Significance
Normal cells harbor protective mechanisms to sense DNA damage, halt cell growth, and repair chromatin lesions to maintain genomic stability. How mitogen signaling in proliferating cells is affected upon BRCA1 loss as part of these protective checkpoints is an intriguing question. This study reveals a unique finding of linking BRCA1 to Hippo signaling pathway to explain this conundrum. Our work shows that serum-responsive expression of BRCA1 is required for YAP1 stability. Ubiquitination of NF2 by BRCA1/BARD1 in proliferating cells inhibits NF2/LATS association and Hippo signaling. These findings suggest Hippo signaling activation as a protective barrier in BRCA1-deficient cells, which upon inactivation, promotes cell proliferation and tumorigenesis.
Keywords: Hippo, BRCA1, NF2, ubiquitination, cancer
Abstract
Coordination of growth and genomic stability is critical for normal cell physiology. Although the E3 ubiquitin ligase BRCA1 is a key player in maintenance of genomic stability, its role in growth signaling remains elusive. Here, we show that BRCA1 facilitates stabilization of YAP1 protein and turning “off” the Hippo pathway through ubiquitination of NF2. In BRCA1-deficient cells Hippo pathway is “turned On.” Phosphorylation of YAP1 is crucial for this signaling process because a YAP1 mutant harboring alanine substitutions (Mt-YAP5SA) in LATS1 kinase recognition sites not only resists degradation but also rescues YAP1 transcriptional activity in BRCA1-deficient cells. Furthermore, an ectopic expression of the active Mt-YAP5SA, but not inactive Mt-YAP6SA, promotes EGF-independent proliferation and tumorigenesis in BRCA1−/− mammary epithelial cells. These findings establish an important role of BRCA1 in regulating stability of YAP1 protein that correlates positively with cell proliferation.
Inherited mutations in breast cancer early onset gene BRCA1 predispose individuals to highly aggressive form of breast and ovarian cancers (1, 2). Studies in a knockout mouse model have shown that mouse BRCA1 plays a key role in cell proliferation during embryonic development (3). Mouse BRCA1 function can be compensated by replacing it with human BRCA1 gene despite poor sequence homology (4, 5). BRCA1 is involved in multiple cellular functions, e.g., transcription, heterochromatin structure formation, replication fork stability, homologous recombination repair, centrosome regulation, and mitotic spindle formation (6–11). These diverse roles are specified by interaction of BRCA1 with its heterodimeric partner BARD1 that greatly enhances the E3 ubiquitin ligase activity of the complex (12). How loss of BRCA1 activity in heterozygous carriers leads to tumorigenesis remains only partially understood (13–15).
Ubiquitination is a highly regulated and reversible event induced by various stimuli that not only regulate protein stability but also functional interaction, localization, and signaling dynamics (16). These changes in protein activity by ubiquitination are governed by number of ubiquitin molecules attached and nature of linkage involved. The enzymatic activity of BRCA1/BARD1 ubiquitin ligase complex is unique because it can generate different kinds (mono and poly) of atypical ubiquitin linkages (K6, K29, K48, and K63) depending on the substrate and interacting E2 subunit (17–19). Several cellular proteins have been identified as substrates of this ubiquitin ligase activity, e.g., NPM1, RPB1, CtiP, RPB8, PR-A, TFIIE, Histones H2A, H2B, H3, H4, Ƴ-Tubulin, ER-α, Aurora A/B, and BRCA1 itself (20–23). These proteins are known to be involved in regulating key signaling steps in dividing cells, e.g., in regulating gene expression, proliferation, chromosome maintenance, duplication, repair, and segregation. However, unlike substrates of K48 ubiquitination, which are directed for proteasomal degradation, many substrates with other atypical ubiquitin linkages are not degraded but involved in signaling transduction. Thus, multiple pathways are integrated by BRCA1/BARD1 activity to ensure genomic stability in normal cells. Not only is BRCA1 highly expressed in proliferating cells, it is transcriptionally regulated by serum stimulation and changes in cell density (24). BRCA1 activity is further regulated via changes in its levels, phosphorylation state, and subnuclear distribution, depending on different stages of cell cycle and signaling (25–27). Furthermore, BRCA1/BARD1 activity is also regulated by proteosomal degradation (28). Therefore, BRCA1/BARD1 activity is tightly controlled to coordinate growth-factor-stimulated downstream signaling and genomic stability. Although BRCA1 is known to play key roles in maintenance of genomic stability, whether it cooperates with signaling pathways to regulate physiological processes such as cell growth is not fully explored.
The Hippo pathway transducer, YAP1 protein, is a mitogen-responsive transcriptional coactivator. YAP1 is stabilized in the presence of serum which turns “Off” the Hippo pathway (29). Upon serum stimulation, nuclear YAP1 in association with TEAD proteins promotes expression of genes involved in cell proliferation. In absence of serum, Hippo signaling kinases LATS1/2 are activated which, in turn, phosphorylate YAP1 to cause its cytoplasmic localization and degradation (30), thereby enabling homeostatic growth-restrictive regulation. Although YAP1/TAZ activation has been reported in various cancers (31), mutations involving core Hippo signaling components are not frequent (32), suggesting possible involvement of upstream regulators which are yet to be identified. In a recent analysis performed using 9,125 tumors profiled by The Cancer Genome Atlas (TCGA) from 33 cancer types, multiple alterations in Hippo pathway members were identified per tumor samples (33), suggesting that cooccurring copy number alteration events correlate with oncogenic activation of YAP1. Among core pathway components (scaffold protein: NF2; kinases: MST1, MST2, LATS1, LATS2; accessory molecules: SAV1, MOB1, MOB2; transcriptional coactivators: YAP1, TAZ), NF2 is critical for Hippo signaling as its inactivation impairs LATS1/2 activation and subsequent phosphorylation of YAP1 (34). The current model in the field suggests that NF2 activates Hippo signaling pathway via binding LATS1/2 to facilitate LATS1/2 activation by MST1/2 (35). Also, NF2 activity is regulated by intramolecular interactions between N-terminal Ferm domain and C-terminal coil domains, directing interconversion between open to closed conformation (36). What molecular mechanism restricts NF2 activity during mitogen signaling and assembly of these signaling complexes however remains unclear. Recently reported interactions of Hippo pathway components with various ubiquitin ligases and deubiquitinase enzymes (37–41) suggest existence of ubiquitin signaling switches during Hippo signaling regulation.
In this article, we show an important role of BRCA1 expression in Hippo signaling pathways. During mitogen signaling, NF2 is inhibited by BRCA1/BARD1-mediated ubiquitination, leading to YAP1 stabilization. Our findings suggest a previously uncharacterized protective mechanism in normal cells for fine-tuning growth-factor signaling and genomic stability, which when bypassed in BRCA1-deficient mammary epithelial cells, promote cell growth and tumorigenesis.
Results
BRCA1/BARD1 Promote NF2 Ubiquitination and Inhibits Hippo Signaling.
We first tested if ubiquitin signaling could be involved in Hippo signaling regulation in proliferating cells. We observed that among all of the core Hippo components, only NF2 and to a lesser extent LATS1 were heavily ubiquitinated in serum-stimulated cells (Fig. 1A). LATS1/2 were previously shown to be ubiquitinated by Itch and CRLA4(DCAF1) ubiquitin ligase complex that direct LATS1/2 for degradation (38, 39). The nature of ubiquitination and ligase(s) specific for NF2 as well as its possible implication in Hippo signaling has not been previously explored. Enrichment of ubiquitinated NF2 in denaturing conditions followed by mass spectrometry analyses revealed that NF2 ubiquitination is K63-linked and involves multiple lysines (K159, K269, K274, K364, K387, K396, K439, and K449) residues located not only in unstructured coiled coil domain but also highly ordered N-terminus FERM domain (Fig. 1B and SI Appendix, Fig. S1 A and B). Since FERM domain is known to mediate interaction with LATS1 kinase (35), we examined binding of equal amounts of free and ubiquitinated NF2 with LATS1 from cell extract. Compared with free NF2 that is efficiently bound to LATS1 (Fig. 1C; lane 1), ubiquitination of NF2 inhibited LATS1 binding (Fig. 1C; lane 2). Enrichment of ubiquitinated proteins after cell fractionation revealed that ubiquitinated NF2 resided mainly in the nucleus (Fig. 1D). Using mutants of ubiquitin with single lysine available for linkage (Ub-K6, Ub-K11, Ub-K27, Ub-K29, Ub-K33, Ub-K48, and Ub-K63), we found that NF2 ubiquitination preferentially incorporates Ub-K6, Ub-K27, Ub-K29, and Ub-K63 but not Ub-K48, Ub-K11, and Ub-K33 (Fig. 1E) ubiquitin mutants, suggesting its possible involvement in nonproteosomal regulation. These results are in agreement with the long half-life (exceeding 24 h) time of NF2 in cells (42). Expression of E3 ligase BRCA1 in cultured cell is serum responsive and known to catalyze formation of K6, K29, K48, and K63 linked ubiquitin chains (17–19, 24). To test involvement of BRCA1/BARD1 with NF2 ubiquitination, we inhibited BRCA1/BARD1 activity in cells by expressing shRNA against BRCA1 and BARD1. As shown (Fig. 1F; lane 3 compared with lane 2), ubiquitination of NF2 was greatly enhanced upon stimulation of cell with serum. On the other hand, NF2 ubiquitination was significantly reduced in BRCA1/BARD1-deficient cells (Fig. 1F; lane 5 compared with 3). We further tested if BRCA1 overexpression can also augment NF2 ubiquitination. Overexpression of BRCA1 greatly enhanced NF2 ubiquitination (Fig. 1G; lane 3 compared with 2). Also, to test involvement of BARD1 in this process we coexpressed shRNA against BARD1 in the same cells, which reversed enhanced NF2 ubiquitination (Fig. 1G; lane 4 compared with 3). Similarly, ubiquitination of endogenous NF2 was confirmed to be sensitive to shRNA-mediated inhibition of BRCA1/BARD1 (Fig. 1H). We also inhibited BRCA1 expression in HEK293T-Cas9 cells (stably transduced with Cas9 gene) by transient transfection of single guide RNA (sgRNA) targeting BRCA1 and observed inhibition of NF2 ubiquitination (SI Appendix, Fig. S1C). Mutations in BRCA1 N-terminus ring finger and C-terminus BRCT domains have been identified in human patients (43). We therefore tested the pathogenic mutant defective in BARD1 interaction and ubiquitin ligase activity (BRCA1 C61G) or BRCT domain mutants (BRCA1 M1755R) for their ability to promote NF2 ubiquitination. Both of the pathogenic mutants BRCA1 C61G or BRCA1 M1755R failed to enhance NF2 ubiquitination (Fig. 1I, lanes 5 and 6). On the contrary, expression of ring-finger domain mutant, BRCA1-I31M that does not alter ubiquitin ligase activity, was found to be equally potent as wild-type protein in promoting NF2 ubiquitination (Fig. 1I, lanes 3 and 4). Based on these observations, we speculated that BRCA1-dependent ubiquitination of NF2 in proliferating cells may inhibit Hippo signaling.
Fig. 1.
BRCA1/BARD1 promotes NF2 ubiquitination to inhibit Hippo signaling. (A) HEK293T cells were cotransfected with His-Ub with HA-MOB, HA-SAV, HA-MST1, HA-NF2, HA-LATS1, HA-YAP1, or HA-TAZ. After 36 h cells were treated with MG132 for 8 h followed by lysis in denaturation buffer, and then total ubiquitinated proteins were pulled down with the use of Ni-NTA beads, and ubiquitination was checked by immunoblotting with anti-HA antibody. (B) A schematic structure of NF2, indicating the positions of the ubiquitination sites and peptide sequences identified by mass spectrometry analysis. (C) HEK293T cells were cotransfected with HA-NF2 alone or in combination with His-Ub followed by purification of free and ubiquitinated NF2 using HA-binding beads. The HA-bead–bound proteins were then incubated with cell lysate for LATS1 binding as described in Materials and Methods. (D) NF2 ubiquitination was checked by Ni-NTA pull-downs from HEK293T cells transfected with His–Ub and HA-NF2, after an initial cell fractionation step using MF, CF, and NF. MF, membrane fraction; CF, cytoplasmic fraction; NF, nuclear fraction. Successful cell fractionation was confirmed by immunoblotting for specific markers of MF (E-cadherine), CF (GAPDH), and NF (BRG1). (E) Indicated mutants of HA-ubiquitin were cotransfected into HEK293T cells with Flag-NF2. HA pull-downs from transfected cells were then analyzed by immunoblotting using NF2 antibody. (F) HEK293T cells transfected with ubiquitin and HA-NF2 constructs were first starved for 12 h, then stimulated with serum for 24 h and tested for NF2 ubiquitination as described above. (G) HEK293T cells were cotransfected with His-Ub, HA-NF2, BRCA1 and shBRCA1, and shBARD1 as indicated and NF2 ubiquitination was checked. (H and I) HEK293T cells transfected with His-Ub and indicated plasmids, to measure NF2 ubiquitination. (J) HEK293T cells were cotransfected with UAS-luciferase/TEAD-GAL4, renilla luciferase (normalization control), shBRCA1, and BRCA1 as indicated, followed by measurement of luciferase activity which is normalized to control renilla luciferase reading. Results are expressed as means ± SEMs. Only values of P < 0.05 were considered significant (****P < 0.0001), by ANOVA test.
To dissect molecular consequences of BRCA1-mediated inhibition of Hippo signaling, we examined effects of BRCA1 expression or knockdown on YAP1 transcriptional activity. Measured by YAP1-responsive UAS-luciferase/TEAD-Gal4 reporter (30), we observed a profound increase in YAP1 transcriptional output upon BRCA1 coexpression (Fig. 1J). On the other hand, we observed strong inhibition of reporter activity in BRCA1 knockdown cells. These data suggest that BRCA1 directly regulates Hippo signaling through NF2 ubiquitination and promotes YAP1 activation.
BRCA1 Binds NF2 Through FERM and C-Terminal Domains.
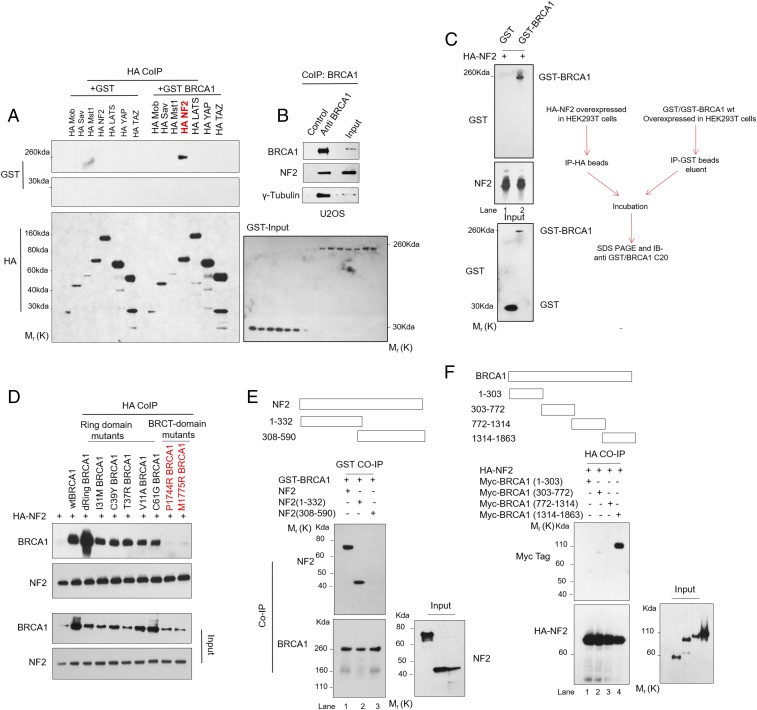
We further analyzed direct role of BRCA1 protein in suppressing Hippo signaling through protein interactions. We tested interaction of BRCA1 with core Hippo pathway components by pull-down assays. HA-tagged NF2, but not any other core component (MOB, SAV, MST1, LATS, YAP1, and TAZ), was able to interact with GST-BRCA1 fusion protein (Fig. 2A). The intracellular interaction of BRCA1 with NF2 was further confirmed by immunoprecipitation of endogenous BRCA1, which also recovered NF2 protein in eluted fractions (Fig. 2B). Also, HA-NF2 was able to specifically bind to GST-BRCA1 fusion protein but not GST negative control in vitro (Fig. 2C), confirming direct interaction between the two proteins. We tested pathogenic mutants in BRCT-domain (P1744R and M1775R) or ring-finger domain (I31M, C39Y, T37R, C61G, V11A, and ring-domain deleted dRing), for their ability to interact with NF2. As shown, both wild-type and all ring-finger mutants were equally potent in NF2 binding (Fig. 2D). However, interaction with NF2 was abolished in BRCT-domain mutants. We also compared patient-derived BRCA1-mutant HCC1937 cell line (harboring mutation in BRCT domain and loss of WT allele) with HCC1937-WTBRCA1 cells (reconstituted with WTBRCA1), for their ability to bind NF2. Consistent with our observations, endogenous NF2 binds BRCA1 in WT-BRCA1 reconstituted HCC1937 cells, but not Mt-BRCA1–expressing HCC1937 cells (SI Appendix, Fig. S2A). We next mapped the protein domain involved in BRCA1/NF2 interaction. The N-terminal half of NF2, which possesses FERM domain, is required for BRCA1 binding (Fig. 2E). Reciprocally, the C-terminal fragment of BRCA1, but not its N-terminal ring-finger domain or central intrinsically disordered region, is required for NF2 binding (Fig. 2F). These results suggested that BRCA1 could regulate Hippo signaling through direct NF2 interaction.
Fig. 2.
BRCA1 binds NF2 through FERM and BRCT domains. (A) HEK293T cells expressing HA-tag Hippo proteins and GST or GST-BRCA1 fusion proteins were subjected to immunoprecipitation as described in Materials and Methods. (B) Endogenous NF2 was detected in anti-BRCA1 beads, but not control, immunoprecipitates. (C) HA-NF2 directly binds GST-BRCA1, as revealed by immunoprecipitation upon in vitro binding experiment, performed as described in Materials and Methods. (D) HEK293T cells expressing various mutants of BRCA1 and HA-NF2 were subjected to immunoprecipitation using HA-binding beads. The resulting eluent was used to measure HA-NF2–bound BRCA1 by immunoblotting with anti-BRCA1 antibody. (E) HEK293T cells expressing either full-length NF2, N-terminus fragment (1–332) or C-terminus fragment (308–590), and GST-BRCA1 were subjected to immunoprecipitation using GST-binding beads. The resulting eluent was used to measure GST-BRCA1–bound NF2 by immunoblotting with anti-NF2 antibody. (F) HEK293T cells expressing the indicated BRCA1 Myc-tag fragments (1–303, 303–772, 772–1314, and 1314–1863) were tested for interaction with HA-NF2.
Serum-Responsive Expression of BRCA1 Contributes to YAP1 Stabilization Through Regulation of Hippo Signaling.
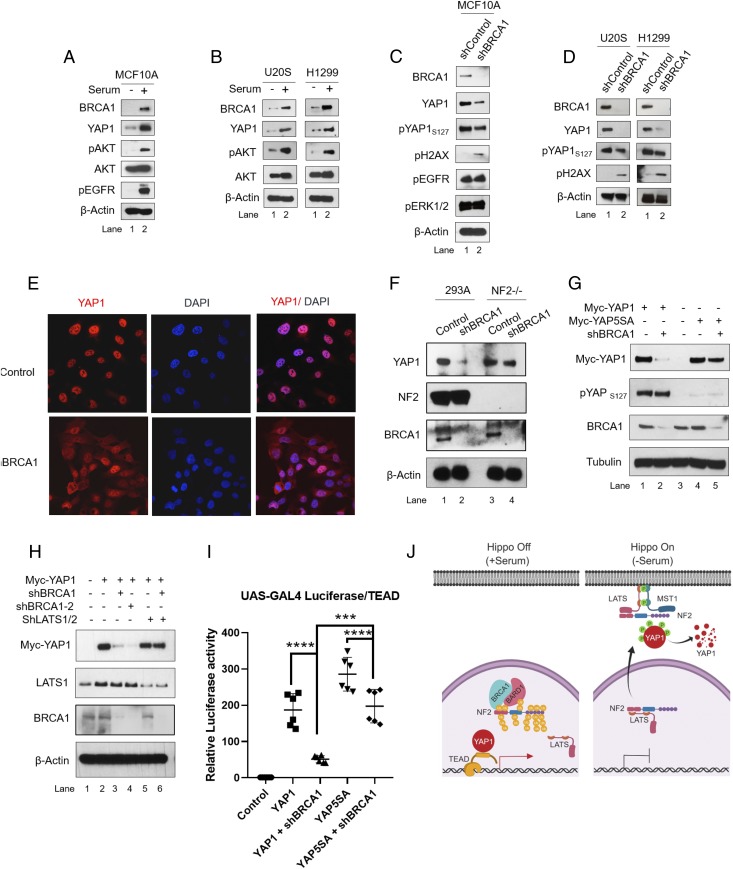
Because NF2-mediated Hippo signaling is involved in regulation of YAP1 stability, we further probed a possible relationship between BRCA1 expression and YAP1 protein. As shown, stimulation of various cell lines with serum led to significant increase in BRCA1 expression (Fig. 3 A and B; lane 2 compared with lane 1). As expected, protein levels of YAP1, pAKT, and pEGFR, which are growth signaling markers, were also elevated in these cells. In contrast, BRCA1 knockdown by shRNA suppressed YAP1 levels in multiple cell types (Fig. 3 C and D; lane 2 compared with lane 1). As anticipated, BRCA1 knockdown activated DNA damage signaling as judged by levels of ɣH2AX. All of the other serum-responsive markers tested using specific antibodies, e.g., pAKT, pEGFR, and pERK1/2, remained unchanged. We examined the cellular localization of YAP1, which is regulated upon phosphorylation through Hippo signaling. As expected, control cells with Hippo signaling suppressed, show strong nuclear localization of YAP1 protein (Fig. 3E). On the other hand, BRCA1 knockdown promoted cytoplasmic localization of YAP1 protein. In contrast, the YAP1 protein level remained unaffected in NF2-null HEK293A cells (Fig. 3F; lane 4 compared with lane 2), suggesting that NF2 downstream Hippo signaling is required for suppression of YAP1 levels in BRCA1-deficient cells. Suppression of YAP1 protein levels by Hippo signaling requires phosphorylation of YAP1 at multiple serine residues by LATS1/2 kinases. We therefore compared cellular levels of WT-YAP1 with Mt-YAP5SA protein that has mutations in all five LATS phosphorylation sites. As expected, expression of shBRCA1 strongly suppressed total levels of WT-YAP1 (Fig. 3G; lane 2 compared with lane 1). Also, the pYAP1 levels in WT-YAP1–expressing cell remained comparable to control cells, suggesting accumulation of phosphorylated WT-YAP1 in BRCA1 knockdown cells. However, BRCA1 knockdown had little or no effect on Mt-YAP5SA levels (Fig. 3G; lane 5 compared with lane 4). Correspondingly, shRNA-mediated inhibition of LATS1/2 kinases rescued WT-YAP1 protein degradation in BRCA1 knockdown cells (Fig. 3H; lane 6 compared with lane 3). YAP1 degradation was also rescued by treatment of cells with proteasome inhibitor MG132 or by coexpression of dominant negative mutant of ubiquitin (SI Appendix, Fig. S2 B and C). Furthermore, we tested rescue of YAP1 transactivation activity by Mt-YAP5SA using UAS-Luciferase/TEAD-Gal4/YAP1 reporter system. Expression of WT-YAP1 and Mt-YAP5SA protein promoted luciferase output from UAS-driven luciferase/Gal-4-TEAD reporter. As expected, BRCA1 knockdown suppressed reporter output in cells expressing WT-YAP1 (Fig. 3I). However, cells expressing Mt-YAP5SA exhibited significantly higher reporter activity compared with WT-YAP1–expressing cells. These results show that BRCA1 deficiency activates Hippo signaling and leads to subsequent proteosomal degradation of WT-YAP1 protein (Fig. 3J).
Fig. 3.
BRCA1 expression regulates YAP1 activity. (A and B) Cells were starved of serum for 12–16 h followed by stimulation with serum in complete medium for 24 h. (C and D) Cells were transduced with lentiviral vectors expressing shRNA against BRCA1 for 72 h, and immunoblot analysis was done. (E) Immunofluorescence analysis to check localization of YAP1 protein in BRCA1 knockdown U2OS cells 36-h postinfection. (F) HEK293A cells or NF2 knockout HEK293A cells (NF2−/−) were infected with lentiviral vectors expressing shRNA against BRCA1 for 72 h and harvested for immunoblotting. (G) HEK293T cells were cotransfected with plasmids encoding myc-tagged WT-YAP1 (Myc-YAP1) or Mt-YAP5SA (Myc-YAP5SA) and shRNA against BRCA1 followed by immunoblot analysis. Both WT- and Mt-YAP1s were detected by anti-Myc tag antibody. (H) HEK293T cells were cotransfected with WT-YAP1, and plasmids expressing two different shRNAs against BRCA1 (shBRCA1-1 and 2) and LATS1/2. (I) HEK293T cells were cotransfected with UAS-luciferase/TEAD-GAL4, renilla luciferase (normalization control), shBRCA1, and WT-YAP1 or Mt-YAP5SA followed by measurement of luciferase activity normalized to renilla luciferase reading. (J) A schematic model describing BRCA1-mediated NF2 ubiquitination, which promotes YAP1 stability in Hippo Off state via suppressing Hippo signaling. Results are expressed as means ± SEM. Only values of P < 0.05 were considered significant (****P < 0.0001), by ANOVA test.
Mt-YAP5SA Expression in BRCA1-Deficient Mammary Epithelial Cells Confers EGF-Independent Proliferation and Tumorigenesis.
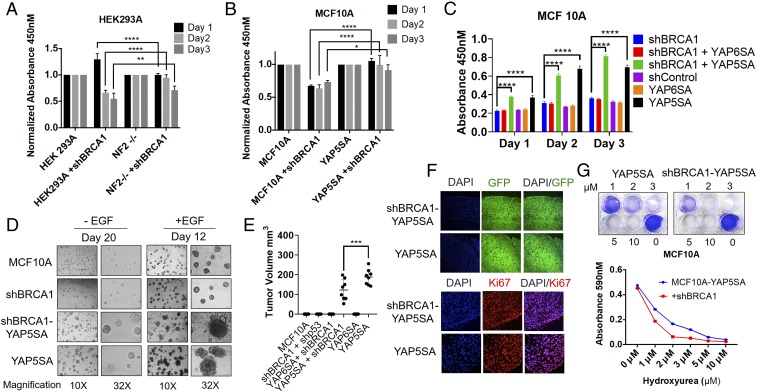
Having established that BRCA1 deficiency activates Hippo signaling, we next examined whether Hippo signaling contributes to growth inhibition observed in BRCA1-deficient cells. As expected, knockdown of BRCA1 expression suppressed cell growth in HEK293A cells (Fig. 4A). In comparison, NF2-null HEK293A cells exhibited partial but significant rescue of growth inhibition caused by BRCA1 knockdown. Similarly, serum stimulation of MCF10A cells expressing shRNA-targeting BRCA1 resulted in delayed growth (Fig. 4B) in comparison with control cells. In contrast, cells with ectopic expression of Mt-YAP5SA exhibited rescue of cell growth. These observations suggest that YAP1 reactivation could confer growth advantage for BRCA1-deficient cells. Previously, YAP1 activation was shown to promote growth of mammary epithelial cells in reduced growth factor conditions (44). We therefore evaluated whether Mt-YAP5SA can promote EGF-independent growth of BRCA1-deficient cells. As expected, MCF10A cells alone showed no growth in reduced serum and EGF-free media (Fig. 4C). Cells expressing null Mt-YAP6SA (with S94A mutation disrupting TEAD binding) behaved similarly with no growth advantage. However, we observed significantly higher cell growth in active Mt-YAP5SA–expressing cells. These results were further confirmed using 3D–culture-based assays in Matrigel where Mt-YAP5SA but not Mt-YAP6SA promoted EGF-independent growth as well as invasive structure formation in BRCA1-deficient mammary epithelial cells (Fig. 4D). We also observed loss of contact inhibition and increase in pAKT levels in Mt-YAP5SA–expressing MCF10A cells, which was significantly up-regulated even in reduced growth factor conditions (SI Appendix, Fig. S3 A and B; lanes 9 and 10). Furthermore, we examined if YAP5A expression is sufficient to initiate tumorigenesis in BRCA1-deficient MCF10A cells. MCF10A cells were injected into mammary fatpad of immunocompromised mice and monitored for visible tumor growth for 45 d. As anticipated, MCF10A cells which are nontumorigenic failed to generate tumors during these experimental periods (Fig. 4E). Similar observation was made with Mt-YAP6SA–expressing MCF10A cells, which also failed to originate tumors. It is important to note that p53 knockdown using shRNA was also not sufficient to directly initiate tumorigenesis in BRCA1-deficient MCF10A cells under the observation period. In contrast, BRCA1-deficient cells expressing Mt-YAP5SA were able to produce tumors in all of the injected mice (Fig. 4E and SI Appendix, Fig. S3C). We concluded that YAP activation but not p53 inhibition alone can directly transform BRCA1-deficient mammary epithelial cells.
Fig. 4.
Mt-YAP5SA expression in BRCA1-deficient mammary epithelial cells confers EGF-independent proliferation and tumorigenesis. (A–C) HEK293A or MCF10A cells were infected with lentivirus encoding shBRCA1 and indicated viruses. Then after 48 h, cells were seeded in 96-well plates, and treated with wst1 reagent to compare cell growth as described in Materials and Methods. (D) MCF10A cells were infected as described in A and then grown on matrigel in presence or absence of EGF. Fresh medium lacking EGF was added every 4 d. (E and F) Representative immunohistochemistry pictures and relative tumor volume. (G) MCF10A cells were treated with hydroxyurea as described in Materials and Methods, washed, and then incubated in six-well plates. Then after 10–12 d, cells were fixed, stained with crystal violet, and cell survival was determined by measuring absorbance at 590 nM. Results are expressed as means ± SEMs. Only values of P < 0.05 were considered significant (****P < 0.0001), by ANOVA test.
The BRCA1-deficient tumors also showed a significant reduction of tumor volume, compared with control BRCA1-proficient tumors (Fig. 3E). We examined the cellular levels of 53BP1, Mt-YAP5SA, and BRCA1 in tumor lysates (SI Appendix, Fig. S3D). All of the samples showed comparable amount of Mt-YAP5SA, 53BP1 proteins. Intracellular expression of BRCA1 was monitored and as expected, tumor-cells–expressing shBRCA1 had efficient knockdown of BRCA1 protein. GFP protein expressed from lentivirus (Fig. 4F) and β-Actin levels were monitored as internal control, which were unchanged. Also, analysis of tumor samples, revealed expression of Vimentin, basal markers Cytokeratin-5, and lack of E-cadherin expression in all of the tumors (SI Appendix, Fig. S3E). Moreover, cell proliferation was analyzed by Ki-67 staining (Fig. 4F) and we observed no significant difference in proliferation index between the two groups of tumors. We then wanted to determine whether higher sensitivity to replication stress could be involved in this phenomenon. As shown, BRCA1-deficient Mt-YAP5SA–expressing cells were highly sensitive to treatment with replication stress agent hydroxyurea (Fig. 4G). These observations highlight important implications of YAP1 reactivation in BRCA1-deficient cells.
Discussion
The cellular levels of oncogene YAP1 are regulated by phosphorylation through Hippo signaling. Growth factors inhibit Hippo signaling and promote YAP1 stabilization. Although the interaction of NF2 with LATS1 and their recruitment to plasma membrane initiate Hippo-signaling activation, it remains unclear what mechanisms dictate assembly of these signaling complexes. The findings here establish an important role of ubiquitination signaling during Hippo pathway regulation. Ubiquitination profiling of core Hippo members revealed that NF2 is heavily ubiquitinated in proliferating cells (Fig. 1A). We found that ubiquitination of NF2 involves multiple lysine residues located in both FERM and coiled-coil domain. Interestingly, point mutations in FERM domain have been identified in human patients and shown to inactivate NF2 protein by inhibiting LATS binding and its recruitment to plasma membrane (35). We speculated that similar molecular modification could be involved in reversible interconversion of NF2 protein to inactive state via ubiquitination switch. Indeed, ubiquitinated NF2 exhibits poor LATS1 binding and resides predominately in nucleus. NF2 ubiquitination was found to be greatly enhanced in cells upon serum stimulation. However, changes in NF2 ubiquitination profile do not alter total NF2 protein levels, suggesting a mechanism of regulation that does not involve degradation. Furthermore, NF2 was found to be ubiquitinated by ubiquitin mutants with only K-6, K-27, K-29, and K-63 available. It is to be noted that expression of BRCA1 ubiquitin ligase is also serum responsive and known to generate ubiquitin chains with K-6, K-29, K-48, and K-63 (17–19, 24). We have shown that BRCA1/BARD1 ubiquitin ligase complex contributes to NF2 ubiquitination (Fig. 1). Furthermore, BRCA1/BARD1 knockdown using shRNA was found to inhibit endogenous NF2 ubiquitination. We also tested whether BRCA1 overexpression promotes NF2 ubiquitination by coexpressing NF2 and BRCA1 protein. Expression of only WT but not ring-finger-domain mutant of BRCA1 was found to enhance NF2 ubiquitination, which was also reversed by BARD1 knockdown. Reduced NF2 ubiquitination observed with BRCT-domain mutant M1755R could be attributed to its ability to compete with endogenous BRCA1 and heterodimerize BARD1 and subsequently inhibit NF2 binding by complex. Among core Hippo-signaling components tested, NF2 was found to interact with BRCA1. Furthermore, interaction between the two proteins was found to be direct in in vitro binding assays, which further supports the hypothesis that NF2 activity is regulated by BRCA1 interaction.
Inherited mutations or reduced BRCA1 activity are known to increase the risk of human breast and ovarian cancers. Previously, we demonstrated aberrant expression of satellite RNAs in BRCA1-deficient tumors, which itself alone can lead to tumorigenesis by promoting genomic instability (13, 14). In agreement with mutational theory, genomic instability can foster tumorigenesis by selecting cells with growth advantage, oncogene activation, or tumor suppressor inactivation. As a protective mechanism, normal cells have multiple checkpoints involving ATM/ATR, CHK1/2, p53, p21, Bax, and 53BP1 activation to restrict growth in the absence of BRCA1 (45, 46). Evolution of signaling adaptation(s) that allow BRCA1-deficient cells to override these and similar protective checkpoints may thus become rate-limiting steps during the process of cell transformation. In support of this notion, development of mammary tumors is accelerated by introduction of p53 null allele in conditional mouse model with mammary-specific deletion of BRCA1. These tumors also exhibited numerous other somatic alterations because of genomic instability (46, 47). Not all BRCA1-deficient human tumors however have p53 mutations, suggesting existence of alternative checkpoint(s). Previous genetic screen using tissue-specific BRCA1 deletion in a p53 heterozygous mouse model identified amplification of YAP1 encoding locus in resulting tumors (44). A recent report also identified YAP1/TAZ activation as a frequent event in human breast cancer, which also correlates with higher histological grade of tumors (ref. 48 and Fig. 1; analysis on 993 primary tumor samples). Additionally, TCGA data indicated that mutation and shallow deletions of BRCA1 are also highly frequent (mutations: 11.71%, 37 cases and shallow deletion: 66.46%, 210 cases; of 316 patients, respectively) in patients with ovarian serous cystadenocarcinoma, supporting its tumor suppressor function. Furthermore, TCGA data analysis revealed coexistence of multiple alterations in Hippo pathway members (shallow deletion or deletion: NF2, LATS1, and LATS2; gain of copy or amplification: YAP1 and TAZ) in these patients (SI Appendix, Fig. S3F). Former analysis performed from 33 cancer types (33) also highlighted multiple cooccurring alteration in Hippo pathway components which may contribute to YAP1/TAZ activation in human tumors.
Both BRCA1 expression and YAP1 stabilization are known to be sensitive to serum stimulation (24, 29). We found that BRCA1 expression is essential for YAP1 stabilization in multiple cell lines with intact Hippo signaling. Furthermore, cytoplasmic localization of YAP1 protein in BRCA1 knockdown cells suggested activation of Hippo signaling, which was then found to degrade YAP1 through proteosomal degradation. The transcriptional program control by YAP1/TEAD1–4 is involved in leading cell-cycle progression. Critical among the known transcriptional target are protein involved in replication licensing, DNA synthesis and damage repair (CDC6, GINS1, MCM3, MCM7, POLA2, POLE3, TOP2A, and RAD18), transcriptional regulators (ETS1, MYC, and MYBL1), cyclins and their activators (CCNA2 and CDC25A), as well as protein involved in mitosis (CENPF, CDCA5, and KIF23) (49). These observations are in agreement with the known role of BRCA1 in maintaining growth and genomic stability. The specific degradation of WT-YAP1 but not phosphorylation defective Mt-YAP5SA mutant upon BRCA1 knockdown clearly established the inhibitory role of BRCA1 in Hippo signaling. We also investigated the physiological consequences of YAP1 degradation in BRCA1-deficient cells. We compared cell growth in cells constituted with Mt-YAP5SA or with NF2 deletion. Compared with WT cells, cells with NF2 deletion or Mt-YAP5SA expression were found to be less sensitive to BRCA1 knockdown with respect to growth inhibition. Since YAP1-mediated cell proliferation also involves AKT activation (44), we also tested AKT phosphorylation in active Mt-YAP5SA or inactive Mt-YAP6SA–expressing MCF10A cells. We found that AKT phosphorylation was significantly up-regulated in MCF10A cells expressing Mt-YAP5SA. The pAKT levels remained up-regulated even in reduced growth-factor conditions, which also correlated with EGF-independent cell growth in both 2D and 3D culture assay. Furthermore, we demonstrated the role of YAP1 activation in promoting tumorigenesis in BRCA1-deficient MCF10A cells. It is important to note that we observe smaller tumors formed in BRCA1-deficient cells and, on treatment with hydroxyurea, the same cells exhibited higher sensitivity toward replication stress compared with BRCA1-proficient cells. BRCA1-deficient cells are known to be highly sensitive to replication stress (50), which is experienced among other stresses by cells in growing tumors. Thus, we concluded that tumor size might correlate with replication stress in these tumors. Higher sensitivity of Mt-YAP5SA–transformed BRCA1-deficient cells to replication stress agent presented here also confirms that these cells are still sensitive to genotoxic agents and combination with YAP1 inhibitors may enhance the therapeutic outcome.
Taken together, here we describe a homeostatic mechanism where expression of chromosomal custodian protein, BRCA1, inhibits Hippo signaling via NF2 ubiquitination to stabilize YAP1. These results identified a ubiquitination switch involving BRCA1-NF2/Hippo-YAP signaling axis, which possibly engages cell growth and genomic stability in normal cells.
Materials and Methods
All protocols involving animal experiments were approved by the Institutional Animal Care and Use, Committee of the Salk institute for Biological studies. HEK293T, HEK293A (human embryonic kidney cell), U2OS (human osteosarcoma cell), and H-1299 (human non–small-cell lung carcinoma cell) were maintained in DMEM (Gibco, Invitrogen) supplemented with glutamine, 10% FCS, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen) at 37 °C with 5% CO2. MCF10A cells (human mammary epithelial cell) were cultured in DMEM/F12 (Invitrogen) supplemented with 5% horse serum (Invitrogen), 20 ng/mL EGF, 0.5 μg/mL hydrocortisone, 10 μg/mL insulin, 100 ng/mL cholera toxin, and 100 μg/mL streptomycin (Invitrogen) at 37 °C with 5% CO2. Generation of HEK293A cells knockout for NF2 gene has been previously described (34). The constructs expressing full length BRCA1 and its deletion fragments were described previously (51). Details of materials and methods including antibodies, plasmids, ubiquitination (52), and all cell-based assays are described in SI Appendix, Material and Methods.
Supplementary Material
Acknowledgments
We thank Duojia Pan, Department of Molecular Biology and Genetics, Johns Hopkins University School of Medicine, for pGal4-TEAD4 and UAS-Luc construct. We also thank Mark Schmitt, Beth Coyne, Mie Soda, and I.M.V. Laboratory members for their help. S.V. was supported by the “The George E Hewitt Foundation for Medical Research” Newport Beach, CA, USA (Salk Institute, La Jolla, CA). This work was supported by the Mass Spectrometry Core of the Salk Institute with funding from NIH-NCI CCSG: P30 014195 and the Helmsley Center for Genomic Medicine. K.-L.G. is supported by grants from NIH (CA196878 and GM51586).
Footnotes
Conflict of interest statement: K.-L.G. is a cofounder and has equity interest in Vivace Therapeutics, Inc. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1822155116/-/DCSupplemental.
References
- 1.Miki Y, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 2.King MC, Marks JH, Mandell JB. New York Breast Cancer Study Group Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 3.Hakem R, et al. The tumor suppressor gene Brca1 is required for embryonic cellular proliferation in the mouse. Cell. 1996;85:1009–1023. doi: 10.1016/s0092-8674(00)81302-1. [DOI] [PubMed] [Google Scholar]
- 4.Chandler J, Hohenstein P, Swing DA, Tessarollo L, Sharan SK. Human BRCA1 gene rescues the embryonic lethality of Brca1 mutant mice. Genesis. 2001;29:72–77. doi: 10.1002/1526-968x(200102)29:2<72::aid-gene1007>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 5.Szabo CI, et al. Human, canine and murine BRCA1 genes: Sequence comparison among species. Hum Mol Genet. 1996;5:1289–1298. doi: 10.1093/hmg/5.9.1289. [DOI] [PubMed] [Google Scholar]
- 6.Chapman MS, Verma IM. Transcriptional activation by BRCA1. Nature. 1996;382:678–679. doi: 10.1038/382678a0. [DOI] [PubMed] [Google Scholar]
- 7.Scully R, et al. BRCA1 is a component of the RNA polymerase II holoenzyme. Proc Natl Acad Sci USA. 1997;94:5605–5610. doi: 10.1073/pnas.94.11.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willis NA, et al. BRCA1 controls homologous recombination at Tus/Ter-stalled mammalian replication forks. Nature. 2014;510:556–559. doi: 10.1038/nature13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunting SF, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sankaran S, Starita LM, Groen AC, Ko MJ, Parvin JD. Centrosomal microtubule nucleation activity is inhibited by BRCA1-dependent ubiquitination. Mol Cell Biol. 2005;25:8656–8668. doi: 10.1128/MCB.25.19.8656-8668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joukov V, et al. The BRCA1/BARD1 heterodimer modulates ran-dependent mitotic spindle assembly. Cell. 2006;127:539–552. doi: 10.1016/j.cell.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 12.Xia Y, Pao GM, Chen HW, Verma IM, Hunter T. Enhancement of BRCA1 E3 ubiquitin ligase activity through direct interaction with the BARD1 protein. J Biol Chem. 2003;278:5255–5263. doi: 10.1074/jbc.M204591200. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Q, et al. BRCA1 tumour suppression occurs via heterochromatin-mediated silencing. Nature. 2011;477:179–184. doi: 10.1038/nature10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Q, et al. Heterochromatin-encoded satellite RNAs induce breast cancer. Mol Cell. 2018;70:842–853. doi: 10.1016/j.molcel.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkitaraman AR. Cancer suppression by the chromosome custodians, BRCA1 and BRCA2. Science. 2014;343:1470–1475. doi: 10.1126/science.1252230. [DOI] [PubMed] [Google Scholar]
- 16.Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 17.Wu W, Koike A, Takeshita T, Ohta T. The ubiquitin E3 ligase activity of BRCA1 and its biological functions. Cell Div. 2008;3:1. doi: 10.1186/1747-1028-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen DE, Brzovic PS, Klevit RE. E2-BRCA1 RING interactions dictate synthesis of mono- or specific polyubiquitin chain linkages. Nat Struct Mol Biol. 2007;14:941–948. doi: 10.1038/nsmb1295. [DOI] [PubMed] [Google Scholar]
- 19.Nishikawa H, et al. Mass spectrometric and mutational analyses reveal Lys-6-linked polyubiquitin chains catalyzed by BRCA1-BARD1 ubiquitin ligase. J Biol Chem. 2004;279:3916–3924. doi: 10.1074/jbc.M308540200. [DOI] [PubMed] [Google Scholar]
- 20.Starita LM, et al. BRCA1-dependent ubiquitination of gamma-tubulin regulates centrosome number. Mol Cell Biol. 2004;24:8457–8466. doi: 10.1128/MCB.24.19.8457-8466.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakar A, Parvin J, Zlatanova J. BRCA1/BARD1 E3 ubiquitin ligase can modify histones H2A and H2B in the nucleosome particle. J Biomol Struct Dyn. 2010;27:399–406. doi: 10.1080/07391102.2010.10507326. [DOI] [PubMed] [Google Scholar]
- 22.Eakin CM, Maccoss MJ, Finney GL, Klevit RE. Estrogen receptor alpha is a putative substrate for the BRCA1 ubiquitin ligase. Proc Natl Acad Sci USA. 2007;104:5794–5799. doi: 10.1073/pnas.0610887104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starita LM, et al. BRCA1/BARD1 ubiquitinate phosphorylated RNA polymerase II. J Biol Chem. 2005;280:24498–24505. doi: 10.1074/jbc.M414020200. [DOI] [PubMed] [Google Scholar]
- 24.Rajan JV, Wang M, Marquis ST, Chodosh LA. Brca2 is coordinately regulated with Brca1 during proliferation and differentiation in mammary epithelial cells. Proc Natl Acad Sci USA. 1996;93:13078–13083. doi: 10.1073/pnas.93.23.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruffner H, Verma IM. BRCA1 is a cell cycle-regulated nuclear phosphoprotein. Proc Natl Acad Sci USA. 1997;94:7138–7143. doi: 10.1073/pnas.94.14.7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruffner H, Jiang W, Craig AG, Hunter T, Verma IM. BRCA1 is phosphorylated at serine 1497 in vivo at a cyclin-dependent kinase 2 phosphorylation site. Mol Cell Biol. 1999;19:4843–4854. doi: 10.1128/mcb.19.7.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scully R, et al. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell. 1997;90:425–435. doi: 10.1016/s0092-8674(00)80503-6. [DOI] [PubMed] [Google Scholar]
- 28.Choudhury AD, Xu H, Baer R. Ubiquitination and proteasomal degradation of the BRCA1 tumor suppressor is regulated during cell cycle progression. J Biol Chem. 2004;279:33909–33918. doi: 10.1074/jbc.M403646200. [DOI] [PubMed] [Google Scholar]
- 29.Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plouffe SW, Hong AW, Guan KL. Disease implications of the Hippo/YAP pathway. Trends Mol Med. 2015;21:212–222. doi: 10.1016/j.molmed.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez-Vega F, et al. Cancer Genome Atlas Research Network Oncogenic signaling pathways in The Cancer Genome Atlas. Cell. 2018;173:321–337.e10. doi: 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plouffe SW, et al. Characterization of Hippo pathway components by gene inactivation. Mol Cell. 2016;64:993–1008. doi: 10.1016/j.molcel.2016.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin F, et al. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sher I, Hanemann CO, Karplus PA, Bretscher A. The tumor suppressor merlin controls growth in its open state, and phosphorylation converts it to a less-active more-closed state. Dev Cell. 2012;22:703–705. doi: 10.1016/j.devcel.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma B, et al. Hypoxia regulates Hippo signalling through the SIAH2 ubiquitin E3 ligase. Nat Cell Biol. 2015;17:95–103. doi: 10.1038/ncb3073. [DOI] [PubMed] [Google Scholar]
- 38.Li W, et al. Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DCAF1)-mediated inhibition of the hippo pathway kinases Lats1 and 2 in the nucleus. Cancer Cell. 2014;26:48–60. doi: 10.1016/j.ccr.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho KC, et al. Itch E3 ubiquitin ligase regulates large tumor suppressor 1 stability. Proc Natl Acad Sci USA. 2011;108:4870–4875, and erratum (2016) 113:E5776. doi: 10.1073/pnas.1101273108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim Y, et al. Deubiquitinase YOD1 potentiates YAP/TAZ activities through enhancing ITCH stability. Proc Natl Acad Sci USA. 2017;114:4691–4696. doi: 10.1073/pnas.1620306114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim Y, Jho EH. Regulation of the Hippo signaling pathway by ubiquitin modification. BMB Rep. 2018;51:143–150. doi: 10.5483/BMBRep.2018.51.3.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li W, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010;140:477–490. doi: 10.1016/j.cell.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruffner H, Joazeiro CA, Hemmati D, Hunter T, Verma IM. Cancer-predisposing mutations within the RING domain of BRCA1: Loss of ubiquitin protein ligase activity and protection from radiation hypersensitivity. Proc Natl Acad Sci USA. 2001;98:5134–5139. doi: 10.1073/pnas.081068398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Overholtzer M, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng CX. BRCA1: Cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Res. 2006;34:1416–1426. doi: 10.1093/nar/gkl010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dine J, Deng CX. Mouse models of BRCA1 and their application to breast cancer research. Cancer Metastasis Rev. 2013;32:25–37. doi: 10.1007/s10555-012-9403-7. [DOI] [PubMed] [Google Scholar]
- 47.Brodie SG, et al. Multiple genetic changes are associated with mammary tumorigenesis in Brca1 conditional knockout mice. Oncogene. 2001;20:7514–7523. doi: 10.1038/sj.onc.1204929. [DOI] [PubMed] [Google Scholar]
- 48.Cordenonsi M, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 49.Zanconato F, et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17:1218–1227. doi: 10.1038/ncb3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Densham RM, et al. Human BRCA1-BARD1 ubiquitin ligase activity counteracts chromatin barriers to DNA resection. Nat Struct Mol Biol. 2016;23:647–655. doi: 10.1038/nsmb.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pao GM, Janknecht R, Ruffner H, Hunter T, Verma IM. CBP/p300 interact with and function as transcriptional coactivators of BRCA1. Proc Natl Acad Sci USA. 2000;97:1020–1025. doi: 10.1073/pnas.97.3.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verma S, Ali A, Arora S, Banerjea AC. Inhibition of β-TrcP-dependent ubiquitination of p53 by HIV-1 Vpu promotes p53-mediated apoptosis in human T cells. Blood. 2011;117:6600–6607. doi: 10.1182/blood-2011-01-333427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.