Abstract
Background
A decreased physical fitness has been reported in patients and survivors of childhood cancer. This is influenced by the negative effects of the disease and the treatment of childhood cancer. Exercise training for adult cancer patients has frequently been reported to improve physical fitness. In recent years, literature on this subject has also become available for children and young adults with cancer, both during and after treatment. This is an update of the original review that was performed in 2011.
Objectives
To evaluate the effect of a physical exercise training intervention on the physical fitness (i.e. aerobic capacity, muscle strength, or functional performance) of children with cancer within the first five years from their diagnosis (performed either during or after cancer treatment), compared to a control group of children with cancer who did not receive an exercise intervention.
To determine whether physical exercise within the first five years of diagnosis has an effect on fatigue, anxiety, depression, self efficacy, and HRQoL and to determine whether there are any adverse effects of the intervention.
Search methods
We searched the electronic databases of Cochrane Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, and PEDro; ongoing trial registries and conference proceedings on 6 September 2011 and 11 November 2014. In addition, we performed a handsearch of reference lists.
Selection criteria
The review included randomized controlled trials (RCTs) and clinical controlled trials (CCTs) that compared the effects of physical exercise training with no training, in people who were within the first five years of their diagnosis of childhood cancer.
Data collection and analysis
Two review authors independently identified studies meeting the inclusion criteria, performed the data extraction, and assessed the risk of bias using standardized forms. Study quality was rated by the Grading of Recommendation Assessment, Development and Evaluation (GRADE) criteria.
Main results
Apart from the five studies in the original review, this update included one additional RCT. In total, the analysis included 171 participants, all during treatment for childhood acute lymphoblastic leukaemia (ALL).
The duration of the training sessions ranged from 15 to 60 minutes per session. Both the type of intervention and intervention period varied in all the included studies. However, the control group always received usual care.
All studies had methodological limitations, such as small numbers of participants, unclear randomization methods, and single‐blind study designs in case of one RCT and all results were of moderate to very low quality (GRADE).
Cardiorespiratory fitness was evaluated by the 9‐minute run‐walk test, timed up‐and‐down stairs test, the timed up‐and‐go time test, and the 20‐m shuttle run test. Data of the 9‐minute run‐walk test and the timed up‐and‐down stairs test could be pooled. The combined 9‐minute run‐walk test results showed significant differences between the intervention and the control groups, in favour of the intervention group (standardized mean difference (SMD) 0.69; 95% confidence interval (CI) 0.02 to 1.35). Pooled data from the timed up‐and‐down stairs test showed no significant differences in cardiorespiratory fitness (SMD ‐0.54; 95% CI ‐1.77 to 0.70). However, there was considerable heterogeneity (I2 = 84%) between the two studies on this outcome. The other two single‐study outcomes, 20‐m shuttle run test and the timed up‐and‐go test, also showed positive results for cardiorespiratory fitness in favour of the intervention group.
Only one study assessed the effect of exercise on bone mineral density (total body), showing a statistically significant positive intervention effect (SMD 1.07; 95% CI 0.48 to 1.66). The pooled data on body mass index showed no statistically significant end‐score difference between the intervention and control group (SMD 0.59; 95% CI ‐0.23 to 1.41).
Three studies assessed flexibility. Two studies assessed ankle dorsiflexion. One study assessed active ankle dorsiflexion, while the other assessed passive ankle dorsiflexion. There were no statistically significant differences between the intervention and control group with the active ankle dorsiflexion test; however, in favour of the intervention group, they were found for passive ankle dorsiflexion (SMD 0.69; 95% CI 0.12 to 1.25). The third study assessed body flexibility using the sit‐and‐reach distance test, but identified no statistically significant difference between the intervention and control group.
Three studies assessed muscle strength (knee, ankle, back and leg, and inspiratory muscle strength). Only the back and leg strength combination score showed statistically significant differences on the muscle strength end‐score between the intervention and control group (SMD 1.41; 95% CI 0.71 to 2.11).
Apart from one sub‐scale of the cancer scale (Worries; P value = 0.03), none of the health‐related quality of life scales showed a significant difference between both study groups on the end‐score. For the other outcomes of fatigue, level of daily activity, and adverse events (all assessed in one study), there were no statistically significant differences between the intervention and control group.
None of the included studies evaluated activity energy expenditure, time spent on exercise, anxiety and depression, or self efficacy as an outcome.
Authors' conclusions
The effects of physical exercise training interventions for childhood cancer participants are not yet convincing. Possible reasons are the small numbers of participants and insufficient study designs, but it can also be that this type of intervention is not as effective as in adult cancer patients. However, the first results show some positive effects on physical fitness in the intervention group compared to the control group. There were positive intervention effects for body composition, flexibility, cardiorespiratory fitness, muscle strength, and health‐related quality of life (cancer‐related items). These were measured by some assessment methods, but not all. However, the quality of the evidence was low and these positive effects were not found for the other assessed outcomes, such as fatigue, level of daily activity, and adverse events. There is a need for more studies with comparable aims and interventions, using a higher number of participants that also include diagnoses other than ALL.
Keywords: Adolescent; Child; Female; Humans; Male; Exercise; Physical Fitness; Antineoplastic Agents; Antineoplastic Agents/therapeutic use; Body Mass Index; Bone Density; Controlled Clinical Trials as Topic; Muscle Strength; Muscle Strength/physiology; Muscle, Skeletal; Muscle, Skeletal/physiology; Neoplasms; Neoplasms/therapy; Physical Endurance; Physical Endurance/physiology; Precursor Cell Lymphoblastic Leukemia‐Lymphoma; Precursor Cell Lymphoblastic Leukemia‐Lymphoma/drug therapy; Quality of Life; Randomized Controlled Trials as Topic; Range of Motion, Articular; Range of Motion, Articular/physiology
Plain language summary
Physical exercise training interventions for children and young adults during and after treatment for childhood cancer
Background
Childhood cancer is less common than adult cancer at a rate of 144 to 148 cases per one million children. An intensive treatment, including combined treatment modalities such as surgery, chemotherapy, radiotherapy, or a combination, is often needed for cure. These treatment modalities are frequently accompanied by side effects, such as feeling sick (nausea), serious infections, organ damage (heart, lung, kidney, liver), decreased bone mineral density (lower minerals, such as calcium, in the bones making the them more fragile), but also decreased muscle strength and physical fitness.
In the past, children were advised to recover in bed, and to take as much rest as possible. Nowadays, it is considered that too much immobility may result in a further decrease of physical fitness and physical functioning. These side effects might be prevented or reduced by introducing a physical exercise training programme during, or shortly after, childhood cancer treatment.
Study characteristics
We searched scientific databases for studies of comparing the effects of physical exercise training within the first five years following the diagnosis of childhood cancer compared with no training. Participants were under 19 years of age with any type of childhood cancer. The evidence is current to November 2014.
Key results
This review included five randomized controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) and one clinical controlled trial (clinical studies where people are put into one of two or more treatment groups but this is not done in a random way) that evaluated the effects of a physical exercise training programme in children during cancer treatment. Childhood acute lymphoblastic leukaemia (ALL) is a cancer of the white blood cells and is the most common type of childhood cancer. For that reason, researchers often focus on this type of cancer since it will provide the largest number of patients in the shortest time‐span. In total, our analysis included 171 participants with ALL. The results of the review showed that there were some small benefits of physical exercise training on body composition (percentage of fat mass, muscles, and bones), flexibility, cardiorespiratory fitness (how effective your heart and lungs are at delivering oxygen to your body), muscle strength and quality of life, but the evidence was limited. This can be related to an unsuitable programmes for children with cancer, or due to poorly designed studies. More studies assessing the effects of exercise are needed in a variety of childhood cancer populations. Furthermore, the current findings do not provide enough evidence to identify an optimal physical exercise training programme for children with cancer, neither do they provide information on the characteristics of people who will, or will not, benefit from such a programme. These important issues still need to be clarified.
Summary of findings
Summary of findings for the main comparison. Physical exercise training compared to usual care for children and young adults during and after treatment for childhood cancer.
| Physical exercise training compared to usual care for children and young adults during and after treatment for childhood cancer | |||||
|
Patient or population: children and young adults during and after treatment for childhood cancer Settings: hospital and non‐hospital Intervention: physical exercise training Comparison: usual care | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Usual care group | Exercise group | ||||
| Cardiorespiratory outcomes | |||||
| 9‐minute run‐walk test wheeled distance counter Follow‐up: mean 3‐4 months | The mean 9‐minute run‐walk test in the control group was 3304.5 feet (1007.2 m) and 2676 feet (816 m) in the 2 studies | The mean difference between the study groups on 9‐minute run‐walk test was 681 feet (95% CI 132 to 1230) in favour of the intervention group | 68 (2 studies) | ⊕⊕⊝⊝ low1 | SMD 0.69 (95% CI 0.02 to 1.35) |
| Timed up‐and‐down stairs test stopwatch Follow‐up: mean 3‐4 months | The mean time used for timed up‐and‐down stairs test in the control groups was 9.0 sec and 8.6 sec in the 2 studies | The mean difference between the study groups on timed up‐and‐down stairs was ‐0.94 sec (95% CI ‐2.94 to 1.06) in favour of the intervention group | 68 (2 studies) | ⊕⊝⊝⊝ very low2 | SMD ‐0.54 (95% CI ‐1.77 to 0.70) |
|
Timed up‐and‐go time test stopwatch Follow‐up: mean 3 months |
The mean timed up‐and‐go time test in the control group was 8.3 sec | The mean timed up‐and‐go time test in the intervention group was 6.6 sec (1.3 SD); showing a mean difference of ‐1.8 (95% CI ‐2.7 to ‐0.8) | 40 (1 study) |
⊕⊕⊝⊝ low1 | SMD ‐1.15 (95% CI ‐1.83 to ‐0.48) |
| Body composition outcomes | |||||
| Bone mineral density (total body) DXA scan Follow‐up: mean 24 months | The control group had a mean SDS on (total body) bone mineral density of ‐1.1 (95% CI ‐0.8 to ‐1.4) | After the 24 months' intervention the mean SDS of the intervention group on total body bone mineral density was 0.3 better than in the control group (‐0.8 SDS (95% CI ‐0.6 to ‐1.2) | 51 (1 study) | ⊕⊕⊕⊝ moderate3 | SMD 1.07 (95% CI 0.48 to 1.66) |
|
BMI Quetelet Index Follow‐up: mean 18 months |
The 2 studies reported a mean change in Quetelet index of the control groups of 1.0 and 0.6 in the 2 studies | The intervention group had a mean change in Quetelet index of 1.2 and 0.7 in the 2 studies The mean difference between the study groups on BMI was 0.18 points (95% CI 0.07 to 0.30) in favour of the intervention group |
64 (2 studies) | ⊕⊕⊝⊝ low4 | SMD 0.59 (95% CI ‐0.23 to 1.41) (BMI increased in the intervention group) |
| Muscle endurance/strength outcomes | |||||
| Ankle dorsiflexion strength Hand‐held dynamometer Follow‐up: mean 4 months | The mean ankle dorsiflexion strength in the control group was 0.22 kg (normalized for participant's weight) | The mean ankle dorsiflexion strength in the intervention groups was 0.25 kg; showing a mean difference of 0.03 (95% CI ‐0.04 to 0.1) | 28 (1 study) | ⊕⊕⊝⊝ low5 | SMD 0.29 (95% CI ‐0.46 to 1.04) |
| Health‐related quality of life | |||||
| Health‐related quality of life PedsQL ‐ General questionnaire (range: 0‐100) (version 4.0) Follow‐up: mean 4 months | The mean health‐related quality of life in the control group was 17.5 points | The mean health‐related quality of life in the intervention group was 15 points; the mean difference between the study groups was ‐2.5 (95% CI ‐10.1 to 5.1) | 28 (1 study) | ⊕⊕⊝⊝ low5 | SMD ‐0.23 (95% CI ‐0.98 to 0.51) |
| Fatigue | |||||
|
General fatigue
PedsQL ‐ Fatigue questionnaire (range: 0‐100) Follow‐up: mean 6 weeks |
The mean general fatigue in the control group was 3.4 points | The mean general fatigue in the intervention group was 3.3 points. The mean difference between the study groups was ‐0.15 (95% CI ‐3.2 to 2.9) | 22 (1 study) | ⊕⊝⊝⊝ very low6 | SMD ‐0.04 (95% CI ‐0.88 to 0.8) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the table. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group. BMI: body mass index; CI: confidence interval; DXA: dual‐energy x‐ray absorptiometry; SD: standard deviation; SDS: standard deviation score; sec: seconds; SMD: standardized mean difference | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1. Rated down for imprecision (sample size less than threshold rule‐of‐thumb n = 400, Schünemann 2009) and for high risk of bias in the study of Tanir 2013.
2 Rated down for imprecision (small sample size and confidence intervals include both appreciable benefit and appreciable harm, defined by SMD = ‐0.5 and SMD = 0.5, Schünemann 2009), for inconsistency, and for high risk of bias in the study of Tanir 2013.
3 Rated down for imprecision (small sample size).
4 Rated down by 1 level for imprecision (small sample size) and by 1 level for unclear risk of bias for sequence generation and outcome assessor blinding in study of Moyer‐Mileur 2009.
5 Rated down by 2 levels for imprecision (small sample size and confidence intervals include both appreciable benefit and appreciable harm, defined by SMD = ‐0.5 and SMD = 0.5, Schünemann 2009).
6 Rated down by a total of 3 levels for imprecision (small sample size and confidence intervals include both appreciable benefit and appreciable harm, defined by SMD = ‐0.5 and SMD = 0.5, Schünemann 2009), and for high risk of (selection) bias in the study of Yeh 2011.
Background
Description of the condition
Only a small percentage of the total population experience childhood cancer; approximately 144 to 148 cases per million children (American Cancer Society 2012; Cancer Research UK 2011). However, the impact of childhood cancer is significant. Many studies report a decreased physical fitness (aerobic capacity and muscle strength), in patients and survivors of acute lymphoblastic leukaemia (ALL), which is the most common type of childhood cancer (Aznar 2006; Hartman 2009; Hovi 1993; Marchese 2004; Moyer‐Mileur 2009; San Juan 2008; Warner 1998; Warner 2008; Wright 1998; Wright 2005), and also in children with cancer in general (Arroyave 2008; Cox 2008; De Caro 2006; Hartman 2008; Ness 2005; Ness 2009; Winter 2009). Reduced daily energy expenditure and lower levels of physical activity have been described as the most important cause of this reduced state of physical fitness in children with cancer (Warner 2008). In addition, a considerable number of survivors of childhood cancer experience motor function disability (Geenen 2007; Van Brussel 2006), which is mostly related to negative motor signs, such as insufficient muscle activity or muscle weakness (Hartman 2008; Wright 2005).
Positive effects of exercise training on physical fitness have been reported in studies with adult with cancer (Cramp 2008; Oldervoll 2004; Schmitz 2005; Watson 2004). It is hypothesized that similar results are possible in children with cancer, or survivors of childhood cancer (Moyer‐Mileur 2009).
Description of the intervention
The intervention under consideration was a physical exercise training programme, introduced within the first five years following the diagnosis of childhood cancer. The exercise training should aim to increase physical fitness by aerobic, anaerobic, strength, or mixed fitness training.
How the intervention might work
Cancer and cancer treatment induce lean tissue degeneration and can, therefore, potentially cause abnormalities in the cardiac and skeletal muscle (Schneider 2007). A decline in protein synthesis and protein degeneration by cancer and its treatment can reduce muscle mass. This can result in a decreased oxidative enzyme activity and a decrease in the number of proteins necessary for metabolism (Schneider 2007). People with cancer often experience muscle weakness, a decreased functional capacity, decreased flexibility, reduced mobility, and diminished health‐related quality of life (HRQoL) (Hartman 2008; Schneider 2007). In addition, a decreased psychosocial functioning and HRQoL as a result of cancer has impact on a person's motivational drive and can result in a poorer self perception of one's ability to perform physical activity (Warner 2008; Wright 1998).
Physical activity can prevent or diminish the negative effects of a sedentary life‐style, such as obesity, poor skeletal health, fatigue, and poor mental health, thereby increasing a person's HRQoL. Increasing physical activity is possible by adopting a less inactive life‐style and increasing sports participation. Beneficial effects of physical activity during or shortly after cancer therapy are an increase in muscle mass and plasma volume, improved lung ventilation and lung perfusion, and an increased cardiac reserve.
Dimeo (Dimeo 2001) suggests such beneficial effects of resistance exercise training on muscle mass in patients with cancer who are treated with glucocorticoids, as was seen in adult patients with other diseases treated with glucocorticoids and resistance exercise.
Why it is important to do this review
Despite the positive results of exercise interventions on fatigue and physical fitness in adults with cancer, the evidence for benefits in children with cancer is limited. Studies within the population of children with cancer and survivors of childhood cancer are emerging and the first data have been published. However, the number of participants in the various publications is small and the variety in type of cancer limited, making it difficult to draw more generalized conclusions. In making healthcare management decisions, participants and clinicians must weigh the benefits and drawbacks of supportive care. Pooled data can help in this decision‐making process.
The purpose of this Cochrane review was to summarize the existing literature on the effectiveness of physical exercise training interventions in children with cancer, implemented within the first five years from diagnosis and to provide a best‐evidence synthesis or meta‐analysis of the reported results. This is an update of the original review that was performed in 2011 (Braam 2013).
Objectives
Primary objective
To evaluate the effect of a physical exercise training intervention on the physical fitness (i.e. aerobic capacity, muscle strength, or functional performance) of children with cancer within the first five years from their diagnosis (performed either during or after cancer treatment), compared to a control group of children with cancer who did not receive an exercise intervention.
Secondary objectives
To determine whether physical exercise within the first five years of diagnosis has an effect on fatigue, anxiety, depression, self efficacy, and HRQoL and to determine whether there are any adverse effects of the intervention.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and controlled clinical trials (CCTs) comparing the effects of physical exercise training within the first five years following the diagnosis of childhood cancer with no training.
We included CCTs in the review when the studies included a well‐defined and comparable control group. Factors that were taken into account regarding comparability were: being children with cancer or survivors of childhood cancer, age, sex, and country of origin.
We included cluster‐randomized trials when the intervention and control groups were comparable in each aspect except for the location of cancer treatment and study recruitment.
We included cross‐over trials when the study results were available for each separate intervention period. We then used the data of the first randomization period.
We did not include reviews but used them to check for relevant references. In addition, we excluded observational studies (including case reports, case‐control studies) and surveys from this review.
Types of participants
Study participants were under 19 years of age at diagnosis of any type of childhood cancer. Participants in the physical exercise training programme needed to be no more than five years from diagnosis. We only included studies that also included adults with cancer when the results of the childhood and adult study populations were reported separately.
Types of interventions
Studies that were included compared a physical exercise training intervention for childhood cancer patients or survivors with a control group receiving care as usual. Care as usual was defined as care when needed, but no specific exercise programme or alternative intervention prescribed to increase physical fitness, HRQoL, self perception, or a combination of these, or to decrease adverse events, fatigue, anxiety, depression, or a combination of these in childhood cancer patients.
The physical exercise training interventions that were offered included different types of training or exercise programmes. For instance, muscle strength or stretching exercises; aerobic exercises; or sports such as gymnastics, swimming, running, or bicycling.
The exercise training intervention could have been additional care during therapy or could have been offered after the standard cancer therapy in a form of rehabilitation. The goals of this exercise training intervention were preventing motor disabilities and a decline in physical fitness, or treating motor function problems that developed during childhood cancer therapy.
The exercise training intervention could have taken place in any setting or location: at home, at a physical therapy centre, in a hospital, or elsewhere. It could either have been a group intervention, or an individual programme.
The duration of the exercise training intervention needed to be at least four weeks in order to be able to report on exercise training effects. The upper limit of the training duration was not fixed for this review. In addition, the duration of physical activities (daily time spent on activities or sports) could differ per protocol.
Types of outcome measures
We included studies evaluating the effect of physical exercise training interventions on physical fitness, HRQoL, fatigue, self efficacy, anxiety, and depression. Furthermore, we studied adverse effects of the intervention programme.
Primary outcomes
The primary outcome of this review was physical fitness measured by:
cardiorespiratory fitness (e.g. peak oxygen uptake (VO2peak), peak work rate (Wmax), endurance time): aerobic or anaerobic exercise capacity tested by ergometry on a cycle ergometer or treadmill, the Wingate anaerobic test, the steep‐ramp‐test, maximal anaerobic running/cycling test, the Cooper test, or another valid instrument;
muscle endurance/strength: assessed with a hand‐held dynamometer, the Biodex, the spring scale, the lateral step‐up test, the sit‐to‐stand test, 10 repetitions maximum, the up‐and‐down stairs test, the minimum chair height test, the muscle power sprint test, a 10 x 5‐m sprint test, the six‐minute walk test, the incremental shuttle walking test, or another valid instrument;
body composition: using body mass index (BMI), skin‐fold measurement, a dual energy x‐ray absorptiometry (DXA) scan, waist circumference, or the waist‐to‐hip‐ratio;
flexibility: conducted with a goniometer, flexometer or with the sit‐and‐reach test, V‐sit test, shoulder or trunk rotation test, straight leg raise, the passive and active ankle dorsiflexion test, or another valid instrument;
activity energy expenditure: for example, by using an accelerometer;
level of daily activity: assessed by an exercise diary, questionnaire, or by accelerometry;
time spent exercising (more than daily activity): assessed by an exercise diary, questionnaire, or by accelerometry.
Secondary outcomes
Secondary outcomes of the review were:
HRQoL: measured by the Pediatric Quality of Life Inventory (PedsQL), Child Health Questionnaire (CHQ), and DISABKIDS;
fatigue: assessed by the PedsQL Multidimensional Fatigue Scale, Childhood Cancer Fatigue Scale (CCFS), or the Fatigue Scale for a child (FS‐C), the same scale for adolescents (FS‐A), and for parents (FS‐P);
anxiety and depression: measured by the Childhood Depression Inventory (CDI) and the Center of Epidemiological Studies Depression Scale (CES‐D);
self efficacy: assessed using the Confidence Scale, the Self‐Efficacy Questionnaire for Children (SEQ‐C), or the Children's Self‐Efficacy Scale;
adverse effects during the study period by collecting information on the occurrence of sport injuries, infections, fractures, heart failure, the recurrence of cancer, and fever.
Search methods for identification of studies
Electronic searches
For this review we searched the following electronic databases of the Cochrane Register of Controlled Trials (CENTRAL) (2014, Issue 3), MEDLINE/PubMed (from 1945 to 11 November 2014), EMBASE/Ovid (from 1980 to 11 November 2014), CINAHL (from 1982 to 11 November 2014), and Physiotherapy Evidence Database (PEDro; from 1929 to 11 November 2014) (www.pedro.org.au/).
The search strategies for the different electronic databases (using a combination of controlled vocabulary and text words) are stated in the appendices (Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5).
Searching other resources
We located information about trials not registered in CENTRAL, MEDLINE, EMBASE, CINAHL, and PEDro, either published or unpublished, by searching the reference lists of relevant articles and reviews. We scanned the conference proceedings of the International Society for Pediatric Oncology (SIOP), the American College of Sports Medicine (ACSM), the International Congress on Physical Activity and Public Health (ICPAPH), and the American Physical Therapy Association (APTA) electronically, or otherwise by handsearching from 2005 to 2014.
We performed a search in the ISRCTN register (www.controlled‐trials.com), and ClinicalTrial.gov database (www.clinicaltrials.gov) for ongoing trials on 11 November 2014. We did not impose language restrictions and will update the searches every two years.
The search included "children", "childhood cancer", "cancer", "exercise training therapy", and "outcome" or any related word combination.
Data collection and analysis
Selection of studies
After employing the search strategy, two review authors (KB, PT) independently identified studies meeting the inclusion criteria. We obtained in full any study that seemed to meet the inclusion criteria on title and abstract, for closer inspection. We noted reasons for exclusion on a separate form. The review authors resolved discrepancies by reaching consensus. In one case, a third party arbitrator (TT) was needed: we required another opinion on the study of de Macedo 2010. This discussion resulted in inclusion of that study because the training corresponded with the described criteria of the protocol.
Data extraction and management
Two review authors (KB, PT) independently performed data extraction using standardized forms. For each study, we collected information on the study design, participant baseline characteristics, settings, sample size, number of participants in each study arm, type of intervention(s), duration of intervention, randomization and blinding procedure, type of control group, type and duration of cancer treatment, and stage of cancer treatment (e.g. during or after treatment), and duration of participant follow‐up.
The extracted outcome measures included: changes in cardiorespiratory fitness, muscle strength/endurance, body composition, body flexibility, daily energy expenditure per time period (e.g. day, week, or month), and changes in the level of daily activity and time spent exercising. In addition, we used a separate form to collect information on psychosocial outcomes such as HRQoL, fatigue, anxiety and depression, and the child's self efficacy. To collect data regarding any other adverse effect of the intervention, we collected all information reported on adverse events during the intervention period in the included studies. We contacted authors of the studies of which only an abstract was available for additional study information.
In the process of data extraction, we reached consensus on all items.
Assessment of risk of bias in included studies
Two review authors (KB, PT) independently assessed the risk of bias in the included RCTs and CCT. This was done according to the following criteria: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessor (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias, such as significant baseline imbalance between study groups in pre‐score or baseline outcome data. We also looked at differential diagnostic activity to observe differences in study protocol for the intervention group and the control group.
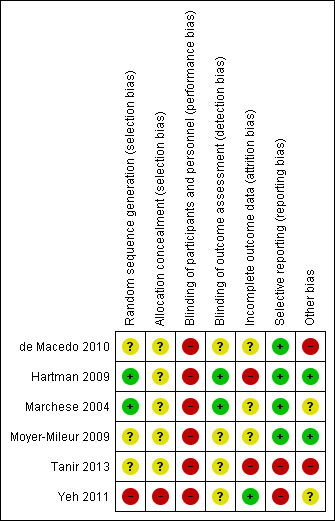
For all 'Risk of bias' items of the included studies, we used the definitions as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We included a 'Risk of bias' summary figure. This figure shows whether a study had a high, low, or unclear risk of bias; a green plus symbol corresponds with a low risk of bias, a red minus symbol corresponds with a high risk of bias, and the yellow question mark symbol corresponds with lack of information or uncertainty over the potential for bias.
We resolved discrepancies by discussion so consensus was reached. We rated quality of the outcomes by using the Grading of Recommendation Assessment, Development and Evaluation (GRADE) criteria (Guyatt 2008a; Guyatt 2008b). For purposes of systematic reviews, GRADE defines the quality of a body of evidence ('high', 'moderate', 'low', or 'very low') as the extent to which we can be confident that an estimate of effect or association is close to the quantity of specific interest. The GRADE system entails an assessment of the quality of a body of evidence for each individual outcome (Guyatt 2008b). Factors that may decrease the quality of evidence are: study limitations, inconsistency of results, indirectness of evidence, imprecision, and publication bias. Factors that may increase the quality of evidence are: large magnitude of effect; plausible confounding, which would reduce a demonstrated effect; and dose‐response gradient (Guyatt 2008a). Two review authors (KB, PT) performed the grading of the quality of evidence in consultation with each other. In case of disagreement, they discussed even minor aspects to reach consensus on that matter.
Measures of treatment effect
The main outcome differences between study groups and pooled data are described in the Table 1. In this table, we provided the illustrative comparative risks (with 95% confidence interval (CI)) and differences in standardized mean difference (SMD). For the Cohen's SMD, we took data from the post‐training/control period measurement. The results of the review also include effect estimates of the intervention per outcome measure. Across the included studies, different outcome assessing scales were used. However, in case of BMI, 9‐minute run‐walk test and the timed up‐and‐down stairs test, we were able to combine data of two studies.
For the interpretation of the Cohen's SMD, we used the following criteria (Higgins 2011):
less than 0.41 represents a small effect;
0.40 to 0.70 represents a moderate effect;
greater than 0.70 represents a large effect.
Dealing with missing data
We sought relevant missing data by contacting the primary study author or the corresponding study author. To optimize the strategy for dealing with missing data, we used an intention‐to‐treat (ITT) analysis when possible. The ITT analysis includes all participants who did not receive the assigned intervention according to the protocol as well as those participants who were lost to follow‐up. We investigated attrition rates, for example, drop‐outs and withdrawals, to optimize data analyses.
Assessment of heterogeneity
We assessed heterogeneity both by visual inspection of the forest plots and by a formal statistical test for heterogeneity, that is, the I2 statistic. We defined significant heterogeneity as I2 greater than 50% (Higgins 2011). In case of heterogeneity, we assessed the following potential sources of clinical heterogeneity: participant characteristics, intervention setting; and stratification methods within studies. When we found heterogeneity, we assessed potential reasons for the differences by examining the study characteristics.
Assessment of reporting biases
In the protocol, we had planned to perform a funnel plot; however, due to an insufficient number of studies (fewer than 10) included in this review, we were not able to do so (Higgins 2011).
Data synthesis
We entered the data of the included studies into Review Manager 5 software (RevMan 2011). We performed the analyses according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). By using the GRADE criteria, the quality of the included studies was taken into account when interpreting the results for the review. We used the random‐effects model throughout the review. When we were unable to perform meta‐analysis, we provided all available effect information from the articles.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses to evaluate whether the outcome was influenced by differences in the age of the participant, the delivered type of physical exercise training intervention, the duration of the exercise training intervention, the exercise training intervention location, type of childhood cancer, and cancer treatment.
On three review outcomes, a meta‐analysis could be performed; that is, on 9‐minute run‐walk test, the timed up‐and‐down stairs test, and BMI. Unfortunately, apart from the intervention and control groups, 9‐minute run‐walk test, the timed up‐and‐down stairs test, and BMI data were not available for other subgroup characteristics (Hartman 2009; Marchese 2004; Moyer‐Mileur 2009; Tanir 2013). Therefore, we could not perform any specific subgroup analyses.
Sensitivity analysis
For those studies that assessed similar outcomes and of which data could be pooled, we performed sensitivity analyses. We assessed whether the outcome would have been different when a study with high or unclear risk of bias would have been excluded from the analyses. This method aimed to assess whether the findings were robust to the decisions made in the process of obtaining them.
Results
Description of studies
Results of the search
Original review in 2011
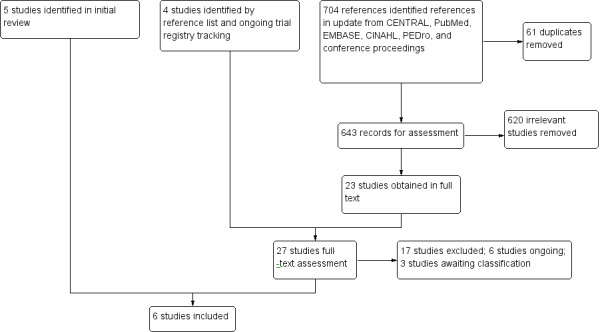
For the original version of the review (Braam 2013), the electronic database searches in CENTRAL, MEDLINE, EMBASE, CINAHL, and PEDro, searches in ongoing trial registries and abstract books from SIOP, ACSM, ICPAPH, and APTA revealed 743 references in 2011.
After removal of duplicates, the search in 2011 resulted in 710 potentially relevant articles. Initial screening of titles and abstracts excluded a further 700 references that did not meet the criteria for inclusion. The 10 remaining references were read in full text. Two out of these 10 studies were ongoing trials, four studies did not meet all eligibility criteria and were thus excluded, and four studies were included.
Reference list tracking led to two additional articles that potentially could be included. One fulfilled the inclusion criteria and was included in the review. Based on the available information in the congress proceeding of the second study, it was not possible to decide if the second study was eligible for inclusion (Elkateb 2007). This study was moved to Characteristics of studies awaiting classification (see Figure 1).
1.
Study flow diagram.
Update in 2014
Running the searches for the update in the aforementioned electronic databases, and searching the abstract books from SIOP, ACSM, ICPAPH, and APTA in November 2014 revealed an additional 704 references.
After removal of duplicates, 643 potentially relevant articles remained. Initial screening of titles and abstracts excluded an additional 620 references that did not meet the inclusion criteria. The remaining 23 references were read in full text. Four of these 23 studies were ongoing trials (see Characteristics of ongoing studies table), 15 did not meet all eligibility criteria, and three out of the 23 studies were congress proceedings (Braam 2014; Sabel 2013; Senn‐Malashonak 2014; see Characteristics of studies awaiting classification table). Braam 2014 combined two congress proceedings from the same study and for that reason were taken together in this review. Only one out of these 23 studies could be included in the update of the original review.
Reference list and trial registry tracking led to four additional articles. Two of these four studies used a study design that did not match the inclusion criteria of this review. For further information, see Excluded studies and Characteristics of excluded studies table (Ruiz 2010; Shore 1999). The other two studies were trial protocols that were moved to the ongoing trial section (Characteristics of ongoing studies table). (See Figure 1).
One of the ongoing trials in the original review of 2011 (that was referred to as 'Braam 2010') reported results in a congress proceeding and, therefore, changed from an ongoing trial to an awaiting classification study and is now referred to as Braam 2014.
In summary, from the original review and the update together we included six studies in this review.
Included studies
Methods
The six studies included in this review were: de Macedo 2010; Hartman 2009; Marchese 2004; Moyer‐Mileur 2009; Tanir 2013; Yeh 2011. Five of these studies were RCTs, and one study used a quasi‐experimental study design, making it a CCT (Yeh 2011). One study performed a power calculation (Hartman 2009). For trial characteristics and outcomes, see the Characteristics of included studies table.
Participants
In total, the analysis included 171 participants. All participants were diagnosed with childhood ALL and studied during chemotherapy (de Macedo 2010; Hartman 2009; Marchese 2004; Moyer‐Mileur 2009; Tanir 2013; Yeh 2011). Of the 171 children, 98 were boys, 70 were girls (de Macedo 2010; Hartman 2009; Marchese 2004; Moyer‐Mileur 2009; Tanir 2013; Yeh 2011); gender was not reported in three children who dropped out. The number of children per study was small. Hartman 2009 included the largest number of children (51 children) in their study, with 26 children in the usual care group and 25 in the intervention group. The 14 children in the study of de Macedo 2010 were divided into nine children who received care as usual and five who received the intervention. Marchese 2004 included 13 children that performed the exercise intervention and 15 who had care as usual. The 13 children analyzed in the study of Moyer‐Mileur 2009 were divided into seven who received care as usual and sic who received the intervention; one child was lost to follow‐up. Tanir 2013 included 41 children, of which one dropped out, resulting in a group distribution of 19 children in the intervention group versus 21 children in the control group. Yeh 2011 included 22 children in the analyses, of which 12 children received the intervention training programme and 10 received care as usual; two children were not taken into analysis because they were lost to follow‐up.
Five studies reported their exclusion criteria; in one study, these data were missing (Moyer‐Mileur 2009). Four studies had cognitive impairment with or without or mental (developmental) impairment an exclusion criterion (Hartman 2009; Marchese 2004; Tanir 2013; Yeh 2011). One study described having difficulties with the national language (Hartman 2009). Four studies excluded children with neurological impairment (de Macedo 2010; Marchese 2004; Tanir 2013; Yeh 2011). Marchese 2004 and Tanir 2013 excluded children with a genetic disorder, as well as children who had received cancer‐related physiotherapy, or children who had participated in a regular exercise programme in the six months before start of the study. Tanir 2013 excluded children with cardiac, pulmonary, renal, or hepatic dysfunction, whereas de Macedo 2010 excluded children with chronic lung disease, neuromuscular disease, or children treated with radiotherapy.
Intervention
The exercise intervention programme of all six studies included at least a home‐based exercise programme with guidance from a therapist of the treating hospital to optimize physical fitness (Hartman 2009; de Macedo 2010; Marchese 2004; Moyer‐Mileur 2009; Tanir 2013; Yeh 2011). However, the duration of the entire intervention, duration of each training session, timing, and type of interventions differed across studies. The duration of the training sessions ranged from 15 minutes to 60 minutes. The intervention period ranged from 10 weeks (de Macedo 2010; Yeh 2011) to two years (Hartman 2009). Five out of six studies introduced the exercise intervention during the maintenance treatment period (de Macedo 2010; Marchese 2004; Moyer‐Mileur 2009; Tanir 2013; Yeh 2011), and in one study it started shortly after diagnosis (Hartman 2009). Five studies determined the effects of an exercise intervention to increase muscle strength of all muscles (Hartman 2009; Marchese 2004; Moyer‐Mileur 2009; Tanir 2013; Yeh 2011). The study of de Macedo 2010 investigated the effect of an inspiratory muscle training programme. They studied the effects of inspiratory muscle training, which was performed with a threshold device using a load of 30% of the maximal inspiratory pressure.
For more details, see the information in the Characteristics of included studies table.
Control
The control groups of the six studies received care as usual, which implies no additional exercise‐related care (de Macedo 2010; Hartman 2009; Marchese 2004; Moyer‐Mileur 2009; Tanir 2013; Yeh 2011). We consider that Tanir 2013 probably made a writing mistake as they reported in the same paper that the control group did and did not receive an exercise intervention. Based on the additional information in the paper, we have now concluded that the control group did not receive an exercise intervention.
With the exception of those of the study of de Macedo 2010, all study participants of the control groups were measured at the same time points as the intervention group. The control group in the study of de Macedo 2010 performed the study assessments during the initial evaluation and after 10 weeks, whereas the intervention group performed the measurements at the end of each training week.
Outcomes
The studied primary outcomes were: cardiorespiratory fitness, muscle endurance/strength, body composition, flexibility, and level of daily activity. Secondary outcomes of this review that were mentioned in the studies were: HRQoL, fatigue, and adverse events. The studies did not address the other secondary outcomes (anxiety, depression, and self efficacy).
Because of the different aims and study methods of the six included studies, there was little overlap in used methods and assessed outcomes. Only two studies performed both the 9‐minute run‐walk test and the timed up‐and‐down stairs test to assess cardiorespiratory fitness (Marchese 2004; Tanir 2013), and, another two studies assessed changes in BMI, as part of changes in body composition (Hartman 2009; Moyer‐Mileur 2009). For further information, see the Characteristics of included studies table and the Data and analyses tables.
Excluded studies
We subsequently excluded 21 publications that had been retrieved. There were four studies that included an adult cancer population instead of a paediatric population (Jarden 2013; Oldervoll 2011; Rief 2011; Steel 2011). We excluded six studies based on the used design; one was a case‐control study (Rosenhagen 2011), one used healthy volunteers as a control population (Shore 1999), one used a cross‐over randomized trial design without presenting data after the first intervention period (before cross‐over) (Speyer 2010), and three were uncontrolled studies (Gohar 2011; Jarden 2013; Ruiz 2010). In another three studies the intervention did not match with the intervention of interest for this review (Geyer 2011; Huang 2014; Kurt 2011), and in one study the aim was to increase motor and process function; this outcome did not correspond with any of the primary or secondary outcomes of this review (Emanuelson 2014).
Another eight studies assessed the effects of a training intervention with duration of less than four weeks (Chamorro‐Vina 2010; Chung 2014; Herbinet 2014; Hinds 2007; Speyer 2010; Speyer 2011; William Li 2013; Winter 2013). Furthermore, there was duplication of information; within these eight excluded studies (on intervention duration), two studies were described in multiple reports: the first study was reported in Chung 2014 and William Li 2013, the second study results were presented in Speyer 2010 and Speyer 2011. The final excluded study was a conference proceeding, presenting data of a pilot study (te Winkel 2008). The full study data were presented separately by Hartman 2009, a study that is included in the review.
The exclusion of two studies was based on two exclusion criteria and therefore mentioned twice in the section above (Jarden 2013; Speyer 2010). Information concerning the excluded studies can be found in the Characteristics of excluded studies table.
Risk of bias in included studies
See the 'Risk of bias' section of the Characteristics of included studies table for the exact scores per study and the support for the judgements made.
Allocation
Two out of the six studies generated random sequence generation adequately (Figure 2; Hartman 2009; Marchese 2004). These two studies used block randomization with sealed envelopes (Hartman 2009; Marchese 2004). Both de Macedo 2010 and Tanir 2013 reported that selection and allocation were random; however, it remained unclear how the randomization procedure was carried out in both studies. A non‐randomised design was used in the study of Yeh 2011, leading to a high risk of selection bias. No information on random sequence generation was available for the fifth study (Moyer‐Mileur 2009). None of the studies described the quality of the envelopes, how the envelopes were sealed, or whether they were coded. Therefore, we judged five out of six studies to have an unclear risk of bias for allocation concealment (de Macedo 2010; Hartman 2009; Marchese 2004; Moyer‐Mileur 2009; Tanir 2013). One study did not use a randomization method and, therefore, had no allocation concealment (Yeh 2011). In summary, five studies had an unclear risk of selection bias and, due to the absence of a randomization procedure, one study had a high risk of selection bias.
2.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Blinding
Blinding of participants and personnel (performance bias)
Due to the nature of the interventions, blinding was virtually impossible: that is, when the participants needed to perform an exercise intervention and the children and their parents were well informed about the study purpose, participants could not be blinded for the study randomization. This could be a potential performance bias in all studies (Higgins 2011). Therefore, we considered all included studies of this review to have a high risk for performance bias.
Blinding of outcome assessors (detection bias)
It is possible to minimize detection bias with blinding the outcome assessor for the randomization. Two studies used outcome assessors who were blinded for study groups (Figure 2; Hartman 2009; Marchese 2004). In the other four studies, the risk was unclear.
Incomplete outcome data
All studies reported withdrawals and drop‐outs during the intervention period. However, only one study used an ITT analysis to deal with missing data and thus had a low risk of attrition bias (Yeh 2011).
In the study of Marchese 2004, the authors reported missing data for daily logs of activity and heart monitor. Yet, no information was reported on methods used for data imputation. For two other studies, it also remained unclear whether they used a method for missing data imputation (de Macedo 2010; Moyer‐Mileur 2009). Therefore, in these three studies, the risk of attrition bias was unclear.
In the study of Hartman 2009, there was a high risk of attrition bias. The authors used a simple imputation technic to include data for those children who dropped out the study. Yet, they included the data from prior to the elimination. This method is very simple and, therefore, increases the risk for bias due to incomplete outcome data.
The study of Tanir 2013 provided no information about missing data. The authors reported that one child died in the intervention group. However, they did not present data on this child. Therefore, in this study the risk of attrition bias was high.
Selective reporting
In two studies, serious selective reporting was detected (Tanir 2013; Yeh 2011). In the first study, results on general quality of life was reported as difference between sexes, instead of difference between the two study groups (Tanir 2013). In the second study, 'adherence' was mentioned to be an extra, or a secondary outcome (Yeh 2011). Yet, in the results, the authors focused on this item as if it was a primary outcome. In the four other studies, the risk of reporting bias was low.
Other potential sources of bias
This review assessed two other biases: baseline outcome data and diagnostic activity.
First, three studies reported the absence of significant differences in baseline outcome data (de Macedo 2010; Hartman 2009; Moyer‐Mileur 2009). One study reported baseline differences on sex, treatment anxiety, and goniometer results were reported (Tanir 2013). In the final two studies, it remained unclear whether all baseline test scores were significantly different between the two study groups (Marchese 2004; Yeh 2011).
Second, the study outcomes were measured at different time points for the intervention and the control group in the study of de Macedo 2010. In the control group, the outcomes were assessed during the initial evaluation and after 10 weeks, whereas in the intervention group measurements were performed at the end of each training week. This could have led to differential diagnostic activity. We judged this study to be of high risk for this other type of bias. The other studies used the same number of measurements, and they were free of 'differential diagnostic activity' (Hartman 2009; Marchese 2004; Moyer‐Mileur 2009; Tanir 2013; Yeh 2011).
In summary, two out of the six studies showed unclear the risk of these 'other biases' (Marchese 2004; Yeh 2011), in another two studies the risk was considered high (de Macedo 2010; Tanir 2013), and in the last two studies the 'other bias' risk was low (Hartman 2009; Moyer‐Mileur 2009).
Effects of interventions
See: Table 1
Because of the different aims and study methods of the six included studies there was little to no overlap in assessed outcomes. We could only pool three outcomes: the timed up‐and‐down stairs test, the 9‐minute run‐walk test (cardiorespiratory fitness), and BMI (body composition).
Cardiorespiratory fitness
In this review, we defined cardiorespiratory fitness as: VO2 peak, Wmax, or endurance time. The included studies assessed physical fitness by the 9‐minute run‐walk test (Marchese 2004; Tanir 2013), timed up‐and‐down stairs test (Marchese 2004; Tanir 2013), timed up‐and‐go test (Tanir 2013), and 20‐m shuttle run test (Moyer‐Mileur 2009).
The 9‐minute run‐walk test showed a significant effect in favour of the intervention (32 children) compared to usual care (36 children) (SMD 0.69 feet; 95% CI 0.02 to 1.35; P value = 0.04). Analysis showed moderate heterogeneity for this item between the studies (I2 = 44%) (Analysis 1.1).
1.1. Analysis.
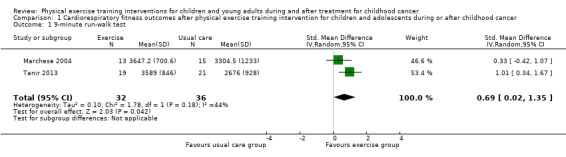
Comparison 1 Cardiorespiratory fitness outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer, Outcome 1 9‐minute run‐walk test.
Two studies assessed the timed up‐and‐down stairs test (Marchese 2004; Tanir 2013). The test results were not significantly different between the intervention (33 children) and the control group (36 children) (SMD ‐0.54 seconds; 95% CI ‐1.77 to 0.70; P value = 0.40). There was considerable heterogeneity for this test between the studies (I2 = 84%) (Analysis 1.2). No ITT analysis could be performed due to missing information on two children who dropped‐out the studies.
1.2. Analysis.
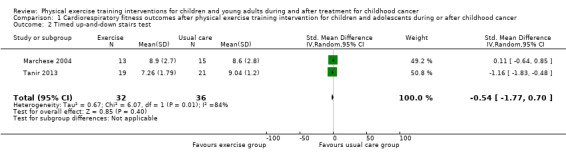
Comparison 1 Cardiorespiratory fitness outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer, Outcome 2 Timed up‐and‐down stairs test.
The timed up‐and‐go test showed a significant positive intervention effect (SMD ‐1.15 seconds; 95% CI ‐1.83 to ‐0.48) (Tanir 2013); children of the intervention group were faster in performing the test.
The 20‐m shuttle run test results showed that children who performed home‐based exercises during their maintenance chemotherapy for ALL (six children) were able to reach higher end‐scores than those in the control group (seven children) (P value = 0.05) (no Review Manager data available). ITT analysis was not performed (Moyer‐Mileur 2009).
Body composition
BMD (Hartman 2009) and BMI (Hartman 2009; Moyer‐Mileur 2009) were assessed as part of the outcome body composition.
The study of Hartman 2009 used a DXA scan to determine BMD (lumbar spine and total body) changes in children with childhood ALL. The assessments were performed at diagnosis, during chemotherapy, and one year after the end of treatment. Analysis showed a significant SMD of 1.07 for total body BMD (95% CI 0.48 to 1.66; P value < 0.001) after the intervention of 24 months (Analysis 2.1). These results revealed a large and significant positive intervention effect on the total body BMD for the intervention group (25 children) compared to the control group (26 children). This analysis was performed according to the principles of the ITT analysis.
2.1. Analysis.
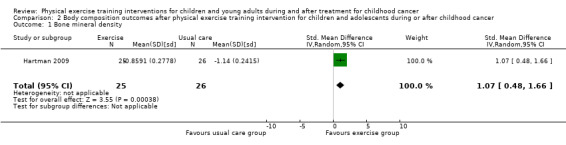
Comparison 2 Body composition outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer, Outcome 1 Bone mineral density.
Two trials studied the differences in BMI between the intervention group and the control group (Hartman 2009; Moyer‐Mileur 2009). The study of Moyer‐Mileur 2009 found no intervention effect on BMI (SMD 0.02; 95% CI ‐1.07 to 1.11). This study compared six children who received a combined nutrition and exercise programme, with seven children who received usual care. Data of one child that dropped out were not reported and we, therefore, could not perform an ITT analysis on BMI. The study of Hartman 2009 showed a statistically significant difference on BMI in favour of the exercise group (25 children) compared to the control group (26 children) (SMD 0.90; 95% CI 0.32 to 1.48). These BMI analyses were performed according to ITT analysis principles (Hartman 2009). Pooled data analysis for BMI showed a non‐significant intervention effect with an SMD of 0.59 on the Quetelet Index (95% CI ‐0.23 to 1.41; P value = 0.16) (Analysis 2.2) in favour of the intervention group. In addition, analysis also showed no substantial heterogeneity (I2 = 48%) for this item between the studies (Analysis 2.2).
2.2. Analysis.
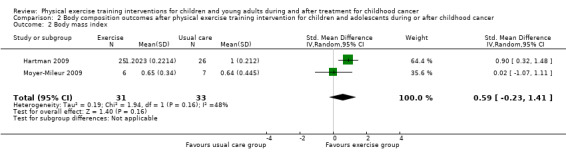
Comparison 2 Body composition outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer, Outcome 2 Body mass index.
Flexibility
Two studies measured the ankle dorsiflexion range of motion. However, in one study this was done in a passive way (Hartman 2009), and in the other by active contraction of the ankle (Marchese 2004). Therefore, we could not pool data.
According to the ITT analysis shown in Analysis 3.1, the passive ankle dorsiflexion showed a moderate significant positive effect for the 25 children in the intervention group compared to the 26 children in the control group (SMD 0.69; 95% CI 0.12 to 1.25; P value = 0.02) (Hartman 2009). Analysis of the ankle dorsiflexion range of motion, measured in active contraction, showed a non‐significant moderate effect in the intervention group (13 children) compared to the control group (15 children) (SMD 0.46; 95% CI ‐0.29 to 1.22; P value = 0.23) (Analysis 3.1) (Marchese 2004). Because Marchese 2004 only provided the data of the children who completed all measurements, we performed no ITT analysis.
3.1. Analysis.
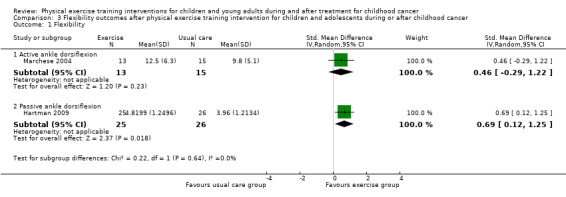
Comparison 3 Flexibility outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer, Outcome 1 Flexibility.
The study of Moyer‐Mileur 2009 assessed body flexibility with the sit‐and‐reach distance test. In this study, there was no difference in the test results between the six children of the intervention and seven children of the control group. P values and ITT analysis were not stated in the text or provided by the authors.
The study of Tanir 2013 used the goniometer to assess the range of motion. Unfortunately, the baseline scores were significantly different, which may have affected the outcomes. This study showed statistically significant differences between the study groups at the end‐measurement with higher scores in the control group, but no significant increase of the goniometer results over time within the study groups. However, the authors did not report the assessment position of the goniometer on the body. Therefore, it was not clear whether a decrease in the goniometry results over time was a positive or a negative study result.
Muscle endurance/strength
Marchese 2004 assessed the knee and ankle strength changes and Tanir 2013 assessed back and leg strength changes by hand‐held dynamometry. In both studies, the authors found a significant effect in favour of the intervention group. Analysis showed that differences between the end scores of the intervention group and the control group were not significantly different for both knee and ankle strength (Analysis 4.1; Analysis 4.2), but that differences were significant for back and leg strength (SMD 1.41; 95% CI 0.71 to 2.11; P value < 0.001) (Analysis 4.3) (Tanir 2013). The SMD of the knee strength was 0.25 (95% CI ‐0.49 to 1.00; P value = 0.51) and the increase of ankle strength was 0.29 (95% CI ‐0.46 to 1.04; P value = 0.44) (Marchese 2004).
4.1. Analysis.
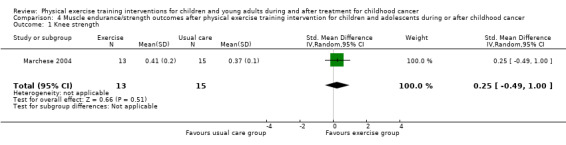
Comparison 4 Muscle endurance/strength outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer, Outcome 1 Knee strength.
4.2. Analysis.
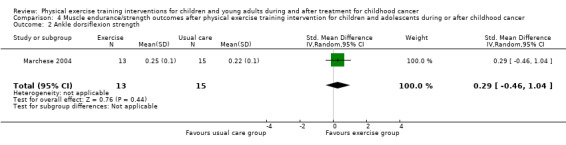
Comparison 4 Muscle endurance/strength outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer, Outcome 2 Ankle dorsiflexion strength.
4.3. Analysis.
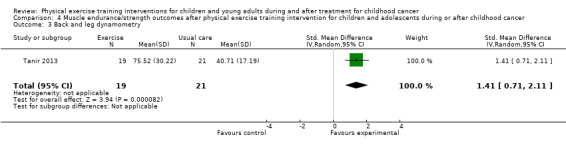
Comparison 4 Muscle endurance/strength outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer, Outcome 3 Back and leg dynamometry.
The study of Moyer‐Mileur 2009 reported differences in the number of completed push‐ups (with knees on the ground) and used a peripheral quantitative computed tomography of the tibia to determine the muscle mass of the participants. According to the original study data, there was no significant change in the maximum number of push‐ups or muscle mass, within or between the intervention (six children) and control group (seven children). The report of this study did not include the data of these results; therefore, we could not perform a Review Manager analysis.
de Macedo 2010 assessed respiratory muscle strength by measuring the maximal inspiratory pressure and maximal expiratory pressure with a digital manometer and a nozzle to dissipate additional pressure caused by the facial muscles and the oropharynx. In the intervention group (five children), the authors found a significant improvement over time compared to the control group (nine children). However, the end score differences were not significant different between the study groups; SMD for inspiratory breathing muscle strength was 0.33 (95% CI ‐0.77 to 1.43; P value = 0.56) and for expiratory breathing muscle strength the SMD was 0.00 (95% CI ‐1.09 to 1.09; P value = 1.00) (Analysis 4.4; Analysis 4.5).
4.4. Analysis.
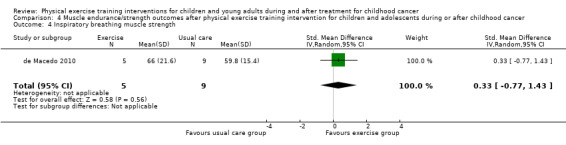
Comparison 4 Muscle endurance/strength outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer, Outcome 4 Inspiratory breathing muscle strength.
4.5. Analysis.
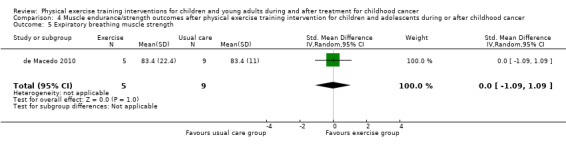
Comparison 4 Muscle endurance/strength outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer, Outcome 5 Expiratory breathing muscle strength.
Due to invalid methods used for missing data imputation, we could not perform an ITT analysis for these outcomes.
Activity energy expenditure
The included studies did not assess activity energy expenditure.
Level of daily activity
One study assessed daily physical activity (Moyer‐Mileur 2009). This study used both the pedometer steps‐per‐day and an activity questionnaire to examine physical activity behaviour. This study showed that the increase in "reported activity in minutes per day" over time was approximately the same for the six children in the intervention group. In the control group, three out of seven children increased in their reported activity in minutes per day. According to the original analyses, the reported activities at baseline and at six months were not statistically significantly different between the intervention group and the control group (Moyer‐Mileur 2009). At 12 months from baseline, there was a higher number of steps recorded in the intervention group compared with the controls, but this difference was of borderline statistical significance (P value = 0.06) (no Review Manager data available) (Moyer‐Mileur 2009). This analysis was not performed according to the ITT procedure.
Time spent exercising (more than daily activity)
The included studies did not assess time spent exercising (more than daily activity).
Health‐related quality of life
One study assessed general HRQoL using the PedsQL Generic Core Scale (version 3.0) (Marchese 2004).
Marchese 2004 found no significant effect on quality of life by the physical exercise training intervention. Overall, the SMD for PedsQL Generic was ‐0.23 points (95% CI ‐0.98 to 0.51; P value = 0.54) (Analysis 5.1). In addition to the participant‐reported data, parent reports also showed no intervention effect: the SMD on the parent general PedsQL questionnaire was 0.38 points (95% CI ‐0.37 to 1.13; P value = 0.32) (Analysis 5.3).
5.1. Analysis.
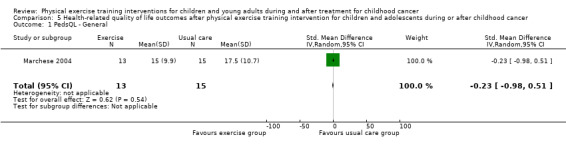
Comparison 5 Health‐related quality of life outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer, Outcome 1 PedsQL ‐ General.
5.3. Analysis.
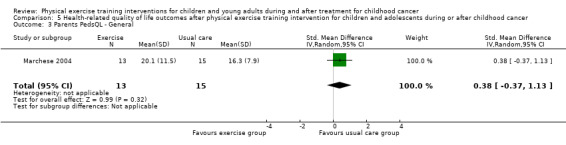
Comparison 5 Health‐related quality of life outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer, Outcome 3 Parents PedsQL ‐ General.
Two studies assessed cancer‐related HRQoL using the PedsQL Cancer Module 3.0 (Marchese 2004; Tanir 2013). However, Tanir 2013 did not report on the total score of the PedsQL Cancer Module, as Marchese 2004 did. On the contrary, Tanir 2013 reported the results of the eight different sub‐scales, which was not available in the study of Marchese 2004. Without the raw data of the questionnaires it was not possible to count the overall sum score from the sub‐scales. For that reason, we could not pool the data on HRQoL (PedsQL Cancer Module). Based on the sub‐scale data, Tanir 2013 found an increase on the HRQoL (cancer‐related items) in both groups by participant‐report on: pain and hurt, nausea, and procedural anxiety scales; without significant differences on the end‐scores. The only significant different end score (P value = 0.03) between the intervention (19 children) and control group (21 children) was found for the sub‐scale assessing worries (in favour of the intervention group) (Tanir 2013).
According to the sum score as assessed in the study of Marchese 2004, the SMD on the PedsQL Cancer Module was 0.16 (95% CI ‐0.58 to 0.91; P value = 0.66) (Analysis 5.2). The parent‐reported intervention effect on cancer‐related HRQoL again showed no intervention effect: the SMD on the parent cancer PedsQL was 0.04 points (95% CI ‐0.70 to 0.79; P value = 0.91) (Analysis 5.4) (Marchese 2004).
5.2. Analysis.
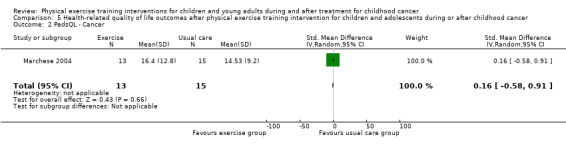
Comparison 5 Health‐related quality of life outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer, Outcome 2 PedsQL ‐ Cancer.
5.4. Analysis.
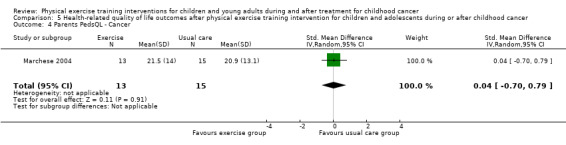
Comparison 5 Health‐related quality of life outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer, Outcome 4 Parents PedsQL ‐ Cancer.
Due to missing data, we could not conduct an ITT analysis.
Fatigue
Yeh 2011 measured the effect of a physical exercise training intervention on fatigue. This study used the PedsQL Multidimensional Fatigue Scale. They compared changes on fatigue between the intervention group (12 children) and the control group (10 children) over eight time points within 10 weeks. There were no significant differences between the intervention and control groups on the sub‐scale general fatigue (SMD ‐0.04; 95% CI ‐0.88 to 0.80; P value = 0.92) (Analysis 6.1). More specifically, there was no intervention effect for sleep and rest (SMD ‐0.01; 95% CI ‐0.85 to 0.83; P value = 0.98) (Analysis 6.2), or for cognitive fatigue (SMD 0.07; 95% CI ‐0.77 to 0.91; P value = 0.86) (Analysis 6.3). Apart from a per‐protocol analysis, the study of Yeh 2011 included an ITT analysis. The ITT analysis revealed no significant intervention effects on fatigue.
6.1. Analysis.
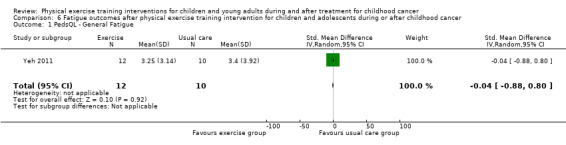
Comparison 6 Fatigue outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer, Outcome 1 PedsQL ‐ General Fatigue.
6.2. Analysis.
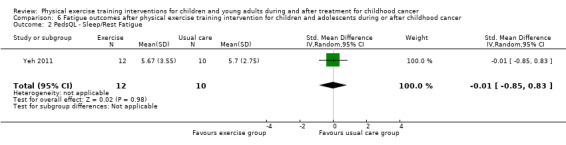
Comparison 6 Fatigue outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer, Outcome 2 PedsQL ‐ Sleep/Rest Fatigue.
6.3. Analysis.
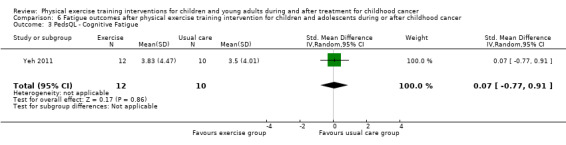
Comparison 6 Fatigue outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer, Outcome 3 PedsQL ‐ Cognitive Fatigue.
Anxiety and depression
The included studies did not assess anxiety and depression.
Self efficacy
The included studies did not assess self efficacy.
Adverse events (due to, or not clearly related to, the intervention)
The study of Marchese 2004 reported that none of the children experienced any negative effects from the exercises or experienced complications attributed to the physical programme. The other studies did not report on adverse events (de Macedo 2010; Hartman 2009; Moyer‐Mileur 2009; Tanir 2013; Yeh 2011).
Sensitivity analysis
We performed sensitivity analyses for those outcomes for which pooling was possible (i.e. 9‐minute walk‐run test, timed up‐and‐down stairs test, and BMI) (Hartman 2009; Marchese 2004; Moyer‐Mileur 2009; Tanir 2013). We assessed whether the outcome would have been different when a study with high or unclear risk was excluded in the review analyses.
Two studies performed both the 9‐minute walk‐run test and the timed up‐and‐down stairs test (Marchese 2004; Tanir 2013). In these studies, there were three bias items: random sequence generation (selection bias), blinding of outcome assessment (detection bias), and selective reporting (reporting bias), in which Tanir 2013 showed high or unclear bias compared to low bias in the study of Marchese 2004. For these three items, we performed sensitivity analyses. For all other risk of bias items, the two studies scored the same (i.e. high or unclear risk) or performed a combination of high and unclear risk.
The outcome of the sensitivity analysis for the 9‐minute walk‐run test of Marchese 2004 without the data of Tanir 2013 showed an SMD of 0.33 (95% CI ‐0.42 to 1.07) whereas the results including Tanir 2013 showed a significant intervention effect with an SMD of 0.69 (95% CI 0.02 to 1.35). This analysis showed the analyses were consistent among the trials.
A sensitivity analysis was performed for the timed‐up‐and‐down‐stairs test. When we assessed data of the study by Marchese 2004 without the data of Tanir 2013, data showed a non‐significant SMD of 0.11 (95% CI ‐0.64 to 0.85). There were comparable results when we included the data of both studies (SMD ‐0.54; 95% CI ‐1.77 to 0.70). Therefore, the results of the trials were consistent among the trials.
Two studies assessed BMI (Hartman 2009; Moyer‐Mileur 2009). In these studies, there were two bias items: random sequence generation (selection bias) and blinding of outcome assessors (detection bias) on which Moyer‐Mileur 2009 showed unclear bias compared to the study of Hartman 2009. For these items, sensitivity analyses were possible. For all other risk of bias items, the two studies scored the same (i.e. low, high, or unclear risk) or performed a combination of high and unclear risk.
The outcome of the sensitivity analysis showed the BMI data of Hartman 2009 without Moyer‐Mileur 2009 (SMD 0.90; 95% CI 0.32 to 1.48). The results of the pooled data revealed an SMD of 0.59 (95% CI ‐0.23 to 1.41). Thus, the results of the sensitivity analyses were consistent among the trials and did not differ from the overall analyses.
Discussion
Summary of main results
Several studies have investigated the effects of physical exercise training interventions on physical fitness in adults with cancer, showing different benefits. Limited studies investigated the effects of such an intervention in a childhood cancer population. In particular, high‐quality studies with an RCT or CCT design are still lacking in this field of research.
This is an update of the original review that was performed in 2011 (Braam 2013). This updated review include six original studies. All studies investigated the effects of a physical exercise training intervention, with a duration of at least four weeks, in children with cancer. They all aimed to improve physical functioning or psychosocial well‐being, and had enrolled children with ALL. However, the studies had two important limitations. First, the total number of participants included in the six studies was limited, and second, the exercise programmes were not always appropriately designed to meet the study goals.
Cardiorespiratory fitness was studied using the 9‐minute run‐walk test, timed up‐and‐down stairs test, timed up‐and‐go time test, and 20‐m shuttle run test. All but the timed up‐and‐down stairs test showed significant positive intervention effects (P value < 0.05).
Bone mineral density increased significantly higher after a physical exercise training intervention when compared with the study control group. Two studies assessed BMI. One study found a significant intervention effect on BMI. However, these results were not found when the data were pooled with the second study.
Four studies assessed flexibility and each study used different test methods. There were no (statistically significant) differences between the study groups in three studies, whereas in the fourth study, there was a statistically significant difference in favour of the exercise group.
One study assessed back and leg strength (muscle strength) and showed a significant intervention effect. The other three studies assessing muscle strength could not report statistically significant intervention effect. There was no statistically significant effect on knee or ankle muscle strength, which were assessed in two studies, or on lung muscle strength (maximal inspiratory and expiratory pressure), which was the primary outcome of the fourth study.
HRQoL assessed by the PedsQL Cancer Module showed some positive effects in the intervention group in comparison to the control group in one study (Tanir 2013). There were no statistically significant differences between the study groups for the level of daily activity and fatigue. In addition, only one study reported no complications attributed to the physical exercise intervention programme, whereas the other studies did not address this item.
None of the six included studies evaluated the outcomes of activity energy expenditure, time spent exercising, anxiety and depression, or self efficacy.
It should be noted that the exercise interventions were not the same and the quality and quantity of the evidence was limited.
For future research, it is advised to assess the effects of one type of exercise intervention in a larger group of children with cancer, preferably in children with ALL as well as other childhood cancer diagnoses. This can be done in well‐designed studies with large sample sizes.
Overall completeness and applicability of evidence
This review provides evidence of modest positive effects of physical exercise training interventions for children with cancer. These modest effects could be due to small sample sizes, various types of interventions provided, and different outcome measures that were used in the six studies. As a result, we could only pool data for 9‐minute walk‐run test, the timed up‐and‐down stairs test, and BMI; therefore, the results of the analysis were instable and weak. However, the meta‐analysis and sensitivity analysis on these three outcomes showed consistent results. Furthermore, the patient population was unintentionally homogeneous since all of the included children had ALL. Therefore, the results of this review are not applicable for other types of childhood cancer.
The Review Manager analyses results of this review were very different to the analyses performed by the authors of some of the studies, which led to different conclusions. For de Macedo 2010, Hartman 2009, and Marchese 2004, the differences were due to different methods of analysis. In this review, we assessed the final outcome differences between the study groups (Analysis 4.1; Analysis 4.4; Analysis 4.5), and did not assess changes over time.
The included studies all had supervised interventions with a duration and intensity in which it was possible to have a physiological response (de Macedo 2010; Hartman 2009; Marchese 2004; Moyer‐Mileur 2009; Yeh 2011). From literature, it is known that supervised exercise interventions in children are more effective compared to non‐supervised programmes (Faigenbaum 2010). It is also known that a well‐designed exercise programme consists of four parameters: mode (type of exercise), intensity, frequency, and duration (ACSM 2010; Ganley 2011). It would be advisable for new studies to first determine if the planned programme includes all elements of these parameters. This will increase the quality of the trials and also increase the comparability.
Appropriate statistical methods are important. The use of incorrect statistical methods can diminish the likelihood of demonstrating the real effects, also in high‐quality interventions. In this review, only one of the included studies used a power calculation (Hartman 2009). In the included studies, the authors assessed baseline (pre‐score) differences between the study groups using the Chi2 test or the Mann‐Whitney U test (Hartman 2009; Moyer‐Mileur 2009; Tanir 2013), the Kruskal‐Wallis test (Moyer‐Mileur 2009; Tanir 2013), and the paired sample T‐test (de Macedo 2010; Tanir 2013). The baseline scores were reported as group mean (de Macedo 2010; Hartman 2009; Marchese 2004; Tanir 2013; Yeh 2011), but also per study participant (Moyer‐Mileur 2009). These baseline differences might have had a large impact on the results and conclusions of this review. It would have been preferable for all authors to have corrected for baseline differences in their analyses. However, this was not done. To increase the quality of evidence of this review, we hoped to be able to pool all raw data (baseline and end of study data) in one database. This would have given us the possibility to correct for these differences. However, not all researchers responded to our request for additional information.
To investigate changes between participants and changes over time the included studies used the paired sample T‐tests (de Macedo 2010; Hartman 2009; Tanir 2013), Friedman two‐way test (Moyer‐Mileur 2009), mixed‐effects model (Yeh 2011), and repeated measure analyses (Hartman 2009; Marchese 2004). The mixed‐effect model and repeated measure analyses are more specific than comparing group mean changes. Therefore, the results of the studies using the better statistical methods are possibly better than the ones using simple statistical techniques. However, in this review, we were unable to use this information in the outcome.
Quality of the evidence
By grading the evidence according to the GRADE criteria (Guyatt 2008b), the overall quality of the individual outcomes varied between moderate and very low. Due to risk of bias, inconsistency, indirectness, imprecision, possible publication bias, or a combination of these, the qualities of the review outcomes were downgraded. None of the individual outcomes were eligible for upgrading. The quality of the evidence is summarized in Table 1. The small number of participants in the trials was the main reason for the low‐quality scores. This is often the case in studies in a paediatric population, and in case of newly introduced interventions. More and larger well‐controlled studies are needed to improve the quality and quantity of evidence. This also emphasizes the need for a core‐set of outcome measures in exercise‐related research in childhood chronic conditions (Van Brussel 2011).
Between the six studies, there was a considerable degree of heterogeneity on mode and intensity of the exercise interventions. When assessing heterogeneity, the 9‐minute walk‐run test and BMI showed no substantial heterogeneity between the two trials (I2 = 44% for 9‐minute walk‐run test and I2 = 48% for BMI). However, the timed up‐and‐down stairs test showed substantial heterogeneity (I2 = 84%).
Potential biases in the review process
The Cochrane Childhood Cancer Group formulated the search strategies for CENTRAL, MEDLINE/PubMed, and EMBASE/Ovid. In addition, we searched two other databases using of a search strategy that we developed ourselves: CINAHL and PEDro. The PEDro database was difficult to search. Therefore, it is possible that we missed one or two studies from this database. However, due to the great overlap between results of the different databases, it is very unlikely that studies were missed.
Agreements and disagreements with other studies or reviews
In 2010, Winter 2010 published a review on childhood cancer and physical activity. This review included 28 studies, and almost half had an uncontrolled study design. Eight studies used healthy controls. Of the four RCTs included in that review, one study included long‐term childhood cancer survivors (mean 12 years from diagnosis). Another RCT offered a two‐ to four‐day intervention, which, therefore, did not match with the inclusion criteria of this Cochrane review (Hinds 2007). The two remaining RCTs of the review by Winter 2010 are also included in this Cochrane review (Hartman 2009; Marchese 2004).
Huang 2011 performed a second review on exercise interventions for childhood cancer patients. They included many of the same studies, but also the study of Chamorro‐Vina 2010, which again introduced an intervention of less than four weeks. Both reviews concluded that results are promising, but that there is a need for more and larger RCTs. Both reviews stated that only a subgroup of the childhood cancer population was tested, since almost all studies concerned children with ALL. These findings are consistent with our findings.
Wolin 2010 performed the third review on exercise intervention for children with cancer. This review studied exercise intervention for adults or children (or both) with cancer. They included 12 studies on children with cancer or survivors of childhood cancer (or both) who did not received a stem cell transplantation and two studies in children with cancer who did receive a haematopoietic stem cell transplantation. Next to the participants who received transplantation, they included also uncontrolled studies and studies with a short intervention duration.
Baumann 2013 performed the fourth review on this subject. This review included 17 studies, of which seven were uncontrolled. They included three RCTs also in our list, but also two others with a short intervention period. Both Baumann 2013 and Wolin 2010 included the study of Shore 1999, which we excluded for this review because it used healthy control participants.
Despite the different studies included in the reviews, all the conclusions were comparable with ours; although studies have limitations in their methodology the results are promising. However, more research is needed to increase the level of evidence.
Authors' conclusions
Implications for practice.
Based on the currently available evidence from the included randomized controlled trials (RCTs) and controlled clinical trials (CCTs), we were unable to draw conclusions regarding the best physical exercise training intervention, neither can we provide information on the best timing of the intervention during or after cancer treatment. However, the six included studies did show that exercise training is feasible in children with acute lymphoblastic leukaemia (ALL).
Effects of the intervention are not yet convincing due to small numbers of participants and insufficient study methodology. Despite that, first results showed more improvements on the outcomes in the intervention group than in the control group. Especially when assessing outcomes such as cardiorespiratory fitness, body composition, flexibility, muscle strength, and HRQoL. However, we identified no significant differences for the level of daily activity, fatigue, and adverse events. Moreover, the included studies did not assess activity energy expenditure, time spent exercising, anxiety, depression, or self efficacy.
Implications for research.
The observed heterogeneity in study findings can be due to differences in the physical exercise training intervention (mode, intensity, frequency, duration, and location), different outcome measures (quantitative, qualitative, physical, or psychosocial), and methods to assess the effects of an intervention. Consensus on these items is needed in order to facilitate comparison of results across different studies.
More and high‐quality evidence is needed in order to be able to draft exercise and physical activity guidelines for this population. We urge the paediatric oncology community to design national or international multicentre studies, while local and small‐scale studies must be discouraged.
In addition, since ‐ even in this update ‐ we could only include six RCTs or CCTs with 171 children, there is a need for additional well‐designed studies with large sample sizes. Results of ongoing trials are awaited, and further trials with adequate power are needed.
What's new
| Date | Event | Description |
|---|---|---|
| 16 March 2017 | Feedback has been incorporated | Feedback regarding reference used in background incorporated. |
History
Protocol first published: Issue 11, 2010 Review first published: Issue 4, 2013
| Date | Event | Description |
|---|---|---|
| 21 August 2015 | New citation required but conclusions have not changed | One new study could be included in the review. By the additional study new information was added to the review; however the update did not change the conclusion of this review. |
| 29 April 2015 | New search has been performed | The search for eligible studies was updated to November 2014 |
| 28 August 2013 | Amended | As part of an audit of reviews by the Cochrane Editorial Unit some comments were received via email; these comments have been incorporated into the discussion and the Summary of Findings table sections of the review. |
Acknowledgements
We would like to thank Edith Leclercq for developing and running the search strategy for CENTRAL, MEDLINE, and EMBASE. We would like to thank Bart Bartels, MSc for translating the Portuguese article and Dr. Annelies Hartman for providing additional data. In addition, we would like to mention that the editorial base of the Cochrane Childhood Cancer Group is funded by Stichting Kinderen Kankervrij (KIKA) Foundation ‐ Children Cancer‐free.
Appendices
Appendix 1. Search strategy for Central Register of Controlled TRials (CENTRAL)
1. For children, we used the following text words for searching Title, Abstract, or Keywords:
infant OR infan* OR newborn OR newborn* OR new‐born* OR baby OR baby* OR babies OR neonat* OR perinat* OR postnat* OR child OR child* OR schoolchild* OR schoolchild OR school child OR school child* OR kid OR kids OR toddler* OR adolescent OR adoles* OR teen* OR boy* OR girl* OR minors OR minors* OR underag* OR under ag* OR juvenil* OR youth* OR kindergar* OR puberty OR puber* OR pubescen* OR prepubescen* OR prepuberty* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR schools OR nursery school* OR preschool* OR pre school* OR primary school* OR secondary school* OR elementary school* OR elementary school OR high school* OR highschool* OR school age OR schoolage OR school age* OR schoolage* OR infancy
2. For childhood cancer, we used the following text words for searching Title, Abstract, or Keywords:
(leukemia OR leukemi* OR leukaemi* OR (childhood ALL) OR AML OR lymphoma OR lymphom* OR hodgkin* OR T‐cell OR B‐cell OR non‐hodgkin OR sarcoma OR sarcom* OR Ewing* OR osteosarcoma OR osteosarcom* OR wilms tumor OR wilms* OR nephroblastom* OR neuroblastoma OR neuroblastom* OR rhabdomyosarcoma OR rhabdomyosarcom* OR teratoma OR teratom* OR hepatoma OR hepatom* OR hepatoblastoma OR hepatoblastom* OR PNET OR medulloblastoma OR medulloblastom* OR PNET* OR neuroectodermal tumors, primitive OR retinoblastoma OR retinoblastom* OR meningioma OR meningiom* OR glioma OR gliom* OR pediatric oncology OR paediatric oncology OR childhood cancer OR childhood tumor OR childhood tumors OR cancer or neoplasms or tumor or cancers or neoplasm or tumors)
3. For cancer, we used the following text words for searching Title, Abstract, or Keywords:
cancer OR oncology OR oncolog* OR neoplasms OR neoplas* OR carcinoma OR carcinom* OR tumor OR tumour OR tumor* OR tumour* OR cancer* OR malignan* OR hematooncological OR hemato oncological OR hemato‐oncological OR hematologic neoplasms OR hematolo* OR bone marrow transplantation OR bone marrow transplant* OR leukaemia OR lymphoma
4. For physical exercise training therapy, we used the following text words for searching Title, Abstract, or Keywords:
exercise OR exercises OR exercis* OR Physical Exercise OR Physical Exercises OR Isometric Exercises OR Isometric Exercise OR Warm‐Up Exercise OR Warm Up Exercise OR Warm‐Up Exercises OR Aerobic Exercises OR Aerobic Exercise OR exercise therapy OR Exercise Therapies OR physical therapy modalities OR Physical Therapy Modality OR Physiotherapy (Techniques) OR Physiotherapies (Techniques) OR Physical Therapy Techniques OR Physical Therapy Technique OR exercise test OR exercise tests OR muscle stretching exercise OR muscle stretching exercises OR physical therapy OR physical therapies OR strengthen* OR stretch* OR physiotherapy OR physiotherap* OR stability training OR training* OR exercise movement technique OR exercise movement techniques OR exercise movement technic OR Exercise Movement Technics OR pilates based exercise OR pilates‐based exercise OR Pilates Based Exercises OR Pilates‐Based Exercises OR pilates OR physical exercise OR gymnastics OR gymnastic OR gymnastic* OR swimming OR locomotion OR locomotions OR locomotion* OR treadmill OR walking OR running OR aerobic OR aerobics OR aerobic* OR cycling OR jogging OR Exertion OR disability of function OR occupational therapy OR occupational therapies OR functional therapy OR functional therapies OR training program OR physical education and training OR Physical Education OR fitness OR cardio training OR weight lifting OR power training OR muscle training OR rowing OR sports OR jump OR jumping
5. For outcome, we used the following text words for searching Title, Abstract, or Keywords:
quality of life OR Qol OR condition* OR physical fitness OR Human Physical Conditioning OR Human Physical Conditionings OR physical effort OR physical skill OR physical activity OR muscle strength OR muscular strength OR lung function OR pulmonary function OR vital capacity OR Depression OR Depressive Disorder OR involutional depression OR fear OR recovery of function OR physical endurance OR range of motion OR VO2 OR VO(2peak) OR ventilatory threshold OR heart rate OR endurance OR activity energy expenditure OR DXA scan OR activity participation OR mets score OR DeltaMetS OR Wingate anaerobic test OR steep ramp test OR dynamometer OR Six Minute Walk Distance OR 6MWD OR lateral step up OR Sit‐to‐Stand OR ten repetition maximum OR minimum chair height OR muscle power OR gross motor function OR GMFCS OR GMFM OR incremental shuttle walking OR sit‐and‐reach
Final search:
1 and (2 or 3) and 4 and 5
[*]=1+ more characters
Appendix 2. Search strategy for MEDLINE/PubMed
1. For children, we used the following MeSH headings and text words:
infant OR infan* OR newborn OR newborn* OR new‐born* OR baby OR baby* OR babies OR neonat* OR perinat* OR postnat* OR child OR child* OR schoolchild* OR schoolchild OR school child OR school child* OR kid OR kids OR toddler* OR adolescent OR adoles* OR teen* OR boy* OR girl* OR minors OR minors* OR underag* OR under ag* OR juvenil* OR youth* OR kindergar* OR puberty OR puber* OR pubescen* OR prepubescen* OR prepuberty* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR schools OR nursery school* OR preschool* OR pre school* OR primary school* OR secondary school* OR elementary school* OR elementary school OR high school* OR highschool* OR school age OR schoolage OR school age* OR schoolage* OR infancy OR schools, nursery OR infant, newborn
2. For cancer and childhood cancer, we used the following MeSH headings and text words:
cancer OR oncology OR oncolog* OR neoplasms OR neoplas* OR carcinoma OR carcinom* OR tumor OR tumour OR tumor* OR tumour* OR cancer* OR malignan* OR hematooncological OR hemato oncological OR hemato‐oncological OR hematologic neoplasms OR hematolo* OR bone marrow transplantation OR bone marrow transplant* OR lymphoma OR (((leukemia OR leukemi* OR leukaemi* OR (childhood ALL) OR AML OR lymphoma OR lymphom* OR hodgkin OR hodgkin* OR T‐cell OR B‐cell OR non‐hodgkin OR sarcoma OR sarcom* OR sarcoma, Ewing's OR Ewing* OR osteosarcoma OR osteosarcom* OR wilms tumor OR wilms* OR nephroblastom* OR neuroblastoma OR neuroblastom* OR rhabdomyosarcoma OR rhabdomyosarcom* OR teratoma OR teratom* OR hepatoma OR hepatom* OR hepatoblastoma OR hepatoblastom* OR PNET OR medulloblastoma OR medulloblastom* OR PNET* OR neuroectodermal tumors, primitive OR retinoblastoma OR retinoblastom* OR meningioma OR meningiom* OR glioma OR gliom*) OR (pediatric oncology OR paediatric oncology)) OR (childhood cancer OR childhood tumor OR childhood tumors)) OR (brain tumor* OR brain tumour* OR brain neoplasms OR central nervous system neoplasm OR central nervous system neoplasms OR central nervous system tumor* OR central nervous system tumour* OR brain cancer* OR brain neoplasm* OR intracranial neoplasm*) OR (leukemia lymphocytic acute) OR (leukemia, lymphocytic, acute[mh])
3. For physical exercise training therapy, we used the following MeSH headings and text words:
exercise OR exercises OR exercis* OR Exercise, Physical OR Exercises, Physical OR Physical Exercise OR Physical Exercises OR Exercise, Isometric OR Exercises, Isometric OR Isometric Exercises OR Isometric Exercise OR Warm‐Up Exercise OR Exercise, Warm‐Up OR Exercises, Warm‐Up OR Warm Up Exercise OR Warm‐Up Exercises OR Exercise, Aerobic OR Aerobic Exercises OR Exercises, Aerobic OR Aerobic Exercise OR exercise therapy OR Therapy, Exercise OR Exercise Therapies OR Therapies, Exercise OR physical therapy modalities OR Modalities, Physical Therapy OR Modality, Physical Therapy OR Physical Therapy Modality OR Physiotherapy (Techniques) OR Physiotherapies (Techniques) OR Physical Therapy Techniques OR Physical Therapy Technique OR Techniques, Physical Therapy OR exercise test OR exercise tests OR muscle stretching exercise OR muscle stretching exercises OR physical therapy OR physical therapies OR strengthen* OR stretch* OR physiotherapy[text] OR physiotherap*[text] OR stability training OR training* OR exercise movement technique OR exercise movement techniques OR Movement Techniques, Exercise OR exercise movement technic OR Exercise Movement Technics OR pilates based exercise OR pilates‐based exercise OR Pilates Based Exercises OR Pilates‐Based Exercises OR Exercises, Pilates‐Based OR pilates OR physical exercise OR gymnastics OR gymnastic OR gymnastic* OR swimming OR locomotion OR locomotions OR locomotion* OR treadmill OR walking OR running OR aerobic OR aerobics OR aerobic* OR cycling OR jogging OR Exertion OR disability of function[text] OR occupational therapy OR occupational therapies OR functional therapy[text] OR functional therapies[text] OR training program OR physical education and training OR Physical Education, Training OR Physical Education OR Education, Physical OR fitness OR cardio training OR weight lifting OR power training OR muscle training OR rowing OR sports OR jump OR jumping
4. For outcome, we used the following MeSH headings and text words:
quality of life OR Qol OR condition* OR physical fitness OR Fitness, Physical OR Physical Conditioning, Human OR Conditioning, Human Physical OR Conditionings, Human Physical OR Human Physical Conditioning OR Human Physical Conditionings OR Physical Conditionings, Human OR physical effort OR physical skill OR physical activity OR muscle strength OR muscular strength OR lung function OR pulmonary function OR vital capacity OR Depression OR Depressive Disorder OR Depression, involutional OR fear OR recovery of function OR physical endurance OR range of motion OR VO2 OR VO(2peak) OR ventilatory threshold OR heart rate OR endurance OR activity energy expenditure OR DXA scan OR activity participation OR mets score OR DeltaMetS OR Wingate anaerobic test OR steep ramp test OR dynamometer OR Six Minute Walk Distance OR 6MWD OR lateral step up OR Sit‐to‐Stand OR ten repetition maximum OR minimum chair height OR muscle power OR gross motor function OR GMFCS OR GMFM OR incremental shuttle walking OR sit‐and‐reach
5. For RCTs and CCTs, we used the following MeSH headings and text words:
(randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) AND humans[mh] (Higgins 2011)
Final search:
1 AND 2 AND 3 AND 4 AND 5
[pt]=publication type [tiab]=title or abstract [sh]=subject heading [mh]=MeSH term [text]=text word [*]=1+ more characters [RCT]= randomised controlled trial [CCT]= controlled clinical trial
Appendix 3. Search strategy for EMBASE/Ovid
1. Forchildren, we used the following Emtree terms and text words:
1. infant/ or infancy/ or newborn/ or baby/ or child/ or preschool child/ or school child/ 2. adolescent/ or juvenile/ or boy/ or girl/ or puberty/ or prepuberty/ or pediatrics/ 3. primary school/ or high school/ or kindergarten/ or nursery school/ or school/ 4. or/1‐3 5. (infant$ or newborn$ or (new adj born$) or baby or baby$ or babies or neonate$ or perinat$ or postnat$).mp. 6. (child$ or (school adj child$) or schoolchild$ or (school adj age$) or schoolage$ or (pre adj school$) or preschool$).mp. 7. (kid or kids or toddler$ or adoles$ or teen$ or boy$ or girl$).mp. 8. (minors$ or (under adj ag$) or underage$ or juvenil$ or youth$).mp. 9. (puber$ or pubescen$ or prepubescen$ or prepubert$).mp. 10. (pediatric$ or paediatric$ or peadiatric$).mp. 11. (school or schools or (high adj school$) or highschool$ or (primary adj school$) or (nursery adj school$) or (elementary adj school) or (secondary adj school$) or kindergar$).mp. 12. or/5‐11 13. 4 or 12
2. For childhood cancer, we used the following Emtree terms and text words:
1. (leukemia or leukemi$ or leukaemi$ or (childhood adj ALL) or acute lymphocytic leukemia).mp. 2. (AML or lymphoma or lymphom$ or hodgkin or hodgkin$ or T‐cell or B‐cell or non‐hodgkin).mp. 3. (sarcoma or sarcom$ or Ewing$ or osteosarcoma or osteosarcom$ or wilms tumor or wilms$).mp. 4. (nephroblastom$ or neuroblastoma or neuroblastom$ or rhabdomyosarcoma or rhabdomyosarcom$ or teratoma or teratom$ or hepatoma or hepatom$ or hepatoblastoma or hepatoblastom$).mp. 5. (PNET or medulloblastoma or medulloblastom$ or PNET$ or neuroectodermal tumors or primitive neuroectodermal tumor$ or retinoblastoma or retinoblastom$ or meningioma or meningiom$ or glioma or gliom$).mp. 6. (pediatric oncology or paediatric oncology).mp. 7. ((childhood adj cancer) or (childhood adj tumor) or (childhood adj tumors) or childhood malignancy or (childhood adj malignancies) or childhood neoplasm$).mp. 8. ((pediatric adj malignancy) or (pediatric adj malignancies) or (paediatric adj malignancy) or (paediatric adj malignancies)).mp. 9. ((brain adj tumor$) or (brain adj tumour$) or (brain adj neoplasms) or (brain adj cancer$) or brain neoplasm$).mp. 10. (central nervous system tumor$ or central nervous system neoplasm or central nervous system neoplasms or central nervous system tumour$).mp. 11. intracranial neoplasm$.mp. 12. LEUKEMIA/ or LYMPHOMA/ or brain tumor/ or central nervous system tumor/ or teratoma/ or sarcoma/ or osteosarcoma/ 13. nephroblastoma/ or neuroblastoma/ or rhabdomyosarcoma/ or hepatoblastoma/ or medulloblastoma/ or neuroectodermal tumor/ or retinoblastoma/ or meningioma/ or glioma/ or childhood cancer/ 14. or/1‐13
3. Forcancer, we used the following Emtree terms and text words:
1. (cancer or cancers or cancer$).mp. 2. (oncology or oncolog$).mp. or exp oncology/ 3. (neoplasm or neoplasms or neoplasm$).mp. or exp neoplasm/ 4. (carcinoma or carcinom$).mp. or exp carcinoma/ 5. (tumor or tumour or tumor$ or tumour$ or tumors or tumours).mp. or exp tumor/ 6. (malignan$ or malignant).mp. 7. (hematooncological or hemato oncological or hemato‐oncological or hematologic neoplasms or hematolo$).mp. or exp hematologic malignancy/ 8. or/1‐7
4. For physical exercise training therapy, we used the following Emtree terms and text words:
1. (exercise or exercises or exercis$).mp. 2. exp exercise/ 3. (physical exercise or physical exercises).mp. 4. exp isometric exercise/ 5. (isometric exercise or isometric exercises).mp. 6. (warm up exercise or warm up exercises or warm‐up exercise or warm‐up exercises).mp. 7. exp aerobic exercise/ 8. (aerobic exercise or aerobic exercises).mp. 9. exp kinesiotherapy/ 10. (exercise therapy or exercise therapies).mp. 11. (physical therapy modality or physical therapy modalities).mp. 12. exp pediatric physiotherapy/ or exp physiotherapy/ 13. (physiotherapy or physiotherapies).mp. 14. (physical therapy technique or physical therapy techniques or physical therapy or physical therapies).mp. 15. exp exercise test/ 16. (exercise test or exercise tests).mp. 17. exp stretching exercise/ 18. (muscle stretching exercise or muscle stretching exercises).mp. 19. (strengthen$ or stretch$).mp. 20. exp muscle exercise/ or stability training.mp. or exp muscle training/ 21. training$.mp. 22. (exercise movement technique or exercise movement techniques).mp. 23. (exercise movement technic or exercise movement technics).mp. 24. (pilates‐based exercise or pilates based exercise or pilates‐based exercises or pilates based exercises).mp. 25. pilates.mp. or exp pilates/ 26. physical exercise.mp. 27. (gymnastic or gymnastics or gymnastic$).mp. 28. exp swimming/ or swimming.mp. 29. exp locomotion/ 30. (locomotion or locomotions or locomotion$).mp. 31. exp treadmill/ or exp treadmill exercise/ 32. treadmill.mp. 33. walking.mp. or exp walking/ 34. exp running/ or running.mp. 35. cycling.mp. or exp cycling/ 36. jogging.mp. or exp jogging/ 37. (aerobic or aerobics or aerobic$).mp. 38. exertion.mp. 39. disability of function.mp. 40. exp occupational therapy/ 41. (occupational therapy or occupational therapies).mp. 42. (functional therapy or functional therapies).mp. 43. training program.mp. 44. (physical education and training).mp. 45. physical education.mp. or exp physical education/ 46. fitness.mp. or exp fitness/ 47. cardio training.mp. 48. weight lifting.mp. or exp weight lifting/ 49. power training.mp. 50. muscle training.mp. 51. rowing.mp. or exp rowing/ 52. sports.mp. or exp sport/ 53. exp jumping/ or (jump or jumping).mp. 54. or/1‐53
5. For outcome, we used the following Emtree terms and text words:
1. exp "quality of life"/ 2. (quality of life or QoL).mp. 3. general condition improvement/ 4. condition$.mp. 5. physical fitness.mp. or exp fitness/ 6. (human physical conditioning or human physical conditionings).mp. 7. physical effort.mp. 8. physical skill.mp. 9. physical activity.mp. or exp physical activity/ 10. (muscle strength or muscular strength).mp. or exp muscle strength/ 11. lung function.mp. or exp lung function/ 12. pulmonary function.mp. 13. vital capacity.mp. or exp vital capacity/ 14. depression.mp. or exp depression/ 15. depressive disorder.mp. 16. involutional depression.mp. or exp involutional depression/ 17. fear.mp. or exp fear/ 18. recovery of function.mp. or exp convalescence/ 19. physical endurance.mp. or exp endurance/ 20. range of motion.mp. or exp "range of motion"/ 21. (VO2 or VO2peak).mp. 22. (VO adj 2peak).mp. 23. ventilatory threshold.mp. 24. heart rate.mp. or exp heart rate/ 25. exp endurance/ or endurance.mp. 26. exp energy expenditure/ or activity energy expenditure.mp. 27. exp dual energy X ray absorptiometry/ or DXA scan.mp. 28. activity participation.mp. 29. mets score.mp. 30. (mets or DeltaMetS).mp. 31. Wingate anaerobic test.mp. 32. exp Steep Ramp Test/ or steep ramp test.mp. 33. dynamometer.mp. or exp dynamometer/ 34. (Six Minute Walk Distance or 6MWD).mp. 35. lateral step up.mp. 36. Sit‐to‐Stand.mp. 37. ten repetition maximum.mp. 38. minimum chair height.mp. 39. muscle power.mp. 40. (gross motor function or GMFCS or GMFM).mp. 41. incremental shuttle walking.mp. 42. sit‐and‐reach.mp. 43. or/1‐42
6. For RCTs and CCTs, we used the following Emtree terms and text words:
1. Randomized Controlled Trial/ 2. Controlled Clinical Trial/ 3. randomized.ti,ab. 4. placebo.ti,ab. 5. randomly.ti,ab. 6. trial.ti,ab. 7. groups.ti,ab. 8. drug therapy.sh. 9. or/1‐8 10. Human/ 11. 9 and 10
Final search
1 and (2 or 3) and 4 and 5 and 6
[mp]=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name [ti,ab]=title, abstract [sh]=subject heading [/]=Emtree term [$]=1+more characters [RCT]= randomized controlled trial [CCT]= controlled clinical trial
Appendix 4. Search strategy for CINAHL
1. For children, we used the following the following MeSH headings (MH) and text words for searching Title, Abstract, or Keywords:
"schoolage" OR (MH "Schools+") OR "peadiatric" OR "paediatric" OR "pediatric" OR (MH "Puberty+") OR "juvenile" OR "underage" OR "under age" OR ("teenager") or (MH "Adolescence+") OR "adolescent" OR "kids" OR "kid" OR "schoolchild" OR ("child*") or (MH "Child") ("newborn") or (MH "Infant, Newborn+") OR ("infant") or (MH "Infant+")
2. For cancer and childhood cancer, we used the following the following MeSH headings (MH) and text words for searching Title, Abstract, or Keywords:
(MH "Central Nervous System Neoplasms+") OR "childhood tumour" OR "childhood tumor" "childhood cancer" OR (MH "Meningioma") OR (MH "Retinoblastoma") OR (MH "Neuroectodermal Tumors+") OR (MH "Ameloblastoma") OR (MH "Teratoma") OR (MH "Rhabdomyosarcoma") OR (MH "Neuroblastoma") OR (MH "Nephroblastoma") OR (MH "Osteosarcoma+") OR (MH "Sarcoma, Ewing's") OR (MH "Sarcoma+") or (MH "Osteosarcoma") OR (MH "Lymphoma+") OR (MH "Leukemia+") OR (MH "Bone Marrow Transplantation+") or (MH "Bone Marrow Neoplasms") OR "hemato oncological" OR ("malignancy") or (MH "Hematologic Neoplasms+") OR "tumour" OR "tumor" OR (MH "Carcinoma+") OR (MH "Neoplasms+") OR ("oncology") or (MH "Oncology+") or (MH "Pediatric Oncology Nursing") or (MH "Oncologic Care") OR ("cancer") or (MH "Neoplasms")
3. For physical exercise training therapy, we used the following the following MeSH headings (MH) and text words for searching Title, Abstract, or Keywords:
("sports") or (MH "Sports+") or (MH "Amateur Sports") or (MH "Aquatic Sports") (MH "Rowing") or (MH "Ergometry") OR ("muscle training") or (MH "Muscle Strengthening") OR "power training" OR (MH "Weight Lifting") OR ("cardio training") or (MH "Athletic Training") or (MH "Athletic Training Programs") OR ("fitness") or (MH "Physical Fitness") OR (MH "Physical Education and Training+") OR "training program" "functional therapies" OR "functional therapy" OR (MH "Occupational Therapy+") or (MH "Pediatric Occupational Therapy") OR "disability of function" OR (MH "Exertion") OR (MH "Cycling") or (MH "Ergometry") OR (MH "Running") or (MH "Running, Distance") OR (MH "Walking") or (MH "Sports") OR (MH "Treadmills") OR (MH "Locomotion") or (MH "Movement") OR (MH "Swimming") OR (MH "Gymnastics") OR ("pilates") or (MH "Pilates") OR (MH "Therapeutic Exercise+") or (MH "Aerobic Exercises") or (MH "Arm Exercises") or (MH "Back Exercises") OR (MH "Stretching") OR (MH "Exercise Test+") or (MH "Exercise Test, Cardiopulmonary") or (MH "Exercise Test, Muscular+") OR "physiotherapy" OR ("exercise therapy") or (MH "Therapeutic Exercise+") or (MH "Exercise Therapy: Ambulation (Iowa NIC)") or (MH "Exercise Therapy: Balance (Iowa NIC)") or (MH "Exercise Therapy: Joint Mobility (Iowa NIC)") or (MH "Exercise Therapy: Muscle Control (Iowa NIC)") OR ("physical therapy") or (MH "Physical Therapy+") or (MH "Pediatric Physical Therapy") or (MH "Physical Therapy Practice, Evidence‐Based") or (MH "Physical Therapy Practice, Research‐Based") OR "therapies" OR (MH "Aerobic Exercises+") or (MH "Therapeutic Exercise+") OR (MH "Warm‐Up Exercise") (MH "Isometric Contraction") or (MH "Isometric Exercises") OR ("physical") or (MH "Education, Physical Therapy") or (MH "Home Physical Therapy") or (MH "Pediatric Physical Therapy") or (MH "Physical Activity") OR ("exercise") or (MH "Exercise+") or (MH "Abdominal Exercises") or (MH "Aerobic Exercises+") or (MH "Anaerobic Exercises") or (MH "Aquatic Exercises") or (MH "Arm Exercises") or (MH "Back Exercises")
4. For outcome, we used the following the following MeSH headings (MH) and text words for searching Title, Abstract, or Keywords:
"shuttle walking test" or ("repetition maximum") or (MH "Anaerobic Threshold") (MH "Rising") OR("lateral step up") or (MH "Step") OR ("six minute walking distance") or (MH "Running, Distance") or (MH "Walking+") OR(MH "Dynamometry") OR "steep ramp test" OR ("anaerobic test") or (MH "Achievement Tests") OR "wingate" OR (MH "Basal Metabolism") or (MH "Glucose Metabolism Disorders") OR (MH "Leisure Participation (Iowa NOC)") or (MH "Play Participation (Iowa NOC)") OR ("DXA scan") or (MH "Biometrics") OR (MH "Energy Metabolism+") or (MH "Activities of Daily Living+") or (MH "Human Activities+") OR ("endurance") OR (MH "Heart Rate+") or (MH "Heart Rate Variability") OR (MH "Respiratory Muscles") OR "VO2" OR "Vo2 peak" OR (MH "Range of Motion") or (MH "Range of Motion (Saba CCC)") or (MH "Motion Therapy, Continuous Passive") or (MH "Motion") OR (MH "Physical Endurance+") OR (MH "Recovery") or (MH "Functional Assessment") OR (MH "Fear+") OR (MH "Depression+") OR ("lung function") or (MH "Respiratory Function Tests+") or (MH "Functional Status") OR ("muscle strength") or (MH "Muscle Strength+") or (MH "Muscle Strengthening+") or (MH "Exercise Test, Muscular+") OR ("physical skill") or (MH "Exercise Test") or (MH "Motor Skills") or (MH "Social Skills") or (MH "Social Skills Training") OR (MH "Exertion") or (MH "Education, Physical Therapy") or (MH "Home Physical Therapy") OR (MH "Physical Fitness+") or (MH "Fitness Centers") OR (MH "Conditioning (Psychology)") or (MH "Conditioning, Cardiopulmonary") OR (MH "Quality of Life+") or (MH "Health and Life Quality (Iowa NOC) (Non‐Cinahl)+")
5. For RCTs and CCTs, we used the following MeSH headings and text words: (MH "randomized controlled trial") or (MH "controlled clinical trial") or (MH "randomized") or (MH "placebo") or ("drug therapy") or (MH "randomly+") or (MH "trial") or (MH "groups+") and (MH "human")
Final search
1 and 2 and 3 and 4 and 5
[MH] = MeSH headings: exploding retrieves all documents containing any of the subject terms below the term selected.
[+] = related terms are also taken into the search: In case of a plus sign (+) next to a narrower or related term, there are narrow terms below the term.
[RCT]= randomised controlled trial [CCT]= controlled clinical trial
Appendix 5. Search strategy for PEDro
1. For children, we used the text word "paediatrics" in <Subdiscipline> field 2. For cancer and childhood cancer, we used the text words "cancer" OR "oncolog" OR "neoplasm" OR "carcinom" or "tumor" OR "malignan" in the <Abstract & Title> field 3. For physical exercise training therapy, we used the text word "exercise" in the <Abstract & Title> field and combined (with OR) with the text words "fitness training" OR "hydrotherapy, balneotherapy" OR "neurodevelopmental therapy, neurofacilitation" OR "skill training" OR "strength training" in the <Therapy> field 4. For RCTs and CCTs, we used the text word "clinical trial" in the <Method> field
Final search
1 and 2 and 3 and 4
For outcome, we defined no search terms
Data and analyses
Comparison 1. Cardiorespiratory fitness outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 9‐minute run‐walk test | 2 | 68 | Std. Mean Difference (IV, Random, 95% CI) | 0.69 [0.02, 1.35] |
| 2 Timed up‐and‐down stairs test | 2 | 68 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.77, 0.70] |
| 3 Timed up‐and‐go test | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐1.83, ‐0.48] |
1.3. Analysis.
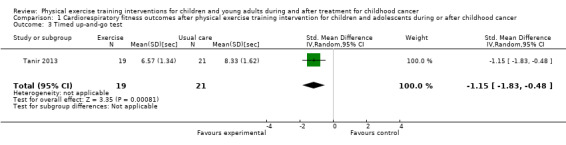
Comparison 1 Cardiorespiratory fitness outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer, Outcome 3 Timed up‐and‐go test.
Comparison 2. Body composition outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bone mineral density | 1 | 51 | Std. Mean Difference (IV, Random, 95% CI) | 1.07 [0.48, 1.66] |
| 2 Body mass index | 2 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [‐0.23, 1.41] |
Comparison 3. Flexibility outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Flexibility | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Active ankle dorsiflexion | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [‐0.29, 1.22] |
| 1.2 Passive ankle dorsiflexion | 1 | 51 | Std. Mean Difference (IV, Random, 95% CI) | 0.69 [0.12, 1.25] |
Comparison 4. Muscle endurance/strength outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Knee strength | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.49, 1.00] |
| 2 Ankle dorsiflexion strength | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.46, 1.04] |
| 3 Back and leg dynamometry | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 1.41 [0.71, 2.11] |
| 4 Inspiratory breathing muscle strength | 1 | 14 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.77, 1.43] |
| 5 Expiratory breathing muscle strength | 1 | 14 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.09, 1.09] |
Comparison 5. Health‐related quality of life outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PedsQL ‐ General | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.98, 0.51] |
| 2 PedsQL ‐ Cancer | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.58, 0.91] |
| 3 Parents PedsQL ‐ General | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.37, 1.13] |
| 4 Parents PedsQL ‐ Cancer | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.70, 0.79] |
Comparison 6. Fatigue outcomes after physical exercise training intervention for children and adolescents during or after childhood cancer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PedsQL ‐ General Fatigue | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.88, 0.80] |
| 2 PedsQL ‐ Sleep/Rest Fatigue | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.85, 0.83] |
| 3 PedsQL ‐ Cognitive Fatigue | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.77, 0.91] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
de Macedo 2010.
| Methods | Design: single‐centre RCT Setting: Brazil Department: paediatric oncology/haematology Randomization: random assignment but no further specifications available Stratification: not mentioned Study duration: 10 weeks Timing: inclusion of the study started during maintenance therapy of the childhood ALL treatment End point measurements: in the intervention group at baseline plus an evaluation every alternate week. In the control group at baseline and 10 weeks thereafter |
|
| Participants | n = 14 Diagnosis: ALL Age at start of study: mean (± SD) age of the whole group was 8.3 ± 2.6 years (range 5‐14 years). The mean age of the intervention group was 7.0 years and that of the control group 9.0 years Sex: 5 boys and 9 girls Exclusion criteria: children with a chronic lung disease, neuromuscular disease, or those receiving or having received radiotherapy |
|
| Interventions | This study investigated an inspiratory muscle training programme. They studied the effects of a domiciliary inspiratory muscle training with a duration of 15 minutes, performed twice a day, for 10 weeks. The training was performed with a threshold device using a load of 30% of the maximal inspiratory pressure The control group received care as usual |
|
| Outcomes |
Physical fitness: Muscle endurance/strength: respiratory muscle strength (maximal inspiratory pressure and maximal expiratory pressure) assessed with a digital manometer Secondary outcomes: None of the secondary outcomes were assessed |
|
| Notes | Article was written in Portuguese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were randomly selected and randomly assigned to 1 of 2 groups, but the exact randomization methods were not reported |
| Allocation concealment (selection bias) | Unclear risk | The exact randomization methods were not reported. It was not clear whether the researchers used sealed envelopes, central allocation, or another method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study did not address the blinding of participants and personnel. However, due to the nature of the interventions, blinding was virtually impossible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study did not address blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting: the authors stated that sample losses occurred; however, they did not report the reasons for these sample losses, neither did they provide information on the used imputation methods |
| Selective reporting (reporting bias) | Low risk | Respiratory muscle strength was the primary outcome. By assessing and reporting on (changes over time of) both the maximal inspiratory pressure and maximal expiratory pressure there was no selective reporting of the study data |
| Other bias | High risk | Differential diagnostic activity: the intervention group and the control group received an unequal number of measurements However, this study was free of baseline imbalance; the baseline differences between the control group and intervention group on outcome‐related items were not significant |
Hartman 2009.
| Methods | Design: single‐centre RCT Setting: the Netherlands Department: paediatric oncology/haematology, paediatric physiotherapy, paediatric endocrinology Randomization: blinded for investigators and treating physicians Stratification: not mentioned Study duration: 3 years. Duration of intervention: 24 months. Follow‐up duration: 12 months Timing: inclusion started directly after diagnosis, at the beginning of chemotherapy treatment End point measurements: at diagnosis, 32 weeks after diagnosis, 1 year after diagnosis, at the end of treatment (and 2 years after diagnosis), 1 year after the end of treatment. There was 1 additional measurement 6 weeks after diagnosis |
|
| Participants | n = 51 Diagnosis: ALL (ALL non‐high risk n = 34, ALL high risk n = 17) Age at start of study: median age: 5.4 years (range 1.3 to 17.1) Sex: 30 boys, 21 girls Exclusion criteria: children with low cognitive impairment and children who could not understand the Dutch language |
|
| Interventions | The intervention consisted of a 2‐year hospital‐based programme performed by paediatric physiotherapists. During these sessions, the physiotherapist measured the motor function to ensure an optimal level of motor functioning. In addition, there was a home‐based exercise programme. Parents were supplied with an exercise list, enabling them to select exercises most appropriate for their child's age and to vary exercises. The exercise programme included exercises to maintain ankle dorsiflexion mobility and short‐burst high‐intensity exercises, to prevent reduction of BMD. In addition, there were exercises to maintain hand and leg function, which were performed once a day; stretching and jumping exercises twice daily. The duration of an exercise session was not mentioned When necessary, the exercise programme was adjusted during these sessions The control group received care as usual |
|
| Outcomes |
Physical fitness: Body composition: BMI, lean body mass, and % body fat. The lean body mass and % body fat were measured by DXA (lumbar spine and total body) Flexibility: passive ankle dorsiflexion; the range of motion past the neutral position received a positive notation and less than neutral a negative notation. Motor performance of children < 3.5 years of age was assessed using the Dutch BSID‐II; ≥ 4 years old using the Dutch version of the Movement‐ABC Secondary outcomes: None of the secondary outcomes were assessed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "At diagnosis randomization into the intervention or the control group was carried out in randomly permuted blocks of randomly chosen size, using sealed envelopes prepared by the statistician" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk'. The use of assignment envelopes was described, but it remained unclear whether envelopes were sequentially numbered, or opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and parents were not blinded for randomization; this was unclear for physiotherapists The investigators and treating physicians were blinded for the study randomization |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors who performed the study outcome tests were blinded for study randomization |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The study authors used a simple imputation method: for children who did not complete the study, data prior to elimination were included. No further information was provided on the imputation of some value for missing data |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcome measures were listed in the methods section and reported in the results section |
| Other bias | Low risk | There was no baseline imbalance as the baseline differences between both groups were not significant. In addition, the number of measurements did not differ for the intervention group or control group |
Marchese 2004.
| Methods | Type of study: single‐centre RCT Setting: USA Department: paediatric rehabilitation, paediatric oncology, paediatric physiotherapy Randomization: primary investigator offered the children an envelope to select assignment into the intervention or control group Stratification: children were stratified according to their childhood cancer risk group and first versus second part of the maintenance therapy Study duration: 4 months Timing: inclusion of the study started during maintenance therapy End point measurements: at baseline and 4 months later |
|
| Participants | n = 28 Diagnosis: ALL Age at start of study: median age of the whole group was 7.7 years (range 4.3‐15.8 years). The median age of the intervention group was 7.6 years (range 4.3‐10.6 years) and of the control group 8.6 years (range 5.1‐15.8 years) Sex: 20 boys and 8 girls Exclusion criteria: a history of antecedent neurological, developmental, or genetic disorders and children receiving a physiotherapy intervention at the start of the study |
|
| Interventions | The intervention programme included 5 hospital‐based physiotherapy sessions (weeks 0, 2, 4, 8, and 12) of 20‐60 minutes. The first session was performed immediately after the baseline testing Next to the hospital‐based programme, the programme also included an individualized home exercise programme. This programme consisted of ankle dorsiflexion stretching exercises (30 seconds, 5 days a week), bilateral lower extremity strengthening exercises (3 sets of 10 repetitions, 3 days a week), and aerobic exercise (daily). The aerobic exercise could be walking, cycling, or swimming; chosen by the participant The control group received care as usual |
|
| Outcomes |
Physical fitness: Cardiorespiratory fitness or peak work rate: 9‐minute run‐walk test and the timed up‐and‐down stairs test Muscle endurance/strength: knee extension strength and ankle dorsiflexion strength both tested with a hand‐held dynamometer. This study also used the time up‐and‐down stairs test and the 9‐minute run‐walk test Flexibility: ankle dorsiflexion range of motion Secondary outcomes: HRQoL: PedsQL version 3.0 Adverse events: any negative effect from the exercises or experienced complications attributed to the physical programme |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The children were stratified by risk group and by whether they were in the first or second half of the maintenance therapy. After that, the primary investigator offered the children an envelope to select assignment into the intervention or control group |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk'. The use of assignment envelopes is described, but it remains unclear whether envelopes were sealed, sequentially numbered, or maybe opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and parents were not blinded for randomization; for personnel, this was unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessors for hand‐held dynamometry, the timed up‐and‐down stairs test, and the 9‐minute run‐walk test were blinded for study randomization. Therefore, these items had a low risk for detection bias The PedsQL questionnaires were filled in by both parents and children. Parents and children were not blinded for the study randomization and, therefore, the quality of life assessment was found to be of high risk for detection bias We judged the overall risk of detection bias for this item to be low because the researchers blinded outcome assessors as much as possible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors reported missing data for daily logs of activity and heart monitor. But no information was reported on methods used for data imputation in case of missing data |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified primary and secondary outcomes of the study were listed in the methods section and reported in the results section |
| Other bias | Unclear risk | The non‐significant baseline differences were reported for patient characteristics but not for study outcome measures. It remains unclear whether the mean differences between the control group and the intervention group at baseline were significant or not Furthermore, we checked for differential diagnostic activity. During the study, all children were pre‐tested and post‐tested. The number of measurements did not differ for the intervention group or control group |
Moyer‐Mileur 2009.
| Methods | Type of study: single‐centre RCT Setting: USA Department: paediatric oncology Randomization: not mentioned Stratification: not mentioned Study duration: 12 months Timing: the inclusion of the study started during the ALL maintenance chemotherapy End point measurements: measures of physical size were obtained at baseline and every 3 months, physical activity was measured at baseline and at 6 and 12 months |
|
| Participants | n = 14 Diagnosis: standard‐risk ALL Age at start of study: mean (± SD) age of the intervention group was 7.2 ± 0.7 years and the mean age of the control group was 5.9 ± 0.7 years Sex: 7 boys and 6 girls; 1 unknown (drop‐out) Exclusion criteria: not mentioned |
|
| Interventions | The intervention included a 12‐month home‐based exercise and nutrition programme Children were prescribed to perform a minimum of 3 'fifteen to twenty‐minute' sessions of moderate‐to‐vigorous activity per week. Activity examples were provided on the pyramid for youth and parents were asked to record the type and amount of physical activity, immediately after the activity was performed Children received nutrition education materials on the basis of the United States Department of Agriculture Food Guide Pyramid and nutrition‐related activities monthly The control group received care as usual |
|
| Outcomes |
Physical fitness: Cardiorespiratory fitness or peak work rate: progressive aerobic cardiovascular endurance run Muscle endurance/strength: push‐ups, the sit‐and‐reach distance test Body composition: BMI, muscle mass (measured by the analysis of the tibia using peripheral quantitative computed tomography Flexibility: sit‐and‐reach distance test Level of daily activity: pedometer combined with an activity diary (monthly, 2 week days, and 1 weekend day) and the ACTIVITY GRAM questionnaire Secondary outcomes: None of the secondary outcomes were assessed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomization was not provided in the article |
| Allocation concealment (selection bias) | Unclear risk | The method of randomization was not provided in the article |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study did not address this item. However, due to the nature of the interventions blinding was virtually impossible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study did not address this item |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although authors reported that 1 child withdrew after 3 months (caused by lack of interest and data of this child were not taken into analysis), the information provided was insufficient to decide whether this withdrawal could have had influence on the study outcomes |
| Selective reporting (reporting bias) | Low risk | The article presented both the mean (plus confidence interval or SD) of all outcome variables and figures including the individual changes of the participants |
| Other bias | Low risk | There was no baseline imbalance, the baseline differences between both groups were not significant Furthermore, we checked for differential diagnostic activity. During the study, all children were pre‐tested and post‐tested. The number of measurements did not differ for the intervention group or control group |
Tanir 2013.
| Methods | Type of study: RCT in 2 university hospitals Setting: Turkey Department: paediatric oncology Randomization: not mentioned Stratification: not mentioned Study duration: 3 months Timing: being in remission (having received a diagnosis of ALL at least 1 year before the study) End point measurements: at baseline and 3 months after baseline |
|
| Participants | n = 40 Diagnosis: ALL Age at start of study: mean (± SD) age of the intervention group was 10.21 ± 1.51 years and the mean age of the control group was 10.72 ± 1.52 years Sex: 24 boys and 16 girls Inclusion criteria:
|
|
| Interventions | The children in the intervention group were offered their first session of training at a designated room in the hospital. One of each child's parents was admitted into the session to serve as a supporting and motivating force. In the session, the exercises that the children would be doing in the next 3 months were demonstrated. The workout comprised active range of motion, leg muscle strengthening and aerobic exercises
The control group received care as usual (see 'Notes' for additional information) |
|
| Outcomes |
Physical fitness: Cardiorespiratory fitness or peak work rate: 9‐minute run‐walk test, timed up‐and‐down stairs test Muscle endurance/strength: leg and back strength tested with a dynamometer. Timed Up and Go Test. This study also used the time up‐and‐down stairs test and the 9‐minute run‐walk test Flexibility: range of motion; joint unclear Secondary outcomes: HRQoL: PedsQL 3.0 Cancer Module Children's Form |
|
| Notes | In the method section of the article authors reported, "The children and parents in the control group were given exercise pamphlets after the monitoring and then provided with 30–60 minute training sessions." Nonetheless, they also reported, "No exercise was recommended to the patients in the control group over the course of the study" We repeatedly contacted the authors for additional information, but we did not receive a response to our requests Without additional information and based on the additional information in the paper, we concluded that the authors made a writing‐mistake in the methods section of their paper. Therefore, we interpreted the results as if the control group received usual care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The two groups were formed by randomized selection." The method used during the randomization was unclear |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The two groups were formed by randomized selection." The method used during the randomization was unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Outcome group: Quote: "Participants were asked to prevent for interaction and not speak about the intervention with children/parents of the control group" However, "it was observed that the children and their families who were subjects of the study did interact, forming loyalties and relationships in a social environment. For this reason, when it is considered that the children and families who received the exercise training had the opportunity to share their knowledge and practice with the control group, we believed that our findings might be the result of this interaction process" Personnel: No information about blinding personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study did not address this item |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Information on all outcomes was provided, yet the authors provided no information about missing data. The authors reported that 1 child died in the intervention group. However, data on that particular child were not provided |
| Selective reporting (reporting bias) | High risk | Data of the PedsQL 4.0 Generic Module was only reported as differences between boys and girls and not as differences between the intervention and control group |
| Other bias | High risk | Baseline differences in sex, treatment anxiety, and on the Goniometer Furthermore, we checked for differential diagnostic activity. The number of measurements did not differ for the intervention group or control group indicating that this study is free of differential diagnostic activity |
Yeh 2011.
| Methods | Type of study: single‐centre CCT feasibility study (quasi‐experimental) Setting: Taiwan Department: paediatric oncology Randomization: not performed Stratification: the intervention group and controls were matched by age and sex Timing: the inclusion of the study started during the ALL maintenance chemotherapy (1 week after completion of the dexamethasone treatment) Study duration: 10 weeks End point measurements: at baseline, once weekly during the 5‐week intervention, at the end of the intervention, and 1 month after the intervention |
|
| Participants | n = 24 Diagnosis: ALL Age at start study: mean (± SD) age intervention group 11.0 ± 3.56 years, mean age of the control group 12.5 ± 3.86 years Sex: 12 boys and 10 girls; 2 unknown (drop‐outs) Exclusion criteria: children who were unwilling to perform an aerobic exercise, or those with physical and developmental impairment |
|
| Interventions | The intervention consisted of a home‐based aerobic exercise instructed by video. 1 session included a warm‐up of 5 minutes, aerobic exercise of 25 minutes and a cooling down period of 5 minutes. The exercises were performed at least 3 times a week, over 6 weeks. In addition, children recorded their physical activity and heart rate data during the exercises in a physical activity log for 3 days with 24 x 1‐hour blocks The aerobic exercise sessions aimed to increase 40‐60% of the child's heart rate reserve The control group received care as usual |
|
| Outcomes |
Physical fitness: None of the physical fitness outcomes were assessed Secondary outcomes: Fatigue: PedsQL Multidimensional Fatigue Scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The researcher team used a quasi‐experimental design that had no random assignment |
| Allocation concealment (selection bias) | High risk | The researcher team used a quasi‐experimental design that had no random assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study did not address this item. However, due to the nature of the interventions, blinding was virtually impossible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study did not address this item |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 types of analyses were conducted: ITT analysis used the data of all children, and the per‐protocol analysis, which included only those children who adhered to the exercise prescription |
| Selective reporting (reporting bias) | High risk | Not all the pre‐specified primary outcomes were reported. In addition, adherence was mentioned to be extra or a secondary outcome. However, in the results the authors focused in this item |
| Other bias | Unclear risk | The non‐significant baseline differences were reported for fatigue study outcomes. However, it remains unclear whether the intervention and control group had different baseline scores on the other study outcomes: physical activity log, OMNI walk/run scale, and the stages of change Furthermore, we checked for differential diagnostic activity. The number of measurements did not differ for the intervention group or control group. Therefore, this study was free from differential diagnostic activity |
ALL: acute lymphoblastic leukaemia; BMD: bone mineral density; BMI: body mass index; BSID‐II: Bayley Scales of Infant development; CCT: controlled clinical trial; DXA: dual energy x‐ray absorptiometry; HRQoL: health‐related quality of life; ITT: intention to treat; Movement‐ABC: Movement Assessment Battery for Children; n: number of participants; OMNI walk/run scale: Omnibus ‐ walk/run scale; PedsQL: Pediatric Quality of Life Inventory; RCT: randomized controlled trial; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chamorro‐Vina 2010 | The exercise intervention was offered < 4 weeks |
| Chung 2014 | The exercise intervention was offered < 4 weeks |
| Emanuelson 2014 | The primary outcome of this study did not correspond with 1 of the primary or secondary outcomes of this review |
| Geyer 2011 | No exercise intervention |
| Gohar 2011 | Uncontrolled study |
| Herbinet 2014 | The exercise intervention was offered < 4 weeks |
| Hinds 2007 | The exercise intervention was offered < 4 weeks |
| Huang 2014 | No exercise intervention |
| Jarden 2013 | Study assessed in adults with cancer and the study was an uncontrolled study |
| Kurt 2011 | No exercise intervention |
| Oldervoll 2011 | Study assessed in adults with cancer |
| Rief 2011 | Study assessed in adults with cancer |
| Rosenhagen 2011 | Case‐control study |
| Ruiz 2010 | Uncontrolled study |
| Shore 1999 | Used health normal volunteer children as a control population |
| Speyer 2010 | The exercise intervention was offered < 4 weeks and cross‐over randomized trial without data presentation after the first intervention period (before cross‐over) |
| Speyer 2011 | The exercise intervention was offered < 4 weeks |
| Steel 2011 | Study assessed in adults with cancer |
| te Winkel 2008 | This study presents pilot data of a study that was reported by Hartman et al. (2009). Hartman et al. was already included in the review (Hartman 2009) |
| William Li 2013 | The exercise intervention was offered < 4 weeks |
| Winter 2013 | The exercise intervention was offered < 4 weeks |
Characteristics of studies awaiting assessment [ordered by study ID]
Braam 2014.
| Methods | Type of study: multicentre RCT Setting: the Netherlands Department: paediatric oncology Randomization: block‐randomization, performed by a blinded independent worker of the paediatric oncology department Stratification: the participants were stratified by cancer (haematological vs. solid cancer), gender and age (boys < 12 vs. ≥ 12 years and girls < 11 years vs. ≥ 11 years), and during or after cancer treatment Timing: children were during or within the first year following childhood cancer therapy. Children who were during treatment were treated and followed up in the outpatient clinic, without overnight hospital staying Study duration: short‐term effects are assessed 4 months from baseline End point measurements: at baseline and after 4 months |
| Participants | n = 68 Diagnosis: childhood cancer (treated with chemotherapy, radiotherapy, or both) Age at start study: 8‐18 years Sex: 37 boys and 31 girls Exclusion criteria: receiving a bone marrow transplant as a part of the childhood cancer treatment, receiving growth hormones as a part of the childhood cancer treatment, permanent wheelchair use/inability to ride a bike, retardation/inability to make a self reflexion and follow sports instructions |
| Interventions | The 12‐week intervention consisted of a combined physical exercise (twice per week) and psychosocial support programme (once every 2 weeks) The physical exercise programme included a protocol with both cardiorespiratory and muscle strength training. The sessions are guided by a paediatric physiotherapist and performed at a local (paediatric) physiotherapist institute The psychosocial support programme (6 child and 2 parent sessions) contained psycho‐education and cognitive‐behavioural therapy (guided by a paediatric psychologist and performed at the academic treatment hospital) The control group received usual care |
| Outcomes |
Primary outcome: Cardiorespiratory fitness: peak oxygen uptake (mL/kg/minute) Secondary outcome: HRQoL: generic core score, cancer module, and multidimensional fatigue module of the PedsQL |
| Notes | This study was published as a conference paper. The total study outcomes were not yet published as a full‐text article Not all provided information was presented in the conference paper. We were able to complete the provided information by own source |
Elkateb 2007.
| Methods | Type of study: single‐centre CCT Setting: Egypt Department: paediatric oncology Randomization: not performed Stratification: not included Timing: children were during chemotherapy treatment for cancer Study duration: not mentioned End point measurements: at baseline, daily in the first week, after the first week, in the third week, and in the sixth week |
| Participants | n = 50 Diagnosis: childhood cancer Age at start study: preschool‐ and school‐aged children Sex: not mentioned Exclusion criteria: not mentioned |
| Interventions | Undefined exercise programme for the intervention group Undefined programme for the control group |
| Outcomes |
Primary outcome: Level of daily activity: observational checklist for recording activities Secondary outcomes: Fatigue: observational checklist for sleeping conditions |
| Notes | This study was published as a conference paper. Based on the currently available information it was not possible to decide if this study was eligible for inclusion in this review |
Sabel 2013.
| Methods | Type of study: RCT including a cross‐over procedure after 10 weeks Setting: Sweden Department: paediatric oncology Randomization: no information in the abstract provided Stratification: no information in the abstract provided Timing: children were 1‐5 years post diagnosis Study duration: no information provided End point measurements: baseline and after 10 weeks |
| Participants | n = 13 Diagnosis: childhood brain tumour survivors (treated with at least radiotherapy); 1‐5 years post diagnosis Age at start study: 7‐17 years Sex: not mentioned Exclusion criteria: not mentioned |
| Interventions | Intervention: an exercise gaming intervention. The 10‐week home‐based motion‐controlled video console (Nintendo Wii) exercise intervention was performed 30 minutes per day, at least 5 days a week. Children had weekly contact with a coach by video‐conferencing Control intervention: waiting list group |
| Outcomes |
Primary outcomes: Activity energy expenditure: energy expenditure and Metabolic Equivalent of Task (METs) assessed by a multisensory activity monitor (SenseWearPro2 Armband) |
| Notes | This study was published as a conference paper. The total study outcomes were not yet published as a full‐text article |
Senn‐Malashonak 2014.
| Methods | Type of study: RCT Setting: Germany Department: stem cell transplantation and immunology Randomization: no information provided in the abstract Stratification: no information provided in the abstract Timing: children were during haematopoietic stem cell transplantation Study duration: 200 days End point measurements: at hospital admission, at discharge, and at 200 days post transplantation |
| Participants | n = 50 Diagnosis: children with cancer who received a haematopoietic stem cell transplantation (SCT) Age at start study: not mentioned Sex: not mentioned Exclusion criteria: not mentioned |
| Interventions | Intervention group: exercise intervention including a standardized resistance, endurance, and flexibility training Control group: performed a mental training and relaxation exercises During inpatient treatment the daily sessions last about 40‐60 minutes for each group |
| Outcomes |
Primary outcome: Cardiorespiratory fitness: peak oxygen uptake (mL/kg/minute) and 6‐minute‐walking‐test) Muscle strength: isometric muscle strength Body composition Secondary outcomes: HRQoL: questionnaire |
| Notes | This study was published as a conference paper. The recruitment continued until December 2014 |
CCT: controlled clinical trial; n: number of participants; RCT: randomized controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Chamorro‐Vina 2012.
| Trial name or title | Exercise in Pediatric Autologous Stem Cell Transplant Patients: a Randomized Controlled Trial |
| Methods | Type of study: multicentre RCT Setting: Alberta Children's Hospital, Canada Department: paediatric oncology and blood and marrow transplantation Randomization: participant allocation is generated by the software Research Randomizer, a research tool provided by the Social Psychology Network (www.socialpsychology.org). Sealed, non‐transparent envelopes are used for the actual randomization, which contains a number that allocate the participant to the control or intervention group. The research co‐ordinator is responsible for the random allocation Stratification: no information provided Timing: children at least 4 weeks before the hospitalization to start autologous stem cell transplantation when the study information is provided Study duration: 180 days End point measurements: at baseline, 30 days, 85 days, and 180 days for general study outcomes. Blood levels at +7, +15, and +56 days post re‐infusion Trial register: NCT01666015 |
| Participants | n = 24 Diagnosis: all cancer types (common types will be: sarcoma, lymphoma, neuroblastoma, germ cell tumour) Inclusion criteria: all children with cancer who undergo autologous stem cell transplantation in Alberta Children's Hospital; who receive myeloablative conditioning; participation approval by treating oncologist; children need to verbally express assent to participate Age at start of study: 5‐18 years Exclusion criteria: evidence for cardiac or pulmonary failure associated with treatment (shortening fraction > 27%; ejection fraction > 49%); functional or cognitive limitations that would prohibit performance of the home‐based training |
| Interventions |
Intervention group: an inpatient and outpatient mixed exercise programme including both resistance and aerobic training Phase 1. Inpatient phase (in Alberta Children's Hospital during conditioning and the isolated phase of the transplantation). Supervised aerobic training (20‐30 minutes) and resistance training of 12‐15 repetitions per exercise training, performed 5 times a week Phase 2. the outpatient phase will take place after discharge. The participants will participate in a 10‐week mixed supervised and home‐based exercise programme utilizing the Nintendo® Wii device. Phase 2 includes both supervised exercise training sessions at the University of Calgary (1/week) and home‐based training (2/week) including 20‐30 minutes of aerobic exercises and 30 minutes of strength and stretching exercises, using the Wii (fit, sports, and dance games) Children will train with an aerobic exercise intensity between 50% and 70% of the heart‐rate reserve No intervention will take place when: platelet counts < 10,000/μL, haemoglobin levels < 8 g/dL, fever > 38 °C, pain, diarrhoea, haemorrhage, or other complications Control group: usual care, waiting list group. During the course of the study, these children will not participate in any scheduled exercise programme and will perform the same battery of tests as the intervention group. After the end of study (phase 2), they will be offered an exercise programme to be held in a separate the University of Calgary |
| Outcomes |
Primary outcomes: Cardiorespiratory fitness: submaximal aerobic test on a treadmill Muscle endurance: partial curl‐up, modified push‐up test and the sit‐to‐stand test Muscle strength: hand‐held dynamometer assessing knee extensions and grip strength Body composition: BMI, fat mass estimation by an equation, and skin‐fold measurement Flexibility: sit‐and‐reach distance test Activity energy expenditure and level of daily activity: accelerometer and exercise diary Secondary outcomes: HRQoL: PedsQL Generic Fatigue: PedsQL Multidimensional Fatigue Scale |
| Starting date | June 2012 |
| Contact information | Carolina Chamorro‐Vina, telephone: 403‐210‐8482; Email: cchamorro@kin.ucalgary.ca |
| Notes | Estimates study completion July 2015 |
Cox 2011.
| Trial name or title | Physical Activity to Modify Sequelae and Quality of Life in Childhood Acute Lymphoblastic Leukaemia (PAQOL) |
| Methods | Type of study: single‐centre RCT Setting: USA Department: paediatric oncology Randomization: not described Stratification: not included Timing: children were in the second to eighth day of the ALL treatment protocol Study duration: 135 weeks End point measurements: at baseline (BMD, HRQoL), after 8 weeks (HRQoL), after 15 weeks (HRQoL), and at completion of therapy (BMD and HRQoL) clinicaltrials.gov/ct2/show/NCT00902213 |
| Participants | n = 208 Diagnosis: newly diagnosed with ALL (immunophenotypic diagnosis of non‐B cell ALL) Age at start of study: 4‐18 years Exclusion criteria: age < 4 years or ≥ 19 years at diagnosis; no parents or legal guardian (≥ 18 years) of the study participant who speaks and understands the English language; diagnosis of cerebral palsy or Down's syndrome; children with a second malignancy, chromosome breakage syndrome, or severe congenital immunodeficiency; inability to obtain written informed consent from parent/young adult and child assent; or pregnant |
| Interventions | Tailored parent‐ and child‐focused physical activity programme An advanced practice nurse will meet twice weekly with the child and family for the first 4 weeks of the intervention to initiate the motivation‐based dialogue and therapeutic interaction; this will be followed by once weekly visits during weeks 5‐8 of the intervention; and monthly visits during weeks 9 through to end of therapy The physiotherapist will meet at least once weekly with the child and family during weeks 1‐4 to initiate the prescriptive tailored exercise programme; subsequent visits to reinforce and modify the programme will occur at least once every other week during weeks 5‐8, and at least once monthly during weeks 9‐135 of the intervention. The physiotherapist will visit at least once weekly during weeks 1‐4, at least once every other week during weeks 5‐8, and at least once monthly during weeks 9‐135. During weeks 9‐135 of the intervention, the advanced practice nurse will call between the monthly in‐person visits to assure fidelity to the intervention and to provide booster support to the intervention where needed |
| Outcomes |
Primary outcomes: Muscle endurance/strength: muscle strength, range of motion, endurance, gross motor skills, used method is not specified Body composition: BMD and bone mineral content Flexibility: range of motion Secondary outcomes: HRQoL: method used not mentioned in the protocol Adverse events |
| Starting date | November 2009 |
| Contact information | Cheyl Cox, info@stjude.org |
| Notes |
Dubnov‐Raz 2010.
| Trial name or title | Physical Activity and Health of Children With Cancer in Remission |
| Methods | Type of study: CCT with parallel assignment Setting: Israel; Sheba medical Center Department: no information provided Randomization: not performed Stratification: not performed Timing: < 3 months from hospitalization Study duration: 6 months End point measurements: at 6 months; there is no information about the performance of a possible baseline measurement Trial register: NCT00839904 |
| Participants | n = 22 Diagnosis: childhood cancer Age at start of study: 6‐16 years Inclusion criteria: in remission from cancer; > 6 months after completion of all therapy Exclusion criteria: no written informed consent; refusal of tests: blood, fitness, questionnaires, or DXA; locomotive handicaps; extreme fatigue, nausea, dyspnoea; concurrent acute illness; recent (< 3 months) hospitalization; documented (echocardiographic or nuclear medicine) decrease in cardiac function; abnormal blood tests: haemoglobin < 10 g/dL, neutropenia < 500/mm3, thrombocytopenia < 50,000/ mm3; additional chronic health conditions unrelated to cancer (e.g. coeliac disease, cerebral palsy, Down's syndrome) |
| Interventions | Intervention group: 2 supervised, 60‐minute weekly exercise sessions and instructions to perform additional physical activities throughout the day Control group: usual care; no intervention |
| Outcomes |
Primary outcomes: Cardiorespiratory fitness Body composition and BMD Secondary outcomes: HRQoL Anxiety and depression: mood |
| Starting date | February 2010 |
| Contact information | No contacts or location information provided |
| Notes | Data collection finished in August 2012 |
Kauhanen 2014.
| Trial name or title | Active Video Games to Promote Physical Activity, Motor Performance and Quality of Life in Children with Cancer: an Intervention Study with 2‐year Follow‐up |
| Methods | Type of study: RCT Setting: Turku University Hospital, Finland Department: paediatric oncology Randomization: is performed by a computer‐generated list ‐ including block randomization with randomly selected block sizes of 2 to 4 Stratification: no information provided Timing: children are asked to participate within 1 week after their cancer diagnosis, or as soon as possible after that Study duration: 30 months End point measurements: baseline; the first week of the intervention, 2 months, 6 months, 1 year and 2.5 years from baseline Trial register: NCT01645436 |
| Participants | n = 40 Diagnosis: ALL, or other diagnoses outside the central nervous system (e.g. M Hodgkin, non‐Hodgkin lymphomas, neuroblastoma, Wilms' tumour, rhabdomyosarcoma, retinoblastoma and Ewing sarcoma) Age at start of study: 3‐16 years Inclusion criteria: chemotherapy treatment with vincristine given for childhood cancer in Turku University Hospital or Tampere University Hospital Exclusion criteria: other diseases limiting their in physical and cognitive function; epilepsy, or not able to communicate (in Finnish, Swedish, or English) |
| Interventions |
Intervention group: the exercise intervention is based on active video gaming on the Nintendo Wii on a light‐to‐moderate activity level. The intervention includes information and recommendation for physical activity games suitable for performing during the intervention period. Exercises are daily for at least 30 minutes per day. The 8‐week intervention is provided during hospitalization and at home, with considerations of the participants' individual conditions The physiotherapist contacts the participants in the intervention group via telephone for consultation during the intervention aiming to increase participation Physical activity is not allowed during fever, vomiting, or nausea episodes, or if the medical conditions change. A cardiologist performs regular echocardiograms after anthracycline therapy Control group: receives general advice for physical activity for 30 minutes per day and no guidance on playing active video games. In relation to usual care, when needed a physiotherapist is consulted |
| Outcomes |
Primary outcomes: Body composition: BMI, waist circumference Activity energy expenditure: 3‐dimensional accelerometer Level of daily activity: questionnaire to assess leisure time physical activity in metabolic equivalents (METs), activity diary, an open question interview about physical activity Secondary outcomes: Fatigue: PedsQL Multidimensional Fatigue Scale Adverse events |
| Starting date | January 2013 |
| Contact information | Mikko Alola, Email: anloka@utu.fi |
| Notes | Estimated completion of the primary outcome data December 2016 |
Mabbott 2013.
| Trial name or title | The Neuro‐protective Effects of Exercise in Children with Brain Tumors |
| Methods | Type of study: RCT Setting: Hospital for Sick Children, Toronto Canada Department: paediatrics Randomization: no specific information provided Stratification: no information provided Timing: children finished the cancer treatment Study duration: 42‐45 weeks End point measurements: Baseline, week 26‐29, and week 42‐45 Trial register: NCT01645436 |
| Participants | n = 30 Diagnosis: childhood brain tumour survivors Age at start study: 9‐14 years Inclusion criteria: native English speaker or at least 2 years of schooling in English at time of the inclusion; diagnosed with a hemispheric or posterior fossa tumour and treated with cranial spinal or focal radiation Exclusion criteria: > 7 years post diagnosis; have a prior history of traumatic brain injury; neurologic disorder, visual or sensory impairment, cerebral palsy, developmental delay or learning disability; requiring sedation for MRI imaging; severe neurological/ motor dysfunction that would preclude safe participation in an exercise programme |
| Interventions |
Intervention group: receive a 12‐week exercise intervention, with 90‐minute sessions provided 3 times a week. Each session includes 30 minutes of circuit training, 30 minutes of organized sports (soccer, floor hockey, basketball, etc.) and 30 minutes of socializing including a healthy snack Control group: receives a delayed intervention. They receive the same 12‐week intervention after the intervention group completed the intervention period |
| Outcomes |
Primary outcomes: Cardiorespiratory fitness: physical fitness: VO2max |
| Starting date | July 2013 |
| Contact information | Donald Mabbott, telephone: 416‐813‐8875; Email: donald.mabbott@sickkids.ca |
| Notes |
Soares‐Miranda 2013.
| Trial name or title | Physical Activity in Pediatric Cancer Patients with Solid Tumors (PAPEC): Trial Rationale and Design |
| Methods | Type of study: RCT Setting: Children's Hospital of Madrid, Hospital Infantil Universitario Nino Jesus, Spain Department: no information provided Randomization: no specific information provided Stratification: on sex Timing: during neoadjuvant chemotherapy Study duration: 2 months End point measurements: baseline, middle measurement during the intervention period, end of the intervention, 2 months after the intervention Trial register: NCT01645436 |
| Participants | n = 60 Diagnosis: children with extra‐cranial primary solid tumours during a course of neoadjuvant chemotherapy Age at start of study: 4‐18 years Inclusion criteria: new diagnosis of an extra‐cranial solid tumour; having a good performance status; receiving treatment in Hospital Infantil Universitario Nino Jesus of Madrid, living in the Madrid province Exclusion criteria: previously receiving cancer therapy |
| Interventions | Intervention group: receives a 60‐ to 70‐minute supervised in‐hospital combined exercise‐training programme (aerobic and strength) 3/week. The intervention includes both 30 minutes of aerobic and 30 minutes of strength training. Muscle strength training is performed with dumbbells and paediatric weight machines; performing 2‐3 sets of 8‐15 repetitions The intervention is offered between the whole period of neoadjuvant chemotherapy treatment. Related to the tumour type, the period can range from 4 to 24 weeks. The intervention is tailored to the patient according to training guidelines or performed in the hospital room when needed, or both Control group: receives usual hospital care, no scheduled training but physical therapy when needed and recommendations for a healthy lifestyle. |
| Outcomes |
Primary outcomes: Cardiorespiratory fitness: cardio‐respiratory capacity: VO2peak Muscle endurance/ strength: 6 repetition maximum of leg and chest (bench) presses and lateral rowing, timed up‐and‐down stairs test, 3‐m and 10‐m timed up‐and‐go test Activity energy expenditure: uni‐axial accelerometer Secondary outcomes: HRQoL: the Child report form of the Child's Health and illness Profile‐Child Edition (CHIP‐CE/CRF), adolescent edition (CHP‐PE/AE), and parents edition (CHP‐CE/PRF) |
| Starting date | September 2012 |
| Contact information | L. Soares‐Miranda, telephone: +351962591421; Email: soaresmiranda@fade.up.pt |
| Notes | Estimated end date September 2015 |
Wiskemann 2012.
| Trial name or title | Physical Activity Intervention Program for Childhood Cancer Patients Under Chemo‐ and/or Radiation Therapy |
| Methods | Type of study: CCT Setting: Hospital of the University Hospital of Heidelberg, Germany Department: no information provided Randomization: no randomization Stratification: no stratification Timing: < 8 weeks after childhood cancer diagnosis Study duration: 1 year End point measurements: at baseline, 3, 6, 9, and 12 months, questionnaires at 6 and 12 months Trial register: NCT02216604 |
| Participants | n = 60 Diagnosis: primary paediatric cancer diagnosis (leukaemia, brain and bone tumours) Age at start of study: 5‐21 years Inclusion criteria: date of diagnosis no longer than 8 weeks before start of the study Exclusion criteria: severe cardiac impairment; bone metastasis inducing skeletal fragility; other orthopaedic diseases or any other circumstances that would impede ability to give informed consent or adherence to study requirements |
| Interventions | Intervention group: multi‐modal exercise intervention The intervention includes a 3‐ to 5‐weekly guided training programme of 15‐ to 30‐minute sessions based on endurance, strength, and balance game training using the Wii (Nintendo) as well as on age‐specific resistance training and sessions of body awareness. During the outpatient phase of the treatment, there is a home‐based exercise training (3‐5 weekly) using a manual. The participants also obtain a movement diary and pedometer Control group: age‐, disease‐, and gender‐matched group receiving no intervention |
| Outcomes |
Primary outcome: Muscle endurance/strength: hand‐held dynamometer, timed up‐and‐down stairs test, one‐leg stand, posturomed and force plate Body composition Flexibility: goniometer Secondary outcome: HRQoL |
| Starting date | December 2012 |
| Contact information | Joachim Wiskemann, telephone: +49‐6221‐565904; Email: joachim@wiskemann‐online.de or andrea.kulozik@med.uni.heidelberg.de |
| Notes |
ALL: acute lymphoblastic leukaemia; BMD: bone mineral density; BMI: body mass index; DXA: dual‐energy x‐ray absorptiometry; HRQoL: health‐related quality of life; n: number of participants; RCT: randomized controlled trial; VO2 peak: maximal oxygen consumption; VO2 max: maximal oxygen uptake.
Differences between protocol and review
The review is an updated version of the review published in 2013. However, the differences between the review and the protocol remain on several aspects.
Instead of using the Cochrane Childhood Cancer Group module for the risk of bias, we used the latest update, which was described in the Cochrane Handbook for Systematic Reviews of Interventions of March 2011 to assess the risk of bias of the included studies (Higgins 2011).
The study of Hartman 2009 included children at diagnosis who were aged one to 18 years. In the protocol, we reported our intention to include studies with participants older than three years of age. We opted to change this because some of the studies introduced a tailored exercise programme that could be adjusted for the child's age. To see changes in outcomes, a child needs to be trainable, co‐operative, and testable. For intensive training, which we had in mind when writing the protocol, children aged under three years will not be able to complete the exercises. However, the study of Hartman 2009 did not assess the effect of a structured intensive training programme, but included physiotherapy sessions with exercises that were appropriate for all ages.
We added possible tests that could have been used to assess the primary outcome.
Finally, we added the ClinicalTrial.gov database as resource for the search of ongoing trials (www.clinicaltrials.gov). We also searched the ClinicalTrial.gov database for missed studies.
Contributions of authors
KB and PT where the principle authors of this Cochrane review and all other authors contributed to the writing of the review.
TT was the primary supervisor, the third‐party arbitrator in case of discrepancies or problems in finding consensus, and the expert on childhood physiology discussions.
MV and ED provided feedback on drafts of the manuscript and were in the general content of the review.
GJK provided feedback on drafts of the manuscript and was responsible for the medical and oncological background of the review protocol.
Sources of support
Internal sources
Pediatric Oncology Hematology, VU University Medical Center, Amsterdam, Netherlands.
Child Development & Exercise Center, Wilhelmina Children's Hospital/University Medical Center Utrecht, Netherlands.
-
Dutch Cochrane Center (DCC), Netherlands.
systematic review course
External sources
Alphe d'HuZes/Dutch Cancer Society, Netherlands.
Roparun, Netherlands.
-
VONK, Netherlands.
VUmc Onderzoek Naar Kinderkanker; a single‐centre research fund for paediatric oncology research programs
Declarations of interest
A few authors of the Braam 2014 congress proceeding are from the same group as of some authors of this review.
KB, PT, TT, MV, ED: None known. GJK: None of my other financial activities relate to marketing or producing interventions used for physical exercise training. Therefore, none of these activities may lead to a conflict of interest.
Joint first authorship with Katja I. Braam
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
de Macedo 2010 {published data only}
- Macedo TMF, Oliveira KMC, Melo JBDC, Medeiros MG, Medeiros Filho WC, Ferreira GMH, et al. Inspiratory muscle training in patients with acute leukemia: preliminary results. Revista Paulista de Pediatria 2010;28:352‐8. [Google Scholar]
Hartman 2009 {published and unpublished data}
- Hartman A, Winkel ML, Beek RD, Muinck Keizer‐Schrama SM, Kemper HC, Hop WC, et al. A randomized trial investigating an exercise program to prevent reduction of bone mineral density and impairment of motor performance during treatment for childhood acute lymphoblastic leukemia. Pediatric Blood and Cancer 2009;53(1):64‐71. [DOI] [PubMed] [Google Scholar]
Marchese 2004 {published data only}
- Marchese VG, Chiarello LA, Lange BJ. Effects of physical therapy intervention for children with acute lymphoblastic leukemia. Pediatric Blood and Cancer 2004;42(2):127‐33. [DOI] [PubMed] [Google Scholar]
Moyer‐Mileur 2009 {published data only}
- Moyer‐Mileur LJ, Ransdell L, Bruggers CS. Fitness of children with standard‐risk acute lymphoblastic leukemia during maintenance therapy: response to a home‐based exercise and nutrition program. Journal of Pediatric Hematology/Oncology 2009;31(4):259‐66. [DOI] [PubMed] [Google Scholar]
Tanir 2013 {published data only (unpublished sought but not used)}
- Tanir MK, Kuguoglu S. Impact of exercise on lower activity levels in children with acute lymphoblastic leukemia: a randomized controlled trial from Turkey. Rehabilitation Nursing 2013;38(1):48‐59. [DOI] [PubMed] [Google Scholar]
Yeh 2011 {published data only}
- Yeh CH, Man Wai JP, Lin US, Chiang YC. A pilot study to examine the feasibility and effects of a home‐based aerobic program on reducing fatigue in children with acute lymphoblastic leukemia. Cancer Nursing 2011;34:3‐12. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chamorro‐Vina 2010 {published data only}
- Chamorro‐Vina C, Ruiz JR, Santana‐Sosa E, Gonzalez Vicent M, Madero L, Perez M, et al. Exercise during hematopoietic stem cell transplant hospitalization in children. Medicine and Science in Sports and Exercise 2010;42(6):1045‐53. [DOI] [PubMed] [Google Scholar]
Chung 2014 {published data only}
- Chung OK. Effectiveness of an adventure‐based training program in promoting regular physical activity among childhood cancer survivors. Pediatric Blood and Cancer. 2014; Vol. 61, issue supplement 2:S162.
Emanuelson 2014 {published data only}
- Emanuelson I, Sabel M, Arvidsson D, Broeren J, Gillenstrand J, Saury JM, et al. Physical activity through home‐based exercise‐gaming after childhood brain tumour treatment. A method to improve motor and process function. Brain Injury. 2014; Vol. 28, issue 5‐6:598.
Geyer 2011 {published data only}
- Geyer R, Lyons A, Amazeen L, Alishio L, Cooks L. Feasibility study: the effect of therapeutic yoga on quality of life in children hospitalized with cancer. Pediatric Physical Therapy 2011;23:375‐9. [DOI] [PubMed] [Google Scholar]
Gohar 2011 {published data only}
- Gohar SF, Comito M, Price J, Marchese V. Feasibility and parent satisfaction of a physical therapy intervention program for children with acute lymphoblastic leukemia in the first 6 months of medical treatment. Pediatric Blood & Cancer 2011;56:799‐804. [DOI] [PubMed] [Google Scholar]
Herbinet 2014 {published data only}
- Herbinet A. Effects of an adapted physical activity program with a playful pedagogy in a service of paediatric oncology. Annals of Physical and Rehabilitation Medicine Conference, Marseille, France. 2014; Vol. 57, issue supplement 1:e371‐2.
Hinds 2007 {published data only}
- Hinds PS, Hockenberry M, Rai SN, Zhang L, Razzouk BI, Cremer L, et al. Clinical field testing of an enhanced‐activity intervention in hospitalized children with cancer. Journal of Pain and Symptom Management 2007;33(6):686‐97. [DOI] [PubMed] [Google Scholar]
Huang 2014 {published data only}
- Huang JS, Dillon L, Terrones L, Schubert L, Roberts W, Finklestein J, et al. Fit4Life: a weight loss intervention for children who have survived childhood leukemia. Pediatric Blood and Cancer 2014;61:894‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarden 2013 {published data only}
- Jarden M, Adansen L, Kjeldsen L, Birgens H, Tolver A, Christensen JF, et al. The emerging role of exercise and health counseling in patients with acute leukemia undergoing chemotherapy during outpatient management. Leukemia Research 2013;37:155‐61. [DOI] [PubMed] [Google Scholar]
Kurt 2011 {published data only}
- Kurt S, Savaser S. Short‐term effects of remission video game in adolescents with cancer ayse. Psycho‐oncology. 2011; Vol. 20, issue supplement 1:82‐3.
Oldervoll 2011 {published data only}
- Oldervoll LM, Loge JH, Lydersen S, Paltiel H, Asp MB, Nygaard UV, et al. Physical exercise for cancer patients with advanced disease: a randomized controlled trial. The Oncologist 2011;16:1649‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rief 2011 {published data only}
- Rief H, Jensen AD, Bruckner T, Herfarth K, Debus J. Isometric muscle training of the spine musculature in patients with spinal bony metastases under radiation therapy. BMC Cancer 2011;11:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenhagen 2011 {published data only}
- Rosenhagen A, Bernhorster M, Vogt L, Weiss B, Senn A, Arndt S, et al. Implementation of structured physical activity in the pediatric stem cell transplantation. Klinische Padiatrie 2011;223:147‐51. [DOI] [PubMed] [Google Scholar]
Ruiz 2010 {published data only}
- Ruiz JR, Fleck SJ, Vingren JL, Ramírez M, Madero L, Fragala MS, et al. Preliminary findings of a 4‐months intrahospital exercise training intervention on IGFs and IGFBPs in children with leukemia. Journal of Strength and Conditioning Research 2010;24(5):1292‐7. [DOI] [PubMed] [Google Scholar]
Shore 1999 {published data only}
- Shore S, Shepard RJ. Immune responses to exercise in children treated for cancer. Journal of Sports Medicine and Physical Fitness 1999;39:240‐3. [PubMed] [Google Scholar]
Speyer 2010 {published data only}
- Speyer E, Herbinet A, Vuillemin A, Briancon S, Chastagner P. Effect of adapted physical activity sessions in the hospital on health‐related quality of life for children with cancer: a cross‐over randomized trial. Pediatric Blood and Cancer 2010;55(6):1160‐6. [DOI] [PubMed] [Google Scholar]
Speyer 2011 {published data only}
- Speyer E, Herbinet A, Vuillemin A, Briacon S. Chastagner P. Adaped physical activity sessions and health‐related quality of life during a hospitalization course for children with cancer: APOP, a cross‐over randomized trial [Activité physique adaptée et qualité de vie liée à la santé lors d'un séjour hospitalier chez des enfants atteints d'un cancer: APOP, un essai randomisé en cross‐over]. Science & Sports 2011;26:202‐6. [Google Scholar]
Steel 2011 {published data only}
- Steel J, Geller DA, Tsung A, Marsh JW, Dew MA, Spring M, et al. Randomized controlled trial of a collaborative care intervention to manage cancer‐related symptoms: lessons learned. Clinical Trials 2011;8(3):298‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
te Winkel 2008 {published data only}
- Winkel M, Beek R, Muinck Keizer‐Schrama S, Hop W, Heuvel M, Pieters R. A randomised trial investigating an exercise program to prevent osteoporosis and motor problems during treatment for childhood acute lymphoblastic leukemia. Pediatric Blood and Cancer 2008;50(5 Suppl):121. [Google Scholar]
William Li 2013 {published data only}
- William Li HC, Chung OKJ, Ho KY, Chiu SY, Lopez V. Effectiveness of an integrated adventure‐based training and health education program in promoting physical activity among childhood cancer survivors. Psycho‐oncology 2013;22:2601‐10. [DOI] [PubMed] [Google Scholar]
Winter 2013 {published data only}
- Winter CC, Muller C, Hardes J, Gosheger G, Boos J, Rosenbaum D. The effect of individualized exercise interventions during treatment in pediatric patients with a malignant bone tumor. Supportive Care in Cancer 2013;21:1629‐36. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Braam 2014 {published and unpublished data}
- Braam KI, Dijk‐Lokkart EM, Takken T, Veening MA, Huisman J, Bierings MB, et al. Short term effects on physical fitness of a 12‐week exercise and psychosocial training program in childhood cancer patients. Pediatric Blood and Cancer. 2014; Vol. 61, issue supplement 2:S242‐3.
- Dijk‐Lokkart EM, Braam KI, Kaspers GJL, Veening MA, Grootenhuis MA, Streg I, et al. Effects on quality of life of participation in a combined physical exercise and psychosocial intervention program for childhood cancer patients. Pediatric Blood and Cancer 2014;61(S2):S160. [Google Scholar]
Elkateb 2007 {published data only}
- Elkateb N, Elsamman G. Effect of exercise on physiological and physical status of cancer children receiving chemotherapy. Pediatric Blood and Cancer 2007;49(4 Suppl):561. [Google Scholar]
Sabel 2013 {published data only}
- Sabel M, Broeren J, Arvidsson D, Sjolund A, Gillenstrand J, Ljungberg C, et al. Physical activity through home‐based exercise‐gaming after childhood brain tumour treatment ‐ a feasibility study [P‐0480]. Pediatric Blood and Cancer 2013;60(S3):165. [Google Scholar]
Senn‐Malashonak 2014 {published data only}
- Senn‐Malashonak A, Arndt S, Siegler K, Rosenhagen A, Klingebiel T, Banzer W, et al. Interim analysis of the randomized prospective exercise therapy study in the pediatric stem cell transplantation (BISON). Bone Marrow Transplantation. 2014:S369.
References to ongoing studies
Chamorro‐Vina 2012 {published data only}
- Chamorro‐Vina C, Guilcher GMT, Khan FM, Mazil K, Schulte F, Wurz A, Williamson T, Reimer RA, Culos‐Reed SN. EXERCISE in pediatric autologous stem cell transplant patients: a randomized controlled trial protocol. BMC Cancer 2012, 12:401. [DOI] [PMC free article] [PubMed]
Cox 2011 {published data only}
- Cox CL. Physical activity to modify sequelae and quality of life in childhood acute lymphoblastic leukemia: a nursing trial. clinicaltrials.gov/ct2/show/NCT00902213 (accessed March 6, 2013).
Dubnov‐Raz 2010 {published data only}
- Dubnov‐Raz G. The effect of physical activity on the mental and physical health of children with cancer in remission. clinicaltrials.gov/ct2/show/NCT00839904 (accessed August 6, 2015).
Kauhanen 2014 {published data only}
- Kauhanen L, Järvelä L, Lähteenmäki P, Arola M, Heinonen OJ, Axelin A, et al. Active video games to promote physical activity in children with cancer: a randomized clinical trial with follow‐up. BMC Pediatrics 2014;14:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mabbott 2013 {published data only}
- Mabbott D, Bartels U, Bouffet E, Laughlin S, Piscione J, Scantlebury N, et al. The neuro‐protective effects of exercise in children with brain tumours. clinicaltrials.gov/ct2/show/NCT01944761 (accessed 12 January 2015) and webapps.cihr‐irsc.gc.ca/cris/detail_e?pResearchId=4732704&p_version=CRIS&p_language=E&p_session_id=1333597 (accessed 13 January 2015).
Soares‐Miranda 2013 {published data only}
- Soares‐Miranda L, Fiuza‐Luces C, Lassaletta A, Santana‐Sosa E, Padilla JR, Fernández‐Casanova L, et al. Physical Activity in Pediatric Cancer patients with solid tumors ( PAPEC ): Trial rationale and design. Contemporary Clinical Trials 2013;36(1):106‐16. [DOI] [PubMed] [Google Scholar]
Wiskemann 2012 {published data only}
- Wiskemann J, Kulozik A. Exercise in Pediatric Cancer Patient Undergoing Anti‐Cancer Treatment (ONKOKIDS‐HD). clinicaltrials.gov/ct2/show/NCT02216604 (accessed August 6, 2015).
Additional references
ACSM 2010
- American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 8th Edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2010. [Google Scholar]
American Cancer Society 2012
- American Cancer Society. Cancer Facts & Figures 2012. www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc‐031941.pdf (accessed 6 March 2013).
Arroyave 2008
- Arroyave WD, Clipp EC, Miller PE, Jones LW, Ward DS, Bonner MJ, et al. Childhood cancer survivors' perceived barriers to improving exercise and dietary behaviors. Oncology Nursing Forum 2008;35(1):121‐30. [DOI] [PubMed] [Google Scholar]
Aznar 2006
- Aznar S, Webster AL, San Juan AF, Chamorro‐Viña C, Maté‐Muñoz JL, Moral S, et al. Physical activity during treatment in children with leukemia: a pilot study. Applied Physiology, Nutrition, and Metabolism 2006;31(4):407‐13. [DOI] [PubMed] [Google Scholar]
Baumann 2013
- Baumann FT, Bloch W, Beulertz J. Clinical exercise interventions in pediatric oncology: a systematic review. Pediatric Research 2013;74(4):366‐74. [DOI] [PubMed] [Google Scholar]
Cancer Research UK 2011
- Cancer Research UK. Childhood cancer incidences. www.cancerresearchuk.org/cancer‐info/cancerstats/childhoodcancer/incidence/ (accessed 8 March 2013).
Cox 2008
- Cox CL, Montgomery M, Oeffinger KC, Leisenring W, Zeltzer L, Whitton JA, et al. Promoting physical activity in childhood cancer survivors: results from the Childhood Cancer Survivor study. Cancer 2008;115:642‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cramp 2008
- Cramp F, Daniel J. Exercise for the management of cancer‐related fatigue in adults. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD006145.pub2] [DOI] [PubMed] [Google Scholar]
De Caro 2006
- Caro E, Fioredda F, Calevo MG, Smeraldi A, Saitta M, Hanau G, et al. Exercise capacity in apparently healthy survivors of cancer. Archives of Disease in Childhood 2006;91(1):47‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dimeo 2001
- Dimeo FC. Effects of exercise on cancer‐related fatigue. Cancer 2001;92(6 Suppl):1689‐93. [DOI] [PubMed] [Google Scholar]
Faigenbaum 2010
- Faigenbaum AD, Myer GD. Pediatric resistance training: benefits, concerns, and program design considerations. Current Sports Medicine Reports 2010;9(3):161‐8. [DOI] [PubMed] [Google Scholar]
Ganley 2011
- Ganley KJ, Paterno MV, Miles C, Stout J, Brawner L, Girolami G, et al. Health‐related fitness in children and adolescents. Pediatric Physical Therapy 2011;23(3):208‐20. [DOI] [PubMed] [Google Scholar]
Geenen 2007
- Geenen MM, Cardous‐Ubbink MC, Kremer LCM, Bos C, Pal HJH, Heinen RC, et al. Medical assessment of adverse health outcomes in long‐term survivors of childhood cancer. JAMA 2007;297(24):2705‐15. [DOI] [PubMed] [Google Scholar]
Guyatt 2008a
- Guyatt GH, Oxman AD, Kunz R, Falck‐Ytter Y, Vist GE, Liberati A, et al. Going from evidence to recommendations. BMJ 2008;336(7652):1049‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008b
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartman 2008
- Hartman A, Bos C, Stijnen T, Pieters R. Decrease in peripheral muscle strength and ankle dorsiflexion as long‐term side effects of treatment for childhood cancer. Pediatric Blood and Cancer 2008;50(4):833‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hislop and Montgomery 2007
- Hislop HJ, Montgomery J. Daniels and Worthingham's Muscle Testing: Techniques of Manual Examination. Eighth. St.Louis, Missouri, USA: Saunders Elsevier, 2007. [Google Scholar]
Hovi 1993
- Hovi L, Era P, Rautonen J, Siimes MA. Impaired muscle strength in female adolescents and young adults surviving leukemia in childhood. Cancer 1993;72(1):276‐81. [DOI] [PubMed] [Google Scholar]
Huang 2011
- Huang TT, Ness KK. Exercise interventions in children with cancer: a review. International Journal of Pediatrics 2011;2011:461512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ness 2005
- Ness KK, Mertens AC, Hudson MM, Wall MM, Leisenring WM, Oeffinger KC, et al. Limitations on physical performance and daily activities among long‐term survivors of childhood cancer. Annals of Internal Medicine 2005;143(9):639‐47. [DOI] [PubMed] [Google Scholar]
Ness 2009
- Ness KK, Leisenring WM, Huang S, Hudson MM, Gurney JG, Whelan K, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer 2009;115(9):1984‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oldervoll 2004
- Oldervoll LM, Kaasa S, Hjermstad MJ, Lund JA, Loge JH. Physical exercise results in the improved subjective well‐being of a few or is effective rehabilitation for all cancer patients?. European Journal of Cancer 2004;40(7):951‐62. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
San Juan 2008
- San Juan AF, Chamorro‐Viña C, Maté‐Muñoz JL, Fernández del Valle M, Cardona C, Hernández M, et al. Functional capacity of children with leukemia. International Journal of Sports Medicine 2008;29(2):163‐7. [DOI] [PubMed] [Google Scholar]
Schmitz 2005
- Schmitz KH, Holtzman J, Courneya KS, Mâsse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta‐analysis. Cancer Epidemiology, Biomarkers & Prevention 2005;14(7):1588‐95. [DOI] [PubMed] [Google Scholar]
Schneider 2007
- Schneider CM, Hsieh CC, Sprod LK, Carter SD, Hayward R. Cancer treatment‐induced alterations in muscular fitness and quality of life: the role of exercise training. Annals of Oncology 2007;18(12):1957‐62. [DOI] [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). GRADE handbook for grading the quality of evidence and the strength of recommendations. Version 3.2 [updated October 2009]. Available from http://www.cc‐ims.net/gradepro.
Van Brussel 2006
- Brussel M, Takken T, Net J, Engelbert RH, Bierings M, Schoenmakers MA, et al. Physical function and fitness in long‐term survivors of childhood leukaemia. Pediatric Rehabilitation 2006;9(3):267‐74. [DOI] [PubMed] [Google Scholar]
Van Brussel 2011
- Brussel M, Net J, Hulzebos E, Helders PJM, Takken T. The Utrecht approach to exercise in chronic childhood conditions: the decade in review. Pediatric Physical Therapy 2011;23:2‐14. [DOI] [PubMed] [Google Scholar]
Warner 1998
- Warner JT, Bell W, Webb DKH, Gregory JW. Daily energy expenditure and physical activity in survivors of childhood malignancy. Pediatric Research 1998;43:607‐13. [DOI] [PubMed] [Google Scholar]
Warner 2008
- Warner JT. Body composition, exercise and energy expenditure in survivors of acute lymphoblastic leukaemia. Pediatric Blood and Cancer 2008;50(2 Suppl):456‐61. [DOI] [PubMed] [Google Scholar]
Watson 2004
- Watson T, Mock V. Exercise as an intervention for cancer‐related fatigue. Physical Therapy 2004;84(8):736‐43. [PubMed] [Google Scholar]
Winter 2009
- Winter C, Müller C, Brandes M, Brinkmann A, Hoffmann C, Hardes J, et al. Level of activity in children undergoing cancer treatment. Pediatric Blood and Cancer 2009;53(3):438‐43. [DOI] [PubMed] [Google Scholar]
Winter 2010
- Winter C, Müller C, Hoffmann C, Boos J, Rosenbaum D. Review, physical activity and childhood cancer. Pediatric Blood and Cancer 2010;54:501‐10. [DOI] [PubMed] [Google Scholar]
Wolin 2010
- Wolin KY, Ruiz JR, Tuchman H, Lucia A. Exercise in adult and pediatric hematological cancer survivors: an intervention review. Leukemia 2010;24:1113‐20. [DOI] [PubMed] [Google Scholar]
Wright 1998
- Wright MJ, Halton JM, Martin RF, Barr RD. Long‐term gross motor performance following treatment for acute lymphoblastic leukemia. Medical and Pediatric Oncology 1998;31(2):86‐90. [DOI] [PubMed] [Google Scholar]
Wright 2005
- Wright MJ, Galea V, Barr RD. Proficiency of balance in children and youth who have had acute lymphoblastic leukemia. Physical Therapy 2005;85(8):782‐90. [PubMed] [Google Scholar]
References to other published versions of this review
Braam 2010
- Braam KI, Torre P, Takken T, Veening MA, Dulmen‐den Broeder E, Kaspers GJL. Exercise interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD008796] [DOI] [Google Scholar]
Braam 2013
- Braam KI, Torre P, Takken T, Veening MA, Dulmen‐den Broeder E, Kaspers GJL. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008796.pub2] [DOI] [PubMed] [Google Scholar]


