Abstract
Background
Pain is prevalent during orthodontics, particularly during the early stages of treatment. To ensure patient comfort and compliance during treatment, the prevention or management of pain is of major importance. While pharmacological means are the first line of treatment for alleviation of orthodontic pain, a range of non‐pharmacological approaches have been proposed recently as viable alternatives.
Objectives
To assess the effects of non‐pharmacological interventions to alleviate pain associated with orthodontic treatment.
Search methods
Cochrane Oral Health’s Information Specialist searched the following databases: Cochrane Oral Health’s Trials Register (to 6 October 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2016, Issue 9), MEDLINE Ovid (1946 to 6 October 2016), Embase Ovid (1980 to 6 October 2016) and EThOS (to 6 October 2016). We searched ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform for ongoing trials. No restrictions were placed on the language or date of publication when searching the electronic databases.
Selection criteria
Randomised controlled trials (RCTs) comparing a non‐pharmacological orthodontic pain intervention to a placebo, no intervention or another non‐pharmacological pain intervention were eligible for inclusion. We included any type of orthodontic treatment but excluded trials involving the use of pre‐emptive analgesia or pain relief following orthognathic (jaw) surgery or dental extractions in combination with orthodontic treatment. We excluded split‐mouth trials (in which each participant receives two or more treatments, each to a separate section of the mouth) and cross‐over trials.
Data collection and analysis
At least two review authors independently assessed risk of bias and extracted data. We used the random‐effects model and expressed results as mean differences (MD) with 95% confidence intervals (CI). We investigated heterogeneity with reference to both clinical and methodological factors.
Main results
We included 14 RCTs that randomised 931 participants. Interventions assessed included: low‐level laser therapy (LLLT) (4 studies); vibratory devices (5 studies); chewing adjuncts (3 studies); brain wave music or cognitive behavioural therapy (1 study) and post‐treatment communication in the form of a text message (1 study). Twelve studies involved self‐report assessment of pain on a continuous scale and two studies used questionnaires to assess the nature, intensity and location of pain.
We combined data from two studies involving 118 participants, which provided low‐quality evidence that LLLT reduced pain at 24 hours by 20.27 mm (95% CI ‐24.50 to ‐16.04, P < 0.001; I² = 0%). LLLT also appeared to reduce pain at six hours, three days and seven days.
Results for the other comparisons assessed are inconclusive as the quality of the evidence was very low. Vibratory devices were assessed in five studies (272 participants), four of which were at high risk of bias and one unclear. Chewing adjuncts (chewing gum or a bite wafer) were evaluated in three studies (181 participants); two studies were at high risk of bias and one was unclear. Brain wave music and cognitive behavioural therapy were evaluated in one trial (36 participants) assessed at unclear risk of bias. Post‐treatment text messaging (39 participants) was evaluated in one study assessed at high risk of bias.
Adverse effects were not measured in any of the studies.
Authors' conclusions
Overall, the results are inconclusive. Although available evidence suggests laser irradiation may help reduce pain during orthodontic treatment in the short term, this evidence is of low quality and therefore we cannot rely on the findings. Evidence for other non‐pharmacological interventions is either very low quality or entirely lacking. Further prospective research is required to address the lack of reliable evidence concerning the effectiveness of a range of non‐pharmacological interventions to manage orthodontic pain. Future studies should use prolonged follow‐up and should measure costs and possible harms.
Plain language summary
Techniques for reducing pain during orthodontics without using painkillers
Review question
Orthodontic treatment (teeth braces) can be painful, particularly following initial brace placement and later adjustments, for a week or more. We examined the merits of methods to reduce pain during orthodontic treatment without the need for painkillers.
Background
Pain is usual during orthodontic treatment and may make some people stop treatment early, meaning that planned benefits do not occur. Painkillers are recommended to reduce pain during orthodontic treatment, but an effective non‐drug solution would lower risks of side effects and help people to continue for the full course of treatment.
Search date
We included studies published before 6 October 2016.
Study characteristics
We included 14 studies that involved a total of 931 teenagers and adults. The studies investigated the effects of using laser irradiation provided by the orthodontist, vibratory devices, changing chewing patterns (patients chewing gum or wafers), brain wave music, cognitive behavioural therapy, and text messages to support people after braces were fitted. The main outcome measured was the intensity of pain over the short term as reported by patients.
Key results
We found insufficient evidence to assess the effectiveness of the interventions, although the available low‐quality evidence suggested that laser irradiation may help to control short‐term orthodontic pain. None of the studies considered side effects of the treatments. We identified relatively few studies, some of which used flawed methods or were not well reported. More research to look at the possible merits of non‐drug methods of pain control would be helpful. Future studies should measure pain over longer time periods and should measure side effects and costs.
Quality of the evidence
The quality of the evidence on the effectiveness of non‐drug ways to ease orthodontic pain was low to very low, so we are not able to rely on the findings.
Summary of findings
Background
Description of the condition
Orthodontics is a specialty within dentistry concerned with the treatment of malocclusion, which can be a result of dento‐alveolar disproportion (most commonly crowding), disproportionate jaws or a combination of the two. The ultimate goal of orthodontics is to create a balanced facial profile with aligned teeth and optimal dental occlusion leading to better aesthetics and function. Tooth movement, which is needed to reach this goal, is possible through the application of light forces in patients of all ages. A wide variety of orthodontic appliances, fixed or removable, can be used for this purpose. Fixed appliances are attached to teeth with adhesive, and cannot be removed by the patient for the duration of the treatment.
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage (Bonica 1979). The forces required for tooth movement are often associated with discomfort or pain, as tooth movement is only possible through a process of inflammation. During inflammation, various biochemical mediators are released which are responsible for the sensation of pain. Pain during orthodontic treatment can be dependent on age (Bergius 2000; Brown 1991; Jones 1985), gender (Bergius 2000; Ngan 1989), psychological well‐being (Bergius 2000; Sergl 1998), culture (Bergius 2000), and previous pain experiences (Bergius 2000; Firestone 1999; Ngan 1989). This makes pain subjective.
Pain has been reported in 70% to 94% of orthodontic patients during treatment (Firestone 1999; Kvam 1987; Oliver 1985; Schreurer 1996); fixed appliances are associated with more pain than removable appliances (Sergl 1998; Stewart 1997). During fixed appliance‐based treatment, orthodontic pain typically gradually increases from two hours after the placement of the first arch wire (Jones 1984; Schreurer 1996; Soltis 1971), peaking at 24 hours and then decreasing gradually, but may last from two days to a week or more (Burstone 1962; Ngan 1989). In terms of severity, orthodontic pain may range from slight discomfort during chewing to a constant, throbbing pain. No specific arch wire or bracket type has consistently been found to cause less pain (Jian 2013).
Description of the intervention
Management of orthodontic pain includes pharmacological and non‐pharmacological interventions. Various drugs are effective for the management of pain during orthodontic treatment (Ngan 1994; Paganelli 1993; Simmons 1992), the most commonly used class is non‐steroidal anti‐inflammatory drugs (NSAIDs). However, pharmacological or drug interventions may have some negative side effects and some patients may be unwilling to use then or may be allergic to them. For these reasons, a large number of non‐pharmacological interventions have also been investigated to alleviate orthodontic pain. Some examples of these are bite wafers and chewing gum, low level laser therapy (LLLT), vibratory stimulation, transcutaneous electrical nerve stimulation (TENS), application of ice/cryotherapy, acupuncture/acupressure, and psychological interventions such as a structured telephone call to patients during treatment.
How the intervention might work
LLLT is defined as laser treatment in which the energy produced by the laser is low enough not to cause an increase in body temperature. The laser produces a pure light with a single wave length that stimulates the biological processes within the tissue being treated. LLLT has anti‐inflammatory effects which can result in pain relief (Hashmi 2010).
The roots of teeth are surrounded by small fibres called periodontal ligament (PDL) fibres that connect the teeth to the jaw bone. Adjunctive vibratory stimulation may increase vascularity and limit ischaemia following orthodontic appliance placement activating large‐diameter sensory nerve fibres. This force is delivered via proprietary devices which the patient bites into for short periods (usually around 20 minutes) on a daily basis. There is limited evidence to support their clinical effectiveness. The theory behind the use of bite wafers (Otasevic 2006) and chewing gum (Benson 2012) is analogous to that underpinning the use of vibratory adjuncts. Chewing on a bite wafer (or chewing gum) is postulated to lead to loosening of the PDL fibres and an increase in blood flow to the areas surrounding the roots. This increase in blood flow may prevent or relieve inflammation, which in turn, relieves pain (Furstman 1972).
Cryotherapy is the use of low temperatures for medical treatment, which also modulates pain transmission from tissues. It enhances capillary contraction and reduces the temperature of damaged areas following trauma or surgery or both. Thus, cryotherapy controls oedema by reducing permeability, haemorrhage and metabolism (Movahedi 2006; Shin 2009). Acupuncture is a form of traditional Chinese medicine. It is believed that the manipulation of thin, solid needles inserted into so‐called 'acupuncture points' in the skin can relieve certain types of pain. Acupressure is based on acupuncture but involves application of physical pressure, by hand, elbow, or with the aid of various devices to acupuncture points on the surface of the body. Although both acupuncture and acupressure are widely used to manage acute and chronic pain, their methods of action and efficacy are not fully understood (Cruccu 2007; Paley 2015; Vachiramon 2005). TENS is a form of stimulation‐produced analgesia. Two electrical conductors (electrodes) are placed in direct contact with the painful teeth. An electrical current is produced between the electrodes, which causes the release of natural products and stimulates the nerves responsible for the transmission of pain (Atamaz 2012).
Another non‐pharmacological intervention mentioned in the literature is the use of a structured telephone call. Some theories imply that psychological factors contribute to the perception of pain (Melzack 1965), and the literature shows a relationship between anxiety and pain (Litt 1996; Schupp 2005; Sergl 1998; Theunissen 2012). A structured telephone call can be used to reassure and encourage patients to reduce anxiety and ultimately lead to pain relief.
Why it is important to do this review
Pain, in general, motivates us to withdraw from potentially damaging situations, protect a damaged body part while it heals, and to avoid those situations in the future. Pain during orthodontic treatment has been shown to be the most common reason for discontinuation of treatment (Kluemper 2002; Oliver 1985; Patel 1989), and accounts for why pain is a significant factor hindering patient compliance (Brown 1991; Patel 1989; Sergl 1998). Orthodontic pain has also been linked with reduced levels of oral hygiene (Soltis 1971; White 1984). To ensure patient comfort and compliance during treatment, the prevention or management of pain is of major importance. This review investigated non‐pharmacological interventions for alleviating pain during orthodontic treatment. Pain relief following tooth extraction or surgical procedures associated with orthodontic treatment was not included.
Objectives
To assess the effects of non‐pharmacological interventions to alleviate pain associated with orthodontic treatment.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) in which a non‐pharmacological pain intervention was compared concurrently to a placebo, no intervention, or another non‐pharmacological pain intervention. RCTs that compared pharmacological and non‐pharmacological interventions to a placebo or no intervention were included but only data for non‐pharmacological interventions were used. We excluded split‐mouth studies (in which each participant receives two or more treatments, each to a separate section of the mouth), owing to the lack of independence of pain‐relieving interventions in different intra‐oral sites.
Types of participants
We included people of any age undergoing any type of orthodontic treatment. We excluded trials involving the use of pre‐emptive analgesia or pain relief following orthognathic (jaw) surgery or dental extractions, or both in combination with orthodontic treatment.
Types of interventions
We included the following active interventions to alleviate pain either alone or in combination.
Low‐level laser therapy (LLLT).
Vibratory adjuncts.
Experimental chewing adjuncts, e.g. bite wafers and chewing gum.
Psychosocial and other interventions, e.g. verbal follow‐up and reassurance in the form of a structured telephone call, brain wave music or cognitive behavioural therapy.
Physical interventions such as transcutaneous electric nerve stimulation (TENS), ice/cryotherapy and acupuncture/acupressure.
Control: Any form of orthodontic treatment without the use of a non‐pharmacological technique to reduce subjective pain experience. Comparisons were made with placebo, or with the same intervention but at a different dose or intensity, or at a different time interval.
Types of outcome measures
Primary outcomes
Patient‐reported pain intensity or pain relief, as measured on a visual analogue scale (VAS), numerical rating scale, or any categorical scale.
Secondary outcomes
Dose/intensity and frequency of pain relief needed.
Any rescue medication (alternative pain relief taken or prescribed, including dose and time, following the last treatment).
Adverse effects of pain treatment (ideally recorded both at person and event level within each trial arm).
Quality of life or satisfaction, or both.
Time off school or work or both.
Response to treatment (defined as reduction in pain by at least 50%).
Search methods for identification of studies
Electronic searches
Cochrane Oral Health’s Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions:
Cochrane Oral Health’s Trials Register (searched 6 October 2016) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 9) in the Cochrane Library (searched 6 October 2016) (Appendix 2);
MEDLINE Ovid (1946 to 6 October 2016) (Appendix 3);
Embase Ovid (1980 to 6 October 2016) (Appendix 4);
EThOS (http://ethos.bl.uk/) (to 6 October 2016) (Appendix 5).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. The Embase search was combined with an adapted version of the Cochrane Embase Project filter for identifying RCTs in Embase Ovid (see http://www.cochranelibrary.com/help/central‐creation‐details.html for information).
Searching other resources
Cochrane Oral Health's Information Specialist searched the following trial registries for ongoing studies, see Appendix 6 for details of the search strategy:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 6 October 2016);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 6 October 2016).
We examined the reference lists of relevant articles to identify additional published and unpublished relevant studies.
We did not perform a separate search for adverse effects of interventions used; we considered adverse effects described in included studies only.
Data collection and analysis
Selection of studies
Two review authors (Hardus Strydom (HS) and Piotr Fudalej (PF)) independently assessed the titles and abstracts of studies identified through the searches. We obtained full copies of all studies appearing to meet the inclusion criteria and those for which there were insufficient data in the title and abstract to make a definitive decision. Two review authors (HS and Padhraig Fleming (PSF)) assessed the full‐text papers independently and resolved any disagreement on the eligibility of included studies through discussion with a third review author (Nikolaos Pandis (NP)). From this group of studies, we recorded the studies that did not meet the inclusion criteria in the Characteristics of excluded studies section of the review and reported the reasons for exclusion.
Data extraction and management
We designed data extraction forms to record year of publication and study setting, as well as details of the participants including demographic characteristics and criteria for inclusion. We entered study details into the Characteristics of included studies tables in Review Manager 5 (RevMan 2014). Two review authors (PSF and PF) extracted data independently, with disagreements resolved by consulting with a third review author. We extracted the following details where available.
Trial methods: (a) method of allocation; (b) masking of participants, trialists and outcome assessors; (c) exclusion of participants after randomisation; and proportion of, and reasons for, losses at follow‐up.
Participants: (a) country of origin and study setting; (b) sample size; (c) age; (d) gender; (e) inclusion and exclusion criteria.
Intervention: (a) type; (b) materials and techniques used; (c) time of follow‐up.
Control: (a) type; (b) materials and techniques used; (c) time of follow‐up.
Outcomes: (a) primary and secondary outcomes mentioned in the Types of outcome measures section of this review. If stated, we recorded the sources of funding. We planned to use this information to aid assessment of heterogeneity and the external validity of any included trial.
Assessment of risk of bias in included studies
Two review authors (PSF and NP) independently assessed risk of bias in the included trials using Cochrane’s tool for assessing risk of bias as described in section 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We compared the assessments and resolved any disagreements through discussion. We assessed the following domains as at low, high or unclear risk of bias.
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias), and outcome assessors (detection bias)
Incomplete outcome data addressed (attrition bias)
Selective outcome reporting (reporting bias)
Other bias
We categorised and reported the overall risk of bias of each included study according to the following.
Low risk of bias (plausible bias unlikely to seriously alter the results) if all domains were assessed as at low risk of bias
Unclear risk of bias (plausible bias that raises some doubt about the results) if one or more domains were assessed as at unclear risk of bias
High risk of bias (plausible bias that seriously weakens confidence in the results) if one or more domains were assessed as at high risk of bias
Measures of treatment effect
For continuous outcomes including pain scores on 100 mm scales, we calculated mean differences with 95% confidence intervals (CI). In our protocol, we had planned to dichotomise pain results, but as most studies measured pain on a VAS, we decided to use continuous data in order not to lose information. For dichotomous outcomes such as presence or absence of pain or use of painkillers, we planned to calculate risk ratios with 95% CI.
Unit of analysis issues
We did not include split‐mouth or cross‐over trials. Where studies had more than one treatment group, we made necessary adjustments to the control group numbers in order to avoid double counting participants.
Dealing with missing data
Where data were unclear or incomplete, we contacted the corresponding authors. If missing data were unavailable, we followed the advice outlined in section 16.1.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In case of the use of 'rescue medication', we had planned to use two imputation methods to calculate estimate of pain relief.
Baseline observation carried forward (BOCF) ‐ the pain relief score is set to zero for all remaining time points from rescue medication until the end of the observation period.
Last observation carried forward (LOCF) ‐ the last pain relief measurement, at the observation immediately preceding remedication, is used for all remaining assessments.
Assessment of heterogeneity
We assessed clinical heterogeneity by considering the characteristics of the studies, similarity between the types of participants, and interventions and outcomes assessed. We assessed statistical heterogeneity using a Chi² test and the I² statistic, where I² values of 30% to 60% might indicate moderate heterogeneity, 50% to 90% substantial heterogeneity, and 75% to 100% very substantial ('considerable') heterogeneity. We considered heterogeneity to be significant when the P value was below 0.10 (Higgins 2011).
Assessment of reporting biases
If a sufficient number of studies assessing similar interventions were to be identified for inclusion in future review updates, we would assess publication bias based on the recommendations for testing funnel plot asymmetry as described in section 10.4.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If asymmetry was to be identified, we would attempt to assess other possible causes and explore these in the discussion if appropriate.
Data synthesis
We pooled data from studies with similar participants, interventions and outcomes. We calculated a weighted treatment effect with the results expressed as mean difference (MD), when different scales for the same outcome were used and 95% CI for continuous outcomes. We used random‐effects models for meta‐analyses.
Subgroup analysis and investigation of heterogeneity
Where we found significant heterogeneity, we had planned to conduct the following subgroup analyses to explore the source, including:
type of interventions;
dose or intensity of interventions;
participants' characteristics: age, gender, ethnicity, psychological well‐being and previous pain experienced; and
type of orthodontic appliance used.
We will include these in future updates of this review if there are sufficient data.
Sensitivity analysis
We undertook sensitivity analysis based on risk of bias (low risk of bias versus high or unclear risk of bias) to investigate the robustness of conclusions.
Summary of results
We produced 'Summary of findings' tables for the main comparisons and primary outcomes of this review using the GRADE system (Guyatt 2008) with GRADEpro software.
We assessed the quality of the body of evidence with reference to the following.
Overall risk of bias of the included studies.
Indirectness of the evidence.
Inconsistency of the results.
Imprecision of the estimates.
Risk of publication bias.
Magnitude of the effect.
We categorised the quality of the body of evidence for each of the primary outcomes as high, moderate, low, or very low.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The electronic searches yielded 739 records, and two were found from other sources. After removal of duplicates, 471 records were screened by title and abstract for eligibility. We identified 28 potentially relevant studies and obtained the full‐text articles. After assessment of the full texts, we excluded 13 studies (see Characteristics of excluded studies). We are waiting for more information about one study (see Characteristics of studies awaiting classification). We included 14 studies in this review (Figure 1).
1.
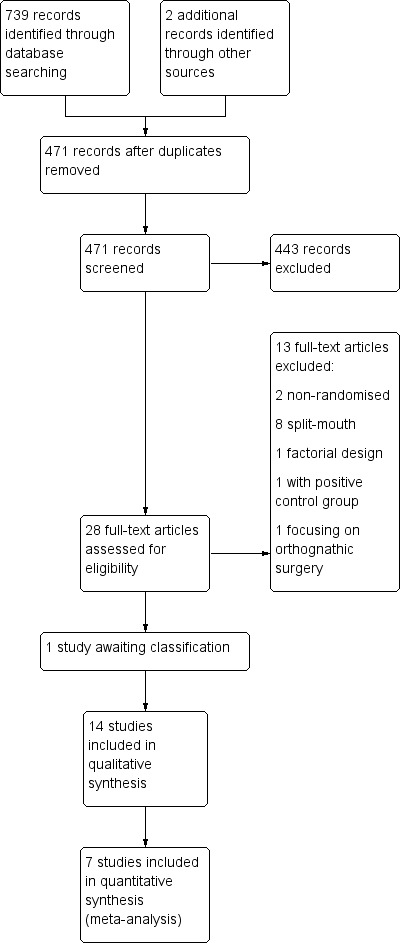
Study flow diagram
Included studies
Characteristics of the trial settings and investigators
Twelve studies were carried out in university and hospital settings and two were undertaken in a private practice setting (Miles 2012; Miles 2016).
Nine studies were two‐group parallel studies; one study was a three‐arm trial (Huang 2016); three studies involved four parallel groups (Harazaki 1997; Kim 2013; Woodhouse 2015); and one study involved five groups (Farzanegan 2012).
Characteristics of the participants
A total of 931 participants were randomised in the 14 studies. Around 860 of the participants were analysed (this is an estimate as one study did not specify the number of evaluated participants). The studies involved both adolescents and adults, with participants under the age of 16 years included in two studies (Miles 2012; Otasevic 2006) and adolescents up to 18 years included in three studies (Benson 2012; Farzanegan 2012; Keith 2013). Participants were deemed to require orthodontic treatment with fixed appliances. Comorbidity, chronic pain conditions and regular consumption of pain medications were common exclusion criteria. Participants required orthodontic extraction of four premolars in Farzanegan 2012, mandibular first premolar extraction in Woodhouse 2015, while suitability for non‐extraction treatment in the mandibular arch was a requirement for inclusion in Miles 2012 and Miles 2016. Participants had recently commenced fixed appliance treatment, with pain assessment undertaken over the first week of appliance therapy in 10 studies (Benson 2012; Farzanegan 2012; Huang 2016; Keith 2013; Kim 2013; Lobre 2015; Marie 2003, Miles 2012; Miles 2016; Otasevic 2006). Pain experience was assessed in the first week following placement of orthodontic separators in one study (Nobrega 2013). In two studies (Benson 2012; Lobre 2015), assessment was undertaken both following separator placement, following initial fixed appliance placement, and subsequent to later fixed appliance adjustments either over the initial four months of appliance therapy (Lobre 2015) or until working stainless steel arch wires were engaged (Benson 2012). In a further study, pain experienced following the initial two adjustments was considered (Woodhouse 2015).
Characteristics of the interventions
The interventions assessed four main approaches: low‐level laser therapy (LLLT) irradiation; vibratory adjuncts; experimental chewing adjuncts; and psychosocial approaches (post‐treatment text messaging, brain wave music and cognitive behavioural therapy).
LLLT was used in four studies (Harazaki 1997; Kim 2013; Nobrega 2013; Turhani 2006).
Vibratory devices were used in five studies (Lobre 2015; Marie 2003; Miles 2012; Miles 2016; Woodhouse 2015), using the AcceleDent Aura micropulse device, used for 20 minutes daily throughout the study period (Lobre 2015; Woodhouse 2015; Miles 2016), the Tooth Masseusefor 20 minutes daily (Miles 2012) or Good Vibrations for 15 minutes daily (Marie 2003).
The influence of experimental changes in chewing behaviour was assessed in three studies (Benson 2012; Farzanegan 2012; Otasevic 2006), with chewing gum used in two throughout the study period (Benson 2012; Farzanegan 2012). Bite wafers were used in two intervention groups in Farzanegan 2012 and by Otasevic 2006 throughout the seven‐day study periods.
Pain reduction using either brain wave music or cognitive behavioural therapy was assessed in one trial (Huang 2016)
Post‐treatment communication in the form a text message was carried out in one study (Keith 2013).
Control conditions
In all studies, control group participants received conventional fixed appliance‐based orthodontic treatment without the use of non‐pharmacological approaches to reduce pain.
Placebo control groups were used in six studies. Farzanegan 2012 incorporated consumption of a vitamin B₆ tablet immediately after arch wire placement and at eight‐hour intervals for a week if pain persisted. Nobrega 2013 used placebo irradiation with infrared light administered in an identical fashion to that received by intervention group participants. Turhani 2006 reports using placebo laser without active irradiation. Kim 2013 incorporated a group submitted to LED irradiation in a manner similar to the LLLT intervention group. The LED device worked on a wave length of 635 nM with 12.9 mW output from a device that looked the same as the LLLT design. Harazaki 1997 included a placebo group whose treatment involved use of a laser probe positioned intra‐orally to simulate delivery of LLLT. Woodhouse 2015 incorporated a sham used in the same way as the active AcceleDent micropulse device (as well as a control group undergoing standard treatment without use of either an active vibratory adjunct or a sham).
Dietary changes were recommended for control group participants in two studies. Benson 2012 suggested avoiding chewing gum and Otasevic 2006 recommended that participants avoid both chewing for three hours following appliance placement and hard foods for the seven‐day study period.
No alternative interventions or placebos were included in six studies (Huang 2016; Keith 2013; Lobre 2015; Marie 2003; Miles 2012; Miles 2016). Miles 2012 and Miles 2016 used no vibration for control groups participants; Keith 2013 used no text messaging; Huang 2016Lobre 2015 and Marie 2003 did not use any interventions.
Characteristics of the outcomes
Twelve studies assessed pain scores on a continuous scale (Benson 2012; Farzanegan 2012; Huang 2016; Keith 2013; Kim 2013; Lobre 2015; Marie 2003, Miles 2012; Miles 2016; Nobrega 2013; Otasevic 2006; Woodhouse 2015). In the study report, Marie 2003 included only one figure and no usable data. Otasevic 2006 presented median values only, without a measure of dispersion. Questionnaires assessing pain experience, quality, intensity and location were used in two studies (Harazaki 1997; Turhani 2006). The use of analgesics was recorded in three studies (Benson 2012; Keith 2013; Otasevic 2006). Associated morbidity related to pain was considered in two studies with the total impact score of the appliance (Benson 2012) and the impact of the appliance on oral function assessed (Farzanegan 2012). Pain assessments were recorded at multiple time intervals during the first week of appliance therapy in all studies. In Lobre 2015, assessments were undertaken over the initial four months of appliance placement on a daily basis for the first week following appliance adjustment and then weekly over the remainder of the month. Pain was also assessed both at the beginning of treatment and throughout the alignment phase in Benson 2012.
Excluded studies
We excluded 13 studies; eight were split‐mouth studies (Abtahi 2013; Artés‐Ribas 2013; Bicacki 2012; Domínguez 2013; Doshi‐Mehta 2012; Eslamian 2014; Lim 1995; Marini 2013); three applied ineligible study designs (Bartlett 2005; Esper 2011; Roth 1986); and two studied populations that were not relevant to this review (Gasperini 2014; Murdock 2010). See Characteristics of excluded studies.
Risk of bias in included studies
Only one study was assessed at low risk of bias (Nobrega 2013); six studies were graded at unclear risk of bias (Farzanegan 2012; Harazaki 1997; Huang 2016; Kim 2013; Turhani 2006; Woodhouse 2015); and seven studies were judged at high risk of bias (Benson 2012; Keith 2013; Lobre 2015; Marie 2003; Miles 2012; Miles 2016; Otasevic 2006). Further details of risk of bias assessments are presented in the Characteristics of included studies section. Overall ratings are graphically presented in Figure 2 and Figure 3.
2.
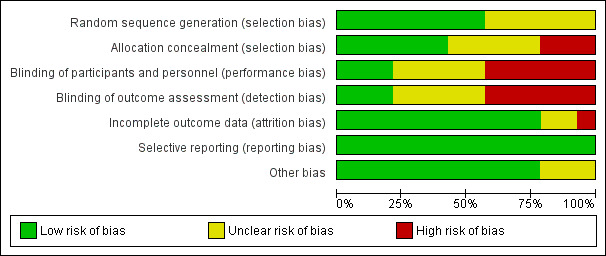
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
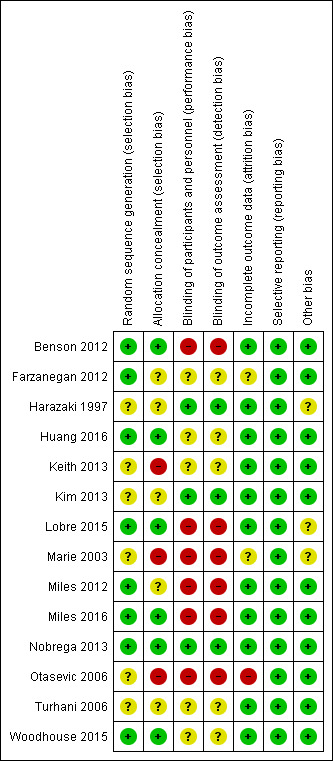
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Random sequence generation
The methods used to generate the allocation sequence and the method of concealing the sequence, such that participants and investigators enrolling participants could not foresee the next assignment, are key indicators for minimising bias in a clinical trial (Schulz 1995). The method was clear and adequate in eight studies (Benson 2012; Farzanegan 2012; Huang 2016; Lobre 2015; Miles 2012; Miles 2016; Nobrega 2013; Woodhouse 2015) and unclear in six studies (Harazaki 1997; Keith 2013; Kim 2013; Marie 2003; Otasevic 2006; Turhani 2006).
Allocation concealment
Concealment of the allocation sequence was undertaken and described in six of the included studies (Benson 2012; Huang 2016; Lobre 2015; Miles 2016; Nobrega 2013; Woodhouse 2015). We assessed allocation concealment as unclear in five studies (Farzanegan 2012; Harazaki 1997; Kim 2013; Miles 2012; Turhani 2006) and at high risk of bias in three studies (Keith 2013; Marie 2003; Otasevic 2006).
Blinding
Blinding of participants was important for this review because the main outcome was self‐assessed pain; however, the complexity of blinding both participants and personnel to the interventions is acknowledged. Some studies stated that participants and personnel were blinded, but the means used to attempt blinding had potential to be discerned by participants; for example, some studies used a control that could potentially be distinguished from the intervention, or attempted blinding by withholding some details about the study (see Characteristics of included studies). In the five studies where these situations were reported, we assessed risk of bias for blinding as unclear (Farzanegan 2012; Huang 2016; Keith 2013; Turhani 2006; Woodhouse 2015). Placebos likely to provide effective blinding were provided in four studies, which we assessed as low risk of bias (Harazaki 1997; Kim 2013; Nobrega 2013; ). In the six studies where blinding was not attempted (for example, blinding of participants to the use of adjuncts to simulate chewing was not possible) or could be very easily broken, we assessed the risk of bias as high (Benson 2012; Lobre 2015; Marie 2003; Miles 2012; Miles 2016; Otasevic 2006).
Incomplete outcome data
We judged risk of attrition bias to be at low in 11 of the included studies; there were no drop‐outs reported in seven studies (Harazaki 1997; Huang 2016; Keith 2013; Kim 2013; Miles 2016; Nobrega 2013; Turhani 2006). High drop‐out rates were reported, but reasons were not provided in Otasevic 2006, which we judged to be at high risk of bias. Farzanegan 2012 and Marie 2003 were assessed as unclear risk of bias for this domain.
Selective reporting
Although a study protocol was available for only one study (Nobrega 2013), in general, outcomes listed in the studies 'Methods’ sections were comparable to the reported results. We therefore assessed the included studies as being at low risk of reporting bias.
Other potential sources of bias
There was no reason for concern about other potential sources of bias in 11 of the included studies; the risk of other bias was considered unclear in three studies due to a lack of detail relating to baseline characteristics and the nature of the interventions and outcomes in the methods section (Harazaki 1997; Lobre 2015; Marie 2003).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Low‐level laser therapy versus placebo.
| Low‐level laser therapy versus placebo | ||||||
| Patient or population: adolescents and adults undergoing orthodontic treatment Setting: university Intervention: low‐level laser therapy Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Absolute effect in control | Mean difference (MD) low‐level laser therapy compared to control | |||||
|
Patient‐reported pain intensity or pain relief VAS (1 mm to 100 mm) ‐ 24 hours |
36 to 55.47 | Mean pain intensity in the intervention group was 20.27 mm lower (24.50 lower to 16.04 lower) | ‐ | 118 (2 RCTs) | ⊕⊕⊝⊝ lowa,b | At 6 hours, a sensitivity analysis removing the study at unclear risk of bias showed effectiveness of laser therapy: MD ‐17.90 mm, 95% CI ‐28.80 to ‐7.00 At 3 days, MD was ‐10.76 mm, 95% CI ‐13.80 to 7.73 mm At 7 days, MD was ‐6.39 mm, 95% CI ‐8.65 to ‐4.13 (1 study, 58 participants) |
| Adverse effects | Not measured | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
a Downgraded one level for imprecision b Downgraded one level for risk of bias
Summary of findings 2. Vibratory stimulation versus control.
| Vibratory stimulation versus control | ||||||
| Patient or population: adolescents and adults undergoing orthodontic treatment Setting: university and private practice Intervention: vibratory stimulation Comparison: no intervention or placebo vibration | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Absolute effect in control | Mean difference (MD) with vibratory stimulation compared to control | |||||
|
Patient‐reported pain intensity or pain relief VAS (1 mm to 100 mm) ‐ 24 hours |
47.6 to 57.65 | Mean pain intensity in the intervention group was 1.32 mm higher (11.79 lower to 14.43 higher) | ‐ | 154 (3 RCTs) | ⊕⊕⊝⊝ very lowa,b | Insufficient evidence to determine whether this intervention was effective or not at all timepoints |
| Adverse effects | Not measured | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
a Downgraded two levels for imprecision b Downgraded one level for risk of bias
Summary of findings 3. Chewing gum or bite wafer versus control.
| Chewing gum or bite wafer versus control | ||||||
| Patient or population: adolescents undergoing orthodontic treatment Setting: university and hospital Intervention: chewing gum or wafer Comparison: placebo or no chewing gum | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Absolute effect in control | Mean difference (MD) with chewing gum or wafer compared to control | |||||
|
Patient‐reported pain intensity or pain relief VAS upon chewing (1 mm to 100 mm) ‐ 24 hours |
41.6 to 74.7 | Mean pain intensity in the intervention group was15.38 mm lower (28.90 lower to 1.86 lower) | ‐ | 96 (2 RCTs) | ⊕⊝⊝⊝ very lowa,b,c | Insufficient evidence to determine whether this intervention was effective or not at all time points |
| Adverse effects | Not measured | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; VAS: visual analogue scale; mm: millimetre | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
a Downgraded one level for imprecision b Downgraded one level for risk of bias c Downgraded one level for inconsistency. Otasevic 2006 did not provide data suitable for meta‐analysis but reported higher pain in intervention group than control.
Summary of findings 4. Brain wave music or cognitive behavioural therapy versus control.
| Brain wave music (BWM) or cognitive behavioural therapy (CBT) versus control | ||||||
|
Patient or population: adults undergoing orthodontic treatment Settings: university Intervention: BWM or CBT Comparison: no special instructions | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Absolute effect in control | Mean difference (MD) with BMW or CBT compared to control | |||||
| Patient‐reported pain intensity or pain relief: VAS (1 mm to 100 mm) ‐ BWM vs control ‐ 24 hours | 53.83 | Mean patient‐reported pain intensity in the intervention group was26.65 mm lower (39.06 lower to 14.24 lower) | 24 (1 RCT) | ⊕⊝⊝⊝ very lowa,b | Insufficient evidence to determine whether this intervention was effective or not at all timepoints | |
| Adverse effects for BWM | Not measured | |||||
| Patient‐reported pain intensity or pain relief: VAS (1 mm to 100 mm) ‐ CBT vs control ‐ 24 hours | 53.83 | Mean patient‐reported pain intensity in the intervention group was20.67 mm lower (32.12 lower to 9.22 lower) | 24 (1 RCT) | ⊕⊝⊝⊝ very lowa,b | Insufficient evidence to determine whether this intervention was effective or not at all timepoints | |
| Adverse effects for CBT | Not measured | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; VAS: visual analogue scale; BWM: brain wave music; CBT: cognitive behavioural therapy | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
a Downgraded two levels for imprecision b Downgraded one level for unclear risk of bias
Summary of findings 5. Post‐treatment text message versus no text.
| Post‐treatment text message compared with no text message for alleviating orthodontic pain | ||||||
|
Patient or population: people undergoing orthodontic treatment Settings: university Intervention: text message Comparison: no text message | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Patient‐reported pain intensity or pain relief: VAS (1 mm to 100 mm) ‐ 24 hours | Not measured | 39 (1 RCT) | ⊕⊝⊝⊝ very lowa,b | Insufficient evidence to determine whether this intervention was effective or not at all timepoints | ||
| Adverse effects | Not measured | |||||
a Downgraded two levels for imprecision b Downgraded one level for high risk of bias
See: Summary of findings table 1; Table 2; Table 3; Table 4. No studies evaluated TENS; ice orcryotherapy; acupuncture or acupressure.
Low‐level laser therapy versus placebo
Patient‐reported pain intensity or pain relief measured on a VAS or other scale
Low‐level laser therapy (LLLT) versus placebo was assessed in two studies with 118 participants (Kim 2013, assessed at unclear risk of bias, and Nobrega 2013, assessed at low risk of bias) (Analysis 1.1). We assessed the evidence for this comparison as low quality owing to imprecision and the risk of bias. Time points included in the meta‐analyses were six hours, one day, three days and seven days. LLLT reduced pain compared to placebo at most time points. At six hours, the mean reduction on the VAS for irradiation was MD ‐10.63 mm (95% CI ‐22.33 to 1.07), although heterogeneity was substantial (I² = 78%). A sensitivity analysis conducted by removing Kim 2013 showed effectiveness of irradiation (MD ‐17.90, 95% CI ‐28.80 to ‐7.00; P < 0.001). LLLT also reduced pain at one day (MD ‐20.27 mm, 95% CI ‐24.50 to ‐16.04; P < 0.001; I² = 9%; 2 studies, 118 participants); three days (MD ‐10.76 mm, 95% CI ‐13.80 to ‐7.73; P < 0.001; I² = 0%; two studies, 118 participants); and seven days (MD ‐6.39 mm, 95% CI ‐8.65 to ‐4.13; P < 0.001; one study, 58 participants).
1.1. Analysis.
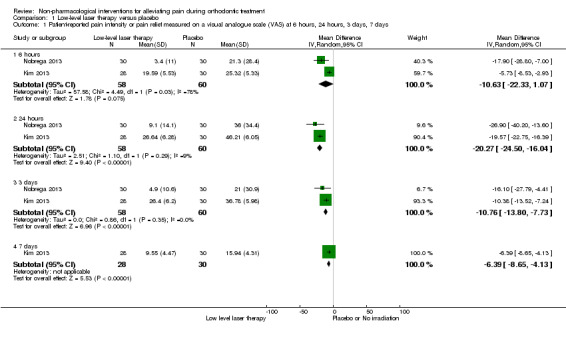
Comparison 1 Low‐level laser therapy versus placebo, Outcome 1 Patient‐reported pain intensity or pain relief measured on a visual analogue scale (VAS) at 6 hours, 24 hours, 3 days, 7 days.
Two other studies (160 participants in total) assessing this comparison used bespoke assessments and different interventions. They were assessed at unclear risk of bias. Harazaki 1997 (84 participants) used a laser and assessed the onset of pain based on a five‐point scale, the proportion of patients experiencing severe pain, the level of pain at the outset, and the day at which pain disappeared. The percentage of participants reporting severe pain upon appliance activation was slightly lower in the intervention group than the placebo group, although inferential statistical analysis was not undertaken. Turhani 2006 (76 participants) also assessed the effectiveness of LLLT and reported fewer participants experiencing pain at 6 hours (P < 0.05) and 30 hours (P < 0.05), although no effect was observed at 54 hours.
Secondary outcomes
The secondary outcomes were not assessed for this comparison.
Vibratory stimulation versus placebo vibration or no vibration
Patient‐reported pain intensity or pain relief measured on a VAS or other scale
Three studies (two at high risk of bias and one unclear) involving 154 participants provided short‐term data for this comparison (Miles 2012; Miles 2016; Woodhouse 2015) (Analysis 2.1). Lobre 2015 provided longer‐term data. Marie 2003 (a study at high risk of bias) also assessed vibratory devices but did not provide usable data. We assessed the evidence for this comparison as very low quality owing to imprecision and the risk of bias. There was no evidence that vibratory stimulation reduced pain at any of the time points assessed (Analysis 2.1; Analysis 2.2). At six hours, the mean reduction on the 100 mm VAS was ‐0.52 mm (95% CI ‐9.41 to 8.36; P = 0.91; 2 trials, 115 participants). No statistical heterogeneity was found (I² = 0%). Similar findings were observed at 24 hours (MD 1.32 mm, 95% CI ‐11.79 to 14.43; P = 0.84; 3 trials, 154 participants; I² = 59%), three days (MD .82 mm; 95% CI ‐5.12 to 8.76; P = 0 .61; 3 trials, 154 participants; I² = 7%) and also at seven days (MD 1.28 mm; 95% CI ‐3.16 to 5.71; P = 0.57; 3 trials, 154 participants; I² = 39%).
2.1. Analysis.
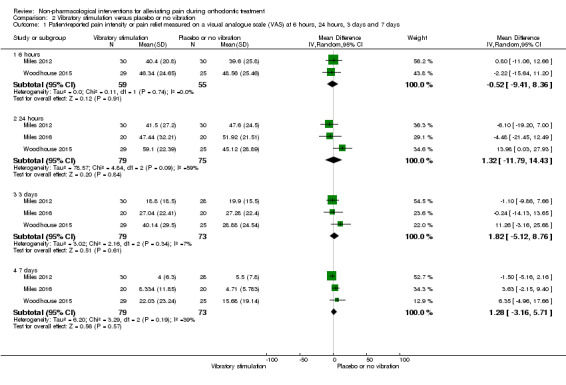
Comparison 2 Vibratory stimulation versus placebo or no vibration, Outcome 1 Patient‐reported pain intensity or pain relief measured on a visual analogue scale (VAS) at 6 hours, 24 hours, 3 days and 7 days.
2.2. Analysis.
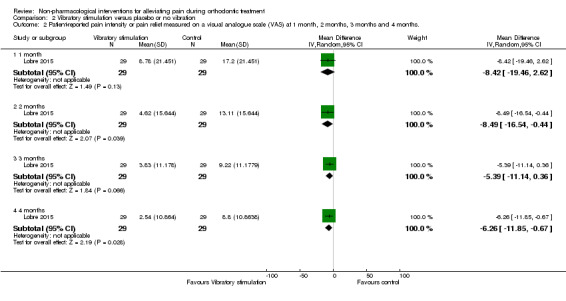
Comparison 2 Vibratory stimulation versus placebo or no vibration, Outcome 2 Patient‐reported pain intensity or pain relief measured on a visual analogue scale (VAS) at 1 month, 2 months, 3 months and 4 months..
Longer follow‐up was carried out in Lobre 2015 (high risk of bias) and Woodhouse 2015 (unclear risk of bias). Lobre 2015 found mean pain during the first four months of treatment appeared to be lower in the intervention group at two time points: mean overall pain intensity was 8.49 mm lower on the VAS during the second month (P = 0.04) and 6.26 mm lower during the fourth month (P = 0.03), with no evidence of benefit at month one and month three (Analysis 2.2). Woodhouse 2015 assessed pain experience for seven days following engagement of two arch wires (0.014 inch and 0.018 inch NiTi) and did not find evidence of a benefit for the intervention at any time point (Analysis 2.3).
2.3. Analysis.
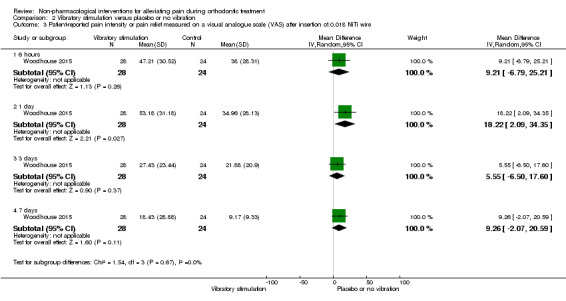
Comparison 2 Vibratory stimulation versus placebo or no vibration, Outcome 3 Patient‐reported pain intensity or pain relief measured on a visual analogue scale (VAS) after insertion of 0.018 NiTi wire.
Secondary outcomes
Dose/intensity and frequency of pain relief needed
Woodhouse 2015 assessed analgesic consumption over a one‐week period after both visits and found no statistical difference between the intervention groups either after visit 1 (P = 0.533) or visit 2 (P = 0.901) with 72%, 60% and 73% of participants requiring analgesia in the AcceleDent Aura, sham, and control groups following the first visit, respectively. These findings were mirrored following the second visit, although the prevalence of analgesic use was much lower (32% to 38%). Specifically for the comparison between AccelDent Aura and control the results for visit 1 and visit 2 were RR 0.99, 95% CI 0.72 to 1.37, P = 0.96, and RR 1.00, 95% CI 0.46 to 2.20, P = 0.99.
Data in relation to analgesic consumption was provided by Miles 2016 (see Analysis 2.4), however data were presented at specific timepoints (6 hours, 24 hours, 3 days and 7 days) after placement of the appliance. Miles 2016 noted statistically less analgesic use in the intervention group at 24 hours (RR 0.63, 95% CI 0.44 to 0.92; P < 0.01), however no statistically significant differences were identified at six hours (RR 1.00, 95% CI 0.43 to 2.33; P = 0.72), three days (RR 0.75, 95% CI 0.19 to 2.93; P = 0.68) or seven days (RR 0.33, 95% CI 0.02 to 7.02; P = 0.31).
2.4. Analysis.
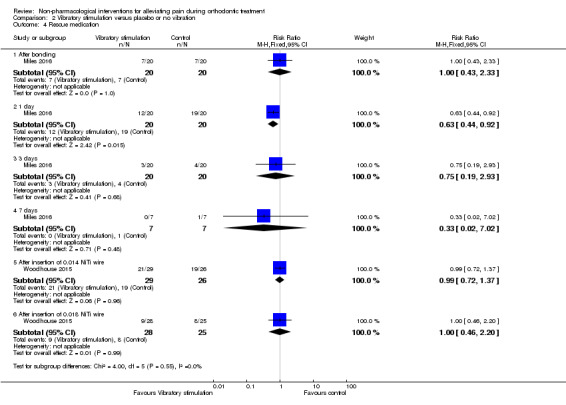
Comparison 2 Vibratory stimulation versus placebo or no vibration, Outcome 4 Rescue medication.
None of the other secondary outcomes were assessed.
Chewing adjuncts (chewing gum or wafer) versus no chewing gum or placebo
Patient‐reported pain intensity or pain relief measured on a VAS or other scale
The effect of chewing adjuncts was assessed in three trials (Benson 2012, high risk of bias; Farzanegan 2012, unclear risk of bias; Otasevic 2006, high risk of bias) that enrolled a total of 191 participants. We assessed the evidence for this comparison as very low quality owing to inconsistency, imprecision and the risk of bias. Otasevic 2006 analysed 84 participants and presented median values only with no measure of dispersion. Otasevic 2006 stated finding higher median pain in the bite wafer group compared to the group who avoided chewing at one day (P = 0.006). Farzanegan 2012 included three relevant intervention groups versus control (we adjusted the control sample size downwards in our three comparisons to avoid double counting participants). Based on a single study with evaluable data from 39 participants (Farzanegan 2012), the mean reduction in the VAS score at six hours was not statistically significant (VAS 1.39 mm, 95% CI ‐3.24 to 0.64; P = 0.18; I² = 0%). However, at 24 hours and three days, statistically significant differences were found. At 24 hours, there was a mean decrease of 15.38 mm (95% CI ‐28.90 to ‐1.86; P = 0.03; I² = 14%), based on two trials involving data for 96 participants (Benson 2012; Farzanegan 2012). At three days, the mean decrease was 29.16 mm (95% CI ‐51.67 to ‐6.65), although this result was based on a single study (Farzanegan 2012). At seven days, the mean reduction was not statistically significant (VAS 15.17 mm, 95% CI ‐32.44 to 2.11; P = 0.09; I² = 46%; 2 trials, 96 participants). See Analysis 3.1.
3.1. Analysis.
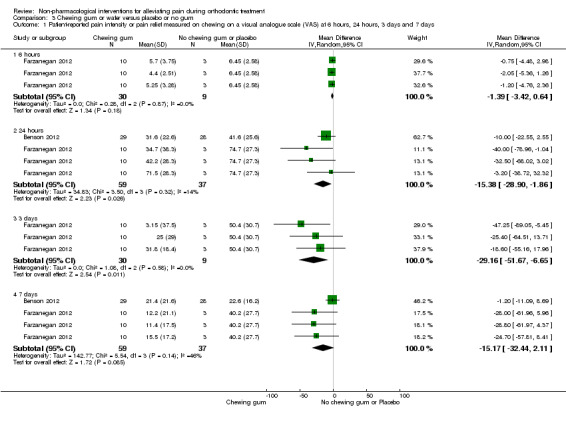
Comparison 3 Chewing gum or wafer versus placebo or no gum, Outcome 1 Patient‐reported pain intensity or pain relief measured on chewing on a visual analogue scale (VAS) at 6 hours, 24 hours, 3 days and 7 days.
Secondary outcomes
Dose/intensity and frequency of pain relief needed
Analgesic consumption was assessed in Benson 2012 (57 participants). There were no statistically significant differences between the groups at 24 hours (P = 0.903) or at one week (P = 0.104).
Quality of life or patient satisfaction
The impact of the appliances and associated pain on oral function was assessed in two studies (Benson 2012; Farzanegan 2012; 107 participants in total). The severity of pain was recorded during four oral functions including chewing, occlusion of posterior teeth, and occlusion of anterior teeth (Farzanegan 2012), with little difference observed among groups. Benson 2012 reported that the global impact of the appliance was lower in the chewing gum group 24 hours after appliance placement (RR 0.45, 95% CI 0.22 to 0.94; P = 0.03), although this difference dissipated by seven days (RR 0.68, 95% CI 0.30 to 1.53; P = 0.35) (Analysis 3.2). In terms of total impact scores, the median score was 16 lower in the chewing gum group at 24 hours (P = 0.031). By seven days, the between‐groups difference was not statistically significant (P = 0.185).
3.2. Analysis.
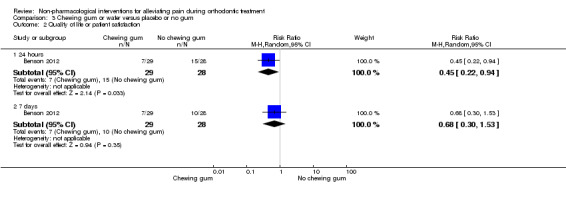
Comparison 3 Chewing gum or wafer versus placebo or no gum, Outcome 2 Quality of life or patient satisfaction.
No data were available for other secondary outcomes.
Brain wave music or cognitive behavioural therapy versus no special instructions
Patient‐reported pain intensity or pain relief measured on a VAS or other scale
The potential benefits of brain wave music and cognitive behavioural therapy on pain experience was assessed in one study at unclear risk of bias, which involved 36 participants (Huang 2016; Analysis 4.1). We assessed the quality of evidence for this comparison as very low owing to imprecision and the possible risk of bias. Brain wave music was shown to reduce pain at 24 hours (MD ‐26.65 mm, 95% CI ‐39.06 to ‐14.24; P < 0.001) and three days (MD ‐23.44 mm, 95% CI ‐36.82 to ‐10.06; P < 0.001). No statistically significant effect was observed at seven days (MD ‐4.72 mm, 95% CI ‐15.83 to 6.39; P = 0.41).
4.1. Analysis.
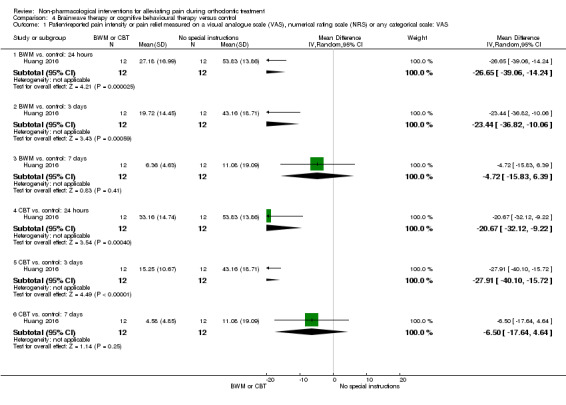
Comparison 4 Brainwave therapy or cognitive behavioural therapy versus control, Outcome 1 Patient‐reported pain intensity or pain relief measured on a visual analogue scale (VAS), numerical rating scale (NRS) or any categorical scale: VAS.
Similarly, cognitive behavioural therapy was also shown to be effective at 24 hours (MD ‐20.67 mm, 95% CI ‐32.12 to ‐9.22; P < 0.001) and three days (MD ‐27.91 mm, 95% CI ‐40.10 to ‐15.72; P < 0.001), but had no statistically significant effect at seven days (MD ‐6.50 mm, 95% CI ‐17.64 to 4.64; P = 0.25).
Secondary outcomes
No data were available for the secondary outcomes.
Post‐treatment communication (text messaging) versus no communication
Patient‐reported pain intensity or pain relief measured on a VAS or other scale
Pain experience was assessed over a seven‐day period in one study at high risk of bias, with 39 participants (Keith 2013). We assessed the evidence for this comparison as very low quality owing to imprecision and high risk of bias. Less pain was observed in the intervention group at two, three, four and five days (P < 0.05) following appliance placement, although no difference was found between groups at four hours, six days or seven days (Analysis 5.1).
5.1. Analysis.
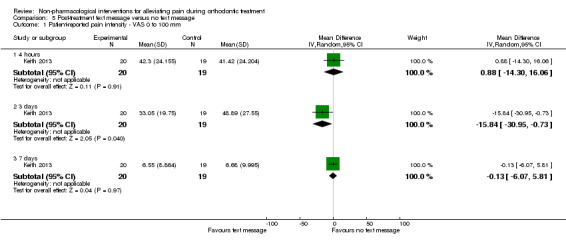
Comparison 5 Post‐treatment text message versus no text message, Outcome 1 Patient‐reported pain intensity ‐ VAS 0 to 100 mm.
Secondary outcomes
No data were available for the secondary outcomes.
Discussion
Summary of main results
We included 14 randomised controlled trials (RCTs), one at low risk of bias, six at unclear risk of bias, and seven at high risk of bias. The 14 studies enrolled 931 participants, of whom around 860 were included in analyses. The studies investigated a range of interventions including low‐level laser therapy (LLLT), vibratory stimulation, chewing adjuncts, brain wave music, cognitive behavioural therapy, and text messaging. Pain analyses were confined to the first week following separator or fixed appliance placement in 11 studies. in terms of the other three studies, Lobre 2015 followed‐up for up to four months, Woodhouse 2015 included more than one appliance adjustment, and Benson 2012) considered overall duration of orthodontic alignment.
Low‐level laser therapy versus placebo or no irradiation
There is low‐quality evidence that the use of LLLT reduced self‐reported pain levels on a visual analogue scale (VAS) at six hours, 24 hours, three days and seven days after appliance placement. There was insufficient evidence to determine effects on the secondary outcomes of this review.
Vibratory stimulation versus placebo vibration or no vibration
There is very low‐quality evidence for vibratory stimulation that does not allow us to draw firm conclusions. We did not find evidence that vibratory devices reduce pain, though the results are imprecise and may be compatible with no difference in pain or an increase in pain or a decrease in pain. We did not find evidence that vibratory stimulation leads to a significant difference in analgesic use, although one study reported a reduced requirement 24 hours after appliance placement but not at other time points (Miles 2016).
Chewing adjuncts (chewing gum or bite wafer) versus no chewing gum or placebo
On the basis of very low‐quality evidence, we found inconsistent results relating to alleviation of self‐reported pain associated with the use of chewing adjuncts. We found insufficient evidence to enable conclusions to be drawn relating to the secondary outcomes of this review.
Psychosocial and other interventions
On the basis of one trial, we found very low‐quality evidence of decreased pain experience with brain wave music or cognitive behavioural therapy, although effects were inconsistent. There was no evidence relating to the secondary outcomes of this review. The effectiveness of post‐treatment text messaging was assessed in one study assessed at high risk of bias, with a reduction in pain experience observed at four of the seven time intervals assessed. We assessed the quality of this evidence as very low.
Overall completeness and applicability of evidence
We included and assessed patient‐centred outcomes, including levels of pain experience, requirement for rescue medications and functional impacts of orthodontic treatment. Assessments were primarily undertaken over the initial week following appliance placement. More prolonged assessment was undertaken in three of the included studies (Benson 2012; Lobre 2015; Woodhouse 2015). Pain, however, is known to arise throughout orthodontic treatment, albeit typically being less severe after the initial period. Harms associated with the alternatives to anti‐inflammatories assessed in the studies were not considered. While these harms are likely to be minimal, given the conservative nature of the interventions, it is important that they are considered.
A 100 mm VAS was the most common pain assessment method used in the included studies. Given the relatively low number of included studies, it is important that outcomes assessed in future clinical trials use similar outcome measures; the 100 mm VAS appears to be the most accepted approach at present. Notwithstanding, a threshold level of pain reduction in terms of intensity or duration has yet to be established.
There were insufficient data to consider the impact of different participant characteristics on the effectiveness of the interventions to reduce pain; for example, we could not investigate whether there was a differential response between males and females.
The lack of evidence identified for interventions in this review may reflect the relative infancy of a variety of the approaches to address orthodontic pain and that use of analgesics is an established practice. It is important that some of the more promising approaches are subjected to further prospective analysis with prolonged follow‐up and that the secondary outcomes including potential harms of these novel interventions are assessed. Further research should also address the cost implications of these interventions as they do represent additional procedures; for example, using proprietary devices to facilitate delivery of vibratory stimulation may have significant associated costs for clinicians, patients, or both.
Quality of the evidence
Limitations in study design and implementation
The design of the included studies was generally adequate; however, only one included study was assessed at low risk of bias. Reporting was generally poor; methods applied to conceal allocation, and to blind investigators and participants were unclear in a number of studies (Figure 2). Blinding of participants was attempted in a number of studies with the use of placebo interventions. Blinding participants was not possible for some interventions, such as use of altered chewing.
Indirectness of the evidence
The primary objective of this review ‐ subjective assessment of pain experience ‐ was considered in all the included studies. However, there were very few studies for each of the intervention types assessed, with some interventions only assessed in one study. Differing protocols and proprietary brands of interventions such as LLLT or vibratory stimulation were used, making direct comparisons more difficult. Moreover, pain assessments were recorded over the initial week following appliance placement or manipulation in each study; extended follow‐up was performed in only two studies. Therefore, the relative effectiveness of non‐pharmacological interventions over the course of orthodontic treatment remains unclear. Data relating to harms or other impacts of interventions or compliance‐based procedures were not reported in the included studies. However, the research settings were representative; most included studies were undertaken in either hospital or university centres and involved both adolescent and adult participants.
Inconsistency of results
Assessment of the consistency of reported outcomes in the included studies was challenging because of the small number of included studies, variation among interventions, and insufficient usable data being available. For example, studies that reported on chewing adjuncts reported findings in opposite directions.
Imprecision of results
The quality of the evidence was downgraded for imprecision because of the lack of similar studies, low numbers of participants and wide confidence intervals.
Publication bias
We undertook a detailed search for both published and unpublished studies, with no restrictions on language to limit the risk of publication bias. We searched the reference lists of included studies and contacted many study authors to obtain information that was not included in the published reports. Given that few studies comparing similar interventions were found, funnel plot assessment of publication bias was not possible (Higgins 2011).
Potential biases in the review process
Efforts were made to reduce bias in the review process by ensuring a comprehensive search for potentially eligible studies. The independent, duplicate assessments of study eligibility and data extraction, limited the likelihood of additional bias. We also chose broad inclusion criteria, leading to a clinically heterogeneous group of studies presenting a range of interventions. We made changes to the review methods following publication of the protocol (see Differences between protocol and review). We acknowledge that post hoc changes to the review methods may have introduced a risk of bias.
Agreements and disagreements with other studies or reviews
No previous systematic review has assessed the impact of non‐pharmacological interventions to alleviate pain during orthodontic treatment. Other systematic reviews have addressed the potential value of some of these interventions, such as adjunctive vibratory stimulation on the rate of orthodontic tooth movement have (El‐Angbawi 2015).
Authors' conclusions
Implications for practice.
There is a lack of reliable evidence concerning the effectiveness of a range of non‐pharmacological interventions to manage orthodontic pain. A small number of studies provided low‐quality evidence that orthodontic pain may be reduced in the short term by use of low‐level laser irradiation; however, further prospective research considering pain experiences both during the initial stages and throughout orthodontic treatment are required.
Implications for research.
There is need for further comprehensive clinical trials that assess the effectiveness of non‐pharmacological interventions for orthodontic pain. Future trials should be robust, well‐designed and reported in accordance with the CONSORT statement or extensions of the CONSORT statement. Clear methodological conduct and reporting would help with appraisal of study results, accurate judgements about risk of bias, and the overall quality of the evidence. Moreover, studies with unclear methodology have been shown to produce biased estimates of treatment effects (Schulz 1995). Detailed reporting of methods, such as generation of allocation concealment, and numbers and reasons for participants' withdrawals and exclusions, is required. Where possible the use of a placebo to enable blinding would also be helpful.
Further research should evaluate emerging techniques in relation to pain experienced throughout treatment. It is important that these assessments also incorporate a holistic evaluation of both the potential benefits and possible harms associated with these interventions. Costs should be also be considered. Some of the more novel techniques require prolonged daily use of an appliance (such as vibratory stimulation), which has implications for cost, compliance and impact on daily life.
A limitation of a number of the included studies was the short‐term nature of the assessment. Orthodontic treatment is lengthy and pain is known to arise both after the initial visit and following regular adjustment appointments. It would be helpful if future studies evaluated pain experience over prolonged periods. If potential benefits associated with clinician‐delivered pain alleviation procedures are proven, there may be potential value in repeating these procedures throughout the course of treatment.
While most clinical trials used continuous scales as a means of recording pain experience, it is accepted that many outcome measures used in clinical trials are not standardised patient‐oriented outcome measurements. A need remains for the development of an accepted set of patient‐oriented outcomes within many specialties, including orthodontics.
Acknowledgements
We would like to thank Anne Littlewood for all her help developing the search strategy and running the searches. We also thank the editorial team (Luisa Fernandez‐Mauleffinch, Scott Deacon, Anne Littlewood, Tanya Walsh and Helen Worthington) and the external referees Sheena Deery, Christine Feinmann, Anne Marie Kuijpers‐Jagtman and Mohammad Owaise Sharif for their advice. We thank Ann Jones for final copy editing.
Appendices
Appendix 1. Cochrane Oral Health’s Trials Register search strategy
#1 (orthodontic*) AND (INREGISTER) #2 (((tooth or teeth or dental or oral*) AND (bracket* or brace* or wire* or headgear* or "head gear*" or facemask* or "face mask*" or face‐mask* or head‐gear* or chincap* or facebow* or "chin cap*" or chin‐cap* or "face bow*" or face‐bow*))) AND (INREGISTER) #3 (((tooth or teeth or dental or oral*) AND (appliance* or device*))) AND (INREGISTER) #4 (((intraoral or "intra oral" or intra‐oral or extraoral or "extra oral" or extra‐oral) and (appliance* or device*) and (tooth or teeth or dental))) AND (INREGISTER) #5 ("activator appliance*") AND (INREGISTER) #6 (#1 or #2 or #3 or #4 or #5) AND (INREGISTER) #7 (laser*) AND (INREGISTER) #8 ((vibrat* or acceledent)) AND (INREGISTER) #9 (("transcutaneous electric nerve stimulation" or TENS or electrostimulat* or electro‐stimulat* or "electro stimulat*")) AND (INREGISTER) #10 ((electroanalgesia or "percutaneous electric nerve stimulat*" or "percutaneous electrical nerve stimulat*")) AND (INREGISTER) #11 ((telemedicine or teledentistry or phone or telephone or call* or communicat*)) AND (INREGISTER) #12 (("bite wafer*" or "therapy wafer*" or "Elastobite wafer*" or "flex* wafer*" or "masticatory wafer*" or thera‐bite*)) AND (INREGISTER) #13 (gum*) AND (INREGISTER) #14 ((ice* or cryotherap* or "cold therap*")) AND (INREGISTER) #15 (acupunc*) AND (INREGISTER) #16 (#7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15) AND (INREGISTER) #17 (#6 and #16) AND (INREGISTER) #18 ((pain* or analgesi* or discomfort* or ache* or tender* or sore* or odontalg*)) AND (INREGISTER) #19 (#17 and #18) AND (INREGISTER)
Appendix 2. Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search strategy
#1 MeSH descriptor: [Orthodontics] explode all trees #2 orthodontic* #3 ((tooth or teeth or dental or oral*) and (bracket* or brace* or wire* or headgear* or "head gear*" or facemask* or "face mask*" or face‐mask* or head‐gear* or chincap* or facebow* or "chin cap*" or chin‐cap* or "face bow*" or face‐bow*)) #4 ((tooth or teeth or dental or oral*) and (appliance* or device*)) #5 ("activator appliance*") #6 ((intraoral or "intra oral" or intra‐oral or extraoral or "extra oral" or extra‐oral) near/5 (appliance* or device*) and (tooth or teeth or dental)) #7 #1 or #2 or #3 or #4 or #5 or #6 #8 MeSH descriptor: [Lasers] this term only #9 laser* #10 MeSH descriptor: [Vibration] this term only #11 ((vibrat* near/5 stimulat*) or (mechanic* near/5 vibrat*) or acceledent) #12 MeSH descriptor: [Transcutaneous Electric Nerve Stimulation] this term only #13 TENS:ti,ab,kw #14 (electrostimulat* or electro‐stimulat* or "electro stimulat*") #15 (electroanalgesia or "percutaneous electric nerve stimulat*" or "percutaneous electrical nerve stimulat*") #16 MeSH descriptor: [Telemedicine] this term only #17 (telemedicine or teledentistry or phone or telephone or call* or communicat*) #18 ((bite near/5 wafer*) or (therapy near/5 wafer*) or "Elastobite wafer*" or (flex* near/5 wafer*) or (masticatory near/5 wafer*) or thera‐bite*) #19 MeSH descriptor: [Chewing Gum] this term only #20 gum* #21 MeSH descriptor: [Ice] this term only #22 ice* #23 MeSH descriptor: [Cryotherapy] this term only #24 (cryotherap* or "cold therap*") #25 MeSH descriptor: [Acupuncture] explode all trees #26 acupuncture* #27 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 #28 #7 and #27 #29 MeSH descriptor: [Pain] explode all trees #30 (pain* or analgesi* or discomfort* or ache* or tender* or sore* or odontalg*) #31 #29 or #30 #32 #28 and #31
Appendix 3. MEDLINE Ovid search strategy
1. exp Orthodontics/ 2. orthodontic$.mp. 3. ((tooth or teeth or dental$ or oral$) and (bracket$ or brace$ or wire$ or headgear$ or "head gear$" or facemask$ or "face mask$" or head‐gear$ or face‐mask$ or chincap$ or facebow$ or "chin cap$" or chin‐cap$ or face‐bow$ or "face bow$")).mp. 4. ((tooth or teeth or dental or oral) and ((function$ adj5 applianc$) or (fix$ adj5 applianc$) or (remov$ adj5 applianc$) or (function$ adj5 device$) or (fix$ adj5 device$) or (remov$ adj5 applianc$))).mp. 5. "activator appliance$".mp. 6. (((intraoral or "intra oral" or intra‐oral or extraoral or "extra oral" or extra‐oral) adj5 (applianc$ or devic$)) and (tooth or teeth or dental)).mp. 7. or/1‐6 8. Lasers/ 9. laser$.mp. 10. Vibration/ 11. ((vibrat$ adj5 stimulat$) or (mechanic$ adj5 vibrat$) or acceledent).mp. 12. Transcutaneous Electric Nerve Stimulation/ 13. TENS.mp. 14. (electrostimulat$ or electro‐stimulat$ or "electro stimulat$" or "electric nerve stimulat$" or "electrical nerve stimulat$").mp. 15. (electroanalgesia or "percutaneous electric nerve stimulat$" or "percutaneous electrical nerve stimulat$").mp. 16. Telemedicine/ 17. (telemedicine or teledentistry or phone or telephone or call$ or communicat$).mp. 18. ((bite$ adj5 wafer$) or (therapy adj5 wafer$) or "Elastobite wafer$" or (flex$ adj5 wafer$) or (masticatory adj5 wafer$) or Thera‐bite$).mp. 19. or/8‐18 20. 7 and 19 21. Pain/ 22. (pain$ or analgesi$ or discomfort$ or ache$ or tender$ or sore$ or odontalg$).mp. 23. 21 or 22 24. 20 and 23
Appendix 4. Embase Ovid search strategy
1. exp Orthodontics/ 2. orthodontic$.mp. 3. ((tooth or teeth or dental$ or oral$) and (bracket$ or brace$ or wire$ or headgear$ or "head gear$" or facemask$ or "face mask$" or head‐gear$ or face‐mask$ or chincap$ or facebow$ or "chin cap$" or chin‐cap$ or face‐bow$ or "face bow$")).mp. 4. ((tooth or teeth or dental or oral) and ((function$ adj5 applianc$) or (fix$ adj5 applianc$) or (remov$ adj5 applianc$) or (function$ adj5 device$) or (fix$ adj5 device$) or (remov$ adj5 applianc$))).mp. 5. "activator appliance$".mp. 6. (((intraoral or "intra oral" or intra‐oral or extraoral or "extra oral" or extra‐oral) adj5 (applianc$ or devic$)) and (tooth or teeth or dental)).mp. 7. or/1‐6 8. Lasers/ 9. laser$.mp. 10. Vibration/ 11. ((vibrat$ adj5 stimulat$) or (mechanic$ adj5 vibrat$) or acceledent).mp. 12. Transcutaneous Electric Nerve Stimulation/ 13. TENS.mp. 14. (electrostimulat$ or electro‐stimulat$ or "electro stimulat$" or "electric nerve stimulat$" or "electrical nerve stimulat$").mp. 15. (electroanalgesia or "percutaneous electric nerve stimulat$" or "percutaneous electrical nerve stimulat$").mp. 16. Telemedicine/ 17. (telemedicine or teledentistry or phone or telephone or call$ or communicat$).mp. 18. ((bite$ adj5 wafer$) or (therapy adj5 wafer$) or "Elastobite wafer$" or (flex$ adj5 wafer$) or (masticatory adj5 wafer$) or Thera‐bite$).mp. 19. or/8‐18 20. 7 and 19 21. Pain/ 22. (pain$ or analgesi$ or discomfort$ or ache$ or tender$ or sore$ or odontalg$).mp. 23. 21 or 22 24. 20 and 23 This subject search was linked to an adapted version of the Cochrane Embase Project filter for identifying RCTs in Embase Ovid (see http://www.cochranelibrary.com/help/central‐creation‐details.html for information):
1. Randomized controlled trial/ 2. Controlled clinical study/ 3. Random$.ti,ab. 4. randomization/ 5. intermethod comparison/ 6. placebo.ti,ab. 7. (compare or compared or comparison).ti. 8. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 9. (open adj label).ti,ab. 10. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 11. double blind procedure/ 12. parallel group$1.ti,ab. 13. (crossover or cross over).ti,ab. 14. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 15. (assigned or allocated).ti,ab. 16. (controlled adj7 (study or design or trial)).ti,ab. 17. (volunteer or volunteers).ti,ab. 18. trial.ti. 19. or/1‐18 20. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 21. 19 not 20
Appendix 5. EThOS search strategy
orthodontic AND pain
Appendix 6. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and World Health Organization International Clinical Trials Registry Platform search strategy
ClinicalTrials.gov: orthodontic AND pain WHO International Clinical Trials Registry Platform: orthodontic pain
Data and analyses
Comparison 1. Low‐level laser therapy versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient‐reported pain intensity or pain relief measured on a visual analogue scale (VAS) at 6 hours, 24 hours, 3 days, 7 days | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6 hours | 2 | 118 | Mean Difference (IV, Random, 95% CI) | ‐10.63 [‐22.33, 1.07] |
| 1.2 24 hours | 2 | 118 | Mean Difference (IV, Random, 95% CI) | ‐20.27 [‐24.50, ‐16.04] |
| 1.3 3 days | 2 | 118 | Mean Difference (IV, Random, 95% CI) | ‐10.76 [‐13.80, ‐7.73] |
| 1.4 7 days | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐6.39 [‐8.65, ‐4.13] |
Comparison 2. Vibratory stimulation versus placebo or no vibration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient‐reported pain intensity or pain relief measured on a visual analogue scale (VAS) at 6 hours, 24 hours, 3 days and 7 days | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6 hours | 2 | 114 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐9.41, 8.36] |
| 1.2 24 hours | 3 | 154 | Mean Difference (IV, Random, 95% CI) | 1.32 [‐11.79, 14.43] |
| 1.3 3 days | 3 | 152 | Mean Difference (IV, Random, 95% CI) | 1.82 [‐5.12, 8.76] |
| 1.4 7 days | 3 | 152 | Mean Difference (IV, Random, 95% CI) | 1.28 [‐3.16, 5.71] |
| 2 Patient‐reported pain intensity or pain relief measured on a visual analogue scale (VAS) at 1 month, 2 months, 3 months and 4 months. | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 1 month | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐8.42 [‐19.46, 2.62] |
| 2.2 2 months | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐8.49 [‐16.54, ‐0.44] |
| 2.3 3 months | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐5.39 [‐11.14, 0.36] |
| 2.4 4 months | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐6.26 [‐11.85, ‐0.67] |
| 3 Patient‐reported pain intensity or pain relief measured on a visual analogue scale (VAS) after insertion of 0.018 NiTi wire | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 6 hours | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 9.21 [‐6.79, 25.21] |
| 3.2 1 day | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 18.22 [2.09, 34.35] |
| 3.3 3 days | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 5.55 [‐6.50, 17.60] |
| 3.4 7 days | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 9.26 [‐2.07, 20.59] |
| 4 Rescue medication | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 After bonding | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.43, 2.33] |
| 4.2 1 day | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.44, 0.92] |
| 4.3 3 days | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.19, 2.93] |
| 4.4 7 days | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.02] |
| 4.5 After insertion of 0.014 NiTi wire | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.72, 1.37] |
| 4.6 After insertion of 0.018 NiTi wire | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.46, 2.20] |
Comparison 3. Chewing gum or wafer versus placebo or no gum.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient‐reported pain intensity or pain relief measured on chewing on a visual analogue scale (VAS) at 6 hours, 24 hours, 3 days and 7 days | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6 hours | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐3.42, 0.64] |
| 1.2 24 hours | 2 | 96 | Mean Difference (IV, Random, 95% CI) | ‐15.38 [‐28.90, ‐1.86] |
| 1.3 3 days | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐29.16 [‐51.67, ‐6.65] |
| 1.4 7 days | 2 | 96 | Mean Difference (IV, Random, 95% CI) | ‐15.17 [‐32.44, 2.11] |
| 2 Quality of life or patient satisfaction | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 24 hours | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.22, 0.94] |
| 2.2 7 days | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.30, 1.53] |
Comparison 4. Brainwave therapy or cognitive behavioural therapy versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient‐reported pain intensity or pain relief measured on a visual analogue scale (VAS), numerical rating scale (NRS) or any categorical scale: VAS | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 BWM vs. control: 24 hours | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐26.65 [‐39.06, ‐14.24] |
| 1.2 BWM vs. control: 3 days | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐23.44 [‐36.82, ‐10.06] |
| 1.3 BWM vs. control: 7 days | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐4.72 [‐15.83, 6.39] |
| 1.4 CBT vs. control: 24 hours | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐20.67 [‐32.12, ‐9.22] |
| 1.5 CBT vs. control: 3 days | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐27.91 [‐40.10, ‐15.72] |
| 1.6 CBT vs. control: 7 days | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐6.5 [‐17.64, 4.64] |
Comparison 5. Post‐treatment text message versus no text message.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient‐reported pain intensity ‐ VAS 0 to 100 mm | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 4 hours | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.88 [‐14.30, 16.06] |
| 1.2 3 days | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐15.84 [‐30.95, ‐0.73] |
| 1.3 7 days | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐6.07, 5.81] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Benson 2012.
| Methods | Trial design: RCT, 2 groups Location: Charles Clifford Hospital, Sheffield, UK Number of centres: 1 |
|
| Participants | SELECTION CRITERIA • Aged up to 18 years The following exclusion criteria were applied. • Patients with a cleft of the lip or palate • Patients with phenylketonuria (who must avoid products containing aspartame or artificial sweeteners which contain phenylalanine) • Significant medical history • Poor dental or periodontal health precluding the use of fixed appliances • Undergoing orthodontic treatment with fixed upper and lower appliances Participants: 68 Number randomised: 68 (intervention: 37; control: 31) Number evaluated: 57 (intervention: 29; control: 28) (31 male, 26 female) Mean age: 14.7 (SD 1.5) years intervention group, 13.9 (SD 1.6) control group |
|
| Interventions | INTERVENTION: Chewing gum (Wrigley's Orbit Complete) for as‐required use at the bonding/separator appointment and subsequent appointments up to the visit after the placement of the working arch wire (0.019 × 0.025 mm stainless steel) CONTROL: Non‐chewing gum group, specifically asked not to chew gum for the duration of the study |
|
| Outcomes | The primary outcome was the Total Impact Score (TIS) reported by the participants at 24 hours and 1 week after placement of the brace. Secondary outcomes included assessment of pain using the VAS measurements at 24 hours and 1 week and reported use of oral analgesics | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...using computer‐generated random numbers" (p. 180) |
| Allocation concealment (selection bias) | Low risk | "The allocations were concealed in consecutively numbered opaque sealed envelopes, which were opened only after the patient and parent had agreed to enter the trial and had signed the consent form." (p. 180) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Efforts to blind operators to group allocation was undertaken where possible: "Masking of the patient to group allocation was not possible because they were either asked to chew gum or not. Masking of the operator was undertaken where practical; however this was not always possible." (p. 180) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of chief assessors (participants) "Masking of the operator was undertaken where practical; however this was not always possible" (p. 180) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 57 of 68 randomised participants were analysed. Reasons for failure to complete including drop‐out, failure to complete diaries and an administrative error were outlined Comment: Failure to complete was reported with the reason given and these represented fewer than 20% of the sample |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but outcomes mentioned in the methods section appeared to have been reported |
| Other bias | Low risk | Appeared to be free of other forms of bias |
Farzanegan 2012.
| Methods | Trial design: RCT, 5 groups Location: Dental School, Mashhad University of Medical Sciences, Iran Number of centres: 1 |
|
| Participants | SELECTION CRITERIA • Scheduled for fixed appliance treatment • Moderate crowding (4 to 8 mm) according to Little's irregularity index • Requiring extraction of 4 first premolars for orthodontic reasons • Extractions scheduled to be finished at least 2 weeks before placement of the orthodontic appliances • No systemic diseases and not receiving analgesic therapy • Undergoing orthodontic treatment with fixed upper and lower appliances. Participants: 50 (50 female) Number randomised: 50 (Group 1: 10; Group 2: 10; Group 3: 10; Group 4: 10; Group 5: 10) Number evaluated: not mentioned Age range: 13 years to 18 years |
|
| Interventions | INTERVENTION: 1. Ibuprofen group, participants took a 400 mg ibuprofen tablet immediately after arch wire placement and at 8‐hourly intervals for 1 week if pain persisted. 2. Chewing gum group, participants chewed a sugar‐free gum (Orbit, The Wrigley Company) for 5 minutes immediately after arch wire placement and at 8‐hour intervals for 1 week if they experienced pain. 3. Soft‐viscoelastic group, participants used horseshoe‐shaped viscoelastic polyvinyl siloxane bite wafers with low toughness. Participants in these 2 groups chewed or bit down on the bite wafers for 5 minutes at 8‐hour intervals for 1 week if pain persisted. 4. Hard‐viscoelastic group, participants used horseshoe‐shaped viscoelastic polyvinyl siloxane bite wafers with moderate toughness. Participants in these 2 groups chewed or bit down on the bite wafers for 5 minutes at 8‐hour intervals for 1 week if pain persisted. CONTROL: Placebo: Participants asked to take a B₆ vitamin tablet immediately after arch wire placement and at 8‐hour intervals for 1 week if pain persisted |
|
| Outcomes | Pain intensity (measured on a 100 mm VAS) after 2 hours, 6 hours and at bedtime on the day of arch wire placement, and at 24 hours, 2 days, 3 days and 7 days after first appointment. Severity of pain was recorded for 4 oral functions including chewing, biting, fitting back teeth and fitting front teeth | |
| Notes | We compared intervention groups 2, 3 and 4 against control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...according to their clinic entrance number and a random number table" (p. 170). This was confirmed by e‐mail communication (22 August 2015) |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in paper and clarification not given in e‐mail communication |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | A placebo was used but as it was a tablet, it is uncertain if this was an effective placebo for the three chewing intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants in the control group received a placebo intervention but this was not identical so it is uncertain if it was effective |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐outs or numbers completing the study were not mentioned |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but outcomes mentioned in the methods section appeared to have been reported |
| Other bias | Low risk | Appeared to be free of other forms of bias |
Harazaki 1997.
| Methods | Trial design: RCT, 3 groups Location: Department of Orthodontics, Tokyo Dental College, Japan Number of centres: 1 |
|
| Participants | SELECTION CRITERIA • Receiving edgewise orthodontic therapy • Undergoing orthodontic treatment with fixed upper and lower appliances Participants: 84 (27 male, 57 female) Number randomised: 84 (Group 1: 44 ; Group 2: 20; Group 3: 20) Number evaluated: 84 (Group 1: 44 ; Group 2: 20; Group 3: 20) Age range: 11 years to 34 years |
|
| Interventions | INTERVENTION: Either a laser irradiation group receiving laser therapy for 30 seconds at the apical region of each tooth from a labial or lingual direction (Group 2) or a blind irradiation group receiving the same therapy without irradiation (Group 3) CONTROL: No irradiation (Group 1)| |
|
| Outcomes | Questionnaire involving 5 questions exploring the timing at which the pain commenced, when pain peaked and abated, and exploring the nature and severity of the discomfort | |
| Notes | We could not extract any usable data from this paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A blind irradiation placebo group was included in the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were blinded in groups 1 and 2 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but outcomes mentioned in the methods section appeared to have been reported |
| Other bias | Unclear risk | Not enough detail provided to allow an assessment |
Huang 2016.
| Methods | Trial design: RCT, 3 groups Location: Orthodontic Department of the West China Hospital of Stomatology, Chengdu, China. Number of centres: 1 |
|
| Participants | SELECTION CRITERIA Recruited from among 54 right‐handed healthy young medical college students after they provided written informed consent. (i) aged 22 ± 3 years; (ii) mild‐to‐moderate malocclusion and no previous orthodontic treatment; (iii) no oral diseases which may lead to pain perception (i.e. toothache, periodontitis, oral ulcer, pulpitis) within 1 week; (iv) no infectious diseases or systemic diseases or both; (v) pain threshold from 3 to 60 seconds; and (vi) pain tolerance < 5 min, as reflected by the cold pressor test (CPT). EXCLUSION CRITERIA Psychiatric issues, abnormal pain perception, and excessive anxiety or depression based on screening by the CPT with EEG monitoring and a series of questionnaires, including the Short‐form McGill Pain Questionnaire, the Trait‐Anxiety Inventory, the State‐Anxiety Inventory, and the Self‐Rating Depression Scale. Participants: 36 (gender distribution not given) Number randomised: 36 (cognitive behavioural therapy: 12; brain wave music: 12; control: 12) Number evaluated: 36 (cognitive behavioural therapy: 12; brain wave music: 12; control: 12) Mean age: 22 ± 3 years |
|
| Interventions | INTERVENTIONS: Cognitive behavior therapy or brain wave music both lasted for approximately 3 minutes. There was a verbal introduction (2 minutes) before and a silent period (5 minutes) after each intervention, the intervention therefore lasted 10 minutes overall. CONTROL: No special instructions |
|
| Outcomes | VAS scores recorded daily 1 to 10 days, then at 14 and 30 days after initial orthodontic appliance placement | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A stratified block randomisation was performed before the treatment... One individual in each block was randomly assigned to the BWM group, the CBT group or the control group, via a computer‐generated sequence." (p. 2) |
| Allocation concealment (selection bias) | Low risk | "Group allocation was performed by a Chinese Clinical Trial Registry statistician" (p. 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Operators were blinded to group allocation. "One individual in each block was randomly assigned to the BWM group, the CBT group or the control group, via a computer‐generated sequence performed by a Chinese Clinical Trial Registry statistician. The clinicians and data analysts were blinded to the allocation. Separately, in isolated rooms, the three groups received the same 15‐min instruction regarding orthodontic treatment, tooth‐movement pain, oral hygiene maintenance and the respective study procedures." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data analysts were blinded. As described in blinding section above, trial authors considered participants to be blinded but it is unclear if this would have been effective as placebo was not used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but outcomes mentioned in the methods section appeared to have been reported |
| Other bias | Low risk | Appeared to be free of other forms of bias |
Keith 2013.
| Methods | Trial design: RCT, 2 groups Location: Seton Hill University, Pennsylvania, USA Number of centres: 1 |
|
| Participants | SELECTION CRITERIA • Undergoing orthodontic treatment with fixed upper and lower appliances • Aged 10 years to 18 years • Access to a cellular telephone • No previous orthodontic treatment • No reported chronic usage of analgesic medications • No pain‐related pathology or disease Participants: 39 (14 male, 25 female) Number randomised: 39 (intervention: 20; control: 19) Number evaluated: 39 (intervention: 20; control: 19) Mean age: 12.6 years intervention group, 14.2 years control group |
|
| Interventions | INTERVENTION: Text message following appointment offering encouragement and concern CONTROL: No text message |
|
| Outcomes | Outcomes: Pain intensity was measured on a 100 mm VAS; the use of analgesia at baseline, 4 hours following appliance placement and at the same time daily for 7 days | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "CONSORT 2010 and randomised sequencing guidelines, subject group assignment was done" (p. 606) |
| Allocation concealment (selection bias) | High risk | "...assigned to the experimental and control groups in an attempt to closely approximate the trial arms based on a minimization protocol as described by Pandis" (p. 606) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of researchers was not mentioned: "Subjects were blinded as to group status and were not made aware that a text message was part of the study." (p. 606). It is uncertain this would have been an effective method of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Trial authors considered participants (outcome assessors for pain) to be blinded as they were not aware that a text message was part of the study; however, it is uncertain that this would have been an effective method of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but outcomes mentioned in the methods section appeared to have been reported |
| Other bias | Low risk | Appeared to be free of other forms of bias |
Kim 2013.
| Methods | Trial design: RCT, 3 groups Location: Catholic University of Korea, South Korea Number of centres: 1 |
|
| Participants | SELECTION CRITERIA • Complete eruption of the second molars • No open interproximal contacts of the first molar • No previous orthodontic treatment, metabolic and periodontal diseases • Not on medication • Undergoing orthodontic treatment with fixed upper and lower appliances Participants: 88 (23 male, 65 female) Number randomised: 88 (Group 1: 28; Group 2: 30; Group 3: 30) Number evaluated: 88 (Group 1: 28; Group 2: 30; Group 3: 30) Mean age: 22.7 years |
|
| Interventions | INTERVENTION: Participants in the (1) laser irradiation group (N = 28) were asked to use the laser for 30 seconds on each area immediately then every 12 hours for 1 week with close contact between the tip and mucosa to irradiate the mesiobuccal, mesiolingual, distobuccal, and distolingual areas of the molars. The laser was a low‐level medical semiconductor laser device with an AlGaInP diode, wave length of 635 nM, energy of 10 m mJ, field diameter of 5.6 mm, and output potency of 6 mW CONTROL: LED was a placebo applied using the same regime as the intervention group. The LED device had a wave length of 635 NM and output of 12.9 mW of the same exterior design (Group 2). Another control group received no irradiation or placebo (Group 3) |
|
| Outcomes | Pain intensity (measured on a 100 mm VAS) at 11 intervals: 5 minutes, 1 hour, 6 hours, 12 hours and then at days 1, 2, 3, 4, 5, 6 and 7 after separator placement | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomly assigned" (p. 612) |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Subjects assigned to the laser and LED groups were blinded to their assignment." (p. 612) Blinding of the investigators was not mentioned but a placebo LED device was used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but outcomes mentioned in the methods section appeared to have been reported |
| Other bias | Low risk | Appeared to be free of other forms of bias |
Lobre 2015.
| Methods | Trial design: RCT, 2 groups Location: Tri‐service orthodontic program, Texas, USA Number of centres: 1 |
|
| Participants | SELECTION CRITERIA • Healthy child (aged 10 years and over) and adult patients offering consent and approved for comprehensive orthodontic treatment • Participants were excluded from recruitment if they currently had any pre‐existing pain conditions or if they were unable to comply with the restriction on using any analgesic drugs during the course of the study Participants: 70 Number randomised: 70 (intervention: 35; control: 35) Number evaluated: 58 (intervention: 29; control: 29) Baseline characteristics: not reported, although authors state (p. 627): "Stratified analysis was used for gender and age; however, the study was not powered adequately to look at subgroup differences." |
|
| Interventions | INTERVENTION: AcceleDent Aura micropulse vibration device. Participants assigned to the experimental group were instructed to use the device for 20 minutes daily beginning on the day separators were placed and continuing daily for the first 4 months of levelling and aligning. CONTROL: No intervention to alleviate pain |
|
| Outcomes | Pain intensity measured on a VAS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were assigned to comparison groups using a block allocation sequence. This sequence was concealed from the investigators. Participants were randomised in blocks of 10 with five patients being allocated to each arm of the trial until all 70 patients were randomised. For participant allocation, a computer‐ generated list of random numbers was used. The randomization sequence was created using Stata 9.0 statistical software (StataCorp, College Station, Tex) with a 1:1 allocation using a random block size of 10." (p. 2) |
| Allocation concealment (selection bias) | Low risk | "This sequence was concealed from the investigators... A designated individual (not part of the investigative team) performed the allocation." (p. 2) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants was not undertaken |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of participants was not undertaken |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "In each group, 29 of 35 participants (83%) remained in the study after the 4‐month trial. Six patients from each group were excluded from the study. Four of six patients from the device groups used a quantity of rescue medication that was considered excessive, mostly for non dental pain. The other two patients were noncompliant with their pain diary. In the control group, three patients used rescue medication too often (headache, body pain) and three others were noncompliant with respect to the pain diary." (p. 2) Comment: Failure to complete was reported with the reason given and these represented fewer than 20% of the sample |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but outcomes mentioned in the methods section appeared to have been reported |
| Other bias | Unclear risk | Baseline characteristics were not given; imbalances between the groups were possible |
Marie 2003.
| Methods | Trial design: RCT, 2 groups Location: Louisiana State University, Louisiana, USA Number of centres: 1 |
|
| Participants | SELECTION CRITERIA Inclusion criteria • No previous pain • Undergoing orthodontic treatment with fixed upper and lower appliances Exclusion criteria • None reported Participants: 48 (21 male, 27 female) Number randomised: 48 (intervention: 24; control: 24) Number evaluated: not mentioned Mean age: 25.3 years (SD not reported) in control group, 25.2 years (SD not reported) in intervention group |
|
| Interventions | INTERVENTION: vibratory stimulation for 15 minutes after wire placement CONTROL: no intervention to alleviate pain |
|
| Outcomes | Pain intensity measured on a VAS | |
| Notes | No usable data provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly divided" (p. 206) |
| Allocation concealment (selection bias) | High risk | Concealment of allocation was not undertaken |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants was not undertaken |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of participants was not undertaken |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐outs or numbers completing the study were not mentioned |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but outcomes mentioned in the methods section appeared to have been reported |
| Other bias | Unclear risk | Inadequate description of baseline characteristics of the sample and little information on the nature of the intervention |
Miles 2012.
| Methods | Trial design: RCT, 2 groups Location: Private practice, Caloundra, Australia Number of centres: 1 |
|
| Participants | SELECTION CRITERIA Inclusion criteria • Aged 11 years to 15 years • Non‐extraction in the mandibular arch • No impactions or unerupted teeth • Fixed appliance from 6 to 6 in both arches • Residencing locally • Undergoing orthodontic treatment with fixed upper and lower appliances Exclusion criteria • None reported Participants: 66 (26 males, 40 females) Number randomised: 66 (intervention: 33; control: 33) Number evaluated: 60 (intervention: 30; control: 28) Mean age: 13.1 years (SD 0.2) in control group, 13 years (SD 0.2) in intervention group |
|
| Interventions | INTERVENTION: vibratory stimulation (Tooth Masseuse) for 20 minutes daily CONTROL: no intervention to alleviate pain |
|
| Outcomes | Discomfort intensity measured on a 100 mm VAS at 5 time points: immediately after placement, 6 to 8 hours, 1, 3 and 7 days later | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomly assigned in blocks of six to ensure even numbers in the control and experimental groups" (p. 214) |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The clinician was blinded to the study participants at all appointments." (p. 216) However, the participants were not blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants (the outcome assessors for pain) were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 58 of 66 randomised participants were analysed. Reasons for failure to complete and time points at which drop‐outs occurred were given. Comment: Failure to complete was reported with the reason given and these represented fewer than 20% of the sample |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but outcomes mentioned in the methods section appeared to have been reported |
| Other bias | Low risk | Appeared to be free of other forms of bias |
Miles 2016.
| Methods | Trial design: RCT, 2 groups Location: Private practice, Caloundra, Australia Number of centres: 1 |
|
| Participants | SELECTION CRITERIA Eligibility for inclusion consisted of (1) children aged up to 16 years, (2) a fully erupted dentition from first molar forward, (3) erupted or erupting second molars, (4) no missing or previously extracted permanent teeth, (5) undergoing comprehensive orthodontic treatment with full fixed appliances, and (6) a Class II malocclusion requiring extraction of 2 maxillary premolars but no mandibular extractions. Number randomised: 40 (20 males, 20 females) Number evaluated: 40 (intervention: 20; control: 20) Mean age: 12.7 (SD 1.2) years intervention group, 13.0 (SD 1.5) control group Sex: 14 female/6 male intervention group, 12 female/8 male control group |
|
| Interventions | INTERVENTION: AcceleDent Aura appliance for 20 minutes per day CONTROL: no vibration appliance All patients were indirectly bonded with conventional 0.018‐in slot, MBT prescription brackets (Victory Series; 3M Unitek, Monrovia, CA, USA) on all mandibular teeth and the maxillary premolars and molars, whereas the maxillary incisors and canines were bonded with MBT equivalent prescription self‐ligating In‐Ovation C ceramic brackets (GAC International, Bohemia, NY, USA). The arch wires were identical in both groups during the 10‐week experimental period: a 0.014‐in thermal nickel‐titanium wire (G&H Wire, Franklin, IN. USA) |
|
| Outcomes | The primary outcome was the change in mandibular anterior arch perimeter over the 10 weeks of the trial. Secondary outcomes were the change in the mandibular arch irregularity index over the 10 weeks and amounts of discomfort and analgesic use during the first week of the trial | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using permuted blocks of 10 randomly generated numbers with the random generation function in Excel (Microsoft, Redmond, WA, USA);..." (p. 929) |
| Allocation concealment (selection bias) | Low risk | "...the numbers were sealed in opaque envelopes and shuffled by a staff member. A clinical assistant opened an envelope for the group assignment after a patient's brackets were bonded and gave routine instructions in a closed consultation room to ensure that the operator (P.M.) was blinded" (p. 929) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Patients were aware of their treatment group... The operator was blinded to the group assignment" (p. 929) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were not blinded (see above) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but outcomes mentioned in the methods section appeared to have been reported |
| Other bias | Low risk | Appeared to be free of other forms of bias |
Nobrega 2013.
| Methods | Trial design: RCT, 2 groups Location: orthodontic clinic, São José dos Campos, Brazil Number of centres: 1 |
|
| Participants | SELECTION CRITERIA Inclusion criteria • Aged over 12 years • Presence of erupted permanent first and second lower molars • Presence of erupted first and second premolars • Voluntary participation in the study confirmed by signing the informed consent form Exclusion criteria • Using antibiotics or analgesics • Being pregnant or breastfeeding • Cardiac disease • Systemic diseases • Contraindications to NSAID use • Having undergone any type of surgical procedure during the preceding 2 weeks • Gastrointestinal illness (gastritis, gastric ulcer, lactose intolerance, chronic diarrhoea or intestinal inflammatory illness) • Presence of melanin pigmentation in the gingiva in the area to be irradiated • Presence of treated or untreated apical bone lesions • Presence of one or more diastema in the region of the molars or premolars, or both Participants: 60 (22 males, 38 females) Number randomised: 60 (intervention: 30; control: 30) Number evaluated: 60 (intervention: 30; control: 30) Mean age: 17.9 (SD 3.9) years intervention group, 17.1 (SD 3.9) years control group |
|
| Interventions | INTERVENTION: immediately after placement of separators, irradiation with low‐level laser therapy (LLLT) using an AlGaAs diode (with a single spot application to the region of the root apex at a dose of 2 J/cm², and along the root axis buccally with three spot applications of 1 J/cm² at the infrared wave length of 830 nM) CONTROL: placebo irradiation with infrared light radiation in the same locations taking the same amount of time for the procedure as was used for the intervention group |
|
| Outcomes | Pain intensity on a VAS. The primary outcome was mean pain intensity in intervention and control groups at 5 time points: 2, 6 and 24 hours, and 3 and 5 days after separator placement. The secondary outcome was frequency of absence of pain in intervention and control groups | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The procedures were codified as A and B, and for allocation of the participants, a computer generated list of random letter was used (programme available at: http/www.dave‐reed.com/Nifty/randSeq.html) with blocked randomization to ensure the ratio 1:1." (p. 12) |
| Allocation concealment (selection bias) | Low risk | "The randomization sequence was protected in opaque envelopes, sealed, and consecutively numbered, and the entire procedure was performed by another person, and not the investigator." (p.12) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "During the phase of procedures and data collection, only the manufacturer had knowledge of the respective functions of the laser probes and not only the patients, but also the operator/researcher were blinded, and in all the cases, the researcher also acted as the operator." (p.12) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "During the phase of procedures and data collection, only the manufacturer had knowledge of the respective functions of the laser probes and not only the patients, but also the operator/researcher were blinded, and in all the cases, the researcher also acted as the operator." (p.12) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Study protocol mentioned (http://ecgovbr.bvsalud.org/, registration number RBR‐8v3tkq) but inaccessible. Outcomes mentioned in the methods section appeared to have been reported |
| Other bias | Low risk | Appeared to be free of other forms of bias |
Otasevic 2006.
| Methods | Trial design: RCT, 2 groups Location: Royal London Hospital, UK Number of centres: 1 |
|
| Participants | SELECTION CRITERIA Inclusion criteria • Aged up to 16 years • Undergoing orthodontic treatment with fixed upper and lower appliance Exclusion criteria • None reported Participants: 125 Number randomised: 125. No further details given Number evaluated: 84 (intervention: 38; control: 46). These included 47 females and 37 males, with 21 and 26 females in the intervention and control groups, respectively Mean age: 14 (SD 1.7) years intervention group, 14.1 (SD 1.7) years control group |
|
| Interventions | INTERVENTION: A wafer was chewed under supervision for 10 minutes immediately after placement of the fixed appliance. Additional wafers were given to the patients to take home. These were to be chewed if they experienced pain CONTROL: Participants were instructed not to chew for 3 hours following placement of the appliance and to avoid chewing hard foods for the next 7 days |
|
| Outcomes | Pain intensity on a 100 mm VAS each morning, lunch time and evening for 7 days | |
| Notes | Only figures with medians without variance or precision were provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated" (p. e10) |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not undertaken |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants was not undertaken |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of participants was not undertaken |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 84 of 125 randomised participants were analysed. Comment: Failure to complete was at a high level, 33% of the sample, with no explanation |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but outcomes mentioned in the methods section appeared to have been reported |
| Other bias | Low risk | Appeared to be free of other forms of bias |
Turhani 2006.
| Methods | Trial design: RCT, 2 groups Location: Medical University of Vienna, Austria Number of centres: 1 |
|
| Participants | SELECTION CRITERIA Inclusion criteria • None reported • Undergoing orthodontic treatment with fixed upper and lower appliances Exclusion criteria • Chronic pain • History of neurological and psychiatric disorders Participants: 76 (30 males, 46 females) Number randomised: 76 (intervention: 38; control: 38) Number evaluated: 76 (intervention: 38; control: 38) Mean age: 25.1 years intervention group, 21 years control group (SD not reported for either group) |
|
| Interventions | INTERVENTION: Immediately after placement of one arch wire, irradiation with low‐level laser therapy (LLLT) using a dental version of Mini Laser 2075 (Helbo Photodynamic Systems GmbH & Co KG, Linz, Austria; 670 nM, 75 mW). CONTROL: Placebo laser therapy without active irradiation (participants were blinded) |
|
| Outcomes | Prevalence (item 1), quality (item 2), intensity (items 3 and 4), localisation (item 3), and the time course (item 4) of subjectively perceived pain. Items 3 and 4 were evaluated with a 5‐point scale (0, no pain; 5, unbearable pain). Measured at 6, 30 and 54 hours | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | P. 371: "...randomly selected and received placebo laser treatment" (p. 371) |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The operator who applied the laser treatment and the placebo could distinguish between them, but the patients were blinded to the difference" (p. 372). On page 375, authors state the knowledge of the operator may have been subconsciously transferred to the participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The operator who applied the laser treatment and the placebo could distinguish between them, but the patients were blinded to the difference" (p. 372). On page 375, authors state the knowledge of the operator may have been subconsciously transferred to the participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but outcomes mentioned in the methods section appeared to have been reported |
| Other bias | Low risk | Appeared to be free of other forms of bias |
Woodhouse 2015.
| Methods | Trial design: RCT, 3 groups Location: King’s College London Dental Institute (Guy’s Hospital); Royal Alexander Children’s Hospital, Brighton and William Harvey Hospital, Ashford; UK Number of centres: 3 |
|
| Participants | SELECTION CRITERIA Inclusion criteria • Aged up to 20 years at treatment start • No medical contra‐indications, including regular medication • In permanent dentition • Mandibular arch incisor irregularity • Extraction of mandibular first premolars as part of the orthodontic treatment plan • Undergoing orthodontic treatment with fixed upper and lower appliances Exclusion criteria • None reported Participants: 81 (40 males, 41 females) Number randomised: 81 (Group 1: 29; Group 2: 25; Group 3: 27) Number evaluated at visit 1: 80 (Group 1: 29; Group 2: 25; Group 3: 26) Number evaluated at visit 2: 77 (Group 1: 28; Group 2: 24; Group 3: 25) Mean age: 14.1 (SD 1.7) years |
|
| Interventions | INTERVENTION: Pre‐adjusted edgewise fixed‐appliance treatment with adjunctive daily use of a functional AcceleDent (OrthoAccel Technologies, Inc, Houston, TX, USA) vibrational device (Group 1) CONTROL: Pre‐adjusted edgewise fixed‐appliance treatment with adjunctive use of a non‐functional (sham) AcceleDent device (Group 2) and pre‐adjusted edgewise fixed‐appliance treatment alone (Group 3) Participants allocated to both the working and sham devices were instructed to use the device for 20 minutes daily The bonding method and fixed appliance was standardised between groups (3M Victory series, 3M Unitek, Monrovia, CA, USA) with a pre‐determined sequence used in each group during the period of study. Arch wires were inserted and ligated from first molar to first molar using conventional elastomerics. Arch wire progression occurred only if full bracket engagement was achievable, which required the relevant arch wire to be fully tied into the base of the bracket slot adjacent to each tie wing using elastomeric ligation. No bite planes, auxiliary arches, inter‐maxillary elastics, headgears or temporary anchorage devices were used during the period of investigation |
|
| Outcomes | Pain intensity on a VAS. The primary outcome measure was maximum pain experience during initial alignment. Secondary outcomes were mean pain at each time point (4 hours, 24 hours, 3 days and 7 days) after placement of the brace and the first arch wire adjustment; alignment rate; and oral analgesic consumption during the study period | |
| Notes | Used Group 1 vs Group 2 in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...sequence was generated by one investigator (MTC) using GraphPad online software (http://www.graphpad.com/quickcalcs/index.cfm) with unrestricted equal participant allocation (1:1:1)" (p. 3) |
| Allocation concealment (selection bias) | Low risk | Central allocation was used: "...undertaken centrally at King’s College London, independently from the clinical operators, following recruitment (allocation concealment)" (p. 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Page 3: 'Treating clinicians and subjects could not be blinded to the use of AcceleDent; however, subjects were blinded to the allocation of functional or sham appliances, as both were identical in appearance (with the exception that the sham appliance did not vibrate).' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Treating clinicians and subjects could not be blinded to the use of AcceleDent; however, subjects were blinded to the allocation of functional or sham appliances, as both were identical in appearance (with the exception that the sham appliance did not vibrate)." (p. 3) "The pain questionnaires and extracted data were coded appropriately, so that both outcome assessor (NRW) and statistician (SNP) were blinded to subject allocation. The coding of the data was broken after the end of the analysis and no breach of blinding was identified." (p. 3) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 80 of 81 randomised participants were analysed at the first time point and 77 at the second. Reasons for failure to complete and the time point at which drop‐outs occurred were given and these represented fewer than 20% of the sample |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes and those mentioned in the methods section appeared to have been reported |
| Other bias | Low risk | Appeared to be free of other forms of bias |
RCT = randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abtahi 2013 | Split‐mouth study |
| Artés‐Ribas 2013 | Split‐mouth study |
| Bartlett 2005 | Non‐randomised study |
| Bicacki 2012 | Split‐mouth study |
| Domínguez 2013 | Split‐mouth study |
| Doshi‐Mehta 2012 | Split‐mouth study |
| Eslamian 2014 | Split‐mouth study |
| Esper 2011 | Non‐randomised study |
| Gasperini 2014 | Assessed effects of low‐level laser on swelling and pain related to orthognathic surgery rather than orthodontics |
| Lim 1995 | Split‐mouth study |
| Marini 2013 | Split‐mouth study |
| Murdock 2010 | Used positive control group involving analgesic use |
| Roth 1986 | Four‐factor repeated measures design |
Characteristics of studies awaiting assessment [ordered by study ID]
Tortamano 2009.
| Methods | Trial design: RCT, 6 groups Location: University of São Paulo, Brazil Number of centres: 1 |
| Participants | SELECTION CRITERIA Inclusion criteria • Enrolled at the orthodontic clinic at the School of Dentistry of the University of São Paulo, Brazil • About to start orthodontic treatment with the MBT straight‐wire technique • Signed informed consent agreeing to the research procedures •Undergoing orthodontic treatment with fixed upper and lower appliances Exclusion criteria • None reported Participants: 60 (18 males, 42 females) Number randomised: 60 (Group 1: 10; Group 2: 10; Group 3: 10; Group 4: 10; Group 5: 10; Group 6: 10) Number evaluated: 60 (Group 1: 10; Group 2: 10; Group 3: 10; Group 4: 10; Group 5: 10; Group 6: 10) Mean age: 15.9 (SD not reported) years, range 12 to 18 years |
| Interventions | INTERVENTION: Immediately after placement of 1 arch wire, irradiation with low‐level laser therapy (LLLT) using an AlGaAs diode was done with a fixed appliance in place in the maxilla (Group 1) or mandible (Group 2) CONTROL: Placebo irradiation groups had a laser probe positioned into the mouth overlying the root and could hear a sound every 10 seconds in the maxilla (Group 3) or mandible (Group 4). In the 'no intervention' groups irradiation was not simulated but a fixed appliance was in place in the maxilla (Group 5) or mandible (Group 6) |
| Outcomes | Pain intensity on a 10‐point numerical scale over a 7‐day period |
| Notes | No usable data. Scale not given. Survey was completed for a week but no breakdown by day. Unclear how the intervention was applied and whether one patient received in the same or different interventions in two jaws. E‐mail 30 October 2015: "Dear Dr Santos, We are conducting a Cochrane systematic review on non‐pharmacological methods for pain reduction in orthodontics.Your trial is eligible for inclusion: Low‐level laser therapy for pain caused by placement of the first orthodontic arch wire: A randomised clinical trial Tortamano et al AJODO 2009;136:662‐7. I was wondering if it would be possible to provide some details that would allow me to include your study in the meta‐analysis. Ideally, I would like to have individual patient data as it is unclear from the methods and Table II if the patients received the same intervention in both jaws. If patients received the same intervention in both jaws I can get average values per group per patient by averaging the values for maxilla and mandible from Table II. However, if patient received different intervention I would be grateful if you could provide individual patient data so I can somehow account for the existing within patient correlations, which I cannot extract now from the reported values. Your help will be greatly appreciated. Many thanks, Nick |
Differences between protocol and review
We edited some wording in the Background and Methods sections.
We included interventions delivered either before or after the onset of orthodontic pain as non‐pharmacological approaches are commonly prescribed prior to the onset of pain.
We incorporated a number of different novel non‐pharmacological interventions that had not been described at the protocol stage.
We updated the grey literature searches and the text in the Methods in relation to the 'Risk of bias' assessment and 'Assessment of reporting bias' in line with the current version of the Cochrane Handbook for Systematic Reviews of Interventions, and we added more details about the process.
We modified the list of data extraction items.
We had planned to dichotomise pain results, but because most studies measured pain on a visual analogue scale, we decided to use the continuous data so not to lose information.
We used risk ratio (RR) rather than odds ratio (OR) for binary data, in line with current Cochrane Oral Health policy, to facilitate interpretation of the results.
We used the random‐effects model even if there were fewer than three studies because we considered this to be more appropriate than the fixed‐effect model.
We added the outcomes reported in the 'Summary of findings tables' to the Methods section.
Contributions of authors
Draft the protocol: Hardus Strydom, Piotr Fudalej Obtain copies of trials: Hardus Strydom, Padhraig Fleming Selection of trials: Padhraig Fleming, Hardus Strydom, Piotr Fudalej, Nikolaos Pandis Data extraction: Piotr Fudalej, Padhraig Fleming, Nikolaos Pandis Enter data into RevMan: Nikolaos Pandis Carry out analysis: Nikolaos Pandis Interpret the analysis: Padhraig Fleming, Hardus Strydom, Piotr Fudalej, Nikolaos Pandis, Laura MacDonald, Christos Katsaros, Michele Curatolo Draft the final review: Padhraig Fleming, Laura MacDonald Update the review: Padhraig Fleming, Nikolaos Pandis, Laura MacDonald
Sources of support
Internal sources
No sources of support supplied
External sources
-
Cochrane Oral Health Global Alliance, UK.
Through our Global Alliance (ohg.cochrane.org/partnerships‐alliances), Cochrane Oral Health has received support from: British Association for the Study of Community Dentistry, UK; British Association of Oral Surgeons, UK; British Orthodontic Society, UK; British Society of Paediatric Dentistry, UK; British Society of Periodontology, UK; Canadian Dental Hygienists Association, Canada; Centre for Dental Education & Research (CDER), India; Mayo Clinic, USA; National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland (NES), UK; and Royal College of Surgeons of Edinburgh, UK
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
Declarations of interest
Padhraig S Fleming: none known Nikolaos Pandis: none known Hardus Strydom: none known Christos Katsaros: none known Laura MacDonald: none known Michele Curatolo: none known Piotr Fudalej: none known
New
References
References to studies included in this review
Benson 2012 {published data only}
- Benson PE, Razi RM, Al‐Bloushi RJ. The effect of chewing gum on the impact, pain and breakages associated with fixed orthodontic appliances: a randomized clinical trial. Orthodontics & Craniofacial Research 2012;15(3):178‐87. [DOI] [PubMed] [Google Scholar]
Farzanegan 2012 {published data only}
- Farzanegan F, Zebarjad SM, Alizadeh S, Ahrari F. Pain reduction after initial archwire placement in orthodontic patients: a randomized clinical trial. American Journal of Orthodontics and Dentofacial Orthopedics 2012;141( 2 ):169‐73. [DOI] [PubMed] [Google Scholar]
Harazaki 1997 {published data only}
- Harazaki M, Isshiki Y. Soft laser irradiation effects on pain reduction in orthodontic treatment. Bulletin of Tokyo Dental College 1997;38(4):291‐5. [PubMed] [Google Scholar]
Huang 2016 {published data only}
- Huang R, Wang J, Wu D, Long H, Yang X, Liu H, et al. The effects of customised brainwave music on orofacial pain induced by orthodontic tooth movement. Oral Diseases 2016;22(8):766‐74. [DOI] [PubMed] [Google Scholar]
Keith 2013 {published data only}
- Keith DJ, Rinchuse DJ, Kennedy M, Zullo T. Effect of text message follow‐up on patient's self‐reported level of pain and anxiety. Angle Orthodontist 2013;83(4):605‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2013 {published data only}
- Kim WT, Bayome M, Park JB, Park JH, Baek SH, Kook YA. Effect of frequent laser irradiation on orthodontic pain. A single‐blind randomized clinical trial. Angle Orthodontist 2013;83(4):611‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lobre 2015 {published data only}
- Lobre WD, Callegari BJ, Gardner G, Marsh CM, Bush AC, Dunn WJ. Pain control in orthodontics using a micropulse vibration device: A randomized clinical trial. Angle Orthodontist 2016;86(4):625‐30. [DOI: 10.2319/072115-492.1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marie 2003 {published data only}
- Marie SS, Powers M, Sheridan JJ. Vibratory stimulation as a method of reducing pain after orthodontic appliance adjustment. Journal of Clinical Orthodontics 2003;37(4):205‐8. [PubMed] [Google Scholar]
Miles 2012 {published data only}
- Miles P, Smith H, Weyant R, Rinchuse DJ. The effects of a vibrational appliance on tooth movement and patient discomfort: a prospective randomised clinical trial. Australian Orthodontic Journal 2012;28(2):213‐8. [PubMed] [Google Scholar]
Miles 2016 {unpublished data only}
- Miles P, Fischer E. Assessment of the changes in arch perimeter and irregularity in the mandibular arch during initial alignment with the AcceleDent Aura appliance vs no appliance in adolescents: a single‐blind randomized clinical trial. American Journal of Orthodontics and Dentofacial Orthopedics 2016;150(6):928‐36. [DOI] [PubMed] [Google Scholar]
Nobrega 2013 {published data only}
- Nóbrega C, Silva EM, Macedo CR. Low‐level laser therapy for treatment of pain associated with orthodontic elastomeric separator placement: a placebo‐controlled randomized double‐blind clinical trial. Photomedicine and Laser Surgery 2013;31:10‐16. [DOI] [PubMed] [Google Scholar]
Otasevic 2006 {published data only}
- Otasevic M, Naini FB, Gill DS, Lee RT. Prospective randomized clinical trial comparing the effects of a masticatory bite wafer and avoidance of hard food on pain associated with initial orthodontic tooth movement. American Journal of Orthodontics and Dentofacial Orthopedics 2006;130(1):e9‐15. [DOI] [PubMed] [Google Scholar]
Turhani 2006 {published data only}
- Turhani D, Scheriau M, Kapral D, Benesch T, Jonke E, Bantleon HP. Pain relief by single low‐level laser irradiation in orthodontic patients undergoing fixed appliance therapy. American Journal of Orthodontics and Dentofacial Orthopedics 2006;130(3):371‐7. [DOI] [PubMed] [Google Scholar]
Woodhouse 2015 {published data only}
- Woodhouse NR, DiBiase AT, Papageorgiou SN, Johnson N, Slipper C, Grant J, et al. Supplemental vibrational force does not reduce pain experience during initial alignment with fixed orthodontic appliances: a multicenter randomized clinical trial. Scientific Reports 2015;5:17224. [DOI: 10.1038/srep17224] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abtahi 2013 {published data only}
- Abtahi SM, Mousavi SA, Shafaee H, Tanbakuchi B. Effect of low‐level laser therapy on dental pain induced by separator force in orthodontic treatment. Dental Research Journal 2013;10(5):647‐51. [PMC free article] [PubMed] [Google Scholar]
Artés‐Ribas 2013 {published data only}
- Artés‐Ribas M, Arnabat‐Dominguez J, Puigdollers A. Analgesic effect of a low‐level laser therapy (830 nm) in early orthodontic treatment. Lasers in Medical Science 2013;28(1):335‐41. [DOI] [PubMed] [Google Scholar]
Bartlett 2005 {published data only}
- Bartlett BW, Firestone AR, Vig KW, Beck FM, Marucha PT. The influence of a structured telephone call on orthodontic pain and anxiety. American Journal of Orthodontics and Dentofacial Orthopedics 2005;128(4):435‐41. [DOI] [PubMed] [Google Scholar]
Bicacki 2012 {published data only}
- Bicakci AA, Kocoglu‐Altan B, Toker H, Mutaf I, Sumer Z. Efficiency of low‐level laser therapy in reducing pain induced by orthodontic forces. Photomedicine and Laser Surgery 2012;30(8):460‐5. [DOI] [PubMed] [Google Scholar]
Domínguez 2013 {published data only}
- Domínguez A, Velásquez SA. Effect of low‐level laser therapy on pain following activation of orthodontic final archwires: a randomized controlled clinical trial. Photomedicine and Laser Surgery 2013;31(1):36‐40. [DOI] [PubMed] [Google Scholar]
Doshi‐Mehta 2012 {published data only}
- Doshi‐Mehta G, Bhad‐Patil WA. Efficacy of low‐intensity laser therapy in reducing treatment time and orthodontic pain: a clinical investigation. American Journal of Orthodontics and Dentofacial Orthopedics 2012;141(3):289‐97. [DOI] [PubMed] [Google Scholar]
Eslamian 2014 {published data only}
- Eslamian L, Borzabadi‐Farahani A, Hassanzadeh‐Azhiri A, Badiee MR, Fekrazad R. The effect of 810‐nm low‐level laser therapy on pain caused by orthodontic elastomeric separators. Lasers in Medical Science 2014;29(2):559‐64. [DOI] [PubMed] [Google Scholar]
Esper 2011 {published data only}
- Esper MA, Nicolau RA, Arisawa EA. The effect of two phototherapy protocols on pain control in orthodontic procedure ‐ a preliminary clinical study. Lasers in Medical Science 2011;26(5):657‐63. [DOI] [PubMed] [Google Scholar]
Gasperini 2014 {published data only}
- Gasperini G, Rodrigues de Siqueira IC, Rezende Costa L. Does low‐level laser therapy decrease swelling and pain resulting from orthognathic surgery?. International Journal of Oral and Maxillofacial Surgery 2014;43( 7 ):868‐73. [DOI] [PubMed] [Google Scholar]
Lim 1995 {published data only}
- Lim HM, Lew KK, Tay DK. A clinical investigation of the efficacy of low level laser therapy in reducing orthodontic postadjustment pain. American Journal of Orthodontics and Dentofacial Orthopedics 1995;108(6):614‐22. [DOI] [PubMed] [Google Scholar]
Marini 2013 {published data only}
- Marini I, Bartolucci ML, Bortolotti F, Innocenti G, Gatto MR, Alessandri Bonetti G. The effect of diode superpulsed low‐level laser therapy on experimental orthodontic pain caused by elastomeric separators: a randomized controlled clinical trial. Lasers in Medical Science 2015;30(1):35‐41. [DOI] [PubMed] [Google Scholar]
Murdock 2010 {published data only}
- Murdock S, Phillips C, Khondker Z, Hershey HG. Treatment of pain after initial archwire placement: a noninferiority randomized clinical trial comparing over‐the‐counter analgesics and bite‐wafer use. American Journal of Orthodontics and Dentofacial Orthopedics 2010;137(3):316‐23. [DOI] [PubMed] [Google Scholar]
Roth 1986 {published data only}
- Roth PM, Thrash WJ. Effect of transcutaneous electrical nerve stimulation for controlling pain associated with orthodontic tooth movement. American Journal of Orthodontics and Dentofacial Orthopedics 1986;90(2):132‐8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Tortamano 2009 {published data only}
- Tortamano A, Lenzi DC, Haddad AC, Bottino MC, Dominguez GC, Vigorito JW. Low‐level laser therapy for pain caused by placement of the first orthodontic archwire: a randomized clinical trial. American Journal of Orthodontics and Dentofacial Orthopedics 2009;136(5):662‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Atamaz 2012
- Atamaz FC, Durmaz B, Baydar M, Demircioglu OY, Iyiyapici A, Kuran B, et al. Comparison of the efficacy of transcutaneous electrical nerve stimulation, interferential currents, and shortwave diathermy in knee osteoarthritis: a double‐blind, randomized, controlled, multicenter study. Archives of Physical Medicine and Rehabilitation 2012;93(5):748‐56. [DOI] [PubMed] [Google Scholar]
Bergius 2000
- Bergius M, Kiliaridis S, Berggren U. Pain in orthodontics. A review and discussion of the literature. Journal of Orofacial Orthopedics 2000;61(2):125‐37. [DOI] [PubMed] [Google Scholar]
Bonica 1979
- Bonica JJ. The need of a taxonomy. Pain 1979;6(3):247‐8. [DOI] [PubMed] [Google Scholar]
Brown 1991
- Brown DF, Moerenhout RG. The pain experience and psychological adjustment to orthodontic treatment of preadolescents, adolescents, and adults. American Journal of Orthodontics and Dentofacial Orthopedics 1991;100(4):349‐56. [DOI] [PubMed] [Google Scholar]
Burstone 1962
- Burstone CJ. The biomechanics of tooth movement. In: Kraus BS, Riedel RA editor(s). Vistas in Orthodontics. Philadelphia: Lea & Febiger, 1962:197‐213. [Google Scholar]
Cruccu 2007
- Cruccu G, Aziz TZ, Garcia‐Larrea L, Hansson P, Jensen TS, Lefaucheur JP, et al. EFNS guidelines on neurostimulation therapy for neuropathic pain. European Journal of Neurology 2007;14(9):952‐70. [DOI] [PubMed] [Google Scholar]
El‐Angbawi 2015
- El‐Angbawi A, McIntyre GT, Fleming PS, Bearn DR. Non‐surgical adjunctive interventions for accelerating tooth movement in patients undergoing fixed orthodontic treatment. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD010887.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Firestone 1999
- Firestone AR, Scheurer PA, Burgin WB. Patients' anticipation of pain and pain‐related side effects, and their perception of pain as a result of orthodontic treatment with fixed appliances. European Journal of Orthodontics 1999;21(4):387‐96. [DOI] [PubMed] [Google Scholar]
Furstman 1972
- Furstman L, Bernick S. Clinical considerations of the periodontium. American Journal of Orthodontics 1972;61(2):138‐55. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hashmi 2010
- Hashmi JT, Huang YY, Osmani BZ, Sharma SK, Naeser MA, Hamblin MR. Role of low‐level laser therapy in neurorehabilitation. PM & R : the Journal of Injury, Function, and Rehabilitation 2010;2(12 Suppl 2):S292‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from: www.cochrane‐handbook.org.
Jian 2013
- Jian F, Lai W, Furness S, McIntyre GT, Millett DT, Hickman J, et al. Initial arch wires for tooth alignment during orthodontic treatment with fixed appliances. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD007859.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1984
- Jones ML. An investigation into the initial discomfort caused by placement of an archwire. European Journal of Orthodontics 1984;6(1):48‐54. [DOI] [PubMed] [Google Scholar]
Jones 1985
- Jones ML, Richmond S. Initial tooth movement: force application and pain‐‐a relationship?. American Journal of Orthodontics 1985;88(2):111‐6. [DOI] [PubMed] [Google Scholar]
Kluemper 2002
- Kluemper GT, Hiser DG, Rayens MK, Jay MJ. Efficacy of a wax containing benzocaine in the relief of oral mucosal pain caused by orthodontic appliances. American Journal of Orthodontics and Dentofacial Orthopedics 2002;122(4):359‐65. [DOI] [PubMed] [Google Scholar]
Kvam 1987
- Kvam E, Gjerdet NR, Bondevik O. Traumatic ulcers and pain during orthodontic treatment. Community Dentistry and Oral Epidemiology 1987;15(2):104‐7. [DOI] [PubMed] [Google Scholar]
Litt 1996
- Litt MD. A model of pain and anxiety associated with acute stressors: distress in dental procedures. Behaviour Research and Therapy 1996;34(5‐6):459‐76. [DOI] [PubMed] [Google Scholar]
Melzack 1965
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150(3699):971‐9. [DOI] [PubMed] [Google Scholar]
Movahedi 2006
- Movahedi AF, Rostami S, Salsali M, Keikhaee B, Moradi A. Effect of local refrigeration prior to venipuncture on pain related responses in school age children. Australian Journal of Advanced Nursing 2006;24(2):51‐5. [PubMed] [Google Scholar]
Ngan 1989
- Ngan P, Kess B, Wilson S. Perception of discomfort by patients undergoing orthodontic treatment. American Journal of Orthodontics and Dentofacial Orthopedics 1989;96(1):47‐53. [DOI] [PubMed] [Google Scholar]
Ngan 1994
- Ngan P, Wilson S, Shanfeld J, Amini H. The effect of ibuprofen on the level of discomfort in patients undergoing orthodontic treatment. American Journal of Orthodontics and Dentofacial Orthopedics 1994;106(1):88‐95. [DOI] [PubMed] [Google Scholar]
Oliver 1985
- Oliver RG, Knapman YM. Attitudes to orthodontic treatment. British Journal of Orthodontics 1985;12(4):179‐88. [DOI] [PubMed] [Google Scholar]
Paganelli 1993
- Paganelli C. Pharmacological support during orthodontic therapy with a topical anti‐inflammatory. Minerva Stomatologica 1993;42(6):271‐4. [PubMed] [Google Scholar]
Paley 2015
- Paley CA, Johnson MI, Tashani OA, Bagnall AM. Acupuncture for cancer pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD007753.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 1989
- Patel V. Non‐completion of Orthodontic Treatment: A Study of Patient and Parental Factors Contributing to Discontinuation in the Hospital Service and Specialist Practice [Thesis]. Cardiff (UK): University of Wales, 1989. [Google Scholar]
RevMan 2014 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
Schreurer 1996
- Scheurer PA, Firestone AR, Burgin WB. Perception of pain as a result of orthodontic treatment with fixed appliances. European Journal of Orthodontics 1996;18(4):349‐57. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schupp 2005
- Schupp CJ, Berbaum K, Berbaum M, Lang EV. Pain and anxiety during interventional radiologic procedures: effect of patients' state anxiety at baseline and modulation by nonpharmacologic analgesia adjuncts. Journal of Vascular and Interventional Radiology 2005;16(12):1585‐92. [DOI] [PubMed] [Google Scholar]
Sergl 1998
- Sergl HG, Klages U, Zentner A. Pain and discomfort during orthodontic treatment: causative factors and effects on compliance. American Journal of Orthodontics and Dentofacial Orthopedics 1998;114(6):684‐91. [DOI] [PubMed] [Google Scholar]
Shin 2009
- Shin YS, Lim NY, Yun SC, Park KO. A randomised controlled trial of the effects of cryotherapy on pain, eyelid oedema and facial ecchymosis after craniotomy. Journal of Clinical Nursing 2009;18(21):3029‐36. [DOI] [PubMed] [Google Scholar]
Simmons 1992
- Simmons KE, Brandt M. Control of orthodontic pain. Journal IDA 1992;71(4):8‐10. [PubMed] [Google Scholar]
Soltis 1971
- Soltis JE, Nakfoor PR, Bowman DC. Changes in ability of patients to differentiate intensity of forces applied to maxillary central incisors during orthodontic treatment. Journal of Dental Research 1971;50(3):590‐6. [DOI] [PubMed] [Google Scholar]
Stewart 1997
- Stewart FN, Kerr WJ, Taylor PJ. Appliance wear: the patient's point of view. European Journal of Orthodontics 1997;19(4):377‐82. [DOI] [PubMed] [Google Scholar]
Theunissen 2012
- Theunissen M, Peters ML, Bruce J, Gramke HF, Marcus MA. Preoperative anxiety and catastrophizing: A systematic review and meta‐analysis of the association with chronic postsurgical pain. Clinical Journal of Pain 2012;28(9):819‐41. [DOI] [PubMed] [Google Scholar]
Vachiramon 2005
- Vachiramon A, Wang WC. Acupuncture and acupressure techniques for reducing orthodontic post‐adjustment pain. Journal of Contemporary Dental Practice 2005;6(1):163‐7. [PubMed] [Google Scholar]
White 1984
- White LW. Pain and cooperation in orthodontic treatment. Journal of Clinical Orthodontics 1984;18(8):572‐5. [PubMed] [Google Scholar]


