Abstract
Background:
Persistent deficits in arm function are common after stroke. An improved understanding of the factors that contribute to the performance of skilled arm movements is needed. One such factor may be self-efficacy.
Objective:
To determine the level of self-efficacy for skilled, goal-directed reach actions in individuals with mild motor impairment after stroke and whether self-efficacy for reach performance correlated with actual reach performance.
Methods:
Twenty individuals with chronic stroke (months post-stroke, mean 58.1 ± 38.8) and mild motor impairment (upper extremity Fugl-Meyer motor score, mean 53.2, range 39 to 66) and six age-matched controls reached to targets presented in two directions (ipsilateral, contralateral). Prior to each block (24 reach trials), individuals rated their confidence on reaching to targets accurately and quickly on a scale that ranged from 0 (‘not very confident’) to 10 (‘very confident’).
Results:
Overall reach performance was slower and less accurate in the more affected arm compared to both the less affected arm and controls. Self-efficacy for both reach speed and reach accuracy was lower for the more affected arm compared with the less affected arm. For reaches with the more affected arm, self-efficacy for reach speed and age significantly predicted movement time to ipsilateral targets (R2=0.352) while self-efficacy for reach accuracy and Fugl-Meyer motor score significantly predicted endpoint error to contralateral targets (R2=0.291).
Conclusions:
Self-efficacy relates to measures of reach control and may serve as a target for interventions to improve proximal arm control after stroke.
Keywords: stroke, self-efficacy, confidence, arm, kinematics
Introduction
Persistent deficits in arm function are common after stroke. While rehabilitation interventions can improve arm function, recovery is often incomplete with large variation in response to training across individuals.1,2 Additionally, improvements in functional capacity do not always translate into increased arm use in everyday life.3,4 Further understanding the factors that contribute to the performance of skilled arm movements after stroke may help inform the development of novel approaches to arm rehabilitation.
Skilled reach performance, as measured by kinematic measures of speed and accuracy, can characterize sensorimotor recovery after stroke5, providing important information about proximal arm control6,7, especially in individuals with milder motor deficits 8,9. The ability to effectively and efficiently transport the arm is important for a variety of functional tasks, including placement of the hand to allow for object manipulation. An understanding of the variables that contribute to the control of skilled reaching may inform the content and design of interventions aimed at improving proximal arm control after stroke. One important variable may be self-efficacy.
Self-efficacy is one of several personal factors that are proposed to combine to determine an individual’s motivation, affect and performance related to a given behavior.10 Specifically, self-efficacy refers to an individual’s confidence or perception of the prospective capability to perform a behavior at a specific level or in a given situation.10,11 This perception goes beyond holding a belief that engaging in a behavior will lead to a specific outcome (outcome expectations). Self-efficacy relates to whether the individual believes she can actually perform the required behavior in order to achieve the desired outcome.12 Self-efficacy is not a general or trait variable but is specific to a domain of activities or behaviors and is predictive of future behavior; higher confidence can both reflect and lead to higher levels of accomplishment and behavioral engagement.10,12,13
After stroke, self-efficacy has been shown to be a significant predictor of falls14,15, physical function16,17, gait function18, and walking activity19,20. Overall, individuals with higher self-efficacy have fewer falls, better physical and gait function, and increased levels of walking activity than those with lower self-efficacy. Self-efficacy for skilled upper extremity tasks after stroke has not been extensively studied to date. Recently, self-efficacy and feedback indicating successful performance has been shown to relate to arm choice in a reaching task in individuals post-stroke.21,22 However, it is currently not known if self-efficacy for skilled reaching predicts reach performance.
The purpose of this study was to determine the level of self-efficacy for skilled, goal-directed reach actions in individuals with mild motor impairment after stroke and whether self-efficacy for reach performance correlates with actual reach performance. We hypothesized that self-efficacy would be lower for reaches with the more affected arm compared to the less affected arm and nondisabled controls. Additionally, we hypothesized that self-efficacy would significantly correlate with reach performance such that individuals with lower self-efficacy would reach more slowly and with less accuracy than individuals with higher confidence.
Methods
Participants
Individuals in this study were part of a parent study that investigated the control of reach extent after stroke.23,24 Participants had to be pre-morbidly right-hand dominant25, have a history of stroke at least 3 months prior, and have some movement capability in the more affected arm (upper extremity Fugl-Meyer (UE FM) motor score26 ≥ 28). Potential participants were excluded if they presented with cognitive impairment (score <25 on the Mini-Mental State Exam27), hemispatial neglect (score <52 on the BIT star cancellation test28), current pain in either arm, botulinum toxin injection in the more affected arm within the previous three months, or surgical intervention in either arm within the previous six months. All participants provided written informed consent prior to participation through a protocol approved by the Health Sciences Institutional Review Board at the University of Southern California.
Several clinical measures were performed to determine arm motor status. The UE FM motor score26 and Action Research Arm Test (ARAT)29 were used to determine level of arm motor impairment and arm motor function, respectively. The Stroke Impact Scale (SIS) Hand domain30, a patient-reported outcome measure, was used to determine perceived hand function. Self-efficacy for UE functional activities was assessed using the Confidence in Arm and Fland Movements Questionnaire (CAHM). The CAHM is a 20-item questionnaire that asks the individual to rate his or her level of confidence to perform a series of functional tasks that involve the weaker arm or both arms on a scale of 0 to 100; a rating of 0 indicates ‘very uncertain/unconfident’ about being able to successfully perform a task while a rating of 100 indicates ‘very certain/confident’.
Reaching Task
The reaching task has been described in detail previously.24 Briefly, individuals performed three-dimensional reach movements to six targets (3.8 cm sphere) presented in two directions (+45°, −45°) and three distances (8, 16, 24 cm) (Fig. 1) in an immersive virtual environment (VE) (Innovative Sports Training, Inc., Chicago, IL). Finger position was represented in the VE as a 2 cm white sphere, or cursor, that moved in real-time as the participant moved the finger. At the start of each trial, the participant placed the cursor onto a Home position (2.5 cm blue sphere) that aligned with a physical start switch. After a variable foreperiod (1.3, 1.6, 1.9 s), the Home position and the cursor position disappeared and a single target appeared, at which time the participant reached to the target; the trial ended when velocity dropped below 3 cm/sec. The target was visible while reaching but the arm and virtual cursor that represented finger position were not, thereby eliminating on-line visual feedback during movement. Visual post-response feedback was provided after each trial which showed the proximity of end point finger position to the target. If the cursor overlapped with the target (error tolerance of 2.9 cm based on object radius), the target turned green on feedback, indicating successful hit of the target on that trial. If the cursor did not overlap with the target, the target remained red during feedback. Participants were instructed to “Reach to the target as fast as possible when ready”; instructions prioritized speed of movement over accuracy as one goal of this study was to investigate anticipatory planning of ballistic, goal-directed reaches to targets that varied in distance.23,24 However, the task did not impose any external time constraints on the speed of reach performance for an individual trial.
Figure 1.
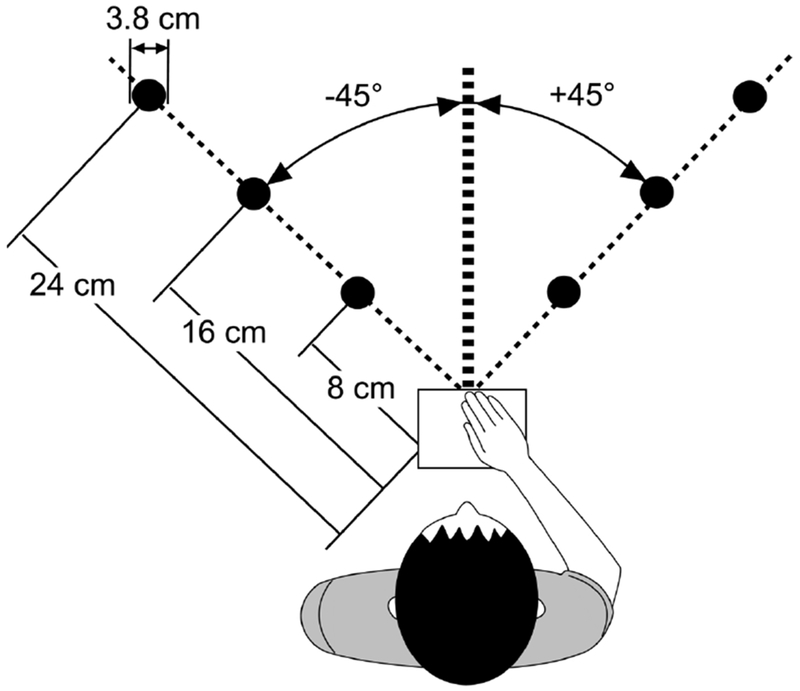
Schematic of reach paradigm. Six targets were presented in 2 directions (+45°,−45°) and 3 distances (8, 16, 24 cm) in an immersive virtual environment. The start switch (open square) was aligned with the sternum.
Index finger position was collected from an electromagnetic sensor at a sampling rate of 120 Hz throughout each reach trial and analyzed using a custom script in MATLAB (Mathworks, Inc., Natick, MA). Position data were filtered with a low-pass 2nd order Butterworth filter with a 10 Hz cut-off and differentiated to determine velocity and acceleration.31 For each trial, peak velocity (first peak after movement onset), movement time, and endpoint error (three-dimensional linear distance between target and finger position at movement offset) were extracted using previously described methods.24 Due to the well-described effect of movement direction on the magnitude of kinematic variables32,33, data for each target direction were analyzed separately. Target direction was converted to indicate either an ipsilateral reach (right arm reaching to +45° targets, left arm reaching to −45° targets) or contralateral reach (right arm reaching to −45°, left arm reaching to +45° targets) for group analyses (see Fig 1). The effect of target distance on measures of reach performance has been previously reported in detail.24 In the current analysis, we utilized the mean of each kinematic variable across all target distances to quantify overall reach performance similar to previous analyses.23
Reach Self-Efficacy
Self-efficacy (SE) for reach accuracy and reach speed were collected prior to each block of reach trials. Before the start of data collection, participants were shown a visual analog scale for rating SE that ranged from 0 (‘not very confident’) to 10 (‘very confident’) for two questions: “How confident are you that you will reach to the target accurately on the next set of trials?” and “How confident are you that you will reach to the target quickly on the next set of trials?”. ‘Accurately’ was defined as receiving green on feedback (an indication of being on target); ‘Quickly’ was defined as reaching to the target within the allotted trial time. Participants rated accuracy SE and speed SE verbally prior to each of the seven blocks of reach trials.
Experimental Procedure
Participants completed the reaching task with the ipsilesional arm first followed by the contralesional arm with a 30 to 60 minute break between arm sessions. For ease of discussion and presentation of results, we will refer to these as the less affected and more affected arms, respectively. The order of arm used first in control participants was counterbalanced. Each session began with 24 practice trials. Participants then completed 7 blocks of 24 trials (total of 168 reach trials) with each arm; the first 2 blocks were dropped from data analysis to eliminate any effects related to adjusting to the virtual environment similar to previous analyses23,24 leaving 120 reach trials (5 blocks) for analyses. Within each block, targets were presented in a pseudorandom order such that no consecutive trials were to the same target and each target was presented four times. A rest period of 2 to 5 minutes was provided between blocks.
Verbal cues related to accuracy and speed were provided throughout data collection. Cues were general in nature and primarily aimed at keeping the participant engaged in the task for the primary purpose of the experiment to investigate the planning of reach actions. Examples of accuracy verbal cues provided include ‘Good job’ and ‘Close’. Examples of speed verbal cues include ‘Keep moving quickly’ and ‘Good speed’.
Statistical Analysis
All data or their log transform met the criteria for normality using the Shapiro-Wilk test (p>0.05). Measures of reach performance (endpoint error, movement time, peak velocity) and self-efficacy (accuracy SE, speed SE) did not change over blocks in either group (p>0.185 for main effect of block; see Supplemental Figures). Therefore, for group analyses, we used a mean value for reach performance and SE across blocks for each participant. A mixed model analysis of variance with within-group factors of arm and SE type (accuracy, speed) and a between-group factor of group (control, stroke) was used to examine differences in SE. Significance level was set at p<0.05 for all comparisons.
Correlation analyses between SE and kinematic measures of reach performance were performed using Pearson’s r. Due to the proposed specificity of SE, accuracy SE was correlated with a measure of reach accuracy (endpoint error) and speed SE with measures of reach speed (peak velocity, movement time). Additionally, the relationship between reach SE and clinical measures of arm function (UEFM, ARAT, SIS hand, CAHM) was investigated. In the control group, both arms were combined in a single comparison. Significance level for correlations was set at a corrected p<0.0167.
Forward step-wise linear regression modeling was conducted to examine what variables combined to best predict reach performance with the more affected arm. Possible predictors included SE (accuracy, speed), arm motor status (UEFM, ARAT, SIS hand, CAHM), and demographic variables (age, time post-stroke). Variables that showed a bivariate correlation with reach performance (p<0.1) were entered into the model (p<0.1 to enter, p>0.15 to leave). SPSS 22 (IBM Corp., Armonk, NY) statistical software was used for all analyses.
Results
Participants
Twenty individuals with chronic stroke and six age-matched nondisabled, controls participated in this study (Table 1). Individuals in the stroke group presented with a mix of right and left arm hemiparesis and, overall, had mild motor impairment (UE FM motor score range 39-66).34 Participants post-stroke reported continued difficulty performing functional tasks with the more affected hand (Hand SIS) and reduced confidence in completing functional tasks with the more affected arm (CAHM). As expected, reaches with the more affected arm were slower and less accurate then reaches with both the less affected and control arms (Table 2). Additionally, reaches to contralateral targets were slower than reaches to ipsilateral targets with both arms (p<0.001) while reaches to contralateral targets were less accurate than reaches to ipsilateral targets for the more affected arm only (p=0.02).
Table 1.
Participant Demographics
| Stroke | Control | |
|---|---|---|
| Age | 62.5 ± 12.2 | 63.8 ± 14.4 |
| Gender | 11M/9F | 2M/4F |
| Time post-stroke (months) | 58.1 ± 38.8 | |
| More affected side | 8R/12L | |
| UEFM Motor (max 66) | 53.2 ± 7.6 | |
| ARAT (max 57) | 46.2 ± 9.2 | |
| SIS Hand (max 100) | 65.8 ± 22.1 | |
| CAHM (max 100) | 65.7 ± 24.0 |
Values represent group mean ± standard deviation. M=Male; F=Female; R=Right; L=Left; UEFM=Upper Extremity Fugl-Meyer; ARAT=Action Research Arm Test; SIS=Stroke Impact Scale; CAHM=Confidence in Arm and Hand Movements.
Table 2.
Summary of Reach Performance
| Peak Velocity (cm/sec) | Movement Time (sec) | Error (cm) | |
|---|---|---|---|
| Ipsilateral Targets | |||
| Control | 110.3 ± 11.8 | 0.361 ± 0.034 | 3.6 ± 0.3 |
| Less Affected | 87.3 ± 7.1 | 0.458 ± 0.038 | 3.8 ± 0.3 |
| More Affected | 58.2 ± 5.8*+ | 0.570 ± 0.038*+ | 4.6 ± 0.4*+ |
| Contralateral Targets | |||
| Control | 75.7 ± 6.4 | 0.463 ± 0.032 | 4.2 ± 0.4 |
| Less Affected | 62.0 ± 5.4 | 0.565 ± 0.041 | 3.9 ± 0.2 |
| More Affected | 46.2 ± 4.5*+ | 0.710 ± 0.038*+ | 5.2 ± 0.3*+ |
Values represent group mean ± standard error.
p<0.01 for differences between more affected and less affected arms;
p<0.04 for differences between more affected arm and controls
Self-Efficacy for Skilled Reach Actions
Mean self-efficacy for accuracy SE and speed SE is shown in Figure 2 for both groups. Initial analyses indicated a significant arm by SE type by group interaction (p=0.002). Therefore, a repeated measures ANOVA was conducted separately for each group. In the control group, speed SE (right arm: 7.6 ± 1.2; left arm: 7.3 ± 1.4) was higher than accuracy SE (right arm: 5.6 ± 1.8; left arm: 5.6 ± 7.8) in both arms (p=0.027 for main effect of SE type). SE did not differ between the right and left arms (p=0.461 for main effect of arm) and there was no arm by SE type interaction (p=0.327). In the stroke group, analyses revealed a significant main effect for arm (p<0.001) and for SE type (p=0.007). Overall, SE was lower in the more affected arm (speed SE: 5.7 ± 2.0; accuracy SE: 5.0 ± 2.4) compared to the less affected arm (speed SE: 7.4 ± 1.5; accuracy SE: 6.5 ± 1.8), and speed SE was higher than accuracy SE across arms. Accuracy SE in the stroke group did not differ from the control group for either the less affected (p=0.185) or the more affected (p=0.457) arm. Speed SE was lower in the stroke group compared to the control group for the more affected arm (p=0.01) but not the less affected arm (p=0.933).
Figure 2.
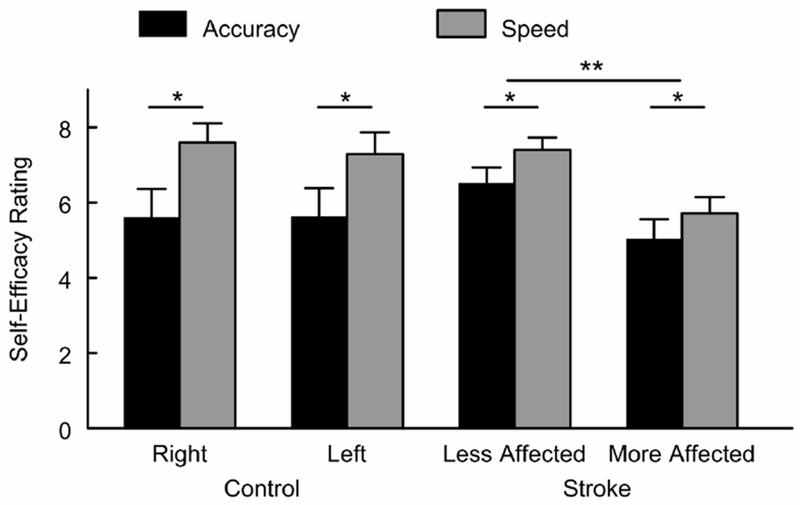
Mean self-efficacy by group and arm. Each bar represents group mean with standard error bar for accuracy self-efficacy and speed self-efficacy. *p<0.05 for differences between accuracy and speed self-efficacy; **p<0.05 for differences between arms.
Relationship between Self-Efficacy and Kinematic Variables of Reach Performance
In the control group, accuracy SE showed a negative correlation with endpoint error that was statistically significant for reaches to contralateral targets (across midline); individuals with higher accuracy SE tended to have lower error (Table 3). Speed SE showed a positive correlation with peak velocity and a negative correlation with movement time; individuals with higher speed SE tended to have higher peak velocity and shorter movement time. Similar relationships between SE and reach performance were found for the less affected arm in the stroke group (Table 3, Fig. 3). For reaches with the more affected arm, accuracy SE showed a negative relationship with endpoint error for contralateral targets. Speed SE did not significantly correlate with peak velocity or movement time although there was a trend for a negative correlation between speed SE and movement time to ipsilateral targets (p=0.038). Within the stroke group, neither accuracy SE nor speed SE correlated with UE FM, ARAT, SIS hand, or CAHM score (r<0.250, p>0.289).
Table 3.
Correlation between Self-Efficacy and Reach Performance
| Accuracy Self-Efficacy | Speed Self-Efficacy | |||||
|---|---|---|---|---|---|---|
| Control | Less Affected | More Affected | Control | Less Affected | More Affected | |
| Ipsilateral Targets | ||||||
| Error | −0.611 | −0.321 | −0.395 | |||
| Peak Velocity | 0.808* | 0.540* | 0.341 | |||
| Movement Time | −0.689* | −0.558* | −0.467 | |||
| Contralateral Targets | ||||||
| Error | −0.733* | −0.638* | −0.557* | |||
| Peak Velocity | 0.814* | 0.482 | 0.302 | |||
| Movement Time | −0.720* | −0.513* | −0.364 | |||
p<0.0167
Figure 3.
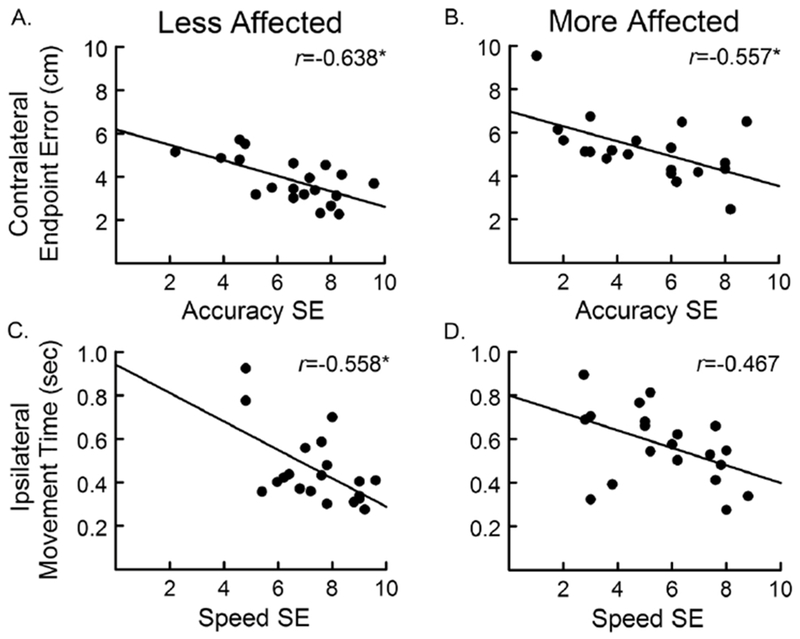
Relationship between self-efficacy and reach performance. The relationship between endpoint error to contralateral targets and accuracy self-efficacy shown for the less affected (A) and more affected (B) arms. The relationship between movement time to ipsilateral targets and speed self-efficacy shown for the less affected (C) and more affected (D) arms. *p<0.0167 for correlation. Note that the r value in C was determined using the log of movement time; raw data presented here for ease of interpretation.
Based on the results of the correlation analysis for the more affected arm, the regression models focused on endpoint error to the contralateral targets and movement time to the ipsilateral targets as dependent variables. For endpoint error, only accuracy SE (r=−0.557, p=0.011) and UE FM motor score (r=0.459, p=0.042) showed a significant bivariate correlation with error and were entered as possible predictors. In the first step, UE FM score was entered into the regression model and was a significant predictor of endpoint error (R2=0.167, F=4.80, (p=0.042). In the second step, accuracy SE was added to the model and accounted for additional variance in endpoint error (R2=0.352, F=6.15, (p=0.01). For movement time, speed SE (r=0.467, (p=0.038) and age (r=0.463, (p==0.04) were included as possible predictors. In the first step, age was a significant predictor of movement time (R2=0.170, F=4.90, (p=0.04). In the second step, speed SE accounted for additional variance in movement time (R2=0.291, F=1.89, (p=0.021).
Discussion
This study examined self-efficacy, a patient-reported measure, for skilled, goal-directed reach actions after stroke. Overall, self-efficacy for reach performance was lower for reaches with the more affected arm compared to the less affected arm; this decrease in self-efficacy corresponded to a decrease in actual reach performance with the more affected arm consistent with previous studies.35–37 Self-efficacy for reach accuracy and reach speed appeared distinct, with accuracy SE reported as being lower than speed SE in both the more affected and less affected arms as well as in controls. Additionally, reach SE significantly correlated with actual reach performance in the more affected arm, suggesting that individuals with mild motor impairment due to stroke have insight into their ability to perform a skilled reach task.
There have been limited studies that have investigated the relationship between SE and reach performance after stroke [see Rowe et al, 201738 for an exception]. Self-efficacy related to measures of reach performance for the more affected arm and remained a predictor of performance even when other clinical and demographic variables were considered. There is a growing body of evidence that supports a significant role for SE in several domains of function after stroke including fall risk14,15, physical function16,17, gait function18, and walking activity19,20. The consistency of these results across studies and task domains suggest that patient-reported confidence may be an important factor in motor skill performance and function after stroke. Self-efficacy for upper extremity function has received limited attention in the literature to date. An understanding of an individual’s confidence to successfully perform goal-directed upper extremity tasks may provide insight into the reported disconnect between arm movement capacity and arm use after stroke.3,4 Decreased SE to successfully complete a functional task using the more affected arm may lead an individual to choose a different strategy, such as using the less affected arm instead.39 Self-efficacy is a quick, inexpensive, patient-reported outcome measure that can easily be performed in both the research laboratory and the clinic. Future studies seeking to understand the predictors of skilled arm function should consider including a measure of SE.
Self-efficacy combined with age to predict reach speed to ipsilateral targets while self-efficacy combined with UE FM motor score to predict reach accuracy to contralateral targets. Decreases in movement speed during goal-directed reaching have frequently been associated with increases in age.40–42 In general, increased age shows a positive correlation with movement time to complete a reach task. As many individuals post-stroke are often older, the finding that age also relates to reach speed is consistent with this previous work. In contrast, UE FM motor score correlated with reach accuracy. The UE FM is a measure of motor impairment that asks individuals to move in specific movement patterns in and out of synergy.26 The motor score reflects how well an individual can perform each pattern, and there is no time constraint for most items. This finding suggests that the UE FM provides information about the ability of individuals with mild stroke-related motor impairment to control arm movements in a manner that supports accurate completion of a reach task.
For both endpoint error and movement time, the relationship between SE and reach performance was direction specific. Speed SE correlated with movement time for reaches to ipsilateral targets while accuracy SE correlated with endpoint error for reaches to contralateral targets. Reach direction had an effect on measures of reach performance for the more affected arm (shorter movement time to ipsilateral targets, higher endpoint error to contralateral targets) consistent with previous reports in nondisabled individuals.32,33 Previous research has suggested an effect of target direction on reach SE after stroke.43 Chen et al (2012) found that confidence was lower for targets in the contralateral workspace that required a reach across midline than for targets in the ipsilateral workspace. However, in that study, SE was not separated into speed and accuracy and the relationship between measures of reach self-efficacy and reach performance was not examined. Our findings suggest that accuracy SE and speed SE may also be affected by reach direction although this was not directly examined in the current study (i.e. SE was not reported separately by target direction). Future studies could investigate the interaction of SE type and reach direction in individuals with and without stroke.
Overall, in both groups, participants felt more confident with respect to reach speed than they did for reach accuracy for reaches with both arms. This difference in SE may have been driven by the demands of the task. Participants were encouraged to move quickly but no time constraints on movement time were present, whereas visual feedback related to accuracy was presented after each trial. This feedback may have increased participants’ focus on accuracy which may have impacted the SE rating. Alternatively, given that the task did not impose any constraints on speed or accuracy, each participant may have selected a reach strategy that optimized both variables for task completion44,45; the self-selected strategy may have impacted SE ratings. While the current study cannot determine why speed SE was rated higher than accuracy SE, the findings suggest that these variables can be rated distinctly by participants with and without stroke. Future studies could examine the effect of task manipulations on speed or accuracy on reach SE.
In general, self-reported measures of SE are thought to be specific to the task or domain reflected in the question posed.10,12 This may explain the finding that measures of reach SE correlated with measures of reach performance but not with clinical measures of motor impairment or function. One might expect reach SE to correlate with a general measure of SE for UE functional activities (CAHM), however, this was not the case. The CAHM asks the individual to rate his or her confidence in performing a variety of unimanual and bimanual functional tasks, many of which require some degree of hand function in addition to transport of the arm. Reach SE in the current study related to one’s ability to transport the arm accurately and quickly to targets in a horizontal plane and no hand function was required. The lack of relationship between the CAHM and reach SE may suggest that each measure focuses on a specific task domain, functional tasks and reach control, respectively.
Interventions that seek to improve proximal arm control after stroke should consider the role of self-efficacy for reach control found in the current study. Self-efficacy is thought to play an important role in other domains of health13, including disease self-management46–49 and physical activity50,51. Strategies that aim to improve self-efficacy may be particularly important for translation of motor rehabilitation interventions targeting proximal arm function and ultimately arm use in the natural environment after stroke. Such strategies may include a combination of physical practice, social support, and techniques to enhance intrinsic motivation including goal setting.11,52–54 In fact, recent work has shown that confidence to perform UE functional tasks can change in response to training designed to target both skill performance and confidence.54,55 Future work could examine whether self-efficacy for reach control changes with repetitive practice or whether interventions that target improvements in SE benefit arm control, arm use, and overall function.
The frequency and type of verbal feedback provided to participants during reach trials was not controlled or logged in the current study. Comments were general in nature and aimed to maintain participant engagement in the reach task. Instructions and feedback can impact levels of SE. 10,12,52 However, we did not see a significant change in SE across the data collection session (no change over blocks), suggesting that the verbal feedback provided had a limited effect on SE in the current study. Future work could examine the effect of instruction and feedback content and frequency on SE during the performance of a reach task or other skilled upper extremity tasks after stroke.
This study only included individuals with chronic stroke who had mild motor impairment but continued deficits in arm function and confidence. The relationship between self-efficacy and reach control in individuals with more severe motor impairment or who are in the acute/subacute period of recovery may be different. The current study was designed to assess confidence during reach performance but not during learning. Neither reach SE nor reach performance changed over blocks in a single session of practice. Future studies could investigate how self-efficacy changes over time or in response to a period of motor training and how these changes correspond to changes in reach performance. In the current analysis, reach performance was summarized across target distances similar to our previous analysis.23 While target distance may impact the level of SE after stroke43, our study design did not allow us to examine the effect of distance on speed SE or accuracy SE. The findings in the current study support continued investigation into the role of SE in reach control after stroke, including the influence of variables such as target distance. The task used in the current study did not require individuals to grasp or manipulate an object. Self-efficacy may be different for tasks that required reaching for the purpose of engaging the hand in a manipulation task.
Conclusions
Self-efficacy for skilled reaching with the more affected arm was decreased in this group of individuals with mild motor impairment due to stroke. Self-efficacy for reach speed and reach accuracy appeared distinct and correlated with kinematic measures of reach performance with the more affected arm; individuals with higher self-efficacy tended to reach faster and with less error. Self-efficacy should be considered as a predictor of reach control in studies seeking to understand reach deficits and may be a target,13 in combination with performance, of interventions aimed at improving proximal arm control after stroke.
Supplementary Material
Acknowledgements
Funding for this research was provided in part through a Mary McMillan Doctoral Scholarship and a Promotion of Doctoral Studies II Scholarship from the Foundation for Physical Therapy, a grant from the California Physical Therapy Fund, and NIH T32GM081740. The virtual reality system used in this study was provided by Innovative Sports Training, Inc.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Prabhakaran S, Zarahn E, Riley C, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22:64–71. [DOI] [PubMed] [Google Scholar]
- 2.Stinear C Prediction of recovery of motor function after stroke. Lancet Neurol. 2010;9:1228–1232. [DOI] [PubMed] [Google Scholar]
- 3.Waddell KJ, Strube MJ, Bailey RR, et al. Does task-specific training improve upper limb performance in daily life poststroke? Neurorehabil Neural Repair. 2017;31:290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doman CA, Waddell KJ, Bailey RR, Moore JL, Lang CE. Changes in upper-extremity functional capacity and daily performance during outpatient occupational therapy for people with stroke. Am J Occup Ther. 2016;70:7003290040p7003290041–7003290040p7003290011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwakkel G, Lannin NA, Borschmann K, et al. Standardized measurement of sensorimotor recovery in stroke trials: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Neurorehabil Neural Repair. 2017;31:784–792. [DOI] [PubMed] [Google Scholar]
- 6.Kitago T, Liang J, Huang VS, et al. Improvement after constraint-induced movement therapy: recovery of normal motor control or task-specific compensation? Neurorehabil Neural Repair. 2013;27:99–109. [DOI] [PubMed] [Google Scholar]
- 7.Subramanian SK, Yamanaka J, Chilingaryan G, Levin MF. Validity of movement pattern kinematics as measures of arm motor impairment poststroke. Stroke. 2010;41:2303–2308. [DOI] [PubMed] [Google Scholar]
- 8.Platz T, Bock S, Prass K. Reduced skilfulness of arm motor behaviour among motor stroke patients with good clinical recovery: does it indicate reduced automaticity? Can it be improved by unilateral or bilateral training? A kinematic motion analysis study. Neuropsychologia. 2001;39:687–698. [DOI] [PubMed] [Google Scholar]
- 9.Platz T, Prass K, Denzler P, Bock S, Mauritz KH. Testing a motor performance series and a kinematic motion analysis as measures of performance in high-functioning stroke patients: reliability, validity, and responsiveness to therapeutic intervention. Arch Phys Med Rehabil. 1999;80:270–277. [DOI] [PubMed] [Google Scholar]
- 10.Cervone D, & Scott WD. Self-efficacy theory of behavioral change: foundations, conceptual issues, and therapeutic implications In: O’Donahue WT, & Krasner L, ed. Theories of behavior therapy: Exploring behavior change. Washington, DC: American Psychological Association; 1995:349–383. [Google Scholar]
- 11.Lewthwaite R Motivational considerations in physical activity involvement. Phys Ther. 1990;70:808–819. [DOI] [PubMed] [Google Scholar]
- 12.Bandura A Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. [DOI] [PubMed] [Google Scholar]
- 13.Sheeran P, Maki A, Montanaro E, et al. The impact of changing attitudes, norms, and self-efficacy on health-related intentions and behavior: A meta-analysis. Health psychol. 2016;35:1178–1188. [DOI] [PubMed] [Google Scholar]
- 14.Landers MR, Oscar S, Sasaoka J, Vaughn K. Balance confidence and fear of falling avoidance behavior are most predictive of falling in older adults: prospective analysis. Phys Ther. 2016;96:433–442. [DOI] [PubMed] [Google Scholar]
- 15.Pang MY, Eng JJ. Fall-related self-efficacy, not balance and mobility performance, is related to accidental falls in chronic stroke survivors with low bone mineral density. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2008;19:919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellstrom K, Lindmark B, Wahlberg B, Fugl-Meyer AR. Self-efficacy in relation to impairments and activities of daily living disability in elderly patients with stroke: a prospective investigation. J Rehabil Med. 2003;35:202–207. [DOI] [PubMed] [Google Scholar]
- 17.Salbach NM, Mayo NE, Robichaud-Ekstrand S, Hanley JA, Richards CL, Wood-Dauphinee S. Balance self-efficacy and its relevance to physical function and perceived health status after stroke. Arch Phys Med Rehabil. 2006;87:364–370. [DOI] [PubMed] [Google Scholar]
- 18.LeBrasseur NK, Sayers SP, Ouellette MM, Fielding RA. Muscle impairments and behavioral factors mediate functional limitations and disability following stroke. Phys Ther. 2006;86:1342–1350. [DOI] [PubMed] [Google Scholar]
- 19.Danks KA, Pohlig RT, Roos M, Wright TR, Reisman DS. Relationship between walking capacity, biopsychosocial factors, self-efficacy, and walking activity in persons poststroke. J Neurol Phys Ther. 2016;40:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durcan S, Flavin E, Horgan F. Factors associated with community ambulation in chronic stroke. Disabil Rehabil. 2016;38:245–249. [DOI] [PubMed] [Google Scholar]
- 21.Chen SY. The influence of self-efficacy on recovery of spontaneous arm use in hemiparetic stroke. Dissertation Abstracts International: Section B: The Sciences and Engineering. 2012;73:278. [Google Scholar]
- 22.Ballester BR, Maier M, San Segundo Mozo RM, Castaneda V, Duff A, PF MJV. Counteracting learned non-use in chronic stroke patients with reinforcement-induced movement therapy. J Neuroeng Rehabil. 2016;13:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart JC, Gordon J, Winstein CJ. Control of reach extent with the paretic and nonparetic arms after unilateral sensorimotor stroke II: planning and adjustments to control movement distance. Exp Brain Res. 2014;232:3431–3443. [DOI] [PubMed] [Google Scholar]
- 24.Stewart JC, Gordon J, Winstein CJ. Control of reach extent with the paretic and nonparetic arms after unilateral sensorimotor stroke: kinematic differences based on side of brain damage. Exp Brain Res. 2014;232:2407–2419. [DOI] [PubMed] [Google Scholar]
- 25.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 26.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 28.Hartman-Maeir A, Katz N. Validity of the Behavioral Inattention Test (BIT): relationships with functional tasks. Am J Occup Ther. 1995;49:507–516. [DOI] [PubMed] [Google Scholar]
- 29.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4:483–492. [DOI] [PubMed] [Google Scholar]
- 30.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The Stroke Impact Scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. [DOI] [PubMed] [Google Scholar]
- 31.Winter D. Biomechanics and motor control of human movement. 3 ed. Hoboken: John Wiley & Sons, Inc; 2005. [Google Scholar]
- 32.Stewart JC, Gordon J, Winstein CJ. Planning and adjustments for the control of reach extent in a virtual environment. J Neuroeng Rehabil. 2013;10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon J, Ghilardi MF, Ghez C. Accuracy of planar reaching movements. I. Independence of direction and extent variability. Experimental Brain Research. 1994;99:97–111. [DOI] [PubMed] [Google Scholar]
- 34.Woytowicz EJ, Rietschel JC, Goodman RN, et al. Determining levels of upper extremity movement impairment by applying a cluster analysis to the Fugl-Meyer Assessment of the upper extremity in chronic stroke. Arch Phys Med Rehabil. 2017;98:456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner JM, Lang CE, Sahrmann SA, Edwards DF, Dromerick AW. Sensorimotor impairments and reaching performance in subjects with poststroke hemiparesis during the first few months of recovery. Phys Ther. 2007;87:751–765. [DOI] [PubMed] [Google Scholar]
- 36.Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123(Pt 5):940–953. [DOI] [PubMed] [Google Scholar]
- 37.Roby-Brami A, Fuchs S, Mokhtari M, Bussel B. Reaching and grasping strategies in hemiparetic patients. Motor Control. 1997;1:72–91. [Google Scholar]
- 38.Rowe JB, Chan V, Ingemanson ML, Cramer SC, Wolbrecht ET, Reinkensmeyer DJ. Robotic assistance for training finger movement using a Hebbian model: A randomized controlled trial. Neurorehabil Neural Repair. 2017;31:769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han CE, Kim S, Chen S, et al. Quantifying arm nonuse in individuals poststroke. Neurorehabil Neural Repair. 2013;27:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ketcham CJ, Seidler RD, Van Gemmert AW, Stelmach GE. Age-related kinematic differences as influenced by task difficulty, target size, and movement amplitude. J Gerontol B Psychol Sci Soc Sci. 2002;57:P54–64. [DOI] [PubMed] [Google Scholar]
- 41.Pohl PS, Winstein CJ, Fisher BE. The locus of age-related movement slowing: sensory processing in continuous goal-directed aiming. J Gerontol B Psychol Sci Soc Sci. 1996;51:P94–102. [DOI] [PubMed] [Google Scholar]
- 42.Welford AT, Norris AH, Shock NW. Speed and accuracy of movement and their changes with age. Acta Psychol (Amst). 1969;30:3–15. [DOI] [PubMed] [Google Scholar]
- 43.Chen S, Lewthwaite R, Schweighofer N, Winstein CJ. Discriminant validity of a new measure of self-efficacy for reaching movements after stroke-induced hemiparesis. Journal of hand therapy: official journal of the American Society of Hand Therapists. 2013;26:116–122; quiz 123. [DOI] [PubMed] [Google Scholar]
- 44.Manohar SG, Chong TT, Apps MA, et al. Reward pays the cost of noise reduction in motor and cognitive control. Curr Biol. 2015;25:1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Missenard O, Fernandez L. Moving faster while preserving accuracy. Neuroscience. 2011; 197:233–241. [DOI] [PubMed] [Google Scholar]
- 46.Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient education and counseling. 2004;52:271–277. [DOI] [PubMed] [Google Scholar]
- 47.Clark AM, Wiens KS, Banner D, et al. A systematic review of the main mechanisms of heart failure disease management interventions. Heart (British Cardiac Society). 2016;102:707–711. [DOI] [PubMed] [Google Scholar]
- 48.Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Effective clinical practice : ECP. 2001;4:256–262. [PubMed] [Google Scholar]
- 49.Plevinsky JM, Greenley RN, Fishman LN. Self-management in patients with inflammatory bowel disease: strategies, outcomes, and integration into clinical care. Clinical and experimental gastroenterology. 2016;9:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nezami BT, Lang W, Jakicic JM, et al. The effect of self-efficacy on behavior and weight in a behavioral weight-loss intervention. Health psychol. 2016;35:714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Becofsky K, Baruth M, Wilcox S. Psychosocial mediators of two community-based physical activity programs. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2014;48:125–129. [DOI] [PubMed] [Google Scholar]
- 52.Wulf G, Lewthwaite R. Optimizing performance through intrinsic motivation and attention for learning: The OPTIMAL theory of motor learning. Psychon Bull Rev. 2016;23:1382–1414. [DOI] [PubMed] [Google Scholar]
- 53.Winstein CJ, Kay DB. Translating the science into practice: shaping rehabilitation practice to enhance recovery after brain damage. Prog Brain Res. 2015;218:331–360. [DOI] [PubMed] [Google Scholar]
- 54.Winstein C, Lewthwaite R, Blanton SR, Wolf LB, Wishart L. Infusing motor learning research into neurorehabilitation practice: a historical perspective with case exemplar from the accelerated skill acquisition program. J Neurol Phys Ther. 2014;38:190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lewthwaite R, Winstein CJ, Lane CJ, et al. Accelerating stroke recovery: body structures and functions, activities, participation, and quality of life outcomes from a large rehabilitation trial. Neurorehabil Neural Repair. 2018;32:150–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


