Abstract
Background
Vitamin C is an essential micronutrient and powerful antioxidant. Observational studies have shown an inverse relationship between vitamin C intake and major cardiovascular events and cardiovascular disease (CVD) risk factors. Results from clinical trials are less consistent.
Objectives
To determine the effectiveness of vitamin C supplementation as a single supplement for the primary prevention of CVD.
Search methods
We searched the following electronic databases on 11 May 2016: the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library; MEDLINE (Ovid); Embase Classic and Embase (Ovid); Web of Science Core Collection (Thomson Reuters); Database of Abstracts of Reviews of Effects (DARE); Health Technology Assessment Database and Health Economics Evaluations Database in the Cochrane Library. We searched trial registers on 13 April 2016 and reference lists of reviews for further studies. We applied no language restrictions.
Selection criteria
Randomised controlled trials of vitamin C supplementation as a single nutrient supplement lasting at least three months and involving healthy adults or adults at moderate and high risk of CVD were included. The comparison group was no intervention or placebo. The outcomes of interest were CVD clinical events and CVD risk factors.
Data collection and analysis
Two review authors independently selected trials for inclusion, abstracted the data and assessed the risk of bias.
Main results
We included eight trials with 15,445 participants randomised. The largest trial with 14,641 participants provided data on our primary outcomes. Seven trials reported on CVD risk factors. Three of the eight trials were regarded at high risk of bias for either reporting or attrition bias, most of the 'Risk of bias' domains for the remaining trials were judged as unclear, with the exception of the largest trial where most domains were judged to be at low risk of bias.
The composite endpoint, major CVD events was not different between the vitamin C and placebo group (hazard ratio (HR) 0.99, 95% confidence interval (CI) 0.89 to 1.10; 1 study; 14,641 participants; low‐quality evidence) in the Physicians Health Study II over eight years of follow‐up. Similar results were obtained for all‐cause mortality HR 1.07, 95% CI 0.97 to 1.18; 1 study; 14,641 participants; very low‐quality evidence, total myocardial infarction (MI) (fatal and non‐fatal) HR 1.04 (95% CI 0.87 to 1.24); 1 study; 14,641 participants; low‐quality evidence, total stroke (fatal and non‐fatal) HR 0.89 (95% CI 0.74 to 1.07); 1 study; 14,641 participants; low‐quality evidence, CVD mortality HR 1.02 (95% 0.85 to 1.22); 1 study; 14,641 participants; very low‐quality evidence, self‐reported coronary artery bypass grafting (CABG)/percutaneous transluminal coronary angioplasty (PTCA) HR 0.96 (95% CI 0.86 to 1.07); 1 study; 14,641 participants; low‐quality evidence, self‐reported angina HR 0.93 (95% CI 0.84 to 1.03); 1 study; 14,641 participants; low‐quality evidence.
The evidence for the majority of primary outcomes was downgraded (low quality) because of indirectness and imprecision. For all‐cause mortality and CVD mortality, the evidence was very low because more factors affected the directness of the evidence and because of inconsistency.
Four studies did not state sources of funding, two studies declared non‐commercial funding and two studies declared both commercial and non‐commercial funding.
Authors' conclusions
Currently, there is no evidence to suggest that vitamin C supplementation reduces the risk of CVD in healthy participants and those at increased risk of CVD, but current evidence is limited to one trial of middle‐aged and older male physicians from the USA. There is limited low‐ and very low‐quality evidence currently on the effect of vitamin C supplementation and risk of CVD risk factors.
Plain language summary
Vitamin C supplementation to prevent cardiovascular disease
Background
Cardiovascular diseases (CVD) are a group of conditions affecting the heart and blood vessels. CVD is a global burden and varies between regions, and this variation has been linked in part to dietary factors. Such factors are important because they can be modified to help with CVD prevention and management.This review assessed the effectiveness of vitamin C supplementation as a single supplement at reducing cardiovascular death, all‐cause death, non‐fatal endpoints (such as heart attacks, strokes and angina) and CVD risk factors in healthy adults and adults at high risk of CVD .
Study characteristics
We searched scientific databases for randomised controlled trials (clinical trials where people are allocated at random to one of two or more treatments) looking at the effects of vitamin C supplementation in healthy adults or those at high risk of developing CVD. We did not include people who already had CVD (e.g. heart attacks and strokes). The evidence is current to May 2016.
Key results
Eight trials fulfilled our inclusion criteria. One large trial looked at the effects of vitamin C supplements on the risk of major CVD events (fatal and non‐fatal) and found no beneficial effects. This trial was however conducted in middle‐aged and older male doctors in the USA and so its not certain that the effects are the same in other groups of people. Seven trials looked at the effects of vitamin C supplements on CVD risk factors. We could not combine these trials as there was lots of missing information and differences between the trials in terms of the participants recruited, the dose of vitamin C and the duration of trials. Overall, there were inconsistent effects of vitamin C supplements on lipid levels and blood pressure and more research is needed. Four of the included studies did not mention sources of funding of the study, two had non‐commercial (grants) funding and two had both commercial (industries) and non‐commercial funding (grants).
Quality of the evidence
The evidence was of low or very low quality for major CVD events (myocardial infraction, stroke, angina and coronary artery bypass grafting), all‐cause mortality and CVD mortality. The evidence was of low quality because it was not applicable to the general population (included only USA male physicians) and limited studies of vitamin C on the prevention of CVD.
Summary of findings
Summary of findings for the main comparison. Vitamin C supplementation versus placebo for primary prevention of cardiovascular disease.
| Vitamin C supplementation versus placebo for primary prevention of cardiovascular disease | ||||||
|
Patient or population: middle‐aged US male physicians
Settings: Not clear
Intervention: Vitamin C supplementation Comparision: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Vitamin C supplementation | |||||
| Major cardiovascular event Physicians Follow‐up: mean 8 years | 86 per 1000 | 85 per 1000 (77 to 94) | HR 0.99 (0.89 to 1.10) | 14,641 (1 study) | ⊕⊕⊝⊝ low1,2 | Inconsistency was difficult to evaluate given that one trial assessed the primary outcome. Grey literature search is unlikely to introduce publication bias. See Appendix 2 for major cardiovascular event checklist |
| Cardiovascular mortality Physicians Follow‐up: mean 8 years | 35 per 1000 | 35 per 1000 (29 to 42) | HR 1.02 (0.85 to 1.22) | 14,641 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | Inconsistency was difficult to evaluate given that one trial assessed the primary outcome. Grey literature search is unlikely to introduce publication bias. See Appendix 2 for major cardiovascular event checklist |
| All‐cause mortality Physicians Follow‐up: mean 8 years | 110 per 1000 | 117 per 1000 (107 to 128) | HR 1.07 (0.97 to 1.18) | 14,641 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | Inconsistency was difficult to evaluate given that one trial assessed the primary outcome. Grey literature search is unlikely to introduce publication bias. See Appendix 2 for major cardiovascular event checklist |
| Total myocardial infarction (fatal and non‐fatal) Physicians Follow‐up: mean 8 years | 34 per 1000 | 36 per 1000 (30 to 42) | HR 1.04 (0.87 to 1.24) | 14,641 (1 study) | ⊕⊕⊝⊝ low1,2 | Inconsistency was difficult to evaluate given that one trial assessed the primary outcome. Grey literature search is unlikely to introduce publication bias. See Appendix 2 for major cardiovascular event checklist |
| Total stroke (fatal and non‐fatal) Physicians Follow‐up: mean 8 years | 34 per 1000 | 30 per 1000 (25 to 36) | HR 0.89 (0.74 to 1.07) | 14,641 (1 study) | ⊕⊕⊝⊝ low1,2 | Inconsistency was difficult to evaluate given that one trial assessed the primary outcome. Grey literature search is unlikely to introduce publication bias. See Appendix 2 for major cardiovascular event checklist |
| Self‐reported CABG/PTCA Participant self‐reports Follow‐up: mean 8 years | 97 per 1000 | 93 per 1000 (84 to 103) | HR 0.96 (0.86 to 1.07) | 14,641 (1 study) | ⊕⊕⊝⊝ low1,2 | Inconsistency was difficult to evaluate given that one trial assessed the primary outcome. Self‐reported outcomes are unlikely to introduce bias in this trial. Grey literature search is unlikely to introduce publication bias. See Appendix 2 for major cardiovascular event checklist |
| Self‐reported angina Participant self‐reports Follow‐up: mean 8 years | 105 per 1000 | 98 per 1000 (89 to 108) | HR 0.93 (0.84 to 1.03) | 14,641 (1 study) | ⊕⊕⊝⊝ low1,2 | Inconsistency was difficult to evaluate given that one trial assessed the primary outcome. Self‐reported outcomes are unlikely to introduce bias in this trial. Grey literature search is unlikely to introduce publication bias. See Appendix 2 for major cardiovascular event checklist |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HR: Hazard ratio; CABG: coronary artery bypass grafting; PTCA: percutaneous transluminal coronary angioplasty | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Middle‐aged US male physicians and is therefore not highly applicable to the decision context (downgraded by one for indirectness). 2 Small number of included studies (n = 1) for these outcomes (downgraded by one for imprecision). 3 8 years follow‐up (timeframe) may not be sufficient to detect mortality (downgraded by one for indirectness).
Background
Description of the condition
Cardiovascular disease (CVD) remains the number one cause of death globally (WHO 2011a). CVDs are the result of disorders of the heart and blood vessels and include cerebrovascular disease, coronary heart disease (CHD), and peripheral arterial disease (PAD) (WHO 2011b). In 2008, an estimated 17.3 million people died from CVDs, representing 30% of all global deaths. Of these deaths, an estimated 7.3 million were due to CHD and 6.2 million were due to stroke (WHO 2011a). Over 80% of CVD deaths occur in low‐ and middle‐income countries, and the number of CVD deaths is expected to increase to 23.3 million by 2030 (Mathers 2006; WHO 2011a).
One of the main mechanisms thought to cause CVD is atherosclerosis, in which the arteries become narrowed by plaques or atheromas (NHS 2012). Atherosclerosis can cause CVD when the arteries are completely blocked by a blood clot or when blood flow is restricted by a narrowed artery, limiting the amount of blood and oxygen that can be delivered to organs or tissue (British Heart Foundation 2012). Whilst arteries may naturally become harder and narrower with age, this process may be accelerated by factors such as smoking, high cholesterol, high blood pressure, obesity, a sedentary lifestyle, and ethnicity (NHS 2012). Prevention of CVD by targeting modifiable factors remains a key public health priority. Diet plays a major role in the aetiology of many chronic diseases including CVD, thereby contributing to a significant geographical variability in morbidity and mortality rates across different countries and populations worldwide (WHO 2003). A number of dietary factors have been found to be associated with CVD risk, such as a low consumption of fruit and vegetables (Begg 2007), a high intake of saturated fat (Siri‐Tarino 2010) and a high consumption of salt (He 2011). Dietary factors are important since they can be modified in order to lower CVD risk, making them a prime target for interventions aimed at primary prevention and management of CVD.
Description of the intervention
The intervention examined in this review is vitamin C supplementation as a single ingredient. No limit was placed on the dose or frequency at which vitamin C is taken. Vitamin C (ascorbic acid or ascorbate) is an essential micronutrient that acts as a powerful water‐soluble antioxidant, reducing oxidative stress. It cannot be synthesised in the body and is acquired primarily through the consumption of fruit, vegetables, supplements, fortified beverages, and fortified breakfast or 'ready‐to‐eat' cereals (Frei 1989; WHO 2006).
Adults need 40 mg/day of vitamin C, which can be obtained from a healthy diet. Supplementation of vitamin C up to 1000 mg per day is unlikely to cause side effects (NHS choices 2015), whereas larger amounts can cause stomach pain, diarrhoea and flatulence. The pharmacokinetics of vitamin C are complex where the relationship between the amount ingested and plasma and tissue levels is dependent on absorption, tissue transport, renal reabsorption and excretion and rate of utilisation (Levine 2011). The dose concentration curve is sigmoidal with its steep portion between 30 mg and 100 mg of vitamin C daily. At doses greater than 100 mg/day, plasma concentrations reach a plateau between 70 μmol/L and 80 μmol/L. At doses greater than 400 mg/day, further increases in plasma concentrations are minimal (Levine 2011).
Data on the adverse effects of vitamin C supplementation show that these are relatively rare. A survey of 9328 patients who used high‐dose intravenous vitamin C during the preceding 12 months revealed that only 101 had side effects, mostly minor, including lethargy/fatigue in 59 patients, change in mental status in 21 patients and vein irritation/phlebitis in six patients (Padayatty 2010). In a recent meta‐analysis of the effects of vitamin C supplementation, alone and in combination with other agents (such as vitamin E, magnesium, zinc, selenium) on blood pressure (Juraschek 2012), few trials (six of 29) reported adverse effects, however details of these were not provided in the paper.
How the intervention might work
Population‐based observational studies have shown an inverse association between plasma vitamin C concentrations and vitamin C intake with blood pressure (McCarron 1984; Moran 1993). Observational studies have also shown an inverse relationship between vitamin C intake and mortality due to CVD (Jacques 1995; Simon 1998). However, the results from randomised controlled trials (RCTs) have not observed beneficial effects of vitamin C supplementation in the prevention of cardiovascular events (Cook 2007; The Physicians Health Study II), or mortality outcomes (Bjelakovic 2007).
Low‐density lipoprotein (LDL) cholesterol in the blood may be importantly atherogenic only after oxidative modification, which allows it to be taken up by macrophages in the artery walls. These macrophages, which are attracted to regions where oxidised LDL is being taken up, become loaded with cholesterol (and are then described as "foam cells" in the artery walls), leading to the development of "fatty streaks". Oxidised LDL can also be cytotoxic. Antioxidants such as vitamin C can protect LDL from oxidative modification and may help avoid CVD (Steinberg 1989).
A recent review has summarised the important functions of vitamin C in the vascular bed in support of endothelial cells (May 2013). These functions include increasing the synthesis and deposition of type IV collagen in the basement membrane, stimulating endothelial proliferation, inhibiting apoptosis, scavenging radical species, and sparing endothelial cell‐derived nitric oxide to help modulate blood flow. Endothelial dysfunction is an early sign of inflammatory disease such as atherosclerosis and vitamin C could have a part to play in preventing these early stages.
In the early stages of atherosclerosis, monocytes adhere to the walls of the endothelium, causing the vessel walls to thicken and lose their elasticity. Research has shown that vitamin C supplementation can reduce the rate of monocyte adhesion to the endothelial cell wall. A study looked at the effects of vitamin C (250 mg per day, six weeks duration) in healthy adults with normal and below‐average plasma vitamin C concentration at baseline. Before the study, participants with below average levels of vitamin C had 30% greater monocyte adhesion than normal, putting them at higher risk for atherosclerosis. After six weeks of vitamin C supplementation, the rate of monocyte adhesion fell by 37% (Woollard 2002).
Furthermore, intercellular adhesion molecule‐1 (ICAM‐1) is an inducible surface glycoprotein that mediates the adhesion of monocytes to the endothelium. The researchers went on to demonstrate that the same dose and duration of vitamin C supplementation was able to reduce monocyte ICAM‐1 expression by 50% in participants with below‐average plasma vitamin C concentration (Rayment 2003). Vitamin C supplementation might improve nitric oxide bioactivity (Huang 2000), as well as endothelial function of brachial and coronary arteries, as suggested by short‐term interventions among high‐risk individuals (Grebe 2006; McNulty 2007; Silvestro 2002; Solzbach 1997).
Why it is important to do this review
A systematic review of the effects of individual vitamins and minerals, and multivitamins, on clinical endpoints has been conducted (Fortmann 2013). This review was conducted for the US Task Force for Preventative Services. The authors found two trials of vitamin C supplementation reporting clinical endpoints relevant for CVD prevention, where no effect of the intervention was found. In terms of effects on CVD risk factors, from preliminary searching of the Cochrane Library, we identified five systematic reviews in the Database of Abstracts of Reviews of Effects (DARE), which assessed the effects of vitamin C supplementation on blood pressure (Juraschek 2012; McRae 2006a; Ness 1997), low‐density lipid (LDL) cholesterol and triglycerides (McRae 2008), and total cholesterol (McRae 2006b). Only two of these included only RCTs (Juraschek 2012; McRae 2008), the reminder include also non‐randomised experimental studies and observational studies. The first review of RCTs covered both primary and secondary prevention and the effects of vitamin C supplementation alone and in combination with other agents (such as vitamin E, magnesium, zinc, selenium) in trials between two and 26 weeks duration (Juraschek 2012). The authors concluded that vitamin C supplementation reduced systolic and diastolic blood pressure in short‐term trials. The second review concluded that supplementation with at least 500 mg/day of vitamin C, for a minimum of four weeks, can result in a significant decrease in serum LDL cholesterol and triglyceride concentrations. However, the lack of quality assessment and analysis of statistical heterogeneity, and the small sample sizes of the included trials, limit the reliability of the authors' conclusions (McRae 2008).
For the current review we examined evidence from RCTs of vitamin C as a single supplement in the general population and those at moderate to high risk of CVD. This review will update and build on the existing systematic reviews discussed above by assessing vitamin C supplementation (as a single supplement only) in populations relevant for the primary prevention of CVD, in trials of at least three months duration and assessing a wider range of outcomes.
Objectives
To determine the effectiveness of vitamin C supplementation as a single supplement for the primary prevention of cardiovascular disease (CVD).
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) including cross‐over trials,studies reported as full‐text, those published as abstract only, and unpublished data were eligible for inclusion.
Types of participants
Healthy adults (18 years old or over) from the general population and those at moderate to high risk of CVD (e.g. hypertension, hyperlipidaemia, overweight/obesity). As the review focuses on the primary prevention of CVD, we excluded those who have experienced a previous myocardial infarction (MI), stroke, revascularisation procedure (coronary artery bypass grafting (CABG) or percutaneous transluminal coronary angioplasty (PTCA)), and those with angina or angiographically‐defined coronary heart disease (CHD). If participants were at high risk of CVD they were included if less than 25% of participants had CVD at baseline.
We also planned to exclude trials involving participants with type 2 diabetes, although this is a major risk factor for CVD, as interventions for the treatment and management of type 2 diabetes are covered by reviews registered with the Cochrane Metabolic and Endocrine Disorders Group.
Types of interventions
The intervention was vitamin C supplements alone as a single ingredient. No limit was placed on the dose or frequency of vitamin C taken. Trials were only considered where the comparison group was placebo or no intervention. Multifactorial intervention studies (including other additional interventions such as dietary changes and exercise) were not included in this review, in order to avoid confounding. If there had been a sufficient number of trials, we also planned to stratify results by dose of vitamin C.
Types of outcome measures
We included studies with follow‐up periods of at least three months. Follow‐up is considered to be the time elapsed since the start of the intervention.
Primary outcomes
Major cardiovascular events
Cardiovascular mortality
All‐cause mortality
Non‐fatal endpoints such as MI, CABG, PTCA, angina, or angiographically‐defined CHD, stroke, carotid endarterectomy, peripheral arterial disease (PAD)
Secondary outcomes
Changes in blood pressure (BP) (systolic (SBP) and diastolic (DBP) and blood lipids (total cholesterol, high‐density lipid (HDL) cholesterol, low‐density lipid (LDL) cholesterol, triglycerides)
Occurrence of type 2 diabetes as a major CVD risk factor
Validated health‐related quality of life measures
Adverse effects
Costs
Search methods for identification of studies
Electronic searches
We identified trials through systematic searches of the following bibliographic databases on 11 May 2016:
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (2016, Issue 4 of 12)
Health Technology Assessment (HTA) in the Cochrane Library (2016, Issue 2 of 4)
Database of Abstracts of Reviews of Effects (DARE) in the Cochrane Library (2015, Issue 2 of 4)
NHS Economic Evaluation Database (NEED) in the Cochrane Library (2015, Issue 2 of 4)
MEDLINE (Ovid, 1946 to April week 4 2016)
Embase Classic and Embase (Ovid, 1947 to 2016 Week 19)
Web of Science Core Collection (Thomson Reuters, 1970 to 11 May 2016)
We used Medical subject headings (MeSH) or equivalent and text word terms. Searches were designed in accordance with the Cochrane Heart Group methods and guidance.
The search strategies are detailed in Appendix 1. The Cochrane sensitivity‐maximising RCT filter (Lefebvre 2011) was applied to MEDLINE (Ovid) and adaptations of it to the other databases, except CENTRAL.
We searched all databases from their inception to the present, and we imposed no restriction on language of publication.
Searching other resources
We checked reference lists of reviews for additional studies. We searched ClinicalTrials.gov (http://www.clinicaltrials.gov/) and the World Health Organization (WHO) International Clinical Trials Registry platform (ICTRP) search portal (http://apps.who.int/trialsearch/) for ongoing trials on 13 April 2016 using the search terms Vitamin C OR ascorbic acid AND cardio*. Where necessary we contacted authors for any additional information.
Data collection and analysis
Selection of studies
Two review authors (LA, LH, NF, RW, OG or KR) independently screened for inclusion titles and abstracts of all the studies we identified as a result of the search, and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publication and two review authors (LA, LH, NF, RW, OG or KR) independently screened the full‐text and identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion or, if required, we consulted a third author (KR/SS). We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) and 'Characteristics of excluded studies' table.
1.
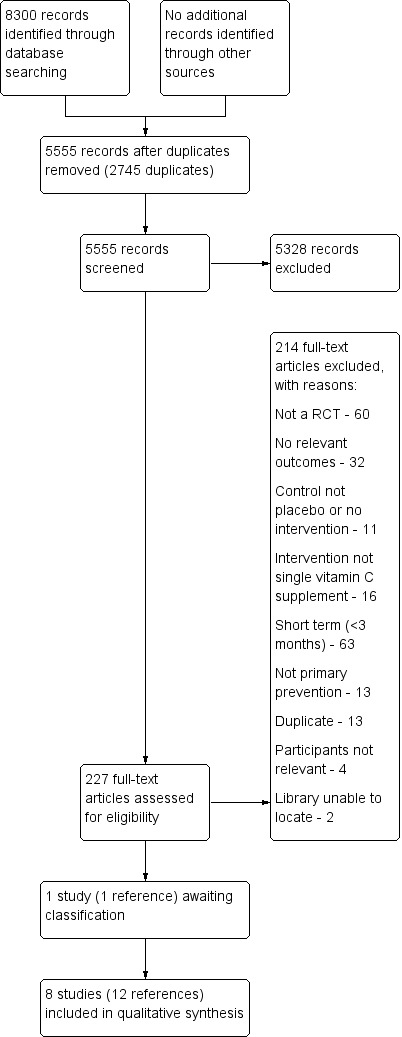
Study flow diagram.
Data extraction and management
Two review authors (LA, LH, NF, RW) independently extracted study characteristics from included studies using a pre‐standardised data extraction form, and contacted chief investigators to request additional relevant information if necessary. We extracted details of the study design, participant characteristics, study setting, intervention (including dose and duration), and outcome data including details of outcome assessment, adverse effects, and methodological quality (randomisation, blinding, attrition) from each of the included studies. We resolved disagreements by consensus or by involving a third author (KR/SS). One author (NF) transferred data into the Review Manager (RevMan 2012) file. We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second author (KR/LA) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (NF, RW) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another author (KR/SS). We assessed the risk of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias (bias due to problems not covered elsewhere, e.g. industry funding)
We graded each potential source of bias as having a 'low risk of bias', a 'high risk of bias' or an 'unclear risk of bias'. Studies were regarded as at high risk of bias if any of the domains listed above were regarded at high risk of bias.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and report any deviations from it in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
Data were processed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We expressed dichotomous outcomes as hazard ratios (HRs), with 95% confidence intervals (CIs). For continuous outcomes, net changes were compared (i.e. intervention group minus control group differences) and a mean difference (MD) and 95% CIs calculated for each study.
Unit of analysis issues
Cross‐over trials
We used data only from the first half of the trial as a parallel group design. We only considered risk factor changes (i.e. blood pressure, lipid levels) before patients crossed over to the other therapy and where the duration was a minimum of three months before cross‐over occurred.
Studies with multiple intervention groups
Data for the control group were used for each intervention group comparison. We reduced the weight assigned to the control group by dividing the control group number (N) by the number of intervention groups.
Cluster‐randomised trials
If identified, we intended to analyse cluster‐randomised trials using the unit of randomisation (cluster) as the number of observations. Where necessary, individual‐level means and standard deviations (SDs) adjusted for clustering would be utilised together with the number of clusters in the denominator, in order to weight the trials appropriately. We did not find any cluster‐RCTs that met the inclusion criteria for our review.
Dealing with missing data
We contacted investigators in order to verify key study characteristics and obtain missing numerical outcome data where possible.
Missing data were captured in the data extraction form and reported in the 'Risk of bias' table. If a trial collected an outcome measure at more than one time point, the longest period of follow‐up with 20% or fewer dropouts was utilised.
Assessment of heterogeneity
For each outcome, we conducted tests of heterogeneity using the Chi2 test of heterogeneity and I2 statistic. Where there was no heterogeneity, a fixed‐effect meta‐analysis was performed. If moderate to substantial heterogeneity was detected (40% to 100%), we looked for possible explanations for this (e.g. participants and intervention). If the source of heterogeneity could not be explained, we considered the following options: provide a narrative overview and not aggregate the studies at all or use a random‐effects model with appropriate cautious interpretation.
Assessment of reporting biases
Had there been sufficient studies (10 or more), we intended to plot the trial effect against the standard error and present the results as funnel plots (Sterne 2011). Since asymmetry could be caused by a relationship between effect size and sample size or by publication bias, we planned to examine any observed effect for clinical heterogeneity and carry out additional sensitivity tests (Sterne 2011). There were insufficient trials to conduct this analysis.
Data synthesis
Statistical analysis was carried out using the Cochrane Collaboration’s statistical software, (RevMan 2012). Dichotomous data were entered as events and the number of participants and continuous data were entered as means and SDs. In the absence of moderate to substantial heterogeneity (40% to 100%) and provided that there were sufficient trials, we combined the results, using a fixed‐effect model. In the presence of substantial heterogeneity we plotted the effects for individual trials in the forest plot but have not pooled them statistically.
Subgroup analysis and investigation of heterogeneity
If there were sufficient trials (10 or more) we intended to stratify results by high risk of CVD versus the general population, and also by dose of vitamin C.
Sensitivity analysis
We planned to carry out sensitivity analyses with studies of six months or more follow‐up, and excluding studies at a high risk of bias. Studies were regarded as at high risk of bias if any of the domains in the risk of bias tool were regarded at high risk of bias.
Quality of evidence
We present the overall quality of the evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias), but also to external validity such as directness of results. Two review authors (LA, KR) rated the quality for each outcome. We presented a summaries of the evidence in Table 1, which provides key information about the best estimate of the magnitude of the effect, in relative terms for each relevant comparison of alternative management strategies, numbers of participants and trials addressing each important outcome, and the rating of the overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We presented results on the outcomes as described in Types of outcome measures.
In addition, we established an appendix 'Checklist to aid consistency and reproducibility of GRADE assessments' (Meader 2014) to help with standardisation of 'Summary of findings' tables (Appendix 2).
Results
Description of studies
Results of the search
The searches generated 5555 hits after duplicates were removed. Screening of titles and abstracts identified 227 papers to go forward for formal inclusion and exclusion. Of these, nine randomised controlled trials (RCTs) met the inclusion criteria. There is one trial in abstract form awaiting classification. Details of the flow of studies through the review are shown in the PRISMA flow diagram in Figure 1.
Included studies
Details of the methods, participants, intervention, comparison group and outcome measures for each of the studies included in the review are shown in the Characteristics of included studies. Eight trials were included randomising a total of 15,445 participants. The largest trial recruited males only (14,641 randomised) (The Physicians Health Study II), six trials recruited male and female participants, and one trial did not specify the gender of participants ( Mostafa 1989). The trials varied in the participants recruited. Three trials recruited patients with hypercholesterolaemia (ASAP Study; Cerna 1992; Jacques 1995), one trial recruited patients with hypertension (Schindler 2003), one trial recruited older participants aged 60 to 80 years, some with borderline or newly diagnosed hypertension (Fotherby 2000), one trial recruited healthy young medical students aged 18 to 25 years (Menne 1975), another recruited from a US University campus, but no details of age were provided (Mostafa 1989), and the largest trial recruited US male physicians aged 50 years or older at the start of the study (The Physicians Health Study II), where some participants had CVD risk factors (see Characteristics of included studies).
Two trials were conducted in Boston, MA, USA (Jacques 1995; The Physicians Health Study II). The remaining studies were conducted in Bratislava, Czechoslovakia (Cerna 1992), the UK (Fotherby 2000), South Africa (Menne 1975), Mississippi, USA (Mostafa 1989), Kuopio, Eastern Finland (ASAP Study), and for one trial this was unclear (Schindler 2003).
The duration of the intervention and follow‐up periods varied considerably from three months to eight years. The trial with the longest intervention and follow‐up period was eight years (The Physicians Health Study II). This was followed by three years (ASAP Study); two years (Schindler 2003), 18 months (Cerna 1992), eight months ( Jacques 1995), six months (Mostafa 1989), four months (Menne 1975), and three months (Fotherby 2000).
In five of the trials the dose of vitamin C supplementation was 500 mg/day (ASAP Study; Cerna 1992; Fotherby 2000; Mostafa 1989; The Physicians Health Study II); in two trials the dose was 1 g/day (Jacques 1995; Menne 1975), and in the remaining trial the dose was 2 g/day (Schindler 2003).
Details of the trial awaiting assessment is presented in the Characteristics of studies awaiting classification table. This study is only available as an abstract and we are awaiting responses from the authors to our requests asking for further information.
Excluded studies
Details and reasons for exclusion for studies that closely missed the inclusion criteria are provided in the Characteristics of excluded studies table. Reasons for exclusion for the majority of studies included alternative designs (not RCTs), short‐term studies (< three months), and no relevant outcomes (see Figure 1).
Risk of bias in included studies
Details are presented for the included trial in the 'Risk of bias' tables in the Characteristics of included studies table and in Figure 2; Figure 3.
2.
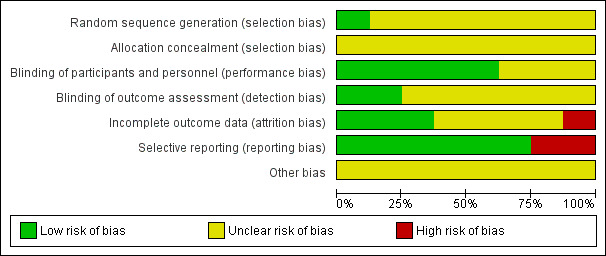
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
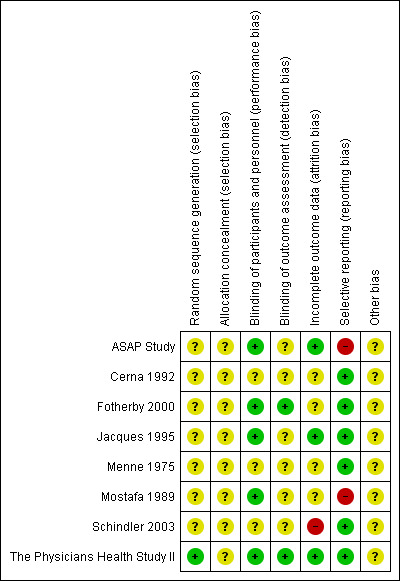
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only one study reported the method of random sequence generation which was regarded as at low risk of bias (The Physicians Health Study II); for the remaining eight studies this was unclear. No details were provided for the method of allocation concealment in all eight trials so this was judged to be at unclear risk of bias.
Blinding
Five trials reported blinding participants and personnel and were judged to be at low risk of performance bias (ASAP Study; Fotherby 2000; Jacques 1995; Mostafa 1989; The Physicians Health Study II). The remaining three studies were at unclear risk of performance bias as blinding or participants and personnel were not reported (Cerna 1992; Menne 1975; Schindler 2003). Two trials were judged to be at low risk of detection bias as outcome assessors were blind to group allocation (Fotherby 2000; The Physicians Health Study II). For the remaining six trials blinding of outcome assessors was not stated and this was judged to be at unclear risk of bias (ASAP Study; Cerna 1992; Jacques 1995; Menne 1975; Mostafa 1989; Schindler 2003).
Incomplete outcome data
There was a low risk of attrition bias in three trials (ASAP Study; Jacques 1995; The Physicians Health Study II). In one trial there was a high risk of attrition bias as no reasons for loss to follow‐up were given and the authors did not perform an intention‐to‐treat analysis (Schindler 2003). For the remaining four studies this was judged as unclear (Cerna 1992; Fotherby 2000; Menne 1975; Mostafa 1989).
Selective reporting
Two studies were judged to be at high risk of reporting bias (ASAP Study; Mostafa 1989). The first because no outcome data were provided for total cholesterol, LDL cholesterol, triglycerides or blood pressure (ASAP Study), the second because outcome data were not provided for the control group (Mostafa 1989).
Other potential sources of bias
There was insufficient information to judge other potential sources of bias and all studies were regarded as unclear risk of bias.
Effects of interventions
See: Table 1
Primary outcomes
One study provided data for all our primary outcomes (The Physicians Health Study II). This was the largest study randomising 7329 US physicians to vitamin C supplementation and 7312 to the placebo group, with a mean follow‐up period of eight years. This trial was a factorial 2 x 2 trial of vitamin E and vitamin C supplementation and it is therefore possible to compare two vitamin C arms (active vitamin C and placebo vitamin E and active vitamin C and active vitamin E) with two non‐vitamin C arms (placebo vitamin C and active vitamin E and placebo vitamin C and E). The hazard ratios reported in this trial were adjusted for a number of variables including age, study cohort (the original Physicians study I or II), and vitamin E assignment. Results are presented below using the inverse variance method.
A composite measure of major cardiovascular events was the primary end point in this trial including non‐fatal myocardial infarction (MI), non‐fatal stroke, and cardiovascular mortality. Reported end points were confirmed in medical records and registers. The adjusted hazard ratios for this composite measure, all‐cause mortality, cardiovascular mortality, total MI (fatal and non‐fatal) and total stroke (fatal and non‐fatal) are presented below and graphically in Analysis 1.1; Analysis 1.3; Analysis 1.2; Analysis 1.4; Analysis 1.7) The authors of The Physicians Health Study II concluded that there was no evidence that vitamin C supplementation reduced the risk of major cardiovascular events and that these data provide no support for the use of vitamin C supplements for the prevention of cardiovascular disease (CVD) in middle‐aged and older men.
1.1. Analysis.
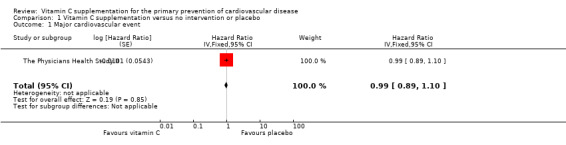
Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 1 Major cardiovascular event.
1.3. Analysis.
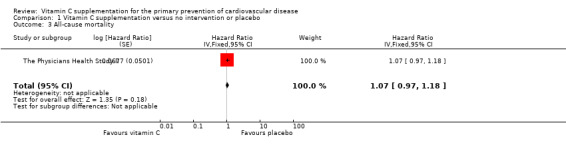
Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 3 All‐cause mortality.
1.2. Analysis.
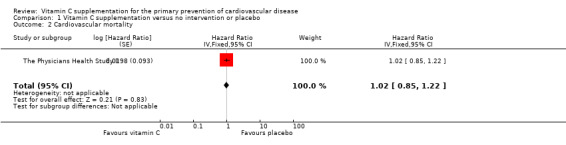
Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 2 Cardiovascular mortality.
1.4. Analysis.
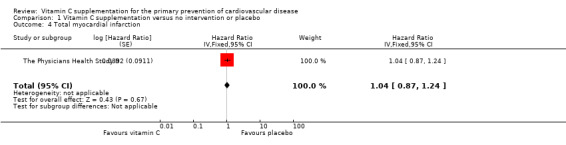
Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 4 Total myocardial infarction.
1.7. Analysis.
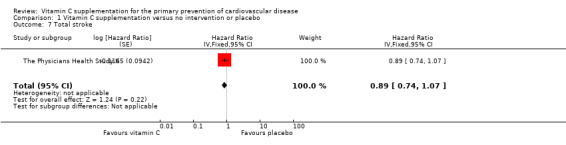
Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 7 Total stroke.
Major cardiovascular events
There was no effect of vitamin C supplementation on major cardiovascular events at eight years follow‐up hazard ratio (HR) 0.99 (95% confidence interval (CI) 0.89 to 1.10); 1 study; 14,641 participants; low quality of evidence (graphically presented in Analysis 1.1).
Cardiovascular mortality
There was no substantial effect of vitamin C supplementation on all cardiovascular morality HR 1.02 (95% CI 0.85 to 1.22); 1 study; 14,641 participants; very low quality of evidence (graphically presented in Analysis 1.2).
Two deaths were reported in the ASAP Study, one in the vitamin C group (subarachnoid haemorrhage) and one in the placebo group (cardiac dysrhythmia). These data have not been incorporated in the meta‐analysis as the inverse variance method was used for the The Physicians Health Study II to take account of the adjusted hazard ratios for this study. In a separate analysis, incorporation of these two deaths had no effect on the estimate as the The Physicians Health Study II had 99.9% of the weight.
All‐cause mortality
There was no effect of vitamin C supplementation on all‐cause morality at eight years follow‐up HR 1.07 (95% CI 0.97 to 1.18); 1 study; 14,641 participants; very low quality of evidence (graphically presented in Analysis 1.3).
Total myocardial infraction
There was no effect of vitamin C on total myocardial infarction (fatal and non‐fatal events) HR 1.04 (95% CI 0.87 to 1.24); 1 study; 14,641 participants; low quality of evidence (graphically presented in Analysis 1.4).
Self‐reported revascularisation
There was nor effect of vitamin C supplementation on revascularisation (coronary artery bypass grafting (CABG) and percutaneous transluminal coronary angioplasty (PTCA)) at eight years follow‐up HR 0.96, 95% CI 0.86 to 1.07; 1 study, 14,641 participants; low quality of evidence (graphically presented in Analysis 1.5).
1.5. Analysis.
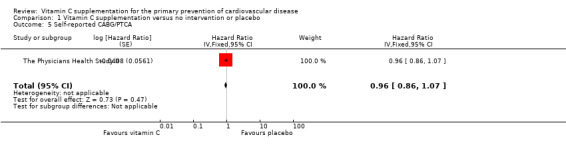
Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 5 Self‐reported CABG/PTCA.
Self‐reported angina
There were no considerable effects of vitamin C supplementation on self‐reported angina symptoms HR 0.93, 95% CI 0.84 to 1.03, 1 study, 14,641 participants, low quality of evidence (graphically presented in Analysis 1.6).
1.6. Analysis.
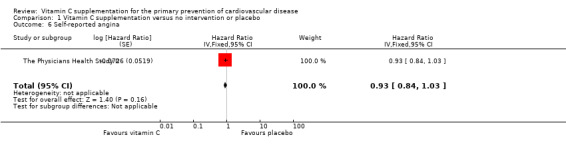
Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 6 Self‐reported angina.
Total stroke
There was no effect of vitamin C supplementation on total stroke (fatal and non‐fatal events) at eight years follow‐up HR 0.89 (95% CI 0.74 to 1.07); 1 study; 14,641 participants; low quality of evidence (graphically presented in Analysis 1.7).
Secondary outcomes
Cardiovascular risk factors
Seven studies (ASAP Study; Cerna 1992; Fotherby 2000; Jacques 1995; Menne 1975; Mostafa 1989; Schindler 2003) looked at our secondary outcomes, but only three provided clear outcome data that could be used in meta‐analyses (Cerna 1992; Jacques 1995; Schindler 2003). These three trials looked at the effect of vitamin C on cholesterol (total cholesterol, HDL‐cholesterol and LDL‐cholesterol), however, due to significant heterogeneity between the trials (I2 = 93% for total cholesterol, I2 = 95% for LDL‐cholesterol, I2 = 66% for HDL‐cholesterol, I2 = 47% for triglycerides), meta‐analyses were not performed. Data from these three trials were not pooled but have been plotted to only show the graphical display. For three studies (Cerna 1992; Jacques 1995; Schindler 2003), we imputed standard deviation differences from baseline to follow‐up as these data were not available in the papers. To do this, we followed the guidelines in the Cochrane Handbook for Systematic Reviews of interventions for obtaining standard deviations from standard errors (Higgins 2011, chapter 7.3.3) and have used a correlation coefficient in these calculations of 0.5 as recommended by Follman (Follman 1992 ). Results are described narratively and the authors reports of the remaining four trials without usable data are also described below.
Blood pressure
One trial reported data that could be used in a meta‐analysis (Analysis 1.8; Analysis 1.9). This trial reported no effects of vitamin C supplementation on either systolic blood pressure (SBP) (mean difference (MD) ‐1.00 mmHg, 95% CI ‐4.94 to 2.94; 16 participants) or diastolic blood pressure (DBP) (MD ‐2.00 mmHg, 95% CI ‐6.82 to 2.82; 16 participants) (Schindler 2003). However, this study was extremely small, at high risk of attrition bias so results should be treated with caution. Antihypertensive medication was also used by some participants, which may have impacted on the results obtained.
1.8. Analysis.
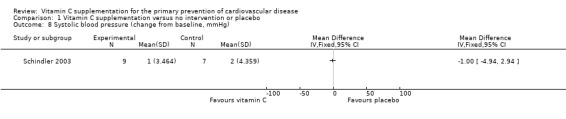
Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 8 Systolic blood pressure (change from baseline, mmHg).
1.9. Analysis.
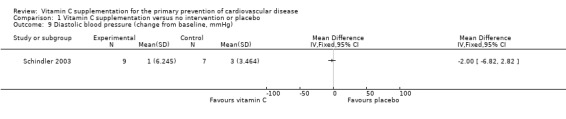
Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 9 Diastolic blood pressure (change from baseline, mmHg).
No control group data were provided for one trial so these were not usable in a meta‐analysis (Mostafa 1989).
One cross‐over trial reported results over the whole trial period and not in phases and so we were unable to incorporate the results in the meta‐analysis (Fotherby 2000). The authors reported that clinic blood pressure did not change between the placebo and vitamin C stages. Daytime ambulatory blood pressure showed a smaller decrease in SBP of 2 ± 5.2 mmHg in comparison to DBP 1 ± 4.7 mmHg for 40 participants (Fotherby 2000).
For one study, blood pressure was reported at baseline but not at follow‐up (ASAP Study).
Lipid levels
For total cholesterol (Analysis 1.10), one of the three trials (Cerna 1992), showed that there was a reduction in total cholesterol with vitamin C supplementation (MD ‐1.17 mmol/L. 95% CI ‐1.60 to ‐0.74; 1 study; 140 participants). The other two trials showed no evidence of an effect of vitamin C supplementation on total cholesterol (MD 0.02 mmol/L. 95% CI ‐0.18 to 0.22; 1 study; 138 participants) (Jacques 1995) and (MD 0.05 mmol/L. 95% CI ‐0.14 to 0.24; 1 study; 16 participants) (Schindler 2003). The reduction in lipid levels seen in the Cerna 1992 study compared to others may be attributable to the high baseline levels of total cholesterol and LDL‐cholesterol.
1.10. Analysis.
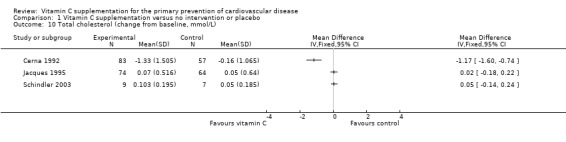
Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 10 Total cholesterol (change from baseline, mmol/L).
For LDL‐cholesterol (Analysis 1.11), one trial (Cerna 1992), showed that there was a significant reduction in LDL‐cholesterol with vitamin C supplementation (MD ‐1.27 mmol/L. 95% CI ‐1.67 to ‐0.87; 136 participants). The other two trials showed no evidence of effect of vitamin C supplementation on LDL‐cholesterol (MD 0.00 mmol/L. 95% CI ‐0.15 to 0.15; 1 study; 138 participants) (Jacques 1995) and (MD 0.16 mmol/L. 95% CI ‐0.02 to 0.33; 1 study; 16 participants) (Schindler 2003).
1.11. Analysis.
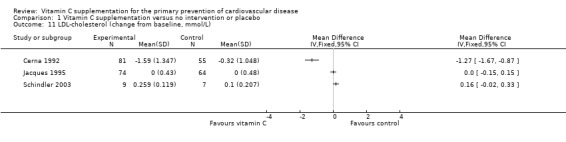
Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 11 LDL‐cholesterol (change from baseline, mmol/L).
For HDL‐cholesterol (Analysis 1.12), two trials showed no effect of vitamin C supplementation (MD 0.03 mmol/L. 95% CI ‐0.09 to 0.15; 1 study; 136 participants Cerna 1992, and MD 0.00 mmol/L. 95% CI ‐0.06 to 0.06; 1 study; 138 participants Jacques 1995), whilst the third showed a decrease in HDL with the intervention (MD ‐0.13 mmol/L. 95% CI ‐0.23 to ‐0.03; 1 study; 16 participants Schindler 2003). This very small study was however regarded at high risk of attrition bias (Schindler 2003). Data were also provided for the ASAP Study (ASAP Study) for men and women reported separately at three years follow‐up. We were unable to combine these data in the meta‐analysis as the numbers randomised to each group were unclear in the report. The mean HDL cholesterol increased in three years more among men who received vitamin C supplement than in men who received placebo (P = 0.025). In women, vitamin C had no effect on serum HDL cholesterol at three years.
1.12. Analysis.
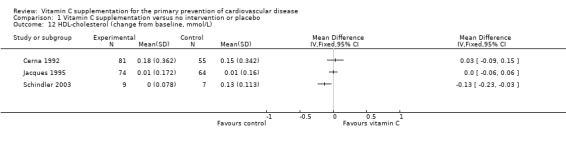
Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 12 HDL‐cholesterol (change from baseline, mmol/L).
Three trials looked at the effect of vitamin C on triglycerides (Fotherby 2000; Jacques 1995; Schindler 2003) and heterogeneity between studies was 47%. One trial showed no effect of vitamin C supplementation on triglyceride levels (MD 0.05 mmol/L, 95% CI ‐0.14 to 0.24; 138 participants) (Jacques 1995), whilst the other trial showed an increase in triglyceride levels with the intervention (MD 0.23 mmol/L, 95% CI 0.07 to 0.38, 16 participants) (Schindler 2003). This very small study was however regarded at high risk of attrition bias (Schindler 2003) (Analysis 1.13). Pooling these studies using a random effects model favoured the placebo group but this did not reach statistical significance (MD 0.15 mmol/L, 95% CI ‐0.02 to 0.32) (Analysis 1.13).
1.13. Analysis.
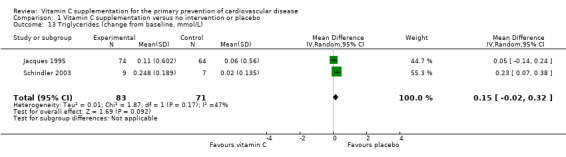
Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 13 Triglycerides (change from baseline, mmol/L).
One cross‐over trial reported results over the whole trial period and not in phases and so we were unable to incorporate the results in the meta‐analysis (Fotherby 2000). For the total group of 40 participants, there was no difference between placebo and vitamin C phases of the study for mean total cholesterol (6.2 ± 0.9 versus 6.2 ± 1.0 mmol/L; 40 participants), LDL cholesterol (3.6 ± 0.8 versus 3.5 ± 0.9 mmol/L) and HDL cholesterol (1.53 ± 0.35 versus 1.56 ± 0.36 mmol/L). The authors did however find that when they stratified by sex, there was an increase in HDL cholesterol with vitamin C in women by 0.08 ± 0.36 mmol/L, but not in men (Fotherby 2000).
For one study, total cholesterol, LDL cholesterol and triglycerides are reported at baseline but not at follow‐up (ASAP Study).
Type 2 diabetes
None of the included studies reported the occurrence of type 2 diabetes as a major CVD risk factor.
Health‐related quality of life
None of the included studies reported validated health‐related quality of life measures.
Adverse effects
The Physicians Health Study II examined a series of adverse effects of both vitamin C and vitamin E supplementation compared to placebo. No differences were seen in any of the following outcomes: bleeding (because vitamin E may potentially inhibit platelet function), gastrointestinal tract symptoms (peptic ulcer, constipation, diarrhoea, gastritis, and nausea), fatigue, drowsiness, skin discolouration or rashes and migraine (The Physicians Health Study II). Adverse effects were also reported in the ASAP Study. These were described as death, serious adverse event and adverse event with no further details given. No differences were seen between the vitamin C and placebo groups.
Cost
None of the included studies reported costs.
Subgroup analyses
There were insufficient trials (less than 10) to stratify results by high risk of CVD versus the general population, and by dose of vitamin C.
Sensitivity analyses
There were insufficient trials to conduct sensitivity analyses.
Discussion
Summary of main results
We included eight trials (15,445 participants randomised) from the 5555 papers screened. The largest trial with 14,641 participants provided data on our primary outcomes, cardiovascular disease (CVD) clinical events (The Physicians Health Study II). One smaller trial reported all‐cause mortality as adverse events. Seven trials reported on CVD risk factors. Three of these trials provided data in a useable format for meta‐analyses (Cerna 1992; Jacques 1995; Schindler 2003); the remaining four did not (ASAP Study; Fotherby 2000; Menne 1975; Mostafa 1989). We attempted to contact authors to provide missing details but were unsuccessful despite repeated attempts. Heterogeneity in the three trials precluded meta‐analysis and we provide a narrative synthesis. Three trials were regarded at high risk of bias for reporting bias (Schindler 2003), or attrition bias (ASAP Study; Mostafa 1989); most of the risk of bias domains for the remaining trials were judged as unclear, with the exception of the largest trial where most domains were judged to be at low risk of bias (The Physicians Health Study II).
The composite endpoint major CVD events was not different between the vitamin C and placebo group in the Physicians Health Study II and similar results were obtained for all‐cause mortality, total myocardial infarction (MI) (fatal and non‐fatal) and total stroke (fatal and non‐fatal). The authors of this trial concluded that vitamin C supplementation does not reduce the risk of major CVD events over eight years of follow‐up and should not be recommended for use in this group of participants ‐ middle‐aged and older men (The Physicians Health Study II). Adverse events were reported in this study and the ASAP Study with no significant differences between the vitamin C and placebo groups.
There were variable and inconsistent effects of vitamin C supplementation on CVD risk factors (lipid levels and blood pressure). None of the trials reported our other secondary outcomes occurrence of type 2 diabetes as a major risk factor for CVD, health‐related quality of life and costs.
Overall completeness and applicability of evidence
One large trial dominated this review and reports on our primary outcomes (The Physicians Health Study II). Whilst the study is adequately powered, the findings are limited to middle‐aged and older male physicians from the USA. The participants had variable baseline CVD risk but no significant effect modification was found between vitamin C and baseline risk.
Seven trials reported on CVD risk factors (lipid levels and blood pressure) with inconsistent findings. There were limitations in the available data as only three trials provided data in a useable format for meta‐analyses, and for the remaining studies we were unable to obtain additional information from the study authors. The findings to date for these outcomes are inconclusive.
Quality of the evidence
We have presented the overall quality of the evidence for each primary outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias), but also to external validity such as directness of results. All of the studies included in this review were randomised controlled trials (RCTs). There was no serious risk of bias detected for study limitations, inconsistency and publication bias. Grey literature was not searched but a comprehensive search across major databases and reference lists of relevant studies was carried out, therefore we could not formally assess these domains and they were not downgraded. The evidence for major CVD event, total MI, total stroke, self‐reported angina and self‐reported coronary artery bypass grafting (CABG)/percutaneous transluminal coronary angioplasty (PTCA) was of low quality. The outcomes were downgraded by one for indirectness (the populations were poorly applicable) and downgraded by one for imprecision (small magnitude of the number of included studies < five studies). The evidence for all‐cause mortality and CVD mortality was very low quality. The outcomes were downgraded by two levels for indirectness (the populations were poorly applicable, and the timeframe was insufficient), and downgraded by one level for imprecision (small magnitude of the number of included studies < five studies). Overall, inconsistency was difficult to evaluate because one trial was evaluated and therefore heterogeneity and the forest plot can not be evaluated (Table 1).
Potential biases in the review process
Although OpenGrey was not screened due to limited resources, a comprehensive search across major databases for interventions involving vitamin C supplementation was carried out for this review. In addition, the reference lists of systematic reviews were screened and authors contacted for information when needed. All screening, inclusion and exclusion and data abstraction were carried out independently by two review authors.
Multivitamins and mineral preparations including vitamin C were excluded from this review because it would not be possible to disentangle the specific effects of vitamin C. Multifactorial interventions were excluded to avoid confounding. This did however limit the number of trials that were eligible for inclusion.
Agreements and disagreements with other studies or reviews
In terms of clinical events, only one trial was identified to examine the effects of vitamin C on CVD events for primary prevention (The Physicians Health Study II). These results are however restricted to middle‐aged and older male physicians in the USA. One further smaller trial reported all‐cause mortality as adverse events (ASAP Study), with only one event reported in each of the vitamin C and placebo groups. Other trials have looked at the effects of vitamin C supplementation in women, but for secondary rather than primary prevention of CVD (Cook 2007). There were similar findings in terms of major CVD events. A recent systematic review of the effects of individual vitamins and minerals, and multivitamins, on clinical endpoints has been conducted for the US Task Force for Preventative Services. The authors found two trials of vitamin C supplementation reporting clinical endpoints relevant for CVD prevention, where no effect of the intervention was found (Fortmann 2013). These two trials are the same trials reported in the current review (ASAP Study; The Physicians Health Study II).
We were unable to determine the effectiveness of vitamin C supplementation on major CVD risk factors (lipid levels and blood pressure) with the trials included in the current version of the review due to missing information, heterogeneity of participants, dose of supplementation, duration of intervention and follow‐up, and methodological quality.
Previous systematic reviews of RCTs have examined the effects of vitamin C supplementation alone and in combination with other agents (such as vitamin E, magnesium, zinc, selenium) in trials between two and 26 weeks duration (Juraschek 2012). The authors concluded that vitamin C supplementation reduced systolic and diastolic blood pressure in short‐term trials. Another review of RCTs concluded that supplementation with at least 500 mg/day of vitamin C, for a minimum of four weeks, can result in a significant decrease in serum LDL cholesterol and triglyceride concentrations. However, the lack of quality assessment and analysis of statistical heterogeneity, and the small sample sizes of the included trials, limit the reliability of the authors' conclusions (McRae 2008).
Authors' conclusions
Implications for practice.
Currently, there is no evidence to suggest that vitamin C supplementation reduces the risk of cardiovascular disease (CVD). However, the results of this review should be interpreted with caution as the evidence was rated as low quality mainly for indirectness (downgraded by one level) and imprecision (downgraded by one level) for major CVD event, total myocardial infarction (MI), total stroke, self‐reported angina and self‐reported coronary artery bypass grafting (CABG)/percutaneous transluminal coronary angioplasty (PTCA). The evidence was rated as very low quality mainly for very serious indirectness (downgraded by two levels) and imprecision (downgraded by one level) for all‐cause morality and CVD mortality. Inconsistency of the evidence was difficult to evaluate because only one trial was evaluated and therefore heterogeneity and the forest plot can not be evaluated.
Implications for research.
Whilst a large adequately powered RCT reporting our primary outcomes clearly showed no effect of vitamin C supplementation on major CVD endpoints, these data are limited to middle‐aged and older male physicians from the USA. Future research should report the effect of vitamin C supplements on type 2 diabetes and use validated measures for quality of life. Future research should report economic data, costs of vitamin C supplements should be measured and reported as non of the trials reported costs. Future research and reports should provide adequate and transparent methodological details such as sequence generation, allocation concealment, blinding of outcomes assessors and report all outcomes. Higher‐quality trials are required as the current evidence is of low and very low methodological quality. There is limited evidence to date on the effects of vitamin C supplements on CVD risk factors.
Acknowledgements
We are grateful to Nicole Martin for conducting the searches for this review and for some duplicate screening. We are also grateful Cornelia Kunz and Mariana Dyakova at Warwick Medical School for their help with German and Russian translations.
Appendices
Appendix 1. Search strategies
Cochrane Library
#1 MeSH descriptor: [Ascorbic Acid] this term only #2 ascorb* #3 vit* near/6 c #4 magnorbin #5 hybrin #6 #1 or #2 or #3 or #4 or #5 #7 MeSH descriptor: [Cardiovascular Diseases] explode all trees #8 cardio* #9 cardia* #10 heart* #11 coronary* #12 angina* #13 ventric* #14 myocard* #15 pericard* #16 isch?em* #17 emboli* #18 arrhythmi* #19 thrombo* #20 atrial next fibrillat* #21 tachycardi* #22 endocardi* #23 (sick next sinus) #24 MeSH descriptor: [Stroke] explode all trees #25 (stroke or stokes) #26 cerebrovasc* #27 cerebral next vascular #28 apoplexy #29 (brain near/2 accident*) #30 ((brain* or cerebral or lacunar) near/2 infarct*) #31 MeSH descriptor: [Hypertension] explode all trees #32 hypertensi* #33 (peripheral next arter* next disease*) #34 ((high or increased or elevated) near/2 blood pressure) #35 MeSH descriptor: [Hyperlipidemias] explode all trees #36 hyperlipid* #37 hyperlip?emia* #38 hypercholesterol* #39 hypercholester?emia* #40 hyperlipoprotein?emia* #41 hypertriglycerid?emia* #42 MeSH descriptor: [Arteriosclerosis] explode all trees #43 MeSH descriptor: [Cholesterol] explode all trees #44 cholesterol #45 "coronary risk factor*" #46 MeSH descriptor: [Blood Pressure] this term only #47 "blood pressure" #48 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 #49 #6 and #48
MEDLINE
1. Ascorbic Acid/ 2. ascorb*.tw. 3. (vit* adj6 c).tw. 4. magnorbin.tw. 5. hybrin.tw. 6. or/1‐5 7. exp Cardiovascular Diseases/ 8. cardio*.tw. 9. cardia*.tw. 10. heart*.tw. 11. coronary*.tw. 12. angina*.tw. 13. ventric*.tw. 14. myocard*.tw. 15. pericard*.tw. 16. isch?em*.tw. 17. emboli*.tw. 18. arrhythmi*.tw. 19. thrombo*.tw. 20. atrial fibrillat*.tw. 21. tachycardi*.tw. 22. endocardi*.tw. 23. (sick adj sinus).tw. 24. exp Stroke/ 25. (stroke or stokes).tw. 26. cerebrovasc*.tw. 27. cerebral vascular.tw. 28. apoplexy.tw. 29. (brain adj2 accident*).tw. 30. ((brain* or cerebral or lacunar) adj2 infarct*).tw. 31. exp Hypertension/ 32. hypertensi*.tw. 33. peripheral arter* disease*.tw. 34. ((high or increased or elevated) adj2 blood pressure).tw. 35. exp Hyperlipidemias/ 36. hyperlipid*.tw. 37. hyperlip?emia*.tw. 38. hypercholesterol*.tw. 39. hypercholester?emia*.tw. 40. hyperlipoprotein?emia*.tw. 41. hypertriglycerid?emia*.tw. 42. exp Arteriosclerosis/ 43. exp Cholesterol/ 44. cholesterol.tw. 45. "coronary risk factor* ".tw. 46. Blood Pressure/ 47. blood pressure.tw. 48. or/7‐47 49. randomized controlled trial.pt. 50. controlled clinical trial.pt. 51. randomized.ab. 52. placebo.ab. 53. drug therapy.fs. 54. randomly.ab. 55. trial.ab. 56. groups.ab. 57. 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 58. exp animals/ not humans.sh. 59. 57 not 58 60. 6 and 48 and 59
Embase
1. ascorbic acid/ 2. ascorb*.tw. 3. (vit* adj6 c).tw. 4. magnorbin.tw. 5. hybrin.tw. 6. or/1‐5 7. exp cardiovascular disease/ 8. cardio*.tw. 9. cardia*.tw. 10. heart*.tw. 11. coronary*.tw. 12. angina*.tw. 13. ventric*.tw. 14. myocard*.tw. 15. pericard*.tw. 16. isch?em*.tw. 17. emboli*.tw. 18. arrhythmi*.tw. 19. thrombo*.tw. 20. atrial fibrillat*.tw. 21. tachycardi*.tw. 22. endocardi*.tw. 23. (sick adj sinus).tw. 24. exp cerebrovascular disease/ 25. (stroke or stokes).tw. 26. cerebrovasc*.tw. 27. cerebral vascular.tw. 28. apoplexy.tw. 29. (brain adj2 accident*).tw. 30. ((brain* or cerebral or lacunar) adj2 infarct*).tw. 31. exp hypertension/ 32. hypertensi*.tw. 33. peripheral arter* disease*.tw. 34. ((high or increased or elevated) adj2 blood pressure).tw. 35. exp hyperlipidemia/ 36. hyperlipid*.tw. 37. hyperlip?emia*.tw. 38. hypercholesterol*.tw. 39. hypercholester?emia*.tw. 40. hyperlipoprotein?emia*.tw. 41. hypertriglycerid?emia*.tw. 42. exp Arteriosclerosis/ 43. exp Cholesterol/ 44. cholesterol.tw. 45. "coronary risk factor*".tw. 46. Blood Pressure/ 47. blood pressure.tw. 48. or/7‐47 49. 6 and 48 50. random$.tw. 51. factorial$.tw. 52. crossover$.tw. 53. cross over$.tw. 54. cross‐over$.tw. 55. placebo$.tw. 56. (doubl$ adj blind$).tw. 57. (singl$ adj blind$).tw. 58. assign$.tw. 59. allocat$.tw. 60. volunteer$.tw. 61. crossover procedure/ 62. double blind procedure/ 63. randomized controlled trial/ 64. single blind procedure/ 65. 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 66. (animal/ or nonhuman/) not human/ 67. 65 not 66 68. 49 and 67 69. limit 68 to embase
Web of Science
# 12 #11 AND #10 # 11 TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*) # 10 #9 AND #8 # 9 TS=(ascorb* or (vit* near/6 c) or magnorbin or hybrin) # 8 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 # 7 TS=(hyperlipid* OR hyperlip?emia* OR hypercholesterol* OR hypercholester?emia* OR hyperlipoprotein?emia* OR hypertriglycerid?emia*) # 6 TS=("high blood pressure") # 5 TS=(hypertensi* OR "peripheral arter* disease*") # 4 TS=(stroke OR stokes OR cerebrovasc* OR cerebral OR apoplexy OR (brain SAME accident*) OR (brain SAME infarct*)) # 3 TS=("atrial fibrillat*" OR tachycardi* OR endocardi*) # 2 TS=(pericard* OR isch?em* OR emboli* OR arrhythmi* OR thrombo*) # 1 TS=(cardio* OR cardia* OR heart* OR coronary* OR angina* OR ventric* OR myocard*)
Appendix 2. Checklist to aid consistency and reproducibility of GRADE assessments
| Major CVD event | All‐cause mortality | CVD mortality | Total MI | Total stroke | Self‐reported angina | Self‐reported CABG/PTCA | ||
| Study limitations (risk of bias) | 1. Was random sequence generation used (i.e. no potential for selection bias)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
| 3. Was there blinding of participants and personnel (i.e. no potential for performance bias)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 4. Was there blinding of outcome assessment (i.e. no potential for detection bias)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 5. Was an objective outcome used? | Yes (end points were examined by physicians) | Yes (end points were examined by physicians) | Yes (end points were examined by physicians) | Yes (end points were examined by physicians) | Yes (end points were examined by physicians) | No (unlikely to introduce bias because it was a double‐blinded trial) | No (unlikely to introduce bias because it was a double‐blinded trial) | |
| 6. Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 7. Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 8. No other biases reported (i.e. no potential of other bias)? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
| 9. Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Inconsistency | 1. Point estimates did not vary widely? | Not applicable (one trial in forest plot) | Not applicable (one trial in forest plot) | Not applicable (one trial in forest plot) | Not applicable (one trial in forest plot) | Not applicable (one trial in forest plot) | Not applicable (one trial in forest plot) | Not applicable (one trial in forest plot) |
| 2. To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | Not applicable (one trial in forest plot) | Not applicable (one trial in forest plot) | Not applicable (one trial in forest plot) | Not applicable (one trial in forest plot) | Not applicable (one trial in forest plot) | Not applicable (one trial in forest plot) | Not applicable (one trial in forest plot) | |
| 3. Was the direction of effect consistent? | Not applicable (one trial in forest plot) | Not applicable (one trial in forest plot) | Not applicable (one trial in forest plot) | Not applicable (one trial in forest plot) | Not applicable (one trial in forest plot) | Not applicable (one trial in forest plot) | Not applicable (one trial in forest plot) | |
| 4. What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40% to 60%), high I² > 60%)? | Not applicable (cannot calculate I2 for one trial) | Not applicable (cannot calculate I2 for one trial) | Not applicable (cannot calculate I2 for one trial) | Not applicable (cannot calculate I2 for one trial) | Not applicable (cannot calculate I2 for one trial) | Not applicable (cannot calculate I2 for one trial) | Not applicable (cannot calculate I2 for one trial) | |
| 5. Was the test for heterogeneity statistically significant (P < 0.1)? | Not applicable (one trail meta‐analysed) | Not applicable (one trail meta‐analysed) | Not applicable (one trail meta‐analysed) | Not applicable (one trail meta‐analysed) | Not applicable (one trail meta‐analysed) | Not applicable (one trail meta‐analysed) | Not applicable (one trail meta‐analysed) | |
| Indirectness | 1. Were the populations in included studies applicable to the decision context? | Poorly applicable (middle aged US male physicians) (↓) | Poorly applicable (middle aged US male physicians) (↓) | Poorly applicable (middle aged US male physicians) (↓) | Poorly applicable (middle aged US male physicians) (↓) | Poorly applicable (middle aged US male physicians) (↓) | Poorly applicable (middle aged US male physicians) (↓) | Poorly applicable (middle aged US male physicians) (↓) |
| 2. Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | |
| 3. Was the included outcome not a surrogate outcome? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 4. Was the outcome timeframe sufficient? | Sufficient | Insufficient (longer timeframe may be necessary to cover the critical etiologic window or provide a sufficient cumulative dose capable of preventing cardiovascular disease) (↓) | Insufficient (longer timeframe may be necessary to cover the critical etiologic window or provide a sufficient cumulative dose capable of preventing cardiovascular disease) (↓) | Sufficient | Sufficient | Sufficient | Sufficient | |
| 5. Were the conclusions based on direct comparisons? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Imprecision | 1. Was the confidence interval for the pooled estimate not consistent with benefit? | Difficult to judge (one trial) | Difficult to judge (one trial) | Difficult to judge (one trial) | Difficult to judge (one trial) | Difficult to judge (one trial) | Difficult to judge (one trial) | Difficult to judge (one trial) |
| 2. What is the magnitude of the median sample size (high: 300 participants, intermediate: 100‐300 participants, low: < 100 participants)?a | High | High | High | High | High | High | High | |
| 3. What was the magnitude of the number of included studies (large: >10 studies, moderate: 5‐10 studies, small: <5 studies)?a | Small (↓) | Small (↓) | Small (↓) | Small (↓) | Small (↓) | Small (↓) | Small (↓) | |
| 4. Was the outcome a common event (e.g. occurs more than 1/100)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Publication bias | 1. Was a comprehensive search conducted? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Was grey literature searched? | No (OpenGrey search was not conducting due to limited resources) | No (OpenGrey search was not conducting due to limited resources) | No (OpenGrey search was not conducting due to limited resources) | No (OpenGrey search was not conducting due to limited resources) | No (OpenGrey search was not conducting due to limited resources) | No (OpenGrey search was not conducting due to limited resources) | No (OpenGrey search was not conducting due to limited resources) | |
| 3. Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 4. There was no industry influence on studies included in the review? | Yes (intervention providers had no role in the study design; conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript) | Yes (intervention providers had no role in the study design; conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript) | Yes (intervention providers had no role in the study design; conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript) | Yes (intervention providers had no role in the study design; conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript) | Yes (intervention providers had no role in the study design; conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript) | Yes (intervention providers had no role in the study design; conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript) | Yes (intervention providers had no role in the study design; conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript) | |
| 5. There was no evidence of funnel plot asymmetry? | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable | |
| 6. There was no discrepancy in findings between published and unpublished trials? | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable | |
|
aDepends on the context of the systematic review area (↓): key item for potential downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); GRADE: Grading of Recommendations Assessment, Development and Evaluation; N/A: not applicable | ||||||||
Data and analyses
Comparison 1. Vitamin C supplementation versus no intervention or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major cardiovascular event | 1 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.89, 1.10] | |
| 2 Cardiovascular mortality | 1 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.85, 1.22] | |
| 3 All‐cause mortality | 1 | Hazard Ratio (Fixed, 95% CI) | 1.07 [0.97, 1.18] | |
| 4 Total myocardial infarction | 1 | Hazard Ratio (Fixed, 95% CI) | 1.04 [0.87, 1.24] | |
| 5 Self‐reported CABG/PTCA | 1 | Hazard Ratio (Fixed, 95% CI) | 0.96 [0.86, 1.07] | |
| 6 Self‐reported angina | 1 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.84, 1.03] | |
| 7 Total stroke | 1 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.74, 1.07] | |
| 8 Systolic blood pressure (change from baseline, mmHg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Diastolic blood pressure (change from baseline, mmHg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Total cholesterol (change from baseline, mmol/L) | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 LDL‐cholesterol (change from baseline, mmol/L) | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 HDL‐cholesterol (change from baseline, mmol/L) | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13 Triglycerides (change from baseline, mmol/L) | 2 | 154 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.02, 0.32] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ASAP Study.
| Methods | RCT of parallel group design | |
| Participants | ASAP trial. 520 smoking and non‐smoking men and postmenopausal women, aged 45 to 69 years, with hypercholesterolaemia (≥ 5.0 mmol/L) were recruited in Kuopio, Eastern Finland. Recruitment was by multiple advertisements in the main local newspaper Exclusion criteria: · Premenopausal · Had regular oestrogen substitution therapy · Regular intake of antioxidants · Acetosalicyclic acid or any other drug with antioxidative properties · Severe obesity (BMI > 32k g/m2) · Type 1 diabetes · Uncontrolled hypertension (DBP > 105 mmHg) · Any condition limiting mobility, making study visits impossible · Severe disease shortening life expectancy · Other disease or condition worsening the adherence to the measurements or treatment. |
|
| Interventions | Intervention (Vitamin C, n = 130): Slow‐release ascorbic acid (250 mg) taken twice daily. Control (Placebo, n = 130): No details provided. Follow‐up: 3 years. |
|
| Outcomes | Total cholesterol, LDL‐cholesterol, HDL‐cholesterol, triglycerides, cardiovascular mortality. | |
| Notes | This was a 4‐arm trial (placebo, vitamin E, vitamin C, vitamin C + E). We used the placebo and vitamin C arms only. The focus of the study was to explore the effects of vitamins C and E on atherosclerotic progression using the common carotid artery mean intima media thickness (IMT). Deaths from cardiovascular causes were specified as reasons for "drop‐out", however, death from cardiovascular causes was not a pre‐specified outcome measure. Men randomised to take vitamin C took more angina pectoris drugs than men in the other groups (< 25% of participants had CVD at baseline so it met our criteria for primary prevention). Outcome data for total cholesterol, LDL‐cholesterol, HDL‐cholesterol, triglycerides and cardiovascular mortality were not reported in the paper. Review author NF attempted to contact the authors using the email address provided in the paper but this was undeliverable (email address did not exist). A later publication (Salonen 2003) reported change in HDL cholesterol in men and women separately after 3 years of supplementation. Funding source: non‐commercial (grants from the Academy of Finland Nos. 41258 and 52668) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised separately in four strata. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The study was double‐masked. The masking was carried out by the provider of the supplements (Ferrosan A/S, Denmark) and delivered to the data centre of the Field Centre, Research Institute of Public Health, University of Kuopio, after the completion of reading of the videotapes of ultrasonographic examinations". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of dropouts specified and reasons provided. |
| Selective reporting (reporting bias) | High risk | The authors state that they will measure total cholesterol, LDL, triglycerides and blood pressure but do not report outcomes. |
| Other bias | Unclear risk | Insufficient information to judge. Non‐commercial funding. |
Cerna 1992.
| Methods | RCT of parallel group design | |
| Participants | 140 participants were recruited. Participants were employees of matador, ZTS Works and the Retirement Office (Industrial plants and an organisation) situated in Bratislava, Slovakia. Inclusion criteria: · Men (mean age, 48 years) and women (mean age, 47 years) · No signs of clinical problems · Cholesterol > 6.2 mmol/L, Triacylglycerols >1.7 mmol/L, or mixed hyperlipaemia. |
|
| Interventions | Intervention (n = 83; 33 men and 50 women): Participants were provided Celaskon effervescens Spofa at a dose of 500 mg/day, for 18 months. Control (n = 57; 24 men and 33 women): No details provided. |
|
| Outcomes | Total cholesterol, LDL cholesterol, HDL cholesterol. | |
| Notes | Review author NF could only find contact details for one of the authors, Emil Ginter. NF contacted Emil Ginter to ask for further information on the control group, but who was unable to help. Funding: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as specified. |
| Other bias | Unclear risk | Insufficient information to judge. Did not state funding. |
Fotherby 2000.
| Methods | RCT cross‐over design | |
| Participants | 40 participants were recruited in the UK, from general practice lists. Inclusion criteria: · Men and women · Aged 60‐80 years · Non‐smokers for >.10 years · No history of vascular disease, i.e. no known stroke, myocardial infarction, angina or peripheral vascular disease · Not taking prescribed medications, including aspirin, non‐steroidal anti‐inflammatory drugs or vitamin supplements. · Normotensive individuals and those with a history of borderline hypertension or newly diagnosed hypertension, but who had never received treatment were included. |
|
| Interventions | Intervention: Vitamin C capsules (250 mg) twice daily, for 3 months. Control: Placebo capsules, twice daily, for 3 months. After a 1 week ‘wash‐out’ participants crossed to the alternative treatment for a further 3 months. |
|
| Outcomes | Systolic blood pressure, diastolic blood pressure, total cholesterol, LDL‐cholesterol, HDL cholesterol. | |
| Notes | Our intention was to analyse this as parallel group, using data only from the first 3‐month intervention before patients crossed over to the other therapy, but insufficient data were reported for us to be able to do this. Review author NF attempted to contact the first author, Dr Martin Fotherby, using the email address provided in the paper but this was undeliverable (email address does not exist). NF also contacted the last author, Dr Gordon Ferns who provided location details for Dr Fotherby. An alternative email address was sought and NF attempted to contact Dr Martin Fotherby on two occasions via email. We have not had a response and so the results as reported in the paper are described narratively in text. Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators involved with blood pressure measurements and sample analysis were blinded to the treatment each individual was receiving. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and investigators involved with blood pressure measurements and sample analysis were blinded to the treatment each individual was receiving. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of dropouts is unclear. The main paper says 40 completed the study but two of the abstracts state 37 completed. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as specified. |
| Other bias | Unclear risk | Insufficient information to judge Did not state funding. |
Jacques 1995.
| Methods | RCT of parallel group design. | |
| Participants | 155 participants (men and women, 20 to 65 years old) were recruited and screened between December 1989 and September 1991 from two sources in Boston. Employees of a large manufacturing complex were recruited by work site posters and presentations, and Boston area residents were recruited by printed advertisements. Exclusion criteria: · Age ˂ 20 or > 65 years · Plasma ascorbic acid > 80 µmol/L for men or > 90 µmol/L for women · HDL cholesterol > 1.4 mmol/L for men or >1.7 mmol/L for women · Total cholesterol > 6.7 mmol/L · Body mass index > 31 kg/m2 for men or > 33 kg/m2 for women · Current smokers · History of diabetes, heart disease or liver disease · Vitamin C supplement use (> 60 mg/day) within the last 3 months · Use of lipid altering medication · On a weight modifying diet |
|
| Interventions | Intervention (n = 80): 2 x 500 mg vitamin C tablets per day (one each morning and one each evening) for 8 months. Control (n = 75): 2 x placebo tablets per day (one each morning and one each evening) for 8 months. |
|
| Outcomes | Total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides. | |
| Notes | Funding: commercial and non‐commercial (Hoffman‐La Roche and US Department of Agriculture). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States "double‐blind" but provide no further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers lost to follow‐up provided, and reasons for exclusions provided. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as specified. |
| Other bias | Unclear risk | Insufficient information to judge Non‐commercial and commercial funding. Authors did state that the content of the study does not necessarily represent the views of the US Department of Agriculture. |
Menne 1975.
| Methods | RCT of parallel group design | |
| Participants | 122 healthy, young medical students, (108 males and 14 females, 18‐25 years of age) were recruited from a South African University between April 1974 and August 1974. No further inclusion/exclusion criteria for participants was specified. |
|
| Interventions | Intervention (n = 62): Two 500 mg tablets of ascorbic acid, daily for four months Control (n = 60): Two placebo tablets (500 mg citric acid), daily for four months |
|
| Outcomes | Serum cholesterol, serum triglycerides. | |
| Notes | Outcome data were only displayed graphically and not reported in tables, therefore insufficient for use in a meta‐analysis. NF could not find any contact details for the authors of this paper. Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. Just says "divided at random". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States only participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as specified but in graphical not numerical form. |
| Other bias | Unclear risk | Insufficient information to judge. Did not state funding. |
Mostafa 1989.
| Methods | RCT of parallel group design | |
| Participants | 67 volunteers, recruited at the University of Mississippi Campus, Mississippi, USA. No further inclusion/exclusion criteria for participants was specified. |
|
| Interventions | Intervention (n = 37): One 500 mg vitamin C tablet per day for 6 months. Particpants were instructed to avoid taking vitamin C from other sources and it was emphasised that they will not alter their diet habits or their lifestyle. Control (n = 30): Placebo, for 6 months (no further details provided). |
|
| Outcomes | Systolic blood pressure, diastolic blood pressure, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides. | |
| Notes | Age, gender and ethnicity were not stated in the paper. Outcome data for the control group were not provided in the paper and so data are insufficient for use in meta‐analyses. Before and after results where provided by the authors are described in the results text. Review author NF could not find any contact details for the authors of this paper. Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double blind techniques were used to exclude the possibility of bias. Neither the subjects, nor staff knew which subjects had taken the vitamin C or placebo regimens". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details provided. |
| Selective reporting (reporting bias) | High risk | Outcome data were not reported for the control group. |
| Other bias | Unclear risk | Insufficient information to judge. Did not state funding. |
Schindler 2003.
| Methods | RCT of parallel group design | |
| Participants | 20 participants with normal coronary angiograms (12 males, 8 females), with hypertension (SBP ≥ 145 mmHg) Exclusion criteria: · History of unstable angina pectoris · Previous myocardial infarction · Malignant hypertension · Diabetes mellitus · Evidence of glucose intolerance · Cardiac autonomic neuropathy · Valvular heart disease · Peripheral vascular disease · Significant endocrine, hepatic, renal, and inflammatory disease · Regular dietary intake of antioxidants Only patients not on vasoactive medication, such as angiotensin‐converting enzyme inhibitors, calcium channel blockers, or statins at baseline and/or throughout the study period of two years, were recruited. In hypertensive patients, a regular intake of diuretics and beta‐blockers was allowed for blood pressure control. Each hypertensive patient treated with beta‐blockers discontinued the medication seven days before each study. In addition, during this period, patients were asked to use a short‐term calcium antagonist to control high blood pressure. In these patients, study sessions were only performed if patients did not use a short‐acting calcium antagonist for at least 24 hours before the investigation. |
|
| Interventions | Intervention (n = 12): 2 g vitamin C daily, for 2 years Control (n = 8): Placebo supplementation daily, for two years |
|
| Outcomes | Systolic blood pressure, diastolic blood pressure, total cholesterol, LDL cholesterol, very‐LDL cholesterol, HDL cholesterol, triglycerides. | |
| Notes | 50 patients in total were included in the study, but only the hypertensive patients (n = 20) were randomised into a vitamin C and placebo group. The remaining participants (chronic smokers, hypercholesterolaemic patients, and control participants) were assigned to an open‐label treatment with 2 g vitamin C daily during 2‐year follow‐up. During the study, hypertensive patients (n = 20, vitamin C and placebo groups) received diuretics and three patients received combined therapy with beta‐blockers. Paper does not state where participants were recruited from. Funding source: non‐commercial (grant from the German Research Foundation [So 241/2‐2], the government of Baden‐Württemberg for the “Center of Clinical Research II: Cardiovascular Diseases—Analysis and Integration of Form und Function” at the Albert‐Ludwig‐ University Freiburg [Project: Sch‐A1/A2 and EUN‐A2] and a grant from the Basel Heart Foundation of Switzerland). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to judge (paper states that investigators were unaware of patients assignment to vitamin C or placebo, but does not state whether patients were aware, or not). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No reasons for loss to follow‐up. No ITT analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as specified. |
| Other bias | Unclear risk | Insufficient information to judge. Non‐commercial funding. |
The Physicians Health Study II.
| Methods | RCT of parallel group design | |
| Participants | 14,641 male participants were recruited in two phases. Starting in July 1997, 7641 participants were recruited from Physicians Health Study I. In July 1999 recruitment of physicians started. Invitational letters were sent to US male physicians identified from a list provided from the American Medical Association. 7000 willing and eligible men were recruited. Inclusion criteria: · Men · Age 50 years and older · Willing to forego any current use of multivitamins or individual supplements containing more than 100% RDA of vitamin E, vitamin C, β‐carotene or vitamin A Exclusion criteria: · History of cirrhosis, active liver disease, taking anticoagulants · A serious illness that would preclude participation 5.1% of participants had prevalent CVD at baseline (non‐fatal MI and stroke). *Some participants had a history of diabetes (approximately 6%), a history of high cholesterol (approximately 36%), a history of hypertension (approximately 42%), a BMI ≥30 kg/m2 (approximately 11%), were smokers (approximately 4%). Approximately 77% of participants were taking aspirin at baseline. *approximate percentages due to aggregated data |
|
| Interventions | Intervention (Vitamin C (500 mg) taken daily, n = 7329) Control (Placebo, n = 7312) Mean follow‐up was 8 years. |
|
| Outcomes | Major cardiovascular events, total MI, MI death, total stroke, stroke death, Ischaemic stroke, haemorrhagic stroke, cardiovascular death, congestive heart failure, angina, revascularisation, all‐cause mortality. | |
| Notes | This trial was a factorial 2 x 2 trial of vitamin E and vitamin C supplementation and it is therefore possible to compare two vitamin C arms (active vitamin C and placebo vitamin E and active vitamin C and active vitamin E) with two non‐vitamin C arms (placebo vitamin C and active vitamin E and placebo vitamin C and E). Funding source: commercial and non‐commercial (grants from the National Institutes of Health, investigator‐initiated grant from BASF Corporation. Study agents and packaging were provided by BASF Corporation, Wyeth Pharmaceuticals and DSM Nutritional Products Inc) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated list of random numbers randomised in blocks of 16, stratified by age, prior diagnosis of CVD and cancer, and for the PHSI subjects, by their original β‐carotene assignment". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Treatment and follow‐up continued in a blinded fashion", "All tablets were identical in appearance, size, and color". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | End points were examined by physicians blinded to randomised treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers for participants dead, alive and unknown status provided. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as specified. |
| Other bias | Unclear risk | Insufficient information to judge. Commercial and non‐commercial funding, authors clearly mention that commercial funding had no role in the study. |
BMI: body mass index CVD: cardiovascular disease DBP: diastolic blood pressure HDL: high‐density lipoprotein ITT: intention‐to‐treat LDL: low‐density lipoprotein MI: myocardial infarction RCT: randomised controlled trial RDA: recommended daily allowance SBP: systolic blood pressure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bassuk 2004 | Secondary prevention trial (The Women's Antioxidant Cardiovascular Study) |
| Boushehri 2012 | Not a RCT |
| Bunpo 2015 | Multifactorial intervention |
| Calzada 1995 | Short term (8 weeks) |
| Dobson 1984 | Not a RCT |
| Ghosh 1994 | Short term (6 weeks) |
| Jayachandran 2000 | Not a RCT |
| Osilesi 1991 | Short term (6 weeks) |
| Schutte 2004 | Intervention not vitamin C supplementation as a single supplement |
| Shidfar 2003 | Short term (10 weeks) |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Nicolaides 2002.
| Methods | RCT of parallel group design |
| Participants | 1032 participants |
| Interventions | Intervention (n = unspecified): Vitamin C (1g/day) Control (n = unspecified): No treatment Follow‐up: 10 years |
| Outcomes | Cardiovascular events |
| Notes | This study is awaiting classification because only an abstract of the trial is available. The baseline health of participants is unclear and more data are also needed on the cardiovascular events. Review author NF sought an email address of the author, Andrew Nicolaides to ask if a full report had been published. Andrew Nicolaides suggested to contact Dr Gianni Belcaro. NF emailed Dr Gianni Belcaro on two occasions. No response has been received. |
RCT: randomised controlled trial
Differences between protocol and review
Citation searches for key articles and OpenGrey searches were not conducted due to limited resources. Authors were contacted where necessary for additional information.
Contributions of authors
Review authors LA, NF, RW, LH, KR and OG screened titles and abstracts and assessed studies for formal inclusion and exclusion. LA, NF and RW abstracted data and assessed methodological rigour. NF wrote the first draft of the results and KR updated this section and wrote the other sections of the review. LA conducted the GRADE analysis, which was checked by KR and updated sections of the review. SS subject expertise, and assisted with the literature and writing the background for the review and assisted with interpretation of findings.
Sources of support
Internal sources
Warwick Medical School, University of Warwick, UK.
External sources
NIHR Cochrane Programme Grant, UK.
Karen Rees is also supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care West Midlands at University Hospitals Birmingham NHS Foundation Trust, UK.
Declarations of interest
None known.
New
References
References to studies included in this review
ASAP Study {published data only}
- Salonen JT, Nyyssonen K, Salonen R, Lakka HM, Kaikkonen J, Porkkala‐Sarataho E, et al. Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) study: a randomized trial of the effect of vitamins E and C on 3‐year progression of carotid atherosclerosis. Journal of Internal Medicine 2000;248:377‐86. [DOI] [PubMed] [Google Scholar]
- Salonen RM, Nyyssönen K, Kaikkonen J, Porkkala‐Sarataho E, Voutilainen S, Rissanen TH, et al. Six‐year effect of combined vitamin C and E supplementation on atherosclerotic progression. The Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) Study. Circulation 2003;107:947‐53. [DOI] [PubMed] [Google Scholar]
Cerna 1992 {published data only}
- Cerna O, Ramacsay L, Ginter E. Plasma lipids, lipoproteins and atherogenic index in men and women administered vitamin C. Cor et Vasa 1992;34:246‐54. [PubMed] [Google Scholar]
Fotherby 2000 {published data only}
- Fotherby MD, Williams J, Ferns G. Effect of Vitamin C on Ambulatory Blood Pressure (Bp) in Older Hypertensive and Normotensive Persons. Age and Ageing. 1998; Vol. 27:5‐a.
- Fotherby MD, Williams JC, Forster LA, Craner P, Ferns GA. Effect of vitamin C on ambulatory blood pressure and plasma lipids in older persons. Journal of Hypertension 2000;18:411‐5. [DOI] [PubMed] [Google Scholar]
- Nicolaides A, Cesarone M R, Belcaro G, Sanctis M, Incandela L, Griffin M. Effect of vitamin c on atherosclerosis progression and cardiovascular events in a ten‐year randomized study (San Valentino). Journal of the American College of Cardiology. 2002; Vol. 39:265.
Jacques 1995 {published data only}
- Jacques PF, Sulsky SI, Perrone GE, Jenner J, Schaefer EJ. Effect of vitamin C supplementation on lipoprotein cholesterol, apolipoprotein, and triglyceride concentrations. Annals of Epidemiology 1995;5:52‐9. [DOI] [PubMed] [Google Scholar]
Menne 1975 {published data only}
- Menne IV, Grey PC, Kotze JP, Sommers DK, Brown JM, Spies JH. Ascorbic acid and blood lipid and uric acid levels of students. South African Medical Journal. Suid‐Afrikaanse Tydskrif Vir Geneeskunde 1975;49:2225‐8. [PubMed] [Google Scholar]
Mostafa 1989 {published data only}
- Mostafa S el‐D, Garner DD, Garrett L, Whaley RF, el‐Sekate M, Kiker M. Beneficial effects of vitamin C on risk factors of cardiovascular diseases. Journal of the Egyptian Public Health Association 1989;64(1‐2):123‐33. [PubMed] [Google Scholar]
Schindler 2003 {published data only}
- Schindler TH, Nitzsche EU, Munzel T, Olschewski M, Brink I, Jeserich M, et al. Coronary vasoregulation in patients with various risk factors in response to cold pressor testing: contrasting myocardial blood flow responses to short‐ and long‐term vitamin C administration. Journal of the American College of Cardiology 2003;42:814‐22. [DOI] [PubMed] [Google Scholar]
The Physicians Health Study II {published data only}
- Christen WG, Gaziano M, Hennekens CH, for the steering committee of the Physicians Health Study II. Design of Physicians’ Health Study II—A randomized trial of beta‐carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Annals of Epidemiology 2000;10:125–34. [DOI] [PubMed] [Google Scholar]
- Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA 2008;300(18):2123‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bassuk 2004 {published data only}
- Bassuk SS, Albert CM, Cook NR, Zaharris E, MacFadyen JG, Danielson E, et al. The Women's Antioxidant Cardiovascular Study: design and baseline characteristics of participants. Journal of Women's Health 2004;13:99‐117. [DOI] [PubMed] [Google Scholar]
Boushehri 2012 {published data only}
- Boushehri SN, Yusof RM, Taib MNM, Mirzaei K, Yazdekhasti N, Akbarzadeh S. Effect of vitamin supplementation on serum oxidised low‐density lipoprotein levels in male subjects with cardiovascular disease risk factors. Iranian Journal of Basic Medical Sciences 2012;15:958‐64. [PMC free article] [PubMed] [Google Scholar]
Bunpo 2015 {published data only}
- Bunpo P, Anthony TG. Ascorbic acid supplementation does not alter oxidative stress markers in healthy volunteers engaged in a supervised exercise program. Applied Physiology, Nutrition, and Metabolism 2015;41(2):175‐80. [DOI] [PubMed] [Google Scholar]
Calzada 1995 {published data only}
- Calzada C, Bruckdorfer KR, Rice‐Evans CA. The influence of antioxidant nutrients on platelet function in healthy volunteers. Atherosclerosis 1997;128:97‐105. [DOI] [PubMed] [Google Scholar]
Dobson 1984 {published data only}
- Dobson HM, Muir MM, Hume R. The effect of ascorbic acid on the seasonal variations in serum cholesterol levels. Scottish Medical Journal 1984;29:176‐82. [DOI] [PubMed] [Google Scholar]
Ghosh 1994 {published data only}
- Ghosh SK, Ekpo EB, Shah IU, Girling AJ, Jenkins C, Sinclair AJ. A double‐blind, placebo‐controlled parallel trial of vitamin C treatment in elderly patients with hypertension. Gerontology 1994;40:268‐72. [DOI] [PubMed] [Google Scholar]
Jayachandran 2000 {published data only}
- Jayachandran M, Arivazhagan P, Panneerselvam C. Age‐associated plasma lipids, lipid peroxidation, and antioxidant systems in relation to vitamin C supplementation in humans. Journal of Anti‐Aging Medicine 2000;3:437‐45. [Google Scholar]
Osilesi 1991 {published data only}
- Osilesi O, Trout DL, Ogunwole JO, Glover EE. Blood pressure and plasma lipids during ascorbic acid supplementation in borderline hypertensive and normotensive adults. Nutrition Research 1991;11:405‐12. [Google Scholar]
Schutte 2004 {published data only}
- Schutte AE, Huisman HW, Oosthuizen W, Rooyen JM, Jerling JC. Cardiovascular effects of oral Supplementation of vitamin C, E and folic acid in young healthy males. International Journal for Vitamin and Nutrition Research 2004;74:285‐93. [DOI] [PubMed] [Google Scholar]
Shidfar 2003 {published data only}
- Shidfar F, Keshavarz A, Jallali M, Miri R, Eshraghian M. Comparison of the effects of simultaneous administration of vitamin C and omega‐3 fatty acids on lipoproteins, apo A‐I, apo B, and malondialdehyde in hyperlipidemic patients. International Journal for Vitamin and Nutrition Research 2003;73:163‐70. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Nicolaides 2002 {published data only}
- Nicolaides A, Cesarone MR, Belcaro G, Sanctis M, Incandela L, Griffin M. Effect of vitamin C on atherosclerosis progression and cardiovascular events in a ten‐year randomised study (San Valentino). Journal of the American College of Cardiology 2002;39:265A. [Google Scholar]
Additional references
Begg 2007
- Begg S, Vos T, Barker B, Stevenson C, Stanley L, Lopez A. The Burden of Disease and Injury in Australia 2003. Vol. Cat. no. PHE 82, Canberra: Australian Institute of Health and Welfare, May 2007. [Google Scholar]
Bjelakovic 2007
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta‐analysis. JAMA 2007;297:842–57. [DOI] [PubMed] [Google Scholar]
British Heart Foundation 2012
- British Heart Foundation 2012. Cardiovascular Disease. http://www.bhf.org.uk/heart‐health/conditions/cardiovascular‐disease.aspx (Accessed September 2013).
Cook 2007
- Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women’s Antioxidant Cardiovascular Study. Archives of Internal Medicine 2007;167:1610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Follman 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45:769‐73. [DOI] [PubMed] [Google Scholar]
Fortmann 2013
- Fortmann SP, Burda BU, Senger CA, Lin JS, Beil TL, O'Connor E, et al. Vitamin, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: a systematic evidence review for the U.S. Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US) Nov 2013. [PubMed]
Frei 1989
- Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proceedings of the National Academy of Sciences of the United States of America 1989;86:6377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grebe 2006
- Grebe M, Eisele HJ, Weissmann N, Schaefer C, Tillmanns H, Seeger W, et al. Antioxidant vitamin C improves endothelial function in obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine 2006;173:897–901. [DOI] [PubMed] [Google Scholar]
He 2011
- He F, Burnier M, MacGregor G. Nutrition in cardiovascular disease: salt in hypertension and heart failure. European Heart Journal 2011;32:3073‐80. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of interventions Version 5.1 [updated March 2011]. The Cochrane Library, The Cochrane Collaboration (2011). Available from www.cochrane‐handbook.org. [Google Scholar]
Huang 2000
- Huang A, Vita JA, Venema RC, Keaney JF Jr. Ascorbic acid enhances endothelial nitric‐oxide synthase activity by increasing intracellular tetrahydrobiopterin. The Journal of Biological Chemistry 2000;275:17399–406. [DOI] [PubMed] [Google Scholar]
Juraschek 2012
- Juraschek SP, Guallar E, Appel LJ, Miller ER. Effects of vitamin C supplementation on blood pressure: a meta‐analysis of randomized controlled trials. American Journal of Clinical Nutrition 2012;95(5):1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org). [Google Scholar]
Levine 2011
- Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration‐function approach yields pharmacology and therapeutic discoveries. Advances in Nutrition 2011;2:78‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathers 2006
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine 2006;3(11):e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
May 2013
- May JM, Harrison FE. Role of vitamin C in the function of the vascular endothelium. Antioxidants and Redox Signaling 2013;19(17):2068‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarron 1984
- McCarron DA, Morris CD, Henry HJ, Stanton JL. Blood pressure and nutrient intake in the United States. Science 1984;224:1392–8. [DOI] [PubMed] [Google Scholar]
McNulty 2007
- McNulty PH, Robertson BJ, Tulli MA, Hess J, Harach LA, Scott S, et al. Effect of hyperoxia and vitamin C on coronary blood flow in patients with ischemic heart disease. Journal of Applied Physiology 2007;102:2040–5. [DOI] [PubMed] [Google Scholar]
McRae 2006a
- McRae M P. Is vitamin C an effective antihypertensive supplement: a review and analysis of the literature. Journal of Chiropractic Medicine 2006;5(2):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
McRae 2006b
- McRae M P. The efficacy of vitamin C supplementation on reducing total serum cholesterol in human subjects: a review and analysis of 51 experimental trials. Journal of Chiropractic Medicine 2006;5(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
McRae 2008
- McRae MP. Vitamin C supplementation lowers serum low‐density lipoprotein cholesterol and triglycerides: a meta‐analysis of 13 randomised controlled trials. Journal of Chiropractic Medicine 2008;7(2):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systemic Reviews 2014;3:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moran 1993
- Moran JP, Cohen L, Greene JM, Xu G, Feldman EB, Hames CG, et al. Plasma ascorbic acid concentrations relate inversely to blood pressure in human subjects. American Journal of Clinical nutrition 1993;57:213–7. [DOI] [PubMed] [Google Scholar]
Ness 1997
- Ness AR, Chee D, Elliott P. Vitamin C and blood pressure: an overview. Journal of Human Hypertension 1997;11(6):343. [DOI] [PubMed] [Google Scholar]
NHS 2012
- NHS, 2012. Atherosclerosis. http://www.nhs.uk/conditions/Atherosclerosis/Pages/Introduction.aspx#commentCountLink Accessed September 2013.
NHS choices 2015
- Vitamin C. http://www.nhs.uk/Conditions/vitamins‐minerals/Pages/Vitamin‐C.aspx.
Padayatty 2010
- Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, Levine M. Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One 2010;5(7):e11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rayment 2003
- Rayment SJ, Shaw J, Woollard KJ, Lunec J, Griffiths HR. Vitamin C supplementation in normal subjects reduces constitutive ICAM‐1 expression. Biochemical and Biophysical Research Communications 2003;308(2):339‐45. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Silvestro 2002
- Silvestro A, Scopacasa F, Oliva G, Cristofaro T, Iuliano L, Brevetti G. Vitamin C prevents endothelial dysfunction induced by acute exercise in patients with intermittent claudication. Atherosclerosis 2002;165:277–83. [DOI] [PubMed] [Google Scholar]
Simon 1998
- Simon JA, Hudes ES. Relation of serum ascorbic acid to serum lipids and lipoproteins in US adults. Journal of the American College of Nutrition 1998;17:250–5. [DOI] [PubMed] [Google Scholar]
Siri‐Tarino 2010
- Siri‐Tarino P, Sun Q, Hu F, Krauss R. Saturated fat, carbohydrate, and cardiovascular disease. American Journal of Clinical Nutrition 2010;91:502‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solzbach 1997
- Solzbach U, Hornig B, Jeserich M, Just H. Vitamin C improves endothelial dysfunction of epicardial coronary arteries in hypertensive patients. Circulation 1997;96:1513–9. [DOI] [PubMed] [Google Scholar]
Steinberg 1989
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modification of low‐ density lipoprotein that increases its atherogenicity. New England Journal of Medicine 1989;320:915‐924. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
WHO 2003
- WHO Study Group. Diet, nutrition and the prevention of chronic diseases. World Health Organization, Geneva 2003; Vol. Report Series 916. [PubMed]
WHO 2006
- WHO. Guidelines on food fortification with micronutrients. The World Health Organization, 2006. [Google Scholar]
WHO 2011a
- WHO. Global status report on non communicable diseases 2010. Geneva: The World Health Organization, 2011. [Google Scholar]
WHO 2011b
- World Health Organization. Cardiovascular diseases (CVDs). Factsheet Number 317 Updated March 2013.
Woollard 2002
- Woollard KJ, Loryman CJ, Meredith E, Bevan R, Shaw JA, Lunec J, et al. Effects of oral vitamin C on monocyte: endothelial cell adhesion in healthy subjects. Biochemical and Biophysical Research Communications 2002;294:1161‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Flowers 2014
- Flowers N, Wheelhouse R, Stranges S, Rees K. Vitamin C supplementation for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD011114] [DOI] [PMC free article] [PubMed] [Google Scholar]


