Abstract
Introduction
Human Immunodeficiency Virus (HIV) infection is associated with hypochlorhydria but the mechanism is unknown. The objective of this study was to determine effects of anti-retroviral therapy (ART) on gastric physiology as measured by validated markers.
Methods
We studied HIV infected individuals who were either ART-naïve or on treatment with undetectable viral loads. We measured H.pylori IgG antibodies, pepsinogen (PG) 1 and 2 levels and fasting gastrin-17 using Biohit GastroPanel®. Gastric antral biopsies and juice were obtained for histology and pH respectively. Also included were historical data from HIV negative participants (n = 72) in a previous study, for reference.
Results
We enrolled 84 HIV positive individuals with a median age 42 years (IQR 37-40 years). 55(66%) were female, 32(38%) were ART naïve, and 52(62%) were on ART. Hypochlorhydria (pH>4) was present in 48(57%) of the HIV positive and 18(25%) of the HIV negative individuals (OR 4: 95% CI 1.9-8.5, P<0.001) with no significant effect of ART (OR 0.9: 95% CI 0.3-2.3, P = 0.82). Hypochlorhydria was not associated with the serological detection of corpus atrophy using low PG 1:2 ratio (OR 2.1: 95% CI 0.5-10.2, P = 0.37) or GastroPanel® algorithm, (OR 0.7: 95% CI 0.01-60.1, P = 1.0). ART reduced the frequency of low PG 1:2 ratio (P = 0.001), but not the histological detection in the antrum of atrophy or non-atrophic gastritis.
Conclusion
ART use is associated with reduced serological evidence of corpus atrophy but has no effect on fasting pH, supporting earlier data that suggest that the mechanism of HIV-associated hypochlorhydria is multifactorial.
Keywords: HIV, intestinal metaplasia, hypochlorhydria, gastric atrophy, pepsinogen
Introduction
Sub-Saharan Africa bears over two-thirds of the global Human Immunodeficiency Virus (HIV) burden. The prevalence of HIV in Zambia is 12.9% with 63% of these individuals being on antiretroviral therapy (ART) [1]. The use of ART has resulted in HIV infected individuals living longer and healthier lives and the classical opportunistic infections becoming less common [2]. The profile of health conditions in general and in HIV infection is beginning to change with a notable shift towards non-communicable diseases [2]. The gut plays an important role in HIV infection and it has been established that gut-associated lymphoid tissue continues to harbour HIV even when there is complete serological viral load suppression [3, 4]. Hypochlorhydria (gastric pH greater than 4) is twice as common in HIV infection probably due to the presence of the virus [5, 6]. Hypochlorhydria is typically a consequence of the loss of gastric parietal cell mass resulting from atrophy, a known risk factor for gastric cancer. There is no evidence to date of parietal cell loss in HIV infection [5]. In addition, the long-term consequences of persistent hypochlorhydria in HIV infection, including gastric cancer are unknown [7]. It is however known that hypochlorhydria predisposes to intestinal infections [5], micronutrient deficiencies [8] and other health outcomes. Over 15 years ago ART was rolled out to the general public in sub-Saharan Africa. Many HIV infected persons have benefited from this therapy, whose influence on gastric physiology remains uninvestigated. For the evaluation of gastric histology, endoscopy with biopsies is the gold standard but it is invasive and costly [9]. The option of serological assessment, "serological biopsy2 is therefore attractive. The GastroPanel® (Biohit Helsinki, Finland) is a non-invasive diagnostic test for gastric pathology including four-biomarkers, pepsinogen 1 (PG 1), pepsinogen 2 (PG 2), Helicobacter pylori IgG (H.pylori IgG) antibodies and Gastrin-17. The utility of the GastroPanel® may prove to be a useful tool in detection of atrophic gastritis and gastric cancer risk assessment [10, 11]. However, some studies have questioned its usefulness [12, 13]. The aim of this study was to compare these markers of gastric physiology in HIV infected ART-naïve individuals and those who have taken treatment long enough to completely suppress HIV in blood. In addition, we included data from HIV negative individuals as a point of reference. To our knowledge, this tool has not been applied to the problem of hypochlorhydria in HIV infected individuals.
Methods
Participant enrollment: We enrolled participants from the University Teaching Hospital (UTH) in Lusaka, Zambia between October 2014 and July 2015. This was a case control study which included asymptomatic HIV infected individuals who were either ART-naïve as cases or on ART with full viral suppression ("ART-treated") as controls. Only individuals above the age of 18 years and who had given written consent were included in the study. Excluded were those with evidence of ART failure, use of gastric acid suppressing medication, presence of active gastrointestinal disease or significant co-morbidities such as chronic liver or kidney disease. For comparison, all proven HIV negative community volunteers from a previous study were included [14]. The university of Zambia biomedical research ethics committee (Ref no. 004-04-14) and the national health research authority approved this study.
Upper gastrointestinal endoscopy: Using a Pentax EG-2990i instrument, we conducted upper gastrointestinal endoscopies on HIV positive participants after an overnight fast. A full assessment of the upper gastrointestinal tract was carried out first according to a standard protocol. After flushing the biopsy channel, gastric juice was aspirated for pH determination, using pH paper test strips (Sigma Chemical Company St Louis, USA), which measure pH to the nearest 0.5. Hypochlorhydria was defined as gastric pH>4.0. Four gastric biopsies were obtained from the gastric antrum (three of which were from the region of the incisura) and immediately placed in formalin and sent to the histopathology laboratory for analysis. The biopsies were stained with hematoxylin and eosin and Giemsa special staining for H.pylori. The pathologist (AS) characterized gastric histology by considering infiltration of the lamina propria by acute or chronic inflammatory cells to diagnose active or chronic non-atrophic gastritis (NAG). The presence of gastritis with increased distance between the individual glands (glandular loss) and the condensation of reticulin fibers in the lamina propria was used to diagnose chronic atrophic gastritis (CAG) [15]. The complete Operative Link on Gastritis Assessment (OLGA) could not be employed as no corpus biopsies were obtained.
Laboratory analysis: Using the Biohit GastroPanel® ELISA kits, (Biohit Helsinki, Finland), serum samples were analysed for pepsinogens 1 and 2, gastrin-17 and H.pylori IgG antibodies. A PG 1:2 ratio of less than 3.0 was considered low, indicative of gastric body atrophy. To designate a result as low PG 1 or gastrin-17, we used the cut-offs 30 μg/L and 1 μg/L respectively. Samples with enzyme immunounits greater than or equal to 30 were reported to be positive for the presence of H.pylori IgG antibodies. Using the GastroPanel® algorithm [13] and GastroSoft® analysis panel available online (Gastrosoft®) [16], we were able to designate each participant as having corpus or antral atrophy, multifocal atrophy, non-atrophic gastritis or normal mucosa. The GastroPanel® algorithm utilizes results for H.pylori, PG 1 and gastrin-17, while in the Gastrosoft®, PG 2 is included as well. To measure the HIV viral load in plasma, we used the COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, version 2 (Roche Molecular Systems, Branchburg, USA).
Statistical analysis and sample size calculation: We used previously published data on HIV positive patients with hypochlorhydria. In that study, 25% of the HIV negative individuals had hypochlorhydria while the proportion among those with HIV infection (with a high CD4) count was 50%, giving an odds ratio of 4 (P<0.001) [5]. Using these proportions at a power of 80%, 66 patients would be needed in each group. In included in this study were 84 HIV positive and 72 HIV negative individuals. Statistical analysis was done in STATA 13 (College Station, TX, USA). Medians and interquartile ranges were obtained for continuous variables while proportions were employed for categorical variables. The Fisher's exact test was used to compare binary variables and presented as odds ratios with 95% confidence intervals. For the comparison of continuous variables, Kruskal-Wallis test and the Spearman rank's correlation were used. In all instances, a two-sided P value of <0.05 was considered statistically significant.
Results
We enrolled 84 clinically asymptomatic HIV positive participants. 32(38%) were ART-naïve and 52(62%) had been on ART for 2-14 years (median 10 years). The median age was 43 years (IQR 37-40 years); 66% were female. All individuals in the ART-treated group had undetectable viral loads and significantly higher CD4 counts than the ART-naïve group (medians 522 cells/μL and 297 cells/μL respectively, P=0.001). Our comparison group included 72 clinically asymptomatic HIV negative participants from a previous study (Table 1).
Table 1.
Demographic and biological characteristics of the three groups: HIV positive and ART naive, HIV positive and ART-treated, and HIV negative
| HIV positive, ART naïve n=32 | HIV positive, ART treated n=52 | HIV negative n=72 | P | |
|---|---|---|---|---|
| Age in years, median (IQR) | 36(30-44) | 45(40-52) | 41(30-53) | 0.001 |
| Female, n(%) | 20(63) | 35(67) | 39(54) | 0.35 |
| BMI, (median IQR) | 22(20-23) | 23(21-27) | 21(20-25) | 0.08 |
| Gastric pH, Median (IQR) | 4.8(2.0-6.0) | 5.5(2.0-6.0) | 1.5(1.0-4.4) | <0.001 |
| Hypochlorhydria, n(%) | 19(59) | 29(56) | 18(25) | <0.001 |
| PG 1, Median (IQR) | 73.1(55.8-110) | 69.7(45.2-104.1) | 80.7(53.2-107.3) | 0.15 |
| *Low PG 1, μg/L, n(%) | 3(10) | 4(8) | 2(3) | 0.21 |
| PG 2, μg/L Median (IQR) | 14.7(9.8-38.6) | 12.7(6.9-20.7) | 14.0(9.9-22.0) | 0.25 |
| PG 1:2 ratio, Median (IQR) | 4.3(2.2-9.1) | 5.4(4.2-6.9) | 5.9(4.3-7.7) | 0.36 |
| **Low PG 1:2 ratio, n(%) | 10(37) | 4(7.6) | 6(8) | 0.001 |
| Gastrin-17 μg/L, Median (IQR) | 10.4(4.9-14.4) | 6.0(3.6-14.7) | *** | 0.08 |
| Low gastrin-17, n(%) | 1(3) | 5(10) | *** | 0.40 |
| H.pylori IgG antibody EIU, Median (IQR) | 61.4(38.2-86) | 76.4(38.2-99.1) | *** | 0.12 |
| H.pylori positive | 26(81) | 41(79) | *** | 0.10 |
Using 30 μg/L as the cut-off
Using 3.0 as the cut-off
In the previous study, gastrin-17 and H.pylori IgG antibodies were not measured
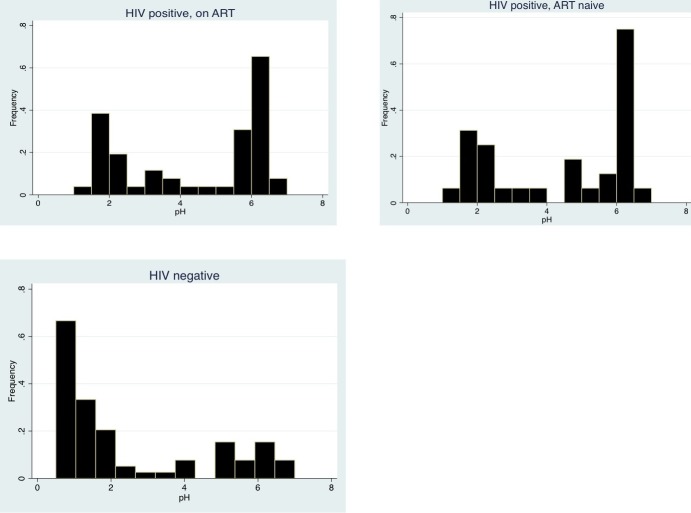
Fasting gastric pH: Gastric pH was bimodally distributed in all three groups, with two peaks of frequency between 1 to 3 and 5 to 6 (Figure 1). Hypochlorhydria was present in 48(57%) of the HIV positive and 18(25%) of the HIV negative individuals (OR 4.0: 95% CI 1.9-8.5, P<0.001) with no significant difference between the ART-naïve and ART-treated groups (OR 0.9: 95% CI 0.3-2.3, P = 0.82). The median age among those with hypochlorhydria was significantly higher, 44(36-53) years versus 40(30-49) years, P = 0.02. Hypochlorhydria was associated with the presence of H.pylori IgG antibodies in the ART-treated group (OR 8.6: 95% CI 1.4-89.3, P = 0.007) but not in the ART-naïve group (OR 1.6: 95% CI 0.2-14.2, P = 0.67). In addition, there was no link between hypochlorhydria and BMI (P = 0.13) or CD4 count (P = 0.50) (data not shown in tables).
Figure 1.
Gastric pH observed in three groups; HIV positive and ART naive, HIV positive and ART-treated and HIV negative
Pepsinogen 1:2 ratio: The median values of PG 1, PG 2 and PG 1/PG 2 ratio was not significantly different in the three groups (Table 1). However, 37% of ART-naïve group had a low PG 1:2 ratio while in both the ART-treated and HIV negative groups the proportion was around 8% (Table 1). Using an unsupervised stepwise logistic regression, we found that being HIV positive increased the odds of having a low PG 1:2 ratio. However, individuals who were female, on ART and less than 40 years had significantly lower odds of having a low PG 1:2 ratio (Table 2).
Table 2.
Factors associated with pepsinogen 1:2 ratio of less than 3.0; data for all
| Low PG 1:2 ratio, n=20 | Normal PG 1:2 ratio, n=131 | Univariable | Multivariable, n=125 | |||
|---|---|---|---|---|---|---|
| OR(95% CI) | P | OR(95% CI) | P | |||
| Female | 8(40) | 85(65) | 0.4(0.1-1.0) | 0.047 | 0.15(0.03-0.65) | 0.01 |
| HIV positive | 14(70) | 65(50) | 2.1(0.8-8.0) | 0.099 | 24(4.0-154) | 0.001 |
| On ART | 4(20) | 48(37) | 0.4(0.1-1.5) | 0.207 | 0.02(0.0-0.24) | 0.001 |
| Age less than 40 years | 7(35) | 61(47) | 0.6(0.2-1.8) | 0.470 | 0.1(0.02-0.73) | 0.02 |
the three groups included, n=151
GastroPanel® algorithm: Analysis using the GastroPanel® algorithm indicated that 44(52%) of HIV positive individuals had NAG. Antral atrophy was detected in 19(23%), corpus atrophy in 2(2%) and multifocal atrophy in 4(5%). There was no significant difference in the occurrence of these lesions in the ART-naïve and treated groups. The proportion of NAG on histology was 72(95%) with that of antral atrophy being 6(8%). All individuals with NAG detected using the GastroPanel® also had it on histology, with a positive predictive value (PPV) of 100%, sensitivity of 71% and 100% specificity. The PPV for GastroPanel® detection of antral atrophy was 26%, with a sensitivity of 50% and a specificity of 80%.
GastroSoft® online software: Using this alternative online software, 50(80%) of all the HIV positive individuals were positive for H.pylori infection, 2(3%) had antral atrophy and 10(13%) had corpus atrophy. Similarly, there was no difference between the ART-naïve and treated groups (data not shown). The PPV of this test for antral atrophy was 75%, with a sensitivity of 25% and a specificity of 97%.
Serological levels of pepsinogens in comparison with histological diagnoses of the antrum in HIV infection: individuals with histological diagnosis of antral atrophy were significantly older (median 55, IQR 52-59 years) than those without (median 41, IQR 37-49 years; P=0.009). HIV positive individuals with non-atrophic gastritis had increased levels of both PG 1 and 2. However, those with atrophic gastritis reduced levels, although the difference was not statistically significant (Table 3).
Table 3.
Serological levels of pepsinogens in individuals with atrophic gastritis, non-atrophic gastritis and H.pylori infection diagnosed on histology
| Histological diagnosis | PG 1(μg/L) Median (IQR) | PG 2(μg/L) Median (IQR) | PG 1:2 ratio Median (IQR) | Fasting gastrin-17 Median (IQR) | |
|---|---|---|---|---|---|
| Atrophic gastritis | Present | 44.9(37.2-114) | 8.6(4.5-10.7) | 5.8(3.6-7.5) | 16.9(5.9-22.1) |
| Absent | 73.1(50.1-102) | 14.3(8.5-22.5) | 5.5(4.0-7.3) | 6.6(4.1-13.6) | |
| P value | 0.23 | 0.24 | 0.84 | 0.11 | |
| Non-atrophic gastritis | Present | 74.6(50.9-110) | 14.7(9.1-21.9) | 5.3(3.6-7.0) | 7(4.3-14.7) |
| Absent | 39.7(30.4-43.6) | 5.3(3.9-6.3) | 8.4(5.5-10.3) | 4.5(1.9-11.8) | |
| P value | 0.008 | 0.007 | 0.19 | 0.34 | |
| H.pylori seen | Present | 86.4(60.3-114.2) | 17.1(8.7-21.3) | 5.4(4.6-6.6) | 7.4(4.7-15.2) |
| Absent | 43.5(30.2-59.5) | 6.2(4.5-9.8) | 7.8(6.1-9.3) | 2.9(0.9-10.8) | |
| P value | 0.02 | 0.48 | 0.42 | 0.02 |
Discussion
In this study, we have shown that HIV-associated hypochlorhydria [5, 17] is similar between ART-naïve and ART-treated individuals but not any of the serological indicators of corpus atrophy [18]. In contrast, ART reduced the occurrence of low PG 1:2 ratio, a reported serological indicator of gastric corpus atrophy. This apparently paradoxical finding suggests that hypochlorhydria in HIV infection is not a consequence of gastric atrophy. The pathogenesis of hypochlorhydria in HIV infection remains obscure. In 1988, Lake-Bakaar et al. suggested that it could be due to the presence of parietal cell autoantibodies [17]. Their conclusions were however based on a very small number of patients and there has been no further studies supporting this theory. Another explanation could be that persistent gastric inflammation drives the low acid production despite the lower prevalence of H.pylori in HIV infection [19]. Our results showed an association between hypochlorhydria and H.pylori in the ART-treated group but not the ART-naïve group. This could be a suggestion of H.pylori re-colonization with ART use without any effect on hypochlorhydria. Quantification of parietal cell mass with direct measure of maximal gastric acid output in HIV infection is one possible approach to understanding HIV associated hypochlorhydria. Transcriptomic or proteomic analysis of gastric mucosal tissue may also be useful. Histological detection of gastric premalignant lesions such as atrophy is not without sampling error. To increase sensitivity, we obtained four antral biopsies using ordinary white light endoscopy. We were able to establish that the occurrence of gastric atrophy was not affected by ART use. We then evaluated the influence of ART on PG 1, PG 2 and gastrin-17 compared to histological diagnoses. PG 1 is produced in the corpus and its levels are reduced in corpus atrophy. When the corpus is inflamed without glandular loss, levels of PG 1 in blood increase [20]. PG 2 is produced in the corpus, antrum and duodenum. Similar to PG 1 its levels will increase in non-atrophic gastritis [20]. In this study, we report consistent trends showing a significant increase in both PG 1 and 2 with non-atrophic gastritis and a decrease of both markers in atrophy. Similarly, Kitamura et al concluded that serum pepsinogen analysis could be used to predict the presence of H.pylori gastritis [21].
However, on histology only antral atrophy was analysed. We were therefore not surprised that that the decrease of pepsinogens in antral atrophy was insignificant. The third serological marker evaluated in this study was fasting gastrin-17. The G cells of the antrum produce basal gastrin-17 [20]. It is expected that levels of gastrin-17 would decrease when the antral mucosa is atrophic. Paradoxically our results did not show any association between antral atrophy and gastric-17. In addition, we did not find any effect of HIV disease progression on the levels of fasting gastrin-17 (which would be indicative of antral inflammation) as determined by some other investigators. The levels of serum pepsinogens were similar regardless of the presence of H.pylori on histology. Histology is specific for H.pylori yet its sensitivity is hampered by the patchy colonization of the stomach [22]. The median age of participants in the ART-naïve group was significantly lower than in the ART-treated or the HIV negative groups. As the incidence of gastric atrophy increases with advancing age, we would expect the occurrence of low PG 1:2 ratio to be less in the ART-naïve group. However, our results showed a significantly higher proportion of low PG 1:2 ratio in the younger ART-naïve group, an effect that seem to be reversed on ART. We therefore concluded that HIV infection could be directly or indirectly influencing the production of pepsinogens, through a mechanism similar to that causing hypochlorhydria. Several investigators have published reports supporting the use of "serological biopsy" to evaluate gastric physiology, but this has not yet been examined in HIV infection. It is clear that HIV is associated with physiological changes and therefore any such investigative strategies would warrant specific validation. We therefore explored the utility of the GastroPanel® kit for detection of gastric atrophy in HIV infection. To analyse our data, we used both the algorithm for GastroPanel and the online software GastroSoft®. Results from both analyses were very similar, with both showing no significant difference between the ART-naïve and ART-treated groups. All the individuals designated as having NAG using the GastroPanel® also had it on histology, giving the test a very good specificity. However, the sensitivity was much lower as the GastroPanel® missed some cases of NAG. From our findings, the GastroPanel® would not be helpful for detecting antral atrophy in this population. We were unable to fully assess its usefulness for corpus atrophy, as there were no histology results available. This is the first study to examine the influence of ART on gastric physiology. The notable limitation of this study was the lack of gastric corpus biopsies. Secondly, measuring the maximal gastric acid output as done by Welage et al. would have allowed us to better understand hypochlorhydria in HIV infection [23]. Conversely, this study draws its strength from the fact that the analysis was done in healthy HIV infected volunteers with HIV negative controls.
Conclusion
We have shown that ART does not reverse HIV associated hypochlorhydria. The mechanism of low acid output in HIV infection is yet to be established.
What is known about this topic
HIV infection is associated with hypochlorhydria;
Anti-retroviral therapy can suppress the viral load to undetectable levels;
Use of the GastroPanel® has not been validated in HIV infection.
What this study adds
Anti-retroviral therapy does not reverse HIV associated hypochlorhydria;
The occurrence of gastric atrophy is not affected by anti-retroviral therapy;
Anti-retroviral therapy reduces the occurrence of low pepsinogen 1:2 ratio.
Competing interests
The authors declare no competing interests.
Acknowledgments
We would like to acknowledge endoscopy nurses Themba Banda, Rose Soko and Joyce Sibwani for their assistance rendered during all the endoscopic procedures.
Authors’ contributions
Violet Kayamba and Paul Kelly designed the study, with later contributions from Douglas Corbett Heimburger and Douglas Morgan. Patient enrolment was done by Violet Kayamba. Histopathological analysis of the biopsies was done by Aaron Shibemba while Kanekwa Zyambo was responsible for sample storage and running of ELISAs. All authors contributed toward manuscript preparation.
References
- 1.Global information and education on HIV and AIDS . HIV and AIDS in Zambia. Accessed on 15thOctober 2016. [Google Scholar]
- 2.GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tee-Wook Chun, David Nickle, Jesse Justement, Jennifer Meyers, Gregg Roby, Claire Hallahan, Shyam Kottilil, Susan Moir, JoAnn Mican, James Mulins, Douglas Ward, Joseph Kovacs, Peter Mannon, Anthony Fauci. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis. 2008;197(5):714–20. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 4.Jason Micheal Brenchley, Daniel Douek. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1(1):23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul Kelly, Tamar Shawa, Styner Mwanamakondo, Rose Soko, Goeffrey, Robin Barclay, Ian Ralph Sanderson. Gastric and intestinal barrier impairment in tropical enteropathy and HIV: limited impact of micronutrient supplementation during a randomised controlled trial. BMC Gastroenterol. 2010;10:72. doi: 10.1186/1471-230X-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joe Geraghty, Alexander Thumbs, Anstead Kankwatira, Tim Andrews, Andrew Moore, Rose Malamba, Neema Mtunthama, Kai Hellberg, Lughano Kalongolera, Paul Toole, Andrea Varro, Mark Pritchard, Melita Gordon. Helicobacter pylori, HIV and Gastric Hypochlorhydria in the Malawian Population. PLoS One. 2015 Aug 5;10(8):e0132043. doi: 10.1371/journal.pone.0132043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Violet Kayamba, Akwi Wasi Asombang, Victor Mudenda, Mpala Mwanza Lisulo, Edford Sinkala, Styner Mwanamakondo, Isaac Mweemba, Paul Kelly. Gastric adenocarcinoma in Zambia: a case-control study of HIV, lifestyle risk factors and biomarkers of pathogenesis. S Afr Med J. 2013;103(4):255–9. doi: 10.7196/SAMJ.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrea Betesh, Carol Santa Ann, Jason Cole, John Fordtran. Is achlorhydria a cause of iron deficiency anemia. Am J Clin Nutr. 2015;102(1):9–19. doi: 10.3945/ajcn.114.097394. [DOI] [PubMed] [Google Scholar]
- 9.Atsushi Tashiro, Masatoshi Sano, Koichi Kinameri, Kazutaka Fujita, Yutaka Takeuchi. Comparing mass screening techniques for gastric cancer in Japan. World J Gastroenterol. 2006;12(30):4873–4. doi: 10.3748/wjg.v12.i30.4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucio Lombardo, Rosalia Leto, Giancarlo Molinaro, Marco Migliardi, Nicoletta Ravarino, Rodolfo Rocca, Bruno Torchio. Prevalence of atrophic gastritis in dyspeptic patients in Piedmont: a survey using the GastroPanel test. Clin Chem Lab Med. 2010;48(9):1327–32. doi: 10.1515/CCLM.2010.256. [DOI] [PubMed] [Google Scholar]
- 11.Svetlana Kurilovich, Anna Belkovets, Oleg Reshetnikov, Tatyana Openko, Sofja Malyutina, Ylija Ragino, Lilija Scherbakova, Marcis Leja, Lea Paloheimo, Kari Syrjänen, Mikhail Voevoda. Stomach-specific Biomarkers (GastroPanel) Can Predict the Development of Gastric Cancer in a Caucasian Population: a Longitudinal Nested Case-Control Study in Siberia. Anticancer Res. 2016;36(1):247–53. [PubMed] [Google Scholar]
- 12.Shigeto Mizuno, Masao Kobayashi, Shohken Tomita, Ikuya Miki, Atsuhiro Masuda, Mitsuko Onoyama, Yasuki Habu, Hideto Inokuchi, Yoshiyuki Watanabe. Validation of the PG test method for gastric cancer screening using a follow-up study. Gastric Cancer. 2009;12(3):158–63. doi: 10.1007/s10120-009-0522-y. [DOI] [PubMed] [Google Scholar]
- 13.Adrian McNicholl, Montserrat Forné, Jesus Barrio, Cristobal De la Coba, Begoña González, Robin Rivera, Maria Esteve, Fernando Fernandez-Bañares, Beatriz Madrigal, Beatriz Gras-Miralles, Angeles Perez-Aisa, Jose Viver-Pi-Sunyer, Felipe Bory, Merce Rosinach, Carmen Loras, Carlos Esteban, Santos Santolaria, Fernando Gomollon, Julio Valle, Javier Gisbert. Helicobacter pylori Study Group of Asociación Española de Gastroenterología (AEG): Accuracy of GastroPanel for the diagnosis of atrophic gastritis. Eur J Gastroenterol Hepatol. 2014;26(9):941–8. doi: 10.1097/MEG.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Violet Kayamba, Mumba Chomba, Paul Kelly. Short-term micronutrient-antioxidant supplementation has no impact on a serological marker of gastric atrophy in Zambian adults: retrospective analysis of a randomised controlled trial. BMC Gastroenterol. 2014;14:52. doi: 10.1186/1471-230X-14-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richard Hunt, Micheal Camilleri, Sheila Crowe, Emad El-Omar, James Fox, Enerst Kuipers, Peter Malfertheiner, Kenneth McColl, Mark Pritchard, Massimo Rugge, Amnon Sonnenberg, Kokichi Sugano, Jan Tack. The stomach in health and disease. Gut. 2015;64(10):1650–68. doi: 10.1136/gutjnl-2014-307595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GastroPanel . GastroPanel report (GastroSoft) Accessed 15thOctober 2016. [Google Scholar]
- 17.Gerond Lake-Bakaar, Elizabeth Quadros, Sary Beidas, Magdy Elsakr, Winston Tom, Donald Wilson, Hosoon Dincsoy, Paul Cohen, Eugene Straus. Gastric secretory failure in patients with the acquired immunodeficiency syndrome (AIDS) Ann Intern Med. 1988;109(6):502–4. doi: 10.7326/0003-4819-109-6-502. [DOI] [PubMed] [Google Scholar]
- 18.Peter Malfertheiner, Francis Megraud, Colm O'Morain, Javier Gisbert, Enerst Kuipers, Anthony Axon, Franco Bazzoli, Antonio Gasbarrini, John Atherton, David Graham, Richard Hunt, Paul Moayyedi, Theodore Rokkas, Massimo Rugge, Micheal Selgrad, Sebastian Suerbaum, Kokichi Sugano, Emad El-Omar. European Helicobacter and Microbiota Study Group and Consensus panel: management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2016;66(1):6–30. [Google Scholar]
- 19.Daniel Nevin, Christopher Morgan, David Graham, Robert Genta. Helicobacter pylori gastritis in HIV-infected patients: a review. Helicobacter. 2014;19(5):323–9. doi: 10.1111/hel.12131. [DOI] [PubMed] [Google Scholar]
- 20.Alexander Loor, Dan Dumitrascu DL. Helicobacter pylori Infection, Gastric Cancer and Gastropanel. Rom J Intern Med. 2016;54(3):151–156. doi: 10.1515/rjim-2016-0025. [DOI] [PubMed] [Google Scholar]
- 21.Yoko Kitamura, Masaharu Yoshihara, Masanori Ito, Tomoyuki Boda, Taiji Matsuo, Takahiro Kotachi, Shinji Tanaka, Kazuaki Chayama. Diagnosis of Helicobacter pylori-induced gastritis by serum pepsinogen levels. J Gastroenterol Hepatol. 2015;30(10):1473–7. doi: 10.1111/jgh.12987. [DOI] [PubMed] [Google Scholar]
- 22.Saurabh Kumar Patel, Chandra Bhan Pratap, Ashok Kumar Jain, Anil Kumar Gulati, Gopal Nath. Diagnosis of Helicobacter pylori: what should be the gold standard. World J Gastroenterol. 2014;20(36):12847–59. doi: 10.3748/wjg.v20.i36.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welage LS, Carver PL, Revankar S, Pierson C, Kauffman CA. Alterations in gastric acidity in patients infected with human immunodeficiency virus. Clin Infect Dis. 1995;21(6):1431–8. doi: 10.1093/clinids/21.6.1431. [DOI] [PubMed] [Google Scholar]