Abstract
Background
Guidelines have provided positive recommendations for pulmonary rehabilitation after exacerbations of chronic obstructive pulmonary disease (COPD), but recent studies indicate that postexacerbation rehabilitation may not always be effective in patients with unstable COPD.
Objectives
To assess effects of pulmonary rehabilitation after COPD exacerbations on hospital admissions (primary outcome) and other patient‐important outcomes (mortality, health‐related quality of life (HRQL) and exercise capacity).
Search methods
We identified studies through searches of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, PEDro (Physiotherapy Evidence Database) and the Cochrane Airways Review Group Register of Trials. Searches were current as of 20 October 2015, and handsearches were run up to 5 April 2016.
Selection criteria
Randomised controlled trials (RCTs) comparing pulmonary rehabilitation of any duration after exacerbation of COPD versus conventional care. Pulmonary rehabilitation programmes had to include at least physical exercise (endurance or strength exercise, or both). We did not apply a criterion for the minimum number of exercise sessions a rehabilitation programme had to offer to be included in the review. Control groups received conventional community care without rehabilitation.
Data collection and analysis
We expected substantial heterogeneity across trials in terms of how extensive rehabilitation programmes were (i.e. in terms of number of completed exercise sessions; type, intensity and supervision of exercise training; and patient education), duration of follow‐up (< 3 months vs ≥ 3 months) and risk of bias (generation of random sequence, concealment of random allocation and blinding); therefore, we performed subgroup analyses that were defined before we carried them out. We used standard methods expected by Cochrane in preparing this update, and we used GRADE for assessing the quality of evidence.
Main results
For this update, we added 11 studies and included a total of 20 studies (1477 participants). Rehabilitation programmes showed great diversity in terms of exercise training (number of completed exercise sessions; type, intensity and supervision), patient education (from none to extensive self‐management programmes) and how they were organised (within one setting, e.g. pulmonary rehabilitation, to across several settings, e.g. hospital, outpatient centre and home). In eight studies, participants completed extensive pulmonary rehabilitation, and in 12 studies, participants completed pulmonary rehabilitation ranging from not extensive to moderately extensive.
Eight studies involving 810 participants contributed data on hospital readmissions. Moderate‐quality evidence indicates that pulmonary rehabilitation reduced hospital readmissions (pooled odds ratio (OR) 0.44, 95% confidence interval (CI) 0.21 to 0.91), but results were heterogenous (I2 = 77%). Extensiveness of rehabilitation programmes and risk of bias may offer an explanation for the heterogeneity, but subgroup analyses were not statistically significant (P values for subgroup effects were between 0.07 and 0.11). Six studies including 670 participants contributed data on mortality. The quality of evidence was low, and the meta‐analysis did not show a statistically significant effect of rehabilitation on mortality (pooled OR 0.68, 95% CI 0.28 to 1.67). Again, results were heterogenous (I2 = 59%). Subgroup analyses showed statistically significant differences in subgroup effects between trials with more and less extensive rehabilitation programmes and between trials at low and high risk for bias, indicating possible explanations for the heterogeneity. Hospital readmissions and mortality studies newly included in this update showed, on average, significantly smaller effects of rehabilitation than were seen in earlier studies.
High‐quality evidence suggests that pulmonary rehabilitation after an exacerbation improves health‐related quality of life. The eight studies that used St George's Respiratory Questionnaire (SGRQ) reported a statistically significant effect on SGRQ total score, which was above the minimal important difference (MID) of four points (mean difference (MD) ‐7.80, 95% CI ‐12.12 to ‐3.47; I2 = 64%). Investigators also noted statistically significant and important effects (greater than MID) for the impact and activities domains of the SGRQ. Effects were not statistically significant for the SGRQ symptoms domain. Again, all of these analyses showed heterogeneity, but most studies showed positive effects of pulmonary rehabilitation, some studies showed large effects and others smaller but statistically significant effects. Trials at high risk of bias because of lack of concealment of random allocation showed statistically significantly larger effects on the SGRQ than trials at low risk of bias. High‐quality evidence shows that six‐minute walk distance (6MWD) improved, on average, by 62 meters (95% CI 38 to 86; I2 = 87%). Heterogeneity was driven particularly by differences between studies showing very large effects and studies showing smaller but statistically significant effects. For both health‐related quality of life and exercise capacity, studies newly included in this update showed, on average, smaller effects of rehabilitation than were seen in earlier studies, but the overall results of this review have not changed to an important extent compared with results reported in the earlier version of this review.
Five studies involving 278 participants explicitly recorded adverse events, four studies reported no adverse events during rehabilitation programmes and one study reported one serious event.
Authors' conclusions
Overall, evidence of high quality shows moderate to large effects of rehabilitation on health‐related quality of life and exercise capacity in patients with COPD after an exacerbation. Some recent studies showed no benefit of rehabilitation on hospital readmissions and mortality and introduced heterogeneity as compared with the last update of this review. Such heterogeneity of effects on hospital readmissions and mortality may be explained to some extent by the extensiveness of rehabilitation programmes and by the methodological quality of the included studies. Future researchers must investigate how the extent of rehabilitation programmes in terms of exercise sessions, self‐management education and other components affects the outcomes, and how the organisation of such programmes within specific healthcare systems determines their effects after COPD exacerbations on hospital readmissions and mortality.
Plain language summary
Pulmonary rehabilitation for people who have been in hospital with an exacerbation of chronic obstructive pulmonary disease
Review question: We wished to compare the impact of pulmonary rehabilitation after an exacerbation of chronic obstructive pulmonary disease (COPD) on hospital readmissions and other patient‐important outcomes such as quality of life versus usual post‐exacerbation care.
Study characteristics: We included 20 studies involving 1477 participants with COPD. Rehabilitation programmes started in hospital in some trials and after discharge in others. These programmes showed great diversity in terms of exercise training (e.g. number of completed exercise sessions, type and intensity of exercise training), patient education (none to extensive self‐management programmes) and how programmes were organised (within one setting, e.g. pulmonary rehabilitation, to across several settings, e.g. hospital, outpatient centre and home).
Key results: Quality of life and exercise capacity were improved by rehabilitation, and the effect was substantially larger than the minimal important difference. Results for hospital readmissions and mortality were diverse, with some studies showing that pulmonary rehabilitation reduced hospital admissions and mortality compared with usual community care (no rehabilitation), and other studies not showing such effects.
Quality of the evidence: Uncertainty about reasons for differences across trials in terms of hospital readmissions and mortality led to downgrading of the quality of evidence (moderate‐quality evidence for reduction in hospital readmissions and low‐quality evidence for reduction in mortality). The quality of evidence was high for quality of life and exercise capacity.
Conclusion: Pulmonary rehabilitation improves quality of life and exercise capacity and is a safe intervention for patients with COPD after they have experienced an exacerbation. The reasons for diverse effects on hospital readmissions and mortality, however, are not fully clear. Future studies should explore whether the extent of the rehabilitation programme and the organisation of such programmes within specific healthcare systems (e.g. within the rehabilitation setting vs embedded in the continuum of care from hospital to home to outpatient care) determines the effects of rehabilitation after COPD exacerbations.
Summary of findings
Summary of findings for the main comparison. Pulmonary rehabilitation versus usual care.
| Pulmonary rehabilitation versus usual care for patients with COPD | ||||||
| Population: participants with COPD who had experienced a recent exacerbation Setting: inpatient, outpatient or home‐based Intervention: rehabilitation Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with rehabilitation | |||||
| Hospital readmission (to end of follow‐up, median 9 months) | High risk for 1‐year readmission | OR 0.44 (0.21 to 0.91) | 810 (8 RCTs) | ⊕⊕⊕⊝ Moderatea | ||
| 500 per 1000 | 306 per 1000 (174 to 476) | |||||
| Mortality (to end of follow‐up, median 12 months) | High risk for 1‐year mortality | OR 0.68 (0.28 to 1.67) | 670 (6 RCTs) | ⊕⊕⊝⊝ Lowa,b | None of the trials used mortality as a primary outcome, and none of the trials was powered to detect a meaningful effect of rehabilitation on mortality. | |
| 150 per 1000 | 107 per 1000 (47 to 228) | |||||
| Health‐related quality of life: St George's Respiratory Questionnaire ‐ SGRQ: total score (to end of follow‐up, median 5 months) | SGRQ score at beginning of rehabilitation was typically around 65 | Mean change from baseline in SGRQ Total score in the intervention group was 7.80 units lower (95% CI ‐12.12 to ‐3.47) | ‐ | 1003 (8 RCTs) | ⊕⊕⊕⊕ Highc | A lower score indicates better quality of life. |
| Change from baseline in 6‐minute walking test (to end of follow‐up, median 3 months) | 6‐Minute walking distance at beginning of rehabilitation was typically around 300 metres | Mean change from baseline in 6‐minute walking test in the intervention group was 62.38 metres more (95% CI 38.45 to 86.31) | ‐ | 819 (13 RCTs) | ⊕⊕⊕⊕ Highd | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded because of heterogeneity of treatment effects with unclear reasons.
bDowngraded because of large 95% CI crossing 1.0.
cStatistical testing of heterogeneity showed significant differences in results across trials, but we did not downgrade the quality because the heterogeneity does not affect interpretation of results. Studies did not have an active control, and participants were aware of group assignment, but we did not downgrade because this did not lower our confidence in the estimate of effect.
dUnexplained substantial statistical heterogeneity detected (I2 = 87%), but we did not downgrade the quality because the pooled effect is large and well above the minimal important difference for the 6‐minute walking test of 30 metres.
Background
Clinical guidelines and documents of the American Thoracic Society (ATS) and the European Respiratory Society (ERS) include positive recommendations for pulmonary rehabilitation after chronic obstructive pulmonary disease (COPD) exacerbations based on earlier versions of this systematic review and its included trials (BTS 2013; ERS ATS Statement 2013; GOLD 2016). However, recent studies indicate that post exacerbation rehabilitation may not always be effective. In addition, concerns have arisen that pulmonary rehabilitation may not be safe shortly after exacerbations of COPD. Therefore, our aim is to update our previous systematic review by assessing the effectiveness and safety of pulmonary rehabilitation after exacerbations of COPD.
The protocol for this Cochrane review was based on a previously published non‐Cochrane systematic review (Puhan 2005).
Description of the condition
Exacerbations and hospitalisations in patients with COPD represent a major health burden for both patients and healthcare systems in industrialised and developing countries (Chan‐Yeung 2004; Kessler 2006; Seemungal 1998; Sin 2002; Sullivan 2000). Acute exacerbations are the most common reason for hospital admissions and death among patients with COPD (Aaron 2014; Garcia‐Aymerich 2003; Mannino 2002; Piquet 2013; Soler‐Cataluna 2005). In addition, patients with COPD have reported reduced health‐related quality of life (HRQL) (Kessler 2006; Schlenk 1998) compared with the healthy population, which is further impaired by acute and repeated exacerbations (Seemungal 1998). Patients are at risk of early death and continued exacerbations requiring hospitalisation (Aaron 2014; Piquet 2013; Soler‐Cataluna 2005). Mortality rates during the year following a hospitalisation are around 35% (Almagro 2002; Connors 1996; Groenewegen 2003; Seneff 1995; Vitacca 2001), and rehospitalisation rates are around 60% (Connors 1996; Cydulka 1997; Escarrabill 2014; Groenewegen 2003; Martin 1982).
From the healthcare provider's perspective, COPD is resource‐consuming (Ford 2015; Jansson 2013; Sullivan 2000). Acute exacerbations are the cost drivers for COPD care, accounting for more than 70% of COPD‐related costs incurred as the result of emergency visits and hospitalisations (NHLBI 2001; Oostenbrink 2004; Sullivan 2000).
Description of the intervention
Position papers of the American College of Physicians, the American College of Chest Physicians, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) and the National Institute for Health and Care Excellence (NICE) have provided recommendations on acute care and follow‐up management for acute exacerbations (Amir 2011; GOLD 2016; NICE 2010). Pulmonary rehabilitation could play an important role in peri‐exacerbation management (management around the time of an exacerbation) because it combines several interventions that are known to improve health status and prognosis, such as physical exercise, smoking cessation, self‐management education, optimisation of medications and psychological and social support (BTS 2013; ERS ATS Statement 2013; Maddocks 2015; Puhan 2014). A large body of evidence on patients with stable COPD shows that pulmonary rehabilitation improves exercise capacity and HRQL (McCarthy 2015), and that it may be cost‐effective (ERS ATS Statement 2013; Griffiths 2001).
How the intervention might work
A multi‐disciplinary approach to pulmonary rehabilitation addresses multiple risk factors for hospital readmission and determinants of poor exercise capacity and quality of life. This combined effect may accelerate recovery from exacerbations and lower the risk of hospital readmission by improving exercise capacity, alleviating symptoms and promoting better self‐management.
Why it is important to do this review
COPD exacerbations are a major burden for patients, caregivers and society. Evaluation of the effectiveness and safety of post exacerbation strategies such as pulmonary rehabilitation could substantially lower the disease burden.
Objectives
To assess effects of pulmonary rehabilitation after COPD exacerbations on hospital admissions (primary outcome) and other patient‐important outcomes (mortality, HRQL and exercise capacity).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing pulmonary rehabilitation with conventional community care after acute exacerbations of COPD. We included studies reported as full text, those published as abstract only and unpublished data.
Types of participants
Participants with COPD after inpatient or outpatient care for acute exacerbation. This review required that more than 90% of study participants were patients with COPD.
Types of interventions
Any inpatient and/or outpatient pulmonary rehabilitation programme, including at least physical exercise (endurance or strength exercise, or both), delivered to patients who have received acute care for an exacerbation of COPD. The rehabilitation programme must commence immediately after initiation of exacerbation treatment or within three weeks of initiation of exacerbation treatment. We did not apply a criterion for the minimum number of exercise sessions to be included in the review because guideline recommendations provide no definition for when a programme qualifies as rehabilitation based on the number or type of exercise sessions. Rehabilitation programmes could include additional components such as self‐management education, psychological support, dietary advice and breathing exercises. We excluded from the review studies on pulmonary rehabilitation programmes that included only neuromuscular stimulation or inspiratory muscle training but no physical exercise programme. We included usual care control groups.
Types of outcome measures
Primary outcomes
Hospital admissions (at least one hospital admission during follow‐up)
Secondary outcomes
HRQL as measured by generic (e.g. Short Form (SF)‐36) or disease‐specific questionnaires (e.g. Chronic Respiratory Questionnaire (CRQ), St George's Respiratory Questionnaire (SGRQ))
Exacerbation rates (after discharge)
Number of outpatient visits
Length of readmissions
Mortality
Functional exercise capacity as measured by two‐, three‐, four‐, six‐ or 12‐minute‐walk test, or by a shuttle walk test
Maximal exercise capacity
Exercise endurance
Withdrawals
Adverse events
Costs
Search methods for identification of studies
Electronic searches
We detailed in Appendix 1 search methods used in the previous version of this review. The previously published version included searches up to March 2010. The search period for this update is March 2010 to October 2015.
For this update, we identified trials from the Cochrane Airways Review Group Specialised Register (CAGR), which is maintained by the Information Specialist for the Group. This Register contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED) and PsycINFO, and by handsearching of respiratory journals and meeting abstracts (see Appendix 2 for details). We searched all records in the CAGR using the search strategy presented in Appendix 3.
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/). We searched all databases from their inception to October 2015, with no restriction on language of publication. We screened the list of papers on pulmonary rehabilitation that is prepared bimonthly by the Rehabilitation and Chronic Care Group of the European Respiratory Society (ERS) and sent to its members (MP). We completed handsearching on 5 April 2016.
Searching other resources
We screened reference lists from included primary studies, review articles and conference proceedings of the American Thoracic Society (ATS) and the European Respiratory Society (ERS) (ERS ATS Statement 2013), and we contacted experts in the field to ask about additional published and unpublished studies. We applied no restrictions on the language of articles and completed handsearching on 5 April 2016.
Data collection and analysis
Selection of studies
Three review authors/contributors (MP, EGS, MS) independently assessed the titles and abstracts of all identified citations. Review authors recorded and then compared decisions (to order full‐text article or reject). We resolved disagreements by consensus with close attention to the inclusion/exclusion criteria. Three review authors/contributors (MP, EGS, MS) evaluated the full text of all potentially eligible papers and made a decision whether to include or exclude each study according to the inclusion and exclusion criteria specified above. We again resolved disagreements by consensus with close attention to the inclusion/exclusion criteria. We excluded all studies that did not fulfil all of the criteria and listed their bibliographic details, along with reasons for exclusion. A third review author (CC or TT) resolved discrepancies if two review authors disagreed.
Data extraction and management
Three independent review authors/contributors (MP, EGS, MS) independently screened the full texts of included studies and recorded details about study design, interventions, participants and outcome measures in a predefined Windows Excel form. We tested the data collection forms on a small sample of studies with strong likelihood for inclusion and exclusion. A third review author resolved disagreements. We registered bibliographic details such as study author, journal, year of publication and language.
Assessment of risk of bias in included studies
We assessed risk of bias in included studies as high, low or unclear using the Cochrane 'Risk of bias' tool (Higgins 2011) and the following risk types.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We recorded the initial degree of discordance between review authors and corrected discordant scores based on obvious errors. We resolved discordant scores based on real differences in interpretation through consensus or third party arbitration. Review authors were not blinded to names of study authors, institutions or journals nor to trial outcomes.
Measures of treatment effect
When possible, estimates and confidence limits were related to the minimal important difference (MID) (Schunemann 2005) for each outcome. We assessed whether estimates and 95% confidence limits for differences between study groups exceeded the MID (Chronic Respiratory Questionnaire ± 0.5 on seven‐point scales and St George’s Respiratory Questionnaire ± 4 points; Schunemann 2003) or represented an important effect (six‐minute walk distance ≥ 30 meters, which is based on a broad consensus and is less than the previous definition, and incremental shuttle walk test ≥ 47.5 meters; Holland 2014; Singh 2014).
Unit of analysis issues
The unit of analysis was the participant. We neither encountered nor expected any non‐standard study designs.
Dealing with missing data
We contacted study authors to obtain missing information.
Assessment of heterogeneity
We used forest plots to compare results across trials and the I2 statistic to measure heterogeneity among them. When we identified substantial heterogeneity, we reported this and explored possible causes by performing prespecified subgroup analyses (extent of rehabilitation programme, length of follow‐up (< 3 months vs ≥ 3 months)) and by analysing methodological items derived from the quality assessment (generation of random sequence, concealment of random allocation and blinding (low risk vs unclear or high risk). Previous versions of this review used length of follow‐up and methodological items (Puhan 2011). Compared with earlier versions of this review, investigators created extent of rehabilitation programmes as a new explanatory variable for heterogeneity (see below) on the basis of recent discussions (Hopkinson 2014; Maddocks 2015; Spruit 2014) and before meta‐analyses were carried out.
Pulmonary rehabilitation programmes can differ in many aspects, which may influence their effectiveness. Such programmes take place in inpatient, outpatient or home‐based settings; are of short (e.g. six weeks) or long (e.g. six months) duration and involve different intensity (e.g. training twice per week, daily training). Exercise training can include both endurance and strength training or either of the two. and many types of exercise training can be chosen to match the needs of patients. Pulmonary rehabilitation programmes also differ in terms of patient education offered, from basic advice to extensive self‐management programmes. Finally, adherence to a pulmonary rehabilitation programme determines the amount of training and education actually received by participants (e.g. attendance at 60% of planned exercise sessions).
Given the increasing diversity of pulmonary rehabilitation programmes and various ways to implement them in real‐world practice, we introduced a new reason to explain heterogeneity as part of the update of this systematic review. We assessed how extensive rehabilitation programmes were as a possible source of heterogeneity of trial results, and we stratified meta‐analyses by studies that offered an extensive pulmonary rehabilitation programme and studies that offered only moderately, slightly or not extensive pulmonary rehabilitation programmes (summarised as "less extensive" rehabilitation programmes). Review authors developed and used an approach not used before for assessment of the extent of rehabilitation programmes. When possible, we followed the statements and guidelines of national (British Thoracic Society; BTS 2013) and international societies (ERS and American Thoracic Society (ATS); ERS ATS Statement 2013). We did not upgrade or downgrade the extent of rehabilitation programmes if programme characteristics were in line with these statements and guidelines, but we downgraded or upgraded, respectively, the extent of programmes if some components were less than or exceeded what these guidance documents recommend. We considered pulmonary rehabilitation programmes to be extensive if:
participants followed, on average, at least 16 exercise training sessions, calculated as the total number of possible exercise training sessions times the (average) attendance rate. For example, if a programme was designed to include at least five exercise training sessions in the hospital, followed by a standard eight‐week outpatient programme with three sessions per week, 5 + 24 = 29 sessions were possible. If the attendance rate was 80%, participants followed, on average, 23 exercise training sessions. We selected a cut‐off of 16 exercise sessions based on duration of outpatient programmes of at least eight weeks, with two to three sessions per week and an attendance rate of 80% (thus 8*2.5 ‐ 4 = 16 sessions), as recommended by ERS and ATS (ERS ATS Statement 2013), rather than on the lower minimum number of sessions (≥ 12) recommended by BTS (BTS 2013);
they included two to three exercise training sessions per week, as recommended by ERS, ATS and BTS (ERS ATS Statement 2013, BTS 2013);
exercise training included at least endurance exercise (± strength exercise), as recommended by ERS, ATS and BTS (ERS ATS Statement 2013, BTS 2013); or
most exercise training sessions were supervised by physiotherapists or other trained health professionals, as recommended by ERS, ATS and BTS (ERS ATS Statement 2013, BTS 2013).
Similar to the GRADE approach, we downgraded the extent of pulmonary rehabilitation programmes for the following reasons (e.g. by ‐1 from extensive to moderately extensive).
By ‐1 if the total number of exercise training sessions was between 10 and 15, and by ‐2 if the total number of exercise training sessions was less than 10.
By ‐1 if fewer than 2 training sessions were provided per week.
By ‐1 if training was offered that is unlikely to modify the risk for hospital admissions and mortality, and is unlikely to improve health‐related quality of life and exercise capacity (e.g. only outdoor walking without the use of tests or parameters that would ensure training of at least moderate intensity, only strength exercise, less than 20 minutes of endurance training per session, other reasons).
By ‐1 if most exercise training sessions were not supervised by physiotherapists or other trained health professionals, and by ‐2 if most exercise training sessions (> 80%) were not supervised at all.
We upgraded the extent of pulmonary rehabilitation programmes for the following reasons.
By +1 if the total number of exercise training sessions was greater than 30.
By +1 if pulmonary rehabilitation programmes included an extensive self‐management programme (i.e. patient education about COPD, self‐monitoring, early action when exacerbations develop, written action plan, etc.).
Two review authors (EGS and MP) independently graded the pulmonary rehabilitation programmes of all included trials and resolved discrepancies in grading by discussion. If discrepancies remained, a third review author made the final decision.
Finally, we assessed how results changed with the addition of new studies and stratified analyses by studies included in the earlier version of this review versus studies added in this update.
Assessment of reporting biases
When we were able to pool more than 10 studies, we created and examined a funnel plot to explore possible small study and publication biases.
Data synthesis
We pooled trial results by calculating mean differences (MDs) and pooled odds ratios (ORs) using random‐effects models in Review Manager 5 (RevMan 2014).
'Summary of findings' table
We included a 'Summary of findings' table for the 2016 update of the review. We selected the following outcomes in consultation with the Cochrane Airways Review Group editorial team: hospital readmissions, mortality, SGRQ total score and six‐minute walk test.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to studies that contributed data to the meta‐analyses for prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) along with GRADEpro software (December 2015 version). We justified all decisions to downgrade or upgrade the quality of studies by using footnotes and made comments to aid the reader's understanding of the review when necessary.
Subgroup analysis and investigation of heterogeneity
We performed prespecified subgroup analyses when extent of the rehabilitation programme (extensive vs less extensive), length of follow‐up (< 3 months vs ≥ 3 months) and methodological items from the quality assessment (generation of random sequence, concealment of random allocation and blinding (low risk vs unclear or high risk) served as stratification variables (see Assessment of heterogeneity for details).
We used the formal test for subgroup interactions provided in Review Manager 5.3 (RevMan 2014).
Sensitivity analysis
We considered using a fixed‐effect model for sensitivity analyses but, given the heterogeneity of results across studies, we decided to use only a random‐effects model.
Results
Description of studies
Results of the search
In the original search, we identified 1759 citations through searches of electronic databases. We excluded 1740 citations after screening titles and abstracts and retrieved a total of 22 studies for detailed evaluation (19 obtained through searches of electronic databases and three via handsearching). We included six reports in the original review (Behnke 2000; Kirsten 1998; Man 2004; Murphy 2005; Nava 1998; Troosters 2002).
The search for the first update covered the period from July 2008 to March 2010. We identified 62 references through the electronic database search. We retrieved for full‐text assessment three articles from electronic databases and one via handsearching. We included three additional references (Carr 2009; Eaton 2009; Seymour 2010) in the review update.
The search for the most recent and current update covered the period from April 2010 to October 2015, with handsearches run to 5 April 2016. We identified 449 references through the electronic database search. We retrieved for full‐text assessment 20 references from electronic databases and two via handsearching. Figure 1 shows a study flow diagram. We included 11 additional studies (Borges 2014; Deepak 2014; Greening 2014; He 2015; Ko 2011; Tang 2012; Torres‐Sánchez 2014; Torres‐Sánchez 2015; Troosters 2010; Ko 2016; Liao 2015) in this review update.
1.
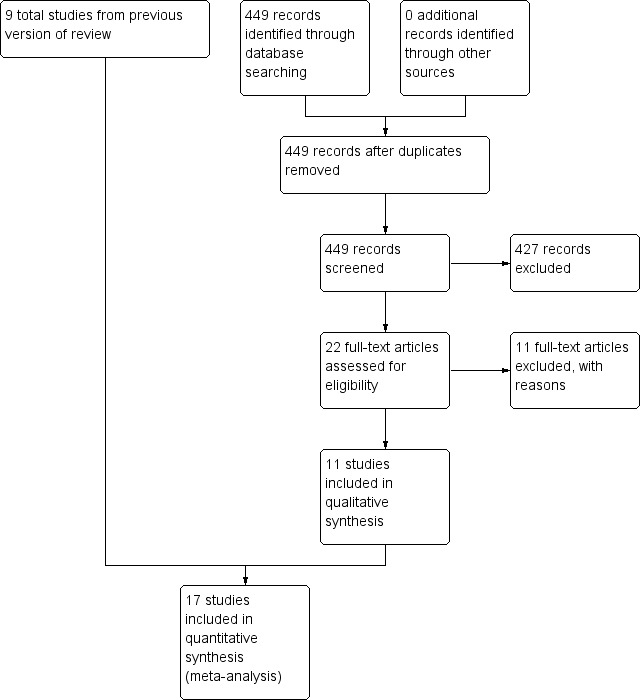
Study flow diagram.
Included studies
Twenty studies (drawn from 22 citations) met the eligibility criteria of this review. Eighteen studies were published in peer‐reviewed journals, one study as an abstract (Torres‐Sánchez 2014) and one as an abstract and as part of a full publication (Troosters 2002). The studies involved a total of 1477 participants who were in the recovery phase of a recent COPD exacerbation. Greening 2014 involved people with different respiratory conditions, however we were able to include the ITT data from COPD patients only; these data were provided to us by the authors upon request.
In 12 studies (Behnke 2000; Borges 2014; Eaton 2009; Greening 2014; He 2015; Kirsten 1998; Liao 2015; Nava 1998; Tang 2012; Torres‐Sánchez 2014; Torres‐Sánchez 2015; Troosters 2010), participants started inpatient pulmonary rehabilitation within two to eight days of hospital admission; in one study (Carr 2009), participants started an inpatient or outpatient rehabilitation programme; in six studies (Deepak 2014; Ko 2011; Ko 2016; Man 2004; Seymour 2010; Troosters 2002), outpatient rehabilitation was initiated after inpatient exacerbation treatment; and in one study (Murphy 2005), outpatient rehabilitation was started after "home from hospital care programme" for the exacerbation. Thirteen studies reported rehabilitation programme completion rates ranging from 40% to 94% (median, 77%). Only one study (Troosters 2010) provided details about the exacerbation treatment provided to participants (i.e. 32 mg oral corticosteroids for one week). For eight studies, we found that participants followed extensive pulmonary rehabilitation (Behnke 2000; Man 2004; Ko 2011; Ko 2016; He 2015; Nava 1998; Seymour 2010; Troosters 2002), and in seven studies, they completed moderately extensive pulmonary rehabilitation (Carr 2009; Eaton 2009; Greening 2014; Kirsten 1998; Liao 2015; Murphy 2005; Torres‐Sánchez 2015), whereas participants followed slightly extensive pulmonary rehabilitation in one study (Tang 2012) and pulmonary rehabilitation that was not extensive in two studies (Borges 2014; Troosters 2010). For two studies, we could not determine the extensiveness of the pulmonary rehabilitation programme (Deepak 2014; Torres‐Sánchez 2014). See Assessment of heterogeneity and Characteristics of included studies for details of the assessment of each included study (Table 2).
1. Extensiveness of pulmonary rehabilitation programmes of included trials.
| Study | Number of sessions | Trainings per week | Rehabilitation programme | Supervision of training | Extent of rehabilitation programme |
| Behnke 2000 | +1a (p 1185) | ‐ | ‐ | ‐1b (p 1185) | Extensive |
| Borges 2014 | ‐2c (p 1642) | ‐ | ‐1d (p 1639) | ‐ | Not extensive |
| Carr 2009 | ‐1e (p 320‐1) | ‐ | ‐ | ‐ | Moderately extensive |
| Deepak 2014 | Unclearf | Unclear | ‐ | ‐ | Unclear |
| Eaton 2009 | ‐2c (p 231‐2) | ‐ | +1g (p 231) | ‐ | Moderately extensive |
| Greening 2014 | ‐ | ‐ | +1g (p 3) | ‐2h (p 3‐4) | Moderately extensive |
| He 2015 | ‐ | Extensive | |||
| Kirsten 1998 | ‐ | ‐ | ‐ | ‐1b (p 1193) | Moderately extensive |
| Ko 2011 | ‐ | ‐ | ‐ | ‐ | Extensive |
| Ko 2016 | ‐ | ‐ | +1g (p 6) | ‐1b (p 7) | Extensive |
| Liao 2015 | ‐2c (p 1706) | ‐ | +1g (p 1705) | ‐ | Moderately extensive |
| Man 2004 | ‐ | ‐ | +1f (p 2) | ‐ | Extensive |
| Murphy 2005 | ‐1e (p 1298) | ‐ | ‐ | ‐ | Moderately extensive |
| Nava 1998 | +1a (p 850‐1) | ‐ | ‐ | ‐ | Extensive |
| Seymour 2010 | ‐1e (p 423 & 425) | ‐ | +1f (p 423) | ‐ | Extensive |
| Tang 2012 | ‐2c (p 164 & 167) | ‐ | ‐ | ‐ | Slightly extensive |
| Torres‐Sánchez 2014 | Unclear | Unclear | Unclear | Unclear | Unclear |
| Torres‐Sánchez 2015 | ‐1e (p 3) | ‐ | ‐ | ‐ | Moderately extensive |
| Troosters 2002 | +1a (p 208‐9) | ‐ | ‐ | ‐ | Extensive |
| Troosters 2010 | ‐2c (p 1073‐4) | ‐ | ‐1d (p 1073) | ‐ | Not extensive |
Explanations for downgrading and upgrading
a(> 30 sessions).
bSome training sessions unsupervised.
c< 10 exercise training sessions.
dOnly strength training.
e10 to 15 exercise training sessions.
f14 weeks, but unclear number of sessions per week.
gComprehensive self‐management training.
hMostly unsupervised training (> 80% of all sessions).
Excluded studies
The main reason for study exclusion was that the study population did not have COPD. We recorded reasons for exclusion of 10 studies in the Characteristics of excluded studies table.
Risk of bias in included studies
For details about risk of bias judgements and an overview of judgements across studies, see the Characteristics of included studies tables (Figure 2).
2.
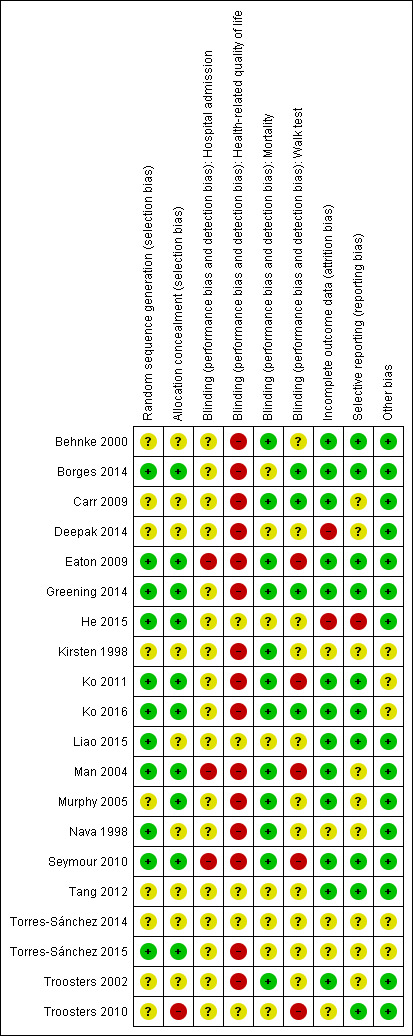
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
When reported, available information regarding treatment group assignment and allocation concealment indicated low risk of bias.
Blinding
Participants could not be blinded in these studies; this fact may have introduced bias for outcomes such as health‐related quality of life, but it is less likely to be an important source of bias for mortality and hospital readmission. Outcome assessors could be blinded for outcomes such as exercise endurance or six‐minute walk distance, and three studies described such blinding (Borges 2014; Carr 2009; Greening 2014).
Incomplete outcome data
Some studies did not assess the outcomes of participants who dropped out of rehabilitation programmes or were lost to follow‐up. However, reported study flows suggest that the extent of attrition bias is likely to be small.
Selective reporting
We found no evidence of reporting bias.
Other potential sources of bias
We identified no other potential sources of bias. We did not create a funnel plot for the primary outcome, as fewer than 10 studies contributed to this outcome.
Effects of interventions
See: Table 1
Hospital readmissions
Eight studies involving 810 participants (Behnke 2000; Eaton 2009; Greening 2014; Ko 2011; Ko 2016; Man 2004; Murphy 2005; Seymour 2010) contributed data on hospital readmissions. The follow‐up period for these studies ranged from three to 18 months, with a median duration of nine months. Moderate‐quality evidence (Table 1) shows that pulmonary rehabilitation reduced hospital readmission (pooled odds ratio (OR) 0.44, 95% confidence interval (CI) 0.21 to 0.91; Figure 3). However, the results were heterogenous (I2 = 77%), with four studies showing large and statistically significant reductions in the risk of hospital admission associated with pulmonary rehabilitation, and four studies showing no effect. Although subgroup analyses performed to investigate heterogeneity showed no statistical significance (P < 0.05), extensiveness of rehabilitation programmes and methodological quality may explain heterogeneity, and length of follow‐up may not (Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11). Figure 4 shows that studies newly included in this update reported, on average, smaller effects of rehabilitation than were noted in earlier studies.
3.
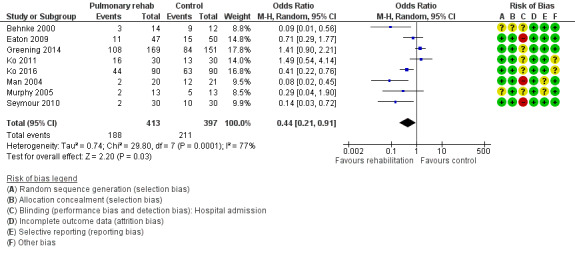
Forest plot of comparison: 1 Rehabilitation versus control, outcome: 1.1 Hospital readmission (to end of follow‐up).
1.7. Analysis.
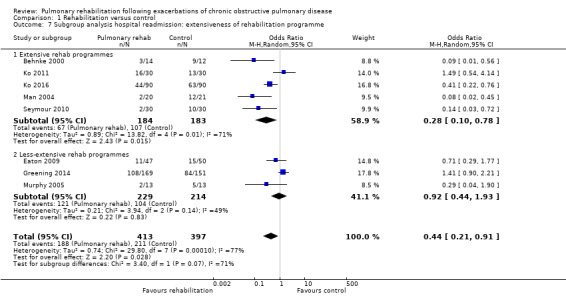
Comparison 1 Rehabilitation versus control, Outcome 7 Subgroup analysis hospital readmission: extensiveness of rehabilitation programme.
1.8. Analysis.
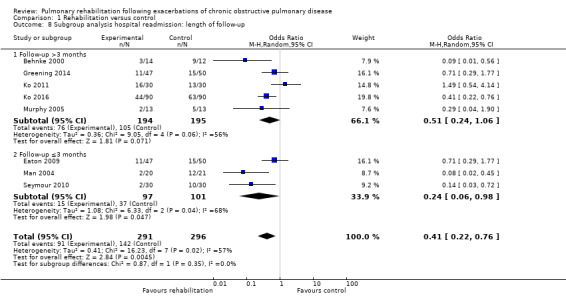
Comparison 1 Rehabilitation versus control, Outcome 8 Subgroup analysis hospital readmission: length of follow‐up.
1.9. Analysis.
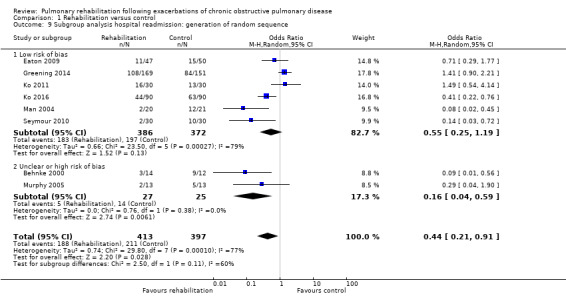
Comparison 1 Rehabilitation versus control, Outcome 9 Subgroup analysis hospital readmission: generation of random sequence.
1.10. Analysis.
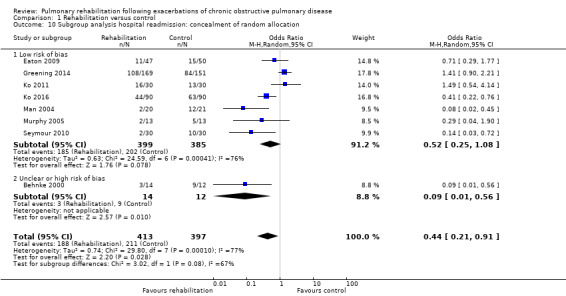
Comparison 1 Rehabilitation versus control, Outcome 10 Subgroup analysis hospital readmission: concealment of random allocation.
1.11. Analysis.
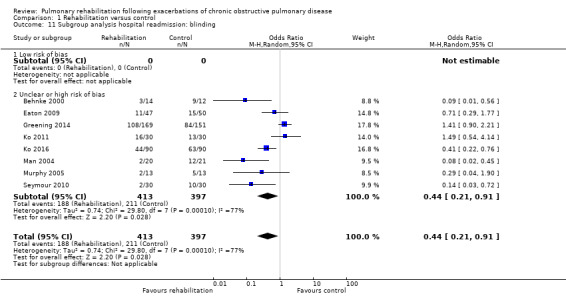
Comparison 1 Rehabilitation versus control, Outcome 11 Subgroup analysis hospital readmission: blinding.
4.
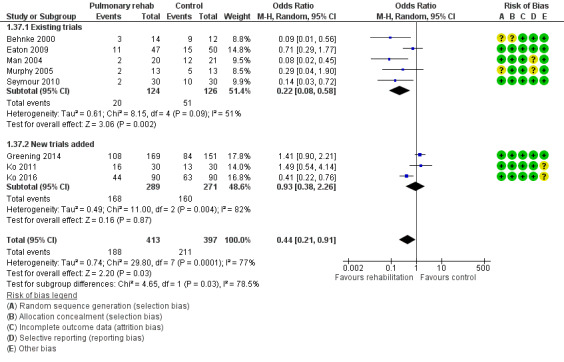
Forest plot of comparison: 1 Rehabilitation versus control, outcome: 1.37 Hospital readmission (to end of follow‐up) with separated new trial data.
Mortality
Six studies including 670 participants contributed data on mortality (Behnke 2000; Greening 2014; Ko 2011; Ko 2016; Man 2004; Troosters 2002). The follow‐up period for these studies ranged from three to 48 months, with a median duration of 12 months. The quality of evidence was low (Table 1), and meta‐analysis showed no statistically significant effects of rehabilitation on mortality (pooled OR 0.68, 95% CI 0.28 to 1.67; Figure 5). Again, results were heterogenous (I2 = 59%), with one study showing reduced mortality, one study excessive mortality and four no effect. Subgroup analyses showed statistically significant differences in subgroup effects between studies with more and less extensive rehabilitation programmes (Analysis 1.12) and between studies at low and high risk of bias (Analysis 1.14; Analysis 1.15), suggesting explanations for the heterogeneity, but length of follow‐up did not explain heterogeneity (Analysis 1.13). As for hospital readmissions, Figure 6 shows that studies newly included in this update reported, on average, smaller effects of rehabilitation on mortality than were noted in earlier studies.
5.
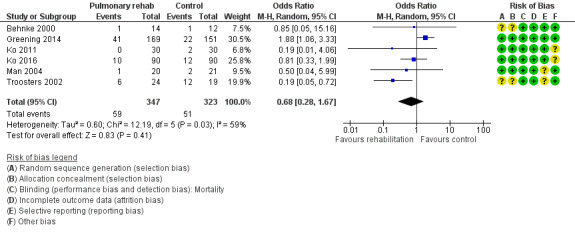
Forest plot of comparison: 1 Rehabilitation versus control, outcome: 1.2 Mortality.
1.12. Analysis.
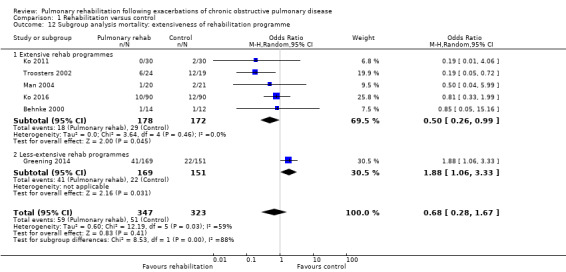
Comparison 1 Rehabilitation versus control, Outcome 12 Subgroup analysis mortality: extensiveness of rehabilitation programme.
1.14. Analysis.
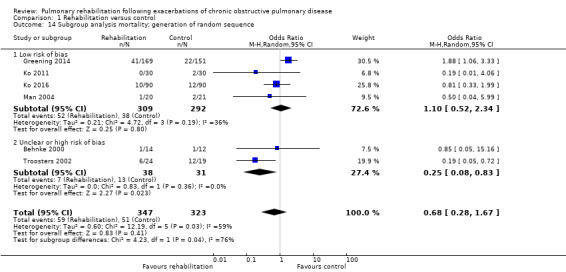
Comparison 1 Rehabilitation versus control, Outcome 14 Subgroup analysis mortality: generation of random sequence.
1.15. Analysis.
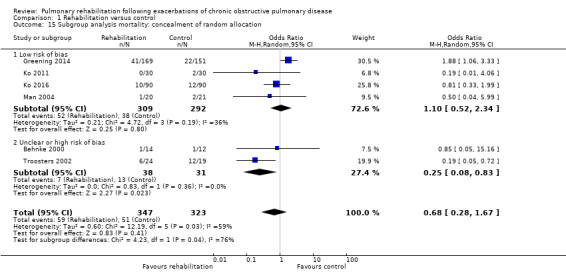
Comparison 1 Rehabilitation versus control, Outcome 15 Subgroup analysis mortality: concealment of random allocation.
1.13. Analysis.
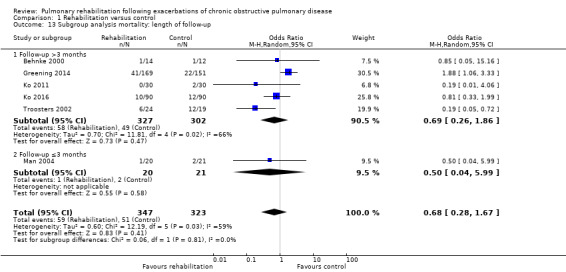
Comparison 1 Rehabilitation versus control, Outcome 13 Subgroup analysis mortality: length of follow‐up.
6.
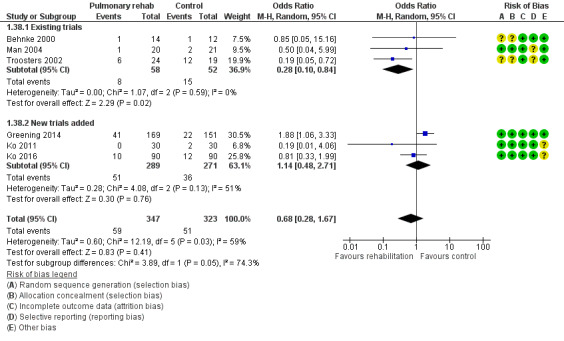
Forest plot of comparison: 1 Rehabilitation versus control, outcome: 1.38 Mortality with separated new trial data.
Health‐related quality of life
Two instruments were used to measure HRQL: The CRQ was used in five studies involving 259 participants (Behnke 2000; Carr 2009; Eaton 2009; Man 2004; Seymour 2010), and the SGRQ was used in eight studies involving 846 participants (Borges 2014; Deepak 2014; Greening 2014; Ko 2011; Ko 2016; Man 2004; Murphy 2005; Seymour 2010).
High‐quality evidence indicates that pulmonary rehabilitation after an exacerbation improves health‐related quality of life (Table 1). The eight studies that used the SGRQ reported a statistically significant effect on total score, which was above the MID of four points (mean difference (MD) ‐7.80, 95% CI ‐12.12 to ‐3.47; Figure 7). Statistically significant and important effects (greater than MID) were also observed for the impact and activities domains of the SGRQ and for the dyspnoea, fatigue and emotional function domains of the CRQ (Analysis 1.3). Effects were not statistically significant for SGRQ symptoms nor for CRQ mastery domains. Again, heterogeneity was evident in all of these analyses, but most studies showed positive effects of pulmonary rehabilitation, with some studies observing large effects and others smaller but statistically significant effects. Extensive rehabilitation programmes showed larger effects than less extensive rehabilitation programmes, but differences between subgroups of trials (extensive vs less extensive programmes) were not statistically significant for CRQ (Analysis 1.17) nor for SGRQ (Analysis 1.22). Subgroup analyses comparing trials with respect to length of follow‐up were inconsistent. Although trials of short duration noted a smaller effect on the CRQ (Analysis 1.18), investigators reported a larger effect on the SGRQ (Analysis 1.23). Trials at high risk of bias with respect to concealment of random allocation showed statistically significantly larger effects on the SGRQ (Analysis 1.25), but other subgroup analyses revealed no statistically significant effects. Studies newly included in this update showed, on average, smaller effects of rehabilitation than were noted in earlier trials (Figure 8), but overall results did not change to an important extent compared with the earlier version of this review. One study involving 49 obese COPD participants (Torres‐Sánchez 2015) used the EuroQol 5D instrument and found statistically significant effects of rehabilitation for the domains of self‐care, usual activities, anxiety and depression, and for the visual analogue scale, but no effect for mobility and pain/discomfort domains.
7.
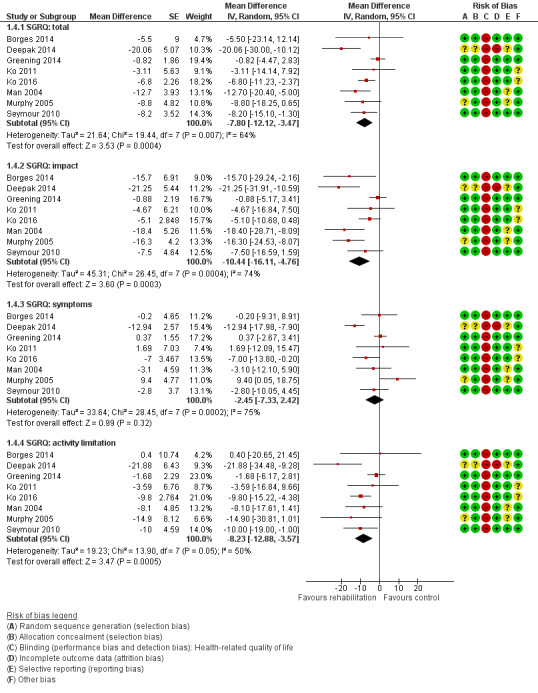
Forest plot of comparison: 1 Rehabilitation versus control, outcome: 1.4 Health‐related quality of life: St George's Respiratory Questionnaire.
1.3. Analysis.
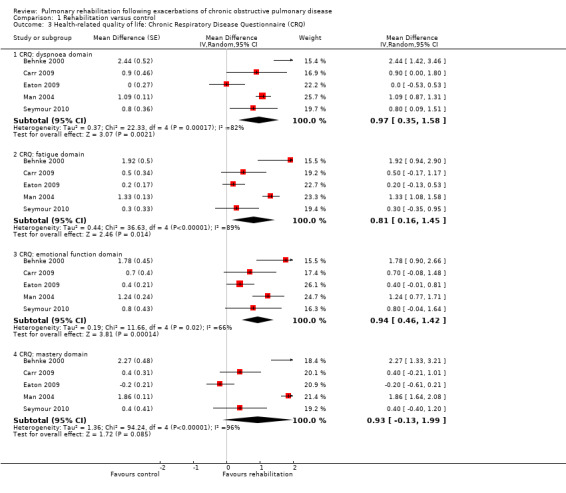
Comparison 1 Rehabilitation versus control, Outcome 3 Health‐related quality of life: Chronic Respiratory Disease Questionnaire (CRQ).
1.17. Analysis.
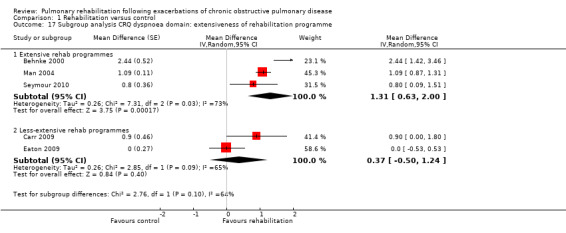
Comparison 1 Rehabilitation versus control, Outcome 17 Subgroup analysis CRQ dyspnoea domain: extensiveness of rehabilitation programme.
1.22. Analysis.
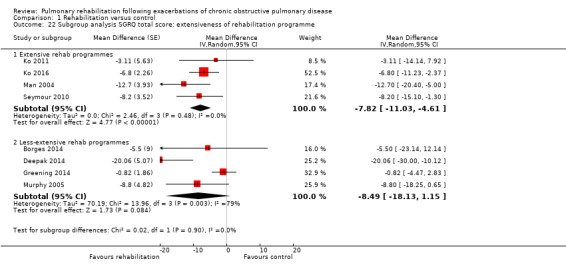
Comparison 1 Rehabilitation versus control, Outcome 22 Subgroup analysis SGRQ total score: extensiveness of rehabilitation programme.
1.18. Analysis.
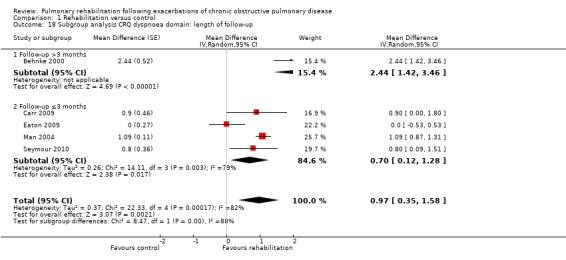
Comparison 1 Rehabilitation versus control, Outcome 18 Subgroup analysis CRQ dyspnoea domain: length of follow‐up.
1.23. Analysis.
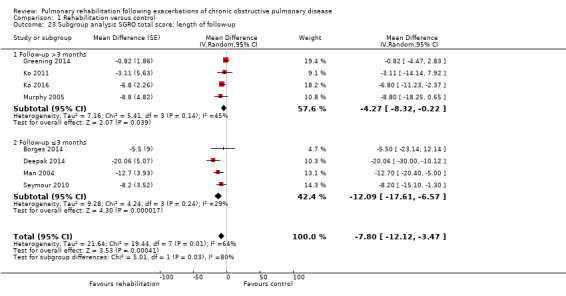
Comparison 1 Rehabilitation versus control, Outcome 23 Subgroup analysis SGRQ total score: length of follow‐up.
1.25. Analysis.
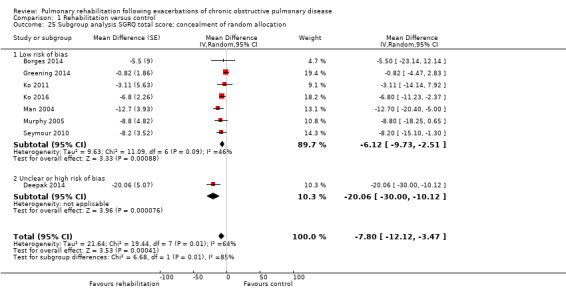
Comparison 1 Rehabilitation versus control, Outcome 25 Subgroup analysis SGRQ total score: concealment of random allocation.
8.
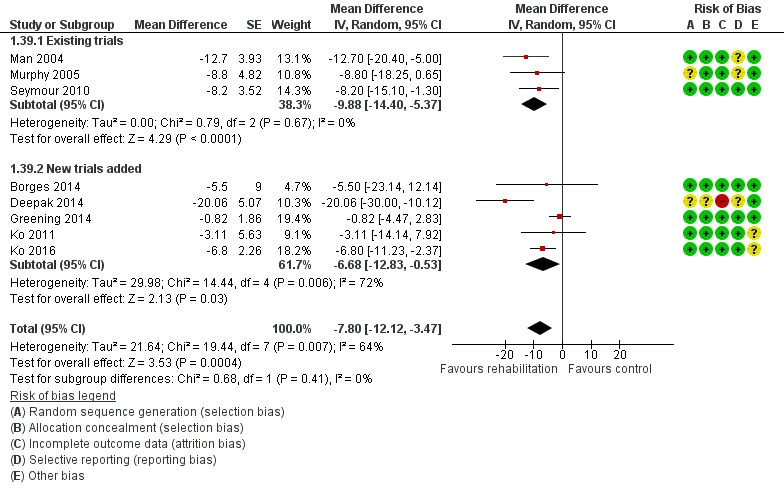
Forest plot of comparison: 1 Rehabilitation versus control, outcome: 1.39 Health‐related quality of life: SGRQ total with separated new trial data.
Exercise capacity
Thirteen studies involving 819 participants used the six‐minute walk test (Behnke 2000; Borges 2014; Carr 2009; Deepak 2014; Eaton 2009; He 2015; Kirsten 1998; Ko 2011; Ko 2016; Liao 2015; Nava 1998; Troosters 2002; Troosters 2010), and four studies involving 448 participants used the shuttle walk test to measure exercise capacity (Greening 2014; Man 2004; Murphy 2005; Seymour 2010). One study used the three‐minute walk test (Tang 2012).
High‐quality evidence (Table 1) shows that six‐minute walk distance (6MWD) improved, on average, by 62 meters (95% CI 38 to 86; Figure 9) and shuttle walk test distance by 48 meters (95% CI ‐1 to 97; Analysis 1.6); these findings were not statistically significant. Again, much heterogeneity was evident, but most studies showed positive effects of pulmonary rehabilitation, and heterogeneity was driven particularly by differences between studies showing very large effects and studies showing smaller but statistically significant effects. Subgroup analysis comparing trials at low and high risk for bias with respect to concealment of random allocation (Analysis 1.30) showed statistically significantly smaller effects in trials at low risk of bias. Studies at high risk of bias, because they lacked blinding, showed statistically significantly larger effects on the shuttle walk test (Analysis 1.36), but no other subgroup analyses revealed a reason for heterogeneity (Analysis 1.27; Analysis 1.28; Analysis 1.29; Analysis 1.31 for 6MWD; Analysis 1.32; Analysis 1.33; Analysis 1.34; Analysis 1.35 for shuttle walk test). Three‐minute walk distance increased more in the low‐intensity exercise group than in the control group (effect size 0.4, 95% CI ‐0.5 to 1.3) or the high‐intensity exercise group (effect size 0.6, 95% CI ‐0.3 to 1.5), but the differences were not statistically significant (Tang 2012). One study involving 49 obese patients with COPD (Torres‐Sánchez 2015) used the EuroQol 5D instrument and found statistically significant effects of rehabilitation for the domains of self‐care, usual activities, anxiety and depression and for the two‐minute step‐in‐place test performed to assess exercise capacity, as well as a statistically significant effect of rehabilitation on the number of repetitions performed (increase of 17.6 vs 4.9 repetitions, with 47 repetitions reported at baseline (both groups)).
9.
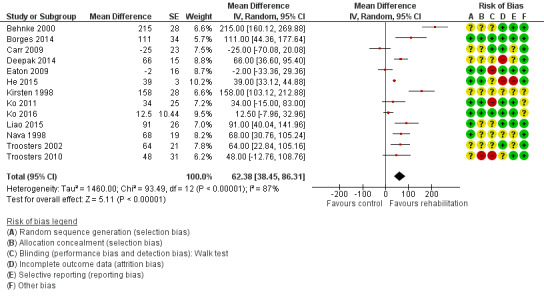
Forest plot of comparison: 1 Rehabilitation versus control, outcome: 1.5 Change from baseline in 6‐minute walking test.
1.6. Analysis.
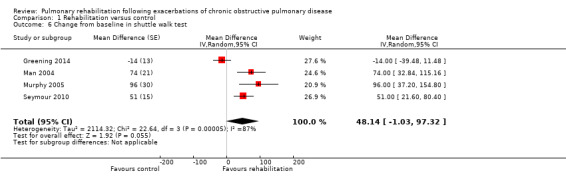
Comparison 1 Rehabilitation versus control, Outcome 6 Change from baseline in shuttle walk test.
1.30. Analysis.
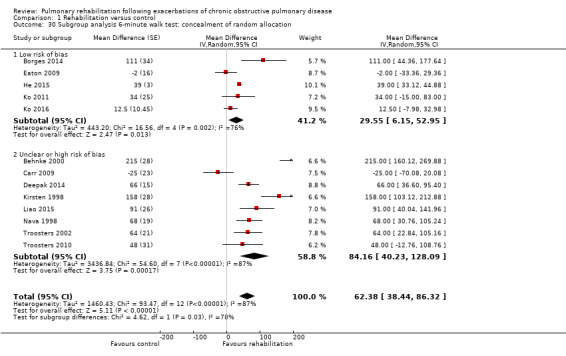
Comparison 1 Rehabilitation versus control, Outcome 30 Subgroup analysis 6‐minute walk test: concealment of random allocation.
1.36. Analysis.
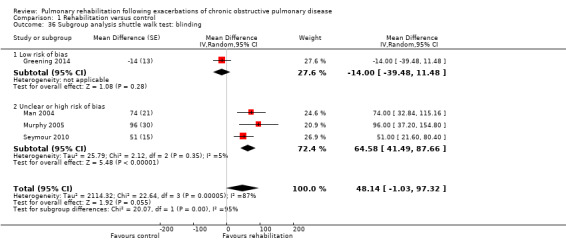
Comparison 1 Rehabilitation versus control, Outcome 36 Subgroup analysis shuttle walk test: blinding.
1.27. Analysis.
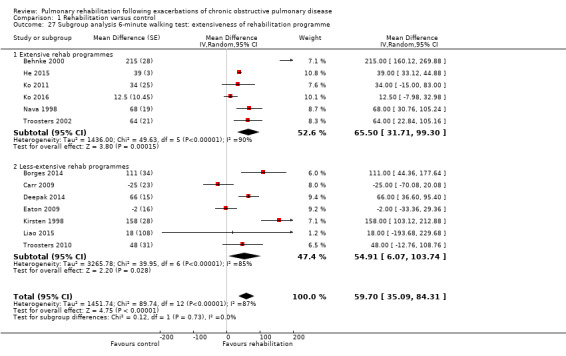
Comparison 1 Rehabilitation versus control, Outcome 27 Subgroup analysis 6‐minute walking test: extensiveness of rehabilitation programme.
1.28. Analysis.
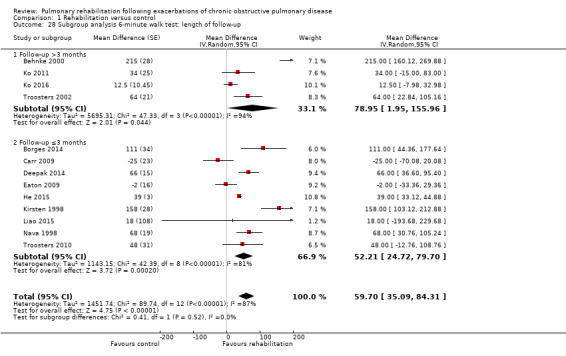
Comparison 1 Rehabilitation versus control, Outcome 28 Subgroup analysis 6‐minute walk test: length of follow‐up.
1.29. Analysis.
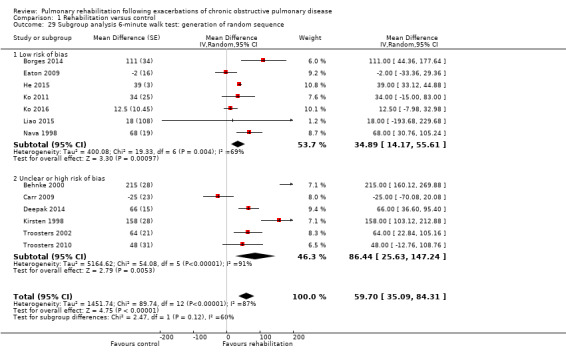
Comparison 1 Rehabilitation versus control, Outcome 29 Subgroup analysis 6‐minute walk test: generation of random sequence.
1.31. Analysis.
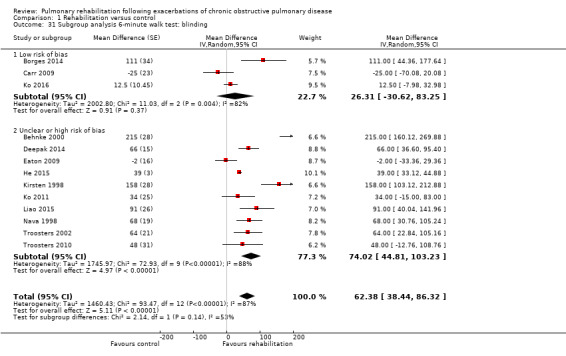
Comparison 1 Rehabilitation versus control, Outcome 31 Subgroup analysis 6‐minute walk test: blinding.
1.32. Analysis.
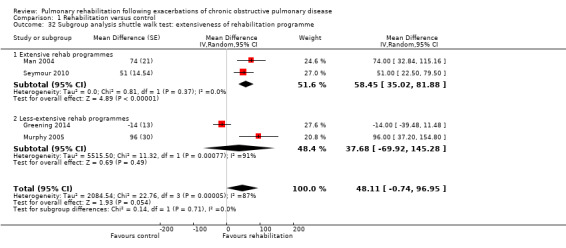
Comparison 1 Rehabilitation versus control, Outcome 32 Subgroup analysis shuttle walk test: extensiveness of rehabilitation programme.
1.33. Analysis.
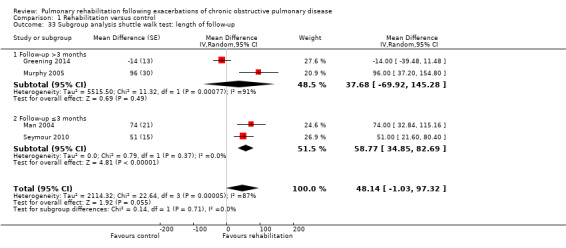
Comparison 1 Rehabilitation versus control, Outcome 33 Subgroup analysis shuttle walk test: length of follow‐up.
1.34. Analysis.
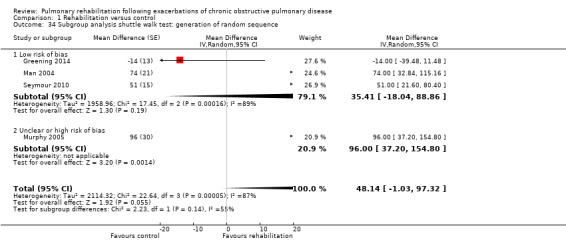
Comparison 1 Rehabilitation versus control, Outcome 34 Subgroup analysis shuttle walk test: generation of random sequence.
1.35. Analysis.
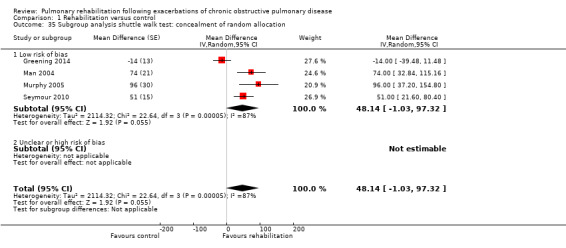
Comparison 1 Rehabilitation versus control, Outcome 35 Subgroup analysis shuttle walk test: concealment of random allocation.
Adverse events
Five studies involving 278 participants explicitly recorded adverse events (Behnke 2000;Eaton 2009; He 2015; Man 2004; Tang 2012). Four studies reported no adverse events during rehabilitation programmes. whereas one study (Tang 2012) reported one serious event that occurred when a participant felt unwell, but symptoms resolved within one hour and the participant continued with the rehabilitation programme.
Discussion
Summary of main results
Overall evidence of high quality shows moderate to large effects of rehabilitation on health‐related quality of life and exercise capacity in participants with chronic obstructive pulmonary disease (COPD) that are well above the minimal important difference (MID) for the Chronic Respiratory Questionnaire (CRQ), St George's Respiratory Questionnaire (SGRQ), the six‐minute walk distance test (6MWD) and the shuttle walk distance test (Holland 2014; Jones 2005; Schunemann 2003; Schunemann 2005; Singh 2014). Some recent studies showed no significant effect of rehabilitation on hospital readmissions and mortality. and introduced heterogeneity as compared with the last update of this review. Such heterogeneity of effects on hospital readmissions and mortality is not fully understood at this point, which explains why review authors assigned only moderate quality to evidence showing statistically significant effects of rehabilitation on hospital readmissions, and low quality to evidence revealing its not statistically significant effect on mortality.
Overall completeness and applicability of evidence
The update of this systematic review was substantial in that review authors included 11 additional studies, and this more than doubled the number of included study participants. Updated meta‐analyses that include a diverse set of trials informed the recent debate about how pulmonary rehabilitation has to be delivered to be beneficial for patients after acute exacerbations of COPD (Maddocks 2015). This debate began because more recent trials (Carr 2009; Eaton 2009; Greening 2014; Ko 2011) showed smaller or no effects of pulmonary rehabilitation after acute exacerbations of COPD compared with earlier versions of this systematic review (Puhan 2011). As we argued earlier (Puhan 2011), small trials tend to overestimate the effect of an intervention compared with large trials (Cappelleri 1996; Ioannidis 1998; Kjaergard 2001; LeLorier 1997). This phenomenon may be attributed in part to a publication bias, that is, the fact that small trials are more likely to be published if they show statistically significant treatment effects (Egger 1998). On the other hand, methodological shortcomings of small trials such as inadequate generation of the randomisation code, insufficient concealment of random allocation and lack of blinding may contribute to discrepancies between the results of single large trials and pooled estimates based on small trials (Kjaergard 2001). In our systematic review, included trials had methodological limitations, and some subgroup analyses revealed that risk of bias explains some of the heterogeneity noted for different outcomes. Hence, it cannot be excluded that estimates provided by the meta‐analyses may represent overestimations of the effect of pulmonary rehabilitation after an acute exacerbation.
Indeed, the largest trial, which included 320 participants, showed no benefit of pulmonary rehabilitation for people with COPD as per separate data provided on this population (Greening 2014). However, this trial has been criticized for not offering an extensive pulmonary rehabilitation programme (Hopkinson 2014; Spruit 2014). Participants in the intervention group followed, on average, 2.6 supervised sessions during hospital admission, then received largely unsupervised training after discharge. Some may argue that we should not have included this trial in this systematic review because the intervention was not designed or implemented as a rehabilitation programme that is extensive enough to have an effect on hospital readmissions, mortality and other outcomes. It is difficult to draw a line to show when a programme qualifies as a pulmonary rehabilitation programme in accordance with international standards (ERS ATS Statement 2013), so we decided to use rather inclusive trial eligibility criteria. Such an approach offers the opportunity to explore reasons for heterogeneity across trials, which may be highly informative for practice. For this purpose, we applied a scoring approach to assess the extensiveness of a pulmonary rehabilitation programme (using addition and subtraction of points in a way that is similar to the GRADE approach). When developing this approach, we recognised that multiple criteria should be used rather than a single criterion, such as the number of completed training sessions or the combination of endurance and strength exercise. A single criterion is not sufficient for evaluation of complex interventions such as pulmonary rehabilitation, wherein multiple components act synergistically and introduce the risk of mis‐classifying studies. Therefore, we considered the number of exercise training sessions, the frequency of exercise training and type and supervision of training, as well as self‐management education, in assessing how extensive pulmonary rehabilitation programmes were (Assessment of heterogeneity). As much as possible, we aligned the cut‐offs for upgrading and downgrading the extensiveness of rehabilitation programmes with the recent European Respiratory Society (ERS)‐American Thoracic Society (ATS) statement (ERS ATS Statement 2013) and British Thoracic Society (BTS) guidelines on pulmonary rehabilitation (BTS 2013). Although two independent review authors assessed programmes and sought consensus, we cannot exclude that others may classify some programmes differently. However, Table 2 presents all reasons for downgrading or upgrading of evidence for each study.
Results of this systematic review suggest that it may matter how pulmonary rehabilitation is delivered. The eight trials that offered and implemented an extensive programme showed mostly large and consistent effects on readmissions, health‐related quality of life and exercise capacity while also suggesting an effect on mortality. Although the programmes of these eight trials differed (see Characteristics of included studies and Table 2), all offered many training sessions (Behnke 2000; Nava 1998; Troosters 2002) or programmes long in duration (Behnke 2000; Troosters 2002), or they added extensive self‐management education to the exercise programme (Ko 2016; Man 2004; Seymour 2010). The results of less extensive programmes are also important because some reflect barriers for implementation and uptake of pulmonary rehabilitation after acute exacerbations of COPD. For example, today's hospital admission for a COPD exacerbation is often too short in duration to permit initiation of a programme. Also, patients who are admitted are often old and have multiple conditions, which may render the uptake of pulmonary rehabilitation difficult. The transition from the inpatient to the outpatient setting and the organisation required along the continuum of care are challenging, and patients may not continue with rehabilitation or may not start at all. In some countries, reimbursement schemes do not allow for extensive rehabilitation programmes. All of these challenges have been recently summarised and discussed (ERS ATS Statement 2013).
The applicability of current evidence also requires consideration that the group of patients willing or motivated by their healthcare professionals to participate in rehabilitation is probably quite a select one. This does not preclude that patients with COPD in general would benefit from rehabilitation after an exacerbation, but one should be cautious in judging the applicability of the results of this systematic review and should consider local circumstances and barriers. Conducting trials on pulmonary rehabilitation after an exacerbation is challenging. First, recruitment of participants is difficult because many may not wish to be randomly allocated to different types of post exacerbation management in a situation of poor health status (Benzo 2015). One trial on pulmonary rehabilitation after an exacerbation was stopped because only a few participants could be recruited (Van den Berg 2015). Recruitment was very slow in one trial comparing rehabilitation after exacerbation with rehabilitation in a stable pulmonary state (Puhan 2012), and another trial had to be stopped before the recruitment target was reached (Spaar 2009). Second, individuals willing to participate in a trial are likely to have a preference for pulmonary rehabilitation. If they are randomised to the control group or to rehabilitation after a period of time, they might ask for pulmonary rehabilitation at any time during follow‐up. Given the clear benefits of this intervention for patients in a stable condition as confirmed in meta‐analyses (McCarthy 2015), patients who experience an exacerbation can hardly be refused access to rehabilitative strategies. Whatever design investigators choose, a careful discussion of ethical and methodological issues is necessary before large trials are under way.
Quality of the evidence
The quality of the evidence was moderate for hospital readmissions, low for mortality and high for health‐related quality of life and exercise capacity. The main reason for downgrading the quality of evidence for hospital readmissions and mortality is the heterogeneity of results, with some trials showing positive effects of rehabilitation, some no effects and one even revealing a negative impact of rehabilitation on mortality (Greening 2014). In addition, none of the trials included mortality as a primary outcome, and most reported durations of follow‐up that were too short for an effect of pulmonary rehabilitation on mortality to be detected. Reasons for downgrading or upgrading the quality of evidence are given in Table 1.
Potential biases in the review process
Strengths of this systematic review include the extensive literature search, rigorous adherence to a predefined protocol and successful contact with authors of the included studies, all of whom provided additional information about their data.
We split the studies into subgroups before we reviewed the results, but we defined the extensiveness of rehabilitation programmes in a somewhat arbitrary way.
Agreements and disagreements with other studies or reviews
Compared with pulmonary rehabilitation in patients with COPD in stable condition, the effect size of rehabilitation on health‐related quality of life is similar among patients who have recently had an exacerbation of COPD. Mean differences between rehabilitation and control groups for CRQ dyspnoea, fatigue, emotional function and mastery domains in this Cochrane review were close to those observed in the Cochrane review on pulmonary rehabilitation for people with stable COPD (McCarthy 2015). Compared with the earlier version of this Cochrane review (Puhan 2011), the current evidence base is more diverse because different pulmonary rehabilitation programmes have been tested across a wide range of participants and settings around the world. Also, effect estimates became smaller with the addition of new trials (Figure 4; Figure 6; Figure 8; Figure 10)
10.
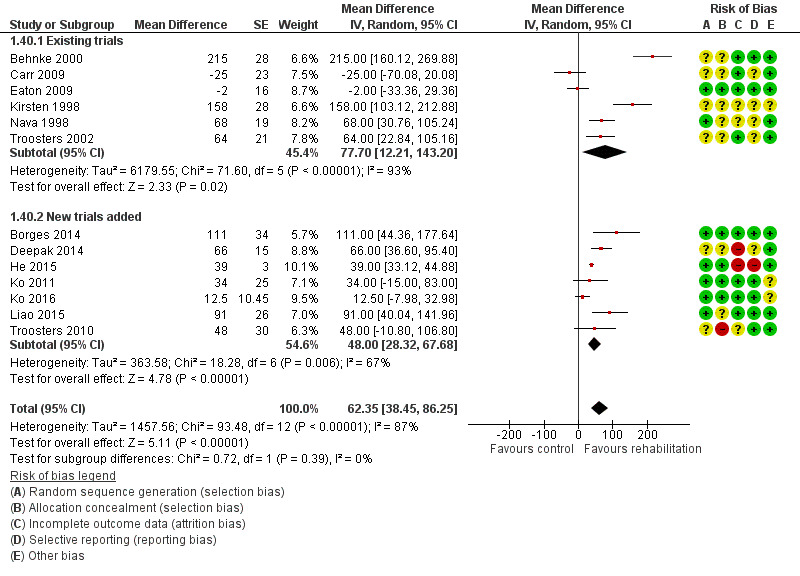
Forest plot of comparison: 1 Rehabilitation versus control, outcome: 1.40 Change from baseline in 6‐minute walking test with separated new trial data.
When only trials with an extensive rehabilitation programme were considered (Behnke 2000; He 2015; Ko 2011; Ko 2016; Man 2004; Nava 1998; Seymour 2010; Troosters 2002), the effects were larger than those seen in stable patients. Together with large improvements in exercise capacity and, in particular, substantial risk reduction for hospital admissions, pulmonary rehabilitation appears to be a particularly attractive addition to the treatment of patients after an exacerbation if an extensive rehabilitation programme can be implemented. Several possible explanations have been proposed for these large effects. First, as mentioned above, exacerbations lead to significant reductions in muscle function (Spruit 2003) and physical activity (Pitta 2006). This initial deterioration may render patients more likely to improve following pulmonary rehabilitation. Pulmonary rehabilitation is a particularly potent intervention for reverting physical inactivity (Troosters 2010a), and it has been shown that patients whose physical activity levels improve have less chance of being readmitted (Garcia‐Aymerich 2006; Pitta 2006). Second, because eligible participants had been hospitalised for a COPD exacerbation, a deficiency in self‐management or education may be evident among this group. This deficiency may be targeted in part by the rehabilitation intervention, and patient education may be of particular benefit for modifying behaviour in these patients. Indeed, a major study of a patient management programme that included home exercise for patients with COPD after an acute exacerbation reported impressive results (Bourbeau 2003). In this study, the mean number of hospital admissions per participant was reduced from 1.6 to 0.9 during the year following hospital admission for an acute exacerbation. It is well known from earlier studies that the recovery period is long, even for patients who have no further exacerbations, and that another exacerbation within six months can markedly limit recovery (Spencer 2003). A final explanation for the attractiveness of pulmonary rehabilitation programmes may be the effect of pulmonary rehabilitation on depressive symptoms after exacerbations. Depression is a significant risk factor for readmission, and pulmonary rehabilitation has been shown to improve depressive symptoms among depressed patients (Coventry 2007; Trappenburg 2005). Our meta‐analyses show that pulmonary rehabilitation during the recovery period is superior to usual care in terms of prognosis and health‐related quality of life.
Do we need more trials on pulmonary rehabilitation after COPD exacerbations?
A large body of available evidence from the systematic review on stable patients with COPD and from this systematic review shows large effects of pulmonary rehabilitation among patients with COPD (McCarthy 2015). Recently available trial findings show that many different exercise protocols are feasible and effective for patients with COPD, even if patients have poor health status, as is often the case during and after rehabilitation (ERS ATS Statement 2013). Exercise modalities include various forms of endurance and strength training, specific resistance training during hospital admission (Troosters 2010a), neuromuscular electrical stimulation and interval training, among others (ERS ATS Statement 2013; Sillen 2009).
Questions now may be focused less on the effectiveness of pulmonary rehabilitation after a COPD exacerbation in principle and more on how rehabilitation programmes should be designed and implemented, and how practitioners can foster patient uptake (ATS ERS Policy statement 2015). Uptake of pulmonary rehabilitation by patients is often low. In the Eaton trial, for example, 97 of 288 participants agreed to enrol in the trial; 47 were randomised to pulmonary rehabilitation, but only 19 of these 47 participants adhered to the rehabilitation programme (Eaton 2009). Those who adhered to the programme had substantially lower risk of readmission than participants who did not adhere to the rehabilitation programme, which corroborates the results of this Cochrane review showing that extensive rehabilitation programmes may be effective. Researchers should explore new ways of motivating patients to participate in pulmonary rehabilitation. For example, practitioners can explore the preferences of patients in terms of setting and type of exercise training, so the programme can be individualised according to both medical criteria and patient preferences. Also, the best timing for rehabilitation remains uncertain. Should rehabilitation start during an admission or shortly thereafter, or should it start when a patient's condition is stable again? An advantage of immediate rehabilitation after exacerbation is that it may provide a window of opportunity for patient education because patients may be more willing to change their health behaviour after an exacerbation. Also, continuity of care is possible if patients are immediately referred to pulmonary rehabilitation. A disadvantage of rehabilitation after exacerbation is that patients often re‐exacerbate within weeks, so that the rehabilitation process is interrupted or even discontinued. Also, initiation of physical exercise is challenging for patients after an exacerbation, and more time may be needed to find the appropriate exercise protocol for an individual patient (Puhan 2005a). One trial addressed the comparison of early versus late rehabilitation after an exacerbation but failed to recruit enough participants (Puhan 2012).
The studies included in this Cochrane review had a median follow‐up of three months. Given that physical exercise and self‐management should be based on a long‐term perspective, it is important for researchers to gather more data on health outcomes and costs over longer periods. Large and long‐term randomised trials would be ideal for addressing these important questions, but they may not be feasible because of lack of funding, slow participant recruitment and other reasons, as explained above. Therefore, advanced observational study methods and analyses may be employed. Finally, more evidence on the cost‐effectiveness of pulmonary rehabilitation in the post exacerbation setting is needed to inform policy decisions about pulmonary rehabilitation.
Authors' conclusions
Implications for practice.
Evidence of moderate quality (on average) from 20 studies (1477 participants) suggests that pulmonary rehabilitation is an effective intervention for post exacerbation treatment of patients with COPD. Effects leading to improved health‐related quality of life and exercise capacity are large. Effects on hospital readmission were statistically significant in the meta‐analysis but heterogenous across trials, and investigators need to explore whether the extensiveness of rehabilitation programmes explains such heterogeneity.
Implications for research.
The decision to begin new trials of pulmonary rehabilitation should be made against the background of perceived ethics about the benefit of pulmonary rehabilitation after exacerbation and against the methodological and logistical challenges of such trials if comparisons include a no‐exercise intervention. Studies should investigate how care providers can design and implement extensive rehabilitation programmes with a long‐term perspective that are feasible, reimbursable and attractive enough for patients and healthcare providers. Trials should assess the best timing of pulmonary rehabilitation. Finally, formal cost‐effectiveness analyses should be conducted to estimate the financial benefit derived from rehabilitation after COPD exacerbations.
Feedback
Details of interventions administered in the studies, 6 July 2009
Summary
Thanks for a very helpful review. I am interested in using for my patients, but am puzzled by which program of "rehabilitation" to adopt. The table of characteristics shows considerable variation, with several combinations, although most seem to be endurance exercise only rather than a more complex "rehabilitation" program. I was interested in any advice on what program I should implement with my patients. Could this (and a sample program) be included with the updated review?
Reply
Thank you for this comment. Based on our review, we cannot make any statements about which rehabilitation programmes work best. However, there are systematic reviews on trials comparing different exercise programs that may help you defining your rehabilitation programme (e.g. Puhan et al. Comparison of exercise modalities and intensities to treat skeletal muscle dysfunction during respiratory rehabilitation in COPD patients ‐ a systematic review. Thorax 2005;60(5):367‐75).
Contributors
Paul Glasziou
Use of data from Greening 2014 trial, 13 September 2019
Summary
Dear authorship team, I wish to comment on some ambiguities regarding the reporting of data from Greening 2014. This study has had an important impact on the field, hence accurate reporting of its findings is essential. I note the following ambiguities that I feel should be addressed:
The source of data are not currently clear. As the publicly available paper comprises patients with conditions other than COPD, one presumes the data reported in this review relate specifically to those patients with a diagnosis of COPD. Data related to most (but not all) outcomes for the COPD patient subset are unavailable in the original paper and its accompanying online supplement. If data were sourced directly from the authors for the purpose of this review, the study reference should be changed from its current label of ‘published data only’.
It would be helpful for the authors to clarify which one of Greening's analyses was used as the source of data for this Cochrane review, as the paper describes outcome data from I) an intention‐to‐treat analysis; ii) a per protocol analysis; and iii) a customised sub‐analysis of participants who were not readmitted during the follow‐up period.
Is there a data entry error related to the subgroup analyses related to this study by Greening and colleagues? I note, for example, that the outcome of shuttle walk distance (presumably measured in metres) is reported in Analyses 1.32 to 1.35. I question whether the data reported in Analysis 1.32 are correct, as it is the only analysis that demonstrates a different effect estimate for this individual study (MD ‐19.00; 95% CI‐44.48 to 6.48) and the overall outcome (MD 47.10; 95% CI ‐4.53 to 98.74), compared to Analyses 1.33 to 1.35 (Greening 2014, MD ‐14.00; 95% CI ‐39.48 to 11.48) and overall outcome (MD 48.14; 95% CI ‐1.03 to 97.32). The nature of the different subgroup analyses (intervention extensiveness, length of follow‐up, random sequence, allocation concealment, blinding) should not account for this difference. While I don't feel these changes will significantly alter the implications of this review, I feel this amendment is important as other clinicians and researchers may look to this Cochrane review (as I did) to identify the data that are not publicly available in the Greening paper. Many thanks for considering these comments.
Christain Osadnik, Department of Physiotherapy, Monash University, Melbourne Victoria, Australia
Do you have any affiliation with or involvement in any organisation with a financial interest in the subject matter of your comment?: No
Reply
Thank you for the feedback and the opportunity to add clarity to the review.
We received the data (aggregated) for COPD patients only from Greening et al. We have changed the reference as requested and noted this in the text of the review.
For the readmission and mortality outcomes we used ITT (as far as we understood when getting the data, but there was an imbalance in numbers, with 169 participants with COPD in the intervention group and 151 participants with COPD in the control group) and for ISWT and SGRQ we received ITT data. But since some participants withdrew or died, these did not match up to those randomized. We did not get the IPD and other variables to allow us to do any imputation procedures to deal with imbalance. Therefore the analyses are ITT with some missing data.
There is a mistake in the Analysis 1.32. The correct value is ‐14 instead of ‐19. All the analysis (1.6, and 1.33‐1.35) we used ‐14 for the difference in the ISWT. We updated analysis Analysis 1.32 with the correct value. This resulted in a small, but unimportant, change in the total analysis result from (MD 47.10; 95% CI ‐4.53 to 98.74) to (MD 48.11, 95% CI ‐0.74 to 96.95).
Contributors
Feeddback submitter: Christian Osadnik
Feedback Editor: Sally Spencer
Authors: Milo Puhan, Elena Gimeno, Chris Cates, Thierry Troosters.
What's new
| Date | Event | Description |
|---|---|---|
| 18 November 2019 | Feedback has been incorporated | We provided the information that we received the ITT data for COPD patients only from the authors of Greening 2014. In analysis 1.32, we corrected a mistake: We now use, as in all other analyses, the correct between group difference on ‐14 instead of ‐19. The results did not change materially. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 20 October 2015 | New citation required and conclusions have changed | Analyses were stratified for how extensive rehabilitation programmes were because they differed substantially. The impact of new evidence on patient‐important outcomes gathered for this review update is emphasised in the revised abstract and review. |
| 20 October 2015 | New search has been performed | This review updates the review published in 2010. We ran a search on 8 October 2014, and again on 20 October 2015, and ran handsearches up to 5 April 2016. This update identified 11 additional studies (Borges 2014; Deepak 2014; Greening 2014; He 2015; Ko 2011; Tang 2012; Torres‐Sánchez 2014; Torres‐Sánchez 2015; Troosters 2010; Ko 2016; Liao 2015) that added 1045 participants. We included in this update a 'Summary of findings' table that was based on GRADE and revised the Discussion section substantially because additional evidence became available. |
| 10 August 2011 | New citation required but conclusions have not changed | This review has been published as a new citation version to correct an error by which we omitted this at the last update. We changed the review author byline at the last update. |
| 12 July 2010 | New search has been performed | We incorporated posted comments into the review. We ran a new literature search and included 3 new studies (Eaton 2009; Carr 2009; Seymour 2010), increasing the total number of participants from 219 to 432. We made no changes to the review conclusions. |
| 8 April 2008 | Amended | We converted the review to new review format. |
| 20 February 2005 | New citation required and major changes | We made substantive amendments. |
Acknowledgements
We thank Liz Stovold of the Cochrane Airways Review Group, and Dr Pius Estermann, Information Specialist at the hospital library, University Hospital, Zurich, Switzerland, for performing the literature searches. We thank Dr Emma Dennett, Managing Editor of the Cochrane Airways Review Group, for great support and careful editing of this review. We thank Madlaina Scharplatz and Johann Steurer for contributions to earlier versions of this review.
Rebecca Normansell was the Contact Editor for this review and commented critically on it.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding provided to the Cochrane Airways Review Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search methods up to October 2010
We performed literature searches in the following electronic databases: MEDLINE Embase PEDro (Physiotherapy Evidence Database) The Cochrane Central Register of Controlled Trials (CENTRAL; 2008, Issue 2)
We performed a very broad literature search to identify any randomised controlled studies on pulmonary rehabilitation in patients with COPD. The search strategy for MEDLINE and Embase can be found in Appendix 1. In addition, we used the PubMed "related articles" function for included studies to identify further studies. Also, we performed a Science Citation Index search for studies that cite included studies, as well as for studies that are cited by included studies.
In addition, we carried out a search of the Cochrane Airways Review Group Specialised Register of COPD trials, using the following terms:
(rehabilitat* or fitness or exercis* or physical* or train* or kinesio* or endurance*) and (acute* or exacerb* or emerg* or hospital* or admit* or admis* or discharg*)
| Combined search strategy for MEDLINE (Ovid) and Embase (Ovid) |
| 1 lung diseases obstructive.af. 2 chronic obstructive lung disease.af. 3 chronic obstructive pulmonary disease.af. 4 exp pulmonary disease chronic obstructive/ 5 or/1‐4 6 rh.fs. 7 rehabilitation.de. 8 exp exercise movement techniques/ 9 exp exercise test/ 10 exp physical endurance/ 11 exp muscle training/ 12 exp kinesiotherapy/ 13 exp exercise/ 14 or/6‐13 15 5 and 14 16 clinical trial.pt. 17 exp epidemiologic methods/ 18 exp controlled study/ 19 exp major clinical study/ 20 exp evidence based medicine/ 21 or/16‐20 22 15 and 21 23 comment.pt. 24 editorial.pt. 25 exp editorial/ 26 or/23‐25 27 22 not 26 28 remove duplicates from 27 |
Appendix 2. Sources and search methods for the Cochrane Airways Review Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (the Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and the RCT filter are adapted to identify trials in other electronic databases.
Appendix 3. Search strategy to identify relevant trials from the CAGR for 2016 update
#1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All
#2 MeSH DESCRIPTOR Bronchitis, Chronic
#3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)
#4 COPD:MISC1
#5 (COPD OR COAD OR COBD):TI,AB,KW
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 MeSH DESCRIPTOR Rehabilitation Explode All
#8 rehabilitat* or fitness or exercis* or physical* or train* or kinesio* or endurance*
#9 #7 or #8
#10 MeSH DESCRIPTOR Acute Disease
#11 MeSH DESCRIPTOR Disease Progression
#12 (acute* or exacerb* or emerg* or admit* or admis* or discharg*) or hospital*:ti,ab,kw
#13 #10 or #11 or #12
#14 #6 and #9 and #13
#15 (#14) AND (INREGISTER)
[Note: in field #4, MISC1 denotes the field in which the reference has been coded for condition, in this case, COPD.]
Data and analyses
Comparison 1. Rehabilitation versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospital readmission (to end of follow‐up) | 8 | 810 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.21, 0.91] |
| 2 Mortality | 6 | 670 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.28, 1.67] |
| 3 Health‐related quality of life: Chronic Respiratory Disease Questionnaire (CRQ) | 5 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 CRQ: dyspnoea domain | 5 | Mean Difference (Random, 95% CI) | 0.97 [0.35, 1.58] | |
| 3.2 CRQ: fatigue domain | 5 | Mean Difference (Random, 95% CI) | 0.81 [0.16, 1.45] | |
| 3.3 CRQ: emotional function domain | 5 | Mean Difference (Random, 95% CI) | 0.94 [0.46, 1.42] | |
| 3.4 CRQ: mastery domain | 5 | Mean Difference (Random, 95% CI) | 0.93 [‐0.13, 1.99] | |
| 4 Health‐related quality of life: St George's Respiratory Questionnaire | 8 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 4.1 SGRQ: total | 8 | Mean Difference (Random, 95% CI) | ‐7.80 [‐12.12, ‐3.47] | |
| 4.2 SGRQ: impact | 8 | Mean Difference (Random, 95% CI) | ‐10.44 [‐16.11, ‐4.76] | |
| 4.3 SGRQ: symptoms | 8 | Mean Difference (Random, 95% CI) | ‐2.45 [‐7.33, 2.42] | |
| 4.4 SGRQ: activity limitation | 8 | Mean Difference (Random, 95% CI) | ‐8.23 [‐12.88, ‐3.57] | |
| 5 Change from baseline in 6‐minute walking test | 13 | Mean Difference (Random, 95% CI) | 62.38 [38.45, 86.31] | |
| 6 Change from baseline in shuttle walk test | 4 | Mean Difference (Random, 95% CI) | 48.14 [‐1.03, 97.32] | |
| 7 Subgroup analysis hospital readmission: extensiveness of rehabilitation programme | 8 | 810 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.21, 0.91] |
| 7.1 Extensive rehab programmes | 5 | 367 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.10, 0.78] |
| 7.2 Less‐extensive rehab programmes | 3 | 443 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.44, 1.93] |
| 8 Subgroup analysis hospital readmission: length of follow‐up | 8 | 587 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.22, 0.76] |
| 8.1 Follow‐up >3 months | 5 | 389 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.24, 1.06] |
| 8.2 Follow‐up ≤3 months | 3 | 198 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.06, 0.98] |
| 9 Subgroup analysis hospital readmission: generation of random sequence | 8 | 810 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.21, 0.91] |
| 9.1 Low risk of bias | 6 | 758 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.25, 1.19] |
| 9.2 Unclear or high risk of bias | 2 | 52 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.04, 0.59] |
| 10 Subgroup analysis hospital readmission: concealment of random allocation | 8 | 810 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.21, 0.91] |
| 10.1 Low risk of bias | 7 | 784 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.25, 1.08] |
| 10.2 Unclear or high risk of bias | 1 | 26 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.56] |
| 11 Subgroup analysis hospital readmission: blinding | 8 | 810 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.21, 0.91] |
| 11.1 Low risk of bias | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Unclear or high risk of bias | 8 | 810 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.21, 0.91] |
| 12 Subgroup analysis mortality: extensiveness of rehabilitation programme | 6 | 670 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.28, 1.67] |
| 12.1 Extensive rehab programmes | 5 | 350 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.26, 0.99] |
| 12.2 Less‐extensive rehab programmes | 1 | 320 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [1.06, 3.33] |
| 13 Subgroup analysis mortality: length of follow‐up | 6 | 670 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.28, 1.67] |
| 13.1 Follow‐up >3 months | 5 | 629 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.26, 1.86] |
| 13.2 Follow‐up ≤3 months | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 0.5 [0.04, 5.99] |
| 14 Subgroup analysis mortality: generation of random sequence | 6 | 670 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.28, 1.67] |
| 14.1 Low risk of bias | 4 | 601 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.52, 2.34] |
| 14.2 Unclear or high risk of bias | 2 | 69 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.08, 0.83] |
| 15 Subgroup analysis mortality: concealment of random allocation | 6 | 670 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.28, 1.67] |
| 15.1 Low risk of bias | 4 | 601 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.52, 2.34] |
| 15.2 Unclear or high risk of bias | 2 | 69 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.08, 0.83] |
| 16 Subgroup analysis mortality: blinding | 6 | 670 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.28, 1.67] |
| 16.1 Low risk of bias | 6 | 670 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.28, 1.67] |
| 16.2 Unclear or high risk of bias | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Subgroup analysis CRQ dyspnoea domain: extensiveness of rehabilitation programme | 5 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 17.1 Extensive rehab programmes | 3 | Mean Difference (Random, 95% CI) | 1.31 [0.63, 2.00] | |
| 17.2 Less‐extensive rehab programmes | 2 | Mean Difference (Random, 95% CI) | 0.37 [‐0.50, 1.24] | |
| 18 Subgroup analysis CRQ dyspnoea domain: length of follow‐up | 5 | Mean Difference (Random, 95% CI) | 0.97 [0.35, 1.58] | |
| 18.1 Follow‐up >3 months | 1 | Mean Difference (Random, 95% CI) | 2.44 [1.42, 3.46] | |
| 18.2 Follow‐up ≤3 months | 4 | Mean Difference (Random, 95% CI) | 0.70 [0.12, 1.28] | |
| 19 Subgroup analysis CRQ dyspnoea domain: generation of random sequence | 5 | Mean Difference (Random, 95% CI) | 0.97 [0.35, 1.58] | |
| 19.1 Low risk of bias | 3 | Mean Difference (Random, 95% CI) | 0.65 [‐0.07, 1.37] | |
| 19.2 Unclear or high risk of bias | 2 | Mean Difference (Random, 95% CI) | 1.65 [0.14, 3.16] | |
| 20 Subgroup analysis CRQ dyspnoea domain: concealment of random allocation | 5 | Mean Difference (Random, 95% CI) | 0.97 [0.35, 1.58] | |
| 20.1 Low risk of bias | 3 | Mean Difference (Random, 95% CI) | 0.65 [‐0.07, 1.37] | |
| 20.2 Unclear or high risk of bias | 2 | Mean Difference (Random, 95% CI) | 1.65 [0.14, 3.16] | |
| 21 Subgroup analysis CRQ dyspnoea domain: blinding | 5 | Mean Difference (Random, 95% CI) | 0.97 [0.35, 1.58] | |
| 21.1 Low risk of bias | 0 | Mean Difference (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.2 Unclear or high risk of bias | 5 | Mean Difference (Random, 95% CI) | 0.97 [0.35, 1.58] | |
| 22 Subgroup analysis SGRQ total score: extensiveness of rehabilitation programme | 8 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 22.1 Extensive rehab programmes | 4 | Mean Difference (Random, 95% CI) | ‐7.82 [‐11.03, ‐4.61] | |
| 22.2 Less‐extensive rehab programmes | 4 | Mean Difference (Random, 95% CI) | ‐8.49 [‐18.13, 1.15] | |
| 23 Subgroup analysis SGRQ total score: length of follow‐up | 8 | Mean Difference (Random, 95% CI) | ‐7.80 [‐12.12, ‐3.47] | |
| 23.1 Follow‐up >3 months | 4 | Mean Difference (Random, 95% CI) | ‐4.27 [‐8.32, ‐0.22] | |
| 23.2 Follow‐up ≤3 months | 4 | Mean Difference (Random, 95% CI) | ‐12.09 [‐17.61, ‐6.57] | |
| 24 Subgroup analysis SGRQ total score: generation of random sequence | 8 | Mean Difference (Random, 95% CI) | ‐7.80 [‐12.12, ‐3.47] | |
| 24.1 Low risk of bias | 6 | Mean Difference (Random, 95% CI) | ‐5.87 [‐9.87, ‐1.88] | |
| 24.2 Unclear or high risk of bias | 2 | Mean Difference (Random, 95% CI) | ‐14.32 [‐25.35, ‐3.29] | |
| 25 Subgroup analysis SGRQ total score: concealment of random allocation | 8 | Mean Difference (Random, 95% CI) | ‐7.80 [‐12.12, ‐3.47] | |
| 25.1 Low risk of bias | 7 | Mean Difference (Random, 95% CI) | ‐6.12 [‐9.73, ‐2.51] | |
| 25.2 Unclear or high risk of bias | 1 | Mean Difference (Random, 95% CI) | ‐20.06 [‐28.00, ‐10.12] | |
| 26 Subgroup analysis SGRQ total score: blinding | 8 | Mean Difference (Random, 95% CI) | ‐7.80 [‐12.12, ‐3.47] | |
| 26.1 Low risk of bias | 0 | Mean Difference (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.2 Unclear or high risk of bias | 8 | Mean Difference (Random, 95% CI) | ‐7.80 [‐12.12, ‐3.47] | |
| 27 Subgroup analysis 6‐minute walking test: extensiveness of rehabilitation programme | 13 | Mean Difference (Random, 95% CI) | 59.70 [35.09, 84.31] | |
| 27.1 Extensive rehab programmes | 6 | Mean Difference (Random, 95% CI) | 65.50 [31.71, 99.30] | |
| 27.2 Less‐extensive rehab programmes | 7 | Mean Difference (Random, 95% CI) | 54.91 [6.07, 103.74] | |
| 28 Subgroup analysis 6‐minute walk test: length of follow‐up | 13 | Mean Difference (Random, 95% CI) | 59.70 [35.09, 84.31] | |
| 28.1 Follow‐up >3 months | 4 | Mean Difference (Random, 95% CI) | 78.95 [1.95, 155.96] | |
| 28.2 Follow‐up ≤3 months | 9 | Mean Difference (Random, 95% CI) | 52.21 [24.72, 79.70] | |
| 29 Subgroup analysis 6‐minute walk test: generation of random sequence | 13 | Mean Difference (Random, 95% CI) | 59.70 [35.09, 84.31] | |
| 29.1 Low risk of bias | 7 | Mean Difference (Random, 95% CI) | 34.89 [14.17, 55.61] | |
| 29.2 Unclear or high risk of bias | 6 | Mean Difference (Random, 95% CI) | 86.44 [25.63, 147.24] | |
| 30 Subgroup analysis 6‐minute walk test: concealment of random allocation | 13 | Mean Difference (Random, 95% CI) | 62.38 [38.44, 86.32] | |
| 30.1 Low risk of bias | 5 | Mean Difference (Random, 95% CI) | 29.55 [6.15, 52.95] | |
| 30.2 Unclear or high risk of bias | 8 | Mean Difference (Random, 95% CI) | 84.16 [40.23, 128.09] | |
| 31 Subgroup analysis 6‐minute walk test: blinding | 13 | Mean Difference (Random, 95% CI) | 62.38 [38.44, 86.32] | |
| 31.1 Low risk of bias | 3 | Mean Difference (Random, 95% CI) | 26.31 [‐30.62, 83.25] | |
| 31.2 Unclear or high risk of bias | 10 | Mean Difference (Random, 95% CI) | 74.02 [44.81, 103.23] | |
| 32 Subgroup analysis shuttle walk test: extensiveness of rehabilitation programme | 4 | Mean Difference (Random, 95% CI) | 48.11 [‐0.74, 96.95] | |
| 32.1 Extensive rehab programmes | 2 | Mean Difference (Random, 95% CI) | 58.45 [35.02, 81.88] | |
| 32.2 Less‐extensive rehab programmes | 2 | Mean Difference (Random, 95% CI) | 37.68 [‐69.92, 145.28] | |
| 33 Subgroup analysis shuttle walk test: length of follow‐up | 4 | Mean Difference (Random, 95% CI) | 48.14 [‐1.03, 97.32] | |
| 33.1 Follow‐up >3 months | 2 | Mean Difference (Random, 95% CI) | 37.68 [‐69.92, 145.28] | |
| 33.2 Follow‐up ≤3 months | 2 | Mean Difference (Random, 95% CI) | 58.77 [34.85, 82.69] | |
| 34 Subgroup analysis shuttle walk test: generation of random sequence | 4 | Mean Difference (Random, 95% CI) | 48.14 [‐1.03, 97.32] | |
| 34.1 Low risk of bias | 3 | Mean Difference (Random, 95% CI) | 35.41 [‐18.04, 88.86] | |
| 34.2 Unclear or high risk of bias | 1 | Mean Difference (Random, 95% CI) | 96.0 [37.20, 154.80] | |
| 35 Subgroup analysis shuttle walk test: concealment of random allocation | 4 | Mean Difference (Random, 95% CI) | 48.14 [‐1.03, 97.32] | |
| 35.1 Low risk of bias | 4 | Mean Difference (Random, 95% CI) | 48.14 [‐1.03, 97.32] | |
| 35.2 Unclear or high risk of bias | 0 | Mean Difference (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 36 Subgroup analysis shuttle walk test: blinding | 4 | Mean Difference (Random, 95% CI) | 48.14 [‐1.03, 97.32] | |
| 36.1 Low risk of bias | 1 | Mean Difference (Random, 95% CI) | ‐14.0 [‐39.48, 11.48] | |
| 36.2 Unclear or high risk of bias | 3 | Mean Difference (Random, 95% CI) | 64.58 [41.49, 87.66] | |
| 37 Hospital readmission (to end of follow‐up) with separated new trial data | 8 | 810 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.21, 0.91] |
| 37.1 Existing trials | 5 | 250 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.08, 0.58] |
| 37.2 New trials added | 3 | 560 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.38, 2.26] |
| 38 Mortality with separated new trial data | 6 | 670 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.28, 1.67] |
| 38.1 Existing trials | 3 | 110 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.10, 0.84] |
| 38.2 New trials added | 3 | 560 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.48, 2.71] |
| 39 Health‐related quality of life: SGRQ total with separated new trial data | 8 | Mean Difference (Random, 95% CI) | ‐7.80 [‐12.12, ‐3.47] | |
| 39.1 Existing trials | 3 | Mean Difference (Random, 95% CI) | ‐9.88 [‐14.40, ‐5.37] | |
| 39.2 New trials added | 5 | Mean Difference (Random, 95% CI) | ‐6.68 [‐12.83, ‐0.53] | |
| 40 Change from baseline in 6 minute walking test with separated new trial data | 13 | Mean Difference (Random, 95% CI) | 62.35 [38.45, 86.25] | |
| 40.1 Existing trials | 6 | Mean Difference (Random, 95% CI) | 77.70 [12.21, 143.20] | |
| 40.2 New trials added | 7 | Mean Difference (Random, 95% CI) | 48.00 [28.32, 67.68] |
1.1. Analysis.
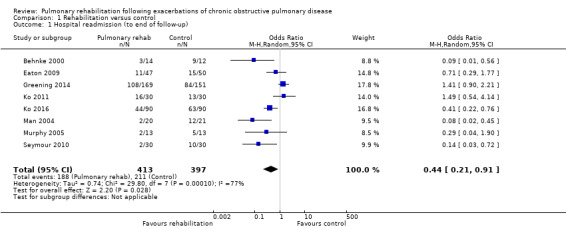
Comparison 1 Rehabilitation versus control, Outcome 1 Hospital readmission (to end of follow‐up).
1.2. Analysis.
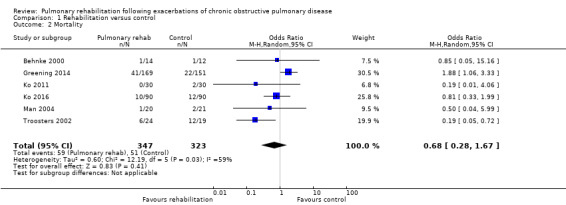
Comparison 1 Rehabilitation versus control, Outcome 2 Mortality.
1.4. Analysis.
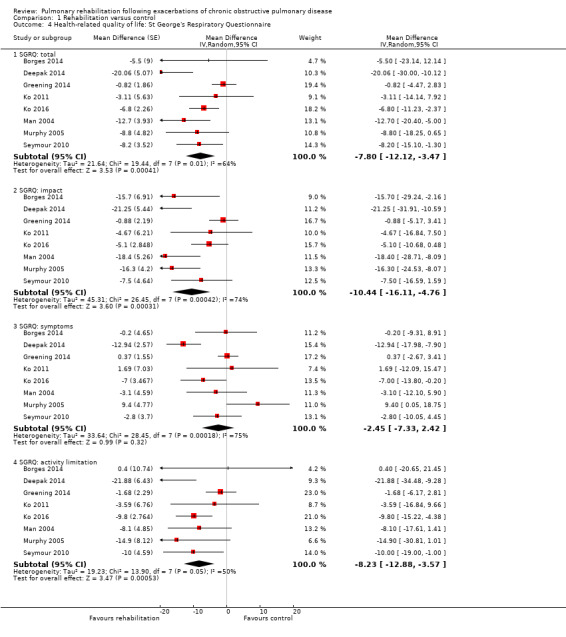
Comparison 1 Rehabilitation versus control, Outcome 4 Health‐related quality of life: St George's Respiratory Questionnaire.
1.5. Analysis.
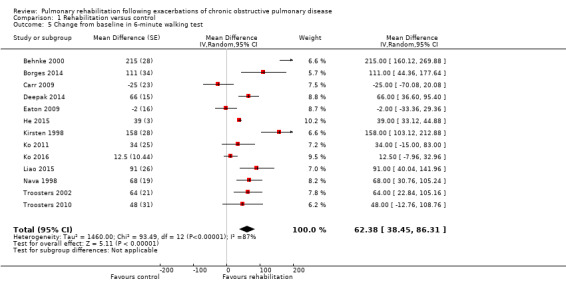
Comparison 1 Rehabilitation versus control, Outcome 5 Change from baseline in 6‐minute walking test.
1.16. Analysis.
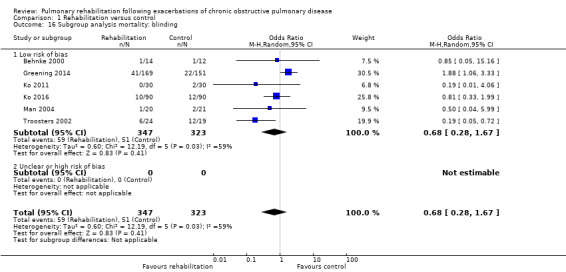
Comparison 1 Rehabilitation versus control, Outcome 16 Subgroup analysis mortality: blinding.
1.19. Analysis.
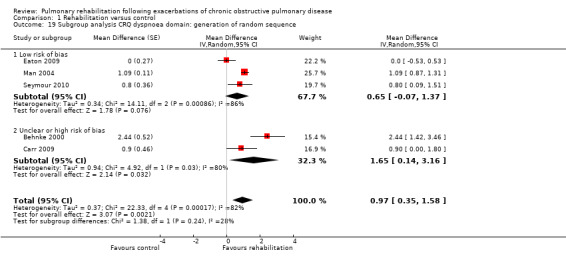
Comparison 1 Rehabilitation versus control, Outcome 19 Subgroup analysis CRQ dyspnoea domain: generation of random sequence.
1.20. Analysis.
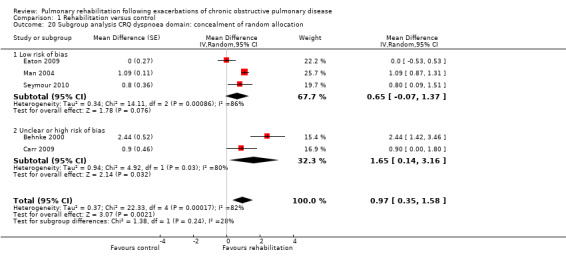
Comparison 1 Rehabilitation versus control, Outcome 20 Subgroup analysis CRQ dyspnoea domain: concealment of random allocation.
1.21. Analysis.
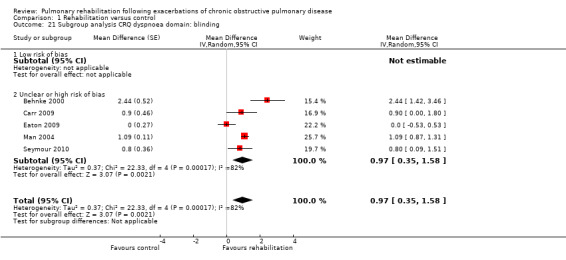
Comparison 1 Rehabilitation versus control, Outcome 21 Subgroup analysis CRQ dyspnoea domain: blinding.
1.24. Analysis.
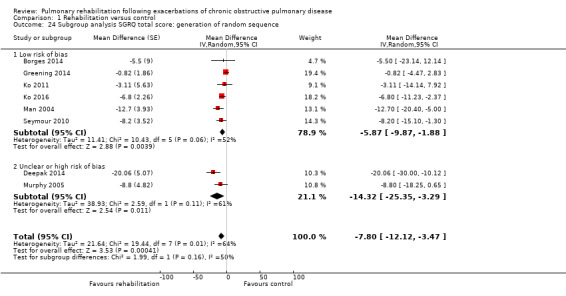
Comparison 1 Rehabilitation versus control, Outcome 24 Subgroup analysis SGRQ total score: generation of random sequence.
1.26. Analysis.
Comparison 1 Rehabilitation versus control, Outcome 26 Subgroup analysis SGRQ total score: blinding.
1.37. Analysis.
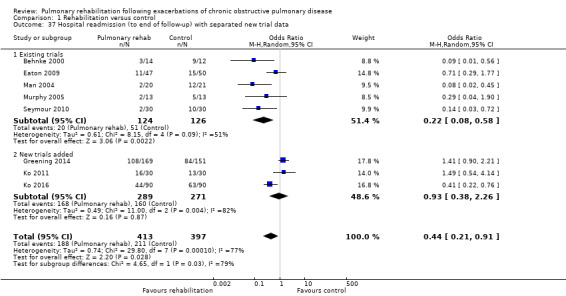
Comparison 1 Rehabilitation versus control, Outcome 37 Hospital readmission (to end of follow‐up) with separated new trial data.
1.38. Analysis.
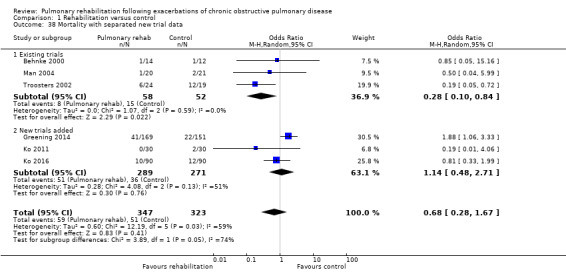
Comparison 1 Rehabilitation versus control, Outcome 38 Mortality with separated new trial data.
1.39. Analysis.
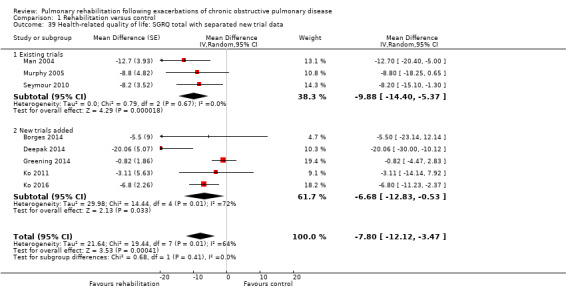
Comparison 1 Rehabilitation versus control, Outcome 39 Health‐related quality of life: SGRQ total with separated new trial data.
1.40. Analysis.
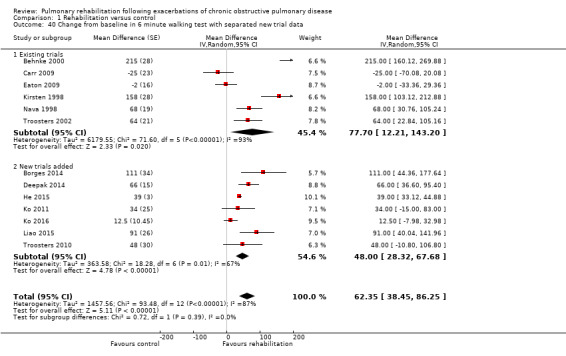
Comparison 1 Rehabilitation versus control, Outcome 40 Change from baseline in 6 minute walking test with separated new trial data.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Behnke 2000.
| Methods | Randomised parallel‐group trial | |
| Participants | 26 participants with COPD (mean age 67 years, 77% males, mean FEV1 36% predicted) after inpatient treatment for acute exacerbation | |
| Interventions |
Rehabilitation: within 4‐7 days after admission, inpatient pulmonary rehabilitation with endurance exercise (5 walking sessions/d for 10 days), followed by 6 months of supervised home‐based endurance exercise (3 walking sessions/d for 6 months). Completion rate of pulmonary rehabilitation: 65.2% (15/23 participants) Usual care: standard inpatient care without exercise and standard community care with respirologist. Follow‐up: 76 weeks |
|
| Outcomes | CRQ, 6MWD, hospital readmission, mortality | |
| Notes | Pulmonary rehabilitation programme was considered extensive (upgraded by +1 for > 30 exercise sessions and downgraded by ‐1 for unsupervised training). Financial support was provided by the Verein zur FoÈ rderung der Rehabilitationsforschung in Schleswig‐Holstein e.V., Deutsche Gesellschaft fuer Medizinische Rehabilitation and Landesversicherungsanstalt Freie und Hansestadt, Hamburg, Germany. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available from trial report |
| Blinding (performance bias and detection bias) Hospital admission | Unclear risk | Information not available from trial report. Outcome may be affected by knowledge of treatment group assignment. |
| Blinding (performance bias and detection bias) Health‐related quality of life | High risk | Although unavoidable by definition, lack of blinding may affect outcome. |
| Blinding (performance bias and detection bias) Mortality | Low risk | Information not available from trial report. Outcome unlikely to be affected by knowledge of treatment group assignment |
| Blinding (performance bias and detection bias) Walk test | Unclear risk | Information not provided in trial report. Potential lack of blinding likely to affect outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up are detailed for both groups, and the final number of participants is balanced between comparison groups. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting is sufficiently complete and transparent. Study authors provided individual participant data. Clinical trial registration number not reported |
| Other bias | Low risk | No indication of other biases |
Borges 2014.
| Methods | Randomised parallel‐group trial | |
| Participants | 29 participants with COPD (CG: n = 14, mean age 68 years, 71% males, mean FEV1 39% predicted; IG: n = 15, mean age 64 years, 53% male, mean FEV1 42% predicted) admitted to the hospital for treatment of COPD exacerbation | |
| Interventions |
Rehabilitation: exercise training started on third day of hospitalisation, inpatient pulmonary rehabilitation (completed 5.6 sessions on average) with whole‐body resistance training for upper and lower limbs (session every morning with free weights in 2 sets of 8 repetitions). Completion rate of pulmonary rehabilitation: 95%. Follow‐up: 1 month Usual care: Participants received normative daily care, including chest physiotherapy, non‐invasive ventilation if needed and verbal instructions to carry on with their normative daily physical activities. Participants did not receive an exercise programme or a recommendation to exercise after hospital discharge. Follow‐up: 1 month |
|
| Outcomes | SGRQ, 6MWD | |
| Notes | Pulmonary rehabilitation programme was considered not extensive (downgraded by ‐2 for < 10 exercise training sessions and by ‐1 for strength training only). Financial support was provided by the Sao Paulo Research Foundation (Grant no. 2007/51‐354‐7) and the Brazilian Scientific Foundation (Grant no. 305987/2010‐0). Clinical trial identifier: NCT01786928 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | "Allocation was concealed in sequentially numbered, sealed, opaque envelopes" |
| Blinding (performance bias and detection bias) Hospital admission | Unclear risk | Information not available from trial report |
| Blinding (performance bias and detection bias) Health‐related quality of life | High risk | Information not available from trial report. Although unavoidable by definition, lack of blinding may affect outcome. |
| Blinding (performance bias and detection bias) Mortality | Unclear risk | Information not available from trial report |
| Blinding (performance bias and detection bias) Walk test | Low risk | "Evaluation[s] were performed by a blinded evaluator." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up are detailed for both groups, and the final number of participants is balanced between comparison groups. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting is sufficiently complete and transparent. Clinical trial registration number is not reported. |
| Other bias | Low risk | No indication of other biases |
Carr 2009.
| Methods | Randomised parallel‐group trial | |
| Participants | 34 participants with COPD (mean age 68 years, 44% males, mean FEV1 0.91 L) after inpatient treatment for acute exacerbation | |
| Interventions |
Rehabilitation: inpatient or outpatient pulmonary rehabilitation (based on participant preference or location of initial PR) (2 hours/session over 3 weeks, completed between 9 and 15 sessions) with breathing exercise, strength and interval training and corridor and treadmill walking or cycling; patient education (energy conservation, lung health, drugs and stress management). Completion rate of pulmonary rehabilitation: 94% (16/17 participants). Follow‐up: 12 weeks Usual care: standard inpatient and community care without exercise (not further specified). Follow‐up: 12 weeks |
|
| Outcomes | CRQ (primary outcome), 6MWD (secondary outcome) | |
| Notes | Pulmonary rehabilitation programme was considered moderately extensive (downgraded by ‐1 for 10‐15 exercise training sessions). Financial support provided by the Ontario Thoracic Society |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; additional information not available from trial report |
| Allocation concealment (selection bias) | Unclear risk | Information not available from trial report |
| Blinding (performance bias and detection bias) Hospital admission | Unclear risk | Information not reported |
| Blinding (performance bias and detection bias) Health‐related quality of life | High risk | Although unavoidable by definition, lack of blinding may affect outcome. |
| Blinding (performance bias and detection bias) Mortality | Low risk | Information not available from trial report. Outcome unlikely to be affected by knowledge of treatment group assignment |
| Blinding (performance bias and detection bias) Walk test | Low risk | "The investigator responsible for collecting outcome measures was unaware of group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up are detailed for both groups, and the final number of participants is balanced between comparison groups. |
| Selective reporting (reporting bias) | Unclear risk | Outcome reporting is sufficiently complete and transparent: Results for all listed primary and secondary outcomes are reported. Clinical trial registration number not reported |
| Other bias | Low risk | No indication of other biases |
Deepak 2014.
| Methods | Randomised parallel‐group trial | |
| Participants | 60 participants with COPD (CG: mean age 58 years, 93% males, FEV1 53% predicted; IG: mean age 59 years, 93% male, FEV1 47% predicted) after admission for treatment of an acute exacerbation | |
| Interventions |
Rehabilitation: within 2 weeks after discharge, supervised outpatient pulmonary rehabilitation with limb strengthening and aerobic activities, education, nutrition and psychosocial rehabilitation for 12 weeks, including chest physiotherapy for drainage of secretions, breathing retraining techniques, techniques to control dyspnoea. Adherence to pulmonary rehabilitation not reported. Follow‐up: 3 months Usual care: conventional treatment. Follow‐up: 3 months |
|
| Outcomes | SGRQ, 6MWD | |
| Notes | Extensiveness of pulmonary rehabilitation programme could not be assessed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described by study authors as follows: "randomisation was done by block randomisation technique"; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available from trial report |
| Blinding (performance bias and detection bias) Hospital admission | Unclear risk | Information not reported |
| Blinding (performance bias and detection bias) Health‐related quality of life | High risk | Information not available from trial report. Although unavoidable by definition, lack of blinding may affect outcome. |
| Blinding (performance bias and detection bias) Mortality | Unclear risk | Information not reported |
| Blinding (performance bias and detection bias) Walk test | Unclear risk | Information not provided in trial report. Potential lack of blinding likely to affect outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal and drop‐out rates are not reported. |
| Selective reporting (reporting bias) | Unclear risk | Outcome reporting is sufficiently complete and transparent: Results for all listed outcomes are reported. Clinical trial registration number not reported |
| Other bias | Low risk | No indication of other biases |
Eaton 2009.
| Methods | Randomised parallel‐group trial | |
| Participants | 97 participants with COPD (mean age 70 years, 44% males, mean FEV1 36% predicted) | |
| Interventions |
Rehabilitation: The patient started inpatient programme as soon as medically appropriate, as determined by the attending medical team. Inpatient programme: supervised walking and upper/lower limb‐strengthening exercise at least 30 minutes/d until discharge, followed by outpatient programme: supervised exercise for 8 weeks (1‐hour session, twice weekly) and patient education (coping with dyspnoea, the importance of a regular daily home exercise programme, management of activities of daily living, drugs, vaccines, airway clearance techniques, nutritional advice, self‐management and action plans for exacerbations, stress and panic management, relaxation techniques, mood disturbance, adapting to a chronic illness and end‐of‐life care). Only 19 (40%) patients assigned to early rehabilitation satisfied the a priori definition of adherence (attendance at 75% of rehabilitation sessions) Follow‐up: 12 weeks Usual care: standardised care in accordance with ATS/ERS COPD guidelines and standardised advice on exercise and maintaining daily activities, but not further specified. Follow‐up: 12 weeks |
|
| Outcomes | Hospital readmission and hospital days (primary outcomes); 6MWD, CRQ (secondary outcomes) | |
| Notes | Pulmonary rehabilitation programme was considered moderately extensive (downgraded by ‐2 for < 10 exercise training sessions but upgraded by +1 for extensive self‐management training) Financial support provided by the Green Lane Research and Educational Foundation Clinical trial identifier: ACTRNO12605000372684 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Only information from computer available at time of randomisation |
| Blinding (performance bias and detection bias) Hospital admission | High risk | Outcome may be affected by knowledge of treatment group assignment. |
| Blinding (performance bias and detection bias) Health‐related quality of life | High risk | Although unavoidable by definition, lack of blinding may affect outcome. |
| Blinding (performance bias and detection bias) Mortality | Low risk | Information not available from trial report. Outcome unlikely to be affected by knowledge of treatment group assignment |
| Blinding (performance bias and detection bias) Walk test | High risk | Lack of blinding likely to affect outcome assessment. "The nature of intervention precluded blinding of participants and health care providers." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up are detailed for both groups, and the final number of participants is balanced between comparison groups. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting is sufficiently complete and transparent. Clinical trial registration number reported ACTRNO12605000372684 |
| Other bias | Low risk | No indication of other biases |
Greening 2014.
| Methods | Randomised parallel‐group trial | |
| Participants | 389 participants (CG: n = 193, mean age 71 years, 44% males, mean FEV1 57% predicted; IG: n = 196, mean age 71 years, 45% males, mean FEV1 52% predicted) admitted to hospital for an exacerbation of chronic respiratory disease (320 (82%) patients with COPD; CG: n = 151; IG: n = 169) | |
| Interventions |
Rehabilitation: within 48 hours of hospital admission, supervised volitional (strength and aerobic training) and non‐volitional (neuromuscular electrical stimulation) techniques (median duration of hospital admission 5 days). The mean number of sessions during the hospital admission was 2.7 (SD 2.6) for aerobic training, 2.5 (SD 1.9) for resistance training and 3.6 (SD 3.2) for neuromuscular electrical stimulation training. In addition, a self‐management and educational package was offered. Completion rate of pulmonary rehabilitation: 86% for inpatient aerobic training, 90% for strength training and 90% for neuromuscular electrical stimulation training After discharge, participants received instructions on how to follow a progressive walking‐based home exercise programme, to continue daily neuromuscular electrical stimulation and to follow the self‐management programme. The postdischarge training was supported by telephone consultations from the pulmonary rehabilitation intervention team, using motivational interviewing techniques, at 48 hours, 2 weeks and 4 weeks. Continued daily adherence to the home programme was reported by 54% of participants for aerobic training and by 61% for resistance training. Follow‐up: 12 months Usual care: standard care during hospital admission (median duration of hospital admission 5 days) including airway clearance techniques, mobility and advice on smoking cessation, dietetic advice and nutritional support if appropriate. No supervised or progressive exercise programme was provided during the admission or immediately after discharge, but outpatient pulmonary rehabilitation was offered to all participants 3 months after discharge. Follow‐up: 12 months |
|
| Outcomes | Hospital readmissions, mortality (primary outcomes); SGRQ, ISWT and ESWT (secondary outcomes) | |
| Notes | Pulmonary rehabilitation programme was considered moderately extensive (downgraded by ‐2 for mostly unsupervised training and upgraded by +1 for extensive self‐management training). Financial support provided by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care in Leicestershire, Northamptonshire and Rutland (CLAHRC LNR), by the NIHR Leicester Respiratory Biomedical Research Unit and CLAHRC East Midlands, and by the University of Leicester Clinical Trials Unit Clinical trial identifier: ISRCTN05557928 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Automated Internet‐based service (www.sealedenvelope.com) |
| Allocation concealment (selection bias) | Low risk | No details but can be assumed because of use of automated Internet‐based service (www.sealedenvelope.com) |
| Blinding (performance bias and detection bias) Hospital admission | Unclear risk | Hospital admissions were captured through hospital databases and general practice records. Unclear if group assignment was known while hospital admissions were ascertained |
| Blinding (performance bias and detection bias) Health‐related quality of life | High risk | Although unavoidable by definition, lack of blinding may affect outcome. |
| Blinding (performance bias and detection bias) Mortality | Low risk | Information not available from trial report. Outcome unlikely to be affected by knowledge of treatment group assignment |
| Blinding (performance bias and detection bias) Walk test | Low risk | "All investigators performing the outcome measures were blinded to treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up are detailed for both groups, and the final number of participants is balanced between comparison groups. "We used an intention to treat analysis to assess the primary outcome." |
| Selective reporting (reporting bias) | Low risk | Information is reported in a sufficiently complete and transparent way. Clinical trial registration number reported |
| Other bias | Low risk | Supplemental details of methods and analysis reported |
He 2015.
| Methods | Randomised parallel‐group trial | |
| Participants | 94 participants (CG: n = 28, mean age 74 years, 82% males, mean FEV1 39% predicted; IG: n = 66, mean age 69 years, 91% males, mean FEV1 38% predicted) admitted to hospital for an exacerbation of chronic respiratory disease. 101 enrolled; 7 withdrew after randomisation (not included in analyses) | |
| Interventions |
Rehabilitation: from the second day of hospital admission, exercise training (endurance + strength, twice daily), relaxation and breathing retraining and education. The mean number of days of pulmonary rehabilitation was 9.1, which results in an average of 18 exercise sessions. Completion rate of pulmonary rehabilitation: not explicitly reported. Follow‐up: in‐hospital period (average, 9 days) Usual care: standard care during hospital admission (median duration of hospital admission, 10 days). Follow‐up: in‐hospital period (average, 10 days) |
|
| Outcomes | 6MWD, CRQ, SGRQ, adverse events | |
| Notes | Pulmonary rehabilitation programme was considered extensive. Financial support provided by grants from National Natural Science Foundation of China (81200044) and Shanghai Pujiang Program (12PJ1407800) and Research Fund for the Doctoral Program of Higher Education of China (20120072120070) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not available from trial report. Information requested of study authors: "method of random allocation by using a computer random number generator" |
| Allocation concealment (selection bias) | Low risk | Information not available from trial report. Information requested of study authors: "randomisation process was concealed from those responsible for recruiting patients using central telephone randomisation system" |
| Blinding (performance bias and detection bias) Hospital admission | Unclear risk | Information not reported |
| Blinding (performance bias and detection bias) Health‐related quality of life | Unclear risk | Although unavoidable by definition, lack of blinding may affect outcome. |
| Blinding (performance bias and detection bias) Mortality | Unclear risk | Information not reported |
| Blinding (performance bias and detection bias) Walk test | Unclear risk | Information not available from trial report. Although unavoidable by definition, lack of blinding may affect outcome. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up are not specified. Final number of participants is not balanced between comparison groups (intervention group, n = 66; control group. n = 28). |
| Selective reporting (reporting bias) | High risk | CRQ domain scores not reported. Clinical trial registration number not reported |
| Other bias | Low risk | No indication of other biases |
Kirsten 1998.
| Methods | Randomised parallel‐group trial | |
| Participants | 29 participants with COPD (mean age 64 years, 90% males, mean FEV1 36% predicted) after inpatient treatment for acute exacerbation | |
| Interventions |
Rehabilitation: within 6 to 8 days after admission, inpatient pulmonary rehabilitation with endurance exercise (5 walking sessions/d for 10 days). Completion rate of pulmonary rehabilitation: not reported Usual care: standard inpatient care without exercise (not further specified). Follow‐up: 11 days |
|
| Outcomes | 6MWD | |
| Notes | Pulmonary rehabilitation programme was considered moderately extensive (downgraded by ‐1 for partly unsupervised training) Financial support provided by the Landesversicherungsanstalt (LVA) Freie und Hansestadt Hamburg |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; additional information not available from trial report |
| Allocation concealment (selection bias) | Unclear risk | Information not available from trial report |
| Blinding (performance bias and detection bias) Hospital admission | Unclear risk | Information not reported |
| Blinding (performance bias and detection bias) Health‐related quality of life | High risk | Information not available from trial report. Although unavoidable by definition, lack of blinding may affect outcome. |
| Blinding (performance bias and detection bias) Mortality | Low risk | Information not available from trial report. Outcome unlikely to be affected by knowledge of treatment group assignment |
| Blinding (performance bias and detection bias) Walk test | Unclear risk | Information not provided in trial report. Potential lack of blinding likely to affect outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up are not specified, although the final number of participants is balanced between comparison groups. |
| Selective reporting (reporting bias) | Unclear risk | Outcome reporting is sufficiently complete and transparent. Clinical trial registration number not reported |
| Other bias | Unclear risk | Unclear further potential bias |
Ko 2011.
| Methods | Randomised parallel‐group trial | |
| Participants | 60 participants with COPD (CG: n = 30, mean age 74 years, 1 female, mean FEV1 41% predicted; IG: n = 30, mean age 73 years, 100% males, FEV1 46% predicted) admitted with COPD exacerbation | |
| Interventions |
Rehabilitation: outpatient pulmonary rehabilitation (within 2‐3 weeks after discharge) with endurance training for 8 weeks (3 sessions/wk, 2 hours each session), advice to perform home exercises for at least 20 minutes/d and education on breathing techniques and how to cope with daily activities. Completion rate of pulmonary rehabilitation: 73% (22/30). Follow‐up: 12 months Usual care: instructions to have regular exercise. Follow‐up: 12 months |
|
| Outcomes | SGRQ, 6MWD, hospital readmissions, emergency admissions, mortality | |
| Notes | Pulmonary rehabilitation programme was considered extensive. Only a small proportion of participants received long‐acting bronchodilators, which may have limited their ability to exercise. Financial support provided by the Hong Kong Lung Foundation Grant and the Respiratory Research Fund of the Chinese University of Hong Kong Clinical trial identifier: NCT00287625 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer programme (allocation by minimization) was used to assist the randomisation of subjects equally into each group taking into account five factors; age (< 70 or ≥ 70 years), gender, length of hospital admission (< 7 or ≥ 7 days), 6 min walk (6MW) test (< 100 or ≥ 100 m) and predicted FEV1 (< 30 or ≥ 30%)." |
| Allocation concealment (selection bias) | Low risk | Used minimisation when allocation was concealed |
| Blinding (performance bias and detection bias) Hospital admission | Unclear risk | Information not available from trial report |
| Blinding (performance bias and detection bias) Health‐related quality of life | High risk | Information not available from trial report. Although unavoidable by definition, lack of blinding may affect outcome. |
| Blinding (performance bias and detection bias) Mortality | Low risk | Information not available from trial report. Outcome unlikely to be affected by knowledge of treatment group assignment |
| Blinding (performance bias and detection bias) Walk test | High risk | Information not available from trial report. Although unavoidable by definition, lack of blinding may affect outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up are detailed for both groups, and the final number of participants is balanced between comparison groups. "We used intention‐to‐treat analyses for all subjects who had been randomised." |
| Selective reporting (reporting bias) | Low risk | Information is reported sufficiently complete and in a transparent way. Clinical trial number reported |
| Other bias | Unclear risk | Unclear further potential bias |
Ko 2016.
| Methods | Randomised parallel‐group trial | |
| Participants | 180 participants with COPD (mean age 75 years, 96% males, mean FEV1 45% predicted) admitted for COPD exacerbation | |
| Interventions | Rehabilitation: comprehensive programme with multi‐disciplinary approach at discharge from hospital, which included respiratory nurse education, exercise training programme at home or a short course of outpatient pulmonary rehabilitation and management of COPD according to international guidelines. Participants were provided a telephone number of a healthcare provider for seeking advice. In addition, they received 3‐monthly telephone calls. Completion rate of pulmonary rehabilitation sessions: 71%. Follow‐up: 12 months Usual care: conventional medical treatment and follow‐up as per normal practice. Follow‐up: 12 months |
|
| Outcomes | Hospital readmission rate (primary outcome); SGRQ, 6MWD, mortality (secondary outcomes) | |
| Notes | Pulmonary rehabilitation programme was considered extensive (downgraded by ‐1 for some unsupervised training and upgraded by +1 for comprehensive self‐management programme). Clinical trial identifier: NCT01108835 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation was used that considered 5 potential confounders. |
| Allocation concealment (selection bias) | Low risk | By using minimisation, investigators ensured allocation concealment. |
| Blinding (performance bias and detection bias) Hospital admission | Unclear risk | Information about who collected the data not available from trial report |
| Blinding (performance bias and detection bias) Health‐related quality of life | High risk | Although unavoidable by definition, lack of blinding may affect outcome. |
| Blinding (performance bias and detection bias) Mortality | Low risk | Information about who collected the data not available from trial report. Outcome unlikely to be affected by knowledge of treatment group assignment |
| Blinding (performance bias and detection bias) Walk test | Low risk | "the research assistant performing walking tests was neither involved in the delivery of the patient care nor aware of the randomization process/" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up are detailed for both groups, and the final number of participants is balanced between comparison groups. "Analyses were conducted according to intention‐to‐treat principle." |
| Selective reporting (reporting bias) | Low risk | Information is reported in a sufficiently complete and transparent way. Clinical trial number reported |
| Other bias | Unclear risk | Unclear further potential bias |
Liao 2015.
| Methods | Randomised parallel‐group trial | |
| Participants | 61 participants with COPD (mean age 70 years, 61% males, no FEV1 data) admitted to hospital for a COPD exacerbation | |
| Interventions | Rehabilitation: inpatient respiratory rehabilitation exercise training package consisting of walk training (2 sessions/d, 10 to 30 minutes per session), disease awareness, sputum clearance treatment, pursed lip breathing, upper limb exercise with deep breathing and nutrition management and health education. Follow‐up: 4 days Usual care: health education. Follow‐up: 4 days |
|
| Outcomes | 6MWD | |
| Notes | Pulmonary rehabilitation programme was considered moderately extensive (downgraded by ‐2 for < 10 exercise sessions; upgraded by +1 for comprehensive self‐management training) Financial support provided by the Chest Hospital, Ministry of Health and Welfare, Taiwan (DOH100‐HO‐3053) Clinical trial identifier: NCT02329873 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomised via a coin toss |
| Allocation concealment (selection bias) | Unclear risk | Information not available from trial report |
| Blinding (performance bias and detection bias) Hospital admission | Unclear risk | Information not reported. Outcome not assessed |
| Blinding (performance bias and detection bias) Health‐related quality of life | Unclear risk | Information not reported. Outcome not assessed |
| Blinding (performance bias and detection bias) Mortality | Unclear risk | Information not reported. Outcome not assessed |
| Blinding (performance bias and detection bias) Walk test | Unclear risk | Information not available from trial report. Although unavoidable by definition, lack of blinding may affect outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up are detailed for both groups, and the final number of participants is balanced between comparison groups. |
| Selective reporting (reporting bias) | Low risk | Information is reported in a sufficiently complete and transparent way. Clinical trial number reported |
| Other bias | Low risk | No indication of other biases |
Man 2004.
| Methods | Randomised parallel‐group trial | |
| Participants | 42 participants with COPD (mean age 70 years, 41% males, FEV1 39% predicted) after inpatient treatment for acute exacerbation | |
| Interventions |
Rehabilitation: multi‐disciplinary outpatient pulmonary rehabilitation (within 10 days of discharge) with endurance and strength exercise and patient education for 8 weeks (2 sessions/wk). Completion rate of pulmonary rehabilitation: 85.7% (18/21 participants) Usual care: standard community care with respirologist. Follow‐up: 12 weeks |
|
| Outcomes | CRQ, SGRQ, ISWT, hospital readmission, hospital days, emergency admissions, mortality | |
| Notes | Pulmonary rehabilitation programme was considered extensive (upgraded by +1 for extensive self‐management training). Financial support provided by the British Lung Foundation Trevor Clay Memorial Grant. and by “Pursuing Perfection,” co‐ordinated by the NHS Modernisation Agency |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random number generator was our tool to assign an intervention to the first patient entering the study. We used the minimisation method to assign patients further to the intervention group, taking into account five factors: age (< 70 years or 70 years), sex, length of hospital admission (< 7 days or 7 days), incremental shuttle walk distance at discharge (< 100 metres or 100 metres), and predicted forced expiratory volume in one second (FEV1; < 30% or 30%)." |
| Allocation concealment (selection bias) | Low risk | Used minimisation when allocation was concealed |
| Blinding (performance bias and detection bias) Hospital admission | High risk | Lack of blinding may affect outcome assessment. "Owing to the nature of the intervention... it was not possible to blind the patients or the assessors (investigator responsible and members of the pulmonary rehabilitation team)." |
| Blinding (performance bias and detection bias) Health‐related quality of life | High risk | Although unavoidable by definition, lack of blinding may affect outcome. |
| Blinding (performance bias and detection bias) Mortality | Low risk | Information not available from trial report. Outcome unlikely to be affected by knowledge of treatment group assignment. |
| Blinding (performance bias and detection bias) Walk test | High risk | Lack of blinding likely to affect outcome assessment. "Owing to the nature of the intervention... it was not possible to blind the patients or the assessors (investigator responsible and members of the pulmonary rehabilitation team)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up are detailed for both groups, and the final number of participants is balanced between comparison groups. "We analysed data on intention to treat basis. We made no attempt to impute missing data from those participants who were lost to follow up." |
| Selective reporting (reporting bias) | Unclear risk | Information is reported in a sufficiently complete and transparent way. Clinical trial number not reported |
| Other bias | Low risk | No indication of other biases |
Murphy 2005.
| Methods | Randomised parallel‐group trial | |
| Participants | 26 participants with COPD (mean age 66 years, 65% males, mean FEV1 40% predicted) after home‐for‐hospital treatment for acute exacerbation | |
| Interventions |
Rehabilitation: supervised home‐based pulmonary rehabilitation with endurance and strength exercise for 6 weeks (2 supervised sessions/wk and daily unsupervised sessions). Completion rate of pulmonary rehabilitation: 76.9% (10/13 participants) Usual care: standard community care with respirologist. Follow‐up: 26 weeks |
|
| Outcomes | SGRQ, EQ‐5D, ISWT, 3‐minute step test, hospital readmission | |
| Notes | Dr Murphy provided standard deviations for SGRQ measurements. Pulmonary rehabilitation programme was considered moderately extensive (downgraded by ‐1 for 10 to 15 exercise training sessions). Financial support not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" ‐ Although the process of generating the randomisation schedule was not specified, it was presumed done because of efforts made with allocation concealment. |
| Allocation concealment (selection bias) | Low risk | "...each patient was randomly assigned in a 1:1 ratio for the home exercise group or a control group (standard care group) using blinded sealed envelopes." |
| Blinding (performance bias and detection bias) Hospital admission | Unclear risk | Information not reported |
| Blinding (performance bias and detection bias) Health‐related quality of life | High risk | Although unavoidable by definition, lack of blinding may affect outcome. |
| Blinding (performance bias and detection bias) Mortality | Low risk | Information not available from trial report. Outcome unlikely to be affected by knowledge of treatment group assignment |
| Blinding (performance bias and detection bias) Walk test | Unclear risk | Information not provided in trial report. Potential lack of blinding likely to affect outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up are detailed for both groups, and the final number of participants is balanced between comparison groups. |
| Selective reporting (reporting bias) | Unclear risk | Information is reported in a sufficiently complete and transparent way. Clinical trial number not reported |
| Other bias | Low risk | No indication of other biases |
Nava 1998.
| Methods | Randomised parallel‐group trial | |
| Participants | 70 participants with COPD (mean age 66 years, 73% males, mean FEV1 32% predicted, 76% needed mechanical ventilation) admitted to inpatient care for treatment of acute exacerbation | |
| Interventions |
Rehabilitation: within 3 to 5 days after admission, inpatient pulmonary rehabilitation with 4 steps of increasing intensity Step I, if unable to walk: mobilisation and strength training for lower extremities Step II, if able to walk: endurance exercise (walking) Step III, if possible: endurance exercise (cycling and stair climbing) and respiratory muscle training Step IV, if possible: endurance exercise (cycling at highest tolerated intensity, 2 sessions/d for 3 weeks) Completion rate of pulmonary rehabilitation: 85.4% (41/48 participants) Usual care: only steps I and II. Follow‐up: 6 weeks |
|
| Outcomes | 6MWD, mortality | |
| Notes | Pulmonary rehabilitation programme was considered extensive (upgraded by +1 for > 30 exercise sessions). Financial support not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomised via a computer programme; additional information not available from trial report |
| Allocation concealment (selection bias) | Unclear risk | Information not available from trial report |
| Blinding (performance bias and detection bias) Hospital admission | Unclear risk | Information not available from trial report |
| Blinding (performance bias and detection bias) Health‐related quality of life | High risk | Information not available from trial report. Although unavoidable by definition, lack of blinding may affect outcome. |
| Blinding (performance bias and detection bias) Mortality | Low risk | Information not available from trial report. Outcome unlikely to be affected by knowledge of treatment group assignment |
| Blinding (performance bias and detection bias) Walk test | Unclear risk | Information not provided in trial report. Potential lack of blinding likely to affect outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up are not specified. The final number of participants is balanced between groups according to 3:1 randomisation (intervention group n = 60 and control group n = 20). |
| Selective reporting (reporting bias) | Unclear risk | Outcome reporting is sufficiently complete and transparent. Clinical trial registration number not reported |
| Other bias | Low risk | No indication of other biases |
Seymour 2010.
| Methods | Randomised parallel‐group trial | |
| Participants | 60 participants with COPD (mean age 66 years, 82% males, mean FEV1 52% predicted) after inpatient treatment of acute exacerbation | |
| Interventions |
Rehabilitation: within a week after hospital discharge, outpatient pulmonary rehabilitation twice‐weekly exercise (limb strengthening and aerobic activities) and education sessions, during 8 weeks. Completion rate of pulmonary rehabilitation: 77% (23/30). Participants were provided general information about COPD and were offered outpatient appointments with general practitioner or respiratory team. Follow‐up: 12 weeks Usual care: Participants were provided general information about COPD and were offered outpatient appointments with general practitioner or respiratory team. Not referred further Follow‐up: 12 weeks |
|
| Outcomes | Exacerbation with hospitalisation (primary outcome), ISWT, ESWT, CRQ and SGRQ (secondary) | |
| Notes | Pulmonary rehabilitation programme was considered extensive (downgraded by ‐1 for 10 to 15 exercise training sessions, and upgraded by +1 for extensive self‐management training). Financial support provided by the British Lung Foundation Clinical trial identifier: NCT00557115 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were allocated by concealed randomisation by a statistician. The minimisation method matched groups for age (< 70 years or ≥ 70 years), sex (male or female), predicted FEV1 (< 30% or ≥ 30%), duration of admission (< 7 or ≥ 7 days) and baseline ISWT (< 100 m or ≥ 100 m)." |
| Allocation concealment (selection bias) | Low risk | Used minimisation when allocation was concealed |
| Blinding (performance bias and detection bias) Hospital admission | High risk | Authors state: "Due to the nature of the intervention, it was not possible to blind subjects to their allocation." |
| Blinding (performance bias and detection bias) Health‐related quality of life | High risk | "Due to the nature of the intervention, it was not possible to blind subjects to their allocation." Although unavoidable by definition, lack of blinding may affect outcome. |
| Blinding (performance bias and detection bias) Mortality | Low risk | Information not available from trial report. Outcome unlikely to be affected by knowledge of treatment group assignment |
| Blinding (performance bias and detection bias) Walk test | High risk | Authors state: "Due to the nature of the intervention, it was not possible to blind subjects to their allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up are well specified, and the final number of participants is balanced between comparison groups. "Participants were analysed on an intention‐to‐treat basis regardless of compliance." |
| Selective reporting (reporting bias) | Low risk | Outcome reporting is sufficiently complete and transparent: Results for all listed primary and secondary outcomes were reported. Clinical trial registration number reported |
| Other bias | Low risk | No indication of other biases |
Tang 2012.
| Methods | Randomised parallel‐group trial | |
| Participants | 32 participants with COPD (CG: mean age 78 years, 55% males, mean FEV1 47% predicted; low‐Intensity IG: mean age 68 years, 45% males, mean FEV1 45% predicted; high‐Intensity IG: mean age 74 years, 20% males, mean FEV1 46% predicted) after inpatient treatment of acute exacerbation | |
| Interventions | Rehabilitation: Within 2 days after admission, inpatient exercise programme followed twice‐daily 15‐minute exercise sessions, in addition to standard physical therapy treatment. Low‐intensity group walked at 40% and high‐intensity group at 70% of the 3‐minute walk test for 7.5 minutes; upper and lower limb resistance exercise was also done. Adherence to pulmonary rehabilitation was 78% in the low‐intensity group and 71% in the high‐intensity group. Follow‐up: until hospital discharge Usual care: once‐daily physical therapy (sputum clearance techniques, mobility and functional training) | |
| Outcomes | Adverse events (primary outcome), 3MWD (secondary outcome) | |
| Notes | Pulmonary rehabilitation programme was considered slightly extensive (downgraded by ‐2 for < 10 exercise training sessions). Financial support not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A block randomisation allocation sequence was generated using a web‐based program." |
| Allocation concealment (selection bias) | Unclear risk | "Principal investigator unsealed envelopes sequentially and allocated patients after baseline assessment." |
| Blinding (performance bias and detection bias) Hospital admission | Unclear risk | Information not reported |
| Blinding (performance bias and detection bias) Health‐related quality of life | Unclear risk | Infromation not reported |
| Blinding (performance bias and detection bias) Mortality | Unclear risk | Information not available from trial report. Outcome unlikely to be affected by knowledge of treatment group assignment |
| Blinding (performance bias and detection bias) Walk test | Unclear risk | "RCT blinded to baseline and discharge assessments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up are specified, and the final number of participants is balanced between comparison groups. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting is sufficiently complete and transparent: Results for all listed primary and secondary outcomes were reported. Clinical trial registration number not reported |
| Other bias | Low risk | No indication of other biases |
Torres‐Sánchez 2014.
| Methods | Randomised parallel‐group trial | |
| Participants | 60 participants with COPD (mean age 71 years, 93% males, FEV1 not reported) admitted for treatment of non‐infectious exacerbation | |
| Interventions |
Rehabilitation: daily resistance lower limbs and controlled breathing exercises for 45 minutes. No other information reported Usual care: standard medical treatment |
|
| Outcomes | SGRQ | |
| Notes | Information extracted from conference meeting abstract. Study authors have not published the data. Extensiveness of pulmonary rehabilitation programme could not be assessed. Financial support not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "were randomly allocated to a control or an intervention group" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Hospital admission | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Health‐related quality of life | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Mortality | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Walk test | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
Torres‐Sánchez 2015.
| Methods | Randomised parallel‐group trial | |
| Participants | 49 participants with COPD and body mass index (BMI) ≥ 30 kg/cm2 and admitted to the hospital for ≥ 7 days for a COPD exacerbation (CG: mean age 74 years, 91% males, mean FEV1 41% predicted, mean BMI 34 kg/cm2; IG: mean age 72 years, 100% males, mean FEV1 39% predicted, mean BMI 34 kg/cm2 | |
| Interventions | Rehabilitation: twice‐daily individualised and supervised multi‐modal PR during 30 to 45 minutes. Programme included deep breathing, range of motion and upper and lower limb muscle strengthening exercises and 20 to 30 minutes of limb exercises. Adherence to PR not reported, but participants needed ≥ 7 training sessions to be considered for the analyses. It is unclear how many participants were excluded because they completed fewer than 7 sessions. Usual care: standard medical therapy, including systemic steroids, inhaled bronchodilators and oxygen | |
| Outcomes | 2‐Minute step‐in‐place test, EuroQol (EQ‐5D) | |
| Notes | Pulmonary rehabilitation programme was considered moderately extensive (downgraded by ‐1 for 10 to 15 exercise training sessions). Financial support provided by the professional association of physiotherapists of Andalusia, Spain (Colegio Profesional de Fisioterapeutas de Andalucía) and the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) and the Spanish Foundation of the Lung (Fundación Respira) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | An independent nurse assigned participants to IG or CG according to a computer‐generated randomisation list. The nurse informed the physiotherapist once participants had given their approval to participate in the study. |
| Blinding (performance bias and detection bias) Hospital admission | Unclear risk | NA |
| Blinding (performance bias and detection bias) Health‐related quality of life | High risk | Although unavoidable by definition, lack of blinding may affect outcome. |
| Blinding (performance bias and detection bias) Mortality | Unclear risk | NA |
| Blinding (performance bias and detection bias) Walk test | Unclear risk | Unclear who supervised the 2‐minute step‐in‐place test |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Adherence to PR not reported, but participants needed ≥ 7 training sessions to be considered for the analyses. It is unclear how many participants were excluded because they completed fewer than 7 sessions. |
| Selective reporting (reporting bias) | Unclear risk | Unclear if some measures taken at baseline (e.g. SGRQ) were not used as outcomes |
| Other bias | Unclear risk | No indication of other biases |
Troosters 2002.
| Methods | Randomised parallel‐group trial | |
| Participants | 43 participants with COPD (mean age 62 years, 85% males, FEV1 39% predicted) after inpatient treatment for acute exacerbation | |
| Interventions |
Rehabilitation: outpatient pulmonary rehabilitation with endurance and strength exercise for 6 months (3 sessions/wk in first 3 months, then 2 sessions/wk). Completion rate of pulmonary rehabilitation: 70.8% (17/24 participants) Usual care: standard community care with respirologist (not further specified). Follow‐up: 208 weeks |
|
| Outcomes | 6MWD, mortality | |
| Notes | Pulmonary rehabilitation programme was considered extensive (upgraded by +1 for > 30 exercise sessions). Financial support provided by the Fonds voor Wetenschappelijk Onderzoek‐Vlaanderen (G0189.97 and G0175.99), Levenslijn Grant 7.0002.94, and Onderzoeksfonds, KU Leuven, Grant 27/98 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; additional information not available from trial report |
| Allocation concealment (selection bias) | Unclear risk | Information not available from trial report |
| Blinding (performance bias and detection bias) Hospital admission | Unclear risk | Information not available from trial report. Outcome unlikely to be affected by knowledge of treatment group assignment |
| Blinding (performance bias and detection bias) Health‐related quality of life | High risk | Information not provided in trial report. Although unavoidable by definition, lack of blinding may affect outcome. |
| Blinding (performance bias and detection bias) Mortality | Low risk | Information not available from trial report. Outcome unlikely to be affected by knowledge of treatment group assignment |
| Blinding (performance bias and detection bias) Walk test | Unclear risk | Information not provided in trial report. Potential lack of blinding likely to affect outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up are specified, and the final number of participants is balanced between comparison groups. |
| Selective reporting (reporting bias) | Unclear risk | Outcome reporting is sufficiently complete and transparent. Clinical trial registration number not reported |
| Other bias | Low risk | No indication of other biases |
Troosters 2010.
| Methods | Randomised parallel‐group trial | |
| Participants | 36 participants with COPD (CG: n = 19, mean age 69 years, 74% males, FEV1 50% predicted; IG: n = 17, mean age 67 years, 76% males, FEV1 40% predicted) admitted for treatment for acute exacerbation | |
| Interventions |
Rehabilitation: daily quadriceps resistance training for 7 days. Follow‐up: 8 days Usual care: medical usual care plus mucous secretion clearance techniques and breathing exercises. Follow‐up: 8 days |
|
| Outcomes | 6MWD | |
| Notes | Pulmonary rehabilitation programme was considered not extensive (downgraded by ‐2 for < 10 exercise training sessions, and by ‐1 when only strength training was offered). Financial support provided by the Research Foundation, Flanders grants KAN 1.5.139.06N and G.0386.05N Clinical trial identifier: NCT00877084 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised via "opaque envelopes prepared by an independent secretary" |
| Allocation concealment (selection bias) | High risk | "Tests were performed by researchers who were not blind to the allocation of the patients." |
| Blinding (performance bias and detection bias) Hospital admission | Unclear risk | Information not reported |
| Blinding (performance bias and detection bias) Health‐related quality of life | Unclear risk | Information not reported |
| Blinding (performance bias and detection bias) Mortality | Unclear risk | Information not reported |
| Blinding (performance bias and detection bias) Walk test | High risk | Researchers and participants were not blind to the allocation group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up are not specified, although the final number of participants is balanced between comparison groups. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting is sufficiently complete and transparent. Clinical trial registration number reported |
| Other bias | Low risk | Supplemental material on methods and analysis reported. No indication of other biases |
3MWD: three‐minute walking distance; 6MWD: six‐minute walking distance; ATS: American Thoracic Society; BMI: body mass index; BODE index: body mass index, airflow obstruction, dyspnoea and exercise capacity index; CG: control group; COPD: chronic obstructive pulmonary disease; CRQ: Chronic Respiratory Questionnaire; EQ‐5D: EuroQoL questionnaire; ERS: European Respiratory Society; ESWT: endurance shuttle walk test; FEV1: forced expiratory volume in one second; h: hour; IG: intervention group; ISWT: incremental shuttle walk test; SF‐36: short‐form health survey; SGRQ: St George's Respiratory Questionnaire.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ali 2014 | Not a randomised trial |
| Aljassem 2012 | Not a randomised trial |
| Babu 2010 | No control group without exercise training |
| Benzo 2015 | Did not study intervention but provided reasons for non‐participation in a trial |
| Puhan 2012 | No control group without rehabilitation |
| Rasekaba 2009 | Not a randomised trial |
| Saey 2011 | Comment on Troosters 2010 trial |
| Tang 2013 | Qualitative results from Tang 2012 trial |
| Torres‐Sánchez 2013 | Not a randomised trial |
| Zheng 2012 | Not a randomised trial |
Characteristics of ongoing studies [ordered by study ID]
Beekman 2014.
| Trial name or title | Reducing Exacerbations in Patients With Chronic Obstructive Pulmonary Disease With Physiotherapy |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Study ongoing |
Castelain 2008.
| Trial name or title | Early Rehabilitation of COPD Patients in ICU |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Study has been terminated. No data published |
Hughes 2015.
| Trial name or title | Pulmonary Rehabilitation and ACTIvity after COPD Exacerbations: the PRACTICE trial |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Recruiting participants |
Knaut 2014.
| Trial name or title | Evaluation of Aerobic Exercise Program During Hospitalization in Quality of Life and in Exercise Capacity After One Month of Discharge in Exacerbated COPD |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Abstracts/22nd Annual Congress, Munich, Germany, 6‐10 September 2014 |
Morante 2013.
| Trial name or title | Impact of Early Respiratory Rehabilitation in the Exacerbations of Re‐admitted COPD Patients |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Recruiting participants |
Spielmanns 2015.
| Trial name or title | Effect of Pneumological Rehabilitation After an Acute Exacerbation of COPD (Chronic Obstructive Pulmonary Disease) |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Not recruiting |
Stickland 2015.
| Trial name or title | Examining Pulmonary Rehabilitation on Discharged COPD Patients |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Recruiting participants |
Differences between protocol and review
Review authors added risk of bias tables for the 2010 update of this review. We added a 'Summary of findings' table, along with specified subgroup analyses on extensiveness of pulmonary rehabilitation programmes, length of follow‐up and three indicators of methodological quality.
We added clarification regarding types of studies: We did not include studies on pulmonary rehabilitation programmes that included only neuromuscular stimulation or inspiratory muscle training but no physical exercise programme.
In the original protocol, we planned to attempt to obtain data from intention‐to‐treat (ITT) and per‐protocol populations, and to perform a sensitivity analysis to see whether this made a difference in meta‐analysis results; however, the number of trials and the quality of their reporting did not allow us to compare ITT and per‐protocol analyses.
Contributions of authors
Protocol writing: Puhan, Scharplatz, Gimeno‐Santos. Acquisition of data: Puhan, Gimeno‐Santos, Scharplatz. Analysis and interpretation of data: Puhan, Gimeno‐Santos, Scharplatz, Troosters, Cates. Drafting of manuscript: Puhan. Critical revision of manuscript for important intellectual content: Puhan, Gimeno‐Santos, Scharplatz, Troosters, Cates.
Dr Madlaina Scharplatz (MS) and helped with the previous version of this review, but is not an author of the current version of the review.
We thank Prof Johann Steurer and Prof Haydn Walters for contributions to previous versions.
Sources of support
Internal sources
The review authors declare that no internal funding was received for this systematic review, Other.
External sources
The study authors declare that no external funding was received for this systematic review, Other.
Declarations of interest
MA Puhan, E Gimeno‐Santos, CJ Cates: no conflicts of interest to declare.
T Troosters conducts research in this field and recruits participants with acute exacerbations into rehabilitation programmes.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Behnke 2000 {published data only}
- Behnke M, Jorres RA, Kirsten D, Magnussen H. Clinical benefits of a combined hospital and home‐based exercise programme over 18 months in patients with severe COPD. Monaldi Archives for Chest Disease 2003;59(1):44‐51. [PubMed] [Google Scholar]
- Behnke M, Taube C, Kirsten D, Lehnigk B, Jorres RA, Magnussen H. Home‐based exercise is capable of preserving hospital‐based improvements in severe chronic obstructive pulmonary disease. Respiratory Medicine 2000;94(12):1184‐91. [DOI] [PubMed] [Google Scholar]
Borges 2014 {published data only}
- Borges RC, Carvalho CR. Impact of resistance training in chronic obstructive pulmonary disease patients during periods of acute exacerbation. Archives of Physical Medicine and Rehabilitation 2014;95(9):1638‐45. [DOI] [PubMed] [Google Scholar]
Carr 2009 {published data only}
- Carr SJ, Hill K, Brooks D, Goldstein RS. Pulmonary rehabilitation after acute exacerbation of chronic obstructive pulmonary disease in patients who previously completed a pulmonary rehabilitation program. Journal of Cardiopulmonary Rehabilitation and Prevention 2009;29(5):318‐24. [DOI] [PubMed] [Google Scholar]
Deepak 2014 {published data only}
- Deepak TH, Mohapatra PR, Janmeja AK, Sood P, Gupta M. Outcome of pulmonary rehabilitation in patients after acute exacerbation of chronic obstructive pulmonary disease. Indian Journal of Chest Diseases & Allied Sciences 2014;56(1):7‐12. [PubMed] [Google Scholar]
Eaton 2009 {published data only}
- Eaton T, Young P, Fergusson W, Moodie L, Zeng I, O'Kane F, et al. Does early pulmonary rehabilitation reduce acute health‐care utilization in COPD patients admitted with an exacerbation? A randomized controlled study. Respirology 2009;14(2):230‐8. [DOI] [PubMed] [Google Scholar]
Greening 2014 {published and unpublished data}
- Greening NJ, Williams JE, Hussain SF, Harvey‐Dunstan TC, Bankart MJ, Chaplin EJ, et al. An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trial. BMJ 2014;349:g4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey‐Dunstan T, Greening N, Williams J, Bankart J, Hussain S, Chaplin E, et al. Early pulmonary rehabilitation for exacerbations of chronic respiratory disease (CRD): functional results. European Respiratory Journal 2013; Vol. 42, issue Suppl 57:4854.
He 2015 {published data only}
- He M, Yu S, Wang L, Lv H, Qiu Z. Efficiency and safety of pulmonary rehabilitation in acute exacerbation of chronic obstructive pulmonary disease. Medical Science Monitor 2015;21:806‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirsten 1998 {published data only}
- Kirsten DK, Taube C, Lehnigk B, Jorres RA, Magnussen H. Exercise training improves recovery in patients with COPD after an acute exacerbation. Respiratory Medicine 1998;92(10):1191‐8. [DOI] [PubMed] [Google Scholar]
Ko 2011 {published data only}
- Ko FW, Dai DL, Ngai J, Tung A, Ng S, Lai K, et al. Effect of early pulmonary rehabilitation on health care utilization and health status in patients hospitalized with acute exacerbations of COPD. Respirology 2011;16(4):617‐24. [DOI] [PubMed] [Google Scholar]
Ko 2016 {published data only}
- Ko F, Cheung NK, Rainer TH, Lum C, Wong I, Hui D. Comprehensive care programme for patients with chronic obstructive pulmonary disease: a randomized controlled trial. Thorax 2016 July 28 [Epub ahead of print]. [DOI: 10.1136/thoraxjnl-2016-208396] [DOI] [PubMed] [Google Scholar]
Liao 2015 {published data only}
- Liao LY, Chen KM, Chung WS, Chien JY. Efficacy of a respiratory rehabilitation exercise training package in hospitalized elderly patients with acute exacerbation of COPD: a randomized control trial. International Journal of Chronic Obstructive Pulmonary Disease 2015;10(1):1703‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Man 2004 {published data only}
- Man WD, Polkey MI, Donaldson N, Gray BJ, Moxham J. Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled study. BMJ 2004;329:1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2005 {published data only}
Nava 1998 {published data only}
- Nava S. Rehabilitation of patients admitted to a respiratory intensive care unit. Archives of Physical Medicine & Rehabilitation 1998;79(7):849‐54. [DOI] [PubMed] [Google Scholar]
Seymour 2010 {published data only}
- Seymour JM, Moore L, Jolley CJ, Ward K, Creasey J, Steier JS, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax 2010;65(5):423‐8. [DOI] [PubMed] [Google Scholar]
Tang 2012 {published data only}
- Tang CY, Blackstock FC, Clarence M, Taylor NF. Early rehabilitation exercise program for inpatients during an acute exacerbation of chronic obstructive pulmonary disease: a randomized controlled trial. Journal of Cardiopulmonary Rehabilitation and Prevention 2012;32(3):163‐9. [DOI] [PubMed] [Google Scholar]
Torres‐Sánchez 2014 {published data only}
- Torres‐Sánchez I, Valenza MC, Valenza‐Demet G, Cabrera‐Martos I, Flores‐Barba MJ, Ruíz‐Sáez A. Quality of life in hospitalized patients for exacerbation of COPD included in a physical therapy program. Chest 2014;145(3_MeetingAbstracts):372A. [Google Scholar]
Torres‐Sánchez 2015 {published data only}
- Torres‐Sánchez I, Valenza MC, Sáez‐Roca G, Cabrera‐Martos I, López‐Torres I, Rodríguez‐Torres J. Results of a multimodal program during hospitalization in obese COPD exacerbated patients. COPD 2015;13(1):19‐25. [DOI] [PubMed] [Google Scholar]
Troosters 2002 {published data only}
- Troosters T, Gosselink R, Paepe K, Decramer M. Pulmonary rehabilitation improves survival in COPD patients with a recent severe acute exacerbation. American Journal of Respiratory and Critical Care Medicine 2002;165(8):A16. [Google Scholar]
- Troosters T, Gosselink R, Decramer M. Short‐ and long‐term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. American Journal of Medicine 2000;109(3):207‐12. [DOI] [PubMed] [Google Scholar]
Troosters 2010 {published data only}
- Troosters T, Probst VS, Crul T, Pitta F, Gayan‐Ramirez G, Decramer M, et al. Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2010;181(10):1072‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ali 2014 {published data only}
- Ali MS, Talwar D, Jain SK. The effect of a short‐term pulmonary rehabilitation on exercise capacity and quality of life in patients hospitalised with acute exacerbation of chronic obstructive pulmonary disease. Indian Journal of Chest Diseases & Allied Sciences 2014;56(1):13‐9. [PubMed] [Google Scholar]
Aljassem 2012 {published data only}
- Aljassem A, Fodor E, LaBan MM, Ravikrishnan KP, Riutta JC, Sherman S, et al. Poster 77 ‐ Pulmonary rehabilitation: its role in decreasing length of stay, cost of hospitalization, and 30‐day readmission rates for chronic obstructive pulmonary disease. PM & R: Journal of Injury, Function, and Rehabilitation 2012;4(10 Suppl):S215‐6. [Google Scholar]
Babu 2010 {published data only}
- Babu AS, Noone MS, Haneef M, Samuel P. The effects of 'on‐call/out of hours' physical therapy in acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Clinical Rehabilitation 2010;24(9):802‐9. [DOI] [PubMed] [Google Scholar]
Benzo 2015 {published data only}
- Benzo R, Wetzstein M, Neuenfeldt P, McEvoy C. Implementation of physical activity programs after COPD hospitalizations: lessons from a randomized study. Chronic Respiratory Disease 2015;12:5‐10. [DOI] [PubMed] [Google Scholar]
Puhan 2012 {published data only}
- Puhan MA, Spaar A, Frey M, Turk A, Brändli O, Ritscher D, et al. Early versus late pulmonary rehabilitation in chronic obstructive pulmonary disease patients with acute exacerbations: a randomized trial. Respiration Epub 2011 Aug 16;83(6):499‐506. [DOI] [PubMed] [Google Scholar]
Rasekaba 2009 {published data only}
- Rasekaba TM, Williams E, Hsu‐Hage B. Can a chronic disease management pulmonary rehabilitation program for COPD reduce acute rural hospital utilization?. Chronic Respiratory Disease 2009;6(3):157‐63. [DOI] [PubMed] [Google Scholar]
Saey 2011 {published data only}
- Saey D, Ribeiro F. Resistance training preserves skeletal muscle function in patients with COPD who are hospitalised with an acute exacerbation. Journal of Physiotherapy 2011;57(3):194. [DOI] [PubMed] [Google Scholar]
Tang 2013 {published data only}
- Tang CY, Taylor NF, Blackstock FC. Patients admitted with an acute exacerbation of chronic obstructive pulmonary disease had positive experiences exercising from the beginning of their hospital stay: a qualitative analysis. Chronic Respiratory Disease 2013;10(4):197‐205. [DOI] [PubMed] [Google Scholar]
Torres‐Sánchez 2013 {published data only}
- Torres‐Sánchez I, Valenza MC, Cabrera‐Martos I, López‐Pacheco ML, Valenza‐Demet G. Physical therapy effects on psychological functions in patients with acute COPD exacerbation. European Respiratory Journal 2013;42(Suppl 57):P1297. [Google Scholar]
Zheng 2012 {published data only}
- Zheng Z, Liang A, Cen HH, Chen Q. Early versus late pulmonary rehabilitation on anxiety and depression in chronic obstructive pulmonary disease patients with acute exacerbations. European Respiratory Journal 2012;40(Suppl 56):P3546. [Google Scholar]
References to ongoing studies
Beekman 2014 {published data only}
- Reducing Exacerbations in Patients With Chronic Obstructive Pulmonary Disease With Physiotherapy. Ongoing study Starting date of trial not provided. Contact author for more information.
Castelain 2008 {published data only}
- Early Rehabilitation of COPD Patients in ICU. Ongoing study Starting date of trial not provided. Contact author for more information.
Hughes 2015 {published data only}
- Pulmonary Rehabilitation and ACTIvity after COPD Exacerbations: the PRACTICE trial. Ongoing study Starting date of trial not provided. Contact author for more information.
Knaut 2014 {published data only}
- Evaluation of Aerobic Exercise Program During Hospitalization in Quality of Life and in Exercise Capacity After One Month of Discharge in Exacerbated COPD. Ongoing study Starting date of trial not provided. Contact author for more information.
Morante 2013 {published data only}
- Impact of Early Respiratory Rehabilitation in the Exacerbations of Re‐admitted COPD Patients. Ongoing study Starting date of trial not provided. Contact author for more information.
Spielmanns 2015 {published data only}
- Effect of Pneumological Rehabilitation After an Acute Exacerbation of COPD (Chronic Obstructive Pulmonary Disease). Ongoing study Starting date of trial not provided. Contact author for more information.
Stickland 2015 {published data only}
- Examining Pulmonary Rehabilitation on Discharged COPD Patients. Ongoing study Starting date of trial not provided. Contact author for more information.
Additional references
Aaron 2014
- Aaron SD. Management and prevention of exacerbations of COPD. BMJ 2014;349:g5237. [DOI] [PubMed] [Google Scholar]
Almagro 2002
- Almagro P, Calbo E, Ochoa DE, Barreiro B, Quintana S, Heredia JL, et al. Mortality after hospitalization for COPD. Chest 2002;121(5):1441‐8. [DOI] [PubMed] [Google Scholar]
Amir 2011
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Annals of Internal Medicine 2011;155(3):179‐91. [DOI] [PubMed] [Google Scholar]
ATS ERS Policy statement 2015
- Rochester CL, Vogiatzis I, Holland AE, Lareau SC, Marciniuk DD, Puhan MA, et al. An Official American Thoracic Society/European Respiratory Society Policy Statement: Enhancing implementation, use, and delivery of pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine 2015;192(11):1373‐86. [DOI] [PubMed] [Google Scholar]
Bourbeau 2003
- Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupre A, Begin R, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease‐specific self‐management intervention. Archives of Internal Medicine 2003;163(5):585‐91. [DOI] [PubMed] [Google Scholar]
BTS 2013
- British Thoracic Society Pulmonary Rehabilitation Guideline Group. BTS guideline on pulmonary rehabilitation in adults. Thorax 2013;68:S2. [Google Scholar]
Cappelleri 1996
- Cappelleri JC, Ioannidis JP, Schmid CH, Ferranti SD, Aubert M, Chalmers TC, et al. Large trials vs meta‐analysis of smaller trials: how do their results compare?. JAMA 1996;276(16):1332‐8. [PubMed] [Google Scholar]
Chan‐Yeung 2004
- Chan‐Yeung M, Ait‐Khaled N, White N, Ip MS, Tan WC. The burden and impact of COPD in Asia and Africa. International Journal of Tuberculosis and Lung Disease 2004;8(1):2‐14. [PubMed] [Google Scholar]
Connors 1996
- Connors AF Jr, Dawson NV, Thomas C, Harrell FE Jr, Desbiens N, Fulkerson WJ, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT Investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). American Journal of Respiratory and Critical Care Medicine 1996;154(4 Pt 1):959‐67. [DOI] [PubMed] [Google Scholar]
Coventry 2007
- Coventry PA, Hind D. Comprehensive pulmonary rehabilitation for anxiety and depression in adults with chronic obstructive pulmonary disease: systematic review and meta‐analysis. Journal of Psychosomatic Research 2007;63(5):551‐65. [DOI] [PubMed] [Google Scholar]
Cydulka 1997
- Cydulka RK, McFadden ER Jr, Emerman CL, Sivinski LD, Pisanelli W, Rimm AA. Patterns of hospitalization in elderly patients with asthma and chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1997;156(6):1807‐12. [DOI] [PubMed] [Google Scholar]
Egger 1998
- Egger M, Smith GD. Bias in location and selection of studies. BMJ 1998;316(7124):61‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
ERS ATS Statement 2013
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An Official American Thoracic Society/European Respiratory Society Statement: key concepts and advances in pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine 2013;188(8):e13–e64. [DOI] [PubMed] [Google Scholar]
Escarrabill 2014
Ford 2015
- Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state‐specific medical and absenteeism costs of COPD among adults aged ≥ 18 years in the United States for 2010 and projections through 2020. Chest 2015;147(1):31‐45. [DOI] [PubMed] [Google Scholar]
Garcia‐Aymerich 2003
- Garcia‐Aymerich J, Farrero E, Felez MA, Izquierdo J, Marrades RM, Anto JM. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax 2003;58(2):100‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia‐Aymerich 2006
- Garcia‐Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006;61(9):772‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
GOLD 2016
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016. http://www.goldcopd.org/ (accessed 5 December 2016).
Griffiths 2001
- Griffiths TL, Phillips CJ, Davies S, Burr ML, Campbell IA. Cost effectiveness of an outpatient multidisciplinary pulmonary rehabilitation programme. Thorax 2001;56(10):779‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Groenewegen 2003
- Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality‐related factors after hospitalization for acute exacerbation. Chest 2003;124(2):459‐67. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Holland 2014
- Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, et al. An Official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. European Respiratory Journal 2014;44(6):1428‐46. [DOI] [PubMed] [Google Scholar]
Hopkinson 2014
- Hopkinson N. What is and what is not post exacerbation pulmonary rehabilitation?. BMJ 2014;349:g4315. [Google Scholar]
Ioannidis 1998
- Ioannidis JP, Cappelleri JC, Lau J. Issues in comparisons between meta‐analyses and large trials. JAMA 1998;279(14):1089‐93. [DOI] [PubMed] [Google Scholar]
Jansson 2013
- Jansson SA, Backman H, Stenling A, Lindberg A, Ronmark E, Lundback B. Health economic costs of COPD in Sweden by disease severity. Has it changed during a ten years period?. Respiratory Medicine 2013;107(12):1931–8. [DOI] [PubMed] [Google Scholar]
Jones 2005
- Jones PW. St. George's Respiratory Questionnaire: MCID. Journal of COPD 2005;2(1):75‐9. [DOI] [PubMed] [Google Scholar]
Kessler 2006
- Kessler R, Ståhl E, Vogelmeier C, Haughney J, Trudeau E, Löfdahl CG, et al. Patient understanding, detection, and experience of COPD exacerbations: an observational, interview‐based study. Chest 2006;130:133–42. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
LeLorier 1997
- LeLorier J, Gregoire G, Benhaddad A, Lapierre J, Derderian F. Discrepancies between meta‐analyses and subsequent large randomized, controlled trials. New England Journal of Medicine 1997;337(8):536‐42. [DOI] [PubMed] [Google Scholar]
Maddocks 2015
- Maddocks M, Kon SS, Singh SJ, Man WD. Rehabilitation following hospitalization in patients with COPD: can it reduce readmissions?. Respirology 2015;20(3):395‐404. [DOI] [PubMed] [Google Scholar]
Mannino 2002
- Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest 2002;121(90050):121S‐6S. [DOI] [PubMed] [Google Scholar]
Martin 1982
- Martin TR, Lewis SW, Albert RK. The prognosis of patients with chronic obstructive pulmonary disease after hospitalization for acute respiratory failure. Chest 1982;82(3):310‐4. [DOI] [PubMed] [Google Scholar]
McCarthy 2015
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD003793.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
NHLBI 2001
- National Heart LaBI, US Department of Health and Human Services NIoH. Data Fact Sheet ‐ Chronic obstructive pulmonary disease (COPD). http://www.cancer.org/acs/groups/cid/documents/webcontent/003143‐pdf.pdf (accessed 2 June 2015).
NICE 2010
- National Institute for Health and Care Excellence (NICE). Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. www.nice.org.uk/guidance/cg101 (accessed 2 June 2015).
Oostenbrink 2004
- Oostenbrink JB, Rutten‐van Molken M. Resource use and risk factors in high‐cost exacerbations of COPD. Respiratory Medicine 2004;98:883‐91. [DOI] [PubMed] [Google Scholar]
Piquet 2013
- Piquet J, Chavaillon JM, David P, Martin F, Blanchon F, Roche N, et al. High‐risk patients following hospitalisation for an acute exacerbation of COPD. European Respiratory Journal 2013;42(4):946‐55. [DOI] [PubMed] [Google Scholar]
Pitta 2006
- Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest 2006;129(3):536‐44. [DOI] [PubMed] [Google Scholar]
Puhan 2005a
- Puhan MA, Schunemann HJ, Frey M, Scharplatz M, Bachmann LM. How should COPD patients exercise during respiratory rehabilitation? Comparison of exercise modalities and intensities to treat skeletal muscle dysfunction. Thorax 2005;60(5):367‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Puhan 2008
Puhan 2014
- Puhan MA, Lareau SC. Evidence‐based outcomes from pulmonary rehabilitation in the chronic obstructive pulmonary disease patient. Clinics in Chest Medicine 2014;35(2):295‐301. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3.5. The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schlenk 1998
- Schlenk EA, Erlen JA, Dunbar‐Jacob J, McDowell J, Engberg S, Sereika SM, et al. Health‐related quality of life in chronic disorders: a comparison across studies using the MOS SF‐36. Quality of Life Research 1998;7(1):57‐65. [DOI] [PubMed] [Google Scholar]
Schunemann 2003
- Schunemann HJ, Griffith L, Jaeschke R, Goldstein R, Stubbing D, Guyatt GH. Evaluation of the minimal important difference for the feeling thermometer and the St. George's Respiratory Questionnaire in patients with chronic airflow obstruction. Journal of Clinical Epidemiology 2003;56(12):1170‐6. [DOI] [PubMed] [Google Scholar]
Schunemann 2005
- Schunemann H, Puhan M, Goldstein R, Jaeschke R, Guyatt G. Measurement properties and interpretability of the Chronic Respiratory Disease Questionnaire (CRQ). Journal of Chronic Obstructive Pulmonary Disease 2005;2(1):81‐9. [DOI] [PubMed] [Google Scholar]
Seemungal 1998
- Seemungal T, Donaldson G, Paul E, Bestall J, Jeffries D, Wedzicha J. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1998;157(5):1418‐22. [DOI] [PubMed] [Google Scholar]
Seneff 1995
- Seneff MG, Wagner DP, Wagner RP, Zimmerman JE, Knaus WA. Hospital and 1‐year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. Journal of the American Medical Association 1995;274(23):1852‐7. [PubMed] [Google Scholar]
Sillen 2009
- Sillen MJ, Speksnijder CM, Eterman RM, Janssen PP, Wagers SS, Wouters EF, et al. Effects of neuromuscular electrical stimulation of muscles of ambulation in patients with chronic heart failure or COPD: a systematic review of the English‐language literature. Chest 2009;136(1):44–61. [DOI] [PubMed] [Google Scholar]
Sin 2002
- Sin DD, Stafinski T, Ng YC, Bell NR, Jacobs P. The impact of chronic obstructive pulmonary disease on work loss in the United States. American Journal of Respiratory and Critical Care Medicine 2002;165(5):704‐7. [DOI] [PubMed] [Google Scholar]
Singh 2014
- Singh SJ, Puhan MA, Andrianopoulos V, Hernandes NA, Mitchell KE, Hill CJ, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. European Respiratory Journal 2014;44(6):1447‐78. [DOI] [PubMed] [Google Scholar]
Soler‐Cataluna 2005
- Soler‐Cataluña JJ, Martínez‐García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2006;60(1):925‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spaar 2009
- Spaar A, Frey M, Turk A, Karrer W, Puhan MA. Recruitment barriers in randomized controlled trials from the physicians' perspective: a postal survey. BMC Medical Research Methodology 2009;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spencer 2003
- Spencer S, Jones PW. Time course of recovery of health status following an infective exacerbation of chronic bronchitis. Thorax 2003;58(7):589‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spruit 2003
- Spruit MA, Gosselink R, Troosters T, Kasran A, Gayan‐Ramirez G, Bogaerts P, et al. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF‐I. Thorax 2003;58(9):752‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spruit 2014
- Spruit M, Rochester CL, Pitta F, Goldstein R, Troosters T, Nici L, et al. A 6‐week, home‐based, unsupervised exercise training program is not effective in patients with chronic respiratory disease directly following a hospital admission. BMJ 2014;349:g4315. [Google Scholar]
Sullivan 2000
- Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest 2000;117(2 Suppl):5S‐9S. [DOI] [PubMed] [Google Scholar]
Trappenburg 2005
- Trappenburg JC, Troosters T, Spruit MA, Vandebrouck N, Decramer M, Gosselink R. Psychosocial conditions do not affect short‐term outcome of multidisciplinary rehabilitation in chronic obstructive pulmonary disease. Archives of Physical Medicine and Rehabilitation 2005;86(9):1788‐92. [DOI] [PubMed] [Google Scholar]
Troosters 2010a
- Troosters T, Gosselink T, Janssens W, Decramer M. Exercise training and pulmonary rehabilitation: new insights and remaining challenges. European Respiratory Review 2010;19(115):24‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van den Berg 2015
- Berg JWK. Pulmonary rehabilitation for chronic obstructive pulmonary disease (COPD) exacerbation. https://clinicaltrials.gov/ct2/show/record/NCT00413543 (accessed 5 December 2016). [NCT00413543]
Vitacca 2001
- Vitacca M. Exacerbations of COPD: predictive factors, treatment and outcome. Monaldi Archives of Chest Disease 2001;56(2):137‐43. [PubMed] [Google Scholar]
References to other published versions of this review
Puhan 2005
- Puhan MA, Scharplatz M, Troosters T, Steurer J. Respiratory rehabilitation after acute exacerbation of COPD may reduce risk for readmission and mortality – a systematic review. Respiratory Research 2005;6(1):54. [DOI: 10.1186/1465-9921-6-54] [DOI] [PMC free article] [PubMed] [Google Scholar]
Puhan 2011
- Puhan MA, Gimeno‐Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD005305.pub3] [DOI] [PubMed] [Google Scholar]


