Abstract
Objective:
To review clinical and pre-clinical evidence supporting the role of visual pathways, from the eye to the cortex, in the development of photophobia in headache disorders.
Background:
Photophobia is a poorly understood light-induced phenomenon that emerges in a variety of neurological and ophthalmological conditions. Over the years, multiple mechanisms have been proposed to explain its causes; however, scarce research and lack of systematic assessment of photophobia in patients has made the search for answers quite challenging. In the field of headaches, significant progress has been made recently on how specific visual networks contribute to photophobia features such as light-induced intensification of headache, increased perception of brightness and visual discomfort, which are frequently experienced by migraineurs. Such progress improved our understanding of the phenomenon and points to abnormal processing of light by both cone/rod-mediated image-forming and melanopsin-mediated non-image-forming visual pathways, and the consequential transfer of photic signals to multiple brain regions involved in sensory, autonomic and emotional regulation.
Conclusion:
Photophobia phenotype is diverse, and the relative contribution of visual, trigeminal and autonomic systems may depend on the disease it emerges from. In migraine, photophobia could result from photic activation of retina-driven pathways involved in the regulation of homeostasis, making its association with headache more complex than previously thought.
Keywords: Migraine, retina, photoreceptors, thalamus, hypothalamus, autonomic
Introduction
Abundant visual symptomatology in some headaches has driven scientists and clinicians to seek understanding of the intriguing association between the visual system and headache disorders. Historically, the most compelling association between these comes from patients’ descriptions of visual hallucinations and scotomas, known today as visual aura (1–3). The phenomenon behind these symptoms is widely accepted to originate in the cortex as a result of altered, genetically-driven ionic imbalances capable of disrupting the dynamic equilibrium between excitatory and inhibitory networks (4). Existing evidence supports the notion that abnormal cortical excitability is not only able to produce such visual or sensory hallucinations through cortical spreading depression (CSD) but also influences the way it processes sensory information from the outside world (5,6). Indeed, one of the hallmarks of migraine is multisensory dysfunction, which expresses itself in the form of increased sensory perception in what is classically referred to as migraine phobias or sensory allodynias. The common notion is that these sensory disturbances originate from abnormal processing in different cortical areas, including the visual cortex, but recent data suggest that pathological processing may occur downstream at lower levels of the visual pathways (7,8).
The pioneering description on the nature of photophobia by Lebensohn emphasizes the duality of the phenomenon by differentiating light sensitivity due to glare from that associated with induction or exacerbation of pain by light, which he called true photophobia. He suggested that painful photophobia depends on the functional integrity of both the optic and trigeminal nerves, and that light-induced pain may originate from iris constriction and vasodilatation driven by sensitized trigeminal axon reflexes. His conclusions were mostly made, however, from patients with ocular conditions, and did not distinguish these from photophobia in migraine (9,10). An effort to differentiate photophobia features in migraine was made by Drummond, who developed a photophobia questionnaire (11) and performed a series of psychophysical studies in headache sufferers. His findings expand on Lebensohn’s view and suggest that migraine is associated with a subcortical inability to suppress glare and light-induced pain, and that trigeminal-autonomic reflexes further contribute to pain by promoting vasodilatation in pain-sensitized extra/intracranial vessels (12–14).
Significant progress has been made since then, particularly in the last two decades, towards a better scientific understanding of how specific visual pathways contribute to photophobia. This progress also requires us to reshape the definition of photophobia as a neurological symptom, according to the disease or group of diseases it emerges from. There is, however, a growing body of evidence supporting the notion that neural pathways mediating light-induced intensification of headache in migraine may differ from the pathways mediating increased sensitivity to light or from those mediating ocular discomfort in prechiasmal conditions (8,15,16). For example, photophobia perception in migraine has been recently shown to be influenced by activation of retinal photoreceptors and the photic signals they transmit to multiple brain regions involved in sensory, autonomic and emotional regulation (17,18). Therefore, in migraineurs, light could act as a strong modulator of a vast, multidimensional neuronal network involved in the regulation of homeostasis, making its association with headache more complex than previously thought.
In this review, we will first describe photophobia as a phenomenon, and then discuss clinical and preclinical evidence supporting the role of visual pathways, from the eye to the cortex, in the pathophysiology of migraine photophobia.
Definition of photophobia and mechanistic theories
The current definition of photophobia in medical literature is at best incomplete, as it fails to precisely describe the many forms this light-induced phenomenon can take. The strict meaning of the word – fear (phobia) of light (photo) – distance it from the common use of the term by clinicians and patients (photophobia sufferers) when attempting to describe light-induced neurological symptoms, which usually emerge in the form of (i) increased sensitivity to light or glare, (ii) intensification of headache and (iii) ocular pain or discomfort. Many neurological conditions have been associated with photophobia including migraine, traumatic brain injury, concussion, meningitis, intracranial tumors and subarachnoid hemorrhage. Similarly, a variety of neuro-ophthalmic disorders such as uveitis, iritis, cyclitis, keratitis, retinitis pigmentosa, cone-rod dystrophy, corneal damage and blepharitis present with photophobia as well (19–22). Controversy on the causes of photophobia and its use as a medical term remains, likely due to the scarcity of data and lack of thorough assessment of specific clinical features, particularly in the headache population. The long list of pathologies that are accompanied with photophobia, irrespective of the type, and the complexity of the interaction between visual and trigeminal systems, has made the search for answers quite challenging.
In the past, many theories on the mechanisms of photophobia have been proposed and were focused on whether photophobia depends on the functional integrity of either the optic nerve, the trigeminal nerve, or both. Many of these proposals implied that both systems should interact with each other somewhere along their paths to produce the painful form of photophobia. Altogether, these proposals included abnormal processing of visual stimuli by the visual cortex due to hyperexcitability or reduced habituation (23–29); by the thalamus or its alteration by top-down cortico-thalamic input from the striate cortex (30); due to parenchymal lesions in the occipital lobe, thalamus or anywhere in the central course of the trigeminal nerve leading to non-painful hypersensitivity to light, termed ‘‘central dazzle’’ (31); due to direct activation of pain fibers in the eye (e.g. cornea, iris, uvea) by light, which consequently activate the spinal trigeminal nucleus, midbrain and thalamus in migraine or ocular diseases such as corneal damage (32,33); due to indirect activation of trigeminal pain fibers during parasympathetically-mediated vasodilation of ocular blood vessels (34,35); due to mechanical compression and irritation of the basal meninges by tumors near the optic chiasm (20); and sympathetic dysfunction leading to suboptimal control over the pupil and increased retinal illumination in migraine (12,36) and blepharospasm (37).
Due to the many anatomical structures and medical conditions that are associated with photophobia, efforts to identify the underlying neurobiological mechanisms behind its different forms must continue to focus on the relationship between visual networks, trigeminal pain pathways and autonomic regulation at multiple levels, from the eye to the cerebral cortex, in a pathology-specific manner.
Visual pathways for exacerbation of headache by light
The vast majority of migraineurs with normal eyesight report that exposure to ambient light renders their headache more intense and that lights appear brighter than usual during an attack (11,38,39). These clinical features may also in some cases be accompanied with ocular discomfort or pain, known as photo-oculodynia, especially when exposed to bright lights (15). Migraine patients experiencing these light-induced symptoms of photophobia usually stop performing mundane tasks, wear glasses that diminish the amount of light reaching the eye and, when possible, seek the comfort of darkness. The common notion is that exposure to light that is otherwise comfortable to healthy people exacerbates the intensity of the headache. However, the identity of photophobia in migraine is particularly intriguing because it may express itself as light-induced exacerbation of headache alone, as increased brightness alone, or both, at any given attack, and in some cases with the additional presence of ocular discomfort (15).
Despite the lack of distinction between these photophobia features, the high prevalence in migraine has placed it as one of the main criteria (besides headache characteristics) for the diagnosis of migraine (with and without aura) in the International Classification of Headache Disorders (40), where photophobia is simply defined as ‘‘hypersensitivity to light, usually causing avoidance’’. Although less frequent, photophobia has also been reported in tension-type headache (TTH), headaches associated with traumatic brain injury, unilaterally in cervicogenic and cluster headache and other trigeminal autonomic cephalalgias (TACs) (11,19,39,41). However, there is virtually no research attempting to identify the cause of photophobia in these other headache conditions.
Insight into the role of visual networks for light-induced intensification of headache emerged in a recent translational study with blind migraineurs (42). The authors hypothesized that photic information carried through the optic nerve must be transmitted, at least partially, to retino-recipient brain targets for this phenomenon to occur. Accordingly, intensification of headache was perceived only by blind migraineurs with an intact optic nerve who were able to partially perceive ambient illumination but unable to form images due to degeneration of rod and cone photoreceptors (i.e. patients diagnosed with retinitis pigmentosa, retinal prematurity or cone/rod dystrophy). Conversely, totally blind migraineurs lacking any type of visual perception due to damage of the optic nerve (i.e. those with optic neuropathy, retinal cancer or enucleation), which translates to complete absence of photic information reaching their brain, testified that light does not hurt them during migraine. These optic nerve-compromised subjects also presented fragmented or irregular sleep patterns and deficient pupillary light response. These findings provided evidence that in the absence of visual signaling from rods and cones, light activation of the newly discovered intrinsically photosensitive retinal ganglion cells (ipRGCs) expressing melanopsin photopigment was sufficient to produce photophobia during migraine headaches (43–45). Along with these clinical observations, anatomical and functional data obtained in rats further support this scenario (42). Experiments using in vivo electrophysiological recordings of dura-sensitive (trigeminovascular) neurons in the thalamus showed that shining light into the contralateral eye induces robust and sustained increase in their activity. These light/dura-sensitive neurons, which are located in the lateral posterior (LP) and posterior (Po) nuclei, receive input from ipRGCs. In addition, the axons of these thalamic trigeminovascular neurons project to multiple cortical areas including somatosensory (S1/S2) and visual (V1/V2) cortices (42,46) (Figure 1). Further evidence supporting the existence of such a pathway in humans comes from imaging studies showing blood oxygen-level dependent (BOLD) responses in the pulvinar (LP/Po area in the posterior thalamus of the rat) of patients undergoing a migraine attack with extra-cephalic allodynia (47), and from diffusion MR tractography showing direct connections from the optic nerve to the pulvinar, and from the pulvinar to several associative cortical brain regions (48). (see ‘‘thalamo-cortical role in photophobia’’ section below).
Figure 1.
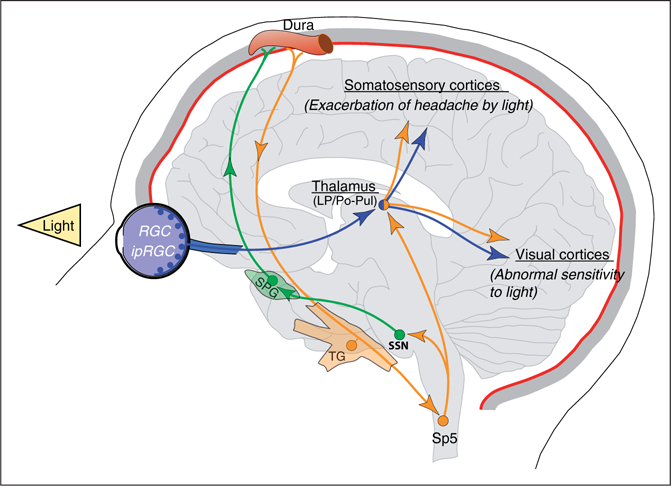
Schematic representation of the retino-thalamo-cortical pathways proposed to explain the most common light-induced sensory symptoms in migraine when patients are exposed to ambient light during attacks. LP: lateral posterior thalamic; Po: posterior nucleus of the thalamus; Pul: pulvinar; RGC: retinal ganglion cells; ipRGC: intrinsically sensitive retinal ganglion cells; SSN: superior salivatory nucleus; SPG: sphenopalatine ganglion; TG: trigeminal ganglion; Sp5: spinal trigeminal nucleus.
These studies suggest that the convergence of photic signals from ipRGCs onto the trigeminovascular thalamo-cortical pathway has the potential to explain how light intensifies the perception of headache during migraine. Additional evidence supporting the role of ipRGCs in migraine photophobia comes from a better understanding of their physiological role (49–52), as well as from patients’ testimonies and research reporting that migraineurs are more sensitive to certain wavelengths of visible light (53), and from the significant benefit they obtain when using lenses blocking bands of spectral light (54–56). It is important to note however, that the sensitivity to selective light stimulation of the ipRGCs is not the only responsible for explaining the effects of blocking specific wavelengths, and therefore a single-player conclusion must be tempered by the knowledge that rods and cones provide visual signals to the ipRGCs under dim to moderately bright illumination.
Role of ipRGCs in migraine photophobia
As mentioned above, it is well established that activation of melanopsinergic ipRGCs under conditions when rod and cone visual signaling is compromised allows regulation of biological functions such as circadian photoentrainment, pupillary light reflex (PLR), melatonin release and eye development (44,45,57–60). Comprehensive reviews on the structural and functional properties of these photoreceptors highlight their role as an illumination detector system, optimally-driven by ~480 nm wavelength photons producing blue in the visible spectrum (49,61). In fact, a study with blind individuals who were unable to form images due to the absence of cone-mediated visual signaling and were nominally unaware of light perception, yet with intact ability to photoentrain and regulate melatonin release, showed that they were able to correctly identify the interval period of a 481 nm test light but failed to detect it at other wavelengths. Such perception, described by them as ‘‘brightness’’, led to the conclusion that these photoreceptors might also contribute to some awareness of light (62). This possibility is further supported by anatomical and functional evidence in primates and rodents showing direct ipRGC projections to the lateral geniculate nucleus (LGN), which in turn projects to the V1 cortex (63,64). Also, in neonatal rodents whose rods and cones are not fully developed, light stimulation induces a robust behavioral response of avoidance associated with aversion, which was completely abolished in mice lacking melanopsin photoreceptors (65–67). Altogether, these studies unveiled previously unknown functions for non-image-forming pathways and suggest that the perceptual experience induced by direct activation of ipRGCs likely includes awareness and emotional reactions through continuous sensing of background illumination to regulate functional adaptation of the retina (49,68–71).
In the context of migraine, further support for the role of non-image-forming pathways in photophobia emerged in a proof-of-concept study designed to maximally block blue light (480 nm) using notch filter lenses during 2-week periods in chronic migraineurs (54). Using the headache impact test (HIT-6) and a photophobia questionnaire, the authors found that not only blue but also sham lenses filtering red light (620 nm) were beneficial for the headache and photophobia. The expected benefit of blocking blue and the surprising benefit of blocking red light was interpreted as due to the intrinsic bistable properties of melanopsin photopigment, by which red light could photoswitch melanopsin photopigment to a more sensitive state. Accordingly, under resting state (dark), melanopsin can be maximally activated by short wavelengths of light (~480 nm blue) through photoconversion of 11-cis to all-trans retinaldehyde isoform, which leads to ipRGC depolarization and transmission of photic information to the brain (49). Direct application of long wavelengths (~620 nm red) was proposed to activate an intrinsic photorecovery mechanism (72,73); however, direct recordings of ipRGCs under red wavelengths failed to show a response (74), thus opposing the bistability hypothesis described above. Nevertheless, despite the mechanisms involved, blocking specific wavelengths of light with tinted glasses has proven effective in decreasing migraine frequency in children and adults (55,56), and photophobia symptoms in blepharospasm (75).
From another therapeutic perspective, normalizing photic neurotransmission along visual pathways remains a potential and attractive target to treat migraine photophobia emerging from visual processes. Along this line, ipRGCs projecting to thalamic, hypothalamic and midbrain nuclei contain glutamate and pituitary adenylate cyclase-activating polypeptide (PACAP) as neurotransmitters (76–79), two molecules that have been largely implicated in migraine pathophysiology. For example, PACAP can trigger migraine-like headaches in migraineurs and healthy controls when injected intravenously (80,81), and it has been associated with a variety of functions relevant to migraine biology including stress, feeding, circadian rhythms and pain (82,83). Its receptors are also strategically located in a number of anatomical structures linked to migraine such as cranial vasculature, mast cells, sensory and autonomic neurons in the periphery, and brain areas including ipRGC-projecting targets and brainstem trigeminal nuclei (77,84,85). Although, nitroglycerin-induced photophobic behaviors are greatly attenuated in transgenic mice lacking PACAP (86), the specific role of this peptide in migraine photophobia remains to be determined.
As mentioned above, additional retinal mechanisms are also involved in the type of photophobia experienced by migraineurs with normal eyesight, and although the neural mechanism unraveled by the Burstein’s group (42) suggests an association between intensification of headache by light and ipRGCs (especially in blind subjects), activation of ipRGCs is also achieved extrinsically by rods and cones (87) through amacrine and bipolar retinal cells (88,89).
Role of cone/rod-driven retinal pathways in migraine photophobia
The classic image-forming photoreceptor system is at the forefront in color formation and contributes to light intensity processing at all times in activities of our daily life. For migraineurs with normal eyesight, virtually all luminous intensities can trigger uncomfortable features of photophobia (19). They are hypersensitive to light in plain daylight or while facing a screen, in which S- M- and L-cones are more sensitive to color and high illumination conditions (photopic vision); in low illumination conditions where rods are more sensitive (scotopic vision); or when driving at night using both photoreceptors (mesopic vision) (90,91), which is a serious challenge for photophobic migraineurs. Given that migraine has also been associated with abnormal color vision and color discrimination (92–94), maladaptive dysfunction of cones and/or rods’ photoreceptors could play an additional role in migraine photophobia.
In a recent translational study, Noseda et al. (2016) documented classic photoreceptor contribution to migraine photophobia. A psychophysical study was performed in migraineurs with normal eyesight to test the effects of colors in headache intensity and other headache features. White, blue (447 ± 10 nm), green (530 ± 10 nm), amber (590 ± 10 nm) and red (627 ± 10 nm) lights were applied using a full-field Ganzfeld stimulator calibrated to deliver narrow bands of visible light at equal intensity to the human eye for 3 minutes. Surprisingly, green light significantly attenuated the headache intensity, as opposed to the exacerbating effect of white, blue, amber and red lights. Headache features such as throbbing, location and spread were also affected selectively. Mechanistic support was obtained by using color-selective electroretinography (ERG) and visual evoked potentials (VEP) in migraineurs, and electrophysiological recordings of dura/light-sensitive neurons in the rat LP/Po thalamic region. Green light evokes the smallest a-wave signal in the light-adapted flash ERG of migraineurs as compared to white, blue, amber and red. Similarly, neuronal responses of rat dura/light-sensitive neurons were milder under green illumination, and cortical P2 amplitude generated by green in patients interictally was significantly smaller than P2 evoked by other colors. These results suggest that the perception of headache intensity is selectively modulated by spectral light through photoactivation of cone-driven retinal pathways. The unexpected soothing effects of green light observed in the psychophysical study add to the previously known hypersensitivity to blue and red (53,56,75), which is further supported by this study.
In the same cohort of patients, other light-induced non-sensory symptoms were studied under the premise that migraineurs also describe exposure to light as an unpleasant or uncomfortable experience. Psychophysical assessment showed that light, independent of the wavelength, triggered more changes in symptoms associated with autonomic functions during migraine than interictally or in control subjects (18). These symptoms included parasympathetically-mediated lacrimation, salivation, nausea and rhinorrhea, and sympathetically-mediated dry mouth, shortness of breath, chest tightness and palpitations, among other autonomic symptoms. In addition, the association between light and negative emotions such as feeling angry, scared, sad or stressed was stronger in migraineurs, as opposed to the association between light and positive emotions described as relaxing, happy, calming and soothing, which was stronger in control subjects than in migraineurs. Interestingly, green light was the only color able to trigger positive emotions during migraine, and red was most commonly associated to negative emotions. To delineate the neural mechanism behind these observations, a complimentary anatomical study in rats showed RGC axons interacting with hypothalamic neurons that project directly to the brainstem (superior salivatory nucleus, SSN) and spinal cord (intermediolateral nucleus, IML) nuclei involved in parasympathetic and sympathetic regulation, respectively. Hypothalamic neurons receiving retinal input were also shown to contain dopamine, histamine, orexin, melanin-concentrating hormone, oxytocin, or vasopressin. Altogether, the variety of autonomic symptoms contributing to the unpleasant sensations described by migraineurs could be associated with photic activation of these retinohypothalamic-parasympathetic (RHP) and retinohypothalamic-sympathetic (RHS) pathways (Figure 2). The emotions triggered by colors of light, however, may originate from a more complex interplay between these retino-hypothalamic networks and limbic structures, as well as direct retinal input to the amygdala (64,95,96). In addition, these findings raise the possibility that the hypothalamus is the culprit in abnormal parasympathetic and sympathetic functions during migraine. This possibility is further supported by the pivotal role played by the hypothalamus in migraine pathophysiology (see review on the hypothalamus in this same special issue).
Figure 2.
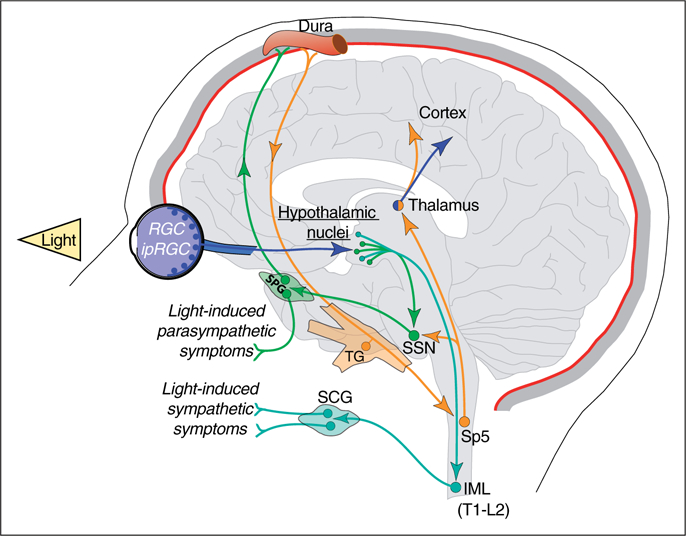
Schematic representation of the retino-hypothalamo-parasympathetic (SSN) and sympathetic (IML) pathways proposed to explain a host of light-induced autonomic responses during migraine attacks, and to a lesser extent, between attacks. SCG: superior cervical ganglion; IML: intermediolateral nucleus.
Role of retinal pathways to midbrain and brainstem in migraine photophobia
Independent of the contribution of hypothalamic dysfunction, the role of the autonomic nervous system in photophobia is undisputable. Other potential mechanisms causing dysregulation of autonomic functions in migraine, in addition to the RHP/RHS pathways described above, could be linked to dysfunction of retinal pathways controlling pupillary light reflex (PLR) through autonomically-driven midbrain circuits (12,97–99) (Figure 3). Along this line, further contribution of autonomic dysregulation in migraine photophobia was recently tested in a remarkable study by Brennan’s group (100). Quantitative measurement of PLR together with clinical evaluation and psychophysical assessment of photophobia threshold was performed in migraineurs and healthy controls. They showed that abnormal PLR was associated with low photophobia thresholds interictally, and that this abnormality was correlated with migraine severity. Chronic migraineurs and patients experiencing the most severe migraines (i.e. those exhibiting the highest HIT-6 and Migraine Disability Assessment (MIDAS) scores) had the lowest photophobia thresholds and displayed the greatest alteration in constriction latency (parasympathetic) and pupillary re-dilation (sympathetic) measurements. In addition to validating the pupillary function as a biomarker of migraine photophobia, the authors conclude that abnormal PLR is central in origin and reflects autonomic maladaptation to chronic light sensitivity. This is consistent with the notion of altered excitability in CNS structures in migraine pathophysiology (27,101).
Figure 3.
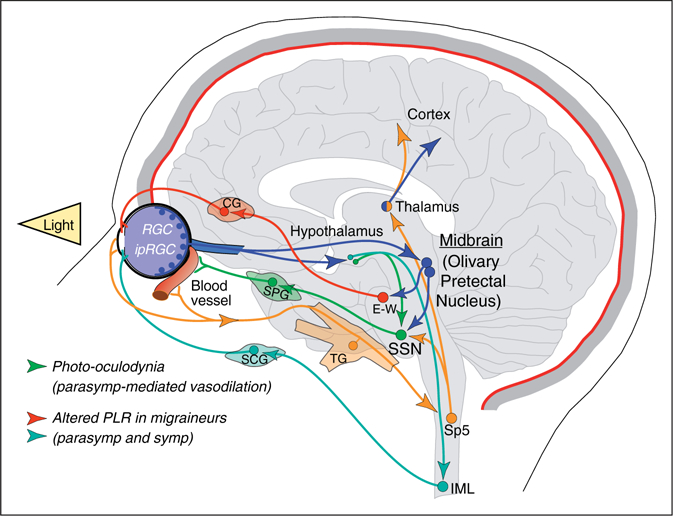
Schematic representation of the retino-midbrain-parasympathetic (SSN) pathway proposed to explain bright light-induced ocular pain. Pupillary light reflex circuit, which is abnormal in migraine, is also depicted. CG: ciliary ganglion; E-W: Edinger-Westphal nucleus.
Pupillary light reflex involves transfer of photic information by cone/rod-ipRGC photoreceptors to the pretectal area in the midbrain, and from there to preganglionic parasympathetic (E-W) and sympathetic (IML) nuclei controlling iris movement through ciliary and superior cervical ganglia, respectively (102). Interestingly, another mechanism involving retinal input to the pretectal area and autonomic regulation of the ophthalmic region has been proposed to contribute to photophobia. In this case, excess of light may lead to ocular pain or photo-oculodynia, which is another clinical manifestation falling into the definition of photophobia. Accordingly, bright light could induce ocular pain by activation of a retinal-olivary pretectal (OPN)-SSN pathway that drives vasodilation of choroid blood vessels. Ocular pain may then result from mechanical stimulation of sensitized trigeminal nociceptors innervating dilated eye vasculature (34,35) (Figure 3). Activation of these trigeminal nociceptors can further amplify photophobia through trigeminal-autonomic reflex (12) and hypothalamic facilitation of trigeminal activity (103,104). These complementary mechanisms are thought to play a major role in the type of photophobia associated with inflammation of the uvea (iris, ciliary body and choroid), corneal damage and other prechiasmal pathologies (13,15,32,105).
These ocular structures are richly innervated by trigeminal sensory neurons containing CGRP and, given that CGRP plays a fundamental role in migraine pathophysiology, its contribution to photophobia is beyond doubt (106). Indeed, photophobic behaviors such as increased light aversion or sensitivity to light have been shown in a transgenic mouse model of migraine presenting increased sensitivity to CGRP due to overexpression of the human receptor activity–modifying protein 1 (hRAMP1) (107,108). Behavioral data obtained using this model in combination with selective blockage of CGRP suggest that CGRP may contribute to photophobia through both peripheral and central, yet unknown, mechanisms (109).
Role of thalamo-cortical loop in migraine photophobia
A large body of evidence points to structural and functional abnormalities in the thalamus of migraineurs (47,110,111). How these abnormalities impact the perception of photophobia is unknown, but as described above, thalamic neurons outside the main visual pathway can also process photic and nociceptive trigeminovascular signals and transfer them to the cortex (17,42). This scenario places light as another modulator of nociception at the thalamic level. Indeed, thalamic neurons are subjected to a variety of modulatory inputs from cortical, intrathalamic, hypothalamic and brainstem origin (112,113). In rats, thalamic LP/Po nuclei implicated in photophobia are influenced by a variety of neuropeptides and neurotransmitters from different sources such as GABA from the reticular thalamic nucleus; noradrenaline from the locus coeruleus; serotonin from the raphe nuclei; and histamine, MCH, orexin, acetylcholine and dopamine from a variety of hypothalamic and forebrain nuclei (18,114). Also, besides glutamatergic afferents from ascending trigeminal afferents (115,116), glutamatergic input from ipRGCs to LP but not Po has been shown in mice (64,117), suggesting that activation of thalamic trigeminovascular neurons in these nuclei by light may occur directly through retinal input and indirectly through other structures such as the superior colliculus (95,118–120) and cortex (115). Accordingly, thalamic LP/Po neurons not only send axonal projections to a variety of cortical areas involved in sensory processing (42,46) but also receive massive projections from the cortex (112,121). Indeed, a single Po neuron in the thalamus was shown to integrate sensory ascending information with powerful cortical signals and transfer the integrated activity back to cortical networks (122). In humans and primates, structural and functional homology of the LP/Po area in rodents is found in the pulvinar (123,124), which has been associated with migraine photophobia (48) and projects to several cortical regions including the cingulate, visual association, somatosensory, parietal and prefrontal cortices (47,125–127), suggesting that this region of the posterior thalamus and its connectivity with the cortex play a wider role in sensory processing.
Observations that migraineurs are also more sensitive to light between attacks (11,39,128) and that lower thresholds for visual detection were measured in migraine with aura (129), led to the hypothesis of altered excitability of cortical areas involved in the processing of photic signals. Indeed, a recent PET imaging study with migraine patients and healthy controls showed that migraineurs have an intensity-dependent increased sensitivity to light correlated with increased activation of the visual cortex (25,130). Therefore, a hyperexcitable visual cortex incapable of habituating to visual stimuli was proposed as the neural substrate of migraine photophobia. Abnormally high neuronal excitability is not limited to the visual cortex; however, it may also include cortical regions involved in the perception of pain, sound, smell and taste, suggesting that other sensory hypersensitivities are mediated by abnormal excitability of cortical regions (23,24,27,131–133), or alternatively, by dysrhythmias in thalamo-cortical networks (134).
Article highlights.
Photophobia is a poorly defined multi-symptomatic light-induced phenomenon.
The relative contribution of visual, trigeminal and autonomic mechanisms in photophobia may depend on the condition it emerges from.
Both cone/rod-mediated image-forming and melanopsin-mediated non-image-forming visual pathways contribute to photophobia in migraineurs with normal eyesight.
Migraine photophobia emerges from activation of retinal photoreceptors and the photic signals they transmit to multiple brain regions involved in sensory, autonomic and emotional regulation.
Blocking all colors of light but green may alleviate photophobia symptoms in migraine.
Photophobia’s association with headache appears more complex than previously thought.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grants R21-NS090254, R01-NS104296 (R.N.) and R37-NS079678 (R.B.).
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Lauritzen M, Dreier JP, Fabricius M, et al. Clinical relevance of cortical spreading depression in neurological disorders: Migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab 2011; 31: 17–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolff HG, Tunis MM and Goodell H. Studies on headache: Evidence of tissue damage and change in pain sensitivity in subjects with vascular headaches of the migraine type. AMA Arch Intern Med 1953; 92: 478–484. [DOI] [PubMed] [Google Scholar]
- 3.Lashley KS. Patterns of cerebral integration indicated by the scotomas of migraine.. Arch Neurol Psychiatry 1941; 46: 331–339. [Google Scholar]
- 4.Pietrobon D and Moskowitz MA. Chaos and commotion in the wake of cortical spreading depression and spreading depolarizations. Nat Rev Neurosci 2014; 15: 379–393. [DOI] [PubMed] [Google Scholar]
- 5.Theriot JJ, Toga AW, Prakash N, et al. Cortical sensory plasticity in a model of migraine with aura. J Neurosci 2012; 32: 15252–15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwedt TJ. Multisensory integration in migraine. Curr Opin Neurol 2013; 26: 248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans RW and Digre KB. Light sensitivity in migraineurs. Headache 2003; 43: 917–920. [DOI] [PubMed] [Google Scholar]
- 8.Noseda R and Burstein R. Advances in understanding the mechanisms of migraine-type photophobia. Curr Opin Neurol 2011; 24: 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebensohn JE and Bellows J. The nature of photophobia. Arch Ophthalmol 1934; 12: 380–390. [Google Scholar]
- 10.Lebensohn JE. Photophobia: Mechanism and implications. Am J Ophthalmol 1951; 34: 1294–1300. [DOI] [PubMed] [Google Scholar]
- 11.Drummond PD. A quantitative assessment of photophobia in migraine and tension headache. Headache 1986; 26: 465–469. [DOI] [PubMed] [Google Scholar]
- 12.Drummond PD. Disturbances in ocular sympathetic function and facial blood flow in unilateral migraine headache. J Neurol Neurosurg Psychiatr 1990; 53: 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drummond PD and Woodhouse A. Painful stimulation of the forehead increases photophobia in migraine sufferers. Cephalalgia 1993; 13: 321–324. [DOI] [PubMed] [Google Scholar]
- 14.Drummond PD. Photophobia and autonomic responses to facial pain in migraine. Brain 1997; 120: 1857–1864. [DOI] [PubMed] [Google Scholar]
- 15.Digre KB and Brennan KC. Shedding light on photophobia. J Neuroophthalmol 2012; 32: 68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn AH and Brennan KC. Unanswered questions in headache: How does a migraine attack stop? Headache 2012; 52: 186–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noseda R, Bernstein CA, Nir R-R, et al. Migraine photophobia originating in cone-driven retinal pathways. Brain 2016; 139: 1971–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noseda R, Lee AJ, Nir R-R, et al. Neural mechanism for hypothalamic-mediated autonomic responses to light during migraine. Proc Natl Acad Sci USA 2017; 114: E5683–E5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz BJ and Digre KB. Diagnosis, pathophysiology, and treatment of photophobia. Surv Ophthalmol 2016; 61: 466–477. [DOI] [PubMed] [Google Scholar]
- 20.Kawasaki A and Purvin VA. Photophobia as the presenting visual symptom of chiasmal compression. J Neuroophthalmol 2002; 22: 3–8. [DOI] [PubMed] [Google Scholar]
- 21.Welty TE and Horner TG. Pathophysiology and treatment of subarachnoid hemorrhage. Clin Pharm 1990; 9: 35–39. [PubMed] [Google Scholar]
- 22.Lamonte M, Silberstein SD and Marcelis JF. Headache associated with aseptic meningitis. Headache 1995; 35: 520–526. [DOI] [PubMed] [Google Scholar]
- 23.Aurora SK, Cao Y, Bowyer SM, et al. The occipital cortex is hyperexcitable in migraine: Experimental evidence. Headache 1999; 39: 469–476. [DOI] [PubMed] [Google Scholar]
- 24.Mulleners WM, Chronicle EP, Palmer JE, et al. Visual cortex excitability in migraine with and without aura. Headache 2001; 41: 565–572. [DOI] [PubMed] [Google Scholar]
- 25.Denuelle M, Boulloche N, Payoux P, et al. A PET study of photophobia during spontaneous migraine attacks. Neurology 2011; 76: 213–218. [DOI] [PubMed] [Google Scholar]
- 26.Sand T, White L, Hagen K, et al. Visual evoked potential and spatial frequency in migraine: A longitudinal study. Acta Neurol Scand 2009; 120: 33–37. [DOI] [PubMed] [Google Scholar]
- 27.Coppola G and Schoenen J. Cortical excitability in chronic migraine. Curr Pain Headache Rep 2011; 16: 93–100. [DOI] [PubMed] [Google Scholar]
- 28.Coppola G, Pierelli F and Schoenen J. Habituation and migraine. Neurobiol Learn Mem 2009; 92: 249–259. [DOI] [PubMed] [Google Scholar]
- 29.Sand T, Zhitniy N, White LR, et al. Brainstem auditoryevoked potential habituation and intensity-dependence related to serotonin metabolism in migraine: A longitudinal study. Clin Neurophysiol 2008; 119: 1190–1200. [DOI] [PubMed] [Google Scholar]
- 30.Coleston DM, Chronicle E, Ruddock KH, et al. Precortical dysfunction of spatial and temporal visual processing in migraine. J Neurol Neurosurg Psychiatr 1994; 57: 1208–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cummings JL and Gittinger JW. Central dazzle A thalamic syndrome? Arch Neurol 1981; 38: 372–374. [DOI] [PubMed] [Google Scholar]
- 32.Moulton EA, Becerra L and Borsook D. An fMRI case report of photophobia: Activation of the trigeminal nociceptive pathway. Pain 2009; 145: 358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckhardt LB, McLean JM and Goodell H. Experimental studies on headache: The genesis of pain from the eye. Proc Assoc Res Nerv Ment Dis 1943; 23: 209–227. [Google Scholar]
- 34.Okamoto K, Tashiro A, Chang Z, et al. Bright light activates a trigeminal nociceptive pathway. Pain 2010; 149: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okamoto K, Tashiro A, Thompson R, et al. Trigeminal interpolaris/caudalis transition neurons mediate reflex lacrimation evoked by bright light in the rat. Eur J Neurosci 2012; 36: 3492–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fine PG and Digre KB. A controlled trial of regional sympatholysis in the treatment of photo-oculodynia syndrome. J Neuro-Ophthalmol 1995; 15: 90–94. [PubMed] [Google Scholar]
- 37.McCann JD, Gauthier M, Morschbacher R, et al. A novel mechanism for benign essential blepharospasm. Ophthal Plast Reconstr Surg 1999; 15: 384–389. [DOI] [PubMed] [Google Scholar]
- 38.Selby G and Lance JW. Observations on 500 cases of migraine and allied vascular headache. J Neurol Neurosurg Psychiatry 1960; 23: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanagaite J, Pareja JA, Støren O, et al. Light-induced discomfort and pain in migraine. Cephalalgia 1997; 17: 733–741. [DOI] [PubMed] [Google Scholar]
- 40.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed] [Google Scholar]
- 41.Irimia P, Cittadini E, Paemeleire K, et al. Unilateral photophobia or phonophobia in migraine compared with trigeminal autonomic cephalalgias. Cephalalgia 2008; 28: 626–630. [DOI] [PubMed] [Google Scholar]
- 42.Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci 2010; 13: 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Provencio I, Jiang G, De Grip WJ, et al. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA 1998; 95: 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berson DM. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002; 295: 1070–1073. [DOI] [PubMed] [Google Scholar]
- 45.Lucas RJ, Hattar S, Takao M, et al. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science 2003; 299: 245–247. [DOI] [PubMed] [Google Scholar]
- 46.Noseda R, Jakubowski M, Kainz V, et al. Cortical projections of functionally identified thalamic trigeminovascular neurons: Implications for migraine headache and its associated symptoms. J Neurosci 2011; 31: 14204–14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burstein R, Jakubowski M, Garcia-Nicas E, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol 2010; 68: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maleki N, Becerra L, Upadhyay J, et al. Direct optic nerve pulvinar connections defined by diffusion MR tractography in humans: Implications for photophobia. Hum Brain Mapp 2011; 33: 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berson DM. Phototransduction in ganglion-cell photoreceptors. Pflugers Arch – Eur J Physiol 2007; 454: 849–855. [DOI] [PubMed] [Google Scholar]
- 50.Graham DM, Wong KY, Shapiro P, et al. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J Neurophysiol 2008; 99: 2522–2532. [DOI] [PubMed] [Google Scholar]
- 51.Ecker JL, Dumitrescu ON, Wong KY, et al. Melanopsin-expressing retinal ganglion-cell photoreceptors: Cellular diversity and role in pattern vision. Neuron 2010; 67: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ksendzovsky A, Pomeraniec IJ, Zaghloul KA, et al. Clinical implications of the melanopsin-based non–image-forming visual system. Neurology 2017; 88: 1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Main A, Vlachonikolis I and Dowson A. The wavelength of light causing photophobia in migraine and tension-type headache between attacks. Headache 2000; 40: 194–199. [DOI] [PubMed] [Google Scholar]
- 54.Hoggan RN, Subhash A, Blair S, et al. Thin-film optical notch filter spectacle coatings for the treatment of migraine and photophobia. J Clin Neurosci 2016; 28: 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilkins AJ, Patel R, Adjamian P, et al. Tinted spectacles and visually sensitive migraine. Cephalalgia 2002; 22: 711–719. [DOI] [PubMed] [Google Scholar]
- 56.Good PA, Taylor RH and Mortimer MJ. The use of tinted glasses in childhood migraine. Headache 1991; 31: 533–536. [DOI] [PubMed] [Google Scholar]
- 57.Hattar S, Liao HW, Takao M, et al. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 2002; 295: 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rao S, Chun C, Fan J, et al. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature 2013; 494: 243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci 2001; 21: 6405–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thapan K, Arendt J and Skene DJ. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol 2001; 535: 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lucas RJ, Peirson SN, Berson DM, et al. Measuring and using light in the melanopsin age. Trends Neurosci 2014; 37: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zaidi FH, Hull JT, Peirson SN, et al. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol 2007; 17: 2122–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dacey DM, Liao H-W, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 2005; 433: 749–754. [DOI] [PubMed] [Google Scholar]
- 64.Delwig A, Larsen DD, Yasumura D, et al. Retinofugal projections from melanopsin-expressing retinal ganglion cells revealed by intraocular injections of Cre-dependent virus. PLoS ONE 2016; 11: e0149501–e0149514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Panda S, Provencio I, Tu DC, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science 2003; 301: 525–527. [DOI] [PubMed] [Google Scholar]
- 66.Johnson J, Wu V, Donovan M, et al. Melanopsin-dependent light avoidance in neonatal mice. Proc Natl Acad Sci USA 2010; 107: 17374–17378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delwig A, Logan AM, Copenhagen DR, et al. Light evokes melanopsin-dependent vocalization and neural activation associated with aversive experience in neonatal mice. PLoS ONE 2012; 7: e43787–e43788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Warthen DM and Provencio I. The role of intrinsically photosensitive retinal ganglion cells in nonimage-forming responses to light. Eye Brain 2012; 4: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown TM, Tsujimura S-I, Allen AE, et al. Melanopsin-based brightness discrimination in mice and humans. Curr Biol 2012; 22: 1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hankins MW and Hughes S. Vision: Melanopsin as a novel irradiance detector at the heart of vision. Curr Biol 2014; 24: R1055–R1057. [DOI] [PubMed] [Google Scholar]
- 71.Provencio I, Rollag MD and Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature 2002; 415: 493–493. [DOI] [PubMed] [Google Scholar]
- 72.Mure LS, Rieux C, Hattar S, et al. Melanopsin-dependent nonvisual responses: Evidence for photopigment bistability in vivo. J Biol Rhythms 2007; 22: 411–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mure LS, Cornut P-L, Rieux C, et al. Melanopsin bistability: A fly’s eye technology in the human retina. PLoS ONE 2009; 4: e5991–e6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mawad K and van Gelder RN. Absence of long-wave-length photic potentiation of murine intrinsically photosensitive retinal ganglion cell firing in vitro. J Biol Rhythms 2008; 23: 387–391. [DOI] [PubMed] [Google Scholar]
- 75.Blackburn MK, Lamb RD, Digre KB, et al. FL-41 tint improves blink frequency, light sensitivity, and functional limitations in patients with benign essential blepharospasm. Ophthalmology 2009; 116: 997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hannibal J. Melanopsin is expressed in PACAP-containing retinal ganglion cells of the human retinohypothalamic tract. Invest Ophthalmol Vis Sci 2004; 45: 4202–4209. [DOI] [PubMed] [Google Scholar]
- 77.Hannibal J and Fahrenkrug J. Target areas innervated by PACAP-immunoreactive retinal ganglion cells. Cell Tissue Res 2004; 316: 99–113. [DOI] [PubMed] [Google Scholar]
- 78.Engelund A, Fahrenkrug J, Harrison A, et al. Vesicular glutamate transporter 2 (VGLUT2) is co-stored with PACAP in projections from the rat melanopsin-containing retinal ganglion cells. Cell Tissue Res 2010; 340: 243–255. [DOI] [PubMed] [Google Scholar]
- 79.Gompf HS, Fuller PM, Hattar S, et al. Impaired circadian photosensitivity in mice lacking glutamate transmission from retinal melanopsin cells. J Biol Rhythms 2015; 30: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schytz HW, Birk S, Wienecke T, et al. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain 2009; 132: 16–25. [DOI] [PubMed] [Google Scholar]
- 81.Amin FM, Hougaard A, Schytz HW, et al. Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain 2014; 137: 779–794. [DOI] [PubMed] [Google Scholar]
- 82.Hashimoto H, Shintani N and Baba A. New insights into the central PACAPergic system from the phenotypes in PACAP- and PACAP receptor-knockout mice. Ann NY Acad Sci 2006; 1070: 75–89. [DOI] [PubMed] [Google Scholar]
- 83.Schytz HW, Olesen J and Ashina M. The PACAP receptor: A novel target for migraine treatment. Neurotherapeutics 2010; 7: 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uddman R, Tajti J, Hou M, et al. Neuropeptide expression in the human trigeminal nucleus caudalis and in the cervical spinal cord C1 and C2. Cephalalgia 2002; 22: 112–116. [DOI] [PubMed] [Google Scholar]
- 85.Condro MC, Matynia A, Foster NN, et al. High-resolution characterization of a PACAP-EGFP transgenic mouse model for mapping PACAP-expressing neurons. J Comp Neurol 2016; 524: 3827–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Markovics A, Kormos V, Gaszner B, et al. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol Dis 2012; 45: 633–644. [DOI] [PubMed] [Google Scholar]
- 87.Gu¨ ler AD, Ecker JL, Lall GS, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature 2008; 453: 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Belenky MA, Smeraski CA, Provencio I, et al. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol 2003; 460: 380–393. [DOI] [PubMed] [Google Scholar]
- 89.Wong KY, Dunn FA, Graham DM, et al. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol 2007; 582: 279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stockman A and Sharpe LT. Into the twilight zone: The complexities of mesopic vision and luminous efficiency. Ophthalmic Physiol Opt 2006; 26: 225–239. [DOI] [PubMed] [Google Scholar]
- 91.Barrionuevo PA, Colombo EM and Issolio LA. Retinal mesopic adaptation model for brightness perception under transient glare. J Opt Soc Am A Opt Image Sci Vis 2013; 30: 1236–1247. [DOI] [PubMed] [Google Scholar]
- 92.Shepherd AJ. Local and global motion after-effects are both enhanced in migraine, and the underlying mechanisms differ across cortical areas. Brain 2006; 129: 1833–1843. [DOI] [PubMed] [Google Scholar]
- 93.Tibber MS, Guedes A and Shepherd AJ. Orientation discrimination and contrast detection thresholds in migraine for cardinal and oblique angles. Invest Ophthalmol Vis Sci 2006; 47: 5599–5604. [DOI] [PubMed] [Google Scholar]
- 94.Berger A, Findler M, Korach T, et al. Is male migraine associated with color vision deficiency? Findings among Israeli adolescents between 2007 and 2013. J Child Neurol 2016; 31: 593–596. [DOI] [PubMed] [Google Scholar]
- 95.Hattar S, Kumar M, Park A, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol 2006; 497: 326–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pessoa L and Adolphs R. Emotion processing and the amygdala: From a ‘‘low road’’ to ‘‘many roads’’ of evaluating biological significance. Nat Rev Neurosci 2010; 11: 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mylius V, Braune HJ and Schepelmann K. Dysfunction of the pupillary light reflex following migraine headache. Clin Auton Res 2003; 13: 16–21. [DOI] [PubMed] [Google Scholar]
- 98.Harle DE, Wolffsohn JS and Evans BJW. The pupillary light reflex in migraine. Ophthalmic Physiol Opt 2005; 25: 240–245. [DOI] [PubMed] [Google Scholar]
- 99.Cambron M, Maertens H, Paemeleire K, et al. Autonomic function in migraine patients: Ictal and interictal pupillometry. Headache 2014; 54: 655–662. [DOI] [PubMed] [Google Scholar]
- 100.Cortez MM, Rea NA, Hunter LA, et al. Altered pupillary light response scales with disease severity in migrainous photophobia. Cephalalgia 2016; 37: 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stankewitz A and May A. Cortical excitability and migraine. Cephalalgia 2007; 27: 1454–1456. [DOI] [PubMed] [Google Scholar]
- 102.McDougal DH and Gamlin PD. Autonomic control of the eye. Compr Physiol 2015; 5: 439–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Katagiri A, Okamoto K, Thompson R, et al. Posterior hypothalamic modulation of light-evoked trigeminal neural activity and lacrimation. Neuroscience 2013; 246: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Katagiri A, Okamoto K, Thompson R, et al. Posterior hypothalamic modulation of ocular-responsive trigeminal subnucleus caudalis neurons is mediated by Orexin-A and Orexin1 receptors. Eur J Neurosci 2014; 40: 2619–2627. [DOI] [PubMed] [Google Scholar]
- 105.Ahn AH and Brennan KC. Unanswered questions in headache: so what is photophobia, anyway? Headache 2013; 53: 1673–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Russo AF. Calcitonin gene-related peptide (CGRP): A new target for migraine. Annu Rev Pharmacol Toxicol 2015; 55: 533–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Recober A, Kuburas A, Zhang Z, et al. Role of calcitonin gene-related peptide in light-aversive behavior: Implications for migraine. J Neurosci 2009; 29: 8798–8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kaiser EA, Kuburas A, Recober A, et al. Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT1B/D agonist. Journal of Neuroscience 2012; 32: 15439–15449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mason BN, Kaiser EA, Kuburas A, et al. Induction of migraine-like photophobic behavior in mice by both peripheral and central CGRP mechanisms. J Neurosci 2017; 37: 204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Afridi SK, Matharu MS, Lee L, et al. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain 2005; 128: 932–939. [DOI] [PubMed] [Google Scholar]
- 111.Amin FM, Hougaard A, Magon S, et al. Altered thalamic connectivity during spontaneous attacks of migraine without aura: A resting-state fMRI study. Cephalalgia 2018; 38: 1237–1244. [DOI] [PubMed] [Google Scholar]
- 112.Sherman SM and Guillery RW. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond, B, Biol Sci 2002; 357: 1695–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol 1992; 39: 337–388. [DOI] [PubMed] [Google Scholar]
- 114.Kagan R, Kainz V, Burstein R, et al. Hypothalamic and basal ganglia projections to the posterior thalamus: Possible role in modulation of migraine headache and photophobia. Neuroscience 2013; 248: 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Landisman CE and Connors BW. VPM and PoM nuclei of the rat somatosensory thalamus: Intrinsic neuronal properties and corticothalamic feedback. Cereb Cortex 2007; 17: 2853–2865. [DOI] [PubMed] [Google Scholar]
- 116.Graziano A, Liu XB, Murray KD, et al. Vesicular glutamate transporters define two sets of glutamatergic afferents to the somatosensory thalamus and two thalamocortical projections in the mouse. J Comp Neurol 2008; 507: 1258–1276. [DOI] [PubMed] [Google Scholar]
- 117.Delwig A, Majumdar S, Ahern K, et al. Glutamatergic neurotransmission from melanopsin retinal ganglion cells is required for neonatal photoaversion but not adult pupillary light reflex. PLoS ONE 2013; 8: e83974–e83978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gooley JJ, Lu J, Fischer D, et al. A broad role for melanopsin in nonvisual photoreception. J Neurosci 2003; 23: 7093–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Donnelly JF, Thompson SM and Robertson RT. Organization of projections from the superior colliculus to the thalamic lateral posterior nucleus in the rat. Brain Res 1983; 288: 315–319. [DOI] [PubMed] [Google Scholar]
- 120.Wei P, Liu N, Zhang Z, et al. Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nature Communications 6: 66756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Descheˆ nes M, Veinante P and Zhang ZW. The organization of corticothalamic projections: Reciprocity versus parity. Brain Res Brain Res Rev 1998; 28: 286–308. [DOI] [PubMed] [Google Scholar]
- 122.Groh A, Bokor H, Mease RA, et al. Convergence of cortical and sensory driver inputs on single thalamocortical cells. Cereb Cortex 2014; 24: 3167–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marion R, Li K, Purushothaman G, et al. Morphological and neurochemical comparisons between pulvinar and V1 projections to V2. J Comp Neurol 2013; 521: 813–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou N, Masterson SP, Damron JK, et al. The mouse pulvinar nucleus links the lateral extrastriate cortex, striatum, and amygdala. J Neurosci 2018; 38: 347–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Glendenning KK, Hall JA, Diamond IT, et al. The pulvinar nucleus of Galago senegalensis. J Comp Neurol 1975; 161: 419–458. [DOI] [PubMed] [Google Scholar]
- 126.Raczkowski D and Diamond IT. Cortical connections of the pulvinar nucleus in Galago. J Comp Neurol 1980; 193: 1–40. [DOI] [PubMed] [Google Scholar]
- 127.Grieve KL, Acun˜ a C and Cudeiro J. The primate pulvinar nuclei: Vision and action. Trends Neurosci 2000; 23: 35–39. [DOI] [PubMed] [Google Scholar]
- 128.Main A, Dowson A and Gross M. Photophobia and phonophobia in migraineurs between attacks. Headache 1997; 37: 492–495. [DOI] [PubMed] [Google Scholar]
- 129.Chronicle EP, Wilkins AJ and Coleston DM. Thresholds for detection of a target against a background grating suggest visual dysfunction in migraine with aura but not migraine without aura. Cephalalgia 1995; 15: 117–122. [DOI] [PubMed] [Google Scholar]
- 130.Boulloche N, Denuelle M, Payoux P, et al. Photophobia in migraine: An interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatr 2010; 81: 978–984. [DOI] [PubMed] [Google Scholar]
- 131.Ambrosini A and Schoenen J. The electrophysiology of migraine. Curr Opin Neurol 2003; 16: 327–331. [DOI] [PubMed] [Google Scholar]
- 132.Aurora SK and Wilkinson F. The brain is hyperexcitable in migraine. Cephalalgia 2007; 27: 1442–1453. [DOI] [PubMed] [Google Scholar]
- 133.de Tommaso M, Ambrosini A, Brighina F, et al. Altered processing of sensory stimuli in patients with migraine. Nat Rev Neurol 2014; 10: 144–155. [DOI] [PubMed] [Google Scholar]
- 134.Coppola G, Ambrosini A, Di Clemente L, et al. Interictal abnormalities of gamma band activity in visual evoked responses in migraine: An indication of thalamocortical dysrhythmia? Cephalalgia 2007; 27: 1360–1367. [DOI] [PubMed] [Google Scholar]


