Abstract
Background
Dysarthria is an acquired speech disorder following neurological injury that reduces intelligibility of speech due to weak, imprecise, slow and/or unco‐ordinated muscle control. The impact of dysarthria goes beyond communication and affects psychosocial functioning. This is an update of a review previously published in 2005. The scope has been broadened to include additional interventions, and the title amended accordingly.
Objectives
To assess the effects of interventions to improve dysarthric speech following stroke and other non‐progressive adult‐acquired brain injury such as trauma, infection, tumour and surgery.
Search methods
We searched the Cochrane Stroke Group Trials Register (May 2016), CENTRAL (Cochrane Library 2016, Issue 4), MEDLINE, Embase, and CINAHL on 6 May 2016. We also searched Linguistics and Language Behavioral Abstracts (LLBA) (1976 to November 2016) and PsycINFO (1800 to September 2016). To identify further published, unpublished and ongoing trials, we searched major trials registers: WHO ICTRP, the ISRCTN registry, and ClinicalTrials.gov. We also handsearched the reference lists of relevant articles and contacted academic institutions and other researchers regarding other published, unpublished or ongoing trials. We did not impose any language restrictions.
Selection criteria
We selected randomised controlled trials (RCTs) comparing dysarthria interventions with 1) no intervention, 2) another intervention for dysarthria (this intervention may differ in methodology, timing of delivery, duration, frequency or theory), or 3) an attention control.
Data collection and analysis
Three review authors selected trials for inclusion, extracted data, and assessed risk of bias. We attempted to contact study authors for clarification and missing data as required. We calculated standardised mean difference (SMD) and 95% confidence interval (CI), using a random‐effects model, and performed sensitivity analyses to assess the influence of methodological quality. We planned to conduct subgroup analyses for underlying clinical conditions.
Main results
We included five small trials that randomised a total of 234 participants. Two studies were assessed as low risk of bias; none of the included studies were adequately powered. Two studies used an attention control and three studies compared to an alternative intervention, which in all cases was one intervention versus usual care intervention. The searches we carried out did not find any trials comparing an intervention with no intervention. The searches did not find any trials of an intervention that compared variations in timing, dose, or intensity of treatment using the same intervention. Four studies included only people with stroke; one included mostly people with stroke, but also those with brain injury. Three studies delivered interventions in the first few months after stroke; two recruited people with chronic dysarthria. Three studies evaluated behavioural interventions, one investigated acupuncture and another transcranial magnetic stimulation. One study included people with dysarthria within a broader trial of people with impaired communication.
Our primary analysis of a persisting (three to nine months post‐intervention) effect at the activity level of measurement found no evidence in favour of dysarthria intervention compared with any control (SMD 0.18, 95% CI ‐0.18 to 0.55; 3 trials, 116 participants, GRADE: low quality, I² = 0%). Findings from sensitivity analysis of studies at low risk of bias were similar, with a slightly wider confidence interval and low heterogeneity (SMD 0.21, 95% CI ‐0.30 to 0.73, I² = 32%; 2 trials, 92 participants, GRADE: low quality). Subgroup analysis results for stroke were similar to the primary analysis because few non‐stroke participants had been recruited to trials (SMD 0.16, 95% CI ‐0.23 to 0.54, I² = 0%; 3 trials, 106 participants, GRADE: low quality).
Similar results emerged from most of the secondary analyses. There was no evidence of a persisting effect at the impairment (SMD 0.07, 95% CI ‐0.91 to 1.06, I² = 70%; 2 trials, 56 participants, GRADE: very low quality) or participation level (SMD ‐0.11, 95% CI ‐0.56 to 0.33, I² = 0%; 2 trials, 79 participants, GRADE: low quality) but substantial heterogeneity on the former. Analyses of immediate post‐intervention outcomes provided no evidence of any short‐term benefit on activity (SMD 0.29, 95% CI ‐0.07 to 0.66, I² = 0%; 3 trials, 117 participants, GRADE: very low quality); or participation (SMD ‐0.24, 95% CI ‐0.94 to 0.45; 1 study, 32 participants) levels of measurement.
There was a statistically significant effect favouring intervention at the immediate, impairment level of measurement (SMD 0.47, 95% CI 0.02 to 0.92, P = 0.04, I² = 0%; 4 trials, 99 participants, GRADE: very low quality) but only one of these four trials had a low risk of bias.
Authors' conclusions
We found no definitive, adequately powered RCTs of interventions for people with dysarthria. We found limited evidence to suggest there may be an immediate beneficial effect on impairment level measures; more, higher quality research is needed to confirm this finding.
Although we evaluated five studies, the benefits and risks of interventions remain unknown and the emerging evidence justifies the need for adequately powered clinical trials into this condition.
People with dysarthria after stroke or brain injury should continue to receive rehabilitation according to clinical guidelines.
Plain language summary
Interventions for speech problems (dysarthria) after stroke or other non‐progressive brain injury
Review question Does any type of treatment help people who have difficulty speaking clearly after a stroke or other types of brain injury acquired during adulthood?
Background Brain damage caused by stroke, injury or other non‐progressive disease can make speech unclear and difficult for listeners to understand. This condition is known as dysarthria and it occurs when face, tongue, and throat muscles are weak, slow, and unco‐ordinated. Dysarthria can cause people who are affected to lose confidence when talking and become socially isolated, even if others see symptoms as mild. People with dysarthria do not have difficulties thinking, remembering, or retrieving words.
Treatment is usually provided by a speech and language therapist or speech pathologist and involves advice and education plus strategies and exercises to increase clarity of speech and to cope with social interaction. Other types of treatment used include acupuncture or brain stimulation.
We wanted to find out if any treatments work, if the effects are long lasting, and if so, which works best, when treatment should start, how frequent treatment should be, and for how long. To find out we searched for, evaluated, and summarised the quality of the existing research on this topic.
Search date
We searched the literature up to May 2016.
Study characteristics We included five small trials that randomised only 234 people, almost all with stroke. Two trials investigated dysarthria treatment versus an attention control and three compared one treatment with usual care. There were no trials that compared one treatment to no treatment.
Key results We found few randomised controlled trials of dysarthria treatment, and those that have been conducted involved small numbers of participants, or were not adequately designed or had serious reporting flaws.
We compared many different measures at various time points after treatment, so caution is recommended when interpreting results. We found no evidence of effectiveness on most measures, including long‐lasting improvement in every day communication abilities. A positive finding was short‐term improvement in muscle movement, such as tongue and lip control. However, this result is not reliable because it was based on small numbers of people, and we found concerns about the conduct and reporting of some trials. This finding needs to be investigated in a bigger, better designed trial.
We found insufficient evidence to tell us whether any one treatment is better than any other or whether treatment is better than general support, or no treatment. We found no studies that examined timing, duration, or intensity of treatment. This is a clinically important question and should be considered in future trials.
Quality of the evidence The included trials varied in quality but all included small numbers of participants. Overall, studies were rated as low to very low quality evidence.
Summary of findings
Summary of findings for the main comparison. Dysarthria intervention compared with another intervention, attention control, placebo or no intervention for people with dysarthria after stroke or other adult‐acquired, non‐progressive brain injury.
| Dysarthria intervention compared with another intervention, attention control, placebo or no intervention for people with dysarthria after stroke or other adult‐acquired, non‐progressive brain injury | ||||
|
Patient or population: adults with dysarthria following stroke or other adult‐acquired, non‐progressive brain injury Settings: any Intervention: dysarthria intervention Comparison: another intervention, attention control, placebo or no intervention | ||||
| Outcomes | Standardised mean difference (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Dysarthria intervention versus any control: persisting effects, activity level | 0.18 [‐0.18, 0.55] | 116 participants 3 RCTs | ⊕⊕⊝⊝ low | Very small numbers and none of the studies are adequately powered. Only two of the three studies considered low risk of bias |
| Dysarthria intervention versus any control: persisting effects, impairment level | 0.07 [‐0.91, 1.06] | 56 participants 2 RCTs |
⊕⊝⊝⊝ very low | Very small numbers, none of the studies are adequately powered. Only one of the two studies considered low risk of bias |
| Dysarthria intervention versus any control: persisting effects, participation level | ‐0.11 [‐0.56, 0.33] | 79 participants 2 RCTs |
⊕⊕⊝⊝ low | Both studies considered low risk of bias but very small numbers and neither study adequately powered. |
| Dysarthria intervention versus any control for stroke subgroup: persisting effects, activity level | 0.16 [‐0.23, 0.54] | 106 participants 3 RCTs |
⊕⊕⊝⊝ low | Very small numbers and none of the studies are adequately powered. Only two of the three studies considered low risk of bias |
| Dysarthria intervention versus any control: immediate effects, activity level | 0.29 [‐0.07, 0.66] | 117 participants 3 RCTs |
⊕⊝⊝⊝ very low | Very small participant numbers, not adequately powered. Only one of the three studies considered to be low risk of bias |
| Dysarthria intervention versus any control: immediate effects, impairment level | 0.47 [0.02, 0.92] | 99 participants 4 RCTs |
⊕⊝⊝⊝ very low | Very small participant numbers, not adequately powered. Only one of the four studies considered to be low risk of bias. This comparison shows a significant effect |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
Background
Description of the condition
Dysarthria is a speech disorder affecting intelligibility due to disturbances in neuromuscular control. Dysarthria affects approximately 20% to 30% of stroke survivors (Lawrence 2001; Lubart 2005; Warlow 2008) and 10% to 60% of those who survive traumatic brain injury. It can occur in adults as an outcome of meningitis, encephalitis, post‐surgical meningioma, and acoustic neuroma (Sellars 2005).
Dysarthria is defined as a neurologic motor speech impairment causing the speech musculature to be slow, weak and/or imprecise (Duffy 2013). This causes poor co‐ordination of movements involving breathing, voice production, resonance, and oral articulation (Yorkston 1996). People with dysarthric speech typically sound less intelligible or slurred because of poor oral control of articulators, particularly the tongue. Speech can also be quiet, underpowered, and lacking expressiveness because of respiratory control or impaired vocal cord function. Dysarthria includes a wide severity range; some people may be mostly unintelligible to the listener; people at the milder end of the range may experience lapses in speech accuracy, or fatigue, but speech is generally intelligible.
Dysarthria impacts beyond impaired communication. It can negatively affect psychological wellbeing, social participation, and rehabilitation (Brady 2011; Dickson 2008; Tilling 2001). Brady 2011 found that the psychological impact can be influenced by pre‐morbid levels of communication demands. An individual with mild dysarthria, but high levels of communication before their illness, may experience psychological impairment as severe as someone with more severe dysarthria.
Description of the intervention
Behavioural interventions by a speech and language therapist or speech language pathologist are the mainstay of dysarthria treatment. The primary aim is to maximise the patient's ability to communicate with others. UK treatment guidelines for dysarthria (Taylor‐Goh 2005) recommend that behavioural interventions address all dimensions of the International Classification of Functioning, Disability and Health (ICF) Framework; impairment, activity and participation (WHO 2001). Impairment level exercises to improve the strength, speed, or function of the impaired musculature may be used. These are usually non‐speech and oro‐motor movements of affected muscles or muscle groups. This may include external stimulation of the muscles such as applying ice packs, brushing the skin, acupuncture (traditional and electrical), or transcranial magnetic stimulation of the brain. At the activity level, compensatory strategies to increase intelligibility through purposeful speech production such as over‐articulation or slowing rate of speech may be used. In addition alternative ways to communicate, or support speech, may be used such as an alphabet chart or computers with artificial voice software. Participation level approaches may use facilitated group work, education, and feedback to support the psychological health of people living with dysarthria or advice to a communication partner may be implemented.
How the intervention might work
The interventions at the impairment level in the Description of the intervention are likely to be focused on the recovery of impaired movement through exercises to increase strength, range, precision and speed of movement required for speech. Treatment can utilise non‐speech or more typically speech‐focused movement tasks. Intervention for limb rehabilitation indicates some association between muscle strength and function of movement (Langhorne 2009) but it is not known whether this is the case for muscles involved in speech. Interventions may examine intensity of intervention and may compare quantity, duration and frequency of input. We know from post‐stroke research more generally that increased intensity of treatment may be a key element in recovery but the optimum frequency, duration and quantity of intervention is not known (Intercollegiate Stroke Working Party 2016).
The interventions at the activity and participation level as outlined in the Description of the intervention are likely to focus on strategies or patient specific goals to improve speech intelligibility that relate to a meaningful communication activity for that person. Stroke guidance suggests that goal setting should be used as a rehabilitation tool (Intercollegiate Stroke Working Party 2016).This may include reducing rate of speech when talking on the phone, employing purposeful use of speech intonation to distinguish statements from questions in conversation, or advice to the key communication partner. Group or individual work to target confidence in use of communication is another treatment approach, which may incorporate principles of psychological interventions such as motivational interviewing. Environmental modification and education can also be utilised to optimise communication ease and success in a given context such as a family, hospital or nursing home setting.
Why it is important to do this review
The previous version of this review found no studies that met inclusion criteria (Sellars 2005). Further trials have since been published, and this update broadened the scope of the search strategy applied by Sellars 2005 to include all interventions carried out by any health professional, people with dysarthria, a trained individual, or any other new approaches to treatment.
Objectives
To assess the effects of interventions to improve dysarthric speech following stroke and other non‐progressive adult‐acquired brain injury such as trauma, infection, tumour and surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of interventions to improve non‐progressive dysarthric speech in adults with acquired brain injuries, including comparisons with no intervention, another intervention (which may be the same intervention approach but alternative method, theory, timing, duration or frequency), attention control, or placebo. We included data only from the first phase of cross‐over trials to avoid contamination.
Types of participants
Adults (aged over 18 years) diagnosed with non‐progressive dysarthria following acquired brain injury, principally stroke and traumatic brain injury, at any time since stroke onset or trauma event.
Types of interventions
We considered any type of intervention for acquired dysarthria including behavioural or psychological approaches, use of devices and medication, excluding surgical interventions. Interventions could be carried out by any healthcare professional, healthcare staff, trained volunteer, family member or carer, or the person with dysarthria.
Interventions addressed any level of the International Classification of Functioning Disability and Health (ICF) (WHO 2001) including the following.
Impairment level: interventions specifically targeting the impairment of function, e.g. non‐speech and oro‐motor exercises to improve speed, range, strength, accuracy of speech/respiratory musculature, external stimulation of the muscles such as applying ice packs, brushing the skin, transcranial magnetic stimulation of the brain, acupuncture (traditional and electrical).
Activity level: interventions to increase intelligibility by modifying existing speech (e.g. modifying rate of speech) or the use of augmentative or alternative communication devices e.g. light tech aids (non‐technical materials such as an alphabet chart) and high tech aids (such as text‐to‐talk computer devices).
Participation level: interventions aimed at support or education for the individual with dysarthria or programmes for people with dysarthria and their conversational partners or conversational training as well as any psychological approaches to treatment that focus on increasing social participation.
We did not place any restrictions on frequency, intensity, or duration of the interventions.
Types of outcome measures
Primary outcomes
The primary outcome measure for this review was the long‐term effectiveness of the dysarthria intervention on everyday speech (activity level, persisting effect) compared with any control (another intervention, attention control or placebo, or no intervention). Attempts to objectively measure everyday speech are usually based on listener perception grading scales such as dysarthria therapy outcome measures (Enderby 1997) or the communication effectiveness measure (Mackenzie 2007). We defined evidence of a persistent beneficial effect as around six months post‐intervention extracted as measures taken between three and nine months post‐intervention.
When trials used more than one outcome measure at the activity level, we took the primary outcome as specified by the trial investigators. If a trial had not specified a primary outcome measure, we checked if a measure of functional communication had been used at the specified time points.
Secondary outcomes
Secondary outcomes included exploring effects:
at other measurement levels (e.g. impairment, participation);
at other time points (e.g. immediate post‐intervention);
compared with specific control groups (e.g. another intervention, attention control or placebo, or no intervention);
for clinical subgroups (e.g. stroke, brain injury);
for studies assessed at low risk of bias.
Secondary outcome measures were as follows.
Communication at impairment level (immediate and persisting): speech impairment measure e.g. Frenchay Dysarthria Assessment edition I or II (Enderby 1983), Iowa Oral Performance Instrument (IOPI) (IOPI 2005), measures of intelligibility (e.g. Assessment of intelligibility of Dysarthric Speech) (Yorkston 1984), acoustic and perceptual measures of voice and speech (e.g. vocal profile analysis, pitch, loudness, air flow, sound spectography).
Communication at activity level (immediate): activity measure (e.g. Dysarthria Therapy Outcome Measure) (Enderby 1997), listener acceptability measures.
Communication‐related quality of life (immediate and persisting participation level): patient perception of impact (e.g. Dysarthria Impact Profile) (Walshe 2009); Communication Outcomes after Stroke Scale (Long 2008).
Generic quality of life measures: mood scales (e.g. Hospital Anxiety and Depression Scale) (Zigmond 1983); subjective health scales (e.g. EuroQol, SF‐36) (Herdman 2011).
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We did not impose any language restrictions and we sought translations for non‐English language studies.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (last searched by the Managing Editor to May 2016), the Cochrane Central Register of Controlled Trials (CENTRAL, Cochrane Library 2016, Issue 4; Appendix 1), MEDLINE (1946 to May 2016; Appendix 2), Embase (1974 to May 2016; Appendix 3), CINAHL (1937 to May 2016; Appendix 4), PsycINFO (1800 to September 2016; Appendix 5) and LLBA (1976 to November 2016; Appendix 6) using comprehensive search strategies.
We searched major trials registers for ongoing trials including the World Health Organization International Clinical Trials Registry Platform (who.int/ictrp/search/en/), the ISRCTN registry (isrctn.com/), ClinicalTrials.gov (clinicaltrials.gov/) and the Stroke Trials Registry (strokecenter.org/trials/).
Searching other resources
In an effort to identify other published, unpublished, and ongoing trials we handsearched the reference lists of relevant articles and contacted academic institutions and other researchers.
Data collection and analysis
Selection of studies
Our selection criteria were as follows.
Research participants with dysarthria following stroke or other adult‐acquired, non‐progressive brain injury.
Interventions designed to reduce the dysarthria or its impact on living with dysarthria.
RCTs.
One author (CM) excluded any obviously irrelevant reports from the titles and abstracts retrieved in the search. Three authors (CM, AB, PC) independently examined the remaining abstracts and then the full‐text to determine eligibility and exclude irrelevant reports. We resolved disagreements through discussion. No review author examined their own study. We pursued finding conference proceedings and dissertations that were difficult to retrieve using email contacts, university alumni societies, and conference committees. We arranged for reports published in languages other than English to be translated where required. Where possible, we contacted authors of studies for clarification to inform discussions around eligibility. All authors agreed final decisions on included studies and proceeded to data collection. The studies we judged as ineligible for inclusion are listed with reasons for exclusion in Characteristics of excluded studies.
Data extraction and management
Three authors (CM, AB, PC) independently carried out data extraction from trial reports in pairs (avoiding authors' own trials), and extracted the following data.
Methods: study design, study duration, sequence generation, allocation sequence concealment, blinding.
Participants: total number, attrition, setting, diagnostic criteria, age, gender, country of research.
Interventions: total number of intervention groups, specific intervention and details.
Outcomes: outcomes and time points, outcome definition and measurement.
Results: number of participants allocated to each intervention, sample size, missing participants, summary data.
We attempted to contact trial authors for further information where risk of bias was unclear or data were missing. We reconciled the independent data extraction between pairs of review authors and would have resolved any disagreements by discussion or with reference to an independent arbitrator (ST) if required.
Assessment of risk of bias in included studies
Three authors (CM, AB, PC) independently carried out the assessment of risk of bias and methodological quality within the pairs assigned for data extraction. The authors used Cochrane's 'Risk of bias' tool (Higgins 2011). We examined the studies for the following quality criteria: random sequence generation, allocation concealment, blinding of outcome assessors, incomplete outcome data, and selective reporting.
For random sequence generation (selection bias), we considered trials to be low risk if the random component was clearly described, at high risk of bias where randomisation was influenced by the availability of the intervention, or an unclear risk where there was insufficient information to decide. For allocation concealment (selection bias), we considered trials adequately concealed if the process made clear that participants and investigators could not possibly predict allocation. We considered a study to be at high risk if there was a possibility that allocation could be predicted (e.g. open random allocation schedule, open computer systems potentially accessible to the investigator), or where concealment was unclear and the study author was unable to provide sufficient information or did not respond.
It was accepted that the participants and the therapists delivering the intervention could not be blinded to the intervention. Thus, we considered blinding in terms of outcome assessment (performance bias and detection bias) and we considered studies to be at a low risk of bias if the outcome assessor was clearly blinded to the intervention; we considered studies to be at a high risk of bias if this was not the case, the blinding could be broken and an unclear risk of bias if there was insufficient information provided.
We considered incomplete outcome data (attrition bias) a low risk if there were:
no missing outcome data;
missing outcome data that were unlikely to be related to true outcome;
missing outcome data that were balanced in numbers across intervention groups;
similar reasons for missing data across groups; and
missing data that had been imputed using appropriate methods that did not affect outcome and were reported as such.
We considered studies to be at a high risk of bias if they did not address:
incomplete outcome data adequately;
missing outcome data likely to be related to the true outcome;
imbalance of numbers or reasons for missing data across the intervention groups;
effect size among missing outcomes to induce clinically relevant bias;
an intention‐to‐treat analysis done with substantial differences of the intervention received.
We considered selective reporting (reporting bias) within studies included in the review. We considered whether studies had reported all outcome data compared with their planned protocols (published or unpublished) where possible. Where this was not possible, we asked study authors for additional information on planned outcome reporting prior to the study. We considered study authors who did not respond to this request an unclear risk.
Measures of treatment effect
We treated the measures of functional speech as a continuous measure. We abstracted, calculated or requested means and standard deviations. We calculated standardised mean differences (SMDs) and confidence intervals (CIs), using a random‐effects model for the primary outcome and for any secondary outcomes measures included.
Unit of analysis issues
For continuous data we requested or calculated the mean and standard deviation (SD) data. We analysed outcomes as SMD and 95% CI. We used inverse variance and random‐effects models. We entered data so that a higher score represented a favourable outcome.
We used RevMan 5 for all analyses (RevMan 2014).
Dealing with missing data
We requested missing data from study authors as needed; this is reported in Characteristics of included studies.
Assessment of heterogeneity
We assessed heterogeneity between trials with the selected comparisons and outcomes comparing measures, time points, trial design and clinical subgroups. We determined statistical heterogeneity based on the statistic with Chi² distribution. We quantified heterogeneity using the I² statistic, which describes the proportion of total variance across trials. We considered heterogeneity of 40% or more as considerable and 70% or more as substantial (Deeks 2011). Heterogeneity below 40% was considered low.
Assessment of reporting biases
We planned to explore reporting bias if 10 or more trials were included in the review as outlined in The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data synthesis
The primary analysis pooled all trials in the meta‐analysis, using a random‐effects model, including the dysarthria intervention versus any control (another intervention, attention control, placebo or no intervention). We considered primary outcome data measures and secondary outcome measures at various time points (immediate and persistent) and various levels of functioning.
GRADE and 'Summary of findings' table
We created Table 1 for the main comparison and included the following outcomes:
dysarthria intervention versus any control: persisting effects, activity level;
dysarthria intervention versus any control: persisting effects, impairment level;
dysarthria intervention versus any control: persisting effects, participation level;
dysarthria intervention versus any control for stroke subgroup: persisting effects, activity level;
dysarthria intervention versus any control: immediate effects, activity level; and
dysarthria intervention versus any control: immediate effects, impairment level.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence as it related to the included studies (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEproGDT software (GRADEproGDT 2015). We justified all decisions to down‐ or upgrade the quality of studies in footnotes, and provided comments to aid readers' understanding where necessary.
Subgroup analysis and investigation of heterogeneity
We carried out subgroup analysis to explore the effect of comparison with all controls (another intervention, attention control, placebo or no intervention). We carried out clinical subgroup analysis of stroke or brain injury and a subgroup sensitivity analysis where studies had low risk of bias.
Sensitivity analysis
We carried out sensitivity analysis to explore methodological heterogeneity including studies with adequate allocation concealment and adequate blinding, these were the studies we considered to be at low risk of bias.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies; and Characteristics of studies awaiting classification.
Results of the search
Our searches identified 17,313 records; the screening process is shown in the PRISMA flow diagram (Figure 1). Five papers met our inclusion criteria (Bowen 2012; Kwon 2015; Mackenzie 2014; Wenke 2010; Xu 2010) and are described in Characteristics of included studies. We also identified two ongoing studies (Peng 2015; ReaDySpeech; see Characteristics of ongoing studies). Both ReaDySpeech, and Peng 2015 presented insufficient detail to inform assessment, and will be assessed for inclusion in a future review update. The study authors of Peng 2015 have been contacted for further information; we will monitor for publication of the study. You 2010 included an English language abstract, but presents insufficient information to make a decision regarding inclusion; this study is presented in Characteristics of studies awaiting classification.
1.
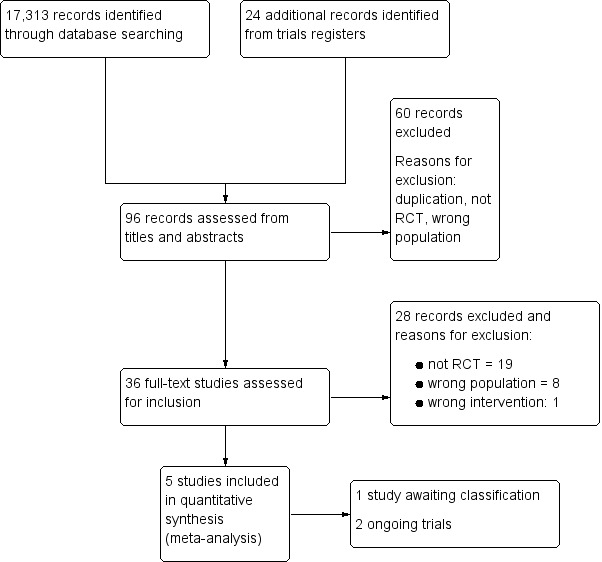
Study flow diagram
Included studies
The included trials randomised a total of 234 participants, ranging from 25 (Kwon 2015) to 66 (Bowen 2012). The five trials are detailed in the Characteristics of included studies table and we have included the comparison data below . All included studies were RCTs and each contributed to more than one comparison. We present data that compared one dysarthria intervention with another dysarthria intervention and a dysarthria intervention with an attention control. We found no studies that compared dysarthria intervention with nothing or the same dysarthria interventions with variations in timing, duration, or frequency of delivery. Further information on intervention characteristics and the main comparisons are presented in Characteristics of included studies and Table 1.
The previous version of this review did not include any studies (Sellars 2005).
Participant characteristics
All five included trials recruited men and women; the proportion of men ranged from 56% (Bowen 2012) to 85% (Kwon 2015). The average age ranged from 49 years (Wenke 2010) to 70 years (Bowen 2012). Four studies included only people with stroke (Bowen 2012; Kwon 2015; Mackenzie 2014; Xu 2010); one study included people with stroke and a small number with traumatic brain injury (Wenke 2010). Two studies tested interventions that were provided in the first four months (Bowen 2012) and two months following stroke (Kwon 2015). Two studies involved participants who were in the chronic stage of recovery (Mackenzie 2014; Wenke 2010). Xu 2010 included people between one and 12 months after stroke.
Participants were recruited from hospital (Bowen 2012; Xu 2010), the community (Mackenzie 2014), or the source of recruitment location was not specified (Wenke 2010) or not clear (Kwon 2015). Three studies reported dysarthria severity assessed and reported as part of study characteristics (Bowen 2012; Mackenzie 2014; Wenke 2010). People with severe dysarthria were excluded in Xu 2010 and severity was not reported in Kwon 2015. Co‐occurring communication impairment or cognitive problems were excluded by two studies (Kwon 2015; Xu 2010). Co‐occurring aphasias were described in Bowen 2012 and Mackenzie 2014 but not mentioned in Wenke 2010; however, Wenke 2010 identified co‐existing cognitive impairment. Bowen 2012 recruited people with communication difficulties after stroke including aphasia, dysarthria, or both. People with dysarthria were a planned subgroup within the study by Bowen 2012 and we extracted dysarthria data from the trial data.
Intervention and control interventions
None of the included studies compared dysarthria interventions with no intervention. Two trials compared an intervention with an attention control (Bowen 2012; Kwon 2015). Bowen 2012 investigated enhanced best practice speech and language therapy delivered by speech and language therapists supported by assistants compared with an attention control (employees offering an equivalent amount of time and social contact but no therapy or therapist input). Kwon 2015 investigated repetitive transcranial magnetic stimulation versus sham repetitive transcranial magnetic stimulation; both groups received the same speech therapy intervention.
Three trials compared dysarthria interventions with usual dysarthria care (Mackenzie 2014; Wenke 2010; Xu 2010). Mackenzie 2014 examined oro‐motor exercises compared with usual care. Wenke 2010 investigated Lee Silverman Voice Treatment (LSVT), an approach that focusses on increased volume of speech, with usual care. Xu 2010 compared acupuncture with usual care. Usual care was described as behavioural strategies that address impairment and activity levels of functioning (Mackenzie 2014; Wenke 2010; Xu 2010). Wenke 2010 and Mackenzie 2014 reported that usual care was based on existing literature and best practice guidelines; Wenke 2010 also included consensus agreement. Components of usual care were not reported in Xu 2010.
There were no comparisons of one intervention versus the same intervention with variations in timing, intensity, or duration of treatment.
We referred to the template for intervention description and replication checklist (TiDier) when extracting the information on the interventions for each study (Hoffmann 2014).
Intervention compared with attention control
Two studies assessed dysarthria interventions compared with attention controls (Bowen 2012; Kwon 2015; 86 participants). Bowen 2012 Investigated enhanced, flexible, best practice behavioural speech therapy, and Kwon 2015 examined repetitive transcranial magnetic stimulation. The enhanced, best practice intervention in Bowen 2012 was described in sufficient detail to enable replication from the manual provided and was agreed by consensus of speech and language therapists to address impairment, activity, and participation levels of functioning. Kwon 2015 described the repetitive transcranial magnetic stimulation intervention, equipment used, and how motor‐evoked potentials were calculated and established for each participant. The intervention was to be led by an experienced speech and language therapist in Bowen 2012, and in Kwon 2015, the intervention was carried out by a physiatrist (physicians specialising in physical medicine and rehabilitation). The attention control applied in Bowen 2012 was structured social contact, carried out by employed, part‐time, visitors; five of nine visitors had high levels of educational attainment. In Kwon 2015 the attention control was sham repetitive transcranial magnetic stimulation, carried out by the same physiatrist using the same methods as the intervention, but holding the coil perpendicular to the skull rather than tangential to the skull surface.
The population in both studies was people with stroke, both interventions and attention control were delivered at the same time, soon after stroke, within the first two months (Kwon 2015), and within the first four months (Bowen 2012).
Repetitive transcranial magnetic stimulation treatment duration was five days per week for two weeks (Kwon 2015). Enhanced speech therapy was conducted for a maximum of 16 weeks, with duration and frequency as clinically indicated up to a maximum of three times per week (Bowen 2012). Bowen 2012 mentioned homework, which was given as appropriate to people in the intervention arm, but not to the attention control arm participants. The unpublished intervention manual provided by the Bowen 2012 study authors, includes a sheet to encourage documentation of homework by participants, but there is no further description of whether homework was carried out or completed. Participants in the intervention arm discussed homework and its impact during interviews conducted as part of the qualitative aspect of this study. Kwon 2015 describes that both groups had the same speech therapy intervention carried out for 30 minutes, five days per week for the two weeks of rTMS treatment. The content of the speech therapy intervention was not described, although it was carried out by a skilled speech therapist. There was no mention of homework in Kwon 2015. Participants in the study by Kwon 2015 were not aware of the intervention type they were randomised to receive ‐ either the active repetitive transcranial magnetic stimulation or the attention control sham therapy.
The outcome measure for Kwon 2015 was a blinded assessment of impairment level immediately post intervention. Participants in Bowen 2012 were aware of the intervention type they were randomised to receive; the primary outcome was a blinded assessment of activity level functioning at six months post‐entry to the study.
Intervention A compared with intervention B
Three trials, involving a total of 117 randomised participants, compared one intervention with another intervention (Mackenzie 2014; Wenke 2010; Xu 2010). All three studies compared usual care versus an alternative intervention (Mackenzie 2014; Wenke 2010; Xu 2010). There were no trials that compared one intervention with the same intervention but with variations in timing, duration, or intensity of delivery.
Intervention A in Wenke 2010 was Lee Silverman Voice Treatment (LSVT) which aims to increase vocal loudness. In Xu 2010, intervention A was acupuncture; and in Mackenzie 2014 10 minutes of non‐speech oro‐motor exercises (tongue and lip movements) replaced 10 minutes word and sentence practice.
Intervention A was delivered by the same speech pathologist trained in LSVT in Wenke 2010; traditional Chinese medical specialists carried out acupuncture in Xu 2010; and the same experienced speech and language therapist provided treatment in Mackenzie 2014.
Intervention B in all three studies was usual care.
Wenke 2010 and Mackenzie 2014 described intervention B as behavioural therapy, addressing impairment and activity levels of functioning. Both studies provided sufficient information to enable replication of the therapy. Xu 2010, did not describe intervention B in sufficient detail to enable replication; there was no information around the content of the therapy, level of impairment, or how therapy was delivered.
Intervention B was delivered by an experienced speech pathologist in Wenke 2010; the same hearing and speech specialist delivered the usual care to participants in both arms in Xu 2010; and the same experienced speech and language therapist delivered both intervention A and B in Mackenzie 2014.
Treatment timing was for people in the chronic phase of recovery following stroke or brain injury of more than six months or more than three months in Wenke 2010 and Mackenzie 2014 respectively. In Xu 2010 timing ranged for people with acute to chronic dysarthria of between one and 12 months post stroke.
Treatment duration ranged from four weeks (Wenke 2010), to eight weeks (Mackenzie 2014) and nine weeks (Xu 2010).
Treatment frequency for interventions A and B was the same for Wenke 2010, at one hour per day, four days a week, and the same for Mackenzie 2014 at 40 minutes once a week. Xu 2010 differed, with both arms receiving speech therapy for 30 minutes, five times per week but intervention A was delivered for four weeks, with a week long break followed by four weeks of intervention A.
Independent practice of homework was described in Wenke 2010 and Mackenzie 2014 but was not used in Xu 2010. In Wenke 2010, independent, daily homework was suggested between sessions for intervention B group participants only, but whether this was carried out and recorded was not described. In Mackenzie 2014, participants in both intervention A and B were encouraged to carry out independent practice of their allocated intervention of around 30 minutes, five days a week during the seven between session practice weeks for a total of 1050 minutes. This was documented by participants in a diary and the results reported and analysed.
All participants in the three studies were aware of which intervention they were randomised to, none of the three studies had a primary outcome measure.
All three studies carried out an activity level measure, with this being considered to show persistent change for Wenke 2010 at six months post treatment, and Mackenzie 2014 at two months post intervention in a chronic population, but was only carried out immediately post intervention in Xu 2010.
Outcomes
All five studies used different outcome measures and at various time points. The primary outcome for this review was to examine the persisting effect of the intervention at the activity level of functioning.
Four studies carried out activity level measures (Bowen 2012; Mackenzie 2014; Wenke 2010; Xu 2010). Kwon 2015 did not carry out a measure of activity level of functioning.
Wenke 2010 and Xu 2010 used a measure of perceived intelligibility by a speech and language therapist, Bowen 2012 used the dysarthria therapy outcome measures (Enderby 1997), and Mackenzie 2014 used the communication effectiveness measure (Mackenzie 2007) and the Speech Intelligibility Test (Yorkston 1996). The only study that specified the primary outcome measure was Bowen 2012.
For our analyses of persisting outcome, we took data from measures carried out at three to nine months post intervention; this included Wenke 2010 (six months post treatment) and Bowen 2012 (measured at six months post randomisation). Mackenzie 2014 carried out the final outcome measure at two months (eight weeks) post intervention. The review authors discussed if these data should be included, because this was a chronic population with proximity to the proposed minimum time point of three months (12 weeks). We decided that the proposed time criterion (three months to nine months) in the review protocol was too tight, and agreed to relax timings to include the study data as a persisting effect. This change is reported in Differences between protocol and review. The latest time point for the primary outcome measure, taken by Xu 2010, was immediately post intervention, which did not meet our requirement of three to nine months post intervention to examine persistent change.
The secondary outcomes were other measures at various time points. This meant we examined data from the activity level measures at immediate time point post‐intervention, and this had been carried out by Wenke 2010, Xu 2010 and Mackenzie 2014. We considered 'immediate' measure to have been carried out at the end of the treatment period or the time period nearest to the end of treatment.
Communication impairment measures were used in four studies (Kwon 2015; Mackenzie 2014; Wenke 2010; Xu 2010). These were articulatory precision (Wenke 2010), maximum phonation time (Xu 2010), lip and tongue movements from the Frenchay dysarthria assessment (FDA‐2) (Mackenzie 2014), and an articulation test (Kwon 2015). These impairment measures were carried out to show persistent effect between the three month and nine month time points by Wenke 2010 and Mackenzie 2014, but not Xu 2010 or Kwon 2015. These measures were carried out immediately post‐intervention by all four studies (Kwon 2015; Mackenzie 2014; Wenke 2010; Xu 2010). Measures at the participation level were used by Bowen 2012, which used the Communication Outcomes after Stroke Scale (COAST; Long 2008), and Mackenzie 2014, which used the Communicative Effectiveness Survey (CES; Donovan 2007). Both studies applied this participation level measure as a persistent measure of change between three month and nine months, but only Mackenzie 2014 applied this immediately post treatment.
Excluded studies
See: Characteristics of excluded studies
We excluded 28 studies primarily because they were not RCTs (Fitzgerald‐DeJean 2008; Fukusako 1989; Garcia 1998; Huffman 1978; Huh 2014; Hustad 2003; Ince 1973; Jones 1972; Katić 1973; Li 2013; Markov 1973; Nagasawa 1970; Palmer 2004; Palmer 2007; Robertson 2001; Rosenbek 2006; Sakharov 2013; Togher 2014; Varma 2004). In several studies, participants were not dysarthric (Behn 2011; Behn 2012; Braverman 1999; Sze 2002; Togher 2004), or had mixed aetiologies including progressive and congenital conditions (Cohen 1993; Kelly 2000; Main 1998), or a surgical intervention was investigated (Qinglan 2002).
Risk of bias in included studies
Overall risk of bias for the five included studies is depicted in Figure 2 and Figure 3.
2.
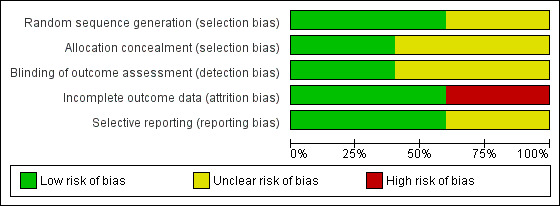
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
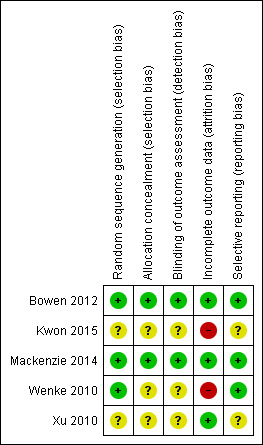
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Three review authors independently assessed the included studies for methodological quality (avoiding their own studies) and discussed any discrepancies. We intended to carry out sensitivity analysis according to studies at low risk of bias for each domain. We considered that two studies were at low risk of bias overall, and these were included in the sensitivity analysis (Bowen 2012; Mackenzie 2014). All five included studies reported inclusion and exclusion criteria.
Allocation
We assessed two RCTs at low risk of bias for both random sequence generation and allocation concealment (Bowen 2012; Mackenzie 2014). One study, while demonstrating random sequence generation, provided insufficient details to determine adequacy of allocation concealment (Wenke 2010). Two studies provided insufficient details around random sequence generation and allocation concealment and we considered them to have unclear risk of bias without further clarification (Kwon 2015; Xu 2010). All included studies demonstrated adequate matching between randomised groups at baseline with no obvious concerns around risk in this area.
Blinding
Blinded outcome assessment on all measures was clearly described by Bowen 2012 and Mackenzie 2014. It is not clear in Wenke 2010, Xu 2010 or Kwon 2015 whether those involved in the outcome assessments were blind to the intervention. Although it was implied that those carrying out the outcome measures were not involved in the study, reporting was not sufficiently clear for this to be assessed as low risk without further information and evidence that the blinding process was not easy to break.
Incomplete outcome data
Not all studies described completion of intervention, those that did reported a total of 14 (from 112 randomised participants) withdrawals, with no differences between intervention and control group participants (Bowen 2012; Kwon 2015; Mackenzie 2014).
All five studies reported the number of participants lost to some or all of the follow‐up assessments and across all five studies 33 out of the 234 randomised had either no follow up assessment or incomplete follow up assessment. We considered Xu 2010 to be at low risk of bias; there was no attrition from recruitment to follow‐up. Bowen 2012 was assessed as low risk of bias for incomplete data; detailed explanations were provided in the study's data analysis. Missing data from Mackenzie 2014 was discussed with the study authors, who provided additional information about their analysis using imputed results and multiple imputations had made no difference to the findings; we rated this study as low risk of bias. Wenke 2010 reported treating missing data in a standard statistical way; however, implications were not fully addressed and without further information, this study was assessed at high risk of bias. Reporting in Kwon 2015 raised significant concerns about incomplete outcome data: five participants were randomised to both treatment arms, but three withdrew from the active treatment arm and two from the sham treatment. Data for these participants were withdrawn from the study; no intention‐to‐treat analysis was carried out or discussion included around the implications of these withdrawn data on conclusions. We assessed Kwon 2015 at high risk of bias for this domain.
Adherence to intervention and dropout rates by included study are described in Characteristics of included studies.
Selective reporting
Bowen 2012, Mackenzie 2014 and Wenke 2010 reported studies in full with specified outcome measures at specified time points. Bowen 2012 also published a protocol and analyses.
Possible presence of selective reporting was harder to ascertain for Xu 2010 and Kwon 2015. Both studies were assessed at unclear risk of bias for selective reporting. This assessment will be revised following confirmation of methods applied and clarification from the study authors.
Effects of interventions
See: Table 1
See: Table 1
The results of this review are presented below to show the evidence for the objectives of the review. The main objective was to find whether there was an effect on dysarthric speech of any intervention and this is presented below under the three comparisons. In summary there was no evidence of a long‐term effect of the dysarthria intervention on everyday speech compared to any control.
Results are described for comparisons in each outcome.
Dysarthria intervention compared with another intervention, attention control, placebo or no intervention: persisting effects.
Dysarthria Intervention compared with another intervention, attention control, placebo or no intervention: immediate effects.
Dysarthria intervention A versus dysarthria intervention B (whether this is two different interventions or the same intervention with varying timing, duration, and frequency of delivery): persisting and immediate effects.
We included five studies that involved a total of 234 randomised participants. Comparisons were analysed according to our primary outcome of persisting effects of communication at activity level (three RCTs, 116 participants). Comparisons were further analysed for measurement of impairment and participation at immediate and persistent time points. Data were also considered for one subgroup of people with stroke because there were insufficient data for any other clinical subgroups.
We calculated standardised mean difference (SMD) and 95% confidence intervals (CI) because different measures were used of the same underlying construct. We used a random‐effects model.
Comparison 1: dysarthria intervention versus any control: persisting effects (three to nine months post intervention), activity level
We found no evidence of an effect for persisting effects at communication activity level for any control (Bowen 2012; Mackenzie 2014; Wenke 2010; 116 participants): SMD 0.18, (95% CI ‐0.18 to 0.55, Tau² = 0.00; Chi² = 1.47, df = 2, P = 0.48; I² = 0%; GRADE: low quality). Findings were very similar for each study, with narrow CIs, but very small numbers of participants. None of the studies were adequately powered to find an effect (Analysis 1.1). We considered two of the three studies to be at low risk of bias.
1.1. Analysis.
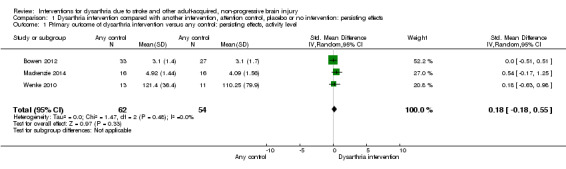
Comparison 1 Dysarthria intervention compared with another intervention, attention control, placebo or no intervention: persisting effects, Outcome 1 Primary outcome of dysarthria intervention versus any control: persisting effects, activity level.
Secondary outcomes of dysarthria intervention versus any control: persisting effects (three to nine months), impairment or participation level
We found no evidence of a persisting effect on impairment level measures in favour of any treatment (Mackenzie 2014; Wenke 2010; 56 participants, SMD 0.07, 95% CI ‐0.91 to 1.06; Tau² = 0.35; Chi² = 3.32, df = 1 (P = 0.07); I² = 70%; GRADE: very low quality). There was substantial heterogeneity between the trials (Analysis 1.2). Both studies had small numbers of participants, and neither study was adequately powered. We considered one study at low risk of bias.
1.2. Analysis.
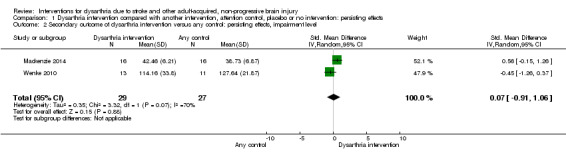
Comparison 1 Dysarthria intervention compared with another intervention, attention control, placebo or no intervention: persisting effects, Outcome 2 Secondary outcome of dysarthria intervention versus any control: persisting effects, impairment level.
These two RCTs (79 participants) found no evidence of a persisting effect at the participation level (Bowen 2012; Mackenzie 2014): SMD ‐0.11 (95% CI ‐0.56 to 0.33) and Heterogeneity: Tau² = 0.00; Chi² = 0.16, df = 1 (P = 0.69); I² = 0%; GRADE: low quality (Analysis 1.3). These two studies have small numbers, they are not adequately powered, and only one has a low risk of bias.
1.3. Analysis.
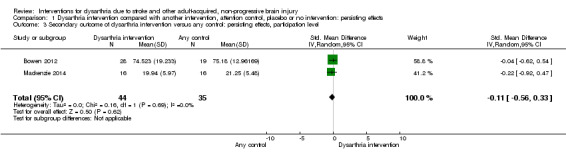
Comparison 1 Dysarthria intervention compared with another intervention, attention control, placebo or no intervention: persisting effects, Outcome 3 Secondary outcome of dysarthria intervention versus any control: persisting effects, participation level.
Sensitivity analysis of dysarthria intervention versus any control (persisting effects, activity level) included two studies with adequate allocation concealment/adequate blinding (Bowen 2012; Mackenzie 2014). The data from the sensitivity analysis of these two studies with 92 participants showed no effect and slight heterogeneity (SMD 0.21, 95% CI ‐0.30 to 0.73, heterogeneity: Tau² = 0.05; Chi² = 1.47, df = 1 (P = 0.23); I² = 32%; GRADE: low quality) (Analysis 1.4).
1.4. Analysis.
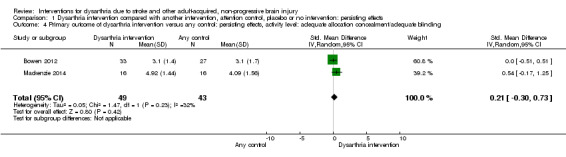
Comparison 1 Dysarthria intervention compared with another intervention, attention control, placebo or no intervention: persisting effects, Outcome 4 Primary outcome of dysarthria intervention versus any control: persisting effects, activity level: adequate allocation concealment/adequate blinding.
Only one of the studies had a comparison of dysarthria intervention versus attention control with a measure of persisting effects at the activity level. This one study with 60 participants (SMD 0.00, 95% CI ‐0.51 to 0.51), indicated no evidence of an effect when comparing the intervention with an attention control (Bowen 2012) (Analysis 1.5).
1.5. Analysis.
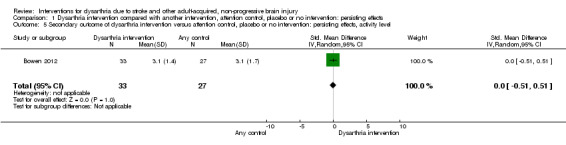
Comparison 1 Dysarthria intervention compared with another intervention, attention control, placebo or no intervention: persisting effects, Outcome 5 Secondary outcome of dysarthria intervention versus attention control, placebo or no intervention: persisting effects, activity level.
The stroke subgroup for comparison 1 included three studies (Bowen 2012; Mackenzie 2014; Wenke 2010; 106 participants) and showed no evidence of effect (SMD 0.16, 95% CI ‐0.23 to 0.54, Chi² = 1.61, df = 2, P = 0.45; I² = 0%; GRADE: low quality; Analysis 1.6).
1.6. Analysis.
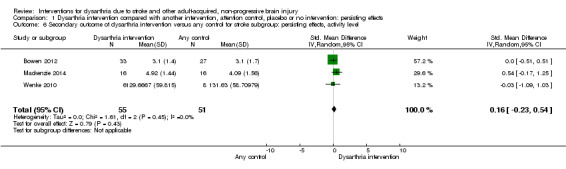
Comparison 1 Dysarthria intervention compared with another intervention, attention control, placebo or no intervention: persisting effects, Outcome 6 Secondary outcome of dysarthria intervention versus any control for stroke subgroup: persisting effects, activity level.
Comparison 2: dysarthria intervention compared with another intervention, attention control, placebo or no intervention: immediate effects at activity, impairment and participation level
Three included studies, with 117 participants, had measures of activity level immediately post intervention but found no evidence of an effect: (SMD 0.29, 95% CI ‐0.07 to 0.66) (Mackenzie 2014; Wenke 2010; Xu 2010). The heterogeneity among studies was low but included very small numbers (Chi² = 0.64, df = 2 (P = 0.73); I² = 0%) GRADE: very low quality) (Analysis 2.1).
2.1. Analysis.
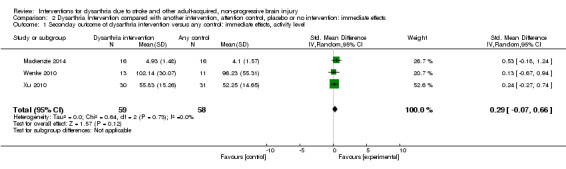
Comparison 2 Dysarthria Intervention compared with another intervention, attention control, placebo or no intervention: immediate effects, Outcome 1 Seconday outcome of dysarthria intervention versus any control: immediate effects, activity level.
Four studies measured impairment level immediately post intervention (Kwon 2015; Mackenzie 2014; Wenke 2010; Xu 2010). These studies had a total of 99 participants, so each included small numbers of participants but there was a statistically significant effect favouring intervention (P value = 0.04), SMD 0.47 (95% CI 0.02 to 0.92) with low heterogeneity (Chi² = 0.73, df = 2 (P = 0.69); I² = 0%). Only one study was low risk of bias, GRADE: very low quality (Analysis 2.2).
2.2. Analysis.
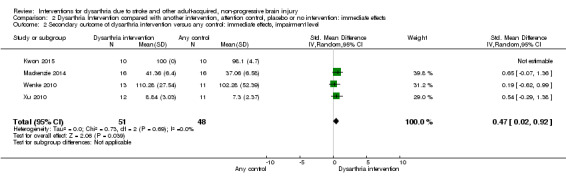
Comparison 2 Dysarthria Intervention compared with another intervention, attention control, placebo or no intervention: immediate effects, Outcome 2 Secondary outcome of dysarthria intervention versus any control: immediate effects, impairment level.
One study measured participation level immediately post intervention (Mackenzie 2014). This single study had 32 participants: SMD ‐0.24 (95% CI ‐0.94 to 0.45) indicating no effect of the intervention (Analysis 2.3).
2.3. Analysis.
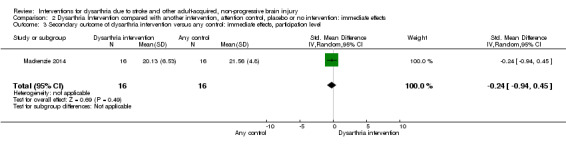
Comparison 2 Dysarthria Intervention compared with another intervention, attention control, placebo or no intervention: immediate effects, Outcome 3 Secondary outcome of dysarthria intervention versus any control: immediate effects, participation level.
Comparison 3: dysarthria intervention A versus dysarthria intervention B: persisting and immediate effects at activity, impairment and participation level
Due to the small number of studies in this review there are only two comparisons in this section that have not already been carried out in the earlier analysis. It may be possible to populate this section more fully in the future as more trials are carried out.
Analysis 3.1 included two studies of 56 participants comparing intervention A versus B, with a measure of persisting effects at the activity level: SMD 0.38 (95% CI ‐0.15 to 0.91) indicating no effect of intervention (Mackenzie 2014; Wenke 2010). These studies have low heterogeneity (Heterogeneity: Tau² = 0.00; Chi² = 0.43, df = 1 (P = 0.51); I² = 0%; GRADE: very low quality).
3.1. Analysis.
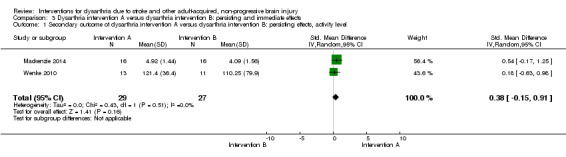
Comparison 3 Dysarthria intervention A versus dysarthria intervention B: persisting and immediate effects, Outcome 1 Secondary outcome of dysarthria intervention A versus dysarthria intervention B: persisting effects, activity level.
The second analysis of intervention A versus intervention B that has a measure of persisting effect at the participation level included one study: Mackenzie 2014. This study has 32 participants: SMD ‐0.22 (95% CI ‐0.92 to 0.47) and indicates no effect of the intervention (Analysis 3.2).
3.2. Analysis.
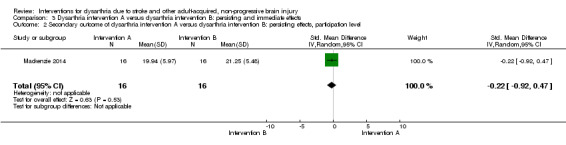
Comparison 3 Dysarthria intervention A versus dysarthria intervention B: persisting and immediate effects, Outcome 2 Secondary outcome of dysarthria intervention A versus dysarthria intervention B: persisting effects, participation level.
We would also have carried out analysis on intervention A versus intervention B, persisting effects at the impairment level but this has been carried out in Analysis 1.2.
We would have looked at intervention A versus intervention B, immediate effects; activity level (Analysis 2.1), impairment level (Analysis 2.2), participation level (Analysis 2.3) but these have already been carried out in the earlier comparisons.
Discussion
We examined the effectiveness of dysarthria interventions for people with speech problems due to stroke and other adult‐acquired, non‐progressive brain injury. We have built on the work of Sellars 2005 presented in the previous version of this review, by amending and updating objectives and review outcomes to reflect a more global perspective, and to consider new evidence. We considered whether dysarthria interventions were effective when compared with any control, whether the dysarthria intervention was more effective than an attention control, whether one type of dysarthria intervention was more effective than another, or whether one type of dysarthria intervention was more effective than the same intervention when delivered in a different way. We included five studies and presented data from 234 randomised participants.
Summary of main results
See: Table 1. Meta‐analyses demonstrated no evidence of a statistically significant persisting effect of dysarthria intervention compared with any control when communication was measured at either the activity (three studies, 116 participants), impairment (two studies, 56 participants), or participation level (two studies, 79 participants). This lack of effect did not change in the sensitivity analyses of only the studies with a low risk of bias (two studies, 92 participants), when the analysis was restricted to those with an attention control/placebo (one study, 60 participants), or to the subgroup of those with an underlying condition of stroke (three trials, 106 participants). Similarly, there was no evidence for the immediate effect of dysarthria intervention at the activity level (three studies, 117 participants) or participation level (one study, 32 participants). The one significant finding at the impairment level immediately post‐intervention, (four trials, 99 participants) means that clinically there may be some improvement of tongue and lip movement for example but there is no evidence that these persist long‐term and the very small numbers and very low quality of the evidence make this an uncertain estimate.
Key findings from this review
Despite one positive finding, there was insufficient evidence to enable firm conclusions to be drawn due to quality of the evidence.
Evidence quality was graded as low or very low.
There was low risk of bias in only two studies.
There was no consensus on outcome measures or time points for measurement.
Overall completeness and applicability of evidence
We only identified five, small trials which indicates the evidence base is limited. In addition to the limited number of trials there were only small numbers of participants within the trials and there were also issues around quality and risk of bias. There is clearly much more that needs to be done before the objectives of the review can be fully addressed. The wide variety of outcome measures, where none of the five trials used any of the same outcome measures, indicates a need for consensus amongst researchers, people with dysarthria and clinicians to identify which measures should be used in future research. However the included studies were all relevant to the review question in that they were all RCTs of dysarthria intervention for stroke and brain injury. The review set out to establish the evidence for all clinical groups who may have dysarthria but we found no RCTs for other types of non‐progressive brain injury that may cause dysarthria. One of the studies excluded people with severe dysarthria and one did not report severity so generalisation to the wider dysarthric population could be affected.
There were variable amounts of information relating to intervention and control description and replicability according to the TIDieR checklist that we used when evaluating the studies (Hoffmann 2014). In two of the studies this was clearly described in sufficient detail for replication (Bowen 2012; Mackenzie 2014). There was less detail in Wenke 2010, although the LSVT intervention used in this study cannot be described as the treatment is trademarked and not available publicly. Xu 2010 gave minimal information about the usual care interventions in both arms, and this could not be replicated from the information given but they provided much more detail about the acupuncture delivery. Kwon 2015 gave detail around the transcranial magnetic stimulation intervention and how the sham/attention control was carried out. There was no detail around the speech therapy that was given to both groups to ensure they had the same treatment alongside the transcranial magnetic stimulation intervention and sham. There was variation in reporting whether the intervention was provided as intended by the protocol and this is detailed in Characteristics of included studies . Fidelity of the intervention and how this was monitored was not described in Wenke 2010, Xu 2010 or Kwon 2015 which is important when considering applicability of the evidence. Fidelity to the interventions and attention control was described in detail, including information about how this was monitored, who carried this out, when and how, in Bowen 2012 and Mackenzie 2014. Whether participants completed the intervention in the arm to which they were allocated was described in Bowen 2012 and Mackenzie 2014. Current practice in the UK around rehabilitation continues to focus on early intervention and the review included three studies of early intervention whereas the other two considered intervention with a chronic population.
Quality of the evidence
This review shows that we do not have a robust enough body of evidence to draw firm conclusions about the objectives of this review. It is a measure of progress that there were recent studies that could be included in the meta‐analyses however we rated evidence quality for the key outcomes as low or very low (Table 1). The primary objective of this review was reported by only three of the studies (116 participants; Analysis 1.1). However, none of the three studies were adequately powered to enable comparisons of the interventions because of the small numbers of participants. Bowen 2012, while adequately powered to look at early communication intervention in aphasia and dysarthria, was not adequately powered to evaluate dysarthria intervention only. All secondary outcomes were downgraded due to small participant numbers and imprecision.
Only Bowen 2012 and Mackenzie 2014 had low risk of bias; the other three studies all had areas of unclear risk or high risk. We carried out sensitivity analyses to remove any studies with high or unclear risk of bias but this did not alter the direction or the significance of the results (Analysis 1.4). The one significant finding was from four studies where we considered the overall quality of the evidence to be very low, which raises concerns around how confident we can feel about this estimate of effect (Analysis 2.2). The main message about the quality of the evidence found in this review is that, in addition to being adequately powered, the reporting of RCTs must adhere to the CONSORT guidelines (Schulz 2010) and follow the template for intervention description and replication (TIDieR; Hoffmann 2014).
Potential biases in the review process
The search strategy was broadened for this review to include trials that may have been carried out by a range of professionals or non‐professionals and we felt confident that we used search terms to reflect this broad scope. However, not knowing what potential professional or non‐professional groups may be carrying out research may introduce the possibility of bias particularly where unpublished literature or ongoing trials were sought, as only those who have worked or are working in the field of dysarthria were approached.
The search strategy was in line with this broad approach and we documented reasons for study exclusions. We carried out searches with no time restrictions: the searches were all carried out in English language databases, and although we imposed no language restrictions, and had a paper published in Chinese (Xu 2010) translated, this may have restricted our search method. It is highly probable that papers published in other languages were not identified, and this review may be biased toward English‐speaking research studies. Xu 2010 was published in Chinese and data extraction was carried out by two independent Chinese‐speaking individuals, but neither were involved in the review team; discrepancies with data extraction may have occurred. There was some need for interpretation of information, which may not be entirely as intended by the author. Where clarification could not be obtained from study authors, it is possible that information may have been interpreted incorrectly, and that the review is biased until information can be clarified.
Data collection was carried out by individual review authors and then compared in an attempt to reduce any bias around particular methodologies or intervention approaches. To ensure risk of bias judgements were carried out fairly this was considered independently and then compared and discussed by the review team.
The review team was conscious that a review author (AB) was also the lead author of an included study. We considered how to approach this before starting the review, should the study be eligible for inclusion. The review was structured to ensure the study author was not involved in assessing or making judgements about her own study. However, AB provided additional information and data when requested, and contributed her opinion to wider discussions where this was relevant. We were very conscious of the potential for bias in this particular situation and took steps to reduce bias as much as possible.
Agreements and disagreements with other studies or reviews
A previous Cochrane review of dysarthria intervention found no suitable studies for inclusion at that time (Sellars 2005). There are no other systematic reviews of non‐progressive dysarthria.
Authors' conclusions
Implications for practice.
Research evidence is not yet sufficiently robust to guide clinical practice. It is therefore important for clinicians to continue to offer rehabilitation for people with dysarthria in line with current clinical guidelines.
Implications for research.
Further research will need to be appropriately designed to avoid risk of bias, and evaluate persisting effects on activity level measures.
The absence of evidence for dysarthria interventions highlights the paucity of research for this distressing condition, and need for adequately‐powered, methodologically‐sound and well‐reported studies.
Although inclusion of five studies (from none 10 years ago) is to be celebrated, much more needs to be done. Dysarthria research activity is in striking contrast to aphasia research, which has now amassed 57 trials of speech and language therapy interventions for aphasia following stroke (Brady 2016).
Future dysarthria trials should clearly report methods governing randomisation, allocation concealment, clarity around attrition, and include evidence of full reporting of all outcomes. Where possible, blinding of outcome assessment is desirable, but is not always possible to achieve in rehabilitation research. When considering methodological approaches, researchers may want to consider a range of control groups such as comparing interventions with no treatment, or alternative treatment, or an attention control. These control arms answer different but important questions.
It is important to consider follow‐up and intention‐to‐treat analysis: these are important factors in minimising bias.
Rehabilitation trialists will find it helpful to adhere to the CONSORT guidelines for all future studies. Future definitive trials must have adequate statistical power to detect clinically meaningful differences and this may be informed by feasibility and pilot trials.
It would be helpful if researchers could agree core outcome sets and timing of measurements. Interventions should be clearly described and replicable, and researchers would benefit from adherence to the TIDieR checklist.
Future studies should include patients' and carers' views on the available interventions and the most meaningful way of measuring treatment effects. Patients' and carers' views on acceptability of available interventions and acceptability measures (adherence or satisfaction scales) should be considered in future studies. The involvement of patients and carers in commissioning and designing research would greatly increase the quality of the research discussion especially related to potential interventions and possible outcome measures. We found no studies considering timing, intensity, and duration of interventions, which are concepts of clinical importance that need to be considered in future research.
What's new
| Date | Event | Description |
|---|---|---|
| 12 May 2016 | New citation required and conclusions have changed | This updated review has found that although the evidence was not robust enough to indicate whether one treatment was better than another it does describe future research directions in more detail. |
| 12 May 2016 | New search has been performed | The review title and scope of searches have been updated since the last review. The review objectives have also been amended since the review was last published. The previous review found no studies suitable for inclusion. Five new studies (234 participants) have been included in the review. This review includes risk of bias assessment, grading of the quality of evidence and a 'Summary of findings' table. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 1 April 2015 | Amended | Amendments to update the protocol agreed with the Cochrane Stroke Group Editorial Board |
| 3 December 2014 | Amended | New first author and co‐author team with previous lead author remaining involved |
| 2 October 2008 | Amended | Converted to new review format. |
| 4 February 2005 | New search has been performed | All literature searches for this review have been updated. No new trials for inclusion have been uncovered by these searches. |
Acknowledgements
Cameron Sellars, Thomas Hughes and Peter Langhorne, authors of the original review, and the contribution this review made to the field.
Hazel Fraser, Cochrane Stroke Group Managing Editor, for her support and suggestions as well as providing us with details of trials from the Cochrane Stroke Group's Trials Register.
Brenda Thomas, Cochrane Stroke Group Information Specialist, for support in reviewing the search strategy.
Jo Whitcombe (Clinical Outreach Librarian), Naomi Leech (Assistant Librarian) and Steven Glover (Head of Library Services), Central Manchester University Hospitals NHS Foundation Trust for writing and carrying out the search strategies.
Trialists who responded to emails and provided various additional information.
Xu Xiaoguang, statistician at the University of Manchester, who translated and extracted information from the Chinese study and contacted the author.
Luo Haiying, Chinese speaker, who translated and extracted information from the Chinese study.
Dr Emma Patchick who helped to track down various potential papers, abstracts and theses for the review.
Thanks to Brian Stafford, Cochrane consumer reviewer, who provided helpful and considered comments from a lay‐person's perspective, which shaped the final review, particularly the plain language summary.
Thanks to Joshua Cheyne, Cochrane Stroke Group Information Specialist, who provided helpful comments and supported further detailed guidance on the search strategies.
Thanks to the Cochrane Stroke Group editors and referees who provided detailed, helpful comments on the draft version of this review, in particular Peter Langhorne, Valentina Assi, and Marian Brady.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
Cochrane Library databases (CDSR, DARE, CENTRAL, HTA) searched to May 2016
1. MeSH descriptor: [Cerebrovascular Disorders] this term only
2. MeSH descriptor: [Basal Ganglia Cerebrovascular Disease] explode all trees
3. MeSH descriptor: [Brain Ischemia] explode all trees
4. MeSH descriptor: [Carotid Artery Diseases] explode all trees
5. MeSH descriptor: [Cerebrovascular Trauma] explode all trees
6. MeSH descriptor: [Intracranial Arteriovenous Malformations] explode all trees
7. MeSH descriptor: [Intracranial Arterial Diseases] explode all trees
8. MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees
9. MeSH descriptor: [Intracranial Hemorrhages] explode all trees
10. MeSH descriptor: [Stroke] this term only
11. MeSH descriptor: [Brain Infarction] explode all trees
12. MeSH descriptor: [Stroke, Lacunar] this term only
13. MeSH descriptor: [Vasospasm, Intracranial] this term only
14. MeSH descriptor: [Vertebral Artery Dissection] this term only
15. MeSH descriptor: [Hypoxia, Brain] explode all trees
16. stroke* or "post stroke" or poststroke or post‐stroke or apoplex* or cerebrovasc* or CVA or SAH or "cerebral vasc*" (Word variations have been searched)
17. (brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or "middle cerebr*" or mca* or "anterior circulaion" or "basilar artery" or "vertebral artery") and (ischaemi* or ischemi* or thrombos* or thromboem* or emboli* or occlus* or hypoxi*) (Word variations have been searched)
18. (brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or "basal gangli*" or putaminal or putamen or "posterior fossal" or hemisphere* or subarachnoid) and (haemorrhag* or hemorrhag* or haematoma* or bleed*) (Word variations have been searched)
19. MeSH descriptor: [Hemiplegia] explode all trees
20. MeSH descriptor: [Paresis] explode all trees
21. MeSH descriptor: [Aphasia] explode all trees
22. MeSH descriptor: [Gait Disorders, Neurologic] explode all trees
23. (hemipar* or hemipleg* or paresis or paretic or aphasi* or dysphasi*) (Word variations have been searched)
24. MeSH descriptor: [Brain Damage, Chronic] explode all trees
25. MeSH descriptor: [Brain Injuries] this term only
26. MeSH descriptor: [Brain Concussion] explode all trees
27. MeSH descriptor: [Brain Hemorrhage, Traumatic] explode all trees
28. MeSH descriptor: [Brain Injury, Chronic] this term only
29. MeSH descriptor: [Diffuse Axonal Injury] this term only
30. MeSH descriptor: [Craniocerebral Trauma] this term only
31. MeSH descriptor: [Head Injuries, Closed] explode all trees
32. MeSH descriptor: [Intracranial Hemorrhage, Traumatic] explode all trees
33. MeSH descriptor: [Brain Abscess] explode all trees
34. MeSH descriptor: [Central Nervous System Infections] explode all trees
35. MeSH descriptor: [Encephalitis] explode all trees
36. MeSH descriptor: [Meningitis] explode all trees
37. (encephalitis or meningitis or "head injur*") (Word variations have been searched)
38. MeSH descriptor: [Brain Neoplasms] explode all trees
39. (brain or cerebr*) and (injur* or hypoxi* or damage* or concussion or trauma* or neoplasm* or lesion* or tumor* or tumour* or cancer* or infection) (Word variations have been searched)
40.{or #1‐#39}
41. MeSH descriptor: [Dysarthria] this term only
42. MeSH descriptor: [Articulation Disorders] this term only
43. MeSH descriptor: [Speech Articulation Tests] this term only
44. MeSH descriptor: [Speech Disorders] this term only
45. MeSH descriptor: [Voice Disorders] this term only
46. MeSH descriptor: [Aphonia] this term only
47. MeSH descriptor: [Dysphonia] this term only
48. MeSH descriptor: [Communication Disorders] this term only
49. (dysarth* or dysphon* or anarth* or dyspros* or aphon* or dysfluen* or stutter* or stammer*) (Word variations have been searched)
50. (speech or articul* or disarticul* or phonat* or phonolog* or voice or vocal or prosod* or intonat* or respirat* or communicat* or fluen*) and (disorder* or impair* or problem* or difficult*) (Word variations have been searched)
51. speech and (slow* or weak* or imprecis* or intelligibil* or unintelligibil* or accuracy or fatigue) (Word variations have been searched)
52. {or #41‐51}
53. MeSH descriptor: [Mouth] explode all trees
54. MeSH descriptor: [Larynx] explode all trees
55. MeSH descriptor: [Laryngeal Muscles] explode all trees
56. MeSH descriptor: [Pharynx] explode all trees
57. MeSH descriptor: [Pharyngeal Muscles] explode all trees
58. MeSH descriptor: [Facial Muscles] this term only
59. MeSH descriptor: [Palatal Muscles] this term only
60. (mouth or tongue or lingual or palat* or laryn* or pharyn* or orofacial or oro‐facial or "face musc*" or facial musc*) (Word variations have been searched)
61. {or #53‐#60}
62. MeSH descriptor: [Movement Disorders] this term only
63. MeSH descriptor: [Ataxia] this term only
64. MeSH descriptor: [Dystonia] this term only
65. MeSH descriptor: [Dystonic Disorders] this term only
66. MeSH descriptor: [Hyperkinesis] this term only
67. MeSH descriptor: [Hypokinesia] explode all trees
68. MeSH descriptor: [Muscle Hypertonia] this term only
69. MeSH descriptor: [Muscle Hypotonia] this term only
70. MeSH descriptor: [Muscle Weakness] this term only
71. MeSH descriptor: [Muscular Diseases] this term only
72. MeSH descriptor: [Muscle Spasticity] this term only
73.( atax* or dyston* or hyperkin* or hypokin* or hypoton* or hyperton* or flaccid* or spastic*) (Word variations have been searched)
74. {or #62‐#73}
75. #61 and #74
Appendix 2. MEDLINE (PubMed) search strategy
MEDLINE (PubMed) from 1946 to May 2016
1. Search (("Cerebrovascular Disorders"[Mesh:noexp]) OR "Basal Ganglia Cerebrovascular Disease"[Mesh]) OR "Brain Ischemia"[Mesh]) OR "Carotid Artery Diseases"[Mesh]) OR "Cerebrovascular Trauma"[Mesh]) OR "Intracranial Arteriovenous Malformations"[Mesh]) OR "Intracranial Arterial Diseases"[Mesh]) OR "Intracranial Embolism and Thrombosis"[Mesh]) OR "Intracranial Hemorrhages"[Mesh]) OR "Stroke"[Mesh:noexp]) OR "Brain Infarction"[Mesh]) OR "Stroke, Lacunar"[Mesh:noexp]) OR "Vasospasm, Intracranial"[Mesh:noexp]) OR "Vertebral Artery Dissection"[Mesh:noexp]) OR "Hypoxia, Brain"[Mesh])
2. Search (stroke*[Text Word] OR "post stroke"[Text Word] OR poststroke[Text Word] OR post‐stroke[Text Word] OR apoplex*[Text Word] OR cerebrovasc*[Text Word] OR CVA[Text Word] OR SAH[Text Word] OR cerebral vasc*[Text Word])
3. Search ((brain[Text Word] OR cerebr*[Text Word] OR cerebell*[Text Word] OR vertebrobasil*[Text Word] OR hemispher*[Text Word] OR intracran*[Text Word] OR intracerebral[Text Word] OR infratentorial[Text Word] OR supratentorial[Text Word] OR middle cerebr*[Text Word] OR mca*[Text Word] OR anterior circulation[Text Word] OR basilar artery[Text Word] OR vertebral artery[Text Word])) AND (Ischemi*[Text Word] OR infarct*[Text Word] OR thrombos*[Text Word] OR thromboem*[Text Word] OR emboli*[Text Word] OR occlus*[Text Word] OR hypoxi*[Text Word]))
4. Search (((Brain*[Text Word] OR cerebr*[Text Word] OR cerebell*[Text Word] OR intracerebral[Text Word] OR intracran*[Text Word] OR parenchymal[Text Word] OR intraparenchymal[Text Word] OR intraventricular[Text Word] OR infratentorial[Text Word] OR supratentorial[Text Word] OR basal gangli*[Text Word] OR putaminal[Text Word] OR putamen[Text Word] OR posterior fossa[Text Word] OR hemisphere*[Text Word] OR subarachnoid[Text Word])) AND (haemorrhag*[Text Word] OR hemorrhag*[Text Word] OR haematoma*[Text Word] OR hematoma*[Text Word] OR bleed*[Text Word]))
5. Search (("Hemiplegia"[Mesh]) OR "Paresis"[Mesh]) OR "Aphasia"[Mesh]) OR "Gait Disorders, Neurologic"[Mesh])
6. Search (Hemipar*[Text Word] OR hemipleg*[Text Word] OR paresis[Text Word] OR paretic[Text Word] OR aphasi*[Text Word] OR dysphasi*[Text Word])
7. Search (("Brain Damage, Chronic"[Mesh]) OR "Brain Injuries"[Mesh:noexp]) OR "Brain Concussion"[Mesh]) OR "Brain Hemorrhage, Traumatic"[Mesh]) OR "Brain Injury, Chronic"[Mesh:noexp]) OR "Diffuse Axonal Injury"[Mesh:noexp])
8. Search (("Craniocerebral Trauma"[Mesh:noexp]) OR "Head Injuries, Closed"[Mesh]) OR "Intracranial Hemorrhage, Traumatic"[Mesh])
9. Search (("Brain Abscess"[Mesh]) OR "Central Nervous System Infections"[Mesh]) OR "Encephalitis"[Mesh]) OR "Meningitis"[Mesh])
10. Search (encephalitis[Text Word] OR meningitis[Text Word] OR head injur*[Text Word])
11. Search "Brain Neoplasms"[Mesh]
12. Search (((brain[Text Word] OR cerebr*[Text Word])) AND (injur*[Text Word] OR hypoxi*[Text Word] OR damage*[Text Word] OR concussion[Text Word] OR trauma*[Text Word] OR neoplasm*[Text Word] OR lesion*[Text Word] OR tumor*[Text Word] OR tumour*[Text Word] OR cancer*[Text Word] OR infection[Text Word]))
13. Search (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12)
14. Search (("Dysarthria"[Mesh:noexp]) OR "Articulation Disorders"[Mesh:noexp]) OR "Speech Articulation Tests"[Mesh:noexp])
15. Search ("Speech Disorders"[Mesh:noexp]) OR "Voice Disorders"[Mesh:noexp]) OR "Aphonia"[Mesh:noexp]) OR "Dysphonia"[Mesh:noexp]) OR "Communication Disorders"[Mesh:noexp])
16. Search (dysarth*[Text Word] OR dysphon*[Text Word] OR anarth*[Text Word] OR dyspros*[Text Word] OR aphon*[Text Word] OR dysfluen*[Text Word] OR stutter*[Text Word] OR stammer*[Text Word])
17. Search (((speech[Text Word] OR articul*[Text Word] OR disarticul*[Text Word] OR phonat*[Text Word] OR phonolog*[Text Word] OR voice[Text Word] OR vocal[Text Word] OR prosod*[Text Word] OR intonat*[Text Word] OR respirat*[Text Word] OR communicat*[Text Word] OR fluen*[Text Word])) AND (disorder*[Text Word] OR impair*[Text Word] OR problem*[Text Word] OR difficult*[Text Word]))
18. Search (speech[Text Word]) AND (slow*[Text Word] OR weak*[Text Word] OR imprecis*[Text Word] OR intelligibil*[Text Word] OR unintelligibil*[Text Word] OR accuracy[Text Word] OR fatigue[Text Word])
19. Search ("Mouth"[Mesh]) OR "Larynx"[Mesh]) OR "Laryngeal Muscles"[Mesh]) OR "Pharynx"[Mesh:noexp]) OR "Pharyngeal Muscles"[Mesh]) OR "Facial Muscles"[Mesh:noexp]) OR "Palatal Muscles"[Mesh:noexp])
20. Search (mouth[Text Word] OR tongue[Text Word] OR lingual[Text Word] OR palat*[Text Word] OR laryn*[Text Word] OR pharyn*[Text Word] OR orofacial[Text Word] OR oro‐facial[Text Word] OR face musc*[Text Word] OR facial musc*[Text Word])
21. Search (#19 OR #20)
22. Search ("Movement Disorders"[Mesh:noexp]) OR "Ataxia"[Mesh:noexp]) OR "Dystonia"[Mesh:noexp]) OR "Dystonic Disorders"[Mesh:noexp]) OR "Hyperkinesis"[Mesh:noexp]) OR "Hypokinesia"[Mesh:noexp]) OR "Muscle Hypertonia"[Mesh:noexp]) OR "Muscle Hypertonia"[Mesh]) OR "Muscle Hypotonia"[Mesh:noexp]) OR "Muscle Weakness"[Mesh:noexp]) OR "Muscular Diseases"[Mesh:noexp]) OR "Muscle Spasticity"[Mesh:noexp])
23. Search (atax*[Text Word] OR dyston*[Text Word] OR hyperkin*[Text Word] OR hypokin*[Text Word] OR hypoton*[Text Word] OR hyperton*[Text Word] OR flaccid*[Text Word] OR spastic*[Text Word])
24. Search (#22 OR #23)
25. Search (#21 AND #24)
26. Search (#14 OR #15 OR #16 OR #17 OR #18 OR #25)
27. Search "Randomized Controlled Trials as Topic"[Mesh:noexp]
28. Search "Random Allocation"[Mesh:noexp]
29. Search "Controlled Clinical Trials as Topic"[Mesh:noexp]
30. Search "Control Groups"[Mesh:noexp]
31. Search ("Clinical Trials as Topic"[Mesh:noexp]) OR "Clinical Trials, Phase I as Topic"[Mesh:noexp]) OR "Clinical Trials, Phase II as Topic"[Mesh:noexp]) OR "Clinical Trials, Phase III as Topic"[Mesh:noexp]) OR "Clinical Trials, Phase IV as Topic"[Mesh:noexp])
32. Search "Double‐Blind Method"[Mesh:noexp]
33. Search "Single‐Blind Method"[Mesh:noexp]
34. Search "Placebos"[Mesh:noexp]
35. Search "Placebo Effect"[Mesh:noexp]
36. Search "Cross‐Over Studies"[Mesh:noexp]
37. Search randomized controlled trial[Publication Type]
38. Search controlled clinical trial[Publication Type]
39. Search (clinical trial[Publication Type] OR clinical trial, phase i[Publication Type] OR clinical trial, phase ii[Publication Type] OR clinical trial, phase iii[Publication Type] OR clinical trial, phase iv[Publication Type])
40. Search (random*[Text Word] OR RCT[Text Word] OR RCTs[Text Word])
41. Search (controlled[Text Word]) AND (trial*[Text Word] OR stud*[Text Word])
42. Search (clinical*[Text Word] AND trial*[Text Word])
43. Search (control[Text Word] OR treatment[Text Word] OR experiment*[Text Word] OR intervention[Text Word])) AND (group*[Text Word] OR subject*[Text Word] OR patient*[Text Word])
44. Search (quasi‐random*[Text Word] OR quasi random*[Text Word] OR pseudo‐random*[Text Word] OR pseudo random*[Text Word])
45. Search (control[Text Word] OR experiment*[Text Word] OR conservative[Text Word])) AND (treatment[Text Word] OR therapy[Text Word] OR procedure[Text Word] OR manage*[Text Word])
46. Search (singl*[Text Word] OR doubl*[Text Word] OR tripl*[Text Word] OR trebl*[Text Word])) AND (blind*[Text Word] OR mask*[Text Word])
47. Search (cross‐over[Text Word]) OR cross over[Text Word]) OR crossover[Text Word])
48. Search (placebo*[Text Word] OR sham[Text Word])
49. Search trial[Title]
50. Search (assign*[Text Word] OR allocat*[Text Word])
51. Search controls[Text Word]
52. Search (#27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51)
53. Search (#13 AND #26 AND #52)
54. Search ("Animals"[Mesh]) NOT "Humans"[Mesh:noexp])
55. Search (#53 NOT #54)
Appendix 3. Embase (Ovid) search strategy
Embase (Ovid) from 1974 to May 2016
1. CEREBROVASCULAR DISEASE/ or exp BASAL GANGLION DISEASE/ or exp BASAL GANGLION HEMORRHAGE/ or exp BRAIN ISCHEMIA/ or exp CAROTID ARTERY DISEASE/ or exp CEREBROVASCULAR ACCIDENT/ or exp CEREBRAL ARTERY DISEASE/ or exp BRAIN ARTERIOVENOUS MALFORMATION/ or exp BRAIN EMBOLISM/ or exp OCCLUSIVE CEREBROVASCULAR DISEASE/ or exp BRAIN HEMORRHAGE/ or exp BRAIN INFARCTION/ or LACUNAR STROKE/ or STROKE/ or BRAIN VASOSPASM/ or ARTERY DISSECTION/ or exp BRAIN HYPOXIA/
2. (stroke$ or post stroke or poststroke or post‐stroke or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or SAH).ti,ab
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation or basilar artery or vertebral artery) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).ti,ab.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).ti,ab
5. exp HEMIPLEGIA/ or exp PARESIS/ or exp APHASIA/ or exp NEUROLOGIC GAIT DISORDER/
6. (hemipar$ or hemipleg$ or paresis or paretic or aphasi$ or dysphasi$).ti,ab
7. exp BRAIN DAMAGE, CHRONIC/ or BRAIN INJURY/ or exp BRAIN CONCUSSION/ or exp BRAIN HAEMORRHAGE, TRAUMATIC/ or BRAIN INJURY, CHRONIC/ or DIFFUSE AXONAL INJURY/
8. HEAD INJURY/ or exp HEAD INJURIES, CLOSED/ or exp INTRACRANIAL HEMORRHAGE, TRAUMATIC/
9. exp BRAIN ABSCESS/ or exp CENTRAL NERVOUS SYSTEM INFECTION/ or exp ENCEPHALITIS/ or exp MENINGITIS
10. (encephalitis or meningitis or head injur$).ti,ab.
11. exp BRAIN TUMOR/
12. ((brain or cerebr$) adj5 (injur$ or hypoxi$ or damage$ or concussion or trauma$ or neoplasm$ or lesion$ or tumor$ or tumour$ or cancer$ or infection$)).ti,ab.
13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12
14. DYSARTHRIA/ or SPEECH SOUND DISORDER/ or SPEECH ARTICULATION TESTS/
15. SPEECH DISORDER/ or VOICE DISORDER/ or APHONIA/ or DYSPHONIA/ or COMMUNICATION DISORDER/
16. (dysarth$ or dysphon$ or anarth$ or dyspros$ or aphon$ or dysfluen$ or stutter$ or stammer$).ti,ab
17. ((speech or articul$ or disarticul$ or phonat$ or phonolog$ or voice or vocal or prosod$ or intonat$ or respirat$ or communicat$ or fluen$) adj5 (disorder$ or impair$ or problem$ or difficult$)).ti,ab
18. (speech adj5 (slow$ or weak$ or imprecis$ or intelligibil$ or unintelligibil$ or accuracy or fatigue)).ti,ab
19. exp MOUTH/ or exp LARYNX/ or exp LARYNX MUSCLE/ or PHARYNX/ or exp PHARYNGEAL MUSCLE/ or FACE MUSCLE/ or PALATE/
20. (mouth or tongue or lingual or palat$ or laryn$ or pharyn$ or orofacial or oro‐facial or face musc$ or facial musc$).ti,ab
21. 19 or 20
22. MOTOR DYSFUNCTION/ or ATAXIA/ or DYSTONIC DISORDER/ or HYPERKINESIA/ or HYPOKINESIA/ or MUSCLE HYPOTONIA/ or exp MUSCLE HYPOTONIA/ or MUSCLE WEAKNESS/ or MUSCLE DISEASE/ or SPASTICITY/
23. (atax$ or dyston$ or hyperkin$ or hypokin$ or hypoton$ or hyperton$ or flaccid$ or spastic$).ti,ab
24. 22 or 23
25. 21 and 24
26. 14 or 15 or 16 or 17 or 18 or 25
27. "RANDOMIZED CONTROLLED TRIAL (TOPIC)"/
28. RANDOMIZATION/
29. "CONTROLLED CLINICAL TRIAL (TOPIC)"/
30. CONTROL GROUP/
31. "CLINICAL TRIAL (TOPIC)"/ or "PHASE 1 CLINICAL TRIAL (TOPIC)"/ or "PHASE 2 CLINICAL TRIAL (TOPIC)"/ or "PHASE 3 CLINICAL TRIAL (TOPIC)"/ or "PHASE 4 CLINICAL TRIAL (TOPIC)"/
32. DOUBLE BLIND PROCEDURE/
33. SINGLE BLIND PROCEDURE/
34. PLACEBO/
35. PLACEBO EFFECT/
36. CROSSOVER PROCEDURE/
37. RANDOMIZED CONTROLLED TRIAL/
38. CLINICAL TRIAL/
39. PHASE 1 CLINICAL TRIAL/ or PHASE 2 CLINICAL TRIAL/ or PHASE 3 CLINICAL TRIAL/ or PHASE 4 CLINICAL TRIAL/
40. (random$ or RCT or RCTs).ti,ab
41. (controlled adj5 (trial$ or stud$)).ti,ab
42. (clinical$ adj5 trial$).ti,ab.
43. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).ti,ab
44. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).ti,ab
45. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).ti,ab.
46. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).ti,ab
47. (cross‐over or cross over or crossover).ti,ab
48. (placebo$ or sham).ti,ab.
49. trial.ti
50. (assign$ or allocat$).ti,ab
51. controls.ti,ab.
52. 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51
53. 13 and 26 and 52
54. exp ANIMALS/ not HUMANS/
55. 53 not 54
Appendix 4. CINAHL (NICE Evidence Services Portal HDAS) search strategy
CINAHL (Ovid) from 1937 to May 2016
1. CEREBROVASCULAR DISORDERS/ OR exp BASAL GANGLIA CEREBROVASCULAR DISEASE/ OR exp HYPOXIA‐BRAIN,ISCHEMIA/ OR exp CAROTID ARTERY DISEASES/ OR exp CEREBROVASCULAR CIRCULATION/ OR exp INTRACRANIAL ARTERIAL DISEASES/ OR exp ARTERIOVENOUS MALFORMATIONS/ OR exp INTRACRANIAL EMBOLISM AND THROMBOSIS/ OR exp INTRACRANIAL HEMORRHAGE/ OR STROKE/ OR STROKE,LACUNAR/ OR CEREBRAL VASOSPASM/ OR VERTEBRAL ARTERY DISSECTIONS/ OR exp HYPOXIA,BRAIN
2. (stroke* OR "post stroke" OR poststroke OR post‐stroke OR apoplex* OR "cerebral vasc*" OR cerebrovasc* OR cva OR SAH OR "brain infarction" OR "cerebrovascular trauma").ti,ab
3. ((brain OR cerebr* OR cerebell* OR vertebrobasil* OR hemispher* OR intracran* OR intracerebral OR infratentorial OR supratentorial OR "middle cerebr*" OR mca* OR "anterior circulation" OR "basilar artery" OR "vertebral artery") adj5 (ischemi* OR ischaemi* OR infarct* OR thrombo* OR emboli* OR occlus* OR hypoxi*)).ti,ab;
4. ((brain* OR cerebr* OR cerebell* OR intracerebral OR intracran* OR parenchymal OR intraparenchymal OR intraventricular OR infratentorial OR supratentorial OR "basal gangli*" OR putaminal OR putamen OR "posterior fossa" OR hemispher* OR subarachnoid) adj5 (hemorrhag* OR haemorrhag* OR hematoma* OR haematoma* OR bleed*)).ti,ab;
5. exp HEMIPLEGIA/ OR exp PARALYSIS/ OR exp APHASIA/ OR exp GAIT DISORDERS,NEUROLOGIC/;
6. (hemipar* OR hemipleg* OR paresis OR paretic OR aphasi* OR dysphasi*).ti,ab;
7. exp BRAIN DAMAGE,CHRONIC/ OR BRAIN INJURIES/ OR exp BRAIN CONCUSSION/ OR exp INTRACRANIAL HEMORRHAGE/
8. ("chronic brain injury" OR "diffuse axonal injury" OR "craniocerebral trauma" OR "closed head injur*" OR "intracranial hemorrhag*").ti,ab
9. exp BRAIN ABSCESS/ OR exp CENTRAL NERVOUS SYSTEM INFECTIONS/ OR exp ENCEPHALITIS/ OR exp MENINGITIS/
10. (encephalitis OR meningitis OR "head injur*" OR "traumatic brain hemorrhag*" OR "chronic brain injury" OR "diffuse axonal injury" OR "craniocerebral trauma" OR "closed head injur*" OR "intracranial hemorrhag*").ti,ab
11. exp BRAIN NEOPLASMS/
12. ((brain OR cerebr*) adj5 (injur* OR hypoxi* OR damage* OR concussion OR trauma* OR neoplas* OR lesion* OR tumor* OR tumour* OR cancer* OR infection*)).ti,ab
13. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12
14. DYSARTHRIA/ OR ARTICULATION DISORDERS/ OR SPEECH ARTICULATION TESTS/
15. SPEECH DISORDERS/ OR VOICE DISORDERS/ OR APHONIA/ OR DYSPHONIA,SPASMODIC/ OR DYSPHONIA,MUSCLE TENSION/ OR COMMUNICATIVE DISORDERS/
16. (dysarth* OR dysphon* OR anarth* OR dyspros* OR aphon* OR dysfluen* OR stutter* OR stammer*).ti,ab
17. ((speech OR articul* OR disarticul* OR phonat* OR phonolog* OR voice OR vocal OR prosod* OR intonat* OR respirat* OR communicat* OR fluen*) adj5 (disorder* OR impair* OR problem* OR difficult*))
18. (speech adj5 (slow* OR weak* OR imprecis* OR intelligibil* OR unintelligibil* OR accuracy OR fatigue)).ti,ab
19. exp MOUTH/ OR exp LARYNX/ OR exp LARYNGEAL MUSCLES/ OR PHARYNX/ OR exp PHARYNGEAL MUSCLES/ OR FACIAL MUSCLES/ OR PALATAL MUSCLES/
20. (mouth OR tongue OR lingual OR palat* OR laryn* OR pharyn* OR orofacial OR oro‐facial OR "face musc*" OR "facial musc*").ti,ab
21. 19 OR 20
22. MOVEMENT DISORDERS/ OR ATAXIA/ OR DYSTONIA/ OR DYSTONIC DISORDERS/ OR HYPERKINESIS/ OR HYPOKINESIA/ OR MUSCLE HYPOTONIA/ OR exp MUSCLE HYPERTONIA/ OR MUSCLE WEAKNESS/ OR MUSCULAR DISEASES/ OR MUSCLE SPASTICITY/
23. (atax* OR dyston* OR hyperkin* OR hypokin* OR hypoton* OR hyperton* OR flaccid* OR spastic*).ti,ab
24. 22 OR 23
25. 21 AND 24
26. 14 OR 15 OR 16 OR 17 OR 18 OR 25
27. RANDOMIZED CONTROLLED TRIALS/
28. RANDOM ASSIGNMENT/
29. CLINICAL TRIALS/
30. CONTROL GROUP/
31. ("clinical trials" OR "clinical trials,phase i" OR "clinical trials,phase ii" OR "clinical trials,phase iii" OR "clinical trials,phase iv").ti,ab
32. DOUBLE‐BLIND STUDIES/
33. SINGLE‐BLIND STUDIES/
34. PLACEBOS/
35. PLACEBO EFFECT/
36. CROSSOVER DESIGN/
37. "randomized controlled trial".pt
38. "controlled clinical trial".pt
39. ("clinical trial" OR "clinical trial phase i" OR "clinical trial phase ii" OR "clinical trial phase iii" OR "clinical trial phase iv").pt
40. (random* OR RCT OR RCTs).ti,ab
41. (controlled adj5 (trial* OR stud*)).ti,ab
42. (clinical* adj5 trial*).ti,ab
43. ((control OR treatment OR experiment* OR intervention) adj5 (group* OR subject* OR patient*)).ti,ab
44. (quasi‐random* OR "quasi random*" OR pseudo‐random* OR "pseudo random*").ti,ab
45. ((control OR experiment* OR conservative) adj5 (treatment OR therapy OR procedure OR manage*)).ti,ab
46. ((singl* OR doubl* OR tripl* OR trebl*) adj5 (blind* OR mask*)).ti,ab
47. (cross‐over OR "cross over" OR crossover).ti,ab
48. (placebo* OR sham).ti,ab
49. trial.ti
50. (assign* OR allocat*).ti,ab
51. controls.ti,ab
52. 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42 OR 43 OR 44 OR 45 OR 46 OR 47 OR 48 OR 49 OR 50 OR 51
53. 13 AND 26 AND 52
54. exp ANIMALS/ NOT HUMAN/
55. 53 NOT 54
Appendix 5. PsycINFO search strategy
PsycINFO (Ovid) from 1800 to September 2016
1. cerebrovascular disorders/ or cerebral hemorrhage/ or exp cerebral ischemia/ or cerebral small vessel disease/ or cerebrovascular accidents/ or subarachnoid hemorrhage/
2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
5. hemiparesis/ or hemiplegia/
6. (hemipleg$ or hemipar$ or paresis or paretic).tw.
7. head injuries/ or exp brain concussion/ or brain damage/ or exp traumatic brain injury/
8. ((brain or cerebr$) adj5 (injur$ or hypoxi$ or damage$ or concussion or trauma$ or neoplasm$ or lesion$ or tumor$ or tumour$ or cancer$ or infection$)).tw.
9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
10. dysarthria/ or articulation disorders/
11. dysphonia/ or speech disorders/
12. (dysarth$ or dyphon$ or anarth$ or dyspros$ or aphon$ or dysfluen$ or stutter$ or stammer$).tw.
13. ((speech or articul$ or disarticul$ or phonat$ or phonolog$ or voice or vocal or prosod$ or intonat$ or respirat$ or communicat$ or fluen$) adj5 (disorder$ or impair$ or problem$ or difficult$)).tw.
14. (speech adj5 (slow$ or weak$ or imprecis$ or intelligibil$ or unintelligibil$ or accuracy or fatigue)).tw.
15. "mouth (anatomy)"/ or exp tongue/ or larynx/ or pharynx/ or vocal cords/ or facial muscles/
16. (mouth or tongue or lingual or palat$ or laryn$ or pharyn$ or orofacial or oro‐facial or face musc$ or facial musc$).tw.
17. 14 or 15
18. muscular disorders/ or movement disorders/ or ataxia/ or bradykinesia/ or dyskinesia/ or hyperkinesis/ or neuromuscular disorders/ or spasms/ or muscle spasms/
19. (atax$ or dyston$ or hyperkin$ or hypokin$ or hypoton$ or hyperton$ or flaccid$ or spastic$).tw.
20. 18 or 19
21. 17 and 20
22. 10 or 11 or 12 or 13 or 14 or 21
23. clinical trials/ or treatment effectiveness evaluation/ or placebo/
24. (random$ or RCT or RCTs).tw.
25. (controlled adj5 (trial$ or stud$)).tw.
26. (clinical$ adj5 trial$).tw.
27. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
28. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
29. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
30. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
31. (cross‐over or cross over or crossover).tw.
32. (placebo$ or sham).tw.
33. trial.ti.
34. (assign$ or allocat$).tw.
35. controls.tw.
36. 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35
37. 9 and 22 and 36
Appendix 6. Linguistics and Language Behavior Abstracts (LLBA) search strategy
LLBA (ProQuest) 1976 to November 2016
(((dysarth* OR dysphon* OR anarth* OR dyspros* OR aphon* OR dyston*) OR ((speech OR articulat* OR voice OR vocal OR communicat*) AND (disorder* OR impair* OR problem* OR difficult*)) OR ((phonat* OR prosod* OR intonat* OR respirat*) AND (disorder* OR impair* OR problem* OR difficult*)) OR SU("Articulation Disorders" OR "Dysarthria"))) AND (SU("Brain Damage" OR "Stroke") OR (stroke* OR "post stroke" OR poststroke OR post‐stroke OR apoplex* OR cerebrovasc* OR CVA OR SAH OR "cerebral vasc*"))
Data and analyses
Comparison 1. Dysarthria intervention compared with another intervention, attention control, placebo or no intervention: persisting effects.
Comparison 2. Dysarthria Intervention compared with another intervention, attention control, placebo or no intervention: immediate effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seconday outcome of dysarthria intervention versus any control: immediate effects, activity level | 3 | 117 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.07, 0.66] |
| 2 Secondary outcome of dysarthria intervention versus any control: immediate effects, impairment level | 4 | 99 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.02, 0.92] |
| 3 Secondary outcome of dysarthria intervention versus any control: immediate effects, participation level | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.94, 0.45] |
Comparison 3. Dysarthria intervention A versus dysarthria intervention B: persisting and immediate effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Secondary outcome of dysarthria intervention A versus dysarthria intervention B: persisting effects, activity level | 2 | 56 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.15, 0.91] |
| 2 Secondary outcome of dysarthria intervention A versus dysarthria intervention B: persisting effects, participation level | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.92, 0.47] |
Characteristics of studies
Characteristics of included studies [author‐defined order]
Bowen 2012.
| Methods |
Study design: RCT Study duration: December 2006 to end of follow up July 2010 Pragmatic, parallel, superiority RCT with blinded outcome assessment This was a larger trial of all communication impairments following stroke and the dysarthria population was a planned subgroup from this larger trial. We were able to extract the data for the dysarthria population |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Treatment group
Attention Control group
|
|
| Outcomes | Outcomes used in this review:
Secondary outcomes:
Methods to measure outcomes: Primary outcome: blinded, functional communicative ability assessed on the TOM activity sub scale. A conversation with an unfamiliar conversation partner was rated using the TOM by an expert independent expert speech and language therapist Outcomes were evaluated at baseline and 6 months post randomisation, with 2‐month gap between completion of intervention and final assessment Numbers lost to follow up: intervention lost 4/34; attention control lost 8/32 |
|
| Notes | Funding source: this project was funded by the NIHR Health Technology Assessment programme. The Stroke Association funded part of the excess treatment costs Contact with study authors for additional information: primary outcome reported for subgroups of diagnosis (i.e. aphasia, dysarthria); secondary outcomes not reported separately Contacted the statistician involved in this paper for the dysarthria specific data of all outcomes; this was provided in full Other: we have ensured AB, author of this trial and involved in this Cochrane review, has had no involvement in the review of this study but she contributed her opinion and provided additional information when requested |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by an external, independent, web‐based randomisation service using a computer‐generated string of random permuted blocks. Participants were randomised using a 1:1 allocation ratio in blocks of 2, 4, and 6 with different combinations depending on site and stratified according to severity and study centre. |
| Allocation concealment (selection bias) | Low risk | External, independent, web‐based |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment carried out by an independent speech and language therapist, blinded to treatment allocation and not involved in treating study participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used and dropouts specified in report |
| Selective reporting (reporting bias) | Low risk | Study protocol available and all statistical data included in the report |
Kwon 2015.
| Methods |
Study design: RCT: single centre, prospective, randomised, double‐blind, sham stimulation‐controlled trial Study duration: June 2013 to April 2015 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes | Outcomes used in this review. No primary outcome identified
Dysarthria was evaluated by a single skilled speech therapist who was blind to the study protocol before and after the rTMS sessions These 4 measures were carried out prior to and immediately at the end of the 2‐week treatment period |
|
| Notes | Funding source: not known We were unsuccessful in contacting the first author of the study for further information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation using a random numbers table; odd numbers went to the repetitive transcranial magnetic stimulation group and even numbers went to the sham stimulation group although it does not specify if this was equal randomisation. Insufficient information available |
| Allocation concealment (selection bias) | Unclear risk | No description of what method was used to ensure allocation concealment so this indicates a potential risk in the absence of further information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study reports the outcome assessor was blinded to protocol but insufficient detail as to how this was ensured; it may have been easy to break this blinding process |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 participants were randomised to treatment groups but then failed to complete the treatment. These participants and their data were withdrawn from all the analysis and no consideration evident as to how this missing data was dealt with |
| Selective reporting (reporting bias) | Unclear risk | In the absence of a protocol this remains unclear |
Mackenzie 2014.
| Methods |
Study design: a feasibility RCT Study duration: enrolment within 1 year |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention A group
Intervention B group
|
|
| Outcomes | Outcomes used in this review. No primary outcome measure identified
Intervention A lost 4/19 to follow‐up Intervention B lost 4/20 to follow‐up |
|
| Notes | Funding source: Dunhill Medical Trust We requested further information, which was provided, as well as a telephone consultation We were able to classify incomplete outcome data as low risk following discussion with the study author. They clarified that they had statistically analysed their findings appropriately and this had not affected the results: "Group A versus Group B difference was not indicated on any of the four measures, based on data for 32 completing participants: SIT F(1, 30)=1.46, p=0.24; CEM F(1, 30) = 2.39, p = 0.13, CES F(1, 30) = 0.58, p = 0.45; FDA‐2 F(1, 30) = 2.61, p = 0.12. There was no significant interaction between group allocation and assessment point on any of the four measures for these participants: SIT F(3, 90) = 0.88, p = 0.97; CEM F(3, 90) = 0.34, p = 0.80; CES F(3, 90) = 0.16, p = 0.92; FDA F(3, 90) = 0.12, p = 0.95. In view of the scale nature of the CEM measure, non‐parametric analysis was also undertaken and provided similar results. Imputation of results for seven additional cases with incomplete intervention and/or post‐intervention assessments, by last observation carried forward and multiple imputation provided similar results for all measures." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer generated and the block system was employed to facilitate the logistics of recruitment and intervention. This would not affect sequence generation. Patrticipants were referred in batches of 8 and then randomised within each block so 4 to group A and 4 to group B |
| Allocation concealment (selection bias) | Low risk | This was provided in opaque envelopes after the initial assessment by the 'assessor' and just before the intervention treatment started by the 'intervention' researcher |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Single blinded experienced speech and language therapy research assessor collected the outcome measurements. These were rated or transcribed by groups of blinded graduating speech and language therapy students |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome not likely to clinically impact, discussed with study author and confirmed all data included and adjusted where appropriate |
| Selective reporting (reporting bias) | Low risk | Feasibility study but all data and outcomes reported |
Wenke 2010.
| Methods |
Study design: RCT; an experimental research design was used to investigate the effects of 2 treatments at multiple follow‐up time points Study duration: not known |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Treatment group A
Treatment group B
|
|
| Outcomes | 26 randomised Intervention A lost 4/13 to some follow‐up assessments Intervention B lost 4/13 to some follow‐up assessments No primary outcome measure specified.
We used intelligibility measure as the primary outcome measure at activity level and articulatory precision as the secondary impairment level measure The data presented in the paper analysed the vowels and consonants separately, which meant data extraction was not possible without further information from the authors |
|
| Notes | Funding source: not known Contact with study authors: the study authors responded to 1 email answering questions relating to randomisation. We were unable to pursue a telephone consultation with the authors to discuss further |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation according to severity levels was carried out and allocation based on the results of this clinical judgement. Computer generated randomisation confirmed by author |
| Allocation concealment (selection bias) | Unclear risk | Further information suggested a pre‐generated list was used and stored on a computer in an Excel file, but it was not clear who had access to this list and how easily accessible this list was |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | 2 certified speech‐language pathologists served as independent listeners. This implies they are not involved in the study but does not specify whether they were blind or not to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unable to find out more from study author; missing outcome data showing imbalance across the 2 groups |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported at all time points |
Xu 2010.
| Methods |
Study design: RCT to observe the effect of acupuncture combined with speech therapy for dysarthria versus speech therapy only Study duration: not known |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Treatment group (acupuncture)
Control group (usual care)
|
|
| Outcomes | No primary outcome measure identified Outcome measures used were:
Outcome measures carried out immediately post treatment when the 9‐week treatment period ended. The outcome measures were carried out before and immediately after the trial by the hearing and speech specialists who did not know the details of the trial No participants were lost to follow up from either group |
|
| Notes | Funding source: not known We were unsuccessful in contacting the first author of the study for further information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Refers to a random number table but limited information make this judgment difficult |
| Allocation concealment (selection bias) | Unclear risk | There is no information about allocation concealment without further discussion with the author of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The participants were tested before and after the treatment by the same hearing and speech therapist who did not know the detail of the trial. This implies they were blinded to the intervention but no further information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to have no missing data with all participants recruited remaining in the trial to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | We were unable to verify selective reporting after an unsuccessful attempt to contact the authors |
CT: computer tomography ITT: intention‐to‐treat MRI: magnetic resonance imaging RCT: randomised controlled trial SLT: speech and language therapist
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Behn 2011 | Excluded people with dysarthria |
| Behn 2012 | Intervention for carers not people with dysarthria |
| Braverman 1999 | RCT; included people with communication problems other than dysarthria Intervention for cognition not dysarthria |
| Cohen 1993 | Mixed aetiology of progressive and non‐progressive adult‐acquired and congenital brain injury |
| Fitzgerald‐DeJean 2008 | Not an RCT; wrong intervention (language) |
| Fukusako 1989 | Not an RCT |
| Garcia 1998 | Not an RCT |
| Huffman 1978 | Not an RCT |
| Huh 2014 | Not an RCT |
| Hustad 2003 | Not an RCT |
| Ince 1973 | Not an RCT |
| Jones 1972 | Not an RCT |
| Katić 1973 | Not an RCT |
| Kelly 2000 | Mixed aetiology of participants, progressive and non‐progressive |
| Li 2013 | Not an RCT |
| Main 1998 | Mixed aetiology of participants, progressive and non‐progressive |
| Markov 1973 | Not an RCT |
| Nagasawa 1970 | Not an RCT |
| Palmer 2004 | Not an RCT |
| Palmer 2007 | Not an RCT |
| Qinglan 2002 | Wrong intervention (surgical) |
| Robertson 2001 | Not an RCT |
| Rosenbek 2006 | Not an RCT |
| Sakharov 2013 | Not an RCT |
| Sze 2002 | Intervention not for people with dysarthria |
| Togher 2004 | Intervention not for people with dysarthria |
| Togher 2014 | Not an RCT |
| Varma 2004 | Not an RCT |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
You 2010.
| Methods | The effects of transcranial direct stimulation (tDCS) on dysarthria in stroke patients In a prospective, double blinded, randomised case control study performed between January 2007 and December 2008, 6 people were randomised to anodal tDCS application and conventional speech therapy, and 6 participants were randomised to the sham group, which received only conventional speech therapy. tDCS was delivered for 30 minutes at 2 milliampere (mA) with 25 cm², five times/week, for a total of 2 weeks. The effects were assessed in maximal phonation time (MPT), alternative motion rates (AMR)‐Pa, AMR‐Ta, AMR‐Ka, and sequential motion rates (SMR)‐PaTaKa using the Multi‐Media Dimension Voice Program |
| Participants | 12 participants who developed dysarthria after acute middle cerebral artery infarction were included in this study |
| Interventions | Experimental intervention: anodal tDCS application and conventional speech therapy Usual care intervention: conventional speech therapy only |
| Outcomes | Pre‐treatment patient evaluation showed no significant difference between the 2 groups for all parameters. The MPT, AMR‐Pa, AMR‐Ta, AMR‐Ka, and SMR‐PaTaKa were improved pre‐ and post‐treatment in the stimulation group, while MPT, SMR‐PaTaKa were improved in the sham group (P < 0.05). The AMR‐Pa significantly improved in the stimulation group compared with the sham group (P < 0.05) |
| Notes | This study is in Korean and needs to be translated and data extracted before it can be considered for inclusion in the review. We were unsuccessful in contacting the first author for further information |
Characteristics of ongoing studies [ordered by study ID]
Peng 2015.
| Trial name or title | Modified VitalStim electroacupuncture improves the speech function in people with spastic dysarthria after stroke |
| Methods | 32 people with spastic dysarthria after stroke within 1 month were randomly divided into VitalStim group (n = 16) and control group (n = 16). Basic medical therapy, physical therapy, occupational therapy, and speech therapy were used in both groups. Additionally, modified VitalStim electroacupuncture at acupoints of Yiming (EXHN14), Fengchi (GB20), Dazhui (BU14), Lianquan (RN23), Baihui (DU20), and lateral Jinjinyuye was performed in Vitalstim group. Participants in VitalStim group received extra 30‐minute VitalStim therapy once a day, for a total of 28 days. The outcomes were evaluated by using modified Barthel index (MBI) and Frenchay Dysarthria Assessment (FDA), and the practical significance of VitalStim electroacupuncture were statistical analysed |
| Participants | 32 participants with spastic dysarthria after stroke within 1 month |
| Interventions | Basic medical therapy, physical therapy, occupational therapy, and speech therapy were used in both groups. Additionally, modified VitalStim electroacupuncture at acupoints of Yiming (EXHN14), Fengchi (GB20), Dazhui (BU14), Lianquan (RN23), Baihui (DU20) and lateral Jinjinyuye was performed in Vitalstim group. Participants in the VitalStim group received extra 30‐minute VitalStim therapy once a day, for a total of 28 days |
| Outcomes | The outcomes were evaluated by using modified Barthel index (MBI) and Frenchay Dysarthria Assessment (FDA). MBI increased significantly after treatment in both groups (P < 0.01). Compared with both groups, MBI increased more significantly in VitalStim group (P < 0.05). Significant improvements were found in VitalStim group in relation to 20 FDA items, such as lips spread, tongue at rest and palate maintenance (P < 0.05). The performance of the patients in VitalStim group on the rest of FDA items also showed an improvement trend compared with that of control (P > 0.05) except for the two items in relation to tongue alternate and jaw in speech. |
| Starting date | Not known |
| Contact information | YN Peng, Y Yin, BT Tan, W Jiang, B Zheng, YY Deng, LH Yu The Second Affiliated Hospital of Chongqing Medical University, Rehabilitation Medicine, Chongqing, China Chongqing Medical University, Rehabilitation Therapy, Chongqing, China |
| Notes | This study is available as an abstract only and no full report can be found. We unsuccessfully attempted to contact the authors to obtain further information about this study, including if the full study has been published. WCPT Congress 2015/Physiotherapy 2015; 101 (Suppl 1): eS833–eS1237 eS1189 Ethics approval: Ethical approval obtained from the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University. http://dx.doi.org/10.1016/j.physio.2015.03.2113 Research Report Poster Presentation |
ReaDySpeech.
| Trial name or title | ReaDySpeech for people with dysarthria after stroke: protocol for a feasibility RCT |
| Methods | A feasibility RCT will recruit 36 people with post‐stroke dysarthria who are more than 1 week post‐stroke. Participants will be externally randomised in a 2:1 ratio to receive either ReaDySpeech and usual care (24 participants) or usual care only (12 participants). This study is single blind with the researcher carrying out the baseline and outcome measures blinded to treatment allocation. The primary objective is to assess the feasibility of conducting a definitive trial. Secondary objectives include recruitment rate, and determining: numbers of eligible patients recruited and reasons for non‐recruitment; loss of participants to follow‐up and reasons; acceptability of randomisation and the intervention; adherence to the intervention; acceptability of outcome measures; defining 'usual' care; and the implications of the intervention for the patient/family/carer |
| Participants | The study population includes adults (aged ≥18 years) with dysarthria as a result of stroke |
| Interventions | ReaDySpeech is an online programme which delivers articulation exercises to improve breathing; intonation; facial expression; rate of speech; and oro‐motor control (including range of movement, strength and speed). ReaDySpeech is set up and amended by the treating therapist according to the participant's progress. The participant accesses these exercises online, via any Wi‐Fi enabled device (smart phone, tablet computer, laptop computer or personal computer). It can be used in a variety of ways: as part of face‐to‐face therapy during a session with a speech and language therapist or a therapy assistant, or the participant can use it independently outside of the therapy sessions, with or without the support of family or carers. The therapists select clinically relevant exercises and negotiate agreed intensity and duration of use with the participant, adherence to which is monitored by the software programme which will record the exercises selected by the therapist. Therapists will have an instruction booklet with screen shots to support their use of ReaDySpeech. The proof of concept work has shown that ReaDySpeech can be delivered by any qualified speech and language therapist of any level of experience. In this trial, participating therapists will use ReaDySpeech with participants who meet the inclusion criteria alongside 'usual' care for a maximum of 10 weeks. No specifications about the intensity of ReaDySpeech care will be made and this will be decided according to the therapist's clinical judgement in consultation with the participant |
| Outcomes | Primary outcome: Dysarthria Therapy Outcome Measure (Therapist‐reported activity level measure) Secondary outcomes: COAST (communication outcome after stroke scale), Dysarthria Impact Profile (patient‐reported outcome measure, activity and participation level), Frenchay Dysarthria Assessment 2nd edition (therapist‐reported impairment level measure); Euroquol 5D‐5L (patient‐reported generic health outcome measure) |
| Starting date | September 2015 |
| Contact information | claire.mitchell@manchester.ac.uk |
| Notes | ISRCTN84996500 |
Differences between protocol and review
The title of this review was changed from "Speech and language therapy for dysarthria due to non‐progressive brain damage" to reflect the broader scope of the search, which is intended to have a more global reach. The search terms for this review now include interventions carried out by any health professional, people with dysarthria, or a trained individual (whether voluntary, employed, or family member) or any other possible approaches to delivery. This review has considered any type of intervention for acquired dysarthria including behavioural or psychological approaches, use of devices and medication, with the exception of surgical intervention. This review was also designed to reflect the international levels of functioning including impairment, activity, and participation level effects (WHO 2001). We included an examination of risk of bias in this review in accordance with current Cochrane methodology (Higgins 2011). This review now has a summary of findings table which includes the five GRADE considerations to assess the quality of the body of evidence of the studies included in the meta‐analysis using GRADEproGDT software (GRADEproGDT 2015). The primary outcome in the protocol was to examine long‐term, persistent effectiveness between three and nine months post‐intervention, but during the review process, we found this time criterion was too restrictive. Following discussion among review authors the timings were relaxed to include Mackenzie 2014, which was felt to be the most appropriate way forward, but this was a change from the original protocol.
Contributions of authors
Claire Mitchell initiated and designed the review, conducted the searches, screened and retrieved references, contacted relevant authors, obtained translations for non‐English publications, requested ongoing and unpublished study information, extracted data from included trials, evaluated methodological quality, entered and analysed the data, interpreted the findings, and wrote the review. Audrey Bowen designed the review, screened references for inclusion, extracted data from included trials, evaluated methodological quality, analysed the data, interpreted the findings and contributed to the writing of the review. Sarah Tyson supported decision‐making for inclusion, contributed to the writing of the review, and commented on review drafts. Zoe Butterfint commented on the final versions of the updated review. Paul Conroy designed the review, screened references for inclusion, extracted data from included trials, evaluated methodological quality, analysed the data, interpreted the findings and contributed to the writing of the review.
Sources of support
Internal sources
-
Jo Whitcombe (Clinical Outreach Librarian), Naomi Leech (Assistant Librarian) and Steven Glover (Head of Library Services) Central Manchester University Hospitals NHS Foundation Trust, UK.
Search terms and searching
External sources
-
National Institute for Health Research, UK.
Claire Mitchell is funded by a National Institute for Health Research Doctoral Research Fellowship DRF‐2014‐07‐043
Audrey Bowen’s salary is part funded by Stroke Association and partly by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) Greater Manchester. The funders had no role in the design of the study, data collection and analysis, decision to publish, or preparation of the manuscript. However, the project outlined in this article may be considered to be affiliated to the work of the NIHR CLAHRC Greater Manchester.
Declarations of interest
Claire Mitchell is a speech and language therapist and is funded by a National Institute for Health Research Doctoral Research Fellowship (DRF‐2014‐07‐043) and is registered with the Health and Care Professions Council, UK.
Audrey Bowen’s salary is part funded by Stroke Association and partly by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) Greater Manchester. Audrey Bowen has been involved in a study included in this review (Bowen 2012). She did not contribute to the assessment or interpretation of this study.
Sarah Tyson: none known.
Zoe Butterfint: none known.
Paul Conroy is a speech and language therapist, member of the Royal College of Speech and Language Therapists, and is registered with the Health and Care Professions Council, UK.
This report presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the review authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Bowen 2012 {published data only}
- Bowen A. Assessing the effectiveness of communication therapy in the north west Current Controlled Trials. http://www.controlled‐trials.com 2004.
- Bowen A, Hesketh A, Patchick E, Young A, Davies L, Vail A, et al. Clinical effectiveness, cost‐effectiveness and service users' perceptions of early, well‐resourced communication therapy following a stroke: a randomised controlled trial (the ACT NoW Study). Health Technology Assessment 2012; Vol. 16, issue 26:1‐160. [DOI] [PubMed]
- Bowen A, Hesketh A, Pathchick A, Young A, Davies L, Vail A, et al. Effectiveness of enhanced communication therapy in the first four months after stroke for aphasia and dysarthria: a randomised controlled trial. BMJ 2012;345:e4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen A, on behalf of ACTNoW investigators. The ACTNoW study: a randomised controlled trials of speech and language therapy early after stroke. Neurorehabilitation and Neural Repair 2012;26(6):680. [Google Scholar]
- Hesketh A, Long AF, Bowen A, on behalf of the ACT NoW Study. Agreement on outcome: speaker, carer and therapist perspectives on functional communication after stroke. Aphasiology 2011;25(3):291‐308. [Google Scholar]
- Hesketh A, Long AF, Patchick E, Lee J, Bowen A. The reliability of rating conversation as a measure of functional communication following stroke. Aphasiology 2008;9:970‐84. [Google Scholar]
- Long AF, Hesketh A, Bowen A. Communication outcome after stroke: a new measure of the carer's perspective. Clinical Rehabilitation 2009;23:846‐56. [DOI] [PubMed] [Google Scholar]
- Long AF, Hesketh A, Paszek G, Booth M, Bowen A. Development of a reliable self‐report outcome measure for pragmatic trials of communication therapy following stroke: the Communication Outcome after Stroke (COAST) scale. Clinical Rehabilitation 2008;22(12):1083‐94. [DOI] [PubMed] [Google Scholar]
- Young A, Gomersall T, Bowen A. Trial participants' experiences of early, enhanced speech and language therapy after stroke compared with employed visitor support: a qualitative study nested within a RCT. Clinical Rehabilitation 2013;27(2):174‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AM, Pearl G, Lee J. The ACT NoW study, involving service users in research. www.researchgate.net/profile/Alys_Young/publication/265189672_The_ACT_NoW_Study‐Involving_Service_Users_in_Research/links/548afaa30cf214269f1dcbdb.pdf. The Stroke Network publications, (Accessed prior to 12 December 2016).
Kwon 2015 {published data only}
- Kwon YG, Do KH, Park SJ, Chang MC, Chun MH. Effect of repetitive transcranial magnetic stimulation on patients with dysarthria after subacute stroke. Annals of Rehabilitation Medicine 2015;39(5):793‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mackenzie 2014 {published data only}
- Mackenzie C, Muir M, Allen C, Jensen A. Non‐speech oro‐motor exercises in post‐stroke dysarthria intervention: a randomised feasibility trial. International Journal of Language and Communication Disorders 2014;49(5):602‐17. [DOI] [PubMed] [Google Scholar]
Wenke 2010 {published data only}
- Wenke R, Theodoros D, Cornwell P. The short‐ and long‐term effectiveness of the LSVT for dysarthria following TBI and stroke. Brain Injury 2008;22(4):339‐52. [DOI] [PubMed] [Google Scholar]
- Wenke R, Theordoros D, Cornwell P. Effectiveness of Lee Silverman Voice Treatment (LSVT) on hypernasality in non‐progressive dysarthria: the need for further research. International Journal of Language and Communication Disorders 2010;45(1):31‐46. [DOI] [PubMed] [Google Scholar]
- Wenke RJ, Cornwell P, Theodoros DG. Changes to articulation following LSVT and traditional dysarthria therapy in non‐progressive dysarthria. International Journal of Speech‐Language Pathology 2010;12(3):203‐20. [DOI] [PubMed] [Google Scholar]
- Wenke RJ, Theodoros D, Cornwell P. A comparison of the effects of the Lee Silverman Voice Treatment and traditional therapy on intelligibility, perceptual speech features, and everyday communication in nonprogressive dysarthria. Journal of Medical Speech‐Language Pathology 2011;19(4):1‐24. [Google Scholar]
Xu 2010 {published data only}
- Xu J, Li H, Chen Z, Liu L, Jing S. Effect of acupuncture on the speech and acoustics level in patients with dysarthria. Chinese Acupuncture and Moxibustion 2010;30(7):537‐41. [PubMed] [Google Scholar]
References to studies excluded from this review
Behn 2011 {published data only}
- Behn N. Communication Training for Paid Caregivers of People with Traumatic Brain Injury (TBI). Master of Applied Sciences Thesis 2011.
Behn 2012 {published data only}
- Behn N, Togher L, Power E, Heard R. Evaluating communication training for paid carers of people with traumatic brain injury. Brain Injury 2012;26(13‐14):1702‐15. [DOI] [PubMed] [Google Scholar]
Braverman 1999 {published data only}
- Braverman SE, Spector J, Warden DL, Wilson BC, Ellis TE, Bamdad MJ. A multidisciplinary TBI inpatient rehabilitation programme for active duty service members as part of a randomized controlled trial. Brain Injury 1999;13(6):405‐15. [DOI] [PubMed] [Google Scholar]
Cohen 1993 {published data only}
- Cohen NS, Masse R. The application of singing and rhythmic instruction as a therapeutic intervention for persons with neurogenic communication disorders. Journal of Music Therapy 1993;30(2):81‐99. [Google Scholar]
Fitzgerald‐DeJean 2008 {published data only}
- Fitzgerald‐DeJean D. The Investigation of Treatment Outcomes for Adults with Chronic Brain Injury Following Intensive Multidisciplinary Treatment. Baton Rouge (USA): Louisiana State University, 2008. [Google Scholar]
Fukusako 1989 {published data only}
- Fukusako Y, Endo K, Konno K, Hasegawa K, Tatsumi IF, Masaki S. Changes in the speech of spastic dysarthric patients after treatment based on perceptual analysis. Annual Bulletin of the Research Institute of Logopedics and Phoniatrics 1989;23:119‐40. [Google Scholar]
Garcia 1998 {published data only}
- Garcia JM, Dagenais PA. Dysarthric sentence intelligibility: contribution of iconic gestures and message predictiveness. Journal of Speech Language and Hearing Research 1998;41(6):1282‐93. [DOI] [PubMed] [Google Scholar]
Huffman 1978 {published data only}
- Huffman AL. Biofeedback treatment of orofacial dysfunction ‐ preliminary study. American Journal of Occupational Therapy 1978;32(3):149‐54. [PubMed] [Google Scholar]
Huh 2014 {published data only}
- Huh MJ, Park HM, Kim NS, Jung JH. Phonation performance in chronic stroke patients with inspiratory muscle training. European Respiratory Journal 2014;44(Suppl 58):P4321. [Google Scholar]
Hustad 2003 {published data only}
- Hustad K, Jones T, Dailey S. Implementing speech supplementation strategies: effects on intelligibility and speech rate of individuals with chronic severe dysarthria. Journal of Speech, Language and Hearing Research 2003;46(2):462‐74. [PubMed] [Google Scholar]
Ince 1973 {published data only}
- Ince LP, Rosenberg DN. Modification of articulation in dysarthria. Archives of Physical Medicine and Rehabilitation 1973;54(5):233‐6. [PubMed] [Google Scholar]
Jones 1972 {published data only}
- Jones W. Speech rehabilitation following a stroke. British Journal of Disorders of Communication 1972;7(1):82‐6. [DOI] [PubMed] [Google Scholar]
Katić 1973 {published data only}
- Katić M. Rehabilitation of speech disorders in the patient after cerebrovascular stroke. Neuropsihijatrija 1973;21(1):166‐7. [PubMed] [Google Scholar]
Kelly 2000 {published data only}
- Kelly S, Main A, Manley G, McLean C. Electropalatography and the Linguagraph system. Medical Engineering & Physics 2000;22(1):47‐58. [DOI] [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li M, Wang X, Li S, Pan C, Chen Y, Zhang Z, et al. Rehabilitation effect of Cantonese training for Cantonese motility dysarthria. Chinese Journal of Cerebrovascular Disease 2013;10:2. [Google Scholar]
Main 1998 {published data only}
- Main A. The Use of Electropalatography in the Treatment of Acquired Dysarthria. Canterbury (UK): University of Kent, 1998. [Google Scholar]
Markov 1973 {published data only}
- Markov G. The treatment of aphasia and dysarthria using psychoforin (Tofranil) and myocalm. Zhurnal Nevpatologii i Psikhatrii imeni SS Korsakova 1973;73(4):512‐3. [PubMed] [Google Scholar]
Nagasawa 1970 {published data only}
- Nagasawa T, Kamiyama G. Speech training after cerebrovascular accidents, with special reference to dysarthria. Saishin igaku Modern Medicine 1970;25(7):1474‐8. [PubMed] [Google Scholar]
Palmer 2004 {published data only}
- Palmer R, Enderby P, Cunningham S. The effect of three practice conditions on the consistency of chronic dysarthric speech. Journal of Medical Speech‐Language Pathology 2004;12:183‐8. [Google Scholar]
Palmer 2007 {published data only}
- Palmer R, Enderby P, Hawley M. Addressing the needs of speakers with longstanding dysarthria: computerized and traditional therapy compared.. International Journal of Language and Communication Disorders 2007;42(Suppl 1):61‐79. [DOI] [PubMed] [Google Scholar]
Qinglan 2002 {published data only}
- Qinglan Y, Zhiwei H, Feng L, Qingjuan Y, Shan G, Junrong H. Treatment of pseudobulbar paralysis with acupuncture and sublingual blood‐letting. International Journal of Clinical Acupuncture 2002;13(4):251‐4. [Google Scholar]
Robertson 2001 {published data only}
- Robertson S. The efficacy of oro‐facial and articulation exercises in dysarthria following stroke. International Journal of Language and Communication Disorders 2001;36(Suppl):292‐7. [DOI] [PubMed] [Google Scholar]
Rosenbek 2006 {published data only}
- Rosenbek JC, Rodriguez AD, Hieber B, Leon SA, Crucian GP, Ketterson TU. Effects of two treatments for aprosodia secondary to acquired brain injury. Journal of Rehabilitation Research and Development 2006;43(3):379‐90. [DOI] [PubMed] [Google Scholar]
Sakharov 2013 {published data only}
- Sakharov VI, Isanova VA. The rehabilitation treatment of patients with motor and cognitive disorders after stroke. Zhurnal Nevpatologii i Psikhatrii imeni SS Korsakova 2013;114(8 Vypusk 2 Insul't):39‐41. [PubMed] [Google Scholar]
Sze 2002 {published data only}
- Sze FKH, Wong E, Yi X, Woo J. Does acupuncture have additional value to standard post‐stroke motor rehabilitation?. Stroke 2002;33(1):186‐94. [DOI] [PubMed] [Google Scholar]
Togher 2004 {published data only}
- Togher L, McDonald S, Code C, Grant S. Training communication partners of people with traumatic brain injury: a randomised controlled trial. Aphasiology 2004;18(4):313‐35. [Google Scholar]
Togher 2014 {published data only}
- Togher L, McDonald S, Tate R, Power E, Rietdijk R. Training everyday communication partners is efficacious in improving the communication of people with severe TBI: findings from a single‐blind multi‐centre clinical trial. Brain Injury 2014;28(5‐6):723‐4. [Google Scholar]
Varma 2004 {published data only}
- Varma AK. The effect of motor control on oro‐facial dysfunctions in stroke patients under Indian conditions. Stroke 2004;35(6):e319. [Google Scholar]
References to studies awaiting assessment
You 2010 {published data only}
- You DS, Chun MH, Kim DY, Han EY, Jung SE. The effects of transcranial direct current stimulation on dysarthria in stroke patients. Journal of Korean Academy of Rehabilitation Medicine 2010;34:10‐4. [Google Scholar]
References to ongoing studies
Peng 2015 {published data only}
- Peng YN, Yin Y, Tan BT, Jiang W, Zheng B, Deng YY, et al. Modified vitalstim electroacupuncture improves the speech function in patients with spastic dysarthria after stroke. Physiotherapy 2015;101(Suppl 1):eS833‐eS1237. [Google Scholar]
ReaDySpeech {unpublished data only}
- Mitchell C. ReaDySpeech for people with dysarthria after stroke. www.isrctn.com/ (accessed prior to 12 December 2016).
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brady 2011
- Brady M, Clark A, Dickson S, Paton G, Barbour R. The impact of stroke‐related dysarthria on social participation and implications for rehabilitation. Disability & Rehabilitation 2011;33(3):178‐86. [DOI] [PubMed] [Google Scholar]
Brady 2016
- Brady MC, Kelly H, Godwin J, Enderby P, Campbell P. Speech and language therapy for aphasia following stroke. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD000425.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org 2011.
Dickson 2008
- Dickson S, Barbour RS, Brady M, Clark AM, Paton G. Patients' experiences of disruptions associated with post‐stroke dysarthria. International Journal of Language and Communication Disorders 2008;43(2):135‐53. [DOI] [PubMed] [Google Scholar]
Donovan 2007
- Donovan NJ, Velozo CA, Rosenbek JC. The communicative effectiveness survey: Investigating its item‐level psychometrics. Journal of Medical Speech‐Language Pathology 2007;15:433‐47. [Google Scholar]
Duffy 2013
- Duffy JR. Motor speech disorders: Substrates, differential diagnosis, and management. Third Edition. Vol. 1, St Louis, Missouri: Elsevier Health Sciences, 2013. [Google Scholar]
Enderby 1983
- Enderby PM. Frenchay dysarthria assessment. Pro‐ed Austin, TX, 1983. [Google Scholar]
Enderby 1997
- Enderby P, John A. Therapy Outcome Measures: Speech‐language pathology technical manual. Singular, 1997. [Google Scholar]
GRADEproGDT 2015 [Computer program]
- GRADEproGDT. GRADEproGDT: GRADEpro Guideline Development Tool [www.guidelinedevelopment.org]. Hamilton: McMaster University, 2015.
Herdman 2011
- Herdman M, Gudex C, Lloyd A, Janssen MF, Kind P, Parkin D, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Quality of Life Research 2011;20:1727‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updatedMarch 2011]. 5.1.0. The Cochrane Collaboration 2011. Available from www.cochrane‐handbook.org, 2011. [Google Scholar]
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Ruairidh M, Perera R, Moher D. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Intercollegiate Stroke Working Party 2016
- Intercollegiate Stroke Working Party. National clinical guideline for stroke. National clinical guideline for stroke. 5th Edition. London: Royal College of Physicians, October 2016. [Google Scholar]
IOPI 2005
- IOPI. Iowa Oral Performance Instrument. Iowa Oral Performance Instrument: Users' Manual 2005.
Langhorne 2009
- Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurology August 2009;8(8):741‐754. [DOI] [PubMed] [Google Scholar]
Lawrence 2001
- Lawrence ES, Coshall C, Dundas R, Stewart J, Rudd AG, Howard R. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke 2001;32:1279‐84. [DOI] [PubMed] [Google Scholar]
Long 2008
- Long AF, Hesketh A, Paszek G, Booth M, Bowen A. Development of a reliable self‐report outcome measure for pragmatic trials of communication therapy following stroke: the Communication Outcome after Stroke (COAST) scale. Clinical Rehabilitation 2008;22(12):1083‐94. [DOI] [PubMed] [Google Scholar]
Lubart 2005
- Lubart E, Leibovitz A, Baumoehl Y, Klein C, Gil I, Abramovitz L. Progressing stroke with neurological deterioration in a group of Israeli elderly. Archives of Gerontology and Geriatrics 2005;41:95‐100. [DOI] [PubMed] [Google Scholar]
Mackenzie 2007
- Mackenzie C, Lowit A. Behavioural intervention effects in dysarthria following stroke: communication effectiveness, intelligibility and dysarthria impact. International Journal of Language and Communication Disorders 2007;42(2):131‐53. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor‐Goh 2005
- Taylor‐Goh S. Royal College of Speech and Language Therapists Clinical Guidelines. Speechmark, 2005. [Google Scholar]
Tilling 2001
- Tilling K, Sterne JAC, Rudd AG, Glass TA, Wityk RJ, Wolfe CDA. A new method for predicting recovery after stroke. Stroke 2001;32:2867‐73. [DOI] [PubMed] [Google Scholar]
Walshe 2009
- Walshe M, Peach RK, Miller N. Dysarthria Impact Profile: development of a scale to measure psychosocial effects. International Journal of Language and Communication Disorders 2009;44:693‐715. [DOI] [PubMed] [Google Scholar]
Warlow 2008
- Warlow CP, Gijn J, Dennis MS, Wardlaw JM, Bamford J, Hankey GJ, et al. Stroke: Practical Management. 3rd Edition. Oxford: Blackwell Science Ltd, 2008. [Google Scholar]
WHO 2001
- World Health Organization. ICF: International Classification of Functioning Disability and Health. Geneva: World Health Organization, 2001. [Google Scholar]
Yorkston 1984
- Yorkston KM, Beukelman DR. Assessment of intelligibility of dysarthric speech. Assessment Manual. Austin, Texas: Pro‐ed, 1984. [Google Scholar]
Yorkston 1996
- Yorkston KM. Treatment efficacy: dysarthria. Journal of Speech and Hearing Disorders 1996;39:S46‐S57. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith PR. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Sellars 2000
- Sellars C. [Determining the availability of good quality evidence for the effectiveness of speech and language therapy interventions for dysarthria post‐stroke (Abstract)]. Proceedings of the Consensus Conference on Stroke Treatment and Service Delivery. Edinburgh, UK: Royal College of Physicians of Edinburgh, 2000.
Sellars 2001
- Sellars C, Legg L, Langhorne P, Pollock A. Determining the availability of good quality evidence for the effectiveness of speech and language therapy interventions for dysarthria post‐stroke (Abstract). Cerebrovascular Diseases 2001;11(Suppl 4):43. [Google Scholar]
Sellars 2002
- Sellars C, Hughes T, Langhorne P. Speech and language therapy for dysarthria due to non‐progressive brain damage: a systematic Cochrane review. Clinical Rehabilitation 2002;16(1):61‐8. [DOI] [PubMed] [Google Scholar]
Sellars 2002a
- Sellars C, Hughes T, Langhorne P. Speech and language therapy for dysarthria due to non‐progressive brain damage. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD002088] [DOI] [PubMed] [Google Scholar]
Sellars 2005
- Sellars C, Hughes T, Langhorne P. Speech and language therapy for dysarthria due to non‐progressive brain damage. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD002088.pub2] [DOI] [PubMed] [Google Scholar]


