Abstract
Background:
No comparative review of Vaccine Adverse Event Reporting System (VAERS) submissions following pandemic influenza A (H1N1) 2009 and seasonal influenza vaccinations during the pandemic season among U.S. military personnel has been published.
Methods:
We compared military vs. civilian adverse event reporting rates. Adverse events (AEs) following vaccination were identified from VAERS for adults aged 17–44 years after pandemic (monovalent influenza [MIV], and seasonal (trivalent inactivated influenza [IIV3], live attenuated influenza [LAIV3]) vaccines. Military vaccination coverage was provided by the Department of Defense’s Defense Medical Surveillance System. Civilian vaccination coverage was estimated using data from the National 2009 H1N1 Flu Survey and the Behavioral Risk Factor Surveillance System survey.
Results:
Vaccination coverage was more than four times higher for MIV and more than twenty times higher for LAIV3 in the military than in the civilian population. The reporting rate of serious AE reports following MIV in service personnel (1.19 per 100,000) was about half that reported by the civilian population (2.45 per 100,000). Conversely, the rate of serious AE reports following LAIV3 among service personnel (1.32 per 100,000) was more than twice that of the civilian population. Although fewer military AEs following MIV were reported overall, the rate of Guillain–Barré Syndrome (GBS) (4.01 per million) was four times greater than that in the civilian population. (1.04 per million).
Conclusions:
Despite higher vaccination coverage in service personnel, the rate of serious AEs following MIV was about half that in civilians. The rate of GBS reported following MIV was higher in the military.
Keywords: Pandemic influenza A H1N1 (2009), influenza vaccine, Vaccine safety
1. Introduction
In 2009, monovalent vaccines were rapidly developed and dispensed to prevent the spread of pandemic influenza A 2009 (H1N1) virus. In the United States, as the licensure and manufacturing processes for these novel vaccines were comparable to those of the seasonal vaccines for that year [1], similar vaccine safety profiles post licensure were anticipated. Subsequently, European studies which assessed safety among military personnel during the 2009–2010 influenza season found much higher reporting rates after MF59 and AS03 adjuvanted pandemic vaccines than after the seasonal trivalent inactivated influenza (IIV3) seasonal vaccines [2,3]. Similarly, an assessment of the adverse event (AE) profile in the U.S. civilian population following pandemic influenza A (H1N1) 2009 monovalent vaccine (MIV) using the Vaccine Adverse Event Reporting System (VAERS) was consistent with that of the seasonal influenza vaccines, although the reporting rate was higher [4]. In addition, one study assessing reporting rates to VAERS found reporting of hypersensitivity reactions following the pandemic influenza A (H1N1) 2009 vaccine to be elevated among adult civilian women compared with adult civilian men [5].
VAERS is a passive surveillance system for vaccine safety implemented in 1990 and is jointly administered by the Centers for Disease Control and Prevention and the Food and Drug Administration [6,7]. Healthcare providers are required to report vaccine AEs specified in the Vaccine Injury Table and manufacturers are required to report all AEs for licensed U.S. vaccines [8–10]. In addition, members of the public (vaccinees, parents of vaccine recipients, and others) may report suspected AEs to the system voluntarily. With the initiation of the national pandemic influenza A (H1N1) 2009 vaccination program, reporting to VAERS generally was enhanced by providing VAERS contact information on influenza vaccination record cards and advertising in medical journals. The military provided contact information for reporting to VAERS if there were problems after vaccination affecting its personnel [11]. Additionally, state vaccine safety coordinators were hired and trained on reporting requirements and more VAERS personnel were hired to code reports and obtain/review medical records. Finally, VAERS capacity to analyze additional reports was also improved so that potential safety signals could be rapidly identified.
To date, no comparative review of reports of AEs following pandemic influenza A (H1N1) 2009 and seasonal influenza vaccinations in the active component U.S. military in 2009–2010 influenza season has been published. It is also unclear whether elevated reporting rates to VAERS during the 2009–2010 influenza season found with the civilian vaccination program also affected the military population. The goals of this study were to identify potential differences between the U.S. military and civilian populations following pandemic influenza A (H1N1) 2009 and seasonal influenza vaccinations during the 2009–2010 influenza season related to: (1) vaccination coverage; (2) types of AEs reported to VAERS, and (3) a possible sex difference in hypersensitivity reactions reported following MIV among the military population.
2. Methods
2.1. Data sources
Military vaccination coverage among active duty personnel was determined using data from the Defense Medical Surveillance System (DMSS), an active surveillance system administered by the Department of Defense (DoD) to integrate data from medical treatment facilities, vaccination centers, and military personnel offices worldwide [12]. Data from DMSS also were used to validate active-duty military VAERS reports.
Civilian vaccination coverage was estimated using data from the National 2009 H1N1 Flu Survey (NHFS) and the Behavioral Risk Factor Surveillance System (BRFSS) survey [13–15]. Interview data collected for BRFSS and NHFS between November 2009 and June 2010 were used to measure pandemic influenza A (H1N1) 2009 vaccination coverage for October 2009 through May 2010. The population in each subgroup was estimated using the NHFS. Kaplan–Meier (KM) survival analysis was used to estimate the cumulative proportion of persons vaccinated separately for BRFSS and NHFS [15,16]. Monthly estimates from the two surveys were then combined in order to derive final monthly estimates of cumulative vaccination coverage [16].
2.2. Study design and population
We conducted a retrospective review of military and civilian VAERS reports to investigate possible differences in AE reporting rates for both the 2009 pandemic H1N1 and 2009–2010 seasonal influenza vaccines. The study population included all persons aged 17–44 years for whom a VAERS report was filed following either or both the pandemic (H1N1) 2009 or 2009–2010 seasonal influenza vaccines from August 1, 2009 through December 31, 2010. Service personnel were then identified if the VAERS report indicated that the vaccine (s) was administered in a military clinic and/or was purchased with military funds and included active personnel in the Army, Air Force, Marines and Navy.
2.3. Clinical review of reports
VAERS reports are classified as serious or non-serious. Reports are classified as serious based on the Code of Federal Regulations (21 CFR 600.80) if death, life-threatening illness, hospitalization or prolongation of hospitalization, permanent disability or a congenital anomaly is reported. For serious reports from sources other than manufacturers, medical records are routinely requested. All VAERS reports were reviewed by a CDC medical officer who classified the AEs based on information in the text of the report and in medical records (when available) according to one of the following body system categories [4]: hypersensitivity (e.g., anaphylaxis, angioedema, dyspnea, urticaria, wheezing), cardiovascular (e.g., arrhythmia, hypertension, hypotension, myocarditis), ENT (ears, nose, throat), gastrointestinal (e.g., vomiting, diarrhea), local reaction (e.g., pain, tenderness, erythema), musculoskeletal (e.g., arthralgia, arthritis), neurologic (e.g., paresthesia, peripheral neuropathy, Bell’s palsy, Guillain–Barré syndrome, convulsion), pregnancy-specific outcomes (e.g., spontaneous abortion, fetal death), psychiatric, respiratory (e.g., influenza-like illness, rhinorrhea, sore throat, cough), other infectious, other non-infectious conditions (e.g., diabetes, thrombocytopenia, multiple symptoms), and death. We used Brighton Collaboration criteria to verify the diagnosis for all reports suggestive of Guillain–Barré Syndrome (GBS) [17] and anaphylaxis [18]. We also considered GBS verified if medical records included a neurologist’s diagnosis of GBS with no contradictory information and, for anaphylaxis, a documented physician’s diagnosis of anaphylaxis within 24 h of vaccination. Cause of death was determined from the available autopsy report, death certificate, or medical record. Vaccine administration errors without an adverse health event and foreign reports were excluded.
2.4. Statistical analyses
We compared rates of AEs per doses administered following MIV, seasonal IIV3 and trivalent live attenuated influenza vaccine (LAIV3). To account for variability in the civilian estimation from the complex survey designs of NHFS and BRFSS in comparison with the data from the active military personnel, we used the delta method to calculate confidence intervals around the reporting rate ratios. We did not assess live attenuated monovalent vaccine (LAMV) further in our analysis as information on LAMV vaccination coverage for both military personnel and civilians was unavailable. We report descriptive analyses only consisting of the overall rates of AEs reported by the military and civilian populations. We could not perform statistical tests to assess reasons for the differences between the civilian and military populations because data for important confounding variables were unavailable.
Because VAERS is a routine surveillance program and does not meet the definition of research, it is not subject to Institutional Review Board (IRB) review and informed consent requirements. Similarly, NHFS was considered a non-research function and therefore not subject to IRB review.
3. Results
Of the total 434 military reports to VAERS for individuals who received MIV and/or seasonal influenza vaccine, 262 recipients were confirmed to be active component military in the DMSS; other reports denoted that the patient was a dependent or military retiree vaccinated at a military clinic and were therefore included in the civilian population. Vaccination coverage in the military was higher than that of the civilian population for all influenza vaccines: more than four times higher for MIV (73.6% vs. 16.8% vaccinated); slightly higher for seasonal IIV3 (34.0% vs. 28.0%); and more than twenty times higher for LAIV3 (47.7% vs. 2.1%) (Table 1).
Table 1.
Reports to the Vaccine Adverse Event Reporting System (VAERS) following influenza A (H1N1) 2009 monovalent vaccine and 2009–2010 seasonal influenza vaccines among adults aged 17–44 years, United States, August 1, 2009-December 31, 2010a.
| Serious reports | |||||||
|---|---|---|---|---|---|---|---|
| Influenza vaccine administered | Total Reports | Serious reports | Age in years median | Onset interval in days median (range) | Population vaccinated N (%) | Reporting Rate per 100,000 doses administered | Reporting Rate Ratio Military to Civilian population (95% CI) |
| Total US population | |||||||
| 2009-H1N1 MIV | 4,107 | 469 | 33 | 2 (0, 257) | 19,163,035 (16.8) | 2.45 | Ref |
| Seasonal: | |||||||
| IIV3 | 2,140 | 192 | 34 | 2 (0, 149) | 31,938,392 (28.0) | 0.60 | Ref |
| LAIV3 | 150 | 12 | 32.5 | 4 (0, 178) | 2,395,379 (2.1) | 0.50 | Ref |
| Total U.S. active military population | |||||||
| 2009-H1N1 MIV | 175 | 21 | 27 | 9 (0, 84) | 1,757,801 (73.6) | 1.19 | 0.49 (0.06, 0.92) |
| Seasonal: | |||||||
| IIV3 | 41 | 6 | 23.5 | 1.5 (0, 261) | 811,406 (34.0) | 0.74 | 1.23 (0.43, 2.03) |
| LAIV3 | 64 | 15 | 27 | 24 (1, 211) | 1,138,209 (47.7) | 1.32 | 2.63 (2.18, 3.09) |
Vaccinated by July 31, 2010.
The rates of serious AE reports following the study vaccines varied by both military/civilian status and sex. For MIV, the rate reported by military personnel (1.19 per 100,000) was approximately half that reported in the civilian population (2.45 per 100,000, reporting rate ratio (RRR): 0.49 (95% CI: 0.06, 0.92). Conversely, the rate of serious AE reports following LAIV3 among service personnel (1.32 per 100,000) was more than twice that of the civilian population (0.50 per 100,000, RRR: 2.63 [95% CI: 2.18, 3.09]).
Among men, the reporting rate of all AE reports following MIV in service personnel (6.83 per 100,000) was significantly less than that reported by the civilian population (10.67 per 100,000, RRR: 0.64 [95% CI: 0.44, 0.83]) (Table 2). Conversely, among women, the rate of all AE reports following LAIV3 among service personnel (13.63 per 100,000) was significantly higher than that of the civilian population (8.74 per 100,000 RRR: 1.56 [95% CI: 1.14, 1.97]).
Table 2.
Reports to the Vaccine Adverse Event Reporting System (VAERS) following influenza A (H1N1) 2009 monovalent vaccine and 2009–2010 seasonal influenza vaccines among adults aged 17–44 years, by Sex, United States, August 1, 2009-December 31, 2010a.
| Influenza vaccine administered | Total reports | Age in years median | Onset interval in days median (range) | Population vaccinated | Reporting Rate per100,000 doses administered | Reporting Rate Ratio Military to Civilian population (95% CI) | Reporting Rate Ratio Women to Men by population (95% CI) |
|---|---|---|---|---|---|---|---|
| US population: Men | |||||||
| 2009-H1N1 MIV | 917 | 32 | 1 (0, 306) | 8,592,313 (15.1) |
10.67 | Ref | Ref |
| Seasonal: | |||||||
| IIV3 | 489 | 33 | 0 (0, 306) | 13,954,883 (24.6) |
3.50 | Ref | Ref |
| LAIV3 | 56 | 27.5 | 1 (0, 39) | 1,331,303 (2.3) |
4.21 | Ref | Ref |
| U.S. active military population: Men | |||||||
| 2009-H1N1 MIV | 102 | 28 | 0 (0, 84) | 1,494,377 (74.4) |
6.83 | 0.64 (0.44, 0.83) | Ref |
| Seasonal: | |||||||
| IIV3 | 29 | 32 | 1 (0, 14) | 672,800 (33.5) |
4.31 | 1.23 (0.87, 1.59) | Ref |
| LAIV3 | 42 | 27 | 7 (0, 211) | 976,768 (48.6) |
4.30 | 1.02 (0.73, 1.32) | Ref |
| US population: Women | |||||||
| 2009-H1N1 MIV | 3169 | 32 | 0 (0, 316) | 10,570,722 (18.4) |
29.98 | Ref | 2.80 (2.76, 2.83) |
| Seasonal: | |||||||
| IIV3 | 1630 | 33 | 0 (0, 394) | 17,983,509 (31.4) |
9.06 | Ref | 2.59 (2.54, 2.64) |
| LAIV3 | 93 | 31 | 0 (0, 178) | 1,064,076 (1.9) |
8.74 | Ref | 2.08 (1.88, 2.27) |
| U.S. active military population: Women | |||||||
| 2009-H1N1 MIV | 73 | 30 | 0 (0, 24) | 263,422 (69.5) |
27.71 | 0.93 (0.70, 1.15) | 3.96 (3.74, 4.19) |
| Seasonal: | |||||||
| IIV3 | 12 | 27 | 0 (0, 261) | 138,606 (36.6) |
8.66 | 0.96 (0.39,1.52) | 2.01 (1.46, 2.56) |
| LAIV3 | 22 | 27.5 | 1 (0, 24) | 161,439 (42.6) |
13.63 | 1.56 (1.14,1.97) | 3.10 (2.83, 3.37) |
Note: the comparisons for the last column are women vs men within the military population and women vs. men in the civilian population.
Vaccinated by July 31, 2010.
3.1. Types of AE reported by the civilian population
The most commonly reported AEs (serious or non-serious) following MIV in civilians were allergic reactions (n = 747, 24.7% of total AE), other non-infectious outcomes (n = 577, 19.1%), and neurological outcomes (n = 429, 14.2%; including 9 GBS cases all in males which met the Brighton Collaboration case definition). (Fig. 1, Table 3) None of the GBS cases had received another vaccine at the time of the MIV inoculation. The order of the most frequently reported AEs differed slightly for IIV3, in which musculoskeletal outcomes (n = 303, 14.9%), were more common than neurological outcomes (n = 215, 10.6%), and for LAIV3, in which respiratory/ILI outcomes (n = 23, 21.3%), were as common as other non-infectious outcomes (n = 23, 21.3%), followed by neurological outcomes (n = 9, 8.3%).
Fig. 1.
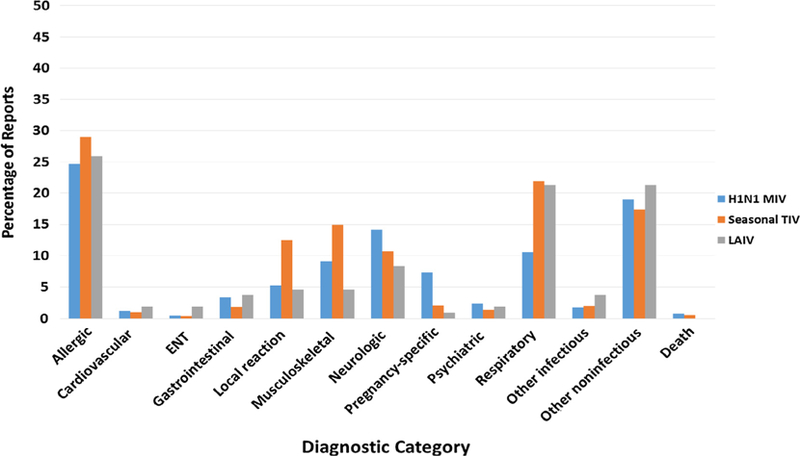
Distribution of diagnostic categories for VAERS reports received by December 31, 2010 for civilian individuals aged 17–44 years vaccinated with pandemic influenza A (H1N1) 2009 monovalent vaccine and/or seasonal influenza vaccine between August 1, 2009 and July 31, 2010.
Table 3.
Selected clinical findings of Guillain Barré Syndrome cases after pandemic influenza A (H1N1) 2009 monovalent vaccine, VAERS, 2009–2010.
| Case | Age (yr) Race Sex | Vaccines | Onset after vaccination (days) | PMH | Illness/other in 4 wks prior to onset of GBS Symptoms | Symptoms | CSF | EMG | Other | Neurologist’s Diagnosis/ Brighton level |
Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Civilian cases (n = 10) | |||||||||||
| 1 | 39 M | MIV (Sanofi Pasteur) | 2 | DM1 on insulin pump | Clostridium Difficile Infection (detected as inpatient) | Paresthesias (whole body) and cramps in legs | ND | Normal exam | Brain scan, MRI cervical spine | ‘‘Sensory GBS” (4) vs. small fiber neuropathy | Supportive |
| 2 | 39 M | MIV (UNK) | 0 (4 h) | Asthma | Viral URTI (onset 1 wk Before vaccination) | Ascending weakness, numbness, paresthesia in limbs | +ve albumin cytologic dissociation | ND | CBC normal | GBS (2) | IVIG |
| 3 | 24 M | MIV (Novartis) | 2 | Prader-Willi Syndrome, HTN, obesity, sleep apnea, leg cellulitis, anxiety/depression | ND | Lower leg weakness & numbness | Rare wbcs | +ve for acute progressive neuropathy | MRI cervical & lumbar spine normal, serology for HSV2 and EBV -ve | GBS (2) | Intubated, plasmapheresis |
| 4 | 35 M | MIV (Sanofi Pasteur) | 36 | Good health | Back injury after lifting 100 lb object, Herpes zoster left side of Back | Ascending numbness, and weakness all limbs, unable to walk | +ve albumin cytologic dissociation | Absent F waves c/w early GBS | CBC, CMP, CRP, ANA, MRI head, cervical and thoracic spine | ‘‘Atypical GBS” (2) | Plasmapheresis but suffered relapse on discharge, readmitted repeat plasmapheresis followed by course of IVIG with marked +ve clinical response |
| 5 | 32 M | MIV (Novartis) | Approx. 10 | ND | ND | Soreness legs & lower back, sweats, chest cramps, eye twitching | ND | ND | ND | Mild Miller Fisher variant of GBS (4) |
ND |
| 6 | 40 M | MIV (Sanofi Pasteur) | 3 | ND (MRI brain = showed no acute abnormality but frontal lobe encephalomalacia possibly remote trauma) | Weakness bilaterally arms and legs, numbness and paresthesias, metallic taste | +ve albumin cytologic dissociation | +ve for demyelination | CTs & MRIs brain, cervical & lumbar spine, USD abdomen, heavy metal screen, EBV serology-ve, CMV IgM/IgG +ve | GBS (1) ‘‘likely 2° to recent CMV infection with acute on chronic hepatitis, also monoclonal gammopathy of unknown significance | IVIG & SoluMedrol, Gangcyclovir for CMV | |
| 7 | 38 M | MIV (Novartis) | 7 | ND | URTI (symptoms began 7 days After vaccination) | Low back pain, fever cough, fatigue, ascending weakness, paresthesias numbness limbs face and tongue | +ve albumin cytologic dissociation | Abnormal supportive of AIDP | CBC, CMP, ESR, CRP, TFT, vitamin B-12, CXR, CT brain & abdomen | GBS (1) | IVIG |
| 8 | 44 M | MIV (Sanofi Pasteur) | 31 | Depression | URTI (onset 1 wk after vaccination) | Ascending paresthesias, numbness, weakness in all limbs | +ve albumin cytologic dissociation | ND | CBC, CMP, CRP, TFT, CXR, MRI lumbar spine |
GBS (2) | IVIG |
| 9 | 18 M | H1N1 (UNK) | 97 | Migraine, depression, PCN allergy | ND | Paresthesia & numbness in upper & lower limbs, areflexia, photophobia | +ve albumin cytologic dissociation | ND | ND | GBS (2) | Plasmapheresis IVIG |
| 10 | 41 M | MIV (Novartis) | 52 | ND | Febrile illness + mild Hepatomegaly 1–2 wk prior to symptom Onset | Ascending weakness all limbs, resp failure, transient delirium | +ve albumin cytologic dissociation | +ve for demyelination | Hep A, B, C serol -ve, monospot +ve = infectious mononucleosis | GBS (2) | Plasmapheresis intubated in ICU, antibiotics, prolonged Rehab |
| Military cases (n = 7) | |||||||||||
| 1 | 19 M | MIV (Sanofi Pasteur) Hep A/B (1wk earlier) | 9 | ND | Nausea (1 week) & cough, vomiting, Diarrhea (1 day) | Numbness, paresthesias | Protein normal, 0 wbcs | +ve for mild acute demyelination | MRI brain, cervical & thoracic spine, drug screen, CBC | GBS (2) | IVIG |
| 2 | 43 M | MIV (Sanofi Pasteur) | 10 | NHL (in remission 3.5 yr), allergic rhinitis | URTI | Paresthesias, numbness, L side facial droop, weakness in all limbs | ND | +ve for mild acute demyelination | CBC, CMP, Mg, Phosphorus, serum IgA, IgG, ESR, ANA | GBS (2) | Prednisolone & acyclovir (for presumed Bell’s palsy), IVIG |
| 3 | 28 M | MIV (Novartis), MCV4, LAIV3, Tdap, Hep A/B | 46 | ND | URTI | Headache, numbness in legs and face, blurred vision, areflexia and ataxia on exam, dysarthria | ND | +ve for mild acute demyelination | CBC, CMP | Miller Fisher variant of GBS (2) | IVIG |
| 4 | 40 M | H1N1 (UNK) | 36 | GERD, OSA, seasonal allergies | ND | Ascending weakness, paresthesias in all limbs | +ve albumin cytologic dissociation | ND | MRI cervical spine & brain | GBS (1) | IVIG |
| 5 | 18 M | MIV (Novartis) Hep A/B, MPS, PPV23 | 30 | ND | URTI/otitis media (3 days) | Ascending weakness in limbs | ND | +ve for mild acute demyelination | MRI cervical, thoracic, lumbar spine & brain | GBS (2) | IVIG |
| 6 | 21 M | MIV (Novartis), LAIV3, Tdap, IPV | 32 | ND | Pneumonia (1 week) (RBL infiltrate on CXR) | Ascending weakness all limbs | ND | +ve for mild acute demyelination | CBC, ESR, Blood cx | GBS (2) | IVIG, azithromycin, cefdinir |
| 7 | 26 M | MIV (Novartis), Hep A/B | 2 | Headaches & vertigo | Sinusitis (2 weeks) | Lightheaded, diplopia, numbness in limbs, areflexia on exam, ataxia, ophthalmoparesis | ND | ND | CT brain, Blood cx | Miller Fisher variant of GBS (3) | Azithromycin, Vitamin B12 |
MIV = monovalent H1N1 vaccine; DM1 = type 1 diabetes mellitus; ND = not documented; MRI = magnetic resonance imaging; GBS = Guillain Barré syndrome; URTI = upper respiratory tract infection; CBC = complete blood count; IVIG = intravenous immunoglobulin; HTN = systemic hypertension; wbcs = white blood cells; HSV2 = herpes simplex virus type 2; EBV = Epstein-barr virus; CMP = comprehensive metabolic panel; CRP = C-reactive protein; ANA = antinuclear antibody; USD = ultrasound exam; CMV = cytomeglaovirus; IgM = immunoglobulin M; IgG = immunoglobulin G; AIDP = acute inflammatory demyelinating polyradiculopathy; TFT = thyroid funtion tests; CT = computerized tomographic scan; H1N1 (UNK) = H1N1 vaccine unknown type; PCN = penicillin; Hep A, B, C serol = hepatitis A, B, C serology; NHL = Non-Hodgkins lymphoma; IgA = immunoglobulin A; IgG = immunoglobulin G; ESR = erythrocyte sedimentation rate; MCV4 = meningococcal conjugate vaccine; LAIV = live attenuated influenza vaccine; Tdap = tetanus diphtheria acellular pertussis vaccine; Hep A/B = hepatitis A & B vaccine; GERD = gastroesophageal reflux disease; OSA = obstructive sleep apnea; MPS = meningococcal polysaccharide vaccine; PPV23 = pneumococcal polysaccharide vaccine; IPV = inactivated polio vaccine; RBL = right basal lobe; CXR = chest X-ray; CT = computerized tomographic scan; Blood cx = blood culture.
To calculate the rate of post-MIV GBS we excluded civilian Case #9 and military Case #4 as the vaccine they received was reported as ‘‘H1N1 unknown”.
3.2. Types of AE reported by the military
In order of frequency, the most commonly reported AEs (serious or non-serious) following MIV in service personnel were allergic reactions (n = 57, 33.1% of total AE), other non-infectious reactions (n = 35, 20.4%), and neurological outcomes (n = 26, 15.1%; including 6 Brighton-confirmed GBS cases, all in males) (Fig. 2, Table 3). Two-thirds (4 of 6) of the GBS cases received other vaccines at the same time as the MIV. Reported AE profiles were in the same order of frequency for LAIV3 as MIV, but for IIV3, after allergic reactions and other non-infectious reactions, respiratory/ILI outcomes (n = 8, 20.0%), were more common than neurological outcomes (n = 2, 5.0%).
Fig. 2.
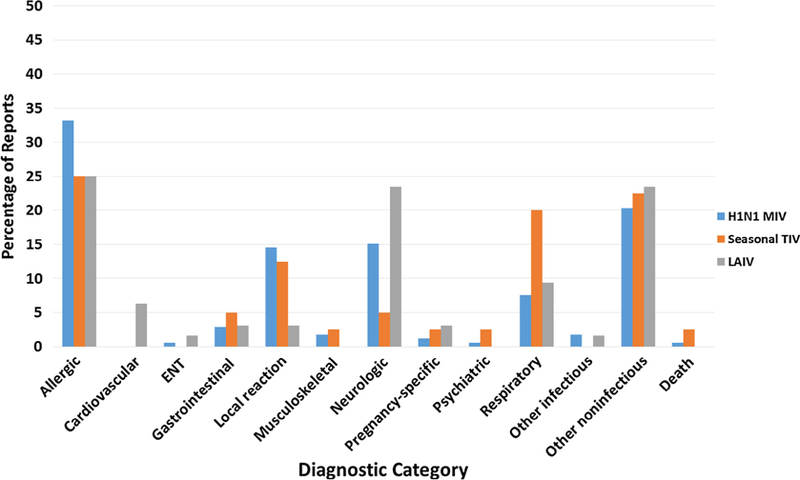
Distribution of diagnostic categories for VAERS reports received by December 31, 2010 for confirmed active military individuals aged 17–44 years vaccinated with pandemic influenza A (H1N1) 2009 monovalent vaccine and/or seasonal influenza vaccine between August 1, 2009 and July 31, 2010.
3.3. Hypersensitivity following MIV by sex among military personnel
There was a slightly higher proportion of women reporting hypersensitivity reactions following MIV than among their male counterparts (17.8% versus 14.7%); however, this difference was not statistically significant (p = 0.58).
4. Discussion
Overall, our study found that influenza vaccination coverage was higher in the military than among the civilian population, and that women, whether they were civilian or military, reported AEs for pandemic influenza A (H1N1) 2009 monovalent and 2009–2010 seasonal vaccines more frequently than men. Service personnel reported significantly fewer serious AEs following MIV, yet significantly more serious AEs following LAIV3 than did the civilian population. Although a higher proportion of women reported hypersensitivity reactions following MIV than their male counterparts, this difference was not statistically significant.
Vaccination coverage in the military population was more than four times higher for MIV compared to the civilian population. The higher vaccination coverage among service personnel is not surprising, given that the DoD has a mandatory immunization program providing service personnel with protection from a variety of pathogenic threats. Although the higher vaccination coverage may result in more potential AEs, the rate of serious AE reports following MIV by service personnel was roughly half that reported by the civilian population.
The third most commonly reported type of AE following MIV in both the military and civilian populations was neurological, including GBS. GBS, is a disorder in which the body’s immune system attacks part of the peripheral nervous system. Estimates of the incidence of GBS range from 0.8 to 1.9 cases per 100,000 person-years; rates are higher in males and increase with age. Risk factors for GBS include antecedent gastrointestinal or respiratory infection (including influenza). GBS has been associated with the 1976 swine–influenza vaccine [19], though no elevated risk was found in the military then [20]. Subsequently, several studies have assessed the risk of GBS following seasonal inactivated influenza vaccines. The data have been variable, demonstrating either no or a small increased risk (1–2 additional cases per 1 million vaccine doses administered). One study assessing risk of GBS in the military following seasonal influenza vaccines during 1980–1988 found no significant increased risk [21]. During the 2009 H1N1 influenza pandemic, results of epidemiologic studies from several monitoring systems for GBS were variable and inconsistent [22–25]. Results of a meta-analysis of data from six of the systems identified a modest increased risk (IRR 2.35, 95% CI 1.42–4.01, P = 0.0003) or approximately 1.6 excess cases of GBS per million people vaccinated; however, potential for confounding by seasonality could not be ruled out [26].
In our study, although few AEs following MIV were reported among male service personnel, six individuals met the Brighton Collaboration case definition for GBS (4.01 per million doses administered), compared with 9 (1.04 ± 0.22 per million doses administered) reported in the male civilian population. Two-thirds of the military GBS cases had received other vaccinations at the same time as the MIV, whereas none of the civilian GBS cases received concomitant vaccines. It is unknown if differences between civilian and military populations in the number and types of concomitant vaccines may increase or decrease AEs. One study found no evidence that concurrent receipt of multiple vaccinations is related to hospitalization risk among US service personnel [27]. Though not specifically assessing concurrent receipt of multiple vaccinations, in another study assessing VAERS from 2005 through 2013, a higher proportion of reports of GBS cases following LAIV3 was observed in the military population compared with the civilian population [28]. One other study found the incidence of GBS in the U.S. military to be slightly higher than that found in the general population [29]. A possible explanation for the finding of more GBS in the military population is the association between GBS and certain antecedent infections, such as with Campylobacter jejuni [30], and a number of studies have documented C. jejuni as a leading cause of travelers’ diarrhea in U.S. military personnel [31–33]. Several of our GBS cases had an antecedent respiratory or other infection (although we did not identify any C. jejuni-associated GBS cases among the military or civilian MIV VAERS reports we reviewed, but stool culture for this pathogen is rarely performed).
The higher rate of GBS following MIV among military personnel found in our study may also have been related to differences in AE reporting between military and civilian populations. Military policy states that VAERS reports can be filed by physicians or patients when events appear to be unexpected in nature or severity but that reports must be filed for events resulting in hospitalization, lost duty time (>24 h), or death [34]. Thus, more serious AEs are highly likely to be reported by military personnel.
Compared with the civilian population, the proportion of service personnel who received LAIV3 was more than twenty times higher with double the rate of serious AEs reported, the majority of whom were women. Although one study found higher rates of AEs following smallpox vaccination among military women compared with civilian women, these differences were not statistically significant [34]. Though military women reported AEs more frequently, the types of AE reported were similar to to those for civilian women and the majority were allergic reactions. Other studies have also found higher rates of AE reports among female service personnel compared with their male counterparts for the anthrax vaccine adsorbed (AVA) [35,36].
The most commonly reported AEs (serious or non-serious) following MIV in both service personnel and civilians were allergic reactions. A previous study in VAERS assessing immediate hypersensitivity reactions following pandemic influenza A (H1N1) 2009 monovalent vaccines in the general population found a higher reporting rate among adult females [5]. The highest female to male reporting ratio was among persons aged 20–39 years, which closely matches the 17–44 age group included in our study population. However, we did not find a significant sex difference in service personnel reporting hypersensitivity reactions following MIV.
Our civilian population demonstrated the same pattern as one European study [2] that found much higher reporting rates of AEs following the AS03-adjuvanted MIV vaccine than those reported after the seasonal IIV3 vaccinations among their military personnel during the 2009–2010 influenza season. Similarly, higher rates of serious AEs following MIV (1.19 per 100.000 doses administered) were reported compared with IIV3 (0.74 per 100,000 doses administered) in our military population. Yet, the rates of serious AEs following MIV were comparable to those reported following LAIV3 (1.32 per 100,000 doses administered) among service personnel. Though the similarity in reporting rates of serious AEs following MIV and LAIV3 may be due to stimulated reporting in the military population, the rate of AEs following MIV by the military was less than that reported by civilians.
VAERS has a number of strengths, such as its broad national scope, timely reporting and early detection of possible new, rare, or unusual patterns of AEs, which may be further investigated [11]. Still it is a spontaneous reporting system that has important limitations, including underreporting of less serious AEs, incomplete information, varying quality of reports, and lack of a control or unexposed comparison group. Additionally, it is not known to what degree the underreporting varies across populations and/or healthcare providers. Vaccine doses distributed are typically used as proxy for doses administered to obtain crude AE reporting rates. However, a strength of our analyses was that instead of using the vaccine doses distributed in calculating the rates of AEs, we had the actual number of doses administered by the military and we estimated the number of civilians vaccinated from the combined surveys, NHFS and BRFSS. Yet the influenza vaccination coverage estimates based on these latter surveys tend to be overestimated [37] (i.e., estimated doses received exceeds actual doses administered), so that the denominators of our AE rates could be too high, and thus our AE rates may be too low. Due to these limitations, we cannot determine causal associations between the vaccines and AEs from this study. A further limitation of our analysis is that we could not compare AE reporting rates between the civilian and military populations by receipt of concomitant vaccinations; although those data are available in the military, they are not available for civilians. Additionally, concerning military reports, the VAERS form does not collect standardized data on deployment. Therefore, we used data from DMSS to validate active-duty military VAERS reports; however, there is potential for misclassification if VAERS reports failed to indicate vaccines were given in a military facility.
We found higher reporting of GBS following MIV among male service personnel compared with civilian men, even though the rate of serious AEs following MIV by military personnel was about half that reported by the civilian population. The reason for the differences in GBS reporting is not clear but it may be related to differences in reporting practices between the military and civilian sectors, preceding infections and other potential environmental exposures. Of note, during the 2009–2010 influenza season, the CDC and the Food and Drug Administration actively solicited reports of serious events such as GBS which may have been linked with the pandemic influenza A (H1N1) 2009 monovalent vaccine and the seasonal 2009–2010 influenza vaccine [38]. Specifically, a partnership was established with the American Academy of Neurology, which includes physicians who are most likely to provide care for people with GBS. Nevertheless, we confirmed results from other studies that found more frequent AE reporting by women than men and higher reporting rates of AEs among women in the military compared with civilian women. The reasons for this observed difference are not known and may benefit from additional studies, including whether the administration of different combinations of vaccines may impact specific AEs rates and types of AEs, and, in particular, if there is a sex effect.
Our findings may be useful in interpreting VAERS data when comparing AEs associated with other vaccines between the military and civilian populations.
Acknowledgements
The authors gratefully acknowledge Dr. Limone C. Collins, Chief, and Ms. Christina Spooner, MS, Program Manager, Immunization Healthcare Branch, Public Health Command, for technical assistance, and Angelia A. Eick-Cost, PhD, ScM, Armed Forces Health Surveillance Center, Silver Spring, MD for assistance providing study data. The authors also thank James Singleton, PhD, MS, for assistance providing vaccination coverage data, and Mario Cano, MD MPH, Pedro Moro, MD MPH and Frank DeStefano, MD MPH for their expert review of the manuscript.
Disclaimer
The views, findings and conclusions in this report are those of the authors and do not reflect the official policy or position of the Centers for Disease Control and Prevention, the Departments of the Army/Navy/Air Force, Department of Defense, nor the US Government. Use of trade names and commercial sources is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention, the US Department of Health and Human Services, the Departments of the Army/Navy/Air Force, the Department of Defense, or the US Government. This study was supported by CDC and no external funding was secured.
Footnotes
Conflict of interest
None of the authors has conflicts of interest.
References
- [1].Centers for Disease Control and Prevention. Safety of influenza A (H1N1) 2009 monovalent vaccines – United States, October 1-November 24, 2009. MMWR Morbidity and mortality weekly report, vol. 58, 2009, pp. 1351–1356. [PubMed] [Google Scholar]
- [2].Mayet A, Ligier C, Gache K, Manet G, Nivoix P, Dia A, et al. Adverse events following pandemic influenza vaccine Pandemrix(R) reported in the French military forces–2009–2010. Vaccine 2011;29:2576–81. [DOI] [PubMed] [Google Scholar]
- [3].Banzhoff A, Haertel S, Praus M. Passive surveillance of adverse events of an MF59-adjuvanted H1N1v vaccine during the pandemic mass vaccinations. Human Vaccines 2011;7:539–48. [DOI] [PubMed] [Google Scholar]
- [4].Vellozzi C, Broder KR, Haber P, Guh A, Nguyen M, Cano M, et al. Adverse events following influenza A (H1N1) 2009 monovalent vaccines reported to the Vaccine Adverse Event Reporting System, United States, October 1, 2009-January 31, 2010. Vaccine 2010;28:7248–55. [DOI] [PubMed] [Google Scholar]
- [5].Halsey NA, Griffioen M, Dreskin SC, Dekker CL, Wood R, Sharma D, et al. Immediate hypersensitivity reactions following monovalent 2009 pandemic influenza A (H1N1) vaccines: reports to VAERS. Vaccine 2013;31:6107–12. [DOI] [PubMed] [Google Scholar]
- [6].Chen RT, Rastogi SC, Mullen JR, Hayes SW, Cochi SL, Donlon JA, et al. The Vaccine Adverse Event Reporting System (VAERS). Vaccine 1994;12:542–50. [DOI] [PubMed] [Google Scholar]
- [7].Singleton JA, Lloyd JC, Mootrey GT, Salive ME, Chen RT. An overview of the vaccine adverse event reporting system (VAERS) as a surveillance system VAERS Working Group. Vaccine 1999;17:2908–17. [DOI] [PubMed] [Google Scholar]
- [8]. Administration FaD. 21 CFR Part 600.80 Postmarketing reporting of adverse experiences. National Childhood Vaccine Injury Act of 1986 ed: Federal Register; 1997. p. (42 USC 300aa-25).
- [9]. Regulations USCoF. 21 CFR Part 600.80 Postmarketing reporting of adverse experiences. 21: Federal Register; 2014.
- [10]. VAERS Table of Reportable Events Following Vaccination.
- [11].Salmon DA, Akhtar A, Mergler MJ, Vannice KS, Izurieta H, Ball R, et al. Immunization-safety monitoring systems for the 2009 H1N1 monovalent influenza vaccination program. Pediatrics 2011;127(Suppl 1):S78–86. [DOI] [PubMed] [Google Scholar]
- [12].Rubertone MV, Brundage JF. The Defense Medical Surveillance System and the Department of Defense serum repository: glimpses of the future of public health surveillance. Am J Public Health 2002;92:1900–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Centers for Disease Control and Prevention. Interim results: state-specific influenza A (H1N1) 2009 monovalent vaccination coverage – United States, October 2009-January 2010. MMWR Morbidity and mortality weekly report, vol. 59, 2010, pp. 363–368. [PubMed] [Google Scholar]
- [14].Centers for Disease Control and Prevention. Interim results: influenza A (H1N1) 2009 monovalent and seasonal influenza vaccination coverage among health-care personnel – United States, August 2009-January 2010. MMWR Morbidity and mortality weekly report, vol. 59, 2010, pp. 357–362. [PubMed] [Google Scholar]
- [15].Centers for Disease Control and Prevention. Interim results: influenza A (H1N1) 2009 monovalent vaccination coverage — United States, October-December 2009. MMWR Morbidity and mortality weekly report, vol. 59, 2010, pp. 44–48. [PubMed] [Google Scholar]
- [16].Furlow-Parmley C, Singleton JA, Bardenheier B, Bryan L. Combining estimates from two surveys: an example from monitoring 2009 influenza A (H1N1) pandemic vaccination. Stat Med 2012;31:3285–94. [DOI] [PubMed] [Google Scholar]
- [17].Brighton Collaboration Definitions & Guidelines; 2008. https://brightoncollaboration.org/public/what-we-do/setting-standards/case-definitions/available-definitions.html; cited 2015.
- [18].Ruggeberg JU, Gold MS, Bayas JM, Blum MD, Bonhoeffer J, Friedlander S, et al. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2007;25:5675–84. [DOI] [PubMed] [Google Scholar]
- [19].Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, Keenlyside RA, Ziegler DW, Retailliau HF, et al. Guillain-Barré syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am J Epidemiol 1979;110:105–23. [DOI] [PubMed] [Google Scholar]
- [20].Johnson DE. Guillain-Barre syndrome in the US Army. Arch Neurol 1982;39:21–4. [DOI] [PubMed] [Google Scholar]
- [21].Roscelli JD, Bass JW, Pang L. Guillain-Barre syndrome and influenza vaccination in the US Army, 1980–1988. Am J Epidemiol 1991;133:952–5. [DOI] [PubMed] [Google Scholar]
- [22].Greene SK, Rett M, Weintraub ES, Li L, Yin R, Amato AA, et al. Risk of confirmed Guillain-Barré syndrome following receipt of monovalent inactivated influenza A (H1N1) and seasonal influenza vaccines in the Vaccine Safety Datalink Project, 2009–2010. Am J Epidemiol 2012;175:1100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tokars JI, Lewis P, DeStefano F, Wise M, Viray M, Morgan O, et al. The risk of Guillain-Barré syndrome associated with influenza A (H1N1) 2009 monovalent vaccine and 2009–2010 seasonal influenza vaccines: results from self-controlled analyses. Pharmacoepidemiol Drug Saf 2012;21:546–52. [DOI] [PubMed] [Google Scholar]
- [24].Wise ME, Viray M, Sejvar JJ, Lewis P, Baughman AL, Connor W, et al. Guillain-Barré syndrome during the 2009–2010 H1N1 influenza vaccination campaign: population-based surveillance among 45 million Americans. Am J Epidemiol 2012;175:1110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Burwen DR, Sandhu SK, MaCurdy TE, Kelman JA, Gibbs JM, Garcia B, et al. Surveillance for Guillain-Barré syndrome after influenza vaccination among the Medicare population, 2009–2010. Am J Public Health 2012;102:1921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Salmon DA, Proschan M, Forshee R, Gargiullo P, Bleser W, Burwen DR, et al. Association between Guillain-Barré syndrome and influenza A (H1N1) 2009 monovalent inactivated vaccines in the USA: a meta-analysis. Lancet 2013;381:1461–8. [DOI] [PubMed] [Google Scholar]
- [27].Payne DC, Aranas A, McNeil MM, Duderstadt S, Rose CE Jr. Concurrent vaccinations and U.S. military hospitalizations. Ann Epidemiol 2007;17:697–703. [DOI] [PubMed] [Google Scholar]
- [28].Haber P, Moro PL, McNeil MM, Lewis P, Woo EJ, Hughes H, et al. Post-licensure surveillance of trivalent live attenuated influenza vaccine in adults, United States, Vaccine Adverse Event Reporting System (VAERS), July 2005-June 2013. Vaccine 2014;32:6499–504. [DOI] [PubMed] [Google Scholar]
- [29].Nelson L, Gormley R, Riddle MS, Tribble DR, Porter CK. The epidemiology of Guillain-Barré Syndrome in U.S. military personnel: a case-control study. BMC Research Notes 2009;2:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Van Der Meche F. The Guillain-Barré Syndrome; pathogenesis and treatment. Rev Neurol Paris 1996;152:355–8. [PubMed] [Google Scholar]
- [31].Petruccelli BP, Murphy GS, Sanchez JL, Walz S, DeFraites R, Gelnett J, et al. Treatment of traveler’s diarrhea with ciprofloxacin and loperamide. J Infect Dis 1992;165:557–60. [DOI] [PubMed] [Google Scholar]
- [32].Murphy GS, Bodhidatta L, Echeverria P, Tansuphaswadikul S, Hoge CW, Imlarp S, et al. Ciprofloxacin and loperamide in the treatment of bacillary dysentery. Ann Intern Med 1993;118:582–6. [DOI] [PubMed] [Google Scholar]
- [33].Echeverria P, Jackson LR, Hoge CW, Arness MK, Dunnavant GR, Larsen RR. Diarrhea in U.S. troops deployed to Thailand. J Clin Microbiol 1993;31:3351–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].McMahon AW, Zinderman C, Ball R, Gupta G, Braun MM. Comparison of military and civilian reporting rates for smallpox vaccine adverse events. Pharmacoepidemiol Drug Saf 2007;16:597–604. [DOI] [PubMed] [Google Scholar]
- [35].Sever JL, Brenner AI, Gale AD, Lyle JM, Moulton LH, Ward BJ, et al. Safety of anthrax vaccine: an expanded review and evaluation of adverse events reported to the Vaccine Adverse Event Reporting System (VAERS). Pharmacoepidemiol Drug Saf 2004;13:825–40. [DOI] [PubMed] [Google Scholar]
- [36].McNeil MM, Chiang IS, Wheeling JT, Zhang Y. Short-term reactogenicity and gender effect of anthrax vaccine: analysis of a 1967–1972 study and review of the 1955–2005 medical literature. Pharmacoepidemiol Drug Saf 2007;16:259–74. [DOI] [PubMed] [Google Scholar]
- [37].Centers for Disease Control and Prevention. National Early Season Flu Vaccination Coverage, United States, November 2014. http://www.cdc.gov/flu/fluvaxview/nifs-estimates-nov2014.htm. 2016. [Google Scholar]
- [38].Centers for Disease Control and Prevention. Fact Sheet: Guillain-Barré Syndrome (GBS), 2009. http://www.cdc.gov/h1n1flu/vaccination/factsheet_gbs.htm.


