Abstract
Background
The majority of people with hip fracture are treated surgically, requiring anaesthesia.
Objectives
The main focus of this review is the comparison of regional versus general anaesthesia for hip (proximal femoral) fracture repair in adults. We did not consider supplementary regional blocks in this review as they have been studied in another review.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library; 2014, Issue 3), MEDLINE (Ovid SP, 2003 to March 2014) and EMBASE (Ovid SP, 2003 to March 2014). We reran the search in February 2017. Potential new studies of interest were added to a list of "Studies awaiting Classification" and will be incorporated into the formal review findings during the review update.
Selection criteria
We included randomized trials comparing different methods of anaesthesia for hip fracture surgery in adults. The primary focus of this review was the comparison of regional anaesthesia versus general anaesthesia. The use of nerve blocks preoperatively or in conjunction with general anaesthesia is evaluated in another review. The main outcomes were mortality, pneumonia, myocardial infarction, cerebrovascular accident, acute confusional state, deep vein thrombosis and return of patient to their own home.
Data collection and analysis
Two reviewers independently assessed trial quality and extracted data. We analysed data with fixed‐effect (I2 < 25%) or random‐effects models. We assessed the quality of the evidence according to the criteria developed by the GRADE working group.
Main results
In total, we included 31 studies (with 3231 participants) in our review. Of those 31 studies, 28 (2976 participants) provided data for the meta‐analyses. For the 28 studies, 24 were used for the comparison of neuraxial block versus general anaesthesia. Based on 11 studies that included 2152 participants, we did not find a difference between the two anaesthetic techniques for mortality at one month: risk ratio (RR) 0.78, 95% confidence interval (CI) 0.57 to 1.06; I2 = 24% (fixed‐effect model). Based on six studies that included 761 participants, we did not find a difference in the risk of pneumonia: RR 0.77, 95% CI 0.45 to 1.31; I2 = 0%. Based on four studies that included 559 participants, we did not find a difference in the risk of myocardial infarction: RR 0.89, 95% CI 0.22 to 3.65; I2 = 0%. Based on six studies that included 729 participants, we did not find a difference in the risk of cerebrovascular accident: RR 1.48, 95% CI 0.46 to 4.83; I2 = 0%. Based on six studies that included 624 participants, we did not find a difference in the risk of acute confusional state: RR 0.85, 95% CI 0.51 to 1.40; I2 = 49%. Based on laboratory tests, the risk of deep vein thrombosis was decreased when no specific precautions or just early mobilization was used: RR 0.57, 95% CI 0.41 to 0.78; I2 = 0%; (number needed to treat for an additional beneficial outcome (NNTB) = 3, 95% CI 2 to 7, based on a basal risk of 76%) but not when low molecular weight heparin was administered: RR 0.98, 95% CI 0.52 to 1.84; I2 for heterogeneity between the two subgroups = 58%. For neuraxial blocks compared to general anaesthesia, we rated the quality of evidence as very low for mortality (at 0 to 30 days), pneumonia, myocardial infarction, cerebrovascular accident, acute confusional state, decreased rate of deep venous thrombosis in the absence of potent thromboprophylaxis, and return of patient to their own home. The number of studies comparing other anaesthetic techniques was limited.
Authors' conclusions
We did not find a difference between the two techniques, except for deep venous thrombosis in the absence of potent thromboprophylaxis. The studies included a wide variety of clinical practices. The number of participants included in the review is insufficient to eliminate a difference between the two techniques in the majority of outcomes studied. Therefore, large randomized trials reflecting actual clinical practice are required before drawing final conclusions.
Plain language summary
Regional or general anaesthesia for hip fracture surgery in adults
Background: The majority of people with hip fracture are elderly and are treated surgically, which requires anaesthesia. The fracture usually results from a simple fall. These patients often have many other medical problems associated with ageing, which places them at high risk of mortality after anaesthesia. The most common types of anaesthesia are 'general' and 'regional anaesthesia'. General anaesthesia involves a loss of consciousness (induced sleep). Regional anaesthesia involves an injection of a solution containing local anaesthetic inside the spine (neuraxial block) or around the nerves outside the spine (peripheral nerve block) to prevent pain in the leg with the hip fracture. We reviewed the evidence about the effect of regional anaesthesia on patients undergoing surgery for hip fracture.
Study characteristics: The evidence is current to March 2014. In total, we included 31 studies (with 3231 participants) in our review. Of those 31 studies, 28 (2976 participants) provided data for the meta‐analyses. The mean age of the participants varied from 75 to 86 years. Those studies were published between 1977 and 2013 and so covering a wide range of clinical practices and improvements in techniques over time. Two studies were funded by the anaesthetic drug manufacturer or by an agency with a commercial interest, one received charitable funding, and one was funded by a government agency. We reran the search in February 2017. Potential new studies of interest were added to a list of "Studies awaiting Classification" and will be incorporated into the formal review findings during the review update.
Key results : The trial reports of many of the studies indicated a sub‐suboptimal level of methodological rigour and the number of participants included was often insufficient to allow us to draw a definitive conclusion on many of the outcomes studied. We did not find any difference in mortality at one month (11 trials with 2152 participants) between neuraxial blocks and general anaesthesia. We also did not find a difference for pneumonia, myocardial infarction, cerebrovascular accident, acute confusional state, congestive heart failure, acute kidney injury, pulmonary embolism, number of patients transfused with red blood cells, length of surgery and length of hospital stay between these two anaesthetic techniques in two to twelve studies. Likewise, when potent prophylactic drugs (such as low molecular weight heparin) were used against postoperative clot formation, we did not find a difference in the risk of deep venous thrombosis. Without prophylaxis with potent anticoagulant drugs the risk of deep venous thrombosis was less with neuraxial block.
Quality of the evidence: The level of evidence was very low for mortality, pneumonia, myocardial infarction, cerebrovascular accident, acute confusional state, decrease in the incidence of deep venous thrombosis in the absence of potent prophylaxis, and return of patient to their own home. This means that any estimate of effect is very uncertain.
Summary of findings
Summary of findings for the main comparison. Neuraxial block compared to general anaesthesia for hip fracture repair.
| Neuraxial block compared to general anaesthesia for hip fracture repair | ||||||
| Patient or population: Patients with hip fracture repair Settings: Surgery Intervention: Neuraxial block Comparison: General anaesthesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| General anaesthesia | Neuraxial block | |||||
| Mortality Follow‐up: 0‐1 month | Study population | RR 0.78 (0.57 to 1.06) | 2152 (11 studies) | ⊕⊝⊝⊝ very low1,2,3,4,5,6,7,8 | ||
| 80 per 1000 | 62 per 1000 (46 to 85) | |||||
| Low | ||||||
| 35 per 1000 | 27 per 1000 (20 to 37) | |||||
| High | ||||||
| 95 per 1000 | 74 per 1000 (54 to 101) | |||||
| Pneumonia Follow‐up: 0‐7 days | Study population | RR 0.77 (0.45 to 1.31) | 761 (6 studies) | ⊕⊝⊝⊝ very low1,2,3,4,6,7,9,10 | ||
| 64 per 1000 | 50 per 1000 (29 to 84) | |||||
| Low | ||||||
| 30 per 1000 | 23 per 1000 (13 to 39) | |||||
| High | ||||||
| 80 per 1000 | 62 per 1000 (36 to 105) | |||||
| Myocardial infarction Follow‐up: 0‐7 days | Study population | RR 0.89 (0.22 to 3.65) | 559 (4 studies) | ⊕⊝⊝⊝ very low1,2,3,4,6,7,9,10 | ||
| 10 per 1000 | 9 per 1000 (2 to 38) | |||||
| Low | ||||||
| 5 per 1000 | 4 per 1000 (1 to 18) | |||||
| High | ||||||
| 50 per 1000 | 44 per 1000 (11 to 183) | |||||
| Cerebrovascular accident Follow‐up: 0‐7 days | Study population | RR 1.48 (0.46 to 4.83) | 729 (6 studies) | ⊕⊝⊝⊝ very low1,2,3,4,6,7,9,10 | ||
| 8 per 1000 | 12 per 1000 (4 to 38) | |||||
| Low | ||||||
| 10 per 1000 | 15 per 1000 (5 to 48) | |||||
| High | ||||||
| 50 per 1000 | 74 per 1000 (23 to 241) | |||||
| Acute confusional state Follow‐up: 0‐7 days | Study population | RR 0.85 (0.51 to 1.40) | 624 (6 studies) | ⊕⊝⊝⊝ very low1,3,4,6,7,9,10,11 | ||
| 177 per 1000 | 150 per 1000 (90 to 247) | |||||
| Low | ||||||
| 50 per 1000 | 42 per 1000 (25 to 70) | |||||
| High | ||||||
| 250 per 1000 | 212 per 1000 (127 to 350) | |||||
| Deep vein thrombosis Follow‐up: 0‐10 days | Study population | RR 0.57 (0.41 to 0.78) | 116 (2 studies) | ⊕⊝⊝⊝ very low1,2,4,7,9,10,12,13 | For this outcome, we retained only studies without adequate prophylaxis | |
| 780 per 1000 | 444 per 1000 (320 to 608) | |||||
| Low | ||||||
| 200 per 1000 | 114 per 1000 (82 to 156) | |||||
| High | ||||||
| 900 per 1000 | 513 per 1000 (369 to 702) | |||||
| Return of patient to their own home Follow‐up: 1 year | Study population | RR 0.84 (0.61 to 1.16) | 130 (1 study) | ⊕⊝⊝⊝ very low1,3,4,6,7,10,14 | ||
| 578 per 1000 | 486 per 1000 (353 to 671) | |||||
| Low | ||||||
| 400 per 1000 | 336 per 1000 (244 to 464) | |||||
| High | ||||||
| 800 per 1000 | 672 per 1000 (488 to 928) | |||||
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Allocation concealment and/or blinding of outcome assessors unclear/inadequate in 75% or more of the included studies. 2 I2 smaller than 25%. 3 Direct comparison only: all outcomes were based on direct comparisons, were performed on the population of interest, and were not surrogate markers. 4 Optimal information size not achieved. 5 Correcting for the possibility of publication bias would change the conclusion. 6 RR greater than 0.5 and less than 2.0. 7 We did not identify any confounding factors that could change the effect. 8 We upgraded the quality on absence of effect because the effect seemed to be present only in older studies. 9 Either no evidence of a publication bias or correcting for the possibility of publication bias would not modify the conclusion. 10 No evidence of a dose response effect. 11 I2 statistics close to 50%. 12 This is a surrogate marker for a clinical outcome: systematic venography instead of clinically relevant event. 13 RR 0.57, excluding studies where heparin was given as prophylaxis. 14 Not available due to low number of studies.
Background
Description of the condition
The term 'proximal femoral fracture', or 'hip fracture', refers to a fracture of the femur in the area of bone immediately distal to the articular cartilage of the hip, to a level of about five centimetres below the lower border of the lesser trochanter. The majority of these fractures occur in the elderly population, and more than 30% of the patients are 85 years or older (Brauer 2009). In the United States, while the age‐adjusted incidence of hip fracture increased from 1986 to 1995, a steady decline from 1995 to 2005 has been reported. In women, the incidence increased by 9.0% in 1995 compared to 1986, with a subsequent decline of 24.5% in 2005. In men, the increase in incidence from 1986 to 1995 was 16.4%, and the subsequent decrease to 2005 was 19.2% (Brauer 2009). Despite this improvement, hip fractures in the elderly are still quite common, and this improvement may not apply to developing countries. Between 1986 and 2005, in the United States, the annual mean number of hip fractures was 957.3 per 100,000 (95% confidence interval (CI), 921.7 to 992.9) for women and 414.4 per 100,000 (95% CI, 401.6 to 427.3) for men (Brauer 2009). The injury is usually the result of a simple fall.
The majority of these fractures are treated surgically; thus hip fracture surgery represents one of the most common emergency orthopaedic procedures. Surgical treatment may be either fixation of the fracture or replacement of the femoral head with an arthroplasty. Internal fixation involves using screws or pins, either alone, or in combination with a side plate applied to the femur, or by the use of an intramedullary nail with a cross screw inserted into the femoral head. Arthroplasty involves excision of the fractured area of bone and replacement with a partial or total hip replacement, which may be cemented in place.
Description of the intervention
The term 'regional anaesthesia' may include neuraxial blocks or peripheral nerve blocks. Neuraxial blockade refers to placement of a solution containing local anaesthetics close to the spinal cord. Neuraxial blocks may be executed by spinal or epidural or combined spinal/epidural blockade. For a spinal block, the solution containing the local anaesthetics is placed in the cerebrospinal fluid. For an epidural block, the solution containing the local anaesthetic is placed in the epidural space, outside the dura matter (membrane surrounding the cord and the cerebrospinal fluid). Peripheral nerve blocks refer to placement of local anaesthetics around peripheral nerves or plexus outside the spine. Peripheral nerve blocks for this indication are usually performed by a posterior lumbar plexus block (psoas compartment block), with or without additional blocks (sacral plexus block, iliac crest infiltration). For posterior lumbar plexus blocks and for sacral plexus blocks, the local anaesthetics are placed around the roots of the nerves, immediately after their exit from the spine at the lumbar level (posterior lumbar plexus block) or at the sacral level (sacral plexus block). An iliac crest infiltration is the deposition of local anaesthetics under the skin above the iliac crest along its superior border. Neuraxial or peripheral nerve blocks may be performed by a single shot injection or by incremental doses, with or without a continuous infusion thereafter. When a patient is having an operation under regional anaesthesia alone, he/she remains conscious, but insensitive to pain. The muscle relaxation obtained is also usually sufficient to allow the surgeon to repair the fracture. General anaesthesia refers to the use of a variety of intravenous and or inhalation drugs to render the patient unconscious, amnesic of the procedure and insensitive to pain. Neuromuscular blocking agents are often added for a hip fracture repair under general anaesthesia.
How the intervention might work
Whilst the hip fracture is usually the only injury, the patients frequently have many other medical problems associated with ageing. Indeed, an increase in all comorbidities (except paralysis) in patients with hip fracture was recorded from 1986 to 2005 (Brauer 2009). Advanced age and frequent multiple associated comorbidities put these patients at high risk of mortality after anaesthesia. The 10‐year probability of survival of patients with comorbidities (American Society of Anesthesiologists) physical status (ASA) III or IV undergoing major surgery is lower than that of patients without significant comorbidities (ASA I or II) (Kennedy 2010). A recent Cochrane Overview, found that the 0 to 30‐day mortality of patients undergoing high or moderate cardiac risk procedures is lower in patients having an operation under neuraxial blocks compared to those having an operation under general anaesthesia: risk ratio (RR) 0.71, 95% CI 0.53 to 0.94; moderate quality of evidence (Guay 2014). Major orthopaedic surgeries such as hip fracture repairs are considered moderate cardiac risk procedures (Fleisher 2007).
Why it is important to do this review
In a previous version of this review, we concluded that regional anaesthesia was associated with a borderline decreased mortality at one month: RR 0.69, 95% CI 0.50 to 0.95 (Parker 2004). This finding however, is not corroborated with a recent large retrospective study, where the authors concluded that the use of regional anaesthesia compared with general anaesthesia was not associated with lower 30‐day mortality, but only with a modestly shorter length of hospital stay (Neuman 2014).
This review is an update of previous versions (Parker 2001; Parker 2004; Urwin 2000). We undertook this update to search for new studies and adjust the methodology to the latest Cochrane requirements.
Objectives
The main focus of this review is the comparison of regional versus general anaesthesia for hip (proximal femoral) fracture repair in adults. The scope of this review, originally published in 2000 (Urwin 2000), was expanded in the second update (Parker 2001) to also cover other methods of anaesthesia. We did not consider supplementary regional blocks in this review as they have been studied in another review (Parker 2002).
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs). We excluded cluster and cross‐over trials. We included all RCTs regardless of language of publication or publication status.
Types of participants
We considered studies that included participants ≥ 16 years old undergoing hip fracture surgery (an emergency surgery).
Types of interventions
We included studies that compared any combination of the following interventions.
Neuraxial blocks: epidural (single shots or continuous), spinals (single shots or continuous), or combined spinal/epidural (single shots or continuous), with or without intravenous sedation.
Peripheral nerve blocks: posterior lumbar (psoas) plexus blocks, with or without sacral plexus blocks, or any other peripheral nerve blocks, with or without sedation.
General anaesthesia based on inhalational agents (with or without opioids and/or neuromuscular blocking agents), or on total intravenous anaesthesia (ketamine‐based technique or other). Any technique where an endotracheal tube or a laryngeal mask airway was used was considered as general anaesthesia.
Types of outcome measures
Primary outcomes
Mortality from any cause at 30 days, three months, six months, and one year (cumulative).
Pneumonia (author's definition).
Myocardial infarction (author's definition).
Secondary outcomes
Cerebrovascular accident (author's definition).
Acute confusional state (author's definition).
Deep vein thrombosis.
Return of patient to their own home.
Congestive cardiac failure (author's definition).
Acute kidney injury (author's definition).
Pulmonary embolism.
Unsatisfactory surgical results.
Number of patients transfused.
Length of hospital stay.
Length of surgery (in minutes).
Operative hypotension (author's definition).
Urine retention.
Incomplete or unsatisfactory analgesia.
Please see Table 2 for the definitions and time points of these outcomes.
1. Outcomes: Definitions and time points.
| Outcome | Study | Definition | Time point |
| Pneumonia | Berggren 1987 | "treated for" | "during the postoperative period" |
| Bigler 1985 | Unspecified | "postoperatively" | |
| Davis 1981 | Chest X‐Ray in clinical suspicion | Up to four weeks | |
| Heidari 2011 | "diagnosed by the consultant specialist" | "postoperative" | |
| McLaren 1978 | "The clinical criteria adopted as indicating respiratory problems were productive cough, the presence of rhonchi or crepitations on auscultation or abnormalities on chest X‐ray." However the criteria adopted for the diagnosis of a pneumonia ("respiratory infection") are not clearly mentioned | Up to four weeks | |
| Racle 1986 | "clinical and radiological criteria" | "in hospital" | |
| White 1980 | Unspecified | Within four weeks | |
| Myocardial infarction | Biboulet 2012 | EKG and troponin measurement daily for three days, no definition provided | Within one month |
| Couderc 1977 | Serial preprogrammed EKGs up to postoperative day 10 interpreted by a blinded cardiologist. Q wave |
In hospital | |
| Heidari 2011 | "diagnosed by the consultant specialist" | "postoperative" | |
| Juelsgaard 1998 | World Health Organization criteria applied by a blinded investigator | Within one month | |
| Congestive cardiac failure | Berggren 1987 | "treated for" | "during the postoperative period" |
| Biboulet 2012 | "acute heart failure" | Within one month | |
| Bigler 1985 | "cardiac decompensation" | "postoperatively" | |
| Davis 1981 | "life‐threatening complications" "congestive heart failure" | Within four weeks | |
| Heidari 2011 | "diagnosed by the consultant specialist" | "postoperative" | |
| Racle 1986 | "episode of congestive heart failure" | In hospital | |
| Cerebrovascular accident | Berggren 1987 | "stroke" | All patients developed their stroke on postoperative day one |
| Biboulet 2012 | "stroke" | Within one month | |
| Bigler 1985 | "Neurological sequelae", apoplexy for the sole event reported | "postoperatively" | |
| Davis 1981 | "cerebrovascular accident" | Within four weeks | |
| Heidari 2011 | "cerebrovascular accident" "diagnosed by the consultant specialist" | "postoperative" | |
| Racle 1986 | "cerebrovascular accident" | In hospital | |
| Acute confusional state | Berggren 1987 | Diagnostic and Statistical Manual of Mental Disorders (DSM‐Ill) as criteria for acute confusional state | Within seven days (the period 0‐8 hours after the surgery was excluded) |
| Bigler 1985 | "Mental confusion" | "postoperatively" | |
| Casati 2003 | Mini Mental State Examination test decreased 2 points from baseline | Within seven days | |
| de Visme 2000 | Mini Mental Status Examination lower than 5 | Between the third and fifth postoperative day | |
| Heidari 2011 | Cognitive dysfunction based on time, person, and place disorientation | Up to postoperative day two | |
| Kamitani 2003 | Delirium was judged by floor nurse, using the Inoue's confusion assessment method diagnosis algorithm | Up to postoperative day four | |
| Racle 1986 | Confusion with agitation | In hospital | |
| White 1980 | Unspecified | Within four weeks | |
| Renal failure (or acute kidney injury) | Davis 1981 | "acute renal failure" | Within four weeks |
| Racle 1986 | Blood creatinine > 135 micromol/Liter | In hospital | |
| Deep vein thrombosis | Brichant 1995 | Systematic bilateral contrast venography | Postoperative day ten |
| Davis 1981 | 125‐iodine fibrinogen uptake test performed daily for seven days | Within seven days | |
| Heidari 2011 | "deep veins thrombosis" "diagnosed by the consultant specialist" | "postoperative" | |
| Ibanez 1993 | "thrombosis" | Within seven days | |
| McKenzie 1984 | Systematic venography | Between postoperative day seven and ten | |
| White 1980 | "deep vein thrombosis" | Within four weeks | |
| Pulmonary embolism | Berggren 1987 | "pulmonary embolism" | "postoperatively" |
| Bigler 1985 | "pulmonary embolus" | "postoperatively" | |
| Brichant 1995 | Pulmonary venous angiogram or ventilation‐perfusion lung scanning on clinical suspicion | Unclear | |
| Heidari 2011 | "pulmonary emboli" "diagnosed by the consultant specialist" | "postoperatively" | |
| Racle 1986 | Clinical suspicion confirmed with angiography | In hospital | |
| Unsatisfactory surgical results | Spreadbury 1980 | Either an unstable fixation of the fracture by nail and plate or the dislocation of a prosthesis, which required bedrest on traction and prevented early mobilization | In hospital |
| Operative hypotension | Berggren 1987 | Decrease > 30% from baseline for systolic arterial blood pressure | Intraoperative |
| Biffoli 1998 | Decrease of 30% from baseline for arterial blood pressure | Intraoperative | |
| Brown 1994 | Requiring the administration of a sympathomimetic | Intraoperative | |
| Casati 2003 | Decrease in systolic arterial pressure 20% from baseline | Intraoperative | |
| Couderc 1977 | Decrease of 40 mmHG in systolic arterial blood pressure | Intraoperative | |
| Davis 1987 | Decrease in systolic arterial blood pressure > 20% from baseline | Intraoperative | |
| Eyrolle 1998 | Decrease in mean arterial blood pressure > 20% from baseline | Intraoperative | |
| Juelsgaard 1998 | Decrease in systolic arterial blood pressure > 33% from baseline | Intraoperative | |
| Maurette 1988 | Decrease in mean arterial blood pressure > 20% from baseline | Intraoperative | |
| McLaren 1978 | Decrease in systolic arterial blood pressure > 50% from baseline | Intraoperative | |
| Messina 2013 | Decrease in mean arterial blood pressure of 25% from baseline | Intraoperative | |
| Racle 1986 | Decrease in systolic arterial blood pressure of 20% from baseline | Intraoperative | |
| Svarting 1986 | Decrease in systolic arterial blood pressure > 30% from baseline | Intraoperative | |
| Urine retention | Berggren 1987 | "urinary retention" | "postoperative" |
| Cao 2008 | "that required catheterization" | In hospital |
EKG: electrocardiogram
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library; 2014, Issue 3, see Appendix 1), MEDLINE (Ovid SP, 2003 to March 2014, see Appendix 2) and EMBASE (Ovid SP, 2003 to March 2014, see Appendix 3). We also screened the reference lists of all studies retained. We reran the search in February 2017. We will deal with the two studies of interest when we update the review.’
We imposed no language restriction. We considered articles of all languages and translated them if necessary
Searching other resources
We looked at: http://www.clinicaltrials.gov, http://isrctn.org, http://www.umin.ac.jp/ctr/index.htm, http://www.anzctr.org.au/, http://www.trialregister.nl/, and https://eudract.ema.europa.eu/ for trials in progress in 2014.
Data collection and analysis
Selection of studies
Two review authors (JG and SK) independently screened abstract/titles. We retrieved all potentially relevant studies.We excluded duplicate publications based on the sites and dates of data collection. We noted reasons for exclusion. We resolved disagreements by discussion; involvement of a third review author was never required. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (see Figure 1; Moher 2009), and 'Characteristics of excluded studies' table.’
1.
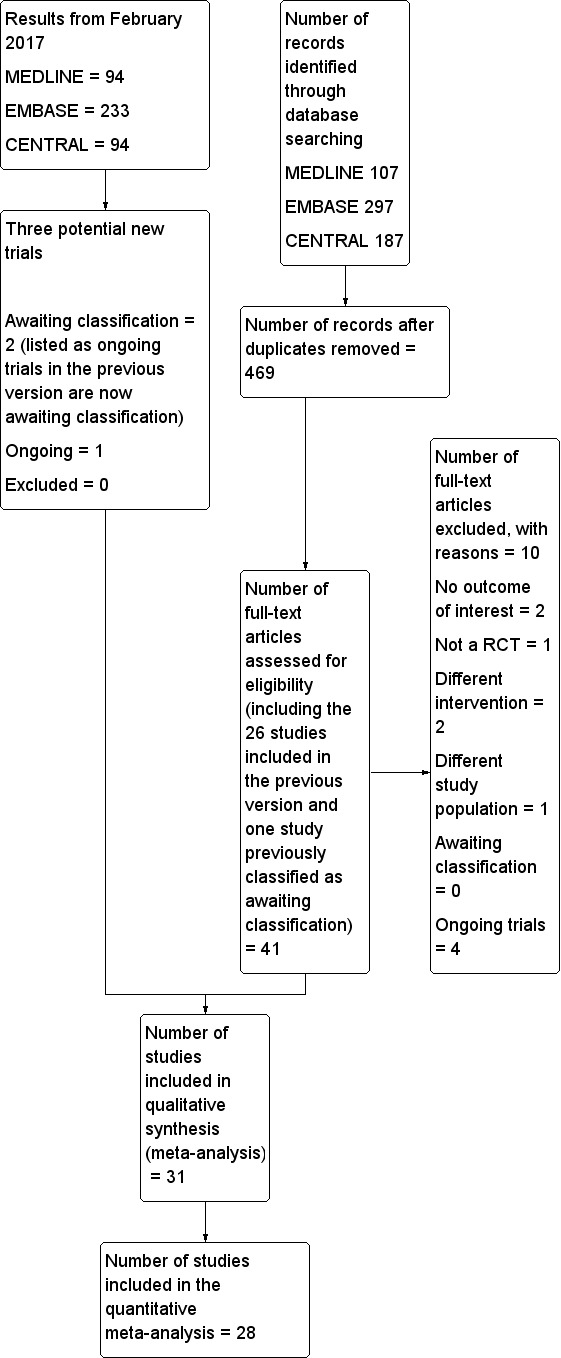
Study flow diagram.
Data extraction and management
When the study was already included in the previous version, one review author (JG) rechecked all entries from the manuscripts. For the five new studies (Biboulet 2012; Cao 2008; Heidari 2011; Hoppenstein 2005; Messina 2013), two review authors independently extracted the data for our selected outcomes. We entered the number of participants with events and total number of patients included in each treatment group in an Excel sheet for mortality from any cause at 30 days, three months, six months, and one year (cumulative), pneumonia, myocardial infarction, congestive heart failure, cerebrovascular accidents, acute confusional state, acute kidney injury, deep venous thrombosis, pulmonary embolism, return of patient to their own home, unsatisfactory surgical results, number of patients transfused, operative hypotension and urine retention. For continuous data (length of surgery and of hospital stay) we entered mean, standard deviation and total number of participants in each group, or P values and number of participants in each group when the former were not available.
We resolved all disagreements by discussion, involvement of a third review author was never required. One review author (JG) also entered in Comprehensive Meta‐Analysis software the information required for heterogeneity exploration (http://www.meta‐analysis.com): year when the study was published, mean age of participants, percentage of participants undergoing arthroplasty, cut‐off point for operative hypotension definition, preinduction administration of fluids, regional anaesthetic technique in the treatment group (type of block [spinal versus epidural versus peripheral nerve block], single shot versus continuous technique, uni‐ versus bilateral spinal), inhalational agent used in the control group, mean ASA physical status, delay before the surgery, thromboprophylaxis, and use of neuromuscular blocking agent in the control group.
Assessment of risk of bias in included studies
Two review authors (JG and SK) independently assessed the quality of the studies with the Cochrane 'Risk of bias' tool (Higgins 2011). We assessed the risk of bias based on the information presented in the reports, with no assumptions: low risk, high risk or unclear risk of bias. When there was not enough information in the report to make an assessment, we judged the item as high risk for blinding (blinding of participants and personnel and blinding of outcome assessment) and as unclear for all other items. We resolved any disagreements by discussion; involvement of a third review author was never required.
Measures of treatment effect
Data are expressed as risk ratios (RRs) (dichotomous), mean difference (MDs) (continuous data) or standardized mean difference (SMDs) and their 95% CI.
Unit of analysis issues
We included only parallel RCTs. When the study contained more than two groups, in order to avoid including duplicate data, we either selected only the groups relevant to the review, or fused two subgroups, or split the control group in half. The choice between the two latter options was made according to the solution that best fitted our criteria for heterogeneity exploration.
Dealing with missing data
We only analysed the available data; we made no imputation.
Assessment of heterogeneity
We measured statistical heterogeneity using the I2 statistic.
Assessment of reporting biases
We judged a study to have used selective reporting when data were collected as stated in the methods section, but not reported in the results section. We mentioned data that were provided as per the protocol (not on an intention‐to‐treat basis) as other risk of bias.
Data synthesis
We analysed data with Comprehensive Meta‐analysis software (http://www.meta‐analysis.com). In addition, we used Review Manager 5 with fixed‐effect (I2 < 25%; Higgins 2003) or random‐effects models (RevMan 2014). We expressed data as RRs (dichotomous), MDs (continuous data) or SMDs and their 95% CI. When we found an effect, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) from the odds ratio (http://www.nntonline.net/visualrx/). When we did not find any effect, we calculated the optimal information size according to Pogue 1997 from http://stat.ubc.ca/˜rollin/stats/ssize/b2.html and power with Power and Precision V3.2 for our major outcomes (http://power‐analysis.com/).
Subgroup analysis and investigation of heterogeneity
We explored any moderate amount of heterogeneity (I2 > 25%; Higgins 2003) by visual inspection of the forest plots, with studies placed in order according to a specific moderator, subgroupings (categorical moderators) or meta‐regressions (continuous moderators). We considered the following factors in the heterogeneity exploration: year when the study was published; fixation versus joint replacement; single shot versus incremental dose for neuraxial block; uni‐ versus bilateral spinal; inhalational agents or not (type); neuromuscular blocking agents or not; ASA physical status; mean age of participants; administration of an intravenous bolus fluid before the neuraxial block; and thromboprophylaxis. We used the Egger's regression intercept to assess the possibility of a small‐study effect (Rucker 2011).
Sensitivity analysis
A sensitivity analysis could be performed when the results of one single study appeared as an outlier on the forest plot or on the basis of the 'Risk of bias' assessment.
Grading the body of evidence
We judged the quality of the body of evidence using GRADEProGDT (http://ims.cochrane.org/revman/gradepro) (Guyatt 2011a), and presented in a 'Summary of findings' table each major outcome: mortality at one month, pneumonia, myocardial infarction, cerebrovascular accident, acute confusional state, deep venous thrombosis, and return of patient to their own home. For risk of bias, we judged the quality of evidence as low risk of bias when most information came from studies at low risk of bias, we downgraded by one level when most information came from studies at low or unclear risk of bias and downgraded by two levels when the proportion of information from studies at high risk of bias was sufficient to affect the interpretation of results.
For inconsistency, we downgraded the quality of evidence by one when the I2 statistic was 50% or higher without satisfactory explanation and by two levels when the I2 statistic was 75% or higher without an explanation. We did not downgrade the quality of evidence for indirectness as all outcomes were based on direct comparisons, were performed on the population at interest and were not surrogate markers (Guyatt 2011b), except for deep venous thrombosis. In the included studies, the latter was evaluated by systematic venographies performed within 10 days after surgery. This was considered as a surrogate marker for clinically relevant events.
For imprecision (Guyatt 2011c), we downgraded the quality of evidence by one when: the CI around the effect size was large or overlapped an absence of effect, and failed to exclude an important benefit or harm; and the number of participants was lower than the optimal information size. We downgraded the quality by two levels when the CI was very wide and included both appreciable benefit and harm.
For publication bias, we downgraded the quality of evidence by one when correcting for the possibility of publication as assessed by the Duval and Tweedie’s fill and trim analysis changed the conclusion. We upgraded the quality of evidence by one when the effect size was large (< 0.5 or > 2.0) and by two when the effect size was very large (RR < 0.2 or > 5) (Guyatt 2011d). We applied the same rules for OR when the basal risk was lower than 20%. For SMD, we used 0.8 as the cut‐off point for a large effect (Pace 2011). We also upgraded the quality by one when evidence of a dose related response was found.
We upgraded the quality by one when the possible effect of confounding factors would reduce a demonstrated effect or suggest a spurious effect when results showed no effect. When the quality of the body of evidence is high quality, further research is very unlikely to change our confidence in the estimate of effect. When the quality is moderate, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. When the quality is low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. When the quality is very low, any estimate of effect is very uncertain (Guyatt 2008).
Results
Description of studies
Results of the search
The flow diagram of the study selection is included in Figure 1 (Moher 2009). From the previous version, we reassessed 26 included studies and one study awaiting classification. We also assessed 14 new studies from the electronic search or from the reference lists of studies. We excluded ten studies for the following reasons: no outcome of interest for this review (Darling 1994; Messaoudi 2009), quasi‐randomized trial (Adams 1990), different intervention (Ungemach 1987; Yao 1997), different study population (Lattermann 2005), and ongoing trials (ISRCTN36381516; NCT00590707; NCT02190903; NCT02213380). We reran the search in February 2017. The search terms were slightly modified from those of the previous searches. The modified strategy used for 2014 and onwards can be found in Appendix 4. Three hundred and eighty‐two new citations were found: 55 in CENTRAL, 233 in Embase and 94 in Medline.
Included studies
In total, we included 31 studies (with 3231 participants) in our review. Of those 31 studies, 28 (2976 participants) provided data for the meta‐analyses. The reasons for not including three studies in the meta‐analysis were: the time point at which mortality was measured was unclear (Tasker 1983); the time point did not correspond to the time points chosen for this review (mortality data provided at two weeks only; Ungemach 1993); and results provided for our study population were mental tests (Wajima 1995). Therefore, we included 28 studies with 2976 participants published between 1977 and 2013 for the quantitative meta‐analysis.
Two studies were funded by the drug manufacturer or by an agency with a commercial interest (Davis 1987; Valentin 1986), one received charitable funding (Davis 1981), and one was funded by a government agency (Berggren 1987). The source of funding was unspecified for the other studies. The mean age of the participants varied from 74.8 to 86 years. Their mean ASA physical status varied from 2.0 to 3.3. The mean delay before the surgery varied from 24 to 240 hours. The percentage of participants undergoing arthroplasty varied from 0% to 100%. The thromboprophylaxis used was: early mobilization (Davis 1981); socks (Valentin 1986); dextran (Berggren 1987); antivitamine K drugs (Couderc 1977); unfractionated heparin (Heidari 2011; Racle 1986); or low molecular heparin (Brichant 1995). The neuraxial blocks used were: spinal (Biboulet 2012; Biffoli 1998; Bigler 1985; Bredahl 1991; Casati 2003; Davis 1981; Davis 1987; de Visme 2000; Eyrolle 1998; Hoppenstein 2005; Ibanez 1993; Juelsgaard 1998; Kamitani 2003; Maurette 1988; McKenzie 1984; McLaren 1978; Messina 2013; Racle 1986; Svarting 1986; Valentin 1986; White 1980); epidural (Berggren 1987; Cao 2008; Couderc 1977; Wajima 1995); or any of these two techniques (Brichant 1995; Heidari 2011). Inhalational agents used for general anaesthesia were: nitrous oxide alone (Bigler 1985; Bredahl 1991; Davis 1981; Davis 1987; McLaren 1978; Spreadbury 1980; Svarting 1986); methoxyflurane (Couderc 1977); halothane (Berggren 1987; Heidari 2011; McKenzie 1984; White 1980); enflurane (Juelsgaard 1998; Maurette 1988; Racle 1986); enflurane or nitrous oxide (Valentin 1986); isoflurane (Biffoli 1998; Hoppenstein 2005); or sevoflurane (Casati 2003; Kamitani 2003; Messina 2013; Wajima 1995).
Table 3 contains the anaesthetic agents used to produce general anaesthesia or sedation. Twenty‐four studies were involved in the comparison neuraxial block versus general anaesthesia (Berggren 1987; Biboulet 2012; Biffoli 1998; Bigler 1985; Bredahl 1991; Brichant 1995; Cao 2008; Casati 2003; Couderc 1977; Davis 1981; Davis 1987; Heidari 2011; Hoppenstein 2005; Ibanez 1993; Juelsgaard 1998; Kamitani 2003; Maurette 1988; McKenzie 1984; McLaren 1978; Messina 2013; Racle 1986; Svarting 1986; Valentin 1986; Wajima 1995). Three studies were involved in the comparison neuraxial block versus peripheral nerve block (Cao 2008; de Visme 2000; Eyrolle 1998). One study compared a neuraxial block added to general anaesthesia versus general anaesthesia alone (White 1980). The same study was also used for a comparison between peripheral nerve block added to general anaesthesia compared to general anaesthesia alone (White 1980), and one study compared intravenous ketamine to classic general anaesthesia (Spreadbury 1980).
2. Anaesthetic agents for sedation or to produce general anaesthesia.
| Study | Sedative drugs for participants of the regional blockade group | Anaesthetic agents for general anaesthesia |
| Berggren 1987 | Premedication: Meperidine No other sedative drugs mentioned as routinely administered for the surgery. |
Premedication: Meperidine Induction: Thiopental and atropine Maintainance: Nitrous oxide, halothane and succinylcholine infusion |
| Biboulet 2012 | None mentioned | Subgroup 1 Induction: Propofol Maintainance: Propofol infusion (for a bispectral index value of 50) and remifentanil Subgroup 2 Induction: Sevoflurane Maintainance: Sevoflurane (for a bispectral index value of 50) and remifentanil |
| Biffoli 1998 | None mentioned | Induction: Propofol Maintainance: Nitrous oxide, isoflurane, fentanyl (intermittent injections) plus an atracurium infusion |
| Bigler 1985 | Premedication: Pethidine Small amounts of diazepam if needed |
Premedication: Pethidine Induction: Diazepam and atropine Maintainance: Nitrous oxide, fentanyl and pancuronium |
| Bredahl 1991 | Premedication: Pethidine Diazepam for mild sedation |
Premedication: Pethidine Induction: Thiopental Maintainance: Nitrous oxide, thiopental and pethidine |
| Brichant 1995 | Not mentioned | According to local practice |
| Brown 1994 | Premedication: Tamazepam or pethidine No drug supplementation during the surgery |
Premedication: Tamazepam or pethidine Induction: Thiopental or propofol Maintainance: Nitrous oxide, isoflurane or enflurane and atracurium 0.5 mg/kg one dose |
| Cao 2008 | Midazolam and fentanyl if required | |
| Casati 2003 | One dose of fentanyl before the block No other sedative drug routinely administered for the surgery. |
Induction: Sevoflurane Maintainance: Nitrous oxide, sevoflurane |
| Couderc 1977 | Premedication: Hydroxyzine and atropine None mentioned for the surgery |
Premedication: Hydroxyzine and atropine Induction: Thiopental Maintainance: Nitrous oxide plus 1) Thiopental and dextromoramide or 2) methoxyflurane. One dose of pancuronium in some participants |
| Davis 1981 | Ketamine 20‐25 mg (for eight participants) before the spinal Ketamine at unspecified total doses during the surgery (two participants) or 25 mg at skin closure (two participants) Diazepam (mean dose 9 mg; range 0‐35 mg) |
Induction: Diazepam (mean dose 9.5 mg; range 2.5 to 30 mg) Maintainance: Nitrous oxide, fentanyl and pancuronium |
| Davis 1987 | Benzodiazepine (optional) | Induction: Thiopental Maintainance: Nitrous oxide, fentanyl and non‐depolarizing neuromuscular blocking agent |
| de Visme 2000 | Alfentanil before the block and as required during the surgery. No mandatory sedative drugs mentioned. One patient in the continuous peripheral nerve block received "sedation repeatedly". | |
| Eyrolle 1998 | Propofol as required | |
| Heidari 2011 | None mentioned | Induction: Thiopental Maintainance: Nitrous oxide, fentanyl, halothane and one dose of pancuronium |
| Hoppenstein 2005 | None mentioned | Induction: Thiopental Maintainance: Nitrous oxide, isoflurane, fentanyl and vecuronium |
| Ibanez 1993 | Not reported | Not reported |
| Juelsgaard 1998 | Premedication: Pethidine None mentioned for the surgery |
Premedication: Pethidine Induction: Thiopental Maintainance: Nitrous oxide, enflurane, fentanyl and one dose of atracurium |
| Kamitani 2003 | No sedative drug | Induction: Propofol Maintainance: Nitrous oxide, fentanyl, sevoflurane and one dose of vecuronium |
| Maurette 1988 | None mentioned | Induction: Thiopental Maintainance: Nitrous oxide, dextromoramide and enflurane |
| McKenzie 1984 | Small doses of diazepam | Induction: Althesin Maintainance: Nitrous oxide and halothane |
| McLaren 1978 | Althesin and nitrous oxide; arousable by ear lobe pressure | Induction: Althesin Maintainance: Nitrous oxide, fentanyl and pancuronium one dose |
| Messina 2013 | None mentioned | Induction: Propofol Maintainance: Sevoflurane, remifentanil and one dose of cisatracurium |
| Racle 1986 | Premedication: Hydroxyzine and atropine Flunitrazepam, verbal contact possible |
Premedication: Hydroxyzine and atropine Induction: Thiopental Maintainance: Nitrous oxide, enflurane, fentanyl and one dose of vecuronium |
| Spreadbury 1980 | No group with regional anaesthesia alone | Ketamine group Induction and maintenance: Ketamine and diazepam (2.5 to 10 mg) Relaxant group Technique at the discretion of the attending anaesthesiologist |
| Svarting 1986 | Premedication: Pethidine and atropine None mentioned for the surgery |
Premedication: Pethidine and atropine Induction: Thiopental Maintainance: Nitrous oxide, fentanyl (repeated injections) and one dose of pancuronium |
| Tasker 1983 | Not reported | Not reported |
| Ungemach 1993 | Not reported | Induction: Not reported Maintainance: Nitrous oxide, isoflurane and fentanyl |
| Valentin 1986 | Premedication: Pethidine and promethazine in some of the participants Small doses of diazepam and fentanyl |
Premedication: Pethidine and promethazine in some of the participants Subgroup 1 Induction: Thiopental or not Maintainance: Nitrous oxide, enflurane and gallamine (not all participants) Subgroup 2 Induction: Not clearly mentioned Maintainance: Nitrous oxide, droperidol, fentanyl and gallamine |
| Wajima 1995 | None mentioned | Induction: Thiopental Maintainance: Nitrous oxide and sevoflurane |
| White 1980 | Premedication: Diazepam Althesin, nitrous oxide and fentanyl (spontaneous breathing) for the two subgroups |
Premedication: Diazepam Induction: Thiopental Maintainance: Nitrous oxide, halothane and fentanyl |
Excluded studies
We excluded 10 studies (see Characteristics of excluded studies for the reasons for their exclusion).
Awaiting classification
There are two studies awaiting classifications (Neuman 2016; Parker 2015). For further details see Characteristics of studies awaiting classification.
Ongoing studies
We found four ongoing trials (ISRCTN36381516; NCT00590707; NCT02190903; NCT02213380; see Characteristics of ongoing studies) in the 2014 search. When the search was reran in February 2017, two of the ongoing trials were now published and are classified as awaiting classification (ISRCTN36381516; NCT02190903). A new ongoing trial (NCT02507505) was found.
Risk of bias in included studies
The risk of bias of included studies can be found in Figure 2 and Figure 3.
2.
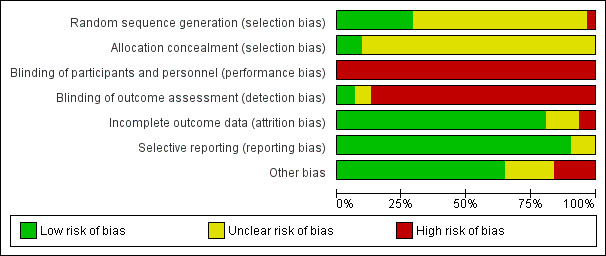
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
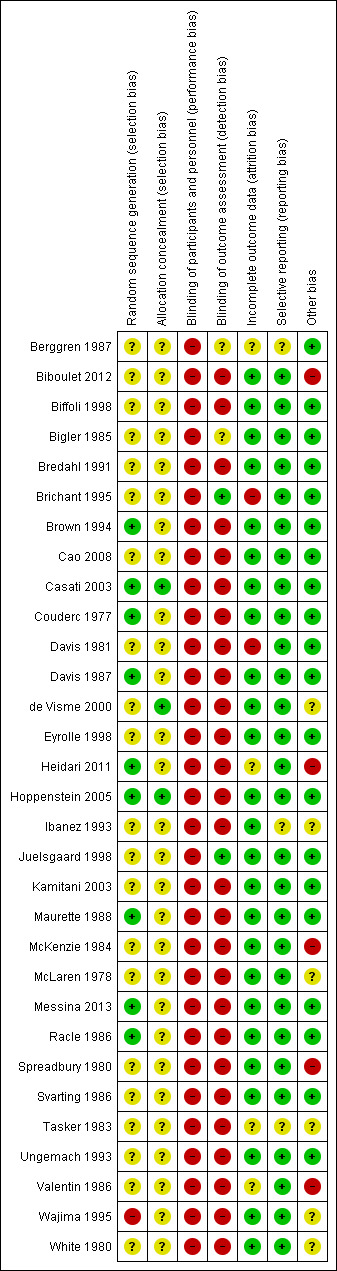
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged allocation concealment to be at low risk of bias for three studies (Casati 2003; de Visme 2000; Hoppenstein 2005), and unclear for all other included studies (Figure 3).
Blinding
For a comparison between neuraxial and general anaesthesia, blinding of participants is not possible, and blinding of personnel taking care of the patient is probably an unrealistic expectation, at least for the personnel taking care of the patient during the first few hours after the surgery. We judged blinding of the outcome assessor to be at unclear risk of bias in Berggren 1987 and Bigler 1985 and at low risk of bias in Brichant 1995 and Juelsgaard 1998 (see Figure 2; Figure 3).
Incomplete outcome data
We classified six studies as unclear or at high risk of bias for this item (Berggren 1987; Brichant 1995; Davis 1981; Heidari 2011; Tasker 1983; Valentin 1986; seeFigure 2; Figure 3).
Selective reporting
We classified three studies as unclear for selective reporting (Berggren 1987; Ibanez 1993; Tasker 1983; see Figure 2; Figure 3).
Other potential sources of bias
We classified 11 studies as unclear or at high risk for other risks of bias (Biboulet 2012; de Visme 2000; Heidari 2011; Ibanez 1993; McKenzie 1984; McLaren 1978; Spreadbury 1980; Tasker 1983; Valentin 1986; Wajima 1995; White 1980; Figure 2; Figure 3).
Effects of interventions
See: Table 1
Neuraxial block versus general anaesthesia
The definition and time points for all outcomes retained, can be found in Table 2.
Primary outcomes
1. Mortality
Mortality at one month
Based on 11 studies that included 2152 participants, we did not find a difference between the two techniques for mortality at one month: risk ratio (RR) 0.78, 95% confidence interval (CI) 0.57 to 1.06; I2 = 24% (fixed‐effect model) (Berggren 1987; Biboulet 2012; Bigler 1985; Davis 1981; Davis 1987; Heidari 2011; Juelsgaard 1998; McKenzie 1984; McLaren 1978; Racle 1986; Valentin 1986; Analysis 1.1). Egger's regression intercept showed no significant evidence of a small‐study effect. Duvall and Tweedie's trim and fill analysis showed that two studies might be missing to the left for an adjusted point of estimate: RR 0.70, 95% CI 0.51 to 0.97. Accepting that a publication bias occurred, and considering a basal rate of mortality of 8%, the number needed to treat for an additional beneficial outcome (NNTB) would be 42, 95% CI 26 to 339. A meta‐regression with the year where the study was published showed that effect size favouring neuraxial blockade compared to general anaesthesia might be higher in older studies (Figure 4). The number of participants included here allows to eliminate a difference of 25% in the risk of mortality at one month with a power of 0.57 (α 0.05; β 0.2; one‐sided test). A number of 4024 (2012 per group) would be required for a power of 0.8 (α 0.05; β 0.2; one‐sided test) if a large study would be done.
1.1. Analysis.
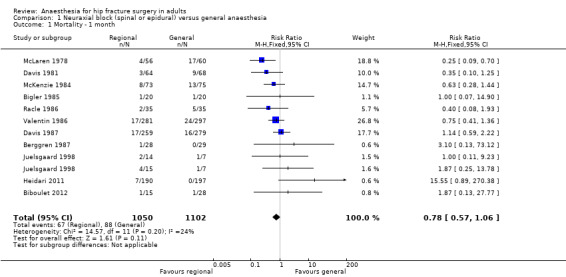
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 1 Mortality ‐ 1 month.
4.
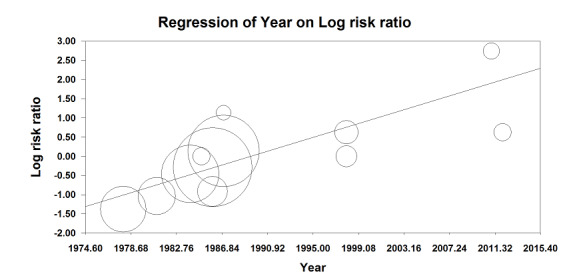
Meta‐regression of mortality at 0‐1 month versus the year when the study was published. The effect size decreases with time: P value = 0.002.
This meta regression plot was not produced in RevMan. The figure was generated automatically by the software, and cannot be amended. The software has expressed the years as decimals.
For mortality at one month, we downgraded the level of evidence by two for risk of bias based on the fact that we judged 75% or more of the included studies as unclear or inadequate for allocation concealment and/or blinding of outcome assessors. We did not downgrade an absence of effect for inconsistency because the I2 statistic was lower than 25%. We included direct comparisons only, and this outcome was not a surrogate marker. We downgraded the level by one for imprecision based on the fact that the optimal information size was not achieved. We downgraded the level by one for publication bias because correcting for this possibility would make the effect present instead of absent (RR after correction 0.70, 95% CI 0.51 to 0.97 versus RR 0.78, 95% CI 0.57 to 1.06 without correction). We did not change the level for amplitude of effect size (RR 0.78 and therefore > 0.5). We did not identify any confounding factors justifying upgrading. We upgraded the level for a dose response effect because we concluded to an absence of effect, and the meta‐regression showed that an effect was present only in older studies. We rated the quality of evidence as very low.
Mortality at three months
Based on five studies that included 953 participants (Berggren 1987; Couderc 1977; McKenzie 1984; Racle 1986; Valentin 1986), we did not find a difference in mortality at 3 months: RR 0.77, 95% CI 0.55 to 1.08; I2 = 0% (Analysis 1.2) . Egger's regression intercept showed no significant evidence of a small‐study effect. Duvall and Tweedie's trim and fill analysis showed that one study might be missing to the left for an adjusted point of estimate: RR 0.78, 95% CI 0.56, 1.09. Considering a basal mortality rate of 13.8%, 2204 participants (1102 per group) would be required to eliminate a difference of 25% (α 0.05; β 0.2; one‐sided test) if a large study would be done.
1.2. Analysis.
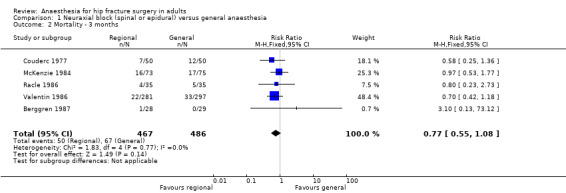
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 2 Mortality ‐ 3 months.
Mortality at six months
Based on two studies that included 726 participants (McKenzie 1984; Valentin 1986), we did not find a difference in mortality at six months: RR 1.00, 95% CI 0.73 to 1.37; I2 = 0% (Analysis 1.3). Considering a basal mortality rate of 17.5%, 1678 participants (839 per group) would be required to eliminate a difference of 25% (α 0.05; β 0.2; one‐sided test) if a large study would be done.
1.3. Analysis.
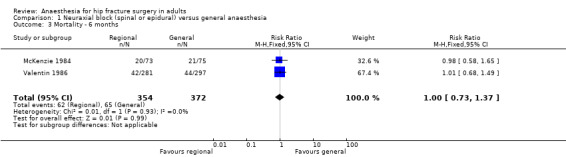
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 3 Mortality ‐ 6 months.
Mortality at one year
Based on two studies that included 726 participants (McKenzie 1984; Valentin 1986), we did not find a difference in mortality at one year after the surgery: RR 1.06, 95% CI 0.81 to 1.39; I2 = 0% (Analysis 1.4). Considering a basal mortality rate of 21.8%, 1310 participants (655 per group) would be required to eliminate a difference of 25% (α 0.05; β 0.2; one‐sided test) if a large study would be done.
1.4. Analysis.
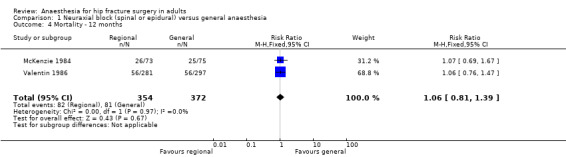
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 4 Mortality ‐ 12 months.
2. Pneumonia
Based on six studies that included 761 participants (Berggren 1987; Bigler 1985; Davis 1981; Heidari 2011; McLaren 1978; Racle 1986), we did not find a difference in the risk of pneumonia: RR 0.77, 95% CI 0.45 to 1.31; I2 = 0% (Analysis 1.5). Egger's regression intercept showed no significant evidence of a small‐study effect. Duvall and Tweedie's trim and fill analysis showed that three studies might be missing to the right for an adjusted point of estimate: RR 1.15, 95% CI 0.71 to 1.86. Considering a pneumonia rate of 6.4%, 5106 participants (2553 per group) would be required to eliminate a difference of 25% (α 0.05; β 0.2; one‐sided test) if a large study would be done. Likewise, the number of participants included here would give a power of 0.29 (α 0.05; β 0.2; one‐sided test).
1.5. Analysis.
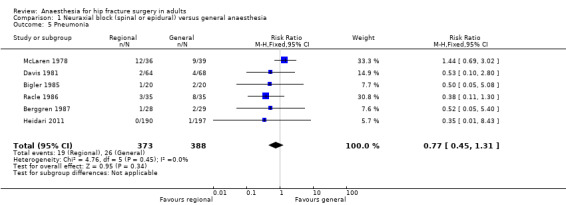
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 5 Pneumonia.
For pneumonia, we downgraded the level of evidence by two for risk of bias based on the fact that we judged 75% or more of the included studies as unclear or inadequate for allocation concealment and/or blinding of outcome assessors. We did not downgrade for inconsistency because the I2 statistic was lower than 25%. We included direct comparisons only, and this outcome was not a surrogate marker. We downgraded the level by one for imprecision based on the fact that the optimal information size was not achieved. We did not downgrade the level for publication bias because correcting for this possibility would not change the conclusion (RR after correction 1.15, 95% CI 0.71 to 1.86 versus RR 0.77, 95% CI 0.45 to 1.31 without correction). We did not change the level for amplitude of effect size (RR 0.77 and therefore > 0.5). We did not identify any confounding factors or dose response effect justifying upgrading. We rated the quality of evidence as very low.
3. Myocardial infarction
Based on four studies that included 559 participants (Biboulet 2012; Couderc 1977; Heidari 2011; Juelsgaard 1998), we did not find a difference in the risk of myocardial infarction: RR 0.89, 95% CI 0.22 to 3.65; I2 = 0% (Analysis 1.6). Egger's regression intercept showed no significant evidence of a small‐study effect. Duvall and Tweedie's trim and fill analysis showed no evidence of a publication bias. Considering a basal myocardial infarction rate of 1%, 34318 participants (17159 per group) would be required to eliminate a difference of 25% (α 0.05; β 0.2; one‐sided test) if a large study would be done. Likewise, the number of participants included here would give a power of 0.09 (α 0.05; one‐sided test).
1.6. Analysis.
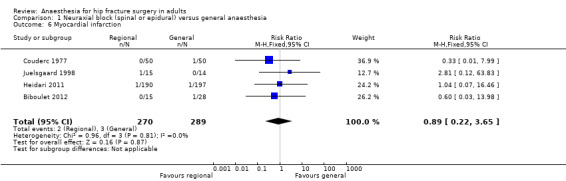
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 6 Myocardial infarction.
For myocardial infarction, we downgraded the level of evidence by two for risk of bias based on the fact that we judged 75% or more of the included studies as unclear or inadequate for allocation concealment and/or blinding of outcome assessors. We did not downgrade for inconsistency because the I2 statistic was lower than 25%. We included direct comparisons only, and this outcome was not a surrogate marker. We downgraded the level by one for imprecision based on the fact that the optimal information size was not achieved. We found no evidence of a publication bias. We found no evidence of a large effect size, confounding factors justifying upgrading, or dose response effect. We rated the quality of evidence as very low.
Secondary outcomes
1. Cerebrovascular accident (stroke)
Based on six studies that included 729 participants (Berggren 1987; Biboulet 2012; Bigler 1985; Davis 1981; Heidari 2011; Racle 1986), we did not find a difference in the risk of cerebrovascular accident: RR 1.48, 95% CI 0.46 to 4.83; I2 = 0% (Analysis 1.7). Egger's regression intercept showed no significant evidence of a small‐study effect. Duvall and Tweedie's trim and fill analysis showed no evidence of a publication bias. Considering a basal cerebrovascular accident rate of 2%, 17,008 participants (8504 per group) would be required to eliminate a difference of 25% (α 0.05; β 0.2; one‐sided test) if a large RCT would be done. Likewise, the power obtained with the number of participants included here is 0.13 (α 0.05; β 0.2; one‐sided test).
1.7. Analysis.
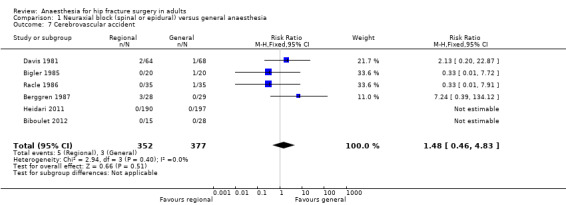
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 7 Cerebrovascular accident.
For cerebrovascular accident, we downgraded the level of evidence by two for risk of bias based on the fact that we judged more than 75% of the included studies as unclear or inadequate for allocation concealment and/or blinding of outcome assessors. We did not downgrade for inconsistency because the I2 statistic was lower than 25%. We included direct comparisons only, and this outcome was not a surrogate marker. We downgraded the level by one for imprecision based on the fact that the optimal information size was not achieved. We found no evidence of a publication bias. We found no evidence of a large effect size, confounding factors justifying upgrading, or dose response effect. We rated the quality of evidence as very low.
2. Acute confusional state
Based on six studies that included 624 participants (Berggren 1987; Bigler 1985; Casati 2003; Heidari 2011; Kamitani 2003; Racle 1986), we did not find a difference in the risk of acute confusional state (see Table 2 for exact definition): RR 0.85, 95% CI 0.51 to 1.40; I2 = 49% (Analysis 1.8). Egger's regression intercept showed no significant evidence of a small‐study effect. Duvall and Tweedie's trim and fill analysis showed no evidence of a publication bias. Excluding the Heidari 2011 study, the RR would be 1.08,95% CI 0.75, 1.55); I2 = 0%. We classified Heidari 2011 at low risk of bias for two items only (randomization and selective reporting). The study enrolled 400 participants and randomized them to either general anaesthesia, maintained with nitrous oxide and halothane, or to a neuraxial block (spinal or epidural (5.7% continuous)). Intravenous morphine was given at participants' request for postoperative analgesia in both groups. Results are provided as a per‐protocol basis with 10 participants excluded from the neuraxial group and three excluded from the general anaesthesia group. Cognitive dysfunctions were noted before discharge from recovery room, and at 24 and 48 hours after the end of the surgery based on time, person, and place disorientation (unclear who assessed the participants and whether or not outcome assessors were blinded for this specific item). In the general anaesthesia group the number of participants who were classified as disorientated were 22 at the recovery room, six on first postoperative day, and three on the second postoperative day. For the neuraxial group, the number of participants classified as disorientated were seven, six, and one for the same periods. Halothane, an inhalational agent slowly eliminated from the body, is no longer in use in the vast majority of developed countries, and the choice of inhalational agent may influence mental function after general anaesthesia, particularly in the elderly (Rortgen 2010). Furthermore, the choice of morphine as the primary mode of analgesia after a major hip surgery may also contribute to postoperative cognitive dysfunction (Hebl 2005). These two factors may have contributed to a higher effect size of regional anaesthesia compared with general anaesthesia in the study of Heidari 2011, especially considering that the main difference was found in the postanaesthesia care unit. Considering a basal rate of 17.7%, 1652 participants (826 per group) would be required to eliminate a 25% difference (α 0.05; β 0.2; one‐sided test) if a large RCT would be done. Likewise, the power obtained with the number of participants included here is 0.46 (α 0.05; β 0.2; one‐sided test).
1.8. Analysis.
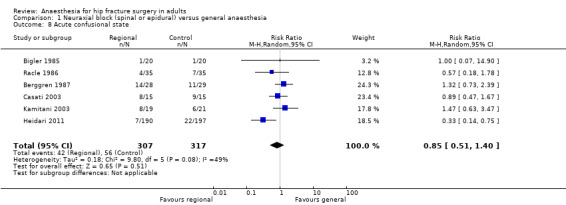
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 8 Acute confusional state.
For acute confusional state, we downgraded the level of evidence by two for risk of bias based on the fact that we judged more than 75% of the included studies as unclear or inadequate for allocation concealment and/or blinding of outcome assessors. We downgraded the level by one for inconsistency because the I2 statistic was 49%%. We included direct comparisons only, and this outcome was not a surrogate marker. We downgraded the level by one for imprecision based on the fact that the optimal information size was not achieved. We found no evidence of a publication bias. We found no evidence of a large effect size, confounding factors justifying upgrading, or dose response effect. We rated the quality of evidence as very low.
3. Deep vein thrombosis
The diagnosis of deep venous thrombosis was done by injection of 125‐iodine fibrinogen (Davis 1981), or venography (Brichant 1995; McKenzie 1984), or as diagnosed by the consultant specialist (Heidari 2011). Two studies including 116 participants did not use any potent thromboprophylaxis (Davis 1981; McKenzie 1984). The risk of deep vein thrombosis decreased when no specific precautions except just use of early mobilization was used (RR 0.57, 95% CI 0.41 to 0.78; I2 = 0%; NNTB = 3, 95% CI 2 to 7; based on a basal risk of 76%), but not when low molecular weight heparin was administered (RR 0.98, 95% CI 0.52 to 1.84; I2 = 58% for heterogeneity between the two subgroups) (Analysis 1.9). On the same basal risk the optimal information size for a large trial would be 152 participants (76 per group) for a 25% decrease (α 0.05; β 0.2; one‐sided test).
1.9. Analysis.
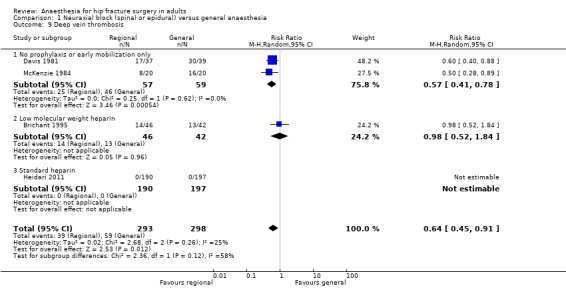
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 9 Deep vein thrombosis.
For deep venous thrombosis in the absence of potent thromboprophylactic agents, we downgraded the level of evidence by two for risk of bias based on the fact that we judged more than 75% of the included studies as unclear or inadequate for allocation concealment and/or blinding of outcome assessors. There was no evidence of inconsistency. We downgraded the level by one based on indirectness due to the fact that systematic venographies and injection of marked fibrinogen are surrogate markers for clinically relevant events. We downgraded the level by one for imprecision based on the fact that the optimal information size was not achieved. We found no evidence of a publication bias. We found no evidence of a large effect size, confounding factors justifying upgrading, or dose response effect. We rated the quality of evidence as very low.
4. Return of patient to their own home
Data were available for one study only: RR 0.84, 95% CI 0.61 to 1.16 (McKenzie 1984; Table 4). Considering a basal rate of 5.3% (Brauer 2009), 7898 (3949 per group) would be required to eliminate a 25% difference (α 0.05; β 0.2; one‐sided test) if a large RCT would be done. Likewise, the power obtained with the number of participants included here is 0.1 (α 0.05; one‐sided test).
3. Results for outcomes from single studies.
| Comparison: Neuraxial block versus general anaesthesia | ||||||
| Study | Outcome | Number of patients | Type of effect size | Effect size | Lower 95% CI | Upper 95% CI |
| McKenzie 1984 | Patient returned to their own home | 130 | RR | 0.84 | 0.61 | 1.16 |
| Berggren 1987 | Urine retention | 57 | RR | 0.86 | 0.30 | 2.51 |
| Comparison: Neuraxial block added to general anaesthesia compared to general anaesthesia alone | ||||||
| Study | Outcome | Number of patients | Type of effect size | Effect size | Lower 95% CI | Upper 95% CI |
| White 1980 | Pneumonia | 30 | RR | 0.80 | 0.20 | 3.20 |
| White 1980 | Acute confusional state | 30 | RR | 1.00 | 0.16 | 6.09 |
| White 1980 | Deep vein thrombosis | 30 | RR | 0.17 | 0.01 | 3.94 |
| White 1980 | Length of surgery (minutes) | 30 | MD | 0.00 | ‐17.96 | 17.96. |
| Comparison: Neuraxial block versus peripheral nerve block | ||||||
| Study | Outcome | Number of patients | Type of effect size | Effect size | Lower 95% CI | Upper 95% CI |
| de Visme 2000 | Acute confusional state | 29 | RR | 0.89 | 0.35 | 2.28 |
| Eyrolle 1998 | Operative hypotension | 50 | RR | 6.00 | 2.02 | 17.83 |
| de Visme 2000 | Length of surgery (minutes) | 29 | MD | 17.00 | ‐0.76 | 34.76 |
| Comparison: Intravenous ketamine alone (without neuromuscular blocking agent) versus general anaesthesia | ||||||
| Study | Outcome | Number of patients | Type of effect size | Effect size | Lower 95% CI | Upper 95% CI |
| Spreadbury 1980 | Unsatisfactory surgical results defined as unstable fixation or prosthesis dislocation | 60 | RR | 2.33 | 0.67 | 8.18 |
| Spreadbury 1980 | Mortality | 60 | RR | 1.00 | 0.46 | 2.17 |
| Spreadbury 1980 | Patient returned home | 60 | RR | 0.95 | 0.66 | 1.38 |
| Spreadbury 1980 | Length of hospital stay | 39* | MD | 12.00 | 5.63 | 18.37 |
CI: confidence interval; MD: mean difference; RR: risk ratio
*: Mean duration of admission refers only to those patients who were discharged home
For this outcome, we downgraded the level by two for risk of bias based on the fact that we judged the sole study included as unclear or inadequate for allocation concealment and blinding of outcome assessors. Inconsistency could not be evaluated. The included study was a direct comparison. We downgraded the level by one for imprecision based on the fact that the optimal information size was not achieved. Publication bias could not be evaluated. We found no evidence of a large effect size, confounding factors justifying upgrading, or dose response effect. We rated the quality of evidence as very low.
5. Congestive cardiac failure
Based on six studies that included 729 participants (Berggren 1987; Biboulet 2012; Bigler 1985; Davis 1981; Racle 1986), we did not find a difference in the risk of congestive heart failure: RR 0.78, 95% CI 0.31 to 1.96; I2 = 0% (Analysis 1.10). Egger's regression intercept showed no significant evidence of a small‐study effect. Duvall and Tweedie's trim and fill analysis showed no evidence of a publication bias. Considering a basal congestive heart failure of 6%, 5466 participants (2733 per group) would be required to eliminate a difference of 25% (α 0.05; β 0.2; one‐sided test) if a large RCT would be done. Likewise, the power obtained with the number of participants included here is 0.23 (α 0.05; β 0.2; one‐sided test).
1.10. Analysis.
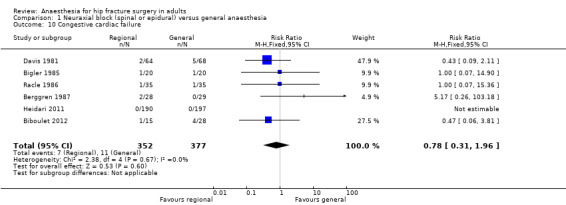
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 10 Congestive cardiac failure.
6. Acute kidney injury
Data were available only for two small studies that included 202 participants: RR 1.02, 95% CI 0.18 to 5.83; I2 = 0% (Davis 1981; Racle 1986; Analysis 1.11).
1.11. Analysis.
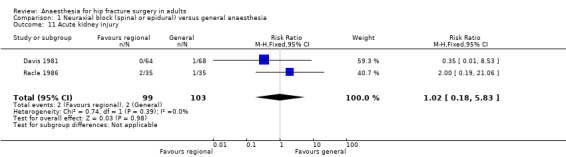
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 11 Acute kidney injury.
7. Pulmonary embolism
Based on five studies that included 642 participants (Berggren 1987; Bigler 1985; Brichant 1995; Heidari 2011; Racle 1986), we did not find a difference in the risk of pulmonary embolism: RR 3.35, 95% CI 0.82 to 13.57; I2 = 0% when data were analysed as risk ratio. However, if the Peto odds ratio method was used (Analysis 1.12), the difference became statistically significant in favour of general anaesthesia: Peto odds ratio 7.51, 95% CI 1.51 to 37.38; I2 = 0%. Egger's regression intercept did not show a small‐study effect. Duvall and Tweedie's trim and fill analysis did not show any evidence of a publication bias. The classical fail‐safe number was three. Because there was no event on the side of general anaesthesia a NNTH could not be calculated. Based on a rate of 1.9% in the treatment group, the NNTB would be 61, 95% CI 55 to 157, when general anaesthesia is used.
1.12. Analysis.
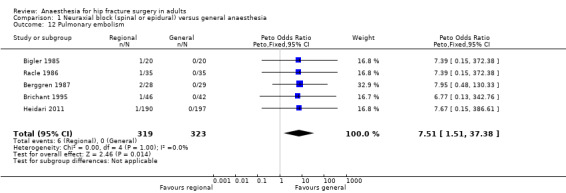
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 12 Pulmonary embolism.
8. Unsatisfactory surgical results
There were no data for this outcome for this comparison.
9. Number of patients transfused
Based on two studies that included 172 participants (Bigler 1985; Davis 1981), we did not find a difference in the number of transfused (red blood cells) participants for femur fixation: RR 0.93, 95% CI 0.76 to 1.15; I2 = 0%. Based on one study that included 30 participants (Svarting 1986), we could not demonstrate a difference in the number of transfused patients for arthroplasty: RR 0.20, 95% CI 0.03 to 1.51. Heterogenity between the two subgroups = 55% (Analysis 1.13).
1.13. Analysis.
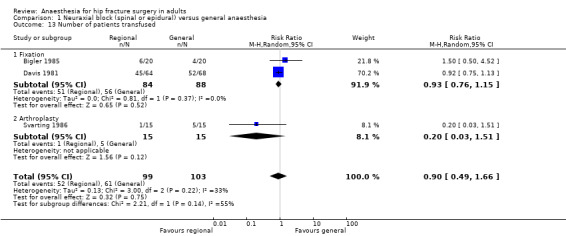
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 13 Number of patients transfused.
10. Length of hospital stay
Based on four studies that included 1143 participants (Davis 1987; Heidari 2011; McKenzie 1984; Racle 1986), we did not find a difference in length of hospital stay: mean difference (MD) ‐ 0.20, 95% CI ‐ 1.05 to 0.65; I2 = 0% (Analysis 1.14). Egger's regression intercept showed no significant evidence of a small‐study effect. Duvall and Tweedie's trim and fill analysis showed that one study might be missing to the left for an adjusted point of estimate of ‐ 0.21, 95% CI ‐ 1.05 to 0.63.
1.14. Analysis.
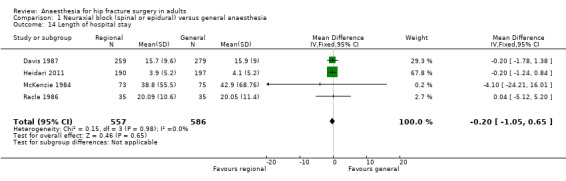
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 14 Length of hospital stay.
11. Length of surgery (in minutes)
Based on 12 studies that included 973 participants (Berggren 1987; Biffoli 1998; Bigler 1985; Bredahl 1991; Heidari 2011; Hoppenstein 2005; Kamitani 2003; Maurette 1988; McKenzie 1984; Messina 2013; Racle 1986; Svarting 1986), a neuraxial block would not reduce the surgical time: MD ‐ 2.73, 95% CI ‐ 8.50 to 3.04; I2 = 62% (Analysis 1.15). Egger's regression intercept showed no significant evidence of a small‐study effect. Duvall and Tweedie's trim and fill analysis showed that two studies might be missing to the right, for an adjusted point of estimate: ‐0.85, 95% CI ‐6.70 to 5.00.
1.15. Analysis.
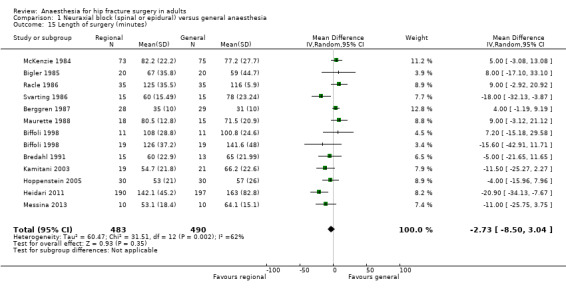
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 15 Length of surgery (minutes).
12. Operative hypotension
The definition for operative hypotension varied from the number of participants who had a decrease of systolic arterial blood pressure of 20% (Casati 2003; Davis 1987; Racle 1986), mean arterial blood pressure of 20% (Maurette 1988), mean arterial blood pressure of 25% (Messina 2013), systolic arterial blood pressure of 30% (Berggren 1987; Svarting 1986), arterial blood pressure of 30% (Biffoli 1998), systolic arterial blood pressure of 33% (Juelsgaard 1998), arterial blood pressure ≥ 40 mm HG (Couderc 1977), systolic arterial blood pressure of 50% (McLaren 1978), or undefined (Brown 1994). The risk of operative hypotension was lower with a neuraxial block when low dose unilateral (RR 0.57, 95% CI 0.37 to 0.89; I2 = 0%) (Casati 2003; Messina 2013), or incremental spinals were performed (RR 0.20, 95% CI 0.05 to 0.78) (Juelsgaard 1998); but not with bilateral sensory/motor blockade single shot spinal anaesthesia (RR 1.31, 95% CI 0.87 to 1.95; I2 = 36%) (Biffoli 1998; Brown 1994; Davis 1987; Juelsgaard 1998; Maurette 1988; McLaren 1978; Racle 1986; Svarting 1986), or epidural anaesthesia (RR 1.01, 95% CI 0.50 to 2.07; I2 = 73%) (Berggren 1987; Couderc 1977; Analysis 1.16). A bolus of fluid was administered before the neuraxial block for six studies (Biffoli 1998; Couderc 1977; Davis 1987; Juelsgaard 1998; Racle 1986; Svarting 1986).
1.16. Analysis.
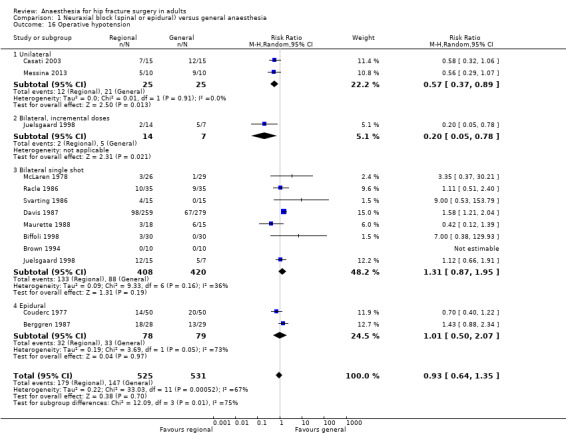
Comparison 1 Neuraxial block (spinal or epidural) versus general anaesthesia, Outcome 16 Operative hypotension.
13. Urine retention
Based on one small study we did not find a difference in the risk of urine retention: RR 0.86, 95% CI 0.30 to 2.51 (Berggren 1987; Table 4).
Neuraxial block added to general anaesthesia compared to general anaesthesia alone
We found only one small study for this comparison (White 1980; Table 4). The study contains three groups including one where a psoas compartment block was added to general anaesthesia. This group was not retained because it was considered outside the scope of the present review: RR 0.80, 95% CI 0.20 to 3.20 for pneumonia, RR 1.00, 95% CI 0.16 to 6.09 for acute confusional state, and RR 0.17, 95% CI 0.01 to 3.94 for deep vein thrombosis. The MD for length of surgery was 0.00, 95% CI ‐17.96 to 17.96 minutes.
Neuraxial block versus peripheral nerve block
Three studies that included 139 participants compared a neuraxial block (spinal or epidural) to peripheral nerve blocks (Cao 2008; de Visme 2000; Eyrolle 1998). For the peripheral nerve blocks, two studies used posterior lumbar (psoas compartment) block alone (Cao 2008; Eyrolle 1998), and one study added sacral plexus block and iliac crest infiltration to the lumbar plexus block (de Visme 2000). Neuraxial blocks reduce the risk of a failed block: RR 0.24, 95% CI 0.12 to 0.49; I2 = 0 %) (Analysis 2.1). However, when the block combination was used (psoas compartment block plus sacral plexus block plus iliac crest infiltration), three out of the four participants (where the block was judged as incomplete), required only one bolus of 250 mcg of alfentanil at skin incision as supplemental analgesia (de Visme 2000).
2.1. Analysis.
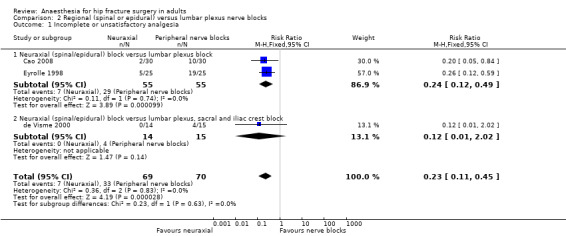
Comparison 2 Regional (spinal or epidural) versus lumbar plexus nerve blocks, Outcome 1 Incomplete or unsatisfactory analgesia.
We did not find a difference for the risk of acute confusional state: RR 0.89, 95% CI 0.35 to 2.28 (de Visme 2000; Table 4). Operative hypotension was more common with neuraxial blocks: RR 6.00, 95% CI 2.02 to 17.83 (Eyrolle 1998; Table 4) with a NNTH = 2 (95% CI 5 to 2) (basal rate of 12% in the peripheral nerve block group). This was also true for urine retention: RR 14.00, 95% CI 1.90 to 103.00; I2 = 0% (basal rate of 0% in the peripheral nerve block groups) (Cao 2008; Eyrolle 1998; Analysis 2.2). We found no difference in the surgical time based on one study: MD 17.00, 95% CI ‐ 0.76 to 34.76 minutes (de Visme 2000; Table 4).
2.2. Analysis.
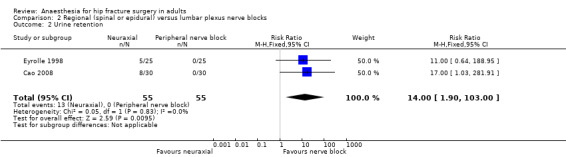
Comparison 2 Regional (spinal or epidural) versus lumbar plexus nerve blocks, Outcome 2 Urine retention.
Intravenous ketamine versus general anaesthesia
We found only one study for this comparison (Spreadbury 1980; Table 4). We could not demonstrate a difference in the risk for unsatisfactory surgical results defined as unstable fixation or prosthesis dislocation by ketamine alone without neuromuscular blocking agents: RR 2.33, 95% CI 0.67 to 8.18; in mortality during hospital stay: RR 1.00, 95% CI 0.46 to 2.17; or the chances of the participant returning home: RR 0.95, 95% CI 0.66 to 1.38. Length of hospital stay for the survivors was longer in the ketamine group: MD 12.00, 95% CI 5.63 to 18.37 days.
Discussion
Neuraxial block versus general anaesthesia
Many of the studies within this review involved small numbers of participants and reported only a few outcome measures. From the actual available RCTs, for adults undergoing hip fracture surgery, regional anaesthesia decreases the risk of deep venous thrombosis only in the absence of potent thromboprophylaxis (very low quality of evidence; Table 1). A unilateral or incremental spinal anaesthesia decreases the incidence of operative hypotension. The optimal information size for an alpha error of 0.05 and a beta error of 0.2 (one‐sided test) to eliminate a difference of 25% was not achieved for: mortality at one month, pneumonia, myocardial infarction, cerebrovascular accident, acute confusional state, and return of patient to their own home. Data were available from only one study for return of patient to their own home. The trial reports of many studies indicated a suboptimal level of methodological rigour, in particular regarding the exact method used for randomization, concealment of allocation, assessor blinding and intention‐to‐treat analysis (Figure 2; Figure 3). Therefore, an absence of difference cannot be stated with certainty for the vast majority of the outcomes included in the present review.
The type of anaesthetic techniques used in many of the studies may not reflect current clinical practice, and this may have prevented us in finding clinically relevant differences between general anaesthesia and regional anaesthesia. For instance, for acute confusional state, one study used diazepam as the induction agent in the general anaesthesia group and as a sedative in the regional anaesthesia group (Bigler 1985). Residual blood concentrations of benzodiazepines have not been shown to correlate with postoperative cognitive dysfunction (Rasmussen 1999). However, some authors reported a clear association between the risk of hip fractures and recently introduced benzodiazepines in the drug regimen of the elderly (Wang 2001), suggesting that newly introduced benzodiazepines will affect the elderly in someway. Therefore it is difficult to be certain that benzodiazepine administration in both groups did not mask an actual difference between general anaesthesia and regional anaesthesia. Likewise, halothane, an inhalational agent slowly eliminated from the body, that is no longer in use in the vast majority of developed countries, was administered in two of the studies and this may have increased the difference between the two anaesthetic techniques, this time favouring regional anaesthesia (Berggren 1987; Heidari 2011). The choice of an inhalational agent may influence mental functions after general anaesthesia, particularly in the elderly (Rortgen 2010).
The definitions of outcomes and the exact time points at which the participants were evaluated varied widely or were unclear (Table 2). For example, we took as "acute confusional state" all various authors' definitions without any discrimination. Although statistical heterogeneity disappeared (I2 = 0%) when Heidari 2011 was withdrawn from the analysis (Analysis 1.8), one can see from Table 4, that the definition of "acute confusional state" varied widely, and included various level of a possible transient decrease in mental function (minus 2 points out of 30 on the Mini Mental Status for Casati 2003 or a Mini Mental Status score lower than 5/30 for de Visme 2000), actual confusion (Berggren 1987; Bigler 1985; Heidari 2011), delirium with or without agitation (Kamitani 2003; Racle 1986), or was unspecified (White 1980). A transient decline in the ability to perform a mathematical test may not be as relevant as mental deterioration preventing the person from participating in their rehabilitation (confusion/delirium/agitation). Finally, the exact time point where the outcome was taken also varies widely (up to four weeks: White 1980). Although, there may not be any clinically relevant difference between general anaesthesia and regional anaesthesia after seven days, when any type of mental evaluation is accepted (Guay 2011), a transient decline in mental function sufficient to affect adequate communication between the patient and personnel taking care of her/him, may affect rehabilitation, or even put her/him at risk of further injury (Wang 2001). Further studies performed with short acting drugs may need to differentiate between the various levels of transient decline in mental function (enough to prevent adequate participation in own care and rehabilitation programme or not) and from delirium, with or without agitation (requiring restraining procedures or potent psychotropic drugs).
Studies included were published between 1977 and 2013 and clinical practice has changed during this period. The vast majority of centres will now preferentially use shorter acting drugs in the hope of decreasing the length of time during which patients may be under the residual influence of the anaesthetic agents. This may influence the person's ability to actively participate in their rehabilitation, and possibly their overall outcome. Therefore, we decided to explore the year when the study was published as a factor of heterogeneity and, indeed, we found a correlation between the effect size for mortality and the year when the study was published (Figure 4). This suggests that a lower mortality rate associated with regional anaesthesia compared with general anaesthesia might have been more pronounced in the oldest trials, before the widespread use of short acting anaesthetic drugs. Many of the included trials are relatively old and may not represent contemporary practice, nor account for the advances in safety in the field of anaesthesia. From 1986 to 2005, the one‐year mortality rate after a hip fracture decreased by 8.8% for women and 20.0% for men (Brauer 2009). This overall decrease in mortality associated with hip fractures probably reflects advances in the global care of this population. Among other factors, a reduction of the delay before surgery, improved surgical devices and movement toward replacement arthroplasty, combined with a push for earlier weight bearing exercise are all possible factors that may have contributed to this higher rate of survival. If the overall rate of mortality is lower, then the number of participants that will be needed to be included to eliminate a difference between regional and general anaesthesia will be higher.
Neuraxial block decreases the incidence of deep venous thrombosis compared to general anaesthesia only when potent antithrombotic agents such as low molecular weight heparin are not used (Analysis 1.9). It is important to note however that none of the three studies included in the present review used clinical symptoms of deep venous thrombosis as an outcome (Brichant 1995; Davis 1981; McKenzie 1984).
In the present review, regional anaesthesia was associated with a higher risk of pulmonary embolism (its statistical significance depended on the technique of analysis used). It is important to note that an absence of event in the general anaesthesia group, such as found here, is quite exceptional. Particluarly considering the fact that the thromboprophylaxis used in these studies was below actual standards for at least four of them: dextran (Berggren 1987), unfractionated heparin (Heidari 2011; Racle 1986), or unspecified (Bigler 1985). The rate of pulmonary embolism found here with regional anaesthesia (1.9%) is consistent with the rate found after lower limb arthoplasty with modern potent thromboprophylaxis: incidence 1.1%, 95% CI 0.3 to 1.9% (Samama 2007). A meta‐analysis performed on the efficacy of various regimen of thromboprophylaxis after hip arthroplasty found that compared with the risk obtained after a placebo (1.51%), only warfarin (0.16%), pneumatic compression (0.26%), and low molecular weight heparin (0.36%) were associated with a significantly lower risk of symptomatic pulmonary embolism (Freedman 2000). Therefore, this finding cannot be considered a clear demonstrated effect of an intervention because the 0% rate of pulmonary embolism found with general anaesthesia and suboptimal prophylaxis is not consistent with the medical literature. We have to attribute this exceptionally low rate to an absence of systematic screening, unclear outcome definitions and/or inadequate period of follow‐up.
Length of hospital stay can be considered as an indirect marker for cost. We did not find a difference in the mean length of hospital stay for hip fracture between these two anaesthetic techniques (Analysis 1.14). The length of hospital stay of patients operated for hip fracture has decreased from a median of 12 days (interquartile range (IQR), 8.0 to 16.0) between 1986 and 1988 to five days (IQR, 4.0 to 12.0) between 2003 and 2005 (Brauer 2009). This reduced length of hospital stay may however be falsely reassuring because it is associated with a decreased percentage of patients returning home after a hip fracture. In the Brauer 2009 study, 34.3% (95% CI 34.0% to 34.6%) of patients were going home with self care after a hip fracture between 1986 and 1988, while only 5.3% (95% CI 5.2% to 5.4%) were doing so between 2003 and 2005. Between 2003 and 2005, 52.8% of patients with hip fracture (95% CI 52.5% to 53.2%) were discharged to a skilled nursing facility. From limited data, we did not find a difference between the two techniques in the percentage of patients returning home after a fracture hip repair (Analysis 1.13).
In the past, operative hypotension associated with neuraxial blocks has been considered a major draw back to the use of neuraxial blocks in the aged population. One can see however, that this problem can be overcome with the use of a small dose unilateral spinal or incremental dose spinal anaesthesia (Analysis 1.16). Epidurals will not affect the incidence of operative hypotension compared to general anaesthesia (Analysis 1.16).
Neuraxial block added to general anaesthesia compared to general anaesthesia alone
The sole study to address this question involved only 20 participants in each group (White 1980). There was no statistically significant difference between techniques for any of the outcome measures reported. Because of the small numbers of participants involved, no conclusions about the lack of difference between the two techniques can be made.
Neuraxial block versus peripheral nerve block
The three included trials involved only 139 participants in total (Cao 2008; de Visme 2000; Eyrolle 1998). The limited results available suggest that the neuraxial anaesthesia is associated with a lower risk of incomplete or unsatisfactory analgesia (RR 0.24, 95% CI 0.12 to 0.47), and this would be particularly true if a posterior lumbar plexus block is used alone, without the addition of a sacral plexus block plus an iliac crest infiltration. Altogether, the information available would suggest that peripheral nerve blocks could be used as the sole anaesthetic technique, but only when both neuraxial block and general anaesthesia pose special risks.
Intravenous ketamine versus general anaesthesia
The sole trial comparing ketamine with general anaesthesia involved only 60 participants (Spreadbury 1980). The numbers of participants were too small to show if the increase in 'unsatisfactory surgical results' in the ketamine group was a significant factor of ketamine use.
Summary of main results
For adults undergoing hip fracture surgery, regional anaesthesia is associated with a decreased incidence of deep venous thrombosis only in the absence of potent thromboprophylaxis. Due to the inclusion of old trials that may not reflect actual clinical practice, an absence of clear definitions for many outcomes, and the small number of participants included for the vast majority of outcomes, we cannot make a clear statement on the presence/absence of difference in outcomes between regional and general anaesthesia for hip fracture repair for any other outcome.
Overall completeness and applicability of evidence
A substantial part of the evidence is derived from old small studies with suboptimal methodology. Rare adverse events related to the anaesthetic techniques cannot be evaluated from these small studies. These studies however, suggest that results obtained with general anaesthesia or neuraxial blocks would be relatively similar.
Quality of the evidence
Details of reasons for upgrading of downgrading the quality of evidence are given in the section effects of interventions (Results).
For neuraxial blocks compared to general anaesthesia, we rated the quality of evidence as very low for mortality at 0 to 30 days, pneumonia, myocardial infarction, cerebrovascular accident, acute confusional state, decreased rate of deep venous thrombosis in the absence of potent thromboprophylaxis, and return of patient to their own home.
Potential biases in the review process
We included all available RCTs. Those studies were published between 1977 and 2013, thus covering a wide range of clinical practices.
Agreements and disagreements with other studies or reviews
The absence of difference in the mortality rate between neuraxial blockade and general anaesthesia is in agreement with a recent retrospective study (Neuman 2014).
Authors' conclusions
Implications for practice.
We did not find a difference between the two anaesthetic techniques, except for deep venous thrombosis in the absence of potent thromboprophylaxis and reduced operative hypotension (if a unilateral or incremental spinal anaesthesia is used). The studies included a wide variety of clinical practices. The number of participants included in the review is insufficient to eliminate a difference between the two anaesthetic techniques for the majority of outcomes studied. These outcomes were often insufficiently defined and the exact time point at which they were measured was often unclear. Therefore, large randomized trials reflecting actual clinical practice are required before drawing final conclusions.
Limited evidence suggests that ketamine anaesthesia without neuromuscular blocking agents may increase the incidence of poor surgical results.
Implications for research.
Research in this population is often limited by the fact that many of these patients may have declined mental function, impeding their ability to give informed consent.
New large high quality trials are required to re‐evaluate the effect of the anaesthetic technique on the mortality rate of patients undergoing surgery for hip fracture repair.
Because aspirin has now become a choice for thromboprophylaxis for hip fracture surgery in some countries (Falck‐Ytter 2012), and bears no contraindication with neuraxial blocks (Horlocker 2010), trials evaluating the effect of a neuraxial block in patients receiving aspirin as their thromboprophylaxis could be relevant.
Finally, future studies should include a measure of the quality of life.
What's new
| Date | Event | Description |
|---|---|---|
| 15 February 2017 | Amended | We reran the search to February 2017. |
History
Protocol first published: Issue 4, 1997 Review first published: Issue 4, 1999
| Date | Event | Description |
|---|---|---|
| 26 January 2016 | New search has been performed | We reran the search to March 2014 |
| 26 January 2016 | New citation required and conclusions have changed | Compared to the previous version (Parker 2004) , we withdraw one study (Adams 1990; quasi‐randomized trial) and added five new studies (Biboulet 2012; Cao 2008; Heidari 2011; Hoppenstein 2005; Messina 2013). Compared to general anaesthesia, using a neuraxial block for the surgery decreases the rate of thrombosis only if a prophylaxis with low molecular weight heparin is not used. We did not find a difference for mortality, pneumonia, myocardial infarction, cerebrovascular accident, acute confusional state or chances of returning home after the surgery. Change in authors: Helen HG Handoll and Richard Griffiths left the review. Joanne Guay, Pushpaj R Gajendragadkar and Sandra Kopp joined the review for this update. |
| 23 January 2014 | Amended | This review has been transferred from the Bone, Joint and Muscle Trauma Group to the Cochrane Anaesthesia Group. |
| 4 September 2008 | Amended | Converted to new review format. |
| 11 June 2004 | New citation required and conclusions have changed | For the third update, first appearing in Issue 4, 2004, the trial search was updated to February 2004. The following changes were made. (1) Five studies comparing spinal versus general anaesthesia were newly included (Biffoli 1998; Casati 2003; Kamitani 2003; Svarting 1986; Wajima 1995). (2) Additional data were obtained for McLaren 1978 resulting in the number of patients being increased from 55 to 116 for some of the outcomes. (3) Seven studies were newly excluded and two added to 'Studies awaiting assessment'. (4) Format changes to conform to the revised Cochrane Style Guide. (5) There were substantive changes to the conclusions of the review, reflecting new findings on postoperative acute confusional state and in response to editorial comments. |
Notes
January 2014: This review has been transferred from the Bone, Joint and Muscle Trauma Group to the Cochrane Anaesthesia Group.
Acknowledgements
We thank Helen Handoll who worked on the first three versions of the review for giving us the opportunity to update this review (Parker 2001; Parker 2004; Urwin 2000), Karl Sales for the translation of Ibanez 1993, Jia Jiang for the translation of Cao 2008, Richard Griffiths who was also an author on the last published version (Parker 2004), and Karen Hovhannisyan for the search for this update.
We thank the Bone, Joint and Muscle Trauma Group for their help in the past with our reviews (Parker 2001; Parker 2004; Urwin 2000).
We thank Mark D Neuman (content editor), Marialena Trivella (statistical editor), Santosh Rath (peer reviewer), Arthur Atchabahian (peer reviewer) and Janet L Wale (consumer reviewer) for their help in this update.
We also thank Janne Vendt who redesigned the search reran in February 2017 for 2014 and onwards.
Appendices
Appendix 1. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor: [Hip Fractures] explode all trees #2 ((hip* or fem?r* or trochant* or pertrochant* or intertrochant* or subtrochant* or intracapsular* or extracapsular*) and fracture*) #3 #1 or #2 #4 MeSH descriptor: [Anesthesia] explode all trees #5 (an?est* near (regional* or local* or general or spinal or epidural or intravenous)) or (endotracheal or laryngeal mask* or ((nerv or neuraxial) near block*)):ti,ab #6 #4 or #5 #7 #3 and #6
Appendix 2. MEDLINE (Ovid SP) search strategy
1. exp Hip Fractures/ or ((hip* or fem?r* or trochant* or pertrochant* or intertrochant* or subtrochant* or intracapsular* or extracapsular*) adj5 fracture*).mp. 2. exp Anesthesia/ or (an?est* adj4 (regional* or local* or general or spinal or epidural or intravenous)).mp. or (endotracheal or laryngeal mask* or ((nerv or neuraxial) adj3 block*)).ti,ab. 3. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3
Appendix 3. EMBASE (Ovid SP) search strategy
1. exp hip fracture/ or ((hip* or fem?r* or trochant* or pertrochant* or intertrochant* or subtrochant* or intracapsular* or extracapsular*) adj5 fracture*).mp. 2. exp anesthesia/ or (an?est* adj4 (regional* or local* or general or spinal or epidural or intravenous)).mp. or (endotracheal or laryngeal mask* or ((nerv or neuraxial) adj3 block*)).ti,ab. 3. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh 4. 1 and 2 and 3
Appendix 4. Modified strategy used when the search was reran in February 2017
Central
#1 MeSH descriptor: [Hip Fractures] explode all trees 1287 #2 ((hip* or fem?r* or trochant* or pertrochant* or intertrochant* or subtrochant* or intracapsular* or extracapsular*) and fracture*) 5605 #3 #1 or #2 5605 #4 MeSH descriptor: [Anesthesia] explode all trees 17293 #5 (an*est* near (regional* or local* or general or spinal or epidural or intravenous)) or (endotracheal or laryngeal mask* or ((nerv* or neuraxial) near block*)):ti,ab 45561 #6 #4 or #5 48386 #7 #3 and #6 452 #8 #7 Publication Year from 2014 to 2017, in Trials 55
Medline
1 Hip Fractures/ or ((hip* or fem?r* or trochant* or pertrochant* or intertrochant* or subtrochant* or intracapsular* or extracapsular*) adj5 fracture*).mp. (47583) 2 exp Anesthesia/ or (an?est* adj4 (regional* or local* or general or spinal or epidural or intravenous)).mp. or (endotracheal or laryngeal mask* or ((nerv* or neuraxial) adj3 block*)).ti,ab. (269134) 3 ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. (3450527) 4 1 and 2 and 3 (321) 5 limit 4 to yr="2014 ‐Current" (94)
Embase
1 exp hip fracture/ or ((hip* or fem?r* or trochant* or pertrochant* or intertrochant* or subtrochant* or intracapsular* or extracapsular*) adj5 fracture*).mp. (67218) 2 exp anesthesia/ or (an?est* adj4 (regional* or local* or general or spinal or epidural or intravenous)).mp. or (endotracheal or laryngeal mask* or ((nerv* or neuraxial) adj3 block*)).ti,ab. (392401) 3 (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. (6637043) 4 1 and 2 and 3 (657) 5 limit 4 to yr="2014 ‐Current" (233)
Data and analyses
Comparison 1. Neuraxial block (spinal or epidural) versus general anaesthesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality ‐ 1 month | 11 | 2152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.57, 1.06] |
| 2 Mortality ‐ 3 months | 5 | 953 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.55, 1.08] |
| 3 Mortality ‐ 6 months | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.73, 1.37] |
| 4 Mortality ‐ 12 months | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.81, 1.39] |
| 5 Pneumonia | 6 | 761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.45, 1.31] |
| 6 Myocardial infarction | 4 | 559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.22, 3.65] |
| 7 Cerebrovascular accident | 6 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.46, 4.83] |
| 8 Acute confusional state | 6 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.51, 1.40] |
| 9 Deep vein thrombosis | 4 | 591 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.45, 0.91] |
| 9.1 No prophylaxis or early mobilization only | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.41, 0.78] |
| 9.2 Low molecular weight heparin | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.52, 1.84] |
| 9.3 Standard heparin | 1 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Congestive cardiac failure | 6 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.31, 1.96] |
| 11 Acute kidney injury | 2 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.18, 5.83] |
| 12 Pulmonary embolism | 5 | 642 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.51 [1.51, 37.38] |
| 13 Number of patients transfused | 3 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.49, 1.66] |
| 13.1 Fixation | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.15] |
| 13.2 Arthroplasty | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.03, 1.51] |
| 14 Length of hospital stay | 4 | 1143 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.05, 0.65] |
| 15 Length of surgery (minutes) | 12 | 973 | Mean Difference (IV, Random, 95% CI) | ‐2.73 [‐8.50, 3.04] |
| 16 Operative hypotension | 12 | 1056 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.64, 1.35] |
| 16.1 Unilateral | 2 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.37, 0.89] |
| 16.2 Bilateral, incremental doses | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.05, 0.78] |
| 16.3 Bilateral single shot | 8 | 828 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.87, 1.95] |
| 16.4 Epidural | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.50, 2.07] |
Comparison 2. Regional (spinal or epidural) versus lumbar plexus nerve blocks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incomplete or unsatisfactory analgesia | 3 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.11, 0.45] |
| 1.1 Neuraxial (spinal/epidural) block versus lumbar plexus block | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.12, 0.49] |
| 1.2 Neuraxial (spinal/epidural) block versus lumbar plexus, sacral and iliac crest block | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 2.02] |
| 2 Urine retention | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.0 [1.90, 103.00] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Berggren 1987.
| Methods | RCT. The study was approved by the Ethics Committee of the University of Umeå. Informed consent | |
| Participants | Orthopaedic hospital in Umea, Sweden
57 patients with a femoral neck fracture
Mean age 77/78 years (range 65‐92 years)
Male: 19%
Number lost to follow‐up: 4 (7%) Length of follow‐up: 12 months Only fully lucid participants were included in the study For fixation, either hookpins or von Bahr screws were used. In two patients with fractures close to the trochanteric area, fixation was achieved with a sliding screw and a plate Forty‐two of the patients (74%) were operated on within 36 hr of sustaining their fractures, the remaining patients were operated on within 72 hr |
|
| Interventions | Both groups premedicated with pethidine 25‐50 mg Treatment group: Neuraxial block (epidural anaesthesia) with 2% prilocaine in the epidural space, mean volume used 12.5 mL. The catheter was removed in the postanaesthesia care unit (n = 28) Control group: General anaesthesia with thiopentone 3‐4 mg/kg, atropine 0.25‐0.5 mg IV, succinylcholine ventilated with nitrous oxide and oxygen and halothane and succinylcholine infusion (n = 29) | |
| Outcomes | Mortality at 30 days and 3 months
Acute confusional state within 7 days (modified Organic Brain Syndrome Scale) (performed by 2 investigators with a 90% interrater reliability) Cerebrovascular accident Pneumonia (requiring treatment) Congestive heart failure (requiring treatment) Pulmonary embolism (requiring treatment) Operative hypotension (> 30% not responsive to treatment) Length of surgery Urine retention |
|
| Notes | This study was designed to evaluate the difference in postoperative acute confusional state after the surgery. No a priori definition or specification time of evaluation was given for all other types of complications. Thromboprophylaxis with dextran plus mobilization on first postoperative day when possible. No mention on the type of drugs used for postoperative analgesia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no detail |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors were blinded for the postoperative acute confusional state but presence/absence of blinding not mentioned for all other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Four participants lost to follow‐up 4 died by 1 year, 1 in the epidural group on 1st postoperative day, the other 3 (group not given) by 5 months. Patients were interviewed at 6 and 12 months regarding living conditions and walking ability ‐ data not presented |
| Selective reporting (reporting bias) | Unclear risk | Some results (see above) not provided |
| Other bias | Low risk | The two groups were comparable with regard to age, sex, pre‐existing diseases, and preoperative medications (Table 2), except for drugs with anticholinergic effects, which were used significantly more frequently in the epidural group. We identified no other serious risk of bias |
Biboulet 2012.
| Methods | RCT Ethics committee approval and written informed consent from all patients obtained |
|
| Participants | Forty‐five patients older than 75 years, with ASA physical status III or IV, and with severe cardiac comorbidities, presenting for hip fracture and undergoing hip nailing or partial hip replacement The exclusion criteria were contraindication to spinal anaesthesia, allergy to any of the anaesthetic drugs used, and total hip replacement Mean delay before the surgery > 72 hours 75% nails 25% hemiarthroplasty |
|
| Interventions |
Treatment group: Continuous spinal anaesthesia performed with a 19G Sprotte needle and a 23G multiple holes catheter inserted 3 cm cephalad ( n = 15). The operated side was placed up at L3‐L4 or L4‐L5 with 2.5 mg boluses of plain bupivacaine to achieve T10 sensory level or 10 mg. Hyperbaric bupivacaine and 20º Trendelenburg position could be used to achieve T10 thereafter. The catheter was withdrawn in the postanaesthesia care unit Control groups: 1. Target control infusion of propofol infusion was started with an initial target plasma concentration set at 1.5 mcg/mL and adjusted for a BIS value of 50 plus remifentanil (n = 15 randomized; 14 analysed) 2. Sevoflurane for a BIS value of 50 plus remifentanil (n = 15 randomized; 14 analysed) On arrival in the operating room, a femoral nerve block was performed in all patients with 30 mL of ropivacaine 0.5%. Before induction, 500 mL of crystalloid was administered for 30 minutes. During surgery, blood loss was compensated by crystalloid and/or 6% hydroxyethyl starch. Blood transfusion was carried out whenever the haemoglobin level dropped to less than 10 g/dL. Paracetamol and morphine for postoperative analgesia |
|
| Outcomes | Mortality at 1 month Cerebrovascular accident Myocardial infarction (EKG preoperatively and daily for 3 days after the surgery) Cardiac heart failure |
|
| Notes | The two treatment groups were fused and compared to the treatment group Follow‐up of 1 month Presence/absence of thromboprophylaxis not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided into 3 groups", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. One patient withdrawn from each of the control groups |
| Selective reporting (reporting bias) | Low risk | No failed spinal mentioned |
| Other bias | High risk | Groups well balanced. Not in intention‐to‐treat |
Biffoli 1998.
| Methods | Randomized trial: method not stated Informed consent obtained from the patient or a relative |
|
| Participants | District hospital in Italy
60 patients ASA 1 or 2, with a femoral neck fracture aged 70 years and above
Mean age 83 years (range not stated)
Male: 13%
Number lost to follow‐up: probably none Patients with psychiatric disease or those taking psychoactive drugs or with visual, auditory or language disturbances were excluded from the study. No patients received meperidine or anticholinergic drugs |
|
| Interventions |
Treatment group: Spinal anaesthesia (L3‐4 or L4‐5) with a mean dose of 12.7 mg hyperbaric bupivacaine 1%. Supplemental oxygen 40% during the surgery. Ephedrine or atropine for HR < 50 b.p.m. or decrease of 30% or more of the arterial blood pressure (n = 30)
Control group: General anaesthesia with propofol 1 mg/kg, atracurium besilate 0.5 mg/kg, nitrous oxide, isoflurane, atracurium infusion of 0.5 mg/kg/hr and fentanyl 1 mcg/kg as required (n = 30) Eight mL/kg of IV fluids before the induction for all patients. postoperative analgesia with IV ketorolac |
|
| Outcomes | Length of surgery Operative hypotension (number of patients > 30%) | |
| Notes | In Italian Patient group split into 2 groups depending on preoperative mental state: 38 who were not confused and 22 who were six points or less were considered orientated and seven or more disorientated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No failed spinal mentioned |
| Other bias | Low risk | Groups well balanced |
Bigler 1985.
| Methods | Randomized trial: method not stated. Informed consent was obtained from all patients and the protocol was approved by the ethical committee of Copenhagen hospitals | |
| Participants | Place and country of study not stated
40 patients with a proximal femoral fracture operated within 48 hours
Mean age 79 years.
Male: 17.5%
Loss to follow‐up: not known Patients in whom there were contraindications to spinal analgesia, with subtrochanteric fractures, severe dementia, cancer and with psychiatric or disseminated neurological disease were not studied Lateral approach to the hip fixation |
|
| Interventions |
Treatment group: Spinal anaesthesia with 3 mL of 0.75% bupivacaine at L3‐4 in the lateral decubitus position on the fractured side (n = 20)
Control group: General anaesthesia using atropine, fentanyl, pancuronium, nitrous oxide/oxygen, diazepam and suxamethonium (n = 20) All were premedicated with intramuscular pethidine 75 mg and normal saline 10 mL/kg was given intravenously before anaesthesia. if systolic arterial blood pressure fall exceeded 30% of the preoperative value, intravenous and intramuscular ephedrine 12.5 and 37.5 mg respectively were given |
|
| Outcomes | Length of surgery Number of patients transfused Mortality at 1 month Acute confusional state within 7 days Pneumonia Cerebrovascular accident Congestive cardiac failure Pulmonary embolism |
|
| Notes | Length of follow‐up: 3 months. This study was designed to evaluate the difference in postoperative acute confusional state after the surgery. No a priori definition or specification time of evaluation was given for all other types of complications. Attempt to early mobilization only as thromboprophylaxis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated to spinal analgesia or general anaesthesia." No details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinded to the anaesthetic technique used for mental tests Unspecified for the other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout or failed block reported |
| Selective reporting (reporting bias) | Low risk | No failed spinal mentioned |
| Other bias | Low risk | The two groups were comparable in relation to age, sex, ASA physical status, preoperative haemoglobin and blood pressure. Preoperative mental scores were slightly (not statistically significantly) lower in the spinal group |
Bredahl 1991.
| Methods | Randomized trial: method not stated Approved by the Ethics Committee and informed consent obtained from each patient |
|
| Participants | Orthopaedic hospital Aalborg, Denmark
30 ASA I or II physical status women with a proximal femoral fracture
Mean age 79 years (range 60‐90)
Male: 0%
Loss to follow‐up: not stated, but 2 excluded due to incomplete data Operated within 24 hours |
|
| Interventions |
Treatment group: Ephedrine 25 mg IM plus IV ephedrine for pre‐spinal hypotension (2 patients). Spinal anaesthesia at L2‐3 or L3‐4 with 2.5‐3 mL of 0.5% plain bupivacaine in lateral decubitus. Oxygen supplement during the surgical intervention, sedation with IV diazepam (n = 15)
Control group: General anaesthesia using thiopentone, pethidine, pancuronium, nitrous oxide/oxygen, IPPV, and suxamethonium (n = 15 randomized; 13 analysed) Premedication with pethidine 0.5 mg/kg for all patients. IM Nicomorphine for postoperative analgesia |
|
| Outcomes | Length of surgery | |
| Notes | Study designed to examine the difference in body temperature between the two anaesthetic technique Length of follow‐up: 3 days Presence/absence of thromboprophylaxis not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two patients excluded from the general anaesthesia group for incomplete data |
| Selective reporting (reporting bias) | Low risk | No failed spinal mentioned |
| Other bias | Low risk | Groups well balanced |
Brichant 1995.
| Methods | RCT Approved by the Ethics Committee and informed consent obtained from each patient |
|
| Participants | Orthopaedic hospital in Brussels, Belgium
106 patients with proximal femoral fracture
Age: not stated
Male: percentage not stated
Number lost to follow‐up: not stated Operated between 10 and 72 hours after admission Length of follow‐up: 10 days |
|
| Interventions | Treatment group: Neuraxial block (spinal or epidural) anaesthesia with bupivacaine (n = 54 randomized; 46 analysed) Control group: General anaesthesia administered according to 'local practice' (n = 52 randomized; 42 analysed) | |
| Outcomes | Deep vein thrombosis (venography) Pulmonary embolism (confirmed with angiography or lung ventilation/perfusion scan) | |
| Notes | Conference abstract only All patients had subcutaneous nadroparin for DVT prophylaxis for 10 days and contralateral stocking | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent panel of experts unaware of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | > 20% excluded from analysis |
| Selective reporting (reporting bias) | Low risk | No failed spinal mentioned |
| Other bias | Low risk | Groups said to be well balanced |
Brown 1994.
| Methods | RCT Approved by the local Ethics Committee and signed written informed consent from each participant |
|
| Participants | Orthopaedic hospital in Hong Kong 20 ASA 2 or 3 patients with a proximal femoral fracture Mean age 77 years (range 66‐91) Male: 50% Number lost to follow‐up: not stated | |
| Interventions | Premedication with pethidine or temazepam Treatment group: Spinal (subarachnoid) anaesthesia with 0.2 mg/kg hyperbaric bupivacaine (n = 10) Control group: General anaesthesia using thiopentone or propofol, isoflurane or enflurane atracurium and nitrous oxide/oxygen (n= 10) |
|
| Outcomes | Operative hypotension (requiring administration of vasopressor) | |
| Notes | Length of follow‐up: 2 days (up to 44 hours) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized trial: use of random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No failed neuraxial block mentioned |
| Other bias | Low risk | Groups well balanced |
Cao 2008.
| Methods | RCT Approved by the Ethics Committee and written informed consents obtained |
|
| Participants | 60 ASA 2 or 3 participants, aged between 65 and 97 years old, weighting 45 to 75kg scheduled for unilateral intertrochanteric femoral fracture repair | |
| Interventions |
Treatment group : Posterior lumbar plexus block with 20 mL of bupivacaine 1.0% and 30 mL of ropivacaine 0.5% injected when a contraction of the quadriceps was obtained at 0.3 mA (disappearing at 0.2 mA) with the side to be blocked uppermost (n = 30) Control group: Lumbar epidural with ropivacaine 0.75% for a sensory level at L1‐L2 (n = 30) |
|
| Outcomes | Urinary retention | |
| Notes | No anaesthesia or local anaesthetic toxicity‐related complications happened in two groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No cross‐over |
| Other bias | Low risk | Groups well balanced |
Casati 2003.
| Methods | RCT Approval was obtained from our Institutional Ethics Committee and written informed consent from each participant |
|
| Participants | Orthopaedic hospital in Milan, Italy 30 patients of ASA grade II or III undergoing hemiarthroplasty for a proximal femoral fracture Mean age 84 years (range 67‐94) Male: 7% Number lost to follow‐up: 0 | |
| Interventions |
Treatment group: Spinal anaesthesia at L3‐4 with 7.5 mg of hyperbaric bupivacaine injected in lateral decubitus with the patient maintained in this position for 15 minutes (n = 15)
Control group: General anaesthesia with sevoflurane inhalation and laryngeal mask airway. No neuromuscular blocking agent (n = 15) All patients received a standardized protocol for preoperative fluid resuscitation, including blood The day before surgery, all patients had a standard preoperative evaluation, including chest radiography, electrocardiography and routine laboratory tests. Preoperative analgesics consisted of IV ketorolac (30 mg every 8 h). Preoperative fluid resuscitation including transfusion if required to maintain haemoglobin concentration 9 G/L. Postoperative analgesia with tramadol and ketorolac Length of follow‐up: 7 days & hospital discharge |
|
| Outcomes | Operative blood loss (taken as P value) Operative hypotension (20% decrease, period undefined). Numbers taken are those requiring a bolus of fluid Acute confusional state within 7 (defined as maximal number of patients with a decrease of 2 points or more on the Mini mental test and this occurred at 24 hours while patients were tested at 24 hours and 7 days) | |
| Notes | Absence/presence of thromboprophylaxis unspecified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated". Probably adequate considering the use of sealed envelopes although not clearly mentioned |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No failed spinal mentioned |
| Other bias | Low risk | Groups well balanced |
Couderc 1977.
| Methods | RCT | |
| Participants | Orthopaedic hospital in Paris, France
100 patients with a proximal femoral fracture (nail and plate n = 56; hemiarthroplasty n = 44)
Mean age 86 years. (Inclusion criterion: 80+ years; range not stated)
Male: 14%
Number lost to follow‐up: not stated Operated within 24 hours after admission |
|
| Interventions |
Treatment group: Five to seven hundreds mL of fluid (LR or colloid). Neuraxial block (epidural anaesthesia) in lateral decubitus consisting of single shot or continuous infusion of 0.5% bupivacaine and adrenaline with or without lidocaine (n = 50)
Control group: General anaesthesia with thiopentone, pancuronium or succinylcholine, dextromoramide or methoxyflurane, nitrous oxide/oxygen (n = 50) Premedicated with hydroxyzine and atropine |
|
| Outcomes | Mortality at 3 months (cumulative) Operative hypotension ( ≥ 40 mm HG; number of patients at induction of anaesthesia) Myocardial infarction (serial preprogrammed EKGs and enzymes) | |
| Notes | In French Length of follow‐up: 3 months Complete data for fatal myocardial infarction, congestive heart failure and pulmonary embolism not provided Early mobilization and anti‐vitamin K from day 3 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized study: by 'drawing of lots' |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | EKG interpreted by a blinded cardiologist. Not blinded for the other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No failed epidural reported |
| Other bias | Low risk | Groups well balanced |
Davis 1981.
| Methods | Randomized trial: method not stated | |
| Participants | Orthopaedic hospital Christchurch, New Zealand
132 patients with a proximal femoral fracture (intertrochanteric, basicapital or subcapital) and coming within 72 hours of the injury
Mean age 81/78 years (Inclusion criterion: 50+, range not given)
Male: 15%
Number lost to follow‐up: 0 Patients < 50 years of age, requiring arthroplasty or with cardiac heart failure were excluded |
|
| Interventions |
Treatment group: Spinal anaesthesia in lateral decubitus on the fractured side using hyperbaric tetracaine 0.5% in 51 patients and hyperbaric 0.5% cinchocaine in 13 patients. Patients were retained in this position for 10 minutes. Ketamine also used for sedation in 8 patients (positioning for the spinal). Sedation also provided with diazepam (mean dose 9 mg). Supplemental oxygen or a mixture of nitrous oxide and oxygen during the surgery (n = 64)
Control group: General anaesthesia with diazepam (2.5‐30 mg) mean dose 9.5 mg, fentanyl 1‐3 mcg/kg, nitrous oxide and oxygen, IPPV, pancuronium (mean dose 6 mg) (n = 68). Infusion of 200‐400 mL of fluids before induction for all patients Length of follow‐up : 1 month |
|
| Outcomes | Mortality ‐ at 1 month
Operative hypotension Number of patients transfused Pneumonia Cerebrovascular accident Congestive heart failure Acute kidney injury Deep vein thrombosis (fibrinogen) (available in 76 patients only) |
|
| Notes | Presence/absence of thromboprophylaxis not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete data for deep venous thrombosis (76/132) |
| Selective reporting (reporting bias) | Low risk | Eight failed spinal |
| Other bias | Low risk | Groups well balanced. Intention‐to‐treat analysis |
Davis 1987.
| Methods | RCT Approved by the five Ethics Committee of the participating hospitals |
|
| Participants | Orthopaedic hospitals in New Zealand ‐ multicentre study
549 (538 analysed) participants with a proximal femoral fracture
Mean age 79.5 years (range not stated)
Male: 22%
Number lost to follow‐up: 0, but 11 excluded 20% subcapital and 80% trochanteric fractures Excluded if < 55 years of age, arthroplasty required, pathological fracture or contraindication to one anaesthetic technique |
|
| Interventions | Treatment group: Spinal anaesthesia with sedation with diazepam. Hyper of isobaric tetracaine, nupercaine or bupivacaine for spinal (n= 259 analysed) Control group: General anaesthesia with pre‐oxygenation, IV induction with thiopentone, IPPV maintained with nitrous oxide/oxygen, non‐depolarizing neuromuscular blocker, fentanyl (n = 279 analysed) | |
| Outcomes | Mortality at 1 month (28 days) Operative hypotension requiring vasopressor Length of hospital stay | |
| Notes | There was 1 non fatal anaphylactoid reaction at induction of general anaesthesia Length of follow‐up: 1 month A longer duration of follow‐up was available for a fraction of the patients only and therefore was not reported to decrease the chances of selective reporting |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized with stratification by sex and hospital |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 excluded out of 549. Exclusion were made on pre‐defined criteria: age less than 55 years, multiple trauma, possible replacement or hemi‐arthroplasty or pathological fractures |
| Selective reporting (reporting bias) | Low risk | 30 failed spinal and 14 incomplete |
| Other bias | Low risk | Groups well balanced. Intention‐to‐treat |
de Visme 2000.
| Methods | RCT Approved by the Research and Ethics Committees and written informed consent from all patients |
|
| Participants | Orthopaedic hospital in Brest, France
29 patients with a proximal femoral fracture (69% trochanteric and 31 % femoral neck)
Mean age 85 years (range 68‐97)
Male: 17%
Number lost to follow‐up: none Evidence of cognitive deficit (MMSE lower than 5), contraindication to SA, or peripheral nerve block, resulted in exclusion Length of follow‐up: not stated but probably 5 days |
|
| Interventions |
Treatment group: Lumber plexus (30 mL Winnie's technique), sacral plexus (10 mL Mansour's technique) with 1.33% lidocaine and adrenaline (1:240,000) and 5 mL 1% lidocaine for the iliac crest block (for lateral cutaneous nerve) (n = 15). Neurostimulation at 0.8 mA accepted Control group: Spinal anaesthesia at L3‐4 with 3 mL 0.5% plain bupivacaine (n = 14) All patients received 250 mcg alfentanil before being turned to the lateral position, with the operated side uppermost The patients were given 5 mL/kg of Hartman solution before the 5 mL/kg/h of the same solution until transferred to the recovery room. Ephedrine for systolic < 90 mmHG or 30% decrease |
|
| Outcomes | Length of surgery Acute confusional state within 7 days |
|
| Notes | There were no complications that could be attributed to the techniques In both groups, adequate muscle relaxation was present for all patients to ensure manipulation for positioning of the injured extremity and fracture reduction Presence/absence of thromboprophylaxis not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly assigned by the hospital pharmacy just before transfer to the operating area to 2 groups |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Patients in the CPNB group required light adjunct of drugs (alfentanil 250 mcg) 3 times for the incision (anaesthesia was judged incomplete) and 1 patient received sedation repeatedly (anaesthesia was judged unsatisfactory). No conversion to GA |
| Other bias | Unclear risk | Groups well balanced |
Eyrolle 1998.
| Methods | RCT Ethics Committee approval |
|
| Participants | Orthopaedic hospital in Paris, France 50 patients with a proximal femoral fracture Mean age 82 years (range not stated) Male: % not stated Number lost to follow‐up: none probably | |
| Interventions |
Treatment group: Lumber plexus block using equal volumes of 2% lidocaine and 0.5% bupivacaine with 1:200,000 epinephrine (n = 25) Control group: Spinal anaesthesia with 0.5% bupivacaine (n = 25) A light sedation with propofol intravenously, as required |
|
| Outcomes | Operative hypotension (mean arterial blood pressure decrease > 20%) | |
| Notes | Conference abstract only presence/absence of thromboprphylaxis not mentioned In the PNB group, 19/25 patients required a propofol infusion > 1 mg/kg/h. A lumbar plexus block alone was considered insufficient to provide adequate anaesthesia for hip fracture repair |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized in two groups", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No failed block mentioned |
| Other bias | Low risk | Groups said to be equivalent |
Heidari 2011.
| Methods | RCT This study was approved by the Ethics Committee |
|
| Participants | Four hundred patients older than 30 years old (in class I, II, and III of ASA classification) who were scheduled for elective operative fixation of fractured hip Patients were included if they had no dementia or cognitive dysfunctions and no history of opioid or psychotic drugs use. Patients with hypersensitivity to blood transfusion, sever reaction to cement implantation, sever bleeding, hypotension needed interventions, prolonged operation changed surgical plan which impressed the study goals, were excluded 54.5% trochanteric 20.9% femoral neck 9.8% subtrochanteric Mean time of surgery > 72 hours after admission |
|
| Interventions |
Treatment group: patients were given Ringer's lactate solution (15 mL/kg) prior to the induction of anaesthesia and received either spinal with plain bupivacaine 0.5% (3 mL) at L3‐L4 or epidural anaesthesia with 25 mL of 0.5% bupivacaine with epinephrine 1:200,00 performed with a 18‐G‐Touhy needle at the L3‐L4 (n = 190) Control group: Patients were given Ringer's lactate solution (10 mL/kg), thiopental, fentanyl, nitrous oxide, halothane and pancuronium. Residual neuro‐muscular block was antagonized (n = 197) All patients were infused Ringer's lactate solution (4 mL/kg) on arrival at the operating room to all patients. postoperative analgesia with morphine |
|
| Outcomes | Operative hypotension (MAP < 70% of baseline or 65 mm HG) Length of surgery Length of hospital stay (after the surgery) Mortality at 1 month Acute confusional state in hospital Myocardial infarction Congestive heart failure Pneumonia Thrombosis Pulmonary embolism Cerebrovascular accident |
|
| Notes | "low dose of heparin as a DVT prophylaxis" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned into two groups using random‐number table" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Three patients of the general anaesthesia group and 10 of the neuraxial block group were excluded because of change in anaesthetic or surgical plan" |
| Selective reporting (reporting bias) | Low risk | Recruitment ended with the completion of the protocol in the 387th patient. |
| Other bias | High risk | "Groups are similar regarding age, weight and gender ratio." Not in intention‐to‐treat: "Three patients of the GA group and 10 of the NA group were excluded because of change in anaesthetic or surgical plan" |
Hoppenstein 2005.
| Methods | RCT Institutional review board–approved and written informed consent was obtained before patient inclusion into the study |
|
| Participants | Sixty ASA physical status I, II, and III geriatric patients at least 60 years of age, undergoing surgical fixation of the neck of femur Patients with known haemoglobinopathy, as well as those with a clinical history of cerebrovascular or carotid artery disease, were excluded from the study. |
|
| Interventions |
Treatment group: spinal anaesthesia performed with the patient in the lateral decubitus position and 4 mg isobaric bupivacaine plus 25 mcg of fentanyl via a 25‐gauge pencil‐point needle (n = 30) Control group: General anaesthesia with thiopental, fentanyl, nitrous oxide, isoflurane and vecuronium (n = 30) |
|
| Outcomes | Length of surgery | |
| Notes | Presence/absence of thromboprophylaxis not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were allocated via computer‐generated randomization schedule to one of two treatment groups |
| Allocation concealment (selection bias) | Low risk | Randomized after enrolment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "open label study" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "open label study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No conversion to general anaesthesia |
| Other bias | Low risk | Groups well balanced |
Ibanez 1993.
| Methods | RCT | |
| Participants | Patients undergoing hip fracture repair by Ender's fixation | |
| Interventions |
Treatment group: spinal anaesthesia (n = 30) Control group: general anaesthesia (n = 30) |
|
| Outcomes | Mortality Deep vein thrombosis |
|
| Notes | Conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "at random" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Unclear risk | Standard deviation not provided |
| Other bias | Unclear risk | Few details, conference abstract |
Juelsgaard 1998.
| Methods | RCT Approved by the Ethics Committee |
|
| Participants | Orthopaedic hospital in Aarhus, Denmark 29 followed‐up out of 54 patients with proximal femoral fracture and known coronary artery disease. Patients with a recent (< 6 months) myocardial infarction excluded For 29 patients included in this review: Age: mean 80.9 years (range 65‐99) Male: 13% Number lost to follow‐up: 0, but 11 excluded from original trial population | |
| Interventions |
Treatment groups: Single shot (n = 15) with 2.5 mL of 0.5% bupivacaine or incremental doses (0.5 mL every 15 minutes) (n = 14) of the same drug. Considered as two subgroups of the same study
Control group: General anaesthesia with fentanyl 1‐2 mcg/kg, 1‐4 mg/kg thiopentone, 0.5 mg/kg atracurium, nitrous oxide and oxygen and enflurane (n = 14). This group was split in half to be compared with each treatment group separately All patients were premedicated with pethidine 1 mg/kg and received supplemental oxygen for 12 hours after the surgery |
|
| Outcomes | Mortality at 1 month Operative hypotension (number of patients with 33% reduction in systolic arterial blood pressure from baseline) Myocardial infarction (World Health Organization criteria) | |
| Notes | Presenc/absence of thromboprophylaxis not mentioned Length of follow‐up: 1 month |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The investigator was blinded to the treatment group" (ischemias) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No failed spinal mentioned |
| Other bias | Low risk | Groups well balanced |
Kamitani 2003.
| Methods | RCT Oral informed consent |
|
| Participants | 40 patients with a femoral neck fracture
Mean age 82 years (range ‐ not stated)
Male: 10%
Number lost to follow‐up: 0 Patients who could not communicate well were excluded in this study |
|
| Interventions |
Treatment group: Spinal anaesthesia with 3 mL of 0.5% isobaric bupivacaine (n = 19)
Control group: General anaesthesia with propofol (0.5‐1 mg), vecuronium (0.5‐1 mg/kg), nitrous oxide, sevoflurane and fentanyl (0.1‐0.2 mg/kg) and local field block with local anaesthesia (n = 21) Diclofenac for postoperative analgesia |
|
| Outcomes | Length of surgery Acute confusional state (maximal incidence within 7 days) (Inoue's " the confusion assessment method diagnosis algorithm" by the nurse on the floor) | |
| Notes | In Japanese Length of follow‐up: 4 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No failed spinal mentioned |
| Other bias | Low risk | Groups well balanced |
Maurette 1988.
| Methods | RCT Consent obtained from each patient. |
|
| Participants | Orthopaedic hospital Bordeaux, France
35 patients with a proximal femoral fracture Garden's screw or Hender's nail Mean age 83 years (> 70 years) Male: % not stated Number lost to follow‐up: not stated, but 2 excluded as they failed to participate in postoperative tests |
|
| Interventions |
Treatment group: Spinal anaesthesia with 1.5 mg/kg prilocaine (n = 19 randomized; 18 analysed)
Control group: General anaesthesia using thiopentone, spontaneous ventilation, nitrous oxide/oxygen, enflurane, dextromoramide (n = 16 randomized; 15 analysed) No premedication |
|
| Outcomes | Operative hypotension (number of patients with 20% decrease in MAP) Length of surgery |
|
| Notes | In French Length of follow‐up: 3 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'random draw' |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient from each group did not participate in the test and was excluded from the analysis |
| Selective reporting (reporting bias) | Low risk | No failed spinal mentioned |
| Other bias | Low risk | Groups well balanced (included similar results for mental tests before the surgery: 52.2 ± 8 and 52.5 ± 8.5 for spinal and GA respectively) |
McKenzie 1984.
| Methods | Randomized trial: use of envelopes containing random numbers | |
| Participants | Orthopaedic hospital in Glasgow, Scotland
150 patients with fractured neck of femur Nail/plate no hemiarthroplasty Mean age 75 years (range not stated) Male: % not stated Mean delay before the surgery 48 hours Number lost to follow‐up: 0, but 2 excluded due to postponement of operation |
|
| Interventions | Treatment group: Spinal anaesthesia with 0.5% hyperbaric cinchocaine 1.3‐1.5 mL at L3‐4 or L4‐5 . Supplemented by small doses of diazepam if required (n = 73) Control group: General anaesthesia induced with althesin 1‐3 mL, suxamethonium 50 mg, nitrous oxide and oxygen, halothane and spontaneous respiration (n = 75) | |
| Outcomes | Mortality at 1, 3, 6 and 12 months Length of surgery Operative blood loss Length of hospital stay Deep vein thrombosis (venography; subgroup of 40) Returned home | |
| Notes | Additional information supplied by Dr McLaren indicated that all the references referred to one study. Additional data on mortality supplied Length of follow‐up: 12 months The venography study for DVT detection involved a subgroup of 40 patients |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | " randomly allocated." No details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. Over the period study, operation was postponed in only two patients (not included in the 150). In two patients, the subarachnoid space could not be identified and these patients received general anaesthesia and were excluded from the study. |
| Selective reporting (reporting bias) | Low risk | The authors provided results for all measurements for 148 patients. |
| Other bias | High risk | Groups well balanced. Not in intention‐to‐treat: two patients randomized, excluded (surgery postponed) |
McLaren 1978.
| Methods | Randomized trial: method not stated | |
| Participants | Orthopaedic hospital in Glasgow, Scotland
116 patients with fractured neck of femur
Mean age 76 years.
Male: % not stated
Number lost to follow‐up: none for the original report of 55 cases. Loss to follow‐up not reported in the later study report (1982) of 116 cases Nail and plate only, hemiarthroplasty excluded from the study |
|
| Interventions | No premedication Treatment group: Spinal anaesthesia with 0.5 mL hyperbaric cinchocaine 0.5% injected in lateral decubitus position with the patient lying on the fractured side. the patient was maintained in that position for 3 minutes. Patients sedated with 10% althesin in 5% dextrose during operation (n = 36/56) Control group: General anaesthesia with althesin 50 mcg/kg, pancuronium bromide 0.1 mg/kg, IPPV, nitrous oxide, oxygen and fentanyl 0.05 mg as needed, reversal of neuromuscular blocking drugs at the end (n = 39/60) | |
| Outcomes | Mortality at 1 month
Operative hypotension (decrease in systolic blood pressure greater than 50% of baseline) Pneumonia |
|
| Notes | The original paper in 1978 reported the results for 55 cases. A later report in 1982 of the same study gave the outcome for 116 patients. The latter report was used for the outcomes of mortality at one month. The original paper was used for the other outcomes for 55 patients. The methodology assessment was based on the 1978 report. Length of follow‐up: 1 month minimum |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No failed spinal mentioned |
| Other bias | Unclear risk | Fourteen patients in the spinal group (54%) and ten patients in the general anaesthetic group (34%) had signs and/or symptoms of respiratory problems preoperatively |
Messina 2013.
| Methods | RCT Approved by the Ethics Committee |
|
| Participants | Patients > 75 years of age admitted with fractured hips not scheduled for hip replacement | |
| Interventions |
Treatment group: Spinal with 7.5 mg of levobupivacaine plus 5 mcg of sufentanil performed in the lateral position with the fractured side uppermost. Patients were kept in this position for 5 minutes (n = 10) Control group: General anaesthesia with propofol, sevoflurane, remifentanil and cisatracurium (n = 10) All patients received Ringer's Lactate solution 500 mL rapidly administered via the peripheral line before either spinal or general anaesthesia induction, Voluven", (hydroxyethylstarch 6%), 20 mL X BMI l3 was used to treat the first episode of hypotension in both groups |
|
| Outcomes | Surgery (min) Operative hypotension (MAP decrease of 25% or more) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No failed spinal mentioned: "All patients in subarachnoid anaesthesia group had an effective intraoperative anaesthesia and analgesia" |
| Other bias | Low risk | Groups well balanced |
Racle 1986.
| Methods | RCT Consent obtained from the family or the patient for the spinal anaesthesia group |
|
| Participants | Orthopaedic hospital in Cedex, France
70 female patients with a proximal femoral fracture
Mean age: 82 years (Inclusion criterion: 75+, range not given)
Male: 0%
Number lost to follow‐up: not state Hemi‐ or total hip arthroplasty |
|
| Interventions | Premedication with IM hydroxyzine and atropine Treatment group: 200‐300 IV fluids before the spinal and 8 mL/kg after. Spinal anaesthesia with 3 mL 0.5% bupivacaine + adrenaline with the patients lying on the fractured side (n = 35). Flunitrazepam for sedation Control group: General anaesthesia using thiopentone, vecuronium, fentanyl, nitrous oxide/oxygen, enflurane (n = 35) |
|
| Outcomes | Mortality at 1 and 3 months
Length of surgery
Operative hypotension (number of patients with > 20% decrease in systolic arterial blood pressure)
Length of hospital stay
Pneumonia Congestive cardiac failure Acute confusional state (maximal number observed) Pulmonary embolism Acute kidney injury Cerebrovascular accident |
|
| Notes | Length of follow‐up: 3 months In French Early mobilization and heparin for thromboprophylaxis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization table Cochran and Cox" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No failed spinal |
| Other bias | Low risk | Groups well balanced |
Spreadbury 1980.
| Methods | RCT No specific consent "as not double‐blind" Protocol changed to restrict the trial to patients > 80 years in view of poor surgical results in the Ketamine group |
|
| Participants | Orthopaedic hospital in Warwick, England
60 previously ambulant female patients with a proximal femoral fracture
Mean age 84 years (range not stated)
Male: % not stated Nail/plate (45%) or arthroplasty (55%) Number lost to follow‐up: none |
|
| Interventions | Atropine 0.6 mg, with analgesics and oral diazepam as required Treatment group: Ketamine anaesthesia with ketamine 2 mg/kg at induction then ketamine 1 mg/kg as required. Also optional diazepam (n = 30) Control group: General anaesthesia using drugs and method chosen by the anaesthetist. Twenty patients received neuromuscular blocking agents (n = 30) Postoperative analgesia with papaveretum or pethidine ans supplemental oxygen for 24 hours |
|
| Outcomes | Mortality during hospital stay Length of hospital stay (for patients discharged home only) Returned home Unsatisfactory surgical results (unstable nail/plate or prosthesis dislocation) | |
| Notes | Length of follow‐up: not stated Presence/absence of thromboprophylaxis not mentioned Total number of patients taken as the denominator for the outcome "return home" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned up to 14 days. |
| Selective reporting (reporting bias) | Low risk | The authors provided results for all measurements, no evidence of selective reporting. |
| Other bias | High risk | More certified (versus in training) surgeons for the older patients in the ketamine group Unclear exactly how many patients came from home. Not in intention‐to‐treat for all results |
Svarting 1986.
| Methods | RCT Approved by the Ethics Committee and informed consent obtained from each patient |
|
| Participants | University hospital in Helsinki, Finland
30 patients with a proximal femoral fracture treated with a Thompson prosthesis. (ASA grade II OR III)
Mean age 77 years (range not stated)
Male: 13% Thompson's prosthesis Number lost to follow‐up: none likely |
|
| Interventions | Both groups premedicated with pethidine and atropine Treatment group : Spinal anaesthesia using 3 mL of 0.5% isobaric bupivacaine into the subarachnoidal space. Patients maintained for 3 min in the lateral decubitus position Balanced salt solution 10 mL/kg (n = 15) Control group: General anaesthesia using fentanyl, thiopental, pancuronium bromide, nitrous oxide/oxygen, then atropine and neostigmine (n = 15) |
|
| Outcomes | Operative hypotension Length of surgery Number of patients transfused |
|
| Notes | Length of follow‐up: not stated Emphasis in the article on the connection of the results with the use of methylmethacrylate cement for the Thompson prosthesis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No failed spinal mentioned |
| Other bias | Low risk | Groups well balanced |
Tasker 1983.
| Methods | RCT | |
| Participants | Orthopaedic hospital in Leicester, England
100 patients with a proximal femoral fracture
Mean age not stated.
Male: % not stated Subcapital fractures Thompson's prosthesis Number lost to follow‐up: not stated |
|
| Interventions |
Treatment group : Spinal Control group: General anaesthesia Exact method of anaesthesia not stated. The number of participants attributed to each treatment group is unspecified. For the purpose of this review, we counted them as 50 participants in each group |
|
| Outcomes | Mortality. This result was not included in the analysis: time point unknown | |
| Notes | Conference abstract only Length of follow‐up: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random selection", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned, conference abstract |
| Selective reporting (reporting bias) | Unclear risk | Few details, conference abstract only |
| Other bias | Unclear risk | No details |
Ungemach 1993.
| Methods | RCT | |
| Participants | Orthopaedic hospital in Mannheim, Germany 114 patients with a proximal femoral fracture Mean age 79 years (range not stated). Male: 16% Number lost to follow‐up: not stated | |
| Interventions |
Treatment group: spinal anaesthesia with 3‐4 mL of 0.5% hyperbaric bupivacaine (n = 57) Control group: general anaesthesia with isoflurane, fentanyl, nitrous oxide/oxygen (n = 57) |
|
| Outcomes | Mortality at 2 weeks only. This result was not included in the analysis | |
| Notes | Conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | No failed spinal mentioned |
| Other bias | Low risk | Groups said to be comparable |
Valentin 1986.
| Methods | RCT "It was decided not to obtain informed consent from the patients as to participation in the study because a large proportion of patients with hip fractures are old, and mentally unable to reach a logical decision." "Human research committees were not established in Denmark at the start of this investigation" |
|
| Participants | Orthopaedic hospital in Hellerup, Denmark
662 patients (578 analysed) with a proximal femoral fracture
Mean age 79 years (range 50 ‐ 100)
Male: 20% internal fixation (63%) or hemiarthroplasty (37%) (Moore) Number lost to follow‐up: 2 (0.3%), 84 patients excluded patients wow‐dose heparin treatment were excluded |
|
| Interventions | No premedication was given to poor‐risk patients; in the others, premedication consisted of pethidine 25‐75 mg, often supplemented with promethazine 12.5‐25 mg, both administered IM, approximately 30 min before the induction of anaesthesia Treatment group: Spinal anaesthesia with 3‐4 mL isobaric bupivacaine injected in the lateral decubitus position. it is unclear whether the fractured side was up or down. Patients were left in that position for 5‐15 minutes until a sensory level to the lower thoracic segments was achieved. Patients were sedated with fentanyl 0.05‐0.1 mg IV and midazolam and some received supplemental oxygen. (n = 281) Control group: General anaesthesia with enflurane and nitrous oxide/oxygen with or without thiopentone at induction or neurolept anaesthesia with droperidol, fentanyl and nitrous oxide/oxygen. All patients having neurolept anaesthesia, and the majority of the patients receiving enflurane received gallamine to provide neuromuscular blockade during the surgical procedure. (n = 297) |
|
| Outcomes | Mortality at 1 month, 3, 6 and 12 months (read from graphs) | |
| Notes | Length of follow‐up: 24 months Mobilization on postoperative day 2 or 3 Antiembolic stockings were provided, but anticoagulant therapy was not given routinely |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 84 patients excluded from the analysis. Their allocation is not mentioned |
| Selective reporting (reporting bias) | Low risk | Not in intention‐to‐treat: "Of the 662 patients admitted to the study, 84 were later excluded by the authors for the reasons listed in table II, leaving data from 578 patients for consideration" |
| Other bias | High risk | "the two anaesthetic groups were comparable with respect to age, sex, type of fracture and surgical procedure. Classification according to the ASA criteria was applied to 508 patients: there were more patients in ASA group III in the general anaesthesia group, and more of group IV in the spinal anaesthesia group (P value < 0.02) (table IV). Not in intention‐to‐treat: "Of the 662 patients admitted to the study, 84 were later excluded by the authors for the reasons listed in table II, leaving data from 578 patients for consideration" |
Wajima 1995.
| Methods | RCT Informed consent of this studies were obtained from the patients and families |
|
| Participants | Hospital in Higashine, Japan
41 (randomized) patients with a femoral neck fracture
Mean age 80 (range: inclusion criteria ages 70‐90 years)
Male: 22% Number lost to follow‐up: Probably none Operated with a mean delay of 7 and 10 days after the surgery |
|
| Interventions | Treatment group: Epidural anaesthesia with continuous infusion of bupivacaine and butorphanol for 72 hours postoperatively (n = 16; 8 between 70 and 80 years of age and 8 > 80 years of age) Control group: General anaesthesia with thiopental, succinylcholine, nitrous oxide and sevoflurane. Postoperative analgesia with diclofenac. (n = 25; 10 between 70 and 80 years of age and 15 > 80 years of age) | |
| Outcomes | Mental tests (Hasegawa dementia scale scores) at 7 days (a high score means good mental function). Not retained in the analysis | |
| Notes | In Japanese Length of follow‐up: 1 week The study contains participants with abdominal and hip fracture surgery (41 randomized and 9 not randomized) for a total of 98 participants. Results in tables are provided for 40 participants (19 for neuraxial blockade and 21 for general anaesthesia). These tables contain results for delirium after the surgery. A letter was sent to authors on January 27, 2016 to obtain number of participants with hip surgery who experienced acute confusional state within seven days. We did not receive any reply so far. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "randomly allocated", no details and size of the groups unequal (16 versus 25) |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No failed epidural mentioned |
| Other bias | Unclear risk | Patients of the GA group in the 70s had higher preoperative scores while those in the 80s had lower ones |
White 1980.
| Methods | RCT "consent was sought for inclusion in the trial" |
|
| Participants | Orthopaedic hospital in Cape Town, South Africa
40 of 60 patients in trial with a proximal femoral fracture of less than 8 days Operated at a mean of 3.5 days from the fracture Zimmer sliding screw (64%) or Moore prosthesis (36%) Mean age 79 years (> 60 years [range not stated]) Male: 8% Number lost to follow‐up: 0 |
|
| Interventions | All patients received diazepam 10 mg orally 2 hours before surgery Treatment group 1: Spinal anaesthesia with 0.6‐0.8 mL hyperbaric cinchocaine and 'light' general anaesthesia with althesin, fentanyl, nitrous oxide/oxygen. Injection in the lateral decubitus position and maintained lying on the fractured side for 5 minutes (n = 20) Treatment group 2: Psoas nerve block with 30 mL 2% mepivacaine and 'light' general anaesthesia with fentanyl and althesin (n = 20 randomized; n = 16 analysed). Chayen's technique with the fracture side uppermost. This group was not retained (outside the scope of the present review) Control group: General anaesthesia with thiopentone, suxamethonium, nitrous oxide/oxygen, halothane, fentanyl. Competitive neuromuscular blocking drugs were not used.This group (n = 20) was split in two for comparison with the two treatment groups |
|
| Outcomes | Mortality at 1 month
Length of surgery
Pneumonia
Acute confusional state
Deep vein thrombosis Total medical complications (pneumonia, deep vein thrombosis) |
|
| Notes | Length of follow‐up: minimum 4 weeks presence/absence of thromboprophylaxis not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No failed block mentioned |
| Other bias | Unclear risk | Groups well balanced except possibly for a lower number of preoperative pneumonia in the Psoas compartment block group |
Abbreviations AMT: Abbreviated mental test; ASA: American Society of Anesthesiologists (ASA) physical status; BIS: bispectral index monitor; b.p.m.: beats per minute; CHF: cardiac heart failure; CPNB: continuous peripheral nerve block; DVT: deep venous thrombosis; EKG: electrocardiogram; GA: general anaesthesia; h: hour; IM: intramuscular; IPPV: intermittent positive pressure ventilation; IV: intravenous; kg: kilogram; L: Lumbar; MAP: mean arterial blood pressure; mg: milligram; MI: myocardial infarction; mL: millilitres; mm HG: millimetre of mercury; MMSE: mini mental state examination; n: number; PE: pulmonary embolism; PNB:; peripheral nerve block; RCT: randomized controlled trial; SA: spinal anaesthesia; T: Thoracic; ug: microgram
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adams 1990 | Quasi‐randomized: allocated according to the day of surgery (even or uneven) |
| Darling 1994 | No outcome of interest |
| Lattermann 2005 | Different population: elective surgery for primary total hip arthroplasty |
| Messaoudi 2009 | No outcome of interest |
| Ungemach 1987 | A randomized trial of 50 hip fracture patients using either enflurane or enflurane and fentanyl. The trial was excluded as it was a comparison of different drugs within one type of anaesthesia (general anaesthesia) and not a comparison of different anaesthetic techniques |
| Yao 1997 | Different intervention. Study on two different speeds of injection of the local anaesthetic for spinal anaesthesia |
Characteristics of studies awaiting assessment [ordered by study ID]
Neuman 2016.
| Methods | RCT Institutional review board approval obtained Written informed consents obtained Between July 2014 and March 2015 |
| Participants | 12 participants aged 18 or older, femoral neck or pertrochanteric hip fracture requiring surgery, and ability to speak English and provide written informed consent. Exclusion criteria were pathological or periprosthetic fracture, concurrent conditions requiring surgery (e.g., multitrauma, acute cholecystitis), Montreal Cognitive Assessment4 score less than 16, delirium at time of screening, known contraindications to spinal anaesthesia or volatile general anaesthetics, and pregnancy. |
| Interventions | Intervention: spinal anaesthesia with sedation (n = 6) Control: general anaesthesia (n = 6) |
| Outcomes |
|
| Notes | ClinicalTrials.gov identifier NCT02190903 |
Parker 2015.
| Methods | RCT (sealed opaque envelope) Approval from Research and Development Committe obtained Written informed consent obtained No external source of funding Between June 2007 and November 2012 |
| Participants | 322 participants aged over 49 years of age presenting to one hospital with an acute hip fracture. Patients with dementia were included if their next was willing to allow their relative to participate in the trial. Patients with more than one injury were also included if spinal anaesthesia was suitable. Exclusion criteria: patients who expressed a preference to a particular method of anaesthesia or for whom the surgeon considered one of the anaesthetic technique being more appropriate |
| Interventions | Intervention: spinal anaesthesia (n = 158 randomised, 13 switched to general anaesthesia and one lost to follow‐up at 68 days) Control: general anaesthesia (n = 164 randomised, 6 switched to spinal anaesthesia) |
| Outcomes |
|
| Notes | Participants were assessed at 6 weeks and by a telephone interview at one year. ISRCTN36381516 |
Characteristics of ongoing studies [ordered by study ID]
NCT00590707.
| Trial name or title | Post‐operative delirium in elderly surgical patients (STRIDE) |
| Methods | RCT; double‐blind (subject, caregiver, outcomes assessor); parallel assignment |
| Participants | 65 years of age or older at admission; has surgical treatment of a traumatic hip fracture; has Mini‐Mental Status Exam score of 15 or higher; able to read/write/speak/hear/understand English; receives spinal anaesthesia |
| Interventions | Patients randomly assigned to this arm will receive enough sedative drugs to keep their level of awareness during the hip fracture repair at a BIS score of 50‐60. This is the "deeper sedation" arm. versus patients randomly assigned to this arm will receive enough sedative drugs to keep their level of awareness during the hip fracture repair at a BIS score of 70‐80. This is the "moderate sedation" arm |
| Outcomes | Primary outcome measures: Presence of delirium (time frame: postoperative days 1‐5; 1 month after surgery; and 1 year after surgery). Memory/thinking testing Secondary outcome measures: Change in functional status (time frame: 1 month and 1 year after surgery). Ability to perform Activities of Daily Living; strength and walking testing |
| Starting date | January 2005 |
| Contact information | Frederick E. Sieber, MD: fsieber1@jhmi.edu and Michael D. Sklar, MA: msklar1@jhmi.edu |
| Notes |
NCT02213380.
| Trial name or title | Effect of Anesthesia on Post‐operative Delirium in Elderly Patients Undergoing Hip Fracture Surgery (RAGADelirium) |
| Methods | RCT; open label; parallel assignment |
| Participants | Older patients (≥ 65 years with hip fracture and planned hip fracture surgery |
| Interventions | General anaesthesia versus regional anaesthesia |
| Outcomes | Primary Outcome Measures: the incidence of postoperative cognitive dysfunction (POD) in 7 days postoperation [ Time Frame: in 7 days postoperation POD diagnosed with CAM
Secondary Outcome Measures:
|
| Starting date | October 2014 |
| Contact information | Contact: Ting LI, M.D.: liting1021@aliyun.com and Contact: Sishi Chen: chensishiwz@163.com |
| Notes |
NCT02507505.
| Trial name or title | Regional versus General Anesthesia for Promoting Independence after Hip Fracture (REGAIN): protocol for a pragmatic, international multicentre trial Ethics boards approval obtained |
| Methods | RCT, multicenter |
| Participants | 1600 previously ambulatory participants aged 50 and older with a clinically or radiographically diagnosed intra‐ or extracapsular hip fracture and planned surgical treatment via hemiarthoplasty, total hip arthroplasty or appropriate fixation procedure Excluion criteria: planned concurrent surgery not amenable to spinal anaesthesia, contraindications to spinal anaesthesia, known susceptibility to malignant hyperthermia, periprosthetic fracture, prior participation to this trial, prisoner status or not being suitable for randomisation |
| Interventions | Intervention: spinal anaesthesia Control: general anaesthesia |
| Outcomes |
|
| Starting date | Recruitment began in February 2016 and will continue until the end of 2019 |
| Contact information | Mark D Neuman |
| Notes | Expected results late 2020 |
BIS: bispectral index; CAM: Confusion Assessment Method ; mcg/kg: micrograms per kilogram of body weight; mL; millilitres; RCT: randomized controlled trial
Differences between protocol and review
We made the following changes to the published protocol (Parker 1997)
Change in title: The first review (Urwin 2000), and update (Parker 2001), were published under the title: "General versus spinal/epidural anaesthesia for surgery for hip fractures in adults". The title was changed in the second update to reflect an expansion in the scope of the review to include comparisons of all forms of anaesthesia (Parker 2004).
Changes made in 2016 updated version
Background was updated
Objectives were reformulated from:
The following null hypotheses were tested within the trials included so far in this review: (1) There is no difference in outcome between regional anaesthesia (spinal or epidural) and general anaesthesia. (2) There is no difference in outcome between regional anaesthesia (spinal or epidural) supplemented with a ’light’ general anaesthetic and general anaesthesia alone. (3) There is no difference in outcome between regional anaesthesia (spinal or epidural) and regional nerve blocks alone. (4) There is no difference in outcome between anaesthesia using ketamine (with or without a benzodiazepine) and inhalation general anaesthesia.
To:
The main focus of this review is the comparison of regional versus general anaesthesia for hip fracture repair. More precisely we tried to determine whether there are any major advantages in using regional anaesthesia compared to general anaesthesia for hip fracture repairs. The scope of this review, originally published in 2000 (Urwin 2000), was expanded in the second update to also cover other methods of anaesthesia (Parker 2001). We did not consider supplementary regional blocks in this review as they have been studied in another review (Parker 2002).
Study selection was changed from:
Types of interventions
(1) Regional anaesthesia (if necessary supplemented by sedatives) achieved by injection of local anaesthetic into the epidural or subarachnoid spaces. This type of anaesthesia is also referred to as 'spinal' or 'epidural' (2) General anaesthesia using intravenous or inhalation agents to render the patient unconscious. Unless otherwise stated, general anaesthesia refers to general anaesthesia using inhalation agents in this review. (3) Intravenous ketamine. (4) Local nerve blocks (if necessary supplemented by sedatives) when used as the primary method of anaesthesia. Trials testing other methods of anaesthesia as the primary method of anaesthesia were considered for inclusion. Trials comparing the use of local nerve blocks in conjunction with general anaesthesia and the use of nerve blocks preoperatively, are evaluated in another Cochrane review (Parker 2001). Also not considered in this review were trials comparing different types of drugs or techniques of individual methods of anaesthesia.
To:
Types of interventions
We included studies that compared any combination of the following interventions:
Neuraxial blocks: epidural (single shots or continuous), spinals (single shots or continuous), or combined spinal/epidural (single shots or continuous) with or without intravenous sedation;
Peripheral nerve blocks: posterior lumbar (psoas) plexus blocks with or without sacral plexus blocks or any other peripheral nerve blocks with or without sedation;
General anaesthesia based on inhalational agents (with or without opioids and/or neuromuscular blocking agents), or on total intravenous anaesthesia (ketamine‐based technique or other). Any technique where an endotracheal tube or a laryngeal mask airway was used was considered as general anaesthesia.
Outcomes: the number was reduced.
Method: methods were brought up to date.
Contributions of authors
Conceiving this update: Martyn Parker (MP), Joanne Guay (JG)
Co‐ordinating the review: JG, MP
Screening search results: JG and PRG
Organizing retrieval of papers: JG
Screening retrieved papers against inclusion criteria: JG and Sandra Kopp (SK)
Appraising quality of papers: JG and SK
Abstracting data from papers: JG and SK
Writing to authors of papers for additional information: JG
Data management for the review: JG
Entering data into Review Manager (RevMan 5.3): JG
RevMan statistical data: JG
Other statistical analysis not using RevMan: JG
Interpretation of data: JG, MP, PRG and SK
Statistical inferences: JG
Writing the review: JG, MP, PRG and SK
Securing funding for the review: Departmental resources only
Performing previous work that was the foundation of the present study: JG and MP
Guarantor for the review (one author): JG
Person responsible for reading and checking review before submission: JG, MP, PRG and SK
Sources of support
Internal sources
University of Teesside, Middlesbrough, UK.
Peterborough and Stamford Hospitals NHS Foundation Trust, Peterborough, UK.
External sources
No sources of support supplied
Declarations of interest
Joanne Guay: I am the editor of a multi authors textbook on anaesthesia (including notions on general and regional anaesthesia).
Martyn J Parker has received expenses and honorarium from a number of commercial companies and organizations for giving lectures on different aspects of hip fracture treatment. In addition he has received royalties from BBraun ltd related to the design and development of an implant used for the internal fixation of intracapsular hip fractures. This implant and fracture type is not considered in this review and none of these payments related directly to this review. He is the author of one ongoing trial (ISRCTN36381516).
Pushpaj R Gajendragadkar: none known.
Sandra Kopp: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Berggren 1987 {published data only}
- Berggren D, Gustafson Y, Eriksson B, Bucht G, Hansson LH, Reiz S, et al. Postoperative confusion after anaesthesia in elderly patients with femoral neck fractures. Anesthesia and Analgesia 1987;66:497‐504. [PUBMED: 3578861] [PubMed] [Google Scholar]
Biboulet 2012 {published data only}
- Biboulet P, Jourdan A, Haevre V, Morau D, Bernard N, Bringuier S, et al. Hemodynamic profile of target‐controlled spinal anaesthesia compared with 2 target‐controlled general anaesthesia techniques in elderly patients with cardiac comorbidities. Regional Anesthesia and Pain Medicine 2012;37(4):433‐40. [PUBMED: 22609644] [DOI] [PubMed] [Google Scholar]
Biffoli 1998 {published data only}
- Biffoli F, Piacentino V, Meconcelli G, Guidi F, Dal Poggetto L, Bacci I, et al. The effect of anesthesiologic technique on the mental state of elderly patients submitted for orthopedic surgery of the lower limbs [Influenza della condotta anestesiologica sullo stato mentale di soggetti anziani sottoposti a chirurgia ortopedica dell'arto inferiore]. Minerva Anestesiologica 1998;64(1‐2):13‐9. [PUBMED: 9658786] [PubMed] [Google Scholar]
Bigler 1985 {published data only}
- Bigler D, Adelhoj B, Petring OU, Pederson NO, Busch P, Kalhke P. Mental function and morbidity after acute hip surgery during spinal and general anaesthesia. Anaesthesia 1985;40:672‐6. [PUBMED: 4025772 ] [DOI] [PubMed] [Google Scholar]
Bredahl 1991 {published data only}
- Bredahl C, Hindsholm KB, Frandsen PC. Changes in body heat during hip fracture surgery: a comparison of spinal analgesia and general anaesthesia. Acta Anaesthesiologica Scandinavica 1991;35:548‐52. [PUBMED: 1897353 ] [DOI] [PubMed] [Google Scholar]
Brichant 1995 {published data only}
- Brichant JF, Blom‐Peters L, Buffels R, Lamy M. Central neural blockage failed to decrease deep venous thrombosis in patients undergoing hip surgery and receiving low molecular weight heparin. [Abstract]. British Journal of Anaesthesia 1995;74 Suppl 1:75. [Google Scholar]
Brown 1994 {published data only}
- Brown AG, Visram AR, Jones RDM, Irwins MG, Bacon‐Shone J. Preoperative and postoperative oxygen saturation in the elderly following spinal or general anaesthesia ‐ an audit of current practice. Anaesthesia and Intensive Care 1994;22:150‐4. [PUBMED: 8210017] [DOI] [PubMed] [Google Scholar]
Cao 2008 {published data only}
- Cao QQ, Xu XZ, Lu YY, Chen LM, Guo XY. [Comparison of lumbar plexus block and epidural block for elderly patients undergoing intertrochanteric femoral fracture surgery]. Zhonghua Yi Xue Za Zhi 2008;88(37):2614‐7. [PUBMED: 19080708] [PubMed] [Google Scholar]
Casati 2003 {published data only}
- Casati A, Aldegheri G, Vinciguerra E, Marsan A, Fraschini G, Torri G. Randomized comparison between sevoflurane anaesthesia and unilateral spinal anaesthesia in elderly patients undergoing orthopaedic surgery. European Journal of Anaesthesiology 2003;20(8):640‐6. [PUBMED: 12932066 ] [DOI] [PubMed] [Google Scholar]
Couderc 1977 {published data only}
- Couderc E, Mauge F, Duvaldestin P, Desmonts JM. Comparative results of general and peridural anaesthesia for hip surgery in the very old patient [Resultats comparatifs de l'anesthésie générale et péridurale chez le grand vieillard dans la chirurgie de la hanche]. Anesthésie, Analgésie, Réanimation 1977;34(5):987‐98. [PUBMED: 613869] [PubMed] [Google Scholar]
Davis 1981 {published data only}
- Davis FM, Laurenson VG. Spinal anaesthesia or general anaesthesia for emergency hip surgery in elderly patients. Anaesthesia and Intensive Care 1981;9:352‐8. [PUBMED: 6797318] [DOI] [PubMed] [Google Scholar]
- Davis FM, Quince M, Laurenson VG. Deep vein thrombosis and anaesthetic technique in emergency hip surgery. BMJ 1980;281:1528‐9. [PUBMED: 7437865] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 1987 {published data only}
- Davis FM, Woolner DF, Frampton C, Wilkinson A, Grant A, Harrison RT, et al. Prospective, multi‐centre trial of mortality following general or spinal anaesthesia for hip fracture surgery in the elderly. British Journal of Anaesthesia 1987;59:1080‐8. [PUBMED: 3311100] [DOI] [PubMed] [Google Scholar]
de Visme 2000 {published data only}
- Visme V, Picard F, Jouan R, Legrand A, Savry C, Morin V. Combined lumbar and sacral plexus block compared with plain bupivacaine spinal anaesthesia for hip fractures in the elderly. Regional Anesthesia and Pain Medicine 2000;25(2):158‐62. [PUBMED: 10746528] [DOI] [PubMed] [Google Scholar]
Eyrolle 1998 {published data only}
- Eyrolle L, Zetlaoui P, Belbachir A, Rosencher N, Conseiller C. Regional anaesthesia for femoral neck fracture surgery: comparison of lumbar plexus block and spinal anaesthesia [Abstract]. British Journal of Anaesthesia 1998;80 Suppl 1:112. [Google Scholar]
Heidari 2011 {published data only}
- Heidari SM, Soltani H, Hashemi SJ, Talakoub R, Soleimani B. Comparative study of two anaesthesia methods according to postoperative complications and one month mortality rate in the candidates of hip surgery. Journal of Research in Medical Sciences: The Official Journal of Isfahan University of Medical Sciences 2011;16(3):323‐30. [PUBMED: 22091252 ] [PMC free article] [PubMed] [Google Scholar]
Hoppenstein 2005 {published data only}
- Hoppenstein D, Zohar E, Ramaty E, Shabat S, Fredman B. The effects of general vs spinal anaesthesia on frontal cerebral oxygen saturation in geriatric patients undergoing emergency surgical fixation of the neck of femur. Journal of Clinical Anaesthesia 2005;17(6):431‐8. [PUBMED: 16171663] [DOI] [PubMed] [Google Scholar]
Ibanez 1993 {published data only}
- Ibanez MT, Martinez‐Navarrete MA, Guitelman AJ, Marti‐Viano JL, Hernandez‐Perez H. Postoperative complications of surgical fixation in hip fracture. Comparative study of general/surgical anaesthesia [Complicaciones potoperatorias en la fijacion quirurgica de la fractura de cadera. Estudio comparativo anestesia general/anestesia quirurgica]. Revista Española de Anestesiología y Reanimación. 1993;40(Suppl 1):81. [CENTRAL: 00746932] [Google Scholar]
Juelsgaard 1998 {published data only}
- Juelsgaard P, Sand NP, Felsby S, Dalsgaard J, Jakobsen KB, Brink O, et al. Perioperative myocardial ischaemia in patients undergoing surgery for fractured hip randomized to incremental spinal, single‐dose spinal or general anaesthesia. European Journal of Anaesthesiology 1998;15(6):656‐63. [PUBMED: 9884850] [DOI] [PubMed] [Google Scholar]
Kamitani 2003 {published data only}
- Kamitani K, Higuchi A, Asashi T, Yoshida H. Postoperative delirium after general anaesthesia vs. spinal anaesthesia in geriatric patients. Masui ‐ Japanese Journal of Anesthesiology 2003;52(9):972‐5. [PUBMED: 14531256] [PubMed] [Google Scholar]
Maurette 1988 {published data only}
- Maurette P, Castagnera L, Vivier C, Erny P. Comparative repercussions of general and spinal anaesthesia on psychological functions of the aged subject [Repercussions comparées de l'anesthésie générale et de la rachianesthésie sur les fonctions psychiques du sujet agé]. Annales Francaises d' Anesthesie et de Reanimation 1988;7:305‐8. [PUBMED: 3202339] [DOI] [PubMed] [Google Scholar]
McKenzie 1984 {published and unpublished data}
- McKenzie PJ, Wishard HY. Anaesthesia for fractured neck of femur (letter). BMJ 1981;282:399‐400. [PUBMED: 6780043] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie PJ, Wishart HY, Dewar KMS, Gray I, Smith G. Comparison of the effects of spinal anaesthesia and general anaesthesia on postoperative oxygenation and perioperative mortality. British Journal of Anaesthesia 1980;52:49‐53. [PUBMED: 6769450] [DOI] [PubMed] [Google Scholar]
- McKenzie PJ, Wishart HY, Gray I, Smith G. Effects of anaesthetic technique on deep vein thrombosis: a comparison of subarachnoid and general anaesthesia. British Journal of Anaesthesia 1985;57:853‐7. [PUBMED: 4027101] [DOI] [PubMed] [Google Scholar]
- McKenzie PJ, Wishart HY, Smith G. Long‐term outcome after repair of fractured neck of femur; comparison of subarachnoid and general anaesthesia. British Journal of Anaesthesia 1984;56:581‐4. [PUBMED: 6721969] [DOI] [PubMed] [Google Scholar]
McLaren 1978 {published data only}
- McLaren AD. Mortality studies. A review. Regional Anesthesia 1982;7(Suppl 4):S172‐4. [Google Scholar]
- McLaren AD, Stockwell MC, Reid VT. Anaesthetic techniques for surgical correction of fractured neck of femur: a comparative study of spinal and general anaesthesia in the elderly. Anaesthesia 1978;33:10‐4. [PUBMED: 626331] [DOI] [PubMed] [Google Scholar]
Messina 2013 {published data only}
- Messina A, Frassanito L, Colombo D, Vergari A, Draisci G, Della Corte F, et al. Hemodynamic changes associated with spinal and general anaesthesia for hip fracture surgery in severe ASA III elderly population: a pilot trial. Minerva anestesiologica 2013;79(9):1021‐9. [PUBMED: 23635998] [PubMed] [Google Scholar]
Racle 1986 {published data only}
- Racle JP, Benkhadra A, Poy JY, Gleizal B, Gaudray A. Comparative study of general and spinal anaesthesia in elderly women in hip surgery [Etude comparative de l'anesthésie générale et de la rachianestésie chez la femme agée dans la chirurgie de la hanche]. Annales Francaises d' Anesthésie et de Réanimation 1986;5:24‐30. [PUBMED: 3706840] [DOI] [PubMed] [Google Scholar]
Spreadbury 1980 {published data only}
- Spreadbury TH. Anaesthetic techniques for surgical correction of fractured neck of femur: a comparative study of ketamine and relaxant anaesthesia in elderly women. Anaesthesia 1980;35:208‐14. [PUBMED: 7386839] [DOI] [PubMed] [Google Scholar]
Svarting 1986 {published data only}
- Svartling N, Lehtinen AM, Tarkkanen, L. The effect of anaesthesia on changes in blood pressure and plasma cortisol levels induced by cementation with methylmethacrylate. Acta Anaesthesiologica Scandinavica 1986;30(3):247‐52. [PUBMED: 3739582] [DOI] [PubMed] [Google Scholar]
Tasker 1983 {published data only}
- Tasker TPB, Raitt DG, Kohn RLJ, Vater M, Crawshaw C. Subarachnoid block or general anaesthesia?: a study of the stress response during and after surgery for prosthetic replacement of fractured neck of femur [abstract]. Journal of Bone and Joint Surgery. British Volume 1983;65:660. [Google Scholar]
Ungemach 1993 {published data only}
- Ungemach JW, Andres FJ, Eggert E, Schoder K. The role of anaesthesia in geriatric patients with hip fractures: A prospective study. European Journal of Anaesthesiology 1993;10(5):380. [Google Scholar]
Valentin 1986 {published data only}
- Valentin N, Lomholt B, Jensen JS, Hejgaard N, Kreiner S. Spinal or general anaesthesia for surgery of the fractured hip? A prospective study of mortality in 578 patients. British Journal of Anaesthesia 1986;58:284‐91. [PUBMED: 3947489] [DOI] [PubMed] [Google Scholar]
Wajima 1995 {published data only}
- Wajima Z, Kurosawa H, Inoue T, Yoshikawa T, Ishikawa G, Shitara T, et al. Changes in dementia rating scale scores of elderly patients with femoral neck fracture during perioperative period [Japanese]. Masui ‐ Japanese Journal of Anesthesiology 1995;44(11):1489‐97. [PUBMED: 8544286 ] [PubMed] [Google Scholar]
White 1980 {published data only}
- White IW, Chappell WA. Anaesthesia for surgical correction of fractured femoral neck: a comparison of three techniques. Anaesthesia 1980;35:1107‐10. [PUBMED: 7446914] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adams 1990 {published data only}
- Adams HA, Wolf C, Michaelis G, Hempelmann G. Postoperative course and endocrine stress response of geriatric patients with fractured neck of femur [Postoperativer verlauf und endokrine streb‐reaktion geriatrischer patienten mit huftnahen frakturen; prospektiv‐randomisierte studie zum vergleich von spinalanasthesin und halothan‐intubatinosnarkosen]. Anasthesie, Intensivtherapie, Notfallmedizin 1990;25:263‐70. [PUBMED: 2171375] [PubMed] [Google Scholar]
Darling 1994 {published data only}
- Darling JR, Murray JM, Hainsworth AM, Trinick TR. The effect of isoflurane or spinal anesthesia on Indocyanine green disappearance rate in the elderly. Anesthesia and Analgesia 1994;78:706‐9. [PUBMED: 8135390] [DOI] [PubMed] [Google Scholar]
Lattermann 2005 {published data only}
- Lattermann R, Belohlavek G, Wittmann S, Fuchtmeier B, Gruber M. The anticatabolic effect of neuraxial blockade after hip surgery. Anesthesia and Analgesia 2005;101(4):1202‐8, table of contents. [PUBMED: 16192545] [DOI] [PubMed] [Google Scholar]
Messaoudi 2009 {published data only}
- Messaoudi K, Azaiez W, Ammous A, Raddaoui K, Cherif A. Combined lumbar and sciatic plexus block compared with unilateral bupivacaine spinal anaesthesia for hip fractures in the elderly: Preliminary results. European Journal of Anaesthesiology 2009;26(Supplement 45):122. [Google Scholar]
Ungemach 1987 {published data only}
- Ungemach JW. Inhalation anaesthesia or "balanced anaesthesia"? A comparative perioperative study in geriatric patients [Inhalationsanaesthesie oder " balancierte anaesthesie "?: Eine vergleichende perioperative studie geriatrischer patienten]. Anaesthesist 1987;36:288‐91. [PUBMED: 3631497 ] [PubMed] [Google Scholar]
Yao 1997 {published data only}
- Yao CZ, Zhang Z, Diao YC, Zhu C, Jin MC. Surgical repair of the fracture of neck of femur using spinal anaesthesia with hypobaric bupivacaine [Original title in Chinese]. Chinese Journal of Anesthesiology 1997;17(5):306‐7. [Google Scholar]
References to studies awaiting assessment
Neuman 2016 {published data only}
- Neuman MD, Mehta S, Bannister ER, Hesketh PJ, Horan AD, Elkassabany NM. Pilot Randomized Controlled Trial of Spinal Versus General Anesthesia for Hip Fracture Surgery. Journal of the American Geriatrics Society 2016; Vol. 64, issue 12:2604‐6. [PUBMED: 27996117] [DOI] [PMC free article] [PubMed]
Parker 2015 {published data only}
- Parker MJ, Griffiths R. General versus regional anaesthesia for hip fractures. A pilot randomised controlled trial of 322 patients. Injury 2015;46(8):1562‐6. [PUBMED: 26049662] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00590707 {published data only}
- NCT00590707. Post‐operative delirium in elderly surgical patients (STRIDE). https://clinicaltrials.gov/ct2/show/NCT00590707 accessed 2014.
NCT02213380 {published data only}
- NCT02213380. Effect of anesthesia on post‐operative delirium in elderly patients undergoing hip fracture surgery (RAGADelirium). https://clinicaltrials.gov/ct2/show/NCT02213380 accessed 2014.
NCT02507505 {published data only}
- Neuman M. Information on our trial. Email to J Guay 23 February 2017.
- Neuman MD, Ellenberg SS, Sieber FE, Magaziner JS, Feng R, Carson JL. Regional versus General Anesthesia for Promoting Independence after Hip Fracture (REGAIN): protocol for a pragmatic, international multicentre trial. BMJ open 2016;6(11):e013473. [PUBMED: 27852723] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Brauer 2009
- Brauer CA, Coca‐Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA 2009;302(14):1573‐9. [PUBMED: 19826027] [DOI] [PMC free article] [PubMed] [Google Scholar]
Falck‐Ytter 2012
- Falck‐Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e278S‐325S. [PUBMED: 22315265] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleisher 2007
- Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation 2007;116(17):e418‐99. [PUBMED: 17901357] [DOI] [PubMed] [Google Scholar]
Freedman 2000
- Freedman KB, Brookenthal KR, Fitzgerald RH Jr, Williams S, Lonner JH. A meta‐analysis of thromboembolic prophylaxis following elective total hip arthroplasty. Journal of Bone and Joint Surgery American 2000;82‐A(7):929‐38. [PUBMED: 10901307] [DOI] [PubMed] [Google Scholar]
Guay 2011
- Guay J. General anaesthesia does not contribute to long‐term post‐operative cognitive dysfunction in adults: A meta‐analysis. Indian Journal of Anaesthesia 2011;55(4):358‐63. [PUBMED: 22013251] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guay 2014
- Guay J, Choi P, Suresh S, Albert N, Kopp S, Pace NL. Neuraxial blockade for the prevention of postoperative mortality and major morbidity: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD010108.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research ed.) 2008;336(7650):924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Hebl 2005
- Hebl JR, Kopp SL, Ali MH, Horlocker TT, Dilger JA, Lennon RL, et al. A comprehensive anesthesia protocol that emphasizes peripheral nerve blockade for total knee and total hip arthroplasty. The Journal of Bone and Joint Surgery. American Volume 2005;87 Suppl 2:63‐70. [PUBMED: 16326725] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horlocker 2010
- Horlocker TT, Wedel DJ, Rowlingson JC, Enneking FK, Kopp SL, Benzon HT, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence‐Based Guidelines (Third Edition). Regional Anesthesia and Pain Medicine 2010;35(1):64‐101. [PUBMED: 20052816] [DOI] [PubMed] [Google Scholar]
Kennedy 2010
- Kennedy RR, Lee A, Frizelle FA. Influence of general health and degree of surgical insult on long‐term survival. The British Journal of Surgery 2010;97(5):782‐8. [PUBMED: 20235082] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Neuman 2014
- Neuman MD, Rosenbaum PR, Ludwig JM, Zubizarreta JR, Silber JH. Anesthesia technique, mortality, and length of stay after hip fracture surgery. JAMA 2014;311(24):2508‐17. [PUBMED: 25058085] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pace 2011
- Pace NL. Research methods for meta‐analyses. Best Practice & Research. Clinical Anaesthesiology 2011;25(4):523‐33. [PUBMED: 22099918] [DOI] [PubMed] [Google Scholar]
Parker 2002
- Parker MJ, Griffiths R, Appadu BN. Nerve blocks (subcostal, lateral cutaneous, femoral, triple, psoas) for hip fractures. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD001159] [DOI] [PubMed] [Google Scholar]
Pogue 1997
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta‐analysis. Controlled clinical trials 1997;18(6):580‐93; discussion 661‐6. [PUBMED: 9408720] [DOI] [PubMed] [Google Scholar]
Rasmussen 1999
- Rasmussen LS, Steentoft A, Rasmussen H, Kristensen PA, Moller JT. Benzodiazepines and postoperative cognitive dysfunction in the elderly. ISPOCD Group. International Study of Postoperative Cognitive Dysfunction. British Journal of Anaesthesia 1999;83(4):585‐9. [PUBMED: 10673874] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rortgen 2010
- Rortgen D, Kloos J, Fries M, Grottke O, Rex S, Rossaint R, et al. Comparison of early cognitive function and recovery after desflurane or sevoflurane anaesthesia in the elderly: a double‐blinded randomized controlled trial. British Journal of Anaesthesia 2010;104(2):167‐74. [PUBMED: 20042477] [DOI] [PubMed] [Google Scholar]
Rucker 2011
- Rucker G, Schwarzer G, Carpenter JR, Binder H, Schumacher M. Treatment‐effect estimates adjusted for small‐study effects via a limit meta‐analysis. Biostatistics (Oxford, England) 2011;12(1):122‐42. [PUBMED: 20656692] [DOI] [PubMed] [Google Scholar]
Samama 2007
- Samama CM, Ravaud P, Parent F, Barre J, Mertl P, Mismetti P. Epidemiology of venous thromboembolism after lower limb arthroplasty: the FOTO study. Journal of Thrombosis and Haemostasis 2007;5(12):2360‐7. [PUBMED: 17908282] [DOI] [PubMed] [Google Scholar]
Wang 2001
- Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Hazardous benzodiazepine regimens in the elderly: effects of half‐life, dosage, and duration on risk of hip fracture. The American Journal of Psychiatry 2001;158(6):892‐8. [PUBMED: 11384896] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Parker 1997
- Parker MJ, Griffiths R, Appadu B. General versus spinal/epidural anaesthesia for hip fracture surgery. Cochrane Database of Systematic Reviews 1997, Issue 4. [DOI: 10.1002/14651858.CD000521] [DOI] [Google Scholar]
Parker 2001
- Parker MJ, Handoll HHG, Griffiths R, Urwin SC. Anaesthesia for hip fracture surgery in adults. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD000521] [DOI] [PubMed] [Google Scholar]
Parker 2004
- Parker MJ, Handoll HHG, Griffiths R. Anaesthesia for hip fracture surgery in adults. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD000521.pub2] [DOI] [PubMed] [Google Scholar]
Urwin 2000
- Urwin SC, Parker MJ, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta‐analysis of randomized trials. British Journal of Anaesthesia 2000;84(4):450‐5. [PUBMED: 10823094] [DOI] [PubMed] [Google Scholar]


