Abstract
Background
This is an update of the review published in Issue 4, 2003. Bone metastasis cause severe pain as well as pathological fractures, hypercalcaemia and spinal cord compression. Treatment strategies currently available to relieve pain from bone metastases include analgesia, radiotherapy, surgery, chemotherapy, hormone therapy, radioisotopes and bisphosphonates.
Objectives
To determine efficacy and safety of radioisotopes in patients with bone metastases to improve metastatic pain, decrease number of complications due to bone metastases and improve patient survival.
Search methods
We sought randomised controlled trials (RCTs) in MEDLINE, EMBASE, CENTRAL, and the PaPaS Trials Register up to October 2010.
Selection criteria
Studies selected had metastatic bone pain as a major outcome after treatment with a radioisotope, compared with placebo or another radioisotope.
Data collection and analysis
We assessed the risk of bias of included studies by their sequence generation, allocation concealment, blinding of study participants, researchers and outcome assessors, and incomplete outcome data. Two review authors extracted data. We performed statistical analysis as an "available case" analysis, and calculated global estimates of effect using a random‐effects model. We also performed an intention‐to‐treat (ITT) sensitivity analysis.
Main results
This update includes 15 studies (1146 analyzed participants): four (325 participants) already included and 11 new (821 participants). Only three studies had a low risk of bias. We observed a small benefit of radioisotopes for complete relief (risk ratio (RR) 2.10, 95% CI 1.32 to 3.35; Number needed to treat to benefit (NNT) = 5) and complete/partial relief (RR 1.72, 95% CI 1.13 to 2.63; NNT = 4) in the short and medium term (eight studies, 499 participants). There is no conclusive evidence to demonstrate that radioisotopes modify the use of analgesia with respect to placebo. Leucocytopenia and thrombocytopenia are secondary effects significantly associated with the administration of radioisotopes (RR 5.03; 95% CI 1.35 to 18.70; Number needed to treat to harm (NNH) = 13). Pain flares were not higher in the radioisotopes group (RR 0.74; 95% CI 0.27 to 2.06). There are scarce data of moderate quality when comparing Strontium‐89 (89Sr) with Samarium‐153 (153Sm), Rhenium‐186 (186Re) and Phosphorus‐32 (32P). We observed no significant differences between treatments. Similarly, we observed no differences when we compared different doses of 153Sm (0.5 versus 1.0 mCi).
Authors' conclusions
This update adds new evidence on efficacy of radioisotopes versus placebo, 89Sr compared with other radioisotopes, and dose‐comparisons of 153Sm and 188Re. There is some evidence indicating that radioisotopes may provide complete reduction in pain over one to six months with no increase in analgesic use, but severe adverse effects (leucocytopenia and thrombocytopenia) are frequent.
Keywords: Humans; Bone Neoplasms; Bone Neoplasms/radiotherapy; Bone Neoplasms/secondary; Fractures, Bone; Fractures, Bone/radiotherapy; Hypercalcemia; Hypercalcemia/radiotherapy; Pain; Pain/radiotherapy; Pain Measurement; Phosphorus Radioisotopes; Phosphorus Radioisotopes/therapeutic use; Radioisotopes; Radioisotopes/therapeutic use; Randomized Controlled Trials as Topic; Ruthenium Radioisotopes; Ruthenium Radioisotopes/therapeutic use; Samarium; Samarium/therapeutic use; Spinal Cord Compression; Spinal Cord Compression/radiotherapy; Strontium Radioisotopes; Strontium Radioisotopes/therapeutic use
Radioisotopes to ease metastatic bone pain
Pain is commonly experienced by people whose cancer has spread to their bones. There are several ways to treat this pain, including the administration of radioisotopes, which are chemical elements that emit radiation and act on the bone to reduce the effects of the cancer. This review looked at the effectiveness of radioisotopes for relieving pain, reducing patients' needs for conventional pain‐killers, improvements in quality of life, and increased survival. There is some evidence that radioisotopes may give pain relief over one to six months, but the treatment also seemed to be associated with adverse effects, notably reducing blood cells (leucocytes). When comparing different radioisotopes or different doses of a radioisotope, we identified no conclusive differences.
Summary of findings
Summary of findings for the main comparison.
Radioisotopes compared to Placebo for metastatic bone pain
| Radioisotopes compared to Placebo for metastatic bone pain | ||||||
| Patient or population: patients with metastatic bone pain Settings: Intervention: Radioisotopes Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Radioisotopes | |||||
| Pain relief ‐ Complete relief | Medium risk population1 | RR 2.1 (1.32 to 3.35) | 296 (4 studies) | ⊕⊕⊕⊝ moderate2,3 | 79 events | |
| 124 per 1000 | 260 per 1000 (164 to 415) | |||||
| Pain relief ‐ Complete/Partial relief | Medium risk population1 | RR 1.72 (1.13 to 2.63) | 119 (3 studies) | ⊕⊕⊕⊝ moderate3,4 | 49 events | |
| 182 per 1000 | 313 per 1000 (206 to 479) | |||||
| Compression of spinal cord | Medium risk population5 | RR 1.1 (0.39 to 3.07) | 270 (2 studies) | ⊕⊕⊝⊝ low3,6 | 16 events | |
| NA | NA | |||||
| Pain flares | Medium risk population5 | RR 0.74 (0.27 to 2.06) | 270 (2 studies) | ⊕⊕⊝⊝ low3,6 | 20 events | |
| NA | NA | |||||
| Thrombocytopenia III‐IV | Medium risk population1 | RR 2.21 (0.98 to 4.99) | 313 (4 studies) | ⊕⊕⊝⊝ low3,7 | 22 events | |
| 33 per 1000 | 73 per 1000 (32 to 165) | |||||
| Leucocytopenia III‐IV | High risk population8 | RR 5.9 (1.62 to 21.47) | 410 (4 studies) | ⊕⊕⊝⊝ low3,6 | 22 events | |
| 59 per 1000 | 348 per 1000 (96 to 1000) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Median baseline risk in the included studies 2 Two out of four studies had moderate risk of bias, while the other two had low risk of bias 3 Low number of events 4 Two out of three studies had low risk of bias 5 Not computed because the intervention has no significant effect 6 All studies with high risk of bias 7 Three out of four studies with high risk of bias 8Buchali 1988 baseline risk
Background
This is an update of the original review published in Issue 4, 2003 on 'Radioisotopes for metastatic bone pain' (Roque 2003). Many oncological patients with advanced disease will develop metastatic deposits in their bones. A high proportion of patients diagnosed with malignant breast, lung and prostate tumours develop bone metastases (80% to 85%). The spine, pelvis and ribs are often the earliest sites of metastases, but most bone metastases (more than 80%) are found in the axial skeleton (Nelson 1991).
Description of the condition
The main consequence of bone involvement is severe pain. Possible mechanisms that may cause pain from bone metastases include: a) damage in the neurons that change their receptors in response to excessive stretch, or excessive mechanical stimulation or inflammation; b) tumor‐derived products as alfa tumor necrosi factor, endothelins, interleukins, epidermal growth factor (GF), transformin GF, platelet GF or prostaglandins that stimulate the nociceptors; c) nerve destruction caused by the increasing size of the tumor; d) and the acidosis produced by the osteoclast’s tissular destruction that stimulate the nociceptors (Sabino 2005). Other clinical manifestations of bone metastases are pathological fractures (8% to 30% of patients), hypercalcaemia (10%) and spinal cord compression (5%) (Nelson 1991). These complications can adversely affect the quality of life and life expectancy of these patients (Scully 2002).
Description of the intervention
Several treatment modalities are used to treat pain caused by bone metastases. These include analgesics as well as treatments to control or reduce the tumour, because pain‐induced impairment can become more significant than the illness itself. An array of treatment strategies is available to treat metastatic bone disease, including analgesia, radiotherapy, surgery, chemotherapy, hormone therapy, radioisotopes and bisphosphonates.
Systemic analgesics: acetaminophen; non‐steroidal anti‐inflammatory drugs (NSAIDs); and opioids (according to the WHO pain ladder).
Local field irradiation or surgery for isolated bone metastases. If the disease is restricted to one region of the body, hemibody irradiation is an option. Radiotherapy is well recognised as an effective treatment for relieving pain and a great number of patients achieve complete resolution of pain (Chow 2007; Sze 2002).
Antineoplastic treatment, e.g., surgery, chemotherapy or hormone therapy (Aaron 1997; McEwan 1998).
When the painful bone involvement is widespread, the use of bone‐seeking agents may be considered, bisphosphonates or radioisotopes, often combined with a bisphosphonate carrier (Wong 2003).
Radiopharmaceuticals are beta emitting agents administered intravenously, with a particular affinity to bone turnover sites (Mertens 1998). Advantages of radioisotopes include the ability to simultaneously treat multiple sites of disease, ease of administration, and the potential integration with other treatments like radiotherapy or chemotherapy (PGR 2004). The most frequently used radioactive substances are strontium‐ 89 (89Sr) and Samarium‐153 (153Sm), approved in Europe and the United States. Other agents considered in clinical trials are rhenium‐186 (186Re) and rhenium‐188 (188Re), which are still in the experimental phase. Strontium‐89 is a pure beta emitter and has a long physical half‐life (50 days), while 153Sm, 188Re and 186Re have much shorter physical half‐lives (less than four days) and are gamma‐emitters, enabling post‐treatment scintigraphic imaging and dosimetry (IAEA 1459) but also having greater implications for radiation protection.
Why it is important to do this review
Many clinical trials have been undertaken in this field and in general they have shown that radioisotopes have a beneficial effect in controlling pain due to metastatic bone lesions. Unfortunately, a majority of these studies either have methodological flaws; utilise small sample sizes; are not head‐to‐head comparison trials; or use different criteria to measure pain (Mertens 1998), so assessment of the available evidence is not straightforward. The main purpose of this review is to determine in a systematic and unbiased fashion the efficacy of radioisotopes to control metastatic bone pain and complications due to bone metastases, as well as to determine the specific agents or doses that are most efficacious. This updated review has introduced some methodological changes with respect to the previous review; we have summarised these in the section on 'Differences between protocol and review'.
Objectives
The main objective of this review is to determine:
the efficacy of radiopharmaceuticals to control pain in patients with metastatic bone lesions, in comparison with either placebo, other radiopharmaceuticals or other dosages of the same radiopharmaceutical.
Secondary objectives are to determine the following.
The efficacy of radiopharmaceuticals in terms of prevention or treatment of complications due to bone metastases (hypercalcaemia, fracture and spinal cord compression).
The efficacy of radiopharmaceuticals in terms of patient survival and quality of life.
The safety of radiopharmaceuticals in terms of patient adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials (RCTs) where the major outcome is metastatic bone pain after treatment with radiopharmaceuticals, compared with placebo or an alternative radiopharmaceutical or different dose of the same radiopharmaceutical.
Types of participants
Participants of any age with metastatic bone pain caused by any primary tumour and defined by Technetium‐99m (99mTc) bone scan, Magnetic Resonance Imaging (MRI), or other imaging modality.
Types of interventions
Radiopharmaceuticals of interest are radioactive isotopes, alone or chelated with a bisphosphonate. Comparisons of interest were:
radiopharmaceutical versus placebo;
comparisons between radiopharmaceuticals (Radiopharmaceutical A versus Radiopharmaceutical B);
dose‐comparison (Dose 1 Radiopharmaceutical A versus Dose 2 Radiopharmaceutical A).
We accepted rescue medication, steroids or palliative radiotherapy as a co‐intervention, if equally available to the two treatment groups.
Types of outcome measures
Primary outcomes
Pain relief.
The main outcome measure was pain, measured by visual analogue scales (VAS) or nominal scales of four to five categories. We included only evaluations of pain rated by the patient; we excluded physician‐rated evaluations.
Whenever pain was assessed as the percentage of people reducing their pain from baseline, we considered the following categories:
complete reduction of pain (reduction from baseline is 100%);
complete/partial reduction of pain (reduction from baseline is greater or equal to 50%, up to and including complete reduction);
any reduction in pain.
Secondary outcomes
Reduction in analgesia consumption.
Rescue medication at baseline and post‐intervention.
Complications due to bone metastases (hypercalcaemia, fracture, nerve root and spinal cord compression and bone marrow infiltration).
Disease progression.
Quality of life.
Side effects (pain flares, leucocytopenia, thrombocytopenia, anemia).
Hospitalisation due to side effects.
Length of improvement.
All secondary outcomes were collected as measured by the study authors, except for hematological and non‐hematological toxicity that had to be grade III‐IV. We excluded the following types of studies.
Studies assessing bone pain as a secondary outcome.
Studies that evaluated only biochemical results, without information on clinical results.
Studies focusing on the prevention of bone metastases, with no data provided for pain or complications due to bone metastases.
Search methods for identification of studies
Electronic searches
We originally searched for RCTs related to this review electronically using MEDLINE (1966 to 2003), EMBASE (1974 to 2003) and the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 1, 2003).
The update of the search covered the following databases and periods: PaPaS Trials Register (up to July 2008), CENTRAL (up to Issue 4 2010), MEDLINE (Limit to 2003 to Oct week 3 2010), EMBASE (Limit to 2003 to 2010 week 43). Please see Appendix 1 for the updated search strategies applied.
Searching other resources
We reviewed identified systematic reviews and meta‐analyses and reference lists from identified RCTs to identify further RCTs. We undertook no additional handsearching of journals. We searched for ongoing trials on the metaRegister of Controlled Trials (mRCT) www.controlled‐trials.com. We searched for unpublished trials funded by the International Atomic Energy Agency on www.iaea.org.
Data collection and analysis
Selection of studies
We critically appraised the studies identified by the search strategy for inclusion in the systematic review. Two independent review authors (MJM and PA, MR or MF) assessed each study in an open fashion (we undertook no blinding of authors, institution, or source of publication of the study). We would have resolved any discrepancy by consensus or by the intervention of a third review author, but there were no discrepancies.
Data extraction and management
We designed a data collection form to extract data on the characteristics of the study participants; the characteristics of the interventions and of the different comparison groups; aspects of trial design related to methodological quality; and the relevant outcomes for each participant subgroup. For cross‐over studies, we planned to collect and analyse only data corresponding to the first period of treatment.
Two review authors independently extracted the data and completed a standard spreadsheet form designed for that purpose. Where information provided in the trial reports was incomplete, we contacted the author(s) of the study to provide the required data.
Assessment of risk of bias in included studies
Two review authors (MR and MJM or PA) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009a). We resolved any disagreement by discussion or by involving a third assessor. The following items were assessed:
Sequence generation (checking for possible selection bias): We described for each included study the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
Allocation concealment (checking for possible selection bias): We described for each included study the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
Blinding of study participants (checking for possible performance bias): We described for each included study all the methods used, if any, to blind participants from knowledge of which intervention a participant received.
Blinding of study researchers (checking for possible performance bias): We described for each included study all the methods used, if any, to blind study researchers from knowledge of which intervention a participant received.
Blinding of outcome assessors (checking for possible performance bias): We described for each included study all the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations): We described for each included study the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported; the numbers included in the analysis at each stage (compared with the total randomised participants); reasons for attrition or exclusion where reported; and whether missing data were balanced across groups or were related to outcomes. We didn't consider that a high attrition rate was a source of bias if an intention‐to‐treat (ITT) analysis could be performed. Where sufficient information was reported or could be supplied by the study authors, we re‐included missing data in the analyses which we undertook.
For each item, we assessed the methods to introduce:
low risk of bias;
high risk of bias;
uncertain risk of bias.
With reference to 1 to 6 above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. If the bias in a study was likely to impact on its findings, we considered the study to have an overall high risk of bias, and low risk of bias otherwise. We explored the impact of the level of bias undertaking sensitivity analyses ‐ see 'Sensitivity analysis'.
We evaluated the quality of the evidence using the GRADE system and from this developed a Summary of Findings table (Guyatt 2008). This table has three elements:
i) the outcomes most relevant to patients (critical and important outcomes according to GRADE),
ii) a summary measure for the quality of the evidence (confidence in estimate of a treatment effect),
iii) a summary estimate for the relative risk (RR) and absolute effect for the interventions of interest.
The outcomes selected as most relevant to patients were: complete pain relief, compete/partial pain relief, compression of spinal cord, pain flares, thrombocytopenia III‐IV and leucocytopenia III‐IV. The summary measure for the quality of the evidence was derived for the authors based on the completeness and robustness of the evidence available for each outcome, taking into account the number and risk of bias of the studies contributing data for each outcome. The relative effect of the intervention for each outcome was taken from the metanalysis results. The calculation of the absolute effects of the intervention for each outcome involves applying the relative effects to one or more basal risks. For each outcome, we selected the median baseline risk observed in the included studies. For leucocytopenia, it was necessary to choose the baseline risk of Buchali 1988, since the rest of the included studies had a null risk. For non significant outcomes, no absolute effect was computed.
Measures of treatment effect
We estimated the effect of treatment by risk ratios (RR) and standardised mean differences (SMD), with their corresponding confidence intervals (CI). For all significant outcomes (complete pain relief, complete/partial pain relief, leucocytopenia, thrombocytopenia), we computed the number needed to treat to benefit one patient (NNT) or the number needed to treat to harm one patient (NNH) as the inverse of the pooled risk differences (RD), since the meta‐analysis of RD were highly homogeneous for these outcomes (I2 = 0%). We computed ranges of plausible values for NNTs applying the pooled RR to the range of control risks observed in the individual studies (see section 12.5.4.2, Schüneman 2009). The previous method could not be applicable to NNH, since the expected control rate for these treatment‐related outcomes would be 0% and numerical problems would ensue.
Unit of analysis issues
We analysed cross‐over trials included in the review (Lewington 1991; Maxon 1991) as parallel trials, including only data from the first period of treatment.
Assessment of heterogeneity
We computed global estimates for each variable effect by conducting a meta‐analysis of single effect measures of the study (RR for dichotomous variables and SMD for continuous variables) applying a random‐effects model (DerSimonian 1986). Prior to calculating estimates of effect, we assessed the presence and degree of heterogeneity by means of I2.
Data synthesis
The review's main analysis was an "available data" analysis, where we analysed data for each included study as provided by the study authors, either per protocol or using ITT.
Sensitivity analysis
We performed several sensitivity analyses to assess how robust the estimate of the global effect was regarding the:
imputation strategies in an ITT analysis of radioisotopes versus placebo trials;
adjuvant role of radioisotope treatment when administered with external beam radiotherapy;
time of pain assessment (30 days versus earlier);
publication status of studies;
risk of bias (low risk versus high risk).
We performed two sensitivity ITT analyses for dichotomous variables related to treatment effect, analysing each participant in their corresponding randomised treatment group, regardless of either completion or withdrawal from the study and imputing missing dichotomous data with two different strategies. In the first imputation strategy, we assumed that all withdrawn participants had not responded or had presented an adverse outcome ("worst case" scenario). We performed the second imputation strategy under the assumption that the incidence of adverse outcomes in withdrawn participants was the same as in the control group ("same as control" scenario).
We did not consider dichotomous variables related to adverse effects in this sensitivity analysis, since the authors considered it wrong to attribute unintended (adverse) effects to a treatment that somebody did not receive (Higgins 2009b).
Results
Description of studies
We screened the references retrieved by bibliographic searches by reading the abstracts and full text of the articles where appropriate.
Results of the search
For this review update the electronic search of the PaPaS register led to the retrieval of 39 references, 89 references from CENTRAL, 130 references from MEDLINE and 176 references from EMBASE. After de‐duplications and excluding records identified previously, we identified 248 new references in this update. Additionally, searching the database of ongoing trials identified one study, and the search of unpublished trials identified one multinational study, published in part in three publications.
Included studies
This update adds 11 new studies and 821 participants to the original review (Baczyk 2007; E13013 2000; Han 2002; Maxon 1991; Nair 1999; Nilsson 2007; Palmedo 2003; Resche 1997; Sartor 2004; Sciuto 2001; Smeland 2003). Thus, the updated review has 15 included studies, although we could include only 14 studies in the statistical analyses (1146 participants: 802 receiving active treatment, 344 receiving placebo). Eight studies assessed the effectiveness of radioisotopes versus placebo (Buchali 1988; Han 2002; Lewington 1991; Maxon 1991; Porter 1993; Sartor 2004; Serafini 1998; Smeland 2003), three studies compared different radioisotopes (Baczyk 2007; Nair 1999; Sciuto 2001), and four studies were dose‐comparison studies (E13013 2000; Palmedo 2003; Resche 1997; Serafini 1998).
The included studies provided experimental data for five different radioisotopes: 89Sr, 153Sm, 186Re, 188Re and phosphorus‐32 (32P). There are data on efficacy versus placebo for three different radioisotopes: 89Sr (Buchali 1988; Lewington 1991; Porter 1993; Smeland 2003), 186Re (Han 2002; Maxon 1991) and 153Sm (Sartor 2004; Serafini 1998). Head‐to‐head comparisons are available for 89Sr versus 153Sm (Baczyk 2007), 89Sr versus 186Re (Sciuto 2001) and 89Sr versus 32P (Nair 1999). Finally, dose‐comparison studies assessed 1.0 and 0.5 mCi of 153Sm (E13013 2000; Resche 1997; Serafini 1998), plus a study on 188Re (Palmedo 2003).
The most studied malignancies were prostate (Baczyk 2007; Buchali 1988; Han 2002; Lewington 1991; Palmedo 2003; Porter 1993; Sartor 2004) and breast cancer (Baczyk 2007; Sciuto 2001). The remaining six studies included participants with different primary cancers, mainly prostate and breast.
Excluded studies
After excluding reviews, uncontrolled studies and studies unrelated to the review topic, we identified 18 potentially eligible studies as not meeting the inclusion criteria; and we have described these in the 'Characteristics of excluded studies' table. Reasons for exclusion of these studies were as follows.
Not a RCT (Dickie 1999; Haesner 1992; Liepe 2007; Storto 2006).
Dose‐escalation trials (De Klerk 1994; Zhang 2003; Morris 2009; Loeb 2009; Zafeirakis 2009).
Control groups other than placebo or radioisotopes (Nilsson 2005; Oosterhof 2003; Quilty 1994; Wang 2003).
Main outcome other than pain (Parker 2009; Malmberg 1997; McEwan 1994 is a retrospective cost‐benefit analysis of a subset of patients in Porter 1993).
Radioisotopes adjuvant to chemotherapy (Porfiri 2010; Tu 2001).
There is one additional study awaiting assessment (Ma 2009).
Risk of bias in included studies
We have provided a graphical representation of the included studies risk of bias in Figure 1 and Figure 2. The risk of bias of the studies included in the review is mostly unclear, due to a lack of detail in the publications regarding the assessed items. Allocation concealment, the item most related to risk of bias, has been poorly described in the included studies, and it is difficult to assess the likelihood of bias associated with it in the included studies.
Figure 1.
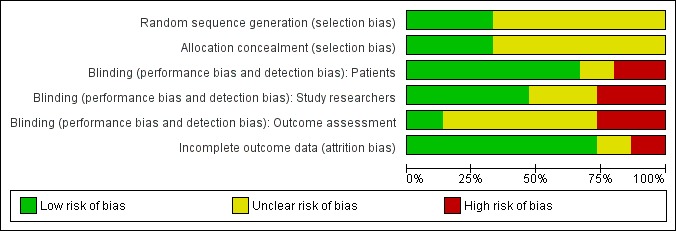
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Figure 2.
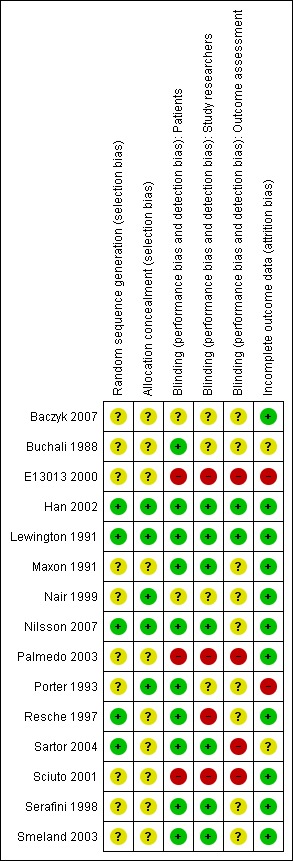
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Blinding of participants and incomplete data assessment are probably the most relevant items besides allocation concealment. Blinding of participants is particularly relevant, given that the main outcome is assessment of pain by the participant. Incomplete data assessment is particularly relevant, since the sample sizes are small and attrition is high because of death and disease progression in a target population that is terminally ill. These two items are the ones for which more information is available from the included studies, and present the highest number of studies with low risk of bias: nine studies had a double‐blind design and one additional study a single blind design, and 11 studies adequately described the number and causes of missing data. Additionally, six studies had high attrition (Buchali 1988; Han 2002; Lewington 1991; Maxon 1991; Resche 1997; Smeland 2003), and one study did not state the number of randomised participants (E13013 2000).
Only two studies presented low risk of bias in all assessed dimensions (Han 2002; Lewington 1991) and were considered to have a global low risk of bias in the sensitivity analysis by risk of bias, as well as a third study that presented low risk of bias in all but one dimension (Nilsson 2007). There were four studies that presented low risk of bias in two dimensions considered to be crucial in a study (blinding of participants and incomplete outcome data) (Maxon 1991; Resche 1997; Serafini 1998; Smeland 2003), but none of them had clear allocation concealment and thus were grouped with the remaining studies in the sensitivity analysis by risk of bias.
Effects of interventions
See: Table 1
Considerations of statistical analysis of individual studies
We analysed the Lewington 1991 and Maxon 1991 studies as parallel, using data from the first treatment period. We considered two participants missing in the Porter 1993 study as dropouts that occurred in each of the treatment groups. Buchali 1988 included participants with no pain at the beginning of the study. Adverse effects and survival were assessed in all the participants of the study. An ITT analysis for pain improvement considered only those participants experiencing pain at the beginning of the study. For the radioisotopes versus placebo comparison, we combined data from the two groups treated with 153Sm in the three‐arm study Serafini 1998.
We were unable to analyse the E13013 2000 study since the number of randomised participants in the Chinese site is unknown. Also, we sought additional information regarding the full multinational trial, unsuccessfully.
Efficacy of radioisotopes
Pain control
Among the eight studies (499 participants) that provided sufficient data for analysis of pain, there were four studies assessing pain control at short‐term (one month; Lewington 1991; Maxon 1991; Sartor 2004; Serafini 1998), three studies at medium‐term (three to six months; Han 2002; Nilsson 2007; Porter 1993) and one study at long‐term (12 months; Buchali 1988). There is evidence suggesting a beneficial effect of radioisotopes for pain control, from studies assessing pain at short and medium term (one to six months). The only study assessing pain at long‐term (12 months) was not significant.
Data on complete (100% relief) and complete/partial (50% to 100%) reduction of pain were homogeneous (I2 = 0%). They showed a small but significant improvement in pain in the radioisotope group, both for complete relief (RR 2.10, 95% CI 1.32 to 3.35) and complete/partial relief (RR 1.72, 95%CI 1.13 to 2.63). The absolute benefit for these outcomes varies widely across baseline risks provided in the SoF table and should be interpreted with caution. The NNT for complete pain relief is five (plausible range two to 44, see Table 9), and for complete/partial pain relief is eight (plausible range one to 54, see Table 10). Results for any reduction of pain were heterogeneous (I2 = 68%) and the meta‐analysis result is non‐significant (RR 1.36, 95% CI 0.77 to 2.40). Two studies also assessed pain control in a continuous fashion: Maxon 1991 as a reduction in a pain index and Han 2002 as the number of response days, defined by 25% pain reduction or 25% improvement in a medication index or daily activity score. This latter study showed a significant effect of radioisotopes (RR 3.14, 95%CI 2.47 to 3.81), while the former was non‐significant.
Table 1.
Range of NNT for complete pain reduction
| RR | Control risk | NNT |
| 2.1 95%CI 1.32 to 3.35 |
7.1% (Lewington 1991) |
13 95%CI 6 to 44 |
| 17.6% (Sartor 2004) |
5 95%CI 2 to 18 |
|
| 23.5% (Porter 1993) |
4 95%CI 2 to 13 |
See section 12.5.4.2 of Cochrane Handbook for Systematic Reviews of Interventions for NNT computation details. To obtain a range of plausible NNTs of radioisotopes versus placebo for the outcome of complete pain reduction, an estimation of the RR and its 95% CI has been taken from meta‐analysis 1.1.1 and a range of control risk values has been built from the studies included in the analysis that have a non‐zero control risk value. A low control risk means that few patients achieve complete pain control while on placebo; this leads to a higher value of NNT meaning that more patients have to be treated with radioisotopes to observe an additional patient with pain control. Paradoxically, it also means that the treatment has a higher clinical impact because it addresses pain that would otherwise be uncontrolled.
Table 2.
Range of NNT for complete/partial pain reduction
| RR | Control risk | NNT |
| 1.72 95%CI 1.13 to 2.63 |
14.3% (Lewington 1991) |
10 95%CI 4 to 54 |
| 18.2% (Nilsson 2007) |
8 95%CI 3 to 42 |
|
| 50.0% (Porter 1993) |
3 95%CI 1 to 15 |
See section 12.5.4.2 of Cochrane Handbook for Systematic Reviews of Interventions for NNT computation details. To obtain a range of plausible NNTs of radioisotopes vs placebo for the outcome of complete/partial pain reduction, an estimation of the RR and its 95% CI has been taken from meta‐analysis 1.1.1 and a range of control risk values has been built from the studies included in the analysis that have a non‐zero control risk value.
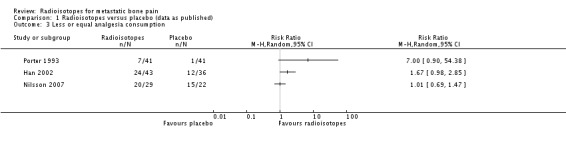
Less or equal analgesia use
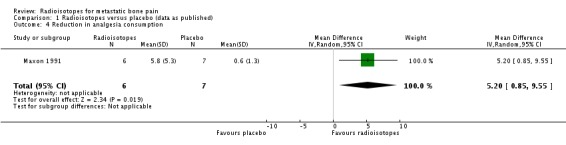
There is no conclusive evidence to demonstrate that radioisotopes modify the use of analgesia with respect to placebo. One study (Porter 1993) provided data on analgesia use and concluded that at three months a greater proportion of participants had stopped taking analgesics altogether in the active group as compared to the placebo group. One study (Han 2002) provided data on request of palliative radiotherapy, which was transformed to "not request of palliative radiotherapy"; for this outcome, a marginally non‐significant greater probability was observed for participants in the active group. A third study (Nilsson 2007) collected data on analgesia consumption classified according to the WHO ladder for cancer pain showing that in participants receiving external beam radiotherapy, addition of either radioisotopes or placebo led to a similar number of participants with increased analgesia consumption with respect to baseline. A fourth small study (Maxon 1991) showed a greater reduction in the analgesia index in the radioisotope group.
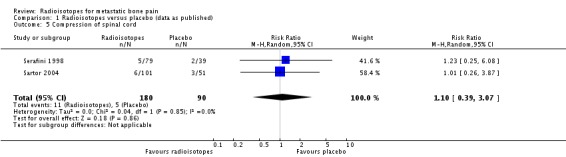
Prevention of spinal cord compression
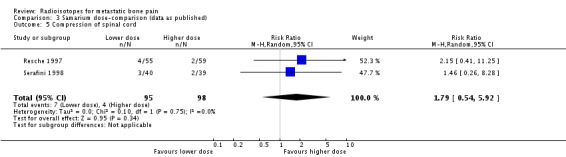
Two studies (270 participants; Sartor 2004; Serafini 1998) did not show a significant effect of radioisotopes on the prevention of spinal cord compression (RR 1.10; 95% CI 0.39 to 3.07; I2 = 0%).
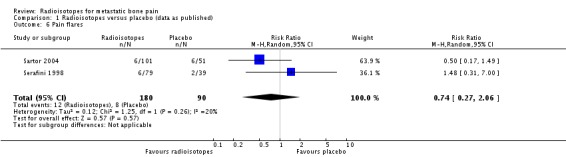
Pain flares
Two studies (270 participants; Sartor 2004; Serafini 1998) did not show a significant effect of radioisotopes on the incidence of pain flares (RR 0.74; 95% CI 0.27 to 2.06; I2 = 20%).
Quality of life
Four studies assessed quality of life (Porter 1993; Smeland 2003) or performance status (Baczyk 2007; Sciuto 2001), but they did not provide adequate data on the analysis of this outcome.
Mortality
Three studies (237 participants) provided mortality data; we found no significant differences in the number of deaths in the treatment and placebo arms (RR 1.14; 95% CI 0.27 to 4.77; I2 = 57%).
Adverse effects
We have shown results for severe adverse effects (grade III to IV). Severe leucocytopenia and thrombocytopenia are adverse effects associated with the administration of radioisotopes. All the included studies and the meta‐analysis showed that the incidence of leucocytopenia is significantly greater in patients treated with radioisotopes (RR 5.90; 95% CI 1.62 to 21.47; I2 = 0%). The NNH to observe one case of leucocytopenia due to radioisotopes is 14 (95% CI 9 to 25). There were also a greater number of thrombocytopenic events in the treatment groups; however, this result did not reach the level of statistical significance (RR 2.21; 95% CI 0.98 to 4.99; I2 = 0%). We found no differences in the number of cases with severe anaemia (RR 1.09; 95% CI 0.47 to 2.56; I2 = 0%).
Other outcomes
There were no data on other potential adverse events, such as hypercalcaemia, fractures and compression of nervous roots.
Head‐to‐head comparisons of radioisotopes
There is little and weak evidence of the relative efficacy of 89Sr with respect to other radioisotopes (153Sm, 186Re and 32P), provided by three small studies (Baczyk 2007; Nair 1999; Sciuto 2001). We have shown results on a graph for each study, but we have not attempted a meta‐analysis due to the scarcity of data and the different comparators tested in the studies.
In the identified study, 89Sr has failed to show statistical differences in the degree of pain alleviation (either in complete or 100% pain relief; in complete/partial or 50% to 100% pain relief; or in pain scores) with respect to the other radioisotopes. The little data gathered on adverse effects do not show any differences in the safety profile of 89Sr compared to the other agents, with equally low incidence of nerve root compression and hypercalcaemia, and no significant differences in the number of participants with pain flares. Also, there are no differences in the median Karnofsky scores of breast cancer patients after receiving 89Sr or 186Re in one study (Sciuto 2001).
Dose‐comparisons
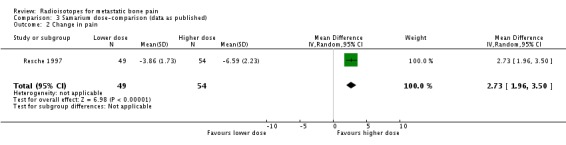
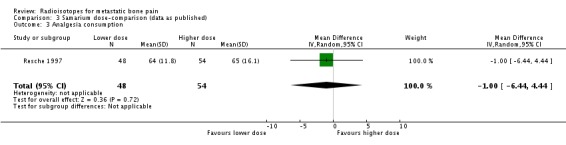
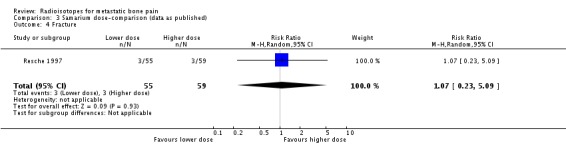
We have shown graphical data only for comparison of doses of 0.5 versus 1.0 mCi of 153Sm (Resche 1997; Serafini 1998). We observed no differences in the frequency of pain relief (any pain relief or 50% to 100% pain relief), although the higher dose showed a greater reduction of pain assessed by means of a visual analogue scale (VAS), without changes in the analgesia intake (pain change mean difference 2.73; 95% CI 1.96 to 3.50). Both doses showed similar incidence of the assessed side effects of 153Sm (fractures, spinal cord compression, pain flares, leucocytopenia, thrombocytopenia, anaemia or death).
A single study comparing a single versus a repeated injection of 188Re (Palmedo 2003) failed to show significant differences among the participants receiving the higher dose and the lower dose for complete pain relief (RR 0.17, 95% CI 0.01 to 3.34) or complete/partial pain relief (50% to 100% pain relief, RR 0.52, 95% CI 0.19 to 1.39), although participants receiving the lower dose tended to achieve some pain relief than participants on the higher dose (RR 0.65, 95% CI 0.48 to 0.89). We observed no differences in the incidence of pain flares (RR 1.44, 95% CI 0.22 to 9.36) and no cases of leucocytopenia or thrombocytopenia in either group.
Sensitivity analysis with imputation of missing data for an intention‐to‐treat analysis
Both the "worst case" scenario and the "same‐as‐control" scenario led to similar results as the main analysis in terms of effect estimation and degree of heterogeneity, for both the pain relief and analgesia consumption outcomes. This finding supports the strength of the analysis and the validity of the results, despite missing data in the original studies.
In the study by Buchali 1988, missing data at 12 months corresponded only to participants who were dead at that timeline and thus were not included in the ITT efficacy analysis, since deaths are unlikely to be related to or prevented by the palliative radioisotope treatment in terminal patients.
Sensitivity analyses by adjuvant role with external beam radiotherapy
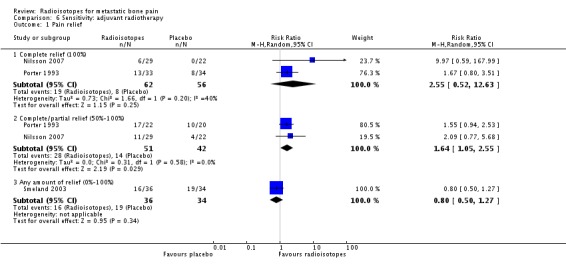
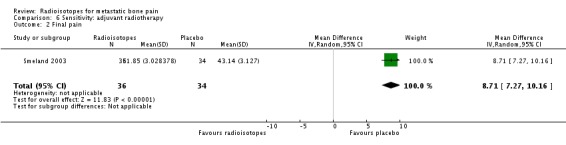
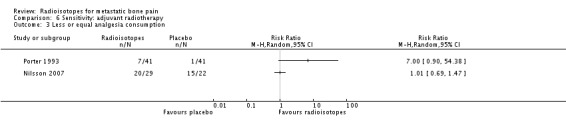
Three studies explored the efficacy of radioisotopes treatment adjuvant to external beam radiotherapy (EBRT) when compared with external beam radiotherapy (Nilsson 2007; Porter 1993; Smeland 2003). When considering only these studies, meta‐analysis results for pain control didn't change substantially: complete relief (RR 2.55; 95% CI 0.52 to 12.63), complete/partial relief (RR 1.64; 95% CI 1.05 to 2.55), any relief (RR 0.80; 95% CI 0.50 to 1.27) and pain relief (MD 8.71; 95% CI 7.27 to 10.16). Also, results for analgesia consumption didn't change.
Sensitivity analysis by time of pain assessment
Although the original review protocol included only studies where pain assessment was performed at least 30 days after treatment, this updated review included a study where assessment was performed at three weeks (Maxon 1991), since it was considered a similar enough time frame. The sensitivity analyses showed no relevant differences with respect to the main analyses.
Sensitivity analysis by publication status of studies
There was a single study partially published (Nair 1999), and it was not included in any meta‐analysis.
Sensitivity analysis by risk of bias
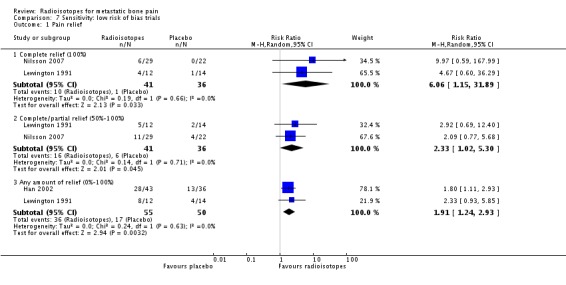
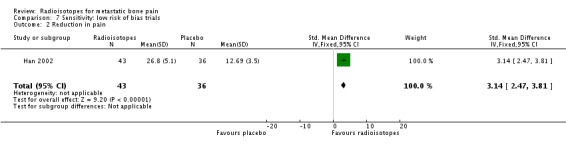
Three studies had globally low risk of bias (Han 2002; Lewington 1991; Nilsson 2007). The analysis restricted to these three studies show a marginally significant effect on all levels of pain relief: complete pain relief (RR 6.06; 95% CI 1.15 to 31.89), complete/partial pain relief (RR 2.33; 95% CI 1.02 to 5.30) and any pain relief (RR 1.91; 95% CI 1.24 to 2.93). At the same time, the results for quantitative reduction in pain also became significant (SMD 3.14; 95% CI 2.47 to 3.81) with no changes in analgesia consumption.
Discussion
Summary of main results
This systematic review was conducted to assess the effectiveness of radioisotopes in the control of pain due to bone metastases and in preventing complications due to bone metastases. The results showed that there is moderate quality evidence supporting a beneficial effect of radioisotopes over placebo for pain control in the short and medium term (one to six months) (NNT for complete pain relief = five, that could be as high as 44; NNT for complete/partial pain relief = four, that could be as high as 54; see Table 9 and Table 10 for a range of plausible values), with no modification of analgesia use. There was no evidence that radioisotopes modify the incidence of complications due to bone metastases (particularly compression of spinal cord) or mortality. There is no available evidence for the effect of radioisotopes on quality of life but a higher incidence of severe secondary effects such as leucocytopenia and thrombocytopenia was observed (NNH for leucocytopenia = 14, 95% CI: 9 to 25). The low quality evidence available on pain flares is inconclusive. We have presented main results and overall quality assessments for this comparison in the Table 1.
This review has identified scarce and low quality evidence on the relative efficacy and safety of 89Sr with respect to other radioisotopes (153Sm, 186Re and 32P). These radioisotopes have similar efficacy and safety profiles as 89Sr.
This review has showed that the existing evidence on the relative efficacy of higher doses versus lower doses of 153Sm is scarce and has low quality. From the data gathered so far, there are no differences between high and low doses in terms of efficacy or safety. Results from a single study with a high risk of bias also failed to show differences in efficacy or safety of a single versus a repeated injection of 188Re.
Overall completeness and applicability of evidence
There are currently three international guidelines available for the use of radioisotopes for metastatic bone pain, published by the European Association of Nuclear Medicine (EANM) (Bodei 2008), the Cancer Care Ontario Program (CCOP) (PGR 2004) and the Society of Nuclear Medicine (SNM) (SNM 2003).
The EANM guideline covers the use of 89Sr, 153Sm and 186Re, whilst the SNM guideline discusses the use of 32P, 89Sr and 153Sm. The guidelines advise the use of radioisotopes for “the treatment of metastatic bone pain that limits normal activities, is not easily controlled by regular analgesics and/or arises from multiple sites not easily controlled by external beam radiotherapy or surgery”, whilst the Cancer Care Ontario Program Guideline goes so far as to advise “The selection of patients for radiopharmaceutical therapy should consider the patient’s unsuitability for alternate palliative interventions (wide field or local field radiotherapy, hormone therapy, chemotherapy, bisphosphonates)”. This guideline states that this treatment should only be given to patients exhibiting increased uptake on bone scintigraphy.
These guidelines report an improvement in pain in 25% to 80% of patients, that pain relief is likely to last two to six months and that retreatment is possible. They report on the common toxicities associated with radioisotopes, particularly haematotoxicity, and provide guidance as to the lower limits of the haematological parameters that can be used to guide treatment (although they do admit that “the precise lower limit is not well defined in literature”). There is agreement that radioisotopes are contraindicated in the management of acute spinal cord compression and pathological fracture.
Despite the previous comments, the EANM guidelines still advise the use of radioisotopes early in the natural history of metastatic bone disease “to increase the rate of therapeutic responses”. The evidence identified so far cannot support or refute this recommendation, since the studies included in this review have recruited specifically participants that have failed conventional treatment. This may be explained in part by the natural process of experiment design, where novel therapeutic agents are first studied as palliative or secondary treatment options on participants that have failed conventional treatment, but it may also reflect current clinical practice. Even with life expectancies of years for patients with bone metastases (particularly for prostate and breast cancer patients when bone is the only site of metastatic disease), clinicians are prescribing several lines of hormone therapy and/or chemotherapy before considering radioisotopes (Australia 2009). Radioisotopes remain as a last option for patients with no other treatments available to them, and often by this stage patients are not suitable for radioisotopes due to poor performance status or insufficient marrow reserve. To consider the administration of radioisotopes earlier in the disease process, radioisotopes should prove to have antitumour activity, besides their pain control effect. Some authors have suggested that this may be the case, and a few sparse trials have been conducted comparing radioisotopes with chemotherapy, radiotherapy or bisphosphonates.
Given the wide number of treatments options available to patients with bone metastases, all of them presenting differential effectiveness and safety profiles, detailed cost effectiveness analyses would be required to evaluate the place of radioisotopes in the array of treatment options. This type of data, so necessary to take informed decisions, is often sorely lacking: a search of cost‐effectiveness radionuclides studies identified a single publication (Velasco 2005), focusing on 153Sm costs. Cost estimates of treatment in UK for the year 2009 give an example of the large cost differences among treatments and the need of economic complementary analyses: Radioisotopes (89Sr and 153Sm) cost in the region of £1300 per injection. This compares with £570 to £1560 for a six‐month course of bisphosphonate (Zolendronate or Pamidronate). Six cycles of cytotoxic chemotherapy cost in the range £1200 (six cycles of single agent Epirubicin) to £6000 (six cycles of single agent Docetaxel), whilst six months of targeted therapy (e.g. Bevacizumab, Trastuzumab, Cetuximab, Erlotinib) cost in the region of £10,000 to £15,000. All of these costs only include the price of the drug, but not the supportive therapies and administration costs. Hormone therapy for six months costs in the range of £15 to £700 (Tamoxifen, Aromatase Inhibitors, Zoladex or Bicalutamide). A single fraction of palliative radiotherapy costs in the range of £50 to £400.
Quality of the evidence
We have analysed 14 studies on the use of radioisotopes in this review, including 1243 participants. This might be considered a fair number of studies and participants, given that the clinical question of interest refers to participants in an advanced stage of a lethal disease and a palliative treatment usually considered after others have failed. Nevertheless the differences between studies with respect to type and dose of studied radioisotopes, comparators used and adjuvant treatments received limit the number of comparable studies from which to extract conclusions. Clinical heterogeneity is further built up due to the differences across studies in outcome scales, endpoint criteria, dose schedules of radioisotopes and adjuvant treatment.
In addition, the moderate risk of bias observed in most included studies reduces the reliability of their results. Only three studies, all of them placebo‐controlled, had a low risk of bias. The sensitivity analysis with these more reliable studies was consistent with respect to the main analysis, although it probably lacked power to detect differences. The overall quality of the evidence according to GRADE about the efficacy of radioisotopes in this population is at best moderate, due to limitations in study design and execution and low number of events (seeTable 1).
Potential biases in the review process
The review authors have tried to be systematic in the identification of eligible studies, including unpublished trials, although no journal handsearching was performed. Authors were contacted to provide and to complete the information published in the original studies in an attempt to overcome some of the problems observed in the included clinical trials. In the case of the E13013 2000 study, even after contacting the IAEA, it was not possible to obtain enough information for a full assessment and analysis of this study. This study was never published fully, despite being a multinational trial recruiting more than 400 participants, funded and promoted by an international agency. This multinational trial was conducted in Argentina, Austria, Brazil, Chile, China and Thailand, and data are only partially available for the Chile and China sub‐studies.
Agreements and disagreements with other studies or reviews
Our review includes two studies (Baczyk 2007; Nilsson 2007; 164 participants) published after the most recent systematic review on this topic (Finlay 2005), providing further evidence on the efficacy of radioisotopes compared to placebo and the first results in the comparison of 89Sr and 153Sm. Our results are in general agreement with the previous review (Finlay 2005). However, while these authors consider the existing evidence to be strongly in favour of radioisotopes, we consider this evidence to be moderate because of the risk of bias of the studies, the limited number of studies and participants studied, and the heterogeneity of their results.
Our review is able to shed more light on the debate of which radioisotope is preferable, although the low number of studies with head‐to‐head comparisons, the heterogeneity between those studies, and the lack of cost‐effectiveness data preclude recommending a particular radioisotope (Brundage 2000; MSAC 1999). The EANM guideline (Bodei 2008) touches on differences between radioisotopes, commenting on their different physical characteristics (half‐life, energy of emissions and type of emissions). This guideline comments that “these differences determine both the clinical benefit and the side effects”, advising that although overall there is “no clear difference in treatment response between 89Sr, 153Sm and 186Re [...] differences in onset of response, duration of response and toxicity do exist”. They state that responses are more rapid and short lived with 153Sm and 186Re whilst the extent and duration of myelosuppression is also less significant. This review has confirmed that there is no evidence for a difference in treatment response between radioisotopes, but did not find any evidence for a difference in toxicities (either temporal or in severity).
The effectiveness of the existing alternatives to palliate pain due to metastatic bone lesions has been reviewed elsewhere (Martinez 2006; McQuay 1999; Pavlakis 2005; Sze 2002; Shelley 2006; Wong 2003; Yuen 2006). Radiotherapy, chemotherapy and bisphosphonates all have a proven effect in reducing bone pain, but it's not clear which is the best treatment or which subgroups of patients will benefit more from each intervention.
While radioisotopes have proven efficacy in pain relief, the relevance of their secondary adverse effects (leucocytopenia and thrombocytopenia) as well as the associated costs have to be carefully considered when compared to other treatment options. As mentioned previously, few trials have compared radioisotopes with radiotherapy, and even fewer trials have compared radioisotopes to chemotherapy (Nilsson 2005; Tu 2001) or bisphosphonates (Wang 2003).
The only radioisotope compared with radiotherapy in RCTs has been 89Sr, either as a stand‐alone treatment or adjuvant to radiotherapy. The relative efficacy of 89Sr is unclear, since the trials present conflicting results regarding which alternative is best for pain control. A previous review noted that 89Sr has similar subjective response rates to external radiotherapy but with no improvement in overall survival (Finlay 2005). Our review identified three studies assessing the efficacy of 89Sr as adjuvant to radiotherapy (Nilsson 2007; Porter 1993; Smeland 2003), and the meta‐analysis restricted to these studies was unable to show differences between treatments for pain control.
The authors of the single study retrieved comparing 89Sr with chemotherapy concluded that 89Sr treatment was preferable since both alternatives had a comparable effect on pain but chemotherapy caused more side effects and need for hospitalisation (Nilsson 2005). It has been hypothesised that cytotoxic drugs can increase the activity of radiation acting as radiosensitisers, although it’s not clear how these two treatments interact. A study of 89Sr plus chemotherapy showed a significant improvement in time to progression and survival in participants assigned 89Sr plus chemotherapy compared to participants receiving chemotherapy alone (Tu 2001). The review of the studies where chemotherapy has been added to 89Sr showed that 89Sr combined with chemotherapy seems to have a substantial therapeutic and palliative advantage over 89Sr alone (Finlay 2005). Nevertheless, current guidelines (Bodei 2008; PGR 2004) recommend that combined treatment with chemotherapy and radioisotopes should still only take place in the context of "experimental clinical trials".
The authors of the single study comparing radioisotopes with respect to bisphosphonates conclude that treatment with 153Sm‐EDTMP had higher efficacy in pain relief, but also higher incidence of blood toxicity than treatment with pamidronate disodium (Wang 2003). The comparison of radioisotopes and bisphosphonates is the most clinically meaningful comparison. However, the frequent use of bisphosphonates for patients with widespread asymptomatic metastatic bone lesions means that this comparison is unlikely to be feasible in the context of a clinical trial.
New agents targeting the action of cancer cells in the bone microenvironment are being developed, like denosumab. Denosumab is a new fully human monoclonal antibody that specifically targets a ligand known as RANKL which is a key mediator of osteoclast formation, function, and survival. Denosumab inhibits osteoclast activity and has been recently authorised in Europe to treat the bone loss associate to the hormonal treatment in men with prostate cancer at risk of vertebral fractures. There are published several clinical trials that assess his efficacy in bone pain metastases but comparative data are only available for bisphosphonates, and no comparison with radioisotopes has been described (Fizazi 2009; Lipton 2007; Stopeck 2010).
Authors' conclusions
This update has included new evidence on the efficacy of radioisotopes versus placebo (five new studies, 462 participants), and two new comparisons: 89Sr compared with other radioisotopes (four studies, 181 participants) and dose‐comparisons of 153Sm (two studies, 193 participants) and 188Re (one study, 64 participants). This updated review has 15 included studies, although we could include only 14 studies in the statistical analyses (1146 participants: 802 receiving active treatment, 344 receiving placebo).
This review main objective was to assess the efficacy of radiopharmaceuticals to control pain in patients with metastatic bone lesions, in comparison with either placebo, other radiopharmaceuticals or other dosages of the same radiopharmaceutical. Also, it aimed to determine their efficacy and safety in terms of prevention or treatment of complications due to bone metastases (hypercalcaemia, fracture and spinal cord compression), patient survival and quality of life, and adverse effects. This review has been able to assess its objectives with respect to the comparison with placebo, in terms of pain control and safety.
There is moderate quality evidence to support a beneficial effect of radioisotopes over placebo for pain control in the short and medium term, with no modification of analgesia use. A higher incidence of severe secondary effects such as leucocytopenia and thrombocytopenia has been observed. Radioisotopes do not modify the incidence of spinal cord compression or mortality. There is no available evidence for the effect of radioisotopes on hypercalcaemia, neutropenia, fractures or quality of life. This review has identified scarce and low quality evidence on the relative efficacy and safety of different radiopharmaceuticals or other dosages of the same radiopharmaceutical. At present radioisotopes remain a secondary option for patients with multifocal pain bone metastases when other more established treatments like radiotherapy, hormone therapy or bisphosphonates have failed
Rigorous parallel, double‐blind clinical trials including long‐term evaluations and larger sample sizes are needed. It is important that future clinical trials include more realistic sample size calculations and properly estimated losses to follow up, due either to mortality or progression of disease, in order to assess their true efficacy.
It is important to determine:
which compounds are most beneficial and safe;
what is the optimal dose and administration route;
what groups benefit the most from therapy;
what is the cost‐effectiveness of each of these compounds;
their adjuvant role with respect to external beam radiotherapy, bisphosphonates or chemotherapy.
Acknowledgements
We are indebted to Caroline Struthers, the Trials Search Co‐ordinator (TSC) of the PaPaS review group, for defining and running the search strategy.
We acknowledge the kindness of Dr Sophie Fossa, Dr Marcuss Thuresson, Dr Klaus Buchali, Dr Inkeri Elomaa and Dr Arthur Porter, who replied to our requests of data clarification and provided help and advice for this review.
We wish to acknowledge the hard work that went in to the original version of this review by Dr Elena Català, Dr Jose Luis Garcia and Dr Marta Ferrandiz. The original review was funded by Agencia Evaluación Tecnologías Sanitarias, Fondo de Investigaciones Sanitarias FIS (grant 00/10011) and Instituto de Salud Carlos III (grants 01/A060 and 01/F070).
We acknowledge the help of the German Cochrane Centre, the Chinese Cochrane Centre, and the Russian Branch of the Nordic Cochrane Centre with the assessment and translation of clinical trials published in German, Chinese and Russian for the first published version of this review.
Our acknowledgement also to Mrs Josefina Figuls, for her invaluable help in developing the review.
Appendices
Appendix 1. Search strategies
PaPaS trials register search strategy
(metasta* AND "bone pain*" AND (radioisotope* OR radionucleotide* OR radionuclide* or strontium or samarium or rhenium))
CENTRAL search strategy
#1 Exp BONE NEOPLASMS sc
#2 Exp NEOPLASM METASTASIS
#3 Exp BONE AND BONES
#4 #2 AND #3
#5 (osseous metasta* or (bone* near metasta*))
#6 #1 OR #4 OR #5
#7 PALLIATIVE CARE (Single term MeSH)
#8 Exp PAIN
#9 (complicat*:ti or complicat*:ab)
#10 hypercalcaemia (Single term MeSH)
#11 (hypercalcaemia or hypercalcaemia)
#12 Exp BONE FRACTURES (Changed from FRACTURES)
#13 (fractur*:ti or fractur:ab)
#14 SPINAL CORD COMPRESSION (Single term MeSH)
#15 ((spin*:ti next cord:ti next compress*:ti) or (spin*:ab next cord:ab next compress*:ab))
#16 Exp NERVE COMPRESSION SYNDROMES
#17 ((nerve near compress*:ti) or (nerve near compress*:ab) or (radicular:ti next compress*:ti) or (radicular:ab next compress*:ab))
#18 (pain*:ti or pain*:ab)
#19 (#7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18)
#20 Exp RADIOISOTOPES
#21 (radionucleotide*:ti or radionucleotide*:ab or radionuclide*:ti or radionuclide*:ab)
#22 (samarium:ti or samarium:ab or strontium:ti or strontium:ab or rhenium:ti or rhenium:ab)
#23 (#20 or #21 or #22)
#24 (#6 and #19 and #23)
MEDLINE via OVID search strategy
1. exp Bone Neoplasms/sc [Secondary]
2. exp Neoplasm Metastasis/
3. exp "Bone and Bones"/
4. 2 and 3
5. (bone$ adj10 metasta$).mp.
6. 1 or 4 or 5
7. PAIN, INTRACTABLE/ or PAIN/
8. Palliative Care/
9. complicat$.ti,ab.
10. complications.hw.
11. hypercalcaemia/
12. hypercalc$.ti,ab.
13. BONE FRACTURES/
14. fractur$.ti,ab.
15. SPINAL CORD COMPRESSION/
16. spin$ compression.ti,ab.
17. NERVE COMPRESSION SYNDROMES/
18. radicular compres$.ti,ab.
19. or/7‐18
20. exp RADIOISOTOPES/
21. (radionucleotide$ or radionuclide$ or radioisotope$).ti,ab.
22. (strontium or samarium or rhenium).ti,ab.
23. or/20‐22
24. 6 and 19 and 23
The subject search was run with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomized trials in MEDLINE: sensitivity maximising version (2008 revision) (OVID format)
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. drug therapy.fs.
6. randomly.ab.
7. trial.ab.
8. groups.ab.
9. or/1‐8
10. humans.sh.
11. 9 and 10
EMBASE via OVID search strategy
1. Bone Metastasis/
2. osseous metasta$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
3. (bone$ adj6 metasta$).ti,ab.
4. or/1‐3
5. exp Palliative Therapy/
6. complication.hw.
7. complicat$.ti,ab.
8. hypercalcaemia/
9. (hypercalcaemia or hypercalcaemia).mp.
10. exp FRACTURE/11. fractur$.ti,ab.
12. Spinal Cord Compression/
13. spin$ cord compress$.ti,ab.
14. "Nerve Root Compression"/
15. radicular compress$.mp. or nerve compress$.ti,ab. or nerve‐root‐compress$.mp.
16. exp PAIN/
17. pain$.ti,ab.
18. or/5‐17
19. exp Radioisotope/
20. radioisotope$.mp. or radionucleotide$.ti,ab. or radionuclide$.ti,ab.
21. (samarium or strontium).ti,ab.
22. rhenium.ti,ab.
23. or/19‐22
24. 4 and 18 and 23
The above subject search was linked to the following Filter for EMBASE via OVID
1. random$.ti,ab.
2. factorial$.ti,ab.
3. (crossover$ or cross over$ or cross‐over$).ti,ab.
4. placebo$.ti,ab.
5. (doubl$ adj blind$).ti,ab.
6. (singl$ adj blind$).ti,ab.
7. assign$.ti,ab.
8. allocat$.ti,ab.
9. volunteer$.ti,ab.
10. CROSSOVER PROCEDURE.sh.
11. DOUBLE‐BLIND PROCEDURE.sh.
12. RANDOMIZED CONTROLLED TRIAL.sh.
13. SINGLE BLIND PROCEDURE.sh.
14. or/1‐13
15. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/
16. HUMAN/
17. 16 and 15
18. 15 not 17
19. 14 not 18
Data and analyses
Comparison 1.
Radioisotopes versus placebo (data as published)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Complete relief (100%) | 4 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [1.32, 3.35] |
| 1.2 Complete/partial relief (50%‐100%) | 3 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [1.13, 2.63] |
| 1.3 Any amount of relief (0%‐100%) | 5 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.77, 2.40] |
| 2 Improvement in pain | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Less or equal analgesia consumption | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Reduction in analgesia consumption | 1 | 13 | Mean Difference (IV, Random, 95% CI) | 5.2 [0.85, 9.55] |
| 5 Compression of spinal cord | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.39, 3.07] |
| 6 Pain flares | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.27, 2.06] |
| 7 Side effects grade III‐IV | 5 | 970 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [0.02, 0.07] |
| 7.1 Leucocytopenia | 4 | 410 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.04, 0.11] |
| 7.2 Thrombocytopenia | 4 | 313 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.03, 0.11] |
| 7.3 Anemia | 2 | 247 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.04, 0.09] |
| 8 Death | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.27, 4.77] |
Analysis 1.1.
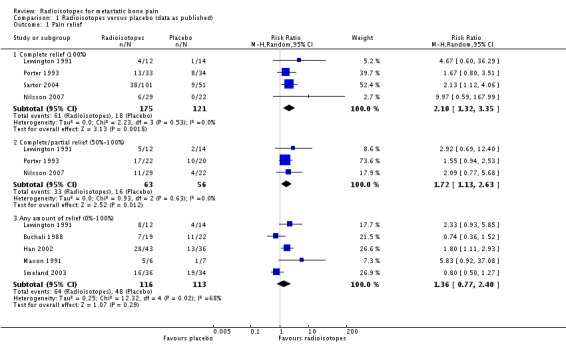
Comparison 1 Radioisotopes versus placebo (data as published), Outcome 1 Pain relief.
Analysis 1.2.
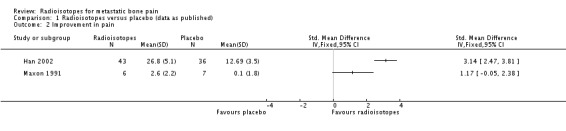
Comparison 1 Radioisotopes versus placebo (data as published), Outcome 2 Improvement in pain.
Analysis 1.3.
Comparison 1 Radioisotopes versus placebo (data as published), Outcome 3 Less or equal analgesia consumption.
Analysis 1.4.
Comparison 1 Radioisotopes versus placebo (data as published), Outcome 4 Reduction in analgesia consumption.
Analysis 1.5.
Comparison 1 Radioisotopes versus placebo (data as published), Outcome 5 Compression of spinal cord.
Analysis 1.6.
Comparison 1 Radioisotopes versus placebo (data as published), Outcome 6 Pain flares.
Analysis 1.7.
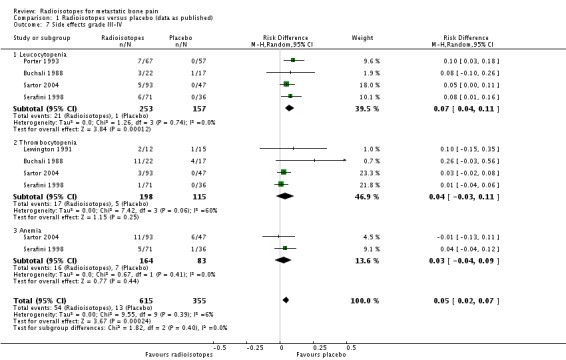
Comparison 1 Radioisotopes versus placebo (data as published), Outcome 7 Side effects grade III‐IV.
Analysis 1.8.
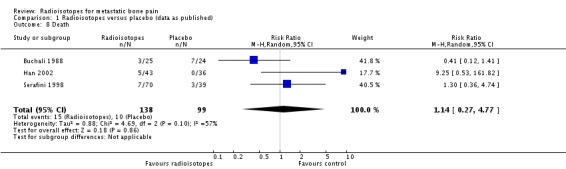
Comparison 1 Radioisotopes versus placebo (data as published), Outcome 8 Death.
Comparison 2.
Different radioisotopes (data as published)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Complete relief (100%) | 3 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Complete/partial relief (50%‐100%) | 3 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Any amount of relief (0%‐100%) | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Compression of nervous roots | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 3 Hypercalcaemia | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.31, 3.24] |
| 4 Pain flares | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5 Final quality of life | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐9.98, 11.98] |
Analysis 2.1.
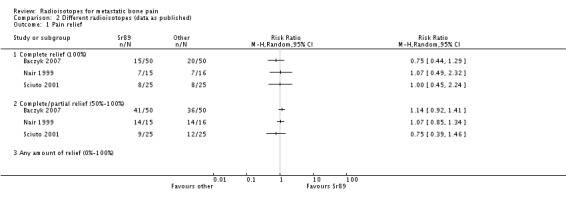
Comparison 2 Different radioisotopes (data as published), Outcome 1 Pain relief.
Analysis 2.2.
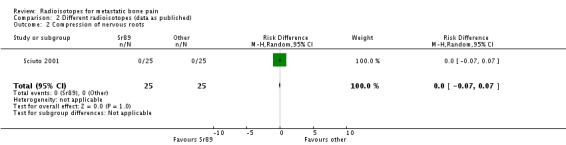
Comparison 2 Different radioisotopes (data as published), Outcome 2 Compression of nervous roots.
Analysis 2.3.
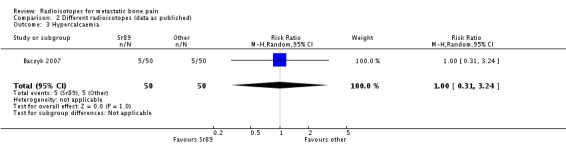
Comparison 2 Different radioisotopes (data as published), Outcome 3 Hypercalcaemia.
Analysis 2.4.
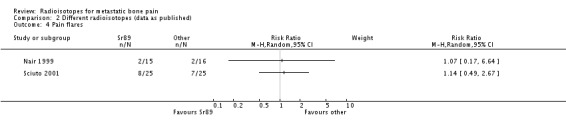
Comparison 2 Different radioisotopes (data as published), Outcome 4 Pain flares.
Analysis 2.5.
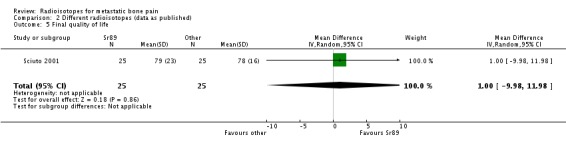
Comparison 2 Different radioisotopes (data as published), Outcome 5 Final quality of life.
Comparison 3.
Samarium dose‐comparison (data as published)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Complete relief (100%) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Complete/partial relief (50%‐100%) | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.59, 1.14] |
| 1.3 Any amount of relief (1%‐100%) | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.74, 1.24] |
| 2 Change in pain | 1 | 103 | Mean Difference (IV, Random, 95% CI) | 2.73 [1.96, 3.50] |
| 3 Analgesia consumption | 1 | 102 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐6.44, 4.44] |
| 4 Fracture | 1 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.23, 5.09] |
| 5 Compression of spinal cord | 2 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [0.54, 5.92] |
| 6 Pain flares | 2 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.47, 2.89] |
| 7 Leucocytopenia III‐IV | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.12, 2.30] |
| 8 Thrombocytopenia III‐IV | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.31, 2.01] |
| 9 Anemia III‐IV | 1 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.26, 8.21] |
| 10 Death | 2 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.63, 2.59] |
Analysis 3.1.
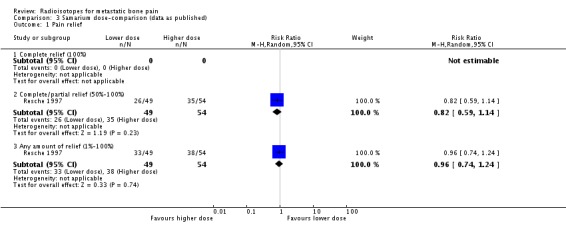
Comparison 3 Samarium dose‐comparison (data as published), Outcome 1 Pain relief.
Analysis 3.2.
Comparison 3 Samarium dose‐comparison (data as published), Outcome 2 Change in pain.
Analysis 3.3.
Comparison 3 Samarium dose‐comparison (data as published), Outcome 3 Analgesia consumption.
Analysis 3.4.
Comparison 3 Samarium dose‐comparison (data as published), Outcome 4 Fracture.
Analysis 3.5.
Comparison 3 Samarium dose‐comparison (data as published), Outcome 5 Compression of spinal cord.
Analysis 3.6.
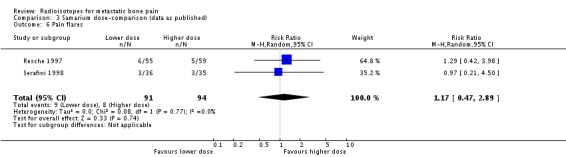
Comparison 3 Samarium dose‐comparison (data as published), Outcome 6 Pain flares.
Analysis 3.7.
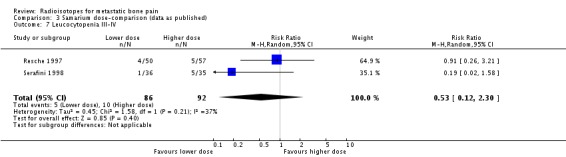
Comparison 3 Samarium dose‐comparison (data as published), Outcome 7 Leucocytopenia III‐IV.
Analysis 3.8.
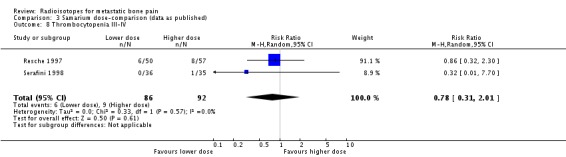
Comparison 3 Samarium dose‐comparison (data as published), Outcome 8 Thrombocytopenia III‐IV.
Analysis 3.9.
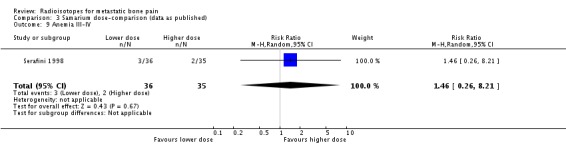
Comparison 3 Samarium dose‐comparison (data as published), Outcome 9 Anemia III‐IV.
Analysis 3.10.
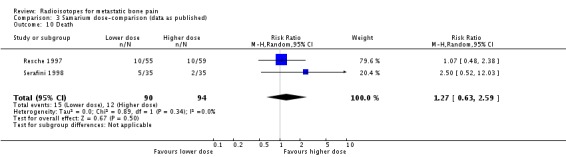
Comparison 3 Samarium dose‐comparison (data as published), Outcome 10 Death.
Comparison 4.
Radioisotopes versus placebo (ITT worst case)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Complete relief (100%) | 4 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.32, 3.37] |
| 1.2 Complete/partial relief (50%‐100%) | 3 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [1.16, 2.77] |
| 1.3 Any amount of relief (0%‐100%) | 5 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.81, 2.49] |
| 2 Less or equal analgesia consumption | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Analysis 4.1.
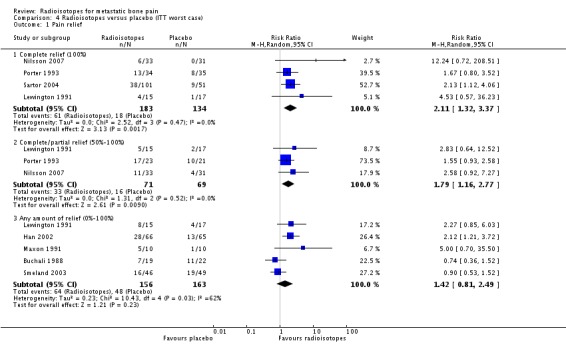
Comparison 4 Radioisotopes versus placebo (ITT worst case), Outcome 1 Pain relief.
Analysis 4.2.
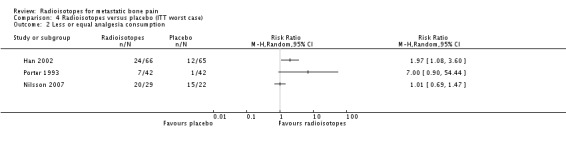
Comparison 4 Radioisotopes versus placebo (ITT worst case), Outcome 2 Less or equal analgesia consumption.
Comparison 5.
Radioisotopes versus placebo (ITT control group rate)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Complete relief (100%) | 4 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.32, 3.37] |
| 1.2 Complete/partial relief (50%‐100%) | 3 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.11, 2.42] |
| 1.3 Any amount of relief (0%‐100%) | 5 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.73, 2.07] |
| 2 Less or equal analgesia consumption | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Analysis 5.1.
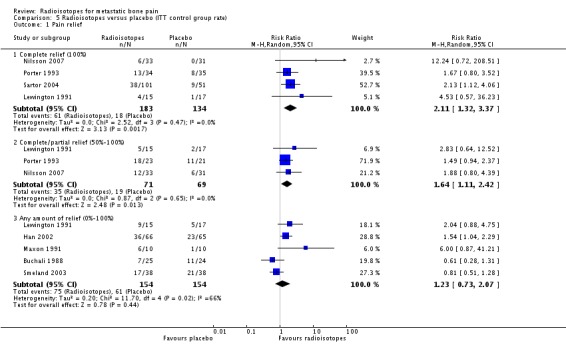
Comparison 5 Radioisotopes versus placebo (ITT control group rate), Outcome 1 Pain relief.
Analysis 5.2.
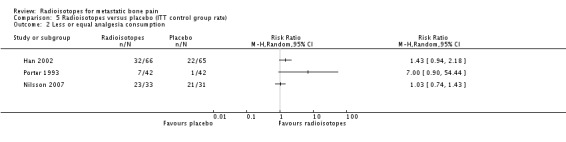
Comparison 5 Radioisotopes versus placebo (ITT control group rate), Outcome 2 Less or equal analgesia consumption.
Comparison 6.
Sensitivity: adjuvant radiotherapy
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Complete relief (100%) | 2 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 2.55 [0.52, 12.63] |
| 1.2 Complete/partial relief (50%‐100%) | 2 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.05, 2.55] |
| 1.3 Any amount of relief (0%‐100%) | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.50, 1.27] |
| 2 Final pain | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 8.71 [7.27, 10.16] |
| 3 Less or equal analgesia consumption | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Analysis 6.1.
Comparison 6 Sensitivity: adjuvant radiotherapy, Outcome 1 Pain relief.
Analysis 6.2.
Comparison 6 Sensitivity: adjuvant radiotherapy, Outcome 2 Final pain.
Analysis 6.3.
Comparison 6 Sensitivity: adjuvant radiotherapy, Outcome 3 Less or equal analgesia consumption.
Comparison 7.
Sensitivity: low risk of bias trials
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Complete relief (100%) | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 6.06 [1.15, 31.89] |
| 1.2 Complete/partial relief (50%‐100%) | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [1.02, 5.30] |
| 1.3 Any amount of relief (0%‐100%) | 2 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [1.24, 2.93] |
| 2 Reduction in pain | 1 | 79 | Std. Mean Difference (IV, Fixed, 95% CI) | 3.14 [2.47, 3.81] |
| 3 Less or equal analgesia consumption | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Analysis 7.1.
Comparison 7 Sensitivity: low risk of bias trials, Outcome 1 Pain relief.
Analysis 7.2.
Comparison 7 Sensitivity: low risk of bias trials, Outcome 2 Reduction in pain.
Analysis 7.3.
Comparison 7 Sensitivity: low risk of bias trials, Outcome 3 Less or equal analgesia consumption.
What's new
Last assessed as up‐to‐date: 10 June 2011.
| Date | Event | Description |
|---|---|---|
| 1 April 2015 | Amended | This review has been withdrawn. See Published notes |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 10 June 2011 | New search has been performed | This update has incorporated two new comparisons (comparing different radioisotopes and dose‐comparisons). It has also introduced several methodological improvements (changes in statistical methods, risk of bias assessment, summary of findings table). Search updated in July 2008 and further up to date in October 2010, and included 11 additional studies (821 additional participants). New studies are: Baczyk 2007, E13013 2000, Han 2002, Maxon 1991, Nair 1999, Nilsson 2007, Palmedo 2003, Resche 1997, Sartor 2004, Sciuto 2001, Smeland 2003. |
| 10 June 2011 | New citation required but conclusions have not changed | Conclusions have not changed significantly but readers of the review should re‐read the update as the conclusions have been revised and the methodology brought up to date. |
| 9 July 2008 | Amended | Converted to new review format. |
| 27 July 2005 | Amended | Minor revisions made to the review. |
Differences between protocol and review
This updated review has introduced some methodological changes with respect to the previous review. First, the inclusion criteria have been modified slightly: requirement of pain assessments at a minimum four weeks after treatment has been eliminated, with the consequence of including one additional study excluded in the first version of the review (Maxon 1991), and comparisons of interest have been expanded to comparisons between radioisotopes (different radioisotopes, or the same radioisotope but at different doses), as well as including radioisotopes chelated with a bisphosphonate.
Secondly, the quality assessment of studies is no longer based on the Jadad and OPVS scores, but on risk of bias tables, assessing the domains of random sequence generation, allocation concealment, blinding and incomplete data assessment.
Thirdly, the main statistical analysis has changed to an available data analysis, and the two imputation ITT analysis have become secondary analysis. Also, the subgroup analyses (by primary tumour location, stage and route of treatment) planned in the first published version of the review were removed.
Finally, a new sensitivity analysis was introduced, to explore whether radioisotopes administered as adjuvant treatment with EBRT had different efficacy than radioisotopes alone.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised trial. | |
| Participants | N = 100 prostate and breast cancer patients (60 with prostate carcinoma, 40 with breast carcinoma). Metastatic bone lesions confirmed by 99mTcMDP bone scan. Participants had to have unsatisfactory pain control with NSAID and/or opioids. (50 to 89Sr, 50 to 153SmEDTMP). |
|
| Interventions | 1. 89Sr at 150 MBq. 2. 153SmEDTMP at 37 MBq/kg of body mass. Follow up: 2 months. |
|
| Outcomes | Pain relief with a 0‐10 VAS assessed by the patient two months after treatment. Analgesic consumption assessed as percent dosage reduction of NSAID and opioids assessed two months after treatment. Karnofsky Performance Score assessed two months after treatment. Adverse effects. |
|
| Notes | 0 patients were lost or withdrawn. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Patients | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Study researchers | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data are provided for all randomised patients. |
| Methods | Randomised double blind trial. | |
| Participants | N = 49 men with prostate cancer. Metastatic bone lesions confirmed by 99mTcEHDP bone scan and radiographs. (25 on 89Sr, 24 on placebo). |
|
| Interventions | 1. 75 MBq/mo for 3 months 89Sr administered iv 2. Placebo. Follow up: 36 months. |
|
| Outcomes | Patient reported subjective pain palliation. Survival up to 2 years. Toxicity assessed at 3, 6 and 12 months. |
|
| Notes | 8 patients had no pain at start of treatment, 10 patients died whilst on treatment, 0 patients withdrawn, 0 patients lost to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Patients | Low risk | "Patients were selected randomly, and the group they were in was not uncovered earlier than one year after the start of treatment". |
| Blinding (performance bias and detection bias) Study researchers | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers described but no causes. High attrition rate. |
| Methods | Published data for the Chinese patients of a multinational International Atomic Energy Agency trial (E13013) that's never been published in full. | |
| Participants | N = 160 cancer patients were enlisted in the Chinese portion of the trial. Total number of randomised patients unclear. 105 patients analysed in the Chinese paper. Patients with any primary tumour histology had to present histologically confirmed bone metastases with pain. | |
| Interventions | 1. 153Sm EDTMP 37 mBq/Kg (1.0 mCi) administered iv 2. 153Sm EDTMP 18.5 mBq/Kg (0.5 mCi) administered iv Follow up: 16 weeks. |
|
| Outcomes | Pain relief with a 5 categories nominal scale. Response to the therapy based on the patient's pain score and the corresponding time after treatment. Analgesia consumption. Karnofsky Performance Score assessed weekly/2 weekly/monthly. Adverse effects assessed weekly/2 weekly/monthly. Analgesic consumption, symptoms and sleeping and eating patterns were recorded by patients and/or family members. |
|
| Notes | This trial is included in the review, but it's not analysed due to insufficient information. Information about full trial accessed from http://www‐crp.iaea.org/html/rifa‐show‐detail.asp?ProgCode=E13013 on 3/12/2008. The original trial compared 3 different doses of 153SM EDTMP, although the Chinese publication states they only tested two doses. Full trial is described summarily in an abstract (Olea 2000). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Patients | High risk | "At the beginning, the dose was kept blind from the patient but not from his/her family and the referring doctor". |
| Blinding (performance bias and detection bias) Study researchers | High risk | "During the later part of the project, no one except the working team was informed of the dose used". |
| Blinding (performance bias and detection bias) Outcome assessment | High risk | "Analgesic consumption, symptoms and sleeping and eating patterns were recorded by patients and/or family members". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It's not clear the total and arm‐wise number of randomised patients, because they only analyse those who have a follow up of 16 weeks in October 1997 from 160 enlisted patients. "During the initial phase of the study, the lower dose was more frequently refused by the referring doctor and/or the patient's family resulting in withdrawal of the patient from the follow‐up". Thus, the patients described in the trial are biased towards the higher dose. |
| Methods | Double blind randomised trial. | |
| Participants | N = 131 men with prostate cancer. All patients had scintigraphic and radiologic evidence of at least 4 bone metastases. Patients with bone metastases from malignancies other than prostate cancer were not included in this study. Patients had to present symptomatic bone metastases no longer responding to any medical or surgical endocrine manipulation treatments. (66 on 186Re, 65 on placebo). |
|
| Interventions | 1. 186Re in doses ranging from 1.295 to 2.960 MBq (35‐80 mCi) administered iv 2. Placebo isotonic sterile saline in 2 ml vials administered iv Follow up: 12 weeks. |
|
| Outcomes | Positive response days assessed as a composite endpoint (number of days pain was reduced 25% compared with the median baseline pain index value, while the medication index and daily activity score remained constant or increased, or on which pain was reduced 25% and the medication index or daily activity score improved 25% compared with baseline levels, without worsening of the remaining factor. Pain relief with a 0‐100 VAS assessed by participants twice daily until 12 wk after treatment or until the request for radiotherapy. Analgesic intake assessed by participants daily with a medication index. Daily activities assessed daily by participants through validated questions. Request of palliative radiotherapy. Survival. PSA levels. |
|
| Notes | 7 patients didn't receive treatment with 186Re, 13 patients didn't receive treatment with placebo. 13 patients were not evaluable on the 186Re group, 16 patients were not evaluable on the placebo group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed by the hospital pharmacist". |
| Allocation concealment (selection bias) | Low risk | "Predetermined randomization list form the Center for Biostatistics". |
| Blinding (performance bias and detection bias) Patients | Low risk | "The hospital pharmacist was the only person during the entire study who knew that therapy was prepared and given". "The infusions were identical in appearance after preparation to ensure blinding. Other study personnel, as well as the patients and the investigators, remained unaware of the treatment assigned during the entire study". |
| Blinding (performance bias and detection bias) Study researchers | Low risk | "The hospital pharmacist was the only person during the entire study who knew that therapy was prepared and given". "The infusions were identical in appearance after preparation to ensure blinding. Other study personnel, as well as the patients and the investigators, remained unaware of the treatment assigned during the entire study". |
| Blinding (performance bias and detection bias) Outcome assessment | Low risk | "The hospital pharmacist was the only person during the entire study who knew that therapy was prepared and given". "The infusions were identical in appearance after preparation to ensure blinding. Other study personnel, as well as the patients and the investigators, remained unaware of the treatment assigned during the entire study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and causes for lost or not evaluated patients are broadly described, but numbers are unequivalent, and the percentage of losses is high". |
| Methods | Crossover randomised double blind trial. | |
| Participants | N = 32 men with prostate cancer metastatic to bone which had escaped control by conventional treatments (hormone manipulation, local radiotherapy or analgesics). All patients had increasing requirements for narcotic analgesics. (15 on 89Sr, 17 on placebo). | |
| Interventions | 1. 150 MBq 89Sr monodose iv 2. Placebo. Follow up: 10 weeks. |
|
| Outcomes | Pain and analgesic assessed daily by patients. Response assessed by the physician every 5 weeks by a 5 categories numerical weighting system. Hematological toxicity assessed after treatment. |
|
| Notes | 5 patients died or too ill to assess, 2 patients withdrawn but assessed, 0 patients lost to follow up, 1 protocol violation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random number tables". |
| Allocation concealment (selection bias) | Low risk | "The radiopharmaceuticals were supplied in coded vials by Amersham International, who, as the central statistical office, were responsible for randomisation". |
| Blinding (performance bias and detection bias) Patients | Low risk | "Both the Strontium‐89 and the placebo were spiked with Strontium‐85 to mask 'white' radiation". |
| Blinding (performance bias and detection bias) Study researchers | Low risk | "Both the Strontium‐89 and the placebo were spiked with Strontium‐85 to mask 'white' radiation". |
| Blinding (performance bias and detection bias) Outcome assessment | Low risk | "Treatments were randomly allocated and assessed blind". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fully described. |
| Methods | Crossover randomised double blind trial. | |
| Participants | N = 20 patients with any primary tumour histology (9 with prostate cancer, 2 with lung cancer, 1 with breast cancer, 1 with thyroid cancer, 7 patients not described). Metastatic bone lesions confirmed by 99mTc bone scan and radiographs. (10 on 186Re, 10 on placebo). |
|
| Interventions | 1. 186Re (sn)HEDP with mean activity 34.0 +‐ 0.8 mCi (1258+‐ 29.6 MBq) administered iv 2. Placebo 99Tc MDP methylene diphosphonate with mean activity 17.7 +‐ 1.5 mCi (654.9 +‐ 55.5 MBq) administered iv Follow up: 12 weeks after initial treatment. |
|
| Outcomes | Pain index based on the patient's daily assessment of the severity of their pain at rest and during activity. Patient's and physicians' subjective impression of pain improvement. Analgesia index based on the number of doses and type of medication used for pain relief assessed daily by the patients. Toxicity. |
|
| Notes | 4 patients were not evaluable on the 186Re group, 3 patients were not evaluable on the placebo group. Outcomes assessed at 3 weeks. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Patients | Low risk | "All physicians, nurses and patients were kept blinded regarding which compound was administered at any time." |
| Blinding (performance bias and detection bias) Study researchers | Low risk | "All physicians, nurses and patients were kept blinded regarding which compound was administered at any time." |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number and reasons of unevaluable patients are described, but due to the small sample size, the losses represent a high percentage. All but one of the losses could be analysed in a first‐period analysis. |
| Methods | Published data for the Indian subset of patients of a multinational International Atomic Energy Agency randomised trial (E13010), that's never been published in full. | |
| Participants | N = 31 cancer patients with any primary tumour histology (13 with prostate cancer, 12 with breast cancer, 3 with lung cancer, 3 with other cancers). Metastatic bone lesions confirmed by radionuclide bone scan. Patients had to present a minimum level of pain from the scan‐positive sites (score of 5 to 10 on a 0‐10 VAS ) not relieved by therapy with non‐narcotic analgesia or with narcotic medication. (15 on 89Sr, 16 on 32P). |
|
| Interventions | 1. 89Sr 4 mCi (148 MBq). 2. 32P 12 mCi. Follow up: 4 months. |
|
| Outcomes | Pain relief assessed daily by patients through a 0‐10 VAS. Analgesic score based on daily assessed analgesic type and administration frequency (0‐20 range). Mobility score assessed every two weeks by a 4‐points score. Pain flares. Toxicity graded as recommended by the IAEA (White blood cell X 109/L:grade 0> = 4.0; grade 1 = 3.0‐3.9; grade 2 2.0‐2.9; grade 3 = 1.0‐1.9; grade 4 < 1.0. Platelets X 109/L:grade 0 = 150‐400; grade 1 = 75‐149.9; grade 2 = 50.0‐74.9; grade 3 = 25.0‐49.9; grade 4 < 25.0. Absolute granulocyte counts of less than 1000 were considered as significant toxicity). Death. |
|
| Notes | Summary description of full trial in http://www‐crp.iaea.org/html/rifa‐show‐detail.asp?ProgCode=E13010. It's not clear whether the outcomes were assessed by the patient or their relatives. "A set of data sheets was given to the patient's relatives to fill out and return every 2 weeks". 0 patients lost to follow up or withdrawn. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | "Randomization of patients to the radionuclide was done at the IAEA, which then informed the center of the radionuclide to be used in a given patient". |
| Blinding (performance bias and detection bias) Patients | Unclear risk | "Two responsible relatives of the patient were taken into confidence and the details of treatment, the stage of the disease and the expected outcome were explained". |
| Blinding (performance bias and detection bias) Study researchers | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Apparently, all randomised patients were assessed. |
| Methods | Double blind randomised trial. | |
| Participants | N = 64 patients with prostate cancer. Patients had to have multiple bone metastases or one painful lesion with two consecutive rising amounts of serum PSA. (33 on 223Ra, 31 on placebo). |
|
| Interventions | 1. 223Ra in dose 50kBq administered iv every 4 weeks during 12 weeks (total 4 injections) + external beam radiotherapy. 2. Placebo administered iv every 4 weeks during 12 weeks (total 4 injections) + external beam radiotherapy. Treatment injection administered on the first day of radiotherapy. External beam radiotherapy administered at the most painful site, either one fraction of 8 Gy or a fractionated course of 20 Gy over 1 week in 5 fractions, or 30 Gy over 2 weeks in 10 fractions. Follow up: 4 months. |
|
| Outcomes | Skeletal related events defined as: • 25% increase in pain severity index, computed from patients' assessment of pain on Brief Pain Inventory forms (scale 0–10). • Increased analgesic consumption based on the WHO ladder for cancer pain. • Neurological symptoms secondary to skeletal manifestations of prostate cancer. • New pathological bone fractures (vertebral and non‐vertebral). • Tumour‐related orthopaedic surgical intervention. • Subsequent external‐beam radiation to relieve skeletal pain. • Use of radioisotopes to relieve new skeletal‐related symptoms. • Use of corticosteroids for skeletal pain palliation. • Use of chemotherapy, bisphosphonates, or hormones to treat progression of skeletal disease. Bone serum alkaline phosphatase. Safty and toxicity assessed 2 weekly up to 4 weeks and at 6, 9, 12, 18 and 24 months. |
|
| Notes | 4 patients were not evaluable on the 223Ra group, 9 patients were not evaluable on the placebo group. All patients withdrawn before week 16 of study (4 weeks of treatment + 12 weeks follow up). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was done centrally with a random number generator stratified by centre". |
| Allocation concealment (selection bias) | Low risk | "Randomisation was done centrally with a random number generator stratified by centre", |
| Blinding (performance bias and detection bias) Patients | Low risk | "An individual from the nuclear medicine department at every centre was responsible for study treatment preparation and labelling". |
| Blinding (performance bias and detection bias) Study researchers | Low risk | "An individual from the nuclear medicine department at every centre was responsible for study treatment preparation and labelling. Researchers remained masked to treatment allocation." |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All patients followed for survival irrespective of study status." "Patients who received at least one dose of study drug were included in the analysis and all results were analyzed by intention to treat." |
| Methods | Randomised open trial. | |
| Participants | N = 64 men with prostate cancer. Metastatic bone lesions confirmed by 99mTcMDP bone scan and x‐ray. Patients had to suffer from multifocal pain and with at least five different sites of bone metastases. (32 on single injection of 188ReHEDP, 32 on two injections of 188ReHEDP). |
|
| Interventions | 1. 188ReHEDP single injection of 1.1 MCi (40.7 MBq)/kg of body weight). 2. 188ReHEDP in two injections separated by 8 weeks. Follow up: until death of patient. |
|
| Outcomes | Pain relief assessed daily by the patient through a 0‐10 VAS. Pain medication index assessed daily by the patient . PSA levels assessed monthly. Toxicity assessed monthly by the National Cancer Institute Common Toxicity Criteria. Survival. |
|
| Notes | Patients were randomised 7 weeks after first injection. 2 patients lost from follow up in single injection group, 4 patients lost to follow up in double injection group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Patients | High risk | Open trial. |
| Blinding (performance bias and detection bias) Study researchers | High risk | Open trial. |
| Blinding (performance bias and detection bias) Outcome assessment | High risk | Open trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patients lost and not assessed are described for each group, with reasons for loss. Number of losses is similar between groups. |
| Methods | Randomised double blind trial. | |
| Participants | N = 126 men with prostate cancer with increasing pain requiring radiotherapy and a worsening bone scan. (68 on 89Sr, 58 on placebo). |
|
| Interventions | 1. 89Sr 10,8 mCi monodose iv + Radiotherapy. 2. Placebo + Radiotherapy. Follow up: 6 months. |
|
| Outcomes | Pain relief graded 0‐5 (RTOG scoring system). Pain score computed as the product of the severity of pain and the frequency of pain. Intake of analgesics graded 0‐4 (RTOG scoring system). Analgesic score computed as the product of the type of pain relief and its frequency of use. QOL assessed by a VAS. Survival. Requirement of further radiotherapy. Development of new pain sites. Toxicity assessed according to RTOG guidelines. |
|
| Notes | 0 patients withdrawn, 2 patients lost to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | "All study material was allocated to individual registered patients by the Cross Cancer Institute in Edmonton, which acted as the study randomization center". |
| Blinding (performance bias and detection bias) Patients | Low risk | "To ensure blinding, both active and placebo materials were spiked with a trace (50 microCi) of strontium‐85." |
| Blinding (performance bias and detection bias) Study researchers | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Numbers described but no causes nor which group did they belong to. |
| Methods | Randomised dose‐controlled trial. | |
| Participants | N = 114 cancer patients with any primary tumour histology (67 with prostate cancer, 36 with breast cancer, 2 with lung cancer, 9 with other cancers). Metastatic bone lesions confirmed by 99mTc bone scan. Participants had to present pain at one or more sites overlying abnormal uptake on the bone scan. (55 to 0.5 MCi dose of 153Sm EDTMP, 59 to 1.0 MCi dose of 153Sm EDTMP). |
|
| Interventions | 1. 153Sm EDTMP 0.5 mCi/Kg administered iv for 1 minute. 2. 153Sm EDTMP 1.0 mCi/Kg administered iv for 1 minute. Follow up: 16 weeks. |
|
| Outcomes | Pain relief with a 0‐10 VAS and nominal scale with 5 categories, assessed weekly by the patient in a diary. Analgesia consumption, assessed weekly by the patient in a diary. Adverse effects and toxicities assessed by weekly/monthly laboratory tests . |
|
| Notes | Efficacy results are presented at 4 weeks. At 4 weeks, 11 patients received concomitant therapies and are excluded from efficacy analyses. At 16 weeks, 58 patients were excluded for requiring alternative therapy, death, disease progression and other causes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were centrally randomised". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Patients | Low risk | "The patients knew they were receiving active treatment but did not know the dose." |
| Blinding (performance bias and detection bias) Study researchers | High risk | "The physicians knew which dose each patient received". |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number and causes of patients lost for assessment at 4 weeks are described. Number of patients included both in efficacy and safety evaluations are provided. |
| Methods | Randomised double blind trial. | |
| Participants | N = 152 men with prostate cancer. Metastatic bone lesions confirmed by a positive bone scan. Patients had to have pain scores greater than 30 on a 0‐100 VAS or use of opioid analgesics in daily doses equivalent to 60 mg oral morphine during the baseline assessment period. (101 on 153Sm, 51 on placebo). |
|
| Interventions | 1. 153Sm‐EDTMP 1 mCi/Kg administered iv for 1 minute. 2. Non‐radioactive 152Sm‐EDTMP 1 MCi/Kg administered iv for 1 minute. Follow up: 4 weeks for the double blind part of the trial. |
|
| Outcomes | Pain relief assessed twice daily by patients through a 0‐100 VAS and nominal assessment with 8 categories. Analgesic consumption assessed twice daily by patients. PSA levels assessed monthly. Toxicity and safety evaluated by laboratory tests, vital sign monitoring, physical examinations, and recording of adverse events. Hematologic tests were performed at baseline and weekly until week 8, or until hematologic recovery. |
|
| Notes | No patients lost to follow up or withdrawn. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Centrally randomized". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Patients | Low risk | "The preparation of all doses was performed by an informed third party at each study site in a manner that "blinded" the clinician‐investigators and patients." |
| Blinding (performance bias and detection bias) Study researchers | Low risk | "The preparation of all doses was performed by an informed third party at each study site in a manner that 'blinded' the clinician‐investigators and patients. All procedures were identical for both drug and placebo, including the use of silent radioactive detection devices and surveillance by technologists for all treatments and placebo." |
| Blinding (performance bias and detection bias) Outcome assessment | High risk | Open assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Apparently, there were no losses. Safety data is presented for a subset of patients. |
| Methods | Open label randomised trial. | |
| Participants | N = 50 patients with breast cancer (48 women, 2 men). Multiple metastatic bone lesions confirmed by 99mTcMDP bone scan. Patients had to have pain resistant to chronic analgesic intake (both opiate and NSAIDs). (25 to 89Sr, 25 to 186ReHEDP). |
|
| Interventions | 1. 89Sr 148 MBq administered as bolus through iv saline drip. 2. 186Re HEDP 1406 MBq administered as bolus through iv saline drip. Follow up: 6 months. |
|
| Outcomes | Pain relief assessed daily by patients. Pain score assessed through a modified Wisconsin test consisting of a five‐point pain‐rating scale (from 0 to 4) recording pain severity, pain frequency, analgesic intake and change in sleep patterns. Analgesic intake. Karnofsky Performance Score assessed monthly. Hematologic toxicity and adverse effects reported monthly according to WHO guidelines. |
|
| Notes | 0 patients were lost or withdrawn. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Patients | High risk | Open label study. |
| Blinding (performance bias and detection bias) Study researchers | High risk | Open label study. |
| Blinding (performance bias and detection bias) Outcome assessment | High risk | Open label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Apparently, all randomised patients were evaluated. |
| Methods | Randomised double blind trial. | |
| Participants | N = 118 patients with any primary tumour histology (80 with prostate cancer, 21 with breast cancer, 6 with lung cancer and 13 with other cancers). Patients had to have painful metastes to bone overlying enhanced uptake on a diagnostic bone scan. (39 1.0 dose of 153Sm, 40 on 0.5 dose of 153Sm, 39 on placebo). |
|
| Interventions | 1. 153Sm 0.5 mCi/Kg iv monodose. 2. 153Sm 1.0 mCi/Kg iv monodose. 3. Placebo iv monodose. Follow up: 16 weeks. |
|
| Outcomes | Pain relief assessed daily by patients on a 0‐10 VAS. Opioid analgesic intake (type and dose) assessed daily by patients. Physician Global Assessment assessed weekly/monthly. Toxicity assessed weekly/monthly according to National Cancer Institut Common Toxicity Criteria. |
|
| Notes | 2 patients unassessable at 4 weeks, 0 patients withdrawn, 0 patients lost to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Patients | Low risk | "Placebo was prepared identically to the active drug, except that it contained nonradioactive 152Sm. The study agents were shipped frozen to each site and stored frozen until dose preparation by an unblinded third party." "Radiation exposure was measured by a radiation safety officer with a silent meter to maintain blinding." |
| Blinding (performance bias and detection bias) Study researchers | Low risk | "Placebo was prepared identically to the active drug, except that it contained nonradioactive 152Sm. The study agents were shipped frozen to each site and stored frozen until dose preparation by an unblinded third party." "Radiation exposure was measured by a radiation safety officer with a silent meter to maintain blinding." |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 patients in the placebo group were excluded from efficacy analyses (but not from safety analyses) due to being considered outliers. Apparently, no other pt was lost on week 4. Number and causes of losses at 16 weeks are described. |
| Methods | Double blind randomised trial. | |
| Participants | N = 95 cancer participants with any primary tumour histology (64 with prostate cancer, 19 with breast cancer and 10 with other cancers). Metastatic bone lesions confirmed by bone scan and radiographs. Patients were referred for painful skeletal metastases. (46 on 89Sr, 49 on placebo). |
|
| Interventions | 1. 89Sr at 150 MBq dose administered iv + external beam radiotherapy. 2. Placebo administered iv + external beam radiotherapy. Treatment injection administered on the first day of radiotherapy. External beam radiotherapy at a maximum of 2 sites, fractionated as 3 Gy/fraction in 10 fractions. Follow up: 3 months. |
|
| Outcomes | Physician‐assessed subjective progression at 3 months and 6 months. Pain scale of the QLQ‐C30 questionnaire assessed at 3 and 6 months. Analgesic score based on the type and dose of all analgesics. Quality of life with the QLQ‐C30 questionnaire assessed at 3 and 6 months. Haematologic toxicity graded according to the WHO recommendations, assessed at 6 weeks, 3 and 6 months. |
|
| Notes | 2 patients ineligible in the 89Sr group, 4 patients didn't receive treatment with placebo. 6 patients were not evaluable on the 89Sr group, 4 patients were not evaluable on the placebo group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Patients | Low risk | The study is described as double blind but no further details are provided. |
| Blinding (performance bias and detection bias) Study researchers | Low risk | The study is described as double blind but no further details are provided. |
| Blinding (performance bias and detection bias) Outcome assessment | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number and reasons of non‐evaluable patients are described, but the number is high with respect to the evaluable patients, particularly for the main outcome where there is only data for 69 out of 95 participants, and thus results are unreliable. |
Gy: Gray iv: intravenous MBq: MegaBecquerel MCi: MegaCurie QoL: quality of life RTOG: Radiation Therapy Oncology Group VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| De Klerk 1994 | Dose‐escalation not randomised trial. |
| Dickie 1999 | Controlled, not randomised trial. |
| Haesner 1992 | Controlled, not randomised trial. |
| Liepe 2007 | Controlled trial comparing 186Re, 186ReHEDP and 89Sr. There are no differences between intervention groups with respect to pain relief (81%, 77% and 80%) or leucocytopenia. |
| Loeb 2009 | Dose‐escalation not randomised trial. |
| Malmberg 1997 | Pain is not main outcome. |
| McEwan 1994 | Retrospective cost‐benefit analysis of a subset of patients in Porter 1993. There are no differences between treatment and placebo groups in indirect costs or total inpatient stay costs. Costs related to tertiary in‐patient stay and to direct therapy are lower on the treatment group. |
| Morris 2009 | Dose‐escalation not randomised trial. |
| Nilsson 2005 | Radioisotopes compared to chemotherapy. |
| Oosterhof 2003 | Radioisotopes compared to radiotherapy. |
| Parker 2009 | Dose‐escalation randomised trial, pain is not main outcome. |
| Porfiri 2010 | Chemotherapy alone or in combination with zoledronic acid (ZA) +/‐ 89Sr. |
| Quilty 1994 | Radioisotopes compared to radiotherapy. |
| Storto 2006 | Non‐randomised trial. |
| Tu 2001 | Radioisotopes plus chemotherapy compared to chemotherapy. |
| Wang 2003 | Radioisotopes compared to bisphosphonates. |
| Zafeirakis 2009 | Dose‐escalation not randomised trial. |
| Zhang 2003 | Dose‐escalation not randomised trial. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomized controlled trial. |
| Participants | Prostate cancer patients with painful bone metastases, who received emasculate. Control group: 73 cases. Combined treatment group: 65 cases. |
| Interventions | Control group: 89Sr treatment by intravenous injection at the dose of 1.48‐2.22 MBq (40‐60 mu Ci)/kg. Combined treatment group: 89Sr at same dose as control group, plus 99Tc‐MDP 200mg by intravenous transfusion per day, total 5 times (the period of treatment). |
| Outcomes | Pain relief. Pain flares. Anodyne reduction. Duration of pain relief. Advance activity ability. |
| Notes | Information obtained from the abstract. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A double‐blind, dose‐response phase II, multicentre study of Radium‐223 (Alpharadin TM) for the palliation of painful bone metastases in hormone refractory prostate cancer patients. |
| Methods | Randomised, double blind (subject, investigator), parallel assignment, safety/efficacy study. |
| Participants | Men with prostate cancer and bone metastases that no longer respond to hormonal treatment. |
| Interventions | Alpharadin (radium‐223) at different dose levels, 5, 25, 50 or 100 kBq/kg body weight. |
| Outcomes | Primary outcome measures: pain assessment, analgesic consumption. Secondary outcome measures: reduction of pain, duration of pain relief, time to pain progression, safety of radium‐223, date of death. |
| Starting date | Study start date: May 2005. Estimated study completion date: December 2010. |
| Contact information | Principal investigator: Sten Nilsson. Radiumhemmet, Karolinska University Hospital, 171 76 Stockholm, Sweden. |
| Notes | Identified through www.controlled‐trials.com, accessed on 3/12/8. |
kBq: kiloBecquerel
Contributions of authors
MR is responsible for the statistical and methodological aspects of the review, and will be primarily responsible for future updates of the review.
MR, MJM and PA performed the duplicate data extraction and the evaluation of the quality of the included studies.
MSB provided clinical experience and expertise to the review.
All authors contributed to the manuscript development.
Declarations of interest
None known
Notes
This review is out of date although it is correct as of the date of publication. The latest version is available in the ‘Other versions’ tab on The Cochrane Library, and may still be useful to readers. However, the review may be misleading as new studies could alter the original conclusions. For further information, please contact the PaPaS team at http://papas.cochrane.org/contact‐us.
Withdrawn from publication for reasons stated in the review
References
References to studies included in this review
- Baczyk M, Czepczynski R, Milecki P, Pisarek M, Oleksa R, Sowinski J. 89Sr versus 153Sm‐EDTMP: comparison of treatment efficacy of painful bone metastases in prostate and breast carcinoma. Nuclear Medicine Communications 2007;28(4):245‐50. [DOI] [PubMed] [Google Scholar]
- Buchali K, Correns HJ, Schuerer M, Schnorr D, Lips H, Sydow K. Results of a double blind study of 89‐strontium therapy of skeletal metastases of prostatic carcinoma. European Journal of Nuclear Medicine 1988;14(7‐8):349‐51. [DOI] [PubMed] [Google Scholar]
- Olea E, Quintana JC, Nagel J, Arenas L, Tomicic M, Gil MC, Araya G. Efficacy and toxicity of samarium‐153‐EDTMP locally produced in the treatment of painful skeletal metastases. IAEA‐TECDOC‐1228. IAEA, 1999. [Google Scholar]; Olea E, Riccabona G, Tian J, Pusuwan P, Parma E, Obaldo JM, et al. Efficacy and toxicity of 153Sm EDTMP in the palliative treatment of painful skeleton metastases: results of an IAEA international multicenter study. Journal of Nuclear Medicine 2000;51:146. [Google Scholar]; Pan Z, Zhu S. Random comparison study of the clinical response to 153SM‐EDTMP 1.0 mg mCi/Kg and 1.5 mg mCi/Kg. IAEA‐TECDOC‐1228. IAEA, 1999. [Google Scholar]; Tian JH, Zhang JM, Hou QT, Oyang QH, Wang JM, Luan ZS, et al. Multicentre trial on the efficacy and toxicity of single‐dose samarium‐153‐ethylene diamine tetramethylene phosphonate as a palliative treatment for painful skeletal metastases in China. European Journal of Nuclear Medicine 1999;26(1):2‐7. [DOI] [PubMed] [Google Scholar]
- Han SH, Klerk JM, Tan S, het Schip AD, Derksen BH, Dijk A, et al. The PLACORHEN study: a double‐blind, placebo‐controlled, randomized radionuclide study with (186) Re‐etidronate in hormone‐resistant prostate cancer patients with painful bone metastases. Placebo Controlled Rhenium Study. Journal of Nuclear Medicine 2002;43(9):1150‐6. [PubMed] [Google Scholar]
- Lewington VJ, McEwan AJ, Ackery DM, Bayly RJ, Keeling DH, Macleod PM, et al. A prospective, randomised double‐blind crossover study to examine the efficacy of strontium‐89 in pain palliation in patients with advanced prostate cancer metastatic to bone. European Journal of Cancer 1991;27(8):954‐8. [DOI] [PubMed] [Google Scholar]
- Maxon HR, Schroder LE, Hertzberg VS, Thomas SR, Englaro EE, Samaratunga R, et al. Rhenium‐186(Sn)HEDP for treatment of painful osseous metastases: results of a double‐blind crossover comparison with placebo. Journal of Nuclear Medicine 1991;32(10):1877‐81. [PubMed] [Google Scholar]
- Nair N. Relative efficacy of 32P and 89Sr in palliation in skeletal metastases. Journal of Nuclear Medicine 1999;40(2):256‐61. [PubMed] [Google Scholar]
- Nilsson S, Franzen L, Parker C, Tyrrell C, Blom R, Tennvall J. Bone‐targeted radium‐223 in symptomatic, hormone‐refractory prostate cancer: a randomised, multicentre, placebo‐controlled phase II study. Lancet Oncology 2007;8:587‐94. [DOI] [PubMed] [Google Scholar]
- Palmedo H, Manka‐Waluch A, Albers P, Schmidt‐Wolf IG, Reinhardt M, Ezziddin S, et al. Repeated bone‐targeted therapy for hormone‐refractory prostate carcinoma: randomized phase II trial with the new, high‐energy radiopharmaceutical rhenium‐188 hydroxyethylidenediphosphonate. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology 2003;21(16):2869‐75. [DOI] [PubMed] [Google Scholar]
- Porter AT, McEwan AJ. Strontium‐89 as an adjuvant to external beam radiation improves pain relief and delays disease progression in advanced prostate cancer: results of a randomized controlled trial. Seminars in Oncology 1993;20(3 Suppl 2):38‐43. [PubMed] [Google Scholar]; Porter AT, McEwan AJ, Powe JE, Reid R, McGowan DG, Lukka H, et al. Results of a randomized Phase‐III trial to evaluate the efficacy of strontium‐89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. International Journal of Radiation, Oncology, Biology and Physics 1993;25(5):805‐13. [DOI] [PubMed] [Google Scholar]
- Resche I, Chatal JF, Pecking A, Ell P, Duchesne G, Rubens R, et al. A dose‐controlled study of 153Sm‐ethylenediaminetetramethylenephosphonate (EDTMP) in the treatment of patients with painful bone metastases. European Journal of Cancer 1997;33(10):1583‐91. [DOI] [PubMed] [Google Scholar]
- Quick D, Reid R, Hoskin P, Duschene G, Sartor O. Efficacy and safety of 153Sm‐EDTMP in alleviating the pain of bone metastases in patients with prostate carcinoma (Meeting abstract). Proceedings of the Annual Meeting American Society of Clinical Oncology. 1996; Vol. 15:A1654. [Google Scholar]; Sartor O, Quick D, Reid R, Hostin P, Duschene G. A double blind placebo controlled study of 153Samarium‐EDTMP for palliation of bone pain in patients with hormone refractory prostate cancer (abstract). Journal of Urology 1997;157(4):321. [Google Scholar]; Sartor O, Reid RH, Hoskin PJ, Quick DP, Ell PJ, Coleman RE, et al. Samarium‐153‐lexidronam complex for treatment of painful bone metastases in hormone‐refractory prostate cancer. Urology 2004;63(5):940‐5. [DOI] [PubMed] [Google Scholar]
- Sciuto R, Festa A, Pasqualoni R, Semprebene A, Rea S, Bergomi S, et al. Metastatic bone pain palliation with 89‐Sr and 186‐re‐HEDP in breast cancer patients. Breast Cancer Research and Treatment 2001 Mar;66(2):101‐9. [DOI] [PubMed] [Google Scholar]
- Serafin ANi, Houston SJ, Resche I, Quick DP, Grund FM, Ell PJ, et al. Palliation of pain associated with metastatic bone cancer using samarium‐153 lexidronam: A double‐blind placebo‐controlled clinical trial. Journal of Clinical Oncology 1998;16(4):1574‐81. [DOI] [PubMed] [Google Scholar]
- Smeland S, Erikstein B, Aas M, Skovlund E, Hess SL, Fosså SD. Role of strontium‐89 as adjuvant to palliative external beam radiotherapy is questionable: results of a double‐blind randomized study. International Journal of Radiation, Oncology, Biology and Physics 2003;56(5):1397‐404. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Klerk JMH, Zonnenberg BA, het Schip AD, van Dijk A, Han SH, Quirijnen JMSR, et al. Dose escalation study of rhenium‐186 hydroxyethylidene diphosphonate in patients with metastatic prostate cancer. Nuclear Medicine and Molecular Imaging 1994;21(10):1114‐20. [DOI] [PubMed] [Google Scholar]
- Dickie GJ, Macfarlane D. Strontium and samarium therapy for bone metastases from prostate carcinoma. Australasian‐Radiology 1999;43(4):476‐9. [DOI] [PubMed] [Google Scholar]
- Haesner M, Buchali K, Pink V, Lips H. [The efficacy of therapy using 89Sr‐strontium chloride in 200 patients with bone metastases of a prostatic cancer]. [German]. Nuklearmedizin 1992;31(2):48‐52. [PubMed] [Google Scholar]
- Liepe K, Franke WG, Kropp J, Koch R, Runge R, Hliscs R. Comparison of rhenium‐188, rhenium‐186‐HEDP and strontium‐89 in palliation of painful bone metastases]. [German] [Vergleich von Rhenium‐188‐HEDP, Rhenium‐186‐HEDP un Strontium‐89 fur die palliative Schmerztherapie von Skelettmetastasen]. Nuclear‐Medizin 2000 Sep;39(6):146‐51. [PubMed] [Google Scholar]; Liepe K, Kotzerke J. A comparative study of 188Re‐HEDP, 186Re‐HEDP, 153Sm‐EDTMP and 89Sr in the treatment of painful skeletal metastases. Nuclear Medicine Communications 2007;28(8):623‐30. [DOI] [PubMed] [Google Scholar]; Liepe K, Runge R, Kotzerke J. Systemic radionuclide therapy in pain palliation. The American Journal of Hospice & Palliative Care 2005;22(6):457‐64. [DOI] [PubMed] [Google Scholar]
- Loeb DM, Garrett‐Mayer E, Hobbs RF, Prideaux AR, Sgouros G, Shokek O, et al. Dose‐finding study of 153Sm‐EDTMP in patients with poor‐prognosis osteosarcoma. Cancer 2009;115(11):2514‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg I, Persson U, Ask A, Tennvall J, Abrahamsson PA. Painful bone metastases in hormone‐refractory prostate cancer: economic costs of strontium‐89 and/or external radiotherapy. Urology 1997;50(5):747‐53. [DOI] [PubMed] [Google Scholar]
- McEwan AJB, Amyotte GA, McGowan DG, MacGillivray JA, Porter AT. A retrospective analysis of the cost effectiveness of treatment with metastron in patients with prostate cancer metastatic to bone. European‐Urology 1994;26(SUPPL. 1):26‐31. [DOI] [PubMed] [Google Scholar]
- Morris MJ, Pandit‐Taskar N, Carrasquillo J, Divgi CR, Slovin S, Kelly WK, et al. Phase I study of samarium‐153 lexidronam with docetaxel in castration‐resistant metastatic prostate cancer. Journal of Clinical Oncology 2009;27(15):2436‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S, Strang P, Ginman C, Zimmermann R, Edgren M, Nordström B, et al. Palliation of bone pain in prostate cancer using chemotherapy and strontium‐89. A randomized phase II study. Journal of Pain and Symptom Management 2005;29:352‐7. [DOI] [PubMed] [Google Scholar]
- Oosterhof GO, Roberts JT, Reijke TM, Engelholm SA, Horenblas S, Maase H, et al. Strontium(89) chloride versus palliative local field radiotherapy in patients with hormonal escaped prostate cancer: a phase III study of the European Organisation for Research and Treatment of Cancer, Genitourinary Group. European Urology 2003;44(5):519‐26. [DOI] [PubMed] [Google Scholar]
- Parker C, Hoskin P, Pascoe S, Chodack A, O'Sullivan JM, Germ JR, et al. A double blind, randomised, dose finding, phase II, multicentre study of radium‐223 for the treatment of patients with metastatic castration‐refractory prostate cancer (CRPC): EudraCT number: 2005‐003680‐22. European Journal of Cancer 2009;7(2‐3):406 Suppl. [Google Scholar]
- Porfiri E, Collins SI, Barton D, Billingham L, McLaren D, Nixon GG, et al. Initial feasibility and safety results from a phase II/III clinical trial to evaluate docetaxel (D) therapy in combination with zoledronic acid (ZA) +/‐ strontium‐89 (Sr89) in hormone‐refractory prostate cancer patients: ISRCTN12808747. Journal of Clinical Oncology 2010;28(15 Suppl 1). [ISRCTN12808747] [Google Scholar]
- Quilty PM, Kirk D, Bolger JJ, Dearnaley DP, Lewington VJ, Mason MD, et al. A comparison of the palliative effects of strontium‐89 and external beam radiotherapy in metastatic prostate cancer. Radiotherapy and Oncology 1994;31(1):33‐40. [DOI] [PubMed] [Google Scholar]
- Storto G, Klain M, Paone G, Liuzzi R, Molino L, Marinelli A, et al. Combined therapy of Sr‐89 and zoledronic acid in patients with painful bone metastases. Bone 2006;39(1):35‐41. [DOI] [PubMed] [Google Scholar]
- Tu SM, Millikan RE, Mengistu B, Delpassand ES, Amato RJ, Pagliaro LC, et al. Bone‐targeted therapy for advanced androgen‐independent carcinoma of the prostate: a randomised phase II trial. Lancet 2001;357(9253):336‐41. [DOI] [PubMed] [Google Scholar]
- Wang RF, Zhang CL, Zhu SL, Zhu M. A comparative study of samarium‐153‐ethylenediaminetetramethylene phosphonic acid with pamidronate disodium in the treatment of patients with painful metastatic bone cancer. Medical Principles & Practice 2003;12(2):97‐101. [DOI] [PubMed] [Google Scholar]
- Zafeirakis A, Zissimopoulos A, Baziotis N, Limouris GS. Management of metastatic bone pain with repeated doses of rhenium 186‐hedp in patients under therapy with zoledronic acid: A safe and additively effective practice. Cancer Biotherapy and Radiopharmaceuticals 2009;24(5):543‐50. [DOI] [PubMed] [Google Scholar]
- Zhang H, Tian M, Li S, Liu J, Tanada S, Endo K. Rhenium‐188‐HEDP therapy for the palliation of pain due to osseous metastases in lung cancer patients. Cancer Biotherapy & Radiopharmaceuticals 2003;18(5):719‐26. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Ma Y, Liu P, Gu A, Xu F, Yuan Q, Wang Z. Combining Strontium‐89 with 99Tc‐MDP for treatment of: Painful bone metastases of prostate cancer. Chinese Journal of Andrology November 2009;23(11):23‐6. [Google Scholar]
References to ongoing studies
- A double‐blind, dose‐response phase II, multicentre study of Radium‐223 (Alpharadin TM) for the palliation of painful bone metastases in hormone refractory prostate cancer patients.. Ongoing study Study start date: May 2005.Estimated study completion date: December 2010..
Additional references
- Aaron AD, Washington DC. Treatment of metastatic adenocarcinoma of the pelvis and the extremities. The Journal of Bone and Joint Surgery 1997;79:917‐32. [DOI] [PubMed] [Google Scholar]
- Australian Cancer Network Management of Metastasic Cancer Prostate Working Party. Clinical Practice Guidelines for the management of locally advanced and metastasic prostate cance. Sidney: Cancer Council Australia and Australian Cancer Network, 2009. [Google Scholar]
- Bodei L, Lam M, Chiesa C, Flux G, Brans B, Chiti A, et al. EANM procedure guideline for treatment of refractory metastatic bone pain. European Journal of Nuclear Medicine and Molecular Imaging 2008 Oct;35(10):1934‐40. [PUBMED: 18649080] [DOI] [PubMed] [Google Scholar]
- Brundage MD, Crook JM, Lukka H and the Genitourinary Cancer Disease Site Group. Use of Strontium‐89 in patients with endocrine‐refractory carcinoma of the prostate metastatic to bone. http://hiru.mcmaster.ca/ccopgi/guidelines/gen/cpg3_6f.html April 2000. [PubMed]
- Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. Journal of Clinical Oncology 2007 Apr 10;25(11):1423‐36. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
- Finlay IG, Mason MD, Shelley M. Radioisotopes for the palliation of metastatic bone cancer: a systematic review. Lancet Oncology 2005;6:392‐400. [DOI] [PubMed] [Google Scholar]
- Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. Journal of Clinical Oncology 2009;27(10):1564‐71. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2008. [Google Scholar]
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009). The Cochrane Collaboration, 2008. [Google Scholar]
- IAEA. Criteria for palliation of bone metastases ‐ Clinical applications. Criteria for palliation of bone metastases ‐ clinical applications. IAEA‐TECDOC‐1549. Vienna: International Atomic Energy Agency, 2007. [Google Scholar]
- Lipton A, Steger GG, Figueroa J, Alvarado C, Solal‐Celigny P, Body JJ et al. Randomized active‐controlled phase II study of denosumab efficacy and safety in patients with breast cancer‐related bone metastases. Journal of Clinical Oncology 2007 Oct 1;25(28):4431‐7. [DOI] [PubMed] [Google Scholar]
- Martinez‐Zapata MJ, Roqué i Figuls M, Alonso‐Coello P, Català E. Calcitonin for metastatic bone pain. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD003223.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwan AJB. Palliative therapy with bone seeking radiopharmaceuticals. Cancer Biotherapy and Radiopharmaceuticals 1998;13:413‐42. [DOI] [PubMed] [Google Scholar]
- McQuay HJ, Collins SL, Carroll D, Moore RA. Radiotherapy for the palliation of painful bone metastases. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001793] [DOI] [PubMed] [Google Scholar]
- Mertens WC, Filipczack LA, Ben‐Josef Edgar, Davis LP, Portes AT. Systemic bone‐seeking Radionuclides for palliation of painful osseous metastases: current concepts. CA: A Cancer Journal for Clinicians 1998;48:361‐74. [DOI] [PubMed] [Google Scholar]
- Medicare Services Advisory Committee. Samarium153‐lexidronam for bone pain due to skeletal metastases. MSAC application 1016. http://www.health.gov.au/haf/msac. August 1999.
- Nelson OS, Munro AJ, Tannock IF. Bone metastatic: pathophysiology and management policy. Journal of Clinical Oncology 1991;9:509‐24. [DOI] [PubMed] [Google Scholar]
- Pavlakis N, Schmidt RL, Stockler MR. Bisphosphonates for breast cancer. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003474.pub2] [DOI] [PubMed] [Google Scholar]
- Therapeutic Radiopharmaceutical Guidelines Group. Radiopharmaceuticals for the palliation of painful bone metastases. Practice Guidelines Report #14‐1. Toronto: Cancer Care Ontario, 2004 Jun 15. [Google Scholar]
- Sabino MAC, Mantyh PW. Pathophysiology of bone cancer pain. The Journal of Supportive Oncology 2005;3(1):15‐24. [PubMed] [Google Scholar]
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.9 (updated September 2009). The Cochrane Collaboration, 2008. [Google Scholar]
- Scully SP, Ghert MA, Zurakowski D, Thompson RC, Gebhardt MC. Pathologic fracture in osteosarcoma: prognostic importance and treatment implications. Journal of Bone and Joint Surgery. American volume 2002;84‐A(1):49‐57. [PubMed] [Google Scholar]
- Shelley M, Harrison C, Coles B, Stafforth J, Wilt T, Mason M. Chemotherapy for hormone‐refractory prostate cancer. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD005247.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein E, Buscombe J, McEwan A, Taylor A. Society of nuclear medicine procedure guideline for palliative treatment of painful bone metastases. version 3.0. Web source January 25, 2003.
- Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double‐blind study. Journal of Clinical Oncology 2010;Nov 8:[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD004721] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latrás VM, Coderch CL, Antoñanzas Villar F, Coya Viña J, Martín Comín J, Martínez Carderón F, et al. Cost‐effectiveness analysis of samario‐153 (Quadramet) for the treatment of patients with prostate cancer and bone metastases [Análisis coste‐efectividad de samario‐153 (Quadramet®) en el tratamiento del dolor en pacientes con cáncer de próstata y metástasis óseas]. Clinical Translational Oncology 2005 Jun;7(5):198‐204. [DOI] [PubMed] [Google Scholar]
- Wong R, Wiffen PJ. Bisphosphonates for the relief of pain secondary to bone metastases. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD002068] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen KY, Shelley M, Sze WM, Wilt T, Mason M. Bisphosphonates for advanced prostate cancer. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006250] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Roqué i Figuls M, Martinez‐Zapata MJ, Alonso‐Coello P, Català E, Garcia JL, Ferrandiz M. Radioisotopes for metastatic bone pain. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003347] [DOI] [PubMed] [Google Scholar]


