Abstract
Background
In the recent years, a variety of bronchoscopic lung volume reduction (BLVR) procedures have emerged that may provide a treatment option to participants suffering from moderate to severe chronic obstructive pulmonary disease (COPD).
Objectives
To assess the effects of BLVR on the short‐ and long‐term health outcomes in participants with moderate to severe COPD and determine the effectiveness and cost‐effectiveness of each individual technique.
Search methods
Studies were identified from the Cochrane Airways Group Specialised Register (CAGR) and by handsearching of respiratory journals and meeting abstracts. All searches are current until 07 December 2016.
Selection criteria
We included randomized controlled trials (RCTs). We included studies reported as full text, those published as abstract only and unpublished data, if available.
Data collection and analysis
Two independent review authors assessed studies for inclusion and extracted data. Where possible, data from more than one study were combined in a meta‐analysis using RevMan 5 software.
Main results
AeriSeal
One RCT of 95 participants found that AeriSeal compared to control led to a significant median improvement in forced expiratory volume in one second (FEV1) (18.9%, interquartile range (IQR) ‐0.7% to 41.9% versus 1.3%, IQR ‐8.2% to 12.9%), and higher quality of life, as measured by the St Georges Respiratory Questionnaire (SGRQ) (‐12 units, IQR ‐22 units to ‐5 units, versus ‐3 units, IQR ‐5 units to 1 units), P = 0.043 and P = 0.0072 respectively. Although there was no significant difference in mortality (Odds Ratio (OR) 2.90, 95% CI 0.14 to 62.15), adverse events were more common for participants treated with AeriSeal (OR 3.71, 95% CI 1.34 to 10.24). The quality of evidence found in this prematurely terminated study was rated low to moderate.
Airway bypass stents
Treatment with airway bypass stents compared to control did not lead to significant between‐group changes in FEV1 (0.95%, 95% CI ‐0.16% to 2.06%) or SGRQ scores (‐2.00 units, 95% CI ‐5.58 units to 1.58 units), as found by one study comprising 315 participants. There was no significant difference in mortality (OR 0.76, 95% CI 0.21 to 2.77), nor were there significant differences in adverse events (OR 1.33, 95% CI 0.65 to 2.73) between the two groups. The quality of evidence was rated moderate to high.
Endobronchial coils
Three studies comprising 461 participants showed that treatment with endobronchial coils compared to control led to a significant between‐group mean difference in FEV1 (10.88%, 95% CI 5.20% to 16.55%) and SGRQ (‐9.14 units, 95% CI ‐11.59 units to ‐6.70 units). There were no significant differences in mortality (OR 1.49, 95% CI 0.67 to 3.29), but adverse events were significantly more common for participants treated with coils (OR 2.14, 95% CI 1.41 to 3.23). The quality of evidence ranged from low to high.
Endobronchial valves
Five studies comprising 703 participants found that endobronchial valves versus control led to significant improvements in FEV1 (standardized mean difference (SMD) 0.48, 95% CI 0.32 to 0.64) and scores on the SGRQ (‐7.29 units, 95% CI ‐11.12 units to ‐3.45 units). There were no significant differences in mortality between the two groups (OR 1.07, 95% CI 0.47 to 2.43) but adverse events were more common in the endobronchial valve group (OR 5.85, 95% CI 2.16 to 15.84). Participant selection plays an important role as absence of collateral ventilation was associated with superior clinically significant improvements in health outcomes. The quality of evidence ranged from low to high.
Intrabronchial valves
In the comparison of partial bilateral placement of intrabronchial valves to control, one trial favoured control in FEV1 (‐2.11% versus 0.04%, P = 0.001) and one trial found no difference between the groups (0.9 L versus 0.87 L, P = 0.065). There were no significant differences in SGRQ scores (MD 2.64 units, 95% CI ‐0.28 units to 5.56 units) or mortality rates (OR 4.95, 95% CI 0.85 to 28.94), but adverse events were more frequent (OR 3.41, 95% CI 1.48 to 7.84) in participants treated with intrabronchial valves. The lack of functional benefits may be explained by the procedural strategy used, as another study (22 participants) compared unilateral versus partial bilateral placement, finding significant improvements in FEV1 and SGRQ when using the unilateral approach. The quality of evidence ranged between moderate to high.
Vapour ablation
One study of 69 participants found significant mean between‐group differences in FEV1 (14.70%, 95% CI 7.98% to 21.42%) and SGRQ (‐9.70 units, 95% CI ‐15.62 units to ‐3.78 units), favouring vapour ablation over control. There was no significant between‐group difference in mortality (OR 2.82, 95% CI 0.13 to 61.06), but vapour ablation led to significantly more adverse events (OR 3.86, 95% CI 1.00 to 14.97). The quality of evidence ranged from low to moderate.
Authors' conclusions
Results for selected BLVR procedures indicate they can provide significant and clinically meaningful short‐term (up to one year) improvements in health outcomes, but this was at the expense of increased adverse events. The currently available evidence is not sufficient to assess the effect of BLVR procedures on mortality. These findings are limited by the lack of long‐term follow‐up data, limited availability of cost‐effectiveness data, significant heterogeneity in results, presence of skew and high CIs, and the open‐label character of a number of the studies.
Keywords: Humans; Bronchi; Bronchi/surgery; Bronchoscopy; Bronchoscopy/methods; Pneumonectomy; Pneumonectomy/adverse effects; Pneumonectomy/methods; Pulmonary Disease, Chronic Obstructive; Pulmonary Disease, Chronic Obstructive/surgery; Randomized Controlled Trials as Topic
Plain language summary
Bronchoscopic lung volume reduction procedures for moderate to severe chronic obstructive pulmonary disease
Review question
Do bronchoscopic lung volume reduction (BLVR) procedures improve health outcomes, without leading to an increased chance of death, higher rates of illness after the procedure, while maintaining acceptable costs for people suffering from moderate to severe chronic obstructive pulmonary disease (COPD)?
Background
BLVR procedures are a collection of innovative non‐surgical procedures that aim to improve the disease status and lung function of participants suffering from moderate to severe COPD, specifically those participants who remain limited despite conventional treatment.
Study characteristics
Fourteen studies including 1979 participants were identified up to December 2016 which studied BVRs (AeriSeal, airway bypass stents, endobronchial coils, endobronchial valves, intrabronchial valves and vapour ablation). Most studies compared a BLVR procedure to optimal medical care or to sham bronchoscopy, while one studied a specific way to place intrabronchial valves: unilaterally or partial bilaterally.
Key results
Evidence for short‐term improvements in disease status were most evident for studies testing endobronchial valves (five studies) and endobronchial coils (three studies), including improvements in lung function and quality of life. Improvements in lung function and quality of life were also found for vapour ablation and AeriSeal, but the quality of that evidence is limited as the study on vapour ablation was small and the study on AeriSeal was terminated early. Neither airway bypass stents (one study) nor partial bilateral placement of intrabronchial valves (two studies) seemed to lead to significant changes in health outcomes, although unilateral placement of intrabronchial valves did lead to better health outcomes as assessed by a small study. Studies that found improvements in health outcomes also found higher rates of potential complications as a result of the procedures, but the current studies did not provide evidence for a higher risk of death after BLVR procedures, although the evidence from the included studies is not conclusive.
Quality of the evidence
The lack of sham bronchoscopy or unclear status of blinding in some studies caused a risk of bias for subjective outcomes (e.g. quality of life and exercise capacity). The lack of long‐term follow‐up, small size of some of the studies, differences in results between trials, and lack of cost‐effectiveness data limits the quality of evidence provided in this review.
Summary of findings
Summary of findings for the main comparison. AeriSeal versus control.
| AeriSeal + optimal medical care versus optimal medical care for the treatment of chronic obstructive pulmonary disease | ||||||
| Patient or population: Participants suffering from chronic obstructive pulmonary disease Setting: Hospital Intervention: AeriSeal + optimal medical care Comparison: Optimal medical care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI or IQR) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with optimal medical care | Risk with AeriSeal | |||||
| % change from baseline in FEV1 | The median % change in FEV1 for optimal medical care was 1.3% (IQR ‐8.2 to 12.9%) | The median % change in FEV1 in the intervention group was 18.9% (IQR ‐0.7% to 41.9%) | ‐ | 34 (1 RCT) | ⊕⊕⊝⊝ LOW1 | |
| Mortality at end of follow‐up | 0 per 1,000 | 21 per 1,000 | OR 2.90 (95% CI 0.14 to 62.15) | 95 (1 RCT) | ⊕⊕⊝⊝ LOW2 | |
| Units SGRQ change from baseline | The median unit change in SGRQ for optimal medical care was ‐3 units (IQR ‐5 to 1 units) | The median unit change in SGRQ for the intervention group was ‐12 units (IQR ‐22 to ‐5 units) | ‐ | 34 (1 RCT) | ⊕⊕⊝⊝ LOW3 | |
| Change from baseline in lung function parameters other than FEV1 | No values reported | Not estimable | Not estimable | Not estimable | ||
| Meters change from baseline in 6MWD | The median meters 6MWD change from baseline was ‐22 meters (IQR ‐41.3 to 9.3 meters) | The median meters 6MWD change from baseline in the intervention group was 31 meters (IQR 0 to 41.3 meters) | ‐ | 34 (1 RCT) | ⊕⊕⊝⊝ LOW4 | |
| Adverse events at end of follow‐up | 176 per 1,000 | 443 per 1,000 (223 to 687) | OR 3.71 (1.34 to 10.24) | 95 (1 RCT) | ⊕⊕⊕⊝ MODERATE5 | Most common respiratory events were pneumonia, COPD exacerbations, post‐acute inflammatory response and pneumothorax. The post‐procedure adverse event rate requiring hospitalization was significantly higher in the treatment condition (44%) compared to the control (18%) condition, P = 0.0098. |
| Cost effectiveness end of follow‐up | No values reported | Not estimable | Not estimable | Not estimable | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; SGRQ: St George's Respiratory Questionnaire; FEV1: Forced Expired Vvolume in one second; RV: Residual Volume; TLC: Total lung capacity; L: Liter; RCT: randomized controlled trial; 6MWD: Six‐Minute Walking Distance; SMD: Standardized Mean Difference; MD: Mean Difference, IQR: Interquartile range | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded 2 levels due to imprecision: very low participant numbers and very wide IQRs
2 Downgraded 2 levels due to imprecision: low participant numbers and very wide CIs
3 Downgraded 2 levels due to risk of performance and detection bias and imprecision: studies were not blinded and SGRQ was dependent on participants' subjective answering, very low participant numbers
4 Downgraded 2 levels due to imprecision and risk of performance bias: low participant numbers, the 6MWD was effort‐dependent and could be influenced in non‐blinded studies
5 Downgraded 1 levels due to imprecision: low participant numbers
Summary of findings 2. Airway bypass stents versus control.
| Airway bypass stents versus sham bronchoscopy for the treatment of chronic obstructive pulmonary disease | ||||||
| Patient or population: Participants suffering from chronic obstructive pulmonary disease Setting: Hospital Intervention: Airway bypass stents + optimal medical care Comparison: Sham bronchoscopy + optimal medical care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with optimal medical care | Risk with bypass stents | |||||
| % Change from baseline in FEV1 | The mean % change in FEV1 for control was ‐1.1% (SD 3) | The mean % change in FEV1 in the intervention group was 0.95% higher (95% CI ‐0.16 to 2.06) | ‐ | 350 (1 RCT) | ⊕⊕⊕⊕ HIGH | |
| Mortality at end of follow‐up | 37 per 1,000 | 29 per 1,000 | OR 0.76 (0.21 to 2.77) | 350 (1 RCT) | ⊕⊕⊕⊝ MODERATE1 | |
| Units SGRQ at end of follow‐up | The mean units of SGRQ for control at end of follow‐up were 58 (SD 15) | The mean units of SGRQ for the intervention group at end of follow‐up was 2 units lower (95% CI ‐5.58 to 1.58) | ‐ | 350 (1 RCT) | ⊕⊕⊕⊕ HIGH | The CI did not reach the MCID used in this review (‐7.1 units), meaning that, with the current state of the treatment, we do not expect the result to change; hence no downgrading of the evidence was performed. |
| Change from baseline in lung function parameters other than FEV1 | The mean L change in RV for control was ‐0.10 L (SD 0.6) | The mean L change in RV for the intervention group at end of follow‐up was 0.04 L more (95% CI ‐0.11 to 0.19) | ‐ | 350 (1 RCT) | ⊕⊕⊕⊕ HIGH | The CI did not reach the MCID used in this review (0.43 L), meaning that, with the current state of the treatment, we do not expect the result to change; hence no downgrading of the evidence was performed. |
| The mean L change in FVC for control was 0.0L (SD 0.4 L) | The mean L change in FVC for intervention was 0.08 L fewer (95% CI ‐0.18 to 0.02) | ‐ | 350 (1 RCT) | ⊕⊕⊕⊕ HIGH | ||
| Meters 6MWD at end of follow‐up | The mean 6MWD for control at end of follow‐up was 297 meters (SD 94) | The mean 6MWD for intervention at end of follow‐up was 16.00 meters fewer (95% CI ‐39.17 to 7.17) | ‐ | 350 (1 RCT) | ⊕⊕⊕⊝ MODERATE2 | |
| Adverse events at end of follow‐up | 112 per 1,000 | 144 per 1,000 (76 to 256) | OR 1.33 (0.65 to 2.73) | 315 (1 study) | ⊕⊕⊕⊝ MODERATE3 | Serious adverse events were reported in 3.4% (n = 7) participants in the treatment group compared to none in the sham control group. Pneumothorax (3 in treatment versus 1 in control), haemoptysis (1 in treatment versus 0 in control) and COPD exacerbations (33 in treatment versus 9 in control) were more frequent in treatment versus sham control. |
| Cost effectiveness | Not reported | not estimable | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; SGRQ: St George's Respiratory Questionnaire; FEV1: forced expired volume in one second; RV: Residual Volume; TLC: Total lung capacity; L: Liter; RCT: randomized controlled trial; 6MWD: Six‐Minute Walking Distance; SMD: Standardized Mean Difference; MD: Mean Difference | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded 1 level for imprecision: the upper end of CI indicates more than 2.7 times the odds of events and lower CI is below 1.
2Downgraded 1 level for imprecision: the lower end of CI indicates crosses the MCID used for this outcome (26 meters).
3Downgraded 1 level for imprecision: the upper end of CI indicates more than 2.7 times the odds of events and lower CI is below 1.
Summary of findings 3. Endobronchial coils versus control.
| Endobronchial coils + optimal medical care versus optimal medical care for the treatment of chronic obstructive pulmonary disease | ||||||
| Patient or population: Participants suffering from chronic obstructive pulmonary disease Setting: Hospital Intervention: Endobronchial coils + optimal medical care Comparison: Optimal medical care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI or SD) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with optimal medical care | Risk with endobronchial coils | |||||
| % change from baseline in FEV1 | The mean % change in FEV1 for optimal medical care ranged between ‐3.0% and 3.6% | The mean change in FEV1 in the intervention group was 10.88 more (95% CI, 5.20 to 16.55) | ‐ | 146 (2 RCTs) | ⊕⊕⊕⊝ MODERATE1 | |
| Mortality at end of follow‐up | 48 per 1,000 | 70 per 1,000 | OR 1.49 (0.67 to 3.29) | 461 (3 RCTs) | ⊕⊕⊕⊝ MODERATE2 | |
| Units SGRQ change from baseline | The mean units SGRQ change for optimal medical care ranged between 0.25 and 1.5 | The mean SGRQ change in the intervention group was 9.14 units fewer (95% CI, ‐11.59 to ‐6.70) | ‐ | 461 (3 RCTs) | ⊕⊕⊕⊝ MODERATE3 | |
| Change from baseline in lung function parameters other than FEV1 | The mean L change in RV for optimal medical care ranged between ‐0.2 L and ‐0.1 L | The mean RV change in the intervention group was 0.32 L fewer (95% CI, ‐0.48 to ‐0.17 L) | ‐ | 461 (3 RCTs) | ⊕⊕⊕⊕ HIGH | |
| The mean L change in TLC for optimal medical care was ‐0.09 L | The mean TLC change in the intervention group was 0.19 L fewer (95% CI, ‐0.43 to ‐0.06) | 146 (2 RCTs) | ⊕⊕⊕⊝ MODERATE4 | |||
| The mean change in RV/TLC for optimal medical care ranged between ‐0.5 to 0 | The mean change in RV/TLC in the intervention group was 3.74 fewer (95% CI ‐5.16 to ‐2.33) | 415 (2 RCTs) | ⊕⊕⊕⊕ HIGH | |||
| Meters change from baseline in 6MWD | The mean 6MWD change from baseline ranged between ‐23 meters and ‐3.2 meters | The mean 6MWD change from baseline in the intervention group was 30.85 meters more (‐1.05 to 62.76 more) | ‐ | 461 (3 RCTs) | ⊕⊕⊝⊝ LOW5 | |
| Adverse events at end of follow‐up | 230 per 1,000 | 391 per 1,000 (297 to 492) | OR 2.14 (1.41 to 3.23) | 461 (3 studies) | ⊕⊕⊕⊕ HIGH | Overall rates of adverse events were higher in the treatment condition compared to control. Lower respiratory tract infections, COPD exacerbations, pneumonia and pneumothorax were the most frequent adverse events. |
| Cost effectiveness at end of follow‐up | The mean costs in USD at end of follow‐up for control was $5,912.00 (SD 3,529.00) | The mean cost at end of follow‐up in the intervention group was $47,908.00 higher ($47,879.00 to $48,073.00) | ⊕⊕⊕⊝ MODERATE6 | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; SGRQ: St George's Respiratory Questionnaire; FEV1: forced expired volume in one second; RV: Residual Volume; TLC: Total lung capacity; L: Liter; RCT: randomized controlled trial; 6MWD: Six‐Minute Walking Distance; SMD: Standardized Mean Difference; MD: Mean Difference | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded 1 level due to imprecision: low participant numbers
2 Downgraded 1 level due to imprecision: the upper end of CI indicated 3.3 times the odds of death
3 Downgraded 1 level due to risk of performance and detection bias: 3 out of 4 studies were not blinded and SGRQ was dependent on participants' subjective answering
4 Downgraded 1 level due to risk of performance error and imprecision: low participant numbers
5 Downgraded 2 levels due to risk of performance bias and inconsistency in results: high heterogeneity and the 6MWD was effort‐dependent and could be influenced in non‐blinded studies
6 Downgraded 1 level due to imprecision: low participant numbers
Summary of findings 4. Endobronchial valves versus control.
| Endobronchial valves + optimal medical care versus optimal medical care for the treatment of chronic obstructive pulmonary disease | ||||||
| Patient or population: Participants suffering from chronic obstructive pulmonary disease Setting: Hospital Intervention: Endobronchial valves + optimal medical care Comparison: Optimal medical care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with optimal medical care | Risk with endobronchial valves | |||||
| % change from baseline in FEV1 | The mean % change in FEV1 for optimal medical care ranged between ‐3.5% and 3.9% | The standardized mean difference in FEV1 in the intervention group was 0.48 (95% CI, 0.32 to 0.64) | ‐ | 703 (5 RCTs) | ⊕⊕⊝⊝ LOW1 | |
| Mortality at end of follow‐up | 30 per 1,000 | 35 per 1,000 | OR 1.07 (0.47 to 2.43) | 703 (5 RCTs) | ⊕⊕⊕⊝ MODERATE2 | |
| Units of SGRQ change from baseline | The mean units of SGRQ change for optimal medical care ranged between ‐3.7 units and 1 unit | The mean SGRQ change in the intervention group was 7.29 units fewer (95% CI, ‐11.12 to ‐3.45 units) | ‐ | 695 (5 RCTs) | ⊕⊕⊝⊝ LOW3 | |
| Change from baseline in lung function parameters other than FEV1 | The mean L change in RV for optimal medical care ranged between ‐0.13 L and 0.05 L | The mean RV change in the intervention group was 0.58 L fewer (95% CI, ‐0.77 to ‐0.39) | ‐ | 200 (3 RCTs) | ⊕⊕⊕⊝ MODERATE4 | |
| The mean L change in TLC for optimal medical care ranged between ‐0.12 L and 0.002 L | The mean TLC change in the intervention group was 0.34 L fewer (95% CI, ‐0.46 to ‐0.23) | ‐ | 107 (2 RCTs) | ⊕⊕⊕⊝ MODERATE5 | ||
| The mean change in RV/TLC for optimal medical care ranged between ‐0.64 and ‐0.4 | The mean change in RV/TLC in the intervention group was 5.76 fewer (95% CI ‐10.45 to ‐1.06) | ‐ | 118 (2 RCTs) | ⊕⊕⊝⊝ LOW6 | ||
| Meters change from baseline in 6MWD | The mean 6MWD change from baseline ranged between ‐17.3 and 10 meters | The mean 6MWD change from baseline in the intervention group was 38.12 meters more (8.68 more to 67.56 more) | ‐ | 379 (4 RCTs) | ⊕⊕⊝⊝ LOW7 | |
| Adverse events at end of follow‐up | 97 per 1,000 | 387 per 1,000 (189 to 631) | OR 5.85 (2.16 to 15.84) | 482 (3 studies) | ⊕⊕⊕⊕ HIGH | Pneumonia distal to the valves was the most common adverse event. Pneumothorax and COPD exacerbations were reported as well. Overall, pneumothorax was associated with higher clinical response. Valve removal or replacement or both were relatively common. |
| Cost‐ effectiveness | The mean modelled costs for control were EUR 15,432 at 10 years (mean QALY = 4.02) |
The mean modelled costs for control were EUR 25,857 at 10 years (mean QALY = 4.43) |
⊕⊕⊕⊝ MODERATE8 | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; SGRQ: St George's Respiratory Questionnaire; FEV1: forced expired volume in one second; RV: Residual Volume; TLC: Total lung capacity; L: Liter; RCT: randomized controlled trial; 6MWD: Six‐Minute Walking Distance; SMD: Standardized Mean Difference; MD: Mean Difference | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded 2 levels due to inconsistency in results and imprecision: high heterogeneity in results and imprecision due to differences in participant‐selection criteria
2 Downgraded 1 level for imprecision: the upper end of CI indicated 2.4 times the odds of death.
3 Downgraded 2 levels due to inconsistency in results and risk of performance and detection bias: high heterogeneity in results and four studies were not blinded (SGRQ is dependent on participants' subjective answering).
4 Downgraded 1 level due to imprecision: low participant numbers
5 Downgraded 1 level due to imprecision: low participant numbers
6 Downgraded 2 levels due to inconsistency in results and imprecision: high heterogeneity in results and low participant numbers
7 Downgraded due to risk of performance bias and inconsistency in results: the 6MWD was effort‐dependent and could be influenced in non‐blinded studies and there was high heterogeneity in results
8 Downgraded 1 level due to imprecision: low participant numbers
Summary of findings 5. Intrabronchial valves versus control.
| Intrabronchial valves versus sham bronchoscopy for the treatment of chronic obstructive pulmonary disease | ||||||
| Patient or population: Participants suffering from chronic obstructive pulmonary disease Setting: Hospital Intervention: Intrabronchial valves + optimal medical care Comparison: Sham bronchoscopy + optimal medical care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with optimal medical care | Risk with intrabronchial valves | |||||
| % change from baseline in FEV1 | The mean % change in FEV1 for optimal medical care was 0.04 (SD 5.74) | The mean change in FEV1 in the intervention group was ‐2.15 less (95% CI, ‐3.47 to ‐0.83) | ‐ | 272 (1 RCT) | ⊕⊕⊕⊝ MODERATE1 | |
| Mortality at end of follow‐up | 6 per 1,000 | 28 per 1,000 | OR 4.95 (0.85 to 28.94) | 350 (2 RCTs) | ⊕⊕⊕⊝ MODERATE2 | |
| Units of SGRQ change from baseline | The mean units of SGRQ change for optimal medical care ranged between ‐1.41 units and ‐3.6 units | The mean SGRQ change in the intervention group was 2.64 units more (95% CI, ‐0.28 to 5.56) | ‐ | 350 (2 RCTs) | ⊕⊕⊕⊕ HIGH | The CI did not reach the MCID used in the review (‐7.1 units), meaning that with the selected treatment strategy (partial bilateral) we did not expect the result to change; hence, no downgrading of the evidence was performed. |
| Change from baseline in lung function parameters other than FEV1 | The mean L change in RV for optimal medical care ranged between ‐0.21 L and 0.07 L | The mean RV change in the intervention group was 0.38 L more (95% CI, 0.12 to 0.65) | ‐ | 312 (2 RCTs) | ⊕⊕⊕⊕ HIGH | |
| The mean L change in TLC for optimal medical care ranged between ‐0.09 L and 0.15 L | The mean TLC change in the intervention group was 0.14 L more (95% CI, ‐0.12 to 0.39) | 312 (2 RCTs) | ⊕⊕⊕⊝ MODERATE3 | |||
| Meters change from baseline in 6MWD | The mean 6MWD change from baseline ranged between ‐3.4 m and 7 m | The mean 6MWD change from baseline in the intervention group was 19.54 meters less (‐37.11 less to ‐1.98 less) | ‐ | 316 (2 RCTs) | ⊕⊕⊕⊝ MODERATE4 | |
| Adverse events at end of follow‐up | 47 per 1,000 | 143 per 1,000 (68 to 278) | OR 3.41 (1.48 to 7.84) | 350 (2 studies) | ⊕⊕⊕⊕ HIGH | Most occurring adverse events were COPD exacerbations, respiratory failure, pneumothorax and pneumonia. |
| Cost‐ effectiveness | Not reported | not estimable | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; SGRQ: St George's Respiratory Questionnaire; FEV1: forced expired volume in one second; RV: Residual Volume; TLC: Total lung capacity; L: Liter; RCT: randomized controlled trial; 6MWD: Six‐Minute Walking Distance; SMD: Standardized Mean Difference; MD: Mean Difference | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded 1 level due to imprecision: low participant numbers
2 Downgraded 1 level for imprecision: the upper end of CI indicated almost 29 times the odds of death.
3 Downgraded 1 level for imprecision: the 95% CI of the intervention group indicated clinically meaningful results
4 Downgraded 1 level for imprecision: high confidence intervals
Summary of findings 6. Vapour ablation versus control.
| Vapour ablation versus optimal medical therapy for the treatment of chronic obstructive pulmonary disease | ||||||
| Patient or population: Participants suffering from chronic obstructive pulmonary disease Setting: Hospital Intervention: Vapour ablation + optimal medical care Comparison: Optimal medical care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with optimal medical care | Risk with vapour ablation | |||||
| % change from baseline in FEV1 | The mean % change in FEV1 for optimal medical care was ‐3.7 (SD 11.1) | The mean change in FEV1 in the intervention group was 14.7% more (95% CI, 7.98 to 21.42) | ‐ | 64 (1 RCT) | ⊕⊕⊝⊝ MODERATE1 | |
| Mortality at end of follow‐up | 0 per 1,000 | 44 per 1,000 | OR 2.82 (95% CI 0.13 to 61.06) | 69 (1 RCT) | ⊕⊕⊝⊝ LOW2 | |
| Units of SGRQ change from baseline | The mean units of SGRQ change for optimal medical care was 0 units (SD 9.8) | The mean SGRQ change in the intervention group was 9.70 units fewer (95% CI, ‐15.62 to ‐3.78) | ‐ | 65 (1 RCT) | ⊕⊕⊝⊝ LOW3 | |
| Lung function parameters other than FEV1 | The absolute between group difference RV in L at end of follow‐up was ‐0.3 L (95% CI ‐0.54 to ‐0.06) | ‐ | 69 (1 RCT) | ⊕⊕⊕⊝ MODERATE4 | ||
| The absolute between group difference TLC in L at end of follow‐up was ‐0.08 L (95% CI ‐0.31 to 0.16) | 69 (1 RCT) | ⊕⊕⊕⊝ MODERATE5 | ||||
| Meters at end of follow‐up in 6MWD | The absolute between group difference 6MWD in meters at end of follow‐up was 30.5 m (95% CI ‐1.5 to 62.4) | ‐ | 69 (1 RCT) | ⊕⊕⊝⊝ LOW6 | ||
| Adverse events at end of follow‐up | 125 per 1,000 | 355 per 1,000 (125 to 681) | OR 3.86 (1.00 to 14.97) | 69 (1 study) | ⊕⊕⊕⊝ MODERATE7 | COPD exacerbations, pneumonia or pneumonitis occurred more often in the treatment group compared to the control group. There were no cases of respiratory failure or ICU admission. All but one adverse events could be resolved by standard care. |
| Cost effectiveness | Not reported | not estimable | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; SGRQ: St George's Respiratory Questionnaire; FEV1: forced expired volume in one second; RV: Residual Volume; TLC: Total lung capacity; L: Liter; RCT: randomized controlled trial; 6MWD: Six‐Minute Walking Distance; SMD: Standardized Mean Difference; MD: Mean Difference | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded 1 level due to imprecision: low participant numbers
2 Downgraded 2 levels due to imprecision: low participant numbers and high CI. Upper bound indicated 61 times the odds of death
3 Downgraded 2 levels due to imprecision and risk of performance and detection bias: low participant numbers and study was not blinded: SGRQ was dependent on participants' subjective answering
4 Downgraded 1 level due to imprecision: low participant numbers
5 Downgraded 1 level due to imprecision: low participant numbers
6 Downgraded 2 levels due to imprecision and risk of performance bias: low participant numbers and the 6MWD was effort‐dependent: can be influenced in non‐blinded studies.
7 Downgraded 1 level due to imprecision: low participant number
Background
Chronic obstructive pulmonary disease (COPD) places a significant burden on healthcare systems and is currently the third leading cause of mortality in the world (Lozano 2013; Mannino 2007). Treatment of early‐stage COPD revolves around managing the disease and slowing its progression via use of short‐ and long‐acting bronchodilators, pulmonary rehabilitation and a focus on smoking cessation (GOLD; Welte 2015). Participants with stable moderate to severe COPD often rely on long‐acting bronchodilators (beta‐agonists and muscarinic antagonists) sometimes in combination with inhaled corticosteroids, with the aim of alleviating dyspnoea while preventing future exacerbations (GOLD; Wedzicha 2012). Furthermore, pulmonary rehabilitation can significantly improve the health status of participants with stable COPD and those with recent exacerbation of the illness (McCarthy 2015; Puhan 2011).
Participants at a more advanced stage of disease respond less to conventional medical treatment and therefore have limited options for treatment of their disease. Lung volume reduction surgery (LVRS) is an exception and can significantly improve exercise capacity, lung function, and quality of life for a specific subset of participants (see Van Agteren 2016 for an extensive review on LVRS). Perceived risks and costs associated with an invasive procedure such as LVRS are often considered to be substantial (McNulty 2014), causing LVRS to be uncommonly performed despite its proven benefit for selected participants (Criner 2011; Zoumot 2014).
Recent advances in the field of nonsurgical bronchoscopic techniques (hereafter referred to as bronchoscopic lung volume reduction, or BLVR) have sparked hope for participants with emphysematous lung tissue who are unresponsive to medical therapy, do not meet the strict criteria for LVRS or do not wish to undergo surgery (Ingenito 2008). Nonsurgical techniques and interventions used to perform BLVR are distinct, but aim to achieve the same result, that is, increased mechanical efficiency of the lung with improved health status of the participant (Fessler 2008; Maxfield 2004). By aiming to achieve similar results as those witnessed in LVRS but without associated short‐term morbidity and mortality, these treatments may prove to be a valuable addition to or substitute for LVRS in the treatment of participants with severe emphysema.
Description of the condition
COPD comprises a heterogeneous group of diseases that show similar symptoms and include contrasting and overlapping underlying disease processes (Stockley 2009). Most participants with COPD are diagnosed with chronic bronchitis, characterised by chronic inflammation of the central airways, emphysema, characterised by impaired and damaged lung parenchyma (the part of the lung involved in gas transfer) epithelium, or show symptoms of both conditions (Kim 2008; Tuder 2003). COPD is a progressive chronic disease that is largely preventable and is characterised by hyperinflation (abnormal inflation or size) and decreased elasticity of the airways resulting from structural degradation and inflammation of lung tissue, impeding efficient gas exchange between the alveoli and the blood (Bourdin 2009; Sharafkhaneh 2008). BLVR focuses specifically on reducing hyperinflation due to damaged and destroyed lung tissue, similar to principles of LVRS, rather than on targeting chronic inflammation of the airways (Fessler 1998; Fessler 2002; Ingenito 2008). This section therefore focuses predominantly on describing the clinical features of emphysema and does not elaborate on the pathophysiology of chronic bronchitis.
Emphysema results from an interplay of various processes and is fuelled predominantly by exposure to cigarette smoke or other noxious particles (e.g. air pollutants) (Stockley 2009; Zeng 2012). Constant exposure to noxious particles leads to oxidative stress, proteinase‐anti‐proteinase imbalance, increased apoptosis, and chronic inflammation ‐ all of which lead to gradual destruction of the lung tissue (Bagdonas 2015; Demedts 2006; Kirkham 2013; Suki 2003; Taraseviciene‐Stewart 2008).
The type of emphysema, determined by identifying specific processes that cause the disease, can be characterised by disease distribution as well as location. An emphysematous lung can show a homogeneous or heterogeneous (regional) pattern of pathological lesions, which can have a different impact on lung parameters characteristic of emphysema (e.g. dynamic lung volume) (Boutou 2015; Mair 2009). Typically, disease heterogeneity refers to heterogeneity between lobes (interlobar), although emphysematous participants often show heterogeneity between areas of each lobe (Intralobar) (Valipour 2015). Weder 1997 developed a more specific classification of emphysema that divides participants into three classes: markedly heterogeneous, intermediately heterogeneous, and homogeneous. Furthermore, emphysema can be divided into subtypes depending on the unit of lung anatomy at which lesions are predominantly present (Hogg 2004):
Centrilobular emphysema: most closely associated with smoking and results from dilation and destruction of respiratory bronchioles. Lesions associated with centrilobular emphysema are located predominantly in the upper lung.
Panlobular emphysema: found mainly in the lower lobes and typical of a genetic (alpha1 anti‐trypsin) deficiency.
Paraseptal emphysema: occurs in the periphery of the lobules, specifically in the subpleural region.
Consistent destruction of healthy lung tissue results in the classic physiological characteristics of severe emphysema: hyperinflation of lungs, loss of elastic recoil, loss of surface area for gas exchange, and flow limitation (Ferguson 2006; Ingenito 2005; Papandrinopoulou 2012). Emphysema causes a decrease in elastic recoil pressure and an increase in lung compliance. This in turn causes static and dynamic hyperinflation of the lungs, which limits airflow and results in clinical outcomes of lower functional capacity, higher levels of dyspnoea, and lower exercise performance. Respiratory symptoms can worsen drastically, leading to physiological deterioration. These respiratory exacerbations can be triggered by a variety of factors and become more frequent in participants with severe emphysema (Celli 2007; Wedzicha 2003).
Description of the intervention
BLVR consists of a combination of nonsurgical techniques for lung volume reduction, performed via bronchoscopy, that allow the proceduralist to gain access to the trachea and lower airways via the nose or the mouth, thereby eliminating the need for a surgical procedure. The proceduralist pre‐identifies parts of the unhealthy lung that need to be targeted via computerised tomography (CT), ventilation/perfusion scintigraphy or magnetic resonance imaging (MRI) (Biederer 2012; Storbeck 2015). Once the target area has been located, lung volume reduction can be performed via a variety of distinct techniques, which are mentioned below.
Endobronchial and intrabronchial valves
BLVR via one‐way valves aims to occlude the most damaged regions of the lung from receiving air during inspiration, while allowing secretions and air to exit the occluded part of the lung (Eberhardt 2015). As this technique targets specific areas of the lung that show emphysematous destruction, it is specifically suitable for heterogeneous emphysema (Fann 2003; Snell 2003; Toma 2003; Venuta 2005; Yim 2004). Currently, two types of valves may be used: endobronchial 'duckbill' (Zephyr, Pulmonx Inc, Redwood City, CA, USA) and intrabronchial 'umbrella' (IBV, Spiration Inc, Redwood, WA, USA) valves.
Endobronchial valves are first measured to fit within the selected lumen; the hollow passageway of the target bronchus. After the size of the target lumen has been determined, the valve is placed by a catheter via flexible or rigid bronchoscopy (Galluccio 2010). Intrabronchial valves are placed via a flexible bronchoscope and seal the airway via support struts (Wood 2007). The umbrella valve is placed into the bronchoscope while in a compressed state. Once the target lumen has been found, a specialised catheter is used to deploy the valve. The struts are covered by a membrane that acts as a barrier to airflow, and anchors keep the valve in place. In cases of valve migration, the procedure can be repeated for both types of valves to restore position and functioning.
When considering treatment with one‐way valves, it is specifically important to pay attention to participant phenotyping and selection, as disease heterogeneity and the presence of collateral ventilation determines treatment response (Milanese 2016; Schuhmann 2015; Shah 2014) (see How the intervention might work for an explanation). Furthermore, the specific treatment strategy used may influence the results, as for instance, Springmeyer 2009 showed that partial bilateral placement of valves may lead to some beneficial results, while leading to lower rates of pneumothorax.
Endobronchial coils
Nitinol coils for BLVR are designed to tether in the airway, thereby restricting diseased parts of the airways in participants with homogeneous and heterogeneous emphysema (Herth 2010; Klooster 2014a; Klooster 2014). The RePneu coil, developed by PneumRx (Mountain View, CA, USA), is inserted via a catheter over a guidewire. The coil sits straight within the bronchoscope, and upon deployment into the target lumen, it returns to its original predetermined coiled shape. In this way, diseased tissue becomes compressed, which shortens the airways and increases elastic recoil. Consideration for treatment using coils relies on CT assessment to rule out contraindications and to determine the extent of lung parenchyma destruction, as coils rely on a minimal amount of healthy parenchyma to be effective (Milanese 2016).
Biological lung volume reduction
Biological lung volume reduction is based on in situ formation of a biodegradable hydrogel that is formed from thrombin and a fibrinogen solution (Criner 2009). The hydrogel, targeting the worst affected lobe of participants suffering from heterogeneous disease, produces an inflammatory response in the airway, causing it to collapse and remodel via scarring and contraction (Refaely 2010). This causes the treated lobe to be reduced in size over the course of three to six weeks, which is hypothesized to lead to improved functional outcomes.
AeriSeal
The AeriSeal system (AerisTherapeutics, Inc., Woburn, MA, USA) is based on the same principle as biological lung volume reduction but uses a synthetic non‐biological foam to induce inflammation, scarring and subsequent shrinkage of lung tissue (Falkenstern‐Ge 2013; Herth 2011). Similar to the biologic hydrogel, the hydrogel foam used in AeriSeal is biodegradable.
Bronchoscopic thermal vapour ablation
Bronchoscopic thermal vapour ablation aims to induce shrinkage of lung tissue via thermal injury through steam in participants with heterogeneous disease (Snell 2009). A bronchoscopic catheter with an occlusion balloon attached is targeted at the area of emphysema predetermined by CT. The balloon is inflated, and a predetermined dose of vapour is targeted to the segments of interest (Emery 2010; Snell 2012). The steam causes an inflammatory response, leading to fibrosis and collapse of the airways distal to this fibrosis (Herth 2012).
Airway bypass stents
Participants with emphysema, especially those with homogeneous emphysema, often show a considerable degree of collateral ventilation due to obstruction of the airways (Cetti 2006; Higuchi 2006). This collateral ventilation causes airflow to bypass obstructed airways via anatomical or artificial channels. By creating artificial openings (fenestrations) between the alveolar space and large airways, trapped air can be allowed to drain. Placement of airway bypass stents allows the fenestrations to be kept open, resulting in improved lung compliance and inspiratory capacity, due to reduced air trapping (Choong 2008).
How the intervention might work
The desired end outcome of BLVR is similar to the outcome expected following LVRS (Cooper 1995; Fessler 2008), that is, improvement in function of the lung attained by:
decreasing the degree of hyperinflation, resulting in improved diaphragm and chest wall mechanics;
increasing elastic recoil pressure, thereby increasing expiratory airflow;
reducing inequalities between ventilation and perfusion, resulting in improved alveolar gas exchange and increased effectiveness of ventilation in maintaining blood gas levels.
Zoumot 2015 adds that BLVR can result in decreased asynchronous movement of different chest wall compartments, leading to improved ventilatory mechanics. Furthermore, BLVR can lead to a reduction in dynamic hyperinflation, which improves ventilatory limitations of exercise (Hopkinson 2005) and BLVR can improve cardiovascular response to exercise (Faisal 2016).
The mechanics involved in achieving this result are not completely the same for BLVR and LVRS and even differ according to the technique of BLVR used. The main aim is to reduce hyperinflation of the lung (Fessler 1998; Fessler 2002; Ingenito 2008). The traditional idea behind BLVR is that blocking emphysematous regions leads to atelectasis, a collapse of the blocked part of the lung or lobe (Toma 2003), similar to the way that LVRS leads to a reduction in diseased tissue via surgical resection. Blocking diseased tissue of the most hyperinflated regions of the lung leads to a reduction in hyperinflation, and thus to improvement in respiratory function, via atelectasis (Ingenito 2001; Venuta 2006). Non‐reversible BLVR techniques (biological lung volume reduction, AeriSeal and thermal vapour ablation) and one‐way valves have traditionally been performed with the primary aim of achieving atelectasis. Occurrence of atelectasis after BLVR is associated with improved survival benefit (Hopkinson 2011) and is desirable for clearly improved functional results after lung volume reduction (Fessler 2005; Hopkinson 2005).
However, atelectasis happens only in a proportion (heterogeneous emphysema) of participants treated with valves (Fann 2003; Snell 2003; Toma 2003; Venuta 2005; Yim 2004). This may be explained by the phenomenon of collateral ventilation, which is described as "the ventilation of alveolar structures through passages or channels that bypass the normal airways" (Cetti 2006; Terry 1978). While resistance to interlobar collateral flow is too high to allow airflow in normal healthy lungs, the extensive damage caused by emphysema leads to increased resistance within segmental airways and, as a result, lower resistance to collateral flow. This means that trapped air can bypass obstructed passages via collateral passages, preventing atelectasis from occurring after BLVR using valves. Collateral ventilation is higher in participants suffering from homogenous emphysema and is related to the integrity of pulmonary fissures; the double‐fold of the membrane (pleura) that covers the lung parenchyma, which forms the distinct lung lobes (Higuchi 2006; Koster 2016).
A number of methods exist to facilitate participant selection (homogenous vs heterogeneous, presence vs absence of collateral ventilation). The proceduralist can rely on visual assessment or uses quantitative assessment of CT to determine disease heterogeneity (Valipour 2015). The potential presence of collateral ventilation can be assessed by determining fissure integrity using CT, complimented by using the Chartis system in case of endobronchial valves (Herth 2013; Schuhmann 2015). The Chartis system (Pulmonx Inc) causes temporary blockage of the target lumen via an inflatable balloon attached to a catheter, effectively mimicking the effect of the placement of a one‐way valve (Aljuri 2009), allowing collateral ventilation to be measured.
Although collateral ventilation reduces the efficacy of methods for BLVR in heterogeneous emphysema, it serves as the foundation for the efficacy of airway bypass stents, which aim to decrease air‐trapping and flow limitation by creating (artificial) collateral ventilation (Lausberg 2003; Rendina 2003). When new airway pathways are created from segmental airways to the lung parenchyma, trapped air can escape the lung, and this in turn can reduce hyperinflation (Choong 2008). As a result of the positive correlation of homogeneous emphysema and collateral ventilation (Higuchi 2006), this technique is especially interesting for participants suffering from homogeneous emphysema.
Why it is important to do this review
The burden of chronic illness is rising (Mannino 2007a). Healthcare costs related to COPD in general rise with disease severity, specifically as the result of (exacerbation‐related) hospitalisations (Dal 2008; Perera 2012). Although the mainstay treatment for moderate to severe emphysema focuses on medical treatment (GOLD; NICE 2010), a proportion of participants are not responsive and will not show functional improvements. Therefore, it is imperative to find effective and cost‐effective ways to improve disease outcomes and quality of life of participants with COPD, thereby preventing visits to the hospital (as the result of exacerbations).
One treatment option may be LVRS as it has been shown to lead to improved functional outcomes, survival and quality of life (Criner 2011, Van Agteren 2016), but selection criteria for this invasive procedure are strict, and procedures are under‐performed. LVRS has been shown to be particularly effective and cost‐effective for participants with upper lobe emphysema and low exercise capacity, as witnessed in the large National Emphysema Treatment Trial (NETT) (Criner 2008), leading to recommendations for this subgroup of participants in COPD treatment guidelines (e.g. NICE 2010). With the exclusion of the above mentioned subgroup, LVRS is generally however associated with higher (early) mortality or costs or both, which offsets some or all functional and palliative improvements over the short or long term (Criner 2008; Miller 2006; Ramsey 2007). This has added to healthcare professionals' misunderstanding of therapeutic risks (McNulty 2014) and overall therapeutic nihilism for an effective treatment for carefully selected emphysematous participants (Zoumot 2014). Furthermore, the traditional approach to LVRS focuses on treating participants with heterogeneous emphysema (Cooper 1995). Effects of LVRS on homogeneous emphysema, therefore, have hardly been studied, despite the potential benefit of this treatment for this group of participants (Weder 2009).
BLVR may be beneficial for participants with heterogeneous or homogeneous emphysema, and it may present a solution for participants who do not meet the stringent criteria for surgical lung resection and for those participants who do not wish to have surgery. Pilot and prospective studies on a variety of BLVR techniques have shown encouraging results in relation to lung function, exercise capacity and quality of life (Choong 2008; Emery 2010; Falkenstern‐Ge 2013; Fann 2003; Herth 2011; Herth 2011; Snell 2003; Snell 2012; Toma 2003; Venuta 2005; Yim 2004). Lack of significant morbidity and mortality encountered in several pilot studies, compared with possible complications and extensive recovery following a surgical procedure like LVRS, drives enthusiasm for BLVR as a suitable treatment option for participants with moderate to severe emphysema. Furthermore, some BLVR methods (valves, but not coils) might function as a less invasive first treatment, after which LVRS can still be considered when continued health status improvement is needed.
It is important to reiterate the crucial role of participant and treatment selection for BLVR (with specific reference to one‐way valves), as this is directly related to treatment effectiveness (Milanese 2016; Shah 2014). By performing subgroup analyses based on disease heterogeneity, the presence of collateral ventilation, and use of different lobar occlusion strategies (e.g. partial bilateral vs complete unilateral), this review furthermore hopes to establish which participants benefit best from each of the BLVR procedures, which may be used to further highlight the importance of participant selection and phenotyping.
Objectives
To assess the effects of BLVR on the short‐ and long‐term health outcomes in people with moderate to severe chronic obstructive pulmonary disease (COPD) and determine the cost‐effectiveness of each individual technique.
Methods
Criteria for considering studies for this review
Types of studies
We included individually or cluster randomized controlled trials (RCTs or cRCTs). We included studies reported as full text, those published as abstract only and unpublished data.
Types of participants
We included studies enrolling participants with moderate to very severe COPD. We excluded studies that recruited participants with giant or bullous emphysema, as giant bullous emphysema is a separate entity pathologically and radiologically (Mura 2005) and is treated by a different surgical procedure known as 'bullectomy'.
Types of interventions
We included studies comparing the following BLVR procedures versus standard medical care or sham bronchoscopy.
Endobronchial valves.
Intrabronchial valves.
Endobronchial coils.
Biologic lung volume reduction.
AeriSeal.
Bronchoscopic thermal vapour ablation.
Airway bypass stents.
Types of outcome measures
We assessed outcome measures per treatment type due to heterogeneity of treatment types and specific participant populations.
Primary outcomes
Percent change in forced expiratory volume in one second (FEV₁) (lung capacity).
Perioperative and postoperative mortality (survival).
Health‐related quality of life (e.g. St Georges Respiratory Questionnaire (SGRQ)).
Secondary outcomes
Improvement in lung function other than FEV₁ (e.g. residual volume (RV), total lung capacity (TLC)).
Exercise capacity (e.g. six‐minute walking distance (6MWD)).
Serious adverse events (e.g. pneumothorax).
Cost‐effectiveness.
Hospital utilisation (readmission, length of stay, and emergency department presentations).
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Information Specialist for the Group. This Register contains trial reports identified through systematic searches of bibliographic databases, including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED) and PsycINFO, and by handsearching of respiratory journals and meeting abstracts (see Appendix 1 for details). We searched all records in the CAGR on 20 April 2016 using the search strategy provided in Appendix 2.
We searched online clinical trials registers, including the ISRCTN registry, the UK Clinical Trials Gateway, ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform, for ongoing and recently completed studies.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references and searched relevant manufacturers' websites for trial information. We searched for errata and retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed) and reported within the review the date this was done.
Data collection and analysis
Selection of studies
Two review authors (JA and KH) independently screened for inclusion the titles and abstracts of all potential studies identified as a result of the search, and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved full‐text study reports or publications. Two review authors (JA and KH) independently screened the full texts, identified studies for inclusion and identified and recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion, or, if required, we consulted a third review author (KC). We identified and excluded duplicates and collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We re‐coded the selection process in sufficient detail to complete a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram (Moher 2009) and a 'Characteristics of excluded studies' table.
Data extraction and management
We used a custom‐made data collection form to record study characteristics and outcome data; this form had been piloted on at least one study in the review. Two review authors (JA and KC) extracted the following study characteristics from the included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study settings, withdrawals and date of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, concomitant medications and excluded medications.
Outcomes: primary and secondary outcomes specified and collected and time points reported.
Notes: funding for study and notable conflicts of interest of study authors.
Two review authors (JA and KH) independently extracted outcome data from included studies. We noted in the 'Characteristics of included studies' table if outcome data were not reported in a useable way. We resolved disagreements by reaching consensus or by involving a third review author (KC). One review author (JA) transferred data into the Review Manager file (RevMan 2014). We double‐checked that data were entered correctly by comparing data presented in the systematic review with those provided in study reports. A second review author (DG) spot‐checked the study characteristics for accuracy against the study report.
Assessment of risk of bias in included studies
Two review authors (JA and KH) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion or by consultation with another review author (KC). We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised 'risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different from that assigned for a participant‐reported pain scale). When information on risk of bias was related to unpublished data or correspondence with a study author, we noted this in the 'Risk of bias' table. When considering treatment effects, we took into account the risk of bias for studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the previously published protocol and reported deviations from it in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
Owing to heterogeneity in treatment approaches, we meta‐analysed outcomes only per treatment type (we did not calculate total effect size for all treatments combined). We analysed outcomes as continuous or dichotomous data using standard statistical techniques with a fixed‐effect model up to the end of follow‐up.
For continuous outcomes, we used mean differences (MDs) and 95% confidence intervals (CIs).
For dichotomous outcomes, we calculated odds ratios (ORs) with 95% CIs.
We attempted to calculate numbers needed to treat for additional harmful outcomes (NNTHs) from potential pooled ORs and reported these alongside the results of outcomes for which we have undertaken this calculation.
Unit of analysis issues
For multi‐arm trials, we planned to include each pair‐wise comparison separately but divided shared intervention groups approximately evenly among the comparators. However, if we deemed that the intervention groups were similar enough to be pooled, we combined these groups by using appropriate formulas, as provided in the Cochrane Handbook for Systematic Reviews of Interventions (Table 7.7a for continuous data and Chapter 16.5.4 for dichotomous data) (Higgins 2011).
For cluster RCTs, we expected to perform the analysis for all studies at the level of participants whilst accounting for clustering of data. For studies that did not adjust for clustering, we replaced the actual sample size with the effective sample size (ESS), calculated by using rho = 0.02, as per Campbell 2000. Studies may use a variety of statistical methods to investigate or compensate for clustering; we recorded whether studies used these methods and whether the significance of any effect was altered. When studies appeared homogeneous via a combination of the statistical I² statistic and homogeneity expressed by visual inspection of the data, we meta‐analysed data using a fixed‐effect model. However, in the presence of significant heterogeneity (as defined below under Data synthesis) we reported both a random and a fixed‐effect meta‐analysis.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible and necessary (e.g. when a completed study was identified as abstract only). When this was not possible, and when missing data were thought to introduce serious bias, we performed a sensitivity analysis to explore the impact of including such studies in the overall assessment of results.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the studies in each analysis. If we identified substantial heterogeneity, we reported this and explored possible causes by performing prespecified subgroup analyses.
Assessment of reporting biases
Provided at least 10 studies were deemed eligible for inclusion, we explored potential reporting biases by using a funnel plot. When we identified fewer than 10 eligible studies, we extrapolated potential reporting biases within the 'Other bias' section in the 'risk of bias' tables.
Data synthesis
We used a fixed‐effect model for data synthesis and performed a sensitivity analysis by using a random‐effects model.
'Summary of findings' table
We created a 'Summary of findings' table that includes the following outcomes.
% change in FEV₁.
Perioperative and postoperative mortality (survival).
Health‐related quality of life (e.g. SGRQ).
Improvement in lung function other than FEV₁ (e.g. RV, TLC).
Exercise capacity (e.g. 6MWD).
Serious adverse events (e.g. pneumothorax).
Cost‐effectiveness.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) (GRADEpro GDT) to assess the quality of a body of evidence as it related to studies that contributed data to meta‐analyses for prespecified outcomes. We adhered to the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and used GRADEpro software (GRADEpro GDT). We justified all decisions to downgrade or upgrade the quality of studies by using footnotes, and we made comments to aid the reader's understanding of the review when necessary.
Subgroup analysis and investigation of heterogeneity
We carried out the following subgroup analyses separated per treatment type.
Time: postoperative (up to six weeks), three months, six months, 12 months, 24 months.
Emphysema pattern: homogeneous versus heterogeneous emphysema.
For valves, we performed two extra subgroup analyses.
Collateral ventilation: present or absent (as determined by fissure integrity or Chartis).
Lobar occlusion strategy: did the trial aim to achieve complete lobar occlusion or not (e.g. partial bilateral vs complete unilateral).
We used the following primary outcomes in the proposed subgroup analyses.
FEV₁.
Mortality.
Quality of life.
The valves subgroup analyses for collateral ventilation were conducted for all outcomes due to the significant influence it had on treatment effectiveness. This was a post‐hoc decision made necessary due to important differences in participant selection in the trials in this comparison.
We used the formal test for subgroup interactions provided in RevMan 2014.
Sensitivity analysis
We performed reanalysis of the data if we observed significant heterogeneity as determined by an I2 statistic exceeding 60% (Higgins 2011) in combination with visual inspection of data indicating heterogeneity. We reported both fixed‐effect and random‐effects analyses when these yielded discordant results. We conducted sensitivity analyses for primary outcomes (FEV₁, mortality, quality of life) among studies reporting high or unclear risk of bias for both sequence generation and allocation concealment.
Results
Description of studies
Results of the search
An overview of the search can be found in Figure 1. The search yielded a total of fourteen RCTs comprising a total of 1979 participants and studying the following BLVR techniques: endobronchial valves (BeLieVeR HIFi 2015; IMPACT 2016; STELVIO 2015; VENT EU 2012; VENT US 2010), intrabronchial valves (Eberhardt 2012; IBV Valve trial 2014; Ninane 2012), AeriSeal (ASPIRE 2015), stents (Ease 2011), endobronchial coils (RENEW 2016; RESET 2015; Revolens 2016) and Vapour Ablation (STEP‐UP 2016). After removal of duplicates and title and abstract screening, the authors assessed 57 full‐text articles. Forty‐four were excluded but deemed relevant for the topic, and 14 trials were included in the qualitative synthesis, with 13 being eligible for quantitative synthesis.
1.
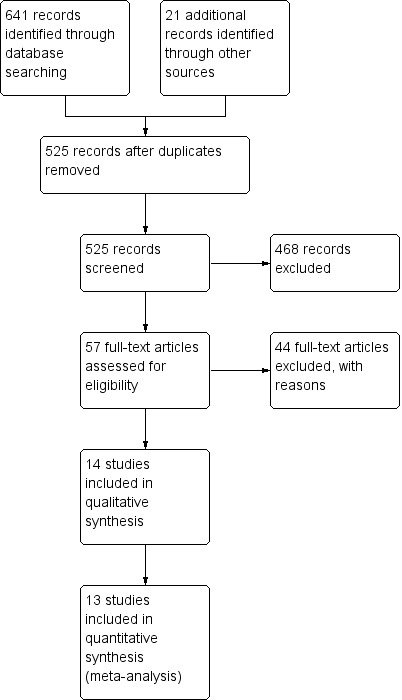
Study flow diagram.
Included studies
A complete overview of the included studies can be found in the Characteristics of included studies table.
Design
All of the included studies were RCTs. A number of studies allowed cross‐over from control to intervention after the initial follow‐up was completed. ASPIRE 2015 planned to follow control participants for 12 months after which the participants would be reassessed for eligibility for treatment with AeriSeal. Due to early termination, this however did not occur. STELVIO 2015 allowed participants who completed six‐month follow‐up in the control condition to cross over to the treatment with endobronchial valves. RESET 2015 followed control participants for 90 days after which these participants received treatment with endobronchial coils. Revolens 2016 indicated that a cross‐over group was planned after the 12‐month follow‐up, but results have not yet been published for that time‐frame. IMPACT 2016 is a currently ongoing randomized cross‐over trial, but results have been published for the three month interval, with extra follow‐up planned. Participants in the control condition were allowed to cross over after six‐month follow‐up. BeLieVeR HIFi 2015, Eberhardt 2012, IBV Valve trial 2014Ease 2011, Ninane 2012, RENEW 2016, STEP‐UP 2016, VENT EU 2012 and VENT US 2010 did not allow for cross‐over. We restricted data extraction to the period before cross‐over.
Three studies reported follow‐up at three months (BeLieVeR HIFi 2015;IMPACT 2016; Ninane 2012) and five studies followed up at six months (ASPIRE 2015; IBV Valve trial 2014; STELVIO 2015; STEP‐UP 2016; VENT EU 2012). The remainder of the studies reported outcomes at 12‐month follow‐up (Ease 2011; RESET 2015; RENEW 2016; Revolens 2016; VENT US 2010).
Sample sizes
The study sample sizes ranged from 22 (Eberhardt 2012: 11 to treatment and 11 to control) to 321 (The United States cohort of the Endobronchial Valve for Emphysema Palliation Trial (VENT US 2010): 220 to treatment and 101 to control). Most studies had a sample size of fewer than 100 participants (ASPIRE 2015; BeLieVeR HIFi 2015; Eberhardt 2012; IMPACT 2016; Ninane 2012; RESET 2015; STELVIO 2015; STEP‐UP 2016). The remaining 6 studies had over 100 participants. (Ease 2011: IBV Valve trial 2014; RENEW 2016; Revolens 2016; VENT EU 2012; VENT US 2010)
Setting
The majority of studies were multi‐centre studies, with the exception of BeLieVeR HIFi 2015, a single‐centre study conducted in the United Kingdom, the STELVIO 2015 study, which took place at a university teaching hospital (University Medical Center Groningen, the Netherlands), and the Eberhardt 2012 study, which was conducted at the University of Heidelberg, Germany. ASPIRE 2015 was a multi‐centre study involving 37 centres in multiple countries including France, Greeece, Italy, the Netherlands, Spain and the US. Ease 2011 was conducted at multiple sites in Australia, Brazil, Canada, Europe (including Austria, Brazil, Germany, Ireland, the Netherlands, Spain and the UK) and the US. RESET 2015 was a multi‐site study involving three centres in the UK, while Revolens 2016 was a multi‐site study conducted in nine French centres. VENT EU 2012 and VENT US 2010 were multi‐site studies belonging to the same overarching study, but were split into a European cohort (VENT EU 2012; 23 centres) and a United States (US) cohort (VENT US 2010; 31 centres). IBV Valve trial 2014 was a multi‐centre study conducted at 26 centres in the US. Ninane 2012 was a multi‐centre study conducted in several European countries including Austria, Belgium, Germany, Italy, Spain and the UK. STEP‐UP 2016 was a multi‐site study involving ten hospitals in Europe and three in Australia. Lastly, RENEW 2016 was a multi‐site study across 21 North American and five European sites, and IMPACT 2016 was conducted in Austria, Germany and the Netherlands.
Participants
Demographics
The average age of the participants ranged between 58 and 65 years of age, with STELVIO 2015 having the youngest average population (58 to 59 years of age) and IBV Valve trial 2014 and VENT US 2010 having the highest average age ranging between 64.7 and 64.8, and 64.9 and 65.3, respectively. The majority of the studies recruited more males than females (ASPIRE 2015; BeLieVeR HIFi 2015; Ease 2011; Eberhardt 2012; IBV Valve trial 2014; Ninane 2012; RESET 2015; Revolens 2016; VENT EU 2012), with only five studies recruiting a majority of females (STELVIO 2015; STEP‐UP 2016; RENEW 2016; IMPACT 2016; VENT US 2010).
Baseline disease status
Inclusion criteria were similar for the included studies, but slight differences were found. Participants needed to be older than 35 or 40 years of age, needed to be non‐smoking (quit smoking time ranged between two and six months) and have low baseline lung function. Specifically, post‐bronchodilator FEV1 inclusion criteria needed to be between 15% and 45% or 50% predicted in all studies except STELVIO 2015 (60% predicted). TLC needed to be > 100% predicted and the inclusion criteria for RV% predicted needed to be > 150% in all studies except for RENEW 2016 (> 175%), Ease 2011 (> 180%), IMPACT 2016 (> 200%) and Revolens 2016 (> 220%). RENEW 2016 initially required a minimum of 225% predicted, but adjusted the criteria midway through the study. The majority of studies furthermore demanded an exercise capacity of > 140 or 150 meters on 6MWD and a score on the modified Medical Research Council (mMRC) dyspnoea scale of > 2, with the exception of STELVIO 2015 who demanded an mMRC score of > 1. These entry criteria led to the following baseline values. Baseline lung function of the included participants was low, with average FEV1 ranging between 23.2% and 33.8% predicted, RV ranging between 179.0% and 258% predicted and TLC ranging between 124.0% and 145.4% predicted. Average scores on the SGRQ ranged between 54.0 units and 70.65 units and average distances on 6MWD between 293.7 and 377.0 meters.
Disease distribution
The majority of the studies on endobronchial valves only included participants with a heterogenous disease distribution (BeLieVeR HIFi 2015; VENT EU 2012; VENT US 2010), the exception being STELVIO 2015, who targeted both homogenous and heterogeneous disease, and IMPACT 2016 who only tested homogenous disease. Two intrabronchial valve studies (IBV Valve trial 2014; Ninane 2012) only targeted participants with upper‐lobe heterogeneous disease, while the other, Eberhardt 2012, recruited participants with upper‐ or lower‐lobe predominant emphysema. As expected, the Ease 2011 study on airway stents only recruited participants suffering from homogenous disease and the studies testing vapour ablation (STEP‐UP 2016) and AeriSeal (ASPIRE 2015) only recruited participants suffering from upper‐lobe predominant heterogenous disease. The three coil studies (RENEW 2016; RESET 2015; Revolens 2016) included both participants with homogenous and heterogeneous disease.
Interventions
AeriSeal
The ASPIRE 2015 study performed BLVR using AeriSeal. All participants were required to have a prerandomization pulmonary rehabilitation (minimum 12 sessions, eight to 10 weeks duration). After randomizations, participants in the treatment group received a 7‐day steroid taper and prophylactic antibiotic course aimed at decreasing the acute inflammatory response after treatment that was found in several pilot studies. The procedure was aimed at treating two upper‐lobe subsegments in each lung in a single session. The most severely damaged segments were treated after they were identified per CT review. After treatment, participants received a 3‐day prophylactic nonsteroidal anti‐inflammatory drug regimen in combination with stress ulcer prophylactics. Both groups received postrandomization pulmonary rehabilitation of at least 10 sessions (eight to nine weeks duration).
Airway stents
Ease 2011 performed a sham‐controlled study on the use of airway stents. All participants had to undergo 16 sessions of prerandomization pulmonary rehabilitation over the course of six to 10 weeks. Participants were then randomly allocated 2:1 to airway bypass or sham control. In the airway bypass group, up to six stents were placed per individual based on pre‐procedure assessment via CT (up to two stents per lobe, excluding the right middle lobe). The sham control group received the same procedure but no airway bypass passages were created or stents placed. All participants had to complete at least 10 sessions of pulmonary rehabilitation for at least eight weeks duration.
Endobronchial coils
RENEW 2016 randomized participants who were on optimal medical therapy to usual care according to the Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (GOLD) criteria, or usual care plus treatment with endobronchial coils. Participants needed to complete a pulmonary rehabilitation program or be performing maintenance pulmonary rehabilitation, and were encouraged to use bronchodilators with or without corticosteroids. The treatment group went on to receive 10 to 14 coils in two sequential treatments in two contralateral lobes separated by four months recovery time. The target lobes were identified in a radiology laboratory and emphysema distribution was assessed using a semi‐quantitative visual assessment.
RESET 2015 randomized participants who were on optimal medical therapy in a 1:1 ratio to treatment with endobronchial coils or best medical care. Optimal medical therapy consisted of previous pulmonary rehabilitation with maintenance of exercises, long acting anticholinergics and beta agonists in combination with inhaler corticosteroids and other pharmacological treatments, as deemed appropriate.The treatment group received bronchoscopy treatment at two time‐points separated by a month (if the participant was stable enough). High resolution CT was used to determine the worst affected lung, which was treated first, followed by the contralateral lung. Up to 14 coils were placed evenly to achieve maximal effect.
Revolens 2016 randomized participants in a 1:1 ratio to endobronchial coils or usual care, usual care being prerandomization pulmonary rehabilitation, inhaled bronchodilators, influenza and pneumococcal vaccination, with or without inhaled corticosteroids and with or without oxygen. Participants in the treatment group received the same care in addition to coil treatment at two time points separated by one to three months. Approximately 10 coils were placed per lobe, starting with the most severely affected lobe. The National Emphysema Treatment Trial (NETT) visual assessment score (NETT 2001) was used to determine disease severity.
Endobronchial valves
BeLieVeR HIFi 2015 was a sham‐controlled parallel group RCT which randomized stable outpatients identified by a multi‐disciplinary COPD team who were on optimal medical care consisting of combined inhaled corticosteroids, long‐acting β2 agonist, and anti‐cholinergic agents. Radiologists identified the worst affected lobe using the NETT study scoring system (NETT 2001), which had to score 1 point over the other lobes and needed to have 90% of fissures intact. After randomizations, participants in the treatment group received unilateral valve placement, while participants in the control group received bronchoscopy and sham valve placement. While Chartis was used in the intervention group, target lobe selection was based on CT scans alone. BeLieVeR HIFi 2015 aimed to achieve complete lobar occlusion.
IMPACT 2016 was a randomized controlled crossover study of participants suffering from homogenous emphysema who were randomized in a 1:1 ratio to treatment with endobronchial valves or standard optimal medical treatment after assessment of collateral ventilation presence. Collateral ventilation was determined via Chartis, and participants who did not show collateral ventilation in either the primary or secondary target lobe, were included in the study. Participants in the treatment group received immediate placement of the valves with the intention of lobar occlusion. Participants in the control group were hospitalized for at least one day post bronchoscopy, while participants in the valve condition were hospitalized for at least 2 days. In case of no functional benefits at three months, participants in the treatment group were checked to see if removal or replacement of valves was necessary. If this was the case, follow‐up was done after removal or replacement of the valve, rather than at the initial follow‐up.
STELVIO 2015 randomized participants in a 1:1 ratio to endobronchial valve treatment or standard medical care in concordance with the 2007 GOLD guidelines. Participants needed to have nearly complete fissures between the target lobe and the adjacent lobe as determined by assessment of high‐resolution CT scans. After baseline assessment, participants got randomized and participants in the treatment group were checked for collateral ventilation using Chartis. If collateral ventilation was present, participants were automatically excluded. Endobronchial valves were placed under either general or conscious sedation. After the procedure was completed and lobar occlusion was verified. participants received a five day course of 25 mg prednisolone (once daily) starting two days before the treatment and a five day course of 250 mg azithromycin (once daily) starting on the procedure day. After randomizations (for control) and the procedure (for treatment), participants continued on standard medical care and physiotherapy.
VENT EU 2012 and VENT US 2010 randomized participants in a 2:1 ratio to unilateral valve placement or standard medical care. All participants underwent a prerandomization 6 to 8 weeks of pulmonary rehabilitation and optimal medical therapy in concordance with the 2001 GOLD guidelines. This included education (smoking cessation), medication (inhaled long‐acting beta‐agonist, an inhaled anticholinergic, or both). A maintenance pulmonary rehabilitation at home was recommended and oxygen therapy was given when deemed necessary. No sham procedure was used. Disease severity and distribution as well as eligibility of participants were assessed via high resolution CT. The target lobe was the most emphysematous lobe (upper or lower); heterogeneity needed to be extensive enough to support preservation of the adjacent ipsilateral lobe (excluding the middle lobe). All participants in the treatment group received prophylactic antibiotics for seven days prior to and following treatment. VENT EU 2012 and VENT US 2010 did not assess collateral ventilation prior to treatment, a major difference in participant selection criteria with the other three trials.
Intrabronchial valves
Eberhardt 2012 randomized participants in a 1:1 ratio to unilateral or bilateral treatment with intrabronchial valves. Unilateral treatment aimed to occlude a full lobe, thereby causing atelectasis, while bilateral treatment left one segment open to avoid atelectasis (and a supposed higher risk of pneumothorax). Participants received general anaesthesia and post treatment received standard postanaesthesia COPD management with bronchodilators and supplemental oxygen. Prophylactic antibiotics and a chest radiograph were received within 24 hours.
IBV Valve trial 2014 recruited participants with stable COPD and randomized them in a 1:1 ratio to partial bilateral treatment with intrabronchial valves or sham valve deployment (control). Partial bilateral valve placement was chosen to reduce the risk of adverse events, while reaching clinical effectiveness. The idea was that partial occlusion allowed redistribution of air to non‐treated lobes. All participants received optimisation of medical care according to American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines for management of stable COPD. Upper‐lobe emphysema and participant selection was determined via CT and perfusion scan.
Ninane 2012 randomized participants in a 1:1 ratio to either partial bilateral treatment with intrabronchial valves or a sham control group. Pulmonary rehabilitation was not a prerequisite for entry in the study, but participants needed to satisfy the ATS/ERS guidelines for management of stable COPD.
Vapour ablation
STEP‐UP 2016 recruited participants with upper‐lobe predominant heterogeneous emphysema. Participants in the control group received optimal medical care comprised of use of bronchodilators, use of inhaled corticosteroids, and recommendation of pulmonary rehabilitation, in line with the 2014 GOLD guidelines. Participants in the treatment group received the same treatment in addition to two‐staged vapour ablation. CT analysis was used to measure the tissue mass and air volume of each segment of the lung, and helped determine the specific treatment time that was needed to achieve the target dose of 8.5 calories per gram of lung tissue. One segment was targeted at the first treatment day, followed by up to two segments 13 weeks later during the second session. After each treatment session, the participants were continued on prophylactic broad spectrum antibiotics for over 14 days and were encouraged to remain active.
Outcomes
Change in FEV1
The majority of studies reported on mean percentage change in post‐bronchodilator FEV1 from baseline to end of follow‐up, either in percentage absolute change from baseline (BeLieVeR HIFi 2015; RESET 2015; Revolens 2016; VENT EU 2012; VENT US 2010) or percentage change in predicted value (Ease 2011; IBV Valve trial 2014; IMPACT 2016; RENEW 2016; STELVIO 2015; STEP‐UP 2016). ASPIRE 2015 and RENEW 2016 however reported median and interquartile ranges (IQR) rather than mean values in absolute change from baseline and change of percentage predicted respectively. Ninane 2012 only mentioned final values for FEV1 in litres. Ease 2011 reports changes from baseline in FEV1 (litres) rather than percentages.
A number of studies reported the percentage of responders to treatment. For FEV1, the studies in this review used different Minimal Clinically Important Differences (MCID) to determine which participant responded and which did not (Cazzola 2008). The specific MCID used to define the responders in FEV1 was reported next to the outcome.
Change in lung function other than FEV1
While FEV1 was by far the most used primary outcome, a number of other outcomes relevant to lung function were reported. Change in static lung volumes (RV and TLC) from baseline was mentioned by a number of studies, including BeLieVeR HIFi 2015, Eberhardt 2012, IBV Valve trial 2014, IMPACT 2016, Ninane 2012, RESET 2015, Revolens 2016, STELVIO 2015, STEP‐UP 2016, VENT US 2010. VENT EU 2012 only reported on TLC and not on RV and Ease 2011 as well as RENEW 2016 only provided information on RV. RV and TLC were reported by BeLieVeR HIFi 2015, RENEW 2016, Revolens 2016 and STELVIO 2015.
BeLieVeR HIFi 2015 reported differences in diffusing capacity of the lung for carbon monoxide (DLCO) between baseline and follow‐up. Ninane 2012 provided information on change from baseline on DLCO, partial pressure of oxygen (PAO2) and partial pressure of carbon dioxide (PACO2). IBV Valve trial 2014 only tested change from baseline in PA02 and PAC02. Ease 2011, IMPACT 2016, RESET 2015; Revolens 2016, STELVIO 2015, STEP‐UP 2016, VENT EU 2012 and VENT US 2010 did not report between‐group difference in gas transfer improvements. ASPIRE 2015 did not present any other lung function parameters other than FEV1.
Change in quality of life
The most popular tool to assess QoL was the SGRQ, which was used by all of the studies. The studies reported a number of additional measures of QoL. ASPIRE 2015, Ease 2011, Eberhardt 2012, IBV Valve trial 2014, IMPACT 2016, RESET 2015, Revolens 2016, STELVIO 2015, VENT US 2010 determined the level of dyspnoea via the modified Medical Research Council (mMRC) dyspnoea scale. BeLieVeR HIFi 2015 and IMPACT 2016 used the COPD assessment test (CAT) to determine QoL of their participants. STELVIO 2015 in addition to the SGRQ and mMRC, used the Clinical COPD Questionnaire (CCQ). Furthermore, the authors indicated that they used the EuroQol 5‐D 3L and the EQ‐VAS score 3L, but did not report the outcomes in the paper. VENT EU 2012 only reported on SGRQ and not on mMRC. IBV Valve trial 2014 and Ninane 2012, in addition to SGRQ and mMRC scores, reported SF‐36 scores. Eberhardt 2012 also used the BODE index (Body‐mass index, airflow Obstruction, Dyspnea, and Exercise).
Change in exercise capacity
Exercise capacity was tested in each of the studies using the 6MWD. Ease 2011, VENT US 2010, and VENT EU 2012, in addition to 6MWD, reported scores on endurance cycle ergometry. Eberhardt 2012 furthermore reported on scores on the Borg Scale of Perceived Exertion (BORG scale).
Adverse events and mortality
All trials reported on mortality and serious adverse events, both pulmonary and adverse events in other areas. Where reported, the proportion of participants experiencing adverse events was added into the meta‐analysis. The definition of adverse events used for the meta‐analysis differed per study and depended on data reported in the studies. All other adverse events were reported narratively.
Cost‐effectiveness and hospital utilization
The only studies reporting information on cost‐effectiveness were Revolens 2016 and, from a combined subgroup of participants, VENT EU 2012 and VENT US 2010. The economic evaluation for Revolens 2016 was conducted to allow for the calculation of quality‐adjusted‐life‐years (QALY) gained over 12 months follow‐up. Health‐related quality of life was measured using the EuroQoL‐5 and its utility values were based on French tariffs. The difference in QALYs was based on the difference between the utility scores of the intervention versus the control group. A two‐tiered approach was used to calculate cost‐effectiveness of endobronchial valves: one tier using costs during the trial (start of trial up to 12 months) and the other tier being a model‐based projection up to 10 years. Costs were acquired via the German diagnosis‐related group (DRG) reimbursement rates and were based on 37 EBV treated participants and 36 matched controls, with an average of 3.08 valves per treatment. The participants were selected on fissure integrity and high heterogeneity, as well as successful lobar occlusion. The official cost‐effectiveness subgroup analysis planned for the full VENT EU 2012 and VENT US 2010 was terminated (clinicaltrials.gov reference: NCT00137956).
ASPIRE 2015, IBV Valve trial 2014, Ninane 2012, RENEW 2016, RESET 2015, Revolens 2016, STELVIO 2015, STEP‐UP 2016 reported on hospital utilization. BeLieVeR HIFi 2015, Ease 2011, Eberhardt 2012, IMPACT 2016, VENT EU 2012 and VENT US 2010 did not specifically report on hospital utilization.
Excluded studies
A number of relevant studies were identified for this review but were excluded as they did not fit the inclusion criteria (see Characteristics of excluded studies). None of the excluded studies were RCTs.
AeriSeal
Falkenstern‐Ge 2013 was an N = 1 study, Fruchter 2016, Kramer 2013 and Magnussen 2012 were retrospective analyses, Kramer 2012 was a prospective single‐arm study and Herth 2011 was a phase‐I dose escalation study.
Airway bypass stents
Two studies were excluded because they were animal studies (Choong 2005; Choong 2006). Cardoso 2007 and Rendina 2003 were case series and Choong 2008 was an in vitro study.
BioLVR
Abumossalam 2016 was a non‐randomized prospective clinical study. Reilly 2007 was an open‐label phase I study while Refaely 2010 and Criner 2009 were phase II studies.
Endobronchial coils
Deslee 2014 was excluded as it was a feasibility study. Slebos 2012 and Herth 2010 were excluded as they were non‐controlled pilot studies. Hartman 2015 was a retrospective analysis of data belonging to Deslee 2014 and Slebos 2012. Kontogianni 2014 was excluded as it was a retrospective analysis.
Endobronchial valves
De Oliveira 2006, Snell 2003, Hopkinson 2005, Yim 2004, Hopkinson 2011 and Venuta 2011 were case series. Toma 2003 and Gompelmann 2010 were pilot and feasibility studies. Venuta 2005 was a prospective, non‐randomized, single centre longitudinal study. Wan 2006 was a retrospective analysis and Kotecha 2011 was a retrospective cohort study. Herth 2012 was a non‐randomized study and Pizarro 2015 was an observational study. Fann 2003 was an animal study.
Intrabronchial valves
Springmeyer 2009 was a case series and Wood 2007 was a non‐randomized multicentre study.
Vapour ablation
Snell 2009 was a feasibility trial and Emery 2010 was an animal study. Snell 2012 was an open‐label, single‐arm safety and efficacy clinical trial. Herth 2012 was a case series, while Gompelmann 2012 and Gompelmann 2013 were retrospective analyses.
Risk of bias in included studies
For an overview of the risk of bias for each study see Figure 2 and Figure 3.
2.
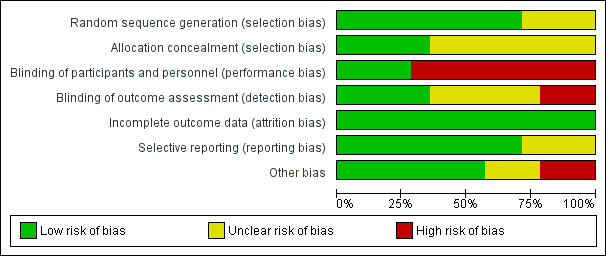
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
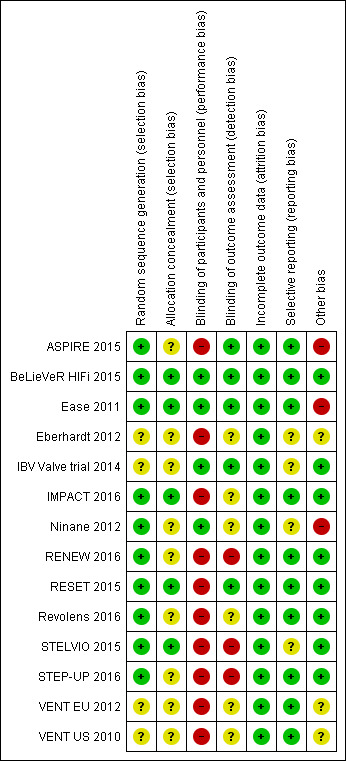
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
ASPIRE 2015, BeLieVeR HIFi 2015, Ease 2011, IMPACT 2016, RENEW 2016RESET 2015, Revolens 2016STELVIO 2015, were deemed to be at low risk of selection bias due to random sequence generation as they conducted random sequence generation via block randomizations. Eberhardt 2012, IBV Valve trial 2014, Ninane 2012, VENT EU 2012 and VENT US 2010 did not provide sufficient information to permit a judgement on whether the study was at high or low risk of selection bias, due to problems related to selection bias.
We judged allocation to be concealed in BeLieVeR HIFi 2015 and Ease 2011 as there was a procedure team and a participant recruitment and assessment team, leading to a classification of a low risk of selection bias. IMPACT 2016, STELVIO 2015 and RESET 2015 used sealed envelopes to which the study personnel did not have access to; thus, these studies were judged at low risk of selection bias. Triallists became aware of group allocation in Ninane 2012 after the participant was anaesthetized in numerical order, but it was not clear whether anyone was aware of the order of allocation and the study was therefore deemed to be at unclear risk of bias. IBV Valve trial 2014 mentioned in‐text that they performed allocation concealment but did not give specific information and was therefore deemed to be at unclear risk of bias. ASPIRE 2015, Eberhardt 2012, RENEW 2016, Revolens 2016, STEP‐UP 2016VENT EU 2012 and VENT US 2010 did not provide sufficient information to permit a judgement on whether the study was at high or low risk of selection bias, due to problems related to allocation concealment.
Blinding
BeLieVeR HIFi 2015, Ease 2011, IBV Valve trial 2014 and Ninane 2012 were sham‐controlled procedures in which the participant was unaware of the group he or she was in. While the proceduralist could not be blinded for obvious reasons, being sham‐controlled caused these studies to be classified as at low risk of bias. ASPIRE 2015 was an open‐label study; a sham procedure was not allowed by FDA mandate, and the study was therefore at high risk of bias. Eberhardt 2012, IMPACT 2016. RENEW 2016, RESET 2015, Revolens 2016, STELVIO 2015, STEP‐UP 2016, VENT EU 2012 and VENT US 2010 were also non‐blinded and were classified as being at a high risk of performance bias.
Outcome assessment was performed by blinded personnel in ASPIRE 2015, BeLieVeR HIFi 2015, Ease 2011, IBV Valve trial 2014 and RESET 2015, leading them to be classified as being at a low risk of detection bias. Eberhardt 2012, IMPACT 2016, Ninane 2012, Revolens 2016, STEP‐UP 2016, VENT EU 2012 and VENT US 2010 did not provide sufficient information to permit judgement and they were therefore deemed to be at an unclear risk of detection bias. STELVIO 2015 only reported that PFT measures were conducted by blind assessors (all other assessments were performed by unblinded assessors) leading this study to be classified as being at a high risk of detection bias. RENEW 2016 stated that lung function and walking distance were measured by blind assessors, but there was no information presented regarding whether the questionnaire was administered by blinded researchers, leading to an unclear risk of bias.
Incomplete outcome data
Attrition was reported in all studies, and was balanced in most studies (ASPIRE 2015; BeLieVeR HIFi 2015; Ease 2011; Eberhardt 2012; IMPACT 2016; Ninane 2012; RENEW 2016; RESET 2015; Revolens 2016; STEP‐UP 2016) and non‐existing in Eberhardt 2012, leading them to be classified as being at a low risk of attrition bias.The IBV Valve trial 2014, STELVIO 2015, VENT EU 2012 and VENT US 2010 had more attrition in the treatment groups, but this was not deemed sufficient to adjust the risk of bias assessment from high to low. .
Selective reporting
The study protocol was available as supplementary material for ASPIRE 2015, RENEW 2016 and RESET 2015, causing them to be classified as being at a low risk of bias. BeLieVeR HIFi 2015, Ease 2011, Revolens 2016, STEP‐UP 2016, VENT EU 2012 and VENT US 2010 published their protocol prior to the study being completed, leading them to be classified as being at a low risk of bias. Eberhardt 2012, IBV Valve trial 2014 and Ninane 2012 did not provide sufficient information to permit judgement and were classified as being at an unclear risk of bias. STELVIO 2015 indicated in their protocol that they also administered the MRC, EuroQol & EQ‐VAS, but did not report on the outcome. All studies were registered at a trial registry portal. IMPACT 2016 was registered on clinicaltrials.gov and deemed at low risk of bias.
Other potential sources of bias
ASPIRE 2015 was terminated due to business reasons, causing this study to be classified as being at a high risk of other bias. Ease 2011 was classified as being at a high risk of other bias, as the study design and coordination of data analysis was performed by the funder (although the trial investigators had access to the data). Ninane 2012 did not reach the intended number of participants and was discontinued for logistical reasons, causing it to be at a high risk of bias. All other studies were deemed to be at a low risk of other biases.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Aeriseal versus standard medical care
Effect on FEV1
Due to the premature termination of ASPIRE 2015, results were presented as median and interquartile range (IQR). Efficacy outcome data was available for 57 patients at 3 month follow‐up and for 34 patients at 6‐month follow‐up. At 3 month follow‐up ASPIRE 2015 reported median absolute improvement of 11.4% (IQR 2.0 to 32.0%) for Aeriseal compared to a reduction of 2.1% (IQR‐4.9 to 9.0%), significantly favouring Aeriseal over control, P = .004. At 6 month follow‐up, ASPIRE 2015 reported a median absolute percentage change from baseline of 18.9% (IQR ‐0.7 to 41.9%) for participants treated with Aeriseal versus 1.3% (IQR ‐8.2 to 12.9%) for participants in the control group, again significantly favouring Aeriseal. p=.043 (low quality evidence). The proportion of participants achieving the MCID, in this study defined as 12% according to American Thoracic Society (ATS) & GOLD standards, was 52.4% of participants in the Aeriseal group compared to 15.4% of the participants in the control group, P = .07.
Mortality (outcome 1.1)
ASPIRE 2015 reported a nonsignificant difference of two deaths in the treatment condition versus no deaths in the control condition (OR 2.90, 95% CI 0.14 to 62.15, low‐quality evidence, see Figure 4). It is important to note the wide confidence intervals for this outcome, with the lower bound reaching 0.14 and the upper bound reaching an OR of 62.15, suggesting that the provided OR of 2.90 is far from conclusive. The two deaths occurred at day 55 and day 65 after the procedure, meaning that none of the deaths took place during the postoperative stage and both deaths took place before the 90‐day follow‐up.
4.
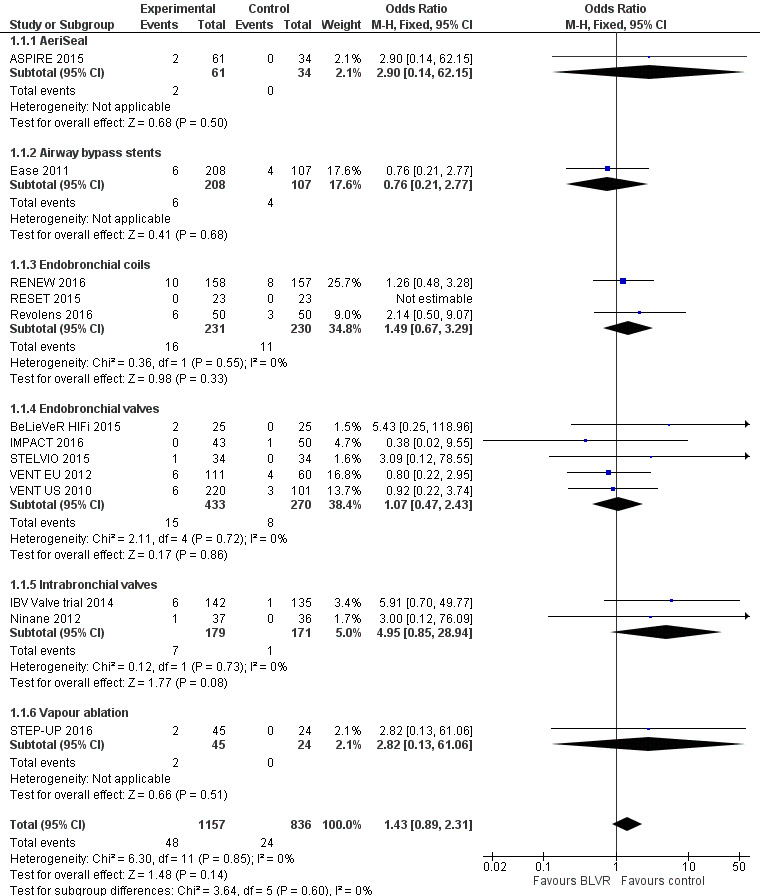
Forest plot of comparison: 1 BLVR to medical therapy, outcome: 1.1 Mortality (All methods, end of follow‐up).
Quality of life
At three‐month follow‐up, ASPIRE 2015 reported a significant difference favouring AeriSeal (‐11 units, IQR ‐18 units to ‐1 units) compared to control (‐4 units, IQR ‐6 units to 3 units) (P = 0.03). At the end of the follow‐up (six months), ASPIRE 2015 reported a significant difference favouring AeriSeal (‐12 units, IQR ‐22 units to ‐5 units) to control (‐3 units, IQR ‐5 units to ‐1 units) (P = 0.007, low‐quality evidence). ASPIRE 2015 furthermore reported a significant change from baseline between‐group difference in dyspnoea measured by the mMRC favouring AeriSeal (‐1.0 units, IQR ‐2.0 units to 0 units) versus control (0 units, IQR ‐0.8 units to 0.8 units) (P = 0.005) at three month follow‐up. This significant difference in mMRC scores did not persist at six months (P = 0.57). The proportion of participants reaching the MCID or higher (defined as a > 4 unit decrease) was not significantly different for control compared to AeriSeal for either SGRQ (76.2% vs 46.2%, P = 0.16) or mMRC (52.4% vs 38.5%, P = 0.66).
Improvement in lung function other than FEV1
ASPIRE 2015 did not test for any differences in RV, TLC, FVC or gas transfer values.
Exercise capacity
At end of follow‐up (six months), ASPIRE 2015 reported a significant (P = 0.02) median change from baseline of 31 meters (IQR 0 meters to 41.3 meters) for AeriSeal versus ‐22 meters (IQR ‐41.3 meters to 9.3 meters) for control (low‐quality evidence). They furthermore indicated that 52% of participants in the treatment group versus 0% of participants in the control group showed an improvement in the MCID (26 meters increase from baseline) (P = 0.003).
Adverse event rate (outcome 1.2)
ASPIRE 2015 reported that a total of 44% of participants treated with AeriSeal and a total of 18% of participants in the control condition suffered from adverse events requiring hospitalization (OR 3.71, 95% CI 1.34 to 10.24; moderate‐quality evidence), see Figure 5. The majority of these adverse events were respiratory. The percentage of adverse events requiring hospitalization was seemingly higher in participants reaching the MCID for FEV1 (67%) compared to those who did not (27%) although this difference did not reach statistical significance (P = 0.14).
5.
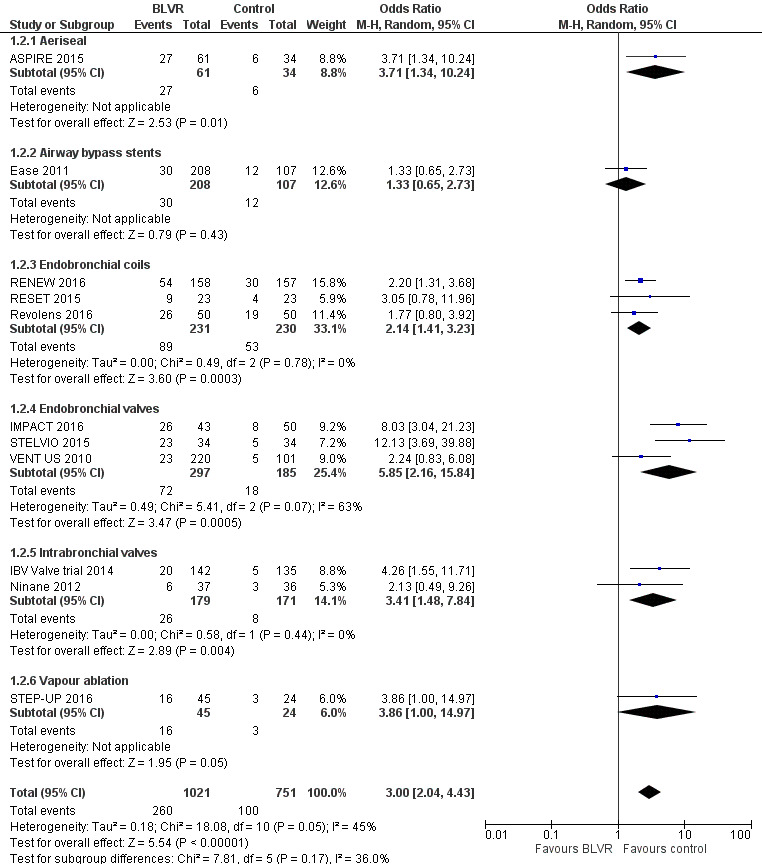
Forest plot of comparison: 1 BLVR to medical therapy, outcome: 1.2 Adverse Events (all methods, end of follow‐up).
ASPIRE 2015 found no serious adverse events in the periprocedural phase. The two deaths in the treatment group were caused by a myocardial infarction and pneumonia/COPD exacerbation at days 55 and 40, respectively. Three participants in the treatment versus none in the control experienced respiratory failure that required non‐invasive ventilation. The most common respiratory events were pneumonia, COPD exacerbations, post acute inflammatory response and pneumothorax. The incidence of respiratory failure and pneumonia in the first 30 days was 10.2% in the treatment group. The post‐procedure adverse event rate requiring hospitalization was significantly higher in the treatment condition (44%) compared to the control (18%) condition (P = .01).
Cost‐effectiveness
ASPIRE 2015 did not report on cost‐effectiveness.
Hospital utilization
ASPIRE 2015 indicated that participants in the treatment group spent at least one night in hospital for observation. Mean hospitalisation duration was 5.3 days (SD 17.9) for the treatment condition.
Airway bypass stents versus standard care
FEV1
Ease 2011 provided detailed data on outcomes at perioperative, three‐month, six‐month and 12‐month follow‐up. There was an initial significant between‐group difference favouring airway bypass stents at one day follow‐up (MD 3.00%, 95% CI 2.01% to 3.99%). This significant between‐group difference did not remain significant at one month, (MD 0.40%, 95% CI ‐0.39% to 1.19%), three‐month (MD 0.50%, 95% CI ‐0.29% to 1.29%), six‐month (MD 0.30%, 95% CI ‐0.49% to 1.09%) or 12‐month follow‐up (MD 0.95%, 95% CI ‐0.16% to 2.06%) (1 study; number of participants = 315, high‐quality evidence). Stents are only used in homogenous emphysema, therefore no differences based on disease distribution were reported.
Mortality (outcome 1.1)
Ease 2011 reported a nonsignificant difference of six deaths in the treatment group (number of participants = 208) versus four in the control (number of participants = 107) (OR 0.76, 95% CI 0.21 to 2.77; moderate‐quality evidence, see Figure 4). Sufficient information was not provided to determine the specific times at which the deaths occurred.
Quality of life
Ease 2011 provided total scores for the SGRQ, indicating no significant mean difference between the two groups at 12‐month follow‐up (‐2.00 units (95% CI ‐5.58 to 1.58), high‐quality evidence). Total scores significantly differed only at the one‐month follow‐up (P = 0.006), but failed to show a significant difference at other endpoint times. Change from baseline scores on the mMRC dyspnoea scale were not significantly different between treatment (‐0.41 units, SD 1.0) and control (‐0.25, SD 1.0) at end of follow‐up (P = 0.21). Change from baseline scores on mMRC significantly differed between groups only at six‐month follow‐up, with scores favouring AeriSeal (‐0.47, SD 1.0) over control (‐0.22, SD 0.9) (P = 0.05).
Improvement in lung function other than FEV1
Ease 2011 did not find a significant reduction from baseline in RV when comparing stents (‐0.06 L, SD 0.7) with control (‐0.10 L, SD 0.6) (P = 0.72, high‐quality evidence). TLC and gas transfer values were not reported. Furthermore, there was no significant change from baseline between‐group difference in FVC at 12‐month follow‐up for stents (‐0.08 L, SD 0.50) compared to control (0.00 L, SD 0.40) (P= 0.21, high‐quality evidence).
Exercise capacity
Ease 2011 did not find a significant difference between airway stents at end of follow‐up (281 meters, SD 109) and control (297 meters, SD 94) (P = 0.26, high‐quality evidence).
Adverse event rate (outcome 1.2)
While the overall rate of composite safety events was higher in participants treated with stents (14.4%) compared to control (11.2%), this figure did not reach statistical significance (OR 1.33, 95% CI 0.65 to 2.73; high‐quality evidence, Figure 5). These composite safety endpoints happened in 30 participants in the treatment versus 12 participants in the control group and entailed five severe adverse events (Ease 2011). Beyond the composite safety events, however, respiratory serious events (pneumothorax, haemoptysis, and COPD exacerbations) were more frequent in participants treated with stents. Major haemoptysis occurred in one participant in the treatment group, while no cases were identified in the control group. Four participants in the treatment condition required ventilation as a result of respiratory failure, versus none in the control group. Pulmonary infection or exacerbation of COPD that required admission for more than seven days happened in 22 participants in the treatment group versus nine in the control group. Two participants suffered a pneumothorax that lasted longer than seven days, while in three participants the pneumothorax was treated within seven days.
Cost‐effectiveness
Ease 2011 did not report on cost‐effectiveness.
Hospital utilization
Ease 2011 reported a mean procedure time of 107 minutes (SD 29) for treatment compared to 60 minutes (SD 5) for control.
Endobronchial coils versus standard care
FEV1
FEV1 at end of follow‐up (outcome 2.1)
Data from the RESET 2015 and Revolens 2016 trials could be meta‐analysed for the effect of coils on absolute percentage change from baseline in FEV1 at end of follow‐up, and results indicated a significant mean difference of 10.88% (95% CI 5.20 to 16.55; number of participants = 146; studies = 2, low‐quality evidence). RESET 2015 furthermore published interim results for the treatment group with the cross‐over group combined, reporting a significant change from baseline in FEV1 of 8.9% (SD 22.1%) (P = 0.02) at 12‐month follow‐up. No results for control were reported. RENEW 2016 provided median values percentage change from baseline, indicating a significant between‐group difference favouring endobronchial coils (3.8%, IQR ‐6.3% to 16.1%) over control (‐2.5%, IQR ‐8.9% to 4.4%) (one sided P < 0.001). At 90 days, RESET 2015 found that 57% of participants treated with coils compared to 26% of participants treated by control reached the MCID of 10% in FEV1 (P = 0.07).
FEV1 stratified per follow‐up period
RENEW 2016 provided final absolute values at different time points. At one month after the first treatment, the authors reported a significant difference of 0.05 L (95% CI 0.00 L to 0.10 L) and after the second treatment that absolute difference remained significant at 0.08 L (95% CI 0.03 L to 0.13 L). At nine‐month follow‐up and twelve month follow‐up, absolute values remained significantly higher in the participants treated with endobronchial coils compared to control, 0.08 L (95% CI 0.02 L to 0.14 L) and 0.06 L (95% CI 0.01 L to 0.11 L), respectively. RESET 2015 reported values for the treatment group, but not for the control group, at 90 days, six months and 12 months. The study found a favourable change from baseline scores for coils at 90 days (13.8%, SD 18.1, P < 0.0001), 180 days (10.0%, SD 21.1, P = 0.005) and 360 days (8.9%, SD 22.2, P = 0.02). Revolens 2016 found an absolute mean difference of 8.00% (95% CI ‐3.91 to 19.91) at six months and an absolute mean difference of 11.00% (95% CI 5.29 to 16.71) at 12 month‐follow‐up.
FEV1 stratified per disease distribution
RESET 2015 did not find a significant difference (P = 0.83) in the effect of endobronchial coils on absolute change in FEV1 from baseline for participants suffering from heterogeneous (6.9%, SD 18.8) versus homogenous (10.1%, SD 24.5) emphysema. Revolens 2016 reported no difference between participants suffering from heterogeneous emphysema and those suffering from homogenous emphysema who were treated with coils (6% (SD 13.6) and 10% (SD 19.7), P = 0.55). RENEW 2016 conducted a post hoc analysis based on four subgroups; where participants had a minimum RV of 225% or higher and heterogenous or homogenous emphysema, but the study was not powered to detect significant subgroup differences. Participants with heterogenous disease who had a baseline RV of more than 225% predicted showed a median between‐group improvement of 12.3% favouring coils (95% CI, 1.6% to 22.1%). A slightly smaller between‐group difference was found for participants who suffered from homogenous disease with an RV of more than 225% (median 8.3%, 95% CI 3.5% to 12.9%). The results for participants showing an RV of less than 225% predicted were smaller, with a median between‐group difference of 2.8% (95% CI ‐19.0% to 16.6%) for participants with heterogeneous disease and 3.5% (95% CI ‐4.0% to 11.1%) for participants with homogenous disease.
Mortality (outcome 1.1)
Data from RENEW 2016, Revolens 2016 and RESET 2015 could be meta‐analysed for effect of endobronchial coils on mortality at end of follow‐up. The authors found no significant difference between the treatment and control groups (OR 1.49 (95% CI 0.67 to 3.29; number of participants = 461; studies = 3, moderate‐quality evidence, see Figure 4). When looking at different time‐points, RENEW 2016 found two cases of mortality in the treatment group compared to none in the control group at one‐month (postoperative) follow‐up, which is a nonsignificant difference (OR 5.03, 95% CI 0.24 to 105.66). RESET 2015 furthermore reported on deaths at a later time period for the treatment group only, indicating that five participants died during the 12‐month follow‐up (all of whom had crossed over from the control group to the treatment group after 90 days), one participant who crossed over died during the 90‐day follow‐up, two died during six‐month follow‐up and two died during 12‐month follow‐up. Revolens 2016 reported mortality for six‐month follow‐up, finding three deaths in the endobronchial group versus one in the control group.
Quality of life (outcome 2.2)
Data from RENEW 2016, RESET 2015 and Revolens 2016 could be meta‐analysed for change from baseline score on the SGRQ, indicating a mean between‐group difference of ‐9.14 units (95% CI ‐11.59 units to ‐6.70 units; number of participants = 461; studies = 3, moderate‐quality evidence) favouring endobronchial coils over ‐control. RESET 2015 furthermore provided longer‐term data for the treatment group, indicating a 6.1 unit reduction (SD 14.0) in SGRQ (P = 0.01) at 12‐month follow‐up. RESET 2015 reported that 65% of participants treated with coils showed a reduction > 4 points on SGRQ and 57% > 8 points on SGRQ compared to 22% and 13% for control, both differences reaching significance (P = 0.01).
When looking at the effect of endobronchial coils for different disease distributions, RESET 2015 did not find a significant difference in change from baseline scores in SGRQ between participants suffering from heterogenous emphysema (‐5.9 units (SD 13.1)) compared to homogenous emphysema (‐6.2 units (SD 15.2), (P = 0.961), nor did Revolens 2016 find a difference between heterogenous and homogenous emphysema on the SGRQ (‐2 units (95% CI ‐11 units to 7 units), P = 0.83).
RENEW 2016 split the results for disease distribution, as mentioned earlier, based on RV% predicted at baseline. Participants with heterogenous disease who had a baseline RV of more than 225% predicted showed a median between‐group decrease of ‐10 units favouring coils (95% CI, ‐16.3 to ‐3.9). Participants who suffered from homogenous disease with an RV of more than 225% showed a between‐group median difference of ‐10.0 units on SGRQ (95% CI ‐13.3 to ‐6.7) favouring coils over control. Participants suffering from heterogeneous disease with an RV% predicted of 225% showed a decrease of 9.9 units (95% CI ‐26.8 to 7.1). Participants suffering from homogenous disease with a baseline RV of < 225% predicted had a between‐group difference of ‐3.3 units (95% CI ‐8.9 to 2.4). Revolens 2016 also found a significant mean difference of ‐0.4 units on the mMRC dyspnoea scale favouring coils over control (P = 0.02), at end of follow‐up. RESET 2015 did not find a significant difference in mMRC scores at 90‐day follow‐up (MD ‐0.15 (‐0.6 to 0.3), P = 0.5).
Improvement in lung function other than FEV1 (outcomes 2.3 to 2.5)
Data from RENEW 2016, RESET 2015 and Revolens 2016 could be meta‐analysed for change from baseline RV; the authors found a significant mean difference of ‐0.32 L (95% CI ‐0.48 to ‐0.17; number of participants = 461; studies = 3, high‐quality evidence) favouring endobronchial coils over control. No significant difference between treatment and control was found after meta‐analysing data for TLC provided by RESET 2015 and Revolens 2016 (MD ‐0.19 L, 95% CI ‐0.43 to 0.06; number of participants = 146; studies = 2, moderate‐quality evidence). Data from RENEW 2016 and Revolens 2016 could also be meta‐analysed for the effect of endobronchial coils on RV/TLC; results indicated a between‐group mean difference of ‐3.74 (95% CI ‐5.16 to ‐2.33; number of participants = 415; studies = 2, high‐quality evidence) favouring coils over control. Revolens 2016 furthermore reported a significant between‐group mean difference in FVC favouring the endobronchial coil group (10% (SD 34.9)) .
RESET 2015 in data on the cross‐over 12‐month follow‐up, reported a significant change from baseline in RV for the treatment group at 12 months of ‐0.32 L (SD 0.77, P = 0.02), but did not provide data on the control group. Similarly, they reported 8.4% (SD 16.3, P = 0.03)) change from baseline in FVC and a 4.3% (SD 10, P = 0.01) reduction in the ratio between RV and TLC, for the treatment condition only. They did not find a significant change in baseline for TLC in the coil group (‐0.13 L (SD 0.48), P = 0.12), at 12 months.
No data on gas transfer values was provided.
Exercise capacity (outcome 2.6)
Data from RENEW 2016, RESET 2015 and Revolens 2016 could be meta‐analysed; the authors found a significant change from baseline difference of 29.50 meters favouring endobronchial coils over control (95% CI 11.94 to 47.06; number of participants = 461; studies = 3; I2 = 69%, low‐quality evidence). The data were re‐analysed using a random‐effects model because there was significant heterogeneity; this resulted in a nonsignificant mean difference of 30.85 meters (95% CI ‐1.05 to 62.76). Visual inspection of the plots showed that heterogeneity was due to the RESET 2015 trial which indicated larger increases in 6MWD than did the other trials. The confidence interval of the result was very large, meaning that caution needs to be taken when interpreting these results.
RENEW 2016 mentioned a between‐group difference of 11.8% in participants reaching the MCID, which favoured endobronchial coils over control P = 0.01), while Revolens 2016 reported 36% of participants treated with coils compared to 18% of those treated by control to had reached the MCID of at least 54 meters (P = 0.03). RESET 2015 found a striking 74% in the intervention condition compared to 17% in the control condition reaching the MCID (P < 0.0003).
Adverse event rate (outcome 1.2)
RENEW 2016 reported a composite score for major complications, which could be meta‐analysed and serious adverse events were reported by RESET 2015 and Revolens 2016. All other adverse events were reported narratively per study (see below). There was an overall higher number of adverse events for participants treated with coils compared to those treated by control (OR 2.14, 95% CI 1.41 to 3.23; number of participants = 458; studies = 3, see Figure 5). RENEW 2016 reported a significant overall higher number of major adverse events (n = 54, 34.8%) in the treatment group compared to the control group (n = 30, 19.1%) (P = 0.002). The authors noted that this was mainly due to an increased number of lower respiratory tract infections (18.7% for intervention versus 4.5% for control, P = 0.001). Two deaths were directly related to the procedure, with one participant developing a pulmonary haemorrhage and respiratory failure which led to cardiac arrest and one participant dying of respiratory failure after the second coil treatment. Participants in the treatment group had significantly more cases of pneumonia (n = 31, 20%) versus control (n = 7, 4, 5%) and pneumothorax (n = 15, 9.7%) versus control (n=1, 0.6%). RESET 2015 reported six serious adverse events in the treatment group (two exacerbations requiring hospitalization, two lower respiratory tract infections and two pneumothoraces) compared to one in the control group (exacerbation) during the treatment recovery period (P = 0.02). In the post‐treatment period, another three exacerbations occurred in the treatment group compared to two exacerbations and a lower respiratory tract infection in the control group (P > 0.99). At 12‐month follow‐up, there were another two exacerbations in the treatment group, a case of chest pain, a lower respiratory tract infection, and a case of pneumonia. Participants with pneumothoraces showed better functional improvements on SGRQ, RV, 6MWD, FEV1, mMRC than those who did not suffer from pneumothorax. Revolens 2016 found that pneumonia was the most frequent adverse event, occurring 11 times in the coil group versus twice in the control group (P = 0.03). Safety composite end‐scores for serious adverse events were significantly higher in treatment (17 events) compared to control groups (eight events) groups (P = 0.05). The most common serious adverse events were COPD exacerbations and pneumonia.
Cost‐effectiveness
Revolens 2016 conducted an extensive health economic evaluation for their preliminary results on endobronchial coils, showing a mean cost difference per participant of USD 47.91 favouring control over endobronchial coils (P < 0.001, moderate‐quality evidence). The biggest proportion of extra costs in the treatment condition were the device cost (average USD 38,609 over two treatments) and costs due to hospitalization (average USD 5,765 over two treatments). Other significant between‐group cost differences stemmed from transportation costs (USD 351 versus USD 160) and costs for imaging (USD 166 versus USD 139) each favouring control. The 12‐month incremental cost‐effectiveness ratio (ICER) determined by the study was USD 782,598 per QALY gained. The majority of the costs was for medical devices (mean cost USD 20,214.00 for first treatment and USD 18,395.00 for the second treatment).
RENEW 2016 and RESET 2015 did not report on cost‐effectiveness.
Hospital utilization
RENEW 2016 found a mean procedure duration of 42 minutes (SD 16) with an average hospital duration of 1.1 nights (range 0 to 15 nights). RESET 2015 reported a mean procedure time of 44.9 minutes (SD 17.4). Most participants were discharged one day after the procedure. Three treatments resulted in hospital stays of two days and one resulted in a hospital stay of three days. Length of stay in Revolens 2016 was 3.1 days for the first treatment and 3.6 days for the second treatment. The mean number of rehospitalizations was 0.5 for treatment versus 0.2 for control (P = 0.21). There was no significant difference in the number of participants requiring more than one rehospitalization (P = 0.16).
Endobronchial valves versus standard care
FEV1
FEV1 at end of follow‐up (outcome 3.1)
Results from BeLieVeR HIFi 2015, IMPACT 2016, STELVIO 2015, VENT EU 2012 and VENT US 2010 could be included in a meta‐analysis for the effect of endobronchial valves on percentage change from baseline in FEV1 at end of follow‐up. The results indicated a significant between‐group standardized mean difference (SMD) of 0.48 (95% CI 0.32 to 0.64; number of participants = 703; studies = 5; low‐quality evidence), favouring treatment with endobronchial valves. A standardized mean difference was required as STELVIO 2015 and IMPACT 2016 reported percentage change of the predicted value, while the remaining studies used absolute percentage change.
While positive, the result of this meta‐analysis needs to be interpreted with caution. Firstly, it lumped together trials that did (BeLieVeR HIFi 2015; IMPACT 2016; STELVIO 2015) and did not (VENT EU 2012; VENT US 2010) determine the presence of intact fissures (and thus tried to exclude participants with collateral ventilation) in the participant sample, which significantly impacted the treatment efficacy (see outcome 3.4 below). Secondly, the mean changes in FEV1 in all studies showed very high standard deviations, ranging from 21.8% to 40.7%, which may suggest considerable skew in the data. This skew became apparent when for instance comparing the median change reported by BeLieVeR HIFi 2015 (8.77% for participants treated with valves and 2.88% for control, with the mean change reported; 24.8% for coils versus 3.9% for control). Thirdly, the results by STELVIO 2015 contributed to significant heterogeneity, as their results were considerably better than the other trials. This may be the result of their treatment strategy: more vigorous monitoring of participants and proactively replacing valves throughout the trial, to ensure better fit of the valves, thereby improving outcomes.
BeLieVeR HIFi 2015 reported that nine participants treated by valves, compared to only one participant treated by control, reached the MCID of 15% or more (P = 0.0022. Similarly, IMPACT 2016 reported significantly more participants treated with valves (34.9%) compared to control (4%) reaching the MCID of 15% or higher improvement in FEV1 (P = 0.0001. STELVIO 2015 found that 72% of participants treated with valves compared to 24% treated by control reached the MCID of 10% (P < 0.001). VENT US 2010 found that 28.6% for participants treated by valves and only 5.4% treated by control reached their MCID of 15% (P<.001).
FEV1 stratified per follow‐up (outcome 3.2)
Data from BeLieVeR HIFi 2015 and IMPACT 2016 could be meta‐analyzed for percentage change from baseline FEV1 at 90‐day follow‐up; the results favoured valves over control (SMD 0.77, 95% CI 0.43 to 1.11; number of participants = 143; studies = 2). STELVIO 2015, VENT EU 2012, VENT US 2010 provided information for six‐month follow‐up and could be meta‐analyzed; results indicated a favourable standardized mean between‐group difference for valves over control of 0.40 (95% CI 0.22 to 0.58; number of participants = 560; studies = 3). Only the European cohort of VENT reported on 12‐month FEV1 change from baseline difference; it showed a mean between‐group difference of 8.00% (95% CI 1.00 to 15.00). Similar to FEV1 at end of follow‐up, results need to be interpreted with caution, as all participants were included (regardless of collateral ventilation presence) and the mean difference may portray an overly positive result due to the high CIs and SDs reported at each time‐frame in all trials using endobronchial valves.
FEV1 stratified per emphysema distribution (outcome 3.3)
STELVIO 2015 and IMPACT 2016, could be meta‐analyzed for the effect of valves on FEV1 percentage predicted in participants with homogenous emphysema; results indicated a significant mean difference of 16.36% (95% CI 9.02 to 23.71; number of participants = 137; studies = 2), favouring valves over control. BeLieVeR HIFi 2015, the study with a similar design, found an absolute mean difference of 20.9% for their heterogeneous population. VENT EU 2012 and VENT US 2010 found mean differences of 8% and 6.8% percent, but, as mentioned earlier, did not assess collateral ventilation in advance. It should be noted that STELVIO 2015 only reported completed case data rather than intention‐to‐treat for this subset of data, which can positively affect the outcome result.
FEV1 stratified per collateral ventilation presence (outcome 3.4)
VENT EU 2012 and VENT US 2010 separated results for participants with complete fissures and those without in a post hoc analysis. These data could be meta‐analysed via generic inverse variance together with the results of BeLieVeR HIFi 2015 which only tested participants with intact fissures and assessed absolute percentage change (rather than predicted percentage change as reported by IMPACT 2016 and STELVIO 2015). Participants with intact fissures showed a significantly better FEV1 absolute percentage change from baseline than controls (18.15%, 95% CI 11.81 to 24.49; number of participants = 542; studies = 3). Participants who did have collateral ventilation did not show a significant improvement compared to control (MD 2.48%, 95% CI ‐2.63 to 7.59). The difference between these two results was highly significant (P = 0.0002). STELVIO 2015 and IMPACT 2016 only tested participants with intact fissures; they found a percentage change in predicted value of 17.80% (95% CI 7.78 to 27.82) and 17.23% (95% CI 8.10 ‐ 26.36), respectively, further strengthening the evidence provided by the meta‐analysis.
FEV1 stratified per lobar occlusion status
VENT EU 2012 and VENT US 2010 reported data for participants with intact fissures who showed complete and incomplete lobar occlusion after treatment. Participants in VENT EU 2012 who showed complete lobar occlusion had a mean absolute percentage change from baseline at 12‐month follow‐up of 28% (SD 32), while participants without complete lobar occlusion showed 2% (SD 10) change from baseline, significantly favouring complete lobar occlusion (P = 0.005). VENT US 2010 found that participants who showed complete lobar occlusion had a mean absolute percentage change from baseline at 12‐month follow‐up of 20.6% (SD 25.1), while participants without complete lobar occlusion showed 5.2% (SD 17.4) change from baseline, significantly favouring complete lobar occlusion (P = 0.006).
Mortality
Mortality at end of follow‐up (outcome 1.1)
Information on mortality was available for each of the studies using endobronchial valves (see Figure 4). At the end of follow‐up, there was no significant difference in mortality between participants treated with endobronchial valves and participants in the control condition (OR 1.07; 95% CI 0.47 to 2.43; number of participants = 703; studies = 5; moderate‐quality evidence).
Mortality stratified per follow‐up (outcome 3.5)
BeLieVeR HIFi 2015 and STELVIO 2015 provided information for the postoperative time‐period and found no significant difference in death rates between endobronchial valves and control (OR 3.12, 95% CI 0.12 to 80.39; number of participants = 118; studies = 2). Information from BeLieVeR HIFi 2015, IMPACT 2016, STELVIO 2015VENT EU 2012; VENT US 2010 was meta‐analysed for 90‐day follow‐up; there was no significant difference in mortality between endobronchial valves and control (OR 2.17, 95% CI 0.67 to 7.02; number of participants = 703; studies = 5). VENT US 2010 did not provide sufficient detail to allow determination of date of death, therefore it was excluded from the six‐month follow‐up meta‐analysis. STELVIO 2015 and VENT EU 2012 found no significant difference in death rates between endobronchial valves and control at six‐month follow‐up (OR 2.04, 95% CI 0.32 to 13.16; number of participants = 239; studies = 2). Finally, VENT EU 2012 and VENT US 2010 did not find a significant difference in mortality at 12‐month follow‐up (OR 0.85, 95% CI 0.33 to 2.22; number of participants = 492; studies = 2).
Mortality stratified for presence of collateral ventilation and lobar occlusion strategy (outcome 3.6)
No specific information was presented to distinguish the mortality rates of participants with and without lobar occlusion. There were no significant differences in mortality between the studies that tested for collateral ventilation by selecting participants with intact fissures (BeLieVeR HIFi 2015; IMPACT 2016; STELVIO 2015; OR 1.93, 95% CI 0.40 to 9.3), and the trials that did not (VENT EU 2012; VENT US 2010; OR 0.85, 95% CI 0.33 to 2.22) (P = 0.38).
Quality of life
SGRQ at end of follow‐up (outcome 3.7)
BeLieVeR HIFi 2015, IMPACT 2016, STELVIO 2015, VENT EU 2012 and VENT US 2010 could be meta‐analysed for quality of life; results indicated a significant difference favouring valves over control in change from baseline scores (MD ‐6.20 units, 95% CI ‐8.19 to ‐4.20; number of participants = 695; studies = 5; I2 = 67%; low‐quality evidence). Due to significant heterogeneity in the results, sensitivity analysis with a random‐effects model was undertaken, which still resulted in a significant difference in quality of life favouring valves over the control (MD 7.29 units; 95% CI ‐11.12 to ‐3.45; number of participants = 695; studies = 5). Visual inspection of the plot indicated that the heterogeneity was due to the STELVIO 2015 study, which showed a visibly bigger decrease in SGRQ scores. Excluding STELVIO 2015 resulted in an I2 of 42% and a mean between‐group decrease of ‐5.34 units (95% CI ‐7.43 to ‐3.24; number of participants = 627; studies = 4).
BeLieVeR HIFi 2015 found no difference between responders with at least a reduction of 4 points in SGRQ for treatment versus control (P =1.0). STELVIO 2015 found that 79% of participants treated by valves and 33% treated by control reached the MCID of ‐4 points SGRQ (P = 0.001). IMPACT 2016 found that 56.8% of participants treated with valves compared to 25% treated by control reached the MCID of < 4 points (P = 0.003) and 45.9% versus 8.3% reached the more stringent MCID of < 8 points (P < 0.0001).
SGRQ stratified per follow‐up period (outcome 3.8)
BeLieVeR HIFi 2015 and IMPACT 2016 were the only studies that reported scores on SGRQ for 90‐day follow‐up; results indicated a mean difference of ‐8.75 (95% CI ‐12.76 to ‐4.74). STELVIO 2015 reported values only for six‐month follow‐up, which could be combined with results from VENT EU 2012 and VENT US 2010. Results indicated a significant mean difference of ‐5.35 units in SGRQ favouring endobronchial valves over control (95% CI ‐7.66 to ‐3.05; number of participants = 560; studies = 3, I2 = 79%). Due to the significant heterogeneity, a random‐effects model was also compared in a sensitivity analysis with a fixed‐effect model; random‐effects analysis indicated a significant mean difference of ‐7.09 (95% CI ‐12.59 to ‐1.60). Visual inspection of the plot indicated that the heterogeneity was due to STELVIO 2015 which reported higher changes in SGRQ. With the exclusion of STELVIO 2015, heterogeneity was eliminated completely and results indicated a mean decrease of ‐4.05 units SGRQ (95% CI ‐6.51 to ‐1.59), favouring endobronchial valves over control.
SGRQ stratified per emphysema distribution
STELVIO 2015 tested participants suffering with homogenous and heterogeneous disease distributions and found (in completed cases) that change from baseline scores on SGRQ between‐group (valves versus control) differences were larger for participants with a heterogenous distribution (‐19 units, 95% CI ‐31 to ‐6), compared to participants suffering from homogenous disease, (‐12 units, 95% CI ‐21 to ‐4) (P = 0.005), although both groups significantly improved from baseline. IMPACT 2016 found a significant mean change from baseline for participants suffering from homogenous disease of ‐9.64 units (95% CI ‐14.09 to ‐5.20, P < 0.0001).
Absolute change in SGRQ stratified for collateral ventilation status (outcome 3.9)
VENT EU 2012 found no difference in SGRQ change from baseline scores between the treatment effect for participants with intact fissures (MD ‐4.00 units, 95% CI ‐10.64 to 2.64) versus participants without intact fissures (MD 0.00 units, 95% CI ‐5.48 to 5.48) at 12‐month follow‐up (P = 0.36). The results from the participants with intact fissures could be combined with the results from BeLieVeR HIFi 2015, STELVIO 2015 and IMPACT 2016, and they showed a between‐group difference in mean change from baseline of ‐9.03 units (95% CI ‐12.07 to ‐5.98; number of participants = 266; studies = 4), favouring endobronchial valves over control. These results were significantly better than the SGRQ score of participants without intact fissures (MD 0.00, 95% CI ‐5.48 to 5.48) and the results from VENT US 2010 from which we could not differentiate between scores from participants with intact and without intact fissures (MD ‐3.40, 95% CI ‐6.43 to ‐0.37)
Absolute change in SGRQ stratified for lobar occlusion status
VENT EU 2012 and VENT US 2010 separated outcomes for participants with and without complete lobar isolation. Participants in VENT EU 2012 who showed complete lobar occlusion had a mean improvement in quality of life from baseline at 12‐month follow‐up of ‐4 units (SD 16), while participants without complete lobar occlusion showed a reduction in quality of life of 2 units (SD 10) change from baseline, which was a nonsignificant difference (P = 0.4). VENT US 2010 found an average reduction of ‐5.4 units in participants with complete fissures who had complete lobar isolation (SD 11.2) compared to an average reduction of ‐0.3 units (SD 12.8) for participants with incomplete lobar isolation (P = 0.12), indicating no difference in QoL for lobar occlusion status.
Other quality of life questionnaires at end of follow‐up
BeLieVeR HIFi 2015 used the CAT in addition to SGRQ to determine quality of life; the authors found no significant difference in change from baseline scores for endobronchial valves (median ‐2, IQR ‐7 to 3) versus control (median 0, IQR ‐2 to 2) (P = 0.23). Change from baseline scores on the mMRC did not significantly differ between endobronchial valves and control (P = 0.40). Similarly, IMPACT 2016 found no difference in CAT score (MD ‐0.9, 95% CI ‐2.9 to 1.1), but did find a significant difference in mMRC score (MD ‐0.57, 95% CI ‐0.98 to ‐0.16). STELVIO 2015 reported scores on the CCQ and found a between‐group difference in change from baseline of ‐0.74 points favouring endobronchial valves over control (P = 0.002). VENT US 2010 reported a significant change from baseline between‐group difference of ‐0.3 units (95% CI ‐0.50 to ‐0.01) on the mMRC, favouring endobronchial valves over control. VENT EU 2012 only reported on the SGRQ.
Improvement in lung function other than FEV1
RV, TLC, RV/TLC and FVC (outcomes 3.10 to 3.12)
Data on the effect of endobronchial valves on RV in litres could be meta‐analysed for BeLieVeR HIFi 2015, IMPACT 2016 and STELVIO 2015; results indicated a significant between‐group difference in change from baseline for valves compared to control (MD ‐0.58, 95% CI ‐0.77 to ‐0.39; number of participants = 200; studies = 3; low‐quality evidence). VENT US 2010 reported mean values for RV, showing a mean of ‐1.29% change from baseline for valves, compared to a mean increase of 0.69% change for control, with the difference between control and intervention being nonsignificant (P = 0.41). VENT EU 2012 did not report on RV. BeLieVeR HIFi 2015 reported a lack of a difference between the numbers of participants reaching the MCID of a 0.35 L reduction in RV between valves (n=11) and control (n=7) (P = 0.24). IMPACT 2016, however, did find a significant difference of 44.2% of participants treated with valves compared to only 18% of those in the control group reaching the MCID of ‐430 mL (P = 0.006). Similar results were found by STELVIO 2015, which indicated that 71% of participants treated by valves compared to 3% treated by control had a reduction of 430 mL in RV (P < 0.001).
Results from BeLieVeR HIFi 2015 and STELVIO 2015 could be meta‐analysed for TLC change from baseline, which significantly favoured endobronchial valves over control (MD ‐0.34 L, 95% CI ‐0.46 to ‐0.23; number of participants = 107; studies = 2, moderate‐quality evidence). VENT EU 2012 split the results for TLC for participants who successfully had lobar occlusion and those who did not, finding a nonsignificant reduction at 12 months of 0.3L (SD 0.7) for participants with lobar occlusion and 0.2 L (SD 1.2) for those without, compared to a 0.4 L reduction in control. VENT US 2010 reports a nonsignificant difference in change from baseline in TLC of ‐1.2% (SD 10.6) for endobronchial valves compared to ‐0.4% (SD 13) for control (P = 0.29).
BeLieVeR HIFi 2015 and STELVIO 2015 provided results for RV/TLC change from baseline indicating a between‐group difference of ‐5.98 (95% CI ‐8.01 to ‐3.95; number of participants = 118; studies = 2; I2 = 81%, low‐quality evidence) using a fixed‐effect model. Due to the significant heterogeneity, results from a random‐effects model were compared with those of a fixed‐effect model; random‐effects model results remained significant (MD ‐5.76, 95% CI ‐10.45 to ‐1.06). STELVIO 2015 also reported on the proportion of participants having a reduction of 4% RV/TLC showing that 63% of participants treated by valves compared to 9% treated by control reached this MCID (P < 0.001).
STELVIO 2015 was the only study reporting information on change in FVC from baseline between treatment and control, indicating a between‐group difference of 14.4% (SD 27.8) favouring valves. STELVIO 2015 furthermore found a significant RV/TLC of ‐8.1% (SD 10.7) favouring valves. BeLieVeR HIFi 2015 also found a significant difference favouring valves of ‐2.75% (SD 1.6). VENT EU 2012 presented split results for participants with lobar occlusion (‐14%, SD 11) and without (0%, SD 13) compared to control (‐2, SD 10), favouring participants who had complete lobar occlusion.
Gas transfer values
BeLieVeR HIFi 2015 found a significant median improvement of 0.30 mmol/min/kPa in DLCO change (IQR 0.03 to 0.43) from baseline for endobronchial valves compared to no improvement, 0 mmol/min/kPa (IQR ‐0.19 to 0.13) (P = 0.003). IMPACT 2016, STELVIO 2015, VENT EU 2012 and VENT US 2010 did not test for between‐group difference in gas transfer improvements.
Exercise capacity
Exercise capacity stratified for end of follow‐up (outcome 3.13)
Effects of valves on 6MWD could be meta‐analysed for BeLieVeR HIFi 2015, IMPACT 2016, STELVIO 2015, and VENT EU 2012; results showed a significant between‐group difference favouring endobronchial valves over control (38.40 meters, 95% CI 24.69 to 52.12; number of participants = 379; studies = 4; I2 = 78%). Due to significant heterogeneity, results were reanalysed using a random‐effects analysis, which also indicated a significantly larger increase in 6MWD for endobronchial valves compared to control (38.12 meters, 95% CI 8.68 to 67.56; number of participants = 379; studies = 4). Visual inspection of the forest plot indicated that heterogeneity was due to STELVIO 2015 which reported larger increases in 6MWD than the other studies. It is important to point out the high SD found in this meta‐analysis, which may be an indicator of skew. VENT US 2010 reported on median change from baseline, indicating a modest increase of 9.3 meters in the intervention group compared to a decrease of ‐10.7 meters in the control condition, leading to a difference of 19.1 meters favouring valves (P = 0.02).
BeLieVeR HIFi 2015 found a significantly higher number of participants being able to walk 26 meter or more for valves (n=12) compared to control (n=4) (P = 0.01). STELVIO 2015 found an 88% response rate in the ability to walk 26 meters or more compared to 6% for control (P < 0.001), while IMPACT 2016 found 50% of participants treated with valves compared to 14% of those in control were able to reach the MCID of 26 meters (P = 0.0002). VENT US 2010 did not find a difference in participants reaching the MCID of 15% improvement between conditions (P = 0.28).
Exercise capacity stratified for collateral ventilation status (outcome 3.14)
VENT EU 2012 separated the results for participants with and without intact fissures, showing that there was no significant difference between valves and control for either two participant groups (P = 0.8 and P = 0.5). The differences between subjects with and without intact fissures were not significant either. VENT US 2010 found similar results showing no difference on the 6MWD at 12‐month follow‐up (P = 0.25 and P = 0.08). When comparing results at a trial level, however, it can be seen that trials that selected participants with intact fissures (BeLieVeR HIFi 2015; IMPACT 2016; STELVIO 2015) showed significantly higher between‐group differences (MD 50.19, 95% CI 24.96 to 75.41; number of participants = 208; studies = 3) than VENT EU 2012 (MD 5.00, 95% CI ‐21.00 to 31.00; number of participants = 171; studies = 1) which did not test for fissure status (P = 0.01).
Adverse event rate (outcome 1.2)
There was an overall higher number of participants suffering a serious adverse event (as defined by the authors) treated with valves compared to those treated by control (OR 5.85, 95% CI 2.16 to 15.84; number of participants = 482; studies = 3). BeLieVeR HIFi 2015 and VENT EU 2012 did not report sufficient information to determine the number of participants with one or more (serious) adverse events and could not be included in the meta‐analysis. Their data, as well as extra data from the other studies (e.g. non‐serious adverse events), were reported on narratively.
BeLieVeR HIFi 2015 did not find a difference in exacerbations of COPD between treatment (n = 23) and control (n = 22) (P = 0.42), nor did they find significant differences in the occurrence of pneumonia (treatment n = 2, control n = 0, P = 0.49) or the occurrence of pneumothorax (treatment n = 2, control n = 1, P = 1.0). Four participants suffered from expectorated valves, which were replaced in three of the participants. Two participants had to have their valves removed.
The total number of respiratory adverse events in the IMPACT 2016 study (including the serious adverse events) was 76.7% in the valve group compared to 40% in the control group, with 44% in the valve group compared to 12% in the control group suffering from serious adverse events leading to death or hospitalization. They found that significantly more participants in the valve group had a pneumothorax (25.6% versus 0% in control, P < 0.001). While there were more participants with COPD exacerbation in the valve group compared to the control group (76.7% vs 40%), there was no significant difference in exacerbation rates requiring hospitalization (16.3% versus 12%). There was only one case of pneumonia in the control group and none in the valve group. Valve migration or replacement or both occurred in five subjects.
STELVIO 2015 found 23 serious adverse events in the treatment group compared to five in the control group (P < 0.001). Six out of 36 participants in the treatment group developed pneumothorax. Pneumonia occurred in two participants, while four participants needed to be hospitalised for a COPD exacerbation. Furthermore, there were 59 non‐serious adverse events in the treatment group compared to 35 in the control group (P < 0.001). Seven out of 34 participants had unacceptable adverse events due to valves, causing valves to be removed in the STELVIO 2015 trial.
VENT EU 2012 found no overall significant differences in rates for serious complications at any of the individual time points studied. While there were no overall differences in occurrence of pneumothorax, participants suffering from a pneumothorax that lasted over seven days, however, were people who had a high lung volume reduction and showed a more positive clinical response. Valve expectoration, migration or aspiration happened 14 times.
The United States cohort of Vent, VENT US 2010, reported no significant difference in composite adverse events for intervention (6.1% and 10.3%) compared to control (1.2% and 4.6%) at six and 12‐month follow‐up (P = 0.08 and 0.17, respectively), although the difference between valves (4.2%) and control (0%) at 90‐day follow‐up approached significance. The most common adverse event at 12‐month follow‐up was pneumonia distal to the valves (nine participants, 4.2%). There were no significant differences in pneumothorax between the two conditions. Exacerbations requiring hospitalization happened significantly more in the valve group compared to control at six, but not at 12 months (P = 0.03 and 0.84, respectively). Hemoptysis occurred more frequently throughout the trial. VENT US 2010 had to remove valves in 31 participants for retrieval of a migrated valve (n = 8), pneumonia management (n = 3), placement in incorrect lobe (n = 3), COPD exacerbations (n = 2), haemoptysis (n = 1) and other reasons (n = 14).
Cost‐effectiveness
The cost‐effectiveness analyses were conducted for participants with high heterogeneity in disease, intact fissures and lobar occlusion in VENT EU 2012 and VENT US 2010. These studies reported that, with average use of 3.08 valves per participant,the initial costs of EBV placement were EUR 9,581.00 per participant (USD 12,742.73, based on 1.33 yearly average conversion rate in 2014) in year one. Projections for the five and ten year total undiscounted costs per participant were EUR 21,478 (USD 28,565.74) for treatment versus EUR 11,180 (USD 14,869.40) for control at five years and EUR 27,841 (USD 37.028.53) for treatment versus EUR 17,383 (USD 23,119.39) for control at ten years. Considering a total incremental QALY gained of 0.24 for treatment at five years and 0.47 at ten years, the undiscounted incremental cost‐effectiveness ratio (ICER) was EUR 46,322 per QALY gained (USD 61,608.26) at five years and EUR 25,142 (USD 33,438.86) at ten years.
BeLieVeR HIFi 2015, IMPACT 2016 and STELVIO 2015 did not report on cost‐effectiveness.
Hospital utilization
BeLieVeR HIFi 2015 reported on several complications after valve placement but did not indicate the precise burden the procedure placed on the hospital compared to control. STELVIO 2015 reported that median post‐treatment hospital stay was 1 day (range, 1 to 13) and that median procedure time was 18 minutes (range 6 to 51). VENT US 2010 indicated a mean procedure time of 33.8 minutes (SD 20.5). VENT EU 2012 reported a mean procedure time of 27 minutes (SD 18).
Intrabronchial valves versus standard medical care
FEV1
FEV1 at end of follow‐up
In the comparison of partial bilateral placement of intrabronchial valves to control, one trial favoured control and one trial found no difference between the two groups in the effects on FEV1 (moderate‐quality evidence). The IBV Valve trial 2014 reported a percentage change from baseline to six‐month follow‐up in the predicted value FEV1 of ‐2.11% for intrabronchial valves versus 0.04% for control, which was a significant difference favouring the control group (P = 0.001). Ninane 2012 only reported final values for FEV1 at three‐month follow‐up, reporting no significant difference for the intervention (0.90 L (SD 0.34) for valves, compared to 0.87 L (SD 0.34 for control, P = 0.065, high‐quality evidence). Both studies used a partial occlusion strategy, so none of the studies aimed to achieve complete lobar occlusion. The IBV Valve trial 2014 aimed to achieve bilateral partial lobe occlusion in order to minimize the risk of pneumothorax. The Ninane 2012 trial did not aim to achieve complete lobar occlusion as, per protocol, they did not treat specific segments of the lobes in order to prevent lobar atelectasis from occurring.
Mortality (outcome 1.1)
With the combination of data on mortality for IBV Valve trial 2014 and Ninane 2012, there was no evidence of a significant difference in risk of mortality between intrabronchial valves and the control at the end of follow‐up (OR 4.95, 95% CI 0.85 to 28.94; moderate‐quality evidence). The studies did not provide sufficient information to meta‐analyse the data on mortality for multiple time‐points or disease distribution.
Quality of life
SGRQ (outcome 4.1)
Results from the IBV Valve trial 2014 and the Ninane 2012 studies could be meta‐analysed for change from baseline, with results indicating no significant difference between intrabronchial valves and control (MD 2.64 units (95% CI ‐0.28 to 5.56); number of participants = 350; studies = 2, high‐quality evidence). IBV Valve trial 2014 did not find a significant difference in the proportion of responders on the SGRQ (32.2% versus 39.8%).
Other questionnaires
IBV Valve trial 2014 did not find a significant between‐group difference in change from baseline scores at six months for MMRC (P = 0.43) and the physical component of the SF‐36 (P = 0.07). Ninane 2012 furthermore did not find a significant difference between groups in change from baseline scores at three months for mMRC (P = 0.64) and two components of the SF‐36 (mental component, P = 0.83, and physical component, P = 0.73).
The IBV Valve trial 2014 did not find a significant difference in change from baseline scores for mMRC (MD ‐0.10, 95% CI ‐0.34 to 0.14; number of participants = 252) or the physical component score on the SF‐36 (MD ‐0.62, 95% CI ‐2.59 to 1.35; number of participants = 240). Ninane 2012 did not find a significant difference in change from baseline values at end of follow‐up (three months) on the mMRC for intrabronchial valves compared to control (MD ‐0.20; 95% CI ‐0.76 to 0.36; number of participants = 73).
Improvement in lung function other than FEV1
RV, TLC and RV/TLC (outcomes 4.2 and 4.3)
Results for the effect of intrabronchial valves on RV could be meta‐analysed for IBV Valve trial 2014 and Ninane 2012 and results indicated a mean difference of 0.38 L change from baseline favouring control (95% CI 0.12 to 0.65; number of participants = 322; studies = 2, high‐quality evidence). Results of those two studies could also be meta‐analysed for TLC and results indicated no significant difference in change from baseline for intrabronchial valves versus control (MD 0.14; 95% CI ‐0.12 to 0.39; number of participants = 322; studies = 2, high‐quality evidence). Ninane 2012 was the only trial reporting on RV/TLC and the authors found a significant MD favouring control (P = 0.01).
Gas transfer values (outcomes 4.4 and 4.5)
Results from IBV Valve trial 2014 and Ninane 2012 could be meta‐analysed for PAO2 and there was no evidence of a significant difference in change from baseline at end of follow‐up (MD 1.95 mm Hg; 95% CI ‐4.20 to 8.10; number of participants = 308; studies = 2). There was also no evidence of a significant difference in change from baseline scores in PACO2 either (MD 1.33 mm Hg; 95% CI 0.27 to 2.39; number of participants = 315; studies = 2). IBV Valve trial 2014 did not test for DLCO differences at end of follow‐up and Ninane 2012 did not find a significant difference between treatment and control in change from baseline (P = 0.53).
Exercise capacity (outcome 4.6)
Results from IBV Valve trial 2014 and Ninane 2012 could be meta‐analysed and results indicated a significant between‐group difference in change from baseline favouring control over intrabronchial valves (MD ‐19.54 meters; 95% CI ‐37.11 to ‐1.98, moderate‐quality evidence).
Adverse event rate (outcome 1.2)
The number of adverse events reported by studies on Intrabronchial valves was higher for the participants in the intervention condition (OR 3.41, 95% CI 1.48 to 7.84; number of participants = 350; studies = 2; high‐quality evidence). IBV Valve trial 2014 reported that the most frequent serious adverse events were COPD exacerbations (treatment, n = 7, control, n = 2). Twenty‐two serious adverse events were counted (death included) for treatment compared to six for control. Non‐fatal serious adverse events in the treatment condition were COPD exacerbation (n = 7), respiratory failure (n = 4), pneumothorax (n = 3), pneumonia (n = 1) and bronchospasm (n = 1). Ninane 2012 found no significant differences in serious adverse events (P = 0.52) or adverse events in general (P = 0.21) at end of follow‐up. Most adverse events were related to COPD exacerbations (treatment, n = 11, control n = 8). Procedural adverse events were predominantly bronchospasms and dyspnoea.
Cost‐effectiveness
IBV Valve trial 2014 and Ninane 2012,did not report on cost‐effectiveness.
Hospital utilization
IBV Valve trial 2014 reported that the median hospital stay was the same for BLVR versus control (one day), but the mean slightly differed with 2.2 days (SD 6) for the BLVR group and 1.0 day (SD 0) for the control group. Ninane 2012 reported a mean procedure time of 62 minutes (SD 17) which was double that of the control group (23 minutes, SD 14, P < 0.0001). There was no difference in days hospitalized (both groups: 1.1 days, SD 0.3, P = 0.26).
Unilateral versus partial bilateral intrabronchial valve treatment
FEV1
At end of follow‐up, Eberhardt 2012 found a significant increase in FEV1 for the unilateral group (21.4%, SD 10.7%), but not for the bilateral group (‐3.1%, SD 15.0) at end of follow‐up. The between‐group difference favoured the unilateral group (MD 24.50%; 95% CI 13.61 to 35.39).
Mortality
There was no reported mortality in the Eberhardt 2012 trial.
Quality of life
At end of follow‐up, Eberhardt 2012 found a significant decrease from baseline in total score of SGRQ (‐11.8 units, SD 10.6) for the unilateral group, and found a nonsignificant increase for participants treated bilaterally (2.12 units, SD 8.5). The between‐group difference significantly favoured unilateral treatment (MD ‐13.92; 95% CI ‐21.95 to ‐5.89). Similar results were found for scores on mMRC (between‐group difference of ‐1.0, P = 0.05) and the BODE index (between‐group difference of ‐3.0, P = 0.003), favouring unilateral treatment.
Improvement in lung function other than FEV1
The unilateral group in the Eberhardt 2012 study had a reduction of ‐872 mL (SD 796) or a percentage change from baseline of ‐14.7% (SD 13.4) (P = 0.005), while no significant change from baseline was noted for the bilateral group (85 mL, SD 446 and 1.5%, SD 7.7). The between‐group difference significantly favoured unilateral treatment (MD ‐16.20; 95% CI ‐25.33 to ‐7.07). TLC was not significantly reduced in either unilateral (% change of ‐4.1, SD 10.1) or bilateral (+1.5%, SD 7.7) and there was no significant between‐group difference (P = 0.47). No data for gas transfer values were presented.
Exercise capacity
The unilateral group in the Eberhardt 2012 study had an improvement of 48.9 meters (SD 53) change from baseline (P = 0.024), while no significant change from baseline was noted for the bilateral group (‐52.3 meters,SD 81.2, P = 0.08). The between‐group difference significantly favoured unilateral treatment (MD 101.20; 95% CI 43.90 to 158.50).
Adverse event rate
Four participants (two in each group) developed an exacerbation in the Eberhardt 2012 trial, but no hospitalization was needed. In the bilateral group, respiratory failure occurred in two extra participants requiring noninvasive ventilation or intubation. One participant developed a pneumothorax which was resolved via a chest drain and this person was discharged after 16 days.
Cost‐effectiveness and hospital utilization
No specific information was mentioned by Eberhardt 2012 on either of the above‐mentioned outcomes.
Vapour ablation versus standard medical care
FEV1
FEV1 at end of follow‐up
STEP‐UP 2016 reported a mean between‐group difference in change from baseline predicted value FEV1 of 14.70% (95% CI 7.98 to 21.42; low‐quality evidence), favouring vapour ablation over control at six‐month follow‐up. The authors also reported that at six‐month follow‐up, 50% of participant treated by vapour ablation versus 13% treated by control reached the MCID used in this study (> 12% increase in FEV1). STEP‐UP 2016 furthermore reported data at the three‐month endpoint, which showed a between‐group mean difference in FEV1 predicted value of 10.1% (95% CI 3.2 to 16.9) favouring vapour ablation over control.
Mortality (outcome 1.1)
STEP‐UP 2016 did not find a significant difference in mortality between treatment (two deaths) and control (0 deaths) (OR 2.93; 95% CI 0.14 to 63.49, number of participants = 70, moderate‐quality evidence). It is important to note the wide CIs, with ORs ranging from 0.14 at the lower bound and 63.49 at the upper bound. The sole death in this study occurred at 84 days after treatment.
Quality of life
STEP‐UP 2016 found a mean difference in change from baseline scores of ‐6.60 units on the SGRQ (95% CI ‐12.4 to ‐0.9) at three months and ‐9.70 units on the SGRQ (95% CI ‐15.62 to ‐3.78) at six months, favouring vapour ablation over control at both follow‐up times (low‐quality evidence). The authors also reported that at six‐month follow‐up, 70% of participants treated by vapour ablation versus 39% treated by control reached the MCID of > 4 points increase in SGRQ, and 53% versus 17% exceeded a reduction of 8 points on the SGRQ.
Improvement in lung function other than FEV1
STEP‐UP 2016 did not provide between‐group comparisons, but rather reported a significant absolute mean difference in RV between control and treatment groups of ‐0.30 L (‐0.54 to ‐0.06, P = 0.02, moderate‐quality evidence), but did not find a significant difference between control and treatment groups for TLC (MD ‐0.08 L; 95% CI –0.32 to 0.16; P = 0.51), or FVC (MD 0.24 L; 95% CI 0.06 to 0.43; P = 0.01, moderate‐quality evidence).
No information on gas transfer values was provided
Exercise capacity
STEP‐UP 2016 found an absolute difference between control and treatment group of 30.5 meters, which did not reach significance (P = 0.06, low‐quality evidence). At six months, 42% of participants treated by vapour ablation versus 23% treated by control exceeded an increase of 26 meters walking distance.
Adverse event rate (outcome 1.2)
The adverse event rate in STEP‐UP 2016 was significantly higher in the intervention group compared to the control condition (OR 3.86; 95% CI 1.00 to 14.97).The study reported 11 (24%) COPD exacerbations in the treatment group compared to 1 (4%) in the control group. Pneumonia or pneumonitis occurred in eight participants (18%) in the treatment group compared to 2 participants (8%) in the control group. There were no cases of respiratory failures or ICU admission. All but one adverse event could be resolved by standard care.
Cost‐effectiveness
STEP‐UP 2016 did not report on cost‐effectiveness
Hospital utilization
No specific information regarding hospital utilization was given by STEP‐UP 2016.
Discussion
Summary of main results
AeriSeal
ASPIRE 2015 was the only RCT published that compared the effect of AeriSeal to treatment with optimal medical care as defined by the GOLD guidelines. This small‐sized open‐label study recruited 95 participants, but only reported efficacy outcomes for 57 participants at three month and 34 participants at six‐month follow‐up, as a result of early termination. The trial found a significant difference favouring AeriSeal compared to control in median change from baseline scores on lung function (FEV1), quality of life (SGRQ, not mMRC) and exercise capacity (6MWD) for participants that completed the trial. There was no significant difference in the odds of death between the two groups, however there were significantly more adverse events that required hospitalization in the treatment group compared to control.
The absolute percentage increase in FEV1 in the treatment group of 18.9% exceeds the recommended MCID of 10% (Donohue 2005). For simplicity sake, this MCID of 10% will be used throughout the discussion, despite noting that different MCIDs exist. Similarly, the decrease in SGRQ of ‐11 units and the 31 meter increase in 6MWD exceed the MCIDs used in this review; a decrease of 7.1 units (Welling 2015) and an increase of 26 meters (Puhan 2011a), respectively. ASPIRE 2015 furthermore dichotomized the results indicating that for each outcome (FEV1, SGRQ, mMRC and 6MWD), over 50% of participants reached the MCID specified in the trial, although only a statistically significant difference between control and intervention existed for 6MWD. The number of adverse events was significantly higher for participants treated with AeriSeal compared to those treated with optimal medical care. These adverse events were predominantly respiratory, more specifically COPD exacerbations and post‐procedural pneumonia and, in the early postoperative interval, post‐treatment acute inflammatory response.
The positive results found, however, need to be placed in the context of the early discontinuation of this single study (the funder pulled out during the trial) and subsequently the low number of participants, the short follow‐up, the lack of important lung function indices (e.g. RV and DLCO) and the sole availability of per protocol data. ASPIRE 2015 joined a range of other registered studies on AeriSeal (e.g. on clinicaltrials.gov) in being terminated (early) and studies are currently back to safety testing to try and improve the safety profile of the technique (see for instance the STAGE study, NCT02877459).
Airway bypass stents
Despite some functional improvements at day one, the only study that looked at the effect of airway bypass stents, Ease 2011, failed to find statistically and clinically significant findings on a range of outcomes including lung function (FEV1, FVC, RV), quality of life (SGRQ and mMRC) and exercise capacity (6MWD). There were no significant differences in mortality between treatment and control. The between‐group difference in composite safety events was only 3.2%. Serious respiratory events were more common in participants treated with stents, but there was no significant difference in composite safety endpoints. While there was an acceptable safety profile, the use of airway bypass stents did not lead to clinically significant short‐term or long‐term results. The lack of results could be attributed to variability between participants or an inadequate silicone polymer dose‐release combination; improvements are needed to determine the desired target areas, participants and improve the procedural efficacy.
Endobronchial coils
Three RCTs (RENEW 2016; RESET 2015; Revolens 2016) tested the effect of endobronchial coils compared to standard care and the results indicated that endobronchial coils led to significantly better results compared to standard medical care for lung function outcomes (FEV1, RV, RV/TLC but not TLC) and quality of life (SGRQ). Exercise capacity (6MWD) did not increase significantly when pooling the results of the three trials. There was no overall higher mortality for endobronchial coils compared to standard medical care, but coils led to higher rates of adverse events, specifically lower respiratory tract infections, COPD exacerbations, pneumothorax and pneumonia.
The overall difference in FEV1 of 10.88% just exceeds the MCID of 10% and the reduction of 9.14 units in SGRQ between groups is clinically and statistically significant. However, the overall reduction of 320 mL RV did not reach the MCID (430 mL, Hartman 2012) for this outcome. There was no conclusive evidence to suggest significant differences between the effectiveness of endobronchial coils for participants with heterogeneous and homogenous disease. Data from prespecified subgroups in RENEW 2016 furthermore seems to suggest that participants with more severe static hyperinflation (RV of higher than 225% predicted) would respond better to treatment than those with lower static hyperinflation, which could provide insight for future trials and participant selection.
Revolens 2016 was the only trial identified in this review that provided a detailed cost‐analysis for endobronchial coils, for a follow‐up period of 12 months. The trial found a mean difference in cost of $US47,908 (favouring control) which, combined with the $782,598 per QALY gained for endobronchial coils, proved that the treatment was costly despite the clinical benefits for individual participants. Some clinical benefits, however, might exceed 12 months and could therefore increase the cost‐effectiveness determined via QALY gained in the long‐run.
Endobronchial valves
A total of five trials provided evidence for the use of endobronchial valves. These trials (BeLieVeR HIFi 2015; IMPACT 2016STELVIO 2015; VENT EU 2012; VENT US 2010), with a total of 703 participants, compared endobronchial valves to standard/optimal medical care. Endobronchial valve placement resulted in significant between‐group improvements in percentage change from baseline for lung function (FEV1, FVC, RV, TLC, RV/TLC), quality of life (SGRQ) and exercise capacity (6MWD). No significant differences in mortality rates were found when comparing endobronchial valves to control, but the number of adverse events was higher for participants treated with endobronchial valves compared to standard medical care.
The two earlier trials (VENT EU 2012 and VENT US 2010) suggested important insights for participant selection when using endobronchial valves after a post hoc analysis showed better response rates in participants who had intact fissures. As a result, the newer trials (BeLieVeR HIFi 2015; IMPACT 2016; STELVIO 2015) altered their inclusion criteria to only select participants with intact fissures, thereby lowering the chance of selecting participants who had collateral ventilation, which resulted in better functional outcomes. For instance, improvements in FEV1 far exceeded the MCID of 10% when participants had intact fissures (a mean difference of 18%), while this was not the case for participants who did not have intact fissures (a mean difference of 2.48%). A similar result was found when looking at quality of life. Participants who had intact fissures showed a significant decrease in SGRQ scores of ‐9.3 units, scores that exceeded the MCID used in this review (‐7.1 units) and was lower than the overall score on quality of life that was found in this review of ‐7.29 units. In comparison, participants who did not have intact fissures had a mean difference of 0.0 units.
The occurrence of high SDs and CIs needs to be taken into account for multiple outcomes (FEV1, SGRQ, 6MWD, and even mortality to a lesser extent) reported on by trials studying endobronchial valves. For instance, the high CIs and SDs found in the mean differences reported for FEV1, indicated the presence of considerable skew, which was further evident by the large differences between median and mean scores in the BeLieVeR HIFi 2015 trial. Interpreting the data provided by the means found in this review should thus be done with caution. On the other hand, throughout the studies that were reported here, the number of participants reaching the MCID for FEV1 was significantly higher in the intervention condition compared to control.
The cost‐effectiveness data for VENT EU 2012 and VENT US 2010 was taken from participants that had high heterogeneity, in complete fissures and lobar occlusion. , The ICER was estimated at EUR 46,322 per QALY gained ($US 61,608.26) at five years and EUR 25,142 ($US 33,438.86) at ten years. While these costs were based on an average valve placement of 3.08 and trials in this review had a slightly higher average (VENT US 2010 mean of 3.8 valves, BeLieVeR HIFi 2015 median of 3 valves and STELVIO 2015 a median of 4 valves) number of valves placed, the costs were considerable and remained around or below the infamous EUR/USD $50,000 per QALY gained cut‐off for cost‐effectiveness ratios.
Intrabronchial valves
The review included two RCTs comparing intrabronchial valves to standard medical care (IBV Valve trial 2014; Ninane 2012) and one trial with a total of 22 participants (Eberhardt 2012) compared unilateral versus partial bilateral valve placement using intrabronchial valves. While there was no significant risk in mortality between treatment with intrabronchial valves and standard medical care, the results showed favourable results for control over intrabronchial valves in lung function (FEV1 and RV) and exercise capacity (6MWD). Furthermore, no significant between‐group differences were found for quality of Life (SGRQ, mMRC and SF‐36 scores), TLC or gas transfer values (PaO2 and PaO2).
The results found by Eberhardt 2012 offer an explanation to the lack of results found by IBV Valve trial 2014 and Ninane 2012, as the former study found that those treated with unilateral valve placement as opposed to partial bilateral treatment showed significantly better results in lung function (FEV1, RV not TLC), quality of life (SGRQ) and exercise capacity (6MWD). IBV Valve trial 2014 and Ninane 2012 tested participants only with upper lobe predominant emphysema and did not specifically address collateral ventilation nor aimed to achieve lobar occlusion (as they aimed to improve quality of life and improve lung function without leading to a higher risk of pneumothorax). This evidence was supported by the results found by studies testing the endobronchial valves, which all aimed to achieve lobar occlusion, and showed better functional results when lobar occlusion was achieved.
Vapour ablation
One trial comprising 70 participants provided evidence for the short‐term effectiveness of vapour ablation as only the six month results have been published. The trial indicated significant favourable results for vapour ablation over standard medical care for lung function (FEV1, RV), quality of life (SGRQ) and exercise capacity (6MWD). As with the other trials, no significant difference in mortality was found. Adverse events were, just like the other methods, more abundant in the treatment compared to the control arm. The between‐group difference of 14.7% FEV1 and ‐9.70 units on the SGRQ change from baseline exceeds the MCID of 10% and ‐7.1 units, respectively. The lack of change in baseline numbers made it impossible to make a judgement on clinically significant changes for 6MWD, FVC, TLC and RV in the same manner as done above.
Overall completeness and applicability of evidence
The results found by this review highlight the short‐term safety of BLVR procedures for COPD. While adverse events were more common in all techniques used compared to optimal medical care or sham bronchoscopy, the large majority of adverse events was resolved using standard medical care (for instance, with antibiotic treatment or via tube drainage) and did not result in death.
Although there was no overall significant difference in mortality between BLVR procedures and control, these findings need to be interpreted with caution. The confidence intervals were too wide to warrant the interpretation that this was a definite conclusive result, as lower and upper bounds of the CI often touch upon clinically meaningful values (e.g. upper bounds ranging from OR = 2.4 to an OR of 63.5). While future trials are thus needed to shed more light on the risk of mortality, post and perioperative procedure‐related deaths were limited in the trials studied in this review. This is an important advantage that BLVR techniques offer over LVRS. While more recent observational studies in LVRS indicate a negligible early death rate due to apparent improved participant selection and ability to perform the surgical procedure (Clark 2014; Ginsburg 2011), the results from all currently available RCTs on LVRS indicate an overall higher risk of early 90‐day mortality for participants treated with LVRS compared to standard medical care, as opposed to a favourable long‐term risk of mortality (Van Agteren 2016).
Despite a potential short‐term survival advantage in specific subsets of participants suffering from emphysema, this advantage needs to be placed in the context of clinical improvements. Lower rates of mortality and morbidity have been associated with less effective results in BLVR procedures (Gompelmann 2013). For instance, a retrospective analysis of three trials on endobronchial valves (including the VENT EU 2012 and VENT US 2010 cohorts) found that pneumothorax was associated with lobar lung volume reduction and superior clinical outcomes (Gompelmann 2014). Similar results were found in other trials in this review (Ease 2011; RENEW 2016), indicating that it may well be that increased risks of morbidity and mortality are necessary for clinical effectiveness to be achieved by BLVR procedures. Specifically for valves, participants might need replacement of valves and additional treatments to achieve long‐term clinical improvements.
The biggest evidence base in this review came from trials studying endobronchial valves and endobronchial coils, trials that found short‐term improvements in lung function, quality of life and exercise capacity surpassing the MCID for several outcomes. With the exception of BeLieVeR HIFi 2015, these trials, however, were not sham‐controlled or included cross‐over participants or both (RESET 2015; Revolens 2016; STELVIO 2015), and they may therefore be at risk of bias for more subjective outcomes such as the SGRQ (Ambrosino 1999; Cooper 2010). This may be reflected in the fact that BeLieVeR HIFi 2015 failed to find clinically significant between‐group scores on the SGRQ and 6MWD, although other aspects of the study (e.g. participant selection methods; Chartis vs CT) may provide an alternative explanation.
While the other trials would have benefited from a sham‐controlled design rather than an open label design, the improvements in RV and RV/TLC are not susceptible to performance bias and indicated favourable outcomes for endobronchial valves and coils. Furthermore, the improvement in SGRQ by endobronchial coils (‐9.78 units) exceeded the already upwardly adjusted MCID for participants suffering form severe COPD (the original MCID was set at ‐4 points (Jones 2005)) by more than 2 points, which does indicate that improvement in quality of life could be expected. In addition, it is important to consider the ethics and practicality behind providing sham‐bronchoscopy to participants at advanced stages of emphysema (Macklin 1999; Miller 2004); the mere act of having a bronchoscopy can already lead to complications in these severely ill participants and complications (e.g. displacement of valves) can lead to unblinding, to name a few.
The evidence presented in this review, in addition, showed promising results for participants suffering from homogenous emphysema and participants without intact fissures. Results by IMPACT 2016 and STELVIO 2015 indicated that endobronchial valves could lead to clinically relevant improvements. In addition, the three trials studying the use of endobronchial coils found significant results for both homogenous and heterogenous disease. This is specifically important in the light of the lack of clinically significant findings by Ease 2011 and the fact that LVRS is not traditionally performed on participants suffering from homogenous disease (Cooper 1995; Weder 2009). Although only tested by single trials with low sample size, the results found by ASPIRE 2015 and STEP‐UP 2016, together with the results by the endobronchial coil studies, ignites hope for participants without intact fissures as these techniques do not rely on fissure integrity to achieve functional results (Gompelmann 2012).
While this review identified a total of fourteen RCTs comprising a total of 1979 participants, the evidence presented leaves a number of important unanswered questions which merit consideration. The large majority of trials only reported on a relatively short follow‐up (ranging between three and 12 months), making it difficult to assess the long‐term benefits and adverse consequences of the treatments. BLVR is increasingly being posited as the less invasive alternative to LVRS, but the results provided by the RCTs in this review do not warrant such conclusions or recommendations yet. Although positive long‐term non‐randomized reports on BLVR techniques are slowly emerging (e.g. 10 year survival data on endobronchial valve placement Garner 2016), LVRS has been tested in several RCTs with longer follow‐up than the trials published in this review (one trial (Pompeo 2012) providing follow‐up until 24 months and two trials (Agzarian 2013; Naunheim 2006) providing follow‐up beyond 36 months). The surgical technique, furthermore, has had time to mature and participant selection has improved over the course of the past 20 years, while BLVR treatment is still young and participant selection criteria are still being investigated and developed. The two treatments, however, may serve different purposes and may be more suitable for different participants; answers and questions that were not within the scope of this review.
Participant selection criteria and maturation of the techniques present a further problem that needs to be addressed. Studies on endobronchial valves have learned from imperfections in the past (for example, not testing for fissure integrity or collateral ventilation) and the Eberhardt 2012 trial further strengthened the results found by trials on endobronchial valves to aim to completely occlude a target lobe, rather than to use a partial bilateral approach, which failed in IBV Valve trial 2014 and Ninane 2012. A trial that is currently still recruiting, the NCT01989182 trial, which has already presented some preliminary positive findings at the European Respiratory Society, might help provide more evidence for intrabronchial valves as it aims to achieve lobar occlusion using intrabronchial valves. Other techniques in this review are still being developed (vapour ablation, most notably) and the evidence for them may change considerably in the next update of this review.
There was a general lack of reporting on gas transfer value improvements, which is disappointing as pO2 and DLCO have been associated with improved survival rates in COPD patients (Boutou 2013) and they could thus provide more information on potential survival rates, specifically in the context of the lack of long‐term follow‐up data. The lack of economic analyses accompanying the current trials poses another limitation. Two trials on LVRS produced detailed cost‐effectiveness analyses based on long‐term follow‐up and modelling. Overall, those two trials found a cost‐effectiveness of USD 140,000 per QALY gained (Criner 2008) and CAN D 133,900 per QALY gained (Miller 2006), with projected costs for a subset of participants in NETT being as low as USD $40,000 per QALY gained. Comparing these figures to the USD 782,598 per QALY gained as found by Revolens 2016, the only trial investigating cost‐effectiveness for endobronchial coils, indicated a significant advantage for LVRS. The projected costs for endobronchial valves of EUR 25,142 per QALY gained were considerably lower at ten years, and despite only coming from a small set of high responding participants, thus indicated that endobronchial valves may become a cost‐effective solution with time. While clinical benefit for participants from any treatment should always outweigh the importance of costs, the adoption of this treatment by agencies, insurers and the government relies on a thorough understanding of the costs and benefits involved, meaning that It is imperative that more trials start publishing cost‐effectiveness data.
The studies presented in this review have almost always been completed in medical centres that were experienced in bronchoscopy and often have had experience with LVRS (e.g. BeLieVeR HIFi 2015). A large proportion of the authors involved in the included reviews have been examining BLVR (and some LVRS) techniques extensively over the past decade or more. On the one hand, this shows that these procedures can be achieved by centres with similar expertise but it also means that the procedures come with a learning curve for those who are attempting to replicate these findings or adopt these techniques in their own institutes, which may result in smaller treatment effects in less experienced institutions.
Quality of the evidence
It was not possible to give an overall indication of the quality of evidence for BLVR due to the distinctiveness of the individual treatments and for which specific subtypes/phenotypes of emphysema the treatments are recommended. It is for this reason that this review included six different summary of findings tables, each focused on a specific method.
AeriSeal
The quality of evidence on the effectiveness of AeriSeal provided by ASPIRE 2015 was rated to be low to moderate, see Table 1. The evidence was downgraded from high to low for the effect of AeriSeal on FEV1, due to imprecision of the results. FEV1 was only reported for 34 participants and the presented confidence intervals were wide. The evidence on mortality was downgraded to low as a result of imprecision in the results: high CIs and low participant numbers. The evidence provided for quality of life (measured by SGRQ) was downgraded to low due to imprecision (as reported above) and risk of performance and detection bias. The evidence for exercise capacity (measured by 6MWD) was downgraded to low due to imprecision (as reported above) and risk of performance bias as the 6MWD is effort‐dependent. Adverse events was downgraded to moderate as a result of the low participant numbers. It was not possible to grade the evidence provided for lung function parameters other than FEV1 or cost‐effectiveness due to a lack of data on those outcomes.
Airway bypass stents
The quality of evidence for the effectiveness of airway bypass stents provided by Ease 2011 was rated as high for all outcomes other than mortality, adverse events and 6MWD see Table 2. The Ease 2011 trial was a well‐powered sham‐controlled study with a very low risk of bias, so no downgrading of the evidence was done for any of the mentioned outcomes. Mortality, adverse events and 6MWD were downgraded for imprecision as the upper bound of the CI touched a potentially clinically relevant OR of 2.7 for adverse events and mortality and crossed the MCID of 26 meters for 6MWD.
Endobronchial coils
The quality of evidence on the effectiveness of endobronchial coils provided by RENEW 2016, RESET 2015 and Revolens 2016 ranged from low to high, see Table 3. The evidence was downgraded from high to moderate for the effect of coils on FEV1 due to imprecision of the results: there were two trials with only 146 participants that could be included in the meta‐analysis. The evidence for TLC and cost‐effectiveness was downgraded due to imprecision in the results stemming from a low participant number reporting on these outcomes. The evidence provided for quality of life (measured by SGRQ) was downgraded to low due to imprecision (as reported above) and risk of performance and detection bias. The evidence for exercise capacity (measured by 6MWD) was downgraded to low due to inconsistency as a result of high heterogeneity and risk of performance bias as the 6MWD is effort‐dependent. Mortality was downgraded to moderate as the upper bound of the CI touched a potentially clinically relevant OR of 3.3.
Endobronchial valves
The quality of evidence on the effectiveness of endobronchial valves provided by BeLieVeR HIFi 2015, IMPACT 2016STELVIO 2015, VENT EU 2012 and VENT US 2010 ranged from low to high, see Table 4. The evidence was downgraded from high to low for the effect of endobronchial valves on FEV1 due to inconsistency as a result of high heterogeneity and differences in participant selection criteria that significantly influenced the result. Scores on the SGRQ were downgraded for inconsistency (as reported above) and risk of performance and detection bias due to the open‐label nature of three out of four of the studies. Scores for RV and TLC were downgraded to moderate due to imprecision in the results stemming from a low participant number reporting on these outcomes. RV/TLC was downgraded to low for imprecision due to low participant numbers and high heterogeneity in results. The evidence for exercise capacity (measured by 6MWD) was downgraded to low due to inconsistency as a result of high heterogeneity and risk of performance bias as the 6MWD is effort‐dependent (and only BeLieVeR HIFi 2015 was sham‐controlled). Mortality was downgraded to moderate as the upper bound of the CI touched a potentially clinically relevant OR of 2.4.
Intrabronchial valves
The quality of evidence on the effectiveness of partial bilateral placement of intrabronchial valves compared to standard medical care provided by IBV Valve trial 2014 and Ninane 2012 ranged from moderate to high, see Table 5. The quality of the evidence provided for change from baseline FEV1 was downgraded as a result of imprecision due to low participant numbers. The quality of the evidence for effect on exercise capacity and mortality was downgraded as a result of wide CIs for the 6MWD and as a result of a potentially clinically meaningful OR of 29 for mortality. The evidence for TLC was downgraded 1 level for imprecision as the 95% CI (touching an increase of 0.39 L) of the intervention group indicated clinically meaningful results. All other variables were rated as high‐quality.
Vapour ablation
The quality of evidence for the effectiveness of vapour ablation provided by STEP‐UP 2016 ranged from low to moderate, see Table 6. The evidence was downgraded from high to moderate for the effect of vapour ablation on FEV1, RV, TLC and adverse events due to imprecision of the results as a result of low participant numbers. The evidence for mortality was downgraded to low due to imprecision as a result of the low participant numbers and very wide CIs indicating an odds of 61. The evidence provided for quality of life (measured by SGRQ) was downgraded to low due to imprecision (as reported above) and risk of performance and detection bias. The evidence for exercise capacity (measured by 6MWD) was downgraded to low due to imprecision (as reported above) and risk of performance bias as the 6MWD is effort‐dependent.
Potential biases in the review process
The quality of any systematic review depends on the quality of the included studies. While all of the studies were RCTs, the majority were not sham‐controlled. Most trials were industry sponsored and the sponsors either had access to or were completely responsible for the data collection and analysis. Furthermore, a number of studies were terminated early due to logistic reasons and therefore did not reach the intended power, leading to the use of per‐protocol rather than intention‐to‐treat data. All of these factors may have contributed to lower quality of the data and therefore may have influenced the quality of this review.
Agreements and disagreements with other studies or reviews
Other studies
The clinical improvements found by ASPIRE 2015 are in line with previous studies on the use of AeriSeal. For instance, Kramer 2012 found long‐term improvements in FEV1, dyspnoea and hyperinflation up to 2 years. Magnussen 2012 found improvements in lung function, quality of life and reduced lung volumes in participants treated by AeriSeal with and without intact fissures, indicating a potential advantage over endo‐ and intrabronchial valves. Studies by Fruchter 2016, Herth 2011 and Kramer 2013, furthermore, provide insight into the potential (long‐term) adverse events that could have been expected in ASPIRE 2015 if it had not been prematurely terminated. Biomarkers of systemic inflammation increased in the first three months but decreased below baseline up until a year after. Furthermore, airway colonisation of bacteria indicated a higher risk of COPD exacerbations, which could be targeted before the trial commenced and could aid in participant selection. The lack of longer‐term data and the lack of power as a result of the low numbers of participants by ASPIRE 2015 could not further confirm these findings.
Rendina 2003, as early as 2003, showed that airway passages could safely be produced. This was then further supported by evidence from animal studies (Choong 2005; Choong 2006) and complemented by evidence from in vitro studies (Choong 2008) and case series (Cardoso 2007), indicating effective lung volume reductions. Cardoso 2007 found that statistically significant reductions were more likely in participants with an RV/TLC of 0.67 or above, which may suggest that future trials may need to look at different, more stringent participant criteria, if airway bypass wants to lead to clinically and statistically significant results.
The results found by the endobronchial coil studies are completely in line with previous research (Kontogianni 2014; Slebos 2012). Deslee 2014 found clinically significant differences in both heterogenous and homogenous emphysematous participants, which is in line with the results found by trials in this review. Furthermore, the significant improvements remained until end of follow‐up at 12 months. The results by long‐term follow‐up of two different cohorts of clinicals trials by Hartman 2015, in addition, provided evidence on long‐term efficiency with 59% of participants reaching the MCID for SGRQ and 40% for 6MWD at 3 year follow‐up.
The results found in the four trials reported in this review complement existing studies on endobronchial valves. Specifically, research on the role of fissure integrity (Venuta 2005) and the necessity for atelectasis to occur as it leads to survival benefit (Hopkinson 2011) are complemented by the results of BeLieVeR HIFi 2015 and STELVIO 2015. Furthermore, endobronchial valves have successfully been tested in a non‐Caucasian population (i.e. Asian) in a open‐label single‐arm trial (Park 2015)
Springmeyer 2009 showed clinically meaningful reduction in lung volume in a study on intrabronchial valves and found that a decrease of 0.35 L of lobar volume was necessary to induce significant improvements in the SGRQ. IBV Valve trial 2014 only showed a decrease of 0.2 L, which may explain the lack of clinically meaning results in other areas. The results found by STEP‐UP 2016 are in line with a pilot study (Snell 2012) and single‐arm studies (Herth 2012), indicating significant changes from baseline at six and 12 months.
Other Reviews
The findings of this review are in line with other reviews (Herth 2016; Gompelmann 2014a) which concluded that the evidence base for BLV techniques is growing and most evidence exists for the use of endobronchial valves. Since these reviews have been published, a number of new trials have been published (IMPACT 2016; RENEW 2016; Revolens 2016; STEP‐UP 2016), strengthening the evidence base for endobronchial coils and vapour ablation, as well as the use of endobronchial valves in homogenous emphysema.
Authors' conclusions
Implications for practice.
Overall, the studies reported in this review did not provide evidence for the clinical effectiveness of airway bypass stents (for homogenous emphysema) and intrabronchial valves, specifically when the latter was performed partially bilaterally, or BioLVR. Ease 2011 was a well‐powered sham‐controlled study which failed to find clinically significant results. Similarly, IBV Valve trial 2014 and Ninane 2012 were sham‐controlled and failed to find substantial clinical improvements at end of follow‐up, which may be attributed to the treatment strategy chosen. No currently ongoing or completed RCTs, and thus no evidence, on the use of BioLVR was found.
Other BLVR interventions, specifically endobronchial coils and valves, led to significant improvements in functional and health status. The trials in this review provide guidance for participant selection criteria by treatment centres. The evidence of this review indicates that heterogeneous and homogenous emphysema participants who show no collateral ventilation or have intact fissures or both may benefit most from endobronchial valves, especially considering the full reversibility of the valves. Participants that suffer from heterogeneous and homogenous emphysema and show collateral ventilation or lack intact fissures or both may be best suited for treatment with endobronchial coils. Participants suffering from upper‐lobe predominant emphysema may in the future be helped by vapour ablation. Using a multidisciplinary team to aid in appropriate participant selection can help find the most suitable participant for each of the techniques (Rathinam 2014).
The lack of long‐term results, the lack of clear cost‐effectiveness data, the lack of results by sham‐controlled studies, the potential presence of skewed data, and heterogeneity in results of some of the outcomes make it difficult to determine a definite answer to the effectiveness of each method for individual COPD patients. This is further emphasized by the strict inclusion and exclusion criteria, and the resulting demographics used in the studies. The age of the participants in the trials in this review was relatively young (averages ranging between 58 and 65) and participants with major comorbidities were excluded. As a large proportion of COPD patients suffer from multiple comorbid disorders (Cavaillès 2013) and the average age of patients in the wards is increasing, potential beneficial results found in the included studies remain limited to a select participant cohort.
Treatment centres that are interested in any of the above mentioned techniques should pay close attention to participant selection, e.g. fissure integrity (Koster 2016), and treatment approach (e.g. lobar occlusion strategy and unilateral approach) (Eberhardt 2015). Furthermore, it is important to be aware of the adverse event rate associated with BLVR and how to deal with it. In general, the more effective the clinical response of the participant, the higher the adverse event rate, which is a trade‐off that both clinicians and patients need to be made aware of.
Implications for research.
Longer‐term follow‐up, combined with the results of current ongoing trials, will help provide a clearer picture on the effectiveness of BLVR in the long‐run, although the ethical problems with leaving participants untreated for long periods of time needs to be noted. Of the fourteen studies, three studies (IMPACT 2016; RESET 2015; STEP‐UP 2016) have not yet published on their prespecified follow‐up endpoints. While several other trials such as the EMPROVE trial (NCT01812447), the LIBERATE trial (NCT01796392) and the TRANSFORM trial (NCT02022683) are planned, only two of these have a planned follow‐up of two years. More importantly however, all of these trials are open‐label, leaving the need for sham‐controlled (or at least double‐blinded) trials unmet, as the large proportion of outcomes used in the BLVR studies were subjective and were thus prone to bias.
Two trials, the REACH trial (NCT01989182) and NCT02823223, are currently still recruiting and investigating the effect of intrabronchial and endobronchial valves in China, which will shed important light on the effectiveness of the systems in an Asian population.
Another focus for future studies should be to compare BLVR techniques head‐to‐head and compare the effectiveness of BLVR methods for heterogeneous emphysema (valves, coils, vapour ablation) directly with LVRS. One unpublished trial comparing endobronchial valves with intrabronchial valves was found (NCT01457833) and one trial, the CELEB trial (ISRCTN19684749), has been identified that aims to directly compare LVRS with endobronchial valve placement aimed to achieve lobar occlusion.
The differences between the use of the Chartis‐system and CT‐assessment on treatment effectiveness of valves were beyond the scope of this review; future reviews and prospective trials should further explore this topic.
Acknowledgements
The Background and Methods sections of this protocol are based on a standard template used by the Cochrane Airways Group.
Chris Cates was the Editor for the protocol of this review and commented critically.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding provided to the Cochrane Airways Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (The Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Appendix 2. Search strategy to identify relevant trial reports from the CAGR
#1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All
#2 MeSH DESCRIPTOR Bronchitis, Chronic
#3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)
#4 COPD:MISC1
#5 (COPD OR COAD OR COBD OR AECOPD):TI,AB,KW
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 bronchoscopic* NEAR reduction*
#8 endoscopic* NEAR reduction*
#9 BLVR
#10 bronchoscopic* NEAR (pneumoplasty OR pneumonectomy)
#11 coil*:ti,ab
#12 valve*:ti,ab
#13 AeriSeal
#14 lung* NEAR sealant
#15 "Biologic Lung Volume Reduction"
#16 bronchoscopic* NEAR ablation
#17 airway* NEAR bypass*
#18 airway* NEAR stent*
#19 MeSH DESCRIPTOR Pneumonectomy
#20 MeSH DESCRIPTOR Bronchoscopy
#21 MeSH DESCRIPTOR Bronchi WITH SU
#22 MeSH DESCRIPTOR Stents
#23 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22
#24 #6 and #23
[Note: in search line #4, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, COPD]
Data and analyses
Comparison 1. BVLR (all methods) versus medical therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (end of follow‐up, all methods) | 13 | 1993 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.89, 2.31] |
| 1.1 AeriSeal | 1 | 95 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.14, 62.15] |
| 1.2 Airway bypass stents | 1 | 315 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.21, 2.77] |
| 1.3 Endobronchial coils | 3 | 461 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.67, 3.29] |
| 1.4 Endobronchial valves | 5 | 703 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.47, 2.43] |
| 1.5 Intrabronchial valves | 2 | 350 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.95 [0.85, 28.94] |
| 1.6 Vapour ablation | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.82 [0.13, 61.06] |
| 2 Adverse events (end of follow‐up, all methods) | 11 | 1772 | Odds Ratio (M‐H, Random, 95% CI) | 3.00 [2.04, 4.43] |
| 2.1 Aeriseal | 1 | 95 | Odds Ratio (M‐H, Random, 95% CI) | 3.71 [1.34, 10.24] |
| 2.2 Airway bypass stents | 1 | 315 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.65, 2.73] |
| 2.3 Endobronchial coils | 3 | 461 | Odds Ratio (M‐H, Random, 95% CI) | 2.14 [1.41, 3.23] |
| 2.4 Endobronchial valves | 3 | 482 | Odds Ratio (M‐H, Random, 95% CI) | 5.85 [2.16, 15.84] |
| 2.5 Intrabronchial valves | 2 | 350 | Odds Ratio (M‐H, Random, 95% CI) | 3.41 [1.48, 7.84] |
| 2.6 Vapour ablation | 1 | 69 | Odds Ratio (M‐H, Random, 95% CI) | 3.86 [1.00, 14.97] |
1.1. Analysis.
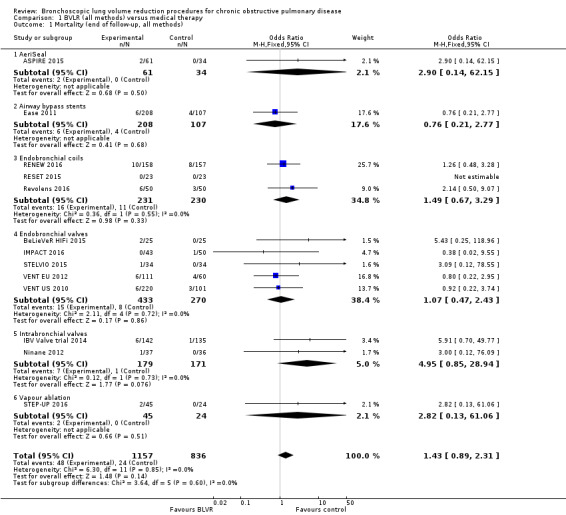
Comparison 1 BVLR (all methods) versus medical therapy, Outcome 1 Mortality (end of follow‐up, all methods).
1.2. Analysis.
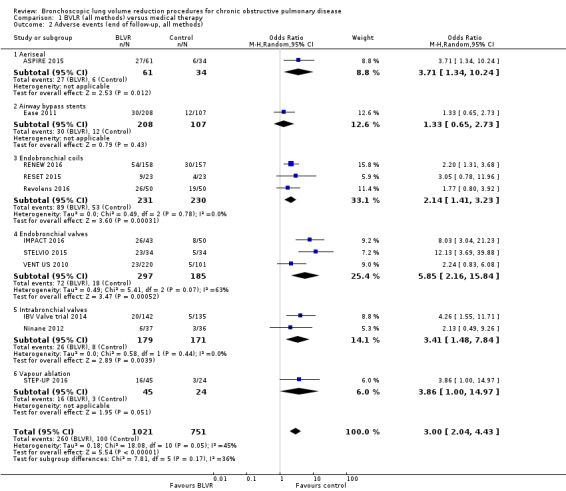
Comparison 1 BVLR (all methods) versus medical therapy, Outcome 2 Adverse events (end of follow‐up, all methods).
Comparison 2. Endobronchial coils versus medical therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Absolute % change in FEV1 (end of follow‐up, endobronchial coils) | 2 | 146 | Mean Difference (IV, Fixed, 95% CI) | 10.88 [5.21, 16.54] |
| 2 SGRQ change from baseline (end of follow‐up, endobronchial coils) | 3 | 461 | Mean Difference (IV, Fixed, 95% CI) | ‐9.14 [‐11.59, ‐6.70] |
| 3 RV change from baseline (L, end of follow‐up, endobronchial coils) | 3 | 461 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.48, ‐0.17] |
| 4 TLC change from baseline (L, end of follow‐up, endobronchial coils) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 RV/TLC change from baseline (end of follow‐up, endobronchial coils) | 2 | 415 | Mean Difference (IV, Fixed, 95% CI) | ‐3.74 [‐5.16, ‐2.33] |
| 6 6MWD change from baseline (end of follow‐up, endobronchial coils) | 3 | 461 | Mean Difference (IV, Random, 95% CI) | 30.85 [‐1.05, 62.76] |
2.1. Analysis.
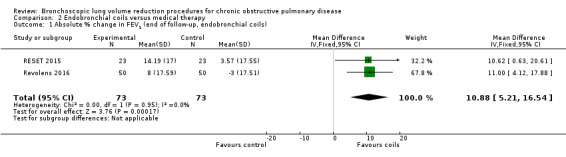
Comparison 2 Endobronchial coils versus medical therapy, Outcome 1 Absolute % change in FEV1 (end of follow‐up, endobronchial coils).
2.2. Analysis.
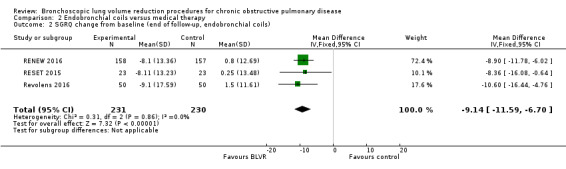
Comparison 2 Endobronchial coils versus medical therapy, Outcome 2 SGRQ change from baseline (end of follow‐up, endobronchial coils).
2.3. Analysis.
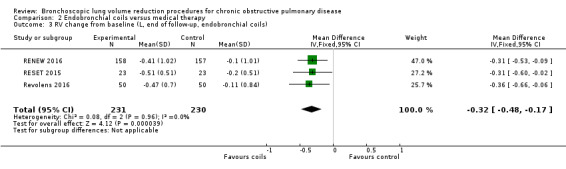
Comparison 2 Endobronchial coils versus medical therapy, Outcome 3 RV change from baseline (L, end of follow‐up, endobronchial coils).
2.4. Analysis.
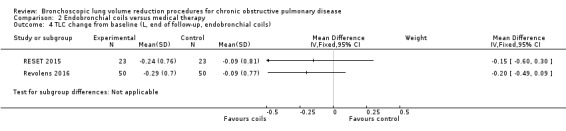
Comparison 2 Endobronchial coils versus medical therapy, Outcome 4 TLC change from baseline (L, end of follow‐up, endobronchial coils).
2.5. Analysis.
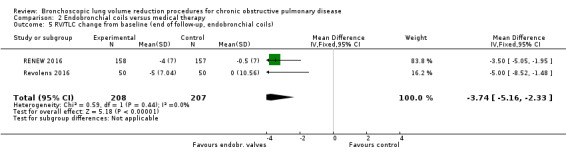
Comparison 2 Endobronchial coils versus medical therapy, Outcome 5 RV/TLC change from baseline (end of follow‐up, endobronchial coils).
2.6. Analysis.
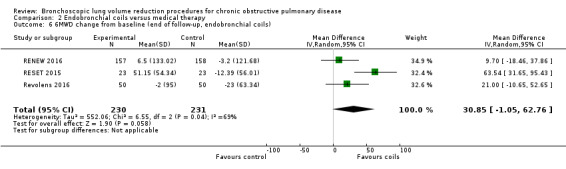
Comparison 2 Endobronchial coils versus medical therapy, Outcome 6 6MWD change from baseline (end of follow‐up, endobronchial coils).
Comparison 3. Endobronchial valves versus medical therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 % change in FEV1 (end of follow‐up, endobronchial valves) | 5 | 703 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.48 [0.32, 0.64] |
| 2 % change in FEV1 (stratified per follow‐up, endobronchial valves) | 5 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 90‐day | 2 | 143 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.77 [0.43, 1.11] |
| 2.2 6‐Month | 3 | 560 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.22, 0.58] |
| 2.3 12‐month | 1 | 171 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.01, 0.65] |
| 3 % change in FEV1 (stratified per disease distribution, endobronchial valves) | 2 | Mean Difference (Fixed, 95% CI) | 16.36 [9.02, 23.70] | |
| 4 % Change in FEV1 (stratified by collateral ventilation, endobronchial valves) | 3 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 No collateral ventilation | 3 | Mean Difference (Fixed, 95% CI) | 18.15 [11.81, 24.48] | |
| 4.2 Collateral ventilation | 2 | Mean Difference (Fixed, 95% CI) | 2.48 [‐2.63, 7.59] | |
| 5 Mortality (stratified per follow‐up, endobronchial valves) | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Postoperative | 2 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.12, 80.39] |
| 5.2 90‐day | 5 | 703 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.67, 7.02] |
| 5.3 6‐months | 2 | 239 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.32, 13.16] |
| 5.4 12‐months | 2 | 492 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.33, 2.22] |
| 6 Mortality (stratified by collateral ventilation, endobronchial valves) | 5 | 703 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.47, 2.43] |
| 6.1 Participants tested for fissure status | 3 | 211 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.40, 9.35] |
| 6.2 Participants not tested for fissure status | 2 | 492 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.33, 2.22] |
| 7 SGRQ change from baseline (end of follow‐up, endobronchial valves) | 5 | 695 | Mean Difference (IV, Random, 95% CI) | ‐7.29 [‐11.12, ‐3.45] |
| 8 SGRQ change from baseline (stratified by follow‐up time, endobronchial valves) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 90‐day | 2 | 135 | Mean Difference (IV, Random, 95% CI) | ‐8.75 [‐12.76, ‐4.74] |
| 8.2 6‐month | 3 | 560 | Mean Difference (IV, Random, 95% CI) | ‐7.09 [‐12.59, ‐1.60] |
| 8.3 12‐month | 2 | 492 | Mean Difference (IV, Random, 95% CI) | ‐4.05 [‐6.51, ‐1.59] |
| 9 SGRQ change from baseline (stratified by collateral ventilation, endobronchial valves) | 5 | 694 | Mean Difference (IV, Fixed, 95% CI) | ‐5.38 [‐7.38, ‐3.38] |
| 9.1 Participants with intact fissures | 4 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐9.03 [‐12.07, ‐5.98] |
| 9.2 participants without intact fissures | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐5.48, 5.48] |
| 9.3 Unable to determine fissure status | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | ‐3.4 [‐6.43, ‐0.37] |
| 10 RV change from baseline (L, end of follow‐up, endobronchial valves) | 3 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐0.77, ‐0.39] |
| 11 TLC change from baseline (L, end of follow‐up, endobronchial valves) | 2 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.46, ‐0.23] |
| 12 RV/TLC change from baseline (end of follow‐up, endobronchial valves) | 2 | 118 | Mean Difference (IV, Random, 95% CI) | ‐5.76 [‐10.45, ‐1.06] |
| 13 6MWD change from baseline (end of follow‐up, endobronchial valves) | 4 | 379 | Mean Difference (IV, Random, 95% CI) | 38.12 [8.68, 67.56] |
| 14 6MWD change from baseline (stratified per collateral ventilation,, endobronchial valves) | 4 | 379 | Mean Difference (IV, Random, 95% CI) | 38.12 [8.68, 67.56] |
| 14.1 Selected participants with intact fissures | 3 | 208 | Mean Difference (IV, Random, 95% CI) | 50.19 [24.96, 75.41] |
| 14.2 Selected participants without intact fissures | 1 | 171 | Mean Difference (IV, Random, 95% CI) | 5.0 [‐21.00, 31.00] |
3.1. Analysis.
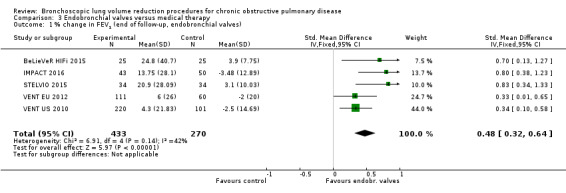
Comparison 3 Endobronchial valves versus medical therapy, Outcome 1 % change in FEV1 (end of follow‐up, endobronchial valves).
3.2. Analysis.

Comparison 3 Endobronchial valves versus medical therapy, Outcome 2 % change in FEV1 (stratified per follow‐up, endobronchial valves).
3.3. Analysis.
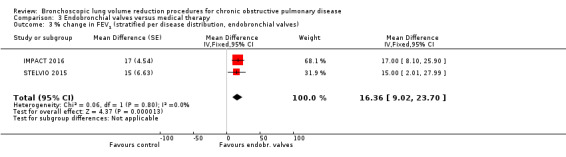
Comparison 3 Endobronchial valves versus medical therapy, Outcome 3 % change in FEV1 (stratified per disease distribution, endobronchial valves).
3.4. Analysis.
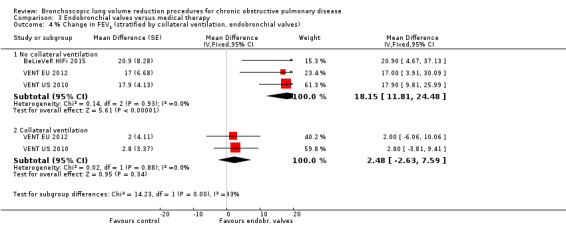
Comparison 3 Endobronchial valves versus medical therapy, Outcome 4 % Change in FEV1 (stratified by collateral ventilation, endobronchial valves).
3.5. Analysis.
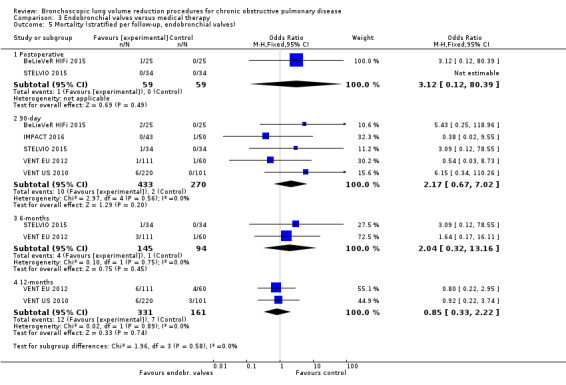
Comparison 3 Endobronchial valves versus medical therapy, Outcome 5 Mortality (stratified per follow‐up, endobronchial valves).
3.6. Analysis.
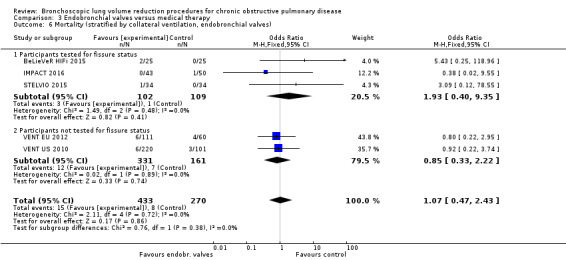
Comparison 3 Endobronchial valves versus medical therapy, Outcome 6 Mortality (stratified by collateral ventilation, endobronchial valves).
3.7. Analysis.
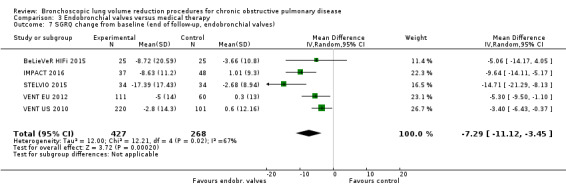
Comparison 3 Endobronchial valves versus medical therapy, Outcome 7 SGRQ change from baseline (end of follow‐up, endobronchial valves).
3.8. Analysis.
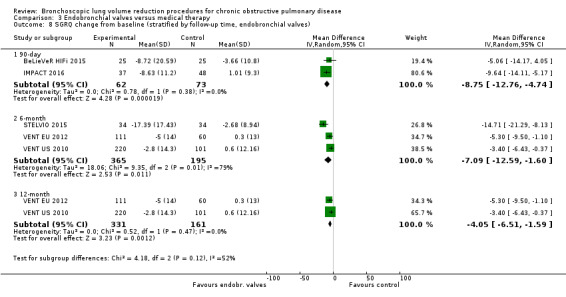
Comparison 3 Endobronchial valves versus medical therapy, Outcome 8 SGRQ change from baseline (stratified by follow‐up time, endobronchial valves).
3.9. Analysis.
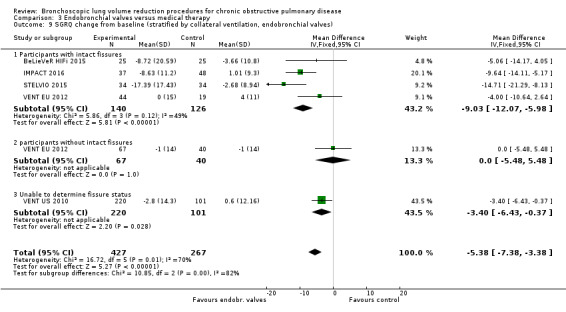
Comparison 3 Endobronchial valves versus medical therapy, Outcome 9 SGRQ change from baseline (stratified by collateral ventilation, endobronchial valves).
3.10. Analysis.
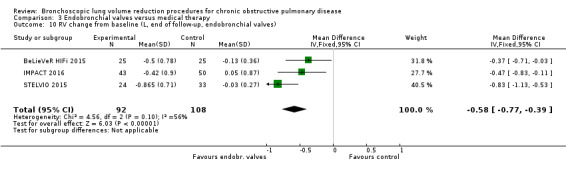
Comparison 3 Endobronchial valves versus medical therapy, Outcome 10 RV change from baseline (L, end of follow‐up, endobronchial valves).
3.11. Analysis.
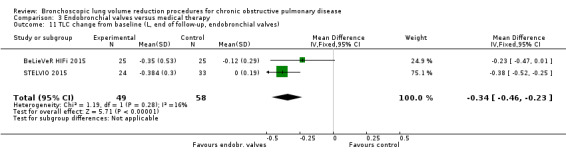
Comparison 3 Endobronchial valves versus medical therapy, Outcome 11 TLC change from baseline (L, end of follow‐up, endobronchial valves).
3.12. Analysis.
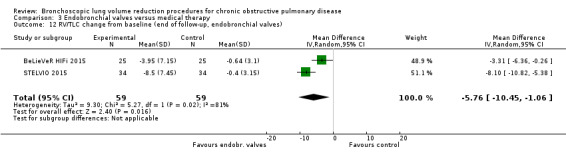
Comparison 3 Endobronchial valves versus medical therapy, Outcome 12 RV/TLC change from baseline (end of follow‐up, endobronchial valves).
3.13. Analysis.
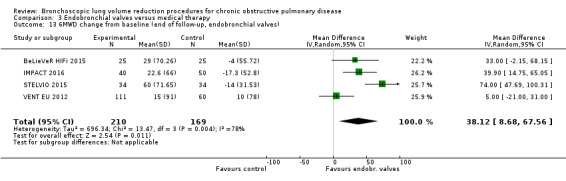
Comparison 3 Endobronchial valves versus medical therapy, Outcome 13 6MWD change from baseline (end of follow‐up, endobronchial valves).
3.14. Analysis.
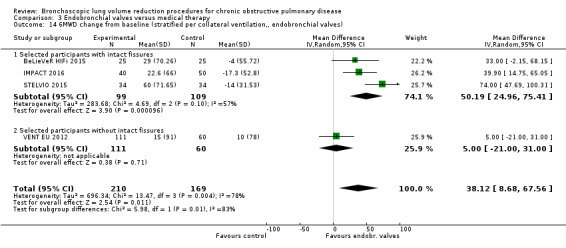
Comparison 3 Endobronchial valves versus medical therapy, Outcome 14 6MWD change from baseline (stratified per collateral ventilation,, endobronchial valves).
Comparison 4. Intrabronchial valves versus medical therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SGRQ change from baseline (end of follow‐up, intrabronchial valves) | 2 | 350 | Mean Difference (IV, Fixed, 95% CI) | 2.64 [‐0.28, 5.56] |
| 2 RV change from baseline (L, end of follow‐up, intrabronchial valves) | 2 | 322 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [0.12, 0.65] |
| 3 TLC change from baseline (L, end of follow‐up, intrabronchial valves) | 2 | 322 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.12, 0.39] |
| 4 PAO2 (end of follow‐up, intrabronchial valves) | 2 | 308 | Mean Difference (IV, Random, 95% CI) | 1.95 [‐4.20, 8.10] |
| 5 PACO2 (end of follow‐up, intrabronchial valves) | 2 | 315 | Mean Difference (IV, Fixed, 95% CI) | 1.33 [0.27, 2.39] |
| 6 6MWD change from baseline (intrabronchial valves) | 2 | 326 | Mean Difference (IV, Fixed, 95% CI) | ‐19.54 [‐37.11, ‐1.98] |
4.1. Analysis.
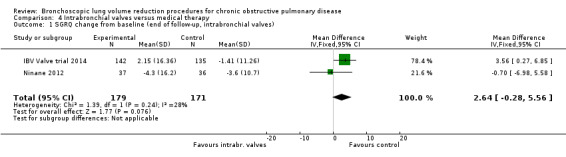
Comparison 4 Intrabronchial valves versus medical therapy, Outcome 1 SGRQ change from baseline (end of follow‐up, intrabronchial valves).
4.2. Analysis.
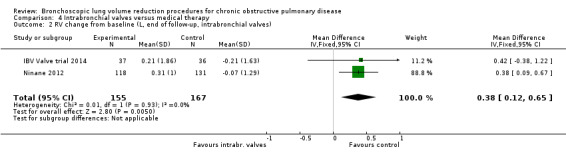
Comparison 4 Intrabronchial valves versus medical therapy, Outcome 2 RV change from baseline (L, end of follow‐up, intrabronchial valves).
4.3. Analysis.
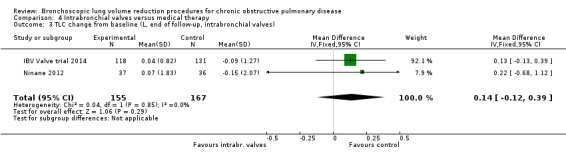
Comparison 4 Intrabronchial valves versus medical therapy, Outcome 3 TLC change from baseline (L, end of follow‐up, intrabronchial valves).
4.4. Analysis.
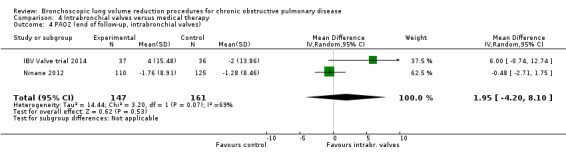
Comparison 4 Intrabronchial valves versus medical therapy, Outcome 4 PAO2 (end of follow‐up, intrabronchial valves).
4.5. Analysis.
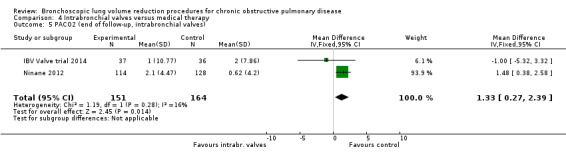
Comparison 4 Intrabronchial valves versus medical therapy, Outcome 5 PACO2 (end of follow‐up, intrabronchial valves).
4.6. Analysis.
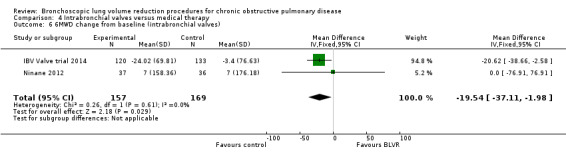
Comparison 4 Intrabronchial valves versus medical therapy, Outcome 6 6MWD change from baseline (intrabronchial valves).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ASPIRE 2015.
| Methods | Randomized clinical trial. 3:2 block randomizations stratified by study site. Allocation concealment not mentioned. Follow‐up until 6 months. | |
| Participants | Baseline Age: treatment 65 years versus control 64 years Participants: treatment n = 61 versus control n = 34 % female: 41% Disease distribution: heterogeneous Baseline score on outcomes: Median FEV1 % predicted (IQR): treatment 29% (23 to 35) versus control 30% (27 to 38) Median QoL in units total score SGRQ (IQR): treatment 54 units (46 to 65) versus control 58 units (45 to 74) Median RV % predicted (IQR): treatment 200% (168 to 231) versus control 179% (168 to 215) Median TLC % predicted (IQR): treatment 124% (115 to 139) versus control 120% (108 to 133) Median DLCO % predicted (IQR): treatment 33% (26 to 39) versus control 36% (18 to 46) Median PaO2 in mm Hg (IQR): treatment 70 mm Hg (64 to 78) versus control 71 mm Hg (63 to 78) Median PaCO2 in mm Hg (IQR): treatment 41 mm Hg (37 to 45) versus control 41 mm Hg (37 to 44) Median 6MWD in meters (IQR): treatment 313 m (236 to 363) versus control 293 m (247 to 420) |
|
| Interventions | Intervention: AeriSeal + optimal medical care. Control: optimal medical care All participants were on or completed optimal medical care |
|
| Outcomes | ‐ Forced expiratory volume in one second (FEV1) ‐ St. George Respiratory Questionnaire (SGRQ) ‐ Medical Research Council Dyspnea score (mMRC) ‐ 6‐minute walk test (6MWT) | |
| Notes | Aeris Therapeutics funded the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomisation sequence was computer‐generated in blocks of five, stratified by site" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As per the FDA mandate, a sham procedure was not used. Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Those conducting pulmonary function tests, 6MWD and questionnaires were blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported: balanced between groups |
| Selective reporting (reporting bias) | Low risk | Protocol available as supplementary material |
| Other bias | High risk | High: study terminated early due to 'business reasons' |
BeLieVeR HIFi 2015.
| Methods | Randomized clinical trial. 1:1 block randomizations stratified by study site. Masking maintained by working with two separate teams. Follow‐up until 3 months. | |
| Participants | Baseline Age: treatment 62 years versus control 63 years Participants: treatment n = 25 versus control n = 25 % female: 38% Disease distribution: heterogeneous Baseline score on outcomes: Mean FEV1 % predicted (SD): treatment 31.6% (10.2) versus control 31.8% (10.5) Mean QoL in units total score on SGRQ (SD): treatment 67.79 units (13.17) versus control 70.65 units (12.48) Mean RV % predicted (SD): treatment 219% (39) versus control 245% (44) Mean TLC % predicted (SD): treatment 132% (12) versus control 142% (15) Mean DLCO % predicted (SD): treatment 33.8% (10.8) versus control 33.7% (44) Mean PaO2 in kPa (SD): treatment 9.74 kPa (1.45) versus control 9.47 kPa (0.89) Mean PaCO2 in kPa (SD): treatment 4.81 kPa (0.86) versus control 4.90 kPa (0.61) Mean 6MWD in meters (SD): treatment 342 m (94) versus control 334 m (81) |
|
| Interventions | Treatment: unilateral valve placement. Control: sham valve placement |
|
| Outcomes | ‐ Percentage change in post‐bronchodilator
FEV1 measured 90 days post procedure ‐ Change in endurance (change in 6MWD) ‐ Change in COPD assessment Test (CAT) ‐ Change in St. George Respiratory Questionnaire (SGRQ) |
|
| Notes | Funded by the Medical Research Council (MRC) and managed by the National Institute for Health Research (NIHR) on behalf of the MRC‐NIHR partnership. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "predetermined block randomisation, with a block size of 10, computer‐generated by the trial statistician" |
| Allocation concealment (selection bias) | Low risk | "Masking was maintained by having two separate teams: one which undertook the randomized procedures and a separate team, masked to study assignment, responsible for recruitment and the assessments " |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Involved sham procedure so participants were blinded. Not possible to blind the proceduralist |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported: balanced between groups |
| Selective reporting (reporting bias) | Low risk | Low: protocol published |
| Other bias | Low risk | No other risk of bias found |
Ease 2011.
| Methods | Randomized clinical trial. 2:1 block randomizations. Allocation concealment was maintained by working with two separate study groups. Follow‐up until 12 months. | |
| Participants | Baseline Age: treatment 64 years versus control 64 years Participants: treatment n = 208 versus control n = 107 % female: 49% Disease distribution: homogenous Baseline score on outcomes: Mean FEV1 % predicted (SD): treatment 23.2% (6.1) versus control 23.6% (7.2) Mean QoL in units total score on SGRQ (SD): treatment 56.6 units (12.9) versus control 58.04 units (13.25) Mean RV % predicted (SD): treatment 244.1 (52.8) versus control 248.5 (51.4) Mean TLC in liter (SD): treatment 7.64 L (1.56) versus control 7.70 L (1.54) Mean DLCO % predicted (SD): treatment 30.6% (11.4) versus control 28.4% (51.4) Mean PaO2: not reported Mean PaCO2: not reported Mean 6MWD in meters (SD): treatment 302 m (88) versus control 297 m (85) |
|
| Interventions | Intervention group receive stent placement while control group received sham bronchoscopy. | |
| Outcomes | ‐ Forced expiratory volume in one second (FEV1)
‐ Modified medical research council dyspnoea scale (mMRC) ‐ Residual Volume (RV) ‐ Forced vital capacity (FVC) ‐ St. George’s Respiratory Questionnaire (SGRQ) ‐ 6‐minute walk test (6MWD) ‐ Cycle ergometry. |
|
| Notes | Funded by Broncus Technologies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization by independent, automated, internet‐based service (Advance Research Associates, Mountain View, CA, USA), with a permuted block size of six and sequential assignment, stratified by investigational site" |
| Allocation concealment (selection bias) | Low risk | "To maintain the study blind, investigators were divided into team A (masked), which completed pre‐procedure and post‐procedure assessments, and team B (unmasked), which only did bronchoscopies without further interaction with patients. We communicated randomisation assignments to members of team B only" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Involved sham procedure so participants were blinded. Not possible to blind the proceduralist |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported: balanced between groups |
| Selective reporting (reporting bias) | Low risk | Protocol published |
| Other bias | High risk | "Funder was responsible for trial design and coordination and data analysis. The corresponding author and writing committee had full access to all data and had final responsibility for the decision to submit for publication" |
Eberhardt 2012.
| Methods | Randomized clinical trial. 1:1 allocation. Not sufficient information to permit judgement on allocation concealment. Follow‐up until 3 months. | |
| Participants | Baseline Age: unilateral 63 years versus bilateral 64 years Participants: unilateral n = 11 versus bilateral n = 11 % female: 45% Disease distribution: heterogeneous Baseline score on outcomes: Mean FEV1 % predicted (SD): unilateral 29.4% (3.95) versus bilateral 31.9 (7.62) Mean QoL in units total score on SGRQ (SD): unilateral 59.0 units (16.3) versus bilateral 58.8 units (14.2) Mean RV % predicted (SD): unilateral 264.8% (47) versus bilateral 269.3 (66.9) Mean TLC % predicted (SD): unilateral 142.2% (15.3) versus bilateral 145.4% (24.9) Mean DLCO: not reported Mean PaO2 in mm Hg (SD): unilateral 67.3 mm Hg (5.95) versus bilateral 62.6 mm Hg (7.99) Mean PaCO2 in mm Hg (SD): unilateral 40.8 mm Hg (5.4) versus bilateral 45.2 mm Hg (4.79) Mean 6MWD in meters (SD): unilateral 305.4 m (68.7) versus control 293.2 m (85.9) |
|
| Interventions | Unilateral (group 1) versus partial bilateral (group 2) placement of intrabronchial valves. | |
| Outcomes | ‐ Pulmonary function tests (PFTs), ‐ 6‐min walk distance (6MWD), ‐ Modified Medical Research Council (mMRC) dyspnoea score ‐ St. George Respiratory Questionnaire (SGRQ) |
|
| Notes | Grants received from Olympus Europe Holding (Germany) for medical education activities. The intrabronchial valves used for this trial and fees associated with the license to use the St. George Respiratory Questionnaire were provided by Olympus Medical Co, Tokyo, Japan . |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported: no attrition |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Insufficient information provided to permit judgement |
IBV Valve trial 2014.
| Methods | Randomized clinical trial. 1:1 allocation. Not sufficient information to permit judgement on allocation concealment. Follow‐up until 6 months. | |
| Participants | Baseline Age: treatment 65 years versus control 65 years Participants: treatment n = 142 versus control n = 135 % female: 43% Disease distribution: heterogeneous Baseline score on outcomes: Mean FEV1 % predicted (SD): treatment 29.8% (7.5) versus control 29.7% (7.9) Mean QoL in units total score on SGRQ (SD): treatment 54.8 units (15.5) versus control 57.1 units (15.2) Mean RV % predicted (SD): treatment 216.0% (50.1) versus control 215.8% (55.9) Mean TLC % predicted (SD): treatment 128.1% (15.9) versus control 128.2% (19.8) Mean DLCO % predicted (SD): treatment 36.4% (12.7) versus control 35.0% (13.1) Mean PaO2 in mm Hg (SD): treatment 67.8 mm Hg (11.3) versus control 67.0 mm Hg (10.7) Mean PaCO2 in mm Hg (SD): treatment 39.8 mm Hg (5.3) versus control 40.8 mm Hg (4.8) Mean 6MWD in meters (SD): treatment 314.1 m (88.6) versus control 308.6 m (81.6) |
|
| Interventions | Partial bilateral placement of Intrabronchial valves compared to sham control | |
| Outcomes | ‐ St. George Respiratory Questionnaire (SGRQ) ‐ Lobar volume changes ‐ Pulmonary function tests ‐ Short Form‐36 ‐ 6‐minute walking distance (6MWD) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment mentioned but not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | " If the patient was randomized to control, a catheter was passed into the target airways and a script read out loud indicating (sham) valve deployment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A separate team without knowledge of the group assignment provided follow‐up evaluations to maintain blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition mentioned: more attrition in treatment group but not deemed sufficient to influence results |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. No protocol published. |
| Other bias | Low risk | No other risk of bias found |
IMPACT 2016.
| Methods | Randomized open‐label one‐way cross‐over RCT. Follow‐up: until 12 months, but only three‐month results published at time of review. | |
| Participants | Baseline Age: treatment 64 years versus control 63 years Participants: treatment n = 43 versus control n = 50 % female: 61% Disease distribution: homogenous Baseline score on outcomes: Mean FEV1 % predicted (SD): treatment 28.4% (6.3) versus control 29.9% (6.6) Mean QoL in units total score on SGRQ (SD): treatment 63.2 units (13.7) versus control 59.3 units (15.6) Mean RV % predicted (SD): treatment 277.3% (55.2) versus control 273.7% (63.4) Mean TLC % predicted (SD): treatment 144.9% (21.2) versus control 144.2% (17.6) Mean DLCO % predicted (SD): not reported Mean PaO2 in mm Hg (SD): not reported Mean PaCO2 in mm Hg (SD): not reported Mean 6MWD in meters (SD): treatment 308 m (91) versus control 328 m (93) |
|
| Interventions | Intervention: Endobronchial valves Control: optimal medical care |
|
| Outcomes | ‐ Forced expiratory volume in one second (FEV1) ‐ St. George Respiratory Questionnaire (SGRQ) ‐ Residual volume (RV) ‐ 6‐minute walking distance (6MWD) ‐ Modified Medical Research Council (mMRC) dyspnoea score ‐ CAT score ‐ BODE index |
|
| Notes | "This study was sponsored and funded by Pulmonx Corporation, Redwood City, CA, USA" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization used a blocked design, although a change in randomization blocks happened during the trial |
| Allocation concealment (selection bias) | Low risk | Concealed envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Well documented reasons for exclusion, drop‐outs and adverse events |
| Selective reporting (reporting bias) | Low risk | NCT02025205 ‐ reported all outcomes |
| Other bias | Low risk | None found |
Ninane 2012.
| Methods | Randomized clinical trial. 1:1 allocation via block randomization. Allocation concealment via sealed envelopes. Follow‐up until 6 months. | |
| Participants | Baseline Age: treatment 61 years versus control 62 years Participants: treatment n = 37 versus control n = 36 % female: 41% Disease distribution: heterogeneous Baseline score on outcomes: Mean FEV1 % predicted (SD): treatment 35% (10) versus control 32% (7) Mean QoL in units total score on SGRQ (SD): treatment 61 units (11) versus control 60 units (13) Mean RV % predicted (SD): treatment 238% (74) versus control 258% (67) Mean TLC % predicted (SD): treatment 130% (19) versus control 136% (18) Mean DLCO mL/min/mm Hg (SD): treatment 9.90 mL/min/mm Hg (4.24) versus control 8.33 mL/min/mm Hg (4.47) Mean PaO2 in mm Hg (SD): treatment 65 mm Hg (10) versus control 66 mm Hg (10) Mean PaCO2 in mm Hg (SD): treatment 40 mm Hg (5) versus control 40 mm Hg (4) Mean 6MWD in meters (SD): treatment 337 m (106) versus control 346 m (123) |
|
| Interventions | Partial bilateral placement of Intrabronchial valves compared to sham control | |
| Outcomes | ‐ St. George Respiratory Questionnaire (SGRQ) ‐ Lobar volume changes ‐ Pulmonary function tests ‐ 6‐minute walking distance (6MWD) ‐ Modified Medical Research Council (mMRC) dyspnoea score |
|
| Notes | Funding provided by Spiration Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "the statistician created blocks of randomization sealed envelopes that were provided to each of the clinical sites." |
| Allocation concealment (selection bias) | Unclear risk | "The envelopes were opened in numerical order only after the patient was anaesthetized and the bronchoscopic evaluation of the airways was completed." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Sham controlled study. Proceduralist could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition mentioned: balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | Did not reach intended number of participants |
RENEW 2016.
| Methods | Randomized clinical trial. 1:1 allocation via block randomization stratified per emphysema type. Follow‐up until 12 months. | |
| Participants | Baseline Age: treatment 63 years versus control 64 years Participants: treatment n = 158 versus control n = 157 % female: 52.4% Disease distribution: both Baseline score on outcomes: Mean FEV1 % predicted (SD): treatment 25.7% (6.3) versus control 26.3% (6.7) Mean QoL in units total score on SGRQ (SD): treatment 60.1 units (12.8) versus control 57.4 units (14.8) Mean RV % predicted (SD): treatment 245.9% (39.1) versus control 244.5% (38.7) Mean TLC % predicted (SD): treatment 139.2% (15.6) versus control 138.8% (16.1) Mean DLCO % predicted (SD): treatment 34.1% (10.5) versus control 34.5% (10.7) Mean PaO2 in mm Hg (SD): treatment 41.6 mm Hg (5.6) versus control 41.5 mm Hg (5.3) Mean PaCO2 in mm Hg (SD): treatment 68.0 mm Hg (10.5) versus control 69.2 mm Hg (10.9) Mean 6MWD in meters (SD): treatment 312.0 m (79.1) versus control 302.7 m (79.3) |
|
| Interventions | Treatment with endobronchial coils versus standard medical care. All participants needed to be treated according to GOLD criteria. | |
| Outcomes | ‐ 6‐Minute Walking distance (6MWD) ‐ Forced expiratory volume in one second (FEV1) ‐ St George’s Respiratory Questionnaire (SGRQ) ‐ Pulmonary function test parameters ‐ Adverse events |
|
| Notes | RENEW was supported by PneumRx Inc, a BTG International group company. Drs Sciurba, Criner, and Slebos received institutional support from Pulmonx. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blinded block randomization (block size of 4) stratified by type of emphysema |
| Allocation concealment (selection bias) | Unclear risk | Unsufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Only walk and spirometry was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported: balanced between groups |
| Selective reporting (reporting bias) | Low risk | Protocol added in supplement and trial registered at clinicaltrials.gov: NCT01608490 |
| Other bias | Low risk | No risk of other bias detected |
RESET 2015.
| Methods | Randomized clinical trial. 1:1 allocation via block randomization, sealed via envelopes. Follow‐up until 12 months. | |
| Participants | Baseline Age: treatment 62 years versus control 65 Participants: treatment n = 23 versus control n = 24 % female: 38% Disease distribution: homogenous and heterogeneous Baseline score on outcomes: Mean FEV1 % predicted (SD): treatment 27.2% (8.0) versus control 28.9% (6.9) Mean QoL in units total score on SGRQ (SD): treatment 65.2 units (8.7) versus control 53.1 units (13.8) Mean RV % predicted (SD): treatment 235.8% (50.3) versus control 217.6% (45.3) Mean TLC % predicted (SD): treatment 136.7% (13.1) versus control 133.6% (15.4) Mean DLCO % predicted (SD): treatment 34.4% (10.2) versus control 38.6% (15.5) Mean PaO2: not reported Mean PaCO2: not reported Mean 6MWD in meters (SD): treatment 293.7 m (75.5) versus control 346.2 m (110.9) |
|
| Interventions | Treatment with endobronchial coils versus optimal medical care. | |
| Outcomes |
Primary safety outcome: number of participants experiencing major complications: death, pneumothorax, haemoptysis, COPD exacerbations, lower respiratory tract infections, respiratory failure, bronchoscopy to remove coils. Primary effectiveness outcome: 6MWD Secondary effectiveness outcome: 6MWD responders, SGRQ, RV and RV/TLC |
|
| Notes | Funded by PneumRx | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated in blocks of four and stratified by treatment centre" |
| Allocation concealment (selection bias) | Low risk | "investigators were unaware of the block sizes. The generated codes were placed in opaque sealed envelopes and opened in sequence when a patient fulfilled all the eligibility criteria" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "all assessments were done by independent research nurses and physiologists who were masked to treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported: balanced between groups |
| Selective reporting (reporting bias) | Low risk | Protocol available |
| Other bias | Low risk | No other risk of bias found |
Revolens 2016.
| Methods | Randomized clinical trial. 1:1 allocation via block randomization. Follow‐up until 6 months. | |
| Participants | Baseline Age: treatment 62 years versus control 63 years Participants: treatment n = 50 versus control n = 50 % female: 39% Disease distribution: homogenous and heterogeneous Baseline score on outcomes: Mean FEV1 % predicted (SD): treatment 25.7% (7.5) versus control 27.4% (6.2) Mean QoL in units total score on SGRQ (SD): treatment 60.8 units (12.8) versus control 57.1 units (14.1) Mean RV % predicted (SD): treatment 271.2% (38.1) versus control 269.3% (44.3) Mean TLC % predicted (SD): treatment 141.7% (16.6) versus control 143.6% (18) Mean DLCO: not reported Mean PaO2: not reported Mean PaCO2: not reported Mean 6MWD in meters (SD): treatment 300 m (112) versus control 326 m (121) |
|
| Interventions | Tretament with endobronchial coils compared to optimal medical care, | |
| Outcomes | ‐ 6‐Minute Walking distance (6MWD) ‐ St George’s Respiratory Questionnaire (SGRQ) ‐ Forced expiratory volume in one second (FEV1) ‐ Other pulmonary function parameters ‐ Modified Medical Research Council (mMRC) dyspnoea score ‐ Adverse events ‐ EuroQoL‐5 |
|
| Notes | Supported by an academic grant from the French Ministry of Health (Direction Générale de l’Offre de Soins, PSTIC‐2012). The coils were purchased from the manufacturer, PneumRx/BTG. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization system with fixed blocks of 4. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported: balanced between groups |
| Selective reporting (reporting bias) | Low risk | Protocol published |
| Other bias | Low risk | No other risk of bias found |
STELVIO 2015.
| Methods | Randomized clinical trial. 1:1 allocation via block randomization, sealed via envelopes. Follow‐up until 6 months. | |
| Participants | Baseline Age: treatment 58 years versus control 59 years Participants: treatment n = 34 versus control n = 34 % female: 68% Disease distribution: homogenous and heterogeneous Baseline score on outcomes: Mean FEV1 % predicted (SD): treatment 29% (7) versus control 29% (8) Mean QoL in units total score on SGRQ (SD): treatment 59.1 units (13.7) versus control 59.3 units (11.6) Mean RV % predicted (SD): treatment 216% (36) versus control 220% (32) Mean TLC % predicted (SD): treatment 130% (13) versus control 133% (10) Mean DLCO % predicted (SD): treatment 38.7% (9.1) versus control 39.0% (9.7) Mean PaO2 in mm Hg (SD): treatment 69 mm Hg (12) versus control 69 mm Hg (9) Mean PaCO2 in mm Hg (SD): treatment 38 mm Hg (6) versus control 38 mm Hg (4) Mean 6MWD in meters (SD): treatment 372 m (90) versus control 377 m (84) |
|
| Interventions | Placement of endobronchial valves compared to standard medical care in concordance with GOLD guidelines. | |
| Outcomes | ‐ Forced expiratory volume in one second (FEV1) ‐ Forced vital capacity (FVC) ‐ 6‐minute walking distance (6MWD) ‐ St. George Respiratory Questionnaire (SGRQ) ‐ Clinical COPD questionnaire ‐ Pulmonary function tests |
|
| Notes | Funded by the Netherlands Organization for Health Research and Development and the University Medical Center Groningen; Netherlands Trial Register number, NTR2876. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Study used randomization list that was computer‐generated in blocks of four" |
| Allocation concealment (selection bias) | Low risk | "The principal investigator and study personnel did not have access to the list. The generated codes were placed in opaque sealed envelopes, which were numbered sequentially" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Only PFT measures were conducted by blind assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported: more attrition in treatment group but not deemed sufficient enough to influence results |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other risk of bias found |
STEP‐UP 2016.
| Methods | Randomized clinical trial. 2:1 allocation via block randomization. Follow‐up until 6 months. | |
| Participants | Baseline Age: treatment 64 years versus control 63 years Participants: treatment n = 46 versus control n = 24 % female: 52% Disease distribution: heterogeneous Baseline score on outcomes: Mean FEV1 % predicted (SD): treatment 33.8% (8.2) versus control 33.7% (8.8) Mean QoL in units total score on SGRQ (SD): treatment 57.7 units (15) versus control 57.3 units (20) Mean RV % predicted (SD): treatment 235% (40.3) versus control 243% (45.1) Mean TLC % predicted (SD): treatment 135.9% (14.6) versus control 136.8% (16.9) Mean DLCO (SD): not reported Mean PaO2 (SD): not reported Mean PaCO2 (SD): not reported Mean 6MWD in meters (SD): treatment 356 m (92) versus control 370 m (111.5) |
|
| Interventions | Treatment with vapour ablation versus optimal medical care in concordance with GOLD. | |
| Outcomes | ‐ Forced expiratory volume in one second (FEV1) ‐ St. George Respiratory Questionnaire (SGRQ) ‐ Adverse events ‐ Other pulmonary function test parameters ‐ 6‐minute walking distance (6MWD) |
|
| Notes | Funded by Uptake Medical | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blinded blocked randomization scheme separated by site |
| Allocation concealment (selection bias) | Unclear risk | Blinding mentioned but no specifics reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label, no mentioning of outcome assessment blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported: balanced between groups |
| Selective reporting (reporting bias) | Low risk | Protocol published |
| Other bias | Low risk | No other risk of bias found |
VENT EU 2012.
| Methods | Randomized clinical trial. 2:1 allocation. Follow‐up until 12 months. | |
| Participants | Baseline Age: treatment 60 years versus control 60 years Participants: treatment n = 111 versus control n = 60 % female: 25% Disease distribution: homogenous and heterogeneous Baseline score on outcomes: Mean FEV1 % predicted (SD): treatment 29% (8) versus control 30% (8) Mean QoL in total score on SGRQ (SD): treatment 59 units (13) versus control 56 units (18) Mean RV % predicted (SD): treatment 240% (51) versus control 240% (47) Mean TLC % predicted (SD): treatment 127% (15) versus control 129% (14) Mean DLCO in mm Hg (SD): treatment 9.77 mL/min/mm Hg (3.83) versus control 9.67 mL/min/mm Hg (3.51) Mean PaO2 in mm Hg (SD): treatment 69.3 mm Hg (10.6) versus control 69.7 mm Hg (11.5) Mean PaCO2 in mm Hg (SD): treatment 38.9 mm Hg (4.6) versus control 38.6 mm Hg (5.3) Mean 6MWD in meters (SD): treatment 341 m (108) versus control 360 m (117) |
|
| Interventions | Unilateral endobronchial valve placement compared to standard medical care. All participants needed to be on optimal medical care according to GOLD guidelines. | |
| Outcomes | ‐ Forced expiratory volume in one second (FEV1)
‐ 6‐minute walking distance (6MWD) ‐ St. George Respiratory Questionnaire (SGRQ) ‐ Medical Research Council Dyspnea score (MRCD) ‐ Oxygen use ‐ Maximum work load |
|
| Notes | Funded by Pulmonx | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Independent data and safety monitoring board mentioned, but not enough information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported: more attrition in treatment group but not deemed sufficient enough to influence results |
| Selective reporting (reporting bias) | Low risk | Protocol published |
| Other bias | Unclear risk | Insufficient information provided to permit judgement |
VENT US 2010.
| Methods | Randomized clinical trial. 2:1 allocation. Follow‐up until 12 months. | |
| Participants | Baseline Age: treatment 65 years versus control 65 years Participants: treatment n = 220 versus control n = 101 % female: 57% Disease distribution: homogenous and heterogeneous Baseline score on outcomes: Mean FEV1 % predicted (SD): treatment 30% (8) versus control 30% (8) Mean QoL: not reported Mean RV % predicted (SD): treatment 216% (44) versus control 212% (47) Mean TLC % predicted (SD): treatment 124% (15) versus control 125% (16) Mean DLCO in % predicted (SD): treatment 33% (9) versus control 36% (16) Mean PaO2 in mm Hg (SD): treatment 69.1 mm Hg (10.3) versus control 68.4 mm Hg (8.1) Mean PaCO2 in mm Hg (SD): treatment 40.5 mm Hg (4.3) versus control 41.6 mm Hg (4.8) Mean 6MWD in meters (SD): treatment 334 m (87) versus control 351 m (83) |
|
| Interventions | Unilateral endobronchial valve placement compared to standard medical care. All participants needed to be on optimal medical care according to GOLD guidelines. | |
| Outcomes | ‐ Forced expiratory volume in one second (FEV1)
‐ 6‐minute walking distance (6MWD) ‐ St. George Respiratory Questionnaire (SGRQ) ‐ Medical Research Council Dyspnea score (MRCD) ‐ Oxygen use ‐ Maximum work load |
|
| Notes | Funded by Pulmonx | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Independent data and safety monitoring board mentioned, but not enough information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported: more attrition in treatment group but not deemed sufficient enough to influence results |
| Selective reporting (reporting bias) | Low risk | Protocol published |
| Other bias | Unclear risk | Insufficient information provided to permit judgement |
BODE: Body‐mass index, airflow Obstruction, Dyspnea, and Exercise; CAT: COPD assessment test score; COPD: Chronic obstructive pulmonary disease; DLCO: Diffusing capacity of the lungs for carbon monoxide; FDA: US Food and Drug Administration; FEV1: Forced expiratory volume in one second; FVC: Forced vital capacity; Hg: mercury; IQR: Interquartile range; kPa: Kilopascal; mMRC: Modified Medical Research Council (mMRC) Dyspnoea Scale; MRC: Medical Research Council; NIHR: National Institute for Health Research; PaCO2: Partial pressure of carbon dioxide; PaO2: Partial pressure of oxygen; PFT: Pulmonary Function Test; QoL: Quality of life; RV: Residual volume; SD: Standard deviation; SGRQ: St. George's Respiratory Questionnaire; TLC: Total lung capacity; 6MWD: 6‐minute walking distance.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abumossalam 2016 | Non‐randomized prospective clinical study |
| Cardoso 2007 | Case series |
| Choong 2005 | Animal study |
| Choong 2006 | Animal study |
| Choong 2008 | In vitro study |
| Criner 2009 | Open‐labeled, multicenter phase 2 dose. Not RCT |
| De Oliveira 2006 | Longitudinal case series |
| Deslee 2014 | Feasibility study |
| Emery 2010 | Animal study |
| Faisal 2016 | Retrospective study |
| Falkenstern‐Ge 2013 | N=1 study |
| Fann 2003 | Animal study |
| Fruchter 2016 | Retrospective analysis |
| Gompelmann 2010 | Feasibility study |
| Gompelmann 2012 | Retrospective analysis |
| Gompelmann 2013 | Retrospective analysis |
| Hartman 2015 | Long‐term follow‐up of pilot studies |
| Herth 2010 | Single‐arm pilot study |
| Herth 2011 | Phase I: Dose escalation study |
| Herth 2012 | Case series |
| Hopkinson 2005 | Case series |
| Hopkinson 2011 | Case series |
| Klooster 2014 | Feasibility trial |
| Kontogianni 2014 | Retrospective analysis |
| Kotecha 2011 | Retrosepctive cohort |
| Kramer 2012 | Single arm, prospective study |
| Kramer 2013 | Retrospective analysis |
| Magnussen 2012 | Retrospective analysis |
| Pizarro 2015 | Observational study |
| Refaely 2010 | Non‐randomized open label phase 2 trial |
| Reilly 2007 | Non‐randomized open label phase 1 trial |
| Rendina 2003 | Case series |
| Slebos 2012 | Single‐arm pilot study |
| Snell 2003 | Case series |
| Snell 2009 | Feasibility trial |
| Snell 2012 | Open‐label, single‐arm safety and efficacy clinical trials |
| Springmeyer 2009 | Case series |
| Toma 2003 | Single‐arm pilot study |
| Venuta 2005 | Prospective, nonrandomized, single‐center longitudinal study |
| Venuta 2011 | Case series |
| Wan 2006 | Retrospective analysis |
| Wood 2007 | Non randomized multicentre study |
| Yim 2004 | Case series |
Characteristics of ongoing studies [ordered by study ID]
ISRCTN19684749.
| Trial name or title | ISRCTN19684749: Comparative effectiveness of lung volume reduction surgery for emphysema and bronchoscopic lung volume reduction with valve placement |
| Methods | Randomized parallel trial. Follow‐up: unknown. Estimated enrolment: 152 participants Sites: hospitals in UK |
| Participants | Participants suffering from heterogeneous emphysema: 1. Adults (aged 18 or over) with COPD 2. FEV1 < 60% predicted 3. Significant hyperinflation (TLC > 100% predicted, RV > 170% predicted) 4. Ex‐smoker > 3 months 5. MRC dyspnoea score of 3 or more 6. CT scan assessed to have intact interlobar fissures (> 90%) and heterogeneous emphysema 7. Provision of informed consent to participate |
| Interventions | Lung volume reduction surgery versus endobronchial valves: Participants will be randomized to either unilateral video assisted thoracoscopic (VATS) lung volume reduction surgery (LVRS) or endobronchial valves (BLVR) placed to achieve lobar occlusion. |
| Outcomes |
Primary: Change in iBODE score (a composite of BMI, FEV1, MRC dyspnoea score and shuttle walk test distance) one year post‐procedure. Secondary:
|
| Starting date | April 2016 |
| Contact information | Dr Nick Hopkinson |
| Notes | ISRCTN19684749 |
NCT01457833.
| Trial name or title | Implantation of endobronchial valves versus intrabronchial valves in patients with severe heterogeneous emphysema |
| Methods | Randomized open‐label study. Follow‐up: until 6 months. Estimated enrolment: n = 50 Site: Thoraxklink Heidelberg (Germany) |
| Participants | COPD patients with severe heterogeneous emphysema Inclusion criteria:
|
| Interventions | Unilateral endobronchial valves versus intrabronchial valve placement |
| Outcomes |
Primary: Improvement in pulmonary function (FEV1 and RV/TLC)
Secondary:
|
| Starting date | August 2011 |
| Contact information | Principal Investigator: Daniela Gompelmann, MD |
| Notes | NCT01457833 |
NCT01796392.
| Trial name or title | Pulmonx endobronchial valves used in treatment of emphysema (NCT01796392) |
| Methods | Randomized open‐label study. Follow‐up: until 12 months. Estimated enrolment: n = 183. Sites: 23 locations (US, Brazil, Netherlands, UK) |
| Participants | Participants suffering from emphysema Inclusion criteria:
|
| Interventions | Intervention: endobronchial valve and optimal medical treatment Control: optimal medical treatment |
| Outcomes | Primary: Forced expiratory volume in 1‐second (FEV1)
Secondary:
|
| Starting date | July 2013 |
| Contact information | Principal Investigator: Gerard Criner, MD and Armin Ernst, MD |
| Notes | NCT01796392 |
NCT01812447.
| Trial name or title | Evaluation of the Spiration® valve system for emphysema to Improve lung function (NCT01812447) |
| Methods | Randomized open‐label study. Follow‐up: 6 months. Estimated enrolment: 270 participants. Sites: multicenter (34 locations in US and Canada) |
| Participants | Inclusion criteria:
|
| Interventions | Intervention: unilateral treatment with IBV valve + optimal medical care. Control: optimal medical care Participants with alpha‐1 antitrypsin will undergo the procedure without randomization. |
| Outcomes |
Primary: mean change in forced expiratory volume in 1 second (FEV1) Secondary:
|
| Starting date | June 2013 |
| Contact information | Spiration Inc |
| Notes | NCT01812447 |
NCT01989182.
| Trial name or title | REACH (Research to assess SVS safety and effectiveness for the treatment of severe EmphysemA in CHina) |
| Methods | Randomized open‐label study. Follow‐up: until 6 months. Estimated enrolment: n = 100 Sites: The First Affiliated Hospital of Guangzhou Medical College |
| Participants | Inclusion criteria:
|
| Interventions | Experimental: treatment with Spiration valve system (SVS) + medical management: procedure to have valves placed in the most diseased lobe of the lung to occlude all segments of the lobe. Control: medical management |
| Outcomes | Primary:
Secondary:
|
| Starting date | September 2013 |
| Contact information | Contact: Yu Chen, MD. Principal Investigator: Shiyue Li, MD |
| Notes | NCT01989182 |
NCT02022683.
| Trial name or title | To Improve lung function and symptoms for emphysema patients using Zephyr endobronchial valve (NCT02022683) |
| Methods | Randomized open‐label study. Follow‐up: up to 24 months. Estimated enrolment: n = 78. Sites: multicenter (Belgium, France, Germany, Netherlands, Sweden, UK) |
| Participants | Participants suffering from heterogeneous emphysema without collateral ventilation Inclusion criteria:
|
| Interventions | Experimental: endoscopic lung volume reduction Control: standard medical care |
| Outcomes | Primary: Change in FEV1
Secondary
|
| Starting date | December 2013 |
| Contact information | Elisabeth Liljensten, DDS, PhD, or Gunnar Hillerdal |
| Notes | NCT02022683 |
NCT02823223.
| Trial name or title | Endobronchial valve in patients with heterogeneous emphysema |
| Methods | Randomized open‐label study. Follow‐up: 6 months. Estimated enrolment: n = 72 participants. Sites: Beijing |
| Participants | Inclusion criteria:
|
| Interventions | Experimental: BLVR with endobronchial valves Participants will have BLVR with endobronchial valves (Zephyr valve) inserted into the target lobe of the lung with the aim of complete lobar exclusion. Control will be standard care: Participants will receive optimal drug therapy and medical management according to clinical practice |
| Outcomes |
Primary: Percentage change in FEV1 at 3 months
Secondary:
(type, incidence, severity, seriousness and relationship to study medications of adverse events (AEs) graded by the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE 4.0)) |
| Starting date | June 2016 |
| Contact information | Liang_an Chen, MD, PhD |
| Notes | NCT02823223 |
ATS: American Thoracic Society; BLVR: Bronchoscopic Lung Volume Reduction; BMI: Body Mass Index; CAT: COPD assessment test score; COPD: Chronic obstructive pulmonary disease; CT: computed tomography; ERS: European Respiratory Society; FEV1: Forced expiratory volume in one second; iBODE: composite score comprising body composition, airway obstruction, dyspnoea and exercise capacity; IBV: Intrabronchial valve; IVC: inspiratory vital capacity; LVRS: Lung volume reduction surgery; mMRC: Modified Medical Research Council (mMRC) Dyspnoea Scale; MRC: Medical Research Council; PFT: Pulmonary Function Test; RV: Residual volume; SAE: Serious adverse event; TLC: Total Lung Capcity; VATS: Video‐assisted thoracoscopic surgery.
Differences between protocol and review
Due to the importance of participant selection and phenotyping, specifically for endobronchial valves, it was decided to report differences between the subgroup of 'collateral ventilation' for secondary outcomes.
Contributions of authors
JEM van Agteren: 'Risk of Bias' assessment, data extraction, data analysis, drafting of review, responsible for final version.
K Hnin: 'Risk of Bias', data extraction, editing of review.
D Grosser: clinical guidance, editing of the review.
KV Carson: RoB conflict resolution, input in protocol.
BJ Smith: supervision of review and editing of the review.
Sources of support
Internal sources
Support provided by the Queen Elizabeth Hospital, Respiratory Medicine Department, Australia.
External sources
No sources of support supplied
Declarations of interest
Dion Grosser has received payment to attend workshops and to provide education and proctoring for placement of endobronchial valves (Pulmonx) and has received flights and accommodation to attend an education session on implantation of coils (PneumRx). None of the other review authors are aware of any conflict of interest.
New
References
References to studies included in this review
ASPIRE 2015 {published data only}
- Come CE, Kramer MR, Dransfield MT, Abu‐Hijleh M, Berkowitz D, Bezzi M, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. European Respiratory Journal 2015;46(3):651‐62. [DOI: 10.1183/09031936.00065715] [DOI] [PMC free article] [PubMed] [Google Scholar]
BeLieVeR HIFi 2015 {published data only}
- Davey C, Zoumot Z, Jordan S, Carr DH, Polkey MI, Shah PL, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR‐HIFi trial): study design and rationale. Thorax 2015;70(3):288‐90. [DOI: 10.1136/thoraxjnl-2014-205127] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey C, Zoumot Z, Jordan S, McNulty WH, Carr DH, Hind MD, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR‐HIFi study): a randomised controlled trial. Lancet 2015;386(9998):1066‐73. [DOI: 10.1016/S0140-6736(15)60001-0] [DOI] [PubMed] [Google Scholar]
Ease 2011 {published data only}
- Shah PL, Slebos D‐J, Cardoso PFG, Cetti E, Voelker K, Levine B, et al. Bronchoscopic lung‐volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham‐controlled, multicentre trial. Lancet 2011;378(9795):997‐1005. [DOI: 10.1016/S0140-6736(11)61050-7] [DOI] [PubMed] [Google Scholar]
- Shah PL, Slebos D‐J, Cardoso PFG, Cetti EJ, Sybrecht GW, Cooper JD. Design of the exhale airway stents for emphysema (EASE) trial: an endoscopic procedure for reducing hyperinflation. BMC Pulmonary Medicine 2011;11(1):1. [DOI: 10.1186/1471-2466-11-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eberhardt 2012 {published data only}
- Eberhardt R, Gompelmann D, Schuhmann M, Reinhardt H, Ernst A, Heussel CP, et al. Complete unilateral vs partial bilateral endoscopic lung volume reduction in patients with bilateral lung emphysema. Chest 2012;142(4):900‐8. [DOI: 10.1378/chest.11-2886] [DOI] [PubMed] [Google Scholar]
IBV Valve trial 2014 {published data only}
- Wood DE, Nader DA, Springmeyer SC, Elstad MR, Coxson HO, Chan A, et al. The IBV Valve trial: a multicenter, randomized, double‐blind trial of endobronchial therapy for severe emphysema. Journal of Bronchology & Interventional Pulmonology 2014;21(4):288‐97. [DOI: 10.1097/LBR.0000000000000110] [DOI] [PubMed] [Google Scholar]
IMPACT 2016 {published data only}
- Valipour A, Slebos D‐J, Herth F, Darwiche K, Wagner M, Ficker JH, et al. Endobronchial valve therapy in patients with homogeneous emphysema: results from the IMPACT study. American Journal of Respiratory and Critical Care Medicine 2016;194 (9):1073‐82. [DOI: 10.1164/rccm.201607-1383OC] [DOI] [PubMed] [Google Scholar]
Ninane 2012 {published data only}
- Ninane V, Bezzi M, Geltner C, Gottlieb J, Seijo L, Munavvar M, et al. Non‐lobar atelectasis approach for the treatment of advanced emphysema with bronchial valves in a European multicenter, single blinded and randomized study. American Journal of Respiratory and Critical Care Medicine 2010;181:A2847. [Google Scholar]
- Ninane V, Bezzi M, Geltner C, Gottlieb J, Seijo L, Munavvar M, et al. The European multicenter, single blinded and randomized study of bronchial valves for the treatment of advanced emphysema: procedural results.. American Journal of Respiratory and Critical Care Medicine 2010;181:A5164. [Google Scholar]
- Ninane V, Geltner C, Bezzi M, Foccoli P, Gottlieb J, Welte T, et al. Multicentre European study for the treatment of advanced emphysema with bronchial valves. European Respiratory Journal 2012;39(6):1319‐25. [DOI: 10.1183/09031936.00019711] [DOI] [PubMed] [Google Scholar]
RENEW 2016 {published data only}
- Sciurba FC, Criner GJ, Strange C, Shah PL, Michaud G, Connolly TA, et al. Effect of endobronchial coils vs usual care on exercise tolerance in patients with severe emphysema: the RENEW randomized clinical trial. JAMA 2016;315(20):2178‐89. [DOI: 10.1001/jama.2016.6261] [DOI] [PubMed] [Google Scholar]
RESET 2015 {published data only}
- Shah PL, Zoumot Z, Singh S, Bicknell SR, Ross ET, Quiring J, et al. Endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET): a randomised controlled trial. Lancet Respiratory Medicine 2013;1(3):233‐40. [DOI: 10.1016/S2213-2600(13)70047-X] [DOI] [PubMed] [Google Scholar]
- Zoumot Z, Kemp SV, Singh S, Bicknell SR, McNulty WH, Hopkinson NS, et al. Endobronchial coils for severe emphysema are effective up to 12 months following treatment: medium term and cross‐over results from a randomised controlled trial. PLOS One 2015;10(4):e0122656. [DOI: 10.1371/journal.pone.0122656] [DOI] [PMC free article] [PubMed] [Google Scholar]
Revolens 2016 {published data only}
- Deslee G, Mal H, Dutau H, Bourdin A, Vergnon JM, Pison C, et al. Lung volume reduction coil treatment vs usual care in patients with severe emphysema: the REVOLENS randomized clinical trial. JAMA 2016;315(2):175‐84. [DOI: 10.1001/jama.2015.17821] [DOI] [PubMed] [Google Scholar]
STELVIO 2015 {published data only}
- Hartman JE, Klooster K, Slebos D‐J, Hacken NHT. Improvement of physical activity after endobronchial valve treatment in emphysema patients. Respiratory Medicine 2016;117:116‐21. [DOI: 10.1016/j.rmed.2016.06.009] [DOI] [PubMed] [Google Scholar]
- Klooster K, Hacken NHT, Hartman JE, Kerstjens HAM, Rikxoort EM, Slebos D‐J. Endobronchial valves for emphysema without interlobar collateral ventilation. New England Journal of Medicine 2015;373(24):2325‐35. [DOI: 10.1056/NEJMoa1507807] [DOI] [PubMed] [Google Scholar]
STEP‐UP 2016 {published data only}
- Bandyopadhyay S, Henne E, Gupta A, Barry R, Snell G, Strange C, et al. Segmental approach to lung volume reduction therapy for emphysema patients. Respiration 2014;89(1):76‐81. [DOI: 10.1159/000369036] [DOI] [PubMed] [Google Scholar]
- Herth FJF, Valipour A, Shah PL, Eberhardt R, Grah C, Egan J, et al. Segmental volume reduction using thermal vapour ablation in patients with severe emphysema: 6‐month results of the multicentre, parallel‐group, open‐label, randomised controlled STEP‐UP trial. Lancet Respiratory Medicine 2016;4(3):185‐93. [DOI: 10.1016/S2213-2600(16)00045-X] [DOI] [PubMed] [Google Scholar]
- Valipour A, Herth FJF, Eberhardt R, Shah PL, Gupta A, Barry R, et al. Design of the randomized, controlled sequential staged treatment of emphysema with upper lobe predominance (STEP‐UP) study. BMC Pulmonary Medicine 2014;14(1):1. [DOI: 10.1186/1471-2466-14-190] [DOI] [PMC free article] [PubMed] [Google Scholar]
VENT EU 2012 {published data only}
- Herth FJF, Noppen M, Valipour A, Leroy S, Vergnon J‐M, Ficker JH, et al. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. European Respiratory Journal 2012;39(6):1334‐42. [DOI: 10.1183/09031936.00161611] [DOI] [PubMed] [Google Scholar]
- Pietzsch JB, Garner A, Herth FJF. Cost‐effectiveness of endobronchial valve therapy for severe emphysema: a model‐based projection based on the VENT study. Respiration 2014;88(5):389‐98. [DOI: 10.1159/000368088] [DOI] [PubMed] [Google Scholar]
VENT US 2010 {published data only}
- Argula RG, Strange C, Ramakrishnan V, Goldin J. Baseline regional perfusion impacts exercise response to endobronchial valve therapy in advanced pulmonary emphysema. Chest 2013;144(5):1578‐86. [DOI] [PubMed] [Google Scholar]
- Brown MS, Kim HJ, Abtin FG, Strange C, Galperin‐Aizenberg M, Pais R, et al. Emphysema lung lobe volume reduction: effects on the ipsilateral and contralateral lobes. European Radiology 2012;22(7):1547‐55. [DOI] [PubMed] [Google Scholar]
- Eberhardt R, Herth FJF, Radhakrishnan S, Gompelmann D. Comparing clinical outcomes in upper versus lower lobe endobronchial valve treatment in severe emphysema. Respiration 2015;90(4):314‐20. [DOI] [PubMed] [Google Scholar]
- Liberator C, Shenoy K, Marchetti N, Criner G. The role of lobe selection on FEV1 response in endobronchial valve therapy. Journal of Chronic Obstructive Pulmonary Disease 2016;13(4):1‐6. [DOI] [PubMed] [Google Scholar]
- Pietzsch JB, Garner A, Herth FJF. Cost‐effectiveness of endobronchial valve therapy for severe emphysema: a model‐based projection based on the VENT study. Respiration 2014;88(5):389‐98. [DOI: 10.1159/000368088] [DOI] [PubMed] [Google Scholar]
- Sciurba FC, Ernst A, Herth FJF, Strange C, Criner GJ, Marquette CH, et al. A randomized study of endobronchial valves for advanced emphysema. New England Journal of Medicine 2010;363(13):1233‐44. [DOI] [PubMed] [Google Scholar]
- Strange C, Herth FJF, Kovitz KL, McLennan G, Ernst A, Goldin J, et al. Design of the endobronchial valve for emphysema palliation trial (VENT): a non‐surgical method of lung volume reduction. BMC Pulomonary Medicine 2007;7(10):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valipour A, Herth FJF, Burghuber OC, Criner G, Vergnon J‐M, Goldin J, et al. Target lobe volume reduction and COPD outcome measures after endobronchial valve therapy. European Respiratory Journal 2014;43(2):387‐96. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abumossalam 2016 {published data only}
- Abumossalam AM, El‐Halaby HA, Abd El‐khalek AM. Poor man medical pneumoplasty: bronchoscopic lung volume reduction with hot saline versus dissolved doxycycline as a neoteric remedy of pulmonary emphysema. Egyptian Journal of Chest Diseases and Tuberculosis 2016;65(1):71‐9. [Google Scholar]
Cardoso 2007 {published data only}
- Cardoso PFG, Snell GI, Hopkins P, Sybrecht GW, Stamatis G, Ng AW, et al. Clinical application of airway bypass with paclitaxel‐eluting stents: early results. Journal of Thoracic and Cardiovascular Surgery 2007;134(4):974‐81. [DOI] [PubMed] [Google Scholar]
Choong 2005 {published data only}
- Choong CK, Haddad FJ, Gee EY, Cooper JD. Feasibility and safety of airway bypass stent placement and influence of topical mitomycin C on stent patency. Journal of Thoracic and Cardiovascular Surgery 2005;129(3):632‐8. [DOI] [PubMed] [Google Scholar]
Choong 2006 {published data only}
- Choong CK, Phan L, Massetti P, Haddad FJ, Martinez C, Roschak E, et al. Prolongation of patency of airway bypass stents with use of drug‐eluting stents. Journal of Thoracic and Cardiovascular Surgery 2006;131(1):60‐4. [DOI] [PubMed] [Google Scholar]
Choong 2008 {published data only}
- Choong CK, Macklem PT, Pierce JA, Das N, Lutey BA, Martinez CO, et al. Airway bypass improves the mechanical properties of explanted emphysematous lungs. American Journal of Respiratory and Critical Care Medicine 2008;178(9):902‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Criner 2009 {published data only}
- Criner GJ, Pinto‐Plata V, Strange C, Dransfield M, Gotfried M, Leeds W, et al. Biologic lung volume reduction in advanced upper lobe emphysema: phase 2 results. American Journal of Respiratory and Critical Care Medicine 2009;179(9):791‐8. [DOI: 10.1164/rccm.200810-1639OC] [DOI] [PubMed] [Google Scholar]
De Oliveira 2006 {published data only}
- Oliveira HG, Macedo‐Neto AV, John AB, Jungblut S, Prolla JC, Menna‐Barreto SS, et al. Transbronchoscopic pulmonary emphysema treatment: 1‐month to 24‐month endoscopic follow‐up. Chest 2006;130(1):190‐9. [DOI] [PubMed] [Google Scholar]
Deslee 2014 {published data only}
- Deslee G, Klooster K, Hetzel M, Stanzel F, Kessler R, Marquette CH, et al. Lung volume reduction coil treatment for patients with severe emphysema: a European multicentre trial. Thorax 2014;69(11):980‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Emery 2010 {published data only}
- Emery MJ, Eveland RL, Eveland K, Couetil LL, Hildebrandt J, Swenson ER. Lung volume reduction by bronchoscopic administration of steam. American Journal of Respiratory and Critical Care Medicine 2010;182(10):1282‐91. [DOI] [PubMed] [Google Scholar]
Faisal 2016 {published data only}
- Faisal A, Zoumot Z, Shah PL, Neder JA, Polkey MI, Hopkinson NS. Effective bronchoscopic lung volume reduction accelerates exercise oxygen uptake kinetics in emphysema. Chest 2016;149(2):435‐46. [DOI] [PubMed] [Google Scholar]
Falkenstern‐Ge 2013 {published data only}
- Falkenstern‐Ge RF, Ingerl H, Kohlhäufl M. Severe emphysema treated by endoscopic bronchial volume reduction with lung sealant (AeriSeal). Case Reports in Pulmonology 2013;2013:.. [DOI: 10.1155/2013/361391] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fann 2003 {published data only}
- Fann JI, Berry GJ, Burdon TA. Bronchoscopic approach to lung volume reduction using a valve device. Journal of Bronchology and Interventional Pulmonology 2003;10(4):253‐9. [Google Scholar]
Fruchter 2016 {published data only}
- Fruchter O, Rosengarten D, Goldberg E, Ben‐Zvi H, Tor R, Kramer MR. Airway bacterial colonization and serum C‐reactive protein are associated with chronic obstructive pulmonary disease exacerbation following bronchoscopic lung volume reduction. Clinical Respiratory Journal 2016;10(2):239‐45. [DOI: 10.1111/crj.12211] [DOI] [PubMed] [Google Scholar]
Gompelmann 2010 {published data only}
- Gompelmann D, Eberhardt R, Michaud G, Ernst A, Herth FJ. Predicting atelectasis by assessment of collateral ventilation prior to endobronchial lung volume reduction: a feasibility study. Respiration 2010;80(5):419‐25. [DOI] [PubMed] [Google Scholar]
Gompelmann 2012 {published data only}
- Gompelmann D, Heussel CP, Eberhardt R, Snell G, Hopkins P, Baker K, et al. Efficacy of bronchoscopic thermal vapor ablation and lobar fissure completeness in patients with heterogeneous emphysema. Respiration 2012;83(5):400‐6. [DOI: 10.1159/000336239] [DOI] [PubMed] [Google Scholar]
Gompelmann 2013 {published data only}
- Gompelmann D, Eberhardt R, Ernst A, Hopkins P, Egan J, Stanzel F, et al. The localized inflammatory response to bronchoscopic thermal vapor ablation. Respiration 2013;86(4):324‐31. [DOI: 10.1159/000354175] [DOI] [PubMed] [Google Scholar]
Hartman 2015 {published data only}
- Hartman JE, Klooster K, Gortzak K, Hacken NH, Slebos DJ. Long‐term follow‐up after bronchoscopic lung volume reduction treatment with coils in patients with severe emphysema. Respirology 2015;20(2):319‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herth 2010 {published data only}
- Herth FJF, Eberhard R, Gompelmann D, Slebos D‐J, Ernst A. Bronchoscopic lung volume reduction with a dedicated coil: a clinical pilot study. Therapeutic Advances in Respiratory Disease 2010;4(4):225‐31. [DOI: 10.1177/1753465810368553] [DOI] [PubMed] [Google Scholar]
Herth 2011 {published data only}
- Herth FJF, Gompelmann D, Stanzel F, Bonnet R, Behr J, Schmidt B, et al. Treatment of advanced emphysema with emphysematous lung sealant (AeriSeal). Respiration 2011;82(1):36‐45. [DOI: 10.1159/000322649] [DOI] [PubMed] [Google Scholar]
Herth 2012 {published data only}
- Herth FJ, Ernst A, Baker KM, Egan JJ, Gotfried MH, Hopkins P, et al. Characterization of outcomes 1 year after endoscopic thermal vapor ablation for patients with heterogeneous emphysema. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:397‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hopkinson 2005 {published data only}
- Hopkinson NS, Toma TP, Hansell DM, Goldstraw P, Moxham J, Geddes DM, et al. Effect of bronchoscopic lung volume reduction on dynamic hyperinflation and exercise in emphysema. American Journal of Respiratory Critical Care Medicine 2005;171(5):453‐60. [DOI] [PubMed] [Google Scholar]
Hopkinson 2011 {published data only}
- Hopkinson NS, Kemp SV, Toma TP, Hansell DM, Geddes DM, Shah PL, et al. Atelectasis and survival after bronchoscopic lung volume reduction for COPD. European Respiratory Journal 2011;37(6):1346‐51. [DOI] [PubMed] [Google Scholar]
Klooster 2014 {published data only}
- Klooster K, Hacken NHT, Franz I, Kerstjens HAM, Rikxoort EM, Slebos D‐J. Lung volume reduction coil treatment in chronic obstructive pulmonary disease patients with homogeneous emphysema: a prospective feasibility trial. Respiration 2014;88(2):116‐25. [DOI: 10.1159/000362522] [DOI] [PubMed] [Google Scholar]
Kontogianni 2014 {published data only}
- Kontogianni K, Gerovasili V, Gompelmann D, Schuhmann M, Heussel CP, Herth FJ, et al. Effectiveness of endobronchial coil treatment for lung volume reduction in patients with severe heterogeneous emphysema and bilateral incomplete fissures: a six‐month follow‐up. Respiration 2014;88(1):52‐60. [DOI] [PubMed] [Google Scholar]
Kotecha 2011 {published data only}
- Kotecha S, Westall GP, Holsworth L, Pham A, Williams TJ, Snell GI. Long‐term outcomes from bronchoscopic lung volume reduction using a bronchial prosthesis. Respirology 2011;16(1):167‐73. [DOI] [PubMed] [Google Scholar]
Kramer 2012 {published data only}
- Kramer MR, Refaely Y, Maimon N, Rosengarten D, Fruchter O. Bilateral endoscopic sealant lung volume reduction therapy for advanced emphysema. Chest 2012;142(5):1111‐7. [DOI] [PubMed] [Google Scholar]
- Kramer MR, Refaely Y, Maimon N, Rosengarten D, Fruchter O. Two‐year follow‐up in patients treated with emphysematous lung sealant for advanced emphysema. Chest 2013;144(5):1677‐80. [DOI: 10.1378/chest.13-0446.] [DOI] [PubMed] [Google Scholar]
Kramer 2013 {published data only}
- Kramer MR, Fruchter O, Eberhardt R, Valipour A, Assadi S, Bonnet R, et al. Emphysematous lung sealant therapy attenuates the chronic inflammatory state of advanced emphysema. Journal of Pulmonary & Respiratory Medicine 2013;3(5):1‐5. [DOI: 10.4172/2161-105X.1000163] [DOI] [Google Scholar]
Magnussen 2012 {published data only}
- Magnussen H, Kramer MR, Kirsten AM, Marquette C, Valipour A, Stanzel F, et al. Effect of fissure integrity on lung volume reduction using a polymer sealant in advanced emphysema. Thorax 2012;67(4):302‐8. [DOI] [PubMed] [Google Scholar]
Pizarro 2015 {published data only}
- Pizarro C, Ahmadzadehfar H, Essler M, Tuleta I, Fimmers R, Nickenig G, et al. Effect of endobronchial valve therapy on pulmonary perfusion and ventilation distribution. PLOS One 2015;10(3):e0118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Refaely 2010 {published data only}
- Refaely Y, Dransfield M, Kramer MR, Gotfried M, Leeds W, McLennan G, et al. Biologic lung volume reduction therapy for advanced homogeneous emphysema. European Respiratory Journal 2010;36(1):20‐7. [DOI] [PubMed] [Google Scholar]
Reilly 2007 {published data only}
- Reilly J, Washko G, Pinto‐Plata V, Velez E, Kenney L, Berger R, et al. Biological lung volume reduction: a new bronchoscopic therapy for advanced emphysema. Chest 2007;131(4):1108‐13. [DOI] [PubMed] [Google Scholar]
Rendina 2003 {published data only}
- Rendina EA, Giacomo T, Venuta F, Coloni GF, Meyers BF, Patterson GA, et al. Feasibility and safety of the airway bypass procedure for patients with emphysema. Journal of Thoracic and Cardiovascular Surgery 2003;125(6):1294‐7. [DOI] [PubMed] [Google Scholar]
Slebos 2012 {published data only}
- Slebos DJ, Klooster K, Ernst A, Herth FJ, Kerstjens HA. Bronchoscopic lung volume reduction coil treatment of patients with severe heterogeneous emphysema. Chest 2012;142(3):574‐82. [DOI] [PubMed] [Google Scholar]
Snell 2003 {published data only}
- Snell GI, Holsworth L, Borrill ZL, Thomson KR, Kalff V, Smith JA, et al. The potential for bronchoscopic lung volume reduction using bronchial prostheses. Chest 2003;124(3):1073‐80. [DOI] [PubMed] [Google Scholar]
Snell 2009 {published data only}
- Snell GI, Hopkins P, Westall G, Holsworth L, Carle A, Williams TJ. A feasibility and safety study of bronchoscopic thermal vapor ablation: a novel emphysema therapy. Annals of Thoracic Surgery 2009;88(6):1993‐8. [DOI] [PubMed] [Google Scholar]
Snell 2012 {published data only}
- Snell G, Herth FJ, Hopkins P, Baker KM, Witt C, Gotfried MH, et al. Bronchoscopic thermal vapour ablation therapy in the management of heterogeneous emphysema. European Respiratory Journal 2012;39(6):1326‐33. [DOI] [PubMed] [Google Scholar]
Springmeyer 2009 {published data only}
- Springmeyer SC, Bolliger CT, Waddell TK, Gonzalez X, Wood DE, Teams IBVVPTR. Treatment of heterogeneous emphysema using the Spiration IBV valves. Thoracic Surgery Clinics 2009;19(2):247‐53, ix‐x. [DOI] [PubMed] [Google Scholar]
Toma 2003 {published data only}
- Toma TP, Hopkinson NS, Hillier J, Hansell DM, Morgan C, Goldstraw PG, et al. Bronchoscopic volume reduction with valve implants in patients with severe emphysema. Lancet 2003;361(9361):931‐3. [DOI] [PubMed] [Google Scholar]
Venuta 2005 {published data only}
- Venuta F, Anile M, Diso D, Carillo C, Giacomo T, D'Andrilli A, et al. Long‐term follow‐up after bronchoscopic lung volume reduction in patients with emphysema. European Respiratory Journal 2012;39(5):1084‐9. [DOI] [PubMed] [Google Scholar]
- Venuta F, Giacomo T, Rendina EA, Ciccone AM, Diso D, Perrone A, et al. Bronchoscopic lung‐volume reduction with one‐way valves in patients with heterogenous emphysema. Annals of Thoracic Surgery 2005;79(2):411‐6; discussion 6‐7. [DOI] [PubMed] [Google Scholar]
Venuta 2011 {published data only}
- Venuta F, Diso D, Anile M, Giacomo T, Rendina EA, Rolla M, et al. Bronchoscopic lung volume reduction as a bridge to lung transplantation in patients with chronic obstructive pulmonary disease. European Journal Cardio‐Thoracic Surgery 2011;39(3):364‐7. [DOI: 10.1016/j.ejcts.2010.06.005] [DOI] [PubMed] [Google Scholar]
Wan 2006 {published data only}
- Wan IY, Toma TP, Geddes DM, Snell G, Williams T, Venuta F, et al. Bronchoscopic lung volume reduction for end‐stage emphysema: report on the first 98 patients. Chest 2006;129(3):518‐26. [DOI] [PubMed] [Google Scholar]
Wood 2007 {published data only}
- Wood DE, McKenna RJ, Yusen RD, Sterman DH, Ost DE, Springmeyer SC, et al. A multicenter trial of an intrabronchial valve for treatment of severe emphysema. Journal of Thoracic and Cardiovascular Surgery 2007;133(1):65‐73. [DOI: 10.1016/j.jtcvs.2006.06.051] [DOI] [PubMed] [Google Scholar]
Yim 2004 {published data only}
- Yim AP, Hwong TM, Lee TW, Li WW, Lam S, Yeung TK, et al. Early results of endoscopic lung volume reduction for emphysema. Journal of Thoracic and Cardiovascular Surgery 2004;127(6):1564‐73. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN19684749 {published data only}
- ISRCTN19684749. The CELEB trial: comparative effectiveness of lung volume reduction surgery for emphysema and bronchoscopic lung volume reduction with valve placement. www.isrctn.com/ISRCTN19684749 (accessed 23 May 2016). [DOI] [PMC free article] [PubMed]
NCT01457833 {published data only}
- NCT01457833. Implantation of endobronchial valves versus intrabronchial valves in patients with severe heterogeneous emphysema. clinicaltrials.gov/ct2/show/NCT01457833 (accessed 19 October 2011).
NCT01796392 {published data only}
- NCT01796392. Pulmonx endobronchial valves used in treatment of emphysema (LIBERATE Study). clinicaltrials.gov/ct2/show/record/NCT01796392 (accessed 20 February 2013).
NCT01812447 {published data only}
- NCT01812447. Evaluation of the Spiration® Valve System for Emphysema to Improve Lung Function (EMPROVE). clinicaltrials.gov/ct2/show/record/NCT01812447 (accessed 7 March 2013).
NCT01989182 {published data only}
- Li S, Wang G, Wang C, Jin F, Gao X, Yang H, et al. LATE‐BREAKING ABSTRACT: The REACH study, a randomized controlled trial assessing the safety and effectiveness of the spiration valve system intra‐bronchial therapy for severe emphysema. European Respiratory Journal 2016;48:OA3013. [Google Scholar]
- NCT01989182. The spiration valve system for the treatment of severe emphysema. clinicaltrials.gov/ct2/show/record/NCT01989182 (accessed 6 November 2013).
NCT02022683 {published data only}
- NCT02022683. To improve lung function and symptoms for emphysema patients Using Zephyr EBV (TRANSFORM). clinicaltrials.gov/ct2/show/record/NCT02022683 (accessed 17 December 2013).
NCT02823223 {published data only}
- NCT02823223. Endobronchial valve in patients With heterogeneous emphysema. clinicaltrials.gov/ct2/show/record/NCT02823223 (accessed 29 June 2016).
Additional references
Agzarian 2013
- Agzarian J, Miller JD, Kosa SD, Malthaner R, Tan L, Canadian Volume Reduction Surgery Study Group. Long‐term survival analysis of the Canadian lung volume reduction surgery trial. Annals of Thoracic Surgery 2013;96(4):1217‐22. [DOI: 10.1016/j.athoracsur.2013.04.077.] [DOI] [PubMed] [Google Scholar]
Aljuri 2009
- Aljuri N, Freitag L. Validation and pilot clinical study of a new bronchoscopic method to measure collateral ventilation before endobronchial lung volume reduction. Applied Physiology 2009;106(3):774‐83. [DOI: 10.3109/15412555.2015.1115007] [DOI] [PubMed] [Google Scholar]
Ambrosino 1999
- Ambrosino N. Field tests in pulmonary disease. Thorax 1999;54(3):191‐3. [DOI: 10.1136/thx.54.3.191] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bagdonas 2015
- Bagdonas E, Raudoniute J, Bruzauskaite I, Aldonyte R. Novel aspects of pathogenesis and regeneration mechanisms in COPD. International Journal of Chronic Obstructive Pulmonary Disease 2015;10:995‐1013. [DOI: 10.2147/COPD.S82518] [DOI] [PMC free article] [PubMed] [Google Scholar]
Biederer 2012
- Biederer J, Beer M, Hirsch W, Wild J, Fabel M, Puderbach M, et al. MRI of the lung (2/3). Why … when … how?. Insights Imaging 2012;3(4):355‐71. [DOI: 10.1007/s13244-011-0146-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bourdin 2009
- Bourdin A, Burgel PR, Chanez P, Garcia G, Perez T, Roche N. Recent advances in COPD: pathophysiology, respiratory physiology and clinical aspects, including comorbidities. European Respiratory Review 2009;18(114):198‐212. [DOI: 10.1183/09059180.00005509] [DOI] [PubMed] [Google Scholar]
Boutou 2013
- Boutou AK, Shrikrishna D, Tanner RJ, Smith C, Kelly JL, Ward SP, et al. Lung function indices for predicting mortality in COPD. European Respiratory Journal 2013;42(3):616‐25. [DOI: 10.1183/09031936.00146012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutou 2015
- Boutou AK, Zoumot Z, Nair A, Davey C, Hansell DM, Jamurtas A, et al. The impact of homogeneous versus heterogeneous emphysema on dynamic hyperinflation in patients with severe COPD assessed for lung volume reduction. Journal of Chronic Obstructive Pulmonary Disease 2015;12(6):598‐605. [DOI: 10.3109/15412555.2015.1020149] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2000
- Campbell M, Grimshaw J, Steen N. Sample size calculations for cluster randomised trials. Journal of Health Services Research & Policy 2000;5(1):12‐16. [DOI] [PubMed] [Google Scholar]
Cavaillès 2013
- Cavaillès A, Brinchault‐Rabin G, Dixmier A, Goupil F, Gut‐Gobert C, Marchand‐Adam S, et al. Comorbidities of COPD. European Respiratory Review 2013;22(130):454‐75. [DOI: 10.1183/09059180.00008612] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cazzola 2008
- Cazzola M, MacNee W, Martinez FJ, Rabe Klaus F, Franciosi LG, Barnes PJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. European Respiratory Journal 2008;31(2):416‐69. [DOI: 10.1183/09031936.00099306] [DOI] [PubMed] [Google Scholar]
Celli 2007
- Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. European Respiratory Journal 2007;29(6):1224‐38. [DOI: 10.1183/09031936.00109906] [DOI] [PubMed] [Google Scholar]
Cetti 2006
- Cetti EJ, Moore AJ, Geddes DM. Collateral ventilation. Thorax 2006;61(5):371‐3. [DOI: 10.1136/thx.2006.060509] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 2014
- Clark SJ, Zoumot Z, Bamsey O, Polkey MI, Dusmet M, Lim E, et al. Surgical approaches for lung volume reduction in emphysema. Clinical Medicine 2014;14(2):122‐7. [DOI: 10.7861/clinmedicine.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 1995
- Cooper JD, Trulock EP, Triantafillou AN, Patterson GA, Pohl MS, Deloney RN, et al. Bilateral pneumectomy (volume reduction) for chronic obstructive pulmonary disease. Journal of Thoracic and Cardiovascular Surgery 1995;109(1):106‐19. [DOI] [PubMed] [Google Scholar]
Cooper 2010
- Cooper JD. "All that glitters": evaluating interventions for emphysema. Chest 2010;138(2):243‐5. [DOI] [PubMed] [Google Scholar]
Criner 2008
- Criner GJ, Sternberg AL. National Emphysema Treatment Trial: the major outcomes of lung volume reduction surgery in severe emphysema. Proceedings of the American Thoracic Society 2008;5(4):393‐405. [DOI: 10.1513/pats.200801-013ET] [DOI] [PMC free article] [PubMed] [Google Scholar]
Criner 2011
- Criner GJ, Cordova F, Sternberg AL, Martinez FJ. The National Emphysema Treatment Trial (NETT) part II: lessons learned about lung volume reduction surgery. American Journal of Respiratory and Critical Care Medicine 2011;184(8):881‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dal 2008
- Dal Negro R. Optimizing economic outcomes in the management of COPD. International Journal of Chronic Obstructive Pulmonary Disease 2008;3(1):1‐10. [PUBMED: PMC2528207] [DOI] [PMC free article] [PubMed] [Google Scholar]
Demedts 2006
- Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respiratory Research 2006;7(1):53. [DOI: 10.1186/1465-9921-7-53] [DOI] [PMC free article] [PubMed] [Google Scholar]
Donohue 2005
- Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005;2(1):111‐24. [DOI] [PubMed] [Google Scholar]
Eberhardt 2015
- Eberhardt R, Gompelmann D, Herth FJF, Schuhmann M. Endoscopic bronchial valve treatment: patient selection and special considerations. International Journal of Chronic Obstructive Pulmonary Disease 2015;10:2147. [DOI: 10.2147/COPD.S63473] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferguson 2006
- Ferguson GT. Why does the lung hyperinflate?. Proceedings of the American Thoracic Society 2006;3(2):176‐9. [DOI: 10.1513/pats.200508-094DO] [DOI] [PubMed] [Google Scholar]
Fessler 1998
- Fessler HE, Permutt S. Lung volume reduction surgery and airflow limitation. American Journal of Respiratory and Critical Care Medicine 1998;157(3):715‐22. [DOI: 10.1164/ajrccm.157.3.9608004] [DOI] [PubMed] [Google Scholar]
Fessler 2002
- Fessler HE, Scharf SM, Permutt S. Improvement in spirometry following lung volume reduction surgery: application of a physiologic model. American Journal of Respiratory and Critical Care Medicine 2002;165(1):34‐40. [DOI] [PubMed] [Google Scholar]
Fessler 2005
- Fessler HE. Collateral ventilation, the bane of bronchoscopic volume reduction. American Journal of Respiratory and Critical Care Medicine 2005;171(5):423‐4. [DOI] [PubMed] [Google Scholar]
Fessler 2008
- Fessler HE, Scharf SM, Ingenito EP, McKenna RJ Jr, Sharafkhaneh A. Physiologic basis for improved pulmonary function after lung volume reduction. Proceedings of the American Thoracic Society 2008;5(4):416‐20. [DOI: 10.1513/pats.200708-117ET] [DOI] [PMC free article] [PubMed] [Google Scholar]
Galluccio 2010
- Galluccio G, Lucantoni G. Bronchoscopic lung volume reduction for pulmonary emphysema: preliminary experience with a new NOVATECHÂ endobronchial silicone one‐way valve. Interactive CardioVascular and Thoracic Surgery 2010;11(2):213‐5. [DOI: 10.1510/icvts.2010.236398] [DOI] [PubMed] [Google Scholar]
Garner 2016
- Garner J, Kemp SV, Toma TP, Hansell DM, Polkey MI, Shah PL, et al. Survival after endobronchial valve placement for emphysema: a 10‐year follow‐up study. American Journal of Respiratory and Critical Care Medicine 2016;194(4):519‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ginsburg 2011
- Ginsburg ME, Thomashow BM, Yip CK, DiMango AM, Maxfield RA, Bartels MN, et al. Lung volume reduction surgery using the NETT selection criteria. Annals of Thoracic Surgery 2011;91(5):1556‐61. [DOI: 10.1016/j.athoracsur.2011.01.054] [DOI] [PubMed] [Google Scholar]
GOLD
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD. http://www.goldcopd.org/ (accessed 2 February 2016).
Gompelmann 2014
- Gompelmann D, Herth FJF, Slebos DJ, Valipour A, Ernst A, Criner GJ, et al. Pneumothorax following endobronchial valve therapy and its impact on clinical outcomes in severe emphysema. Respiration 2014;87(6):485‐91. [DOI] [PubMed] [Google Scholar]
Gompelmann 2014a
- Gompelmann D, Eberhardt R, Herth F. Endoscopic volume reduction in COPD ‐ a critical review. Deutsches Ärzteblatt International 2014;111(49):827‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 2 February 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Hartman 2012
- Hartman JE, Hacken NHT, Klooster K, Boezen HM, Greef MHG, Slebos D‐J. The minimal important difference for residual volume in patients with severe emphysema. European Respiratory Journal 2012;40(5):1137‐41. [DOI: 10.1183/09031936.00219111] [DOI] [PubMed] [Google Scholar]
Herth 2013
- Herth FJF, Eberhardt R, Gompelmann D, Ficker JH, Wagner M, Ek L, et al. Radiological and clinical outcomes of using Chartis to plan endobronchial valve treatment. European Respiratory Journal 2013;41(2):302‐8. [DOI] [PubMed] [Google Scholar]
Herth 2016
- Herth FJF, Slebos D‐J, Rabe KF, Shah PL. Endoscopic lung volume reduction: an expert panel recommendation. Respiration 2016;91(3):241‐50. [DOI: 10.1159/000444090] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (updated March 2011). The Cochrane Collaboration, 2011. available from www.handbook.cochrane.org.
Higuchi 2006
- Higuchi T, Reed A, Oto T, Holsworth L, Ellis S, Bailey MJ, et al. Relation of interlobar collaterals to radiological heterogeneity in severe emphysema. Thorax 2006;61(5):409‐13. [DOI: 10.1136/thx.2005.051219] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hogg 2004
- Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004;364(9435):709‐21. [DOI] [PubMed] [Google Scholar]
Ingenito 2001
- Ingenito EP, Reilly JJ, Mentzer SJ, Swanson SJ, Vin R, Keuhn H, et al. Bronchoscopic volume reduction: a safe and effective alternative to surgical therapy for emphysema. American Journal of Respiratory and Critical Care Medicine 2001;164(2):295‐301. [DOI] [PubMed] [Google Scholar]
Ingenito 2005
- Ingenito EP, Tsai LW, Majumdar A, Suki B. On the role of surface tension in the pathophysiology of emphysema. American Journal of Respiratory and Critical Care Medicine 2005;171(4):300‐4. [DOI: 10.1164/rccm.200406-770PP] [DOI] [PubMed] [Google Scholar]
Ingenito 2008
- Ingenito EP, Wood DE, Utz JP. Bronchoscopic lung volume reduction in severe emphysema. Proceedings of the American Thoracic Society 2008;5(4):454‐60. [DOI: 10.1513/pats.200707‐085ET] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2005
- Jones PW. St. George's respiratory questionnaire: MCID. COPD 2005;2(1):75‐9. [DOI] [PubMed] [Google Scholar]
Kim 2008
- Kim V, Rogers TJ, Criner GJ. New concepts in the pathobiology of chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2008;5(4):478‐85. [PUBMED: 18453359] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkham 2013
- Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest 2013;144(1):266‐73. [DOI: 10.1378/chest.12-2664] [DOI] [PubMed] [Google Scholar]
Klooster 2014a
- Klooster K, Hacken NHT, Slebos D‐J. The lung volume reduction coil for the treatment of emphysema: a new therapy in development. Expert Review of Medical Devices 2014;11(5):481‐9. [DOI: 10.1586/17434440.2014.929490] [DOI] [PubMed] [Google Scholar]
Koster 2016
- Koster TD, Slebos D‐J. The fissure: interlobar collateral ventilation and implications for endoscopic therapy in emphysema. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:765. [DOI: 10.2147/COPD.S103807] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lausberg 2003
- Lausberg HF, Chino K, Patterson GA, Meyers BF, Toeniskoetter PD, Cooper JD. Bronchial fenestration improves expiratory flow in emphysematous human lungs. Annals of Thoracic Surgery 2003;75(2):393‐8. [DOI] [PubMed] [Google Scholar]
Lozano 2013
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013;380(9859):2095‐128. [DOI: 10.1016/S0140-6736(12)61728-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macklin 1999
- Macklin R. The ethical problems with sham surgery in clinical research. New England Journal of Medicine 1999;341(13):992‐6. [DOI: 10.1056/NEJM199909233411312] [DOI] [PubMed] [Google Scholar]
Mair 2009
- Mair G, Miller JJ, McAllister D, Maclay J, Connell M, Murchison JT, et al. Computed tomographic emphysema distribution: relationship to clinical features in a cohort of smokers. European Respiratory Journal 2009;33(3):536‐42. [DOI: 10.1183/09031936.00111808] [DOI] [PubMed] [Google Scholar]
Mannino 2007
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370(9589):765‐73. [DOI: 10.1016/S0140-6736(07)61380-4] [DOI] [PubMed] [Google Scholar]
Mannino 2007a
- Mannino DM, Braman S. The epidemiology and economics of chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2007;4(7):502‐6. [DOI: 10.1513/pats.200701-001FM] [DOI] [PubMed] [Google Scholar]
Maxfield 2004
- Maxfield RA. New and emerging minimally invasive techniques for lung volume reduction. Chest 2004;125(2):777‐83. [DOI] [PubMed] [Google Scholar]
McCarthy 2015
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD003793.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McNulty 2014
- McNulty W, Jordan S, Hopkinson NS. Attitudes and access to lung volume reduction surgery for COPD: a survey by the British Thoracic Society. BMJ Open Respiratory Research 2014;1(1):e000023. [DOI: 10.1136/bmjresp-2014-000023] [DOI] [PMC free article] [PubMed] [Google Scholar]
Milanese 2016
- Milanese G, Silva M, Sverzellati N. Lung volume reduction of pulmonary emphysema: the radiologist task. Current Opinion in Pulmonary Medicine 2016;22(2):179‐86. [DOI: 10.1097/MCP.0000000000000252] [DOI] [PubMed] [Google Scholar]
Miller 2004
- Miller FG, Kaptchuk TJ. Sham procedures and the ethics of clinical trials. Journal of the Royal Society of Medicine 2004;97(12):576‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2006
- Miller JD, Malthaner RA, Goldsmith CH, Goeree R, Higgins D, Cox PG, et al. A randomized clinical trial of lung volume reduction surgery versus best medical care for patients with advanced emphysema: a two‐year study from Canada. Annals of Thoracic Surgery 2006;81(1):314‐21. [PUBMED: 16368389] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mura 2005
- Mura M, Zompatori M, Mussoni A, Fasano L, Pacilli AMG, Ferro O, et al. Bullous emphysema versus diffuse emphysema: a functional and radiologic comparison. Respiratory Medicine 2005;99(2):171‐8. [DOI: 10.1016/j.rmed.2004.06.010] [DOI] [PubMed] [Google Scholar]
Naunheim 2006
- Naunheim KS, Wood DE, Mohsenifar Z, Sternberg AL, Criner GJ, DeCamp MM, et al. Long‐term follow‐up of patients receiving lung‐volume‐reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Annals of Thoracic Surgery 2006;82(2):431‐43. [DOI] [PubMed] [Google Scholar]
NETT 2001
- National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung‐volume‐reduction surgery. New England Journal of Medicine 2001;345(15):1075. [DOI: 10.1056/NEJMoa11798] [DOI] [PubMed] [Google Scholar]
NICE 2010
- The National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. https://www.nice.org.uk/guidance/cg101 (accessed 15 April 2016). [PubMed]
Papandrinopoulou 2012
- Papandrinopoulou D, Tzouda V, Tsoukalas G. Lung compliance and chronic obstructive pulmonary disease. Pulmonary Medicine 2012;2012:1‐6. [DOI: 10.1155/2012/542769] [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2015
- Park TS, Hong Yi, Lee JS, Oh SY, Lee SM, Kim N, et al. Bronchoscopic lung volume reduction by endobronchial valve in advanced emphysema: the first Asian report. International Journal of Chronic Obstructive Pulmonary Disease 2015;10:1501. [DOI: 10.2147/COPD.S85744] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perera 2012
- Perera PN, Armstrong EP, Sherrill DL, Skrepnek GH. Acute exacerbations of COPD in the United States: inpatient burden and predictors of costs and mortality. Journal of Chronic Obstructive Pulmomary Disease 2012;9(2):131‐41. [DOI: 10.3109/15412555.2011.650239] [DOI] [PubMed] [Google Scholar]
Pompeo 2012
- Pompeo E, Rogliani P, Tacconi F, Dauri M, Saltini C, Novelli G, et al. Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery. Journal of Thoracic and Cardiovascular Surgery 2012;143(1):47‐54. [DOI: 10.1016/j.jtcvs.2011.09.050.] [DOI] [PubMed] [Google Scholar]
Puhan 2011
- Puhan MA, Gimeno‐Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD005305.pub3] [DOI] [PubMed] [Google Scholar]
Puhan 2011a
- Puhan MA, Chandra D, Mosenifar Z, Ries A, Make B, Hansel NN, et al. The minimal important difference of exercise tests in severe COPD. European Respiratory Journal 2011;37(4):784‐90. [DOI: 10.1183/09031936.00063810.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramsey 2007
- Ramsey SD, Shroyer AL, Sullivan SD, Wood DE. Updated evaluation of the cost‐effectiveness of lung volume reduction surgery. Chest 2007;131(3):823‐32. [DOI] [PubMed] [Google Scholar]
Rathinam 2014
- Rathinam S, Oey I, Steiner M, Spyt T, Morgan MD, Waller DA. The role of the emphysema multidisciplinary team in a successful lung volume reduction surgery programme. European Journal of Cardio‐Thoracic Surgery 2014;46(6):1021‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schuhmann 2015
- Schuhmann M, Raffy P, Yin Y, Gompelmann D, Oguz I, Eberhardt R, et al. Computed tomography predictors of response to endobronchial valve lung reduction treatment. Comparison with Chartis. American Journal of Respiratory and Critical Care Medicine 2015;191(7):767‐74. [DOI] [PubMed] [Google Scholar]
Shah 2014
- Shah PL, Herth FJF. Current status of bronchoscopic lung volume reduction with endobronchial valves. Thorax 2014;69(3):280‐6. [DOI] [PubMed] [Google Scholar]
Sharafkhaneh 2008
- Sharafkhaneh A, Hanania NA, Kim V. Pathogenesis of emphysema: from the bench to the bedside. Proceedings of the American Thoracic Society 2008;5(4):475‐7. [DOI: 10.1513/pats.200708-126ET] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stockley 2009
- Stockley RA, Mannino D, Barnes PJ. Burden and pathogenesis of chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2009;6(6):524‐6. [DOI: 10.1513/pats.200904-016DS] [DOI] [PubMed] [Google Scholar]
Storbeck 2015
- Storbeck B, Schröder TH, Oldigs M, Rabe KF, Weber C. Emphysema: imaging for endoscopic lung volume reduction [Lungenemphysem: Bildgebung bei endoskopischerLungenvolumenreduktion]. Fortschritte auf dem Gebiet der Röntgenstrahlen und bildgebenden Verfahren 2015;187:543‐54. [DOI: 10.1055/s-0034-1399424] [DOI] [PubMed] [Google Scholar]
Suki 2003
- Suki B, Lutchen KR, Ingenito EP. On the progressive nature of emphysema: roles of proteases, inflammation, and mechanical forces. American Journal of Respiratory and Critical Care Medicine 2003;168(5):516‐21. [DOI: 10.1164/rccm.200208-908PP] [DOI] [PubMed] [Google Scholar]
Taraseviciene‐Stewart 2008
- Taraseviciene‐Stewart L, Voelkel NF. Molecular pathogenesis of emphysema. Journal of Clinical Investigation 2008;118(2):394‐402. [DOI: 10.1172/JCI31811] [DOI] [PMC free article] [PubMed] [Google Scholar]
Terry 1978
- Terry PB, Traystman RJ, Newball HH, Batra G, Menkes HA. Collateral ventilation in man. New England Journal of Medicine 1978;298(1):10‐15. [DOI] [PubMed] [Google Scholar]
Tuder 2003
- Tuder RM, McGrath S, Neptune E. The pathobiological mechanisms of emphysema models: what do they have in common?. Pulmonary Pharmacology and Therapeutics 2003;16(2):67‐78. [DOI] [PubMed] [Google Scholar]
Valipour 2015
- Valipour A, Shah PL, Gesierich W, Eberhardt R, Snell G, Strange C, et al. Patterns of emphysema heterogeneity. Respiration 2015;90(5):402‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Agteren 2016
- Agteren JEM, Carson, KV, Tiong LU, Smith BJ. Lung volume reduction surgery for diffuse emphysema. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD001001.pub3; CD001001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Venuta 2006
- Venuta F, Rendina EA, Giacomo T, Anile M, Diso D, Andreetti C, et al. Bronchoscopic procedures for emphysema treatment. European Journal of Cardio‐thoracic Surgery 2006;29(3):281‐7. [DOI: 10.1016/j.ejcts.2005.12.009] [DOI] [PubMed] [Google Scholar]
Weder 1997
- Weder W, Thurnheer R, Stammberger U, Bürge M, Russi EW, Bloch KE. Radiologic emphysema morphology is associated with outcome after surgical lung volume reduction. Annals of Thoracic Surgery 1997;64(2):313‐20. [DOI] [PubMed] [Google Scholar]
Weder 2009
- Weder W, Tutic M, Bloch KE. Lung volume reduction surgery in nonheterogeneous emphysema. Thoracic Surgery Clinics 2009;19(2):193‐9. [DOI: 10.1016/j.thorsurg.2009.03.002] [DOI] [PubMed] [Google Scholar]
Wedzicha 2003
- Wedzicha JA, Donaldson GC. Exacerbations of chronic obstructive pulmonary disease. Respiratory Care 2003;48(12):1204‐15. [PubMed] [Google Scholar]
Wedzicha 2012
- Wedzicha JA, Decramer M, Seemungal TAR. The role of bronchodilator treatment in the prevention of exacerbations of COPD. European Respiratory Journal 2012;40(6):1545‐54. [DOI: 10.1183/09031936.00048912] [DOI] [PMC free article] [PubMed] [Google Scholar]
Welling 2015
- Welling JBA, Hartman JE, Hacken NHT, Klooster K, Slebos D‐J. The minimal important difference for the St George's Respiratory Questionnaire in patients with severe COPD. European Respiratory Journal 2015;46(6):1598‐604. [DOI: 10.1183/13993003.00535-20151] [DOI] [PubMed] [Google Scholar]
Welte 2015
- Welte T, Vogelmeier C, Papi A. COPD: early diagnosis and treatment to slow disease progression. International Journal of Clinical Practice 2015;69(3):336‐49. [DOI: 10.1111/ijcp.12522] [DOI] [PubMed] [Google Scholar]
Zeng 2012
- Zeng G, Sun B, Zhong N. Non‐smoking‐related chronic obstructive pulmonary disease: A neglected entity?. Respirology 2012;17(6):908‐12. [DOI: 10.1111/j.1440-1843.2012.02152.x.] [DOI] [PubMed] [Google Scholar]
Zoumot 2014
- Zoumot Z, Jordan S, Hopkinson NS. Emphysema: time to say farewell to therapeutic nihilism. Thorax 2014;69(11):973‐5. [DOI: 10.1136/thoraxjnl-2014-205667] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zoumot 2015
- Zoumot Z, LoMauro A, Aliverti A, Nelson C, Ward S, Jordan S, et al. Lung volume reduction in emphysema improves chest wall asynchrony. Chest 2015;148(1):185‐95. [DOI: 10.1378/chest.14-2380] [DOI] [PMC free article] [PubMed] [Google Scholar]


