α were performed by IBT Laboratories (Lenexa, KS). Serum D-dimer testing was performed by Saint Luke's Regional Laboratory (Kansas City, MO). Serum lipopolysaccharide (LPS) testing was performed by Viracor-IBT Laboratories (Lee's Summit, MO). Serum soluble CD14 (sCD14) and serum soluble CD163 (sCD163) levels were determined using the Human Quantikine ELISA assay (R&D Systems, Minneapolis, MN). Serum intestinal-type fatty acid binding protein (sI-FABP) was measured using the Human FABP-2 DuoSet ELISA (R&D Systems, Minneapolis, MN). Unfortunately, LPS, IL-1β, and IL-6 were not detected in most samples (<0.05 EU/ml, <7.1 pg/ml, and <0.6 pg/ml respectively), and therefore not analyzed.
Statistics
Within-group changes from days 0 to 90 were evaluated using paired Student's t-test (two-tailed, nonequal distributions). Between-group comparisons were performed using Wilcoxon rank-sum test. Correlations between variables were evaluated using Spearman rank correlation. A p-value≤0.05 was considered significant (unadjusted for multiple comparisons in this pilot study).
Results
Participant demographics and clinical symptoms
Twenty-four subjects were randomized 1:1 to receive probiotic or placebo for 90 days. Seven did not complete the study (all men), including two receiving probiotic (one withdrew due to lack of transportation and the other did not follow-up) and five receiving placebo (one withdrew because he felt no benefit and suspected he was receiving a placebo and the others did not follow-up); none of these subjects withdrew due to complaints related to probiotic or placebo.
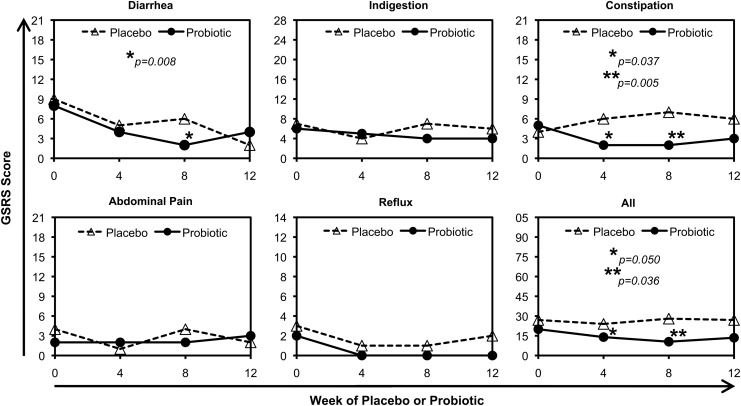
The remaining 17 participants (10 probiotic and seven placebo) who completed the study had reasonably comparable ages, durations of cART including time on their most current cART regimen, nadir CD4+ T cell counts and percentages, and baseline CD4+ T cell counts and percentages (Table 1). No serious adverse events were reported. The GSRS was monitored at baseline (0) and 4, 8, and 12 weeks after the start of probiotic or placebo. Gastrointestinal symptoms were low for both groups at baseline and only mild symptoms were reported during the study. In the probiotic group 3/10 reported bloating. In the placebo group, 2/7 reported increased flatulence and 1/7 reported increased diarrhea. Summed GSRS scores for all categories (diarrhea, indigestion, constipation, abdominal pain, reflux) and the combined totals were examined (Fig. 1). For in-group comparisons of symptoms at 4, 8, or 12 weeks compared to baseline (paired Student's t-test), there were no significant differences in the placebo group. In the probiotic group, there were significant reductions in constipation scores at weeks 4 and 8 (p=0.037 and p=0.005, respectively), and total scores at weeks 4 and 8 (p=0.050 and p=0.036, respectively). Between groups, there were significant differences only at week 8 for the constipation and combined total categories (each p=0.049). Overall, these findings suggest potential benefit in gastrointestinal symptoms due to probiotic administration.
Table 1.
Demographics of the Participants
| Group | ID | Age | Sex | Days on cART | Days current cART | Current cART | CD4+ T cell nadir/mm3 | %CD4+ T cell nadir | Baseline CD4+ T cells/mm3 | Ending CD4+ T cells/mm3 | Baseline %CD4+ T cell | Ending %CD4+ T cell |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Probiotic | GAN02 | 37 | M | 4,506 | 1,161 | TDF/FTC/ATV/r | 17 | 4.0% | 335 | 343 | 23.9% | 26.4% |
| GAN04 | 42 | M | 1,679 | 449 | TDF/FTC/DRV/r | 77 | N/A | 452 | 494 | 21.5% | 24.7% | |
| GAN07 | 59 | M | 5,186 | 771 | RAL/FTC/NVP | 139 | 19.8% | 449 | 366 | 26.4% | 30.5% | |
| GAN09 | 50 | M | 2,499 | 980 | TDF/FTC/LPV/r | 168 | 8.0% | 377 | 377 | 14.5% | 13.0% | |
| GAN11 | 40 | M | 1,542 | 251 | ABC/DRV/r/TDF/FTC | 18 | 3.5% | 278 | 510 | 25.3% | 30.0% | |
| GAN12 | 45 | M | 3,533 | 306 | TDF/FTC/EFV | N/A | N/A | 571 | 757 | 40.8% | 44.5% | |
| GAN13 | 48 | M | 4,336 | 835 | ABC/3TC/NVP | 191 | N/A | 521 | 622 | 41.4% | 44.4% | |
| GAN15 | 72 | M | 5,087 | 589 | TDF/FTC/ATV/r | 51 | 25.4% | 572 | 505 | 28.6% | 29.7% | |
| GAN17 | 59 | M | 3,029 | 1,128 | ATV/r/RAL | 180 | 18.0% | 517 | 686 | 28.7% | 31.2% | |
| GAN23 | 52 | M | 2,170 | 1,450 | ABC/3TC/FPV/r/NVP | 384 | 24.0% | 818 | 709 | 37.2% | 37.3% | |
| Median | 49 | 3,281 | 803 | 139 | 13.0% | 485 | 508 | 27.5% | 30.3% | |||
| SD | 11 | 1,375 | 395 | 115 | 8.8% | 152 | 150 | 8.6% | 9.4% | |||
| Placebo | GAN01 | 53 | M | 262 | 356 | TDF/FTC/EFV | 374 | 17.8% | 600 | 631 | 25.0% | 28.7% |
| GAN03 | 51 | M | 3,771 | 1,552 | TDF/FTC/EFV | 371 | 23.0% | 644 | 580 | 46.0% | 44.6% | |
| GAN10 | 37 | M | 1,066 | 658 | TDF/FTC/RAL | 252 | 19.4% | 432 | 351 | 27.0% | 19.5% | |
| GAN14 | 52 | M | 4,353 | 460 | ABC/3TC/RTV | N/A | N/A | 416 | 486 | 19.8% | 18.0% | |
| GAN18 | 49 | M | 3,784 | 791 | ABC/3TC/TDF/DRV | 7 | 2.0% | 303 | 325 | 27.5% | 25.0% | |
| GAN21 | 46 | M | 3,957 | 692 | ATV/r/RAL | 194 | 19.4% | 290 | 333 | 24.2% | 23.8% | |
| GAN24 | 51 | F | 1,704 | 794 | ABC/3TC/ATV | 34 | 4.3% | 649 | 963 | 29.5% | 27.5% | |
| Median | 51 | 3,771 | 692 | 223 | 18.6% | 432 | 486 | 27.0% | 25.0% | |||
| SD | 6 | 1,645 | 387 | 159 | 8.8% | 155 | 229 | 8.3% | 8.8% |
TDF, tenofovir; FTC, emtricitabine; ATV, atazanavir; /r, low dose ritonavir; DRV, darunavir; RAL, raltegravir; LPV, lopinavir; ABC, abacavir; EFV, efavirenz; NVP, nevirapine; 3TC, lamivudine; FPV, fosamprenavir; RTV, ritonavir; NA, not available.
FIG. 1.
Gastrointestinal symptoms. Median scores from the Gastrointestinal Symptom Rating Scale (GSRS) for five categories of symptoms and all combined are plotted for placebo (n=7) and probiotic (n=9) groups. Subject GAN 009 was excluded because survey information was not provided for two study visits. The maximal value on the y-axis is the highest possible score on the GSRS. Asterisks indicate probiotic group significant differences from week 0 (paired Student's t-test); there were no significant differences from week 0 in the placebo group. Comparisons of placebo versus probiotic groups showed significant differences only for the constipation category at week 8 (p=0.049) and the combination of all symptoms at week 8 (p=0.049).
Increased percentage of CD4+ T cells was observed in the probiotic group
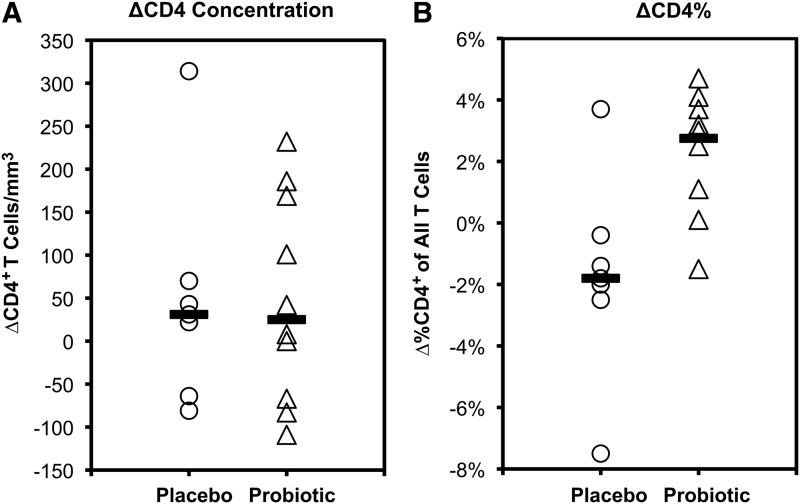
Blood CD4+ T cells at baseline and 90 days were assessed. For both placebo and probiotic groups, median absolute CD4+ T cell counts for both groups were minimally changed, although there was variability between individuals ranging from −81 to +315 (median +31) cells/mm3 for the placebo group and −109 to +232 (median +25) cells/mm3 for the probiotic group (Fig. 2A). However, changes in CD4+ percentage of total T cells were significantly different between placebo and probiotic groups (Fig. 2B), with medians of −1.8% (range −7.5% to +3.7%) versus +2.8% (range −1.5 to +4.7%), respectively (p=0.018). Further taking CD4+ T cell measurements over the prior year before the study into account for the baseline, the slopes of CD4+ T cell counts were similar but the percentages increased more in the probiotic group compared to the placebo group (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/aid). Viremia remained <40 copies HIV-1 RNA/ml plasma in all subjects during the study, indicating that gross changes in viral replication did not explain these differences. These findings suggest that probiotic administration had an immunologic impact.
FIG. 2.
Changes in blood CD4+ T cell counts and percentages during the study. For each group, the changes in blood CD4+ T cell counts (A) and percentages (B) during the 90 days of placebo or probiotic administration are plotted. Each point represents one individual, and the bars indicate the medians for each group.
Proinflammatory blood biomarkers did not change consistently
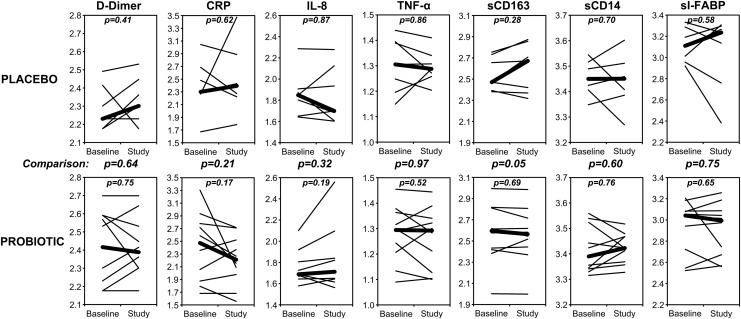
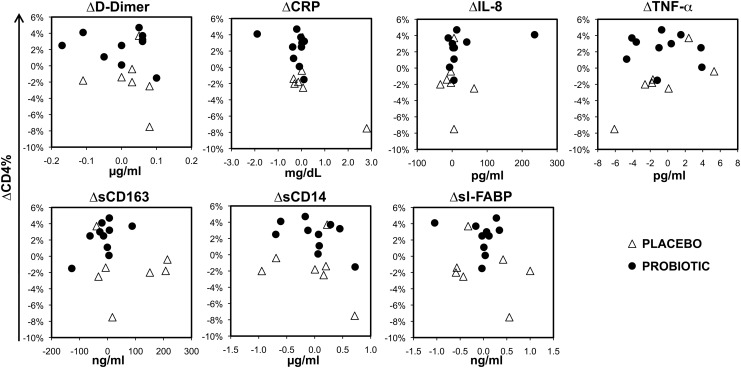
To evaluate for potential inflammatory correlates, several blood biomarkers were evaluated (Fig. 3). There were no statistically significant changes in D-dimer, C-reactive protein (CRP), or intestinal-type fatty acid binding protein (I-FABP) for either the placebo or probiotic group. Between group comparisons of changes in these markers approached statistical significance only for sCD163 (p=0.094). Furthermore, comparisons of changes in these markers to changes in the percentage of CD4+ T cells did not show any significant correlations (Fig. 4). These results indicate that the facets of systemic inflammation reflected by these markers are not overtly affected by administration of probiotic, and suggest that the observed change in percentage of CD4+ T cells in the probiotic group is not simply relatable to changes in the inflammatory processes reflected by these blood markers.
FIG. 3.
Levels of blood biomarkers during the study. For each individual, the blood concentrations of the indicated biomarkers are plotted for values at start to end of placebo/probiotic administration (thin lines), on log10 scales. Thick lines indicate the medians for each group. Not shown are lipopolysaccharide (LPS), interleukin (IL)-1β, and IL-6 because these were not detected (<0.05 EU/ml, <7.1 pg/ml, and <0.6 pg/ml, respectively). Changes in these markers within groups were not statistically significant. Comparisons of changes between groups approached significance (p<0.2) only for soluble (s)CD163 (p=0.05). Units were μg/ml for D-dimer, mg/dl for C-reactive protein (CRP), pg/ml for IL-8, pg/ml for tumor necrosis factor (TNF)-α, ng/ml for sCD163, μg/ml for sCD14, and ng/ml for serum intestinal-type fatty acid binding protein (sI-FABP).
FIG. 4.
Changes in percentage of CD4+ T cells versus changes in proinflammatory blood biomarkers during the study. Changes in the biomarkers and CD4% T cells between the start and end of placebo/probiotic administration are plotted against each other.
Several proinflammatory blood biomarkers were associated with each other
Given the mixed literature regarding the prognostic value of different biomarkers in the pathogenesis and complications of HIV-1 infection, the data were analyzed for associations between biomarkers (Table 2 and Supplementary Fig. S2). Notably, several markers significantly correlated. Across measurements at both time points (Table 2A and Supplementary Fig. S2A), D-dimer was strongly correlated with CRP and TNF-α was strongly correlated with sCD163. Borderline correlations were seen for D-dimer with sI-FABP, sI-FABP with IL-8, TNF-α with sCD14, and TNF-α with sCD163; the two monocyte/macrophage markers sCD14 and sCD163 showed only a trend for a correlation (p=0.078). However, comparison of changes in biomarkers between the start and end of the study (Table 2B and Supplementary Fig. S2B) showed strong correlations for D-dimer with CRP (p=0.00002), CRP with sCD14 (p=0.004), and sCD14 with D-dimer (p=0.008), a borderline correlation for sCD14 with TNF-α, and no correlation for sCD14 with sCD163 (p=0.30). These findings indicate that some of these markers reflect the same inflammatory processes.
Table 2.
Correlations Between Changes in Blood Biomarker Levels Over the Course of the Study
| D-dimer | CRP | IL-8 | TNF-α | sCD163 | sCD14 | I-FABP | |
|---|---|---|---|---|---|---|---|
| A. Absolute Values | |||||||
| D-dimer | N/A | 0.51 (0.002*) | 0.00 (0.98) | 0.21 (0.23) | 0.26 (0.15) | −0.05 (0.80) | −0.35 (0.044*) |
| CRP | 0.51 (0.002*) | N/A | 0.28 (0.11) | 0.30 (0.087) | −0.01 (0.97) | 0.16 (0.37) | 0.13 (0.47) |
| IL-8 | 0.00 (0.98) | 0.28 (0.11) | N/A | 0.28 (0.12) | −0.04 (0.82) | 0.26 (0.12) | 0.36 (0.035*) |
| TNF-α | 0.21 (0.23) | 0.30 (0.087) | 0.28 (0.12) | N/A | 0.51 (0.002*) | 0.34 (0.050*) | 0.12 (0.49) |
| sCD163 | 0.26 (0.15) | −0.01 (0.97) | −0.04 (0.82) | 0.51 (0.002*) | N/A | 0.31 (0.078) | −0.13 (0.46) |
| sCD14 | −0.05 (0.80) | 0.16 (0.37) | 0.26 (0.12) | 0.34 (0.050*) | 0.31 (0.078) | N/A | 0.11 (0.52) |
| I-FABP | −0.35 (0.044*) | 0.13 (0.47) | 0.36 (0.035*) | 0.12 (0.49) | −0.13 (0.46) | 0.11 (0.52) | N/A |
| B. Changes in Values | |||||||
| D-dimer | N/A | 0.84 (0.00002*) | 0.08 (0.77) | −0.26 (0.31) | −0.14 (0.59) | 0.62 (0.008*) | 0.03 (0.90) |
| CRP | 0.84 (0.00002*) | N/A | 0.12 (0.64) | −0.15 (0.56) | −0.12 (0.65) | 0.65 (0.004*) | 0.33 (0.20) |
| IL-8 | 0.08 (0.77) | 0.12 (0.64) | N/A | 0.11 (0.69) | −0.39 (0.13) | 0.10 (0.71) | 0.07 (0.78) |
| TNF-α | −0.26 (0.31) | −0.15 (0.56) | 0.11 (0.69) | N/A | −0.28 (0.27) | −0.50 (0.042*) | −0.06 (0.82) |
| sCD163 | −0.14 (0.59) | −0.12 (0.65) | −0.39 (0.13) | −0.28 (0.27) | N/A | −0.26 (0.30) | 0.46 (0.065) |
| sCD14 | 0.62 (0.008*) | 0.65 (0.004*) | 0.10 (0.71) | −0.50 (0.042*) | −0.26 (0.30) | N/A | 0.02 (0.94) |
| I-FABP | 0.03 (0.90) | 0.33 (0.20) | 0.07 (0.78) | −0.06 (0.82) | 0.46 (0.065) | 0.02 (0.94) | N/A |
For each of the listed biomarkers, Spearman rank correlation rho values (p-value) are given for (A) intraindividual comparisons of blood levels including both time points at the start and finish of the study and (B) intraindividual comparisons of changes of blood levels between the start and finish of placebo/probiotic administration.
CRP, C-reactive protein; IL, interleukin; TNF, tumor necrosis factors sCD163, soluble CD163; sCD14, soluble CD14; I-FABP, intestinal-type fatty acid binding protein.
p≤0.05.
Discussion
The gastrointestinal tract is the major reservoir of activated CD4+ T cells and is centrally involved in HIV-1 pathogenesis. Regardless of infection route, this compartment is the first major site of viral replication in acute infection, sustaining massive loss of CD4+ T cells.2 It is believed that is a major contributor to the inappropriate chronic immune activation that drives progressive immunodeficiency. This activation does not fully normalize with cART, and contributes to increased morbidity such as premature atherosclerotic disease seen in treated persons. At least three mechanisms have been proposed for the role of the gastrointestinal tract: reduced containment of bowel flora and systemic bacterial translocation,4 ongoing viral replication despite cART,6 and loss of key regulatory CD4+ T cells that downmodulate immune activation.5
Bowel flora play a key immunomodulatory role in the gastrointestinal tract. Bacterial components interact with pattern recognition receptors such as TLRs, which give proinflammatory or antiinflammatory immune signals depending on the receptor.17 Certain normal flora promote mucosal integrity and reduce inflammation, and perturbations of flora have been associated with various immune disorders.17 Thus altering the bacterial flora offers a potential avenue to intervene. This pilot study was undertaken to explore the safety and effects of a probiotic in persons with chronic HIV-1 infection who were on cART with viremia to <50 HIV-1 RNA copies/ml, considered to be optimally treated, given that such persons still have residual immune activation that likely mediates the heightened risk of complications such as premature atherosclerotic cardiovascular disease.
Bacillus coagulans is not known to be pathogenic, and the BC30 probiotic utilized in our study was delivered in capsules as inert spores that survive stomach acid and bloom in the gastrointestinal tract,18 and has been safe in human studies.19,20 Moreover, this formulation is stable indefinitely at room temperature, in contrast to some other probiotics. Symptomatically, administration has been shown to improve postprandial gas-related intestinal symptoms21 and to reduce abdominal pain, bloating, and diarrhea in patients with irritable bowel syndrome.22 Given these properties, this probiotic was selected for testing. Our findings were similar in observing this probiotic to be safe and well-tolerated. In this pilot study, the improvement in constipation scores was consistent with prior observations,21,22 although the small sample size and relatively low baseline gastrointestinal symptoms of these generally healthy individuals limited our power to detect a clinical impact.
A handful of prospective studies have examined other probiotics in persons with HIV-1 infection with mixed clinical and immunologic results. A placebo-controlled study of 39 American adults by Wolf et al. administered 3 weeks of freeze-dried Lactobacillus reuteri to persons with CD4+ T cell counts >400 cells/mm3 blood on zidovudine monotherapy or no treatment for HIV-1, finding no clinically significant impact.23 Also negative was a 25-week placebo-controlled trial of oral capsules containing Lactobacillus rhamnosus and Lactobacillus reuteri in 65 Tanzanian women with CD4+ T cell counts >350 cells/mm3 blood not on cART, finding no change in blood CD4+ T cell counts, IgG, IgE, IFN-γ, IL-10, or diarrhea symptoms.24
Another study by the same group included 112 Tanzanian women not on cART administered yogurt supplemented with Lactobacillus rhamnosus for 4 weeks; they demonstrated no change in blood CD4+ T cell counts or clinical symptoms.25 In contrast, a study of 77 Brazilian infected infants mostly not receiving cART given Bifidobacterium bifidum and Streptococcus thermophilus in milk demonstrated an increase in CD4+ T cell counts over 2 months and less diarrhea.26 Another recent pediatric study of 127 Indian children untreated with cART also found significant increases in CD4+ T cell counts over 12 weeks.27 An adult study of 24 cART-untreated Nigerian women receiving yogurt without or with supplementation with Lactobacillus rhamnosus and Lactobacillus reuteri for 30 days similarly noted increased CD4+ T cell counts,28 as well as a larger placebo-controlled study of about 100 Tanzanians administered yogurt supplemented with Lactobacillus rhamnosus and micronutrients.25
These studies, mostly in resource-limited settings and in persons not on effective cART, highlight the challenges of probiotic administration in such settings, such as lack of refrigeration required by many formulations and inadequate clinical study design, as discussed in a consensus statement by Monachese et al.29 Besides the differing patient characteristics, a major caveat to comparing these studies is the diversity of probiotics tested. Although the precise interactions of bacterial flora with the gut immune system are not well understood, the diversity of both innate immune receptors and bacterial proteins indicates that different probiotics likely vary widely in their effects on gut and gut regulation of systemic immunity.
Our study is one of the first to evaluate double-blind placebo-controlled probiotic administration to persons with HIV-1 infection on optimal cART as defined by viremia below the limits of conventional clinical testing. In contrast to a recent probiotic study of Lactobacillus rhamnosus in a similar sized cohort of HIV-1-infected persons on cART,30 we observed no significant rise in blood absolute CD4+ T cell counts. However, we observed an increase in the CD4+ percentage of total T cells, which is a strong independent predictor of immune status and disease progression.31 Unlike prior studies, our subjects were on successful cART treatment with relatively high CD4+ T cell counts, perhaps limiting the dynamic range to observe a rise. Furthermore, we cannot exclude the possibility that this change was not specific to probiotic administration.
Our results are consistent with increasing data suggesting that Bacillus coagulans can have immunomodulatory activity. In vitro, cell wall and metabolites from this bacterium have several effects on immune cells, increasing or decreasing various functions (chemotaxis, phagocytosis, cytokine production).12 Small clinical studies administering this probiotic in vivo have shown increased blood T cell reactivity (CD69 upregulation) and cytokine production (IFN-γ, TNF-α, IL-6, IL-8) after ex vivo exposure to strains of adenovirus and influenza virus,13,14 as well as a reduction of blood CRP in persons with rheumatoid arthritis.15 These apparently contradictory findings of increased inflammatory cytokine production versus reduction of an inflammatory marker suggest that the potential beneficial effects of this probiotic (and probably probiotics in general) are not due to simple down-regulation or up-regulation of immunity, but rather to immunomodulation for more appropriate responses.
Several plasma biomarkers known to be abnormal in HIV-1 infection were examined as potential correlates of immunomodulation by probiotic administration. Those related to gut microbial translocation included sCD14 associated with increased mortality32 and premature cardiovascular disease,33 I-FABP associated with gut mucosal endothelial damage,32 and LPS associated with disease status and T cell activation.34 More general markers of inflammation and immune activation included sCD163 associated with premature cardiovascular disease,35 D-dimer associated with vascular dysfunction/disease36 and increased mortality,37 IL-6 associated with vascular dysfunction36 and increased mortality,37 CRP associated with increased mortality,37 and other proinflammatory cytokines previously shown to be increased in HIV-1 infection including IL-8,38 TNF-α,38,39 and IL-1β.39 Unfortunately, LPS, IL-1 β, and IL-6 were not reliably detectable in the commercial laboratories running those assays, and there were no cells available for direct evaluation of blood T cell activation. For the remaining plasma biomarkers, administration of probiotic was not associated with any significant difference compared to placebo, and none of these markers showed significant correlation with the observed change in percentage of CD4+ T cells. It is unclear whether this lack of observed changes and correlations is related to the small cohort size, relatively short duration of follow-up, immunomodulation in the gut compartment not reflected in the blood, or a biological impact not reflected by these biomarkers.
To our knowledge, the only published data on the effect of probiotics on bacterial translocation and cytokine markers in HIV-1-infected persons involved a recent small study (five persons per arm) of cART-untreated subjects, showing increased CD4+ T cell counts, reduced plasma bacterial RNA, and reduced plasma IL-6 (but unchanged IL-1β and TNF-α) in subjects receiving a “synbiotic” combination of prebiotic (Agave tequilana extract) and probiotic (Lactobacillus rhamnosus plus Bifidobacterium lactis) but not those receiving either prebiotic or probiotic alone.
Incidentally, it was interesting that we observed relatively strong associations between some of these parameters. Sandler et al. and Borges et al. also noted an association of D-dimer with CRP,32,40 with the former additionally noting an association of D-dimer with sCD14. Overall, the biological relationships of these and other biomarkers remain unclear; for example, while D-dimer has been correlated with monocyte activation in HIV-1 infection,41 we saw a correlation of D-dimer only with sCD14 and not CD163, and saw no correlation between sCD14 and sCD163 despite the fact that both are markers on activated monocyte/macrophages.
In summary, the Bacillus coagulans BC30 probiotic preparation in our study was safe and well tolerated in persons with chronic HIV-1 infection on suppressive cART. Administration appeared to increase the percentage of CD4+ T cells, an independent predictor of immune status. A panel of biomarkers of inflammation did not correlate with this change, suggesting either that these markers do not reflect the processes being affected by the probiotic or that the blood compartment does not reflect the processes in the gastrointestinal compartment, a major reservoir of total body T cells. Sampling of the gastrointestinal tract for immune assessment would be a useful adjunct, and further studies will be required to confirm our results and to define the potential clinical benefit and mechanism(s) behind our findings.
Supplementary Material
Acknowledgments
This study was supported by Ganeden Biotech (Mayfield Heights, OH). The funder had no direct role in the design, implementation, or data interpretation of the study. We thank the research volunteers who participated in this study.
Author Disclosure Statement
H.K. serves is a consultant and shareholder of Merck.
References
- 1.Mowat AM. and Viney JL: The anatomical basis of intestinal immunity. Immunol Rev 1997;156:145–166 [DOI] [PubMed] [Google Scholar]
- 2.Brenchley JM, Schacker TW, Ruff LE, et al.: CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 2004;200(6):749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barouch DH, Liu J, Li H, et al.: Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 2012;482(7383):89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenchley JM, Price DA, Schacker TW, et al.: Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12(12):1365–1371 [DOI] [PubMed] [Google Scholar]
- 5.Ibarrondo FJ, Wilson SB, Hultin LE, et al.: Depletion of gut CD4-expressing iNKT cells contributes to systemic immune activation in HIV-1 infection. Mucosal Immunol 2012;61:591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poles MA, Boscardin WJ, Elliott J, et al.: Lack of decay of HIV-1 in gut-associated lymphoid tissue reservoirs in maximally suppressed individuals. J Acquir Immune Defic Syndr 2006;43(1):65–68 [DOI] [PubMed] [Google Scholar]
- 7.Chu H. and Mazmanian SK: Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat Immunol 2013;14(7):668–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roederer M, Keele BF, Schmidt SD, et al. : Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature 2014;505(7484):502–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Brien KL, Liu J, King SL, et al. : Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. Nat Med 2009;15(8):873–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutnick NA, Carnathan DG, Dubey SA, et al. : Baseline Ad5 serostatus does not predict Ad5 HIV vaccine-induced expansion of adenovirus-specific CD4+ T cells. Nat Med 2009;15(8):876–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klatt NR, Canary LA, Sun X, et al. : Probiotic/prebiotic supplementation of antiretrovirals improves gastrointestinal immunity in SIV-infected macaques. J Clin Invest 2013;123(2):903–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen GS, Benson KF, Carter SG, and Endres JR: GanedenBC30 cell wall and metabolites: Anti-inflammatory and immune modulating effects in vitro. BMC Immunol 2010;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimmel M, Keller D, Farmer S, and Warrino DE: A controlled clinical trial to evaluate the effect of GanedenBC(30) on immunological markers. Methods Find Exp Clin Pharmacol 2010;32(2):129–132 [DOI] [PubMed] [Google Scholar]
- 14.Baron M: A patented strain of Bacillus coagulans increased immune response to viral challenge. Postgrad Med 2009;121(2):114–118 [DOI] [PubMed] [Google Scholar]
- 15.Mandel DR, Eichas K, and Holmes J: Bacillus coagulans: A viable adjunct therapy for relieving symptoms of rheumatoid arthritis according to a randomized, controlled trial. BMC Complement Altern Med 2010;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimenas E, Carlsson G, Glise H, et al. : Relevance of norm values as part of the documentation of quality of life instruments for use in upper gastrointestinal disease. Scand J Gastroenterol Suppl 1996;221:8–13 [DOI] [PubMed] [Google Scholar]
- 17.Belkaid Y. and Hand TW: Role of the microbiota in immunity and inflammation. Cell 2014;157(1):121–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maathuis AJ, Keller D, and Farmer S: Survival and metabolic activity of the GanedenBC30 strain of Bacillus coagulans in a dynamic in vitro model of the stomach and small intestine. Benef Microbes 2010;1(1):31–36 [DOI] [PubMed] [Google Scholar]
- 19.Endres JR, Clewell A, Jade KA, et al. : Safety assessment of a proprietary preparation of a novel probiotic, Bacillus coagulans, as a food ingredient. Food Chem Toxicol 2009;47(6):1231–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endres JR, Qureshi I, Farber T, et al. : One-year chronic oral toxicity with combined reproduction toxicity study of a novel probiotic, Bacillus coagulans, as a food ingredient. Food Chem Toxicol 2011;49(5):1174–1182 [DOI] [PubMed] [Google Scholar]
- 21.Kalman DS, Schwartz HI, Alvarez P, et al. : A prospective, randomized, double-blind, placebo-controlled parallel-group dual site trial to evaluate the effects of a Bacillus coagulans-based product on functional intestinal gas symptoms. BMC Gastroenterol 2009;9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hun L: Bacillus coagulans significantly improved abdominal pain and bloating in patients with IBS. Postgrad Med 2009;121(2):119–124 [DOI] [PubMed] [Google Scholar]
- 23.Wolf BW, Wheeler KB, Ataya DG, and Garleb KA: Safety and tolerance of Lactobacillus reuteri supplementation to a population infected with the human immunodeficiency virus. Food Chem Toxicol 1998;36(12):1085–1094 [DOI] [PubMed] [Google Scholar]
- 24.Hummelen R, Changalucha J, Butamanya NL, et al. : Effect of 25 weeks probiotic supplementation on immune function of HIV patients. Gut Microbes 2011;2(2):80–85 [DOI] [PubMed] [Google Scholar]
- 25.Hummelen R, Hemsworth J, Changalucha J, et al. : Effect of micronutrient and probiotic fortified yogurt on immune-function of anti-retroviral therapy naive HIV patients. Nutrients 2011;3(10):897–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trois L, Cardoso EM, and Miura E: Use of probiotics in HIV-infected children: A randomized double-blind controlled study. J Trop Pediatr 2008;54(1):19–24 [DOI] [PubMed] [Google Scholar]
- 27.Gautam N, Dayal R, Agarwal D, et al.: (2014). Role of multivitamins, micronutrients and probiotics supplementation in management of HIV infected children. Indian J Pediatr [Epub ahead of Print]; DOI: 10.1007/s12098-014-1407-6 [DOI] [PubMed] [Google Scholar]
- 28.Anukam KC, Osazuwa EO, Osadolor HB, et al. : Yogurt containing probiotic Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 helps resolve moderate diarrhea and increases CD4 count in HIV/AIDS patients. J Clin Gastroenterol 2008;42(3):239–243 [DOI] [PubMed] [Google Scholar]
- 29.Monachese M, Cunningham-Rundles S, Diaz MA, et al. : Probiotics and prebiotics to combat enteric infections and HIV in the developing world: A consensus report. Gut Microbes 2011;2(3):198–207 [DOI] [PubMed] [Google Scholar]
- 30.Hemsworth JC, Hekmat S, and Reid G: Micronutrient supplemented probiotic yogurt for HIV-infected adults taking HAART in London, Canada. Gut Microbes 2012;3(5):414–419 [DOI] [PubMed] [Google Scholar]
- 31.Moore DM, Hogg RS, Yip B, et al. : CD4 percentage is an independent predictor of survival in patients starting antiretroviral therapy with absolute CD4 cell counts between 200 and 350 cells/μL. HIV Med 2006;7(6):383–388 [DOI] [PubMed] [Google Scholar]
- 32.Sandler NG, Wand H, Roque A, et al. : Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011;203(6):780–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longenecker CT, Jiang Y, Orringer CE, et al. : Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS 2014;28(7):969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunt PW, Brenchley J, Sinclair E, et al. : Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis 2008;197(1):126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burdo TH, Lo J, Abbara S, et al. : Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011;204(8):1227–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker J, Quick H, Hullsiek KH, et al. : Interleukin-6 and d-dimer levels are associated with vascular dysfunction in patients with untreated HIV infection. HIV Med 2010;11(9):608–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuller LH, Tracy R, Belloso W, et al. : Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008;5(10):e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haissman JM, Vestergaard LS, Sembuche S, et al. : Plasma cytokine levels in Tanzanian HIV-1-infected adults and the effect of antiretroviral treatment. J Acquir Immune Defic Syndr 2009;52(4):493–497 [DOI] [PubMed] [Google Scholar]
- 39.Chollet-Martin S, Simon F, Matheron S, et al. : Comparison of plasma cytokine levels in African patients with HIV-1 and HIV-2 infection. AIDS 1994;8(7):879–884 [DOI] [PubMed] [Google Scholar]
- 40.Borges AH, O'Connor JL, Phillips AN, et al. : Factors associated with D-dimer levels in HIV-infected individuals. PLoS One 2014;9(3):e90978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Funderburg NT, Mayne E, Sieg SF, et al. : Increased tissue factor expression on circulating monocytes in chronic HIV infection: Relationship to in vivo coagulation and immune activation. Blood 2010;115(2):161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.