Abstract
Background
Epidural analgesia offers greater pain relief compared to systemic opioid‐based medications, but its effect on morbidity and mortality is unclear. This review was originally published in 2006 and was updated in 2012 and again in 2016.
Objectives
To assess the benefits and harms of postoperative epidural analgesia in comparison with postoperative systemic opioid‐based analgesia for adults undergoing elective abdominal aortic surgery.
Search methods
In the updated review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, and five trial registers in November 2014, together with reference checking to identify additional studies. We reran the search in March 2017. One potential new trial of interest was added to a list of ‘Studies awaiting Classification' and will be incorporated into the formal review findings during the review update.
Selection criteria
We included all randomized controlled trials comparing postoperative epidural analgesia and postoperative systemic opioid‐based analgesia for adults who underwent elective open abdominal aortic surgery.
Data collection and analysis
Two authors independently assessed trial quality and extracted data. We contacted study authors for additional information and data when required. We assessed the level of evidence according to the scale provided by the GRADE working group.
Main results
We included 15 trials published from 1987 to 2009 with 1498 participants in this updated review. Participants had a mean age between 60.5 and 71.3 years. The percentage of women in the included studies varied from 0% to 28.1%. Adding an epidural to general anaesthesia for people undergoing abdominal aortic repair reduced myocardial infarction (risk ratio (RR) 0.54 (95% confidence interval (CI) 0.30 to 0.97); I2 statistic = 0%; number needed to treat for one additional beneficial outcome (NNTB) 28 (95% CI 19 to 1423), visual or verbal analogical scale (VAS) scores up to three days after the surgery (mean difference (MD) ‐1.78 (95% CI ‐2.32 to ‐1.25); I2 statistic = 0% for VAS scores on movement at postoperative day one), time to tracheal extubation (standardized mean difference (SMD) ‐0.42 (95% CI ‐0.70 to ‐0.15); I2 statistic = 83%; equivalent to a mean reduction of 36 hours), postoperative respiratory failure (RR 0.69 (95% CI 0.56 to 0.85); I2 statistic = 0%; NNTB 8 (95% CI 6 to 16)), gastrointestinal bleeding (OR 0.20 (95% CI 0.06 to 0.65); I2 statistic = 0%; NNTB 32 (95% CI 27 to 74)) and time spent in the intensive care unit (SMD ‐0.23 (95% CI ‐0.41 to ‐0.06); I2 statistic = 0%; equivalent to a mean reduction of six hours). We did not demonstrate a reduction in the mortality rate up to 30 days (RR 1.06 (95% CI 0.60 to 1.86); I2 statistic = 0%). The level of evidence was low for mortality and time before tracheal extubation; moderate for myocardial infarction, respiratory failure and intensive care unit length of stay; and high for gastrointestinal bleeding and VAS scores.
Authors' conclusions
Epidural analgesia provided better pain management, reduced myocardial infarction, time to tracheal extubation, postoperative respiratory failure, gastrointestinal bleeding, and intensive care unit length of stay compared with systemic opioid‐based drugs. For mortality, we did not find a difference at 30 days.
Keywords: Adult; Aged; Humans; Middle Aged; Analgesia, Epidural; Analgesia, Epidural/adverse effects; Analgesia, Epidural/methods; Analgesics, Opioid; Analgesics, Opioid/adverse effects; Analgesics, Opioid/therapeutic use; Aorta, Abdominal; Aorta, Abdominal/surgery; Cause of Death; Intubation, Intratracheal; Intubation, Intratracheal/statistics & numerical data; Myocardial Infarction; Myocardial Infarction/prevention & control; Pain Management; Pain Management/methods; Pain Measurement; Pain, Postoperative; Pain, Postoperative/prevention & control; Postoperative Complications; Postoperative Complications/mortality; Randomized Controlled Trials as Topic; Respiration, Artificial; Respiration, Artificial/statistics & numerical data; Time Factors
Plain language summary
Epidural analgesia compared with systemic opioid‐based medicines for people undergoing open abdominal aortic surgery
Background
Open surgery on the abdominal aorta (the main artery to the legs) requires aggressive postoperative pain management. The most commonly used pain management is epidural analgesia. This involves injecting pain‐relieving medicines through a catheter (narrow tube) that is placed in the epidural space (the outermost part of the spinal space). The alternative is systemic opioids (morphine‐like medicines injected into the bloodstream).
Objectives
This review evaluated the effect of these two methods of pain relief and the risks of postoperative complications and deaths after open abdominal aortic surgery. The review was originally published in 2006, updated in 2012, and again in 2015.
Methods
We searched scientific databases for clinical trials comparing epidural analgesia with systemic opioids in adults. Two authors independently assessed the quality of the trials and collected the data. We reran the search in March 2017. We will deal with the new study of interest when we update the review.
Main results
We included 15 trials published from 1987 to 2009 with 1498 participants in this updated review. The evidence is current to November 2014. The trials received financial support from a charitable organization (one study), a governmental organization (four studies) or the pharmaceutical industry (one study). The source of funding was unspecified for nine studies. We found that epidural analgesia reduced heart attacks, postoperative duration of tracheal intubation (a flexible breathing tube that is placed directly into the windpipe), risk of postoperative respiratory failure (requirement of a machine to assist the respiration after the surgery), gastrointestinal bleeding, decreased postoperative pain, and length of stay in the intensive care unit (equivalent to six hours). For death after the surgery, we did not found a difference in the death rate (in hospital or up to 30 days). The quality of evidence was low for mortality and time before tracheal extubation; meaning that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. The quality of evidence was moderate for heart attacks, respiratory failure, and intensive care unit length of stay; meaning that further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. The quality of evidence was high for gastrointestinal bleeding and pain scores; meaning that further research is very unlikely to change our confidence in the estimate of effect.
Authors' conclusions
Epidural analgesia provides better pain management than systemic opioids. It significantly reduces the number of people who will suffer heart damage, time to return of unassisted respiration, gastrointestinal bleeding, and intensive care unit length of stay. We did not find a difference in death rates at 30 days.
Summary of findings
Summary of findings for the main comparison. Epidural pain relief compared to systemic opioid‐based pain relief for abdominal aortic surgery.
| Epidural pain relief compared to systemic opioid‐based pain relief for abdominal aortic surgery | ||||||
| Patient or population: people undergoing abdominal aortic surgery Settings: Intervention: epidural pain relief Comparison: systemic opioid‐based pain relief | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic opioid‐based pain relief | Epidural pain relief | |||||
| Mortality in hospital or up to 30 days Follow‐up: 30 days1 | Study population | RR 1.06 (0.6 to 1.86) | 1383 (14 studies) | ⊕⊕⊝⊝ low2,3,4,5,6,7,8,9 | ‐ | |
| 39 per 1000 | 41 per 1000 (23 to 73) | |||||
| Low | ||||||
| 20 per 1000 | 21 per 1000 (12 to 37) | |||||
| High | ||||||
| 60 per 1000 | 64 per 1000 (36 to 112) | |||||
| Myocardial infarction Follow‐up: 30 days | Study population | RR 0.54 (0.30 to 0.97) | 851 (7 studies) | ⊕⊕⊕⊝ moderate2,3,4,6,8,9,10,11 | ‐ | |
| 76 per 1000 | 41 per 1000 (23 to 74) | |||||
| Low | ||||||
| 20 per 1000 | 11 per 1000 (6 to 19) | |||||
| High | ||||||
| 100 per 1000 | 54 per 1000 (30 to 97) | |||||
| Tracheal intubation duration Follow‐up: 0‐7 days | ‐ | The mean tracheal intubation duration in the intervention groups was 0.42 lower (0.7 to 0.15 lower) | ‐ | 975 (8 studies) | ⊕⊕⊝⊝ low2,4,6,8,9,12,13,14 | Data had to be extracted as P values for 2 studies (Norris 2001; Park 2001). Therefore, results are provided as standardized mean difference |
| Respiratory failure | Study population | RR 0.69 (0.56 to 0.85) | 861 (6 studies) | ⊕⊕⊕⊝ moderate3,4,7,8,9,13,15,16 | ‐ | |
| 315 per 1000 | 217 per 1000 (176 to 267) | |||||
| Low | ||||||
| 150 per 1000 | 104 per 1000 (84 to 128) | |||||
| High | ||||||
| 350 per 1000 | 241 per 1000 (196 to 298) | |||||
| Gastrointestinal bleeding Follow‐up: 30 days | Study population | OR 0.20 (0.06 to 0.65) | 487 (4 studies) | ⊕⊕⊕⊕ high2,3,4,5,6,8,9,15,17 | ‐ | |
| 40 per 1000 | 8 per 1000 (3 to 27) | |||||
| Low | ||||||
| 20 per 1000 | 4 per 1000 (1 to 13) | |||||
| High | ||||||
| 60 per 1000 | 13 per 1000 (4 to 40) | |||||
|
Pain (VAS scores) on movement at postoperative day 1 Scale from: 0 to 10. Follow‐up: mean 1 day |
The mean VAS scores on movement at postoperative day 1 in the intervention groups was 1.78 lower (2.32 to 1.25 lower) | ‐ | 162 (3 studies) | ⊕⊕⊕⊕ high3,4,6,8,9,13,18,19 | ‐ | |
| Intensive care unit length of stay Follow‐up: 0‐7 days | ‐ | The mean intensive care unit length of stay in the intervention groups was 0.23 standard deviations lower (0.41 to 0.06 lower) | ‐ | 523 (3 studies) | ⊕⊕⊕⊝ moderate2,3,4,8,9,13,14,15,16 | Data had to be extracted as P values for 2 studies (Muehling 2009; Park 2001). Therefore, results are provided as standardized mean difference |
| * The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OR: odds ratio; VAS: visual/verbal analogue scale. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 In hospital or up to 30 days. 2 There was uncertainty around allocation concealment for half or more of the studies included in the analysis. 3 I2 statistic < 25%. 4 Direct comparison performed on the population of interest and the outcome was not a surrogate marker. 5 Wide confidence interval. 6 Correcting the publication bias did not change the conclusion. 7 RR > 0.5. 8 No confounding factor justifying upgrading the evidence identified. 9 No dose‐response gradient. 10 Number of participants included lower than the optimal information size, fewer than 2000 and number of events < 400. 11 RR 0.53. 12 I2 statistic = 85%. Egger's regression intercept indicates that there may be a small‐study effect (P value = 0.003 (2‐tailed)). Excluding two studies with 14 participants each (Barre 1989; Broekema 1998), SMD would be ‐0.42 (95% CI ‐0.61 to ‐0.22); I2 statistic = 35%. 13 Optimal information size achieved. 14 Standardized mean difference < 0.8. 15 Uncertainty around blinding of the outcome assessor for half or more of the included studies. 16 No evidence of a publication bias. 17 OR 0.20. 18 We identified no serious risk of bias. 19 Standardized mean difference > 0.8.
Background
Description of the condition
Abdominal aortic aneurysm can be defined as a focal dilation of the abdominal aorta of at least 1.5 times the normal diameter or an absolute value of 3.0 cm or greater (Sampson 2014). Known risk factors include age, male sex, atherosclerosis, smoking, hypertension, and history in a first‐degree relative. In 2010, the incidence varied from 7.9 (95% confidence interval (CI) 6.5 to 9.6) in the 40 to 44 years age group to 2274.8 (95% CI 2149.8 to 2410.2) per 100,000 in the 75 to 79 years age group. These numbers represent a small decrease compared to 1990 figures (8.4 (95% CI 7.0 to 10.1) in the 40 to 44 years age group and 2422.5 (95% CI 2298.6 to 2562.3) per 100,000 in the 75 to 79 years age group (Sampson 2014). Prevalence was higher in high‐income versus low‐income nations. The highest prevalence in 1990 was in the high‐income regions of Australasia and North America. Australasia still had the highest prevalence in 2010 with high‐income North American regions coming third with a prevalence of 256.1 (95% CI 238.6 to 275.0) (Sampson 2014). Contemporary one‐time screening of men for abdominal aortic aneurism appears highly cost‐effective, and seems to remain an effective preventive health‐measure (Svensjo 2014). There actually is insufficient evidence to draw any conclusions about the effectiveness of cardiovascular prophylaxis in reducing mortality and cardiovascular events in people with abdominal aortic aneurisms (Robertson 2014). Traditionally, abdominal aortic aneurisms were treated by open abdominal surgical repair consisting in excision or not of the lesion and interposition of a synthetic graft. Since the early‐1990s, endovascular aneurysm repair has become available. In people considered fit for conventional surgery, endovascular aortic replacement was associated with lower short‐term mortality than open abdominal aortic repair. However, this benefit from endovascular aortic repair did not persist at the intermediate‐ and long‐term follow‐ups. People undergoing endovascular aortic repair had a higher re‐intervention rate than people undergoing open aortic repair (Paravastu 2014).
Description of the intervention
Epidural anaesthesia or analgesia consists of an injection of either local anaesthetic or opioids, or a mixture of both, into the epidural space as a modality to complement general anaesthesia for open abdominal aortic repair (epidural anaesthesia) or to treat postoperative pain (epidural analgesia). Epidural anaesthesia or analgesia is usually achieved through the insertion of an epidural catheter either at the thoracic or lumbar level.
How the intervention might work
Epidural analgesia provides better analgesia than parenteral opioids regardless of the analgesic agent, location of catheter placement, and type and time of pain assessment (Block 2003; Guay 2006). The superior analgesia offers improved postoperative coughing and breathing, and improved pulmonary mechanics thus potentially reducing pulmonary complications (Guay 2014a). The sympathetic blockade provided by epidural anaesthesia and analgesia and the sparing of the total opioid doses required improve bowel motility (Jorgensen 2000). These favourable effects of epidural analgesia may considerably reduce non‐life‐threatening morbidity and may have an important role in a multimodal approach to achieve prompt and expeditious recovery after surgery.
Why it is important to do this review
The original review was conducted because of a lack of consensus regarding the value of epidural anaesthesia and analgesia for this patient population (Nishimori 2006). People undergoing open abdominal aortic surgery typically have vasculopathy, often with a history of diabetes or smoking. They may well be either benefited or harmed by epidural analgesia (Hebl 2006). The most recent version of this review concluded that epidural analgesia provided better pain relief (especially during movement) in the period up to three postoperative days, and reduced the duration of postoperative tracheal intubation, the occurrence of prolonged postoperative mechanical ventilation, myocardial infarction, gastric complications and renal complications (Nishimori 2012).
In the present version of the review, we looked for new studies and updated the methodology.
Objectives
To assess the benefits and harms of postoperative epidural analgesia in comparison with postoperative systemic opioid‐based analgesia for adults undergoing elective abdominal aortic surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) comparing postoperative epidural analgesia and postoperative systemic opioid‐based analgesia for abdominal aortic surgery and assessing at least one of the outcomes of interest (see Types of outcome measures). We excluded from the analysis studies that did not include any outcome of interest.
We excluded all quasi‐randomized trials including one quasi‐randomized trial that was included in the previous version of this review (Lombardo 2009).
We applied no language or publication status restrictions.
Types of participants
We included adults (aged 18 years and older) who had elective, open abdominal aortic surgery, either suprarenal or infrarenal. We excluded people who had emergency surgery.
Types of interventions
We included intraoperative or postoperative (or both) epidural anaesthesia or analgesia added to general anaesthesia (either lumbar or thoracic) compared to general anaesthesia alone. We included all combinations of drugs and all start times (pre‐ or postoperatively). We imposed no restriction regarding the mode of analgesia used in the control group and included systemic opioid‐based pain relief with opioid drugs given by the following routes: intravenous, intramuscular, or subcutaneous. For both groups, bolus dosing, infusion, or patient‐controlled analgesia (PCA) devices were eligible for inclusion.
Types of outcome measures
Primary outcomes
Death from all causes within 30 days of surgery, or death from all causes during hospitalization.
Secondary outcomes
Postoperative cardiovascular complications: myocardial ischaemia, myocardial infarction, congestive heart failure (CHF), ventricular arrhythmias.
Postoperative respiratory complications: tracheal intubation duration, respiratory failure including prolonged mechanical ventilation or need to reinstate mechanical ventilation, pneumonia.
Postoperative cerebrovascular complications.
Postoperative acute kidney injury.
Postoperative gastrointestinal haemorrhage.
Postoperative pain scores (at rest or with movement).
Any indicator for postoperative bowel motility: incidence of ileus, time to first bowel sounds, flatus, bowel movement, or time to first drinking or eating.
Any indicator for postoperative mobilization: any type of functionality score or time to first ambulation.
Length of intensive care unit (ICU) stay.
Length of hospital stay.
We did not pre‐define these outcomes but noted the definitions used by contributing investigators.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 11) via Ovid; Ovid MEDLINE (from inception to week 1, November 2014); and EMBASE (from inception to week 1, November 2014). We limited the search to human studies with no language or date restrictions. For this update, the search was restricted from 2012 to November 2014 (up to 2012 was covered by the previous versions of this review (Nishimori 2006; Nishimori 2012) (Appendix 1). We retrieved full articles of any potential new study. We also checked the references lists of all retained studies. We reran the search in March 2017. We will deal with the one potential new study of interest when we update the review.
Searching other resources
We looked at www.clinicaltrials.gov; isrctn.org; www.umin.ac.jp/ctr/index.htm; www.trialregister.nl/ and eudract.ema.europa.eu/ for trials in progress (December 2014). We also screened conference proceedings of anaesthesiology societies, published in three major anaesthesiology journals, for 2012, 2013, and 2014: British Journal of Anaesthesiology, European Journal of Anaesthesiology and Regional Anesthesia and Pain Medicine. We also checked the website of the American Society of Anesthesiologists for 2012, 2013, and 2014 in December 2014 (www.asaabstracts.com/). We did not contact individuals or organizations.
Data collection and analysis
Selection of studies
We were not blinded to study authors, institutions, journal of publication, or study results. For this update, the two authors independently evaluated the titles and abstracts of trials identified in the literature search for their eligibility. We resolved disagreements through discussion.
Data extraction and management
We recorded information on participants, methods, interventions, and outcomes. We noted the level of epidural catheter placement and the names and doses of the drugs administered, and whether participants had received epidural medication during the surgery. We analysed the data with Review Manager 5 (Review Manager 2014) and Comprehensive Meta Analysis Version 2.2.044 (www.Meta‐Analysis.com; effect size for data extracted as P value, Egger's regression intercept, Duval and Tweedie's trim and fill analysis, meta‐regressions, heterogeneity between subgrouping for some analysis not included in this file).
Assessment of risk of bias in included studies
We evaluated trial quality using the Cochrane 'Risk of bias' tool (Higgins 2011). We assessed risk of bias for the following domains: random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, and selective reporting. We did not use blinding of participants and personnel as a risk of bias domain since it may be considered unethical to use sham epidurals. Instead, we evaluated if care programmes were identical for the intervention and control groups.
Measures of treatment effect
We reported results as risk ratio (RR) and their 95% CI for dichotomous data and mean difference (MD) and 95% CI for continuous data as much as was feasible. If some of the continuous data were given on different scales or if results were not available as mean and standard deviation (SD) or events and total number of participants (P value extraction), we produced the results as standardized mean difference (SMD) and 95% CI. For SMD, we considered 0.2 a small effect, 0.5 a medium effect, and 0.8 a large effect (Pace 2011). For clinical correspondence, we multiplied the SD of the control group of a study at low risk of bias by the SMD. When there was an effect, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) from the odds ratio (OR). We gave results for dichotomous data as RR because OR is not easily understood by clinicians (Deeks 2002; McColl 1998), but used OR for calculation of NNTB and NNTH (www.nntonline.net/visualrx/) (Cates 2002; Deeks 2002). When there was no effect, we calculated the optimal information size in order to make sure that there were enough participants included in the retained studies to justify a conclusion on the absence of effect (Pogue 1998) (www.stat.ubc.ca/˜rollin/stats/ssize/b2.html). We considered a difference of 1% for the mortality rate and 25% (increase or decrease) for the other outcomes as the minimal clinically relevant difference.
Unit of analysis issues
The unit of analysis was a participant who was individually randomized to the treatment group (intervention or control) of the RCTs selected for this review.
Three trials had more than two treatment groups (Broekema 1998; Norris 2001; Reinhart 1989). Broekema 1998 allocated participants to three groups: epidural‐sufentanil group, epidural‐morphine group, and intramuscular morphine (IM) group. Both the epidural‐sufentanil and epidural‐morphine groups received postoperative epidural analgesia; and the IM group received IM for postoperative analgesia. The only difference between the epidural‐sufentanil group and the epidural‐morphine group was the type of opioid they received epidurally (sufentanil or morphine). Otherwise they were similar. We were able to combine these two groups because the author provided raw data.
In Reinhart 1989, participants were allocated into three groups: thoracic epidural anaesthesia group; neurolept anaesthesia group; and halothane group. The thoracic epidural anaesthesia group received postoperative epidural analgesia. The neurolept anaesthesia and halothane groups received postoperative systemic opioid (piritramide analgesia). However, during surgery these two groups received different types of general anaesthesia. The neurolept anaesthesia group received neurolept anaesthesia with fentanyl and droperidol, and the halothane group received halothane with nitrous oxide and oxygen. Data were entered as subgroups, dividing the intervention group (thoracic epidural anaesthesia) by two, allowing us to analyse the data as subgroups or combining them.
In Norris 2001, participants were allocated into four possible combinations of anaesthesia: general anaesthesia (GA) followed by IV morphine patient‐controlled analgesia (IVPCA) (GA‐IVPCA), general anaesthesia followed by epidural patient‐controlled analgesia (EPCA) (GA‐EPCA), general anaesthesia combined with epidural anaesthesia (RSGA) followed by IVPCA (RSGA‐IVPCA) and RSGA followed by EPCA (RSGA‐EPCA). We divided the data from GA‐IVPCA by three to allow us to analyse the data as subgroups compared to the three regimens that included an epidural (GA‐EPCA, RSGA‐IVPCA, and RSGA‐EPCA) or combining them.
Dealing with missing data
We contacted the trial investigators for the data that were missing or unclear. In studies where the surgical population included participants who were not undergoing abdominal aortic surgery, we only included data on the people undergoing abdominal aortic surgery. If separated data were not published, we contacted trial investigators and requested the separated data. If separated data were not available, or were indistinguishable from the group data, we excluded that study.
Assessment of heterogeneity
We assessed clinical heterogeneity through careful evaluation of populations, interventions, and outcomes within each study. We used the I2 statistic to estimate the extent of the heterogeneity.
Assessment of reporting biases
We assessed publication bias with the Duval and Tweedie's trim and fill analysis.
Data synthesis
We conducted meta‐analyses if sufficient data existed from two or more studies. We used random‐effects models for outcomes with I2 statistic greater than 25% and fixed‐effect models for other outcomes.
Subgroup analysis and investigation of heterogeneity
Any amount of heterogeneity was explored but we focused more specifically on comparisons with a moderate or high amount of heterogeneity (I2 statistic greater than 25%) (Higgins 2003), and explored the heterogeneity using Egger's regression intercept (to assess the possibility of a small‐study effect (Rucker 2011), visual inspection of the forest plots with studies placed in order according to a specific moderator, subgroupings (categorical moderators), or meta‐regressions (continuous moderators)). Factors that were considered in the heterogeneity exploration were: year when the study was published, mean age of participants, site of epidural (thoracic versus lumbar), local anaesthetic in the epidural solution or opioids only, duration of epidural use, percentage of women included, and percentage of participants with chronic obstructive pulmonary disease or with CHF.
Sensitivity analysis
We also performed sensitivity analysis in our heterogeneity exploration based on the number of participants included or on the risk of bias assessments (allocation concealment and blinding of the outcome assessor).
Summary of findings
The quality of the body of evidence was judged according to the system developed by the GRADE working group (Guyatt 2011a) and presented in a 'Summary of findings' table (ims.cochrane.org/revman/gradepro) for the following outcomes: mortality in hospital or up to 30 days, myocardial infarction, tracheal intubation duration, respiratory failure, gastrointestinal haemorrhage, visual or verbal analogue scale (VAS) score on movement at postoperative day one and ICU length of stay. For risk of bias, we judged the quality of evidence as low risk of bias when most information came from studies at low risk of bias, and downgraded it by one level when most information came from studies at low or unclear risk of bias or by two levels when the proportion of information from studies at high risk of bias was sufficient to affect the interpretation of results. For inconsistency, we downgraded the quality of evidence by one when the I2 statistic was 50% or higher without satisfactory explanation and by two levels when the I2 statistic was 75% or higher without an explanation. We did not downgrade the quality of evidence for indirectness as all outcomes were based on direct comparisons, were performed on the population at interest, and were not surrogate markers (Guyatt 2011b). For imprecision (Guyatt 2011c), we downgraded the quality of evidence by one when the CI around the effect size was large or overlapped an absence of effect and failed to exclude an important benefit or harm; the number of participants was lower than the optimal information size (unless the sample size was at least of 2000 participants or the number of events included was at least 400); and we downgraded the quality by two levels when the CI was very wide and included both appreciable benefit and harm. For publication bias, we downgraded the quality of evidence by one when correcting for the possibility of publication as assessed by the Duval and Tweedie's fill and trim analysis changed the conclusion. We upgraded the quality of evidence by one when the effect size was large (less than 0.5 or greater than 2.0) and by two when the effect size was very large (RR less than 0.2 or greater than 5) (Guyatt 2011d). We applied the same rules for OR when the basal risk was lower than 20%. For SMD, we used 0.8 as the cutoff point for a large effect (Pace 2011). We also upgraded the quality by one when there was evidence of a dose‐related response. The quality was upgraded by one when possible effect of confounding factors would reduce a demonstrated effect or suggest a spurious effect when results show no effect. When the quality of the body of evidence is high, further research is very unlikely to change our confidence in the estimate of effect. When the quality is moderate, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. When the quality is low, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. When the quality is very low, any estimate of effect is very uncertain (Guyatt 2008).
Results
Description of studies
Results of the search
Figure 1 shows the results of the search for the current update. We reran the search in March 2017. Two‐hundreds and 58 citations were found. From these new citation, and the reference lists of relevant reviews and of the potential new trials, 10 full articles were retrieved. Of these, eight were excluded, one trial is ongoing and one trial was added to the of ‘Characteristics of studies awaiting classification' and will be incorporated into the formal review findings during the review update.
1.
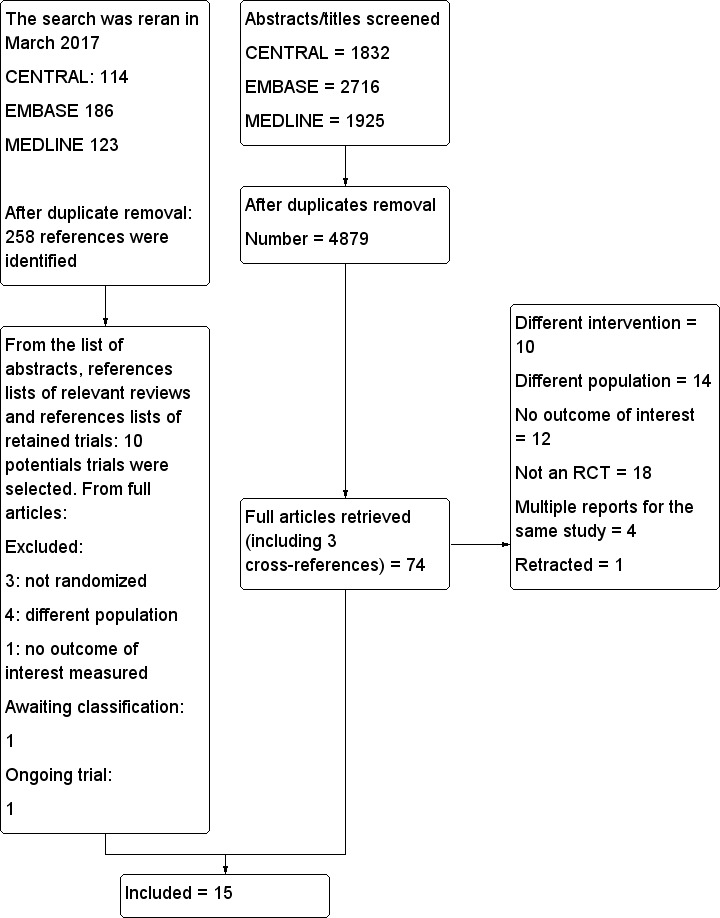
Flow diagram of study selection. We excluded one trial (Lombardo 2009; quasi‐randomized trial; information obtained from the authors by the previous review authors), which was previously included in this review (Nishimori 2012). We added one new trial (Muehling 2009). Therefore, the number of studies included in this review remains unchanged.
The search was reran in March 2017. Seven trials were excluded, one trial (Owczuk 2016) awaits classification and one trial (Li 2015) is ongoing.
RCT: randomized controlled trial.
Included studies
We included 15 trials published from 1987 to 2009 with 1498 participants. Participants had a mean age between 60.5 and 71.3 years. The percentage of women in the included studies varied from 0% to 28.1%. In three included trials, people undergoing aortic surgery were mixed with people undergoing other surgical procedures (Broekema 1998; Park 2001; Yeager 1987). We contacted the authors of all these trials. The authors of Park 2001 published subgroup data on abdominal aortic surgery and also provided the additional data requested for our review. The authors of Broekema 1998 and Yeager 1987 provided additional unpublished data on the aortic abdominal surgery subgroups.
Surgery included both aortic aneurysm repair and surgery for aortic occlusive disease in 10 studies (Barre 1989; Bois 1997; Boylan 1998; Garnett 1996; Kataja 1991; Norris 2001; Park 2001; Peyton 2003; Reinhart 1989; Yeager 1987). Norman 1997 and Muehling 2009 included only repairs of infrarenal aortic aneurysm. de Lis 1990 included only aortic occlusive disease. Two studies did not specify whether the surgical procedure was for aortic aneurysm or occlusive disease (Broekema 1998; Davies 1993). For aortic aneurysm location, only four studies specified supra‐ or infrarenal (Barre 1989; Boylan 1998; Muehling 2009; Norman 1997). Therefore, it was not possible to perform subgroup analysis on either suprarenal versus infrarenal aortic surgery or surgery for aneurysm versus occlusive disease. Appendix 2 provides information about preoperative risks, history of myocardial revascularization, and preoperative medication of participants of the included trials.
Appendix 3 provides the methods of surgical anaesthesia and postoperative analgesia of the included trials. Three trials had more than two treatment groups (Broekema 1998; Norris 2001; Reinhart 1989). The details and how we handled the groups are explained in the Unit of analysis issues section. All other trials had two treatment groups, one group received postoperative epidural analgesia (intervention group) and the other group received postoperative systemic opioid analgesia (control group). People who received postoperative epidural analgesia also received epidural anaesthesia during surgery except for: group II of de Lis 1990, the thoracic epidural anaesthesia (TEA) group of Bois 1997, the GA‐EPCA group of Norris 2001, where epidural infusion was started at the end of surgery. Participants who received postoperative systemic opioid analgesia did not receive epidural anaesthesia during surgery except for the RSGA‐IVPCA group of Norris 2001, where an epidural was used only during the surgery and participants received systemic opioid analgesia postoperatively. All participants received general anaesthesia during surgery. Anaesthetic agents were similar between the control and intervention groups in each study. Inhalational anaesthetics were the main anaesthetics for most of the trials, except for Reinhart 1989 (fentanyl and droperidol for half of the control group) and Yeager 1987 (fentanyl/nitrous oxide in the intervention group versus fentanyl with or without nitrous oxide and with or without inhalational agent in the control group). de Lis 1990 and Muehling 2009 did not specify the anaesthetic agents used.
Eight trials used thoracic epidural analgesia (Barre 1989; Bois 1997; Broekema 1998; Davies 1993; Muehling 2009; Norman 1997; Norris 2001; Reinhart 1989), and two trials used lumbar epidural analgesia (Boylan 1998; Kataja 1991). Four trials used either thoracic or lumbar epidurals (Garnett 1996; Park 2001; Peyton 2003; Yeager 1987). One study did not mention the level of epidural catheter placement (de Lis 1990). Appendix 3 shows the exact solution used in the epidural catheters. For the surgery, nine trials used a local anaesthetic alone: lidocaine (Davies 1993), bupivacaine (Kataja 1991; Norman 1997; Park 2001; Reinhart 1989), ropivacaine (Muehling 2009), lidocaine plus bupivacaine (Barre 1989), lidocaine or bupivacaine (Yeager 1987), and bupivacaine or ropivacaine (Peyton 2003). Four trials used a mixture of local anaesthetics and opioids: bupivacaine plus fentanyl (Norris 2001), lidocaine plus pethidine (Garnett 1996), bupivacaine plus sufentanil or morphine (Broekema 1998), and lidocaine plus bupivacaine plus morphine (Boylan 1998). For postoperative analgesia, eight trials used a mixture of local anaesthetics and opioids: bupivacaine plus fentanyl (Bois 1997; Kataja 1991; Norris 2001), bupivacaine plus morphine (Boylan 1998), bupivacaine plus morphine or sufentanil (Broekema 1998), bupivacaine plus pethidine (Garnett 1996), ropivacaine plus sufentanil (Muehling 2009), and bupivacaine or ropivacaine plus fentanyl or pethidine (Peyton 2003). Two trials used local anaesthetics alone: bupivacaine (Davies 1993; Reinhart 1989). Three trials used opioids alone: morphine (de Lis 1990; Norman 1997; Park 2001). In two trials, the information was unclear (Barre 1989; Yeager 1987).
Appendix 4 summarizes the definitions of postoperative complications. Basically, the outcomes were well defined and were similar among trials.
Excluded studies
From the search for this update and from the study awaiting classification, we excluded seven trials. Two trials were not randomized (Lombardo 2009; Tatsuishi 2012). Two trials compared epidural analgesia to local infiltration (Renghi 2013; Tilleul 2012). One trial did not contain any outcome of interest to this review (Panaretou 2012). One trial studied a different population (no open abdominal aortic surgery included) (Pan 2006). For the last trial, the population studied was participants undergoing lower abdominal surgery. We contacted the authors and did not receive a reply. The reasons for exclusion of the trials can be found in the Characteristics of excluded studies table and in Figure 1. We also changed the reason of exclusion of one article (Piper 2000): this article has now been retracted.
Studies awaiting classification
We reran the search in March 2017. One trial (Owczuk 2016) is awaiting classification. For further details see Characteristics of studies awaiting classification.
Ongoing studies
We reran the search in March 2017. One trial (Li 2015) is ongoing. For further details see Characteristics of ongoing studies
Risk of bias in included studies
Figure 2 shows a 'Risk of bias' graph and Figure 3 shows a 'Risk of bias' summary.
2.
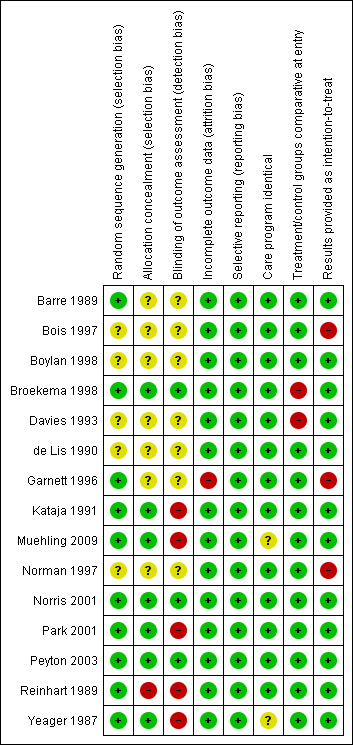
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
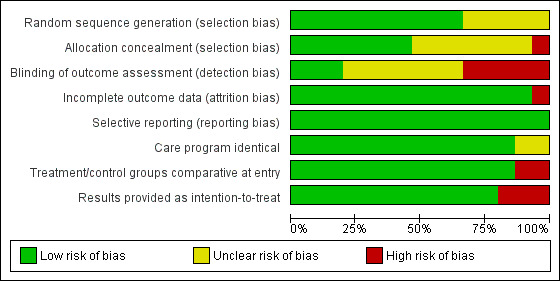
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Seven trials used adequate concealment of allocation (Broekema 1998; Kataja 1991; Muehling 2009; Norris 2001; Park 2001; Peyton 2003; Yeager 1987). Reinhart 1989 did not use allocation concealment. The information was unclear for the remaining seven trials.
Blinding
Three trials blinded outcome assessors. In Norris 2001, all participants had an epidural catheter inserted before surgery and received both masked epidural and intravenous medications in order to blind study participants, anaesthesiologists and outcome assessors. Although the data collectors were not blinded in Peyton 2003, they were not informed about the morbidity endpoints or their definitions. Blinded trial assistants entered the collected data, and a computer algorithm defined whether particular endpoints had occurred at the time of the data entry. Broekema 1998 placed a sham epidural catheter on the skin of the back of participants allocated to control group. The participants were asked not to disclose the route of administration to the observer. If disclosure accidentally occurred, then the observer was replaced. Five trials did not blind outcome assessors (Kataja 1991; Muehling 2009; Park 2001; Reinhart 1989; Yeager 1987), and for seven other studies, the information was unclear.
Incomplete outcome data
We judged only one trial at high risk for attrition bias (Garnett 1996).
Selective reporting
We judged all trials at low risk for reporting bias.
Other potential sources of bias
In Davies 1993, there were significantly more participants with chronic airway disease in the intervention (epidural) group. In Park 2001, there were significantly more smokers in the intervention group. In Broekema 1998, subgroup data for people undergoing aortic surgery that were provided by the author showed that the mean age of the intervention group (72.9 years) was higher than the mean age of the control group (58.0 years). Other baseline data for these three trials and all baseline data for other trials were comparable.
Effects of interventions
See: Table 1
Primary outcome
Death from all causes within 30 days of surgery, or death from all causes during hospitalization
Based on 14 trials that included 1383 participants, we did not find a difference in in‐hospital mortality rate (Bois 1997; Boylan 1998; Broekema 1998; Davies 1993; de Lis 1990; Garnett 1996; Muehling 2009; Norman 1997; Norris 2001; Reinhart 1989; Yeager 1987), or 30‐day mortality rate (Kataja 1991; Park 2001; Peyton 2003) (RR 1.06 (95% CI 0.60 to 1.86); I2 statistic = 0%; Analysis 1.1). Egger's regression intercept showed a small‐study effect (P value 0.03; two‐tailed). Duval and Tweedie's trim and fill analysis showed that three studies might be missing to the right, but the adjusted point of estimate would nevertheless remain not statistically significant if this possible publication bias was corrected. Based on a basal rate of 4.0%, 8352 participants (4176 per group) would have been required to eliminate a difference of 25%, that is, decrease the mortality rate to 3% (alpha 0.05, beta 0.2; one‐sided test; www.stat.ubc.ca/˜rollin/stats/ssize/b2.html). The level of evidence for this outcome was low (Table 1).
1.1. Analysis.
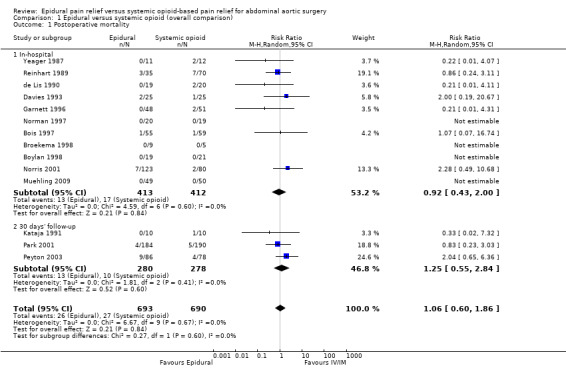
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 1 Postoperative mortality.
One trial gave results for mortality at one year (RR 0.60 (95% CI 0.19 to 1.90)) (Norris 2001).
Secondary outcomes
Postoperative cardiovascular complications
Myocardial ischaemia
Based on five trials that included 503 participants, we did not find a difference in the incidence of myocardial ischaemia (RR 1.05 (95% CI 0.79 to 1.40); I2 statistic = 0%; Analysis 1.2). Four trials used continuous monitoring (Bois 1997; Boylan 1998; Garnett 1996; Norris 2001), one trial performed laboratory investigations on clinical indication only (Muehling 2009). Including or excluding this study did not change the results (Muehling 2009). Egger's regression intercept showed no evidence of a small‐study effect (two‐tailed). Duval and Tweedie's trim and fill analysis showed that three studies might be missing to the right for an adjusted point of estimate of 1.14 (95% CI 0.89 to 1.46).
1.2. Analysis.
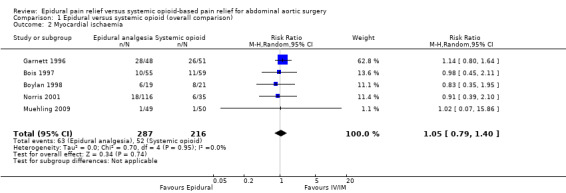
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 2 Myocardial ischaemia.
Myocardial infarction
Based on seven trials that included 851 participants, the effect of the intervention was at the limit for statistical significance (RR 0.54 (95% CI 0.30 to 0.97); I2 statistic = 0%; Analysis 1.3) (Bois 1997; Boylan 1998; Davies 1993; Garnett 1996; Norris 2001; Park 2001; Yeager 1987). Egger's regression intercept showed no evidence of a small‐study effect (two‐tailed). Duval and Tweedie's trim and fill analysis showed that two studies might be missing to left of mean for an adjusted point of estimate RR 0.43 (95% CI 0.24 to 0.84 (random‐effects model)). Based on a basal rate of 7.6%, the NNTB was 28 (95% CI 19 to 1423). The optimal information size for a 25% reduction from a basal rate of 7.6% would be 4252 (2126 per group) (alpha 0.05; beta 0.2; one‐sided test). The level of evidence for this outcome was moderate (Table 1).
1.3. Analysis.
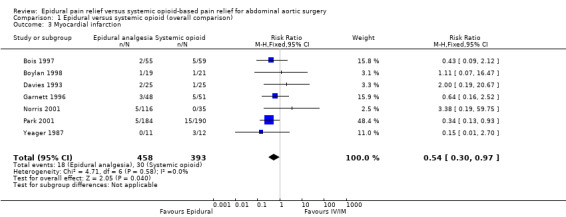
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 3 Myocardial infarction.
Congestive heart failure
Based on six trials that included 811 participants, we did not find a difference in CHF (RR 0.77 (95% CI 0.44 to 1.35): I2 statistic = 0%; Analysis 1.4) (Bois 1997; Davies 1993; Garnett 1996; Norris 2001; Park 2001; Yeager 1987). Egger's regression intercept showed no evidence of a small‐study effect (two‐tailed). Duval and Tweedie's trim and fill analysis showed that one study might be missing to left of mean, but the adjusted point of estimate would nevertheless remain not statistically significant.
1.4. Analysis.
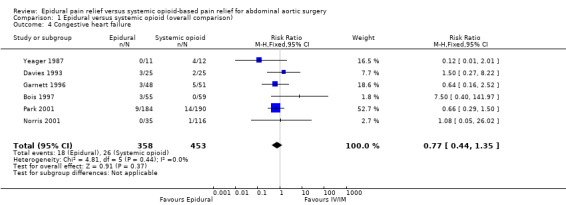
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 4 Congestive heart failure.
Ventricular arrhythmias
Based on four trials that included 689 participants, we did not find a difference in the risk of ventricular arrhythmia (RR 0.55 (95% CI 0.20 to 1.51); I2 statistic = 0%; Analysis 1.5) (Bois 1997; Davies 1993; Norris 2001; Park 2001). Egger's regression intercept showed no evidence of a small‐study effect (two‐tailed). Duval and Tweedie's trim and fill analysis showed that one study might be missing to right of mean, but the adjusted point of estimate would nevertheless remain not statistically significant. Based on a basal rate of 3.2%, 8164 participants (4082 per group) would have been required to eliminate a 25% decrease in the incidence of ventricular arrhythmia.
1.5. Analysis.
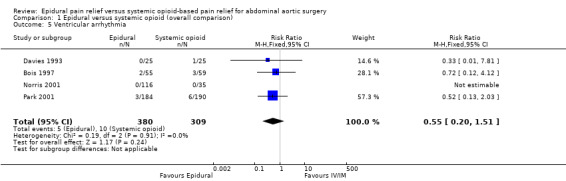
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 5 Ventricular arrhythmia.
Postoperative respiratory complications
Tracheal intubation duration
Based on eight trials that included 975 participants (Appendix 5), adding an epidural to general anaesthesia reduced time to tracheal extubation (SMD ‐0.42 (95% CI ‐0.70 to ‐0.15); I2 statistic = 83%) (Barre 1989; Bois 1997; Boylan 1998; Broekema 1998; Norris 2001; Park 2001; Peyton 2003; Reinhart 1989). Egger's regression intercept indicated that there may be a small‐study effect (P value = 0.001 (two‐tailed)). Excluding two studies with 14 participants (Barre 1989; Broekema 1998), the SMD would be ‐0.39 (95% CI ‐0.54 to ‐0.24; I2 statistic 20%; Analysis 1.6). Still excluding the two small studies, Duval and Tweedie's trim and fill analysis showed that one study might be missing to the right for an adjusted point of estimate (SMD ‐0.41 (95% CI ‐0.61 to ‐0.20); random‐effects model). Based on the SD measured in the control group of the trial with the lowest risk of bias (Barre 1989; SD 85) and where the SD was available for this outcome (Barre 1989; Bois 1997; Boylan 1998; Peyton 2003; Reinhart 1989), the mean reduction would be equivalent to 36 hours. Based on a study with a mean value and SD (Bois 1997), the optimal information size for a 25% reduction from a mean time of 12.9 and an SD of 7.7 hours would be 142 participants (71 per group). The level of evidence for this outcome was low (Table 1).
1.6. Analysis.
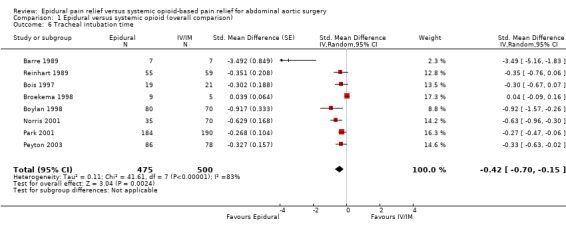
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 6 Tracheal intubation time.
Respiratory failure including prolonged mechanical ventilation or need to reinstate mechanical ventilation
Six trials that included 861 participants reported acute respiratory failure (Davies 1993; Garnett 1996; Norris 2001; Park 2001; Peyton 2003; Yeager 1987). They all defined the outcome as prolonged ventilation after surgery (Appendix 4). The event rate was significantly smaller in the intervention group compared to the control group (RR 0.69 (95% CI 0.56 to 0.85); I2 statistic = 0%; Analysis 1.7). Based on a basal rate of 31.5%, the NNTB was 8 (95% CI 6 to 16). Egger's regression intercept showed no evidence of a small‐study effect (two‐tailed). Duval and Tweedie's trim and fill analysis showed no evidence of a publication bias. The level of evidence for this outcome was high (Table 1). Based on basal rate of 31.5%, the optimal information size for a reduction of 25% was 790 participants (395 per group) (alpha 0.05; beta 0.2; one‐sided test). The level of evidence for this outcome was moderate.
1.7. Analysis.
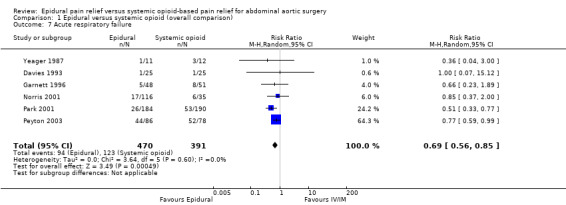
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 7 Acute respiratory failure.
Pneumonia
Based on eight trials that included 1000 participants, we did not find a reduction in the incidence of postoperative pneumonia (RR 0.62 (95% CI 0.37 to 1.04); I2 statistic = 0%; Analysis 1.8) (Boylan 1998; Davies 1993; Garnett 1996; Muehling 2009; Norris 2001; Park 2001; Peyton 2003; Yeager 1987). Egger's regression intercept showed no evidence of a small‐study effect (two‐tailed). Duval and Tweedie's trim and fill analysis showed that two studies might be missing to left of mean for an adjusted point of estimate RR 0.51 (95% CI 0.31 to 0.83). Assuming that a publication bias was present and a basal rate of 7.5%, the NNTB would be 27 (95% CI 19 to 79).
1.8. Analysis.
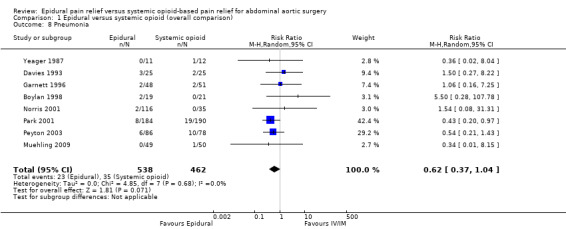
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 8 Pneumonia.
Postoperative cerebrovascular complications
Four trials reported postoperative cerebrovascular complications (Davies 1993; Garnett 1996; Norris 2001; Park 2001). In total, the trials reported 15 cases of cerebrovascular complications (four in intervention group and 11 in control group) in 674 participants. Using Peto OR (rare events), epidural analgesia reduced the risk of stroke (OR 0.33 (95% CI 0.12, 0.93); I2 statistic = 20%; Analysis 1.9). Egger's regression intercept showed no evidence of a small‐study effect (two‐tailed). Duval and Tweedie's trim and fill analysis showed that two studies might be missing to left of mean for an adjusted point of estimate OR 0.21 (95% CI 0.08 to 0.54; fixed‐effect model). Assuming a basal rate of 3.7%, the NNTB would be 41 (95% CI 31 to 400).
1.9. Analysis.
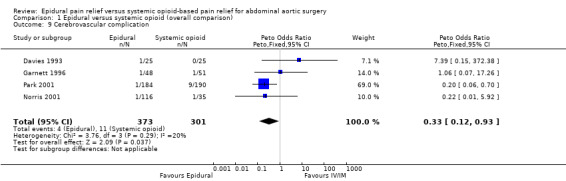
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 9 Cerebrovascular complication.
Postoperative acute kidney injury
Based on nine trials that included 1039 participants we did not find a difference in the incidence of acute kidney injury (RR 0.95 (95% CI 0.59 to 1.53); I2 statistic = 0%; Analysis 1.10) (Davies 1993; de Lis 1990; Garnett 1996; Muehling 2009; Norman 1997; Norris 2001; Park 2001; Peyton 2003; Yeager 1987). Egger's regression intercept showed no evidence of a small‐study effect (two‐tailed). Duval and Tweedie's trim and fill analysis showed that one study might be missing to left of mean, but the adjusted point of estimate would nevertheless remain not statistically significant.
1.10. Analysis.
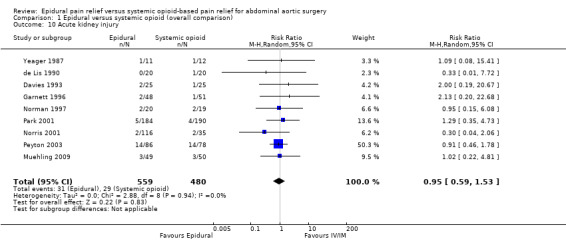
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 10 Acute kidney injury.
Postoperative gastrointestinal haemorrhage
Based on four trials that included 487 participants, and using Peto OR (rare events) adding an epidural to general anaesthesia decreased the risk of postoperative gastrointestinal bleeding (OR 0.20 (95% CI 0.06 to 0.65); I2 statistic = 0%; Analysis 1.11) (Boylan 1998; Davies 1993; Park 2001; Yeager 1987). Egger's regression intercept showed no evidence of a small‐study effect (two‐tailed). Duval and Tweedie's trim and fill analysis showed that two studies might be missing to right of mean for an adjusted point of estimate OR 0.23 (95% CI 0.08 to 0.66). Based on a basal rate of 4%, the NNTB was 32 (95% CI 27 to 74). The level of evidence for this outcome was high (Table 1).
1.11. Analysis.
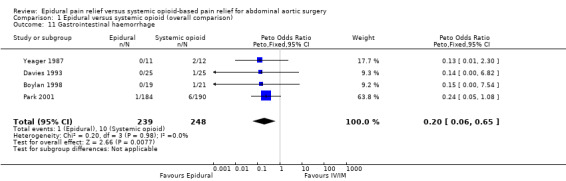
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 11 Gastrointestinal haemorrhage.
Postoperative pain scores (at rest or with movement)
See Appendix 6.
VAS scores were available at rest on postoperative day one for five trials that included 655 participants (Bois 1997; Boylan 1998; Broekema 1998; Park 2001; Peyton 2003). Using an epidural for postoperative analgesia produced a slight decrease in VAS scores at rest on postoperative day one (MD ‐0.85 (95% CI ‐1.46 to ‐0.25); I2 statistic = 61%; Analysis 1.12). Egger's regression intercept showed no evidence of a small‐study effect (two‐tailed). Duval and Tweedie's trim and fill analysis showed no evidence of a publication bias. The effect of the intervention may have decreased with time as shown by a meta‐regression of the MD versus the year when the study was published (Figure 4) (P value = 0.02). However, the effect of the intervention was more consistent on movement as reported in three trials that included 162 participants (MD ‐1.78 (95% CI ‐2.32 to ‐1.25); I2 statistic = 0%; Analysis 1.13) (Boylan 1998; Broekema 1998; Peyton 2003). Egger's regression intercept showed no evidence of a small‐study effect (two‐tailed). Duval and Tweedie's trim and fill analysis showed that two studies might be missing to left of mean for an adjusted point of estimate MD ‐1.78 (95% CI ‐2.26 to ‐1.31). Based on Peyton's value (Peyton 2003), the optimal information size for a decrease of 1.5 from a mean of 5.26 and an SD of 2.88 was 92 participants (46 per group) (alpha 0.05; beta 0.2; one‐sided test). The level of evidence for a reduction of pain on movement at postoperative day one was high (Table 1).
1.12. Analysis.
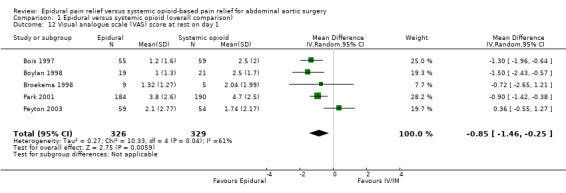
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 12 Visual analogue scale (VAS) score at rest on day 1.
4.
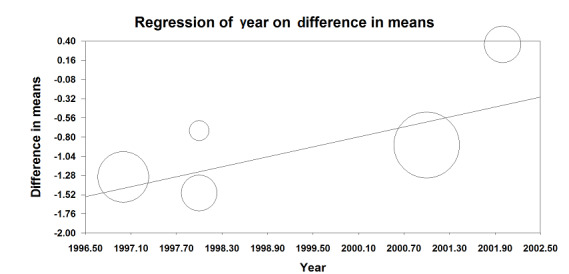
Visual/verbal analogical scores at rest on postoperative day 1. The difference between the intervention is higher in older studies (P value = 0.02).
(This meta regression plot was not produced in RevMan. The figure was generated automatically by the software, and cannot be amended. The software has expressed the years as decimals.)
1.13. Analysis.
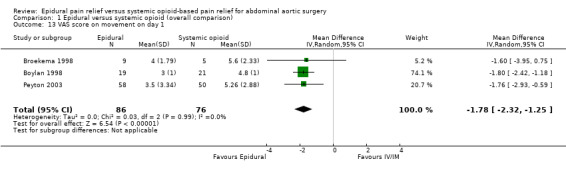
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 13 VAS score on movement on day 1.
VAS scores at rest on postoperative day two were available for three studies with 159 participants (Boylan 1998; Broekema 1998; Peyton 2003). There was a considerable difference in the effect size between the three studies (MD ‐0.38 (95% CI ‐1.34 to 0.57); I2 statistic 58% (Analysis 1.14). Based on the same studies with 155 participants, VAS scores on movement on postoperative day two were lower for people treated with epidural analgesia (MD ‐1.35 (95% CI ‐2.36 to ‐0.35); I2 statistic = 56%; Analysis 1.15) (Boylan 1998; Broekema 1998; Peyton 2003). However, the amplitude of the effect was lower in the more recent and larger study (Peyton 2003). Based on three trials that included 481 participants, we found no difference in VAS scores at rest on postoperative day three (MD ‐0.29 (95% CI ‐0.64 to 0.06); I2 statistic = 0%; Analysis 1.16). The authors of two studies provided data for VAS scores on movement on 105 participants on postoperative day three (MD ‐1.37 (95% CI ‐2.24 to ‐0.51); I2 statistic = 0%; Analysis 1.17) (Broekema 1998; Peyton 2003).
1.14. Analysis.
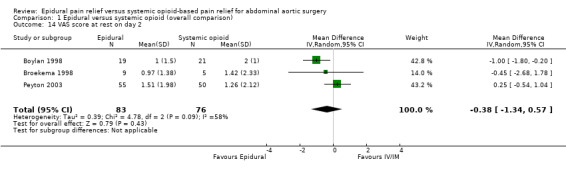
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 14 VAS score at rest on day 2.
1.15. Analysis.
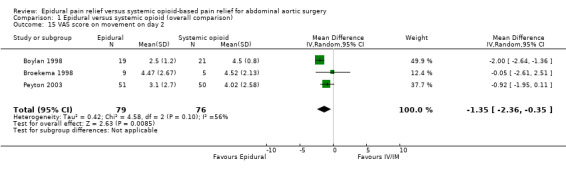
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 15 VAS score on movement on day 2.
1.16. Analysis.
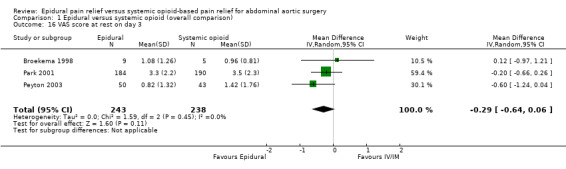
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 16 VAS score at rest on day 3.
1.17. Analysis.
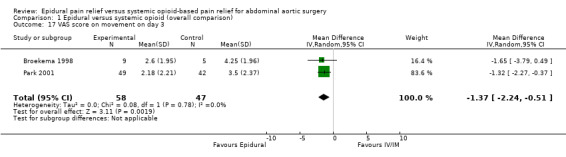
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 17 VAS score on movement on day 3.
Any indicator for postoperative bowel motility: incidence of ileus, time to first bowel sounds, flatus, bowel movement, or time to first drinking or eating
Norris 2001 reported time to postoperative landmarks for feeding and ambulation. They were: first bowel sounds, first flatus, first bowel movements, tolerating clear liquids, tolerating regular diet, and independent ambulation. One author reported on the risk of an ileus after the surgery (Muehling 2009). No significant differences were found among treatment groups.
Any indicator for postoperative mobilization: any type of functionality score or time to first ambulation
Park 2001 reported function scale (0 = unable to perform any tasks to 6 = walk without assistance) on postoperative days one, three, and seven. No significant differences were found among treatment groups.
Length of intensive care unit stay
Based on three trials that included 523 participants, epidural analgesia reduced the ICU length of stay (SMD ‐0.23 (95% CI ‐0.41 to ‐0.06); I2 statistic = 0%; Analysis 1.18) (Davies 1993; Muehling 2009; Park 2001). Egger's regression intercept showed no evidence of a small‐study effect (two‐tailed). Duval and Tweedie's trim and fill analysis showed no evidence of a publication bias. Based on the sole study where mean and SD were available (Davies 1993), the optimal size information for a 25% reduction in the time spent in the ICU would be 522 participants (261 per group) (alpha 0.05, beta 0.2; one‐sided test). The level of evidence for this outcome was moderate (Table 1).
1.18. Analysis.
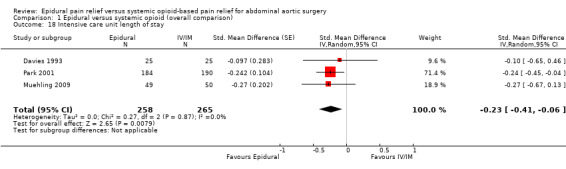
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 18 Intensive care unit length of stay.
Length of hospital stay
Based on five trials that included 676 participants, we did not find a difference in hospital length of stay (SMD ‐0.16 (95% CI ‐0.43 to 0.10); I2 statistic = 55%; Analysis 1.19) (Bois 1997; Davies 1993; Muehling 2009; Norman 1997; Park 2001). Egger's regression intercept showed no evidence of a small‐study effect (two‐tailed). Duval and Tweedie's trim and fill analysis showed no evidence of a publication bias. However, it is possible that effect would be seen only in the more recent trials (Figure 5).
1.19. Analysis.
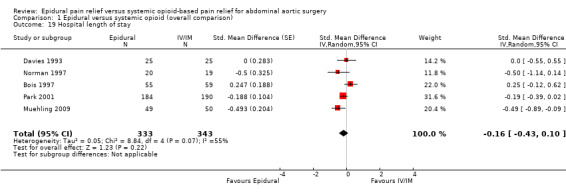
Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 19 Hospital length of stay.
5.
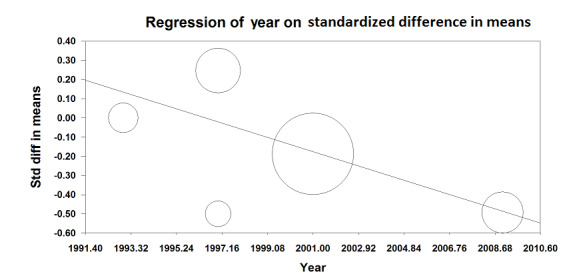
Meta‐regression on the hospital length of stay versus the year where the study was published. The effect seems to be better in the more recent study (P value = 0.04).
Std diff in means: standardized mean difference.
(This meta regression plot was not produced in RevMan. The figure was generated automatically by the software, and cannot be amended. The software has expressed the years as decimals.)
Discussion
Adding an epidural to general anaesthesia does not change the mortality rate at 30 days for people undergoing open abdominal aortic repair (level of evidence low). This is consistent with the results of our overview (Guay 2014a), and with the previous version of this review (Nishimori 2012). However, adding an epidural reduced the rate of myocardial infarction (NNTB 28 (95% CI 19 to 1423); level of evidence moderate). For other interventions (e.g. systematic perioperative administration of beta‐blocking agents or statins), where the intervention reduced myocardial infarction at 30 days, the mortality rate reduction was seen only after a longer period (up to one year) (Guay 2013; Guay 2014b). Here data at one year were very limited. Only one trial gave results for mortality at one year (RR 0.60 (95% CI 0.19 to 1.90)) and the number of participants included in this trial was clearly insufficient to allow us to draw any valid conclusion (Norris 2001). Therefore, studies with a longer follow‐up and a sufficient number of participants are required before a firm conclusion on the effects of adding an epidural to general anaesthesia of people undergoing open abdominal aortic surgery can be drawn.
Adding an epidural to general anaesthesia reduced the time to tracheal extubation (level of evidence low) and postoperative respiratory failure (level of evidence moderate). Therefore it follows that adding an epidural reduced the time spent in the ICU (level of evidence moderate).
We also found a reduction in postoperative gastrointestinal bleeding (level of evidence high). This is consistent with one study showing that people receiving epidural anaesthesia during abdominal aortic reconstruction appeared to have less severe disturbances of sigmoid perfusion compared with people not receiving epidural anaesthesia (Panaretou 2012).
As already clearly demonstrated, epidural analgesia decreased postoperative pain scores and this was particularly evident on pain score on movement. The level of evidence for pain reduction on movement at postoperative day one was high quality.
Summary of main results
Adding an epidural to general anaesthesia for people undergoing abdominal aortic repair reduces VAS scores, time to tracheal extubation, myocardial infarction, postoperative respiratory failure, gastrointestinal bleeding, and time spent in the ICU. We did not find a reduction of mortality (in‐hospital or up to 30 days). The level of evidence was low for mortality and time before tracheal extubation; moderate for myocardial infarction, respiratory failure, and ICU length of stay; and high for gastrointestinal bleeding and VAS scores.
Overall completeness and applicability of evidence
We could not demonstrate a reduction in mortality rate, but the number of participants with an adequate period of follow‐up was clearly insufficient for this outcome. For all other outcomes, we consider that the quality of the studies included in the present review was sufficient to allow us to make conclusions on the effect of adding an epidural to general anaesthesia for people undergoing open abdominal aortic surgery. This is supported by the level of evidence that was rated as moderate quality for a reduction in myocardial infarction, respiratory failure and length of stay in the ICU and high for pain scores and gastrointestinal bleeding . For length of tracheal intubation, there was some inconsistency between the studies and the level of evidence was low. This might be due, in part to a change in practice over the years. Studies included in the present review were published between 1987 and 2009. A change in the clinical practice towards earlier tracheal extubation has clearly taken place during these years.
Quality of the evidence
Table 1 shows the level of evidence.
For mortality, we downgraded the evidence on the basis of risk of bias because seven out of the 14 trials included in the analysis had uncertainty around allocation concealment. There was no inconsistency as measure by a I2 statistic of 0%. Indirectness was not a factor: direct comparison performed on the population at interest and the outcome is not a surrogate marker. We downgraded the level of evidence for imprecision due to a wide CI. Although the possibility of a publication bias was present for this outcome, we did not downgrade the level of evidence on this item because we calculated that applying a correction for the possibility of a publication bias did not change the results. We did not upgrade the evidence based on a large effect size (RR 0.6), We also did not identify confounding factors justifying upgrading the quality of evidence and did not find a dose‐response relationship. We rated the quality of evidence as low.
For myocardial infarction, we downgraded the evidence on the basis of risk of bias because four out of the seven trials included in the analysis had uncertainty around allocation concealment. There was no inconsistency as measure by a I2 statistic of 0%. Indirectness was not a factor: direct comparison performed on the population of interest and the outcome is not a surrogate marker. We downgraded the level of evidence for imprecision because the number of participants included was lower than the optimal information size, fewer than 2000 and the number of events was lower than 400. Although the possibility of a publication bias was present for this outcome, we did not downgrade the level of evidence because we calculated that applying a correction for the possibility of a publication bias did not change the results. We upgraded the level of evidence for this outcome on the basis of a large effect size (RR 0.53). We did not identify confounding factor justifying upgrading the quality of evidence and did not find a dose‐response relationship. We rated the quality of evidence as moderate.
For tracheal intubation, we downgraded the evidence on the basis of risk of bias because four out of the seven trials included in the analysis had uncertainty around allocation concealment. We also downgraded the evidence on the basis of a serious inconsistency (unexplained I2 statistic = 35%). Indirectness was not a factor: direct comparison performed on the population at interest and the outcome was not a surrogate marker. We did not downgrade the level on the basis of imprecision because the optimal information size was achieved. Although the possibility of a publication bias was present for this outcome, we did not downgrade the level of evidence on this item because we calculated that applying a correction for the possibility of a publication bias did not change the results. We did not modify the level of evidence on the basis of the amplitude of the effect size, confounding factors, or dose‐response gradient. We rated the quality of evidence as low.
For respiratory failure, we downgraded the quality of evidence for risk of bias on the basis that there was uncertainty around blinding of the outcome assessor in half or more of the studies. There was no inconsistency as measured by an I2 statistic of 0%. Indirectness was not a factor: direct comparison performed on the population of interest and the outcome was not a surrogate marker. We did not downgrade the level on the basis of imprecision because the optimal information size was achieved. There was no evidence of publication bias. We did not modify the level of evidence on the basis of the amplitude of the effect size, confounding factors, or dose response gradient. We rated the quality of evidence as moderate.
For gastrointestinal bleeding, we downgraded the quality of evidence for risk of bias on the basis that there was uncertainty around allocation concealment and blinding of the outcome assessor in half or more of the studies. There was no inconsistency as measured by an I2 statistic of 0%. Indirectness was not a factor: direct comparison performed on the population of interest and the outcome was not a surrogate marker. We downgraded the level of evidence on the basis of imprecision on the basis of wide CI (95% CI 0.06 to 0.65). Although the possibility of a publication bias was present for this outcome, we did not downgrade the level of evidence on this item because we calculated that applying a correction for the possibility of a publication bias did not change the results. We upgraded the level of the quality of evidence on the basis of a very large effect size (OR 0.20). We did not modify the level of evidence on the basis of confounding factors, or dose‐response gradient. We rated the quality of evidence as high.
For VAS scores on movement at postoperative day one, we found no serious risk of bias. There was no inconsistency as measured by an I2 statistic of 0%. Indirectness was not a factor: direct comparison performed on the population of interest and the outcome was not a surrogate marker. We found no evidence of imprecision. Although the possibility of a publication bias was present for this outcome, we did not downgrade the level of evidence on this item because we calculated that applying a correction for the possibility of a publication bias did not change the conclusion. We upgraded the level of evidence for this outcome on the basis of a large effect size (SMD ‐1.78). We did not modify the level of evidence on the basis of confounding factors or dose‐response gradient. We rated the quality of evidence as high.
For length of stay in the ICU, we downgraded the quality of evidence for risk of bias on the basis of uncertainty for allocation concealment and blinding of the outcome assessor. There was no inconsistency as measured by an I2 statistic of 0%. Indirectness was not a factor: direct comparison performed on the population at interest and the outcome was not a surrogate marker. We found no evidence of imprecision. We found no evidence of a publication bias. We did not modify the level of evidence on the basis of the amplitude of the effect size, confounding factors, or dose‐response gradient. We rated the quality of evidence as moderate.
Potential biases in the review process
We are confident that our search was extensive enough to allow us to include all relevant studies. Additional information provided by some authors also allowed us to include high‐quality unpublished data. Although some of the studies had some item at risk of bias, we think that the quality of included studies is sufficient enough to allow us to draw valid conclusion on our outcomes of interest except for mortality, where studies with a longer period of follow‐up are required, and time to tracheal extubation.
Agreements and disagreements with other studies or reviews
Our results on the absence of effect on the mortality rate at 30 days are consistent with the results of our overview (Guay 2014a), and with the latest previous version of this review (Nishimori 2012). However, as mentioned earlier, for other interventions, such as routine perioperative use of beta‐blocking agents (Guay 2013) or statins (Guay 2014b), a reduction in the rate of myocardial infarction such as seen here may translate into a reduction in the mortality rate only after several months. Therefore, without an appropriate time of follow‐up (up to 30 days only), we cannot conclude on the effect of adding epidural analgesia to general anaesthesia for people undergoing open abdominal aortic surgery for mortality.
Authors' conclusions
Implications for practice.
Compared with systemic opioids, epidural analgesia provided better pain management up to postoperative day three, and reduced time to tracheal extubation, myocardial infarction, postoperative respiratory failure, gastrointestinal bleeding and intensive care unit length of stay. We cannot draw any firm on the effect of adding epidural analgesia to general anaesthesia on the mortality rate of people undergoing open abdominal aortic surgery. These benefits should be balanced with potential risk specific to this population (Hebl 2006; Horlocker 2010).
Implications for research.
For mortality, studies with a longer period of follow‐up (up to one year) could be useful.
Feedback
Feedback to Nishimori 2012
Summary
Summary Analysis 1.12. Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 12 Renal insufficiency) the numbers of the publication Park 2001 are incorrectly stated as "epidural 26/184 ‐ systemic opioid 53/190". Correct would be "epidural 5/184 ‐ systemic opioid 4/190". Following the correction, subtotal and total risk ratio will change. It might also change one of the conclusions, i.e. epidural analgesia would reduce renal complications. Alexander Koch
Reply
I agree with the feedback. The numbers in question is [are] indeed a mistake. The correct number of Park 2001 is 5/184 for epidural group and 4/190 for systemic opioid group. This makes the outcome to be insignificant. The Anaesthesia Group plan to update this review Mina Nishimori
Contributors
Summary author: Dr Alexander Koch. Department of Anesthesiology, Intensive Care and Pain Therapy, University Hospital Frankfurt, Germany. I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback. Reply: Mina Nishimori (lead author)
What's new
| Date | Event | Description |
|---|---|---|
| 3 March 2017 | Amended | Search reran; one potential new study added to awaiting classification (Owczuk 2016) and one to ongoing (Li 2015) |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 5 January 2016 | New search has been performed | One trial (Lombardo 2009) included in the last version (Nishimori 2012) was excluded (quasi‐randomized trial) One new study included (Muehling 2009). |
| 5 January 2016 | New citation required but conclusions have not changed | New authors updated this review (Joanne Guay and Sandra Kopp). Conclusions not changed. |
| 13 March 2014 | Amended | Feedback posted |
| 13 April 2012 | Amended | Erratum corrected. In the plain language summary and abstract, we stated that the duration of intubation after surgery was reduced by roughly 20% in the epidural group. Since this does not correspond with the statement in the result section (48% reduction), we corrected the statement to "approximately half reduction". |
| 13 April 2012 | New search has been performed | In response to the peer reviewers' comments, we amended the following. 1. We reformatted the text according to the recent changes in RevMan.
2. We reworded the text.
3. We revised the statistical aspects of our review.
|
| 13 April 2012 | New search has been performed | We updated our search from 2004 to 2010. We did a full paper review of nine studies from our updated search, and excluded eight (Ali 2010; Beilin 2008; Donatelli 2006; Goldmann 2008; Kawasaki 2007; Murakami 2009; Yarendi 2007; Zhang 2007) and included one (Lombardo 2009a). We also included one study found from our original search (2004), which was waiting for evaluation (de Lis 1990). Two additional papers are awaiting evaluation as they need to be translated (Hu 2006a; Pan 2006a). These new studies did not change our conclusions. |
| 13 April 2012 | New citation required but conclusions have not changed | We included Dr Hui Zheng as our co‐author. |
| 13 April 2012 | New search has been performed | We revised the plain language summary. We also reworded the text. We completed the 'risk of bias tables' and two 'risk of bias figures'. |
| 17 January 2011 | Amended | During the process of updating this review, we found the following errors and corrected them. Erratum corrected: In the text we reported that we found 39 ineligible studies, but in fact, it was 40. There were two duplicate publications and that is why we calculated incorrectly. Erattum corrected: In the previous additional table 3 (now Appendix 4) (definitions of postoperative complications), section of "overall cardiac event" we reported "Yeager 1987" as "Peyton 1987." We corrected it and made a link to the reference. Erattum corrected: Asuero 1990a study characteristics section was completed. We moved the additional tables to the appendices |
| 29 September 2010 | Amended | Contact details updated. |
| 21 June 2008 | Amended | Converted to new review format. |
| 17 May 2006 | Amended | The following changes have been made to the previously published protocol: (1) We edited the wording of the background and methods section. (2) We searched the OVID version of CENTRAL instead of the Cochrane Library CD version of CENTRAL. (3) Because there was a possibility that the search strategy we published in our protocol might miss trials that included aortic abdominal surgery patients as a sub‐group of participants, we removed the search terms indicating aortic abdominal surgery, then did the search again (the amended search strategy is shown in 'Additional Table 02'). |
Notes
Update 2015
We updated the background.
We excluded quasi‐randomized studies.
We amended the interventions (see below).
We rephrased the criteria for inclusion of studies.
We included postoperative epidural analgesia, either lumbar or thoracic. Systemic opioid‐based pain relief included opioid drugs given by the following routes: intravenous, intramuscular, or subcutaneous. Bolus dosing, infusion, or patient‐controlled analgesia devices were eligible for inclusion. For epidural analgesia, we included all combinations of drugs and all start times (pre‐ or postoperatively).
Updated review ‐ Guay 2015
We included intraoperative or postoperative (or both) epidural anaesthesia/analgesia added to general anaesthesia (either lumbar or thoracic) compared to general anaesthesia alone. We included all combinations of drugs and all start times (pre‐ or postoperatively). We imposed no restriction regarding the mode of analgesia used in the control group and included systemic opioid‐based pain relief with opioid drugs given by the following routes: intravenous, intramuscular, or subcutaneous. For both groups, bolus dosing, infusion, or patient‐controlled analgesia devices were eligible for inclusion.
5. We changed the outcomes.
Primary outcomes
Death from all causes within 30 days of surgery, or death from all causes during hospitalization.
Postoperative cardiovascular complication: cardiac death, non‐fatal myocardial infarction, angina, myocardial ischaemia, arrhythmias (supraventricular and ventricular), congestive heart failure, severe hypotensive episode that required treatment.
Postoperative respiratory complications: atelectasis, pneumonia, respiratory failure including prolonged mechanical ventilation or need to reinstate mechanical ventilation.
Postoperative gastrointestinal complication.
Postoperative cerebrovascular complication.
Postoperative renal complication.
Postoperative deep venous thrombosis or pulmonary embolism.
Secondary outcomes
Time to extubation.
Postoperative pain scores (at rest or with movement).
Any indicator for postoperative bowel motility: time to first bowel sounds, flatus, bowel movement, or time to first drinking or eating.
Any indicator for postoperative mobilization: any type of functionality score or time to first ambulation.
Length of intensive care unit (ICU) stay.
Length of hospital stay.
We did not pre‐define these outcomes because definitions for similar outcomes may differ between studies. For example, some studies defined myocardial ischaemia as significant ST change confirmed by electrocardiogram, while others defined it as chest pain requiring nitroglycerin. Rather than defining outcomes strictly and omitting similar outcomes that did not strictly fit the definitions, we noted the definitions used by contributing investigators. Similarly for postoperative pain and mobilization, we noted the chosen measurement tool and whether it was validated or not.
Updated review ‐ Guay 2015
Primary outcomes
Death from all causes within 30 days of surgery, or death from all causes during hospitalization (NOW ONLY ONE PRIMARY OUTCOME).
Secondary outcomes
Postoperative cardiovascular complications: myocardial ischaemia, myocardial infarction, congestive heart failure, ventricular arrhythmias.
Postoperative respiratory complications: tracheal intubation duration, respiratory failure including prolonged mechanical ventilation or need to reinstate mechanical ventilation, pneumonia.
Postoperative cerebrovascular complication.
Postoperative acute kidney injury.
Postoperative gastrointestinal haemorrhage
Postoperative pain scores (at rest or with movement).
Any indicator for postoperative bowel motility: incidence of ileus, time to first bowel sounds, flatus, bowel movement, or time to first drinking or eating.
Any indicator for postoperative mobilization: any type of functionality score or time to first ambulation.
Length of intensive care unit (ICU) stay.
Length of hospital stay.
We did not pre‐define these outcomes but noted the definitions used by contributing investigators.
6. We updated the risk of bias section.
7. We amended the measure of treatment effect section.
8. We changed the statistical methods (in Nishimori 2012 the authors used the Bayesian statistical model).
9. We have changed the subgroup analysis and investigation of heterogeneity section.
10. We changed the sensitivity analysis section.
11. We included a summary of findings table.
12. We excluded one previously included study.
13. We included one new study.
14. We excluded two studies previously awaiting classification.
February 2015
There was an error in Nishimori 2012, Analysis 1.12. Comparison 1 Epidural versus systemic opioid (overall comparison), Outcome 12 Renal insufficiency) the numbers of the publication Park 2001 were incorrectly stated as "epidural 26/184 ‐ systemic opioid 53/190". The correct numbers were: 'epidural 5/184 ‐ systemic opioid 4/190'.
This error was noted, by the feedback function, by Dr Alexander Koch.
This error has been corrected in the updated version.
Authors of the previously published version also had the following acknowledgements (Nishimori 2012):
Dr Peter Choi, Prof Nathan Pace, Dr W Scott Beattie, Dr Soledad Cepeda, Dr Ann Møller, Dr Tom Pedersen, Janet Wale and Kathie Godfrey for their help and editorial advice during the preparation of the protocol and full review;
Prof Stephan Kettner, Dr Cathal Walsh and Prof Nathan Pace for their help and editorial advice during the preparation of the updated review;
Anupa Shah and Carole Foxman for their help in developing search strategies;
Joseph Lau for his comments and advice;
David Shoenfeld for his help in statistical analysis;
the authors of Broekema 1998; Park 2001; Peyton 2003 and Yeager 1987 for kindly providing additional unpublished data; and the following who translated the non‐English studies:
Mr Ivan Solà for translating Fernandez 1990; Dr Oleg Borisenkoor for translating Astakhov 1984; Ajit Kumarfor translating three articles (Borovskikh 1990; Borovskikh 1991a; Borovskikh 1991b); Dr André Muller for translating Thierry 1983; Dr Peter Roos for translating seven articles (Seeling 1985a; Seeling 1985b; Seeling 1986; Seeling 1990; Seeling 1991; Wust 1980; Wust 1982); Dr Bernard Coronel for translating Barre 1989; Dr Karen Hovhannisyan for translating Vabishchevich 1986; Dr Markus Klimek for translating Stelzner 1988 (see Reinhart 1989) and Dr Patrick Brass for translating Brinkmann 1994.
Acknowledgements
We would like to thank Mina Nishimori, James HS Low, Hui Zheng, and Jane C Ballantyne as previous author of this review (Nishimori 2012).
The authors also thank Karen Hovhannisyan who designed the search strategy as well as the University of Quebec in Abitibi‐Temiscamingue and University of Sherbrooke for granting access to electronic data bases and medical journals.
We would also like to thank Stephan Kettner (content editor), Marialena Trivella (statistical editor), and Patricia Tong (consumer referee) for their help and editorial advice during the preparation of this updated systematic review.
Finally, we also thank Mrs. Janne Vendt (Cochrane Information Specialist) who reran the search in March 2017.
Appendices
Appendix 1. The amended search strategy for MEDLINE and Cochrane CENTRAL
The search strategy for Ovid MEDLINE (1966 to July 2004) and Ovid CENTRAL (2004, Issue 3). The original search was performed in 2004 and updated in 2010 and in 2014. 1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized controlled trials.sh. 4. random allocation.sh. 5. double blind method.sh. 6. single blind method.sh. 7. or/1‐6 8. clinical trial.pt. 9. exp clinical trials/ 10. (clin$ adj25 trial$).ti,ab. 11. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 12. placebos.sh. 13. placebo$.ti,ab. 14. random$.ti,ab. 15. research design.sh. 16. or/10‐15 17. comparative study.sh. 18. exp evaluation studies/ 19. follow up studies.sh. 20. prospective studies.sh. 21. (control$ or prospectiv$ or volunteer$).ti,ab. 22. or/17‐21 23. 7 or 16 or 22 24. abdomen/su 25. (abdomin$ or abdomen).ti,ab,sh. 26. (surgery or operat$).ti,ab,sh. 27. 25 and 26 28. 24 or 27 29. analgesia epidural/ 30. exp anesthesia epidural/ 31. (epidural adj2 (analgesi$ or an?esthe$)).ti,ab. 32. 29 or 30 or 31 33. 28 and 32 34. 23 and 33
Appendix 2. Preoperative risk, medication, and prior surgery
| Study ID | Details |
| Barre 1989 | 50% of participants (3/7 vs. 4/7) had ischaemic heart disease. No further information was available |
| Bois 1997 | Preoperative prevalence: angina (16%), prior myocardial infarction (36%), congestive heart failure (2%), diabetes mellitus (11%), hypertension (48%), smoking (69%) History of myocardial re‐vascularization: percutaneous transluminal coronary angioplasty (4%), coronary artery bypass grafting (12%) Preoperative medication: beta‐blockers (21%), calcium channel blockers (36%), nitrates (18%), angiotensin‐converting inhibitors (13%), diuretics (16%) |
| Boylan 1998 | ASA physical status II or III Preoperative prevalence; prior myocardial infarction (15%), coronary artery disease (45%), smoking (65%) Preoperative medication; beta‐blockers (23%), calcium channel blockers (13%), nitrates (10%), angiotensin‐converting inhibitors (15%), diuretics (13%) |
| Broekema 1998 | ASA physical status I, II, or III |
| Davies 1993 | Mean value of ASA physical status was 2.5 for intervention group and 2.4 for the control group Preoperative prevalence: angina (16%), prior myocardial infarction (22%), congestive heart failure (4%), diabetes mellitus (8%), hypertension (50%), smoking (66%), chronic airways disease (42%) |
| de Lis 1990 | Preoperative prevalence: duodenal ulcer (18%), hypertension (31%), ischaemic heart disease (21%), renal insufficiency (creatinine > 3 mg/dL) (13%). No further information was available but the authors mentioned the participants were "high risk" in the discussion section |
| Garnett 1996 | Preoperative prevalence: angina (21%), prior myocardial infarction (23%), congestive heart failure (5%), diabetes mellitus (11%), hypertension (42%), smoking (91%), chronic obstructive pulmonary disease (30%) History of myocardial re‐vascularization: coronary artery bypass grafting (17%) |
| Kataja 1991 | ASA physical status III Preoperative prevalence: angina (40%), prior myocardial infarction (35%), congestive heart failure (10%), hypertension (40%), bronchial asthma (5%), prior stroke (5%) Preoperative medication: beta‐blockers (35%), calcium channel blockers (20%), nitrates (40%), angiotensin‐converting inhibitors (10%), diuretics (10%), digoxin (10%) |
| Muehling 2009 | ASA physical status II‐IV |
| Norman 1997 | Participants underwent "uncomplicated" surgery. No further information was available |
| Norris 2001 | Preoperative prevalence: angina (36%), prior myocardial infarction (26%), congestive heart failure (12%), diabetes mellitus requiring medication (4%), hypertension (63%), renal insufficiency (24%), prior stroke (11%), smoking (90%) History of myocardial re‐vascularization: percutaneous transluminal coronary angioplasty (12%), coronary artery bypass grafting (23%) Preoperative medication: beta‐blockers (26%), calcium channel blockers (40%), nitrates (7%), angiotensin‐converting inhibitors (20%), diuretics (21%), digoxin (13%), aspirin (33%) |
| Park 2001 | Preoperative information was available as the data that represented all the participants, including other types of surgery ASA physical status III or IV (91% were III), Goldman risk index: 0‐5 (49%), 6‐12 (44%), 13‐26 (8%) Preoperative prevalence: angina (14%), prior myocardial infarction (27%), congestive heart failure (10%), diabetes mellitus (22%), hypertension (56%), chronic obstructive pulmonary disease (32%), renal failure (1%), prior stroke (14%), smoking (38%) prior alcoholism (20%), alcoholic liver disease (2%) |
| Peyton 2003 | Authors set an eligibility criteria to exclusively include high‐risk participants Preoperative information was available as the data that represented all the participants, including other types of surgery Preoperative prevalence: angina (20%), myocardial ischaemia (27%), prior myocardial infarction within 2 years (14%), congestive heart failure (12%), diabetes mellitus (44%), severe hepatocellular disease (7%), morbid obesity (4%) |
| Reinhart 1989 | ASA physical status I, II, or III |
| Yeager 1987 | Mean values of ASA physical status and Goldman Index were 2.8 and 9.1 for intervention group (vs. 2.8 and 7.3 for the control group). Eligibility criteria for participating in the study included being scheduled preoperatively by the surgical staff to receive postoperative care in an intensive care unit due either to the severity of pre‐existing disease(s), the magnitude of the anticipated surgical procedure, or both |
| ASA: American Society of Anesthesiologists | |
Appendix 3. Method of anaesthesia during surgery and postoperative analgesia
| Study ID | Group name | Allocation | n | Surgical anaesthesia | Anaesthesia agents | Postoperative analgesia | Analgesia drugs | Duration (postoperative) |
| Barre 1989 | Thoracic epidural anaesthesia | Intervention | 7 | General anaesthesia + epidural |
Nitrous oxide, fentanyl, isoflurane, and vecuronium Epidural lidocaine 2% + bupivacaine 0.5% (14 mL before the surgery + intraoperative infusion at 5 mL/hour) |
Thoracic epidural (level T7) | Not given | Not given |
| Barre 1989 | Opioids | Control | 7 | General anaesthesia | Nitrous oxide, fentanyl, isoflurane, and pancuronium | "Morphinic sedation" The route and method of administration was not clearly stated | Not given | Not given |
| Bois 1997 | Thoracic epidural analgesia | Intervention | 55 | General anaesthesia | Nitrous oxide, fentanyl, isoflurane, and vecuronium | Thoracic epidural (level T6‐7 or T7‐8) | Bupivacaine 0.125% + fentanyl 10 μg/mL Bolus of 0.1 mL/kg of body weight at the end of the surgery + infusion at 0.1 mL/kg/hour Rate adjusted according to pain scores |
48 hours |
| Bois 1997 | IV PCA | Control | 59 | General anaesthesia | Nitrous oxide, fentanyl, isoflurane, and vecuronium | IV PCA | Morphine | 48 hours |
| Boylan 1998 | Lumbar epidural anaesthesia/analgesia | Intervention | 19 | General anaesthesia + epidural |
Nitrous oxide, fentanyl, and isoflurane Epidural lidocaine 2% 10 mL + bupivacaine 0.25% for the surgery Epidural morphine at the end of the surgery |
Lumbar epidural (level L2‐3 or 3‐4) | Infusion of bupivacaine 0.125% + morphine 0.1 mg/mL at 4 mL/hour Rate adjusted according to pain scores |
At least 48 hours |
| Boylan 1998 | IV PCA | Control | 21 | General anaesthesia | Nitrous oxide, fentanyl, and isoflurane IV morphine at the end of surgery |
IV PCA | Morphine | At least 48 hours |
| Broekema 1998 | Thoracic epidural anaesthesia/analgesia (sufentanil) | Intervention | 3 | General anaesthesia + epidural |
Nitrous oxide, sufentanil, isoflurane, and vecuronium Epidural bupivacaine 0.125% 10 mL + sufentanil 50 μg Infusion of bupivacaine 0.125% + sufentanil 1 μg/mL at 6‐10 mL/hour |
Thoracic epidural (level T7‐8 or 8‐9) | Infusion of bupivacaine 0.125% + sufentanil 1 μg/mL at 6‐10 mL/hour Rate adjusted according to pain scores |
Started tapering on postoperative day 3 |
| Broekema 1998 | Thoracic epidural anaesthesia/analgesia (morphine) | Intervention | 6 | General anaesthesia + epidural |
Nitrous oxide, sufentanil, isoflurane, and vecuronium Epidural bupivacaine 0.125% 10 mL + morphine 5 mg Infusion of bupivacaine 0.125% + morphine 0.05 mg/mL at 6‐10 mL/hour |
Thoracic epidural (level T7‐8 or 8‐9) | Infusion of bupivacaine 0.125% + morphine 0.05 mg/mL at 6‐10 mL/hour Rate adjusted according to pain scores |
Started tapering on postoperative day 3 |
| Broekema 1998 | Intramuscular | Control | 5 | General anaesthesia | Nitrous oxide, sufentanil, isoflurane, and vecuronium | Intramuscular morphine | Every 4 hours | Intervals increased after the third postoperative day |
| Davies 1993 | Thoracic epidural anaesthesia/analgesia | Intervention | 25 | General anaesthesia + epidural |
Nitrous oxide, fentanyl, enflurane, and pancuronium Epidural lignocaine 1.5% 5 mL each hour during the surgery |
Lower thoracic epidural (level usually T9‐10) | Infusion of bupivacaine 0.5% | 3 days |
| Davies 1993 | IV analgesia | Control | 25 | General anaesthesia | Nitrous oxide, fentanyl, enflurane, and pancuronium | IV analgesia | IV morphine at 2‐5 mg/hour | 3 days |
| de Lis 1990 | Epidural analgesia | Intervention | 19 | General anaesthesia | No information | Epidural (level is unclear). Inserted under general anaesthesia at the end of the surgery | Epidural morphine 4 mg every 12 hours | Mean 2.8 days |
| de Lis 1990 | IV analgesia | Control | 20 | General anaesthesia | No information | IV bolus | IV morphine (mean 5 mg/day) + IV magnesium (mean 6 g/day) |
Mean 2.8 days |
| Garnett 1996 | Lumbar epidural anaesthesia/analgesia | Intervention | 48 | General anaesthesia + epidural |
Isoflurane, fentanyl, and pancuronium Epidural lidocaine 2% + meperidine 2 mg/mL 10‐15 mL + intraoperative infusion at 5‐10 mL/hour |
Lumbar epidural (level T12‐L1) | Infusion of bupivacaine 0.1% + meperidine 2 mg/mL at 5‐15 mL/hour Rate adjusted according to pain scores |
41‐46 hours |
| Garnett 1996 | IV analgesia | Control | 51 | General anaesthesia | Isoflurane, fentanyl, and pancuronium | IV infusion | IV morphine 2‐10 mg/hour | ‐ |
| Kataja 1991 | Lumbar epidural anaesthesia/analgesia | Intervention | 10 | General anaesthesia + epidural |
Nitrous oxide, isoflurane, fentanyl, and pancuronium Epidural bupivacaine 0.5% 14 mL + 3‐4 mL every 90‐100 minutes |
Lumbar epidural (level T12‐L1) | Infusion of bupivacaine 0.25% + fentanyl 5 μg/mL at 7 mL/hour Rate adjusted on pain scores values |
Unclear |
| Kataja 1991 | IV analgesia | Control | 10 | General anaesthesia | Nitrous oxide, isoflurane, fentanyl, and pancuronium | IV bolus, as needed | IV oxycodone 3‐5 mg on request | Unclear |
| Muehling 2009 | Thoracic epidural anaesthesia/analgesia | Intervention | 49 | General anaesthesia + epidural |
General anaesthesia (no details) Ropivacaine 1% 10 mL preoperatively |
Thoracic epidural (level between T7 and T10) |
Patient‐controlled epidural analgesia Ropivacaine 0.2% + sufentanil 2 μg/mL |
Unclear |
| Muehling 2009 | IV PCA | Control | 50 | General anaesthesia | General anaesthesia (no details) | IV PCA | Piritramide | ‐ |
| Norman 1997 | Thoracic epidural anaesthesia/analgesia | Intervention | 20 | General anaesthesia + epidural |
Nitrous oxide, enflurane, fentanyl, and pancuronium Epidural (drug is not clear) |
Thoracic epidural (level T9‐10, 10‐11) | Bupivacaine for T4 sensory level | At least 48 hours |
| Norman 1997 | IV PCA | Control | 19 | General anaesthesia | Nitrous oxide, enflurane, fentanyl, and pancuronium | IV PCA | Morphine | ‐ |
| Norris 2001 | Thoracic epidural anaesthesia | Intervention | 39 | General anaesthesia + epidural |
Nitrous oxide, enflurane, fentanyl, and pancuronium. Epidural bupivacaine 0.5% 6‐8 mL + fentanyl 50 μg + bupivacaine 0.125% with fentanyl 5 μ/mL at 6 mL/hour |
Thoracic epidural (level T8‐9 or 10‐11) | IV PCA with fentanyl | Mean duration 78 hours |
| Norris 2001 | Thoracic epidural analgesia | Intervention | 38 | General anaesthesia + epidural |
Nitrous oxide, enflurane, fentanyl, and pancuronium | Thoracic epidural (level T8‐9 or 10‐11) | Epidural PCA with bupivacaine 0.0625% and fentanyl 5 μg/mL | Mean duration 78 hours |
| Norris 2001 | Thoracic epidural anaesthesia/analgesia | Intervention | 46 | General anaesthesia + epidural |
Nitrous oxide, enflurane, fentanyl, and pancuronium Epidural bupivacaine 0.5% 6‐8 mL + fentanyl 50 μg + bupivacaine 0.125% with fentanyl 5 μg/mL at 6 mL/hour |
Thoracic epidural (level T8‐9 or 10‐11) | Epidural PCA with bupivacaine 0.0625% and fentanyl 5 μg/mL | Mean duration 79 hours |
| Norris 2001 | IV PCA | Control | 37 | General anaesthesia | Nitrous oxide, enflurane, fentanyl, and pancuronium | IV PCA | IV PCA with fentanyl | Mean duration 81 hours |
| Park 2001 | Epidural anaesthesia/analgesia | Intervention | 184 | General anaesthesia + epidural |
Nitrous oxide, isoflurane, fentanyl, and vecuronium Epidural bupivacaine 0.5% with epinephrine for a sensory level of T6 or higher before the surgery + 3‐5 mL every 3‐5 hours |
Epidural (thoracic or lumbar). "The epidural level sought was Th 6, and this was achieved in about 85% of the patients with a block" | Epidural morphine 3‐6 mg every 12‐24 hours or as an infusion | Mean duration 55 hours (from data of all participants in the trial) |
| Park 2001 | Systemic opioids | Control | 190 | General anaesthesia | Nitrous oxide, isoflurane, fentanyl, and vecuronium | IV boluses or IV PCA or intramuscular (7%) | Morphine or meperidine | ‐ |
| Peyton 2003 | Epidural anaesthesia/analgesia | Intervention | 86 | General anaesthesia + epidural |
General anaesthesia with inhalational agents Epidural bupivacaine or ropivacaine |
Epidural (at a level that would provide epidural block 2 spinal segments above the upper end of the patient's wound) | Infusion of bupivacaine or ropivacaine + fentanyl or pethidine | Intended for ≥ 72 hours (from all participants 225/447 fully compliant to the protocol) |
| Peyton 2003 | IV opioids | Control | 78 | General anaesthesia | General anaesthesia with inhalational agents | Participant or physician controlled IV opioid infusion | Pethidine or fentanyl | ‐ |
| Reinhart 1989 | Thoracic epidural anaesthesia/analgesia | Intervention | 35 | General anaesthesia + epidural |
Nitrous oxide, midazolam, and pancuronium Epidural bupivacaine 0.5% 9‐12 mL + 4.5‐6 mL every 90 minutes |
Thoracic epidural (level T7‐8 or T8‐9) for a sensory level from L2‐3 to T3‐5 | Bupivacaine 0.25% in amounts sufficient to keep the sensory level above T5 | The trial observed participants 24 hours postoperatively |
| Reinhart 1989 | IV analgesia (without inhalational agent during the surgery) | Control | 40 | General anaesthesia | Nitrous oxide, fentanyl, droperidol, and pancuronium | IV bolus as needed | Piritramide | ‐ |
| Reinhart 1989 | IV analgesia (with inhalational agent during the surgery) | Control | 30 | General anaesthesia | Nitrous oxide, halothane, and pancuronium | IV bolus as needed | Piritramide | ‐ |
| Yeager 1987 | Epidural anaesthesia/analgesia | Intervention | 11 | General anaesthesia + epidural |
Nitrous oxide, opioids, and neuromuscular blocking agents Epidural with either bupivacaine 0.75% or lidocaine 1.5% with epinephrine 1 : 200,000 in amounts sufficient to achieve and maintain surgical anaesthesia and muscle relaxation |
Low thoracic or high lumbar epidural | Analgesic concentrations of local anaesthetics narcotics, or both | Mean duration 31 hours (range: 8‐79 hours) (while participants were in ICU) |
| Yeager 1987 | Systemic opioids | Control | 12 | General anaesthesia | Nitrous oxide, fentanyl, and neuromuscular blocking agents or nitrous oxide, inhalational agent (≤ 1.0 minimal alveolar concentration), fentanyl, and neuromuscular blocking agents |
Parenteral narcotics as required for pain relief in the intensive care unit, where 1‐to‐1 or 1‐to‐2 nursing assignments allowed for frequent evaluation of analgesic requirement and immediate administration of titrated doses of analgesics | Parenteral narcotics | While the participants were in ICU |
| ICU: intensive care unit; IV: intravenous; PCA: patient‐controlled analgesia. | ||||||||
Appendix 4. Definitions of postoperative complications
| Outcome | Study ID | Definition |
| Postoperative mortality | Bois 1997 | Cardiac death occurring during the postoperative hospitalization |
| Boylan 1998 | No definition. "No patient died." Taken as in hospital | |
| Broekema 1998 | No definition. They report 2 deaths on day 3 and day 8, but they were not in the aortic surgery subgroup (information from authors). Taken as in hospital | |
| Davies 1993 | No definition. "Two immediate postoperative deaths in the epidural group and one postoperative death because of sepsis in control group". Taken as in hospital | |
| de Lis 1990 | No definition. Authors described 2 deaths in control group. 1 with aspiration on day 4 and the other with acute mesenteric ischaemia (date not shown). Taken as in hospital | |
| Garnett 1996 | Death which occurred in the hospital | |
| Kataja 1991 | No definition. 1 death in hospital. "No mortality was seen within postoperative 30 days" (information from authors) | |
| Muehling 2009 | Death during hospital stay | |
| Norman 1997 | No definition. "There was no perioperative deaths." Taken as in hospital | |
| Norris 2001 | Hospital mortality. Plus death at 1 year after the surgery | |
| Park 2001 | Participant who died within 30 days after surgery | |
| Peyton 2003 | Death from any cause within 30 days of surgery | |
| Reinhart 1989 | Hospital mortality | |
| Yeager 1987 | Death that occurred in the hospital | |
| Myocardial ischaemia | Bois 1997 | Prolonged ischaemia; a new ST segment or T wave abnormality (ST segment depression > 1 mL on at least 2 consecutive daily 12‐leads electrocardiogram recordings |
| Boylan 1998 | Horizontal or downsloping ST segment depression of ≥ 1 mm extending at least 60 mm seconds beyond the J point and lasting ≥ 60 seconds | |
| Garnett 1996 | ST‐segment depression > 1 mm measured at 80 mm seconds beyond the J point or an elevation of 2 mm at 60 mm seconds beyond the J point that lasted > 60 seconds as recorded on the Holter monitor | |
| Muehling 2009 | Myocardial ischaemia was suspected and documented if 2 of the following signs were noticed: chest pain, electrocardiographic changes, elevated heart enzymes | |
| Norris 2001 | Reversible ST segment depression (downward or horizontal sloping) ≥ 1 mm below baseline, or ST segment elevation ≥ 2 mm above baseline at least 1 lead, lasting ≥ 60 seconds, and documented by continuous Holter monitoring | |
| Myocardial infarction | Bois 1997 | New Q waves of ≥ 0.04 second duration and a ≥ 1 mm depth on 12‐leads electrocardiogram, or creatine phosphokinase‐MB > 50 international unit/L |
| Boylan 1998 | Not pre‐defined. They reported 2 cases who developed postoperative myocardial infarction (1 case with non‐fatal myocardial infarction and cardiogenic shock in control group, and 1 case with arm pain and elevated cardiac enzymes in epidural group) | |
| Davies 1993 | Transmural myocardial infarction: new Q waves of Ͱ 0.04 second duration and ≥ 1 mm in amplitude Non‐transmural myocardial infarction: increased creatine phosphokinase‐MB enzyme levels considered to be diagnostic of myocardial damage with or without electrocardiographic changes Recent myocardial infarction at autopsy |
|
| Garnett 1996 | New Q waves on the electrocardiogram or ST segment depression on the electrocardiogram accompanied by an increase in creatine phosphokinase‐MB > 5% and a minimum of 15 international unit/L | |
| Norris 2001 | New Q waves of ≥ 0.04 second duration and a ≥ 1 mm depth on 12‐leads electrocardiogram, or ischaemic electrocardiogram changes associated with an increase in creatine phosphokinase with a > 5% MB fraction | |
| Park 2001 | An increase in the serum concentration of creatine phosphokinase‐MB and lactic dehydrogenase, as evidenced by a ratio of creatine phosphokinase‐MB/creatine phosphokinase being ≥ 5%, with or without the following electrocardiogram changes; a typical new persistent elevation/depression of the ST‐segment with or without a new Q wave of > 0.04 seconds in duration with its depth > 25% of the amplitude of the succeeding R wave in limb leads, or any new Q wave in V1‐V3 | |
| Yeager 1987 | Transmural myocardial infarction: new Q waves of ≥ 0.04 second duration and ≥ 1 mm in amplitude Non‐transmural myocardial infarction: increased lactic acid deshydrogenase enzyme and creatine phosphokinase‐MB enzyme levels considered to be diagnostic of myocardial damage with or without electrocardiographic changes Recent myocardial infarction at autopsy |
|
| Congestive heart failure | Bois 1997 | Pulmonary congestion, classic chest X‐ray changes, and (if catheter was placed) pulmonary capillary wedge pressure > 18 mm Hg |
| Davies 1993 | Pulmonary capillary wedge pressure > 20 mm Hg and classic X‐ray changes, or 1 of above with lung rales or S3 gallop ausculation | |
| Garnett 1996 | Clinical diagnosis (rales, increased pulmonary artery pressure requiring intervention with inotropes or venodilators) and supportive X‐ray findings | |
| Norris 2001 | Clinical diagnosis based on the presence of rales, increased pulmonary capillary wedge pressure, S3 gallop, classic chest X‐ray changes that requires intervention with inotropes or ventilators. Chest X‐ray without clinical signs not included | |
| Park 2001 | Newly developed or significantly worsened congestive heart failure: dyspnoea, basilar rales on lung ausculation with or without an S3 gallop, and confirmation by X‐ray changes of pulmonary congestion with or without a pulmonary capillary wedge pressure > 20 mm Hg, requiring pharmacological therapy | |
| Yeager 1987 | New appearance of classic chest X‐ray changes and a pulmonary artery occlusion pressure > 20 mm Hg, or 1 of the foregoing in conjunction with 1 of the following; the new finding of rales on lung ausculation, S3 gallop on cardiac ausculation, or cardiogenic shock (cardiac index < 2 L/minute/m2 for > 2 hours, despite attempts at correction) | |
| Ventricular tachycardia fibrillation | Bois 1997 | Ventricular tachyarrhythmia: documented ventricular tachycardia; > 5 beats) or ventricular fibrillation on the Holter monitoring |
| Davies 1993 | Ventricular tachycardia or fibrillation | |
| Norris 2001 | Ventricular tachyarrhythmia: documented ventricular tachycardia or ventricular fibrillation | |
| Park 2001 | Persistent ventricular tachyarrhythmia: ventricular tachycardia that lasted > 30 seconds and required pharmacological therapy | |
| Acute respiratory failure | Davies 1993 | Postoperative ventilation for > 24 hours or need to re‐intubate |
| Garnett 1996 | Continued ventilation beyond 12 hours on the day after surgery | |
| Norris 2001 | The need for intubation and mechanical ventilation > 24 hours postoperatively, or need for re‐intubation and mechanical ventilation | |
| Park 2001 | The need for intubation and mechanical ventilation for > 24 hours postoperatively or the need for re‐intubation and mechanical ventilation after 1 hour postoperatively | |
| Peyton 2003 | Mechanical ventilation for a total > 24 hours, either continuously or discrete episodes | |
| Yeager 1987 | The need for intubation and mechanical ventilation > 24 hours postoperatively, or need for re‐intubation and mechanical ventilation | |
| Pneumonia | Boylan 1998 | No definition |
| Davies 1993 | Infiltrations on chest X‐ray + 2 of the following; body temperature > 38 °C, raised white blood cell count, positive sputum culture | |
| Garnett 1996 | Clinical findings suggestive of diagnosis (body temperature, positive sputum culture, pulmonary signs on physical examination) + radiological findings | |
| Muehling 2009 | Pneumonia was confirmed if the participant showed clinical and radiological signs of infection (body temperature > 38 °C, infiltration on chest X‐ray films) that required administration of antibiotics | |
| Norris 2001 | The new appearance of an infiltrate on chest X‐ray combined with the appearance of 2 of the following conditions within 24 hours of the X‐ray abnormality; body temperature > 38.5 °C, leukocyte count > 10,000/mL3, or the identification of a pathogen by sputum gram stain culture | |
| Park 2001 | The presence of a new infiltrate on the chest X‐ray, + 2 of the following 3 clinical findings (a body temperature > 38 °C, an abnormal elevation of white blood cell count, a pathogen identified in the sputum by gram stain and culture), requiring intravenous antibiotic treatment | |
| Peyton 2003 | New chest X‐ray infiltrate + ≥ 2 of the following; body temperature > 38 °C, white blood cell count > 12,000/mL3, positive sputum culture | |
| Yeager 1987 | The new appearance of an infiltrate on chest X‐ray combined with the new appearance of 2 of the following; body temperature > 38.5 °C, an abnormal elevation of white blood cell count, or the identification of a pathogen by sputum gram stain culture | |
| Cerebrovascular complication | Davies 1993 | No definition |
| Garnett 1996 | Stroke: trial authors stated they pre‐defined this outcome, but definition was not found in the text | |
| Norris 2001 | Neurological deficit: new focal neurological deficit | |
| Park 2001 | New cerebral hypoxia/thrombosis/intracranial haemorrhage: the occurrence of new neurologic dysfunction (hemiplegia, hemi‐anaesthesia, hemianopia, or unconsciousness) | |
| Acute kidney injury | Davies 1993 | Postoperative rise in creatinine of 150 μmol/L |
| de Lis 1990 | Polyuric renal insufficiency | |
| Garnett 1996 | Doubling of the serum creatinine concentration at any time in the postoperative period | |
| Muehling 2009 | Urine output was < 0.5 mL/kg per hour or if serum creatinine showed an absolute acute increase in the postoperative course of at least 44 μmol/L, leading to additional volume substitution and administration of diuretics (furosemide) | |
| Norman 1997 | Mild high output renal failure (creatinine > 3.0 mg/dL) without the need of dialysis | |
| Norris 2001 | Renal failure was defined as any postoperative increase in serum creatinine of ≥ 2.0 mg/dL | |
| Park 2001 | A serum creatinine of > 3.0 mg/dL and doubling of baseline value, or the need for dialysis | |
| Peyton 2003 | Rise in serum creatinine of > 100 μmol/L, alkaline phosphatase Ͱ 3 times upper limit of normal, and either lactate dehydrogenase or aspartate transaminase to > 2 times upper limit of normal in the absence of upper abdominal surgery | |
| Yeager 1987 | A postoperative increase of serum creatinine of > 2.0 mg/dL | |
| GI haemorrhage | Boylan 1998 | No definition |
| Davies 1993 | Gastrointestinal bleeding: appearance of blood on nasogastric lavage or rectally, with fall in haemoglobin of ≥ 2 g/dL | |
| Park 2001 | The sudden appearance of frank blood either on nasogastric lavage or per rectum, with a subsequent fall in haemoglobin of ≥ 2 g/dL, with no other known or suspected source of ongoing blood loss | |
| Ileus | Muehling 2009 | Functional bowel obstruction (paralytic ileus) was confirmed if vomiting was present or if the person was unable to take oral food and horizontal fluid levels were documented on erect abdominal x‐ray film |
Appendix 5. Extubation
| Study ID | Extubation criteria | Immediate extubation | Intervention (minutes) | Control (minutes) | Summary | Note |
| Barre 1989 | Participants were extubated "if spontaneous ventilation was satisfactory" | Unclear | Mean: 83, SD: 35 | Mean: 310, SD: 85 | Favours intervention | ‐ |
| Bois 1997 | Weaning and tracheal extubation were performed according to the attending anaesthesiologist and were not controlled by protocol | ‐ | Mean: 636, SD: 450 | Mean 774, SD: 462 | Favours intervention | ‐ |
| Boylan 1998 | Extubation was done in surgical intensive care unit, guided by the unit criteria. Weaning initiated if haemodynamically stable, awake, co‐operative, able to generate adequate vital capacity and negative inspiratory force | Not attempted | Mean: 402, SD: 288 | Mean: 780, SD: 498 | Favours intervention | ‐ |
| Broekema 1998 | Time of extubation at the discretion of the intensive care unit physician | ‐ | ‐ | ‐ | Favours control | Extracted as Cohen's and 95% confidence interval from figure of last version (Nishimori 2012) |
| Norris 2001 | Participants were extubated in intensive care unit. Extubations were controlled by study protocol. Weaning was initiated when rectal temperature > 36 °C, no evidence of active bleeding, fluid requirement < 250 mL/hour, participants could follow simple commands | Not attempted | ‐ | ‐ | Favours intervention | Extracted as number and P values for epidural analgesia vs. intravenous analgesia (P value = 0.03) and for epidural anaesthesia/analgesia vs. intravenous analgesia (P value = 0.002) |
| Park 2001 | Extubation criteria included the ability to generate inspiratory negative pressure > 20 cm H2O and stable cardiopulmonary states. Every effort was made to extubate participants as early as possible | Attempted | ‐ | ‐ | Favours intervention | Extracted as numbers and P value (P value = 0.01) |
| Peyton 2003 | Trial protocol provided guidelines for immediate postoperative management. Extubate as early as possible where vital capacity > 10 mL/kg and body temperature > 35 °C with stable haemodynamic states | Attempted | Mean: 395.4, SD: 946 | Mean: 729, SD: 1094 | Favours intervention | Length of initial intubation |
| Reinhart 1989 | Participants were extubated in intensive care unit. Participants were extubated when they had adequate spontaneous breathing and normal blood gas tensions | Not attempted | Mean: 162, SD: 138 | Mean: 120, SD: 114
(with inhalational agent) Mean: 282, SD: 132 (without inhalational agent) |
Extubation time for thoracic epidural group (intervention) was shorter than without inhalational agent group (control), but longer than with inhalational agent group (control) | ‐ |
| SD: standard deviation. | ||||||
Appendix 6. Pain assessment on verbal/visual analogical pain scale
| Study ID | Type of pain | Evaluation | Result |
| Boylan 1998 | Pain at rest and pain on movement | Every 4 hours until 48 hours postoperatively | Significantly lower pain in intervention group at every time point for both types of pain |
| Bois 1997 | Pain at rest | Every 4 hours until 24 hours postoperatively | Significantly lower pain in intervention group at every time point |
| Norris 2001 | Least pain, pain now, and pain with vigorous cough | 07.00 hours, 13.00 hours, and 19.00 hours for first 3 days postoperatively and then daily to postoperative day 7 | No differences over time among the 4 treatment groups for every type of pain |
| Park 2001 | Pain at rest | Postoperative day 1, 3, and 7 or the day of discharge | Significantly lower pain in intervention group on postoperative day 1. No difference for day 3 and day 7 |
| Peyton 2003 | Pain at rest and pain after cough | Morning and afternoon of postoperative day 1, day 2 and day 3 | Data provided by the authors Results favoured treatment group |
Data and analyses
Comparison 1. Epidural versus systemic opioid (overall comparison).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative mortality | 14 | 1383 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.60, 1.86] |
| 1.1 In‐hospital | 11 | 825 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.43, 2.00] |
| 1.2 30 days' follow‐up | 3 | 558 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.84] |
| 2 Myocardial ischaemia | 5 | 503 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.79, 1.40] |
| 3 Myocardial infarction | 7 | 851 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.30, 0.97] |
| 4 Congestive heart failure | 6 | 811 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.44, 1.35] |
| 5 Ventricular arrhythmia | 4 | 689 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.20, 1.51] |
| 6 Tracheal intubation time | 8 | 975 | Std. Mean Difference (Random, 95% CI) | ‐0.42 [‐0.70, ‐0.15] |
| 7 Acute respiratory failure | 6 | 861 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.56, 0.85] |
| 8 Pneumonia | 8 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.37, 1.04] |
| 9 Cerebrovascular complication | 4 | 674 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.12, 0.93] |
| 10 Acute kidney injury | 9 | 1039 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.59, 1.53] |
| 11 Gastrointestinal haemorrhage | 4 | 487 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.20 [0.06, 0.65] |
| 12 Visual analogue scale (VAS) score at rest on day 1 | 5 | 655 | Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.46, ‐0.25] |
| 13 VAS score on movement on day 1 | 3 | 162 | Mean Difference (IV, Random, 95% CI) | ‐1.78 [‐2.32, ‐1.25] |
| 14 VAS score at rest on day 2 | 3 | 159 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.34, 0.57] |
| 15 VAS score on movement on day 2 | 3 | 155 | Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐2.36, ‐0.35] |
| 16 VAS score at rest on day 3 | 3 | 481 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.64, 0.06] |
| 17 VAS score on movement on day 3 | 2 | 105 | Mean Difference (IV, Random, 95% CI) | ‐1.37 [‐2.24, ‐0.51] |
| 18 Intensive care unit length of stay | 3 | 523 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.41, ‐0.06] |
| 19 Hospital length of stay | 5 | 676 | Std. Mean Difference (Random, 95% CI) | ‐0.16 [‐0.43, 0.10] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barre 1989.
| Methods | Randomized controlled trial | |
| Participants | 14 participants (no information on gender) undergoing surgery for infra‐renal abdominal aortic disease (stenotic atherosclerotic disease or aneurism) were randomized Inclusion criteria not clearly stated | |
| Interventions |
Treatment group: thoracic epidural analgesia (T7) with lidocaine 2% and bupivacaine 0.5% for the surgery (14 mL followed by 5 mL/h) (n = 7). The exact duration of epidural analgesia was unspecified Control group: postoperative IV morphine, both with bolus injections (n = 7) General anaesthesia for all participants (isoflurane, fentanyl, and pancuronium) |
|
| Outcomes | Tracheal intubation duration | |
| Notes | No drop‐outs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "type of anaesthesia decided following a random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Measurements mentioned in the methods section were provided in the results section |
| Care program identical | Low risk | Yes |
| Treatment/control groups comparative at entry | Low risk | Groups well balanced |
| Results provided as intention‐to‐treat | Low risk | No drop‐outs |
Bois 1997.
| Methods | Randomized controlled trial Approved by the ethics committee and informed consents obtained |
|
| Participants | 124 participants scheduled for elective abdominal aortic surgery were randomized. Ten were excluded after randomization. 114 participants (88 men, 27 women) gave outcomes Exclusion: people with a preoperative 12‐lead ECG that was uninterpretable for ischaemia (left bundle‐branch block, left ventricular hypertrophy, atrial fibrillation, digitalis, or paced rhythm), people with contraindications to thoracic epidural anaesthesia, and people with a left ventricular ejection fraction ≤ 35% |
|
| Interventions |
Treatment group: thoracic epidural analgesia (T6‐7 or T7‐8) with bupivacaine 0.125% + fentanyl 10 μg/mL; bolus followed by an infusion started at 0.1 mL/kg/h and adjusted according to VAS scores for 48 h (n = 55) Control group: IV morphine PCA for 48 h (n = 59) All participants had general anaesthesia with isoflurane, fentanyl, and vecuronium |
|
| Outcomes | Mortality (cardiac) during hospitalization Myocardial ischaemia MI CHF VT/VF VAS pain score at rest at postoperative day 1 Time to extubation Hospital stay |
|
| Notes | 10 participants were excluded after randomization because of failure of Holter monitoring or epidural analgesia, or the use of analgesia not included in the protocol. 1 participant who had dural puncture during epidural catheter insertion received PCA but was included in the epidural group. Published data showed total 114, but when numbers of men and women added the total becomes 115 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospective randomized controlled study" No information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/65 participants in control group and 4/59 participants in the intervention group were excluded after randomization. Missing outcome number balanced across intervention and control groups, and well described |
| Selective reporting (reporting bias) | Low risk | Measurements mentioned in the methods section were provided in the results section |
| Care program identical | Low risk | Yes |
| Treatment/control groups comparative at entry | Low risk | Groups well balanced |
| Results provided as intention‐to‐treat | High risk | Not in intention‐to‐treat |
Boylan 1998.
| Methods | Randomized controlled trial Approved by the ethics committee and written informed consents obtained |
|
| Participants | 40 ASA II or III participants (33 men, 7 women) scheduled for elective infrarenal aortic aneurysm repair were randomized Exclusion: coagulopathy or anticoagulant therapy precluding randomization to epidural analgesia; preoperative chronic analgesic use or substance dependence; previous adverse reactions (other than nausea) to narcotic analgesics; and documented cerebrovascular disease or other neuropsychiatric illness, including a history of postoperative confusion. People with preoperative left bundle‐branch block, cardiac glycoside use, or with indwelling pacemakers were excluded from S‐T segment monitoring |
|
| Interventions |
Treatment group: lumbar epidural analgesia (L2‐3 or L3‐4) with lidocaine, bupivacaine, and morphine for the surgery followed by an infusion of bupivacaine 0.125% and morphine 0.1 mg/mL started at 4 mL/h and adjusted according to VAS scores for 48 h (n = 19) Control group: IV morphine PCA for 48 h (n = 21) All participants had general anaesthesia with isoflurane, fentanyl, and neuromuscular blocking agents |
|
| Outcomes | Death in hospital Myocardial ischaemia Tracheal intubation duration Pneumonia GI haemorrhage Hospital length of stay VAS at rest and at movement at postoperative days 1 and 2 |
|
| Notes | 2 participants had their epidural infusions discontinued. 1 participant in each group received naloxone. They were included in intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized open design" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "randomized open design" probably means that both study participants and anaesthesiologists were not blinded. For outcome assessors, it is unclear. They state that patients' S‐T segment recording was assessed by an investigator who was blinded to the anaesthetic technique. But they did not mention whether outcome assessors who evaluated pain, sedation, and respiratory function were blinded. Decisions regarding extubation were made by ICU staff uninvolved in the study, guided by the unit protocol |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | They set withdrawal criteria from the study and included them into intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Measurements mentioned in the methods section were provided in the results section |
| Care program identical | Low risk | Yes |
| Treatment/control groups comparative at entry | Low risk | Groups well balanced |
| Results provided as intention‐to‐treat | Low risk | Intention‐to‐treat analysis was done |
Broekema 1998.
| Methods | Randomized controlled trial Approved by the ethics committee and written informed consents obtained |
|
| Participants | 14 men scheduled for abdominal aortic surgery were randomized | |
| Interventions |
Treatment group: thoracic epidural sufentanil or morphine with bupivacaine infusion Control group: IM morphine All participants had general anaesthesia |
|
| Outcomes | Death Tracheal intubation duration VAS pain score at rest and on movement at postoperative days 1, 2, and 3 |
|
| Notes | Additional information and subgroup data for abdominal aortic surgery provided by authors 1 participant had epidural infusion discontinued because of inadequate analgesia, but was included in intention‐to‐treat analysis No other drop‐outs occurred |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors provided information: statistician prepared randomization with sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Authors provided information: statistician prepared randomization with sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The route of administration was unknown to the outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant with failed epidural was included in the intervention group. No other drop‐outs |
| Selective reporting (reporting bias) | Low risk | Measurements mentioned in the methods section were provided in the results section |
| Care program identical | Low risk | Yes |
| Treatment/control groups comparative at entry | High risk | Subgroup data for people undergoing aortic surgery provided from the author showed that the mean age of intervention group (72.9 years) was higher than the mean age of the control group (58.0 years) |
| Results provided as intention‐to‐treat | Low risk | Intention‐to‐treat analysis was done |
Davies 1993.
| Methods | Randomized controlled trial Approved by the ethic committee and informed consents obtained |
|
| Participants | 50 participants (44 men, 6 women), scheduled for elective abdominal aortic surgery were randomized Exclusion: people with contraindication to an epidural |
|
| Interventions |
Treatment group: thoracic epidural analgesia (T9‐10) with bupivacaine 0.5% for a mean of 62 h (standard deviation 26) (n = 25) Control group: IV morphine infusion (n = 25) All participants also had general anaesthesia with enflurane, fentanyl, and pancuronium |
|
| Outcomes | Death in hospital MI VT/VF Pneumonia Stroke Acute kidney injury GI bleeding ICU stay Hospital stay |
|
| Notes | 1 participant had epidural infusion discontinued because of inadequate analgesia and received IV morphine. This participant was included in intention‐to‐treat analysis. No other drop‐outs occurred | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospective randomized trial" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Patient follow‐up was carried out by an independent anaesthetist at the time of discharge from ICU and at seven days postoperatively". However, it was unclear whether this anaesthesiologist assessed the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant had epidural failure and received IV morphine. This participant was included in the intervention group. No other drop‐outs occurred |
| Selective reporting (reporting bias) | Low risk | Measurements mentioned in the methods section were provided in the results section |
| Care program identical | Low risk | Yes |
| Treatment/control groups comparative at entry | High risk | Significantly more participants with chronic airway disease in the intervention (epidural) group |
| Results provided as intention‐to‐treat | Low risk | Intention‐to‐treat analysis was done |
de Lis 1990.
| Methods | Randomized controlled study Informed consents were obtained |
|
| Participants | 39 participants scheduled for elective infrarenal aortobifemoral graft interposition for aortic occlusive disease were randomized | |
| Interventions |
Treatment group: postoperative epidural morphine 4 mg every 12 h through a catheter placed under general anaesthesia at the end of the surgery and kept for 3‐4 days as required Control group: postoperative IV morphine as required (mean 5 mg/day) plus magnesium All participants were operated on under general anaesthesia and received respiratory physiotherapy after the surgery |
|
| Outcomes | Postoperative death Acute kidney injury |
|
| Notes | No statistically significant differences (mean ± SD) was when comparing the days of postoperative hospital stay (10 ± 3.71 days), postoperative intubation time (3.58 ± 3.7 h), or number of days that required analgesia (2.8 ± 0.82 days) Additional information regarding the study method sought but could not reach the authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were divided into 2 groups randomly" No information about random sequence generation. Unable to reach the authors |
| Allocation concealment (selection bias) | Unclear risk | No statement in the article. Unable to reach the authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement in the article. Unable to reach the authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs/withdrawals are mentioned in the text |
| Selective reporting (reporting bias) | Low risk | Measurements mentioned in the methods section were provided in the results section |
| Care program identical | Low risk | Yes |
| Treatment/control groups comparative at entry | Low risk | Groups with similar co‐morbidities |
| Results provided as intention‐to‐treat | Low risk | No drop‐out |
Garnett 1996.
| Methods | Randomized controlled trial Approved by the ethics committee and informed consents obtained |
|
| Participants | 111 participants scheduled for aortic reconstructive surgery were randomized. Of them, 12 participants were excluded because of failures of Holter monitors. 99 participants (80 men, 19 women) were analysed Exclusion: patient refusal, contraindications to epidural anaesthesia (anticoagulation, skin infections, neurological deficit), or ECG criteria making ischaemia analysis difficult (i.e. left bundle branch block, left ventricular hypertrophy with strain, or paced rhythm) |
|
| Interventions |
Treatment group: T12‐L1 epidural analgesia with bupivacaine 0.1% and meperidine 2 mg/mL (5‐15 mL/h) for 41‐46 h (n = 48) Control group: IV morphine infusion (n = 51) All participants had general anaesthesia with fentanyl isoflurane and pancuronium |
|
| Outcomes | Death in hospital Myocardial ischaemia MI CHF Respiratory failure Pneumonia Stroke Acute kidney injury |
|
| Notes | 12 participants were excluded after randomization | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12/111 participants were excluded because of the failure of the Holter monitor. However, how many of them were allocated to intervention/control group was not shown. They were excluded from the analysis |
| Selective reporting (reporting bias) | Low risk | Measurements mentioned in the methods section were provided in the results section |
| Care program identical | Low risk | Yes |
| Treatment/control groups comparative at entry | Low risk | Groups well balanced |
| Results provided as intention‐to‐treat | High risk | Not in intention‐to‐treat |
Kataja 1991.
| Methods | Randomized controlled trial Approved by the ethics committee and written informed consents obtained |
|
| Participants | 20 ASA III participants (16 men, 4 women) scheduled for abdominal aortic surgery were randomized | |
| Interventions |
Treatment group: T12‐L1 epidural analgesia with bupivacaine 0.25% and fentanyl 5 μg/mL at 7 mL/h (n = 10) Control group: IV oxycodone on request (n = 10) All participants had general anaesthesia with isoflurane, fentanyl, and pancuronium |
|
| Outcomes | Postoperative death within 30 days | |
| Notes | Information about drop‐outs was unclear Additional information about study design provided by authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors provided information: random numbers table |
| Allocation concealment (selection bias) | Low risk | Authors provided information: sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Author provided information that outcome assessor was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No treatment changes, no drop‐outs |
| Selective reporting (reporting bias) | Low risk | Measurements mentioned in the methods section were provided in the results section |
| Care program identical | Low risk | Yes |
| Treatment/control groups comparative at entry | Low risk | Groups well balanced |
| Results provided as intention‐to‐treat | Low risk | The sole outcome retained is mortality and the participant was attributed to its allocated group |
Muehling 2009.
| Methods | Randomized controlled trial Approved by the ethics committee and written informed consents obtained |
|
| Participants | 101 participants with the indication for elective open aneurysm repair of an infrarenal aortic aneurysm Exclusions: withdrawal of informed consent, clinical signs of infection (fever, leukocytosis) on admission, contraindications for epidural anaesthesia (e.g. coagulopathy), neuromuscular disorder that would not allow proper postoperative physiotherapy, planned suprarenal clamping |
|
| Interventions |
Treatment group: thoracic epidural analgesia (T7‐10) with ropivacaine 0.2% and sufentanil 2 μg/mL (patient‐controlled epidural analgesia) (n = 50; 49 analysed). Exact duration unspecified Control group: IV piritramide PCA (n = 51; 50 analysed) General anaesthesia and postoperative non‐steroidal anti‐inflammatory drugs for all participants |
|
| Outcomes | Mortality Myocardial ischaemia Pneumonia Acute kidney injury Paralytic ileus ICU length of stay Postoperative hospital stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After they gave written informed consent, participants were randomly assigned to the 2 treatment arms according to a randomized block design prepared by the Department of Biometry, University of Ulm |
| Allocation concealment (selection bias) | Low risk | After they gave written informed consent, participants were randomly assigned to the 2 treatment arms according to a randomized block design prepared by the Department of Biometry, University of Ulm |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no mention of blinding and the 2 groups had 2 different clinical pathways |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant in each group withdrew written informed consent and was excluded, leaving 50 participants in the traditional group and 49 participants in the fast‐track group, who were studied in an intention‐to‐treat protocol |
| Selective reporting (reporting bias) | Low risk | Measurements mentioned in the methods section were provided in the results section |
| Care program identical | Unclear risk | Epidural group: shorter fasting duration, no bowel washout, enteral feeding and ambulation beginning on the evening of the operation, removal of nasogastric tube at the end of the operation. IV fluids were restricted to 1000 mL/24 h, and participants were allowed to drink up to 2000 mL/24 h |
| Treatment/control groups comparative at entry | Low risk | Groups well balanced |
| Results provided as intention‐to‐treat | Low risk | All participants who gave consent were included in intention‐to‐treat |
Norman 1997.
| Methods | Randomized controlled trial Approved by the ethics committee and written informed consents obtained |
|
| Participants | 42 men scheduled for elective repair of infrarenal aortic aneurysm were randomized. Of them, 3 participants were excluded and 39 participants gave the outcomes Exclusion: malnutrition, chronic renal insufficiency, recent steroid administration, or occluded aorta |
|
| Interventions |
Treatment group: thoracic epidural analgesia (T9‐10 or T10‐11) with bupivacaine intraoperatively and morphine (incremental doses) after the surgery for at least 48 h after the surgery (n = 20) Control group: IV morphine PCA (n = 19) All participants had general anaesthesia with enflurane, fentanyl, and pancuronium |
|
| Outcomes | Perioperative death Acute kidney injury Duration of hospital stay |
|
| Notes | 3 participants were excluded after randomization. 2 were because of protocol violation, and 1 was because of re‐operation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized controlled study" No detailed information |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Postoperative stress hormone assessment, their main outcome of interest, was done blindly, but no information about blinding on other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 excluded participants were excluded from the analysis but were well described in the text |
| Selective reporting (reporting bias) | Low risk | Measurements mentioned in the methods section are provided in the results section |
| Care program identical | Low risk | Yes |
| Treatment/control groups comparative at entry | Low risk | Groups well balanced. Not in intention‐to‐treat |
| Results provided as intention‐to‐treat | High risk | Not in intention‐to‐treat |
Norris 2001.
| Methods | Randomized controlled trial Approved by the ethics committee and informed consents obtained |
|
| Participants | 168 participants waiting for elective abdominal aortic surgery were randomized. Of them, 8 were for the pilot study and only included in mortality data. Of the 160 remaining participants (115 men, 45 women), 9 died during hospitalization. Morbidity data were based on 151 participants surviving to discharge Exclusion: if procedure required clamping of the thoracic aorta; contraindication to any feature of the proposed clinical management, including epidural anaesthesia; previous surgery or severe deformity of the thoraco‐lumbar spine; previous or current neurological disease affecting the lower hemithorax or below; opioid dependence; major surgery in the previous 14 days; and participant refusal |
|
| Interventions |
Treatment group: thoracic epidural analgesia (T8‐9 or T10‐11) (PCA) with bupivacaine 0.125% intraoperatively or 0.0625% after the surgery (or both) plus fentanyl 5 μg/mL, intraoperatively only (n = 39), postoperatively only (n = 38), or intra‐ and postoperatively (n = 46) for at least 72 h Control group: IV fentanyl PCA for at least 72 h All participants had general anaesthesia with fentanyl, enflurane, and pancuronium |
|
| Outcomes | Hospital death and death at 12 months Myocardial ischaemia MI CHF VT/VF Tracheal intubation duration Respiratory failure Pneumonia Neurological deficit (taken as stroke) Acute kidney injury |
|
| Notes | 7 treatment changes. They were included in intention‐to‐treat analysis. 9 participants died in hospital. They were not included in data for major morbidity and other postoperative outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization scheme containing variable‐sized, balanced blocks of treatment assignments |
| Allocation concealment (selection bias) | Low risk | Pharmaceutical company determined the participants treatment assignment and prepared for masked study medications |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All Holter tape recordings were reviewed by a physician who was masked to the treatment regimen to which the participants had been assigned All participants inserted epidural catheter before surgery and received both masked epidural and IV medications. Postoperative pain management was also masked They masked postoperative PCA (single PCA button to activate both epidural and IV pumps) for at least 72 h postoperatively |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant was lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Measurements mentioned in the methods section were provided in the results section |
| Care program identical | Low risk | Yes |
| Treatment/control groups comparative at entry | Low risk | Groups well balanced |
| Results provided as intention‐to‐treat | Low risk | All participants who consented had attempted epidural placement. Randomization occurred after placement of a successful epidural. Failed epidural were not included. Participants whose treatment changed were described and included in intention‐to‐treat analysis |
Park 2001.
| Methods | Randomized, controlled trial in the 15 participating medical centres Approved by the ethics committee and informed consents obtained |
|
| Participants | 374 male high‐risk participants, ASA III or IV, scheduled for abdominal aortic surgery were included in the analysis | |
| Interventions |
Treatment group: thoracic or lumbar epidural bupivacaine 0.5% intraoperatively and morphine bolus every 12‐24 h or infusion after the surgery for a mean duration of 55.2 h (n = 184) Control group: IV or IM (93% IV) morphine or meperidine bolus or PCA (n = 190) |
|
| Outcomes | Death within 30 days MI CHF VT Tracheal intubation Respiratory failure Pneumonia Stroke Acute kidney injury GI bleeding VAS pain score at rest at postoperative day 1 and 3 ICU stay Hospital stay Function score on day 1, 3, and 7 |
|
| Notes | Abdominal aortic surgery group was a subgroup of this large trial. Overall, 1021 participants were randomized. Of them, surgery was cancelled for 26 participants and 11 participants withdrew from the study after randomization. 984 participants underwent surgery. However, outcome was based on 1021 participants Additional data were provided by authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table Quote: "Using an adaptive randomization scheme, we allocated patients to one of two treatment groups to balance between the groups the following prognostic variables; surgical type; age; Goldman index" |
| Allocation concealment (selection bias) | Low risk | Centralized randomization schemes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Abdominal aortic surgery group was a subgroup of this large trial. Overall, 1021 participants were randomized. Of them, surgery was cancelled for 26 participants and 11 participants withdrew from the study after randomization. 984 participants underwent surgery. However, outcome was based on 1021 participants Additional data were provided by authors |
| Selective reporting (reporting bias) | Low risk | Results mentioned in the methods section were found in the results section |
| Care program identical | Low risk | Yes |
| Treatment/control groups comparative at entry | Low risk | There were significantly more smokers in the intervention group. Therefore, the effect of the intervention might have been underestimated |
| Results provided as intention‐to‐treat | Low risk | Intention‐to‐treat |
Peyton 2003.
| Methods | Multicentre, randomized controlled clinical trial Approved by the ethics committees and written informed consents obtained |
|
| Participants | *164 participants (131 men, 33 women) were included in the analysis | |
| Interventions |
Treatment group: epidural local anaesthetic (ropivacaine or bupivacaine) opioid (pethidine or fentanyl for an intended duration of 72 h (n = 86) Control group: participant or physician controlled IV opioid infusion (n = 78) General anaesthesia for all participants |
|
| Outcomes | Death at 30 days Respiratory failure Pneumonia Acute kidney injury VAS at rest and on movement at postoperative days 1, 2, and 3 |
|
| Notes | This is a subgroup analysis of MASTER trial (Rigg 2002). In MASTER trial, 920 participants were randomized and 888 participants gave outcomes. Numbers of drop‐outs or treatment changes were not given for abdominal aortic surgery subgroup Additional data were provided by authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted random blocks with stratification by study centre |
| Allocation concealment (selection bias) | Low risk | By a 24 h randomization service |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Relevant data were collected by study nurses not blinded to allocation. However, they incorporated certain features into the research design to minimize bias in the measurement of outcomes. First, they deliberately did not educate caring clinicians about the morbidity endpoints or their definitions. Second, whether particular endpoints had occurred was defined by a computer algorithm at the time of data entry by staff of the trial secretariat who were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants whose surgery was cancelled after randomization were excluded, but they included 19 participants who were listed for an eligible procedure at the time of randomization subsequently underwent ineligible operation in the analysis. Epidural failure was well described and all those participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Results mentioned in the method section were given in the results section |
| Care program identical | Low risk | Yes |
| Treatment/control groups comparative at entry | Low risk | Groups well balanced |
| Results provided as intention‐to‐treat | Low risk | Intention‐to‐treat analysis |
Reinhart 1989.
| Methods | Randomized prospective study | |
| Participants | 105 participants (77 men, 28 women) scheduled for abdominal aortic surgery for occlusive disease (n = 82) and aneurysm (n = 23) were randomized | |
| Interventions |
Treatment group: thoracic epidural bupivacaine (n = 35) Control group: piritramide IV (n = 40 + 30) |
|
| Outcomes | Hospital mortality Tracheal intubation duration |
|
| Notes | No drop‐outs Additional information on study design provided by author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Results mentioned in the methods section were given in the results section |
| Care program identical | Low risk | Yes |
| Treatment/control groups comparative at entry | Low risk | Groups well balanced |
| Results provided as intention‐to‐treat | Low risk | No drop‐outs, no failed epidural mentioned |
Yeager 1987.
| Methods | Randomized, controlled clinical trial Informed consents obtained |
|
| Participants | We used separated data (n = 23) of participants who underwent abdominal aortic surgery, which were provided by the author | |
| Interventions |
Treatment group: low thoracic or lumbar epidural using lidocaine or bupivacaine for the surgery and analgesic concentration of local anaesthetics or narcotics (or both) after the surgery (n = 11). Duration of epidural analgesia at the discretion of the attending physician Control group: parenteral narcotic analgesics as required (n = 12) General anaesthesia for the surgery for all participants but inhalational agents in the control group only |
|
| Outcomes | Death in hospital MI CHF Tracheal intubation duration Respiratory failure Pneumonia Acute renal injury GI haemorrhage |
|
| Notes | 55 participants were randomized. The surgery for 2 participants was cancelled after randomization (53 gave the outcomes). We used separated data of people undergoing abdominal aortic surgery (n = 23). However, for time to extubation, separated data were not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Low risk | Additional information from the author: adequate |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients who were randomized and received surgery were included in the statistical comparisons" |
| Selective reporting (reporting bias) | Low risk | All results mentioned in the methods section were found in the results section |
| Care program identical | Unclear risk | Nitrous oxide plus epidural and neuromuscular blocking agents for the epidural group versus inhalational agents (≤ 1.0 MAC) for the control group |
| Treatment/control groups comparative at entry | Low risk | Groups well balanced |
| Results provided as intention‐to‐treat | Low risk | 3 failed epidurals included in intention‐to‐treat |
ASA: American Society of Anesthesiologists physical status; CHF: congestive heart failure; ECG: electrocardiogram; GI: gastrointestinal; h: hour; ICU: intensive care unit; IM: intramuscular; IV: intravenous; MAC: minimal alveolar concentration to avoid movement in 50% of the participants at surgical incision; MI: myocardial infarction; n: number; PCA: patient controlled analgesia; VAS: verbal/visual analogue scale; VT/VF: ventricular tachycardia, ventricular fibrillation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahn 2016 | Not a RCT: prospective observational study and different population: 73 participants who had undergone gastrectomy, pylorus‐preserving pancreaticoduodenectomy or hepatectomy in a tertiary care university hospital |
| Akarsu 2015 | Different population: 60 high risk participants undergoing a major abdominal surgical procedure: oesophagogastric (n = 11), hepatobiliary/pancreatic (n = 10), bowel (n = 28), other (n = 1) |
| Ali 2010 | Different population: did not include people who underwent aortic abdominal surgery |
| Astakhov 1984 | Not an RCT. No statement of randomizations |
| Baron 1991 | Not an RCT. Postoperative analgesia was not randomized |
| Beilin 2008 | Different population: did not include people who underwent aortic abdominal surgery |
| Bengtson 1987 | Not an RCT. No statement of randomizations. n = 12 for 1 group, n = 16 for the other group. We contacted the authors but did not receive a reply |
| Bonnet 1989 | Not an RCT. We contacted the author and were informed that postoperative analgesia was not randomized |
| Borovskikh 1990 | Not an RCT. No statement that it was randomized |
| Borovskikh 1991a | Not an RCT. No statement of randomization. This study looked at only physiological measures, and did not look at our outcomes of interest |
| Borovskikh 1991b | Not an RCT. No statement of randomization. Unbalanced groups with regards to age and sex. This study did not look at our outcomes of interest |
| Breslow 1993 | Different population: did not include people who underwent aortic abdominal surgery |
| Brinkmann 1994 | Outcome of interest not measured |
| Brustia 2003 | Not an RCT. Comparison with historical control |
| Bunt 1987 | Not an RCT. High‐risk people (ejection fraction < 0.35) were arbitrarily put into the general anaesthesia group |
| Diebel 1987 | Not an RCT. No information was available for allocation method. Authors did not state random allocation. n = 25 for 1 group, and n = 6 for the other group |
| Dodds 1997 | Different intervention. Both group received postoperative epidural analgesia |
| Donatelli 2006 | Different population. Did not include people who underwent aortic abdominal surgery |
| Dylczyk‐Sommer 2015 | Not a RCT: prospective observational, allocation according to epidural contraindications and participants' preferences |
| Ederoth 2002 | Different population. The participants were mixed with other types of surgery. We were unable to extract data separately |
| Fernandez 1990 | Not an RCT. This was a letter to editor |
| George 1992 | Different intervention. All the participants received postoperative epidural analgesia |
| Gibbs 1992 | No outcome of interest. We contacted the authors. They informed us that there were no postoperative outcomes observed (measured) in this study |
| Gold 1994 | No outcome of interest measured. They did not look at postoperative period |
| Goldmann 2008 | Different population. Did not include people who underwent aortic abdominal surgery |
| Her 1990 | Not an RCT. Allocation was according to each participant's preference |
| Houweling 1993 | Different intervention. Both treatment groups received postoperative epidural analgesia |
| Hu 2006 | Different study population. All types of lower abdominal surgery. Authors contacted on 27 December 2014; did not reply |
| Katz 1992 | Not an RCT. Comparison with historical control |
| Kawasaki 2007 | Different population. Did not include people who underwent aortic abdominal surgery |
| Lombardo 2009 | Quasi‐random allocation (simple alternate procedure). Author provided information (previous version of this review; Nishimori 2012) |
| Lundberg 1990 | Different intervention. All participants received epidural anaesthesia/analgesia |
| Mason 1990 | Not an RCT |
| Misquith 2016 | Different population: 44 ASA 1 or 2 participants aged from 20 to 55 years and scheduled for elective upper abdominal surgery (open cholecystectomies and gastric surgeries) |
| Mohapatra 2016 | Different population: 120 participants planned for elective upper abdominal surgery (partial gastrectomy n = 28, cholecystectomy n = 27, truncal vagotomy plus gastrojejunostomy n = 27, ventral hernia repair n = 18, splenectomy n = 20) |
| Murakami 2009 | Different population. Did not include people who underwent aortic abdominal surgery |
| Pan 2006 | Different study population. All types of abdominal surgery. Authors contacted on 27 December 2014; "Our study did not include patients undergoing open abdominal aortic surgery. They underwent noncardiac, nonvascular surgery" |
| Panaretou 2012 | No outcome of interest measured |
| Pecoraro 1990 | Different intervention. Postoperative analgesia was not randomized |
| Piper 2000 | This article was retracted (Can J Anaesth. 2011 Sep;58(9):889‐90) |
| Renghi 2013 | Different intervention. Compared to local anaesthetic infiltration and not to an opioid‐based regimen |
| Rosseel 1985 | No outcomes of interest measured. Study outcomes did not include our outcomes of interest |
| Saada 1992 | Different intervention. All participants received thoracic epidural anaesthesia/analgesia |
| Salman 2013 | Not a RCT: quasi‐randomised trial: even and odd numbers in order of enrolment |
| Sayed Moawad 2014 | Different population: 100 participants of either sex aged 20 to 60 years scheduled for elective major surgery at a gastroenterology surgical centre |
| Seeling 1985a | No outcomes of interest measured. Describes only the cardiovascular changes during anaesthesia and surgery. It does not look at our outcomes of interest |
| Seeling 1985b | No outcome of interest measured. Uses the same data of patients in Seeling 1985a. Also looked at cardiovascular changes during anaesthesia and surgery. Does not look at our outcomes of interest |
| Seeling 1986 | No outcome of interest measured. This is the third part of the Seeling study (Seeling 1985a; Seeling 1985b). It described the homeostasis and oxygen transport during anaesthesia, with no data about the postoperative course |
| Seeling 1990 | Different population. We were unable to extract aortic abdominal surgery subgroup data from the article. We contacted the author but did not receive a reply |
| Seeling 1991 | Different population. We were unable to extract aortic abdominal surgery subgroup data from the article. We contacted the author but did not receive a reply |
| Smeets 1993 | No outcome of interest measured. We contacted the author. They provided information that they did not measure any postoperative outcomes such as pain, morbidity, or mortality in this study |
| Tatsuishi 2012 | Not randomized |
| Thierry 1983 | Not an RCT. A retrospective study |
| Tilleul 2012 | Different intervention. Compared to local anaesthetic infiltration and not to an opioid‐based regimen |
| Tuman 1991 | Different population. Study population mixed with other surgery. Requested aortic abdominal surgery patient data, but authors did not respond |
| Vabishchevich 1986 | Not an RCT. No statement that participants were randomized. The method of postoperative analgesia for control group was unclear |
| Vaisanen 1998 | Different intervention. Both intervention and control groups received postoperative epidural analgesia |
| Wilhelm 1994 | Different intervention. This study compared postoperative epidural fentanyl and epidural sufentanil. Both groups received postoperative epidural analgesia |
| Wust 1980 | No outcome of interest measured. The data referred exclusively to the intraoperative lactate level under balanced, neurolept, and combined (epidural) anaesthesia, with no outcome data or information on the postoperative course |
| Wust 1982 | No outcome of interest measured. This was not an outcome study, it referred only to the changes in blood volume measured during the operation under different anaesthetic techniques |
| Yardeni 2007 | Different population. Surgery did not include abdominal aortic surgery |
| Yuceyar 2004 | No outcome of interest measured: femoral blood gas and blood concentrations of lipid peroxidation markers |
| Zhang 2007 | No outcome of interest measured. All the study data were collected before surgery |
n: number; RCT: randomized controlled trial.
The authors of all studies already excluded in the previous version (Nishimori 2012) were contacted by the authors of the previously published version.
Characteristics of studies awaiting assessment [ordered by study ID]
Owczuk 2016.
| Methods | RCT Approved by the ethics committee Written informed consents obtained Source of founding: This study was financed by Polish Ministry of Science and Higher Education (grant number N N 403 287334) |
| Participants | 70 participants of both sexes and aged between 40 and 75 years undergoing open abdominal aortic repair |
| Interventions |
Intervention: general and continuous epidural anaesthesia Control: general anaesthesia alone |
| Outcomes |
|
| Notes | Corresponding author: Prof. Radosław Owczuk, MD, PhD |
ASA: American Society of Anesthesiologists physical status
Characteristics of ongoing studies [ordered by study ID]
Li 2015.
| Trial name or title | Effects of two different anesthesia‐analgesia methods on incidence of postoperative delirium in elderly patients undergoing major thoracic and abdominal surgery: study rationale and protocol for a multicenter randomised controlled trial |
| Methods | RCT (open labelled, parallel) |
| Participants | 1800 participants aged 60 to 90 years scheduled to undergo major thoracic or abdominal surgery |
| Interventions |
Intervention: general anaesthesia plus postoperative epidural analgesia Control: general anaesthesia plus postoperative intravenous analgesia |
| Outcomes |
|
| Starting date | Said to be at the stage of participant recruitment and data collection, planned for a 3‐year period of enrolment |
| Contact information | Dong‐Xin Wang |
| Notes | ClinicalTrials.gov NCT01661907 and Chinese Clinical Trial Registry ChiCTR‐TRC‐12002371. |
RCT: randomized controlled trial
Differences between protocol and review
Differences between protocol (Nishimori 2004) and first version of review (Nishimori 2006)
We edited the wording of the background and methods section.
We searched the Ovid version of CENTRAL instead of The Cochrane Library CD version of CENTRAL.
Because there was a possibility that the search strategy we published in our protocol might miss trials that included people undergoing aortic abdominal surgery as a sub‐group of participants, we removed the search terms indicating aortic abdominal surgery, then did the search again (the amended search strategy is shown in Appendix 3).
Contributions of authors
Updated review: Joanne Guay (JG) , Sandra Kopp (SK).
Co‐ordinating the review: JG.
Undertaking manual searches: JG.
Screening search results: JG and SK.
Organizing retrieval of papers: JG.
Screening retrieved papers against inclusion criteria: JG and SK.
Appraising quality of papers: JG and SK.
Abstracting data from papers: JG and SK.
Writing to authors of papers for additional information: JG.
Data management for the review: JG.
Entering data into Review Manager: JG.
Analysis of data: JG.
Interpretation of data: JG and SK.
Writing the review: JG and SK.
Guarantor for the review: JG.
Statistical analysis: JG.
Sources of support
Internal sources
-
University of Sherbrooke, Canada.
University of Sherbrooke granted access to electronic databases and major medical journals
-
University of Quebec in Abitibi‐Temiscamingue, Rouyn‐Noranda, Canada.
University of Quebec in Abitibi‐Temiscamingue provided access to electronic databases and major medical journals
-
Cochrane Anaesthesia Review Group, Denmark.
The authors wish to thank Mr Karen Hovhannisyan who designed the search strategy.
External sources
No sources of support supplied
Declarations of interest
Joanne Guay: I have had no direct relationship with any pharmaceutical company or equipment manufacturer in the past five years. I have not acted as witness expert in the past five years. I am not an author in any of the included or excluded studies. I do not hold any stock other than mutual funds. During the last five years, I have received fees as speaker for two lectures given at the University of Dalhousie: one on regional anaesthesia for carotid endarterectomy and the other on local anaesthetic‐related methaemoglobinaemia. My fees were paid by the University of Dalhousie. I am the editor of a multi authors textbook on anaesthesia (including notions on general and regional anaesthesia). I receive fees for a course on airway management at University of Quebec en Abitibi‐Temiscamingue.
Sandra Kopp: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Barre 1989 {published data only}
- Barre E, Raimbault E, Coriat P, Low Koune JP, Baron JF, Viars P. Monitoring of mixed venous oxygen saturation in aortic surgery: value and limits. Annales Francaises d'Anesthesie et de Reanimation 1989;8(6):688‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bois 1997 {published data only}
- Bois S, Couture P, Boudreault D, Lacombe P, Fuqure F, Girard D, et al. Epidural analgesia and intravenous patient‐controlled analgesia result in similar rates of postoperative myocardial ischemia after aortic surgery. Anesthesia and Analgesia 1997;85(6):1233‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boylan 1998 {published data only}
- Boylan JF, Katz J, Kavanagh BP, Klinck JR, Cheng DC, DeMajo WC, et al. Epidural bupivacaine‐morphine analgesia versus patient‐controlled analgesia following abdominal aortic surgery: analgesic, respiratory, and myocardial effects. Anesthesiology 1998;89(3):585‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Broekema 1998 {unpublished data only}
- Broekema AA, Veen A, Filder V, Gielen M, Hennis PJ. Postoperative analgesia with intramuscular morphine at fixed rate versus epidural morphine or sufentanil and bupivacaine in patients undergoing major abdominal surgery. Anesthesia and Analgesia 1998;87(6):1346‐53. [MEDLINE: ] [PubMed] [Google Scholar]
Davies 1993 {published data only}
- Davies MJ, Silbert BS, Mooney PJ, Dysart RH, Meads AC. Combined epidural and general anaesthesia versus general anaesthesia for abdominal aortic surgery: a prospective randomised trial. Anaesthesia and Intensive Care 1993;21(6):790‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Lis 1990 {published data only}
- Lis AMS, Serrano MN, Alvarez MR, Pena JP, Gauna NM. Effects of postoperative pain on lung volume in patients undergoing aortic surgery. Revista Española de Anestesiología 1990;37(1):15‐8. [PUBMED: 2326519] [PubMed] [Google Scholar]
Garnett 1996 {published data only}
- Garnett RL, MacIntyre A, Lindsay P, Barber GG, Cole CW, Hajjar G, et al. Perioperative ischaemia in aortic surgery: combined epidural/general anaesthesia and epidural analgesia vs general anaesthesia and iv analgesia. Canadian Journal of Anaesthesia 1996;43(8):769‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kataja 1991 {published data only}
- Kataja J. Thoracolumbar epidural anaesthesia and isoflurane to prevent hypertension and tachycardia in patients undergoing abdominal aortic surgery. European Journal of Anaesthesiology 1991;8(6):427‐36. [MEDLINE: ] [PubMed] [Google Scholar]
Muehling 2009 {published data only}
- Muehling B, Schelzig H, Steffen P, Meierhenrich R, Sunder‐Plassmann L, Orend KH. A prospective randomized trial comparing traditional and fast‐track patient care in elective open infrarenal aneurysm repair. World Journal of Surgery 2009;33(3):577‐85. [PUBMED: 19137363] [DOI] [PubMed] [Google Scholar]
- Muehling BM, Ortlieb L, Oberhuber A, Orend KH. Fast track management reduces the systemic inflammatory response and organ failure following elective infrarenal aortic aneurysm repair. Interactive Cardiovascular and Thoracic Surgery 2011;12(5):784‐8. [PUBMED: 21343153] [DOI] [PubMed] [Google Scholar]
Norman 1997 {published data only}
- Norman JG, Fink GW, Strodel WE, Shawary P. The effects of epidural anaesthesia on the neuroendocrine response to major surgical stress: a randomized prospective trial. American Surgeon 1997;63(1):75‐80. [MEDLINE: ] [PubMed] [Google Scholar]
Norris 2001 {published data only}
- Norris EJ, Beattie C, Perler BA, Martinez EA, Meinert CL, Anderson GF, et al. Double‐masked randomized trial comparing alternate combinations of intraoperative anaesthesia and postoperative analgesia in abdominal aortic surgery. Anesthesiology 2001;95(5):1054‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Park 2001 {published and unpublished data}
- Park WY, Thompson JS, Lee KK. Effect of epidural anaesthesia and analgesia on perioperative outcome: a randomized, controlled Veterans Affairs cooperative study. Annals of Surgery 2001;234(4):560‐9; discussion 569‐71. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peyton 2003 {published and unpublished data}
- Peyton PJ, Myles PS, Silbert BS, Rigg JA, Jamrozik K, Parsons R. Perioperative epidural analgesia and outcome after major abdominal surgery in high‐risk patients. Anesthesia and Analgesia 2003;96(2):548‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rigg JR, Jamrozik K, Myles PS, Silbert B, Peyton P, Parsons R, et al. Design of the multicenter Australian study of epidural anaesthesia and analgesia in major surgery: the MASTER trial. Controlled Clinical Trial 2000;21:244‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rigg JR, Jamrozik K, Myles PS, Silbert BS, Peyton PJ, Parsons RW, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet 2002;359:1276‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reinhart 1989 {published data only}
- Reinhart K, Foehring U, Kersting T, Shaefer M, Bredle D, Hirner A, et al. Effects of thoracic epidural anaesthesia on systemic haemodynamic function and systemic oxygen supply‐demand relationship. Anesthesia and Analgesia 1989;69(3):360‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Stelzner J, Reinhart K, Fohring U, Henneberg M, Schafer M, Fitzner R. The effect of thoracic peridural analgesia on the cortisol and glucose response in surgery of the abdominal aorta. Regional Anaesthesie 1988;11(1):16‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Yeager 1987 {published data only}
- Yeager MP, Glass DD, Neff RK, Brinck‐Johnsen T. Epidural anaesthesia and analgesia in high‐risk surgical patients. Anesthesiology 1987;66(6):729‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahn 2016 {published data only}
- Ahn JH, Ahn HJ. Effect of thoracic epidural analgesia on recovery of bowel function after major upper abdominal surgery. Journal of Clinical Anesthesia 2016;34:247‐52. [PUBMED: 27687384] [DOI] [PubMed] [Google Scholar]
Akarsu 2015 {published data only}
- Akarsu Ayazoglu T, Ozensoy A, Geyik FD, Calim M, Duman U, Candan MA. Effects of combined epidural analgesia with total intravenous anesthesia on risky patients underwent major abdominal surgery. Agri 2015;27(4):171‐80. [PUBMED: 26860490] [DOI] [PubMed] [Google Scholar]
Ali 2010 {published data only}
- Ali M, Winter DC, Hanly AM, O'Hagan C, Keaveny J, Broe P. Prospective, randomized, controlled trial of thoracic epidural or patient‐controlled opiate analgesia on perioperative quality of life. British Journal of Anaesthesia 2010;104(3):292‐7. [PUBMED: 20124282] [DOI] [PubMed] [Google Scholar]
Astakhov 1984 {published data only}
- Astakhov AA, Kuvatov GA, Fokin AA, Verbovetskii LP, Kuvatov VA. Choosing the method of anaesthesia in aortofemoral shunting [Vybor metoda anestezii pri aortobedrennom shuntirovanii.]. Vestnik Khirurgii Imeni I. I. Grekova 1984;133(12):73‐5. [PUBMED: 6528452] [PubMed] [Google Scholar]
Baron 1991 {published data only}
- Baron JF, Bertrand M, Barre E, Godet G, Mundler O, Coriat P, et al. Combined epidural and general anaesthesia versus general anaesthesia for abdominal aortic surgery. Anesthesiology 1991;75(4):611‐8. [PUBMED: 1928770] [DOI] [PubMed] [Google Scholar]
Beilin 2008 {published data only}
- Beilin B, Hoofien D, Poran R, Gral I, Grinevich G, Butin B, et al. Comparison of two patient‐controlled analgesia techniques on neuropsychological functioning in the immediate postoperative period. Journal of Clinical and Experimental Neuropsychology 2008;30(6):674‐82. [PUBMED: 18612876] [DOI] [PubMed] [Google Scholar]
Bengtson 1987 {published data only}
- Bengtson A, Lannsjo W, Heideman M. Complement and anaphylatoxin responses to cross‐clamping of the aorta. Studies during general anaesthesia with or without extradural blockade. British Journal of Anaesthesia 1987;59(9):1093‐7. [PUBMED: 3499158] [DOI] [PubMed] [Google Scholar]
Bonnet 1989 {published data only}
- Bonnet F, Touboul C, Picard AM, Vodinh J, Becquemin JP. Neuroleptanesthesia versus thoracic epidural anaesthesia for abdominal aortic surgery. Annals of Vascular Surgery 1989;3(3):214‐9. [PUBMED: 2570604] [DOI] [PubMed] [Google Scholar]
Borovskikh 1990 {published data only}
- Borovskikh NA, Lebedev LV, Strashkov VI, Vinogradov AT. Comparative evaluation of the effectiveness of epidural anesthesia with spontaneous respiration and general anesthesia in aorto‐femoral bifurcation shunt [Sravnitel'naia otsenka effektivnosti epidural'noi anestezii so spontannym dykhaniem i obshchei anestezii pri aortobedrennom bifurkatsionnom shuntirovanii.]. Vestnik Khirurgii Imeni I. I. Grekova 1990;145(7):95‐8. [PUBMED: 2176417] [PubMed] [Google Scholar]
Borovskikh 1991a {published data only}
- Borovskikh NA, Andrushchuk IuV, Levshankov KA. Changes in various indicators of homeostasis after operation on the abdominal aorta in relation to the method of anaesthesia [Izmeneniia nekotorykh pokazatelei gomeostaza posle operatsii na briushnoi aorte v zavisimosti ot metoda anestezii]. Vestnik Khirurgii Imeni I. I. Grekova 1991;146(3):92‐5. [PUBMED: 1654658] [PubMed] [Google Scholar]
Borovskikh 1991b {published data only}
- Borovskikh NA, Filev LV, Luk'ianov IuV. The effect of the methods of anaesthesiological assistance and blood oxygenation on the oxygen‐transport properties of the blood in reconstructive interventions on the abdominal aorta and vessels of the lower extremities [Vliianie metodov anesteziologicheskogo posobiia i oksigenatsii krovi na kislorodtransportnye svoistva krovi pri rekonstruktivnykh vmeshatel'stvakh na briushnoi aorte i sosudakh nizhnikh konechnostei.]. Grudnaia i Serdechno‐Sosudistaia Khirurgiia 1991;11:27‐30. [PUBMED: 1764306] [PubMed] [Google Scholar]
Breslow 1993 {published data only}
- Breslow MJ, Parker SD, Frank SM, Norris EJ, Yates H, Raff H, et al. Determinants of catecholamine and cortisol responses to lower extremity revascularization. The PIRAT Study Group. Anesthesiology 1993;79(6):1202‐9. [PUBMED: 8267195] [DOI] [PubMed] [Google Scholar]
- Christopherson R, Glavan NJ, Norris EJ, Beattie C, Rock P, Frank SM, et al. Control of blood pressure and heart rate in patients randomized to epidural or general anaesthesia for lower extremity vascular surgery. Perioperative Ischemia Randomized Anesthesia Trial (PIRAT) Study Group. Journal of Clinical Anesthesia 1996;8(7):578‐8. [PUBMED: 8910181] [DOI] [PubMed] [Google Scholar]
Brinkmann 1994 {published data only}
- Brinkmann A, Seeling W, Wolf CF, Kneitinger E, Junger S, Rockemann M, et al. The effect of thoracic epidural anaesthesia on the pathophysiology of the eventration syndrome [Einfluss der Thorakalen Epiduralanasthesie auf die Pathophysiologie des Eventrationssyndroms]. Der Anaesthesist 1994;43(4):235‐44. [PUBMED: 8179173] [DOI] [PubMed] [Google Scholar]
Brustia 2003 {published data only}
- Brustia P, Renghi A, Gramaglia L, Porta C, Cassatella R, Angelis R, et al. Mininvasive abdominal aortic surgery. Early recovery and reduced hospitalization after multidisciplinary approach. Journal of Cardiovascular Surgery 2003;44(5):629‐35. [PUBMED: 14735052] [PubMed] [Google Scholar]
Bunt 1987 {published data only}
- Bunt TJ, Manczuk M, Varley K. Continuous epidural anaesthesia for aortic surgery: thoughts on peer review and safety. Surgery 1987;101(6):706‐14. [PUBMED: 3589965] [PubMed] [Google Scholar]
Diebel 1987 {published data only}
- Diebel LN, Lange MP, Schneider F, Mason K, Wilson RF, Jacobs L, et al. Cardiopulmonary complications after major surgery: a role for epidural analgesia?. Surgery 1987;102(4):660‐6. [PUBMED: 3660241] [PubMed] [Google Scholar]
Dodds 1997 {published data only}
- Dodds TM, Burns AK, DeRoo DB, Plehn JF, Haney M, Griffin BP, et al. Effects of anaesthetic technique on myocardial wall motion abnormalities during abdominal aortic surgery. Journal of Cardiothoracic and Vascular Anaesthesia 1997;11(2):129‐36. [PUBMED: 9105980] [DOI] [PubMed] [Google Scholar]
Donatelli 2006 {published data only}
- Donatelli F, Schricker T, Parrella P, Asenjo F, Wykes L, Carli F. Intraoperative infusion of amino acids induces anabolism independent of the type of anaesthesia. Anesthesia and Analgesia 2006;103(6):1549‐56. [PUBMED: 17122238] [DOI] [PubMed] [Google Scholar]
Dylczyk‐Sommer 2015 {published data only}
- Dylczyk‐Sommer J, Owczuk R, Wujtewicz M, Wojciechowski J. Does epidural anaesthesia reduce the incidence of postoperative oxygen desaturation episodes in patients undergoing open abdominal aortic aneurysm repair?. Anaesthesiology Intensive Therapy 2015;47(4):291‐6. [PUBMED: 26401734] [DOI] [PubMed] [Google Scholar]
Ederoth 2002 {published data only}
- Ederoth P, Flisberg P, Ungerstedt U, Nordstrom CH, Lundberg J. Local metabolic changes in subcutaneous adipose tissue during intravenous and epidural analgesia. Acta Anaesthesiologica Scandinavica 2002;46(5):585‐91. [PUBMED: 12027854] [DOI] [PubMed] [Google Scholar]
Fernandez 1990 {published data only}
- Fernandez JA, Casas JI, Villar Landeira JM. Epidural analgesia versus conventional intravenous analgesia after aortic surgery [Analgesia epidural versus analgesia convencional intravenosa, tras cirugia aortica]. Revista Espanola de Anestesiologia y Reanimacion 1990; Vol. 37, issue 5:309‐10. [PUBMED: 2098867] [PubMed]
George 1992 {published data only}
- George KA, Chisakuta AM, Gamble JA, Browne GA. Thoracic epidural infusion for postoperative pain relief following abdominal aortic surgery: bupivacaine, fentanyl or a mixture of both?. Anaesthesia 1992;47(5):388‐94. [PUBMED: 1599061] [DOI] [PubMed] [Google Scholar]
Gibbs 1992 {published data only}
- Gibbs NM, Crawford GP, Michalopoulos N. The effect of epidural blockade on postoperative hypercoagulability following abdominal aortic bypass surgery. Anaesthesia and Intensive Care 1992;20(4):487‐90. [PUBMED: 1463179] [DOI] [PubMed] [Google Scholar]
Gold 1994 {published data only}
- Gold MS, DeCrosta D, Rizzuto C, Ben‐Harari RR, Ramanathan S. The effect of lumbar epidural and general anaesthesia on plasma catecholamines and haemodynamics during abdominal aortic aneurysm repair. Anesthesia and Analgesia 1994;78(2):225‐30. [PUBMED: 8311273] [DOI] [PubMed] [Google Scholar]
Goldmann 2008 {published data only}
- Goldmann A, Hoehne C, Fritz GA, Unger J, Ahlers O, Nachtigall I, et al. Combined vs. isoflurane/fentanyl anaesthesia for major abdominal surgery: effects on hormones and haemodynamics. Medical Science Monitor 2008;14(9):CR445‐52. [PUBMED: 18758414] [PubMed] [Google Scholar]
Her 1990 {published data only}
- Her C, Kizelshteyn G, Walker V, Hayes D, Lees DE. Combined epidural and general anaesthesia for abdominal aortic surgery. Journal of Cardiothoracic Anaesthesia 1990;4(5):552‐7. [PUBMED: 2132133] [DOI] [PubMed] [Google Scholar]
Houweling 1993 {published data only}
- Houweling PL, Ionescu TI, Leguit P, Tweel I, Smalhout B. Comparison of the cardiovascular effects of intravenous, epidural and intrathecal sufentanil analgesia as a supplement to general anaesthesia for abdominal aortic aneurysm surgery. European Journal of Anaesthesiology 1993;10(6):403‐11. [PUBMED: 11767316] [PubMed] [Google Scholar]
Hu 2006 {published data only}
- Hu Y, Wang Y, Li Y. Effects of different analgesic methods on immune function after lower abdominal surgery. Chinese Journal of Clinical Rehabilitation 2006;10(4):62‐4. [Google Scholar]
Katz 1992 {published data only}
- Katz S, Reiten P, Kohl R. The use of epidural anaesthesia and analgesia in aortic surgery. American Surgeon 1992;58(8):470‐3. [PUBMED: 1642382] [PubMed] [Google Scholar]
Kawasaki 2007 {published data only}
- Kawasaki T, Ogata M, Kawasaki C, Okamoto K, Sata T. Effects of epidural anaesthesia on surgical stress‐induced immunosuppression during upper abdominal surgery. British Journal of Anaesthesia 2007;98(2):196‐203. [PUBMED: 17218378] [DOI] [PubMed] [Google Scholar]
Lombardo 2009 {published data only}
- Lombardo L, Ruggia O, Crocella L, Masoero G, Foti M, Mambrini S, et al. Epidural plus general anaesthesia vs general anaesthesia alone for elective aortic surgery: effects on gastric electrical activity and serum gastrin secretion. Minerva Anestesiologica 2009;75(3):109‐15. [PUBMED: 19221543 ] [PubMed] [Google Scholar]
Lundberg 1990 {published data only}
- Lundberg J, Lundberg D, Norgren L, Ribbe E, Thorne J, Werner O. Intestinal haemodynamics during laparotomy: effects of thoracic epidural anaesthesia and dopamine in humans. Anesthesia and Analgesia 1990;71(1):9‐15. [PUBMED: 2194404] [DOI] [PubMed] [Google Scholar]
Mason 1990 {published data only}
- Mason RA, Newton GB, Cassel W, Maneksha F, Giron F. Combined epidural and general anaesthesia in aortic surgery. Journal of Cardiovascular Surgery 1990;31(4):442‐7. [PUBMED: 2211796] [PubMed] [Google Scholar]
Misquith 2016 {published data only}
- Misquith JC, Rao R, Ribeiro KS. Serial peak expiratory flow rates in patients undergoing upper abdominal surgeries under general anaesthesia and thoracic epidural analgesia. Journal of Clinical and Diagnostic Research 2016;10:Uc01‐4. [DOI: 10.7860/JCDR/2016/13942.7327] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohapatra 2016 {published data only}
- Mohapatra, S, Nayak RS, Jena P, Tripathy SK, Das SR. A study of changes in stress factors during elective upper abdominal surgery using different anaesthetic techniques. International Journal of Pharmaceutical Sciences Review and Research 2016;39(1):167‐72. [Google Scholar]
Murakami 2009 {published data only}
- Murakami T, Okuda Y, Ishii M, Kobayashi A, Kawamura M. Comparison of intravenous fentanyl analgesia and epidural analgesia for postoperative pain relief. Masui Japanese Journal of Anesthesiology 2009;58(9):1149‐53. [PubMed] [Google Scholar]
Pan 2006 {published data only}
- Pan LF, Wang DX, Li J. Effects of different methods of anaesthesia and analgesia on early postoperative cognitive dysfunction after non‐cardiac surgery in the elderly. Journal of Peking University 2006;38(5):510‐4. [PubMed] [Google Scholar]
Panaretou 2012 {published data only}
- Panaretou V, Siafaka I, Theodorou D, Manouras A, Seretis C, Gourgiotis S, et al. Combined general‐epidural anaesthesia with continuous postoperative epidural analgesia preserves sigmoid colon perfusion in elective infrarenal aortic aneurysm repair. Saudi Journal of Anaesthesia 2012;6(4):373‐9. [PUBMED: 23493852] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pecoraro 1990 {published data only}
- Pecoraro JP, Dardik H, Mauro A, Wolodiger F, Drascher G, Raccuia S, et al. Epidural anaesthesia as an adjunct to retroperitoneal aortic surgery. American Journal of Surgery 1990;160(2):187‐91. [PUBMED: 2382772] [DOI] [PubMed] [Google Scholar]
Piper 2000 {published data only}
- Miller DR. Retraction note to: Hemodynamics, intramucosal pH and regulators of circulation during perioperative epidural analgesia. Canadian Journal of Anaesthesia 2011;58(9):889‐90. [PUBMED: 2179270] [DOI] [PubMed] [Google Scholar]
- Piper SN, Boldt J, Schmidt CC, Maleck WH, Brosch C, Kumle B. Haemodynamics, intramucosal pH and regulators of circulation during perioperative epidural analgesia. Canadian Journal of Anaesthesia 2000;47(7):631‐7. [PUBMED: 10930202] [DOI] [PubMed] [Google Scholar]
Renghi 2013 {published data only}
- Renghi A, Gramaglia L, Casella F, Moniaci D, Gaboli K, Brustia P. Local versus epidural anaesthesia in fast‐track abdominal aortic surgery. Journal of Cardiothoracic and Vascular Anesthesia 2013;27(3):451‐8. [PUBMED: 23273683] [DOI] [PubMed] [Google Scholar]
Rosseel 1985 {published data only}
- Rosseel P, Marichal P, Lauwers LF, Baute L, Hanegreefs G. A haemodynamic study of epidural versus intravenous anaesthesia for aortofemoral bypass surgery. Acta Anaesthesiologica Belgica 1985;36(4):345‐63. [PUBMED: 4096193] [PubMed] [Google Scholar]
Saada 1992 {published data only}
- Saada M, Catoire P, Bonnet F, Delaunay L, Gormezano G, Macquin‐Mavier I, et al. Effect of thoracic epidural anaesthesia combined with general anaesthesia on segmental wall motion assessed by transoesophageal echocardiography. Anesthesia and Analgesia 1992;75(3):329‐35. [PUBMED: 1510252] [DOI] [PubMed] [Google Scholar]
Salman 2013 {published data only}
- Salman N, Durukan AB, Gurbuz HA, Yamali H, Guler L, Ucar HI, et al. Comparison of effects of epidural bupivacaine and intravenous meperidine analgesia on patient recovery following elective abdominal aortic surgery. Medical Science Monitor 2013;19:347‐52. [PUBMED: 23666275] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sayed Moawad 2014 {published data only}
- Sayed Moawad H, Mokbel EM. Postoperative analgesia after major abdominal surgery: Fentanyl‐bupivacaine patient controlled epidural analgesia versus fentanyl patient controlled intravenous analgesia. Egyptian Journal of Anaesthesia 2014;30:390‐7. [Google Scholar]
Seeling 1985a {published data only}
- Seeling W, Ahnefeld FW, Rosenberg G, Heinrich H, Spilker D. Aortofemoral bifurcation bypass ‐ effect of anaesthesia procedure (NLA, thoracic continuous catheter peridural anaesthesia) on circulation, respiration and metabolism. Hemodynamic changes caused by peridural anaesthesia and anaesthesia induction [Aortofemoraler Bifurkationsbypass ‐ der Einfluss des Anaesthesieverfahrens (NLA, thorakale kontinuierliche Katheterperiduralanaesthesie) auf Kreislauf, Atmung und Stoffwechsel]. Anaesthesist 1985;34(5):217‐28. [PUBMED: 4025792] [PubMed] [Google Scholar]
Seeling 1985b {published data only}
- Seeling W, Ahnefeld FW, Hamann H, Heinrich H, Hutschenreiter S, Rosenberg G, et al. Aortofemoral bifurcation bypass ‐ effect of the anesthesia procedure (NLA, thoracic continuous catheter peridural anesthesia) on circulation, respiration and metabolism. Intraoperative circulatory reactions [Aortofemoraler Bifurkationsbypass ‐ Der Einfluss des Anaesthesieverfahrens (NLA, thorakale kontinuierliche Katheterperiduralanaesthesie) auf Kreislauf, Atmung und Stoffwechsel. Intraoperatives Kreislaufverhalten]. Anaesthesist 1985;34(9):417‐28. [PUBMED: 3909840] [PubMed] [Google Scholar]
Seeling 1986 {published data only}
- Seeling W, Ahnefeld FW, Grunert A, Heinrich H, Lotz P, Rosenberg G, et al. Aortofemoral bifurcation bypass. Effect of the anaesthesia procedure (NLA, thoracic continuous catheter peridural anesthesia) on circulation, respiration and metabolism. Homeostasis and oxygen transport [Aortofemoraler Bifurkationsbypass. Der Einfluss des Anaesthesie‐verfahrens (NLA, thorakale kontinuierliche Katheterperidural‐anaesthesie) auf Kreislauf, Atmung und Stoffwechsel. Homoostase und Sauerstofftransport]. Anaesthesist 1986;35(2):80‐92. [PUBMED: 3963358] [PubMed] [Google Scholar]
Seeling 1990 {published data only}
- Seeling W, Bruckmooser KP, Hufner C, Kneitinger E, Rigg C, Rockemann M. No reduction in postoperative complications by the use of catheterized epidural analgesia following major abdominal surgery [Keine Verminderung postoperativer Komplikationen durch Katheterepiduralanalgesie nach grossen abdominellen Eingriffen]. Anaesthesist 1990;39(1):33‐40. [PUBMED: 2407144] [PubMed] [Google Scholar]
Seeling 1991 {published data only}
- Seeling W, Bothner U, Eifert B, Rockemann M, Schreiber M, Schurmann W, et al. Patient‐controlled analgesia versus epidural analgesia using bupivacaine or morphine following major abdominal surgery. No difference in postoperative morbidity [Patientenkontrollierte Analgesie versus Epiduralanalgesie mit Bupivacain oder Morphin nach grossen abdominellen Eingriffen. Kein Unterschied in der postoperativen Morbiditat]. Anaesthesist 1991;40(11):614‐23. [PUBMED: 1755532] [PubMed] [Google Scholar]
Smeets 1993 {published data only}
- Smeets HJ, Kievit J, Dulfer FT, Kleef JW, Bonnet F. Endocrine‐metabolic response to abdominal aortic surgery: a randomized trial of general anaesthesia versus general plus epidural anaesthesia. World Journal of Surgery 1993;17(5):601‐7. [DOI] [PubMed] [Google Scholar]
Tatsuishi 2012 {published data only}
- Tatsuishi W, Kohri T, Kodera K, Asano R, Kataoka G, Kubota S, et al. Usefulness of an enhanced recovery after surgery protocol for perioperative management following open repair of an abdominal aortic aneurysm. Surgery Today 2012;42(12):1195‐200. [PUBMED: 22797961] [DOI] [PubMed] [Google Scholar]
Thierry 1983 {published data only}
- Thierry M, Corre A, Haddad C, Becade P. Place of peridural anaesthesia in aorto‐iliac surgery. Preliminary comparative study apropos of 32 patients [Place de l'anesthésie péridurale en chirurgie aorto‐iliaque. Etude préliminaire comparative à propos de 32 patients]. Journal des Maladies Vasculaires 1983;8(4):329‐33. [PUBMED: 6663204] [PubMed] [Google Scholar]
Tilleul 2012 {published data only}
- Tilleul P, Aissou M, Bocquet F, Thiriat N, Grelle O, Burke MJ, et al. Cost‐effectiveness analysis comparing epidural, patient‐controlled intravenous morphine, and continuous wound infiltration for postoperative pain management after open abdominal surgery. British Journal of Anaesthesia 2012;108(6):998‐1005. [PUBMED: 22466819] [DOI] [PubMed] [Google Scholar]
Tuman 1991 {published data only}
- Tuman KJ, McCarthy RJ, March RJ, DeLaria GA, Patel RV, Ivankovich AD. Effects of epidural anaesthesia and analgesia on coagulation and outcome after major vascular surgery. Anesthesia and Analgesia 1991;73(6):696‐704. [PUBMED: 1952169] [DOI] [PubMed] [Google Scholar]
Vabishchevich 1986 {published data only}
- Vabishchevich AV, Ginzburg LS, Lemenev VL, Titov AA. Use of epidural anesthesia in middle‐aged and elderly patients for operations in the aortofemoral area [Primenenie epidural'noi anestezii u bol'nykh pozhilogo i starcheskogo vozrasta pri operatsiiakh v aortobedrennoi zone]. Anesteziologiia i Reanimatologiia 1986;5:66‐7. [PUBMED: 3813114] [PubMed] [Google Scholar]
Vaisanen 1998 {published data only}
- Vaisanen O, Parviainen I, Ruokonen E, Hippelainen M, Berg E, Hendolin H, et al. Epidural analgesia with bupivacaine does not improve splanchnic tissue perfusion after aortic reconstruction surgery. British Journal of Anaesthesia 1998;81(6):893‐8. [PUBMED: 10211015] [DOI] [PubMed] [Google Scholar]
Wilhelm 1994 {published data only}
- Wilhelm AJ, Dieleman HG. Epidural fentanyl and sufentanil for intra‐ and postoperative analgesia. A randomized, double‐blind comparison. Pharmacy World and Science 1994;16(1):7‐12. [PUBMED: 8156047] [DOI] [PubMed] [Google Scholar]
Wust 1980 {published data only}
- Wust HJ, Spirgatis WD, Sandmann W, Krian A, Richter O. The effects of different anaesthetic technics on lactate under the course of aorto‐femoral bypass operation [Laktatverhalten wahrend aorto‐femoraler Bypassoperationen unter verschiedenen Narkoseverfahren]. Anasthesie, Intensivtherapie, Notfallmedizin 1980;15(2):87‐98. [PUBMED: 6772048] [PubMed] [Google Scholar]
Wust 1982 {published data only}
- Wust HJ, Feiereis HW, Sandmann W, Florack G. [lood volume in aorto‐femoral bypass operation. Effects of continuous thoracic epidural‐, halothane‐ or neuroleptanaesthesia [Blutvolumen bei aortofemoralen Bypass‐Operationen. Einfluss der kontinuierlichen thorakalen Epiduralanaesthesie, Halothannarkose und Neuroleptanaesthesie]. Anaesthesist 1982;31(9):449‐55. [PUBMED: 7149218] [PubMed] [Google Scholar]
Yardeni 2007 {published data only}
- Yardeni IZ, Shavit Y, Bessler H, Mayburd E, Grinevich G, Beilin B. Comparison of postoperative pain management techniques on endocrine response to surgery: a randomised controlled trial. International Journal of Surgery 2007;5(4):239‐43. [PUBMED: 17660130] [DOI] [PubMed] [Google Scholar]
Yuceyar 2004 {published data only}
- Yuceyar L, Erolcay H, Konukoglu D, Bozkurt AK, Aykac B. Epidural anesthesia may attenuate lipid peroxidation during aorto‐femoral surgery. Canadian Journal of Anaesthesia 2004;51(5):465‐71. [PUBMED: 15128632] [DOI] [PubMed] [Google Scholar]
Zhang 2007 {published data only}
- Zhang J, Zhang W, Li B. The effect of epidural anaesthesia with different concentrations of ropivacaine on sevoflurane requirements. Anesthesia and Analgesia 2007;104(4):984‐6. [PUBMED: 17377119] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Owczuk 2016 {published data only}
- Owczuk R, Dylczyk‐Sommer A, Wojciechowski J, Paszkiewicz M, Wujtewicz M, Stepnowski P, et al. The influence of epidural blockade on gut permeability in patients undergoing open surgical repair of abdominal aortic aneurysm. Anaesthesiology Intensive Therapy 2016;48(2):122‐7. [PUBMED: 26965722] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Li 2015 {published data only}
- Li YW, Li HJ, Li HJ, Feng Y, Yu Y, Guo XY, et al. Effects of two different anesthesia‐analgesia methods on incidence of postoperative delirium in elderly patients undergoing major thoracic and abdominal surgery: study rationale and protocol for a multicenter randomized controlled trial. BMC Anesthesiology 2015;15:144. [PUBMED: 26459347] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Block 2003
- Block BM, Liu SS, Rowlingston AJ, Cowan JA, Wu CL. Efficacy of postoperative epidural analgesia. JAMA 2003;290(18):2455‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guay 2006
- Guay J. The benefits of adding epidural analgesia to general anaesthesia: a meta‐analysis. Journal of Anesthesia 2006;20(4):335‐40. [PUBMED: 17072704] [DOI] [PubMed] [Google Scholar]
Guay 2013
- Guay J, Ochroch EA. Beta‐blocking agents for surgery: influence on mortality and major outcomes. A meta‐analysis. Journal of Cardiothoracic and Vascular Anesthesia 2013;27(5):834‐44. [PUBMED: 23790500] [DOI] [PubMed] [Google Scholar]
Guay 2014a
- Guay J, Choi P, Suresh S, Albert N, Kopp S, Pace NL. Neuraxial blockade for the prevention of postoperative mortality and major morbidity: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD010108.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guay 2014b
- Guay J, Ochroch EA. Effects of adding statins before surgery on mortality and major morbidity: a meta‐analysis. Journal of Cardiothoracic and Vascular Anesthesia 2014;28(2):255‐66. [PUBMED: 24011872] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical Research Ed.) 2008;336(7650):924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines: 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Hebl 2006
- Hebl JR, Kopp SL, Schroeder DR, Horlocker TT. Neurologic complications after neuraxial anaesthesia or analgesia in patients with preexisting peripheral sensorimotor neuropathy or diabetic polyneuropathy. Anesthesia and Analgesia 2006;103(5):1294‐9. [PUBMED: 17056972] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research ed.) 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org, 2011. [Google Scholar]
Horlocker 2010
- Horlocker TT, Wedel DJ, Rowlingson JC, Enneking FK, Kopp SL, Benzon HT, et al. Regional anaesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence‐Based Guidelines (Third Edition). Regional Anesthesia and Pain Medicine 2010;35(1):64‐101. [PUBMED: 20052816] [DOI] [PubMed] [Google Scholar]
Jorgensen 2000
- Jorgensen H, Wetterslev J, Moiniche S, Dahl JB. Epidural local anaesthetics versus opioid‐based analgesic regimens on postoperative gastrointestinal paralysis, PONV and pain after abdominal surgery. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD001893] [DOI] [PubMed] [Google Scholar]
Pace 2011
- Pace NL. Research methods for meta‐analyses. Best practice & research. Clinical Anaesthesiology 2011;25(4):523‐33. [PUBMED: 22099918] [DOI] [PubMed] [Google Scholar]
Paravastu 2014
- Paravastu SC, Jayarajasingam R, Cottam R, Palfreyman SJ, Michaels JA, Thomas SM. Endovascular repair of abdominal aortic aneurysm. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD004178.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robertson 2014
- Robertson L, Atallah E, Stansby G. Pharmacological treatment of vascular risk factors for reducing mortality and cardiovascular events in patients with abdominal aortic aneurysm. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD010447.pub2] [DOI] [PubMed] [Google Scholar]
Rucker 2011
- Rucker G, Schwarzer G, Carpenter JR, Binder H, Schumacher M. Treatment‐effect estimates adjusted for small‐study effects via a limit meta‐analysis. Biostatistics (Oxford, England) 2011;12(1):122‐42. [PUBMED: 20656692] [DOI] [PubMed] [Google Scholar]
Sampson 2014
Svensjo 2014
References to other published versions of this review
Nishimori 2004
- Nishimori M, Ballantyne JC, Low JHS. Epidural pain relief versus systemic opioid‐based pain relief for abdominal aortic surgery. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD005059] [DOI] [PubMed] [Google Scholar]
Nishimori 2006
- Nishimori M, Ballantyne JC, Low JHS. Epidural pain relief versus systemic opioid‐based pain relief for abdominal aortic surgery. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005059.pub2] [DOI] [PubMed] [Google Scholar]
Nishimori 2012
- Nishimori M, Low JHS, Zheng H, Ballantyne JC. Epidural pain relief versus systemic opioid‐based pain relief for abdominal aortic surgery. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD005059.pub3] [DOI] [PubMed] [Google Scholar]


