Abstract
Background
Low molecular weight heparins (LMWHs) have been shown to be effective and safe in preventing venous thromboembolism (VTE). They may also be effective for the initial treatment of VTE. This is the third update of the Cochrane Review first published in 1999.
Objectives
To evaluate the efficacy and safety of fixed dose subcutaneous low molecular weight heparin compared to adjusted dose unfractionated heparin (intravenous or subcutaneous) for the initial treatment of people with venous thromboembolism (acute deep venous thrombosis or pulmonary embolism).
Search methods
For this update the Cochrane Vascular Information Specialist (CIS) searched the Cochrane Vascular Specialised Register (15 September 2016). In addition the CIS searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8) in the Cochrane Library (searched 15 September 2016) and trials' registries.
Selection criteria
Randomised controlled trials comparing fixed dose subcutaneous LMWH with adjusted dose intravenous or subcutaneous unfractionated heparin (UFH) in people with VTE.
Data collection and analysis
Two review authors independently selected trials for inclusion, assessed for quality and extracted data.
Main results
Six studies were added to this update resulting in a total of 29 included studies (n = 10,390). The quality of the studies was downgraded as there was a risk of bias in some individual studies relating to risk of attrition and reporting bias; in addition several studies did not adequately report on the randomisation methods used nor on how the treatment allocation was concealed.
During the initial treatment period, the incidence of recurrent venous thromboembolic events was lower in participants treated with LMWH than in participants treated with UFH (Peto odds ratio (OR) 0.69, 95% confidence intervals (CI) 0.49 to 0.98; 6238 participants; 18 studies; P = 0.04; moderate‐quality evidence). After a follow‐up of three months, the period in most of the studies for which oral anticoagulant therapy was given, the incidence of recurrent VTE was lower in participants treated with LMWH than in participants with UFH (Peto OR 0.71, 95% CI 0.56 to 0.90; 6661 participants; 16 studies; P = 0.005; moderate‐quality evidence). Furthermore, at the end of follow‐up, LMWH was associated with a lower rate of recurrent VTE than UFH (Peto OR 0.72, 95% CI 0.59 to 0.88; 9489 participants; 22 studies; P = 0.001; moderate‐quality evidence). LMWH was also associated with a reduction in thrombus size compared to UFH (Peto OR 0.71, 95% CI 0.61 to 0.82; 2909 participants; 16 studies; P < 0.00001; low‐quality evidence), but there was moderate heterogeneity (I² = 56%). Major haemorrhages occurred less frequently in participants treated with LMWH than in those treated with UFH (Peto OR 0.69, 95% CI 0.50 to 0.95; 8780 participants; 25 studies; P = 0.02; moderate‐quality evidence). There was no difference in overall mortality between participants treated with LMWH and those treated with UFH (Peto OR 0.84, 95% CI 0.70 to 1.01; 9663 participants; 24 studies; P = 0.07; moderate‐quality evidence).
Authors' conclusions
This review presents moderate‐quality evidence that fixed dose LMWH reduced the incidence of recurrent thrombotic complications and occurrence of major haemorrhage during initial treatment; and low‐quality evidence that fixed dose LMWH reduced thrombus size when compared to UFH for the initial treatment of VTE. There was no difference in overall mortality between participants treated with LMWH and those treated with UFH (moderate‐quality evidence). The quality of the evidence was assessed using GRADE criteria and downgraded due to concerns over risk of bias in individual trials together with a lack of reporting on the randomisation and concealment of treatment allocation methods used. The quality of the evidence for reduction of thrombus size was further downgraded because of heterogeneity between studies.
Plain language summary
Fixed daily dose of a low molecular weight heparin compared with an adjusted dose of unfractionated heparin for treating blood clots in the deep veins
Background
Venous thromboembolism (VTE) is a condition in which a blood clot forms in the deep veins of the leg or pelvis (DVT) or the clot travels in the blood and blocks a blood vessel in the lungs (pulmonary embolism (PE)). The chances of getting a VTE can be increased if people have risk factors such as previous clots, prolonged periods of immobility (such as travelling on aeroplanes or bed rest), cancer, exposure to oestrogens (pregnancy, oral contraceptives or hormone replacement therapy), trauma and blood disorders such as thrombophilia (abnormal blood clotting). People with a VTE are treated with an anticoagulant, which prevents further clots from forming. Heparin is an anticoagulant and comes in two forms: low molecular weight heparin (LMWH) or unfractionated heparin (UFH). UFH is an older drug and is given either intravenously or by injection. When administering UFH, clinicians have to monitor blood‐clotting factors carefully and adjust the dose, because of the variability of its effect. LMWH is given by subcutaneous injection once or twice a day and does not need to be monitored as closely as UFH. Study characteristics and key results
This review included 29 randomised controlled trials involving 10,390 participants (current to September 2016), which compared LMWH or UFH for treating people with blood clots. Pooling the results of these trials showed that fewer participants treated with LMWH formed further blood clots and that fewer cases of bleeding occurred. Use of LMWH also reduced the size of the original blood clot when compared to the UFH group. There was no difference in number of deaths between participants treated with LMWH and those treated with UFH.
Quality of the evidence
Results of this review indicate that LMWH may prevent further blood clots and bleeding in people with VTE. However, these findings must be interpreted with caution due to the moderate quality of the evidence as a result of lack of reporting of study methods and problems with study design. Results indicating reduced size of blood clots when taking LMWH also must be interpreted with caution due to the low quality of evidence as results were not similar across the studies.
Summary of findings
Summary of findings for the main comparison. LMWH compared to UFH for initial treatment of venous thromboembolism.
| LMWH compared to UFH for initial treatment of venous thromboembolism | ||||||
| Patient or population: people with venous thromboembolism (VTE) Setting: hospital Intervention: Low molecular weight heparin (LMWH) Comparison: Unfractionated heparin (UFH) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with UFH | Risk with LMWH | |||||
| Incidence of recurrent VTE1 after initial treatment (up to 15 days) |
Study population | OR 0.69 (0.49 to 0.98) | 6238 (18 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | ||
| 24 per 1000 | 17 per 1000 (12 to 24) | |||||
| Incidence of recurrent VTE1 (3 months follow‐up) |
Study population | OR 0.71 (0.56 to 0.90) | 6661 (16 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | ||
| 51 per 1000 | 37 per 1000 (29 to 46) | |||||
| Incidence of recurrent VTE1 (end of follow‐up) |
Study population | OR 0.72 (0.59 to 0.88) | 9489 (22 RCTs) | ⊕⊕⊕⊝ MODERATE 4 | ||
| 50 per 1000 | 36 per 1000 (30 to 44) | |||||
| Reduction in thrombus size (pre‐ and post‐treatment venograms) 5 |
Study population | OR 0.71 (0.61 to 0.82) | 2909 (16 RCTs) | ⊕⊕⊕⊝ LOW 6 | ||
| 423 per 1000 | 342 per 1000 (309 to 375) | |||||
| Incidence of major haemorrhagic episodes (during initial treatment ‐ up to 15 days) 7 |
Study population | OR 0.69 (0.50 to 0.95) | 8780 (25 RCTs) | ⊕⊕⊕⊝ MODERATE 8 | ||
| 21 per 1000 | 15 per 1000 (11 to 20) | |||||
| Overall mortality (end of follow‐up) |
Study population | OR 0.84 (0.70 to 1.01) | 9663 (24 RCTs) | ⊕⊕⊕⊝ MODERATE 9 | ||
| 57 per 1000 | 48 per 1000 (41 to 57) | |||||
| *The basis for the assumed risk for 'study population' was the average risk in the comparison groups (i.e. total number of participants with events in the control group divided by the number of participants in the comparison group). The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; LMWH; low molecular weight heparin; RCTs; randomised controlled trials OR: Peto odds ratio; UFH: unfractionated heparin; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Recurrent venous thromboembolism (VTE) defined as recurrent deep vein thrombosis (DVT) or recurrent pulmonary embolism (PE). The diagnosis of recurrent DVT was accepted if one of the following criteria was met: (a) a new, constant intraluminal‐filling defect not present on the last available venogram; (b) if the venogram was not diagnostic either an abnormal 125I‐fibrinogen leg scan or abnormal impedance plethysmogram or ultrasound result, which had been normal before the suspected recurrent episode (Buller 1991). The diagnosis of PE was accepted if one of the following criteria was met: (a) a segmental defect on the perfusion lung scan that was unmatched on the ventilation scan or chest roentgenogram; (b) positive pulmonary angiography; (c) PE at autopsy. 2 Downgraded as risk of bias serious due to high risk of attrition bias in 4 studies (Fiessinger 1996; Lindmarker 1994; Ninet 1991; Thery 1992), high risk of reporting bias in 2 studies (Lindmarker 1994; Pérez de Llano 2003) and high risk of other bias in 3 studies (Findik 2002; Harenberg 2000a; Lopaciuk 1992). 3 Downgraded as risk of bias serious due to high risk of attrition bias in 1 study (Breddin 2001), high risk of reporting bias in one study (Pérez de Llano 2003), and high risk of other bias in 2 studies (Findik 2002; Lopaciuk 1992). 4 Downgraded as risk of bias serious due to high risk of attrition bias in 2 studies (Breddin 2001; Lindmarker 1994), high risk of reporting bias in 2 studies (Lindmarker 1994; Pérez de Llano 2003), and high risk of other bias in 3 studies (Findik 2002; Harenberg 2000a; Lopaciuk 1992) 5 The number of participants in each group with an improved venographic score, if pre‐ and post‐treatment venograms were obtained and were assessed by persons unaware of treatment assignment. 6 Downgraded as risk of bias serious due to high risk of selection bias in 1 study (Luomanmaki 1996), high risk of attrition bias in 6 studies (Breddin 2001; Fiessinger 1996; Kakkar 2003; Lindmarker 1994; Ninet 1991; Thery 1992), high risk of reporting bias in 1 study (Lindmarker 1994), and high risk of other bias in 4 studies (Harenberg 2000a; Kakkar 2003; Lopaciuk 1992; Luomanmaki 1996). Downgraded further due to moderate heterogeneity (I² = 56%) 7 Haemorrhages were classified as major if they were intracranial, retroperitoneal, led directly to death, necessitated transfusion or they led to the interruption of antithrombotic treatment or (re)operation. 8 Downgraded as risk of bias serious due to high risk of selection bias in 1 study (Luomanmaki 1996), high risk of attrition bias in 5 studies (Fiessinger 1996; Kakkar 2003; Lindmarker 1994; Ninet 1991; Thery 1992), high risk of reporting bias in 2 studies (Lindmarker 1994; Pérez de Llano 2003), and high risk of other bias in 5 studies (Findik 2002; Harenberg 2000a; Kakkar 2003; Lopaciuk 1992; Luomanmaki 1996). 9 Downgraded as risk of bias serious due to high risk of selection bias in 1 study (Luomanmaki 1996), high risk of attrition bias in 4 studies (Breddin 2001; Kakkar 2003; Lindmarker 1994; Thery 1992), high risk of reporting bias in 2 studies (Lindmarker 1994; Pérez de Llano 2003), and high risk of other bias in 5 studies (Findik 2002; Harenberg 2000a; Kakkar 2003; Lopaciuk 1992; Luomanmaki 1996).
Background
Description of the condition
Venous thromboembolism (presence of a blood clot in the veins, VTE) has an incidence in the general population of approximately 0.1% per year. Its main manifestations are leg complaints, due to deep venous thrombosis (DVT), in the lower limb (blood clot in the deep veins of the leg), and signs of dyspnoea (shortness of breath) and pleuritic thoracic pain (chest pain) when a thrombus (clot) becomes dislodged and forms an embolism obstructing blood flow in the pulmonary circulation. Evidence suggests that although people may only complain about either DVT or pulmonary embolism (PE), in many cases the pathological manifestations are shared between these two clinically distinct conditions (Huisman 1989; Hull 1983). Therefore, increasingly they are referred to as one disease and are treated with comparable anticoagulant regimens.
Description of the intervention
Anticoagulant therapy is the treatment of choice for most people with VTE (NICE 2012). Present guidelines recommend initial therapy for DVT with a parenteral anticoagulant (unfractionated heparin (UFH), low molecular weight heparin (LMWH) or fondaparinux) followed by vitamin K antagonist (VKA) therapy (Kearon 2012). Heparin is administered by either continuous intravenous (IV) infusion or twice daily subcutaneous injection (NICE 2012). Heparin dosage is monitored by the activated partial thromboplastin time (APTT) and adjusted to maintain the anticoagulant effect within a defined therapeutic range. For intravenous heparin therapy to achieve its minimal anticoagulant effect, the initial dosing needs to be either weight based (80 units/kg then 18 units/kg/hour) or a fixed dose using a 5000 unit bolus followed by at least 1250 units/hour (Kearon 2012). Laboratory monitoring is necessary because the anticoagulant response to heparin is highly variable among people with VTE. Inadequate heparin dosing is related to an increased risk of VTE recurrence (Turpie 2002).
Why it is important to do this review
A number of LMWH preparations and heparinoids have been developed for clinical use. Compared with UFH, LMWH preparations have a longer plasma half‐life, less inter‐individual variability in anticoagulant response to fixed doses and, in animal models, a more favourable antithrombotic to haemorrhagic ratio (Hirsh 1990; Hirsh 1992). As a result of their pharmacokinetic properties, a stable and sustained anticoagulant effect is achieved when LMWHs are administered subcutaneously once or twice daily, without laboratory monitoring. Although most experience with LMWHs has been in the prevention of VTE, where they have been shown to be safe and effective (Nurmohamed 1992), there is accumulating evidence that these anticoagulants are also safe and effective for the initial treatment of venous thromboembolic events. This is the third update of the Cochrane Review first published in 1999.
Objectives
To evaluate the efficacy and safety of fixed dose subcutaneous low molecular weight heparin compared to adjusted dose unfractionated heparin (intravenous or subcutaneous) for the initial treatment of people with venous thromboembolism (acute deep venous thrombosis or pulmonary embolism).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials (RCTs) with prospective follow‐up.
Types of participants
People with venous thromboembolism (acute deep venous thrombosis or pulmonary embolism) confirmed by objective tests.
Types of interventions
Initial treatment (usually in the first five to 14 days) with fixed dose subcutaneous low molecular weight heparin (LMWH) and adjusted dose unfractionated heparin (UFH) (intravenous or subcutaneous).
Types of outcome measures
Primary outcomes
Incidence of symptomatic recurrent venous thromboembolism (deep venous thrombosis or pulmonary embolism) during the initial treatment and during follow‐up.
Secondary outcomes
Number of participants in whom the thrombus size reduced based on pre‐ and post‐treatment venograms.
Frequency of major haemorrhagic episodes during initial treatment or within 48 hours after treatment cessation.
Overall mortality at the end of follow‐up.
Search methods for identification of studies
There were no language restrictions.
Electronic searches
For this update the Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials.
The Cochrane Vascular Specialised Register (searched 15 September 2016).
The Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8) in the Cochrane Library (searched 15 September 2016).
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies, used are described in the Specialised Register section of the Cochrane Vascular module in the Cochrane Library (www.cochranelibrary.com).
The CIS searched the following trial registries for details of ongoing and unpublished studies.
ClinicalTrials.gov (www.ClinicalTrials.gov).
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch).
ISRCTN Register (www.isrctn.com/).
Searching other resources
We also reviewed the reference lists of relevant papers identified from these searches.
Data collection and analysis
Selection of studies
For this 2016 update, two review authors (LR and LJ) independently assessed studies identified by the searches for eligibility. Any disagreements were resolved by discussion.
Studies were excluded if: (1) they were dose‐ranging studies using higher doses of LMWH than are currently in use; (2) they used LMWH intravenously; (3) they adjusted LMWH dosages after initiation of treatment; (4) the difference in initial treatment was confounded by differences in concomitant medication or long‐term medication; (5) a true LMWH was not used (by true LMWH we mean that no compounds other than heparins were present); (6) the administration of UFH was suboptimal (i.e. not an adjusted dose); (7) the report was an abstract with incomplete data.
Data extraction and management
Data were extracted by two review authors (LR and LJ) and included route of administration, intensity of heparin therapy, intensity of oral anticoagulant therapy and the performance of independent assessment of study outcomes.
In addition, the following data were extracted.
(1) The incidence of symptomatic recurrent DVT and PE during the initial treatment and during follow‐up (if active follow‐up was conducted prospectively at the study centres); whether this incidence was assessed by persons unaware of treatment assignment; and if valid criteria were used for the diagnosis of recurrent VTE.
The diagnosis of recurrent DVT was accepted if one of the following criteria was met. (a) A new, constant intraluminal filling defect not present on the last available venogram. (b) If the venogram was not diagnostic, either an abnormal 125I‐fibrinogen leg scan or abnormal impedance plethysmogram or ultrasound result, which had been normal before the suspected recurrent episode (Buller 1991).
The diagnosis of PE was accepted if one of the following criteria was met. (a) A segmental defect on the perfusion lung scan that was unmatched on the ventilation scan or chest roentgenogram. (b) Positive pulmonary angiography. (c) Pulmonary embolism at autopsy.
(2) The number of participants in each group with an improved venographic score, if pre‐ and post‐treatment venograms were obtained and were assessed by persons unaware of treatment assignment.
(3) The frequency of major haemorrhagic episodes during initial treatment. Haemorrhages were classified as major if they were intracranial, retroperitoneal, led directly to death, necessitated transfusion or they led to the interruption of antithrombotic treatment or (re)operation. All other haemorrhages were classified as minor.
(4) The overall mortality at the end of follow‐up, specified for participants with or without malignant disease, if active follow‐up was prospectively conducted at the study centres.
Assessment of risk of bias in included studies
The risk of bias for all newly included studies was assessed by two review authors (LR and LJ) according to the guidelines given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). The following domains were assessed as being at either a low risk of bias, high risk of bias or unclear risk of bias using the criteria as described in Chapter 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Sequence generation: was the allocation sequence adequately generated?
Allocation treatment: was allocation adequately concealed?
Blinding: was knowledge of the allocated interventions adequately prevented during the study?
Incomplete data: were incomplete outcome data adequately addressed?
Selective outcome reporting: were reports of the study free of suggestion of selective outcome reporting?
Other potential threats to validity: was the study apparently free of other factors that could put it at risk of bias?
We resolved disagreements by discussion and consensus.
Measures of treatment effect
We based reduction in thrombus size on the number of participants whose thrombus size reduced between pre‐ and post‐treatment venograms. We used this outcome and each of the other dichotomous outcomes for the different treatments to calculate an odds ratio (OR) with 95% confidence intervals (CI) separately for each trial. We then combined these ORs across studies, giving due weight to the number of events in each of the two treatment groups in each separate study using the Peto procedure, which assumes a fixed treatment effect (Collins 1987; Mantel 1959). We investigated pulmonary vascular obstruction by calculating the mean difference (MD) between the groups.
We performed all these analyses with the individual LMWH preparations for VTE (that is DVT and PE combined).
We performed an analysis for all LMWH preparations combined if the treatment effects of the individual LMWH preparations were compatible with each other, in view of the biochemical heterogeneity as well as the heterogeneity in animal experiments.
We addressed the validity of combining the trials with a statistical test of homogeneity, which considers whether differences in treatment effect over the individual trials are consistent with natural variation around a constant effect (Collins 1987).
Unit of analysis issues
The unit of analysis in this review was the individual participant.
Dealing with missing data
We sought information about drop‐outs, withdrawals and other missing data and, if not reported, we contacted study authors for this information but did not get a response.
Assessment of heterogeneity
We assessed heterogeneity between the trials by visual examination of the forest plot to check for overlapping CIs, the Chi² test for homogeneity with a 10% level of significance and we used the I² statistic to measure the degree of inconsistency between the studies. An I² result of greater than 50% may represent moderate to substantial heterogeneity (Deeks 2011).
Assessment of reporting biases
We assessed publication bias by funnel plots if a sufficient number of studies (10 or more) were available in the meta‐analyses. There are many reasons for funnel plot asymmetry, and we consulted the Cochrane Handbook for Systematic Reviews of Interventions to aid the interpretation of the results (Sterne 2011).
Data synthesis
One review author (LR) entered the data into Review Manager 5 (RevMan 2014), and the second review author (LJ) cross‐checked data entry. We resolved any discrepancies by consulting the source publication. We used a fixed‐effect model to meta‐analyse the data.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analysis for the different heparin drugs versus unfractionated heparin for all the primary and secondary outcomes of the review.
We also performed the following additional analyses by different groups of interest.
Proximal deep vein thrombosis.
Pulmonary embolism.
Venous thromboembolism with or without malignant disease.
Subcutaneous UFH versus LMWH.
Intravenous UFH versus LMWH.
For these additional analyses, for the outcome 'recurrent VTE' we report the time point 'end of follow‐up' data only.
We also performed a separate analysis to explore any trend over time.
Sensitivity analysis
We performed sensitivity analyses by excluding studies with inadequate concealment of allocation prior to randomisation.
We also performed a sensitivity analysis by excluding studies that did not use the following International Society on Thrombosis and Haemostasis (ISTH) criteria of major bleeding (Schulman 2005).
Fatal bleeding.
Symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial, or intramuscular with compartment syndrome.
Bleeding causing a fall in haemoglobin level of 20 g/L (1.24 mmol/L) or more, or leading to transfusion of two or more units of whole blood or red cells.
Any combination of the above.
Summary of findings
We created 'Summary of findings' tables for LMWH compared with UFH in participants with VTE (Table 1). We used GRADEpro GDT software and the GRADE approach to assess the quality of the evidence for the most clinically relevant outcomes as described in Types of outcome measures. We downgraded the evidence from 'high quality' for serious or very serious study limitations (risk of bias, indirectness and inconsistency of evidence, imprecision of effect estimates or potential publication bias) according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a) and the GRADE Working Group (GRADE Working Group 2008).
Results
Description of studies
Results of the search
See Figure 1.
1.
Study flow diagram.
Included studies
Six additional studies were included in this update (Kakkar 2003; Leizorovicz 2011; Meyer 1995; Moreno‐Palomares 2001; Pérez de Llano 2003; Thery 1992). In total, 29 studies were truly randomised trials, published between 1988 and the end of 2011, with a total of 10,390 participants. Fourteen of the 29 studies included participants with symptomatic deep venous thrombosis of the leg without symptoms of pulmonary embolism. In eight of these 14 studies people with distal deep venous thrombosis were included as well as people with proximal deep venous thrombosis. In nine studies participants were included if they had symptomatic deep venous thrombosis of the leg, with or without symptomatic pulmonary embolism; or asymptomatic deep venous thrombosis of the leg with symptomatic pulmonary embolism; or symptomatic deep venous thrombosis or pulmonary embolism. In four studies participants with pulmonary embolism only were included. All studies used objective diagnostic tests to confirm the diagnosis.
All of the included studies considered fixed dose subcutaneous LMWH once daily (Fiessinger 1996; Hull 1992; Kakkar 2003; Leizorovicz 2011; Lindmarker 1994; Luomanmaki 1996; Simonneau 1997), twice daily (Belcaro 1999; Breddin 2001; Columbus 1997; Decousus 1998; Faivre 1988; Findik 2002; Goldhaber 1998; Harenberg 2000a; Kirchmaier 1998; Koopman 1996; Levine 1996; Lopaciuk 1992; Meyer 1995; Ninet 1991; Pérez de Llano 2003; Prandoni 1992; Prandoni 2004; Riess 2003; Simonneau 1993; Thery 1992), or both (Merli 2001; Moreno‐Palomares 2001) compared with adjusted intravenous dose UFH (Breddin 2001; Columbus 1997; Decousus 1998; Fiessinger 1996; Findik 2002; Goldhaber 1998; Harenberg 2000a; Hull 1992; Kakkar 2003; Kirchmaier 1998; Koopman 1996; Levine 1996; Lindmarker 1994; Luomanmaki 1996; Merli 2001; Meyer 1995; Moreno‐Palomares 2001; Ninet 1991; Pérez de Llano 2003; Prandoni 1992; Simonneau 1993; Simonneau 1997; Thery 1992) or subcutaneous unfractionated heparin (Faivre 1988; Lopaciuk 1992; Prandoni 2004) or both (Belcaro 1999; Leizorovicz 2011). Nine different preparations of LMWH were identified (nadroparin, tinzaparin, enoxaparin, dalteparin, CY 222, certoparin, ardeparin, reviparin and bemiparin). Ten trials did not have any post‐randomisation exclusions or losses to follow‐up. Eleven trials reported the number of participants lost to follow‐up, which ranged from 1.0% to 12.7%. One trial did not report the dropouts (see Characteristics of included studies).
Excluded studies
Five additional studies were excluded for this update (Quiros 2001; Riess 2014; Siguret 2011; Stricker 1999; Ucar 2015). A total of 26 trials were excluded for the following reasons: dosage of UFH was not adjusted (four trials: Kearon 2006; Notarbartolo 1988; Tedoldi 1993; Zanghi 1988); dose‐ranging study (three trials: Banga 1993; de Valk 1995; Handeland 1990); LMWH dosage was adjusted (four trials: Aiach 1989; Bratt 1990; Holm 1986; Ly 1985); intravenous administration of LMWH (four trials: Bratt 1985; Lockner 1985; Lockner 1986; Vogel 1987); results from participants treated for venous thrombosis of the upper limb and for pulmonary embolism could not be distinguished from those of participants with leg vein thrombosis and the outcome was incompletely evaluated (four trials: Albada 1989; Harenberg 1989; Harenberg 1990; Harenberg 2000b); a difference in long‐term treatment between the two treatment regimens (two trials: Monreal 1993; Monreal 1994); no UFH comparison group (Siguret 2011); one study looked at the effect of heparin on haemostatic markers and therefore the outcomes were not relevant for this review (Stricker 1999); a substudy of a study already included in the original review (Riess 2003); not an RCT (Quiros 2001); and treatment with thrombolytic therapy (Ucar 2015).
One ongoing study has been identified (NCT00796692). See Characteristics of ongoing studies.
Risk of bias in included studies
See Figure 2.
2.
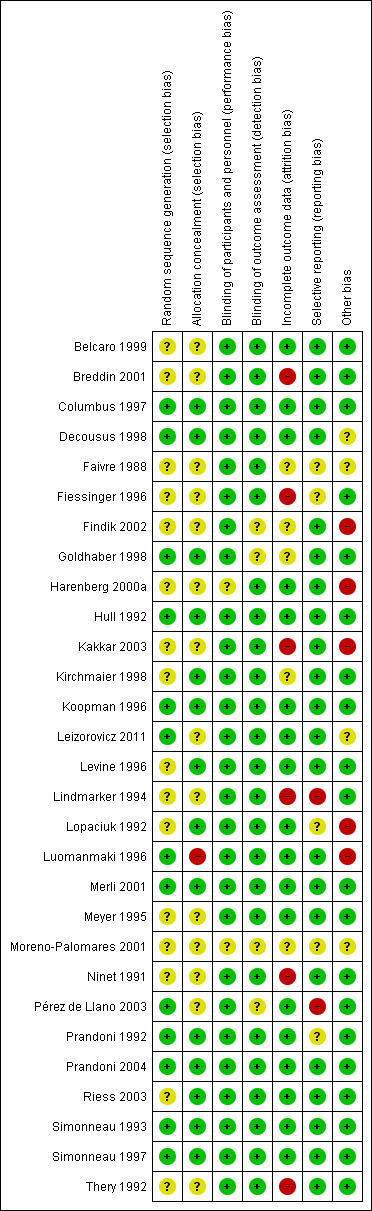
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Thirteen of the 29 included studies adequately described random sequence generation through the use of a computer or telephone system (Columbus 1997; Decousus 1998; Goldhaber 1998; Hull 1992; Koopman 1996; Leizorovicz 2011; Luomanmaki 1996; Merli 2001; Pérez de Llano 2003; Prandoni 1992; Prandoni 2004; Simonneau 1993; Simonneau 1997). In the remaining 16 studies, there was insufficient information about the random sequence generation to permit a judgement of selection bias. In fourteen of the 29 included studies the assigned treatment was adequately concealed prior to allocation (Columbus 1997; Decousus 1998; Goldhaber 1998; Hull 1992; Kirchmaier 1998; Koopman 1996; Levine 1996; Lopaciuk 1992; Merli 2001; Prandoni 1992; Prandoni 2004; Riess 2003; Simonneau 1993; Simonneau 1997), while in the other 14 trials concealment of allocation was unclear, based on the information given in the publication. One study was deemed to be at high risk of selection bias as there was no central allocation (Luomanmaki 1996). Instead, randomisation was conducted separately at each participating centre (see Characteristics of included studies).
Blinding
In two of the studies, authors did not state whether the participants and staff were blinded to the treatment or not and therefore the risk of performance bias for these two studies was unclear (Harenberg 2000a; Moreno‐Palomares 2001). In the remaining 27 included studies treatment allocation was not blinded due to the difference in route of administration between subcutaneous LMWH and intravenous UFH. However, given the clinical outcomes of the study, we judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes and therefore we judged these studies to be at low risk of bias. Even the three studies of subcutaneous UFH versus subcutaneous LMWH were not blinded for treatment allocation due to an initial intravenous bolus in the UFH group (Faivre 1988; Lopaciuk 1992; Prandoni 2004). There was only one double‐blinded clinical trial in which participants received either intravenous UFH with subcutaneous placebo or subcutaneous LMWH with intravenous placebo (Hull 1992).
Four of the 29 included studies did not report whether outcome assessors were blinded to treatment and were therefore judged to be at an unclear risk of detection bias (Findik 2002; Goldhaber 1998; Moreno‐Palomares 2001; Pérez de Llano 2003). In the remaining 25 studies, outcome assessors were blinded to treatment and therefore these studies were judged to be at low risk of detection bias.
Incomplete outcome data
Six studies were judged to be at high risk of attrition bias as data were missing or imbalanced across the groups (Breddin 2001; Fiessinger 1996; Kakkar 2003; Lindmarker 1994; Ninet 1991; Thery 1992); 18 were judged to be at low risk (Belcaro 1999; Columbus 1997; Decousus 1998; Harenberg 2000a; Hull 1992; Koopman 1996; Leizorovicz 2011; Levine 1996; Lopaciuk 1992; Luomanmaki 1996; Merli 2001; Meyer 1995; Pérez de Llano 2003; Prandoni 1992; Prandoni 2004; Riess 2003; Simonneau 1993; Simonneau 1997); while five did not provide enough information to permit a judgement (Faivre 1988; Findik 2002; Goldhaber 1998; Kirchmaier 1998; Moreno‐Palomares 2001).
Selective reporting
Two studies were judged to be at high risk of reporting bias (Lindmarker 1994; Pérez de Llano 2003). In the study by Lindmarker 1994, participants who had died or had a VTE were not included in the analysis. In the study by Pérez de Llano 2003, length of stay was not a prespecified outcome but authors reported data on it in the discussion. Twenty‐two studies were at low risk while the remaining five did not provide enough information to permit judgement on reporting bias (Faivre 1988; Fiessinger 1996; Lopaciuk 1992; Moreno‐Palomares 2001; Prandoni 1992).
Other potential sources of bias
Five studies were judged to be at high risk of bias (Findik 2002; Harenberg 2000a; Kakkar 2003; Lopaciuk 1992; Luomanmaki 1996). Two studies were sponsored by the pharmaceutical companies that provided the study drug (Harenberg 2000a; Kakkar 2003). The study by Findik 2002 had a low statistical power due to low numbers of participants and few outcome events. Lopaciuk 1992 had an imbalance in exclusion of participants at baseline while the study by Luomanmaki 1996 had a higher incidence of malignancy in participants treated with UFH. Twenty studies were judged to be free from other sources of bias; while in the remaining four, there was not enough information to permit judgement (Decousus 1998; Faivre 1988; Leizorovicz 2011; Moreno‐Palomares 2001).
Effects of interventions
See: Table 1
None of the trials individually demonstrated protection from recurrent symptomatic venous thromboembolic complications during the initial treatment period. One trial showed that LMWH conferred protection from recurrent symptomatic venous thromboembolic complications at the end of follow‐up (Breddin 2001). Only Hull 1992 demonstrated a reduction in major haemorrhage after treatment with LMWH. Six studies showed a reduction in thrombus size, between pre‐treatment and post‐treatment venograms, in favour of LMWH (Breddin 2001; Goldhaber 1998; Kakkar 2003; Lopaciuk 1992; Prandoni 1992; Simonneau 1993).
Incidence of symptomatic recurrent venous thromboembolism (Analysis 1.1 to Analysis 1.5) ('Summary of findings' table 1)
The occurrence of symptomatic recurrent venous thromboembolism was evaluated during the initial treatment period (Columbus 1997; Decousus 1998; Fiessinger 1996; Findik 2002; Harenberg 2000a; Kakkar 2003; Kirchmaier 1998; Koopman 1996; Levine 1996; Lindmarker 1994; Lopaciuk 1992; Meyer 1995; Ninet 1991; Pérez de Llano 2003; Prandoni 1992; Riess 2003; Simonneau 1993; Simonneau 1997; Thery 1992); at one month's follow‐up (Columbus 1997; Levine 1996; Pérez de Llano 2003; Prandoni 1992); at three months' follow‐up (Belcaro 1999; Breddin 2001; Columbus 1997; Decousus 1998; Findik 2002; Hull 1992; Koopman 1996; Levine 1996; Lopaciuk 1992; Merli 2001; Meyer 1995; Moreno‐Palomares 2001; Pérez de Llano 2003; Prandoni 1992; Prandoni 2004; Simonneau 1993; Simonneau 1997); and at six months' follow‐up (Harenberg 2000a; Kirchmaier 1998; Koopman 1996; Lindmarker 1994; Pérez de Llano 2003; Prandoni 1992; Riess 2003). Combining all trials with long‐term follow‐up gave a comparison of recurrent thromboembolism at the end of follow‐up. Although Kakkar 2003 reported incidence of recurrent VTE, there was a query regarding the exact number of participants reported to have this outcome. The author was contacted to clarify the data but did not respond and therefore this study was not included in the analysis.
Analysis of the pooled data from these studies demonstrated a reduction in recurrent venous thromboembolic events with LMWH during the initial treatment period (Peto OR 0.69, 95% CI 0.49 to 0.98; moderate‐quality evidence; participants = 6238; studies = 18; P = 0.04); at the end of follow‐up (Peto OR 0.72, 95% CI 0.59 to 0.88; participants = 9489; studies = 22; P = 0.0005), at three months' follow‐up (Peto OR 0.71, 95% CI 0.56 to 0.90; moderate‐quality evidence; participants = 6661; studies = 16; P = 0.005); and at six months' follow‐up (Peto OR 0.68, 95% CI 0.48 to 0.96; participants = 2841; studies = 7; P = 0.03). However, at one month's follow‐up, no difference was found between LMWH and UFH (Peto OR 0.90, 95% CI 0.56 to 1.44; participants = 1741; studies = 4; P = 0.65).
During the initial treatment, 54 (1.7%) of the 3123 participants allocated to LMWH had thrombotic complications versus 76 (2.4%) of the 3115 participants allocated to UFH. After a follow‐up of three months, the period in most of the studies for which oral anticoagulant therapy was given, 122 (3.5%) of the 3440 participants treated with LMWH had a recurrent thrombotic event versus 164 (5.2%) of the 3221 participants treated with UFH.
When different preparations of heparin were compared, a reduction in recurrent VTE was noted during the initial treatment period for enoxaparin (Peto OR 0.51, 95% CI 0.27 to 0.98; participants = 1143; studies = 5; P = 0.04) and at the end of follow‐up for certoparin (Peto OR 0.63, 95% CI 0.40 to 0.99; participants = 2007; 3 studies; P = 0.05) versus UFH. Overall, no differences were observed between the heparin preparations during the initial treatment period and at the end of follow‐up.
Reduction in thrombus size (Analysis 1.6)
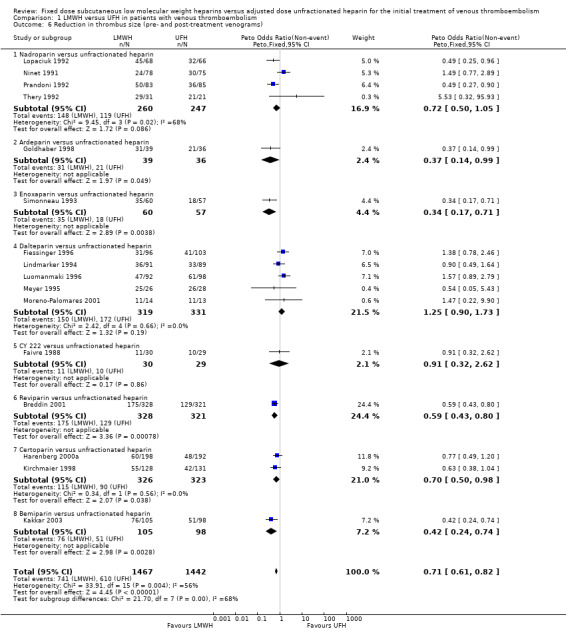
Venograms were obtained before and after heparin treatment in 16 studies (Breddin 2001; Faivre 1988; Fiessinger 1996; Goldhaber 1998; Harenberg 2000a; Kakkar 2003; Kirchmaier 1998; Lindmarker 1994; Lopaciuk 1992; Luomanmaki 1996; Meyer 1995; Moreno‐Palomares 2001; Ninet 1991; Prandoni 1992; Simonneau 1993; Thery 1992). In all studies these venograms were adjudicated by investigators unaware of treatment allocation. The combined results of the 16 studies demonstrated a reduction of thrombus size in 51% of the participants (741 out of 1467) treated with LMWH and in 42% of participants (610 out of 1442) treated with UFH. LMWH was associated with a reduction in thrombus size compared with UFH (Peto OR 0.71, 95% CI 0.61 to 0.82; moderate‐quality evidence; participants = 2909; studies = 16; P < 0.00001). However there was moderate heterogeneity in this analysis (I² = 56%). When we performed analysis by studies reporting on DVT, the heterogeneity was reduced (I² = 34%) (Analysis 2.4). See also below.
2.4. Analysis.
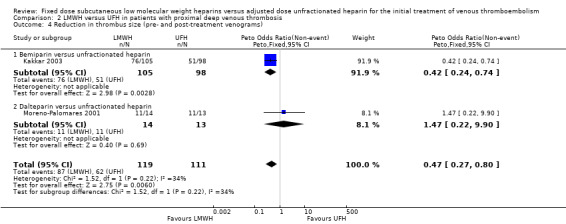
Comparison 2 LMWH versus UFH in patients with proximal deep venous thrombosis, Outcome 4 Reduction in thrombus size (pre‐ and post‐treatment venograms).
Subgroup analysis showed a difference between the LMWH preparations (P = 0.004). Of the individual LMWH preparations, a better venographic outcome was observed for ardeparin (Peto OR 0.37, 95% CI 0.14 to 0.99), enoxaparin (Peto OR 0.34, 95% CI 0.17 to 0.71), reviparin (Peto OR 0.59, 95% CI 0.43 to 0.80), certoparin (Peto OR 0.70, 95% CI 0.50 to 0.98) and bemiparin (Peto OR 0.42, 95% CI 0.24 to 0.74).
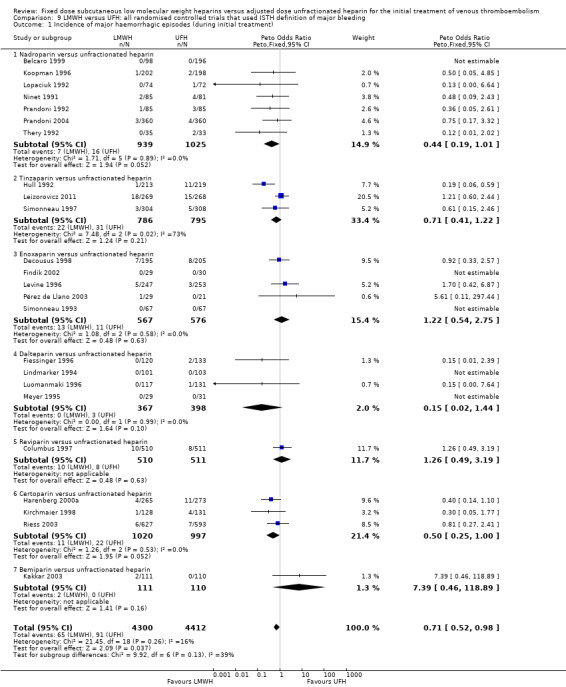
Incidence of major haemorrhage during the initial treatment (Analysis 1.7)
Twenty‐five of the included trials evaluated the occurrence of major haemorrhage during the initial treatment (Belcaro 1999; Columbus 1997; Decousus 1998; Faivre 1988; Fiessinger 1996; Findik 2002; Harenberg 2000a; Hull 1992; Kakkar 2003; Kirchmaier 1998; Koopman 1996; Leizorovicz 2011; Levine 1996; Lindmarker 1994; Lopaciuk 1992; Luomanmaki 1996; Meyer 1995; Ninet 1991; Pérez de Llano 2003; Prandoni 1992; Prandoni 2004; Riess 2003; Simonneau 1993; Simonneau 1997; Thery 1992). Analysis of the pooled data showed a reduction in major haemorrhagic complications in favour of LMWH (Peto OR 0.69, 95% CI 0.50 to 0.95; participants = 8780; studies = 25; moderate‐quality evidence; P = 0.02). At the end of the initial treatment period, 65 (1.5%) of the 4333 participants in the LMWH group versus 94 (2.1%) of the 4447 participants in the UFH group suffered a major haemorrhage.
Subgroup analysis showed no difference between the LMWH preparations (P = 0.10).
Overall mortality at the end of follow‐up (Analysis 1.8)
Twenty‐four studies prospectively evaluated the overall mortality at the end of follow‐up (Breddin 2001; Columbus 1997; Decousus 1998; Findik 2002; Goldhaber 1998; Harenberg 2000a; Hull 1992; Kakkar 2003; Kirchmaier 1998; Koopman 1996; Leizorovicz 2011; Levine 1996; Lindmarker 1994; Lopaciuk 1992; Luomanmaki 1996; Merli 2001; Meyer 1995; Pérez de Llano 2003; Prandoni 1992; Prandoni 2004; Riess 2003; Simonneau 1993; Simonneau 1997; Thery 1992). There was no difference in overall mortality at the end of follow‐up between participants treated with LMWH and UFH (Peto OR 0.84, 95% CI 0.70 to 1.01; moderate‐quality evidence; participants = 9663; studies = 24; P = 0.07). In the LMWH group, 234 (4.7%) of the 5004 participants died versus 265 (5.7%) of the 4659 participants in the UFH group.
When analysed by LMWH preparation, certoparin was the only drug found to be associated with a reduction in overall mortality at the end of follow‐up (Peto OR 0.59, 95% CI 0.36 to 0.97; P = 0.04). Overall, no differences were observed between the heparin preparations in mortality at the end of follow‐up.
Analysis in participants with proximal deep venous thrombosis (Analysis 2.1 to Analysis 2.6)
A total of 4878 participants with proximal deep venous thrombosis were enrolled in eleven studies (Belcaro 1999; Breddin 2001; Harenberg 2000a; Hull 1992; Kakkar 2003; Koopman 1996; Levine 1996; Moreno‐Palomares 2001; Prandoni 1992; Riess 2003; Simonneau 1993). Seven preparations of LMWH were used: nadroparin (three trials, 864 participants), dalteparin (one trial, 30 participants), tinzaparin (one trial, 432 participants), enoxaparin (two trials, 634 participants), reviparin (one trial, 763 participants), certoparin (two trials, 1758 participants) and bemiparin (one trial, 397 participants). In the three‐armed trial by Kakkar 2003 two bemiparin groups were compared with an UFH control group. However, in one of the bemiparin groups, participants did not receive concomitant VKA therapy. All other studies included in this review used concomitant VKA therapy and in order for our results to be comparable, data for this group of participants in the Kakkar 2003 study was not included in the analysis.
At the end of follow‐up, 80 (3.5%) of the 2303 participants treated with LMWH had a symptomatic recurrent venous thromboembolic event versus 143 (6.0%) of the 2369 participants treated with UFH. This reduction was in favour of LMWH (Peto OR 0.57, 95% CI 0.44 to 0.75; participants = 4672; studies = 10; P < 0.0001) (Analysis 2.1). When analysed by LMWH preparation, reviparin was the only drug associated with a reduction in recurrent VTE (Peto OR 0.31, 95% CI 0.15 to 0.63). Overall, no differences were observed between the heparin preparations in symptomatic recurrent venous thromboembolism at the end of follow‐up.
2.1. Analysis.
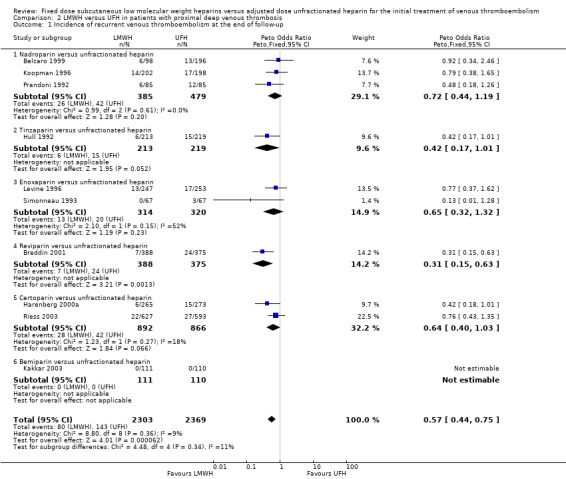
Comparison 2 LMWH versus UFH in patients with proximal deep venous thrombosis, Outcome 1 Incidence of recurrent venous thromboembolism at the end of follow‐up.
LMWH was also associated with a reduction in the incidence of symptomatic, recurrent deep venous thrombosis as well as a reduction in the incidence of pulmonary embolism (respectively Peto OR 0.61, 95% CI 0.41 to 0.91; participants = 2681; studies = 7; P = 0.02; and Peto OR 0.45, 95% CI 0.28 to 0.74; participants = 3024; studies = 7; P = 0.002) (Analysis 2.2; Analysis 2.3). When analysed by type of LMWH preparation, reviparin and certoparin were the only drugs associated with a reduction in the incidence of pulmonary embolism (respectively Peto OR 0.27, 95% CI 0.10 to 0.73; and Peto OR 0.32, 95% CI 0.11 to 0.92). Overall, no differences were observed between the heparin preparations in symptomatic recurrent deep venous thrombosis and pulmonary embolism at the end of follow‐up.
2.2. Analysis.
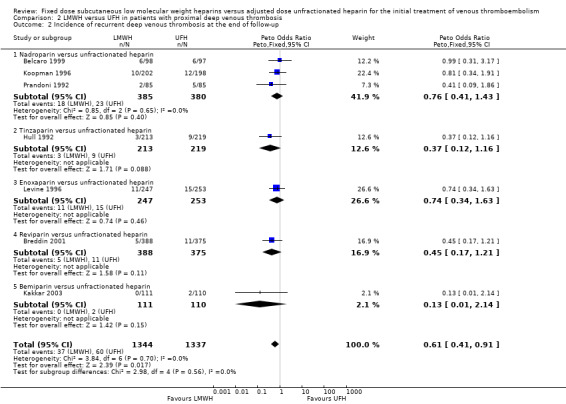
Comparison 2 LMWH versus UFH in patients with proximal deep venous thrombosis, Outcome 2 Incidence of recurrent deep venous thrombosis at the end of follow‐up.
2.3. Analysis.
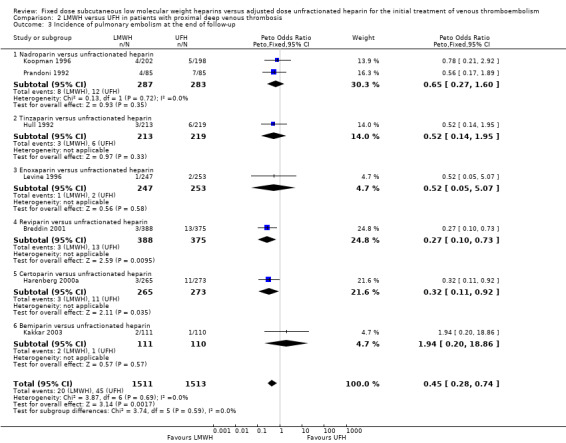
Comparison 2 LMWH versus UFH in patients with proximal deep venous thrombosis, Outcome 3 Incidence of pulmonary embolism at the end of follow‐up.
Pooled analysis of two studies demonstrated a reduction of thrombus size in 73% of the participants treated with LMWH and in 56% of participants treated with UFH (Kakkar 2003; Moreno‐Palomares 2001). LMWH was associated with a better venographic outcome — Peto OR 0.47, 95% CI 0.27 to 0.80; participants = 230; studies = 2; P = 0.006 (Analysis 2.4) — with the result heavily influenced by the Kakkar 2003 study on bemiparin showing a reduction in thrombus size with LMWH (Peto OR 0.42, 95% CI 0.24 to 0.74; participants = 203; studies = 1; P = 0.003) compared with UFH.
Analysis of the pooled data showed a reduction in major haemorrhagic complications in favour of LMWH (Peto OR 0.50, 95% CI 0.29 to 0.85; participants = 3589; studies = 8; P = 0.01) (Analysis 2.5). At the end of the initial treatment period, 18 (1.0%) of the 1804 participants in the LMWH group versus 37 (2.1%) of the 1785 participants in the UFH group suffered a major haemorrhage. Tinzaparin was the only LMWH preparation associated with reduced rates of major haemorrhagic complications (Peto OR 0.19, 95% CI 0.06 to 0.59). Overall, no differences were observed between the heparin preparations in incidence of major haemorrhages during initial treatment.
2.5. Analysis.
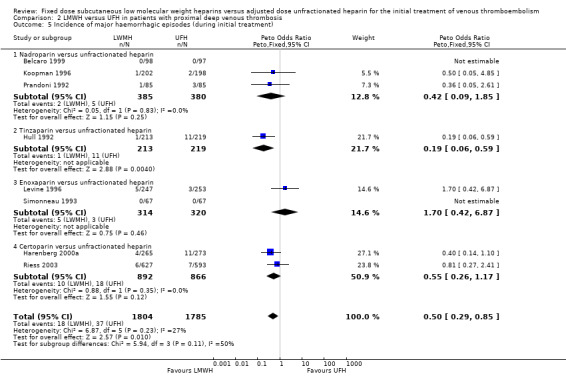
Comparison 2 LMWH versus UFH in patients with proximal deep venous thrombosis, Outcome 5 Incidence of major haemorrhagic episodes (during initial treatment).
Overall mortality at the end of follow‐up demonstrated a reduction in favour of LMWH (Peto OR 0.63, 95% CI 0.47 to 0.85; participants = 4331; studies = 9; P = 0.002) (Analysis 2.6). In the LMWH group, 72 (3.3%) of the 2183 participants died versus 112 (5.2%) of the 2148 participants in the UFH group. Certorparin was the only LMWH preparation associated with a reduction in overall mortality (Peto OR 0.54, 95% CI 0.30 to 0.96). Overall, no differences were observed between the heparin preparations in overall mortality at the end of follow‐up.
2.6. Analysis.
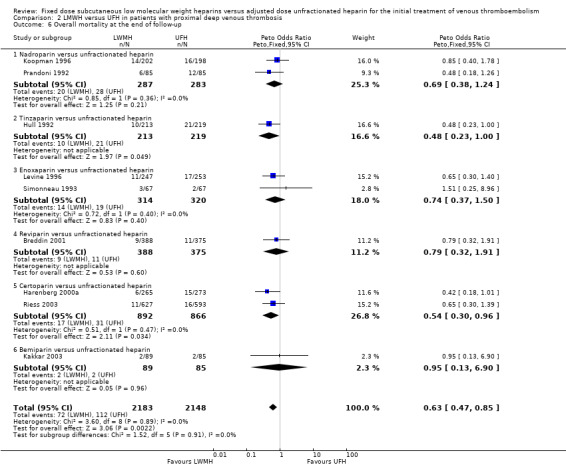
Comparison 2 LMWH versus UFH in patients with proximal deep venous thrombosis, Outcome 6 Overall mortality at the end of follow‐up.
Analysis in participants with pulmonary embolism (Analysis 3.1)
A total of 1407 participants with pulmonary embolism were enrolled in seven studies (Columbus 1997; Findik 2002; Merli 2001; Meyer 1995; Pérez de Llano 2003; Simonneau 1997; Thery 1992). Four preparations of LMWH were used: tinzaparin (one trial, 612 participants), enoxaparin (three trials, 396 participants), dalteparin (two trials, 128 participants), and reviparin (one trial, 271 participants). In the study by Thery 1992, two other treatment groups were given a high dose of nadroparin (600 and 900 anti‐factor Xa IU/kg). Data from these groups were not included in the analysis in this review.
All seven studies measured the rate of recurrent thromboembolic events at the end of follow‐up. Analysis of pooled data showed no difference between participants treated with LMWH and UFH (Peto OR 0.90, 95% CI 0.50 to 1.61; participants = 1407; studies = 7; P = 0.73) (Analysis 3.1). No individual LMWH preparation was associated with a reduction in the rate of recurrent VTE.
3.1. Analysis.
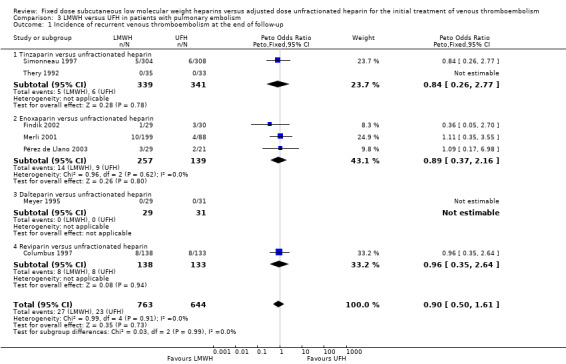
Comparison 3 LMWH versus UFH in patients with pulmonary embolism, Outcome 1 Incidence of recurrent venous thromboembolism at the end of follow‐up.
Two studies measured change in thrombus size (Meyer 1995; Thery 1992). Pooled analysis showed no difference in the number of LMWH and UFH participants whose thrombus size improved (Peto OR 1.36, 95% CI 0.23 to 8.16; participants = 106; studies = 2; P = 0.74) (Analysis 3.2). Both studies also measured change in thrombus size according to improvement in the Miller (Thery 1992) or peripheral vascular obstruction score (PVOS) (Meyer 1995). Pooled analysis showed an improvement (MD −3.14, 95% CI −4.39 to −1.90; participants = 106; studies = 2; P < 0.00001) (Analysis 3.3). No individual LMWH preparation was associated with a change in thrombus size.
3.2. Analysis.
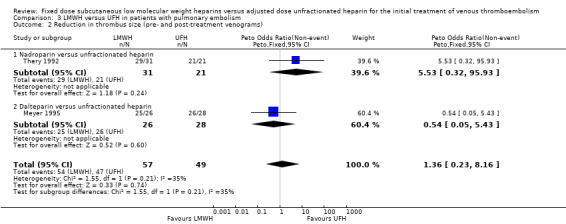
Comparison 3 LMWH versus UFH in patients with pulmonary embolism, Outcome 2 Reduction in thrombus size (pre‐ and post‐treatment venograms).
3.3. Analysis.
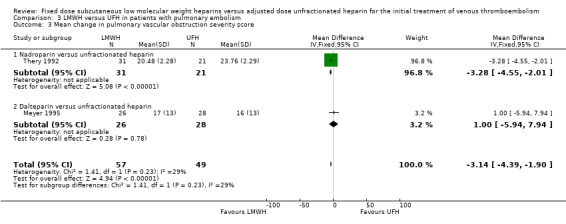
Comparison 3 LMWH versus UFH in patients with pulmonary embolism, Outcome 3 Mean change in pulmonary vascular obstruction severity score.
Three studies measured the incidence of major haemorrhagic complications during initial treatment or within 48 hours after treatment cessation (Meyer 1995; Pérez de Llano 2003; Thery 1992). Pooled analysis showed no difference in the incidence of major bleeding between the LMWH and UFH groups (Peto OR 0.44, 95% CI 0.04 to 4.29; participants = 178; studies = 3; P = 0.48) (Analysis 3.4). However there was significant heterogeneity in this analysis (I² = 58%). No individual LMWH preparation was associated with a reduction in the rate of major haemorrhagic complications.
3.4. Analysis.
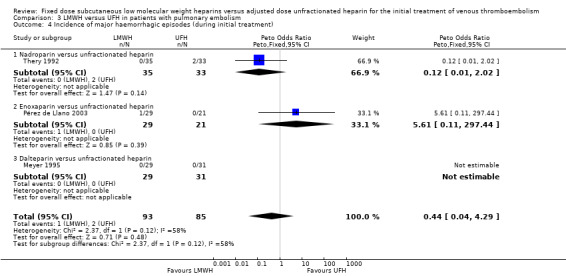
Comparison 3 LMWH versus UFH in patients with pulmonary embolism, Outcome 4 Incidence of major haemorrhagic episodes (during initial treatment).
Three studies measured overall mortality (Meyer 1995; Pérez de Llano 2003; Thery 1992). We found no difference in the overall mortality incidence between the LMWH and UFH groups (Peto OR 1.70, 95% CI 0.17 to 16.71; participants = 178; studies = 3; P = 0.65) (Analysis 3.5). No individual LMWH preparation was associated with reduced overall mortality.
3.5. Analysis.
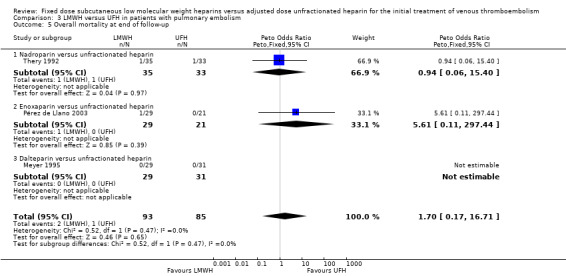
Comparison 3 LMWH versus UFH in patients with pulmonary embolism, Outcome 5 Overall mortality at end of follow‐up.
Analysis in participants with venous thromboembolism with or without malignant disease (Analysis 4.1 to Analysis 5.1)
Six studies evaluated mortality at the end of follow‐up in participants with and without malignant disease (Columbus 1997; Hull 1992; Lindmarker 1994; Lopaciuk 1992; Prandoni 1992; Simonneau 1997). One of these studies individually showed a reduction in deaths at the end of follow‐up with LMWH (Peto OR 0.16, 95% CI 0.03 to 0.72; P = 0.02) (Prandoni 1992). Combining the six studies also demonstrated a reduction in overall mortality in participants with cancer who were treated with LMWH (Peto OR 0.53, 95% CI 0.33 to 0.85; participants = 446; P = 0.009) (Analysis 4.1). In participants without cancer who received LMWH, the reduction in overall mortality of approximately 1% was not different between LMWH and UFH (Peto OR 0.97, 95% CI 0.61 to 1.56; participants = 2139; P = 0.91) (Analysis 5.1).
4.1. Analysis.
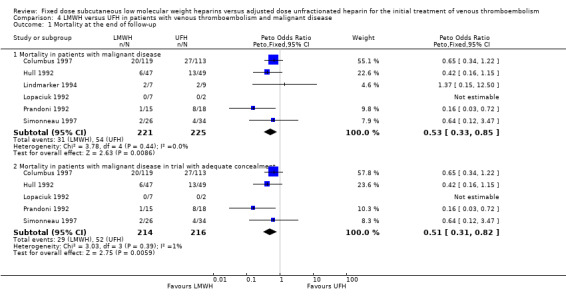
Comparison 4 LMWH versus UFH in patients with venous thromboembolism and malignant disease, Outcome 1 Mortality at the end of follow‐up.
5.1. Analysis.
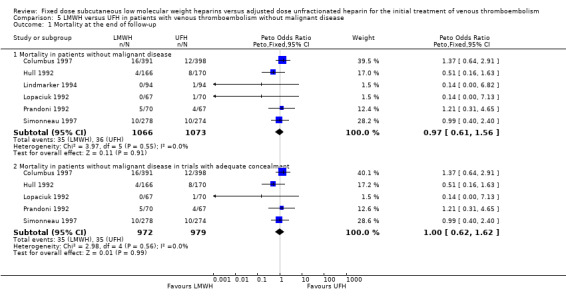
Comparison 5 LMWH versus UFH in patients with venous thromboembolism without malignant disease, Outcome 1 Mortality at the end of follow‐up.
Data on recurrent VTE, reduction in thrombus size and major haemorrhage during initial treatment were not available for the group of participants with or without malignant disease.
Analysis of studies of subcutaneous UFH versus LMWH (Analysis 6.1; Analysis 6.2; Analysis 6.3)
In four studies the UFH in the control group was administered subcutaneously although they did not all report on all outcomes (Faivre 1988; Leizorovicz 2011; Lopaciuk 1992; Prandoni 2004). The analysis of the pooled data from these studies demonstrated no reduction in recurrent venous thromboembolism at the end of follow‐up (Peto OR 1.05, 95% CI 0.56 to 1.95; participants = 1403; studies = 3; P = 0.88). However there was significant heterogeneity (I² = 58%). There was no difference in the incidence of major haemorrhagic complications (Peto OR 0.91, 95% CI 0.50 to 1.67; participants = 1471; studies = 4; P = 0.76), nor overall mortality (Peto OR 1.46, 95% CI 0.91 to 2.35; participants = 1403; studies = 3; P = 0.12), between groups treated with subcutaneous UFH and LMWH.
Data on reduction in thrombus size were not available for the group of participants who received subcutaneous UFH versus LMWH.
Analysis of studies of intravenous UFH versus LMWH (Analysis 7.1; Analysis 7.2; Analysis 7.3)
In the 21 studies which compared LMWH with intravenous UFH we found a reduction in recurrent venous thromboembolism at the end of follow‐up (Peto OR 0.69, 95% CI 0.56 to 0.86; participants = 8375; studies = 21; P = 0.0007); in major haemorrhages (Peto OR 0.62, 95% CI 0.43 to 0.90; participants = 7309; studies = 21; P = 0.01); and in overall mortality (Peto OR 0.77, 95% CI 0.63 to 0.93; participants = 8260; studies = 21; P = 0.008) (Belcaro 1999; Breddin 2001; Columbus 1997; Decousus 1998; Findik 2002; Goldhaber 1998; Harenberg 2000a; Hull 1992; Kakkar 2003; Kirchmaier 1998; Koopman 1996; Levine 1996; Lindmarker 1994; Merli 2001; Meyer 1995; Pérez de Llano 2003; Prandoni 1992; Riess 2003; Simonneau 1993; Simonneau 1997; Thery 1992).
Data on reduction in thrombus size were not available for the group of participants who received intravenous UFH versus LMWH.
Sensitivity analysis of studies with adequate concealment of allocation prior to randomisation (Analysis 8.1 to Analysis 8.6)
Fourteen studies had clear concealment of allocation prior to randomisation based on the information given in the publications (Columbus 1997; Decousus 1998; Goldhaber 1998; Hull 1992; Kirchmaier 1998; Koopman 1996; Levine 1996; Lopaciuk 1992; Merli 2001; Prandoni 1992; Prandoni 2004; Riess 2003; Simonneau 1993; Simonneau 1997). The analysis of the pooled data from these studies demonstrated no difference between LMWH and UFH in recurrent venous thromboembolism during the initial treatment period (Peto OR 0.72, 95% CI 0.50 to 1.05; participants = 4862; studies = 10; P = 0.09) nor at three months (Peto OR 0.79, 95% CI 0.60 to 1.02; participants = 5435; studies = 11; P = 0.07). However, LMWH was associated with both a reduction in the incidence of recurrent VTE at the end of follow‐up (Peto OR 0.76, 95% CI 0.60 to 0.96; participants = 6984; studies = 14; P = 0.02) and overall mortality (Peto OR 0.80, 95% CI 0.65 to 0.99; participants = 6984; studies = 14; P = 0.04). Major haemorrhage (Peto OR 0.68, 95% CI 0.45 to 1.03; participants = 6014; studies = 12; P = 0.07) was not different after treatment with LMWH compared with UFH. The reduction in the thrombus size, however, was in favour of LMWH (Peto OR 0.49, 95% CI 0.37 to 0.66; participants = 753; studies = 5; P < 0.00001). Therefore, while reductions in recurrent venous thromboembolism, major haemorrhages and overall mortality were observed in the LMWH group compared with UFH when all studies were combined, in a sensitivity analysis of studies with adequate concealment of treatment allocation before randomisation, no differences were observed in the incidence of recurrent venous thromboembolism during initial treatment and after three months nor in the incidence of major haemorrhages between LMWH and UFH.
Sensitivity analysis of studies that used the International Society on Thrombosis and Haemostasis (ISTH) definition of major and clinically relevant bleeding (Analysis 9.1)
Only one study did not use the ISTH definition of major bleeding and was excluded for the sensitivity analysis (Faivre 1988). Analysis of the pooled data showed a reduction in major haemorrhagic complications in favour of LMWH (Peto OR 0.71, 95% CI 0.52 to 0.98; participants = 8712; studies = 24; P = 0.04). These results are similar to the results from the analysis including all studies irrespective of their definition of major and clinically relevant bleeding (Analysis 1.7).
1.7. Analysis.
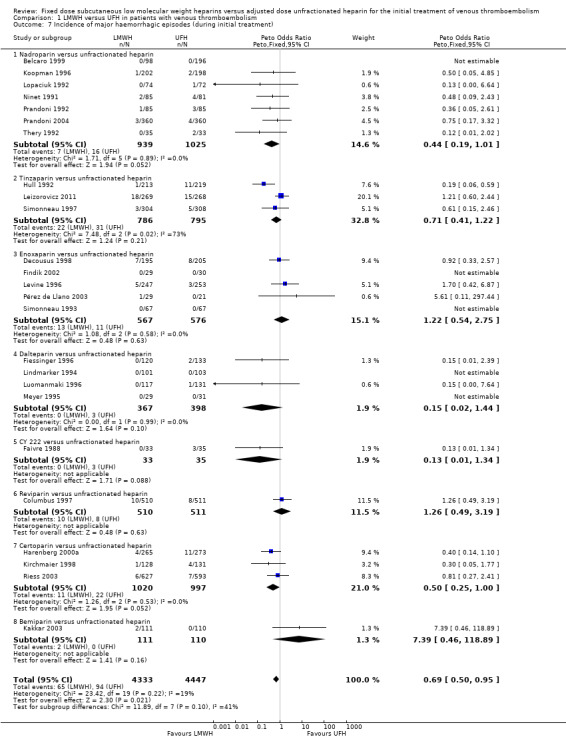
Comparison 1 LMWH versus UFH in patients with venous thromboembolism, Outcome 7 Incidence of major haemorrhagic episodes (during initial treatment).
Trends over time (Analysis 10.1 to Analysis 10.4)
In order to investigate the trend over time, we performed analyses in which all studies were ordered by their date of publication. The forest plots of these analyses did not show an obvious trend over time.
Discussion
Summary of main results
Our review of low molecular weight heparin (LMWH) for the initial treatment of venous thromboembolism (VTE) includes more than 9000 participants and indicates that this drug may be more efficacious than unfractionated heparin (UFH) for preventing recurrent VTE. Many of the included studies reported on other advantages of LMWH over UFH. Firstly, the route of administration (subcutaneous once or twice daily) is more convenient and increases the mobility of participants with VTE. Secondly, the pharmacokinetics are more predictable, which abolishes the need for laboratory monitoring and subsequent dose adjustments. Hence, LMWH can be advocated as the standard therapy for people with confirmed VTE. Treatment in an outpatient setting has been demonstrated to be feasible, safe and cost‐effective for people with DVT (Koopman 1996; Levine 1996; van den Belt 1998).
Analysis of all studies, regardless of methodological quality, showed that LMWH was associated with a lower incidence of recurrent VTE at the end of follow‐up and at three and six months, with 95% CIs less than one (Peto OR 0.72, 95% CI 0.59 to 0.88, Peto OR 0.71, 95% CI 0.56 to 0.90 and Peto OR 0.68, 95% CI 0.48 to 0.96 respectively) but not after one month follow‐up (Peto OR 0.90, 95% CI 0.56 to 1.44). However, when sensitivity analysis was performed on studies that concealed allocation of treatment only, no differences were observed in the incidence of recurrent venous thromboembolism during initial treatment and after three months nor in the incidence of major haemorrhages between LMWH and UFH. We therefore judge that the quality of the evidence is moderate.
When we performed analyses according to the type of VTE index event, the rate of recurrent VTE at the end of follow‐up remained lower in DVT participants treated with LMWH compared with DVT participants treated with UFH (Peto OR 0.57, 95% CI 0.44 to 0.75). However, analysis in participants with PE showed no difference in the rate of recurrent VTE between the two treatment groups (Peto OR 0.90, 95% CI 0.50 to 1.61). When we performed analyses according to mode of delivery of UFH, we found that LMWH was associated with fewer recurrent VTEs than intravenous UFH (Peto OR 0.69, 95% CI 0.56 to 0.86) but that there was no difference when LMWH was compared with subcutaneous UFH (Peto OR 1.05, 95% CI 0.56 to 1.95).
The tendency to improved efficacy with LMWH treatment was not at the cost of a higher rate of major haemorrhage. On the contrary, a reduction in major haemorrhage was demonstrated during the initial treatment period with LMWH. This is largely because the LMWH provides a more stable level of anticoagulation whereas unfractionated heparin dose adjustments may result in more peaks and troughs of anticoagulant effect.
Overall completeness and applicability of evidence
Although these results are promising, there are a number of unresolved issues. Firstly, since only approximately 25% of the participants included in this critical review had a diagnosis of primary pulmonary embolism, it can be argued that more data are required before conclusions can be drawn in this population. Secondly, although the combination of all preparations of LMWH seems logical, and heterogeneity could not be identified, current data do not discriminate between different LMWH preparations. A difference between LMWH preparations was only found for one outcome of the review, reduction in thrombus size. However, studies with large sample sizes and which include comparisons of different preparations are needed to determine whether the efficacy and safety of the individual LMWHs is actually comparable. Thirdly, Prandoni and colleagues noted that the route of administration might be relevant to heparin efficacy (Prandoni 2004). When we limited the analysis to studies that used intravenous UFK, similar results as in the main analyses were observed. When the analysis was confined to those studies that used subcutaneous UFH we found no difference in the incidence of recurrent VTE and major haemorrhages. The lack of difference could be due to the smaller groups in this analysis.
The protocol for this review was published in 1997 and the first version of the review was published in 1998. Initial treatment of VTE has changed since then and, as a result, the current objective of this review is no longer as clinically relevant as before. Therefore, to reflect current practice, future updates of this review will include studies on fixed dose subcutaneous UFH. Additionally, in accordance with current VTE trials on direct‐acting oral anticoagulants, future updates will assess symptomatic PE and symptomatic proximal DVT as the primary outcome. We will also assess side effects of treatment other than bleeding as an additional outcome.
Quality of the evidence
The quality of the evidence was downgraded to moderate due to concerns arising from risk of bias in individual studies. One study was at risk of selection bias (Luomanmaki 1996), six studies were at risk of attrition bias (Breddin 2001; Fiessinger 1996; Kakkar 2003; Lindmarker 1994; Ninet 1991; Thery 1992), two studies were at risk of reporting bias (Lindmarker 1994; Pérez de Llano 2003), and three studies were at risk for other types of bias including baseline differences between the groups (Findik 2002; Lopaciuk 1992; Luomanmaki 1996). A further reason for downgrading the evidence to moderate was that several studies did not adequately report on the methods used to generate the random sequence nor how treatment allocation was kept concealed.
While reductions in recurrent VTE and major haemorrhages were observed in the LMWH group compared with UFH when all studies were combined, in a sensitivity analysis of studies with adequate concealment of treatment allocation before randomisation, no differences were observed in the incidence of recurrent VTE during initial treatment and after three months nor in the incidence of major haemorrhages between LMWH and UFH. An explanation for these differences in effect size could be that the overall reductions are possibly biased by including less adequately performed studies without adequate concealment.
Where there were 10 or more studies in an analysis, we tested for publication bias using funnel plots. We found a suggestion of publication bias for three of the outcomes: incidence of recurrent VTE during initial treatment (Analysis 1.1,Figure 3);incidence of recurrent VTE at three months (Analysis 1.4, Figure 4); and reduction in thrombus size (Analysis 1.6, Figure 5). However, we felt it was insufficient to downgrade for publication bias. For the remaining outcomes, we found no evidence of publication bias for the analyses we tested (Analysis 1.2; Analysis 1.7; Analysis 1.8; Analysis 2.1; Analysis 7.1; Analysis 7.2; Analysis 7.3; Analysis 8.1; Analysis 8.2; Analysis 8.3; Analysis 8.5; Analysis 8.6; Analysis 10.1; Analysis 10.2; Analysis 10.3; Analysis 10.4).
1.1. Analysis.
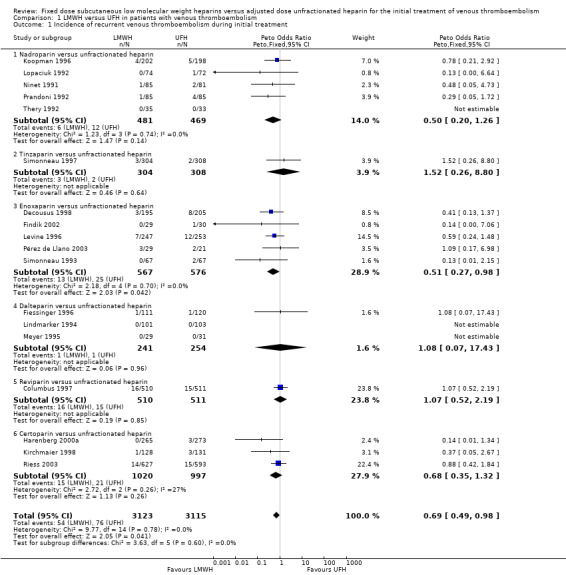
Comparison 1 LMWH versus UFH in patients with venous thromboembolism, Outcome 1 Incidence of recurrent venous thromboembolism during initial treatment.
3.
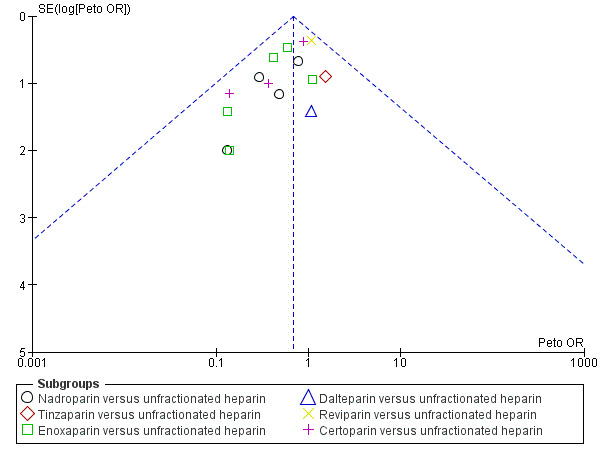
Funnel plot of comparison: 1 LMWH versus UFH in people with venous thromboembolism, outcome: 1.1 Incidence of recurrent venous thromboembolism during initial treatment.
1.4. Analysis.
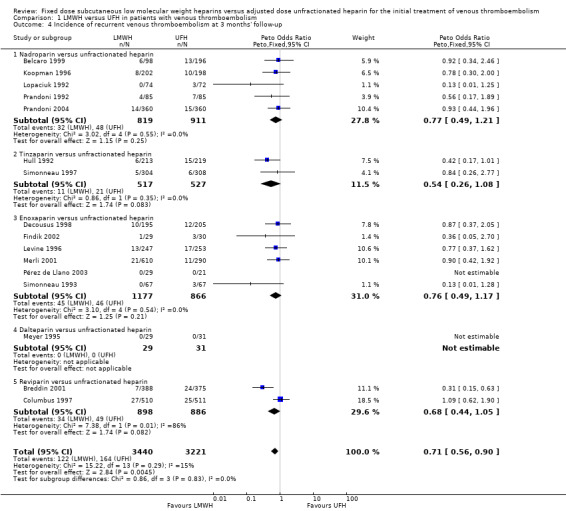
Comparison 1 LMWH versus UFH in patients with venous thromboembolism, Outcome 4 Incidence of recurrent venous thromboembolism at 3 months' follow‐up.
4.
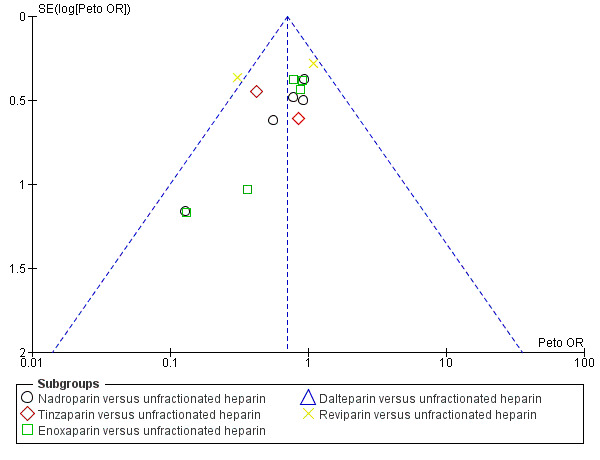
Funnel plot of comparison: 1 LMWH versus UFH in people with venous thromboembolism, outcome: 1.4 Incidence of recurrent venous thromboembolism at 3 months' follow‐up.
1.6. Analysis.
Comparison 1 LMWH versus UFH in patients with venous thromboembolism, Outcome 6 Reduction in thrombus size (pre‐ and post‐treatment venograms).
5.
Funnel plot of comparison: 1 LMWH versus UFH in people with venous thromboembolism, outcome: 1.6 Reduction in thrombus size (pre‐ and post‐treatment venograms).
1.2. Analysis.
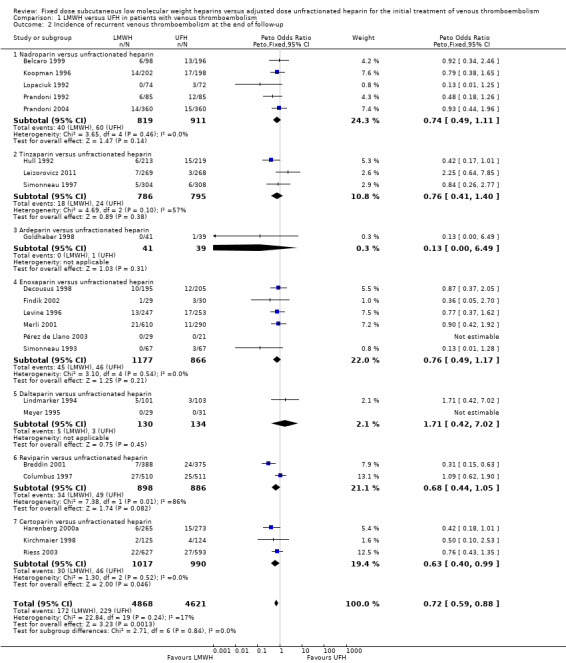
Comparison 1 LMWH versus UFH in patients with venous thromboembolism, Outcome 2 Incidence of recurrent venous thromboembolism at the end of follow‐up.
1.8. Analysis.
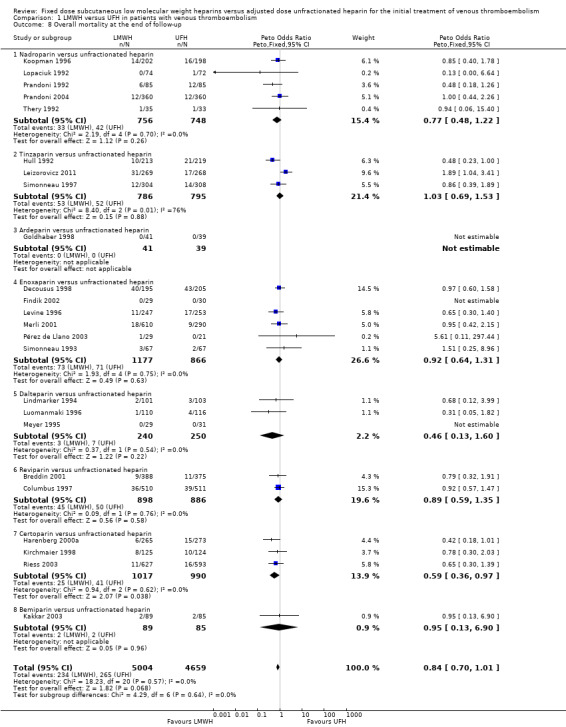
Comparison 1 LMWH versus UFH in patients with venous thromboembolism, Outcome 8 Overall mortality at the end of follow‐up.
7.1. Analysis.
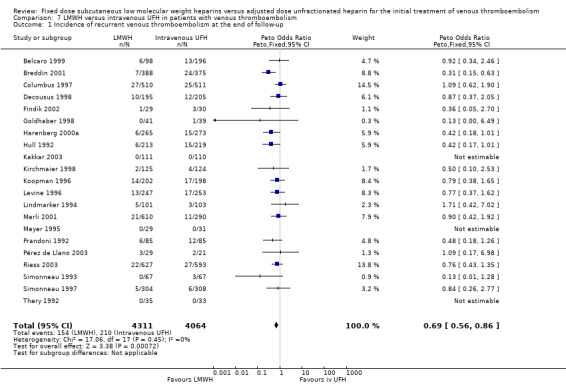
Comparison 7 LMWH versus intravenous UFH in patients with venous thromboembolism, Outcome 1 Incidence of recurrent venous thromboembolism at the end of follow‐up.
7.2. Analysis.
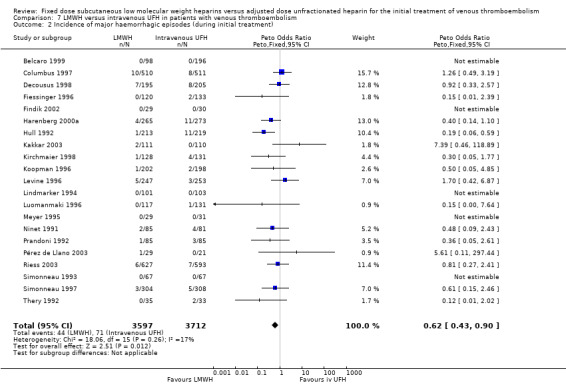
Comparison 7 LMWH versus intravenous UFH in patients with venous thromboembolism, Outcome 2 Incidence of major haemorrhagic episodes (during initial treatment).
7.3. Analysis.
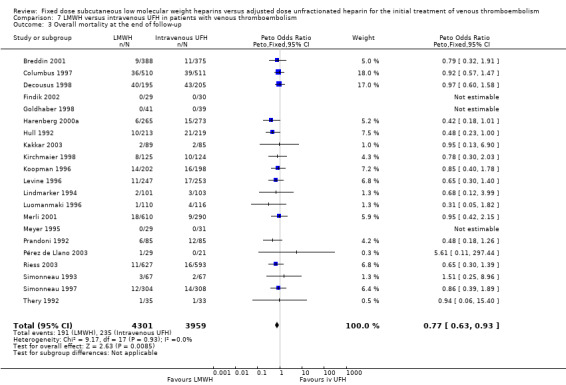
Comparison 7 LMWH versus intravenous UFH in patients with venous thromboembolism, Outcome 3 Overall mortality at the end of follow‐up.
8.1. Analysis.
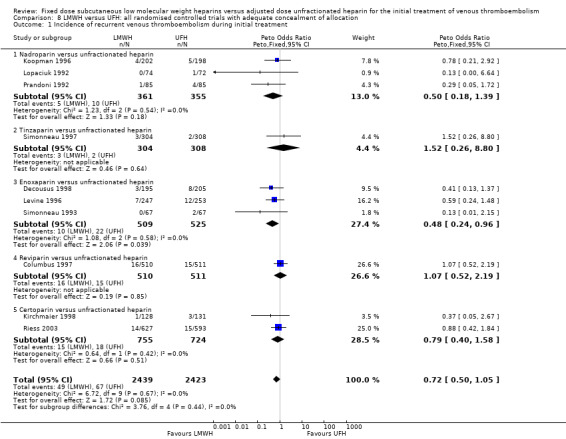
Comparison 8 LMWH versus UFH: all randomised controlled trials with adequate concealment of allocation, Outcome 1 Incidence of recurrent venous thromboembolism during initial treatment.
8.2. Analysis.
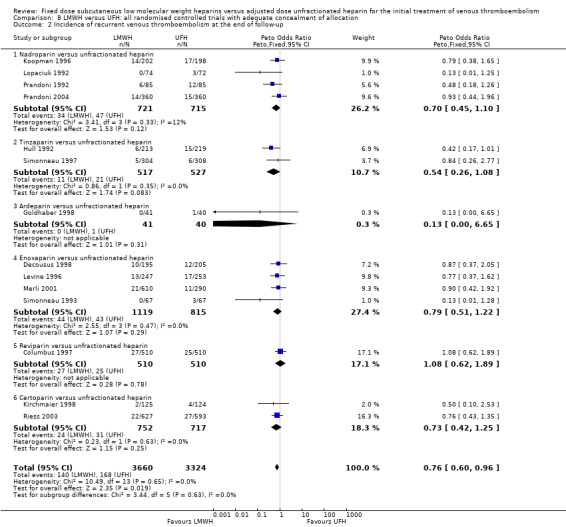
Comparison 8 LMWH versus UFH: all randomised controlled trials with adequate concealment of allocation, Outcome 2 Incidence of recurrent venous thromboembolism at the end of follow‐up.
8.3. Analysis.
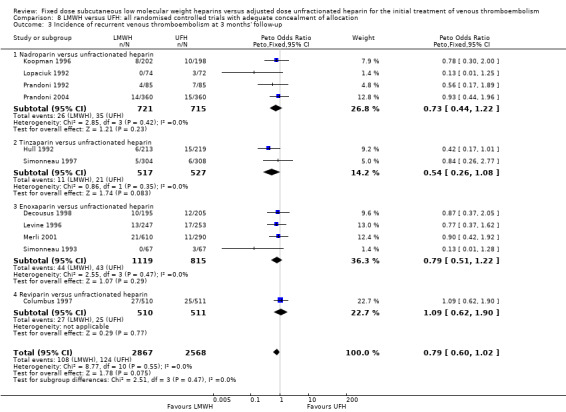
Comparison 8 LMWH versus UFH: all randomised controlled trials with adequate concealment of allocation, Outcome 3 Incidence of recurrent venous thromboembolism at 3 months' follow‐up.
8.5. Analysis.
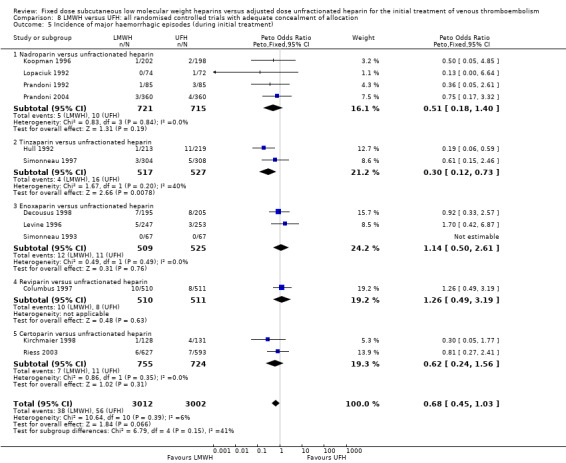
Comparison 8 LMWH versus UFH: all randomised controlled trials with adequate concealment of allocation, Outcome 5 Incidence of major haemorrhagic episodes (during initial treatment).
8.6. Analysis.
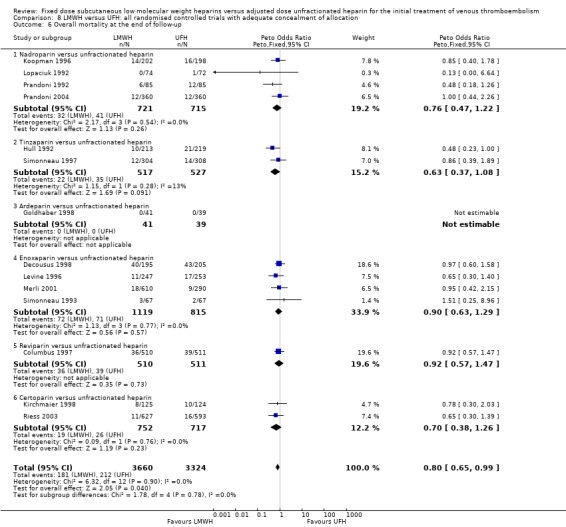
Comparison 8 LMWH versus UFH: all randomised controlled trials with adequate concealment of allocation, Outcome 6 Overall mortality at the end of follow‐up.
10.1. Analysis.
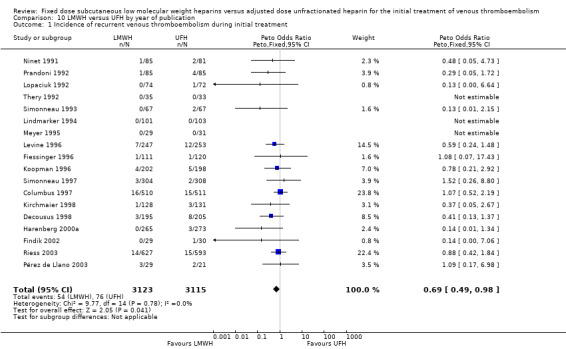
Comparison 10 LMWH versus UFH by year of publication, Outcome 1 Incidence of recurrent venous thromboembolism during initial treatment.
10.2. Analysis.
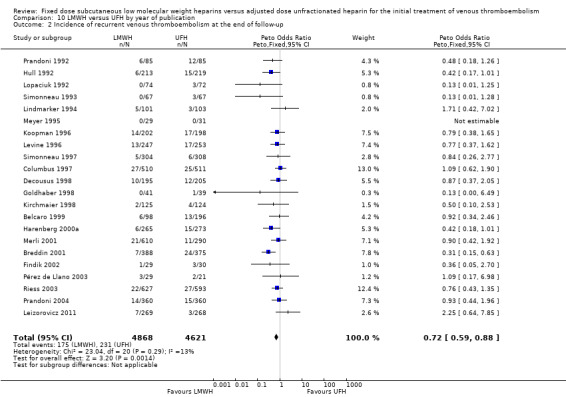
Comparison 10 LMWH versus UFH by year of publication, Outcome 2 Incidence of recurrent venous thromboembolism at the end of follow‐up.
10.3. Analysis.
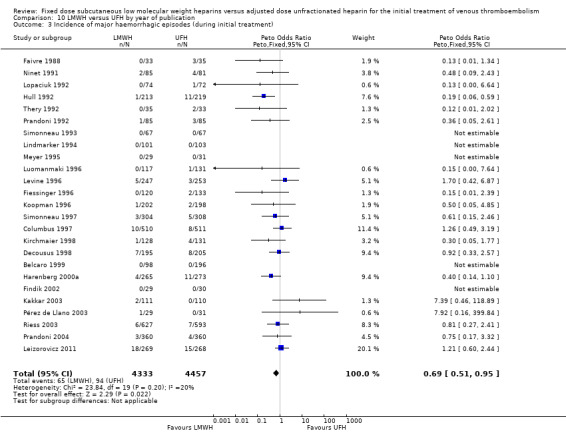
Comparison 10 LMWH versus UFH by year of publication, Outcome 3 Incidence of major haemorrhagic episodes (during initial treatment).
10.4. Analysis.
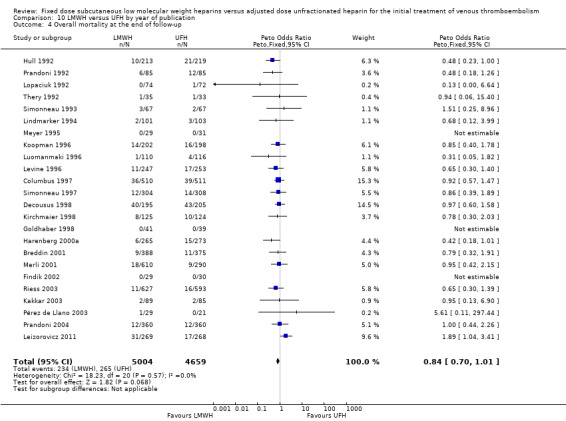
Comparison 10 LMWH versus UFH by year of publication, Outcome 4 Overall mortality at the end of follow‐up.
Potential biases in the review process
Neither of the authors of this review was involved in any of the included or excluded studies. Furthermore, neither has any commercial or other conflict of interest. The search was as comprehensive as possible; and the two review authors independently assessed all studies for inclusion. We are confident that we have included all relevant studies and we have attempted to reduce bias in the review process by performing data extraction and assessing study quality independently. However, the possibility remains that we may have missed studies which have not been published.
The original review did not set out to use the ISTH bleeding definition. However, given that this is now the standard accepted definition for major bleeding, we performed a post hoc sensitivity analysis for ISTH bleeding definitions in order to assess the effect of bleeding definitions used. The results from this sensitivity analysis (Analysis 9.1) are similar to the results from the analysis including all studies (Analysis 1.7) irrespective of their definition of major and clinically relevant bleeding.
9.1. Analysis.
Comparison 9 LMWH versus UFH: all randomised controlled trials that used ISTH definition of major bleeding, Outcome 1 Incidence of major haemorrhagic episodes (during initial treatment).
Agreements and disagreements with other studies or reviews
One network meta‐analysis of four studies compared three LMWH preparations (tinzaparin, nadroparin and enoxaparin) in terms of safety and efficacy for the treatment of deep vein thrombosis (Diaz 2015). Authors found no evidence of differences between tinzaparin, nadroparin and enoxaparin for recurrence of DVT and major bleeding.
Authors' conclusions
Implications for practice.
This review presents moderate‐quality evidence that fixed dose LMWH reduced the incidence of recurrent thrombotic complications and occurrence of major haemorrhage during initial treatment and low‐quality evidence that fixed dose LMWH reduced thrombus size when compared to UFH for the initial treatment of VTE. There was no difference in overall mortality between participants treated with LMWH and those treated with UFH (moderate‐quality evidence).
Implications for research.
Further studies are required to compare LMWH with UFH in the treatment of people with pulmonary embolism. In addition, a large RCT of at least two years' duration should be performed to determine the effects of dosing frequency on long‐term sequelae of venous thromboembolism, such as the development of post‐thrombotic syndrome. Individual low molecular weight heparin preparations could be compared with each other and new drugs should now be compared with LMWH.
Feedback
Anticoagulant feedback, 14 February 2011
Summary
Feedback received on this review, and other reviews and protocols on anticoagulants, is available on the Cochrane Editorial Unit website at http://www.editorial‐unit.cochrane.org/anticoagulants‐feedback.
What's new
| Date | Event | Description |
|---|---|---|
| 15 September 2016 | New search has been performed | Searches rerun. Six new studies included, five new studies excluded and one ongoing study identified. |
| 15 September 2016 | New citation required but conclusions have not changed | Searches rerun. Six new studies included, five new studies excluded and one ongoing study identified. Review updated according to current Cochrane standards. New authors have taken over this review. Conclusions not changed. |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 2, 1998
| Date | Event | Description |
|---|---|---|
| 14 February 2011 | Amended | Link to anticoagulant feedback added |
| 14 July 2010 | New search has been performed | The review was updated, one additional trial was added to the included studies and two additional trials were excluded. |
| 27 April 2010 | New citation required but conclusions have not changed | There was a change in authors in the updated review. |
| 20 October 2008 | Amended | Converted to new review format. |
| 14 November 2005 | Amended | Minor copy edits made. |
| 23 August 2004 | New citation required but conclusions have not changed | Change in authors. |
| 23 August 2004 | New search has been performed | Review substantively updated by the addition of eight new included studies. Conclusions unchanged. |
| 15 February 1999 | New search has been performed | One additional trial included but no change to conclusions. |
Acknowledgements
We acknowledge Cochrane Vascular for their assistance with the literature searches. We would like to thank Prof MH Prins and Dr PMG Erkens for their work on the previous versions of this review.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MESH DESCRIPTOR Thrombosis | 1231 |
| #2 | MESH DESCRIPTOR Thromboembolism | 892 |
| #3 | MESH DESCRIPTOR Venous Thromboembolism | 233 |
| #4 | MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES | 1996 |
| #5 | (thromboprophyla* or thrombus* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol*):TI,AB,KY | 17001 |
| #6 | MESH DESCRIPTOR Pulmonary Embolism EXPLODE ALL TREES | 729 |
| #7 | (PE or DVT or VTE):TI,AB,KY | 4480 |
| #8 | (((vein* or ven*) near thromb*)):TI,AB,KY | 6111 |
| #9 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 | 20325 |
| #10 | MESH DESCRIPTOR Heparin EXPLODE ALL TREES | 3815 |
| #11 | heparin*:TI,AB,KY | 8661 |
| #12 | LMWH:TI,AB,KY | 790 |
| #13 | UFH:TI,AB,KY | 437 |
| #14 | UH:TI,AB,KY | 84 |
| #15 | (nadroparin* or fraxiparin* or enoxaparin or Clexane or klexane or lovenox or dalteparin or Fragmin or ardeparin or normiflo or tinzaparin or logiparin or Innohep or certoparin or sandoparin or reviparin or clivarin* or danaproid or danaparoid):TI,AB,KY | 2405 |
| #16 | (antixarin or ardeparin* or bemiparin* or Zibor or cy 222 or embolex or monoembolex or parnaparin* or "rd 11885" or tedelparin or Kabi‐2165 or Kabi 2165):TI,AB,KY | 149 |
| #17 | (emt‐966 or emt‐967 or "pk‐10 169" or pk‐10169 or pk10169):TI,AB,KY | 8 |
| #18 | (fr‐860 or cy‐216 or cy216 or seleparin* or tedegliparin or seleparin* or tedegliparin*):TI,AB,KY | 51 |
| #19 | ("kb 101" or kb101 or lomoparan or orgaran):TI,AB,KY | 31 |
| #20 | (parnaparin or fluxum or lohepa or lowhepa or "op 2123" or parvoparin or AVE5026):TI,AB,KY | 36 |
| #21 | #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #19 OR #20 | 9580 |
| #22 | #9 AND #21 | 4334 |
Appendix 2. Trials registries searches
Clinicaltrials.gov
134 studies found for: subcutaneous AND heparin
WHO
57 records for 42 trials found for: subcutaneous AND heparin
ISRCTN
13 results subcutaneous AND heparin
Data and analyses
Comparison 1. LMWH versus UFH in patients with venous thromboembolism.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of recurrent venous thromboembolism during initial treatment | 18 | 6238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.49, 0.98] |
| 1.1 Nadroparin versus unfractionated heparin | 5 | 950 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.50 [0.20, 1.26] |
| 1.2 Tinzaparin versus unfractionated heparin | 1 | 612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.52 [0.26, 8.80] |
| 1.3 Enoxaparin versus unfractionated heparin | 5 | 1143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.27, 0.98] |
| 1.4 Dalteparin versus unfractionated heparin | 3 | 495 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.07, 17.43] |
| 1.5 Reviparin versus unfractionated heparin | 1 | 1021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.52, 2.19] |
| 1.6 Certoparin versus unfractionated heparin | 3 | 2017 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.35, 1.32] |
| 2 Incidence of recurrent venous thromboembolism at the end of follow‐up | 22 | 9489 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.59, 0.88] |
| 2.1 Nadroparin versus unfractionated heparin | 5 | 1730 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.49, 1.11] |
| 2.2 Tinzaparin versus unfractionated heparin | 3 | 1581 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.41, 1.40] |
| 2.3 Ardeparin versus unfractionated heparin | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.49] |
| 2.4 Enoxaparin versus unfractionated heparin | 6 | 2043 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.49, 1.17] |
| 2.5 Dalteparin versus unfractionated heparin | 2 | 264 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.71 [0.42, 7.02] |
| 2.6 Reviparin versus unfractionated heparin | 2 | 1784 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.44, 1.05] |
| 2.7 Certoparin versus unfractionated heparin | 3 | 2007 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.40, 0.99] |
| 3 Incidence of recurrent venous thromboembolism at 1 month follow‐up | 4 | 1741 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.56, 1.44] |
| 3.1 Nadroparin versus unfractionated heparin | 1 | 170 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.50 [0.10, 2.55] |
| 3.2 Enoxaparin versus unfractionated heparin | 2 | 550 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.24, 1.48] |
| 3.3 Reviparin versus unfractionated heparin | 1 | 1021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.64, 2.06] |
| 4 Incidence of recurrent venous thromboembolism at 3 months' follow‐up | 16 | 6661 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.56, 0.90] |
| 4.1 Nadroparin versus unfractionated heparin | 5 | 1730 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.49, 1.21] |
| 4.2 Tinzaparin versus unfractionated heparin | 2 | 1044 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.26, 1.08] |
| 4.3 Enoxaparin versus unfractionated heparin | 6 | 2043 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.49, 1.17] |
| 4.4 Dalteparin versus unfractionated heparin | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Reviparin versus unfractionated heparin | 2 | 1784 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.44, 1.05] |
| 5 Incidence of recurrent venous thromboembolism at 6 months' follow‐up | 7 | 2841 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.48, 0.96] |
| 5.1 Nadroparin versus unfractionated heparin | 2 | 570 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.37, 1.19] |
| 5.2 Dalteparin versus unfractionated heparin | 1 | 204 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.71 [0.42, 7.02] |
| 5.3 Certoparin versus unfractionated heparin | 3 | 2007 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.40, 0.99] |
| 5.4 Enoxaparin versus unfractionated heparin | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Reduction in thrombus size (pre‐ and post‐treatment venograms) | 16 | 2909 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.61, 0.82] |
| 6.1 Nadroparin versus unfractionated heparin | 4 | 507 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.50, 1.05] |
| 6.2 Ardeparin versus unfractionated heparin | 1 | 75 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.37 [0.14, 0.99] |
| 6.3 Enoxaparin versus unfractionated heparin | 1 | 117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.17, 0.71] |
| 6.4 Dalteparin versus unfractionated heparin | 5 | 650 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.90, 1.73] |
| 6.5 CY 222 versus unfractionated heparin | 1 | 59 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.32, 2.62] |
| 6.6 Reviparin versus unfractionated heparin | 1 | 649 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.43, 0.80] |
| 6.7 Certoparin versus unfractionated heparin | 2 | 649 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.50, 0.98] |
| 6.8 Bemiparin versus unfractionated heparin | 1 | 203 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.42 [0.24, 0.74] |
| 7 Incidence of major haemorrhagic episodes (during initial treatment) | 25 | 8780 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.50, 0.95] |
| 7.1 Nadroparin versus unfractionated heparin | 7 | 1964 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.19, 1.01] |
| 7.2 Tinzaparin versus unfractionated heparin | 3 | 1581 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.41, 1.22] |
| 7.3 Enoxaparin versus unfractionated heparin | 5 | 1143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [0.54, 2.75] |
| 7.4 Dalteparin versus unfractionated heparin | 4 | 765 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.15 [0.02, 1.44] |
| 7.5 CY 222 versus unfractionated heparin | 1 | 68 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 1.34] |
| 7.6 Reviparin versus unfractionated heparin | 1 | 1021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.49, 3.19] |
| 7.7 Certoparin versus unfractionated heparin | 3 | 2017 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.50 [0.25, 1.00] |
| 7.8 Bemiparin versus unfractionated heparin | 1 | 221 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.46, 118.89] |
| 8 Overall mortality at the end of follow‐up | 24 | 9663 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.70, 1.01] |
| 8.1 Nadroparin versus unfractionated heparin | 5 | 1504 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.48, 1.22] |
| 8.2 Tinzaparin versus unfractionated heparin | 3 | 1581 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.03 [0.69, 1.53] |
| 8.3 Ardeparin versus unfractionated heparin | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Enoxaparin versus unfractionated heparin | 6 | 2043 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.64, 1.31] |
| 8.5 Dalteparin versus unfractionated heparin | 3 | 490 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.13, 1.60] |
| 8.6 Reviparin versus unfractionated heparin | 2 | 1784 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.59, 1.35] |
| 8.7 Certoparin versus unfractionated heparin | 3 | 2007 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.36, 0.97] |
| 8.8 Bemiparin versus unfractionated heparin | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.13, 6.90] |
1.3. Analysis.
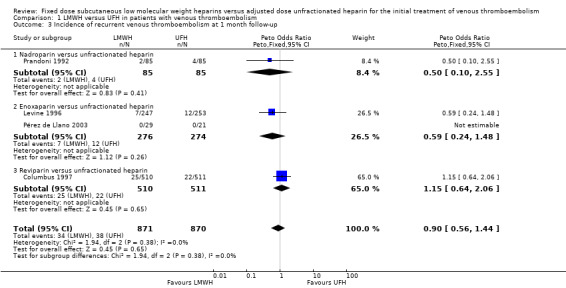
Comparison 1 LMWH versus UFH in patients with venous thromboembolism, Outcome 3 Incidence of recurrent venous thromboembolism at 1 month follow‐up.
1.5. Analysis.
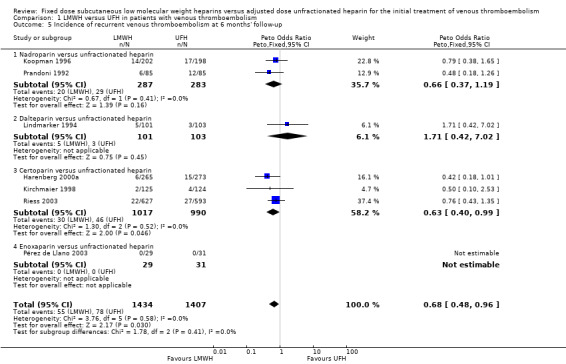
Comparison 1 LMWH versus UFH in patients with venous thromboembolism, Outcome 5 Incidence of recurrent venous thromboembolism at 6 months' follow‐up.
Comparison 2. LMWH versus UFH in patients with proximal deep venous thrombosis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of recurrent venous thromboembolism at the end of follow‐up | 10 | 4672 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.57 [0.44, 0.75] |
| 1.1 Nadroparin versus unfractionated heparin | 3 | 864 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.44, 1.19] |
| 1.2 Tinzaparin versus unfractionated heparin | 1 | 432 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.42 [0.17, 1.01] |
| 1.3 Enoxaparin versus unfractionated heparin | 2 | 634 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.32, 1.32] |
| 1.4 Reviparin versus unfractionated heparin | 1 | 763 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.31 [0.15, 0.63] |
| 1.5 Certoparin versus unfractionated heparin | 2 | 1758 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.40, 1.03] |
| 1.6 Bemiparin versus unfractionated heparin | 1 | 221 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Incidence of recurrent deep venous thrombosis at the end of follow‐up | 7 | 2681 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.61 [0.41, 0.91] |
| 2.1 Nadroparin versus unfractionated heparin | 3 | 765 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.41, 1.43] |
| 2.2 Tinzaparin versus unfractionated heparin | 1 | 432 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.37 [0.12, 1.16] |
| 2.3 Enoxaparin versus unfractionated heparin | 1 | 500 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.34, 1.63] |
| 2.4 Reviparin versus unfractionated heparin | 1 | 763 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.45 [0.17, 1.21] |
| 2.5 Bemiparin versus unfractionated heparin | 1 | 221 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.14] |
| 3 Incidence of pulmonary embolism at the end of follow‐up | 7 | 3024 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.45 [0.28, 0.74] |
| 3.1 Nadroparin versus unfractionated heparin | 2 | 570 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.27, 1.60] |
| 3.2 Tinzaparin versus unfractionated heparin | 1 | 432 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.14, 1.95] |
| 3.3 Enoxaparin versus unfractionated heparin | 1 | 500 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.05, 5.07] |
| 3.4 Reviparin versus unfractionated heparin | 1 | 763 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.10, 0.73] |
| 3.5 Certoparin versus unfractionated heparin | 1 | 538 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.32 [0.11, 0.92] |
| 3.6 Bemiparin versus unfractionated heparin | 1 | 221 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.94 [0.20, 18.86] |
| 4 Reduction in thrombus size (pre‐ and post‐treatment venograms) | 2 | 230 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.27, 0.80] |
| 4.1 Bemiparin versus unfractionated heparin | 1 | 203 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.42 [0.24, 0.74] |
| 4.2 Dalteparin versus unfractionated heparin | 1 | 27 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.47 [0.22, 9.90] |
| 5 Incidence of major haemorrhagic episodes (during initial treatment) | 8 | 3589 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.50 [0.29, 0.85] |
| 5.1 Nadroparin versus unfractionated heparin | 3 | 765 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.42 [0.09, 1.85] |
| 5.2 Tinzaparin versus unfractionated heparin | 1 | 432 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.19 [0.06, 0.59] |
| 5.3 Enoxaparin versus unfractionated heparin | 2 | 634 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.70 [0.42, 6.87] |
| 5.4 Certoparin versus unfractionated heparin | 2 | 1758 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.26, 1.17] |
| 6 Overall mortality at the end of follow‐up | 9 | 4331 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.47, 0.85] |
| 6.1 Nadroparin versus unfractionated heparin | 2 | 570 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.38, 1.24] |
| 6.2 Tinzaparin versus unfractionated heparin | 1 | 432 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.23, 1.00] |
| 6.3 Enoxaparin versus unfractionated heparin | 2 | 634 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.37, 1.50] |
| 6.4 Reviparin versus unfractionated heparin | 1 | 763 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.32, 1.91] |
| 6.5 Certoparin versus unfractionated heparin | 2 | 1758 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.30, 0.96] |
| 6.6 Bemiparin versus unfractionated heparin | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.13, 6.90] |
Comparison 3. LMWH versus UFH in patients with pulmonary embolism.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of recurrent venous thromboembolism at the end of follow‐up | 7 | 1407 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.50, 1.61] |
| 1.1 Tinzaparin versus unfractionated heparin | 2 | 680 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.26, 2.77] |
| 1.2 Enoxaparin versus unfractionated heparin | 3 | 396 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.37, 2.16] |
| 1.3 Dalteparin versus unfractionated heparin | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Reviparin versus unfractionated heparin | 1 | 271 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.35, 2.64] |
| 2 Reduction in thrombus size (pre‐ and post‐treatment venograms) | 2 | 106 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.36 [0.23, 8.16] |
| 2.1 Nadroparin versus unfractionated heparin | 1 | 52 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.53 [0.32, 95.93] |
| 2.2 Dalteparin versus unfractionated heparin | 1 | 54 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.05, 5.43] |
| 3 Mean change in pulmonary vascular obstruction severity score | 2 | 106 | Mean Difference (IV, Fixed, 95% CI) | ‐3.14 [‐4.39, ‐1.90] |
| 3.1 Nadroparin versus unfractionated heparin | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐3.28 [‐4.55, ‐2.01] |
| 3.2 Dalteparin versus unfractionated heparin | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐5.94, 7.94] |
| 4 Incidence of major haemorrhagic episodes (during initial treatment) | 3 | 178 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.04, 4.29] |
| 4.1 Nadroparin versus unfractionated heparin | 1 | 68 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.01, 2.02] |
| 4.2 Enoxaparin versus unfractionated heparin | 1 | 50 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.61 [0.11, 297.44] |
| 4.3 Dalteparin versus unfractionated heparin | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Overall mortality at end of follow‐up | 3 | 178 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.70 [0.17, 16.71] |
| 5.1 Nadroparin versus unfractionated heparin | 1 | 68 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.06, 15.40] |
| 5.2 Enoxaparin versus unfractionated heparin | 1 | 50 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.61 [0.11, 297.44] |
| 5.3 Dalteparin versus unfractionated heparin | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. LMWH versus UFH in patients with venous thromboembolism and malignant disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at the end of follow‐up | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Mortality in patients with malignant disease | 6 | 446 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.33, 0.85] |
| 1.2 Mortality in patients with malignant disease in trial with adequate concealment | 5 | 430 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.31, 0.82] |
Comparison 5. LMWH versus UFH in patients with venous thromboembolism without malignant disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at the end of follow‐up | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Mortality in patients without malignant disease | 6 | 2139 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.61, 1.56] |
| 1.2 Mortality in patients without malignant disease in trials with adequate concealment | 5 | 1951 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.62, 1.62] |
Comparison 6. LMWH versus subcutaneous UFH in patients with venous thromboembolism.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of recurrent venous thromboembolism at the end of follow‐up | 3 | 1403 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.56, 1.95] |
| 2 Incidence of major haemorrhagic episodes (during initial treatment) | 4 | 1471 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.50, 1.67] |
| 3 Overall mortality at the end of follow‐up | 3 | 1403 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.46 [0.91, 2.35] |
6.1. Analysis.
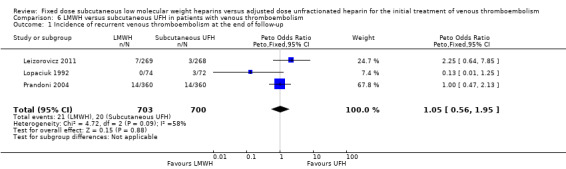
Comparison 6 LMWH versus subcutaneous UFH in patients with venous thromboembolism, Outcome 1 Incidence of recurrent venous thromboembolism at the end of follow‐up.
6.2. Analysis.
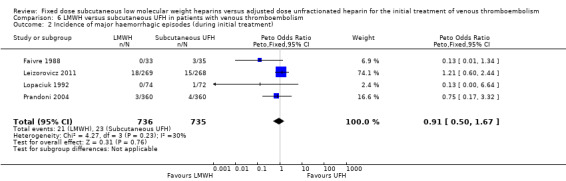
Comparison 6 LMWH versus subcutaneous UFH in patients with venous thromboembolism, Outcome 2 Incidence of major haemorrhagic episodes (during initial treatment).
6.3. Analysis.
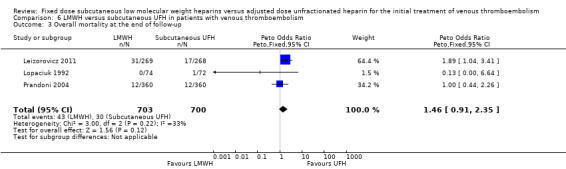
Comparison 6 LMWH versus subcutaneous UFH in patients with venous thromboembolism, Outcome 3 Overall mortality at the end of follow‐up.
Comparison 7. LMWH versus intravenous UFH in patients with venous thromboembolism.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of recurrent venous thromboembolism at the end of follow‐up | 21 | 8375 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.56, 0.86] |
| 2 Incidence of major haemorrhagic episodes (during initial treatment) | 21 | 7309 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.43, 0.90] |
| 3 Overall mortality at the end of follow‐up | 21 | 8260 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.63, 0.93] |
Comparison 8. LMWH versus UFH: all randomised controlled trials with adequate concealment of allocation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of recurrent venous thromboembolism during initial treatment | 10 | 4862 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.50, 1.05] |
| 1.1 Nadroparin versus unfractionated heparin | 3 | 716 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.50 [0.18, 1.39] |
| 1.2 Tinzaparin versus unfractionated heparin | 1 | 612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.52 [0.26, 8.80] |
| 1.3 Enoxaparin versus unfractionated heparin | 3 | 1034 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.24, 0.96] |
| 1.4 Reviparin versus unfractionated heparin | 1 | 1021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.52, 2.19] |
| 1.5 Certoparin versus unfractionated heparin | 2 | 1479 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.40, 1.58] |
| 2 Incidence of recurrent venous thromboembolism at the end of follow‐up | 14 | 6984 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.60, 0.96] |
| 2.1 Nadroparin versus unfractionated heparin | 4 | 1436 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.45, 1.10] |
| 2.2 Tinzaparin versus unfractionated heparin | 2 | 1044 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.26, 1.08] |
| 2.3 Ardeparin versus unfractionated heparin | 1 | 81 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.65] |
| 2.4 Enoxaparin versus unfractionated heparin | 4 | 1934 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.51, 1.22] |
| 2.5 Reviparin versus unfractionated heparin | 1 | 1020 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.62, 1.89] |
| 2.6 Certoparin versus unfractionated heparin | 2 | 1469 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.42, 1.25] |
| 3 Incidence of recurrent venous thromboembolism at 3 months' follow‐up | 11 | 5435 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.60, 1.02] |
| 3.1 Nadroparin versus unfractionated heparin | 4 | 1436 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.44, 1.22] |
| 3.2 Tinzaparin versus unfractionated heparin | 2 | 1044 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.26, 1.08] |
| 3.3 Enoxaparin versus unfractionated heparin | 4 | 1934 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.51, 1.22] |
| 3.4 Reviparin versus unfractionated heparin | 1 | 1021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.62, 1.90] |
| 4 Reduction in thrombus size (pre‐ and post‐treatment venograms) | 5 | 753 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.49 [0.37, 0.66] |
| 4.1 Nadroparin versus unfractionated heparin | 2 | 302 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.49 [0.31, 0.77] |
| 4.2 Ardeparin versus unfractionated heparin | 1 | 75 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.37 [0.14, 0.99] |
| 4.3 Enoxparin versus unfractionated heparin | 1 | 117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.17, 0.71] |
| 4.4 Certoparin versus unfractionated heparin | 1 | 259 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.38, 1.04] |
| 5 Incidence of major haemorrhagic episodes (during initial treatment) | 12 | 6014 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.45, 1.03] |
| 5.1 Nadroparin versus unfractionated heparin | 4 | 1436 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.18, 1.40] |
| 5.2 Tinzaparin versus unfractionated heparin | 2 | 1044 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.12, 0.73] |
| 5.3 Enoxaparin versus unfractionated heparin | 3 | 1034 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.14 [0.50, 2.61] |
| 5.4 Reviparin versus unfractionated heparin | 1 | 1021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.49, 3.19] |
| 5.5 Certoparin versus unfractionated heparin | 2 | 1479 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.24, 1.56] |
| 6 Overall mortality at the end of follow‐up | 14 | 6984 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.65, 0.99] |
| 6.1 Nadroparin versus unfractionated heparin | 4 | 1436 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.47, 1.22] |
| 6.2 Tinzaparin versus unfractionated heparin | 2 | 1044 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.37, 1.08] |
| 6.3 Ardeparin versus unfractionated heparin | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 Enoxaparin versus unfractionated heparin | 4 | 1934 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.63, 1.29] |
| 6.5 Reviparin versus unfractionated heparin | 1 | 1021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.57, 1.47] |
| 6.6 Certoparin versus unfractionated heparin | 2 | 1469 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.38, 1.26] |
8.4. Analysis.
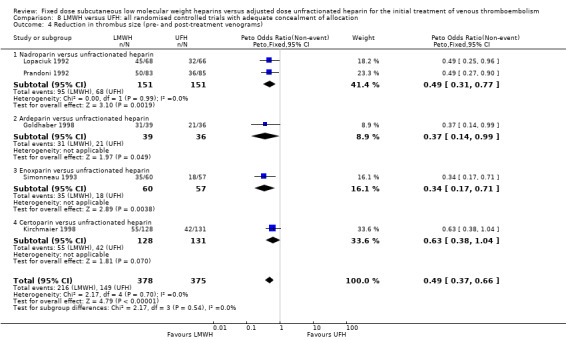
Comparison 8 LMWH versus UFH: all randomised controlled trials with adequate concealment of allocation, Outcome 4 Reduction in thrombus size (pre‐ and post‐treatment venograms).
Comparison 9. LMWH versus UFH: all randomised controlled trials that used ISTH definition of major bleeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of major haemorrhagic episodes (during initial treatment) | 24 | 8712 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.52, 0.98] |
| 1.1 Nadroparin versus unfractionated heparin | 7 | 1964 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.19, 1.01] |
| 1.2 Tinzaparin versus unfractionated heparin | 3 | 1581 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.41, 1.22] |
| 1.3 Enoxaparin versus unfractionated heparin | 5 | 1143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [0.54, 2.75] |
| 1.4 Dalteparin versus unfractionated heparin | 4 | 765 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.15 [0.02, 1.44] |
| 1.5 Reviparin versus unfractionated heparin | 1 | 1021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.49, 3.19] |
| 1.6 Certoparin versus unfractionated heparin | 3 | 2017 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.50 [0.25, 1.00] |
| 1.7 Bemiparin versus unfractionated heparin | 1 | 221 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.46, 118.89] |
Comparison 10. LMWH versus UFH by year of publication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of recurrent venous thromboembolism during initial treatment | 18 | 6238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.49, 0.98] |
| 2 Incidence of recurrent venous thromboembolism at the end of follow‐up | 22 | 9489 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.59, 0.88] |
| 3 Incidence of major haemorrhagic episodes (during initial treatment) | 25 | 8790 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.51, 0.95] |
| 4 Overall mortality at the end of follow‐up | 24 | 9663 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.70, 1.01] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Belcaro 1999.
| Methods | Study design: randomised controlled trial. Method of randomisation: not stated. Concealment of allocation: not blinded for treatment allocation, blinded for outcome assessment. Exclusions post‐randomisation: 31 participants. Lost to follow‐up: none. |
|
| Participants | Country: not stated. Setting: hospital. No.: 197 participants. Age: mean 54 years. Sex: M:F 111:84. Inclusion criteria: informed consent. Exclusion criteria: two or more previous episodes of DVT or PE, currently active bleeding, active ulcers, known familial bleeding or coagulation disorder (i.e. known deficiency of antithrombin III, protein C or protein S), concurrent PE, treatment for the DVT with standard heparin lasting more than 48 hours, or impossibility of being or inability to be treated at home with LMWH or standard heparin. Also excluded were: people with neoplastic disorders requiring surgery or chemotherapy in the following 3 months, and those with likelihood of low or no compliance and/or inability to be included in a follow‐up, pregnancy and a platelet count below 100,000 per mm³. |
|
| Interventions | Treatment: LMWH: administered primarily at home and body weight adjusted (nadroparin 0.1 mL per kg twice daily). Doses were 0.6, 0.8 and 1 mL (respectively equivalent to 6150, 8200 and 10,250 anti‐factor Xa IU). Dose most suitable to the participant's weight was chosen. Control: UFH: i.v. bolus of 5000 IU initially, followed by continuous infusion of 20,000 IU. Dose was adjusted to maintain APTT between 60 and 85 seconds. Treatment duration:
Oral anticoagulation: more than 3 months. |
|
| Outcomes | Primary: symptomatic or asymptomatic (detected by colour duplex scanning) recurrent DVT or DVT extension in 3 months after randomisation. Secondary: bleeding during administration of the study medication or within 48 hours after discontinuation; PE; number of hospital days; number of participants treated directly at home without hospital admission. |
|
| Notes | Follow‐up: 3 months. 2 UFH groups (s.c. and i.v.). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All reported outcome events were reviewed by a central panel unaware of the treatment assigned and participant's identity. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups. |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Breddin 2001.
| Methods | Study design: randomised controlled trial. Method of randomisation: stratified according to site. Concealment of allocation: not blinded for treatment allocation, blinded for outcome assessment. Exclusions post‐randomisation: none. Lost to follow‐up: none. |
|
| Participants | Country: Argentina, Austria, Czech Republic, Denmark, Germany, Hungary, Israel, Poland, Norway, United Kingdom. Setting: hospital. No.: 1137 participants. Age: mean 58 years. Sex: 621 males. Inclusion criteria: acute DVT confirmed by venography without symptoms lasting longer than 14 days. Exclusion criteria: presence of thrombi only in isolated calf veins or isolated muscle veins; clinically symptomatic PE; treatment with UFH, LMWH, or VKA for 24 hours or more before enrolment; uncontrolled hypertension; stroke within 3 weeks of enrolment; cerebral vascular aneurysm or active gastroduodenal ulcer; bacterial endocarditis; thrombocytopenia (< 100,000 platelets/mm³; severe liver or renal insufficiency; receipt of spinal or epidural anaesthesia or lumbar puncture in the 5 days before enrolment; surgery in the 5 days before enrolment; concomitant treatment with fibrinolytic agents or platelet function inhibitors; a body weight of less than 35 kg; pregnancy and known drug abuse. |
|
| Interventions | Treatment: LMWH: Reviparin (Clivarin, Knoll, Ludwigshafen, Germany) twice daily, body weight adjusted (7000 anti‐factor Xa IU for a weight of 35 to 45 kg, 8400 IU for 46 to 60 kg and 12,600 IU for more than 60 kg). Control: 5000 IU i.v. UFH plus continuous i.v. infusion of 1250 IU/hour (dose‐adjusted APTT × 1.5 to 2.5. Treatment duration: LMWH 5 to 7 days, UFH until INR > 2.0 (and maintained). Oral anticoagulation: in both groups (started day 1) for 90 days. |
|
| Outcomes | Primary: change in venographically determined thrombus size (Marder's score) between base line and day 21 (± 2 days). Secondary: Clinical outcomes: recurrent DVT or PE during initial treatment and 3 months' follow‐up; major haemorrhagic events between day 0 and 21. |
|
| Notes | Follow‐up: 90 days. LMWH once daily group (374 participants) not included in analysis because LMWH was given for 28 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for missing second venogram and therefore exclusion for efficacy analysis are not provided and missing outcome data imbalanced in numbers across intervention groups. |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Columbus 1997.
| Methods | Study design: randomised controlled trial. Method of randomisation: stratified according to whether the participant presented with DVT only or with PE, according to clinical centre. Concealment of allocation: not blinded for treatment allocation, blinded for outcome assessment. Exclusions post‐randomisation: none. Lost to follow‐up: none. |
|
| Participants | Country: Netherlands, France, Italy, Germany, Spain, Australia, New Zealand, Canada. Setting: hospital. No.: 1021 participants. Age: mean 60. Sex: 525 males. Inclusion criteria: acute symptomatic DVT and/or PE requiring antithrombotic therapy. DVT documented by ultrasonography or venography and PE by ventilation‐perfusion lung scanning (high probability of PE), pulmonary angiography or, if lung scanning was non‐diagnostic, by demonstrating DVT by compression ultrasonography or venography. Exclusion criteria: therapeutic doses of LMWH, UFH or oral anticoagulant therapy for more than 24 hours; contraindications for anticoagulant therapy; planned thrombolytic therapy; gastrointestinal bleeding in the preceding 14 days; surgery requiring anaesthesia within the previous 3 days; a stroke in the preceding 10 days; platelet count < 100,000/mm³ ; weight < 35 kg; pregnant or of childbearing potential and not using adequate contraception; in a location that made follow‐up difficult. |
|
| Interventions | Treatment: LMWH: Reviparin sodium (Clivarin, Knoll, Luwigshafen, Germany) in body weight adjusted fixed‐dose, s.c., twice daily. Decision to treat participants at home left to treating physician. Control: UFH: APTT‐adjusted dose, continuous i.v. infusion in hospital after initial intravenous bolus of 5000 IU. Treatment duration: at least 5 days; treatment cessation if INR was 2.0 or above for 2 consecutive days. Oral anticoagulation: started on first or second day and continued for a total of 12 weeks; INR 2.0 to 3.0. |
|
| Outcomes | Primary: symptomatic DVT or PE during initial treatment and within 12 weeks of randomisation. Secondary: major haemorrhage during initial treatment and within 12 weeks of randomisation; death within 12 weeks of randomisation. |
|
| Notes | Follow‐up: 12 weeks. DVT only: LMWH 372 (73%) and UFH 378 (74%). PE: 138 (27%) versus 133 (26%). In retrospect, 3 participants with DVT only and 2 with PE should have been excluded at entry as they did not have abnormal test results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed with a computer algorithm. |
| Allocation concealment (selection bias) | Low risk | Central allocation by a 24‐hour telephone service. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Information on all suspected outcome events and deaths was reviewed and classified by a central adjudication committee whose members were unaware of the treatment assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Decousus 1998.
| Methods | Study design: randomised controlled trial. Method of randomisation: stratified according to centre. Concealment of allocation: not blinded for treatment allocation, blinded for outcome assessment. Exclusions post‐randomisation: none. Lost to follow‐up: 4 (1 vital status; 3 for the assessment of non‐fatal events). |
|
| Participants | Country: France (44 centres). Setting: hospital. No.: 400 participants. Age: mean 72. Sex: 190 males. Inclusion criteria: acute proximal DVT confirmed by venography with or without symptomatic PE; at high risk for PE. Exclusion criteria: placement of previous filter; contraindication to or failure of anticoagulant therapy; curative anticoagulant therapy lasting more than 48 hours; indication for thrombolysis; short life expectancy; allergy to iodine; hereditary thrombophilia; severe renal or hepatic failure; pregnancy; likelihood of non‐compliance. |
|
| Interventions | Treatment: LMWH: Enoxaparin (Rhone‐Poulenc Rorer) body weight‐adjusted fixed dose (1 mg per kg body weight), s.c., twice daily (100 anti‐factor Xa IU per mg). Control: UFH: APTT‐adjusted, continuous i.v. infusion (started with 500 IU per kg of body weight per day), after initial i.v. bolus dose of 5000 IU. Treatment duration: 8 to 12 days; discontinuation if INR was 2 or more for 2 consecutive days. Oral anticoagulation: warfarin or acenocoumarol started on day 4 and continued for at least 3 months. |
|
| Outcomes | Primary: symptomatic or asymptomatic PE within the first 12 days after randomisation; all symptomatic recurrent VTE. Secondary: major haemorrhage during the initial treatment period; mortality. |
|
| Notes | Follow‐up: 2 years. The outcome of recurrent VTE was only reported for a follow‐up period of 3 months (also included as the incidence at the end of follow‐up). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by a computer system. |
| Allocation concealment (selection bias) | Low risk | Allocation was performed by a central 24‐hour telephone system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All pulmonary investigations and all documented symptomatic events, including deaths, were validated by an independent adjudication committee whose members were unaware of the treatment assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data unlikely to be related to true outcome. |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes. |
| Other bias | Unclear risk | The study has a potential source of bias due to the fact that 2 interventions (the effectiveness of a vena cava filter and the efficacy of LMWH) are investigated in the same population. There is insufficient Information about the number of participants with a vena cava filter across intervention groups. |
Faivre 1988.
| Methods | Study design: randomised controlled trial. Method of randomisation: not stated. Concealment of allocation: not blinded for treatment allocation, blinded for outcome assessment. Exclusion post‐randomisation: 1 in UFH group (thrombocytopenia). Lost to follow‐up: 9 participants had no second phlebography (3 CY 222, 6 UFH). |
|
| Participants | Country: France. Setting: hospital. No.: 68 participants. Age: mean 66 years. Sex: 39 males. Inclusion criteria: symptomatic DVT and/or symptomatic PE, or symptomatic PE confirmed by ventilation‐perfusion scan and a positive phlebogram. Exclusion criteria: > 2 weeks symptoms of DVT or PE with massive PE; extension of the thrombus into the inferior vena cava. |
|
| Interventions | Treatment: LMWH: CY 222 starting with a bolus injection i.v. 5000 U anti‐factor Xa IU and continued with body weight‐adjusted fixed dose: 155 IU/kg (750 U anti‐factor Xa IU/kg/24 hours), s.c., twice daily. Control: UFH: starting with a bolus injection i.v. 5000 IU of UFH and continued with 500 IU/kg/24 hours s.c., twice daily; dose‐adjusted APTT × 2.0 to 3.0. Treatment duration: 10 days. Oral anticoagulation: not defined for treatment or control groups. |
|
| Outcomes | Primary: change in thrombus size (Marder's score); recurrent DVT and PE. Secondary: major haemorrhage during the initial treatment. |
|
| Notes | Baseline characteristics: difference in presence of PE (66% of participants allocated to LMWH and 34% of participants allocated to UFH had a PE). Repeated venography; participants with thrombotic and bleeding events excluded from venographic evaluation. Unclear from publication whether valid criteria for diagnosis of recurrent VTE were used. No prospective follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded for outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Unclear risk | Insufficient information. ? baseline differences mentioned above? |
Fiessinger 1996.
| Methods | Study design: randomised controlled trial. Method of randomisation: not stated. Concealment of allocation: not blinded for treatment allocation, blinded for outcome assessment. Exclusions post‐randomisation: 10 participants in dalteparin group and 5 participants in UFH group did not have DVT. Lost to follow‐up: 32 participants (13 versus 19) did not have a second phlebogram; 2 (1 versus 1) participants were considered not to have DVT; 20 participants (8 versus 12) were incorrectly included. |
|
| Participants | Country: Austria, France, Spain and Sweden (16 centres). Setting: hospital. No.: 253 participants. Age: mean 61 years. Sex: 115 males. Inclusion criteria: distal and/or proximal DVT with 8 or more days of symptoms. Exclusion criteria: clinical signs suggestive of PE; history of recent DVT (< 1 year) or sequelae of a previous DVT in the same leg; treatment with therapeutic doses of UFH or LMWH prior to randomisation; malignant hypertension; renal or hepatic insufficiency; platelet count < 100 x 10⁹/litre; known hypersensitivity to contrast media; surgery within 5 days of starting treatment; intracerebral bleeding in previous 2 months, gastrointestinal bleeding in previous 2 weeks; pregnancy/lactation. |
|
| Interventions | Treatment: LMWH: 1 mL active substance equivalent to 10,000 anti‐factor Xa IU (Dalteparin, Fragmin) s.c. injection (200 IU/kg) o.d.. Bolus dose of 5000 IU. s.c. if randomisation before phlebography, otherwise a first full‐dose. Control: UFH: before phlebography: bolus dose of 5000 IU i.v. followed by continuous i.v. infusion of 20,000 to 40,000 IU/24 hours APTT‐adjusted (1.5 to 3.0 ×). After phlebography a bolus i.v. injection administered prior to infusion of UFH at discretion of attending physician. Treatment duration: 5 to 10 days, when the prothrombin time (INR) was within therapeutic range (2 to 3) on 2 consecutive days. Oral anticoagulation: started on day of inclusion or day after. Period determined by attending physician; mean period of treatment 5.3 months in both groups. |
|
| Outcomes | Primary: change in thrombus size (Marder's score); recurrent VTE during initial treatment (prospective follow‐up) and at the end of 6 months' follow‐up; PE during initial treatment and at the end of 6 months' follow‐up. Secondary: major haemorrhage during initial treatment; mortality; mortality in participants with malignancy at entry. |
|
| Notes | 20 participants not correctly included; 32 participants without second phlebography. Follow‐up: 6 months, but 23 participants lost to follow‐up; of these 13 were alive. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was blinded and the non‐blinding of others is unlikely to introduce bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing outcome data imbalanced in numbers across intervention groups. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Findik 2002.
| Methods | Study design: randomised controlled trial. Method of randomisation: not stated. Concealment of allocation: not blinded for treatment allocation. Exclusion post‐randomisation: none. Lost to follow‐up: none. |
|
| Participants | Country: Turkey. Setting: hospital. No.: 59 participants. Age: mean 50 years. Sex: 29 males. Inclusion criteria: patients with clinically suspected acute PE, objectively confirmed by ventilation‐perfusion lung scan, showing a high probability or in the case of an indeterminate result accompanied by DVT confirmed by compression ultrasonography. Exclusion criteria: massive PE requiring thrombolytic therapy or embolectomy; contraindication for anticoagulant therapy (active bleeding or haematologic disorders); anticoagulant therapy at a therapeutic dose within 24 hours before study; a life expectancy ≤ 3 months, severe hepatic or renal failure; pregnancy; suspicion of non‐compliance. |
|
| Interventions | Treatment: LMWH: Enoxaparin s.c. 1 mg/kg, 100 anti‐factor Xa IU per kg of body weight twice daily Control: UFH: Starting with a bolus injection i.v. 5000 IU followed by a continuous i.v. infusion of 1000 IU/hour. UFH dose was adjusted (APTT‐1.5 to 2.5 × control value). Treatment duration: approximately 7 days. Oral anticoagulation: started on the second day for a total of 6 months. |
|
| Outcomes | Primary: recurrent VTE, major haemorrhage and mortality during initial treatment and at 3 months. | |
| Notes | Blinding for outcome assessment was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insuffcient information. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information about missing outcome data provided. |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes. |
| Other bias | High risk | The low participant numbers in both LMWH and UFH arms and low event rates reduced the statistical power of the study to detect a significant difference between the arms. |
Goldhaber 1998.
| Methods | Study design: randomised controlled trial. Method of randomisation: computerised, not stratified. Concealment of allocation: not blinded for treatment allocation, blinded for outcome assessment. Exclusions post‐randomisation: none. Lost to follow‐up: not stated. |
|
| Participants | Country: United States. Setting: hospital. No.: 81 participants. Age: mean 54 years. Sex: 43 males. Inclusion criteria: acute (within 14 days) symptomatic DVT of the legs documented by ultrasound and participants had to be deemed appropriate for discharge home. Exclusion criteria: high‐risk DVT involving 3 proximal veins; pelvic vein thrombosis; current symptomatic PE; expected prolonged hospitalisation for other reasons; haemoglobin < 85 g/litre or platelet count < 100 × 10⁹/litre. |
|
| Interventions | Treatment: LMWH: 130 anti‐factor Xa IU/kg ardeparin sodium twice daily subcutaneously for 5 to 15 days. Control: UFH, heparin sodium 5000‐ to 7500‐unit bolus followed by continuous i.v. administration to achieve APTT of 1.5 to 2.5. Titration guided by Cruickshank nomogram. Treatment duration: LMWH 5 to 15 days, UFH 5 days or more to achieve target APTT. Oral anticoagulation: 6 weeks. |
|
| Outcomes | Primary: change in thrombus size; recurrent DVT or PE. Secondary: major and minor haemorrhage. |
|
| Notes | Repeated venography at the end of follow‐up (6 weeks). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation and allocation was accomplished by calling a central computerised service. |
| Allocation concealment (selection bias) | Low risk | Randomisation and allocation was accomplished by calling a central computerised service. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reasons for missing data provided. |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Harenberg 2000a.
| Methods | Study design: randomised controlled trial. Method of randomisation: not stated. Concealment of allocation: blinded for outcome assessment. Exclusions post‐randomisation: not stated. Lost to follow‐up: not stated. |
|
| Participants | Country: Austria, Germany, Switzerland, Czech Republic. Setting: hospital. No.: 541 were eligible of which 3 withdrew informed consent; therefore 538 participants were assigned. Age: 30 years and older. Sex: Males and females (breakdown not supplied). Inclusion criteria: acute symptomatic proximal DVT (thrombosis of the popliteal vein or proximal) documented by ascending venography. Exclusion criteria: indication for surgical or fibrinolytic treatment of DVT; duration of symptoms for more than 3 weeks; ongoing oral anticoagulation; renal failure; severe hypertension (> 200 mmHg systolic and > 105 mmHg diastolic while on antihypertensive treatment); severe hepatic failure; currently active bleeding or disorders contraindicating anticoagulant therapy; contraindication to oral anticoagulants; pregnancy; known intolerance to heparins; intolerance to contrast media; any operation within the past 8 days; acute severe PE; platelet count < 100,000/µL; treatment with heparin > 24 hours before inclusion; treatment with platelet‐inhibiting drugs (100 mg or more acetylsalicylic acid daily allowed). |
|
| Interventions | Treatment: LMWH: fixed dose 8000 anti‐factor Xa IU (Certoparin) s.c., twice daily Control: UFH: adjusted to APTT 2 to 3 × the reference value. Treatment duration: 7 to 15 days. Oral anticoagulation: at least 6 months. |
|
| Outcomes | Primary: change in thrombus size (Marder's score), recurrent VTE, major bleeding and death during treatment and after 6 months' test follow‐up. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data on all potential outcome events were evaluated by an independent committee, which was unaware of the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups. |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes. |
| Other bias | High risk | The study was sponsored by Novartis Pharmacological GmbH, Nuremberg, Germany. |
Hull 1992.
| Methods | Study design: randomised controlled trial. Method of randomisation: computerised and stratified to groups according to study centre. Concealment of allocation: blinded for treatment allocation and outcome assessment. Exclusions post‐randomisation: none. Lost to follow‐up: none. |
|
| Participants | Country: USA, Canada (15 centres). Setting: hospital. No.: 432 participants. Age: 161 participants under 60 years, 270 participants over 60 years. Sex: 140 males, 291 females. Inclusion criteria: symptomatic or asymptomatic proximal DVT with or without symptomatic PE. Exclusion criteria: active bleeding or disorders contraindicating anticoagulant therapy; allergy to heparin, bisulphites or fish; pregnancy; 2 or more previously documented episodes of DVT or PE; history of protein C deficiency; history of heparin‐associated thrombocytopenia; severe malignant hypertension (blood pressure 250 mmHg or more systolic and 130 mmHg or more diastolic); severe hepatic failure (hepatic encephalopathy); severe renal failure; requiring dialysis; geographic inaccessibility preventing attendance at follow‐up visits. Eligible participants were excluded if they had received treatment with warfarin, LMWH or heparinoids within the previous 7 days; treatment with therapeutic s.c. heparin within the preceding 12 hours; received i.v. heparin (265 participants) or declined to give written informed consent (148 participants). |
|
| Interventions | Treatment: LMWH: logiparin body weight adjusted fixed dose 175 anti‐factor Xa IU/kg, s.c., o.d. Control: UFH: dose‐adjusted APTT × 1.5 to 2.5, continuous i.v. infusion starting with 40,320 Units/24 hours; or in people at high risk, 29,760 Units/24 hours. Initial i.v. bolus of 5000 Units. Treatment duration: 6 days provided the INR was 2.0 or more. Oral anticoagulation: warfarin sodium was given for at least 3 months and was started on day 2 of the initial heparin treatment. |
|
| Outcomes | Primary: recurrent DVT and PE; major haemorrhage during or immediately after initial treatment. Secondary: minor haemorrhage; mortality. |
|
| Notes | Placebo controlled. Follow‐up: 3 months. More women in UFH group; no significant effect of gender demonstrated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomised computer‐derived treatment schedule was used to assign the participants to the treatment group. |
| Allocation concealment (selection bias) | Low risk | A randomised computer‐derived treatment schedule was used to assign the participants to the treatment group. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind clinical trial. Participants received either intravenous UFH with subcutaneous placebo or subcutaneous LMWH with intravenous placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A double‐blind clinical trial. The outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes. |
| Other bias | Low risk | There were more women in the intravenous heparin group. To assess the possible effect of this potential gender imbalance, multiple logistic regression was used. No significant effect was found. The study seems to be free of other sources of bias. |
Kakkar 2003.
| Methods | Study design: multicentre, randomised, open‐label, parallel group comparison trial Method of randomisation: not stated Concealment of allocation: not stated Exclusions post‐randomisation: 54 participants Lost to follow‐up: none |
|
| Participants | Country: Spain, Poland and United Kingdom Setting: 27 hospitals No.: 324 participants: 94 bemiparin, 105 bemiparin + VKA, 98 UFH Age: bemiparin mean 63.2 (45.1 to 70.8) years, bemiparin + VKA mean 61.2 (44.4 to 69.5) years, UFH mean 61.2 (49.9 to 70.5) years, Sex: bemiparin 58 M/36 F, bemiparin + VKA 61 M/44 F, UFH 63 M/35 F, Inclusion criteria: people with an acute DVT of the legs, confirmed by venography and who had symptoms for no more than 14 days. Exclusion criteria: people receiving therapeutic doses of heparin or a vitamin K antagonist for more than 48 hours prior to enrolment, clinically symptomatic pulmonary embolism, pregnancy confirmed by urine analysis, ischaemic cerebral vascular accident 1 month prior to enrolment, known cerebral vascular aneurysm, active duodenal ulcer or bacterial endocarditis, severe liver or renal failure, spinal or epidural anaesthesia or lumbar puncture 3 days prior to enrolment, uncontrolled hypertension, allergy to heparin, warfarin, sodium or iodinated contrast medium, history of heparin‐associated thrombocytopenia or platelet count of less than 100,000 platelets per mm³, concurrent treatment with fibrinolytic agents, a body weight of less than 35 kg, treatment with an investigational drug in the last 4 weeks prior to enrolment, inability to attend follow‐up due to geographic inaccessibility and known drug use |
|
| Interventions | Treatment 1: 115 anti‐Xa IU per kg of bemiparin as 1 injection every 24 hours based on participants' weight (5000 anti‐Xa for weight < 50 kg, 7,500 anti‐Xa for weight 50 to 70 kg and 10,000 anti‐Xa IU for more than 70 kg) followed by VKA from day 3 10 mg per day for first 3 days then adjusted to achieve an INR between 2 and 3 for 12 weeks Treatment 2: 115 anti‐Xa IU per kg of bemiparin as 1 injection every 24 hours based on participants' weight (5000 anti‐Xa for weight < 50 kg, 7500 anti‐Xa for weight 50 to 70 kg and 10,000 anti‐Xa IU for more than 70 kg) followed by fixed daily dose of 3500 anti‐Xa units for 90 days. Control: i.v. bolus of 5000 UFH followed by a continuous i.v. infusion at a dose of 40,000 IU per 24 hours in participants at low risk of bleeding and 30,000 IU per 24 hours in participants at high risk of bleeding followed by VKA from day 3 10 mg per day for first 3 days then adjusted to achieve an INR between 2 and 3 for 12 weeks. Treatment duration: 12 weeks. |
|
| Outcomes | Primary: venographically confirmed change in thrombus size between baseline and day 14 assessed with the use of the Marder score and patency of deep venous system determined by venography or Doppler ultrasound at 12 weeks. Secondary: symptomatic recurrence of DVT and PE, major bleeding (clinically overt and associated with a fall in haemoglobin level of at least 2.0 g per decilitre) and death. |
|
| Notes | Follow‐up: 7 days, 14 days, 12 weeks and 28 weeks. In this 3‐armed trial, 2 bemiparin groups were compared with an UFH control group. However, in 1 of the bemiparin groups (treatment 2), participants did not receive concomitant VKA therapy. All other studies included in this review used concomitant VKA therapy and in order for our results to be comparable, data for this group of participants in the Kakkar 2003 study were not included in the analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned". Comment: insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly assigned". Comment: insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Open label Quote: "The venograms were independently assessed by two radiologists of an independent committee who were unaware of the patients treatment assignments". Comment: outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 324 participants in intention‐to‐treat group but only 297 participants included in the per protocol population and only 255 followed up to day 84. Numbers lost to follow‐up not adequately reported. |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes. |
| Other bias | High risk | The study was sponsored by Laboratorios Farmaceuticos Rovi, Madrid, Spain. |
Kirchmaier 1998.
| Methods | Study design: randomised controlled trial. Method of randomisation: computerised. Concealment of allocation: partly blinded for treatment allocation. Exclusions post‐randomisation: 6 participants. Lost to follow‐up: none. |
|
| Participants | Country: Austria, Czech Republic, Germany (total 23 centres). Setting: hospital. No.: 257 participants. Age: median 61 years. Sex: 133 males. Inclusion criteria: symptomatic DVT of the lower leg. Exclusion criteria: thrombi only in 1 or 2 calf veins; treatment with vitamin K antagonists; use of contrast media; surgery in the previous week; thrombocytopenic (< 100,000/µL). |
|
| Interventions | Treatment: subcutaneous LMWH (certoparin) 8000 IU/kg twice daily Control: UFH: initial bolus of 5000 IU followed by 20 IU/kg/hour. In both groups phenprocoumon was started between day 12 and 14. Heparin was stopped until an INR range between 2.0 and 3.5 was reached. Treatment duration: at least 14 days. Oral anticoagulation: Oral anticoagulant therapy was continued for at least 6 months. |
|
| Outcomes | Primary: recurrent VTE; major haemorrhage during initial treatment; change in thrombus size; mortality at the end of follow‐up. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation performed by a statistician, but there is insufficient information about the sequence generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was performed centrally by telephone. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Partly blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment by an investigator, who was blinded to the treatment the participants had received. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for missing phlebograms and perfusion scans were not provided. |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Koopman 1996.
| Methods | Study design: randomised controlled trial. Method of randomisation: stratified according to centre. Concealment of allocation: not blinded for treatment allocation, blinded for outcome assessment. Exclusions post‐randomisation: none. Lost to follow‐up: none. |
|
| Participants | Country: Netherlands, France, Italy, Australia, New Zealand. Setting: hospital. No.: 400 outpatients. Age: Mean 61 years. Sex: 203 males. Inclusion criteria: acute symptomatic proximal DVT documented by venography and/or ultrasonography. Exclusion criteria: VTE in last 2 years; suspected PE; previous treatment with heparin > 24 hours; life expectancy < 6 months; post‐thrombotic syndrome; geographic inaccessibility. |
|
| Interventions | Treatment: LMWH (Nadroparin‐Ca, Fraxiparine) in body weight‐adjusted fixed dose, s.c., twice daily. If appropriate, at home. Control: UFH: APTT‐adjusted dose, continuous i.v. infusion in hospital after initial i.v. bolus of 5000 Units. Treatment duration: at least 5 days; treatment cessation if INR was 2.0 or above in 2 measurements 24 hours apart. Oral anticoagulation: started on first day and continued for 3 months unless persistence of risk factors required its continuation beyond that period. INR 2.0 to 3.0. |
|
| Outcomes | Primary: symptomatic recurrent VTE (DVT or PE) during initial treatment, after 3 months' follow‐up and at the end of follow‐up (6 months); major haemorrhage during initial treatment and after 3 months of follow‐up. Secondary: minor haemorrhage or death during initial treatment, after 3 months of follow‐up and at the end of follow‐up (6 months); other potential outcome events; quality of life. |
|
| Notes | Follow‐up: 6 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation and allocation was achieved by means of a central 24‐hour telephone service. |
| Allocation concealment (selection bias) | Low risk | Randomisation and allocation was achieved by means of a central 24‐hour telephone service. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Documentation of all potential outcome events was submitted to an independent adjudication committee whose members were unaware of the treatment assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups. |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes. |
| Other bias | Low risk | Study appears to be free of other sources of bias. |
Leizorovicz 2011.
| Methods | Study design: international, multicentre, centrally randomised, open, parallel‐group study. Method of randomisation: computer generated randomisation scheme in a 1:1 ratio with central telephone randomisation. Concealment of allocation: no allocation concealment mechanism was attempted. Exclusions post‐randomisation: none Lost to follow‐up: none |
|
| Participants | Country: 8 countries (Belgium, France, Germany, Spain, Serbia, Croatia, Romania and Poland) Setting: 109 hospitals No.: 269 tinzaparin, 270 UFH Age: tinzaparin mean 82.9 ± 5.7 years, UFH mean 82.6 ± 5.8 years Sex: tinzaparin 92 M/177 F, UFH 102 M/168 F Inclusion criteria: people ≥ 70 years with an acute objectively confirmed (compression ultrasonography or venography) symptomatic proximal or distal lower limb DVT or asymptomatic DVT if proximal and associated with a PE. Exclusion criteria: people who had received treatment doses of heparins or thrombolytic agents within the previous 4 weeks prior to randomisation, received oral anticoagulation within the preceding week, planned use of high doses of acetylsalicylic acid (> 300mg/day) or an NSAID, had a requirement for thrombolytic therapy, end stage renal disease requiring dialysis, hepatic insufficiency, bacterial endocarditis, planned epidural or spinal anaesthesia, planned or recent (within 2 weeks) surgery, thrombocytopenia, severe uncontrolled hypertension, overt bleeding or recent stroke. |
|
| Interventions | Treatment: tinzaparin 175 IU/kg subcutaneous injection once daily. Control: UFH (50 IU/kg i.v. bolus followed by twice daily subcutaneous injections in initial doses between 400 to 600 IU/kg/day then adjusted by APTT). Treatment duration: 5 days. Oral anticoagulation: VKA treatment initiated between days 1 and 3 and continued until at least day 90 ± 5 |
|
| Outcomes | Primary: clinically relevant bleeding by day 90 ± 5. Secondary: symptomatic recurrent VTE prior to day 90 ± 5, major and minor bleedings prior to day 90 ± 5, heparin‐induced thrombocytopenia and death. |
|
| Notes | Follow‐up: 90 ± 5 days. Study was unexpectedly terminated early as at a predefined interim analysis conducted after completion of 350 participants, an excess mortality was observed in the tinzaparin group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment assignment was pre‐planned according to a computer generated randomisation scheme in a 1:1 ratio with central telephone randomisation". Comment: low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "No allocation concealment mechanism was attempted as the study was open". Comment: insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was open but care was taken to ensure that outcome assessors and data analysts were kept blinded to the allocation". Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors and data analysts were kept blinded to the allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes. |
| Other bias | Unclear risk | Study sponsored by LEO Pharma. |
Levine 1996.
| Methods | Study design: randomised controlled trial. Method of randomisation: stratified according to centre, mode of diagnosis (venography or ultrasonography), and category of participants (outpatients, admitted at weekend or at night, hospitalised). Concealment of allocation: not blinded for treatment allocation, blinded for outcome assessment. Exclusions post‐randomisation: none. Lost to follow‐up: none. |
|
| Participants | Country: Canada. Setting: hospital. No.: 500 outpatients and inpatients. Age: mean 58 years. Sex: males and females (breakdown not supplied). Inclusion criteria: acute proximal DVT. Exclusion criteria: 2 or more previous episodes of DVT or PE; active bleeding; active peptic ulcer disease; familial bleeding disorder; concurrent symptomatic PE; > 48 hours heparin treatment; inability to be treated with LMWH as outpatient because of coexisting condition (e.g. cancer, infection, stroke) or likelihood of non‐compliance; inability to make follow‐up visits because of geographical inaccessibility; presence of known deficiency of anti‐thrombin III, protein C or protein S; pregnancy. |
|
| Interventions | Treatment: LMWH: enoxaparin (Rhone‐Poulenc Rorer) body weight‐adjusted fixed dose (1 mg/kg body weight), s.c., twice daily, at home. 1 vial: 1 mL/100 mg = 100 anti‐factor Xa IU/mg). Control: UFH: APTT‐adjusted, continuous i.v. infusion (started with 20,000 Units in 500 mL of 5% dextrose solution) in hospital after an initial i.v. bolus of 5000 Units. Treatment duration: at least 5 days; discontinuation if INR was 2 or above and maintained for 2 consecutive days. Oral anticoagulation warfarin sodium started on day 2 and continued for 3 months. |
|
| Outcomes | Primary: symptomatic recurrent VTE within 90 days of follow‐up; major haemorrhage during the initial treatment or 48 hours after treatment cessation. Secondary: minor haemorrhage; mortality. |
|
| Notes | Some participants received 1 or 2 days UFH before randomisation; this was considered part of the overall duration of heparin treatment. Follow‐up: 3 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Low risk | Allocation over the telephone from a central site. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All reported outcome events were reviewed by a central adjudication committee whose members were unaware of the treatment assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Lindmarker 1994.
| Methods | Study design: randomised controlled trial. Method of randomisation: centrally organised using sealed envelopes and stratified for centre. Concealment of allocation: not blinded for treatment allocation, blinded for outcome assessment. Exclusions post‐randomisation: 6 (1 UFH versus 5 fragmin). Lost to follow‐up: for venographic assessment 18 (13 UFH versus 5 fragmin); for clinical outcome assessment 16 participants. |
|
| Participants | Country: Sweden. Setting: hospital. No.: 204 outpatients. Age: mean 61 years. Sex: 116 males. Inclusion criteria: symptomatic distal and proximal DVT. Exclusion criteria: UFH treatment already given for more than 24 hours; surgery < 5 days before; previous DVT in the ipsilateral leg; suspected or verified PE; thrombectomy or thrombolysis indicated; DVT proximal of inguinal arch; intracranial bleeding within previous 2 weeks; known haemorrhagic diathesis or disorders; platelet count below 100 × 10⁹/litre; renal insufficiency (S‐creatinine < 300 µM); hepatic insufficiency with a prothrombin time < 40% (INR > 1.5); allergy to UFH, fragmin or contrast media; pregnancy or breastfeeding; severe hypertension. |
|
| Interventions | Treatment: initial i.v. bolus injection of UFH 5000 Units followed by continuous i.v. infusion of UFH 800 to 1700/hour for a maximum of 24 hours after randomisation: LMWH (fragmin) body weight‐adjusted fixed dose of 200 anti‐factor Xa IU/kg with a maximum of 18,000 IU, s.c., o.d. Control: initial i.v. bolus injection of UFH 5000 Units followed by continuous i.v. infusion of UFH 800 to 1700/hour; after randomisation: continuation of i.v. infusion with UFH dose‐adjusted APTT × 1.5 to 3.0. Treatment duration: at least 5 days; treatment cessation if INR was within therapeutic range (2.0 to 3.0) for 2 consecutive days. Treatment duration no longer than 9 days. Oral anticoagulation: warfarin sodium started on the day that venography was carried out and continued for a minimum of 3 months; INR 2.0 to 3.0. |
|
| Outcomes | Primary: change in thrombus size (Marder's score); recurrent VTE; major haemorrhage. Secondary: mortality; mortality in participants with malignant disease. |
|
| Notes | Repeated venography on day 1 and within 4 days after discontinuation of heparin therapy. Follow‐up: 6 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The participants who died or had a recurrent VTE were not included in the analyses which may result in an underestimation of the number of participants with extended or unchanged thrombosis. |
| Selective reporting (reporting bias) | High risk | Participants who died or had a recurrent VTE were not included in the analyses. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Lopaciuk 1992.
| Methods | Study design: randomised controlled trial. Method of randomisation: sealed envelopes, stratified for site of DVT. Concealment of allocation: not blinded for treatment allocation, blinded for outcome assessment. Exclusions post‐randomisation: 3 participants in UFH group judged to be ineligible (2 with recent history of DVT and 1 deficient in antithrombin III). Lost to follow‐up: 6 in LMWH group and 6 in UFH group (poor phlebogram, 6; absent phlebogram, 4; protocol violation (treatment for 15 days), 1; major bleeding with treatment cessation, 1). |
|
| Participants | Country: Poland (6 centres). Setting: hospital. No.: 149 participants of which 117 participants had proximal DVT. Age: mean 48 years. Sex: 81 males. Inclusion criteria: symptomatic proximal or calf DVT (phlebographically proven). Exclusion criteria: clinically suspected PE; phlegmasia caerulea dolens; treatment with heparin or oral anticoagulants prior to admission; history of VTE in previous 2 years; surgery or trauma within previous 3 days; contraindication to heparin therapy; pregnancy; documented antithrombin III deficiency. |
|
| Interventions | Treatment: LMWH: fraxiparine fixed dose: 92 anti‐factor Xa IU/kg, s.c., twice daily Control: UFH: initial i.v. bolus of 5000 IU followed by 250 IU/kg s.c., twice daily; dose‐adjusted APTT × 1.5 to 2.5 s.c. Treatment duration: 10 days. Oral anticoagulation: acenocoumarol started on day 7 and continued for at least 3 months; INR 2.0 to 3.0. |
|
| Outcomes | Primary: change in thrombus size (Arnesen score); recurrent DVT; PE. Secondary: major and minor haemorrhage; mortality; mortality in participants with malignant disease. |
|
| Notes | Proximal DVT: 58 (LMWH) versus 59 (UFH). Distal DVT: 16 (LMWH) versus 13 (UFH). 12 participants excluded from repeated venography analysis. Follow‐up: 3 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind evaluation of phlebographic results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | High risk | There was an imbalanced exclusion at baseline. |
Luomanmaki 1996.
| Methods | Study design: randomised controlled trial. Method of randomisation: not stated. Concealment of allocation: not blinded for treatment allocation and for clinical outcome assessment; blinded for assessment of venograms at entry to study and at the end of the initial treatment period. Exclusions post‐randomisation: 78 randomised participants excluded because DVT found not to be present after randomisation. Lost to follow‐up: no information given. |
|
| Participants | Country: Sweden and USA (2 centres). Setting: hospital. No.: 248 participants. Age: mean 57.5 years (LMWH); mean 60.5 years (UFH). Sex: 125 males. Inclusion criteria: clinically suspected or verified DVT. Exclusion criteria: none stated. |
|
| Interventions | Treatment: LMWH: dalteparin fixed dose body weight‐adjusted (200 IU/kg), s.c., o.d. Control: UFH: dose‐adjusted APTT × 1.5 to 3.0, continuous i.v. infusion. Treatment duration: 5 to 10 days until therapeutic effect of oral anticoagulants was reached. Oral anticoagulation: started during the initial heparin treatment. |
|
| Outcomes | Primary: change in thrombus size (Marder's score); recurrent VTE (no blind assessment); major haemorrhage; mortality at the end of follow‐up. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted using a Statistical Analysis System Program. |
| Allocation concealment (selection bias) | High risk | No central allocation: randomisation was conducted separately at each participating centre. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded evaluations of venograms. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data unlikely to be related to true outcome. |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes. |
| Other bias | High risk | There was a significantly higher incidence of malignancy in participants randomised to UFH. |
Merli 2001.
| Methods | Study design: randomised controlled trial. Method of randomisation: block randomisation without stratification. Concealment of allocation: partly blinded for treatment allocation, blinded for outcome assessment. Exclusions post‐randomisation: not stated. Lost to follow‐up: not stated. |
|
| Participants | Country: Australia, Austria, Belgium, Denmark, France, Hungary, Ireland, Israel, Italy, Netherlands, Norway, Poland, Spain, Sweden, United Kingdom and USA. Setting: hospital. No.: 900 participants. Age: mean 61 years. Sex: 492 males. Inclusion criteria: symptomatic lower extremity DVT confirmed by venography or ultrasonography (if venography was inconclusive), symptomatic PE confirmed by high probability ventilation‐perfusion scanning or positive pulmonary angiography with confirmation of lower extremity DVT. All those who were eligible underwent baseline lung scanning or angiography. Exclusion criteria: more than 24 hours of previous treatment with heparin or warfarin; need for thrombolytic therapy; known haemorrhagic risk, including active haemorrhage, active intestinal ulcerative disease, known angiodysplasia or eye, spine or central nervous system surgery within the previous month; renal insufficiency (serum creatinine concentration > 180 µmol/litre (2.03 mg/dL)); severe hepatic insufficiency; allergy to heparin, protamine, porcine products, iodine or contrast media; history of heparin‐associated thrombocytopenia or heparin‐ or warfarin‐associated skin necrosis; treatment with other investigational therapeutic agents within the previous 4 weeks; inferior vena cava interruption; known pregnancy or lactation. |
|
| Interventions | Treatment: LMWH: enoxaparin weight‐adjusted s.c. dose (1.0 mg/kg of body weight twice daily or 1.5 mg/kg of body weight o.d.). Control: UFH: initial i.v. bolus injection followed by an infusion based on an approved nomogram. In general: 6 hours after initial bolus an adjusted dose was given to maintain APTT between 55 and 80 seconds. APTT was measured daily. Treatment duration: enoxaparin and heparin treatment were continued for at least 5 days, and warfarin was started within 72 hours of initial study drug administration. 43 participants received phenprocoumon in place of warfarin sodium. INR between 2.0 and 3.0. Oral anticoagulation: oral anticoagulation was continued for at least 3 months. |
|
| Outcomes | Primary: worsening or recurrence of DVT or PE within 3 months. Secondary: clinical overt minor or major haemorrhage. |
|
| Notes | Participants who received LMWH (2 groups; o.d. and twice daily) were analysed as 1 group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors refer to a random number table. |
| Allocation concealment (selection bias) | Low risk | The randomisation numbers were affixed to sealed treatment kits. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Partly blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The Outcome Adjudication Committee provided blinded outcome assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Meyer 1995.
| Methods | Study design: randomised, multicentre pilot study. Method of randomisation: not stated. Concealment of allocation: sealed envelopes, not blinded for treatment allocation, blinded for outcome assessment. Exclusions post‐randomisation: none. Lost to follow‐up: none. |
|
| Participants | Country: France. Setting: hospital. No.: 60 participants: 29 LMWH, 31 UF heparin. Age: mean 60 (range 26 to 84) years LMWH, mean 61 (20 to 88) years UF heparin. Sex: LMWH 9 M/20 F, UF heparin 17 M/14 F Inclusion criteria: men and women > 18 years, weighing 45 to 90 kg and with onset of symptoms suggestive of acute PE within the 5 preceding days. Exclusion criteria: known pregnancy or breastfeeding, major surgical procedure or organ biopsy within the last 5 days, ischaemic cerebrovascular accident within the past 30 days or cerebral haemorrhage within the last 3 months, known haemorrhagic diathesis, active peptic ulcer, pre‐existing significant cardiorespiratory disease, known proliferative diabetic retinopathy, known allergy to heparin or contrast media, platelet count < 100 10⁹/L, chronic renal failure, chronic liver disease, treatment with UFH or LMWH at full dosage for more than 24 hours before randomisation, planned hospital stay < 10 days, oral anticoagulant therapy within 5 days before randomisation and any clinical condition which in the opinion of the physician in charge would not allow safe fulfilment of the protocol. |
|
| Interventions | Treatment: LMWH: fragmin at a fixed dose of 120 anti‐Xa IU/kg subcutaneously twice daily and without any laboratory adjustment. Control: UFH as a continuous intravenous infusion at an initial dosage of 500 IU/kg/24 hours and adjusted daily to maintain APTT between 2 to 3 times the control value. Treatment duration: 10 days Oral anticoagulation: acenocoumarol started on day 7 and continued for at least 3 months |
|
| Outcomes | Primary: incidence of PE recurrence within the first 10 days of treatment Secondary: pulmonary scintigraphic vascular obstruction score (PVOS), major bleeding |
|
| Notes | Follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Treatment was randomly allocated". Comment: insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Treatment was randomly allocated using sealed envelopes". Comment: although the use of assignment envelopes is described, it remains unclear whether envelopes were sequentially numbered and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Open study. All angiograms were reviewed and scored blindly by 3 independent readers unaware of the treatment allocation and clinical events that occurred during the trial. Perfusion lung scans were reviewed and scored blindly by 2 independent readers according to the same procedure". Comment: review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. Furthermore, the blinding of outcome assessment was ensured. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data accounted for. |
| Selective reporting (reporting bias) | Low risk | Study reports data on all pre‐specified outcomes. |
| Other bias | Low risk | Study appears to be free from other sources of bias. |
Moreno‐Palomares 2001.
| Methods | Study design: randomised controlled trial. Method of randomisation: not stated. Concealment of allocation: not stated. Exclusions post‐randomisation: none. Lost to follow‐up: none. |
|
| Participants | Country: Spain. Setting: hospital. No.: 32: 17 LMWH, 15 UFH. Age: mean 70 years LMWH, mean 63 years UFH. Sex: LMWH 5 M/12 F, UFH 6 M/9 F. Inclusion criteria: people with DVT diagnosed by Doppler Exclusion criteria: people with DVT secondary to cancer, hypercoagulability or PE, DVT exclusively in iliac or popliteal vein. |
|
| Interventions | Treatment: LMWH: sodium dalteparin subcutaneously 200 U/kg over 24 hours. If the participant needed more than 180,000 U/day, the doses were divided into 2 and each given over 12 hours. Control: UFH: heparin sodium 400 U/kg as an intravenous continuous infusion. Treatment duration: not stated. Oral anticoagulation: oral dicocoumarol on 2nd day for 3 months. |
|
| Outcomes | Primary: progress of the Doppler. Secondary: post‐phlebitic syndrome. |
|
| Notes | Follow‐up: 3, 6 and 12 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Unclear risk | Insufficient information. |
Ninet 1991.
| Methods | Study design: randomised controlled trial. Method of randomisation: stratified to medical or surgical context in which VTE occurred. Concealment of allocation: not blinded for treatment allocation, blinded for outcome assessment. Exclusions post‐randomisation: none. Lost to follow‐up: 18 participants for assessment of change in thrombus size on venogram. No participants lost to follow‐up for assessment of bleeding events. |
|
| Participants | Country: France (17 centres). Setting: hospital. No.: 166 participants undergoing medical or surgical procedures. Age: estimated overall mean age 63 years. Sex: not stated. Inclusion criteria: recent (< 5 days) proximal DVT. Exclusion criteria: thrombosis affecting inferior vena cava; contraindication to heparin; platelets < 100,000/mm³; blood disease; surgery < 3 days previously; contraindication for isotopic/venographic investigation; pulmonary vascular obstruction 30% or more (lung scan); 24 hours or more heparin or oral anticoagulant therapy; recent history (< 2 years) of cerebrovascular accident or thromboembolic episode; pregnancy. |
|
| Interventions | Treatment: LMWH: fraxiparine body weight‐adjusted fixed dose (± 90 anti‐factor Xa IU/kg, s.c., twice daily) Control: UFH: dose‐adjusted APTT × 1.5 to 2.0, continuous i.v. infusion started with 20 IU/kg/hour. No bolus injection. Treatment duration: 10 days. Oral anticoagulation: not defined for either group. |
|
| Outcomes | Primary: change in thrombus size (Marder's score); recurrent venous thromboembolism (VTE) during initial treatment. Secondary: haemorrhagic episodes during initial treatment; mortality at the end of follow‐up. |
|
| Notes | Repeated venography on day 0 and day 10. Follow‐up was not conducted prospectively at the study centre. 18 (8 versus 10) participants lost to follow‐up. Follow‐up by assessment on information noted and communicated by general practitioners. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Venography was evaluated blind by 2 independent radiologists (coded films). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Recurrences were excluded. |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes. |
| Other bias | Low risk | There are more baseline risk factors in the UFH group compared to the CY 216 group. However, this difference was not statistically significant. |
Prandoni 1992.
| Methods | Study design: randomised controlled trial. Method of randomisation: not stated. Concealment of allocation: not blinded for treatment allocation, blinded for outcome assessment. Exclusions post‐randomisation: none. Lost to follow‐up: none. |
|
| Participants | Country: Italy. Setting: hospital. No.: 170 outpatients. Age: 86 (number over 65 years).. Sex: 86 males. Inclusion criteria: proximal DVT. Exclusion criteria: clinically suspected PE at referral; episode of VTE in same leg within previous 2 years; anticoagulant treatment at referral; contraindication to heparin; pregnancy; allergy to contrast material; residence far from hospital. |
|
| Interventions | Treatment: LMWH: fraxiparine body weight‐adjusted fixed dose; ± 90 anti‐factor Xa IU/kg s.c., twice daily Control: UFH: dose‐adjusted APTT × 1.5 to 2.0, continuous i.v. infusion started with 35,000 Units/24 hours. Initial bolus: 100 Units/kg i.v. Treatment duration: at least 10 days; treatment cessation in INR > 2.0. Oral anticoagulation: Coumarin therapy initial dosage 5 mg started on day 7 of heparin treatment; INR 2.0 to 3.0. |
|
| Outcomes | Primary: change in thrombus size (venogram day 1 and day 10); symptomatic recurrent DVT (including extension) or symptomatic PE; major haemorrhage during initial treatment. Secondary: mortality; change in number of segmental defects on day 10 and day 0 lung scans. |
|
| Notes | Follow‐up: 6 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated treatment by a prescribed randomisation schedule. |
| Allocation concealment (selection bias) | Low risk | Treatment was allocated by sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The venograms and perfusion lung scans of each participant were scored by a panel of 3 experienced observers who were unaware of treatment allocation and the sequence in which the tests were done (before or after treatment). All clinical end points were also reviewed by an adjudication committee unaware of treatment allocation or other details of participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. No participant was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Prandoni 2004.
| Methods | Study design: randomised controlled trial. Method of randomisation: computerised. Stratified according to whether the participants presented with DVT only or with PE, and also stratified according to clinical centre. Concealment of allocation: not blinded for treatment allocation, blinded for outcome assessment. Exclusions post‐randomisation: none. Lost to follow‐up: none. |
|
| Participants | Country: Italy. Setting: hospital. No.: 720. Age: mean 66 years. Sex: 325 male, 395 female. Inclusion criteria: inpatients and outpatients with the clinical suspicion of an acute (less than 3 weeks old) DVT of the lower extremities and/or PE. A positive result of at least 1 of the following tests was required: ascending phlebography, compression ultrasound of the proximal vein system, echo colour Doppler scan of the calf vein system in the case of clinical suspicion of DVT, ventilation‐perfusion scanning, spiral computed tomographic scanning, and pulmonary angiography in the case of clinical suspicion of PE. In the presence of abnormal results of an ultrasound test of the lower extremities, the diagnosis of PE was also accepted if a perfusion lung scan was compatible with a high probability of PE when compared with the chest x‐ray. Exclusion criteria: age less than 18 years, pregnancy, contraindications to anticoagulant treatment, full‐dose anticoagulant treatment (either heparin or oral anticoagulants) for more than 24 hours, haemodynamic instability, previous (less than 1 year earlier) episode of VTE, life expectancy less than 3 months, poor compliance, and geographic inaccessibility for follow‐up. |
|
| Interventions | Treatment: LMWH: nadroparin calcium, subcutaneous administration of nadroparin, 85 IU/kg twice daily Control: UFH: an i.v. bolus of heparin sodium and a s.c. injection of heparin calcium in doses adjusted to body weight (4000 IU i.v. plus 12500 IU s.c. in participants weighing less than 50 kg; 5000 IU plus 15,000 IU, respectively, in participants weighing 50 to 70 kg; and 6000 IU plus 17,500 IU, respectively, in participants weighing more than 70 kg). The first APTT was measured after 6 hours, and subsequent dose adjustments during the first 48 hours were scheduled twice daily. After the first 48 hours, UHF administration was managed on the basis of daily APTT determinations. Treatment duration: At least 5 days; heparin cessation if INR was > 2.0 for 2 consecutive days. Oral anticoagulation: warfarin sodium was started within the first 2 days and continued for a total of 12 weeks. |
|
| Outcomes | Primary: recurrent thromboembolism and mortality during heparin treatment and follow‐up. Secondary: Major bleeding during the period of heparin treatment and the subsequent 48 hours. |
|
| Notes | Follow‐up: 3 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed with a computer algorithm. |
| Allocation concealment (selection bias) | Low risk | Central allocation by a 24‐hour telephone service. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Information on all suspected outcome events and deaths was reviewed and classified by a central adjudication committee blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Pérez de Llano 2003.
| Methods | Study design: multicentre, prospective open study Method of randomisation: SAS statistics computer program Concealment of allocation: none Exclusions post‐randomisation: none Lost to follow‐up: none |
|
| Participants | Country: Spain. Setting: 3 hospitals. No.: enoxaparin 29, UFH 21 Age: enoxaparin mean 66.5 ± 16.2 years, UFH mean 65.9 ± 16.3 years Sex: enoxaparin 20 M/9 F, UFH 14 M/ 7 F Inclusion criteria: people diagnosed with pulmonary thromboembolism (PTE) diagnosed by ventilation‐perfusion scan or plethysmography Exclusion criteria: people with a previous DVT, PTE with haemodynamic repercussion, known factor of hypercoagulability, anticoagulant treatment, pregnancy, formal consideration for anticoagulation or serious concomitant illnesses |
|
| Interventions | Treatment: enoxaparin 1 mg/kg weight every 12 hours. Control: 5% sodium heparin 5000 IU initial bolus through an infusion pump adjusted to the partial thromboplastin time results to an approximated dose of 35,000 IU/day. Treatment duration: until a target INR of 2 to 3 was reached. Oral anticoagulation: acenocoumarol. |
|
| Outcomes | Primary: recurrence of DVT (if plethysmography showed a new venous region affected, if there was a proximal thrombus extension > 5 cm or if arteriography showed new intraluminal defects) or PE (if perfusion scan showed perfusion defects that had not existed in the initial exploration) and major bleeding (intracranial, retroperitoneal, requiring transfusion or haemoglobin < 2 or more points). | |
| Notes | Follow‐up: 1, 3 and 6 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised from the lists of enrolled patients at each centre using the SAS statistics program". Comment: low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated. Comment: insufficient information to permit judgement of low or high risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether the outcome assessors were blinded to treatment and therefore the risk of bias was deemed unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data accounted for. |
| Selective reporting (reporting bias) | High risk | Study authors discuss the length of hospital stay but it was not a prespecified outcome. |
| Other bias | Low risk | Study appears to be free from other sources of bias. |
Riess 2003.
| Methods | Study design: randomised controlled trial. Method of randomisation: not stated. Concealment of allocation: not blinded for treatment allocation, blinded for outcome assessment. Exclusions post‐randomisation: 92 participants. Lost to follow‐up: 22 participants. |
|
| Participants | Country: 121 centres in Germany and the Czech Republic. Setting: hospital and out of hospital. No.: 1220 participants. Age: mean 61 years. Sex: 677 males. Inclusion criteria: men older than 18 years with objectively confirmed acute proximal DVT for fewer than 3 weeks after given written informed consent. Exclusion criteria: isolated calf vein thrombosis; planned fibrinolysis or operation; clinically severe PE; heparin application within 8 days of enrolment (except treatment in the past 24 hours), treatment with VKA for > 24 hours before start of study medication; hypertension with systolic blood pressure > 200 mmHg and diastolic blood pressure > 105 mmHg; known malignant tumour as known cause for the venous occlusion; severe renal or hepatic insufficiency; surgery of the head, chest or abdomen in the past 8 days; intervention in the central nervous system in the past 14 days; evident disseminated intravascular coagulation; clinical condition with an increased risk of bleeding complications during the treatment time; gastrointestinal bleeding or gastric ulcer in the past 4 weeks; contraindication against VKA or known intolerability against heparin; platelet count < 100,000/µL; pregnancy, treatment with platelet inhibitors. |
|
| Interventions | Treatment: LMWH: certoparin fixed unadjusted dose 8000 anti‐factor Xa IU s.c., b.d. for 10 to 14 days. Control: UFH: initial bolus i.v. of 5000 IU followed by continuous infusion starting dose of 20 IU/kg/hour of an adjusted dose UFH to maintain an APTT of 1.5 to 2.5 × the control value. |
|
| Outcomes | Primary: incidence of VTE at the end of follow‐up. Secondary: incidence of recurrent VTE and major bleeding during initial treatment; mortality at the end of follow‐up. |
|
| Notes | Follow‐up: 6 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation. |
| Allocation concealment (selection bias) | Low risk | Randomisation was carried out using a central telephone system. The assignment to 1 of the treatment groups was documented and could not be changed afterwards. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All events were evaluated by an independent end point committee blinded for treatment groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An intent‐to‐treat analysis confirmed the results of the primary 'per protocol' analysis. |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Simonneau 1993.
| Methods | Study design: randomised controlled trial. Method of randomisation: treatment assignments: sealed envelopes, block randomisation using standard random number table and sealed envelopes. Concealment of allocation: not blinded for treatment allocation; blinded for outcome assessment. Exclusion post‐randomisation: 1 (distal DVT). Lost to follow‐up: for qualitative and quantitative venogram assessment: 17 participants lost to follow‐up (treatment cessation before day 10 (5 participants); exclusion post randomisation (1 participant); unassessable venograms due to technical problems (11 participants)). |
|
| Participants | Country: 16 European centres. Setting: hospital. No.: 134 participants. Age: Mean 63 years. Sex: 73 males. Inclusion criteria: proximal DVT with or without suspected PE, but with symptoms < 5 days. Exclusion criteria: active bleeding or disorders contraindicating anticoagulant therapy; surgery in previous 7 days; pregnancy; aspirin, ticlopidine, sulfinpyrazone or non‐steroidal anti‐inflammatory treatment within 7 days before study entry; associated severe PE requiring thrombolytic therapy or surgery; use of curative heparin therapy for > 24 hours or > 25,000 Units of heparin during 24 hours before referral; previous implantation of vena cava filter. |
|
| Interventions | Treatment: LMWH: enoxaparin, clexane body weight‐adjusted fixed dose (1 mg/kg ± 100 anti‐factor Xa IU/kg, s.c., twice daily). Control: UFH: dose‐adjusted APTT × 1.5 to 2.5, continuous i.v. infusion started with 500 Units/kg/24 hours (25,000 Units/5 mL in saline). Treatment duration: 10 days. Oral anticoagulation: started on day 10 for at least 3 months; INR 2.0 to 3.0. |
|
| Outcomes | Primary: change in thrombus size (quantitative venographic score, Marder) between day 0 and day 10; recurrent VTE during 10 days of treatment (asymptomatic and symptomatic DVT and PE); major bleeding during 10 days of treatment. Secondary: minor bleeding; follow‐up at 3 months to record VTE recurrence, bleeding and deaths; qualitative assessment of venogram evolution between day 0 and day 10. |
|
| Notes | Repeated venography on day 10. Follow‐up: 3 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation code was drafted by means of a standard random number table randomising in blocks of 4. |
| Allocation concealment (selection bias) | Low risk | The participants' treatment assignments were taken from sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Venograms, perfusion lung scans and pulmonary angiograms were subsequently reviewed by a central independent panel of 2 consultant specialists unaware of the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data unlikely to be related to true outcome. |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Simonneau 1997.
| Methods | Study design: randomised controlled trial. Method of randomisation: centrally controlled, computerised. Concealment of allocation: not blinded for treatment allocation, blinded for outcome assessment. Exclusions post‐randomisation: none. Lost to follow‐up: none. |
|
| Participants | Country: France, Belgium and Switzerland. Setting: hospital. No.: 612 participants. Age: mean 67 years. Sex: 172 males. Inclusion criteria: clinically suspected acute PE. PE objectively documented by pulmonary angiography or ventilation‐perfusion lung scanning indicating a high probability of PE or showing indeterminate results but accompanied by DVT confirmed by venography or compression ultrasonography. Exclusion criteria: massive PE requiring thrombolytic therapy or pulmonary embolectomy; active bleeding or disorders contraindicating anticoagulant therapy; anticoagulant therapy at a therapeutic dose for > 24 hours; life expectancy < 3 months; severe hepatic or renal failure; likely non‐compliance; pregnancy. |
|
| Interventions | Treatment: LMWH: tinzaparin, innohep in body weight‐adjusted fixed dose, s.c., o.d. Control: UFH: APTT‐adjusted dose, continuous i.v. infusion after an initial i.v. bolus of 50 IU/kg. Treatment duration: at least 5 days; treatment cessation if INR was 2.0 or above on 2 measurements made 24 hours apart. Oral anticoagulation: started between the first and third days of initial treatment and continued for at least 3 months; INR 2.0 to 3.0. |
|
| Outcomes | Primary: symptomatic recurrent VTE during initial treatment (8 days) and at the end of follow‐up (day 90); major haemorrhage during initial treatment (8 days) and at the end of follow‐up (day 90); death at end of follow‐up (day 90). | |
| Notes | Follow‐up: 90 days. 1 participant allocated to UFH and 3 participants allocated to LMWH did not receive the study drug. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation was performed with the use of a 24‐hour computer service. |
| Allocation concealment (selection bias) | Low risk | Central randomisation was performed with the use of a 24‐hour computer service. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data on all potential outcome events were submitted to an independent adjudication committee whose members were unaware of the treatment assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An intention‐to‐treat analysis was performed, but the authors do not give any information about loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Thery 1992.
| Methods | Study design: dose‐finding controlled, randomised trial. Method of randomisation: not stated Concealment of allocation: not blinded for treatment allocation, blinded for outcome assessment. Exclusions post‐randomisation: none. Lost to follow‐up: none. |
|
| Participants | Country: France, Setting: hospital. No.: 68: Fraxparine 35, UFH 33 Age: Fraxiparine mean 60.1 (SD 2.9) years, UFH mean 64.2 (SD 2.5) years Sex: Fraxiparine 17 M/18 F, UFH 14 M/19 F Inclusion criteria: adults > 18 years with a recent angiographically proved PE (within 3 days of the onset of symptoms) and with a pulmonary vascular obstruction assessed by the local radiologists between 15% and 55% (index of severity according to Miller 5 to 18) Exclusion criteria: angiographically determined vascular obstruction < 15% or > 55%, any sign of clinical severity defined as shock, acute cor pulmonale or right heart failure, any contraindication to heparin, active peptic ulcer, recent history of cerebrovascular haemorrhage or ischaemia, known bleeding tendency, previous history of heparin‐induced thrombocytopenia, haemorrhagic diathesis, pre‐existing coagulation disorders, severe renal or hepatic dysfunction, severe systemic hypertension, known pericarditis or endocarditis, pregnancy, pre‐existing DVT or PE within 12 months preceding the inclusion or use of thrombolytic agents, heparin at therapeutic doses for more than 48 hours before inclusion, oral anticoagulants, acetylsalicylic acid or ticlopidine during the 7 days before inclusion, any contraindication to isotopic or angiographic investigations and free‐floating inferior vena cava thrombus. |
|
| Interventions | Treatment: LMWH: Fraxiparine 400 anti‐factor Xa IU U/kg in 2 daily injections Control: UFH: i.v. bolus injection of 50 IU/kg followed by continuous infusion of an initial dose of 600 IU/kg. Treatment duration: 14 days Oral anticoagulation: none |
|
| Outcomes | Primary: pulmonary vascular obstruction Secondary: clinical recurrence of VTE, death and haemorrhagic complications |
|
| Notes | Follow‐up: 8 days Before completion of the trial, enrolment in 2 Fraxiparine groups stopped because of a high incidence of major bleedings. Those 2 groups were given Fraxiparine at a high dose of 600 and 900 anti‐factor Xa IU/kg. Data from these groups were not included in the analyses in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Random treatment allocation schedules were prepared for each clinical centre using sealed treatment allocation envelopes". Comment: insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Random treatment allocation schedules were prepared for each clinical centre using sealed treatment allocation envelopes". Comment: although the use of assignment envelopes is described, it remains unclear whether envelopes were sequentially numbered and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for treatment allocation. Comment: given the clinical outcomes of the study, review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the study could not be performed double‐blind because of the different modes of administration and above all the need for dosage adjustments in the UFH group. However, the main assessment criterion was blindly evaluated by a central independent panel of three radiologists". Comment: review authors judged that the non‐blinding of the participants and staff was unlikely to have affected the outcomes. Furthermore, the blinding of outcome assessment was ensured. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for losses to follow‐up not clearly stated. |
| Selective reporting (reporting bias) | Low risk | Study reports data on all pre‐specified outcomes. |
| Other bias | Low risk | Study appears to be free from other sources of bias. |
APTT: activated partial thromboplastin time cm: centimetre DVT: deep vein thrombosis F: female INR: International normalised ratio IU: International units i.v.: intravenous kg: kilogram LMWH: low molecular weight heparin M: male mg: milligram mL: millilitre mm: millimetre mmHg: millimetres of mercury NSAID: nonsteroidal anti‐inflammatory drug PE: pulmonary embolism PTE: pulmonary thromboembolism o.d.: once daily s.c.: subcutaneous SD: standard deviation UFH: unfractionated heparin VKA: vitamin K antagonists VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aiach 1989 | Low molecular weight heparin dosage was adjusted. |
| Albada 1989 | The results from participants treated for venous thrombosis of the upper limb and for pulmonary embolism could not be distinguished from those of people with leg vein thrombosis, and the outcome was incompletely evaluated. |
| Banga 1993 | This was a dose‐finding study. |
| Bratt 1985 | Intravenous route of administration of low molecular weight heparin, and adjustments were made to dose for this treatment. |
| Bratt 1990 | Low molecular weight heparin dosage was adjusted. |
| de Valk 1995 | This was a dose‐finding study. |
| Handeland 1990 | This was a dose‐finding study. |
| Harenberg 1989 | Abstract with incomplete data. |
| Harenberg 1990 | The results from people treated for venous thrombosis of the upper limb and for pulmonary embolism could not be distinguished from those of participants with leg vein thrombosis, and the outcome was incompletely evaluated. |
| Harenberg 2000b | Abstract with incomplete data. |
| Holm 1986 | Low molecular weight heparin dosage was adjusted. |
| Kearon 2006 | The administration of unfractionated heparin was not in adjusted dose. |
| Lockner 1985 | Intravenous route of administration of low molecular weight heparin. |
| Lockner 1986 | Intravenous route of administration of low molecular weight heparin. |
| Ly 1985 | Adjustment of low molecular weight heparin dosages. |
| Monreal 1993 | The 2 treatment strategies differ with respect to long‐term treatment. |
| Monreal 1994 | The 2 treatment strategies differ with respect to long‐term treatment. |
| Notarbartolo 1988 | Dosage of unfractionated heparin was not adjusted. |
| Quiros 2001 | Not a randomised controlled trial. |
| Riess 2014 | Substudy of Harenberg 1990 and Reiss 2003 studies which are already included in the review. |
| Siguret 2011 | Comparison group were not treated with unfractionated heparin. |
| Stricker 1999 | The main outcome of the study was the effect on haemostatic markers which is not within the scope of our review. |
| Tedoldi 1993 | Dosage of unfractionated heparin was not adjusted. |
| Ucar 2015 | Participants were given thrombolytic treatment. |
| Vogel 1987 | Intravenous route of administration of low molecular weight heparin. |
| Zanghi 1988 | Dosage of unfractionated heparin was not adjusted. |
Characteristics of ongoing studies [ordered by study ID]
NCT00796692.
| Trial name or title | Nadroparin for the Initial Treatment of Pulmonary Thromboembolism (NATSPUTE) |
| Methods | Multicentre, randomised, open‐label, parallel assignment controlled trial |
| Participants | Inclusion criteria: 18 to 75 years of age, symptomatic non‐massive PTE confirmed either by high probability ventilation‐perfusion lung scanning (V/Q scan) or by the presence of intraluminal filling defect on spiral computed tomographic pulmonary angiography (CTPA), haemodynamically stabile, anatomic obstruction no more than 2 lobes on CTPA, or defect no more than 7 segments on V/Q scan, and normal right ventricular function, symptoms within 15 days, written informed consent obtained before randomisation. Exclusion criteria: unfractionated heparin anticoagulation for more than 36 hours prior enrolment, massive PTE or sub‐massive PTE requiring thrombolytic therapy or pulmonary embolectomy, active bleeding or disorders contraindicating anticoagulant therapy, chronic thromboembolism pulmonary hypertension (CTEPH) without evidence of recent episode, severe hepatic or renal failure, allergy to heparin, other components of tinzaparin or acenocoumarol, pregnant status, a life expectancy of less than 3 months, previous thrombocytopaenia induced by heparin, thrombocytopaenia < 100,000/mm³. |
| Interventions | Treatment: LMWH given with a weight‐adjusted dose of 86 international anti‐factor Xa units of nadroparin (Fraxiparine) per kilogram of body weight (86 anti‐factor Xa IU/kg) subcutaneously every 12 hours, which will be used at least 5 to 7 days. Control: UFH is received with an initial bolus dose of 80 IU per kilogram, followed by a continuous intravenous infusion at an initial rate of 18 IU per kilogram per hour. The dose is subsequently adjusted so that the activated partial thromboplastin time (APTT) would be 1.5 to 2.5 times the control value in normal subjects. The tests are performed 4 hours after the start of treatment, whenever a sub‐therapeutic APTT had been measured after a dose adjustment, and otherwise daily. UFH will be used at least 5 to 7 days. Treatment duration: 5 to 7 days. Oral anticoagulation: warfarin. |
| Outcomes | Primary: clinical and image (including V/Q scan and CTPA) improvement at 14 days. Secondary: recurrent venous thromboembolism (VTE), major bleeding, death and heparin‐induced thrombocytopaenia at 3 months. |
| Starting date | June 2002 |
| Contact information | Professor Chen Wang, Beijing Institute of Respiratory Medicine, Beijing Chao Yang Hospital, China |
| Notes | Study authors have been contacted for further information but no response received to date |
IU: international units kg: kilogram LMWH: low molecular weight heparin mm: millimetre PTE: pulmonary thromboembolism UFH: unfractionated heparin
Differences between protocol and review
Included post hoc sensitivity analysis for ISTH bleeding definitions in order to assess the effect of bleeding definitions used.
Contributions of authors
LR: selected studies for inclusion in this update, assessed the quality of studies, carried out data extraction, performed data analysis and wrote the review. LJ: selected studies for inclusion in this update, assessed the quality of the studies and carried out data extraction.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Programme Grant funding to Cochrane Vascular (13/89/23). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular Editorial Base is supported by the Chief Scientist Office.
Declarations of interest
LR: none known. LJ: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Belcaro 1999 {published data only}
- Belcaro G, Nicolaides AN, Cesarone MR, Laurora G, Sanctis MT, Incandela L, et al. Comparison of low‐molecular‐weight heparin, administered primarily at home, with unfractionated heparin, administered in hospital, and subcutaneous heparin, administered at home for deep‐vein thrombosis. Angiology 1999;50(10):781‐7. [DOI] [PubMed] [Google Scholar]
Breddin 2001 {published data only}
- Breddin HK, Hach‐Wunderle V, Nakov R, Kakkar VV. Effects of a low‐molecular‐weight heparin on thrombus regression and recurrent thromboembolism in patients with deep‐vein thrombosis. New England Journal of Medicine 2001;344(9):626‐31. [DOI] [PubMed] [Google Scholar]
Columbus 1997 {published data only}
- Douketis JD, Foster GA, Crowther MA, Prins MH, Ginsberg JS. Clinical risk factors and timing of recurrent venous thromboembolism during the initial 3 months of anticoagulant therapy. Archives of Internal Medicine 2000;160(22):3431‐6. [DOI] [PubMed] [Google Scholar]
- The Columbus Investigators. Low molecular weight heparin is an effective and safe treatment for deep‐vein thrombosis and pulmonary embolism. Blood 1996; Vol. 88:626a.
- The Columbus Investigators. Low‐molecular‐weight heparin in the treatment of patients with venous thromboembolism. New England Journal of Medicine 1997;337(10):657‐62. [DOI] [PubMed] [Google Scholar]
- Cate JW, Büller HR, Gent M, Gallus AS, Ginsberg J, Prins MH, et al. Low‐molecular‐weight heparin in the treatment of patients with venous thromboembolism. Journal of Vascular and Interventional Radiology 1998;9:178. [Google Scholar]
Decousus 1998 {published data only}
- Decousus H, Leizorovicz A, Parent F, Page Y, Tardy B, Girard PH, et al. A clinical trial of vena cava filters in the prevention of pulmonary embolism in patients with proximal deep‐vein thrombosis. New England Journal of Medicine 1998;338(7):409‐15. [DOI] [PubMed] [Google Scholar]
Faivre 1988 {published data only}
- Faivre R, Neuhart E, Kieffer Y, Toulemonde F, Bassand JP, Maurat JP. Subcutaneous administration of a low molecular weight heparin (CY 222) compared with subcutaneous administration of standard heparin in patients with acute deep vein thrombosis. Thrombosis and Haemostasis 1987;58(1):Abstract 430. [Google Scholar]
- Faivre R, Neuhart Y, Kieffer Y, Apfel F, Magnin D, Didier D, et al. A new treatment of deep vein thrombosis: low molecular weight heparin fractions. Randomised study [Un nouveau traitement des thromboses veineuses profondes: les fractions d'heparine de bas poids moleculaire. Etude randomisee]. Presse Medicale 1988;17(5):197‐200. [PubMed] [Google Scholar]
Fiessinger 1996 {published data only}
- Fiessinger JN, Fernandez ML, Gatterer E, Ohlsson CG. Fragmin once daily versus continuous infusion heparin in the treatment of DVT: a European multicentre trial. Haemostasis 1994;24(Suppl 1):Abstract 44. [Google Scholar]
- Fiessinger JN, Lopez‐Fernandez M, Gatterer E, Granqvist S, Kher A, Olsson CG, et al. Once‐daily subcutaneous dalteparin, a low molecular weight heparin, for the initial treatment of acute deep vein thrombosis. Thrombosis and Haemostasis 1996;76(2):195‐9. [PubMed] [Google Scholar]
Findik 2002 {published data only}
- Findik S, Erkan ML, Selçuk MB, Albayrak S, Atici AG, Doru F. Low‐molecular‐weight heparin versus unfractionated heparin in the treatment of patients with acute pulmonary thromboembolism. Respiration 2002;69(5):440‐4. [DOI] [PubMed] [Google Scholar]
Goldhaber 1998 {published data only}
- Goldhaber SZ, Morrison RB, Diran LL, Creager MA, Lee TH Jr. Abbreviated hospitalization for deep venous thrombosis with the use of ardeparin. Archives of Internal Medicine 1998;158(21):2325‐8. [DOI] [PubMed] [Google Scholar]
Harenberg 2000a {published data only}
- Harenberg J, Schmidt JA, Koppenhagen K, Tolle A, Huisman MV, Buller HR. Fixed‐dose, body weight‐independent subcutaneous LMW heparin versus adjusted dose unfractionated intravenous heparin in the initial treatment of proximal venous thrombosis. EASTERN Investigators. Thrombosis and Haemostasis 2000;83(5):652‐6. [PubMed] [Google Scholar]
Hull 1992 {published data only}
- Hull RD, Raskob GE, Brant RF, Pineo GF, Elliott G, Stein PD, et al. Low‐molecular‐weight heparin vs heparin in the treatment of patients with pulmonary embolism. American‐Canadian Thrombosis Study Group. Archives of Internal Medicine 2000;160(2):229‐36. [DOI] [PubMed] [Google Scholar]
- Hull RD, Raskob GE, Pineo GF, Green D, Trowbridge AA, Elliott CG, et al. Subcutaneous low‐molecular‐weight heparin compared with continuous intravenous heparin in the treatment of proximal‐vein thrombosis. New England Journal of Medicine 1992;326(15):975‐82. [DOI] [PubMed] [Google Scholar]
Kakkar 2003 {published data only}
- Kakkar VV, Gebska M, Kadziola Z, Saba N, Carrasco P, Bemiparin Investigators. Low‐molecular‐weight heparin in the acute and long‐term treatment of deep vein thrombosis. Thrombosis and Haemostasis 2003;89(4):674‐80. [PubMed] [Google Scholar]
Kirchmaier 1998 {published data only}
- Kirchmaier CM, Wolf H, Schafer H, Ehlers B, Breddin HK. Efficacy of a low molecular weight heparin administered intravenously or subcutaneously in comparison with intravenous unfractionated heparin in the treatment of deep venous thrombosis. Certoparin‐Study Group. International Angiology 1998;17(3):135‐45. [PubMed] [Google Scholar]
Koopman 1996 {published data only}
- Koopman MMW, Prandoni P, Piovella F, Ockelford PA, Brandjes DPM, Meer J, et al. Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low‐molecular‐weight heparin administered at home. New England Journal of Medicine 1996;334(11):682‐7. [DOI] [PubMed] [Google Scholar]
Leizorovicz 2011 {published data only}
- Leizorovicz A. Tinzaparin compared to unfractionated heparin for initial treatment of deep vein thrombosis in very elderly patients with renal insufficiency ‐ the IRIS trial. Blood 2008; Vol. 112, issue 11:434.
- Leizorovicz A, Siguret V, Mottier D. Safety profile of tinzaparin versus subcutaneous unfractionated heparin in elderly patients with impaired renal function treated for acute deep vein thrombosis: The Innohep((R)) in Renal Insufficiency Study (IRIS). Thrombosis Research 2011;128(1):27‐34. [DOI] [PubMed] [Google Scholar]
- NCT00277394. Innohep© in elderly patients with impaired renal function treated for acute deep vein thrombosis. clinicaltrials.gov/ct/show/NCT00277394 2007.
- Siguret V, Deudon C, Golmard J, Leizorovicz A, Pautas E, Gouin‐Thibault I. Pharmacodynamic response to unfractionated heparin used for initial treatment of acute deep vein thrombosis in elderly patients with renal impairment. A substudy of the IRIS clinical trial. Journal of Thrombosis and Haemostasis 2013;11 (Suppl 2):805. [Google Scholar]
Levine 1996 {published data only}
- Levine M, Gent M, Hirsh J, Leclerc J, Anderson D, Weitz J, et al. A comparison of low‐molecular‐weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep‐vein thrombosis. New England Journal of Medicine 1996;334(11):677‐81. [DOI] [PubMed] [Google Scholar]
Lindmarker 1994 {published data only}
- Holmstrom M, Lindmarker P, Granqvist S, Johnsson H, Lockner D. A 6‐month venographic follow‐up in 164 patients with acute deep vein thrombosis. Thrombosis and Haemostasis 1997;78(2):803‐7. [PubMed] [Google Scholar]
- Lindmarker P, Holmstrom M, Granqvist S, Johnsson H, Lockner D. Comparison of once‐daily subcutaneous Fragmin (TM) with continuous intravenous unfractionated heparin in the treatment of deep venous thrombosis. Thrombosis and Haemostasis 1994;72(2):186‐90. [PubMed] [Google Scholar]
Lopaciuk 1992 {published data only}
- Lopaciuk S, Meissner AJ, Filipecki S, Zawilska K, Sowier J, Ciesielski L, et al. Subcutaneous low molecular weight heparin versus subcutaneous unfractionated heparin in the treatment of deep vein thrombosis: a Polish multicenter trial. Thrombosis and Haemostasis 1992;68(1):14‐8. [PubMed] [Google Scholar]
Luomanmaki 1996 {published data only}
- Luomanmaki K and the Finnish Multicentre Group, et al. Low molecular weight heparin (Fragmin) once daily vs continuous infusion of standard heparin in the treatment of DVT. Haemostasis 1994;24(Suppl 1):Abstract 248. [Google Scholar]
- Luomanmaki K, Grankvist S, Hallert C, Jauro I, Ketola K, Kim HC, et al. A multicentre comparison of once‐daily subcutaneous dalteparin (low molecular weight heparin) and continuous intravenous heparin in the treatment of deep vein thrombosis. Journal of Internal Medicine 1996;240(2):85‐92. [DOI] [PubMed] [Google Scholar]
Merli 2001 {published data only}
- Merli G, Spiro TE, Olsson CG, Abildgaard U, Davidson BL, Eldor A, et al. Subcutaneous enoxaparin once or twice daily compared with intravenous unfractionated heparin for treatment of venous thromboembolic disease. Annals of Internal Medicine 2001;134(3):191‐202. [DOI] [PubMed] [Google Scholar]
- Spiro TE, The Enoxaparin Clinical Trial Group. A multicenter clinical trial comparing once and twice‐daily subcutaneous enoxaparin and intravenous heparin in the treatment of acute deep vein thrombosis. Thrombosis and Haemostasis 1997;374(Suppl):Abstract SC 1527. [Google Scholar]
- Lissovoy G, Yusen RD, Spiro TE, Krupski WC, Champion AH, Sorensen SV. Cost for inpatient care of venous thrombosis: a trial of enoxaparin vs standard heparin. Archives of Internal Medicine 2000;160(20):3160‐5. [DOI] [PubMed] [Google Scholar]
Meyer 1995 {published data only}
- Meyer G, Brenot F, Pacouret G, Simonneau G, Gillet Juvin K, Charbonnier B, et al. Subcutaneous low‐molecular weight heparin Fragmin versus intravenous unfractionated heparin in the treatment of acute non massive pulmonary embolism: an open randomised pilot study. Thrombosis and Haemostasis 1995;74(6):1432‐5. [PubMed] [Google Scholar]
Moreno‐Palomares 2001 {published data only}
- Moreno‐Palomares JJ, Fisac‐Herrero RM, Herrero Domingo A, Ferreira‐Pasos EM, Grasa J, Reverte Cejudo D. [Low molecular weight heparin versus unfractionated heparin in the treatment of deep vein thrombosis]. Anales de Medicina Interna 2001;18(7):364‐8. [PubMed] [Google Scholar]
Ninet 1991 {published data only}
- Ninet J, Bachet P, Prandoni P, Ruol A, Vigo M, Barret A, et al. A randomized trial of subcutaneous low molecular weight heparin (CY216) compared with intravenous unfractionated heparin in the treatment of deep vein thrombosis. Thrombosis and Haemostasis 1991;65(3):251‐6. [PubMed] [Google Scholar]
Pérez de Llano 2003 {published data only}
- Pérez de Llano LA, Baloira Villar A, Veres Racamonde A, Veiga F, Golpe, Gomez R, et al. Multicenter, prospective study comparing enoxaparin with unfractionated heparin in the treatment of submassive pulmonary thromboembolism. Archivos de Bronconeumologia 2003;39(8):341‐5. [DOI] [PubMed] [Google Scholar]
Prandoni 1992 {published data only}
- Prandoni P, Lensing AWA, Büller HR, Carta M, Cogo A, Vigo M, et al. Comparison of subcutaneous low‐molecular‐weight heparin with intravenous standard heparin in proximal deep‐vein thrombosis. Lancet 1992;339(8791):441‐5. [DOI] [PubMed] [Google Scholar]
Prandoni 2004 {published data only}
- Prandoni P, Carnovali M, Marchiori A. Subcutaneous adjusted‐dose unfractionated heparin vs fixed‐dose low‐molecular‐weight heparin in the initial treatment of venous thromboembolism. Archives of Internal Medicine 2004;164:1077‐83. [DOI] [PubMed] [Google Scholar]
Riess 2003 {published data only}
- Riess H, Koppenhagen K, Tolle A, Kemkes‐Matthes B, Grave M, Patek F, et al. Fixed‐dose, body weight‐independent subcutaneous low molecular weight heparin Certoparin compared with adjusted‐dose intravenous unfractionated heparin in patients with proximal deep venous thrombosis. Thrombosis and Haemostasis 2003;90(2):252‐9. [DOI] [PubMed] [Google Scholar]
- Riess H, Koppenhagen K, Tolle AR, Kemkes‐Matthes B, Grave M, Harenberg J, et al. Fixed‐dose body weight‐independent subcutaneous LMW‐heparin (LMWH) certoparin is equally effective to adjusted‐dose intravenous uf‐heparin (UFH) for the initial treatment of proximal deep venous thrombosis (DVT). Annals of Hematology 2001;80 Suppl 1:A56. [Google Scholar]
Simonneau 1993 {published data only}
- Simonneau G, Charbonnier B, Decousus H, Planchon B, Ninet J, Sie P, et al. Subcutaneous low molecular weight heparin compared with continuous intravenous unfractionated heparin in the initial treatment proximal vein thrombosis. Archives of Internal Medicine 1993;153(13):1541‐6. [PubMed] [Google Scholar]
Simonneau 1997 {published data only}
- Simonneau G, Sors H, Charbonnier B, Page Y, Laaban J‐P, Azarian R, et al. for the THESEE Study Group. A comparison of low‐molecular‐weight heparin with unfractionated heparin for acute pulmonary embolism. New England Journal of Medicine 1997;337(10):663‐9. [DOI] [PubMed] [Google Scholar]
Thery 1992 {published data only}
- Thery C, Simonneau G, Meyer G, Helenon O, Bridey F, Armagnac C, et al. Randomized trial of subcutaneous low‐molecular‐weight heparin CY216 (fraxiparine) compared with intravenous unfractionated heparin in the curative treatment of submassive pulmonary embolism. Circulation 1992;85(4):1380‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aiach 1989 {published data only}
- Aiach M, Fiessinger JN, Vitoux JF, Querrec A, Gouault‐Heilmann M, et al. Deep vein thrombosis treatment. A comparative study: subcutaneous Fragmin versus unfractionated heparin administered by continuous infusion. Multicentre trial. [Traitement des thromboses veineuses profondes constituees. Etude comparative d'un fragment d'heparine de bas poids moleculaire (Fragmine) administree par voie sous‐cutanee et de l'heparine standard administree par voie intraveineuse continue. Etude multicentrique. [French]]. Revue de Medecine Interne 1989;10(4):375‐81. [PubMed] [Google Scholar]
Albada 1989 {published data only}
- Albada J, Nieuwenhuis HK, Sixma JJ. Treatment of acute venous thromboembolism with low molecular weight heparin (Fragmin). Circulation 1989;80(4):935‐40. [DOI] [PubMed] [Google Scholar]
Banga 1993 {published data only}
- Banga JD, Valk HW, Wester JWJ, Brouwer CB, Hessen MWJ, Meuwissen OJAT, et al. A dose finding study of subcutaneous heparinoid Oragaran (ORG 10172) twice daily compared to continuous intravenous unfractionated heparin in the treatment of venous thromboembolism. Thrombosis and Haemostasis 1993;69:545 Abstract 20. [Google Scholar]
Bratt 1985 {published data only}
- Bratt G, Tornebohm E, Granqvist S, Aberg W, Lockner D. A comparison between low molecular weight heparin Kabi 2165 and standard heparin in the intravenous treatment of deep vein thrombosis. Thrombosis and Haemostasis 1985;54(4):813‐7. [PubMed] [Google Scholar]
Bratt 1990 {published data only}
- Bratt G, Aberg W, Johansson M, Tornebohm E, Granqvist S, Lockner D. Two daily subcutaneous injections of Fragmin as compared with intravenous standard heparin in the treatment of deep venous thrombosis. Thrombosis and Haemostasis 1990;64(4):506‐10. [PubMed] [Google Scholar]
de Valk 1995 {published data only}
- Valk HW, Banga JD, Wester JWJ, Brouwer CB, Hessen MWJ, Meuwissen OJAT, et al. Comparing subcutaneous danaparoid with intravenous unfractionated heparin for the treatment of venous thromboembolism. A randomised controlled trial. Annals of Internal Medicine 1995;123(1):1‐9. [DOI] [PubMed] [Google Scholar]
Handeland 1990 {published data only}
- Handeland GF, Abildgaard U, Holm HA, Arnesen KE. Dose adjusted heparin treatment of deep venous thrombosis: a comparison of unfractionated and low molecular weight heparin. European Journal of Clinical Pharmacology 1990;39(2):107‐12. [DOI] [PubMed] [Google Scholar]
Harenberg 1989 {published data only}
- Harenberg J, Huck K, Stehle G, Mall K, Schwarz A, Heene DL. Prospective randomized, controlled study on the treatment of deep venous thrombosis using low molecular weight heparin compared with unfractionated heparin. Thrombosis and Haemostasis 1989;62:356 Abstract No. 1106. [Google Scholar]
- Harenberg J, Huisman MV, Tolle AR, Breddin HK, Kirchmaier CM. Reduction in thrombus extension and clinical end points in patients after initial treatment for deep vein thrombosis with the fixed‐dose body weight‐independent low molecular weight heparin certoparin. Seminars in Thrombosis and Hemostasis 2001;27:513‐8. [DOI] [PubMed] [Google Scholar]
- Harenberg J, Riess H, Fischer H, Brom J, Eidinger GW. Initial treatment of patients with acute deep‐vein thrombosis using 8.000 IU bid low‐molecular‐weight heparin certoparin ‐ A 24 months follow up. Journal of Thrombosis and Haemostasis 2005;3(1):Abstract No. P1021. [Google Scholar]
Harenberg 1990 {published data only}
- Harenberg J, Huck K, Bratsch H, Stehle G, Dempfle CE, Mall K, et al. Therapeutic application of subcutaneous low‐molecular‐weight heparin in acute venous thrombosis. Haemostasis 1990;20 Suppl 1:205‐19. [DOI] [PubMed] [Google Scholar]
- Harenberg J, Stehle G, Blauth M, Huck K, Mall K, Heene DL. Dosage, anticoagulant and antithrombotic effects of heparin and low‐molecular‐weight heparin in the treatment of deep vein thrombosis. Seminars in Thrombosis and Hemostasis 1997;23(1):83‐90. [DOI] [PubMed] [Google Scholar]
Harenberg 2000b {published data only}
- Harenberg J, Breddin HK, Kirchmaier CM, Tolle A. Does fixed dose body weight independent subcutaneous low‐molecular‐weight heparin improve the Marder score compared to adjusted dose unfractionated heparin in the treatment of venous thrombosis. Annals of Hematology 2000;79 Suppl 1:A84. [Google Scholar]
Holm 1986 {published data only}
- Holm HA, Ly B, Handeland GF, Abildegaard U, Arnesen KE, Gottschalk P, et al. Subcutaneous heparin treatment of deep vein thrombosis: a comparison of unfractionated and low molecular weight heparin. Haemostasis 1986;16 Suppl 2:30‐7. [DOI] [PubMed] [Google Scholar]
Kearon 2006 {published data only}
- Kearon C, Ginsberg JJ, Julian J, Douketis J, Solymoss S, Ockelford P, et al. Fixed‐dose, weight‐adjusted, unfractionated heparin (UFH) given subcutaneously (sc) without laboratory monitoring for acute treatment of venous thromboembolism (VTE): Randomized comparison with low‐molecular‐weight‐heparin (LMWH). Blood 2004; Vol. 104, issue 11:Abstract 707.
- Kearon C, Ginsberg JS, Julian J, Douketis J, Solymoss S, Ockelford P, et al. Acute treatment of venous thromboembolism (VTE) with fixed‐dose, weight‐adjusted, unfractionated heparin (UFH) given subcutaneously (sc) without laboratory monitoring: randomized comparison with low‐molecular‐weight‐heparin (LMWH). Journal of Thrombosis and Haemostasis 2005; Vol. 3, issue 1:Abstract number: OR147.
- Kearon C, Ginsberg JS, Julian JA, Douketis J, Solymoss S, Ockelford P, et al. Comparison of fixed‐dose weight‐adjusted‐unfractionated heparin and low‐molecular weight heparin for acute treatment of venous thromboembolism. JAMA 2006;296:935‐42. [DOI] [PubMed] [Google Scholar]
Lockner 1985 {published data only}
- Lockner D, Bratt G, Tornebohm E, Aberg W, Granqvist S. A comparison between low molecular weight heparin (LMWH, KABI 2165) and standard heparin in the intravenous treatment of deep vein thrombosis (DVT). Thrombosis and Haemostasis 1985;54(4):813‐7. [PubMed] [Google Scholar]
Lockner 1986 {published data only}
- Lockner D, Bratt G, Tornebohm E, Aberg W, Granqvist S. Intravenous and subcutaneous administration of Fragmin in deep venous thrombosis. Haemostasis 1986;16 Suppl 2:25‐9. [DOI] [PubMed] [Google Scholar]
Ly 1985 {published data only}
- Ly B, Arnesen KE, Holm HA, Handeland GF, Abilgaard U. Subcutaneous LMW or unfractionated heparin in DVT: A randomized, double blind study with dose adjustments according to heparin concentration in plasma. Thrombosis and Haemostasis 1985;54(1):Abstract No. 91. [Google Scholar]
Monreal 1993 {published data only}
- Monreal M, Lafoz E, Vedia C, Roncales J. Comparison of subcutaneous unfractioned heparin with a low molecular weight heparin (Fragmin) in patients with venous thromboembolism and contraindications to coumarin. Thrombosis and Haemostasis 1993;69(649):Abstract No. 383. [PubMed] [Google Scholar]
Monreal 1994 {published data only}
- Monreal M, Lafoz E, Olive A, Rio L. Comparison of subcutaneous unfractioned heparin with a low molecular weight heparin (Fragmin) in patients with venous thromboembolism and contraindications to coumarin. Thrombosis and Haemostasis 1994;71(1):7‐11. [PubMed] [Google Scholar]
Notarbartolo 1988 {published data only}
- Notarbartolo A, Salanitri G, Davi G, Averna M, Barbagallo C, Catalano I. Low molecular weight heparin in the short and long‐term treatment of deep vein thrombosis in diabetic subjects. Praxis Med 1988;9:393‐405. [Google Scholar]
Quiros 2001 {published data only}
- Quiros M. Use of heparin of low molecular weight in the ambulatory handling of patients with deep venous thrombosis. Comparative study: heparin nondivided versus low molecular weight heparin. Revista costarricense de cardiologia 2001;3(2):8‐13. [Google Scholar]
Riess 2014 {published data only}
- Riess H, Becker LK, Melzer N, Harenberg J. Treatment of deep vein thrombosis in patients with pulmonary embolism: Subgroup analysis on the efficacy and safety of certoparin vs. unfractionated heparin. Blood Coagulation & Fibrinolysis 2014;25(8):838‐44. [DOI] [PubMed] [Google Scholar]
Siguret 2011 {published data only}
- Siguret V, Gouin‐Thibault I, Pautas E, Leizorovicz A. No accumulation of the peak anti‐Xa activity of tinzaparin in elderly patients with moderate‐to‐severe renal impairment: the IRIS substudy. Journal of Thrombosis and Haemostasis 2011;9(10):1966‐72. [DOI] [PubMed] [Google Scholar]
Stricker 1999 {published data only}
- Stricker H, Marchetti O, Haeberli A, Mombelli G. Hemostatic activation under anticoagulant treatment: a comparison of unfractionated heparin vs. nadroparin in the treatment of proximal deep vein thrombosis. Thrombosis and Haemostasis 1999;82(4):1227‐31. [PubMed] [Google Scholar]
Tedoldi 1993 {published data only}
- Tedoldi A, Botticella F, Maloberti MR. Antithrombophilic effect of low molecular weight heparins in patients with deep vein thrombosis. Clinical Trials and Meta‐analysis 1993;28(4‐5):215‐25. [Google Scholar]
Ucar 2015 {published data only}
- Ucar EY, Akgun M, Araz O, Tas H, Kerget B, Meral M, et al. Comparison of LMWH versus UFH for hemorrhage and hospital mortality in the treatment of acute massive pulmonary thromboembolism after thrombolytic treatment: randomized controlled parallel group study. Lung. Springer New York LLC, 2015; Vol. 193, issue 1:121‐7. [DOI] [PubMed]
Vogel 1987 {published data only}
- Vogel G, Machulik M. Efficacy and safety of a low molecular weight heparin (LMW‐heparin Sandoz) in patients with deep vein thrombosis. Thrombosis and Haemostasis 1987;58 Suppl:Abstract No. 427. [Google Scholar]
Zanghi 1988 {published data only}
- Zanghi M, Morici V, Costanzo M, Astuto L, Salanitri G. Deep vein thrombosis of the legs: new therapy by means of low molecular weight heparins. Journal of International Medical Research 1988;16(6):474‐84. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00796692 {published data only}
- NCT00796692. Nadroparin for the initial treatment of pulmonary thromboembolism (NATSPUTE). ClinicalTrials.gov (first received 20 November 2008).
Additional references
Buller 1991
- Buller HR, Lensing AWA, Hirsh J, Cate JW. Deep venous thrombosis: new noninvasive tests. Thrombosis and Haemostasis 1991;66(1):133‐9. [PubMed] [Google Scholar]
Collins 1987
- Collins R, Gray R, Godwin J, Peto R. Avoidance of large biases and large random errors in the assessment of moderate treatment effects: the need for systematic overviews. Statistics in Medicine 1987;6(3):245‐50. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Diaz 2015
- Diaz JP, Soto Molina H, Marquez M, Escobar Juarez Y. Low‐molecular‐weight heparin in treatment of deep‐vein thrombosis: A network meta‐analysis. Value in Health 2015;18(3):A31. [Google Scholar]
GRADE Working Group 2008
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. British Medical Journal 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hirsh 1990
- Hirsh J. From unfractionated heparins to low molecular weight heparins. Acta Chirurgica Scandinavica 1990;156 Suppl 556:42‐50. [PubMed] [Google Scholar]
Hirsh 1992
- Hirsh J, Levine MN. Low molecular weight heparin. Blood 1992;79(1):1‐17. [PubMed] [Google Scholar]
Huisman 1989
- Huisman MV, Buller HR, Cate JW, Royen EA, Vreeken J, Kersten MJ, et al. Unexpected high prevalence of silent pulmonary embolism in patients with deep vein thrombosis. Chest 1989;95(3):498‐502. [DOI] [PubMed] [Google Scholar]
Hull 1983
- Hull RD, Hirsh J, Carter CJ, Jay RM, Dodd PE, Ockelford PA, et al. Pulmonary angiography, ventilation lung scanning, and venography for suspected pulmonary embolism with abnormal perfusion lung scan. Annals of Internal Medicine 1983;98(6):891‐9. [DOI] [PubMed] [Google Scholar]
Kearon 2012
- Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e419S‐94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719‐48. [PubMed] [Google Scholar]
NICE 2012
- NICE. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. www.nice.org.uk/guidance/cg144 (accessed 15 November 2016).
Nurmohamed 1992
- Nurmohamed MT, Rosendaal FR, Buller HR, Dekker E, Hommes DW, Vandenbroucke JP, et al. Low‐molecular‐weight heparin versus standard heparin in general and orthopaedic surgery: a meta‐analysis. Lancet 1992;340(8812):152‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulman 2005
- Schulman S, Kearon C, and the Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. Journal of Thrombosis and Haemostasis 2005;3(4):692‐4. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Turpie 2002
- Turpie AGG, Chin BSP, Lip GYH. Venous thromboembolism: treatment strategies. British Medical Journal 2002;325(7370):948‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
van den Belt 1998
- Belt AGM, Bossuyt PMM, Prins MH, Gallus AS, Buller HR, Koopman MMW, et al. Replacing inpatient care by outpatient care in the treatment of deep venous thrombosis. An economic evaluation. Thrombosis and Haemostasis 1998;79(2):259‐63. [PubMed] [Google Scholar]
References to other published versions of this review
Erkens 2010
- Erkens PMG, Prins MH. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD001100.pub3] [DOI] [PubMed] [Google Scholar]
van den Belt 1999
- Belt AGM, Prins MH, Lensing AWA, Castro AA, Clark OAC, Atallah AN, Burihan E. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD001100] [DOI] [PubMed] [Google Scholar]
van Dongen 2004
- Dongen CJ, Belt AGM, Prins MH, Lensing AWA. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD001100.pub2] [DOI] [PubMed] [Google Scholar]


