Abstract
Background
Parkinson’s disease (PD) is a neurodegenerative disorder that is best managed by a combination of medication and regular physiotherapy. In this context, virtual reality (VR) technology is proposed as a new rehabilitation tool with a possible added value over traditional physiotherapy approaches. It potentially optimises motor learning in a safe environment, and by replicating real‐life scenarios could help improve functional activities of daily living.
Objectives
The objective of this review was to summarise the current best evidence for the effectiveness of VR interventions for the rehabilitation of people with PD in comparison with 1) active interventions, and 2) passive interventions. Our primary goal was to determine the effect of VR training on gait and balance. Secondary goals included examining the effects of VR on global motor function, activities of daily living, quality of life, cognitive function, exercise adherence, and the occurrence of adverse events.
Search methods
We identified relevant articles through electronic searches of the Cochrane Movement Disorders Group Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library), MEDLINE, Embase, CINAHL, the Physiotherapy Evidence Database (PEDro), online trials registers, and by handsearching reference lists. We carried out all searches up until 26 November 2016.
Selection criteria
We searched for randomised and quasi‐randomised controlled trials of VR exercise interventions in people with PD. We included only trials where motor rehabilitation was the primary goal.
Data collection and analysis
Two review authors independently searched for trials that corresponded to the predefined inclusion criteria. We independently extracted and assessed all data for methodological quality. A third review author was responsible for conflict resolution when required.
Main results
We included 8 trials involving 263 people with PD in the review. Risk of bias was unclear or high for all but one of the included studies. Study sample sizes were small, and there was a large amount of heterogeneity between trials with regard to study design and the outcome measures used. As a result, we graded the quality of the evidence as low or very low. Most of the studies intended to improve motor function using commercially available devices, which were compared with physiotherapy. The interventions lasted for between 4 and 12 weeks.
In comparison to physiotherapy, VR may lead to a moderate improvement in step and stride length (standardised mean difference (SMD) 0.69, 95% confidence interval (CI) 0.30 to 1.08; 3 studies; 106 participants; low‐quality evidence). VR and physiotherapy interventions may have similar effects on gait (SMD 0.20, 95% CI ‐0.14 to 0.55; 4 studies; 129 participants; low‐quality evidence), balance (SMD 0.34, 95% CI ‐0.04 to 0.71; 5 studies; 155 participants; low‐quality evidence), and quality of life (mean difference 3.73 units, 95% CI ‐2.16 to 9.61; 4 studies; 106 participants). VR interventions did not lead to any reported adverse events, and exercise adherence did not differ between VR and other intervention arms.
The evidence available comparing VR exercise with a passive control was more limited. The evidence for the main outcomes of interest was of very low quality due to the very small sample sizes of the two studies available for this comparison.
Authors' conclusions
We found low‐quality evidence of a positive effect of short‐term VR exercise on step and stride length. VR and physiotherapy may have similar effects on gait, balance, and quality of life. The evidence available comparing VR with passive control interventions was more limited. Additional high‐quality, large‐scale studies are needed to confirm these findings.
Keywords: Aged, Humans, Middle Aged, Gait, Postural Balance, Activities of Daily Living, Parkinson Disease, Parkinson Disease/rehabilitation, Physical Therapy Modalities, Quality of Life, Randomized Controlled Trials as Topic, Virtual Reality Exposure Therapy, Virtual Reality Exposure Therapy/methods
Plain language summary
Virtual reality technology as a useful tool for rehabilitation in Parkinson's disease
Review question
The purpose of this review was to determine the effectiveness of virtual reality (VR) exercise interventions for rehabilitation in Parkinson’s disease (PD). We aimed to investigate whether VR exercise resulted in greater improvements compared to 1) active control interventions, and 2) passive control interventions, on gait, balance, global motor function, activities of daily living, quality of life, cognition, exercise adherence, and the occurrence of adverse events.
Background
PD is a neurodegenerative condition that places a high burden on patient quality of life and independence. As part of a multidisciplinary approach to treatment, regular exercise is encouraged and has been shown to relieve both motor and non‐motor symptoms.
VR technology, a promising new rehabilitation tool, stimulates movement by means of computer‐based games in a VR environment. Both commercial VR systems, such as Nintendo Wii or Xbox Kinect, and customised VR tools specifically designed to address PD symptoms, are frequently used. VR exercise exhibits potential advantages over regular exercise by allowing for individualised skill practice in a motivating and engaging interactive environment.
Study characteristics
We conducted the literature search up until 26 November 2016. We identified 8 studies involving a total of 263 participants with PD. All trials aimed to improve either gait or balance function. Most of the studies compared VR with physiotherapy.
Key results
VR interventions may lead to greater improvements in step and stride length compared with physiotherapy interventions. We found limited evidence that improvements in gait, balance, and quality of life were similar to those found in active control interventions. No adverse events were reported. Fewer studies compared VR with passive control interventions, and evidence was insufficient to determine how VR compares with no active intervention. At present, only a few studies have been done, making generalisation of the findings difficult. Further study is needed to confirm and expand the evidence base for VR in PD.
Quality of the evidence
In general, the quality of the evidence was low or very low. This was the result of small sample sizes and a large amount of heterogeneity between trials with regard to study design and outcome measures used.
Summary of findings
Summary of findings for the main comparison. Virtual reality compared to active intervention (short term) for rehabilitation in Parkinson's disease.
| Virtual reality compared to active intervention (short term) for rehabilitation in Parkinson's disease | ||||||
| Patient or population: rehabilitation in Parkinson's disease Setting: outpatient clinic Intervention: virtual reality Comparison: active intervention (short term) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Score/value with active intervention (short term) | Score/value with virtual reality | |||||
|
Gait (assessed with composite measure: gait speed, step length, stride length, Dynamic Gait Index) (measured in SD units; higher scores mean better outcomes) |
Gait score in the virtual reality groups was on average 0.2 standard deviations higher (0.14 lower to 0.55 higher) than in the control groups. | ‐ | 129 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | As a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate difference, and 0.8 a large difference. | |
|
Gait (assessed with gait speed) (measured in SD units; higher scores mean better outcomes) |
Gait score in the virtual reality groups was on average 0.18 standard deviations higher (0.20 lower to 0.57 higher) than in the control groups. | ‐ | 106 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| Gait (assessed with step and stride length) (measured in SD units; higher scores mean better outcomes) | Gait score in the virtual reality groups was on average 0.69 standard deviations higher (0.30 higher to 1.08 higher) than in the control groups. | ‐ | 106 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
|
Balance (assessed with composite measure: Berg Balance Scale, Timed Up and Go Test, Single‐Leg Stance Test) (measured in SD units; higher scores mean better outcomes) |
Balance score in the virtual reality groups was on average 0.34 standard deviations higher (0.04 lower to 0.71 higher) than in the control groups. | ‐ | 155 (5 RCTs) | ⊕⊕⊝⊝ LOW 2 3 | ||
| Balance (assessed with Berg Balance Scale; from 0 to 56 (best)) | The mean change in balance in the control groups ranged from ‐0.21 to 4.17. | The mean change in balance in the virtual reality groups was on average 0.55 higher (0.48 lower to 1.58 higher) than in the control groups. | ‐ | 86 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | |
|
Quality of life
(assessed with PDQ‐39) (higher values mean better outcomes) |
The mean change in quality of life in the control groups ranged from ‐1.88 to 11.4. | The mean change in the virtual reality groups was on average 3.73 higher (2.16 lower to 9.61 higher) than in the control groups. | ‐ | 106 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | |
| Number of adverse events | All studies reported that no adverse event had taken place in either the virtual reality or the active intervention. | ‐ | 115 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; PDQ‐39: 39‐Item Parkinson’s Disease Questionnaire; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded one level for serious imprecision: total population size was small (< 150). 2Downgraded one level for serious risk of bias: risk of bias was unclear in one or more included trials. 3Downgraded one level for serious inconsistency: heterogeneity was shown in findings across studies.
Summary of findings 2. Virtual reality compared to passive intervention (short term) for rehabilitation in Parkinson's disease.
| Virtual reality compared to passive intervention (short term) for rehabilitation in Parkinson's disease | ||||||
| Patient or population: rehabilitation in Parkinson's disease Setting: not specified in the studies Intervention: virtual reality Comparison: passive intervention (short term) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Score/value with passive intervention (short term) | Score/value with virtual reality | |||||
| Gait (stride length and velocity) | Virtual reality exercise resulted in slight improvement in gait (crossing limb stride length Cohen's d = 1.37, P = 0.003; stride velocity Cohen's d = 1.22, P = 0.011) compared to control intervention. | ‐ | 24 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 1 2 | As a rule of thumb (Cohen's effect size, d), 0.2 standard deviations represents a small difference, 0.5 a moderate difference, and 0.8 a large difference. | |
|
Balance (assessed with composite measure: Berg Balance Scale, Timed Up and Go Test) (higher scores mean better outcome) |
Balance score in the virtual reality group was on average 1.02 standard deviations higher (0.38 higher to 1.65 higher) than in the control group. | ‐ | 44 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
|
Quality of life (assessed with PDQ‐39; higher values mean better outcomes) |
Virtual reality exercise resulted in slight improvement in quality of life (Cohen's d = 1.17, P = 0.004) compared to control intervention. | ‐ | 24 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 1 2 | ||
| Adverse events | No adverse event was reported in the included study. | ‐ | 24 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 1 2 | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; PDQ‐39: 39‐Item Parkinson’s Disease Questionnaire; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded two levels for very serious imprecision (very small sample size, N = 24 participants). 2Downgraded one level for serious risk of bias (risk of bias was unclear for at least one domain in the included studies).
Background
Description of the condition
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders worldwide (Pringsheim 2014). It is mainly associated with a loss of dopaminergic neurons in the substantia nigra pars compacta (Berg 2014; Lees 2009). However, based on differential responses to dopamine uptake, a recent study has suggested involvement of additional neurotransmitter systems, such as cholinergic and noradrenergic circuits (Bohnen 2011).
Bradykinesia, rigidity, rest tremor, and postural instability are the hallmark features of the disease and have a negative impact upon movement quality, gait and balance performance, and fall risk (Canning 2014; Jankovic 2008). In addition, non‐motor features such as cognitive decline, fatigue, apathy, and depression are common and substantially affect patient functioning and quality of life (Rizos 2014).
Multidisciplinary input is increasingly recognised as important in PD management (van der Marck 2013). Physiotherapy is now encouraged as an additional treatment to the well‐established pharmacological and surgical interventions from early disease stages on (Fox 2011). In a review by Tomlinson and colleagues, 39 trials involving a total of 1827 participants with PD were examined to determine the effectiveness of physiotherapy. Significant short‐term benefits were demonstrated for gait, endurance, balance, and global motor function (Tomlinson 2013). Considering the progressive nature of the disease, sustained exercise is considered essential to obtain optimal performance and maintain independence in daily life activities (van Nimwegen 2011).
Description of the intervention
Virtual reality (VR) technology is a promising new rehabilitation tool with a wide range of applications (Riva 2003). Within the context of physiotherapy, VR technology is recommended to optimise motor learning in a safe environment, and may be a worthy alternative to conventional approaches (Burdea 2003; Keshner 2004). By offering augmented feedback about performance, enabling individualised repetitive practice of motor function and stimulating both motor and cognitive processes simultaneously, VR offers opportunities to learn new motor strategies and to relearn motor abilities that were lost as a result of injury or disease (Goble 2014; Mirelman 2013‐1; van Diest 2013).
It is not surprising that VR technology has been proposed as a tool to engage users in long‐term exercise, since it provides training in a challenging and motivating environment. A recent review defined a sense of control, challenge, and success as key components for patient immersion in and enjoyment of a VR system (Lewis 2012). Also, by replicating real‐life scenarios, VR technology provides greater potential for transfer to functional activities of daily living. To date, however, it remains unclear how VR technology may be optimally used and adjusted to the specific needs of various patient populations. High‐quality study is needed to determine the efficacy and added value of this new training approach.
Why it is important to do this review
Conventional physiotherapy aims to maximise functional ability and minimise secondary complications through movement rehabilitation. It has previously been shown to have a positive impact upon gait, endurance, balance, and global motor function in people with PD (Tomlinson 2013; Tomlinson 2014). However, exercise effects decreased after follow‐up periods without training, illustrating the importance of sustained effort (Tomlinson 2013). Although recent studies in PD have demonstrated that prolonged exercise for two years induced sustained benefits on both motor and cognitive outcomes (Corcos 2013; David 2015; Prodoehl 2015), engaging patients in long‐term regular exercise programmes is challenging. Both motor and non‐motor symptom burden may affect the willingness of people with PD to participate. In a recent report, long‐term exercise adherence was shown to be low even with optimal input provided by trainers and coaches (van Nimwegen 2013). Technology‐based exercise interventions may improve adherence by stimulating users to exercise in a personalised, motivating, fun, and engaging manner.
Early pilot studies using uncontrolled designs have explored the effectiveness of VR interventions in PD and have suggested positive effects on gait, balance, and cognitive function after training (Esculier 2012; Gonçalves 2014; Herz 2013; Holmes 2013; Lefaivre 2015; Mhatre 2013; Mirelman 2011; Palacios‐Navarro 2015; Shema 2014). Although full implementation in clinical practice is still to be realised, VR technology has become an increasingly popular tool within physical rehabilitation research. Short‐term improvements following VR exercise have already been demonstrated in healthy older adults and stroke patients based on systematic reviews (Goble 2014; Laver 2015; van Diest 2013).
While VR technology may be beneficial, it also creates additional challenges. By providing distractions in the virtual environment and introducing motor‐cognitive dual tasking, VR technology can create a cognitive overload (Barry 2014). In addition, exercise provided by commercial VR systems may not be specific enough to adequately address PD symptoms. To our knowledge, two reviews have been performed concerning the use of VR technology for rehabilitation in PD, but they included mostly non‐randomised controlled pilot studies (Barry 2014; Mirelman 2013‐1). Given the potential advantages of VR technology, we performed a systematic review including high‐quality trials only with the aim of objectively investigating the effectiveness of VR exercise for people with PD in comparison to regular or no training.
Objectives
The objective of this review was to summarise the current best evidence for the effectiveness of VR interventions for the rehabilitation of people with PD in comparison with 1) active interventions, and 2) passive interventions. Our primary goal was to determine the effect of VR training on gait and balance. Secondary goals included examining the effects of VR on global motor function, activities of daily living, quality of life, cognitive function, exercise adherence, and the occurrence of adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised controlled trials in which at least one of the interventions was an ongoing programme of VR exercise or training for inclusion in the review. We allowed both random and quasi‐random methods of allocation.
Types of participants
We included studies involving participants with a clinically definite diagnosis of idiopathic PD, as defined by the UK Parkinson’s Disease Society Brain Bank or other diagnostic criteria. We made no restrictions with regard to gender, age, disease duration, or disease severity. We included trials reporting an intervention carried out in a mixed sample of participants if data for participants with PD were provided separately.
Types of interventions
We assessed the effectiveness of VR exercise for rehabilitation versus 1) active interventions without a VR component, and 2) passive interventions.
We defined a VR intervention as “a computerized simulation which allows users to interact with images and virtual objects that appear in the virtual environment in real‐time through multiple sensory modalities” (Bisson 2007). All VR interventions needed to have a main focus on exercise and motor rehabilitation. We made no restrictions with regard to frequency and duration of the VR training. To summarise, we included a study if it encompassed:
a user‐computer interface;
interaction in the virtual environment;
feedback on performance; and
a focus on motor rehabilitation.
We excluded trials where the main objective was to study cueing, or to provide visual or auditory references without delivering immediate feedback on motor performance and/or without a virtual environment.
Control interventions needed to involve either passive treatment or active conventional physiotherapy without a VR component. Passive treatment included either educational programmes or a control group receiving no intervention. Active conventional physiotherapy involved usual care or any other exercise intervention without a VR component.
Types of outcome measures
Primary outcomes
Gait. We included both direct measures of gait, such as gait speed or step length, and clinical measures of gait, such as the Dynamic Gait Index or the Two‐ or Six‐Minute Walk Test.
Balance. We took into account direct measures of balance, such as center of pressure behaviour, as well as clinical measures of balance, such as the Berg Balance Scale, Timed Up and Go Test, and Mini‐Balance Evaluation Systems Test (Mini‐BESTest).
If possible, we compared the mean difference as calculated in the meta‐analysis to the minimally important difference (MID) or threshold for appreciable change. The MID for each of the outcome measures was based on the literature.
Secondary outcomes
Global motor function. We used the Unified Parkinson’s Disease Rating Scale (UPDRS) part III to address global motor function changes.
Activities of daily living (ADL). We considered the Physical Activity Scale for the Elderly, UPDRS part II, the Barthel Index of Activities of Daily Living, and other measures of ADL function.
Quality of life. We included two types of quality of life, namely fall‐related quality of life, involving outcome measures such as the Falls Efficacy Scale and Activities‐specific Balance Confidence Scale, and health‐related quality of life, such as determined by the 39‐Item Parkinson’s Disease Questionnaire.
Cognitive function. Measures of cognition consisted of the Mini‐Mental State Examination (MMSE), Montreal Cognitive Assessment, and the Trail Making Test, among others.
Adverse events. We obtained number and type of adverse events.
Exercise adherence. We investigated direct measures of exercise adherence, such as withdrawal or hours of practice, and clinical measures of exercise adherence, such as determined by user satisfaction questionnaires.
Search methods for identification of studies
We used the search strategy recommended by the Cochrane Movement Disorders Group to identify relevant articles.
Electronic searches
We searched the Cochrane Movement Disorders Group Trials Register (November 2016). In addition, we identified relevant articles through electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library; November 2016, Issue 11), MEDLINE (1946 to 26 November 2016), Embase (1947 to 26 November 2016), CINAHL (1982 to 26 November 2016), and the Physiotherapy Evidence Database (PEDro, 1999 to 26 November 2016). We developed search strategies for MEDLINE (OVID) and adapted these for use in the other databases (Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5).
Searching other resources
We attempted to identify other published, ongoing, and planned trials by:
inspecting references of all identified studies;
searching trials registers such as ClinicalTrials.gov (clinicaltrials.gov/) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/); and
handsearching relevant conference proceedings.
Data collection and analysis
Selection of studies
Two review authors (KD, EB) independently screened all search results (title, abstract, and descriptors) to identify studies for possible inclusion in the review. After the initial screening, KD and EB assessed all included trials for eligibility based on the full text. Any disagreements were resolved through discussion or, if necessary, through independent arbitration by PG. Where required, we contacted study authors for additional information.
Data extraction and management
Two review authors (KD, EB) independently extracted data onto a pre‐tested data collection form, including citation details, trial setting, inclusion and exclusion criteria, study population, intervention details, outcome measures, and results. All of the review authors involved in data extraction were provided detailed instructions and a training session. Disagreements were resolved through discussion or, if necessary, through independent arbitration by PG. Where required, we contacted study authors for additional information.
Assessment of risk of bias in included studies
Two review authors (KD, EB) independently assessed the methodological quality of each of the included trials using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We assessed the following items for each included trial: sequence generation (randomisation), allocation concealment, blinding of outcome assessors, incomplete outcome data, and selective outcome reporting. Due to the nature of training interventions, blinding of participants and personnel was not applicable, and was therefore not included in the 'Risk of bias' assessment. Where required, we contacted corresponding authors to retrieve additional information. If we received no response, we judged the 'Risk of bias' criterion as ‘unclear’. All information was collected in the data collection form. and any disagreements were resolved through discussion.
Based on our five 'Risk of bias' items, we determined that studies at:
low risk of bias were those in which all items were assigned a low risk of bias;
unclear risk of bias were studies in which one or more items was found to be at unclear risk of bias; and
high risk of bias were studies in which one or more items was found to be at high risk of bias.
Measures of treatment effect
Two review authors (KD, EB) independently classified outcome measures in terms of the domain assessed (gait, balance, global motor function, activity limitation, quality of life, cognitive function, number and types of adverse events, and exercise adherence). When a study presented more than one outcome measure for the same domain, we employed the most frequently used across studies. We calculated risk ratios (RR) with 95% confidence intervals (CIs) for any dichotomous outcomes. We calculated mean differences (MD) or standardised mean differences (SMD) for continuous outcomes, as appropriate. We used Cochrane's Review Manager 5 (Review Manager 2014) software for all analyses. To support the interpretation of the findings, we performed additional Cohen's d calculations.
Unit of analysis issues
For three‐armed interventions, we used the active control group for the analysis of VR exercise versus an active intervention, and the passive control group for the analysis of VR exercise versus a passive intervention.
Dealing with missing data
We contacted study authors to attempt to retrieve any missing data. We considered studies to be at low risk of bias if an intention‐to‐treat analysis had been performed, and at high risk of bias if not. When dropout was clearly identified for an outcome, we reported the true number of participants contributing to the data. This implied that if postintervention 20 participants performed the UPDRS, but only 18 performed the Berg Balance Scale, then the number of participants contributing to the meta‐analysis would differ between the UPDRS and Berg Balance Scale analyses. The potential impact of missing data was addressed.
Assessment of heterogeneity
We assessed heterogeneity visually by means of forest plots and by reporting the I² statistic. Depending on the degree of heterogeneity found, we decided against data pooling and presented forest plots along with a description of the results. We considered the degree of heterogeneity to be substantial if I² reached 75% or higher.
Data synthesis
We performed a random‐effects model meta‐analysis when possible. If we could not perform a meta‐analysis due to substantial differences between the studies, or when only one study was identified, we provided a narrative review.
If possible, we performed subgroup analyses to determine whether outcomes varied according to age, disease duration, disease severity, frequency of intervention (number of sessions per week), intensity of the intervention (total hours of intervention), and type of intervention (highly specialised programme designed for rehabilitation versus commercial gaming console).
Sensitivity analysis
When applicable, we performed a sensitivity analysis including only studies at low risk of bias. We then compared these results to the main analysis including all of the trials.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
We identified a total of 4576 records through database (4562 studies) and trials register (14 studies) searches. From the 4576 titles and abstracts retrieved, we assessed 63 full‐text articles for eligibility. We excluded studies that did not meet the predefined inclusion criteria, such as non‐randomised controlled trials. Following a thorough screening, we identified nine full‐text articles (Lee 2015; Liao 2015; Pedreira 2013; Pompeu 2012; Shen 2014 ‐ 2015; van den Heuvel 2014; Yang 2015; Yen 2011). The articles by Shen and colleagues were in fact a single study with two bibliographic references (Shen 2014 ‐ 2015). As such, we included eight trials in the qualitative analyses. We included seven studies in the quantitative analyses, as the study from Yen 2011 presented outcomes that were not comparable to the outcomes used in the other studies. A study flow diagram can be found in Figure 1.
1.
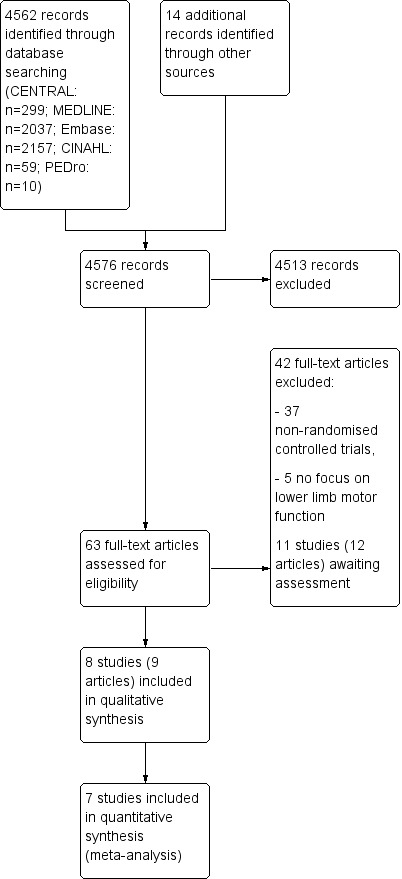
Study flow diagram.
Included studies
All studies were published between 2011 and 2015.
Sample characteristics
A total of 263 participants with PD were included, of which 159 were male (60%) and 104 were female (40%). Reported mean ages ranged between 61.1 and 75.4 years old. All of the included trials had small sample sizes involving fewer than 50 participants, and some (22%) involving fewer than 25 participants (Lee 2015; Yang 2015). Details regarding participant recruitment and withdrawal are presented in Table 3.
1. Participant recruitment and withdrawal.
| Author and year | Screened | Randomised | Allocated virtual reality | Completed trial/analysed at final follow‐up | Completed virtual reality |
| Lee 2015 | Not reported | 20 | 10 | 20 | 10 |
| Liao 2015 | 43 | 36 | 12 | 35 | 12 |
| Pedreira 2013 | 71 | 44 | 22 | 32 | 16 |
| Pompeu 2012 | 50 | 32 | 16 | 32 | 16 |
| Shen 2014 ‐ 2015 | 71 | 51 | 26 | 35 | 18 |
| van den Heuvel 2014 | 59 | 33 | 17 | 31 | 17 |
| Yang 2015 | 44 | 23 | 11 | 20 | 10 |
| Yen 2011 | 67 | 42 | 14 | 32 | 12 |
All but one study clearly specified inclusion and exclusion criteria (Lee 2015). The included participants were comprised of people with PD at different disease stages: one study in the early disease stages only (Hoehn and Yahr I and II) (Pompeu 2012), two studies in the early to moderate disease stages (Hoehn and Yahr I to III) (Liao 2015; Pedreira 2013), three studies in mild to moderate disease stages (Hoehn and Yahr II and III) (van den Heuvel 2014; Yang 2015; Yen 2011), and one study included all disease stages (Hoehn and Yahr I to V) (Shen 2014 ‐ 2015).
Participants were included if they were cognitively intact, as defined by cutoff scores on the MMSE. Different cutoff scores were used, with equal to or greater than 24 in four studies (Liao 2015; Pompeu 2012; Shen 2014 ‐ 2015; van den Heuvel 2014), and equal to or greater than 25 in two studies (Yang 2015; Yen 2011).
Medically unstable participants were excluded, as defined by the presence of:
neurological conditions other than PD (Liao 2015; Pompeu 2012; Shen 2014 ‐ 2015; van den Heuvel 2014; Yang 2015; Yen 2011);
orthopaedic issues (Liao 2015; Pompeu 2012; van den Heuvel 2014; Yen 2011);
cardiopulmonary problems (Liao 2015; Pedreira 2013; Shen 2014 ‐ 2015; van den Heuvel 2014; Yen 2011);
visual impairment (Liao 2015; Pompeu 2012; Shen 2014 ‐ 2015; Yang 2015); and
depression (Pedreira 2013; Pompeu 2012; Yang 2015).
VR interventions
A detailed overview of the contents of the interventions for both VR and control groups is provided in Table 4.
2. Contents of the interventions.
| Author and year | VR intervention | Active control group | Passive control group |
| Lee 2015 |
VR dance exercise neurodevelopment treatment, functional electrical stimulation |
‐ | Neurodevelopment treatment, functional electrical stimulation |
| Liao 2015 |
Wii Fit balance board therapy (yoga, strength, balance) treadmill training |
Conventional physiotherapy (stretching, strength, balance) treadmill training |
Fall prevention education |
| Pedreira 2013 | Nintendo Wii Therapy |
Conventional physiotherapy (mobilisation, balance, strength, rhythmic, postural alignment, dual task, bimanual, cardiorespiratory, gait) |
‐ |
| Pompeu 2012 |
Wii Fit balance board therapy (static balance, dynamic balance, stationary gait) |
Conventional physiotherapy (static balance, dynamic balance, stationary gait) |
‐ |
| Shen 2014 ‐ 2015 |
VR dance exercise SMART EquiTest Balance Master, gait Home: fall‐prone activities |
Conventional physiotherapy (strength and stepping) Home: stepping and walking |
‐ |
| van den Heuvel 2014 |
Motek dynamic balance exercises (body lean, stepping, sit‐to‐stand) |
Conventional physiotherapy (one‐leg stance, dual tasks, stepping, sit‐to‐stand, balancing beam) |
‐ |
| Yang 2015 |
Customised balance board therapy (static posture and dynamic weight shifting) |
Conventional physiotherapy (static posture and dynamic weight shifting) |
‐ |
| Yen 2011 |
Customised balance board therapy (stretching, balance) |
Conventional physiotherapy (stretching, balance) |
‐ |
VR: virtual reality
All of the studies had a main focus on motor rehabilitation, consistent with our predefined inclusion and exclusion criteria. More specifically, three trials focused on the improvement of balance performance (Lee 2015; Yang 2015; Yen 2011), and five trials included both balance and stepping exercises (Liao 2015; Pedreira 2013; Pompeu 2012; Shen 2014 ‐ 2015; van den Heuvel 2014). The total dose of therapy varied between studies, ranging from six to 52 hours of practice spread over a total training period of a minimum of four and a maximum of 12 weeks.
Six studies made use of Wii Fit, Motek, or other commercialised games (Lee 2015; Liao 2015; Pedreira 2013; Pompeu 2012; Shen 2014 ‐ 2015; van den Heuvel 2014), while two studies used customised VR programmes specifically designed for rehabilitation in PD (Yang 2015; Yen 2011). Six studies incorporated a balance board, aimed at training both static and dynamic balance (Liao 2015; Pedreira 2013; Pompeu 2012; van den Heuvel 2014; Yang 2015; Yen 2011). Four studies involved dancing movements, in Lee 2015 and Shen 2014 ‐ 2015, or stepping in place, in Pompeu 2012 and van den Heuvel 2014, in combination with a VR.
The intervention setting differed between studies, with five trials taking place in an outpatient environment (Pedreira 2013; Pompeu 2012; Shen 2014 ‐ 2015; van den Heuvel 2014; Yen 2011), and one in a home‐based setting (Yang 2015). Two trials did not specify the setting of the study (Lee 2015; Liao 2015).
Comparison interventions
All but one trial included an active control group (Lee 2015). Four studies made use of an active control group performing similar exercises as the intervention group, but without a VR (Pedreira 2013; Pompeu 2012; van den Heuvel 2014; Yang 2015). One study made use of an active control group performing exercises that differed from the VR intervention group (Shen 2014 ‐ 2015). In addition, two studies consisted of three‐armed interventions including 1) a VR intervention group, 2) an active control group performing similar exercises within a conventional physiotherapy setting, and 3) a passive control group (Liao 2015; Yen 2011).
Outcomes
An overview of all outcome measures used in the included studies can be found in Table 5. Due to the wide variety of outcome measures among studies, not all outcome measures could be included in the meta‐analyses.
3. Outcome measures.
| Author and year | Gait | Balance | Global Motor Function | Cognitive function | ADL | QoL | Adverse events | Therapy Adherence |
| Lee 2015 | ‐ | Berg Balance Scale | ‐ | ‐ | Modified Barthel Index | Beck Depression Inventory | ‐ | ‐ |
| Liao 2015 | Obstacle crossing: stride length, stride velocity, toe‐obstacle clearance | NeuroCom dynamic posturography system: Limits of Stability, Sensory Organization Test, Timed Up and Go Test | ‐ | ‐ | ‐ | PDQ‐39, Falls Efficacy Scale | Number of adverse events | Withdrawal |
| Pedreira 2013 | ‐ | ‐ | UPDRS total | ‐ | ‐ | PDQ‐39 | ‐ | ‐ |
| Pompeu 2012 | ‐ | Berg Balance Scale, Unipedal Stance Test | ‐ | Montreal Cognitive Assessment | UPDRS II | ‐ | Number of adverse events | Withdrawal |
| Shen 2014 ‐ 2015 | Normal walking: gait velocity, stride length | Limits of Stability, Single‐Leg Stance Test | ‐ | ‐ | ‐ | Activities‐specific Balance Confidence Scale | Number of fallers, fall rate, time to first fall | Withdrawal, number of completed sessions, demographic differences between dropout and non dropout |
| van den Heuvel 2014 | 10‐Metre Walk Test: walking speed, step length | Berg Balance Scale, Single‐Leg Stance Test, Functional Reach Test (Limits of Stability) | UPDRS total, UPDRS III | ‐ | ‐ | PDQ‐39, Falls Efficacy Scale, Hospital Anxiety and Depression Scale, Multidimensional Fatigue Inventory | Number of falls + other adverse events | Withdrawal |
| Yang 2015 | Dynamic Gait Index | Berg Balance Scale, Timed Up and Go Test | UPDRS III | ‐ | ‐ | PDQ‐39 | ‐ | Withdrawal |
| Yen 2011 | ‐ | Sensory Organization Test: single task and dual task (auditory arithmetic subtraction) | ‐ | ‐ | ‐ | ‐ | Number of falls + other adverse events | Withdrawal |
PDQ‐39: 39‐Item Parkinson’s Disease Questionnaire UPDRS: Unified Parkinson’s Disease Rating Scale
Outcome measures were collected at baseline and within the first week following intervention in all trials. Follow‐up periods differed between studies, with most trials reporting a follow‐up period of three months or less (Liao 2015; Pompeu 2012; van den Heuvel 2014; Yang 2015; Yen 2011). One trial reported outcome measures over a longer follow‐up period, namely 12 months (Shen 2014 ‐ 2015).
Excluded studies
We excluded 3985 trials as they did not meet our predefined inclusion and exclusion criteria. We found 49 full‐text articles to be eligible based on title and abstract, and after reading the full text eight trials remained. The excluded full‐text articles consisted of 34 non‐randomised controlled trials (RCTs) using a pre‐post design, four conference abstracts of RCTs for which the authors were contacted but did not reply, and two RCTs without a main focus on lower limb motor rehabilitation. A summary is provided in the Characteristics of excluded studies table.
Risk of bias in included studies
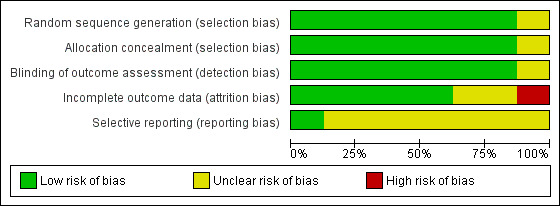
An overview of the methodological quality of the included papers is presented in Figure 2 and Figure 3.
2.
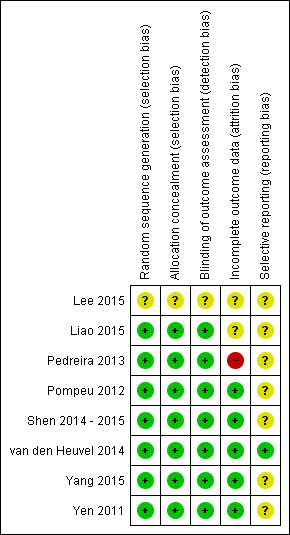
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
We deemed one study to be at low risk of bias (van den Heuvel 2014), five studies at unclear risk of bias (Lee 2015; Pompeu 2012; Shen 2014 ‐ 2015; Yang 2015; Yen 2011), and two studies at high risk of bias (Liao 2015; Pedreira 2013).
Allocation
We judged random sequence allocation and allocation concealment as sufficient in seven trials (Liao 2015; Pedreira 2013; Pompeu 2012; Shen 2014 ‐ 2015; van den Heuvel 2014; Yang 2015; Yen 2011). We judged one trial that did not specify allocation methodology to be at unclear risk of bias (Lee 2015).
Blinding
Seven trials reported adequate blinding of the outcome assessor (Liao 2015; Pedreira 2013; Pompeu 2012; Shen 2014 ‐ 2015; van den Heuvel 2014; Yang 2015; Yen 2011). As mentioned earlier, due to the nature of the VR interventions, blinding of participants and personnel was not applicable and was therefore not included in the 'Risk of bias' assessment.
Incomplete outcome data
Details regarding participant recruitment and withdrawal are presented in Table 3. Most studies dealt with incomplete outcome data adequately by performing an intention‐to‐treat analysis (Shen 2014 ‐ 2015; van den Heuvel 2014; Yang 2015; Yen 2011). However, two trials did not perform an intention‐to treat analysis; we therefore considered one trial to be at high risk of bias (Pedreira 2013), and the other, due to only limited dropout, at unclear risk of bias (Liao 2015). We judged the study from Lee and colleagues as at unclear risk of bias as it did not provide any information regarding participant recruitment or withdrawal (Lee 2015).
Selective reporting
Most studies did not publish a protocol paper and were therefore considered to be at unclear risk of bias regarding selective reporting, with the exception of the study from van den Heuvel and colleagues (van den Heuvel 2014).
Effects of interventions
We included seven trials in the meta‐analyses (Lee 2015; Liao 2015; Pedreira 2013; Pompeu 2012; Shen 2014 ‐ 2015; van den Heuvel 2014; Yang 2015). VR treatments were compared to 1) active interventions, and 2) passive interventions.
Both short‐term and long‐term effects of VR exercise were examined. Short‐term effects were based on performance differences between baseline and immediate postintervention measurements. Long‐term effects included follow‐up periods of at least 12 weeks (Tomlinson 2013).
Comparison 1: Virtual reality versus active intervention
Short‐term outcomes
Primary outcomes
Gait
Four studies investigated the effects of VR exercise on gait (Liao 2015; Shen 2014 ‐ 2015; van den Heuvel 2014; Yang 2015). Different outcome measures were used, namely gait speed, step or stride length, and the Dynamic Gait Index. We performed a meta‐analysis on 1) gait as a composite measure, 2) gait speed, and 3) step and stride length.
Outcome 1: Gait (composite measure)
We carried out a meta‐analysis involving four trials with a total of 129 participants with PD (Liao 2015; Shen 2014 ‐ 2015; van den Heuvel 2014; Yang 2015). We found no significant difference between VR and active control interventions (SMD 0.20, 95% CI ‐0.14 to 0.55) (Analysis 1.1). Gait performance significantly improved irrespective of training allocation in all trials. Cohen's d calculations ranged from ‐0.04 to 0.52, suggesting a minimal difference between VR and control interventions. Statistical heterogeneity was very low (I²=0%; P=0.70).
1.1. Analysis.
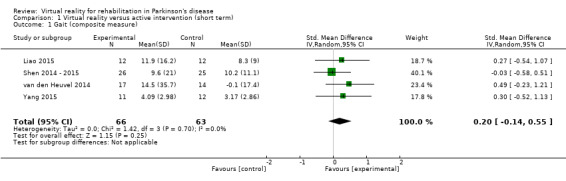
Comparison 1 Virtual reality versus active intervention (short term), Outcome 1 Gait (composite measure).
Outcome 2: Gait speed
Three trials involving a total of 106 participants with PD assessed gait speed. Different walking conditions were used, namely normal walking, in Shen 2014 ‐ 2015 and van den Heuvel 2014, and obstacle walking (Liao 2015). Significant improvements following both VR and active control interventions were found in all trials. A meta‐analysis showed no significant difference between the two training arms (SMD 0.18, 95% CI ‐0.20 to 0.57) (Analysis 1.2). This was confirmed by Cohen's d calculations, which showed a minimal difference between both training arms (range: ‐0.04 to 0.52). There was no statistical heterogeneity (I²=0%; P=0.51).
1.2. Analysis.
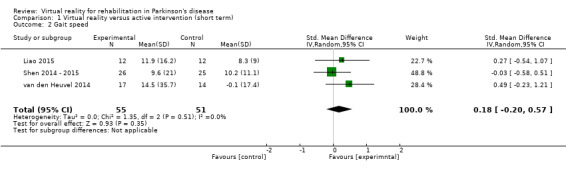
Comparison 1 Virtual reality versus active intervention (short term), Outcome 2 Gait speed.
Outcome 3: Step and stride length
Three studies including a total of 106 participants with PD examined the influence of VR exercise on step, in van den Heuvel 2014, and stride length (Liao 2015; Shen 2014 ‐ 2015). A meta‐analysis indicated a significant difference between VR and active control interventions, whereby VR exercise was shown to be superior (SMD 0.69, 95% CI 0.30 to 1.08) (Analysis 1.3). Based on Cohen's d calculations, the effect was medium to large, with a range from 0.51 to 0.86. There was no statistical heterogeneity between studies (I²=0%; P=0.76).
1.3. Analysis.
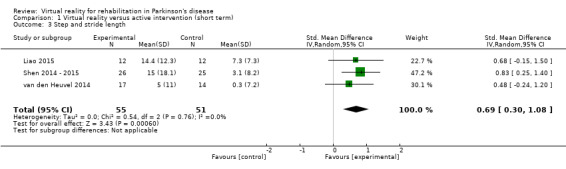
Comparison 1 Virtual reality versus active intervention (short term), Outcome 3 Step and stride length.
Sensitivity analysis
We performed a sensitivity analysis whereby only trials that were deemed to be at low or unclear risk of bias were included. Meta‐analyses of gait as a composite measure, in Shen 2014 ‐ 2015, van den Heuvel 2014, and Yang 2015, and gait speed, in Shen 2014 ‐ 2015 and van den Heuvel 2014, found no significant difference between VR and active control interventions (gait composite measure: SMD 0.19, 95% CI ‐0.20 to 0.58; 3 trials; 105 participants; gait speed: SMD 0.17, 95% CI ‐0.33 to 0.68; 2 trials; 82 participants). Statistical heterogeneity was low in both analyses (gait composite measure: I²=0%; P=0.50; gait speed: I²=23%; P=0.25).
Step and stride length differences remained significant in the sensitivity analysis including two trials and 82 participants with PD (SMD 0.69, 95% CI 0.25 to 1.14) (Shen 2014 ‐ 2015; van den Heuvel 2014). Statistical heterogeneity remained very low (I²=0%; P=0.46).
Balance
Five studies explored the impact of VR exercise on balance (Liao 2015; Pompeu 2012; Shen 2014 ‐ 2015; van den Heuvel 2014; Yang 2015). Balance was measured by means of the Berg Balance Scale, Timed Up and Go Test, and Single‐Leg Stance Test. We executed a meta‐analysis on 1) balance as a composite measure, and 2) balance as measured by the Berg Balance Scale.
Outcome 1: Balance (composite measure)
We included five studies involving 155 participants with PD in the meta‐analysis (Liao 2015; Pompeu 2012; Shen 2014 ‐ 2015; van den Heuvel 2014; Yang 2015). We found no significant difference between VR and active control interventions (SMD 0.34, 95% CI ‐0.04 to 0.71) (Analysis 1.4). All trials showed significant improvements in balance performance, regardless of group allocation. One study found an increased benefit of VR exercise on balance (Shen 2014 ‐ 2015). Cohen's d calculations showed a mixed effect of group allocation, ranging from ‐0.21 to 1.21. The meta‐analysis showed moderate statistical heterogeneity (I²=25%; P=0.26).
1.4. Analysis.
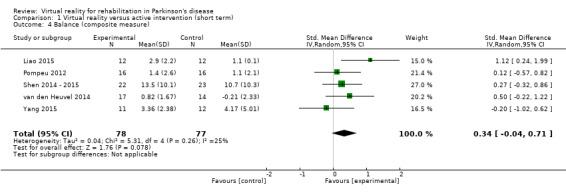
Comparison 1 Virtual reality versus active intervention (short term), Outcome 4 Balance (composite measure).
Outcome 2: Berg Balance Scale
A meta‐analysis involving three trials and a total of 86 participants with PD found no significant difference between VR and active control interventions (MD 0.55, 95% CI ‐0.48 to 1.58) (Pompeu 2012; van den Heuvel 2014; Yang 2015) (Analysis 1.5). All trials demonstrated improvements in balance performance, irrespective of group allocation. Cohen's d calculations indicated small to medium differences between groups (range: ‐0.21 to 0.53). No statistical heterogeneity was present (I²=0%; P=0.54).
1.5. Analysis.
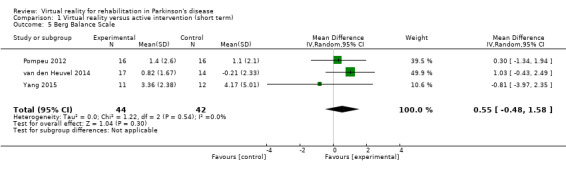
Comparison 1 Virtual reality versus active intervention (short term), Outcome 5 Berg Balance Scale.
Sensitivity analysis
We excluded trials considered to be at high risk of bias from the analyses (Liao 2015). Balance as a composite measure was not differentially affected by VR exercise (balance composite measure: SMD 0.20, 95% CI ‐0.14 to 0.55; 4 trials; 131 participants). Statistical heterogeneity was very low (I²=0%; P=0.64).
Secondary outcomes
Global motor function
Two studies investigated the effects of VR exercise on global motor function (van den Heuvel 2014; Yang 2015). Both trials made use of the Unified Parkinson’s Disease Rating Sale (UPDRS) part III (Analysis 1.6).
1.6. Analysis.
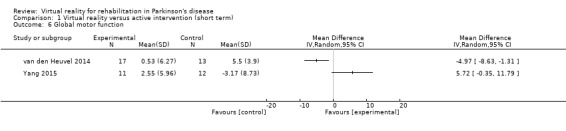
Comparison 1 Virtual reality versus active intervention (short term), Outcome 6 Global motor function.
Outcome 1: UPDRS part III
Due to substantial statistical heterogeneity between trials (I²=88%, P=0.003) (Pompeu 2012; van den Heuvel 2014), we decided against data pooling. In the study from van den Heuvel and colleagues, UPDRS part III scores appeared to be beneficially affected by VR exercise as compared to the active control intervention (P=0.021; Cohen's d=‐0.96). However, the study from Yang and colleagues did not find a significant difference between the two training arms (P=0.35; Cohen's d=0.79).
Activities of daily living
One study involving 32 participants with PD examined the impact of VR exercise on activities of daily living (ADL) (Pompeu 2012), using the UPDRS part II as a measurement of ADL function. A significant improvement was found in both the VR and active control interventions (P<0.001). A significant difference was not observed between the two training arms (Analysis 1.7). This was confirmed by Cohen's d calculations, which showed a small effect of ‐0.13.
1.7. Analysis.
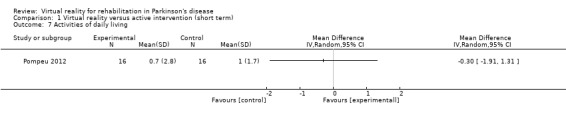
Comparison 1 Virtual reality versus active intervention (short term), Outcome 7 Activities of daily living.
Quality of life
Four trials measured quality of life by means of the 39‐Item Parkinson’s Disease Questionnaire (PDQ‐39) (Liao 2015; Pedreira 2013; van den Heuvel 2014; Yang 2015).
Outcome 1: PDQ‐39
In a meta‐analysis involving four trials and 106 participants with PD, we found no significant difference between VR and active control interventions (MD 3.73, 95% CI ‐2.16 to 9.61) (Analysis 1.8). Most trials described similar improvements in both exercise groups. Only one trial demonstrated greater improvements in the VR exercise group (Pedreira 2013). Cohen's d calculations showed a similar pattern, with most trials ranging from ‐0.06 to 0.32, indicating a minimal effect of group allocation, except for the Pedreira study, which showed a large effect of 1.03. There was moderate heterogeneity between trials (I²=46%; P=0.14).
1.8. Analysis.
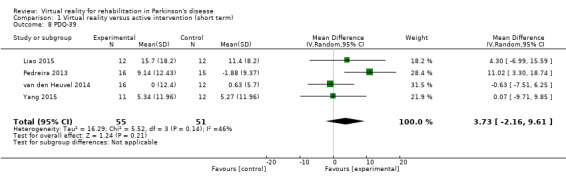
Comparison 1 Virtual reality versus active intervention (short term), Outcome 8 PDQ‐39.
Sensitivity analysis
We performed a sensitivity analysis whereby all trials at high risk of bias were excluded (Liao 2015; Pedreira 2013). Similarly, VR technology was not found to have an added value on quality of life as compared to active control interventions (MD ‐0.40, 95% CI ‐6.03 to 5.23). Statistical heterogeneity was non‐existent (I²=0%).
Cognitive function
One trial reported the effects of VR exercise on cognitive function (Pompeu 2012), measured by the Montreal Cognitive Assessment. In this study including 32 participants with PD, cognitive scores significantly improved in both training interventions equally (P<0.005) (Analysis 1.9). This was confirmed by a Cohen's d calculation, which showed a minimal effect of 0.09.
1.9. Analysis.
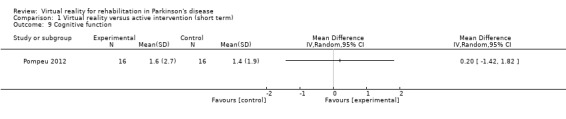
Comparison 1 Virtual reality versus active intervention (short term), Outcome 9 Cognitive function.
Adverse events
Four studies recorded the number and types of adverse events during study participation (Liao 2015; Pompeu 2012; van den Heuvel 2014; Yen 2011). All of these studies reported that no adverse events took place.
Exercise adherence
All trials reported participant withdrawal during training and at follow‐up. Repeated measures analysis of variance (ANOVA) was used to determine differences between interventions and showed no significant effect of VR technology on dropout compared to the control arms.
One study focused additionally on exercise compliance by means of the number of completed training sessions (Shen 2014 ‐ 2015). They showed that the VR group completed more training sessions as compared to the active control intervention during the laboratory‐based training period.
Long‐term outcomes
Primary outcomes
We identified one trial examining the long‐term effects of VR exercise (Shen 2014 ‐ 2015). In this study, gait and balance were measured at three and 12 months' follow‐up. For balance performance, the Limits of Stability Test (SMART EquiTest Balance Master, NeuroCom International Inc, Clackamas, OR) and Single‐Leg Stance Test were used, along with the Activities‐specific Balance Confidence Scale to assess self perceived balance confidence. Gait was examined by means of a 5‐metre instrumented GAITRite walkway (CIR Systems Inc, Havertown, PA), in which gait velocity and stride length were recorded.
At three months' follow‐up, performances on the Activities‐specific Balance Confidence Scale, Single‐Leg Stance Test, and Limits of Stability Test significantly improved in the VR group, but not in the control group. During walking, gait velocity improved equally in both groups, while stride length increased only in the VR exercise intervention.
At 12‐months' follow‐up, the Activities‐specific Balance Confidence Scale, Single‐Leg Stance Test, and stride length were significantly improved in the VR group as compared to the control intervention. Gait velocity improved to the same extent in both interventions. Performances on the Limits of Stability Test were no longer significantly different from baseline performances, and this was true for both the VR and control intervention groups.
Comparison 2: Virtual reality versus passive intervention
Short‐term outcomes
Primary outcomes
Gait
We identified one trial involving 24 participants with PD assessing the effects of VR exercise compared to a passive control group on gait (Liao 2015). In this trial, stride length and stride velocity were measured during obstacle crossing, which was an untrained task in both training cohorts. Stride length (P=0.003; Cohen's d=1.37) and stride velocity (P=0.011; Cohen's d=1.22) of the crossing limb improved significantly more in the VR exercise group as compared to the passive control intervention (Analysis 2.1).
2.1. Analysis.
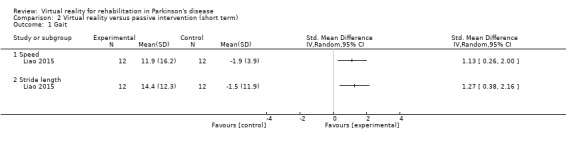
Comparison 2 Virtual reality versus passive intervention (short term), Outcome 1 Gait.
Balance
Two studies examined the effect of VR exercise versus a passive control group on balance performance (Lee 2015; Liao 2015). Different outcome measures were used, namely the Timed Up and Go Test and the Berg Balance Scale. We conducted a meta‐analysis of balance as a composite measure.
Outcome 1: Balance (composite measure)
A meta‐analysis involving 44 participants with PD showed a significant benefit of VR exercise as compared to passive control interventions (SMD 1.02, 95% CI 0.38 to 1.65) (Analysis 2.2). A large effect of VR intervention was confirmed by Cohen's d calculations, with a range from 1.04 to 1.17. No statistical heterogeneity was present (I²=0%; P=0.84).
2.2. Analysis.
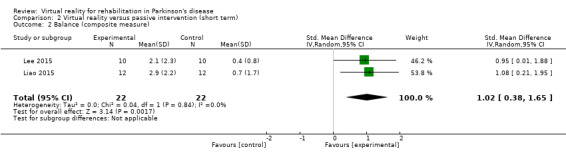
Comparison 2 Virtual reality versus passive intervention (short term), Outcome 2 Balance (composite measure).
Secondary outcomes
Global motor function
We found no trials examining the effects of VR exercise on global motor function as compared to a passive control group.
Activities of daily living
One trial involving 20 participants with PD assessed ADL function by means of the Modified Barthel Index (Lee 2015). This study demonstrated a significant improvement in the VR group (P<0.05; Cohen's d=1.05), which was not the case for the passive control group (Analysis 2.3).
2.3. Analysis.
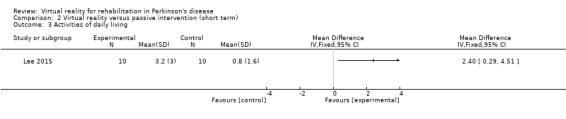
Comparison 2 Virtual reality versus passive intervention (short term), Outcome 3 Activities of daily living.
Quality of life
One study involving 24 participants with PD investigated the effects of VR exercise on quality of life (Liao 2015), using the PDQ‐39 as an outcome measure of quality of life. This study showed a significant difference between VR and passive control interventions (P=0.004; Cohen's d=1.17), whereby VR exercise was found to be superior (Analysis 2.4).
2.4. Analysis.
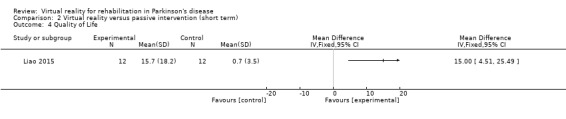
Comparison 2 Virtual reality versus passive intervention (short term), Outcome 4 Quality of Life.
Cognitive function
We found no trials examining the effects of VR exercise on cognitive function as compared to a passive control group.
Adverse events
One study recorded the number and types of adverse events during study participation (Liao 2015), with no adverse events taking place.
Exercise adherence
One trial reported participant withdrawal during training and at follow‐up (Liao 2015), showing no significant differences in dropout between training arms.
Long‐term outcomes
We found no trial addressing the long‐term effects of VR exercise compared to a passive control intervention.
Discussion
Summary of main results
With this review, we investigated the state of the art on the effectiveness of VR exercise for rehabilitation in PD. We identified eight trials involving a total of 263 participants with PD. All studies were published in the last five years, illustrating that VR augmented therapy is a novel research area.
VR exercise was compared to 1) active control interventions, and 2) passive control interventions. Our objective was to investigate whether VR exercise induced greater improvements on gait, balance, global motor function, activities of daily living, quality of life, cognition, exercise adherence, and the occurrence of adverse events. The main results are presented in Table 1 and Table 2.
Based on the current findings, VR therapy induced 1) increased benefits on step and stride length, and 2) similar effects on balance, gait, ADL function, quality of life, and cognitive function as compared to active control interventions in people with PD. In addition, VR exercise elicited greater improvements in gait, balance, ADL function, and quality of life as compared to passive control interventions. Although high‐quality evidence was limited, earlier pilot studies came to similar conclusions, showing positive effects on similar outcomes following VR exercise in PD (Esculier 2012; Gonçalves 2014; Herz 2013; Holmes 2013; Lefaivre 2015; Mhatre 2013; Mirelman 2011; Palacios‐Navarro 2015; Shema 2014).
Both balance and gait measures improved at three and 12 months' follow‐up in the VR group, but not in the control intervention. Additional study is needed to investigate the possible long‐term benefits of VR exercise, as these findings are currently based on one trial only (Shen 2014 ‐ 2015).
In most trials, the active control interventions were closely related to conventional physiotherapy programmes. Physiotherapy is known to improve motor function in people with PD (Hirsch 2009). According to a systematic review by Tomlinson and colleagues, conventional physiotherapy mainly influences gait and balance performance (Tomlinson 2013). In the current review, VR exercise was shown to induce largely similar improvements for both gait and balance. Increased benefits of VR exercise were found for step and stride length only, and balance (composite measure) improvements were approaching significance in favor of VR.
A decrease in step and stride length is characteristic of PD and is associated with a number of other gait‐related symptoms, such as reduced gait speed, increased gait variability, and increased double‐stance time (Hausdorff 2009). While the ability to generate a normal gait pattern as such is not affected in PD, automaticity is reduced and attentional strategies are needed to bypass automatic control mechanisms (Wu 2015). Although our findings are based on a limited body of evidence, it could be that VR technology provided more accurate and complete motor feedback and therefore enabled better stride amplitude correction than traditional physiotherapy. It is important to note that the improvements found were medium to large according to the Cohen's d calculations, indicating a clear difference between VR and active control interventions. Our review did not confirm other increased effects of VR exercise on gait, most notably not on gait speed. The amplitude‐specific effect may be explained by the fact that VR was not used to train gait itself in the current review. A study is currently being conducted that addresses VR‐embedded treadmill training, the results of which may indicate whether gait speed as well as step and stride length may be ameliorated by VR‐enhanced gait training (Mirelman 2013).
Postural instability, on the other hand, is considered to be one of the most disabling motor symptoms of PD (Soh 2011), with a low response to dopaminergic therapy (Bloem 1996; Curtze 2015). It may therefore benefit particularly from physiotherapy interventions both with and without VR technology.
According to a framework by Schoneburg and colleagues, balance is managed by four postural control systems: 1) balance during quiet stance, 2) reactive postural adjustments, 3) anticipatory postural adjustments, and 4) dynamic balance (Schoneburg 2013). All of these systems are likely to be affected in people with PD, often resulting in an increased risk of falls. At present, it is unclear whether VR exercise improves balance performance in general, or whether it influences certain postural control systems more than others. Based on our findings, a mixed effect of group allocation was found with Cohen's d calculations ranging from ‐0.21, indicating a small effect, to 1.21, suggesting a large difference. However, in contrast to passive control interventions, we could observe a large effect of VR. An extensive meta‐analysis on the Berg Balance Scale showed that the improvements did not reach the minimal important difference threshold. Based on the literature, the minimal important difference is set at 2.8 to 6.6 points (Downs 2013), whereas our findings demonstrated an average benefit of merely 0.55 points.
At present, balance performance is mostly measured using clinical outcome measures, such as the Berg Balance Scale. While this is considered to be a robust measure of balance performance (Steffen 2008), it is also characterised by substantial floor and ceiling effects (King 2012). Using more sensitive tools to uncover balance improvements in future studies may aid in clarifying the degree of effectiveness of VR‐based exercise for balance in PD. Objective posturography techniques (McVey 2009; Nonnekes 2013), as well as novel clinical tests such as the Mini‐BESTest (Horak 2009; Vervoort 2015), were shown to reveal subtle balance alterations in PD versus controls.
It has been suggested that VR technology may hold some drawbacks for people with PD, that is cyber‐sickness, cognitive overload, or an inappropriate level and content of exercises for rehabilitation of PD (Barry 2014). Custom‐made VR applications developed to offer a disease‐specific exercise programme are designed to overcome these issues. Such applications may therefore prove to be superior to commercial VR systems. Unfortunately, due to the small body of evidence, we were not able to address these issues or to provide clear suggestions for future treatment.
One of the great advantages of VR exercise is the possibility of exercising in a home‐based setting. Although certain safety issues arise when considering independent, low‐supervised interventions, the practical implications are immense. Home‐based exercise will add a degree of flexibility to patient treatment and might improve long‐term exercise adherence in a population that is prone to dropping out (Ellis 2013). However, future work needs to evaluate if the same quality of treatment can be achieved when limited supervision is provided (King 2015). One of the potential pitfalls of home‐based exercise involves the use of compensatory movements to increase game performance. Patients may start to prioritise game scores over improved quality of movement, thus reducing true training effects. Efforts should be made to ensure that compensatory movements are not beneficial for game performances before implementation of VR exercise in the home environment can be considered.
In conclusion, we found low‐quality evidence suggesting that VR‐enhanced exercise provides a useful alternative to conventional physiotherapy for improving gait, balance, ADL function, quality of life, and cognition in PD. Further study is needed to extend our knowledge of VR technology before wide implementation is warranted. It is of vital importance to unravel which type of VR application results in the best treatment effects for motor rehabilitation and other outcomes important to people with PD.
Overall completeness and applicability of evidence
We identified eight studies, all of which had small sample sizes. Hence, additional study is needed to confirm our findings based on a firm body of evidence. We are encouraged that a number of larger RCTs are currently under way (Mirelman 2013; Straudi 2015; van der Kolk 2015; Whyatt 2015), which are likely to inform the field further.
Our findings must be interpreted with caution, as they are based on a limited number of trials with varying quality. Due to the small number of included trials, it was not feasible to perform subanalyses regarding participant characteristics or study design. More empirical study is needed to determine the applicability of VR interventions in people with PD according to age, cognition, disease severity, and the presence of comorbidity. In addition, further study is needed to define the contents of an ideal VR intervention. While most researchers and clinicians intuitively prefer customised VR interventions targeting specific clinical features of PD (Barry 2014), objective study is desirable to determine whether differential responses exist between commercialised and customised VR interventions. In order to successfully implement VR exercise into daily practice, detailed information on training frequency, duration of the intervention, and targeted motor skills needs to be provided to serve as a guideline for clinicians. Unfortunately, such analyses were not feasible based on the current dataset.
Finally, it was not possible in the context of the available evidence to estimate the effect of VR interventions in the long term. Although VR interventions are often considered to improve exercise adherence, we were not able to validate this assumption based on the current data, as only one trial provided explicit information on compliance (Shen 2014 ‐ 2015).
Quality of the evidence
All of the included studies had small sample sizes, which was reflected in the low certainty in the effects for all of the outcomes of interest. Similarly, we judged the individual risk of bias of most of the included trials as unclear or low. Following an extensive 'Risk of bias' assessment, we found only 11% of the included trials to be at low risk of bias; 67% at unclear risk of bias; and 22% at high risk of bias due to insufficient reporting or lack of intention‐to‐treat analysis. Future trials should endeavor to avoid these methodological shortcomings by abiding to the CONSORT guidelines (Schulz 2010). Most importantly, power‐based studies are needed, and the currently reported studies can be used as a basis for such calculations.
Due to the great diversity in study methodology, a meta‐analysis was not always indicated in this review. While differences in outcome measures resulted in the use of standardised mean differences, follow‐up analyses were not feasible due to high variability between studies in time until first follow‐up. Future studies should pursue a high degree of agreement regarding study design and outcome measures used to ensure a more robust framework for pooled data analysis.
Potential biases in the review process
Although we conducted an extensive literature search, we acknowledge the possibility that we did not identify all relevant studies. Even though we contacted all relevant study authors in accordance with the review methodology, we did not always receive a response. As a result, four conference abstracts that met the inclusion criteria could not be included in the review and may have represented negative trial results. Also, the methodology of some studies remained unclear, as indicated by the 'Risk of bias' assessment.
Agreements and disagreements with other studies or reviews
To our knowledge, two systematic reviews addressing the effectiveness of VR technology for rehabilitation in people with PD have been performed (Barry 2014; Mirelman 2013‐1). These reviews concluded that VR exercise is feasible, but could reach no conclusions regarding the effectiveness of motor rehabilitation due to the very small number of included trials. The present results extend this knowledge by providing further insights into the effectiveness of VR technology for rehabilitation of balance and gait. Our findings seemed to concur with systematic reviews on VR exercise in older adults and stroke patients, in which short‐term motor improvements were reported (Goble 2014; Laver 2015; van Diest 2013).
Authors' conclusions
Implications for practice.
Although the results were inconclusive, low‐quality evidence indicated that virtual reality (VR)‐training was at least as effective as conventional physiotherapy. Whether these improvements were relevant and reached the minimal important difference for gait, balance, and other secondary outcome measures is not clear from this review. Further study is needed before full integration of VR‐based exercise into physiotherapy programs for people with Parkinson's disease can be considered.
Implications for research.
Additional high‐quality studies are needed to provide a deeper insight into the potentially beneficial mechanisms of VR technology and to reveal the differential effects of various VR applications. Future research should standardise the outcome measures and realise adequate follow‐up of at least 12 weeks (preferably 12 months) to examine the long‐term effects of VR.
Furthermore, the examination of VR interventions in different disease stages is recommended to ascertain whether there is a role for technology‐based exercise in the prevention of physical deterioration in early‐stage Parkinson's disease and in the management of disease progression in the moderate to late stages. Finally, empirical evidence is required to provide well‐substantiated recommendations regarding frequency, duration, and content of the VR intervention.
Acknowledgements
We would like to thank Trudy Bekkering and CEBAM for their help with the development of search strategies and guidance in data analyses.
We would also like to thank Margaret Mak, Maarten van den Heuvel, and Caroline Whyatt, who generously provided additional details and analyses from their trials to assist us with the review.
Appendices
Appendix 1. MEDLINE search strategy
Parkinson Disease [mh]
Parkinson* [tiab]
Virtual reality exposure therapy [mh]
VR [tiab]
Virtual [tiab]
Augmented [tiab]
Computer* [tiab]
Software [tiab]
Serious gaming [tiab]
Game [tiab]
User‐computer interface [tiab]
Simulation [tiab]
Exergam* [tiab]
Reality system [tiab]
Interactive [tiab]
1 OR 2
3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15
16 AND 17
Appendix 2. CENTRAL search strategy
Parkinson Disease [ti:ab:kw]
Parkinson* [ti:ab:kw]
VR [ti:ab:kw]
Virtual [ti:ab:kw]
Augmented [ti:ab:kw]
Computer* [ti:ab:kw]
Software [ti:ab:kw]
Serious gaming [ti:ab:kw]
Game [ti:ab:kw]
User‐computer interface [ti:ab:kw]
Simulation [ti:ab:kw]
Exergam* [ti:ab:kw]
Reality system [ti:ab:kw]
Interactive [ti:ab:kw]
1 OR 2
3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14
15 AND 16
Appendix 3. Embase search strategy
Parkinson*
Virtual
Augmented
Gaming
Game
User‐computer interface
Simulation
Exergam*
Reality system
Interactive
2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10
1 AND 11
Appendix 4. CINAHL search strategy
Parkinson (all text)
Virtual (all text)
1 AND 2
Appendix 5. PEDro search strategy
Parkinson
Virtual
1 AND 2
Data and analyses
Comparison 1. Virtual reality versus active intervention (short term).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gait (composite measure) | 4 | 129 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.14, 0.55] |
| 2 Gait speed | 3 | 106 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.20, 0.57] |
| 3 Step and stride length | 3 | 106 | Std. Mean Difference (IV, Random, 95% CI) | 0.69 [0.30, 1.08] |
| 4 Balance (composite measure) | 5 | 155 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.04, 0.71] |
| 5 Berg Balance Scale | 3 | 86 | Mean Difference (IV, Random, 95% CI) | 0.55 [‐0.48, 1.58] |
| 6 Global motor function | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Activities of daily living | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 PDQ‐39 | 4 | 106 | Mean Difference (IV, Random, 95% CI) | 3.73 [‐2.16, 9.61] |
| 9 Cognitive function | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Virtual reality versus passive intervention (short term).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gait | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Speed | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Stride length | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Balance (composite measure) | 2 | 44 | Std. Mean Difference (IV, Random, 95% CI) | 1.02 [0.38, 1.65] |
| 3 Activities of daily living | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Quality of Life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lee 2015.
| Methods | RCT | |
| Participants | 20 people with Parkinson’s disease Inclusion and exclusion criteria not reported |
|
| Interventions | Experimental group (EG: n = 10); control group (CG: n = 10) 5 times per week/6 weeks EG + CG: 30 min neurodevelopment treatment + 15 min functional electrical stimulation EG: additional 30 min VR dance exercise (K‐pop dance festival, Nintendo Inc, Japan) |
|
| Outcomes | Outcomes recorded at baseline and postintervention Primary outcome: Berg Balance Scale Secondary outcomes: Modified Barthel Index, Beck Depression Inventory |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol not publicly available |
Liao 2015.
| Methods | Single‐blinded, stratified RCT | |
| Participants | 36 people with Parkinson’s disease Inclusion criteria: clinical diagnosis of PD, H&Y I to III, independent walking, stable medication, MMSE ≥ 24 Exclusion criteria: history of other neurological, cardiopulmonary, or orthopaedic diseases, history of seizure, use of cardiac pacemaker, vision deficits |
|
| Interventions | Experimental group (VRWii: n = 12); active control group (TE: n = 12); passive control group (CG: n = 12) 2 times per week/6 weeks VRWii: Wii Fit balance board therapy (Nintendo Phuten Co, Ltd, Taiwan) including 10 min yoga, 15 min strengthening exercises, 20 min balance exercises TE: conventional physiotherapy including 10 min stretching, 15 min strengthening exercises, 20 min balance exercises VRWii + TE: additional 15 min treadmill training CG: fall prevention education |
|
| Outcomes | Outcomes recorded at baseline, postintervention, and 1‐month follow‐up Primary outcomes: obstacle‐crossing performance (crossing stride length, crossing stride velocity, vertical toe‐obstacle clearance), dynamic balance performance (Limits of Stability: movement velocity, maximum excursion, directional control) Secondary outcomes: sensory organisation test, PDQ‐39, Falls Efficacy Scale‐International, Timed Up and Go Test |
|
| Notes | The authors declared no potential conflicts of interest. Funding source: National Science Council (NSC 100‐2314‐B‐010‐022‐MY2) and Aim for the Top University Plan (101AC‐P508) of the Ministry of Education of the Republic of China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No intention‐to‐treat analysis, limited dropout |
| Selective reporting (reporting bias) | Unclear risk | Protocol not publicly available |
Pedreira 2013.
| Methods | Single‐blinded RCT | |
| Participants | 44 people with Parkinson’s disease Inclusion criteria: clinical diagnosis of PD, H&Y I to III, 45 to 80 years old Exclusion criteria: dementia, uncontrolled hypertension, heart disease, psychiatric disorders |
|
| Interventions | Experimental group (EG: n = 22); control group (CG: n = 22) 3 times per week/4 weeks EG + CG: 10 min warmup EG: 40 min Nintendo Wii therapy CG: 40 min conventional physiotherapy including trunk and limb mobilisation, balance, muscle strengthening, rhythmic movement, postural alignment, dual task, bimanual, cardiorespiratory, and gait |
|
| Outcomes | Outcomes recorded at baseline, 4 weeks' follow‐up Primary outcome: UPDRS, PDQ‐39 |
|
| Notes | Funding source: Brazilian National Institutes of Science and Technology (CITECS‐MCT‐CNPq) Registered on ClinicalTrials.gov (identifier: NCT01120392) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Computerised randomisation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No intention‐to‐treat analysis, large dropout |
| Selective reporting (reporting bias) | Unclear risk | Protocol not publicly available |
Pompeu 2012.
| Methods | Parallel, prospective, single‐blinded RCT | |
| Participants | 32 people with Parkinson’s disease Inclusion criteria: clinical diagnosis of PD, H&Y I to II, stable medication use, 60 to 85 years old, 5 to 15 years of education, good visual and auditory acuity Exclusion criteria: history of other neurological or orthopaedic diseases, dementia (MMSE < 24), depression (GDS‐15 > 6) |
|
| Interventions | Experimental group (EG: n = 16); control group (CG: n = 16) 2 times per week/7 weeks (EG: 1 additional session at 60 days' follow‐up) EG + CG: 10 min warming, stretching, and active exercises, 10 min resistance training limbs, 10 min trunk, neck, and limbs EG: additional 30 min Wii Fit balance board therapy including static balance, dynamic balance, stationary gait CG: additional 30 min conventional physiotherapy including static balance, dynamic balance, stationary gait |
|
| Outcomes | Outcomes recorded at baseline, postintervention. and 60 days' follow‐up Primary outcome: activities of daily living (UPDRS‐II) Secondary outcomes: Berg Balance Scale, Unipedal Stance Test (single task + dual task with verbal fluency), Montreal Cognitive Assessment |
|
| Notes | The authors declared no potential conflicts of interest. Funding source: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing names |
| Allocation concealment (selection bias) | Low risk | Drawing names |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout |
| Selective reporting (reporting bias) | Unclear risk | Protocol not publicly available |
Shen 2014 ‐ 2015.
| Methods | Single‐blinded RCT | |
| Participants | 51 people with Parkinson’s disease Inclusion criteria: clinical diagnosis of PD, independent walking for 10 m, stable medication, MMSE ≥ 24 Exclusion criteria: history of other neurological or cardiovascular diseases, vision deficits, musculoskeletal disorders affecting balance or locomotion |
|
| Interventions | Experimental group (EG: n = 26); control group (CG: n = 25) 3 times per week/4 weeks laboratory based + 5 times per week/4 weeks home based + 3 times per week/4 weeks laboratory based EG: Laboratory based: 15 min computerised dancing system (KSD Technology Co, Ltd, Shenzhen, China), 15 min use of SMART EquiTest Balance Master (NeuroCom International Inc, Clackamas, OR), 30 min gait training Home based: 20 min exercise of fall‐prone activities CG: Laboratory based: 60 min strength training and stepping exercises Home based: 20 min stepping and walking exercises |
|
| Outcomes | Outcomes recorded at baseline, postintervention, 3 months' follow‐up, 12 months' follow‐up Primary outcome measures: Activities‐specific Balance Confidence Scale, number of fallers, fall rate, time to first fall Secondary outcome measures: Limits of Stability, Single‐Leg Stance Test, self selected walking (gait velocity, stride length), Motor Control Test (NeuroCom) |
|
| Notes | The authors declared no potential conflicts of interest. Funding source: SK Yee Medical Foundation (5‐ZH61) and Hong Kong Parkinson’s Disease Foundation (5‐ZH76) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing lots |
| Allocation concealment (selection bias) | Low risk | Drawing lots |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not publicly available |
van den Heuvel 2014.
| Methods | RCT | |
| Participants | 33 people with Parkinson’s disease Inclusion criteria: clinical diagnosis of PD, H&Y II to III, able to participate Exclusion criteria: history of other neurological, orthopaedic, or cardiopulmonary diseases, MMSE < 24, unstable medication, vision deficits, language problems |
|
| Interventions | Experimental group (EG: n = 17); control group (CG: n = 16) 2 times per week/5 weeks EG: 60 min commercially available interactive dynamic balance exercises (Motek Medical, Amsterdam, the Netherlands) including body lean, stepping, and sit‐to‐stand CG: 60 min conventional balance training including one‐leg stance, dual tasks, stepping exercises, sit‐to‐stand, balancing beam |
|
| Outcomes | Outcomes recorded at baseline, postintervention, 6 weeks' follow‐up Primary outcome: Functional Reach Test Secondary outcomes: Berg Balance Scale, Single‐Leg Stance Test, 10‐Metre Walk Test, UPDRS I, II, III, and IV, Falls Efficacy Scale, PDQ‐39, Hospital Anxiety and Depression Scale, Multidimensional Fatigue Inventory |
|
| Notes | The authors declared no potential conflicts of interest. Funding source: Stichting ParkinsonFonds Registered as an International Standard Randomised Controlled Trial under IS‐RCTN47046299 Protocol paper van den Heuvel 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Opaque, sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analyses performed. |
| Selective reporting (reporting bias) | Low risk | Study protocol available |
Yang 2015.
| Methods | RCT | |
| Participants | 23 people with Parkinson’s disease Inclusion criteria: clinical diagnosis of PD, H&Y II to III, 55 to 85 years old, not engaged in balance or gait training in past 6 months, MMSE > 24 Exclusion criteria: untreated medical conditions affecting balance or gait, depression, vision or auditory deficits |
|
| Interventions | Experimental group (EG: n = 11); control group (CG: n = 12) 2 times per week/6 weeks EG: customised VR balance board therapy including 10 min warming up, 30 min static posture and dynamic weight shifting, 2 x 5 min breaks CG: conventional balance training including 10 min warming up, 30 min static posture and dynamic weight shifting, 2 x 5 min breaks |
|
| Outcomes | Outcomes recorded at baseline, postintervention, and 2 weeks' follow‐up Primary outcome: Berg Balance Scale Secondary outcomes: Dynamic Gait Index, Timed Up and Go Test, PDQ‐39, UPDRS‐III |
|
| Notes | Funding source: National Science Council of Taiwan (Grant No: NSC 97‐2314‐B‐002‐009‐MY3) Registered on ClinicalTrials.gov (identifier: NCT01301651) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation algorithm MATLAB |
| Allocation concealment (selection bias) | Low risk | Randomisation algorithm MATLAB |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not publicly available |
Yen 2011.
| Methods | Prospective, single‐blinded RCT | |
| Participants | 42 people with Parkinson’s disease Inclusion criteria: clinical diagnosis of PD, H&Y II to III, not engaged in balance or gait training, MMSE > 24 Exclusion criteria: history of other neurological, cardiovascular, or orthopaedic diseases, on‐off motor fluctuations, dyskinesia > 3 on UPDRS |
|
| Interventions | Experimental group (EG: n = 14); active control group (TE: n = 14); passive control group (CG: n = 14) 2 times per week/6 weeks EG: customised VR balance board therapy including 10 min stretching, 20 min balance training TE: conventional balance training including 10 min stretching, 20 min balance training |
|
| Outcomes | Outcomes recorded at baseline, postintervention, and 4 weeks' follow‐up Primary outcomes: Sensory Organization Test (equilibrium scores, sensory ratios), auditory arithmetic subtraction task (verbal reaction time), dual task combination of both |
|
| Notes | Funding source: National Science Council of Taiwan (Grant No: NSC 97‐2314‐B‐002‐009‐MY3) Registered on ClinicalTrials.gov (identifier: NCT01301651) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing assignment card (age stratified) |
| Allocation concealment (selection bias) | Low risk | Drawing assignment card (age stratified) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not publicly available |
GDS‐15: 15‐Item Geriatric Depression Scale H&Y: Hoehn and Yahr MMSE: Mini‐Mental State Examination PD: Parkinson's disease PDQ‐39: 39‐Item Parkinson’s Disease Questionnaire RCT: randomised controlled trial UPDRS: Unified Parkinson’s Disease Rating Scale VR: virtual reality
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albani 2009 | No RCT training |
| Alvarez 2010 | No control group ‐ no RCT |
| Alvarez 2012 | No control group ‐ no RCT |
| Assad 2011 | Development of VR ‐ no RCT |
| Barbour 2014 | No control group ‐ no RCT |
| Casserly 2011 | No RCT training |
| dos Santos Mendes 2011 | PD versus healthy control group ‐ no RCT |
| dos Santos Mendes 2012‐1 | No control group ‐ no RCT |
| dos Santos Mendes 2012‐2 | PD versus healthy control group ‐ no RCT |
| dos Santos Mendes 2012‐3 | No control group ‐ no RCT |
| Esculier 2012 | PD versus healthy control group ‐ no RCT |
| Esculier 2014 | PD versus healthy control group ‐ no RCT |
| Gonçalves 2013 | PD+FOG versus PD‐FOG ‐ no RCT |
| Gonçalves 2014 | No control group ‐ no RCT |
| Herz 2013 | No control group ‐ no RCT |
| Holmes 2013 | No control group ‐ no RCT |
| Lefaivre 2015 | No control group ‐ no RCT |
| Loureiro 2012‐1 | No control group ‐ no RCT |
| Loureiro 2012‐2 | No control group ‐ no RCT |
| Ma 2011 | Focus on upper limb function |
| Mey 2010 | No control group ‐ no RCT |
| Mhatre 2013 | No control group ‐ no RCT |
| Milman 2014 | No control group ‐ no RCT |
| Mirelman 2010 | No control group ‐ no RCT |
| Mirelman 2011 | No control group ‐ no RCT |
| Palacios‐Navarro 2015 | No control group ‐ no RCT |
| Pendt 2011 | PD versus healthy control group ‐ no RCT |
| Pompeu 2014 | No control group ‐ no RCT |
| Rochester 2013 | No control group ‐ no RCT |
| Shema 2014 | No control group ‐ no RCT |
| Silva 2013 | No control group ‐ no RCT |
| Summa 2013 | No control group ‐ no RCT |
| Toledo 2011 | No control group ‐ no RCT |
| Zettergren 2011 | Case study ‐ no RCT |
| Zimmermann 2014 | Focus on cognitive performance |
| çömük 2013 | No control group ‐ no RCT |
PD: Parkinson's disease PD+FOG: Parkinson's disease with freezing of gait PD‐FOG: Parkinson's disease without freezing of gaitRCT: randomised controlled trial VR: virtual reality
Characteristics of studies awaiting assessment [ordered by study ID]
Caetano 2016.
| Methods | RCT |
| Participants | 46 people with Parkinson's disease Hoehn and Yahr stage I‐III |
| Interventions | 3 times per week/12 weeks Experimental Group: home‐based step game Control Group: no intervention |
| Outcomes | Outcomes were assessed at baseline and immediately following intervention Usual walking speed, obstacle avoidance, short stepping target, long stepping target, no target/obstacle |
| Notes | ‐ |
Lee 2016.
| Methods | RCT |
| Participants | 36 people with Parkinson's disease |
| Interventions | Experimental group (EG: n=18); Control group (CG: n=18) 3 times per week/8 weeks EG: Wii balance exercise CG: no intervention |
| Outcomes | Outcomes were assessed at baseline and immediately following intervention. Sensory Organizing Test |
| Notes | ‐ |
Liao 2015‐2.
| Methods | RCT |
| Participants | 36 people with Parkinson's disease Hoehn and Yahr stage I‐III |
| Interventions | Experimental group (EG: n=12); active control group (ACG: n=12); passive control group (PCS: n=12) 2 times per week/6 weeks EG + ACG: treadmill training EG: Wii Fit exercise ACG: conventional physiotherapy PCG: fall‐prevention education |
| Outcomes | Outcomes recorded at baseline, immediately after training, and at 1 month follow‐up Lower extremity muscle strength, sensory integration ability, walking velocity, stride length, functional gait assessment |
| Notes | ‐ |
Loureiro 2010.
| Methods | RCT |
| Participants | 12 people with Parkinson's disease Hoehn and Yahr stage II or III |
| Interventions | Experimental group (EG: n = 6); control group (CG: n = 6) 2 times per week/6 weeks EG + CG: conventional physiotherapy EG: additional Wii Fit exercise |
| Outcomes | Timed Up and Go Test and Anterior Functional Reach Test |
| Notes | ‐ |
Piemonte 2011.
| Methods | RCT |
| Participants | 20 people with Parkinson's disease Hoehn and Yahr stage I or II |
| Interventions | Experimental group (EG); control group (CG) 2 times per week/7 weeks EG + CG: 30 minutes of general mobility exercises EG: Wii Fit Plus (10 tasks) CG: motor training |
| Outcomes | Outcomes recorded at baseline, immediately after training, 30 days' and 60 days' follow‐up. Unified Parkinson’s Disease Rating Scale, Montreal Cognitive Assessment, gait performance during single task and dual task, functional gait performance |
| Notes | ‐ |
Pompeu 2012‐2.
| Methods | RCT |
| Participants | 32 people with Parkinson's disease Hoehn and Yahr stage I or II |
| Interventions | Experimental group (EG); control group (CG) 14 sessions of 60 minutes EG: 30 minutes global exercises + 30 minutes VR training CG: 30 minutes global exercises + 30 minutes specific training (no VR) |
| Outcomes | Outcomes were recorded at baseline, immediately after training, and at 60 days' follow‐up. Primary outcome: gait distance in 30 seconds during single task and dual task Secondary outcome: Dynamic Gait Index |
| Notes | ‐ |
Pompeu 2016.
| Methods | RCT |
| Participants | 15 people with Parkinson's disease Hoehn and Yahr stage I‐III |
| Interventions | Experimental group (EG: n=8); control group (VG: n=7) 2 times per week/7 weeks EG: Kinect Adventures‐based training CG: conventional physiotherapy |
| Outcomes | Outcomes were assessed at baseline, immediately following intervention, and at 30 days of follow‐up Limits of Stability, Balance Functional Reserve (eyes open + eyes closed), PDQ‐39, Montreal Cognitive Assessment |
| Notes | ‐ |
Shih 2011.
| Methods | RCT |
| Participants | 29 people with Parkinson's disease Hoehn and Yahr stage III |
| Interventions | Experimental group (EG: n = 15); control group (CG: n = 14) 2 times per week/6 weeks ‐ 40 minutes per session |
| Outcomes | Outcomes were recorded at baseline, immediately after training, and at 2 weeks' follow‐up. Pressure distribution of static and dynamic sitting balance, Trunk Impairment Scale, Modified Functional Reach Test, Berg Balance Scale, Timed Up and Go Test, 39‐Item Parkinson's Disease Questionnaire |
| Notes | ‐ |
Shih 2016.
| Methods | RCT |
| Participants | 20 people with Parkinson's disease Hoehn and Yahr stage I‐III |
| Interventions | Experimental group (EG: n=10); Control group (CG: n=10) 8 weeks EG: balance‐based exergaming CG: conventional balance training |
| Outcomes | Outcomes were assessed at baseline and immediately following intervention. Limits of Stability, One‐leg stance, Berg Balance Scale, Timed Up and Go Test |
| Notes | ‐ |
Van Wegen 2015.
| Methods | RCT |
| Participants | 33 people with Parkinson's disease |
| Interventions | Experimental group (EG: n=17); Control group (CG: n=16) 10 sessions of 60 minutes each EG: augmented visual feedback CG: conventional physiotherapy |
| Outcomes | Outcomes were recorded at baseline, six weeks, and 12 weeks follow‐up. Primary outcome: Functional Reach Test. |
| Notes | ‐ |
Özgönenel 2016.
| Methods | RCT |
| Participants | 33 people with Parkinson's disease Hoehn and Yahr stage I‐III |
| Interventions | Experimental group (EG: n=15); control group (CG: n=18) 3 times per week/5 weeks EG + CG: posture, balance and stretching exercise + electrotherapy EG: Xbox exercise |
| Outcomes | Outcomes were assessed at baseline and immediately post intervention Timed Up and Go Test, Berg Balance Scale, Unified Parkinson's Disease Rating Scale II |
| Notes | ‐ |
RCT: randomised controlled trial VR: virtual reality
Characteristics of ongoing studies [ordered by study ID]
Mirelman 2013.
| Trial name or title | A treadmill training program augmented by virtual reality to decrease fall risk in older adults (V‐TIME) |
| Methods | RCT |
| Participants | 100 people with Parkinson's disease Inclusion criteria: clinical diagnosis of PD, 60 to 85 years old, 2 or more falls 6 months prior to study, stable medication, independent walking, MMSE ≥ 24 Exclusion criteria: history of stroke; traumatic brain injury; rheumatic, orthopaedic, or other neurological diseases; psychiatric comorbidity; acute lower back or lower extremity pain; vision or auditory deficits; interfering therapy |
| Interventions | Experimental group (EG: n = 50); control group (CG: n = 50) 3 times per week/6 weeks EG: treadmill training with virtual reality – obstacle negotiation, progression will include duration, walking speed, orientation, size, frequency of appearance, and shape of the targets CG: treadmill training, progression will include increasing duration and walking speed |
| Outcomes | Outcomes recorded at baseline, postintervention, 1 and 6 months' follow‐up Primary outcome: fall rate Secondary outcomes: gait (usual walking, fast walking, dual task, obstacle negotiation), computerised neuropsychological test battery, Montreal Cognitive Assessment, Trail Making Test, Verbal Fluency Test, Four Square Step Test, Short Physical Performance Battery, Mini‐Balance Evaluation Systems Test, Physical Activity Scale for the Elderly, SF‐36, Falls Efficacy Scale‐International, 7‐days triaxial accelerometer measurement |
| Starting date | January 2013 |
| Contact information | Anat Mirelman: anatmi@tasmc.health.gov.il |
| Notes | Registered on ClinicalTrials.gov (NCT01732653) Protocol paper Mirelman 2013 |
Straudi 2015.
| Trial name or title | Feasibility and effectiveness of virtual reality & use of body weight support treadmill training in Parkinson’s disease |
| Methods | Single‐blinded RCT |
| Participants | 20 people with Parkinson’s disease Inclusion criteria: clinical diagnosis of PD, H&Y II to III, age under 80 years, MMSE ≥ 24 Exclusion criteria: history of other neurological diseases affecting motor function, severe levodopa dyskinesia, pregnancy |
| Interventions | Experimental group (EG); control group (CG) 3 times per week/4 weeks EG: 30 min virtual reality (Xbox Kinect), 30 min treadmill training CG: 60 min conventional physiotherapy |
| Outcomes | Outcomes recorded at baseline, postintervention, 12 weeks' follow‐up Primary outcome: Six‐Minute Walk Test Secondary outcomes: Berg Balance Scale, Timed Up and Go Test, 10‐Metre Walk Test, UPDRS, postural sway (center of pressure), near infrared spectroscopy |
| Starting date | February 2015 |
| Contact information | Sofia Straudi: s.straudi@ospfe.it |
| Notes | Registered on ClinicalTrials.gov (NCT02516644) |
van der Kolk 2015.
| Trial name or title | Park‐in‐Shape study: a phase II double blind randomised controlled trial evaluating the effects of exercise on motor and non‐motor symptoms in Parkinson’s disease |
| Methods | RCT |
| Participants | 130 people with Parkinson’s disease Inclusion criteria: clinical diagnosis of PD, H&Y I to II, 30 to 75 years old, sedentary lifestyle, stable medication, MMSE ≥ 24 Exclusion criteria: history of other neurological, psychiatric, mellitus, pulmonary, or orthopaedic diseases, no Internet at home, unavailability |
| Interventions | Experimental group (EG: n = 65); control group (CG: n = 65) 3 times per week/6 months EG: 30 to 45 min aerobic exercise equipped with gaming elements CG: 30 to 45 min non‐aerobic intervention including stretching, flexibility, and relaxation exercises |
| Outcomes | Outcomes recorded at baseline and postintervention Primary outcome: MDS‐UPDRS motor score (Off) Secondary outcomes: MDS‐UPDRS (On), Mini‐Balance Evaluation Systems Test, Timed Up and Go Test, Dexterity device of Objective Parkinson's Disease Measurement system, falls and near‐falls, Montreal Cognitive Assessment scale, Test of Attentional Performance, Trail Making Test A and B, Hamilton Anxiety and Depression Scale, Scales for Outcomes in Parkinson’s Disease–Sleep and Gastrointestinal, Fatigue Severity Scale, PDQ‐39, Six‐Minute Walk Test, therapy adherence |
| Starting date | ‐ |
| Contact information | Bas R Bloem: bas.bloem@radboudumc.nl |
| Notes | Registered on trialregister.nl (NTR4743) Protocol paper van der Kolk 2015 |
Whyatt 2015.
| Trial name or title | The Nintendo Wii as a balance rehabilitation tool for people with Parkinson’s disease: a preliminary home‐based study |
| Methods | RCT |
| Participants | 28 people with Parkinson’s disease Inclusion criteria: clinical diagnosis of PD, MMSE ≥ 24 Exclusion criteria: vision or auditory deficits |
| Interventions | Experimental group (EG: n = 19); control group (CG: n = 9) 6 weeks, participant chooses frequency and duration EG: Wii Sports and Wii Fit balance board therapy including |
| Outcomes | Outcomes recorded at baseline, postintervention. Primary outcomes: Limits of Stability (NeuroCom Balance Master), static balance, dynamic balance Secondary outcomes: activity diary, questionnaire, focus group |
| Starting date | |
| Contact information | ‐ |
| Notes | Ahead of print |
H&Y: Hoehn and Yahr MDS‐UPDRS: Movement Disorder Society‐Unified Parkinson’s Disease Rating Scale MMSE: Mini‐Mental State Examination PD: Parkinson's disease PDQ‐39: 39‐Item Parkinson’s Disease Questionnaire RCT: randomised controlled trial SF‐36: 36‐Item Short Form Health Survey UPDRS: Unified Parkinson’s Disease Rating ScaleVR: virtual reality
Differences between protocol and review
We performed no subgroup analyses according to participant characteristics (e.g. disease severity) or type of intervention (customised versus commercial VR exercise) due to the low number of included randomised controlled trials. Also, follow‐up analyses were not feasible due to the great diversity in study methodology.
Due to the nature of the interventions, blinding of participants and personnel was deemed not appropriate. This item was therefore not included in the 'Risk of bias' assessment.
Contributions of authors
Kim Dockx was involved in the design, data collection, ‘Risk of bias’ assessment, data extraction, data analysis, and writing of the review. Esther MJ Bekkers was involved in data collection, ‘Risk of bias’ assessment, and data extraction. Veerle Van den Bergh and Pieter Ginis were involved in data collection. Lynn Rochester, Jeffrey M Hausdorff, and Anat Mirelman were involved in interpretation of the results and commented on drafts of the review. Alice Nieuwboer was involved in interpretation of the results, commenting on drafts of the review, and was responsible for overall management and co‐ordination.
Sources of support
Internal sources
No sources of support supplied
External sources
European Commission (FP7 project V‐TIME‐278169), Israel.
European Commission (FP7 project CuPiD‐288516), Italy.
Declarations of interest
Kim Dockx, Esther MJ Bekkers, Lynn Rochester, Jeffery M Hausdorff, Anat Mirelman, and Alice Nieuwboer are involved in the V‐TIME study, which investigates the effectiveness of a VR walking intervention to improve mobility and reduce falls among older people.
Veerle Van den Bergh and Pieter Ginis have no declarations of interest.
New
References
References to studies included in this review
Lee 2015 {published data only}
- Lee NY, Lee DK, Song HS. Effect of virtual reality dance exercise on the balance, activities of daily living, and depressive disorder status of Parkinson's disease patients. Journal of Physical Therapy Science 2015;27:145‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liao 2015 {published data only}
- Liao YY, Yang YR, Cheng SJ, Wu YR, Fuh JL, Wang RY. Virtual reality‐based training to improve obstacle‐crossing performance and dynamic balance in patients with Parkinson's disease. Neurorehabilitation and Neural Repair 2015;29(7):658‐67. [DOI] [PubMed] [Google Scholar]
Pedreira 2013 {published data only}
- Pedreira G, Prazeres A, Cruz D, Gomes I, Monteiro L, Melo A. Virtual games and quality of life in Parkinson's disease: a randomised controlled trial. Advances in Parkinson's Disease 2013;2, No.4:97‐101. [Google Scholar]
Pompeu 2012 {published data only}
- Pompeu JE, dos Santos Mendes FA, Silva KG, Lobo AM, Paula Oliveira T, Zomignani AP, et al. Effect of Nintendo Wii‐based motor and cognitive training on activities of daily living in patients with Parkinson's disease: a randomised clinical trial. Physiotherapy 2012;98:196‐204. [DOI] [PubMed] [Google Scholar]
Shen 2014 ‐ 2015 {published data only}
- Shen X, Mak MKY. Balance and gait training with augmented feedback improves balance confidence in people with Parkinson's disease: a randomized controlled trial. Neurorehabilitation and Neural Repair 2014;28 (6):524‐35. [DOI] [PubMed] [Google Scholar]
- Shen X, Mak MKY. Technology‐assisted balance and gait training reduces falls in patients with Parkinson's disease: a randomised controlled trial with 12 month follow‐up. Neurorehabilitation and Neural Repair 2015;29:103‐11. [DOI] [PubMed] [Google Scholar]
van den Heuvel 2014 {published data only}
- Heuvel MRC, Kwakkel G, Beek PJ, Berendse HW, Daffertshofer A, Wegen EEH. Effects of augmented visual feedback during balance training in Parkinson's disease: a pilot randomised clinical trial. Parkinsonism and Related Disorders 2014;20:1352‐8. [DOI] [PubMed] [Google Scholar]
Yang 2015 {published data only}
- Yang WC, Wang HK, Wu RM, Lo CS, Lin KH. Home‐based virtual reality balance training and conventional balance training in Parkinson's disease: a randomised controlled trial. Journal of the Formosan Medical Association 2015;xx:1‐10. [DOI] [PubMed] [Google Scholar]
Yen 2011 {published data only}
- Yen CY, Lin KH, Hu MH, Wu RM, Lu TW, Lin CH. Effects of virtual reality‐augmented balance training on sensory organization and attentional demand for postural control in people with Parkinson's disease: a randomised controlled trial. Physical Therapy 2011;91:862‐74. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Albani 2009 {published data only}
- Albani G, Menegoni F, Trotti C, Mauro A. Balance in virtual reality in Parkinson's disease: a preliminary study. Journal of Neurology 2009;256 Suppl 2:S144. [Google Scholar]
Alvarez 2010 {published data only}
- Alvarez MV, Grogan PM, Rodriguez M. Ninendo Wii: The role of a youthful game for an aging disease. Movement Disorders 2010;25 Suppl 2:S254‐5. [Google Scholar]
Alvarez 2012 {published data only}
- Alvarez MV, Grogan PM, Rodriguez M. Connecting with Kinect to improve motor and gait function in Parkinson's disease. Movement Disorders 2012;27 suppl 1:S297. [Google Scholar]
Assad 2011 {published data only}
- Assad O, Hermann R, Lilla D, Mellies B, Meyer R, Shevach L, et al. Motion‐based games for Parkinson's disease patients. Parkinsonism & Related Disorders 2013;19:1039‐42. [Google Scholar]
Barbour 2014 {published data only}
- Barbour PJ, Kerstetter A, Tremblay S, Danni A, Hammer I, Weiss MJ. Pilot study to assess benefit of virtual reality game system, wii, on balance and gait in persons with Parkinson's disease. Movement Disorders 2014;29 Suppl 1:S227‐8. [Google Scholar]
Casserly 2011 {published data only}
- Casserly C, Vu Cic V, Kapp G, Jog R, Katchabaw M, Kumar H, et al. Strategy dysfunction in Parkinson's disease in a virtual reality based navigation task. Movement Disorders 2011;26 Suppl 2:S147‐8. [Google Scholar]
çömük 2013 {published data only}
- çömük BN, Tonga E, Gülsen M. The effect of balance training by tetraks interactive balance system on balance and fall risk in Parkinson's patients: a report of four cases. Noropslklyatrl Arslvl 2013;50:3:283‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
dos Santos Mendes 2011 {published data only}
- dos Santos Mendes FA, Pompeu J, Lobo A, Silva K, Piemonte M. Virtual environments and Parkinson's disease: a novel strategy for improving automatic motor control. Physiotherapy 2011;97 Suppl 1:eS801. [Google Scholar]
dos Santos Mendes 2012‐1 {published data only}
- dos Santos Mendes FA, Pegollo F, Pompeu JE, Alencar R, Avanzi R. Use of electronic games as an entertaining and low cost tool for the treatment of patients with Parkinson's disease. Movement Disorders 2012;27 Suppl 1:S301. [Google Scholar]
dos Santos Mendes 2012‐2 {published data only}
- dos Santos Mendes FA, Pompeu JE, Modenesi Lobo A, Guedes de Silva K, Paula Oliveira T, Peterson Zomignani A, et al. Motor learning, retention and transfer after virtual‐reality based training in Parkinson's disease ‐ effect of motor and cognitive demands of games: a longitudinal, controlled clinical study. Physiotherapy 2012 Sep;98 (3):217‐23. [DOI] [PubMed] [Google Scholar]
dos Santos Mendes 2012‐3 {published data only}
- dos Santos Mendes FA, Pegollo F, Alencar R, Avanzi R, Pompeu JE. A new tool for assessment and balance training of patients with Parkinson's disease based on low cost commercial Wii balance board. Movement Disorders 2012;27 Suppl 1:898. [Google Scholar]
Esculier 2012 {published data only}
- Esculier JF, Vaudrin J, Bériault P, Gagnon K, Tremblay LE. Home‐based balance training programme using Wii Fit with balance board for Parkinson's disease: a pilot study. Journal of Rehabilitation Medicine 2012 Feb;44(2):144‐50. [DOI] [PubMed] [Google Scholar]
Esculier 2014 {published data only}
- Esculier JF, Vaudrin J, Tremblay LE. Corticomotor excitability in Parkinson's disease during observation, imagery and imitation of action: effects of rehabilitation using Wii Fit and comparison to healthy controls. Journal of Parkinson's Disease 2014;4(1):67‐75. [DOI] [PubMed] [Google Scholar]
Gonçalves 2013 {published data only}
- Gonçalves GB. Using the Nintendo Wii Fit Plus platform in the sensorimotor training of freezing of gait in Parkinson's disease. Arquivos de Neuro‐Psiquiatria 2013;71:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonçalves 2014 {published data only}
- Gonçalves GB, Leite MA, Orsini M, Pereira JS. Effects of using the Nintendo Wii Fit Plus platform in the sensorimotor training of gait disorders in Parkinson's disease. Neurology International 2014 Jan;17;6(1):5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herz 2013 {published data only}
- Herz NB, Mehta SH, Sethi KD, Jackson P, Hall P, Morgan JC. Nintendo Wii rehabilitation ("Wii‐hab") provides benefits in Parkinson's disease. Parkinsonism & Related Disorders 2013 Nov;19(11):1039‐42. [DOI] [PubMed] [Google Scholar]
Holmes 2013 {published data only}
- Holmes JD, Gi ML, Johnson AM, Jenkins ME. The effects of a home‐based virtual reality rehabilitation program on balance among individuals with Parkinson's disease. Physical & Occupational Therapy in Geriatrics 2013;31(3):241‐53. [Google Scholar]
Lefaivre 2015 {published data only}
- Lefaivre SC, Almeida QJ. Can sensory attention focused exercise facilitate the utilization of proprioception for improved balance control in PD?. Gait and Posture 2015 Feb;41(2):630‐3. [DOI] [PubMed] [Google Scholar]
Loureiro 2012‐1 {published data only}
- Loureiro APC, Ribas CG, Zotz TGG, Chen R, Ribas F. Feasibility of virtual therapy in rehabilitation of Parkinson's disease patients: pilot study. Fisioterapia em movimento 2012 Sep;25(3). [Google Scholar]
Loureiro 2012‐2 {published data only}
- Loureiro APC, Chen R, Stori FR, Ribas CG, Zotz TG. Using interactive virtual rehabilitation for improvement of balance in people with Parkinson's disease: a pilot study. Movement Disorders 2012;27 Suppl 1:S127‐8. [Google Scholar]
Ma 2011 {published data only}
- Ma HI, Hwang WJ, Fang JJ, Kuo JK, Wang CY, Leong IF, et al. Effects of virtual reality training on functional reaching movements in people with Parkinson's disease: a randomised controlled pilot trial. Clinical Rehabilitation 2011 Oct;25(10):892‐902. [DOI] [PubMed] [Google Scholar]
Mey 2010 {published data only}
- Mey M, Herzing K, Braun SM, Rothgangel AS. Virtual rehabilitation in Parkinson's disease: use of a video game console in the physiotherapy treatment. Zeitschrift fur Physiotherapeuten 2010 Jul;62(7):6‐12. [Google Scholar]
Mhatre 2013 {published data only}
- Mhatre PV, Vilares I, Stibb SM, Albert MV, Pickering L, Marciniak CM, et al. Wii Fit balance board playing improves balance and gait in Parkinson disease. Physical Medicine and Rehabilitation 2013 Sep;5(9):769‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milman 2014 {published data only}
- Milman U, Atias H, Weiss A, Mirelman A, Hausdorff JM. Can cognitive remediation improve mobility in patients with Parkinson's disease? Findings from a 12 week pilot study. Journal of Parkinson's Disease 2014;4(1):37‐44. [DOI] [PubMed] [Google Scholar]
Mirelman 2010 {published data only}
- Mirelman A, Maidan I, Jacobs A, Mirelman D, Giladi N, Hausdorff J. Virtual reality for gait training in Parkinson's disease: a feasibility study. Parkinsonism and Related Disorders 2010;16 Suppl 1:S83. [Google Scholar]
Mirelman 2011 {published data only}
- Mirelman A, Maidan I, Herman T, Deutsch JE, Giladi N, Hausdorff JM. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson's disease?. Journal Gerontology Series A Biological Sciences Medical Sciences 2011 Feb;66(2):234‐40. [DOI] [PubMed] [Google Scholar]
Palacios‐Navarro 2015 {published data only}
- Palacios‐Navarro G, García‐Magariño I, Ramos‐Lorente P. A Kinect‐based system for lower limb rehabilitation in Parkinson's disease patients: a pilot study. Journal of Medical Systems 2015 Sep;39(9):103. [DOI] [PubMed] [Google Scholar]
Pendt 2011 {published data only}
- Pendt LK, Reuter I, Müller H. Motor skill learning, retention, and control deficits in Parkinson's disease. PLoS One 2011;6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
Pompeu 2014 {published data only}
- Pompeu JE, Arduini LA, Botelho AR, Fonseca MB, Pompeu SM, Torriani‐Pasin C, et al. Feasibility, safety and outcomes of playing Kinect Adventures! for people with Parkinson's disease: a pilot study. Physiotherapy 2014 Jun;100(2):162‐8. [DOI] [PubMed] [Google Scholar]
Rochester 2013 {published data only}
- Rochester L, Galna B, Olivier P, Jackson D, Barry G, Mhiripiri D, et al. Retraining function with exercise based computer games for people with Parkinson's disease: PD‐kinection. Journal of Parkinson's disease 2013;3 Suppl 1:171‐2. [Google Scholar]
Shema 2014 {published data only}
- Shema SR, Brozgol M, Dorfman M, Maidan I, Sharaby‐Yeshayahu L, Malik‐Kozuch H, et al. Clinical experience using a 5‐week treadmill training program with virtual reality to enhance gait in an ambulatory physical therapy service. Physical Thererapy 2014 Sep;94(9):1319‐26. [DOI] [PubMed] [Google Scholar]
Silva 2013 {published data only}
- Silva FD, Polese JC, Callage Alvarenga LF, Schuster RC. Effect of Wiirehabilitation on trunk mobility of individuals with Parkinson disease: a pilot study. Revista Neurociencias 2013;21:3:364‐8. [Google Scholar]
Summa 2013 {published data only}
- Summa S, Basteris A, Betti E, Sanguineti V. A feasibility study on using Kinect for the rehabilitation in persons with Parkinson's disease. Gait and Posture 2013;37 Suppl 1:S15. [Google Scholar]
Toledo 2011 {published data only}
- Toledo SD, Albert M, Kording K, Marciniak CM, Mhatre PV, Pickering L, et al. Wii video game balance‐board training: does it improve balance and gait in adults with Parkinson disease?. Physical Medicine and Rehabilitation 2011;3:10 Suppl 1:S264. [Google Scholar]
Zettergren 2011 {published data only}
- Zettergren KK, Antunes MS, Canhao JM, Lavallee C. The effects of Nintendo Wii Fit on gait speed, balance and functional mobility on idiopathic Parkinson's disease: a case study. Gerontologist 2011;51:70. [Google Scholar]
Zimmermann 2014 {published data only}
- Zimmermann R, Gschwandtner U, Benz N, Hatz F, Schindler C, Taub E, et al. Cognitive training in Parkinson disease: cognition‐specific vs nonspecific computer training. Neurology 2014 Apr 8;82(14):1219‐26. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Caetano 2016 {published data only}
- Caetano MJD, Menant JC, Canning CG, Song J, Schoene D, Brodie M, et al. Effects of videogame step training on gait adaptability in people with Parkinson's disease: a randomized controlled trial. Movement Disorders 2016;31 Suppl 2:S611. [Google Scholar]
Lee 2016 {published data only}
- Lee GH. Effects of virtual reality exercise program on balance in lower parkinsonism patients. Parkinsonism and Related Disorders 2016;22 Suppl 2:e70. [Google Scholar]
- Lee GH. Virtual‐reality balance training with Nintendo‐Wii games improves dynamic balance in Parkinson's disease patients. Movement Disorders 2016;31 Suppl 2:S656. [Google Scholar]
Liao 2015‐2 {published data only}
- Liao YY, Yang YR, Wu YR, Wang RY. Virtual reality‐based Wii Fit training in improving muscle strength, sensory integration ability, and walking abilities in patients with Parkinson's disease: a randomized control trial. International Journal of Gerontology 2015;9:190‐5. [Google Scholar]
Loureiro 2010 {published data only}
- Loureiro APC, Stori FR, Chen R, Ribas CG, Loureiro CC. Comparative study of conventional physiotherapy and virtual rehabilitation using Wii Fit for improvement of balance in Parkinson's disease patients. Movement Disorders 2010;25 Suppl 3:S714. [Google Scholar]
Özgönenel 2016 {published data only}
- Özgönenel L, çagirici S, çabalar M, Durmusoglu G. Use of game console for rehabilitation of Parkinson's disease. Balkan Medical Journal 2016;33:396‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piemonte 2011 {published data only}
- Pimentel Piemonte ME, Mendes F, Pompeu JE, Lobo AM, Silva KG, Oliveira T, et al. Improvement of gait, functional and cognitive performance in patients with Parkinson's disease after motor and cognitive training. Physiotherapy 2011;97 Suppl 1:eS1002. [Google Scholar]
Pompeu 2012‐2 {published data only}
- Pompeu JE, Mendes FA, Silva KG, Oliveira TP, Lobo AM, Pompeu SMAA, et al. Gait improvement in patients with Parkinson's disease after training in real and virtual environments. Movement Disorders 2012;27 Suppl 1:S134. [Google Scholar]
Pompeu 2016 {published data only}
- Pompeu JE, Silva KG, Freitas TB, Nuvolini RA, Donà F, Torriani‐Pasin C, et al. Effect of European physiotherapy guideline for Parkinson's disease and Microsoft Kinect adventures games training on postural control, cognition and quality of life: randomized clinical trial. Movement Disorders 2016;31 Suppl 2:S184. [Google Scholar]
Shih 2011 {published data only}
- Shih IF, Hu MH, Lu TW, Wu RM, Lin KH. Effects of virtual reality‐augmented sitting balance training on pressure distribution and functional performance in patients with Parkinson's disease. Physiotherapy 2011;97 Suppl 1:eS693‐4. [Google Scholar]
Shih 2016 {published data only}
- Shih MC, Wang RY, Cheng SJ, Yang YR. Effects of a balance‐based exergaming intervention using the Kinect sensor on posture stability in individuals with Parkinson's disease: a single‐blinded randomized controlled trial. Journal of NeuroEngineering and Rehabilitation 2016;13(78). [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Wegen 2015 {published data only}
- Wegen E, Heuvel M, Beek P, Daffertshofer A, Berendse H, Kwakkel G. Balance training with augmented visual feedback in Parkinson's disease: a randomized clinical trial. Physiotherapy 2015;101 Suppl 1:eS1580‐1. [Google Scholar]
References to ongoing studies
Mirelman 2013 {unpublished data only}
- Mirelman A, Rochester L, Reelick M, Nieuwhof F, Pelosin E, Abbruzzese G, et al. V‐TIME: a treadmill training program augmented by virtual reality to decrease fall risk in older adults: study design of a randomized controlled trial. BMC Neurology 2013 feb 6;13(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
Straudi 2015 {unpublished data only}
- Feasibility and effectiveness of virtual reality & use of body weight support treadmill training in Parkinson’s disease. Ongoing study February 2015.
van der Kolk 2015 {unpublished data only}
- Kolk NM, Overeem S, Vries NM, Kessels RPC, Donders R, Brouwer M, et al. Design of the Park‐in‐Shape study: a phase II double blind randomized controlled trial evaluating the effects of exercise on motor and non‐motor symptoms in Parkinson's disease. BMC Neurology 2015;15(56). [DOI] [PMC free article] [PubMed] [Google Scholar]
Whyatt 2015 {unpublished data only}
- The Nintendo Wii as a balance rehabilitation tool for people with Parkinson’s disease: a preliminary home‐based study. Ongoing study Starting date of trial not provided. Contact author for more information.
Additional references
Barry 2014
- Barry G, Galna B, Rochester L. The role of exergaming in Parkinson's disease rehabilitation: a systematic review of the evidence. J Neuroeng Rehabil 2014 Mar 7;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berg 2014
- Berg D, Postuma RB, Bloem B, Chan P, Dubois B, Gasser T, et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson's disease. Movement Disorders 2014 Apr 29;29(4):454‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bisson 2007
- Bisson E, Contant B, Sveistrup H, Lajoie Y. Functional balance and dual‐task reaction times in older adults are improved by virtual reality and biofeedback training. Cyberpsychology & Behavior 2007;10(1):16‐23. [DOI] [PubMed] [Google Scholar]
Bloem 1996
- Bloem BR, Beckley DJ, Dijk JG, Zwinderman AH, Remler MP, Roos RA. Influence of dopaminergic medication on automatic postural responses and balance impairment in Parkinson's disease. Movement Disorders 1996;11(5):509‐521. [DOI] [PubMed] [Google Scholar]
Bohnen 2011
- Bohnen NI, Albin RL. The cholinergic system and Parkinson disease. Behav Brain Res 2011;221:564‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burdea 2003
- Burdea GC. Virtual rehabilitation‐benefits and challenges. Methods Inf Med 2003;42(5):519‐23. [PubMed] [Google Scholar]
Canning 2014
- Canning CG, Paul S, Nieuwboer A. Prevention of falls in Parkinson's disease: a review of fall risk factors and the role of physical interventions. Neurodegenerative Disease Management 2014;4(3):203‐221. [DOI] [PubMed] [Google Scholar]
Corcos 2013
- Corcos DM, Robichaud JA, David FJ, Leurgans SE, Vaillancourt DE, Poon C, et al. A two‐year randomised controlled trial of progressive resistance exercise for Parkinson's disease. Movement Disorders 2013 Aug;28(9):1230‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Curtze 2015
- Curtze C, Nutt JG, Carlson‐Kuhta P, Mancini M, Horak FB. Levodopa is a double‐edged sword for balance and gait in people with Parkinson's disease. Movement Disorders 2015;30(10):1361‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
David 2015
- David FJ, Robichaud JA, Leurgans SE, Poon C, Kohrt WM, Goldman JG, et al. Exercise improves cognition in Parkinson's disease: the PRET‐PD randomised, clinical trial. Movement Disorders 2015 Oct;30(12):1657‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Downs 2013
- Downs S, Marquez J, Chiarelli P. The Berg Balance Scale has high intra‐ and inter‐rater reliability but absolute reliability varies across the scale: a systematic review. Journal of Physiotherapy 2013 Jun;59(2):93‐99. [DOI] [PubMed] [Google Scholar]
Ellis 2013
- Ellis T, Boudreau JK, DeAngelis TR, Brown LE, Cavanaugh JT, Earhart GM, et al. Barriers to exercise in people with Parkinson disease. Phys Ther 2013 May;93(5):628‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 2011
- Fox SH, Katzenschlager R, Lim SY, Ravina B, Seppi K, Coelho M, et al. The Movement Disorder Society Evidence‐Based Medicine Review Update: Treatments for the motor symptoms of Parkinson's disease. Movement Disorders 2011 Oct;23 Suppl 3:S2‐41. [DOI] [PubMed] [Google Scholar]
Goble 2014
- Goble DJ, Cone BL, Fling BW. Using the Wii Fit as a tool for balance assessment and neurorehabilitation: the first half decade of "Wii‐search". J Neuroeng Rehab 2014;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hausdorff 2009
- Hausdorff JM. Gait dynamics in Parkinson's disease: Common and distinct behavior among stride length, gait variability, and fractal‐like scaling. Chaos 2009 Jun;19(2):026113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from handbook.cochrane.org.
Hirsch 2009
- Hirsch MA, Farley BG. Exercise and neuroplasticity in persons living with Parkinson's disease. Eur J Phys Rehabil Med 2009 Jun;45(2):215‐29. [PubMed] [Google Scholar]
Horak 2009
- Horak FB, Wrisley DM, Frank J. The Balance Evaluation Systems Test (BESTest) to differentiate balance deficits. Phys Ther 2009 May;89(5):484‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jankovic 2008
- Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 2008 Apr;79(4):368‐76. [DOI] [PubMed] [Google Scholar]
Keshner 2004
- Keshner EA. Virtual reality and physical rehabilitation: a new toy or a new research and rehabilitation tool?. J Neuroeng Rehabil 2004 Dec;1(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2012
- King LA, Priest KC, Salarian A, Pierce D, Horak FB. Comparing the Mini‐BESTest with the Berg Balance Scale to evaluate balance disorders in Parkinson's disease. Parkinson's Disease 2012;2012:ID375419. [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2015
- King LA, Wilhelm J, Chen Y, Blehm R, Nutt J, Chen Z, et al. Effects of group, individual, and home exercise in persons with Parkinson Disease: a randomized clinical trial. J Neurol Phys Ther 2015 Oct;39(4):204‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laver 2015
- Laver KE, George S, Thomas S, Deutsch JE, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD008349.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lees 2009
- Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet 2009 Jun 13;373(9680):2055‐66. [DOI] [PubMed] [Google Scholar]
Lewis 2012
- Lewis GN, Rosie JA. Virtual reality games for movement rehabilitation in neurological conditions: how do we meet the needs and expectations of the users?. Disabil Rehabil 2012;34(22):1880‐6. [DOI] [PubMed] [Google Scholar]
McVey 2009
- McVey MA, Stylianou AP, Luchies CW, Lyons KE, Pahwa R, Jernigan S, et al. Early biomechanical markers of postural instability in Parkinson's disease. Gait & Posture 2009;30:538‐542. [DOI] [PubMed] [Google Scholar]
Mirelman 2013‐1
- Mirelman A, Maidan I, Deutsch JE. Virtual reality and motor imagery: promising tools for assessment and therapy in Parkinson's disease. Movement Disorders 2013;28(11):1597‐1608. [DOI] [PubMed] [Google Scholar]
Nonnekes 2013
- Nonnekes J, Kam D, Geurts ACH, Weerdesteyn V, Bloem BR. Unraveling the mechanisms underlying postural instability in Parkinson's disease using dynamic posturography. Expert Rev. Neurother. 2013;13(12):1303‐1308. [DOI] [PubMed] [Google Scholar]
Pringsheim 2014
- Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson's disease: a systematic review and meta‐analysis. Movement Disorders 2014 Nov;29(13):1583‐90. [DOI] [PubMed] [Google Scholar]
Prodoehl 2015
- Prodoehl J, Rafferty MR, David FJ, Poon C, Vaillancourt DE, Comella CL, et al. Two‐year exercise program improves physical function in Parkinson's disease: the PRET‐PD randomised clinical trial. Neurorehabilitation and Neural Repair 2015;29(2):112‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riva 2003
- Riva G. Applications of virtual environments in medicine. Methods Inf Med 2003;42:524‐534. [PubMed] [Google Scholar]
Rizos 2014
- Rizos A, Martinez‐Martin P, Odin P, Antonini A, Kessel B, Kozul TK, et al. Characterizing motor and non‐motor aspects of early‐morning off periods in Parkinson's disease: an international multicenter study. Parkinsonism and Related Disorders 2014 Nov;20(11):1231‐5. [DOI] [PubMed] [Google Scholar]
Schoneburg 2013
- Schoneburg B, Mancini M, Horak F, Nutt JG. Framework for understanding balance dysfunction in Parkinson' disease. Mov Disord 2013 Sep;28(11):1474‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soh 2011
- Soh SE, Morris ME, McGinley JL. Determinants of health‐related quality of life in Parkinson's disease: a systematic review. Parkinsonism Relat Disord 2011 Jan;17(1):1‐9. [DOI] [PubMed] [Google Scholar]
Steffen 2008
- Steffen T, Seney M. Test‐retest reliability and minimal detectable change on balance and ambulation tests, the 36‐item short form health survey, and the Unified Parkinson disease rating scale in people with parkinsonism. Physical Therapy 2008 Jun;6:733‐46. [DOI] [PubMed] [Google Scholar]
Tomlinson 2013
- Tomlinson CL, Patel S, Meek C, Herd CP, Clarke CE, Stowe R, et al. Physiotherapy versus placebo or no intervention in Parkinson's disease. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD002817.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomlinson 2014
- Tomlinson CL, Herd CP, Clark CE, Mee C, Patel S, Stowe R, et al. Physiotherapy for Parkinson's disease: a comparison of techniques. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD002815.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
van den Heuvel 2013
- Heuvel MR, Wegen EE, Goede CJ, Burgers‐Bots IA, Beek PJ, Daffertshofer A, Kwakkel G. The effects of augmented visual feedback during balance training in Parkinson's disease: study design of a randomized clinical trial. BMC Neurology 2013 oct 4;13(137). [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Marck 2013
- Marck MA, Munneke M, Mulleners W, Hoogerwaard EM, Borm GF, Overeem S, et al. Integrated multidisciplinary care in Parkinson's disease: a non‐randomised, controlled trial (IMPACT). Lancet Neurology 2013 Oct;12(10):947‐56. [DOI] [PubMed] [Google Scholar]
van Diest 2013
- Diest M, Lamoth CJC, Stegenga J, Verkerke GJ, Postema K. Exergaming for balance training of elderly: state of the art and future developments. J Neuroeng Rehab 2013;10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Nimwegen 2011
- Nimwegen M, Speelman AD, Hofman‐van Rossum EJ, Overeem S, Deeg DJ, Borm GF, et al. Physical inactivity in Parkinson's disease. J Neurol 2011 Dec;258(12):2214‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Nimwegen 2013
- Nimwegen M, Speelman AD, Overeem S, Warrenburg BP, Smulders K, Dontje ML, et al. Promotion of physical activity and fitness in sedentary patients with Parkinson's disease: randomised controlled trial. BMJ 2013;346:f576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vervoort 2015
- Vervoort G, Bengevoord A, Nackaerts E, Heremans E, Vandenberghe W, Nieuwboer A. Distal motor deficit contributions to postural instability and gait disorder in Parkinson's disease. Behavioral Brain Research 2015;287:1‐7. [DOI] [PubMed] [Google Scholar]
Wu 2015
- Wu T, Hallett M, Chan P. Motor automaticity in Parkinson's disease. Neurobiology of Disease 2015 Oct;82:226‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]


