Abstract
Background
Hospitalised newborn neonates frequently undergo painful invasive procedures that involve penetration of the skin and other tissues by a needle. One intervention that can be used prior to a needle insertion procedure is application of a topical local anaesthetic.
Objectives
To evaluate the efficacy and safety of topical anaesthetics such as amethocaine and EMLA in newborn term or preterm infants requiring an invasive procedure involving puncture of skin and other tissues with a needle.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Embase and CINAHL up to 15 May 2016; previous reviews including cross‐references, abstracts, and conference proceedings. We contacted expert informants. We contacted authors directly to obtain additional data. We imposed no language restrictions.
Selection criteria
Randomised, quasi‐randomised controlled trials, and cluster and cross‐over randomised trials that compared the topical anaesthetics amethocaine and eutectic mixture of local anaesthetics (EMLA) in terms of anaesthetic efficacy and safety in newborn term or preterm infants requiring an invasive procedure involving puncture of skin and other tissues with a needle
Data collection and analysis
From the reports of the clinical trials we extracted data regarding clinical outcomes including pain, number of infants with methaemoglobin level 5% and above, number of needle prick attempts prior to successful needle‐related procedure, crying, time taken to complete the procedure, episodes of apnoea, episodes of bradycardia, episodes of oxygen desaturation, neurodevelopmental disability and other adverse events.
Main results
Eight small randomised controlled trials met the inclusion criteria (n = 506). These studies compared either EMLA and placebo or amethocaine and placebo. No studies compared EMLA and amethocaine. We were unable to meta‐analyse the outcome of pain due to differing outcome measures and methods of reporting. For EMLA, two individual studies reported a statistically significant reduction in pain compared to placebo during lumbar puncture and venepuncture. Three studies found no statistical difference between the groups during heel lancing. For amethocaine, three studies reported a statistically significant reduction in pain compared to placebo during venepuncture and one study reported a statistically significant reduction in pain compared to placebo during cannulation. One study reported no statistical difference between the two groups during intramuscular injection.
One study reported no statistical difference between EMLA and the placebo group for successful venepuncture at first attempt. One study similarly reported no statistically significant difference between Amethocaine and the placebo group for successful cannulation at first attempt.
Risk for local redness, swelling or blanching was significantly higher with EMLA (typical risk ratio (RR) 1.65, 95% confidence interval (CI) 1.24 to 2.19; typical risk difference (RD) 0.17, 95% CI 0.09 to 0.26; n = 272; number needed to treat for an additional harmful outcome (NNTH) 6, 95% CI 4 to 11; I2 = 92% indicating considerable heterogeneity) although not for amethocaine (typical RR 2.11, 95% CI 0.72 to 6.16; typical RD 0.05, 95% CI ‐0.02 to 0.11, n = 221). These local skin reactions for EMLA and amethocaine were reported as short‐lasting. Two studies reported no methaemoglobinaemia with single application of EMLA. The quality of the evidence on outcomes assessed according to GRADE was low to moderate.
Authors' conclusions
Overall, all the trials were small, and the effects of uncertain clinical significance. The evidence regarding the effectiveness or safety of the interventions studied is inadequate to support clinical recommendations. There has been no evaluation regarding any long‐term effects of topical anaesthetics in newborn infants.
High quality studies evaluating the efficacy and safety of topical anaesthetics such as amethocaine and EMLA for needle‐related pain in newborn term or preterm infants are required. These studies should aim to determine efficacy of these topical anaesthetics and on homogenous groups of infants for gestational age. While there was no methaemoglobinaemia in the studies that reported methaemoglobin, the efficacy and safety of EMLA, especially in very preterm infants, and for repeated application, need to be further evaluated in future studies.
Plain language summary
Topical anaesthesia for needle‐related pain in newborn infants
Lay title
Topical anaesthesia for newborn infants who require a needle‐related procedure.
Review question
Does the application of a topical anaesthetic applied on the skin reduce pain in newborn infants who require a procedure that punctures the skin?
Background
Some newborn infants require a painful procedure that involves the puncturing of the skin such as heel lancing and venepuncture (puncture of a vein to get blood samples or give medicines) or intramuscular injection. Painful procedures can be stressful for newborn infants and the witnessing of painful procedures can be distressing for parents. One intervention that can be used before a needle insertion procedure is application of a topical local anaesthetic to numb the skin.
Study characteristics
Eight clinical trials enrolling 506 newborn infants met our inclusion criteria.
Results and quality of the evidence
This review of trials found that overall there is not enough good quality evidence to say if topical local anaesthetics applied to the skin help relieve pain during needle‐related pain in newborn infants. Large well‐design clinical trials are needed.
Summary of findings
Summary of findings for the main comparison. EMLA compared with placebo for needle‐related pain in newborn infants.
| EMLA compared with placebo for needle‐related pain in newborn infants | ||||||
|
Patient or population: preterm infants Settings: NICU Intervention: EMLA Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with EMLA | |||||
| Pain using PIPP score | The mean pain using PIPP score was 0 | MD 0.27 higher (1.45 lower to 1.99 higher) |
‐ | 38 (1 study) |
⊕⊕⊝⊝ Low | Single study |
| Pain using NIPS score | The mean pain using NIPS score was 0 | MD 0.27 higher (0.75 lower to 1.29 higher) |
‐ | 38 (1 study) |
⊕⊕⊝⊝ Low | Single study |
| Successful venepuncture first attempt | Study population |
RR 0.98 (0.93 to 1.03) |
111 (1 study) |
⊕⊕⊝⊝ Low | Single study | |
| 1000 per 1000 | 980 per 1000 | |||||
|
Adverse effects (short‐lasting skin reactions) |
Study population |
RR 1.65 (1.24 to 2.19) |
272 (3 studies) |
⊕⊕⊝⊝ Low | High heterogeneity | |
| 269 per 1000 | 444 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EMLA: eutectic mixture of local anaesthetics; MD: mean difference; NICU: neonatal intensive care unit; NIPS: Neonatal Infant Pain Scale; PIPP: Premature Infant Pain Profile; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 2. Amethocaine compared with placebo for needle‐related pain.
| Amethocaine compared with placebo for needle‐related pain | ||||||
|
Patient or population: preterm infants Settings: NICU Intervention: amethocaine Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with amethocaine | |||||
| Pain | The mean pain was 0 | MD 5 lower (17.34 lower to 7.34 higher) |
‐ | 110 (1 study) |
⊕⊕⊝⊝ Low | Single small study |
| Successful cannulation first attempt | Study population |
RR 1.21 (0.82 to 1.81) |
39 (1 study) |
⊕⊝⊝⊝ Very low |
Single small study, risk of selection bias | |
| 650 per 1000 | 787 per 1000 (533 to 1000) | |||||
|
Adverse effects (short‐lasting skin reactions) |
Study population | Not estimable | 181 (3 studies) |
⊕⊕⊕⊝ Moderate | Risk of selection bias | |
| 43 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EMLA: eutectic mixture of local anaesthetics; MD: mean difference; NICU: neonatal intensive care unit; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Hospitalised newborn neonates frequently undergo painful invasive procedures that involve penetration of the skin and other tissues by a needle, often multiple times per procedure. These procedures include needle‐related painful procedures associated with venepuncture, arterial puncture, heel lancing, lumbar puncture, supra‐pubic aspiration of urine, percutaneous venous catheter placement and intramuscular injection. Compared with adults, both preterm and term infants display a hypersensitivity to sensory stimuli (Fitzgerald 2001). Neonates have difficulty communicating the experience of pain to those managing it. Anatomical maturation of nociceptive pathways is complete by mid‐to‐late second trimester and painful stimuli result in immediate hormonal, physiological and behavioural responses (Anand 1987). Prior pain experience in term infants increases subsequent behavioural responses to pain, whereas preterm neonates may have a diminished subsequent behavioural response, but not necessarily altered experience of pain (Whitfield 2000). Ongoing pain and distress may also have long‐term consequences for brain development and behaviour (Anand 2000). Importantly too, several studies have highlighted that one of the most salient sources of stress for parents of hospitalised infants is the witnessing of painful procedures inflicted upon their infant (Foster 2008; Franck 2004).
Assessment of pain and the efficacy of treatment modalities is challenging in non‐verbal neonates. Multi‐dimensional pain measurement tools have been developed including the Neonatal Infant Pain Scale (NIPS) (Lawrence 1993) and the Premature Infant Pain Profile (PIPP) (Stevens 1996). Physiological and behavioural responses to painful stimuli can be minimised and even ameliorated by appropriate analgesia (Anand 2001; Bell 1994).
One intervention that can be used prior to a needle insertion procedure is application of a topical local anaesthetic. Topical anaesthetics are effective in minimising the pain of venepuncture and intravenous cannulation in children (Lander 1996; Lawson 1995; Manner 1987; Maunuksela 1986; Woolfson 1990). One Cochrane Review comparing EMLA (eutectic mixture of local anaesthetics) and amethocaine in children concluded that although EMLA is an effective topical anaesthetic, amethocaine is superior in preventing pain associated with needle procedures (Lander 2006).
Description of the intervention
EMLA and amethocaine are the two most widely used percutaneous topical local anaesthetics for use on intact skin. EMLA is a water‐based cream and each gram contains lidocaine 25 mg (2.5%) and prilocaine 25 mg (2.5%). Lidocaine and prilocaine are amide‐type local anaesthetic agents (AstraZeneca 2008). Prior to the procedure, EMLA is applied using an occlusive dressing to cover the cream and numbing of the skin usually occurs approximately one hour after application and lasts for one to two hours after removal of the cream. Approximately 1 g is sufficient to cover up to 10 cm2 (approximately 3 x 3 cm) of skin (AstraZeneca 2008).
One more recently introduced alternative to EMLA is amethocaine. Amethocaine belongs to the family of local, aminoester‐type anaesthetics and is a white opalescent gel with each gram containing tetracaine base 40 mg (4% amethocaine) (Smith & Nephew 2012). As with EMLA, amethocaine gel is applied to the site and sealed with an occlusive dressing. Amethocaine reportedly has a faster anaesthetic effect than EMLA, and can usually be achieved following a 30‐minute application time for venepuncture, and a 45‐minute application time for venous cannulation. Amethocaine gel should be removed with a gauze swab, and the site prepared with an antiseptic wipe prior to the procedure. Anaesthesia is reported to also last longer than EMLA, remaining for four to six hours in most people after a single application (Smith & Nephew 2012). Approximately 1 g is sufficient to cover and anaesthetise an area of up to 30 cm2 (6 cm x 5 cm) of skin. Smaller areas of anaesthetised skin may be adequate in infants and small children (Smith & Nephew 2012). Numbing of the skin can take between 45 and 60 minutes, an unsuccessful attempt for venous cannulation may require a new application of topical anaesthetic at another site, and thus a further delay in attempting to cannulate until numbing of the skin occurs.
Although the adverse effects of EMLA are usually mild and include transient local skin reactions, potential life‐threatening complications have been reported in infants. Compared with children and adults, infants are believed to be at increased risk of methaemoglobinaemia (MetHb) because they have a deficiency in the enzyme that reduces MetHb, nicotinamide adenine dinucleotide (NADH) cytochrome b5 reductase (Camp 2007; Nilsson 1990; Taddio 1998; Weinberger 2001). The higher body surface area to weight ratio in infants compared with adults may result in higher systemic exposure. In addition, preterm infants may be at even greater risk because immaturity of their skin may enhance drug absorption of drugs (Taddio 1999). As methaemoglobin levels increase, oxygen delivery to tissues is impaired and cellular hypoxia develops (Stephens 2007; Wright 1999; Zaky 2009). Normal physiological levels of methaemoglobin are 0% to 2% but levels less than 5% may be acceptable upon discussion with the medical team. Methaemoglobin levels of 5% and above are generally regarded as clinically significant. The half‐life of methaemoglobin is 55 minutes (Coleman 1996; Sashdeva 2003). MetHb has been shown to inhibit surfactant activity directly (Weinberger 2001). Infants with severe MetHb will exhibit signs such as cyanosis, respiratory distress/increasing ventilator requirements and lethargy (Weinberger 2001).
One Cochrane Review compared EMLA and a range of pain‐relieving measures during circumcision (Brady‐Fryer 2004). One Cochrane Review compared EMLA with placebo or no treatment for boys undergoing circumcision (Taddio 1999). Both reviews found that a single dose of EMLA was safe and did not cause MetHb; however, there were insufficient data to assess the risk of MetHb with multiple doses (Brady‐Fryer 2004; Taddio 1999). One non‐Cochrane systematic review found EMLA to be effective during circumcision, venepuncture, arterial puncture and percutaneous venous catheter placement in newborns but it did not diminish the pain from heel‐lancing (Taddio 1998). One case of MetHb was reported in 1995 by Tse 1995 after injection of prilocaine for a neonatal circumcision. One study by Browne 1999 reported that vasoconstriction following the use of EMLA may render cannulation more difficult in adults. A reported benefit of amethocaine is that it causes vasodilation of the blood vessels and, therefore, may make cannulation easier. Hypersensitivity reactions to amethocaine such as erythema, oedema, pruritus and blistering of the skin have been reported in adults (Smith & Nephew 2012).
How the intervention might work
Both EMLA and amethocaine topical anaesthetics act by causing a reversible block to conduction along nerve fibres. Amethocaine is believed to block nerve conduction mainly by inhibiting sodium ion flux across the axon membrane. Amethocaine achieves this by acting upon specific receptors that control gating mechanisms responsible for conductance changes in specialised proteinaceous sodium channels (Smith & Nephew 2012). EMLA similarly works by stabilising the neuronal membrane preventing the initiation and conduction of nerve impulses (AstraZeneca 2008).
Why it is important to do this review
Invasive procedures such as venepuncture or heel puncture are painful and can cause considerable stress to the neonate and the parents. EMLA (AstraZeneca 2008) and amethocaine (Smith & Nephew 2012) are not recommended for use in preterm or term newborn infants by their respective manufacturing pharmaceutical companies but both topical anaesthetics are used and studied in these populations. This review will help determine which topical anaesthetic preparation (amethocaine or EMLA) is superior in relation to its efficacy and safety for needle‐related procedures in newborn infants.
Objectives
To evaluate the efficacy and safety of topical anaesthetics such as amethocaine and EMLA in newborn term or preterm infants requiring an invasive procedure involving puncture of skin and other tissues with a needle.
Methods
Criteria for considering studies for this review
Types of studies
We considered all published and unpublished randomised controlled trials (RCTs) or quasi‐RCTs for inclusion in this review.
We intended to include cluster and cross‐over randomised trials.
Types of participants
Newborn term or preterm (or both) infants up to a postnatal age of one month requiring an invasive procedure involving puncture of skin and other tissues with a needle (heel lance, venepuncture, arterial puncture, arterial cannulation, supra‐pubic aspiration of urine, lumbar puncture, intramuscular injection, percutaneous venous catheter). We excluded infants previously exposed to a topical anaesthetic prior to enrolment.
Types of interventions
EMLA versus placebo.
EMLA versus amethocaine.
EMLA versus other topical anaesthetic.
Amethocaine versus placebo.
Amethocaine versus other topical anaesthetic.
Amethocaine and EMLA could be given at any dose, location or length of time as determined in each of the included studies. The topical anaesthesia could be applied using any method of delivery and any product (gel, liquid, spray, cream and any other forms).
We excluded topical anaesthesia during circumcision.
Types of outcome measures
Primary outcomes
Pain using validated pain score (measured during the procedure, up to one hour following painful procedure or both) such as:
NIPS (Lawrence 1993);
PIPP (Stevens 1996);
Neonatal Facial Action Coding System (Grunau 1987);
other validated pain measures.
Secondary outcomes
Number of infants with methaemoglobin levels 5% and above (Sashdeva 2003).
Number of needle prick attempts prior to successful needle‐related procedure (e.g. intravenous or arterial cannulation).
Total cry duration (seconds) from beginning of procedure to cessation of crying or within a specified time limit (e.g. up to 180 seconds).
Time taken for completion of procedure (if trial used same procedure, e.g. intravenous cannulation).
Episodes of apnoea ‐ defined as a cessation of breathing for more than 20 seconds or a shorter pause associated with bradycardia or cyanosis.
Episodes of bradycardia ‐ defined as a fall in heart rate of more than 30% below the baseline or less than 100 beats per minute for 10 seconds or longer.
Episodes of oxygen desaturation ‐ defined as a spontaneous fall in peripheral capillary oxygen saturation (SpO2) of 85% for 10 seconds or longer in duration.
Neurodevelopmental disability (after at least 18 months' postnatal age) defined as neurological abnormality including cerebral palsy on clinical examination, developmental delay more than two standard deviations (SDs) below population mean on a standardised test of development.
Other adverse events (as determined by the study).
Search methods for identification of studies
Electronic searches
Two review authors (JF and CT) independently performed the electronic database searches of the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 4), PubMed (to April 2015), Embase via Ovid (1980 to April 2015) and CINAHL via Ovid (1982 to April 2015). Text word fields and, where applicable, database subject heading fields (e.g. MeSH) were searched using the following terms: neonat* OR Infant* OR Newborn AND Venepuncture OR venipuncture OR arterial puncture OR lumbar puncture OR injection OR needle OR percutaneous venous catheter OR heel lanc* OR supra‐pubic aspiration AND Pain OR Analgesia OR Topical anaes* OR topical anesthes* OR Prilocaine OR Amethocaine OR Tetracaine OR EMLA OR Angel OR Ametop OR "eutectic mixture of local anesthetics" OR topical anesthe* OR topical anaesthe*. We applied no language restrictions.
Searching other resources
The search strategy included communication with expert informants, and searches of bibliographies of reviews and trials for references to other trials. We searched previous reviews including cross‐references, abstracts, and conferences and symposia proceedings of the Perinatal Society of Australia and New Zealand and Pediatric Academic Societies (American Pediatric Society, Society for Pediatric Research and European Society for Paediatric Research) from 1990 to April 2015. We intended to contact the corresponding investigators for information if we identified any unpublished trials. We considered unpublished studies or studies only reported as abstracts as eligible for review if methods and data were confirmed by the author. We intended to contact the corresponding authors of identified RCTs for additional information about their studies if we required further data. We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; controlled‐trials.com; and who.int/ictrp). There were no language restrictions.
Data collection and analysis
We used the standard systematic review methods of Cochrane documented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors (JF and CT) independently assessed the potential studies identified as a result of the search strategy for inclusion. We resolved any disagreements through discussion.
Specifically, we:
merged search results using reference management software and remove duplicate records of the same report;
examined titles and abstracts to remove irrelevant reports;
retrieved full text of the potentially relevant reports;
linked multiple reports of the same study;
examined full‐text reports for compliance of studies with eligibility criteria;
corresponded with investigators, when appropriate, to clarify study eligibility;
noted reasons for inclusion and exclusion of articles;
made final decisions on study inclusion and proceeded to data collection;
resolved discrepancies through a consensus process.
Data extraction and management
We used the standardised review methods of the Cochrane Neonatal Review Group (CNRG) to assess the methodological quality of included studies (neonatal.cochrane.org/en/index.html). Two review authors (JF and CT) independently assessed study quality and risk of bias using the following criteria documented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in included studies
Two review authors (JF and CT) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the method as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determined whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment.
We assessed the methods as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk.
(3) Blinding (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We judged studies to be at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results.
We assessed the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate, inadequate or unclear for outcome assessors.
(4) Detection bias (checking for outcome assessment bias)
For each included study, we categorised the methods used to blind outcome assessors from knowledge of which intervention a participant received. As our study population consisted of neonates they would all be blinded to the study intervention. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods used with regards to risk of detection bias as:
low: adequate, follow‐up was performed with assessors blinded to group;
high: inadequate, assessors at follow‐up were aware of group assignment;
unclear: no or unclear information provided.
(5) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total number of randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes.
Where sufficient information was reported, or could be supplied by the trial authors, if applicable, we intended to re‐include missing data in the analyses that we undertook. We assessed methods as:
adequate (less than 20% missing data);
inadequate;
unclear.
(6) Outcome reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk (where it was clear that all the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk.
(7) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias (e.g. early termination of trial due to data‐dependent process, extreme baseline imbalance, etc.). We assessed whether each study was free of other problems that could put it at risk of bias. We assessed other sources of bias as:
yes;
no;
unclear.
(8) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (8) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We intended to explore the impact of the level of bias through undertaking sensitivity analyses.
We judged each criterion as being at 'low risk' of bias, 'high risk' of bias, or 'unclear' risk of bias (for either lack of information or uncertainty over the potential for bias).
Quality of evidence
We assessed the quality of evidence for the main comparison at the outcome level using the GRADE approach (Guyatt 2011a). This methodological approach considers evidence from RCTs as high quality that may be downgraded based on consideration of any of five areas: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias (Guyatt 2011a). The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
high: we are very confident that the true effect lies close to that of the estimate of the effect;
moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different;
low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect;
very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect (Schünemann 2013).
The review authors independently assessed the quality of the evidence found for outcomes identified as critical or important for clinical decision making. These outcomes include: pain, successful venepuncture first attempt and adverse effects.
In cases where we considered the risk of bias arising from inadequate concealment of allocation, randomised assignment, complete follow‐up or blinded outcome assessment to reduce our confidence in the effect estimates, we downgraded the quality of evidence accordingly (Guyatt 2011b). We evaluated consistency by similarity of point estimates, extent of overlap of confidence intervals (CI) and statistical criteria including measurement of heterogeneity (I2 statistic). We downgraded the quality of evidence when large and unexplained inconsistency across studies results was present (i.e. some studies suggest important benefit and others no effect or harm without a clinical explanation) (Guyatt 2011c). Precision was assessed based on the width of the 95% CI and by calculating the optimal information size. If the total number of participants included in the pooled effect estimation was less than the number of participants generated by a conventional sample size calculation for a single adequately powered trial, we considered rating down for imprecision (Guyatt 2011d). When trials were conducted in populations other than the target population, we downgraded the quality of evidence because of indirectness (Guyatt 2011e).
We entered data (i.e. pooled estimates of the effects and corresponding 95% CI) and explicit judgements for each of the above aspects assessed into the Guideline Development Tool, the software used to create 'Summary of findings' tables (GRADEpro 2008). We explained all judgements involving the assessment of the study characteristics described above in footnotes or comments in Table 1; Table 2.
Measures of treatment effect
We analysed the results of the studies using the statistical package Review Manager 5 (RevMan 2014). We summarised data in a meta‐analysis if they were sufficiently statistically homogeneous.
Dichotomous data
We present results as risk ratio (RR) and risk difference (RD) with 95% CI for dichotomous data. If there was a statistically significant reduction in RD, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) and associated 95% CI.
Continuous data
We used the mean difference (MD) if trials reported outcomes in the same way between trials for continuous data. We used the standardised mean difference (SMD) to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
The unit of randomisation and the unit of analysis was the individual infant.
Dealing with missing data
We contacted authors of published studies if we required clarification, or to request additional information. If we had identified missing data, we would have described the number of participants with the missing data in the Effects of interventions section and the Characteristics of included studies table.
Assessment of heterogeneity
We assessed the heterogeneity between the included trials using the formal and commonly applied statistics to assess heterogeneity using the I2 statistic. This test describes the percentage of total variation observed across studies due to heterogeneity rather than sampling (random) error (Higgins 2011). We graded the degree of heterogeneity as non‐existent or minimal for an I2 value of less than 25%, low for an I2 value of 25% to 49%, moderate for an I2 value of 50% to 74% and high for an I2 value of 75% to 100%. Whenever there was evidence of apparent or statistical heterogeneity, we intended to assess the source of the heterogeneity using sensitivity and subgroup analyses looking for sources of bias or methodological differences between the heterogeneous trials.
Assessment of reporting biases
We planned to assess reporting and publication bias by examining the degree of asymmetry of a funnel plot using Review Manager 5 (RevMan 2014).
Data synthesis
We used the fixed‐effect model in Review Manager 5 for meta‐analysis (RevMan 2014). There were limited data available so we performed none of the subgroup analyses. Sensitivity analyses were deemed inappropriate due to the lack of reporting of relevant outcomes in the included studies.
Subgroup analysis and investigation of heterogeneity
We intended to compare the effects of each topical anaesthetic in the following subgroups of participants:
gestational age at birth (term infants 37 weeks' gestation and above); preterm infants (29 to 36 weeks' gestation); very preterm infants (less than 29 weeks' gestation);
type of infants (e.g. healthy newborn infants (those in the normal newborn nursery) versus ill infants in the neonatal intensive care unit (NICU)).
We performed no subgroup analyses because of the small number of included studies.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
The search identified 233 records through database searching and 107 additional records through other sources. After deduplication, we screened 259 records and excluded 225 records leaving 37 potential studies. After reading the full text, we excluded 29 records with reasons (see Characteristics of excluded studies table). We included eight RCTs in the review (see Characteristics of included studies table). See Figure 1 for study flow diagram.
1.
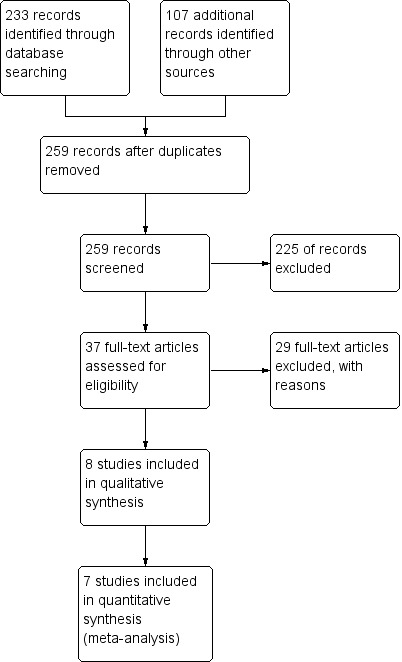
Study flow diagram.
Included studies
Eight small RCTs were eligible for inclusion in this review (n = 506): Bonetto 2008 (n = 38); Jain 2000a (n = 39); Kaur 2003 (n = 60); Larsson 1995 (n = 110); Larsson 1998 (n = 111); Long 2003 (n = 32); Shah 2008 (n = 110); Stevens 1999 (n = 106). We found no studies comparing topical anaesthesia versus topical anaesthesia.
Eutectic mixture of local anaesthetics versus placebo
Bonetto 2008 was a single‐centre study performed in Argentina.
Objective: to investigate the effect of EMLA on pain during heel lancing in newborn infants.
Population: newborn infants, gestation 36 weeks or greater, aged greater than 24 hours after birth to less than 30 days' postnatal age undergoing routine heel lancing.
Intervention: infants in the study group received EMLA cream or placebo cream to the skin and under occlusion for 60 minutes, then removed with a dry cloth with the procedure being performed 10 minutes later.
Outcome: pain.
Kaur 2003 was a single‐centre study performed in India.
Objective: to determine the efficacy of EMLA in alleviating pain associated with lumbar puncture in newborn infants.
Population: newborn infants, gestation 34 weeks or greater and younger than four weeks' postnatal age undergoing diagnostic lumbar puncture.
Intervention: infants received a EMLA cream 1 g or placebo cream to one square in at the site of the procedure and covered with an occlusive dressing for 60 to 90 minutes before the scheduled time of the procedure.
Outcomes: heart rate, transcutaneous oxygen saturation, pain and adverse effects.
Larsson 1995 was a single‐centre study performed in Sweden.
Objective: to evaluate the effect of EMLA when heel lancing for testing for phenylketonuria.
Population: full‐term healthy newborn infants, gestation mean 39.8 weeks, range 36.8 to 42.6 weeks, on their third day of life.
Intervention: infants received EMLA cream 1 g or placebo cream at the site of the procedure and covered with an occlusive dressing for 10, 20, 30, 40, 50, 60, 90 or 120 minutes before the scheduled time of the procedure.
Outcomes: 'pain cry', flexor response, symptoms of MetHb and adverse effects.
Larsson 1998 was a single‐centre study performed in Sweden.
Objective: to determine whether the pain from venepuncture on the dorsal aspect of the hand (for testing for phenylketonuria) in the neonate could be reduced by EMLA.
Population: full‐term newborn infants, gestation mean 39.8 weeks, range 36.8 to 42.6 weeks. Postnatal age was three to eight days.
Intervention: infants received a EMLA cream 0.5 g or placebo cream on the dorsal aspect of the hand and covered with an occlusive dressing for 60 minutes before the scheduled time of the procedure.
Outcomes: pain, duration of first cry, latency to cry, total duration of cry, incidence of crying, number of attempts for successful venepuncture and time required for successful completion of procedure.
Stevens 1999 was a double‐centre study performed in Canada.
Objective: to determine the safety and efficacy of EMLA when undergoing heel lancing.
Population: preterm neonates, gestation 30 to 36 weeks. Postnatal age was one to five days.
Intervention: the study was undertaken in two phases. In phase one, infants in the study group receive EMLA 0.5 g on an occlusive dressing for 30 minutes. Five minutes before heel lancing, the dressing was removed. Infants in the control group received Glaxal base 0.5 g. In phase two, the method was the same as phase one except a larger automated lancet was used because of a change in unit policy. In addition, the placebo cream was changed. It contained all ingredients of EMLA except for the lidocaine and prilocaine, which were substituted with MCT oil, and the creams remained intact for 60 minutes.
Outcomes: pain and methaemoglobin levels.
Amethocaine versus placebo
Jain 2000a was a single‐centre study performed in the UK.
Objective: to investigate the effect of topical amethocaine on the pain of venepuncture in newborn infants.
Population: newborn infants, gestation 27 to 41 weeks, aged two to 17 days' postnatal age undergoing routine venepuncture.
Intervention: infants in the study group received 4% amethocaine gel a 1.5 g or placebo cream to the skin and occlusion for 60 minutes with the procedure being performed five minutes later.
Outcomes: pain, total length of cry, number of attempts to obtain the blood sample and skin reactions.
Long 2003 was a single‐centre study performed in the UK.
Objective: to determine whether the pain from venepuncture (for serum bilirubin measurement and Guthrie tests) in neonates could be reduced by using a tetracaine. (amethocaine)‐containing patch.
Population: newborn infants, gestation 32 to 42 weeks (median 36 weeks). Postnatal age was three to 18 days.
Intervention: infants received a tetracaine patch formulated from hydroxypropyl cellulose discs of uniform area (0.283 cm2) were cut from the dried tetracaine film which, because of the formulation method, was calculated to contain tetracaine base 0.283 mg. The discs were positioned onto pressure‐sensitive adhesive film to sandwich the tetracaine discs between the adhesive film and the release liner. Patches of 16 mm diameter were cut with the 6‐mm disc of tetracaine film at the centre. The tetracaine patch, or a 'placebo device' applied on the dorsal aspect of the hand for 30 minutes before venepuncture.
Outcome: pain.
Shah 2008 was a single‐centre study performed in Canada.
Objective: to evaluate the effectiveness and tolerability of topical amethocaine gel 4% in neonates undergoing intramuscular injection.
Population: full‐term neonates, 37 weeks' gestation or greater undergoing routine antenatal intramuscular injection of vitamin K.
Intervention: infants in the study group received amethocaine gel 4% or placebo to the skin and covered with an adhesive Tegaderm occlusive dressing for 30 minutes before the scheduled time of the procedure.
Outcomes: pain and local adverse events
Excluded studies
We excluded 29 studies from the analysis. These are detailed in the Characteristics of excluded studies table, with reasons for their exclusion.
Risk of bias in included studies
Reviews that compared the topical anaesthetics amethocaine and EMLA in terms of anaesthetic efficacy and safety in newborn term or preterm infants requiring an invasive procedure involving puncture of skin and other tissues with a needle were included in the analysis. Overall, the eight included trials were of high quality (Figure 2; Figure 3). Specific methodological issues regarding the studies included are discussed below.
2.
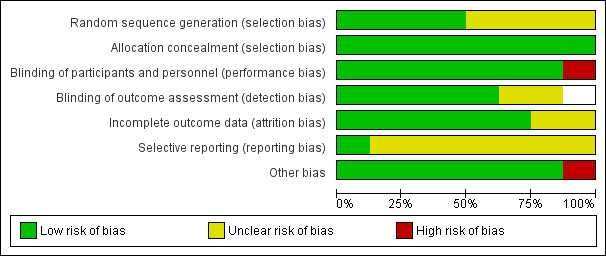
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
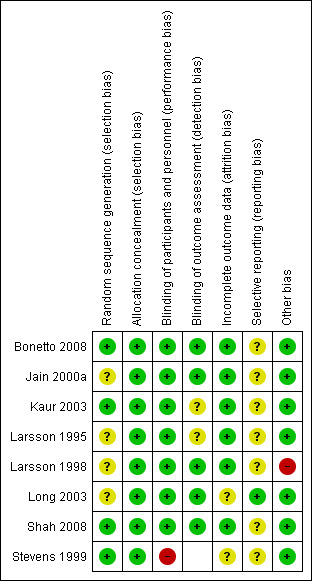
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Randomisation: four studies used computer‐generated randomisation (Bonetto 2008; Kaur 2003; Shah 2008; Stevens 1999). One study used treated identical treatment patches randomly picked from a box (Long 2003). Three studies provided no information on the method of randomisation (Jain 2000a; Larsson 1995; Larsson 1998).
Allocation concealment: one study achieved allocation concealment by keeping the randomisation code in the pharmacy department (Shah 2008). Once an eligible neonate was identified, the pharmacist dispensed the single‐dose study medication. Each participant was identified by a number. In one study, the pharmacist allocated treatment groups (Jain 2000a). One study used creams prepared by the pharmacy department, individually packaged in dark glass ointment jars and labelled by code (Stevens 1999). In one study the neonates received either EMLA or placebo cream 'in a double‐blind manner' (Kaur 2003). Two studies were described as 'double‐blinded' (Bonetto 2008;Larsson 1998). In one study, infants were allocated to eight groups according to the time when EMLA or placebo was applied (Larsson 1995). One study randomly selected patches of identical appearance from a box, equally divided between placebo and active devices (Long 2003).
Blinding of participants and personnel: all eight studies reported that participants and personnel were blinded because the treatment and placebo were identical. One study reported that phase one of the study was not blinded to the research nurse because the colours and consistencies of the creams were different but for phase two, the creams were identical (Stevens 1999).
Blinding of outcome assessment: six studies reported blinding of outcome assessment (Bonetto 2008; Jain 2000a; Larsson 1998; Long 2003; Shah 2008; Stevens 1999). Two studies provided no information (Kaur 2003; Larsson 1995).
Exclusions after randomisation: there were minimal exclusions after randomisation.
Effects of interventions
Results are presented for EMLA versus placebo and amethocaine versus placebo. No studies compared EMLA versus amethocaine, or EMLA versus another active topical anaesthetic, or amethocaine versus another active topical anaesthetic.
Eutectic mixture of local anaesthetics versus placebo
Pain (Outcomes 1.1; 1.2)
Five studies reported on pain but we were unable to perform meta‐analysis due to the different methods used, or reporting of the outcomes (Bonetto 2008; Kaur 2003; Larsson 1995; Larsson 1998; Stevens 1999).
Bonetto 2008 used the 0 to 18 PIPP score for infants over 36 weeks' gestation and Stevens 1999 used the 0 to 21 score for infants of lower gestational age at birth (i.e. less than 28 weeks' gestation). Bonetto 2008 reported no statistical difference between the EMLA and placebo groups from insertion of heel lance and up to three minutes, using the PIPP score (MD 0.27, 95% CI ‐1.45 to 1.99; n = 38). The PIPP score used was a seven‐indicator measure; a score of less than 8 indicated absence or minimal pain and a score greater than 8 indicated moderate pain from a maximum score of 18 (Analysis 1.1). The quality of evidence was low due to results from only one small study.
1.1. Analysis.
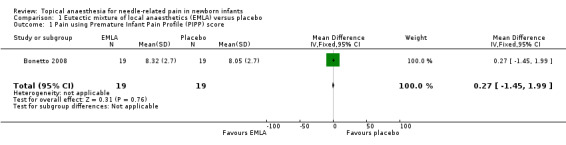
Comparison 1 Eutectic mixture of local anaesthetics (EMLA) versus placebo, Outcome 1 Pain using Premature Infant Pain Profile (PIPP) score.
Bonetto 2008 measured pain during heel lancing using the NIPS and reported no significant difference between the EMLA and placebo groups (MD 0.27, 95% CI ‐0.75 to 1.29; n = 38). A NIPS score of 0 indicated no pain, and a maximum score of 7 indicated moderate‐to‐severe pain (Analysis 1.2). The quality of evidence was low due to results from only one small study.
1.2. Analysis.
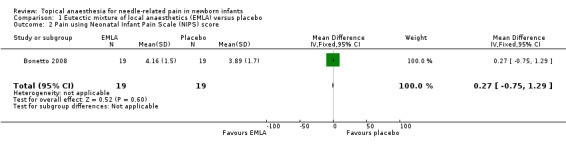
Comparison 1 Eutectic mixture of local anaesthetics (EMLA) versus placebo, Outcome 2 Pain using Neonatal Infant Pain Scale (NIPS) score.
Kaur 2003 reported statistically significant lower pain scores using the simplified Neonatal Facial Coding System (NFCS) for the EMLA group compared to the placebo group during lumbar puncture (mean 4.0 ± standard error (SE) 0.3 with EMLA versus mean 5.0 ± SE 2.7 with placebo; P = 0.004), and needle withdrawal (mean 1.8 ± SE 0.3 with EMLA versus mean 3.9 ± SE 0.3 with placebo; P < 0.001). The NFCS has a maximum score of 5 whereby presence of a pain behaviour scored 1 point and absence of a pain behaviour scored 0 points for each of the five variables.
Larsson 1995 reported no statistical difference between the EMLA and placebo groups for pain during heel lancing which was measured by the utterance of a 'pain cry' (54 out of 56 infants uttered a 'pain cry' with EMLA versus 52 out of 54 infants uttered a 'pain cry' with placebo; P = 0.97).
Larsson 1998 reported pain after venepuncture using the NFCS. There were statistically significant lower pain scores in the EMLA group than the placebo group at 0 to 15 seconds' post venepuncture (median 287 with EMLA versus median 374 with placebo; P = 0.016). There was no statistically significant difference between the scores at 60 to 70 seconds' post venepuncture (median 288 with EMLA versus median 407 with placebo). Each pain variable received a score from 0% to 100% so the total range was 0% to 600% for the six pain variables.
Stevens 1999 used the PIPP score and reported no statistical difference between the EMLA and placebo groups for EMLA applied for 30 minutes (mean 10.19, SD 4.09 with EMLA versus mean 9.45, SD 4.01 with placebo; P = 0.48) and 60 minutes (mean 13.08, SD 4.35 with EMLA versus mean 13.33, SD 3.49 with placebo; P = 0.83) during heel lancing. Pain scores were lower in phase one compared to phase two. The PIPP score used was a seven‐indicator measure, and gave a total range of scores of 0 to 21. A PIPP total score of 6 or less indicated minimal or no pain, whereas scores greater than 12 indicated moderate to severe pain.
Number of infants with methaemoglobin levels 5% and above
One study reported methaemoglobin levels of 5% or greater (Stevens 1999). They reported no methaemoglobin levels of 5% or greater with infants who received two separate doses of EMLA. The mean methaemoglobin concentration for all infants who received EMLA was 1.19%. Larsson 1995 reported no clinical symptoms of MetHb.
Number of needle prick attempts prior to successful needle‐related procedure (successful cannulation first attempt) (Outcome 1.3)
One study reported on successful cannulation at first attempt. There was no statistical difference between the two groups in the successful venepuncture (typical RR 0.98, 95% CI 0.93 to 1.03; typical RD ‐0.02, 95% CI ‐0.07 to 0.03; n = 111) (Analysis 1.3) (Larsson 1998). The quality of evidence was low due to results from only one small study. One study reported that lumbar puncture was traumatic on the first attempt in two cases with EMLA and three cases with placebo (Kaur 2003). No case required more than two attempts.
1.3. Analysis.
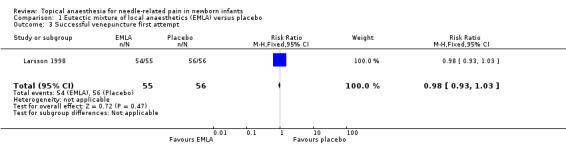
Comparison 1 Eutectic mixture of local anaesthetics (EMLA) versus placebo, Outcome 3 Successful venepuncture first attempt.
Total cry duration
One study reported total cry duration (Larsson 1998). They found no significant difference for median total duration of crying between the two groups during venepuncture (P = 0.89). There were no other data provided.
Time taken for completion of procedure
One study reported on time taken for completion of procedure (Larsson 1998). They reported that it took significantly less time to complete the venepuncture in the placebo group (median 145 seconds with EMLA versus median 125 seconds with placebo; P = 0.01).
Episodes of apnoea
No studies reported episodes of apnoea.
Episodes of bradycardia
No studies reported episodes of bradycardia
Episodes of oxygen desaturation
One study reported that oxygen saturation was comparable between the EMLA and placebo groups during lumbar puncture, with the maximum dip in oxygen saturation level observed at needle insertion (Kaur 2003).
Neurodevelopmental disability
No studies reported neurodevelopmental disability.
Other adverse effects (Outcome 1.4)
Three small studies reported adverse effects (Kaur 2003; Larsson 1995; Stevens 1999). There were statistically significant fewer adverse effects in the placebo group compared to the EMLA group (typical RR 1.65, 95% CI 1.24 to 2.19; typical RD 0.17, 95% CI 0.09 to 0.26; n = 272; NNTH 6, 95% CI 4 to 11). However, there was high heterogeneity (I2 = 92%). The adverse effects reported were: local pallor and redness (Larsson 1995), and temporary blanching (Kaur 2003; Stevens 1999) (Analysis 1.4). The quality of evidence was low due to high heterogeneity. One study reported temporary blanching of the skin during removal of the occlusive dressing but provided no data (Kaur 2003).
1.4. Analysis.
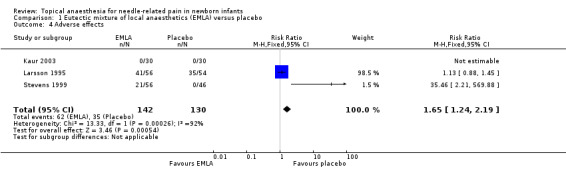
Comparison 1 Eutectic mixture of local anaesthetics (EMLA) versus placebo, Outcome 4 Adverse effects.
All three studies reported the effects as short lasting. One study reported that all the local adverse effects disappeared during the procedure (Larsson 1995). One study reported that overall 10% of the infants had minor skin reactions consisting primarily of redness and swelling at the application site (Stevens 1999). There was no significant difference in adverse reactions indicating that they were most likely the result irritation or sensitivity to the presence or removal of the occlusive dressing. All signs of skin irritation and blanching dissipated within one hour of removing the anaesthetic or placebo cream and dressing.
Subgroup analyses
We performed no subgroup analyses because of the small number of included studies.
Amethocaine versus placebo
Pain (Outcomes 2.1)
Three small studies reported on pain (Jain 2000a; Long 2003; Shah 2008). We were unable to include the following studies for meta‐analysis due to the different methods used, or reporting of the outcomes.
Jain 2000a used the NFCS and reported a significant difference in pain scores during venepuncture between amethocaine (median 3, interquartile range (IQR) 0 to 9) and placebo (median 16, IQR 8 to 16; n = 39). A cumulative NFCS score of 10 or less in the five seconds after insertion of the needle was defined as indicating no or minimal pain. In all, 16 of 19 (84%) amethocaine‐treated infants showed little or no pain in response to insertion of the needle compared with six of 20 (30%) in the placebo group (P = 0.001).
Long 2003 used the NFCS score and reported a significant difference in pain score during venepuncture between amethocaine group (median 0) compared to the placebo group (median 12.5) (P = 0.0002; n = 32) during venepuncture. A cumulative score of 10 or less in the five seconds following the procedure was defined as indicating clinically effective anaesthesia. Fourteen of 15 (93%) amethocaine‐treated neonates presented little or no pain in response to the procedure compared with six of 17 (35%) in the placebo group (P = 0.01), fulfilling the definition of clinically effective local anaesthesia.
Shah 2008 used the Facial Grimacing Score to measure pain during intramuscular injection. The score ranged from 0% to 100%. There was no statistically significant difference between the two groups (MD ‐5.00, 95% CI ‐17.34 to 7.34; n = 110) (Analysis 2.1). The quality of evidence was low due to results from only one small study.
2.1. Analysis.
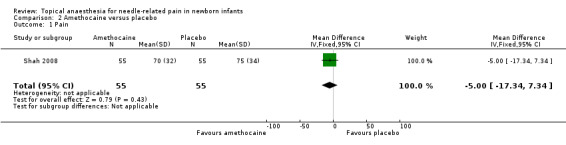
Comparison 2 Amethocaine versus placebo, Outcome 1 Pain.
Number of infants with methaemoglobin levels 5% and above
No studies reported number of infants with methaemoglobin levels 5% and above.
Number of needle prick attempts prior to successful needle‐related procedure (successful cannulation on first attempt) (Outcome 2.2)
One study reported on successful cannulation at first attempt (Jain 2000a). There was no statistical difference between the two groups (typical RR 1.21, 95% CI 0.82 to 1.81; typical RD 0.14, 95% CI ‐0.14 to 0.42; n = 39) (Analysis 2.2). The quality of evidence was low due to results from only one small study and risk of selection bias.
2.2. Analysis.
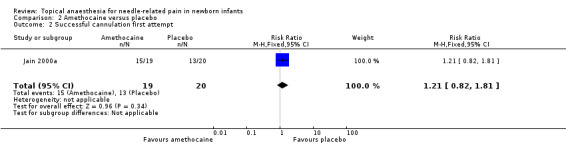
Comparison 2 Amethocaine versus placebo, Outcome 2 Successful cannulation first attempt.
Total cry duration
One small study reported on total cry duration (Jain 2000a). There was no significant difference (in infants that cried) between amethocaine (33 seconds, range 9 to 79; n = 4) and placebo (68 seconds, range 9 to 122; n = 15).
Time taken for completion of procedure
No studies reported time taken for completion of procedure.
Episodes of apnoea
No studies reported episodes of apnoea.
Episodes of bradycardia
No studies reported episodes of bradycardia.
Episodes of oxygen desaturation
No studies reported episodes of oxygen desaturation.
Neurodevelopmental disability
No studies reported neurodevelopmental disability.
Other adverse effects (Outcome 2.3)
Three small studies reported on adverse effects (Jain 2000a; Long 2003; Shah 2008). Jain 2000a and Long 2003 reported no adverse events (local skin reactions). There was no statistical difference between the amethocaine and placebo groups. Shah 2008 found no significant difference between the amethocaine and placebo for local erythema and blanching in the amethocaine and placebo groups but was short‐lasting and mild in nature (typical RR 2.00, 95% CI 0.64 to 6.26; typical RD 0.04, 95% CI ‐0.03 to 0.12); n = 181). There was no heterogeneity (I2 = 0%) (Analysis 2.3). The quality of evidence was moderate due to unclear risk of selection bias.
2.3. Analysis.
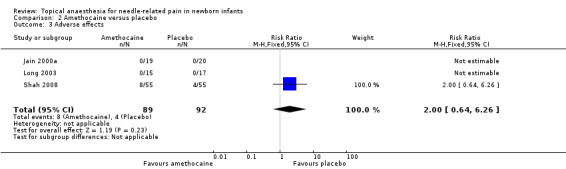
Comparison 2 Amethocaine versus placebo, Outcome 3 Adverse effects.
There were no other adverse effects.
Subgroup analyses
We performed no subgroup analyses because of the small number of included studies.
Discussion
The included studies in this review enrolled infants of 27 weeks' gestation or greater. Thus, there was no evidence on the use of topical anaesthetics in very preterm infants with the majority of the included studies using the preterm and term infant population. All the trials were small, and the effects of uncertain clinical significance. Mostly of the evidence regarding the effectiveness or safety of the interventions studied was inadequate to support clinical recommendations. The different methods of measuring pain and reporting of outcomes meant that the capacity to pool data was limited and this made interpretation difficult. We were unable to determine which topical anaesthetic preparation (amethocaine or EMLA) was superior in relation to its efficacy and safety for needle‐related procedures in newborn infants. None of the studies reported included measures of parental satisfaction or distress, or long‐term outcomes in the infants.
It has been proposed that the effectiveness of topical anaesthetics may be influenced by the type of painful procedure (e.g. intramuscular, heel lance or venepuncture or lumbar puncture). For example, Stevens 1999 and Larsson 1995 argued that topical anaesthetics are less effective during heel lancing because of the increased skin perfusion in the heel. Thus, we intend to include 'type of painful procedure' for subgroup analyses in the review update when there may be more published studies on this topic.
Authors' conclusions
Implications for practice.
Overall, all the trials were small, and the effects of uncertain clinical significance. The evidence regarding the effectiveness or safety of the interventions studied is inadequate to support clinical recommendations. There has been no evaluation regarding any long‐term effects of topical anaesthetics in newborn infants.
Implications for research.
High‐quality studies evaluating the efficacy and safety of topical anaesthetics such as amethocaine and EMLA for needle‐related pain in newborn term or preterm infants are required. These studies should aim to determine efficacy of these topical anaesthetics for the different invasive procedures (especially heel lancing), and on homogeneous groups of infants for gestational age and including long‐term outcomes. While there was no methaemoglobinaemia in the studies that reported methaemoglobin, the efficacy and safety of EMLA, especially in preterm and very preterm infants, and for repeated application, need to be further evaluated in future studies. Studies comparing the efficacy of topical anaesthetics for the different types of needle‐related painful procedures are needed.
Acknowledgements
We acknowledge the support of the Australasian Satellite NRG. We would like to thank Colleen Ovelman and Roger Soll at Cochrane Neonatal for their editorial assistance.
Data and analyses
Comparison 1. Eutectic mixture of local anaesthetics (EMLA) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain using Premature Infant Pain Profile (PIPP) score | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐1.45, 1.99] |
| 2 Pain using Neonatal Infant Pain Scale (NIPS) score | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.75, 1.29] |
| 3 Successful venepuncture first attempt | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.93, 1.03] |
| 4 Adverse effects | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.24, 2.19] |
Comparison 2. Amethocaine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐17.34, 7.34] |
| 2 Successful cannulation first attempt | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.82, 1.81] |
| 3 Adverse effects | 3 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.64, 6.26] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bonetto 2008.
| Methods | Randomised controlled trial performed in Argentina. | |
| Participants | Healthy newborn infants at term requiring heel lancing. Inclusion criteria: gestation ≥ 36 weeks; > 24 hours' postnatal age to < 30 days of life. Exclusion criteria: allergies related to medications, treatment with oxygen, treatment for infection, analgesics or sedatives before procedure, skin infection or disorder. |
|
| Interventions | Placebo: 1. via oral drops; 2. via oral syringe; 3. via cream on skin. Glucose (given via oral syringe) vs 1. placebo via oral drops; 2. placebo cream on skin. Paracetamol (given orally using a syringe) vs 1. placebo via oral drops; 2. placebo via oral syringe. EMLA cream on skin vs 1. placebo via oral drops 2. placebo via oral syringe. No non‐nutritive sucking or any other nourishment. EMLA and placebo cream left on the skin for 60 minutes then removed with a dry cloth and after a further 10 minutes of procedure attended. |
|
| Outcomes | Pain: measured at insertion of heel lance up to 3 minutes using the 0 to 18 PIPP score for infants > 36 weeks' gestation. PIPP scale: a PIPP score < 8 indicated absence or minimal pain; a PIPP score > 8 indicated moderate pain. NIPS scale: a NIPS score of 0 indicated no pain, and a maximum score of 7 indicated moderate‐to‐severe pain. |
|
| Notes | Study translated from Spanish to English via interpreter. Power calculation not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Hydrating cream identical to EMLA; blinding of treating doctor, doctor who performed blood extraction and parents. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Pain measurement performed by 2 observers who were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not obtained. |
| Other bias | Low risk | |
Jain 2000a.
| Methods | Single‐centre randomised controlled trial performed in the UK. | |
| Participants | 40 newborn infants. Gestation 27 to 41 weeks (median 33) at 2 to 17 postnatal age (median 7). Inclusion criteria: infants admitted to the postnatal wards or NICU undergoing routine venepuncture in a hospital. Exclusion criteria: infants who were unwell, ventilated or sedated. |
|
| Interventions | Intervention: amethocaine 1.5 g portion (4%). Placebo: 1.5 g gel. Both gels were applied to the skin under an occlusive dressing for 60 minutes, then wiped off and venepuncture performed 5 minutes later. |
|
| Outcomes | Pain: pain response to needle insertion was assessed using a validated adaptation of the NFCS (NFCS‐short) and by the presence and length of crying. The NFCS‐short assesses each of the following facial characteristics as present (1 point) or absent (0 points): eye squeeze, brow bulge, open
mouth, deepened nasolabial folds and cry. An NFCS score was assigned for each second, starting 5 seconds before the needle insertion and ending 5 seconds after needle insertion (giving a maximum cumulative score of 25 for each 5‐second period). The authors defined a cumulative NFCS score of 10 or less in the 5 seconds after insertion of the needle as indicating no or minimal pain. Incidence of crying. Duration of crying. Successful needle prick at first attempt. Skin reactions. |
|
| Notes | Power calculation not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Infants were randomised to receive amethocaine or placebo gel. Randomisation was stratified within 3 gestation age groups. No other information provided. |
| Allocation concealment (selection bias) | Low risk | Pharmacist allocated treatment groups. The gels were packed in identical tubes by the hospital pharmacy who randomised and coded them. No other information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded to treatment staff and researchers. Gels in identical tubes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The code was only broken at the end of the study after the videotapes had been scored and when the method of defining a painful or non‐painful response had been agreed on. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 infant excluded before analysis before the code was broken because of restlessness before the venepuncture (< 10% attrition). |
| Selective reporting (reporting bias) | Unclear risk | Protocol not obtained. |
| Other bias | Low risk | |
Kaur 2003.
| Methods | Single‐centre randomised controlled trial performed in India. | |
| Participants | 60 newborn infants ≥ 34 weeks' gestation, postnatal age < 4 weeks and undergoing diagnostic lumbar puncture. Uncomplicated vaginal or caesarean section delivery, 5‐minute Apgar score ≥ 7, no history of maternal medication use, absence of structural neurodevelopmental anomalies and a rectal temperature of 37 ± 0.5 °C. Lumbar puncture was performed to rule out meningitis in ill newborns with seizures or sepsis, according to intensive care treatment protocol. Exclusion criteria: infants receiving sedatives or analgesics. |
|
| Interventions | Intervention: EMLA 1.0 g cream. Placebo: 1.0 g cream. Both creams were applied to 1 cm2 of skin under an occlusive dressing for 60 to 90 minutes, then wiped off and the lumbar puncture procedure performed immediately. |
|
| Outcomes | Pain using NFCS score: 4 items of facial action (brow bulge, eye squeeze, nasolabial furrow and open mouth) and the presence of crying used as measures of behavioural response to pain. Each response given a score of 1 if present and 0 if absent, for a possible total ranging from 0 to 5. Heart rate. Oxygen saturation. Methaemoglobinaemia (clinical symptoms). Adverse effects. |
|
| Notes | Power calculation performed: 21 infants per group were needed. Kaur (2003) used 1 total NFCS score of 0 to 5, where presence of a behaviour score = 1 point and absence = 0 points for each of the 5 variables. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated simple random number table. |
| Allocation concealment (selection bias) | Low risk | Neonates received either EMLA or the placebo cream "in a double‐blind manner". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo cream consisted of an inert oil that resembled EMLA. EMLA and placebo were supplied in identical tubes marked only with a number. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes for all infants reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not obtained. |
| Other bias | Low risk | |
Larsson 1995.
| Methods | Single‐centre randomised controlled trial performed in Sweden. | |
| Participants | 112 full‐term healthy newborn infants, range 36.8 to 42.6 weeks' gestation undergoing diagnostic heel prick for phenylketonuria on third day postnatal age in a maternity ward. Exclusion criteria: infants with any illness, abnormality or receiving pharmacotherapy. |
|
| Interventions | Intervention duration groups: 14 newborns to each duration of application group: 10, 20, 30, 40, 50, 60, 90 and 120 minutes. In each group, 7 neonates received EMLA 0.5 g cream and 7 neonates in each group received 0.5 g placebo cream. A silicon adhesive (Tegaderm 3M) with a central opening of approximately 1.0 cm2 was applied to the heel. The opening was marked with a thin soft pen. 500 mg (0.5 mL) of EMLA or placebo was placed on the opening and covered with another occlusive dressing (Tegaderm 3M). After the application time, both the adhesive and the test substance were removed. Sham heel lancing was performed on 10 newborns to evaluate validity of pain assessment ‐ Frey‐hair monofilament was applied before every heel prick to assess baseline flexor reflex response. |
|
| Outcomes | Pain cry defined as present if it included: brow bulge, eye squeeze, naso‐labial furrow and open lips, followed by a strong, high‐pitched cry. Adverse effects (pallor or redness at application site). |
|
| Notes | Sample size calculation not reported. Larsson (1995) combined the behavioural responses to a nominal pain cry or no pain cry response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation procedure was performed by ASTRA, Sweden". No other information provided. |
| Allocation concealment (selection bias) | Low risk | "120 neonates were allocated to 8 groups according to the time when EMLA or placebo was applied (10, 20, 30, 40, 50, 60, 90, 120 min, groups 1‐8). In each of the 8 group, 7 infants received active substance and 7 placebo." Creams prepared by ASTRA, Sweden. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | EMLA and placebo were supplied in identical tubes. "Double‐blinded randomized controlled trial". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/112 infants were excluded because the test substance did not stay in place. The 2 excluded infants were randomised to the placebo group. Data in the study not analysed as intention to treat. Outcomes for other infants reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not obtained. |
| Other bias | Low risk | |
Larsson 1998.
| Methods | Single‐centre randomised controlled trial performed in Sweden. | |
| Participants | 120 neonates, 37 to 43 weeks' gestation undergoing diagnostic venepuncture for phenylketonuria on 3 to 8 days' postnatal age in a maternity ward. Exclusion criteria: none reported. |
|
| Interventions | Intervention: EMLA 0.5 g cream. Placebo: 0.5 g cream. 500 mg (0.5 mL) of EMLA or placebo were applied to the dorsum of the hand under an occlusive dressing (Tegaderm 3M) for 60 minutes, then wiped off, the neonates hand warmed in the nurse's hand for 1 minute, and the venepuncture procedure performed. 1 g EMLA cream corresponded to approximately 1 mL. After the application time of 60 minutes, both the adhesive and the test substance were carefully removed. |
|
| Outcomes | Pain after skin puncture: changes in facial activity were assessed using the NFCS. The facial actions scored included brow bulge, eyes squeezed shut, deepening
of the naso‐labial furrow, open lips, a taut cupped tongue and stretching of the mouth (vertically and horizontally). The facial reactions were analysed during the first 15 seconds after the skin was punctured (0 to 15 seconds) and again during, after a pause of 60 seconds, the first 15 seconds when manipulation took place (60 to 75 seconds). The observation periods were divided into 15 × 1‐second intervals. The presence or absence of the 6 variables during each interval was recorded. Thus, each variable ranged from 0% to 100%. The NFCS score was presented as percent positive (present) scores of the 6 variables and the total range would therefore be 0% to 600%. Cry duration after first skin puncture. Latency to cry after first skin puncture. Duration of first cry. Time to successfully complete procedure. |
|
| Notes | Power calculation not reported. Larsson (1998) gave each variable a score from 0% to 100% so the total range was 0% to 600% for the 6 variables. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation performed by ASTRA, Sweden. No other information provided. |
| Allocation concealment (selection bias) | Low risk | EMLA and placebo were supplied in identical tubes marked only with a number. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | EMLA and placebo were supplied in identical tubes marked only with a number. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 2 blinded observers analysed the results from a video or audio tape. Each observer assessed the data independently and could not communicate findings to the other. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 infants in the EMLA group and 4 infants in the placebo group were excluded post randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not obtained. |
| Other bias | High risk | |
Long 2003.
| Methods | Single‐centre randomised controlled trial performed in the UK. | |
| Participants | 34 newborn infants 32 to 42 weeks' gestation (median 36 weeks) were enrolled at 3 to 18 days' postnatal age (median 6) undergoing diagnostic venepuncture for phenylketonuria or bilirubinaemia aged 3 to 18 days in a hospital. Exclusion criteria: infants with congenital malformations or abnormal neurological state, who needed assisted ventilation and having analgesia or sedation as part of their routine management. |
|
| Interventions | Infants received an amethocaine patch formulated from hydroxypropyl cellulose discs of uniform area (0.283 cm2) were cut from the dried amethocaine film which, because of the formulation method, was calculated to contain amethocaine base (0.283 mg). The discs were positioned onto pressure‐sensitive adhesive film to sandwich the amethocaine discs between the adhesive film and the release liner. Patches of 16 mm diameter were cut with the 6‐mm disc of amethocaine film at the centre. The amethocaine patch, or an identical a 'placebo device' applied on the dorsal aspect of the hand for 30 minutes before venepuncture then wiped off, and the venepuncture procedure performed after 5 minutes. Venepuncture was carried out by a trained neonatal nurse practitioner experienced in performing this procedure. The use of a dummy was not permitted. The area around the vein located on the dorsum of the hand was swabbed with an alcohol wipe and allowed to dry before application of the patch. | |
| Outcomes | Pain: assessed in response to needle insertion using a validated adaptation of the NFCS and the presence of crying. This method scored each of a number of facial characteristics as being present (scoring 1 point) or absent (0 score). These facial characteristics are brow bulge, eye squeeze, naso‐labial furrow, open lips and cry. The median cumulative NFCS scores between the 2 groups scored during the 5 seconds immediately before the venepuncture. A cumulative score of ≤ 10 in the 5 seconds following the procedure was defined as indicating clinically effective anaesthesia. Skin reactions. |
|
| Notes | Power calculation based on the findings of Jain 2000a, a sample size of 34 (power 80%, P = 0.05) was estimated as necessary to show an increase of local anaesthetic action from 17% to 55%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Identical patch randomly picked from a box, equally divided between placebo and active devices. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded to treatment staff and researchers. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Video footage was viewed and scored by nurses not involved in the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 sets of parents withdrew their consent after randomisation and these infants, both in the amethocaine group, were withdrawn from the study. |
| Selective reporting (reporting bias) | Low risk | We were unable to obtain a study protocol. |
| Other bias | Low risk | |
Shah 2008.
| Methods | Single‐centre randomised controlled trial performed in Canada. | |
| Participants | 110 full‐term neonates, ≥ 37 weeks' gestation, neonates who were ≥ 2500 g birth weight undergoing routine post‐natal intramuscular injection of vitamin K 0.5 mL. Exclusion criteria: neonates requiring admission to the NICU, with major congenital or know neurological abnormalities (antenatal diagnosis) or required evaluation for sepsis at birth. |
|
| Interventions | Infants received amethocaine gel 4% (55 infants) or placebo (55 infants) to the skin and covered with an adhesive Tegaderm occlusive dressing for 30 minutes before the scheduled time of the procedure. | |
| Outcomes | Pain: measured using the facial grimacing score. The presence or absence of 3 facial actions (brow bulge, eyes squeeze and deepening of the naso‐labial furrow) were scored in 2‐second intervals for the first 20 seconds (or less if the phase lasted < 20 seconds) of each procedure phase from the videotapes by a trained research assistant who was not involved in the video recording. The research assistant scored each phase sequentially and was unaware of group assignment and study hypothesis. The data were then collapsed for each facial action into the percentage of time the infant expressed the action. An overall pain score was computed by summing the percentage scores for the 3 facial actions and then dividing by 3. The score ranged from 0% to 100%. These 3 facial actions were chosen for the study as they have been observed in 99% of neonates within 6 seconds of an invasive procedure (heel lance) and are believed to be the most sensitive indicators of infant pain. Local adverse events. |
|
| Notes | Sample size based on the pain scores obtained from a previous study. To achieve a clinically significant reduction in pain scores of 20% between groups, with an SD of 7.6, with 80% power and alpha value of < 0.05, authors estimated a sample size of 49 infants in each group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer‐generated list created by a statistician, neonates were consecutively randomised. |
| Allocation concealment (selection bias) | Low risk | "Allocation concealment was achieved by keeping the randomisation code in the pharmacy department." Once an eligible neonate was identified, the research pharmacist dispensed the single‐dose study medication. Each participant was identified by a number. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding parents, carers (nurses), and the research team. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Outcome assessors were blinded to treatment allocation throughout the study. The code was not broken until the database was locked." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes for all infants reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not obtained. |
| Other bias | Low risk | |
Stevens 1999.
| Methods | Double‐centre randomised controlled trial performed in Canada. | |
| Participants | 120 newborn preterm neonates, range 30 to 36 weeks' gestation (56 intervention, 56 placebo) undergoing a heel prick aged 1 to 5 days' postnatal age in 2 NICU. | |
| Interventions | The study was undertaken in 2 phases. For phase 1, 31 infants in the experimental group receive EMLA 0.5 g on an occlusive dressing for 30 minutes. 5 minutes before heel lance, the dressing was removed and any remaining cream was wiped away with a tissue. The heel was then wrapped in a warm moist compress for 5 minutes before heel lance with an automated lancet (Microtainer, 1.4 mm puncture depth. 29 infants in the control group received 0.5 g Glaxal base and the same procedure as the experimental group was used. For phase 2, the method was the same as phase 1, 25 infants in the experimental group receive EMLA 0.5 g on an occlusive dressing for 60 minutes. 21 infants in the control group received 0.5 g placebo cream that was different to that used in phase 1. It contained all ingredients of EMLA except for the lidocaine and prilocaine, which were substituted with MCT oil (different to phase 1 placebo cream) and the creams remained intact for 60 minutes. Also, a larger automated lancet was used in phase 2 than that used in phase 1. This was due to a change in unit practice. A Microtainer, with a 2.2 mm puncture depth was used. |
|
| Outcomes | Pain: used the 0 to 21 PIPP score for infants of lesser gestational age at birth (i.e. < 28 weeks' gestation). The PIPP is a 7‐indicator measure, and gave a total range of scores of 0 to 21. Methaemoglobin levels. Adverse events. |
|
| Notes | Sample size based on previous research results comparing infants who received EMLA vs a placebo, sample size was calculated to show a 20% reduction (alpha = 0.05, beta = 0.20) in pain response scores. A total of 120 infants, or 30 infants per study group, was required. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Infants were randomly assigned using a computer‐generated table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Creams were prepared by the pharmacy department, individually packaged the creams in dark glass ointment jars and labelling the jars by code. Those who coded the data were blinded to infant group and vague to the purpose of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Phase 1 creams were not double blinded as the research nurse applying the creams may have been able to detect group allocation by the cream's colour and consistency. Phase 2 creams were identical in colour and consistency. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In Phase 2, 14 infants were withdrawn after randomisation because they were discharged or transferred, leaving 106 infants available for analysis. Of the 106 infants, 31 were in group 1 (30 minutes' EMLA), 29 in group 2 (30 minutes' Glaxal base), 25 in group 3 (60 minutes' EMLA) and group 4 (60 minutes' placebo). The attrition appeared to be evenly distributed between the intervention and control groups but this is not reported by the authors. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not obtained. |
| Other bias | Low risk | |
EMLA: eutectic mixture of local anaesthetics; NFCS: Neonatal Facial Coding System; NICU: neonatal intensive care unit; NIPS: Neonatal Infant Pain Scale; PIPP: Premature Infant Pain Profile; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abad 2001 | Intervention: oral sucrose vs lidocaine‐prilocaine cream. |
| Acharya 1998 | Median age at inclusion into the study: 3 to 65 days. |
| Ahmed 2009 | Intervention: EMLA vs control in infants undergoing circumcision. |
| Arendts 2008 | Aged 12 months to 12 years. |
| Ballantyne 2003 | Age at time of enrolment into study: 2 to 85 days. |
| Benini 1993 | EMLA vs placebo in infants undergoing circumcision. |
| Biran 2011 | Comparison groups: sucrose and EMLA vs sucrose and placebo cream. |
| Brisman 1998 | Age at enrolment: 1 to 74 days. |
| Choy 1999 | Age of participants 1 to 14 years. |
| Clarke 1986 | Age of participants 1 to 14 years. |
| Essink‐Tebbes 1999 | Non‐randomised controlled trial. |
| Gourrier 1995 | Non‐randomised controlled trial. |
| Jain 2000b | The cutaneous withdrawal reflex was elicited in response to stimulation of the dorsum of the neonate's foot using nylon filaments rather than a needle‐related pain procedure. |
| Joyce 2001 | Intervention: music and EMLA in infants undergoing circumcision. |
| Kucukoglu 2015 | Focus on instrumental touching on infant pain perception. No placebo used. Age at enrolment unclear. |
| Kurien 1985 | Median age 24 to 30 months. |
| Lemyre 2007 | Majority of infants given sucrose in addition to the intervention and placebo. |
| Lindh 2000 | Randomised controlled trial comparing EMLA and placebo. Only outcome measure reported in this study is heart rate variability. |
| Marcatto 2011 | Intervention EMLA vs glucose. |
| McIntosh 1994 | Non‐randomised controlled trial. |
| Moore 2001 | Postnatal age not reported. |
| Nahum 2007 | Age of infants 1 to 60 days. |
| Newbury 2009 | Aged 3 months to 15 years. |
| Nilsson 1990 | Non‐randomised controlled trial. |
| Robieux 1991 | Aged 3 to 36 months. |
| Taddio 1995 | No control group. Randomisation based on site of EMLA application (right or left heel). |
| Taddio 2006 | Interventions: tetracaine vs intravenous morphine vs tetracaine and intravenous morphine vs no treatment (control group not randomised). |
| van Kan 1997 | Aged 1 to 15 years. |
| Weatherstone 1993 | Intervention: 30% lidocaine vs placebo in infants undergoing circumcision. |
EMLA: eutectic mixture of local anaesthetics.
Differences between protocol and review
We added the methodology and plan for the 'Summary of findings' tables and GRADE recommendations, which were not included in the original protocol.
We changed the age of infants eligible for inclusion from postnatal age maximum of 28 days to maximum postnatal age of one month.
Contributions of authors
JPF and SB wrote the protocol.
JPF and CT wrote the first draft of the review, revised subsequent drafts and assessed study eligibility.
KS commented on drafts of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA. Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C, USA.
-
Jorge Alberto Navarro, Australia.
Translation of included study
Declarations of interest
None known.
New
References
References to studies included in this review
Bonetto 2008 {published data only}
- Bonetto G, Salvatico E, Varela N, Cometto C, Gomez PF, Calvo B. Pain prevention in term neonates: randomised trial for three methods. Archives of Argentine Pediatrics 2008;106(5):392‐6. [PUBMED: 19030637] [DOI] [PubMed] [Google Scholar]
Jain 2000a {published data only}
- Jain A, Rutter N. Does topical amethocaine gel reduce the pain of venepuncture in newborn infants? A randomised double blind controlled trial. Archives of Disease in Childhood Fetal and Neonatal Edition 2000;83(3):F207‐10. [PUBMED: 11040170] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaur 2003 {published data only}
- Kaur G, Gupta P, Kumar A. A randomized trial of eutectic mixture of local anesthetics during lumbar puncture in newborns. Archives of Pediatric and Adolescent Medicine 2003;157(11):1065‐70. [PUBMED: 14609894] [DOI] [PubMed] [Google Scholar]
Larsson 1995 {published data only}
- Larsson BA, Lagercrantz LJH, Olsson GL. Does a local anaesthetic cream (EMLA) alleviate pain from heel‐lancing in neonates?. Acta Anaesthesiologica Scandinavica 1995;39(8):1028‐31. [PUBMED: 8607303] [DOI] [PubMed] [Google Scholar]
Larsson 1998 {published data only}
- Larsson BA, Tannfeldt G, Lagercrantz H, Olsson GL. Alleviation of the pain of venepuncture in neonates. Acta Paediatrica 1998;87(7):774‐9. [PUBMED: 9722252] [DOI] [PubMed] [Google Scholar]
Long 2003 {published data only}
- Long CP, McCafferty DF, Sittlington NM, Halliday HL, Woolfson AD, Jones DS. Randomized trial of novel tetracaine patch to provide local anaesthesia in neonates undergoing venepuncture. British Journal of Anaesthesia 2003;91(4):514‐8. [PUBMED: 14504152] [DOI] [PubMed] [Google Scholar]
Shah 2008 {published data only}
- Shah V, Taddio A, Hancock R, Shah P, Ohlsson A. Topical Amethocaine gel 4% for intramuscular injection in term neonates: a double‐blind, placebo‐controlled, randomized trial. Clinical Therapeutics 2008;30(1):166‐74. [PUBMED: 18343253] [DOI] [PubMed] [Google Scholar]
Stevens 1999 {published data only}
- Stevens B, Johnston C, Taddio A, Jack A, Narciso J, Stremler R, et al. Management of pain from heel lance with lidocaine‐prilocaine (EMLA) cream: is it safe and efficacious in preterm infants?. Developmental and Behavioral Pediatrics 1999;20(4):216‐20. [PUBMED: 10475595] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abad 2001 {published data only}
- Abad F, Diaz‐Gomez NM, Domenech E, Gonzalez D, Robayna M, Feria M. Oral sucrose compares favourably with lidocaine‐prilocaine cream for pain during venepuncture in neonates. Acta Paediatrica 2001;90(2):160‐5. [PUBMED: 11236045] [PubMed] [Google Scholar]
Acharya 1998 {published data only}
- Acharya AB, Bustani PC, Phillips JD, Taub NA, Beattie RM. Randomised controlled trial of eutectic mixture of local anaesthetics cream for venepuncture in healthy preterm infants. Archives of Disease in Childhood Fetal and Neonatal Edition 1998;78(2):F138‐42. [PUBMED: 9577286] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmed 2009 {published data only}
- Ahmed K, Shah FA, Ali SA. Neonatal circumcision: with or without anaesthesia. Pakistan Journal of Surgery 2009;25:286‐9. [Google Scholar]
Arendts 2008 {published data only}
- Arendts G, Stevens M, Fry M. Topical anaesthesia and intravenous cannulation success in paediatric patients: a randomized double‐blind trial. British Journal of Anaesthesia 2008;100:521‐4. [DOI] [PubMed] [Google Scholar]
Ballantyne 2003 {published data only}
- Ballantyne M, Ung E, Gibbins S, Stevens B. A randomized controlled trial evaluating the efficacy of tetracaine gel for pain relief from peripherally inserted central catheters in infants. Advances in Neonatal Care 2003;3(6):297‐307. [PUBMED: 14695501] [DOI] [PubMed] [Google Scholar]
Benini 1993 {published data only}
- Benini F, Johnston C, Faucher D, Aranda JV. Topical anesthesia during circumcision in the newborn. JAMA 1993;270(7):850‐3. [PUBMED: 8340985] [PubMed] [Google Scholar]
Biran 2011 {published data only}
- Biran V, Gourrier E, Cimerman P, Walter‐Nicolet E, Mitanchez D, Carbajal R. Analgesic effects of EMLA cream and oral sucrose during venipuncture in preterm infants. Pediatrics 2011;128(1):e63‐70. [PUBMED: 21669894] [DOI] [PubMed] [Google Scholar]
Brisman 1998 {published data only}
- Brisman M, Ljung BML, Otterbom I, Larsson LE, Andreasson SE. Methaemoglobin formation after the use of EMLA cream in term infants. Acta Paediatrica 1998;87(11):1191‐4. [PUBMED: 9846923] [DOI] [PubMed] [Google Scholar]
Choy 1999 {published data only}
- Choy L, Collier J, Watson AR. Comparison of lignocaine‐prilocaine cream and amethocaine gel for local analgesia before venepuncture in children. Acta Paediatrica 1999;88(9):961‐4. [PUBMED: 10519337] [DOI] [PubMed] [Google Scholar]
Clarke 1986 {published data only}
- Clarke S, Radford M. Topical anaesthesia for venepuncture. Archives of Disease in Childhood 1986;61(11):1132‐4. [PUBMED: 3539033] [DOI] [PMC free article] [PubMed] [Google Scholar]
Essink‐Tebbes 1999 {published data only}
- Essink‐Tebbes CM, Wuis EW, Liem KD, Dongen RTM, Hekster YA. Safety of lidocaine‐prilocaine cream application four times a day in premature neonates: a pilot study. European Journal of Pediatrics 1999;158(5):421‐3. [PUBMED: 10333129] [DOI] [PubMed] [Google Scholar]
Gourrier 1995 {published data only}
- Gourrier E, Karoubi P, Hanache A, Merbouche S, Mouchnino G, Dhabhi S, et al. Use of EMLA cream in premature and full‐term newborn infants. Study of efficacy and tolerance. Archives of Pediatrics 1995;2(11):1041‐6. [PUBMED: 8547971] [DOI] [PubMed] [Google Scholar]
Jain 2000b {published data only}
- Jain A, Rutter N. Topical amethocaine gel in the newborn infant: how soon does it work and how long does it last?. Archives of Disease in Childhood Fetal and Neonatal Edition 2000;83(3):F211‐4. [PUBMED: 11040171] [DOI] [PMC free article] [PubMed] [Google Scholar]
Joyce 2001 {published data only}
- Joyce BA, Keck JF, Gerkensmeyer J. Evaluation of pain management interventions for neonatal circumcision pain. Journal of Pediatric Health Care 2001;15(3):105‐14. [PUBMED: 11353359] [DOI] [PubMed] [Google Scholar]
Kucukoglu 2015 {published data only}
- Kucukoglu S, Celebioglu A, Caner I, Ok G, Maden R. The effects of instrumental touching on infant pain perception and the effects of eutectic mixture of local anesthetics (EMLA) on the reduction of pain. Iran Journal of Pediatrics 2015;25:e532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurien 1985 {published data only}
- Kurien L, Lollberg H, Uppfeldt A. Venepuncture pain can be reduced. Journal of Tropical Medicine and Hygiene 1985;88(6):397‐9. [PUBMED: 3837123] [PubMed] [Google Scholar]
Lemyre 2007 {published data only}
- Lemyre B, Hogan DL, Gaboury I, Sherlock R, Blanchard C, Moher D. How effective is tetracaine 4% gel, before venipuncture, in reducing procedural pain in infants: a randomized double‐blind placebo controlled trial. BMC Pediatrics 2007;7:7. [PUBMED: 17288611] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindh 2000 {published data only}
- Lindh V, Wiklund U, Hakansson S. Assessment of the effect of EMLA during venipuncture in the newborn by analysis of heart rate variability. Pain 2000;86(3):247‐54. [PUBMED: 10812254] [DOI] [PubMed] [Google Scholar]
Marcatto 2011 {published data only}
- Marcatto JO, Vasconcelos PC, Araujo CM, Tavares EC, Pereira SY. EMLA versus glucose for PICC insertion: a randomised‐triple masked controlled study. Archives of Disease in Childhood Fetal & Neonatal Edition 2011;96(6):F467‐8. [PUBMED: 21622670] [DOI] [PubMed] [Google Scholar]
McIntosh 1994 {published data only}
- McIntosh N, Veen L, Brameyer H. Alleviation of the pain of heel prick in preterm infants. Archives of Disease in Childhood 1994;70(3):F177‐81. [PUBMED: 8198410] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2001 {published data only}
- Moore J. No more tears: a randomized controlled double‐blind trial of amethocaine gel vs. placebo in the management of procedural pain in neonates. Journal of Advanced Nursing 2001;34(4):475‐82. [PUBMED: 11380714] [DOI] [PubMed] [Google Scholar]
Nahum 2007 {published data only}
- Nahum Y, Tenenbaum A, Isaiah W, Levy‐Khademi F. Effect of eutectic mixture of local anesthetics (EMLA) for pain relief during suprapubic aspiration in young infants: a randomized, controlled trial. Clinical Journal of Pain 2007;23(9):756‐9. [PUBMED: 18075401] [DOI] [PubMed] [Google Scholar]
Newbury 2009 {published data only}
- Newbury C, Herd DW. Amethocaine versus EMLA for successful intravenous cannulation in a children's emergency departments: a randomised controlled trial. Emergency Medicine Journal 2009;26(7):487‐91. [PUBMED: 19546268] [DOI] [PubMed] [Google Scholar]
Nilsson 1990 {published data only}
- Nilsson A, Engberg G, Henneberg S, Danielson K, Verdier CH. Inverse relationship between age‐dependent erythrocyte activity of methaemoglobin reductase and prilocaine‐induced methaemoglobinaemia during infancy. British Journal of Anaesthesia 1990;64(1):72‐6. [PUBMED: 2302379] [DOI] [PubMed] [Google Scholar]
Robieux 1991 {published data only}
- Robieux I, Kumar R, Radhakrishnan S, Koren G. Assessing pain and analgesia with a lidocaine‐prilocaine emulsion in infants and toddlers during venipuncture. Journal of Pediatrics 1991;118(6):971‐3. [PUBMED: 2040936] [DOI] [PubMed] [Google Scholar]
Taddio 1995 {published data only}
- Taddio A, Shennan AT, Stevens B, Leeder JS, Koren G. Safety of lidocaine‐prilocaine cream in the treatment of preterm infants. Journal of Pediatrics 1995;127(6):1002‐5. [PUBMED: 8523173] [DOI] [PubMed] [Google Scholar]
Taddio 2006 {published data only}
- Taddio A, Lee C, Yip A, Parvez B, McNamara PJ, Shah V. Intravenous morphine and topical tetracaine for treatment of pain in preterm neonates undergoing central line placement. Journal of the American Medical Association 2006;295(7):793‐9. [PUBMED: 16478902] [DOI] [PubMed] [Google Scholar]
van Kan 1997 {published data only}
- Kan HJ, Egberts AC, Rijnvos WP, ter Pelkwijk NJ, Lenderink AW. Tetracaine versus lidocaine‐prilocaine for preventing venipuncture‐induced pain in children. American Journal of Health‐System Pharmacy 1997;54(4):388‐92. [PUBMED: 9043560] [DOI] [PubMed] [Google Scholar]
Weatherstone 1993 {published data only}
- Weatherstone KB, Rasmussen LB, Jackson EM, Claflin KS, Leff RD. Safety and efficacy of a topical anesthetic for neonatal circumcision. Pediatrics 1993;92(5):710‐4. [PUBMED: 8414860] [PubMed] [Google Scholar]
Additional references
Anand 1987
- Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. New England Journal of Medicine 1987;317(21):1321‐9. [DOI] [PubMed] [Google Scholar]
Anand 2000
- Anand KJ, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behaviour?. Biology of the Neonate 2000;77(2):69‐82. [DOI] [PubMed] [Google Scholar]
Anand 2001
- Anand KJ. International Evidence‐Based Group for Neonatal Pain. Consensus statement for the prevention and management of pain in the newborn. Archives of Pediatrics & Adolescent Medicine 2001;155(2):173‐80. [DOI] [PubMed] [Google Scholar]
AstraZeneca 2008
- AstraZeneca. EMLA, 2008. www.astrazeneca.com.au/healthcare‐professionals/product‐information (accessed 4 December 2012).
Bell 1994
- Bell SG. The National Pain Management Guideline: implications for neonatal intensive care. Agency for Health Care Policy and Research. Neonatal Network ‐ Journal of Neonatal Nursing 1994;13(3):9‐17. [PubMed] [Google Scholar]
Brady‐Fryer 2004
- Brady‐Fryer B, Wieben N, Lander JA. Pain relief for neonatal circumcision. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004217.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Browne 1999
- Browne J, Awad I, Plant R, McAdoo J, Shorten G. Topical amethocaine (Ametop) is superior to EMLA for intravenous cannulation. Eutectic mixture of local anesthetics. Canadian Journal of Anaesthesia 1999;46(11):1014‐18. [DOI] [PubMed] [Google Scholar]
Camp 2007
- Camp NE. Methemoglobinemia. Journal of Emergency Nursing 2007;33(2):172‐4. [DOI] [PubMed] [Google Scholar]
Coleman 1996
- Coleman MD, Coleman N. Drug induced methaemoglobinaemia. Treatment issues. Drug Safety 1996;14(6):394‐405. [DOI] [PubMed] [Google Scholar]
Fitzgerald 2001
- Fitzgerald M, Beggs S. The neurobiology of pain: developmental aspects. Neuroscientist 2001;7(3):246‐57. [DOI] [PubMed] [Google Scholar]
Foster 2008
- Foster J, Bidewell J, Buckmaster A, Lees S, Henderson‐Smart D. Parental stress and satisfaction in the non‐tertiary special care nursery. Journal of Advanced Nursing 2008;61(5):522‐30. [DOI] [PubMed] [Google Scholar]
Franck 2004
- Franck LS, Cox S, Allen A, Winter I. Parental concern and distress about infant pain. Archives of Disease in Childhood 2004;89(1):F71‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro [Version 3.2 for Windows]. The GRADE Working Group, 2008.
Grunau 1987
- Grunau RV, Craig KD. Pain expression in neonates: facial action and cry. Pain 1987;28:395‐410. [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lander 1996
- Lander J, Hodgins S, Nazarali J, McTavish J, Ouellette J, Friesen E. Determinants of success and failure of EMLA. Pain 1996;64(1):89‐97. [DOI] [PubMed] [Google Scholar]
Lander 2006
- Lander JA, Weltman BJ. EMLA and amethocaine for reduction of children's pain associated with needle insertion. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004236.pub2] [DOI] [PubMed] [Google Scholar]
Lawrence 1993
- Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Network 1993;12(6):59‐66. [PubMed] [Google Scholar]
Lawson 1995
- Lawson RA, Smart NG, Gudgeon AC, Morton NS. Evaluation of an amethocaine gel preparation for percutaneous analgesia before venous cannulation in children. British Journal of Anaesthesia 1995;75(3):282‐5. [DOI] [PubMed] [Google Scholar]
Manner 1987
- Manner T, Kanto J, Iiasalo E, Lindberg R, Vinamaki O, Scheinin M. Reduction of pain at venous cannulation in children with a eutectic mixture of lidocaine and prilocaine (EMLA cream): comparison with placebo cream and no local premedication. Acta Anaesthesiologica Scandinavia 1987;31(8):735‐9. [DOI] [PubMed] [Google Scholar]
Maunuksela 1986
- Maunuksela EL, Korpela R. Double‐blind evaluation of a lignocaine‐prilocaine cream (EMLA) in children: effect on the pain associated with venous cannulation. British Journal of Anaesthesia 1986;58(11):1242‐5. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Centre: The Cochrane Collaboration, 2014.
Sashdeva 2003
- Sachdeva R, Pugeda JG, Casale LR, Meizlish JL, Zarich SW. Benzocaine‐induced methemoglobinemia: a potentially fatal complication of transesophageal echocardiography. Texas Heart Institute Journal 2003;30(4):308‐10. [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). GRADE handbook for grading quality of evidence and strength of recommendations. Available from www.guidelinedevelopment.org/handbook Updated October 2013.
Smith & Nephew 2012
- Smith, Nephew. Ametop gel, 2012. www.smith‐nephew.com/global/assets/en‐gb/pdf/v1‐ametopspc_uk.pdf (accessed 4 December 2012).
Stephens 2007
- Stephens C, Fawcett TN. Nitric oxide and nursing: a review. Journal of Clinical Nursing 2007;16(1):67‐76. [DOI] [PubMed] [Google Scholar]
Stevens 1996
- Stevens B, Johnston CC, Petryshun P, Taddio A. Premature Infant Pain Profile: development and initial validation. Clinical Journal of Pain 1996;12(1):13‐22. [DOI] [PubMed] [Google Scholar]
Taddio 1998
- Taddio A, Ohlsson A, Einarson TR, Stevens B, Koren G. A systematic review of lidocaine‐prilocaine cream (EMLA) in the treatment of acute pain in neonates. Pediatrics 1998;101(2):e1. [DOI] [PubMed] [Google Scholar]
Taddio 1999
- Taddio A, Ohlsson K, Ohlsson A. Lidocaine‐prilocaine cream for analgesia during circumcision in newborn boys. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD000496] [DOI] [PubMed] [Google Scholar]
Tse 1995
- Tse S, Barrington K, Byrne P. Methemoglobinemia associated with prilocaine use in neonatal circumcision. American Journal of Perinatology 1995;12:331‐3. [DOI] [PubMed] [Google Scholar]
Weinberger 2001
- Weinberger B, Laskin DL, Heck DE, Laskin JD. The toxicology of inhaled nitric oxide therapy. Toxicological Sciences 2001;59(1):5‐16. [DOI] [PubMed] [Google Scholar]
Whitfield 2000
- Whitfield MF, Grunau RE. Behavior, pain perception, and the extremely low‐birth weight survivor. Clinics in Perinatology 2000;27(2):363‐79. [DOI] [PubMed] [Google Scholar]
Woolfson 1990
- Woolfson AD, McCafferty DF, Boston V. Clinical experiences with a novel percutaneous amethocaine preparation: prevention of pain due to venipuncture in children. British Journal of Clinical Pharmacology 1990;30(2):273‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 1999
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Annals of Emergency Medicine 1999;34(5):646‐56. [DOI] [PubMed] [Google Scholar]
Zaky 2009
- Zaky A, Siriussawakul A, Tostenrud R, Pauldine R, Lang JD. Clinical update on therapeutic use of nitric oxide. Contemporary Critical Care 2009;7:1‐12. [Google Scholar]


