Abstract
Background
Exacerbations of chronic obstructive pulmonary disease (COPD) are a major driver of decline in health status and impose high costs on healthcare systems. Action plans offer a form of self‐management that can be delivered in the outpatient setting to help individuals recognise and initiate early treatment for exacerbations, thereby reducing their impact.
Objectives
To compare effects of an action plan for COPD exacerbations provided with a single short patient education component and without a comprehensive self‐management programme versus usual care. Primary outcomes were healthcare utilisation, mortality and medication use. Secondary outcomes were health‐related quality of life, psychological morbidity, lung function and cost‐effectiveness.
Search methods
We searched the Cochrane Airways Group Specialised Register along with CENTRAL, MEDLINE, Embase and clinical trials registers. Searches are current to November 2015. We handsearched bibliographic lists and contacted study authors to identify additional studies.
Selection criteria
We included randomised controlled trials (RCT) and quasi‐RCTs comparing use of an action plan versus usual care for patients with a clinical diagnosis of COPD. We permitted inclusion of a single short education component that would allow individualisation of action plans according to management needs and symptoms of people with COPD, as well as ongoing support directed at use of the action plan.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. For meta‐analyses, we subgrouped studies via phone call follow‐up directed at facilitating use of the action plan.
Main results
This updated review includes two additional studies (and 976 additional participants), for a total of seven parallel‐group RCTs and 1550 participants, 66% of whom were male. Participants' mean age was 68 years and was similar among studies. Airflow obstruction was moderately severe in three studies and severe in four studies; mean post bronchodilator forced expiratory volume in one second (FEV1) was 54% predicted, and 27% of participants were current smokers. Four studies prepared individualised action plans, one study an oral plan and two studies standard written action plans. All studies provided short educational input on COPD, and two studies supplied ongoing support for action plan use. Follow‐up was 12 months in four studies and six months in three studies.
When compared with usual care, an action plan with phone call follow‐up significantly reduced the combined rate of hospitalisations and emergency department (ED) visits for COPD over 12 months in one study with 743 participants (rate ratio (RR) 0.59, 95% confidence interval (CI) 0.44 to 0.79; high‐quality evidence), but the rate of hospitalisations alone in this study failed to achieve statistical significance (RR 0.69, 95% CI 0.47 to 1.01; moderate‐quality evidence). Over 12 months, action plans significantly decreased the likelihood of hospital admission (odds ratio (OR) 0.69, 95% CI 0.49 to 0.97; n = 897; two RCTs; moderate‐quality evidence; number needed to treat for an additional beneficial outcome (NNTB) 19 (11 to 201)) and the likelihood of an ED visit (OR 0.55, 95% CI 0.38 to 0.78; n = 897; two RCTs; moderate‐quality evidence; NNTB over 12 months 12 (9 to 26)) compared with usual care.
Results showed no significant difference in all‐cause mortality during 12 months (OR 0.88, 95% CI 0.59 to 1.31; n = 1134; four RCTs; moderate‐quality evidence due to wide confidence interval). Over 12 months, use of oral corticosteroids was increased with action plans compared with usual care (mean difference (MD) 0.74 courses, 95% CI 0.12 to 1.35; n = 200; two RCTs; moderate‐quality evidence), and the cumulative prednisolone dose was significantly higher (MD 779.0 mg, 95% CI 533.2 to 10248; n = 743; one RCT; high‐quality evidence). Use of antibiotics was greater in the intervention group than in the usual care group (subgrouped by phone call follow‐up) over 12 months (MD 2.3 courses, 95% CI 1.8 to 2.7; n = 943; three RCTs; moderate‐quality evidence).
Subgroup analysis by ongoing support for action plan use was limited; review authors noted no subgroup differences in the likelihood of hospital admission or ED visits or all‐cause mortality over 12 months. Antibiotic use over 12 months showed a significant difference between subgroups in studies without and with ongoing support.
Overall quality of life score on St George’s Respiratory Questionnaire (SGRQ) showed a small improvement with action plans compared with usual care over 12 months (MD ‐2.8, 95% CI ‐0.8 to ‐4.8; n = 1009; three RCTs; moderate‐quality evidence). Low‐quality evidence showed no benefit for psychological morbidity as measured with the Hospital Anxiety and Depression Scale (HADS).
Authors' conclusions
Use of COPD exacerbation action plans with a single short educational component along with ongoing support directed at use of the action plan, but without a comprehensive self‐management programme, reduces in‐hospital healthcare utilisation and increases treatment of COPD exacerbations with corticosteroids and antibiotics. Use of COPD action plans in this context is unlikely to increase or decrease mortality. Whether additional benefit is derived from periodic ongoing support directed at use of an action plan cannot be determined from the results of this review.
Keywords: Aged; Female; Humans; Male; Patient Education as Topic; Self Care; Behavior Therapy; Disease Progression; Health Promotion; Health Services Needs and Demand; Health Services Needs and Demand/statistics & numerical data; Hospitalization; Hospitalization/statistics & numerical data; Patient Care Planning; Patient Care Planning/organization & administration; Pulmonary Disease, Chronic Obstructive; Pulmonary Disease, Chronic Obstructive/diagnosis; Pulmonary Disease, Chronic Obstructive/therapy; Quality of Life; Randomized Controlled Trials as Topic
Plain language summary
Review question: Are action plans with brief education to help patients recognise and respond to worsening symptoms effective in COPD?
We reviewed evidence on the effect of action plans for exacerbations in people with chronic obstructive pulmonary disease. We found seven relevant studies. Evidence gathered in this review is current to November 2015.
Background
Chronic obstructive pulmonary disease (COPD) is a disease of the airways that is commonly caused by smoking. People with COPD often experience worsening of symptoms, known as an “exacerbation”, for which they need extra treatment and sometimes a stay in hospital. An action plan is a written or spoken guide that is given, with brief education, to people with COPD to help them recognise symptoms of an exacerbation and start taking extra treatment earlier. Individuals may keep extra medicines at home or may receive a prescription to take to a pharmacist. Sometimes a health professional will make regular phone calls to help patients use the action plan. We conducted this review to find out if having an action plan for COPD exacerbations improves health and reduces hospital visits.
Study characteristics
We found seven relevant studies of 1550 people with COPD. We did not include studies that gave other treatments, such as an exercise programme or longer educational sessions, along with an action plan. People in three studies had ongoing support to help them use the action plan. People in the included studies had moderate to severe symptoms and were followed up for six or 12 months.
Key results
People with COPD who are given an action plan have fewer emergency department visits and hospital stays related to breathing problems over a year. We calculated that for every 19 people given an action plan, one person would avoid a hospital stay for an exacerbation.
People with an action plan took more corticosteroid and antibiotic medicines for exacerbations ‐ on average just under one more course of corticosteroids and two more courses of antibiotics over a year.
Some studies showed that giving people an action plan improved their ability to recognise and self‐start treatment for worsening COPD symptoms.
Giving people an action plan made no difference in their chance of dying from any cause over a year, but this finding showed some variability.
We could not say whether follow‐up phone calls added benefit over following an action plan alone.
Quality of the evidence
The evidence in this review is generally independent and reliable, and we are very or moderately certain about the results.
Conclusions
We believe that people with COPD should be given an individualised action plan with a short educational component so they can benefit from fewer and shorter hospital stays, better understanding of the need to self‐start treatment and appropriate use of medication for exacerbations.
Summary of findings
Summary of findings for the main comparison. Action plan versus usual care for exacerbations of chronic obstructive pulmonary disease.
| Do action plans improve patient outcomes in acute exacerbations of chronic obstructive pulmonary disease | ||||||
| Patient or population: individuals with exacerbations of chronic obstructive pulmonary disease Setting: community and outpatient setting Intervention: action plan Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with action plan | |||||
| Hospitalisations for COPD/100 patient‐years (action plan + phone follow‐up) Follow‐up: 12 months | Rate ratio 0.69 (0.47 to 1.01) | 743 (1 RCT) | ⊕⊕⊕⊝ Moderatea | |||
| Hospitalisations and emergency visits for COPD/100 patient‐years (action plan + phone follow‐up) Follow‐up: 12 months | Rate ratio 0.59 (0.44 to 0.79) | 743 (1 RCT) | ⊕⊕⊕⊕ High | |||
| At least 1 hospital admission Follow‐up: 12 months | 209 per 1000 | 154 per 1000 (114 to 204) | Odds ratio 0.69 (0.49 to 0.97) | 897 (2 RCTs) | ⊕⊕⊕⊝ Moderateb | |
| Mortality (all‐cause) Follow‐up: 12 months | 103 per 1000 | 91 per 1000 (63 to 130) | Odds ratio 0.88 (0.59 to 1.31) | 1134 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| Courses of oral corticosteroids Follow‐up: 12 months | Mean courses of oral corticosteroids were 1.05 | Mean courses of oral corticosteroids in the intervention group were 0.74 more (0.12 more to 1.35 more) | ‐ | 200 (2 RCTs) | ⊕⊕⊕⊝ Moderateb | |
| Courses of antibiotics Follow‐up: 12 months | Mean courses of antibiotics ranged from 1.6 to 3.2 | Mean courses of antibiotics in the intervention group were 2.26 more (1.82 more to 2.7 more) | ‐ | 943 (3 RCTs) | ⊕⊕⊕⊝ Moderatec | Not downgraded for presence of substantial heterogeneity, which is explicable by differences in study design |
| Respiratory‐related quality of life: SGRQ overall score Scale from 0 (best) to 100 (maximum impairment) Follow‐up: 12 months | Mean respiratory‐related quality of life: SGRQ overall score ranged from ‐2 to +6 units | Mean respiratory‐related quality of life: SGRQ overall score in the intervention group was 2.82 units lower (0.83 lower to 4.81 lower) | ‐ | 1009 (3 RCTs) | ⊕⊕⊕⊝ Moderatec | Not downgraded for presence of substantial heterogeneity, which is explicable by differences in study design |
| Depression score assessed with HADS Scale from 0 to 21 (worst) Follow‐up: 12 months | Mean depression score was ‐0.04 | Mean depression score in the intervention group was 0.25 lower (1.14 lower to 0.64 higher) | ‐ | 154 (1 RCT) | ⊕⊕⊝⊝ Lowa,d | |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: rate ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aWide confidence interval; effect size includes null.
bUnclear risk of bias for two studies for allocation and blinding of assessors.
cUnclear risk of bias for three studies for allocation and blinding of assessors.
dUnclear risk of bias for one study for allocation and blinding of assessors.
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is a systemic, progressive, heterogeneous disease with significant worldwide public health importance. COPD is associated with a chronic innate inflammatory response that results from continuous exposure to inhaled noxious particles (GOLD 2016; Hogg 2004). This inflammatory response may induce destruction of lung parenchyma and may disrupt normal repair and defence mechanisms (GOLD 2016). These pathological changes lead to characteristic progressive airflow limitation that is not fully reversible (GOLD 2016).
COPD develops from a combination of genetic and environmental factors and is most commonly linked to cigarette smoking (Halbert 2006). In addition to cigarette smoking, exposures such as burning of wood and other biomass fuels are important risk factors for some populations (GOLD 2016).
COPD is a significant cause of preventable worldwide morbidity and mortality. Estimates have placed COPD as the fourth leading cause of death globally (WHO 2004). The prevalence of COPD is predicted to increase owing to the persisting incidence of smoking and ageing of the global population (GOLD 2016). The World Health Organization (WHO) predicts that COPD will become the third leading cause of death by 2030 (WHO 2008). Other estimates have predicted that COPD will become the seventh leading cause of disability‐adjusted life‐years (DALYs) by 2030 (Mathers 2006). In 2010, the economic burden of COPD in the United States was projected to be $49.9 billion, including $29.5 billion in direct healthcare expenditures (American Lung Association 2014). In Australia, it was estimated that in 2008, COPD cost the economy $98 billion AUD (Access 2008).
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) advises that a postbronchodilator forced expiratory volume of one second (FEV1)/forced vital capacity (FVC) ratio < 0.70 is needed for a diagnosis of COPD (GOLD 2016). Disease severity can be classified by the degree of airflow limitation, although evidence suggests that this is a poor predictor of many negative features of the disease. Patients with similar airflow limitations have been found to belong to different disease phenotypes and to have marked differences in age, symptoms, comorbidities and predicted mortality (Agusti 2010; Burgel 2010). Interest in the potential importance of airway and blood eosinophilia as a predictor of exacerbations and their response to corticosteroids has recently increased (Bafadhel 2012; Pascoe 2015), but this has not been taken into account in most clinical studies, such as those included in this review.
The presentation, progression and pathological abnormalities associated with COPD are variable (Han 2013). COPD can result in an array of systemic physical functional limitations including poor musculoskeletal strength and function, poor exercise performance and self‐reported functional limitations (Eisner 2008). Patients with COPD often have multiple comorbidities spanning both medical and psychiatric illnesses that can have a significant impact on prognosis (Barnes 2009; Hanania 2011; Rennard 2006).
Another important prognostic factor that is a major problem associated with COPD is the occurrence of exacerbations. The GOLD guidelines define a COPD exacerbation as 'an acute event characterised by a worsening of the patient’s respiratory symptoms that is beyond normal day‐to‐day variations and leads to a change in medication' (GOLD 2016). Exacerbations are a major driver of decline in health status and health‐related quality of life (Chhabra 2014; Spencer 2004). They are usually managed with increased bronchodilator medication, oral corticosteroids (Walters 2014) and antibiotics (Vollenweider 2012). People with frequent exacerbations of COPD experience poorer health status, accelerated decline in FEV1, worsened quality of life and increased hospital admissions and mortality (Halpin 2012; Vestbo 2011). COPD exacerbations account for the greatest proportion of the total COPD burden on the healthcare system (GOLD 2016).
Description of the intervention
Management of COPD is complex and should involve a multi‐disciplinary and multi‐modality approach. An action plan is used to encourage early intervention for exacerbations. Action plans provide guidelines detailing self‐initiated actions, such as changing medication regimens or visiting a general practitioner (GP) or hospital, to be undertaken in response to alterations in symptoms of COPD suggesting the start of an exacerbation. A healthcare provider or case manager can develop an action plan by using a template and can personalise the plan for individual patients according to their symptoms and ongoing regular management. Templates for action plans are provided online by some lung support groups, and they can be given to patients in primary care at low cost. Sometimes an action plan is accompanied by prescriptions for prednisolone and an oral antibiotic.
How the intervention might work
Action plans include interventions designed to allow patients to recognise and initiate early treatment for exacerbations. The early warning signs of an exacerbation have been found to be fairly consistent and recognisable within individuals (Kessler 2006). Despite this fact, evidence suggests that patients do not seek medical care for all of the exacerbations that they experience (Langsetmo 2008; Walters 2012). Unreported exacerbations are usually less severe but still impact health status (Langsetmo 2008). Furthermore, some patients may present late for treatment of their exacerbation, and this is associated with slower recovery, worse quality of life and increased healthcare utilisation (Wilkinson 2004). The chronic and progressive nature of COPD underlies the importance of self‐management.
Action plans are frequently incorporated into self‐management interventions for COPD (Bourbeau 2009). A Cochrane systematic review found that comprehensive self‐management interventions improved health‐related quality of life and decreased healthcare utilisation (Zwerink 2014). In this review, 75% of studies incorporated the use of an action plan, and it was hypothesised that the decreased number of respiratory‐related hospitalisations observed in the intervention group may particularly have reflected this (Zwerink 2014).
Why it is important to do this review
Lack of consensus on an operational definition of COPD self‐management has been a barrier to the formulation of clear recommendations (Effing 2012). Heterogeneity among interventions, study populations, follow‐up time and outcome measures made it difficult for review authors in two Cochrane systematic reviews (Kruis 2013; Zwerink 2014) to determine the most effective form and content of self‐management for COPD. Effing et al proposed a conceptual definition of COPD self‐management, stated as follows: "A COPD self‐management intervention is structured but personalised and often multi‐component, with goals of motivating, engaging and supporting the patients to positively adapt their health behaviours and develop skills to better manage their disease" (Effing 2016). Development and evaluation of specific self‐management interventions is important for application of the definition presented by Effing et al. This review is an update of a Cochrane Review first published in 2005 (Turnock 2005). The aim of this review is to determine the role and effectiveness of an action plan as a self‐management intervention provided for patients with COPD without comprehensive self‐management education/training.
Objectives
To compare effects of an action plan for COPD exacerbations provided with a single short patient education component and without a comprehensive self‐management programme versus usual care. Primary outcomes were healthcare utilisation, mortality and medication use. Secondary outcomes were health‐related quality of life, psychological morbidity, lung function and cost‐effectiveness.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs, excluding cross‐over trials.
Types of participants
Participants were patients with a clinical diagnosis of COPD based on spirometric criteria such as those of GOLD (GOLD 2016) for persistent airflow limitation (i.e. postbronchodilator FEV1/FVC < 70%) with a history of smoking. We excluded studies with participants who had received a primary diagnosis of asthma, unless separate results were available for participants with COPD.
Types of interventions
The intervention consisted of an action plan with a single educational component of short duration. The short educational portion allowed time the clinician needed to personalise the action plan according to individual management needs and symptoms. An action plan is defined as a written or oral guideline that details self‐initiated interventions (such as changing medication regimens or visiting a GP or hospital) undertaken in response to alterations in symptoms of COPD (e.g. increased breathlessness, increased amount or purulence of sputum, increased use of a relief inhaler, decreased activity level) (i.e. changes that would suggest commencement of an exacerbation). Investigators permitted ongoing support directed at use of the action plan delivered by telephone or direct contact. We deliberately did not include studies with broader self‐management support interventions, such as individual or group education delivered in multiple sessions over a longer period or exercise programmes, irrespective of whether they included an action plan. Researchers compared the active intervention versus 'usual care' delivered by healthcare providers.
Types of outcome measures
Primary outcomes
Healthcare utilisation, including respiratory‐related hospital admission, treatment in an emergency department (ED) and GP visits for COPD.
Mortality: respiratory‐related and all‐cause.
Use of medication: time to initiation of therapy after symptom onset; courses/duration of antibiotic or corticosteroid use, or both; participant initiation of antibiotic or steroid use, or both.
Secondary outcomes
Health‐related quality of life (HRQoL) measured on validated scales.
Psychological morbidity: anxiety and depression, measured on validated scales.
COPD self‐management knowledge and intended actions (based on participant interview).
Lung function.
Cost‐effectiveness.
Search methods for identification of studies
Electronic searches
We identified trials using the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Information Specialist for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases, including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED) and PsycINFO, and by handsearching of respiratory journals and meeting abstracts (see Appendix 1 for details). We searched all records in the CAGR using the search strategy presented in Appendix 2.
We carried out additional searches of CENTRAL, MEDLINE, Embase, CINAHL, PsycINFO, ClinicalTrials.gov, the WHO trials portal and the Australian New Zealand Clinical Trials Registry (ANZCTR). We have listed in Appendix 3 the search strategies used for these databases. We searched all databases from their inception to November 2015, and we imposed no restrictions on language of publication.
Searching other resources
From full‐text papers obtained, we handsearched bibliographic lists for additional articles. We contacted researchers for information about their ongoing trials and conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the WHO trials portal (www.who.int/ictrp/en/).
Data collection and analysis
Selection of studies
At least two review authors (MH, JW) assessed potentially relevant trials by screening full texts to independently select trials for inclusion and to identify and record reasons for exclusion of ineligible studies. We resolved disagreements through discussion or, if required, we consulted a third review author (RWB). We identified and excluded duplicates and collated multiple reports of the same study, so that each study (rather than each report) was the unit of interest in the review. We recorded the selection process as a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram.
Data extraction and management
We used a data collection form to record study characteristics and outcome data. Two review authors (MH, JW) independently extracted the following characteristics from reports of included studies.
Methods: study design, total duration of study, number of study centres and locations, study setting and duration and date of study.
Participants: N, mean age, age range, gender, withdrawals, inclusion criteria and exclusion criteria.
Interventions: study treatment, comparisons and cointerventions.
Outcomes: primary and secondary outcomes specified and collected and time points reported.
Notes: funding for trial, trial registration and notable conflicts of interest of trial authors.
Two review authors (MH, JW) independently extracted outcome data from reports of included studies. MH entered the data into Review Manager, and JW double‐checked the data. We checked that data were entered correctly by comparing data presented in the systematic review against the study reports.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study (MH, JW) using criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion or by consultation with another review author (RWB). We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias(es).
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised risk of bias judgements across studies for each of the domains listed. When information on risk of bias was related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to those outcomes.
Measures of treatment effect
We analysed dichotomous outcomes using Mantel‐Haenszel odds ratios with a 95% confidence interval (CI). When events were rare, we employed the Peto odds ratio. We entered scale data with a consistent direction of effect.
For continuous variables, we analysed data as mean differences (MDs), with 95% CIs. We used standardised mean difference (SMDs) with 95% CIs when different scales of measurement had been used for a particular outcome. The SMD expresses the difference in means between treatment groups in units of the pooled standard deviation.
We undertook meta‐analyses only when this was meaningful, that is, when treatments, participants and the underlying clinical question were similar.
When skewed data were available (reported as medians and interquartile ranges), we described them narratively.
For 'time‐to‐event' outcomes such as log hazard ratios, we used the fixed‐effect generic inverse variance outcome to combine results. This method gives a weighted average of the effect estimates of separate studies (Deeks 2001). We calculated the number needed to treat for an additional beneficial outcome from the pooled odds ratio and confidence interval, using baseline risk in the control group.
Unit of analysis issues
We analysed dichotomous data by using participants as the unit of analysis.
Dealing with missing data
We contacted investigators to obtain missing numerical outcome data when possible (e.g. when a study was identified as abstract only), or to clarify details of methods. When this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by performing a sensitivity analysis.
If no information on the variability of an effect estimate (confidence interval or P value) was available, we imputed standard deviations. We used one of two methods: borrowing the standard deviation (SD) from another study of similar duration (using the largest value when more than one study provided results), or calculating a correlation coefficient (R value) using data from another study according to methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
We carried out an assessment of possible heterogeneity for pooled effects, when the null hypothesis was that all studies were evaluating the same effect, by using a Breslow‐Day test of heterogeneity; a P value < 0.05 was considered to indicate significant differences between studies. In addition, we used the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than to chance (Higgins 2011). We interpreted statistical heterogeneity as follows: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity and 50% to 90% may represent substantial heterogeneity (Higgins 2011).
We assessed clinical and methodological heterogeneity by recording differences in study design and participant characteristics between individual studies. When we found substantial heterogeneity, we reported this and explored possible causes by performing prespecified subgroup analysis.
Assessment of reporting biases
We tried to minimise reporting bias resulting from non‐publication of studies or selective outcome reporting by using a broad search strategy, checking references of included studies and relevant systematic reviews and contacting study authors for additional outcome data. We planned to visually inspect funnel plots if 10 or more studies contributed to outcome analysis.
Data synthesis
We used a fixed‐effect model and performed a sensitivity analysis with a random‐effects model if we noted unexplained heterogeneity. We presented the findings of our primary outcomes and other important outcomes in a 'Summary of findings' table according to recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (generated with the use of GradePro software) (seven specified a priori in the update).
Hospital admission ‐ respiratory‐related.
Emergency department attendance ‐ respiratory‐related.
Mortality.
Quality of life.
Use of oral corticosteroids.
Use of antibiotics.
Psychological morbidity.
Subgroup analysis and investigation of heterogeneity
In this review update, we planned a priori subgroup analysis based on:
comparison of studies with ongoing support directed at use of an action plan versus those conducted without such support;
severity of COPD: participants with mild to moderate CODP versus those with severe to very severe COPD; and
design of the action plan.
Sensitivity analysis
In assessing heterogeneity, we considered possible causes arising from details of study design. We performed sensitivity analyses by using a random‐effects model versus a fixed‐effect model in assessing risk of bias and in identifying other potential confounders; for studies published only as abstracts, we used various methods to impute a missing standard deviation.
Results
Description of studies
Results of the search
Review authors identified and screened a total of 574 titles and abstracts since the original review was published in 2005. In 2005, two review authors (AT, JW) assessed the full texts of 11 of 199 identified studies, and included three of these studies in the review (McGeoch 2004; Watson 1997; Wood‐Baker 2006). In 2010, review authors included two (Martin 2004; Rootmensen 2008) of 17 studies identified in the search update. From the updated search conducted during 2014, review authors identified 358 studies for screening, of which they assessed 36 full texts for eligibility (Figure 1). Review authors assessed all previously excluded studies for eligibility if the intervention included ongoing support for action plan use. Review authors excluded 33 citations (representing 26 studies) ‐ four owing to wrong comparator, 24 because of the wrong intervention, three as the result of wrong study design, one because the duration of education exceeded eligibility and one because it was a duplicate citation for a study already excluded. One study was ongoing, and review authors included two studies in the review (Rice 2010; Trappenburg 2011). Searches for this update repeated on 21/11/15, before the review update was submitted, yielded no new studies. Two review authors (JW, MH) conducted screening for the most recent update.
1.
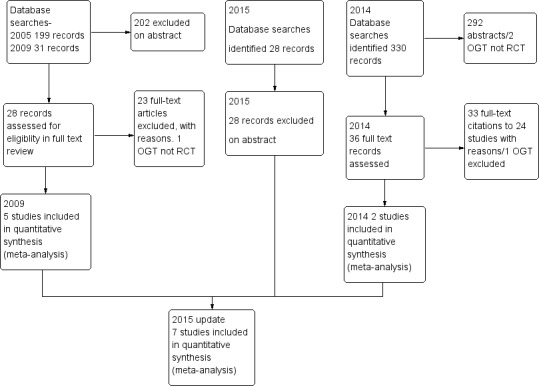
Study flow diagram.
Included studies
See the Characteristics of included studies table.
This review update includes a total of seven parallel‐group RCTs that included 1550 participants with COPD (Table 2). Since the last update appeared in 2010 (Walters 2010), review authors have added two studies (Rice 2010; Trappenburg 2011) with an additional 976 participants. Four trials (Martin 2004; Rice 2010; Rootmensen 2008; Watson 1997) were randomised at patient level, two (McGeoch 2004: Wood‐Baker 2006) were cluster‐randomised at practice level and one (Trappenburg 2011) was randomised by the minimisation technique to control for centre and gender. Four studies recruited participants through GPs. Wood‐Baker 2006 recruited from 54 GPs in 31 practices, and Watson 1997 recruited from 22 GPs in 12 practices. McGeoch 2004 recruited participants attending two groups of general practices but did not specify the number of GPs involved, and Martin 2004 recruited through a consortium of GPs in one region. Rice 2010 recruited participants from a centralised electronic medical record database of a US Veterans Hospital. Trappenburg 2011 recruited participants through scheduled visits to a respiratory nurse at eight regional hospitals and five general practices.
1. Study design.
| Study ID | Dates | Recruitment/Randomisation unit | Follow‐up | Length SME (educator) | RAN, n/WD, n |
Age*, years/ % male |
% current smokers |
FEV1 % pred* INT‐CONT |
QoL INT‐CONT |
| Martin 2004 | Not known | Consortium practices, New Zealand/participants | 12 months | Single interview, length not stated (respiratory nurse) | 96/26 | 70/51 | n/a | 35‐34 | 57‐51 |
| McGeoch 2004 | 7/2002‐ 12/2003 | 2 groups of practices, New Zealand/practice | 12 months | 1 hour (practice nurse or respiratory educator) | 159/9 | 71/59 | 28 | 55‐53 | 43‐37 |
| Rootmensen 2008 (all participants) | Not known | 1 hospital pulmonary outpatient clinic, Netherlands/ participants | 6 months | 45 minutes (pulmonary nurse) | 157 (111 COPD)/17 | 60/55 | 12 | 57‐64 | n/a |
| Rice 2010 | 07/2004‐ 07/2008 | Centralised electronic medical record database/participants | 12 months | 1 to 1.5‐hour group educational session (case manager) | 743/84 | 70/98 | 22 | 36.1‐38.1 | n/a |
| Trappenburg 2011 | 12/2008‐ 12/2010 | 8 regional hospitals and 5 general practices/participants (stratified by gender and centre) | 6 months | Single interview, length not stated (nurse case manager). | 233/41 | 66/57 | 29 | 56.7‐56.5 | n/a |
| Watson 1997 | 1993‐ 07/1994 | 12 practices, 22 GPs, New Zealand/participants | 6 months | Single interview, length not stated (practice nurse) | 69/13 | 68/65 | 28 | 37‐36 | 43‐39 |
| Wood‐Baker 2006 | 2002‐2003 | 54 GPs, 31 practices, Australia/practice | 12 months | 1 hour (respiratory research nurse) | 139/27 | 70/76 | 42 | 46‐44 | 47‐47 |
*: mean; AP: action plan; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in one second; GP: general practitioner; INT‐CONT: intervention group‐control group; QoL: % impairment quality of life 0‐100; RAN: randomisation; SME: self‐management education; WD: withdrawal or death.
All participants had received a diagnosis of COPD as a major functionally limiting disease before inclusion. In line with the GOLD criteria for diagnosis of COPD, all participants showed a postbronchodilator FEV1/FVC ratio < 0.70. However, Rootmensen 2008 recruited participants with a diagnosis of COPD or asthma. We included in this review only results for the subgroup of participants with COPD (111 of 191). Participants in Rice 2010 were also required to have one or more of the following during the previous year: hospitalisation or ED visit for COPD, long‐term home oxygen use or course of systemic corticosteroids for COPD. Trappenburg 2011 recruited participants over the age of 40 who were currently using bronchodilator therapy. Participants in Wood‐Baker 2006 were at least 50 years of age. Both Watson 1997 and Wood‐Baker 2006 also required FEV1 < 65% predicted. McGeoch 2004 stated inclusion criteria of symptoms at least weekly and history of one or more exacerbations in the previous 12 months requiring an increase in therapy. Martin 2004 required at least one hospital admission or two acute exacerbations of COPD requiring GP care during the previous 12 months. Entry criteria for Watson 1997 included current use of bronchodilator therapy.
Assessment of baseline characteristics of participants (Table 2) shows that studies involved people of similar age, with mean age from 60 to 71 years and overall mean age of 68 years. All studies included more male participants, ranging from 51% to 98% with overall mean of 66%. The high incidence of male participants in Rice 2010 (98%) reflected recruiting from Veterans Affairs medical centres. The percentage of current smokers in each study group varied from 28% (Wood‐Baker 2006) to 12% (Rootmensen 2008), with overall mean of 27%. Severity of airflow obstruction, as indicated by the overall mean postbronchodilator FEV1 as percentage of predicted value (staged according to the GOLD classification), was moderate in three studies (McGeoch 2004 (54% predicted); Rootmensen 2008 (61% predicted); Trappenburg 2011 (57% predicted)) and severe in four studies (Martin 2004 (54% predicted); Rice 2010 (54% predicted); Watson 1997 (54% predicted); Wood‐Baker 2006 (54% predicted)). At baseline, mean impairment scores for overall quality of life when available (in four studies) (based on St George's Respiratory Questionnaire maximum impairment = 100) ranged from 37 to 57, with mean overall score of 46. Within studies, impairment in quality of life was similar between intervention and control groups.
Three studies specified exclusion of nursing home residents (McGeoch 2004; Watson 1997; Wood‐Baker 2006). Five studies specified exclusion of participants with other primary limiting diseases such as lung cancer and cardiac disease (Martin 2004; McGeoch 2004; Rice 2010; Trappenburg 2011; Watson 1997). Trappenburg 2011 also excluded participants with a primary diagnosis of asthma. Rice 2010 excluded participants without access to a telephone.
Study follow‐up was six months in three studies (Rootmensen 2008; Trappenburg 2011; Watson 1997) and 12 months in four studies (Martin 2004; McGeoch 2004; Rice 2010; Wood‐Baker 2006). Investigators reported a total of 217 withdrawals from the total 1550 participants enrolled and a drop‐out rate ranging from 5% to 27%.
Action plan intervention
Table 3 presents a comparison of action plan interventions. Three studies used a standard written action plan and information booklet (McGeoch 2004; Watson 1997; Wood‐Baker 2006). Martin 2004, Rice 2010 and Trappenburg 2011 used an individualised action plan intervention. Rootmensen 2008 provided an intervention consisting of additional care that included individual instructions for what to do in case of exacerbations.
2. Action plan (AP) intervention and comparison used in included studies.
| Individualised AP | Standard written AP | Support for AP during study period | SME (individual/group) | Prescription /supply OCS | Prescription /supply ABS | Written COPD educational component | Comparison | |
| Martin 2004 | Written | 3‐Monthly visit regarding use of AP | Individual interview with respiratory nurse, length not stated, individualised action plan according to current treatment and symptoms | All had 7‐day supply | All had 7‐day supply | No | Usual care by own GP | |
| McGeoch 2004 | Yes | No | Individual session by practice nurse or respiratory educator in association with GP 1 hour, covering major points of COPD self‐management plan, and use of validated sputum colour charts | Prescription | Prescription | Educational package | Non‐standard education on COPD according to practice standards | |
| Rice 2010 | Written | Monthly phone call from nurse | Group 1‐1.5 hours, individualised action plan with respiratory nurse | Yes | Prescription | Usual care + 1‐page summary of principles of COPD care according to published guidelines. No AP | ||
| Rootmensen 2008 | Oral | No | Individual protocol‐based educational session covering disease, medications, vaccination, smoking cessation and exacerbation management, 45 minutes in length | Oral medication provided to some, % unknown | Oral medication provided to some, % unknown | No | Usual care | |
| Trappenburg 2011 | Written | Standardised phone calls at 1 and 4 months | Individualised action plan education, length of session not stated | 2%' | 22% | ✓ COPD information | Usual care ‐ pharmacological and non‐pharmacological care according to most recent evidence‐based guidelines, specifically AP denied. All included participants seen by respiratory nurse, who systematically checked and discussed aspects of COPD care, including vaccination, optimisation of medication, inhalation techniques, exercise, nutritional aspects, smoking (cessation) and exacerbation management. | |
| Watson 1997 | Yes | No | Individual session education about use of the action plan with COPD booklet by a senior respiratory outreach nurse; length not stated | Prescription | Prescription | ✓ Guide to living positively with COPD | Usual care by GP, specifically denied access to AP and booklet | |
| Wood‐Baker 2006 | Written | No | Individual educational session with respiratory nurse, covering COPD, smoking cessation, immunisation, nutrition, exercise, sputum clearance, breathing, medication, inhaler use. Individualised action plan developed with GP input. Length not known | 2% | 22% | COPD information booklet | Usual care, COPD information booklet and individual educational session with nurse, but no AP |
ABS: antibiotics; AP: action plan; COPD: chronic obstructive pulmonary disease; GP: general practitioner; OCS: oral corticosteroids; SME: self‐management education.
Wood‐Baker 2006 participants also received an individual educational session with a nurse experienced in managing respiratory disease. Their action plan was a written self‐management plan that was developed in consultation with their treating GP. It listed the participant's maintenance medications and an individualised action plan based on early recognition of symptoms associated with exacerbations of COPD. Seventy‐six per cent received a standard action plan with instructions to self‐initiate a short course of oral corticosteroids and an antibiotic; the other 24% received an action plan with instructions to initiate antibiotics only (N = 10), to double their dose of inhaled corticosteroids and commence an antibiotic (N = 2), to initiate a short course of oral corticosteroids only (N = 1) or to contact their GP (N = 3). Participants following action plans that involved self‐initiation of medication were given prescriptions by their GP. All intervention participants were encouraged to present to their GP early.
Two studies (McGeoch 2004; Watson 1997) used action plans that were identical and provided advice on management of usual care and exacerbations, together with a booklet on self‐management, a prescription from their GP for prednisolone and a broad‐spectrum antibiotic for self‐administration during an exacerbation. Watson 1997 made no attempt to individualise instructions in the action plan, whereas the remaining trials (Martin 2004; McGeoch 2004; Wood‐Baker 2006; Rootmensen 2008) delivered self‐management plan education in an individual session provided by a nurse, a respiratory educator or the participant's GP.
Four trials (Martin 2004; McGeoch 2004; Watson 1997; Wood‐Baker 2006) supplied booklets with action plans that covered topics such as smoking cessation, control of breathlessness, nutrition, exercise, clearance of mucus from the lungs, medications and contact details of community support services. Two trials educated participants on the correct use of inhalers (Rootmensen 2008; Wood‐Baker 2006).
In Rice 2010, participants attended a single 1 to 1.5‐hour group educational session. They received individualised written action plans that included a description of the signs and symptoms of an exacerbation that should prompt initiation of self‐treatment, refillable prescriptions for prednisolone and an oral antibiotic, contact information for a case manager and the telephone number of the 24‐hour VA help line. Participants were instructed to begin action plan medications for symptoms that were substantially worse than usual. A case manager made monthly phone calls to reinforce general principles of COPD management, to review details of the action plan and to answer questions.
In Trappenburg 2011, participants attended an individual educational session with a respiratory nurse, who systematically checked and discussed aspects of COPD care such as vaccination, optimisation of medication, inhalation techniques, exercise, nutritional aspects, smoking (cessation) and exacerbation management. Participants received an individualised action plan that included recognition of symptom changes, use of medication/lifestyle prescriptions, additional medication/breathing exercises and energy preservation in case of symptom increase and a contact person/telephone number in case of an exacerbation. For individual participants, it was optional for the case manager (in consultation with the attending physician) to provide self‐treatment medication (course of corticosteroids and/or antibiotics). Two standardised reinforcement sessions were held by telephone at one and four months to evaluate participants' understanding of and adherence to the action plan; when needed, researchers provided additional information.
Control
Investigators provided all control groups with usual care; although this varied between studies, participants were always specifically denied access to the action plan. Wood‐Baker 2006 provided usual care that included provision of a booklet and an individual nurse educational session. McGeoch 2004 provided non‐standardised education based on routine practice at the time. The remaining three trials (Martin 2004; Rootmensen 2008; Watson 1997) supplied no additional education for participants in control groups. Rice 2010 distributed to usual care participants a one‐page handout containing a summary of principles of COPD care based on published guidelines. In Trappenburg 2011, a nurse case manager assessed participants and systematically checked and discussed aspects of COPD care such as vaccination, optimisation of medication, inhalation techniques, exercise, nutritional aspects, smoking (cessation) and exacerbation management. Participants had no additional contact with the case manager.
Excluded studies
Ten studies were not RCTs, 11 studies involved comprehensive self‐management programmes in which the action plan component could not be isolated and in nine studies, COPD/self‐management education was delivered in multiple sessions or in a single session of several hours' duration. Fifteen studies included no action plan in the intervention.
Risk of bias in included studies
We have provided full details regarding risk of bias assessment for all included studies in the Characteristics of included studies table, along with a summary of grading in Figure 2 and Figure 3.
2.
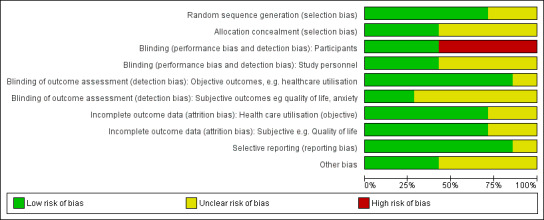
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
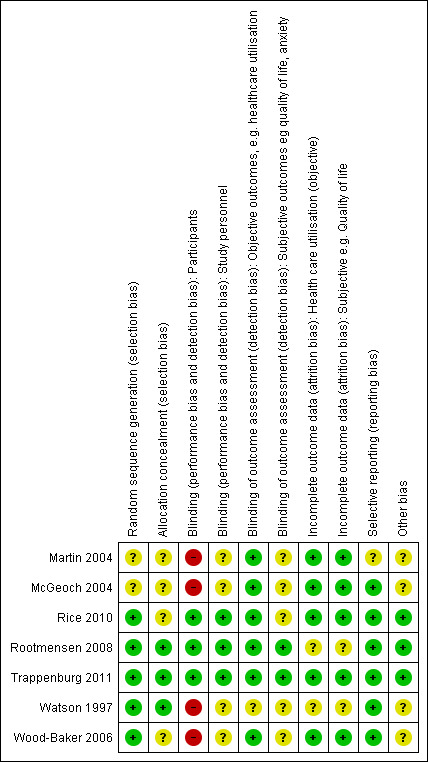
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
With regards to random sequence generation, we assessed five studies as having low risk of bias; two employed permuted block randomisation (Rice 2010; Watson 1997), two the minimisation technique (Rootmensen 2008; Trappenburg 2011) and one a computer‐generated randomised software package (Wood‐Baker 2006). Two studies assessed as having unclear risk reported that they were RCTs but did not describe the method of randomisation used (Martin 2004; McGeoch 2004).
Concerning allocation concealment, we assessed three studies as having low risk of bias (Rootmensen 2008; Trappenburg 2011; Watson 1997). Rootmensen 2008 randomised participants in advance of their clinic attendance and reported these results only to the pulmonary physician just before the visit. Trappenburg 2011 utilised a central web‐based service to conceal the assignment sequence. In Watson 1997, research staff who recruited participants allocated them according to a randomisation list. We assessed four studies as having unclear risk of bias. Three did not report methods of allocation (Martin 2004; Rice 2010; Wood‐Baker 2006), and in McGeoch 2004, researchers allocated participants by practice attendance but did not provide information on allocation of practices.
Blinding
We assessed three studies as having low risk of bias for blinding of participants; two utilised a modified consent procedure by which the major objective of the study was withheld from participants until after the study was completed (Rootmensen 2008; Trappenburg 2011), and in Rice 2010, participants were aware of their allocation, but this awareness was not thought likely to affect primary healthcare utilisation outcomes. Regarding patient‐reported outcomes, we assessed those from Rootmensen 2008 and Trappenburg 2011 as low risk because investigators used a modified consent procedure, and those from Rice 2010 as unclear risk. We assessed four studies as having unclear risk of bias with regards to participants, as they were not blinded (Martin 2004; McGeoch 2004; Watson 1997; Wood‐Baker 2006); this introduced the potential for bias in self‐administered patient assessments, such as quality of life measures and daily diary records. In some practices in McGeoch 2004, GPs may have implemented both intervention and usual care, leading to possible confounding between treatment methods. Martin 2004, McGeoch 2004, Watson 1997 and Wood‐Baker 2006 did not blind outcome assessors, suggesting potential for high bias for subjective outcomes. Rice 2010 adequately blinded assessors.
Incomplete outcome data
Regarding incomplete outcome data for objective healthcare utilisation outcomes, we assessed five studies as having low risk of bias. McGeoch 2004 and Wood‐Baker 2006 reported small numbers lost to follow‐up that were balanced between groups. In Martin 2004, 93 of 96 recruited participants completed follow‐up; three withdrawals occurred for personal reasons, but investigators did not state group allocation. In Rice 2010, the only reason for missing data was death (48 in usual care, 36 in intervention), and study authors were unable to perform intention‐to‐treat analysis. Trappenburg 2011 reported drop‐out rates of 19% in the intervention group and 16% in the control group. For objective healthcare utilisation outcomes, we determined that two studies had unclear risk of bias; Rootmensen 2008 reported on only 90 of 117 participants with COPD, and Watson 1997 noted 13 withdrawals from the 60 participants originally randomised and did not report group allocation for those lost to follow‐up.
Risk of bias assessment concerning incomplete outcome data for subjective outcomes was similar to that for objective outcomes. We assessed five studies as having low risk of bias (Martin 2004; McGeoch 2004; Rice 2010; Trappenburg 2011; Wood‐Baker 2006) and two studies as having unclear risk (Rootmensen 2008; Watson 1997) because researchers did not report withdrawals by group.
Selective reporting
Regarding reporting bias, we assessed six studies as having low risk of bias (McGeoch 2004; Rice 2010; Rootmensen 2008; Trappenburg 2011; Watson 1997; Wood‐Baker 2006). In McGeoch 2004, Rootmensen 2008, Watson 1997 and Wood‐Baker 2006, it was clear that study authors reported all expected outcomes, including those that were prespecified. The protocols for Rice 2010 and Trappenburg 2011 were available, and outcomes reported in these studies were consistent with those prespecified. We assessed Martin 2004 as having unclear risk of bias, as it was not clear that published reports included all expected outcomes and those prespecified.
Other potential sources of bias
We identified no additional sources of bias in Rice 2010, Rootmensen 2008 and Trappenburg 2011. Martin 2004 described a pilot study in which no sample size calculation was performed; study authors did not attempt to examine clustering within practices. McGeoch 2004 reported on statistical analysis to examine the effect of clustering within practices. They analysed the 12‐month change in the outcome variable by using a mixed‐model repeated measures analysis of variance (ANOVA), allowing for cluster‐randomisation of surgeries, and indicated no additional variation from this source beyond that anticipated by between‐subject variation. For this reason, investigators in McGeoch 2004 undertook all analyses by using participants as replicates. In Wood‐Baker 2006, researchers did not perform analyses to examine the effect of clustering within practices. In Watson 1997, baseline analysis showed a statistically significant difference for influenza vaccination in the past year (72% in the intervention group, 44% in the control group).
Effects of interventions
See: Table 1
Results: primary outcomes
Healthcare utilisation, including respiratory‐related hospital admission, treatment in an emergency department (ED) and general practitioner (GP) visits for chronic obstructive pulmonary disease (COPD).
Mortality: respiratory‐related and all‐cause.
Use of medications: time to initiation of therapy after symptom onset; course/duration of antibiotic or corticosteroid use, or both; participant initiation of antibiotic or steroid use, or both
Healthcare utilisation
Analysis 1.1 Rate of hospitalisation for COPD/100 patient‐years: For this outcome, we found one relevant trial with 12‐month follow up (n = 743). The difference between action plan with phone follow‐up and control was not statistically significant (rate ratio (RR) 0.69, 95% confidence interval (CI) 0.47 to 1.01).
1.1. Analysis.
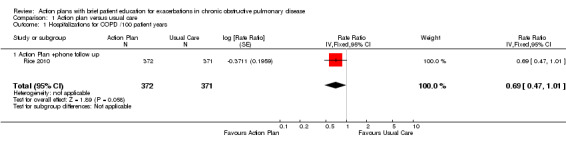
Comparison 1 Action plan versus usual care, Outcome 1 Hospitalizations for COPD /100 patient years.
Analysis 1.2 At least one hospital admission (12‐month follow‐up): For this outcome, we found two relevant trials (n = 897). Results showed a statistically significant difference, with less likelihood for action plan compared with control (subgrouped by phone follow‐up) (odds ratio (OR) 0.69, 95% CI 0.49 to 0.97) and no heterogeneity.
1.2. Analysis.
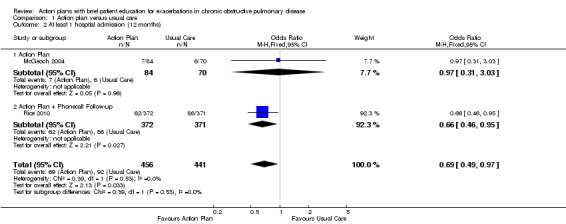
Comparison 1 Action plan versus usual care, Outcome 2 At least 1 hospital admission (12 months).
Analysis 1.3 At least one hospital admission (six‐month follow‐up): For this outcome, we found one relevant trial (n = 227). Results showed no statistically significant difference between action plan with phone follow‐up and control (OR 0.83, 95% CI 0.30 to 2.31).
1.3. Analysis.
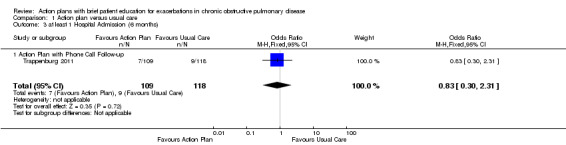
Comparison 1 Action plan versus usual care, Outcome 3 at least 1 Hospital Admission (6 months).
Analysis 1.4 Rate of hospital admission for exacerbation (12‐month follow‐up): For this outcome, we found two relevant trials up (n = 205). Results showed no statistically significant difference between action plan and control (mean difference (MD) 0.23, 95% CI ‐0.03 to 0.49) and no heterogeneity (Chi² = 0.30, df = 1 (P = 0.59), I² = 0%).
1.4. Analysis.
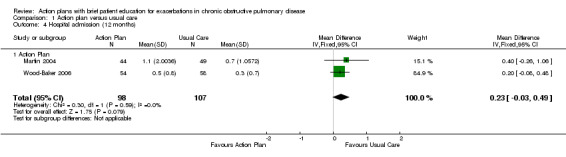
Comparison 1 Action plan versus usual care, Outcome 4 Hospital admission (12 months).
Analysis 1.5 Rate of hospital admission for exacerbation (six‐month follow‐up): For this outcome, we found one relevant trial (n = 227). Results showed no statistically significant difference between action plan with phone follow‐up and control (MD 0.00, 95% CI ‐0.08 to 0.08).
1.5. Analysis.
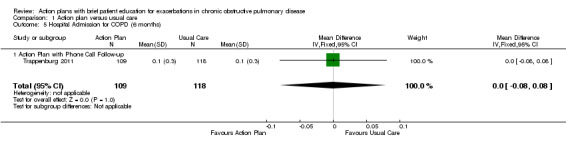
Comparison 1 Action plan versus usual care, Outcome 5 Hospital Admission for COPD (6 months).
Analysis 1.6 Rates of hospitalisation and ED visits for COPD/100 patient‐years: For this outcome, we found one relevant trial with 12‐month follow‐up (n = 743). Results showed a statistically significant difference with less likelihood for action plan with phone follow‐up compared with control (RR 0.59, 95% CI 0.44 to 0.79).
1.6. Analysis.
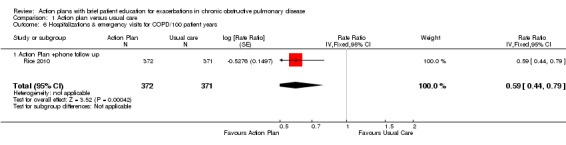
Comparison 1 Action plan versus usual care, Outcome 6 Hospitalizations & emergency visits for COPD/100 patient years.
Analysis 1.7 At least one hospital or ED visit for COPD (12‐month follow‐up): For this outcome, we found one relevant trial (n = 743). Results showed a statistically significant difference with less likelihood for action plan with phone follow‐up compared with control (OR 0.59, 95% CI 0.43 to 0.80).
1.7. Analysis.
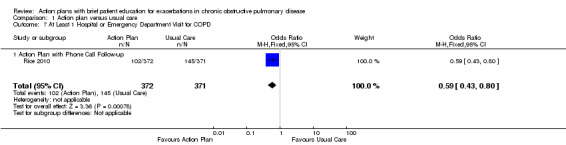
Comparison 1 Action plan versus usual care, Outcome 7 At Least 1 Hospital or Emergency Department Visit for COPD.
Analysis 1.8 Rate of ED visits for COPD/100 patient‐years (12‐month follow‐up): For this outcome, we found one relevant trial (n = 743). Results showed a statistically significant difference with less likelihood for action plan with phone follow‐up compared with control (RR 0.49, 95% CI 0.33 to 0.73).
1.8. Analysis.
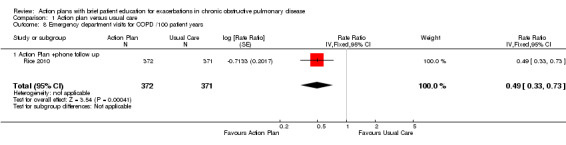
Comparison 1 Action plan versus usual care, Outcome 8 Emergency department visits for COPD /100 patient years.
Analysis 1.9 Rate of ED visits for COPD (12‐month follow‐up): For this outcome, we found two relevant trials (n = 202). Results showed no statistically significant difference between action plan and control (MD 0.37, 95% CI ‐0.50 to 1.24).
1.9. Analysis.
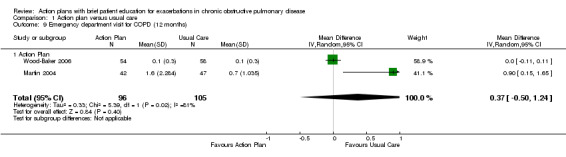
Comparison 1 Action plan versus usual care, Outcome 9 Emergency department visit for COPD (12 months).
Analysis 1.10 At least one ED visit for COPD (12‐month follow‐up): For this outcome, we found two relevant trials (n = 287). Results showed a statistically significant difference with less likelihood for action plan compared with control (subgrouped by phone follow‐up) (OR 0.55, 95% CI 0.38 to 0.78) and no heterogeneity (Chi² = 0.13, df = 1 (P = 0.72), I² = 0%).
1.10. Analysis.
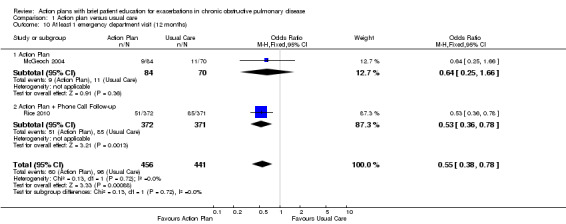
Comparison 1 Action plan versus usual care, Outcome 10 At least 1 emergency department visit (12 months).
Analysis 1.11 Rate of ED visits for COPD (six‐month follow‐up): For this outcome, we found one relevant trial (n = 227). Results showed no statistically significant difference between action plan with phone follow‐up and control (MD 0.00, 95% CI ‐0.09 to 0.09).
1.11. Analysis.
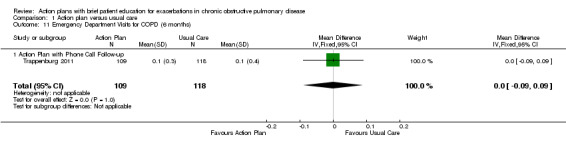
Comparison 1 Action plan versus usual care, Outcome 11 Emergency Department Visits for COPD (6 months).
Analysis 1.12 GP visits/phone contacts for COPD (all or urgent): For this outcome, we found one relevant trial with six‐month follow‐up (n = 56), with no statistically significant difference between action plan and control (MD 1.00, 95% CI ‐0.57 to 2.57), and two relevant trials up (n = 200) with 12‐month follow‐up (MD 0.23, 95% CI ‐1.02 to 1.47).
1.12. Analysis.
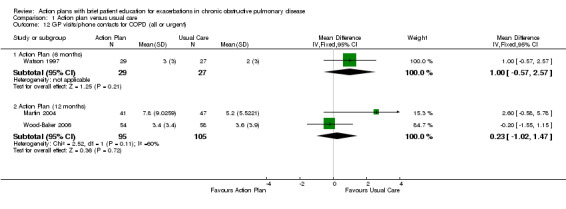
Comparison 1 Action plan versus usual care, Outcome 12 GP visits/phone contacts for COPD (all or urgent).
Analysis 1.13 Rate of non‐COPD GP visits or phone contacts: For this outcome, we found two relevant trials with 12‐month follow‐up (n = 200). Results showed no statistically significant difference between action plan and control (MD 1.25, 95% CI ‐1.54 to 4.03) and moderate heterogeneity (Chi² = 2.38, df = 1 (P = 0.12), I² = 58%).
1.13. Analysis.
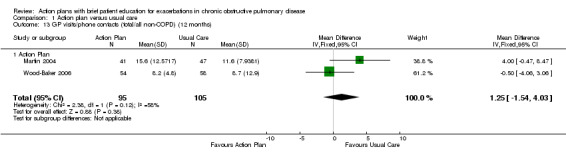
Comparison 1 Action plan versus usual care, Outcome 13 GP visits/phone contacts (total/all non‐COPD) (12 months).
Analysis 1.14 Rate of unscheduled physician visits: For this outcome, we found one relevant trial with six‐month follow‐up (n = 227), with no statistically significant difference between action plan with phone follow‐up and control (MD 0.00, 95% CI ‐0.36 to 0.36).
1.14. Analysis.
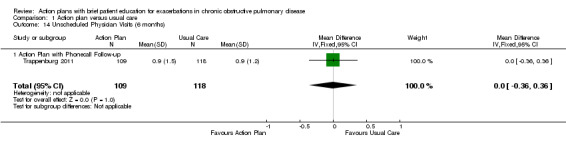
Comparison 1 Action plan versus usual care, Outcome 14 Unscheduled Physician Visits (6 months).
Analysis 1.15 Rate of ambulance calls: For this outcome, we found one relevant trial with six‐month follow‐up (n = 89). Results showed a statistically significant difference between action plan and control, with a higher rate in the action plan group (MD 1.70, 95% CI 0.17 to 3.23).
1.15. Analysis.
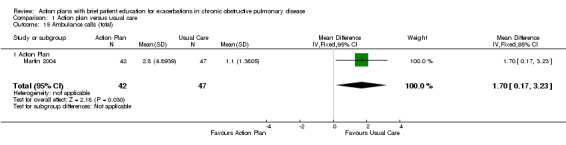
Comparison 1 Action plan versus usual care, Outcome 15 Ambulance calls (total).
Analysis 1.16 Total hospital days: For this outcome, we found one relevant trial with12‐month follow‐up (n = 743). Results showed a statistically significant difference between action plan with phone follow‐up and control, with fewer days spent in hospital in the action plan group (MD ‐1.10, 95% CI ‐2.00 to ‐0.20).
1.16. Analysis.
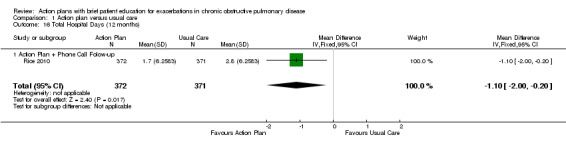
Comparison 1 Action plan versus usual care, Outcome 16 Total Hospital Days (12 months).
Analysis 1.17 Total intensive care unit (ICU) days: For this outcome, we found one relevant trial with 12‐month follow‐up (n = 743). Results showed a statistically significant difference between action plan with phone follow‐up and control, with fewer days spent in the ICU in the action plan group (MD ‐0.30, 95% CI ‐0.60 to ‐0.00).
1.17. Analysis.
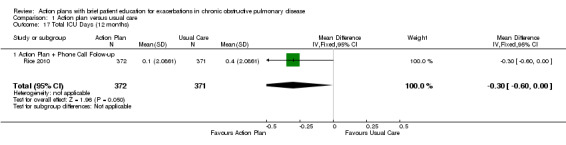
Comparison 1 Action plan versus usual care, Outcome 17 Total ICU Days (12 months).
Mortality
Analysis 1.18 All‐cause mortality: For this outcome, we found four relevant trials with 12‐month follow‐up (n = 1134). Results showed no statistically significant difference between action plan and control (subgrouped by phone follow‐up) (Peto OR 0.88, 95% CI 0.59 to 1.31) and moderate heterogeneity (Chi² = 5.17, df = 3, P = 0.16, I² = 42%) (Figure 4) and imprecision.
1.18. Analysis.
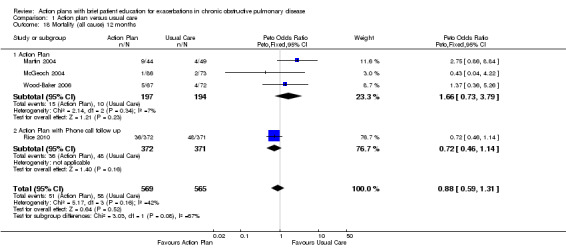
Comparison 1 Action plan versus usual care, Outcome 18 Mortality (all cause) 12 months.
4.
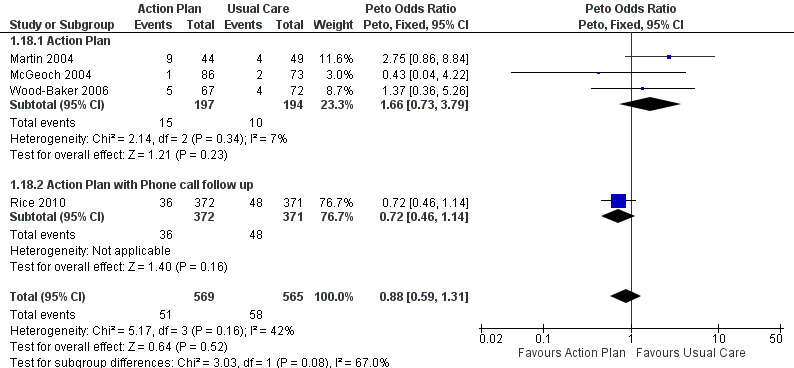
Forest plot of comparison: 1 Action plan versus usual care, outcome: 1.18 Mortality (all cause) 12 months.
Analysis 1.19 Rate of all‐cause mortality per 100 patient‐years: For this outcome, we found one relevant trial with12‐month follow‐up (n = 743). Results showed no statistically significant difference between action plan with phone follow‐up and control (MD ‐3.70, 95% CI ‐8.86 to 1.46), but the result was imprecise.
1.19. Analysis.

Comparison 1 Action plan versus usual care, Outcome 19 Mortality (all cause) per 100 Patient‐Years (12 months).
Analysis 1.20 All‐cause mortality: For this outcome, we found one relevant trial with six‐month follow‐up (n = 229). Results showed no statistically significant difference between action plan with phone follow‐up and control (Peto OR 1.06, 95% CI 0.15 to 7.69), but the result was imprecise.
1.20. Analysis.
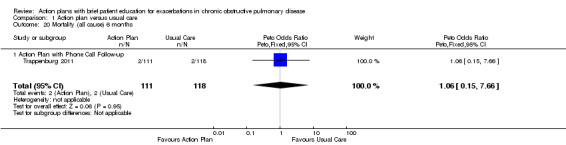
Comparison 1 Action plan versus usual care, Outcome 20 Mortality (all cause) 6 months.
Use of medication for acute exacerbations of COPD
No data were available on time to initiation of therapy after onset of exacerbation symptoms.
Analysis 1.21 Use of one or more courses of oral steroids for exacerbations: For this outcome, we found one relevant trial with six‐month follow‐up (n = 56), with a statistically significant difference between action plan and control and increased odds of steroid use in the action plan group (OR 6.58, 95% CI 1.29 to 33.62), and one relevant trial with 12‐month follow‐up (n = 154), with no statistically significant difference between action plan and control (OR 1.27, 95% CI 0.34 to 4.69).
1.21. Analysis.

Comparison 1 Action plan versus usual care, Outcome 21 At least 1 course oral steroids for exacerbation.
Analysis 1.22 The rate of courses of oral steroids for exacerbations in two relevant trials with 12‐month follow‐up (n = 200) showed a statistically significant difference between action plan and control, with an increased rate of steroid use in the action plan group (MD 0.74, 95% CI 0.12 to 1.35) and no heterogeneity (Chi² = 0.37, df = 1, P = 0.54, I² = 0%).
1.22. Analysis.
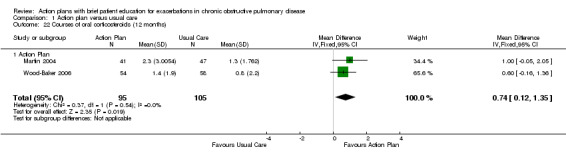
Comparison 1 Action plan versus usual care, Outcome 22 Courses of oral corticosteroids (12 months).
Analysis 1.23 The rate of courses of oral steroids for exacerbations in one relevant trial with six‐month follow‐up (n = 227) showed no statistically significant difference between action plan with phone follow‐up and control (MD 0.00, 95% CI ‐0.23 to 0.23).
1.23. Analysis.
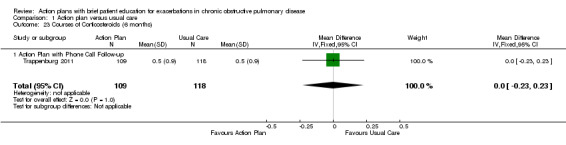
Comparison 1 Action plan versus usual care, Outcome 23 Courses of Corticosteroids (6 months).
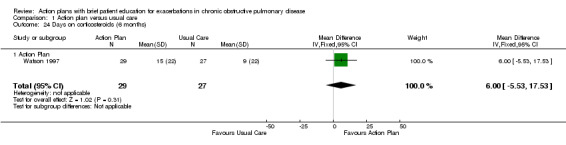
Analysis 1.24 The number of days on oral corticosteroids for exacerbations in one relevant trial with six‐month follow‐up (n = 227) showed no statistically significant difference between action plan and control (MD 6.00, 95% CI ‐5.53 to 17.53).
1.24. Analysis.
Comparison 1 Action plan versus usual care, Outcome 24 Days on corticosteroids (6 months).
Analysis 1.25 Cumulative dose of prednisolone: For this outcome, we found one relevant trial with 12‐month follow‐up (n = 743). Results showed a statistically significant difference between action plan with phone follow‐up and control, with a greater cumulative dose in the action plan group (MD 779.00 mg, 95% CI 533.23 to 1024.77).
1.25. Analysis.

Comparison 1 Action plan versus usual care, Outcome 25 Prednisolone mg (12 months).
Analysis 1.26 Use of one or more courses of antibiotics for exacerbations: For this outcome, we found one relevant trial with six‐month follow‐up (n = 56) that reported a statistically significant difference between action plan and control (Peto OR 6.51, 95% CI 2.02 to 21.05), and two relevant trials with 12‐month follow‐up (n = 293) that reported a statistically significant difference between action plan and control (Peto OR 1.65, 95% CI 1.01 to 2.69); both outcomes show increased odds of antibiotic use in the action plan group.
1.26. Analysis.
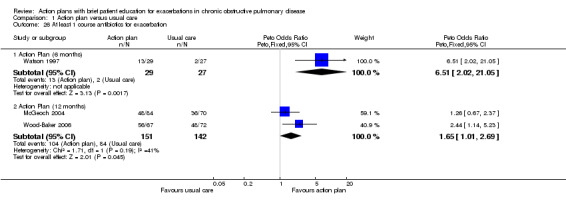
Comparison 1 Action plan versus usual care, Outcome 26 At least 1 course antibiotics for exacerbation.
Analysis 1.27 Rate of courses of antibiotics for exacerbations over 12 months: For this outcome, we found three relevant trials with 12‐month follow‐up (n = 943). Results showed a statistically significant difference between action plan and control, with a higher rate of antibiotic use in the action plan group (subgrouped by phone follow‐up) (MD 2.26, 95% CI 1.82 to 2.70), and a substantial degree of heterogeneity (Chi² = 10.55, df = 2, P = 0.005, I² = 81%) and a statistically significant test for subgroup difference (Chi² = 10.09, df = 1, P = 0.001, I² = 90.1%). In two studies that compared action plan with control, the MD was 0.78 (95% CI ‐0.24 to 1.79), and in one study that compared action plan with phone follow‐up and control, the MD was 2.60 (95% CI 2.12 to 3.08).
1.27. Analysis.
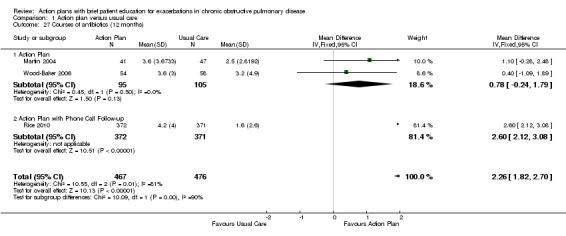
Comparison 1 Action plan versus usual care, Outcome 27 Courses of antibiotics (12 months).
Analysis 1.28 Rate of courses of antibiotics for exacerbations over six months: In one relevant trial with six‐month follow‐up (n = 227), results showed no statistically significant difference between action plan with phone follow‐up and control (MD 0.00, 95% CI ‐0.26 to 0.26).
1.28. Analysis.
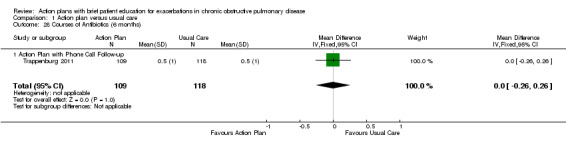
Comparison 1 Action plan versus usual care, Outcome 28 Courses of Antibiotics (6 months).
Analysis 1.29 The number of days on antibiotics over six months for exacerbations in one relevant trial with six‐month follow‐up (n = 56) showed a statistically significant difference between action plan and control, with a greater number of days on antibiotics in the action plan group (MD 6.00 days, 95% CI 1.40 to 10.60).
1.29. Analysis.
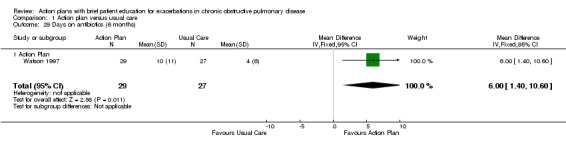
Comparison 1 Action plan versus usual care, Outcome 29 Days on antibiotics (6 months).
Results: secondary outcomes
Respiratory health‐related quality of life: overall scores: St George’s Respiratory Questionnaire (SGRQ), in which a negative direction for the result indicates improvement
Analysis 1.30 SGRQ overall score at 12 months: For this outcome, we found three relevant trials with 12‐month follow‐up (n = 1009). Results showed a statistically significant difference between action plan and control (subgrouped by phone follow‐up), with better quality of life in the action plan group (MD ‐2.79, 95% CI ‐0.82 to ‐4.77), a substantial degree of heterogeneity (Chi² = 7.98, df = 2, P = 0.02, I² = 75%) and a statistically significant test for subgroup difference (Chi² = 7.11, df = 1, P = 0.008, I² = 85.9%). The MD in two studies (n = 264) that compared action plan with control was not significant (0.32, 95% CI 3.34 to ‐2.70), and one study that compared action plan with phone follow‐up and control (n = 743) noted a significant improvement (MD ‐5.10, 95% CI ‐2.50 to ‐7.70).
1.30. Analysis.
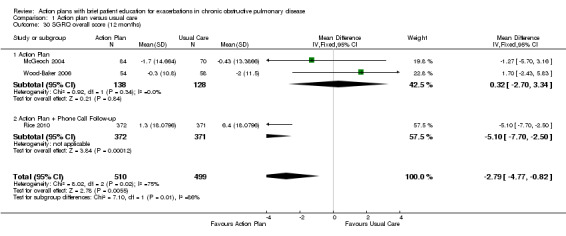
Comparison 1 Action plan versus usual care, Outcome 30 SGRQ overall score (12 months).
Analysis 1.31 SGRQ overall score at six months: For this outcome, we found four relevant trials with six‐month follow‐up (n = 452). Results showed no statistically significant difference between action plan and control (subgrouped by phone follow‐up) (MD ‐0.83, 95% CI ‐2.93 to 1.27), no heterogeneity and no difference between subgroups at this time point.
1.31. Analysis.
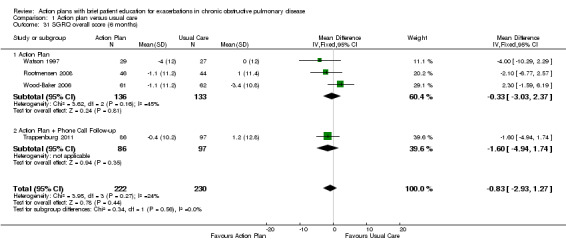
Comparison 1 Action plan versus usual care, Outcome 31 SGRQ overall score (6 months).
Respiratory health‐related quality of life subscales
Analysis 1.32 SGRQ symptom score at 12 months: For this outcome, we found two relevant trials with 12‐month follow‐up (n = 266). Results showed no statistically significant difference between action plan and control (MD ‐2.55, 95% CI ‐6.92 to 1.83) with no heterogeneity.
1.32. Analysis.
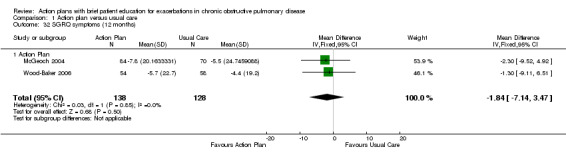
Comparison 1 Action plan versus usual care, Outcome 32 SGRQ symptoms (12 months).
Analysis 1.33 SGRQ symptom score at six months: For this outcome, we found four relevant trials with six‐month follow‐up (n = 448). Results showed no statistically significant difference between action plan and control (subgrouped by phone follow‐up) (MD ‐2.33, 95% CI ‐6.84 to 2.18), with no heterogeneity.
1.33. Analysis.
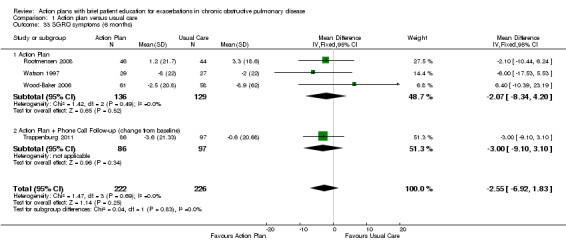
Comparison 1 Action plan versus usual care, Outcome 33 SGRQ symptoms (6 months).
Analysis 1.34 SGRQ activity limitation score at 12 months: For this outcome, we found two relevant trials with 12‐month follow‐up (n = 266). Results showed no statistically significant difference between action plan and control (MD 2.87, 95% CI 7.00 to ‐1.26), with no heterogeneity.
1.34. Analysis.
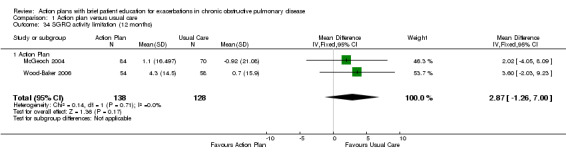
Comparison 1 Action plan versus usual care, Outcome 34 SGRQ activity limitation (12 months).
Analysis 1.35 SGRQ activity limitation score at six months: For this outcome, we found four relevant trials with six‐month follow‐up (n = 452). Results showed no statistically significant difference between action plan and control (subgrouped by phone follow‐up) (MD 0.88, 95% CI ‐1.90 to 3.67), with no heterogeneity.
1.35. Analysis.
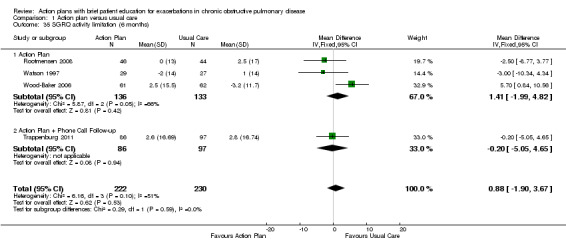
Comparison 1 Action plan versus usual care, Outcome 35 SGRQ activity limitation (6 months).
Analysis 1.36 Change in SGRQ impact score at 12 months: For this outcome, we found two relevant trials with 12‐month follow‐up (n = 266). Results showed no statistically significant difference between action plan and control (MD ‐1.04, 95% CI 2.43 to ‐4.51), with moderate heterogeneity (Chi² = 1.76, df = 1, P = 0.18, I² = 43%).
1.36. Analysis.
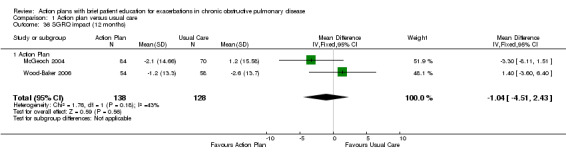
Comparison 1 Action plan versus usual care, Outcome 36 SGRQ impact (12 months).
Analysis 1.37 SGRQ impact score at six months: For this outcome, we found four relevant trials with six‐month follow‐up (n = 452). Results showed no statistically significant difference between action plan and control (subgrouped by phone follow‐up) (MD ‐1.26, 95% CI ‐3.47 to 0.95), with no heterogeneity.
1.37. Analysis.
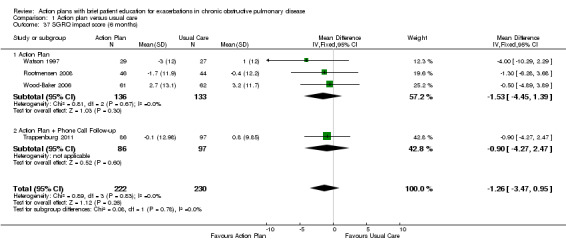
Comparison 1 Action plan versus usual care, Outcome 37 SGRQ impact score (6 months).
Generic health‐related quality of life subdomains: measured by Short Form (SF)‐36
For this outcome, we found one relevant trial with six‐month follow‐up (n = 90) that compared action plan and control. Table 4 shows results for eight domains as mean difference (MD) and 95% confidence interval (CI); a positive result indicates improvement.
3. Generic health‐related quality of life subdomains: measured by Short Form (SF)‐36.
| Outcome | SF‐36 domain | Mean difference | 95% CI |
| Analysis 1.38 | Physical function | 0.30 | ‐7.13 to 7.73 |
| Analysis 1.39 | Role limitation | 9.00 | ‐8.07 to 26.07 |
| Analysis 1.40 | Bodily pain | 18.50 | 6.14 to 30.86 |
| Analysis 1.41 | General health | 2.60 | ‐3.71 to 8.91 |
| Analysis 1.42 | Vitality | 1.60 | ‐4.73 to 7.93 |
| Analysis 1.43 | Social function | 5.30 | ‐4.68 to 15.28 |
| Analysis 1.44 | Role limitation | 7.50 | ‐8.56 to 23.56 |
| Analysis 1.45 | Mental health | 6.30 | 0.64 to 11.96 |
Psychological morbidity: anxiety and depression
Investigators measured these outcomes by using the Hospital Anxiety and Depression Scale (HADS), a 21‐unit scale on which higher score indicates more severe symptoms, in one study that compared action plan with phone follow‐up and control with 12‐month follow‐up (n = 154), and in another study that compared action plan and control with six‐month follow‐up (n = 183). Table 5 shows results for depression and anxiety scores as mean difference (MD) and 95% confidence interval (CI); a negative result indicates fewer symptoms.
4. Psychological morbidity: anxiety and depression.
| Outcome | Domain | Follow‐up: months | MD | 95% CI | n |
| Analysis 1.46 | Depression | 12 | ‐0.25 | ‐1.14 to 0.64 | 154 |
| Analysis 1.47 | Depression | 6 | 0.10 | ‐0.73 to 0.93 | 183 |
| Analysis 1.48 | Anxiety | 12 | 0.14 | ‐1.38 to 1.66 | 154 |
| Analysis 1.49 | Anxiety | 6 | 0.00 | ‐0.83 to 0.83 | 183 |
COPD self‐management for exacerbation and related self‐efficacy
Assessment of these outcomes was based on interviews with participants and use of different questionnaires in three studies that provided relevant data, preventing meta‐analysis of outcomes.
McGeoch 2004 (action plan vs control) used a standardised COPD self‐management questionnaire on which higher score indicates greater self‐efficacy (range 0 to 26), which has been shown to be valid and reliable (Dowson 2004). Rootmensen 2008 (action plan vs control) used a self‐administered self‐management questionnaire that was based on three exacerbation scenarios and included questions adapted from a validated interview‐based questionnaire (Kolbe 1996), on which higher score indicates greater self‐efficacy. Trappenburg 2011 (action plan with phone follow‐up vs control) measured self‐management exacerbation‐related self‐efficacy using a non‐validated questionnaire with 11 items graded on a 5‐point Likert scale. Lower scores indicate greater self‐efficacy for exacerbation‐related self‐management behaviour. Table 6 shows results as mean difference (MD) and 95% confidence interval (CI).
5. COPD self‐management for exacerbation and related self‐efficacy.
| Outcome | Study | Item | Direction improvement | Months | MD | 95% CI | n |
| Analysis 1.50 | McGeoch 2004 | Self‐management knowledge when well | + | 12 | 1.10 | 0.46 to 1.74 | 154 |
| Analysis 1.51 | McGeoch 2004 | Self‐management actions when well | + | 12 | 0.50 | ‐0.24 to 1.24 | 154 |
| Analysis 1.52 | McGeoch 2004 | Self‐management knowledge early exacerbation | + | 12 | 1.80 | 0.75 to 2.85 | 154 |
| Analysis 1.53 | McGeoch 2004 | Self‐management actions early exacerbation | + | 12 | 2.30 | 0.96 to 3.64 | 154 |
| Analysis 1.54 | McGeoch 2004 | Self‐management knowledge severe exacerbation | + | 12 | 2.50 | 0.94 to 4.06 | 154 |
| Analysis 1.55 | McGeoch 2004 | Self‐management action severe exacerbation | + | 12 | 1.50 | 0.47 to 2.53 | 154 |
| Analysis 1.56 | Rootmensen 2008 | Self‐management exacerbation actions | + | 6 | ‐5.10 | ‐15.26 to 5.06 |
90 |
| Analysis 1.57 | Trappenburg 2011 | Self‐efficacy for exacerbation recognition | ‐ | 6 | ‐0.70 | ‐0.98 to ‐0.42 | 183 |
| Analysis 1.58 | Trappenburg 2011 | Self‐efficacy for exacerbation prevention/action | ‐ | 6 | ‐0.90 | ‐1.18 to ‐0.62 | 183 |
Lung function: FEV1 % predicted
For this outcome (Analysis 1.59), we found two relevant trials with six‐month follow‐up (n = 179), in which results showed no statistically significant difference between action plan and control (MD 1.83, 95% CI ‐1.05 to 4.71), and one relevant trial with 12‐month follow‐up (n = 293), in which results showed no statistically significant difference between action plan and control (MD 2.00, 95% CI ‐1.89 to 5.89).
1.59. Analysis.
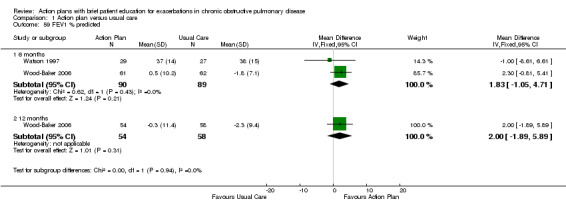
Comparison 1 Action plan versus usual care, Outcome 59 FEV1 % predicted.
Cost‐effectiveness
Analysis 1.60 The cost of hospital admissions (HADM) per participant over 12 months: For this outcome, we found one relevant trial with 12‐month follow up (n = 743). Results showed a statistically significant difference between action plan with phone follow‐up and control, with lower costs in the action plan group (MD ‐1117.00 US$, 95% CI ‐1754.50 to ‐479.50).
1.60. Analysis.

Comparison 1 Action plan versus usual care, Outcome 60 Cost HADM per patient US$ (12 months).
Analysis 1.61 The cost of emergency department visits (EDV) per participant over 12 months: For this outcome, we found one relevant trial with 12‐month follow up (n = 743). Results showed a statistically significant difference between action plan with phone follow‐up and control, with lower costs in the action plan group (MD ‐141.00 US$, 95% CI ‐234.31 to ‐47.69).
1.61. Analysis.

Comparison 1 Action plan versus usual care, Outcome 61 Cost EDV Per Patient US$ (12 months).
Analysis 1.62 The cost of pulmonary drug prescriptions per participant over 12 months: For this outcome, we found one relevant trial with 12‐month follow up (n = 743). Results showed no statistically significant difference between action plan with phone follow‐up and control (MD 15.00 US$, 95% CI ‐6.32 to 36.32).
1.62. Analysis.

Comparison 1 Action plan versus usual care, Outcome 62 Cost Pulmonary Drug Prescriptions per Patient US$ (12 months).
Sensitivity analysis
We performed a sensitivity analysis to examine changes in SGRQ scores (overall and subscales) (Appendix 4). For Watson 1997, we compared results when we used the standard deviation taken from the largest value in another study of similar duration versus the same outcome when we used the standard deviation calculated from the correlation coefficient of data available for the same outcome in Wood‐Baker 2006. The sample size in Watson 1997 was approximately 50% the size of the other studies. Results showed no change in direction or statistical significance of the pooled difference by either method. We have presented in the text the result obtained with the standard deviation for Watson 1997 based on the value obtained from other studies, and a table in Appendix 4 shows corresponding results with use of an imputed standard deviation.
The small number of included studies limited sensitivity analyses performed by risk of bias grading. The increased likelihood of hospital admission for an acute exacerbation remained significant when we excluded studies with unclear risk of bias for randomisation and allocation concealment.
Discussion
Summary of main results
This systematic review update summarises the effects of an action plan (defined as a guideline detailing self‐initiated actions such as changing medication regimens or visiting a general practitioner (GP) or hospital, to be undertaken in response to alterations in symptoms of chronic obstructive pulmonary disease (COPD) suggesting the start of an exacerbation) with an accompanying educational component of short duration only (up to one hour) versus usual clinical care in COPD. Seven relevant randomised studies contributed to the comparison of action plan versus usual care for exacerbations of COPD. We included studies that provided ongoing support directed at use of the action plan, and we excluded studies with broader self‐management interventions.
For the primary outcome of healthcare utilisation for exacerbations, evidence shows benefit over 12 months, with fewer hospitalisations and emergency department (ED) visits for COPD in a large study (n = 743) of action plans with phone support (rate ratio (RR) 0.59, 95% confidence interval (CI) 0.44 to 0.79, moderate‐quality evidence (GRADE)) and decreased likelihood of hospital admission in two studies (n = 897) (odds ratio (OR) 0.69, 95% CI 0.49 to 0.97, moderate‐quality evidence (GRADE)). Thus over 12 months in studies in which participants had relatively low baseline risk, the number needed to treat for an additional beneficial outcome (NNTB) derived by avoiding hospitalisation for an exacerbation was 19 (95% CI 11 to 201).
Over the same follow‐up period, we found benefit for ED visits alone for COPD, with fewer ED visits for COPD in a large study (n = 743) of action plans with phone support (RR 0.49, 95% CI 0.33 to 0.73, high‐quality evidence (GRADE)) and less likelihood of an ED visit for COPD in two studies (n = 897) (OR 0.55, 95% CI 0.38 to 0.78, moderate‐quality evidence (GRADE)). Over 12 months, the NNTB required to avoid an ED visit for an exacerbation was 12 (95% CI 9 to 26). However, two studies (n = 201) that used action plans alone reported no significant reduction in the rate of ED visits for COPD (MD 0.37, 95% CI ‐0.50 to 1.24, very low‐quality evidence (GRADE)). For hospital admissions alone, one study (n = 743) of action plans with phone support reported no significant benefit (RR 0.69, 95% CI 0.47 to 1.01, moderate‐quality evidence (GRADE)). Fewer hospital admissions and ED visits for COPD translated into lower costs for the action plan intervention.
Four studies (n = 1134) found no significant change in all‐cause mortality over 12 months for action plan use, with or without phone support (OR 0.88, 95% CI 0.59 to 1.31, moderate‐quality evidence (GRADE)), but confidence intervals do not rule out important benefit or harm associated with the intervention.
Clear evidence indicates that action plans increased treatment for exacerbations of COPD over 12‐month follow‐up. Two studies (n = 200) reported an increase in courses of oral corticosteroids (MD 0.74, 95% CI 0.12 to 1.35, moderate‐quality evidence (GRADE)), and one study (n = 743) reported an increase in the cumulative dose of oral corticosteroids with phone support for action plan use (779.0 mg prednisolone, 95% CI 533.2 to 1024.8, high‐quality evidence (GRADE)). Three studies (n = 943) reported a significant increase in courses of antibiotics (MD 2.26, 95% CI 1.82 to 2.70, moderate‐quality evidence (GRADE)).
Studies have shown statistically significant benefit for respiratory‐related quality of life with action plan use over 12 months. Using St George's Respiratory Questionnaire (SGRQ) overall score, three studies (n = 1009) reported that the score was 2.8 units lower ‐ from 0.8 lower to 4.8 lower (moderate‐quality evidence (GRADE)). The confidence interval includes the minimum clinically important difference of 4 units. The review found no clear evidence of benefit for psychological morbidity in depression or anxiety as measured by the Hospital Anxiety and Depression Scale (HADS) in a single study over 12 months (low‐quality evidence (GRADE)).
Evidence also shows a positive effect on knowledge of appropriate self‐management for exacerbations in three studies that used different measurement instruments. We found clear evidence that action plans with limited education improved recognition and actions for appropriate self‐management during early stages and in severe exacerbations and led to increased self‐efficacy for exacerbation prevention and actions.
Subgroup analysis: effect of ongoing support directed at use of the action plan delivered by telephone or direct contact
Variation in study measurements limited the ways meta‐analyses could be grouped according to ongoing support for use of an action plan. Two healthcare utilisation outcomes contributed to subgroup analyses. For the likelihood of at least one hospital admission in 12 months (Analysis 1.2), in one study without ongoing support (OR 0.97, 95%CI 0.31 to 3.03) and in one study with ongoing phone support (OR 0.66, 95%CI 0.46 to 0.95), results showed no heterogeneity between subgroup results (Chi² = 0.39, df = 1, P = 0.53, I² = 0%). In the same studies, for the likelihood of at least one ED visit in 12 months (Analysis 1.10), the study without ongoing support (OR 0.64, 95%CI 0.25 to 1.66) and the study with phone support (OR 0.53, 95%CI 0.36 to 0.78) showed no heterogeneity between subgroups (Chi² = 0.13, df = 1, P = 0.72, I² = 0%).
For all‐cause mortality over 12 months (Analysis 1.18), three studies without ongoing support (OR 1.66, 95% CI 0.73 to 3.79) and one study with ongoing phone support (OR 0.72, 95% CI 0.46 to 1.14) showed moderate heterogeneity between subgroup results (Chi² = 5.17, df = 3, P = 0.16, I² = 42%); however, results of the test for subgroup differences were not statistically significant (Chi² = 3.03, df = 1, P = 0.08, I² = 67.0%).
For use of medication for exacerbations, only one outcome permitted subgroup analysis. For courses of antibiotics over 12 months (Analysis 1.27), in two studies with no ongoing support (MD 0.78, 95% CI ‐0.24 to 1.79) and in one study with phone support (MD 2.60, 95% CI 2.12 to 3.08), pooled analysis showed substantial heterogeneity (Chi² = 10.55, df = 2, P = 0.005, I² = 81%), and results of the test for subgroup differences were significant (Chi² = 10.09, df = 1, P = 0.001, I² = 90.1%)
Overall completeness and applicability of evidence
Our searches for this review are current to November 2015, and review results are based on seven studies that included 1550 symptomatic participants with COPD, with fairly typical characteristics of the COPD population at higher risk of exacerbation. In four studies, participants' mean forced expiratory volume at one second (FEV1) was < 50% predicted, and in three studies < 60% predicted. Three studies required a history of exacerbations in the past year (Martin 2004; McGeoch 2004; Rice 2010). Two studies excluded participants receiving home oxygen therapy (Martin 2004; McGeoch 2004), and one study (Watson 1997) excluded those receiving long‐term oral steroid therapy. Study recruitment was community based, and participants had varying numbers of comorbidities. The results of meta‐analyses, including those for healthcare utilisation, hospital admissions and ED presentations, are based on small numbers of studies with similar follow‐up periods, and the included studies have a relatively low baseline risk for hospital admissions. The applicability of findings to populations with high baseline risk is not known.
As shown in Additional Table 3, the format of the intervention had common elements in the self‐management action plan: early recognition of exacerbations based on symptoms, appropriate self‐initiated interventions and directions to seek medical care. Self‐management education was limited to a single short session, and so the intervention excluded multi‐faceted self‐management support programmes. Available information from three studies showed that the length of educational input was 45 minutes (Rootmensen 2008), 60 minutes (McGeoch 2004) and 60 to 90 minutes (Rice 2010). This update included support for implementing the action plan provided up to monthly by direct contact or by phone call. We made this change to reflect clinical practice in which action plans may be delivered in the outpatient setting with some form of ongoing support. Rice 2010 and Trappenburg 2011 included support for action plan use. For combined hospital admissions or ED visits, Rice 2010 reported a rate ratio of 0.78 (95% CI 0.35 to 1.74; P = 0.53) for participants receiving four to eight calls versus zero to three calls. The rate for participants with nine or more calls versus zero to three calls showed a significant reduction with increased phone contacts (RR 0.46, 95% CI 0.22 to 0.96; P = 0.04).
With the addition of new studies, this update has shown greater benefit for patient outcomes in addition to improvement in knowledge and understanding of appropriate actions in the event of an exacerbation. Analysis of mortality data was possible only for all‐cause mortality, as no data were available for respiratory‐related mortality. Studies reported few adverse effects data despite increased use of oral corticosteroids in the action plan group. In Walters 2014, an adverse drug reaction was significantly more likely with corticosteroid treatment of acute exacerbations than with placebo (OR 2.33, 95% CI 1.60 to 3.40), and the number needed to treat for an additional harmful outcome (NNTH) was 6 (95% CI 4 to 10). Hyperglycaemia was the most common adverse event (OR 4.95, 95% CI 2.47 to 9.91). Data on patient‐reported outcomes are few; this review shows benefit for overall respiratory‐related quality of life, but use of different instruments or follow‐up periods precluded meta‐analyses for generic quality of life and psychological morbidity.
Quality of the evidence
Among primary outcomes, this review update, which incorporates two new studies conducted since 2009, shows evidence of benefit for measures of healthcare utilisation of varying strength, generally high or moderate quality of effects on hospital admissions and ED visits when based on greater numbers of studies or on studies with large sample sizes and lower quality when based on smaller studies conducted earlier (before 2004). Review authors downgraded the result for mortality to moderate quality owing to imprecision. We graded the clear benefit derived from greater use of treatment for acute exacerbations as moderate (courses of oral corticosteroids) or high (cumulative doses of oral corticosteroids), and as moderate for courses of antibiotics. For patient‐reported outcomes, we graded respiratory‐related quality of life improvement as showing moderate quality; although health‐related quality of life was improved, the mean effect size was small, so this may not be clinically important for most patients. We graded the quality of evidence for the psychological domain of depression as low and noted no significant differences.
Potential biases in the review process
Confounding may be present in studies based in primary care through self‐selection of general practitioners (GPs) with an interest in COPD (Watson 1997), who might be more likely to treat COPD exacerbations with antibiotics or prednisolone in the absence of an action plan. However, in studies that used cluster‐randomisation (McGeoch 2004; Wood‐Baker 2006) this was less likely. Wood‐Baker 2006 did not account for the effect of clustering. In the meta‐analysis, review authors included available group mean results for individual participants, potentially leading to overestimation of effect. Review authors other than the study author extracted and entered all data from Wood‐Baker 2006. Review authors who were not trialists in a study made decisions on downgrades for Summary of findings tables. McGeoch 2004 reported methods of analysis that accounted for clustering.
Agreements and disagreements with other studies or reviews
As the primary objective of this review is to examine the specific effects of a self‐management action plan for exacerbations of COPD, we restricted included studies to those that excluded broad self‐management training and education, often delivered in a group format, and sometimes as part of a pulmonary rehabilitation programme. In clinical practice in some settings, an action plan may include some form of ongoing support for action plan use, often provided during case management for outpatients or those receiving primary care. For this reason, we decided that this update should include studies with up to monthly ongoing support limited to use of the action plan. We prespecified this change to the inclusion criteria of the old review (Walters 2010) in the updated protocol. Subgroup analysis indicates possibly greater effect for such regular phone support.
In comparison with the previous version of this review, moderate‐quality evidence now suggests benefit in healthcare utilisation for COPD action plans. This development is largely due to inclusion of two new studies (Rice 2010; Trappenburg 2011) that featured ongoing support for action plan use and contributed an additional 976 participants to the pooled analysis.
Other systematic reviews looked at effects of self‐management interventions provided with or without action plans (protocol published (Zwerink 2014)), and examined action plans that form part of a broad self‐management educational programme (Lenferink 2015) or comprehensive pulmonary rehabilitation programme (McCarthy 2015). In Zwerink 2014, 74% of studies (n = 23) included an action plan as part of the self‐management intervention. Similar to our review, Zwerink 2014 found a reduction in the likelihood of respiratory‐related hospitalisation (OR 0.57, 95% CI 0.43 to 0.75) in nine studies reported similar benefit in number needed to treat for an additional beneficial outcome (NNTB) in a population at low baseline risk of avoiding hospital admission (20, 95% CI 15 to 35). It was not possible to create subgroups of at least three studies that did not use an action plan in the intervention; thus review authors for Zwerink 2014 performed no subgroup analyses. The benefit achieved by an action plan with a short patient education component is thus comparable with that achieved by more comprehensive educational programmes and self‐management programmes. A review of pulmonary rehabilitation programmes, in which subgroups compared 'exercise only’ trials (n = 31 trials) and ’exercise plus more comprehensive components’ trials (n = 34), noted no significant differences in effects on respiratory‐related quality of life (McCarthy 2015). The lack of effect on mortality reported in this review is similar to the finding of no effect on mortality in the review of self‐management education (Zwerink 2014), which did not include data from Fan 2012 on a comprehensive care management programme that included action plans. Investigators in Fan 2012 stopped the study prematurely because a higher number of deaths in the intervention group versus the control group could not be explained satisfactorily by study authors.
Authors' conclusions
Implications for practice.
This updated review features new evidence that use of action plans for management of COPD exacerbations, with a single brief COPD educational component and without comprehensive self‐management support, can reduce hospital‐based healthcare utilisation. New evidence also supports use of action plans for increasing self‐efficacy in exacerbation management and for increasing appropriate treatment of COPD exacerbations with corticosteroids and antibiotics. Use of COPD action plans in this context does not increase mortality.
This review update features changes to inclusion criteria that allowed ongoing support limited to delivery of the action plan up to monthly. The review includes two new studies (Rice 2010; Trappenburg 2011), both featuring support for action plan use and contributing significant weight to beneficial effects.
The incidence of exacerbations of COPD is high (O'Reilly 2006), so this review suggests that considerable benefit may result from routine use of action plans with a brief patient education component and with ongoing support for action plan use among individuals with COPD in primary care. Whether additional benefit may be derived from periodic ongoing support for use of an action plan cannot be determined from the results of this review.
Implications for research.
Further research should be conducted to assess whether added benefit in decreasing the impact of COPD exacerbations can result when support for action plans that provide only brief education is optimally delivered. Investigators could also evaluate the utility of action plans that provide brief COPD education at the time of hospital admission for COPD exacerbations.
What's new
| Date | Event | Description |
|---|---|---|
| 18 January 2016 | New search has been performed | This updated review includes 2 new studies (Rice 2010; Trappenburg 2011) and 976 additional participants. In planning this update, before we ran searches, the review author team made changes to the protocol.We prespecified inclusion criteria to permit limited support directed only at use of the action plan (up to monthly). We prespecified that we would perform subgroup analysis to compare studies with and without this limited ongoing support. We updated the outcomes and added to the review information on cost‐effectiveness; we withdrew information on acute exacerbations, functional capacity, symptom scores and days lost from work. In addition, we updated the Methods section to reflect MECIR standards for conduct of a review, and we revised outcomes and the Summary of findings table. |
| 18 January 2016 | New citation required and conclusions have changed | The new evidence included in this updated review now supports action plans with ongoing support. Moderate‐quality evidence suggests benefit derived from COPD action plans for healthcare utilisation. |
History
Protocol first published: Issue 3, 2004 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 25 November 2009 | New citation required and conclusions have changed | We updated this review with inclusion of 2 extra studies (Martin 2004; Rootmensen 2008). We strengthened conclusions by adding new evidence. We incorporated Risk of bias assessments and Summary of findings tables into the review. |
| 7 July 2009 | New search has been performed | We reran the literature search. |
| 16 May 2008 | Amended | We converted the review to new review format. |
| 23 July 2005 | New citation required and conclusions have changed | We made substantive amendments to the review. |
Acknowledgements
C Cates and R Normansell were Editors for this review and commented critically on the review.
We acknowledge contributions made by the original author of the review ‐ A Turnock ‐ in 2005.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
We acknowledge Elizabeth Stovold for assistance provide in searching the CENTRAL database.
We acknowledge and thank the following trialists, who provided missing study information and study data: Geert Rootmensen, Paul Watson, and Richard Wood‐Baker.
We thank the following people who responded to requests for information on studies considered for inclusion or exclusion, and on studies in progress ‐ Jean Bourbeau, Michael Epton, Robyn Maguigan, Samantha Prigmore, Harry Rea, Ian Town, Paul Watson and John Wellingham.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (The Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to identify relevant trials from the CAGR
2014/2015 update
#1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All #2 MeSH DESCRIPTOR Bronchitis, Chronic #3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*) #4 COPD:MISC1 #5 (COPD OR COAD OR COBD):TI,AB,KW #6 #1 OR #2 OR #3 OR #4 OR #5 #7 action* NEXT plan* #8 MeSH DESCRIPTOR Self Care Explode All #9 self* NEXT car* #10 self* NEXT manag* #11 management* NEAR3 (plan* or program*) #12 behaviour* or behavior*:TI,AB,KW #13 MeSH DESCRIPTOR Patient Education as Topic #14 educat*:TI,AB,KW #15 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 #16 #6 and #15
Original review, 2005 and 2009 updates
The records coded as 'COPD' were searched using the following terms:
action plan*" OR action‐plan* OR self‐car* OR "self car*" OR self‐manag* OR "self manag*" OR "management plan*" OR management‐plan* OR "management program*" OR behaviour* OR behavior* OR educat*.
Appendix 3. Strategies for additional searches
CENTRAL was searched using the terms: (Pulmonary disease, chronic obstructive) AND (self care OR self administration OR self‐evaluation programs OR models educational OR cooperative behavior OR health behavior)
MEDLINE via PubMed was searched using : "Pulmonary Disease, Chronic Obstructive"[Mesh] AND ("action plan*" OR action‐plan* OR "management plan*" OR management‐plan* OR "management program*" OR "Self Care"[Mesh] OR "Patient Education as Topic"[Mesh] OR "Patient Education Handout"[Publication Type] OR "Models, Educational"[Mesh] OR behaviour* OR behavior*). Filtered for Randomised Controlled Trial and 2013/12/01 to 2015/11/30 publication date.
Embase was searched using the search terms: 'chronic obstructive lung disease'/exp OR 'chronic obstructive lung disease' AND ('action plan' OR 'action plans' OR 'action‐plan' OR 'action‐plans' OR 'management plan' OR 'management plans' OR 'management‐plan' OR 'management‐plans' OR 'self care'/exp OR 'self care' OR 'patient education'/exp OR 'patient education' OR 'educational model'/exp OR 'educational model' OR 'behavior therapy'/exp OR 'behavior therapy' OR 'behavioral medicine'/exp OR 'behavioral medicine'). Filtered for randomised controlled trial and 01/12/2012 to 30/11/2015 records added to EMBASE.
CINAHL was searched using the search terms: (EXP(“Lung diseases, obstructive”) AND (“self care” OR “self‐care” OR “patient education” OR “behavioral changes” OR “behavioral objectives”) AND EXP(“clinical trials”). Filtered for 12/2013 ‐ 12/2015 published date.
PsycINFO was searched via ProQuest using the search terms: SU.EXACT.EXPLODE("Chronic Obstructive Pulmonary Disease") AND (SU.EXACT("Self Care Skills") OR SU.EXACT("Self Management") OR SU.EXACT("Client Education") OR SU.EXACT("Behavior") OR SU.EXACT("Behavior Therapy") OR SU.EXACT("Cooperation") OR SU.EXACT("Behavioral Medicine") OR SU.EXACT("Health Promotion") OR (action plan*)). Limited to the last 12 months.
The WHO International Clinical Trials Registry Platform (ICTRP) was searched using the search terms: ((pulmonary disease, chronic obstructive) AND ((action plan*) OR action‐plan* OR self‐car* OR (self car*) OR self‐manag* OR (self manag*) OR (management plan*) OR management‐plan* OR (management program*) OR behaviour* OR behavior* OR educat*)). Filtered for 01/12/2013 to 30/11/2015 date of registration.
The Australian New Zealand Clinical Trials Registry (ANZCTR) was searched using the search terms: (pulmonary disease, chronic obstructive) AND ((action plan*) OR action‐plan* OR self‐car* OR (self car*) OR self‐manag* OR (self manag*) OR (management plan*) OR management‐plan* OR (management program*) OR behaviour* OR behavior* OR educat*). Filtered for 01/12/2013 ‐ 30/11/2015 trial start date.
ClinicalTrials.gov (US) was searched using the search terms: (pulmonary disease, chronic obstructive) AND ((action plan*) OR action‐plan* OR self‐car* OR (self car*) OR self‐manag* OR (self manag*) OR (management plan*) OR management‐plan* OR (management program*) OR behaviour* OR behavior* OR educat*). Filtered for received from 01/12/2013 ‐ 30/11/2015.
Appendix 4. Action plan versus usual care ‐ sensitivity analysis for SD in Watson 1997
| Outcome | Watson 1997 SD from correlation imputation | N studies/N participants | Mean difference (IV, fixed, 95% CI) | Watson 1997 SD taken from other studies | N studies/N participants | Mean difference (IV, fixed, 95% CI) (result presented in text) |
| SGRQ overall score 6 MTHS | SD = 22 | 3/269 | ‐2.07 (‐8.34 to 4.20) | SD = 12 | 3/269 | ‐0.33 (‐3.03 to 2.37) |
| SGRQ symptom score 6 MTHS | SD = 28.6 | 3/235 | ‐1.53 (‐8.21 to 5.16) | SD = 22 | 3/235 | ‐2.18 (‐8.36 to 4.00) |
| SGRQ activity limitation score 6 MTHS | SD = 32 | 3/269 | 2.35 (‐1.40 to 6.09) | SD = 14 | 3/269 | 1.41 (‐1.99 to 4.82) |
| SGRQ impact score 6 MTHS | SD = 20 | 3/269 | ‐1.13 (‐4.28 to 2.01) | SD = 12 | 3/269 | ‐1.53 (‐4.45 to 1.39) |
Data and analyses
Comparison 1. Action plan versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospitalizations for COPD /100 patient years | 1 | 743 | Rate Ratio (Fixed, 95% CI) | 0.69 [0.47, 1.01] |
| 1.1 Action Plan +phone follow up | 1 | 743 | Rate Ratio (Fixed, 95% CI) | 0.69 [0.47, 1.01] |
| 2 At least 1 hospital admission (12 months) | 2 | 897 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.49, 0.97] |
| 2.1 Action Plan | 1 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.31, 3.03] |
| 2.2 Action Plan + Phonecall Follow‐up | 1 | 743 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.46, 0.95] |
| 3 at least 1 Hospital Admission (6 months) | 1 | 227 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.30, 2.31] |
| 3.1 Action Plan with Phone Call Follow‐up | 1 | 227 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.30, 2.31] |
| 4 Hospital admission (12 months) | 2 | 205 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.03, 0.49] |
| 4.1 Action Plan | 2 | 205 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.03, 0.49] |
| 5 Hospital Admission for COPD (6 months) | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.08, 0.08] |
| 5.1 Action Plan with Phone Call Follow‐up | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.08, 0.08] |
| 6 Hospitalizations & emergency visits for COPD/100 patient years | 1 | 743 | Rate Ratio (Fixed, 95% CI) | 0.59 [0.44, 0.79] |
| 6.1 Action Plan +phone follow up | 1 | 743 | Rate Ratio (Fixed, 95% CI) | 0.59 [0.44, 0.79] |
| 7 At Least 1 Hospital or Emergency Department Visit for COPD | 1 | 743 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.43, 0.80] |
| 7.1 Action Plan with Phone Call Follow‐up | 1 | 743 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.43, 0.80] |
| 8 Emergency department visits for COPD /100 patient years | 1 | 743 | Rate Ratio (Fixed, 95% CI) | 0.49 [0.33, 0.73] |
| 8.1 Action Plan +phone follow up | 1 | 743 | Rate Ratio (Fixed, 95% CI) | 0.49 [0.33, 0.73] |
| 9 Emergency department visit for COPD (12 months) | 2 | 201 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.50, 1.24] |
| 9.1 Action Plan | 2 | 201 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.50, 1.24] |
| 10 At least 1 emergency department visit (12 months) | 2 | 897 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.38, 0.78] |
| 10.1 Action Plan | 1 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.25, 1.66] |
| 10.2 Action Plan + Phone Call Follow‐up | 1 | 743 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.36, 0.78] |
| 11 Emergency Department Visits for COPD (6 months) | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.09, 0.09] |
| 11.1 Action Plan with Phone Call Follow‐up | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.09, 0.09] |
| 12 GP visits/phone contacts for COPD (all or urgent) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 Action Plan (6 months) | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.57, 2.57] |
| 12.2 Action Plan (12 months) | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐1.02, 1.47] |
| 13 GP visits/phone contacts (total/all non‐COPD) (12 months) | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.25 [‐1.54, 4.03] |
| 13.1 Action Plan | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.25 [‐1.54, 4.03] |
| 14 Unscheduled Physician Visits (6 months) | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.36, 0.36] |
| 14.1 Action Plan with Phonecall Follow‐up | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.36, 0.36] |
| 15 Ambulance calls (total) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [0.17, 3.23] |
| 15.1 Action Plan | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [0.17, 3.23] |
| 16 Total Hospital Days (12 months) | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [0.00, ‐0.20] |
| 16.1 Action Plan + Phone Call Folow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [0.00, ‐0.20] |
| 17 Total ICU Days (12 months) | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.60, ‐0.00] |
| 17.1 Action Plan + Phone Call Folow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.60, ‐0.00] |
| 18 Mortality (all cause) 12 months | 4 | 1134 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.59, 1.31] |
| 18.1 Action Plan | 3 | 391 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.66 [0.73, 3.79] |
| 18.2 Action Plan with Phone call follow up | 1 | 743 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.46, 1.14] |
| 19 Mortality (all cause) per 100 Patient‐Years (12 months) | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐8.86, 1.46] |
| 19.1 Action Plan with Phone Call Follow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐8.86, 1.46] |
| 20 Mortality (all cause) 6 months | 1 | 229 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.15, 7.66] |
| 20.1 Action Plan with Phone Call Follow‐up | 1 | 229 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.15, 7.66] |
| 21 At least 1 course oral steroids for exacerbation | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 Action Plan (6 months) | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.58 [1.29, 33.62] |
| 21.2 Action Plan (12 months) | 1 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.34, 4.69] |
| 22 Courses of oral corticosteroids (12 months) | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [0.12, 1.35] |
| 22.1 Action Plan | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [0.12, 1.35] |
| 23 Courses of Corticosteroids (6 months) | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.23, 0.23] |
| 23.1 Action Plan with Phone Call Follow‐up | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.23, 0.23] |
| 24 Days on corticosteroids (6 months) | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐5.53, 17.53] |
| 24.1 Action Plan | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐5.53, 17.53] |
| 25 Prednisolone mg (12 months) | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | 779.0 [533.23, 1024.77] |
| 25.1 Action Plan with Phone Call Follow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | 779.0 [533.23, 1024.77] |
| 26 At least 1 course antibiotics for exacerbation | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 26.1 Action Plan (6 months) | 1 | 56 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.51 [2.02, 21.05] |
| 26.2 Action Plan (12 months) | 2 | 293 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [1.01, 2.69] |
| 27 Courses of antibiotics (12 months) | 3 | 943 | Mean Difference (IV, Fixed, 95% CI) | 2.26 [1.82, 2.70] |
| 27.1 Action Plan | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.78 [‐0.24, 1.79] |
| 27.2 Action Plan with Phone Call Follow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | 2.6 [2.12, 3.08] |
| 28 Courses of Antibiotics (6 months) | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.26, 0.26] |
| 28.1 Action Plan with Phone Call Follow‐up | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.26, 0.26] |
| 29 Days on antibiotics (6 months) | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [1.40, 10.60] |
| 29.1 Action Plan | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [1.40, 10.60] |
| 30 SGRQ overall score (12 months) | 3 | 1009 | Mean Difference (IV, Fixed, 95% CI) | ‐2.79 [‐4.77, ‐0.82] |
| 30.1 Action Plan | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐2.70, 3.34] |
| 30.2 Action Plan + Phone Call Follow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐7.70, ‐2.50] |
| 31 SGRQ overall score (6 months) | 4 | 452 | Mean Difference (IV, Fixed, 95% CI) | ‐0.83 [‐2.93, 1.27] |
| 31.1 Action Plan | 3 | 269 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐3.03, 2.37] |
| 31.2 Action Plan + Phone Call Follow‐up | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐1.6 [‐4.94, 1.74] |
| 32 SGRQ symptoms (12 months) | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐1.84 [‐7.14, 3.47] |
| 32.1 Action Plan | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐1.84 [‐7.14, 3.47] |
| 33 SGRQ symptoms (6 months) | 4 | 448 | Mean Difference (IV, Fixed, 95% CI) | ‐2.55 [‐6.92, 1.83] |
| 33.1 Action Plan | 3 | 265 | Mean Difference (IV, Fixed, 95% CI) | ‐2.07 [‐8.34, 4.20] |
| 33.2 Action Plan + Phone Call Follow‐up (change from baseline) | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐9.10, 3.10] |
| 34 SGRQ activity limitation (12 months) | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | 2.87 [‐1.26, 7.00] |
| 34.1 Action Plan | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | 2.87 [‐1.26, 7.00] |
| 35 SGRQ activity limitation (6 months) | 4 | 452 | Mean Difference (IV, Fixed, 95% CI) | 0.88 [‐1.90, 3.67] |
| 35.1 Action Plan | 3 | 269 | Mean Difference (IV, Fixed, 95% CI) | 1.41 [‐1.99, 4.82] |
| 35.2 Action Plan + Phone Call Follow‐up | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐5.05, 4.65] |
| 36 SGRQ impact (12 months) | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐4.51, 2.43] |
| 36.1 Action Plan | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐4.51, 2.43] |
| 37 SGRQ impact score (6 months) | 4 | 452 | Mean Difference (IV, Fixed, 95% CI) | ‐1.26 [‐3.47, 0.95] |
| 37.1 Action Plan | 3 | 269 | Mean Difference (IV, Fixed, 95% CI) | ‐1.53 [‐4.45, 1.39] |
| 37.2 Action Plan + Phone Call Follow‐up | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐0.9 [‐4.27, 2.47] |
| 38 SF36 physical function (6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 38.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐7.13, 7.73] |
| 39 SF36 role limitation physical (6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 39.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [‐8.07, 26.07] |
| 40 SF36 bodily pain (6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 40.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 18.5 [6.14, 30.86] |
| 41 SF36 general health (6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 41.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐3.71, 8.91] |
| 42 SF36 vitality (6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 42.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 1.6 [‐4.73, 7.93] |
| 43 SF36 mental health (6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 43.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 6.3 [0.64, 11.96] |
| 44 SF36 role limitation emotional (6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 44.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 7.5 [‐8.56, 23.56] |
| 45 SF36 social function (6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 45.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 5.30 [‐4.68, 15.28] |
| 46 HADS ‐ depression score (12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 46.1 Action Plan | 1 | 154 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐1.14, 0.64] |
| 47 HADS ‐ depression score (6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 47.1 Action Plan + Phone Call Folow‐up | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.73, 0.93] |
| 48 HADS ‐ anxiety score (12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 48.1 Action Plan | 1 | 154 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐1.38, 1.66] |
| 49 HADS ‐ anxiety score (6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 49.1 Action Plan + Phone Call Follow‐up (change from baseline) | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.83, 0.83] |
| 50 Exacerbation knowledge when well (12 months) | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 50.1 Action Plan | 1 | 154 | Mean Difference (Fixed, 95% CI) | 1.1 [0.46, 1.74] |
| 51 Exacerbation actions when well (12 months) | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 51.1 Action Plan | 1 | 154 | Mean Difference (Fixed, 95% CI) | 0.5 [‐0.24, 1.24] |
| 52 Early exacerbation knowledge (12 months) | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 52.1 Action Plan | 1 | 154 | Mean Difference (Fixed, 95% CI) | 1.80 [0.75, 2.85] |
| 53 Early exacerbation actions (12 months) | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 53.1 Action Plan | 1 | 154 | Mean Difference (Fixed, 95% CI) | 2.3 [0.96, 3.64] |
| 54 Severe exacerbation knowledge (12 months) | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 54.1 Action Plan | 1 | 154 | Mean Difference (Fixed, 95% CI) | 2.5 [0.94, 4.06] |
| 55 Severe exacerbation actions (12 months) | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 55.1 Action Plan | 1 | 154 | Mean Difference (Fixed, 95% CI) | 1.5 [0.47, 2.53] |
| 56 Self‐management exacerbation actions (6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 56.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐5.1 [‐15.26, 5.06] |
| 57 Self‐efficacy for Exacerbation Recognition (6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 57.1 Action Plan + Phone Call Follow‐up | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐0.98, ‐0.42] |
| 58 Self‐efficacy for Exacerbation Prevention/Action (6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 58.1 Action Plan + Phone Call Follow‐up | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.18, ‐0.62] |
| 59 FEV1 % predicted | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 59.1 6 months | 2 | 179 | Mean Difference (IV, Fixed, 95% CI) | 1.83 [‐1.05, 4.71] |
| 59.2 12 months | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [‐1.89, 5.89] |
| 60 Cost HADM per patient US$ (12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 60.1 Action Plan with Phone Call Folow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐1117.0 [‐1754.50, ‐479.50] |
| 61 Cost EDV Per Patient US$ (12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 61.1 Action Plan with Phone Call Follow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐141.0 [‐234.31, ‐47.69] |
| 62 Cost Pulmonary Drug Prescriptions per Patient US$ (12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 62.1 Action Plan with Phone Call Follow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | 15.00 [‐6.32, 36.32] |
1.38. Analysis.
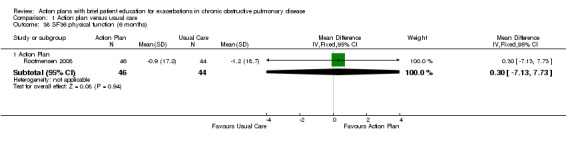
Comparison 1 Action plan versus usual care, Outcome 38 SF36 physical function (6 months).
1.39. Analysis.
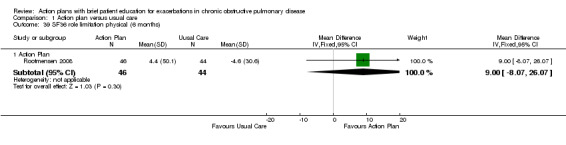
Comparison 1 Action plan versus usual care, Outcome 39 SF36 role limitation physical (6 months).
1.40. Analysis.
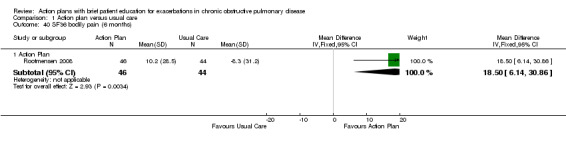
Comparison 1 Action plan versus usual care, Outcome 40 SF36 bodily pain (6 months).
1.41. Analysis.
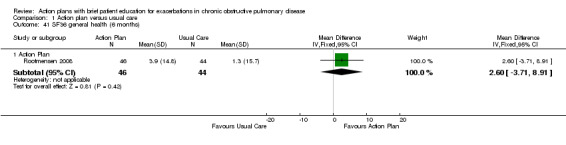
Comparison 1 Action plan versus usual care, Outcome 41 SF36 general health (6 months).
1.42. Analysis.
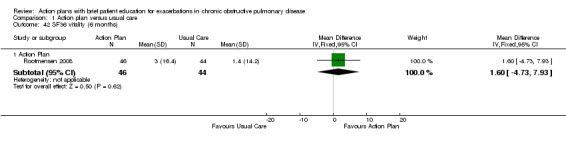
Comparison 1 Action plan versus usual care, Outcome 42 SF36 vitality (6 months).
1.43. Analysis.
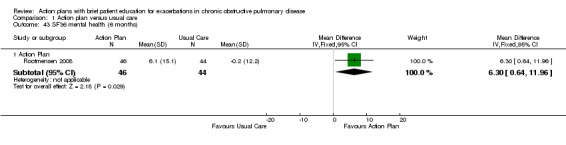
Comparison 1 Action plan versus usual care, Outcome 43 SF36 mental health (6 months).
1.44. Analysis.
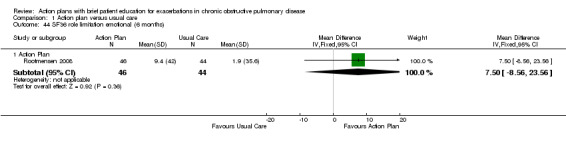
Comparison 1 Action plan versus usual care, Outcome 44 SF36 role limitation emotional (6 months).
1.45. Analysis.
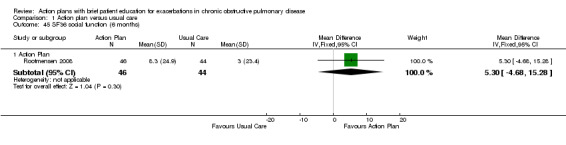
Comparison 1 Action plan versus usual care, Outcome 45 SF36 social function (6 months).
1.46. Analysis.

Comparison 1 Action plan versus usual care, Outcome 46 HADS ‐ depression score (12 months).
1.47. Analysis.

Comparison 1 Action plan versus usual care, Outcome 47 HADS ‐ depression score (6 months).
1.48. Analysis.
Comparison 1 Action plan versus usual care, Outcome 48 HADS ‐ anxiety score (12 months).
1.49. Analysis.
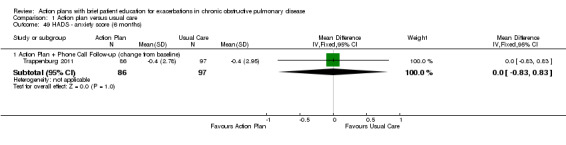
Comparison 1 Action plan versus usual care, Outcome 49 HADS ‐ anxiety score (6 months).
1.50. Analysis.
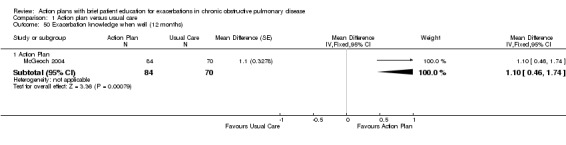
Comparison 1 Action plan versus usual care, Outcome 50 Exacerbation knowledge when well (12 months).
1.51. Analysis.
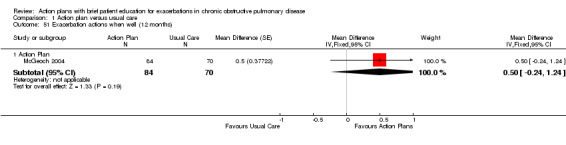
Comparison 1 Action plan versus usual care, Outcome 51 Exacerbation actions when well (12 months).
1.52. Analysis.
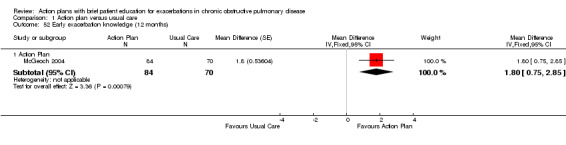
Comparison 1 Action plan versus usual care, Outcome 52 Early exacerbation knowledge (12 months).
1.53. Analysis.
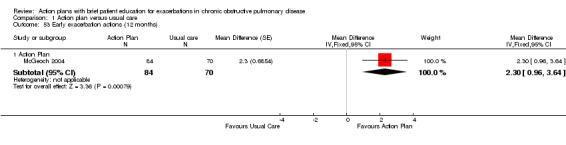
Comparison 1 Action plan versus usual care, Outcome 53 Early exacerbation actions (12 months).
1.54. Analysis.
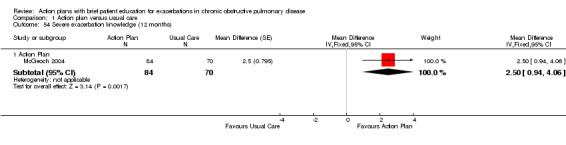
Comparison 1 Action plan versus usual care, Outcome 54 Severe exacerbation knowledge (12 months).
1.55. Analysis.
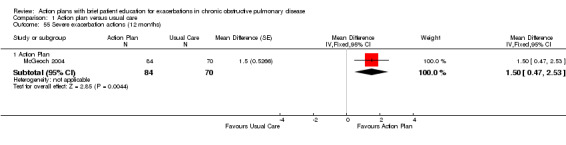
Comparison 1 Action plan versus usual care, Outcome 55 Severe exacerbation actions (12 months).
1.56. Analysis.
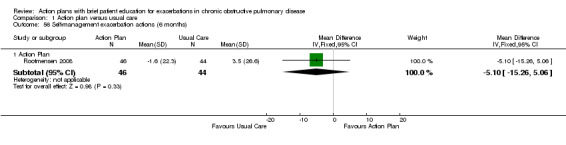
Comparison 1 Action plan versus usual care, Outcome 56 Self‐management exacerbation actions (6 months).
1.57. Analysis.
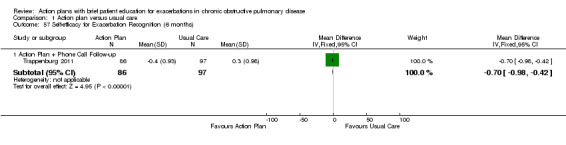
Comparison 1 Action plan versus usual care, Outcome 57 Self‐efficacy for Exacerbation Recognition (6 months).
1.58. Analysis.

Comparison 1 Action plan versus usual care, Outcome 58 Self‐efficacy for Exacerbation Prevention/Action (6 months).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Martin 2004.
| Methods |
Study design: parallel group Location, number of centres: participants recruited through their general practitioners and district nurses in catchment area of single hospital in New Zealand Duration of study: 12 months |
|
| Participants |
N screened: not available N randomised: 96 N completed: 93 (44 INT, 49 UC) M = INT 15 (34%), UC 32 (65%) F = INT 29 (66%), UC 17 (35%) (P < 0.1) Age: INT 71.1 (95% CI 68.7 to 73.5), UC 69.1 (95% CI 63.5 to 74.7) Baseline details: FEV1 % PRED 35.4 (95% CI 31.6 to 39.2), UC 34.3 (95% CI 31.2 to 37.4) Smoking exposure PYH: INT 35.4 (95% CI 29.4 to 41.4), UC 48.2 (95% CI 39.1 to 57.3) (P = 0.03) Inclusion criteria: diagnosis of moderate or severe COPD, aged 55 years or older,at least 1 hospital admission or 2 acute exacerbations of COPD requiring GP care during previous 12 months. Mini Mental State Examination (MMSE) score ≥ 23 Exclusion criteria: terminal illness, coexisting lung cancer, admission to hospital with cardiac disease within previous 12 months, receiving home oxygen therapy |
|
| Interventions |
Intervention: A generic care plan was developed by a group comprising a general practitioner, a community‐based respiratory nurse, a respiratory physician, an emergency department consultant, the local St John's Ambulance paramedical staff director and the after hours GP service director. This results in 5 separate sections within the plan with specific instructions for patient and/or career, GP and/or community nurse, ambulance service, and emergency department and medical staff of Dunedin Hospital. Although sections showed significant overlap, it was recognised that the language and content of each section had to be appropriate for different users of the plan. Thereafter, the care plan was individualised and was 'signed off' for each participant allocated to the intervention group. This was done on the basis of an interview between participant and respiratory nurse (FRS), a review of hospital notes in relation to previous admissions by the respiratory specialist (DRT) and a review by the participant's own GP. Control: UC = usual care by own GP Treatment period: 12 months Follow‐up time points: 3, 6, 9 and 12 months |
|
| Outcomes | Primary outcomes: utilisation of primary care services and hospital admissions; quality of life as measured by St George's Respiratory Questionnaire (SGRQ) | |
| Notes | Not stated if hospital admissions were COPD‐related or all‐cause Funding: Study was supported by South Link Health Inc., a non‐profit consortium of general practitioners. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to the intervention (care plan) or control (usual care) groups". No method of randomisation was described. |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation was published. |
| Blinding (performance bias and detection bias) Participants | High risk | Participants were not blinded to the care plan intervention. Lack of blinding may have affected participants' perception for quality of life measurements. |
| Blinding (performance bias and detection bias) Study personnel | Unclear risk | Study personnel were not blinded to the care plan intervention. "All patients (both intervention and control groups) were visited by the research nurse (DMcN) at the study start and thereafter at three, six and 12 months to provide routine support, and, for the care plan group, further education regarding use of the plan." |
| Blinding of outcome assessment (detection bias) Objective outcomes, e.g. healthcare utilisation | Low risk | All participants (both intervention and control groups) were visited by the research nurse (DMcN) at the study start and thereafter at 3, 6 and 12 months. |
| Blinding of outcome assessment (detection bias) Subjective outcomes eg quality of life, anxiety | Unclear risk | Research nurse who administered quality of life questionnaires was not blinded. |
| Incomplete outcome data (attrition bias) Health care utilisation (objective) | Low risk | GP visits: data for 41/44 INT, 47/49 UC participants. Ambulance call data for 42/44 INT, 47/49 UC. Hospital admission data for 44/44 INT, 49/49 UC |
| Incomplete outcome data (attrition bias) Subjective e.g. Quality of life | Low risk | 96 participants were recruited, 93 completed the study, 3 withdrew for personal reasons (group allocation unknown). |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, and it is not clear whether published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Unclear risk | Number of practices from which participants were recruited is not available. Pilot study, no sample size calculation performed and no attempt made to examine clustering within practices |
McGeoch 2004.
| Methods |
Study design: parallel‐group cluster‐randomised study in an intervention group of practices and a control group of practices Location, number of centres: participants attending 2 groups of general practices in Christchurch, New Zealand Duration of study: 12 months. Year study performed: July 2002‐December 2003 |
|
| Participants |
N screened: 257 N randomised: 159 N completed: 152. INT 84, 1 died, 1 withdrew consent; CONTROL 68, 2 died, 2 withdrew consent, 1 unable to be contacted M = INT 45 (52%), CONTROL 49 (67%) Age: INT 69.8 (11.6), CONTROL 72.1 (9.9) Baseline details: current smoker INT 27 (31%), CONTROL 17 (23%); ex‐smoker INT 59 (69%), CONTROL 56 (77%); pneumococcal vaccination (last 5 years) INT 34 (40%), CONTROL 30 (43%); FEV1 % predicted INT 54.6 (18.7), CONTROL 53.1 (18.1); BMI INT 25.9 (4.6), CONTROL 25.4 (4.1); HADS anxiety INT 6.2 (4.2), CONTROL 5.3 (3.6); HADS depression INT 4.6 (3.7), CONTROL 4.1 (2.9); SGRQ total INT 43.3 (18.8), CONTROL 36.8 (17.6); P = 0.03 Inclusion criteria: GP database searched for diagnosis or use of bronchodilator and inhaled corticosteroid prescriptions. COPD according to ATS criteria (history of cough, sputum, SOB, > 10 pack‐year smoking); plus FEV1/FVC < 70%, weekly symptoms, history or 1+ exacerbations in previous 12 months requiring increased therapy Exclusion criteria: unable/unwilling to sign consent, primary diagnosis asthma, other primary functionally limiting disease, other medical condition likely to affect patient mortality, hospital level residential care, already using self‐management plan, on domiciliary O2, attending GP who already uses self‐management plans more than occasionally, exacerbation of COPD requiring increased treatment within 6 weeks or admission to general hospital within 3 months, cognitive impairment as per 3 MS < 75%, alpha1‐antitrypsin deficiency |
|
| Interventions |
Intervention: AP intervention: usual care and individual standardised educational session from practice nurse or respiratory educator on the use of a self‐management plan, which includes methods of early recognition of exacerbations and appropriate self‐initiated interventions including antibiotics and short course oral corticosteroids; instruction to make early contact with GP. Control: usual care, specifically denied access to written self‐management plan. Non‐standard education on smoking cessation, exercise, controlling breathlessness, nutrition, use of inhaled therapy and immunisation was given according to practice standards. Treatment period: 12 months Follow‐up time points: assessments at baseline, 12 months; telephone interviews at 3, 6 and 9 months |
|
| Outcomes |
Medications: % people used courses of antibiotics and oral steroids at 6 and 12 months
HRQoL: SGRQ measured at 6 and 12 months
Healthcare utilisation: % participants who attended GP visits, ED visits and hospital admissions at 6 and 12 months;
% participants who took courses of antibiotics/prednisone at 12 months
Hospital Anxiety and Depression Scale (HADS): recorded at baseline and at 12 months
COPD Self‐Management Interview (COPD‐SMI): 30‐minute structured interview at baseline and at 12 months, comprising 3 written descriptions of situations (read to participants) based on stages of an exacerbation.
In each scenario, investigators assessed 3 self‐management domains of medication use, healthcare‐seeking decisions and self‐care. They scored each of 13 items per situation on a 3‐point scale (0–2), separately scoring responses for knowledge (knowing what to do) and actions (whether participants would actually do the task and when they would do it), yielding a maximum possible score of 26 for each in all 3 situations. Study visits at baseline and at 12 months, with telephone interviews at 3, 6 and 9 months |
|
| Notes | Funding: Study was funded by Pegasus Health, an independent practitioner association, The Canterbury Respiratory Research Trust and The Asthma and Respiratory Foundation of New Zealand. No funding was received from any pharmaceutical company. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not described. Practices were randomised via 1 investigator. Individual participants were also randomised by a random numbers table if too many were included in a single practice. Participants were screened after randomisation by standardised history and spirometry. |
| Allocation concealment (selection bias) | Unclear risk | Participants were allocated by practice attendance, but information on allocation of practices was not available. If too many patients were identified in each practice, a random numbers table was used to allocate individual participants. An aspect of concern regarding this method was that if the same GP was implementing both intervention and usual care, confounding between treatment methods may occur, possibly diluting effects of active intervention. |
| Blinding (performance bias and detection bias) Participants | High risk | Researchers were unable to blind participants to educational intervention; patient questionnaire outcomes may be influenced by perception of receiving extra intervention. |
| Blinding (performance bias and detection bias) Study personnel | Unclear risk | Nursing staff administering assessments were not blinded to whether participants were included in intervention or control groups. |
| Blinding of outcome assessment (detection bias) Objective outcomes, e.g. healthcare utilisation | Low risk | Although it was not clear how healthcare utilisation data were collected, this was unlikely to be affected by bias. |
| Blinding of outcome assessment (detection bias) Subjective outcomes eg quality of life, anxiety | Unclear risk | Nursing staff administering assessments were not blinded to whether participants were included in intervention or control groups; this may potentially affect collection of questionnaire data. |
| Incomplete outcome data (attrition bias) Health care utilisation (objective) | Low risk | Analysis: INT 84/86 (1 death, 1 WD consent), CONTROL 70/73 (2 WD consent, 1 no contact). Small losses to follow‐up, balanced across groups |
| Incomplete outcome data (attrition bias) Subjective e.g. Quality of life | Low risk | Analysis: INT 84/86 (1 death, 1 WD consent), CONTROL 70/73 (2 WD consent, 1 no contact). Small losses to follow‐up, balanced across groups |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available, but all expected outcomes were reported. |
| Other bias | Unclear risk | Sample size calculation was based on the assumption that about 10 patients would be recruited for each surgery, and that no additional between‐participant variation would be due to clustered‐randomisation of surgeries. Analysis of the 12‐month change in outcome variables was based on a mixed‐model repeated measures ANOVA. This analysis enabled estimation of any additional variation in outcome measures as a consequence of clustered‐randomisation of surgeries rather than individuals. Analyses of outcome variables showed no additional variation from this source beyond that anticipated by between‐participant variation. Analysis of the 12‐month change in outcome variables was based on a mixed‐model repeated measures ANOVA. This analysis enabled estimation of any additional variation in outcome measures as a consequence of clustered‐randomisation of surgeries rather than individuals. Analyses of outcome variables showed no additional variation from this source beyond that anticipated by between‐participant variation. For this reason, all analyses were based on use of participants as replicates. When baseline differences in outcome measures were evident, ANCOVA for repeated measures was used to test the relative effects of treatments. |
Rice 2010.
| Methods |
Study design: parallel‐group randomised controlled trial Location, number of centres: United States of America. Five Veteran Affairs medical centres Duration of study: 12 months |
|
| Participants |
N screened: 1739 eligible, 1316 attempted telephone contact N randomised: 743 (AP 372, UC 371) N completed: AP 336 completed 1 year, 36 deaths; UC 323 completed 1 year, 48 deaths Baseline characteristics: mean age, years (SD) AP 69.1 (9.4), UC 70.7 (9.7); male, n (%) AP 363 (97.6), UC 365 (98.4); mean FEV1, % predicted (SD) AP 36.1 (14.5), UC 38.1 (14.4); current smoker, n (%) AP 80 (21.6), UC 85 (23.0); hospitalised for COPD in the past year, n (%) AP 133 (35.8), UC 145 (39.1); ED visit for COPD in the past year, n (%) AP 218 (58.6), UC 195 (52.6); systemic steroid for COPD in the past year, n (%) AP 210 (56.6), UC 197 (53.5); home oxygen, n (%) AP 200 (53.9), UC 209 (56.6); number in group AP 372, UC 371 Inclusion criteria: diagnosis of COPD and 1 or more of the following during previous year: (1) hospital admission or ED visit for COPD; (2) long‐term home oxygen use; (3) course of systemic corticosteroids for COPD. Additional inclusion criteria: ability to complete the consent process, postbronchodilator spirometry showing FEV1 < 70% predicted, FEV1/FVC < 0.70 Exclusion criteria: any condition that might preclude effective participation in the study or that would reduce life expectancy to less than a year. No access to a telephone |
|
| Interventions |
AP group: education: attended a single 1 to 1.5‐hour group educational session conducted by a case manager; respiratory therapist completed a 1‐day training session. Educational content: ACCP material on general information about COPD, causes, symptoms and treatment of exacerbations, direct observation of inhaler techniques, review and adjustment of outpatient COPD medications, smoking cessation counselling when appropriate, recommendations concerning influenza and pneumococcal vaccinations, encouragement of regular exercise, instruction in hand hygiene. Telephone call follow‐up: case manager monthly phone calls to reinforce general principles of COPD management, review details of the action plan and answer questions. Action plan: individualised written action plan including: (1) description of signs and symptoms of an exacerbation that should prompt initiation of self‐treatment, (2) refillable prescriptions for prednisone and an oral antibiotic, (3) contact information for a case manager, and (4) telephone number of the 24‐hour VA help line. Participants were instructed to begin action plan medications for symptoms that were substantially worse than usual. UC group: education: received 1‐page handout containing a summary of the principles of COPD care according to published guidelines. Telephone call follow‐up: given telephone number for 24‐hour VA nursing help line, a service available to all VA patients. No action plan Follow‐up time points: assessment at baseline and at 12 months. Educational session for AP participants only at the start of the trial, monthly phone calls by a case manager to participants in the AP group; participants were encouraged to contact case manager when they used action plan medications or if they had questions regarding their action plan. |
|
| Outcomes |
Primary outcome: combined number of hospital admissions and ED visits for COPD All outcomes
|
|
| Notes | Details of method, intervention and usual care obtained from online supplement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Online data supplement reports methods of sequence generation as "assigned subjects in equal proportions to each of the two treatment arms by permuted‐block randomisation". |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation concealment were given in the paper or in the trial registration entry. |
| Blinding (performance bias and detection bias) Participants | Low risk | Participants were not blinded, but this is not likely to affect mortality or primary outcomes of healthcare utilisation measures (objective). |
| Blinding (performance bias and detection bias) Study personnel | Low risk | Assessors were blinded: "Blinded pulmonologists independently reviewed all discharge summaries and ED reports and assigned a primary cause for each". |
| Blinding of outcome assessment (detection bias) Objective outcomes, e.g. healthcare utilisation | Low risk | Assessors were blinded: "Blinded pulmonologists independently reviewed all discharge summaries and ED reports and assigned a primary cause for each". Mortaility, healthcare utilisation measures, objective data. Thus low risk of bias |
| Blinding of outcome assessment (detection bias) Subjective outcomes eg quality of life, anxiety | Unclear risk | SGRQ self‐administered patient assessment, with greater potential for bias with lack of blinding |
| Incomplete outcome data (attrition bias) Health care utilisation (objective) | Low risk | The status of all 743 participants was determined after 1 year. |
| Incomplete outcome data (attrition bias) Subjective e.g. Quality of life | Low risk | Only reason for missing data was death (48 in usual care, 36 in intervention). Investigators were unable to perform intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported in trial registration. |
| Other bias | Low risk | No other issues of bias are known. |
Rootmensen 2008.
| Methods |
Study design: parallel group Location, number of centres: single centre, pulmonary outpatient recruitment, Netherlands Duration of study: outcome assessment after 6 months |
|
| Participants |
N screened: 805 outpatient files screened, 386 excluded on previous respiratory nurse contact, 187 patients did not attend outpatient appointment, 19 refused to participate (2 because information on purpose of study was postponed), 22 other reasons given N randomised: 191 (111 COPD) N completed: 157 COPD and asthma. INT 11 did not receive intervention, 13 withdrew consent, 4 died. CONTROL 14 withdrew consent, 3 died M = 105 (55%) F = 86 (45%) Age: AP asthma and COPD mean 60 (SD 15), CONTROL asthma and COPD mean 61 (SD 15) Baseline details: COPD severity GOLD classification ‐ AP GOLD 1/2 = 33 (57%), 3/4 = 22 (39%), CONTROL GOLD 0 = 6 (11%), 1/2 = 30 (55%), 3/4 = 18 (33%); mean FEV1 % predicted AP 57 (SD 19), CONTROL 64 (SD 26); mean FEV1/IVC AP = 0.47 (SD 0.12), CONTROL = 0.50 (SD 0.16) Inclusion criteria: diagnosis of asthma or COPD by respiratory physician, age over 18, ability to understand Dutch questionnaires, never consulted a pulmonary nurse Exclusion criteria: none listed |
|
| Interventions |
Intervention: AP = protocol‐based 45‐minute educational programme on individual basis given by experienced pulmonary nurse. Content (in checklist): information on COPD, underlying pathophysiology, action and proper use of medications and oxygen, avoiding triggers, influenza vaccination, self‐monitoring instructions, smoking cessation. Individual instructions on how to prevent and act for management of exacerbation. Inhalation technique checked. Emergency oral steroids and antibiotics provided to some participants Control: usual care |
|
| Outcomes |
Primary specified outcomes • Knowledge ‐ self‐administered 18‐item questionnaire designed by trialists, including items from 4 previously used questionnaires referenced plus self‐formulated questions. Response true/false/do not know. Score 0‐100% • Inhalation technique ‐ scored by blinded well‐trained observer from videotape demonstration by patient. Score 0‐100% from previously validated criteria • Self‐management knowledge ‐ self‐administered questionnaire on 3 exacerbation scenarios, questions adapted from validated interview‐based questionnaire • Exacerbation incidence ‐ definition exacerbation = worsening of respiratory symptoms that required treatment with oral steroids as judged and prescribed by general practitioner or pulmonary physician Outpatient Clinic Satisfaction Questionnaire ‐ Pulmonology (OCSQ‐P) was used to measure satisfaction with care ‐ general and pulmonary physician subscales |
|
| Notes | Funding: Netherlands Asthma Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation procedure was based on a minimisation procedure. Minimisation factors were diagnosis (asthma or COPD), treated or not by pulmonary physician in previous 2 years |
| Allocation concealment (selection bias) | Low risk | Randomised in advance of clinic attendance. Randomisation results were reported to pulmonary physician just before the participant's visit. |
| Blinding (performance bias and detection bias) Participants | Low risk | Participants were masked for the trial objective to avoid more favourable assessment of participants in additional care group. Participants were told they would be informed about the additional research question only after follow‐up because informing during recruitment would affect the results. Participants asked after visit about length of consultation to detect potential differences in attention between groups. "The number of visits and duration of the first visit were the same for both groups”. |
| Blinding (performance bias and detection bias) Study personnel | Low risk | Investigators "used blind observers to assess adequacy of inhalational techniques”. |
| Blinding of outcome assessment (detection bias) Objective outcomes, e.g. healthcare utilisation | Low risk | Outcome assessors were blinded to outcomes. |
| Blinding of outcome assessment (detection bias) Subjective outcomes eg quality of life, anxiety | Low risk | Outcome assessors were blinded to outcomes. |
| Incomplete outcome data (attrition bias) Health care utilisation (objective) | Unclear risk | No data were measured for participants with COPD. Exacerbation frequency was measured but was not available for COPD only. |
| Incomplete outcome data (attrition bias) Subjective e.g. Quality of life | Unclear risk | Data were available for only 90 of 117 participants with COPD randomised. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available, but it is clear that published reports include all expected outcomes, including those prespecified. |
| Other bias | Low risk | No other issues of bias are known. |
Trappenburg 2011.
| Methods |
Study design: parallel‐group randomised controlled trial Location, number of centres: Netherlands, University Medical Centre Ultrecht. Participants were recruited from 7 regional hospitals and 5 general practices in the Netherlands. Duration of study: 6 months |
|
| Participants |
N screened: 391 N randomised: 233 (AP 111, UC 122) N completed: AP 91 completed 6 months, 21 dropped out (11 withdrew consent, 2 died, 5 comorbidity, 2 moved/logistics, 1 invalid); UC 102 completed 6 months, 20 dropped out (15 withdrew consent, 2 died, 2 comorbidity, 1 invalid) Baseline characteristics: mean age, years (SD) AP 66.1 (11.2), UC 65.1 (10.0); male, n (%) AP 65 (59), UC 69 (57); mean FEV1, % predicted (SD) AP 56.7 (20.3), UC 56.5 (20.6); current smoker, n (%) AP 31 (28), UC 37 (30); hospitalised for COPD in past year, n (%) AP 22 (20), UC 21 (18); number in group AP 111, UC 122; BMI (SD) AP 26.1 (5.5), UC 26.7 (6.5); living alone, n (%) AP 27 (23), UC 22 (18); education: lower secondary or less, n (%) AP 69 (62), UC 83 (68); higher secondary, n (%) AP 29 (26), UC 31 (25); college/university, n (%) AP 13 (12), UC 8 (7); GOLD stage: I, n (%) AP 14 (13), UC 13 (11); II, n (%) AP 55 (50), UC 58 (47); III, n (%) AP 30 (27), UC 38 (31); IV, n (%) AP 11 (10), UC 12 (10); FEV1, mean (SD) AP 1.55 (0.60), UC 1.59 (0.71); FVC, mean (SD) AP 3.03 (0.79), UC 3.17 (0.91); recruited from: GP, n (%) AP 18 (16), UC 17 (14); outpatient clinic, n (%) AP 93 (84), UC 105 (86) Inclusion criteria: postbronchodilator ratio of forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC) < 70%. Age > 40 years. Smoking history > 20 years or 15 pack‐years. Diagnosis of COPD as a major functionally limiting disease. Current use of bronchodilator therapy Exclusion criteria: primary diagnosis of asthma. Primary diagnosis of cardiac disease. Presence of disease that could affect mortality or participation in the study (e.g. confusional states) |
|
| Interventions |
AP group: At inclusion, participants were seen by the nurse case manager (respiratory nurse), who systematically checked and discussed; aspects of COPD care: vaccination, optimisation of medication, inhalation techniques, exercise, nutritional aspects, smoking (cessation) and exacerbation management. Participants in the AP group were encouraged to contact their case manager if they needed further information or wanted to ask a question. Two standardised reinforcement sessions were held by telephone at 1 and 4 months to evaluate participant understanding of and adherence to AP and, when needed, additional information was provided. An action plan for participants was individualised by a respiratory nurse and included: (1) a list of important contact persons and telephone numbers; resource persons: family physician, respiratory physician and respiratory nurse; (2) stable symptom severity (individual stable/normal green zone symptom status); (3) regular medication/lifestyle prescriptions (green zone); (4) additional medication/breathing exercises and energy preservation in case of symptom increase (yellow zone, orange zone); (5) a name contact person/telephone number in case of an exacerbation (orange zone). For individual participants, it was optional for the case manager (in consultation with the attending physician) to provide self‐treatment medication (course of corticosteroids and/or antibiotics). Participants also received usual care, which included pharmacological and non‐pharmacological care according to the most recent evidence‐based guidelines. UC group: At inclusion, participants were seen by a nurse case manager (respiratory nurse), who systematically checked and discussed aspects of COPD care: vaccination, optimisation of medication, inhalation techniques, exercise, nutritional aspects, smoking (cessation) and exacerbation management. No additional contacts with nurse educator. Participants in control group did not receive additional telephone sessions. Participants did not receive an action plan. Received usual care including pharmacological and non‐pharmacological care according to the most recent evidence‐based guidelines Follow‐up time points: assessments at baseline and at 6 months. All participants were contacted by telephone monthly; participants in the AP group received additional telephone follow‐up at 1 and 4 months to evaluate understanding and adherence to the action plan. |
|
| Outcomes |
Primary outcome: time to recovery of health status in the event of an exacerbation All outcomes • Number of exacerbations • Time to recovery from exacerbation • Exacerbation rates • Anthonisen classification of COPD exacerbations • Percentage of exacerbations reported to a healthcare provider • Number respiratory‐related hospital admissions • Hospital days • Emergency room visits • Scheduled visits • Unscheduled visits • Telephone calls to respiratory or family physicians • Symptom diary • Health‐related quality of life • Anxiety and depression • Self‐management exacerbation‐related self‐efficacy* |
|
| Notes |
Funding: not declared in protocol/trial registration or in results publication *Exacerbation‐related self‐efficacy measured by study‐developed questionnaire, consisting of 11 items for which confidence in self‐management capability in the occurrence of an exacerbation is graded on a 5‐point Likert scale. Lower scores indicate high confidence in adequate exacerbation‐related self‐management behaviour. No validity or responsiveness data published for this questionnaire |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was carried out using the minimisation technique to balance the control and intervention groups for centre and gender." Probably done, as earlier reports from the same study authors clearly describe randomisation stratified by centre and gender |
| Allocation concealment (selection bias) | Low risk | "To conceal the assignment sequence, a central web‐based service was used." Probably done, as earlier reports from the same investigators clearly describe use of a central web‐based service for allocation concealment |
| Blinding (performance bias and detection bias) Participants | Low risk | "The modified informed consent procedure (postponed information) meant that patients were unaware of the major aim of the study." Probably done. Postponing receipt of information from participants allowed for adequate blinding of participants. Risk of cross‐contamination between members of intervention and control groups was reduced by stratification of randomisation by centre |
| Blinding (performance bias and detection bias) Study personnel | Low risk | Health professionals would have been aware of which participants were receiving the intervention. This is unlikely to be a significant source of bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes, e.g. healthcare utilisation | Low risk | "All patients were contacted for monthly evaluation by telephone to assess healthcare utilisation and to evaluate proper use of the diary (figure 1)" (healthcare utilisation). Assessors were not blinded, as participants may have disclosed whether or not they were receiving an action plan. "To ensure rigorous and complete exacerbation counts, all diaries were reviewed by three blinded investigators who adjudicated events by consensus" (exacerbations). Unclear from information in the diary whether assessors would have been aware if the participant was receiving an action plan |
| Blinding of outcome assessment (detection bias) Subjective outcomes eg quality of life, anxiety | Low risk | "All patients were instructed to record daily in a diary whether symptoms were increased over their baseline condition" (patient‐reported outcomes). Participants were unaware of the major aim of the study, hence self‐reported outcomes were unlikely to be biased. |
| Incomplete outcome data (attrition bias) Health care utilisation (objective) | Low risk | Drop‐outs 19% intervention and 16% control group. Reasons for withdrawals were given and were balanced in both groups. |
| Incomplete outcome data (attrition bias) Subjective e.g. Quality of life | Low risk | Drop‐outs 19% intervention and 16% control group. Reasons for withdrawals were given and were balanced in both groups. |
| Selective reporting (reporting bias) | Low risk | Medical Research Council Dyspnoea Scale (MRC scale) was reported as a secondary outcome in the protocol but is not listed in the report. All other outcomes listed in the protocol are reported. |
| Other bias | Low risk | No other issues of bias |
Watson 1997.
| Methods |
Study design: Parallel‐group randomised study Location, number of centres: New Zealand, 12 practices, 22 GPs Duration of study: 6‐month follow‐up. Year study performed: 1993‐July 1994 Time points: follow‐up at 6 and 12 months |
|
| Participants |
Diagnosis: COPD defined according to American Thoracic Society: diagnosis of COPD as major functionally limiting disease; smoking history > 10 pack‐years; FEV1 < 65%; FEV1/FVC < 70%; current use of bronchodilator therapy
Screened: 93 patients screened for possible inclusion; 24 did not meet inclusion criteria
Randomised: 69
Completed: 56. Intervention 29; CONTROL 27
Drop‐outs: 13. 4 offended by questionnaire; 3 experienced complications from concurrent medical problems; 3 felt study protocol was too demanding; 1 left the country; 2 died
M = INT 62%, CONTROL 67%
Age: INT 68, CONTROL 67
Inclusion criteria: COPD by ATS criteria, smoking history > 10 pack‐years
COPD severity: FEV1 < 65% predicted, current use of bronchodilator therapy
Exclusion criteria: primary diagnosis of asthma (onset < 35 years), primary diagnosis of cardiac disease (uncontrolled heart failure); primary or secondary diagnosis of another functionally limiting disease (except cor pulmonale) that could significantly affect patient mortality within 6 months of entry to the study (malignant neoplasm) or participation in the study (psychoses); continuous use of oral corticosteroid; long‐term antibiotic therapy; rest home residents Baseline details Intervention: age 68 (SD 10); male 62%; married 52%; current smoker 24%; FEV1 % predicted 37 (SD 14); access to nebuliser 17%; own a peak flow meter 76%; influenza vaccine in last year 72% Control: age 67 (SD 8), male 67%; married 37%; current smoker 33%; FEV1 % predicted 36 (SD 16); access to nebuliser 26%; own a peak flow meter 70%; influenza vaccine in last year 44% Participation in study Intervention group: days in study: 186 (SD 13); days recorded in symptom diary: 144 (SD 62) Control group: days in study: 187 (SD 7); days recorded in symptom diary: 160 (SD 51) |
|
| Interventions | Action plan (AP) intervention: AP = recognition of respiratory symptoms when well and during exacerbations of COPD and medication instructions for worsening symptoms, a booklet on self‐management; supply of prednisone and antibiotic from GP. The booklet, "A Guide to Living Positively With COPD", was developed and circulated among participants' GPs and family. Covered smoking cessation, control of breathlessness, exercise, daily activities, diet, sleep, clearing of mucus, planning for future, medications, O2 and contact details for support services Control: usual care; access to AP and booklet specifically denied | |
| Outcomes | Daily diary cards, which rated respiratory status as usual, mild, moderate or severe; prednisone use, antibiotic use and contact with GP, PN, hospital specialist, pharmacist. Participants were interviewed about access to and use of treatments, services and self‐management strategies. FEV1 and FVC spirometry HRQoL: SGRQ
Outcomes were reported as absolute means and standard deviations from baseline. |
|
| Notes |
Funding: Study was funded in part by the Southern Regional Health Authority. Additional funding and resources were provided by The Canterbury Respiratory Research Group. 85% of participants were given AP by practice nurse (PN), 15% by GP. 90% positive acceptability for AP. Time to provide AP 10‐20 minutes 40%, 20‐30 minutes 35%. 94% GPs and PNs had no difficulty explaining action plan use to participants. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants meeting entry criteria were randomly allocated to the intervention or control group. Permuted block randomisation was used, in blocks of 10. Order within the block was randomly generated by a computer. |
| Allocation concealment (selection bias) | Low risk | Participant level allocated by research staff according to randomisation list. GPs and PNs recruited participants and were blind to group allocation. |
| Blinding (performance bias and detection bias) Participants | High risk | Participants could not be blinded to allocation. Participants completed daily diary cards recording healthcare utilisation and symptoms. Knowledge of allocation to intervention may have biased reporting. |
| Blinding (performance bias and detection bias) Study personnel | Unclear risk | Study staff was not blinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes, e.g. healthcare utilisation | Unclear risk | Participants completed daily diary cards recording healthcare utilisation. |
| Blinding of outcome assessment (detection bias) Subjective outcomes eg quality of life, anxiety | Unclear risk | Exit study visit in clinic for QoL was provided by study staff who were not blinded. |
| Incomplete outcome data (attrition bias) Health care utilisation (objective) | Unclear risk | 60 randomised, 56 completed. Group allocation status of 13 withdrawals was not given. |
| Incomplete outcome data (attrition bias) Subjective e.g. Quality of life | Unclear risk | 60 randomised, 56 completed. Group allocation status of 13 withdrawals was not given. Reasons: 4 participants offended by questionnaires; 3 experienced complications associated with concurrent medical problems; 3 believed the study protocol was too demanding; 1 left the country; 2 died. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available, but it appears that published reports include all expected outcomes, including those prespecified. |
| Other bias | Unclear risk | Baseline access to and use of a variety of treatments, services and self‐management strategies showed no statistically significant differences between groups, except for influenza vaccination in last year: 72% INT, 44% CONTROL |
Wood‐Baker 2006.
| Methods |
Study design: parallel‐group cluster‐randomised trial Location: All GPs registered with Southern Tasmanian Division of General Practitioners (N = 255) were contacted and invited to participate. Duration of study: 12 months. Year study performed: 2002 |
|
| Participants |
N screened: 262
N randomised: 139
N completed: 112 (54 in intervention group and 58 in control group). Drop‐outs: intervention group: 5 deaths; 8 withdrawals. Control group: 4 deaths; 8 withdrawals; 2 lost to follow‐up Inclusion criteria: diagnosis of COPD as primary functionally limiting illness, aged > 50 years, tobacco smoking history > 10 pack‐years, FEV1 < 65% predicted and/or FEV1/FVC ratio < 70% Exclusion criteria: nursing home residents Baseline characteristics Intervention : N = 67: age 69 (SD 7.8); 49 male; 46 married; 37 widowed; 12 separated/divorced; 5 never married; 40 labourers; 19 clerical, sales and service industry workers; 16 tradespersons; 11 managers, admin and professional workers; 9 production and transport; 5 never worked; 36 current smokers; smoking history: 55 (SD 26) pack‐years; BMI 25.9 (SD 5.8); COPD severity: FEV1 % predicted 46.3 (SD 16), FEV1/FVC 56.8 (SD 15.7). Daily steps 4751 (IQR 4473); SGRQ symptoms 59.9 (SD 22.7), activity 62.3 (SD 25.2), impacts 33.4 (SD 21.3), total 46.5 (SD 20.4); participation in pulmonary rehab 30; medications prescribed at enrolment: SABA 97, LABA 36, ipratropium 67, methylxanthine 8, inhaled corticosteroid 60, oral corticosteroid 8, O2 10 Control : N = 72: Age 71 ± 8.4; 67 males; 51 married; 33 widowed; 10 separated/divorced; 6 never married; 27 labourers; 28 clerical, sales and service industry workers; 27 tradespersons; 11 managers, admin and professional workers; 7 production and transport; 0 never worked; 22 current smokers; smoking history: 59 (SD 33.7) pack‐years ; BMI 25.2 ± 5.4; COPD severity: FEV1% predicted 44.2 (SD 15.8), FEV1/FVC 50.9 (SD12.2). Daily steps 3454 (IQR = 3041); SGRQ symptoms ‐ 62.7 (SD 20.6), activity ‐ 66.4 (SD 20.2), impacts 32.1 (SD 17.3), total 47.3 (SD 16.6); participation in pulmonary rehab 24; medications prescribed at enrolment: SABA 78, LABA 24, ipratropium 57, methylxanthine 7, inhaled corticosteroid 43, oral corticosteroid 7, O2 4. |
|
| Interventions | Intervention: Action plan (AP) ‐ COPD information booklet and individual educational session with respiratory nurse (covered basic COPD pathology, smoking cessation, immunisations, nutrition, exercise, clearing of mucus from lungs, control of breathlessness during ADLs, stress management, medications, correct use of inhalers and contact details of community support services). Also written self‐management plan listing maintenance medications and individual AP based on early recognition of exacerbations. 76% of participants received instructions to start short course oral corticosteroids and an antibiotic; remaining 24% received instructions to initiate antibiotics only (N = 10), double dose of inhaled corticosteroids and start antibiotic (2), initiate short course oral corticosteroids only (1) or contact GP (3). Prescriptions were provided as necessary. All were encouraged to present to GP early during exacerbation. Control: usual care, action plan specifically denied Number intervention group: 54 Number control group: 58 | |
| Outcomes |
Health‐related QoL: absolute mean and standard deviation at baseline and mean change in SGRQ and standard deviation at 6 and 12 months
Physiological impairment: lung function spirometry at baseline, at 6 and 12 months
Physical activity measured on digital pedometer over 7 day period at baseline, at 6 and 12 months
Healthcare utilisation: diary used to record GP consults, hospitalisations and attendances to ER, exacerbations
Medications: diary to record antibiotic use, use of short course corticosteroids
Mortality Outcome measurement: 3, 6, 9 and 12 months, 6 and 12 month assessments were face‐to‐face at GP, surgery or participant's home, 3 and 9 months by standardised telephone interviews |
|
| Notes | Not stated if hospitalisation or ED visits were related to COPD or all‐cause Funding: not known |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Practices were randomised to intervention (action plan) or control group by a computer‐generated randomisation software package. |
| Allocation concealment (selection bias) | Unclear risk | Practice level was allocated but no information was published on method of allocation to groups. |
| Blinding (performance bias and detection bias) Participants | High risk | Participants could not be blinded to allocation. Participants completed daily diary cards to record healthcare utilisation and symptoms. Knowledge of allocation to intervention may have biased reporting. |
| Blinding (performance bias and detection bias) Study personnel | Unclear risk | Study staff were not blinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes, e.g. healthcare utilisation | Low risk | Objective assessments were not likely to be affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes eg quality of life, anxiety | Unclear risk | Study visits for QoL were handled by study staff who were not blinded. |
| Incomplete outcome data (attrition bias) Health care utilisation (objective) | Low risk | INT 67 randomised, 5 died, 8 withdrew for personal reasons. 61 completed 6‐month and 54 completed 12‐month assessment. CONTROL 72 randomised, 4 died, 8 withdrew for personal reasons, 2 lost to follow‐up. 62 completed 6‐month and 58 completed 12‐month assessment. Similar proportions in both groups completed. |
| Incomplete outcome data (attrition bias) Subjective e.g. Quality of life | Low risk | INT 67 randomised, 5 died, 8 withdrew for personal reasons. 61 completed 6‐month and 54 completed 12‐month assessment. CONTROL 72 randomised, 4 died, 8 withdrew for personal reasons, 2 lost to follow‐up. 62 completed 6‐month and 58 completed 12‐month assessment. Similar proportions in both groups completed. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and published reports include all expected outcomes, including those prespecified. |
| Other bias | Unclear risk | Unit of randomisation was participant's GP. Intervention and control groups were similar in terms of age, smoking history, airways limitation and QoL scores. Analysis did not take into account clustering by GP. |
ACCP: American College of Chest Physicians; ADLs: activities of daily living; ANCOVA: analysis of covariance; ANOVA: analysis of variance; AP: action plan; ATS: American Thoracic Society; BMI: body mass index;CI: confidence interval; COPD: chronic obstructive pulmonary disease; COPD‐SMI: COPD Self‐Management Interview; ED: emergency department; F: female; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; GP: general practitioner; HADS: Hospital Anxiety and Depression Scale; HRQoL: health‐related quality of life; INT: intervention; IQR: interquartile range; IVC: inspiratory vital capacity; LABA: long‐acting beta‐agonist; M: male; MMSE: Mini Mental State Examination; MRC: Medical Research Council; OCSQ‐P: Outpatient Clinic Satisfaction Questionnaire ‐ Pulmonology; PN: practice nurse; PRED: prednisone; PYH: pack year history; QoL: quality of life; SABA: short‐acting beta‐agonist; SD: standard deviation; SGRQ: St George's Respiratory Questionnaire; SOB: shortness of breath; UC: usual care; VA: Veterans Administration; WD: withdrawal.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Apps 2008 | Intervention did not include an action plan. |
| Benzo 2013 | Action plan was part of a broader self‐management intervention. |
| Bischoff 2011 | This was not a randomised controlled trial (RCT). |
| Bischoff 2013 | Action plan was part of a broader self‐management intervention. |
| Bosch 2007 | Intervention did not include an action plan. |
| Botvinikova 2010 | Intervention did not include an action plan. |
| Bourbeau 2003 | Action plan was part of a broader self‐management intervention, and educational intervention was too long (weekly visits over a 2‐month period). |
| Bucknall 2012 | Educational intervention was too long (4× 40‐minute individual training sessions). |
| Cave 2010 | Intervention did not involve an action plan. |
| Chavannes 2009 | Action plan was part of a broader self‐management intervention. |
| Choi 2014 | This was not a randomised controlled trial (RCT). |
| Chuang 2011 | Educational intervention was too long (4 weekly telephone sessions 20 minutes each). |
| Coultas 2012 | Intervention did not include an action plan. |
| Davies 2014 | This was not a randomised controlled trial (RCT). |
| Dhein 2003 | This was not a randomised controlled trial (RCT). |
| Effing 2009 | Control group was not given usual care. Action plan was part of a broader self‐management intervention. |
| Efraimsson 2008 | Educational intervention was too long (2× 1 hour sessions). |
| Fan 2012 | Educational intervention was too long (4 weekly 90‐minute individual sessions). |
| Hesselink 2004 | Study participants included those with a diagnosis of asthma or COPD. Intervention did not include an action plan. |
| Jarab 2012 | Intervention did not include an action plan. |
| Khdour 2009 | Action plan was part of a broader self‐management intervention. |
| Kiser 2012 | Intervention did not include an action plan. |
| Lawlor 2007 | This was not a randomised controlled trial (RCT). |
| Lenferink 2013 | Educational intervention was too long (4× 2.5‐hour sessions). |
| Maltais 2008 | Action plan was part of a broader self‐management intervention. Control group was not given usual care. |
| Miller 2010 | Educational intervention was too long (4× 40‐minute individual sessions). |
| Monninkhof 2003 | Action plan was part of a broader self‐management intervention. |
| Newman 1995 | Intervention did not include an action plan. |
| Parenteau 2003 | This was not a randomised controlled trial (RCT). |
| Rea 2004 | Action plan was part of a broader self‐management intervention. |
| Roberts 2007 | This was not a randomised controlled trial (RCT). This was a pilot study of the acceptability of a pictorial action plan. |
| Rowett 2005 | Intervention did not include an action plan. |
| Sedeno 2006 | Educational intervention was too long (8 sessions exceeding 1 hour). |
| Sedeno 2009 | Citation to study was already excluded; educational sessions exceeded 1 hour. |
| Siddique 2012 | Intervention did not include an action plan. |
| Song 2014 | Intervention did not include an action plan. |
| Sridhar 2008 | Action plan was part of a broader self‐management intervention. |
| Uijen 2012 | Intervention did not include an action plan. |
| Wakabayashi 2006 | Intervention did not include an action plan. |
| Wittmann 2007 | Control group was not given usual care. Educational intervention was too long (4× 1.5‐hour sessions). |
| Worth 2004 | It was not possible to extract outcome data regarding action plan (AP) only. |
| Yu 2014 | Intervention did not include an action plan. |
Characteristics of ongoing studies [ordered by study ID]
Doheny 2013.
| Trial name or title | The effectiveness of pharmacist‐provided self‐management education to patients with chronic obstructive pulmonary disease |
| Methods |
Study design: randomised controlled trial Location, number of centres: United States of America. 2 community pharmacies in Worcester, Massachusetts Duration of study: proposed to run for 12 months |
| Participants |
N screened: not available N randomised: not available N completed: not available Baseline characteristics: not available Inclusion criteria: current use of an inhaled bronchodilator, aged 40 years or older, smoking history of 10 or more years, diagnosis of chronic obstructive pulmonary disease (COPD) confirmed through spirometry Exclusion criteria: not available |
| Interventions | AP group: education: medication therapy management session that includes a comprehensive medication review (CMR), inhaler technique and correction, presentation of self‐management techniques for COPD, distribution of educational materials about COPD. Action plan: after CMR is completed, the pharmacy will contact the participant's primary care provider to recommend 2 prescriptions: an oral corticosteroid and an antibiotic to keep on file to fill in the event of a COPD exacerbation. Once approval or denial is received, a written action plan is developed and given for each participant, along with a pulse oximeter and digital thermometer. UC group: typical care Follow‐up time points: proposed for participants to be contacted monthly for 12 months to ask questions related to their respiratory health and any exacerbations they may have experienced. At baseline and at 6 and 12 months, participants will be administered the COPD assessment test. |
| Outcomes | Primary outcomes: COPD‐related hospital admissions, COPD‐related unscheduled healthcare visits, health‐related quality of life |
| Starting date | Not available |
| Contact information | Massachusetts College of Pharmacy and Health Sciences. E‐mail: Scott.Doheny@mcphs.edu |
| Notes | Efforts to contact first study author regarding details on progress of the study were unsuccessful. No data are available. |
Differences between protocol and review
In planning this 2016 update, before searches were run, the review author team made changes to the protocol. We prespecified inclusion criteria to permit limited support directed only at use of the action plan (up to monthly). We also prespecified that subgroup analysis would be performed by comparing studies with and without this limited ongoing support.
We made changes to the outcomes; we prespecified these changes before commencing the update on the basis of consensus reached by two review authors (MH, JW) on which outcomes were clinically important. We added information on cost‐effectiveness and withdrew information on acute exacerbations, functional capacity, symptom scores and days lost from work.
Contributions of authors
J Walters: author of original review in 2005 and update in 2009. Collaborating review author in 2016 update: undertook study selection, data extraction and risk of bias assessment, as well as meta‐analysis and revision of review drafts.
M Howcroft: collaborating review author in 2016 update: undertook study selection, data extraction and risk of bias assessment, as well as meta‐analysis and revision of review drafts.
R Wood‐Baker: original review 2005: formulated review topic, advised on search strategy, extracted data and performed meta‐analysis; also revised review drafts. 2009 update: assisted in study selection, checked data, conducted analysis and revised drafts. Contributed to discussion and revision of review drafts in 2016 update.
EH Walters. edited protocol and review drafts in 2005 and 2009; contributed to discussion and revised review drafts in 2016 update.
A Turnock: served as original review author in 2005.
Sources of support
Internal sources
-
MH, RWB, EHW, JAEW, Australia.
University of Tasmania
External sources
-
Commonwealth Department of Health and Ageing, Australia.
JAEW Co‐ordinator Support, Cochrane Airways Australia
-
Asthma Foundation Tasmania, Australia.
Cochrane Airways Australia Scholarship to MH
Declarations of interest
One review author (RWB) was an investigator in an included study (Wood‐Baker 2006).
MH: none known.
JW: none known.
EHW: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Martin 2004 {published data only}
- Martin IR, McNamara D, Sutherland FR, Tilyard MW, Taylor DR. Care plans for acutely deteriorating COPD: a randomized controlled trial. Chronic Respiratory Disease 2004;1(4):191‐5. [DOI] [PubMed] [Google Scholar]
McGeoch 2004 {unpublished data only}
- McGeoch GR, Willsman KJ, Dowson CA, Town GI, Frampton CM, McCartin FJ, et al. Self‐management plans in the primary care of patients with chronic obstructive pulmonary disease. Respirology 2006;11(5):611‐8. [DOI] [PubMed] [Google Scholar]
Rice 2010 {published data only}
- Dewan NA, Rice KL, Caldwell M, Hilleman DE. Economic evaluation of a disease management program for chronic obstructive pulmonary disease. COPD 2011;8(3):153‐9. [DOI] [PubMed] [Google Scholar]
- Rice KL, Dewan N, Bloomfield HE, Grill J, Schult TM, Nelson DB, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2010;182(7):890‐6. [DOI] [PubMed] [Google Scholar]
- Rice KL, Dewan N, Bloomfield HE, Grill MJ, Schult TE, Nelson DB, et al. Case/self management for COPD: a randomized controlled trial [Abstract]. American Journal of Respiratory and Critical Care Medicine 2008;177:A868. [DOI] [PubMed] [Google Scholar]
Rootmensen 2008 {published data only}
- Rootmensen GN, Keimpema ARJ, Looysen EE, Schaaf L, Haan RJ, Jansen HM. The effects of additional care by a pulmonary nurse for asthma and COPD patients at a respiratory outpatient clinic: results from a double blind, randomized clinical trial. Patient Education and Counseling 2008;70(2):179‐86. [DOI] [PubMed] [Google Scholar]
Trappenburg 2011 {published data only}
- Trappenburg J, Heijneman J, Monninkhof E, Bourbeau J, Troosters T, Schrijvers G, et al. Effectiveness of an individualized action plan on health status recovery in patients with COPD: a randomized controlled trial [Abstract]. European Respiratory Society 20th Annual Congress; 2010 Sep 18‐22; Barcelona. 2010:[E2168].
- Trappenburg J, Monninkhof E, Bourbeau J, Troosters T, Schrijvers A, Verheij T, et al. Effect of an action plan with ongoing support by a case manager on exacerbation‐related outcome in patients with COPD: a multicentre randomised controlled trial. Thorax 2011;66:977‐84. [DOI] [PubMed] [Google Scholar]
- Trappenburg JCA, Koevoets L, Weert‐van Oene GH, Monninkhof EM, Bourbeau J, Troosters T, et al. Action plan to enhance self‐management and early detection of exacerbations in COPD patients: a multicenter RCT. BMC Pulmonary Medicine 2009;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 1997 {published data only}
- Watson PB, Town GI, Holbrook N, Dwan C, Toop LJ, Drennan CJ. Evaluation of a self‐managment plan for chronic obstructive pulmonary disease. European Respiratory Journal 1997;10:1267‐71. [DOI] [PubMed] [Google Scholar]
Wood‐Baker 2006 {published and unpublished data}
- McGlone S, Wood‐Baker R, Walters EH. The effect of a written action plan in COPD [Abstract]. Respirology 2004;9(2 Suppl):A46. [Google Scholar]
- Wood‐Baker R, McGlone S, Venn A, Walters EH. Written action plans in chronic obstructive pulmonary disease increase appropriate treatment for acute exacerbations. Respirology 2006;11(5):619‐26. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Apps 2008 {published data only}
- Apps LD, Revitt O, Sewell L, Williams J, Singh SJ. An independent self‐management programme for chronic obstructive pulmonary disease: does it work? A pilot study. Thorax 2008;62(Suppl VII):A137. [Google Scholar]
Benzo 2013 {published data only}
- Benzo R, Vickers K, Ernst D, Tucker S, McEvoy C, Lorig K. Development and feasibility of a self‐management intervention for chronic obstructive pulmonary disease delivered with motivational interviewing strategies. Journal of Cardiopulmonary Rehabilitation and Prevention 2013;33(2):113‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bischoff 2011 {published data only}
- Bischoff EW, Hamd DH, Sedeno M, Benedetti A, Schermer TRJ, Bernard S, et al. Effects of written action plan adherence on COPD exacerbation recovery. Thorax 2011;66(1):26‐31. [DOI] [PubMed] [Google Scholar]
Bischoff 2013 {published data only}
- Bischoff E, Akkermans R, Bourbeau J, Vercoulen J, Weel C, Schermer T. Comprehensive self management and routine monitoring in chronic obstructive pulmonary disease patients in general practice: randomised controlled trial [Abstract]. European Respiratory Society 23rd Annual Congress; 2013 Sep 7‐11; Barcelona. 2013:42. [DOI] [PMC free article] [PubMed]
Bosch 2007 {published data only}
- Bosch D, Feierabend M, Becker A. COPD outpatient education programme (ATEM) and BODE index [article in German]. Pneumologie 2007;61(10):629‐35. [DOI] [PubMed] [Google Scholar]
Botvinikova 2010 {published data only}
- Botvinikova L, Konopkina L, Garagulya A. Efficacy of long‐term educational program (EP) of patients with COPD [Abstract]. European Respiratory Society 20th Annual Congress; 2010 Sep 18‐22; Barcelona. 2010:P4035.
Bourbeau 2003 {published data only}
- Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupre A, Begin R, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease‐specific self‐management intervention. Archives of Internal Medicine 2003;163(5):585‐91. [DOI] [PubMed] [Google Scholar]
- Bourbeau J, Nault D, Dang‐Tan T. Self‐management and behaviour modification in COPD. Patient Education and Counseling 2004;52:271‐7. [DOI] [PubMed] [Google Scholar]
Bucknall 2012 {published data only}
- Bucknall CE, Miller G, Lloyd SM, Cleland J, McCluskey S, Cotton M, et al. Glasgow supported self‐management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ 2012;344(7849):e1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cave 2010 {published data only}
- Cave AJ, Makarows C, Ahmadi E. Effect of respiratory educators in family physicians' offices on COPD [Abstract]. Primary Care Respiratory Journal 2010;19(2):A18 [68]. [Google Scholar]
Chavannes 2009 {published data only}
- Chavannes NH, Grijsen M, Akker M, Schepers H, Nijdam M, Tiep B, et al. Integrated disease management improves one‐year quality of life in primary care COPD patients: a controlled clinical trial. Primary Care Respiratory Journal 2009;18(3):171‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2014 {published data only}
- Choi CJ, Chung HIC, Han G. Patient outcomes according to COPD action plan adherence. Journal of Clinical Nursing 2014;23(5‐6):883‐91. [DOI] [PubMed] [Google Scholar]
Chuang 2011 {published data only}
- Chuang C, Levine SH, Rich J. Enhancing cost‐effective care with a patient‐centric coronary obstructive pulmonary disease program. Population Health Management 2011;14(3):133‐6. [DOI] [PubMed] [Google Scholar]
Coultas 2012 {published data only}
- Coultas DB, Russo R, Peoples J, Ashmore J, Sloan J, Verdan P. Six month results of behavioral self‐management effectiveness trial to enhance lifestyle physical activity among patients with COPD [Abstract]. American Thoracic Society International Conference; 2012 May 18‐23; San Francisco. 2012; Vol. 185:A4871.
Davies 2014 {published data only}
- Davies F, Risør MB, Melbye H, Spigt M, Brookes‐Howell L, O’Neill C, et al. Primary and secondary care clinicians’ views on self‐treatment of COPD exacerbations: a multinational qualitative study. Patient Education and Counseling 2014;96(2):256‐63. [DOI] [PubMed] [Google Scholar]
Dhein 2003 {published data only}
- Dhein Y, Munks‐Lederer C, Worth H. Evaluation of a structured education programme for patients with COPD under outpatients conditions ‐ a pilot study [article in German]. Medizinische Klinik 2003;57:591‐7. [DOI] [PubMed] [Google Scholar]
Effing 2009 {published data only}
- Effing T, Kerstjens H, Valk P, Zielhuis G, Palen J. (Cost)‐effectiveness of self‐treatment of exacerbations on the severity of exacerbations in patients with COPD: The COPE II study. Thorax 2009;64(11):956‐62. [DOI] [PubMed] [Google Scholar]
- Effing TW, Zielhuis GA, Kerstjens HAM, Valk PDLPM, Palen J. A community based reactivation program incorporated in a COPD self‐management program: the COPE II‐Study [Abstract]. American Thoracic Society International Conference; 2009 May 15‐20; San Diego. 2009:A2381 [Poster #519].
Efraimsson 2008 {published data only}
- Efraimsson EO, Hillervik C, Ehrenberg A. Effects of COPD self‐care management education at a nurse‐led primary health care clinic. Scandinavian Journal of Caring Sciences 2008;22(2):178‐85. [DOI] [PubMed] [Google Scholar]
Fan 2012 {published data only}
- Fan VS, Gaziano JM, Lew R, Bourbeau J, Adams SG, Leatherman S, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Annals of Internal Medicine 2012;156(10):673‐83. [DOI] [PubMed] [Google Scholar]
- Fan VS, Niewoehner DE, Lew R. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations. Annals of Internal Medicine 2012;157(7):530‐1. [DOI] [PubMed] [Google Scholar]
Hesselink 2004 {published data only}
- Hesselink A, Penninx B, Windt D, Duin B, Vries P, Twisk J, et al. Effectiveness of an education programme by a general practice assistant for asthma and COPD patients: results from a randomised controlled trial. Patient Education and Counseling 2004;55:121‐8. [DOI] [PubMed] [Google Scholar]
Jarab 2012 {published data only}
- Jarab AS, Al Qudah SG, Khdour M, Shamssain M, Mukattash TL. Impact of pharmaceutical care on health outcomes in patients with COPD. International Journal of Clinical Pharmacy 2012;34(1):53‐62. [DOI] [PubMed] [Google Scholar]
Khdour 2009 {published data only}
- Al‐Khdour M, McCourt B, Kidney J, Crealey GE, McElnay JC. Humanistic and economic outcomes of a disease and medicine management programme for patients with chronic obstructive pulmonary disease (COPD). International Journal of Pharmacy Practice 2008;16(Suppl 3):C60. [Google Scholar]
- Khdour M, Smyth B, Kidney J, McElnay J. Education on disease and medicine management programme for patients with chronic obstructive pulmonary disease. Irish Journal of Medical Science. 2008; Vol. 177(Suppl 13):S447‐8.
- Khdour MR, Agus AM, Kidney JC, Smyth BM, Elnay JC, Crealey GE. Cost‐utility analysis of a pharmacy‐led self‐management programme for patients with COPD. International Journal of Clinical Pharmacy 2011;33(4):665‐73. [DOI] [PubMed] [Google Scholar]
- Khdour MR, Kidney JC, Smyth BM, McElnay JC. Clinical pharmacy‐led disease and medicine management programme for patients with COPD. British Journal of Clinical Pharmacology 2009;68(4):588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiser 2012 {published data only}
- Kiser K, Jonas D, Warner Z, Scanlon K, Bryant SB, DeWalt DA. A randomized controlled trial of a literacy‐sensitive self‐management intervention for chronic obstructive pulmonary disease patients. Journal of General Internal Medicine 2012;27(2):190‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawlor 2007 {published data only}
- Lawlor M, Kealy S, O‘Connell F. Provision of self‐management plans for treatment of COPD exacerbations reduces hospital admissions. European Respiratory Journal 2007;30(Suppl):Abstract 3390. [Google Scholar]
Lenferink 2013 {published data only}
- Lenferink A, Frith P, Valk P, Buckman J, Sladek R, Cafarella P, et al. A self‐management approach using self‐initiated action plans for symptoms with ongoing nurse support in patients with chronic obstructive pulmonary disease (COPD) and comorbidities: the COPE‐III study protocol. Contemporary Clinical Trials 2013;36(1):81‐9. [DOI] [PubMed] [Google Scholar]
Maltais 2008 {published data only}
- Maltais F, Bourbeau J, Shapiro S, Lacasse Y, Perrault H, Baltzan M, et al. Effects of home‐based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine 2008;149(12):869‐78. [DOI] [PubMed] [Google Scholar]
- NCT00169897. Pulmonary rehabilitation at home versus at the gymnasium. NCT00169897. https://clinicaltrials.gov/ct2/show/NCT00169897 (accessed 11 March 2016).
Miller 2010 {published data only}
- Miller GA, Lloyd S, McConnachie A, Bucknall C. Glasgow supported self management trial (GSuST) for moderate to severe COPD: a description of the patient cohort. American Journal of Respiratory and Critical Care Medicine. 2010; Vol. 181, issue Meeting Abstracts:A1512.
Monninkhof 2003 {published data only}
- Monninkhof E, Valk P, Palen J, Herwaarden C, Zielhuis G. Effects of a comprehensive self‐management programme in patients with chronic obstructive pulmonary disease. European Respiratory Journal 2003;22(5):815‐20. [DOI] [PubMed] [Google Scholar]
Newman 1995 {published data only}
- Newman AM, Smith MJ, Wiggins J. A study of disease comprehension in COPD patients and the effects of a simple education programme. European Respiratory Journal 1995;8(Suppl 19):525S. [Google Scholar]
Parenteau 2003 {published data only}
- Parenteau S, Scott AS, McKnight J, Menzies D, Bourbeau J. Impact of an action plan that emphasizes the prompt use of oral prednisone and antibiotics in COPD exacerbation [Abstract]. American Thoracic Society 99th International Conference; 2003 May 16‐21; Seattle. 2003:A108 [Poster:C62].
Rea 2004 {published data only}
- Rea H, McAuley S, Stewart A, Lamont C, Roseman P, Didsbury P. A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Internal Medicine Journal 2004;34:608‐14. [DOI] [PubMed] [Google Scholar]
- Wellingham J, Rea H. Reducing hospital demand through a single chronic disease management programme for COPD and associated co‐morbidity [Abstract]. IPCRG Congress; 2002 June 7‐9; Amsterdam. 2002:28.
Roberts 2007 {published data only}
- Roberts NJ, Partridge MR. Design and test of a pictorial COPD self‐management action plan. European Respiratory Journal 2007;30(Suppl):Abstract 3394. [Google Scholar]
Rowett 2005 {published data only}
- Rowett D, Cafarella S, Simmons S, Frith P. Increased self‐management capacity leads to improved health outcomes for chronic lung disease patients. European Resiratory Journal 2005;26(Suppl 49):72s. [Google Scholar]
Sedeno 2006 {published data only}
- Sedeno MF, Nault D, Hamd DH, Bourbeau J. A written action plan for early treatment of COPD exacerbations: an important component to the reduction of hospitalizations. Proceedings of the American Thoracic Society 2006;3:A603. [Google Scholar]
Sedeno 2009 {published data only}
- Sedeno MF, Nault D, Hamd DH, Bourbeau J. A self‐management education program including an action plan for acute COPD exacerbations. COPD 2009;6(5):352‐8. [DOI] [PubMed] [Google Scholar]
Siddique 2012 {published data only}
- Siddique HH, Olson RH, Parenti CM, Rector TS, Caldwell M, Dewan NA, et al. Randomized trial of pragmatic education for low‐risk COPD patients: impact on hospitalizations and emergency department visits. International Journal of Chronic Obstructive Pulmonary Disease 2012;7(1):719‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2014 {published data only}
- Song HY, Yong SJ, Hur HK. Effectiveness of a brief self‐care support intervention for pulmonary rehabilitation among the elderly patients with chronic obstructive pulmonary disease in Korea. Rehabilitation Nursing 2014;39(3):147‐56. [DOI] [PubMed] [Google Scholar]
Sridhar 2008 {published data only}
- Roberts NJ, Taylor R, Dawson S, Sridhar M, Partridge MR. Self management education in COPD leads to a cost effective reduction in need for unscheduled primary care consultations [Abstract]. American Journal of Respiratory and Critical Care Medicine 2007;175:A283. [Google Scholar]
- Sridhar M, Taylor R, Dawson S, Roberts NJ, Partridge MR. A nurse led intermediate care package in patients who have been hospitalised with an acute exacerbation of chronic obstructive pulmonary disease. Thorax 2008;63(3):194‐200. [DOI] [PubMed] [Google Scholar]
Uijen 2012 {published data only}
- Uijen AA, Bischoff E, Schellevis FG, Bor HHJ, Bosch W, Schers HJ. Continuity in different care modes and its relationship to quality of life: a randomised controlled trial in patients with COPD. British Journal of General Practice 2012;62:e422‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wakabayashi 2006 {published data only}
- Wakabayashi R, Kida K, Yamada K, Jones RCM, Hyland ME. A randomised controlled trial of a patient education programme versus normal care for COPD using the lung information needs questionnaire (LINQ) [Abstract]. European Respiratory Journal. 2006;28(Suppl 50):554s [P3192]. [Google Scholar]
Wittmann 2007 {published data only}
- Wittmann M, Spohn S, Schultz K, Pfeifer M, Petro W. Patient education in COPD during inpatient rehabilitation improves quality of life and morbidity [article in German]. Pneumologie 2007;10:636‐42. [DOI] [PubMed] [Google Scholar]
Worth 2004 {published data only}
- Worth H, Dhein Y. Does patient education modify behaviour in the management of COPD?. Patient Education and Counselling 2004;52(3):267‐70. [DOI] [PubMed] [Google Scholar]
Yu 2014 {published data only}
- Yu SH, Guo AM, Zhang XJ. Effects of self‐management education on quality of life of patients with chronic obstructive pulmonary disease. International Journal of Nursing Sciences 2014;1(1):53‐7. [Google Scholar]
References to ongoing studies
Doheny 2013 {published data only}
- Doheny S, Lynch A, Dunican K, Cabrera A, Silva M. The effectiveness of pharmacist‐provided self‐management education to patients with chronic obstructive pulmonary disease. Journal of the American Pharmacists Association 2013;53(2):e107. [Google Scholar]
Additional references
Access 2008
- Access Economics Pty Limited for The Australian Lung Foundation. Economic impact of COPD and cost effective solutions. The Australian Lung Foundation, 2008:1‐70. [Google Scholar]
Agusti 2010
- Agusti A, Calverley P, Celli B, Coxson H, Edwards L, Lomas D, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respiratory Research 2010;11:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
American Lung Association 2014
- American Lung Association. Chronic obstructive pulmonary disease (COPD) facts. http://www.lung.org/lung‐health‐and‐diseases/lung‐disease‐lookup/copd/learn‐about‐copd/what‐is‐copd.html (accessed 11 March 2016).
Bafadhel 2012
- Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo‐controlled trial. American Journal of Respiratory and Critical Care Medicine 2012;186(1):48‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnes 2009
- Barnes P, Celli B. Systemic manifestations and comorbidities of COPD. European Respiratory Journal 2009;33:1165‐85. [DOI] [PubMed] [Google Scholar]
Bourbeau 2009
- Bourbeau J, Palen J. Promoting effective self‐management programmes to improve COPD. European Respiratory Journal 2009;33:461‐3. [DOI] [PubMed] [Google Scholar]
Burgel 2010
- Burgel P, Paillasseur J, Caillaud D, Tillie‐Leblond I, Chanez P, Escamilla R, et al. Clinical COPD phenotypes: a novel approach using principal component and cluster analyses. European Respiratory Journal 2010;36:531‐9. [DOI] [PubMed] [Google Scholar]
Chhabra 2014
- Chhabra S, Dash D. Acute exacerbations of chronic obstructive pulmonary disease: causes and impacts. Indian Journal of Chest Diseases and Allied Sciences 2014;56:93‐104. [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ. Systematic reviews of evaluations of diagnostic and screening tests. British Medical Journal 2001;323(7305):157‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dowson 2004
- Dowson CA, Town GI, Frampton C, Mulder RT. Psychopathology and illness beliefs influence COPD self‐management. Journal of Psychosomatic Research 2004;56(3):333‐40. [DOI] [PubMed] [Google Scholar]
Effing 2012
- Effing T, Bourbeau J, Vercoulen J, Apter A, Coultas D, Meek P, et al. Self‐management programmes for COPD: moving forward. Chronic Respiratory Disease 2012;9:27‐35. [DOI] [PubMed] [Google Scholar]
Effing 2016
- Effing TW, Vercoulen JH, Bourbeau J, Trappenburg J, Lenferink A, Cafarella P, et al. Definition of a COPD self‐management intervention: International Expert Group consensus. European Respiratory Journal 2016;48(1):46‐54. [DOI] [PubMed] [Google Scholar]
Eisner 2008
- Eisner M, Blanc P, Yelin E, Sidney S, Katz P, Ackerson L, et al. COPD as a systemic disease: impact on physical functional limitations. The American Journal of Medicine 2008; Vol. 121:789‐96. [DOI] [PMC free article] [PubMed]
GOLD 2016
- Global Initiative for Chronic Obstructive Pulmonary Lung Disease. From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016. http://www.goldcopd.org/ (accessed 1 December 2016).
Halbert 2006
- Halbert R, Natoli J, Gano A, Badamgarav E, Buist A, Mannino D. Global burden of COPD: systematic review and meta‐analysis. European Respiratory Journal 2006;28:523‐32. [DOI] [PubMed] [Google Scholar]
Halpin 2012
- Halpin D, Decramer M, Celli B, Kesten S, Liu D, Tashkin D. Exacerbation frequency and course of COPD. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:653‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2013
- Han M. Clinical correlations of computed tomography imaging in chronic obstructive pulmonary disease. Annals of the American Thoracic Society 2013;10:131‐7. [DOI] [PubMed] [Google Scholar]
Hanania 2011
- Hanania N, Müllerova H, Locantore N, Vestbo J, Watkins M, Wouters E, et al. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. American Journal of Respiratory and Critical Care Medicine 2011;183:604‐11. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hogg 2004
- Hogg J. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. The Lancet 2004;364:709‐21. [DOI] [PubMed] [Google Scholar]
Kessler 2006
- Kessler R, Stǻhl E, Vogelmeier C, Haughney J, Trudeau E, Löfdahl C, et al. Patient understanding, detection, and experience of COPD exacerbations: an observational, interview‐based study. Chest 2006;130:133‐42. [DOI] [PubMed] [Google Scholar]
Kolbe 1996
- Kolbe J, Vamos M, Fergusson W, Elkind G, Garrett J. Differential influences on asthma self‐management knowledge and self‐management behavior in acute severe asthma. Chest 1996;110(6):1463‐8. [DOI] [PubMed] [Google Scholar]
Kruis 2013
- Kruis A, Smidt N, Assendelft W, Gussekloo J, Boland M, Molken M, et al. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2013;130(1):133‐42. [DOI] [PubMed] [Google Scholar]
Langsetmo 2008
- Langsetmo L, Platt R, Ernst P, Bourbeau J. Underreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohort. American Journal of Respiratory and Critical Care Medicine 2008;177:396‐401. [DOI] [PubMed] [Google Scholar]
Lenferink 2015
- Lenferink A, Brusse‐Keizer M, Valk PD, Frith PA, Zwerink M, Monninkhof EM, et al. Self management interventions including action plans for exacerbations versus usual care in people with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011682] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathers 2006
- Mathers C, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine 2006;3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 2015
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD003793.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Reilly 2006
- O'Reilly JF, Williams AE, Holt K, Rice L. Defining COPD exacerbations: impact on estimation of incidence and burden in primary care. Primary Care Respiratory Journal 2006;15(6):346‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pascoe 2015
- Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. The Lancet Respiratory Medicine 2015;3(6):435‐42. [DOI] [PubMed] [Google Scholar]
Rennard 2006
- Rennard S, Vestbo J. COPD: the dangerous underestimate of 15%. The Lancet 2006;367:1216‐9. [DOI] [PubMed] [Google Scholar]
Spencer 2004
- Spencer S, Calverley P, Burge P, Jones P. Impact of preventing exacerbations on deterioration of health status in COPD. European Respiratory Journal 2004;23:698‐702. [DOI] [PubMed] [Google Scholar]
Vestbo 2011
- Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. Changes in forced expiratory volume in 1 second over time in COPD. New England Journal of Medicine 2011;365(13):1184‐92. [DOI] [PubMed] [Google Scholar]
Vollenweider 2012
- Vollenweider DJ, Jarrett H, Steurer‐Stey CA, Garcia‐Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD010257] [DOI] [PubMed] [Google Scholar]
Walters 2012
- Walters EH, Walters J, Wills KE, Robinson A, Wood‐Baker R. Clinical diaries in COPD: compliance and utility in predicting acute exacerbations. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:427‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walters 2014
- Walters JAE, Gibson PG, Wood‐Baker R, Hannay M, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD001288.pub3] [DOI] [PubMed] [Google Scholar]
WHO 2004
- Epidemiology and Burden of Disease. The global burden of disease. http://www.who.int/topics/global_burden_of_disease/en/ (accessed 11 March 2016).
WHO 2008
- Global Health Observatory. World Health Statistics. http://www.who.int/gho/en/ (accessed 11 March 2016).
Wilkinson 2004
- Wilkinson TM, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2004;169:1298‐303. [DOI] [PubMed] [Google Scholar]
Zwerink 2014
- Zwerink M, Brusse‐Keizer M, Valk PD, Zielhuis GA, Monninkhof EM, Palen J, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD002990.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Turnock 2005
- Turnock AC, Walters EH, Walters JJAE, Wood‐Baker R. Action plans for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD005074; CD005074] [DOI] [PubMed] [Google Scholar]
Walters 2010
- Walters JAE, Turnock AC, Walters EH, Wood‐Baker R. Action plans with limited patient education only for exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2010, Issue 5 10.1002/14651858.CD005074.pub3. [DOI: 10.1002/14651858.CD005074.pub3] [DOI] [PubMed] [Google Scholar]


