Abstract
OBJECTIVE
Understanding the real-world impacts of lifestyle modification (LSM) for diabetes prevention is imperative to inform resource allocation. The purpose of this study was to synthetize global evidence on the impact of LSM strategies on diabetes incidence and risk factors in one parsimonious model.
RESEARCH DESIGN AND METHODS
PubMed, Embase, Cochrane Library, and ClinicalTrials.gov were searched for studies published between January 1990 and April 2015. Effectiveness/translation studies of any design testing LSM strategies, targeting high-risk populations (with prediabetes or diabetes risk factors), and reporting diabetes incidence, weight, or glucose outcomes were included. We extracted number of diabetes cases/incidence rates and mean changes in weight (kg), fasting blood glucose (FBG, mmol/L), 2-h postload glucose (mmol/L), and hemoglobin A1c (%). Pairwise random-effects and frequentist random-effects network meta-analyses were used to obtain pooled effects.
RESULTS
Sixty-three studies were pooled in the meta-analysis (n = 17,272, mean age 49.7 years, 28.8% male, 60.8% white/European). In analyses restricted to controlled studies (n = 7), diabetes cumulative incidence was 9% among intervention participants and 12% among control participants (absolute risk reduction 3%; relative risk 0.71 [95% CI 0.58, 0.88]). In analyses combining controlled and uncontrolled studies (n = 14), participants receiving group education by health care professionals had 33% lower diabetes odds than control participants (odds ratio 0.67 [0.49, 0.92]). Intervention participants lost 1.5 kg more weight [−2.2, −0.8] and achieved a 0.09 mmol/L greater FBG decrease [−0.15, −0.03] than control participants. Every additional kilogram lost by participants was associated with 43% lower diabetes odds (β = 0.57 [0.41, 0.78]).
CONCLUSIONS
Real-world LSM strategies can reduce diabetes risk, even with small weight reductions.
Introduction
Type 2 diabetes has reached epidemic levels worldwide, affecting 425 million people and accounting for 4 million deaths in 2017 (1). Because lifestyle modification (LSM) has been proven to prevent or at least delay type 2 diabetes and its complications in large randomized trials (2–6), efforts have been directed toward translating these findings for delivery via community and clinical programs. The number of effectiveness or translation studies in the field of type 2 diabetes prevention is growing, but there is a lack of consolidated data describing the outcomes of these studies overall and by intervention delivery method. Summary statistics, such as those derived from meta-analyses, are important tools to guide the development and delivery of community- and clinic-based interventions and can assist decision makers in determining how and where to allocate resources.
Existing meta-analyses show that LSM strategies to prevent type 2 diabetes that are delivered under real-world conditions are effective for promoting weight loss (7–11), a strong diabetes risk factor (12,13). Effects on glucose outcomes are mixed, with both positive and null estimates having been obtained (7,10,11). Regarding type 2 diabetes incidence, one meta-analysis commissioned by the Community Preventive Services Task Force showed LSM can reduce incidence, although estimates were derived from combined translation and proof-of-principle studies (11). Another meta-analysis from Public Health England estimated type 2 diabetes incidence in single-arm and controlled translation studies but did not compare the effect of different intervention strategies (e.g., technology, group education, counseling) against that of control conditions (14).
To understand which specific intervention strategies work under real-world conditions, their effect should be explored separately and compared against that of control conditions. Identifying effective intervention strategies can guide policy-making, especially at a time when several countries are embarking on national diabetes prevention programs (15–18). We thus aim to synthetize and compare the effect of real-world LSM strategies to prevent type 2 diabetes incidence in one parsimonious model using an arm-based network meta-analysis. This approach allows for inclusion and analysis of a larger amount of evidence, inclusion of single-arm studies to compare against control conditions, and estimation of relative effectiveness between intervention strategies (19). This meta-analysis explores the external validity of LSM strategies for type 2 diabetes (hereafter referred to as diabetes) prevention and expands the evidence base by including heterogeneous studies from around the world.
Research Design and Methods
Data Sources and Search
We systematically searched PubMed, Embase, Cochrane Library, and ClinicalTrials.gov electronic databases for articles reporting on the effectiveness of real-world LSM strategies to prevent diabetes from across the world, published between January 1990 and April 2015. Our literature search strategy included a combination of search and Medical Subject Headings (MeSH) terms related to diabetes prevention, lifestyle modification, and translation research (search string example available in Supplementary Data). We did not search for unpublished data. We adhered to PRISMA guidelines for reporting systematic reviews incorporating network meta-analyses of health care interventions (20).
Study Selection
To be eligible, studies had to 1) be effectiveness or translation intervention studies (i.e., implemented under real-world conditions or translations of proven interventions) of any design; 2) test an LMS strategy (defined as any strategy focused on improving physical activity and/or diet to prevent diabetes through weight loss); 3) be directed at high-risk populations (i.e., people with objectively measured prediabetes or diabetes risk factors [e.g., BMI ≥25 kg/m2, African American race]); and 4) report type 2 diabetes incidence rates, weight, or glucose outcomes (fasting blood glucose [FBG], 2-h postprandial glucose [PPG], or hemoglobin A1c [A1C]) measured before and after the intervention. We excluded studies involving samples with a prevalence of diabetes over 20%, gestational diabetes mellitus, metabolic syndrome, participants under 18 years of age, or type 1 diabetes as well as efficacy-type studies (i.e., proof of principle, randomized controlled trials implemented under highly controlled conditions). Two reviewers independently screened titles and abstracts, resolved discrepancies through discussions, and identified a set of studies for full-text review. These reviewers then screened full texts, resolved any discrepancies through discussions, and agreed upon a final set of studies to be included.
Data Extraction and Quality Assessment
The primary outcome of interest was the relative risk, and likelihood, of developing diabetes. For this, we extracted the number of participants who developed diabetes by the end of the study period and crude incidence rates reported. For secondary outcomes, we extracted the preintervention/postintervention (pre/post) changes in weight (kg), FBG (mmol/L), PPG (mmol/L), and A1C (percentage points and mmol/mol). We also extracted information about participant-level characteristics (i.e., age, proportion of male participants, baseline BMI, ethnicity, proportion with objectively detected prediabetes), program-level characteristics (i.e., number of intervention sessions participants attended and intervention duration), and study-level characteristics (design, country, and follow-up length). These data were extracted using a standardized extraction form designed for this study. If studies did not report the data of interest, we contacted authors to request the information or computed it from the baseline and postintervention values reported.
Since translation studies are often conducted under real-world conditions, they often include heterogeneous samples; in addition, quasi-experiments are often more feasible in these settings, which is why we included both uncontrolled and controlled studies. Because of these factors, the quality of translational studies must be assessed using specifically designed metrics. In this meta-analysis, we assessed each study using a set of quality indicators previously used in meta-analyses of translation studies (8,10). The first indicator was whether diabetes risk was defined using blood glucose testing (2 points) or self-reported risk factors or anthropometric measurements (1 point). The second quality indicator was whether reported study attrition was <20% (2 points), 20–40% (1 point), or different between study arms (0 points). The third indicator examined whether intent-to-treat analysis was used to minimize the impact of attrition (2 points) or if per-protocol analysis was used (1 point). The fourth indicator was whether the study described the intervention sufficiently to allow transferability of the program to other settings by reporting the following (1 point each): description of the intervention program; costs and resources used to deliver the program; the qualification of intervention delivery agents; and the acceptability of the program among participants and/or providers. Each study was scored on a 1–10 scale and categorized as low (≤5), medium (6 or 7), or high (≥8) quality.
Data Synthesis and Analyses
We first used random-effects meta-analysis techniques to obtain a pooled pre/post mean difference for weight and glucose outcomes among intervention participants alone. For studies with control groups, we conducted pairwise random-effects meta-analyses to estimate between-group mean difference for weight and glucose outcomes and the relative risk for diabetes at the end of the study.
To obtain both direct and indirect (i.e., mixed) evidence of the effects of different intervention strategies, we conducted a frequentist arm-based network meta-analysis. This approach permits multiple treatment comparisons, even indirect comparisons of treatments that have not been directly compared with a control treatment (e.g., uncontrolled studies) using arm-level data (rather than between-group differences) (21). For this analysis, we grouped similar study arms according to the intervention strategy employed, estimating a pooled effect for each group, and compared the effects of intervention arms against that of control arms. A multivariate random-effects meta-analysis method was used to fit this model, where we obtained pooled effects for each intervention strategy arm compared with control arms.
We explored effect heterogeneity across studies by visually examining forest plots and by computing I2, where I2 greater than 75% indicated significant effect heterogeneity. Meta-regressions were then used to explore whether participant- or intervention-level characteristics explained heterogeneity in treatment effects. To explore transitivity (i.e., similarity in clinical/methodological characteristics across studies), we conducted subgroup meta-analyses stratified by participant-level characteristics and obtained subgroup pooled effects for all outcomes. Consistency was explored by examining whether indirect treatment effects (i.e., those that were not directly compared within studies) were similar or different from direct treatment effects (i.e., those that were directly compared within studies). We conducted sensitivity analyses to compare treatment effects between groups of studies according to their quality classification. Finally, publication bias was assessed using the Egger test and by visually exploring funnel plots.
We used the Metafor package (22) in R programming language (version 3.2.1) to conduct the analyses described. Statistical significance was assessed by examining 95% CIs, where CIs not including the value 0 for mean changes or 1 for relative risk and odds ratio (OR) were deemed statistically significant.
Results
Seventy-seven articles met the inclusion criteria for the systematic review and 63 articles reported complete data used in the meta-analysis (Supplementary Fig. 1). Fourteen articles were not included in the meta-analysis because they provided insufficient data for analyses (i.e., did not report SEs for outcomes and authors did not respond to requests for these data) (23–27) or because these articles reported outcomes from a trial already included in the analysis but with data from other follow-up times (28–36). Participant and intervention characteristics across the 77 studies included in the systematic review are presented in Supplementary Table 1.
The 63 pooled studies enrolled 30,524 participants and data were available for 17,272 of those. Study and participant characteristics across the pooled studies are presented in Table 1. Participant mean age was 49.7 years (range 28.9–68.3), 28.8% were male, and 60.8% were white/European. High-risk participants were identified by assessing diabetes risk factors in 59% of the studies and by objectively assessing prediabetes in 41% of the studies. Forty percent of the studies used pre/post, single-group designs, 35% randomized controlled designs, and 25% nonrandomized controlled designs. Control groups either received no intervention, standard of care, enhanced standard of care, written materials, or a delayed intervention. Most of the studies (67%) were conducted in North America, 19% in Europe, 11% in Oceania, and 3% in Asia. Most interventions were implemented in community settings (62%) and were delivered by health care professionals (52%). Interventions lasted on average 23 weeks (range 1–130 weeks) and offered on average 18 intervention sessions (range 1–58 sessions). Over half of the studies (56%) were modeled after the U.S. Diabetes Prevention Program (DPP).
Table 1.
Characteristics of participants (n = 17,272) and studies (n = 63) included in meta-analyses
| Value | ||
|---|---|---|
| Baseline participant-level characteristics* | ||
| Age, years | 49.7 (7.9) | |
| Male | 28.8% | |
| White/European | 60.8% | |
| Pacific Islander/Indigenous | 29.7% | |
| Asian | 10.6% | |
| Weight, kg | 85.0 (11.0) | |
| BMI, kg/m2 | 31.3 (4.2) | |
| FBG, mmol/L | 5.7 (0.6) | |
| 2-h PPG, mmol/L | 6.4 (1.7) | |
| A1C, % | 5.6 (0.5) | |
| A1C, mmol/mol |
37.7 |
|
| Study-level characteristics | ||
| Country of study | ||
| U.S. | 41 (65) | |
| U.K. | 5 (8) | |
| Australia | 4 (6) | |
| New Zealand | 3 (5) | |
| The Netherlands | 2 (3) | |
| Finland | 2 (3) | |
| Canada, Greece, Israel, Japan, Spain, and Norway (one each) | 6 (10) | |
| Design | ||
| Pre/post single group study | 25 (40) | |
| Nonrandomized controlled study | 16 (25) | |
| Randomized controlled study | 22 (35) | |
| Intervention strategy† | ||
| Group diabetes education from community member | 28 (44) | |
| Group diabetes education from health care professional | 31 (49) | |
| Individual counseling from health care professional | 4 (6) | |
| Education/counseling delivered using technology‡ | 8 (13) | |
| Delivery Setting† | ||
| Remote delivery | 8 (13) | |
| Community setting | 39 (62) | |
| Clinical setting | 25 (40) | |
| Provider Type† | ||
| Lay community member | 28 (44) | |
| Health care professional | 33 (52) | |
| Technology assisted | 7 (11) | |
Data are mean or mean (SD) for participant-level characteristics, unless otherwise noted, and n (%) for study-level characteristics. Individual study characteristics are presented in Supplementary Table 1.
*Baseline participant-level characteristics are weighted averages by the number of participants in each study.
†Some studies used more than one of these strategies/setting/provider, which makes the study count go over 63.
‡Technologies used to provide group/individual education or counseling include interactive voice response calls, video conference, internet, cable television, and DVDs.
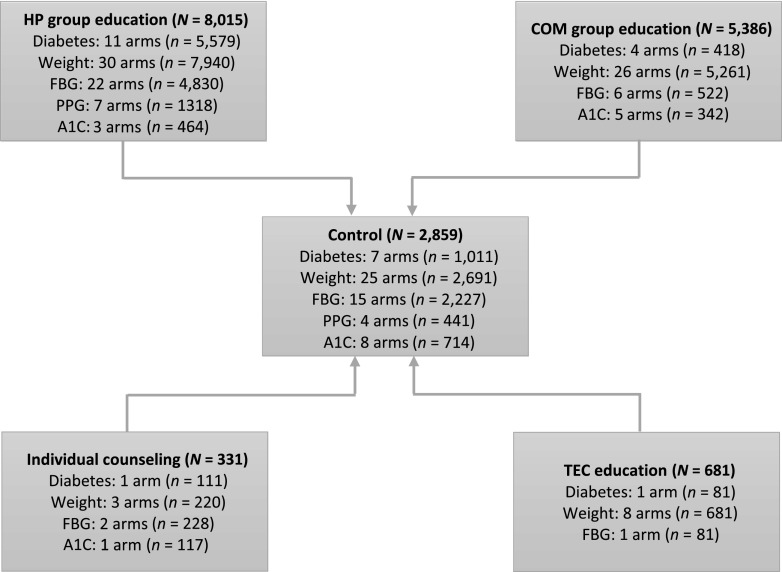
Based on the intervention strategies employed, we created four intervention groups (group education by community members, group education by health care professionals, individual counseling by health care professionals, and education/counseling delivered via technology) and tested the network presented in Fig. 1. The figure shows the eligible comparisons available in the star-shaped network for all outcomes of the direct and indirect meta-analysis. Consistent with the pragmatic nature of translation studies, body weight was the most frequently assessed outcome, reported in 67 intervention arms (n = 14,102). FBG followed with 34 intervention arms (n = 9,189) and diabetes incidence was reported in 17 intervention arms (n = 9,189) reporting this outcome. The least assessed outcomes were A1C, reported in 9 arms (n = 1,577), and PPG, reported in 7 arms (n = 1,318). The most frequently tested intervention strategy was group education delivered by health care professionals (73 arms), followed by group education delivered by lay community members (41 arms). For all outcomes, the least tested intervention strategies were individual counseling by health care professionals (7 arms) and education/counseling delivered via technology (10 arms).
Figure 1.
Network structure of intervention trials assessed in the arm-based network meta-analysis for all outcomes. The figure shows the eligible comparisons available in the star-shaped network for all outcomes (diabetes incidence, weight, FBG, PPG, and A1C) of the direct and indirect meta-analysis. Each node in the network is depicted; the lines show the comparisons that contributed to the analysis. All treatment arms were compared directly or indirectly against control arms. Direct comparisons were those conducted as part of a study, whereas indirect comparisons were those conducted in the network meta-analysis. Although some intervention strategies were compared directly in some studies, these were not indirectly compared in the present meta-analysis. COM, community member; HP, health care professional; TEC, technology.
Diabetes Incidence
Fourteen studies (seven controlled and seven pre/post studies) reported number of participants that developed diabetes at the end of the study period. The prevalence of prediabetes at baseline ranged from 35 to 100% across studies (mean [SD] 62% [24]); none of these studies included participants with diabetes. Follow-up length across studies ranged from 6 to 48 months (19.5 [12.7]) with an observed attrition rate ranging from 0 to 40% (12.3% [13.4]). Diabetes development was ascertained at the end of the follow-up period on the basis of an FBG measure (n = 3), an oral glucose tolerance test (n = 5), or both (n = 4), or based on an A1C measure (n = 2). In controlled studies (n = 7), diabetes cumulative incidence was 9% among intervention participants and 12% among controls (absolute risk reduction 3%). Overall, 6.0 new diabetes cases per 100 persons per year were observed in intervention participants compared with 8.5 in control participants (risk difference 2.5). A pairwise meta-analysis including these studies showed that participants receiving an intervention had a 29% lower risk of developing diabetes than control participants (relative risk 0.71 [95% CI 0.58, 0.88]; I2 = 0%).
The arm-based network meta-analysis including the 14 controlled and uncontrolled studies showed participants in the group education by health care professionals arms (n = 11) had 33% lower odds of having diabetes at the end of the study than participants in the seven control arms (OR = 0.67 [95% CI 0.49, 0.92]). Participants receiving education/counseling delivered via technology (n = 1; OR = 0.21 [0.1, 4.27]), group education by community members (n = 4; OR = 0.71 [0.37, 1.35]), or individual counseling by health care professionals (n = 1; OR = 0.71 [0.37, 1.36]) did not exhibit significantly lower odds of developing diabetes than control participants (Table 2).
Table 2.
Arm-based network meta-analysis comparing the effect on weight loss, FBG, and diabetes incidence between intervention and control arms, by intervention strategy
| Comparison |
Weight* (kg) | FBG† (mmol/L) | Diabetes incidence‡ | |||
|---|---|---|---|---|---|---|
| n | Change [95% CI] | n | Change [95% CI] | n | OR [95% CI] | |
| Group education by COM vs. control | 26 | −2.1 [–3.1, −1.0] | 9 | −0.04 [–0.17, 0.10] | 4 | 0.71 [0.37, 1.35] |
| Group education by HP vs. control | 30 | −1.1 [–2.3, 0.1] | 22 | −0.09 [–0.19, 0.01] | 11 | 0.67 [0.49, 0.92] |
| Individual counseling by HP vs. control | 3 | −0.3 [–3.0, 2.4] | 2 | −0.19 [–0.43, 0.04] | 1 | 0.71 [0.37, 1.36] |
| Education/counseling by TEC vs. control | 8 | −0.14 [–2.8, 2.5] | 1 | −0.08 [–0.40, 0.23] | 1 | 0.21 [0.1, 4.27] |
This was a multiple treatment comparison meta-analysis where arms across studies were grouped according to the intervention employed, and a pooled effect for each group, compared with control arms, was estimated. n = number of intervention arms. Mean (SD) duration of active intervention: 23 (22) weeks; range 1–130. Average (SD) number of sessions offered: 18 (25); range 1–58. COM, community member; HP, health care professional; TEC, technology.
*61 studies including 67 interventions arms and 25 control arms.
†29 studies including 34 interventions arms and 17 control arms.
‡14 studies including 17 interventions arms and 7 control arms.
In subgroup analyses, pooled ORs for diabetes were significant only in some participant groups (Supplementary Table 2). Compared with control participants, significantly lower odds of developing diabetes were observed only in studies where participants were older than 50 years of age (OR = 0.65 [0.48, 0.87]), in studies where >60% of participants were female (OR = 0.61 [0.43, 0.86]), in studies where participant baseline mean BMI was ≥30 kg/m2 (OR = 0.62 [0.44, 0.87]), in studies where participants were white/European (OR = 0.65 [0.48, 0.87]), and in studies with a prediabetes prevalence <50% (OR = 0.60 [0.42, 0.87].
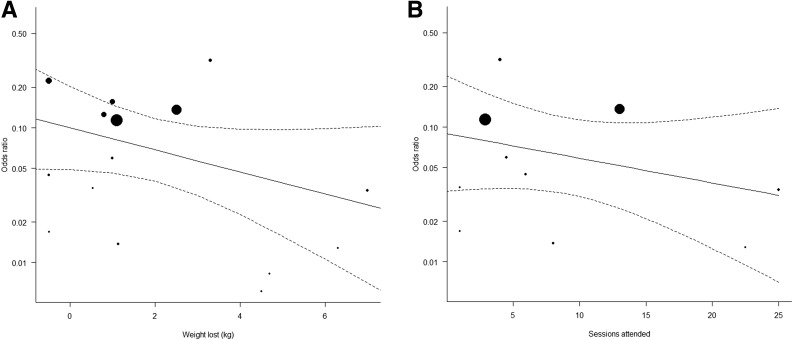
A multivariate meta-regression with participant age, weight lost, average number of sessions attended, and follow-up length as predictors of diabetes risk accounted for 87.8% of effect heterogeneity (P < 0.001). Each additional kilogram of weight lost by participants (β = 0.57 [95% CI 0.41, 0.78]) (Fig. 2A) and each additional intervention session they attended (β = 0.82 [0.74, 0.90]) (Fig. 2B) was associated with 43% and 18% lower odds of developing diabetes, respectively. Conversely, each additional month of follow-up was associated with higher odds of developing diabetes (β = 1.06 [1.02, 1.09]). Participant age was not a significant predictor in the model (β = 0.95 [0.91, 1.00]).
Figure 2.
A: Meta-regression exploring the association between weight loss and odds of having diabetes at the end of the intervention among intervention participants (N = 15 studies). The meta-regression showed the independent effect of weight loss on diabetes risk; every additional kilogram participants lost was associated with 43% lower odds of developing diabetes than among control participants (β = 0.57 [95% CI 0.41, 0.78]). B: Meta-regression exploring the association between number of intervention sessions received and odds of having diabetes at the end of the intervention among intervention participants (N = 10 studies). The meta-regression showed the independent effect of number of intervention sessions received on diabetes risk; every additional session participants received was associated with 18% lower odds of developing diabetes than among control participants (β = 0.82 [0.74, 0.90]). In both panels, the middle line represents the regression line and the two dotted lines the 95% CIs for the regression line.
Weight Loss
From 61 studies with relevant data, the pooled pre/post weight change among intervention participants was −2.5 kg [95% CI−3.0, −1.9], although effects widely varied between studies (τ2 = 4.2, I2 = 99%). In controlled studies (n = 29), intervention participants lost 1.5 kg [−2.2, −0.8] more than control participants (I2 = 90%). The arm-based network meta-analysis showed that, compared with control arms (n = 25), only intervention arms employing group education by community members (n = 26) achieved a significant weight loss (−2.1 kg [–3.1, −1.0]). The weight change observed in studies using group education by health care professionals (n = 30; −1.1 kg [−2.3, 0.1)], individual counseling by health care professionals (n = 3; −0.3 kg [−3.0, 2.4]), and education/counseling delivered via technology (n = 8; −0.1 kg [−2.8, 2.5]) was not significant (Table 2).
In subgroup analyses, weight loss differed by certain participant characteristics (Supplementary Table 2). Studies in which participants were white/European had a larger weight loss (−3.5 kg [−4.6, −2.4]) than studies in which participants were Hispanic (−1.2 kg [−2.1, −0.3]) or Asian (−1.3 kg [−2.2, −0.3]). Similarly, studies in which participant baseline mean BMI was ≥30 kg/m2 showed significantly greater weight loss (−2.9 kg [−3.5, −2.3]) than studies in which participant baseline mean BMI was <30 kg/m2 (−1.1 kg [−1.8, −0.4]).
A multivariate meta-regression with participant baseline BMI and age, proportion of male participants, average number of intervention sessions attended, and follow-up length as predictors of weight change accounted for 65% of effect heterogeneity (R2 = 0.64, P < 0.0001). Higher baseline BMI and higher average number of sessions attended were the only significant predictors of higher weight loss in the model. Specifically, every additional intervention session participants received was associated with 0.15 kg more weight lost (β = −0.15, P < 0.0001) (Supplementary Fig. 2A), while a point higher baseline BMI was associated with 0.37 kg higher weight loss (β = −0.37, P < 0.0001) (Supplementary Fig. 2B).
Glycemic Outcomes
Among studies reporting FBG outcomes (n = 29), a pre/post reduction in FBG of 0.09 mmol/L [95% CI −0.17, −0.02] was observed, although effects widely varied across studies (τ2 = 31.5, I2 = 98%). In controlled studies (n = 14), intervention participants achieved a 0.09 mmol/L [−0.15, −0.03] greater FBG reduction than control participants (I2 = 84%). The arm-based network meta-analysis showed that, compared with control arms (n = 17), intervention arms (n = 34) achieved a 0.07 mmol/L [−0.14, −0.04] reduction in FBG. However, when explored separately, group education by community members (n = 9; −0.03 mmol/L [−0.17, 0.1]), group education by health care professionals (n = 22; −0.09 mmol/L [−0.19, 0.01]), individual counseling by health care professionals (n = 2; −0.19 mmol/L [−0.43, 0.04]), and education/counseling delivered via technology (n = 1; −0.08 mmol/L [−0.39, 0.23]) did not have significant effects (Table 2).
In subgroup analyses, FBG effects were significant only in certain participant groups (Supplementary Table 2). Significant FBG reductions were observed in studies where participants were over 50 years old (−0.16 mmol/L [−0.23, −0.08]), in studies where participants were African American (−0.43 mmol/L [−0.58, −0.28]), and in studies where >60% of participants were female (−0.11 mmol/L [−0.22, −0.00]). Significant FBG reductions were also observed in studies where ≥50% of participants had prediabetes (−0.20 mmol/L [−0.31, −0.08]) and in studies where participant mean BMI was <30 kg/m2 (−0.14 mmol/L [−0.28, −0.00]). A multivariate meta-regression model with participant age, proportion of male participants, average number of intervention sessions attended, follow-up length, and weight change did not explain effect heterogeneity in FBG.
Studies reporting PPG (n = 7) or A1C outcomes (n = 9) did not achieve significant improvements in these outcomes: effects were −0.19 mmol/L [−0.54, 0.16] for PPG and −0.01% [–0.1, 0.1] (−23.6 mmol/mol [–24.6, −22.4]) for A1C.
Quality and Consistency Assessment
Of the 77 studies included in the systematic review, 15 were classified as high quality, 47 as medium quality, and 15 as low quality. Overall, high-quality studies were better than low- or medium-quality studies at balancing aspects of internal validity (e.g., employing objective glucose measures) and external validity (e.g., reporting information to promote intervention translation) (Supplementary Table 1). Of the 63 studies included in the meta-analysis, 12 were considered of high quality, 41 of medium quality, and 10 of low quality. In sensitivity analyses, high-quality studies showed the greatest effects on weight (−3.0 kg [−4.7, −1.3]) and on FBG (−0.17 mmol/L [−0.31, −0.04]). For diabetes incidence, only high quality studies achieved significantly lower odds for developing diabetes (OR = 0.64 [0.48, 0.87]).
Egger tests suggested publication bias was present among studies reporting diabetes incidence (P < 0.0001) and weight outcomes (P < 0.0001). A visual examination of funnel plots confirmed that smaller studies with null effects on weight and diabetes incidence were less likely to be published than studies with positive effects (Supplementary Fig. 3).
Estimates from direct and indirect evidence were consistent (i.e., in the same direction), although significance of effects differed. For diabetes risk, direct evidence showed that only group education by health care professionals lowered the odds of developing diabetes (OR = 0.66 [0.48, 0.91]), while indirect evidence showed no significant effects. For weight change, direct evidence showed that group education by community members (−2.9 kg [−3.3, −2.6]), group education (−1.4 kg [–1.6, −1.2]), and individual counseling (−0.6 kg [−1.1, −0.8]) by health care professionals promoted weight loss. Indirect evidence showed no statistically significant weight loss for any of these strategies. For FBG, direct evidence showed that group education (−0.10 mmol/L [−0.10, −0.00]) and individual counseling (−0.30 mmol/L [−0.50, −0.02]) by health care professionals promoted FBG reductions, while indirect evidence showed no significant effects.
Conclusions
In this systematic review and meta-analysis, we summarize the effects of LSM strategies for type 2 diabetes prevention implemented under real-world conditions. We used a network meta-analysis approach to determine how much evidence is available, identify where evidence is lacking, understand heterogeneity and inconsistency of evidence, and explore what treatments are the most promising. We found that participants receiving an intervention had a 29% lower risk of developing diabetes, lost 1.5 kg more body weight, and reduced FBG by 0.09 mmol/L more than participants not receiving one. Overall, this meta-analysis shows that LSM strategies implemented under real-world conditions are promising approaches for preventing diabetes, though more studies from low- and middle-income countries are still needed.
The diabetes relative risk reduction effect we observed aligns with a previous meta-analytic estimate of translation studies (14) but is smaller than that reported in a meta-analysis from the Community Preventive Services Task Force (11). This may be explained by the fact that the former analysis included efficacy-type studies, which are implemented in optimal conditions and may result in larger effect sizes (37). Regarding intervention strategies, we found that only group education delivered by health care professionals was associated with lower diabetes incidence among those receiving the intervention. This may be due to the fact that more studies conducted in clinical settings have access to glucose data and testing and thus are better able to detect diabetes than those delivered in community settings. Since 9 out of the 14 studies analyzed were conducted in clinical settings, data are still needed to determine the effects of community-based interventions on diabetes incidence.
Intensity of the LSM interventions and length of study follow-up were both important factors influencing diabetes incidence. Specifically, greater attendance at intervention sessions and greater weight loss were associated with larger reductions in diabetes incidence. However, we also noted smaller reductions in diabetes incidence in studies with longer follow-up. This may be related to challenges in maintaining the intensity of the intervention or to continued pathophysiogical progression toward diabetes with aging. Indeed, in our previous meta-analysis, we showed the effects of LSM strategies wane with time (38), suggesting strategies to maintain intervention effects in the long term are needed. Furthermore, longer follow-up studies which examine the relative costs and benefits of LSM are needed to estimate the value of investing in and implementing these programs more widely.
Regarding weight loss, our findings are consistent with those from other meta-analyses showing a pre/post weight loss of 2.3–3.8 kg (9,10) and a between-group weight loss difference of 1.6–2.7 kg (7,9,10,14). When exploring different intervention strategies, we found that group education by community members achieved significant weight reductions. This aligns with findings from a previous meta-analysis (8) and shows that nonmedical personnel are effective intervention delivery agents, achieving similar health benefits at lower costs. We also found that every additional kilogram of weight lost was associated with lower diabetes incidence, supporting the premise that weight loss is important for diabetes prevention (12,13). However, previous meta-analyses of translation studies show that, on aggregate, LSM strategies were associated with a mean weight loss of 2–4% from baseline (8–10), and our findings suggest that even a 1% reduction in body weight significantly reduces risk. This challenges the current practice of reimbursement based on a threshold of 5% weight loss from baseline (36).
We found significant pre/post reductions in FBG within intervention participants and larger reductions among intervention than among control participants, which aligns with previous findings (10,11). When all intervention arms were compared against control arms, significant reductions in FBG were observed but not when intervention arms were explored separately, suggesting lack of statistical power. As in a previous meta-analysis (7), null effects were observed for PPG, which may be due to lack of power given that PPG is an expensive glucose measure requiring a larger time commitment for participants and staff and thus is seldom used in translation studies. Indeed, only seven studies included a PPG measure compared with 29 that included FBG. Null effects were also observed for the nine studies reporting A1C, while positive effects have been observed in a previous meta-analysis of translation studies (14) and another one synthesizing interventions translating the U.S. DPP model (10). The small or no improvements we observed in A1C may be related to the fact that two-thirds of the studies included people with normal glucose regulation.
This meta-analysis identified several gaps in the global translational evidence base. First, even though low- and middle-income countries bear the greatest diabetes burden and mortality (1), ∼90% of the intervention studies included in this review were implemented in high-income countries. Similarly, even though there are ethnic groups that are highly susceptible to develop diabetes (e.g., South Asians, Pacific Islanders), few studies were focused on such groups. Second, 80% of the studies were of low to medium quality, meaning most of the studies included in this meta-analysis had both internal and external validity limitations. Third, we found evidence of publication bias, suggesting small studies with null findings were less likely to be published. There was also imprecision in effect estimates and lack of association between most intervention strategies and outcomes in the arm-based network meta-analysis; this is likely due to small sample sizes and lack of power. For instance, only eight studies tested education/counseling delivered via technology, for which no significant effects were observed. Fourth, only half of the studies measured glucose outcomes, while only 14 reported diabetes incidence; cost and feasibility of measuring these outcomes may explain this low use. Also, most of the studies reporting diabetes incidence had a short study follow-up length (i.e., 1–2 years), which prevents us from exploring a true diabetes prevention effect. This may also have resulted in an overestimation of preventive effects. Finally, since most of the studies reporting on diabetes incidence were conducted in clinical settings, data are still needed to determine the effects of community-based interventions on diabetes incidence.
Strategies that can help address these gaps include the following. To start, more diabetes prevention interventions should be implemented in low- and middle-income countries and more should be offered to those ethnic groups that are prone to develop diabetes. Good quality, controlled studies that balance features of internal and external validity are also needed; frameworks to balance internal and external validity (e.g., Reach, Effectiveness, Adoption, Implementation, and Maintenance [RE-AIM]) (39), feasible controlled study designs (e.g., rollout designs, hybrid designs) (40,41), and pragmatic measures (42) can be used in this endeavor. To better assess diabetes prevention, studies with long-term follow-up periods (e.g., over 5 years) should be pursued. Also, studies with null findings should be published to better understand which diabetes prevention interventions work, for whom, and under which conditions. Last, although measuring glucose outcomes and diabetes incidence may be costly and take time, feasible ways to assess such outcomes should be devised (e.g., using readily available data), especially in community-based interventions, to truly assess diabetes prevention in the real world.
Our findings should be interpreted in light of the present limitations. Transitivity, or similarity between studies, was not achieved given that our meta-analysis examined the external validity of diabetes prevention interventions and included studies that differed in design, methods, populations, and interventions. This probably contributed to the high percentage of statistical heterogeneity we observed (over 90%) in intervention effects, which was partially explained in meta-regressions and subgroup analyses. Forty percent of the studies included used a pre/post single-group design, which may be subject to confounding. To overcome this, we separately examined studies that had control groups and observed larger benefits in intervention groups than control groups. Estimates from direct and indirect evidence were consistent (i.e., in the same direction), which we tested by comparing direct and indirect effects rather than by conducting formal consistency tests (e.g., node splitting, back calculation). We grouped interventions according to two major characteristics (i.e., delivery agent and modality) and did not explore the effect of other features included in some interventions (e.g., incentives, pedometers). Thus, similar but not identical interventions were grouped together. We tested a star-shaped network that did not compare intervention strategies against each other and did not rank intervention strategies according to their probability of being the best. A fully connected network is required to explore the comparative effectiveness of different strategies.
Summary
Even though low- and middle-income countries bear the greatest diabetes burden and mortality, the majority of translational diabetes prevention interventions have been implemented in high-income countries. Available evidence shows LSM strategies implemented under real-world conditions can lower diabetes risk and promote weight loss. Group-based education delivered by lay community members or by health care professionals are promising real-world diabetes prevention strategies. We also found a 1% weight loss associated with lower diabetes incidence, which challenges the current practice of reimbursement based on a threshold of 5% weight loss from baseline. Good quality translational diabetes prevention interventions are needed, mainly in low- and middle-income countries. The burden of diabetes worldwide is only growing and efforts should be directed at implementing proven LSM interventions globally.
Supplementary Material
Article Information
Funding. This project was funded by the Institute for Health Metrics and Evaluation (Disease Control Priorities Network grant UWSC7007 from the Bill & Melinda Gates Foundation). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. M.K.A., K.M.V.N., and M.B.W. were partially supported by the Georgia Center for Diabetes Translation Research (P30DK111024).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. K.I.G. conducted the systematic review, completed data extraction, conducted data analyses, provided interpretation of study findings, and wrote the manuscript. M.B.W. helped conceptualize the study, helped with interpretation of study findings, and helped write the manuscript. A.S. helped conduct the systematic review, helped complete data extraction, and reviewed and edited the manuscript. J.S.H. helped with interpretation of study findings and reviewed and edited the manuscript. K.M.V.N. helped conceptualize and design the study, helped with interpretation of study findings, and reviewed and edited the manuscript. M.K.A. conceptualized and designed the study, helped with data analyses and the interpretation of study findings, and helped write the manuscript. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-2222/-/DC1.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas, 8th edition [Internet], 2017. Brussels, Belgium, International Diabetes Federation. Available at http://www.diabetesatlas.org/. Accessed 15 February 2018
- 2.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramachandran A, Snehalatha C, Mary S, et al.; Indian Diabetes Prevention Programme (IDPP) . The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297 [DOI] [PubMed] [Google Scholar]
- 4.Diabetes Prevention Program Research Group Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015;3:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindström J, Ilanne-Parikka P, Peltonen M, et al.; Finnish Diabetes Prevention Study Group . Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673–1679 [DOI] [PubMed] [Google Scholar]
- 6.Li G, Zhang P, Wang J, et al. . The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–1789 [DOI] [PubMed] [Google Scholar]
- 7.Cardona-Morrell M, Rychetnik L, Morrell SL, Espinel PT, Bauman A. Reduction of diabetes risk in routine clinical practice: are physical activity and nutrition interventions feasible and are the outcomes from reference trials replicable? A systematic review and meta-analysis. BMC Public Health 2010;10:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood) 2012;31:67–75 [DOI] [PubMed] [Google Scholar]
- 9.Dunkley AJ, Bodicoat DH, Greaves CJ, et al. . Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care 2014;37:922–933 [DOI] [PubMed] [Google Scholar]
- 10.Mudaliar U, Zabetian A, Goodman M, et al. . Cardiometabolic risk factor changes observed in diabetes prevention programs in US settings: a systematic review and meta-analysis. PLoS Med 2016;13:e1002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pronk NP. Structured diet and physical activity programmes provide strong evidence of effectiveness for type 2 diabetes prevention and improvement of cardiometabolic health. Evid Based Med 2016;21:18. [DOI] [PubMed] [Google Scholar]
- 12.Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract 2010;89:309–319 [DOI] [PubMed] [Google Scholar]
- 13.Cloostermans L, Wendel-Vos W, Doornbos G, et al. . Independent and combined effects of physical activity and body mass index on the development of type 2 diabetes—a meta-analysis of 9 prospective cohort studies. Int J Behav Nutr Phys Act 2015;12:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashra NB, Spong R, Carter P, et al. A systematic review and meta-analysis assessing the effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes mellitus in routine practice [article online], August 2015. London, Public Health England. Available from https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/456147/PHE_Evidence_Review_of_diabetes_prevention_programmes-_FINAL.pdf. Accessed 13 March 2017
- 15.Torjesen I. NHS England rolls out world’s first national diabetes prevention programme. BMJ 2016;352:i1669. [DOI] [PubMed] [Google Scholar]
- 16.Albright AL, Gregg EW. Preventing type 2 diabetes in communities across the U.S.: the National Diabetes Prevention Program. Am J Prev Med 2013;44(Suppl. 4):S346–S351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saaristo T, Moilanen L, Korpi-Hyövälti E, et al. . Lifestyle intervention for prevention of type 2 diabetes in primary health care: one-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care 2010;33:2146–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ely EK, Gruss SM, Luman ET, et al. . A national effort to prevent type 2 diabetes: participant-level evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care 2017;40:1331–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bafeta A, Trinquart L, Seror R, Ravaud P. Reporting of results from network meta-analyses: methodological systematic review. BMJ 2014;348:g1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutton B, Salanti G, Caldwell DM, et al. . The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–784 [DOI] [PubMed] [Google Scholar]
- 21.Hawkins N, Scott DA, Woods B. ‘Arm-based’ parameterization for network meta-analysis. Res Synth Methods 2016;7:306–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1–48 [Google Scholar]
- 23.Coppell KJ, Tipene-Leach DC, Pahau HL, et al. . Two-year results from a community-wide diabetes prevention intervention in a high risk indigenous community: the Ngati and Healthy project. Diabetes Res Clin Pract 2009;85:220–227 [DOI] [PubMed] [Google Scholar]
- 24.Oba N, McCaffrey R, Choonhapran P, Chutug P, Rueangram S. Development of a community participation program for diabetes mellitus prevention in a primary care unit, Thailand. Nurs Health Sci 2011;13:352–359 [DOI] [PubMed] [Google Scholar]
- 25.Treadwell H, Holden K, Hubbard R, et al. . Addressing obesity and diabetes among African American men: examination of a community-based model of prevention. J Natl Med Assoc 2010;102:794–802 [DOI] [PubMed] [Google Scholar]
- 26.Rowley KG, Daniel M, Skinner K, Skinner M, White GA, O’Dea K. Effectiveness of a community-directed ‘healthy lifestyle’ program in a remote Australian aboriginal community. Aust N Z J Public Health 2000;24:136–144 [DOI] [PubMed] [Google Scholar]
- 27.Zyriax BC, Letsch B, Stock S, Windler E. DELIGHT (delay of impaired glucose tolerance by a healthy lifestyle trial)—a feasibility study on implementing a program of sustainable diabetes prevention in German companies. Exp Clin Endocrinol Diabetes 2014;122:20–26 [DOI] [PubMed] [Google Scholar]
- 28.Yates T, Davies MJ, Sehmi S, Gorely T, Khunti K. The Pre-diabetes Risk Education and Physical Activity Recommendation and Encouragement (PREPARE) programme study: are improvements in glucose regulation sustained at 2 years? Diabet Med 2011;28:1268–1271 [DOI] [PubMed] [Google Scholar]
- 29.Kaholokula JK, Mau MK, Efird JT, et al. . A family and community focused lifestyle program prevents weight regain in Pacific Islanders: a pilot randomized controlled trial. Health Educ Behav 2012;39:386–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackermann RT, Finch EA, Caffrey HM, Lipscomb ER, Hays LM, Saha C. Long-term effects of a community-based lifestyle intervention to prevent type 2 diabetes: the DEPLOY extension pilot study. Chronic Illn 2011;7:279–290 [DOI] [PubMed] [Google Scholar]
- 31.Katula JA, Vitolins MZ, Morgan TM, et al. . The Healthy Living Partnerships to Prevent Diabetes study: 2-year outcomes of a randomized controlled trial. Am J Prev Med 2013;44(Suppl. 4):S324–S332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao L, Yank V, Wilson SR, Lavori PW, Ma J. Two-year weight-loss maintenance in primary care-based Diabetes Prevention Program lifestyle interventions. Nutr Diabetes 2013;3:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piatt GA, Seidel MC, Chen HY, Powell RO, Zgibor JC. Two-year results of translating the diabetes prevention program into an urban, underserved community. Diabetes Educ 2012;38:798–804 [DOI] [PubMed] [Google Scholar]
- 34.Kramer MK, McWilliams JR, Chen HY, Siminerio LM. A community-based diabetes prevention program: evaluation of the group lifestyle balance program delivered by diabetes educators. Diabetes Educ 2011;37:659–668 [DOI] [PubMed] [Google Scholar]
- 35.Amundson HA, Butcher MK, Gohdes D, et al.; Montana Cardiovascular Disease and Diabetes Prevention Program Workgroup . Translating the diabetes prevention program into practice in the general community. Diabetes Educ 2009;35:209–223 [DOI] [PubMed] [Google Scholar]
- 36.Vojta D, Koehler TB, Longjohn M, Lever JA, Caputo NF. A coordinated national model for diabetes prevention: linking health systems to an evidence-based community program. Am J Prev Med 2013;44(Suppl. 4):S301–S306 [DOI] [PubMed] [Google Scholar]
- 37.Glasgow RE, Emmons KM. How can we increase translation of research into practice? Types of evidence needed. Annu Rev Public Health 2007;28:413–433 [DOI] [PubMed] [Google Scholar]
- 38.Haw JS, Galaviz KI, Straus AN, et al. . Long-term sustainability of diabetes prevention approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med 2017;177:1808–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glasgow RE, Klesges LM, Dzewaltowski DA, Estabrooks PA, Vogt TM. Evaluating the impact of health promotion programs: using the RE-AIM framework to form summary measures for decision making involving complex issues. Health Educ Res 2006;21:688–694 [DOI] [PubMed] [Google Scholar]
- 40.Brown CH, Curran G, Palinkas LA, et al. . An overview of research and evaluation designs for dissemination and implementation. Annu Rev Public Health 2017;38:1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012;50:217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glasgow RE, Riley WT. Pragmatic measures: what they are and why we need them. Am J Prev Med 2013;45:237–243 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.