Abstract
Background
This review is an update of 'Topical capsaicin (high concentration) for chronic neuropathic pain in adults' last updated in Issue 2, 2013. Topical creams with capsaicin are used to treat peripheral neuropathic pain. Following application to the skin, capsaicin causes enhanced sensitivity, followed by a period with reduced sensitivity and, after repeated applications, persistent desensitisation. High‐concentration (8%) capsaicin patches were developed to increase the amount of capsaicin delivered; rapid delivery was thought to improve tolerability because cutaneous nociceptors are 'defunctionalised' quickly. The single application avoids noncompliance. Only the 8% patch formulation of capsaicin is available, with a capsaicin concentration about 100 times greater than conventional creams. High‐concentration topical capsaicin is given as a single patch application to the affected part. It must be applied under highly controlled conditions, often following local anaesthetic, due to the initial intense burning sensation it causes. The benefits are expected to last for about 12 weeks, when another application might be made.
Objectives
To review the evidence from controlled trials on the efficacy and tolerability of topically applied, high‐concentration (8%) capsaicin in chronic neuropathic pain in adults.
Search methods
For this update, we searched CENTRAL, MEDLINE, Embase, two clinical trials registries, and a pharmaceutical company's website to 10 June 2016.
Selection criteria
Randomised, double‐blind, placebo‐controlled studies of at least 6 weeks' duration, using high‐concentration (5% or more) topical capsaicin to treat neuropathic pain.
Data collection and analysis
Two review authors independently searched for studies, extracted efficacy and adverse event data, and examined issues of study quality and potential bias. Where pooled analysis was possible, we used dichotomous data to calculate risk ratio and numbers needed to treat for one additional event, using standard methods.
Efficacy outcomes reflecting long‐duration pain relief after a single drug application were from the Patient Global Impression of Change (PGIC) at specific points, usually 8 and 12 weeks. We also assessed average pain scores over weeks 2 to 8 and 2 to 12 and the number of participants with pain intensity reduction of at least 30% or at least 50% over baseline, and information on adverse events and withdrawals.
We assessed the quality of the evidence using GRADE and created a 'Summary of findings' table.
Main results
We included eight studies, involving 2488 participants, two more studies and 415 more participants than the previous version of this review. Studies were of generally good methodological quality; we judged only one study at high risk of bias, due to small size. Two studies used a placebo control and six used 0.04% topical capsaicin as an 'active' placebo to help maintain blinding. Efficacy outcomes were inconsistently reported, resulting in analyses for most outcomes being based on less than complete data.
For postherpetic neuralgia, we found four studies (1272 participants). At both 8 and 12 weeks about 10% more participants reported themselves much or very much improved with high‐concentration capsaicin than with 'active' placebo; the point estimates of numbers needed to treat for an additional beneficial outcome (NNTs) were 8.8 (95% confidence interval (CI) 5.3 to 26) at 8 weeks and 7.0 (95% CI 4.6 to 15) at 12 weeks (2 studies, 571 participants; moderate quality evidence). More participants (about 10%) had average 2 to 8‐week and 2 to 12‐week pain intensity reductions over baseline of at least 30% and at least 50% with capsaicin than control, with NNT values between 10 and 12 (2 to 4 studies, 571 to 1272 participants; very low quality evidence).
For painful HIV‐neuropathy, we found two studies (801 participants). One study reported the proportion of participants who were much or very much improved at 12 weeks (27% with high‐concentration capsaicin and 10% with 'active' placebo). For both studies, more participants (about 10%) had average 2 to 12‐week pain intensity reductions over baseline of at least 30% with capsaicin than control, with an NNT of 11 (very low quality evidence).
For peripheral diabetic neuropathy, we found one study (369 participants). It reported about 10% more participants who were much or very much improved at 8 and 12 weeks. One small study of 46 participants with persistent pain following inguinal herniorrhaphy did not show a difference between capsaicin and placebo for pain reduction (very low quality evidence).
We downgraded the quality of the evidence for efficacy outcomes by one to three levels due to sparse data, imprecision, possible effects of imputation methods, and susceptibility to publication bias.
Local adverse events were common, but not consistently reported. Serious adverse events were no more common with active treatment (3.5%) than control (3.2%). Adverse event withdrawals did not differ between groups, but lack of efficacy withdrawals were somewhat more common with control than active treatment, based on small numbers of events (six to eight studies, 21 to 67 events; moderate quality evidence, downgraded due to few events). No deaths were judged to be related to study medication.
Authors' conclusions
High‐concentration topical capsaicin used to treat postherpetic neuralgia, HIV‐neuropathy, and painful diabetic neuropathy generated more participants with moderate or substantial levels of pain relief than control treatment using a much lower concentration of capsaicin. These results should be interpreted with caution as the quality of the evidence was moderate or very low. The additional proportion who benefited over control was not large, but for those who did obtain high levels of pain relief, there were usually additional improvements in sleep, fatigue, depression, and quality of life. High‐concentration topical capsaicin is similar in its effects to other therapies for chronic pain.
Keywords: Adult; Humans; Administration, Topical; Analgesics; Analgesics/administration & dosage; Analgesics/adverse effects; Capsaicin; Capsaicin/administration & dosage; Capsaicin/adverse effects; Chronic Pain; Chronic Pain/drug therapy; Diabetic Neuropathies; Diabetic Neuropathies/drug therapy; HIV Infections; HIV Infections/complications; Neuralgia; Neuralgia/drug therapy; Neuralgia, Postherpetic; Neuralgia, Postherpetic/drug therapy; Numbers Needed To Treat; Ointments; Pain, Postoperative; Pain, Postoperative/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Capsaicin applied to the skin for chronic neuropathic pain in adults
Bottom line
There is moderate quality evidence that high‐concentration (8%) capsaicin patches can give moderate pain relief, or better, to a minority of people with postherpetic neuralgia, and very low quality evidence that it benefits those with HIV‐neuropathy and peripheral diabetic neuropathy.
Background
Neuropathic pain is caused by damage to nerves, either from injury or disease. Pain is described as chronic if it has been experienced on most days for at least three months. Capsaicin is what makes chilli peppers hot. It is thought to reduce chronic neuropathic pain by making nerves insensitive to pain messages. This review is an update of one last published in 2013, and is about a highly concentrated preparation of capsaicin (8%) that must be administered in carefully controlled conditions in a clinic or hospital, often following local anaesthetic, because without special precautions it can initially cause pain a feeling of burning on the skin. It is used only to treat localised areas of pain. The single application is designed to produce relief of pain for up to three months.
Study characteristics
We searched scientific databases for studies that looked at the effects of high‐concentration capsaicin in adults who had moderate or severe neuropathic pain. The treatment had to have effects measured for at least 8 weeks. The evidence is current to June 2016.
Eight studies satisfied our inclusion criteria, including two new studies for this update. The studies were well conducted.
Key results
In seven studies, involving 2442 participants, we found that the treatment gave good levels of pain relief to a small number of participants with some types of neuropathic pain (pain after shingles, and nerve injury pain associated with HIV infection), and probably also in another type (painful feet because of damaged nerves caused by diabetes). About 4 in 10 people had at least moderate pain relief with capsaicin compared with 3 in 10 with control. The control was a treatment that looked the same but did not contain high levels of capsaicin, with either nothing added, or very small amounts of capsaicin added. In one small study (46 participants) in people with persistent pain after hernia surgery, it did not seem better than control.
In all people who have this treatment there can be short‐lived localised skin problems such as redness, burning, or pain. Serious problems seem to be uncommon, and were no more frequent in these trials with high‐concentration capsaicin than with control using very low‐concentration capsaicin or placebo.
Slightly more people treated with control rather than capsaicin dropped out of the studies because of lack of benefit, but there was no difference between the groups for drop‐outs because of side effects.
Quality of the evidence
We judged the quality of the evidence as moderate or very low for pain relief outcomes, mainly because only a small number of studies and moderate number of participants provided information for each outcome. We judged the quality of the evidence as moderate for harmful effects. Moderate quality means that further research may change the result. Very low quality means we are very uncertain about the results.
Summary of findings
Summary of findings 1. High‐concentration (8%) capsaicin patch compared with control patch (0.4%) for postherpetic neuralgia.
| High‐concentration (8%) capsaicin patch compared with control patch (0.4%) for postherpetic neuralgia | ||||||
|
Patient or population: adults with postherpetic neuralgia Settings: community Intervention: high‐concentration (8%) capsaicin patch, single application Comparison: control patch (0.4% capsaicin), single application | ||||||
| Outcomes | Outcome with intervention | Outcome with comparator | RR, NNT, NNH, NNTp (95% CI) | Number of studies, participants, events | Quality of the evidence (GRADE) | Comments |
| Substantial benefit | ||||||
| PGICvery much improved, week 8 and week 12 | No data | No data | ‐ | ‐ | Very low | No data |
| Moderate benefit | ||||||
| PGICmuch or very much improved, week 8 | 360 in 1000 | 250 in 1000 | RR 1.4 (1.1 to 1.8) NNT 8.8 (5.3 to 26) |
2 studies, 571 participants, 178 events | Moderate | Downgraded 1 level due to susceptibility to publication bias |
| PGIC much or very much improved, week 12 | 390 in 1000 | 250 in 1000 | RR 1.6 (1.2 to 2.0) NNT 7.0 (4.6 to 15) |
2 studies, 571 participants, 189 events | Moderate | Downgraded 1 level due to susceptibility to publication bias |
| Harm ‐ all conditions combined | ||||||
| Withdrawals due to lack of efficacy | 15 in 1000 | 31 in 1000 | RR 0.58 (0.32 to 1.04) NNTp 64 (34 to 610) |
6 studies, 2073 participants, 44 events | Moderate | Downgraded 1 level due to imprecision (few events, wide CI) |
| Withdrawals due to adverse events | 8.0 in 1000 | 9.2 in 1000 | RR 0.80 (0.36 to 1.8) NNTp not calculated |
8 studies, 2487 participants, 21 events | Moderate | Downgraded 1 level due to sparse data (few events) |
| Serious adverse events | 35 in 1000 | 32 in 1000 | RR 1.1 (0.70 to 1.8) NNH not calculated |
7 studies, 1993 participants, 67 events | Moderate | Downgraded 1 level due to sparse data (few events) |
| Death | 4 events | 2 events | Not calculated | 8 studies, 2487 participants | Very low | Downgraded 3 levels as only six events, so no better grading possibleNo death was judged related to study medication by study authors |
| CI: confidence interval; NNH: number needed to treat for one additional harmful outcome; NNT: number needed to treat for one additional beneficial outcome; NNTp: number needed to treat to prevent one withdrawal event; PGIC: Patient Global Impression of Change; RR: risk ratio. | ||||||
| Descriptors for levels of evidence (EPOC 2015):
High quality: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low.
Moderate quality: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate.
Low quality: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high.
Very low quality: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high. † Substantially different: a large enough difference that it might affect a decision. | ||||||
Background
This is an update of a review of high‐concentration (8%) capsaicin for relief of neuropathic pain, published in 2013 (Derry 2013). Low‐concentration capsaicin, usually as a cream or spray that requires regular application, is considered in a separate review (Derry 2012).
Description of the condition
The 2011 International Association for the Study of Pain definition of neuropathic pain is "pain caused by a lesion or disease of the somatosensory system" (Jensen 2011), based on an earlier consensus meeting (Treede 2008). Neuropathic pain is a consequence of a pathological maladaptive response of the nervous system to 'damage' from a wide variety of potential causes. It is characterised by pain in the absence of a noxious stimulus and may be spontaneous (continuous or paroxysmal) in its temporal characteristics or be evoked by sensory stimuli (dynamic mechanical allodynia where pain is evoked by light touch of the skin). Neuropathic pain is associated with a variety of sensory loss (numbness) and sensory gain (allodynia) clinical phenomena, the exact pattern of which vary between person and disease, perhaps reflecting different pain mechanisms operating in an individual person and therefore potentially predictive of response to treatment (Demant 2014; Helfert 2015; von Hehn 2012). Preclinical research hypothesises a bewildering array of possible pain mechanisms that may operate in people with neuropathic pain, which largely reflect pathophysiological responses in both the central and peripheral nervous systems, including neuronal interactions with immune cells (Baron 2012; Calvo 2012; von Hehn 2012). Overall, even the most effective of available drugs provide only modest benefit in treating neuropathic pain (Finnerup 2015; Moore 2013a), and a robust classification of neuropathic pain is not yet available (Finnerup 2013).
Neuropathic pain is usually divided according to the cause of nerve injury. There may be many causes, but common causes of neuropathic pain include diabetes (painful diabetic neuropathy (PDN)), shingles (postherpetic neuralgia (PHN)), amputation (phantom limb pain), neuropathic pain after surgery or trauma, stroke or spinal cord injury, trigeminal neuralgia, and HIV infection. Sometimes the cause is not known.
Many people with neuropathic pain conditions are significantly disabled with moderate or severe pain for many years. Chronic pain conditions comprised five of the 11 top‐ranking conditions for years lived with disability in 2010 (Vos 2012), and are responsible for considerable loss of quality of life, employment, and increased healthcare costs (Bouhassira 2012; Moore 2014a).
In systematic reviews, the overall prevalence of neuropathic pain in the general population is reported to be between 7% and 10% (van Hecke 2014), and about 7% in one systematic review of studies published since 2000 (Moore 2014a). In individual countries, prevalence rates have been reported as 3.3% in Austria (Gustorff 2008), 6.9% in France (Bouhassira 2008), and up to 8% in the UK (Torrance 2006). Some forms of neuropathic pain are increasing, particularly PDN and postsurgical chronic pain (which is often neuropathic in origin)(Hall 2008). The prevalence of PHN is likely to fall if vaccination against the herpes virus becomes widespread.
Estimates of incidence vary between individual studies for neuropathic pain associated with particular conditions, often because of small numbers of cases. In primary care in the UK between 2002 and 2005, the incidences (per 100,000 person‐years' observation) were 28 (95% confidence interval (CI) 27 to 30) for PHN, 27 (95% CI 26 to 29) for trigeminal neuralgia, 0.8 (95% CI 0.6 to 1.1) for phantom limb pain, and 21 (95% CI 20 to 22) for PDN (Hall 2008). Other research groups have estimated an incidence of 4 in 100,000 per year for trigeminal neuralgia (Katusic 1991; Rappaport 1994), and of 12.6 per 100,000 person‐years for trigeminal neuralgia and 3.9 per 100,000 person‐years for PHN in one study of facial pain in the Netherlands (Koopman 2009).
Neuropathic pain is difficult to treat effectively, with only a minority of people experiencing a clinically relevant benefit from any one intervention. A multidisciplinary approach is now advocated, with pharmacological interventions being combined with physical or cognitive interventions, or both. Conventional analgesics are usually thought to be ineffective, but without evidence to support or refute that view. Some people with neuropathic pain may derive some benefit from a topical lidocaine patch or low‐concentration topical capsaicin, though evidence about benefits is uncertain (Derry 2012; Derry 2014). The earlier review of high‐concentration topical capsaicin indicated benefit in some people with PHN (Derry 2013). Treatment for neuropathic pain is more usually with so‐called unconventional analgesics (pain modulators), for example, with antidepressants such as duloxetine and amitriptyline (Lunn 2014; Moore 2012a; Sultan 2008), or antiepileptic drugs such as gabapentin or pregabalin (Moore 2009; Moore 2014b; Wiffen 2013).
The proportion of people who achieve worthwhile pain relief (typically at least 50% pain intensity reduction (PIR); Moore 2013b) with any one intervention is small, generally only 10% to 25% more than with placebo, with numbers needed to treat for an additional beneficial outcome (NNT) usually between 4 and 10 (Kalso 2013; Moore 2013a; Moore 2014c). Neuropathic pain is not particularly different from other chronic pain conditions in that only a small proportion of trial participants have a good response to treatment (Moore 2013a).
The current National Institute for Health and Care Excellence (NICE) guidance for the pharmacological management of neuropathic pain suggests offering a choice of amitriptyline, duloxetine, gabapentin, or pregabalin as initial treatment for neuropathic pain (with the exception of trigeminal neuralgia), with switching if first, second, or third drugs tried are not effective or not tolerated (NICE 2013). This concurs with other recent guidance (Finnerup 2015).
Topical agents are most likely to be used for localised, peripheral neuropathies.
Description of the intervention
Topical medications are applied externally and are taken up through the skin. They exert their effects close to the site of application, and there is no substantial systemic uptake or distribution. This compares with transdermal application, where the medication is applied externally and is taken up through the skin, but relies on systemic distribution for its effect.
Low‐concentration capsaicin creams have not convincingly been shown to be effective for neuropathic pain (Derry 2012). The initial burning sensation felt on application of capsaicin limits the amount of active substance that can be applied at one time, which necessitates frequent (four times per day) application, and reduces compliance with treatment. The high‐concentration (8%) patch was developed to increase the amount of capsaicin delivered to the skin, and improve tolerability. Rapid delivery is thought to improve tolerability because cutaneous nociceptors are 'defunctionalised' quickly, and the single application avoids both noncompliance and contamination of the home environment with particles of dried capsaicin cream (Anand 2011). At the time of this review, the 8% patch is the only high‐strength formulation of capsaicin commercially available, although different strengths and formulations have been investigated in clinical trials. For the purposes of this review, we considered 5% or greater to be a high concentration.
The treatment is usually applied as a single application dermal patch over the area where painful symptoms are felt. Each patch (280 cm2) contains capsaicin 640 μg/cm2, and can be cut to treat smaller areas and irregular shapes, or up to four patches can be used simultaneously to treat large areas, such as the back (eMC 2012). The skin to which patches are applied should not be broken or irritated. The skin is usually treated with a topical local anaesthetic (e.g. topical lidocaine 4% for 60 minutes) before application because the capsaicin may cause an intense burning sensation, and the anaesthetic is then washed off thoroughly, and the skin dried, before the patch is applied. Studies suggest that skin cooling is as effective as topical anaesthetic for relieving initial burning (Knolle 2013), or that any form of pretreatment in unnecessary (Kern 2014). The patch is left in place for 30 minutes when applied to the feet, or 60 minutes for other areas, before removal and careful cleansing of the skin with a specially formulated cleanser, to remove any residual capsaicin. Application must be carried out in a healthcare centre by trained personnel, and patients are usually monitored for up to two hours after treatment. Stringent conditions are required, and as well as using trained healthcare professionals, the treatment setting needs to be well ventilated and spacious due to the vapour of the capsaicin, and cough due to inhalation of capsaicin particles or dust is a hazard for both the healthcare professionals and the patients. Treatment can be repeated after 12 weeks if necessary.
High‐concentration capsaicin is available by prescription only; it was first licensed in Europe and the US in 2009. It was originally licensed in the European Union (EU) to treat neuropathic pain in patients without diabetes, but in 2015 the restriction on patients with diabetes was lifted. In the US, it is licensed only to treat PHN. The US Food and Drug Administration refused a license for neuropathic pain in HIV in 2012. We could not find information about marketed products outside Europe and the US.
How the intervention might work
Capsaicin is the active compound present in chilli peppers, responsible for making them hot when eaten. It binds to nociceptors (sensory receptors responsible for sending signals that cause the perception of pain) in the skin, and specifically to the TRVP1 receptor, which controls movement of sodium and calcium ions across the cell membrane. Initially, binding opens the ion channel (influx of sodium and calcium ions), causing depolarisation and the production of action potentials, which are usually perceived as itching, pricking, or burning sensations. Repeated applications or high concentrations give rise to a long‐lasting effect, which has been termed 'defunctionalisation', probably owing to a number of different effects that together overwhelm the cell's normal functions, and can lead to reversible degeneration of nerve terminals (Anand 2011).
Adverse events from capsaicin are mainly at the application site (burning, stinging, erythema), and systemic events are rare. Achieving double‐blind conditions in placebo‐controlled trials using capsaicin can therefore be difficult.
Why it is important to do this review
Since the review in 2013, there have been new studies involving high‐dose capsaicin in different neuropathic pain conditions and in different formulations. The licensed indications have also changed for Europe (and possibly other jurisdictions). It is important, therefore, to update the review to include the latest information to inform clinical practice.
Objectives
To review the evidence from controlled trials on the efficacy and tolerability of topically applied, high‐concentration (8%) capsaicin for neuropathic pain in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, double‐blind trials comparing high‐concentration (typically 8%) topical capsaicin with placebo or other active treatment for neuropathic pain, with at least 10 participants per treatment arm. We excluded studies published only as short abstracts (usually meeting reports) or studies of experimentally induced pain.
Types of participants
Adults (aged 16 years or more) with neuropathic pain of at least moderate intensity (Collins 1997) resulting from any cause, with a duration of at least 12 weeks and as defined in the study using accepted diagnostic criteria.
Types of interventions
Included studies had at least one treatment arm using a single application of high‐concentration (8%) topical capsaicin, and a comparator arm using placebo or other active treatment.
Types of outcome measures
We anticipated that studies would use a variety of outcome measures, with most studies using standard subjective scales (numerical rating scale or visual analogue scale) for pain intensity or pain relief, or both. We were particularly interested in Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) definitions for moderate and substantial benefit in chronic pain studies (Dworkin 2008). These are defined as:
at least 30% pain relief over baseline (moderate);
at least 50% pain relief over baseline (substantial);
much or very much improved on Patient Global Impression of Change scale (PGIC; moderate);
very much improved on PGIC (substantial).
These dichotomous outcomes are important where pain responses do not follow a normal (Gaussian) distribution. People with chronic pain desire high levels of pain relief, ideally more than 50% PIR, and ideally having no worse than mild pain (Moore 2013b; O'Brien 2010).
Primary outcomes
Participant‐reported pain intensity reduction (PIR) of 30% or greater.
Participant‐reported PIR of 50% or greater.
Patient Global Impression of Change (PGIC) much or very much improved.
PGIC very much improved.
Secondary outcomes
Any pain‐related outcome indicating some improvement.
Withdrawals due to lack of efficacy and adverse events.
Participants experiencing local adverse events (application site events) and systemic adverse events.
Participants experiencing any serious adverse event. Serious adverse events typically include any untoward clinical occurrence or effect that at any dose results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, is a congenital anomaly or birth defect, is an 'important medical event' that may jeopardise the person, or may require an intervention to prevent one of the above characteristics or consequences.
Specific adverse events, particularly local skin reactions.
We anticipated that outcomes would be reported after different durations of treatment, and extracted data reported around 8 to 12 weeks as this is the expected duration of a single‐dose administration of high‐concentration topical capsaicin, but not generally to examine outcomes at less than 6 weeks because that would be considered an inadequate duration of effect. Where longer‐duration outcomes were available, we also extracted these. We also anticipated that reporting of adverse events would vary between trials with regard to the terminology used, method of ascertainment, and categories that were reported (e.g. occurring in at least 5% of participants or where there was a statistically significant difference between treatment groups). Care was taken to identify these details.
Search methods for identification of studies
Electronic searches
For this update, we searched the following databases, without language restriction.
Cochrane Central Register of Controlled Trials (CENTRAL, via the Cochrane Register of Studies Online database) to 10 June 2016.
MEDLINE via Ovid (January 2012 to 10 June 2016).
Embase via Ovid (January 2012 to 10 June 2016).
See Appendix 1 for the CENTRAL search strategy, Appendix 2 for the MEDLINE search strategy, and Appendix 3 for the Embase search strategy.
Searching other resources
We also searched the reference lists of review articles and included studies, together with two clinical trials databases (ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (ICTRP); www.who.int/ictrp/en/), and the Astellas clinical trials website. We did not search grey literature and short abstracts, or directly contact manufacturers and license holders for unpublished clinical trial data for this update.
Data collection and analysis
Two review authors independently selected the studies for inclusion, assessed risk of bias, and extracted data. One review author entered data for analyses, which was checked by another review author. We resolved disagreements through discussion.
Selection of studies
We reviewed the titles and abstracts of studies identified by the searches on‐screen to eliminate those that clearly did not satisfy the inclusion criteria. We obtained full reports of the remaining studies to determine inclusion in the review. We considered cross‐over studies only if data from the first treatment period were reported separately.
We did not anonymise the studies before assessment. We have included a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart (Figure 1).
1.
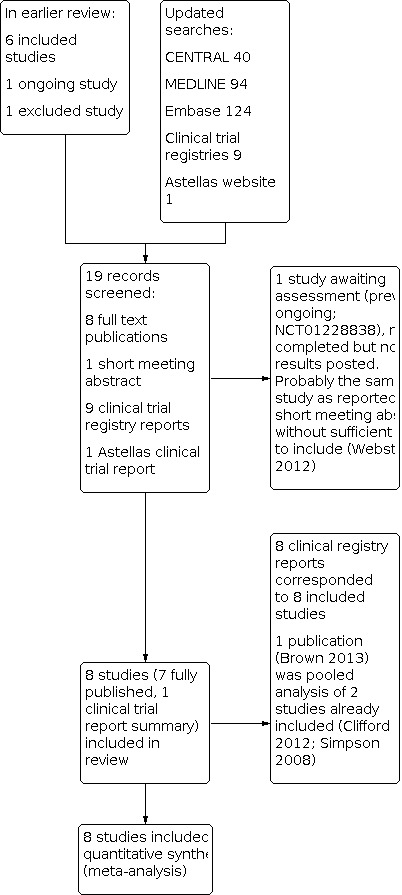
Study flow diagram.
Data extraction and management
We abstracted information on participants, interventions, and outcomes from the original reports into a standard data extraction form and checked for agreement before entry into Review Manager 5 (RevMan 2014) or any other analysis tool. We included information about the pain condition and number of participants treated, drug and dosing regimen, study design (placebo or active control), study duration and follow‐up, analgesic outcome measures and results, withdrawals, and adverse events. We did not contact authors for further information.
Assessment of risk of bias in included studies
We used the Oxford Quality Score (Jadad 1996) as the basis for inclusion, limiting inclusion to studies that, as a minimum, were randomised and double‐blind.
The review authors independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 8, Higgins 2011), and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We assessed the following for each study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, such as random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies at a high risk of bias that used a nonrandom process (odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment assessed whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as: low risk of bias (telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation and were therefore at a high risk of bias (open list).
Blinding of participants and personnel, and outcome assessment (checking for possible performance and detection bias). We assessed the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, identical tablets; matched in appearance and smell); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved). We excluded studies at a high risk of bias that were not double‐blind.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (fewer than 10% of participants did not complete the study or used 'baseline observation carried forward' analysis, or both); unclear risk of bias (used 'last observation carried forward' (LOCF) analysis); high risk of bias (used 'completer' analysis).
Size (checking for possible biases confounded by small size). Small studies have been shown to overestimate treatment effects, probably due to methodological weaknesses (Dechartres 2013; Nüesch 2010). We assessed studies as: low risk of bias if they had at least 200 participants per treatment arm; unclear risk of bias if they had 50 to 200 participants per treatment arm; high risk of bias if they had fewer than 50 participants per treatment arm.
Measures of treatment effect
We used risk ratio (RR) to establish statistical difference. We used numbers needed to treat for an additional beneficial outcome (NNT) and pooled percentages as an absolute measure of benefit.
We used the following terms to describe adverse outcomes in terms of harm or prevention of harm:
When significantly fewer adverse outcomes occurred with capsaicin than with control (placebo or active) we used the term the number needed to treat to prevent one event (NNTp).
When significantly more adverse outcomes occurred with capsaicin compared with control (placebo or active) we used the term the number needed to treat for an additional harmful outcome or cause one event (NNH).
Unit of analysis issues
We accepted randomisation to the individual participant only. In the event of a study having more than one active treatment arm, in which data were not combined for analysis, we planned to split the control treatment arm between active treatment arms. For cross‐over studies, we planned to use only the first period data.
Dealing with missing data
The most likely source of missing data was expected to be from participants dropping out from the studies. We looked specifically for evidence of LOCF and used a dichotomous responder analysis, where a responder was defined as a participant who experienced the predefined outcome and remained in the study (e.g. did not withdraw due to adverse events). LOCF is a potential source of major bias in chronic pain studies (Moore 2012b).
For all outcomes, we carried out analyses, as far as possible, on a modified intention‐to‐treat (ITT) basis (we included all participants who were randomised and received an intervention). Where sufficient information was reported, we added back missing data in the analyses we undertook.
Assessment of heterogeneity
We planned to deal with clinical heterogeneity by combining studies that examined similar conditions, and to assess statistical heterogeneity visually (L'Abbé 1987), and with using the I2 statistic. When the I2 value was greater than 50%, we considered possible reasons for this.
Assessment of reporting biases
We planned to assess publication bias by examining the number of participants in trials with zero effect (RR 1.0) needed for the point estimate of the NNT to increase beyond a clinically useful level (Moore 2008a). In this case, we specified a clinically useful level as an NNT of 10 for clinical improvement at 8 or 12 weeks.
Data synthesis
We conducted analyses of all efficacy outcomes according to type of painful condition, because interventions are known to have different effects in different types of neuropathic pain (Moore 2009). For adverse events, we combined all conditions.
At least 200 participants had to be available for any outcome before we pooled studies (Moore 1998). Where appropriate, we calculated RR with 95% CI using a fixed‐effect model (Morris 1995). We calculated NNT and NNH with 95% CIs using the pooled number of events, using the method devised by Cook and Sackett (Cook 1995). We assumed a statistically significant difference from control when the 95% CI of the RR did not include the number one.
If we had found significant clinical heterogeneity and considered it appropriate to combine studies, we would have investigated it using a random‐effects model.
Quality of the evidence
We used the GRADE system to assess the quality of the evidence related to the key outcomes listed in Types of outcome measures, as appropriate (Appendix 4). Two review authors independently rated the quality of each outcome.
We paid particular attention to inconsistency, where point estimates vary widely across studies or CIs of studies show minimal or no overlap (Guyatt 2011), and potential for publication bias, based on the amount of unpublished data required to make the result clinically irrelevant (Moore 2008a).
In addition, there may be circumstances where the overall rating for a particular outcome needs to be adjusted as recommended by GRADE guidelines (Guyatt 2013a). For example, if there are so few data that the results are highly susceptible to the random play of chance, or if studies use LOCF imputation in circumstances where there are substantial differences in adverse event withdrawals, one would have no confidence in the result, and would need to downgrade the quality of the evidence by three levels, to very low quality. In circumstances where there were no data reported for an outcome, we would have reported the level of evidence as very low quality (Guyatt 2013b).
'Summary of findings' table
We included a 'Summary of findings' table, as set out in the author guide (PaPaS 2012), and recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 4, Higgins 2011; Table 1). We included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes of 'substantial benefit' (PGIC very much improved from weeks 2 to 8 and weeks 2 to 12), 'moderate benefit' (PGIC much or very much improved from weeks 2 to 8 and weeks 2 to 12), withdrawals due to adverse events, withdrawals due to lack of efficacy, serious adverse events, and death (a particular serious adverse event).
For the 'Summary of findings' table we used the following descriptors for levels of evidence (EPOC 2015).
High: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low.
Moderate: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate.
Low: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high.
Very low: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high.
† Substantially different: a large enough difference that it might affect a decision.
Subgroup analysis and investigation of heterogeneity
We planned all efficacy analyses to be according to individual painful conditions. We did not plan further subgroup analyses since experience of previous reviews indicated that there would be too few data for any meaningful subgroup analysis.
Sensitivity analysis
We did not plan any sensitivity analyses for this update.
Results
Description of studies
Results of the search
The earlier review included six studies, excluded one study, and identified one ongoing study. Updated searches identified 40 articles in CENTRAL, 94 in MEDLINE, and 124 in Embase. After screening titles and abstracts, we obtained full copies of two published reports. We also identified nine relevant clinical study reports in trial registries and one on the Astellas website. One study was reported in a short meeting abstract.
One of the published reports was a new study that satisfied our inclusion criteria (Bischoff 2014). The other published report was a pooled analysis of two studies already included in the review (Brown and colleagues, reporting on Clifford 2012 and Simpson 2008). The unpublished study on the Astellas website satisfied our inclusion criteria (STEP 2014); this has since been published, and checked against our data extraction (Simpson and colleagues, 2016, see under STEP 2014). Eight reports identified in clinical trial registries related to the six previously included studies, the new published study, and the study identified on the Astellas website. The remaining report was previously identified as an ongoing study, which has now completed, but no results have been posted (NCT01228838). It is likely that this study is the one reported in a short meeting abstract (Webster and colleagues, see under NCT01228838). There was insufficient information to include this study in the review, and it has been placed in 'Studies awaiting assessment'. See Figure 1.
Included studies
We included eight studies, with 2488 participants. Six were in the earlier review (Backonja 2008; Clifford 2012; Irving 2011; Simpson 2008; Webster 2010a; Webster 2010b) and two were new studies (Bischoff 2014; STEP 2014).
Participants had pain due to PHN (Backonja 2008; Backonja 2010; Irving 2011; Webster 2010a; Webster 2010b), HIV‐neuropathy (Clifford 2012; Simpson 2008), painful PDN (STEP 2014), and persistent pain after inguinal herniorrhaphy (Bischoff 2014). In all studies, pain was of at least moderate severity and was frequently unresponsive to, or poorly controlled by, conventional therapy. In studies of PHN, the mean age of participants was 70 to 71 years and men and women were enrolled in approximately equal numbers. In studies of HIV‐neuropathy, the mean age of participants was 48 and 50 years and about 90% were men. For PDN, the mean age of participants was 63 years and 58% were men, while for persistent pain after inguinal herniorrhaphy, the mean age was 54 years and over 90% were men.
The duration of application of high‐concentration topical capsaicin varied between 30 and 90 minutes, with most participants treated for 60 minutes. Clifford 2012; Simpson 2008 and Webster 2010a tested different durations in participants with HIV‐neuropathy and PHN, while STEP 2014 treated the feet of all participants with PDN for 30 minutes. Bischoff 2014 treated participants with persistent pain after inguinal herniorrhaphy for 60 minutes.
All the included studies used a 'placebo' comparator. Because application of capsaicin to the skin, particularly at this high concentration, initially causes erythema (redness) and a burning or stinging sensation in many people, maintaining the double‐blind status of studies is problematic. Most studies used a low dose (0.04%) of capsaicin in the control patch to produce some degree of skin irritation without effective analgesia, in an attempt to prevent participants from guessing their treatment allocation, but two studies did not (Bischoff 2014; STEP 2014). We refer to these control patches as 'low‐concentration capsaicin control' and 'placebo control', respectively.
Most studies permitted stable treatment with concomitant oral or transdermal drugs (opioids of morphine equivalent 60 mg/day or less) to be continued for neuropathic pain without change in dose or frequency, but all topical medications were discontinued at least seven days before the study. STEP 2014 did not allow any oral, transdermal, or parenteral opioids at any dose in the seven days preceding patch application.
Details of included studies are in the Characteristics of included studies table.
Excluded studies
We excluded one study after reading the full report, because study duration was only 4 weeks (Backonja 2010; see the Characteristics of excluded studies table).
Risk of bias in included studies
We scored each study for methodological quality using the Oxford Quality Score; all studies scored 4/5 except Bischoff 2014, which scored 5/5, and Simpson 2008, which scored 3/5.
We completed a 'Risk of bias' table for all studies for sequence generation, allocation concealment, blinding, incomplete outcome data, and size. We judged all the studies to be low or unclear risk of bias for all criteria except Bischoff 2014, which we judged at high risk of bias for size. In most cases where the risk was assessed as 'unknown' it was likely that the methods were rigorous, but the reporting inadequate (e.g. randomisation, allocation concealment). Where there was incomplete outcome data due to missing values, the most relevant outcomes were reported without LOCF imputation.
Full details can be found in the Characteristics of included studies table and Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only Bischoff 2014 clearly reported the method of randomisation, but since these studies were carried out under rigorous conditions by the pharmaceutical company it is likely that the schedule was computer‐generated. Only Backonja 2008 and Irving 2011 adequately described the method used to conceal the sequence allocation.
Blinding
Studies were all described as double‐blind, and this was generally well described.
Incomplete outcome data
The nature of the studies was that all participants received the single application of topical high‐concentration capsaicin at the start of the study, but there were some withdrawals or losses to follow‐up thereafter, although these were generally small. Modified LOCF analysis was used for some efficacy outcomes, but no imputation was used for weekly pain scores or patient global assessment of treatment, where nonreporting was regarded as nonresponse. All participants were included for safety analyses.
Selective reporting
All relevant outcomes were reported according to the study methods, although there was inconsistency between studies in the exact outcomes reported.
Other potential sources of bias
Bischoff 2014 had small treatment groups, which can be associated with overestimation of effect. Otherwise, studies were generally large and apparently well conducted, so there were no other obvious sources of bias.
Effects of interventions
See: Table 1
Types of efficacy outcomes reported
In these studies, participants with chronic pain were given a single 30‐ to 90‐minute intervention with high‐concentration topical capsaicin, and their pain was then measured over the following 8 to 12 weeks. Because the intervention itself could cause localised pain at the application site, no pain measurements were generally made in the first post‐treatment week. The outcomes then reported were of two distinct types.
We assessed the longevity of benefit from a PGIC made at specific points, usually 8 and 12 weeks after drug administration. Responses of much or very much improved equated to 'moderate benefit', and very much improved to 'substantial benefit'. We considered these outcomes to provide the most reliable evidence. The expected pattern would be early, but not later, differences between active and control interventions.
Most studies calculated average pain scores over weeks 2 to 8 and 2 to 12, and recorded the number of participants with PIR of at least 30% or at least 50% over baseline. We considered these outcomes to provide less reliable evidence because they used data averaged over the study duration, and these studies used LOCF imputation for most missing data. These outcomes might be regarded as assessing whether the intervention 'worked' in providing a larger proportion of participants with adequate pain relief with the intervention than with control. Because the largest difference between active treatment and control typically occurred in the first 4 to 6 weeks after treatment, these measures did not adequately address for how long the benefits lasted.
Not all studies reported all of these outcomes, so data were inconsistently available for pooling and analysis. We have used what we consider to be the most reliable evidence for the 'Summary of findings' table.
Details of study efficacy outcomes are in Appendix 5, adverse events and withdrawals in Appendix 6, and patch tolerability in Appendix 7. Preliminary analyses demonstrated that duration of administration of high‐concentration topical capsaicin of 30 and 90 minutes for PHN and HIV‐neuropathy resulted in no discernible difference in efficacy from 60 minutes (Analysis 1.1; Analysis 1.3), so in the following analyses we combined results for different durations of patch application.
1.1. Analysis.

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 1: Postherpetic neuralgia (PHN) ‐ at least 50% pain intensity reduction over weeks 2 to 8
1.3. Analysis.

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 3: PHN ‐ at least 30% pain intensity reduction over weeks 2 to 8
Postherpetic neuralgia
PHN was the neuropathic pain condition in four studies involving 1272 participants (742 exposed to high‐concentration topical capsaicin, 530 to low‐concentration 0.04% capsaicin control) (Backonja 2008; Irving 2011; Webster 2010a; Webster 2010b). Not all outcomes were reported in all studies, with the exception of at least 30% PIR over 2 to 8 weeks compared with baseline pain, which all four studies reported.
Pain intensity reduction of 30% or greater, or 50% or greater
Results for the different levels of PIR are shown in the 'Summary of results A' table. The magnitude of the treatment effect was similar for at least 30% reduction (moderate benefit) and at least 50% reduction (substantial benefit) over baseline for the average weekly pain intensity over 2 to 8 (at least 30% PIR 2 to 8 weeks; at least 50% PIR 2 to 8 weeks) and 2 to 12 weeks (at least 30% PIR 2 to 12 weeks; at least 50% PIR 2 to 12 weeks), with NNT point estimates of between 10 and 12 in comparisons with low‐concentration capsaicin controls (Figure 3; Analysis 1.2; Analysis 1.3; Analysis 1.4).
3.

Forest plot of comparison: 1 High‐concentration (8%) capsaicin versus control (single dose), outcome: 1.1 Postherpetic neuralgia ‐ at least 50% pain intensity reduction over weeks 2 to 8.
1.2. Analysis.

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 2: PHN ‐ at least 50% pain intensity reduction over 2 to 12 weeks
1.4. Analysis.

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 4: PHN ‐ at least 30% pain intensity reduction over weeks 2 to 12
We downgraded the quality of the evidence to very low because of imprecision (wide CIs), because of the uncertain effects of the use of LOCF imputation, and because of susceptibility to publication bias with point estimates for NNT of 10 or above.
Patient Global Impression of Change much or very much improved
Results for PGIC are shown in the 'Summary of results A' table.
There were no data reported for PGIC very much improved (substantial benefit).
Only two of the four studies reported PGIC outcomes of much or very much improved (moderate benefit) at 8 and 12 weeks (Irving 2011; Webster 2010b). At both 8 and 12 weeks, there was a significant benefit for high‐concentration over low‐concentration topical capsaicin, with point estimates of the NNTs of 8.8 for high‐concentration and 7.0 for low‐concentration (Figure 4).
4.

Forest plot of comparison: 1 High‐concentration (8%) capsaicin versus control (single dose), outcome: 1.5 Postherpetic neuralgia ‐ Patient Global Impression of Change much or very much improved at 8 and 12 weeks.
We downgraded the quality of the evidence to moderate due to susceptibility to publication bias as null effect data from only about 250 participants would be needed to raise the NNT above 10 (Moore 2008a). See Table 1.
Summary of results A
| Outcome | Number | Per cent with outcome | RR (95% CI) | NNT (95% CI) | ||
| Trials | Participants | 8% Capsaicin | Control | |||
| Postherpetic neuralgia | ||||||
| ≥ 30% PIR 2 to 8 weeks | 4 | 1272 | 43 | 34 | 1.3 (1.1 to 1.5) | 11 (6.8 to 26) |
| ≥ 30% PIR 2 to 12 weeks | 3 | 973 | 46 | 37 | 1.3 (1.1 to 1.5) | 10 (6.3 to 28) |
| ≥ 50% PIR 2 to 8 weeks | 3 | 870 | 29 | 20 | 1.4 (1.1 to 1.9) | 12 (7.2 to 41) |
| ≥ 50% PIR 2 to 12 weeks | 2 | 571 | 33 | 24 | 1.3 (1.0 to 1.7) | 11 (6.1 to 62) |
| PGIC much/very much improved at 8 weeks | 2 | 571 | 36 | 25 | 1.4 (1.1 to 1.8) | 8.8 (5.3 to 26) |
| PGIC much/very much improved at 12 weeks | 2 | 571 | 39 | 25 | 1.6 (1.2 to 2.0) | 7.0 (4.6 to 15) |
| HIV‐neuropathy | ||||||
| ≥30% PIR 2 to 12 weeks | 2 | 801 | 39 | 30 | 1.4 (1.1 to 1.7) | 11 (6.2 to 47) |
| PGIC much/very much 12 weeks* | 1 | 307 | 27 | 10 | 2.8 (1.4 to 5.6) | 5.8 (3.8 to 12) |
| Peripheral diabetic neuropathy | ||||||
| ≥ 30% PIR 2 to 8 weeks* | 1 | 369 | 40 | 33 | 1.2 (0.92 to 1.6) | Not calculated |
| ≥ 30% PIR 2 to 12 weeks* | 1 | 369 | 41 | 32 | 1.3 (0.98 to 1.7) | Not calculated |
| ≥ 50% PIR 2 to 8 weeks* | 1 | 369 | 21 | 18 | 1.2 (0.77 to 1.8) | Not calculated |
| ≥ 50% PIR 2 to 12 weeks* | 1 | 369 | 22 | 19 | 1.2 (0.77 to 1.7) | Not calculated |
| PGIC much/very much 8 weeks* | 1 | 369 | 38 | 28 | 1.3 (1.0 to 1.8) | 10 (5.2 to 520) |
| PGIC much/very much 12 weeks* | 1 | 369 | 36 | 28 | 1.2 (0.92 to 1.7) | Not calculated |
| * Note that these results are from > 200 participants, but from a single study and so should be treated with caution. They are reported for comparative purposes only. | ||||||
| CI: confidence interval; NNT: number needed to treat for an additional beneficial outcome; PGIC: Patient Global Impression of Change; PIR: pain intensity reduction; RR: risk ratio | ||||||
HIV‐neuropathy
Painful HIV‐neuropathy was the neuropathic pain condition in two studies involving 801 participants (557 exposed to high‐concentration topical capsaicin, 244 to low‐concentration 0.04% capsaicin control) (Clifford 2012; Simpson 2008). All outcomes were not reported in both studies, with the exception of at least 30% PIR over 2 to 12 weeks compared with baseline pain.
Pain intensity reduction of 30% or greater, or 50% or greater
Neither study reported at least 50% PIR over baseline (substantial benefit).
Both studies reported at least 30% PIR (moderate benefit) over 2 to 12 weeks compared with baseline ('Summary of results A' table) (Figure 5). The point estimate of the NNT was 11.
5.

Forest plot of comparison: 1 High‐concentration (8%) capsaicin versus control (single dose), outcome: 1.6 HIV‐neuropathy ‐ at least 30% pain intensity reduction over weeks 2 to 12.
We downgraded the quality of the evidence to very low because of imprecision (wide CIs), because of the uncertain effects of the use of LOCF imputation, and because of susceptibility to publication bias with a point estimate for NNT above 10.
Patient Global Impression of Change much or very much improved
We found no data for PGIC very much improved (substantial benefit).
One study reported PGIC of much or very much improved (moderate benefit) at 12 weeks (Simpson 2008). Results are shown in the 'Summary of results A' table for comparison, but they derive from a single study and should be interpreted with caution. There was a significant benefit for high‐concentration topical capsaicin over low‐concentration control, with a point estimate for the NNT of 5.8.
We downgraded the quality of the evidence to very low due to sparse data, imprecision, and susceptibility to publication bias.
Peripheral diabetic neuropathy
STEP 2014 treated 369 participants with painful PDN (186 exposed to high‐concentration topical capsaicin, 183 to placebo), and reported outcomes over 8 and 12 weeks.
Pain intensity reduction of 30% or greater, or 50% or greater
The study reported both 30% and 50% PIR over 2 to 8 and 2 to 12 weeks compared with baseline. About 10% more participants had at least a 30% reduction with high‐concentration capsaicin than with placebo at both time points. The response rate was lower for at least 50% PIRs, and only 3% higher with capsaicin than with placebo ('Summary of results A' table).
We downgraded the quality of the evidence to very low because of imprecision (wide CIs), uncertain effects of the use of LOCF imputation, and susceptibility to publication bias.
Patient Global Impression of Change much or very much improved
We found no data for PGIC very much improved (substantial benefit).
About 10% more participants reported PGIC outcomes of much or very much improved (moderate benefit) at 8 and 12 weeks with capsaicin than with placebo ('Summary of results A' table).
We downgraded the quality of the evidence to very low due to sparse data, imprecision, and susceptibility to publication bias.
Persistent pain after inguinal herniorrhaphy
Bischoff 2014 treated 45 participants with persistent pain after inguinal herniorrhaphy (23 exposed to high‐concentration capsaicin, 22 to placebo).
The study did not report any responder analyses, but did report no significant difference in the summed pain intensity difference (from baseline) between groups at 4, 8, or 12 weeks after treatment. We downgraded the quality of the evidence to very low due to sparse data.
Subgroup analysis
Dose and condition
Analysis by pain condition has been carried out in the primary analysis above.
Adverse events
Reporting of adverse events was inconsistent and incomplete (Appendix 6). Most studies did not report the precise methods used to collect adverse event data, such as use of direct or indirect questioning or participant diaries, or the timing of data collection, but they did consistently classify adverse events and serious adverse events according to the Medical Dictionary for Regulatory Activities. Most adverse events were transient and mild to moderate in intensity. Five studies reported adverse events occurring in at least 3% (Backonja 2008; Clifford 2012; Irving 2011; Webster 2010a; Webster 2010b), and two in at least 2% (Simpson 2008; STEP 2014), of participants in any treatment arm, together with any serious adverse events. Bischoff 2014 reported all adverse events. The most common events were application site (skin) reactions.
Local skin reactions
All included studies reported on local skin reactions. Two studies used placebo control patches (Bischoff 2014; STEP 2014), but in the other studies the control patches contained a low concentration (0.04%) of capsaicin to mimic the burning sensation of capsaicin without providing effective pain relief. It was not possible to determine the number of participants experiencing any type of local skin reaction since more than one symptom may appear in an individual participant. We chose to analyse 'erythema, pain, papules, and pruritus' as these were fairly consistently reported in individual studies. For analysis, we combined studies in the different pain conditions and all durations of application since there were no obvious differences or trends and the number of events was small.
Some studies captured all adverse events following application. We defined these as Group 1 studies, which comprised Backonja 2008; Bischoff 2014; Clifford 2012; and Irving 2011 (Analysis 1.7). The other studies reported adverse events differently; these Group 2 studies comprised Simpson 2008, STEP 2014, Webster 2010a, and Webster 2010b (Analysis 1.8; 'Summary of results B' table). Two Group 2 studies specifically stated that "treatment associated erythema, discomfort and pain on the day of treatment were not captured as adverse events but reported as dermal assessment scores or 'Pain Now' scores" (Webster 2010a; Webster 2010b). They reported very much lower rates of skin adverse events, presumably because events in the first day were not included. The other Group 2 studies did not specify whether they included skin reactions on the first day as adverse events, but they also had a very much lower rate (Simpson 2008; STEP 2014), and are analysed with the Webster studies (Webster 2010a; Webster 2010b).
1.7. Analysis.

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 7: Local skin reactions ‐ group 1
1.8. Analysis.

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 8: Local skin reactions ‐ group 2
We downgraded the quality of the evidence to moderate due to inconsistent reporting and assumptions made in pooling studies for analysis.
Summary of results B
| Outcome | Number | Per cent with outcome |
RR (95% CI) |
NNH (95% CI) |
||
| Studies | Participants | 8% Capsaicin | Control | |||
| Group 1 | ||||||
| Erythema | 4 | 1355 | 75 | 57 | 1.4 (1.3 to 1.5) | 5.5 (4.3 to 7.7) |
| Pain | 4 | 1355 | 69 | 29 | 2.3 (2.0 to 2.6) | 2.5 (2.2 to 2.8) |
| Papules | 3 | 1312 | 6.3 | 2.0 | 3.6 (1.9 to 6.9) | 23 (16 to 46) |
| Pruritus | 3 | 1312 | 3.7 | 2.0 | 2.0 (0.98 to 4.0) | Not calculated |
| Oedema | 3 | 1312 | 3.9 | 1.2 | 3.0 (1.4 to 6.2) | 38 (23 to 110) |
| Group 2 | ||||||
| Erythema | 1 | 129 | 5.3 | 0 | Not calculated | Not calculated |
| Pain | 4 | 1005 | 9.9 | 3.8 | 2.4 (1.4 to 4.1) | 16 (11 to 31) |
| Papules | 3 | 735 | 3.4 | 2.4 | 1.6 (0.59 to 4.2) | Not calculated |
| Pruritus | 3 | 735 | 14 | 9.4 | 1.6 (0.98 to 2.5) | Not calculated |
| Oedema | 3 | 735 | 8.0 | 6.1 | 1.3 (0.75 to 2.4) | Not calculated |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; RR: risk ratio | ||||||
Patch tolerability
Use of local anaesthetic before application, local cooling, and availability of short‐acting opioids for pain relief in the first few days following treatment all help to increase tolerability of the treatment. Most of the studies assessed tolerability by the number of participants able to complete at least 90% of the intended application time, the degree of dermal irritation two hours after application (FDA 1999), and the numbers of participants using medication for treatment‐related discomfort on days zero to five ('Summary of results C' table). For analysis, we have combined studies in different neuropathic pain conditions and for all durations of application since there were no obvious differences or trends and the number of events was small for most outcomes (Analysis 1.9). Bischoff 2014 reported only that one participant experienced severe pain at the application site, which necessitated patch removal, and was withdrawn from the study. STEP 2014 reported the number of participants with dermal irritation scores of 4 or greater (scale 0 to 7) at 15 and 60 minutes after patch removal (0/186 with capsaicin and 2/183 with placebo at both time points).
1.9. Analysis.

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 9: Patch tolerability
We downgraded the quality of the evidence to moderate due to inconsistent reporting and assumptions made in pooling studies for analysis.
Summary of results C
| Outcome | Number | Per cent with outcome |
RR (95% CI) |
NNH (95% CI) |
||
| Studies | Participants | 8% Capsaicin | Control | |||
| < 90% application time | 6 | 2074 | 1.7 | 0.3 | 3.3 (1.2 to 9.2) | 77 (45 to 260) |
| DIS > 2 at 2 hours | 3 | 1065 | 11 | 0.7 | 12 (4.0 to 34) | 9.6 (7.7 to 13) |
| DIS > 0 at 2 hours | 2 | 606 | 40 | 18 | 2.3 (1.6 to 3.2) | 4.5 (3.3 to 6.7) |
| Pain medication 0 to 5 days | 7 | 2442 | 43 | 17 | 2.5 (2.2 to 2.9) | 3.8 (3.4 to 4.4) |
| CI: confidence interval; DIS: dermal irritation score; NNH: number needed to treat for an additional harmful outcome; RR: risk ratio | ||||||
The estimate for NNH for achieving less than 90% of the scheduled patch application time should be interpreted with caution since the numbers of participants with this outcome were very small (22/1300 (1.9%) with capsaicin and 2/774 (0.58%) with control).
Systemic adverse events
Systemic adverse events included diarrhoea, nausea, vomiting, fatigue, infections, musculoskeletal disorders, hypertension, dizziness, and headache. Individual events generally occurred in fewer than 5% of participants in each treatment arm, with no obvious differences between different doses and control arms (Appendix 6). Three studies specifically reported on cough, which occurred in 2% to 3% of participants treated with high‐concentration capsaicin and 0% to 4% of participants treated with control (Simpson 2008; Webster 2010a; Webster 2010b). No further analysis of systemic adverse events was carried out.
Serious adverse events
Serious adverse events were uncommon. Seven studies provided data for analysis (Backonja 2008; Bischoff 2014; Irving 2011; Simpson 2008; STEP 2014; Webster 2010a; Webster 2010b); 41/1175 (3.5%) of participants treated with high‐concentration capsaicin and 26/818 (3.2%) of participants treated with control experienced serious adverse events, giving an RR of 1.1 (95% CI 0.70 to 1.8) (Analysis 1.10; Figure 6). The NNH was not calculated. The remaining study reported that serious adverse events occurred with similar frequency in both treatment groups (6%), and judged none to be treatment‐related (Clifford 2012).
1.10. Analysis.

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 10: Serious adverse events
6.

Forest plot of comparison: 1 High‐concentration (8%) capsaicin versus control (single dose), outcome: 1.10 Serious adverse events.
One event was judged possibly related to study medication. This participant experienced increased blood pressure on the day of treatment following treatment with high‐concentration capsaicin (Backonja 2008).
We downgraded the quality of the evidence to moderate due to few events.
There were six deaths, four following treatment with high‐concentration capsaicin (one each in Clifford 2012; Irving 2011; Simpson 2008; Webster 2010a), and two following low‐concentration capsaicin control (both in Simpson 2008). None were judged related to study medication.
We downgraded the quality of the evidence to very low due to the very small number of events (six in total).
Withdrawals
Adverse events
There were 12 withdrawals due to adverse events in 1507 participants (0.80%) treated with high‐concentration capsaicin and nine withdrawals in 980 participants (0.92%) treated with control, giving an RR of 0.80 (95% CI 0.36 to 1.8); the NNH was not calculated (Analysis 1.11).
1.11. Analysis.

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 11: Withdrawals
We downgraded the quality of the evidence to moderate (few events, wide CIs).
Lack of efficacy
There were 20 withdrawals due to lack of efficacy in 1298 participants (1.5%) treated with high‐concentration capsaicin and 24 withdrawals in 775 participants (3.1%) treated with control, giving an RR of 0.58 (95% CI 0.32 to 1.04); the NNTp was 64 (95% CI 34 to 610) (Analysis 1.11).
Withdrawals for other reasons (such as lost to follow‐up) were generally below 10% and evenly distributed between treatment arms (Appendix 6). No further statistical analysis of withdrawals was carried out.
We downgraded the quality of the evidence to moderate (few events, wide CIs).
Discussion
Summary of main results
A single application of a high‐concentration (8%) capsaicin patch for 30 to 90 minutes provides significant pain relief for up to 12 weeks in some people with chronic pain arising from PHN or HIV‐neuropathy. The evidence we considered most reliable and trustworthy generated NNTs between about 6 and 9 measured at 8 or 12 weeks; for every seven to nine people treated, one will experience improvement in pain over 12 weeks who would not have done with control. These results for an outcome at a specific point in time were supported by positive benefits with a similar order of magnitude for outcomes that we considered less reliable, of people with average PIR of at least 50% or at least 30% measured over periods of time between 2 and 12 weeks.
There were insufficient data to draw any conclusions about treatment of PDN or persistent pain after inguinal herniorrhaphy. For PDN, there was a similar difference in response rate (about 10%) between capsaicin and placebo as was seen in studies in PHN and HIV‐neuropathy for the outcomes of much or very much improved and at least 30% PIR, but the difference was only 3% for at least 50% PIR. For persistent pain after inguinal herniorrhaphy, there was no difference between capsaicin and placebo for summed pain intensity difference from baseline at any time point.
These results might be compared with an NNT of 5.4 (95% CI 3.9 to 9.2) over 12 weeks for 600 mg pregabalin compared with placebo in 702 participants with PHN (Moore 2009). The NNT for much or very much improved in 1121 participants treated with gabapentin (any dose) compared with placebo yielded an NNT of 5.5 (95% CI 4.3 to 7.7) (Moore 2011a). No other drug therapies have comparable data sets for estimation of efficacy in PHN.
Painful HIV‐neuropathy is a condition in which there are no large comparable data sets, but where few therapies appear to demonstrate any benefit (Phillips 2010); topical high‐concentration capsaicin is therefore notable for providing some evidence of effective pain relief.
The annual incidence of PDN appears to be increasing, at least in the UK, and its incidence is similar to that of PHN; topical capsaicin is not a common initial treatment (Hall 2013). A number of oral therapies have NNTs of 5 or 6 for at least 50% PIR after 12 weeks (duloxetine 60 mg or 120 mg, gabapentin at doses of 1200 mg or above, and pregabalin 600 mg daily; Kalso 2013).
Treatments for chronic pain are characterised by the small proportion of people who obtain high degrees of treatment‐specific pain relief. However, the benefits of good pain relief go far beyond pain itself, with associated benefits in terms of improved sleep, reduced fatigue and depression, an overall improvement in quality of life, and even the ability to spend more time in employment or looking after the family (Azevedo 2016; Gülfe 2010; Hoffman 2010; Ikenberg 2012; Moore 2009; Straube 2011).
Use of capsaicin at the high concentration of 8% is associated with increased local skin reactions, primarily burning, stinging, and erythema, that affects many people, whether or not they obtain good pain relief, but these effects can be managed and resolve quickly after the single application.
Overall completeness and applicability of evidence
The earlier review identified studies in only two neuropathic pain conditions (PHN and HIV‐neuropathy), and, while this update found additional studies in PDN and postsurgical (inguinal herniorrhaphy) pain, there were too few data to draw any sensible conclusions in these conditions. This leaves a gap in our knowledge of the utility of high‐concentration capsaicin in a considerable proportion of people with localised neuropathic pain.
We found no double‐blind studies using an active comparator, so no direct comparisons with other treatments can be made.
The decision to exclude studies of less than 6 weeks' duration reduced the amount of evidence available to us; we excluded a single study with only 38 participants amounting to only about 3% of the total number of participants (Backonja 2010). We feel this was justified because benefits extending to only 4 weeks are unlikely to outweigh the considerable efforts associated with high‐concentration capsaicin use, at least at the moment.
The largest deficiencies resulted from inconsistent reporting, especially of efficacy outcomes. For example, for five of the six efficacy outcomes reported from the four PHN studies, complete data were available for analysis for only one outcome, and for the other five the amount available varied between 45% and 76% of the total participants. Importantly, both of the most reliable outcomes for benefit at 8 or 12 weeks after application were calculated using only 45% of participants. For HIV‐neuropathy, only two of the six outcomes were reported, and only one less‐reliable outcome reported in both studies. For PDN, all six efficacy outcomes were reported, but for inguinal herniorrhaphy pain none of our desired outcomes were reported.
This represents a considerable loss of evidence from otherwise high‐quality, well‐conducted, and mainly large studies. It is a deficiency that could affect the applicability of the results we have, and probably reflects the difficulties in reporting large, detailed, and complex clinical trials within the severe constraints of the allowable size of papers for publication. The deficiency should be rectified. Rectification would require no more studies, but rather better access to trial data, perhaps in the form of clinical trial reports, as has been done before (Moore 2005; Moore 2008b; Moore 2011b; Straube 2010).
Adverse event reporting was also limited by different ways of reporting data. This is a problem that has been commented upon previously in pain studies, and more generally (Edwards 1999; Loke 2001). The review did not specifically look for, or find, safety data relating to quantitative sensory testing or intra‐epidermal nerve fibre density.
The studies reviewed provided no information about long‐term efficacy and safety with repeated applications, but this has been investigated in a number of longer‐duration (52 weeks) studies, in a variety of neuropathic pain conditions (PACE 2014; Simpson 2014; STRIDE 2014). Generally, these studies have not demonstrated any additional safety issues with up to seven patch applications, and in some participants the therapeutic efficacy is maintained, or even improved, over time.
Quality of the evidence
The included studies were of generally high methodological quality, although with deficiencies in describing the process of randomisation and allocation concealment. Data handling of missing data did not adversely affect quality or involve any possible biases in studies that contributed efficacy data for meta‐analyses, but imputation methods were unclear in the two studies added to this update.
Because topical capsaicin is associated with erythema, burning, and local pain, a 'true' placebo was thought likely to lead to immediate unblinding. Six studies used 0.04% topical capsaicin as an 'active' placebo control, one that would mimic the local adverse effects of capsaicin without longer‐term pain relief. Responses to various outcomes with control were of the order of 25% of participants benefiting for the outcome of much or very much improved using PGIC in PHN, compared with 15% to 20% for the same outcome with placebo in trials of pregabalin (Moore 2009) and gabapentin (Moore 2011a). That might suggest that the low‐concentration control had some very small longer‐term benefit that would work to diminish the apparent efficacy of high‐concentration topical capsaicin, but this should not be considered more than speculation with the evidence available, especially relating to benefits from low‐concentration capsaicin creams (Derry 2012). Moreover, in the single study of PDN that used a 'true' placebo patch and reported PGIC, the placebo response (28%) was remarkably similar to the 'active' placebo response.
The use of stable concomitant medication throughout the studies may also have reduced their sensitivity to demonstrate an effect of high‐concentration capsaicin over placebo.
We downgraded the quality of the evidence for efficacy outcomes to moderate or very low because of the small number of studies and events, because use of LOCF imputation meant possible bias for some outcomes, imprecision, and susceptibility to publication bias. For harms, we downgraded the evidence to moderate because, despite adequate numbers of studies and participants, there were few events.
Potential biases in the review process
We used an extensive search strategy, and examined bibliographies, reference lists, clinical trial registries, and a pharmaceutical company database. High‐concentration capsaicin is a relatively recent therapy and it is unlikely that the search overlooked relevant high‐quality large studies. However, the relatively high NNTs for high‐concentration topical capsaicin combined with incomplete reporting of PGIC outcomes means that, for substantial benefit in PHN, null effect data from only about 250 participants would be needed to raise the NNT above 10 (Moore 2008a), at which point the efficacy of the therapy might be regarded as very limited. For moderate benefit, null effect data from only about 80 participants would be required.
Agreements and disagreements with other studies or reviews
Several reviews of high‐concentration capsaicin have been published since our earlier review. Burness 2016 and Űçeyler 2014 were narrative reviews, including different study types and all conditions; neither carried out any pooled analyses. Mou 2013 included the four‐week study that we excluded, but not the two new studies in this update; pooled analysis for percentage change and responder outcomes was performed, generating odds ratios and RRs, but not NNTs. An abstract of a network meta‐analysis in PDN showed no difference in efficacy between capsaicin 8% and pregabalin, gabapentin, and duloxetine, but reduced systemic adverse events; there were too few details to assess the methods used (van Nooten 2015). There was general agreement between these reviews that high‐concentration capsaicin can provide moderate or substantial benefit to a minority of people with localised, peripheral neuropathic pain, and that some people continue to derive benefit with repeated applications.
One open‐label study compared a single application of high‐concentration capsaicin with oral gabapentin titrated to a maximum of 600 mg daily, and did not demonstrate superiority of capsaicin over 8 weeks (ELEVATE 2014). Slightly more participants achieved 'optimal therapeutic effect' with capsaicin (147/282, 52%) than with pregabalin (124/277, 45%). 'Optimal therapeutic effect' was defined as at least 30% reduction in average pain for the past 24 hours from baseline to week eight, no discontinuation due to lack of efficacy or tolerability, no change in background chronic medication, and no moderate or severe adverse events during the stable treatment period). Withdrawals due to adverse events, lack of efficacy, and participant choice were all higher with pregabalin than with capsaicin.
Authors' conclusions
Implications for practice.
For people with chronic neuropathic pain
High‐concentration topical capsaicin is better than very low‐concentration capsaicin in people with postherpetic neuralgia. Good pain relief (moderate or substantial benefit for 2 to 12 weeks) is achieved by about 10% more people with high‐concentration capsaicin than control, after a single application. There is limited evidence that a similar proportion benefit in painful diabetic neuropathy and HIV‐neuropathy. What is less clear is how well repeated applications work, as the therapy needs to be repeated several times a year.
For clinicians
High‐concentration topical capsaicin is better than very low‐concentration capsaicin in people with postherpetic neuralgia. Good pain relief (moderate or substantial benefit for 2 to 12 weeks) is achieved by about 10% more people with high‐concentration capsaicin than control, after a single application. There is limited evidence that a similar proportion of people benefit in painful diabetic neuropathy and HIV‐neuropathy. What is less clear is how well repeated applications work, as the therapy needs to be repeated several times a year.
High‐concentration topical capsaicin is therefore similar to other therapies for chronic pain. The high cost of single and repeated applications suggest that high‐concentration topical capsaicin is likely to be used when other available therapies have failed, and that it should probably not be used repeatedly without substantial documented pain relief. Even when efficacy is established, there are unknown risks, especially on epidermal innervation, of repeated application over long periods.
Some clinicians would prefer to see more information on safety data relating to quantitative sensory testing or intra‐epidermal nerve fibre density.
For policy makers
It is clear that high‐concentration topical capsaicin works well in a small proportion of people with various forms of neuropathic pain, although the evidence is not of the highest quality. The problem is that there is no way of knowing in whom the therapy will work before using it, and there is little evidence about efficacy in repeated dosing, which is needed in chronic pain conditions. This therapy would probably be tried only after other therapies have been shown not to work. Ongoing use is probably only worthwhile in people with demonstrably high levels of pain intensity reduction.
For funders
It is clear that high‐concentration topical capsaicin works well in a small proportion of people with various forms of neuropathic pain, although the evidence is not of the highest quality. The problem is that there is no way of knowing in whom the therapy will work before using it, and there is little evidence about efficacy in repeated dosing, which is needed in chronic pain conditions. This therapy would probably be tried only after other therapies had been shown not to work. Ongoing use is probably only worthwhile in people with demonstrably high levels of pain intensity reduction.
Implications for research.
General
The general thrust of these findings is that high‐concentration topical capsaicin can provide good levels of pain relief in people with chronic neuropathic pain, but only about 1 in 10 more will benefit. It is now recognised that chronic pain generally, and chronic neuropathic pain in particular, is difficult to treat and that the most effective therapies give very good results to only a minority of people with these painful conditions.
For high‐concentration topical capsaicin, there are four important questions that are not addressed by this review, and are probably not captured by the extant clinical literature.
Is it possible to predict which people will benefit? The answer is almost certainly not, but there is ongoing research.
What do we know about long‐term use? Extension studies from randomised controlled trials have demonstrated that some people who benefited initially continue to benefit over up to seven applications, but the evidence is limited. There may be questions about whether the nature of pain changes with repeated use.
What do we know about the area of pain over the long term with repeated treatment? Very little, but there are suggestions at least that this may reduce in size.
What are the effects on quantitative sensory testing or intra‐epidermal nerve fibre density, or other measures of nerve function?
Practitioners might well supplement these observations with other questions or observations of their own, but in terms of evidence there are many known, and some unknown, unknowns.
Design
Trial designs would need to be radically different to capture answers to the research questions.
Measurement (endpoints)
A major issue is not in the measurement of pain, as most studies, especially modern ones, have used standard pain intensity and pain relief scales. However, reporting of average pain changes is inadequate, and the use of responder analyses (at least 50% pain intensity reduction, or participants experiencing mild or no pain) is preferred.
The inclusion of safety data relating to quantitative sensory testing or intra‐epidermal nerve fibre density may be both interesting and illuminating.
Comparison between active treatments
Indirect comparisons with carrier are probably as informative as use of an active comparator.
What's new
| Date | Event | Description |
|---|---|---|
| 29 July 2021 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 4, 2009
| Date | Event | Description |
|---|---|---|
| 18 February 2020 | Amended | Clarification added to Declarations of interest. |
| 29 May 2019 | Amended | Contact details updated. |
| 8 June 2017 | Amended | Small correction to wording in Abstract Main results. |
| 13 January 2017 | Review declared as stable | See Published notes. |
| 1 July 2016 | New citation required but conclusions have not changed | Additional data were for different neuropathic pain conditions. |
| 1 July 2016 | New search has been performed | New searches conducted on 10 June 2016; two new studies (415 participants) identified for inclusion. We have used GRADE used to assess the quality of the evidence, and added a Summary of findings table. |
| 18 February 2013 | Amended | Contact details updated. |
| 7 September 2012 | New citation required and conclusions have changed | Review of new and different pharmaceutical formulation. Original review split according to concentration of capsaicin in the product; this review considers high‐concentration (8%) capsaicin, while another review considers low‐concentration (< 1%) capsaicin (Derry 2012). We analysed the data to take account of revised guidelines for systematic reviews in pain. This new formulation is very different from previous low‐concentration capsaicin creams, with modern high‐quality, large studies, and with efficacy in postherpetic neuralgia and painful HIV‐neuropathy. |
| 7 September 2012 | New search has been performed | Search updated and four new studies identified. |
Notes
Assessed for updating in 2019
A new search within two years is not likely to identify any potentially relevant studies likely to change the conclusions. Therefore, following discussion with the authors and editors, this review has now been stabilised until 2021, at which point we will assess the review for updating. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Assessed for updating in 2021
An updated search did not identify any potentially relevant studies likely to change the conclusions. Therefore, following discussion with the authors and editors, this review has now been stabilised until 2026, at which point we will assess the review for updating. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
Support was provided by the National Health Service) (NHS) Cochrane Collaboration Programme Grant Scheme, and the National Institute for Health Research (NIHR) Biomedical Research Centre Programme. The Oxford Pain Relief Trust provided infrastructure support for this review.
The NIHR is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group.
Disclaimer: the views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
MeSH descriptor Capsaicin (400)
(capsaicin OR capsaicine OR capsici OR axsain OR capsidol OR capsig OR capsin OR capsina OR capsiplast OR capzasin‐P OR dolorac OR gelcen OR katrum OR "No pain‐HP" OR priltam OR "R‐gel" OR zacin OR zostrix OR capsicum):TI,AB,KY (763)
1 OR 2 (763)
exp MeSH descriptor Administration, topical (13012)
(topical* OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR crème OR lotion OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster):TI,AB,KY (79078)
4 OR 5 (81831)
MeSH descriptor Diabetic neuropathies EXPLODE ALL TREES (1017)
MESH DESCRIPTOR Peripheral Nervous System Diseases EXPLODE ALL TREES (2878)
MESH DESCRIPTOR Neuralgia EXPLODE ALL TREES (694)
((neuropath* OR diabet* post‐herpetic OR neuralgia OR phantom OR stump)):TI,AB,KY (7096)
7 OR 8 OR 9 OR 10 (8623)
3 AND 6 AND 11 (108)
2012 TO 2016:YR (192843)
12 AND 13 (40)
Appendix 2. MEDLINE search strategy (via Ovid)
Capsaicin/ (1149)
(capsaicin OR capsaicine OR capsici OR axsain OR capsidol OR capsig OR capsin OR capsina OR capsiplast OR capzasin‐P OR dolorac OR gelcen OR katrum OR "No pain‐HP" OR priltam OR "R‐gel" OR zacin OR zostrix OR capsicum).mp. (2436)
1 or 2 (2436)
exp Administration, topical/ (10094)
(topical* OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR creme OR lotion OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster).mp. (191041)
4 or 5 (193874)
exp Diabetic neuropathies/ (2906)
exp Peripheral Nervous System Diseases/ (166699)
exp Neuralgia/ (3473)
(neuropath* OR diabet* post‐herpetic OR neuralgia OR phantom OR stump).mp. (28080)
7 or 8 or 9 or 10 (36908)
randomized controlled trial.pt. (83324)
controlled clinical trial.pt. (6282)
randomized.ab. (75790)
placebo.ab. (25769)
drug therapy.fs. (304825)
randomly.ab. (49098)
trial.ab. (78401)
groups.ab. (275092)
12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 (637615)
3 AND 6 AND 11 AND 20 (94)
Appendix 3. EMBASE search strategy (via Ovid)
Capsaicin/ (13552)
(capsaicin or capsaicine or capsici or axsain or capsidol or capsig or capsin or capsina or capsiplast or capzasin‐P or dolorac or gelcen or katrum or "No pain‐HP" or priltam or "R‐gel" or zacin or zostrix or capsicum).mp. (17641)
1 or 2 (17641)
exp Topical Drug Administration/ (28821)
(topical* or cutaneous or dermal or transcutaneous or transdermal or percutaneous or skin or massage or embrocation or gel or ointment or aerosol or cream or creme or lotion or foam or liniment v spray or rub or balm or salve or emulsion or oil or patch or plaster).mp. (1410353)
4 or 5 (1417324)
exp Diabetic Neuropathies/ (14444)
exp Peripheral Nervous System Diseases/ (43680)
exp Neuralgia/ (63962)
(neuropath* or diabet* post‐herpetic or neuralgia or phantom or stump).mp. (252239)
7 or 8 or 9 or 10 (276770)
Randomized controlled trial/ (348956)
Double‐blind procedure/ (102583)
Crossover‐procedure/ (41951)
(random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or (doubl* adj blind*) or assign* or allocat*).tw. (1194868)
12 or 13 or 14 or 15 (1262910)
3 and 6 and 11 and 16 (359)
limit 17 to yr="2012‐Current" (124)
Appendix 4. GRADE: criteria for assigning grade of evidence
The GRADE system uses the following criteria for assigning a quality level to a body of evidence (Chapter 12, Higgins 2011).
High: randomised trials; or double‐upgraded observational studies.
Moderate: downgraded randomised trials; or upgraded observational studies.
Low: double‐downgraded randomised trials; or observational studies.
Very low: triple‐downgraded randomised trials; or downgraded observational studies; or case series/case reports.
Factors that may decrease the quality level of a body of evidence are:
limitations in the design and implementation of available studies suggesting high likelihood of bias;
indirectness of evidence (indirect population, intervention, control, outcomes);
unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses);
imprecision of results (wide confidence intervals);
high probability of publication bias.
Factors that may increase the quality level of a body of evidence are:
large magnitude of effect;
all plausible confounding would reduce a demonstrated effect or suggest a spurious effect when results show no effect;
dose‐response gradient.
Appendix 5. Summary of outcomes in individual studies: efficacy
| Study ID | Treatment | Clinical improvement |
| Backonja 2008 | (1) Capsaicin patch 8%, n = 206 (2) Control patch, n = 196 |
Over 2 to 12 weeks ≥ 30% pain reduction from baseline: (1) 91/206, (2) 69/196 (Participants with ≥ 50% pain reduction from baseline ‐ no significant difference between groups) ≥ 2 points reduction in pain from baseline: (1) 87/206, (2) 55/196 At 12 weeks PGIC (slightly/much/very much improved): (1) 114/206, (2) 85/196 Over 2 to 8 weeks ≥ 30% pain reduction from baseline: (1) 87/206, (2) 63/196 ≥ 2 points reduction in pain from baseline: (1) 82/206, (2) 51/196 At 8 weeks PGIC (slightly/much/very much improved): (1) 109/206, (2) 83/196 |
| Bischoff 2014 | (1) Capsaicin patch 8% 60 min, n = 24 (23 treated) (2) Placebo patch 60 min, n = 22 |
No responder outcomes reported. No significant difference in the summed pain intensity difference (from baseline) between groups at 4, 8, or 12 weeks after treatment in completers |
| Clifford 2012 | (1) Capsaicin patch 8% 30 min, n = 167 (2) Capsaicin patch 8% 60 min, n = 165 (3) Control patch 30 min, n = 73 (4) Control patch 60 min, n = 89 |
Over 2 to 12 weeks ≥ 30% pain reduction from baseline: (1) 65/167, (2) 79/165, (3) 19/73, (4) 40/89 At 12 weeks PGIC (slightly, much, very much improved): (1) 109/167, (2) 114/165, (3) 33/73, (4) 56/89 |
| Irving 2011 | (1) Capsaicin patch 8%, n = 212 (2) Control patch, n = 204 |
At 12 weeks ≥ 50% pain reduction from baseline: (1) 64/212, (2) 43/204 ≥ 30% pain reduction from baseline: (1) 100/212, (2) 71/204 At 12 weeks PGIC (much and very much improved): (1) 83/212, (2) 50/204 ≥ 2 points reduction in pain from baseline: (1) 91/212, (2) 59/204 Over 2 to 8 weeks ≥ 50% pain reduction from baseline: (1) 61/212, (2) 41/204 ≥ 30% pain reduction from baseline: (1) 98/212, (2) 69/204 At 8 weeks PGIC (much and very much improved): (1) 71/212, (2) 49/204 ≥ 2 points reduction in pain from baseline: (1) 89/212, (2) 53/204 |
| Simpson 2008 | (1) Capsaicin patch 8% 30 min, n = 72 (2) Capsaicin patch 8% 60 min, n = 78 (3) Capsaicin patch 8% 90 min, n = 75 (4) Control patch, n = 82 |
Over 2 to 12 weeks ≥ 30% pain reduction from baseline: (1) 30/72, (2) 19/78, (3) 27/75, (4) 15/82 (capsaicin combined 76/225) At 12 weeks PGIC (much and very much improved): (1) 23/72, (2) 18/78, (3) 20/75, (4) 9/82 (capsaicin combined 61/225) |
| STEP 2014 | (1) Capsaicin patch 8%, 30 min, n = 186 (2) Placebo patch, n = 183 |
Over 2 to 8 weeks ≥ 50% pain reduction from baseline: (1) 39/186, (2) 33/183 ≥ 30% pain reduction from baseline: (1) 74/186, (2) 60/183 At 8 weeks PGIC (much and very much improved), using ITT denominators: (1) 71/186, (2) 52/183 Over 2 to 12 weeks ≥ 50% pain reduction from baseline: (1) 41/186, (2) 35/183 ≥ 30% pain reduction from baseline: (1) 76/186, (2) 58/183 At 12 weeks PGIC (much and very much improved), using ITT denominators: (1) 68/186, (2) 51/183 |
| Webster 2010a | (1) Capsaicin patch 8% 30 min, n = 72 (2) Capsaicin patch 8% 60 min, n = 77 (3) Capsaicin patch 8% 90 min, n = 73 (4) Control patch, 30, 60, 90 min pooled for analysis, n = 77 |
Over 2 to 8 weeks ≥ 50% pain reduction from baseline: (1) 17/72, (2) 21/77, (3) 17/73, (4) 8/77 ≥ 30% pain reduction from baseline: (1) 27/72, (2) 27/77, (3) 29/73, (4) 22/77 At 12 weeks PGIC (slight, much and very much improved): (1+2+3) 122/222 (capsaicin combined), (4) 32/77 |
| Webster 2010b | (1) Capsaicin patch 8%, n = 102 (2) Control patch, n = 53 |
Over 2 to 12 weeks ≥ 50% pain reduction from baseline: (1) 40/102, (2) 19/53 ≥ 30% pain reduction from baseline: (1) 50/102, (2) 26/53 At 12 weeks PGIC (much and very much improved): (1) 41/102, (2) 15/53 Over 2 to 8 weeks ≥ 50% pain reduction from baseline: (1) 37/102, (2) 19/53 ≥ 30% pain reduction from baseline: (1) 50/102, (2) 24/53 At 8 weeks PGIC (much and very much improved): (1) 43/102, (2) 14/53 |
| ITT: intention‐to‐treat; min: minute; n: number of participants in treatment arm; PGIC: Patient Global Impression of Change. | ||
Appendix 6. Summary of outcomes in individual studies: adverse events and withdrawals
| Study ID | Treatment | Local AEs | Systemic AEs | Serious AEs | Withdrawals/exclusions |
| Backonja 2008 | (1) Capsaicin patch 8%, n = 206 (2) Control patch, n = 196 |
Mostly transient, mild to moderate Erythema: (1) 193/205, (2) 128/197 Pain: (1) 114/205, (2) 43/197 Papules: (1) 20/205, (2) 6/197 Pruritus: (1) 10/205, (2) 6/197 Oedema: (1) 12/205, (2) 2/197 |
Nausea, vomiting, nasopharyngitis, sinusitis, back pain, dizziness, headache, worsening of PHN, hypertension ‐ all reported at < 5% per group, with no clear difference between groups | (1) 10/205, (2) 6/197 (1 in capsaicin group judged related to medication) |
AE: (1) 1/205, (2) 0/197 LoE: (1) 10/205, (2) 9/197 Lost to follow‐up: (1) 3/205, (2) 2/197 Other: (1) 5/205, (2) 7/197 |
| Bischoff 2014 | (1) Capsaicin patch 8% 60 min, n = 24 (23 treated) (2) Placebo patch 60 min, n = 22 |
Pain: (1) 12/23, (2) 6/20 Erythema: (1) 9/23, (2) 3/20 Burning sensation: (1) 12/23, (2) 1/20 |
None | None | AE: (1) 1/23 (due to pain during application), (2) 0/22 LoE: (1) 0.23, (2) 2/22 (began new analgesic treatment) Lost to follow‐up: (1) 0/23, (2) 2/22 |
| Clifford 2012 | (1) Capsaicin patch 8% 30 min, n = 167 (2) Capsaicin patch 8% 60 min, n = 165 (3) Control patch 30 min, n = 73 (4) Control patch 60 min, n = 89 |
Generally mild or moderate. Groups combined Erythema: (1+2) 176/332, (3+4) 58/162 Pain: (1+2) 274/332, (3+4) 62/162 Papules: (1+2) 12/332, (3+4) 0/162 Pruritus: (1+2) 12/332, (3+4) 2/162 Oedema: (1+2) 4/332, (3+4) 5/162 |
Diarrhoea, nausea, respiratory tract infection, pain, worsening neuropathy ‐ all reported, generally < 5% per group | Approximately 6% in all groups with "infections and infestations" 1 death in capsaicin 60 min group (judged unrelated) |
AE: (1) 0/167, (2) 2/165 (1 death), (3) 0/72, (4) 1/90 All judged unrelated LoE: (1) 0/167, (2) 1/165, (3) 0/73, (4) 1/89 Lost to follow‐up: (1) 3/167, (2) 2/165, (3) 2/73, (4) 0/89 Other: (1) 8/167, (2) 6/165, (3) 0/73, (4) 6/89 |
| Irving 2011 | (1) Capsaicin patch 8%, n = 212 (2) Control patch, n = 204 |
Most mild or moderate Erythema: (1) 194/212, (2) 141/204 Pain: (1) 134/212, (2) 57/204 Papules: (1) 15/212, (2) 5/204 Pruritus: (1) 6/212, (2) 3/204 Oedema: (1) 13/212, (2) 0/204 |
Nausea, vomiting, sinusitis, respiratory tract infection, musculoskeletal disorders, dizziness, headache ‐ all reported, most < 5% per group | (1) 11/212 (1 death), (2) 8/204 None considered drug‐related |
AE: (1) 4/212 (1 death), (2) 3/304 All SAE and considered not related to treatment LoE: (1) 1/212, (2) 5/204 Lost to follow‐up: (1) 4/212, (2) 5/204 Other: (1) 11/212, (2) 5/204 |
| Simpson 2008 | (1) Capsaicin patch 8% 30 min, n = 72 (2) Capsaicin patch 8% 60 min, n = 78 (3) Capsaicin patch 8% 90 min, n = 75 (4) Control patch, n = 82 |
Self‐limiting and mild to moderate Pain: (1) 47/225, (2) 7/82 Papules: (1) 11/225, (2) 1/82 Pruritus: (1) 39/225, (2) 5/82 Swelling: (1) 29/225, (2) 7/82 |
Diarrhoea, nausea, vomiting, fatigue, infections, musculoskeletal disorders, dizziness, headache, psychiatric disorders ‐ all reported, < 5% per group | (1) 1/225, (2) 2/82 All deaths, all judged unrelated to study medication |
AE: (1) 3/225 (1 death), (2) 3/82 (2 deaths) LoE: (1) 1/225, (2) 2/82 Lost to follow‐up: (1) 13/225, (2) 4/82 Other: (1) 5/225, (2) 2/82 |
| STEP 2014 | (1) Capsaicin patch 8%, 30 min, n = 186 (2) Placebo patch, n = 183 |
Mostly mild or moderate Application site pain and reactions overall: (1) 63/186, (2) 15/183 Pain: (1) 18/186, (2) 4/183 Burning sensation: (1) 26/186, (2) 5/183 |
Musculoskeletal disorders, infections, respiratory disorders, gastrointestinal disorders | (1) 2/186, (2) 7/183 | AE: (1) 0/186, (2) 1/183 Lost to follow‐up: (1) 2/186, (2) 1/183 Participant decision: (1) 7/186, (2) 6/183 |
| Webster 2010a | (1) Capsaicin patch 8% 30 min, n = 72 (2) Capsaicin patch 8% 60 min, n = 77 (3) Capsaicin patch 8% 90 min, n = 73 (4) Control patch, 30, 60, 90 min pooled for analysis, n = 77 |
Transient and mild to moderate Pain: (1) 1/222, (2) 2/77 Papules: (1) 3/222, (2) 2/77 Pruritus: (1) 17/222, (2) 9/77 Swelling: (1) 3/222, (2) 5/77 |
Diarrhoea, nausea, vomiting, infections, musculoskeletal disorders, dizziness, headache, cough ‐ all reported, mostly < 5% per group | (1+2+3) 10/222 (1 death), (4) 3/77 None considered related to study medication |
AE: (1+2+3) 2/222, (4) 1/77 (death) LoE: (1+2+3) 4/222, (4) 0/77 Lost to follow‐up (1+2+3) 7/222, (4) 1/77 Other: (1+2+3) 9/222, (4) 2/77 |
| Webster 2010b | (1) Capsaicin patch 8%, n = 102 (2) Control patch, n = 53 |
Transient and mild to moderate Erythema: (1) 4/102, (2) 0/53 Pain: (1) 4/102, (2) 2/53 Papules: (1) 4/102, (2) 2/53 Pruritus: (1) 17/102, (2) 6/53 Swelling: (1) 10/102, (2) 1/53 |
Nausea, infections, musculoskeletal disorders, dizziness, cough, nasopharyngitis, hypertension ‐ all reported, mostly < 5% per group | (1) 7/102, (2) 0/53 None considered related to study medication |
AE: None in either group LoE: (1) 3/102, (2) 7/53 Lost to follow‐up: (1) 5/102, (2) 0/53 Other: (1) 3/102, (2) 3/53 |
| AE: adverse event; LoE: lack of efficacy; n: number of participants in treatment arm; PHN: postherpetic neuralgia; SAE: serious adverse event | |||||
Appendix 7. Patch tolerability
| Study ID | Treatment | Completed < 90% application time | Dermal irritation score > 2 at 2 hours | Rescue medication days 0 to 5 |
| Backonja 2008 | (1) Capsaicin patch 8%, n = 206 (2) Control patch, n = 196 |
(1) 1/206, (2) 2/196 |
"Common but mild, transient and self‐limited" | (1) 99/206, (2) 32/196 |
| Bischoff 2014 | (1) Capsaicin patch 8% 60 min, n = 24 (23 treated) (2) Placebo patch 60 min, n = 22 |
Not reported 1 participant in (1) had patch removed early |
No data | No data |
| Clifford 2012 | (1) Capsaicin patch 8% 30 min, n = 167 (2) Capsaicin patch 8% 60 min, n = 165 (3) Control patch 30 min, n = 73 (4) Control patch 60 min, n = 89 |
(1+2) 10/332, (3+4) 0/162 |
(1+2) 13/332, (3+4) 0/162 |
(1+2) 246/332, (3+4) 53/162 |
| Irving 2011 | (1) Capsaicin patch 8%, n = 212 (2) Control patch, n = 204 |
(1) 4/212, (2) 0/204 |
(1) 6/212, (2) 1/204 |
(1) 112/212, (2) 43/204 |
| Simpson 2008 | (1) Capsaicin patch 8% 30 min, n = 72 (2) Capsaicin patch 8% 60 min, n = 78 (3) Capsaicin patch 8% 90 min, n = 75 (4) Control patch, n = 82 |
(1) 0/72, (2) 0/78, (3) 2/75, (4) 0/82 |
> 0 at 2 hours: (1+2+3) 92/225, (4) 23/82 |
(1+2+3) 124/225, (4) 19/82 |
| STEP 2014 | (1) Capsaicin patch 8%, 30 min, n = 186 (2) Placebo patch, n = 183 |
No data | Dermal irritation (scale 0 to 7) score ≥ 4 (definite oedema) at 15 and 60 min after patch removal (1) 0/186, (2) 2/183 |
(1) 35/186, (2) 10/183 |
| Webster 2010a | (1) Capsaicin patch 8% 30 min, n = 72 (2) Capsaicin patch 8% 60 min, n = 77 (3) Capsaicin patch 8% 90 min, n = 73 (4) Control patch, 30, 60, 90 min pooled for analysis, n = 77 |
(1) 0/73, (2) 1/77, (3) 0/73, (4) 0/77 |
> 0 at 2 hours: (1+2+3) 87/222, (4) 5/77 |
(1+2+3) 12/222, (4) 3/77 |
| Webster 2010b | (1) Capsaicin patch 8%, n = 102 (2) Control patch, n = 53 |
(1) 4/102, (2) 0/53 |
(1) 53/102, (2) 2/53 | (1) 12/102, (2) 1/53 |
| min: minutes; n: number of participants in treatment arm | ||||
Data and analyses
Comparison 1. High‐concentration (8%) capsaicin versus control (single dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Postherpetic neuralgia (PHN) ‐ at least 50% pain intensity reduction over weeks 2 to 8 | 3 | 870 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.12, 1.86] |
| 1.1.1 Using 30‐minute application | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [0.73, 11.88] |
| 1.1.2 Using 60‐minute application | 3 | 674 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.03, 1.75] |
| 1.1.3 Using 90‐minute application | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.64, 6.33] |
| 1.2 PHN ‐ at least 50% pain intensity reduction over 2 to 12 weeks | 2 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.00, 1.71] |
| 1.3 PHN ‐ at least 30% pain intensity reduction over weeks 2 to 8 | 4 | 1268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.13, 1.52] |
| 1.3.1 Using 30‐minute application | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.67, 2.69] |
| 1.3.2 Using 60‐minute application | 4 | 1072 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.12, 1.52] |
| 1.3.3 Using 90‐minute application | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.74, 2.95] |
| 1.4 PHN ‐ at least 30% pain intensity reduction over weeks 2 to 12 | 3 | 973 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.07, 1.45] |
| 1.5 PHN ‐ Patient Global Impression of ChangePGIC much or very much improved at 8 and 12 weeks | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 At 8 weeks | 2 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.10, 1.84] |
| 1.5.2 At 12 weeks | 2 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.20, 1.99] |
| 1.6 HIV‐neuropathy ‐ at least 30% pain intensity reduction over weeks 2 to 12 | 2 | 801 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.09, 1.68] |
| 1.6.1 Using 30‐minute application | 2 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.14, 2.46] |
| 1.6.2 Using 60‐minute application | 2 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.84, 1.44] |
| 1.6.3 Using 90‐minute application | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.83, 4.53] |
| 1.7 Local skin reactions ‐ group 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 Erythema | 4 | 1355 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.32, 1.54] |
| 1.7.2 Pain | 4 | 1355 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [1.98, 2.59] |
| 1.7.3 Papules | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.58 [1.87, 6.85] |
| 1.7.4 Pruritus | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.98, 4.03] |
| 1.7.5 Oedema | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [1.44, 6.18] |
| 1.8 Local skin reactions ‐ group 2 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.8.1 Erythema | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.31 [0.35, 114.82] |
| 1.8.2 Pain | 4 | 1105 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [1.41, 4.05] |
| 1.8.3 Papules | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.59, 4.24] |
| 1.8.4 Pruritus | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.98, 2.50] |
| 1.8.5 Oedema | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.75, 2.39] |
| 1.9 Patch tolerability | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.9.1 < 90% of application time | 6 | 2074 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [1.17, 9.15] |
| 1.9.2 Dermal irritation score > 2 at 2 hours | 3 | 1065 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.80 [4.04, 34.48] |
| 1.9.3 Dermal irritation score > 0 at 2 hours | 2 | 606 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.60, 3.26] |
| 1.9.4 Pain medication 0 to 5 days | 7 | 2442 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [2.18, 2.92] |
| 1.10 Serious adverse events | 7 | 1993 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.70, 1.86] |
| 1.11 Withdrawals | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.11.1 Adverse events | 8 | 2487 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.36, 1.78] |
| 1.11.2 Lack of efficacy | 6 | 2073 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.32, 1.02] |
1.5. Analysis.

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 5: PHN ‐ Patient Global Impression of ChangePGIC much or very much improved at 8 and 12 weeks
1.6. Analysis.

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 6: HIV‐neuropathy ‐ at least 30% pain intensity reduction over weeks 2 to 12
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Backonja 2008.
| Study characteristics | ||
| Methods | RCT, DB, multicentre, parallel groups, single application, 12‐week duration. Patch applied to painful area, up to 1000 cm2 Oral pain medication continued without change. Transdermal opioids (morphine equivalent ≤ 60 mg/day) permitted, but not topical analgesics Rescue medication: after application participants allowed hydrocodone/paracetamol (5/500 mg) for ≤ 5 days Pain assessed daily (average pain for last 24 hours). PGIC assessed at endpoint. Clinic visits at 4, 8, 12 weeks |
|
| Participants | Postherpetic neuropathy with at least moderate pain, ≥ 6 months since vesicle crusting. Exclusion: pain in/around facial area N = 402 M = 190, F = 212 Mean age: 71 years Baseline pain: 30 mm to 90 mm (mean 60 mm) |
|
| Interventions | (1) Capsaicin patch 8%, n = 206 (2) Control patch, n = 196 Topical local anaesthetic applied for 60 min, then patch applied for 60 min Control patch contained 0.04% capsaicin to mimic AEs |
|
| Outcomes | PI: 11‐point numeric pain rating scale (responder: ≥ 30% and ≥ 2‐point reduction from baseline) PGIC: 7‐point scale (responder: much and very much improved) AEs Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Remote treatment assignment, using unique number on printed labels affixed to outside of patch envelope |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Low concentration of capsaicin in "identically formulated" control patch to mimic local skin reaction of active treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Modified (no details) LOCF analysis for primary outcome, but no imputation for weekly scores. All participants included for safety analysis |
| Size | Low risk | 206 participants in capsaicin arm,196 participants in control arm |
Bischoff 2014.
| Study characteristics | ||
| Methods | RCT, DB, PC, parallel group, single application, 12‐week duration. Pain assessment twice daily in 3 days before treatment and clinical visits at 1, 2, 3 months. |
|
| Participants | Persistent pain after inguinal herniorrhaphy score ≥ 5/10 for > 6 months Exclusion: bilateral groin pain, allergy to any component of treatment, comorbidity that might interfere with treatment or assessment N = 46 M = 42, F = 4 Mean age: 54 years Baseline pain on movement: 5.5/10 (range 3 to 7) |
|
| Interventions | (1) Capsaicin patch 8%, n = 24 (23 treated) (2) Placebo patch, n = 22 Topical local anaesthetic (EMLA; lidocaine + prilocaine) applied for 60 min, then patch applied to groin area for 60 min Cool packs applied to skin for 45 to 60 min after patch removal and cleansing Stable (≥ 4 weeks) analgesic medication continued without change |
|
| Outcomes | SPID ‐ difference between groups at 4, 8, 12 weeks AEs Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 Study terminated early due to expiry of placebo patch |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization list" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "placebo patches were identical in appearance and composition (in regard to vehicle substances)". 70% of capsaicin participants and 80% of placebo participants correctly guessed assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation unclear |
| Size | High risk | < 50 participants per treatment arm |
Clifford 2012.
| Study characteristics | ||
| Methods | RCT, DB, parallel groups, single application, 12‐week duration. Patches applied to both feet, up to 1120 cm2 Oral pain medication continued without change. Transdermal opioids (morphine equivalent ≤ 80 mg/day) permitted, but not topical analgesics, or implanted medical device for pain relief Rescue medication: during application participants allowed oral oxycodone solution (1 mg/mL) and local cooling; after application allowed hydrocodone/paracetamol (5/500 mg) for ≤ 5 days, and paracetamol (≤ 3 g/day) throughout Pain assessed daily (average pain for last 24 hours). PGIC assessed at 12 weeks. Clinic visits at 4, 8, 12 weeks |
|
| Participants | HIV‐associated distal sensory neuropathy for ≥ 2 months Exclusion: previous use of NGX‐4010 (capsaicin) N = 494 M = 432, F = 62 Mean age: 50 years Baseline pain: 30 mm to 90 mm (mean 60 mm) |
|
| Interventions | (1) Capsaicin patch 8% 30 min, n = 167 (2) Capsaicin patch 8% 60 min, n = 165 (3) Placebo patch 30 min, n = 73 (4) Placebo patch 60 min, n = 89 Topical local anaesthetic applied for 60 min, then patch applied for 30 or 60 min Control patch contained 0.04% capsaicin to mimic AEs |
|
| Outcomes | PI: 11‐point numeric pain rating scale (responder: ≥ 30% reduction from baseline) PGIC: 7‐point scale (reporting: slightly, much and very much improved) AEs Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described; "allocation scheme prepared by Fisher Clinical Services" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described as identical; "low‐dose capsaicin control patches were used instead of placebo to provide effective blinding ..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Modified LOCF analysis for primary outcome, but no imputation for weekly scores. All participants included for safety analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm. |
Irving 2011.
| Study characteristics | ||
| Methods | RCT, DB, multicentre, parallel‐group, single application, 12‐week duration. Patch applied to painful area, up to 1120 cm2 Oral pain medication continued without change. Transdermal opioids (morphine equivalent ≤ 60 mg/day) permitted, but not topical analgesics Pain assessed daily (average pain for last 24 hours). PGIC assessed at 4, 8, 12 weeks. Clinic visits at 4, 8, 12 weeks |
|
| Participants | Postherpetic neuropathy with at least moderate pain, ≥ 6 months since vesicle crusting. Exclusion: pain above neck area N = 416 M = 190, F = 226 Mean age: 70 years Baseline pain: 30 mm to 90 mm (mean 57 mm) |
|
| Interventions | (1) Capsaicin patch 8%, n = 212 (2) Control patch, n = 204 Topical local anaesthetic applied for 60 min, then patch applied for 60 min Control patch contained 0.04% capsaicin to mimic AEs |
|
| Outcomes | PI: 11‐point numeric pain rating scale (responder: ≥ 30%, ≥ 50%, and ≥ 2‐point reduction from baseline) PGIC: 7‐point scale (responder: much and very much improved) AEs Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described; "allocation scheme prepared by Fisher Clinical Services" |
| Allocation concealment (selection bias) | Low risk | Each kit "designated by a unique kit number, which was printed on the investigational drug label affixed to the outer bag enclosure and on each individual patch envelope" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The NGX‐4010 [capsaicin] and control patches were identical in appearance, as were the blinded study kits" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Modified LOCF analysis for primary outcome, but no imputation for weekly scores. All participants included for safety analysis. |
| Size | Low risk | > 200 participants per treatment arm |
Simpson 2008.
| Study characteristics | ||
| Methods | RCT, DB, multicentre, parallel groups, single application, 12‐week duration. Patches applied to both feet, up to maximum 1000 cm2 Oral pain medication continued without change. No topical analgesics During application participants allowed oral oxycodone solution (1 mg/mL) or equivalent, after application allowed hydrocodone/paracetamol (5/500 mg) for ≤ 7 days Pain assessed daily (average pain for last 24 hours). PGIC assessed at 12 weeks. Clinic visits at 4, 8, 12 weeks |
|
| Participants | HIV‐associated distal sensory polyneuropathy with ≥ 2 months' moderate to severe pain in both feet N = 307 M = 286, F = 21 Mean age: 48 years (range 29 to 74) Baseline pain: 30 mm to 90 mm (mean ~ 60 mm) |
|
| Interventions | (1) Capsaicin patch 8% 30 min, n = 72 (2) Capsaicin patch 8% 60 min, n = 78 (3) Capsaicin patch 8% 90 min, n = 75 (4) Control patch, n = 82 Topical local anaesthetic applied for 60 min, then patch applied for 30, 60, or 90 min Control patch contained 0.04% capsaicin to mimic AEs |
|
| Outcomes | PI: 11‐point numeric pain rating scale (responder: ≥ 30% reduction from baseline) PGIC: 7‐point scale (responder: much and very much improved) AEs Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Control patch contained a low concentration of capsaicin to mimic local skin reaction of active treatment. Although it does not say "identical" or use similar wording, we judged this to be low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | BOCF or 'no improvement' imputed for missing values for dichotomous data analyses |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
STEP 2014.
| Study characteristics | ||
| Methods | R, DB, multicentre, parallel group, PC, single application, 12‐week duration. Pain assessed daily | |
| Participants | Painful diabetic neuropathy, distal, symmetrical, > 1 year (score > 3 on Michigan Neuropathy Screening Instrument), glycated haemoglobin ≤ 11% and history indicating control, 24‐hour PI ≥ 4/10 in screening period, stable doses of analgesics for ≥ 4 weeks before screening N = 369 M = 215, F = 154 Mean age: 63 years (range 33 to 89) Mean baseline pain: 6.5/10 |
|
| Interventions | (1) Capsaicin patch 8%, n =186 (2) Placebo patch, n = 183 Up to 4 patches applied to painful areas of feet Topical anaesthetic cream applied according to prescribing information, then patch applied for 30 min Stable concomitant neuropathic pain medication (antiepileptic or antidepressant drugs) allowed if unchanged |
|
| Outcomes | ≥ 30% and ≥ 50% PI reduction over weeks 2 to 8 and 2 to 12 compared with baseline PGIC much and very much improved at 8 and 12 weeks AEs Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The placebo patches were visually and cosmetically indistinguishable from the active capsaicin patches." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation unclear |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Webster 2010a.
| Study characteristics | ||
| Methods | RCT, DB, multicentre, parallel‐group, single application, 12‐week duration. Patch applied to painful area, up to 1120 cm2 Oral pain medication continued without change. Transdermal opioids (morphine equivalent ≤ 60 mg/day) permitted, but not topical analgesics Rescue medication: during application participants allowed oral oxycodone solution (1 mg/mL) and local cooling; after application allowed hydrocodone/paracetamol (5/500 mg) for ≤ 5 days, and paracetamol (≤ 2 g/day) throughout Pain assessed daily (average pain for last 24 hours). PGIC assessed at 4, 8, 12 weeks. Clinic visits at 4, 8, 12 weeks |
|
| Participants | Postherpetic neuropathy with at least moderate pain, ≥ 6 months since vesicle crusting. Exclusion: pain in/around facial area N = 299 M = 150, F = 149 Mean age: 71 years Baseline pain: 30 mm to 90 mm (mean 55 mm) |
|
| Interventions | (1) Capsaicin patch 8% 30 min, n = 72 (2) Capsaicin patch 8% 60 min, n = 77 (3) Capsaicin patch 8% 90 min, n = 73 (4) Control patch, 30, 60, 90 min pooled for analysis, n = 77 Topical local anaesthetic applied for 60 min, then patch applied for 30, 60 or 90 min Control patch contained 0.04% capsaicin to mimic AEs |
|
| Outcomes | PI: 11‐point numeric pain rating scale (responder: ≥ 30%, ≥ 50%, and ≥ 2‐point reduction from baseline) PGIC: 7‐point scale (reporting: slightly, much and very much improved) AEs Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described; "randomisation scheme prepared by Cardinal Health" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identically appearing control patches" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Modified LOCF analysis for primary outcome, but no imputation for weekly scores. All participants included for safety analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Webster 2010b.
| Study characteristics | ||
| Methods | RCT, DB, multicentre, parallel‐group, single application, 12‐week duration. Patch applied to painful area, up to 1000 cm2 Oral pain medication continued without change. Transdermal opioids (morphine equivalents ≤ 60 mg/day) permitted, but not topical analgesics Rescue medication: during application participants allowed oral oxycodone solution (1 mg/mL) and local cooling; after application allowed hydrocodone/paracetamol (5/500 mg) for ≤ 5 days, and paracetamol (≤ 2 g/day) throughout Pain assessed daily (average pain for last 24 hours). PGIC assessed at 4, 8, 12 weeks. Clinic visits at 4, 8, 12 weeks |
|
| Participants | Postherpetic neuropathy with at least moderate pain, ≥ 6 months since vesicle crusting. Exclusion: pain in/around facial area N = 155 M = 72, F = 83 Mean age: 70 years Baseline pain: 30 mm to 90 mm (mean 53 mm) |
|
| Interventions | (1) Capsaicin patch 8%, n = 102 (2) Control patch, n = 53 Topical local anaesthetic applied for 60 min, then patch applied for 60 min Control patch contained 0.04% capsaicin to mimic AEs |
|
| Outcomes | PI: 11‐point numeric pain rating scale (responder: ≥ 30%, ≥ 50%, and ≥ 2‐point reduction from baseline) PGIC: 7‐point scale (responder: much and very much improved) AEs Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described; "randomisation scheme prepared by Cardinal Health" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identically‐appearing ...... control patch" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Modified LOCF analysis for primary outcome, but no imputation for weekly scores. All participants included for safety analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
AE: adverse event; BOCF: baseline observation carried forward; DB: double‐blind(ing); F: female; LOCF: last observation carried forward; M: male; min: minute; N: number of participants in study; n: number of participants in treatment arm; PC: placebo‐controlled; PGIC: Patient Global Impression of Change; PI: pain intensity; R: randomisation; RCT: randomised controlled trial; W: withdrawals.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Backonja 2010 | Study duration only 4 weeks |
Characteristics of studies awaiting classification [ordered by study ID]
NCT01228838.
| Methods | RCT, DB, multicentre, parallel groups, single application, 12‐week duration Treatment applied for 5 minutes |
| Participants | Postherpetic neuropathy with > 6 months of pain since vesicle crusting Baseline pain: 4/10 to 9/10 Age: 18 to 90 years |
| Interventions | Capsaicin topical liquid 10% Capsaicin topical liquid 20% Placebo Stable pain medications continued unchanged throughout study |
| Outcomes | Participants with ≥ 30% decrease in pain from baseline at weeks 8 and 12 Participants with ≥ 2‐unit decrease in pain from baseline at weeks 8 and 12 |
| Notes | Primary completion date September 2011. No results posted as of March 2016 |
DB: double‐blind; RCT: randomised controlled trial.
Differences between protocol and review
For the first update in 2013, we used revised guidelines for reviews in pain, which took into account our better understanding of potential biases both in studies and in the review process (PaPaS 2012). Moreover, the very different nature of the treatment with high‐concentration capsaicin meant that somewhat different outcomes were used, but those reflect the basic principles outlined in the PaPaS author guide.
For this 2017 update, we included an assessment of the quality of the evidence using GRADE and created a 'Summary of findings' table, in line with current standards for Cochrane Reviews. We have removed tiers of evidence from our analysis since these are largely replaced by GRADE. We also removed the prespecified sensitivity analyses since there were insufficient data to formally examine these issues in the earlier review, and it was thought unlikely that this situation would have changed.
Contributions of authors
For the original review, SD and RL carried out searches for studies, data extraction, and analyses. RAM was involved with analysis and HJM acted as arbitrator. All authors were involved with writing the review.
For the first update, SD and TT searched for studies and carried out data extraction; RAM checked data extraction. SD and RAM carried out analyses and wrote the initial draft review. All authors were involved with writing the full review.
For this update, SD and RAM searched for studies, carried out data extraction, and revised analyses. All authors were involved with writing the full review.
Sources of support
Internal sources
The Oxford Pain Relief Trust, UK
External sources
-
The National Institute for Health Research (NIHR), UK
NIHR Cochrane Programme Grant: 13/89/29 ‐ Addressing the unmet need of chronic pain: providing the evidence for treatments of pain
Declarations of interest
SD: none known.
ASCR undertakes consultancy and advisory board work for Imperial College Consultants ‐ since June 2013 this has included remunerated work for: Spinifex, Abide, Astellas, Neusentis, Merck, Medivir, Mitsubishi, Aquilas, Asahi Kasei, Relmada, Novartis, and Orion. All consultancy activity relates to consultancy advice on the preclinical/clinical development of drugs for neuropathic pain. Neusentis was a subsidiary of Pfizer. He owned share options in Spinifex Pharmaceuticals which was acquired by Novartis in July 2015. ASCR was a Principal Investigator in the EuroPain consortium. EuroPain has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement number 115007, resources for which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/20072013) and European Federation of Pharmaceutical Industries and Associations (EFPIA) companies (www.imieuropain.org). Specifically, research funding for ASCR's laboratory has been received by Imperial College from Pfizer (manufacturer of gabapentin) and Astellas ‐ both these grants were for projects related to improving the validity of animal models of neuropathic pain. ASCR is a site investigator for the Neuropain project, funded by Pfizer via Kiel University ‐ Chief Investigator Prof Ralf Baron. He is Vice‐Chair of the International Association for the Study of Pain (IASP) Special Interest Group on Neuropathic Pain (www.neupsig.org) and serves on the Executive Committee of ACTTION (Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks; www.acttion.org).
PC received support from Boston Scientific (2014) for travel and accommodation at a scientific meeting; Boston Scientific does not market drugs. PC is a specialist pain physician and manages patients with chronic pain.
TT: none known.
RAM has received grant support from Grünenthal relating to individual patient level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has received honoraria for attending boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015). He has received honoraria from Omega Pharma (2016) and Futura Pharma (2016) for providing advice on trial and data analysis methods.
This review was identified in a 2019 audit as not meeting the current definition of the Cochrane Commercial Sponsorship policy. At the time of its publication it was compliant with the interpretation of the existing policy. As with all reviews, new and updated, at update this review will be revised according to 2020 policy update.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Backonja 2008 {published data only}
- Backonja M, Wallace MS, Blonsky ER, Cutler BJ, Malan P Jr, Rauck R, et al, NGX-4010 C107 Study Group. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet Neurology 2008;7(12):1106-12. [DOI: 10.1016/S1474-4422(08)70228-X] [DOI] [PubMed] [Google Scholar]
Bischoff 2014 {published data only}
- Bischoff JM, Ringsted TK, Petersen M, Sommer C, Uçeyler N, Werner MU. A capsaicin (8%) patch in the treatment of severe persistent inguinal postherniorrhaphy pain: a randomized, double-blind, placebo-controlled trial. PLoS One 2014;9(10):e109144. [DOI: 10.1371/journal.pone.0109144] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clifford 2012 {published data only}
- Brown S, Simpson DM, Moyle G, Brew BJ, Schifitto G, Larbalestier N, et al. NGX-4010, a capsaicin 8% patch, for the treatment of painful HIV-associated distal sensory polyneuropathy: integrated analysis of two phase III, randomized, controlled trials. AIDS Research and Therapy 2013;10(1):5. [DOI: 10.1186/1742-6405-10-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford DB, Simpson DM, Brown S, Moyle G, Brew BJ, Conway B, et al, NGX-4010 C119 Study Group. A randomized, double-blind, controlled study of NGX-4010, a capsaicin 8% dermal patch, for the treatment of painful HIV-associated distal sensory polyneuropathy. Journal of Acquired Immune Deficiency Syndromes 2012;589(2):126-33. [DOI: 10.1097/QAI.0b013e31823e31f7] [DOI] [PubMed] [Google Scholar]
Irving 2011 {published data only}
- Irving GA, Backonja MM, Dunteman E, Blonsky ER, Vanhove GF, Lu SP, et al, NGX-4010 C117 Study Group. A multicenter, randomized, double-blind, controlled study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. Pain Medicine 2011;12(1):99-109. [DOI: 10.1111/j.1526-4637.2010.01004.x] [DOI] [PubMed] [Google Scholar]
Simpson 2008 {published data only}
- Brown S, Simpson DM, Moyle G, Brew BJ, Schifitto G, Larbalestier N, et al. NGX-4010, a capsaicin 8% patch, for the treatment of painful HIV-associated distal sensory polyneuropathy: integrated analysis of two phase III, randomized, controlled trials. AIDS Research and Therapy 2013;10(1):5. [DOI: 10.1186/1742-6405-10-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DM, Brown S, Tobias J, NGX-4010 C107 Study Group. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology 2008;70(24):2305-13. [DOI: 10.1212/01.wnl.0000314647.35825.9] [DOI] [PubMed] [Google Scholar]
STEP 2014 {unpublished data only}
- Astellas PharmaEurope BV (Sponsor). A phase III, double-blind, randomized, placebo-controlled, multicenter study evaluating the efficacy and safety of QUTENZA® in subjects with painful diabetic peripheral neuropathy (clinical study results). www.astellasclinicalstudyresults.com/hcp/study.aspx?ID=76 Date first received: 6 February 2012. [ASTELLAS ID: E05-CL-3004]
- Astellas Pharma Inc (Responsible party). A phase III, double-blind, randomized, placebo-controlled, multicenter study evaluating the efficacy and safety of QUTENZA® in subjects with painful diabetic peripheral neuropathy. clinicaltrials.gov/ct2/show/NCT01533428 Date first received: 12 February 2012. [ASTELLAS ID: E05-CL-3004] [CTG: ]
- Simpson DM, Robinson-Papp J, Van J, Stoker M, Jacobs H, Snijder RJ, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebo-controlled study. Journal of Pain 2016 Oct 13 [Epub ahead of print]. [DOI: 10.1016/j.jpain.2016.09.008] [DOI] [PubMed] [Google Scholar]
Webster 2010a {published data only}
- Webster LR, Tark M, Rauck R, Tobias JK, Vanhove GF. Effect of duration of postherpetic neuralgia on efficacy analyses in a multicenter, randomized, controlled study of NGX-4010, an 8% capsaicin patch evaluated for the treatment of postherpetic neuralgia. BMC Neurology 2010;10:92. [DOI: 10.1186/1471-2377-10-92] [DOI] [PMC free article] [PubMed] [Google Scholar]
Webster 2010b {published data only}
- Webster LR, Malan TP, Tuchman MM, Mollen MD, Tobias JK, Vanhove GF. A multicenter, randomized, double-blind, controlled dose finding study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. Journal of Pain 2010;11(10):972-82. [DOI: 10.1016/j.jpain.2010.01.270] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Backonja 2010 {published data only}
- Backonja MM, Malan TP, Vanhove GF, Tobias JK, C102/106 Study Group. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomized, double-blind, controlled study with an open-label extension. Pain Medicine 2010;11(4):600-8. [DOI: 10.1111/j.1526-4637.2009.00793.x] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT01228838 {published data only}
- Vanhove T. A multicenter randomized, double-blind, controlled study to evaluate safety, tolerability and preliminary efficacy of two capsaicin concentration variations of NGX-1998 (10% or 20% w/w) in subjects with postherpetic neuralgia (PHN). clinicaltrials.gov/ct2/show/NCT01228838 Date first received: 25 October 2010. [CTG: ] [NEUROGESX ID: C204]
- Webster L, Bhattacharya S, Wallace M, Wells B, Tobias J, Babbar S. Efficacy and safety of NGX-1998, a novel topical liquid formulation of capsaicin, in patients with postherpetic neuralgia: results of a multi-center, placebo-controlled trial. Journal of Pain 2012;13 (4 Suppl 1):S72. [DOI: 10.1016/j.jpain.2012.01.300] [DOI] [Google Scholar]
Additional references
Anand 2011
- Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. British Journal of Anaesthetics 2011;107(4):490-502. [DOI: 10.1093/bja/aer260] [DOI] [PMC free article] [PubMed] [Google Scholar]
Azevedo 2016
- Azevedo LF, Costa-Pereira A, Mendonça L, Dias CC, Castro-Lopes JM. The economic impact of chronic pain: a nationwide population-based cost-of-illness study in Portugal. European Journal of Health Economics 2016;17(1):87-98. [DOI: 10.1007/s10198-014-0659-4] [DOI] [PubMed] [Google Scholar]
Baron 2012
- Baron R, Wasner G, Binder A. Chronic pain: genes, plasticity, and phenotypes. Lancet Neurology 2012;11(1):19-21. [DOI: 10.1016/S1474-4422(11)70281-2] [DOI] [PubMed] [Google Scholar]
Bouhassira 2008
- Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008;136(3):380-7. [DOI: 10.1016/j.pain.2007.08.013] [DOI] [PubMed] [Google Scholar]
Bouhassira 2012
- Bouhassira D, Chassany O, Gaillat J, Hanslik T, Launay O, Mann C, et al. Patient perspective on herpes zoster and its complications: an observational prospective study in patients aged over 50 years in general practice. Pain 2012;153(2):342-9. [DOI: 10.1016/j.pain.2011.10.026] [DOI] [PubMed] [Google Scholar]
Burness 2016
- Burness CB, McCormack PL. Capsaicin 8% patch: a review in peripheral neuropathic pain. Drugs 2016;76:123-34. [DOI: 10.1007/s40265-015-0520-9] [DOI] [PubMed] [Google Scholar]
Calvo 2012
- Calvo M, Dawes JM, Bennett DL. The role of the immune system in the generation of neuropathic pain. Lancet Neurology 2012;11(7):629-42. [DOI: 10.1016/S1474-4422(12)70134-5] [DOI] [PubMed] [Google Scholar]
Collins 1997
- Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain 1997;72(1-2):95-7. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ (Clinical Research Ed) 1995;310:452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dechartres 2013
- Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ 2013;346:f2304. [DOI: 10.1136/bmj.f2304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Demant 2014
- Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain 2014;155(11):2263-73. [DOI: 10.1016/j.pain.2014.08.014] [DOI] [PubMed] [Google Scholar]
Derry 2012
- Derry S, Moore RA. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews 2012, Issue 9. Art. No: CD010111. [DOI: 10.1002/14651858.CD010111] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2014
- Derry S, Wiffen PJ, Moore RA, Quinlan J. Topical lidocaine for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2014, Issue 7. Art. No: CD010958. [DOI: 10.1002/14651858.CD010958.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105-21. [DOI: 10.1016/j.jpain.2007.09.005] [DOI] [PubMed] [Google Scholar]
Edwards 1999
- Edwards JE, McQuay HJ, Moore RA, Collins SL. Reporting of adverse effects in clinical trials should be improved: lessons from acute postoperative pain. Journal of Pain and Symptom Management 1999;18(6):427-37. [DOI: 10.1016/S0885-3924(99)00093-7] [DOI] [PubMed] [Google Scholar]
ELEVATE 2014
- Astellas PharmaEurope BV (Sponsor). QUTENZATM versus pregabalin in subjects with peripheral neuropathic pain: an open-label, randomized, multicenter, non-inferiority efficacy and tolerability study. www.astellasclinicalstudyresults.com/hcp/study.aspx?ID=83 Date first received: 11 July 2012. [EUDRACT NUMBER: 2011-005872-41]
eMC 2012
- eMC. Qutenza 179mg cutaneous patch. www.medicines.org.uk/emc/medicine/23156/SPC/qutenza 179mg cutaneous patch/ (accessed 28 April 2012).
EPOC 2015
- Effective Practice and Organisation of Care (EPOC). 23. Worksheets for preparing a Summary of Findings using GRADE. Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services. Available at: epoc.cochrane.org/epoc-specific-resources-review-authors (accessed 30 November 2016) 2015.
FDA 1999
- US Department of Health and Human Services. Guidance for industry: skin irritation and sensitization testing of generic transdermal drug products. www.fda.gov/ohrms/dockets/98fr/990236Gd.pdf (accessed 13 December 2016).
Finnerup 2013
- Finnerup NB, Scholz J, Attal N, Baron R, Haanpää M, Hansson P, et al. Neuropathic pain needs systematic classification. European Journal of Pain 2013;17(7):953-6. [DOI: 10.1002/j.1532-2149.2012.00282.x] [DOI] [PubMed] [Google Scholar]
Finnerup 2015
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurology 2015;14(2):162-73. [DOI: 10.1016/S1474-4422(14)70251-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gülfe 2010
- Gülfe A, Kristensen LE, Saxne T, Jacobsson LT, Petersson IF, Geborek P. Utility-based outcomes made easy: the number needed per quality-adjusted life year gained. An observational cohort study of tumor necrosis factor blockade in inflammatory arthritis from Southern Sweden. Arthritis Care and Research 2010;62(10):1399-406. [DOI: 10.1002/acr.20235] [DOI] [PubMed] [Google Scholar]
Gustorff 2008
- Gustorff B, Dorner T, Likar R, Grisold W, Lawrence K, Schwarz F, et al. Prevalence of self-reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiological Scandinavica 2008;52(1):132-6. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence - inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294-302. [DOI: 10.1016/j.jclinepi.2011.03.017] [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151-7. [DOI: 10.1016/j.jclinepi.2012.01.006] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables - binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158-72. [DOI: 10.1016/j.jclinepi.2012.01.012] [DOI] [PubMed] [Google Scholar]
Hall 2008
- Hall GC, Carroll D, McQuay HJ. Primary care incidence and treatment of four neuropathic pain conditions: a descriptive study, 2002-2005. BMC Family Practice 2008;9:26. [DOI: 10.1186/1471-2296-9-26] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2013
- Hall GC, Morant SV, Carroll D, Zahava LG, McQuay HJ. An observational descriptive study of the epidemiology and treatment of neuropathic pain in a UK general population. BMC Family Practice 2013;14:28. [DOI: 10.1186/1471-2296-14-28] [DOI] [PMC free article] [PubMed] [Google Scholar]
Helfert 2015
- Helfert SM, Reimer M, Höper J, Baron R. Individualized pharmacological treatment of neuropathic pain. Clinical Pharmacology and Therapeutics 2015;97(2):135-42. [DOI: 10.1002/cpt.19] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hoffman 2010
- Hoffman DL, Sadosky A, Dukes EM, Alvir J. How do changes in pain severity levels correspond to changes in health status and function in patients with painful diabetic peripheral neuropathy? Pain 2010;149(2):194-201. [DOI: 10.1016/j.pain.2009.09.017] [DOI] [PubMed] [Google Scholar]
Ikenberg 2012
- Ikenberg R, Hertel N, Moore RA, Obradovic M, Baxter G, Conway P, et al. Cost-effectiveness of tapentadol prolonged release compared with oxycodone controlled release in the UK in patients with severe non-malignant chronic pain who failed 1st line treatment with morphine. Journal of Medical Economics 2012;15(4):724-36. [DOI: 10.3111/13696998.2012.670174] [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials 1996;17:1-12. [DOI: 10.1016/0197-2456(95)00134-4] [DOI] [PubMed] [Google Scholar]
Jensen 2011
Kalso 2013
- Kalso E, Aldington DJ, Moore RA. Drugs for neuropathic pain. BMJ 2013;347:f7339. [DOI: 10.1136/bmj.f7339] [DOI] [PubMed] [Google Scholar]
Katusic 1991
- Katusic S, Williams DB, Beard CM, Bergstralh EJ, Kurland LT. Epidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: similarities and differences, Rochester, Minnesota, 1945-1984. Neuroepidemiology 1991;10:276-81. [DOI: 10.1159/000110284] [DOI] [PubMed] [Google Scholar]
Kern 2014
- Kern KU, Nowack W, Poole C. Treatment of neuropathic pain with the capsaicin 8% patch: is pretreatment with lidocaine necessary? Pain Practice 2014;14(2):E42-50. [DOI: 10.1111/papr.12143] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knolle 2013
- Knolle E, Zadrazil M, Kovacs GG, Medwed S, Scharbert G, Schemper M. Comparison of cooling and EMLA to reduce the burning pain during capsaicin 8% patch application: a randomized, double-blind, placebo-controlled study. Pain 2013;154(12):2729-36. [DOI: 10.1016/j.pain.2013.08.001] [DOI] [PubMed] [Google Scholar]
Koopman 2009
- Koopman JS, Dieleman JP, Huygen FJ, Mos M, Martin CG, Sturkenboom MC. Incidence of facial pain in the general population. Pain 2009;147(1-3):122-7. [DOI: 10.1016/j.pain.2009.08.023] [DOI] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta-analysis in clinical research. Annals of Internal Medicine 1987;107:224-33. [DOI: 10.7326/0003-4819-107-2-224] [DOI] [PubMed] [Google Scholar]
Loke 2001
- Loke YK, Derry S. Reporting of adverse drug reactions in randomised controlled trials - a systematic survey. BMC Clinical Pharmacology 2001;1:3. [DOI: 10.1186/1472-6904-1-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lunn 2014
- Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No: CD007115. [DOI: 10.1002/14651858.CD007115.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything - large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209-16. [DOI: 10.1016/S0304-3959(98)00140-7] [DOI] [PubMed] [Google Scholar]
Moore 2005
- Moore RA, Derry S, Makinson GT, McQuay HJ. Tolerability and adverse events in clinical trials of celecoxib in osteoarthritis and rheumatoid arthritis: systematic review and meta-analysis of information from company clinical trial reports. Arthritis Research and Therapy 2005;7(3):R644-65. [DOI: 10.1186/ar1704] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2008a
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA, editors(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15-23. [ISBN: 978-0-931092-69-5] [Google Scholar]
Moore 2008b
- Moore RA, Derry S, McQuay HJ. Discontinuation rates in clinical trials in musculoskeletal pain: meta-analysis from etoricoxib clinical trial reports. Arthritis Research and Therapy 2008;10(3):R53. [DOI: 10.1186/ar2422] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2009
- Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD007076. [DOI: 10.1002/14651858.CD007076.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2011a
- Moore RA, Wiffen PJ, Derry S, McQuay HJ. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2011, Issue 3. Art. No: CD007938. [DOI: 10.1002/14651858.CD007938.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2011b
- Moore RA, Gaskell H, Rose P, Allan J. Meta-analysis of efficacy and safety of intravenous ferric carboxymaltose (Ferinject) from clinical trial reports and published trial data. BMC Blood Disorders 2011;11:4. [DOI: 10.1186/1471-2326-11-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2012a
- Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD008242. [DOI: 10.1002/14651858.CD008242.pub2] [DOI] [PubMed] [Google Scholar]
Moore 2012b
- Moore RA, Straube S, Eccleston C, Derry S, Aldington D, Wiffen P, et al. Estimate at your peril: imputation methods for patient withdrawal can bias efficacy outcomes in chronic pain trials using responder analyses. Pain 2012;153(2):265-8. [DOI: 10.1016/j.pain.2011.10.004] [DOI] [PubMed] [Google Scholar]
Moore 2013a
- Moore A, Derry S, Eccleston C, Kalso E. Expect analgesic failure; pursue analgesic success. BMJ 2013;346:f2690. [DOI: 10.1136/bmj.f2690] [DOI] [PubMed] [Google Scholar]
Moore 2013b
- Moore RA, Straube S, Aldington D. Pain measures and cut-offs - 'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400-12. [DOI: 10.1111/anae.12148] [DOI] [PubMed] [Google Scholar]
Moore 2014a
- Moore RA, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non-cancer pain and chronic neuropathic pain. Pain Practice 2014;14(1):79-94. [DOI: 10.1111/papr.12050] [DOI] [PubMed] [Google Scholar]
Moore 2014b
- Moore RA, Wiffen PJ, Derry S, McQuay HJ. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2014, Issue 4. Art. No: CD007938. [DOI: 10.1002/14651858.CD007938.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2014c
- Moore RA, Cai N, Skljarevski V, Tölle TR. Duloxetine use in chronic painful conditions - individual patient data responder analysis. European Journal of Pain 2014;18(1):67-75. [DOI: 10.1002/j.1532-2149.2013.00341.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 1995
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG, editors(s). Statistics with Confidence - Confidence Intervals and Statistical Guidelines. London: British Medical Journal, 1995:50-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mou 2013
- Mou J, Paillard F, Turnbull B, Trudeau J, Stoker M, Katz NP. Efficacy of Qutenza® (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain 2013;154(9):1632-9. [DOI: 10.1016/j.pain.2013.04.044] [DOI] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence (NICE). Neuropathic pain - pharmacological management: the pharmacological management of neuropathic pain in adults in non-specialist settings, 2013. www.nice.org.uk/guidance/cg173 (accessed 30 November 2016).
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ 2010;341:c3515. [DOI: 10.1136/bmj.c3515] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 2010
- O'Brien EM, Staud RM, Hassinger AD, McCulloch RC, Craggs JG, Atchison JW, et al. Patient-centered perspective on treatment outcomes in chronic pain. Pain Medicine 2010;11(1):6-15. [DOI: 10.1111/j.1526-4637.2009.00685] [DOI] [PubMed] [Google Scholar]
PACE 2014
- Astellas PharmaEurope BV (Sponsor). A randomized, controlled, long-term safety study evaluating the effect of repeated applications of QUTENZATM plus standard of care versus standard of care alone in patients with painful diabetic peripheral neuropathy. www.astellasclinicalstudyresults.com/hcp/study.aspx?ID=75 Date first received: 10 November 2011. [ASTELLAS ID: E05-CL-3002] [CTG: ] [EUDRACT NUMBER: 2009-016458-42]
PaPaS 2012
- PaPaS author and referee guidance. papas.cochrane.org/papas-documents (accessed 30 November 2016).
Phillips 2010
- Phillips TJ, Cherry CL, Cox S, Marshall SJ, Rice AS. Pharmacological treatment of painful HIV-associated sensory neuropathy: a systematic review and meta-analysis of randomised controlled trials. PLoS One 2010;5(12):e14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rappaport 1994
- Rappaport ZH, Devor M. Trigeminal neuralgia: the role of self-sustaining discharge in the trigeminal ganglion. Pain 1994;56:127-38. [DOI: 10.1016/0304-3959(94)90086-8] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Simpson 2014
- Simpson DM, Brown S, Tobias JK, Vanhove GF, NGX-4010 C107 Study Group. NGX-4010, a capsaicin 8% dermal patch, for the treatment of painful HIV-associated distal sensory polyneuropathy: results of a 52-week open-label study. Clinical Journal of Pain 2014;30(2):134-42. [DOI: 10.1097/AJP.0b013e318287a32f] [DOI] [PubMed] [Google Scholar]
Straube 2010
- Straube S, Derry S, Moore RA, McQuay HJ. Pregabalin in fibromyalgia: meta-analysis of efficacy and safety from company clinical trial reports. Rheumatology 2010;49(4):706-15. [DOI: 10.1093/rheumatology/kep432] [DOI] [PubMed] [Google Scholar]
Straube 2011
- Straube S, Moore RA, Paine J, Derry S, Phillips CJ, Hallier E, et al. Interference with work in fibromyalgia: effect of treatment with pregabalin and relation to pain response. BMC Musculoskeletal Disorders 2011;12:125. [DOI: 10.1186/1471-2474-12-125] [DOI] [PMC free article] [PubMed] [Google Scholar]
STRIDE 2014
- Astellas PharmaEurope BV (Sponsor). A multicentre, single-arm, open-label study of the repeated administration of QUTENZATM for the treatment of peripheral neuropathic pain. www.astellasclinicalstudyresults.com/hcp/study.aspx?ID=81 Date first received: 28 October 2010. [EUDRACT NUMBER: 2009-016457-18]
Sultan 2008
- Sultan A, Gaskell H, Derry S, Moore RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC Neurology 2008;8:29. [DOI: 10.1186/1471-2377-8-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Torrance 2006
- Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. Journal of Pain 2006;7(4):281-9. [DOI: 10.1016/j.jpain.2005.11.008] [DOI] [PubMed] [Google Scholar]
Treede 2008
- Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008;70(18):1630-5. [DOI: 10.1212/01.wnl.0000282763.29778.59] [DOI] [PubMed] [Google Scholar]
Űçeyler 2014
- Űçeyler N, Sommer C. High-dose capsaicin for the treatment of neuropathic pain: what we know and what we need to know. Pain and Therapy 2014;3(2):73-84. [DOI: 10.1007/s40122-014-0027-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Hecke 2014
- Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 2014;155(4):654-62. [DOI: 10.1016/j.pain.2013.11.013] [DOI] [PubMed] [Google Scholar]
van Nooten 2015
- Nooten FE, Charokopou M, Poole C, Treur M. A systematic literature review and network meta-analysis of capsaicin 8% patch versus oral neuropathic pain medications for the treatment of painful diabetic peripheral neuropathy. Value in Health 2015;18(7):A659. [DOI: 10.1016/j.jval.2015.09.2388] [DOI] [PubMed] [Google Scholar]
von Hehn 2012
- Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012;73(4):638-52. [DOI: 10.1016/j.neuron.2012.02.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163-96. [DOI: 10.1016/S0140-6736(12)61729-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiffen 2013
- Wiffen PJ, Derry S, Moore RA, Aldington D, Cole P, Rice ASC, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia - an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2013, Issue 11. Art. No: CD010567. [DOI: 10.1002/14651858.CD010567.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Derry 2009
- Derry S, Lloyd R, Moore RA, McQuay HJ. Topical capsaicin for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD007393. [DOI: 10.1002/14651858.CD007393.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2013
- Derry S, Sven-Rice A, Cole P, Tan T, Moore RA. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews 2013, Issue 2. Art. No: CD007393. [DOI: 10.1002/14651858.CD007393.pub3] [DOI] [PubMed] [Google Scholar]
Mason 2004
- Mason L, Moore RA, Derry S, Edwards JE, McQuay HJ. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ (Clinical Research Ed) 2004;328(7446):991. [10.1136/bmj.38042.506748.EE] [DOI] [PMC free article] [PubMed] [Google Scholar]


