Abstract
Background
Kidney transplantation is the preferred form of kidney replacement therapy for patients with end‐stage kidney disease (ESKD) and is often complicated by worsening or new‐onset diabetes. Management of hyperglycaemia is important to reduce post‐transplant and diabetes‐related complications. The safety and efficacy of glucose‐lowering agents after kidney transplantation is largely unknown.
Objectives
To evaluate the efficacy and safety of pharmacological interventions for lowering glucose levels in patients who have undergone kidney transplantation and have diabetes.
Search methods
We searched the Cochrane Kidney and Transplant Specialised Register to 15 April 2016 through contact with the Information Specialist using search terms relevant to this review. Studies contained in the Specialised Register are identified through search strategies specifically designed for CENTRAL, MEDLINE, and EMBASE; handsearching conference proceedings; and searching the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
All randomised controlled trials (RCTs), quasi‐RCTs and cross‐over studies examining head‐to‐head comparisons of active regimens of glucose‐lowering therapy or active regimen compared with placebo/standard care in patients who have received a kidney transplant and have diabetes were eligible for inclusion.
Data collection and analysis
Two authors independently assessed study eligibility and quality and performed data extraction. Continuous outcomes were expressed as post‐treatment mean differences (MD) or standardised mean difference (SMD). Adverse events were expressed as post‐treatment absolute risk differences (RD). Dichotomous clinical outcomes were presented as risk ratios (RR) with 95% confidence intervals (CI).
Main results
We included seven studies that involved a total of 399 kidney transplant recipients. All included studies had observed heterogeneity in the patient population, interventions and measured outcomes or missing data (which was unavailable despite correspondence with authors). Many studies had incompletely reported methodology preventing meta‐analysis and leading to low confidence in treatment estimates.
Three studies with 241 kidney transplant recipients examined the use of more intensive compared to less intensive insulin therapy in kidney transplant recipients with pre‐existing type 1 or 2 diabetes. Evidence for the effects of more intensive compared to less intensive insulin therapy on transplant graft survival, HbA1c, fasting blood glucose, all cause mortality and adverse effects including hypoglycaemia was of very low quality. More intensive versus less intensive insulin therapy resulted in no difference in transplant or graft survival over three to five years in one study while another study showed that more intensive versus less intensive insulin therapy resulted in more rejection events over the three year follow‐up (11 events in total; 9 in the more intensive group, P = 0.01). One study showed that more intensive insulin therapy resulted in a lower mean HbA1c (10 ± 0.8% versus 13 ± 0.9%) and lower fasting blood glucose (7.22 ± 0.5 mmol/L versus 13.44 ± 1.22 mmol/L) at 13 months compared with standard insulin therapy. Another study showed no difference between more intensive compared to less intensive insulin therapy on all‐cause mortality over a five year follow‐up period. All studies showed either an increased frequency of hypoglycaemia or severe hypoglycaemia episodes.
Three studies with a total of 115 transplant recipients examined the use of DPP4 inhibitors for new‐onset diabetes after transplantation. Evidence for the treatment effect of DPP4 inhibitors on transplant or graft survival, HbA1c and fasting blood glucose levels, all cause mortality, and adverse events including hypoglycaemia was of low quality. One study comparing vildagliptin to placebo and another comparing sitagliptin to placebo showed no difference in transplant or graft survival over two to four months of follow‐up. One study comparing vildagliptin to placebo showed no significant change in estimated glomerular filtration rate from baseline (1.9 ± 10.3 mL/min/1.73 m2, P = 0.48 and 2.1 ± 6.1 mL/min/1.73 m2, P = 0.22) and no deaths, in either treatment group over three months of follow‐up. One study comparing vildagliptin to placebo showed a lower HbA1c level (mean ± SD) (6.3 ± 0.5% versus versus 6.7 ± 0.6%, P = 0.03) and trend towards a greater lowering of fasting blood glucose (‐0.91 ± ‐0.92 mmol/L versus vs ‐0.19 ± 1.16 mmol/L, P = 0.08) with vildagliptin. One study comparing sitagliptin to insulin glargine showed an equivalent lowering of HbA1c (‐0.6 ± 0.5% versus ‐0.6 ± 0.6%, P = NS) and fasting blood glucose (4.92 ± 1.42 versus 4.76 ± 1.09 mmol/L, P = NS) with sitagliptin. For the outcome of hypoglycaemia, one study comparing vildagliptin to placebo reported no episodes of hypoglycaemia, one study comparing sitagliptin to insulin glargine reported fewer episodes of hypoglycaemia with sitagliptin (3/28 patients; 10.7% versus 5/28; 17.9%) and one cross‐over study of sitagliptin and placebo reported two episodes of asymptomatic moderate hypoglycaemia (2 to 3.9 mmol/L) when sitagliptin was administered with glipizide. All three studies reported no drug interactions between DPP4 inhibitors and the immunosuppressive agents taken.
Evidence for the treatment effect of pioglitazone for treating pre‐existing diabetes was of low quality. One study with 62 transplant recipients compared the use of pioglitazone with insulin to insulin alone for treating pre‐existing diabetes. Pioglitazone resulted in a lower HbA1c level (mean ± SD) (‐1.21 ± 1.2 versus 0.39 ± 1%, P < 0.001) but had no effects on fasting blood glucose (6.58 ± 2.71 versus 7.28 ± 2.78 mmol/L, P = 0.14 ), and change in creatinine (3.54 ± 15.03 versus 10.61 ± 18.56 mmol/L, P = 0.53) and minimal adverse effects (no episodes of hypoglycaemia, three dropped out due to mild to moderate lower extremity oedema, cyclosporin levels were not affected).
Authors' conclusions
Evidence concerning the efficacy and safety of glucose‐lowering agents for treating pre‐existing and new‐onset diabetes in kidney transplant recipients is limited. Existing studies examine more intensive versus less intensive insulin therapy, and the use of DPP4 inhibitors and pioglitazone. The safety and efficacy of more intensive compared to less intensive insulin therapy is very uncertain and the safety and efficacy of DPP4 inhibitors and pioglitazone is uncertain, due to data being limited and of poor quality. Additional RCTs are required to clarify the safety and efficacy of current glucose‐lowering agents for kidney transplant recipients with diabetes.
Keywords: Humans; Kidney Transplantation; Adamantane; Adamantane/adverse effects; Adamantane/analogs & derivatives; Adamantane/therapeutic use; Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 1/blood; Diabetes Mellitus, Type 1/drug therapy; Diabetes Mellitus, Type 2; Diabetes Mellitus, Type 2/blood; Diabetes Mellitus, Type 2/drug therapy; Dipeptidyl Peptidase 4; Dipeptidyl‐Peptidase IV Inhibitors; Dipeptidyl‐Peptidase IV Inhibitors/adverse effects; Dipeptidyl‐Peptidase IV Inhibitors/therapeutic use; Fasting; Fasting/blood; Glycated Hemoglobin A; Glycated Hemoglobin A/metabolism; Graft Survival; Graft Survival/drug effects; Hypoglycemia; Hypoglycemia/chemically induced; Hypoglycemic Agents; Hypoglycemic Agents/adverse effects; Hypoglycemic Agents/therapeutic use; Insulin; Insulin/adverse effects; Insulin/therapeutic use; Nitriles; Nitriles/adverse effects; Nitriles/therapeutic use; Pyrrolidines; Pyrrolidines/adverse effects; Pyrrolidines/therapeutic use; Randomized Controlled Trials as Topic; Sitagliptin Phosphate; Sitagliptin Phosphate/adverse effects; Sitagliptin Phosphate/therapeutic use; Thiazolidinediones; Thiazolidinediones/adverse effects; Thiazolidinediones/therapeutic use
Glucose‐lowering agents for treating pre‐existing or new‐onset diabetes in kidney transplant recipients
What is the issue?
Kidney transplantation is often complicated by worsening or new‐onset diabetes. The safety and effectiveness of drugs used to lower glucose in this setting is largely not known.
What did we do?
We evaluated the effectiveness and safety of glucose‐lowering drugs in people with diabetes who have received a kidney transplant by searching the Cochrane Kidney and Transplant Specialised Register. We included all randomised controlled and cross‐over studies examining this question to 15 April 2016.
What did we find?
We found seven studies which together included 399 kidney transplant recipients. Four studies were undertaken in patients with pre‐existing type 1 or type 2 diabetes; three of these examined more versus less intensive insulin treatment, and one compared pioglitazone and insulin treatment to insulin treatment alone. Three studies were undertaken in patients with new‐onset diabetes after transplantation, and studied the effectiveness and safety of DPP‐4 inhibitors. From these studies, the effects of more compared to less intensive insulin treatment on survival of the kidney transplant, control of diabetes, and survival of the patient, as well as treatment side‐effects, are not well understood. The effects of using DPP4 inhibitors and pioglitazone on survival of the kidney transplant, control of diabetes and survival of the patient and possible side‐effects are also uncertain.
Conclusion
Available research concerning glucose‐lowering treatment for diabetes in people who have received kidney transplants is limited. More studies are required to confirm the effectiveness and safety of glucose‐lowering agents in this population.
Summary of findings
Summary of findings for the main comparison.
More intensive versus less intensive insulin therapy for treating pre‐existing and new‐onset diabetes in kidney transplant recipients
| More intensive versus less intensive insulin therapy for treating pre‐existing and new‐onset diabetes in kidney transplant recipients | |||
| Patient or population: kidney transplant recipients with pre‐existing type 1 or type 2 diabetes Setting: inpatients and outpatients Intervention: more intensive insulin therapy Comparison: less intensive insulin therapy | |||
| Outcomes | Impact | No. of participants (studies) | Quality of the evidence (GRADE) |
| Transplant or graft survival Follow‐up: range 3 years to 5 years | While one study showed that more intensive versus less intensive therapy resulted in no difference between groups, the other study showed that more intensive versus less intensive insulin therapy resulted in more rejection events over the 3 year follow‐up (11 events in total; 9 in the more intensive group, P = 0.01) | 192 (2) | ⊕⊝⊝⊝ VERY LOW 1 2 3 4 |
| Glycated haemoglobin Follow‐up: 13 months | One study compared more intensive to a less intensive insulin regiment, which resulted in a lower mean HbA1c (± SEM) at 13 months 10 ± 0.8% compared with standard insulin (13 ± 0.9%). This result was difficult to interpret given that participants' baseline HbA1c was not reported | 49 (1) | ⊕⊝⊝⊝ VERY LOW 2 5 |
| Fasting blood glucose Follow‐up: 13 months | One study showed that intensive insulin therapy achieved a lower FBG (mean ± SEM = 7.22 ± 0.5 mmol/L) compared with standard insulin therapy (13.44 ± 1.22 mmol/L) at 13 months despite both arms having similar baseline FBG levels | 49 (1) | ⊕⊝⊝⊝ VERY LOW 2 5 |
| Kidney function: creatinine level, eGFR, albuminuria | Not reported by any study | ‐ | ‐ |
| All‐cause mortality Follow‐up: 5 years | One study showed no difference between more intensive versus less intensive insulin therapy | 99 (1) | ⊕⊝⊝⊝ VERY LOW 2 6 |
| Macrovascular and microvascular events: cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke, new or worsening kidney disease, retinopathy | Not reported by any study | ‐ | ‐ |
| Safety: hypoglycaemia, discontinuation of medication due to adverse events, gastrointestinal side effects, congestive heart failure, oedema, lactic acidosis, liver abnormalities/failure, cancer events, other adverse events as described by authors Follow‐up: range 3 months to 5 years | One study showed that intensive insulin therapy resulted in more frequent and severe episodes of hypoglycaemia compared with standard insulin therapy. Another study showed that more intensive insulin therapy resulted in a higher rate of severe hypoglycaemia compared with less intensive insulin therapy (1.7 episodes/patient/year versus < 0.1 episodes/patient/year, P < 0.001). Of the 29 episodes of severe hypoglycaemia resulting in hospital admission, 26 occurred in the intensive insulin group. A patient in the intensive insulin group remained comatose for 6 days and required a 2 week hospital admission. The last study showed that more intensive insulin therapy resulted in more patients experiencing hypoglycaemic episodes (BGL < 3.88 mmol/L) compared with less intensive insulin therapy (84% versus 25%, P < 0.001). However, more intensive insulin therapy did not result in significantly more episodes of severe hypoglycaemia (defined as BGL < 2.22 mmol/L) than less intensive insulin therapy (8 events in 7 patients compared with 2 events in 2 patients, P = 0.08) | 241 (3) | ⊕⊝⊝⊝ VERY LOW 2 7 8 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | |||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1 Both studies had a very high risk of bias. One study did not report the method of random sequence generation and did not describe allocation concealment or blinding and had incomplete outcome data and potential selection bias. The other study did not report the method of random sequence generation, did not report the method of allocation concealment, was unblinded and had incomplete outcome data.
2 Narrative synthesis was conducted. Estimates are not precise.
3 While one study involved people mostly with type 1 diabetes, the other study involved people mainly with type 2 diabetes with 10% having type 1 diabetes
4 While one study demonstrated that more intensive versus less intensive insulin therapy did not change graft survival, the other study demonstrated that more versus less intensive insulin therapy resulted in more rejection events.
5 This study had a very high risk of bias. The method of random sequence generation was unavailable as well as the allocation concealment method. There was no blinding reported in the study, there was incomplete outcome data, selective reporting, and there was a change in selection criteria during the study
6 This study had a very high risk of bias. The method of random sequence generation was not reported, there was no blinding mentioned in the study, and there was evidence of incomplete outcome data and possible selection bias.
7 While two studies involved people mostly with type 1 diabetes, the other study involved people mainly with type 2 diabetes with 10% having type 1 diabetes
8 All three studies had a very high risk of bias. One study did not report the method of random sequence generation, did not report allocation concealment, did not report blinding, had incomplete outcome data, had evidence of selective reporting, and had additional instances of selection bias. One study did not report the method of random sequence generation and did not describe allocation concealment or blinding and had incomplete outcome data and potential selection bias. The other study did not report the method of random sequence generation, did not report the method of allocation concealment, was unblinded and had incomplete outcome data.
Summary of findings 2.
DPP4 inhibitors versus placebo or other glucose‐lowering agents for treating pre‐existing or new‐onset diabetes in kidney transplant recipients
| DPP4 inhibitors versus placebo or other glucose‐lowering agents for treating pre‐existing or new‐onset diabetes in kidney transplant recipients | |||
| Patient or population: kidney transplant recipients with new‐onset diabetes Setting: inpatients and outpatients Intervention: DPP4 inhibitors Comparison: placebo or other glucose‐lowering agents | |||
| Outcomes | Impact | No. of participants (studies) | Quality of the evidence (GRADE) |
| Transplant or graft survival Follow‐up: range 2 months to 3 months | One study compared vildagliptin to placebo on patients with NODAT and there was no difference in transplant or graft survival on correspondence with the authors. The other study was a cross‐over RCT with 100% graft survival reported by the authors on correspondence | 70 (2) | ⊕⊕⊝⊝ LOW 1 2 |
| Glycated haemoglobin Follow‐up: 3 months | One study compared vildagliptin to placebo and the mean (± SD) absolute HBA1c level remained significantly lower in the vildagliptin group compared with placebo (6.3 ± 0.5% versus 6.7 ± 0.6%, P = 0.03). The other study compared sitagliptin with insulin glargine and found that sitagliptin was equivalent in lowering HbA1c from baseline compared with insulin glargine therapy (‐0.6 ± 0.5% versus ‐0.6 ± 0.6%, P = NS) | 77 (2) | ⊕⊕⊝⊝ LOW 2 3 |
| Fasting blood glucose Follow‐up: 3 months | One study compared vildagliptin to placebo and there was a trend towards greater mean lowering of fasting blood glucose in the vildagliptin group (‐0.91 ± 0.92 mmol/L versus ‐0.19 ± 1.16 mmol/L, P = 0.08). The other study showed that sitagliptin therapy had an equivalent effect to insulin glargine on fasting blood glucose (4.92 ± 1.42 versus 4.76 ± 1.09 mmol/L, P = NS) | 77 (2) | ⊕⊕⊝⊝ LOW 2 3 |
| Kidney function: creatinine levels, eGFR, albuminuria Follow‐up: 3 months | One study compared vildagliptin to placebo and there was no significant change in eGFR from baseline in either group (1.9 ±10.3 mL/min/1.73 m2, P = 0.48 and 2.1 ± 6.1 mL/min/1.73 m2, P = 0.22). Both groups did not develop proteinuria or albuminuria. The other study was a cross‐over RCT comparing sitagliptin to placebo and no proteinuria or albuminuria developed in either intervention group during the study | 70 (2) | ⊕⊕⊝⊝ LOW 1 2 |
| All‐cause mortality Follow‐up: range 3 months | One study compared vildagliptin to placebo and there were no deaths in either group. The other study was a cross‐over RCT comparing sitagliptin to placebo with no deaths reported in either group | 70 (2) | ⊕⊕⊝⊝ LOW 1 2 |
| Macrovascular and microvascular events: cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke, new or worsening kidney disease, retinopathy. | Not reported | ‐ | ‐ |
| Safety events: hypoglycaemia, discontinuation of medication due to adverse events, gastrointestinal side effects, congestive heart failure, oedema, lactic acidosis, liver abnormalities/failure, cancer events, other adverse events as described by authors. Follow‐up: range 2 months to 3 months | One study compared vildagliptin to placebo. Vildagliptin did not result in any episodes of hypoglycaemia, however a hypoglycaemic episode was not defined. There were similar rates of adverse events (5/16; 31.3%) in the vildagliptin compared to the placebo group (6/16; 37.5%) with small but similar numbers of episodes of angina (1 versus 0) elevated liver enzymes (2 versus 1), elevated pancreatic enzymes (0 versus 1), elevated triglycerides (0 versus 1), cough (0 versus 1), conjunctivitis (1 versus 0), urinary tract infections (1 versus 1) and leukopenia (0 versus 1). There were no reported drug interactions with any immunosuppressive agents being taken. One study compared sitagliptin with insulin glargine. There were fewer episodes of hypoglycaemic events in the sitagliptin group (3/28 patients; 10.7%) compared with the insulin glargine group (5/28; 17.9%). Hypoglycaemia was not defined and there were no severe episodes reported. There was an overall low incidence of adverse events which were mild in both the sitagliptin and insulin glargine groups. The last study compared sitagliptin to placebo in a cross‐over RCT. There were no episodes of severe hypoglycaemia due to sitagliptin (BGL < 2 mmol/L and unconsciousness or the need of assistance). However, 2 patients each had episodes of asymptomatic moderate hypoglycaemia (BGL 2 to 3.9 mmol/L) while receiving glipizide and sitagliptin. Sitagliptin was associated with one discontinuation due to night sweats and an increase in uric acid levels, but no episodes of gout were reported. There was no interaction between sitagliptin and other immunosuppressive agents | 115 (3) | ⊕⊕⊝⊝ LOW 2 4 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | |||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1 1 study had an unclear risk of bias for blinding of participants and outcomes, and the other study was a randomised cross‐over trial and had a high risk of bias because there was no allocation concealment and the participants and outcome assessment was unblinded, the study was not placebo controlled, and there was no wash out period. Both studies were equal in size
2 Narrative synthesis was conducted. Estimates are not precise
3 1 study had an unclear risk of bias for blinding of participants and outcomes, and the other study had a high risk of bias as it had a high risk of allocation concealment was open label and had incomplete outcome data.
4 1 study had an unclear risk of bias for blinding of participants and outcomes. Another study was a randomised cross‐over trial and had a high risk of bias because there was no allocation concealment and the participants and outcome assessment was unblinded, the study was not placebo controlled, and there was no wash out period. The third study also had a high risk of bias because it was open label, there was no allocation concealment reported and had incomplete outcome data.
Summary of findings 3.
Pioglitazone versus placebo for treating pre‐existing or new‐onset diabetes in kidney transplant recipients
| Pioglitazone versus placebo for treating pre‐existing or new‐onset diabetes in kidney transplant recipients | |||
| Patient or population: kidney transplant recipients with pre‐existing diabetes Setting: inpatients and outpatients Intervention: pioglitazone Comparison: placebo | |||
| Outcomes | Impact | No. of participants (studies) | Quality of the evidence (GRADE) |
| Transplant or graft survival | Not reported | ‐ | ‐ |
| Glycated Haemoglobin Follow‐up: 4 months |
Pioglitazone and insulin therapy significantly lowered HbA1c from baseline to a greater extent compared with insulin therapy alone (‐1.21 ± 1.2 versus 0.39 ± 1%, P < 0.001) | 62 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| Fasting blood glucose Follow‐up: 4 months |
Pioglitazone and insulin therapy had equivalent effects on post intervention FBG compared with insulin therapy alone (mean ± SD = 6.58 ± 2.71 versus 7.28 ± 2.78 mmol/L, P = 0.14) | 62 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| Kidney function: creatinine level, eGFR, albuminuria Follow‐up: 4 months |
Pioglitazone and insulin therapy had similar effects when compared with insulin therapy on change in creatinine levels from baseline (3.54 ± 15.03 versus 10.61 ± 18.56 mmol/L, P = 0.53) | 62 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| All‐cause mortality | Not reported | ‐ | ‐ |
| Macrovascular and microvascular complications: cardiovascular death, non‐fatal myocardial infarction (MI), non‐fatal stroke, new or worsening kidney disease, retinopathy | Not reported | ‐ | ‐ |
| Safety: hypoglycaemia, discontinuation of medication due to adverse events, gastrointestinal side effects, congestive heart failure, oedema, lactic acidosis, liver abnormalities/failure, cancer events, other adverse events as described by authors Follow‐up: 4 months |
No episodes of hypoglycaemia in comparing pioglitazone and insulin therapy versus insulin therapy alone. Four participants dropped out due to adverse effects of pioglitazone (3 had mild to moderate lower extremity oedema and one had insomnia). No study participants had transient elevation of liver enzyme levels two or more times the upper limits or episodes of liver toxicity, severe cardiac failure, or change in creatinine levels . Pioglitazone did not affect cyclosporin levels or dose | 62 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | |||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1 Narrative synthesis was conducted. Estimates are not precise.
2 Only 1 study found.
Background
Description of the condition
Diabetes is increasing in global prevalence and is projected to affect 592 million people by 2035 (IDF Atlas 2013). Diabetes is now the most common cause of end‐stage kidney disease (ESKD) accounting for 50% of disease globally (Collins 2007; McDonald 2009), and often coexists in patients with non‐diabetic kidney disease (McDonald 2009). People with diabetes and chronic kidney disease (CKD) have increased morbidity and mortality mainly due to cardiovascular disease. Together, both conditions pose an additive risk to cardiovascular disease with this risk increasing as nephropathy progresses (Radbill 2008).
Where possible, kidney transplantation is the preferred management option for many patients with diabetes and ESKD. Kidney transplantation is associated with 25% to 65% better patient survival than continuing on dialysis (Meier‐Kriesche 2001; Rabbat 2000; Schnuelle 1998; Wolfe 1999), and this is mainly attributable to decreased cardiovascular disease risk (Lentine 2005; Meier‐Kriesche 2004). People with type 1 diabetes and ESKD may undergo kidney or kidney‐pancreas transplantation. Where successful kidney‐pancreas transplantation occurs, the need for glucose‐lowering intervention is often negated. People with type 2 diabetes and ESKD receive kidney transplants alone. The immunosuppressive agents required after transplantation, such as mammalian target of rapamycin inhibitors or prednisolone, often result in hyperglycaemia by decreasing insulin sensitivity and impairing insulin secretion (Duijnhoven 2001; Dumler 2007; Guerra 2012). In patients with non‐diabetic kidney disease, the risk of developing new‐onset diabetes after transplantation (NODAT) is markedly increased (Davidson 2003; Diabetes Care 2003). Estimates of NODAT incidence one year after kidney transplantation range from 2% to 52% (Kesiraju 2014).
Description of the intervention
Over the past five to 10 years the range of pharmacological interventions to treat diabetes has increased and now includes oral agents, insulin and non‐insulin injectables. Pharmacological interventions for people with type 2 diabetes have traditionally been used in combination and introduced in a stepwise fashion beginning with oral agents, followed by injectables. The commonly available classes of glucose‐lowering medications are biguanides, thiazolidinediones, second generation sulphonylureas, glucosidase inhibitors, dipeptidyl peptidase‐4 (DPP‐4) inhibitors, glucagon‐like peptide‐1 analogues and insulins. Newer and emerging classes of glucose‐lowering medications are sodium glucose co‐transporter 2 (SGLT2) inhibitors, dual peroxisome proliferator‐activated receptor agonists, amylin analogues, bromocriptine and GPR40 or free fatty acid receptor 1 agonists.
How the intervention might work
Epidemiological studies show that poor glycaemic control is associated with increased incidence of both macrovascular and microvascular complications, including kidney function decline (Bash 2008; Khaw 2004; Klein 1994; Meigs 1997; Selvin 2004). Pre‐existing diabetes in the transplant population has been associated with increased risk of post‐transplant and wound infections, delayed graft function, early onset of acute rejection, increased cardiovascular disease and decreased survival (Cosio 2008; Klein 1994; Kyriakides 1975; Lansang 2006; Parekh 2010; Thomas 2001; Wiesbauer 2010). Similarly, NODAT is associated with increased risk of diabetes‐related complications, cardiovascular disease, graft failure, and mortality (Burroughs 2007; Cole 2008; Hjelmesaeth 2006; Kasiske 2003).
Large scale studies of people with diabetes and preserved kidney function have provided strong evidence that intensive glucose control reduces the incidence and progression of microvascular outcomes and particularly kidney complications (ADVANCE 2008; Ismail‐Beigi 2010; CONTROL Group 2009; DCCT Group 1993; DCCT Group 1995; DCCT Group 2005; Duckworth 2009; Holman 2008; UKPDS Group 1998a; UKPDS 1998b).
Why it is important to do this review
There are significant knowledge gaps surrounding the efficacy and safety of glucose‐lowering interventions in patients with kidney transplantation. The choice of drug therapy is often complicated by potential interactions with immunosuppressive medication, and the associated problem of kidney insufficiency. Recommendations are either dated (Davidson 2003), extrapolated from randomised controlled trials (RCTs) of non‐transplant patients (Diabetes Care 2003; KDIGO 2009; Inzucchi 2012), or extrapolated from cohort studies of transplant patients (Wiesbauer 2010).
Given the uncertainty surrounding the efficacy and safety of contemporary pharmacological interventions for improving glucose control in the kidney transplant population, we performed a systematic review to inform clinical practice and highlight areas requiring further research.
The original scope of this review was investigation of the efficacy and safety of contemporary glucose‐lowering agents for diabetes in both people with CKD (including people with ESKD) and kidney transplant recipients. However, as the specific challenges of managing blood glucose levels were deemed different in kidney transplant recipients compared with other patients with CKD, we decided to examine the efficacy and safety of contemporary glucose‐lowering in these different populations in separate reviews. This review examined glucose‐lowering in the kidney transplant population. The complementary review, "Insulin and glucose‐lowering agents for treating people with diabetes and chronic kidney disease" will be completed in 2017 (Lo 2015).
Objectives
To evaluate the efficacy and safety of pharmacological interventions for lowering glucose levels in patients who have undergone kidney transplantation and have diabetes.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs
Quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at glucose‐lowering therapy
Cross‐over studies (only first phase data considered).
Types of participants
Inclusion criteria
Adults (aged 18 years or over) and children (aged up to 18 years) who are kidney transplant recipients with type 1 or type 2 diabetes, or NODAT.
Exclusion criteria
Treatment with first generation sulphonylureas
Pancreas transplantation
Islet cell transplantation.
Types of interventions
Head‐to‐head comparisons of active regimens (including comparisons of monotherapy or combination therapy with two or more pharmacological glucose‐lowering interventions, comparisons of different doses and durations of the same intervention) or active regimen compared with placebo/control/standard care were eligible for inclusion.
Metformin
Insulin
Sulphonylurea (excluding first generation)
Glucagon‐like peptide‐1 agonists
Glinides
Glitazones
ɑ‐glucosidase inhibitors
DPP‐4 inhibitors
SGLT2 inhibitors
Dual peroxisome proliferator‐activated receptor agonists
Amylin analogues
Bromocriptine
GPR40 or free fatty acid receptor 1 agonists.
Types of outcome measures
Efficacy
Safety.
Primary outcomes
Transplant or graft survival
Glycated haemoglobin A1c (HbA1c)
Fasting blood glucose (FBG)
Kidney function markers including creatinine, estimated glomerular filtration rate (eGFR), albuminuria
Systolic and diastolic blood pressure (BP)
Lipids (HDL, LDL, triglyceride)
Body weight.
Secondary outcomes
All‐cause mortality
Macrovascular events (cardiovascular death, non‐fatal myocardial infarction (MI), non‐fatal stroke)
Microvascular events (new or worsening kidney disease, retinopathy)
-
Safety:
Hypoglycaemia
Discontinuation of medication due to adverse events
Gastrointestinal side effects
Congestive heart failure, oedema
Lactic acidosis
Liver abnormalities/failure
Cancer events
Other adverse events as described by authors.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register to 15 April 2016 through contact with the information Specialist using search terms relevant to this review. The Specialised Register contains studies identified from the following sources.
Quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE (OVID SP)
Handsearching of renal‐related journals and the proceedings of major renal conferences
Searching of the current year of EMBASE (OVID SP)
Weekly current awareness alerts for selected renal journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies and a list of handsearched journals, conference proceedings and current awareness alerts are available in the Specialised Register section of information about the Cochrane Kidney and Transplant.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete trials to investigators known to be involved in previous studies.
See Appendix 1 for search strategies
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies potentially relevant to the review. Titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however, studies and reviews thought to include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts, and if necessary the full text, to determine which studies satisfied inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standardised data extraction forms. Studies reported in non‐English language journals were to be translated before assessment. Where more than one publication of one study was found, reports were grouped together and the publication with the most complete data was included. When relevant outcomes were only published in earlier versions these data were used. Any discrepancy between published versions was to be highlighted. Disagreements were resolved by consultation with all authors.
Assessment of risk of bias in included studies
The following items were assessed using the risk of bias assessment tool (Higgins 2003) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (e.g. all‐cause mortality, macrovascular and microvascular events or safety events such as hypoglycaemic episodes) results were to be expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. HbA1c, fasting plasma glucose, creatinine, eGFR, albuminuria, blood pressure, lipids, body weight), the mean difference (MD) was to be used, or the standardised mean difference (SMD) if different scales had been used.
Dealing with missing data
Any further information required from the original author was requested by written correspondence and any relevant information obtained included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population were carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (e.g. last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
Assessment of heterogeneity
Heterogeneity was to be analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
If possible, funnel plots were to be used to assess for the potential existence of small study bias (Higgins 2011). Reporting bias could not be assessed: only two studies could be pooled for one outcome.
Data synthesis
Data were to be pooled using the random‐effects model but the fixed‐effect model was also to be used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was to be used to explore possible sources of heterogeneity (e.g. participants, interventions and study quality). Heterogeneity among participants could be related to age, sex, type 1 diabetes versus type 2 diabetes, duration of diabetes, baseline HbA1c level, primary cause of kidney disease (diabetes versus others), and CKD stage. Heterogeneity in treatments could be related to prior or concomitant agent(s) used (such as insulin therapy), and the agent, dose and duration of therapy. Adverse effects were to be tabulated and assessed with descriptive techniques, because they were likely to differ for various agents. Where possible, the risk difference with 95% CI was to be calculated for each adverse effect, either compared with no treatment or another agent.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors on effect size.
Repeating the analysis excluding unpublished studies
Repeating the analysis taking account of risk of bias, as specified
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
'Summary of findings' tables
We presented the main results of the review in Table 1; Table 2; Table 3. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We have presented the following outcomes in the 'Summary of findings' tables.
Transplant or graft survival
HbA1c
FBG
Kidney function markers including creatinine, eGFR, albuminuria
All‐cause mortality, macrovascular events (cardiovascular death, non‐fatal MI, non‐fatal stroke)
Microvascular events (new or worsening kidney disease, retinopathy)
Safety: hypoglycaemia; discontinuation of medication due to adverse events; gastrointestinal side effects; congestive heart failure; oedema; lactic acidosis; liver abnormalities/failure; cancer events; other adverse events as described by authors.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The search yielded 41 records (20 studies). Following assessment of the titles, abstracts and full‐text, we included seven studies (16 records), excluded 11 studies (23 records) and two studies are awaiting assessment (Figure 1).
Figure 1.
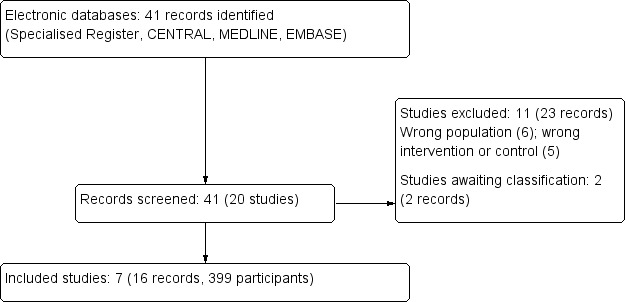
Study flow diagram
Included studies
We included seven studies (Barbosa 1983; Barbosa 1994; Haidinger 2010; Hermayer 2012; Kharazmkia 2014; Soliman 2013; Strom Halden 2014) that involved 399 participants. Study sample size ranged from 19 (Strom Halden 2014) to 99 (Barbosa 1994) and most were conducted in single centre hospital clinics associated with kidney transplant units. Study follow‐up ranged from two months (Strom Halden 2014) to five years (Barbosa 1994).
Six studies were parallel RCTs (Barbosa 1983; Barbosa 1994; Haidinger 2010; Hermayer 2012; Kharazmkia 2014; Soliman 2013) and one was a cross‐over RCT (Strom Halden 2014). Only Haidinger 2010 and Kharazmkia 2014 were double blinded.
Three studies compared more versus less intensive regimens of insulin from the immediate post‐transplant period onwards in patients with pre‐existing type 1 diabetes (Barbosa 1983; Barbosa 1994) or both type 1 and type 2 diabetes (Hermayer 2012).
Three studies compared DPP‐4 inhibitors either with placebo or glargine insulin in patients with NODAT. Haidinger 2010 compared vildagliptin with placebo; Strom Halden 2014 compared sitagliptin and placebo in a cross‐over RCT; and Soliman 2013 compared sitagliptin to glargine.
Kharazmkia 2014 compared pioglitazone and insulin therapy with insulin therapy alone in patients with diabetic kidney disease who had received kidney transplants.
Excluded studies
Reasons for exclusion were as follows (see Characteristics of excluded studies).
Wrong population (Han 2010; Hecking 2012; Re 2010; Vienna SAPT‐NODAT 2012; Werzowa 2013; Yates 2014)
Wrong intervention or control group (Kim 1997a; LANDMARK 2 2009; Orazio 2011; Sharif 2009; Yates 2012).
Risk of bias in included studies
Overall, one study was judged to be have an unclear risk of bias (Haidinger 2010), and all other included studies were found to be at high risk of bias (Barbosa 1983; Barbosa 1994; Hermayer 2012; Kharazmkia 2014; Soliman 2013; Strom Halden 2014) (Figure 2; Figure 3).
Figure 2.
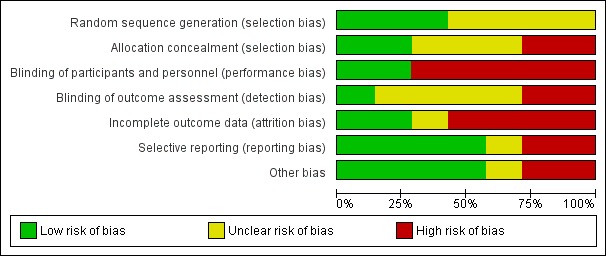
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
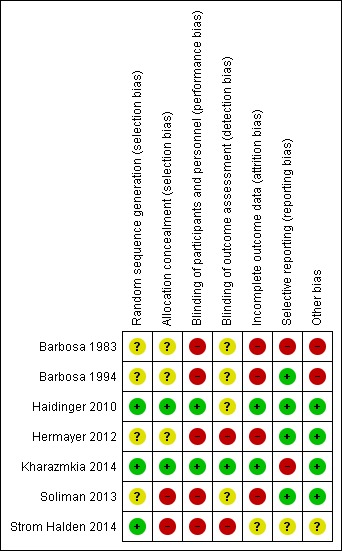
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Three studies were judged to be at low risk of bias (Haidinger 2010; Kharazmkia 2014; Strom Halden 2014) and four studies were judged unclear (Barbosa 1983; Barbosa 1994; Hermayer 2012; Soliman 2013).
Allocation concealment
Two studies were judged to be at low risk of bias (Haidinger 2010; Kharazmkia 2014), two were judged to be at high risk of bias (Soliman 2013; Strom Halden 2014) and three studies were judged unclear (Barbosa 1983; Barbosa 1994; Hermayer 2012).
Blinding
Performance bias
Five studies were judged to be at high risk of bias (Barbosa 1983; Barbosa 1994; Hermayer 2012; Soliman 2013; Strom Halden 2014) and two studies were at low risk of bias (Haidinger 2010; Kharazmkia 2014).
Detection bias
One study was judged to be a low risk of bias (Kharazmkia 2014); two were at high risk of bias (Hermayer 2012; Strom Halden 2014), and four studies were judged unclear (Barbosa 1983; Barbosa 1994; Haidinger 2010; Soliman 2013).
Incomplete outcome data
Two studies were judged to be at low risk of bias (Haidinger 2010; Kharazmkia 2014), four were at high risk of bias (Barbosa 1983; Barbosa 1994; Hermayer 2012; Soliman 2013), and one study was judged unclear (Strom Halden 2014).
Selective reporting
Four studies were judged to be at low risk of bias (Barbosa 1994; Haidinger 2010; Hermayer 2012; Soliman 2013). Two studies were at high risk of bias. Barbosa 1983 had a high risk of reporting bias, and did not list any pre‐specified primary outcomes, but had pre‐specified objectives, none of which were reported, and Kharazmkia 2014 had a high risk of reporting bias as several errors were detected in the published report. One study was judged unclear (Strom Halden 2014).
Other potential sources of bias
Four studies were judged to be at low risk of bias (Haidinger 2010; Hermayer 2012; Kharazmkia 2014; Soliman 2013), two studies were at high risk of bias (Barbosa 1983; Barbosa 1994), and one study was judged unclear (Strom Halden 2014). Strom Halden 2014 was a cross‐over RCT and there was no washout period between the four weeks of sitagliptin and non‐sitagliptin treatment. For patients randomised to sitagliptin treatment first and then crossed‐over to the non‐sitagliptin treatment, the perceived effectiveness of sitagliptin might have been attenuated by the lack of a wash‐out period.
Effects of interventions
See: Table 1; Table 2; Table 3
Differences concerning interventions and outcomes measured, poor availability of additional data from authors, and poor study reporting meant that outcome data could not be pooled for meta‐analysis.
Primary outcomes
Transplant or graft survival
Treatment effects of intensive versus less intensive insulin therapy and DPP4 inhibitors on transplant or graft survival were very uncertain and uncertain, respectively. Two studies reported transplant or graft survival as an outcome of more intensive versus less intensive insulin therapy (Barbosa 1994; Hermayer 2012), and two studies reported transplant or graft survival as an outcome of DPP4 therapy (sitagliptin Strom Halden 2014 and vildagliptin Haidinger 2010). However, results could not be pooled due to observed heterogeneity in the therapy regimens and the measures of transplant or graft survival .
Hermayer 2012 reported graft rejections and incidence of delayed graft function (serum creatinine > 221 µmol/L 10 days post‐transplant or the need for dialysis within the first seven days post‐transplant). More intensive compared with less intensive insulin therapy resulted in a greater incidence of rejection events during the three year follow‐up period (9/11 versus 3/11 respectively, P = 0.01). However, more intensive compared with less intensive insulin therapy resulted in a similar incidence of delayed graft function affecting 8/44 participants (18%) and 12/49 (24%) participants, respectively (P = 0.46). Of note, there was no association between graft rejection and experience of severe hypoglycaemia (blood glucose level (BGL) < 2.22 mmol/L).
Barbosa 1994 reported transplant or graft survival indirectly. In this study, patients who experienced graft loss or chronic rejection were excluded from the results. There was no significant difference in numbers of graft losses between patients receiving more intensive (two events) compared with less intensive insulin therapy (two events) (P = 0.70).
In response to our enquiry, Strom Halden 2014 reported 100% transplant and graft survival during the eight week cross‐over study. Haidinger 2010 also confirmed in correspondence that there was no difference in graft and kidney transplant function between vildagliptin and placebo group participants.
HbA1c
Five studies reported HbA1c: Barbosa 1983 compared more intensive with less intensive insulin therapy; Haidinger 2010, Soliman 2013 and Strom Halden 2014 examined the efficacy of DPP‐4 inhibitors; and Kharazmkia 2014 investigated pioglitazone. Treatment effects of more intensive versus less intensive insulin therapy were very uncertain, and the treatment effects of DPP‐4 inhibitors and pioglitazone were uncertain.
More compared with less intensive insulin therapy resulted in a lower mean HbA1c (± standard error of the mean (SEM)) at 13 months 10 ± 0.8% compared with standard insulin (13 ± 0.9%). This result was difficult to interpret given that participants' baseline HbA1c was not reported (Barbosa 1983).
In Haidinger 2010 the group receiving vildagliptin demonstrated a greater reduction in mean (± SD) HbA1c at three months (0.6 ± 0.5%) compared with the placebo group (0.1 ± 0.5%, P = 0.02). At four months the changes in HbA1c levels were similar: HbA1c reduction in the vildagliptin group (0.5 ± 0.4%) was no longer significantly greater than HbA1c reduction in placebo group participants (0 ± 0.8%, P = 0.08). However, the mean (± SD) absolute HbA1c level remained significantly lower in the vildagliptin group compared with placebo (6.3 ± 0.5% versus 6.7 ± 0.6% P = 0.03). This effect occurred in a setting of similar baseline HbA1c levels in the vildagliptin and placebo groups (6.7 ± 0.73% versus 6.7 ± 0.82 %, P = 0.95).
In Soliman 2013, sitagliptin was equivalent in lowering HbA1c from baseline compared with insulin glargine therapy (‐0.6 ± 0.5% versus ‐0.6 ± 0.6%, P = NS). First phase data for the sitagliptin and placebo groups were unavailable for Strom Halden 2014.
Pioglitazone and insulin therapy significantly lowered HbA1c from baseline to a greater extent compared with insulin therapy alone (‐1.21 ± 1.2 versus 0.39 ± 1, P < 0.001) (Kharazmkia 2014).
HbA1 rather than HbA1c was reported as a measure of long‐term glycaemic control by Barbosa 1994. Patients receiving more intensive insulin therapy had significantly lower mean (± SD) HbA1 at the end of follow‐up compared with standard insulin group participants (0.096 ± 0.016 versus 0.117 ± 0.013, P < 0.001) although baseline HbA1 was not reported.
Fasting blood glucose and mean blood glucose level
Five studies reported FBG. Barbosa 1983 compared more intensive with less intensive insulin therapy with the treatment effect being very uncertain. Four studies examined the efficacy of oral glucose‐lowering agents, three investigated DPP‐4 inhibitors (Haidinger 2010; Soliman 2013; Strom Halden 2014), and Kharazmkia 2014 looked at pioglitazone. Treatment effects were uncertain.
Barbosa 1983 reported that intensive insulin therapy achieved a lower FBG (mean ± SEM: 7.22 ± 0.5 mmol/L) compared with standard insulin therapy (13.44 ± 1.22 mmol/L) at 13 months (the study lasted for two years) despite both arms having similar baseline FBG levels (13.39 ± 0.78 versus 13.67 ± 0.67 mmol/L).
Haidinger 2010 and Strom Halden 2014 compared the effects of DPP‐4 inhibitors on FBG compared with placebo. Soliman 2013 compared the effect of DPP‐4 inhibitor treatment on FBG with insulin glargine. However, only data from Haidinger 2010 were available; first phase data from the cross‐over study by Strom Halden 2014 were unavailable. Haidinger 2010 showed that vildagliptin use trended toward lowering FBG more than placebo (mean ± SD: ‐0.91 ± 0.92 mmol/L versus ‐0.18 ± 1.16 mmol/L, P = 0.08). Sitagliptin therapy had an equivalent effect to insulin glargine on post‐intervention FBG (mean ± SD: 4.92 ± 1.42 versus 4.76 ± 1.09, P = NS) (Soliman 2013).
Pioglitazone and insulin therapy had equivalent effects on post intervention FBG compared with insulin therapy alone (mean ± SD: 6.58 ± 2.71 versus 7.28 ± 2.78, P = 0.14) (Kharazmkia 2014) .
Hermayer 2012 reported mean BGL rather than FBG. Intensive insulin therapy achieved a significantly lower mean BGL (mean ± SEM: 6.81 ± 0.19 mmol/L) compared with less intensive insulin therapy (9.85 mmol/L ± 0.19, P < 0.001). However these results were heavily influenced by the intensive group having more frequent BGL assessments in the first 72 hours post‐transplant. When only BGL values after 72 hours were considered, there were no significant differences in BGL between the intensive and standard groups (8.71 ± 0.51 mmol/L versus 8.61 ± 0.49 mmol/L, P = 0.90).
Kidney function markers
Three studies reported creatinine, eGFR and/or albuminuria/proteinuria. Treatment effects of DPP4 inhibitors and pioglitazone on kidney function markers were uncertain.
The effect of DPP‐4 inhibitor use on eGFR and creatinine were compared with placebo by Haidinger 2010 and Strom Halden 2014. The results could not be pooled because data from the first phase of Strom Halden 2014 were unavailable. In Haidinger 2010, vildagliptin, like placebo, had no significant change on eGFR from baseline (1.9 ± 10.3 mL/min/1.73 m2, P = 0.48 and 2.1 ± 6.1 mL/min/1.73 m2, P = 0.22, respectively).
Pioglitazone and insulin therapy had similar effects when compared with insulin therapy on change in creatinine levels from baseline (3.54 ± 15.03 versus 10.61 ± 18.56, P = 0.53) (Kharazmkia 2014).
In correspondence, Strom Halden 2014, reported no development of proteinuria or albuminuria during the study; Haidinger 2010 reported no significant change in albuminuria and proteinuria in both vildagliptin and placebo groups during follow‐up.
Systolic and diastolic blood pressure
The effect of more intensive compared with less intensive insulin therapy on systolic and diastolic blood pressure was very uncertain and the effects of DPP‐4 inhibitors and pioglitazone were uncertain.
Barbosa 1994 reported the effect of more intensive compared with less intensive insulin therapy on systolic and diastolic BP. At baseline, mean (± SD) baseline systolic BP was higher in the more intensive insulin therapy group (132 ± 9 mm Hg) compared with the less intensive insulin therapy group (126 ± 10 mm Hg, P = 0.03). However, there was no significant difference between mean (± SD) baseline diastolic BP in the more intensive (78 ± 4.9 mm Hg) compared with the less intensive group (78 ± 5.2 mm Hg, P = 0.99). During the study there was no significant difference in mean (± SD) systolic (131 ± 7 versus 129 ± 9 mm Hg, P = 0.39) or diastolic (77 ± 6 versus 75 ± 8 mm Hg, P = 0.88) BP between the intensive and standard groups.
Haidinger 2010 and Strom Halden 2014 reported the effects of DPP‐4 inhibitors versus placebo on BP. Results could not be pooled, because first phase data from Strom Halden 2014 were unavailable. Haidinger 2010 reported no significant difference between vildagliptin and placebo groups at baseline in mean (± SD) systolic (131.9 ± 6 versus 127.9 ± 10.2 mm Hg, P = 0.19) or diastolic BP (72.6 ± 7.6 versus 77.3 ± 9.7 mm Hg, P = 0.14). At three months, there was no significant change from baseline in mean (± SD) systolic/diastolic BP in the vildagliptin (‐0.4/1.1 ± 6.6/6.4 mm Hg both P > 0.1) or placebo group (2.5/1.9 ± 11.3/10.1 mm Hg both P > 0.1).
Lipid profile
The effects of DPP‐4 inhibitors and pioglitazone on lipid profile were uncertain
Two studies reported the effect of DPP‐4 inhibitors on lipid profile. Results could not be pooled because Haidinger 2010 compared the effect of the DPP‐4 inhibitor vildagliptin and placebo on lipid levels and Soliman 2013 compared the DPP‐4 inhibitor sitagliptin with insulin glargine.
Haidinger 2010 reported that at baseline there was no significant difference between the vildagliptin and placebo groups in terms of mean (± SD) total cholesterol (5.61 ± 1.27 versus 5.06 ± 0.85 mmol/L, P = 0.16); LDL (3.28 ± 1.17 versus 2.82 ± 0.73 mmol/L, P = 0.35); HDL (1.45 ± 0.6 versus 1.47 ± 0.6 mmol/L, P = 0.61); and triglycerides (2.03 ± 0.89 versus 1.95 ± 0.97 mmol/L P = 0.80). At three months there was no significant change in any lipid parameter in both the vildagliptin and the placebo groups.
Soliman 2013 reported that post‐intervention, in a comparison of sitagliptin and glargine, total cholesterol was lower (mean ± SD: 3.65 ± 0.50 versus 4.35 ± 0.56 mmol/L P < 0.05); HDL was higher (1.15 ± 0.21 versus 1.05 ± 0.14 mmol/L P < 0.05); and triglyceride levels lower (1.49 ± 0.20 versus 1.71 ± 0.23 mmol/L P < 0.05).
Kharazmkia 2014 reported the effect of pioglitazone and insulin therapy compared with insulin therapy alone on lipid profile. Pioglitazone and insulin therapy compared with insulin therapy alone lowered total cholesterol (mean ± SD: ‐0.47 ± 0.73 versus 0.11 ± 0.79 mmol/L, P = 0.004); LDL (‐ 0.40 ± 0.43 versus 0.004 ± 0.84 mmol/L, P = 0.002) and triglyceride levels (‐ 0.32 ± 0.09 versus 0.04 ± 0.15, P = 0.04) from baseline to a greater extent, and raised HDL (0.11 ± 0.18 versus ‐0.01 ± 0.30, P = 0.007) to a greater extent.
Weight
The treatment effects of DPP4 inhibitors and pioglitazone on weight was uncertain.
Compared with insulin glargine, use of the DPP‐4 inhibitor sitagliptin resulted in statistically greater weight loss (‐0.4 kg versus 0.8 kg, P < 0.05) (Soliman 2013).
Pioglitazone and insulin treatment compared with insulin treatment alone had a similar effect on weight (0.61 ± 2.63 kg versus 0.6 ± 2.16 kg, P = 0.73) (Kharazmkia 2014).
In correspondence, Haidinger 2010 confirmed no difference in body weight between vildagliptin and placebo groups.
Secondary outcomes
All‐cause mortality
Treatment effects of more intensive versus less intensive insulin therapy and DPP‐4 inhibitors on all‐cause mortality were very uncertain and uncertain, respectively.
Barbosa 1994 reported all‐cause mortality. More intensive compared with less intensive insulin therapy did not significantly reduce all‐cause mortality compared with the standard group (7 versus 8 deaths, P = 0.79).
In correspondence Haidinger 2010, confirmed that there were no deaths in the vildagliptin or placebo groups and Strom Halden 2014 reported no deaths over eight weeks of follow‐up.
Macrovascular and microvascular events
None of the studies reported macrovascular (defined as cardiovascular death, non‐fatal MI or non‐fatal stroke) or microvascular (new or worsening kidney disease or retinopathy) events. This was confirmed in correspondence with Haidinger 2010 who reported no macrovascular events in either the vildagliptin or placebo groups.
Safety
The safety of more compared to less intensive insulin therapy was very uncertain, and the safety of DPP‐4 inhibitors and pioglitazone was uncertain.
Three studies reported the effect of more intensive versus less intensive insulin therapy on episodes of hypoglycaemia (Barbosa 1983; Barbosa 1994; Hermayer 2012). Results could not be pooled because of observed heterogeneity in the intervention, definition of hypoglycaemia, and presentation of data.
Barbosa 1983 reported in the narrative that intensive insulin therapy resulted in more frequent and severe episodes of hypoglycaemia compared with standard insulin therapy.
Barbosa 1994 reported only severe hypoglycaemic episodes, that is, those requiring third party assistance. More intensive insulin therapy resulted in a higher rate of severe hypoglycaemia compared with less intensive insulin therapy (1.7 episodes/patient/year versus < 0.1/patient/year, P < 0.001). Of the 29 episodes of severe hypoglycaemia resulting in hospital admission, 26 occurred in the intensive insulin group. A patient in the intensive insulin group remained comatose for six days and required a two week hospital admission.
Hermayer 2012 reported that more intensive insulin therapy resulted in more patients experiencing hypoglycaemic episodes (BGL < 3.88 mmol/L) compared with less intensive insulin therapy (84% versus 25% P < 0.001). However, more intensive insulin therapy did not result in significantly more episodes of severe hypoglycaemia (defined as BGL < 2.22 mmol/L) than less intensive insulin therapy (eight events in seven patients compared with two events in two patients, P = 0.08).
Three studies reported the effect of DPP‐4 use on hypoglycaemic episodes (Haidinger 2010; Soliman 2013; Strom Halden 2014). Data could not be pooled due to observed heterogeneity in definition of hypoglycaemia, presentation of data, and interventions. Haidinger 2010 reported that vildagliptin use did not result in any episodes of hypoglycaemia; however, hypoglycaemia was not defined. Strom Halden 2014 reported no episodes of severe hypoglycaemia due to sitagliptin (BGL < 2 mmol/L and unconsciousness or the need of assistance). However, two patients each had episodes of asymptomatic moderate hypoglycaemia (BGL 2 to 3.9 mmol/L) while receiving glipizide and sitagliptin. Soliman 2013 reported fewer episodes of hypoglycaemia events in the sitagliptin group (3/28 patients; 10.7%) compared with insulin glargine group participants (5/28; 17.9%). Hypoglycaemia was not defined and no severe episodes were reported (Soliman 2013).
One study examined the effect of pioglitazone on hypoglycaemia. Kharazmkia 2014 reported no episodes of hypoglycaemia in comparing pioglitazone and insulin therapy versus insulin therapy alone.
Concerning other safety parameters, among studies that compared more intensive versus less intensive insulin therapy, Hermayer 2012 reported more episodes of severe hyperglycaemia (BGL > 19.44 mmol/L) in the less compared with more intensive insulin therapy (12 participants (24%) in the less intensive arm compared with five participants (11%) in the intensive arm). This was however not statistically significant (P = 0.10). There were no reports of discontinuation of the study intervention due to adverse effects.
Among studies involving DPP‐4 inhibitors, the only study reporting discontinuation of the study intervention due to adverse effects was Strom Halden 2014, where sitagliptin use was associated with one discontinuation due to night sweats. Haidinger 2010 reported similar rates of adverse events (5/16; 31.3%) in the vildagliptin group compared with the placebo group (6/16; 37.5%), with small but similar numbers of episodes of angina (1 versus 0), elevated liver enzymes (2 versus 1), elevated pancreatic enzymes (0 versus 1), elevated triglycerides (0 versus 1), cough (0 versus 1), conjunctivitis (1 versus 0), urinary tract infections (1 versus 1) and leukopenia (0 versus 1). There were no reported drug interactions with any immunosuppressive agents being taken. Soliman 2013 reported an overall low incidence of adverse events which were mild in both the sitagliptin and insulin glargine groups. Strom Halden 2014 reported an association between sitagliptin use and an increase in uric acid level (median, IQR: 25, ‐2 to 45 µmol/L) but no episodes of gout were reported. Sitagliptin did not interact with any immunosuppressive agents ‐ there were no significant changes in trough concentrations of cyclosporin A (P = 0.45), tacrolimus (P= 0.07), everolimus (P = 1.00) and mycophenolate mofetil (P = 0.65) following sitagliptin treatment.
Kharazmkia 2014 reported that four participants dropped out due to adverse effects of pioglitazone (three had mild to moderate lower extremity oedema and one had insomnia). No study participants had transient elevation of liver enzyme levels two or more times the upper limits or episodes of liver toxicity, severe cardiac failure, or change in creatinine levels. Pioglitazone did not affect cyclosporin levels or dose.
Subgroup analysis
Prespecified subgroup analyses were not possible due to the limited number of included studies.
Sensitivity analysis and publication bias
Sensitivity analyses and assessment for publication bias via a funnel plot were not possible due to the limited number of included studies.
Other outcomes reported
Barbosa 1994 reported glomerular mesangial expansion on electron microscopy (as an indicator of development of diabetic nephropathy). Intensive glycaemic control retarded onset and development of diabetic nephropathy; the standard group had a two‐fold greater increase in mesangial matrix volume fraction on kidney biopsy compared with the intensive group (P = 0.02) on kidney biopsy.
Discussion
Summary of main results
This review provides a contemporary comprehensive overview of efficacy and safety of glucose‐lowering agents for patients with kidney transplantation and diabetes. Evidence for the current use of glucose‐lowering agents was limited; we identified only six parallel RCTs and one cross‐over RCT for inclusion. Three studies compared more intensive with less intensive insulin regimens in people with pre‐existing diabetes, three examined the efficacy and safety of DPP‐4 inhibitors in people with NODAT, and one study compared pioglitazone and insulin therapy with insulin therapy alone.
We found that the treatment effect of more intensive compared to less intensive insulin regimens in kidney transplant recipients with type 1 and 2 diabetes was very uncertain. Three studies investigating more intensive compared with less intensive insulin therapy improved glycaemic control but resulted in an increased rate of hypoglycaemia or severe hypoglycaemia, especially when aiming for normoglycaemia (Barbosa 1983; Barbosa 1994; Hermayer 2012). Compared with less intensive, more intensive insulin therapy decreased development of diabetic nephropathy lesions as seen histologically in the kidney allografts of patients with type 1 diabetes in one study (Barbosa 1994). Intensive peri‐ and post‐transplant insulin therapy (IV insulin transitioning to a basal plus bolus regimen aiming for BGL < 6.7 mmol/L on discharge) in kidney transplant recipients with type 1 or 2 diabetes did not reduce incidence of delayed graft function but increased the incidence of hypoglycaemia, but not severe hypoglycaemia. This regimen was associated with an increased number of rejection episodes compared with standard insulin therapy (subcutaneous insulin with a basal plus bolus regiment aiming for a BGL < 10 mmol/L on discharge) (Hermayer 2012). All studies were assessed at high risk of bias
The treatment effect of DPP4 inhibitors in kidney transplant recipients with type 2 diabetes was uncertain. The DPP‐4 inhibitors vildagliptin and sitagliptin appeared to offer effective and safe alternate methods of lowering glucose in people with NODAT, with low risk of hypoglycaemia, and no reported interaction with immunosuppressant drugs (Haidinger 2010; Soliman 2013; Strom Halden 2014). These results are limited by the small number of participants studied (96 across all 3 studies), the overall quality of the studies (only one was assessed at low risk of bias; Haidinger 2010) and the inclusion of a cross‐over study with no available phase 1 data (Strom Halden 2014).
The treatment effect of pioglitazone in kidney transplant recipients with type 2 diabetes was uncertain. In one study, compared with insulin therapy, pioglitazone and insulin therapy for kidney transplant recipients with pre‐existing diabetes achieved lower HbA1c, better lipid profile, equivalent weight gain and no reported interaction with immunosuppressant drugs (Kharazmkia 2014). However, there was a higher incidence of lower extremity oedema. This result is limited by the small study population (62) and errors identified in the published report, leading to a high risk of reporting bias.
Due to the limited number and low quality of studies, treatment effects are uncertain or very uncertain, the clinical application of results is limited and presentation of definitive findings and conclusions concerning the efficacy and safety of glucose‐lowering agents in kidney transplant recipients with diabetes is not possible.
Overall completeness and applicability of evidence
Our review was limited by suboptimal study quality; most included studies were assessed at high risk of bias. Consequently, the applicability of findings to clinical practice is limited.
Another limiting factor was that most studies were conducted in Caucasian or African American patients, affecting the generalisability of findings to other populations. This is especially relevant given that DPP‐4 inhibitors have been reported to lower FBG to a greater extent in Asian compared with non‐Asian patients (Kim 2013). As a result, the efficacy of DPP‐4 inhibitors may also differ in Asian compared with non‐Asian kidney transplant patients.
Quality of the evidence
Evidence for all treatment effects was of low or very low quality. We found that all seven included studies were limited in terms of study quality. Only one study was assessed at low risk of bias (Haidinger 2010). Although all studies were described as RCTs (one was a cross‐over RCT) (Strom Halden 2014), only three studies adequately reported randomisation methods (Haidinger 2010; Kharazmkia 2014; Strom Halden 2014); one confirmed on correspondence (Haidinger 2010). Additionally, most studies had poorly described or had no allocation concealment, and had an unclear or high risk of performance and detection bias.
Potential biases in the review process
Publication bias could not be assessed due to the limited number of studies. Despite a comprehensive search, we were unable to contact authors of two studies, and we were unable to exclude the possibility that studies with negative findings remained unpublished.
Agreements and disagreements with other studies or reviews
The Transplantation Society NODAT guidelines have previously provided recommendations extrapolated from the American Diabetes Association guidelines (which describes the utility of glucose‐lowering agents in the non‐transplant population) (Davidson 2003). Similarly, the 2009 KDIGO guidelines on kidney transplantation (KDIGO 2009) and other experts (Ghisdal 2012; Hecking 2013; Sarno 2012; Therasse 2013; Wissing 2014; Yates 2012b) have provided guidance on the use of glucose‐lowering agents in this population, but without the stringent methodology of a systematic review. Our review highlights the lack of RCTs, and suboptimal reporting and data in existing studies examining glucose‐lowering interventions in kidney transplant recipients with diabetes.
Most reviews and experts recommend use of insulin in the peri‐ and post‐ kidney transplant period for the management of uncontrolled hyperglycaemia in the setting of the physiological stress of surgery and high dose corticosteroid requirements (Yates 2012). They propose inpatient glycaemic targets based on the AACE/ADA guidelines for inpatient management of hyperglycaemia (Moghissi 2009). Insulin is also recommended as a step‐up therapy from oral hypoglycaemic agents in the setting of treatment failure in the subacute and chronic outpatient setting (Wissing 2014). A general glycaemic target of 7% is recommended to prevent onset and progression of microvascular complications (ADA 2015). However, most of these recommendations are extrapolated from studies which are not specific for people with diabetes and a kidney transplant. Our systematic review support these recommendations, and suggest that a random BGL < 10 mmol/L is appropriate (Hermayer 2012). Additionally, our systematic review suggests that intensive glucose control may decrease development of nephropathy (as observed histologically on biopsy) but increase rates of hypoglycaemia (Barbosa 1994). However, our review is limited by the high risk of bias amongst included studies, and the quality of evidence reviewed was very low.
The studies examining vildagliptin versus placebo (Haidinger 2010) and sitagliptin and glargine versus glargine (Soliman 2013), are the only published RCTs (at the time of our literature search in April 2016) studying DPP‐4 inhibitors in this population. The other included sitagliptin study (Strom Halden 2014) was a cross‐over RCT. Although the treatment effects of DPP4‐inhibitors from our review are uncertain as all three studies were relatively small and short‐term with follow‐up to a maximum of four months, and only one was assessed at low risk of bias, they do support the growing body of literature on the efficacy and safety of DPP‐4 inhibitors for kidney transplant recipients. Lane 2011 previously reported an HbA1c improvement of 0.5% and no episodes of hypoglycaemia or evidence of interaction with immunosuppressant therapy (tacrolimus and sirolimus) in a single‐arm pilot study of 15 patients treated with sitagliptin. More studies of DPP‐4 inhibitor use in the kidney transplant population are required.
Kharazmkia 2014 was the only published RCT (at the time of our literature search in April 2016) studying the efficacy and safety of pioglitazone in kidney transplant recipients with type 2 diabetes. Over four months, pioglitazone demonstrated effective HbA1c lowering without interaction with immunosuppressant agents. This is coherent with results of previous studies, including a retrospective single‐arm pilot study of 10 kidney transplant recipients with pre‐existing diabetes or NODAT treated with pioglitazone (Luther 2004) and a three‐arm parallel RCT of 48 stable kidney transplant recipients comparing pioglitazone, vildagliptin and placebo in kidney transplant recipients with impaired glucose tolerance (Werzowa 2013). However, in contrast to Kharazmkia 2014, Luther 2004 did not show any beneficial effect on lipid profile, or significant fluid retention, possibly due to the small cohort, the non‐randomised design of the study, and the fact that insulin was not used concurrently with pioglitazone. There is an increased risk of oedema when thiazolidinediones are used concurrently with insulin (Iglesias 2006). Similarly, in Werzowa 2013 the pioglitazone arm did not show a beneficial effect on lipid profile or an increased rate of oedema compared with placebo, possibly due to the small cohort, insulin not being used concurrently with pioglitazone, and the fact that the study population had IGT not diabetes.
Authors' conclusions
The evidence concerning the efficacy and safety of glucose‐lowering agents in kidney transplant recipients with diabetes is limited and of low or very low quality. Additional RCTs are needed to examine the safety and efficacy of contemporary glucose‐lowering agents for kidney transplant recipients with diabetes.
There is limited evidence for the safety and use of contemporary glucose‐lowering agents for kidney transplant recipients with diabetes.
More RCTs investigating different insulin regimens are required to determine appropriate acute inpatient treatment in the peri‐transplant period and appropriate long‐term outpatient treatment to improve outcomes such as graft survival and graft function.
DPP‐4 inhibitors and Pioglitazone appear to be a promising treatment for use in kidney transplant recipients, but larger double blind RCTs are required to confirm safety and efficacy, especially with respect to graft survival outcomes. Appropriately blinded RCTs are needed to evaluate the safety and efficacy of other classes of contemporary glucose‐lowering agents in the kidney transplant population such as sulphonylureas or SGLT2 inhibitors.
Acknowledgements
We wish to thank Cochrane Kidney and Transplant. We also thank the referees for their comments and feedback during the preparation of this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement. | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
This review has no analyses.
Differences between protocol and review
The original protocol and scope of this review was to study the efficacy and safety of contemporary glucose‐lowering agents for diabetes in both chronic kidney disease and kidney transplant recipients. Due to inherent differences in the clinical presentation and management challenges of the kidney transplant and the chronic kidney disease populations, the authors decided to examine glucose‐lowering in these two populations as two separate systematic reviews. This is the first of two reviews, examining the efficacy and safety of glucose‐lowering pharmacotherapy in people with kidney transplants with new‐onset or pre‐existing diabetes. The second review will examine the efficacy and safety of glucose‐lowering pharmacotherapy in people with diabetes and chronic kidney disease, insulin and glucose‐lowering agents in people with diabetes and chronic kidney disease.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
Co‐interventions
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible due to the nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were a much larger number of patients who dropped out of the intensive group (5/33 or 15.15%) due to noncompliance compared with the standard group (1/16 or 6.25%). Conversely there was a larger number of patients who dropped out of the standard group due to kidney graft rejection (3/16 or 18.75%) compared with the intensive group (4/33 or 12.12%) However, overall the proportion of people dropping out due to all causes (including kidney rejection, death and lack of compliance) was equivalent between the standard (31.25%) and the intensive groups (30.61%). Furthermore, although not stated, it seemed that a per protocol analysis was done, increasing the risk of attrition bias |
| Selective reporting (reporting bias) | High risk | The study did not list any pre‐specified primary outcomes, but had prespecified objectives, none of which were reported |
| Other bias | High risk | There was the an additional risk of selection or allocation bias as the authors mentioned that during the study, due to the high attrition rate in the first year of the study, they introduced more stringent selection criteria which led to fewer dropouts in subsequent years Funding source: NIH grant, Eli Lilly, Ames Company, Minnesota Medical Foundation, Squibb/Novo, Minnesota Mining and Manufacturing |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
Co‐interventions
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible due to the nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A large number of patients did not complete the study, however, all the withdrawals and losses to follow‐up were described. Although dropout rate was high, the authors wrote that there was no significant difference in dropout rates between groups, and no obvious source of bias that would affect conclusions, regarding maximised versus standard glycaemic control. However, with regard to the breakdown of causes of drop‐out, the intensive insulin therapy group had a higher rate of voluntary withdrawals 9/27 or 33.33% (i.e. people who could not tolerate the intensive insulin therapy) compared with the less intensive insulin therapy group 4/24 or 16.67%. This could lead to attrition bias, given that the authors did a per protocol analysis for outcomes show |
| Selective reporting (reporting bias) | Low risk | The prespecified primary outcome of glomerular mesangial expansion as determined by electron microscopy was reported |
| Other bias | High risk | There was an additional risk of selection bias because there was a change in the randomisation method ‐ initially investigators randomised 2:1 in favour of the intensive group as investigators hypothesised that this group would have more withdrawals from the study than the standard group. As this was not the case, in the seventh year of recruiting, they randomised 2:1 in favour of the standard group Funding source: Grants from the National Institute of Health and the National Centre for Research Resources. Also funding from Eli Lilly and Boehringer Mannheim Diagnostics, Minnesota Medical Foundation and the American Diabetes Association |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
Co‐interventions
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | On correspondence, randomisation was reported to be performed by the pharmaceutical department via a computer randomisation program |
| Allocation concealment (selection bias) | Low risk | On correspondence, allocation concealment and blinding was reported to be maintained until the end of the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was done at the local pharmacy; study was also double blind and a placebo pill was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout rate, with 1/33 not completing the study |
| Selective reporting (reporting bias) | Low risk | The main prespecified primary and secondary outcomes of interest were reported |
| Other bias | Low risk | Funding source: Medical University of Vienna, Austria |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
Co‐interventions
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The study was unblinded and subjects were randomised before transplant. Losses to follow‐up mainly occurred if people were not transplanted. Reasons for not being transplanted included a positive cross match, poor graft quality, patient health, and “other” and “unspecified”. There was a higher dropout rate i.e. 8/52 or 15.38% rate in the intensive group and 3/52 = 5.77% in the standard group. Given that there was no allocation concealment, the higher dropout rate in the intensive group could potentially be related to investigator bias |
| Selective reporting (reporting bias) | Low risk | All the prespecified primary and secondary outcomes were reported |
| Other bias | Low risk | Funding source: American Diabetes Association Clinical Investigator Award |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
Co‐interventions
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number method |
| Allocation concealment (selection bias) | Low risk | "...concealment of randomisation was adhered to" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double blind; clinician researchers, participants and data analysts remained blinded to the random allocations during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as double blind; clinician researchers, participants and data analysts remained blinded to the random allocations during the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10/71 participants (14.29%) did not complete the study. 5/35 (11.4%) pioglitazone group participants dropped out because they did not receive the intervention; 5/36 placebo arm participants (11.1%) dropped out because they did not receive the intervention |
| Selective reporting (reporting bias) | High risk | Reported all major outcomes. Did not report eGFR. Several errors were detected in the published report |
| Other bias | Low risk | Funding source: grant from the Deputy of Research and Technology of Shahid Behesti University of Medical Sciences |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
Co‐interventions
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | High risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open‐label study but many endpoints requiring assay were performed by technicians blinded to the treatment sequence. Therefore most efficacy outcomes were blinded except for weight. For safety outcomes, it was unclear if researchers were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17/62 (27.4%) participants dropped out. Rates were higher in the glargine group (insulin) (14/31; 45%) compared with sitagliptin (3/31; 9.68%) In the insulin group 6 patients who required multiple short acting dosages of insulin were excluded; 3 were lost to follow‐up, and 5 did not want to continue insulin due to the fear of the long‐term need for insulin. In the sitagliptin group, all drop outs occurred 1 week after randomisation. 3/31 patients needed multiple short acting dosages of insulin and were excluded A PP analysis was conducted |
| Selective reporting (reporting bias) | Low risk | Prespecified primary outcomes of interest were reported |
| Other bias | Low risk | Funding source: no grants were received for the study |
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
Co‐interventions
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by use of a pre‐made list of treatment sequence, and the consecutively included patients were assigned the next number on the list, which was not available to the physicians recruiting the patients |
| Allocation concealment (selection bias) | High risk | No allocation concealment as confirmed by the authors. The study was unblinded and not placebo controlled. This was confirmed with the authors |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Reported to be unblinded and not placebo controlled |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Reported to be unblinded and not placebo controlled |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One patient (5.26%) dropped out and did not complete the study due to side effects (night sweats) from sitagliptin |
| Selective reporting (reporting bias) | Unclear risk | All prespecified primary and secondary outcomes were reported, however first phase data were not available |
| Other bias | Unclear risk | The authors confirmed in writing that there was no washout period for patients who stopped sitagliptin and then crossed‐over to no treatment. This may dilute out the effect of sitagliptin compared with no treatment Funding source: investigator initiated; grant from Southeast Norway Regional Health Authority and Laboratory for renal physiology at Rikshospitalet |
ALT ‐ alanine aminotransferase; AST ‐ aspartate aminotransferase; AZA ‐ azathioprine; BGL ‐ blood glucose level; BP ‐ blood pressure; CHF ‐ congestive heart failure; CSA ‐ cyclosporin; eGFR ‐ estimated glomerular filtration rate; FBG ‐ fasting blood glucose. HBA1c ‐ glycated haemoglobin A1c; HDL ‐ high density lipoprotein; LDL ‐ low density lipoprotein; M/F ‐ male/female; MI‐ myocardial infarction; MMF ‐ mycophenolate mofetil; MPA ‐ mycophenolic acid; NODAT ‐ new‐onset diabetes after transplant; PP ‐ per protocol; SC ‐ subcutaneous; SD ‐ standard deviation; T1DM ‐ type 1 diabetes mellitus; T2DM ‐ type 2 diabetes mellitus; TAC ‐ tacrolimus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Han 2010 | Wrong population: did not involve patients with diabetes |
| Hecking 2012 | Wrong population: did not involve patients with diabetes |
| Kim 1997a | Wrong intervention/control: did not use glucose‐lowering agents |
| LANDMARK 2 2009 | Wrong intervention/control: did not use glucose‐lowering agents |
| Orazio 2011 | Wrong intervention/control: did not use glucose‐lowering agents |
| Re 2010 | Wrong population: did not involve patients with diabetes |
| Sharif 2009 | Wrong intervention/control: did not use glucose‐lowering agents |
| Vienna SAPT‐NODAT 2012 | Wrong population: did not involve patients with diabetes |
| Werzowa 2013 | Wrong population: did not involve patients with diabetes |
| Yates 2012 | Wrong intervention/control: did not use glucose‐lowering agents |
| Yates 2014 | Wrong population: did not involve patients with diabetes |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
Co‐interventions
|
| Outcomes | Primary outcomes
Secondary outcomes
|
| Notes |
|
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Notes |
|
BP ‐ blood pressure; eGFR ‐ estimated glomerular filtration rate; FBG ‐ fasting blood glucose; HBA1c ‐ glycated haemoglobin A1c; HDL ‐ high density lipoprotein. LDL ‐ low density lipoprotein; M/F ‐ male/female; MI‐ myocardial infarction; SD ‐ standard deviation
Contributions of authors
Draft the protocol: MJ, CL, SZ
Study selection: CL, MJ
Extract data from studies: CL, MJ
Enter data into RevMan: CL
Carry out the analysis: CL
Interpret the analysis: CL, MJ, SB, HP, SW, CH, AC, VP, SZ
Draft the final review: CL, MJ, SZ
Disagreement resolution: SZ
Update the review: CL, MJ, SZ
Declarations of interest
Clement Lo: none known
Min Jun: none known
Sunil Badve: none known
Helen Pilmore: none known
Sarah White: none known
Carmel Hawley has received fees from Amgen, Shire, Roche, Abbott, Bayer, Fresenius, Baxter, Gambro, Janssen‐Cilag and Genzyme in relation to consultancy, speakers' fees, education, and grants for activities unrelated to this review
Alan Cass: Chronic kidney disease Advisory Board for Merck; receiving grant support from Baxter, Servier, Roche, and Gambro; and receiving lecture fees and travel reimbursements from Servier, Amgen, and Fresenius. The George Institute for Global Health conducted the ADVANCE Study, which was supported by a grant from Servier
Vlado Perkovic reports being a member of steering committees for and receiving honoria for scientific presentations from Boehringer Ingleheim, being a consultant and receiving honoraria for speaking at scientific symposia for Eli Lilly, receiving grants from and being a steering committee member and advisory board member for Janssen, being an advisory board member and receiving speaking honoraria from Astra Zeneca, receiving personal fees from Merck, and receiving speaking honoraria from Novo Nordisk.
Sophia Zoungas has previously received speaker honoraria from Servier, MSD, Sanofi Aventis, Eli Lilly, Astra Zeneca, BMS, Takeda and Janssen. She has previously served on external advisory boards for MSD, Novo Nordisk, Sanofi Aventis, Amgen, Novartis, Janssen, Astra Zeneca and Eli Lilly.
New
References
References to studies included in this review
- Barbosa J, Connett J, Fryd D, Sutherland D, Rao V, Anderson R, et al. The Minnesota diabetes complications clinical trial. The first three years. Acta Diabetologica Latina 1983;20(2):165‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Barbosa J, Johnson S. Severe hypoglycemia during maximized insulin treatment of diabetes in a randomized clinical trial. Diabetes Care 1983;6(1):62‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Barbosa J, Steffes MW, Sutherland DE, Connett JE, Rao KV, Mauer SM. Effect of glycemic control on early diabetic renal lesions. A 5‐year randomized controlled clinical trial of insulin‐dependent diabetic kidney transplant recipients. JAMA 1994;272(8):600‐6. [MEDLINE: ] [PubMed] [Google Scholar]; Chang SS, Luiza M, Caramori A, Steffes MW, Barbosa J, Mauer M. Effect of cyclosporine (CSA) on early diabetic renal lesions in type 1 diabetic kidney transplant (TX) recipients [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):113A. [CENTRAL: CN‐00550411] [Google Scholar]
- Haidinger M, Werzowa J, Hecking M, Antlanger M, Stemer G, Pleiner J, et al. Efficacy and safety of vildagliptin in new‐onset diabetes after kidney transplantation‐‐a randomized, double‐blind, placebo‐controlled trial. American Journal of Transplantation 2014;14(1):115‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Haidinger M, Werzowa J, Hecking M, Pacini G, Antlanger M, Kovarik J. Efficacy and safety of vildagliptin in newly diagnosed diabetes after kidney transplantation: Results from the Vienna VINODAT trial [abstract]. Wiener Klinische Wochenschrift 2013;125(2 Suppl 1):S19‐20. [EMBASE: 71350674] [Google Scholar]; Haidinger M, Werzowa J, Hecking M, Stemer G, Pleiner J, Kopecky C, et al. Efficacy and safety of vildagliptin in newly diagnosed diabetes after kidney transplantation [abstract no: O308]. Transplant International 2013;26(Suppl 2):175. [EMBASE: 71359610] [Google Scholar]; Haidinger M, Werzowa J, Hecking M, Stemer G, Pleiner J, Kopecky C, et al. Vildagliptin in new‐onset diabetes after kidney transplantation is safe and efficient‐results from the Vienna VINODAT trial [abstract]. Nephrology Dialysis Transplantation 2013;28(Suppl 1):i77. [EMBASE: 71075201] [Google Scholar]; Haidinger M, Werzowa J, Voigt HC, Pleiner J, Stemer G, Hecking M, et al. A randomized, placebo‐controlled, double‐blind, prospective trial to evaluate the effect of vildagliptin in new‐onset diabetes mellitus after kidney transplantation. Trials [Electronic Resource] 2010;11:91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermayer KL, Egidi MF, Finch NJ, Baliga P, Lin A, Kettinger L, et al. A randomized controlled trial to evaluate the effect of glycemic control on renal transplantation outcomes. Journal of Clinical Endocrinology & Metabolism 2012;97(12):4399‐406. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Hermayer KL, Egidi MF, Finch NJ, Baliga P, Lin A, Kettinger LD, et al. A randomized controlled trial to evaluate the effect of glycemic control on renal transplantation outcomes [abstract no: 171‐OR]. Diabetes 2011;60(Suppl 1):A47. [EMBASE: 70627955] [DOI] [PubMed] [Google Scholar]; Li P, Hunt KJ, Taber DJ, Carter RE, Kettinger L, Luttrell D, et al. Inflammatory biomarkers, glycemic variability, hypoglycemia, and renal transplant outcomes: results of a randomized controlled trial. Transplantation 2014;98(6):632‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kharazmkia A, Ahmadpoor P, Ziaie S, Salamzadeh J, Pour‐Reza‐Gholi F, Khoshdel A, et al. Effects of pioglitazone on blood glucose and inflammatory markers of diabetic kidney transplant patients: a randomized controlled trial. Iranian Journal of Kidney Diseases 2014;8(5):408‐16. [MEDLINE: ] [PubMed] [Google Scholar]
- Soliman AR, Fathy A, Khashab S, Shaheen N, Soliman MA. Sitagliptin might be a favorable antiobesity drug for new onset diabetes after a renal transplant.[Erratum appears in Exp Clin Transplant. 2014 Feb;12(1):87]. Experimental & Clinical Transplantation: Official Journal of the Middle East Society for Organ Transplantation 2013;11(6):494‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Strom Halden TA, Aasberg A, Hartmann A, Jenssen T. The effect of sitagliptin treatment on hyperglycemia and insulin secretion after renal transplantation [abstract no: O309]. Transplant International 2013;26(Suppl 2):175‐6. [EMBASE: 71359611] [Google Scholar]; Strom Halden TA, Asberg A, Vik K, Hartmann A, Jenssen T. Short‐term efficacy and safety of sitagliptin treatment in long‐term stable renal recipients with new‐onset diabetes after transplantation. Nephrology Dialysis Transplantation 2014;29(4):926‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Han SJ, Hur KY, Kim YS, Kang ES, Kim SI, Kim MS, et al. Effects of pioglitazone on subclinical atherosclerosis and insulin resistance in nondiabetic renal allograft recipients. Nephrology Dialysis Transplantation 2010;25(3):976‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hecking M, Haidinger M, Doller D, Werzowa J, Tura A, Zhang J, et al. Early basal insulin therapy decreases new‐onset diabetes after renal transplantation. Journal of the American Society of Nephrology 2012;23(4):739‐49. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Hecking M, Haidinger M, Doller D, Werzowa J, Tura A, Zhang J, et al. Early basal insulin therapy decreases new‐onset diabetes after renal transplantation by protecting endogenous insulin secretion [abstract no: MO‐008]. Transplant International 2011;24(Suppl 2):108. [EMBASE: 70527443] [Google Scholar]; Hecking M, Werzowa J, Haidinger M, Do D, Pacini G, Saemann M. Early basal insulin therapy prevents new‐onset diabetes after transplantation by improving endogenous insulin secretion [abstract]. Diabetes 2011;60:A19‐20. [EMBASE: 70627857] [Google Scholar]
- Kim YS, Kim MS, Kim SI, Lim SK, Lee HY, Han DS, et al. Post‐transplantation diabetes is better controlled after conversion from prednisone to deflazacort: a prospective trial in renal transplants. Transplant International 1997;10(3):197‐201. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kaisar M, Armstrong K, Prins J, Marwick T, Johnson D, Hawley C, et al. The impact of aggressive cardiovascular risk modification on carotid intima media thickness and brachial artery reactivity in renal transplant recipients [abstract no: 132]. Nephrology 2008;13(Suppl 3):A134. [CENTRAL: CN‐00766731] [Google Scholar]; Kaisar MO, Armstrong K, Hawley C, Campbell S, Mudge D, Johnson DW, et al. Adiponectin is associated with cardiovascular disease in male renal transplant recipients: baseline results from the LANDMARK 2 study. BMC Nephrology 2009;10:29. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orazio LK, Isbel NM, Armstrong KA, Tarnarskyj J, Johnson DW, Hale RE, et al. Evaluation of dietetic advice for modification of cardiovascular disease risk factors in renal transplant recipients. Journal of Renal Nutrition 2011;21(6):462‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Re LS, Pilotti R, Petroni J, Yagupski D, Goldberg J, Casadei D. Impact of the strict glycemic control in the post‐operative period of transplantation with ECD kidneys. Preliminary results of a prospective and randomized trial [abstract]. Transplantation 2010;90(Suppl):221. [EMBASE: 71531522]20644455 [Google Scholar]; Re LS, Pilotti RE, Petroni JN, Yagupski D, Goldberg JC, Casadei DH. Impact of the strict glycemic control in the post‐operative period of kidney transplantation with ECD kidneys. Preliminary results of a prospective and randomized trial [abstract no: 307]. American Journal of Transplantation 2010;10(Suppl 4):131‐2. [EMBASE: 70463668] [Google Scholar]
- Sharif A, Ravindran V, Moore R, Baboolal K. The effects of rosuvastatin on 51CR‐EDTA measured glomerular filtration rate and urinary albumin excretion in non‐diabetic renal transplant recipients [abstract no: 1386]. Transplantation 2008;86(2 Suppl):466. [CENTRAL: CN‐00766701] [Google Scholar]; Sharif A, Ravindran V, Moore R, Baboolal K. The effects of rosuvastatin on 51CR‐EDTA measured glomerular filtration rate and urinary albumin excretion in non‐diabetic renal transplant recipients [abstract no: P50]. British Transplantation Society (BTS).11th Annual Congress; 2008 Apr 16‐18; Glasgow, UK. 2008. [Google Scholar]; Sharif A, Ravindran V, Moore R, Dunseath G, Luzio S, Owens D, et al. The effect of rosuvastatin on insulin sensitivity and pancreatic beta‐cell function in nondiabetic renal transplant recipients. American Journal of Transplantation 2009;9(6):1439‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Sharif A, Ravindran V, Moore R, Dunseath G, Luzio S, Owens D, et al. The effects of rosuvastatin on insulin sensitivity and secretion in non‐diabetic renal transplant recipients [abstract no: 611]. Transplantation 2008;86(2 Suppl):214. [CENTRAL: CN‐00766702] [DOI] [PubMed] [Google Scholar]; Sharif A, Ravindran V, Moore R, Dunseath G, Luzio S, Owens D, et al. The effects of rosuvastatin on insulin sensitivity and secretion in non‐diabetic renal transplant recipients [abstract no: P67]. British Transplantation Society (BTS). 11th Annual Congress; 2008 Apr 16‐18; Glasgow, UK. 2008. [Google Scholar]; Sharif A, Ravindran V, Moore R, Luzio S, Dunseath G, Owens D, et al. Influence of rosuvastatin on attenuation of the metabolic syndrome in non‐diabetic renal transplant recipients [abstract no: 60]. American Journal of Transplantation 2009;9(Suppl 2):208. [CENTRAL: CN‐00775209] [DOI] [PubMed] [Google Scholar]; Sharif A, Ravindran V, Moore R, Luzio S, Dunseath G, Owens D, et al. Rosuvastatin is associated with few improvements in components of the metabolic syndrome, with no change in insulin resistance, in renal transplant recipients. Journal of Diabetes 2009;1:A92‐3. [EMBASE: 70212602] [Google Scholar]
- Hecking M, Haidinger M, Werzowa J, Kainz A, Antlanger M, Tura A, et al. Sensor‐augmented insulin‐pump therapy in new‐onset diabetes after transplantation (SAPT‐NODAT): Study protocol for a randomized controlled trial [abstract no: 58]. European Surgery [Acta Chirurgica Austriaca] 2012;44(3 Suppl). [EMBASE: 71645704] [Google Scholar]
- Werzowa J, Hecking M, Haidinger M, Lechner F, Doller D, Pacini G, et al. Vildagliptin and pioglitazone in patients with impaired glucose tolerance after kidney transplantation: a randomized, placebo‐controlled clinical trial. Transplantation 2013;95(3):456‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Werzowa J, Hecking M, Lechner F, Haidinger M, Saemann MD, Pleiner J, et al. Vildagliptin and pioglitazone in impaired glucose tolerance after renal transplantation: Results from the Glucose Control in Pre‐diabetic Renal Transplant Patients (GCPD) study [abstract]. European Surgery [Acta Chirurgica Austriaca] 2011;43(242 Suppl):15‐6. [EMBASE: 70582304] [Google Scholar]
- Yates CJ, Fourlanos S, McWhinney B, Fullinfaw R, Colman PG, Cohney S. Free prednisolone exposure correlates with peak glucose early after kidney transplantation [abstract no: 64]. 30th Transplantation Society of Australia & New Zealand [TSANZ]; 2012 Jun 27‐29; Canberra, ACT. 2012:81. [Google Scholar]
- Cohney S, Fourlanos S, Colman P, Yates C. Divided daily prednisolone dosing reduces hyperglycemia after kidney transplantation [abstract no: D1480]. American Journal of Transplantation 2013;13(Suppl S5):467. [EMBASE: 71058056]23205765 [Google Scholar]; Yates CJ, Fourlanos S, Colman PG, Cohney SJ. Divided dosing reduces prednisolone‐induced hyperglycaemia and glycaemic variability: a randomized trial after kidney transplantation. Nephrology Dialysis Transplantation 2014;29(3):698‐705. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Arashnia R, Roohi‐Gilani K, Karimi‐Sari H, Nikjoo N, Bahramifar A. Effect of pioglitazone therapy on high sensitive C‐reactive protein and lipid profile in diabetic patients with renal transplantation: a randomize clinical trial. Journal of Nephropathology 2015;4(2):48‐53. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross P. Influence of pioglitazone for renal transplant function in diabetics ‐ a double blind randomised placebo controlled cross over study. www.clinicaltrials.gov/ct2/show/NCT00507494 (accessed 19 October 2016).
Additional references
- American Diabetes Association. Standards of medical care in diabetes ‐ 2015. Diabetes Care 2015;38(Suppl 1):S1‐S94. [PubMed] [Google Scholar]
- ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New England Journal of Medicine 2008;358(24):2560‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bash LD, Selvin E, Steffes M, Coresh J, Astor BC. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminuria and retinopathy: Atherosclerosis Risk in Communities (ARIC) Study. Archives of Internal Medicine 2008;168(22):2440‐7. [PUBMED: 19064828] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burroughs TE, Swindle J, Takemoto S, Lentine KL, Machnicki G, Irish WD, et al. Diabetic complications associated with new‐onset diabetes mellitus in renal transplant recipients. Transplantation 2007;83(8):1027‐34. [PUBMED: 17452891] [DOI] [PubMed] [Google Scholar]
- Cole EH, Johnston O, Rose CL, Gill JS. Impact of acute rejection and new‐onset diabetes on long‐term transplant graft and patient survival. Clinical Journal of The American Society of Nephrology: CJASN 2008;3(3):814‐21. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AJ, Kasiske B, Herzog C, Chavers B, Foley R, Gilbertson D, et al. Excerpts from the United States Renal Data System 2006 Annual Data Report. American Journal of Kidney Diseases 2007;49(1 Suppl 1):A6‐7, S1‐296. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- CONTROL Group, Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes.[Erratum appears in Diabetologia. 2009 Nov;52(1):2470 Note: Control Group [added]]. Diabetologia 2009;52(11):2288‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cosio FG, Hickson LJ, Griffin MD, Stegall MD, Kudva Y. Patient survival and cardiovascular risk after kidney transplantation: the challenge of diabetes. American Journal of Transplantation 2008;8(3):593‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Davidson J, Wilkinson A, Dantal J, Dotta F, Haller H, Hernandez D, et al. New‐onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation 2003;75(10 Suppl):SS3‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. New England Journal of Medicine 1993;329(14):977‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. The Diabetes Control and Complications (DCCT) Research Group. Kidney International 1995;47(6):1703‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. New England Journal of Medicine 2005;353(25):2643‐53. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26 Suppl 1:S5‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. [Erratum appears in N Engl J Med. 2009 Sep 3;361(10):1028], [Erratum appears in N Engl J Med. 2009 Sep 3;361(10):1024‐5; PMID: 19726779]. New England Journal of Medicine 2009;360(2):129‐39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Duijnhoven EM, Boots JM, Christiaans MH, Wolffenbuttel BH, Hooff JP. Influence of tacrolimus on glucose metabolism before and after renal transplantation: a prospective study. Journal of the American Society of Nephrology 2001;12(3):583‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dumler F, Kilates C. Metabolic and nutritional complications of renal transplantation. Journal of Renal Nutrition 2007;17(1):97‐102. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ghisdal L, Laecke S, Abramowicz MJ, Vanholder R, Abramowicz D. New‐onset diabetes after renal transplantation: risk assessment and management. Diabetes Care 2012;35(1):181‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra G, Ilahe A, Ciancio G. Diabetes and kidney transplantation: past, present, and future. Current Diabetes Reports 2012;12(5):597‐603. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hecking M, Werzowa J, Haidinger M, Horl WH, Pascual J, Budde K, et al. Novel views on new‐onset diabetes after transplantation: development, prevention and treatment. Nephrology Dialysis Transplantation 2013;28(3):550‐66. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Hjelmesaeth J, Hartmann A, Leivestad T, Holdaas H, Sagedal S, Olstad M, et al. The impact of early‐diagnosed new‐onset post‐transplantation diabetes mellitus on survival and major cardiac events. Kidney International 2006;69(3):588‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. New England Journal of Medicine 2008;359(15):1577‐89. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation, IDF Diabetes Atlas, 6th ed. Brussels, Belgium: International Diabetes Federation, 2013.
- Iglesias P, Díez JJ. Peroxisome proliferator‐activated receptor gamma agonists in renal disease. European Journal of Endocrinology 2006;154(5):613‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycaemia in type 2 diabetes: a patient‐centered approach. Position statement of the American Diabetes Association(ADA) and the European Association for the Study of Diabetes(EASD).[Erratum appears in Diabetologia. 2013 Mar;56(3):680]. Diabetologia 2012;55(6):1577‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ismail‐Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial.[Erratum appears in Lancet. 2010 Oct 30;376(9751):1466]. Lancet 2010;376(9739):419‐30. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. American Journal of Transplantation 2003;3(2):178‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. American Journal of Transplantation 2009;9 Suppl 3:S1‐155. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kesiraju S, Paritala P, Rao Ch UM, Sahariah S. New onset of diabetes after transplantation ‐ an overview of epidemiology, mechanism of development and diagnosis. Transplant Immunology 2013;30(1):52‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Annals of Internal Medicine 2004;141(6):413‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose‐lowering efficacy of dipeptidyl peptidase‐4 inhibitors between Asians and non‐Asians: a systematic review and meta‐analysis. Diabetologia 2013;56(4):696‐708. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE, Cruickshanks KJ. Relationship of hyperglycemia to the long‐term incidence and progression of diabetic retinopathy. Archives of Internal Medicine 1994;154(19):2169‐78. [MEDLINE: ] [PubMed] [Google Scholar]
- Kyriakides GK, Simmons RL, Najarian JS. Wound infections in renal transplant wounds: pathogenetic and prognostic factors. Annals of Surgery 1975;182(6):770‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane JT, Odegaard DE, Haire CE, Collier DS, Wrenshall LE, Stevens RB. Sitagliptin therapy in kidney transplant recipients with new‐onset diabetes after transplantation. Transplantation 2011;92(10):e56‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lansang MC, Ma L, Schold JD, Meier‐Kriesche HU, Kaplan B. The relationship between diabetes and infectious hospitalizations in renal transplant recipients. Diabetes Care 2006;29(7):1659‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. Journal of the American Society of Nephrology 2004;16(2):496‐506. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lo C, Toyama T, Hirakawa Y, Jun M, Cass A, Hawley C, et al. Insulin and glucose‐lowering agents for treating people with diabetes and chronic kidney disease. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD011798] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther P, Baldwin D Jr. Pioglitazone in the management of diabetes mellitus after transplantation. American Journal of Transplantation 2004;4(12):2135‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- McDonald S, Excell L, Dent H. ANZDATA 32nd Annual Report. 2009 Report: Data to 2008. Chapter 2: New patients commencing treatment in 2008. www.anzdata.org.au/anzdata/AnzdataReport/32ndReport/Ch02.pdf (accessed 19 October 2016).
- Meier‐Kriesche HU, Ojo AO, Port FK, Arndorfer JA, Cibrik DM, Kaplan B. Survival improvement among patients with end‐stage renal disease: trends over time for transplant recipients and wait‐listed patients. Journal of the American Society of Nephrology 2001;12(6):1293‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Meier‐Kriesche HU, Schold JD, Srinivas TR, Reed A, Kaplan B. Kidney transplantation halts cardiovascular disease progression in patients with end‐stage renal disease. American Journal of Transplantation 2004;4(10):1662‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Meigs JB, Singer DE, Sullivan LM, Dukes KA, D'Agostino RB, Nathan DM, et al. Metabolic control and prevalent cardiovascular disease in non‐insulin‐dependent diabetes mellitus (NIDDM): The NIDDM Patient Outcome Research Team. American Journal of Medicine 1997;102(1):38‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009;32(6):1119‐31. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh J, Bostrom A, Feng S. Diabetes mellitus: a risk factor for delayed graft function after deceased donor kidney transplantation. American Journal of Transplantation 2010;10(2):298‐303. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rabbat CG, Thorpe KE, Russell JD, Churchill DN. Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. Journal of the American Society of Nephrology 2000;11(5):917‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Radbill B, Murphy B, LeRoith D. Rationale and strategies for early detection and management of diabetic kidney disease. Mayo Clinic Proceedings 2008;83(12):1373‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sarno G, Muscogiuri G, Rosa P. New‐onset diabetes after kidney transplantation: prevalence, risk factors, and management. Transplantation 2012;93(12):1189‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schnuelle P, Lorenz D, Trede M, Woude FJ. Impact of renal cadaveric transplantation on survival in end‐stage renal failure: evidence for reduced mortality risk compared with hemodialysis during long‐term follow‐up. Journal of the American Society of Nephrology 1998;9(11):2135‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancat FL, Powe NR, et al. Meta‐analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Annals of Internal Medicine 2004;141(6):421‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Therasse A, Wallia A, Molitch ME. Management of post‐transplant diabetes. Current Diabetes Reports 2013;13(1):121‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thomas MC, Mathew TH, Russ GR, Rao MM, Moran J. Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study. Transplantation 2001;72(7):1321‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352(9131):854‐65. [MEDLINE: ] [PubMed] [Google Scholar]
- Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group.[Erratum appears in Lancet 1999 Aug 14;354(9178):602]. Lancet 1998;352(9131):857‐53. [MEDLINE: ] [PubMed] [Google Scholar]
- Wiesbauer F, Heinze G, Regele H, Horl WH, Schernthaner GH, Schwarz C, et al. Glucose control is associated with patient survival in diabetic patients after renal transplantation. Transplantation 2010;89(5):612‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wissing KM, Pipeleers L. Obesity, metabolic syndrome and diabetes mellitus after renal transplantation: prevention and treatment. Transplantation Reviews 2014;28(2):37‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. New England Journal of Medicine 1999;341(23):1725‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yates CJ, Fourlanos S, Hjelmesaeth J, Colman PG, Cohney SJ. New‐onset diabetes after kidney transplantation‐changes and challenges. American Journal of Transplantation 2012;12(4):820‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Jun M, Lo C, Badve SV, Pilmore H, White SL, Hawley C, et al. Glucose lowering therapies for chronic kidney disease and kidney transplantation. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD009966] [DOI] [Google Scholar]


