Abstract
The presence of Fusobacterium nucleatum (F. nucleatum) in the gut is associated with the development of colorectal cancer (CRC). F. nucleatum promotes tumor development by inducing inflammation and host immune response in the CRC microenvironment. Adhesion to the intestinal epithelium by the cell surface proteins FadA, Fap2 and RadD expressed by F. nucleatum can cause the host to produce inflammatory factors and recruit inflammatory cells, creating an environment which favors tumor growth. Furthermore, F. nucleatum can induce immune suppression of gut mucosa by suppressing the function of immune cells such as macrophages, T cells and natural killer cells, contributing the progression of CRC.
Introduction
The human intestine is home to more than 100 trillion microbes, forming a unique genome that changes with human nutrition status, geographic location, and even age [1], [2]. Gut microbiota is in harmony with the human body, affecting human health and shaping the immune system of the host [3]. Imbalance in gut microbiota can lead to a variety of diseases, such as colitis, colorectal cancer (CRC), infection, food allergies, obesity, diabetes, cardiovascular atherosclerosis, bone metabolic diseases, Parkinson's and neurodegenerative diseases [4].
The colon has been colonized by the largest density of microorganisms [5]. Recent studies have reported that certain pathogenic bacteria in the colon are associated with CRC [5], [6]. It's possible to screen CRC by detecting tumor-associated microorganisms in the feces [7]. CRC is the third most common tumor in the world, causing significant morbidity and mortality [3]. Unfortunately, the mechanism of this malignancy has not been fully explained, but inflammation is a recognized risk factor [8]. CRC is a chronic disease which can arise from other intestinal inflammatory conditions [6].
In recent years, many studies have been conducted on the correlation between inflammatory microorganisms and CRC. The occurrence of intestinal inflammatory diseases such as colitis is related to the metastasis of intestinal microbes [2]. Colitis occurs when microbes turn from a ‘eubiotic’ to a ‘dysbiotic’ state [2]. Chronic bacterial infection of the colon is a driving factor in tissue inflammation, increasing the risk of developing CRC [9].
Gut Microbiota and Host Immunity
Human bodies are constantly exposed to a diverse array of microbes, as well as their metabolite byproducts [10]. The gut microbiome influences the development of the immune system of the host, and conversely the immune system regulates the microbe composition in the gut [5], [11]. Microorganisms in the gut play a key role in the activation, training and regulation of the host immune system [12]. The communication between the intestinal microflora and the host's immune system begins at a very early stage of development [13]. Intestinal microbes can recruit immune cells and initiate inflammatory reactions, which play a direct role in promoting the maturation of the immune system [10], [14]. The intestinal mucosal immune system is a protective barrier system composed of cellular (i.e. epithelial- and mesoderm-derived immune cells) and non-cellular components (e.g. antimicrobial peptides, cytokines and antibodies), which can resist microbial attack [15].
On the one hand, intestinal microbes affect the host immune system through secretion of metabolites (e.g. butyrate, L-tryptophan, indole, bile acids and retinoic acid), signaling pathways (e.g. Toll-like receptors and Nod-like receptors), and small noncoding RNAs (e.g. miRNA) [9], [16]. On the other hand, the intestinal immune system exposes bacteria to the host, reducing pathological outcomes, and modulating the stratification of bacteria in the epithelial barrier [17]. The intestinal immune system affects the composition of bacteria, whereas the bacteria promote the development of the intestinal immune system. Disruption of the relationship between intestinal bacteria and the host immune system will affect the overall health of the host [15], [17]. Intestinal epithelial cells recognize pathogen-associated molecular structures (such as lipopolysaccharides and flagella) through their surface Toll-like receptors. This triggers the maturation of antigen-presenting cells (such as dendritic cells), the initiation of immune responses, and the release of inflammatory factors, which are associated with the development of CRC [18]. This dysregulation of the host immune system represents a potential mechanism for the effect of intestinal microbes on the development and progression of CRC [19].
Invasion of Fusobacterium nucleatum Contributes to the Carcinogenesis of CRC
Fusobacterium nucleatum (F. nucleatum) is a Gram-negative, anaerobic oral commensal bacterium that is associated with a variety of human diseases, including periodontal disease [20], Alzheimer's disease [21], brain abscess [22], cardiovascular disease [23], miscarriage [24] and inflammatory bowel disease [25]. Recently, F. nucleatum has been proposed to be associated with CRC [26]. F. nucleatum promotes the occurrence of CRC through several virulence mechanisms: colonization, invasion, and modulation of host immune response [27].
F. nucleatum bacteria interact with each other by expressing a variety of different virulence factors, and can adhere to many different mammalian cell types, including epithelial and endothelial cells, polymorph nuclear neutrophils, monocytes, erythrocytes, fibroblasts, and natural killer (NK) cells [28], [29]. The cell surface protein FadA is a key virulence factor in F. nucleatum which regulates adhesion and invasion of the bacterium. The expression of FadA gene in human CRC specimens was significantly higher than that in adjacent normal tissues [30]. This protein enables F. nucleatum to bind E-cadherin in CRC and epithelial cells, activate the β-catenin pathway, and induce the expression of transcription factors lymphoid enhancer factor (LEF)/T cell factor (TCF) which promote tumor cell growth [30], [31]. It's recently reported that FadA can up-regulate Wnt/β-catenin modulator Annexin A1 expression through E-cadherin [32]. FadA can also bind to endothelial cells VE-cadherin, which is a linker molecule on endothelial cells [33]. This combination alters the integrity of the endothelium, increases the permeability of the endothelium, and allows the bacteria to overcome the blood brain barriers, placental barriers, and colonize different parts of the body [34]. Outer membrane vesicles (OMVs) from F. nucleatum can degrade E-cadherin, thus promoting bacterial invasion and tumor metastasis [35].
In addition, F. nucleatum also has two other outer membrane proteins, Fap2 and RadD [36]. The lectin Fap2 can bind Gal-GalNAc, a polysaccharide overexpressed in CRC. This binding of Fap2 facilitates colonization of F. nucleatum and explicates fusobacteria abundance in CRC [37]. RadD can mediate communication between F. nucleatum and other bacterial species, contributing to the formation of multispecies biofilms [36], [38], which has been shown to be associated with proximal colon cancer [39].
F. nucleatum-Induced Inflammation Contributes to CRC Development
There has been a growing body of literature suggesting a link between chronic inflammation and CRC, in which gastrointestinal inflammation may promote CRC development [30]. Increased evidence suggests that F. nucleatum can shape the inflammatory microenvironment in the CRC, promoting tumor growth and metastasis [40]. For example, F. nucleatum can stimulate reactive oxygen species (ROS) production, inducing inflammatory responses in CRC cells [41]. Infection of CRC cells with F. nucleatum increased the expression of miR21, a pathogenic role in chronic inflammation and colitis-associated colon cancer, therefore promoting tumor cell proliferation and invasive activity [8], [42].
Adherence of F. nucleatum to CRC cells via FadA stimulates the release of inflammatory factors, such as NF-κB, IL-6, IL-8, IL-10 and IL-18, which promote cell proliferation in CRC [30]. In patients with F. nucleatum infection, strong humoral immunity is induced, and antibodies against F. nucleatum, IgA and IgG are present [43]. F. nucleatum infection increases the infiltration of inflammatory cells, such as macrophages, dendritic cells, and granulocytes, which create a pro-inflammatory microenvironment that is conducive to the occurrence of CRC [44]. Macrophages infected with F. nucleatum can induce the release of inflammatory cytokines [45], [46]. Natural cytotoxic receptor NKp46 of NK cell can directly recognize F. nucleatum through its surface ligand, secreting TNF-α to aggravate inflammation [20]. Some inflammatory response signatures were specific to F. nucleatum but not to other bacteria founded in CRC tissues, such as IL1β, IL24, PTGS2 (COX-2), IL8, IL6 and TNF, which were enriched in F. nucleatum-infected CRCs [44].
F. nucleatum is prevalent in gastrointestinal inflammation diseases especially inflammatory bowel disease (IBD) [25]. F. nucleatum strains originating from IBD patients were significantly more invasive than strains isolated from healthy tissues [25]. Highly invasive F. nucleatum isolates derived from the inflamed area of human Crohn's disease triggered high expression of MUC2 and TNF-α in colon cancer cells [47]. In IBD patients, the release of IL-1β and TNF-α can damage colon cells and impair epithelial integrity, which increases the chance of contact between F. nucleatum and the colon epithelium [48]. This may partially explain why patients with IBD are susceptible to CRC. Mechanisms of adhesion, invasion and inflammation mediated by F. nucleatum in CRCs were summarized in Table 1.
Table 1.
Fusobacterium nucleatum induced invasion and inflammation contributes to colorectal cancer
| Virulence factor | Function | Mechanisms | References |
|---|---|---|---|
| Fn infection | Pro-inflammatory microenvironment | Inducing inflammatory cells infiltration | [49] |
| Inflammatory cytokines production | Accumulation of reactive oxygen species | [41] | |
| Cell proliferation and invasion | Increasing the expression of miR21 | [8], [42] | |
| FadA | Cell proliferation | Activating the β-catenin pathway | [30], [31] |
| Up-regulating Wnt/β-catenin modulator Annexin A1 | [32] | ||
| Bacterial colonization | Binding endothelial cell VE-cadherin | [33], [34] | |
| Fap2 | Bacterial colonization | Binding Gal-GalNAc overexpressed in CRC | [37] |
| RadD | Biofilms formation | Mediating communication between Fn and other bacteria | [36], [38] |
| LPS | Inflammatory cytokines production | Activating immune cells | [55] |
| OMVs | Bacterial invasion and tumor metastasis | Degrading E-cadherin | [35] |
Fn, Fusobacterium nucleatum; CRC, colorectal cancer; OMV, outer membrane vesicles.
F. nucleatum-Induced Immune Suppression Promotes CRC Development
Macrophages
F. nucleatum modulates the tumor immune environment by amplifying bone marrow-derived cells [49] such as tumor-associated macrophages, which play an important role in tumor invasion and metastasis [50]. Meanwhile, the tumor microenvironment can affect the heterogeneity of macrophages, which can differentiate from pro-inflammatory M1-phenotype to a tumor-promoting M2-phenotype [51], [52].
Our recent study revealed that F. nucleatum displayed an immunosuppressive effect by promoting M2 polarization of macrophages in F. nucleatum-related CRCs, possibly through the TLR4/IL-6/p-STAT3/c-MYC signaling pathway [53]. F. nucleatum induces infiltration of M2 macrophages in the colorectal environment, thus forming a tumor-promoting microenvironment [54], [55]. The metabolite of F. nucleatum, butyric acid, can induce apoptosis in monocytes/macrophages and lymphocytes by activating free fatty acid receptors [56]. In addition, F. nucleatum can invade macrophages and induce the expression of indoleamine2,3-dioxygenase on the cell surface, creating a toxic microenvironment which impairs the function of peripheral blood lymphocytes, thereby allowing macrophages to escape cytotoxic T lymphocyte attack [55].
T cells
T cell activity can be inhibited by the virulence factors of F. nucleatum [36], [57]. F. nucleatum abundance is negatively correlated with CD3+T cell density in CRC [1]. Additionally, we have shown that a high abundance of F. nucleatum in CRC is associated with lower numbers of CD4+T cells [58]. Decreased T cell density in CRC could be explained by apoptotic cell death and arrested proliferation of T cells induced by F. nucleatum [59], [60], [61]. For example, F. nucleatum inhibitory protein (FIP) can inhibit human T cell activation by arresting cells in the G1 phase of the cell cycle [60]. A recent study revealed that the association of F. nucleatum with tumor-infiltrating lymphocytes (TIL) differed by MSI status of CRC. The presence of F. nucleatum was negatively associated with TIL in MSI-high tumors, but positively in non-MSI-high tumors [62].
In the colorectal tumor microenvironment, F. nucleatum can release short-peptides (formylmethionyl-leucyl-phenylalanine) and short-chain fatty acids (butyrate, propionate, and acetate) which lead to recruitment of myeloid-derived suppressor cells (MDSCs) [48]. MDSCs can regulate immune response by suppressing CD4+ T helper cell function, inhibiting T cell proliferation, and inducing T cell apoptosis [48], [63]. F. nucleatum can also induce human lymphocyte death through Fap2 and RadD [36]. Fap2 of F. nucleatum can inhibit human T cell activation by directly interacted with TIGIT, an inhibitory receptor present on various T cells [57]. Moreover, F. nucleatum can interact directly with monocytes, which may recruit T helper 17 cells and T regulatory cells through CCL20/CCR6 pathway, promoting CRC formation [64].
NK Cells
All human NK cells express the Fap2 receptor TIGIT, which recognizes poliovirus receptor (PVR) and nectin-2 as ligands [65]. This binding of NK cells to F. nucleatum inhibits the killing activity of NK cells, thereby promoting the formation of colorectal tumors [35], [57], [66].
Dendritic Cells
The infiltration of CD103+ dendritic cells (DCs) was increased in tumors from F. nucleatum-fed mice compared with control group [44]. This population of DCs can promote the expansion of Foxp3+ regulatory T cells, a CD4+ T cell subset that inhibits cytotoxic and effector T cells, therefore diminishing anti-tumor immunity [67].
Tumor-Associated Neutrophils
Amount of tumor-associated neutrophils (TANs) in intestinal tumors of F. nucleatum-fed mice was significantly increased compared with controls [44]. It's recently reported that TANs play a role in tumor progression and in the regulation of anti-tumor immunity [68]. Increased TANs in CRC associate with malignant phenotype and predict poor prognosis of patients with CRC [69]. These findings suggest that F. nucleatum may suppress antitumor immunity through inducing the infiltration of TANs in CRC.
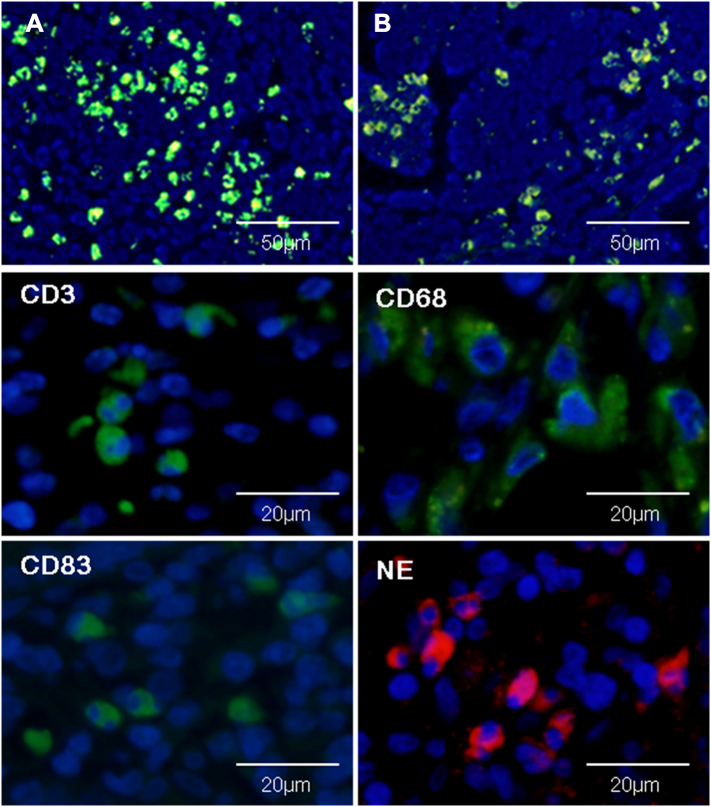
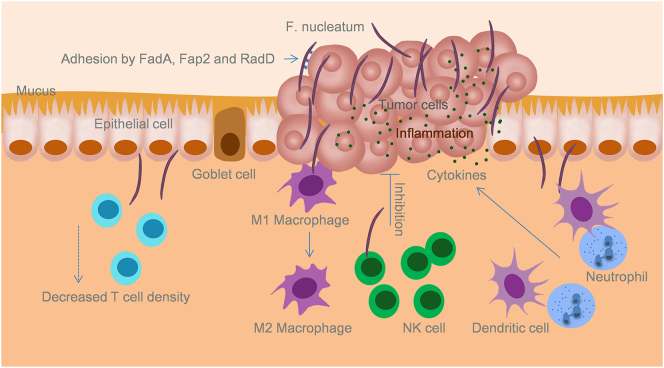
Representative images of infiltrating immune cells in F. nucleatum-related CRC were shown in Figure 1. Adhesion, invasion, inflammation and immune suppression mediated by F. nucleatum in CRC was sketched in Figure 2. Mechanisms of immune suppression induced by F. nucleatum in CRC were summarized in Table 2.
Figure 1.
Infiltrating immune cell populations in human F. nucleatum related colorectal cancer. High abundance of F. nucleatum within colon cancer tissue (A) and matched metastatic lymph nodes (B) detected by immunofluorescence. High density of immune cells (CD3+, CD68+, CD83+, and NE cells) within the environment of F. nucleatum-positive colon cancers (immunofluorescence). NE, neutrophils.
Figure 2.
F. nucleatum induces a pro-inflammatory microenvironment and suppression of host immunity that favor tumor growth within the gut mucosa. F. nucleatum binds to colon epithelium through FadA, Fap2 and RadD, and invades the mucosa. This invasion by F. nucleatum increases the infiltration of inflammatory cells and the release of cytokines which stimulate cell proliferation. Moreover, invasive F. nucleatum interacts with the immune cells in the colon mucosa, resulting in the decrease of T cell density, increased M2 macrophage polarization, inhibition of NK cell activity, and the increase of dendritic cells and tumor-associated neutrophils that diminish anti-tumor immunity. The inhibition of mucosa immunity favors tumor progression.
Table 2.
Fusobacterium nucleatum induces immune suppression in colorectal cancer
| Immune cells | Function | Mechanism | References |
|---|---|---|---|
| Macrophages | M2 polarization | Activating TLR4 signaling pathway | [53] |
| Apoptosis | Butyric acid activating free fatty acid receptors | [56] | |
| Escape T lymphocyte attack | Impairing the function of peripheral blood lymphocytes | [55] | |
| Lymphocytes | Reducing CD3 + T cells | Unknown | [1] |
| Reducing CD4 + T cells | Correlated with TOX expression | [58] | |
| Inhibiting proliferation | Arresting cells in the G1 phase | [60] | |
| Inhibiting activation | Interaction with TIGIT | [57] | |
| Apoptosis | Recruitment of MDSCs | [48], [63] | |
| Butyric acid | [56] | ||
| NK cells | Inhibition of NK cell cytotoxicity | Fap2 binding TIGIT molecule | [35], [57] |
| DCs | Dampening anti-tumor immunity | Promoting the expansion of regulatory T cells | [67] |
| TANs | Dampening anti-tumor immunity | Increasing the number of TANs | [44] |
TOX, thymocyte selection-associated high-mobility group box; MDSCs, myeloid-derived suppressor cells; NK, natural killer; DCs, dendritic cells; TANs, tumor-associated neutrophils.
Conclusion
The presence of F. nucleatum as symbiotic bacteria in the human intestinal tract has been confirmed to be related to the development of CRC. F. nucleatum promotes CRC through different virulence mechanisms, such as adhesion to the intestinal epithelium and inducing inflammatory and immune responses in the host. The resistance reactions induced in the host by F. nucleatum induce an inflammatory environment in the host, and promote the recruitment of inflammatory cells as well as the secretion of inflammatory factors. This response to F. nucleatum creates a microenvironment which favors tumor growth. Furthermore, F. nucleatum can induce immune suppression of gut mucosa by suppressing the function of immune cells such as macrophages, T cells and NK cells, contributing to the progression of CRC.
Conflicts of Interest
Authors declare no Conflict of Interests for this article.
Funding
This work was supported by Natural Science Foundation of Sichuan Science and Technology Agency under Award No. 2018JY0167.
References
- 1.Mima K., Sukawa Y., Nishihara R., Qian Z.R., Yamauchi M., Inamura K., Kim S.A., Masuda A., Nowak J.A., Nosho K. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 2015;1(5):653–661. doi: 10.1001/jamaoncol.2015.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sethi V., Kurtom S., Tarique M., Lavania S., Malchiodi Z., Hellmund L., Zhang L., Sharma U., Giri B., Garg B. Gut microbiota promotes tumor growth in mice by modulating immune response. Gastroenterology. 2018;155(1):33–37.e6. doi: 10.1053/j.gastro.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10):661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 4.Hall AB, Tolonen AC, Xavier RJ. Human genetic variation and the gut microbiome in disease. Nat Rev Genet. 2017;18(11):690–699. doi: 10.1038/nrg.2017.63. [DOI] [PubMed] [Google Scholar]
- 5.Tilg H, Adolph TE, Gerner RR, Moschen AR. The intestinal microbiota in colorectal cancer. Cancer Cell. 2018;33(6):954–964. doi: 10.1016/j.ccell.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Flemer B., Lynch D.B., Brown J.M., Jeffery I.B., Ryan F.J., Claesson M.J., O'Riordain M., Shanahan F., O'Toole P.W. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut. 2017;66(4):633–643. doi: 10.1136/gutjnl-2015-309595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eklof V., Lofgren-Burstrom A., Zingmark C., Edin S., Larsson P., Karling P., Alexeyev O., Rutegard J., Wikberg M.L., Palmqvist R. Cancer-associated fecal microbial markers in colorectal cancer detection. Int J Cancer. 2017;141(12):2528–2536. doi: 10.1002/ijc.31011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y., Weng W., Peng J., Hong L., Yang L., Toiyama Y., Gao R., Liu M., Yin M., Pan C. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-kappaB, and up-regulating expression of microRNA-21. Gastroenterology. 2017;152(4):851–866.e24. doi: 10.1053/j.gastro.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Yang Y, Huycke MM. Microbiome-driven carcinogenesis in colorectal cancer: models and mechanisms. Free Radic Biol Med. 2017;105:3–15. doi: 10.1016/j.freeradbiomed.2016.10.504. [DOI] [PubMed] [Google Scholar]
- 10.Kurilshikov A, Wijmenga C, Fu J, Zhernakova A. Host genetics and gut microbiome: challenges and perspectives. Trends Immunol. 2017;38(9):633–647. doi: 10.1016/j.it.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Zeng MY, Inohara N, Nunez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10(1):18–26. doi: 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falony G., Joossens M., Vieira-Silva S., Wang J., Darzi Y., Faust K., Kurilshikov A., Bonder M.J., Valles-Colomer M., Vandeputte D. Population-level analysis of gut microbiome variation. Science. 2016;352(6285):560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 14.Ost KS, Round JL. A few good commensals: gut microbes use ifn-gamma to fight salmonella. Immunity. 2017;46(6):977–979. doi: 10.1016/j.immuni.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Sina C, Kemper C, Derer S. The intestinal complement system in inflammatory bowel disease: Shaping intestinal barrier function. Semin Immunol. 2018;37:66–73. doi: 10.1016/j.smim.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Postler TS, Ghosh S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab. 2017;26(1):110–130. doi: 10.1016/j.cmet.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao J., Xu K., Liu H., Liu G., Bai M., Peng C., Li T., Yin Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. 2018;8:13. doi: 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cremonesi E., Governa V., Garzon J.F.G., Mele V., Amicarella F., Muraro M.G., Trella E., Galati-Fournier V., Oertli D., Daster S.R. Gut microbiota modulate T cell trafficking into human colorectal cancer. Gut. 2018;67(11):1984–1994. doi: 10.1136/gutjnl-2016-313498. [DOI] [PubMed] [Google Scholar]
- 19.De Arcangelis A., Hamade H., Alpy F., Normand S., Bruyere E., Lefebvre O., Mechine-Neuville A., Siebert S., Pfister V., Lepage P. Hemidesmosome integrity protects the colon against colitis and colorectal cancer. Gut. 2017;66(10):1748–1760. doi: 10.1136/gutjnl-2015-310847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaushu S., Wilensky A., Gur C., Shapira L., Elboim M., Halftek G., Polak D., Achdout H., Bachrach G., Mandelboim O. Direct recognition of Fusobacterium nucleatum by the NK cell natural cytotoxicity receptor NKp46 aggravates periodontal disease. PLoS Pathog. 2012;8(3) doi: 10.1371/journal.ppat.1002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sparks Stein P., Steffen M.J., Smith C., Jicha G., Ebersole J.L., Abner E., Dawson D. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer's disease. Alzheimers Dement. 2012;8(3):196–203. doi: 10.1016/j.jalz.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kai A, Cooke F, Antoun N, Siddharthan C, Sule O. A rare presentation of ventriculitis and brain abscess caused by Fusobacterium nucleatum. J Med Microbiol. 2008;57:668–671. doi: 10.1099/jmm.0.47710-0. Pt 5. [DOI] [PubMed] [Google Scholar]
- 23.Genco R, Offenbacher S, Beck J. Periodontal disease and cardiovascular disease: epidemiology and possible mechanisms. J Am Dent Assoc. 2002;(133 Suppl):14S–22S. doi: 10.14219/jada.archive.2002.0375. [DOI] [PubMed] [Google Scholar]
- 24.Chanomethaporn A., Chayasadom A., Wara-Aswapati N., Kongwattanakul K., Suwannarong W., Tangwanichgapong K., Sumanonta G., Matangkasombut O., Dasanayake A.P., Pitiphat W. Association between periodontitis and spontaneous abortion: A case-control study. J Periodontol. 2018 doi: 10.1002/JPER.18-0174. [DOI] [PubMed] [Google Scholar]
- 25.Strauss J., Kaplan G.G., Beck P.L., Rioux K., Panaccione R., Devinney R., Lynch T., Allen-Vercoe E. Invasive potential of gut mucosa-derived Fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm Bowel Dis. 2011;17(9):1971–1978. doi: 10.1002/ibd.21606. [DOI] [PubMed] [Google Scholar]
- 26.Sears CL. The who, where and how of fusobacteria and colon cancer. Elife. 2018 doi: 10.7554/eLife.28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bullman S., Pedamallu C.S., Sicinska E., Clancy T.E., Zhang X., Cai D., Neuberg D., Huang K., Guevara F., Nelson T. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017;358(6369):1443–1448. doi: 10.1126/science.aal5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23:141–147. doi: 10.1016/j.mib.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Baba Y., Ishimoto T., Iwatsuki M., Hiyoshi Y., Miyamoto Y., Yoshida N., Wu R., Baba H. Progress in characterizing the linkage between Fusobacterium nucleatum and gastrointestinal cancer. J Gastroenterol. 2019;54(1):33–41. doi: 10.1007/s00535-018-1512-9. [DOI] [PubMed] [Google Scholar]
- 30.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14(2):195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y., Peng Y., Yu J., Chen T., Wu Y., Shi L., Li Q., Wu J., Fu X. Invasive Fusobacterium nucleatum activates beta-catenin signaling in colorectal cancer via a TLR4/P-PAK1 cascade. Oncotarget. 2017;8(19):31802–31814. doi: 10.18632/oncotarget.15992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubinstein M.R., Baik J.E., Lagana S.M., Han R.P., Raab W.J., Sahoo D., Dalerba P., Wang T.C., Han Y.W. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/beta-catenin modulator Annexin A1. EMBO Rep. 2019 doi: 10.15252/embr.201847638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vander Haar EL, So J, Gyamfi-Bannerman C, Han YW. Fusobacterium nucleatum and adverse pregnancy outcomes: Epidemiological and mechanistic evidence. Anaerobe. 2018;50:55–59. doi: 10.1016/j.anaerobe.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fardini Y., Wang X., Temoin S., Nithianantham S., Lee D., Shoham M., Han Y.W. Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Mol Microbiol. 2011;82(6):1468–1480. doi: 10.1111/j.1365-2958.2011.07905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashemi Goradel N., Heidarzadeh S., Jahangiri S., Farhood B., Mortezaee K., Khanlarkhani N., Negahdari B. Fusobacterium nucleatum and colorectal cancer: A mechanistic overview. J Cell Physiol. 2019;234(3):2337–2344. doi: 10.1002/jcp.27250. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan C.W., Ma X., Paranjpe A., Jewett A., Lux R., Kinder-Haake S., Shi W. Fusobacterium nucleatum outer membrane proteins Fap2 and RadD induce cell death in human lymphocytes. Infect Immun. 2010;78(11):4773–4778. doi: 10.1128/IAI.00567-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abed J., Emgard J.E., Zamir G., Faroja M., Almogy G., Grenov A., Sol A., Naor R., Pikarsky E., Atlan K.A. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe. 2016;20(2):215–225. doi: 10.1016/j.chom.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaplan CW, Lux R, Haake SK, Shi W. The Fusobacterium nucleatum outer membrane protein RadD is an arginine-inhibitable adhesin required for inter-species adherence and the structured architecture of multispecies biofilm. Mol Microbiol. 2009;71(1):35–47. doi: 10.1111/j.1365-2958.2008.06503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu J., Chen Y., Fu X., Zhou X., Peng Y., Shi L., Chen T., Wu Y. Invasive Fusobacterium nucleatum may play a role in the carcinogenesis of proximal colon cancer through the serrated neoplasia pathway. Int J Cancer. 2016;139(6):1318–1326. doi: 10.1002/ijc.30168. [DOI] [PubMed] [Google Scholar]
- 40.Brennan CA, Garrett WS. Gut microbiota, inflammation, and colorectal cancer. Annu Rev Microbiol. 2016;70:395–411. doi: 10.1146/annurev-micro-102215-095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang B., Wang K., Jia Y.P., Zhu P., Fang Y., Zhang Z.J., Mao X.H., Li Q., Zeng D.Z. Fusobacterium nucleatum-induced impairment of autophagic flux enhances the expression of proinflammatory cytokines via ROS in Caco-2 cells. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0165701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi C., Yang Y., Xia Y., Okugawa Y., Yang J., Liang Y., Chen H., Zhang P., Wang F., Han H. Novel evidence for an oncogenic role of microRNA-21 in colitis-associated colorectal cancer. Gut. 2016;65(9):1470–1481. doi: 10.1136/gutjnl-2014-308455. [DOI] [PubMed] [Google Scholar]
- 43.Wang H.F., Li L.F., Guo S.H., Zeng Q.Y., Ning F., Liu W.L., Zhang G. Evaluation of antibody level against Fusobacterium nucleatum in the serological diagnosis of colorectal cancer. Sci Rep. 2016;6 doi: 10.1038/srep33440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kostic A.D., Chun E., Robertson L., Glickman J.N., Gallini C.A., Michaud M., Clancy T.E., Chung D.C., Lochhead P., Hold G.L. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14(2):207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park S.R., Kim D.J., Han S.H., Kang M.J., Lee J.Y., Jeong Y.J., Lee S.J., Kim T.H., Ahn S.G., Yoon J.H. Diverse Toll-like receptors mediate cytokine production by Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans in macrophages. Infect Immun. 2014;82(5):1914–1920. doi: 10.1128/IAI.01226-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noh E.J., Kang M.J., Jeong Y.J., Lee J.Y., Park J.H., Choi H.J., Oh S.M., Lee K.B., Kim D.J., Shin J.A. Withaferin A inhibits inflammatory responses induced by Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans in macrophages. Mol Med Rep. 2016;14(1):983–988. doi: 10.3892/mmr.2016.5326. [DOI] [PubMed] [Google Scholar]
- 47.Dharmani P, Strauss J, Ambrose C, Allen-Vercoe E, Chadee K. Fusobacterium nucleatum infection of colonic cells stimulates MUC2 mucin and tumor necrosis factor alpha. Infect Immun. 2011;79(7):2597–2607. doi: 10.1128/IAI.05118-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bashir A, Miskeen AY, Hazari YM, Asrafuzzaman S, Fazili KM. Fusobacterium nucleatum, inflammation, and immunity: the fire within human gut. Tumour Biol. 2016;37(3):2805–2810. doi: 10.1007/s13277-015-4724-0. [DOI] [PubMed] [Google Scholar]
- 49.Keku TO, McCoy AN, Azcarate-Peril AM. Fusobacterium spp. and colorectal cancer: cause or consequence? Trends Microbiol. 2013;21(10):506–508. doi: 10.1016/j.tim.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edin S., Wikberg M.L., Dahlin A.M., Rutegard J., Oberg A., Oldenborg P.A., Palmqvist R. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0047045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edin S, Wikberg ML, Oldenborg PA, Palmqvist R. Macrophages: Good guys in colorectal cancer. Oncoimmunology. 2013;2(2) doi: 10.4161/onci.23038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen W., Xu Y., Zhong J., Wang H., Weng M., Cheng Q., Wu Q., Sun Z., Jiang H., Zhu M. MFHAS1 promotes colorectal cancer progress by regulating polarization of tumor-associated macrophages via STAT6 signaling pathway. Oncotarget. 2016;7(48):78726–78735. doi: 10.18632/oncotarget.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen T., Li Q., Wu J., Wu Y., Peng W., Li H., Wang J., Tang X., Peng Y., Fu X. Fusobacterium nucleatum promotes M2 polarization of macrophages in the microenvironment of colorectal tumours via a TLR4-dependent mechanism. Cancer Immunol Immunother. 2018;67(10):1635–1646. doi: 10.1007/s00262-018-2233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park HE, Kim JH, Cho NY, Lee HS, Kang GH. Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma. Virchows Arch. 2017;471(3):329–336. doi: 10.1007/s00428-017-2171-6. [DOI] [PubMed] [Google Scholar]
- 55.Xue Y., Xiao H., Guo S., Xu B., Liao Y., Wu Y., Zhang G. Indoleamine 2,3-dioxygenase expression regulates the survival and proliferation of Fusobacterium nucleatum in THP-1-derived macrophages. Cell Death Dis. 2018;9(3):355. doi: 10.1038/s41419-018-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abe K. Butyric acid induces apoptosis in both human monocytes and lymphocytes equivalently. J Oral Sci. 2012;54(1):7–14. doi: 10.2334/josnusd.54.7. [DOI] [PubMed] [Google Scholar]
- 57.Gur C., Ibrahim Y., Isaacson B., Yamin R., Abed J., Gamliel M., Enk J., Bar-On Y., Stanietsky-Kaynan N., Coppenhagen-Glazer S. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42(2):344–355. doi: 10.1016/j.immuni.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen T., Li Q., Zhang X., Long R., Wu Y., Wu J., Fu X. TOX expression decreases with progression of colorectal cancers and is associated with CD4 T-cell density and Fusobacterium nucleatum infection. Hum Pathol. 2018;79:93–101. doi: 10.1016/j.humpath.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 59.Huynh T., Kapur R.V., Kaplan C.W., Cacalano N., Kinder Haake S., Shi W., Sieling P., Jewett A. The role of aggregation in Fusobacterium nucleatum-induced immune cell death. J Endod. 2011;37(11):1531–1535. doi: 10.1016/j.joen.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 60.Shenker BJ, Datar S. Fusobacterium nucleatum inhibits human T-cell activation by arresting cells in the mid-G1 phase of the cell cycle. Infect Immun. 1995;63(12):4830–4836. doi: 10.1128/iai.63.12.4830-4836.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shenker BJ, DiRienzo JM. Suppression of human peripheral blood lymphocytes by Fusobacterium nucleatum. J Immunol. 1984;132(5):2357–2362. [PubMed] [Google Scholar]
- 62.Hamada T., Zhang X., Mima K., Bullman S., Sukawa Y., Nowak J.A., Kosumi K., Masugi Y., Twombly T.S., Cao Y. Fusobacterium nucleatum in colorectal cancer relates to immune response differentially by tumor microsatellite instability status. Cancer Immunol Res. 2018;6(11):1327–1336. doi: 10.1158/2326-6066.CIR-18-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268.64. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye X., Wang R., Bhattacharya R., Boulbes D.R., Fan F., Xia L., Adoni H., Ajami N.J., Wong M.C., Smith D.P. Fusobacterium nucleatum subspecies animalis influences proinflammatory cytokine expression and monocyte activation in human colorectal tumors. Cancer Prev Res (Phila) 2017;10(7):398–409.65. doi: 10.1158/1940-6207.CAPR-16-0178. [DOI] [PubMed] [Google Scholar]
- 65.Stanietsky N., Simic H., Arapovic J., Toporik A., Levy O., Novik A., Levine Z., Beiman M., Dassa L., Achdout H. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2009;106(42):17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guevarra L.A., Jr., Afable A.C.F., Belza P.J.O., Dy K.J.S., Lee S.J.Q., Sy-Ortin T.T., Albano P. Immunogenicity of a Fap2 peptide mimotope of Fusobacterium nucleatum and its potential use in the diagnosis of colorectal cancer. Infect Agent Cancer. 2018;13:11. doi: 10.1186/s13027-018-0184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coombes J.L., Siddiqui K.R., Arancibia-Carcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204(8):1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 69.Rao H.L., Chen J.W., Li M., Xiao Y.B., Fu J., Zeng Y.X., Cai M.Y., Xie D. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients' adverse prognosis. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0030806. [DOI] [PMC free article] [PubMed] [Google Scholar]