Abstract
Evidence in support of links between type-2 diabetes mellitus (T2DM) and Alzheimer’s disease (AD) has increased considerably in recent years. AD pathological hallmarks include the accumulation of extracellular amyloid-β (Aβ) and intracellular hyperphosphorylated tau in the brain, which are hypothesized to promote inflammation, oxidative stress, and neuronal loss. T2DM exhibits many AD pathological features, including reduced brain insulin uptake, lipid dysregulation, inflammation, oxidative stress, and depression; T2DM has also been shown to increase AD risk, and with increasing age, the prevalence of both conditions increases. In addition, amylin deposition in the pancreas is more common in AD than in normal aging, and although there is no significant increase in cerebral Aβ deposition in T2DM, the extent of Aβ accumulation in AD correlates with T2DM duration. Given these similarities and correlations, there may be common underlying mechanism(s) that predispose to both T2DM and AD. In other studies, an age-related gradual loss of testosterone and an increase in testosterone resistance has been shown in men; low testosterone levels can also occur in women. In this review, we focus on the evidence for low testosterone levels contributing to an increased risk of T2DM and AD, and the potential of testosterone treatment in reducing this risk in both men and women. However, such testosterone treatment may need to be long-term, and would need regular monitoring to maintain testosterone at physiological levels. It is possible that a combination of testosterone therapy together with a healthy lifestyle approach, including improved diet and exercise, may significantly reduce AD risk.
Keywords: Alzheimer’s disease, men, testosterone, type-2 diabetes, women
INTRODUCTION
The prevalence of type 2 diabetes (T2DM) and Alzheimer’s disease (AD) both increase with age. There is also an age-related gradual loss of testosterone levels in men and women, together with increasing testosterone resistance due to factors such as rising sex hormone-binding globulin (SHBG) levels and hypothalamic-pituitary-gonadal axis insensitivity. Mounting evidence, originally from epidemiological studies and now also from pre-clinical and clinical studies, indicates that people with T2DM are at increased risk of developing AD and that hallmarks of T2DM such as insulin resistance and hyperinsulinemia can lead to cognitive impairment [1]. A recent animal study that crossed two well-established mouse models of T2DM and AD showed that diabetes exacerbates memory impairment [2]. T2DM and AD also have similar pathological features of amyloid accumulation, indicating common pathogenic mechanisms; in the brain in AD (amyloid-β (Aβ) peptide) and in islets of the pancreas in T2DM (islet amyloid peptide, or amylin). Other similarities include the fact that they can both be degraded by the same protease, insulin degrading enzyme (IDE), the fact that they can both bind to the amylin receptor, and the common β-sheet secondary structures of these two amyloid proteins [3]. Aβ has no established physiological functions, yet amylin, which is co-secreted with insulin from the pancreas, can readily cross the blood-brain barrier and mediate improvements in glucose metabolism and reduce inflammation. Furthermore, amylin and its analogs have been shown to reduce Aβ pathology in an AD animal model [4], and the deposition of amylin in the pancreas has been found to be more common in AD compared with healthy controls. Despite no significant increase in Aβ amyloid in the brains of T2DM individuals versus controls, for those with amyloid plaques, the extent of plaque accumulation correlates with the duration of T2DM [5]. Furthermore, impairments in insulin signaling, glucose metabolism, lipid metabolism, and inflammation have all been observed in both conditions [6]. Given these similarities, there may be common underlying mechanism(s) via testosterone that predispose susceptible individuals to T2DM and AD. In this review, we focus on the age-related changes in circulating testosterone levels in men and women and discuss how these changes may increase the risk of both T2DM and AD. We also evaluate the potential of testosterone therapy as a modifier of both conditions.
TESTOSTERONE AND DIABETES
Total testosterone circulating in the plasma comprises ~2% free testosterone, ~20–40% albumin- bound testosterone, and ~60–80% SHBG-bound testosterone [7]. The levels of total and free testosterone are significantly lower in men with T2DM [8]. Mounting evidence from animal models, epidemiological studies and human clinical trials have indicated a strong association between low testosterone levels, which result from the age-related gradual decline in testosterone levels and insulin resistance, one of the hallmarks of T2DM [8, 9]. Testosterone levels in men appear to influence glycemic status, and to be inversely proportional to the risk of having T2DM [10]. It has been shown that men with T2DM and low testosterone can benefit significantly from testosterone treatment [11–13]. Furthermore, testosterone therapy for the treatment of androgen deficiency has been shown to reduce features of T2DM such as insulin resistance, adiposity, inflammation, and hypercholesterolemia while improving glucose levels [14, 15]. In contrast, a reduction in testosterone levels due to androgen deprivation therapy [16] in the treatment of prostate cancer magnifies the incidence and prevalence of T2DM by increasing blood insulin levels and lowering insulin sensitivity and glycemic control [17–20]. Moreover, ADT has been associated with increased insulin resistance [21, 22]. In summary, the above findings indicate that low testosterone increases the prevalence of insulin resistance and T2DM in men.
In contrast to men, increased testosterone levels (excess testosterone levels) induces insulin resistance in pre-menopausal women [23], thus increasing the risk of T2DM [10]. This is exemplified by excessive testosterone in women with polycystic ovary syndrome, which is associated with insulin resistance, predisposing these women to higher risk of T2DM [24]. However, in healthy women, testosterone administration is associated with increased insulin resistance [25]. Compared to men, women generally tend to have all the factors that would be predicted to promote insulin resistance such as having more adipose mass, abrupt loss of estrogen and persistent lower testosterone levels post-menopause, while having only two thirds the skeletal muscle mass of men. Some studies report that women are equally as sensitive to insulin as men [25, 26], whereas one study found women to be more insulin sensitive than men; however this study was done in a young, healthy population (average age of 34 years) in which the levels of sex hormones would have fluctuated according to the reproductive cycle, thus the contribution of sex hormones toward insulin sensitivity cannot be determined from these latter studies [25, 26].
There is no doubt that the actions of sex hormones (testosterone and estrogen) on metabolic homeostasis are implicated in diabetes both in males and females (extensively reviewed in [27]). Interestingly, the sex difference in the prevalence of diabetes is reversed based on the stage of reproductive life, that is, there are more males with T2DM before puberty whereas more females with T2DM post-menopause, based on a survey of global diabetic populations [28]. Furthermore, there are conflicting reports about the effects of hormone replacement therapy on insulin sensitivity. For instance, estrogen replacement therapy has been reported to have no effect on insulin sensitivity in post-menopausal women [29, 30], but it increased insulin sensitivity when administered in post-menopausal women with diabetes [31]. In addition, the association of sex hormones (testosterone and estrogen) with insulin resistance differs depending on the estrogen status [32].
Testosterone replacement therapy in postmenopausal women has shown the potential to increase insulin sensitivity through indirect effects on lipid metabolism and body fat distribution (reviewed in [33]). Furthermore, a small but significant reduction in insulin resistance was observed in post-menopausal women treated with the testosterone replacement therapy, as well as with a combination of testosterone and estrogen treatment, but not with only estrogen treatment [34]. Despite the potential value of the metabolic effects of testosterone treatment in women, research is significantly lacking in this area.
The literature on animal studies (mostly rodent) that addresses sex hormone effects on activity has demonstrated that female rodents are not only more active than male rodents [35], but also protected against high fat diet induced metabolic syndrome [36]. For example, the prevalence of T2DM has been found to be significantly higher in male compared to female transgenic mice overexpressing human amylin; however, in cell culture studies aimed at determining whether the hormones testosterone and estrogen themselves influenced the amylin-induced cytotoxicity, it was found that the sex steroids were not able to reduce the cytotoxicity caused by amylin in any of the three cell culture models tested [37]. In other studies, amylin deficiency was shown to modify bone development during growth, and this effect was found to be gender-dependent [38], suggesting that sex steroid levels (testosterone in particular) can influence amylin metabolism. In summary, further investigation is required to elucidate the influence of sex hormones on glucose metabolism and insulin resistance, and to clarify to what extent risk factors and treatment guidelines should be gender-specific. There is a need for both animal studies and human clinical trials to be designed specifically to evaluate gender differences when determining efficacy and outcomes of the available treatments.
TESTOSTERONE AND AD
Modest changes in testosterone levels can be detected in many men during mid-life, and yet with age, testosterone levels decrease further, and the incidence of such changes also increases with advancing age. Furthermore, it should be noted that decreases in testosterone levels are even more significant in men who are overweight or obese, conditions which are linked to development of diabetes, and which result in turn in a higher risk of AD. The gradual decline in testosterone levels begins in early adult life in both men and women. This decline occurs many decades before the accumulation of cerebral Aβ begins. Therefore, testosterone replacement therapy aimed at restoring normal physiological levels of testosterone in both men and women should be considered as an important prevention strategy for T2DM and AD. Furthermore, relatively low dose testosterone treatment close to age and gender appropriate physiological testosterone levels should be sufficient, obviating the need for higher testosterone doses and thus avoiding the associated side-effects.
Experiments using numerous animal and cellular models have demonstrated a variety of neuroprotective effects of testosterone, which include improving cognitive performance and synaptic plasticity [39, 40], improving synapse density on hippocampal dendritic spines [41–43], maintaining hippocampal function during aging [39, 44], improving cerebral blood flow and glucose metabolism in specific brain regions [45], reducing the aggregation of Aβ and its associated neurotoxicity [46, 47], and reducing tau hyperphosphorylation [47]. We were the first to report that low testosterone levels in elderly men are associated with increased plasma levels of Aβ [48], the peptide that aggregates and deposits in the brain as amyloid in AD.
Low testosterone levels have been linked to a reduction in cognitive skills [49]. In both rodents and humans, the depletion of testosterone has been shown to reduce cognitive performance, and it has been found that this effect can be reversed by testosterone supplementation [50]. Research from several groups including our own have shown that men with low testosterone in their brain and blood are at increased risk of AD [51, 52]. Similarly, low testosterone levels have been shown to precede the cognitive impairment and neuropathological outcomes of AD [53, 54], suggesting that low testosterone may partially be a cause rather than a consequence of AD. Recently, a large retrospective cohort study which utilized data from hospital medical records indicated that long term androgen deprivation treatment [16] for prostate cancer is associated with an increased risk of AD, and that this increased risk correlated with the duration of ADT [55]. This risk of AD may also be influenced by apolipoprotein E (APOE) ε4 allele status, the strongest genetic risk factor for AD, to our knowledge this has not yet been examined, and it should be incorporated in future analyses [56].
Testosterone treatment has been trialed as an approach to prevent cognitive decline and the development of AD, and although clinical trials have shown some promise in improving cognition in men with low testosterone [57], clinical efficacy has shown mixed outcomes [58]. However, it is important to bear in mind that there is a paucity of data on the relationship between testosterone and AD in older men, such as whether it is reduced total or free (bioavailable) testosterone that might predispose men to AD. Based on two recent meta-analyses and a review of the significance of low testosterone as a risk factor for AD, it is clear that this concept needs further research. The review, which is similar to ours, looked at mechanisms that link AD and T2DM and highlighted the evidence that age-related endocrine changes, particularly low testosterone levels, precede the cognitive and neuropathological changes seen in AD, and increase the risk of both T2DM and AD [59]. One meta-analysis that supports the concept that testosterone levels influence the risk of AD takes into account all forms of testosterone [60], whereas another meta-analysis opposes it: this review only takes into account total testosterone levels, yet also considers estrogen and SHBG [61], and comes to the conclusion that SHBG levels are higher in AD, thus influencing bioavailable testosterone. This leads back to the same concept that bioavailable testosterone is the key factor influencing AD risk, though indicating SHBG levels are also relevant and should be measured in future studies. Thus, as proof of concept, testosterone trials need to not only utilize an accurate testosterone (free and total) measurement assay by mass-spectrometry, but also require correction for SHBG levels as SHBG increases with aging and the majority of testosterone is SHBG-bound. As mentioned above, consideration of the APOE genotype is also of the utmost importance.
The prevalence of AD is significantly higher in women compared to men (almost two-thirds of people with AD are women), which cannot be attributed simply to the higher longevity of women compared to men. There must be other factors, biological explanations, that account for this gender difference; factors that may include genetics and in particular, differences in sex hormones [62].
Unlike andropause in men, testosterone levels are largely unaffected by natural menopause in women. However, circulating levels of testosterone produced by the ovaries and the adrenal glands decline gradually with age starting from late reproductive years [63, 64]; a decline which persists during and after menopause [65]. This may result in decreased libido, diminished well-being, and lowered mood [65]. Despite the fact that men produce more testosterone than women, testosterone is actually the most abundant sex steroid in women, circulating in the micro and nanomolar range, compared with a picomolar concentration range of estrogen [66]. Estrogen is made from testosterone and other adrenal hormones in women, thus women can experience disease-related symptoms due to lack of testosterone. However, gender-specific clinical studies in AD research have not been a major focus, including assessments of gender-specific effects of testosterone replacement therapy in men and women. The role of testosterone is much more clearly defined in men than it is in women, and the diagnosis of testosterone deficiency in women at present is lacking because there are no defined levels of testosterone below which a woman can be diagnosed as androgen deficient [67]. Despite the fact that low doses of testosterone have been widely used in women for the symptomatic treatment of sexual dysfunction and premenstrual syndrome, clinical trials addressing the effect on cognition are still lacking. Testosterone administration in women with low testosterone levels has resulted in small but statistically significant improvements on verbal learning and memory [68], yet other studies found neither an improvement or worsening of cognitive performance [69, 70], However, most of these studies were done over a short term (≤26 weeks) thus it is not known whether the effects of testosterone replacement on cognition are long-lasting; and furthermore, little is known about the long term safety of testosterone use in women.
MECHANISMS PROPOSED TO LINK T2DM AND AD: TESTOSTERONE TREATMENT AS A MODIFIER
Cell culture and animal model studies indicate several mechanisms may potentially link T2DM and AD. Impairments in insulin signaling, inadequate Aβ clearance by IDE, neprilysin (NEP), and ApoE, disrupted glucose metabolism, inflammation, and altered lipid metabolism are all thought to contribute to both T2DM and AD. The possible role of testosterone in mediating changes to these potential mechanisms will be discussed in this section.
Impaired insulin receptor signaling and testosterone action
Insulin resistance is characterized by the inability to respond to insulin, which results in reduced downstream signaling following insulin stimulation, and eventually an increase in production of insulin (hyperinsulinemia) by the islet beta cells of the pancreas, in compensation. The insulin receptors [34] are tyrosine kinases that facilitate several signaling pathways, including glucose transport and are expressed not only in the peripheral insulin-sensitive organs such as the liver and muscles, but also in the central nervous system (CNS), such as in the hippocampus and cortex [71]. Brain insulin, however, is thought to originate mostly from endogenous production [72], and the high levels of insulin in the brain are reported to be independent of peripheral insulin, since circulating plasma insulin levels do not influence the levels in the brain [73,74]. In the brain, insulin binds to IRs, then auto-phosphorylation occurs on multiple substrates including insulin receptor substrate-1 (IRS-1) and IRS-2. Phosphorylated IRS activates downstream signaling pathways linked to phosphatidylinositol 3-kinase (PI3K), which is important for synaptic plasticity, learning, and memory [75]. The activation of PI3K converts membrane phospholipid phosphatidylinositol 4,5-biphosphate (PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3), leading to the activation of Akt which phosphorylates glycogen synthase kinase (GSK)3β [76]. It has been known for a long time that GSK3β regulates tau phosphorylation in AD [77], and recent evidence has shown that the enzyme similarly regulates tau phosphorylation in the T2DM brain, thereby leading to neurofibrillary tangle formation [78].
There is evidence that impairments in insulin signaling and function in the brain are linked to AD. However, the underlying mechanisms are not clear. Cell culture and animal model studies have demonstrated that insulin plays a role in Aβ and tau metabolism. For example, insulin has been shown to affect both the production and degradation of Aβ in neurons as well as tau hyperphosphorylation [79]. Furthermore, IR knockout mice exhibit a significant reduction in both Akt and GSK3p phosphorylation, which in turn can lead to the hyperphosphorylation of tau. However, there were no changes observed in memory or glucose metabolism in these mice, suggesting that either there are other mechanisms involved, or increased tau phosphorylation per se is not sufficient to explain the link between T2DM and AD [80]. Other links between insulin signaling dysfunction and AD involve vascular remodeling, at least partly via increased RAGE (receptor for advanced glycation endproducts) expression, which increases reactive oxygen species levels and vascular inflammation [81].
Testosterone levels have also been linked to insulin signaling and downstream events; for example, cell culture and animal experimental data have shown that testosterone depletion via castration reduces IR expression and increases IRS-1 serine phosphorylation [82]. Conversely, testosterone treatment has been shown to increase the expression of IR in both liver and skeletal muscle, but not adipose tissue; the three major insulin responsive target organs which also express androgen receptors (AR) [83–85]. It is important to remember that peripheral testosterone can also reach the brain via the blood brain barrier using a receptor-mediated transport system [86, 87], and it has been shown that testosterone treatment prevents the heat-shock induced over-activation of GSK3β and subsequently diminishes tau hyperphosphorylation in female rats [88, 89], which may be effected via aromatization of testosterone to estrogen [47]. In other studies, male mice bred with pancreatic cells lacking AR (βARKO) fed a high fat diet have been shown to secrete less insulin and to develop glucose intolerance [90]. Further work by this same group, demonstrated that testosterone enhanced glucose-stimulated insulin secretion via AR in human pancreatic β cells and male mouse islet, an effect that is eliminated in βARKO(–/y) islets as well as human islets treated with an AR antagonist, suggesting that AR is a novel receptor that enhances pancreatic β cells function. The mechanism of this testosterone activated AR-enhancing insulin secretion is through the interaction between extranuclear AR and the glucagon-like peptide 1, which in turn increases islet cAMP and activates protein kinase A [90].
Clinical trials have shown that anti-androgen treatment decreases insulin resistance in women with excessive testosterone [91, 92]. However, most studies investigating the effect of testosterone on peripheral insulin sensitivity have been done in an excessive testosterone (hyperandrogenic) setting, and concerned with reducing testosterone levels. Studies of testosterone administration that investigate peripheral insulin sensitivity have been restricted to men, and there are no clinical trials examining brain insulin sensitivity to date. Nevertheless, these findings concerning testosterone’s potential effects on insulin signaling pathways support the concept that testosterone therapy may be valuable in the prevention and treatment of T2DM and AD.
Impaired Aβ clearance via insulin degrading enzyme and testosterone
Cell culture and animal model studies have demonstrated that insulin and related proteins affect both the production and degradation of Aβ [93, 94]. For example, there are several Aβ degrading enzymes, one of which is IDE. This enzyme plays a major role in the degradation of both insulin and Aβ [95], yet IDE has a preferential affinity for insulin such that it can inhibit IDE-mediated degradation of Aβ. This is likely to be one of the reasons that higher levels of Aβ have been found in the brains of diabetic patients and animals, in a brain region-dependent manner [96–99]. Furthermore, IDE defects are linked to the development of T2DM [100] and IDE has been identified as a T2DM and AD susceptibility gene [101].
Testosterone levels are likely to influence Aβ degradation, as testosterone has been shown to be an endogenous regulator of several Aβ-degrading enzymes, including IDE and NEP. For example, the new generation selective androgen receptor modulators (SARMs) exhibit potential therapeutic value by reducing Aβ levels in the brain via increased levels and activity of IDE in AD animal models [102]. While SARMs are efficacious in the treatment of osteoporosis and sarcopenia, arecent study found that one SARM—NEP28—increased the activity of NEP which may reduce brain Aβ levels [103]. Similarly, other studies have shown that testosterone strongly elevates neuronal expression of NEP through an AR-dependent mechanism which in turn decreases Aβ, demonstrated in both castrated rats treated with DHT, and APP23 mice crossed with aromatase knockout mice [104, 105]. Furthermore, testosterone supplementation has been shown to elevate IDE levels in hyperinsulinemic castrated rats [106]. The upregulation of IDE and NEP may also have a beneficial effect on the levels, toxicity or fibrillization of amylin [107, 108]. Thus, the effect of testosterone in modulating IDE and NEP expression and activity in both T2DM and AD may improve these conditions.
Impaired glucose metabolism and testosterone
Defective insulin secretion or action results in reduced insulin-stimulated glucose uptake. This eventually leads to persistently elevated blood glucose levels, thereby predisposing to serious complications that contribute to AD pathology [109,110]. There is now also accumulating evidence that testosterone has a significant direct effect in modulating glucose metabolism [111]. For example, many studies have demonstrated that testosterone may improve glucose metabolism by modification of the glucose transporter GLUT4 and increased expression of insulin receptor protein (reviewed in [112]). Testosterone also mediates sex-specific activation of the AR in the brain, skeletal muscle, liver, adipose tissue, and pancreatic islet β-cells for maintenance in glucose homeostasis (reviewed in [27]).
However, recent studies have also shown that glucose uptake and GLUT4 translocation is stimulated by testosterone via liver kinase B1/AMP-activated protein kinase (LKB1/AMPK) signaling in adipocytes, a mechanism that is independent of AR [113]. AMPK plays a key role in glucose metabolism and is a master regulator of cellular energy homeostasis for gonadal function as well as the biogenesis of GLUT4 and mitochondria in adipose tissue, skeletal muscle, and liver [114, 115]. It is activated by LKB1 and in response to metabolic stresses, exercise, sex hormones, and insulin sensitizing agents such as Metformin [116–118]. In T2DM, dysregulation of AMPK has been observed in both animals and humans and AMPK activation improves glucose uptake [119]. AMPK activation has also been shown to decrease mTOR signaling activity which facilitates autophagy and promotes lysosomal degradation of Ap. However, LKB1/AMPK signaling can also lead to greater Aβ generation and tau phosphorylation [116]. Thus the potential of AMPK activation in AD in reducing development of AD is still unclear, as both beneficial and detrimental effects have been observed [116]. Nevertheless, the LKB1/AMPK pathway, and the influence of testosterone on this pathway, are worth investigating further as a potential treatment avenue for T2DM and AD.
Testosterone deprivation has been shown to increase blood glucose levels, an effect accompanied by the inhibition of Akt phosphorylation, GLUT4 protein expression, and glucose uptake. The inhibition of these parameters was found to be reversed following testosterone treatment [83]. In young men with idiopathic hypogonadotropic hypogonadism, acute testosterone withdrawal reduced insulin sensitivity in the blood [120]. Long-term effects may also occur, however, as there is also much evidence that insulin sensitivity is indirectly influenced by testosterone via its effects on body composition (reviewed in [121, 122]). Interestingly, a reciprocal relationship also exists in which acute and chronic hyperglycemia can lower testosterone levels, suggesting that low testosterone is a consequence of impaired glucose metabolism [123, 124]. Thus, a bi-directional relationship exists between testosterone and glucose control, at least mediated in part by increased visceral fat [125]. This suggests that the normalization of testosterone levels, either by testosterone treatment or a weight loss approach (by diet, exercise, or surgery) has therapeutic potential in the prevention and treatment of T2DM and thus possibly also AD.
Studies of the effects of testosterone supplementation in hypogonadal men on glucose homeostasis have so far produced mixed results [126]; though interestingly, positive outcomes of testosterone replacement therapy on glucose uptake have been measured by FDG-PET scans in elderly patients with AD [127]. Testosterone replacement in hypogonadal men with T2DM improved glycemic control in some studies [13, 14] but no improvements were found in other studies [15, 128, 129]. Thereasons for these discrepancies were in part related to the cut-off value to measure insulin resistance, HOMA-IR1 or 2, indicating that participants need to be selected to have insulin resistance at baseline to be able to assess the effect of testosterone on this parameter. Another likely reason for discrepancies in the results is the difference in duration of these studies; those of long term duration (~6 years) resulted in improvements in insulin resistance, whereas shorter term studies (≤ 1 year) resulted in no improvements [130]. Consideration should also have been given to the forms of testosterone supplementation used in these studies; a recently published retrospective cohort study reported significant variation in metabolic outcomes that were dependent on the administration method [131]. In accordance, only one (testosterone implant) had significantly beneficial effects on glucose levels after 12 months, despite almost unanimously beneficial effects on lipid profile.
Both rodent and human data has shown that there are gender-specific differences in peripheral glucose metabolism; that is, healthy females have higher glucose effectiveness compared to males [132]. In the CNS, similar gender differences exist in the hypothalamic circuitry controlling brain glucose homeostasis [133]. These differences are clearly attributed to the sex hormones. However, once low levels of testosterone manifest due to aging, the resulting impairment in glucose metabolism does not differ significantly between men and women. Thus, it is believed that testosterone therapy can improve glucose metabolism in elderly men and women [45, 127, 134], despite findings from randomized clinical trials so far being inconsistent [125].
Impaired lipid homeostasis and testosterone
Another feature that links T2DM and AD is lipid dysregulation both in the brain and circulation. Commonly seen in both conditions are hypercholesterolemia and perturbations of the high-density lipoprotein (HDL) and low-density lipoprotein (LDL) ratio, as well as elevated very low density lipoproteins (VLDL), triglycerides, and free fatty acids [135–137]. These factors may contribute to the development of insulin resistance and cardiovascular disease, but also AD-related pathology [138, 139]. Testosterone depletion has been shown to induce dyslipidemia in men [140, 141], while studies of testosterone replacement demonstrate favorable outcomes on lipid parameters such as decreasing total and LDL cholesterol, and reductions in triglyceride levels, although not all studies report changes in LDL levels [131, 142]. Conversely, testosterone replacement also appears to reduce levels of HDL cholesterol which could be deleterious due to increased cardiovascular disease risk. However, testosterone replacement does not lower levels below the normal range (if at all), when testosterone levels are maintained at a eugonadal level [131]. In addition, differences in the length of the CAG repeat polymorphism in the AR gene which is known to influence AR sensitivity, may mediate the effect on HDL cholesterol or other parameters [141]. Dietary and lifestyle factors could also be relevant. Despite the mixed findings, most studies indicate that testosterone elicits beneficial effects on blood lipid profiles.
In humans, gender-specific differences are associated with distinct body fat distribution and energy substrate utilization patterns; that is women store more lipids and have higher whole-body insulin sensitivity than men, whereas men tend to oxidize lipids more than women. These conditions are influenced by the menstrual phase in females, and by nutritional status and exercise intensity in both genders (reviewed in [132]). In contrast to the vast amount of evidence regarding the effect of estrogen on lipid metabolism, randomized clinical trials, investigating testosterone therapy-induced changes to lipid parameters in women with low testosterone, are still lacking. One study showed that a low dose of testosterone caused a modest increase in plasma HDL and VLDL levels [143]. However, a meta-analysis concerning peri- and post-menopausal women found that adding testosterone to hormone therapy caused a reduction in HDL [144]. There is a need for more randomized clinical trials of testosterone therapy in both men and women to elucidate gender-specific differences that may occur in lipid homeostasis.
Impaired Aβ clearance via ApoE and testosterone
Apolipoprotein E (ApoE) is another important agent involved in Aβ clearance. The APOE ε4 allele is the strongest genetic risk factor for AD, and interestingly, the ApoE ε4 protein is less efficient than ApoE ε2 and ApoE ε3 in promoting Aβ clearance [145]. Furthermore, differences in lipidation status between the ApoE isoforms influence the ability of ApoE to promote Ap degradation, and the lipidation of ApoE is influenced by its lipid transporter ABCA1, whereby higher lipidation increases the clearance of Aβ [146]. It has also been found that ApoE in APOE ε4 carriers has a lower level of lipidation, an effect that is worsened by a high fat diet [147], and possession of APOE ε4 alleles has been reported to be associated with T2DM [148] whereas APOE ε2 confers a moderate risk for T2DM [149]. ApoE facilitates Aβ proteolysis by IDE, and another APOE characteristic that provides a link to both AD and T2DM is its negative correlation with IDE expression and activity in the brain; for example, it has been shown that AD patients have lower hippocampal IDE levels if carrying an APOE ε4 allele [97] and similarly, APOE ε4 mice which are known to have increased Aβ deposition exhibit lower IDE levels [150, 151]. Clinical trials have shown that AD patients exhibit impaired insulin metabolism, as shown by elevated plasma insulin levels, lower CSF insulin levels, and a reduced CSF to plasma ratio when compared to healthy aged controls; however, in APOE ε4 allele homozygotes, plasma insulin levels and the CSF- to-plasma insulin ratios have been found to be in the normal range, indicating metabolic differences in APOE ε4 allele carriers [152, 153]. Other studies have shown that APOE ε4 allele carriers with AD are less likely to benefit from insulin-related therapeutics [151] and that gender as well as APOE ε4 status influence insulin resistance and therapeutic responses [154].
Less is known about the interaction between ApoE and tau pathology in AD; however, one study has shown that although APOE allele status does show a correlation with Aβ amyloid deposition levels, it does not appear to predict tau pathology in a cognitively normal elderly cohort [155].
In men, it has been found that both low serum testosterone and the interaction between testosterone and APOE ε4 are associated with AD [156]. Moreover, there is compelling evidence of a significant interaction between testosterone levels and APOE genotype, with respect to brain function. For example, in people with APOE ε4 alleles, brain structure and cognition appear to be particularly sensitive to circulating testosterone levels. Furthermore, free testosterone levels have been found to be positively correlated with verbal episodic memory performance in APOE ε4 carriers but not in non-APOE ε4 individuals. Testosterone levels have also been found to influence hippocampal volume in APOE ε4 allele carriers, in the Vietnam Era Twin Study of Aging, such that those with low testosterone and who are also APOE ε4 carriers have the smallest hippocampal volumes [157]. In other studies, the depletion of testosterone following castration in APOE ε4 mice resulted in behavioral deficits in some tasks, which were not apparent in APOE ε3 or APOE knockout mice [158]; furthermore, testosterone treatment appeared to reverse these behavioral deficits by stimulating AR-dependent pathways [159]. Interestingly, a study by Raber et al. showed that female APOE ε4 mice responded to testosterone treatment with improved memory compared to male mice [159].
Inflammation and testosterone
Insulin resistance, which is recognized as a major T2DM hallmark, is associated with inflammation, which can be stimulated by increased levels of inflammatory mediators such as interleukin-6 (IL-6) and C-reactive protein (CRP) [160]. AD has also been shown to be associated with inflammation. For example inflammatory molecules, including acute-phase inflammatory reactants and pro-inflammatory cytokines, have been found in the CSF and amyloid plaques of AD patient brains [161–164], and increased levels of IL-6 have been found in the lumbar and ventricular CSF of patients with AD [165]. There is also evidence that circulating levels of inflammatory markers, particularly IL-6, are raised before dementia symptoms appear [166]. Although higher levels of these inflammatory mediators alone are not sufficient to serve as a diagnostic marker of AD, it should be noted that the levels of these inflammatory markers are highly variable when compared to cognitively healthy people. For example, elevated CRP levels been shown to indicate an increased risk of AD in a subset of Japanese American people and also in a European cohort [167, 168], but not in Mexican Americans [169].
Many features of the peripheral and CNS inflammation that can be detected in both T2DM and AD are worsened in people with low testosterone levels. For example, many males with T2DM have low testosterone levels, and it has been found that this subgroup of patients also have high CRP levels. Furthermore, it has now been found that these low-testosterone T2DM males also have a low hematocrit, which correlates inversely with the CRP concentration [170]. Some studies suggest that elevated CRP levels can predict increased severity of AD, although findings have been inconsistent [171–173]. Increased IL-6 together with low testosterone has also been observed in older men with T2DM [174] and AD [175, 176] and evidence from experimental studies indicates that testosterone secretion is inhibited by IL-6 [177]. Interestingly, testosterone treatment appears to be able to reduce some of these inflammatory signs: testosterone has been found to lower the inflammatory state through decreased CRP levels in a study of hypogonadal aging men who were treated with testosterone supplementation for at least 15 months [178]. However, the effect was not found in hypogonadal men with T2DM, although this may be due to the short treatment duration [11]. In in vitro and in vivo studies, it has been shown that testosterone treatment can downregulate IL-6 production [179]. However, in some clinical studies the effect of testosterone on IL-6 have produced conflicting results; for example, testosterone has been found to partially reverse the increasing IL-6 levels caused by anti-androgen treatment, but two clinical studies did not find any changes in IL-6 levels post-testosterone treatment [180, 181].
Closely linked to inflammation is oxidative stress, which again is associated with both T2DM and AD pathology [182–185]. Testosterone treatment has been shown to reduce indices of oxidative stress and improve cognition in the SAMP8 mouse model of aging, potentially via upregulating expression of the class III protein deacetylase, SIRT1 [184]. This appears to occur partly by induction of antioxidants and reduced release of reactive oxygen species [186]. However, testosterone may also be pro-oxidant in some circumstances, suggesting a role in redox balance [187]. In naturally-aging rats, testosterone produces positive effects on oxidative markers in the kidney [188]. However, a recent human study in the Texas Alzheimer’s Research & Care Consortium (TARCC) cohort showed that the effects of testosterone may be beneficial on cognition in men with low oxidative stress, but detrimental for those with high oxidative stress as determined by homocysteine levels <12 μM [189]. It is also possible these differences may only occur in some ethnic groups.
Less is known about the effects of testosterone on redox processes in T2DM; however, the beneficial effects seen in generalized models and the link between oxidative stress and insulin resistance suggests testosterone may also be able to reduce T2DM-related oxidative stress [190]. Supporting this, testosterone has been observed to reduce oxidative stress in human aortic endothelial cells via AR-mediated activation of PI3K/signaling, leading to activation of endothelial nitric oxide synthase (eNOS) and increased nitric oxide (NO) production [191]. While direct evidence is lacking, eNOS activation and NO upregulation have also been demonstrated to reduce oxidative stress indices in diabetic mice [192]. Conversely, in humans, supra-physiological doses of testosterone have been observed to downregulate expression of eNOS and NO production, as well as reducing antioxidant capacity [193], highlighting the importance of dosage. Overall, these studies indicate that testosterone and changes in inflammatory and oxidative markers are causally linked; however more longitudinal and interventional studies are needed.
TESTOSTERONE REGULATES BACE 1 ACTION IN AD AND T2DM
Accumulating evidence shows that T2DM causes brain insulin resistance, which increases the risk of AD (reviewed in [194]). Moreover, the extent of Aβ accumulation in the brains of people with T2DM correlates with the duration of T2DM [5]. Interestingly, it has been shown that people with T2DM are more likely to develop neurofibrillary tangles in their brain, irrespective of whether they have AD or cognitive impairment [195]. In fact, AD has been termed “type 3 diabetes”, which represents the chronic insulin resistance and insulin deficiency in the brain. An early piece of evidence for the links to T2DM came from a postmortem study that showed striking reductions in gene expression for insulin, insulin growth factor (IGF)-1 and IGF-II as well as its receptors in the AD CNS, that resembled but was distinct from changes seen in T2DM [196]. While these studies linked T2DM with the risk of developing AD, recent studies showed that this link may also work in the reverse order, such that people with AD may be more likely to develop T2DM. In an animal study that investigated mice expressing human β-site amyloid precursor protein cleaving enzyme 1 (BACE1), the knock-in mice showed signs of systemic poor glucose control compared to control, such as increased glucose levels and reduced glucose metabolism in the brains of animals, commencing at 4 months of age; the mice also showed systemic changes, including a fatty liver phenotype and impaired liver glycogen storage [197]. BACE1 is a novel aspartic protease that is highly expressed in neuronal cells; it initiates Aβ formation by cleaving amyloid-β protein precursor (AβPP), with the second cleavage being carried out by γ-secretase [198]. BACE1 activity increases with age and to an even greater extent (two to five-fold) in sporadic AD [199]. Besides the brain, high mRNA levels of BACE1 are also found in the pancreatic β-cells, although the β-secretase activity in these cells is comparatively low due to alternative splicing [200]. This pancreatic isoform of BACE1 may not cleave AβPP, but other newly identified substrates such as enteropeptidase [200]. Some studies have demonstrated that the lack of BACE1 is able to prevent Aβ synthesis [201–203], and may also protect against obesity and T2DM [204]. Further evidence of links between AD and T2DM come from the findings that the BACE1 gene is present in a region associated with a high risk of diabetes in Pima Indians, which led to the finding (in a Caucasian population) of the T2DM-linked functionally relevant rs535860 SNP [205]. This study also showed that a reduction in BACE1 expression (in a cell culture model) results in much lower insulin mRNA expression, and that Bace1(–/–) mice have lower plasma insulin concentrations, lower body weight, though normal glucose tolerance and insulin sensitivity [205].
The first study to indicate that endogenous testosterone can downregulate BACE1 activities, via a mechanism independent from estrogen, was done by Li and colleagues by genetically targeting aromatase in male APP23 transgenic mice (ArKO × APP23). The authors found that the brains of aromatase-deficient AD mice had reduced pathology and improved cognitive function than the brains of control AD mice. The aromatase-deficient AD mice had lower levels of BACE1 activity, indicating that testosterone may prevent the production of Aβ by inhibiting BACE1 activity [104]. On the other hand, in female APP23 mice with a genetic deficiency of aromatase (APP/Ar+/–), in which the brain contains non-detectable levels of estrogen, the administration of estrogen caused the downregulation of BACE1 [206], suggesting that estrogen, but not testosterone, may prevent the Aβ production by inhibiting BACE1 activity in female mice. Taken together, these studies highlight BACE1 as a major driver of the impaired glucose and insulin regulation, and suggest that BACE1 inhibition is an attractive therapeutic strategy for AD and T2DM. However, caution about potential mechanism-based toxicities resulting from complete BACE1 inhibition should be considered, as data generated from BACE1 deficient mouse models (BACE1 –/–) have revealed abnormalities in specific cognitive functions such as spatial and reference memories [207]. In addition, BACE1 is known to cleave a large number of substrates, so it is not surprising complete BACE1 ablation has been associated with a higher mortality shortly after birth, whereas surviving mice are smaller and display hyperactive behavior [203]. However, partial reduction in a BACE1 and AD mouse model (BACE1 +/−) resulted in normal spatial memory function, alongside improvements in AD neuropathology [208]. The degree to which BACE1 inhibition may provide beneficial Aβ-lowering effects and improvements in insulin resistance remains to be determined in preclinical studies, not only in animal models of AD and T2DM, but also in wild-type animals. More research is also needed to investigate the BACE1-lowering effects of testosterone.
COMBINATION OF TESTOSTERONE REPLACEMENT THERAPY AND EXERCISE AS PREVENTION AND TREATMENT FOR T2DM AND AD
Biologically, the sex hormones, in particular testosterone, play a substantial role in regulating various physiological parameters in both males and females and therefore would be the natural target for investigations into a possible involvement in regulating the effects of physical activity. This section describes the beneficial effect of exercise either on its own or in combination with testosterone supplementation in terms of prevention and management of T2DM and AD.
Beneficial effects of exercise alone in the prevention and management of T2DM and AD
With respect to T2DM, few studies have compared the efficacy of exercise interventions in men versus women [209]. Women with T2DM have been shown to have a greater risk of cardiovascular disease than men, and greater difficulties in physical activity adherence may exist for women compared to men in the context of T2DM [210]. However, intensive exercise intervention has been shown to be equally effective in preventing T2DM in both men and women [211]. Thus, exercise is important to reduce morbidity and mortality in individuals with T2DM, regardless of gender. Furthermore, given that increased intensity could determine the effect of exercise in the prevention and management of T2DM, studies have shown that endurance (aerobic) and strength (resistance) exercise trainings are most effective in improving insulin action and reducing blood glucose rapidly [212], however, the mechanistic difference between the two training types are unclear [213, 214]. Whether testosterone promotes peripheral and brain insulin sensitivity via exercise is less well studied. In mice, testosterone has been shown to increase metabolic rate via an AR-dependent action on skeletal muscle [215], and AR knockout mice exhibit decreased physical activity [216]. Unfortunately, clinical trials of T2DM that measure changes in physical activity in a careful and comprehensive manner are still lacking [125].
With respect to AD, few studies have compared the efficacy of exercise in men versus women, and there are clear differences between men and women regarding cognitive improvement in association with exercise [217, 218]. The biological explanation for this is partly related to the abrupt loss of the neuroprotective sex hormone estrogen and the decline of testosterone in postmenopausal women [219]. Animal studies and clinical trials have shown the benefit of exercise as a non-pharmacological option in reducing Aβ amyloid pathology, maintaining cognition, as well as preventing and postponing cognitive decline in the aging brain (extensively reviewed in [220–222]). More recent studies have expanded the research question by investigating exercise mediated effects on hippocampal neurogenesis [220, 223] and anti-inflammatory effects [224, 225]. Of particular interest with the link to T2DM, exercise not only improves peripheral insulin sensitivity, but also brain insulin signaling in rodents [226–228] as well as increased cerebral blood flow in men [229]. It has also been shown that depending on the intensity of the exercise, brain glucose uptake is altered [230]. A single bout of exercise has also been shown to reduced cortical BACE1 expression and activity, independent of changes in adiposity, and which is accompanied by the reductions in AMPK, Akt, and MAPK signaling in the cortex of mice fed a high fat diet [231].
The efficacy of testosterone replacement therapy is enhanced by exercise in T2DM and AD: the role of the muscle-brain axis
Whether exercise may provide benefits to testosterone replacement therapy alone still needs to be validated in studies of longer duration and larger sample size. Interestingly, recent studies have shown that exercise accentuates testosterone replacement therapy in men with low testosterone levels [232] and that these elevated testosterone levels persist, even after cessation of therapy [233]. In fact, different intensities of exercise may change the response of circulating testosterone levels both in animal models and in humans [234]. For example, acute exercise elevated testosterone in rodents, and resistance training induced elevated muscular dihydrotestosterone (DHT) thereby improving glucose metabolism in T2DM rats [235]. Clinical studies have shown that high intensity exercise (strength training), as opposed to low to moderate intensity, increases testosterone levels in older T2DM patients which may help to reduce the risk of AD (reviewed in [234, 236]). The molecular mechanism(s) underlying the effect of exercise on improving testosterone levels may be mediated by one of the most prominent trophic factors in the brain, brain-derived neurotrophic factor (BDNF) involved in neurogenesis and synaptogenesis. BDNF is expressed not only throughout the CNS, but also in skeletal muscle [237]. The link between BDNF and testosterone levels has been demonstrated in the development of the adolescent brain and in the pathogenesis of neurological disorders, with a role in cell proliferation. For instance, castration and testosterone administration in C57BL/6 mice during adolescence results in an AR-dependent effect on BDNF-tyrosine kinase (Trk) B signaling in the hippocampal and forebrain regions of the brain [238] and similarly in macaque monkeys and rats [239]. Previous studies suggest that neurotrophins such as BDNF act as mediators of testosterone action in the survival of new neurons in adult brain [240, 241]. Moreover, testosterone regulates BDNF levels in the skeletal muscle, and in turn androgenic action at the muscle regulates BDNF levels in motoneurons [242, 243]. In humans, plasma BDNF has been shown to be positively correlated with circulating testosterone in the plasma of older men [244]. In both animal models and patients of T2DM and AD, BDNF has been shown to be depleted and these low levels of BDNF may be one of the pathogenic factors that cluster T2DM and AD together [245].
Exercise increases BDNF levels as a stimulus for the induction of neurogenesis to improve synaptic plasticity [246, 247], possibly in a testosterone dependent manner as explained above. However, acute endurance exercise can only transiently increase BDNF in the blood, while strength training does not alter BDNF levels [246].
Another mechanism of an exercise-mediated effect on neurogenesis via testosterone is through interaction with IGF-1. IGF-1 levels are reduced in the aging brain, and testosterone has been shown to increase IGF-1 levels in vitro [248] and in both hypogonadal and healthy men [249]. In addition, testosterone interacts with IGF-1 to control IGF-1-binding protein in androgen-responsive skeletal muscle fibroblasts [250]. Exercise has been shown to enhance IGF-1 expression in the brain [223] as well as induce uptake of blood IGF-1 by specific groups of neurons in the rat brain [251], which could possibly be attributed to the involvement of testosterone levels. Thus, on activation by exercise, skeletal muscles may release growth factors such as BDNF and IGF-1 into the circulation to communicate with the brain, an effect possibly mediated by testosterone.
CONCLUSION
The relationship between low testosterone, T2DM, and AD is complex, and appears to involve a number of mechanisms such as insulin resistance, glucose metabolism, lipid regulation, Aβ clearance, inflammation, oxidative stress, and increased BACE1 activity. Even though these relationships are complex in nature, and our understanding of these relationships is currently incomplete, the evidence so far suggests that testosterone-based therapy has the potential to affect some of the metabolic pathways common to both T2DM and AD in a favorable manner, as well as modify interactions between some of these pathways (Fig. 1). Testosterone may reduce insulin resistance, improve glucose metabolism, improve several indices of dyslipidemia, increase Aβ clearance by increasing IDE and NEP expression and activity, and reduce inflammation (with many of these changes influenced by APOE allele status and also potentially by AR-CAG repeat polymorphism length). Therefore, testosterone may serve as a modifier of several metabolic pathways, disruptions of which have been linked to both T2DM and AD. However, testosterone treatment-induced improvements in the metabolic disturbances that occur in one or both of these conditions can also be achieved to some extent by adopting a healthy lifestyle, involving a better diet, exercise, and weight loss. It may eventuate that lifestyle changes combined with testosterone treatment will provide optimum benefits. Further basic research and adequately designed clinical trials need to be carried out to determine the value and efficacy of testosterone treatment on the metabolic disruptions and pathological changes common to T2DM, AD and related metabolic conditions. In addition, since gender-specific (and reproductive stage-dependent) differences related to T2DM and AD have been demonstrated in humans and animal models, both genders need to be studied in order to elucidate these fundamental differences so that gender-appropriate testosterone treatment regimens can be established.
Fig. 1.
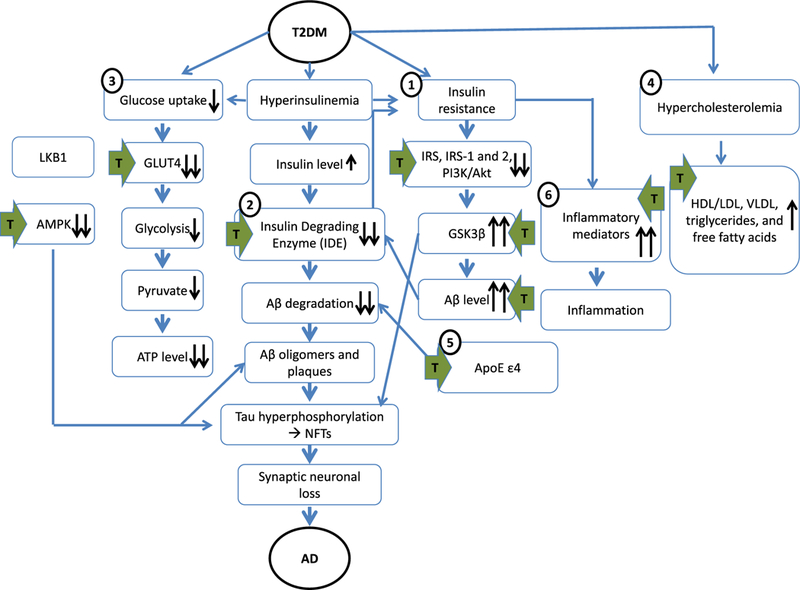
Common pathogenic mechanisms linking T2DM and AD under low testosterone conditions, showing points (green arrows) at which testosterone treatment modifies this relationship. 1) T2DM patient have insulin resistance which downregulates the expression of phosphorylated IRS and downstream signaling of PI3K activation in the brain. As a result, GSK3β activity increases, and leads to Aβ and NFT deposition. 2) Hyperinsulinemia and IDE defects in T2DM patients lead to decreased clearance of Aβ in the brain. 3) Impairment in insulin-stimulated glucose uptake in the skeletal muscle of T2DM correlates with downregulated expression of GLUT-4 which in turn affects the glycolysis pathway. Although glucose is transported from skeletal muscle to the brain via GLUT-1, the role of testosterone in this pathway is not known. Testosterone also promotes GLUT4 translocation via LKB1/AMPK signaling and stimulates glucose transport in adipocytes, in which dysregulation of AMPK occurs in T2DM and AD. 4) Hypercholesterolemia and perturbations to the HDL:LDL ratio, as well as elevated VLDL, triglycerides and free fatty acids in T2DM contribute to the development of AD-related pathology and testosterone treatment has been shown to reduce all these parameters. 5) Possession of APOE ε4 genes causes impairments in lipoproteins and Aβ clearance, which confers a greater risk of T2DM and AD. 6) Peripheral and brain inflammation is associated with T2DM and AD and can exacerbate the underlying pathologies through increasing pro-inflammatory mediators. Inflammation is also closely linked to oxidative stress that causes reduction of eNOS and NO. T2DM, type 2 diabetes; AD, Alzheimer’s disease; IRS, insulin receptors; eNOS, endothelial nitric oxide synthase; NO, nitric oxide; GSK3β, glycogen synthase kinase 3p; PI3K, phosphatidylinositol 3-kinase; Ap, amyloid-β; NFT, neurofibrillary tangles; ApoE ε4, apolipoprotein E4; GLUT4, glucose transporter 4.
Footnotes
DISCLOSURE STATEMENT
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/16–1259r2).
REFERENCES
- [1].Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM (1999) Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 53, 1937–1942. [DOI] [PubMed] [Google Scholar]
- [2].Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M, Rakugi H, Morishita R (2010) Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci USA 107, 7036–7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Qiu WQ, Zhu H (2014) Amylin and its analogs: A friend or foe for the treatment of Alzheimer’s disease? Front Aging Neurosci 6, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhu H, Wang X, Wallack M, Li H, Carreras I, Dedeoglu A, Hur JY, Zheng H, Li H, Fine R, Mwamburi M, Sun X, Kowall N, Stern RA, Qiu WQ (2015) Intraperitoneal injection of the pancreatic peptide amylin potently reduces behavioral impairment and brain amyloid pathology in murine models of Alzheimer’s disease. Mol Psychiatry 20, 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC (2004) Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 53, 474–481. [DOI] [PubMed] [Google Scholar]
- [6].Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB (2011) Diabetes mellitus and Alzheimer’s disease: Shared pathology and treatment? Br J Clin Pharmacol 71, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vermeulen A, Verdonck L, Kaufman JM (1999) A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84, 3666–3672. [DOI] [PubMed] [Google Scholar]
- [8].Grossmann M, Thomas MC, Panagiotopoulos S, Sharpe K, Macisaac RJ, Clarke S, Zajac JD, Jerums G (2008) Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 93, 1834–1840. [DOI] [PubMed] [Google Scholar]
- [9].Kapoor D, Aldred H, Clark S, Channer KS, Jones TH (2007) Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: Correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 30, 911–917. [DOI] [PubMed] [Google Scholar]
- [10].Ding EL, Song Y, Malik VS, Liu S (2006) Sex differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 295, 1288–1299. [DOI] [PubMed] [Google Scholar]
- [11].Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH (2007) The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol 156, 595–602. [DOI] [PubMed] [Google Scholar]
- [12].Bhasin S, Parker RA, Sattler F, Haubrich R, Alston B, Umbleja T, Shikuma CM, Team ACTGPAS (2007) Effects of testosterone supplementation on whole body and regional fat mass and distribution in human immunodeficiency virus-infected men with abdominal obesity. J Clin Endocrinol Metab 92, 1049–1057. [DOI] [PubMed] [Google Scholar]
- [13].Kapoor D, Goodwin E, Channer KS, Jones TH (2006) Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 154, 899–906. [DOI] [PubMed] [Google Scholar]
- [14].Boyanov MA, Boneva Z, Christov VG (2003) Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male 6, 1–7. [PubMed] [Google Scholar]
- [15].Corrales JJ, Almeida M, Burgo R, Mories MT, Miralles JM, Orfao A (2006) Androgen-replacement therapy depresses the ex vivo production of inflammatory cytokines by circulating antigen-presenting cells in aging type-2 diabetic men with partial androgen deficiency. J Endocrinol 189, 595–604. [DOI] [PubMed] [Google Scholar]
- [16].Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging 21,383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lage MJ, Barber BL, Markus RA (2007) Association between androgen-deprivation therapy and incidence of diabetes among males with prostate cancer. Urology 70, 1104–1108. [DOI] [PubMed] [Google Scholar]
- [18].Keating NL, O’Malley AJ, Smith MR (2006) Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 24, 4448–4456. [DOI] [PubMed] [Google Scholar]
- [19].Haidar A, Yassin A, Saad F, Shabsigh R (2007) Effects of androgen deprivation on glycaemic control and on cardiovascular biochemical risk factors in men with advanced prostate cancer with diabetes. Aging Male 10, 189–196. [DOI] [PubMed] [Google Scholar]
- [20].Nead KT, Gaskin G, Chester C, Swisher-McClure S, Leeper NJ, Shah NH (2016) Association between androgen deprivation therapy and risk of dementia. JAMA Oncol 34, 566–571. [DOI] [PubMed] [Google Scholar]
- [21].Keating NL, O’Malley A, Freedland SJ, Smith MR (2012) Diabetes and cardiovascular disease during androgen deprivation therapy: Observational study of veterans with prostate cancer. J Natl Cancer Inst 104, 1518–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Grossmann M (2011) Low testosterone in men with type 2 diabetes: Significance and treatment. J Clin Endocrinol Metab 96, 2341–2353. [DOI] [PubMed] [Google Scholar]
- [23].Polderman KH, Gooren LJ, Asscheman H, Bakker A, Heine RJ (1994) Induction of insulin resistance by androgens and estrogens. J Clin Endocrinol Metab 79, 265–271. [DOI] [PubMed] [Google Scholar]
- [24].De Leo V, Musacchio MC, Morgante G, La Marca A, Petraglia F (2004) Polycystic ovary syndrome and type 2 diabetes mellitus. Minerva Ginecol 56, 53–62. [PubMed] [Google Scholar]
- [25].Mauvais-Jarvis F (2015) Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ 6, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Samaan MC, Anand SS, Sharma AM, Samjoo IA, Tarnopolsky MA (2015) Sex differences in skeletal muscle phosphatase and tensin homolog deleted on chromosome 10 (PTEN) levels: A cross-sectional study. Sci Rep 5, 9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Navarro G, Allard C, Xu W, Mauvais-Jarvis F (2015) The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity (Silver Spring) 23, 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 27, 1047–1053. [DOI] [PubMed] [Google Scholar]
- [29].Cagnacci A, Soldani R, Carriero PL, Paoletti AM, Fioretti P, Melis GB (1992) Effects of low doses of transdermal 17 beta-estradiol on carbohydrate metabolism in postmenopausal women. J Clin Endocrinol Metab 74, 1396–1400. [DOI] [PubMed] [Google Scholar]
- [30].O’Sullivan AJ, Ho KK (1995) A comparison of the effects of oral and transdermal estrogen replacement on insulin sensitivity in postmenopausal women. J Clin Endocrinol Metab 80, 1783–1788. [DOI] [PubMed] [Google Scholar]
- [31].Bitoska I, Krstevska B, Milenkovic T, Subeska-Stratrova S, Petrovski G, Mishevska SJ, Ahmeti I, Todorova B (2016) Effects of hormone replacement therapy on insulin resistance in postmenopausal diabetic women. Open Access Maced J Med Sci 4, 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Matsui S, Yasui T, Tani A, Kunimi K, Uemura H, Yamamoto S, Kuwahara A, Matsuzaki T, Irahara M (2013) Associations of estrogen and testosterone with insulin resistance in pre- and postmenopausal women with and without hormone therapy. Int J Endocrinol Metab 11, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Corbould A (2008) Effects of androgens on insulin action in women: Is androgen excess a component of female metabolic syndrome? Diabetes Metab Res Rev 24, 520–532. [DOI] [PubMed] [Google Scholar]
- [34].Zang H, Carlstrom K, Arner P, Hirschberg AL (2006) Effects of treatment with testosterone alone or in combination with estrogen on insulin sensitivity in postmenopausal women. Fertil Steril 86, 136–144. [DOI] [PubMed] [Google Scholar]
- [35].Lightfoot JT (2008) Sex hormones’ regulation of rodent physical activity: A review. Int J Biol Sci 4, 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pettersson US, Walden TB, Carlsson PO, Jansson L, Phillipson M (2012) Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One 7, e46057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schwingshackl A, Blasko I, Steiner E, Pozzilli P, Cavallo MG, Berger P, Grubeck-Loebenstein B (1998) Sex steroids do not prevent amylin-induced apoptosis in human cells. Exp Cell Res 241, 265–268. [DOI] [PubMed] [Google Scholar]
- [38].Davey RA, Moore AJ, Chiu MW, Notini AJ, Morris HA, Zajac JD (2006) Effects of amylin deficiency on trabecular bone in young mice are sex-dependent. Calcif Tissue Int 78,398–403. [DOI] [PubMed] [Google Scholar]
- [39].Schulz K, Korz V (2010)Hippocampal testosterone relates to reference memory performance and synaptic plasticity in male rats. Front Behav Neurosci 4, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hajszan T, MacLusky NJ, Leranth C (2008) Role of androgens and the androgen receptor in remodeling of spine synapses in limbic brain areas. Horm Behav 53, 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Leranth C, Hajszan T, MacLusky NJ (2004) Androgens increase spine synapse density in the CA1 hippocampal sub field of ovariectomized female rats.J Neuro sci 24,495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jia JX, Cui CL, Yan XS, Zhang BF, Song W, Huo DS, Wang H, Yang ZJ (2016) Effects of testosterone on synaptic plasticity mediated by androgen receptors in male SAMP8 mice. J Toxicol Environ Health A 79, 849–855. [DOI] [PubMed] [Google Scholar]
- [43].Hamson DK, Wainwright SR, Taylor JR, Jones BA, Watson NV, Galea LA (2013) Androgens increase survival of adult-born neurons in the dentate gyrus by an androgen receptor-dependent mechanism in male rats. Endocrinology 154, 3294–3304. [DOI] [PubMed] [Google Scholar]
- [44].Atwi S, McMahon D, Scharfman H, MacLusky NJ (2016) Androgen modulation of hippocampal structure and function. Neuroscientist 22, 46–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Moffat SD, Resnick SM (2007) Long-term measures of free testosterone predict regional cerebral blood flow patterns in elderly men. Neuro biol Aging 28, 914–920. [DOI] [PubMed] [Google Scholar]
- [46].Rosario ER, Pike CJ (2008) Androgen regulation of betaamyloid protein and the risk of Alzheimer’s disease. Brain Res Rev 57, 444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rosario ER, Carroll J, Pike CJ (2010) Testosterone regulation of Alzheimer-like neuropathology in male 3xTg-AD mice involves both estrogen and androgen pathways. Brain Res 1359, 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gandy S, Almeida OP, Fonte J, Lim D, Waterrus A, Spry N, Flicker L, Martins RN (2001) Chemical andropause and amyloid-beta peptide. JAMA 285, 2195–2196. [DOI] [PubMed] [Google Scholar]
- [49].Fuller SJ, Tan RS, Martins RN (2007) Androgens in the etiology of Alzheimer’s disease in aging men and possible therapeutic interventions. J Alzheimers Dis 12, 129–142. [DOI] [PubMed] [Google Scholar]
- [50].Leuner B, Shors TJ (2004) New spines, new memories. Mol Neurobiol 29, 117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Verdile G, Laws SM, Henley D, Ames D, Bush AI, Ellis KA, Faux NG, Gupta VB, Li QX, Masters CL, Pike KE, Rowe CC, Szoeke C, Taddei K, Villemagne VL, Martins RN, Group AR (2014) Associations between gonadotropins, testosterone and beta amyloid in men at risk of Alzheimer’s disease. Mol Psychiatry 19, 69–75. [DOI] [PubMed] [Google Scholar]
- [52].Pike CJ, Carroll JC, Rosario ER, Barron AM (2009) Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol 30, 239–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rosario ER, Chang L, Stanczyk FZ, Pike CJ (2004) Age- related testosterone depletion and the development of Alzheimer disease. JAMA 292, 1431–1432. [DOI] [PubMed] [Google Scholar]
- [54].Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ (2011) Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol Aging 32, 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Nead KT, Gaskin G, Chester C, Swisher-McClure S, Dudley JT, Leeper NJ, Shah NH (2016) Androgen deprivation therapy and future Alzheimer’s disease risk. J Clin Oncol 34, 566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Martins RN, Gandy S (2016) Prostate cancer: Increased dementia risk following androgen deprivation therapy? Nat Rev Urol 13, 188–189. [DOI] [PubMed] [Google Scholar]
- [57].Wahjoepramono EJ, Asih PR, Aniwiyanti V, Taddei K, Dhaliwal SS, Fuller SJ, Foster J, Carruthers M, Verdile G, Sohrabi HR, Martins RN (2016) The effects of testosterone supplementation on cognitive functioning in older men. CNS Neurol Disord Drug Targets 15, 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bassil N, Alkaade S, Morley JE (2009) The benefits and risks of testosterone replacement therapy: A review. Ther Clin Risk Manag 5, 427–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Jayaraman A, Pike CJ (2014) Alzheimer’s disease and type 2 diabetes: Multiple mechanisms contribute to interactions. Curr Diab Rep 14, 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lv W, Du N, Liu Y, Fan X, Wang Y, Jia X, Hou X, Wang B (2016) Low testosterone level and risk of Alzheimer’s disease in the elderly men: A systematic review and metaanalysis. Mol Neurobiol 53, 2679–2684. [DOI] [PubMed] [Google Scholar]
- [61].Xu J, Xia LL, Song N, Chen SD, Wang G (2016) Testosterone, estradiol, and sex hormone-binding globulin in Alzheimer’s disease: A meta-analysis. Curr Alzheimer Res 13, 215–222. [DOI] [PubMed] [Google Scholar]
- [62].Mielke MM, Vemuri P, Rocca WA (2014) Clinical epidemiology of Alzheimer’s disease: Assessing sex and gender differences. Clin Epidemiol 6, 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Davison SL, Bell R, Donath S, Montalto JG, Davis SR (2005) Androgen levels in adult females: Changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab 90, 3847–3853. [DOI] [PubMed] [Google Scholar]
- [64].Haring R, Hannemann A, John U, Radke D, Nauck M, Wallaschofski H, Owen L, Adaway J, Keevil BG, Brabant G (2012) Age-specific reference ranges for serum testosterone and androstenedione concentrations in women measured by liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab 97, 408–415. [DOI] [PubMed] [Google Scholar]
- [65].Glaser R, Dimitrakakis C (2013) Testosterone therapy in women: Myths and misconceptions. Maturitas 74, 230–234. [DOI] [PubMed] [Google Scholar]
- [66].Papalia MA, Davis SR (2003) What is the rationale for androgen therapy for women? Treat Endocrinol 2, 77–84. [DOI] [PubMed] [Google Scholar]
- [67].Wierman ME, Basson R, Davis SR, Khosla S, Miller KK, Rosner W, Santoro N (2006) Androgen therapy in women: An Endocrine Society Clinical Practice guideline. J Clin Endocrinol Metab 91, 3697–3710. [DOI] [PubMed] [Google Scholar]
- [68].Davis SR, Jane F, Robinson PJ, Davison SL, Worsley R, Maruff P, Bell RJ (2014) Transdermal testosterone improves verbal learning and memory in postmenopausal women not on oestrogen therapy. Clin Endocrinol (Oxf) 81, 621–628. [DOI] [PubMed] [Google Scholar]
- [69].Huang G, Wharton W, Travison TG, Ho MH, Gleason C, Asthana S, Bhasin S, Basaria S (2015) Effects of testosterone administration on cognitive function in hysterectomized women with low testosterone levels: A dose-response randomized trial. J Endocrinol Invest 38, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shah S, Bell RJ, Savage G, Goldstat R, Papalia MA, Kulkarni J, Donath S, Davis SR (2006) Testosterone aromatization and cognition in women: A randomized, placebo-controlled trial. Menopause 13, 600–608. [DOI] [PubMed] [Google Scholar]
- [71].Cole AR, Astell A, Green C, Sutherland C (2007) Molecular connexions between dementia and diabetes. Neurosci Biobehav Rev 31, 1046–1063. [DOI] [PubMed] [Google Scholar]
- [72].Maher PA, Schubert DR (2009) Metabolic links between diabetes and Alzheimer’s disease. Expert Rev Neurother 9, 617–630. [DOI] [PubMed] [Google Scholar]
- [73].Havrankova J, Roth J, Brownstein MJ (1979) Concentrations of insulin and insulin receptors in the brain are independent of peripheral insulin levels. Studies of obese and streptozotocin-treated rodents. J Clin Invest 64, 636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Blazquez E, Velazquez E, Hurtado-Carneiro V, Ruiz- Albusac JM (2014) Insulin in the brain: Its pathophysiological implications for States related with central insulin resistance, type 2 diabetes and Alzheimer’s disease. Front Endocrinol (Lausanne) 5, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Saltiel AR (2001) New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell 104, 517–529. [DOI] [PubMed] [Google Scholar]
- [76].Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789. [DOI] [PubMed] [Google Scholar]
- [77].Hooper C, Killick R, Lovestone S (2008) The GSK3 hypothesis of Alzheimer’s disease. J Neurochem 104, 1433–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A (2009) Glycogen synthase kinase 3: More than a namesake. Br J Pharmacol 156, 885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Yang Y, Ma D, Wang Y, Jiang T, Hu S, Zhang M, Yu X, Gong CX (2013) Intranasal insulin ameliorates tau hyperphosphorylation in a rat model of type 2 diabetes. J Alzheimers Dis 33, 329–338. [DOI] [PubMed] [Google Scholar]
- [80].Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, Kondo T, Alber J, Galldiks N, Kustermann E, Arndt S, Jacobs AH, Krone W, Kahn CR, Bruning JC (2004) Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci USA 101, 3100–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Takeuchi M, Yamagishi S (2008) Possible involvement of advanced glycation end-products (AGEs) in the pathogenesis of Alzheimer’s disease. Curr Pharm Des 14, 973–978. [DOI] [PubMed] [Google Scholar]
- [82].Muthusamy T, Murugesan P, Srinivasan C, Balasubramanian K (2011) Sex steroids influence glucose oxidation through modulation of insulin receptor expression and IRS-1 serine phosphorylation in target tissues of adult male rat. Mol Cell Biochem 352, 35–45. [DOI] [PubMed] [Google Scholar]
- [83].Muthusamy T, Murugesan P, Balasubramanian K (2009) Sex steroids deficiency impairs glucose transporter 4 expression and its translocation through defective Akt phosphorylation in target tissues of adult male rat. Metabolism 58, 1581–1592. [DOI] [PubMed] [Google Scholar]
- [84].Parthasarathy C, Renuka VN, Balasubramanian K (2009) Sex steroids enhance insulin receptors and glucose oxidation in Chang liver cells. Clin Chim Acta 399, 49–53. [DOI] [PubMed] [Google Scholar]
- [85].Sesti G, Marini MA, Briata P, Tullio AN, Montemurro A, Borboni P, De Pirro R, Gherzi R, Lauro R (1992) Androgens increase insulin receptor mRNA levels, insulin binding, and insulin responsiveness in HEp-2 larynx carcinoma cells. Mol Cell Endocrinol 86, 111–118. [DOI] [PubMed] [Google Scholar]
- [86].Schwartz MW, Porte D Jr (2005) Diabetes, obesity, and the brain. Science 307, 375–379. [DOI] [PubMed] [Google Scholar]
- [87].Chiu SL, Chen CM, Cline HT (2008) Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron 58, 708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Papasozomenos SC (1997) The heat shock-induced hyperphosphorylation of tau is estrogen-independent and prevented by androgens: Implications for Alzheimer disease. Proc Natl Acad Sci USA 94, 6612–6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Papasozomenos S, Shanavas A (2002) Testosterone prevents the heat shock-induced overactivation of glycogen synthase kinase-3 beta but not of cyclin-dependent kinase 5 and c-Jun NH2-terminal kinase and concomitantly abolishes hyperphosphorylation of tau: Implications for Alzheimer’s disease. Proc Natl Acad Sci USA 99, 1140–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Navarro G, Xu W, Jacobson DA, Wicksteed B, Allard C, Zhang G, De Gendt K, Kim SH, Wu H, Zhang H, Verho-even G, Katzenellenbogen JA, Mauvais-Jarvis F (2016) Extranuclear actions of the androgen receptor enhance glucose-stimulated insulin secretion in the male. Cell Metab 23, 837–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Moghetti P, Tosi F, Castello R, Magnani CM, Negri C, Brun E, Furlani L, Caputo M, Muggeo M (1996) The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: Evidence that androgens impair insulin action in women. J Clin Endocrinol Metab 81, 952–960. [DOI] [PubMed] [Google Scholar]
- [92].Nohara K, Laque A, Allard C, Munzberg H, Mauvais-Jarvis F (2014) Central mechanisms of adiposity in adult female mice with androgen excess. Obesity (SilverSpring) 22, 1477–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Gasparini L, Gouras GK, Wang R, Gross RS, Beal MF, Greengard P, Xu H (2001) Stimulation of beta-amyloid precursor protein trafficking by insulin reduces intraneuronal beta-amyloid and requires mitogen-activated protein kinase signaling. J Neurosci 21, 2561–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Gasparini L, Netzer WJ, Greengard P, Xu H (2002) Does insulin dysfunction play a role in Alzheimer’s disease? Trends Pharmacol Sci 23, 288–293. [DOI] [PubMed] [Google Scholar]
- [95].Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S (2003) Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA 100, 4162–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Caccamo A, Oddo S, Sugarman MC, Akbari Y, LaFerla FM (2005) Age- and region-dependent alterations in Abeta-degrading enzymes: Implications for Abeta-induced disorders. Neurobiol Aging 26, 645–654. [DOI] [PubMed] [Google Scholar]
- [97].Cook DG, Leverenz JB, McMillan PJ, Kulstad JJ, Ericksen S, Roth RA, Schellenberg GD, Jin LW, Kovacina KS, Craft S (2003) Reduced hippocampal insulin-degrading enzyme in late-onset Alzheimer’s disease is associated with the apolipoprotein E-epsilon4 allele. Am J Pathol 162, 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Farris W, Mansourian S, Leissring MA, Eckman EA, Bertram L, Eckman CB, Tanzi RE, Selkoe DJ (2004) Partial loss-of-function mutations in insulin- degrading enzyme that induce diabetes also impair degradation of amyloid beta-protein. Am J Pathol 164, 1425–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Liu Y, Liu L, Lu S, Wang D, Liu X, Xie L, Wang G(2011) Impaired amyloid beta-degrading enzymes in brain of streptozotocin-induced diabetic rats. J Endocrinol Invest 34, 26–31. [DOI] [PubMed] [Google Scholar]
- [100].Tang WJ (2016) Targeting insulin-degrading enzyme to treat type 2 diabetes mellitus. Trends Endocrinol Metab 27, 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Costes S, Butler PC (2014) Insulin-degrading enzyme inhibition, a novel therapy for type 2 diabetes? Cell Metab 20, 201–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].George S, Petit GH, Gouras GK, Brundin P, Olsson R (2013) Nonsteroidal selective androgen receptor modulators and selective estrogen receptor beta agonists moderate cognitive deficits and amyloid-beta levels in a mouse model of Alzheimer’s disease. ACS Chem Neurosci 4, 1537–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Akita K, Harada K, Ichihara J, Takata N, Takahashi Y, Saito K (2013) A novel selective androgen receptor modulator, NEP28, is efficacious in muscle and brain without serious side effects on prostate. Eur J Pharmacol 720, 107–114. [DOI] [PubMed] [Google Scholar]
- [104].McAllister C, Long J, Bowers A, Walker A, Cao P, Honda S, Harada N, Staufenbiel M, Shen Y, Li R (2010) Genetic targeting aromatase in male amyloid precursor protein transgenic mice down-regulates beta-secretase (BACE1) and prevents Alzheimer-like pathology and cognitive impairment. J Neurosci 30, 7326–7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Yao M, Nguyen TV, Rosario ER, Ramsden M, Pike CJ (2008) Androgens regulate neprilysin expression: Role in reducing beta-amyloid levels. J Neurochem 105, 2477–2488. [DOI] [PubMed] [Google Scholar]
- [106].Vieira JS, Saraiva KL, Barbosa MC, Porto RC, Cresto JC, Peixoto CA, Wanderley MI, Udrisar DP (2011) Effect of dexamethasone and testosterone treatment on the regulation of insulin-degrading enzyme and cellular changes in ventral rat prostate after castration. Int J Exp Pathol 92, 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Bennett RG, Duckworth WC, Hamel FG (2000) Degradation of amylin by insulin-degrading enzyme. J Biol Chem 275, 36621–36625. [DOI] [PubMed] [Google Scholar]
- [108].Guan H, Chow KM, Shah R, Rhodes CJ, Hersh LB (2012) Degradation of islet amyloid polypeptide by neprilysin. Diabetologia 55, 2989–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Verdile G, Keane KN, Cruzat VF, Medic S, Sabale M, Rowles J, Wijesekara N, Martins RN, Fraser PE, Newsholme P (2015) Inflammation and oxidative stress: The molecular connectivity between insulin resistance, obesity, and Alzheimer’s disease. Mediators Inflamm 2015, 105828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Verdile G, Fuller SJ, Martins RN (2015) The role of type 2 diabetes in neurodegeneration. Neurobiol Dis 84, 22–38. [DOI] [PubMed] [Google Scholar]
- [111].Kim C, Halter JB (2014) Endogenous sex hormones, metabolic syndrome, and diabetes in men and women. Curr Cardiol Rep 16, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Rao PM, Kelly DM, Jones TH (2013) Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol 9, 479–493. [DOI] [PubMed] [Google Scholar]
- [113].Mitsuhashi K, Senmaru T, Fukuda T, Yamazaki M, Shinomiya K, Ueno M, Kinoshita S, Kitawaki J, Katsuyama M, Tsujikawa M, Obayashi H, Nakamura N, Fukui M (2016) Testosterone stimulates glucose uptake and GLUT4 translocation through LKB1/AMPK signaling in 3T3-L1 adipocytes. Endocrine 51, 174–184. [DOI] [PubMed] [Google Scholar]
- [114].Bertoldo MJ, Faure M, Dupont J, Froment P (2015) AMPK: A master energy regulator for gonadal function. Front Neurosci 9, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Yamada E, Lee TW, Pessin JE, Bastie CC (2010) Targeted therapies of the LKB1/AMPK pathway for the treatment of insulin resistance. Future Med Chem 2, 1785–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Cai Z, Yan LJ, Li K, Quazi SH, Zhao B (2012) Roles of AMP-activated protein kinase in Alzheimer’s disease. Neuromolecular Med 14, 1–14. [DOI] [PubMed] [Google Scholar]
- [117].McInnes KJ, Brown KA, Hunger NI, Simpson ER (2012) Regulation of LKB1 expression by sex hormones in adipocytes. IntJ Obes (Lond) 36, 982–985. [DOI] [PubMed] [Google Scholar]
- [118].Borgquist A, Meza C, Wagner EJ (2015) The role of AMP- activated protein kinase in the androgenic potentiation of cannabinoid-induced changes in energy homeostasis. Am J Physiol Endocrinol Metab 308, E482–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Coughlan KA, Valentine RJ, Ruderman NB, Saha AK (2014) AMPK activation: A therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes 7, 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Yialamas MA, Dwyer AA, Hanley E, Lee H, Pitteloud N, Hayes FJ (2007) Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 92, 4254–4259. [DOI] [PubMed] [Google Scholar]
- [121].Volpi E, Lieberman SA, Ferrer DM, Gilkison CR, Rasmussen BB, Nagamani M, Urban RJ (2005) The relationships between testosterone, body composition, and insulin resistance: A lesson from a case of extreme hyperandrogenism. Diabetes Care 28, 429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Seidell JC, Bjorntorp P, Sjostrom L, Kvist H, Sannerstedt R (1990) Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism 39, 897–901. [DOI] [PubMed] [Google Scholar]
- [123].Allan CA (2014) Sex steroids and glucose metabolism. Asian J Androl 16, 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Caronia LM, Dwyer AA, Hayden D, Amati F, Pitteloud N, Hayes FJ (2013) Abrupt decrease in serum testosterone levels after an oral glucose load in men: Implications for screening for hypogonadism. Clin Endocrinol (Oxf) 78, 291–296. [DOI] [PubMed] [Google Scholar]
- [125].Grossmann M (2014) Testosterone and glucose metabolism in men: Current concepts and controversies. J Endocrinol 220, R37–R55. [DOI] [PubMed] [Google Scholar]
- [126].Saad F (2009) The role of testosterone in type 2 diabetes and metabolic syndrome in men. Arq Bras Endocrinol Metabol 53, 901–907. [DOI] [PubMed] [Google Scholar]
- [127].Tan RS (2013) Testosterone effect on brain metabolism in elderly patients with Alzheimer’s disease: Comparing two cases at different disease stages. Aging Clin Exp Res 25, 343–347. [DOI] [PubMed] [Google Scholar]
- [128].Grossmann M, Hoermann R, Wittert G, Yeap BB (2015) Effects of testosterone treatment on glucose metabolism and symptoms in men with type 2 diabetes and the metabolic syndrome: A systematic review and meta-analysis of randomized controlled clinical trials. Clin Endocrinol (Oxf) 83, 344–351. [DOI] [PubMed] [Google Scholar]
- [129].Lee CH, Kuo SW, Hung YJ, Hsieh CH, He CT, Yang TC, Lian WC, Chyi-Fan S, Pei D (2005) The effect of testosterone supplement on insulin sensitivity, glucose effectiveness, and acute insulin response after glucose load in male type 2 diabetics. Endocr Res 31, 139–148. [DOI] [PubMed] [Google Scholar]
- [130].Haider A, Yassin A, Doros G, Saad F (2014) Effects of long-term testosterone therapy on patients with “diabesity”: Results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol 2014, 683515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Carruthers M, Cathcart P, Feneley MR (2015) Evolution of testosterone treatment over 25 years: Symptom responses, endocrine profiles and cardiovascular changes. Aging Male 18, 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Varlamov O, Bethea CL, Roberts CT Jr (2014) Sex- specific differences in lipid and glucose metabolism. Front Endocrinol (Lausanne) 5, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Morford J, Mauvais-Jarvis F (2016) Sex differences in the effects of androgens acting in the central nervous system on metabolism. Dialogues Clin Neurosci 18, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Miller KK, Deckersbach T, Rauch SL, Fischman AJ, Grieco KA, Herzog DB, Klibanski A (2004) Testosterone administration attenuates regional brain hypometabolism in women with anorexia nervosa. Psychiatry Res 132,197–207. [DOI] [PubMed] [Google Scholar]
- [135].Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA (2000) Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis 7,321–331. [DOI] [PubMed] [Google Scholar]
- [136].Krauss RM (2004) Lipids and lipoproteins inpatients with type 2 diabetes. Diabetes Care 27, 1496–1504. [DOI] [PubMed] [Google Scholar]
- [137].Boden G (2008) Obesity and free fatty acids. Endocrinol Metab Clin North Am 37, 635–646, viii-ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Martins AR, Nachbar RT, Gorjao R, Vinolo MA, Festuccia WT, Lambertucci RH, Cury-Boaventura MF, Silveira LR, Curi R, Hirabara SM (2012) Mechanisms underlying skeletal muscle insulin resistance induced by fatty acids: Importance of the mitochondrial function. Lipids Health Dis 11, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Shie FS, Jin LW, Cook DG, Leverenz JB, LeBoeuf RC (2002) Diet-induced hypercholesterolemia enhances brain A beta accumulation in transgenic mice. Neuroreport 13, 455–459. [DOI] [PubMed] [Google Scholar]
- [140].Mitsuzuka K, Kyan A, Sato T, Orikasa K, Miyazato M, Aoki H, Kakoi N, Narita S, Koie T, Namima T, Toyoda S, Fukushi Y, Habuchi T, Ohyama C, Arai Y, Tohoku Evidence-Based Medicine Study G, Michinoku Urological Cancer Study G (2016) Influence of 1 year of androgen deprivation therapy on lipid and glucose metabolism and fat accumulation in Japanese patients with prostate cancer. Prostate Cancer Prostatic Dis 19, 57–62. [DOI] [PubMed] [Google Scholar]
- [141].Stanworth RD, Kapoor D, Channer KS, Jones TH (2011) Dyslipidaemia is associated with testosterone, oestra- diol and androgen receptor CAG repeat polymorphism in men with type 2 diabetes. Clin Endocrinol (Oxf) 74, 624–630. [DOI] [PubMed] [Google Scholar]
- [142].Corona G, Monami M, Rastrelli G, Aversa A, Tishova Y, Saad F, Lenzi A, Forti G, Mannucci E, Maggi M (2011) Testosterone and metabolic syndrome: A meta-analysis study. J Sex Med 8, 272–283. [DOI] [PubMed] [Google Scholar]
- [143].Buckler HM, McElhone K, Durrington PN, Mackness MI, Ludlam CA, Wu FC (1998) The effects of low-dose testosterone treatment on lipid metabolism, clotting factors and ultrasonographic ovarian morphology in women. Clin Endocrinol (Oxf) 49, 173–178. [DOI] [PubMed] [Google Scholar]
- [144].Somboonporn W, Davis S, Seif MW, Bell R (2005) Testosterone for peri- and postmenopausal women. Cochrane Database Syst Rev, CD004509. [DOI] [PubMed] [Google Scholar]
- [145].Verghese PB, Castellano JM, Garai K, Wang Y, Jiang H, Shah A, Bu G, Frieden C, Holtzman DM (2013) ApoE influences amyloid-beta (Abeta) clearance despite minimal apoE/Abeta association in physiological conditions. Proc Natl Acad Sci USA 110, E1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Wildsmith KR, Holley M, Savage JC, Skerrett R, Landreth GE (2013) Evidence for impaired amyloid beta clearance in Alzheimer’s disease. Alzheimers Res Ther 5, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Hanson AJ, Bayer-Carter JL, Green PS, Montine TJ, Wilkinson CW, Baker LD, Watson GS, Bonner LM, Callaghan M, Leverenz JB, Tsai E, Postupna N, Zhang J, Lampe J, Craft S (2013) Effect of apolipoprotein E genotype and diet on apolipoprotein E lipidation and amyloid peptides: Randomized clinical trial. JAMA Neurol 70, 972–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].El-Lebedy D, Raslan HM, Mohammed AM (2016) Apolipoprotein E gene polymorphism and risk of type 2 diabetes and cardiovascular disease. Cardiovasc Diabetol 15, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Anthopoulos PG, Hamodrakas SJ, Bagos PG (2010) Apolipoprotein E polymorphisms and type 2 diabetes: A meta-analysis of 30 studies including 5423 cases and 8197 controls. Mol Genet Metab 100, 283–291. [DOI] [PubMed] [Google Scholar]
- [150].Du J, Chang J, Guo S, Zhang Q, Wang Z (2009) ApoE 4 reduces the expression of Abeta degrading enzyme IDE by activating the NMDA receptor in hippocampal neurons. Neurosci Lett 464, 140–145. [DOI] [PubMed] [Google Scholar]
- [151].Reger MA, Watson GS, Frey WH 2nd, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD, Cherrier MM, Craft S (2006) Effects of intranasal insulin on cognition in memory-impaired older adults: Modulation by APOE genotype. Neurobiol Aging 27, 451–458. [DOI] [PubMed] [Google Scholar]
- [152].Craft S, Asthana S, Schellenberg G, Cherrier M, Baker LD, Newcomer J, Plymate S, Latendresse S, Petrova Raskind M, Peskind E, Lofgreen C, Grimwood K (1999) Insulin metabolism in Alzheimer’s disease differs according to apolipoprotein E genotype and gender. Neuroendocrinology 70, 146–152. [DOI] [PubMed] [Google Scholar]
- [153].Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D Jr (1998) Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: Relationship to severity of dementia and apolipoprotein E genotype. Neurology 50, 164–168. [DOI] [PubMed] [Google Scholar]
- [154].Hanson AJ, Banks WA, Hernandez Saucedo H, Craft S (2016) Apolipoprotein E genotype and sex influence glucose tolerance in older adults: A cross-sectional study. Dement Geriatr Cogn Dis Extra 6, 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA (2010) APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 67, 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Hogervorst E, Lehmann DJ, Warden DR, McBroom J, Smith AD (2002) Apolipoprotein E epsilon4 and testosterone interact in the risk of Alzheimer’s disease in men. Int J Geriatr Psychiatry 17, 938–940. [DOI] [PubMed] [Google Scholar]
- [157].Panizzon MS, Hauger R, Dale AM, Eaves LJ, Eyler LT, Fischl B, Fennema-Notestine C, Franz CE, Grant MD, Jak AJ, Jacobson KC, Lyons MJ, Mendoza SP, Neale MC, Prom-Wormley EC, Seidman LJ, Tsuang MT, Xian H, Kremen WS (2010) Testosterone modifies the effect of APOE genotype on hippocampal volume in middle-aged men. Neurology 75, 874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Pfankuch T, Rizk A, Olsen R, Poage C, Raber J (2005) Role of circulating androgen levels in effects of apoE4 on cognitive function. Brain Res 1053, 88–96. [DOI] [PubMed] [Google Scholar]
- [159].Raber J, Bongers G, LeFevour A, Buttini M, Mucke L (2002) Androgens protect against apolipoprotein E4- induced cognitive deficits. J Neurosci 22, 5204–5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Watson GS, Craft S (2003) The role of insulin resistance in the pathogenesis of Alzheimer’s disease: Implications for treatment. CNS Drugs 17, 27–45. [DOI] [PubMed] [Google Scholar]
- [161].Hotamisligil GS (2003) Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord 27(Suppl 3), S53–S55. [DOI] [PubMed] [Google Scholar]
- [162].Pickup JC, Mattock MB, Chusney GD, Burt D (1997) NIDDM as a disease of the innate immune system: Association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 40,1286–1292. [DOI] [PubMed] [Google Scholar]
- [163].van de Ree MA, Huisman MV, Princen HM, Meinders AE, Kluft C, Group DA-S (2003) Strong decrease of high sensitivity C-reactive protein with high-dose atorvastatin in patients with type 2 diabetes mellitus. Atherosclerosis 166, 129–135. [DOI] [PubMed] [Google Scholar]
- [164].Hull M, Strauss S, Berger M, Volk B, Bauer J (1996) The participation of interleukin-6, a stress-inducible cytokine, in the pathogenesis of Alzheimer’s disease. Behav Brain Res 78, 37–41. [DOI] [PubMed] [Google Scholar]
- [165].Rosler N, Wichart I, Jellinger KA (2001) Intra vitam lumbar and post mortem ventricular cerebrospinal fluid immunoreactive interleukin-6 in Alzheimer’s disease patients. Acta Neurol Scand 103, 126–130. [DOI] [PubMed] [Google Scholar]
- [166].Uchoa MF, Moser VA, Pike CJ (2016) Interactions between inflammation, sex steroids, and Alzheimer’s disease risk factors. Front Neuroendocrinol 43, 60–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ (2002) Early inflammation and dementia: A 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol 52, 168–174. [DOI] [PubMed] [Google Scholar]
- [168].Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruiten-berg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM (2004) Inflammatory proteins in plasma and the risk of dementia: The Rotterdam Study. Arch Neurol 61, 668–672. [DOI] [PubMed] [Google Scholar]
- [169].O’Bryant SE, Johnson L, Edwards M, Soares H, Devous MD, Ross S, Rohlfing G, Hall J, Texas Alzheimer’s R, Care C (2013) The link between C-reactive protein and Alzheimer’s disease among Mexican Americans. J Alzheimers Dis 34, 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Bhatia V, Chaudhuri A, Tomar R, Dhindsa S, Ghanim H, Dandona P (2006) Low testosterone and high C-reactive protein concentrations predict low hematocrit in type 2 diabetes. Diabetes Care 29, 2289–2294. [DOI] [PubMed] [Google Scholar]
- [171].Yarchoan M, Louneva N, Xie SX, Swenson FJ, Hu W, Soares H, Trojanowski JQ, Lee VM, Kling MA, Shaw LM, Chen-Plotkin A, Wolk DA, Arnold SE (2013) Association of plasma C-reactive protein levels with the diagnosis of Alzheimer’s disease. J Neurol Sci 333, 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].O’Bryant SE, Waring SC, Hobson V, Hall JR, Moore CB, Bottiglieri T, Massman P, Diaz-Arrastia R (2010) Decreased C-reactive protein levels in Alzheimer disease. J Geriatr Psychiatry Neurol 23, 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Kravitz BA, Corrada MM, Kawas CH (2009) Elevated C-reactive protein levels are associated with prevalent dementiainthe oldest-old. Alzheimers Dement 5,318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Kristiansen OP, Mandrup-Poulsen T (2005) Interleukin-6 and diabetes: The good, the bad, or the indifferent? Diabetes 54(Suppl 2), S114–S124. [DOI] [PubMed] [Google Scholar]
- [175].Cojocaru IM, Cojocaru M, Miu G, Sapira V (2011) Study of interleukin-6 production in Alzheimer’s disease. Rom J Intern Med 49, 55–58. [PubMed] [Google Scholar]
- [176].Rubio-Perez JM, Morillas-Ruiz JM (2012) A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Scientific World Journal 2012, 756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Maggio M, Basaria S, Ceda GP, Ble A, Ling SM, Bandinelli S, Valenti G, Ferrucci L (2005) The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest 28, 116–119. [PubMed] [Google Scholar]
- [178].Giltay EJ, Haider A, Saad F, Gooren LJ (2008) C-reactive protein levels and ageing male symptoms in hypogonadal men treated with testosterone supplementation. Andrologia 40, 398–400. [DOI] [PubMed] [Google Scholar]
- [179].Maggio M, Guralnik JM, Longo DL, Ferrucci L (2006) Interleukin-6 in aging and chronic disease: A magnificent pathway. J Gerontol A Biol Sci Med Sci 61, 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Lambert CP, Sullivan DH, Evans WJ (2003) Effects of testosterone replacement and/or resistance training on interleukin-6, tumor necrosis factor alpha, and leptin in elderly men ingesting megestrol acetate: A randomized controlled trial. J Gerontol A Biol Sci Med Sci 58, 165–170. [DOI] [PubMed] [Google Scholar]
- [181].Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH (2004) The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab 89, 3313–3318. [DOI] [PubMed] [Google Scholar]
- [182].Folli F, Corradi D, Fanti P, Davalli A, Paez A, Giaccari A, Perego C, Muscogiuri G (2011) The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: Avenues for a mechanistic- based therapeutic approach. Curr Diabetes Rev 7, 313–324. [DOI] [PubMed] [Google Scholar]
- [183].Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA (2001) Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol 60, 759–767. [DOI] [PubMed] [Google Scholar]
- [184].Ota H, Akishita M, Akiyoshi T, Kahyo T, Setou M, Ogawa S, Iijima K, Eto M, Ouchi Y (2012) Testosterone deficiency accelerates neuronal and vascular aging of SAMP8 mice: Protective role of eNOS and SIRT1. PLoS One 7, e29598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Lovell MA, Xiong S, Xie C, Davies P, Markesbery WR (2004) Induction of hyperphosphorylated tau in primary rat cortical neuron cultures mediated by oxidative stress and glycogen synthase kinase-3. J Alzheimers Dis 6, 659–671; discussion 673–681. [DOI] [PubMed] [Google Scholar]
- [186].Marin DP, Bolin AP, dos Santos Rde C, Curi R, Otton R (2010) Testosterone suppresses oxidative stress in human neutrophils. Cell Biochem Funct 28, 394–402. [DOI] [PubMed] [Google Scholar]
- [187].Peltola V, Huhtaniemi I, Metsa-Ketela T, Ahotupa M (1996) Induction of lipid peroxidation during steroidogenesis in the rat testis. Endocrinology 137, 105–112. [DOI] [PubMed] [Google Scholar]
- [188].Yanar K, Atukeren P, Cebe T, Kunbaz A, Ozan T, Kansu AD, Durmaz S, Gulec V, Belce A, Aydin S, Cakatay U, Rizvi SI (2015) Ameliorative effects of testosterone administration on renal redox homeostasis in naturally aged rats. Rejuvenation Res 18, 299–312. [DOI] [PubMed] [Google Scholar]
- [189].Cunningham RL, Singh M, O’Bryant SE, Hall JR, Barber RC (2014) Oxidative stress, testosterone, and cognition among Caucasian and Mexican-American men with and without Alzheimer’s disease. J Alzheimers Dis 40, 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Park K, Gross M, Lee DH, Holvoet P, Himes JH, Shikany JM, Jacobs DR Jr (2009) Oxidative stress and insulin resistance: The coronary artery risk development in young adults study. Diabetes Care 32, 1302–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Yu J, Akishita M, Eto M, Ogawa S, Son BK, Kato S, Ouchi Y, Okabe T (2010) Androgen receptor-dependent activation of endothelial nitric oxide synthase in vascular endothelial cells: Role of phosphatidylinositol 3-kinase/akt pathway. Endocrinology 151, 1822–1828. [DOI] [PubMed] [Google Scholar]
- [192].Cheang WS, Wong WT, Tian XY, Yang Q, Lee HK, He GW, Yao X, Huang Y (2011) Endothelial nitric oxide synthase enhancer reduces oxidative stress and restores endothelial function in db/db mice. Cardiovasc Res 92, 267–275. [DOI] [PubMed] [Google Scholar]
- [193].Skogastierna C, Hotzen M, Rane A, Ekstrom L (2014) A supraphysiological dose of testosterone induces nitric oxide production and oxidative stress. Eur J Prev Cardiol 21, 1049–1054. [DOI] [PubMed] [Google Scholar]
- [194].de la Monte SM, Wands JR (2008) Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol 2, 1101–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Moran C, Beare R, Phan TG, Bruce DG, Callisaya ML, Srikanth V, Alzheimer’s Disease Neuroimaging I (2015) Type 2 diabetes mellitus and biomarkers of neurodegeneration. Neurology 85, 1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM (2005) Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease-is this type 3 diabetes? J Alzheimers Dis 7, 63–80. [DOI] [PubMed] [Google Scholar]
- [197].Plucinska K, Dekeryte R, Koss D, Shearer K, Mody N, Whitfield PD, Doherty MK, Mingarelli M, Welch A, Riedel G, Delibegovic M, Platt B (2016) Neuronal human BACE1 knockin induces systemic diabetes in mice. Diabetologia 59, 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Cole SL, Vassar R (2008) BACE1 structure and function in health and Alzheimer’s disease. Curr Alzheimer Res 5, 100–120. [DOI] [PubMed] [Google Scholar]
- [199].Cole SL, Vassar R (2007) The basic biology of BACE1: A key therapeutic target for Alzheimer’s disease. Curr Genomics 8, 509–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Dislich B, Lichtenthaler SF (2012) The membrane- bound aspartyl protease BACE1: Molecular and functional properties in Alzheimer’s disease and beyond. Front Physiol 3, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, Fan W, Kha H, Zhang J, Gong Y, Martin L, Louis JC, Yan Q, Richards WG, Citron M, Vassar R (2001) Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci 4, 231–232. [DOI] [PubMed] [Google Scholar]
- [202].Roberds SL, Anderson J, Basi G, Bienkowski MJ, Branstetter DG, Chen KS, Freedman SB, Frigon NL, Games D, Hu K, Johnson-Wood K, Kappenman KE, Kawabe TT, Kola I, Kuehn R, Lee M, Liu W, Motter R, Nichols NF, Power M, Robertson DW, Schenk D, Schoor M, Shopp GM, Shuck ME, Sinha S, Svensson KA, Tatsuno G, Tintrup H, Wijsman J, Wright S, McConlogue L (2001) BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: Implications for Alzheimer’s disease therapeutics. Hum Mol Genet 10, 1317–1324. [DOI] [PubMed] [Google Scholar]
- [203].Dominguez D, Tournoy J, Hartmann D, Huth T, Cryns K, Deforce S, Serneels L, Camacho IE, Marjaux E, Craessaerts K, Roebroek AJ, Schwake M, D’Hooge R, Bach P, Kalinke U, Moechars D, Alzheimer C, Reiss K, Saftig P, De Strooper B (2005) Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J Biol Chem 280, 30797–30806. [DOI] [PubMed] [Google Scholar]
- [204].Meakin PJ, Harper AJ, Hamilton DL, Gallagher J, McNeilly AD, Burgess LA, Vaanholt LM, Bannon KA, Latcham J, Hussain I, Speakman JR, Howlett DR, Ashford ML (2012) Reduction in BACE1 decreases body weight, protects against diet-induced obesity and enhances insulin sensitivity in mice. Biochem J 441, 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Hoffmeister A, Tuennemann J, Sommerer I, Mossner J, Rittger A, Schleinitz D, Kratzsch J, Rosendahl J, Kloting N, Stahl T, Rossner S, Paroni F, Maedler K, Kovacs P, Bluher M (2013) Genetic and biochemical evidence for a functional role of BACE1 in the regulation of insulin mRNA expression. Obesity (Silver Spring) 21, E626–633. [DOI] [PubMed] [Google Scholar]
- [206].Li R, He P, Cui J, Staufenbiel M, Harada N, Shen Y(2013) Brain endogenous estrogen levels determine responses to estrogen replacement therapy via regulation of BACE1 and NEP in female Alzheimer’s transgenic mice. Mol Neurobiol 47, 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Ohno M, Sametsky EA, Younkin LH, Oakley H, Younkin SG, Citron M, Vassar R, Disterhoft JF (2004) BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer’s disease. Neuron 41, 27–33. [DOI] [PubMed] [Google Scholar]
- [208].Singer O, Marr RA, Rockenstein E, Crews L, Coufal NG, Gage FH, Verma IM, Masliah E (2005) Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat Neurosci 8, 1343–1349. [DOI] [PubMed] [Google Scholar]
- [209].Arnetz L, Ekberg NR, Alvarsson M (2014) Sex differences in type 2 diabetes: Focus on disease course and outcomes. Diabetes Metab Syndr Obes 7, 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Miller TM, Gilligan S, Herlache LL, Regensteiner JG Sex differences in cardiovascular disease risk and exercise in type 2 diabetes. J Investig Med 60, 664–670. [DOI] [PubMed] [Google Scholar]
- [211].Perreault L, Ma Y, Dagogo-Jack S, Horton E, Marrero Crandall J, Barrett-Connor E, Diabetes Prevention P (2008) Sex differences in diabetes risk and the effect of intensive lifestyle modification in the Diabetes Prevention Program. Diabetes Care 31, 1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Riddell M, Perkins BA (2009) Exercise and glucose metabolism in persons with diabetes mellitus: Perspectives on the role for continuous glucose monitoring. J Diabetes Sci Technol 3, 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Olver TD, Laughlin MH (2016) Endurance, interval sprint, and resistance exercise training: Impact on microvascular dysfunction in type 2 diabetes. Am J Physiol Heart Circ Physiol 310, H337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B, American College of Sports Medicine, American Diabetes Association (2010) Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 33,e147–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Fernando SM, Rao P, Niel L, Chatterjee D, Stagljar M, Monks DA (2010) Myocyte androgen receptors increase metabolic rate and improve body composition by reducing fat mass. Endocrinology 151, 3125–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Rana K, Fam BC, Clarke MV, Pang TP, Zajac JD, MacLean HE (2011) Increased adiposity in DNA binding-dependent androgen receptor knockout male mice associated with decreased voluntary activity and not insulin resistance. Am J Physiol Endocrinol Metab 301, E767–E778. [DOI] [PubMed] [Google Scholar]
- [217].Balsamo S, Willardson JM, Frederico Sde S, Prestes J, Balsamo DC, Dahan da CN, Dos Santos-Neto L, Nobrega OT (2013) Effectiveness of exercise on cognitive impairment and Alzheimer’s disease. Int J Gen Med 6, 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Kivipelto M, Solomon A, Ahtiluoto S, Ngandu T, Lehtisalo J, Antikainen R, Backman L, Hanninen T, Jula A, Laatikainen T, Lindstrom J, Mangialasche F, Nissinen A, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H (2013) The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): Study design and progress. Alzheimers Dement 9, 657–665. [DOI] [PubMed] [Google Scholar]
- [219].Liu M, Kelley MH, Herson PS, Hurn PD (2010) Neuroprotection of sex steroids. Minerva Endocrinol 35, 127–143. [PMC free article] [PubMed] [Google Scholar]
- [220].Ryan SM, Kelly AM (2016) Exercise as a pro-cognitive, pro-neurogenic and anti-inflammatory intervention in transgenic mouse models of Alzheimer’s disease. Ageing Res Rev 27, 77–92. [DOI] [PubMed] [Google Scholar]
- [221].Liang KY, Mintun MA, Fagan AM, Goate AM, Bugg JM, Holtzman DM, Morris JC, Head D (2010) Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann Neurol 68, 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC (2011) Physical exercise as a preventive or disease- modifying treatment of dementia and brain aging. Mayo Clin Proc 86, 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Intlekofer KA, Cotman CW (2013) Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol Dis 57, 47–55. [DOI] [PubMed] [Google Scholar]
- [224].Tapia-Rojas C, Aranguiz F, Varela-Nallar L, Inestrosa NC (2016) Voluntary running attenuates memory loss, decreases neuropathological changes and induces neurogenesis in a mouse model of Alzheimer’s disease. Brain Pathol 26, 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Ke HC, Huang HJ, Liang KC, Hsieh-Li HM (2011) Selective improvement of cognitive function in adult and aged APP/PS1 transgenic mice by continuous non-shock treadmill exercise. Brain Res 1403, 1–11. [DOI] [PubMed] [Google Scholar]
- [226].Kang EB, Cho JY (2014) Effects of treadmill exercise on brain insulin signaling and beta-amyloid in intracerebroventricular streptozotocin induced-memory impairment in rats. J Exerc Nutrition Biochem 18, 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Muller AP, Gnoatto J, Moreira JD, Zimmer ER, Haas CB, Lulhier F, Perry ML, Souza DO, Torres-Aleman I, Portela LV (2011) Exercise increases insulin signaling in the hippocampus: Physiological effects and pharmacological impact of intracerebroventricular insulin administration in mice. Hippocampus 21, 1082–1092. [DOI] [PubMed] [Google Scholar]
- [228].Flores MB, Fernandes MF, Ropelle ER, Faria MC, Ueno M, Velloso LA, Saad MJ, Carvalheira JB (2006) Exercise improves insulin and leptin sensitivity in hypothalamus of Wistar rats. Diabetes 55, 2554–2561. [DOI] [PubMed] [Google Scholar]
- [229].Lucas SJ, Cotter JD, Brassard P, Bailey DM (2015) High- intensity interval exercise and cerebrovascular health: Curiosity, cause, and consequence. J Cereb Blood Flow Metab 35, 902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Kemppainen J, Aalto S, Fujimoto T, Kalliokoski KK, Langsjo J, Oikonen V, Rinne J, Nuutila P, Knuuti J (2005) High intensity exercise decreases global brain glucose uptake in humans. J Physiol 568, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].MacPherson RE, Baumeister P, Peppler WT, Wright DC, Little JP (2015) Reduced cortical BACE1 content with one bout of exercise is accompanied by declines in AMPK, Akt, and MAPK signaling in obese, glucose-intolerant mice. J Appl Physiol (1985) 119, 1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Heufelder AE, Saad F, Bunck MC, Gooren L (2009) Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl 30, 726–733. [DOI] [PubMed] [Google Scholar]
- [233].Cho DY, Yeo JK, Cho SI, Jung JE, Yang SJ, Kong DH, Ha JK, Kim JG, Park MG (2016) Exercise improves the effects of testosterone replacement therapy and the durability of response after cessation of treatment: A pilot randomized controlled trial. Asian J Androl. doi: 10.4103/1008-682X.184269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Sato K, Iemitsu M, Katayama K, Ishida K, Kanao Y, Saito M (2016) Responses of sex steroid hormones to different intensities of exercise in endurance athletes. Exp Physiol 101, 168–175. [DOI] [PubMed] [Google Scholar]
- [235].Horii N, Sato K, Mesaki N, Iemitsu M (2016) Increased muscular 5alpha-dihydrotestosterone in response to resistance training relates to skeletal muscle mass and glucose metabolism in type 2 diabetic rats. PLoS One 11, e0165689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Bertram S, Brixius K, Brinkmann C (2016) Exercise for the diabetic brain: How physical training may help prevent dementia and Alzheimer’s disease in T2DM patients. Endocrine 53, 350–363. [DOI] [PubMed] [Google Scholar]
- [237].Matthews VB, Astrom MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O, Akerstrom T, Yfanti C, Broholm C, Mortensen OH, Penkowa M, Hojman P, Zankari A, Watt MJ, Bruunsgaard H, Pedersen BK, Febbraio MA (2009) Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 52, 1409–1418. [DOI] [PubMed] [Google Scholar]
- [238].Hill RA, Wu YW, Kwek P, van den Buuse M (2012) Modulatory effects of sex steroid hormones on brain- derived neurotrophic factor-tyrosine kinase B expression during adolescent development in C57Bl/6 mice. J Neuroendocrinol 24, 774–788. [DOI] [PubMed] [Google Scholar]
- [239].Allen KM, Purves-Tyson TD, Fung SJ, Shannon Weickert C (2015) The effect of adolescent testosterone on hippocampal BDNF and TrkB mRNA expression: Relationship with cell proliferation. BMC Neurosci 16, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Rasika S, Alvarez-Buylla A, Nottebohm F (1999) BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron 22, 53–62. [DOI] [PubMed] [Google Scholar]
- [241].Verhovshek T, Cai Y, Osborne MC, Sengelaub DR (2010) Androgen regulates brain-derived neurotrophic factor in spinal motoneurons and their target musculature. Endocrinology 151, 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Verhovshek T, Rudolph LM, Sengelaub DR (2013) Brain- derived neurotrophic factor and androgen interactions in spinal neuromuscular systems. Neuroscience 239, 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Verhovshek T, Sengelaub DR (2013) Androgen action at the target musculature regulates brain-derived neurotrophic factor protein in the spinal nucleus of the bulbocavernosus. Dev Neurobiol 73, 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Golden E, Emiliano A, Maudsley S, Windham BG, Carlson OD, Egan JM, Driscoll I, Ferrucci L, Martin B, Mattson MP (2010) Circulating brain-derived neurotrophic factor and indices of metabolic and cardiovascular health: Data from the Baltimore Longitudinal Study of Aging. PLoS One 5, e10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, Fischer CP, Lindegaard B, Petersen AM, Taudorf S, Secher NH, Pilegaard H, Bruunsgaard H, Pedersen BK (2007) Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 50, 431–438. [DOI] [PubMed] [Google Scholar]
- [246].Huang T, Larsen KT, Ried-Larsen M, Moller NC, Andersen LB (2014) The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand J Med Sci Sports 24, 1–10. [DOI] [PubMed] [Google Scholar]
- [247].Cotman CW, Berchtold NC, Christie LA (2007) Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci 30, 464–472. [DOI] [PubMed] [Google Scholar]
- [248].Ashton WS, Degnan BM, Daniel A, Francis GL (1995) Testosterone increases insulin-like growth factor-1 and insulin-like growth factor-binding protein. Ann Clin Lab Sci 25, 381–388. [PubMed] [Google Scholar]
- [249].Hobbs CJ, Plymate SR, Rosen CJ, Adler RA (1993) Testosterone administration increases insulin-like growth factor-I levels in normal men. J Clin Endocrinol Metab 77, 776–779. [DOI] [PubMed] [Google Scholar]
- [250].Yoshizawa A, Clemmons DR (2000) Testosterone and insulin-like growth factor (IGF) I interact in controlling IGF-binding protein production in androgen-responsive foreskin fibroblasts. J Clin Endocrinol Metab 85, 1627–1633. [DOI] [PubMed] [Google Scholar]
- [251].Carro E, Nunez A, Busiguina S, Torres-Aleman I (2000) Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci 20, 2926–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]


