Abstract
Background & Aims:
The most prescribed non-nucleoside reverse transcriptase inhibitor efavirenz has been associated with elevated risk for dyslipidemia and hepatic steatosis in HIV-infected patients but the underlying mechanisms remain elusive. Here we investigated the role of pregnane X receptor (PXR) in mediating the adverse effects of efavirenz on lipid homeostasis.
Methods:
Cell-based reporter assays, primary cell culture, and multiple mouse models including conditional knockout and humanized mice were combined to study the impact of efavirenz on PXR activities and lipid homeostasis in vitro and in vivo. A novel liver-specific PXR knockout mouse model was also generated to determine the contribution of hepatic PXR signaling to efavirenz-elicited dyslipidemia and hepatic steatosis.
Results:
We found that efavirenz is a potent PXR-selective agonist that can efficiently activate PXR and induce its target gene expression in vitro and in vivo. Treatment with efavirenz induced hypercholesterolemia and hepatic steatosis in mice but deficiency of hepatic PXR abolished these adverse effects. Interestingly, efavirenz-mediated PXR activation regulated the expression of several key hepatic lipogenic genes including fatty acid transporter CD36 and cholesterol biosynthesis enzyme squalene epoxidase (SQLE), leading to increased lipid uptake and cholesterol biosynthesis in hepatic cells. While CD36 is a known PXR target gene, we identified a DR-2-type of PXR-response element in the SQLE promoter and established SQLE as a direct transcriptional target of PXR. Since PXR exhibits considerable pharmacology differences across species, we also confirmed these findings in PXR-humanized mice and human primary hepatocytes.
Conclusions:
The widely prescribed anti-retroviral drug efavirenz induces hypercholesterolemia and hepatic steatosis by activating PXR signaling. Activation of PXR should be taken into consideration for patients undergoing long-term treatment with PXR agonistic anti-retroviral drugs.
Keywords: HIV, anti-retroviral drugs, dyslipidemia, hepatic steatosis, pregnane X receptor, squalene epoxidase
Lay Summary
Efavirenz is widely prescribed for HIV-infected patients but has some side effects. It can increase lipid levels in patients’ blood and liver. Here we show that efavirenz can activate a unique liver protein called PXR which mediates the adverse effects of efavirenz on lipid levels in mouse models.
Introduction
As the average lifespan of HIV-infected patients receiving anti-retroviral therapy (ART) lengthens, morbidity and mortality from cardiovascular disease (CVD) pose considerable challenges [1–4]. Not only does HIV infection elevate the risk for CVD, but the use of anti-retroviral (ARV) drugs has also been associated with dyslipidemia and increased risk of CVD, thereby exacerbating a serious health challenge for long-term survivors [1, 2, 4]. Current optimal ART options consist of a combination of several drug classes including protease inhibitors (PIs), nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitor (NNRTIs), and integrase strand transfer inhibitors (INSTIs, also known as integrase inhibitors) [2, 3]. Although several first-generation PIs such as amprenavir and ritonavir with documented dyslipidemic properties have been used on a limited basis, many currently recommended first-line ARV drugs have also been associated with dyslipidemia and increased CVD risk [2–6]. For example, the NNRTI efavirenz, one of the most prescribed anti-retroviral drugs worldwide, has been consistently associated with dyslipidemia including increased total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol levels in multiple clinical studies [2, 5–7]. Despite the strong evidence linking certain ARV drugs with dyslipidemia and CVD risk, the underlying mechanisms responsible for the adverse effects of ARV drugs remain elusive.
We and others have previously identified several HIV PIs including amprenavir and ritonavir as potent agonists of pregnane X receptor (PXR; also known as steroid and xenobiotic receptor, or SXR) [8, 9]. PXR is a nuclear receptor activated by numerous endogenous hormones, dietary steroids, pharmaceutical agents, and xenobiotic chemicals [10–12]. PXR functions as a xenobiotic sensor that induces the expression of genes required for xenobiotic metabolism in the liver and intestine [11, 12]. We and others have recently demonstrated that PXR signaling also plays an important role in lipid homeostasis [9, 12–15]. PXR signaling has also been implicated in contributing to ARV drugs’ dyslipidemic effects. For example, treatment with ritonavir, a potent PXR activator [8], caused hyperlipidemia and was associated with increased risk of CVD in HIV patients [16]. We also demonstrated that another PI, amprenavir can activate PXR to elevate plasma cholesterol levels in mice [9].
In the present study, we tested currently recommended ARV drugs, and identified several widely-prescribed ARV drugs such as efavirenz as potent PXR agonists. Interestingly, treatment with efavirenz stimulated hypercholesterolemia and hepatic steatosis in mice in a PXR-dependent manner. Efavirenz-mediated PXR activation altered the expression of several key hepatic lipogenic genes including fatty acid transporter CD36 and cholesterol biosynthesis enzyme squalene epoxidase (SQLE), leading to increased lipid uptake and cholesterol synthesis in hepatic cells.
Materials and Methods
Animals
C57BL/6 wild type (WT) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The PXR-humanized (hPXR•mPXR−/−) and PXR-null (mPXR−/−) mice on C57BL/6 background have been described before [17, 18]. The hPXR•mPXR−/− and mPXR−/− mice used in this study were littermates which had the same background (mPXR null alleles) except for one allele of hPXR•mPXR−/− mice carrying the human PXR gene. To investigate the tissue-specific role of PXR in xenobiotic and lipid metabolism, we have obtained mouse embryonic stem cell clones containing conditional PXR flox allele from International Knockout Mouse Consortium (EUMMCR, EPD0141_1_G04) and generated mice carrying PXR flox alleles (PXRF/F) on C57BL/6 background. PXRF/F mice were further crossed with Albumin-Cre transgenic mice (The Jackson Laboratory) to generate hepatocyte-specific PXR-deficient mice (termed as PXRΔHep). The PXRF/F and PXRΔHep mice used in this study were also littermates and PXRΔHep mice carried heterozygous knock-in for Albumin-Cre. All animals were housed in a pathogen-free environment with a light-dark cycle, under a protocol approved by the University of Kentucky Institutional Animal Care and Use Committee. For the treatment, 8-week-old male mice were fed a semisynthetic low-fat AIN76 diet containing 0.02% cholesterol (Research Diet; New Brunswick, NJ) [9, 13] and treated by oral gavage with vehicle (corn oil), 25, 50, or 100 mg/kg body weight efavirenz or emtricitabine daily for 1 week. On the day of euthanization, mice were fasted for 6 hr following the dark cycle (feeding cycle) and blood and tissues were then collected as previously described [9, 13].
Statistical analysis
All data are presented as the mean ± SEM. Individual pairwise comparisons were analyzed by two-sample, two-tailed Student’s t-test unless otherwise noted, with P<0.05 was regarded as significant. One-way analysis of variance (ANOVA) was used when multiple comparisons were made, followed by Dunnett’s t test for multiple comparisons to a control. Two-way analysis of variance was used when multiple comparisons were made followed by a Bonferroni multiple comparisons test. N numbers are listed in figure legends.
For further details regarding other materials and methods, please refer to the CTAT table and supplementary information.
Results
Currently recommended ARV drugs including efavirenz are potent PXR agonists
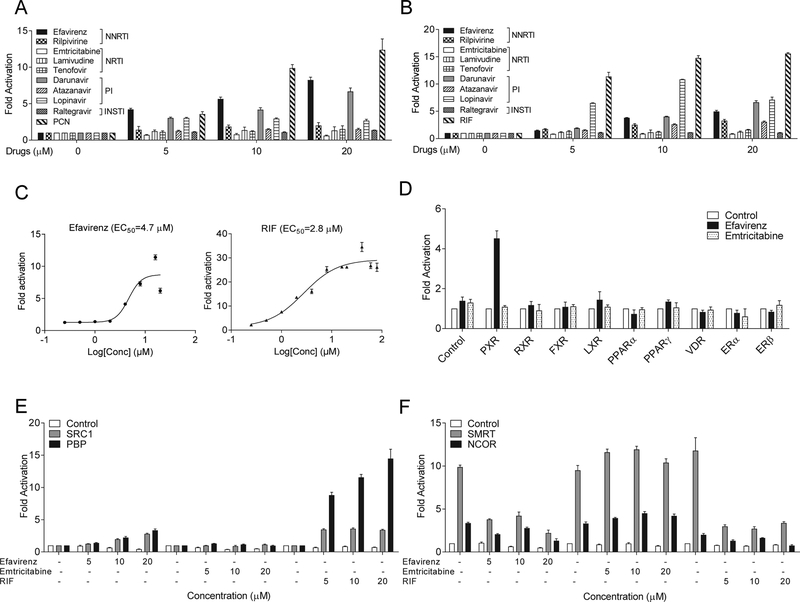
We first tested currently recommended ARV drugs from commonly used drug classes including NNRTI, NRTI, PI, and INSTI by transfections assays (Fig. 1, A and B). Since PXR exhibits considerable differences in its pharmacology across species [11], the potent PXR ligands pregnenolone 16α-carbonitrile (PCN) and rifampicin (RIF) were used as the positive control for mouse (m) and human (h) PXR, respectively. We found that several widely-prescribed ARV drugs, including NNRTI efavirenz and PIs darunavir and lopinavir can potently activate both human and mouse PXR (Fig. 1, A and B). Rilpivirine and lopinavir can also affect PXR activity but they are relatively weak agonists for PXR. By contrast, the NRTIs including emtricitabine, lamivudine, and tenofovir, as well as INSTI raltegravir had no effects on either mouse or human PXR activities. Efavirenz is one of the most prescribed ARV drugs to treat HIV infection worldwide and dose-response analysis showed that efavirenz can activate hPXR at concentrations at low μM range with an EC50 of 4.7 μM, which is comparable to potent PXR agonist RIF (Fig. 1C).
Figure 1. Non-nucleoside reverse transcriptase inhibitor efavirenz is a potent PXR-selective agonist.
(A and B) HepG2 cells were transfected with (A) full-length mPXR together with a mPXR reporter ((CYP3A2)3-luc) or (B) full-length hPXR together with hPXR reporter (CYP3A4-luc) and CMX-β-galactosidase control plasmid. Cells were then treated with DMSO control, ARV drugs, and PCN (mPXR ligand) or RIF (hPXR ligand) at the indicated concentrations for 24 hr. (C) HepG2 cells were transfected with hPXR and CYP3A4-luc reporter together with CMX-b-galactosidase plasmid. Cells were then treated with efavirenz or RIF at the indicated concentrations for 24 hr. (D) HepG2 cells were transfected with a GAL4 reporter and a series of GAL4 plasmids in which the GAL4 DNA-binding domain is linked to the indicated nuclear receptor ligand-binding domain. Cells were treated with DMSO control or 10 μM efavirenz or emtricitabine for 24 hr. (E and F) HepG2 cells were transfected with a GAL4 reporter, VP16-hPXR vector, and expression vector for GAL4 DBD or GAL4 DBD linked to the receptor interaction domains of PXR co-activators (GAL4-SRC1 or GAL4-PBP) (E) or PXR corepressors (GAL4-SMRT or GAL4-NCoR) (F). Cells were treated with DMSO control, efavirenz, emtricitabine, or RIF at the indicated concentrations for 24 hr. Data are shown as fold induction of normalized luciferase activity compared with DMSO treatment and represent the mean of triplicate experiments.
Efavirenz is a PXR-selective agonist that modulates the interactions between PXR and co-regulators
We next tested the ability of efavirenz to activate a panel of other nuclear receptors (Fig. 1D). Efavirenz can activate PXR but was unable to activate any of the other nuclear receptors such as liver X receptor (LXR) and peroxisome proliferator-activated receptor (PPAR). The NRTI emtricitabine did not affect the activities of PXR or the other nuclear receptors. Nuclear receptor co-regulators play key roles in nuclear receptor signaling. We found that efavirenz but not emtricitabine promoted the specific interactions between PXR and the co-activators steroid receptor coactivator-1 (SRC-1) and PPAR binding protein (PBP) (Fig. 1E). Unliganded PXR interacted with PXR co-repressors including nuclear receptor co-repressor (NCoR) and silencing mediator of retinoid and thyroid hormone (SMRT) (Fig. 1F), and efavirenz disrupted this interaction as did the potent human PXR ligand RIF. By contrast, emtricitabine did not affect the PXR-co-repressor interactions. Taken together, binding of efavirenz to PXR inhibits PXR-co-repressor interaction and promotes PXR-co-activator recruitment, thereby inducing PXR transcriptional activation in a concentration-dependent manner.
Efavirenz stimulates PXR target gene expression in vitro and in vivo
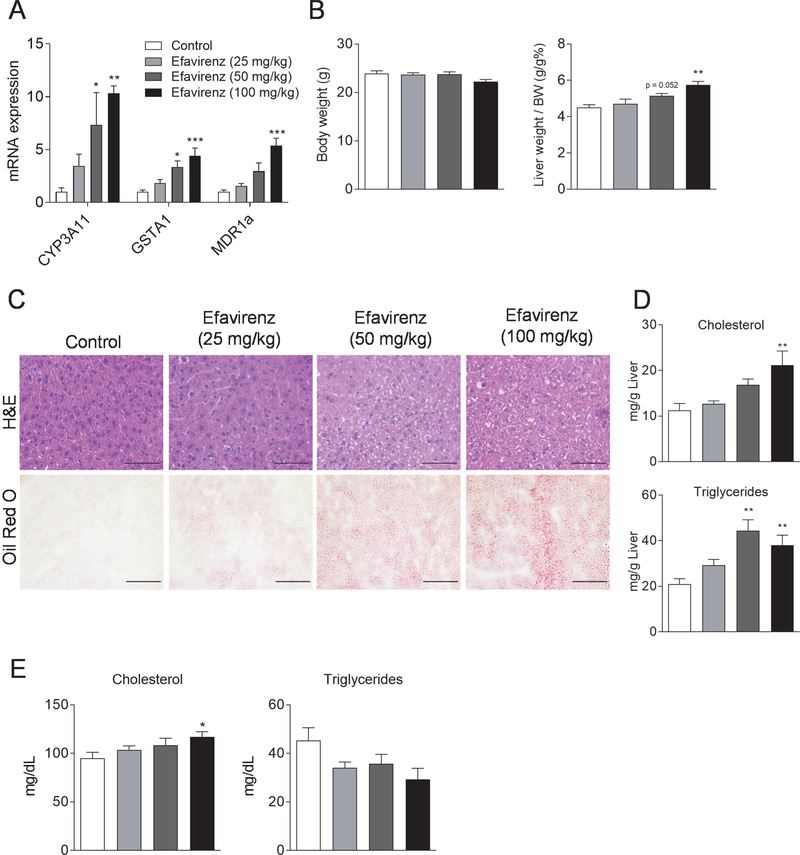
Using mouse primary hepatocytes and human hepatoma HepaRG cells [9], we found that efavirenz induced the expression of bona fide PXR target genes in these hepatic cells (Fig. S1). As expected, treatment with emtricitabine had no effects on PXR target gene expression in those cells. To investigate the effects of efavirenz on PXR activity in vivo, WT mice were treated with vehicle control, 25, 50 or 100 mg/kg body weight of efavirenz daily by oral gavage for 1 week. Consistent with in vitro results, efavirenz treatment resulted in significantly increased hepatic expression of PXR target genes in a dose-dependent manner (Fig. 2A).
Figure 2. Exposure to efavirenz induces hypercholesterolemia and liver steatosis in wild-type mice.
Eight-week-old male WT mice were treated with vehicle, 25, 50, or 100 mg/kg/day of efavirenz by oral gavage for 1 week. (A) QPCR analysis of the hepatic expression of PXR target genes. (B) Body weight and the ratio of liver weight to body weight. (C) Hematoxylin and eosin (top, bar=50 μm) and Oil red O (bottom, bar=100 μm) stained liver sections. (D) Hepatic total cholesterol and triglyceride levels. (E) Plasma total cholesterol and triglyceride levels. Significance was determined by one-way ANOVA (A, B, D, and E). (n=5–6, *P<0.05, **P<0.01, and ***P<0.001 compared to control group).
Efavirenz induces hypercholesterolemia and hepatic steatosis in WT mice.
Efavirenz treatment has also been associated with increased hepatic steatosis and dyslipidemia in humans [5–7, 19, 20]. We found that efavirenz treatment did not affect body weight but induced hepatomegaly (Fig. 2B) and hepatic steatosis in mice (Fig. 2C). Consistently, hepatic cholesterol and triglyceride contents were significantly increased in efavirenz-treated mice in a dose-dependent manner (Fig. 2D). WT mice treated with efavirenz also had increased plasma total cholesterol levels but unchanged triglycerides as compared with control WT mice (Fig. 2E). Thus, efavirenz treatment can induce hypercholesterolemia and hepatic steatosis in WT mice.
Generation of liver-specific PXR knockout mice
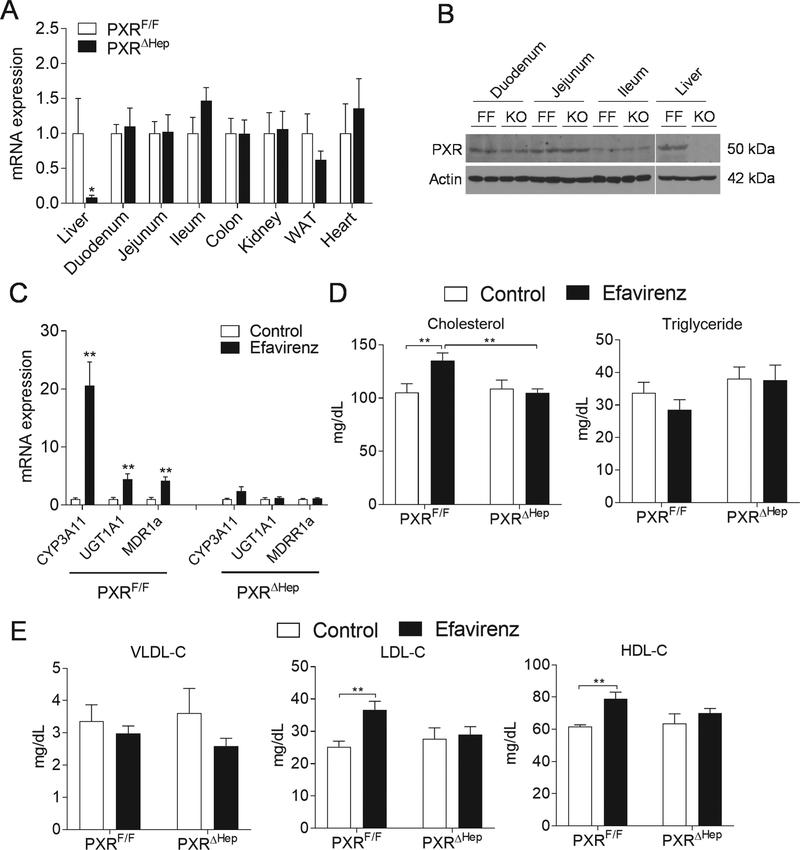
PXR is expressed at high levels in the liver, an organ central for maintaining whole-body lipid homeostasis. To define the liver-specific role of PXR in xenobiotic and lipid metabolism, we have successfully generated mice carrying PXR flox alleles (PXRF/F) (Fig. S2). PXRF/F mice were further crossed with Albumin-Cre transgenic mice to generate hepatocyte-specific PXR-deficient mice (termed as PXRΔHep) (Fig. S2A). PCR analysis of genomic DNA indicated that the recombination was specific to the liver of PXRΔHep mice (Fig. S2B). As expected, the mRNA levels and protein levels of PXR were significantly decreased in liver but not in other major tissues of PXRΔHep mice as compared with PXRF/F mice (Fig. 3A and3B), which demonstrated the specific and efficient PXR deletion in liver of PXRΔHep mice.
Figure 3. Efavirenz elicits hypercholesterolemia in PXRF/F mice but not in PXRΔHep mice.
(A) QPCR analysis of PXR mRNA levels in major tissues of PXRF/F and PXRΔHep mice (n=4–6, *P<0.05). (B) Western blot analysis of PXR proteins in liver and intestine of PXRF/F and PXRΔHep mice. (C-F) Eight-week-old male PXRF/F and PXRΔHep littermates were treated with vehicle control or 100 mg/kg/day of efavirenz by oral gavage for 1 week. Hepatic gene expression was analyzed by QPCR (C). Plasma total cholesterol and triglyceride levels were measured by standard methods (D). Lipoprotein fractions (VLDL, LDL, and HDL) were isolated and the cholesterol levels of each fraction were measured (E). Significance was determined by Student’s t test (A and C) or two-way ANOVA (D and E). (n=5–7, **P<0.01).
Efavirenz stimulates hypercholesterolemia and hepatic steatosis through hepatic PXR signaling
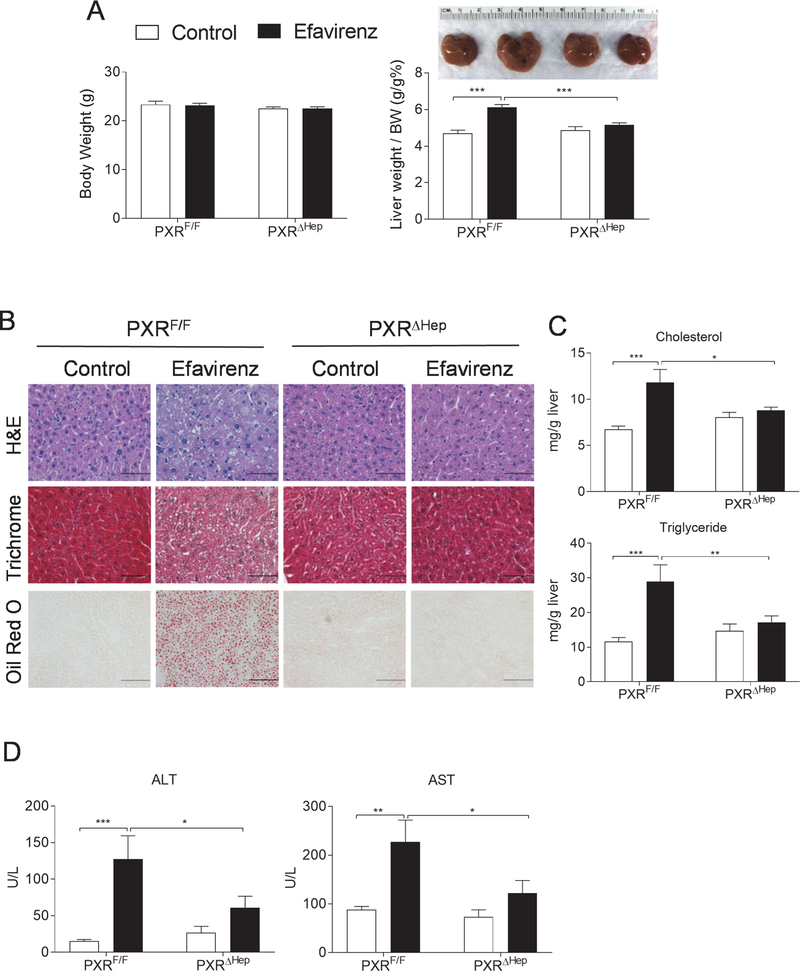
To determine whether efavirenz induces dyslipidemia and hepatic steatosis through hepatic PXR signaling, PXRF/F and PXRΔHep littermates were treated with efavirenz (100 mg/kg/day) or vehicle control for 1 week. The 100 mg/kg/day dose was selected based on previously studies which suggest that this dose is a mouse equivalent dose to those used clinically in humans (600 mg/day) when considering the interspecies scaling factor between mice and human (12.3:1) [21]. As expected, efavirenz treatment also stimulated the expression of PXR target genes such as CYP3A11 in the liver of PXRF/F mice but not in PXRΔHep mice (Fig. 3C). Similar to WT mice, efavirenz treatment did not affect fasting plasma triglyceride levels but significantly increased total cholesterol levels in PXRF/F mice (Fig. 3D). Lipoprotein fraction analysis showed that efavirenz treatment significantly increased both LDL and HDL cholesterol levels but did not affect VLDL cholesterol levels in PXRF/F mice (Fig. 3E). Interestingly, deficiency of hepatic PXR abolished the impact of efavirenz treatment on plasma total and lipoprotein cholesterol levels in PXRΔHep mice, suggesting that hepatic PXR signaling is required for efavirenz-elicited hypercholesterolemia. PXRF/F mice treated with efavirenz also had liver enlargement and increased hepatic steatosis (Fig. 4, A and B). However, short-term efavirenz treatment did not significantly affect liver fibrosis assessed by trichrome staining (Fig. 4B). Hepatic cholesterol and triglyceride levels were also significantly elevated in efavirenz-treated PXRF/F mice (Fig. 4C). Deficiency of PXR abolished the impact of efavirenz on hepatic steatosis in PXRΔHep mice (Fig. 4, B and C). Consistent with increased hepatic steatosis, efavirenz also increased plasma ALT and AST levels in a PXR-dependent manner (Fig. 4D).
Figure 4. Efavirenz treatment leads to increased hepatic steatosis in PXRF/F mice but not in PXRΔHep mice.
Eight-week-old male PXRF/F and PXRΔHep littermates were treated with vehicle control or 100 mg/kg/day of Efavirenz for 1 week. (A) Body weight, the ratio of liver weight to body weight, and representative liver image (n=6–7, ***P<0.001). (B) Representative H&E (bar=50 μm), Trichrome (bar=50 μm), and Oil red O (bottom, bar =100 μm) stained liver sections. (C and D) Hepatic total cholesterol and triglyceride levels (C) and plasma ALT and AST levels (D) (n=7, *P<0.05, **P<0.01 and ***P<0.001). Significance was determined by two-way ANOVA (A, C, and D).
In addition to efavirenz, PXRF/F and PXRΔHep mice were also treated with emtricitabine, another widely prescribed ARV drug that does not activate PXR (Fig. 1). As expected, emtricitabine did not affect hepatic PXR target gene expression in both PXRF/F and PXRΔHep mice (Fig. S3A). Plasma lipid levels and hepatic lipid accumulation was not affected by emtricitabine treatment in either PXRF/F or PXRΔHep mice (FigS3, B–F). Taken together, these results suggest that adverse effects of efavirenz on plasma cholesterol levels and hepatic steatosis are mediated through hepatic PXR signaling in mice.
Efavirenz-mediated PXR activation alters the key hepatic lipogenic gene expression in mice
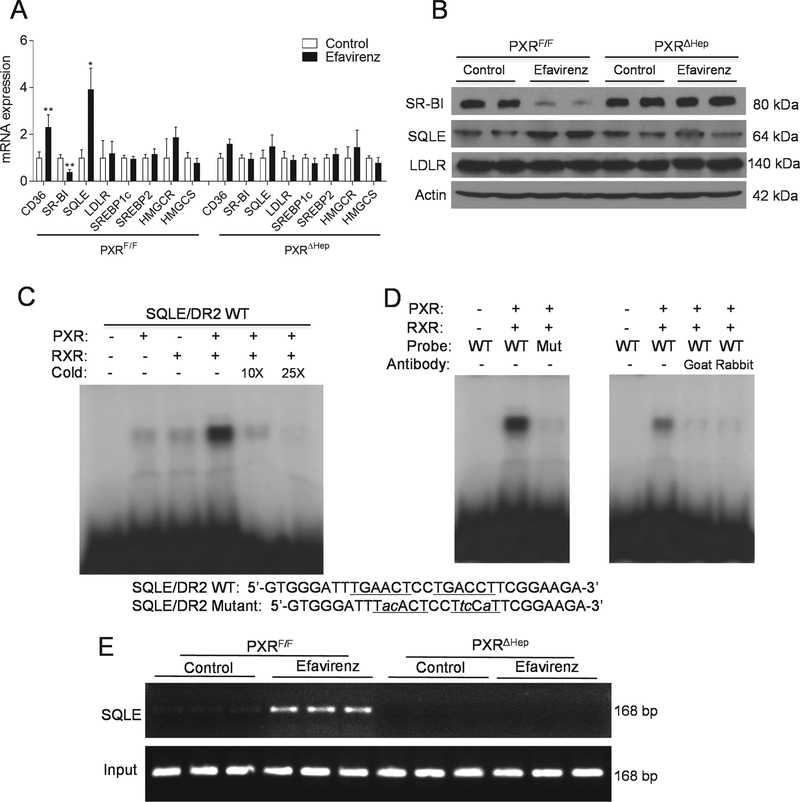
We next analyzed the expression of key hepatic genes mediating lipid homeostasis to elucidate potential molecular mechanisms for the adverse effects of efavirenz. While efavirenz-mediated PXR activation did not affect the expression of several well-studied lipogenic genes including HMGCR, SREBP1c, SREBP2, DGATs, and FAS (Fig. 5A and S4A), exposure to efavirenz led to altered expression of key lipid transporters SR-BI and CD36, and cholesterol synthesize enzyme SQLE (Fig. 5A). In addition, efavirenz treatment also induced a couple of genes involved β-oxidation, Acox1 and Cpt2 in the liver of PXRF/F but not PXRΔHep mice (Fig. S4A). We also measured several hepatic inflammatory gene expression and found that MCP-1 expression was also elevated in efavirenz-treated PXRF/F mice (Fig. S4B). It is possible that increased β-oxidation and inflammatory gene expression was due to the increased hepatic steatosis in these mice.
Figure 5. Squalene epoxidase is a direct transcriptional target of PXR.
(A and B) Eight-week-old male PXRF/F and PXRΔHep littermates were treated with vehicle control or 100 mg/kg/day of efavirenz for 1 week. (A) Hepatic gene expression was analyzed by QPCR (n=5–7, *P<0.05, **P<0.01, and ***P<0.001). (B) Western blot analysis of hepatic SR-BI, SQLE, and LDLR proteins in control or efavirenz-treated mice. (C) In vitro translated human PXR and RXR, as indicated, were incubated with [32P]-labeled SQLE/DR-2 probes for EMSA analysis. (D) PXR/RXR proteins were incubated with [32P]-labeled WT or mutated SQLE/DR-2 probes for EMSA analysis (left panel). PXR/RXR proteins were incubated with two different anti-PXR antibodies, goat anti-PXR or rabbit anti-PXR antibodies for 1 hr prior to the addition of the WT [32P]-labeled SQLE/DR-2 probes for EMSA analysis (right panel). (E) Primary hepatocytes isolated from PXRF/F and PXRΔHep mice were treated with control or 20 μM efavirenz for 24 hr. ChIP analysis was then performed to determine the recruitment of PXR onto the SQLE promote. Significance was determined by Student’s t test (A).
SR-BI is a major receptor for HDL uptake in the liver [22], and decreased expression or loss-of-function of SR-BI has been associated with elevated plasma HDL levels in animals and humans [22, 23]. PXR signaling has been previously shown to downregulate hepatic expression of SR-BI in vitro and in vivo [14, 24]. Consistently, efavirenz treatment decreased hepatic SR-BI mRNA levels and protein levels in PXRF/F but not PXRΔHep mice (Fig. 5, A and B). It is likely that PXR-mediated downregulation of SR-BI expression contributed to the increased plasma HDL levels in efavirenz-treated mice. In addition to SR-BI, efavirenz significantly increased hepatic expression of fatty acid transporter CD36 in PXRF/F but not PXRΔHep mice (Fig. 5, A and B). CD36 is a direct transcriptional target of PXR that contributes significantly to PXR ligand-mediated hepatic steatosis [15]. Thus, the adverse effects of efavirenz on hepatic steatosis may be mediated, at least in part, through PXR-CD36 axis in mice.
Squalene epoxidase is a direct transcriptional target of PXR
The role of PXR in regulating hepatic SR-BI and CD36 expression has been previously demonstrated [15, 24]. However, the impact of PXR activation on SQLE expression has not been reported. Efavirenz-mediated PXR activation increased both mRNA and protein levels of SQLE in liver (Fig. 5, A and B). While the role of HMGCR in cholesterol homeostasis has been extensively studied by many groups, little is known about the transcriptional regulation of SQLE which is another rate-limiting enzyme involved in the first oxidative step in cholesterol biosynthesis [25, 26]. We then analyzed the promoter of SQLE gene and identified a DR-2-type (direct repeat spaced by two nucleotides) of nuclear receptor response element (TGAACTCCTGACCT), similar to DR-2 element found in other PXR target genes [10]. Electrophoretic mobility shift assay (EMSA) confirmed that PXR and RXR heterodimer can bind to this DR-2 element. The binding of SQLE/DR-2 by PXR-RXR was specific as excessive cold probe decreased PXR-RXR binding to this element (Fig. 5C). In addition, mutations of the DR-2 element can abolish the binding of PXR-RXR dimers to the mutant DR-2 site (Fig. 5D). Two different anti-PXR antibodies also disrupted the protein-DNA complex (Fig. 5D), suggesting that PXR is a component of the protein complex that binds to the SQLE/DR2 element. Next, chromatin immunoprecipitation (ChIP) assays demonstrated that efavirenz treatment can increase the recruitment of PXR onto the SQLE promoter region containing DR-2 element in primary hepatocytes isolated from PXRF/F mice but deficiency of PXR abolished these effects (Fig. 5E). Taken together, these results demonstrated that SQLE is a direct transcriptional target of PXR.
Efavirenz-mediated PXR activation stimulates fatty acid uptake and cholesterol synthesis in hepatic cells
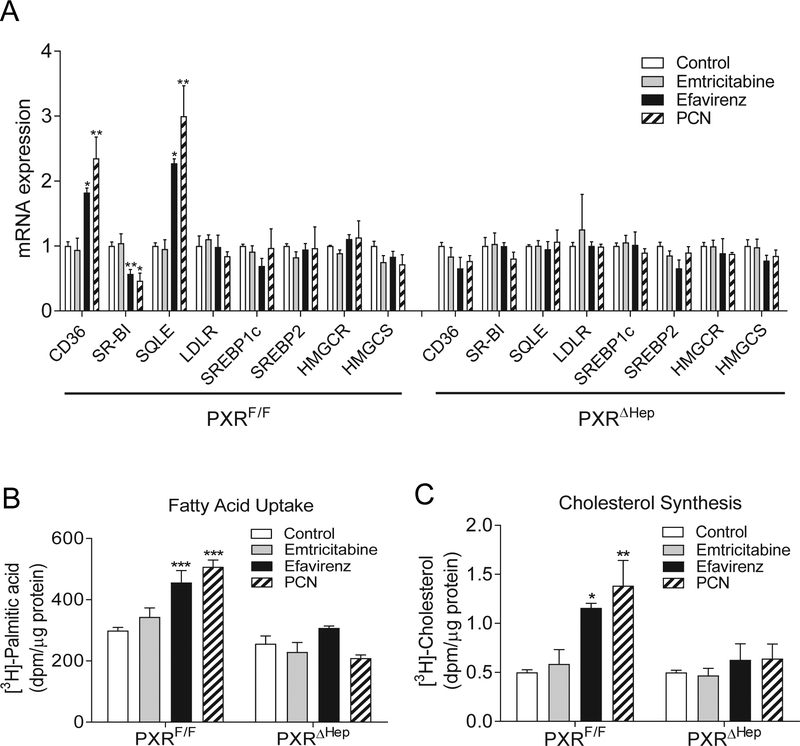
To further investigate the impact of efavirenz-mediated PXR activation on hepatic lipogenesis, we isolated primary hepatocytes from PXRF/F and PXRΔHep mice. Consistent with in vivo results, treatment with efavirenz and the known PXR ligand PCN altered the expression of bona fide PXR target genes (Fig. S5) and key lipogenic genes including CD36, SQLE, and SR-BI in control primary hepatocytes but not in PXR-deficient hepatocytes (Fig. 6A). By contrast, the NRTI emtricitabine did not affect the expression of any of those genes in control or PXR-deficient hepatocytes.
Figure. 6. Efavirenz-mediated PXR activation stimulates fatty acid uptake and cholesterol synthesis in hepatocytes.
Primary hepatocytes isolated from PXRF/F or PXRΔHep mice were treated with 20μM emtricitabine, efavirenz, or PCN for 24 hr. (A) QPCR analysis of lipogenic gene expression. (B) Fatty acid uptake assays were performed using [3H]-palmitic acid. (C) Cholesterol synthesis assays were performed using [3H]-acetate. Significance was determined by one-way ANOVA (A-C). (n=3, **P<0.01 and ***P<0.001 compared to control group).
CD36 plays an important role in regulating fatty acid uptake [15] and we then performed fatty acid uptake assays using [3H]-palmitic acid to determine the effects of efavirenz on hepatocyte fatty acid uptake. Indeed, activation of PXR by efavirenz or PCN significantly increased [3H]-palmitic acid uptake by control hepatocytes and deficiency of PXR abolished PXR ligand-mediated fatty acid uptake in hepatocytes of PXRΔHep mice (Fig. 6B). By contrast, emtricitabine treatment did not affect fatty acid uptake by either control or PXR-deficient hepatocytes (Fig. 6B).
Since SQLE is a rate-limiting enzyme in cholesterol biosynthesis, we also performed cholesterol synthesis assays to determine whether activation of PXR can directly affect cholesterol synthesis in hepatocytes. Consistently, efavirenz and PCN treatment significantly increased [3H]-acetate incorporation into [3H]-cholesterol in control hepatocytes but did not affect cholesterol biosynthesis in PXR-deficient hepatocytes (Fig. 6C). As expected, emtricitabine did not affect cholesterol biosynthesis in control or PXR-deficient hepatocytes. Collectively, these results confirmed that efavirenz-mediated PXR activation can stimulate fatty acid uptake and cholesterol synthesis in hepatic cells.
Efavirenz induces dyslipidemia and hepatic lipid accumulation in PXR-humanized mice
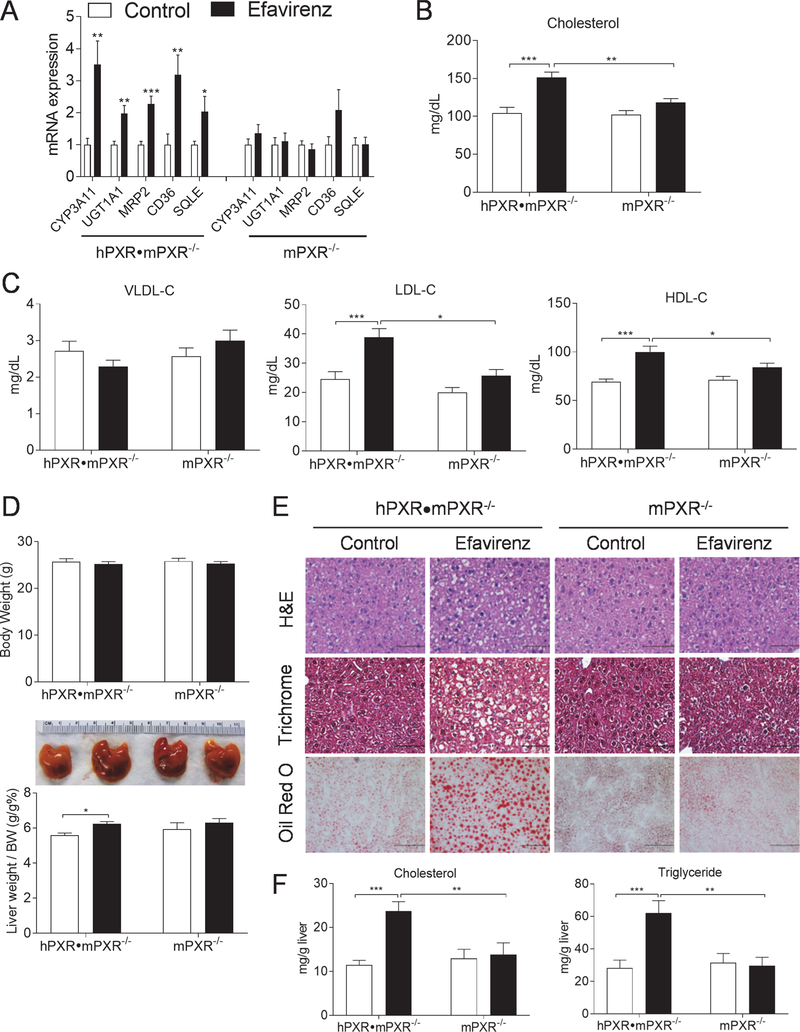
PXR exhibits considerable differences in its pharmacology and its ligand-binding domain is divergent across species [10, 11]. We next evaluated the effects of efavirenz on hepatic lipogenesis and plasma lipid levels in PXR-humanized (hPXR•mPXR−/−) mice that express the human PXR gene in place of mouse PXR gene [17, 18]. Efavirenz treatment also significantly increased the hepatic expression of known PXR target genes and newly identified SQLE in hPXR•mPXR−/− but not mPXR−/− mice (Fig. 7A). By contrast, efavirenz-stimulated PXR target gene induction was abolished in liver of mPXR−/− littermates. Consistent with results obtained from WT and PXRF/F mice, exposure to efavirenz also led to elevated plasma total, LDL, and HDL cholesterol levels; hepatomegaly; and hepatic steatosis and lipid accumulation in hPXR•mPXR−/− mice but not in mPXR−/− mice (Fig. 7, B-F).
Figure 7. Efavirenz induces hypercholesterolemia and hepatic steatosis in PXR-humanized mice.
Eight-week-old male hPXR•mPXR−/− and mPXR−/− littermates were treated with vehicle control or 100 mg/kg/day of efavirenz for 1 week. (A) QPCR analysis of hepatic gene expression. (B and C) Plasma total cholesterol (B) and VLDL, LDL, and HDL cholesterol levels (C). (D) Body weight, the ratio of liver weight to body weight, and representative liver image. (E) Representative H&E (bar=50 μm), Trichrome (bar=50 μm), and Oil red O (bar=100 μm) stained liver sections. (F) Hepatic total cholesterol and triglyceride levels. Significance was determined by Student’s t test (A) or two-way ANOVA (B-D, and F). (n=5–7, *P<0.05, **P<0.01 and ***P<0.001).
Efavirenz-mediated PXR activation regulates lipogenic gene expression in primary hepatocytes from PXR-humanized mice and humans
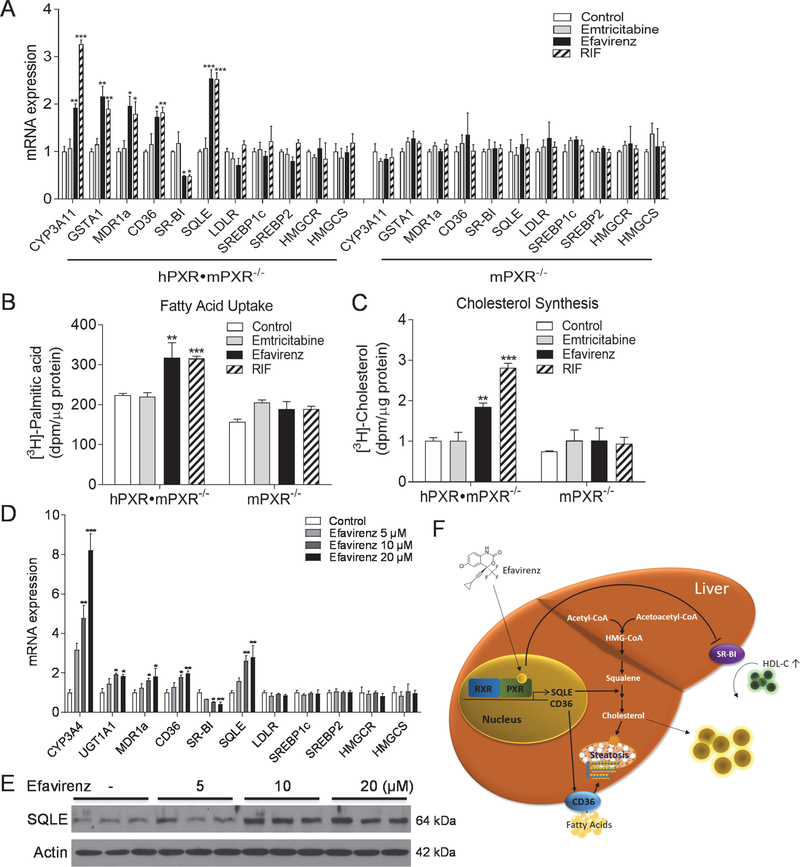
In addition to in vivo studies, we also performed in vitro assays using primary hepatocytes of hPXR•mPXR−/− and mPXR−/− mice (Fig. 8, A-C). In addition to regulating known PXR target genes, both efavirenz and RIF increased SQLE and CD36 expression and suppressed SR-BI expression in hepatocytes isolated from hPXR•mPXR−/− mice but not in PXR-deficient hepatocytes (Fig. 8A). As expected, emtricitabine did not affect any of those genes in hepatocytes of those mice (Fig. 8A). Further, efavirenz and RIF, but not emtricitabine, significantly increased both fatty acid uptake and cholesterol biosynthesis in hepatocytes isolated from hPXR•mPXR−/− mice, and deficiency of PXR abolished these effects in hepatocytes of mPXR−/− mice (Fig. 8, B and C).
Figure 8. Efavirenz-mediated PXR activation regulates lipogenic gene expression in primary hepatocytes from PXR-humanized mice and humans.
(A-C) Primary hepatocytes isolated from hPXR•mPXR−/− or mPXR−/− mice were treated with 20μM emtricitabine, efavirenz, or RIF for 24 hr. QPCR analysis (A), fatty acid uptake assays using [3H]-palmitic acid (B), cholesterol synthesis assays using [3H]-acetate (C). (n=3, *P<0.05, **P<0.01 and ***P<0.001). (D and E) Human primary hepatocytes were treated with efavirenz at indicated concentrations for 24 hr. QPCR analysis of lipogenic genes (D) and Western blot analysis of SQLE proteins (E) in control or efavirenz-treated hepatocytes (n=3, *P<0.05, **P<0.01 and ***P<0.001). (F) Schematic representation of the mechanism through which efavirenz-mediated PXR activation elicits hypercholesterolemia and hepatic steatosis. Significance was determined by one-way ANOVA (A-D).
Lastly, human primary hepatocytes were cultured and treated with efavirenz at different doses. Efavirenz treatment also resulted in increased SQLE and CD36 but decreased SR-BI expression in a dose-dependent manner (Fig. 8D). Immunoblotting results also confirmed elevated SQLE protein levels in efavirenz-treated human primary hepatocytes (Fig. 8E). Therefore, efavirenz-mediated PXR activation may have clinically relevant impact on hepatic lipogenic pathways in humans.
Discussion
Despite significant reduction in the morbidity and mortality associated with HIV infection over the past few decades, HIV-infected patients treated with effective ART have elevated risk for dyslipidemia and CVD [1–4]. In the current study, we identified currently recommended ARV drugs including efavirenz as potent PXR agonists. Short-term exposure to efavirenz significantly increased plasma cholesterol levels and elicited hepatic steatosis in mice in a hepatic PXR-dependent manner. Interestingly, PXR can regulate the expression of several key hepatic lipogenic genes including CD36, SR-BI, and SQLE which play important roles in hepatic lipid uptake and cholesterol biosynthesis (Fig. 8F). While current ART uses a combination of several drug classes, we found that only PIs and NNTRIs including efavirenz, darunavir, and lopinavir can activate PXR by transfection assays. By contrast, none of the NRTIs or INSTI we tested activated PXR. Interestingly, all of these PXR agonistic drugs have been associated with dyslipidemia in HIV-infected patients [2, 5, 6]. For example, clinical studies have demonstrated that both efavirenz and lopinavir can cause hyperlipidemia in HIV-infected patients, and the combination of efavirenz and lopinavir has more profound effects on elevating lipid levels in patients [2, 6]. On the contrary, several NRTIs including emtricitabine and tenofovir that do not activate PXR, have low risk of dyslipidemia [27–29]. Consistently, we found that efavirenz but not emtricitabine was able to induce hypercholesterolemia and hepatic steatosis in mice, and deficiency of PXR abolished efavirenz-elicited adverse effects. These results strongly suggest the involvement of PXR in certain ARV drug-associated dyslipidemia.
Previous studies have demonstrated that activation of PXR by various ligands can affect the expression of hepatic lipogenic genes including CD36, SR-BI, S14, and lipin-1 [11, 12, 14, 15, 24, 30]. For example, CD36, an important fatty acid transporter in the liver, has been demonstrated as a direct target gene of PXR [15]. In the current study, efavirenz-mediated PXR activation stimulated CD36 expression and promoted fatty acid uptake by hepatocytes. Therefore, the increased CD36 expression likely contributed significantly to the efavirenz-elicited hepatic steatosis in vivo. Efavirenz treatment also suppressed the hepatic expression of another scavenger receptor, SR-BI which is a major receptor mediating selective uptake of HDL. PXR can suppress hepatic SR-BI expression [14, 24] and decreased SR-BI expression has been well-established to increase plasma HDL levels in animal models [22]. Although HDL cholesterol has been considered to have beneficial effects on CVD, SR-BI loss of function mutations significantly elevated plasma HDL levels in humans, but paradoxically increased their risk of CVD, which is likely due to the impaired reverse cholesterol transport [23]. Therefore, the efavirenz-induced HDL levels may also have deleterious instead of beneficial effects on CVD in HIV-infected patients.
In addition to altered CD36 and SR-BI expression, efavirenz-mediated PXR activation also stimulated the expression of SQLE, a member of flavoprotein monooxygenase family. SQLE catalyzes the epoxidation of squalene to generate 2,3-oxidosqualene, which is the first oxidative step in cholesterol biosynthesis and also in this process [25]. Squalene epoxidation is a rate-limiting step and also an important regulatory point in cholesterol synthesis. Thus, SQLE has been considered as a promising target for hypercholesterolemia treatment in addition to HMGCR [25]. We then identified a PXR binding site in SQLE promoter, and both EMSA and ChIP assays confirmed SQLE as a directly transcriptional target of PXR. Consistent with the known function of SQLE in cholesterol biosynthesis, efavirenz-medicated PXR activation can indeed elevate cholesterol synthesis in cultured primary hepatocytes.
In summary, we have identified several widely-used ARV drugs including the most prescribed NNRTI efavirenz as potent agonists of PXR. Efavirenz can efficiently activate PXR and stimulate its target gene expression in vitro and in vivo. Interestingly, efavirenz treatment led to hypercholesterolemia and hepatic steatosis in mice, and deficiency of hepatic PXR abolished those adverse effects. Efavirenz-mediated PXR activation altered the hepatic expression of several key genes including CD36, SR-BI, and SQLE that mediate lipid uptake and cholesterol synthesis in the liver. These findings provide mechanistic insights and new understandings of ART-associated dyslipidemia and CVD risk, and activation of PXR should be taken into consideration for patients undergoing long-term treatment with ARV drugs.
Supplementary Material
Highlights:
Efavirenz is a widely prescribed HIV drug for patients.
Efavirenz increases the risk for dyslipidemia and hepatic steatosis in patients.
Efavirenz is a potent agonist for nuclear receptor PXR.
Efavirenz activates PXR to elicit the adverse effects on lipid homeostasis in mice.
PXR regulates the expression of several key lipogenic genes in liver.
Acknowledgement
We thank Dr. Frank Gonzalez at National Cancer Institute for the PXR-humanized mice and Dr. Chingwen Yang at Rockefeller University for the help with PXR-flox mouse generation.
Financial support: This work was supported by NIH grants, R01HL123358, R01ES023470, R01HL131925, and R21ES022745 (to C.Z.). The authors also acknowledge the core services (supported by NIH grant P30GM127211).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no conflict of interest to declare.
References
- [1].Friis-Moller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003;349:1993–2003. [DOI] [PubMed] [Google Scholar]
- [2].Feeney ER, Mallon PW. HIV and HAART-Associated Dyslipidemia. The open cardiovascular medicine journal 2011;5:49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Estrada V, Portilla J. Dyslipidemia related to antiretroviral therapy. AIDS reviews 2011;13:49–56. [PubMed] [Google Scholar]
- [4].Desai M, Joyce V, Bendavid E, Olshen RA, Hlatky M, Chow A, et al. Risk of cardiovascular events associated with current exposure to HIV antiretroviral therapies in a US veteran population. Clin Infect Dis 2015;61:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].van Leth F, Phanuphak P, Stroes E, Gazzard B, Cahn P, Raffi F, et al. Nevirapine and efavirenz elicit different changes in lipid profiles in antiretroviral-therapy-naive patients infected with HIV-1. PLoS medicine 2004;1:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Haubrich RH, Riddler SA, DiRienzo AG, Komarow L, Powderly WG, Klingman K, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS 2009;23:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sinxadi PZ, McIlleron HM, Dave JA, Smith PJ, Levitt NS, Haas DW, et al. Plasma Efavirenz Concentrations Are Associated With Lipid and Glucose Concentrations. Medicine (Baltimore) 2016;95:e2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dussault I, Lin M, Hollister K, Wang EH, Synold TW, Forman BM. Peptide mimetic HIV protease inhibitors are ligands for the orphan receptor SXR. J Biol Chem 2001;276:33309–33312. [DOI] [PubMed] [Google Scholar]
- [9].Helsley RN, Sui Y, Ai N, Park SH, Welsh WJ, Zhou C. Pregnane X receptor mediates dyslipidemia induced by the HIV protease inhibitor amprenavir in mice. Mol Pharmacol 2013;83:1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Blumberg B, Sabbagh W Jr., Juguilon H, Bolado J Jr., van Meter CM, Ong ES, et al. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev 1998;12:3195–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhou C, Verma S, Blumberg B. The steroid and xenobiotic receptor (SXR), beyond xenobiotic metabolism. Nucl Recept Signal 2009;7:e001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhou C Novel functions of PXR in cardiometabolic disease. Biochim Biophys Acta 2016;1859:1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhou C, King N, Chen KY, Breslow JL. Activation of PXR induces hypercholesterolemia in wild-type and accelerates atherosclerosis in apoE deficient mice. J Lipid Res 2009;50:2004–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].de Haan W, de Vries-van der Weij J, Mol IM, Hoekstra M, Romijn JA, Jukema JW, et al. PXR agonism decreases plasma HDL levels in ApoE3-Leiden.CETP mice. Biochim Biophys Acta 2009;1791:191–197. [DOI] [PubMed] [Google Scholar]
- [15].Zhou J, Zhai Y, Mu Y, Gong H, Uppal H, Toma D, et al. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J Biol Chem 2006;281:15013–15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet 1998;351:1881–1883. [DOI] [PubMed] [Google Scholar]
- [17].Ma X, Shah Y, Cheung C, Guo GL, Feigenbaum L, Krausz KW, et al. The PREgnane X receptor gene-humanized mouse: a model for investigating drug-drug interactions mediated by cytochromes P450 3A. Drug Metab Dispos 2007;35:194–200. [DOI] [PubMed] [Google Scholar]
- [18].Sui Y, Park SH, Helsley RN, Sunkara M, Gonzalez FJ, Morris AJ, et al. Bisphenol A increases atherosclerosis in pregnane X receptor-humanized ApoE deficient mice. Journal of the American Heart Association 2014;3:e000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Macías J, Mancebo M, Merino D, Téllez F, Montes-Ramírez ML, Pulido F, et al. Changes in Liver Steatosis After Switching From Efavirenz to Raltegravir Among Human Immunodeficiency Virus–Infected Patients With Nonalcoholic Fatty Liver Disease. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2017;65:1012–1019. [DOI] [PubMed] [Google Scholar]
- [20].Macias J, Berenguer J, Japon MA, Giron-Gonzalez JA, Rivero A, Lopez-Cortes LF, et al. Hepatic steatosis and steatohepatitis in human immunodeficiency virus/hepatitis C virus-coinfected patients. Hepatology 2012;56:1261–1270. [DOI] [PubMed] [Google Scholar]
- [21].Stoddart CA, Bales CA, Bare JC, Chkhenkeli G, Galkina SA, Kinkade AN, et al. Validation of the SCID-hu Thy/Liv mouse model with four classes of licensed antiretrovirals. PLoS One 2007;2:e655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Varban ML, Rinninger F, Wang N, Fairchild-Huntress V, Dunmore JH, Fang Q, et al. Targeted mutation reveals a central role for SR-BI in hepatic selective uptake of high density lipoprotein cholesterol. Proc Natl Acad Sci USA 1998;95:4619–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zanoni P, Khetarpal SA, Larach DB, Hancock-Cerutti WF, Millar JS, Cuchel M, et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science 2016;351:1166–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sporstol M, Tapia G, Malerod L, Mousavi SA, Berg T. Pregnane X receptor-agonists down-regulate hepatic ATP-binding cassette transporter A1 and scavenger receptor class B type I. Biochem Biophys Res Commun 2005;331:1533–1541. [DOI] [PubMed] [Google Scholar]
- [25].Belter A, Skupinska M, Giel-Pietraszuk M, Grabarkiewicz T, Rychlewski L, Barciszewski J. Squalene monooxygenase - a target for hypercholesterolemic therapy. Biol Chem 2011;392:1053–1075. [DOI] [PubMed] [Google Scholar]
- [26].Gill S, Stevenson J, Kristiana I, Brown AJ. Cholesterol-dependent degradation of squalene monooxygenase, a control point in cholesterol synthesis beyond HMG-CoA reductase. Cell Metab 2011;13:260–273. [DOI] [PubMed] [Google Scholar]
- [27].Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med 2006;354:251–260. [DOI] [PubMed] [Google Scholar]
- [28].Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 2004;292:191–201. [DOI] [PubMed] [Google Scholar]
- [29].Smith KY, Patel P, Fine D, Bellos N, Sloan L, Lackey P, et al. Randomized, double-blind, placebo-matched, multicenter trial of abacavir/lamivudine or tenofovir/emtricitabine with lopinavir/ritonavir for initial HIV treatment. AIDS 2009;23:1547–1556. [DOI] [PubMed] [Google Scholar]
- [30].Moreau A, Teruel C, Beylot M, Albalea V, Tamasi V, Umbdenstock T, et al. A novel pregnane X receptor and S14-mediated lipogenic pathway in human hepatocyte. Hepatology 2009;49:2068–2079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.