Abstract
Background
Prolonging kidney transplant survival is an important clinical priority. Induction immunosuppression with antibody therapy is recommended at transplantation and non‐depleting interleukin‐2 receptor monoclonal antibodies (IL2Ra) are considered first line. It is suggested that recipients at high risk of rejection should receive lymphocyte‐depleting antibodies but the relative benefits and harms of the available agents are uncertain.
Objectives
We aimed to: evaluate the relative and absolute effects of different antibody preparations (except IL2Ra) when used as induction therapy in kidney transplant recipients; determine how the benefits and adverse events vary for each antibody preparation; determine how the benefits and harms vary for different formulations of antibody preparation; and determine whether the benefits and harms vary in specific subgroups of recipients (e.g. children and sensitised recipients).
Search methods
We searched the Cochrane Kidney and Transplant's Specialised Register to 29 August 2016 through contact with the Information Specialist using search terms relevant to this review.
Selection criteria
Randomised controlled trials (RCTs) comparing monoclonal or polyclonal antibodies with placebo, no treatment, or other antibody therapy in adults and children who had received a kidney transplant.
Data collection and analysis
Two authors independently extracted data and assessed risk of bias. Dichotomous outcomes are reported as relative risk (RR) and continuous outcomes as mean difference (MD) together with their 95% confidence intervals (CI).
Main results
We included 99 studies (269 records; 8956 participants; 33 with contemporary agents). Methodology was incompletely reported in most studies leading to lower confidence in the treatment estimates.
Antithymocyte globulin (ATG) prevented acute graft rejection (17 studies: RR 0.63, 95% CI 0.51 to 0.78). The benefits of ATG on graft rejection were similar when used with (12 studies: RR 0.61, 0.49 to 0.76) or without (5 studies: RR 0.65, 0.43 to 0.98) calcineurin inhibitor (CNI) treatment. ATG (with CNI therapy) had uncertain effects on death (3 to 6 months, 3 studies: RR 0.41, 0.13 to 1.22; 1 to 2 years, 5 studies: RR 0.75, 0.27 to 2.06; 5 years, 2 studies: RR 0.94, 0.11 to 7.81) and graft loss (3 to 6 months, 4 studies: RR 0.60, 0.34 to 1.05; 1 to 2 years, 3 studies: RR 0.65, 0.36 to 1.19). The effect of ATG on death‐censored graft loss was uncertain at 1 to 2 years and 5 years. In non‐CNI studies, ATG had uncertain effects on death but reduced death‐censored graft loss (6 studies: RR 0.55, 0.38 to 0.78). When CNI and older non‐CNI studies were combined, a benefit was seen with ATG at 1 to 2 years for both all‐cause graft loss (7 studies: RR 0.71, 0.53 to 0.95) and death‐censored graft loss (8 studies: RR 0.55, 0.39 to 0.77) but not sustained longer term. ATG increased cytomegalovirus (CMV) infection (6 studies: RR 1.55, 1.24 to 1.95), leucopenia (4 studies: RR 3.86, 2.79 to 5.34) and thrombocytopenia (4 studies: RR 2.41, 1.61 to 3.61) but had uncertain effects on delayed graft function, malignancy, post‐transplant lymphoproliferative disorder (PTLD), and new onset diabetes after transplantation (NODAT).
Alemtuzumab was compared to ATG in six studies (446 patients) with early steroid withdrawal (ESW) or steroid minimisation. Alemtuzumab plus steroid minimisation reduced acute rejection compared to ATG at one year (4 studies: RR 0.57, 0.35 to 0.93). In the two studies with ESW only in the alemtuzumab arm, the effect of alemtuzumab on acute rejection at 1 year was uncertain compared to ATG (RR 1.27, 0.50 to 3.19). Alemtuzumab had uncertain effects on death (1 year, 2 studies: RR 0.39, 0.06 to 2.42; 2 to 3 years, 3 studies: RR 0.67, 95% CI 0.15 to 2.95), graft loss (1 year, 2 studies: RR 0.39, 0.13 to 1.30; 2 to 3 years, 3 studies: RR 0.98, 95% CI 0.47 to 2.06), and death‐censored graft loss (1 year, 2 studies: RR 0.38, 0.08 to 1.81; 2 to 3 years, 3 studies: RR 2.45, 95% CI 0.67 to 8.97) compared to ATG. Creatinine clearance was lower with alemtuzumab plus ESW at 6 months (2 studies: MD ‐13.35 mL/min, ‐23.91 to ‐2.80) and 2 years (2 studies: MD ‐12.86 mL/min, ‐23.73 to ‐2.00) compared to ATG plus triple maintenance. Across all 6 studies, the effect of alemtuzumab versus ATG was uncertain on all‐cause infection, CMV infection, BK virus infection, malignancy, and PTLD. The effect of alemtuzumab with steroid minimisation on NODAT was uncertain, compared to ATG with steroid maintenance.
Alemtuzumab plus ESW compared with triple maintenance without induction therapy had uncertain effects on death and all‐cause graft loss at 1 year, acute rejection at 6 months and 1 year. CMV infection was increased (2 studies: RR 2.28, 1.18 to 4.40). Treatment effects were uncertain for NODAT, thrombocytopenia, and malignancy or PTLD.
Rituximab had uncertain effects on death, graft loss, acute rejection and all other adverse outcomes compared to placebo.
Authors' conclusions
ATG reduces acute rejection but has uncertain effects on death, graft survival, malignancy and NODAT, and increases CMV infection, thrombocytopenia and leucopenia. Given a 45% acute rejection risk without ATG induction, seven patients would need treatment to prevent one having rejection, while incurring an additional patient experiencing CMV disease for every 12 treated. Excluding non‐CNI studies, the risk of rejection was 37% without induction with six patients needing treatment to prevent one having rejection.
In the context of steroid minimisation, alemtuzumab prevents acute rejection at 1 year compared to ATG. Eleven patients would require treatment with alemtuzumab to prevent 1 having rejection, assuming a 21% rejection risk with ATG.
Triple maintenance without induction therapy compared to alemtuzumab combined with ESW had similar rates of acute rejection but adverse effects including NODAT were poorly documented. Alemtuzumab plus steroid withdrawal would cause one additional patient experiencing CMV disease for every six patients treated compared to no induction and triple maintenance, in the absence of any clinical benefit. Overall, ATG and alemtuzumab decrease acute rejection at a cost of increased CMV disease while patient‐centred outcomes (reduced death or lower toxicity) do not appear to be improved.
Plain language summary
Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients
What is the issue?
A kidney transplant is the best treatment for many people who have severe kidney disease to allow patients to return to work and feel better. Patients who receive a kidney transplant receive drugs to prevent their own body from rejecting the transplant ‐ the aim of treatment is to prolong the function of the kidney transplant while minimising common long‐term side effects of treatment such as cancer, infection, and diabetes. For some patients who have a much higher risk of rejection, additional treatment is given at the time of the operation (which may lower the body's ability to attack the kidney transplant and increase kidney function but can increase the risk of complications such as infection and cancer).
What did we do?
We searched the Cochrane Kidney and Transplant's Specialised Register to 29 August 2016 for randomised controlled trials (RCTs) comparing monoclonal or polyclonal antibodies with placebo, no treatment, or other antibody therapy in adults and children who had received a kidney transplant.
What did we find?
We identified 99 studies (265 records; 8956 participants; 33 with contemporary agents). From the available studies in this area, an antibody against human immune cells (ATG) reduces the chances of a patient having a kidney rejection by one‐third, but it is uncertain whether this prolongs the function of the kidney transplant or survival for the patient. ATG significantly increases viral infections including cytomegalovirus. In addition, the effects of ATG treatment on cancer are not well understood. Alemtuzumab is another treatment which has been compared to ATG in patients who have received less or no steroid therapy as part of their transplant treatment. Treatment with alemtuzumab with lower steroid doses or no steroid treatment at all may lower a patient's risk of kidney rejection within a year after transplantation when compared to ATG but overall the information about treatment benefits and harms of alemtuzumab in many clinical situations are not certain. This means we are not confident about the effects of alemtuzumab on kidney function, patient survival or treatment side‐effects.
Conclusions
Overall the available research on antibody treatment for kidney transplantation is limited when clinicians and patients make joint decisions about antibody therapy at the time of a kidney transplant because of the uncertain long term benefits and hazards of these treatments.
Summary of findings
Background
Description of the condition
Kidney transplantation is the treatment of choice for many patients with end‐stage kidney disease (ESKD) but demand exceeds supply from organ donors. Increasing this supply and prolonging kidney transplant survival are therefore important for patients and health systems (Tonelli 2011).
Description of the intervention
Immunosuppressive therapy consists of initial induction and maintenance regimens to prevent rejection. Induction may be defined as treatment with a biologic agent either before, at the time of, or immediately after transplantation to deplete or modulate T cell responses at the time of antigen presentation. Maintenance immunosuppression protocols usually involve three drugs acting on different parts of the T‐cell activation or proliferation cascade: calcineurin inhibitors (CNI) (e.g. cyclosporin (CSA), tacrolimus), antiproliferative agents (e.g. azathioprine, mycophenolate mofetil) and corticosteroids (e.g. prednisolone) (Denton 1999; Hong 2000).
Induction immunosuppression with antibody therapy is now recommended at the time of transplantation for all patients (KDIGO 2009). Antibody therapies are monoclonal or polyclonal, and depleting or non‐depleting of lymphocytes. Non‐depleting interleukin‐2 receptor monoclonal antibodies (IL2Ra) are considered first line but it is suggested that recipients at high risk of rejection (e.g. children, subsequent transplants, certain racial groups such as African‐Americans, and other sensitised patients) should receive lymphocyte‐depleting antibodies. Depleting antibodies are also used for those at risk of delayed graft function to delay the introduction of full dose CNI, which can prolong the duration of acute tubular necrosis (Denton 1999). Depleting antibodies include polyclonal antibodies against the human lymphocyte (antilymphocyte globulin (ALG); antithymocyte globulin (ATG)).
How the intervention might work
Depleting antibodies bind to target immune effector cells leading to complement mediated destruction. Non‐depleting antibodies bind to targets on effector cells preventing their interaction with other cells rendering them ineffective, but do not lead to cell destruction.
Most antibodies used in transplantation have been directed at T cells. Significant reduction in circulating T‐effector cells is rapidly observed, leading to impaired cell mediated immunity (the desired effect to prevent kidney transplant rejection). A number of different preparations of ATG have been produced over the last few decades. These can be broadly divided into horse ATG (hATG), derived from horse serum after immunisation of horses with human thymocytes, and rabbit ATG (rATG), derived from rabbit serum. There are currently two or three standardised preparations available globally. Historical ATG preparations used in early studies were less standardised compared to the preparations currently available. Even though both hATG and rATG contain antibodies to a wide variety of T‐cell antigens and MHC antigens, it is likely that the effects are not equal given that the two types are prepared differently. One study assessing both efficacy and safety clearly showed differences between these two preparations (Brennan 1999).
Monomurab‐CD3 is a murine monoclonal antibody against the CD3 receptor on activated T cells (Orthoclone OKT3) which became available in the late 1980s. OKT3 removes the functional T‐cell population from circulation, producing immunosuppression useful for both induction therapy and the management of acute rejection. However, this profound immunosuppression is associated with immediate toxicity (cytokine release syndrome) and higher rates of infection and malignancy than standard triple therapy (Soulillou 2001). Use of these preparations may also be limited by the development of neutralising antibodies to their xenogeneic components (Kreis 1992). Use of OKT3 for both induction and treatment of acute rejection has declined in many countries over recent years due to the side effect profile. Janssen‐Cilag discontinued the manufacture of OKT3 in 2010 due to a combination of declining sales and evidence from a Cochrane review on treatment of acute rejection confirming that OKT3 was associated with increased side effects compared to newer biologic agents (Webster 2006).
More recently, the IL2Ra basiliximab and daclizumab have been used in the induction phase. IL2Ra are IgG monoclonal antibodies to the interleukin‐2 receptor found only on activated T cells. IL2Ra are more specific immunosuppressants, with no immediate toxicity, and are increasingly used as induction agents, but not for treating acute rejection (Cibrik 2001). These agents are investigated in a separate Cochrane review (Webster 2010) and so will not be considered here.
Other antibodies have also been introduced for kidney transplantation induction such as alemtuzumab. This humanised CD‐52 specific complement fixing monoclonal antibody was first used for induction by Calne 1999. Alemtuzumab causes profound depletion of T‐cells from peripheral blood and also less marked depletion of other mononuclear cells.
Although the majority of current anti‐rejection therapies are targeted at T‐cell mechanisms, there is increasing evidence that B‐cells may have a role due to their ability to act as antigen presenting cells and T‐cell activators (Zand 2007). For this reason the B‐cell depleting anti‐CD20 antibody, rituximab is also being used in kidney transplantation. Initially this was used in studies for ABO‐incompatible kidney transplants at induction (Tyden 2003) but is now being considered for selected patients in some centres.
Why it is important to do this review
Favoured antibody preparations and rates of use differ from country to country and among transplant units. In 2007 in the USA, 78% of recipients received an antibody preparation as part of induction immunosuppression. Forty five per cent of kidney recipients received ATG, 1% OKT3, 27% IL2Ra and 10% received alemtuzumab (UNOS 2011). In Australia, 93% of patients received an IL2Ra in 2008 and 5% to 10% received an additional or alternative antibody preparation (ANZDATA 2009). There has clearly been an increase in use of antibody induction therapy over the last decade (ANZDATA 2009; UNOS 2011) but there is still a large amount of variability in the type of antibody preparation used. This reflects local policies to some extent but there is also uncertainty, in particular in patients at high risk of rejection, as to whether one agent is superior to another. In patients at higher risk of rejection, increased risk of side effects may be acceptable if a treatment is more effective at reducing the risk of acute rejection, leading to improved rates of allograft and patient survival.
The aim of this systematic review is to summarise the relative short and long‐term beneficial and adverse effects of different antibody preparations (except IL2Ra) used as induction in kidney transplant recipients. A previous Cochrane review looks at the use of antibodies for treatment of acute rejection episodes (Webster 2006).
Objectives
To evaluate the relative and absolute effects of different antibody preparations (except IL2Ra) when used as induction therapy in kidney transplant recipients.
To determine how the benefits and adverse events vary for each antibody preparation.
To determine how the benefits and harms vary for different formulations of antibody preparation.
To determine whether the benefits and harms vary in specific subgroups of recipients (e.g. children and sensitised recipients).
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at different antibody preparations (except IL2Ra) used as induction in kidney transplant recipients.
Types of participants
Adults and children who are kidney transplant recipients.
Recipients of multi‐organ transplants were excluded from this review.
Types of interventions
We included studies using antibody preparations given in combination with any other immunosuppressive agents for induction therapy.
Exclusions were IL2Ra, as they are the subject of a separate Cochrane Review (Webster 2010). The authors also note that the manufacture of OKT3 was discontinued in January 2010 but have decided to include this agent in the interventions for historical purposes.
We examined the following comparisons.
ATG versus placebo/no treatment
ATG versus ALG
ATG versus a different ATG (e.g. rabbit versus horse)
ATG versus monomurab‐CD3
ALG versus placebo/no treatment
ALG versus monomurab‐CD3
Monomurab‐CD3 versus placebo/no treatment
Alemtuzumab/anti‐CD52 versus placebo/no treatment
Alemtuzumab/anti‐CD52 versus other poly‐ or monoclonal antibody
Rituximab/anti‐CD20 versus placebo/no treatment
Rituximab/anti‐CD20 versus other poly‐ or monoclonal antibody
Other poly‐ or monoclonal antibody versus placebo/no treatment
Other poly‐ or monoclonal antibody versus other poly‐ or monoclonal antibody
Antibody versus non‐antibody intervention
The 'class effect' of anti‐lymphocyte preparations was initially assumed but differences in formulation were also examined (e.g. rabbit versus horse‐based ATG formulations). All dosage regimens were included and low versus high dose regimens were examined.
Types of outcome measures
Where possible, outcome events were assessed at one, three and six months, and at one, two, three and five years post‐transplantation.
Primary outcomes
Death (all cause)
Graft loss (defined as dependence on dialysis, graft loss censored for death with a functioning allograft)
Graft loss including death with a functioning graft
Incidence of acute rejection of kidney (analysed as combined outcome for clinical suspicion, biopsy‐proven and steroid resistant).
Secondary outcomes
Kidney allograft function: glomerular filtration rate (GFR), serum creatinine (SCr), creatinine clearance (CrCl), or as defined by authors
Incidence of delayed graft function
Incidence of bacterial, fungal and viral infectious complications specifically including cytomegalovirus (CMV) (both asymptomatic CMV viraemia and true cases of CMV infection with tissue invasion were analysed as reported by the individual studies) and Polyoma BK virus
Incidence of new‐onset diabetes after transplantation (NODAT)
Incidence of any malignancy
Incidence of post‐transplant lymphoproliferative disorders (PTLD) and lymphoma
Incidence of treatment‐related adverse reactions (gastrointestinal, neurological, haematological, biochemical) and recognised syndromes (e.g. serum sickness, cytokine release syndrome).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register up to 29 August 2016 through contact with the Information Specialist using search terms relevant to this review. The Cochrane Kidney and Transplant Specialised Register contains studies identified from the following sources
Quarterly searches of the Cochrane Central Register of Controlled Trials CENTRAL
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals & the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney‐journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal & ClinicalTrials.gov
Studies contained in the Specialised register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane kidney and Transplant. Details of these strategies as well as a list of handsearched journals, conference proceedings and current awareness alerts are available in the 'Specialised Register' section of information about the Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that might have been relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable. However, studies and reviews that might include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and, if necessary the full text, of these studies to determine which studies satisfy the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, records were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions these data were used. Any discrepancy between published versions was to be highlighted. Where duplicate publication was suspected authors were contacted for clarification and if duplication was confirmed the initial full publication together with any subsequent publication which adds additional information (e.g. longer term follow‐up data) was included in the review.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors(detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (e.g. rejection) results were expressed as risk ratios (RR) with 95% confidence intervals (95% CI).
Where continuous scales of measurement were used to assess the effects of treatment (e.g. CrCl), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales were used. For count data (such as total number of infections/person‐year of follow‐up) the rate ratio was used. Where time‐to‐event data could not be dichotomised, survival analysis methods were used and the results expressed as hazard ratio (HR).
Where outcomes were not amenable to meta‐analysis, i.e. if reported idiosyncratically (e.g. drug‐related specific adverse reactions), they were tabulated and assessed with descriptive techniques, and the risk difference (RD) with 95% CI was calculated. Quality of life and economic data was analysed using descriptive techniques.
Assessment of heterogeneity
Clinical and methodological heterogeneity was analysed using a Cochran Q test (Chi² with N‐1 degrees of freedom and a P value of 0.05 used for statistical significance) and with the I² test (Higgins 2003). I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
Funnel plots were used to assess for the potential existence of small study bias (Higgins 2011).
Data synthesis
Data was pooled using the random effects model (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was used to explore possible clinical sources of heterogeneity.
Baseline maintenance immunosuppression
Antibody formulation (e.g. rabbit versus horse ATG)
Duration and dose of antibody treatment.
'Summary of findings' tables
We have presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We presented the following outcomes in the 'Summary of findings' tables.
Death
Graft loss
Delayed graft function
Acute rejection
CMV infection
Malignancy
NODAT
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
After searching the Specialised Register we identified 452 records. After duplicates were removed and titles and abstracts screened we retrieved 285 full‐text articles for further assessment. Of these, 99 studies (268 records) were included and five studies (8 records) were excluded. Three ongoing studies (NCT00733733; NCT01154387; ReMIND Study 2013) were identified, and five studies (NCT00089947; NCT00861536; NCT01046955; NCT01354301; Stevens 2016) were identified prior to publication. These eight studies and will be assessed in a future update of this review (Figure 1).
1.

Flow diagram of included and excluded studies.
Included studies
Of the 99 included studies, 92 had data that could be used for meta‐analysis and these combined studies represented a total of 8802 randomised participants. ATG was used in 41 studies, alemtuzumab in 11, OKT3 in 27, ALG in 26, rituximab in 3 and other antibodies in 5 studies.
There were 19 comparisons of an antibody versus placebo or antibody versus other antibody that were studied in a single study only. These are briefly discussed in the text below but have not been meta‐analysed.
Interventions
Number of studies (participants) in included studies by comparison
| ATG | ALG | Alemtuzumab | Rituximab | OKT3 | Othera | Placebo | |
| ATG | 9 (513)b | 1 (50) | 6 (446) | ‐ | 6 (571) | 2 (141) | 17 (2044) |
| ALG | ‐ | 3 (254)b | ‐ | ‐ | 7 (644) | ‐ | 16 (1809) |
| Alemtuzumab | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 4 (296) |
| Rituximab | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 3 (447) |
| OKT3 | ‐ | ‐ | ‐ | ‐ | 2 (55)b | ‐ | 12 (1184) |
| Othera | ‐ | ‐ | ‐ | ‐ | 1 (20) | ‐ | 3 (328) |
a Includes the following; anti CD2 rat monoclonal antibody, anti CD7 monoclonal antibody, anti‐LFA‐1 monoclonal antibody, anti‐ICAM‐1 monoclonal antibody, rituximab combined with ATG, bortezomib combined with ATG, both rituximab and bortezomib combined with ATG.
b Indicates studies comparing different doses or formulations of same agent.
ATG versus placebo/no treatment
Twelve studies (1491 participants) compared ATG with placebo or no treatment in a CNI‐based regimen (Banhegyi 1991; Charpentier 2002; Kasiske 1997; Khosroshahi 2008; Martins 2004; Mourad 1998; Samsel 1999; Sheashaa 2008; Thibaudin 1998; TRIMS Study 2010; van den Hoogen 2013; Yussim 2000), and a further five studies (553 participants) in a non‐CNI‐based regimen (Cosimi 1976; Diethelm 1979; Kountz 1977; Kreis 1986; Wechter 1979).
Rabbit ATG versus horse ATG
Three studies (155 participants) compared rATG with hATG in a CNI‐based regimen (Bock 1999; Brennan 1999; Rostaing 2010).
ATG versus alemtuzumab
Six studies (446 participants) compared ATG with alemtuzumab. Four studies had early steroid withdrawal (ESW) or steroid minimisation in both arms in a CNI‐based regimen (Farney 2008; Hanaway 2011; Lu 2011; Thomas 2007) and two studies had ESW in the alemtuzumab arm only (Ciancio 2005; Ciancio 2010) and triple maintenance in the ATG groups.
Alemtuzumab versus placebo/no treatment
Four studies (296 participants) compared alemtuzumab with placebo or no treatment. Three of four studies used ESW with either single or double agent maintenance immunosuppression in the alemtuzumab group (CAMPASIA Study 2005; Margreiter 2008; Sharaf El Din 2006) versus triple therapy maintenance in the control group, and one study (Friend 1987) used ESW and single agent CSA maintenance in both groups.
Rituximab versus placebo
Three studies (447 participants) compared rituximab with placebo (Smeekens 2013; Tsai 2012; Tyden 2009).
ATG versus OKT3
Six studies (571 participants) compared ATG with OKT3 (Bock 1995; Cole 1994; Fukuuchi 1996; Kumar 1998a; Perez‐Tamajon 1996; Raffaele 1991). Maintenance immunosuppression was CNI‐based triple therapy and the same in both arms for all six studies.
OKT3 versus placebo/no treatment
Twelve studies (1184 participants) compared OKT3 with placebo or no treatment (Abramowicz 1992; Ackermann 1988; Benfield 1999; Debure 1987; De Pauw 1990; Henry 2001; Kreis 1986; Morales 1994a; Norman 1988; Norman 1993; Shield 1993; Vigeral 1986).
ALG versus OKT3
Six studies (593 participants) compared ALG with OKT3 (Broyer 1993; Frey 1991; Grino 1991; Hanto 1991; Niaudet 1990; Vela 1994).
ALG versus placebo/no treatment
Sixteen studies (1809 participants) compared ALG with placebo or no treatment (Belitsky 1991; Bell 1983; Cantarovich 2008; Condie 1985; Gianello 1987; Grundmann 1984; Halloran 1982; Jakobsen 1981; Grino 1990; Launois 1977; Maiorca 1984; Minnesota Study 1982; Novick 1983; Sansom 1976; Slakey 1993; Taylor 1976).
Other antibodies
Five studies looked at single antibody comparisons each: anti‐CD2 rat monoclonal antibody versus placebo (40 participants, Squifflet 1997), anti‐CD7 monoclonal antibody versus OKT3 (20 participants, Lazarovits 1993), anti‐LFA‐1 monoclonal antibody versus placebo (22 participants, Spillner 1998), anti‐LFA‐1 monoclonal antibody versus ATG (101 participants, Hourmant 1996), and anti‐ICAM‐1 monoclonal antibody versus placebo (266 participants, EARTS Study 1999). One small pilot study compared ATG with 3 other combination induction regimens; ATG + rituximab, ATG + bortezomib; ATG + rituximab + bortezomib (40 participants, Ejaz 2013).
Other comparisons
A further thirteen studies looked at other ATG, OKT3 or ALG comparisons but each of these had only a single study for each comparison. The ATG studies were:
Single versus divided dose ATG (142 participants, Stevens 2008)
Two versus four doses (same total) of ATG (17 participants, Buchler 2013)
rATG Fresenius versus rATG Merieux (90 participants, Norrby 1997)
ATG adjusted for CD3 count versus fixed dose (45 participants, Abouna 1995)
ATG adjusted for CD3 count versus adjusted for total lymphocyte count (21 participants, Ata 2013)
standard versus low dose ATG (43 participants, Grafals 2014)
ATG versus ALG (50 participants, Toledo‐Pereyra 1985).
The OKT3 studies were:
Standard versus low dose (26 participants, Norman 1993a)
Standard versus high dose (29 participants, Abramowicz 1994)
OKT3 versus ALG given only for delayed graft function (51 participants, Steinmuller 1991).
The remaining ALG studies were:
Low versus high dose (83 participants, Sakhrani 1992)
Low potency versus high potency ALG (71 participants, Thomas 1977)
Fourteen versus 7 days induction (100 participants, Grundmann 1987).
Reported outcome measures
The reporting of outcome measures was variable across studies: 83 reported patient death, 70 reported all‐cause graft loss and 24 death‐censored graft loss while 84 reported acute rejection and 42 reported delayed graft function (see Figure 1). Acute rejection was reported in a further seven studies but could not be used in meta‐analysis as rejection was either reported without actual figures or reported as total number of episodes rather than number of participants. Graft function was reported at a variety of time points in 33 studies. Some studies reporting graft function could not be included in meta‐analysis as there was no SD or SE reported. Reporting of harms was more limited and inconsistent among studies. Participants with any serious infection were reported in 61 (66%) studies, however a further 7 studies also assessed infection, but expressed their results as ‘infectious episodes’, or reported no actual figures and so this data could not be easily meaningfully combined. CMV infection was reported in 35 studies and BKV infection in only 7 studies. Malignancy and PTLD were reported in only 30 studies and NODAT in 12. Haematological effects were reported in very few studies; 16 reported leucopenia and 12 thrombocytopenia. Very small numbers of studies reported other adverse outcomes including serum sickness, tremor, headache, chronic allograft nephropathy (on biopsy) and failure to complete induction therapy.
Excluded studies
Five studies were excluded (Alloway 1993; Kirsch 2006; Kumar 2002b; NCT00000936; NCT01312064). The reasons for exclusion were:
Mixed population and data could not be separated (Alloway 1993)
No outcomes of interest were reported (Kirsch 2006)
Not a true randomisation (Kumar 2002b)
Study terminated and no results published (NCT00000936; NCT01312064).
Risk of bias in included studies
Reporting of details of study methodology was incomplete for the majority of studies. Details are summarised in Figure 2 and Figure 3.
2.
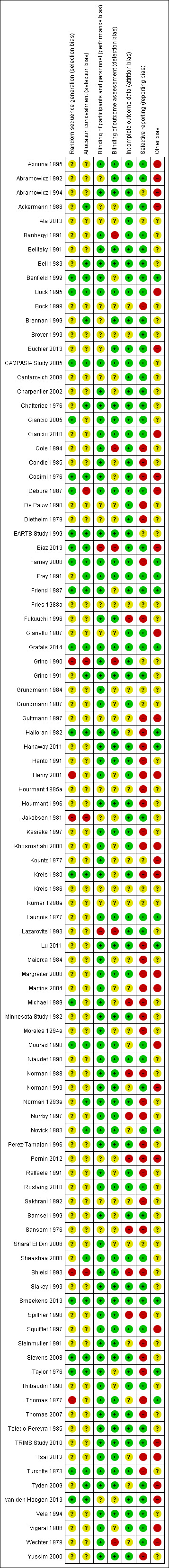
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Twenty studies reported adequate sequence generation, and 27 reported adequate allocation concealment. Five studies used inadequate methods of sequence generation and four used inadequate allocation concealment. The remainder (74 studies for sequence generation and 68 for allocation concealment) used unclear methodology.
Blinding
Seventy‐six studies adequately reported blinding of participants and personnel, and 54 studies adequately reported blinding of outcome assessment. Two studies had inadequate blinding of participants and personnel and six studies had inadequate blinding of outcome assessment. The remainder had unclear methods.
Incomplete outcome data
Incomplete outcome data was adequately addressed in 68 studies, and inadequately in eight studies. The remainder were unclear.
Selective reporting
Forty‐five studies were free of selective reporting, 43 studies were inadequate, while the remainder of studies were unclear.
Other potential sources of bias
Thirteen studies declared their funding source to be independent or academic funding body, and so were judged free of other potential biases. Twenty‐eight studies were deemed to be high risk of other bias due to funding from a pharmaceutical company or author links to industry or other reasons not covered by above bias assessments. Others did not disclose the funding source of the study or gave limited information about funding and were judged unclear.
Effects of interventions
for the main comparison.
| ATG compared with placebo or no induction for kidney transplant recipients | ||||||
|
Patient or population: kidney transplant recipients Settings: Intervention: ATG Comparison: placebo/no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo/no treatment | ATG | |||||
|
Death (including CNI) Follow‐up: median 24 months (IQR 12‐24) |
Medium risk population |
RR 0.75 (0.27 to 2.06) |
632 (5) | ⊕⊕⊝⊝ low1,2 | ||
| 31 per 1000 | 23 per 1000 (8 to 64) | |||||
|
All‐cause graft loss (including CNI) Follow‐up: median 1 year (IQR 12‐24) |
Medium risk population |
RR 0.65 (0.36 to 1.19) |
549 (3) | ⊕⊕⊝⊝ low1,2 | ||
| 109 per 1000 | 71 per 1000 (39 to 129) | |||||
|
Delayed graft function Follow‐up: N/A (immediate) |
Medium risk population | RR 0.93 (0.78 to 1.10) | 1304 (9) | ⊕⊕⊝⊝ low1,2 | ||
| 283 per 1000 | 263 per 1000 (221 to 311) | |||||
|
Acute rejection (including CNI) Follow‐up: median 1 year (IQR 6‐24) |
Medium risk population |
RR 0.61 (0.49 to 0.76) |
1491 (12) | ⊕⊕⊕⊝ moderate1 | ||
| 365 per 1000 | 222 per 1000 (179 to 277) | |||||
|
Infection: CMV infection Follow‐up: median 1 year (IQR 4.5‐13.5) |
Medium risk population |
RR 1.55 (1.24 to 1.95) |
1072 (6) | ⊕⊕⊕⊝ moderate1 | ||
| 176 per 1000 | 273 per 1000 (218 to 343) | |||||
|
Malignancy Follow‐up: median 18 months (IQR 12‐60) |
Medium risk population |
RR 0.94 (0.30 to 2.94) |
891 (7) | ⊕⊕⊝⊝ low1,2,3 | ||
| 15 per 1000 | 14 per 1000 (5 to 44) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval: RR: Risk Ratio; IQR: interquartile range. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 At risk of selection bias as more than 50% of studies rated as allocation concealment and/or random sequence generation unclear or high risk of causing bias.
2 Confidence interval includes range of plausible values below clinical significance or including harm.
3Based on few events across all studies.
2.
| Alemtuzumab plus ESW or steroid minimisation versus ATG for induction therapy for kidney transplant recipients | ||||||
|
Patient or population: kidney transplant recipients Settings: Intervention: alemtuzumab plus ESW or steroid minimisation Comparison: ATG ± ESW or steroid minimisation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| ATG | Alemtuzumab | |||||
|
Death (ESW both arms) Follow‐up: median 1 year (IQR 12‐36) |
Medium risk population | RR 0.27 (0.07 to 1.06) | 180 (3) | ⊕⊕⊝⊝ low1,2 | ||
| 102 per 1000 | 27 per 1000 (7 to 108) | |||||
|
All‐cause graft loss (ESW both arms) Follow‐up: median 18 months (IQR 12‐30) |
Medium risk population |
RR 0.60 (0.34 to 1.08) |
360 (4) | ⊕⊕⊝⊝ low1,2 | ||
| 148 per 1000 | 89 per 1000 (50 to 160) | |||||
|
Acute rejection (ESW both arms) Follow‐up: median 18 months (IQR 12‐30) |
Medium risk population |
RR 0.57 (0.35 to 0.93) |
360 (4) | ⊕⊕⊕⊝ moderate1 | ||
| 208 per 1000 | 119 per 1000 (73 to 193) | |||||
|
Biopsy‐proven CAN (ESW with alemtuzumab only) Follow‐up: median 30 months (IQR 24‐36) |
Medium risk population |
RR 2.45 (1.02 to 5.94) |
86 (2) | ⊕⊕⊝⊝ low1,2 | ||
| 116 per 1000 | 284 per 1000 (118 to 689) | |||||
|
CMV infection (all studies) Follow‐up: median 30 months (IQR 24‐36) |
Medium risk population |
RR 1.08 (0.46 to 2.56) |
225 (3) | ⊕⊕⊝⊝ low1,2 | ||
| 80 per 1000 | 86 per 1000 (37 to 205) | |||||
|
NODAT (ESW alemtuzumab only) Follow‐up: median 30 months (IQR 24‐36) |
Medium risk population |
RR 0.41 (0.12 to 1.40) |
69 (2) | ⊕⊕⊝⊝ low1,2 | ||
| 237 per 1000 | 97 per 1000 (28 to 332) | |||||
|
Malignancy (all studies) Follow‐up: median 36 months (IQR 12‐36) |
Medium risk population |
RR 4.93 (0.59 to 41.11) |
187 (3) | ⊕⊝⊝⊝ very low1,2,3 | All reported events from single study (other 2 studies reported 0 events) | |
| 11 per 1000 | 54 per 1000 (6 to 452) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval: RR: Risk Ratio; IQR: interquartile range. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 At risk of selection bias as more than 50% of studies rated as allocation concealment and/or random sequence generation unclear or high risk of causing bias.
2 Confidence interval includes range of plausible values below clinical significance or including harm.
3Based on few events across all studies.
ATG versus placebo/no induction treatment
ATG had little or no effect on death at 1 to 2 years compared to placebo or no treatment in older studies without CNI maintenance (Analysis 1.1.3 (6 studies, 621 participants): RR 1.03, 95% CI 0.86 to 1.22; I2 = 0%) and uncertain effect in more contemporary studies including CNI maintenance (Analysis 1.1.2 (5 studies, 632 participants): RR 0.75, 95% CI 0.27 to 2.06; I2 = 0%). In the CNI studies, there was also uncertain effect on death at 3 to 6 months (Analysis 1.1.1 (3 studies, 523 participants): RR 0.41, 95% CI 0.13 to 1.22; I2 = 0%) and at 5 years (Analysis 1.1.4 (2 studies, 159 participants): RR 0.94, 95% CI 0.11 to 7.81; I2 = 48%).
1.1. Analysis.
Comparison 1 ATG versus placebo/no treatment, Outcome 1 Death.
Treatment with ATG had uncertain effect on all‐cause graft loss in CNI studies at 3 to 6 months (Analysis 1.2.1 (4 studies, 638 participants): RR 0.60, 95% CI 0.34 to 1.05; I2 = 0%), at 1 to 2 years (Analysis 1.2.2 (3 studies, 549 participants): RR 0.65, 95% CI 0.36 to 1.19; I2 = 6%) and at 5 years (Analysis 1.2.4 (2 studies, 159 participants): RR 1.13, 95% CI 0.62 to 2.05; I2 = 0%). However, ATG reduced graft loss in the non‐CNI studies at 1 to 2 years (Analysis 1.2.3 (4 studies, 500 participants): RR 0.70, 95% CI 0.49 to 1.01; I2 = 50%). When CNI and non‐CNI studies were combined, ATG reduced all‐cause graft loss at 1 to 2 years (Analysis 1.2.5 (7 studies, 1049 participants): RR 0.71, 95% CI 0.53 to 0.95; I2 = 35%).
1.2. Analysis.
Comparison 1 ATG versus placebo/no treatment, Outcome 2 Graft loss (all cause).
Death‐censored graft loss was reduced at 1 to 2 years in non‐CNI studies (Analysis 1.3.2 (6 studies, 299 participants): RR 0.55, 95% CI 0.38 to 0.78; I2 = 0%) but there was uncertain effect in CNI studies at 2 years (Analysis 1.3.1 (2 studies, 82 participants): RR 0.57, 95% CI 0.19 to 1.75; I2 = 0%) and at 5 years (Analysis 1.3.3 (2 studies, 148 participants): RR 1.64, 95% CI 0.20 to 13.18; I2 = 71%). Again, if CNI and non‐CNI studies were combined then death censored graft loss was significantly reduced with ATG at 1 to 2 years (Analysis 1.3.4 (8 studies, 381 participants): RR 0.55, 95% CI 0.39 to 0.77; I2 = 0%).
1.3. Analysis.
Comparison 1 ATG versus placebo/no treatment, Outcome 3 Graft loss (death censored).
ATG prevented acute rejection (Analysis 1.4 (17 studies, 2044 participants): RR 0.63, 95% CI 0.51 to 0.78; I2 = 65%). The relative reduction in risk of rejection was similar in studies including CNI maintenance (Analysis 1.4.1 (12 studies, 1491 participants): RR 0.61, 95% CI 0.49 to 0.76; I2 = 35%) compared to non‐CNI studies (Analysis 1.4.2 (5 studies, 553 participants): RR 0.65, 95% CI 0.43 to 0.98; I2 = 73%) (P = 0.79; I2 = 0% for subgroup analysis).
1.4. Analysis.
Comparison 1 ATG versus placebo/no treatment, Outcome 4 Acute rejection.
ATG had little or no effect on delayed graft function (Analysis 1.5 (9 studies, 1304 participants): RR 0.93, 95% CI 0.78 to 1.10; I2 = 0%).
1.5. Analysis.
Comparison 1 ATG versus placebo/no treatment, Outcome 5 Delayed graft function.
ATG increased CMV infection (Analysis 1.6.2 (6 studies, 1072 participants): RR 1.55, CI 1.24 to 1.95; I2 = 0%) but had uncertain effects on all‐cause viral infection (Analysis 1.6.4 (3 studies, 197 participants): RR 1.38, 95% CI 0.56 to 3.39; I2 = 46%) and bacterial infection (Analysis 1.6.5 (5 studies, 775 participants): RR 1.15, 95% CI 0.96 to 1.37; I2 = 0%).
1.6. Analysis.
Comparison 1 ATG versus placebo/no treatment, Outcome 6 Infection.
Leucopenia (Analysis 1.7 (4 studies, 920 participants): RR 3.86, 95% CI 2.79 to 5.34; I2 = 0%) and thrombocytopenia (Analysis 1.8 (4 studies, 848 participants): RR 2.41, 95% CI 1.61 to 3.61; I2 = 0%) were both increased by ATG.
1.7. Analysis.
Comparison 1 ATG versus placebo/no treatment, Outcome 7 Leucopenia.
1.8. Analysis.
Comparison 1 ATG versus placebo/no treatment, Outcome 8 Thrombocytopenia.
ATG had uncertain effects on both early malignancy at 1 to 2 years (Analysis 1.9.1 (3 studies, 611 participants): RR 0.94, 95% CI 0.22 to 3.94; I2 = 0%) and on late malignancy at 5 years (Analysis 1.9.2 (2 studies, 159 participants): RR 0.94, 95% CI 0.14 to 6.23; I2 = 0%). The single study (151 participants) that reported PTLD had no events at 1 year in either arm (Analysis 1.9).
1.9. Analysis.
Comparison 1 ATG versus placebo/no treatment, Outcome 9 Malignancy or PTLD.
ATG had uncertain effect on development of NODAT (Analysis 1.10.1 (6 studies, 935 participants): RR 1.01, 95% CI 0.56 to 1.84; I2 = 39%).
1.10. Analysis.
Comparison 1 ATG versus placebo/no treatment, Outcome 10 Other adverse outcomes.
There was no difference in SCr at 6 months (Analysis 1.11.1 (2 studies, 503 participants): MD ‐5.34 µmol/L, 95% CI ‐13.44 to 2.75; I2 = 0%), 1 year (Analysis 1.11.2 (2 studies, 222 participants): MD ‐10.56 µmol/L, 95% CI ‐21.81 to 0.69) or 5 years (Analysis 1.11.5 (1 study, 55 participants): MD ‐32.70 µmol/L, 95% CI ‐68.98 to 3.58) following ATG therapy in studies including CNI maintenance. There was also no difference in SCr at 1 year in the single non CNI study that assessed graft function (Turcotte 1973). Graft function measured by eGFR was only assessed in one study (Sheashaa 2008) and was similar between treatment groups at 5 years (1 study, 71 participants: MD 4.80 mL/min, 95% CI ‐2.57 to 12.17).
1.11. Analysis.
Comparison 1 ATG versus placebo/no treatment, Outcome 11 Serum creatinine.
Rabbit ATG versus horse ATG
There was sparse data for meta‐analyses comparing rATG versus hATG. rATG had uncertain effects on death at 1 year (Analysis 2.1.1 (2 studies, 139 participants): RR 0.41, 95% CI 0.07 to 2.30; I2 = 0%) and on long‐term death at 10 years (Analysis 2.2.2 (1 study, 72 participants): RR 0.75, 95% CI 0.35 to 1.59) compared to hATG. The effect on all‐cause graft loss was also uncertain at both 1 year (Analysis 2.1.3 (2 studies, 139 participants: RR 0.31, 95% CI 0.08 to 1.27; I2 = 14%) and at 10 years (Analysis 2.1.4 (1 study, 72 participants: RR 0.96, 95% CI 0.58 to 1.58).
2.1. Analysis.
Comparison 2 Rabbit ATG versus horse ATG, Outcome 1 Main outcomes.
2.2. Analysis.
Comparison 2 Rabbit ATG versus horse ATG, Outcome 2 Other adverse outcomes.
rATG prevented acute rejection (2 studies, 88 participants: RR 0.17, 95% CI 0.04 to 0.76) compared to hATG although one study reported no events (Rostaing 2010).
Single studies reported uncertain effects of rATG compared to hATG with respect to delayed graft function (Rostaing 2010) (Analysis 2.1.7, 16 participants: RR 0.50, 95% CI 0.06 to 4.47), all‐cause infection (Rostaing 2010) (Analysis 2.2.1, 16 participants: RR 1.67, 95% CI 0.59 to 4.73), and malignancy (Brennan 1999) (Analysis 2.2.4, 72 participants: RR 0.40, 95% CI 0.12 to 1.35).
Brennan 1999 reported CMV disease was reduced with rATG at 1 year (Analysis 2.2.2, 72 participants: RR 0.38, 95% CI 0.15 to 0.96), more leucopenia with rATG compared to hATG (analysis 2.2.3, 72 participants: RR 13.50, 95% CI 1.95 to 93.46), and graft function was better at 10 years with a lower SCr in the hATG group (Analysis 2.3, 35 participants: MD 44.0 µmol/L, 95% CI 20.41 to 67.59).
2.3. Analysis.
Comparison 2 Rabbit ATG versus horse ATG, Outcome 3 Serum creatinine.
Alemtuzumab versus ATG
The effects of alemtuzumab (with ESW or minimisation) compared to ATG on death were uncertain both at 1 year (Analysis 3.1.1 (2 studies, 41 participants): RR 0.39, 95% CI 0.06 to 2.42; I2 = 0%) and at 2 to 3 years (Analysis 3.1.2 (3 studies, 225 participants): RR 0.67, 95% CI 0.15 to 2.95; I2 = 33%). Similarly, alemtuzumab had uncertain effect on all‐cause graft loss at 1 year (Analysis 3.1.3 (2 studies, 41 participants): RR 0.39, 95% CI 0.12 to 1.30; I2 = 0%) and at 2 to 3 years (Analysis 3.1.4 (3 studies, 379 participants): RR 0.98, 95% CI 0.47 to 2.06; I2 = 42%) and on death‐censored graft loss at 1 year (Analysis 3.1.5 (2 studies, 37 participants): RR 0.38, 95% CI 0.08 to 1.81; I2 = 0%) and at 2 to 3 years (Analysis 3.1.6 (2 studies, 186 participants): RR 2.45, 95% CI 0.67 to 8.97; I2 = 17%) compared to ATG. There was also uncertain effect of alemtuzumab versus ATG on delayed graft function (Analysis 3.1.7 (2 studies, 86 participants): RR 0.62, 95% CI 0.13 to 3.07; I2 = 0%).
3.1. Analysis.
Comparison 3 Alemtuzumab + early steroid withdrawal (ESW) or minimisation versus ATG ± ESW, Outcome 1 Death and graft loss.
Alemtuzumab had uncertain effect on acute rejection in the first 6 months (Analysis 3.2.1 (3 studies, 341 participants): RR 0.47, 95% CI 0.17 to 1.30; I2 = 32%) and at 1 year or more (Analysis 3.2.2 (6 studies, 446 participants: RR 0.68, 95% CI 0.44 to 1.05; I2 = 0%). Two of these 6 studies favoured ATG (Ciancio 2005; Ciancio 2010) while the other four favoured alemtuzumab (Farney 2008; Hanaway 2011; Lu 2011; Thomas 2007). This difference may be explained by ESW in the alemtuzumab group but not the ATG group in two studies (Ciancio 2005; Ciancio 2010), compared to ESW in both arms in the other four studies. Subgroup analysis of these four studies showed acute rejection was reduced at 1 year and beyond by alemtuzumab compared to ATG in studies with ESW in both arms (Analysis 3.2.3 (4 studies, 360 participants: RR 0.57, 95% CI 0.35 to 0.93; I2 = 0%) (test for subgroup differences, P = 0.13). Subgroup analysis of the two studies with alemtuzumab plus ESW versus ATG and steroid continuation showed the effect of alemtuzumab and ESW on acute rejection at 1 year was uncertain (Analysis 3.2.4 (2 studies, 86 participants): RR 1.27, 95% CI 0.50 to 3.19; I2 = 0%). The results of all outcomes other than acute rejection were not significantly altered when subgroup analysis was done including only studies with steroid avoidance in both the alemtuzumab and ATG arms.
3.2. Analysis.
Comparison 3 Alemtuzumab + early steroid withdrawal (ESW) or minimisation versus ATG ± ESW, Outcome 2 Rejection.
There was an increased rate of chronic allograft nephropathy (CAN) on biopsy with alemtuzumab plus ESW but this was only assessed in the 2 studies that had triple maintenance immunosuppression in the ATG arms (Analysis 3.2.5 (2 studies, 86 participants): RR 2.64, 95% CI 1.09 to 6.36; I2 = 0%). The classification of CAN is a historical one, present in the original Banff 1997 diagnostic categories (Racusen 1999) but removed in the 2005 update (Solez 2007).
Alemtuzumab had uncertain effect on all‐cause infection (Analysis 3.3.1 (4 studies, 247 participants): RR 0.94, 95% CI 0.63 to 1.41; I2 = 0%), CMV infection (Analysis 3.3.2 (3 studies, 225 participants): RR 1.08, 95% CI 0.46 to 2.56; I2 = 0%), and BKV infection (Analysis 3.3.3 (2 studies, 86 participants: RR 3.00 95% CI 0.13 to 70.83; I2 = 0%), when compared to ATG.
3.3. Analysis.
Comparison 3 Alemtuzumab + early steroid withdrawal (ESW) or minimisation versus ATG ± ESW, Outcome 3 Infection.
Risk of leucopenia was assessed in one study (Ciancio 2005) and was increased at one month with alemtuzumab compared to ATG (Analysis 3.4.1 (60 participants): RR 21.00, 95% CI 1.29 to 342.93) but not at two years (Analysis 3.4.2 (53 participants): RR 3.12, 95% CI 0.13 to 70.83).
3.4. Analysis.
Comparison 3 Alemtuzumab + early steroid withdrawal (ESW) or minimisation versus ATG ± ESW, Outcome 4 Other adverse effects.
The effect of alemtuzumab plus ESW and dual maintenance (tacrolimus and mycophenolate) versus ATG and triple maintenance (CNI, steroid and either azathioprine or mycophenolate) on NODAT was uncertain (Analysis 3.4.3 (2 studies, 69 participants): RR 0.41, 95% CI 0.12 to 1.40; I2 = 0%).
There was uncertain effect of alemtuzumab compared to ATG for other harms including malignancy (Analysis 3.4.4 (3 studies, 187 participants): RR 4.93, 95% CI 0.59 to 41.11), PTLD (Analysis 3.4.5 (2 studies, 165 participants): no events), cytokine release syndrome (Analysis 3.4.6 (1 study, 22 participants): RR 0.20, 95% CI 0.01 to 3.74), or occurrence of any serious adverse event (Analysis 3.4.7 (1 study, 139 participants): RR 0.81, 95% CI 0.59 to 1.12).
Graft function measured by CrCl was lower with alemtuzumab plus ESW and dual maintenance at six months (Analysis 3.5.1 (2 studies, 83 participants): MD ‐13.35 mL/min, 95% CI ‐23.91 to ‐2.80; I2 = 0%) and two years (Analysis 3.5.2 (2 studies, 77 participants): MD ‐12.86 mL/min, 95% CI ‐23.73 to ‐2.00; I2 = 0%) compared to ATG plus triple maintenance.
3.5. Analysis.
Comparison 3 Alemtuzumab + early steroid withdrawal (ESW) or minimisation versus ATG ± ESW, Outcome 5 Creatinine clearance.
Alemtuzumab (and ESW) versus no induction
Three of the four studies used triple maintenance immunosuppression including steroids in the control group (CAMPASIA Study 2005; Margreiter 2008; Sharaf El Din 2006), Friend 1987 used only CSA. Sensitivity analyses excluding Friend 1987 did not significantly alter the summary risk ratio for any outcomes for the remaining studies. Results are therefore reported including all four studies.
Alemtuzumab and ESW had uncertain effect on death (Analysis 4.1.1 (4 studies, 296 participants): RR 1.54, 95% CI 0.60 to 4.00; I2 = 0%) and all‐cause graft loss (Analysis 4.1.2 (4 studies, 296 participants): RR 0.86, 95% CI 0.47 to 1.59; I2 = 0%) at 6 to 12 months compared to no induction.
4.1. Analysis.
Comparison 4 Alemtuzumab + early steroid withdrawal (ESW) versus no induction, Outcome 1 Main outcomes.
Alemtuzumab and ESW had little or no effect on acute rejection within 6 months compared with no induction (Analysis 4.1.3 (3 studies, 213 participants): RR 0.72, 95% CI 0.48 to 1.08; I2 = 0%) and had uncertain effect at 1 year or later (Analysis 4.1.4 (4 studies, 244 participants): RR 0.89, 95% CI 0.42 to 1.87; I2 = 32%).
CAMPASIA Study 2005 showed uncertain effects of alemtuzumab on delayed graft function (Analysis 4.1.5 (30 participants): RR 2.00, 95% CI 0.26 to 15.62)
The risk of CMV infection was increased with alemtuzumab (Analysis 4.2.1 (2 studies, 161 participants): RR 2.28, 95% CI 1.18 to 4.40; I2 = 0%) compared with control.
4.2. Analysis.
Comparison 4 Alemtuzumab + early steroid withdrawal (ESW) versus no induction, Outcome 2 Other adverse outcomes.
The effect of alemtuzumab was imprecise for all‐cause infection (Analysis 4.2.2 (3 studies, 213 participants): RR 1.15, 95% CI 0.46 to 2.89; I2 = 71%), NODAT (Analysis 4.2.3 (2 studies, 161 participants): RR 0.57, 95% CI 0.13 to 2.46; I2 = 0%), and thrombocytopenia (Analysis 4.2.4 (1 study, 30 participants): RR 1.33, 95% CI 0.45 to 3.96). Malignancy and PTLD were assessed in CAMPASIA Study 2005 and there were no events reported in either group.
There was little or no effect on graft function measured by SCr with alemtuzumab and ESW compared to no induction both at 6 months (Analysis 4.3.1 (1 study, 27 participants): MD ‐5.00 µmol/L, 95% CI ‐28.90 to 18.90) and 1 year (Analysis 4.3.2 (2 studies, 108 participants): MD ‐2.89 µmol/L, 95% CI ‐43.29 to 37.52; I2 = 0%).
4.3. Analysis.
Comparison 4 Alemtuzumab + early steroid withdrawal (ESW) versus no induction, Outcome 3 Serum creatinine.
Rituximab versus placebo
Only death and acute rejection were reported in all three studies comparing rituximab versus placebo.
Rituximab had uncertain effect on death both at 6 months (Analysis 5.1.1 (3 studies, 447 participants): RR 0.55, 95% CI 0.18 to 1.71; I2 = 0%) and at 3 to 4 years (Analysis 5.1.2 (2 studies, 381 participants): RR 2.06, 95% CI 0.27 to 15.64; I2 = 74%) when compared to placebo.
5.1. Analysis.
Comparison 5 Rituximab versus placebo, Outcome 1 Main outcomes.
There was uncertain effects of rituximab on all‐cause graft loss (Analysis 5.1.3 (2 studies, 416 participants): RR 0.58, 95% CI 0.26 to 1.28; I2 = 0%) and death‐censored graft loss (Analysis 5.1.4 (2 studies, 405 participants): RR 0.55, 95% CI 0.21 to 1.46; I2 = 0%) at 6 months.
Acute rejection was not reduced at 6 months with rituximab compared to placebo (Analysis 5.1.5 (3 studies, 447 participants): RR 0.73, 95% CI 0.48 to 1.10; I2 = 0%).
Leucopenia at 6 months was increased (Analysis 5.2.4 (2 studies, 416 participants): RR 8.15, 95% CI 2.00 to 33.15; I2 = 21%) with rituximab compared to placebo.
5.2. Analysis.
Comparison 5 Rituximab versus placebo, Outcome 2 Other adverse outcomes.
The effect of rituximab on CMV infection, BKV infection, fungal infection and malignancy was also uncertain (Analysis 5.2).
There was little or no effect of rituximab on graft function (eGFR) at 6 months (Analysis 5.3 (2 studies, 388 participants): MD 0.32 mL/min, 95% CI ‐3.34 to 3.97; I2 = 0%).
5.3. Analysis.
Comparison 5 Rituximab versus placebo, Outcome 3 Graft function at 6 months (eGFR).
ATG versus OKT3
ATG had uncertain effect on death at 6 to 12 months compared with OKT3 (Analysis 6.1.1 (5 studies, 451 participants): RR 1.29, 95% CI 0.64 to 2.60; I2 = 0%) and no effect on death‐censored graft loss at 6 to 12 months (Analysis 6.1.2 (5 studies, 439 participants): RR 1.00, 95% CI 0.64 to 1.57; I2 = 0%).
6.1. Analysis.
Comparison 6 ATG versus OKT3, Outcome 1 Main outcomes.
There was little or no effect on acute rejection with ATG compared to OKT3 at 1 year (Analysis 6.1.3 (4 studies, 450 participants): RR 0.76, 95% CI 0.53 to 1.09; I2 = 67%) and on delayed graft function (Analysis 6.1.4 (3 studies, 235 participants): RR 0.80, 95% CI 0.52 to 1.24; I2 = 0%).
ATG had no effect compared to OKT3 on CMV infection (Analysis 6.2.1 (3 studies, 274 participants): RR 1.13, 95% CI 0.88 to 1.46; I2 = 4%) and uncertain effects on bacterial infection (Analysis 6.2.2 (1 study, 50 participants): RR 0.51, 95% CI 0.20 to 1.32), leucopenia (Analysis 6.2.3 (1 study, 104 participants): RR 1.92, 95% CI 0.78 to 4.74), thrombocytopenia (Analysis 6.2.4 (1 study, 104 participants): RR 4.81, 95% CI 0.24 to 97.91), and the inability to complete induction due to side effects (Analysis 6.2.6 (2 studies, 131 participants): RR 1.96, 95% CI 0.10 to 39.72; I2 = 50%). Malignancy was only reported in Bock 1995 and there were no events reported in either group (Analysis 6.2.5).
6.2. Analysis.
Comparison 6 ATG versus OKT3, Outcome 2 Other adverse outcomes.
Bock 1995 reported ATG had uncertain effects compared to OKT3 on graft function at 1 year (SCr) (Analysis 6.3 (88 participants): MD 0.00 µmol/L, 95% CI ‐3.56 to 3.56).
6.3. Analysis.
Comparison 6 ATG versus OKT3, Outcome 3 Serum creatinine at 1 year.
OKT3 versus placebo/no treatment
A reduction in death was seen with OKT3 compared to no induction at 1 to 2 years (Analysis 7.1.1 (6 studies, 491 participants): RR 0.41, 95% CI 0.18 to 0.97; I2 = 0%) but the benefit was uncertain at 3 to 5 years (Analysis 7.1.2 (5 studies, 768 participants): RR 0.72, 95% CI 0.37 to 1.44; I2 = 38%).
7.1. Analysis.
Comparison 7 OKT3 versus placebo/no induction, Outcome 1 Main outcomes.
The effect of OKT3 compared to no induction on graft loss was uncertain both at 1 to 2 years (Analysis 7.1.3 (7 studies, 416 participants): RR 0.55, 95% CI 0.30 to 1.02; I2 = 0%) and at 3 to 5 years (Analysis 7.1.4 (5 studies, 768 participants): RR 0.73, 95% CI 0.47 to 1.14; I2 = 65%).
Acute rejection was decreased with OKT3 compared to no induction for CNI studies (Analysis 7.1.5 (8 studies, 968 participants): RR 0.60, 95% CI 0.43 to 0.83; I2 = 79%) but the effect was uncertain in non CNI studies (Analysis 7.1.6 (3 studies, 85 participants): RR 0.70, 95% CI 0.33 to 1.46; I2 = 86%).
The effect of OKT3 compared to placebo on delayed graft function was uncertain (Analysis 7.1.7 (6 studies, 494 participants): RR 1.08, 95% CI 0.70 to 1.65; I2 = 63%)
Abramowicz 1992 showed an increased risk of all‐cause infection with OKT3 (Analysis 7.2.1 (108 participants): RR 1.38, 95% CI 1.04 to 1.82). OKT3 had uncertain effects on all other infection subtypes including bacterial infection (Analysis 7.2.2 (3 studies, 366 participants): RR 1.01, 95% CI 0.76 to 1.34; I2 = 0%), all‐cause viral infection (Analysis 7.2.3 (2 studies, 353 participants: RR 0.99, 95% CI 0.72 to 1.37; I2 = 0%), CMV infection (Analysis 7.2.4 (3 studies, 332 participants): RR 1.52, 95% CI 0.82 to 2.84; I2 = 0%), Herpes Simplex virus infection (Analysis 7.2.5 (1 study, 215 participants): RR 1.45, 95% CI 0.89 to 2.38), and fungal infection (Analysis 7.2.6 (3 studies, 568 participants): RR 1.26, 95% CI 0.33 to 4.89; I2 = 68%).
7.2. Analysis.
Comparison 7 OKT3 versus placebo/no induction, Outcome 2 Other adverse effects.
The effect of OKT3 compared to placebo on malignancy and PTLD was uncertain (Analysis 7.2.7 (3 studies, 610 participants): RR 1.34, 95% CI 0.52 to 3.50; I2 = 0%).
There was no difference in graft function measured by SCr with OKT3 compared to placebo both at 3 months (Analysis 7.3.1 (3 studies, 226 participants): MD ‐0.93 µmol/L, 95% CI ‐15.78 to 13.93; I2 = 0%) and at 1 year (Analysis 7.3.2 (2 studies, 261 participants): MD ‐6.22 µmol/L, 95% CI ‐18.21 to 5.76; I2 = 0%). The effect on graft function at 3 to 4 years was uncertain with only 2 studies reporting for a total of 38 participants at this time point (Analysis 7.3.3 (2 studies, 38 participants): ‐21.10 µmol/L, 95% CI ‐49.81 to 7.61; I2 = 60%).
7.3. Analysis.
Comparison 7 OKT3 versus placebo/no induction, Outcome 3 Serum creatinine.
ALG versus OKT3
ALG had uncertain effects on death at 1 to 2 years (Analysis 8.1.1 (3 studies, 300 participants): RR 2.00, 95% CI 0.62 to 6.47; I2 = 0%) and 3 years (Analysis 8.1.2 (2 studies, 265 participants): RR 1.03, 95% CI 0.13 to 8.09; I2 = 41%) and also uncertain effect on all‐cause graft loss at 1 to 2 years (Analysis 8.1.3 (3 studies, 300 participants): RR 1.01, 95% CI 0.57 to 1.80; I2 = 18%) and 3 years (Analysis 8.1.4 (2 studies, 265 participants): RR 1.08, 95% CI 0.68 to 1.70 ; I2 = 0%) compared with OKT3.
8.1. Analysis.
Comparison 8 ALG versus OKT3, Outcome 1 Main outcomes.
There was little or no effect on acute rejection with ALG compared to OKT3 (Analysis 8.1.5 (6 studies, 593 participants): RR 0.97, 95% CI 0.83 to 1.13; I2 = 0%).
Delayed graft function was less with ALG compared to OKT3 (Analysis 8.1.6 (3 studies, 310 participants): RR 0.78, 95% CI 0.61 to 0.99; I2 = 0%)
ALG had uncertain effect on CMV infection (Analysis 8.2.1 (4 studies, 431 participants): RR 1.53, 95% CI 0.82 to 2.85; I2 = 57%) and all other infection outcomes (Analysis 8.2).
8.2. Analysis.
Comparison 8 ALG versus OKT3, Outcome 2 Other adverse outcomes.
ALG treatment was associated with lower SCr values at 1 year (Analysis 8.3.1 (2 studies, 245 participants): MD ‐15.85 µmol/L, 95% CI ‐28.55 to ‐3.15; I2 = 0%) but this was not sustained at 2 years (Analysis 8.3.2 (2 studies, 223 participants): MD 12.50 µmol/L, 95% CI ‐13.52 to 38.52; I2 = 59%).
8.3. Analysis.
Comparison 8 ALG versus OKT3, Outcome 3 Serum creatinine.
ALG versus placebo/no treatment
ALG had little or no effect on all‐cause death or all‐cause graft loss at any time point after transplantation compared to placebo or no induction (Analysis 9.1).
9.1. Analysis.
Comparison 9 ALG versus placebo/no induction, Outcome 1 Main outcomes.
Acute rejection was prevented with ALG compared to placebo or no induction (Analysis 9.1.7 (13 studies, 1575 participants): RR 0.69, 95% CI 0.53 to 0.92; I2 = 87%) and ALG reduced delayed graft function (Analysis 9.1.8 (5 studies, 615 participants): RR 0.55, 95% CI 0.31 to 0.97; I2 = 73%).
ALG markedly increased both CMV infection (Analysis 9.2.1 (3 studies, 289 participants): RR 2.45, 95% CI 1.23 to 4.85; I2 = 0%) and all‐cause viral infections (Analysis 9.2.2 (2 studies, 324 participants): RR 2.71, 95% CI 1.86 to 3.95; I2 = 0%), and may increase bacterial infection rates (Analysis 9.2.3 (4 studies, 742 participants): RR 1.18, 95% CI 0.92 to 1.52; I2 = 43%). The treatment effect on fungal infection rates was uncertain (Analysis 9.2.4 (1 study, 230 participants): RR 1.11, 95% CI 0.63 to 1.95).
9.2. Analysis.
Comparison 9 ALG versus placebo/no induction, Outcome 2 Other adverse outcomes.
ALG markedly increased thrombocytopenia (Analysis 9.2.5 (1 study, 67 participants): RR 12.19, 95% CI 3.10 to 47.92) and leucopenia (Analysis 9.2.6 (2 studies, 297 participants): RR 20.31, 95% CI 0.61 to 676.54; I2 = 83%). ALG had uncertain effects on malignancy or PTLD (Analysis 9.2.7 (4 studies, 623 participants): RR 0.60, 95% CI 0.27 to 1.31; I2 = 0%) and NODAT (Analysis 9.2.8 (1 study, 105 participants): RR 0.93, 95% CI 0.22 to 3.93).
ALG had uncertain effect on both early graft function at 1‐2 years and long term graft function at 10‐20 years compared to placebo or no induction (Analysis 9.3).
9.3. Analysis.
Comparison 9 ALG versus placebo/no induction, Outcome 3 Serum creatinine.
Other studies
The remainder of comparisons (Figure 1) involved only a single study and therefore could not be used for meta‐analysis. The results are summarised briefly below.
Dose comparisons
Stevens 2008 assessed single versus divided dose ATG. There were no differences in any reported outcomes. Abouna 1995 compared ATG adjusted for the CD3 count with fixed dose ATG and again there was no difference in outcomes. One very small study by Ata 2013 compared ATG with dose adjusted by CD3 count compared to dose adjusted for total lymphocyte count and there was no difference in outcomes. Grafals 2014 compared 'standard' dose ATG (3.75 mg/kg total) with low dose ATG (2.25 mg/kg total) and found no significant difference in outcomes. Another very small study by Buchler 2013 compared a split of four versus two doses of ATG (same total dose of 6 mg/kg) and found no difference in outcomes. Two studies compared different OKT3 dose regimens: standard versus low dose (Norman 1993a) and standard versus high dose (Abramowicz 1994). There were no significant differences in either of these small studies. Low versus high dose ALG was also assessed in Sakhrani 1992 and seven days versus 14 days ALG was addressed in Grundmann 1987. There were no differences in the low versus high dose study. Treatment was frequently stopped early in the 14 day group but there were no other differences in outcomes. One older study by Thomas 1977 comparing low potency ALG with high potency ALG found increased acute rejection at three months (RR 4.14, 95% CI 1.55 to 11.00) and increased graft loss at 1 year (RR 2.53, 95% CI 1.30 to 4.90) with the low potency ALG.
Table summarising single studies of different dose comparisons
| Comparison / Study ID (number of participants) | Outcome | RR |
95% CI lower limit |
95% CI upper limit |
| rATG: single 6 mg/kg versus 4 x 1.5 mg/kg doses (same total dose) | ||||
| Stevens 2008 (142) | Death at 6 months | 0.34 | 0.01 | 8.27 |
| Graft loss (all cause) at 6 months | 0.21 | 0.01 | 4.21 | |
| Acute rejection | 0.69 | 0.26 | 1.83 | |
| Delayed graft function | 2.40 | 0.65 | 8.91 | |
| Malignancy/PTLD | 0.21 | 0.01 | 4.21 | |
| BKV | 0.15 | 0.01 | 2.79 | |
| Severe febrile reaction (anaphylaxis requiring ICU) | 1.03 | 0.15 | 7.10 | |
| Serum sickness | 0.21 | 0.01 | 4.21 | |
| NODAT | 0.82 | 0.47 | 1.42 | |
| ATG: 2 x3 mg/kg versus 4 x 1.5 mg/kg doses (same total) | ||||
| Buchler 2013 (17) | ** | ‐ | ‐ | ‐ |
| ATG: adjusted for CD3 count versus fixed dose of 15 mg/kg/d | ||||
| Abouna 1995 (45) | Death at 2 years | 0.96 | 0.06 | 14.37 |
| Graft loss (all cause) 2 years | 0.72 | 0.18 | 2.85 | |
| Acute rejection | 0.96 | 0.5 | 1.84 | |
| Leucopenia | 0.36 | 0.11 | 1.18 | |
| Thrombocytopenia | 0.14 | 0.01 | 2.51 | |
| Viral infection (all cause) | 0.96 | 0.15 | 6.21 | |
| Bacterial infection (all cause) | 0.64 | 0.21 | 1.96 | |
| ATG: adjusted by CD3 count versus adjusted by total lymphocytes | ||||
| Ata 2013 (21) | ** | ‐ | ‐ | ‐ |
| ATG: standard (3.75 mg/kg total) versus low dose (2.25 mg/kg total) | ||||
| Grafals 2014 (43) | Acute rejection at 1 year | 0.57 | 0.12 | 2.81 |
| Leucopenia | 0.69 | 0.31 | 1.56 | |
| Severe infection | 0.77 | 0.14 | 4.14 | |
| CMV infection | 0.23 | 0.01 | 4.50 | |
| BKV infection | 0.38 | 0.02 | 8.86 | |
| Death at 1 year | 8.00 | 0.44 | 146.08 | |
| Delayed graft function | 3.07 | 0.94 | 10.02 | |
| Malignancy at 1 year | 2.30 | 0.23 | 23.51 | |
| PTLD at 1 year | 0 events | not estimable | ||
| Graft function at 1 year (SCr, µmol/L) | 6.00* | 1.07 | 10.93 | |
| OKT3: standard dose (5 mg) versus low dose (2 mg) | ||||
| Norman 1993a (26) | Death at 1 year | 0 events | not estimable | |
| Graft loss at 1 year | 3 | 0.13 | 67.51 | |
| Acute rejection | 0.2 | 0.01 | 3.8 | |
| Delayed graft function | 1.25 | 0.43 | 3.63 | |
| CMV | 4 | 0.51 | 31.13 | |
| Herpes Simplex virus | 0.5 | 0.05 | 4.86 | |
| Bacterial | 0.86 | 0.4 | 1.86 | |
| Fungal | 1 | 0.16 | 6.07 | |
| Malignancy | 4.72 | 0.23 | 96.59 | |
| OKT3: standard dose (5 mg) versus high dose (10 mg) | ||||
| Abramowicz 1994 (29) | Death at 3 months | 0 events | not estimable | |
| Graft loss at 3 months | 4.69 | 0.24 | 89.88 | |
| Acute rejection to 3 months | 0.47 | 0.1 | 2.16 | |
| Delayed graft function | 0.93 | 0.34 | 2.54 | |
| ALG: low versus high dose | ||||
| Sakhrani 1992 (83) | Death at 1 year | 0.89 | 0.41 | 1.97 |
| Acute rejection | 0.86 | 0.48 | 1.55 | |
| Leucopenia | 0.5 | 0.18 | 1.41 | |
| Severe infection | 1.05 | 0.52 | 2.11 | |
| ALG: 14 days versus 7 days | ||||
| Grundmann 1987 (100) | Death 1 year | 0 events | not estimable | |
| Graft loss (all cause) 1 year | 0.29 | 0.06 | 1.31 | |
| Acute rejection | 0.5 | 0.05 | 5.34 | |
| Delayed graft function | 0.62 | 0.28 | 1.35 | |
| Pneumonia | 3 | 0.13 | 71.92 | |
| Wound infection | 0 events | not estimable | ||
| Treatment stopped due to side effects | 63 | 3.96 | 1002.01 | |
| Graft function at 1 year (SCr, µmol/L) | ‐35.4* | ‐78.72 | 7.92 | |
| ALG: high versus low potency | ||||
| Thomas 1977 (71) | Acute rejection at 3 months | 4.14 | 1.55 | 11.00 |
| Graft loss at 1 year | 2.53 | 1.30 | 4.90 | |
* MD and SD for continuous variables (not RR and 95% CI).
** Results not converted to RR for extremely small studies with 10 or fewer participants in each group.
Significant results shown in bold.
Other antibody preparations
Anti‐CD2 rat monoclonal antibody was compared with no induction treatment in Squifflet 1997. This small study (40 participants) showed acute rejection was decreased by anti‐CD2 (RR 0.42, 95% CI 0.18 to 0.96) but no difference in any other outcomes. Another small study compared anti CD7 with OKT3 (Lazarovits 1993) and there were no differences. Two studies assessed anti‐LFA‐1 monoclonal antibody: one in comparison with no induction agent (Spillner 1998) and the other in comparison with ATG (Hourmant 1996). Other than decreased fever with anti‐LFA‐1 compared to ATG, differences were not significant in either of these studies. One small pilot study (Ejaz 2013) comparing four different interventions (ATG versus ATG + rituximab versus ATG + bortezomib versus ATG + rituximab + bortezomib) did not show any significant differences in outcomes other than an increase in new‐onset peripheral neuropathy in the bortezomib groups. There were only 10 participants in each group and follow‐up only reported to one year at the time of this review. One final study compared anti‐ICAM‐1 monoclonal antibody with placebo (EARTS Study 1999) but again there were no differences in outcomes.
Norrby 1997 compared two rabbit ATG preparations made by different manufacturers. There was no difference for the only reported outcomes of acute rejection and CMV infection. One small (51 participants) study by Steinmuller 1991 compared OKT3 with ALG but antibody therapy was only given for patients with delayed graft function. For this reason it was considered separately from the other studies comparing OKT3 and ALG. Side effects were reduced with ALG compared to OKT3 (RR 0.41, 95% CI 0.24 to 0.72) but there were no other significant differences in outcomes. Finally Toledo‐Pereyra 1985 compared ATG with ALG also showed no significant differences in outcomes.
Table summarising single studies of other antibody preparations
| Comparison / Study ID (number of participants) | Outcome | RR |
95% CI lower limit |
95% CI upper limit |
| Rabbit ATG Fresenius versus rabbit ATG Merieux | ||||
| Norrby 1997 (90) | Acute rejection | 0.87 | 0.63 | 1.20 |
| CMV infection | 0.56 | 0.29 | 1.07 | |
| ALG versus ATG | ||||
| Toledo‐Pereyra 1985 (50) | Death at 1 year | 0.5 | 0.10 | 2.49 |
| Graft loss at 1 year | 0.92 | 0.50 | 1.67 | |
| Acute rejection | 0.95 | 0.73 | 1.24 | |
| Thrombocytopenia | 1 | 0.15 | 6.55 | |
| Leucopenia | 0.07 | 0 | 1.11 | |
| HSV infection | 2 | 0.19 | 20.67 | |
| ALG vs OKT3 (given only if delayed graft functionpost‐operatively) | ||||
| Steinmuller 1991 (51) | Death at 6 months | 0.48 | 0.05 | 4.98 |
| Graft loss (all cause) at 6 months | 0.96 | 0.27 | 3.43 | |
| Acute rejection | 0.61 | 0.28 | 1.32 | |
| Side effects (any reported) | 0.41 | 0.24 | 0.72 | |
| Any infection | 0.89 | 0.51 | 1.55 | |
| CMV | 0.89 | 0.51 | 1.55 | |
| Anti‐CD7 versus OKT3 | ||||
| Lazarovits 1993 (20) | Death 5 years | 1 | 0.07 | 13.87 |
| Graft loss 5 years | 0.11 | 0.01 | 1.83 | |
| Acute rejection | 1.4 | 0.67 | 2.94 | |
| Serious infection | 0.25 | 0.03 | 1.86 | |
| Anti‐CD2 rat monoclonal antibody versus no induction | ||||
| Squifflet 1997 (40) | Death at 6 months | 0.2 | 0.01 | 3.92 |
| Graft loss (death censored) at 6 months | 0 events | Not estimable | ||
| Acute rejection | 0.42 | 0.18 | 0.96 | |
| Delayed graft function | 0.17 | 0.02 | 1.26 | |
| Bacterial infection | 0.25 | 0.03 | 2.05 | |
| CMV | 0.5 | 0.05 | 5.08 | |
| EB virus | 3 | 0.13 | 69.52 | |
| Herpes Simplex virus | 4 | 0.49 | 32.72 | |
| Other viral infection | 0.33 | 0.01 | 7.72 | |
| Malignancy | 3 | 0.13 | 69.52 | |
| Graft function at 6 months (SCr, µmol/L) | 8* | ‐20.99 | 36.99 | |
| Anti‐LFA‐1 monoclonal antibody versus no induction1 | ||||
| Spillner 1998 (22) | Death at 1 year | 3 | 0.14 | 66.53 |
| Graft loss (all cause) at 1 year | 1 | 0.17 | 5.89 | |
| Serious infection | 1 | 0.07 | 14.05 | |
| CMV infection | 1 | 0.17 | 5.89 | |
| Delayed graft function | 1.5 | 0.31 | 7.3 | |
| Graft function at 1 year (SCr, µmol/L) | ‐17.6* | ‐62.69 | 27.49 | |
| Anti‐LFA‐1 monoclonal antibody versus ATG | ||||
| Hourmant 1996 (101) | Death at 1 year | 4.72 | 0.23 | 95.86 |
| Graft loss (death censored) at 1 year | 0.39 | 0.08 | 1.93 | |
| Acute rejection | 1.05 | 0.62 | 1.78 | |
| Delayed graft function | 0.55 | 0.28 | 1.09 | |
| Any episode of infection | 1.05 | 0.74 | 1.48 | |
| CMV disease | 0.94 | 0.5 | 1.77 | |
| Treatment stopped due to side effects | 0.24 | 0.03 | 2.04 | |
| Leucopenia | 0.4 | 0.11 | 1.47 | |
| Thrombocytopenia | 0.57 | 0.22 | 1.44 | |
| Fever (1st 10 days) | 0.58 | 0.36 | 0.94 | |
| Anti‐ICAM‐1 monoclonal antibody versus placebo | ||||
| EARTS Study 1999 (266) | Death at 1 year | 1.71 | 0.7 | 4.22 |
| Graft loss at 1 year | 1.4 | 0.76 | 2.59 | |
| Acute rejection at 3 months | 1.18 | 0.88 | 1.57 | |
| Acute rejection at 1 year | 1.07 | 0.82 | 1.41 | |
| Primary non function | 1.2 | 0.38 | 3.83 | |
| Delayed graft function | 1.21 | 0.82 | 1.77 | |
| Any infection | 1.13 | 0.98 | 1.3 | |
| Sepsis | 1.3 | 0.59 | 2.86 | |
| Malignancy | 0.5 | 0.05 | 5.45 | |
| ATG versus ATG + rituximab vs ATG + bortezomib versus ATG + rituximab + bortezomib | ||||
| Ejaz 2013 (40) | ** | ‐ | ‐ | ‐ |
* MD and SD for continuous variables (not RR and 95% CI).
** Results not converted to RR for extremely small studies with 10 or fewer participants in each group
Significant results shown in bold.
1. Acute rejection was reported for anti‐LFA 1 versus no induction but was reported as total number of episodes rather than total number of patients with any episode (results were 5 episodes with anti‐LFA 1 versus 12 episodes with no induction)
Discussion
Summary of main results
Many antibody preparations are now available for induction immunosuppression in kidney transplantation and we sought to summarise the evidence in this review to help inform clinical decision making and policy. Our inclusion criteria were deliberately broad resulting in 28 different pairwise comparisons and studies spanning over many decades. This review provides the best summary available of all RCTs (excluding IL2Ra) and highlights several issues.
Firstly, the evidence basis for decision making is poorly informed by studies in this area. The effects of polyclonal antibody induction remain uncertain for many important outcomes including graft loss and death. Many relevant, well recognised potential harms are not reported frequently in RCTs and more well designed studies reporting patient‐centred outcomes (benefits and harms) are required. Some effects of antibody induction could be quantified.
ATG reduced acute rejection rates by roughly one third when compared to placebo or no treatment, at the cost of approximately 50% increase in the risk of CMV complications and an uncertain impact on future malignancy risk. rATG reduces acute rejection compared to hATG but data supporting this is weak as all events were only reported in a single study. The only significant difference seen in comparisons between alemtuzumab and ATG in steroid avoidance studies was that alemtuzumab reduces rejection at one year; in comparison alemtuzumab increased CMV infection but had similar rejection rates when compared to no induction and triple maintenance. NODAT was not reduced with alemtuzumab plus ESW compared to triple maintenance. OKT3 decreases acute rejection compared to placebo or no treatment but has been withdrawn from clinical use due to a poor side effect profile. ALG prevented acute rejection and led to better kidney function at one year post‐transplant compared with placebo or no treatment but increased the rates of all viral infections.
Overall completeness and applicability of evidence
A decision was made to include any co‐intervention immunosuppression regimens to ensure all relevant studies were included. As a result, a large number of studies from the pre‐CNI era were included which may not be relevant to clinical practice today. Where possible, results were separated into CNI or non‐CNI maintenance as combining these groups was not felt to be clinically useful. As a result, there were multiple subgroups for outcomes of death and graft loss for most comparisons as studies frequently reported these outcomes at a variety of time points. There were no benefits seen for improved patient or graft survival with ATG despite decreased rejection rates when CNI and non‐CNI studies were separated. This lack of benefit may be due to small numbers of studies in each subgroup. When CNI and non‐CNI studies were combined, a reduction in both all‐cause graft loss and death‐censored graft loss was seen at one to two years post‐transplant. This benefit was not sustained however in the studies that assessed longer term graft survival at five years. Results for acute rejection were generally more robust as this was reported in nearly all studies and time points were more standardised resulting in larger subgroups and greater statistical power. New studies are required to see if the absence of benefit is due to a lack of power or whether there really is no effect of one antibody compared to another antibody or placebo on patient and graft survival.
The main aim of using alemtuzumab has been to try to reduce the doses of maintenance immunosuppression required to prevent rejection, especially steroids. It is hoped that this will reduce some of the long term side effects of steroids, including NODAT. However, NODAT was not reduced with alemtuzumab plus ESW compared to ATG and triple maintenance or with alemtuzumab plus ESW compared to triple maintenance alone. This may be partly due to small numbers in these studies or may be due to the role of CNI, especially tacrolimus also causing increased rates of NODAT. Other steroid side effects have generally not been well reported in these studies. In the absence of any data to confirm a reduction in side effects, it is difficult to support the use of alemtuzumab and ESW currently compared to another antibody with triple maintenance.
The applicability of the results of this meta‐analysis to the general transplant population may be limited by the individual studies. The majority of studies included patient groups with mixed immunological risk and a small number studied higher risk groups. Benefits and harms of individual treatments generally seemed homogenous across studies despite these apparent differences in risk. Harms are frequently under‐reported in clinical studies compared to benefits and this review may therefore underestimate some of the potential harms of treatments due to possible under‐reporting in the individual studies.
Quality of the evidence
Overall, the quality of the evidence was generally low to only moderately good by GRADE criteria. Figure 2 shows the individual biases for each study. The most common problem was potential selection bias due to unclear methods of both randomisation and allocation concealment. Only 20% to 27% of included studies were low risk of bias for either random sequence generation or allocation concealment (see Figure 3).
For the main comparison of ATG versus placebo, quality of evidence according to GRADE criteria was moderate for outcomes of acute rejection and CMV infection but low for all other outcomes. The evidence for acute rejection and CMV was graded as moderate rather than high as more than 50% of studies rated methods of allocation concealment and/or random sequence generation as ‘unclear’ or ‘high risk’ as a potential source of bias. For the comparison of alemtuzumab plus ESW versus ATG with and without ESW, the evidence for acute rejection was rated as moderate quality but evidence for all other outcomes was either low or very low quality by GRADE criteria. Again the main reason for acute rejection evidence being graded as moderate rather than high was a significant risk of selection bias due to poor reporting of randomisation and allocation concealment.
Potential biases in the review process
The review was conducted with standard Cochrane methodology and there were no changes from the original protocol.
Agreements and disagreements with other studies or reviews
One study of registry data of transplant recipients in the US also failed to show any improvement in all‐cause graft survival despite decreasing rates of acute rejection (Meier‐Kriesche 2004). More alarmingly, this study showed a trend towards worsening death censored graft survival, despite more potent maintenance immunosuppression. However, given these trends are taken from registry data, it is hard to interpret what this really means, especially with older and more co‐morbid patients being transplanted in recent years.
Many antibody therapies have now shown a reduction in acute rejection but it remains uncertain as to whether this translates into increased patient or graft survival for any of the antibodies in this review. In comparison, there was a reduction in graft loss at one year (but not after) for IL2Ra compared to placebo (24 studies, 4672 participants: RR 0.75, 95% CI 0.62 to 0.90) in a systematic review by Webster 2010. However, there was no difference for graft loss when IL2Ra and ATG were compared in the same review and clinically diagnosed acute rejection rates were also similar for IL2Ra and ATG. However, ATG increased early malignancy at one year compared to IL2Ra (7 studies, 1067 participants: RR 0.25, 95% CI 0.07 to 0.87) but had no effect on malignancy at other time points (Webster 2010). It is possible that malignancy is influenced more by maintenance immunosuppression than induction agents given it is a relatively late complication after transplantation. However, under‐reporting of late harms is common in RCTs and malignancy rates may therefore be grossly underestimated in existing studies of induction agents leading to insufficient power to determine true cancer risk.
In steroid avoidance studies, alemtuzumab reduced acute rejection compared to ATG when ESW was used in both arms. These results would support using alemtuzumab over ATG in patients deemed to be at particularly high risk of steroid side effects and where maintenance with ESW is planned. Further studies of alemtuzumab and ESW compared to no induction and triple maintenance showed similar rates of acute rejection but an increased risk of CMV infection with alemtuzumab. There was no other difference in harms but this may need larger studies to show potential benefits of alemtuzumab relating to steroid avoidance. Reduction of maintenance immunosuppression certainly has theoretical benefits, including reduction in antihypertensives, antihyperlipidaemics, cholesterol, cataracts and NODAT requiring treatment as well as possible reduction of late complications such as malignancy. However, none of the studies to date have been long enough duration or large enough to confirm any of these suggested benefits.
Authors' conclusions
Implications for practice.
Given a 45% acute rejection risk with no induction (assumed risk from control group in Analysis 1.4), seven patients would need ATG to prevent one from experiencing acute transplant rejection, while one additional patient would experience CMV disease for every 12 patients treated with ATG. Where only studies including CNI maintenance were assessed, the acute rejection rate was 37% with no induction and six patients would need treatment with ATG to prevent one person having acute rejection. In steroid withdrawal studies, 11 patients would require alemtuzumab to prevent one patient experiencing rejection given a 21% rejection risk with ATG. Alemtuzumab treatment combined with steroid withdrawal would cause one additional patient experiencing CMV disease for every six patients treated when compared with no antibody induction and triple maintenance, and without apparent benefits to patient‐centred outcomes. ATG and alemtuzumab decreased acute rejection at a cost of increased CMV while patient‐centred outcomes including survival or side effects do not appear to be improved.
In kidney transplant recipients deemed to be at high risk of rejection, the evidence remains unclear as to whether one particular antibody preparation is better than any other at preventing acute rejection. However, this review does suggest that the perceived benefit of induction immunosuppression in reducing acute rejection may not actually lead to any long‐term benefits or improvements in patient‐centred outcomes.
Implications for research.
Longer term follow‐up is always a problem when assessing study data. Although some of the studies in this review have reported fairly long‐term data, the numbers are generally too small to draw conclusions. Longer term follow‐up is needed to really establish whether the benefit of reduced acute rejection with ATG has a significant impact on graft survival or indeed patient survival. In the absence of this information, is it possible to say that decreasing acute rejection is truly a benefit? Reducing the risk of acute rejection becomes less important to an individual patient if this fails to improve long‐term graft or patient survival, especially if the treatment causes potential severe side effects and other harms. We need to find better ways of monitoring long‐term harmful outcomes such as malignancy in any future studies. This may require an ongoing observational cohort study of patients once the initial RCT phase of a study is completed. Another response to this issue is follow up within established registries combined with core patient outcome sets.
If ESW or steroid minimisation is planned in an individual patient, the data in this review would support use of alemtuzumab over ATG due to a reduction in acute rejection. Further studies with long‐term follow‐up or ongoing follow‐up of existing studies are needed to show if there is sustained benefit to steroid reduction therapy and indeed if the benefits outweigh risks of increased chronic rejection and potential increased long‐term graft loss.
When assessing outcomes in transplantation it is difficult to separate the contribution of induction immunosuppression versus maintenance immunosuppression. The appropriate question for future studies may relate to maintenance rather than induction immunosuppression. Increasing knowledge in the field of transplant immunology has led to continual reassessment of the Banff diagnostic criteria and a much greater understanding of antibody‐mediated rejection over recent years. Future studies comparing different immunosuppression regimens need to assess for not only differences in all cause rejection but also differences in the different subgroups of rejection. Ideally study designs should also include some measure of adherence to maintenance immunosuppression as this is particularly relevant for antibody‐mediated rejection in the presence of de novo donor‐specific antibodies. Adherence can be difficult to measure and is generally poorly reported or not measured at all in studies. However, this may be the area that really needs to be studied if we want to increase long term patient and graft survival in kidney transplantation.
Acknowledgements
We wish to thank the referees for the comments and feedback during the preparation of this review. We would also like to Jonathan Craig who contributed to the protocol of this review.
NB acknowledges the support of the Medical Research Council (MRC) Centre for Transplantation, King's College London, UK ‐ MRC grant no. MR/J006742/1. His research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
SP is supported by a Rutherford Discovery Fellowship from the Royal Society of New Zealand.
PH acknowledges support from staff at the Department of Renal Medicine, Whangarei Hospital, New Zealand.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE (OvidSP) |
|
| EMBASE (OvidSP) |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. ATG versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 At 3 to 6 months (+ CNI) | 3 | 523 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.13, 1.22] |
| 1.2 At 1 to 2 years (+ CNI) | 5 | 632 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.27, 2.06] |
| 1.3 At 1 to 2 years (no CNI) | 6 | 621 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.86, 1.22] |
| 1.4 At 5 years (+ CNI) | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.11, 7.81] |
| 2 Graft loss (all cause) | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 At 3 to 6 months (+ CNI) | 4 | 638 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.34, 1.05] |
| 2.2 At 1 to 2 years (+ CNI) | 3 | 549 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.36, 1.19] |
| 2.3 At 1 to 2 years (no CNI) | 4 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.49, 1.01] |
| 2.4 At 5 years (+ CNI) | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.62, 2.05] |
| 2.5 At 1 to 2 years (all studies) | 7 | 1049 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.53, 0.95] |
| 3 Graft loss (death censored) | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 At 1 to 2 years (+ CNI) | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.19, 1.75] |
| 3.2 At 1 to 2 years (no CNI) | 6 | 299 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.38, 0.78] |
| 3.3 at 5 years (+ CNI) | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.20, 13.18] |
| 3.4 At 1 to 2 years (all studies) | 8 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.39, 0.77] |
| 4 Acute rejection | 17 | 2044 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.51, 0.78] |
| 4.1 At 1 to 2 years (+ CNI) | 12 | 1491 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.49, 0.76] |
| 4.2 At 1 to 2 years (no CNI) | 5 | 553 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.43, 0.98] |
| 5 Delayed graft function | 9 | 1304 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.78, 1.10] |
| 6 Infection | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Any infection | 7 | 824 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.88, 1.26] |
| 6.2 CMV infection | 6 | 1072 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.24, 1.95] |
| 6.3 Other viral infection (not CMV) | 4 | 664 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.43, 2.87] |
| 6.4 Viral infection (all cause) | 3 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.56, 3.39] |
| 6.5 Bacterial infection | 5 | 775 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.96, 1.37] |
| 7 Leucopenia | 4 | 920 | Risk Ratio (M‐H, Random, 95% CI) | 3.86 [2.79, 5.34] |
| 8 Thrombocytopenia | 4 | 848 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [1.61, 3.61] |
| 9 Malignancy or PTLD | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Malignancy at 1 to 2 years (+ CNI) | 3 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.22, 3.94] |
| 9.2 Malignancy at 5 years (+ CNI) | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.14, 6.23] |
| 9.3 Malignancy at 1 to 2 years (no CNI) | 2 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 PTLD at 1 to 2 years (+ CNI) | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Other adverse outcomes | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 NODAT | 6 | 935 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.56, 1.84] |
| 10.2 Serum sickness | 1 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 60.67 [3.74, 984.93] |
| 10.3 Tremor | 1 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.46, 1.87] |
| 11 Serum creatinine | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 At 6 months (+ CNI) | 2 | 503 | Mean Difference (IV, Random, 95% CI) | ‐5.34 [‐13.44, 2.75] |
| 11.2 At 1 year (+ CNI) | 2 | 222 | Mean Difference (IV, Random, 95% CI) | ‐10.56 [‐21.81, 0.69] |
| 11.3 At 1 year: LD recipients (no CNI) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐9.70 [‐67.32, 47.92] |
| 11.4 At 1 year: DD recipients (no CNI) | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐23.0 [‐62.70, 16.70] |
| 11.5 At 5 years (+ CNI) | 1 | 55 | Mean Difference (IV, Random, 95% CI) | ‐32.70 [‐68.98, 3.58] |
Comparison 2. Rabbit ATG versus horse ATG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Main outcomes | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death at 1 year | 2 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.07, 2.30] |
| 1.2 Death at 10 years | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.35, 1.59] |
| 1.3 Graft loss (all cause) at 1 year | 2 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.08, 1.27] |
| 1.4 Graft loss (all cause) at 10 years | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.58, 1.58] |
| 1.5 Acute rejection at 1 month | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Acute rejection at 1 year | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.04, 0.76] |
| 1.7 Delayed graft function | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.06, 4.47] |
| 2 Other adverse outcomes | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Infection (all cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 CMV disease at 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Leucopenia | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Malignancy at 10 years | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Headache | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Serum creatinine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 At 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 At 10 years | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Alemtuzumab + early steroid withdrawal (ESW) or minimisation versus ATG ± ESW.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death and graft loss | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death at 1 year | 2 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.06, 2.42] |
| 1.2 Death at 2 to 3 years | 3 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.15, 2.95] |
| 1.3 Graft loss (all cause) at 1 year | 2 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.12, 1.30] |
| 1.4 Graft loss (all cause) at 2 to 3 years | 3 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.47, 2.06] |
| 1.5 Graft loss (death censored) at 1 year | 2 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.08, 1.81] |
| 1.6 Graft loss (death censored) at 2 to 3 years | 2 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 2.45 [0.67, 8.97] |
| 1.7 Delayed graft function | 2 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.13, 3.07] |
| 2 Rejection | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Acute rejection at 3 to 6 months (ESW both arms) | 3 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.17, 1.30] |
| 2.2 Acute rejection ≥ 1 year (all studies) | 6 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.44, 1.05] |
| 2.3 Acute rejection ≥ 1 year (ESW both arms) | 4 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.35, 0.93] |
| 2.4 Acute rejection ≥ 1 year (ESW with alemtuzumab only) | 2 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.50, 3.19] |
| 2.5 CAN (biopsy proven) (ESW with alemtuzumab only) | 2 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 2.45 [1.02, 5.94] |
| 3 Infection | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 All cause (moderate‐severe) | 4 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.63, 1.41] |
| 3.2 CMV infection | 3 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.46, 2.56] |
| 3.3 BK virus infection | 2 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 70.83] |
| 4 Other adverse effects | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Leucopenia at 1 month | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 21.0 [1.29, 342.93] |
| 4.2 Leucopenia at 2 years | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 3.12 [0.35, 28.06] |
| 4.3 NODAT | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.12, 1.40] |
| 4.4 Malignancy | 3 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 4.93 [0.59, 41.11] |
| 4.5 PTLD | 2 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Cytokine release syndrome | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 3.74] |
| 4.7 Any serious adverse event | 1 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.59, 1.12] |
| 5 Creatinine clearance | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 At 6 months | 2 | 83 | Mean Difference (IV, Random, 95% CI) | ‐13.35 [‐23.91, ‐2.80] |
| 5.2 At 24 months | 2 | 77 | Mean Difference (IV, Random, 95% CI) | ‐12.86 [‐23.73, ‐2.00] |
Comparison 4. Alemtuzumab + early steroid withdrawal (ESW) versus no induction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Main outcomes | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death at 6 to 12 months | 4 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.60, 4.00] |
| 1.2 Graft loss (all cause) at 6 to 12 months | 4 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.47, 1.59] |
| 1.3 Acute rejection at 6 months | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.48, 1.08] |
| 1.4 Acute rejection ≥ 1 year | 3 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.42, 1.87] |
| 1.5 Delayed graft function | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.26, 15.62] |
| 2 Other adverse outcomes | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 CMV infection | 2 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 2.28 [1.18, 4.40] |
| 2.2 Infection (all cause) | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.46, 2.89] |
| 2.3 NODAT | 2 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.13, 2.46] |
| 2.4 Thrombocytopenia | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.45, 3.96] |
| 2.5 Malignancy or PTLD | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Serum creatinine | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 6 months | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐28.90, 18.90] |
| 3.2 1 year | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐2.89 [‐43.29, 37.52] |
Comparison 5. Rituximab versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Main outcomes | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death at 6 months | 3 | 447 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.18, 1.71] |
| 1.2 Death at 3 to 4 years | 2 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.27, 15.64] |
| 1.3 Graft loss (all cause) at 6 months | 2 | 416 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.26, 1.28] |
| 1.4 Graft loss (death censored) at 6 months | 2 | 405 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.21, 1.46] |
| 1.5 Acute rejection at 6 months | 3 | 447 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.48, 1.10] |
| 1.6 Delayed graft function | 1 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.65, 1.76] |
| 2 Other adverse outcomes | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 CMV infection | 2 | 416 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.75, 2.47] |
| 2.2 BK virus infection | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.18] |
| 2.3 Fungal infection at 6 months | 3 | 447 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.50, 1.27] |
| 2.4 Leucopenia at 6 months | 2 | 416 | Risk Ratio (M‐H, Random, 95% CI) | 8.15 [2.00, 33.15] |
| 2.5 Malignancy at 2 years | 1 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.40, 2.66] |
| 3 Graft function at 6 months (eGFR) | 2 | 388 | Mean Difference (IV, Random, 95% CI) | 0.32 [‐3.34, 3.97] |
Comparison 6. ATG versus OKT3.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Main outcomes | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death at 6 to 12 months | 5 | 451 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.64, 2.60] |
| 1.2 Graft loss (death censored) at 6 to 12 months | 5 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.64, 1.57] |
| 1.3 Acute rejection at 1 year | 4 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.53, 1.09] |
| 1.4 Delayed graft function | 3 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.52, 1.24] |
| 2 Other adverse outcomes | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 CMV infection | 3 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.88, 1.46] |
| 2.2 Bacterial infection | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.20, 1.32] |
| 2.3 Leucopenia | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [0.78, 4.74] |
| 2.4 Thrombocytopenia | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 4.81 [0.24, 97.91] |
| 2.5 Malignancy at 1 year | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Unable to complete induction due to side effects | 2 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [0.10, 39.72] |
| 3 Serum creatinine at 1 year | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 7. OKT3 versus placebo/no induction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Main outcomes | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death at 1 to 2 years | 6 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.18, 0.97] |
| 1.2 Death at 3 to 5 years | 5 | 768 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.37, 1.44] |
| 1.3 Graft loss (all cause) at 1 to 2 years | 7 | 416 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.30, 1.02] |
| 1.4 Graft loss (all cause) at 3 to 5 years | 5 | 768 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.47, 1.14] |
| 1.5 Acute rejection, any episode (+ CNI) | 8 | 968 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.43, 0.83] |
| 1.6 Acute rejection at 3 months (no CNI) | 3 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.33, 1.46] |
| 1.7 Delayed graft function | 6 | 494 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.70, 1.65] |
| 2 Other adverse effects | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Infection (all cause) | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.04, 1.82] |
| 2.2 Bacterial infection | 3 | 366 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.76, 1.34] |
| 2.3 Viral infection (all cause) | 2 | 353 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.72, 1.37] |
| 2.4 CMV infection | 3 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.82, 2.84] |
| 2.5 HSV infection | 1 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.89, 2.38] |
| 2.6 Fungal infection | 3 | 568 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.33, 4.89] |
| 2.7 Malignancy or PTLD | 3 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.52, 3.50] |
| 3 Serum creatinine | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 3 months | 3 | 226 | Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐15.78, 13.93] |
| 3.2 1 year | 2 | 261 | Mean Difference (IV, Random, 95% CI) | ‐6.22 [‐18.21, 5.76] |
| 3.3 3 to 4 years | 2 | 38 | Mean Difference (IV, Random, 95% CI) | ‐21.10 [‐49.81, 7.61] |
Comparison 8. ALG versus OKT3.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Main outcomes | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death at 1 to 2 years | 3 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.62, 6.47] |
| 1.2 Death at 3 years | 2 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.13, 8.09] |
| 1.3 Graft loss (all cause) at 1 to 2 years | 3 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.57, 1.80] |
| 1.4 Graft loss (all cause) at 3 years | 2 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.68, 1.70] |
| 1.5 Acute rejection (any episode) | 6 | 593 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.83, 1.13] |
| 1.6 Delayed graft function | 3 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.61, 0.99] |
| 2 Other adverse outcomes | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 CMV infection | 4 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.82, 2.85] |
| 2.2 Viral infection (not CMV) | 1 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.34, 1.65] |
| 2.3 Serious infection | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.19, 3.43] |
| 2.4 Viral infection (all cause) | 2 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.69, 2.64] |
| 2.5 PCP | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.05, 4.90] |
| 2.6 PTLD | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Serum creatinine | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 1 year | 2 | 245 | Mean Difference (IV, Random, 95% CI) | ‐15.85 [‐28.55, ‐3.15] |
| 3.2 2 years | 2 | 223 | Mean Difference (IV, Random, 95% CI) | 12.50 [‐13.52, 38.52] |
Comparison 9. ALG versus placebo/no induction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Main outcomes | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death at 1 to 2 years | 12 | 1180 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.96, 1.69] |
| 1.2 Death at 3 to 5 years | 2 | 406 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.67, 1.50] |
| 1.3 Death at 15 to 20 years | 2 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.40, 2.10] |
| 1.4 Graft loss (all cause) at 1 to 2 years | 11 | 1049 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.75, 1.09] |
| 1.5 Graft loss (all cause) at 3 to 5 years | 3 | 527 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.47, 1.39] |
| 1.6 Graft loss (all cause) at 15 to 20 years | 2 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.67, 1.34] |
| 1.7 Acute rejection | 13 | 1575 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.53, 0.92] |
| 1.8 Delayed graft function | 5 | 615 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.31, 0.97] |
| 2 Other adverse outcomes | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 CMV infection | 3 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 2.45 [1.23, 4.85] |
| 2.2 Any viral infection | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 2.71 [1.86, 3.95] |
| 2.3 Bacterial infection | 4 | 742 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.92, 1.52] |
| 2.4 Fungal infection | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.63, 1.95] |
| 2.5 Thrombocytopenia | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 12.19 [3.10, 47.92] |
| 2.6 Leucopenia | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 20.31 [0.61, 676.54] |
| 2.7 Malignancy or PTLD | 4 | 623 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.27, 1.31] |
| 2.8 NODAT | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.22, 3.93] |
| 3 Serum creatinine | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 At 1 to 2 years | 4 | 369 | Mean Difference (IV, Random, 95% CI) | ‐16.94 [‐50.86, 16.97] |
| 3.2 At 10 to 20 years | 2 | 221 | Mean Difference (IV, Random, 95% CI) | ‐3.77 [‐41.06, 33.53] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abouna 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes included |
| Other bias | High risk | Study supported by Upjohn Company |
Abramowicz 1992.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Baseline imbalance. PRA, donor age and HLA mismatch all higher in OKT3 group; funded by Cilag |
Abramowicz 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Dose adjustment as per level from day 3 post‐op
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding but the review authors judge that the outcomes are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Unclear risk | Short‐term follow‐up reported only |
| Other bias | High risk | Supported by Cilag Bennelux |
Ackermann 1988.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | 'Randomized by sealed envelope draw' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unblinded but not likely to influence most outcomes; may influence reporting of infections |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not certain if acute rejection was biopsy‐proven or clinically diagnosed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Grant from Ortho Pharmaceutical Corp (OKT3 manufacturer) |
Ata 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Maintenance immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported but likely not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported but likely not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Unclear risk | Brief report only |
| Other bias | Unclear risk | Funding not reported |
Banhegyi 1991.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
CSA adjusted according to levels in both groups |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Acute rejection episodes were not biopsy‐proven |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | 7 patients excluded: vascular complications (2), trauma (1), ABO‐incompatible transplant (1), ‘therapy protocol not followed’ (2). Not clear which group these patients were from; possibly all from one group This appears to be a preliminary report, however no further publication has been identified |
Belitsky 1991.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Funding source not reported |
Bell 1983.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Random number code' stated, no other information provided |
| Allocation concealment (selection bias) | Low risk | Pharmacy controlled; fluids supplied by pharmacists, under double‐blind conditions |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Funding not reported |
Benfield 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization in a 1:1 ratio occurred preoperatively by contacting the central data center" |
| Allocation concealment (selection bias) | Low risk | "Central data center" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear for outcome of acute rejection; not all episodes were biopsy‐proven |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | Appears free of other biases |
Bock 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequences established prior to start of study such that within each set of 4 consecutive patients 2 received ATGF and 2 received OKT3 |
| Allocation concealment (selection bias) | Low risk | "assigned treatments were kept in sealed envelopes that were opened when the patient was admitted to the hospital for transplantation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Funded by Cilag and Fresenius |
Bock 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Abstract only, limited outcomes reported and not able to be included in meta‐analyses |
| Other bias | Unclear risk | Insufficient information to permit judgement; funding source not reported |
Brennan 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence used but not reported |
| Allocation concealment (selection bias) | Low risk | Not reported, however appears to be coordinated by the pharmacist |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Only the pharmacist was unblinded and responsible for maintaining that the investigator, staff, laboratory, and pathologists remained blinded to patient study drug group for greater than 1 year after transplantation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Only the pharmacist was unblinded and responsible for maintaining that the investigator, staff, laboratory, and pathologists remained blinded to patient study drug group for greater than 1 year after transplantation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Funding not reported |
Broyer 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Most outcomes unlikely to influence outcomes but unclear whether acute rejection was biopsy proven or clinically diagnosed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement; funding source not reported |
Buchler 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Maintenance immunosuppression (both groups)
Prophylaxis (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported but likely unblinded, possible bias for some outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Likely unblinded but low risk in view of hard outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Two authors from Genzyme (manufacturer of ATG); however ATG dose same in both groups |
CAMPASIA Study 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence used in balanced blocks of 3 |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes placed with the principal investigator of each centre; the envelopes were opened in serial order within 5 hr post‐transplant |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported (to 6 months) |
| Other bias | Unclear risk | Partially funded by ILEX pharmaceuticals (Alemtuzumab manufacturer) |
Cantarovich 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors probably not blinded but unlikely to influence outcome however, less than 50% of acute rejection was biopsy‐proven. Therefore, possible source of bias in making ‘clinical’ diagnosis of acute rejection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Funding source not reported |
Charpentier 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Appears free from other bias except that funding source not reported |
Chatterjee 1976.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients assigned a number, however method not described |
| Allocation concealment (selection bias) | Low risk | "sealed envelope containing directions for randomization to the treated (HAHTG) or nontreated (non‐HAHTG) group." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Upjohn prepared and supplied hATG |
Ciancio 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using a standard randomized block design with block sizes of three or six patients (ordering of the block sizes was also randomized), ensuring a balance of patients across treatment arms after each block of patients was randomized" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Funded by National Institutes of Health grant No. R01DK25243‐24, Miami Veterans Affairs Medical Center research support, and Fujisawa Pharmaceuticals, Tokyo, Japan |
Ciancio 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label, unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Open‐label, unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Investigators funded by Roche |
Cole 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Clinically diagnosed acute rejection (no biopsy‐proven acute rejection) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | Expected outcomes reported, however infection data cannot be included in our meta‐analysis |
| Other bias | Unclear risk | Funding source not reported |
Condie 1985.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not blinded, may affect some but not all outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | Expected outcomes reported, however acute rejection rates and SCr not fully reported (short‐term only) |
| Other bias | Unclear risk | Funding not reported |
Cosimi 1976.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used |
| Allocation concealment (selection bias) | Low risk | Central allocation via Upjohn company – list kept by "Hypersensitivity Diseases Research’s co‐ordinating center for ATG studies" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label; unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Most outcomes not likely to be affected but not all acute rejection was biopsy‐proven acute rejection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | Side effects not reported for controls. Authors felt likely to be under‐reported in controls as not double blinded study, therefore data not given (likely to be much higher rate of side effects in ATG group, even if double blinded, therefore, probably not acceptable reason for not reporting Also, some hard outcomes such as WCC and platelets could be easily collected for both groups |
| Other bias | High risk | Authors on Wechter paper are from Upjohn Co (suppliers of ATG). Cosimi paper– supported in part by research grants from the Upjohn Co and from General Research Support Grants RR‐05486‐12 and HL1‐18646‐01, both from US Public health service |
De Pauw 1990.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if acute rejection episodes were biopsy‐proven acute rejection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | Death not reported at all and only limited reporting of some other outcomes; data for graft function and infectious complications not available to meta‐analyse |
| Other bias | Unclear risk | Insufficient information to permit judgement and funding source not specified |
Debure 1987.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '...the randomisation schedule was computer generated’ |
| Allocation concealment (selection bias) | High risk | No comment in paper about whether treatment allocations were concealed Imbalance in HLA mismatches (see above) favouring controls suggesting problems with randomisation, but would potentially bias results in favour of controls |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded, unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Funding source not declared, however 1 author was an employee of Ortho Pharmaceuticals |
Diethelm 1979.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unblinded; unlikely to influence most outcomes but may influence some |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear if acute rejection was biopsy‐proven acute rejection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing patients unlikely to affect results (2 with never functioned kidneys excluded) |
| Selective reporting (reporting bias) | High risk | Acute rejection, death and graft loss reported but could not be included in meta‐analyses |
| Other bias | Unclear risk | Funding source not reported |
EARTS Study 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Placebo
17 patients across both groups got ATG for DGF |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Performed in blocks of 6 to ensure balanced distribution of treatment per centre' |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded; pathologist reviewing biopsies for suspected acute rejection was also blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes reported |
| Other bias | Unclear risk | Appears free from other bias but funding source not declared |
Ejaz 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
Immunosuppression (all groups)
Prophylaxis (all groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computerised block randomisation, generated by independent statistician |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, sequential order as consented |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label, some outcomes (e.g. reporting of side effects) likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label, may affect assessment of toxicities |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Two authors received research funds from both Genzyme and Millennium Research grant support from Genzyme, Bortezomib provided by Millennium Pharm |
Farney 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
Prophylaxis (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '2 distinct randomly generated lists' |
| Allocation concealment (selection bias) | Low risk | Allocation done independently by research co‐ordinator. Co‐ordinator informed transplant surgeon just before surgery which agent to use. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | Unable to meta‐analyse death, DGF, infection due to combined data |
| Other bias | Low risk | Appears free from other bias; study self‐funded (by Wake Forest University Baptist Medical Center) |
Frey 1991.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes, all acute rejection was biopsy‐proven |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | Supported by NIH research grant |
Friend 1987.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
If steroid‐resistant acute rejection (after 2 or more courses of steroids) switched to either:
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Mainly low risk but not all acute rejection was biopsy‐proven acute rejection. Some was diagnosed and treated even when no evidence on biopsy but high clinical suspicion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | Appears free from other bias |
Fries 1988a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement, abstract only |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement, abstract only |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement, abstract only |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement, abstract only |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement, abstract only |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement, abstract only |
| Other bias | Unclear risk | Insufficient information to permit judgement, abstract only |
Fukuuchi 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Change in eligibility for randomisation part way through |
| Selective reporting (reporting bias) | High risk | Change in eligibility for randomisation part way through |
| Other bias | Unclear risk | Funding source not reported |
Gianello 1987.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported but likely not blinded; low risk of bias for hard outcomes but bias possible for some outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Likely not blinded; some acute rejection was biopsy‐proven acute rejection but some was clinical |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Seems results are combined here for 2 separate studies; one study of 1st DD transplant recipients, another study of 2nd DD transplant recipients. ‘we have concurrently conducted a similar studyin secondary cadaver grafts…we analyse in this report the outcome of both…..’. |
Grafals 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated protocols used for randomisation |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by research coordinator, sealed envelopes used (see clinical trials website) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open label but low risk in view of outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Open label but low risk in view of outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | all expected outcomes reported |
| Other bias | Low risk | Appears free of other biases |
Grino 1990.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | 'the allocation to treatment groups was done alternately' |
| Allocation concealment (selection bias) | High risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Acute rejection episodes diagnosed clinically (no biopsy) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Unclear risk | Some expected outcomes not reported such as infection and other adverse outcomes |
| Other bias | Unclear risk | Funding source not reported |
Grino 1991.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | "randomly allocated by a closed‐envelope technique" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Funding source not reported |
Grundmann 1984.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Outcomes not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | May affect some outcomes and not reported if blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists; funding source not reported |
Grundmann 1987.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified if diagnosis of acute rejection was biopsy proven or clinical |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Unclear risk | Unsure why acute rejection not reported beyond 3 weeks if there were any incidences of any other side effects such as malignancy/PTLD |
| Other bias | Unclear risk | Funding source not reported |
Guttmann 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement; abstract only |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement; abstract only |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement; abstract only |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement; abstract only |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement; abstract only |
| Selective reporting (reporting bias) | High risk | No actual figures reported for any outcomes |
| Other bias | High risk | Abstract only publication, no full‐text publication identified |
Halloran 1982.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balance, restricted randomisation according to treatment centre; randomised block of varying size was generated |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes held by the research pharmacist at each participating centre |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 patients switched groups from control to mALG, not clear how analysed |
| Selective reporting (reporting bias) | High risk | Unable to include acute rejection results in the meta‐analysis |
| Other bias | Low risk | Appears free of other bias. Funded by grant from Medical Research council of Canada. Also grant from Conacher foundation |
Hanaway 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Maintenance immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Automated system' used but not really clear. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated but no information on method used is available. Even after reading supplementary appendix, info is still vague. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | All expected outcomes reported however SCr similar at 1 year but actual figures not given and cannot be meta‐analysed |
| Other bias | Low risk | Study appears free form other bias. Funding by Astellas Pharma Global Development |
Hanto 1991.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Maintenance immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | All expected outcomes reported; unable to meta‐analyse infection data |
| Other bias | Unclear risk | Funding source not reported |
Henry 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomised according to whether patient record number ended in odd or even number |
| Allocation concealment (selection bias) | Unclear risk | Randomised according to whether patient record number ended in odd or even number. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unlikely to influence outcomes although uncertain if acute rejection episodes were biopsy proven or clinically diagnosed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | All expected outcomes reported however unable to included graft function in the meta‐analyses as no SD or SE reported |
| Other bias | High risk | Potential bias due to funding from OKT3 (Grant from Ortho Bio‐Tech – OKT3 manufacturer) |
Hourmant 1985a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Control group
2nd randomisation at 3 months of the control group only to continue with standard treatment or switch to low dose CSA monotherapy (6 mg/kg/d) (treatment group 2 ‐ Cys group I) |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some outcomes reported with insufficient detail to fully assess e.g. infection |
| Selective reporting (reporting bias) | High risk | Some outcomes reported with insufficient detail to fully assess |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Hourmant 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Maintenance immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes; all acute rejection was biopsy‐proven |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | Graft survival at 3 months not reported |
| Other bias | Unclear risk | Funding source not reported |
Jakobsen 1981.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | ‘patients allotted by drawing cards marked yes or no’; Half patients in each group in each centre |
| Allocation concealment (selection bias) | High risk | Drawing cards |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if acute rejection was biopsy‐proven acute rejection or clinical diagnosis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Funding source not reported |
Kasiske 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes; all acute rejection was biopsy proven |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | Expected outcomes reported; unable to meta‐analyse graft function |
| Other bias | Unclear risk | Funding source not reported |
Khosroshahi 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Acute rejection was both biopsy‐proven acute rejection and clinical |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | Limited outcomes reported and only very short‐term follow‐up |
| Other bias | High risk | Exclusion criteria included intra‐op and post‐op problems; patients would already have been entered prior to this. Therefore, were patients withdrawn after randomisation? No real details about this. Funding source not reported |
Kountz 1977.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Limited info about how acute rejection was diagnosed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Results seem to be a mixture of 2 studies. Initial study had 4 groups, including 2 x low dose ATG (1 x IV, 1 x IM). These 2 groups excluded after 15 patients in each group. Results combined with this study. Upjohn company funded study and provided the ATG; result in favour of ATG |
Kreis 1980.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table used |
| Allocation concealment (selection bias) | Low risk | 'Physicians in charge of the patients were not aware of the list kept at the Upjohn Company' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear for 'reversible renal failure episodes' or acute rejection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | Expected outcomes reported, however unsure if reversible kidney failure episodes is acute rejection and therefore results were not used |
| Other bias | High risk | ATG provided by Upjohn co and computer analysis also done by them |
Kreis 1986.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement if acute rejection episodes were biopsy proven or not |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement and funding source not declared |
Kumar 1998a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Funding source not specified |
Launois 1977.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | Supported by a grant from University of Rennes |
Lazarovits 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and therefore high risk for certain outcomes, e.g. tolerance of antibody therapy |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and acute rejection could be diagnosed clinically |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Funded by grants from Kidney Foundation of Canada and Sandoz Canada Inc (CD7 manufacturer) |
Lu 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Maintenance immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open label but probably low risk given hard outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above; all acute rejection was biopsy‐proven acute rejection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | All expected outcomes reported; graft function, WCC count could not be included in our meta‐analyses |
| Other bias | Low risk | None apparent. Supported by grant from Fujian Key Laboratory of Transplant Biology (No. 2008J1006) |
Maiorca 1984.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Acute rejection, infection could not be used in our meta‐analyses |
| Other bias | Unclear risk | Insufficient information to permit judgement, funding not reported |
Margreiter 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes but not clear if the biopsy reviewer was blinded to the treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | Outcomes reported as per protocol (as per Clinicaltrials.gov); however unable to meta‐analyse the graft function results (no SD) |
| Other bias | High risk | Supported by Astellas Pharma GmbH, Munich (Tacrolimus supplier) |
Martins 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unlikely to influence outcomes but unclear if acute rejection was clinical diagnosis or biopsy‐proven acute rejection |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement; 2 patients excluded from analyses due to death with a functioning graft; probably should have been included |
| Selective reporting (reporting bias) | High risk | SCr and infection could not be included in our meta‐analyses |
| Other bias | High risk | Funding not reported, but one of the co‐authors is from Fresenius Biotech |
Michael 1989.
| Methods |
|
|
| Participants |
|
|
| Interventions | Initial treatment (both groups)
Treatment group
Control group
Reassessment after 2 weeks |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via computerised random number generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether all acute rejection was biopsy proven (likely yes while patient had DGF but unclear if diagnosed after graft started functioning) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Patients whose grafts never functioned were excluded from the analyses |
| Selective reporting (reporting bias) | High risk | No extractable data available for review outcomes, SD and SE not reported; several results only presented as figures |
| Other bias | Unclear risk | Funding not reported |
Minnesota Study 1982.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified but method not specified |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | All expected outcomes reported, however patient numbers vary in the different reports of this study |
| Other bias | Unclear risk | Funding not fully disclosed. Supported in part by a grant from NIH |
Morales 1994a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement if acute rejection episodes were biopsy‐proven acute rejection |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | SD and SE not reported for graft function; complications such as infection or malignancy not well reported |
| Other bias | Unclear risk | Funding source not reported |
Mourad 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list generated centrally. Patients randomised 1:1 and stratified by centre |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes opened post‐op |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes (all acute rejection was biopsy‐proven acute rejection) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Relatively large drop out numbers in each group; ITT results reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Funded by Fujisawa GmbH (TAC manufacturers) |
Niaudet 1990.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Funding source not reported |
Norman 1988.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 patients excluded from analyses; 6 excluded as received grafts form donor under age 5 years (historically poor outcomes); 2 excluded in OKT3 group as only received 1 or 2 doses of OKT3 (reasons not reported) |
| Selective reporting (reporting bias) | High risk | Some expected outcomes not reported |
| Other bias | Unclear risk | 'Supported by Ortho Pharmaceutical' (OKT3 manufacturers) |
Norman 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stated but insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Safety analyses 111 versus 104 included (215 total) Efficacy analyses 105 versus 102 included (207 total) 224 patients entered into the study 9 patients excluded after randomisation as 'not treated' (whether this means not transplanted or not treated as per protocol is not reported) Additional 8 patients excluded from efficacy analyses and therefore included only in safety analyses (6 paediatric patients and 2 patients who did not follow randomisation schedule) |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Funded by RW Johnson pharmaceutical research institute; corresponding author is an employee of RW Johnson |
Norman 1993a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | limited info. ‘The patients were randomised in blocks of four patients’. |
| Allocation concealment (selection bias) | Low risk | Randomisation schedule kept by pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients, nurses and doctors all blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | SD/SE not reported for graft function and cannot be meta‐analysed |
| Other bias | Unclear risk | Funding source not reported |
Norrby 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Denominators sometimes unclear |
| Selective reporting (reporting bias) | High risk | Outcomes reported but actual numbers not given, therefore difficult to verify data |
| Other bias | Unclear risk | Unclear due to limited information. Funding from 4 different groups: Gothenburg University, Riksforbundet Njursjuka, Njursjukas forening i Vast Sverige, and Gelins Minnesfond. |
Novick 1983.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Previously numbered drug vials’ but not clear how sequence generated |
| Allocation concealment (selection bias) | Low risk | Randomised via a central office at the University of Minnesota |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Patients only receiving 50% of ALG total dose or less were excluded from results (4/35; 10% of group (2 withdrew, 2 unable to tolerate due to side effects) Not certain if these patients would have altered outcomes if included |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | University of Minnesota ALG lab provided the ALG |
Perez‐Tamajon 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | All expected outcomes reported however unable to use acute rejection data |
| Other bias | Unclear risk | Insufficient information to permit judgement and funding source not clear |
Pernin 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Limited reporting of outcomes |
| Selective reporting (reporting bias) | High risk | Has not been published as full paper |
| Other bias | High risk | Abstract only |
Raffaele 1991.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified whether acute rejection episodes were biopsy‐proven acute rejection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | Expected outcomes reported given only short‐term follow‐up. However, graft loss and death not reported. (may be none but would expect these outcomes to be reported) |
| Other bias | Unclear risk | Insufficient information to permit judgement to assess and funding source not declared |
Rostaing 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Immunosuppression (both groups)
Prophylaxis (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported given short follow‐up only |
| Other bias | Unclear risk | Funding not declared |
Sakhrani 1992.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated how acute rejection was determined |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Kidneys did not function in 4 patients (2 in each group) and 1 patient from each group moved out of the country |
| Selective reporting (reporting bias) | High risk | Death not reported; results reported as percentages and could not be meta‐analysed |
| Other bias | Unclear risk | Funding not reported |
Samsel 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
Prophylaxis (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not all acute rejection was biopsy‐proven |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported; 1 patient excluded in control group as immunosuppression was withdrawn however was included in the safety analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Funding source unclear; ATG supplied by Fresenius Pharma Support |
Sansom 1976.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'randomised numbers consecutively...' insufficient to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unlikely to influence reported outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if acute rejection was biopsy‐proven |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Higher immunological risk patients excluded (2nd transplant patients) after randomisation; no results given for these 15 patients |
| Selective reporting (reporting bias) | High risk | As above; acute rejection results could not be included in the meta‐analysis |
| Other bias | Unclear risk | Funding source unclear "gift of rabbit ALG and financial assistance" provided by GD Searle; Queen Elizabeth Hospital Renal Research Fund provided some funding |
Sharaf El Din 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear if acute rejection was biopsy‐proven |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Sheashaa 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcomes reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | No obvious source but funding source not declared |
Shield 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Limited information but no reason for severe imbalance in LD vs DD patients and unequal numbers in intervention and treatment groups. Likely selection bias; possibly post‐hoc report of unpublished RCT |
| Allocation concealment (selection bias) | High risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Percentages given but no actual numbers for survival and no causes of patient or graft loss |
| Selective reporting (reporting bias) | High risk | Acute rejection not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement and funding not declared |
Slakey 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcomes reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Funding not declared |
Smeekens 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Pre‐med, immunosuppression and prophylaxis (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list of random numbers, prepared by independent investigator |
| Allocation concealment (selection bias) | Low risk | Study numbers only available to authorised nurses who signed confidentiality statements. Medication prepared by authorised nurses |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medication in identical bags for rituximab and placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | None apparent. 'Both companies were informed of the results and had no role in study design, data collection, analysis, interpretation or writing of the report.' |
Spillner 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | Unable to meta‐analyse acute rejection results |
| Other bias | Unclear risk | Funding not reported |
Squifflet 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Supported by the manufacturers of BTI‐322 |
Steinmuller 1991.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some outcomes reported in an unclear way (e.g. graft losses seems to include some deaths but not all deaths) |
| Selective reporting (reporting bias) | High risk | Most expected outcomes reported but some not clear. Some patients had early acute rejection but were treated by course of antibody therapy (therefore, rates of acute rejection may be lower than expected) Not clear if all patients were biopsied or only those whose SCr continued to rise post antibody treatment or SCr fell then rose again Graft function: documented at 6 months but not included in meta‐analysis as no SD or SE given |
| Other bias | Unclear risk | Funding not reported |
Stevens 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Pre‐meds, immunosuppression, prophylaxis (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ‘Randomly generated treatment group assignments’ after stratification into 6 different groups |
| Allocation concealment (selection bias) | Low risk | ‘Sequentially numbered sealed envelopes’. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All patient outcome data reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | High risk | Primary outcomes not well reported (graphs only, no figures reported for kidney function) |
| Other bias | Unclear risk | Partly funded by Genzyme with unrestricted grant. (but ATG in both arms) |
Taylor 1976.
| Methods |
RCT Multicentre ‐ 12 centres across Canada 12 month follow up |
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers used |
| Allocation concealment (selection bias) | Low risk | sealed envelopes with patient allocations, only opened during operation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement, in particular, not clear how acute rejection episodes were diagnosed and what made them a minor versus a major acute rejection episode |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | All expected outcomes reported; graft function reported at 60 days but not able to be used in analyses of this review as no SD or SE given |
| Other bias | Low risk | Appears free of other bias; funding by Medical Research Council, Canada |
Thibaudin 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unlikely to influence outcomes Not all acute rejection was biopsy proven (72% in ATG group and 90% in control) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Funding source not reported |
Thomas 1977.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | 'randomisation usually on an alternate basis but not necessarily so'. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as double blind "neither medical nor nursing staff aware of which letter group was high potency (H.P.‐A.L.G.) and which was moderate potency (M.P.‐A.L.G.)" Labelled group A and group B – low risk given hard outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Five patients excluded due to inadvertent major deviations from standard protocol |
| Selective reporting (reporting bias) | High risk | Deaths not fully reported. Infection not fully reported |
| Other bias | Low risk | None apparent. Funded in part by 2 x NIH grants |
Thomas 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | Unable to analyse infection data |
| Other bias | Unclear risk | Funding source not reported |
Toledo‐Pereyra 1985.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Funding source not reported |
TRIMS Study 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
Prophylaxis (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Sponsored by Genzyme (rATG manufacturers) NB: enrolment stopped early at 151 patients (planned to enrol 200) by study sponsor – due to ‘budget reasons’ |
Tsai 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Full study not reported |
| Other bias | High risk | Abstract only. Funding source unknown |
Turcotte 1973.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Separate sets of random cards for DD and LD recipients |
| Allocation concealment (selection bias) | Low risk | Cards in sealed envelopes, not opened until the time of surgery |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | All expected outcomes reported, however unable to use acute rejection or infection data |
| Other bias | Unclear risk | Unclear: hATG provided by Upjohn Co (therefore partially funded by them) Also funded by Maud T. Lane Fund and research grant from Public Health Service |
Tyden 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
Prophylaxis (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement ("...in randomization blocks of four") |
| Allocation concealment (selection bias) | Low risk | Randomisation performed at hospital pharmacy department |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Infusion bags marked ‘Mantra study medication’ with content blinded to both the patient and the investigator |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Most expected outcomes reported but no mention of malignancy in the study; poor follow‐up at 3 years |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | High risk | Grants from Roche, Sweden and Astellas Pharma 'Had advisory input into study design, collected data via electronic reporting and monitored study conduct' |
van den Hoogen 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
Prophylaxis (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer derived algorithm at coordinating centre |
| Allocation concealment (selection bias) | Low risk | Printed on paper and put into sealed, numbered envelopes. patients assigned a consecutive number in the order in which they entered the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | DGF was primary outcome and decision regarding need for dialysis post‐op may be quite subjective; unlikely to influence other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Terminated early due to poor recruitment 'This study was financially supported by Fresenius Biotech GmbH, Gräfelfing, Germany. The company had no input in study design, data collection, data analysis, and writing or editing of the manuscript'. |
Vela 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported however unable to use graft function data |
| Other bias | Unclear risk | Funding source not reported |
Vigeral 1986.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Most cases of acute rejection were biopsy‐proven acute rejection but not all. Clinical decision for acute rejection without biopsy could be prone to bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported given short term follow‐up only |
| Other bias | High risk | Funding source not declared; one of the authors is from Ortho Pharmaceutical Corporation (OKT3 manufacturer) |
Wechter 1979.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control:
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Acute rejection episodes mainly diagnosed clinically; lack of blinding may have influenced reporting of adverse outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Side effects not well reported for control group |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Contact author an employee of Upjohn company |
Yussim 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Immunosuppression (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | High risk | All expected outcomes reported however SD/SE not reported for graft function |
| Other bias | Unclear risk | Insufficient information to permit judgement |
ALG ‐ antilymphocyte globulin; ANC ‐ absolute neutrophil count; ATG ‐ antithymocyte globulin; ATGAM ‐ horse ATG; ATN ‐ acute tubular necrosis; AZA ‐ azathioprine; BKV ‐ BK virus; CAN ‐ chronic allograft nephropathy; CMV ‐ cytomegalovirus; CNI ‐ calcineurin inhibitor; CSA ‐ cyclosporin A; DD ‐ deceased donor; DGF ‐ delayed graft function; DEX ‐ dexamethasone; EBV ‐ Epstein–Barr virus; eGFR ‐ estimated glomerular filtration rate; ESKD ‐ end‐stage kidney disease; GI ‐ gastrointestinal; hATG ‐ horse ATG; Hep ‐ hepatitis; HIV ‐ human immunodeficiency virus; HLA ‐ human leukocyte antigen; IL‐2RA ‐ interleukin 2 receptor antagonist; IV ‐ intravenous; LD ‐ living donor; mALG ‐ Minnesota ALG; M/F ‐ male/female; MMF ‐ mycophenolate mofetil; MP ‐ methylprednisolone; NODAT ‐ new‐onset diabetes after transplantation; post‐op ‐ post‐operative; PRA ‐ panel reactive antibodies; PRED ‐ prednisone; PTLD ‐ post‐transplant lymphoproliferative disease; rATG ‐ rabbit ATG; RBC ‐ red blood cell; RCT‐ randomised controlled trial; SCr ‐ serum creatinine; SD ‐ standard deviation; SE ‐ standard error; SEM ‐ standard error of the mean; WCC ‐ white cell count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alloway 1993 | Study includes kidney‐pancreas recipients, results not reported separately for kidney only recipients |
| Kirsch 2006 | No outcomes relevant to this review (critical circulating DC subsets, i.e. myeloid (DC1) versus lymphoid (DC2) DC) |
| Kumar 2002b | "Due to financial constraints randomization was based on affordability to bear the cost of ATG. Those who could afford the cost were included in the study group and those who couldn't became the control" |
| NCT00000936 | Study terminated; no data available |
| NCT01312064 | Study terminated; no data available |
ATG ‐ antilymphocyte globulin
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00089947.
| Methods | Randomised, open‐label, parallel assignment (phase 2) |
| Participants | 150 participants, ≥18 years, LD kidney transplant recipients |
| Interventions | rATG with rapid discontinuation of steroids versus steroids per hospital standards for at least 1st 90 days after transplant |
| Outcomes | Primary: kidney transplant rejection, organ loss and death at 6 months Secondary: kidney function after transplantation and overall safety of rATG |
| Notes | This study has been completed but no study results have been posted on Clinicaltrials.gov |
NCT00861536.
| Methods | Randomised, open‐label, parallel assignment (phase 4) |
| Participants | 40 participants, ≥18 years, recipients of kidney transplants of high immunological risk |
| Interventions | ATG (Fresenius) versus thymoglobulin |
| Outcomes | Primary: adverse events Secondary: rejection, graft function, patient survival, graft survival |
| Notes | This study has been completed but no study results have been posted on Clinicaltrials.gov |
NCT01046955.
| Methods | Randomised, open‐label, parallel assignment (phase 4) |
| Participants | 38 participants, age > 14 years, 1st LD kidney transplant recipients |
| Interventions | ATG versus alemtuzumab versus daclizumab |
| Outcomes | Primary: effectiveness and toxicity at 3 years, patient and graft survival at 1 and 3 years Secondary: incidence of adverse reactions at 1 and 3 years |
| Notes | This study has been completed but no study results have been posted on Clinicaltrials.gov |
NCT01354301.
| Methods | Randomised, open‐label, parallel assignment (Phase 4) |
| Participants | 300 participants, ≥ 18 years, low risk kidney transplant recipients |
| Interventions | Single dose ATG and everolimus versus basiliximab and everolimus versus basiliximab and MMF |
| Outcomes | Primary: incidence of CMV infection or disease at 1 year Secondary: incidence of treatment failure at 1 year (composite of biopsy‐confirmed acute rejection, graft loss, death, loss to follow‐up) |
| Notes | This study has been completed but no study results have been posted on Clinicaltrials.gov |
Stevens 2016.
| Methods | Double‐blind, double‐dummy RCT |
| Participants | 18 to 65 years DD or LD kidney transplant recipients |
| Interventions | Single dose rATG versus divided dose rATG |
| Outcomes | Primary: composite endpoint of fever, hypotension, hypoxia, cardiac events, DGF Secondary: patient survival; graft survival acute rejection; incomplete ATG infusion; eGFR |
| Notes | Results yet to be incorporated |
CMV ‐ cytomegalovirus; DGF ‐ delayed graft function; LD ‐ living donor; MMF ‐ mycophenolate mofetil; rATG ‐ rabbit antilymphocyte globulin
Characteristics of ongoing studies [ordered by study ID]
NCT00733733.
| Trial name or title | Anti‐T‐lymphocyte globulin (ATG) in renal transplantation of kidneys with a non‐heart‐beating (NHB) donor |
| Methods | Randomised, open‐label, parallel assignment (Phase 3) |
| Participants | 180 participants, recipients of DD kidney transplants |
| Interventions | rATG versus no intervention |
| Outcomes | Primary: incidence of initial DGF (defined as need for dialysis) within 3 months Secondary: duration of initial DGF, incidence of primary never‐functioning grafts, incidence of biopsy‐proven acute rejection within 3 months, kidney function (MDRD) at 1,2 and 3 months, proteinuria at 1, 2 and 3 months, % of patients with arterial hypertension at 3 months, % of patients with antihypertensive drugs at 3 months, % of hyperlipidaemic patients at 3 months, % of post‐transplant DM at 3 months, incidence of CMV infection at 3 months, incidence of tumours/PTLD at 3 months, patient and graft survival at 3 months, incidence of other infections at 3 months, microalbuminuria at 1, 2 and 3 months |
| Starting date | January 2008 |
| Contact information | Radboud University (Prof. Dr Andries Hoitsma, UMC St Radboud Hospital) |
| Notes | Estimated study completion date was June 2010; recruitment status unknown; study details last verified in August 2008 |
NCT01154387.
| Trial name or title | Evaluating safety and efficacy of TOL101 induction versus anti‐thymocyte globulin to prevent kidney transplant rejection |
| Methods | Randomised, open‐label, parallel assignment (Phase 1 and Phase 2) |
| Participants | 85 participants, age 18‐60, first kidney transplant recipients |
| Interventions | ATG versus TOL101 dose A versus TOL101 dose B |
| Outcomes | Primary: safety and tolerability of ascending doses of TOL101 and effectiveness of TOL101 to target and down regulate T cells at 6 months Secondary: effects of ascending doses of TOL101 on CD3+ T lymphocyte numbers and other immune cell subsets at 14 days and 6 months, pharmacokinetic profile of TOL101 and exposure‐response relationship over time at 14 days, biopsy‐proven acute organ rejection at 6 months, graft survival at 6 months, patient survival at 6 months, kidney function by measured GFR at 6 months and urine protein to creatinine ration at 3 and 6 months, DGF at 7 days, immunogenicity of TOL101 by measurement of anti‐TOL101 antibodies at 14 and 28 days, presence of DSA at 3 months and 6 months |
| Starting date | July 2010 |
| Contact information | Tolera Therapeutics Inc (Stuart Flechner MD, The Cleveland Clinic) |
| Notes | Estimated study completion date was June 2013; recruitment status was active; not recruiting; study details last verified in June 2013 |
ReMIND Study 2013.
| Trial name or title | RituxiMab INDuction in renal transplantation (ReMIND) |
| Methods | Randomised, open‐label, parallel assignment (phase 4) |
| Participants | 612 participants, ≥18 years, recipients of LD kidney transplants |
| Interventions | Rituximab and 1 week prednisolone versus continued prednisolone |
| Outcomes | Primary: eGFR at 1 year Secondary: biopsy proven acute rejection at 1, 2, 3, 4 and 5 years, allograft survival at 1, 2, 3, 4 and 5 years, patient survival at 1, 2, 3, 4 and 5 years, infection rate at 1 year, changes in B and T cell repertoire |
| Starting date | November 2010 |
| Contact information | Guy's and St Thomas' NHS Foundation Trust (Nizam Mamode, MD, FRCS(Gen) |
| Notes | Estimated study completion date is October 2023; active, recruiting participants; study details last verified August 2016 |
ATG ‐ antilymphocyte globulin; CMV ‐ cytomegalovirus; DD ‐ deceased donor; DGF ‐ delayed graft function; DM‐ diabetes mellitus; GFR ‐ glomerular filtration rate; LD ‐ living donor: MDRD ‐ Modification of Diet in Renal Disease; PTLD ‐ post‐transplant lymphoproliferative disease; rATG ‐ rabbit ATG
Contributions of authors
Study selection: PH, NC, NB, SP
Screening of articles: PH, NC, NB, SP
Disagreement resolution: PH, NC, NB, SP
Data extraction: PH, NC, NB, SP
Data entry: PH
Carry out the analysis: PH, NC, NB, SP
Interpret the analysis: PH, NC, NB, SP
Draft the final review: AW, PH, NC, NB, SP
Update the review: AW, PH, NC, NB, SP
Declarations of interest
AW: Nothing to declare
NC: Nothing to declare
PH: Nothing to declare
NB: NB is a co‐investigator of the ongoing randomised, controlled clinical trial, ReMIND (RituxiMab INDuction in renal transplantation, NCT01095172).
SP: Nothing to declare
New
References
References to studies included in this review
Abouna 1995 {published data only}
- Abouna GM, Kumar MS, al‐Abdullah IH, Loose J, Sullivan DK, Phillips K, et al. Induction immunosuppression with antithymocyte globulin in renal transplantation using a variable dose according to the absolute number of CD3+ T cells. Transplantation Proceedings 1995;27(5):2676‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Abouna GM, al‐Abdullah IH, Kelly‐Sullivan D, Kumar MS, Loose J, Phillips K, et al. Randomized clinical trial of antithymocyte globulin induction in renal transplantation comparing a fixed daily dose with dose adjustment according to T cell monitoring. Transplantation 1995;59(11):1564‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Abramowicz 1992 {published data only}
- Abramowicz D, Goldman M, Pauw L, Vanherweghem JL, Kinnaert P, Vereerstraeten P. The long‐term effects of prophylactic OKT3 monoclonal antibody in cadaver kidney transplantation‐‐a single‐center, prospective, randomized study. Transplantation 1992;54(3):433‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Abramowicz D, Norman DJ, Goldman M, Pauw L, Kinnaert P, Kahana L, et al. OKT3 prophylaxis improves long‐term renal graft survival in high‐risk patients as compared to cyclosporine: combined results from the prospective, randomized Belgian and US studies. Transplantation Proceedings 1995;27(1):852‐3. [MEDLINE: ] [PubMed] [Google Scholar]
- Abramowicz D, Norman DJ, Vereerstraeten P, Goldman M, Pauw L, Vanherweghem JL, et al. OKT3 prophylaxis in renal grafts with prolonged cold ischemia times: association with improvement in long‐term survival. Kidney International 1996;49(3):768‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Goldman M, Abramowicz D, Pauw L, Marecaux G, Vanderwinden JM, Kinnaert P, et al. Beneficial effects of prophylactic OKT3 in cadaver kidney transplantation: comparison with cyclosporin A in a single‐center prospective randomized study. Transplantation Proceedings 1991;23(1 Pt 2):1046‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Abramowicz 1994 {published data only}
- Abramowicz D, Goldman M, Mat O, Estermans G, Crusiaux A, Vanherweghem JL, et al. OKT3 serum levels as a guide for prophylactic therapy: a pilot study in kidney transplant recipients. Transplant International 1994;7(4):258‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ackermann 1988 {published data only}
- Ackermann JR, Lefor WM, Kahana L, Weinstein S, Shires DL. Prophylactic use of OKT3 in renal transplantation: Part of a prospective randomized multicenter trial. Transplantation Proceedings 1988;20(1 Suppl 1):242‐4. [EMBASE: 1988111881] [Google Scholar]
- Kahana L, Ackermann J, Lefor W, Weinstein S, Wright C, DeQuesada A, et al. Uses of orthoclone OKT3 for prophylaxis of rejection and induction in initial nonfunction in kidney transplantation. Transplantation Proceedings 1990;22(4):1755‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Kahana L, Narvarte J, Ackermann J, Lefor W, Weinstein S, Wright C, et al. OKT3 prophylaxis versus conventional drug therapy: single‐center perspective, part of a multicenter trial. American Journal of Kidney Diseases 1989;14(5 Suppl 2):5‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Ata 2013 {published data only}
- Ata P, Kara M, Ozdemir E, Canbakan M, Gokce AM, Bayraktar FA, et al. Monitoring of CD3(+) T‐cell count in patients receiving antithymocyte globulin induction after cadaveric renal transplantation. Transplantation Proceedings 2013;45(3):929‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Banhegyi 1991 {published data only}
- Banhegyi C, Rockenschaub S, Muhlbacher F, Kovarik J, Balcke P, Gotzinger P, et al. Preliminary results of a prospective randomized clinical trial comparing cyclosporine A to antithymocyte globulin immunosuppressive induction therapy in kidney transplantation. Transplantation Proceedings 1991;23(4):2207‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Belitsky 1991 {published data only}
- Belitsky P, MacDonald AS, Cohen AD, Crocker J, Hirsch D, Jindal K, et al. Comparison of antilymphocyte globulin and continuous i.v. cyclosporine A as induction immunosuppression for cadaver kidney transplants: a prospective randomized study. Transplantation Proceedings 1991;23(1 Pt 2):999‐1000. [MEDLINE: ] [PubMed] [Google Scholar]
- Gulanikar AC, MacDonald AS, Sungurtekin U, Belitsky P. The incidence and impact of early rejection episodes on graft outcome in recipients of first cadaver kidney transplants. Transplantation 1992;53(2):323‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gulanikar AC, Sungurtekin U, MacDonald AS, Belitsky P, Bitter‐Suermann H, Cohen A, et al. Sequential discontinuation of azathioprine and prednisone in renal transplantation. Transplantation Proceedings 1991;23(4):2226‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Bell 1983 {published data only}
- Bell PR, Blamey RW, Briggs JD, Castro JE, Hamilton DN, Knapp MS, et al. Medical research council trial of antilymphocyte globulin in renal transplantation. A multicenter randomized double‐blind placebo controlled clinical investigation. Transplantation 1983;35(6):539‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Benfield 1999 {published data only}
- Benfield MR, Herrin J, Feld L, Rose S, Stablein D, Tejani A. Safety of kidney biopsy in pediatric transplantation: a report of the Controlled Clinical Trials in Pediatric Transplantation Trial of Induction Therapy Study Group. Transplantation 1999;67(4):544‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Benfield MR, Symons JM, Bynon S, Eckhoff D, Herrin J, Harmon W, et al. Mycophenolate mofetil in pediatric renal transplantation. Pediatric Transplantation 1999;3(1):33‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Benfield MR, Tejani A, Harmon WE, McDonald R, Stablein DM, McIntosh M, et al. A randomized multicenter trial of OKT3 mAbs induction compared with intravenous cyclosporine in pediatric renal transplantation. Pediatric Transplantation 2005;9(3):282‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tejani A. A randomized prospective multicenter trial of T‐cell antibody induction therapy in pediatric renal transplantation [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000.
- Tejani A, Harmon W, Benfield M, Elshihabi I, McDonald R, Stablein D, et al. A randomized prospective multicenter trial of t‐cell antibody induction therapy in pediatric renal transplantation [abstract]. Transplantation 2000;69(8 Suppl):S111. [CENTRAL: CN‐00402826] [Google Scholar]
- Tejani A, Harmon W, Benfield M, Rose S, Stablein D, Strom T, et al. A randomized prospective multicenter trial of t‐cell antibody induction therapy in pediatric renal transplantation [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):709A. [Google Scholar]
Bock 1995 {published data only}
- Bock HA, Gallati H, Zurcher RM, Bachofen M, Mihatsch MJ, Landmann J, et al. A randomized prospective trial of prophylactic immunosuppression with ATG‐Fresenius versus OKT3 after renal transplantation. Transplantation 1995;59(6):830‐40. [MEDLINE: ] [PubMed] [Google Scholar]
- Bock HA, Zurcher R, Mihatsch M, Landmann J, Thiel G. A randomized prospective trial of prophylactic immunosuppression with OKT3 vs. ATG‐Fresenius (ATG‐F) after renal transplantation [abstract]. 12th International Congress of Nephrology; 1993 June 13‐18; Jerusalem, Israel. 1993:165. [CENTRAL: CN‐00550381]
- Bock HA, Zurcher R, Mihatsch M, Landmann J, Thiel G. A randomized prospective trial of prophylactic therapy with OKT3 vs ATG‐Fresenius (ATG‐F) after renal transplantation [abstract]. Journal of the American Society of Nephrology 1992;3(3):852. [CENTRAL: CN‐00460418] [Google Scholar]
Bock 1999 {published data only}
- Bock HA, Tsinalis D, Nickeleit V, Mihatsch M, Thiel G. Randomized prospective study of polyclonal antilymphocyte globulin induction after renal transplantation: ATGAM vs ATG‐Fresenius [abstract no: A3646]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):720A. [CENTRAL: CN‐00583882] [Google Scholar]
Brennan 1999 {published data only}
- Brennan DC, Flavin K, Burgess S, Dolan S, Kano JM, Mahon M, et al. A randomized, double‐blinded comparison of thymoglobulin vs ATGAM for induction in adult renal transplant recipients [abstract no: 710]. Transplantation 1998;65(12):S180. [CENTRAL: CN‐00602088] [DOI] [PubMed] [Google Scholar]
- Brennan DC, Flavin K, Lowell JA, Howard TK, Shenoy S, Burgess S, et al. A randomized, double‐blinded comparison of Thymoglobulin versus Atgam for induction immunosuppressive therapy in adult renal transplant recipients. [erratum appears in Transplantation 1999 May 27;67(10):1386.]. Transplantation 1999;67(7):1011‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brennan DC, Flavin K, Lowell JA, Howard TK, Shenoy S, Burgess S, et al. Leukocyte response to thymoglobulin or ATGAM for induction immunosuppression in a randomized, double‐blind clinical trial in renal transplant recipients. Transplantation Proceedings 1999;31(3B Suppl):16S‐8S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hardinger KL, Rhee S, Buchanan P, Koch M, Miller B, Enkvetchakul D, et al. A prospective, randomized, double‐blinded comparison of thymoglobulin versus ATGAM for induction immunosuppressive therapy: 10‐year results. Transplantation 2008;86(7):947‐52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardinger KL, Schnitzler MA, Miller B, Lowell J, Shenoy S, Brennan DC. Long‐term comparison of thymoglobulin versus ATGAM for induction in adult renal transplantation: evidence of improved allograft survival at 5 years [abstract]. American Journal of Transplantation 2003;3(Suppl 5):556. [CENTRAL: CN‐00445642] [Google Scholar]
- Hardinger KL, Schnitzler MA, Miller B, Lowell JA, Shenoy S, Koch MJ, et al. Five‐year follow up of thymoglobulin versus ATGAM induction in adult renal transplantation. Transplantation 2004;78(1):136‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Broyer 1993 {published data only}
- Broyer M, Gagnadoux MF, Guest G, Arsan A, Beurton D, Revillon Y, et al. Prophylactic OKT3 monoclonal antibody versus antilymphocyte globulins: a prospective, randomized study in 148 first cadaver kidney grafts. Transplantation Proceedings 1993;25(1 Pt 1):570‐1. [MEDLINE: ] [PubMed] [Google Scholar]
Buchler 2013 {published data only}
- Buchler M, Longuet H, Lemoine R, Herr F, Gatault P, Thibault G, et al. Pharmacokinetic and pharmacodynamic studies of two different rabbit antithymocyte globulin dosing regimens: results of a randomized trial. Transplant Immunology 2013;28(2‐3):120‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Longuet H, Buchler M, Lemoine R, Herr F, Gatault P, Thibault G, et al. Pharmacokinetic and pharmacodynamic studies of two different rabbit antithymocyte antiglobulin dosing regimens [abstract no: O069]. Transplant International 2013;26(Suppl 2):44‐5. [EMBASE: 71359107] [DOI] [PubMed] [Google Scholar]
CAMPASIA Study 2005 {published data only}
- Munoz AS, Cabanayan‐Casasola CB, Danguilan RA, Padua FB, Ona ET. Campath‐1H (alemtuzumab) as an induction agent for the prevention of graft rejection and preservation of renal function in kidney transplant patients: Philippine 3‐year follow‐up. Transplantation Proceedings 2008;40(7):2230‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vathsala A, CAMPASIA Study Group. Safety and efficacy of campath‐1h (mabcampath®) with low dose cyclosprorine monotherapy in patients receiving kidney transplants ‐ 6 month analysis of the pilot randomised controlled [abstract]. Transplantation 2004;78(2 Suppl):56. [CENTRAL: CN‐00509538] [Google Scholar]
- Vathsala A, Campasia Study Group. One year results of a pilot randomised controlled trial of the effectiveness of alemtuzumab as an induction agent for prevention of graft rejection and preservation of renal function in patients receiving kidney transplants [abstract no: T‐PO50029]. Nephrology 2005;10(Suppl):A215. [CENTRAL: CN‐00583367] [Google Scholar]
- Vathsala A, Ona ET, Tan S, Suresh S, Chan Y, Lou H, et al. Campasia: a pilot randomised controlled trial of the effectiveness of campath‐1h (mabcampath®) as an induction agent for prevention of graft rejection and preservation of renal function in patients receiving kidney transplants [abstract]. American Journal of Transplantation 2004;4(Suppl 8):406. [CENTRAL: CN‐00509539] [Google Scholar]
- Vathsala A, Ona ET, Tan SY, Suresh S, Lou HX, Cabanayan Casasola CB, et al. Randomized trial of Alemtuzumab for prevention of graft rejection and preservation of renal function after kidney transplantation. Transplantation 2005;80(6):765‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vathsala A, Ona ET, Tan SY, Suresh S, Lou HX, Cabanayan‐Casasola CB, et al. Induction therapy with alemtuzumab together with low dose cyclosporine monotherapy permits steroid‐free immunosuppression, mitigates drug‐related, non‐immune toxicities and improves quality of life [abstract no: TH‐PO550]. Journal of the American Society of Nephrology 2006;17(Abstracts):224A. [CENTRAL: CN‐00644285] [Google Scholar]
- Vathsala A, Ona ET, Tan SY, Suresh S, Lou HX, Cabanayan‐Casasola CB, et al. Lymphocyte recovery after depletion by alemtuzumab in renal transplant recipients: impact on outcome [abstract no: 1015]. American Journal of Transplantation 2005;5(Suppl 11):415. [CENTRAL: CN‐00644284] [Google Scholar]
Cantarovich 2008 {published data only}
- Cantarovich M, Durrbach A, Hiesse C, Benoit G, Charpentier B. Short and long‐term impact of anti‐thymocyte globulin induction on clinical outcomes in renal transplant patients: 15‐year results of a prospective and randomized trial [abstract no: 1034]. American Journal of Transplantation 2002;2(Suppl 3):398. [CENTRAL: CN‐00415382] [Google Scholar]
- Cantarovich M, Durrbach A, Hiesse C, Ladouceur M, Benoit G, Charpentier B. 20‐year follow‐up results of a randomized controlled trial comparing antilymphocyte globulin induction to no induction in renal transplant patients. Transplantation 2008;86(12):1732‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cantarovich M, Durrbach A, Ladouceur M, Hiesse C, Benoit G, Charpentier B. 20 year follow‐up results of a randomized controlled trial comparing anti‐lymphocyte globulin‐induction to no induction in renal transplant patients [abstract no: 535]. American Journal of Transplantation 2008;8(Suppl 2):321. [DOI] [PubMed] [Google Scholar]
- Cantarovich M, Durrbach A, Ladouceur M, Hiesse C, Benoit G, Charpentier B. 20 year follow‐up results of a randomized controlled trial comparing anti‐lymphocyte globulin‐induction to no induction in renal transplant patients [abstract no: 879]. Transplantation 2008;86(2S):307. [DOI] [PubMed] [Google Scholar]
- Durrbach A, Cantarovich M, Hiesse C, Benoir G, Charpentier B. Short and long‐term impact of anti‐lymphocyte globulin induction on clinical outcomes in renal transplant patients: 15‐year results of a prospective and randomized trial [abstract no: 2341]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00420825]
Charpentier 2002 {published data only}
- Charpentier B, European Tacrolimus vs Microemulsified Cyclosporin Study Group. A three arm study comparing immediate tacrolimus therapy with ATG induction therapy followed by either tacrolimus or cyclosporine in adult renal transplant recipients. Transplantation Proceedings 2002;34(5):1625‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Charpentier B, Rostaing L, Berthoux F, Lang P, Civati G, Touraine JL, et al. A three‐arm study comparing immediate tacrolimus therapy with antithymocyte globulin induction therapy followed by tacrolimus or cyclosporine A in adult renal transplant recipients. Transplantation 2003;75(6):844‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rostaing L, Tacrolimus versus Microemulsified Cyclosporin Study Group. Comparison of ATG induction followed by tacrolimus therapy with ATG induction followed by cyclosporine therapy, and immediate tacrolimus‐based triple therapy [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):916A. [CENTRAL: CN‐00433645] [Google Scholar]
Chatterjee 1976 {published data only}
- Chatterjee SN. Antithymocyte globulin in renal transplant recipients. Report of a prospective randomized controlled trial. Archives of Surgery 1976;111(6):680‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ciancio 2005 {published data only}
- Baruqui JA, Ciancio G, Gaynor J, Guerra G, Sageshima J, Chen L, et al. Randomized trial of three induction antibodies in kidney transplantation: long‐term results [abstract no: P‐37]. American Journal of Transplantation 2014;14(Suppl 3):80. [EMBASE: 71388259] [DOI] [PubMed] [Google Scholar]
- Carreno MR, Ciancio G, Burke GW, Rosen A, Ricordi C, Tzakis A, et al. Cellular phenotypes affected by induction therapy with campath‐1h vs thymoglobulin vs zenapax in kidney allograft recipients [abstract]. American Journal of Transplantation 2004;4(Suppl 8):405. [CENTRAL: CN‐00509121] [Google Scholar]
- Ciancio G, Burke GW, Gaynor JJ, Carreno MR, Cirocco RE, Mathew JM, et al. A randomized trial of three renal transplant induction antibodies: early comparison of tacrolimus, mycophenolate mofetil, and steroid dosing, and newer immune‐monitoring. Transplantation 2005;80(4):457‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ciancio G, Burke GW, Gaynor JJ, Mattiazzi AD, Carreno MR, Rosen A, et al. Randomized trial of three different induction regimens to prevent acute renal allograft rejection: early results [abstract]. American Journal of Transplantation 2004;4(Suppl 8):266. [CENTRAL: CN‐00509135] [Google Scholar]
- Ciancio G, Burke GW, Gaynor JJ, Roth D, Kupin W, Rosen A, et al. A randomized trial of thymoglobulin vs. alemtuzumab (with lower dose maintenance immunosuppression) vs. daclizumab in renal transplantation at 24 months of follow‐up. Clinical Transplantation 2008;22(2):200‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ciancio G, Burke GW, Mattiazzi AD, Illanes HG, Gaynor JJ, Carreno M, et al. A randomized trial of three different antibody induction regimens in renal transplantation [abstract no: 1625]. American Journal of Transplantation 2005;5(Suppl 11):569. [CENTRAL: CN‐00644195] [Google Scholar]
- Ciancio G, Gaynor JJ, Guerra G, Sageshima J, Chen L, Mattiazzi A, et al. Randomized trial of three induction antibodies in kidney transplantation: long‐term results. Transplantation 2014;97(11):1128‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ciancio G, Gaynor JJ, Sageshima J, Chen L, Roth D, Kupin W. Machine perfusion in kidney transplantation: better outcomes in the presence of longer pump time [abstract no: 1011]. American Journal of Transplantation 2010;10(Suppl 4):333. [EMBASE: 70464387] [Google Scholar]
- Ciancio G, Gaynor JJ, Sageshima J, Roth D, Kupin W, Guerra G, et al. Machine perfusion following static cold storage preservation in kidney transplantation: donor‐matched pair analysis of the prognostic impact of longer pump time. Transplant International 2012;25(1):34‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ciancio 2010 {published data only}
- Baruqui JA, Ciancio G, Gaynor J, Guerra G, Sageshima J, Chen L, et al. Randomized trial of three induction antibodies in kidney transplantation: long‐term results [abstract no: P‐37]. American Journal of Transplantation 2014;14(Suppl 3):80. [EMBASE: 71388259] [DOI] [PubMed] [Google Scholar]
- Ciancio G, Gaynor JJ, Guerra G, Sageshima J, Chen L, Mattiazzi A, et al. Randomized trial of three induction antibodies in kidney transplantation: long‐term results. Transplantation 2014;97(11):1128‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ciancio G, Gaynor JJ, Roth D, Kupin W, Hanson L, Tueros L, et al. Randomized trial of thymoglobulin versus alemtuzumab (with lower dose maintenance immunosuppression) versus daclizumab in living donor renal transplantation. Transplantation Proceedings 2010;42(9):3503‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cole 1994 {published data only}
- Cole EH, Cattran DC, Farewell VT, Aprile M, Bear RA, Pei YP, et al. A comparison of rabbit antithymocyte serum and OKT3 as prophylaxis against renal allograft rejection. Transplantation 1994;57(1):60‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Condie 1985 {published data only}
- Condie RM, Waskosky KE, Hall BL. Efficacy of Minnesota antilymphoblast globulin in renal transplantation: a multicenter, placebo‐controlled, prospective, randomized, double‐blind study. Transplantation Proceedings 1985;17(1 Pt 2):1304‐10. [EMBASE: 1985078723] [Google Scholar]
Cosimi 1976 {published data only}
- Cosimi AB. The clinical value of antilymphocyte antibodies. Transplantation Proceedings 1981;13(1 Pt 1):462‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Cosimi AB, Wortis HH, Delmonico FL, Russell PS. Randomized clinical trial of antithymocyte globulin in cadaver renal allograft recipients: importance of T cell monitoring. Surgery 1976;80(2):155‐63. [MEDLINE: ] [PubMed] [Google Scholar]
- Wechter WJ, Brodie JA, Morrell RM, Rafi M, Schultz JR. Antithymocyte globulin (ATGAM) in renal allograft recipients. Multicenter trials using a 14‐dose regimen. Transplantation 1979;28(4):294‐302. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Debure 1987 {published data only}
- Debure A, Chkoff N, Chatenoud L, Lacombe M, Campos H, Noel LH, et al. One‐month prophylactic use of OKT3 in cadaver kidney transplant recipients. Transplantation 1988;45(3):546‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Debure A, Chkoff N, Chatenoud L, Lacombe M, Campos H, Noel LH, et al. Preventive treatment of rejection by the prolonged administration of OKT3: decrease of the immune response of the host [Traitement prophylactique du rejet par l'administration prolongee d'OKT3: diminution de la reponse immune de l'hote]. Nephrologie 1987;8(3):87‐94. [MEDLINE: ] [PubMed] [Google Scholar]
De Pauw 1990 {published data only}
- Pauw L, Abramowicz D, Goldman M, Vereerstraeten P, Kinnaert P, Toussaint C. Comparison between prophylactic use of OKT3 and cyclosporine in cadaveric renal transplantation. Transplantation Proceedings 1990;22(4):1759‐60. [MEDLINE: ] [PubMed] [Google Scholar]
Diethelm 1979 {published data only}
- Diethelm AG, Blackstone E, Whelchel JD, Pass RF, Chambers L, Phillips SJ, et al. The adjunctive value of equine antithymocyte membrane globulin in a randomized study of patients undergoing cadaveric renal transplantation. Transplantation Proceedings 1979;11(1):27‐30. [MEDLINE: ] [PubMed] [Google Scholar]
EARTS Study 1999 {published data only}
- Salmela K, Wramner L, Ekberg H, Hauser I, Bentdal O, Lins LE, et al. A randomized multicenter trial of the anti‐ICAM‐1 monoclonal antibody (enlimomab) for the prevention of acute rejection and delayed onset of graft function in cadaveric renal transplantation: a report of the European Anti‐ICAM‐1 Renal Transplant Study Group. Transplantation 1999;67(5):729‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ejaz 2013 {published data only}
- Ejaz N, Shields A, Alloway R, Sadaka B, Girnita A, Mogilishetty G, et al. A prospective, randomized pilot study of B‐cell targeted induction therapy in sensitized kidney transplant recipients: final report [abstract no: 175]. American Journal of Transplantation 2013;13(Suppl S5):84. [EMBASE: 71056751] [DOI] [PubMed] [Google Scholar]
- Ejaz NS, Shields AR, Alloway RR, Sadaka B, Girnita AL, Mogilishetty G, et al. Randomized controlled pilot study of B cell‐targeted induction therapy in HLA sensitized kidney transplant recipients. American Journal of Transplantation 2013;13(12):3142‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schmidt N, Shields AR, Alloway RR, Sadaka B, Mogilishetty G, Kremer J, et al. Randomized controlled trial of B‐cell targeted induction therapy in HLA sensitized kidney transplant recipients: preliminary results [abstract no: 100]. American Journal of Transplantation 2012;12(Suppl S3):56. [EMBASE: 70746047] [DOI] [PubMed] [Google Scholar]
Farney 2008 {published data only}
- Doares W, Ashcraft E, Singh R, Hart L, Farney A, Hartmann E, et al. Prospective, randomized, single‐center trial of alemtuzumab vs rabbit anti‐thymocyte globulin induction in renal and pancreas transplant: infectious complications update [abstract no: 315]. American Journal of Transplantation 2009;9(Suppl 2):282‐3. [EMBASE: 70010188] [Google Scholar]
- Farney A, Rogers J, Doares W, Iskandar S, Orlando G, Adams P, et al. 7 year results of a prospective randomized study of alemtuzumab vs rabbit anti‐thymocyte globulin induction in kidney and kidney pancreas transplantation [abstract]. Transplantation 2014;98(Suppl 1):90. [EMBASE: 71543817] [Google Scholar]
- Farney A, Rogers J, Hart L, Doares W, Iskandar S, Orlando G, et al. Long‐term results of a prospective randomized study of alemtuzumab vs rabbit anti‐thymocyte globulin induction in kidney and kidney pancreas transplantation [abstract no: 101]. American Journal of Transplantation 2012;12(Suppl S3):56. [EMBASE: 70746048] [Google Scholar]
- Farney A, Singh R, Rogers J, Ashcroft E, Hartmann E, Hart L, et al. A prospective randomized trial of alemtuzumab versus rabbit anti‐thymocyte globulin induction in kidney and pancreas transplantation: minimum 6 months follow up [abstract no: 796]. Transplantation 2008;86(2S):278. [CENTRAL: CN‐00740550] [Google Scholar]
- Farney A, Sundberg A, Moore P, Hartmann E, Rogers J, Doares W, et al. A randomized trial of alemtuzumab vs. anti‐thymocyte globulin induction in renal and pancreas transplantation. Clinical Transplantation 2008;22(1):41‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Farney AC, Doares W, Rogers J, Singh R, Hartmann E, Hart L, et al. A randomized trial of alemtuzumab versus antithymocyte globulin induction in renal and pancreas transplantation. Transplantation 2009;88(6):810‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Farney AC, Singh R, Rogers J, Ashcroft E, Hartmann E, Hart L, et al. A prospective randomized study of alemtuzumab vs rabbit anti‐thymocyte globulin induction in kidney and pancreas transplantation [abstract no: 532]. American Journal of Transplantation 2008;8(Suppl 2):320. [CENTRAL: CN‐00653719] [Google Scholar]
- Hartmann EL, Doares W, Reeves‐Daniel A, Rogers J, Singh R, Hart L, et al. Safety and efficacy of lymphocyte‐depleting induction therapy in older transplant recipients: should grandpa receive campath? [abstract no: 1755]. American Journal of Transplantation 2009;9(Suppl 2):675. [CENTRAL: CN‐00795687] [Google Scholar]
- Singh R, Farney A, Rogers J, Doares W, Hartmann E, Reeves‐Daniel A, et al. A randomized, prospective trial of alemtuzumab versus rabbit anti‐thymocyte globulin induction in kidney‐pancreas transplantation: a single center experience [abstract no: 87]. American Journal of Transplantation 2009;9(Suppl 2):216. [CENTRAL: CN‐00790946] [Google Scholar]
- Stratta R, Ashcraft E, Hartmann E, Rogers J, Doares W, Hart L, et al. A prospective randomized comparison of alemtuzumab versus rabbit anti‐thymocyte globulin induction in renal and pancreas transplantation [abstract no: P132]. Transplant International 2007;20(Suppl 2):127. [CENTRAL: CN‐00740553] [Google Scholar]
- Stratta R, Rogers J, Orlando G, Farooq U, Al‐Shraideh Y, Doares W, et al. 5 year results of a prospective, randomized single center study of alemtuzumab compared to rabbit anti‐thymocyte globulin induction in simultaneous kidney‐pancreas transplantation [abstract]. Transplantation 2014;98(Suppl 1):215. [EMBASE: 71544175] [Google Scholar]
- Stratta R, Singh R, Farney A, Rogers J, Doares W, Hartman E. A randomized, prospective trial of alemtuzumab versus rabbit anti‐thymocyte globulin induction in kidney‐pancrease transplantation: a single center experience [abstract no: O‐289]. Transplant International 2009;22(Suppl 2):76‐7. [Google Scholar]
- Stratta RJ, Rogers J, Hart L, Doares W, Kaczmorski S, Reeves‐Daniel A, et al. 5 year results of a prospective randomized single center study of alemtuzumab compared to rabbit anti‐thymocyte globulin induction in kidney‐pancreas transplantation [abstract]. Transplantation 2013;96(Suppl 6):S86. [EMBASE: 71249227] [Google Scholar]
- Stratta RJ, Rogers J, Orlando G, Farooq U, Al‐Shraideh Y, Doares W, et al. Depleting antibody induction in simultaneous pancreas‐kidney transplantation: a prospective single‐center comparison of alemtuzumab versus rabbit anti‐thymocyte globulin. Expert Opinion on Biological Therapy 2014;14(12):1723‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stratta RJ, Rogers J, Orlando G, Farooq U, Al‐Shraideh Y, Farney AC. 5‐year results of a prospective, randomized, single‐center study of alemtuzumab compared with rabbit antithymocyte globulin induction in simultaneous kidney‐pancreas transplantation. Transplantation Proceedings 2014;46(6):1928‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Frey 1991 {published data only}
- Frey DJ, Matas AJ, Gillingham KJ, Canafax D, Payne WD, Dunn DL, et al. MALG vs OKT3 following renal transplantation: a randomized prospective trial. Transplantation Proceedings 1991;23(1 Pt 2):1048‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Frey DJ, Matas AJ, Gillingham KJ, Canafax D, Payne WD, Dunn DL, et al. Sequential therapy‐‐a prospective randomized trial of MALG versus OKT3 for prophylactic immunosuppression in cadaver renal allograft recipients. Transplantation 1992;54(1):50‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Friend 1987 {published data only}
- Friend PJ, Calne RY, Hale G, Waldmann H, Evans DB, Rolles K, et al. Prophylactic use of an antilymphocyte monoclonal antibody following renal transplantation: a randomized controlled trial. Transplantation Proceedings 1987;19(1 Pt 3):1898‐900. [MEDLINE: ] [PubMed] [Google Scholar]
- Friend PJ, Hale G, Waldmann H, Gore S, Thiru S, Joysey V, et al. Campath‐1M‐‐prophylactic use after kidney transplantation. A randomized controlled clinical trial. Transplantation 1989;48(2):248‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Friend PJ, Waldmann H, Hale G, Tighe H, Calne R. The use of anti‐lymphocyte monoclonal antibodies following organ transplantation [abstract]. Nephrology Dialysis Transplantation 1988;3(1):97‐8. [CENTRAL: CN‐00796675] [Google Scholar]
Fries 1988a {published data only}
- Fries D. Optimal results in cadaver renal transplantation using prophylactic ALG, cyclosporin (CsA) and prednisone (P) [abstract]. Nephrology Dialysis Transplantation 1988;3(1):95. [CENTRAL: CN‐00260355] [PubMed] [Google Scholar]
- Fries D, Hiesse C, Charpentier B, Lantz O, Bensadoun H, Benoit G. A single center experience with "low‐dose" cyclosporine in cadaveric renal transplantation. Clinical Transplants 1988:115‐29. [MEDLINE: ] [PubMed] [Google Scholar]
Fukuuchi 1996 {published data only}
- Fukuuchi F, Lefrancois N, Bosshard S, Chapuis F, Dubernard JM, Touraine JL. Comparison of prophylactic OKT3 versus ATG in immunologic high risk cadaver renal transplant recipients [abstract]. Journal of the American Society of Nephrology 1995;6(3):1084. [CENTRAL: CN‐00484030] [Google Scholar]
- Fukuuchi F, Lefrancois N, Chapuis F, Gebuhrer L, Bosshard S, Dubernard JM, et al. Comparative efficacy of prophylactic monoclonal (OKT3) and polyclonal antibodies (ATG) in immunologic high‐risk renal transplant recipients. Transplantation Proceedings 1996;28(5):2808‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Gianello 1987 {published data only}
- Gianello P, Squifflet JP, Pirson Y, Stoffel M, Dereme T, Alexandre GP. Cyclosporine‐steroids versus conventional therapy in cadaver kidney transplantation: analysis of a randomized trial at two years. Transplantation Proceedings 1987;19(1 Pt 3):1867‐72. [MEDLINE: ] [PubMed] [Google Scholar]
Grafals 2014 {published data only}
- Grafals M, Simpson M, Gilligan H, Pomposelli J, Akoad M, Kwaja K, et al. Prospective randomized study of low dose antithymocyte globulin as induction in non sensitized adult renal transplant recipients [abstract no: C1351]. American Journal of Transplantation 2013;13(Suppl 5):430. [EMBASE: 71057927] [Google Scholar]
- Grafals M, Smith B, Murakami N, Trabucco A, Hamill K, Marangos E, et al. Immunophenotyping and efficacy of low dose ATG in non‐sensitized kidney recipients undergoing early steroid withdrawal: a randomized pilot study. PLoS ONE [Electronic Resource] 2014;9(8):e104408. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B, Grafals M, Murakami N, Trabucco A, Hamill K, Marangos E, et al. Immune phenotyping and efficacy of low dose ATG in non‐sensitized kidney recipients undergoing early steroid withdrawal: a randomized pilot study [abstract]. Transplantation 2014;98(Suppl 1):580. [EMBASE: 71545491] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grino 1990 {published data only}
- Grino JM, Alsina J, Sabater R, Castelao AM, Gil‐Vernet S, Andres E, et al. Antilymphoblast globulin, cyclosporine, and steroids in cadaveric renal transplantation. Transplantation 1990;49(6):1114‐7. [2360253] [DOI] [PubMed] [Google Scholar]
- Koga A, Moreso FJ, Seron D, Gil‐Vernet S, Cruzado JM, Castelao AM, et al. Beneficial effect of concomitant induction with antilymphoblast globulin, cyclosporine, and steroids on long‐term renal allograft outcome. Transplantation Proceedings 2004;36(5):1305‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grino 1991 {published data only}
- Gonzalez C, Grino JM, Castelao AM, Seron D, Gil‐Vernet S, Andres E, et al. Pre‐transplant ALG, low dose cyclosporine (CsA) and steroids versus pre‐transplant OKT3, CsA and steroids in kidney cadaveric transplantation [abstract]. Kidney International 1990;37(6):1601. [CENTRAL: CN‐00601919] [Google Scholar]
- Grino JM, Castelao AM, Gonzalez C, Seron D, Gil‐Vernet S, Andres E, et al. Pre‐transplant ALG, low dose cyclosporine (CYA) and steroids in kidney cadaveric transplantation [abstract]. 11th International Congress of Nephrology; 1990 Jul 15‐20; Tokyo, Japan. 1990:513A. [CENTRAL: CN‐00601920]
- Grino JM, Castelao AM, Seron D, Gonzalez C, Galceran JM, Gil‐Vernet S, et al. Antilymphocyte globulin versus OKT3 induction therapy in cadaveric kidney transplantation: a prospective randomized study. American Journal of Kidney Diseases 1992;20(6):603‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grino JM, Castelao AM, Seron D, Gonzalez C, Galceran JM, Gil‐Vernet S, et al. Prophylactic OKT3, CyA, and steroids versus antilymphoblast globulin, CyA, and steroids in cadaveric kidney transplantation. Transplantation Proceedings 1992;24(1):39‐41. [MEDLINE: ] [PubMed] [Google Scholar]
- Grino JM, Castelao AM, Seron D, Gonzalez C, Gil‐Vernet S, Andres E, et al. Antilymphocyte serum, cyclosporine and corticoids, versus OKT3, cyclosporine, and corticoids in kidney transplantation [Serum anti‐lymphocyte, ciclosporine et corticoides, versus OKT3, ciclosporine et corticoides en transplantation renale]. Presse Medicale 1991;20(40):2039‐42. [MEDLINE: ] [PubMed] [Google Scholar]
- Mestre M, Gonzalez C, Grino JM, Valls A, Bonete J, Mane E, et al. Sequential monitoring of immunoregulatory T cell subsets in renal transplantation. Transplantation Proceedings 1992;24(1):73‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Grundmann 1984 {published data only}
- Grundmann R. Infectious diseases under prophylactic ALG treatment and their prevention in a prospectively randomized trial. Scandinavian Journal of Urology & Nephrology Supplementum 1985;92:33‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Grundmann R, Wienand P, Hesse U. Improvement in cyclosporine handling by anti‐lymphocyte globulin in the early post‐operative period. Proceedings of the European Dialysis & Transplant Association ‐ European Renal Association 1985;21:987‐91. [MEDLINE: ] [PubMed] [Google Scholar]
- Grundmann R, Wienand P, Hesse U. Improvement of the cyclosporin A handling by ALG in the early postoperative period [abstract]. Kidney International 1984;26(4):637. [CENTRAL: CN‐00601926] [Google Scholar]
- Grundmann R, Wienand P, Meider G, Vlaho V, Pichlmaier H. Use and limits of preventive antilymphocyte globulin therapy following kidney transplantation. A prospective randomized study [Nutzen und grenzen einer prophylaktischen antilymphozytenglobulin‐therapie nach nierentransplantation. Eine prospektiv randomisierte studie]. Klinische Wochenschrift 1984;62(20):979‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grundmann 1987 {published data only}
- Grundmann R, Hesse U, Wienand P, Baldamus C, Arns W. Graft survival and long‐term renal function after sequential conventional cyclosporin A therapy in cadaver kidney transplantation‐‐a prospective randomized trial. Klinische Wochenschrift 1987;65(18):879‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grundmann R, Wienand P, Hesse U. Sequential conventional and cyclosporine therapy in cadaver renal transplantation‐‐a prospective randomized trial. Transplantation Proceedings 1987;19(5):4033‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Guttmann 1997 {published data only}
- Guttmann RD, TEAMS1 Group (Transplant European Antilfa Multicenter Study Group). Randomized clinical trial of anti‐lfa‐1 monoclonal antibody in cadaveric renal transplantation: a European multicenter study [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:258. [CENTRAL: CN‐00509222]
Halloran 1982 {published data only}
- Halloran P, Ludwin D, Aprile M. Randomized comparison between cyclosporine and conventional therapy plus Minnesota antilymphocyte globulin in cadaveric renal transplantation. Transplantation Proceedings 1983;15(4 Suppl 1‐2):2513‐6. [EMBASE: 1984089086] [Google Scholar]
- Halloran PF, Lien J, Aprile M, White N. Preliminary results of a randomized comparison of cyclosporine and Minnesota antilymphoblast globulin. Transplantation Proceedings 1982;14(4):627‐30. [MEDLINE: ] [PubMed] [Google Scholar]
Hanaway 2011 {published data only}
- Hanaway M, Woodle ES, Mulgaonkar S, Peddi R, Harrison G, Vandeputte K, et al. 12 month results of a multicenter, randomized trial comparing three induction agents (alemtuzumab, thymoglobulin and basiliximab) with tacrolimus, mycophenolate mofetil and a rapid steroid withdrawal in renal transplantation [abstract no: 135]. American Journal of Transplantation 2008;8(Suppl 2):215. [Google Scholar]
- Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi VR, Kaufman DB, First MR, et al. Alemtuzumab induction in renal transplantation. New England Journal of Medicine 2011;364(20):1909‐19. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Holman J, Harrison G, Vandeputte K, First R, Fitzsimmons W. Immune cell activation comparing three induction agents (alemtuzumab, thymoglobulin and basiliximab) with tacrolimus, mycophenolate mofetil and a rapid steroid withdrawal in renal transplantation [abstract no: 553]. Transplantation 2008;86(2 Suppl):194. [CENTRAL: CN‐00676047] [Google Scholar]
- Mulgaonkar S, Hanaway M, Woodle ES, Peddi R, Harrison G, Vandeputte K, et al. Continuing 24 month results of a multicenter, randomized trial comparing three induction agents (alemtuzumab, thymoglobulin and basiliximab) with tacrolimus, mycophenolate mofetil and a rapid steroid withdrawal in renal transplantation [abstract no: 312]. American Journal of Transplantation 2009;9(Suppl 2):282. [EMBASE: 70010185] [Google Scholar]
- Peddi R, Hanaway M, Woodle S, Mulgaonkar S, Harrison G, Vandeputte K, et al. Final 36 month results of a randomized trial comparing three induction agents (alemtuzumab, thymoglobulin and basiliximab) with tacrolimus, mycophenolate mofetil and rapid steroid withdrawal in renal transplantation [abstract no: 35]. American Journal of Transplantation 2010;10(Suppl 4):49. [EMBASE: 70463396] [Google Scholar]
- Woodle S, Hanaway M, Mulgaonkar S, Peddi R, Harrison G, Vandeputte K, et al. 12 month results of a multicenter, randomized trial comparing three induction agents (alemtuzumab, thymoglobulin and basiliximab) with tacrolimus, mycophenolate mofetil and a rapid steroid withdrawal in renal transplantation [abstract no: 876]. Transplantation 2008;86(2 Suppl):306. [CENTRAL: CN‐00653740] [Google Scholar]
Hanto 1991 {published data only}
- Hanto DW, Jendrisak MD, McCullough CS, So SK, Marsh JW, Rush T, et al. A prospective randomized comparison of prophylactic ALG and OKT3 in cadaver kidney allograft recipients. Transplantation Proceedings 1991;23(1 Pt 2):1050‐1. [MEDLINE: ] [PubMed] [Google Scholar]
- Hanto DW, Jendrisak MD, So SK, McCullough CS, Rush TM, Michalski SM, et al. Induction immunosuppression with antilymphocyte globulin or OKT3 in cadaver kidney transplantation. Results of a single institution prospective randomized trial. Transplantation 1994;57(3):377‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Henry 2001 {published data only}
- Henry ML, Elkhammas EA, Bumgardner G, Davies EA, Pelletier RP, et al. A prospective randomized trial of neoral and cellcept with and without OKT3 induction [abstract]. Transplantation 1998;65(12):S191. [CENTRAL: CN‐00445692] [Google Scholar]
- Henry ML, Pelletier RP, Elkhammas EA, Bumgardner GL, Davies EA, Ferguson RM. A randomized prospective trial of OKT3 induction in the current immunosuppression era. Clinical Transplantation 2001;15(6):410‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hourmant 1985a {published data only}
- Hourmant M, Soulillou JP, Guenel J. Comparison of three immuno‐suppressive regimens in kidney transplantation: CyA, ATG and CyA, ATG and conventional treatment ‐ a one‐center randomized study [abstract]. Kidney International 1984;26(4):639. [CENTRAL: CN‐00626072] [Google Scholar]
- Hourmant M, Soulillou JP, Guenel J. Comparison of three immunosuppressive regimens in kidney transplantation: a single‐centre randomised study. Proceedings of the European Dialysis & Transplant Association ‐ European Renal Association 1985;21:982‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Hourmant M, Soulillou JP, Guenel J. Comparison of three immunosuppressive strategies in kidney transplantation: Antithymocyte globulin and conventional treatment, antithymocyte globulin and cyclosporine, and cyclosporine. A one‐center randomized study. Transplantation Proceedings 1985;17(1 Pt 2):1158‐61. [EMBASE: 1985079646] [Google Scholar]
Hourmant 1996 {published data only}
- Hourmant M. Multicenter comparative study of an anti‐lfa1 adhesion molecule monoclonal antibody and thymoglobulin in prophylaxis of acute rejection in kidney transplantation. [abstract]. ISN XIII International Congress of Nephrology; 1995 Jul 2‐6; Madrid, Spain. 1995:336. [CENTRAL: CN‐00509239]
- Hourmant M, Bedrossian J, Durand D, Kessler M, Branchu Y, Caudrelier P, et al. Multicenter comparative study of an anti‐LFA‐1 adhesion molecule monoclonal antibody and antithymocyte globulin in prophylaxis of acute rejection in kidney transplantation. Transplantation Proceedings 1995;27(1):864. [MEDLINE: ] [PubMed] [Google Scholar]
- Hourmant M, Bedrossian J, Durand D, Kessler M, Lebranchu Y, Caudrelier P, et al. Multicenter study of an anti‐LFA1 adhesion molecule monoclonal antibody (Moab) and anti‐thymocyte globulin (ATG) in prophylaxis of acute rejection (AR) in kidney transplantation (KT) [abstract]. 14th Annual Meeting. American Society of Transplant Physicians (ASTP); 1995 May 14‐17; Chicago (ILL). 1995.
- Hourmant M, Bedrossian J, Durand D, Lebranchu Y, Renoult E, Caudrelier P, et al. A randomized multicenter trial comparing leukocyte function‐associated antigen‐1 monoclonal antibody with rabbit antithymocyte globulin as induction treatment in first kidney transplantations. Transplantation 1996;62(11):1565‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jakobsen 1981 {published data only}
- Jakobsen A, Flatmark A, Lundgren G, Solheim B, Groth CG. A controlled trial with AHLG Behring: lack of effect on cadaveric renal graft survival. Scandinavian Journal of Urology & Nephrology Supplementum 1981;64:205‐12. [MEDLINE: ] [PubMed] [Google Scholar]
Kasiske 1997 {published data only}
- Kasiske BL, Goerdt PJ, Heim‐Duthoy K, Rao KV, Dahl DC, Ney AL, et al. Interim results of a randomized controlled trial comparing antithymocyte globulin (ATG) with cyclosporine (CSA) induction [abstract]. Journal of the American Society of Nephrology 1995;6(3):1097. [CENTRAL: CN‐00484599] [Google Scholar]
- Kasiske BL, Johnson HJ, Goerdt PJ, Heim‐Duthoy KL, Rao VK, Dahl DC, et al. A randomized trial comparing cyclosporine induction with sequential therapy in renal transplant recipients. American Journal of Kidney Diseases 1997;30(5):639‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Khosroshahi 2008 {published data only}
- Khosroshahi HT, Tubbs RS, Shoja MM, Ghafari A, Noshad H, Ardalan MR. Effect of prophylaxis with low‐dose anti‐thymocyte globulin on prevention of acute kidney allograft rejection. Transplantation Proceedings 2008;40(1):137‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kountz 1977 {published data only}
- Butt KM, Zielinski CM, Parsa I, Elberg AJ, Wechter W, Kountz SL. Trends in immunosuppression for kidney transplantation. Kidney International ‐ Supplement 1978;13(Suppl 8):S95‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Kountz SL, Butt KH, Rao TK, Zielinski CM, Rafi M, Schultz JR. Antithymocyte globulin (ATG) dosage and graft survival in renal transplantation. Transplantation Proceedings 1977;9(1):1023‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Kreis 1980 {published data only}
- Crosnier J, Kreis H, Descamps JM, Mansouri R. Are there non‐steroid‐dependent rejection episodes?. Proceedings of the European Dialysis & Transplant Association 1980;17:391‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Kreis H, Mansouri R, Descamps JM, Dandavino R, N'Guyen AT, Bach JF, et al. Antithymocyte globulin in cadaver kidney transplantation: a randomized trial based on T‐cell monitoring. Kidney International 1981;19(3):438‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kreis 1986 {published data only}
- Kreis H, Chkoff N, Chatenoud L. Prolonged administration of a monoclonal anti‐T3 cell antibody (ORTHOCLONE OKT3) to kidney allograft recipients. Transplantation Proceedings 1986;18(4):954‐6. [EMBASE: 1986223864] [Google Scholar]
Kumar 1998a {published data only}
- Kumar MS, Cahill K, Kumar AM, Panigrahi D, Seirka D, Singleton R, et al. ATGAM versus OKT3 induction therapy in cadaveric kidney transplantation: patient and graft survival, CD3 subset, infection, and cost analysis. Transplantation Proceedings 1998;30(4):1351‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kumar MS, Laskow DA, Panigrahi D, Kumar AM, Cahill K, Pankewyez O, et al. Correlation of CD3 counts and serum IL‐10 levels during induction to incidence of rejections and infections in cadaveric kidney recipients [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):683A. [CENTRAL: CN‐00446225] [Google Scholar]
Launois 1977 {published data only}
- Launois B, Campion JP, Fauchet R, Kerbaol M, Cartier F. Prospective randomized clinical trial in patients with cadaver‐kidney transplants. Transplantation Proceedings 1977;9(1):1027‐30. [MEDLINE: ] [PubMed] [Google Scholar]
Lazarovits 1993 {published data only}
- Lazarovits AI, Rochon J, Banks L, Hollomby DJ, Muirhead N, Jevnikar AM, et al. Human mouse chimeric CD7 monoclonal antibody (SDZCHH380) for the prophylaxis of kidney transplant rejection. Journal of Immunology 1993;150(11):5163‐74. [MEDLINE: ] [PubMed] [Google Scholar]
- Lazarovits AI, Rochon J, Banks L, Hollomby DJ, Muirhead N, Jevnikar AM, et al. Human mouse chimeric CD7 monoclonal antibody (SDZCHH380) for the prophylaxis of kidney transplant rejection. Transplantation Proceedings 1993;25(1 Pt 1):820‐2. [MEDLINE: ] [PubMed] [Google Scholar]
- Sharma L, Muirhead N, Lazarovits AI. Human mouse chimeric Cd7 monoclonal antibody (SCZCHH380) for the prophylaxis of kidney transplant rejection: no transplant losses and absence of chronic rejection at 4 years [abstract]. 15th Annual Meeting American Society of Transplant Physicians (ASTP); 1996 May 10‐14; Chicago Ill. 1996.
Lu 2011 {published data only}
- Lu TM, Yang SL, Wu WZ, Tan JM. Alemtuzumab induction therapy in highly sensitized kidney transplant recipients. Chinese Medical Journal 2011;124(5):664‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Maiorca 1984 {published data only}
- Maiorca R, Cristinelli L, Scolari S, Sandrini S, Brunori G, Tonini G, et al. Prospective controlled trial with antilynfocytic globulin (A.L.G.), in first cadaveric renal transplants treated with low‐dose steroids both in prophylaxis and rejection therapy [abstract]. Kidney International 1984;26(4):644. [CENTRAL: CN‐00775915] [Google Scholar]
Margreiter 2008 {published data only}
- Margreiter R, Klempnauer J, Neuhaus P, Muehlbacher F. Alemtuzumab (Campath‐1H) induction followed by tacrolimus monotherapy vs tacrolimus based triple drug immunosuppression in cadaveric renal transplantation ‐ results of a multicenter trial [abstract no: 333]. American Journal of Transplantation 2007;7(Suppl 2):234. [CENTRAL: CN‐00644175] [Google Scholar]
- Margreiter R, Klempnauer J, Neuhaus P, Muehlbacher F, Boesmueller C, Calne RY. Alemtuzumab (Campath‐1H) and tacrolimus monotherapy after renal transplantation: results of a prospective randomized trial. American Journal of Transplantation 2008;8(7):1480‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Martins 2004 {published data only}
- Martins LS, Sarmento AM, Dias L, Henriques AC, Borner G, Cabrita A. Results of safety and efficacy of two ATG bolus‐therapy regimen vs standard therapy in prophylaxis after renal transplantation in end stage renal disease patients [abstract]. Transplantation 2004;78(2 Suppl):266. [CENTRAL: CN‐00509341] [Google Scholar]
Michael 1989 {published data only}
- Michael H, Francos G, Burke J, Besarab A, Jarrell B, Moritz M, et al. Effect of cyclosporine (CYA) versus antilymphocyte globulin (ALG) on delayed graft function (DGF) in renal transplant patients [abstract]. Kidney International 1989;35(1):520. [CENTRAL: CN‐00626110] [DOI] [PubMed] [Google Scholar]
- Michael HJ, Francos GC, Burke JF, Besarab A, Moritz M, Gillum D, et al. A comparison of the effects of cyclosporine versus antilymphocyte globulin on delayed graft function in cadaver renal transplant recipients. Transplantation 1989;48(5):805‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Minnesota Study 1982 {published data only}
- Boudreaux JP, McHugh L, Canafax DM, Ascher N, Sutherland DE, Payne W, et al. The impact of cyclosporine and combination immunosuppression on the incidence of posttransplant diabetes in renal allograft recipients. Transplantation 1987;44(3):376‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Canafax DM, Min DI, Gruber SA, Matas AJ, Payne WD, Dunn DL, et al. Immunosuppression for cadaveric renal allograft recipients: a risk‐factor matched comparison of the Minnesota randomized trial with an antilymphoblast globulin, azathioprine, cyclosporine, and prednisone protocol. Clinical Transplantation 1989;3(2):110‐9. [EMBASE: 1989138098] [Google Scholar]
- Canafax DM, Simmons RL, Sutherland DE, Fryd DS, Strand MH, Ascher NL, et al. Early and late effects of two immunosuppressive drug protocols on recipients of renal allografts: results of the Minnesota randomized trial comparing cyclosporine versus antilymphocyte globulin‐azathioprine. Transplantation Proceedings 1986;18(2 Suppl 1):192‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Canafax DM, Torres A, Fryd DS, Heil JE, Strand MH, Ascher NL, et al. The effects of delayed function on recipients of cadaver renal allografts. A study of 158 patients randomized to cyclosporine or ALG‐azathioprine. Transplantation 1986;41(2):177‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ferguson RM, Rynasiewicz JJ, Sutherland DE, Simmons RL, Najarian JS. Cyclosporin A in renal transplantation: a prospective randomized trial. Surgery 1982;92(2):175‐82. [MEDLINE: ] [PubMed] [Google Scholar]
- Frick T, Fryd DS, Goodale RL, Simmons RL, Sutherland DE, Najarian JS. Incidence and treatment of candida esophagitis in patients undergoing renal transplantation. Data from the Minnesota prospective randomized trial of cyclosporine versus antilymphocyte globulin‐azathioprine. American Journal of Surgery 1988;155(2):311‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Frick TW, Fryd DS, Goodale RL, Simmons RL, Sutherland DE, Najarian JS. Lack of association between azathioprine and acute pancreatitis in renal transplantation patients. Lancet 1991;337(8735):251‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Frick TW, Fryd DS, Sutherland DE, Goodale RL, Simmons RL, Najarian JS. Hypercalcemia associated with pancreatitis and hyperamylasemia in renal transplant recipients. Data from the Minnesota randomized trial of cyclosporine versus antilymphoblast azathioprine. American Journal of Surgery 1987;154(5):487‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gores PF, Fryd DS, Sutherland DE, Najarian JS, Simmons RL. Hyperuricemia after renal transplantation. American Journal of Surgery 1988;156(5):397‐400. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gruber S, Pescovitz M, Simmons R, Fryd D. Cyclosporine use lowers risk of thromboembolism in diabetic renal allograft recipients [abstract]. Kidney International 1987;31(1):458. [CENTRAL: CN‐00550666] [Google Scholar]
- Gruber SA, Chavers B, Payne WD, Fryd DS, Canafax DM, Simmons RL, et al. Allograft renal vascular thrombosis‐‐lack of increase with cyclosporine immunosuppression. Transplantation 1989;47(3):475‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gruber SA, Chavers B, Payne WD, Fryd DS, Canafax DM, Simmons RL, et al. Frequency of allograft renal vascular thrombosis under three immunosuppressive regimens at a single institution. Transplantation Proceedings 1989;21(1 Pt 2):2139‐40. [MEDLINE: ] [PubMed] [Google Scholar]
- Gruber SA, Pescovitz M, Simmons RL, Najarian JS, Ascher NL, Payne WD, et al. Thromboembolic complications after renal transplantation: results from the randomized trial of cyclosporine v azathioprine‐antilymphocyte globulin. Transplantation Proceedings 1987;19(1 Pt 2):1815‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Gruber SA, Pescovitz MD, Simmons RL, Najarian JS, Ascher NL, Payne WD, et al. Thromboembolic complications in renal allograft recipients. A report from the prospective randomized study of cyclosporine versus azathioprine‐antilymphocyte globulin. Transplantation 1987;44(6):775‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gruber SA, Simmons RL, Najarian JS, Vercellotti G, Ascher NL, Dunn DL, et al. Erythrocytosis and thromboembolic complications after renal transplantation: results from a randomized trial of cyclosporine versus azathioprine‐antilymphocyte globulin. Transplantation Proceedings 1988;20(3 Suppl 3):948‐50. [MEDLINE: ] [PubMed] [Google Scholar]
- Hesse UJ, Fryd DS, Chatterjee SN, Simmons RL, Sutherland DE, Najarian JS. Pulmonary infections. The Minnesota randomized prospective trial of cyclosporine vs azathioprine‐antilymphocyte globulin for immunosuppression in renal allograft recipients. Archives of Surgery 1986;121(9):1056‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Johnson CP, Simmons RL, Sutherland DE, Canafax DM, Ascher NL, Payne WD, et al. A randomized trial comparing cyclosporine with antilymphoblast‐globulin‐azathioprine for renal allograft recipients. Results at 2 1/2‐6 years. Transplantation 1988;45(2):380‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Najarian JS, Ferguson RM, Sutherland DE. A prospective trial of the efficacy of cyclosporine in renal transplantation at the University of Minnesota. Transplantation Proceedings 1983;15(1):438‐41. [EMBASE: 1983156214] [Google Scholar]
- Najarian JS, Fryd DS, Strand M, Canafax DM, Ascher NL, Payne WD, et al. A single institution, randomized, prospective trial of cyclosporin versus azathioprine‐antilymphocyte globulin for immunosuppression in renal allograft recipients. Annals of Surgery 1985;201(2):142‐57. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescovitz MD, Gruber SA, Ascher NL, Kruse LV, Najarian JS, Payne WD, et al. Frequency of diabetes‐related complications in renal allograft recipients prospectively randomized to cyclosporine or azathioprine. Transplantation Proceedings 1987;19(1 Pt 2):1537‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Rynasiewicz JJ, Sutherland DE, Ferguson RM, Squifflet JP, Morrow CE, Goetz FC, et al. Cyclosporin A for immunosuppression: observations in rat heart, pancreas, and islet allograft models and in human renal and pancreas transplantation. Diabetes 1982;31 Suppl 4:92‐108. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Simmons RL, Canafax DM, Strand M, Ascher NL, Payne WD, Sutherland DE, et al. Management and prevention of cyclosporine nephrotoxicity after renal transplantation: use of low doses of cyclosporine, azathioprine, and prednisone. Transplantation Proceedings 1985;17(4 Suppl 1):266‐75. [MEDLINE: ] [PubMed] [Google Scholar]
- Sutherland DE, Fryd DS, Strand M, Ascher N, Simmons RL, Najarian JS. Results of renal transplantation in diabetics at the University of Minnesota since 1979, including a comparison of outcome in diabetic and nondiabetic recipients randomized to cyclosporine versus azathioprine for immunosuppression. Transplantation Proceedings 1984;16(3):629‐32. [MEDLINE: ] [PubMed] [Google Scholar]
- Sutherland DE, Fryd DS, Strand MH, Canafax DM, Ascher NL, Payne WD, et al. Results of the Minnesota randomized prospective trial of cyclosporine versus azathioprine‐antilymphocyte globulin for immunosuppression in renal allograft recipients. American Journal of Kidney Diseases 1985;5(6):318‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sutherland DE, Payne WD, Fryd DS. Comparison of cyclosporine and azathioprine for immunosuppression in diabetic and nondiabetic renal allograft recipients. Transplantation Proceedings 1985;17(1 Pt 2):1204‐11. [EMBASE: 1985079661] [Google Scholar]
- Sutherland DE, Strand M, Fyd DS, Ferguson RM, Simmons RL, Ascher NL, et al. Comparison of azathioprine‐antilymphocyte globulin versus cyclosporine in renal transplantation. American Journal of Kidney Diseases 1984;3(6):456‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morales 1994a {published data only}
- Anonymous. Cyclosporine monotherapy versus OKT3 and cyclosporine versus prednisone and cyclosporine as induction therapy in older renal transplant patients: a multicenter randomized study. Spanish Monotherapy Study Group. Transplantation Proceedings 1994;26(5):2522‐4. [MEDLINE: ] [PubMed] [Google Scholar]
- Morales JM, Marcen R, Grino JM, Arias M, Vilardell J. Comparison of three induction protocols in older renal transplant patients: cyclosporine (CYA) monotherapy (M) vs OKT3 and CYNA vs prednisone (P) and CYA. A multicenter randomized study [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):950. [CENTRAL: CN‐00485149] [Google Scholar]
Mourad 1998 {published data only}
- Anonymous. Tacrolimus in renal transplantation: a comparison of induction vs noninduction therapy (triple therapy): three‐month results. Transplantation Proceedings 1999;31(1‐2):330‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Antoine C, Thakur S, Daugas E, Fraoui R, Boudjeltia S, Julia P, et al. Vascular microthrombosis in renal transplant recipients treated with tacrolimus. Transplantation Proceedings 1998;30(6):2813‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Charpentier B. An induction versus no‐induction protocol in anticalcineurin‐based immunosuppression using very low‐dose steroids. Transplantation Proceedings 2001;33(4 Suppl):3S‐10S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Charpentier B. Induction versus non‐induction protocols in anti‐calcineurin‐based immunosuppression. Transplantation Proceedings 2001;33(7‐8):3334‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mourad G, French and Belgian Tacrolimus Renal Transplantation Study Group. ATG‐Induction vs. non‐induction with tacrolimus‐based immunosuppression in renal transplantation [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000. [CENTRAL: CN‐00446848]
- Mourad G, Garrigue V, Squifflet JP, Besse T, Berthoux F, Alamartine E, et al. Induction versus noninduction in renal transplant recipients with tacrolimus‐based immunosuppression. Transplantation 2001;72(6):1050‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Niaudet 1990 {published data only}
- Niaudet P, Murcia I, Jean G, Broyer M. A comparative trial of OKT3 and antilymphocyte serum in the preventive treatment of rejection after kidney transplantation in children [Essai comparatif de l'OKT3 et du serum antilymphocytaire dans le traitement preventif du rejet apres transplantation renale chez l'enfant]. Annales de Pediatrie 1990;37(2):83‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Norman 1988 {published data only}
- Norman DJ, Shield CF 3rd, Barry J, Bennett WM, Henell K, Kimball J, et al. Early use of OKT3 monoclonal antibody in renal transplantation to prevent rejection. American Journal of Kidney Diseases 1988;11(2):107‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Norman 1993 {published data only}
- Abramowicz D, Norman DJ, Goldman M, Pauw L, Kinnaert P, Kahana L, et al. OKT3 prophylaxis improves long‐term renal graft survival in high‐risk patients as compared to cyclosporine: combined results from the prospective, randomized Belgian and US studies. Transplantation Proceedings 1995;27(1):852‐3. [MEDLINE: ] [PubMed] [Google Scholar]
- Abramowicz D, Norman DJ, Vereerstraeten P, Goldman M, Pauw L, Vanherweghem JL, et al. OKT3 prophylaxis in renal grafts with prolonged cold ischemia times: association with improvement in long‐term survival. Kidney International 1996;49(3):768‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Norman DJ, Kahana L, Stuart FP Jr, Thistlethwaite JR Jr, Shield CF 3rd, Monaco A, et al. A randomized clinical trial of induction therapy with OKT3 in kidney transplantation. Transplantation 1993;55(1):44‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shield CF 3rd, Jacobs RJ, Wyant S, Das A. A cost‐effectiveness analysis of OKT3 induction therapy in cadaveric kidney transplantation. American Journal of Kidney Diseases 1996;27(6):855‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Norman 1993a {published data only}
- Norman DJ, Kimball JA, Barry JM. Cytokine‐release syndrome: differences between high and low doses of OKT3. Transplantation Proceedings 1993;25(2 Suppl 1):35‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Norman DJ, Kimball JA, Bennett WM, Shihab F, Batiuk TD, Meyer MM, et al. A prospective, double‐blind, randomized study of high‐versus low‐dose OKT3 induction immunosuppression in cadaveric renal transplantation. Transplant International 1994;7(5):356‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Norrby 1997 {published data only}
- Norrby J, Olausson M. A randomized clinical trial using ATG Fresenius or ATG Merieux as induction therapy in kidney transplantation. Transplantation Proceedings 1997;29(7):3135‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Novick 1983 {published data only}
- Novick AC, Braun WE, Steinmuller D, Buszta C, Greenstreet R, Kiser W. A controlled randomized double‐blind study of antilymphoblast globulin in cadaver renal transplantation. Transplantation 1983;35(2):175‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Perez‐Tamajon 1996 {published data only}
- Perez‐Tamajon L, González JM, Torres A, Rodriguez A, Hernandez D, Losada M, et al. Induction treatment with sequential quadruple therapy in renal transplantation: polyclonal vs. monoclonal AC at low doses [abstract]. Nefrología 1996;16:97. [CENTRAL: CN‐00602023] [Google Scholar]
Pernin 2012 {published data only}
- Pernin V, Portales P, Szwarc I, Garrigue V, Vetromile F, Delmas S, et al. Lymphocyte reconstitution after induction with thymoglobulin in renal transplantation: Impact of the mode of administration (daily vs intermittent treatment based on T lymphocyte monitoring) [abstract no: SAP671]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii533‐4. [EMBASE: 70766907] [Google Scholar]
Raffaele 1991 {published data only}
- Raffaele P, Pouteil‐Noble C, Lefrancois N, Bosshard S, Betuel H, Aymard M, et al. Influence of a randomized monoclonal or polyclonal program of therapy on cytomegalovirus infection in kidney transplantation. Transplantation Proceedings 1991;23(1 Pt 2):1361‐2. [MEDLINE: ] [PubMed] [Google Scholar]
Rostaing 2010 {published data only}
- Rostaing L, Lavayssiere L, Kamar N. Hematologic adverse effects of 2 different polyclonal antilymphocyte preparations in de novo kidney transplant patients. Experimental & Clinical Transplantation 2010;8(2):178‐80. [MEDLINE: ] [PubMed] [Google Scholar]
Sakhrani 1992 {published data only}
- Sakhrani L, Aswad S, Obispo E, Mendez RG, Khetan U, Asai P, et al. Optimal dose of Minnesota antilymphocyte globulin for induction immunosuppression in cadaveric renal transplants. Transplantation Proceedings 1992;24(5):1730‐1. [MEDLINE: ] [PubMed] [Google Scholar]
Samsel 1999 {published data only}
- Samsel R, Chmura A, Korczak‐Kowalska G, Wlodarczyk Z, Pliszczynski J, Adadynski L, et al. Long term results of single perioperative high dose of ATG as induction in kidney transplantation [abstract]. American Journal of Transplantation 2004;4(Suppl 8):351. [CENTRAL: CN‐00509456] [Google Scholar]
- Samsel R, Chmura A, Wlodarczyk Z, Wyzgal J, Cieciura T, Lagiewska B, et al. Perioperative single high dose ATG‐Fresenius S administration as induction immunosuppressive therapy in cadaveric renal transplantation‐‐preliminary results. Annals of Transplantation 1999;4(2):37‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Samsel R, Pliszczynski J, Chmura A, Korczak G, Wlodarczyk Z, Cieciura T, et al. Safety and efficacy of high dose ATG bolus administration on rewascularization in kidney graft patients‐‐long term results. Annals of Transplantation 2008;13(1):32‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Samsel R, Rowinski W, Chmura A, Wlodarczyk Z, Wyzgal J, Cieciura T, et al. Perioperative administration of single, high‐dose of ATG‐Fresenius‐S as an induction immunosuppressive therapy in cadaveric renal transplantation: preliminary results. Transplantation Proceedings 2001;33(6):2952‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sansom 1976 {published data only}
- Sansom JR, Barnes AD, Hall CL. A randomized prospective clinical trial of antilymphocyte globulin in 100 cadaveric renal transplants. Postgraduate Medical Journal 1976;52(5 Suppl):75‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Sharaf El Din 2006 {published data only}
- Sharaf EI Din UA, Sharaf EI Din BA, EI Damanhoury HA. Alemtuzumab induction as steroid sparing agent in living kidney transplantation: a randomized prospective controlled trial [abstract no: SA‐PO449]. Journal of the American Society of Nephrology 2006;17(Abstracts):670A. [CENTRAL: CN‐00644281] [Google Scholar]
Sheashaa 2008 {published data only}
- Sheashaa HA, Hamdy AF, Bakr MA, Abdelbaset SF, Ghoneim MA. Long‐term evaluation of single bolus high dose ATG induction therapy for prophylaxis of rejection in live donor kidney transplantation. International Urology & Nephrology 2008;40(2):515‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shield 1993 {published data only}
- Shield CF 3rd, Beilman G. Safety of OKT3 use in the operating room. Transplantation Proceedings 1993;25(2 Suppl 1):43‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Slakey 1993 {published data only}
- Johnson CP, Slakey DP, Callaluce RD, Browne BJ, Roza AM, Adams MB. Prospective randomized comparison of quadruple vs triple therapy for first cadaver transplants with immediate function. Transplantation Proceedings 1993;25(1 Pt 1):585‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Slakey DP, Johnson CP, Callaluce RD, Browne BJ, Zhu YR, Roza AM, et al. A prospective randomized comparison of quadruple versus triple therapy for first cadaver transplants with immediate function. Transplantation 1993;56(4):827‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smeekens 2013 {published data only}
- Joosten I, Baas MC, Kamburova EG, Hoogen MW, Koenen HJ, Hilbrands LB. Anti‐B cell therapy with rituximab as induction therapy in renal transplantation. Transplant Immunology 2014;31(4):207‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kamburova E, Koenen H, Hoogen M, Joosten I, Hilbrands L. A single dose of rituximab results in a long lasting B‐cell depletion in peripheral blood, without affecting the peripheral T‐cell compartment [abstract no: 186]. American Journal of Transplantation 2013;13(Suppl S5):87. [EMBASE: 71056762] [Google Scholar]
- Kamburova EG, Koenen HJ, Hoogen MW, Baas MC, Joosten I, Hilbrands LB. Longitudinal analysis of T and B cell phenotype and function in renal transplant recipients with or without rituximab induction therapy. PLoS ONE [Electronic Resource] 2014;9(11):e112658. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamburova EG, Hoogen MW, Koenen HJ, Baas MC, Hilbrands LB, Joosten I. Cytokine release after treatment with rituximab in renal transplant recipients. Transplantation 2015;99(9):1907‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Smeekens SP, Hoogen MW, Kamburova EG, Veerdonk FL, Joosten I, Koenen HJ, et al. The effects of in vivo B‐cell depleting therapy on ex‐vivo cytokine production. Transplant Immunology 2013;28(4):183‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hoogen M, Kamburova E, Baas M, Steenbergen E, Florquin S, Koenen H, et al. Type of rejection and biopsy findings after induction therapy with a single dose of rituximab [abstract]. Transplantation 2014;98(Suppl 1):463. [EMBASE: 71545077] [Google Scholar]
- Hoogen M, Steenbergen E, Hoitsma A, Hilbrands L. Placebo‐controlled trial [abstract no: 266.1]. American Journal of Transplantation 2013;13(Suppl S5):112‐3. [EMBASE: 71056843] [Google Scholar]
- Hoogen MW, Kamburova EG, Baas MC, Steenbergen EJ, Florquin S, Koenen H, et al. Rituximab as induction therapy after renal transplantation: a randomized, double‐blind, placebo‐controlled study of efficacy and safety. American Journal of Transplantation 2015;15(2):407‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Spillner 1998 {published data only}
- Spillner J, Kohnle M, Albrecht KH, Heemann U. Anti‐LFA‐1 monoclonal antibody in renal transplantation: renal function, infections, and other complications. Transplantation Proceedings 1998;30(5):2163. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Squifflet 1997 {published data only}
- Squifflet JP, Besse T, Malaise J, Mourad M, Delcorde C, Hope JA, et al. BTI‐322 for induction therapy after renal transplantation: a randomized study. Transplantation Proceedings 1997;29(1‐2):317‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Squifflet JP, Besse T, Mourad M, Malaise J, Delcorde C, Pirson Y, et al. A randomized study of BTI‐322 for the prevention of renal allograft rejection: one year follow up [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:258. [CENTRAL: CN‐00509490]
Steinmuller 1991 {published data only}
- Steinmuller DR, Hayes J, Novick AC, Streem SB, Hodge E, Slavis S, et al. Comparison of OKT3 to ALG for prophylaxis for patients with acute renal failure after cadaver renal transplantation [abstract]. 11th International Congress of Nephrology; 1990 Jul 15‐20; Tokyo, Japan. 1990:512A. [CENTRAL: CN‐00716029] [DOI] [PubMed]
- Steinmuller DR, Hayes JM, Novick AC, Streem SB, Hodge E, Slavis S, et al. Comparison of OKT3 with ALG for prophylaxis for patients with acute renal failure after cadaveric renal transplantation. Transplantation 1991;52(1):67‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stevens 2008 {published data only}
- Stevens R, Foster K, Miles C, Rigley T, Kalil A, Wrenshall L. A randomized 2X2 factorial trial: Part 1, single‐dose rATG induction improves long‐term renal transplant outcomes [abstract]. Transplantation 2014;98(Suppl 1):91. [EMBASE: 71543822] [Google Scholar]
- Stevens R, Foster K, Miles C, Rigley T, Wrenshall L. A randomized trial of renal transplantation: Steroid and calcineurin‐inhibitor withdrawal after rabbit anti‐thymocyte globulin induction [abstract]. Transplantation 2014;98(Suppl 1):476. [EMBASE: 71545124] [Google Scholar]
- Stevens RB. Modern approaches to combining sirolimus with calcineurin inhibitors. Transplantation Proceedings 2008;40(10 Suppl):S21‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stevens RB, Foster KW, Miles CD, Kalil AC, Florescu DF, Sandoz JP, et al. A randomized 2x2 factorial clinical trial of renal transplantation: steroid‐free maintenance immunosuppression with calcineurin inhibitor withdrawal after six months associates with improved renal function and reduced chronic histopathology. PLoS ONE [Electronic Resource] 2015;10(10):e0139247. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RB, Foster KW, Miles CD, Lane JT, Kalil AC, Florescu DF, et al. A randomized 2x2 factorial trial, part 1: single‐dose rabbit antithymocyte globulin induction may improve renal transplantation outcomes. Transplantation 2015;99(1):197‐209. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RB, Kalil AC, Miles CD, Florescu DF, Lane JT, Skorupa AJ, et al. Reduced patient mortality and infectious complications, and superior graft function, in renal transplant patients after single‐dose RATG induction; 5‐year data from a prospective, randomized trial [abstract no: 38]. American Journal of Transplantation 2010;10(Suppl 4):49‐50. [EMBASE: 70463399] [Google Scholar]
- Stevens RB, Lane JT, Boerner BP, Miles CD, Rigley TH, Sandoz JP, et al. Single‐dose rATG induction at renal transplantation: superior renal function and glucoregulation with less hypomagnesemia. Clinical Transplantation 2012;26(1):123‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stevens RB, Lane JT, Boerner BP, Miles CD, Rigley TH, Sandoz JP, et al. Single‐dose rabbit anti‐thymocyte globulin (rATG) induction reduces tubular injury after renal transplantation, resulting in superior renal function, serum Mg++ retention, and glucose regulation [abstract no: 1703]. American Journal of Transplantation 2010;10(Suppl 4):522. [EMBASE: 70465078] [Google Scholar]
- Stevens RB, Mercer D, Rigley T, Nielsen K, Henning M, Skorupa A, et al. Single‐dose induction with rabbit anti‐thymocyte globulin (rATG) safely improves renal allograft function and reduces chronic allograft nephropathy [abstract no: 538]. American Journal of Transplantation 2008;8(Suppl 2):322. [CENTRAL: CN‐00653788] [Google Scholar]
- Stevens RB, Mercer DF, Grant WJ, Freifeld AG, Lane JT, Groggel GC, et al. Randomized trial of single‐dose versus divided‐dose rabbit anti‐thymocyte globulin induction in renal transplantation: an interim report. Transplantation 2008;85(10):1391‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stevens RB, Rigley T, Skorupa J, Nielsen K, Wrenshall L. Successful calcineurin‐inhibitor (CI) discontinuation following early steroid withdrawal (ESW) in renal allograft recipients: reduced chronic allograft nephropathy (CAN) and improved renal function without increased rejection or graft loss [abstract no: 2123]. Transplantation 2008;86(2S):695. [Google Scholar]
- Stevens RB, Rigley TH, Nielsen KJ, Skorupa A, Sandoz J, Kellogg A, et al. Single‐dose induction with rabbit anti‐thymocyte globulin (rATG; 6 mg/kg over 24 hours) is safer and improves immediate and long‐term renal allograft function [abstract no: 616]. American Journal of Transplantation 2009;9(Suppl 2):369. [Google Scholar]
- Stevens RB, Rigley TH, Nielsen KJ, Skorupa JY, Skorupa A, Sandoz J, et al. Calcineurin‐inhibitor (CI) discontinuation following early steroid withdrawal (ESW) in renal allograft recipients without increase rejection or graft loss [abstract no: 1100]. American Journal of Transplantation 2009;9(Suppl 2):501. [Google Scholar]
- Stevens RB, Skorupa JY, Rigley TH, Sandoz JP, Kellogg A, Miller N, et al. Calcineurin‐inhibitor withdrawal vs minimization after kidney transplantation is safe but does not improve renal function; 5‐year results of a prospective, randomized trial [abstract no: 1643]. American Journal of Transplantation 2010;10(Suppl 4):505. [Google Scholar]
- Stevens RB, Wrenshall L, Mercer D, Rigley T, Nielsen K, Henning M, et al. Successful calcineurin‐inhibitor (CI) discontinuation following early steroid withdrawal (ESW) in renal allograft recipients; reduced chronic allograft nephropathy (CAN) and improved renal function without increased rejection or graft loss [abstract no: 289]. American Journal of Transplantation 2008;8(Suppl 2):255. [CENTRAL: CN‐00653790] [Google Scholar]
Taylor 1976 {published data only}
- Ackman CF, Taylor HE, Schual RS. Long‐term follow up of Canadian clinical trial of antilymphocyte globulin in renal transplantation [abstract]. Kidney International 1979;15(6):709. [CENTRAL: CN‐00444100] [Google Scholar]
- MacDonald AS, Belitsky P, Lannon SG, Cohen A, White J. Antithymocyte globulin in cadaver renal grafts: prophylaxis and antirejection therapy with three different preparations. Transplantation Proceedings 1982;14(4):631‐4. [MEDLINE: ] [PubMed] [Google Scholar]
- Taylor HE, Ackman CF, Horowitz I. Canadian clinical trial of antilymphocyte globulin in human cadaver renal transplantation. Canadian Medical Association Journal 1976;115(12):1205‐8. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Thibaudin 1998 {published data only}
- Thibaudin D, Alamartine E, Filippis JP, Diab N, Laurent B, Berthoux F. Advantage of antithymocyte globulin induction in sensitized kidney recipients: a randomized prospective study comparing induction with and without antithymocyte globulin. Nephrology Dialysis Transplantation 1998;13(3):711‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thomas 1977 {published data only}
- Thomas F, Mendez‐Picon G, Thomas J, Peace K, Flora R, Lee HM. Effect of antilyphocyte‐globulin potency on survival of cadaver renal transplants. Prospective randomised double‐blind trial. Lancet 1977;2(8040):671‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thomas 2007 {published data only}
- Thomas PG, Woodside KJ, Lappin JA, Vaidya S, Rajaraman S, Gugliuzza KK. Alemtuzumab (Campath 1H) induction with tacrolimus monotherapy is safe for high immunological risk Renal transplantation. Transplantation 2007;83(11):1509‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Toledo‐Pereyra 1985 {published data only}
- Toledo‐Pereyra LH, Bergren C, Mittal VK, Whitten JI, Baskin S, McNichol L. A prospective randomized comparison of antilymphoblast globulin versus antithymocyte globulin for cadaver kidney transplantation. Transplantation 1985;40(4):448‐50. [MEDLINE: ] [PubMed] [Google Scholar]
- Toledo‐Pereyra LH, Bergren C, Whitten J. Comparison of ALG and ATG for renal transplantation [abstract]. Kidney International 1985;28(2):388. [CENTRAL: CN‐00583280] [Google Scholar]
TRIMS Study 2010 {published data only}
- Woodle ES, Peddi VR, Tomlanovich S, Mulgaonkar S, Kuo PC, TRIMS Study Investigators. A prospective, randomized, multicenter study evaluating early corticosteroid withdrawal with thymoglobulin in living‐donor kidney transplantation. Clinical Transplantation 2010;24(1):73‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Woodle ES, TRIMS Study Group. A randomized, prospective, multicenter comparative study evaluating a thymoglobulin‐based early corticosteroid cessation regime in renal transplantation [abstract no: 673]. American Journal of Transplantation 2006;6(Suppl 2):294. [CENTRAL: CN‐00716028] [Google Scholar]
- Woodle ES, TRIMS Study Group. A randomized, prospective, multicenter study of thymoglobulin in renal transplantation for induction and minimization of steroids (TRIMS) [abstract no: 1632]. American Journal of Transplantation 2005;5(Suppl 11):571. [CENTRAL: CN‐00716027] [Google Scholar]
Tsai 2012 {published data only}
- Tsai MK, Lee CY, Yang CY, Yeh CC, Hu RH, Lee PH. Rituximab induction therapy provided additional immunosuppressive effect and functional benefit to non‐sensitized renal transplant recipients: an interim report [abstract no: 997]. American Journal of Transplantation 2012;12(Suppl S3):319. [EMBASE: 70746950] [Google Scholar]
Turcotte 1973 {published data only}
- Turcotte JG, Feduska NJ, Haines RF, Freier DT, Gikas PW, McDonald FD, et al. Antithymocyte globulin in renal transplant recipients. A clinical trial. Archives of Surgery 1973;106(4):484‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tyden 2009 {published data only}
- Tyden G, Genberg H, Tollemar J, Ekberg H, Persson NH, Tufveson G, et al. A randomized, doubleblind, placebo‐controlled, study of single‐dose rituximab as induction in renal transplantation. Transplantation 2009;87(9):1325‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tyden G, Mjornstedt L, Ekberg H, Tufveson G. A prospective, randomised, placebo controlled, multicenter study of the efficacy and safety of rituximab as induction therapy together with tacrolimus, mycophenolate mofetil and steroids in renal transplantation [abstract no: 861]. Transplantation 2008;86(2S):300. [CENTRAL: CN‐00763744] [Google Scholar]
- Tyden G, Mjornstedt L, Ekberg H, Tufveson G. Is rituximab safe to use in kidney transplant patients?. American Journal of Transplantation 2010;10(8):1949. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tydén G, Ekberg H, Tufveson G, Mjörnstedt L. A randomized, double‐blind, placebo‐controlled study of single dose rituximab as induction in renal transplantation: a 3‐year follow‐up. Transplantation. 2012;94(3):e21‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van den Hoogen 2013 {published data only}
- Hoogen MW, Kho MM, Abrahams AC, Zuilen AD, Sanders JS, Dijk M, et al. Effect of a single intraoperative high‐dose ATG‐Fresenius on delayed graft function in donation after cardiac‐death donor renal allograft recipients: a randomized study. Experimental & Clinical Transplantation 2013;11(2):134‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vela 1994 {published data only}
- Vela C, Cristol JP, Chong G, Okamba A, Lorho R, Mion C, et al. Antilymphocyte globulins versus OKT3 as prophylactic treatment in highly sensitized renal transplant recipients. Transplant International 1994;7 Suppl 1:S259‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vigeral 1986 {published data only}
- Vigeral P, Chkoff N, Chatenoud L, Campos H, Lacombe M, Droz D, et al. Prophylactic use of OKT3 monoclonal antibody in cadaver kidney recipients. Utilization of OKT3 as the sole immunosuppressive agent. Transplantation 1986;41(6):730‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wechter 1979 {published data only}
- Wechter WJ, Morrell RM, Bergan J, Rosenberg JC, Turcotte J, Schultz JR. Extended treatment with antithymocyte globulin (ATGAM) in renal allograft recipients. Transplantation 1979;28(5):365‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yussim 2000 {published data only}
- Yussim A, Shapira Z. Single‐bolus high‐dose ATG for prophylaxis of rejection in renal transplantation‐‐a prospective, randomized study. Transplant International 2000;13 Suppl 1:S293‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alloway 1993 {published data only}
- Alloway R, Kotb M, Hathaway D, Ohman M, Strain S, Gaber AO. The pharmacokinetic profile of standard and low‐dose OKT3 induction immunosuppression in renal transplant recipients. Transplantation 1994;58(2):249‐53. [MEDLINE: ] [PubMed] [Google Scholar]
- Alloway R, Kotb M, Hathaway DK, Gaber LW, Vera SR, Gaber AO. Randomized double‐blind study of standard versus low‐dose OKT3 induction therapy in renal allograft recipients. American Journal of Kidney Diseases 1993;22(1):36‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Alloway R, Kotb M, Hathaway DK, Gaber LW, Vera SR, Gaber AO. Results of a prospective, randomized double‐blind study comparing standard vs low‐dose OKT3 induction therapy. Transplantation Proceedings 1993;25(1 Pt 1):550‐2. [MEDLINE: ] [PubMed] [Google Scholar]
- Chaballa M, Alloway RR, Kotb M, Hathaway DK, Gaber L, Vera SR, et al. Five year follow up of prospective, randomized double‐blind study comparing standard versus low dose OKT3 induction therapy in renal allograft recipients [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:259. [CENTRAL: CN‐00509127]
Kirsch 2006 {published data only}
- Kirsch BM, Haidinger M, Zeyda M, Bohmig GA, Tombinsky J, Muhlbacher F, et al. Alemtuzumab (Campath‐1H) induction therapy and dendritic cells: impact on peripheral dendritic cell repertoire in renal allograft recipients. Transplant Immunology 2006;16(3‐4):254‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kumar 2002b {published data only}
- Kumar A, Zaman W, Chaurasia D, Gupta A, Sharma RK, Gulati S. Prospective randomized trial to evaluate the efficacy of single low dose ATG induction in renal transplant recipient with spousal kidney. Indian Journal of Urology 2002;19(1):58‐62. [EMBASE: 36347895] [Google Scholar]
NCT00000936 {unpublished data only}
- Blechman‐Krom I. Controlled trial of induction therapy in renal transplantation. www.clinicaltrials.gov/ct2/show/NCT00000936 (accessed 3 November 2016).
NCT01312064 {unpublished data only}
- Tsai MK. Clinical outcome of de novo everolimus‐based immunosuppressive therapy for renal transplantation using rituximab induction. www.clinicaltrials.gov/ct2/show/NCT01312064 (accessed 22 August 2016).
References to studies awaiting assessment
NCT00089947 {published data only}
- NCT00089947. Randomized, prospective, phase 2 study comparing thymoglobulin in a rapid discontinuation of corticosteroids protocol with standard corticosteroid therapy in living donor renal transplantation using mycophenolate mofetil and tacrolimus maintenance therapy. clinicaltrials.gov/ct2/show/NCT00089947 (accessed 22 August 2016).
NCT00861536 {published data only}
- Steiger J. An open, multicenter, randomised, parallel group pilot study to investigate two different polyclonal rabbit immunoglobulin preparations for safety and efficacy: a comparison of ATG‐fresenius to thymoglobulin in prophylaxis for immunological high risk patients following renal transplantation. www.clinicaltrials gov/ct2/show/NCT00861536 (accessed 22 August 2016).
NCT01046955 {unpublished data only}
- Burke GW. Head‐to‐head comparison of thymoglobulin vs. campath‐1h vs. our standard center treatment protocol in living donor renal transplantation ‐ a study to evaluate the avoidance of long‐term nephrotoxic calcineurin inhibitor therapy. www.clinicaltrials.gov/ct2/show/NCT01046955 (accessed 3 November 2016).
NCT01354301 {unpublished data only}
- Tedesco H. Efficacy and safety of induction strategies combined with low tacrolimus exposure in kidney transplant recipients receiving everolimus or sodium mycophenolate. www.clinicaltrials.gov/ct2/show/NCT01354301 (accessed 3 November 2016).
Stevens 2016 {published data only}
- Stevens RB, Wrenshall LE, Miles CD, Farney AC, Jie T, Sandoz JP, et al. A double‐blind, double‐dummy, flexible‐design randomized multicenter trial: early safety of single‐ versus divided‐dose rabbit anti‐thymocyte globulin induction in renal transplantation. American Journal of Transplantation 2016;16(6):1858‐67. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT00733733 {published data only}
- Hoitsma AJ. A prospective, randomized, open, multicenter study to evaluate the efficacy and tolerability of induction therapy with a single high‐dose anti‐t‐lymphocyte globulin (ATG) in renal transplant patients with a kidney from a non‐heart‐beating donor and tacrolimus, mycophenolate mofetil, and steroids as basic immunosuppression. www.clinicaltrials.gov/ct2/show/NCT00733733 (accessed 22 August 2016).
NCT01154387 {unpublished data only}
- Flechner S. A two part, phase 1/2, safety, PK and PD study of TOL101, an anti‐TCR monoclonal antibody for prophylaxis of acute organ rejection in patients receiving renal transplantation. www.clinicaltrials.gov/ct2/show/NCT01154387 (accessed 22 August 2016).
ReMIND Study 2013 {unpublished data only}
- Mamode M. A randomized trial of rituximab in induction therapy for living donor renal transplantation. www.clinicaltrials.gov/ct2/show/NCT01095172 (accessed 22 August 2016).
Additional references
ANZDATA 2009
- ANZDATA. [direct communication]. Australian and New Zealand Dialysis and Transplant Registry 2009.
Calne 1999
- Calne R, Moffatt SD, Friend PJ, Jamieson NV, Bradley JA, Hale G, et al. Campath IH allows low‐dose cyclosporine monotherapy in 31 cadaveric renal allograft recipients. Transplantation 1999;68(10):613‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cibrik 2001
- Cibrik DM, Kaplan B, Meier‐Kriesche HU. Role of anti‐interleukin‐2 receptor antibodies in kidney transplantation. Biodrugs 2001;15(10):655‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Denton 1999
- Denton MD, Magee CC, Sayegh MH. Immunosuppressive strategies in transplantation. Lancet 1999;353(9158):1083‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated February 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hong 2000
- Hong JC, Kahan BD. Sirolimus‐induced thrombocytopenia and leukopenia in renal transplant recipients: risk factors, incidence, progression, and management. Transplantation 2000;69(10):2085‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDIGO 2009
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. American Journal of Transplantation 2009;9 Suppl 3:S1‐155. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kreis 1992
- Kreis H. Antilymphocyte globulins in kidney transplantation. Kidney International ‐ Supplement 1992;38:188‐92. [MEDLINE: ] [PubMed] [Google Scholar]
Meier‐Kriesche 2004
- Meier‐Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. American Journal of Transplantation 2004;4(3):378‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Racusen 1999
- Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney international 1999;55(2):713‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Solez 2007
Soulillou 2001
- Soulillou JP, Giral M. Controlling the incidence of infection and malignancy by modifying immunosuppression. Transplantation 2001;72(12 Suppl):S89‐93. [MEDLINE: ] [PubMed] [Google Scholar]
Tonelli 2011
- Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. American Journal of Transplantation 2011;11(10):2093‐109. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tyden 2003
- Tyden G, Kumlien G, Fehrman I. Successful ABO‐incompatible kidney transplantations without splenectomy using antigen specific immunoadsorption and rituximab. Transplantation 2003;76(4):730‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
UNOS 2011
- United Network for Organ Sharing. OPTN data [direct communication]. www.unos.org (accessed 4 November 2016).
Webster 2006
- Webster A, Pankhurst T, Rinaldi F, Chapman JR, Craig JC. Polyclonal and monoclonal antibodies for treating acute rejection episodes in kidney transplant recipients. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004756.pub3] [DOI] [PubMed] [Google Scholar]
Webster 2010
- Webster AC, Ruster LP, McGee R, Matheson SL, Higgins GY, Willis NS, et al. Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003897.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zand 2007
- Zand MS. Therapeutic antibody agents for B‐cell immunomodulation in renal transplantation. Transplantation 2007;84(11 S Suppl):S11‐9. [EMBASE: 350294707] [Google Scholar]
References to other published versions of this review
Morgan 2004
- Morgan P, Cross NB, Barnett AN, Craig JC, Webster AC. Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD004759] [DOI] [PMC free article] [PubMed] [Google Scholar]


