Abstract
Background:
To address the need for disseminable, evidence-based depression treatment options for Latinx adults with limited English proficiency (LEP), our team developed ¡Aptívate!, a Spanish-language Behavioral Activation self-help mobile application. Primary aims of this study were to: 1) examine feasibility and uptake of ¡Aptívate! among depressed Latinx adults with LEP and 2) preliminarily examine ¡Aptívate! efficacy for depression treatment.
Methods:
Participants (N=42) with elevated depressive symptoms were randomized 2:1:1 to: 1) ¡Aptívate! (n=22), 2) an active control Spanish-language app (“iCouch CBT”; n=9), or 3) Treatment As Usual (i.e., no app; n=11). Feasibility was assessed via self-reported app utilization and app analytics data. Depressive symptoms were assessed weekly for eight weeks via self report.
Results:
All ¡Aptívate! participants used the app at least once, 81.8% of participants used the app ≥8 times, and 36.4% of participants used the app ≥56 times. Weekly retention was strong: 72.7% and 50% of participants continued to use the app at one- and two-months post-enrollment, respectively. Generalized Estimating Equation models indicated a significant interaction between time and treatment, such that ¡Aptívate! participants reported significantly lower depressive symptoms over time than TAU. Depressive symptoms did not differ on average across time between the iCouch and TAU conditions, nor between iCouch and ¡Aptívate!.
Limitations:
Limitations include small sample size, limited follow-up, and lack of analytics data for the active control condition.
Conclusions:
With further research, ¡Aptívate! may offer a feasible, efficacious approach to extend the reach of evidence-based depression treatment for Latinx adults with LEP.
Keywords: Depression, Latinx health, Mhealth, Behavioral activation
1. Introduction
United States Latinx adults experience similar or higher rates of depression as non-Latinx White adults (Pratt and Brody, 2016), but are less likely to engage in treatment (Lagomasino et al., 2005). Language proficiency is one key barrier to the receipt of evidence-based depression treatment. In the United States, more than 37 million individuals speak Spanish at home and 16.3 million of these individuals report speaking English less than very well (US Census Bureau, 2013). As compared to their English proficient counterparts, Latinx adults with limited English proficiency (LEP) have significantly lower odds of receiving mental health treatment (Bauer et al., 2010; Kim et al., 2011; Sentell et al., 2007). When treatment is received, Latinx adults with LEP tend to receive lower quality care and are subsequently at increased risk for treatment dropout (Cabassa et al., 2012). mHealth platforms, and specifically mobile applications (apps) that can be delivered via smartphone, can be leveraged to disseminate evidence-based psychological depression treatments in Spanish to Latinx adults (Muñoz et al., 2018). Smartphone ownership rates among Latinx adults now mirror that of the general population such that 77% of this group own smartphones (Pew Research Center, 2018). As compared to other racial/ethnic groups, Latinx individuals are more likely to be smartphone dependent (i.e., reliance on smartphones for online access), suggesting that mHealth interventions delivered via smartphone, rather than via computer or tablet, may hold greatest promise (Pew Research Center, 2018). Yet, few studies to date have developed and/or tested psychological depression interventions delivered via mobile app for this population. One recent study that did examine the feasibility and efficacy of several available depression treatment apps among this group found poor treatment engagement and limited efficacy (Pratap et al., 2018). Clearly, there is need for additional research focused on development and testing of app-based depression treatments for Latinx adults with LEP.
Because U.S. Latinx are a highly heterogeneous group with varied sociocultural and historical origins (González Burchard et al., 2005), an appropriate depression psychotherapy to adapt for mobile app delivery must be idiographic to accommodate personal beliefs/values on a case-by-case basis. Brief Behavioral Activation Treatment for Depression (Brief BA; Collado et al., 2014b; Lejuez et al., 2011) is an idiographic, straightforward, concise, yet evidence-based psychotherapy for depression. Collado et al. (2014a; 2014b) developed a direct Spanish language translation of Brief BA, delivered in person using paper forms, for Latinx adults with LEP. Treatment efficacy was evaluated as compared to a supportive counseling control condition, also delivered in Spanish. As compared to supportive counseling, Spanish-language Brief BA led to significantly greater decreases (68% vs. 59%) in depressive symptoms as well as greater remission of Major Depressive Disorder (93% vs. 50%) by treatment end, with treatment gains sustained through one-month post-treatment follow-up. However, this treatment approach is limited because public health need exceeds the number of mental health treatment providers who are trained in evidence-based practice and can deliver services in Spanish (Alegria et al., 2002; Aponte-Rivera et al., 2014). Adapting and testing Brief BA, delivered in Spanish, for mobile app delivery holds promise for reaching those Latinx individuals with LEP in need of evidence-based depression treatment who do not access traditional services.
To address this need, our group developed a Spanish language mobile app (“¡Aptívate!”; Dahne et al., 2018) informed by Brief BA. Although ¡Aptívate! is based on an evidence-based treatment for depression, the feasibility and efficacy of the app has not yet been examined. The aims of the present study were two-fold: 1) to examine the feasibility and uptake of ¡Aptívate! among Latinx adults with LEP and elevated depressive symptoms and 2) to preliminarily examine the efficacy of ¡Aptívate! as compared to both an active control depression treatment mobile app and to treatment as usual (TAU).
2. Methods
2.1. Participants
Study participants were recruited between September 2017 and June 2018. Participants were initially recruited locally via primary care/family medicine clinics and community centers that serve the Charleston, South Carolina Latinx community. Because of slow recruitment, in January 2018 we expanded to remote recruitment and recruited study participants nationwide using postings in Spanish on websites including Craigslist, Facebook, and Twitter. Those interested completed a brief screening either via phone or online via REDCap to establish preliminary study eligibility. All participants who screened eligible following the preliminary screening were contacted to schedule a time for a baseline visit, at which time final study eligibility was confirmed. Participants who scheduled a baseline visit but did not attend were given one opportunity to reschedule before being considered lost to follow-up. Baseline visits occurred in-person for those recruited locally and via doxy.me video (Welch et al., 2016) for those recruited remotely. Study participants had to meet the following criteria: 1) age 18+, 2) currently own a smartphone, 3) if not an iPhone owner, report willingness to utilize a study iPhone, 4) report willingness to utilize a mobile app for the treatment of depressed mood, 5) have a current, valid e-mail address that is checked at least once per day, 6) report Spanish language preference, 7) able to read fluently in Spanish, 8) score of ≥ 10 on the Patient Health Questionnaire-8, Spanish language version at screening (PHQ-8; Kroenke et al., 2009), and 9) have been seen by a doctor within the last year. The criterion of being seen by a doctor within the last year was included because we view primary care as one potential avenue for future treatment dissemination (Dahne et al., 2018).
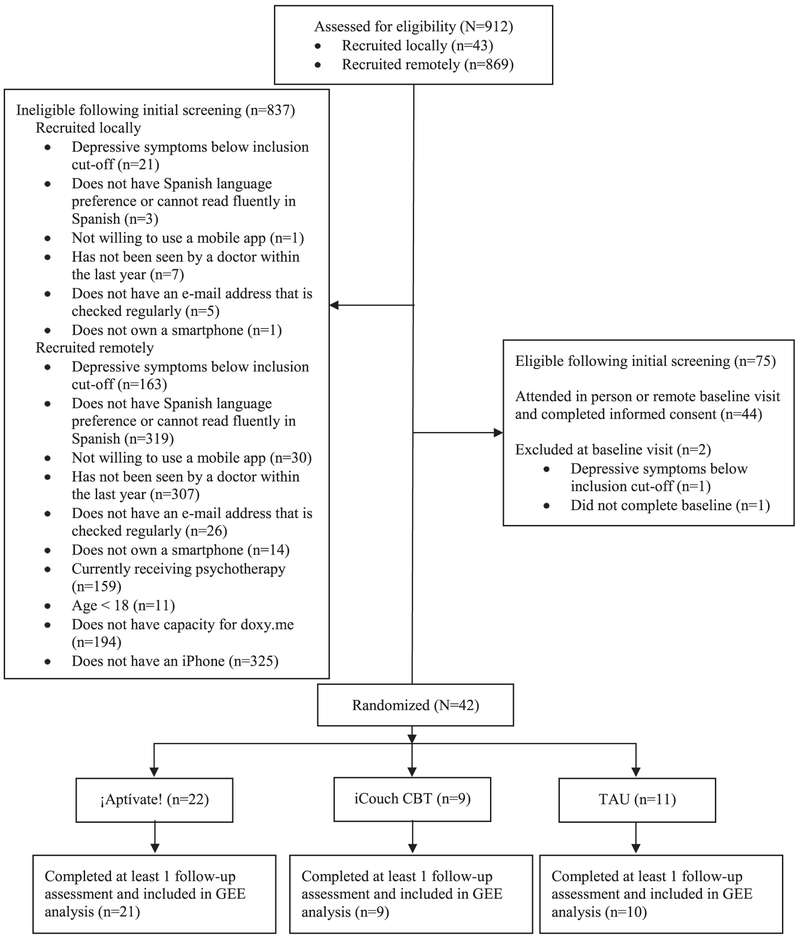
Participants were excluded if they were currently receiving psychotherapy, if they were visually impaired, if they scored ≤ 13 on the Spanish language Beck Depression Inventory-II (BDI-II; Beck et al., 1996; Wiebe and Penley, 2005) at baseline, and/or if they endorsed suicidality during the baseline visit. Because both apps utilized in this trial were only accessible via iOS, participants who lived local to the Charleston area but did not own an iPhone capable of running iOS 8 or higher were lent one for the duration of the trial. Participants recruited remotely were required to own a study compatible iPhone in order to be included in the trial. Remote participants were also required to have capacity for doxy.me (used for the baseline video session and consent), specifically 1) access to a computer with a webcam and speakers and 2) a compatible internet browser (at the time of this trial, doxy.me was optimized for Google Chrome and Firefox). See Fig. 1 for CONSORT diagram.
Fig. 1.
CONSORT Flow Diagram.
2.2. Procedures
All study procedures were approved by the Medical University of South Carolina Institutional Review Board. Upon consent, participants completed self-report assessments and were subsequently randomized 2:1:1 to receive either the Spanish language Behavioral Activation mobile app (¡Aptívate!), an active control Spanish language Cognitive Behavioral Therapy (CBT) mobile app (iCouch CBT), or Treatment as Usual (i.e., no app). We utilized 2:1:1 randomization because a primary aim of this trial was to examine ¡Aptívate! feasibility. Thus, we wanted to ensure enough participants within that arm while still keeping sample size fairly low ( ~ 20 participants in the ¡Aptívate! condition), consistent with a pilot trial. Randomization was stratified based on whether the participant reported currently taking psychiatric medications for the treatment of mental/emotional problems. Because clinical data are limited on other available mobile apps that purport to treat depressive symptoms (Chan et al., 2015; Leigh and Flatt, 2015; Neary and Schueller, 2018; Price et al., 2014), we opted to compare ¡Aptívate! efficacy to a commercially available active control mobile app in addition to TAU to extend the literature on efficacy data for other clinical apps. We selected iCouch CBT as the active control app because, at the time of trial onset, it was one of the only existing mobile apps that was available in Spanish and informed by Cognitive Behavioral Therapy (CBT), the most evaluated depression treatment among Latinx adults (Collado et al., 2016).
¡Aptívate! and iCouch CBT were provided to study participants at no cost. At the time of this study, ¡Aptívate! was available via the iTunes App Store (United States) for $4.99 and iCouch CBT was available for $2.99. If randomized to either mobile app condition, study staff helped the participant download the app and provided a brief, scripted overview ( ~ 10 min) on how to use the app. Following this overview, participants were provided with time ( ~ 5 min) to use the app freely and ask any questions. App proficiency was not formally evaluated. Those provided with an app were instructed to use the app regularly, at least once per day, for the duration of the study. Following the baseline visit, participants were e-mailed assessment measures weekly for eight weeks. Thus, participants completed study assessments at nine time-points in total (i.e., baseline and eight weekly follow-up assessments). Participants were compensated for any follow-up assessments completed within 72 h of receiving the e-mailed link. All measures were completed via REDCap, a secure, web-based research data capture system (Harris et al., 2009). Participants were compensated up to $250 for completion of all assessments. Participants who borrowed an iPhone as part of the trial were compensated an additional $50 for return of the iPhone in working, non-damaged order within two-weeks of their trial completion date.
2.3. Interventions
Spanish Language Behavioral Activation Mobile App (¡Aptívate!).
Details of the ¡Aptívate! app have been published previously, including suggested instructions for integrating the app into primary care practice (Dahne et al., 2018). Briefly, ¡Aptívate! is a self-help Spanish language adaptation of Brief BA (Collado et al., 2014b; Lejuez et al., 2011). Within ¡Aptívate!, the user identifies individualized values and associated activities across five life areas, including relationships, daily responsibilities, recreation, career and education, and health. Values then become a framework for generating activities. For example, within the relationships life area, a user might create a value of “develop a strong family unit.” Activities consistent with this value might include “have family dinners twice a week” and “call my children three times a week” (Dahne et al., 2018). These activities are scheduled and tracked within a daily calendar and users can view fluctuations in mood as a function of changes in activity level. For activities that are difficult to complete, the user can identify an individual who may assist in completing that activity and ways to ask that person to help complete the activity. Users are provided with psychoeducation regarding the association between mood and activity and are reinforced for continued app utilization and completion of activities via badges that can be earned within the app.
Cognitive Behavioral Therapy (CBT) Spanish Language Mobile App (iCouch CBT; active control).
iCouch CBT prompts users to explain distressing situations and associated emotions, identify corresponding thoughts and cognitive distortions, and ultimately identify alternative thoughts and corresponding emotions. These logs can be saved so that the user can review them at a later time. A prior review of mental health mobile apps which included iCouch CBT noted that the app is CBT-based, addresses both low mood and anxiety, includes reporting of thoughts, feelings, or behaviors, and includes a log of past app use (Bakker et al., 2016). To our knowledge, there are no published efficacy studies of iCouch CBT for the treatment of depression.
Treatment as Usual (TAU; inactive control).
Participants randomized to the TAU condition did not receive a mobile app. However, they were not precluded from seeking out additional mental health care (i.e., the care they would have received had they not been enrolled in the trial).
2.4. Measures
Primary outcomes for this trial include: 1) feasibility of ¡Aptívate! as indicated by self-report and app utilization analytics and 2) change in depressive symptoms over time as a function of treatment condition.
App Feasibility.
Participants in both app conditions self-reported on a weekly basis whether they used the app they were assigned during the prior week. Those who did not complete the follow-up assessment were coded as having not used the app during the preceding week. In addition to self-report data, user analytics data for participants randomized to the ¡Aptívate! condition were available via Yahoo's Flurry Analytics system. Specific analytics data captured included: 1) total number of app sessions across the trial duration and within each trial week, 2) average time per app session, 3) total time spent using the app across the trial duration, 4) number of times the participant added a new value within the app, 5) number of times the participant created a value-driven activity within the app, 6) number of activities completed across the trial duration, 7) badges earned across the trial duration, 8) number of times the participant rated their daily mood across the trial duration, and 9) weekly retention, defined as the percentage of study participants who utilized ¡Aptívate! at least once during each week after study enrollment. We did not have access to similar data for participants randomized to the iCouch CBT condition because this app was not developed by our research team.
Depressive Symptoms.
A Spanish language version of the PHQ-8 (Kroenke et al., 2009; Wulsin et al., 2002) was utilized as a preliminary study screening measure to determine whether participants were experiencing at least minimal symptoms of depression. The PHQ-8 was selected as the initial study screening measure because it is brief and can be quickly completed either via phone or online. Across the study follow-up (including baseline), depressive symptoms were assessed via the Spanish language BDI-II (Beck et al., 1996; Wiebe and Penley, 2005). Scores on the BDI-II are interpreted as follows: 0–13=minimal depression, 14–19=mild depression, 20–28=moderate depression, 29–63=severe depression. The BDI-II was also used as a study screening measure at baseline to exclude those with current suicidality, defined as response of “I would like to kill myself’ or “I would kill myself if I had the chance” on the suicidal thoughts or wishes item of the BDI-II, although no participants were excluded at baseline due to this criterion.
2.5. Statistical analysis plan
Chi-square and ANOVA analyses were used to determine baseline differences in participant demographics as a function of treatment condition. Chi-square analyses were utilized to compare self-reported app utilization across groups and descriptive statistics were utilized to examine ¡Aptívate! feasibility and acceptability as indicated by app analytics data. Subsequently, a generalized estimating equation (GEE) model assuming a normal distribution, identity link function, and exchangeable correlation matrix (i.e., within-subject observations are equally correlated) was used to examine the interaction between time and treatment condition on BDI-II depressive symptoms adjusting for the main effects of baseline depressive symptoms, time, treatment condition, and whether the participant was recruited locally or remotely. Baseline depressive symptoms were included as a covariate in order to adjust for between-group differences in depression that may have existed prior to the study being implemented. As GEE was utilized to examine change in depression over time as a function of treatment condition, participants were only included in the GEE model if at least one follow-up assessment was completed (n=2 excluded from GEE model). Because GEE accommodates within-subject correlations across repeated measures, unbalanced designs, and incomplete data, it provides less biased estimates than traditional regression approaches in analyzing longitudinal data (Hardin and Hilbe, 2002).
3. Results
3.1. Participant characteristics
In total, 42 participants were enrolled in the trial (1=22 ¡Aptívate!, n=9 iCouch CBT, n=11 TAU). See Table 1 for participant demographics for the full sample as well as by treatment group. There were no significant demographic differences as a function of treatment condition. At baseline, participants on average reported experiencing depressive symptoms in the moderate to severe depression range [BDI-II baseline M(SD)=31.24(9.71); ¡Aptívate!=32.18(9.22), iCouch CBT=28.33(7.71), TAU=31.73(12.27)]. Study retention was high, with 90.5% of participants across treatment groups completing at least six of the eight follow-up assessments. There were no significant differences in study retention between treatment groups.
Table 1.
Demographics for the full sample and by Treatment Group.
| Full Sample (N=42) |
¡Aptívate! (n=22) |
iCouch CBT (n=9) |
TAU (n=11) |
|
|---|---|---|---|---|
| Age (M(SD)) | 36.05 (11.44) | 32.68 (9.18) | 40.56 (14.16) | 39.09 (12.07) |
| Gender (% Female) | 66.7% | 77.3% | 44.4% | 63.6% |
| Local vs Remote (% Local) | 28.6% | 27.3% | 22.2% | 36.4% |
| Race | ||||
| White | 23.8% | 27.3% | 33.3% | 9.1% |
| Black | 2.4% | 0.0% | 0.0% | 9.1% |
| Native Hawaiian/Pacific Islander | 2.4% | 0.0% | 0.0% | 9.1% |
| Native American | 7.1% | 4.5% | 0.0% | 18.2% |
| Multiracial | 11.9% | 18.2% | 0.0% | 9.1% |
| Other | 52.4% | 50.0% | 66.7% | 45.5% |
| Ethnicity (% Hispanic) | 100% | 100% | 100% | 100% |
| Relationship Status | ||||
| In Relationship | 61.9% | 54.6% | 66.7% | 72.8% |
| Single | 38.1% | 45.4% | 33.3% | 27.3% |
| Education | ||||
| ≤ High School diploma | 14.3% | 9.1% | 11.1% | 27.3% |
| ≥ High School diploma | 85.8% | 90.9% | 88.8% | 72.8% |
| Annual Household Income | ||||
| < $50k | 62.0% | 63.7% | 77.7% | 45.5% |
| ≥$50k | 21.4% | 22.7% | 11.1% | 27.3% |
| Don't know/Not sure/Refused | 16.7% | 13.6% | 11.1% | 27.3% |
| Employment Status | ||||
| Unemployed | 47.6% | 36.4% | 77.8% | 45.5% |
| Employed ≥ part time | 40.5% | 50.0% | 22.2% | 36.4% |
| Other | 11.9% | 13.6% | 0.0% | 18.2% |
| Phone Ownership | ||||
| iPhone | 81.0% | 81.8% | 88.9% | 72.7% |
| Android | 19.0% | 18.2% | 11.1% | 27.3% |
| Currently Taking Meds for Mental or Emotional Problems (% Yes) | 12.2% | 13.6% | 11.1% | 10.0% |
| Number of Follow-Up Assessments Completed (M(SD)) | 7.12 (2.16) | 7.50 (1.71) | 7.78 (0.67) | 5.82 (3.16) |
Note: There were no baseline differences as a function of study group.
3.2. App feasibility
Self-Reported App Utilization.
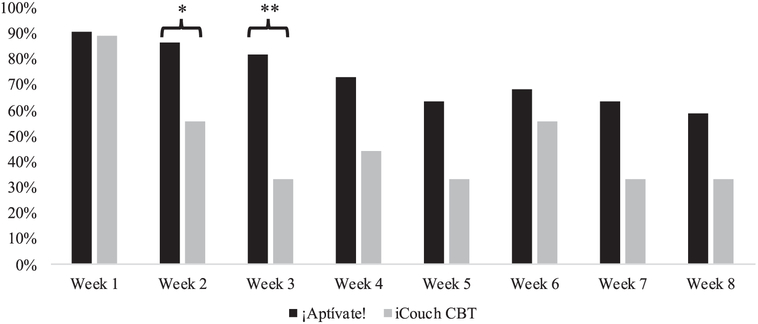
See Fig. 2 for self-reported app utilization rates (i.e., whether the assigned app was used during the week prior) as a function of treatment condition. Self-reported incidence of app utilization was significantly higher for the ¡Aptívate! as compared to the iCouch CBT condition at Week 3, χ2=6.87, p=0.009, with a trend toward significance at Week 2, χ2=3.47, p=0.06.
Fig. 2.
Self-Reported App Utilization as a Function of Treament Group.
Note: Self-reported app utilization was defined as any app use within the preceding week. Those who did not complete the weekly assessment were coded as not using the app. *p < 0.10; **p < 0.05.
¡Aptívate! Analytics.
As indicated by analytics data, all ¡Aptívate! participants used the app at least once during the trial, 81.8% of participants used the app at least 8 times (i.e., at least once per week on average), 45.5% of participants used the app at least 28 times (i.e., every other day on average), and 36.4% of participants used the app 56 or more times (i.e., at least once per day on average). Participants on average had 61.41(91.73) app sessions throughout the eight-week trial duration, spent 87.73(63.08) seconds using the app per session, and spent 65.77(82.76) minutes using the app in total throughout the trial. Participants created on average 4.68(3.54) unique values within the app, 11.77(17.02) activities across values, and completed 21.73(45.60) activities (Table 2).
Table 2.
¡Aptívate! Analytics Over Eight Weeks of Study Duration.
| Metric | M(SD) | Median | Range |
|---|---|---|---|
| Total Number of Sessions Across Study Duration | 61.41(91.73) | 22.50 | 3.00 – 433.00 |
| Week 1 | 25.23(49.39) | 15.00 | 2.00–242.00 |
| Week 2 | 7.91(14.47) | 4.00 | 0.00–68.00 |
| Week 3 | 6.05(9.39) | 3.00 | 0.00–42.00 |
| Week 4 | 5.73(6.65) | 3.00 | 0.00–25.00 |
| Week 5 | 4.14(4.64) | 2.00 | 0.00–16.00 |
| Week 6 | 4.86(6.23) | 1.50 | 0.00–17.00 |
| Week 7 | 4.18(5.39) | 2.00 | 0.00–19.00 |
| Week 8 | 3.27(4.67) | 0.50 | 0.00–17.00 |
| Average Time per Session (sec) | 87.73(63.08) | 78.00 | 19.00–271.00 |
| Total Time Spent Using ¡Aptívate! (min) | 65.77(82.76) | 34.00 | 3.00–325.00 |
| Add Value Event Occurrences | 4.68(3.54) | 5.00 | 0.00–10.00 |
| Add Value-Driven Activity Event Occurrences | 11.77(17.02) | 5.50 | 0.00–68.00 |
| Complete Activity Event Occurrences | 21.73(45.60) | 0.00 | 0.00–186.00 |
| Badge Earned Event Occurrences | 4.55(3.50) | 4.00 | 0.00–13.00 |
| Set Mood Event Occurrences | 24.23(21.02) | 22.00 | 0.00–56.00 |
Some analytics outcomes significantly differed as a function of whether a participant was recruited locally vs. remotely. Local participants as compared to remote participants had a significantly greater number of app sessions during Week 2 of the trial (Local (M(SD)=18.00(25.61), Remote=4.13(4.30); F(1, 20)=4.73, p=0.04), spent on average more time per session using the app (Local (M(SD)=149.83(77.57) seconds, Remote=64.44(38.00) seconds; F(1, 20)=12.30, p=0.002), spent more time in total using the app (Local (M(SD)=147.50(119.65) minutes, Remote=35.13(33.80) minutes; F(1, 20)=12.42, p=0.04), and scheduled more activities on average (Local (M(SD)=51.83(57.33), Remote=9.75(18.37); F(1, 20)=7.19, p=0.01).
¡Aptívate! Retention.
Weekly app retention, defined as any app use within each week following trial enrollment, was examined for ¡Aptívate! participants. No procedures to increase app retention (e.g., contacting participants who were not using the app to remind them to login) were instituted by the study team during the trial. In general, retention was high across the study duration: 100% of participants utilized ¡Aptívate! at least once during the first week following trial enrollment, 77.3% during the second week, 77.3% during the third week, 72.7% during the fourth week, 59.1% during the fifth week, 54.5% during the sixth week, 59.1% during the seventh week, and 50.0% during the eighth week. Chi-square analyses revealed no significant differences in study retention across weeks as a function of whether study participants were recruited locally vs. remotely.
3.3. Change in depressive symptoms over time as function of treatment condition
The GEE model indicated a significant main effect of baseline depressive symptoms (χ2=34.66, df=1, p<0.001) as well as a significant interaction between time and treatment condition (χ2=35.06, df=14, p=0.001). Post-hoc pairwise comparisons both within- and between-groups were utilized to understand the nature of the significant time by treatment interaction. Regarding within-group comparisons, adjusting for baseline, depressive symptoms among participants in the ¡Aptívate! condition were significantly lower as compared to the Week 1 assessment (i.e., the first follow-up) at Weeks 2, 5, 6, 7, and 8. Depressive symptoms among participants in the iCouch CBT condition were significantly lower as compared to the Week 1 assessment at Weeks 3, 4, 5, 6, 7, and 8. Depressive symptoms among participants in the TAU condition were only significantly different from the Week 1 assessment at Week 8. When comparing follow-up BDI scores to baseline BDI scores, among those in the ¡Aptívate! and iCouch CBT conditions, all follow-up BDI scores were significantly lower than baseline BDI scores, whereas among those in the TAU condition, BDI scores at Weeks 3 through 8 were significantly lower than baseline BDI scores.
Between-group mean differences when adjusting for baseline depressive symptoms and remote vs. local were significantly different on average across time points only between the ¡Aptívate! and TAU conditions (¡Aptívate! vs. TAU mean difference across time=−5.39(2.11), p=0.01). Within time points, significant mean group differences between ¡Aptívate! vs. TAU were evident at Week 2 (¡Aptívate! vs. TAU mean difference=−10.17(3.26), p=0.002), Week 6 (¡Aptívate! vs. TAU mean difference=−7.48(3.24), p=0.02), and Week 7 (¡Aptívate! vs. TAU mean difference=−8.08(3.80), p=0.03). There were no significant mean differences in depressive symptoms between the iCouch CBT and TAU conditions or between the iCouch CBT and ¡Aptívate! conditions across time points (iCouch CBT vs. TAU p=0.16; iCouch CBT vs. ¡Aptívate! p=0.66) or within any individual follow-up week (all p’s > 0.08).
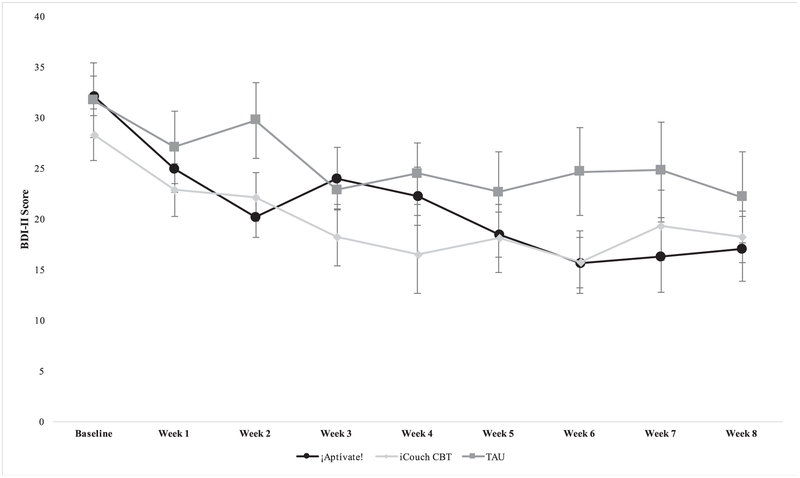
On average, participants in the ¡Aptívate! condition had a 13.79(12.33)-point decrease on the BDI-II from pre- to post-treatment, participants in the iCouch CBT condition had a 11.25(8.19)-point decrease, and participants in the TAU condition had a 8.00(5.44) point decrease. In addition, 54.5% of participants in the ¡Aptívate! condition, 77.8% of participants in the iCouch CBT condition, and 36.4% of participants in the TAU condition evidenced less than minimal symptoms of depression on the BDI-II (i.e., score ≥ 13) at some point during the study. Among those who completed the Week 8 assessment (n=19 ¡Aptívate!, n=8 iCouch CBT, n=6 TAU), 42.1% of ¡Aptívate! participants, 25.0% of iCouch CBT participants, and 16.7% of TAU participants had less than minimal symptoms of depression at that time. See Fig. 3 for a graph of depressive symptoms by time as a function of treatment condition.
Fig. 3.
BDI-II Depressive Symptoms by Treatment Condition.
Note: Mean BDI-II scores with corresponding standard errors are plotted above on the y-axis.
4. Discussion
This study offers preliminary feasibility and efficacy of ¡Aptívate! for the treatment of depressive symptoms among Latinx adults with LEP. Nearly all participants used the app on average at least once per week and a third of participants used the app on average once per day. Although these utilization rates are promising, particularly as compared to prior research (Arean et al., 2016; Pratap et al., 2018; Rosa et al., 2015), most participants utilized the app less frequently than instructed (at least once per day). Thus, future studies might consider additional support to promote more frequent app utilization among study participants, such as by sending additional reminders to continue to use the app, implementing a contingency management approach to reinforce continued app engagement, or integrating with existing care sites, such as primary care.
Retention was high across the eight-week trial duration, with 72.7% and 50% of participants continuing to use the app one- and two-months following trial enrollment respectively. Self-reported retention was also generally higher for the ¡Aptívate! condition as compared to the iCouch CBT condition. These retention rates are in contrast to most app-based mental health treatment trials in the general population, wherein few users continue to use the app beyond two-weeks (Arean et al., 2016; Rosa et al., 2015).
¡Aptívate! participants utilized all core app features, including mood monitoring, values identification, and activity selection, scheduling, and completion. However, there was considerable variability across participants in frequency of app use and specific app features utilized. Some of this variability is explained by local vs. remote recruitment: those who were recruited locally and attended in person baseline visits used the app with greater frequency, for longer duration, and scheduled more activities. Reduced mHealth engagement among remotely recruited Latinx adults with depressive symptoms has been found by other research groups as well. For example, one recent study found that only 18.7% of Latinx participants with depressive symptoms who enrolled in a remote app-based intervention study actually downloaded their assigned treatment app (Pratap et al., 2018). Thus, for this population, and perhaps for other populations, in person recruitment via community centers, primary care, or other venues may be associated with higher app utilization. Our team's original recruitment plan was to recruit in person, locally via primary care/family medicine clinics and community centers that serve the Charleston, South Carolina Latinx community. However, recruitment via these outlets was slow, which may speak to limited feasibility of this intervention within Charleston primary care/family medicine clinics and community centers. Future studies should examine the feasibility of recruiting LEP Latinx adults with depressive symptoms from these venues in areas that have larger Latinx populations. Future studies may also consider further evaluating the impact of remote vs. local recruitment on mHealth treatment utilization. Prioritization of in person recruitment via either single- or multi-center study designs may maximize participant engagement with a mobile app treatment among this population.
Regarding efficacy, this pilot trial suggests preliminary efficacy for ¡Aptívate! among a sample of Latinx adults with LEP recruited nationwide. After accounting for baseline depressive symptoms as well as local/remote recruitment, participants in the ¡Aptívate! condition had significantly lower symptoms of depression over time relative to TAU participants. Within treatments, as compared to depressive symptoms at Week 1, participants in both the ¡Aptívate! and iCouch CBT conditions had significantly lower symptoms at most follow-up assessment time points. In comparison, symptoms of depression among TAU participants were only lower at Week 8 as compared to Week 1. Participants in both the ¡Aptívate! and iCouch CBT conditions also had clinically significant decreases in depressive symptoms on average (i.e., BDI-II decrease of ≥ 10 points) from pre- to post-treatment. Thus, both ¡Aptívate! and iCouch CBT may be associated with more rapid and more clinically significant decreases in depression over time relative to TAU.
Results of this study should be interpreted with limitations in mind. This was a small pilot study and the sub-samples within the iCouch and TAU conditions were particularly small. Although there were no baseline demographic differences between groups, it is possible that outcomes may have been unduly influenced by outliers. Remotely recruited participants were required to own an iPhone capable of running ¡Aptívate! or iCouch CBT and were also required to have capacity for teleconsent procedures. Thus, this sample may not be representative of all Latinx adults with LEP and depressive symptoms. Analytics data were not available for iCouch CBT because this app was not developed by our team. Thus, we could not compare app feasibility using analytics data between the two app conditions. However, this is a limitation that would be faced by any research team that uses a commercially available mobile app as an active control treatment condition. Finally, participants did not complete follow-up assessments beyond eight-weeks and as such it is unclear whether treatment gains would be sustained beyond this timeframe. An eight-week assessment period was selected for this trial because Brief BA is typically delivered over the course of eight-to twelve-sessions (Lejuez et al., 2011). Identifying the appropriate length of follow-up duration for any app-based mental health treatment study is difficult because, unless app use is somehow restricted, participants can continue to have access to treatment content beyond the initially prescribed period of time.
This trial extends the literature on app-based interventions for depression treatment among United States Latinx adults with LEP and elevated depressive symptoms. App-based interventions such as ¡Aptívate! may help to address the currently unmet need for evidence-based, disseminable psychological treatments delivered in Spanish for this population. Future larger scale examinations of ¡Aptívate! efficacy and effectiveness as well as examination of treatment outcomes beyond the eight-week window studied herein will be needed prior to broad recommendation of this app as a treatment for depression among Latinx adults with LEP. Furthermore, although a strength of app-based psychological interventions is that there are fewer barriers to use as compared to traditional in-person psychological treatment, not all Latinx adults with LEP and elevated depressive symptoms will respond to a self-help depression treatment app such as ¡Aptívate!. Future research should consider examinations of moderators of treatment response (e.g., severity of depressive symptoms, comfort with technology) which may help to determine which subpopulations may benefit the most from ¡Aptívate!. With further research, ¡Aptívate! may offer a feasible and efficacious approach to extend the reach of evidence-based depression treatment for this undertreated group.
Acknowledgements
The authors would like to thank MountainPass Technology LLC, including Zachary Gavin, Jim Nichols, Bryan Hobbs, Brian Cordyack, and Tamara Wiesen, for their contributions to the development of ¡Aptívate!.
Financial Support and Role of the Funding Source
Funding for this research was provided by the National Institute of Minority Health and Health Disparities(R41 MD010491) and by the National Institute on Drug Abuse (T32 DA007288, K23 DA045766). The funding sources had no role in study design, data collection, data analysis, data interpretation, in writing this report, or in the decision to submit this article for publication.
Footnotes
Conflict Of Interest Statement
Declaration of Interest: The authors (JD, JK, CWL) are co-owners of Behavioral Activation Tech, LLC which owns the rights to ¡Aptívate!. The authors report no other actual or potential conflicts of interest that may bias the present work.
References
- Alegria M, Canino G, Rios R, Vera M, Calderon J, Rusch D, Ortega AN, 2002. Inequalities in use of specialty mental health services among Latinos, African Americans, and non-Latino whites. Psychiatry Serv. 53, 1547–1555. [DOI] [PubMed] [Google Scholar]
- Aponte-Rivera V, Dunlop BW, Ramirez C, Kelley ME, Schneider R, Blastos B, Larson J, Mercado F, Mayberg H, Craighead WE, 2014. Enhancing Hispanic participation in mental health clinical research: development of a Spanish-speaking depression research site. Depress. Anxiety 31, 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arean PA, Hallgren KA, Jordan JT, Gazzaley A, Atkins DC, Heagerty PJ, Anguera JA, 2016. The use and effectiveness of mobile apps for depression: results from a fully remote clinical trial. J. Med. Internet Res 18, e330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker D, Kazantzis N, Rickwood D, Rickard N, 2016. Mental health smartphone apps: review and evidence-based recommendations for future developments. JMIR Ment. Health 3, e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AM, Chen C-N, Alegría M, 2010. English language proficiency and mental health service use among Latino and Asian Americans with mental disorders. Med. Care 48, 1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Beck Depression Inventory-II (BDI-II). Psychological Corporation, San Antonio, TX. [Google Scholar]
- Cabassa LJ, Molina GB, Baron M, 2012. Depression fotonovela: development of a depression literacy tool for Latinos with limited English proficiency. Health Promot. Pract. 13, 747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S, Torous J, Hinton L, Yellowlees P, 2015. Towards a framework for evaluating mobile mental health apps. Telemed J. E. Health 21, 1038–1041. [DOI] [PubMed] [Google Scholar]
- Collado A, Castillo S, Maero F, Lejuez C, MacPherson L, 2014a. Pilot of the brief behavioral activation treatment for depression in Latinos with limited English proficiency: preliminary evaluation of efficacy and acceptability. Behav. Therapy 45, 102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado A, Lim AC, MacPherson L, 2016. A systematic review of depression psychotherapies among Latinos. Clin. Psychol. Rev 45, 193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado A, Long KE, MacPherson L, Lejuez CW, 2014b. The efficacy of a behavioral activation intervention among depressed US Latinos with limited English language proficiency: study protocol for a randomized controlled trial. Trials 15, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahne J, Collado A, Lejuez CW, Risco C, Diaz VA, Kustanowitz J, Zvolensky M, Carpenter MJ, 2018. ¡Aptívate!: a Spanish-language behavioral activation mobile application for delivery via primary care. Psychol. Serv. 10.1037/ser0000304. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González Burchard E, Borrell LN, Choudhry S, Naqvi M, Tsai H-J, Rodriguez-Santana JR, Chapela R, Rogers SD, Mei R, Rodriguez-Cintron W, 2005. Latino populations: a unique opportunity for the study of race, genetics, and social environment in epidemiological research. Am. J. Public Health 95, 2161–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin JW, Hilbe JM, 2002. Generalized Estimating Equations. Chapman and Hall/CRC. [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, 2009. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf 42, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Loi CXA, Chiriboga DA, Jang Y, Parmelee P, Allen RS, 2011. Limited English proficiency as a barrier to mental health service use: a study of Latino and Asian immigrants with psychiatric disorders. J. Psychiatry Res 45, 104–110. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH, 2009. The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord 114, 163–173. [DOI] [PubMed] [Google Scholar]
- Lagomasino IT, Dwight-Johnson M, Miranda J, Zhang L, Liao D, Duan N, Wells KB, 2005. Disparities in depression treatment for Latinos and site of care. Psychiatry Serv. 56, 1517–1523. [DOI] [PubMed] [Google Scholar]
- Leigh S, Flatt S, 2015. App-based psychological interventions: friend or foe. Evid.-Based Mental Health 18, 97–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Hopko DR, Acierno R, Daughters SB, Pagoto SL, 2011. Ten year revision of the brief behavioral activation treatment for depression: revised treatment manual. Behav. Modif. 35, 111–161. [DOI] [PubMed] [Google Scholar]
- Munoz RF, Chavira DA, Himle JA, Koerner K, Muroff J, Reynolds J, Rose RD, Ruzek JI, Teachman BA, Schueller SM, 2018. Digital apothecaries: a vision for making health care interventions accessible worldwide. mHealth 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary M, Schueller SM, 2018. State of the field of mental health apps. Cogn. Behav. Pract 25, 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew Research Center, 2018. Mobile Fact Sheet. Pew Internet & American Life Project. [Google Scholar]
- Pratap A, Renn BN, Volponi J, Mooney SD, Gazzaley A, Arean PA, Anguera JA, 2018. Using Mobile Apps to Assess and Treat Depression in Hispanic and Latino Populations: fully Remote Randomized Clinical Trial. J. Med. Internet Res. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L, Brody D, 2016. Depression in the U.S. household population, 2009-2012, NCHS data brief, no 172. [PubMed] [Google Scholar]
- Price M, Yuen EK, Goetter EM, Herbert JD, Forman EM, Acierno R, Ruggiero KJ, 2014. mHealth: a mechanism to deliver more accessible, more effective mental health care. Clin. Psychol. Psychother 21, 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa C, Campbell AN, Miele GM, Brunner M, Winstanley EL, 2015. Using e-technologies in clinical trials. Contemp. Clin. Trials 45, 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentell T, Shumway M, Snowden L, 2007. Access to mental health treatment by English language proficiency and race/ethnicity. J. Gen. Intern. Med 22 (Suppl 2), 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Census Bureau, 2013. Detailed languages spoken at home and ability to speak English for the population 5 years and over: 2009–2013. https://www.census.gov/data/tables/2013/demo/2009-2013-lang-tables.html.
- Welch BM, Marshall E, Qanungo S, Aziz A, Laken M, Lenert L, Obeid J, 2016. Teleconsent: a Novel approach to obtain informed consent for research. Contemp. Clin. Trials Commun. 3, 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe JS, Penley JA, 2005. A psychometric comparison of the Beck Depression Inventory-II in English and Spanish. Psychol. Assess 17, 481. [DOI] [PubMed] [Google Scholar]
- Wulsin L, Somoza E, Heck J, 2002. The feasibility of using the Spanish PHQ-9 to screen for depression in primary care in Honduras. Prim. Care Companion J. Clin. Psychiatry 4, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]