Abstract
Background
The link between body mass index (BMI) and disease characteristics in rheumatoid arthritis (RA) remains controversial. Body composition (BC) has been more frequently recommended to be used instead of BMI for more accurate assessment. Our study aimed to investigate the characteristics of BC in RA patients and their associations with disease characteristics.
Methods
Body composition was assessed in consecutive Chinese RA patients and control subjects by bioelectric impedance analysis. Overfat was defined by body fat percentage (BF%) as ≥25% for men and ≥35% for women. Myopenia was defined by appendicular skeletal muscle mass index (ASMI) ≤7.0 kg/m2 in men and ≤5.7 kg/m2 in women. BMI and clinical data including disease activity, function, and radiographic assessment were collected. Active disease was defined by disease activity score in 28 joints with four variables including C‐reactive protein (DAS28‐CRP) ≥2.6. Functional limitation was defined as Stanford health assessment questionnaire disability index (HAQ‐DI) >1. Radiographic joint damage (RJD) was defined as the Sharp/van der Heijde modified sharp score (mTSS) >10.
Results
There were 457 RA patients (mean age 49.5 ± 13.1 years old with 82.7% women) and 1860 control subjects (mean age 34.3 ± 9.9 years old with 51.2% women) recruited. Comparisons of BMI and BC between RA patients and control subjects in age and gender stratification showed that lower BMI with 17.7% underweight and lower ASMI with 45.1% myopenia are the main characteristics in RA patients. Compared with those without myopenia, RA patients with myopenia had significantly higher DAS28‐CRP (median 3.5 vs. 3.0), higher HAQ‐DI (median 0.38 vs. 0.13) with higher rate of functional limitation (24.8% vs. 7.6%), and higher mTSS (median 22.3 vs. 9.0) with more RJD (71.8% vs. 45.8%) (all P < 0.001). Multivariate logistic regression analysis showed myopenia were positively associated with functional limitation (OR = 2.546, 95% CI: 1.043–6.217) and RJD (OR = 2.660, 95% CI: 1.443–4.904). All RA patients were divided into four BC subgroups according to overfat and myopenia. Those with both overfat and myopenia had the worst disease characteristics. After adjustment for confounding factors, significant additive interactions were observed between overfat and myopenia in active disease (AP = 0.528, 95% CI: 0.086–0.971), functional limitation (AP = 0.647, 95% CI: 0.356–0.937), and RJD (AP = 0.514, 95% CI: 0.139–0.890).
Conclusions
Myopenia is very common in RA patients that is associated with functional limitation and joint damage in RA. Further research on the underlying mechanism and the effect of skeletal muscle mass improvement in RA management are worth exploring in the future.
Keywords: rheumatoid arthritis, body composition, myopenia, overfat, radiographic joint damage
Introduction
Rheumatoid arthritis (RA) is a common inflammatory autoimmune disease leading to irreversible erosive joint destruction, disability, and impaired quality of life.1 During the last decades, research on metabolic status in the pathophysiology of RA has increased dramatically. Some researchers indicated that increasing body mass index (BMI) in RA patients was associated with higher risk for RA development1 and worse disease outcomes,2, 3, 4 while others showed no association with disease outcomes,5, 6 or even contrasting results such as reduced risks of RA development,7 radiographic damage,8, 9 and early death.10 The link between BMI and RA disease characteristics remains controversial. In addition, BMI has been challenged for the limitation of failure to differentiate comprising tissues of the body. Fat mass and other tissues are components of total weight and can vary enormously among individuals. People with low lean mass but high fat mass may still have a normal BMI. Skeletal muscle mass was found to be decreased in RA by dual X‐ray absorptiometry (DXA) measurement.11 Rheumatoid cachexia is characterized by loss of muscle with or without weight loss in the presence of stable or increased fat mass. Although there is no consensus criterion for diagnosis of rheumatoid cachexia, it has been reported ranging from 17% to 60% in RA.12, 13, 14
Instead of BMI, more accurate body composition (BC), including fat mass, muscle mass, and other tissues, has been frequently recommended to assess metabolic status. There are four main techniques commonly used to evaluate BC in clinical practice: DXA, computed tomography, magnetic resonance imaging, and bioelectric impedance analysis (BIA). Because DXA, computed tomography, and magnetic resonance imaging are radioactive or expensive and time‐consuming techniques requiring trained professionals, BIA was adopted in this study. BIA is reliable, objective, practical, inexpensive, and does not require highly trained personnel.15 In this cross‐sectional study, we investigated the characteristics of BC in consecutive RA patients comparing with control subjects in terms of their associated disease characteristics including RA disease activity, functional limitation, and radiographic joint damage (RJD).
Materials and Methods
Study patients and controls
Consecutive Chinese patients with RA aged older than 16 years who fulfilled the 1987 revised criteria of the American College of Rheumatology16 or 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for RA17 were recruited from September 2015 to July 2017 at the Department of Rheumatology, Sun Yat‐Sen Memorial Hospital, Guangzhou, China. Control subjects were white‐collar employees in Zhangjiang InnoPark of Shanghai voluntarily participating in this study from June 2015 to August 2016. Exclusion criteria included cancer, current infection or pregnancy, severe mental disorders, and implanted electronic devices. This study was conducted in compliance with the Declaration of Helsinki, and the protocol was approved by the Medical Ethics Committee of Sun Yat‐Sen Memorial Hospital (SYSEC‐2009‐06). All participants gave their written informed consent before clinical data collection.
Body mass index and body composition
Demographic data and anthropometric measurements of RA patients and control subjects were collected. Weight was measured to the nearest 0.1 kg without shoes, socks, bulky clothing, and other accessories. Height was measured to the nearest 0.01 m without shoes and socks using a stadiometer. BMI (kg/m2) was calculated as weight (kg) divided height (m) squared. As recommended by the Working Group on Obesity in China,18 subjects were categorized by BMI as underweight (BMI < 18.5 kg/m2), normal weight (18.5 kg/m2 ≤ BMI < 24 kg/m2), overweight (24 kg/m2 ≤ BMI < 28 kg/m2), and obese (BMI ≥ 28 kg/m2).
Body composition was assessed by BIA using an InBody 230 device19 (Biospace Co., Shanghai, China), which measures BC indicators including body fat percentage (BF%), the mass and distribution of muscle and fat in trunk, and appendicular extremities. Appendicular skeletal muscle mass index (ASMI) was defined as appendicular skeletal muscle mass/height2 (kg/m2). Overfat was defined by BF% as ≥25% for men and ≥35% for women.20 Myopenia was defined by ASMI ≤7.0 kg/m2 in men and ≤5.7 kg/m2 in women according to Asian Working Group for Sarcopenia.21
Clinical and radiographic assessment
Clinical data of RA patients were collected simultaneously as in our previous report22 and modified according to 2017 European League Against Rheumatism recommendation23 including disease duration, time of morning stiffness, 28‐joint tender and swollen joint count (28TJC and 28SJC), patient and provider global assessment of disease activity (PtGA and PrGA, range 0–10), pain visual analogue scale (Pain VAS), erythrocyte sedimentation rate [ESR, mm/h, 0–20 mm/h (female), 0–15 mm/h (male)], C‐reactive protein (CRP, mg/L, 0–5 mg/L), rheumatoid factor (RF, mg/L, 0–20 mg/L), and anti‐cyclic citrullinated peptide antibody (ACPA, IU/mL, 0–18 IU/mL). Disease activity was assessed with simplified disease activity index (SDAI), clinical disease activity index (CDAI), and disease activity score in 28 joints with four variables including CRP (DAS28‐CRP). Disease activity defined by DAS28‐CRP was divided into four categories: high disease activity (DAS28‐CRP > 5.1), moderate disease activity (3.2 ≤ DAS28‐CRP ≤ 5.1), low disease activity (2.6 ≤ DAS28‐CRP < 3.2), and remission (DAS28‐CRP < 2.6). Active disease was defined by DAS28‐CRP ≥ 2.6.24 Chinese language version of Stanford health assessment questionnaire disability index (HAQ‐DI) was used to assess physical activity function in eight categories (dressing, rising, eating, walking, hygiene, reaching, griping, and activities). Functional limitation was defined as HAQ‐DI > 1.25
Conventional radiographs of bilateral hands and wrists (anteroposterior view) were performed on all RA patients and assessed with the Sharp/van der Heijde modified Sharp score by two experienced observers (C. M. H. from radiology and M. J. D. from rheumatology) who were blinded to clinical data as we described previously.26 Sixteen areas for joint erosion (JE) and 15 for joint space narrowing (JSN) of hands were assessed in each hand/wrist. The maximum score per single joint for JE is 5 and for JSN is 4, with the sum of JE (0–160) and JSN (0–120) subscores constituting total modified Sharp score (mTSS, 0–280).27 Reliability and agreement were assessed using an intra‐class correlation coefficient and the mean intra‐class correlation coefficient for inter‐observer agreement was 0.977. Subjects with mTSS>10 were considered having RJD.25
Statistical analysis
Statistical analyses were performed with spss for Windows 20.0 statistical software (IBM, Armonk, NY, USA). Data were presented as frequencies and percentages for categorical variables, and mean and standard deviation or median and interquartile range for continuous variables according to distributions. Two independent samples t‐test or Mann–Whitney U‐test were used to compare the differences of continuous variables according to distributions between two groups. Fisher's exact test and χ2 test were used for categorical variables in two groups. Kruskal–Wallis test was used for comparison in four BMI categorizations and four BC subgroups. Bonferroni correction was used for multiple comparisons in four BC subgroups. Univariate logistic regression analyses were used to identify the association of BMI and BC with RA disease characteristics including active disease, functional limitation, and RJD. Further multivariate logistic regression analyses were performed to confirm their significant associations, adjusted for potential confounders including age, gender, smoking habits, disease duration, RF status, ACPA status, and previous treatment [treatment naïve (yes/no), glucocorticoid use (yes/no), methotrexate use (yes/no), leflunomide use (yes/no), biologic agents use (yes/no)], as well as adding BMI and BC indicators. Interaction, defined as departure from additivity of effects, was evaluated between overfat and myopenia in RA disease characteristics including active disease, functional limitation, and RJD. The attributable proportion (AP) due to interaction with 95% CI was calculated as Andersson et al. described.28 Multivariate logistic regression analyses were performed to confirm their interactions, adjusted for age, gender, BMI, smoking habits, disease duration, RF status, ACPA status, and previous treatment [treatment naïve (yes/no), glucocorticoid use (yes/no), methotrexate use (yes/no), leflunomide use (yes/no), biologic agents use (yes/no)]. All significance tests were two‐tailed and were conducted at the 5% significance level.
Results
Baseline characteristics of rheumatoid arthritis patients and control subjects
There were 457 RA patients and 1860 control subjects recruited, and the baseline characteristics of RA patients were shown in Table 1. In RA group, the mean age was 49.5 ± 13.1 years old with 82.7% women. The median disease duration was 54 months (range 1–480), 3.5% with short disease duration (<6 months), and 72.0% with long disease duration (>24 months). There were 35.4% RA patients in remission, 14.2% in low disease activity, 36.1% in moderate disease activity, and 14.2% in high disease activity defined by DAS28‐CRP. There were 93.2% RA patients with bony erosion and 16.8% patients without previous glucocorticoids or disease modifying anti‐rheumatic drugs therapy for 6 months before enrolment (treatment naïve). There were 65 (14.2%) RA patients with hypertension and 37 (8.1%) RA patients with type 2 diabetes mellitus. In control group, the mean age was 34.3 ± 9.9 years old with 51.2% women. Compared with control subjects, RA patients were older (49.5 ± 13.1 years vs. 34.3 ± 9.9 years, P < 0.001) with the predominance of female (82.7% vs. 51.2%, P < 0.001).
Table 1.
Baseline characteristics of rheumatoid arthritis patients
| Baseline characteristics | RA patients (n = 457) |
|---|---|
| Female, n (%) | 378 (82.7) |
| Age, years, mean ± SD | 49.5 ± 13.1 |
| Disease duration, month, median (IQR) | 54 (24–118) |
| BMI, kg/m2, mean ± SD | 21.8 ± 3.4 |
| Smoking habits | |
| Active smoking, n (%) | 68 (14.9) |
| Exposure to second hand smoke, n (%) | 139 (30.4) |
| Without exposure to smoke, n (%) | 250 (54.7) |
| Positive RF, n (%) | 300 (65.6) |
| Positive ACPA, n (%) | 317 (69.4) |
| Core disease activity indicators | |
| 28TJC, median (IQR) | 2 (0–5) |
| 28SJC, median (IQR) | 1 (0–4) |
| ESR, (mm/h), median (IQR) | 27 (15–47) |
| CRP, (mg/L), median (IQR) | 4.6 (3.3–14.5) |
| DAS28‐CRP, median (IQR) | 3.2 (2.0–4.3) |
| SDAI, median (IQR) | 11.0 (4.3–21.3) |
| CDAI, median (IQR) | 10 (4–19) |
| Functional indicators | |
| HAQ‐DI, median (IQR) | 0.25 (0–0.75) |
| Functional limitation, n (%) | 70 (15.3) |
| Radiographic assessment | |
| mTSS, median (IQR) | 13.0 (4.5–38.3) |
| Bony erosion, n (%) | 426 (93.2) |
| RJD, n (%) | 263 (57.5) |
| Previous medications | |
| Treatment naïve, n (%) | 77 (16.8) |
| Glucocorticosteroids, n (%) | 243 (53.2) |
| Methotrexate, n (%) | 299 (65.4) |
| Leflunomide, n (%) | 236 (51.6) |
| Biologic agents, n (%) | 30 (6.6) |
Functional limitation, HAQ‐DI > 1; Bony erosion, JE subscore > 0; RJD, radiographic joint damage (mTSS > 10); treatment naïve, without previous glucocorticosteroids or DMARDs therapy for 6 months before enrolment; IQR, interquartile range; SD, standard deviation.
Body mass index and body composition in rheumatoid arthritis patients
The mean BMI in RA and control groups were 21.8 ± 3.4 kg/m2 vs. 22.6 ± 3.2 kg/m2, in line with 17.7% vs. 7.8% with underweight, 58.0% vs. 61.8% with normal weight, 20.1% vs. 25.2% with overweight, and 4.2% vs. 5.2% with obese, respectively. In view of the significant differences in gender and age between two groups, BMI and BC were compared between RA patients and control subjects in age and gender stratification (Figures 1 and 2). The male RA patients had significantly lower BMI than controls in all age subgroups except age 31–40 years old, together with greater proportion of underweight and less proportion of overweight and obesity. The female RA patients had lower BMI than controls in age ≤30 and 51–60 years old subgroups and had greater proportion of underweight and less proportion of normal weight in age ≤30 and 31–40 years old subgroups (all P < 0.05, Figure 1).
Figure 1.
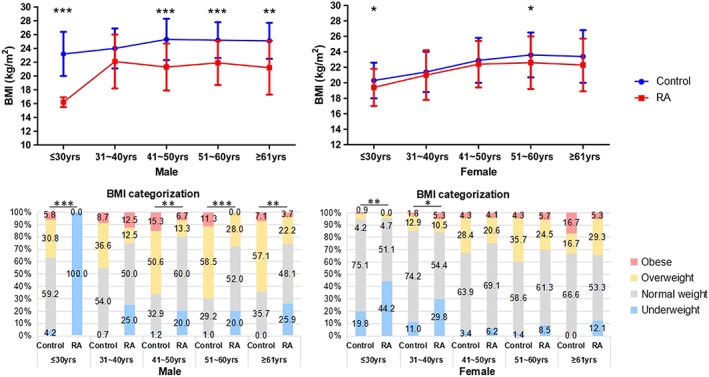
Comparisons of BMI between RA patients and control subjects in age and gender stratification. Underweight, BMI < 18.5 kg/m2; Normal weight, 18.5 kg/m2 ≤ BMI < 24 kg/m2; Overweight, 24 kg/m2 ≤ BMI < 28 kg/m2; Obese, BMI ≥ 28 kg/m2; * P < 0.05, ** P < 0.01, *** P < 0.001. Male: ≤30 years old, control n = 380, RA n = 4; 31–40 years old, control n = 322, RA n = 8; 41–50 years old, control n = 85, RA n = 15; 51–60 years old, control n = 106, RA n = 25; ≥61 years old, control n = 14, RA n = 27. Female: ≤30 years old, control n = 429, RA n = 43; 31–40 years old, control n = 326, RA n = 57; 41–50 years old, control n = 116, RA n = 97; 51–60 years old, control n = 70, RA n = 106; ≥61 years old, control n = 12, RA n = 75.
Figure 2.
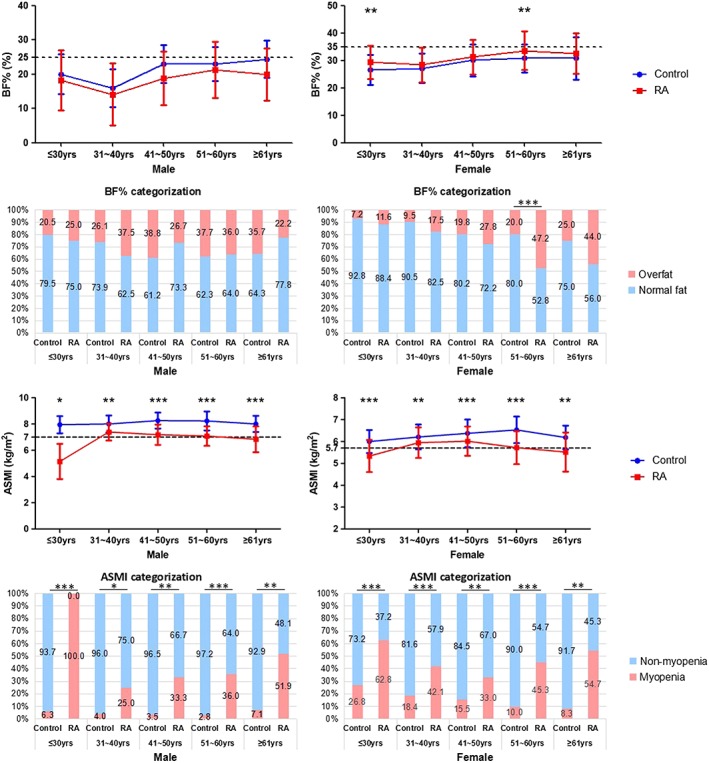
Comparisons of BC between RA patients and control subjects in age and gender stratification. Overfat, BF% ≥ 25% for men and ≥ 35% for women; Myopenia, ASMI ≤ 7.0 kg/m2 in men and ≤5.7 kg/m2 in women; * P < 0.05, ** P < 0.01, *** P < 0.001. Male: ≤30 years old, control n = 380, RA n = 4; 31–40 years old, control n = 322, RA n = 8; 41–50 years old, control n = 85, RA n = 15; 51–60 years old, control n = 106, RA n = 25; ≥61 years old, control n = 14, RA n = 27. Female: ≤30 years old, control n = 429, RA n = 43; 31–40 years old, control n = 326, RA n = 57; 41–50 years old, control n = 116, RA n = 97; 51–60 years old, control n = 70, RA n = 106; ≥61 years old, control n = 12, RA n = 75.
The mean BF% in RA and control groups were 29.6 ± 8.3% vs. 24.7 ± 6.5%, in line with 32.4% vs. 18.4% with overfat, respectively. The mean ASMI in RA and control groups were 6.0 ± 0.9 kg/m2 vs. 7.1 ± 1.1 kg/m2, in line with 45.1% (43.0% in male RA patients and 45.5% in female RA patients) vs. 13.2% with myopenia, respectively. Comparisons of the BC between RA patients and control subjects in age and gender stratification were shown in Figure 2. The male RA patients had significantly lower ASMI and higher prevalence of myopenia than controls in all age subgroups, while no differences in BF% and the prevalence of overfat were found in all age subgroups. The female RA patients also had significantly lower ASMI and higher prevalence of myopenia in all age subgroups, together with significantly higher BF% in age ≤30 and 51–60 years old subgroups and higher prevalence of overfat in age 51–60 years old subgroup (all P < 0.05).
Comparisons of disease characteristics among rheumatoid arthritis patients in body mass index or body composition categorizations
The comparisons of disease characteristics among RA patients in BMI categorization showed no significant difference except for age, morning stiffness, and JSN subscore (Supporting Information, Table S1 ). Compared with RA patients with normal weight, RA patients with underweight were younger (median 40 years vs. 50 years, P = 0.001) with higher JSN subscore (median 7.0 vs. 3.0, P = 0.042), while RA patients with overweight were older (median 54 years vs. 50 years, P = 0.003) with longer morning stiffness.
The comparisons of disease characteristics between RA patients in BF% or ASMI subgroups were showed in Table 2. Compared with RA patients with normal fat, RA patients with overfat were older with longer disease duration, higher RA disease activity indicators, including PtGA, PrGA, CRP, DAS28‐CRP, SDAI, and CDAI, higher functional indicators including HAQ‐DI and the rate of functional limitation, and higher radiographic assessment index including mTSS, JE subscore, and the rate of bony erosions as well as RJD. In contrast to RA patients without myopenia, RA patients with myopenia had longer disease duration, higher disease activity including almost all core disease activity indicators, higher functional indicators including HAQ‐DI and the rate of functional limitation, and higher radiographic scores including mTSS, JSN and JE subscores, and the rate of RJD (all P < 0.05).
Table 2.
Comparisons of disease characteristics between rheumatoid arthritis patients in body fat percentage or appendicular skeletal muscle mass index subgroups
| Characteristics | BF% | ASMI | ||||
|---|---|---|---|---|---|---|
| Normal fat (n = 309) | Overfat (n = 148) | P | Non‐myopenia (n = 251) | Myopenia (n = 206) | P | |
| Female, n (%) | 253 (81.9) | 125 (84.5) | 0.494 | 206 (82.1) | 172 (83.5) | 0.689 |
| Age, years, median (IQR) | 49 (37–58) | 54 (48–61) | <0.001 | 50 (42–57) | 52 (38–61) | 0.370 |
| Disease duration, month, median (IQR) | 48 (24–96) | 64 (27–141) | 0.005 | 48 (24–96) | 72 (24–120) | 0.013 |
| BMI, kg/m2, mean ± SD | 20.3 ± 2.4 | 24.8 ± 3.1 | <0.001 | 23.4 ± 3.2 | 19.9 ± 2.6 | <0.001 |
| Smoking habits | 0.905 | 0.108 | ||||
| Active smoking, n (%) | 46 (14.9) | 22 (14.9) | 38 (15.1) | 30 (14.6) | ||
| Exposure to second hand smoke, n (%) | 92 (29.8) | 47 (31.8) | 86 (34.3) | 53 (25.7) | ||
| Without exposure to smoke, n (%) | 171 (55.3) | 79 (53.4) | 127 (50.6) | 123 (59.7) | ||
| Positive RF, n (%) | 203 (65.7) | 97 (65.5) | 0.974 | 158 (62.9) | 142 (68.9) | 0.180 |
| Positive ACPA, n (%) | 212 (68.6) | 105 (70.9) | 0.612 | 179 (71.3) | 138 (67.0) | 0.318 |
| Core disease activity indicators | ||||||
| Morning stiffness, min, median (IQR) | 0 (0–10) | 0 (0–15) | 0.191 | 0 (0–10) | 0 (0–15) | 0.921 |
| 28TJC, median (IQR) | 2 (0–5) | 2 (0–8) | 0.145 | 1 (0–4) | 3 (0–7) | 0.005 |
| 28SJC, median (IQR) | 1 (0–4) | 1 (0–4) | 0.248 | 1 (0–3) | 2 (0–6) | 0.016 |
| PtGA, median (IQR) | 3 (1–5) | 4 (2–6) | 0.049 | 2 (0–5) | 4 (1–6) | <0.001 |
| PrGA, median (IQR) | 3 (1–5) | 4 (2–6) | 0.016 | 2 (0–5) | 4 (1–6) | <0.001 |
| Pain VAS, median (IQR) | 2 (2–4) | 3 (2–4) | 0.131 | 2 (1–4) | 3 (2–4) | <0.001 |
| ESR, (mm/h), median (IQR) | 26 (15–44) | 32 (15–52) | 0.133 | 24 (14–40) | 32 (16–63) | <0.001 |
| CRP, (mg/L), median (IQR) | 3.8 (3.3–12.9) | 5.4 (3.3–20.0) | 0.008 | 3.4 (3.3–10.4) | 7.2 (3.3–23.9) | <0.001 |
| DAS28‐CRP, median (IQR) | 3.2 (2.0–4.1) | 3.4 (2.3–4.8) | 0.043 | 3.0 (1.9–4.0) | 3.5 (2.4–5.0) | <0.001 |
| SDAI, median (IQR) | 10.3 (3.8–18.3) | 12.3 (5.2–25.6) | 0.023 | 9.3 (3.3–17.1) | 13.8 (5.1–26.0) | 0.001 |
| CDAI, median (IQR) | 10 (3–17) | 12 (4–24) | 0.047 | 9 (2–16) | 12 (4–24) | 0.001 |
| Functional indicators | ||||||
| HAQ‐DI, median (IQR) | 0.13 (0–0.50) | 0.38 (0–1.00) | <0.001 | 0.13 (0–0.50) | 0.38 (0–1.03) | <0.001 |
| Functional limitation, n (%) | 38 (12.3) | 32 (21.6) | 0.010 | 19 (7.6) | 51 (24.8) | <0.001 |
| Radiographic assessment | ||||||
| mTSS, median (IQR) | 11.0 (3.8–32.5) | 16.5 (6.6–50.8) | 0.005 | 9.0 (3.0–25.0) | 22.3 (8.0–59.8) | <0.001 |
| JSN subscore, median (IQR) | 3.0 (0–11.8) | 3.0 (0–20.0) | 0.158 | 1.0 (0–7.0) | 8.0 (1.0–30.3) | <0.001 |
| JE subscore, median (IQR) | 9.0 (2.5–21.0) | 11.8 (6.0–29.8) | 0.001 | 6.5 (2.5–16.5) | 15.0 (5.9–32.3) | <0.001 |
| Bony erosion, n (%) | 282 (91.3) | 144 (97.3) | 0.016 | 233 (92.8) | 193 (93.7) | 0.716 |
| RJD, n (%) | 168 (54.4) | 95 (64.2) | 0.047 | 115 (45.8) | 148 (71.8) | <0.001 |
Overfat, BF% ≥ 25% for men and ≥35% for women; Myopenia, ASMI ≤ 7.0kg/m2 in men and ≤5.7kg/m2 in women; functional limitation, HAQ‐DI > 1; Bony erosion, JE subscore > 0; RJD, radiographic joint damage (mTSS > 10); IQR, interquartile range.
Associations of body mass index and body composition with rheumatoid arthritis disease characteristics
To explore the associations of BMI and BC with RA disease characteristics including active disease, functional limitation, and RJD, univariate and multivariate logistic regression analyses were performed (Table 3). There were 64.6% RA patients with active disease. Univariate logistic regression analysis showed that myopenia was positively associated, and ASMI was negatively associated with active disease in RA patients, while BMI, BMI categorization, BF%, or overfat showed no association. After adjustment for age, gender, smoking habits, disease duration, RF status, ACPA status, and previous treatment, ASMI was still associated with active disease. After further adjustment for the effects of BMI and BF% and confounding factors above, multivariate logistic regression showed that ASMI was still associated with active disease (OR = 0.454, 95% CI: 0.224–0.920, P = 0.028).
Table 3.
Logistic regression analysis of the associations of body mass index and body composition with rheumatoid arthritis disease characteristics
| BMI and BC | Active disease | Functional limitation | RJD | |||
|---|---|---|---|---|---|---|
| OR 95% CI | P | OR 95% CI | P | OR 95% CI | P | |
| Univariate | ||||||
| BMI | 0.982 (0.927–1.040) | 0.526 | 0.931 (0.860–1.009) | 0.080 | 0.964 (0.913–1.019) | 0.196 |
| Normal weight | 1.0 NA | NA | 1.0 NA | NA | 1.0 NA | NA |
| Underweight | 0.938 (0.551–1.597) | 0.815 | 1.040 (0.537–2.014) | 0.907 | 1.517 (0.903–2.548) | 0.115 |
| Overweight | 1.119 (0.670–1.870) | 0.667 | 0.660 (0.325–1.338) | 0.249 | 1.036 (0.644–1.665) | 0.884 |
| Obese | 0.463 (0.176–1.217) | 0.118 | 0.277 (0.036–2.126) | 0.217 | 1.102 (0.429–2.828) | 0.840 |
| BF% | 1.006 (0.983–1.030) | 0.622 | 1.018 (0.987–1.050) | 0.266 | 1.023 (1.000–1.046) | 0.047 |
| Overfat | 1.366 (0.888–2.100) | 0.156 | 1.967 (1.172–3.303) | 0.010 | 1.504 (1.005–2.253) | 0.047 |
| ASMI | 0.732 (0.592–0.904) | 0.004 | 0.429 (0.315–0.584) | <0.001 | 0.576 (0.463–0.718) | <0.001 |
| Myopenia | 1.666 (1.125–2.466) | 0.011 | 4.018 (2.284–7.066) | <0.001 | 3.018 (2.039–4.467) | <0.001 |
| Multivariatea | ||||||
| BF% | NA | NA | NA | NA | 0.998 (0.969–1.028) | 0.907 |
| Overfat | NA | NA | 1.659 (0.911–3.021) | 0.098 | 1.068 (0.675–1.689) | 0.779 |
| ASMI | 0.743 (0.564–0.978) | 0.034 | 0.341 (0.229–0.509) | <0.001 | 0.491 (0.364–0.662) | <0.001 |
| Myopenia | 1.490 (0.967–2.295) | 0.071 | 3.957 (2.078–7.532) | <0.001 | 3.057 (1.969–4.746) | <0.001 |
| Multivariateb | ||||||
| ASMI | 0.454 (0.224–0.920) | 0.028 | 0.123 (0.050–0.307) | <0.001 | 0.356 (0.177–0.713) | 0.004 |
| Myopenia | 1.461 (0.781–2.730) | 0.235 | 2.546 (1.043–6.217) | 0.040 | 2.660 (1.443–4.904) | 0.002 |
Active disease, DAS28‐CRP ≥ 2.6; functional limitation, HAQ‐DI > 1; RJD, radiographic joint damage (mTSS>10); OR, odds ratio in logistic regression; 95% CI, 95% confidence interval; NA, not applicable.
Adjusted for age, gender, smoking habits, disease duration, RF status, ACPA status and previous treatment [treatment naïve (yes/no), glucocorticosteroids use (yes/no), methotrexate use (yes/no), leflunomide use (yes/no), biologic agents use (yes/no)].
Adjusted for age, gender, BMI, BF%, smoking habits, disease duration, RF status, ACPA status and previous treatment [treatment naïve (yes/no), glucocorticosteroids use (yes/no), methotrexate use (yes/no), leflunomide use (yes/no), biologic agents use (yes/no)].
There were 15.3% RA patients with functional limitation. Univariate logistic regression analysis showed that overfat and myopenia were positively associated with functional limitation, while ASMI was associated negatively. After adjustment for age, gender, smoking habits, disease duration, RF status, ACPA status, and previous treatment, myopenia was positively associated with functional limitation, while ASMI was associated negatively. After further adjustment for the effects of BMI and BF% and confounding factors above, multivariate logistic regression showed that ASMI (OR = 0.123, 95% CI: 0.050–0.307, P < 0.001) and myopenia (OR = 2.546, 95% CI: 1.043–6.217, P = 0.040) were still associated with functional limitation.
There were 57.5% RA patients with RJD. Univariate logistic regression analysis showed that BF%, overfat, and myopenia were positively associated with RJD, while ASMI was associated negatively. After adjustment for age, gender, smoking habits, disease duration, RF status, ACPA status, and previous treatment, myopenia was positively associated with RJD, while ASMI was associated negatively. After adjustment for the effect of BMI and BF% and confounding factors above, multivariate logistic regression showed that ASMI (OR = 0.356, 95% CI: 0.177–0.713, P = 0.004) and myopenia (OR = 2.660, 95% CI: 1.443–4.904, P = 0.002) were still associated with RJD.
Interaction between overfat and myopenia
According to overfat and myopenia, all RA patients were divided into four BC subgroups, and their disease characteristics were compared in Table 4. There were 167 (36.5%) RA patients with normal fat and non‐myopenia, 84 (18.4%) with overfat but non‐myopenia, 142 (31.1%) with normal fat but myopenia, and 64 (14.0%) with both overfat and myopenia. RA patients with both overfat and myopenia had approximate BMI compared with those with normal fat and non‐myopenia (22.4 ± 2.4 kg/m2 vs. 21.7 ± 2.1 kg/m2, P = 0.113). There were significant differences in age, disease duration, almost all core disease activity indicators, functional indicators, and radiographic assessment indicators among four subgroups. Compared with normal fat and non‐myopenia subgroup, RA patients with overfat but non‐myopenia were older, while RA patients with normal fat but myopenia had higher functional indicators including HAQ‐DI and the rate of functional limitation, higher radiographic assessment index including mTSS, subscores of JSN and JE, and the rate of RJD. Moreover, RA patients with both overfat and myopenia had the highest ESR, CRP, HAQ‐DI, the highest rate of functional limitation, mTSS, and JE subscore in comparison with other three subgroups, respectively. In addition, RA patients with both overfat and myopenia had significantly higher core disease activity indicators including PtGA, PrGA, Pain VAS, DAS28‐CRP, SDAI, CDAI, and higher rate of active disease, higher JSN subscore, and higher rate of RJD, when compared with normal fat and non‐myopenia subgroup as well as with overfat but non‐myopenia subgroup, respectively (all P < 0.0083, Bonferroni correction).
Table 4.
Comparisons of disease characteristics among rheumatoid arthritis patients in body composition subgroups
| Characteristics | Normal fat and non‐myopenia (n = 167) | Overfat but non‐myopenia (n = 84) | Normal fat but myopenia (n = 142) | Overfat and myopenia (n = 64) | P a |
|---|---|---|---|---|---|
| Female, n (%) | 136 (81.4) | 70 (83.3) | 117 (82.4) | 55 (85.9) | 0.876 |
| Age, years, median (IQR) | 48 (40–56) | 53 (46–61) b | 50 (35–61) | 55 (49–62) b | <0.001 |
| Disease duration, month, median (IQR) | 40 (18–84) | 60 (29–120) | 60 (24–108) | 96 (24–153) b | 0.002 |
| BMI, kg/m2, mean ± SD | 21.7 ± 2.1 | 26.6 ± 2.2 b | 18.7 ± 1.7 b , c | 22.4 ± 2.4 c , d | <0.001 |
| Smoking habits | 0.588 | ||||
| Active smoking, n (%) | 25 (15.0) | 13 (15.5) | 21 (14.8) | 9 (14.1) | |
| Exposure to second hand smoke, n (%) | 56 (33.5) | 30 (35.7) | 36 (25.4) | 17 (26.6) | |
| Without exposure to smoke, n (%) | 86 (51.5) | 41 (48.8) | 85 (59.9) | 38 (59.4) | |
| Positive RF, n (%) | 108 (64.7) | 50 (59.5) | 95 (66.9) | 47 (73.4) | 0.349 |
| Positive ACPA, n (%) | 121 (72.5) | 58 (69.0) | 91 (64.1) | 47 (73.4) | 0.374 |
| Core disease activity indicators | |||||
| Morning stiffness, min, median (IQR) | 0 (0–10) | 0 (0–10) | 0 (0–10) | 0 (0–30) | 0.626 |
| 28TJC, median (IQR) | 1 (0–4) | 2 (0–5) | 2 (0–7) | 4 (1–10) b | 0.012 |
| 28SJC, median (IQR) | 1 (0–4) | 1 (0–3) | 1 (0–4) | 2 (0–8) b | 0.024 |
| PtGA, median (IQR) | 2 (0–5) | 3 (1–5) | 3 (1–6) | 5 (2–7) b , c | 0.001 |
| PrGA, median (IQR) | 2 (0–4) | 2 (1–5) | 3 (1–5) | 5 (2–7) b , c | <0.001 |
| Pain VAS, median (IQR) | 2 (1–4) | 2 (1–4) | 2 (2–4) | 4 (2–6) b , c | 0.001 |
| ESR, (mm/h), median (IQR) | 25 (15–38) | 24 (12–40) | 28 (14–52) | 45 (24–75) b , c , d | <0.001 |
| CRP, (mg/L), median (IQR) | 3.3 (3.3–10.8) | 4.0 (3.3–7.3) | 4.8 (3.3–19.1) | 13.1 (3.4–42.4) b , c , d | <0.001 |
| DAS28‐CRP, median (IQR) | 2.9 (1.9–3.9) | 3.1 (2.0–4.2) | 3.3 (2.1–4.3) | 3.9 (2.7–5.5) b , c | <0.001 |
| DAS28‐CRP ≥ 2.6, n (%) | 99 (59.3) | 50 (59.5) | 94 (66.2) | 52 (81.2) b , c | 0.012 |
| SDAI, median (IQR) | 8.3 (3.3–16.7) | 10.5 (4.3–20.7) | 11.3 (4.3–20.6) | 18.7 (6.1–33.9) b , c | <0.001 |
| CDAI, median (IQR) | 8.0 (2.0–16.0) | 9.5 (2.5–19.8) | 10 (4–20) | 16 (4–33) b , c | 0.001 |
| Functional indicators | |||||
| HAQ‐DI, median (IQR) | 0 (0–0.38) | 0.19 (0–0.72) | 0.25 (0–0.88) b | 0.75 (0.12–1.47) b , c , d | <0.001 |
| Functional limitation, n (%) | 12 (7.2) | 7 (8.3) | 26 (18.3) b | 25 (39.1) b , c , d | <0.001 |
| Radiographic assessment | |||||
| mTSS, median (IQR) | 7.5 (2.5–25.0) | 10.3 (4.6–26.8) | 20.3 (7.4–51.0) b | 26.5 (11.9–114.9) b , c , d | <0.001 |
| JSN subscore, median (IQR) | 1.0 (0–7.5) | 1.5 (0–6.5) | 6.0 (0.5–22.0) b , c | 12.8 (1.6–46.4) b , c | <0.001 |
| JE subscore, median (IQR) | 5.5 (2.0–15.5) | 7.5 (4.1–18.0) | 13.0 (4.0–27.3) b | 19.8 (10.0–65.3) b , c , d | <0.001 |
| Bony erosion, n (%) | 152 (91.0) | 81 (96.4) | 130 (91.5) | 63 (98.4) | 0.110 |
| RJD, n (%) | 73 (43.7) | 42 (50.0) | 95 (66.9) b | 53 (82.8) b , c | <0.001 |
Overfat, BF% ≥ 25% for men and ≥ 35% for women; Myopenia, ASMI≤7.0 kg/m2 in men and ≤ 5.7 kg/m2 in women; Functional limitation, HAQ‐DI > 1; Bony erosion, JE subscore>0; RJD, radiographic joint damage (mTSS>10); IQR, interquartile range.
Comparison in four groups by Kruskal–Wallis test.
Compared with Normal fat + Non‐myopenia patients in Bonferroni correction, P < 0.0083.
Compared with Overfat + Non‐myopenia patients in Bonferroni correction, P < 0.0083.
Compared with Normal fat + Myopenia patients in Bonferroni correction, P < 0.0083.
Additive interaction between overfat and myopenia in RA disease characteristics were shown in Table 5. After adjustment for age, gender, BMI, smoking habits, disease duration, RF status, ACPA status, and previous treatment, multivariate logistic regression analysis showed that myopenia with normal fat had a higher probability accompanied by RJD (OR = 2.339, 95% CI: 1.283–4.263) when compared with normal fat without myopenia, while overfat without myopenia showed no association with RA disease characteristics. In addition, overfat in conjunction with myopenia had higher probabilities accompanied by active disease (OR = 2.185, 95% CI: 1.021–4.673), functional limitation (OR = 7.611, 95% CI: 3.065–18.901), and RJD (OR = 4.736, 95% CI: 2.141–10.480). Moreover, significant additive interactions were observed between overfat and myopenia in active disease (AP = 0.528, 95% CI: 0.086–0.971), functional limitation (AP = 0.647, 95% CI: 0.356–0.937), and RJD (AP = 0.514, 95% CI: 0.139–0.890).
Table 5.
Additive interaction between overfat and myopenia in rheumatoid arthritis disease characteristics
| Factors | Active disease (OR 95% CI) | Functional limitation (OR 95% CI) | RJD (OR 95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Overfat | Myopenia | Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate |
| − | − | 1.0 NA | 1.0 NA | 1.0 NA | 1.0 NA | 1.0 NA | 1.0 NA |
| + | − | 1.010 (0.592–1.723) | 0.011 (0.455–2.245) | 1.174 (0.445–3.102) | 1.886 (0.497–7.150) | 1.288 (0.761–2.178) | 0.961 (0.436–2.117) |
| − | + | 1.345 (0.845–2.141) | 1.020 (0.548–1.898) | 2.895 (1.402–5.978) | 1.803 (0.727–4.476) | 2.603 (1.636–4.141) | 2.339 (1.283–4.263) |
| + | + | 2.976 (1.479–5.991) | 2.185 (1.021–4.673) | 8.280 (3.823–17.931) | 7.611 (3.065–18.901) | 6.204 (3.027–12.718) | 4.736 (2.141–10.480) |
| AP 95% CI | 0.545 (0.146–0.943) | 0.528 (0.086–0.971) | 0.629 (0.358–0.901) | 0.647 (0.356–0.937) | 0.534 (0.183–0.885) | 0.514 (0.139–0.890) | |
Active disease, DAS28‐CRP ≥ 2.6; functional limitation, HAQ‐DI > 1; RJD, radiographic joint damage (mTSS>10); OR, odds ratio in logistic regression; 95% CI, 95% confidence interval; AP, attributable proportion due to additive interaction; NA, not applicable.
Multivariate, adjusted for age, gender, BMI, smoking habits, disease duration, RF status, ACPA status and previous treatment [treatment naïve (yes/no), glucocorticosteroids use (yes/no), methotrexate use (yes/no), leflunomide use (yes/no), biologic agents use (yes/no)].
Discussion
This cross‐sectional study compared BMI and BC between 457 Chinese RA patients and 1860 control subjects in age and gender stratification and found that lower BMI with 17.7% underweight and lower ASMI with 45.1% myopenia are their main characteristics of metabolic status in Chinese RA patients. RA patients with myopenia had worse disease characteristics including significantly higher indicators of disease activity, higher rate of functional limitation, and radiographic scores. Further multivariate regression analysis confirmed the association of ASMI and myopenia with functional limitation and RJD. This is the first report of the association of myopenia with RA joint destruction, which implies that further research on the underlying mechanism and the effect of skeletal muscle mass improvement in RA management are worth exploring in future.
Asian patients with RA reported less obesity but more underweight than that of Western countries. In contrast to published RA data from the US and Germany National Database for Rheumatic Diseases, our data of Chinese RA patients confirmed the lower prevalence of obesity (China 4.2% vs. Western 21.4–34.7%) and higher prevalence of underweight (China 17.7% vs. Western 1.1–2.2%).29, 30 Furthermore, our RA patients also showed lower prevalence of obesity (4.2% vs. 13.1%) but higher prevalence of underweight (17.7% vs. 3.8%) compared with Chinese adults from Chinese Center for Disease Control and Prevention.31 RA individuals reported almost 2 kg/m2 BMI values lower than those of healthy controls for the same level of body fat.32 Previous studies reported the association between low BMI and joint damage,25 and rapid weight loss was a strong predictor of death in RA patients.33 In our study, RA patients had significantly lower BMI and higher prevalence of underweight than those of control subjects. In contrast, our data showed no significant difference of disease characteristics among RA patients in BMI categorization and no association between BMI and RA disease characteristics including joint damage in logistic regression analyses.
There were 32.4% RA patients with overfat in this study. However, no association was found in BF% or overfat with RA disease characteristics in multivariate regression. The fat tissue can produce a wide variety of adipokines. Elevated leptin, adiponectin, resistin, and visfatin have been reported in serum and synovial fluid of RA patients that are correlated with disease parameters, such as ESR, CRP, DAS28, HAQ score, and radiographic damage.34 These results are supported by experiments of mouse. Leptin‐deficient mice had less severe arthritis than wild‐type mice.35 Adiponectin injection in collagen‐induced arthritis mice resulted in more severe arthritic progression.36 Mouse cartilage explants treated with resistin released increased levels of inflammatory cytokines.37 Visfatin inhibitor effectively reduced mice arthritis severity and decreased pro‐inflammatory cytokine secretion.38 However, clinical data showed a surprising protective action of obesity on RJD in RA patients.8, 9 The controversial effect of obesity and fat tissue on RA disease characteristics remains elusive.
Chronic inflammatory response in RA is a highly energy‐consuming process, inducing a hypermetabolic and catabolic state with increased resting energy expenditure, which results in loss of muscle mass despite adequate dietary intake.12 Previous studies showed that decreased muscle mass is associated with worse outcomes even higher mortality in some circumstance, such as liver cirrhosis, cancer, and older adults.39, 40, 41 Thus, valid, reliable, accurate, and cost‐effective tools for BC measurement, instead of BMI, are necessary for assessing RA patients. Previous studies reported 37–57% male RA patients and 13–43.3% female RA patients having myopenia under different definitions.11, 42, 43 Decreased skeletal muscle mass especially appendicular muscle was shown to be associated with low physical function in RA.12 However, the association of myopenia with RA joint destruction has not been reported. Our study showed that RA patients had lower ASMI and higher prevalence of myopenia (45.1% vs. 13.2%) than controls. In RA patients, less ASMI was associated with worse disease characteristics including active disease, functional limitation, and RJD. Each 1 kg/m2 increase in ASMI, the risk of active disease, functional limitation, and RJD decreased 54.6%, 87.7%, and 64.4% respectively after adjustment for age, gender, BMI, BF%, smoking habits, disease duration, RF status, ACPA status, and previous treatment. Myopenia was associated with increased risks of both functional limitation by 2.546‐fold and RJD by 2.660‐fold.
The concept of biological interaction as originally described by Rothman44 is commonly used in the context of disease aetiology, for example, when examining gene–environment interaction. Biological interaction between two risk factors is defined as a deviation from additivity of the absolute effects of the two factors in question. Synergy, known as additive interaction, is defined as the resulting effect is more than the sum of the absolute effects of the two factors. In our study, there were 14.0% patients with both overfat and myopenia, showing approximate BMI compared with those with normal fat and non‐myopenia. These characteristics were similar to rheumatoid cachexia. Rheumatoid cachexia was reported to be associated with higher CRP, worse disease activity, and physical disability by a Swedish RA case–control study.45 However, another study failed to confirm the association.46 Sarcopenic obesity and ectopic fatty infiltration of skeletal muscles have been observed in other diseases such as liver cirrhosis and diffuse large B‐cell lymphoma, where its presence is associated with particularly poor disease control and prognosis.39, 40 In our study, compared with three other BC subgroups based on overfat and myopenia, RA patients with both overfat and myopenia had the worst indicators of almost all disease characteristics. There were significant additive interactions between overfat and myopenia in all RA disease characteristics with AP 52.8% in active disease, 64.7% in functional limitation, and 51.4% in RJD, respectively, indicating the resulting effects of active disease, functional limitation, and RJD were more than the sum of the absolute effects of overfat and myopenia. This is the first report of the additive interaction between overfat and myopenia in RA disease characteristics.
The low mobility caused by functional limitation may lead to the loss of muscle mass and vice versa. However, the causality and underlying mechanism of myopenia and joint destruction remain elusive. Elevated pro‐inflammatory cytokines, such as TNF‐α, IL‐1β, and IL‐6, can promote osteoclastic bone resorption and inhibit osteoblast differentiation, meanwhile suppress myogenic proliferation and differentiation as well as increase muscle degradation, resulting in joint destruction and muscle wasting. Although the effect of TNF‐α inhibitors on muscle mass is conflict in different studies,12 RA patients reported a significant gain in appendicular lean mass after 1 year treatment with IL‐6 receptor antagonist tocilizumab.47 Mechanical factors play significant roles in both muscle and bone through affecting on the differentiation of mesenchymal stem cells into osteoblasts and myotubes.48 Interestingly, recent studies indicated that both muscle and bone are considered as mutually impacted endocrine organs.48 Myostatin, a skeletal muscle‐derived endocrine factor, is a negative regulator of muscle growth and regeneration, which leads to muscle hypertrophy. Myostatin not only induces IL‐1β expression in RA synovial fibroblasts through the activin‐like kinase receptor signalling pathways, resulting in severe inflammation,49 but also enhances RANKL‐induced osteoclastogenesis contributing to bone degradation in inflammatory arthritis.50 Whether myostatin play roles in the pathogenesis of myopenia‐related joint damage in RA is worth further exploration.
The practical implication of our finding about the effect of skeletal muscle mass improvement in RA management are worth exploring. Some adjuvant treatments had been tried to attenuate muscle loss in RA patients. Wilkinson et al.51 investigated the effectiveness of oral creatine supplementation for 24 weeks. They found that creatine increased muscle mass but not strength or objective physical function. Another study found diet addition of essential pro‐anabolic amino acids had no better effect on BC than a mixture of non‐essential amino acids.52 Resistance training is known as a treatment for muscle mass increase and improves BC. Morsley et al.53 reported that BF% in 83 patients with RA and other inflammatory arthritis decreased by 1.78 ± 8.22% and HAQ‐DI decreased by 0.24 ± 0.62 (both P < 0.0001) after 6‐week progressive resistance training. Several studies supported the effectiveness of mixed programme of aerobic and resistance training on BC.54 Stavropoulos‐Kalinoglou et al.55 also found that the mixed programme of aerobic and resistance training is effective in reducing cardiovascular risk in RA.
There are several limitations of our study. Firstly, it was designed as a cross‐sectional investigation at single centre. Risk factors and outcome measurement at the same timeframe made it scientifically inappropriate to determine the causality between myopenia and RA joint destruction. The baseline characteristics of recruited RA patients in our study were similar to the Chinese Registry of Rheumatoid Arthritis56 including 13 210 RA patients (80.6% female, mean age 52.9 years, and median disease duration 4.0 years) from 173 centres in 31 provinces all over China. Secondly, the controls in our study comprising mainly white‐collar volunteer employees were younger than RA group featuring with lower prevalence of obesity (5.2% vs. 13.1%) and slightly higher prevalence of underweight (7.8% vs. 3.8%) than that of Chinese adults in the registry.31 For adjusting selection bias, we stratified RA patients and control subjects by age and gender for statistical analysis. Epidemiological data have showed that the incidence rates of RA are about three‐fold higher in women than men, with predominance (approximate 80%) of middle‐aged disease onset.57 Although in line with the features of RA incidence, our data showed only few male RA patients in age ≤30 (n = 4) and 31–40 (n = 8) years old subgroups. More male RA patients, especially younger patients, are needed in further study. Thirdly, cut‐offs from different BIA manufacturers are not interchangeable. Because the lack of an available InBody device cut‐off derived from the Asian population, we applied AGWS cut‐offs established by Tanimoto in 2012 which used a different Tanita device.21 BIA method itself has some limitations, including its accuracy mainly due to the problem of the individual prediction error and availability of population‐specific equations to predict lean mass according to the reference standard used to validate the BIA equation. Fortunately, there are several reliable and evidence‐based equations available for the more precise assessment of metabolic status in RA patients.58 A future large scale of multi‐community based epidemiological survey on general population and multi‐centre prospective studies on RA patients are needed to address these limitations in our study and make potentially novel discoveries.
In conclusion, myopenia is very common in Chinese RA patients, which is associated with functional limitation and joint damage. Further research on the underlying mechanism and the effect of skeletal muscle mass improvement in RA management are worth exploring in large study.
Author contributions
L. J. Z. and L. J. J. contributed equally to this work, including conceiving and designing the study, reading and analysing documents, performing the statistical analysis, and drafting the manuscript. Corresponding authors X. D. and D. L. also conceived and participated in its design, advised on the search, read and analysed documents, and edited the paper. L. Q. H. and M. Y. Q. participated in clinical assessment and BC measurement of RA patients and critically revised the manuscript. C. W. M. and H. X. L. participated in BC measurement of control subjects. L. N. participated in the design, provided statistical advice, and critically revised the manuscript. M. J. D. and C. M. H. carried out the radiographic assessment and critically revised the manuscript. All authors read and approved the final manuscript.
Conflict of interest
None declared.
Ethical approval
This study was conducted in compliance with the Declaration of Helsinki. The Medical Ethics Committee of Sun Yat‐Sen Memorial Hospital approved the protocol (SYSEC‐2009‐06). All patients agreed to participate in this study and signed written informed consent. This study has obtained consent to publish from the participants (or legal parent or guardian for children) to report individual patient data. Details that might disclose the identity of the participants under study have been omitted.
Funding
This work was supported by National Natural Science Foundation of China (grant nos 81671612 and 81601427), Natural Science Foundation of Guangdong Province (grant nos 2016A030313307 and 2017A030313576), and the Fundamental Research Funds for the Central Universities (grant no. 17ykjc12).
Supporting information
Table S1. Comparisons of disease characteristics among RA patients in BMI categorization
Acknowledgements
We thank all subjects and medical staff who generously contributed to this study. We thank Yan‐Fang Ye from Clinical Research Design Division, Sun Yat‐Sen Memorial Hospital, who kindly provided statistical advice for this manuscript. The authors certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017.59
Lin, J.‐Z. , Liang, J.‐J. , Ma, J.‐D. , Li, Q.‐H. , Mo, Y.‐Q. , Cheng, W.‐M. , He, X.‐L. , Li, N. , Cao, M.‐H. , Xu, D. , and Dai, L. (2019) Myopenia is associated with joint damage in rheumatoid arthritis: a cross‐sectional study. Journal of Cachexia, Sarcopenia and Muscle, 10: 355–367. 10.1002/jcsm.12381.
Contributor Information
Dan Xu, Email: daniel.xu@curtin.edu.au.
Lie Dai, Email: dailie@mail.sysu.edu.cn.
References
- 1. Qin B, Yang M, Fu H, Ma N, Wei T, Tang Q, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose‐response meta‐analysis. Arthritis Res Ther 2015;17:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ajeganova S, Andersson ML, Hafström I, BARFOT Study Group . Association of obesity with worse disease severity in rheumatoid arthritis as well as with comorbidities: a long‐term followup from disease onset. Arthritis Care Res (Hoboken) 2013;65:78–87. [DOI] [PubMed] [Google Scholar]
- 3. Heimans L, van den Broek M, le Cessie S, Siegerink B, Riyazi N, Han KH, et al. Association of high body mass index with decreased treatment response to combination therapy in recent‐onset rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2013;65:1235–1242. [DOI] [PubMed] [Google Scholar]
- 4. Stavropoulos‐Kalinoglou A, Metsios GS, Panoulas VF, Nevill AM, Jamurtas AZ, Koutedakis Y, et al. Underweight and obese states both associate with worse disease activity and physical function in patients with established rheumatoid arthritis. Clin Rheumatol 2009;28:439–444. [DOI] [PubMed] [Google Scholar]
- 5. Choe JY, Bae J, Lee H, Park SH, Kim SK. Lack association of body mass index with disease activity composites of rheumatoid arthritis in Korean population: cross‐sectional observation. Clin Rheumatol 2014;33:485–492. [DOI] [PubMed] [Google Scholar]
- 6. Pers YM, Godfrin‐Valnet M, Lambert J, Fortunet C, Constant E, Mura T, et al. Response to tocilizumab in rheumatoid arthritis is not influenced by the body mass index of the patient. J Rheumatol 2015;42:580–584. [DOI] [PubMed] [Google Scholar]
- 7. Turesson C, Bergström U, Pikwer M, Nilsson JÅ, Jacobsson LT. A high body mass index is associated with reduced risk of rheumatoid arthritis in men, but not in women. Rheumatology (Oxford) 2016;55:307–314. [DOI] [PubMed] [Google Scholar]
- 8. Baker JF, Ostergaard M, George M, Shults J, Emery P, Baker DG, et al. Greater body mass independently predicts less radiographic progression on X‐ray and MRI over 1‐2 years. Ann Rheum Dis 2014;73:1923–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Helm‐van Mil AH, van der Kooij SM, Allaart CF, Toes RE, Huizinga TW. A high body mass index has a protective effect on the amount of joint destruction in small joints in early rheumatoid arthritis. Ann Rheum Dis 2008;67:769–774. [DOI] [PubMed] [Google Scholar]
- 10. George MD, Baker JF. The obesity epidemic and consequences for rheumatoid arthritis care. Curr Rheumatol Rep 2016;18:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doğan SC, Hizmetli S, Hayta E, Kaptanoğlu E, Erselcan T, Güler E. Sarcopenia in women with rheumatoid arthritis. Eur J Rheumatol 2015;2:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Challal S, Minichiello E, Boissier MC, Semerano L. Cachexia and adiposity in rheumatoid arthritis. Relevance for disease management and clinical outcomes. Joint Bone Spine 2016;83:127–133. [DOI] [PubMed] [Google Scholar]
- 13. Santillán‐Díaz C, Ramírez‐Sánchez N, Espinosa‐Morales R, Orea‐Tejeda A, Llorente L, Rodríguez‐Guevara G, et al. Prevalence of rheumatoid cachexia assessed by bioelectrical impedance vector analysis and its relation with physical function. Clin Rheumatol 2018;37:607–614. [DOI] [PubMed] [Google Scholar]
- 14. Pineda‐Juárez JA, Lozada‐Mellado M, Ogata‐Medel M, Hinojosa‐Azaola A, Santillán‐Díaz C, Llorente L, et al. Body composition evaluated by body mass index and bioelectrical impedance vector analysis in women with rheumatoid arthritis. Nutrition 2018;53:49–53. [DOI] [PubMed] [Google Scholar]
- 15. Kim M, Shinkai S, Murayama H, Mori S. Comparison of segmental multifrequency bioelectrical impedance analysis with dual‐energy X‐ray absorptiometry for the assessment of body composition in a community‐dwelling older population. Geriatr Gerontol Int 2015;15:1013–1022. [DOI] [PubMed] [Google Scholar]
- 16. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–324. [DOI] [PubMed] [Google Scholar]
- 17. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–2581. [DOI] [PubMed] [Google Scholar]
- 18. Zhou BF. Cooperative Meta‐Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—study on optimal cut‐off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci 2002;15:83–96. [PubMed] [Google Scholar]
- 19. Lee LW, Liao YS, Lu HK, Hsiao PL, Chen YY, Chi CC, et al. Validation of two portable bioelectrical impedance analyses for the assessment of body composition in school age children. PLoS One 2017;12:e0171568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oliveros E, Somers VK, Sochor O, Goel K, Lopez‐Jimenez F. The concept of normal weight obesity. Prog Cardiovasc Dis 2014;56:426–433. [DOI] [PubMed] [Google Scholar]
- 21. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 22. Ma JD, Zhou JJ, Zheng DH, Chen LF, Mo YQ, Wei XN, et al. Serum matrix metalloproteinase‐3 as a noninvasive biomarker of histological synovitis for diagnosis of rheumatoid arthritis. Mediators Inflamm 2014;2014:179284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Radner H, Chatzidionysiou K, Nikiphorou E, Gossec L, Hyrich KL, Zabalan C, et al. 2017 EULAR recommendations for a core data set to support observational research and clinical care in rheumatoid arthritis. Ann Rheum Dis 2018;77:476–479. [DOI] [PubMed] [Google Scholar]
- 24. Anderson J, Caplan L, Yazdany J, Robbins ML, Neogi T, Michaud K, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken) 2012;64:640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baker JF, George M, Baker DG, Toedter G, Von Feldt JM, Leonard MB. Associations between body mass, radiographic joint damage, adipokines and risk factors for bone loss in rheumatoid arthritis. Rheumatology (Oxford) 2011;50:2100–2107. [DOI] [PubMed] [Google Scholar]
- 26. Ma JD, Wei XN, Zheng DH, Mo YQ, Chen LF, Zhang X, et al. Continuously elevated serum matrix metalloproteinase‐3 for 3~6 months predict one‐year radiographic progression in rheumatoid arthritis: a prospective cohort study. Arthritis Res Ther 2015;17:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 2000;27:261–263. [PubMed] [Google Scholar]
- 28. Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol 2005;20:575–579. [DOI] [PubMed] [Google Scholar]
- 29. Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:1471–1479. [DOI] [PubMed] [Google Scholar]
- 30. Albrecht K, Richter A, Callhoff J, Huscher D, Schett G, Strangfeld A, et al. Body mass index distribution in rheumatoid arthritis: a collaborative analysis from three large German rheumatoid arthritis databases. Arthritis Res Ther 2016;18:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deng Q, Jiang Y, Wang L. Association between body mass index and quality of life in Chinese adults. Zhonghua Liu Xing Bing Xue Za Zhi 2014;35:557–561, (Article in Chinese). [PubMed] [Google Scholar]
- 32. Stavropoulos‐Kalinoglou A, Metsios GS, Koutedakis Y, Nevill AM, Douglas KM, Jamurtas A, et al. Redefining overweight and obesity in rheumatoid arthritis patients. Ann Rheum Dis 2007;66:1316–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baker JF, Billig E, Michaud K, Ibrahim S, Caplan L, Cannon GW, et al. Weight loss, the obesity paradox, and the risk of death in rheumatoid arthritis. Arthritis Rheumatol 2015;67:1711–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev 2014;13:981–1000. [DOI] [PubMed] [Google Scholar]
- 35. Busso N, So A, Chobaz‐Péclat V, Morard C, Martinez‐Soria E, Talabot‐Ayer D, et al. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol 2002;168:875–882. [DOI] [PubMed] [Google Scholar]
- 36. Sun X, Feng X, Tan W, Lin N, Hua M, Wei Y, et al. Adiponectin exacerbates collagen‐induced arthritis via enhancing Th17 response and prompting RANKL expression. Sci Rep 2015;5:11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee JH, Ort T, Ma K, Picha K, Carton J, Marsters PA, et al. Resistin is elevated following traumatic joint injury and causes matrix degradation and release of inflammatory cytokines from articular cartilage in vitro. Osteoarthr Cartil 2009;17:613–620. [DOI] [PubMed] [Google Scholar]
- 38. Busso N, Karababa M, Nobile M, Rolaz A, Van Gool F, Galli M, et al. Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin enzymatic activity identifies a new inflammatory pathway linked to NAD. PLoS One 2008;3:e2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Montano‐Loza AJ, Angulo P, Meza‐Junco J, Prado CM, Sawyer MB, Beaumont C, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle 2016;7:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chu MP, Lieffers J, Ghosh S, Belch A, Chua NS, Fontaine A, et al. Skeletal muscle density is an independent predictor of diffuse large B‐cell lymphoma outcomes treated with rituximab‐based chemoimmunotherapy. J Cachexia Sarcopenia Muscle 2017;8:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tyrovolas S, Koyanagi A, Olaya B, Ayuso‐Mateos JL, Miret M, Chatterji S, et al. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: a multi‐continent study. J Cachexia Sarcopenia Muscle 2016;7:312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ngeuleu A, Allali F, Medrare L, Madhi A, Rkain H, Hajjaj‐Hassouni N. Sarcopenia in Rheumatoid arthritis: prevalence, influence of disease activity and associated factors. Rheumatol Int 2017;37:1015–1020. [DOI] [PubMed] [Google Scholar]
- 43. Baker JF, Long J, Ibrahim S, Leonard MB, Katz P. Are men at greater risk of lean mass deficits in rheumatoid arthritis? Arthritis Care Res (Hoboken) 2015;67:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rothman KJ. Epidemiology. An introduction. New York: Oxford University Press; 2002. [Google Scholar]
- 45. Engvall IL, Elkan AC, Tengstrand B, Cederholm T, Brismar K, Hafstrom I. Cachexia in rheumatoid arthritis is associated with inflammatory activity, physical disability, and low bioavailable insulin‐like growth factor. Scand J Rheumatol 2008;37:321–328. [DOI] [PubMed] [Google Scholar]
- 46. Lemmey AB, Wilkinson TJ, Clayton RJ, Sheikh F, Whale J, Jones HS, et al. Tight control of disease activity fails to improve body composition or physical function in rheumatoid arthritis patients. Rheumatology (Oxford) 2016;55:1736–1745. [DOI] [PubMed] [Google Scholar]
- 47. Tournadre A, Pereira B, Dutheil F, Giraud C, Courteix D, Sapin V, et al. Changes in body composition and metabolic profile during interleukin 6 inhibition in rheumatoid arthritis. J Cachexia Sarcopenia Muscle 2017;8:639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kawao N, Kaji H. Interactions between muscle tissues and bone metabolism. J Cell Biochem 2015;116:687–695. [DOI] [PubMed] [Google Scholar]
- 49. Hu SL, Chang AC, Huang CC, Tsai CH, Lin CC, Tang CH. Myostatin promotes interleukin‐1β expression in rheumatoid arthritis synovial fibroblasts through inhibition of miR‐21‐5p. Front Immunol 2017;8:1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dankbar B, Fennen M, Brunert D, Hayer S, Frank S, Wehmeyer C, et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat Med 2015;21:1085–1090. [DOI] [PubMed] [Google Scholar]
- 51. Wilkinson TJ, Lemmey AB, Jones JG, Sheikh F, Ahmad YA, Chitale S, et al. Can creatine supplementation improve body composition and objective physical function in rheumatoid arthritis patients? A randomized controlled trial. Arthritis Care Res (Hoboken) 2016;68:729–737. [DOI] [PubMed] [Google Scholar]
- 52. Marcora S, Lemmey A, Maddison P. Dietary treatment of rheumatoid cachexia with beta‐hydroxy‐beta‐methylbutyrate, glutamine and arginine: a randomised controlled trial. Clin Nutr 2005;24:442–454. [DOI] [PubMed] [Google Scholar]
- 53. Morsley K, Berntzen B, Erwood L, Bellerby T, Williamson L. Progressive resistance training (PRT) improves rheumatoid arthritis outcomes: a district general hospital (DGH) model. Musculoskeletal Care 2018;16:13–17. [DOI] [PubMed] [Google Scholar]
- 54. Strasser B, Leeb G, Strehblow C, Schobersberger W, Haber P, Cauza E. The effects of strength and endurance training in patients with rheumatoid arthritis. Clin Rheumatol 2011;30:623–632. [DOI] [PubMed] [Google Scholar]
- 55. Stavropoulos‐Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, Nightingale P, Kitas GD, Koutedakis Y. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis 2013;72:1819–1825. [DOI] [PubMed] [Google Scholar]
- 56. Jin S, Li M, Fang Y, Li Q, Liu J, Duan X, et al. Chinese Registry of Rheumatoid Arthritis (CREDIT): II. Prevalence and risk factors of major comorbidities in Chinese patients with rheumatoid arthritis. Arthritis Res Ther 2017;19:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Doran MF, Pond GR, Crowson CS, O'Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty‐year period. Arthritis Rheum 2002;46:625–631. [DOI] [PubMed] [Google Scholar]
- 58. Buckinx F, Landi F, Cesari M, Fielding RA, Visser M, Engelke K, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle 2018;9:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the journal of cachexia, sarcopenia and muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparisons of disease characteristics among RA patients in BMI categorization


