Abstract
Background
Fine-needle aspiration (FNA) is the most accurate and cost-effective method for evaluating thyroid nodules. However, FNA-induced secondary changes completely replacing thyroid tumors (vanishing tumors) may create a novel problem. In this study, we highlight the diagnostic and management issues associated with the unintended consequences of ultrasonography (US)-guided FNA.
Methods
Fourteen thyroid glands (11 women and 3 men, ages 33–64 years) with vanishing tumors were prospectively identified between 2009 and 2012 upon surgical resection. Cytology and histopathology slides were reviewed, and second opinions were obtained when necessary.
Results
The cytology of the 14 vanishing tumors was suspicious/positive for papillary thyroid carcinoma (PTC) in 5, indeterminate (atypia of unknown significance) in 5, benign in 2, follicular neoplasm in 1, and nondiagnostic in 1 nodule. Upon thyroidectomy, the vanishing tumors ranged in size from 0.4 to 3.5 cm (median 0.7 cm). Microscopically, the nodules showed cystic degeneration, organizing hemorrhage, granulation tissue, fibrosis, and microcalcifications. In seven tumors, a few residual malignant cells (PTC in five) or residual benign follicles (hemorrhagic cyst in two) at the periphery of the vanishing tumors helped with the final diagnosis. The remaining seven tumors were completely replaced by FNA-induced secondary changes, and had the cytology diagnosis of benign in one, follicular neoplasm in one, and suspicious/positive for PTC in five. Of the latter five, two showed additional separate foci of PTC, while three vanishing tumors (0.5, 1.2, and 1.6 cm) had no residual malignant cells and no additional carcinoma leading to a final diagnosis of negative for malignancy.
Conclusions
US-guided FNA may lead to complete obliteration of thyroid nodules, rendering final diagnosis upon thyroidectomy difficult or impossible. In these unusual circumstances, the possibility that the surgical pathology may be nonrepresentative should be considered if the cytologic features on FNA are sufficient by themselves to support a definitive diagnosis of PTC.
Introduction
The American Thyroid Association (ATA) recently revised the management guidelines for thyroid nodules, and recommends ultrasonographic (US) assessment of nodules ≥1 cm in size (1). This has increased the detection of subclinical thyroid nodules by high-resolution US (2). US predictors of thyroid malignancy include hypoechogenicity, coarse, central or peripheral calcification, irregular margins, a taller-than-wide dimension, increased vascularity, lymph node metastasis, and extracapsular extension (1,3). US-guided fine-needle aspiration (FNA) is recommended for nodules with suspicious sonographic characteristics, nodules larger than 1–1.5 cm in size, and in the presence of clinical risk factors for malignancy (4,5).
However, FNA-induced reactive changes in thyroid nodules can be worrisome (6–9) and include atypical nuclei, hemorrhage, infarction, fibrinoid necrosis, fibrosis, cystic degeneration, pseudocapsular invasion, and squamous metaplasia. Rarely, these changes completely or extensively replace a thyroid nodule, so as to render the final surgical pathology diagnosis on thyroidectomy difficult or impossible (vanishing tumors). Should the surgical pathology diagnosis of negative for malignancy rendered in the absence of any residual malignant cells be considered final when the FNA cytology is unequivocally malignant? Here we discuss the management dilemma encountered with these vanishing tumors.
Methods
We defined vanishing tumors as thyroid nodules with at least one FNA before surgery that upon resection showed complete or near-complete replacement by reactive changes with no or minimal residual malignant or benign thyroid follicles. Cases were prospectively identified on thyroidectomies performed between July 2009 and January 2012. All FNAs were performed under US guidance using a 25-gauge needle with two to three passes. All thyroids were entirely submitted for microscopy, and all vanishing tumors were further examined at multiple levels until they were finished (consumed, cut through). When multiple nodules were present within the thyroid, all nodules with worrisome features at the initial section were leveled (at least three levels) until a definitive diagnosis was reached. The cytology slides were sent to an expert endocrine cytopathologist (Z.B.) blinded to the surgical pathology findings, and the histopathology slides were sent to an expert endocrine pathologist (A.K.) blinded to the cytopathology diagnosis to remove any bias. FNA-induced changes were differentiated from spontaneous degenerative changes as follows: (i) reactive changes tended to be in a linear needle-track-like distribution in the former, and irregular, usually associated with larger nodules in the latter; (ii) FNA in the specific nodule was confirmed by the ultrasonographers' drawings designating the sampled nodule (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/thy).
BRAFV600E mutation on four vanishing tumors was analyzed by single-strand conformational polymorphism after extraction of DNA from the needle rinse, and the BRAFV600E region was amplified by PCR, as described elsewhere (10).
Results
Fourteen thyroids (lobectomy 4 and total thyroidectomy 10 [5 with and 5 without central neck dissection]) with vanishing tumors were identified among 1516 thyroid resections (0.9%). The patients were aged 33–68 years (11 women and 3 men). The most common presentation was thyroid nodule. The relevant clinicopathological and US findings are shown in Figure 1 and Table 1.
FIG. 1.
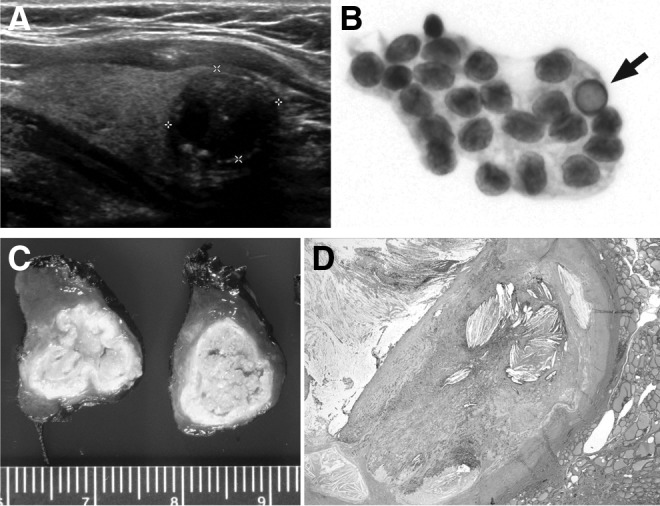
(A, D) Case 6, a 56-year-old woman with incidental thyroid nodule. (A) Ultrasonographic examination showing a 1.6-cm hypoechoic mass of mixed echogenicity with peripheral calcification. (D) Microscopic examination shows a fibrotic encapsulated cyst with its contents replaced by organizing hemorrhage, cholesterol granuloma, and granulation tissue consistent with FNA-induced secondary changes (×2). (B) Case 2, a 50-year-old woman with 0.5-cm nodule: fine-needle aspiration (FNA) showing a sheet of tumor cells of follicular derivation with moderate amount of cytoplasm, large oval nuclei with powdery chromatin, irregular nuclear membrane, nuclear grooves, and an intranuclear inclusion (arrow). (C) Case 3, left thyroid lobe from a 36-year-old woman showing a 1.4-cm encapsulated tumor with wrinkled collapsed capsule and yellow pasty contents. The tumor was subjected to three FNA procedures in the 4 years preceding surgery.
Table 1.
Clinicopathologic Information on Vanishing Tumors
| Surgical pathology | |||||||
|---|---|---|---|---|---|---|---|
| Case | Clinical presentation | US findings | Cytologya | Surgery | Initial pathology | Initial diagnosis | External expert opinion |
| 1 | 56M Right thyroid nodule (on warfarin for pulmonary embolism) |
Right lobe, 3.9-cm nodule in the mid- to lower lobe with a 2.8-cm solid component |
FNA: 1×(3 weeks before surgery) Initial Dx: Nondiagnostic 2nd opinion: Nondiagnostic |
Right lobectomy for rapidly enlarging cyst after FNA |
Gross: 3.5-cm hemorrhagic cyst Microscopy: Hemorrhagic cyst with squamous metaplasia, hemosiderin deposition, granulation tissue, and a minute focus of residual benign follicles |
Hemorrhagic nodule with squamous metaplasia. Remaining thyroid with benign colloid nodules—negative for malignancy | Hemorrhagic nodule with squamous metaplasia. Remaining thyroid with benign colloid nodules—negative for malignancy |
| 2 | 50F Hyperthyroidism and goiter |
Right lobe, 0.5-cm hypoechoic nodule with peripheral and central calcification and extracapsular extension |
FNA: 1×(2 months before surgery) Initial Dx: Suspicious for PTC 2nd opinion: Positive for PTC |
Total thyroidectomy |
Gross: 0.4-cm tan white, cystic nodule Microscopy: Encapsulated cyst; focal ossification and degenerative nuclear atypia |
Encapsulated 0.4-cm cystic nodule—negative for malignancy | Colloid nodule with cystic degeneration, fibrosis, and ossification—negative for malignancy |
| 3 | 36F Long standing left thyroid nodule |
Left lobe, 2-cm indeterminate nodule with calcification |
FNA: 3×(4, 2, and 1.9 years before surgery) Initial Dx: 2004, Minimal colloid and very rare, somewhat atypical follicular cells; 2005, Paucicellular specimen—no evidence of malignancy; 2008, Nondiagnostic 2nd opinion: 2004, Benign; 2005, Nondiagnostic; 2008, Benign |
Left lobectomy |
Gross: 1.4-cm encapsulated nodule with yellow pasty contents Microscopy: Degenerative cyst with calcified/ossified capsule filled with granulation tissue, hemosiderin, cholesterol crystals, and histiocytes |
Infarcted 1.4-cm nodule with hyalinization and focal dystrophic calcification, remaining thyroid parenchyma without significant abnormalities | Hemorrhage, necrosis, granulation tissue, fibrosis, calcification, and ossification—favor benign colloid nodule—negative for malignancy |
| 4 | 53M Right thyroid nodule |
Right lobe, 1-cm indeterminate hypoechoic nodule with possible calcifications |
FNA: 2×(2 years and 6 weeks before surgery) Initial Dx: Indeterminate (AUS) 2nd opinion: Benign |
Total thyroidectomy and right central neck dissection |
Gross:Well-circumscribed 0.7-cm nodule in middle of right lobe Microscopy:Collapsed thick capsule with granulation tissue, cholesterol crystals, and hemorrhage |
Right lobe with 0.7-cm nodule: consistent with FNA-induced reactive changes (separate incidental 0.6- and 0.2-cm PTC [pT1a pN1a]) | Hemorrhage, cholesterol granuloma, organizing granulation tissue suggestive of benign colloid nodule (separate incidental focus of PTC) |
| 5 | 45F Right thyroid nodule |
Right lobe, 1.2-cm nodule |
FNA: 1×(2 months before surgery) Initial Dx: Suspicious for PTC 2nd opinion:b Positive for PTC |
Total thyroidectomy and right central neck dissection |
Gross: 0.5-cm nodule in the right mid- to upper pole Microscopy: Cyst with granulation tissue, hemosiderin, macrophages, and histiocytes |
Multinodular goiter with cystic degeneration. FNA site with chronic inflammation and cystic change identified | Hemorrhage and organizing granulation tissue—benign colloid nodule—negative for malignancy |
| 6 | 56F Incidental right thyroid nodule, history of breast carcinoma |
Right lobe, 1.6-cm indeterminate hypoechoic nodule with internal calcification |
FNA: 3×(within 6 months) Initial Dx: Indeterminate (AUS) 2nd opinion: Suspicious for PTC |
Right lobectomy |
Gross: Thickly encapsulated 1.5-cm cyst Microscopy: Cyst with organizing hemorrhage, granulation tissue, acute, and chronic inflammation with macrophages and eosinophils, squamous metaplasia, and degenerative changes, including focal calcifications and occasional worrisome psammoma-like concretions |
1.5-cm cyst with degenerative and reactive changes—definitive diagnosis difficult | Extensively sclerotic and partially calcified, follicular-patterned nodule, predominantly degenerated, and partially infarcted with focal nuclear atypia; no definite malignancy |
| 7 | 68F Hyperthyroidism and goiter, history of colon cancer |
Left lobe, 0.8-cm indeterminate nodule with calcification; multiple nodules in both lobes |
FNA: 1×(4 months before surgery) Initial Dx: Left lobe 0.8-cm nodule—indeterminate (AUS), negative for BRAFV600E mutation 2nd opinion: (none) |
Total thyroidectomy |
Gross: Large multinodular and cystic thyroid Microscopic:Left lobe with 0.6-cm encapsulated nodule entirely replaced byorganizing hematoma with a small focus of viable tumor cells in the capsule |
Multifocal (×3) PTC (left lobe 1.2 cm and 0.6 cm; right lobe 0.1 cm) | (none) |
| 8 | 48F Difficulty swallowing, choking sensation, frequently clearing throat—goiter |
Left lobe, 1.8-cm dominant cystic nodule |
FNA: 1×(3 months before surgery) Initial Dx: Cystic degeneration in a hyperplastic nodule 2nd opinion: (none) |
Total thyroidectomy |
Gross: 1-cm well-circumscribed hemorrhagic cystic nodule in left lobe Microscopy: Benign colloid nodule with organizing hemorrhage, granulation tissue, and squamous metaplasia |
Nodular goiter with hemorrhagic cyst | (none) |
| 9 | 54F 0.8-cm right thyroid nodule diagnosed as PTC on outside cytology |
Right lobe, 0.8-cm thyroid nodule with calcification |
FNA: 1×(2 months before surgery) Initial Dx: Positive for PTC 2nd opinion: (none) |
Total thyroidectomy and right central neck dissection |
Gross: Right lobe with 0.7-cm encapsulated cystic nodule Microscopy: Cyst with extensive reactive changes (fibrosis, granulation tissue, and squamous metaplasia), and minute foci of residual PTC in the capsule |
Multifocal (×2) PTC (right lobe 0.7 cm cystic, left lobe 0.2 cm) | (none) |
| 10 | 33F Multinodular goiter |
Left lobe, suspicious 1.3-cm indeterminate nodule; isthmus, 1.6-cm nodule |
FNA:1×(14 months before surgery) Initial Dx: Left lobe nodule: suspicious for PTC 2nd opinion: (none) |
Total thyroidectomy |
Gross: Left lobe with 1.1-cm hemorrhagic cystic nodule Microscopy: Left lobe with encapsulated 1.1-cm cystic nodule with granulation tissue, extensive squamous metaplasia, and minute foci of residual PTC |
Multifocal (×4) PTC (left lobe 1.1 cm and 0.3 cm, isthmus 1.4 cm, right lobe 0.7 cm) | (none) |
| 11 | 49F Incidental left thyroid nodule |
Left lobe, 0.6-cm nodule with calcification in mid-lobe; 1.6-cm cystic nodule in lower lobe |
FNA:1×(2 months before surgery) Initial Dx: Left mid-lobe: 0.6-cm nodule positive for PTC (positive for BRAFV600E mutation) 2nd opinion: (none) |
Total thyroidectomy and central neck dissection |
Gross: 0.6-cm calcified encapsulated in left lobe Microscopy: Left lobe with predominantly encapsulated 0.6-cm nodule with extensive squamous metaplasia, granulation tissue, and minute foci of residual PTC in the capsule |
0.6-cm PTC in left lobe; multinodular goiter, negative lymph nodes (0/5) | (none) |
| 12 | 64F Multinodular goiter |
Right lobe, 1.1-cm indeterminate calcified nodule; multiple other nodules |
FNA: 2×(5 and 2 months before surgery) Initial Dx: Right lobe: indeterminate (AUS × 2) with atypical nuclear features (negative for BRAFV600E mutation) 2nd opinion: (none) |
Total thyroidectomy |
Gross: 0.6-cm partially cystic nodule in right lobe Microscopy: Right lobe with predominantly encapsulated cystic nodule with calcified capsule, hemorrhage, granulation tissue, and a few papillae consistent with PTC |
0.6-cm PTC in right lobe, multinodular goiter | (none) |
| 13 | 64M Multinodular goiter |
Multiple nodules in both lobes: left lobe, 0.7-cm indeterminate nodule; right lobe, 1.2-cm suspicious nodule |
FNA:1×(left lobe; 2 months before surgery); 1×(right lobe; 2 months before surgery) Initial Dx:Left lobe nodule: indeterminate (AUS); positive forBRAFV600Emutation 2nd opinion: Right lobe: positive for PTC |
Total thyroidectomy with central neck dissection |
Gross: Left lobe: 0.7-cm firm tan nodule Microscopy: Left lobe fibrotic partially cystic nodule with hemosiderin and granulation tissue |
Left lobe:0.7-cm fibrotic partially cystic nodule with FNA induced reactive changes—no PTC identified; right lobe: 1.2-cm PTC | (none) |
| 14 | 37F Right thyroid nodule |
2-cm nodule |
FNA: 1×(1 month before surgery) Initial Dx: Right lobe: Hürthle cell neoplasm 2nd opinion:b Follicular neoplasm |
Right lobectomy |
Gross: 2-cm hemorrhagic cyst Microscopy: Hemorrhagic cyst with cyst wall showing granulation tissue and squamous metaplasia |
Hemorrhagic cyst with reactive changes—negative for malignancy (no residual follicular or Hürthle cells identified) | (none) |
In case of multiple nodules, the vanishing tumor is highlighted in bold.
Number of times (and when) FNA was performed, initial diagnosis (Dx), and second opinion.
Second opinion from our institution (initially diagnosed at the referring institution).
AUS, atypia of undetermined significance; F, female; FNA fine-needle aspiration; M, male; PTC papillary thyroid carcinoma.
The FNA diagnosis was suspicious (n=3) or positive (n=2) for papillary thyroid carcinoma (PTC) in 5, follicular neoplasm in 1, indeterminate (atypia of unknown significance [AUS]) in 5, benign in 2, and nondiagnostic in 1 vanishing tumor. Upon second opinion (cases 1–6, and 14), an indeterminate (AUS) cytology was upgraded to suspicious (case 6), and suspicious was changed to positive for PTC (cases 2 and 5). In four cases, multiple FNAs were performed on the same nodule. The FNA needle rinse tested positive (cases 11 and 13) and negative (cases 7 and 12) for BRAFV600E mutation in 2 cases each.
The vanishing tumors ranged from 0.4 to 3.5 cm (median 0.7 cm) in size. A decrease of 0.1–0.7 cm was noted compared to the size measured by US. The nodules showed variable degrees of cystic change, organizing hemorrhage with hemosiderin pigment-laden macrophages, granulation tissue, fibrosis, sclerosis, and microcalcifications in all vanishing tumors in proportion to the temporal relationship of FNA to surgery (Fig. 1C, D). Acute and chronic inflammatory cells, including eosinophils, foamy macrophages, cholesterol granulomas with multinucleated giant cells, and squamous metaplasia frequently lining the cyst, were seen. Two vanishing tumors showed focal ossification.
Seven tumors (cases 2–6, 13, and 14) were completely replaced by reactive changes. These included 3 tumors considered suspicious/positive for PTC on FNA (cases 2, 5, and 6), but negative for malignancy on surgical pathology, one with indeterminate/benign FNA (case 4), one with indeterminate (AUS), but BRAFV600E-positive FNA (case 13) that also had multifocal PTC, one with benign (case 3), and one with Hürthle cell/follicular neoplasm (case 14) on FNA. PTC was diagnosed in five tumors (cases 7, 9–12), based on a few residual malignant cells at the periphery of the vanishing tumors of which three (cases 7, 9, and 10) had multifocal PTC. Cases 1 and 8 had a few residual benign thyroid follicles in the wall of the hemorrhagic cyst.
Discussion
Inability to find malignancy on thyroid resections is typically documented in the final pathology report as negative for malignancy. We demonstrate that the final diagnosis may be compromised when no representative follicular cells remain in a suspicious nodule that is completely replaced by secondary reactive changes after single or repeated US-guided FNA. Certain patterns emerged among the vanishing tumors. Five of the nodules were ≤1 cm, the smallest being 0.5 cm by US. Four nodules ranging in size from 1 to 2 cm were subjected to multiple FNA before surgery. FNA-induced hemorrhage completely obliterated a large 3.9-cm thyroid nodule in a patient on warfarin (case 1). One nodule was likely a Hürthle cell neoplasm (case 14), which are known to undergo infarction or ischemic necrosis when subjected to FNA. We considered two hypotheses regarding the vanishing tumors: (i) the cytology may have been false positive, or (ii) the surgical pathology may be false negative. The value of second opinion in cytopathology is well established. Second opinions in cytopathology may change management in 13% to 30% of cases (11,12). Multiple reviews and expert consultations suggest that the FNA diagnosis was not false positive, and therefore at least three patients in our series (cases 2, 5, and 6) may have had 0.5-cm (pT1a), 1.2-cm and 1.6-cm (pT1b) PTC. Thus, the final surgical pathology diagnosis of negative for malignancy indicates nonrepresentation of the original tumor rather than true negative.
The vanishing-cancer phenomenon has been described in prostates where minute cancers identified on biopsies disappeared on radical prostatectomies (13). Unlike prostate, where a final diagnosis of cancer is rendered on a needle core biopsy, the Bethesda system of reporting thyroid FNA implies cancer risk varying from 1–4% in the nondiagnostic category to 97–99% in the positive for malignancy category (14). Thus, the final confirmation of malignancy is made on surgical pathology. This general wisdom may be open to question in the rare event of vanishing tumor. Furthermore, a reduction in the size of the vanishing tumors after US-guided FNA could potentially lead to understaging of malignant tumors. There is significant distress and morbidity associated with total thyroidectomy for a BRAFV600E mutation-positive thyroid carcinoma diagnosed on FNA that is finally deemed negative for malignancy. By reporting our experience, we hope to raise awareness to these unexpected events. Currently, there are no consensus guidelines for the management of vanishing tumors. In a recent publication, Marchetti et al. macrodissected tumor cells from the FNA cytology slides and performed BRAF mutational analysis that helped improve their suspicious diagnosis to definite for carcinoma by 37% (15). A search for conclusive evidence of malignancy on FNA cytology may need to be undertaken when no residual tumor cells remain in a thyroid nodule.
This observational study has several limitations, the absence of follow-up being one. The issue of mixed identity or mixed specimens was not explored due to technical reasons. We are also unable to comment on the incidence of vanishing tumors after US-guided FNA, since only a subset of the thyroids subjected to FNA undergoes thyroidectomy. However, we noted a prevalence of <1% among all thyroid resections performed during the same period for various reasons.
In conclusion, vanishing tumors of the thyroid may be an unintended consequence of US-guided FNA. With wider acceptance of high-resolution US and US-guided FNA for thyroid nodules, there may be an increase in their incidence leading to diagnostic and management dilemmas.
Supplementary Material
Acknowledgments
The funding for this work is provided by the Doris Duke Charitable Foundation and the Yale Office of Student Research.
Disclosure Statement
The authors have no competing financial interests.
References
- 1.Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Pacini F. Schlumberger M. Sherman SI. Steward DL. Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 2.Ezzat S. Sarti DA. Cain DR. Braunstein GD. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994;154:1838–1840. doi: 10.1001/archinte.154.16.1838. [DOI] [PubMed] [Google Scholar]
- 3.Kim EK. Park CS. Chung WY. Oh KK. Kim DI. Lee JT. Yoo HS. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002;178:687–691. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- 4.Frates MC. Benson CB. Charboneau JW. Cibas ES. Clark OH. Coleman BG. Cronan JJ. Doubilet PM. Evans DB. Goellner JR. Hay ID. Hertzberg BS. Intenzo CM. Jeffrey RB. Langer JE. Larsen PR. Mandel SJ. Middleton WD. Reading CC. Sherman SI. Tessler FN. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005;237:794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 5.Cibas ES. Alexander EK. Benson CB. de Agustin PP. Doherty GM. Faquin WC. Middleton WD. Miller T. Raab SS. White ML. Mandel SJ. Indications for thyroid FNA and pre-FNA requirements: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:390–399. doi: 10.1002/dc.20827. [DOI] [PubMed] [Google Scholar]
- 6.LiVolsi VA. Merino MJ. Worrisome histologic alterations following fine-needle aspiration of the thyroid (WHAFFT) Pathol Annu. 1994;29:99–120. [PubMed] [Google Scholar]
- 7.Ersoz C. Soylu L. Erkocak EU. Tetiker T. Gumurdulu D. Histologic alterations in the thyroid gland after fine-needle aspiration. Diagn Cytopathol. 1997;16:230–232. doi: 10.1002/(sici)1097-0339(199703)16:3<230::aid-dc7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Baloch ZW. LiVolsi VA. Post fine-needle aspiration histologic alterations of thyroid revisited. Am J Clin Pathol. 1999;112:311–316. doi: 10.1093/ajcp/112.3.311. [DOI] [PubMed] [Google Scholar]
- 9.Pandit AA. Phulpagar MD. Worrisome histologic alterations following fine needle aspiration of the thyroid. Acta Cytol. 2001;45:173–179. doi: 10.1159/000327273. [DOI] [PubMed] [Google Scholar]
- 10.Adeniran AJ. Theoharis C. Hui P. Prasad ML. Hammers L. Carling T. Udelsman R. Chhieng DC. Reflex BRAF testing in thyroid fine-needle aspiration biopsy with equivocal and positive interpretation: a prospective study. Thyroid. 2011;21:717–723. doi: 10.1089/thy.2011.0021. [DOI] [PubMed] [Google Scholar]
- 11.Park JH. Kim HK. Kang SW. Jeong JJ. Nam KH. Chung WY. Park CS. Second opinion in thyroid fine-needle aspiration biopsy by the Bethesda System. Endocr J. 2012;59:205–212. doi: 10.1507/endocrj.ej11-0274. [DOI] [PubMed] [Google Scholar]
- 12.Tan YY. Kebebew E. Reiff E. Caron NR. Ogilvie JB. Duh QY. Clark OH. Ljung BM. Miller T. Does routine consultation of thyroid fine-needle aspiration cytology change surgical management? J Am Coll Surg. 2007;205:8–12. doi: 10.1016/j.jamcollsurg.2007.02.075. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein NS. Begin LR. Grody WW. Novak JM. Qian J. Bostwick DG. Minimal or no cancer in radical prostatectomy specimens. Report of 13 cases of the “vanishing cancer phenomenon.”. Am J Surg Pathol. 1995;19:1002–1009. doi: 10.1097/00000478-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Cibas ES. Ali SZ. The Bethesda system for reporting thyroid cytopathology. Thyroid. 2009;19:1159–1165. doi: 10.1089/thy.2009.0274. [DOI] [PubMed] [Google Scholar]
- 15.Marchetti I. Iervasi G. Mazzanti CM. Lessi F. Tomei S. Naccarato AG. Aretini P. Alberti B. Di Coscio G. Bevilacqua G. Detection of the BRAF(V600E) mutation in fine needle aspiration cytology of thyroid papillary microcarcinoma cells selected by manual macrodissection: an easy tool to improve the preoperative diagnosis. Thyroid. 2012;22:292–298. doi: 10.1089/thy.2011.0107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


