Abstract
Background
According to current recommendations a multimodal approach is believed to be the gold standard for postoperative pain treatment in children. However, several surveys in the last few years demonstrated that postoperative pain in children is still a serious problem, mainly because opioids are avoided. One of the reasons for this is the fear of severe adverse events following opioid administration. Tramadol is a weak mu‐opioid agonist and inhibits reuptake of noradrenaline and serotonin (5HT). Because of a relatively wide therapeutic window and a ceiling effect with a lower risk for severe adverse events (for example respiratory depression) tramadol is a widely used opioid in children. However, the exact efficacy and occurrence of adverse events following tramadol (in comparison with placebo or other opioids) for postoperative pain treatment in children and adolescents are currently not clear.
Objectives
To assess the effectiveness and side effect profile of tramadol for postoperative pain relief in children and adolescents undergoing different surgical procedures.
Search methods
We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 6), MEDLINE via PubMed (January 1966 to July 2014) and EMBASE via Ovid (January 1947 to July 2014). There were no restrictions regarding language or date of publication. The reference lists of all included trials were checked for additional studies.
Selection criteria
All randomised controlled clinical trials investigating the perioperative administration of tramadol compared to placebo or other opioids for postoperative pain treatment in children and adolescents were included.
Data collection and analysis
Three review authors independently assessed the study eligibility, performed the data extraction and assessed the risk of bias of included trials.
Main results
Twenty randomised controlled trials involving 1170 patients were included in this systematic review. The overall risk of bias in included trials was assessed as unclear, because concealment of allocation processes and blinding of outcome assessors were poorly described. Due to inconsistent outcome reporting, data from 17 included trials could be pooled for some endpoints only. Eight trials compared tramadol administration with placebo and five trials found that the need for rescue analgesia in the postoperative care unit (PACU) was reduced in children receiving tramadol (RR 0.40; 95% CI 0.20 to 0.78; low quality evidence). Only one trial investigated the number of patients with moderate to severe pain, but a non‐validated pain scale was used (very low quality evidence). Four trials compared morphine with tramadol administration. There was no clear evidence of difference in the need for rescue analgesia in the PACU (RR 1.25; 95% CI 0.83 to 1.89; low quality evidence) with tramadol compared with morphine. No trials could be pooled for the outcome 'number of patients with moderate to severe pain'. Three trials were included for the comparison of tramadol with nalbuphine. There was no clear evidence for the need for rescue analgesia in the PACU (RR 0,63; 95% CI 0.16 to 2.45; low quality evidence). Only one trial reported the number of patients with moderate to severe pain, but used a non‐validated pain scale (very low quality evidence). Two out of six included trials, which compared pethidine with tramadol, reported the number of children with a need for rescue analgesia within the PACU and showed no clear evidence (RR 0.93; 95% CI 0.43 to 2.02; very low quality evidence). Two trials reported the number of patients with moderate to severe pain and showed a lower RR in patients treated with tramadol (RR 0.64; 95% CI 0.36 to 1.16; low quality evidence). Only one trial was included, which compared tramadol with fentanyl, reporting the number of patients with the need for rescue analgesia (very low quality evidence). Generally, adverse events were poorly reported. Most data could be pooled for the comparison with placebo focusing on the RR for postoperative nausea and vomiting (PONV) in the postoperative care unit and 24 h postoperation. Children treated with tramadol, compared to placebo, did not show clear evidence of benefit for PONV in the postoperative care unit (RR 0.84; 95% CI 0.28 to 2.52; moderate quality evidence) and 24 h postoperation (RR 0.78; 95% CI 0.54 to 1.12; moderate quality evidence).
Authors' conclusions
The overall evidence regarding tramadol for postoperative pain in children is currently low or very low and should be interpreted with caution due to small studies and methodological problems (different validated and non‐validated pain scales with different pain triggers, missing sample size calculations and missing intention‐to‐treat analysis). Nevertheless, we demonstrated that tramadol administration might provide appropriate analgesia when compared to placebo; this is based on results showing reduced rescue analgesia in children treated with tramadol compared to placebo. In contrast, the evidence regarding the comparison with other opioids (for example morphine) was uncertain. Adverse events were only poorly reported, so an accurate risk‐benefit analysis was not possible.
Plain language summary
Does the perioperative administration of tramadol provide effective and safe postoperative analgesia in children?
Children experience pain after surgery (‘postoperative pain’) and according to recently published trials the management of this pain is of major concern. A combination of drugs may be the best way to treat postoperative pain, for example drugs called ‘opioids’, like morphine and codeine. However, there are concerns about severe side effects (adverse events) when using opioids. Tramadol is a weak opioid that is used worldwide to treat children with moderate to severe acute or chronic pain. Tramadol can be given to children before surgery to help reduce pain afterwards. It is believed that tramadol administration might be associated with a lower risk for respiratory or haemodynamic depression and might therefore be the ideal analgesic drug for children in the perioperative period. Our systematic review assessed the efficacy and adverse events of tramadol administration compared to placebo or other opioids. In July 2014 we found 20 small randomised controlled trials involving 1170 patients. These small trials had limited data but tramadol may be better than placebo. In five trials, mostly preschool children undergoing minor surgery (for example tonsillectomy) were treated with tramadol or placebo before the start of surgery. Children needed less rescue medication in the postoperative care unit when given tramadol, indicating better analgesia with tramadol. Due to the low amount of usable data, the evidence focusing on the comparison of tramadol with other opioids (for example morphine, nalbuphine, pethidine, fentanyl) is currently unclear. Adverse events were generally only poorly reported in the trials so that the side effects as a result of tramadol administration were not clear.
Summary of findings
Background
Description of the condition
Results of several surveys in different European countries demonstrated that postoperative pain in children is still a relevant problem (Segerdahl 2008; Taylor 2008). In contrast, there is currently clear evidence that an adequate postoperative pain treatment may reduce the risk of subsequent morbidity (Kharasch 2003) and delayed wound healing (Glaser 1999). Furthermore, effective treatment of postoperative pain during infancy may be associated with a reduced incidence of persistent chronic postoperative pain (Page 2013) and a decreased sensitivity to subsequent painful stimuli in adulthood (Andrews 2002; Walker 2009; Weisman 1998). Finally, several studies have demonstrated that sufficient pain reduction or prevention is an important expectation of parents (Franck 2004; Romsing 1996). Improved postoperative pain management in children is therefore not only an ethical but also an essential healthcare issue in perioperative care.
Description of the intervention
In the last decade the number of outpatient procedures in children has increased in most western countries (Segerdahl 2008) because they are associated with a lower incidence of infection and reduced costs (Scaife 1988). Due to major advances in general and regional anaesthetic techniques a wide range of procedures can be performed as day‐case surgery. However, unrelieved pain is one of the most common reasons for unanticipated hospital re‐admission (Awad 2004). The success of day‐case surgery is therefore dependent on effective postoperative pain management. Tramadol is a widely used weak opioid in the treatment of chronic and acute pain in children; it has one‐tenth the potency of morphine and a ceiling effect with high doses (Bamigbade 1998; Dayer 1994). Its use (compared to other opioids) is believed to be associated with a lower incidence of side effects including respiratory or haemodynamic depression, nausea and vomiting, constipation and sedation (Bosenberg 1998; Scott 2000; Tarkkila 1998; Vickers 1992). However in the USA and the UK, tramadol has recently been placed in the drug abuse and dependence schedules, indicating a high risk for abuse. Like other opioids, tramadol does not inhibit prostaglandin synthesis so that patients with a high risk of postoperative bleeding or with renal impairment or asthma and undergoing surgery might benefit from pain therapy with tramadol.
How the intervention might work
Tramadol is a synthetic 4‐phenyl‐piperidine analogue of codeine with a central analgesic effect. It is available as a racemic mixture of (+) and (‐) enantiomers. The following mechanisms have been described for its mode of action. The (+) enantiomer has only a weak affinity to µ‐opioid receptors and inhibits serotonin reuptake, while the (‐) enantiomer inhibits norepinephrine reuptake in the spinal cord (Bozkurt 2005; Scott 2000). Additionally, a local anaesthetic action has been described for tramadol. The latter different modes of action might explain, in part, the extended analgesic efficacy and the lower incidence of typical opioid related side effects (for example postoperative nausea and vomiting (PONV)). Nevertheless, almost 40% of the analgesic action is provided by O‐desmethyl tramadol (M1) following the rapid metabolism of tramadol in the liver via the cytochrome P450 enzyme CYP2D6 (Bozkurt 2005; Hennies 1988; Lintz 1998). After oral administration tramadol is rapidly absorbed with an absolute bioavailability of 65% to 70% due to extensive first‐pass metabolism after absorption (Lintz 1998; Scott 2000). Generally, there are no significant differences in the pharmacokinetics (elimination half‐life, distribution, serum clearance and concentration of metabolites) of tramadol between children and adults after oral application or intravenous injection (Murthy 2000; Payne 2002). However, recently published data suggest that the plasma time‐concentration following tramadol administration might be different in infants due to immature metabolite elimination via the kidneys (Allegaert 2011). Therefore, higher adverse events rates might be possible within this age group following tramadol administration (Allegaert 2011). Apart from age, genetic variances might influence the analgesic efficacy, because around 8% of the Caucasian population has a cytochrome P450 enzyme (CYP2D6) deficiency, which impairs the analgesic properties of tramadol (see above). Furthermore, the important metabolism in the liver might be influenced by other drugs which are also metabolised by CYP2D6 enzymes, for example ondansetron.
Why it is important to do this review
Results from several clinical studies published in the last few years have suggested that tramadol might be an effective analgesic for moderate to severe pain in children. However, results from these studies seem inconsistent and a quantitative systematic review analysing the analgesic efficacy and adverse effects of tramadol in the treatment of postoperative pain in children and adolescents has not been available.
Objectives
To assess the efficacy and adverse events of tramadol for postoperative pain treatment in children and adolescents undergoing surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) investigating tramadol for postoperative pain in children.
Types of participants
We included all children and adolescents (0 to 18 years old) irrespective of sex or type of surgery.
Types of interventions
We included all RCTs investigating tramadol for postoperative pain in children and adolescents in comparison to placebo or any other opioid.
Types of outcome measures
We included studies when they reported any of the following outcome measures.
Primary outcomes
Number of patients requiring rescue analgesia (in postoperative care unit (PACU), 24 h postoperation)
Number of patients with moderate to severe pain (assessed with a postoperative pain scale) (in PACU, 24 h postoperation)
Secondary outcomes
Time to rescue analgesic (minutes) (in PACU, 24 h postoperation)
Total required dose of rescue analgesic (in PACU, 24 h postoperation)
Number of rescue analgesic doses (in PACU, 24 h postoperation)
Number of participants with adverse events (postoperative nausea and vomiting (PONV) (in PACU, 24 h postoperation), hypotension, bradycardia, respiratory depression, pruritus, sedation, obstipation)
Search methods for identification of studies
The general principle of the search strategy used for all databases consisted of a combination of indexed and free‐text terms to reflect the concepts of 'tramadol', 'postoperative pain' and 'children'. We modified the search terms according to the constraints of each database (Appendix 1; Appendix 2; Appendix 3).
Electronic searches
We searched the following data sources:
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 7);
MEDLINE (PubMed) (January 1966 to July 2014);
EMBASE (Ovid) (January 1947 to July 2014).
Searching other resources
In addition, the reference lists of all included trials were checked for additional studies. If there were any data that were missing within included trials, the corresponding authors were contacted in order to receive additional information. Furthermore, we searched within the trials database ClinicalTrials.gov in order to identify addional studies.
Data collection and analysis
Two review authors (AS, SR) independently scanned the titles retrieved by the initial search to exclude irrelevant studies. The systematic search was not limited to any specific language. Two review authors (AS, SR) identified the studies which were included in this review by using a standardised study eligibility form developed by the review authors. If there were disagreements, we contacted a third review author (EPZ).
Selection of studies
Three review authors (AS, SR, CMF) independently performed the study quality assessment using the 'risk of bias' tables provided in The Cochrane Collaboration's Review Manager (RevMan 5) software (RevMan 2014). There were no restrictions concerning the language of the article or publication type. We resolved all differences by discussion among the review authors.
Data extraction and management
Three review authors (AS, SR, CMF) independently extracted the data using a standardised data extraction form developed by the review authors. We defined the number of participants with moderate to severe pain as participants with a pain score of > 3 (Hirschfeld 2013). If different pain scales (Visual Analogue Scale (VAS), Numerical Rating Scale (NRS), Faces Pain Scale ‐ Revised (FPS‐R)) were used in the included studies, we considered all pain scales to be equivalent as long as they were based on a 0 to 10 scale (Standing 2009). Additionally, when postoperative pain intensity was assessed with non‐validated scales (for example three point scales: low, moderate, severe pain), we extracted these results for the outcome 'number of patients with moderate to severe pain', but reported the results within the text. If multi‐arm trials were included, data were extracted for each comparison (for example tramadol versus morphine). Furthermore, if different doses of tramadol were applied, we only extracted the results following the highest doses. If necessary, we retrieved additional data, missing in the published studies, by contacting the authors of the relevant articles. We resolved all differences in data extraction by discussion among the review authors at each step of the data extraction process.
Assessment of risk of bias in included studies
We assessed the risk of bias of included studies using the Cochrane Collaboration's tool for assessing risk of bias (RevMan 2014). The standard components of the domains included adequacy of allocation generation, allocation concealment, blinding, completeness of outcome data, possible selected outcome reporting and any other potential sources of bias. We judged each component as 'low risk' of bias, 'high risk' of bias or 'unclear'. We included a 'Risk of bias' table as part of the 'Characteristics of included studies' table and a 'Risk of bias' summary figure, which detailed all of the judgements made for all studies included in the review. Two review authors (AS, SR) independently carried out an assessment of the risk of bias. We resolved any disagreements by discussion between the review authors and with a further review author acting as arbiter (EPZ).
1. Allocation of intervention
We considered allocation of the intervention as adequate if it was generated by a random system (for example computer, random number table algorithm, tossing of a coin). We considered allocation inadequate if a non‐random system was used (for example names, dates).
2. Concealment of allocation
We considered concealment adequate if an acceptable method, such as a central allocation system, sequentially‐numbered sealed opaque envelopes or an on‐site locked compute,r was used, ensuring that the group assignment was not revealed to participant recruiters, investigators or participants prior to the final allocation into the respective group. We considered concealment inadequate if it allowed the participant recruiters, investigators or participants to know the treatment allocation in advance, or if the concealment procedure was not reported.
3. Blinding of participants and personnel
We considered blinding adequate if participants and persons responsible for participants' care (for example nurses) were all blinded to the intervention. We considered blinding inadequate if participants or persons responsible for participants' care were not blinded to the intervention.
4. Blinding of outcome assessment
We considered blinding adequate if the outcome assessors were blinded to the intervention. We considered blinding inadequate if outcome assessors were not blinded to the intervention.
5. Incomplete outcome data reporting
We considered outcome data reporting adequate if all withdrawals and dropouts were described. We considered outcome data reporting inadequate if the numbers of dropouts and withdrawals were lacking, or if the reasons for withdrawals and dropouts were not given.
6. Selective reporting
We considered data reporting as adequate if all relevant pain outcomes (at least the validated pain scores) were reported. We considered outcome data reporting as inadequate if only selected pain outcomes or non‐validated measurements were used.
Measures of treatment effect
For proportions (dichotomous outcomes) we calculated the risk ratio (RR) with 95% confidence interval (CI), while for continuous data we estimated the mean difference (MD) with 95% CI. To estimate the statistical significance of the results we calculated the 95% CI for each item. Furthermore, we calculated the number needed to treat to benefit (NNTB) for efficacy outcomes, and the number needed to treat to harm (NNTH) for adverse events if enough trials could be pooled (> four trials per outcome) and the result was significant.
Dealing with missing data
If there were doubts about missing data (patient dropouts, selective outcome reporting), we contacted the relevant authors to obtain further information. If the complete information could be obtained, we would perform intention‐to‐treat (ITT) analyses. If the complete information could not be obtained, we performed complete‐case analyses. We planned to perform sensitivity analyses by imputing missing data (best case, worst case) in order to test the robustness of the retrieved results.
Assessment of heterogeneity
We assessed clinical and methodological differences in the included studies to decide if studies were sufficiently homogeneous to be combined. We analysed statistical heterogeneity using the I2 statistic. We calculated this value for each of the outcomes listed above for the comparisons with sham interventions. We assumed relevant heterogeneity if the I2 statistic was ≥ 30%.
Data synthesis
For dichotomous data we used the Mantel‐Haenszel fixed‐effect or random‐effects models, while for continuous data the inverse variance fixed‐effect or random‐effects models were used, in RevMan 5.3. If the test for heterogeneity (I2 statistic ≥ 30%) was positive, we carried out the analysis using the random‐effects model. However, taking into account that random‐effects models sometimes did not deliver more conservative results, especially in the presence of small‐study effects, we reported the summary statistics in conjunction with a sensitivity analysis (results obtained with a fixed‐effect model). If random‐effects model estimates demonstrated larger effect sizes, we discussed whether it is reasonable to conclude that the intervention was more effective in the smaller studies. We reported summary RRs and MDs with 95% CIs. We considered RRs with the range of the lower and upper bounds of the 95% CI not crossing one, and MDs with the range of the lower and upper bounds of the 95% CI not crossing zero, to be statistically significant. Where there were differences in methodological quality, we performed sensitivity analyses (high quality studies and /low quality studies).
Subgroup analysis and investigation of heterogeneity
We gave consideration to the magnitude of clinical and methodological heterogeneity. To evaluate the effects of clinical heterogeneity, we planned to perform subgroup analyses calculating the RR or MD in conjunction with the corresponding CI for each subgroup, if more than four trials were included for the outcome. We used a fixed‐effect model Chi2 test of heterogeneity to compare subgroups. Additionally, we considered non‐overlapping subgroup CIs as consistent with a statistically significant difference.
We further analysed the data concerning the following subgroups, if available.
Type of surgery (head and neck, thoracic, abdominal, extremity).
Different doses of tramadol and routes of administration (oral, intravenous, intramuscular).
Summary of findings tables
We used the principles of the GRADE system (Schunemann 2008) to assess the quality of the body of evidence associated with specific outcomes (number of participants requiring rescue, number of patients with moderate to severe pain, PONV) in our review and constructed a 'Summary of findings' (SoF) table using the GRADE software. The GRADE approach appraised the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflected the item being assessed.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies
Results of the search
The literature search identified 95 studies at the first stage. After exclusion by title and abstract 43 potentially relevant studies were identified. After reading the full texts 20 RCTs were finally included in the present systematic review (Figure 1). There were no significant disagreements between review authors during the extraction process.
1.

Study flow diagram.
Included studies
Summary details of the 20 included studies are given in the Characteristics of included studies table.
Design
All 20 included studies were parallel group RCTs. Tramadol was administrated intravenously (Ali 2008; Antila 2006; Bösenberg 1998; Chiaretti 2000; Cocelli 2012; Ekemen 2008; Engelhardt 2003; Hullett 2006; Moyado Garcia 2009; Ozalevli 2005; Ozer 2003; Ozköse 2000; Umuroglu 2004; van den Berg 1999; Viitanen 2001), orally (Payne 1999; Roelofse 1999) or by intramuscular injection (Barsoum 1995; Ertugrul 2006; Schäffer 1986). In four studies tramadol was applied before induction of general anaesthesia (Ali 2008; Chiaretti 2000; Payne 1999; Roelofse 1999), while in 12 trials tramadol was administered directly after induction (Antila 2006; Bösenberg 1998; Cocelli 2012; Ekemen 2008; Engelhardt 2003; Ertugrul 2006; Hullett 2006; Ozer 2003; Ozköse 2000; Umuroglu 2004; van den Berg 1999; Viitanen 2001). Four trials specifically mentioned that tramadol was given before (Moyado Garcia 2009) or after the end of surgery (Barsoum 1995; Ozalevli 2005; Schäffer 1986). Apart from two trials (Barsoum 1995; Ozalevli 2005) that applied tramadol specifically for postoperative pain treatment, in the other 18 trials tramadol was always applied as a prophylactic analgesic drug. Four trials applied tramadol as a bolus followed by a continuous infusion (Chiaretti 2000; Moyado Garcia 2009; Ozalevli 2005), while the remaining trials applied a bolus injection alone.
Sample sizes
The number of children and adolescents analysed in the 20 studies ranged from 24 to 152 (1170 in total). Sample size calculation and intention‐to‐treat analysis were not reported in any of the included studies.
Participants
Studies involved children aged from 1 to 18 years of age, with each study having a different age range. One trial reported that young adults ( to 21 years) were also included; but a specific analysis of the demographic data of the different study groups showed that the mean age in all study groups was below 18 years (van den Berg 1999). Therefore, the review authors decided to include the data of this RCT. Another trial investigated the administration of tramadol only in neonates (up to 28 days old) that were undergoing surgery, as a postoperative continuous infusion (Alencar 2012). Due to this specific age, the authors decided to exclude this study because only this trial focused explicitly on neonates.
Common paediatric surgical procedures were performed within the included trials: ear‐nose‐throat (ENT) surgery (for example adeno‐tonsillectomy, tonsillectomy) (Ali 2008; Antila 2006; Cocelli 2012; Engelhardt 2003; Ertugrul 2006; Hullett 2006; Ozalevli 2005; Ozer 2003; Ozköse 2000; Umuroglu 2004; van den Berg 1999; Viitanen 2001), lower abdominal surgery (Barsoum 1995; Bösenberg 1998; Ekemen 2008; Schäffer 1986) and dental extraction (Payne 1999; Roelofse 1999). In one study children underwent neurosurgical interventions for various disorders (Chiaretti 2000), while in another trial the surgical procedures were not explicitly described (Moyado Garcia 2009).
Interventions
Eleven trials were performed as multi‐arm trials. The following interventions were compared in the included studies.
1. Tramadol versus placebo (Ali 2008; Antila 2006; Bösenberg 1998; Cocelli 2012; Ozköse 2000; Payne 1999; Roelofse 1999; Umuroglu 2004; van den Berg 1999; Viitanen 2001).
2. Tramadol versus morphine (Engelhardt 2003; Hullett 2006; Ozalevli 2005; Umuroglu 2004).
3. Tramadol versus nalbuphine (Barsoum 1995; Moyado Garcia 2009; Schäffer 1986; van den Berg 1999).
4. Tramdadol versus pethidine (Barsoum 1995; Bösenberg 1998; Ekemen 2008; Ertugrul 2006; Ozer 2003; van den Berg 1999).
5. Tramadol versus fentanyl (Chiaretti 2000).
Outcomes
All trials investigated the efficacy and adverse effects following perioperative (before or after anaesthesia induction, before or after the end of surgery) administration of tramadol in comparison with placebo or other opioids (morphine, nalbuphine, pethidine, fentanyl). A validated pain scale (Visual Analogue Scale (VAS), Numeric Rating Scale (NRS), Faces Pain Scale (FPS), Toddler‐Preschooler Postoperative Pain Scale (TPPPS), Children's Hospital of Eastern Ontario Pain Scale (CHEOPS)) was used in nine studies (Ali 2008; Chiaretti 2000; Ertugrul 2006; Moyado Garcia 2009; Ozalevli 2005; Ozköse 2000; Payne 1999; Schäffer 1986; Umuroglu 2004); however, the pain‐trigger used for rescue analgesia differed significantly between the trials. The number of patients requiring rescue analgesia was recorded in 13 studies (Ali 2008; Barsoum 1995; Bösenberg 1998; Chiaretti 2000; Engelhardt 2003; Hullett 2006; Moyado Garcia 2009; Ozalevli 2005; Ozköse 2000; Schäffer 1986; Umuroglu 2004; van den Berg 1999; Viitanen 2001), while only three studies reported the time to rescue analgesic (Bösenberg 1998; Umuroglu 2004; Viitanen 2001). Outcome variables were either ordinal (for example severity of pain) or nominal in nature (for example presence of PONV). Methods used for statistical analysis included both nonparametric and parametric tests.
Excluded studies
Reasons for the exclusion of 23 studies are given in the Characteristics of excluded studies table. Five trials were excluded because they were not RCTs (Griessinger 1997; Mikhel'son 2002; Oliveira 2000; Ostreikov 1993; Sun 2010). Two trials were only available as conference abstracts, without including relevant outcome data (Bedirli 2011; Metodiev 2011). Three trials were retrospective studies (Apiliogullari 2011; Macarone 1998; Osifo 2008) and were excluded. Another trial tested different doses of tramadol and was therefore excluded (Finkel 2002). One trial explored only the pain from propofol injection and was therefore excluded (Borazan 2012). Two trials were excluded (Aguirre Córcoles 2003; Chu 2006) because they investigated tramadol delivered by a patient‐controlled intravenous pain pump. Three trials investigated the caudal (Günes 2004), epidural (Demiraran 2005) or peritonsillar (Akkaya 2009) application of tramadol, while single trials compared tramadol with paracetamol (Pendeville 2000) and with ilioinguinal and iliohypogastric block (Khosravi 2006). Within the Courtney 2000 trial the adult and paediatric data were mixed, while in another trial no data from children were reported (Altunkaya 2003). Finally, Alencar 2012 studied the continuous infusion of tramadol in 28 day old neonates as a sedating agent and was therefore excluded.
Risk of bias in included studies
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We assessed the methodological quality of included trials using the Cochrane Collaboration's tool for assessing risk of bias (Higgins 2011).
Allocation
Sequence generation
Eight trials were rated as being at low risk of bias because a computer‐generated random sequence (Antila 2006; Bösenberg 1998; Cocelli 2012; Engelhardt 2003; Viitanen 2001) or a predesigned table of random numbers (Moyado Garcia 2009; Ozköse 2000; van den Berg 1999) was used. The other included studies did not give clear information on how the randomisation sequence was generated; they were assessed as being at unclear risk of bias.
Concealment of allocation
In contrast, only three trials explicitly described the method of allocation concealment and were rated as being at low risk of bias (Ali 2008; Engelhardt 2003; Umuroglu 2004). The majority of included studies did not describe allocation concealment and thus were assessed as being at unclear risk of bias.
Blinding
Performance bias
Eight trials were performed as double‐blinded studies, with the participant and provider of the intervention blinded to therapy (Bösenberg 1998; Ekemen 2008; Ertugrul 2006; Moyado Garcia 2009; Ozalevli 2005; Ozköse 2000; Roelofse 1999; Viitanen 2001). In three studies the participants and observers were blinded (Barsoum 1995; Ozer 2003; van den Berg 1999); the participants and investigators were blinded in six studies (Ali 2008; Antila 2006; Engelhardt 2003; Payne 1999; Schäffer 1986; Umuroglu 2004). Three studies were assessed as being at unclear risk of bias because the blinding was unclear (Chiaretti 2000; Cocelli 2012; Hullett 2006).
Detection bias
Only three studies explicitly noted that the observers were blinded to the investigation (Barsoum 1995; Ozer 2003; van den Berg 1999). One trial was rated as being at high risk for detection bias because the triallists stated that the drug doses were noted within the patients´records (Barsoum 1995). All other trials did not describe the blinding of outcome assessment and were rated as being at unclear risk of bias.
Incomplete outcome data
All trials reported that all participants were included in the analysis. Therefore, in all included trials this item was assessed as being at low risk of bias.
Selective reporting
Fourteen trials were rated as being at low risk of bias because all outcomes were measured and reported in full (Ali 2008; Antila 2006; Barsoum 1995; Chiaretti 2000; Cocelli 2012; Engelhardt 2003; Ertugrul 2006; Ozer 2003; Ozköse 2000; Payne 1999; Roelofse 1999; Schäffer 1986; Umuroglu 2004; van den Berg 1999). In contrast, in three trials postoperative pain was measured but was only selectively reported (Bösenberg 1998; Ekemen 2008; Hullett 2006). In another three trials postoperative pain was not measured (Moyado Garcia 2009; Ozalevli 2005; Viitanen 2001).
Other potential sources of bias
There was only one trial that was rated as being at high risk of bias, because the triallists also used a self‐reporting pain scale in the two year old children that was not validated for this age group (Barsoum 1995). We did not find any further potential sources of bias in all other trials.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Tramadol compared with placebo.
| Tramadol compared with placebo for postoperative pain in children | ||||
|
Patient or population: children undergoing surgery Settings: hospital Intervention: 1 to 3 mg/kg tramadol intravenously Comparison: placebo | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Number of patients requiring rescue analgesia (PACU) | RR 0.40 (0.20 to 0.78) | 289 (5) | ⊕⊕⊝⊝ low | double‐downgraded due to unexplained heterogeneity and limitations in the study design (use of different pain scales and triggers for rescue analgesia) |
| Number of patients with moderate to severe pain (PACU) | 44 (1) | ⊕⊝⊝⊝ very low | triple‐downgraded, due to high risk of publication bias, limitations in the study design (use of non‐validated pain scale) and imprecision of results (wide CIs) | |
| Number of patients with PONV (PACU) | RR 0.84 (0.28 to 2.52) | 215 (3) | ⊕⊕⊕⊝ moderate | downgraded due to imprecision of results (wide CIs) |
| Number of patients with PONV (24 hours postoperatively) | RR 0.78 (0.54 to 1.12) | 150 (4) | ⊕⊕⊕⊝ moderate | downgraded due to imprecision of results (wide CIs) |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
RR = relative risk
CI = confidence interval
PONV = postoperative nausea and vomiting
PACU = postoperative care unit
Summary of findings 2. Tramadol compared with morphine.
| Tramadol compared with morphine for postoperative pain in children | ||||
|
Patient or population: children undergoing surgery Settings: hospital Intervention: 1 to 2 mg/kg tramadol intravenously Comparison: 0.1 mg/kg morphine intravenously | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Number of patients requiring rescue analgesia (PACU) | RR 1.25 (0.83 to 1.89) | 127 (3) | ⊕⊕⊝⊝ low | double‐downgraded due to unexplained heterogeneity and limitations in the study design (use of different pain scales and triggers for rescue analgesia) |
| Number of patients with moderate to severe pain (PACU) | no data available | |||
| Number of patients requiring rescue analgesia (24 hours postoperatively) | RR 1.62 (0.65 to 4.04) | 151 (3) | ⊕⊕⊝⊝ low | double‐downgraded due to unexplained heterogeneity and limitations in the study design (use of different pain scales and triggers for rescue analgesia) |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
RR = relative risk
CI = confidence interval
PONV = postoperative nausea and vomiting
PACU = postoperative care unit
Summary of findings 3. Tramadol compared with nalbuphine.
| Tramadol compared with nalbuphine for postoperative pain in children | ||||
|
Patient or population: children undergoing surgery Settings: hospital Intervention: 0.75 to 3 mg/kg tramadol intravenously or intramuscularly Comparison: 0.1 to 0.3 mg/kg nalbuphine intravenously or intramuscularly | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Number of patients requiring rescue analgesia (24 hours postoperatively) | RR 0.63 (0.16 to 2.45) | 110 (2) | ⊕⊝⊝⊝ very low | triple‐downgraded due to unexplained heterogeneity, limitations in the study design (use of different pain scales and triggers for rescue analgesia) and imprecision of results (wide CIs) |
| Number of patients with moderate to severe pain (PACU) | 50 (1) | ⊕⊝⊝⊝ very low | triple‐downgraded, due to high risk of publication bias, limitations in the study design (use of non‐validated pain scale) and imprecision of results (wide CIs) | |
| Number of patients with PONV (PACU) | RR 1.00 (0.50 to 2.01) | 137 (2) | ⊕⊕⊝⊝ low | double‐downgraded due to unexplained heterogeneity and imprecision of results (wide CIs) |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
RR = relative risk
CI = confidence interval
PONV = postoperative nausea and vomiting
PACU = postoperative care unit
Summary of findings 4. Tramadol compared with pethidine.
| Tramadol compared with pethidine for postoperative pain in children | ||||
|
Patient or population: children undergoing surgery Settings: hospital Intervention: 1 to 3 mg/kg tramadol intravenously, per mouth or intramuscularly Comparison: 1 to 1.5 mg/kg pethidine intravenously, per mouth or intramuscularly | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Number of patients requiring rescue analgesia (PACU) | RR 0.93 (0.43 to 2.02) | 120 (2) | ⊕⊝⊝⊝ very low | triple‐downgraded due to unexplained heterogeneity, limitations in the study design (use of different pain scales and triggers for rescue analgesia) and imprecision of results (wide CIs) |
| Number of patients with moderate to severe pain (PACU) | RR 0.64 (0.36 to 1.16) | 94 (2) | ⊕⊕⊝⊝ low | double‐downgraded due to unexplained heterogeneity and limitations in the study design (use of different pain scales and triggers for rescue analgesia) |
| Number of patients with PONV (PACU) | RR 0.75 (0.28 to 2.02) | 156 (3) | ⊕⊕⊝⊝ low | double‐downgraded due to unexplained heterogeneity and imprecision of results (wide CIs) |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
RR = relative risk
CI = confidence interval
PONV = postoperative nausea and vomiting
PACU = postoperative care unit
Summary of findings 5. Tramadol compared with fentanyl.
| Tramadol compared with fentanyl for postoperative pain in children | ||||
|
Patient or population: children undergoing surgery Settings: hospital Intervention: 0.5 mg/kg bolus followed by 150 μg/kg/h tramadol intravenously Comparison: 2 µg/kg/h fentanyl intravenously | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Number of patients requiring rescue analgesia (24 hours postoperatively) | 28 (1) | ⊕⊝⊝⊝ very low | triple‐downgraded, due to high risk of publication bias, limitations in the study design and imprecision of results (wide CIs) | |
| Number of patients with moderate to severe pain (PACU) | no data available | |||
| Number of patients with PONV (24 hours postoperatively) | 28 (1) | ⊕⊝⊝⊝ very low | triple‐downgraded, due to high risk of publication bias, limitations in the study design and imprecision of results (wide CIs) | |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
RR = relative risk
CI = confidence interval
PONV = postoperative nausea and vomiting
PACU = postoperative care unit
Tramadol versus placebo (Comparison 1)
Ten studies investigated the comparison tramadol versus placebo (Ali 2008; Antila 2006; Bösenberg 1998; Cocelli 2012; Ozköse 2000; Payne 1999; Roelofse 1999; Umuroglu 2004; van den Berg 1999; Viitanen 2001) (Table 1).
Primary outcomes
Five studies including 289 patients investigated the number of children requiring rescue analgesia in the PACU (Ali 2008; Bösenberg 1998; Ozköse 2000; van den Berg 1999; Viitanen 2001). All the included trials for this outcome used different pain scales with different pain triggers. The RR for the need for rescue analgesia was significantly reduced in children treated with 1 to 3 mg/kg tramadol (administered during induction for adeno‐tonsillectomy) compared to placebo (RR 0.40; 95% CI 0.20 to 0.78; I2 = 87%; Analysis 1.1). We explored the observed heterogeneity in subgroup analyses. The study quality was comparable and was therefore not further explored. There was only one trial which applied tramadol in children undergoing abdominal surgery (Bösenberg 1998), while the other trials investigated children undergoing ENT surgery (Ali 2008; Ozköse 2000; van den Berg 1999; Viitanen 2001). The subgroup analysis focusing on the influence of surgery revealed that following ENT surgery the RR for the need for rescue analgesia was lower (RR 0.39; 95% CI 0.3 to 0.52; Analysis 1.2) compared to abdominal surgery (RR 0.60; 95% CI 0.34 to 1.07; Analysis 1.2), but this result was influenced by heterogeneity (I2 = 38.8%) and the 95% CIs overlapped, which assumed a non‐significant influence of surgery. The subgroup analysis (Analysis 1.3) focusing on the influence of tramadol dose revealed that the RR for the need for rescue analgesia was lower in children undergoing ENT surgery following 1 mg/kg tramadol (RR 0.07; 95% CI 0.02 to 0.21) (Ali 2008; Ozköse 2000) compared to 2 mg/kg (RR 0.63; 95% CI 0.48 to 0.83) (Bösenberg 1998; Viitanen 2001) or 3 mg/kg tramadol (RR 0.62; 95% CI 0.38 to 1.02) (van den Berg 1999). However, this subgroup analysis was influenced by heterogeneity (I2 = 86.2%) and the 95% CIsoverlapped, which indicated a non‐significant influence of tramadol dose on this outcome. In contrast, the number of patients with moderate to severe pain was only reported in one trial, which used a non‐validated five point verbal pain scale (none, mild, moderate, severe, very severe) (Bösenberg 1998). Fewer children undergoing adeno‐tonsillectomy (9 out of 22) who received 2 mg/kg tramadol during induction, compared to placebo (15 out of 22), complained about moderate to severe pain in the PACU (P > 0.05).
1.1. Analysis.

Comparison 1 Tramadol versus placebo, Outcome 1 Number of patients requiring rescue analgesia (PACU).
1.2. Analysis.

Comparison 1 Tramadol versus placebo, Outcome 2 Number of patients requiring rescue analgesia (PACU) (Subgroup: Surgery).
1.3. Analysis.

Comparison 1 Tramadol versus placebo, Outcome 3 Number of patients requiring rescue analgesia (PACU) (Subgroup: Dose).
Secondary outcomes
Three included trials (Bösenberg 1998; Umuroglu 2004; Viitanen 2001) investigated the outcome time to first rescue analgesic'. Children treated with 1.5 (Umuroglu 2004) to 2 mg/kg tramadol (Bösenberg 1998; Viitanen 2001) for adeno‐tonsillectomy did not show a significantly longer time to first rescue analgesic compared to placebo (MD 44.66; 95% CI ‐24.26 to 113.58; I2 = 71%; Analysis 1.4). Two included trials investigated the cumulative postoperative analgesic consumption of pethidine (relative potency compared to morphine 0.1) (Viitanen 2001) or fentanyl (relative potency compared to morphine 100) (Antila 2006) following administration of 1 to 2 mg/kg tramadol (during induction) or placebo in children undergoing adenoid‐tonsillectomy. In the PACU, children treated with 2 mg/kg tramadol showed a significantly lower opioid consumption (calculated as morphine equivalents) (tramadol 0.4 mg/kg ± 0.5 versus placebo 0.7 mg/kg ± 0.39) (Viitanen 2001); however, 24 h postoperation, this difference was not confirmed in another trial following 1 mg/kg tramadol (tramadol 1.52 mg/kg ± 0.84 versus placebo 1.39 mg/kg ± 0.79) (Antila 2006). Adverse events were poorly reported. Six included trials reported data regarding the RR for PONV (Ali 2008; Antila 2006; Cocelli 2012; Ozköse 2000; Umuroglu 2004; van den Berg 1999). The pooled data analysis demonstrated that following tramadol administration the RR for PONV was not significantly higher in the PACU (RR 0.84; 95% CI 0.28 to 2.52; I2 = 15%; Analysis 1.5) or 24 h postoperation (RR 0.78; 95% CI 0.54 to 1.12; I2 = 0%; Analysis 1.6). In three included trials no children suffering from respiratory depression were noted following tramadol administration (Ali 2008; Umuroglu 2004; van den Berg 1999) (Analysis 1.7). Other adverse events were not reported.
1.4. Analysis.

Comparison 1 Tramadol versus placebo, Outcome 4 Time to first rescue analgesic (min).
1.5. Analysis.

Comparison 1 Tramadol versus placebo, Outcome 5 Number of patients with PONV (PACU).
1.6. Analysis.

Comparison 1 Tramadol versus placebo, Outcome 6 Number of patients with PONV (24h postop).
1.7. Analysis.

Comparison 1 Tramadol versus placebo, Outcome 7 Number of patients with respiratory depression.
Tramadol versus morphine (Comparison 2)
The comparison tramadol versus morphine was assessed by four included trials (Engelhardt 2003; Hullett 2006; Ozalevli 2005; Umuroglu 2004) (Table 2).
Primary outcomes
All trials reported data focusing on the outcome number of patients requiring rescue analgesia (Engelhardt 2003; Hullett 2006; Ozalevli 2005; Umuroglu 2004). All included trials used different pain scales with different pain triggers for this outcome. In the PACU and 24 h postoperation, children undergoing adeno‐tonsillectomy who were treated with intraoperative tramadol 1 mg/kg to 2 mg/kg showed a non‐significantly higher RR for the need for rescue analgesia compared to children treated with morphine 0.1 mg/kg (RR (PACU) 1.25; 95% CI 0.83 to 1.89; I2 = 0%; Analysis 2.1 (Engelhardt 2003; Hullett 2006; Umuroglu 2004); RR (2 4h postoperation) 1.62; 95% CI 0.65 to 4.04; I2 = 0%; Analysis 2.2 (Engelhardt 2003; Hullett 2006; Ozalevli 2005)). Three trials applied tramadol during induction (Engelhardt 2003; Hullett 2006; Umuroglu 2004) while only one specifically mentioned that tramadol was applied postoperation (Ozalevli 2005;). Due to a lack of data the outcome number of patients with moderate to severe pain could not be assessed.
2.1. Analysis.
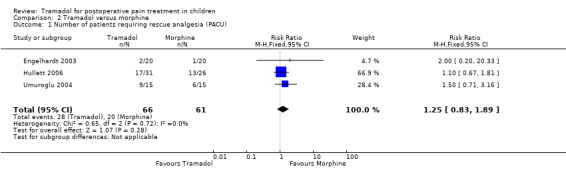
Comparison 2 Tramadol versus morphine, Outcome 1 Number of patients requiring rescue analgesia (PACU).
2.2. Analysis.
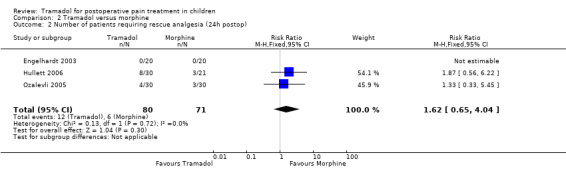
Comparison 2 Tramadol versus morphine, Outcome 2 Number of patients requiring rescue analgesia (24h postop).
Secondary outcomes
Only one trial investigated the outcome time to first rescue analgesic and reported that 15 children undergoing adeno‐tonsillectomy and treated with 1.5 mg/kg tramadol during induction (155.6 min ± 172.7) required the first rescue analgesic 112.4 min earlier than 15 children receiving 0.1 mg/kg morphine during induction (268 min ± 157.9) (Umuroglu 2004). All trials reported data on adverse events. Two trials investigated children with PONV in the PACU (Umuroglu 2004) and 24 h postoperation (Ozalevli 2005). In the PACU 3 out of 15 patients in each group complained about PONV following 1.5 mg tramadol compared to 0.1 mg/kg morphine during induction (Umuroglu 2004). In contrast, another study comparing 1 mg/kg tramadol versus 0.1 mg/kg morphine after surgery in children undergoing adeno‐tonsillectomy reported that less children suffered from PONV following tramadol administration 24 h postoperation (3 out of 30 versus 11 out of 30) (Ozalevli 2005). Four trials reported the number of children with respiratory depression (Engelhardt 2003; Hullett 2006; Ozalevli 2005; Umuroglu 2004) but only one trial mentioned two children suffering from respiratory problems following 0.1 mg/kg morphine (Hullett 2006) (Analysis 2.3). Only one trial assessed the outcomes bradycardia, hypotension, pruritus and urinary retention following tramadol or morphine administration postoperation. Generally, either no children (in both groups) suffered from these adverse events (bradycardia, hypotension) or the results (pruritus: 0 in tramadol group versus 1 in morphine group, and urinary retention: 1 patient in each group) were comparably low (Ozalevli 2005).
2.3. Analysis.

Comparison 2 Tramadol versus morphine, Outcome 3 Number of patients with respiratory depression.
Tramadol versus nalbuphine (Comparison 3)
Four trials were available for the comparison tramadol versus nalbuphine (Barsoum 1995; Moyado Garcia 2009; Schäffer 1986; van den Berg 1999) (Table 3).
Primary outcomes
The number of patients with a need for rescue analgesia was assessed in three included trials. All trials used different pain scales with different pain triggers. One trial reported that a higher number of patients treated with tramadol (3 mg/kg) compared to nalbuphine (0.3 mg/kg) required rescue analgesia in the PACU (14 out of 38 versus 11 out of 34) (van den Berg 1999); however, 24 h postoperation the latter result was not confirmed (RR 0.63; 95% CI 0.16 to 2.45; I2 = 54%; Analysis 3.1) (Barsoum 1995; Schäffer 1986). Only one included trial, which investigated drug administration for postoperative pain treatment following surgery, reported data on the outcome number of patients with moderate to severe pain (PACU) assessed on a four point verbal scale (no pain, mild, moderate, severe pain) following drug administration (Barsoum 1995); there was only one child in the nalbuphine group (0.1 mg/kg) that complained about moderate to severe pain 0.5 h after drug administration.
3.1. Analysis.

Comparison 3 Tramadol versus nalbuphine, Outcome 1 Number of patients requiring rescue analgesia (24h postop).
Secondary outcomes
In the PACU the RR for PONV was not significantly different in both groups (RR (PACU) 1.00; 95% CI 0.50 to 2.01; Analysis 3.2) (Schäffer 1986; van den Berg 1999). Only one trial reported that at 24 h postoperation 1 child out of 25 children treated with tramadol suffered from PONV compared to no child treated with nalbuphine (Barsoum 1995). No patient with bradycardia (Analysis 3.4) or with respiratory depression (Analysis 3.3) was mentioned in two included trials (Barsoum 1995; Moyado Garcia 2009). Only one trial reported patients with sedation or hypotension following 1 mg/kg tramadol compared to 0.1 mg/kg nalbuphine given at the end of surgery (Moyado Garcia 2009); whereas no patient suffered from hypotension, one patient in the tramadol group and two patients in the nalbuphine group complained about sedation postoperatively.
3.2. Analysis.

Comparison 3 Tramadol versus nalbuphine, Outcome 2 Number of patients with PONV (PACU).
3.4. Analysis.

Comparison 3 Tramadol versus nalbuphine, Outcome 4 Number of patients with bradycardia.
3.3. Analysis.

Comparison 3 Tramadol versus nalbuphine, Outcome 3 Number of patients with respiratory depression.
Tramadol versus pethidine (Comparison 4)
Six trials investigated the comparison tramadol versus pethidine (Barsoum 1995; Bösenberg 1998; Ekemen 2008; Ertugrul 2006; Ozer 2003; van den Berg 1999). However, data could only be pooled for a low number of outcomes (Table 4).
Primary outcomes
Only two trials reported the number of patients requiring rescue analgesia and demonstrated that children treated with 2 to 3 mg/kg tramadol versus 1 to 1.5 mg/kg pethidine during induction showed a lower but non‐significant RR for the need for rescue analgesia (RR 0.93; 95% CI 0.43 to 2.02; I2 = 66%; Analysis 4.1) (Bösenberg 1998; van den Berg 1999). All included trials used different pain scales and pain triggers. At 24 h postoperation only one included trial reported that 3 out of 25 patients treated with tramadol required rescue analgesia compared to 8 out of 25 patients receiving pethidine; this trial compared the application of tramadol versus pethidine postoperation (Barsoum 1995). A similar result was seen in two trials regarding the number of patients with moderate to severe pain (RR 0.64; 95% CI 0.36 to 1.16; Analysis 4.2) (Barsoum 1995; Bösenberg 1998).
4.1. Analysis.

Comparison 4 Tramadol versus pethidine, Outcome 1 Number of patients requiring rescue analgesia (PACU).
4.2. Analysis.

Comparison 4 Tramadol versus pethidine, Outcome 2 Number of patients with moderate/severe pain (PACU).
Secondary outcomes
Only one trial investigated the time to first rescue analgesic and reported that 22 children treated with tramadol (251 min ±143) instead of pethidine (223 min ± 123) during induction required their first rescue analgesic 28 minutes later (Bösenberg 1998). Adverse events were poorly reported. The most commonly reported outcome was PONV in the PACU. The results of three included trials could be pooled (Ertugrul 2006; Ozer 2003; van den Berg 1999) and revealed that the RR for PONV in the PACU was lower in children treated with tramadol compared to pethidine, but this result failed to reach significance (RR 0.75; 95% CI 0.28 to 2.02; I2 = 0%; Analysis 4.3). In one trial (van den Berg 1999) out of three included trials, two children treated with 1.5 mg/kg pethidine suffered from respiratory depression after surgery (RR 0.20; 95% CI 0.01 to 4.03; Analysis 4.4) (Barsoum 1995; Ekemen 2008; van den Berg 1999). Furthermore, no child with bradycardia (Analysis 4.5) (Barsoum 1995; Ekemen 2008) or hypotension (Ekemen 2008) was noted in the included trials.
4.3. Analysis.

Comparison 4 Tramadol versus pethidine, Outcome 3 Number of patients with PONV (PACU).
4.4. Analysis.

Comparison 4 Tramadol versus pethidine, Outcome 4 Number of patients with respiratory depression.
4.5. Analysis.

Comparison 4 Tramadol versus pethidine, Outcome 5 Number of patients with bradycardia.
Tramadol versus fentanyl (Comparison 5)
Only one included trial investigated the comparison tramadol versus fentanyl in children for postoperative pain treatment (Chiaretti 2000) (Table 5).
Primary outcomes
The included trial reported data on the postoperative pain outcome in children treated with tramadol (bolus during induction followed by continuous infusion during surgery) or fentanyl (same regimen as in the tramadol group) for neurosurgical procedures (Chiaretti 2000). During the observation period (around 18 h) only 2 out of 14 children in the tramadol group compared to 0 out of 14 children in the fentanyl group required additional rescue analgesia.
Secondary outcomes
No other postoperative pain outcomes were reported. Adverse events were also poorly reported. More patients in the fentanyl group complained about PONV (6 out of 14 compared to 0 out of 14 in the tramadol group). No child suffering from respiratory depression or bradycardia was reported.
Discussion
Summary of main results
The present quantitative systematic review included 20 randomised controlled trials (RCTs) including 1170 children undergoing surgery, which investigated the efficacy and adverse events following tramadol versus placebo or other opioids for postoperative pain treatment. The overall quality of the evidence is low and it should be interpreted with caution because all included trials used different validated and non‐validated pain scales in children over a wide range of ages. Furthermore, different pain triggers were used for administration of rescue analgesia, which made it difficult to compare the results. Nevertheless, we performed a quantitative analysis on the need for rescue analgesia taking into account that, despite the use of different pain scales and triggers, children with significant postoperative pain were treated within all trials. In comparison with placebo, children receiving 1 to 2 mg/kg tramadol during induction for adeno‐tonsillectomy showed a significantly lower RR for the need for rescue analgesia in the PACU. In addition, the number of children with moderate to severe pain in the PACU was lower following tramadol administration, but this result failed to reach significance. Further non‐significant results were seen for the outcomes time to first rescue analgesic and cumulative postoperative analgesic consumption. The comparison of tramadol with other commonly used opioids is currently difficult to assess due to the lack of appropriate data for single opioids as comparators. Comparison with morphine suggested a similar or slightly lower analgesic efficacy of tramadol in the PACU and 24 h postoperation. Due to conflicting results and the lack of pooled outcome data the evidence for the comparisons with nalbuphine, pethidine and fentanyl is currently not clear. Generally, adverse events were only poorly and insufficiently reported. Most data could be pooled for PONV; and children treated with tramadol compared to placebo did not show a significantly higher RR for PONV in the PACU or 24 h postoperation. However, an appropriate risk‐benefit analysis was not possible.
Overall completeness and applicability of evidence
Despite growing evidence in this field, postoperative pain in children is still a relevant health problem and cause for concern (Groenewald 2012; Stanko 2013). This is problematic because there is evidence that untreated or insufficiently treated postoperative pain may increase suffering, complications, rehabilitation, cost and, most recently addressed, lead to persistent postsurgical pain in children (Page 2013). A multimodal postoperative pain treatment including nonsteroidal analgesic drugs, regional anaesthesia and opioids is currently believed to be the gold standard in perioperative care (Michelet 2012; Russell 2013). However, opioids are still insufficiently administered in children, mainly due to the fear of side effects (Dadure 2009). The present review is significantly limited by the fact that different validated and non‐validated pain scores with different pain triggers were used in children over a wide range of ages. This fact makes it difficult to compare the results of the included trials. Nevertheless, the review authors performed a quantitative analysis of 17 included trials taking into account that, despite different pain scores and pain triggers, children with significant postoperative pain were treated in all included trials. We demonstrated a significantly reduced RR for the need for rescue analgesia in children treated with 1 to 2 mg/kg tramadol compared to placebo. However, due to insufficient data, this could not be confirmed for the outcomes number of patients with moderate to severe pain and the time to first rescue analgesic. Additionally, evidence for the comparison with other opioids was sparse, due to limited data; we were only able to address one single outcome. The need for rescue analgesia was non‐significantly increased in children treated with tramadol compared to morphine as well as compared to nalbuphine in the PACU and 24 h postoperation. Generally, adverse events for the comparison with placebo or other opioids were insufficiently addressed. The most commonly reported outcome parameter was PONV; tramadol, compared to placebo, did not significantly increase the RR for PONV in the PACU and 24 h postoperation. Furthermore, no children treated with tramadol suffered from respiratory depression. The latter result is important because respiratory depression might be the most feared adverse event following opioid administration in children (Niesters 2013). However, the total number of patients that was assessed was low and insufficient to address safety. Due to limited data, the comparison with other opioids regarding adverse events could not be sufficiently analysed; thus the risk‐benefit profile of tramadol compared to placebo and to other common opioids remains unclear.
Quality of the evidence
Although the present review included 20 RCTs including 1170 participants, data could only be pooled for a low number of comparisons and relevant outcomes. Therefore, the presented results should be interpreted with caution due to the high risk for reporting bias. Furthermore, many trials included only a low number of participants, so that many results were imprecise and had wide confidence intervals. Another relevant problem was the use of different validated and non‐validated pain scales, which might have particularly influenced the outcome number of patients with moderate to severe pain. Furthermore, different pain triggers were used for application of rescue analgesia, which might have influenced the outcomes number of patients requiring rescue analgesia, time to first rescue analgesic, total required dose of rescue analgesic and number of rescue analgesic doses. Additionally, the age range of included participants was wide and therefore different pain scores were used, but they were assessed as equivalent. Despite these limitations, data were analysed quantitatively in a similar way to another Cochrane Review focusing on postoperative pain in children (Standing 2009), but the evidence was downgraded due to heterogeneous methodology between the studies. Additionally, the impact of subgroup and sensitivity analyses investigating the identified heterogeneity was limited due to the low number of included trials per outcome. Finally, the overall methodological quality of the included trials was assessed as moderate, because concealment of allocation processes and blinding of outcome assessors were poorly described. In conclusion, according to the GRADE approach (Schunemann 2008), the evidence for the comparisons with placebo (Table 1), morphine (Table 2), pethidine (Table 4), nalbuphine (Table 3) and fentanyl (Table 5) was double or triple downgraded due to the limited study design (use of different pain scales and triggers for rescue analgesia), imprecision of results and unexplained heterogeneity.
Potential biases in the review process
We planned to investigate the outcome postoperative pain scores, but this outcome could not be sufficiently analysed due to a lack of data and large heterogeneity in the pain scales used. Furthermore, these pain scales were mostly non‐validated, and different pain triggers were used (even if the same pain scales were used). Because the outcome number of patients with the need for rescue analgesia was reported more widely within the included trials, the authors decided to analyse this outcome as the primary outcome. However, this variation to the initial protocol might have influenced the results, and the retrieved results should be interpreted with caution.
Agreements and disagreements with other studies or reviews
The current available evidence focusing on opioid treatment in children for postoperative pain is sparse. Our review group recently published another Cochrane review investigating nalbuphine for postoperative pain in children. The problems, which we addressed in the latter review, were almost the same as in the present review; limited evidence due to a low number of available trials and limited quality of the available evidence due to limitations in the study design (use of different pain scales and triggers for rescue analgesia), imprecision of results and unexplained heterogeneity (Schnabel 2014).
Authors' conclusions
Implications for practice.
The overall quality of evidence regarding postoperative analgesia following tramadol use in children compared to placebo or other opioids is currently low or very low. Nevertheless, analysis of five included trials demonstrated that the prophylactic administration of tramadol (during induction of anaesthesia) might provide effective postoperative analgesia in children undergoing adeno‐tonsillectomy, compared to placebo. The ideal tramadol dose is currently unclear. The latter finding might be important because adeno‐tonsillectomies are one of the most painful surgical procedures during childhood (Groenewald 2012; Stanko 2013) and the use of other opioids (for example morphine) is feared due to an increased risk of postoperative respiratory problems (for example due to an underdiagnosed risk of sleep apnoea within this population) (Subramanyam 2014). One included trial specifically compared the risk of postoperative desaturation following tramadol or morphine administration in children with obstructive sleep apnoea who were undergoing adeno‐tonsillectomy. They reported 26% significantly fewer episodes of postoperative oxygen desaturation in the tramadol group (Hullett 2006). In contrast, a recently published case report revealed that a child with a ultrarapid CYP2D6 genotype and obstructive sleep apnoea syndrome suffered from severe postoperative respiratory depression following day‐case tonsillectomy (Orliaguet 2015). The latter finding highlights the possible influence of genetic variations on the efficacy (8% to 10% possibility of a cytochrome P450 enzyme deficiency causing an inability to metabolize the prodrug tramadol into the active component (M1)) and the adverse effects of tramadol administration, which are currently unknown. To conclude, although tramadol administration might be an interesting option, for example for post‐tonsillectomy pain, the current evidence is very limited and further research is urgently needed to further define a possible role of tramadol for postoperative pain treatment in children.
Implications for research.
The following implications for research could be defined based on the results of this review.
Because the evidence for tramadol use in children is currently sparse, due to incomplete and insufficient outcome reporting (including adverse events), additional randomised controlled trials comparing tramadol with other commonly used opioids are needed in order to enable an appropriate risk‐benefit analysis. Postoperative pain should be assessed with validated pain scales and, accordingly, rescue analgesia should be given following clearly defined pain triggers (e.g. NRS > 3 based on a 0 to 10 point scale (Hirschfeld 2013)).
Tramadol administration should be investigated in children undergoing different common paediatric surgical procedures in order to detect possible procedure specific differences.
The possible influence of genetic variability on efficacy and adverse events should be assessed in children treated with tramadol for postoperative pain.
Tramadol administration should be studied in patient groups with a high risk for opioid related adverse events (e.g. children with obstructive sleep apnoea undergoing adeno‐tonsillectomy).
Finally, tramadol administration should be studied in different age groups and dosages to further evaluate the clinical consequences of the reported pharmacokinetic findings (e.g. ceiling effect, higher risk for specific adverse events in younger age groups (Allegaert 2011)).
What's new
| Date | Event | Description |
|---|---|---|
| 26 June 2019 | Review declared as stable | See Published notes |
History
Protocol first published: Issue 1, 2012 Review first published: Issue 3, 2015
| Date | Event | Description |
|---|---|---|
| 27 January 2017 | Review declared as stable | See Published notes. |
Notes
2017
A restricted search in January 2017 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
2019
A full updated search in March 2019 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be reassessed for updating in five years. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Readers should be aware that the Food and Drug Administration (FDA) issued an alert on 20 April 2017 restricting the use of codeine and tramadol medicines in children. Codeine is approved to treat pain and cough, and tramadol is approved to treat pain. These medicines carry serious risks, including slowed or difficult breathing and death, which appear to be a greater risk in children younger than 12 years, and should not be used in these children. These medicines should also be limited in some older children. Single‐ingredient codeine and all tramadol‐containing products are FDA‐approved only for use in adults.
Acknowledgements
We would like to thank the peer reviewers for their valuable comments and the editorial office of the Cochrane Pain, Palliative and Supportive Group for the support. The present review was not sponsored by any drug company or any institutional organisation.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. MEDLINE search strategy
Tramadol/
tramadol.mp.
k‐315.mp.
ralivia er.mp.
rybix.mp.
ryzolt.mp.
tramahexal.mp.
tramake.mp.
tramalgin.mp.
tramedo.mp.
ultram.mp.
zamadol.mp.
zydol.mp.
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13
Pain, Postoperative/
(pain* and (postoperative or post operative or surg*)).mp.
15 or 16
exp infant/
exp child/
adolescent/
(infant* or child* or adoles*).mp.
18 or 19 or 20 or 21
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
23 or 24 or 25 or 26 or 27 or 28 or 29 or 30
14 and 17 and 22 and 31
key:
mp = title, original title, abstract, name of substance word, subject heading word, unique identifier
pt = publication type
ab = abstract
fs = floating subheading
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor: [Tramadol] this term only
#2 tramadol
#3 k‐315
#4 ralivia
#5 rybix
#6 ryzolt
#7 tramahexal
#8 tramake
#9 tramalgin
#10 tramedo
#11 ultram
#12 zamadol
#13 zydol
#14 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13
#15 MeSH descriptor: [Pain, Postoperative] this term only
#16 (pain* and (postoperative or post operative or surg*))
#17 #15 or #16
#18 MeSH descriptor: [Infant] explode all trees
#19 MeSH descriptor: [Child] explode all trees
#20 MeSH descriptor: [Adolescent] explode all trees
#21 (infant* or child* or adoles*)
#22 #18 or #19 or #20 or #21
#23 #14 and #17 and #22
Appendix 3. EMBASE search strategy
EMBASE (Ovid)
1 Tramadol/
2 tramadol.mp.
3 k‐315.mp.
4 ralivia er.mp.
5 rybix.mp.
6 ryzolt.mp.
7 tramahexal.mp.
8 tramake.mp.
9 tramalgin.mp.
10 tramedo.mp.
11 ultram.mp.
12 zamadol.mp.
13 zydol.mp.
14 or/1‐13
15 Pain, Postoperative/
16 (pain* and (postoperative or post operative or surg*)).mp.
17 15 or 16
18 exp infant/
19 exp child/
20 adolescent/
21 (infant* or child* or adoles*).mp.
22 or/18‐21
23 random$.tw.
24 factorial$.tw.
25 crossover$.tw.
26 cross over$.tw.
27 cross‐over$.tw.
28 placebo$.tw.
29 (doubl$ adj blind$).tw.
30 (singl$ adj blind$).tw.
31 assign$.tw.
32 allocat$.tw.
33 volunteer$.tw.
34 Crossover Procedure/
35 double‐blind procedure.tw.
36 Randomized Controlled Trial/
37 Single Blind Procedure/
38 or/23‐37
39 (animal/ or nonhuman/) not human/
40 38 not 39
41 14 and 17 and 22 and 40
Data and analyses
Comparison 1. Tramadol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients requiring rescue analgesia (PACU) | 5 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.20, 0.78] |
| 2 Number of patients requiring rescue analgesia (PACU) (Subgroup: Surgery) | 5 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.33, 0.54] |
| 2.1 Abdominal surgery | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.34, 1.07] |
| 2.2 ENT Surgery | 4 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.30, 0.52] |
| 3 Number of patients requiring rescue analgesia (PACU) (Subgroup: Dose) | 5 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.33, 0.54] |
| 3.1 Tramadol 1mg iv. | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.21] |
| 3.2 Tramadol 2mg iv. | 2 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.48, 0.83] |
| 3.3 Tramadol 3mg iv. | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 1.02] |
| 4 Time to first rescue analgesic (min) | 3 | 154 | Mean Difference (IV, Random, 95% CI) | 44.66 [‐24.26, 113.58] |
| 5 Number of patients with PONV (PACU) | 3 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.28, 2.52] |
| 6 Number of patients with PONV (24h postop) | 4 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.54, 1.12] |
| 7 Number of patients with respiratory depression | 3 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Tramadol versus morphine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients requiring rescue analgesia (PACU) | 3 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.83, 1.89] |
| 2 Number of patients requiring rescue analgesia (24h postop) | 3 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.65, 4.04] |
| 3 Number of patients with respiratory depression | 4 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.51] |
Comparison 3. Tramadol versus nalbuphine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients requiring rescue analgesia (24h postop) | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.16, 2.45] |
| 2 Number of patients with PONV (PACU) | 2 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.50, 2.01] |
| 3 Number of patients with respiratory depression | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number of patients with bradycardia | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Tramadol versus pethidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients requiring rescue analgesia (PACU) | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.43, 2.02] |
| 2 Number of patients with moderate/severe pain (PACU) | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.36, 1.16] |
| 3 Number of patients with PONV (PACU) | 3 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.28, 2.02] |
| 4 Number of patients with respiratory depression | 3 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.03] |
| 5 Number of patients with bradycardia | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ali 2008.
| Methods | RCT Parallel design |
|
| Participants | Adeno‐tonsillectomy, n = 90 (46 males, 44 females), children aged 4 to 10 years Group 1: 16 males, 14 females; mean age 7.46 years (SD 1.85) Group 2: 15 males, 15 females; mean age 7.53 years (SD 1.88) Group 3: 15 males, 15 females; mean age 7.61 years (SD 1.93) |
|
| Interventions | Group 1: placebo (saline) po + placebo (saline) iv (n = 30) Group 2: dextromethorphan 1 mg/kg po + placebo (saline) iv (n = 30) Group 3: placebo (saline) plus tramadol 1 mg/kg iv (n = 30) Administration time: 30 minutes before arrival in the operating room and during induction of anaesthesia |
|
| Outcomes | ‐ Postoperative pain scales (FPS) (1, 2, 3, 4, 5, 6 h postoperation) ‐ Analgesic and antiemetic requirements during 6‐h observation period ‐ Number of patients with adverse events (vomiting, respiratory parameters) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "Each patient was randomly assigned to one of three groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "A sealed envelope method was used for randomization." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "randomized, prospective, doubleblind and placebo‐controlled study design" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes are measured and fully reported. |
| Other bias | Unclear risk | No apparent bias |
Antila 2006.
| Methods | RCT Parallel design |
|
| Participants | Tonsillectomy, n = 45 (20 males, 25 females), children aged 5 to 15 years Group 1: 7 males, 8 females; mean age 12.5 years (SD 1.9) Group 2: 6 males, 9 females; mean age 12.5 years (SD 2.3) Group 3: 7 males, 8 females; mean age 11.9 years (SD 2.4) |
|
| Interventions | Group 1: placebo iv (n=15) Group 2: 2 mg/kg ketoprofen iv (n = 15) Group 3: 1 mg/kg tramadol iv (n = 15) Administration time: after the induction of anaesthesia and before surgical incision; after surgery the randomised study treatment was continued as a 6‐h iv infusion in which the child received either another dose of saline control, ketoprofen (2 mg/kg) or tramadol (1 mg/kg) |
|
| Outcomes | ‐ Postoperative pain scales (VAS) (30 min, 60 min, 90 min, 2 h, 6 h, and 24 h postoperation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization to treatment groups was performed by computer in blocks of nine patients." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Children were prospectively randomized to one of the three treatment groups" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The double‐blind nature of the study was confirmed by having a nurse, not otherwise participating in the treatment of the patient, to prepare the coded study treatment syringes and infusions." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
Barsoum 1995.
| Methods | Open trial Parallel design |
|
| Participants | Lower abdominal surgery (appendectomy, hernia repair, testicular or urethral surgery), n = 75 (61 males, 14 females), children aged 2 to 12 years Group 1: 22 males, 9 females; mean age 5.4 years (SD 2.6) Group 2: 20 males, 5 females; mean age 6.3 years (SD 1.9) Group 3: 19 males, 6 females; mean age 6.2 years (SD 2.8) |
|
| Interventions | Group 1: 0.1 mg/kg nalbuphine im (n = 25) Group 2: 2 mg/kg tramadol im (n = 25) Group 3: 1 mg/kg pethidine im (n = 25) Administration time: after the end of surgery (first expression of pain) an additional injection of the half initial dose was administrated 30 and 60 min, if inadequate analgesia |
|
| Outcomes | ‐ Postoperative pain scales (VRS) (0.5, 1, 3, 6, 12, 24 h postoperation) ‐ Number of patients with no or slight pain 1 h postoperation ‐ Total analgesic consumption 24 h postoperation ‐ Frequency of administration 24 h postoperation ‐ Investigators' assessment of overall pain relief (excellent, very good, good, satisfactory, poor) at 24 h postoperation; number, nature, time of onset of adverse events (vomiting, haemodynamic, respiratory parameters); overall assessment of tolerability 24 h postoperation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "The children were randomised into 3 groups of 25,..." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was intended to be a double‐blind (observer blinded),.." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "drug dosages were recorded in the patient records,..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | High risk | Verbal rating scale (VRS) was also used in the youngest children, which were 2 years old. However, at this age, this scale is not validated |
Bösenberg 1998.
| Methods | RCT Parallel design |
|
| Participants | Inguinal surgery (orchidopexy, herniotomy), n = 88 (87 males, 1 female), children aged 2 to 10 years Group 1: 22 males, mean age 6 years (SD 3) Group 2: 22 males, mean age 5 years (SD 2) Group 3: 21 males, 1 female; mean age 5 years (SD 2) Group 4: 22 males, mean age 5 years (SD 2) |
|
| Interventions | Group 1: tramadol 1 mg/kg iv (n = 22) Group 2: tramadol 2 mg/kg iv (n = 22) Group 3: pethidine 1 mg/kg iv (n = 22) Group 4: placebo (n = 22) Administration time: after induction of anaesthesia |
|
| Outcomes | ‐ Documentation of respiratory and haemodynamic parameters during anaesthesia ‐ Postoperative pain scales (five‐point verbal rating scale) (1, 2, 3, 4, 5,6 h postoperation) ‐ Recovery from anaesthesia ‐ Number of patients with the need for rescue analgesia ‐ Number of patients with moderate to severe pain |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computer‐generated randomisation." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "preserve blinding, the study medication was prepared in identical syringes by a designated third party" "Randomisation codes were masked to the patient, the investigator and nursing staff." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | High risk | Postoperative pain was measured, but was only selectively reported. An overall pain intensity score was described, but no values at time points were specified |
| Other bias | Unclear risk | No apparent bias |
Chiaretti 2000.
| Methods | RCT Parallel design |
|
| Participants | Major neurosurgical surgery, n = 42 (23 males, 20 female) Group 1: 10 males, 8 females; mean age 67.8 months (SD 66.5) Group 2: 6 males, 5 females; mean age 62 months (SD 50) Group 3: 7 males, 7 female; mean age 74 months (SD 47.5) |
|
| Interventions | Group 1: tramadol 1 mg/kg iv (n = 14) Group 2: tramadol 0.5 mg/kg iv followed by continuous infusion at the rate of 150 μg/kg/h (n = 14) Group 3: fentanyl by continuous infusion at the rate of 2 µg/kg/h (n = 14) Administration time: at the induction of general anaesthesia |
|
| Outcomes | ‐ Postoperative pain scales (AFS scale and CHEOPS score) (every 4 h for 12 to 36 h postoperation) ‐ Documentation of respiratory and haemodynamic parameters postoperation ‐ Number of patients with adverse events (vomiting, nausea, apnoea, and bradycardia) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "Patients were allocated randomly to three intraoperative treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detailed information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
Cocelli 2012.
| Methods | RCT Parallel design |
|
| Participants | Tonsillectomy, n = 90 (42 males, 48 female), children aged 2 to 9 years Group 1: 14 males, 16 females; mean age 6.3 years (SD 0.002) Group 2: 14 males, 16 females; mean age 6.17 years (SD 1.599) Group 3: 14 males, 16 female; mean age 6.1 years (SD 1.517) |
|
| Interventions | Group 1: placebo (saline) iv (n = 30) Group 2: 1.5 ml 0.75% ropivacaine to the tonsil lodge (n = 30) Group 3: tramadol 1 mg/kg iv (n = 30) Administration time: after induction of anaesthesia |
|
| Outcomes | ‐ Documentation of haemodynamic parameters for 24 h postoperation ‐ Postoperative pain scales at resting and swallowing (Maunuksela pain scores) (1, 2, 3, 6, 9, 12, 15, 18, 21, 24 h postoperation) ‐ Number of patients with adverse events (hypotension, breathing difficulties and bleeding, vomiting, nausea) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... paediatric tonsillectomies: A randomized placebo controlled study", "Randomization was provided by the use of a random number generator" |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detailed information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
Ekemen 2008.
| Methods | RCT Parallel design |
|
| Participants | Lower abdominal surgery (e.g. hernia repair, appendicectomy, testicular or ureteral surgery), n = 110, children aged 2 to 12 years Group 1: mean age 5.7 years (SD 0.39) Group 2: mean age 5.65 years (SD 0.56) |
|
| Interventions | Group 1: pethidine 1 mg/kg iv (n = 60) Group 2: tramadol 2 mg/kg iv (n = 50) Administration time: at the induction of anaesthesia |
|
| Outcomes | ‐ Documentation of haemodynamic parameters and sedation score for 24 h postoperation ‐ Postoperative pain scales (4‐point restlessness–pain protocol) (5, 10, 20, 30 min, 6 and 24 h postoperation) ‐ Number of patients with adverse events (episodes of vomiting, nausea, apnoea and bradycardia) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: " ... we designed prospective, randomized, controlled study." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information Quote: "Patients were allocated randomly to receive either.." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: " ...were recorded by an assistant investigating anaesthetist, who was blind to test drug ..." "Physicians in recovery room, blind to the test drug...assessed residual analgesia..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | High risk | All outcomes were measured, but pain scores values were not specified |
| Other bias | Unclear risk | No apparent bias |
Engelhardt 2003.
| Methods | RCT Parallel design |
|
| Participants | Tonsillectomy or adeno‐tonsillectomy, n = 60, children aged 2 to 14 years Group 1: mean age 8.1 years (SD 4.2) Group 2: mean age 7.1 years (SD 3.6) Group 3: mean age 7 years (SD 4.2) |
|
| Interventions | Group 1: morphine 0.1 mg/kg iv (n = 20) Group 2: tramadol 1 mg/kg iv (n = 20) Group 3: tramadol 2 mg/kg iv (n = 20) Administration time: after induction of anaesthesia All patients received diclofenac (1 mg/kg) rectally prior to commencing surgery |
|
| Outcomes | ‐ Postoperative pain scales (1 = pain free, 5 = worst possible pain) (every 4 hours for 24 h postoperation) ‐ Sedation scores, analgesic requirements, respiratory rate, nausea and episodes of vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We conducted a prospective, double‐blind, randomized controlled trial in children ..." "A computer‐generated randomization table ..." |
| Allocation concealment (selection bias) | Low risk | Quote: "Each patient volunteer was given a subsequent study number, which determined the drug and dose to be given. The code was not broken during the study." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All study drugs were diluted in water for injection to the total volume of 10 ml and given to the attending anaesthetist without disclosing the drug." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
Ertugrul 2006.
| Methods | RCT Parallel design |
|
| Participants | Adeno‐tonsillectomy, n = 45 (18 male, 27 female) children aged 1 to 7 years Group 1: 7 male, 8 female; median age 4.26 years (SD 1.57) Group 2: 6 male, 9 female; mean age 4.07 years (SD 1.54) Group 3: 5 male, 10 female; mean age 3.93 years (SD 1.66) |
|
| Interventions | Group 1: ketamine 0.5 mg/kg po (n = 15) Group 2: tramadol 1 mg/kg po (n = 15) Group 3: meperidine 1 mg/kg po (n = 15) Administration time: after induction of anaesthesia |
|
| Outcomes | ‐ Postoperative pain scales (Toddler–Preschooler Postoperative Pain Scale) (30, 60, 120 and 240 min after extubation) ‐ Postoperative agitation scores ‐ Time to opening eyes upon command (min) ‐ Number of patients with vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "After induction of anaesthesia and before tracheal intubation, children were randomly allocated to receive.." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Post‐operative pain was scored blind ..."; "Post‐operative pain was assessed in the recovery room 30, 60, 120 and 240 min after tracheal extubation by a blinded observer ..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
Hullett 2006.
| Methods | RCT Parallel design |
|
| Participants | Adeno‐tonsillectomy, n = 60, children aged 1 to 8 years Group 1: median age 5.0 years (P25 to P75 4.5 to 7.0) Group 2: mean age 5.0 years (P25 to P75 3.0 to 7.0) |
|
| Interventions | Group 1: morphine 0.1 mg/kg iv (n = 28) Group 2: tramadol 1 mg/kg iv (n = 32) Administration time: at induction of anaesthesia |
|
| Outcomes | ‐ Postoperative pain scales (PMH Pain Assessment Tool) (0, 15, 30 min; 1, 2, 3, 4, 5, 6 h postoperation) ‐ Sedation score ‐ Time to first analgesic requirement and total numbers of doses ‐ Number of patients with respiratory depression |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "... in this double blinded, prospective, controlled trial." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detailed information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Six other patients were excluded because of protocol violations or inadequate data collection" |
| Selective reporting (reporting bias) | High risk | All outcomes were measured, but pain scores values were only incompletely specified: ‐ only description of number of patient with pain score > 6 at the first three study time points ‐ no pain score values |
| Other bias | Unclear risk | No apparent bias |
Moyado Garcia 2009.
| Methods | RCT Parallel design |
|
| Participants | Surgical procedures with expected moderate to severe postoperative pain (e.g. perineal fistula and bladder neck closure, bilateral oblique pelvic osteotomy, exploratory laparotomy, bilateral elongation of Achilles tendon, etc), n = 24 (14 males, 10 females; children aged 1 to 10 years) Group 1: 7 males, 5 females; mean age 6.2 years (2.5 to 10) Group 2: 7 males, 5 females; mean age 4.4 years (1.6 to 10) |
|
| Interventions | Group 1: nalbuphine iv (bolus 100 μg/kg, 0.2 μg/kg/min for 72 h) (n = 12) Group 2: tramadol iv (bolus 1 mg/kg, 2.0 μg/kg/min for 72 h) (n = 12) Administration time: before the end of surgery |
|
| Outcomes | ‐ Postoperative pain scales (FPS, VAS) (every 1 h for the first 24 h, then every 4 h until the end of the 72 h study period) ‐ Documentation of haemodynamic and respiratory parameters during 72 h study period ‐ Sedation scores, episodes of vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "By means of a predesigned table of random numbers, children were allocated to receive..." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "In order to preserve the double‐blind design of the study, drugs were prepared on a case‐by‐case basis, according to the predesigned table of random numbers, by one of the investigators of the study (JCR‐M) who did not participate in the evaluation of patients’ eligibility or study outcomes. Evaluation of patients’ eligibility, pain score in younger children, application of the visual analogue scale (VAS) in older children, and evaluation of adverse events during the study period were performed by an investigator (JCH‐P) who was unaware of the patients’ allocation group. Adherence to the protocol was also monitored by another investigator (DM‐G)." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | High risk | All outcomes were measured, pain intensities were not mentioned within the text |
| Other bias | Unclear risk | No apparent bias |
Ozalevli 2005.
| Methods | RCT Parallel design |
|
| Participants | Adeno‐tonsillectomy, n = 60 (37 males, 23 females), children aged 6 to 12 years Group 1: 18 males, 12 females; mean age 7 years (6 to 11) Group 2: 19 males, 11 females; mean age 6 years (6 to 12) |
|
| Interventions | Group 1: tramadol iv (loading dose 1 mg/kg, bolus 0.2 mg/kg/10min) (n = 30) Group 2: morphine iv (loading dose 0.1 mg/kg, bolus 0.02 mg/kg/10min) (n = 30) Administration time: after surgery |
|
| Outcomes | ‐ Documentation of haemodynamic and respiratory parameters during and after anaesthesia ‐ Postoperative pain scales (CHEOPS) (0, 15, 30, 60 min; 2, 4, 6, 24 h postoperation) ‐ Documentation of sedation score ‐ Cumulative opioid consumption ‐ Number of patients with adverse events (urinary retention, nausea) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "This prospective, randomized, double‐blind study was designed..." "Randomization was performed consecutively." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information Quote: "The patients were then randomly allocated to receive..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Thus both observers and patients were blinded to the drug used." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | High risk | All outcomes were measured. Pain intensity score (CHEOPS) was described, but no values were reported |
| Other bias | Unclear risk | No apparent bias |
Ozer 2003.
| Methods | RCT Parallel design |
|
| Participants | Tonsillectomy with or without adenoidectomy, n = 50 (23 males, 27 females), children aged 4 to 7 years Group 1: 10 males, 15 females; mean age 5.7 years (SD 1.5) Group 2: 13 males, 12 females; mean age 6.8 years (SD 1.6) |
|
| Interventions | Group 1: tramadol 1 mg/kg iv (n = 25) Group 2: meperidine 1 mg/kg iv (n = 25) Administration time: after induction of anaesthesia |
|
| Outcomes | ‐ Documentation of MAP perioperatively ‐ Postoperative pain score (FPS) at 0 min, 10 min, 20 min, 45 min postoperation) ‐ Opioid related adverse events like nausea and vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "Fifty children ... were randomly assigned... ." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: " The nurse in the PACU who was unaware of the nature of the test drug assessed the agitation score. The same nurse evaluated analgesia..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
Ozköse 2000.
| Methods | RCT Parallel design |
|
| Participants | Tonsillectomy with or without adenoidectomy, n = 45 (27 males, 18 females), children aged 2 to 11 years) Group 1: 8 males, 7 females; mean age 5.1 years (SD 2.8) Group 2: 10 males, 5 females; mean age 5.3 years (SD 2.9) Group 3: 9 males, 6 females; mean age 5.6 years (SD 3.2) |
|
| Interventions | Group 1: tramadol 0.5 mg/kg iv (n = 15) Group 2: tramadol 1 mg/kg iv (n = 15) Group 3: placebo iv (n = 15) Administration time: after induction of anaesthesia |
|
| Outcomes | ‐ Postoperative pain score (FPS ≤ 6 years, VAS > 7 years; 15 min, 30 min, 60 min postoperation) ‐ Number of patients with need for additional analgesia ‐ Opioid related adverse events ‐ Documentation of HR and MAP perioperatively |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "... a randomized, double‐blind and placebo‐controlled protocol." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information Quote: "...these syringes were consecutively used." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The syringes were marked only with a coded label ... ." "...the author who was unaware of group assignment... assessed pain ... . " |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
Payne 1999.
| Methods | RCT Parallel design |
|
| Participants | Dental extraction, n = 50 (26 males, 24 females), children aged 4 to 7 years Group 1: 21 males, 19 females; mean age 5.3 years (SD 1.1) Group 2: 5 males, 5 females; mean age 4.8 years (SD 1.2) |
|
| Interventions | Group 1: tramadol 3 mg/kg + 0.5 mg/kg midazolam po (n = 40) Group 2: placebo + 0.5 mg/kg midazolam po (n = 10) Administration time: after induction of anaesthesia All children received midazolam |
|
| Outcomes | ‐ Postoperative pain score (Oucher face pain scale) (15, 30, 60, and 120 min postoperation) ‐ Documentation of oxygen saturation ‐ Opioid related adverse events (respiratory, cardiovascular, or emetic incidents) ‐ Documentation of HR and MAP perioperatively |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "...were included in a double‐blind, randomized, placebo‐controlled trial..." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The anaesthetist who did the anaesthetic assessments and the research sister who did the postanaesthetic recovery and pain assessments were not informed which study medication was administered." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
Roelofse 1999.
| Methods | RCT Parallel design |
|
| Participants | Dental extraction, n = 60 (31 males, 29 females), children aged 4 to 7 years Group 1: 18 males, 14 females, mean age 5.3 years (SD 1.1) Group 2: 13 males, 15 females, mean age 5.1 years (SD 1.2) |
|
| Interventions | Group 1: tramadol 1.5 mg/kg + midazolam po (n = 31) Group 2: placebo + midazolam po (n = 29) Administration time: after induction of anaesthesia All children received midazolam |
|
| Outcomes | ‐ Postoperative pain score (Oucher face pain scale) (15, 30, 60, and 120 min postoperation) ‐ Opioid related adverse events (emesis) ‐ Documentation of postoperative recovery ‐ Number of patients with the need for rescue analgesia |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "... were included in a double‐blind, randomized, placebo‐controlled trial.." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither, anaesthetist, ..., nor research sister who did the post‐anaesthetic recovery and pain assessments, were informed which study medication ..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
Schäffer 1986.
| Methods | RCT Parallel design |
|
| Participants | Herniotomy, orchidopexy, circumcision, hydrocelectomy, n = 60, children aged 1 to 9 years Group 1: 25 males, 5 females; mean age 4.5 years (SD 1.9) Group 2: 26 males, 4 females; mean age 4.87 years (SD 2.4) |
|
| Interventions | Group 1: 0.15 to 0.2 mg/kg nalbuphine im Group 2: 0.75 to 1 mg/kg tramadol im Administration time: after surgery (at the arrival in the recovery area) |
|
| Outcomes | ‐ VAS pain scale (0 to 10 cm) (1, 2, 3, 4, 6, 8, 24 h postoperation) ‐ Number of adverse events (vomiting, headache, singultus, hallucination, dry mouth) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...each group received in a randomized and double‐blind manner ..."; "... received by an randomization plan..." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...each group received in a randomized and double‐blind manner ..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
Umuroglu 2004.
| Methods | RCT Parallel design |
|
| Participants | Adeno‐tonsillectomy, n = 60 (32 males, 28 females), children aged 5 to 12 years Group 1: 5 males, 10 females; mean age 6.9 (SD 2.1) Group 2: 9 males, 6 females; mean age 7.13 (SD 2.51) Group 3: 7 males, 8 females; mean age 6.06 (SD 2.51) Group 4: 7 males, 8 females; mean age 6.96 (SD 2.08) |
|
| Interventions | Group 1: ketamine 0.5 mg/kg iv (n = 15) Group 2: morphine 0.1 mg/kg iv (n = 15) Group 3: tramadol 1.5 mg/kg iv (n = 15) Group 4: saline iv (n = 15) Administration time: during induction of anaesthesia |
|
| Outcomes | ‐ Postoperative pain score (5‐P‐NRS, CHEOPS); 1 min, 5 min, 10 min, 15 min, 20 min, 30 min, 45 min, 60 min, 120 min, 240 min, 360 min postoperation ‐ Number of patients with need for additional analgesia ‐ Duration of postoperative analgesia, time to first rescue analgesia ‐ Opioid related adverse events (nausea, vomiting, sedation) ‐ Documentation of heart rate and oxygen saturation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "A randomized, prospective, double‐blind and placebo‐controlled study design was used." |
| Allocation concealment (selection bias) | Low risk | Quote: "A sealed envelope method was used for randomization." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A randomized, prospective, double‐blind and placebo‐controlled study design was used." "pain scores... were assessed by an independent observer." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "pain scores... were assessed by an independent observer." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
van den Berg 1999.
| Methods | RCT Parallel design |
|
| Participants | Tonsillectomy with and without adenoidectomy; n = 152 (99 males, 53 females), children and young adults aged 8 to 21 years Group 1: 25 males, 12 females; mean age 13 (SD 15) Group 2: 25 males, 13 females; mean age 14 (SD 15) Group 3: 26 males, 12 females; mean age 16 (SD 14) Group 4: 23 males, 16 females; mean age 15 (SD 14) |
|
| Interventions | Group 1: saline (n = 37) Gropu 2: tramadol 3 mg/kg iv (n = 38) Group 3: pethidine 1.5 mg/kg iv (n = 38) Group 4: nalbuphine 0.3 mg/kg iv (n = 39) Administration time: 30 sec before induction of anaesthesia |
|
| Outcomes | ‐ Postoperative pain score (restlessness‐pain score in PACU) ‐ Number of patients with need for postoperative additional analgesia ‐ Time to extubation ‐ Documentation of heart rate, blood pressure ‐ Opioid related adverse events (emesis, sedation) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A prospective, double‐blind, randomized, controlled study ... .", "Each patient was block randomized ... ." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The test drugs were injected by the investigating anaesthetist, who was not blind to the test drug injected." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Nurses in the PACU, blind to the test drug administered ..., assessed residual analgesia ... ." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
Viitanen 2001.
| Methods | RCT Parallel design |
|
| Participants | Adenoidectomy, n = 80, children aged 1 to 3 years | |
| Interventions | Group 1: tramadol 2 mg/kg iv (n = 40) Group 2: saline iv (n = 40) Administration time: after induction of anaesthesia All children received rectal ibuprofen (10 mg/kg) before start of surgery |
|
| Outcomes | ‐ Postoperative cumulative pethidine consumption ‐ Number of patients with the need for rescue analgesia ‐ Duration of postoperative analgesia, time to first rescue analgesia ‐ Opioid related adverse events (nausea, vomiting) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eighty children were allocated randomly... .", "According to a computer‐generated table of random numbers... ." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both study drugs were prepared and administered by a nurse who did not otherwise participate in the care of the child." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "... assessed by a trained nurse who was blinded... ." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | High risk | All outcomes were measured, but pain score values were not reported |
| Other bias | Unclear risk | No apparent bias |
iv = intravenously
po = per mouth
im = intramuscularly
SD = standard deviation
PONV = postoperative nausea and vomiting
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aguirre Córcoles 2003 | Intravenous patient‐controlled analgesia |
| Akkaya 2009 | Peritonsillar infiltration of tramadol |
| Alencar 2012 | Only neonates were studied, continuous application of tramadol on an intensive care unit |
| Altunkaya 2003 | No children included |
| Apiliogullari 2011 | Retrospective study |
| Bedirli 2011 | Conference abstract |
| Borazan 2012 | Propofol injection pain was assessed |
| Chu 2006 | Intravenous patient‐controlled analgesia |
| Courtney 2000 | Children and adult included, no differentiation possible |
| Demiraran 2005 | Epidural injection |
| Finkel 2002 | Tested different dosages of tramadol (1 mg/kg versus 2 mg/kg) |
| Griessinger 1997 | No RCT |
| Günes 2004 | Caudal application |
| Khosravi 2006 | Comparison with ilioinguinal and iliohypogastric block |
| Macarone 1998 | Retrospective study |
| Metodiev 2011 | Conference abstract |
| Mikhel'son 2002 | No RCT |
| Oliveira 2000 | No RCT |
| Osifo 2008 | Retrospective study |
| Ostreikov 1993 | No RCT |
| Pendeville 2000 | Versus paracetamol |
| Sun 2010 | No RCT |
RCT = randomized controlled trial
iv = intravenous
Differences between protocol and review
Postoperative pain scores could not be sufficiently analysed, because there was a large heterogeneity and mainly non validated pain scales were used. Accordingly, other outcomes were included in the summary of findings tables. The definition for moderate to severe pain has been changed from NRS ≥ 3 to > 3. Therefore, the authors decided to assess the outcome 'number of patients with the need for rescue analgesia' as the primary outcome. The systematic search also included the trial database ClinicalTrials.gov, but no other studies were identified. The authors calculated dichotomous data by using the Mantel‐Haenszel method instead of the inverse‐variance method as iniatially proposed. Within the risk of bias section the selective reporting domain has been clarified and we added criteria for the calculation of NNT/NNH (at least four studies). Finally, we reduced the number of planned subgoup analysis (no subgroup analysis focusing on the age of included children, duration of postoperative care (PACU/24h/48h))
Contributions of authors
| Draft the protocol | AS,SR, PZ |
| Develop a search strategy | AS |
| Search for studies (usually 2 authors) | AS, SR, CMF |
| Obtain copies of studies | AS, SR, CMF |
| Select which studies to include (2 + 1 arbiter) | AS, SR, CMF, EPZ |
| Extract data from studies (2 authors) | AS, SR, CMF |
| Enter data into RevMan 5 | AS, SR, CMF |
| Carry out the analysis | AS, SR |
| Interpret the analysis | AS, PZ |
| Draft the final write‐up of the review | AS, PZ, EPZ |
| Update the review | AS, SR, EPZ |
| Content expert name | AS, EPZ |
| Methodologist name | AS |
Declarations of interest
AS has no relevant conflicts of interests.
SR has no relevant conflicts of interests.
CMF has no relevant conflicts of interests.
PZ has no relevant conflicts of interests.
EPZ has no relevant conflicts of interests.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Ali 2008 {published data only}
- Ali SM, Shahrbano S, Ulhaq TS. Tramadol for pain relief in children undergoing adenotonsillectomy a comparison with dextromethorphan. Laryngoscope 2008 Sep;118(9):1547‐9. [DOI] [PubMed] [Google Scholar]
Antila 2006 {published data only}
- Antila H, Manner T, Kuurila K, Salanterä S, Kujala R, Aantaa R. Ketoprofen and tramadol for analgesia during early recovery after tonsillectomy in children. Paediatric Anaesthesia 2006 May;16(5):548‐53. [DOI] [PubMed] [Google Scholar]
Barsoum 1995 {published data only}
- Barsoum MW. Comparison of the efficacy and tolerability of tramadol, pethidine and nalbuphine in children with postoperative pain. Clinical Drug Investigation 1995;9(4):183‐90. [Google Scholar]
Bösenberg 1998 {published data only}
- Bösenberg AT, Ratcliffe S. The respiratory effects of tramadol in children under halothane an anaesthesia. Anaesthesia. 1998 Oct;53(10):960‐4. [DOI] [PubMed] [Google Scholar]
Chiaretti 2000 {published data only}
- Chiaretti A, Viola L, Pietrini D, Piastra M, Savioli A, Tortorolo L, et al. Preemptive analgesia with tramadol and fentanyl in pediatric neurosurgery. Child's Nervous System 2000 Feb;16(2):93‐9. [DOI] [PubMed] [Google Scholar]
Cocelli 2012 {published data only}
- Cocelli LP, Ugur BK, Durucu C, Kul S, Arik H, Mumbuc S. Comparison of pre‐emptive tonsillar lodge infiltration with ropivacaine versus intravenous tramadol in pediatric tonsillectomies a randomized placebo‐controlled study. International Journal of Pediatric Otorhinolaryngology 2012 May;76(5):653‐7. [DOI] [PubMed] [Google Scholar]
Ekemen 2008 {published data only}
- Ekemen S, Yelken B, Ilhan H, Tokar B. A comparison of analgesic efficacy of tramadol and pethidine for management of postoperative pain in children a randomized, controlled study. Pediatric Surgery International 2008 Jun;24(6):695‐8. [DOI] [PubMed] [Google Scholar]
Engelhardt 2003 {published data only}
- Engelhardt T, Steel E, Johnston G, Veitch DY. Tramadol for pain relief in children undergoing tonsillectomy a comparison with morphine. Paediatric Anaesthesia 2003 Mar;13(3):249‐52. [DOI] [PubMed] [Google Scholar]
Ertugrul 2006 {published data only}
- Ertugrul F, Akbas M, Karsli B, Kayacan N, Bulut F, Trakya A. Pain relief for children after adenotonsillectomy. Journal of International Medical Research 2006 Nov‐Dec;34(6):648‐54. [DOI] [PubMed] [Google Scholar]
Hullett 2006 {published data only}
- Hullett BJ, Chambers NA, Pascoe EM, Johnson C. Tramadol vs morphine during adenotonsillectomy for obstructive sleep apnea in children. Paediatric Anaesthesia 2006 Jun;16(6):648‐53. [DOI] [PubMed] [Google Scholar]
Moyado Garcia 2009 {published data only}
- Moyao‐García D, Hernández‐Palacios JC, Ramírez‐Mora JC, Nava‐Ocampo AA. A pilot study of nalbuphine versus tramadol administered through continuous intravenous infusion for postoperative pain control in children. Acta Biomedica 2009 Aug;80(2):124‐30. [PubMed] [Google Scholar]
Ozalevli 2005 {published data only}
- Ozalevli M, Unlügenç H, Tuncer U, Güneş Y, Ozcengiz D. Comparison of morphine and tramadol by patient‐controlled analgesia for postoperative analgesia after tonsillectomy in children. Paediatric Anaesthesia 2005 Nov;15(11):979‐84. [DOI] [PubMed] [Google Scholar]
Ozer 2003 {published data only}
- Ozer Z, Görür K, Altunkan AA, Bilgin E, Camdeviren H, Oral U. Efficacy of tramadol versus meperidine for pain relief and safe recovery after adenotonsillectomy. European Journal of Anaesthesiology 2003 Nov;20(11):920‐4. [DOI] [PubMed] [Google Scholar]
Ozköse 2000 {published data only}
- Ozköse Z, Akçabay M, Kemaloğlu YK, Sezenler S. Relief of posttonsillectomy pain with low‐dose tramadol given at induction of anesthesia in children. International Journal of Pediatric Otorhinolaryngology 2000 Jul 14;53(3):207‐14. [DOI] [PubMed] [Google Scholar]
Payne 1999 {published data only}
- Payne KA, Roelofse JA. Tramadol drops in children: analgesic efficacy, lack of respiratory effects, and normal recovery times. Anesthesia Progress 1999 Summer;46(3):91‐6. [PMC free article] [PubMed] [Google Scholar]
Roelofse 1999 {published data only}
- Roelofse JA, Payne KA. Oral tramadol analgesic efficacy in children following multiple dental extractions. European Journal of Anaesthesiology 1999 Jul;16(7):441‐7. [DOI] [PubMed] [Google Scholar]
Schäffer 1986 {published data only}
- Schäffer J, Piepenbrock S, Kretz FJ, Schönfeld C. Nalbuphine and tramadol for the control of postoperative pain in children. Anaesthesist 1986 Jul;35(7):408‐13. [PubMed] [Google Scholar]
Umuroglu 2004 {published data only}
- Umuroğlu T, Eti Z, Ciftçi H, Yilmaz Göğüş F. Analgesia for adenotonsillectomy in children: a comparison of morphine, ketamine and tramadol. Paediatric Anaesthesia 2004 Jul;14(7):568‐73. [DOI] [PubMed] [Google Scholar]
van den Berg 1999 {published data only}
- Berg AA, Montoya‐Pelaez LF, Halliday EM, Hassan I, Baloch MS. Analgesia for adenotonsillectomy in children and young adults a comparison of tramadol, pethidine and nalbuphine. European Journal of Anaesthesiology 1999 Mar;16(3):186‐94. [DOI] [PubMed] [Google Scholar]
Viitanen 2001 {published data only}
- Viitanen H, Annila P. Analgesic efficacy of tramadol 2 mg kg(‐1) for paediatric day‐case adenoidectomy. British Journal of Anaesthesia 2001 Apr;86(4):572‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aguirre Córcoles 2003 {published data only}
- Aguirre Córcoles E, Durán González ME, Zambudio GA, González Celdrán R, Castaño Collado I, Cárceles Barón MD, et al. [Post‐surgical paediatric pain Nursing‐ PCA vs continuous I.V. infusion of tramadol]. Cir Pediatria 2003 Jan;16(1):30‐3. [PubMed] [Google Scholar]
Akkaya 2009 {published data only}
- Akkaya T, Bedirli N, Ceylan T, Matkap E, Gulen G, Elverici O, et al. Comparison of intravenous and peritonsillar infiltration of tramadol for postoperative pain relief in children following adenotonsillectomy. European Journal of Anaesthesiology 2009 Apr;26(4):333‐7. [DOI] [PubMed] [Google Scholar]
Alencar 2012 {published data only}
- Alencar AJ, Sanudo A, Sampaio VM, Góis RP, Benevides FA, Guinsburg R. Efficacy of tramadol versus fentanyl for postoperative analgesia in neonates. Archives of Diseases in Childhood. Fetal and Neonatal Edition 2012 Jan;97(1):F24‐9. [DOI] [PubMed] [Google Scholar]
Altunkaya 2003 {published data only}
- Altunkaya H, Ozer Y, Kargi E, Babuccu O. Comparison of local anaesthetic effects of tramadol with prilocaine for minor surgical procedures. British Journal of Anaesthesia 2003 Mar;90(3):320‐2. [DOI] [PubMed] [Google Scholar]
Apiliogullari 2011 {published data only}
- Apiliogullari S, Kara I, Senaran H, Duman A, Celik JB. Combined spinal and light general anaesthesia for children undergoing developmental hip dysplasia surgery: Single centre experience. Regional Anesthesia and Pain Medicine 2011. [Google Scholar]
Bedirli 2011 {published data only}
- Bedirli N, Akcabay M, Emik U. The effects of intraoperative single dosage tramadol or dexmedetomidine on postoperative analgesia, sedation and emerge reactions in pediatric patients undergoing adenotonsillectomy. Regional Anesthesia and Pain Medicine 2011. [Google Scholar]
Borazan 2012 {published data only}
- Borazan H, Sahin O, Kececioglu A, Uluer MS, Et T, Otelcioglu S. Prevention of propofol injection pain in children: A comparison of pre‐treatment with tramadol and propofol‐lidocaine mixture. International Journal of Medical Sciences 2012;9(6):492‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chu 2006 {published data only}
- Chu YC, Lin SM, Hsieh YC, Chan KH, Tsou MY. Intraoperative administration of tramadol for postoperative nurse‐controlled analgesia resulted in earlier awakening and less sedation than morphine in children after cardiac surgery.. Anesthesia and Analgesia 2006 Jun;102(6):1668‐73. [DOI] [PubMed] [Google Scholar]
Courtney 2000 {published data only}
- Courtney MJ, Cabraal D. Tramadol vs diclofenac for posttonsillectomy analgesia. Archives of Otolaryngology‐‐Head & Neck Surgery 2001 Apr;127(4):385‐8. [DOI] [PubMed] [Google Scholar]
Demiraran 2005 {published data only}
- Demiraran Y, Kocaman B, Akman RY. A comparison of the postoperative analgesic efficacy of single‐dose epidural tramadol versus morphine in children. British Journal of Anaesthesia 2005 Oct;95(4):510‐3. [DOI] [PubMed] [Google Scholar]
Finkel 2002 {published data only}
- Finkel JC, Rose JB, Schmitz ML, Birmingham PK, Ulma GA, Gunter JB, et al. An evaluation of the efficacy and tolerability of oral tramadol hydrochloride tablets for the treatment of postsurgical pain in children. Anesthesia and Analgesia 2002 Jun;94(6):1469‐73. [DOI] [PubMed] [Google Scholar]
Griessinger 1997 {published data only}
- Griessinger N, Rösch W, Schott G, Sittl R. Tramadol infusion for pain therapy following bladder exstrophy surgery in pediatric wards. Urologe A 1997 Nov;36(6):552‐6. [DOI] [PubMed] [Google Scholar]
Günes 2004 {published data only}
- Güneş Y, Gündüz M, Unlügenç H, Ozalevli M, Ozcengiz D. Comparison of caudal vs intravenous tramadol administered either preoperatively or postoperatively for pain relief in boys. Paediatric Anaesthesia 2004 Apr;14(4):324‐8. [DOI] [PubMed] [Google Scholar]
Khosravi 2006 {published data only}
- Khosravi MB, Khezri S, Azemati S. Tramadol for pain relief in children undergoing herniotomy: a comparison with ilioinguinal and iliohypogastric blocks. Paediatric Anaesthesia 2006 Jan;16(1):54‐8. [DOI] [PubMed] [Google Scholar]
Macarone 1998 {published data only}
- Macarone Palmieri A, Meglio M, Testa D, Salafia M, Iasiello A. Anesthesiologic and surgical problems in adenotonsillectomy in pediatric patients. Our current trend. Minerva Anestesiologica 1998 Dec;64(12):545‐52. [PubMed] [Google Scholar]
Metodiev 2011 {published data only}
- Metodiev Y, Gavrilova N. Analgesic efficacy of ultrasound‐guided transversus abdominis plane block after open appenedectomy in children. Regional Anesthesia and Pain Medicine 2011. [Google Scholar]
Mikhel'son 2002 {published data only}
- Mikhel'son VA, Zhirkova IuV, Beliaeva ID, Stepanenko SM, Manerova AF, Butyleva OIu. [Postoperative analgesia with tramadol in newborn children using the method of continuous intravenous infusion]. Anesteziologiia i Reanimatologiia 2003 Jan‐Feb;1:24‐8. [PubMed] [Google Scholar]
Oliveira 2000 {published data only}
- Oliveira EDS, Micheletti LG, Sakata RK, Iwata NM, Do Amaral JLG. A comparative study of postoperative analgesia of tramadol or fentanyl in pediatric patients anesthetic induction. Pediatric Anesthesia 2000;413(1‐2):89‐92. [Google Scholar]
Osifo 2008 {published data only}
- Osifo OD, Aghahowa ES. Safety profile and efficacy of commonly used analgesics in surgical neonates in Benin City, Nigeria. American Journal of Perinatology 2008 Nov;25(10):617‐22. [DOI] [PubMed] [Google Scholar]
Ostreikov 1993 {published data only}
- Ostreĭkov IF, Sen'kov VN, Kurilova ES, Bershinskiĭ VP, Pivovarov SA, Babaev BD. [Use of tramadol during minor surgical interventions in children]. Anesteziologiia i Reanimatologiia 1993 May‐Jun;3:28‐31. [PubMed] [Google Scholar]
Pendeville 2000 {published data only}
- Pendeville PE, Montigny S, Dort JP, Veyckemans F. Double‐blind randomized study of tramadol vs. paracetamol in analgesia after day‐case tonsillectomy in children. European Journal of Anaesthesiology 2000 Sep;17(9):576‐82. [DOI] [PubMed] [Google Scholar]
Sun 2010 {published data only}
- Sun Y, Xu W, Hu J, Xu W, Bai J, Zhang M. Combination effect of tramadol and low dose propofol on emergence agitation in children receiving sevoflurane for adenotonsillectomy procedure. Journal of Shanghai Jiaotong University 2010;1. [Google Scholar]
Additional references
Allegaert 2011
- Allegaert K, Rochette A, Veyckemans F. Developmental pharmacology of tramadol during infancy: ontogeny, pharmacogenetics and elimination clearance. Paediatric Anaesthesia 2011;21(3):266‐73. [PUBMED: 20723094] [DOI] [PubMed] [Google Scholar]
Andrews 2002
- Andrews K, Fitzgerald M. Wound sensitivity as a measure of analgesic effects following surgery in human neonates and infants. Pain 2002;99(1‐2):185‐95. [PUBMED: 12237196] [DOI] [PubMed] [Google Scholar]
Awad 2004
- Awad IT, Moore M, Rushe C, Elburki A, O'Brien K, Warde D. Unplanned hospital admission in children undergoing day‐case surgery. European Journal of Anaesthesiology 2004;21(5):379‐83. [PUBMED: 15141796] [DOI] [PubMed] [Google Scholar]
Bamigbade 1998
- Bamigbade TA, Langford RM. Tramadol hydrochloride: an overview of current use. Hospital Medicine 1998;59(5):373‐6. [PUBMED: 9722388] [PubMed] [Google Scholar]
Bosenberg 1998
- Bosenberg AT, Ratcliffe S. The respiratory effects of tramadol in children under halothane an anaesthesia. Anaesthesia 1998;53(10):960‐4. [PUBMED: 9893539] [DOI] [PubMed] [Google Scholar]
Bozkurt 2005
- Bozkurt P. Use of tramadol in children. Paediatric Anaesthesia 2005;15(12):1041‐7. [PUBMED: 16324021] [DOI] [PubMed] [Google Scholar]
Dadure 2009
- Dadure C, Bringuier S, Raux O, Rochette A, Troncin R, Canaud N, et al. Continuous peripheral nerve blocks for postoperative analgesia in children: feasibility and side effects in a cohort study of 339 catheters. Canadian Journal of Anaesthesia = Journal Canadien d'Anesthesie 2009;56(11):843‐50. [PUBMED: 19697092] [DOI] [PubMed] [Google Scholar]
Dayer 1994
- Dayer P, Collart L, Desmeules J. The pharmacology of tramadol. Drugs 1994;47 Suppl 1:3‐7. [PUBMED: 7517823] [DOI] [PubMed] [Google Scholar]
Franck 2004
- Franck LS, Cox S, Allen A, Winter I. Parental concern and distress about infant pain. Archives of Disease in Childhood. Fetal and Neonatal Edition 2004;89(1):F71‐5. [PUBMED: 14711862] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glaser 1999
- Glaser R, Kiecolt‐Glaser JK, Marucha PT, MacCallum RC, Laskowski BF, Malarkey WB. Stress‐related changes in proinflammatory cytokine production in wounds. Archives of General Psychiatry 1999;56(5):450‐6. [PUBMED: 10232300] [DOI] [PubMed] [Google Scholar]
Groenewald 2012
- Groenewald CB, Rabbitts JA, Schroeder DR, Harrison TE. Prevalence of moderate‐severe pain in hospitalized children. Paediatric Anaesthesia 2012;22(7):661‐8. [PUBMED: 22332912] [DOI] [PubMed] [Google Scholar]
Hennies 1988
- Hennies HH, Friderichs E, Schneider J. Receptor binding, analgesic and antitussive potency of tramadol and other selected opioids. Arzneimittel‐Forschung 1988;38(7):877‐80. [PUBMED: 2849950] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration.
Hirschfeld 2013
- Hirschfeld G, Zernikow B. Variability of "optimal" cut points for mild, moderate, and severe pain: neglected problems when comparing groups. Pain 2013;154(1):154‐9. [PUBMED: 23182623] [DOI] [PubMed] [Google Scholar]
Kharasch 2003
- Kharasch S, Saxe G, Zuckerman B. Pain treatment: opportunities and challenges. Archives of Pediatrics & Adolescent Medicine 2003;157(11):1054‐6. [PUBMED: 14609892] [DOI] [PubMed] [Google Scholar]
Lintz 1998
- Lintz W, Barth H, Osterloh G, Schmidt‐Bothelt E. Pharmacokinetics of tramadol and bioavailability of enteral tramadol formulations. 3rd Communication: suppositories. Arzneimittel‐Forschung 1998;48(9):889‐99. [PUBMED: 9793614] [PubMed] [Google Scholar]
Michelet 2012
- Michelet D, Andreu‐Gallien J, Bensalah T, Hilly J, Wood C, Nivoche Y, et al. A meta‐analysis of the use of nonsteroidal antiinflammatory drugs for pediatric postoperative pain. Anesthesia and Analgesia 2012;114(2):393‐406. [PUBMED: 22104069] [DOI] [PubMed] [Google Scholar]
Murthy 2000
- Murthy BV, Pandya KS, Booker PD, Murray A, Lintz W, Terlinden R. Pharmacokinetics of tramadol in children after i.v. or caudal epidural administration. British Journal of Anaesthesia 2000;84(3):346‐9. [PUBMED: 10793594] [DOI] [PubMed] [Google Scholar]
Niesters 2013
- Niesters M, Overdyk F, Smith T, Aarts L, Dahan A. Opioid‐induced respiratory depression in paediatrics: a review of case reports. British Journal of Anaesthesia 2013;110(2):175‐82. [PUBMED: 23248093] [DOI] [PubMed] [Google Scholar]
Orliaguet 2015
- Orliaguet G, Hamza J, Couloigner V, Denoyelle F, Loriot MA, Broly F, et al. A case of respiratory depression in a child with ultrarapid CYP2D6 metabolism after tramadol. Pediatrics 2015. [PUBMED: 25647677] [DOI] [PubMed] [Google Scholar]
Page 2013
- Page MG, Stinson J, Campbell F, Isaac L, Katz J. Identification of pain‐related psychological risk factors for the development and maintenance of pediatric chronic postsurgical pain. Journal of Pain Research 2013;6:167‐80. [PUBMED: 23503375] [DOI] [PMC free article] [PubMed] [Google Scholar]
Payne 2002
- Payne KA, Roelofse JA, Shipton EA. Pharmacokinetics of oral tramadol drops for postoperative pain relief in children aged 4 to 7 years‐‐a pilot study. Anesthesia Progress 2002;49(4):109‐12. [PUBMED: 12779111] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Romsing 1996
- Romsing J, Walther‐Larsen S. Postoperative pain in children: a survey of parents' expectations and perceptions of their children's experiences. Paediatric Anaesthesia 1996;6(3):215‐8. [PUBMED: 8732613] [DOI] [PubMed] [Google Scholar]
Russell 2013
- Russell P, Ungern‐Sternberg BS, Schug SA. Perioperative analgesia in pediatric surgery. Current Opinion in Anaesthesiology 2013;26(4):420‐7. [PUBMED: 23756911] [DOI] [PubMed] [Google Scholar]
Scaife 1988
- Scaife JM, Campbell I. A comparison of the outcome of day‐care and inpatient treatment of paediatric surgical cases. Journal of Child Psychology and Psychiatry, and Allied Disciplines 1988;29(2):185‐98. [PUBMED: 3372615] [DOI] [PubMed] [Google Scholar]
Schnabel 2014
- Schnabel A, Reichl SU, Zahn PK, Pogatzki‐Zahn E. Nalbuphine for postoperative pain treatment in children. Cochrane Database of Systematic Reviews 2014;7:CD009583. [PUBMED: 25079857] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schunemann 2008
- Schunemann HJ, Oxman AD, Brozek J, Glasziou P, Bossuyt P, Chang S, et al. GRADE: assessing the quality of evidence for diagnostic recommendations. Evidence‐Based Medicine 2008;13(6):162‐3. [PUBMED: 19043023] [DOI] [PubMed] [Google Scholar]
Scott 2000
- Scott LJ, Perry CM. Tramadol: a review of its use in perioperative pain. Drugs 2000;60(1):139‐76. [PUBMED: 10929933] [DOI] [PubMed] [Google Scholar]
Segerdahl 2008
- Segerdahl M, Warren‐Stomberg M, Rawal N, Brattwall M, Jakobsson J. Children in day surgery: clinical practice and routines. The results from a nation‐wide survey. Acta Anaesthesiologica Scandinavica 2008;52(6):821‐8. [PUBMED: 18498436] [DOI] [PubMed] [Google Scholar]
Standing 2009
- Standing JF, Savage I, Pritchard D, Waddington M. Diclofenac for acute pain in children. Cochrane Database of Systematic Reviews 2009, (4):CD005538. [PUBMED: 19821348] [DOI] [PubMed] [Google Scholar]
Stanko 2013
- Stanko D, Bergesio R, Davies K, Hegarty M, Ungern‐Sternberg BS. Postoperative pain, nausea and vomiting following adeno‐tonsillectomy ‐ a long‐term follow‐up. Paediatric Anaesthesia 2013;23(8):690‐6. [PUBMED: 23668258] [DOI] [PubMed] [Google Scholar]
Subramanyam 2014
- Subramanyam R, Chidambaran V, Ding L, Myer CM 3rd, Sadhasivam S. Anesthesia‐ and opioids‐related malpractice claims following tonsillectomy in USA: LexisNexis claims database 1984‐2012. Paediatric Anaesthesia 2014;24(4):412‐20. [PUBMED: 24417679] [DOI] [PubMed] [Google Scholar]
Tarkkila 1998
- Tarkkila P, Tuominen M, Lindgren L. Comparison of respiratory effects of tramadol and pethidine. European Journal of Anaesthesiology 1998;15(1):64‐8. [PUBMED: 9522144] [DOI] [PubMed] [Google Scholar]
Taylor 2008
- Taylor EM, Boyer K, Campbell FA. Pain in hospitalized children: a prospective cross‐sectional survey of pain prevalence, intensity, assessment and management in a Canadian pediatric teaching hospital. Pain Research & Management 2008;13(1):25‐32. [PUBMED: 18301813] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vickers 1992
- Vickers MD, O'Flaherty D, Szekely SM, Read M, Yoshizumi J. Tramadol: pain relief by an opioid without depression of respiration. Anaesthesia 1992;47(4):291‐6. [PUBMED: 1519677] [DOI] [PubMed] [Google Scholar]
Walker 2009
- Walker SM, Franck LS, Fitzgerald M, Myles J, Stocks J, Marlow N. Long‐term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain 2009;141(1‐2):79‐87. [PUBMED: 19026489] [DOI] [PubMed] [Google Scholar]
Weisman 1998
- Weisman SJ, Bernstein B, Schechter NL. Consequences of inadequate analgesia during painful procedures in children. Archives of Pediatrics & Adolescent Medicine 1998;152(2):147‐9. [PUBMED: 9491040] [DOI] [PubMed] [Google Scholar]


