ABSTRACT
Nuclear factor 90 (NF90) is a dual DNA- and RNA-binding protein expressed ubiquitously in mammalian cells, including monocytes. Here, to elucidate the function of NF90 in the immune response, we analyzed systematically its influence on gene expression programs in the human monocytic cell line THP-1 expressing normal or reduced NF90 levels. RNA sequencing analysis revealed many mRNAs showing differential abundance in NF90-silenced cells, many of them encoding proteins implicated in the response to immune stimuli and malaria infection. The transcription of some of them (e.g. TNF, LILRB1, and CCL2 mRNAs) was modulated by silencing NF90. Ribonucleoprotein immunoprecipitation (RIP) analysis further revealed that a subset of these mRNAs associated directly with NF90. To understand how NF90 influenced globally the immune response to malaria infection, lysates of red blood cells infected with Plasmodium falciparum (iRBC lysates) or uninfected/mock-infected (uRBC lysates) were used to treat THP-1 cells as a surrogate of malaria infection. NF90 affected the stability of a few target mRNAs, but influenced more generally the translation and secretion of the encoded cytokines after treatment with either uRBC or iRBC lysates. Taken together, these results indicate that NF90 contributes to repressing the immune response in cells responding to P. falciparum infection and suggest that NF90 can be a therapeutic target in malaria.
KEYWORDS: NF90, RNA-binding protein, ribonucleoprotein complex, monocytes, translational control, malaria
Introduction
Changes in gene expression programs enable organisms to adapt to developmental cues and to changes in the environment. Transcriptional and post-transcriptional mechanisms of gene regulation are critically controlled by proteins that bind DNA, RNA, or both [1,2]. NF90 is a DNA- and RNA-binding protein (DRBP) encoded by the Interleukin Enhancer-Binding Factor 3 (ILF3) gene localized on human chromosome 19 [3,4]. NF90 is found in both the nucleus and the cytoplasm, and bears two double-stranded RNA-binding motifs, a zinc finger domain, and a nucleic acid-binding arginine/glycine-rich (RGG) motif [4]. NF90 can bind DNA and regulate the transcription of specific genes, and it can also bind single- and double-stranded RNA to control mammalian and viral RNA metabolism [4,5]. Accordingly, NF90 has been found to participate in many levels of gene regulation, including mRNA stabilization, translation control, viral RNA replication and transcription, and biogenesis of microRNAs and circular RNAs [6–11].
The activity of NF90 as a transcription factor was initially identified in the immune response. NF90 was found as a large subunit of the constitutive human transcription complex NFAT (nuclear factor of activated T cells) involved in the transcription of the IL2 and IL13 genes [12,13]. More recent studies characterizing systematically the genomic regions with which NF90 associates have uncovered many active promoters and enhancers with NF90-binding sites, including those in genes encoding proliferative proteins [5].
Through its RNA-binding function, NF90 was found to influence the post-transcriptional lives of many target transcripts, particularly a subset of mRNAs bearing AU-rich sequences in their 3' untranslated regions (UTRs) [14]. Some NF90 targets, including IL2 and DUSP1/MKP-1 mRNAs, were stabilized upon NF90 binding [6,14,15]. However, NF90 was also found to control the translation of other target mRNAs. Translational repression by NF90 was reported in actively dividing fibroblasts, which expressed high levels of NF90 and low levels of MCP1, GROA, IL6, and IL8; conversely, senescent fibroblasts expressed low levels of NF90 and thus translation of the same factors, including inflammatory cytokines, was elevated [16]. In addition, NF90 promoted the translation of vascular endothelial growth factor (VEGF) and cyclin T1 (CCNT1) by enhancing the recruitment of the corresponding mRNAs to polysomes [17,18].
We recently identified NF90 as a repressor of the translation of cytokine BAFF (encoded by the TNFSF13B gene) in THP-1 cells. NF90 cooperated with microRNA miR-15a in binding the wild-type isoform of BAFF mRNA and inhibited BAFF translation [19]. In the presence of a genetic variant (BAFF-var), the binding site for NF90 and one of the two miR-15a sites were lost, leading to increased production of soluble BAFF and raising susceptibility to autoimmune diseases like multiple sclerosis and systemic lupus erythematosus [19,20].
Although these lines of evidence indicated that NF90 influenced the production of different immune response proteins at both the transcriptional and post-transcriptional levels, a global analysis of NF90 in the immune response program has not been carried out. We thus sought to study how NF90 influenced the expression of immune proteins using monocytes, which play key roles in innate immunity and inflammation, as well as in diseases in which these processes are dysregulated. We systematically analyzed the association of NF90 with RNAs in the human monocytic cell line THP-1 [21]. RNA sequencing analysis identified mRNAs differentially abundant in THP-1 cells expressing low levels of NF90, including several mRNAs encoding proteins critical for the response to malaria infection, a disease caused by Plasmodium parasites [22]. Malaria infection is strongly influenced by the release of inflammatory mediators from innate immune cells and is a major public health problem, causing hundreds of thousands of deaths each year [23]. Analysis of THP-1 cells treated with lysates of red blood cells infected or not with Plasmodium falciparum revealed that NF90 influenced the levels of immune response proteins in response to malaria antigens on different levels: transcription, mRNA turnover, and translation. In line with previous results [24], the number of CD14+ cells declined after treatment with lysates of red blood cells infected with P. falciparum, while NF90 silencing restored CD14+ cells. We propose that NF90 regulates gene expression programs in THP-1 cells and influences the ability of these cells to respond to malaria antigens.
Results
Lowering NF90 levels alters the transcriptome in monocytic THP-1 cells
The DRBP NF90 regulates gene expression in response to diverse signals, including immune stimuli. To begin to assess its global impact on immune cells, we first investigated how NF90 modulated total RNA expression patterns in the human monocytic cell line THP-1. After stably silencing NF90 in THP-1 cells using a short hairpin (sh)RNA (Figure 1(a)), we compared total RNA levels in cells expressing low levels of NF90 (NF90 shRNA) and cells expressing normal levels of NF90 (Ctrl shRNA). RNA sequencing (RNA-seq) analysis (Supplementary Table S1) identified 183 RNAs significantly less abundant (green dots) and 118 significantly more abundant (red dots) in NF90 shRNA cells compared to Ctrl cells (Figure 1(b)). Most transcripts identified in this analysis were protein-coding messenger (m)RNAs (90%), while 6% were long non-coding (lnc)RNAs, and 3% were RNAs encoded by pseudogenes (Figure 1(c)).
Figure 1.
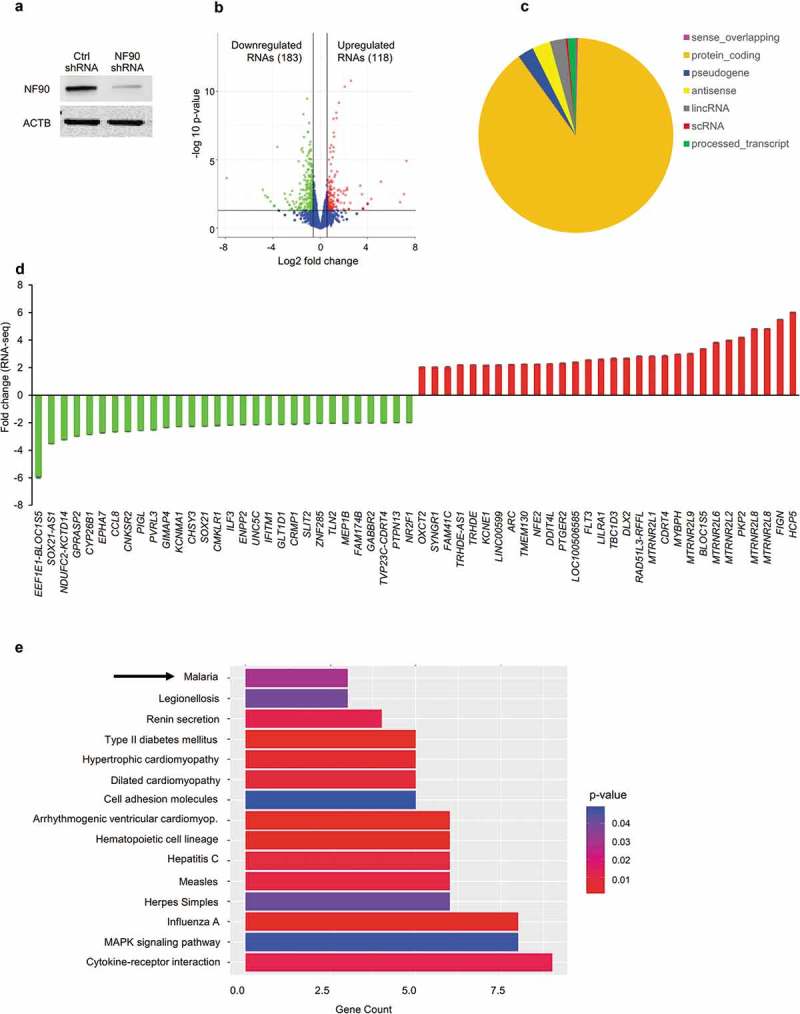
RNA-seq analysis of THP-1 cells expressing different levels of NF90. (a) Western blot analysis of THP-1 cell populations with stably reduced NF90 levels (NF90 shRNA) or normal levels (Ctrl shRNA). (b) Volcano plot representation of the differential abundance of RNAs in THP-1 cells (Ctrl shRNA vs NF90 shRNAs) in the two different comparison groups; reduced (green) and increased (red) mRNAs are highlighted. (c) Classification of the THP-1 cell transcriptome based on the coding potential of the transcripts expressed. (d) Among the mRNAs showing differential abundance, those displaying the greatest fold increases (red) and decreases (green) by RNA-seq analysis were plotted. (e) KEGG pathway enrichment analysis of the mRNAs differentially expressed in THP-1 cells from panel (b), containing normal or reduced NF90 levels.
For the RNAs most differentially abundant, relative (fold) levels were calculated by comparing RNA-seq results from NF90 shRNA cells with Ctrl shRNA. Quantification of these signals revealed that GPRASP2, CCL8, and NF90/ILF3 mRNAs, as well as antisense lncRNA SOX21-AS1 decreased in NF90 shRNA cells, while ARC, FLT3, LILRA1, FIGN and HCP5 mRNAs increased (Figure 1(d)); see Supplementary Table S1 for a full list. KEGG pathway analysis of the mRNAs differentially regulated in NF90 shRNA cells (Figure 1(e)) revealed that the encoded proteins were implicated in biological processes related to cytokine receptor interaction and infectious diseases. Interestingly, several of the mRNAs differentially expressed were implicated in the response to malaria infection, a disease caused by Plasmodium parasites and a major public health problem worldwide. We thus focused on the role of NF90 in regulating mRNAs implicated in the immune response to malaria infection.
NF90 binds to and regulates mRNAs encoding immune-related proteins
Proteins such as fms-related tyrosine kinase 3 (FLT3), leukocyte immunoglobulin-like receptor 1 (LILRB1), complement receptor 1 (CR1), tumor necrosis factor (TNF), the acute-phase-regulated monocyte/macrophage membrane receptor CD163, and the inflammatory/inducible chemokines CXCL10, CXCL11, CCL2 and CCL8 are involved in the response to malaria infection [25–30] and were selected for further analysis (Figure 2(a)).
Figure 2.
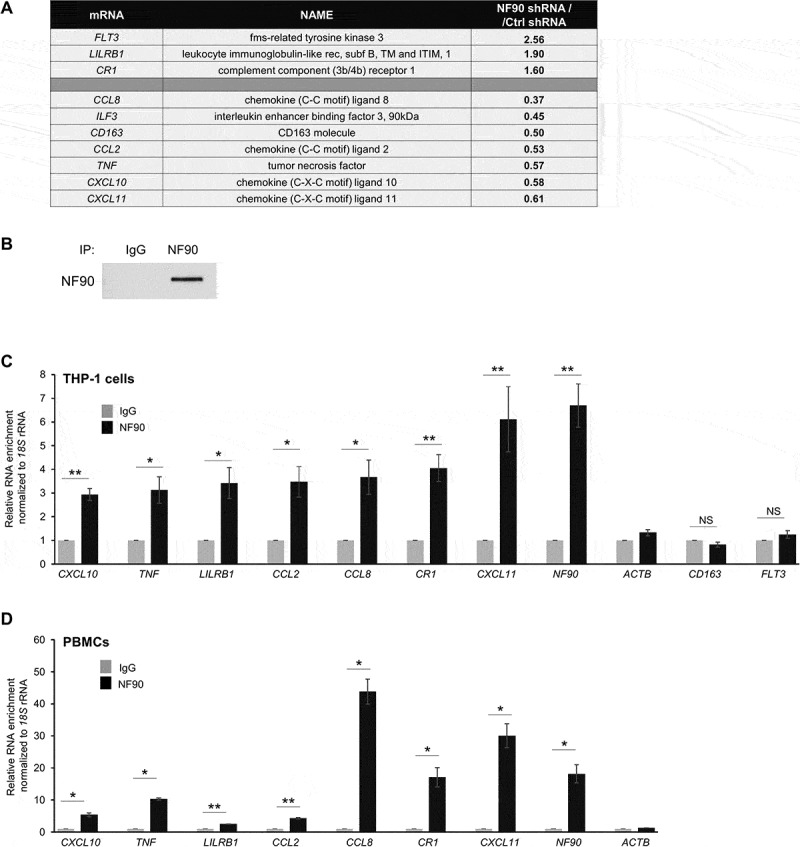
In THP-1 cells, NF90-associated mRNAs encode proteins implicated in the immune response to malaria infection. (a) Major NF90 targets implicated in the immune response to malaria infection; fold changes in abundance after silencing NF90 are indicated in the right column. (b) After immunoprecipitation (IP) using anti-NF90 or IgG antibodies, the presence of NF90 in the IP material was assayed by Western blot analysis. (c,d) Ribonucleoprotein immunoprecipitation (RIP) analysis was conducted using lysates from THP-1 cells (c) and primary PBMCs (d) using either anti-NF90 or IgG antibodies. The levels of target mRNAs in the ribonucleoprotein (RNP) complexes present in the IgG IP and NF90 IP samples were measured by RT-qPCR analysis. The levels of enrichment of the mRNAs in NF90 IP relative to IgG IP were calculated after normalization to 18S rRNA. Data in (c,d) are the means and standard deviation (+SD) from at least three independent experiments. *, P < 0.05; **, P < 0.01, ***, P < 0.005.
Given that NF90 binds a range of RNAs, we hypothesized that NF90 might directly associate with and regulate the fate of several of the selected mRNAs. We performed ribonucleoprotein (RNP) immunoprecipitation (RIP) analysis using lysates of THP-1 cells and anti-NF90 antibody or normal mouse IgG as control, under conditions that preserved the integrity of NF90 RNPs complexes; NF90 was effectively enriched in the IP material, as assessed by Western blot analysis (Figure 2(b)). The RNA present in the RIP samples was isolated and subjected to reverse transcription (RT) followed by quantitative real-time (q)PCR analysis to monitor the levels of mRNAs in NF90 IP compared to IgG IP. Most mRNAs tested were significantly enriched in the NF90 RIP from THP-1 lysates, supporting the notion that these mRNAs interacted with NF90 in THP-1 cells; CD163 and FLT3 mRNAs were not enriched, suggesting that these RNAs did not associate with NF90 (Figure 2(c)). To validate these results and further determine if the same complexes might exist in human primary cells, we performed RIP analysis using purified peripheral blood mononuclear cells (PBMCs) from human donors. Binding of the target mRNAs (Figure 2(c)) was confirmed by NF90 RIP analysis using primary PBMC lysates (Figure 2(d)). ACTB mRNA levels in the same IP samples were measured to monitor differences in sample input [31] (Figure 2(c,d)). Collectively, these findings indicate that NF90 associates with several mRNAs encoding proteins involved in the immune response to malaria.
To test if NF90 modulated the levels of these mRNAs, we used RT-qPCR analysis to measure the steady-state levels of mature mRNAs (Figure 3(a)). As shown, NF90 ablation in THP-1 cells lowered the steady-state abundance of CXC10, TNF, CCL2, CCL8, CXCL11, and NF90 mRNAs, while it modestly elevated the abundance of LILRB1 and CR1 mRNAs. We also measured the levels of the corresponding pre-mRNAs (Figure 3(b)) as surrogate indicators of de novo transcription; several mRNAs showed trends in steady-state levels that mirrored the changes observed in pre-mRNA levels, suggesting that NF90 might help control their transcription. However, for others (e.g. NF90 and CCL8 mRNAs), changes in the pre-RNA levels did not match the observed changes in steady-state mRNA levels, suggesting that NF90 may not control their transcription and instead may influence the stability of these mRNAs (Figure 3(a,b)). To investigate this possibility directly, we measured the half-lives of the same subset of mRNAs by treating cells with actinomycin D (which inhibits RNA polymerase II and thus blocks de novo transcription), collecting RNA at different times thereafter, and measuring the time required to reduce mRNAs to one-half of their initial abundance (t1/2 ; Figure 3(c)). The loss of CCL8, TNF, CXCL10 and CXCL11 mRNAs was significantly greater (P < 0.05) at some time points in NF90-silenced cells, suggesting that NF90 increased their stability at least moderately (Figure 3(c)). The relative stabilities of other mRNAs were comparable between groups.
Figure 3.
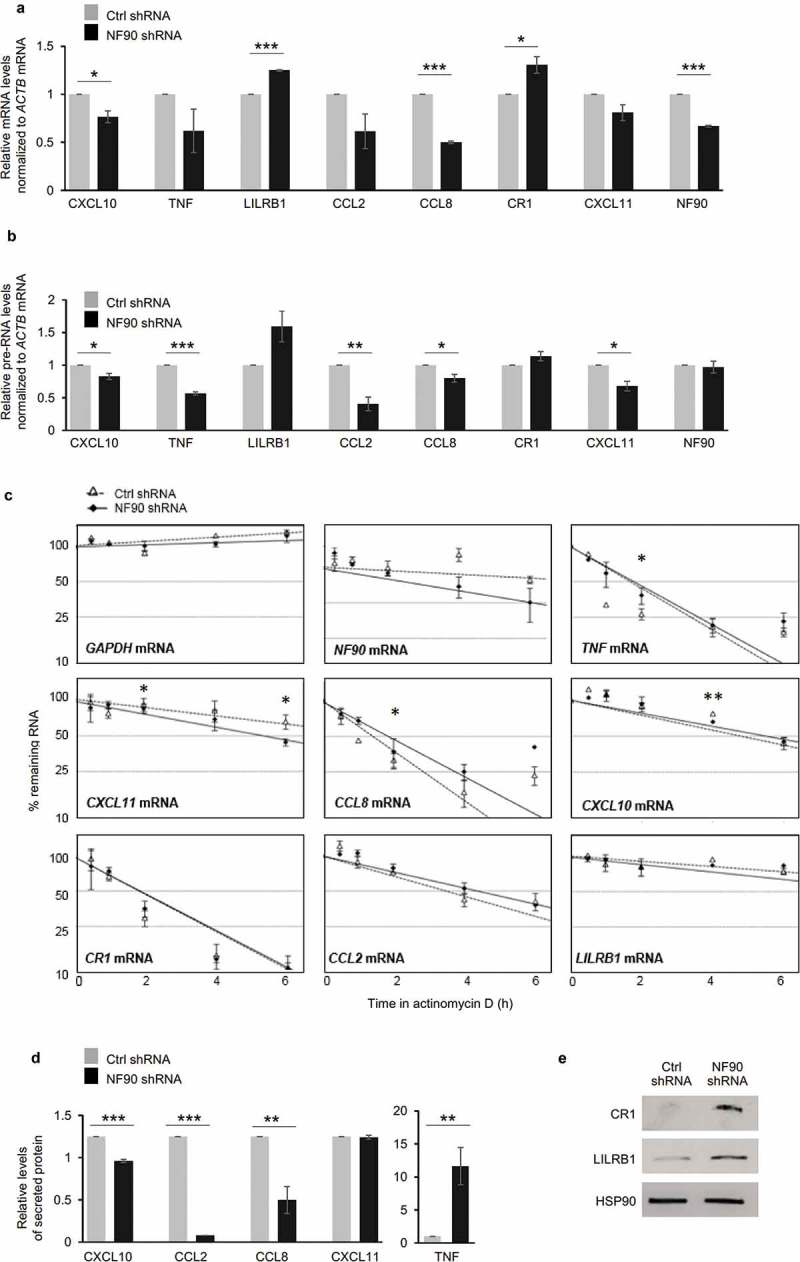
Influence of NF90 on target mRNAs and encoded proteins. (a,b) THP-1 cell populations expressing normal (Ctrl shRNA) or reduced NF90 (NF90 shRNA) (as in Figure 1(a)) were used to prepare total RNA, and the levels of mature mRNAs (a) or pre-mRNAs (b) identified as targets of NF90 (Figure 2(a)) were measured by RT-qPCR analysis. (c) In the cells described in panels (a,b), the relative decay rates of NF90-target mRNAs were assessed by RT-qPCR analysis after treatment with actinomycin D for the indicated times; GAPDH mRNA, a stable transcript, was included as negative control, and mRNA levels were normalized to 18S rRNA levels. Data were plotted on semi-logarithmic scales using Prism. (d,e) The THP-1 cells described in panel (a) were used to quantify by ELISA the levels of protein secreted (d) and to assess by Western blot analysis the levels of protein in the cell lysates (e). In (a-d) data are the means and standard deviation (+SD) from at least three independent experiments. *, P < 0.05; **, P < 0.01, ***, P < 0.005.
To study if NF90-elicited changes in mRNA levels were reflected in changes in protein production, we employed ELISA and Western blot analysis, according to the detection methods available for each protein. ELISA data indicated that lowering NF90 levels reduced significantly the secretion of CCL2, CCL8 and CXCL10 (Figure 3(d)), while it increased the levels of secreted TNF (Figure 3(d)). Proteins CR1 and LILRB1, detectable by Western blot analysis in whole-cell lysates, showed strong upregulation after NF90 silencing (Figure 3(e)). Together, these results indicate that NF90 modulates the transcription and stability of several mRNAs encoding factors that influence the response to malaria proteins. With the exception of TNF, NF90-elicited changes in mRNA levels were reflected in changes in protein production and secretion in THP-1 cells.
NF90-regulated response to treatment with P. falciparum antigens
In light of the aforementioned effects of NF90 on immune response factors, we investigated whether the cellular response to P. falciparum antigens was influenced by NF90. THP-1 cells expressing normal or reduced NF90 levels were incubated with lysates prepared from red blood cells (RBCs) (Methods) that had been mock-infected or infected with P. falciparum (Figure 4(a)). Lysates contained hemozoin, a dark aggregate of free heme released after digestion of hemoglobin by P. falciparum; hemozoin was readily visible in treated THP-1 cells but not in control cells (Figure 4(b)). Twelve lysate equivalents from infected and uninfected RBCs (iRBCs and uRBCs, respectively) were used for each THP-1 cell line, and RNA and protein were extracted 48 h later. Western blot analysis revealed that NF90 levels did not change following exposure to P. falciparum antigens (Figure 4(c)). Treatment with P. falciparum antigens influenced the expression of the subset of mRNAs and pre-mRNAs encoding the cytokines and chemokines of interest (Figure 4(d,e)). Interest-ingly, however, cells expressing reduced NF90 levels showed lower inductions of CXCL10, TNF, CCL2, CCL8, and CXCL11 mRNAs in response to the P. falciparum antigens; these trends were mirrored by changes in pre-mRNAs in most cases, but not for LILRB1 or CCL2 mRNAs (Figure 4(e)).
Figure 4.
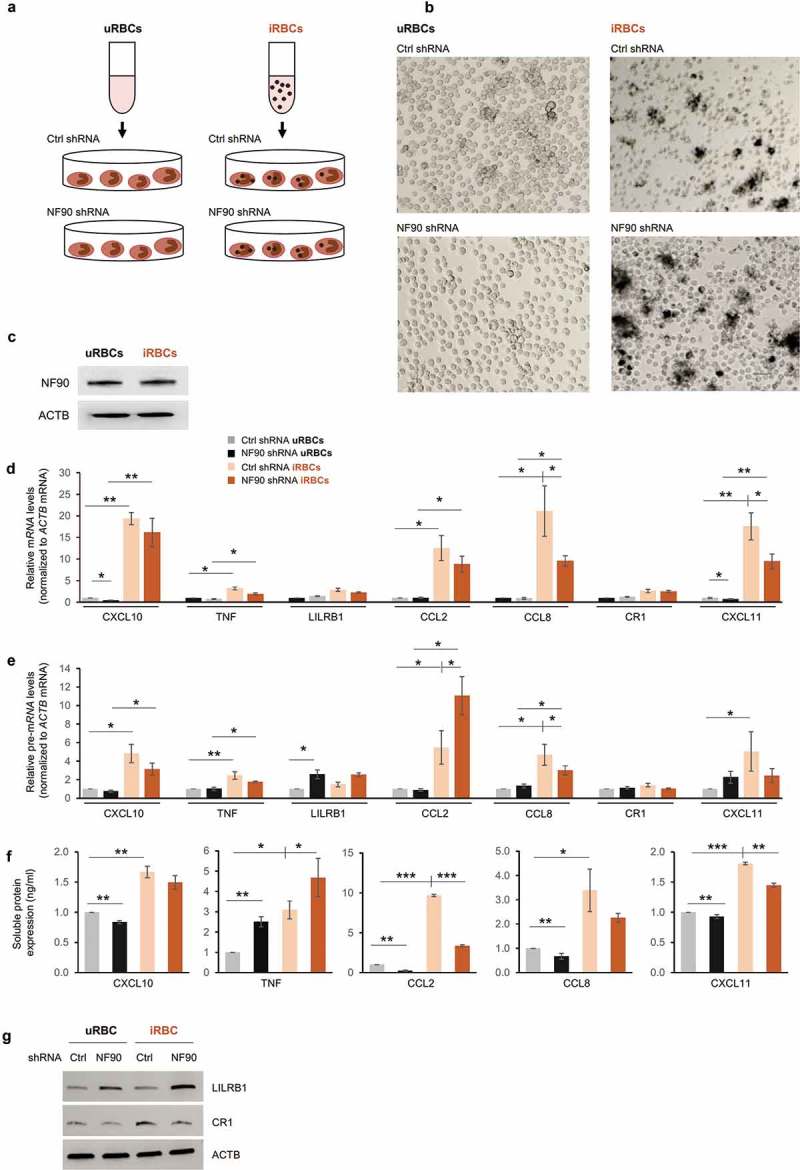
NF90 modulates the response to exposure to P. falciparum antigens. (a, b) Schematic representation (a) and micrographs (b) of THP-1 cells treated with lysates from red blood cells uninfected or infected with P. falciparum (uRBCs, iRBCs), as prepared by lysis of mycoplasma-free culture of RBCs at the schizont stage. THP-1 cells expressing normal (Ctrl shRNA) or reduced NF90 (NF90 shRNA) were treated with iRBCs or uRBCs for 48 h; conditioned media and cell pellets were then collected and used for downstream applications. (c-g) In THP-1 cells treated as in (b), the levels of NF90 and ACTB were assessed by Western blot analysis (c) and the levels of the indicated mRNAs (d) and pre-mRNAs (e) were measured by RT-qPCR analysis. The levels of protein secreted were quantified by ELISA (f) and the levels of protein produced were assessed by Western blot analysis (g). Data in (d-f) are the means and standard deviation (+SD) from at least three independent experiments. *, P < 0.05; **, P < 0.01, ***, P < 0.005.
We then assessed the impact of P. falciparum antigens on protein production and whether NF90 affected this process. We used ELISA to measure the levels of soluble protein in the supernatants of THP-1 cells treated with iRBC or uRBC lysates for 48 h. As shown, treatment with P. falciparum lysates (iRBCs) increased significantly the levels of all secreted cytokines compared with treatment with uRBCs (Figure 4(f)), with TNF, CCL2 and CCL8 showing the strongest augmentations. Importantly, silencing NF90 suppressed the iRBC-elicited increase in CCL2 and CXCL11 levels and lowered the basal levels of CXCL10, CCL2, CCL8, and CXCL11. Silencing NF90 increased TNF levels in cells treated with either iRBC or uRBC, supporting the notion that NF90 is a general repressor of TNF production (Figure 4(f)). Western blot analysis revealed that LILRB1 levels similarly increased in NF90-silenced cells treated with uRBC lysates and even more in cells treated with iRBC lysates, although LILRB1 levels did not rise following iRBC treatment alone (Figure 4(g)). By contrast, CR1 levels increased after treatment with iRBC, but NF90 silencing prevented this elevation (Figure 4(g)).
Given the discrepancies between mRNA levels and protein levels for the NF90 targets studied, we investigated if silencing NF90 and/or treating with P. falciparum lysates (iRBC groups) affected the translation of some of these mRNAs. A measure of the rates of translation was ascertained by fractionating cytoplasmic lysates through linear gradients (10–50% sucrose) and evaluating the sizes of polysomes associated with the mRNAs; shifts to larger polysomes would indicate more active translation and shifts to smaller polysomes would be consistent with reduced translation. The overall polysome populations were comparable in Ctrl relative to NF90 shRNA cells, indicating that the relative abundance of free ribosomal subunits (40S, 60S), monosomes (80S), and low- and high-molecular-weight (LMW and HMW) polysomes did not change substantially after silencing NF90 (Figure 5(a); compare the polysome traces on the left to those on the right). By contrast, exposure to P. falciparum (iRBC) lysates triggered changes consistent with reduced widespread translation, including an overall rise in 80S peaks; in Ctrl shRNA cells, the sizes of LMW and HMW polysome peaks were reduced, although these changes were less apparent in NF90 shRNA cells.
Figure 5.
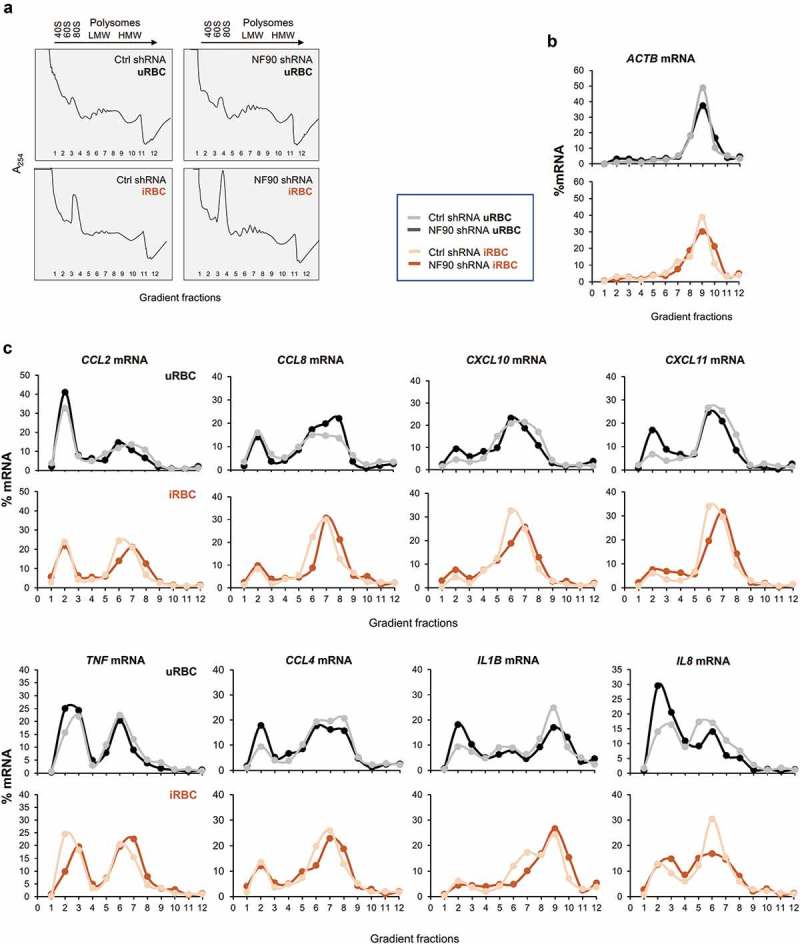
Polysome distribution analysis of NF90 target mRNAs in response to P. falciparum antigens. (a) Cytoplasmic lysates from the THP-1 cell populations described in Figure 1(a) that had been treated with uRBC or iRBC lysates for 48 h were fractionated through sucrose gradients (10–50%), revealing the polysome traces shown. RNA was then extracted from fractions 1 and 2 (free RNA/RNPs), fractions 3 and 4 [ribosomal subunits (40S, 60S) and monosomes (80S)], fractions 5–8 [low-molecular-weight (LMW) polysomes], and fractions 9–11 [high-molecular-weight (HMW) polysomes]; fraction 12 typically had little or no RNA. (b,c) The relative distribution of ACTB mRNA, expressing a housekeeping protein (β-Actin), was assessed by RT-qPCR analysis and the relative levels of ACTB mRNA in each fraction (%) relative to the total ACTB mRNA in the gradient was plotted in order to identify global changes in translation as a function of NF90 levels and exposure to P. falciparum (b). The relative distribution from NF90 target mRNAs was assessed as described in (b) and plotted individually for the mRNAs shown. The data are representative of two independent analyses yielding similar results.
Following isolation of RNA from each polysome fraction, relative mRNA distributions were assayed by quantifying specific mRNAs in each fraction using RT-qPCR analysis, and calculating their relative distribution (%) along the sucrose gradient. The distribution of ACTB mRNA, encoding the housekeeping protein ACTB (β-Actin), was monitored to test overall translation in THP-1 cells treated with either control lysates or P. falciparum lysates (uRBC, iRBC), as well as under normal or silenced NF90 levels (Ctrl shRNA, NF90 shRNA) (Figure 5(b)). For many of the mRNAs tested (Figure 5(c)), cells in the iRBC lysate group displayed increased polysome sizes and decreased 40S/60S/80S fractions. For example, for CCL2 mRNA, the robust nonpolysomal peak seen in fraction 2 of the uRBC group was diminished in the iRBC group, while there was a compensatory rise in the polysomal peaks at fractions 6 and 7 from the uRBC group to the iRBC group. Comparable shifts were seen for example for CCL8, CCL4, IL1B, and IL8 mRNAs. These data suggested that exposure to P. falciparum lysates enhanced the translation of several key NF90 target mRNAs. In addition, for many mRNAs, silencing NF90 shifted polysome sizes to moderately larger forms; this was somewhat apparent after uRBC treatment (e.g. for CCL8 mRNA), but it was more apparent after iRBC treatment (e.g. for CCL2, CCL8, CXCL10, TNF, CCL4, and IL1B mRNAs). Together, these results suggested that exposure to malaria antigens in iRBC lysates increased the translation of several mRNAs, while NF90 prevented excess translation.
Since the expression of these cytokines fundamentally affects the response of THP-1 cells to P. falciparum antigens, we studied the role of NF90 in regulating the expression of key players of the immune response to malaria.
NF90 regulated CD14 expression in THP-1 cells
P. Falciparum antigens induce modifications of the clusters of differentiation (CD) proteins expressed by antigen-presenting cells, such as monocytes. Surface antigens CD14 and CD16, expressed on monocytes/macrophages to elicit the innate immune response, are widely used as monocyte-macrophage differentiation markers [32]. The percentages of CD14+ and CD16+ THP-1 cells expressing normal or reduced NF90 levels were assessed 48 h after treatment with iRBC or uRBC lysates (Figure 6(a)). As determined by measuring the populations in quadrant Q1, lower percentages of CD14+ THP-1 cells were recovered after treatment with iRBC lysates compared with uRBC lysates; interestingly, however, silencing of NF90 increased the abundance of CD14+ THP-1 cells, suggesting a role for NF90 in this process (Figure 6(a,b)). CD16+ THP-1 cells were low or absent, as previously reported [32], in keeping with the fact that CD16 identifies macrophages and is low in monocytes.
Figure 6.
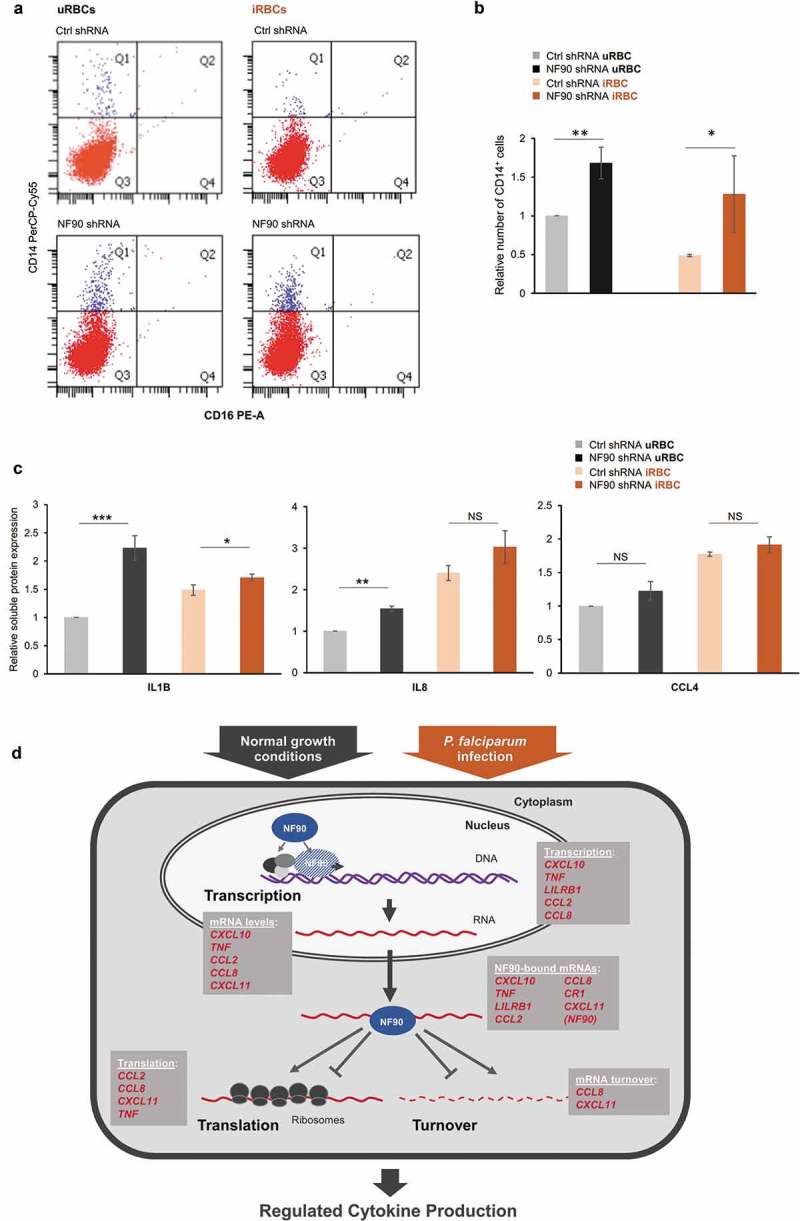
NF90 regulates CD14 expression. (a) Analysis of CD14+ and CD16+ THP-1 cells (Ctrl or NF90 shRNA) 48 h after treatment with iRBC or uRBC lysates. (b) Relative number of CD14+ THP-1 cells following treatment with P. falciparum antigens in the presence of normal or reduced NF90 levels. (c) Bioplex analysis of the levels of IL1B, IL8, and CCL4 expressed by the cells in panel (a). (d) Proposed model. In untreated cells or cells treated with P. falciparum antigens, NF90 controls the production of key immune response genes by modulating transcription, mRNA stability, and translation. Collectively, these actions contribute to mounting an adaptive gene expression program. In (b,c), data are the means and standard deviation (+SD) from at least three independent experiments. *, P < 0.05; **, P < 0.01, ***, P < 0.005.
Finally, we analyzed the general impact of NF90 on the response of THP-1 cells. Supernatants from cells exposed to malaria antigens (iRBCs vs uRBCs) were used to measure cytokines and chemokines more globally using a Bioplex instrument (Methods). Exposure to malaria antigens increased the levels of IL1B, IL8 and CCL4, in line with the pro-inflammatory phenotype triggered by P. falciparum antigens. Cells expressing reduced NF90 levels produced higher basal levels of IL1B and IL8 following exposure to uRBC lysates and generally increased the levels of IL1B, IL8 and CCL4, although the differences only reached significance for IL1B (Figure 6(c)). Unfortunately, other interesting cytokines were below the detection level of the multiplex assay.
In summary, these results support a key role for NF90 in regulating the production of immune proteins in response to malaria antigens. Directly or indirectly, NF90 modulates the expression of these proteins by influencing different aspects of RNA metabolism, including transcription, mRNA turnover, and translation (Figure 6(d)). The RNAs regulated by NF90 during the immune response to malaria antigens are indicated (Figure 6(d)).
Discussion
We have investigated the impact of NF90, a protein that binds RNA and DNA, on the gene expression program of the monocytic leukemia cell line THP-1. By RNA-seq analysis of THP-1 cells expressing normal or reduced levels of NF90, we found that NF90 influenced the abundance of a subset of mRNAs encoding proteins that were strongly implicated in the response of innate immune cells to malaria infection (Figure 1). The impact of NF90 on the abundance of immune response factors reflected the various types of control of gene expression, transcriptional and post-transcriptional, elicited by NF90 on previously reported targets [4,14].
Among those NF90-regulated mRNAs involved in the response to malaria, NF90 appeared to be necessary for the transcriptional changes of several mRNAs, including TNF, LILRB, and CCL2 mRNA (Figure 3(a,b)). NF90 was reported to influence the transcription of other immune genes, including IL2 and IL13; the effect of NF90 on transcription was likely via direct binding to promoter elements or through indirect influence on other transcriptional regulators. Whether NF90 influences the transcription of immune-related genes in THP-1 cells by directly binding to regulatory regions or by indirectly influencing the activities of other transcription factors remains to be studied in molecular detail.
NF90 was also found to form a complex with some of these mRNAs and influenced their stability and translation, two connected processes. In this regard, NF90 was previously shown to alter the turnover of IL2 and VEGF mRNAs [6,14,15,17,18] and repressed the translation of CCL16 and IL8 mRNAs in fibroblasts [16]. In this study, NF90 appeared to stabilize some target mRNAs with which it interacted, including CCL8 and CXCL11 mRNAs (Figure 3), although at present we do not know the molecular details of this influence. NF90 repressed the translation of several interacting mRNAs (e.g. CCL2, CCL8, CXCL10, TNF, CCL4, and IL1B mRNAs) (Figure 5), but this effect also awaits mechanistic analysis. Whether NF90 modulates the half-lives and translation rates of these mRNAs via direct binding and whether it competes or cooperates with other decay/translation factors (e.g. microRNAs or other RBPs) remains to be investigated for each mRNA. In this regard, we recently reported that BAFF mRNA was subject to translational repression by NF90 in cooperation with miR-15a [19], whereas NF90 and HuR jointly increased the stability of MKP-1 (DUSP1) mRNA [15]. Like other RBPs, the different post-transcriptional outcomes of NF90 target mRNAs are likely a reflection of its combinatorial interaction with other RBPs and microRNAs in the cell; these interactions themselves depend on the cell type and the specific RNP complexes forming on a given mRNA during particular growth conditions.
To understand how NF90 influenced the immune response to malaria infection, lysates of red blood cells that had been infected (iRBC) or not (uRBCs) with P. falciparum were used as a surrogate of malaria infection in THP-1 cells expressing normal or reduced levels of NF90. The results (Figures 4–6) indicate that NF90 affects the expression levels of several cytokines in response to P. falciparum antigens, underscoring the key role of NF90 in controlling the patterns of proteins produced in the presence of malaria antigens. For a number of genes (CXCL10, CXCL11, CCL2, CCL8, and TNF), pre-mRNA, mRNA, and protein levels were elevated by exposure to P. falciparum lysates. The influence of NF90 and P. falciparum lysates followed the trends in protein production for most of these cytokines – CXCL10, CCL2, CCL8, and CXCL11 (Figure 4(f)) – but for TNF, protein secretion did not follow the trend predicted by the changes in TNF mRNA levels, since suppressing NF90 reduced TNF mRNA levels but increased TNF secretion (Figure 4).
The influence of NF90 on the expression of TNF and CCL2 was particularly interesting. For TNF, both the presence of NF90 and exposure to P. falciparum antigens increased TNF mRNA levels [likely via transcription, as TNF pre-mRNA followed the same abundance pattern (Figure 3(a,b))], but TNF secretion was instead lower in the presence of NF90 regardless of P. falciparum antigens (Figure 4). Analysis of polysomes associated with TNF mRNA helped to reconcile these observations, as polysome sizes increased after silencing NF90 in the P. falciparum antigen groups (iRBC, Figure 5). For CCL2, NF90 similarly appeared to promote gene transcription, as silencing NF90 lowered CCL2 pre-mRNA and mRNA levels (Figure 3(a,b)), while NF90 seemed to promote CCL2 translation, as CCL2 mRNA polysomes were slightly smaller when NF90 was silenced in uninfected cells (Figure 5(c)) and CCL2 protein levels were strongly reduced (Figure 3(d)). These sets of findings suggest that for TNF and CCL2, NF90 may first control transcription, and then possibly was loaded co-transcriptionally onto the TNF and CCL2 pre-mRNAs to modulate their post-transcriptional fates. This dual regulation was previously shown in T cells for another NF90 target, IL2 [6,33]. NF90 associated with the IL2 proximal promoter to promote transcription [34], and it bound the 3’UTR of the transcribed IL2 mRNA [33] to control the nuclear export of mature IL2 mRNA to the cytoplasm [4].
These molecular changes were translated into changes in cellular responses. The reduction of CD14+ THP-1 cells treated with iRBC lysates, compared with uRBC lysates, mimicked the decline in circulating CD14+ monocytes observed previously in individuals infected with P. falciparum [24,35], as monocytes differentiate into macrophages and dendritic cells to respond to the infection. In this scenario, NF90 appears to contribute to reducing CD14+ cells, since silencing NF90 elevated the CD14+ pool.
Collectively, our results indicate that NF90 can function as both a positive and a negative regulator of the transcription, mRNA stability, translation, and secretion of key immune factors implicated in the response to malaria antigens. Accordingly, NF90 can regulate positively some cytokines (e.g. CCL2, CR1) and negatively other cytokines (e.g. TNF, LILRB1). This versatility is likely conferred by the ability of NF90 to bind DNA and RNA, and interact functionally with other RBPs and microRNAs. Whether NF90 phosphorylation or other post-translational modifications affect NF90 binding to target DNA/RNA or its downstream function is unknown [2,4]. It seems that NF90 may have a key role in fine-tuning the production of immune factors in basal conditions as well as in response to immune modulators such as malaria antigens. The findings in this report provide a framework for deeper investigation of the impact of NF90 on the immune response to malaria antigens and for efforts to identify therapeutic modulators of NF90 function.
Methods
Cell culture
The human acute monocytic leukemia cell line THP-1 was cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and antibiotics. THP-1 cells expressing normal levels or low levels of NF90 (Ctrl or NF90 shRNA) used in this study were described previously [19]. Primary PBMCs were purified from genotyped healthy human donors from the Sardinia cohort [20] and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and antibiotics.
Western blot analysis
Whole-cell lysates were prepared in RIPA buffer, size-fractionated through 4–12% gradient polyacrylamide gels (Thermo Fisher Scientific), and transferred to a nitrile membranes (Biorad). Membranes were incubated for 16 h with primary antibodies recognizing NF90 (BD Transduction Laboratories), HSP90, ACTB (Santa Cruz Biotechnology), LILRB1, CR1, CXCL10 or TNF (Abcam). Secondary antibodies conjugated with horseradish peroxidase (HRP) (Kindle Biosciences) were detected by enhanced chemiluminescence (Kevik).
Analysis of polysome gradients
For polysome analysis, lysates were prepared from THP-1 cells (Ctrl or NF90 shRNA; treated with lysates from uRBCs or iRBCs) and incubated with 0.1 mg/ml cycloheximide for 10 min. Cytoplasmic extracts were then fractionated through a linear sucrose gradient [10–50% (w/v)] as previously reported [36]. Fractions were collected using a fraction collector and monitored by optical density measurement (A254) (Brandel). The RNA in each fraction was isolated and the relative enrichment analyzed using RT-qPCR and the primers listed (Supplementary Table S2).
Preparation of P. falciparum-infected red blood cell (RBC) lysates
Lysates were prepared from red blood cells that had been infected with P. falciparum (iRBC) or were left uninfected (uRBC), as previously described [37]. Briefly, P. falciparum parasites were maintained in fresh human ORh+ erythrocytes at 3% hematocrit in RPMI 1640 medium (KD Medical) supplemented with 10% heat-inactivated ORh+ human serum, 7.4% sodium bicarbonate (GIBCO, Invitrogen) and 25 µg/ml of gentamycin (GIBCO, Invitrogen), at 37°C in the presence of a gas mixture containing 5% O2, 5% CO2 and 90% N2. Mycoplasma-free cultures of iRBCs at schizont stage were isolated using magnetic columns (LD MACS Separation Columns, Miltenyi Biotec). Lysates of P. falciparum (iRBCs) were obtained by three freeze-thaw cycles in liquid nitrogen and 37°C water bath. THP-1 cells expressing normal levels (Ctrl shRNA) or low levels of NF90 (NF90 shRNA) were treated with iRBC or uRBC lysates for 48 h; conditioned media and cell pellets were collected for analysis.
Conditioned media were used for human cytokine analysis with the Bio-Rad Bio-Plex Pro Human Cytokine 17-plex assay according to the manufacturer’s instructions (Bio-Rad Laboratories). The abundance of CCL2, CCL8, TNF, CXCL10, CXCL11 was quantified from conditioned media using specific ELISA kits (R&D Systems and Lifespan Biosciences).
RNA isolation, reverse transcription (RT)-quantitative (q)PCR analysis and RNA sequencing
RNA was isolated from THP-1 cells using the TriPure isolation reagent (Roche) following the manufacturer’s protocol. Total RNA was reverse-transcribed (RT) into cDNA using Maxima reverse transcriptase (Thermo Fisher) and random hexamers, and subsequently analyzed by quantitative (q)PCR analysis using SYBR Green mix (Kapa Biosystems). The relative levels of RNA were calculated by the 2-ΔCt method and 18S rRNA levels were calculated for normalization. Gene-specific primer pairs are listed in Supplementary Table S2. For RNA sequencing, RNA integrity was assessed by using the Agilent Bioanalyzer 2100, and only samples with clean rRNA peaks were used for subsequent steps. Libraries for RNA-seq were prepared according to KAPA Stranded RNA-Seq Kit with RiboErase (KAPA Biosystems, Wilmington, MA) system. Paired-end sequencing was performed on an Illumina HighSeq 4000 instrument (Illumina Inc., San Diego, CA).
Immunoprecipitation of ribonucleoprotein (RNP) complexes (RIP analysis)
Lysates of THP-1 cells were prepared using PEB buffer (10 mM Hepes, 100 mM KCl, 5 mM MgCl2, 25 mM EDTA, 0.5% IGEPAL, 2 mM DTT, 50 U/ml RNase out and protease inhibitors); after clarification, 200 μg of lysates were incubated (2 h, 4°C) with a suspension of protein-A Sepharose beads precoated overnight with 5 μg of a specific antibody or normal IgG (BD Transduction Laboratories) as a control. Following incubation, the beads were washed with NT2 buffer (50 mM Tris–HCl pH 7.5, 150 mM NaCl, 1 mM MgCl2, 0.05% IGEPAL) and then treated with RNase-free DNase I (15 min, 30°C). RNA levels were assessed by RT-qPCR analysis using the primers listed in Supplementary Table S2; after normalization to 18S rRNA levels, enrichments were calculated as the relative abundance of a given mRNA in NF90 IP compared to IgG IP.
Analysis of mRNA stability by actinomycin D assays
THP-1 cells were serum-starved overnight, then incubated with Actinomycin D (Act D; 2.5 µg/ml) to block de novo transcription. Cells were harvested at subsequent time intervals (0, 2, 4 and 6 h) and total RNA was extracted and processed as described by RT-qPCR analysis. Data from Act D assays were processed using the Prism3.03 software to assess mRNA decay curves. Error bars represent the SEM of three independent experiments.
FACS analysis
THP-1 cells were washed in PBS with 1% BSA (bovine serum albumin) and incubated for 20 min at 4°C with Fc receptor Blocker (BD Biosciences). Subsequently cells were incubated 20 min at 4°C with fluorescently labeled antibodies specific for CD14-PerCP/Cy5.5 (M5E) and CD16-PE purchased from BD Biosciences. FACS analyses were performed on a BD FACSCanto™ (BD Biosciences) and analyzed using FlowJo software (Tree Star, Inc).
Statistical analysis
Statistical significance was determined using two-tailed t-tests, as indicated in the Figure legends. Values were considered significant when p < 0.05.
Supplementary Material
Funding Statement
This work was supported by the NIA Z01-AG000511-19.
Acknowledgments
This work was supported by the NIA-IRP and NIAID-IRP, NIH. MLI, VL, FC were supported by the Fondazione di Sardegna (ex Fondazione Banco di Sardegna, Prot. U1301.2015/AI.1157.BE Prat. 2015-1651). We thank Peter D. Crompton (NIAID, NIH) for assistance with the preparation of RBC lysates. We thank Giuseppe Delogu for technical support and critical comments on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Cassiday LA, Maher LJ.. Having it both ways: transcription factors that bind DNA and RNA. Nucleic Acids Res. 2002;30:4118–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hudson WH, Ortlund EA.. The structure, function and evolution of proteins that bind DNA and RNA. Nat Rev Mol Cell Biol. 2014;15:749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Duchange N, Pidoux J, Camus E, et al. Alternative splicing in the human interleukin enhancer binding factor 3 (ILF3) gene. Gene. 2000;261:345–353. [DOI] [PubMed] [Google Scholar]
- [4].Castella S, Bernard R, Corno M, et al. Ilf3 and NF90 functions in RNA biology. Wiley Interdiscip Rev RNA. 2015;6:243–256. [DOI] [PubMed] [Google Scholar]
- [5].Wu T-H, Shi L, Adrian J, et al. NF90/ILF3 is a transcription factor that promotes proliferation over differentiation by hierarchical regulation in K562 erythroleukemia cells. PLoS ONE. 2018;13:e0193126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shim J, Lim H, Yates JR, et al. Nuclear export of NF90 is required for interleukin-2 mRNA stabilization. Mol Cell. 2002;10:1331–1344. [DOI] [PubMed] [Google Scholar]
- [7].Xu YH, Grabowski GA. Molecular cloning and characterization of a translational inhibitory protein that binds to coding sequences of human acid beta-glucosidase and other mRNAs. Mol Genet Metab. 1999;68:441–454. [DOI] [PubMed] [Google Scholar]
- [8].Isken O, Grassmann CW, Sarisky RT, et al. Members of the NF90/NFAR protein group are involved in the life cycle of a positive-strand RNA virus. Embo J. 2003;22:5655–5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Isken O, Baroth M, Grassmann CW, et al. Nuclear factors are involved in hepatitis C virus RNA replication. Rna. 2007;13:1675–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li X, Liu C-X, Xue W, et al. Coordinated circRNA biogenesis and function with NF90/NF110 in viral infection. Mol Cell. 2017;67:214–227.e7. [DOI] [PubMed] [Google Scholar]
- [11].Sakamoto S, Aoki K, Higuchi T, et al. The NF90-NF45 complex functions as a negative regulator in the microRNA processing pathway. Mol Cell Biol. 2009;29:3754–3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Corthésy B, Kao PN. Purification by DNA affinity chromatography of two polypeptides that contact the NF-AT DNA binding site in the interleukin 2 promoter. J Biol Chem. 1994;269:20682–20690. [PubMed] [Google Scholar]
- [13].Kiesler P, Haynes PA, Shi L, et al. NF45 and NF90 regulate HS4-dependent interleukin-13 transcription in T cells. J Biol Chem. 2010;285:8256–8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kuwano Y, Pullmann R, Marasa BS, et al. NF90 selectively represses the translation of target mRNAs bearing an AU-rich signature motif. Nucleic Acids Res. 2010;38:225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kuwano Y, Kim HH, Abdelmohsen K, et al. MKP-1 mRNA stabilization and translational control by RNA-binding proteins HuR and NF90. Mol Cell Biol. 2008;28(14):4562–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tominaga-Yamanaka K, Abdelmohsen K, Martin-dale JL, et al. NF90 coordinately represses the senescence-associated secretory phenotype. Aging (Albany NY). 2012;4:695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vumbaca F, Phoenix KN, Rodriguez-Pinto D, et al. Double-stranded RNA-binding protein regulates vascular endothelial growth factor mRNA stability, translation, and breast cancer angiogenesis. Mol Cell Biol. 2008;28:772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hoque M, Shamanna RA, Guan D, et al. HIV-1 replication and latency are regulated by translational control of cyclin T1. J Mol Biol. 2011;410:917–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Idda ML, Lodde V, McClusky WG, et al. Cooperative translational control of polymorphic BAFF by NF90 and miR-15a. Nucleic Acids Res. 2018;108:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Steri M, Orrù V, Idda ML, et al. Overexpression of the cytokine BAFF and autoimmunity risk. N Engl J Med. 2017;376:1615–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tsuchiya S, Yamabe M, Yamaguchi Y, et al. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980;26:171–176. [DOI] [PubMed] [Google Scholar]
- [22].Garcia LS. Malaria. Clin Lab Med. 2010;30:93–129. [DOI] [PubMed] [Google Scholar]
- [23].Chua CLL, Brown G, Hamilton JA, et al. Monocytes and macrophages in malaria: protection or pathology? Trends Parasitol. 2013;29:26–34. [DOI] [PubMed] [Google Scholar]
- [24].Noone C, Parkinson M, Dowling DJ, et al. Plasma cytokines, chemokines and cellular immune responses in pre-school Nigerian children infected with plasmodium falciparum. Malar J. 2013;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stoute JA. Complement receptor 1 and malaria. Cell Microbiol. 2011;13:1441–1450. [DOI] [PubMed] [Google Scholar]
- [26].Tamura T, Kimura K, Yuda M, et al. Prevention of experimental cerebral malaria by Flt3 ligand during infection with plasmodium berghei ANKA. Infect Immun. 2011;79:3947–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nasr A, Allam G, Hamid O, et al. IFN-gamma and TNF associated with severe falciparum malaria infection in Saudi pregnant women. Malar J. 2014;13:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Saito F, Hirayasu K, Satoh T, et al. Immune evasion of plasmodium falciparum by RIFIN via inhibitory receptors. Nature. 2017;9:725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Imani JP. Levels of soluble CD163 and severity of malaria in children in Ghana. Clin Vaccine Immunol. 2008;15:1456–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ioannidis LJ, Nie CQ, Hansen DS. The role of chemokines in severe malaria: more than meets the eye. Parasitology. 2014;141:602–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pullmann R, Kim HH, Abdelmohsen K, et al. Analysis of turnover and translation regulatory RNA-binding protein expression through binding to cognate mRNAs. Mol Cell Biol. 2007;27:6265–6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Aldo PB, Craveiro V, Guller S, et al. Effect of culture conditions on the phenotype of THP-1 monocyte cell line. Am J Reprod Immunol. 2013;70:80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pei Y, Zhu P, Dang Y, et al. Nuclear export of NF90 to stabilize IL-2 mRNA is mediated by AKT-dependent phosphorylation at Ser647 in response to CD28 costimulation. J Immunol. 2008;180:222–229. [DOI] [PubMed] [Google Scholar]
- [34].Shi L, Qiu D, Zhao G, et al. Dynamic binding of Ku80, Ku70 and NF90 to the IL-2 promoter in vivo in activated T-cells. Nucleic Acids Res. 2007;35:2302–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Loharungsikul S, Troye-Blomberg M, Amoudruz P, et al. Expression of toll-like receptors on antigen-presenting cells in patients with falciparum malaria. Acta Trop. 2008;105:10–15. [DOI] [PubMed] [Google Scholar]
- [36].Panda AC, Martindale JL, Gorospe M. Polysome Fractionation to Analyze mRNA Distribution Profiles. Bio Protoc. 2017;7:pii: e2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Portugal S, Moebius J, Skinner J, et al. Exposure-dependent control of malaria-induced inflammation in children. PLoS Pathog. 2014;10:e1004079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


