Abstract
Serum chemotactic activity is important in regulating neutrophil migration. The ability of neutrophils to migrate to infectious site is crucial for host effective pathogen control, but unregulated neutrophil activation can also cause tissue damage. During bacterial sepsis, the complement alternative pathway (AP) is massively activated in blood and tissues and reportedly contributes to sepsis pathogenesis. Complement factor B (FB) is an essential component of the AP activation. However, the impact of FB/AP activation on blood chemotactic activity during bacterial infection is unclear. In this study, we found that sera of septic mice following cecal ligation and puncture (CLP) had much higher chemotactic activities on neutrophils than those of sham animals. Compared with wild-type (WT) mice, FB−/− mice had significantly attenuated serum chemotactic activity, under both non-septic and septic conditions. Moreover, sera with the activated AP by zymosan and cobra venom factor (CVF) in vitro induced a significant increase in neutrophil migration compared to sera without the AP activation. Complement activation generates complement cleavage fragment such as Ba, C3a and C5a. To delineate the contribution of these downstream effectors, we incubated AP-active sera (AP activated by zymosan/CVF) or sera from sham and septic mice with anti-C5a or mAb1379 (anti-Ba) neutralizing antibody. We found that anti-C5a, but not mAb1379, markedly attenuated the neutrophil chemotactic effect of the AP-activated sera and that of septic sera. Taking together, these data suggest that the complement alternative pathway activation during bacterial sepsis plays a pivotal role in promoting blood chemotactic activity through a C5a-dependent mechanism.
Keywords: Chemotaxis, complement factor B, Cecal ligation and puncture
INTRODUCTION
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection (1). Neutrophils are a major effector cell during the host defense and neutrophil migration plays a critical role for pathogen clearance and host survival during sepsis. For example, it is well documented that neutrophil migration from vascular to infection site in early stage of sepsis is critical for pathogen clearance (2) and the excessive migration to tissues in the late stage of sepsis may be associated with organ injury (3, 4). The neutrophil migration from vessels to extravascular spaces is termed forward migration. Recent studies have shown that neutrophils can also leave sites of tissue infection and migrate back into the circulation, a process termed reverse migration (5). The reverse migration may contribute to systemic inflammation and tissue damage at distal organs (6). In either case, serum chemotactic activity is important for neutrophil migration and potentially impacts on systemic and tissue inflammation.
The host complement system is activated via three pathways during bacterial sepsis: 1) classic, 2) lectin, and 3) alternative pathway (AP) (7), all leading to cleavage and activation of multiple complement factors such as C3a, C5a, and factor B (FB). These complements are an important part of the host defense, but excessive and uncontrolled complement activation is harmful and may contribute to tissue damage and mortality in sepsis. Brandtzaeg, et al. demonstrate that the overwhelming complement activation seen in those with severe sepsis, is due to an uncontrolled AP amplification (8). Targeted deletion of FB, a key component of the AP (9, 10), leads to reduced organ injury and improved survival during bacterial sepsis (10). However, how FB and the AP activation affect serum chemotactic activity during bacterial sepsis is unclear.
In the current study, we tested WT and FB KO serum chemotactic activity ex vivo under sham and septic conditions, as well as the chemotactic ability of WT and FB-deficient neutrophils in vitro. Moreover, we examined the impact of the serum AP activation by zymosan/CVF in vitro on its chemotactic ability and delineated the contribution of the downstream effectors of the AP activation to serum chemotactic activity.
MATERIALS AND METHODS
Animals
C57BL/6J Wild-type (WT) mice were purchased from the Jackson Laboratory and housed in an animal facility at the Massachusetts General Hospital (MGH) for at least one week before experiments. FB−/− mice were constructed as previously described (9) and kindly provided by Xiaobo Wu at Washington University School of Medicine in St. Louis. Mice with an age of 8–12 weeks were used in current study. Both male and female mice were used to isolate neutrophils for in vitro experiments, but only male mice used for in vivo cecal ligation and puncture surgery. All animals were housed in a pathogen-free, temperature-controlled, and air-conditioned facility with 12-h/12-h light/dark cycles and fed with the same bacteria-free diet. All animal care and procedures were performed according to the protocols approved by the Institutional Animal Care and Use Committee at MGH and are in compliance with the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health.
Neutrophil isolation
Mice were euthanized and disinfected. Bone marrow cells were harvested from tibias and femurs and neutrophils were purified by gradient density as described previously (11). In short, cells were washed once and suspended in 3ml cold Dulbecco’s phosphate-buffered saline (DPBS) without Ca2+. Cell suspension was then layered on the top of 3 ml of Histopaque 1077 and 3ml Histopaque 1119 (Sigma-Aldrich, St. Louis, MO, USA) in a 15 ml tube and centrifuged for 30 min at 700 g without break. Neutrophils were collected at the interface of Histopaque 1119 and 1077 layers, washed once with 10ml DPBS and suspended in RPMI-1640 medium supplemented with 0.05% bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO, USA) and 1% penicillin /streptomycin (Invitrogen, Grand Island, NY, USA). The purity of the neutrophils collected using this method was > 90% as determined by flow cytometry using the neutrophil markers Gr-1 and Ly-6G in our previous report (11).
Neutrophil migration assay
High-throughput screening (HTS) 96-Transwell plates (Corning, NY, USA) were used for neutrophil migration assay. Chemoattractant such as recombinant murine keratinocyte chemoattractant (KC) (Peprotech, Rocky Hill, NJ, USA), recombinant murine C5a (R&D Systems Inc., Minneapolis, MN, USA) or AP-active sera were loaded in the bottom reservoir of the well. Neutrophils (1 × 105/well) were added to the top insert transwell with polycarbonate membrane of pore size 3 μm and allowed to migrate in a cell culture incubator (37 °C, 5% CO2) for 3 hours. After assay completed, neutrophils that migrated to the lower compartments were quantified by CyQUANT® Direct Cell Proliferation Assay Kit (Life technologies, Carlsbad, CA, USA), a system based on a cell-permeable DNA-binding dye in combination with a background suppression reagent. In detail, after removal of the upper compartments, the detection reagent was added to cells in the lower compartments and incubated at 37 °C for 30 minutes. Cells were then suspended and spun down at 30 g for 2 minutes to evenly distribute at the bottom of well. Cell-associated fluorescence was then measured at excitation/emission wavelength of 480/535 nm with background correction using SpectraMax M5 Multi-Mode Microplate Reader (Sunnyvale, CA, USA). The fluorescence intensity is linearly dependent on neutrophil numbers ranging between 40 and 100,000 cells using CyQUANT Direct Cell proliferation assay kit. Neutrophil migration percentage was determined as fluorescence intensity of migratory cells in the reservoir divided by fluorescence intensity of total loaded cells in the top insert of a transwell system, which is equal to the ratio of neutrophil numbers.
Mouse model of polymicrobial sepsis
A clinically relevant rodent model of sepsis was created by cecal ligation and puncture (CLP) as described previously (12–14). Briefly, mice were anesthetized with ketamine (100 mg/kg) and xylazine (4 mg/kg). The abdominal cavity was opened in layers. The feces were gently pushed to fill the distal part of the cecum. The cecum was ligated 1.0 cm from the tip. A puncture was made with an 18-gauge needle through the cecum, and a small amount (droplet) of feces was extruded to ensure the patency of the puncture site before returning it back to the abdominal cavity. The sham-operated mice underwent laparotomy but without CLP. The abdominal wall incision was closed in layers. After surgery, pre-warmed (37°C) normal saline (50 ml/kg body weight) was administered subcutaneously. Postoperative pain control was managed with subcutaneous injection of bupivacaine (3 mg/kg) and buprenorphine (0.1 mg/kg). Bupivacaine was given once right after surgery at the incision site and buprenorphine given right after surgery and once more 12 hours later.
Serum Preparation
Sera instead of plasma were used in the current project to avoid the use of anticoagulants such as EDTA, heparin, or citrate, all of which can interfere with in vitro alternative complement activation assay (15, 16). Twenty-four hours after sham or CLP surgery, the blood was collected when the mice were still in deep anesthesia via cardiac puncture without open chest. Then the mice were euthanized by overdose sodium pentobarbital intraperitoneal injection. The harvested whole blood was kept at room temperature for 1 hour to form clots and then centrifuged at 4 °C 1000 g for 10 minutes. The serum was then collected and centrifuged at 4°C 20,000 g for 10 minutes to remove cell pellets. The clean sera were stored at −80 °C for further analysis.
Neutralizing antibody
Mouse monoclonal anti-mouse FB antibody, mAb1379, was developed by Dr. Joshua M. Thurman, the University of Colorado School of Medicine, which has been demonstrated to bind to FB (specifically to Ba fragment) and inhibit the alternative pathway activation in vitro and in vivo (17). The binding of mAb1379 to Ba fragment was also confirmed by immunoprecipitation in current study (see Results). Mouse IgG was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA) for control. Monoclonal rat anti-mouse neutralizing C5a antibody and control rat IgG were purchased from R&D systems (Minneapolis, MN, USA). Neutralizing antibodies or corresponding control IgGs were incubated with the AP activated serum suspension at room temperature for 60 min or 2 hours before loaded in the bottom reservoir for neutrophil migration assay.
AP activation assay in vitro
Zymosan-mediated AP activation
Zymosan-induced AP activation in mouse serum was measured by C3 deposition on zymosan with FITC-labeled antibody to C3 (MP Biomedicals, Solon, OH, USA) as described previously with minor modifications (10, 17–19). Briefly, 1.5 × 107 activated zymosan particles (Sigma-Aldrich, St. Louis, MO, USA) were added into15 μl of serum in the presence of 10 mM EGTA (to block the Classic and Lectin complement pathways) and 5 mM MgCl2. All samples were then brought to 150 μl with RPMI 1640 culture medium supplemented with 0.05% BSA. Assay mixtures were incubated at a 37˚C water bath for 30 min and reactions stopped by placing the tubes on ice. After centrifuge at 4 °C with 1700 g for 10 minutes followed by 20,000 g for 10 minutes, the zymosan particle pellets were measured for C3 deposition with flow cytometry. The particle-free supernatants that contained proteins such as FB, C3, C5 and their cleaved fragments were transferred to a new Eppendorf tube and stored at −80 °C for future neutrophil migration assay.
CVF-mediated AP activation
CVF is a functional analog of C3b and forms a complex with FB in a metal-dependent interaction. Once the complex is activated by factor D, Ba and Bb fragments are generated from the cleavage of FB, an indication of the AP activation. CVF-mediated AP activation was performed as previously described with minor modifications (20). Different doses of CVF (Complement Technology, Tyler, TX, USA) at 0, 2, 10, 50, 100 units/ml serum was added to 3 μl of serum containing 5 mM MgCl2. All samples were then brought up to a final volume of 30 μl with RPMI 1640 culture medium supplemented with 0.05% BSA and then incubated at 37°C water bath for 60 min. Reactions were stopped by placing the tubes on ice and supernatants were subjected to SDS/PAGE.
Immunoprecipitation
To confirm the binding site of mAb1379 to Ba fragment, we performed immunoprecipitation experiment. Control mouse IgG or mAb1379 was added into CVF activated serum suspension containing Ba/Bb fragments and rotated overnight at 4°C. Thirty μl protein G sepharose beads (GE Healthcare Bio-sciences Corp, Piscataway, NJ, USA) at 50% slurry were then added into each sample and rotated 2 hours at 4 °C. After spun down, the supernatant was transferred to a new Eppendorf tube. Immunoprecipitates were boiled in SDS-sample buffer for 10 min to release G sepharose beads. The supernatant and eluted immunoprecipitates were subjected to SDS/PAGE and blotted with antibody as described below.
Western Blot
CVF-active mixtures were fractionated by SDS-PAGE under reducing conditions and blotted with goat anti-human FB antibody (Complement Technology, Tyler, TX, USA) or goat anti-mouse C3 antibody (MP Biomedicals, Santa Ana, CA, USA). HRP-conjugated donkey anti-goat IgG (Sigma-Aldrich, St. Louis, MO, USA) was used as the secondary antibody. Proteins were visualized in a chemiluminescence detection system (Bio-Rad ChemiDOC™ XRS+) using Luminata Western HRP substrate (Millipore, Billerica, MA).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA). The distribution of the continuous variables was expressed as the mean ± SE. All data were analyzed by t-test or two-way ANOVA with Bonferroni post hoc tests for statistical significance unless stated otherwise. The null hypothesis was rejected for P<0.05 with the two-tailed test.
RESULTS
Complement AP activation enhances serum chemotactic activity
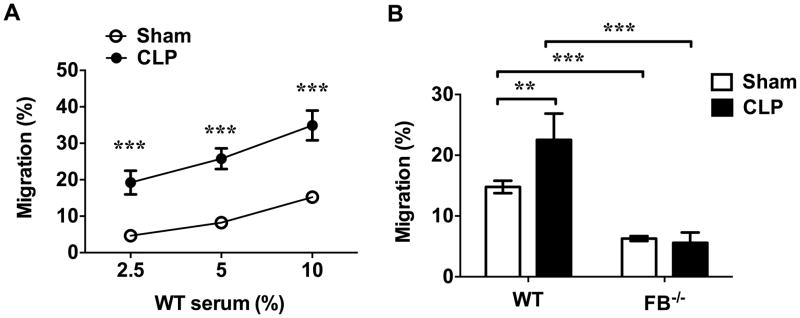
We have previously demonstrated that polymicrobial infection leads to both systemic and local tissue complement AP activation and an increase in the blood complements such as FB, C3 and their cleavage products (10). To test the impact of the AP activation on serum chemotactic activity, we performed neutrophil transwell migration assay. As shown in Fig. 1A, sera from sham mice exhibited a chemotactic effect on neutrophils in a dose-dependent fashion. When the serum concentration in the medium increased from 2.5% to 10%, neutrophil migration increased from 4.7% to 15.2%. In comparison, septic sera, prepared from blood harvested 24 hours after CLP, markedly increased neutrophil migration by 2.3–4.1 fold. To determine the role of the AP activation to the observed serum chemotactic activities, we tested and compared the serum of FB−/− mice with that of WT mice for their neutrophil chemotactic activities. FB is an essential component of the AP and the absence of FB completely abrogated AP activation (9). As noted in Fig. 1B, serum from FB−/− mice had a marked reduction in its chemotactic activity compared with that of WT mice. The serum neutrophil chemotactic activity significantly reduced from 14.8% to 6.3% and from 22.5% to 5.6% in sham and CLP groups, respectively. Importantly, unlike WT mice, FB−/− mice failed to increase their serum neutrophil chemotactic activity in response to polymicrobial infection (Fig. 1B). These ex-vivo data clearly demonstrated an important contribution of serum FB and the AP activation to serum chemotactic activity during polymicrobial sepsis. Of note, the levels of septic serum-induced neutrophil migration varied among different mouse samples in a range between 22.5% (Fig. 1B) to 34.9% (Fig. 1A) at 10% of serum.
Figure 1. AP activation in septic serum enhances neutrophils migration.
Serum was prepared from blood harvested 24 h after sham or CLP surgery. Neutrophil migration was tested using transwell plate. (A) Dose-dependent increase of neutrophil migration in response to different concentrations of serum. n=11. (B) FB deficiency markedly reduces serum chemotactic activity in both sham and CLP mice. Serum concentration: 10%. n= 30 in WT-sham serum group, n=31 in FB−/− -sham serum group, n=7 in WT-CLP serum group, n=7 in FB−/− -CLP serum group), ** P<0.01, *** P<0.001.
Neutrophil FB expression has no impact on its chemotactic ability
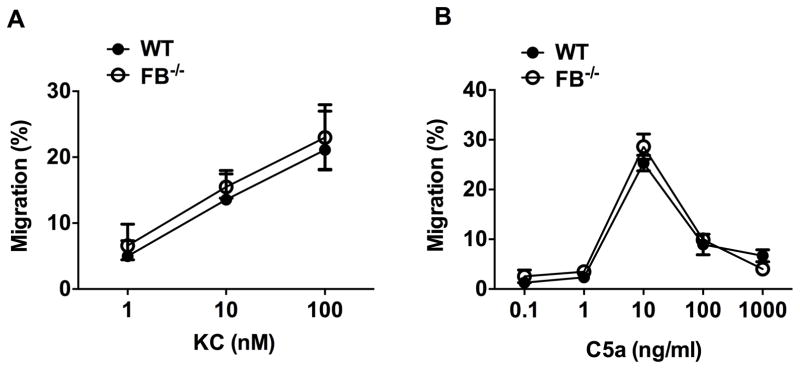
To determine whether or not neutrophil FB expression plays a role in regulating its migratory function, we tested neutrophil migration in response to KC and C5a, two neutrophil chemoattractants. As shown in Fig. 2, WT neutrophils exhibited a dose-dependent migratory response to KC between 1 and 100 nM. Interestingly, the dose-response of neutrophils to C5a was bell-shaped with the peak response at 10 ng/ml (Fig. 2B). In both cases, neutrophils lacking FB exhibited the same dose-response as WT neutrophils (Fig. 2 A–B). These in vitro data suggesting that the neutrophil FB expression play no role in regulating its migratory function in response to these chemoattractants.
Figure 2. FB expression in neutrophil has no impact on its chemotactic capability to KC or C5a.
WT and FB−/− neutrophils were exposed to the different concentrations of KC (A, n=6) or C5a (B, n=3) in a transwell system for 3 hours and neutrophil migration was tested.
Serum AP activation by zymosan enhances neutrophils migration
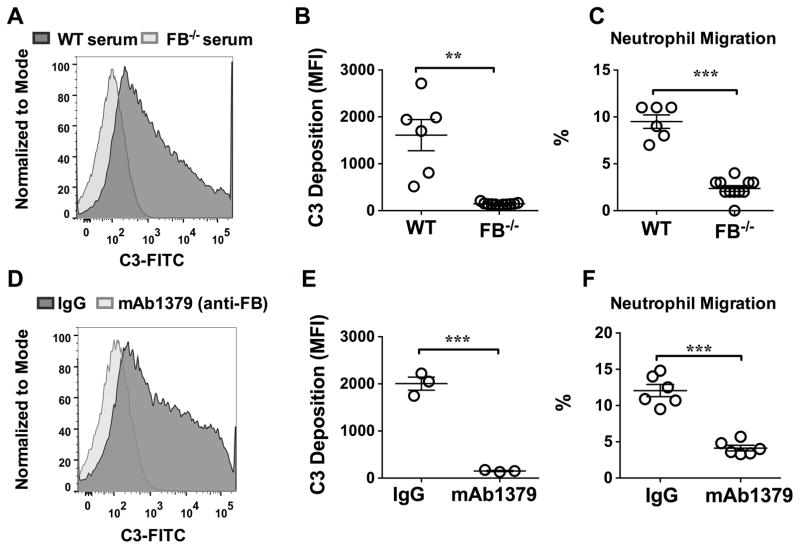
Since the above data has demonstrated the essential role of serum FB/AP activation in serum chemotactic activity during bacterial infection, we wondered if the specific AP activation in vitro could simulate and enhance the serum chemotactic activity on neutrophils. As seen in Fig. 3A–B, in the presence of Mg2+ and EGTA (to block the classic and lectin pathways), zymosan treatment of WT serum led to a robust AP activation as demonstrated by C3 deposition on zymosan. In contrast, zymosan treatment of FB-deficient serum failed to induce the AP activation. Importantly, the inhibition of AP activation by FB deficiency markedly attenuated neutrophil migration (9.5±0.7% vs. 2.4±0.3%, P<0.001) as illustrated in Fig. 3C). Of note, while the presence of EGTA in these experiments could reduce the serum-induced neutrophil migration (data not shown), it should not impact on the result that FB-deficiency reduced zymosan-activated neutrophil migration, as both groups were compared in the presence of EGTA. Moreover, treatment of WT serum with mAb1379, a FB neutralizing antibody, but not control IgG, effectively blocked the zymosan-induced AP activation (2006±139 vs. 152±12, P<0.01) (Fig. 3D–E) and markedly reduced the neutrophil migration capability induced by zymosan-treated serum (12.1±0.8% vs. 4.1±0.4%, P<0.001) (Fig. 3F).
Figure 3. Zymosan-mediated AP activation increases serum chemotactic activity.
Total 15 μl of serum from WT or FB−/− mice was incubated with 1.5×107 zymosan at 37˚C for 30 min in the presence of Mg2+/EGTA. After incubation, zymosan was stained with FITC-labeled anti-C3 and tested for C3 deposition using flow cytometry as detailed in the Materials and Methods. (A) Representative histogram of C3 deposition on zymosan detected by flow cytometry: effect of FB-deficiency on AP activation. (B) Mean fluorescence intensity (MFI) of C3 deposition in zymosan after incubation with WT and FB−/− serum as described above. n= 6–11. (C) Neutrophil migration in response to zymosan-treated serum from WT and FB−/− mice. n=6–11. (D) Representative histogram of C3 deposition on zymosan detected by flow cytometry: effect of anti-FB. Control IgG or mAb1379 (anti-mouse FB antibody) was incubated with WT serum at room temperature for 30 min and then tested for the zymosan-mediated AP activation. (E) Mean fluorescence intensity (MFI) of C3 deposition on zymosan from control IgG and mAb1379 (anti-FB neutralizing antibody)-treated WT serum. n= 3. (F) Effect of mAb1379 (anti-FB antibody) on neutrophil migration in response to zymosan-treated serum. n= 6. ** P<0.01, *** P<0.001.
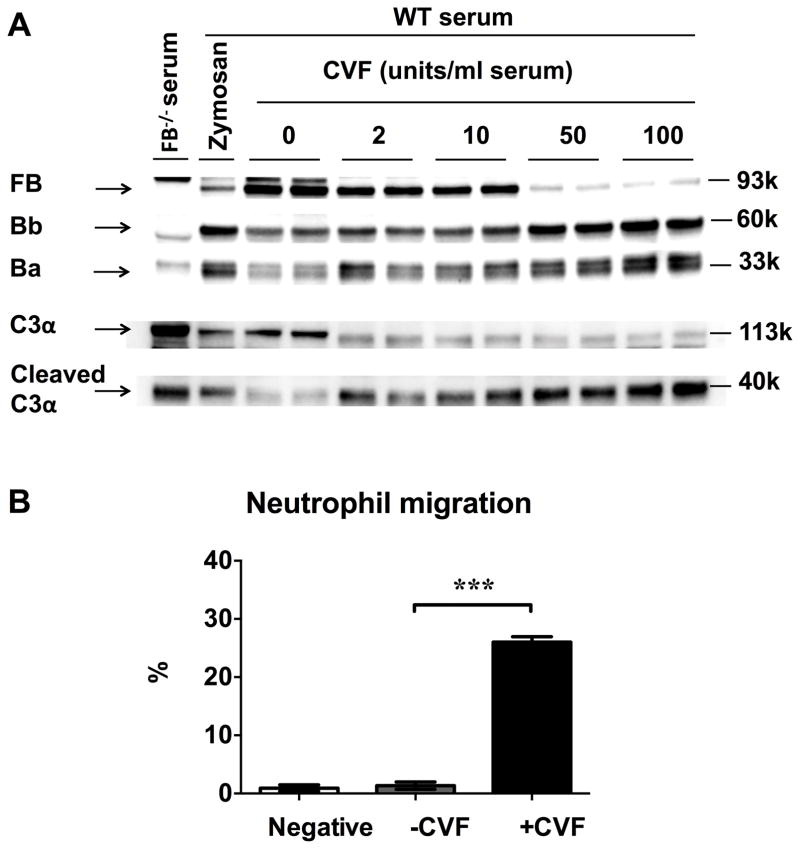
To further confirm the role of the complement AP activation in mediating serum neutrophil chemotactic activity, we tested the serum chemotactic activity in a different model where the AP is activated by cobra venom factor (CVF). CVF is an analog of C3b and binds to FB to activate the complement AP. As shown in Fig. 4A, treatment of serum with CVF (2–100 units/ml serum) led to a dose-dependent cleavage of FB and C3 as demonstrated by increased expression of Ba/Bb and cleaved C3α. Importantly, CVF-treated serum had a marked increase in its chemotactic activity towards neutrophils compared with those of untreated serum (26.0±0.9% vs. 1.4±0.6%, P<0.001) (Fig. 4B). Collectively, these data suggest that the complement AP activation in serum, either induced by zymosan or by CVF, leads to a marked increase in serum chemotactic activity on neutrophils.
Figure 4. CVF-induced AP activation increases serum chemotactic activity.
(A) CVF induces the serum complement AP activation. WT serum was incubated with zymosan (1.5×107) or various concentrations of CVF (0, 2, 10, 50, 100 unit/ml serum) in medium. Serum proteins were separated in 4–12% SDS-PAGE and blotted with anti-FB and anti-C3 antibody. Reduction in FB (93 kDa) and C3 (C3α chain-113kDa) or increase in Ba (33 kDa), Bb (60 kDa) and cleaved C3α (40 kDa) fragments indicate the CVF-induced AP activation. FB−/− serum was served as negative control and zymosan activated WT serum was served as positive control. (B) CVF-activated serum has enhanced chemotactic activity towards neutrophils. 1% WT mice serum was incubated with or without CVF (100 unit/ml serum) at 37˚C for 60 min, and then tested for its chemotactic activity toward neutrophils. n=5–6. *** P<0.001. Negative control is the treatment medium (RMPI 1640 with 0.05% BSA/5mM MgCl2) without serum.
Complements play a key role in the AP-activated serum chemotactic activity
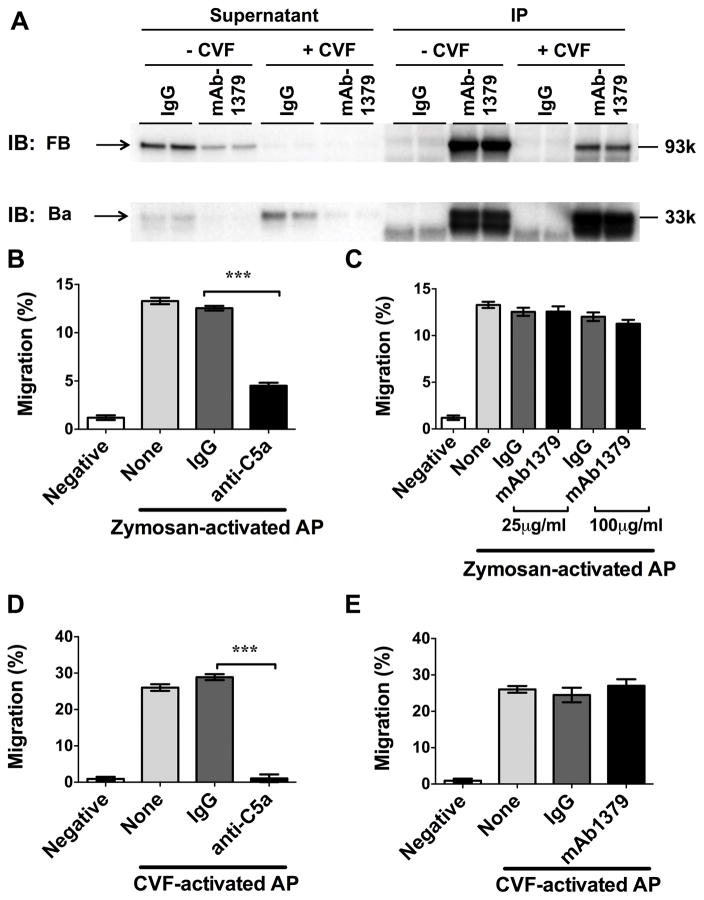
The three complement activation pathways (classic, lectin, alternative) converge on C3 and lead to the cleavage of C3 and C5 and the generation of C3a, C3b, C5a, and C5b. In addition, the AP activation leads to the FB cleavage and Ba generation. Both C5a and Ba reportedly have a chemotactic effect on neutrophils (21, 22). To delineate the contribution of C5a and Ba to the neutrophil chemotactic activity, we tested the impact of anti-C5a and mAb1379 on the AP-activated serum-induced neutrophil migration. First, we confirmed the binding of mAb1379 to Ba fragment as it is reportedly to inhibit the AP activation in sera by binding to an epitope in the SCR3 region of Ba (17). As illustrated in Fig. 5A, serum was treated with CVF to activate the AP and generate the Ba fragment. Immunoprecipitation of the AP-active serum with mAb1379, but not control IgG, was able to pull down the majority of FB and Ba fragments from the supernatant, demonstrating the ability of mAb1379 to bind to FB as well as to Ba fragment.
Figure 5. C5a, but not Ba, plays a key role in neutrophil migration induced by the AP-active serum.
(A) mAb1379, an anti-FB neutralizing antibody, is able to bind to and pull down the Ba fragment. Control IgG or mAb1379 was added into CVF activated serum suspension containing Ba/Bb fragments. The supernatant and immunoprecipitates (IP) were subjected to Western blot. (B–C) Effect of anti-C5a or mAb1379 (anti-Ba) on neutrophil migration in response to zymosan-activated serum. (D–E) Effect of anti-C5a or mAb1379 (anti-Ba) on neutrophil migration in response to CVF-activated serum. n= 4–12. *** P<0.001. Negative control is the treatment medium (RMPI 1640 with 0.05% BSA/5mM MgCl2) without serum. None: serum alone without IgG or antibody.
We next tested contribution of C5a and Ba to the AP activation-mediated serum chemotactic activity using the neutralizing antibodies to C5a and Ba. As shown in Fig. 5B, in the presence of Mg2+ /EGTA, zymosan-treated serum induced much stronger neutrophil chemotactic activity, indicating a contribution of the AP. Anti-C5a treatment of the AP-active serum led to 64% reduction in its chemotactic activity compared with the IgG control (4.5±0.3% vs. 12.5±0.2%, P<0.001). In contrast, mAb1379 at 25 μg/ml and 100 μg /ml failed to impact on the AP-induced neutrophil migration (Fig. 5C). The similar effects were observed in the CVF-activated serum. Anti-C5a completely abolished the neutrophil chemotactic effect of the CVF-treated serum (1.1±0.4% vs. 28.9±0.8%, P<0.001), whereas mAb1379 failed to impact on neutrophil migration induced by CVF-activated sera (Fig. 5D–E).
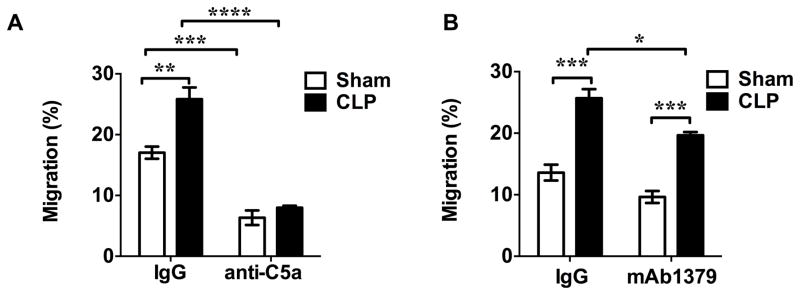
C5a plays a key role in neutrophils migration in sepsis
Next we tested the possible contribution of C5a and Ba in vivo to serum neutrophil chemotactic activity of sham and septic mice. As shown in Fig. 6, while both sham and septic serum possessed chemotactic activity towards neutrophils, septic mice exhibited significantly higher chemotactic activity compared with sham mice. Importantly, treatment with anti-C5a antibody, not the control IgG, led to a completely blockage of this increase in neutrophil chemotactic activities mediated by septic serum compared to that of sham serum (anti-C5a: sham group vs. CLP group, 6.3±1.2% vs. 8.0±0.3%) (Fig. 6A). In contrast, mAb1379 failed to block the septic serum-induced increase in serum chemotactic activity (mAb1379: sham group vs. CLP group, 9.6±0.9 % vs. 19.7±0.5%, P<0.001) (Fig. 6B).
Figure 6. C5a, but not Ba, plays a key role in serum chemotactic effect on neutrophils in sepsis.
10 % WT sham or CLP serum was incubated with corresponding control IgG or anti-C5a antibody (A) or mAb1379 (anti-FB/Ba) (B) at room temperature for 2 h before tested for chemotactic activity. n=3.* P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001.
DISCUSSION
The most notable finding of this study is the importance of FB and the alternative pathway in serum chemotactic activity during bacterial sepsis. The loss of function studies using FB−/− mice show an important contribution of FB and the AP activation to the serum neutrophil chemotactic activity under normal and infectious conditions. Mice lacking FB exhibited markedly reduced serum chemotactic activity than WT mice. Interestingly, there was no difference in neutrophil’s chemotactic ability to KC or C5a between WT and FB−/− neutrophils. These data suggest that the FB expression in neutrophils per se plays no role in its migratory function. On the other hand, reduction in chemokines or other serum chemotactic factors in FB−/− mice during sepsis could have in part explained the attenuated serum chemotactic activity in response to polymicrobial infection. We have shown previously that FB−/− septic mice had decreased plasma level of KC compared with WT septic mice (10). Thus, it is possible that reduced chemokine production, at least KC, in FB−/−, is also responsible for the observed reduction in the serum chemotactic activity.
We demonstrate that the complement AP activation in vitro by means other than bacterial infection can also enhance serum chemotactic activity. In the presence of Mg2+ and EGTA, C3 deposition reflects the AP activation in vitro by zymosan. Both FB genetic deletion and antibody neutralization effectively blocked the AP activation by zymosan and markedly attenuated the neutrophil chemotaxis. In consistent with these data is that CVF, a potent C3b analog and the AP activator, markedly increases serum neutrophil chemotactic activity. The data provide a direct evidence that the complement AP activation plays an important role in regulating serum neutrophil chemotactic activity.
C3a and C5a are the common products of all three complement pathways, whereas Ba is the product of the AP activation. Since C3a is largely inactive or only slightly active as a chemotactic factor (21), we decided to focus on the potential role of C5a and Ba. We found that anti-C5a, but not anti-FB/Ba, neutralizing antibody markedly inhibits the AP (CVF or zymosan)-stimulated neutrophil chemotactic activity in vitro. Anti-C5a also markedly reduced the serum chemotactic activity of both sham and septic mice and completely prevented the increase in chemotactic activity associated with septic serum. These data suggest that C5a, but probably not Ba, plays a key role in the AP activation-induced neutrophil chemotactic activity. Consistent with the current finding, a previous study demonstrates that C5aR mediates neutrophil recruitment in a non-infectious disease (i.e., murine arthritis) and plays an important role in disease progression (23, 24). Also, inhibition of C5a significantly reduced the neutrophil accumulation in the lung in E.coli sepsis model of Baboon (25). The current study provides direct evidence that C5a, which is specifically derived from the AP activation, plays the key role in determining the serum chemotactic activity during bacterial sepsis.
In summary, the current study clearly demonstrates that the complement AP activity plays a pivotal role in maintaining serum neutrophil chemotactic function under both normal and septic conditions. While FB expression in neutrophils is not involved in their chemotactic ability, the robust AP activation and subsequent C5a generation in serum appear to be essential for the increased serum neutrophil chemotactic activity observed in septic animals.
Acknowledgments
Disclosure of Funding Support: This work was supported in part by National Institutes of Health (Bethesda, Maryland) grants GM097259 (WC) and a mentored research award from International Anesthesia Research Society (San Francisco, California) (LZ).
We would like to thank Dr. Joshua M. Thurman from University of Colorado School of Medicine for providing the anti-FB/Ba antibody (mAb1379).
Footnotes
Disclosure of Conflict of Interest: The authors declare no conflict of interest.
Authorship
G.Q.X. designed, performed experiments, analyzed data and wrote the manuscript; Y.F. performed experiments; D.L. performed experiments; Q.C.Z. revised the manuscript; W.C. designed experiments and revised the manuscript. L.Z. designed, performed experiments and wrote the manuscript and all authors revised it.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alves-Filho JC, Spiller F, Cunha FQ. Neutrophil paralysis in sepsis. Shock. 2010;34(Suppl 1):15–21. doi: 10.1097/SHK.0b013e3181e7e61b. [DOI] [PubMed] [Google Scholar]
- 3.Kovach MA, Standiford TJ. The function of neutrophils in sepsis. Current opinion in infectious diseases. 2012;25(3):321–327. doi: 10.1097/QCO.0b013e3283528c9b. [DOI] [PubMed] [Google Scholar]
- 4.Zemans RL, Colgan SP, Downey GP. Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. American journal of respiratory cell and molecular biology. 2009;40(5):519–535. doi: 10.1165/rcmb.2008-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nature reviews. Immunology. 2016;16(6):378–391. doi: 10.1038/nri.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colom B, Bodkin JV, Beyrau M, Woodfin A, Ody C, Rourke C, Chavakis T, Brohi K, Imhof BA, Nourshargh S. Leukotriene B4-Neutrophil Elastase Axis Drives Neutrophil Reverse Transendothelial Cell Migration In Vivo. Immunity. 2015;42(6):1075–1086. doi: 10.1016/j.immuni.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement System Part I - Molecular Mechanisms of Activation and Regulation. Frontiers in immunology. 2015;6:262. doi: 10.3389/fimmu.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandtzaeg P, Hogasen K, Kierulf P, Mollnes TE. The excessive complement activation in fulminant meningococcal septicemia is predominantly caused by alternative pathway activation. J Infect Dis. 1996;173(3):647–655. doi: 10.1093/infdis/173.3.647. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto M, Fukuda W, Circolo A, Goellner J, Strauss-Schoenberger J, Wang X, Fujita S, Hidvegi T, Chaplin DD, Colten HR. Abrogation of the alternative complement pathway by targeted deletion of murine factor B. Proc Natl Acad Sci U S A. 1997;94(16):8720–8725. doi: 10.1073/pnas.94.16.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou L, Feng Y, Li Y, Zhang M, Chen C, Cai J, Gong Y, Wang L, Thurman JM, Wu X, et al. Complement factor B is the downstream effector of TLRs and plays an important role in a mouse model of severe sepsis. J Immunol. 2013;191(11):5625–5635. doi: 10.4049/jimmunol.1301903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Y, Zou L, Si R, Nagasaka Y, Chao W. Bone marrow MyD88 signaling modulates neutrophil function and ischemic myocardial injury. Am J Physiol Cell Physiol. 2010;299(4):C760–769. doi: 10.1152/ajpcell.00155.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou L, Feng Y, Chen YJ, Si R, Shen S, Zhou Q, Ichinose F, Scherrer-Crosbie M, Chao W. Toll-like receptor 2 plays a critical role in cardiac dysfunction during polymicrobial sepsis. Crit Care Med. 2010;38(5):1335–1342. doi: 10.1097/CCM.0b013e3181d99e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou L, Feng Y, Zhang M, Li Y, Chao W. Nonhematopoietic toll-like receptor 2 contributes to neutrophil and cardiac function impairment during polymicrobial sepsis. Shock. 2011;36(4):370–380. doi: 10.1097/SHK.0b013e3182279868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4(1):31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mollnes TE, Garred P, Bergseth G. Effect of time, temperature and anticoagulants on in vitro complement activation: consequences for collection and preservation of samples to be examined for complement activation. Clinical and experimental immunology. 1988;73(3):484–488. [PMC free article] [PubMed] [Google Scholar]
- 16.Kirschfink M, Mollnes TE. Modern complement analysis. Clinical and diagnostic laboratory immunology. 2003;10(6):982–989. doi: 10.1128/CDLI.10.6.982-989.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thurman JM, Kraus DM, Girardi G, Hourcade D, Kang HJ, Royer PA, Mitchell LM, Giclas PC, Salmon J, Gilkeson G, et al. A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol Immunol. 2005;42(1):87–97. doi: 10.1016/j.molimm.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 18.Leinhase I, Rozanski M, Harhausen D, Thurman JM, Schmidt OI, Hossini AM, Taha ME, Rittirsch D, Ward PA, Holers VM, et al. Inhibition of the alternative complement activation pathway in traumatic brain injury by a monoclonal anti-factor B antibody: a randomized placebo-controlled study in mice. J Neuroinflammation. 2007;4:13. doi: 10.1186/1742-2094-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quigg RJ, Kozono Y, Berthiaume D, Lim A, Salant DJ, Weinfeld A, Griffin P, Kremmer E, Holers VM. Blockade of antibody-induced glomerulonephritis with Crry-Ig, a soluble murine complement inhibitor. J Immunol. 1998;160(9):4553–4560. [PubMed] [Google Scholar]
- 20.Edens RE, Linhardt RJ, Bell CS, Weiler JM. Heparin and derivatized heparin inhibit zymosan and cobra venom factor activation of complement in serum. Immunopharmacology. 1994;27(2):145–153. doi: 10.1016/0162-3109(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez HN, Henson PM, Otani A, Hugli TE. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978;120(1):109–115. [PubMed] [Google Scholar]
- 22.Hamuro J, Hadding U, Bitter-Suermann D. Fragments Ba and Bb derived from guinea pig factor B of the properdin system: purification, characterization, and biologic activities. J Immunol. 1978;120(2):438–444. [PubMed] [Google Scholar]
- 23.Sadik CD, Kim ND, Iwakura Y, Luster AD. Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and FcgammaR signaling. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(46):E3177–3185. doi: 10.1073/pnas.1213797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu R, Lin F, Bao C, Huang H, Ji C, Wang S, Jin L, Sun L, Li K, Zhang Z, et al. Complement 5a receptor-mediated neutrophil dysfunction is associated with a poor outcome in sepsis. Cellular & molecular immunology. 2016;13(1):103–109. doi: 10.1038/cmi.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keshari RS, Silasi R, Popescu NI, Patel MM, Chaaban H, Lupu C, Coggeshall KM, Mollnes TE, DeMarco SJ, Lupu F. Inhibition of complement C5 protects against organ failure and reduces mortality in a baboon model of Escherichia coli sepsis. Proceedings of the National Academy of Sciences of the United States of America. 2017 doi: 10.1073/pnas.1706818114. [DOI] [PMC free article] [PubMed] [Google Scholar]